- 1Graduate Institute of Acupuncture Science, China Medical University, Taichung, Taiwan
- 2Department of Photonics and Communication Engineering, Asia University, Taichung, Taiwan
- 3Chinese Medicine Research Center, China Medical University, Taichung, Taiwan
Neuropsychiatric disorders, including depression, anxiety, schizophrenia, and Alzheimer's disease (AD), are diseases that are directly or indirectly associated with cerebral dysfunction and contribute significantly to disability in adult populations worldwide. Important limitations surround the currently available pharmacologic agents for neuropsychiatric disorders and, moreover, many patients fail to respond to these therapies. Acupuncture might be a complementary therapy for neuropsychiatry disorders. In this review, we investigate the current evidence for the treatment efficacy of acupuncture in depression, anxiety, schizophrenia, and AD. Secondly, we review recent advances in understanding of the dysregulated glutamate system underlying the pathophysiology of these disorders. Finally, we discuss the ways in which acupuncture treatment can potentially modulate glutamate receptors and excitatory amino acid transporters. We conclude that the treatment effects of acupuncture may be underpinned by its intervention in the dysregulated glutamate system. Further preclinical and clinical studies are needed to clarify the possible mechanisms of acupuncture in these neuropsychiatric disorders and to establish protocols for treatment guidelines.
Introduction
Neuropsychiatry focuses on illness relating to altered cognition, mood, or behavior caused by cerebral dysfunction with neuronal pathological changes (e.g., dysregulated neurotransmitter systems or tissue damage), or abnormal physiological conditions (e.g., hyper/hypoglycemia or hypoxia). These changes in health conditions have profound effects upon individuals and society (1). Neuropsychiatric disorders contribute to over 10% of disability worldwide, exceeding the morbidity rates associated with cardiovascular disease or cancer (2). In developed-market economies, 25% of all disability has been attributed to neuropsychiatric disorders (2). In 2016, ~18% of adults aged ≥18 years in the United States of America had any mental illness in the past year and ~4% had a serious mental illness in that period (3, 4). A serious mental illness is defined as a diagnosable mental, behavioral, or emotional disorder (e.g., major depressive disorder [MDD], schizophrenia) causing serious functional impairment and substantially interfering with or limiting one or more major life activities, such as maintaining interpersonal relationships, activities of daily living, self-care, employment, and recreation (3, 4). Similarly, a systematic review of epidemiological data from 16 European countries estimated in 2005 that 27% of the adult population (18–65 years of age) had experienced at least one mental disorder (e.g., substance use, psychosis, depression, anxiety, or eating disorder) in the past 12 months (5). Despite the high burden of psychiatric illness, only a subset of these people receive the mental health services that they need. For instance, according to data from the World Health Organization (WHO) European Region, 3 out of 4 people with MDD are inadequately treated (6). The majority of psychiatric disorders are mild or moderate, but if left untreated, the evidence suggests that they can develop into more serious illness (2).
Important limitations surround the currently available treatments for neuropsychiatric disorders. For instance, first-line pharmacotherapy for MDD typically consists of a selective serotonin reuptake inhibitor, a serotonin and norepinephrine reuptake inhibitor, or the norepinephrine-dopamine reuptake inhibitor bupropion, alone or in combination with psychotherapy. These therapies are associated with low remission rates and high dropout rates (7). As for the treatment of schizophrenia, the typical and atypical antipsychotics are mostly effective for the positive symptoms (hallucinations, delusions); cognitive and negative symptoms (deficits in working memory and attention, negative affect, and anhedonia) are largely unresponsive to current pharmacologic therapies. Moreover, serious side effects limit the use of some otherwise effective medications (8).
Acupuncture in Neuropsychiatry Treatment
Acupuncture has long been used in Chinese medicine to treat numerous neuropsychiatric conditions, from acute delirium to post-stroke spasticity. The two forms of acupuncture manipulation that are used clinically are manual acupuncture (MA) and electroacupuncture (EA) (9). Traditional acupuncturists commonly use MA, whereby they insert the acupuncture needle into the acupoint to a certain depth and rotate it by hand. In EA, the needles are connected to an electrical stimulator that delivers a stimulating current to the acupoints. Another method that is also described as EA is the positioning of a surface electrode on the skin at the acupoint, without insertion of acupuncture needles. For this review, the evidence on the use of acupuncture in neuropsychiatry treatment is limited to investigations using MA and EA, with acupuncture needle insertion.
Numerous clinical reports from various sources, including the non-Western scientific literature, attest to the efficacy of acupuncture in depression (10–13), anxiety disorders (14, 15), schizophrenia (16–19), and Alzheimer's disease (AD) (20–23). Although much of this evidence is widely acknowledged to be of varying quality, many reports attest to the efficacy and safety of acupuncture treatment (24–27). In experienced hands, acupuncture is a safe therapy with a low risk of adverse events. Serious and potentially life-threatening acupuncture-related complications, including transmission of infections, pneumothorax, cardiovascular lesions, and hemorrhage or hematomas in the central nervous system (CNS), are very rarely reported (28). In a large study from Germany that included 2.2 million acupuncture sessions in 229,230 patients, the overall incidence of acupuncture-related adverse events was 8.6%, among which 2.2% of the patients required medical treatment (29). The vast majority of adverse events were due to minor bleeding/hematomas (6.1%), pain (1.7%), or vegetative symptoms such as vertigo, or nausea (0.7%). A review of three Chinese trials involving nearly 2,000 treatments identified instances of subcutaneous hematoma, bleeding and needle site pain, and reported that elderly people seem to be at greater risk of such adverse events (30).
Acupuncture for the Treatment of Depression
The effectiveness of acupuncture in depression has been extensively investigated with various sets of acupuncture points and treatment parameters (e.g., duration, frequency, and number of treatment sessions). A recent Cochrane systematic review that included 64 studies (7,104 participants) examined the effectiveness of acupuncture for the treatment of depression (10). The evidence indicated that acupuncture treatment may moderately or slightly lower the severity of depression compared with treatment as usual and control acupuncture (invasive, non-invasive sham controls), respectively, although the quality of the evidence was judged as being mostly low or very low. Other reviews have also described evidence in support of MA and EA as generally beneficial, safe and well-tolerated as monotherapy in MDD and post-stroke depression, but the evidence is insufficient to support the use of acupuncture in combination with antidepressants (31, 32). Two Chinese studies examined the effectiveness of acupuncture and moxibustion (a treatment involving burning of the dried Chinese herb mugwort or Artemesia vulgaris to apply heat onto or very close to an acupoint) in relieving psychological distress in 163 patients with depression and sought to determine whether gender-related differences exist in response to acupuncture and moxibustion (12, 13). The authors reported that acupuncture and moxibustion can significantly improve distress at even as late as 3 months after the completion of treatment, and that the level of efficacy is higher among females than males. Hence, acupuncture treatment in depression may improve depressive symptoms of depression and endure for some months.
