- 1Department of Psychiatry, University of Oxford, Oxford, United Kingdom
- 2Pediatric University Hospital-Department, Bambino Gesù Children's Hospital, Rome, Italy
- 3Department of Epidemiology, Lazio Regional Health Service, Rome, Italy
- 4Department of Pediatrics, School of Medicine, University of Colorado Anschutz Medical Campus, JFK, Aurora, CO, United States
- 5Brain and Body Integration - Mental Health Clinic, Denver, CO, United States
- 6Department of Biomedicine and Prevention, University of Rome Tor Vergata, Rome, Italy
- 7Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, L'Aquila, Italy
- 8Child Neurology and Psychiatry Unit, System Medicine Department, University of Rome Tor Vergata, Rome, Italy
- 9Developmental Imaging and Psychopathology Lab, Department of Psychiatry, School of Medicine, University of Geneva, Geneva, Switzerland
Background: Recent studies have examined the association between autism spectrum disorder and schizophrenia spectrum disorders, describing a number of cognitive features common to both conditions (e.g., weak central coherence, difficulties in set-shifting, impairment in theory of mind). Several studies have reported high levels of autistic symptoms in population with schizophrenia spectrum disorders. Our study systematically reviews and quantitatively synthetizes the current evidence on the presence of autistic symptoms in individuals with schizophrenia spectrum disorders.
Methods: A comprehensive literature search of the PubMed/MEDLINE, Cochrane Library, CINHAL, and Embase databases was performed from the date of their inceptions until March 2018. The primary outcome measure was the Autism Spectrum Quotient (AQ). As secondary outcome measures, we analyzed the AQ subscales. Data were extracted and analyzed by using a conservative model and expressed by standardized mean difference (SMD).
Results: Thirteen studies comprising a total of 1,958 individuals were included in the analysis. Results showed that individuals with schizophrenia spectrum disorders have higher levels of autistic symptoms compared to healthy controls [SMD: 1.39, 95% confidence interval (CI): 1.11 to 1.68] and lower levels of autistic symptoms compared to individuals with autism (SMD: −1.27, 95% CI: −1.77 to −0.76).
Conclusions: Current findings support that individuals with schizophrenia spectrum disorders have higher autistic symptoms than healthy controls. Therefore, further studies are needed in order to shed light on the association between these two conditions.
Introduction
According to the current diagnostic classification systems, Schizophrenia Spectrum Disorders (SSDs) include schizophrenia, schizophreniform disorder, and other psychotic disorders (1). These disorders are defined by abnormalities in one or more of the following five domains: delusions, hallucinations, disorganized thinking (speech), grossly disorganized or abnormal motor behavior (including catatonia), and negative symptoms. The onset of schizophrenia spectrum disorders is usually between late teens and mid-30s. Onset prior to adolescence is rare (2).
By contrast, Autism Spectrum Disorder (ASD) is an early onset lifelong condition characterized by persistent deficits in social communication, as well as restricted and repetitive patterns of behavior (3). Until a century ago, autism was considered as an early expression of schizophrenia. Later on, different studies have demonstrated that these conditions are separate (3–5). Despite their differences, SSDs and ASD appear to show several similar symptoms and research into the possible link between these disorders has grown considerably with studies showing shared genetic risk factors as well as potential links in specific clinical characteristics of these two disorders [for a systematic review see Kincaid et al. (6)]. Recent studies have shown a genetic overlap between ASD and SSDs (7). For example, studies assessing copy number variation (CNV) in ASD and SSDs have repeatedly observed heterozygous deletions eliminating exons of the neurexin-1α gene (but not the neurexin-1β gene) in patients with ASD and SSDs (8–10). Furthermore, several studies have reported high number of shared CNV deletions and duplications, including 1q21, 15q11.2, 15q13.3, 16p11.2, 22q12, and Neurexin 1 loci, in ASD and SSDs (8, 11).
Moreover, an overlap between early autistic symptoms and psychotic experiences during adolescence was reported in longitudinal studies reporting that 20–50% of individuals with childhood-onset schizophrenia met criteria for premorbid ASD (12–16). In addition, social difficulties and language impairment are common to both conditions. Specifically, deficits in reciprocal social interactions are considered part of the core clinical symptoms of ASD. In fact, individuals with ASD exhibit deficits in eye contact, non-verbal communication (e.g., descriptive, conventional, and emphatic gestures), and difficulties to develop age-appropriate relationships (17). Similarly, social withdrawal is documented in individuals with SSDs. Indeed, impairment in social functioning common to both conditions may be due to underlying mechanisms (e.g., deficits in theory of mind) that are common to both conditions (18–24). Studies investigating social functioning deficits in these conditions have shown contrasting findings. For example, Couture et al. (23) completed a battery of social cognitive measures in 44 individuals with schizophrenia, 36 individuals with high functioning autism, and 41 non-clinical controls and reported that individuals with schizophrenia and individual with high functioning autism were both impaired on a variety of social cognitive tasks. By contrast, Sasson et al. (24) comparing the visual scanning patterns and emotion judgments of individuals with autism, individuals with schizophrenia, and controls, suggested that both individuals with autism and individuals with schizophrenia fixate faces less than controls. However, their results also found that only individuals with autism fail to orient to faces more rapidly based on the presence of facial information (24).
In clinical practice, distinguishing between these conditions has proved to be challenging given the symptom overlap between ASD and SSDs. For example, social communication deficits and restricted and repetitive behaviors typical of ASD can be misinterpreted as possible signs of a SSD (25). Some perceptions reported by individuals with ASD are misinterpreted as hallucinations (26). Deficits in emotion recognition leading to misinterpretations of the actions of others is a core symptom of ASD and is also common in SSDs (26). Moreover, difficulties with emotional reciprocity or speech delay in ASD can be misinterpreted as blunted affect or alogia (poverty of speech) in SSDs (26). Furthermore, catatonic features are present in both disorders (26, 27). Previous systematic reviews suggested elevated rates of co-occurrence of 28, ASD and SSDs (29). For example, Kincaid et al. (6) in a recent systematic review reported a prevalence rate of ASD that ranged from <1 to 52% across outpatient and inpatient populations with a diagnosis of schizophrenia or other psychotic disorders. However, considering ASD as dimensional disorder, separate from the issue of ASD diagnosis is the matter of autistic traits. Autistic traits refer to symptoms that are typical of ASD at the time of the assessment; however, these symptoms are generally not reported during childhood, which is essential for an ASD diagnosis (6).