Moreover, sleep status may improve in patients with depression after acupuncture treatment. A recent meta-analysis that included 18 randomized clinical trials (RCTs) involved 1,678 adults given acupuncture for depression-related insomnia and found significant improvements in sleep quality with acupuncture compared with Western medicine (11). When acupuncture was given as an adjunctive therapy with Western medicine, both depression and insomnia were improved (11). However, in another RCT involving 150 patients with residual insomnia associated with MDD, traditional acupuncture needling produced only mild treatment effects that were similar to those of minimal acupuncture and placebo acupuncture (33). There were no significant group-by-time interactions during the 5-week post-treatment period. Thus, the psychological effect of acupuncture might play an important role in the treatment of acupuncture in depression-related insomnia.
Acupuncture for the Treatment of Anxiety
The effectiveness of acupuncture in anxiety has been widely investigated, using various sets of acupuncture points and treatment parameters. A narrative review published by the British Acupuncture Council reported that regular acupuncture and EA treatments improved anxiety symptoms (34). However, significant differences between the protocols used in regular acupuncture and EA made it hard to rule out a general beneficial or possible placebo effect. A more recent systematic literature review that included 32 English-language clinical and preclinical studies published between 2000 and 2010 also reported significant, positive results with acupuncture treatment for anxiety (14). Although the quality of these studies was variable, the authors suggested that patients who are resistant to conventional interventions (e.g., cognitive behavioral therapy) may prefer acupuncture treatment. Thus, acupuncture treatment may have positive effects on the symptoms of anxiety.
Acupuncture may also be of benefit in anxiety-related insomnia. A Canadian study reported that acupuncture significantly improved sleep quality in patients with anxiety and insomnia (15). At the end of acupuncture treatment, urine 6-sulfatoxymelatonin (a metabolite of melatonin) levels were normalized and several polysomnographic measures, as well as self-reported fatigue, sleepiness, anxiety, and level of depression, were significantly improved. Combined with the evidence of treatment efficacy in depression-related insomnia, acupuncture may have broader utility in neuropsychiatric disorders with impaired quality of sleep.
Interestingly, acupuncture treatment may also reduce anxiety levels in medical conditions other than neuropsychiatry. An early study reported that auricular acupuncture reduced state anxiety in patients waiting for surgery by a significantly greater extent than either body or sham acupuncture (35). Another study has reported that both body and auricular acupuncture effectively reduce preoperative anxiety; self-rated anxiety scores were significantly reduced from baseline in both groups (36). Possible underlying mechanisms remain unclear. One plausible explanation is that acupuncture regulates the autonomic nervous system. For instance, acupuncture appears to modulate heart rate variability, a non-invasive indicator of changes in autonomic state. In patients with mild depression or anxiety, verum acupuncture but not sham acupuncture was associated with significant reductions from baseline in mean resting heart rate at 5 and 15 min after needle application, with a trend toward an increase in high frequency (HF; 0.15–0.4 Hz) and a decrease in low frequency (LF; 0.04–0.15 Hz) spectral power (37). These results suggest that verum acupuncture modulates autonomic activity in response to alterations of internal and external environments, and thus reduces overall anxiety in patients with depression or anxiety.
However, it should be recognized that the interpretation of results from clinical trials investigating acupuncture interventions in depression and anxiety disorders is complicated by different interventions, different comparators used against acupuncture interventions, and the small sample sizes in many trials (38). These shortcomings prevent any accurate assessment of acupuncture for these conditions or a true comparison of the relative effectiveness of different treatment regimens. Moreover, the data are difficult to interpret from those studies where needling at specific and non-specific points have yielded similar outcomes (38).
The evidence consulted for depression and anxiety is summarized in Table 1.
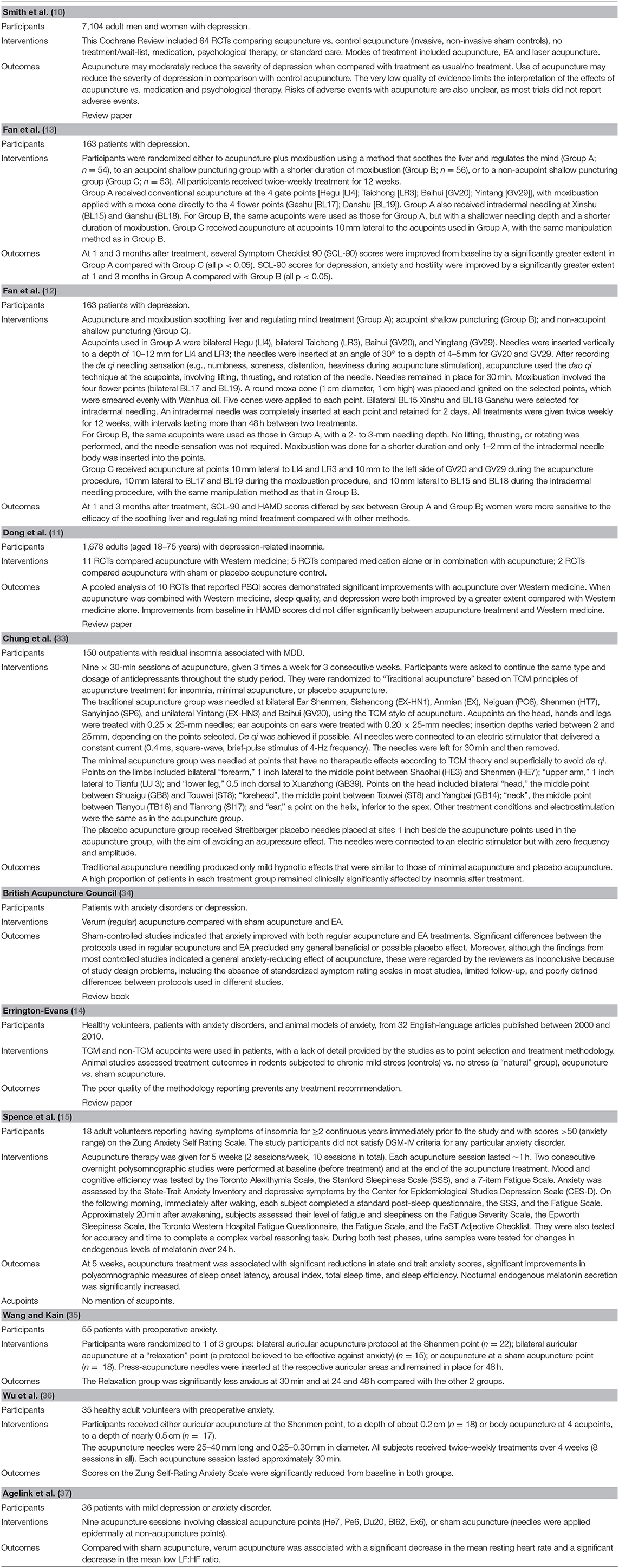
Table 1. Overview of characteristics of included studies evaluating acupuncture for depression and anxiety.