Studying autistic symptoms in individuals with SSDs can give further insight to understand the overlap and distinction between these conditions, which can have important diagnostic and treatment implications (22). For example, previous studies reported that individuals with SSDs with autistic features had a longer duration of illness compared to individuals with SSDs without autistic symptoms (30, 31). Moreover, these studies also suggest that a longer duration of illness is associated with poorer long-term outcomes and a higher symptom severity in this clinical population (32). Therefore, an early screening of autistic symptoms in individuals with SSDs might be able to inform both psychological and pharmacological treatments, and possibly modify the clinical outcome in this clinical population (6). Kincaid et al. (6) systematic review reported prevalence rates of autistic symptoms across outpatient and inpatient populations with a diagnosis of schizophrenia or other psychotic disorders ranging from 9.6 to 61%. However, to our knowledge, no previous meta-analysis was conducted in order to quantify the presence of autistic symptoms in SSD populations. Therefore, the aim of this systematic review and meta-analysis was to systematically review and quantitatively synthetize the current evidence on the presence of autistic symptoms in individuals with SSDs compared to healthy controls and individuals with autism.
Methods
Literature Search
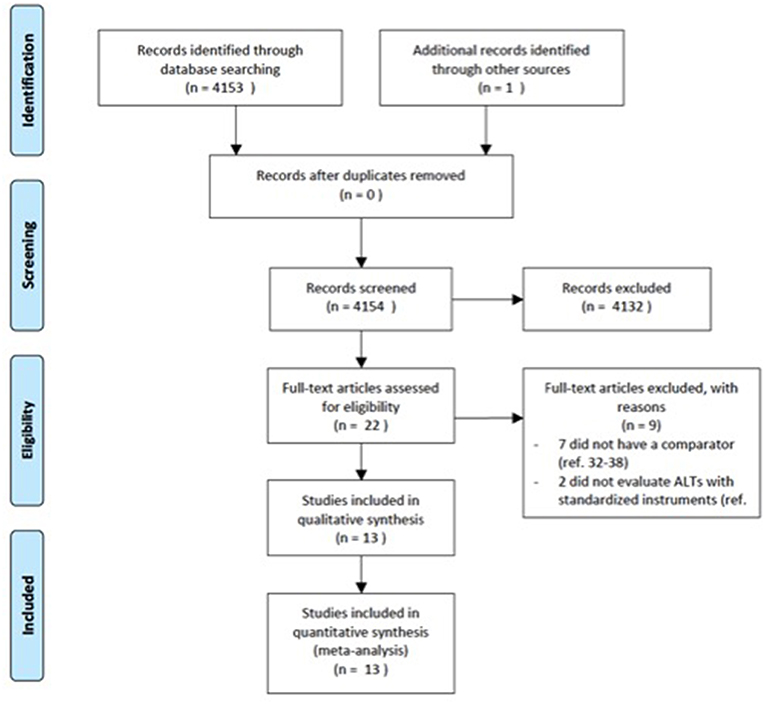
The electronic databases of PubMed, Medline, CINAHL, ISI web of knowledge were searched up from the date of their inceptions until March 2018. We used a search algorithm based on a combination of the terms: autist*, “autistic disorder,” “autism,” “child development disorders, pervasive,” “Asperger syndrome,” and “schizophrenia,” “schizophrenia spectrum and other psychotic disorders,” “schizophrenia, paranoid,” “schizophrenia, disorganized,” “schizophrenia, childhood,” “schizotypal personality disorder.” Reference lists of eligible papers were also screened for relevant studies. No language limit was used. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (33) (Figure 1).
Study Selection
All studies using recognized assessment scales to measure autistic symptoms in individuals with SSDs, compared to healthy controls or autism, were eligible for inclusion. We included study-defined diagnosis of first-episode psychosis or SSDs [i.e., schizophrenia, psychotic disorder not otherwise specified (NOS), schizoaffective disorder, schizophreniform disorder, delusional disorder]. The exclusion criteria were: (a) articles not within the field of interest of this review; (b) review articles, editorials, or letters, comments, conference proceedings; (c) case reports or case series; (d) studies dated before 1990 if the system used for the diagnosis of schizophrenia did not use operationalized criteria, but only disease names with no diagnostic criteria (i.e., ICD-9); (e) We excluded mood disorders with psychotic features (e.g., major depression with psychotic symptoms, bipolar disorder with psychotic symptoms). Two researchers (MS, VP) independently identified potential titles from all databases and screened the abstracts for relevance. Full-texts were then retrieved and read to determine eligibility. Disagreements were resolved by consensus.