Acupuncture for the Treatment of Schizophrenia
Compared with depression and anxiety, relatively few studies have addressed the efficacy of acupuncture in schizophrenia. A meta-analysis of 13 RCTs including 954 patients, all from China, provides positive evidence for the effectiveness of acupuncture (with or without EA or moxibustion) in treating the symptoms of schizophrenia (19). Some of the RCTs reported that acupuncture plus drug therapy significantly improved auditory hallucinations, positive symptoms and response rates compared with antipsychotics alone or in combination with sham EA. In a recent systematic review of data from 26 studies (1,181 participants) reporting limited evidence for the use of adjunctive acupuncture therapy in the treatment of positive, negative and cognitive symptoms, the authors point out the importance of differences between quantitative and qualitative changes (16). The limited evidence for treatment efficacy may be partly due to the fact that positive symptoms still exist, but patients are suffering less. Other evidence suggests that individualized acupuncture is beneficial for patients with schizophrenia as an adjunctive treatment with routine care (18). After completing individualized acupuncture sessions, patients reported improvements in symptoms of schizophrenia, side effects of medication, energy, motivation, sleep, addictions and other associated physical problems. A case study has reported that positive and negative symptoms can be improved for up to 3 months after add-on acupuncture treatment (17). A more recent case study describes how add-on acupuncture treatment improved general psychopathology and negative symptoms but not positive symptoms (39). Thus, individualized add-on acupuncture treatment may help to alleviate symptoms of schizophrenia.
Interestingly, the review by van den Noort et al. also describes beneficial effects with add-on acupuncture in accompanying sleep disorders (16). Several of the studies in that review reported improvements in subjective and objective sleep measurements. Acupuncture treatment appears to have similar effects to but is safer than zopiclone, a prescription medication for sleep disorder. Thus, acupuncture treatment may improve sleep dysregulation in schizophrenia.
As with the clinical evidence for acupuncture in depression and anxiety disorders, the clinical evidence is limited for the effectiveness of acupuncture as a treatment for schizophrenia. The meta-analysis performed by Lee and colleagues found that all of the included studies were limited by low methodological quality, the low overall number, and the small sample sizes (19). Moreover, as all of the studies were conducted in China, international trials are needed to investigate whether the effects can be replicated in other ethnicities.
The evidence consulted for schizophrenia is summarized in Table 2.
Acupuncture for the Treatment of Alzheimer's Disease
Another disorder that commonly exhibits neuropsychiatric symptoms is dementia. Neuropsychiatric symptoms are amongst the earliest signs and symptoms of neurocognitive disorders and incipient cognitive decline, and can be challenging to treat (40). Evidence shows that acupuncture can increase a patient's verbal and motor skills and improve mood and cognitive function. In an early Chinese trial, 38 patients with senile dementia were treated with acupuncture and acupoint-injection with aceglutamide (23). After treatment, symptoms had improved in 16 patients. In a small pilot study, 1 month of acupuncture treatment improved cognitive function in 8 patients with mild-to-moderate AD (22). Mini-Mental State Examination subscores assessing verbal orientation and motor coordination, as well as overall scores, were significantly improved from baseline. In a US study that included 11 patients with dementia (10 with AD and 1 with vascular dementia), depression and anxiety scores improved significantly after acupuncture treatment (21). These early pilot studies indicated that acupuncture treatment may be of benefit for neuropsychiatric symptoms in dementia, especially in AD.
More recently, a larger clinical trial involving 87 patients with mild-to-moderate AD reported that acupuncture may significantly improve cognitive function (20). When used as monotherapy, acupuncture treatment was associated with significantly greater decreases from baseline in Alzheimer's disease Assessment Scale-Cognitive (ADAS-cog) scores compared with donepezil. The improvement in cognitive function was observed for up to 12 weeks after the end of acupuncture treatment. Moreover, no patients discontinued treatment because of adverse events in the acupuncture group, but four patients did so in the donepezil group. In addition, a small-scale functional magnetic resonance imaging study explored the relationships between de qi sensations (a special needling sensation evoked by acupuncture) induced by different needling depths of acupuncture and their differential effects on the reorganizations of whole-brain networks in 12 patients with mild cognitive impairment (41). The results show that as compared with superficial needling, acupuncture with deep needling induces stronger, wider-ranging de qi sensations and enhances nodal centrality, primarily in the abnormal regions of the brain implicated in mild cognitive impairment. Hence, acupuncture treatment in mild-to-moderate AD appears to be not only beneficial but also safe, with the capacity to improve dysfunctional neural mechanisms involved in mild cognitive impairment.
The evidence consulted for AD is summarized in Table 3.