Data Extraction
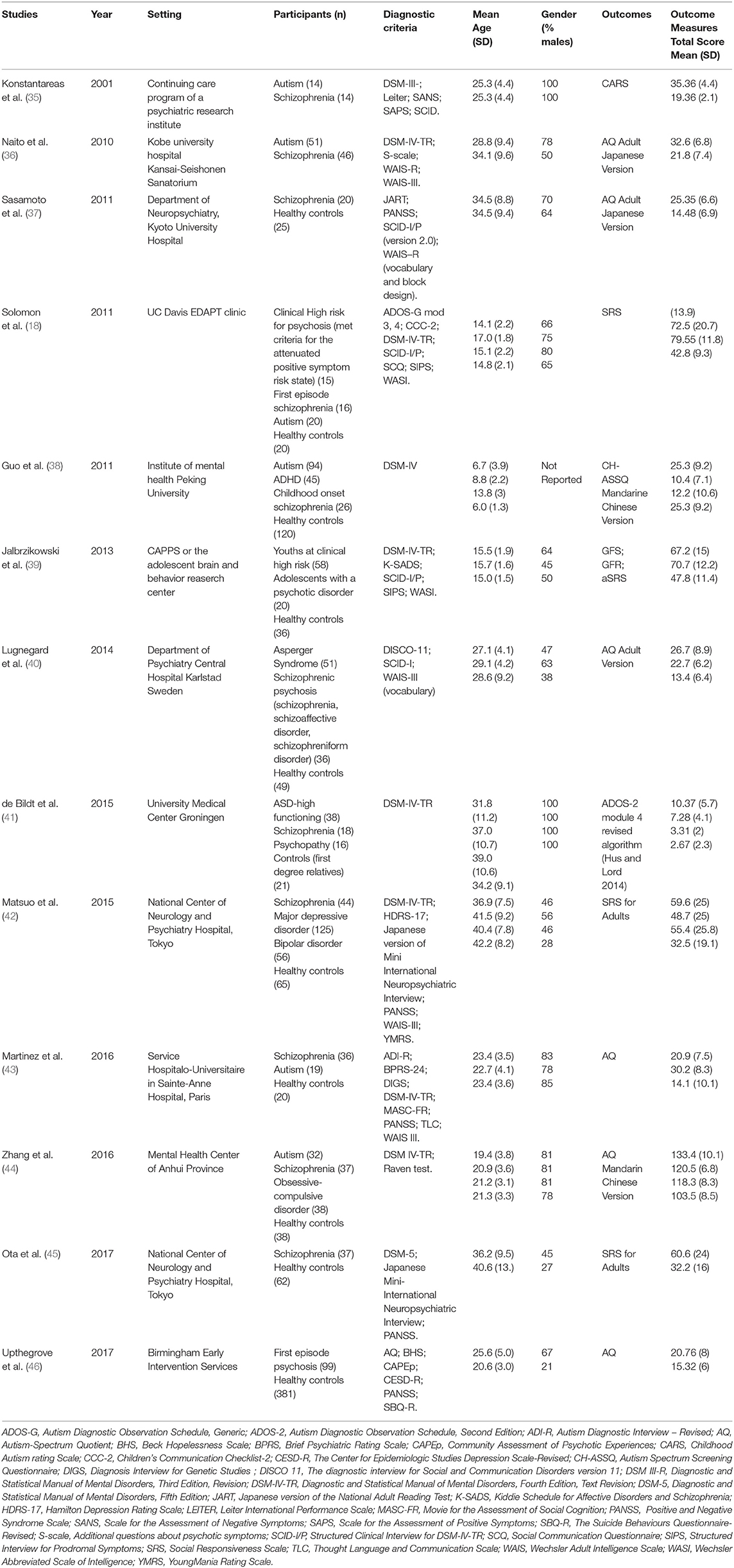
For each included study, the same two reviewers independently documented information about the publication (i.e., author's names, journal, year of publication, setting), patients' and comparison's characteristics (i.e., gender, age, diagnostic criteria, outcomes). We assessed the quality and potential sources of bias for each study by using the Newcastle Ottawa scale (NOS) (34) (Table 1).
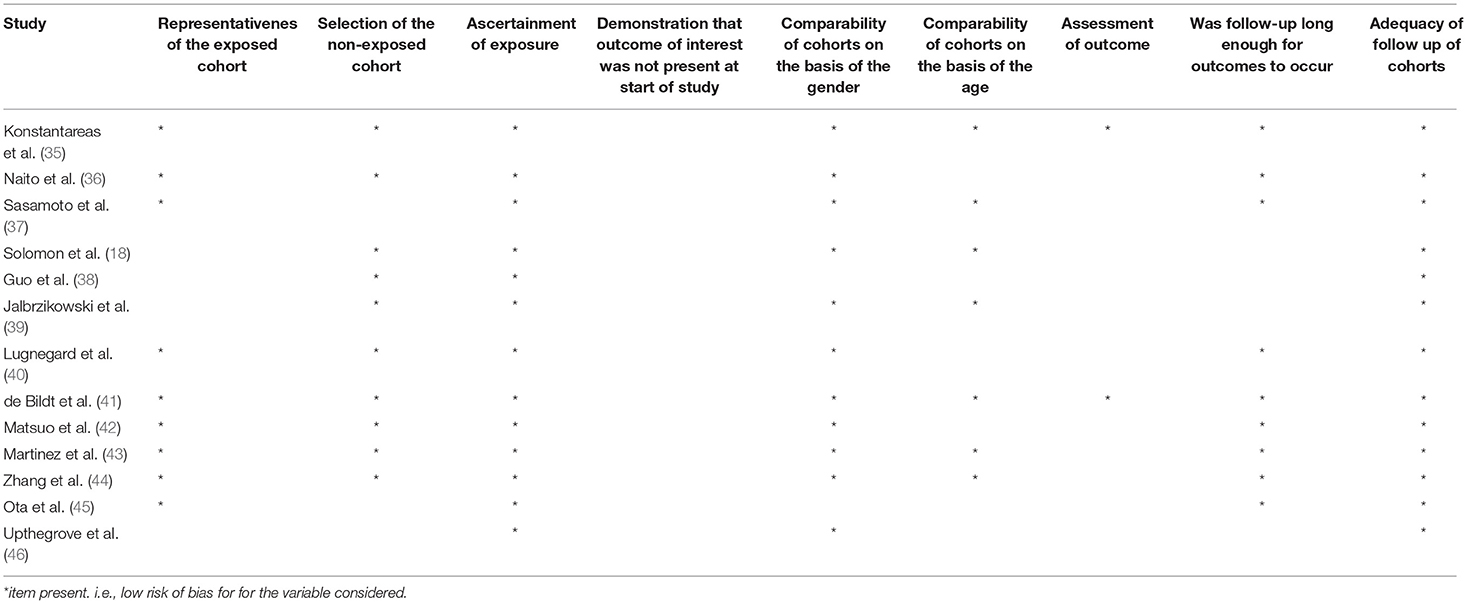
Table 1. Risk of bias table for assessing the quality of cohort studies by using the Newcastle-Ottawa Scale.
Outcome Measures
The Autism Questionnaire (AQ), expressed as continuous variable was used as the primary outcome measure. AQ subscales were used as secondary outcome measures. The AQ is a 50-item questionnaire consisting of five different areas: social skill, attention switching, attention to detail, communication, and imagination (47). Whenever the AQ was not measured or reported, the following scales measuring symptoms of autism were used: the Social Responsiveness Scale (SRS) (48), the Autism Diagnostic Observation Schedule-Second Edition (ADOS-2) (49, 50), the Autism Spectrum Screening Questionnaire (ASSQ) (51), and the Childhood Autism Rating Scale (CARS) (52, 53).
The SRS is a 65-item rating scale focusing on social awareness and avoidance (54, 55).
The ADOS-2 is a semi-structured, standardized assessment of communication, social interaction, play, and repetitive behaviors. The ADOS-2 includes five modules, The choice of the module is based on the level of expressive language and chronological age of the individual being evaluated (i.e., Module Toddler: from 12 to 30 months of age who do not consistently use phrase speech; Module 1: children 31 months and older who do not consistently use phrase speech; Module 2: children of any age who use phrase speech, but are not verbally fluent; Module 3: verbally fluent children or adolescents; Module 4: verbally fluent older adolescents and adults) (49, 50).
The ASSQ is a teacher's or parent's rating scale that investigates four main fields: social difficulties, tic/motor/obsessive-compulsive disorders, and autistic style (51).
The CARS is a clinician-rated observation scale evaluating socialization, communication, emotional responses, and sensory sensitivities. This scale has shown good reliability, a high degree of correlation with the Diagnostic and Statistical Manual of Mental Disorders (DSM)- Fourth Edition-Text Revised criteria, and a good agreement with diagnoses made using the ADOS-2 and the Autism Diagnostic Interview-Revised (ADI-R) (56).
Data Analysis
We synthesized study included in the current review consistently with meta-analytic recommendations (57). Data analysis were performed using STATA (version 13.1). data analysis involved the following steps: (a) calculating standardized mean difference (SMD) for each comparison with confidence intervals (CI) (95%); (b) determining an overall average SMD; (c) estimating heterogeneity. We considered SMD “small” if <0.40, “moderate” from 0.40 to 0.70, and “large” if >0.7 (58). The combined use of slightly different scales suggested application of the random effects model, which is more conservative than the fixed-effects model. Qualitative data were presented descriptively. The I2 statistic was used to assess the heterogeneity of effect sizes (57). The I2 statistic rages from 0 to 100 and measures the percent of variation across effect sizes due to heterogeneity compared to chance. A high I2 index indicates greater heterogeneity and greater variation in effect size across studies. We used I2 thresholds of 25, 50, and 75% to differentiate low, moderate and high heterogeneity. We also undertook subgroup analyses for studies on adults, = studies on children, studies on adolescents, studies including only patients diagnosed with schizophrenia, studies including also individuals diagnosed with SSDs, and studies including also individuals with an unspecified psychotic episode.
Results
Selected Studies
The literature search generated 4,153 articles. 4,131 articles were excluded due to the fact that they did not meet the inclusion criteria. Twenty-two articles were screened for eligibility by full-text review. Of these, seven did not have a comparator group (32, 59–64) and two did not evaluate autistic symptoms with standardized instruments (65, 66). A total of 13 studies comprising 1,958 individuals were included in the current meta-analysis.
Study Characteristics
The characteristics of the included studies are presented in Table 2. The study population mean age was 25.4 years. Three studies included only children and adolescents (18, 38, 39). Four studies were undertaken in Japan (36, 37, 42, 45), four in Europe (35, 40, 41, 46), three in the U.S. (18, 39, 43), and two in China (38, 44). Most of the studies included a high percentage of males, with the exception of three studies (40, 42, 45). Most of the studies included patients with a diagnosis of schizophrenia. Nine studies included a healthy control group, one study included first degree relatives as control group (41), and eight studies included a group with a diagnosis of autism. Seven studies assessed the presence of autistic symptoms in other clinical groups (i.e., psychopathy, attention-deficit/hyperactivity disorder, ultra-high risk (UHR) to develop psychosis, first episode psychosis, obsessive compulsive disorder, major depressive disorder, bipolar disorder) (18, 38, 39, 41, 42, 44, 46). To support the diagnosis, one study used the DSM-Third Edition-Text Revised criteria (35), one study used the DSM-Fourth Edition criteria (38), four studies used the DSM-Fourth Edition-Text Revised criteria (18, 36, 39, 44), and one study used the DSM-Fifth Edition criteria in one study (45). The quality assessment is described in Table 1. Of note, we used self-reported scales which may introduce a bias for the assessment of the outcome.
Meta-Analysis