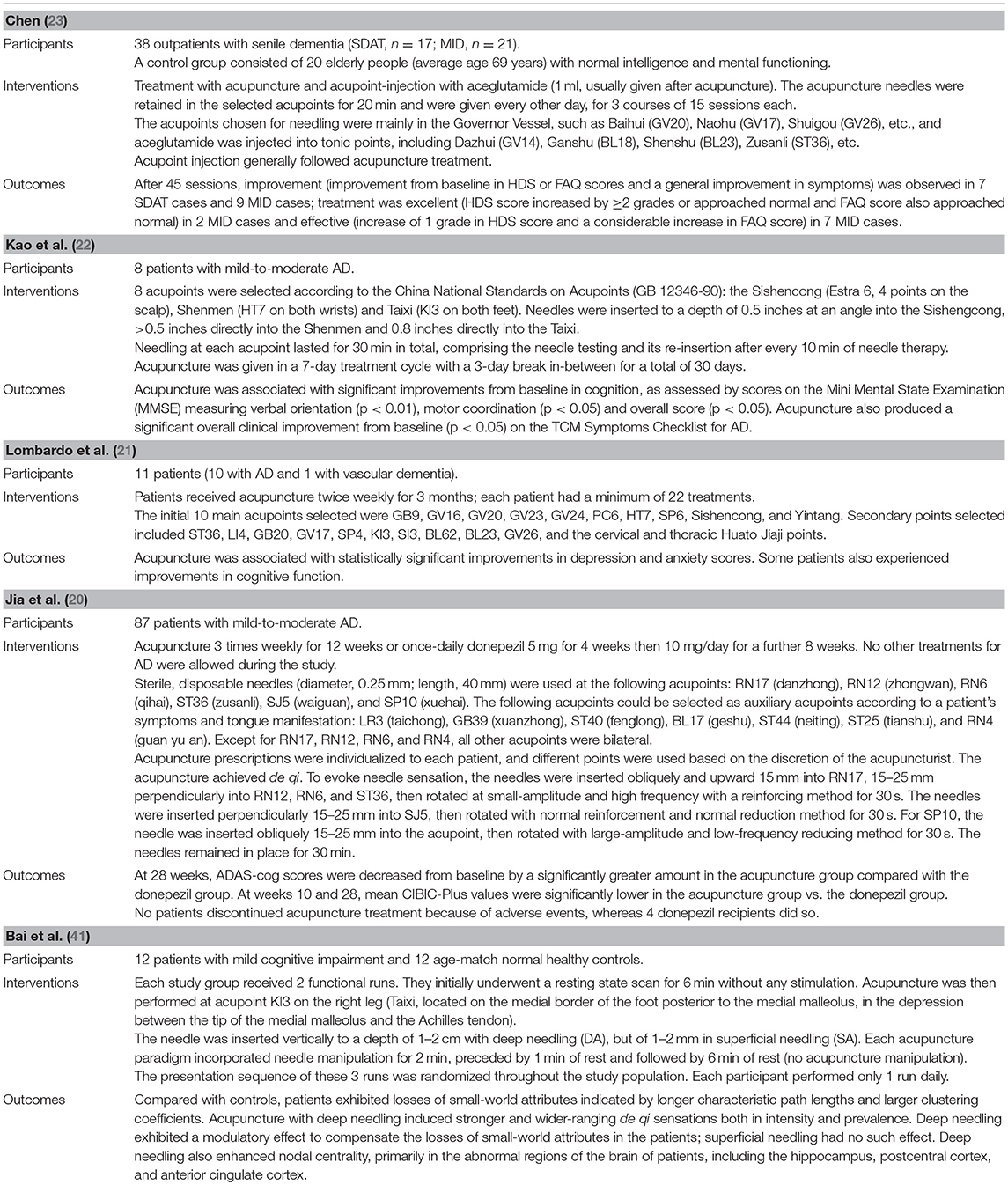
Table 3. Overview of characteristics of included studies evaluating acupuncture for Alzheimer's disease.
Glutamate and Glycine in CNS Disorders
In the past decade, increasing evidence implicates the important role of glutamate in the pathophysiology of many CNS disorders (42, 43). L-Glutamate, the most common excitatory neurotransmitter in the CNS, is involved in synaptic plasticity and cognition (42, 44, 45). Glutamate receptors, synaptic receptors located primarily on the membranes of neuronal cells, are responsible for the glutamate-mediated postsynaptic excitation of neural cells and are important for neural communication, memory formation, learning, and regulation. Neural glutamate signaling is accommodated by two receptor families: ionotropic glutamate receptors (iGluRs; e.g., the N-methyl-D-aspartate [NMDA] receptor and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA] receptor) and metabotropic glutamate receptors (mGluRs) (46). The iGluRs produce excitatory glutamate-evoked currents, whereas the mGluRs are G protein-coupled receptors that control cellular processes through the G protein signaling cascades. Evidence implicates iGluRs, and/or mGluRs, as potential drug targets in neuropsychiatric disorders, including depression, anxiety, schizophrenia, and AD (46, 47).
One of the iGluRs is the NMDA receptor, which is activated by glutamate as well as glycine as a co-agonist. Its activation allows cations to flow through the cell membrane. The NMDA receptor is very important for controlling synaptic plasticity and memory function (48). Recent studies indicate that the dysregulation of the NMDA receptor may play an important role in schizophrenia (49, 50) and depression (51). Conversely, altered AMPA receptor functioning is a feature of many CNS disorders, including amyotrophic lateral sclerosis (ALS), ischemia, traumatic brain injury, epilepsies, and AD (47). The AMPA receptor is responsible for mediating most of the fast synaptic transmission in the CNS (47). Modulation of AMPA receptor numbers explains much of the plasticity of excitatory transmission in the brain, whereby increasing or decreasing AMPA receptors alters synaptic strength, which may be linked to neuropsychiatric disorders such as schizophrenia and depression (52, 53).
The release of glutamate in the synaptic cleft at a particular concentration is maintained by either glutamine synthetase or excitatory amino acid proteins, i.e., excitatory amino acid transporters (EAATs), which re-uptake excessive glutamate from the synapse. A high density of EAATs near the synapse ensures quick removal or transportation of any unbound glutamate. Of the five different membrane-bound transporters, EAAT2 performs more than 90% of the clearance of extracellular glutamate into crude synaptosomes to prevent neuronal excitotoxicity and hyperexcitability. When this process is impaired, it can allow large amounts of glutamate to spill out from the synapse, which may be a pathophysiological mechanism in CNS disorders (54) including schizophrenia, cognitive deficits, dementia and AD, and other disorders. Evidence suggests that EAAT2 activation is a promising therapeutic approach in many neuropsychiatric disease models (45).
Throughout the CNS, glycine acts as a co-agonist with L-glutamate at NMDA receptors. Glycine fluxes are regulated by two specific glycine transporters: GlyT1 and GlyT2. Whereas, GlyT2 is expressed in the spinal cord, brainstem and cerebellum, GlyT1 is expressed in these regions as well as in the forebrain areas such as the cortex, hippocampus, septum, and thalamus (55). GlyT2 is expressed by glycinergic nerve endings in rat spinal cord, while GlyT1 appears to be preferentially expressed by glial cells. Preclinical investigations suggest that GlyT2 is predominantly responsible for glycine uptake at glycinergic synapses, and that GlyT1 is involved in monitoring glycine concentration surrounding NMDA receptor-expressing synapses. In rats, GlyT1 inhibition potentiates NMDA receptor activity and affects NMDA receptor-dependent long-term potentiation (55). It is possible to modulate the function of the NMDA receptor by varying the availability of the glycine co-agonist. This has potential in the treatment of schizophrenia: the NMDA hypofunction hypothesis of schizophrenia postulates that increasing glutamatergic transmission via the NMDA receptors and inhibiting GlyT1 on glial cells enhances NMDA receptor neurotransmission by slowing the process of removal of glycine from the synapse and thus elevates synaptic glycine levels (55, 56).
Glutamate, Depression, and Anxiety
The monoamine hypothesis of depression contends that the underlying pathophysiology is due to depleted levels of serotonin, norepinephrine, and/or dopamine in the CNS (57). However, no direct evidence supports a primary dysfunction of a specific monoamine system in patients with MDDs. Moreover, not only do many patients fail to respond to monoamine antidepressants, but residual symptoms, relapses and recurrences are common even with adequate dosing of these medications (58) and it can take up to several days or weeks for core depressive symptoms to begin to lift after monoamine antidepressants have elevated synaptic monoamine levels (57–59). MDD pathogenesis appears to involve something else beyond the monoamine system.
Glutamatergic modulation shows potential in antidepressant treatment. A single subanesthetic intravenous dose of the NMDA receptor antagonist ketamine acts rapidly in treatment-resistant depression within hours of administration, with effects that are typically sustained for 7–14 days (60). Moreover, the ketamine metabolite (2R,6R)-hydroxynorketamine [(2R,6R)-HNK] appears to have the antidepressant effects of ketamine and lacks the psychiatric, psychotomimetic, cardiovascular, neurological, and other side effects associated with acute dosing of ketamine in patients with depression (52). Unlike ketamine, (2R,6R)-HNK is not a NMDA receptor antagonist but is associated with the modulation of the AMPA receptor. Thus, both NMDA and AMPA receptors may be involved in the pathophysiological changes of depression and potentially represent new targets for the development of rapid-acting antidepressants.
The glutamatergic system also plays a major role in the pathogenesis of anxiety (61–63). Long-term administration of various antidepressant agents including selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs) lowers glutamatergic activity in some regions, such as the hippocampus (61, 64), while the acute administration of NMDA receptor antagonists produces anti-anxiety and antidepressant effects in preclinical and clinical models (43, 62). Lamotrigine, used in epilepsy and bipolar depression, inhibits glutamate release and has proven efficacy in certain symptoms of post-traumatic stress disorder (PTSD), namely, re-experiencing and avoidance/numbing (65). Similarly, topiramate, which acts partly as an AMPA/kainate blocker, decreases re-experiencing symptoms in PTSD (66).
Glutamate Receptor Dysregulation Contributes to Schizophrenia
Animal models of schizophrenia have implicated glutamate receptor dysregulation and other proteins relating to glutamate transmission, including EAAT2 (54). The glutamate hypothesis of schizophrenia is supported by the finding that the NMDA receptor antagonist phencyclidine blocks glutamate-activated postsynaptic currents and induces schizophrenia-like symptoms, including psychosis and cognitive impairment (50). Moreover, phencyclidine impairs prepulse inhibition (PPI) of the startle reflex, a simple form of information processing that is consistently reduced in schizophrenia. In rats treated with ceftriaxone and acute phencyclidine, PPI was more impaired compared with rats given either treatment alone (67). A broad-spectrum cephalosporin antibiotic, ceftriaxone stimulates EAAT2 expression and lessens neurotoxicity by inhibiting neuronal cell death associated with glutamate excitotoxicity (68). Upregulation of EAAT2 expression by ceftriaxone is thought to be via presynaptic activation of the mGluR, as mGluR2/3 agonist treatment prevented the PPI impairment associated with ceftriaxone-induced upregulation of EAAT2 in rats (69). EAAT2 overexpression causing PPI impairment may be due to glutamate spillover, which mGluR2/3 relies on for activation due to its perisynaptic localization (54). In patients with schizophrenia, oral clozapine 25–35 mg/kg/day for 3 weeks downregulated astrocytic EAAT2 levels and increased extracellular glutamate levels (70).
Interestingly, expression of the presynaptic protein synaptophysin, which is involved in neurotransmitter release, is significantly increased in the same anatomical areas where EAAT2 levels are downregulated by clozapine (71). This suggests that glutamate release may assist clozapine in potentiation of the excitatory synapse. More support for glutamate receptor dysregulation in schizophrenia is seen with the selective GlyT1 inhibitor sarcosine (N-methylglycine), which has shown promise in the treatment of cognitive impairment in patients with chronic schizophrenia (56, 72).
The Dysregulated Glutamate System and AD
It is well-known that AD is associated with reductions in glutamate transporter capacity and protein expression, as well as a selective loss of the vesicular glutamate transporter (73–75). Recent evidence further points to impairment of EAAT2 function in AD, with findings of significantly lower levels of EAAT2 gene expression in the cortex and decreased EAAT2 immunoreactivity in the motor cortex of patients with AD (76). Although EAAT2b expression did not vary significantly according to disease severity, significantly upregulated levels of exon-skipping variant mRNA expression have been found, which reduces wild-type EAAT2 protein expression in primary astrocytes and inhibits glutamate transport (76). The correlation of EAAT2 expression with increasing neurodegeneration, in combination with the ability of exon-skipping variants to reduce glutamate reuptake, suggests that increased glutamate levels may propagate excitotoxic processes implicated in AD pathogenesis.
Moreover, iGluRs may also contribute to the excitotoxic processes implicated in AD pathogenesis. It is well-established that the NMDA receptor plays an important role in excitotoxicity (77). Hyperactivity of the NMDA receptor may result in a flooding of cations (e.g., Ca2+) into the neuron leading to degeneration of the dendritic spines or even the death of the neuron. In patients with AD, treatment with the uncompetitive NMDA receptor antagonist memantine can benefit the cognitive symptoms of AD (78). Notably, in preclinical models of AD, AMPA-mediated transmission, and altered synapse morphology correlated with cognitive decline (47). An initial loss of dendritic spines and synapses is accompanied by a concomitant increase in presynaptic release probability, as the neuronal circuit attempts to compensate for synaptic dysfunction and loss (47). This evidence suggests that increased synaptic glutamate levels induced by a reduction in reuptake may trigger iGluR hyperactivity and lead to the excitotoxic processes relating to the cognitive symptoms of AD.
Acupuncture Modulates Glutamate Neurotransmission
To date, very little neuroscience research has explored the effects of acupuncture on the role of glutamate in neuropsychiatric disorders. While studies of acupuncture analgesia indicate that acupuncture stimulation may modulate levels of expression of glutamate expression and its receptor, as well as EAAT expression (79, 80), no existing studies have reported on acupuncture-induced modulation of the glycine transporter or other upstream regulatory mechanisms (e.g., D-amino acid oxidase and the amino acid transporter system).
Research has reported that both high- and low-frequency EA significantly decreases upregulated levels of NMDA receptor 1 and 2A and AMPA receptor 1 expression in the spinal cord in an inflammatory pain animal model (81). These findings are corroborated by other research reporting that EA (10 Hz) inhibited phosphorylation of NMDA receptor 1 in spinal cord and alleviated pain in a rat model of inflammatory pain (82). At the supraspinal level, alternating high- and low-frequency EA decreased levels of NMDA receptor 1 and c-fos expression in the rostral ventromedial medulla in an animal model of visceral pain (83). Thus, the expression of glutamate and NMDA receptors may be modulated by acupuncture stimulation in the CNS.