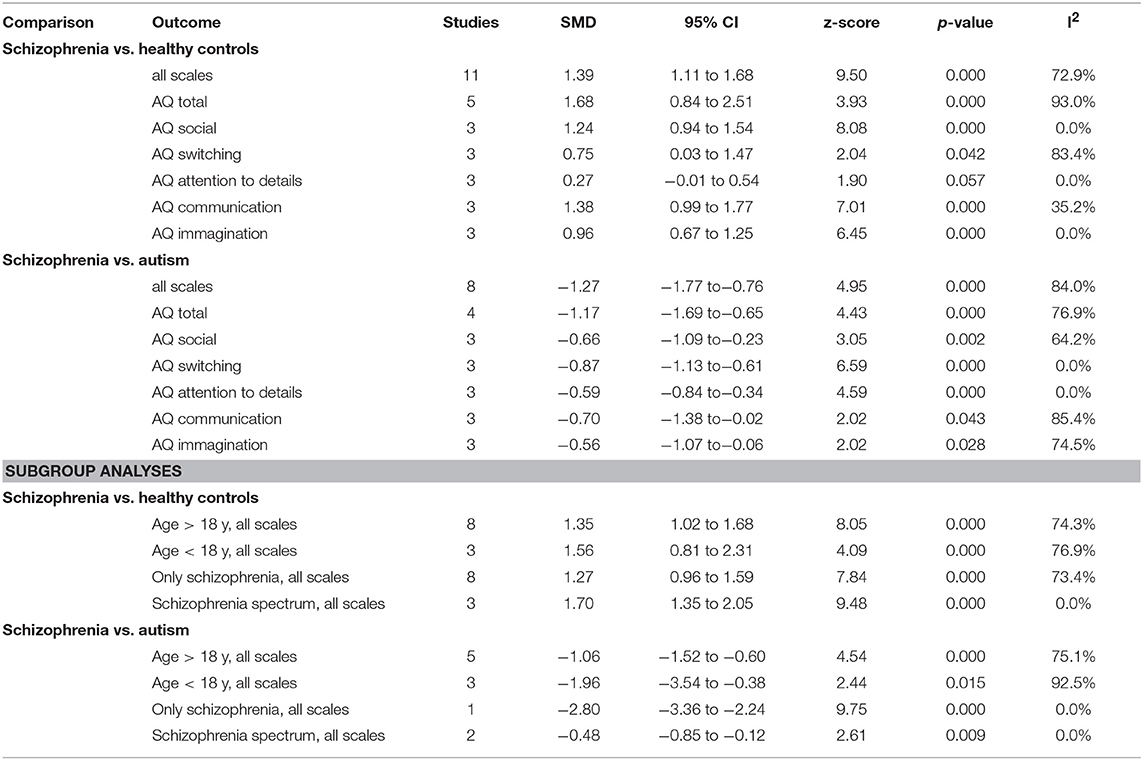
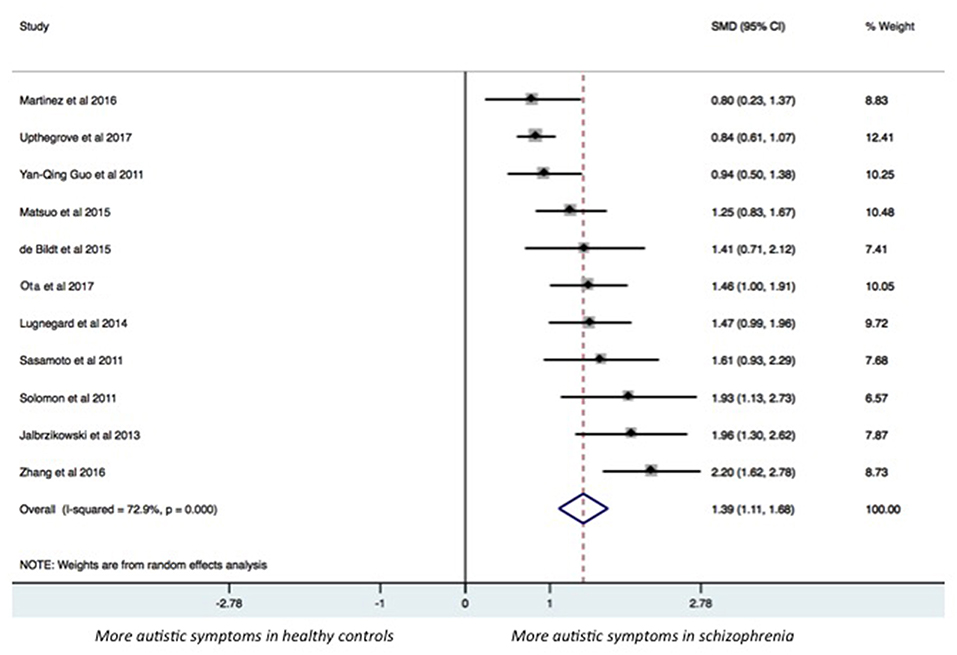
The results of the pairwise meta-analysis for individuals with SSDs vs. healthy controls and individuals with autism compared for the presence of autistic symptoms in our study are presented in Table 3 (See Forest Plot, Figure 2).
Individuals with SSDs have significantly higher autistic symptoms than healthy controls (SMD: 1.39; 95% CI: 1.11 to 1.68) and lower autistic symptoms than individuals with autism (SMD: −1.27; 95% CI: −1.77 to −0.76). Individuals with SSDs have significantly higher autistic symptoms than healthy controls on the AQ total (SMD: 1.68; 95% CI: 0.84 to 2.51), the AQ Social subscale (SMD: 1.24; 95% CI: 0.94 to 1.54), the AQ Attention Switching subscale (SMD: 0.75; 95% CI: 0.03 to 1.47), the AQ Communication subscale (SMD: 1.38; 95% CI: 0.99 to 1.77), and the AQ Imagination subscale (SMD: 0.96; 95% CI: 0.67 to 1.25). However, no significant difference was found between individuals with SSDs and healthy controls on the AQ Attention to Details subscale (SMD: 0.27; 95% CI: −0.01 to 0.54).
Individuals with SSDs have significantly lower autistic symptoms than individuals with autism on the AQ total (SMD: −1.17; 95% CI: −1.69 to−0.65) and on all the AQ subscales (see Table 3).
We undertook subgroup analyses on studies on children, on studies on adolescents, and on studies reporting a diagnosis of SSD, and we found that results did not substantially change (see Table 3).
Discussion
The aim of this meta-analysis was to provide a synthesis of existing literature on the presence of autistic symptoms in individuals with SSDs. The meta-analysis results show that individuals with SSDs have significantly higher autistic symptoms than healthy controls and lower autistic symptoms than individuals with autism. The results of this meta-analysis support a shared symptomatology between these conditions. For example, language deficits often found in individuals with autism are also frequently found in prodromal symptoms of SSDs and were highlighted by the difference on the AQ Communication subscale between individuals with SSDs and healthy controls (SMD: 1.38) (67). Moreover, tangential thought, formal language, and focus on favorite subjects are often present in individuals with autism, and these language symptoms are similar to formal thought disorder which is characterized by disorganized speech (22). Furthermore, individuals with autism may present with language deficits characterized by a lack of verbal initiation and poverty of content similar to individuals with schizophrenia and negative symptoms (22). Likewise, social deficits seem to be present in both conditions (18, 22). For example, social isolation and difficulties to maintain age-appropriate peer relationships are observed in individuals with autism as well as in individuals with schizophrenia. Therefore, it is not surprising that the AQ Communication subscale, which measure the communication skills, and the AQ Socialization subscale, which measure the socialization ability, reported that highest scores in this group. On the other hand, it has to be noted that the level of autistic symptoms changes among the AQ subscales. Indeed, the difference in the AQ sub-scale scores between individuals with SSDs and healthy controls showed higher levels of autistic symptoms in individuals with SSDs on the AQ Communication subscale (SMD: 1.38), on the AQ Social subscale (SMD: 1.24), the AQ Imagination subscale (SMD: 0.96), and the AQ Attention Switching subscale (SMD: 0.75). Notably, no significant difference was found between individuals with SSDs and healthy controls on the AQ Attention to Details subscale (SMD: 0.27), thus suggesting that in this clinical population the difficulties on some autistic symptoms are not as severe. In general, in clinical practice it is difficult to discern between positive symptoms of SSDs and autism symptoms. For example, sensory issues present in individuals with autism may be misdiagnosed as hallucinations in schizophrenia with significant treatment implications. Similarly, it can be also difficult to discern between negative symptoms of schizophrenia and autism symptoms. For example, the lack of emotional reciprocity that is present in individuals with autism resemble the blunt affect or alogia (i.e., poverty of speech) in schizophrenia. Therefore, it is possible that individuals with SSDs develop autistic-like symptoms as a result of their negative symptoms. Our results are consistent with previous research in this area reporting that autistic symptoms seem to be prevalent in SSDs, ranging from 9.6 to 61% (6).