Acupuncture also modulates EAAT2 expression. A recent study examined the effect of acupuncture on depressive behaviors and EAAT2 in rats subjected to chronic unpredictable mild stress (44). Both acupuncture therapy and drug treatment with the glutamate reuptake enhancer riluzole significantly increased sucrose consumption in the sucrose preference test paradigm. This increase in sucrose consumption was associated with an elevated food intake and shortened latency in the novelty-suppressed feeding test paradigm. The amelioration of depressive behavioral actions was consistent with increasing numbers of EAAT2-positive cells and protein expression in the hippocampus and prefrontal cortex. EAAT2 mRNA expression was also increased in the prefrontal cortex, but there was no change in the hippocampus. Moreover, the antidepressant effect was observed later with acupuncture than with riluzole, indicating that repeated acupuncture stimulation may be needed to accumulate EAAT2 expression. Thus, acupuncture-mediated modulation of EAAT2 expression may ameliorate depression.
Conclusions
In summary, evidence indicates that acupuncture treatment may be of benefit in several neuropsychiatric disorders, including depression, anxiety, schizophrenia, and AD. The pathophysiology of these disorders may be associated with glutamate dysregulation, marked by a high rate of glutamate release and elevated expression of glutamate receptors and glutamate transporters in the CNS. The ability of acupuncture stimulation to modulate glutamate receptor and EAAT expression suggests that the treatment effects of acupuncture are underpinned by its intervention in the dysregulated glutamate system. Further preclinical and clinical studies are needed to clarify the possible mechanisms of acupuncture in these neuropsychiatric disorders and to establish protocols for treatment guidelines.
Author Contributions
C-HT and IM: collecting and analyzing literature and writing the manuscript; Y-HC: designing and coordinating the study as well as writing the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology, Taiwan (MOST 107-2320-B-039−031 -MY3, 106-2320-B-039−019, 107-2314-B-039-058-MY2, and 106-2314-B-039-012) and China Medical University (CMU 105-S-25). This work was also supported by the Chinese Medicine Research Center, China Medical University from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project under the Ministry of Education (MOE) in Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
1. Stein DJ, Phillips KA, Bolton D, Fulford KW, Sadler JZ, Kendler KS. What is a mental/psychiatric disorder? From DSM-IV to DSM-V. Psychol Med. (2010) 40:1759–65. doi: 10.1017/S0033291709992261
2. Juul SH, Nemeroff CB. Psychiatric epidemiology. Handb Clin Neurol. (2012) 106:167–89. doi: 10.1016/B978-0-444-52002-9.00010-3
3. Park-Lee E, Lipari RN, Hedden SL, Kroutil LA, Porter JD. Receipt of Services for Substance Use Andmental Health Issues Among Adults: Results From the 2016 National Survey on Drug Use and Health CBHSQ Data Review. Rockville, MD: Substance Abuse and Mental Health Services Administration (2012–2017).
4. Substance Abuse and Mental Health Services Administration (SAMHSA). Mental Health and Substance Use Disorders. Rockville, MD: Substance Abuse and Mental Health Services Administration (2017).
5. Wittchen HU, Jacobi F. Size and burden of mental disorders in Europe–a critical review and appraisal of 27 studies. Eur Neuropsychopharmacol. (2005) 15:357–76. doi: 10.1016/j.euroneuro.2005.04.012
6. World Health Organization Regional Office for Europe. Prevalence of Mental Disorders. World Health Organization Regional Office for Europe (2018).
7. Clevenger SS, Malhotra D, Dang J, Vanle B, IsHak WW. The role of selective serotonin reuptake inhibitors in preventing relapse of major depressive disorder. Ther Adv Psychopharmacol. (2018) 8:49–58. doi: 10.1177/2045125317737264
8. Winsky L, Driscoll J, Brady L. CHAPTER 2 - drug discovery and development initiatives at the National Institute of Mental Health: from cell-based systems to proof of concept. In: McArthur RA and Borsini F, Editors. Animal and Translational Models for CNS Drug Discovery. San Diego, CA: Academic Press (2008). p. 59–74.
9. Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. (2008) 85:355–75. doi: 10.1016/j.pneurobio.2008.05.004
10. Smith CA, Armour M, Lee MS, Wang LQ, Hay PJ. Acupuncture for depression. Cochrane Database Syst Rev. (2018) 3:CD004046. doi: 10.1002/14651858.CD004046.pub4
11. Dong B, Chen Z, Yin X, Li D, Ma J, Yin P, et al. The efficacy of acupuncture for treating depression-related insomnia compared with a control group: a systematic review and meta-analysis. Biomed Res Int. (2017) 2017:9614810. doi: 10.1155/2017/9614810
12. Fan L, Gong J, Fu W, Chen Z, Xu N, Liu J, et al. Gender-related differences in outcomes on acupuncture and moxibustion treatment among depression patients. J Altern Complement Med. (2015) 21:673–80. doi: 10.1089/acm.2015.0068
13. Fan L, Fu W-B, Xu N-G, Liu J-H, Li Z-P, Ou A-H. [Impacts of acupuncture and moxibustion on outcome indices of depression patients' subjective reports]. World J Acupunct Moxibust. (2013) 23:22–8.
14. Errington-Evans N., Acupuncture for anxiety. CNS Neurosci Ther. (2012) 18:277–84. doi: 10.1111/j.1755-5949.2011.00254.x
15. Spence DW, Kayumov L, Chen A, Lowe A, Jain U, Katzman MA, et al. Acupuncture increases nocturnal melatonin secretion and reduces insomnia and anxiety: a preliminary report. J Neuropsychiatry Clin Neurosci. (2004) 16:19–28. doi: 10.1176/jnp.16.1.19
16. van den Noort M, Yeo S, Lim S, Lee SH, Staudte H, Bosch P. Acupuncture as add-on treatment of the positive, negative, and cognitive symptoms of patients with schizophrenia: a systematic review. Medicines (2018) 5:29. doi: 10.3390/MEDICINES5020029
17. Bosch P, Staudte H, van den Noort M, Lim S. A case study on acupuncture in the treatment of schizophrenia. Acupunct Med. (2014) 32:286–9. doi: 10.1136/acupmed-2014-010547
18. Ronan P, Robinson N, Harbinson D, Macinnes D. A case study exploration of the value of acupuncture as an adjunct treatment for patients diagnosed with schizophrenia: results and future study design. Zhong Xi Yi Jie He Xue Bao (2011) 9:503–14. doi: 10.3736/jcim20110507
19. Lee MS, Shin BC, Ronan P, Ernst E. Acupuncture for schizophrenia: a systematic review and meta-analysis. Int J Clin Pract. (2009) 63:1622–33. doi: 10.1111/j.1742-1241.2009.02167.x
20. Jia Y, Zhang X, Yu J, Han J, Yu T, Shi J, et al. Acupuncture for patients with mild to moderate Alzheimer's disease: a randomized controlled trial. BMC Complement Altern Med. (2017) 17:556. doi: 10.1186/s12906-017-2064-x
21. Lombardo NBE, Dresser MVB, Malivert M, McManus CA, Vehvilainen L, Ooi WL, et al. Acupuncture as treatment for an21 doixiety and depression in persons with dementia: results of a feasibility and effectiveness study. Alzheimers Care Today (2001) 2:28–41. doi: 10.1016/j.ctim.2007.07.005
22. Kao H, Wang M, Yu S, Yuan S, Mao W, Zhang W, et al. Acupuncture enhancement in clinical symptoms and cognitive motor abilities of the Alzheimer's disease patients. Neurobiol Aging (2000) 21:79 doi: 10.1016/S0197-4580(00)82578-6
23. Chen Y. Clinical research on treating senile dementia by combining acupuncture with acupoint-injection. Acupunct Electrother Res. (1992) 17:61–73. doi: 10.3727/036012992816357800
24. Lao L, Hamilton GR, Fu J, Berman BM. Is acupuncture safe? A systematic review of case reports. Altern Ther Health Med. (2003) 9:72–83.
26. White A. A cumulative review of the range and incidence of significant adverse events associated with acupuncture. Acupunct Med. (2004) 22:122–33. doi: 10.1136/aim.22.3.122
27. Yang C, Hao Z, Zhang LL, Guo Q. Efficacy and safety of acupuncture in children: an overview of systematic reviews. Pediatr Res. (2015) 78:112–9. doi: 10.1038/pr.2015.91
28. He W, Zhao X, Li Y, Xi Q, Guo Y. Adverse events following acupuncture: a systematic review of the Chinese literature for the years 1956-2010. J Altern Complement Med. (2012) 18:892–901. doi: 10.1089/acm.2011.0825
29. Witt CM, Pach D, Brinkhaus B, Wruck K, Tag B, Mank S, et al. Safety of acupuncture: results of a prospective observational study with 229,230 patients and introduction of a medical information and consent form. Forsch Komplementmed. (2009) 16:91–7. doi: 10.1159/000209315
30. Zhao L, Zhang FW, Li Y, Wu X, Zheng H, Cheng LH, et al. Adverse events associated with acupuncture: three multicentre randomized controlled trials of 1968 cases in China. Trials (2011) 12:87. doi: 10.1186/1745-6215-12-87
31. Wu J, Yeung AS, Schnyer R, Wang Y, Mischoulon D. Acupuncture for depression: a review of clinical applications. Can J Psychiatry (2012) 57:397–405. doi: 10.1177/070674371205700702
32. Zhang ZJ, Chen HY, Yip KC, Ng R, Wong VT. The effectiveness and safety of acupuncture therapy in depressive disorders: systematic review and meta-analysis. J Affect Disord. (2010) 124:9–21. doi: 10.1016/j.jad.2009.07.005
33. Chung KF, Yeung WF, Yu YM, Yung KP, Zhang SP, Zhang ZJ, et al. Acupuncture for residual insomnia associated with major depressive disorder: a placebo- and sham-controlled, subject- and assessor-blind, randomized trial. J Clin Psychiatry (2015) 76:e752–60. doi: 10.4088/JCP.14m09124
34. British Acupuncture Council, Acupuncture Research Resource Council. Depression, Anxiety and Acupuncture: The Evidence for Effectiveness. Briefing paper No. 9 (2002).
35. Wang SM, Kain ZN. Auricular acupuncture: a potential treatment for anxiety. Anesth Analg. (2001) 92:548–53. doi: 10.1213/00000539-200102000-00049
36. Wu S, Liang J, Zhu X, Liu X, Miao D. Comparing the treatment effectiveness of body acupuncture and auricular acupuncture in preoperative anxiety treatment. J Res Med Sci. (2011) 16:39–42.
37. Agelink MW, Sanner D, Eich H, Pach J, Bertling R, Lemmer W, et al. [Does acupuncture influence the cardiac autonomic nervous system in patients with minor depression or anxiety disorders?]. Fortschr Neurol Psychiatr. (2003) 71:141–9. doi: 10.1055/s-2003-37756
38. Pilkington K. Anxiety, depression and acupuncture: a review of the clinical research. Auton Neurosci. (2010) 157:91–5. doi: 10.1016/j.autneu.2010.04.002
39. Bosch P, Staudte H, Yeo SJ, Lee SH, Lim S, van den Noort M. Acupuncture treatment of a male patient suffering from long-term schizophrenia and sleep disorders. J Tradit Chin Med. (2017) 37:862–7. doi: 10.1016/S0254-6272(18)30052-9
40. Lanctot KL, Amatniek J, Ancoli-Israel S, Arnold SE, Ballard C, Cohen-Mansfield J, et al. Neuropsychiatric signs and symptoms of Alzheimer's disease: new treatment paradigms. Alzheimers Dement. (2017) 3:440–9. doi: 10.1016/j.trci.2017.07.001
41. Bai L, Zhang M, Chen S, Ai L, Xu M, Wang D, et al. Characterizing acupuncture de qi in mild cognitive impairment: relations with small-world efficiency of functional brain networks. Evid Based Complement Alternat Med. (2013) 2013:304804. doi: 10.1155/2013/304804
42. Soni N, Reddy BV, Kumar P. GLT-1 transporter: an effective pharmacological target for various neurological disorders. Pharmacol Biochem Behav. (2014) 127:70–81. doi: 10.1016/j.pbb.2014.10.001
43. Pilc A, Wieronska JM, Skolnick P. Glutamate-based antidepressants: preclinical psychopharmacology. Biol Psychiatry (2013) 73:1125–32. doi: 10.1016/j.biopsych.2013.01.021
44. Luo D, Ma R, Wu Y, Zhang X, Liu Y, Wang L, et al. Mechanism underlying acupuncture-ameliorated depressive behaviors by enhancing glial glutamate transporter in Chronic Unpredictable Mild Stress (CUMS) rats. Med Sci Monit. (2017) 23:3080–7. doi: 10.12659/MSM.902549
45. Takahashi K, Foster JB, Lin CL. Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cell Mol Life Sci. (2015) 72:3489–506. doi: 10.1007/s00018-015-1937-8
46. Reiner A, Levitz J. Glutamatergic signaling in the central nervous system: ionotropic and metabotropic receptors in concert. Neuron (2018) 98:1080–98. doi: 10.1016/j.neuron.2018.05.018
47. Chang PK, Verbich D, McKinney RA. AMPA receptors as drug targets in neurological disease–advantages, caveats, and future outlook. Eur J Neurosci. (2012) 35:1908–16. doi: 10.1111/j.1460-9568.2012.08165.x
48. Newcomer JW, Farber NB, Olney JW. NMDA receptor function, memory, and brain aging. Dialogues Clin Neurosci. (2000) 2:219–32.