However, the findings of this meta-analysis should be interpreted with some caution. Limitations include the paucity of studies analyzed, different diagnostic criteria and measures, and differences in the age of the samples.
In our meta-analysis, we included only 13 studies. The pool of 13 studies was small and did not allow meta-regression analyses to examine how participants characteristics (e.g., gender, age, illness duration) can predict autistic symptoms. However, the total number of participants included in the current meta-analysis is large (n = 1,958) and the results are strong, with high SMDs, and precise 95%CIs.
Furthermore, we found studies with different diagnostic criteria, which is a reason for increased heterogeneity. However, it has to be noted that all the diagnostic criteria used by studies included on the current meta-analysis were well-validated and all studies used either a DSM based diagnosis, or the Structured Interview for Prodromal Symptoms (SIPS), or the Positive and Negative Syndrome Scale (PANSS) for the diagnosis of SSDs. Indeed, the AQ is a self-report scale, which may introduce reporting bias. Moreover, this measure was not always available, and we needed to use other instruments measuring autistic symptoms. Therefore, given the different scales used in the current meta-analysis, we analyzed data by SMDs as opposed to mean differences.
Three studies included only children and adolescents (18, 38, 39), which may differ from adults in terms of diagnosis stability and autistic symptoms. Therefore, we decided to undertake subgroup analyses taking into account the age. ASD by definition is a disorder present from early childhood. It is important to consider the individual's developmental history in order to distinguish between the presence of an ASD and autistic symptoms. However, it is still unclear whether difficulties such as those present in individuals with autism are present before the onset of schizophrenia or whether they are dependent by the schizophrenic state itself (e.g., resulting from thought dysfunction), thus not truly autistic.
Further studies investigating the etiology of autism symptoms in children and adolescents with SSDs are needed in order to shed light on this issue. Furthermore, longitudinal studies investigating autistic symptoms in high-ultra risk state populations can clarify whether these symptoms are stable features of this clinical population or are dependent by the schizophrenic state of illness. Indeed, it has to be noted that in the present meta-analysis only one study (41) used a diagnostic tool for autism (i.e., ADOS-2). All other studies used instruments that were designed as screening measures for autism. Therefore, in order to distinguish between trait and state, future studies would benefit by the use of diagnostic measures (e.g., ADOS-2) along with developmental history in order to confirm whether any deficit associated with autism was present before the onset of SSDs. Indeed, it is well-known that individual with autism are at an increased risk to develop other mental-health conditions and a ASD diagnosis is particularly difficult in adults, especially when knowledge of early developmental history is missing. Therefore, longitudinal studies investigating autistic symptoms in individuals with SSDs in different psychopathological phases could clarify whether these characteristics persist after recovery from SSDs.
Conclusion
In conclusion, we found that individuals with SSDs have higher autistic symptoms than healthy controls and lower autistic symptoms than individuals with autism. To our knowledge, this is the first systematic review and meta-analysis trying to quantitatively pool all evidences on the topic. Our study has some limitations, including the use of self-report scales, which may introduce reporting bias. Therefore, further studies investigating the etiology of autism symptoms are needed to shed light on the association between these conditions.
Author Contributions
MS and VP overviewed and examined the literature. FD performed the statistical analysis. FD, VP, AR, and MS wrote the manuscript. LM, MA, and PC designed the study, contributed to theoretical interpretation read and final proof reading. Each author read and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-5. Washington, DC: American Psychiatric Association (2013).
2. Miettunen J, Immonen J, McGrath J, Isohanni M, Jääskeläinen E. The age of onset of schizophrenia spectrum disorders. Schizophr Bull. (2018) 44(Suppl.1):S270. doi: 10.1093/schbul/sby017.659
3. Bender L. Childhood schizophrenia; clinical study on one hundred schizophrenic children. Am J Orthopsychiatry (1947) 17:40–56.
4. Kolvin I. Studies in the childhood psychoses: diagnostic criteria and classification. Br J Psychiatry (1971) 118:381–4.
6. Kincaid DL, Doris M, Shannon C, Mulholland C. What is the prevalence of autism spectrum disorder and ASD traits in psychosis? A systematic review. Psychiatry Res. (2017) 250:99–105. doi: 10.1016/j.psychres.2017.01.017
7. Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science (2018) 359:693–7. doi: 10.1126/science.aad6469
8. Carroll LS, Owen MJ. Genetic overlap between autism, schizophrenia and bipolar disorder. Genome Med. (2009) 1:102. doi: 10.1186/gm102
9. Burbach JP, van der Zwaag B. Contact in the genetics of autism and schizophrenia. Trends Neurosci. (2009) 32:69–72. doi: 10.1016/j.tins.2008.11.002
10. Reichelt AC, Rodgers RJ, Clapcote SJ The role of neurexins in schizophrenia and autistic spectrum disorder. Neuropharmacology (2012) 62:1519–26. doi: 10.1016/j.neuropharm.2011.01.024
11. St Clair D. Copy number variation and schizophrenia. Schizophr Bull. (2009) 35:9–12. doi: 10.1093/schbul/sbn147
12. Bevan Jones R, Thapar A, Lewis G, Zammit S. The association between early autistic traits and psychotic experiences in adolescence. Schizophr Res. (2012) 135:164–9. doi: 10.1016/j.schres.2011.11.037
13. Sullivan S, Rai D, Golding J, Zammit S, Steer C. The association betweenautism spectrum disorder and psychotic experiences in the Avon longitudinalstudy of parents and children (ALSPAC) birth cohort. J Am Acad Child Adolesc Psychiatry (2013) 52:806–14.e2. doi: 10.1016/j.jaac.2013.05.010
14. Volkmar FR, Cohen DJ. Comorbid association of autism and schizophrenia. Am J Psychiatry (1991) 148:1705–7.
15. Leyfer O, Folstein S, Bacalman S, Davis N, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. J Autism Dev Disord. (2006) 36:849–61. doi: 10.1007/s10803-006-0123-0
16. Rapoport J, Chavez A, Greenstein D, Addington A, Gogtay N. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J Am Acad Child Adolesc. Psychiatry (2009) 48:10–8. doi: 10.1097/CHI.0b013e31818b1c63
17. Losh M, Adolphs R, Poe MD, Couture S, Penn D, Baranek GT, et al. Neuropsychological profile of autism and the broad autism phenotype. Arch Gen Psychiatry (2009) 66:518–26. doi: 10.1001/archgenpsychiatry.2009.34
18. Solomon M, Olsen E, Niendam T, Ragland JD, Yoon J, Minzenberg M, et al. From lumping to splitting and back again: atypical social and language development in individuals with clinical-high-risk for psychosis, first episode schizophrenia, and autism spectrum disorders. Schizophr Res. (2011) 131(1–3):146–151. doi: 10.1016/j.schres.2011.03.005
19. Larson FV, Wagner AP, Jones PB, Tantam D, Lai MC, Baron-Cohen S, et al. Psychosis in autism: a comparison of the features of both conditions in a dually-affected cohort. Br J Psychiatry (2017) 210:269–75. doi: 10.1192/bjp.bp.116.187682
20. Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. (2009) 109:1–9. doi: 10.1016/j.schres.2008.12.020
21. Sprong M, Becker H, Schothorst P, Swaab H, Ziermans T, Dingemans P, et al. Pathways to psychosis: a comparison of the pervasive developmental disorder subtype multiple complex developmental disorder and the “at risk mental state”. Schizophr Res. (2008) 99:38–47. doi: 10.1016/j.schres.2007.10.031
22. Chisholm K, Lin A, Abu-Akela A, Wood SJ. The association between autism and schizophrenia spectrum disorders: a review of eight alternate models of co-occurrence. Neurosci Biobehav Rev. (2015) 55:173–83. doi: 10.1016/j.neubiorev.2015.04.012
23. Couture SM, Penn DL, Losh M, Adolphs R, Hurley R, Piven J. Comparison of social cognitive functioning in schizophrenia and high functioning autism: more convergence than divergence. Psychol Med. (2010) 40:569–79. doi: 10.1017/S003329170999078X
24. Sasson N, Tsuchiya N, Hurley R, Couture SM, Penn DL, Adolphs R, et al. Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia (2007) 45:2580–8. doi: 10.1016/j.neuropsychologia.2007.03.009
25. Cochran DM, Dvir Y, Frazier JA. “Autism-plus” spectrum disorders: intersection with psychosis and the schizophrenia spectrum. Child Adolesc Psychiatr Clin N Am. (2013) 22:609–27. doi: 10.1016/j.chc.2013.04.005
26. Chisholm K, Lin A, Armando M. Schizophrenia Spectrum Disorder and ASD. In: Mazzone L, Vitiello B, editors. The Psychiatric Comorbidities in Autism Spectrum Disorder. Springer (2016). p. 51–66. doi: 10.1007/978-3-319-29695-1
27. Mazzone L, Postorino V, Valeri G, Vicari S. Catatonia in patients with autism: prevalence and management. CNS Drugs (2014) 28:205–15. doi: 10.1007/s40263-014-0143-9
28. Padgett FE, Miltsiou E, Tiffin PA. The co-occurrence of nonaffective psychosis and the pervasive developmental disorders: a systematic review. J Intellect Dev Disabil. (2010) 35:187–98. doi: 10.3109/13668250.2010.494596
29. Skokauskas N, Gallagher L. Psychosis, affective disorders and anxiety in autistic spectrum disorder: prevalence and nosological considerations. Psychopathology (2010) 43:8–16. doi: 10.1159/000255958
30. Rabinowitz J, Levine SZ, Häfner H. A population based elaboration of the role of age of onset on the course of schizophrenia. Schizophr Res. (2006) 88:96–101. doi: 10.1016/j.schres.2006.07.007
31. Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. (2014) 16:505–24. Availabe online at: https://academicworks.medicine.hofstra.edu/articles/978
32. Barlati S, Deste G, Gregorelli M, Vita A. Autistic traits in a sample of adult patients with schizophrenia: prevalence and correlates. Psychol Med. (2018) 20:1–9. doi: 10.1017/S0033291718000600
33. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
34. Wells GA, Shea B, O'Connell D, et al. The Newcastle Ottawa scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analysis. (2011). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
35. Konstantareas MM, Hewitt T. Autistic disorder and schizophrenia: diagnostic overlaps. J Autism Dev Disord. (2001) 31:19–28. doi: 10.1023/A:1005605528309
36. Naito K, Matsui Y, Maeda K, Tanaka K. Evaluation of the validity of the Autism Spectrum Quotient (AQ) in differentiating high-functioning autistic spectrum disorder from schizophrenia. Kobe J Med Sci. (2010) 56:E116–24.
37. Sasamoto A, Miyata J, Hirao K, Fujiwara H, Kawada R, Fujimoto S, et al. Social impairment in schizophrenia revealed by Autism-Spectrum Quotient correlated with gray matter reduction. Soc Neurosci. (2011) 6:548–58. doi: 10.1080/17470919.2011.575693
38. Guo YQ, Tang Y, Rice C, Lee LC, Wang YF, Cubells JF. Validation of the Autism Spectrum Screening Questionnaire, Mandarin Chinese Version (CH-ASSQ) in Beijing, China. Autism (2011) 15:713–27. doi: 10.1177/1362361310396383
39. Jalbrzikowski M, Krasileva KE, Marvin S, Zinberg J, Andaya A, Bachman P, et al. Reciprocal social behavior in youths with psychotic illness and those at clinical high risk. Dev Psychopathol. (2013) 25(4 Pt 1):1187–97. doi: 10.1017/S095457941300045X
40. Lugnegård T, Hallerbäck MU, Gillberg C. Asperger syndrome and schizophrenia: overlap of self-reported autistic traits using the Autism-spectrum Quotient (AQ). Nord J Psychiatry (2015) 69:268–74. doi: 10.3109/08039488.2014.972452
41. De Bildt A, Sytema S, Meffert H, Bastiaansen JACJ. The Autism Diagnostic Observation Schedule, Module 4: Application of the Revised Algorithms in an Independent, Well-Defined, Dutch Sample (n = 93). J Autism Dev Disord. (2016) 46:21–30. doi: 10.1007/s10803-015-2532
42. Matsuo J, Kamio Y, Takahashi H, Ota M, Teraishi T, Hori H, et al. Autistic-like traits in adult patients with mood disorders and schizophrenia. PLoS ONE (2015) 10:e0122711. doi: 10.1371/journal.pone.0122711
43. Martinez G, Alexandre C, Mam-Lam-Fook C, Bendjemaa N, Gaillard R, Garel P, et al. Phenotypic continuum between autism and schizophrenia: Evidence from the Movie for the Assessment of Social Cognition (MASC). Schizophr Res. (2017) 185:161–166. doi: 10.1016/j.schres.2017.01.012
44. Zhang L, Sun Y, Chen F, Wu D, Tang J, Han X. Psychometric properties of the Autism-Spectrum Quotient in both clinical and non-clinical samples: Chinese version for mainland China. BMC Psychiatry (2016) 16:213. doi: 10.1186/s12888-016-0915
45. Ota M, Matsuo J, Sato N, Teraishi T, Hori H, Hattori K, et al. Correlation of reduced social communicational and interactional skills with regional grey matter volumes in schizophrenia patients. Acta Neuropsychiatr (2017) 29:374–81. doi: 10.1017/neu.2017.9
46. Upthegrove R, Abu-Akel A, Chisholm K, Lin A, Zahid S, Pelton M, et al. Autism and psychosis: clinical implications for depression and suicide. Schizophr Res. (2018) 195:80–85. doi: 10.1016/j.schres.2017.08.028
47. Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. (2001) 31:5–17. doi: 10.1023/A:1005653411471
48. Constantino J, Gruber C. Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services (2005).
49. Lord C, Luyster RJ, Gotham K, Guthrie W. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part II): Toddler Module. Torrance, CA: Western Psychological Services (2012).
50. Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part I): Modules 1-4. Torrance, CA: Western Psychological Services (2012).
51. Ehlers S, Gillberg C, Wing L. A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. J Autism Dev Disord. (1999) 29:129–41.
52. Garfin DG, McCallon D, Cox R. Validity and reliability of the Childhood Autism Rating Scale with autistic adolescents. J Autism Dev Disord. (1988) 18:367–78.
53. Perry A, Condillac RA, Freeman N, Dunn-Geier J, Belair J. Multi-site study of the Childhood Autism Rating Scale (CARS) in five clinical groups of young children. J Autism Dev Disord. (2005) 35:625–34. doi: 10.1007/s10803-005-0006-9
54. Bölte S, Poustka F, Constantino JN. Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS). Autism Res. (2008) 1:354–63. doi: 10.1002/aur.49
55. Constantino JN. The Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services (2002).
56. Ventola PE, Kleinman J, Pandey J, Barton M, Allen S, Green J, et al. Agreement among four diagnostic instruments for autism spectrum disorders in toddlers. J Autism Dev Disord. (2006) 36:839–47. doi: 10.1007/s10803-006-0128-8
57. Higgins JP, Thompson SG, Deeks JJ, Altman DG Measuring inconsistency in meta-analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
58. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane collaboration (2011). Available online at: www.cochrane-handbook.org
59. Chang HL, Juang YY, Wang WT, Huang CI, Chen CY, Hwang YS. Screening for autism spectrum disorder in adult psychiatric outpatients in a clinic in Taiwan. Gen Hosp Psychiatry (2003) 25:284–8. doi: 10.1016/S0163-8343(03)00053-7
60. Fraser R, Cotton S, Gentle E, Angus B, Allott K, Thompson A. Non-expert clinicians' detection of autistic traits among attenders of a youth mental health service. Early Interv Psychiatry (2012) 6:83–6. doi: 10.1111/j.1751-7893.2011.00288.x
61. Mandell DS, Lawer LJ, Branch K, Brodkin ES, Healey K, Witalec R, et al. Prevalence and correlates of autism in a state psychiatric hospital. Autism (2012) 16:557–67. doi: 10.1177/1362361311412058
62. Hallerback MU, Lugnegard T, Gillberg C. Is autism spectrum disorder common in schizophrenia? Psychiatry Res. (2012) 198:12–7. doi: 10.1016/j.psychres.2012.01.016
63. Waris P, Lindberg N, Kettunen K, Tani P. The relationship between Asperger's syndrome and schizophrenia in adolescence. Eur Child Adolesc Psychiatry (2013) 22:217–23. doi: 10.1007/s00787-012-0338-x
64. Davidson C, Greenwood N, Stansfield A, Wright S. Prevalence of Asperger syndrome among patients of an early intervention in psychosis team. Early Interv Psychiatry (2014) 8:138–46. doi: 10.1111/eip.12039
65. Kästner A, Begemann M, Michel TM, Everts S, Stepniak B, Bach C, et al. Autism beyond diagnostic categories: characterization of autistic phenotypes in schizophrenia. BMC Psychiatry (2015) 13:115. doi: 10.1186/s12888-015-0494-x
66. Morrison KE, Pinkham AE, Penn DL, Kelsven S, Ludwig K, Sasson NJ. Distinct profiles of social skill in adults with autism spectrum disorder and schizophrenia. Autism Res. (2017) 10:878–87. doi: 10.1002/aur.1734
Keywords: schizophrenia, autism, development, comorbidity, meta-analysis
Citation: De Crescenzo F, Postorino V, Siracusano M, Riccioni A, Armando M, Curatolo P and Mazzone L (2019) Autistic Symptoms in Schizophrenia Spectrum Disorders: A Systematic Review and Meta-Analysis. Front. Psychiatry 10:78. doi: 10.3389/fpsyt.2019.00078
Received: 18 September 2018; Accepted: 04 February 2019;
Published: 21 February 2019.
Edited by:
Richard Eugene Frye, Phoenix Children's Hospital, United StatesReviewed by:
Stephanie Ameis, Hospital for Sick Children, CanadaKaren Muller Smith, University of Louisiana at Lafayette, United States
Copyright © 2019 De Crescenzo, Postorino, Siracusano, Riccioni, Armando, Curatolo and Mazzone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luigi Mazzone, Z2lnaW1henpvbmVAeWFob28uaXQ=
†These authors have contributed equally to this work
 Franco De Crescenzo
Franco De Crescenzo Valentina Postorino
Valentina Postorino Martina Siracusano6,7
Martina Siracusano6,7 Marco Armando
Marco Armando Paolo Curatolo
Paolo Curatolo Luigi Mazzone
Luigi Mazzone