49. Lin CH, Lane HY, Tsai GE. Glutamate signaling in the pathophysiology and therapy of schizophrenia. Pharmacol Biochem Behav. (2012) 100:665–77. doi: 10.1016/j.pbb.2011.03.023
50. Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. (2007) 78:69–108. doi: 10.1016/S0074-7742(06)78003-5
51. Serafini G, Howland RH, Rovedi F, Girardi P, Amore M. The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol. (2014) 12:444–61. doi: 10.2174/1570159X12666140619204251
52. Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature (2016) 533:481–6. doi: 10.1038/nature17998
53. Rubio MD, Drummond JB, Meador-Woodruff JH. Glutamate receptor abnormalities in schizophrenia: implications for innovative treatments. Biomol Ther. (2012) 20:1–18. doi: 10.4062/biomolther.2012.20.1.001
54. O'Donovan SM, Sullivan CR, McCullumsmith RE. The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. npj Schizophrenia (2017) 3:32. doi: 10.1038/s41537-017-0037-1
55. Abdel-Magid AF. Glycine transporter 1 inhibitors and the promise of a new schizophrenia therapy. ACS Med Chem Lett. (2016) 7:549–51. doi: 10.1021/acsmedchemlett.6b00183
56. Hashimoto K. Glycine transport inhibitors for the treatment of schizophrenia. Open Med Chem J. (2010) 4:10–9. doi: 10.2174/1874104501004010010
57. Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry (2000) 61(Suppl. 6):7–11.
58. Pomara N, Sidtis JJ. Brain neurotoxic amyloid-beta peptides: their potential role in the pathophysiology of depression and as molecular therapeutic targets. Br J Pharmacol. (2010) 161:768–70. doi: 10.1111/j.1476-5381.2010.00948.x
59. Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry (2007) 64:327–37. doi: 10.1001/archpsyc.64.3.327
60. Ionescu DF, Papakostas GI. Experimental medication treatment approaches for depression. Transl Psychiatry (2017) 7:e1068. doi: 10.1038/tp.2017.33
61. Riaza Bermudo-Soriano C, Perez-Rodriguez MM, Vaquero-Lorenzo C, Baca-Garcia E. New perspectives in glutamate and anxiety. Pharmacol Biochem Behav. (2012) 100:752–74. doi: 10.1016/j.pbb.2011.04.010
62. Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med. (2006) 73:941–9.
63. Bergink V, van Megen HJ, Westenberg HG. Glutamate and anxiety. Eur Neuropsychopharmacol. (2004) 14:175–83. doi: 10.1016/S0924-977X(03)00100-7
64. Bonanno G, Giambelli R, Raiteri L, Tiraboschi E, Zappettini S, Musazzi L, et al. Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci. (2005) 25:3270–9. doi: 10.1523/JNEUROSCI.5033-04.2005
65. Zavodnick AD, Ali R. Lamotrigine in the treatment of unipolar depression with and without comorbidities: a literature review. Psychiatr Q. (2012) 83:371–83. doi: 10.1007/s11126-012-9208-4
66. Berlant J, van Kammen DP. Open-label topiramate as primary or adjunctive therapy in chronic civilian posttraumatic stress disorder: a preliminary report. J Clin Psychiatry (2002) 63:15–20. doi: 10.4088/JCP.v63n0104
67. Melone M, Bellesi M, Gubbini A, Conti F. GLT-1 up-regulation enhances the effect of PCP on prepulse inhibition of the startle reflex in adult rats. Schizophr Res. (2009) 109:196–7. doi: 10.1016/j.schres.2009.02.010
68. Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, et al. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. (2008) 283:13116–23. doi: 10.1074/jbc.M707697200
69. Bellesi M, Conti F. The mGluR2/3 agonist LY379268 blocks the effects of GLT-1 upregulation on prepulse inhibition of the startle reflex in adult rats. Neuropsychopharmacology (2010) 35:1253–60. doi: 10.1038/npp.2009.225
70. Melone M, Vitellaro-Zuccarello L, Vallejo-Illarramendi A, Pérez-Samartin A, Matute C, Cozzi A, et al. The expression of glutamate transporter GLT-1 in the rat cerebral cortex is down-regulated by the antipsychotic drug clozapine. Mol Psychiatry (2001) 6:380. doi: 10.1038/sj.mp.4000880
71. Bragina L, Melone M, Fattorini G, Torres-Ramos M, Vallejo-Illarramendi A, Matute C, et al. GLT-1 down-regulation induced by clozapine in rat frontal cortex is associated with synaptophysin up-regulation. J Neurochem. (2006) 99:134–41. doi: 10.1111/j.1471-4159.2006.04030.x
72. Lin CY, Liang SY, Chang YC, Ting SY, Kao CL, Wu YH, et al. Adjunctive sarcosine plus benzoate improved cognitive function in chronic schizophrenia patients with constant clinical symptoms: a randomised, double-blind, placebo-controlled trial. World J Biol Psychiatry (2017) 18:357–68. doi: 10.3109/15622975.2015.1117654
73. Kirvell SL, Esiri M, Francis PT. Down-regulation of vesicular glutamate transporters precedes cell loss and pathology in Alzheimer's disease. J Neurochem. (2006) 98:939–50. doi: 10.1111/j.1471-4159.2006.03935.x
74. Li S, Mallory M, Alford M, Tanaka S, Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol. (1997) 56:901–11. doi: 10.1097/00005072-199708000-00008
75. Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann Neurol. (1996) 40:759–66. doi: 10.1002/ana.410400512
76. Scott HA, Gebhardt FM, Mitrovic AD, Vandenberg RJ, Dodd PR. Glutamate transporter variants reduce glutamate uptake in Alzheimer's disease. Neurobiol Aging (2011) 32:553 e1–11. doi: 10.1016/j.neurobiolaging.2010.03.008
78. Parsons CG, Stoffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system–too little activation is bad, too much is even worse. Neuropharmacology (2007) 53:699–723. doi: 10.1016/j.neuropharm.2007.07.013
79. Cui L, Ding Y, Zeng J, Feng Y, Li M, Ding M. Spinal glutamate transporters are involved in the development of electroacupuncture tolerance. Int J Mol Sci. (2016) 17:357. doi: 10.3390/ijms17030357
80. Gao P, Gao XI, Fu T, Xu D, Wen Q. Acupuncture: emerging evidence for its use as an analgesic (Review). Exp Ther Med. (2015) 9:1577–81. doi: 10.3892/etm.2015.2348
81. Choi BT, Kang J, Jo UB. Effects of electroacupuncture with different frequencies on spinal ionotropic glutamate receptor expression in complete Freund's adjuvant-injected rat. Acta Histochem. (2005) 107:67–76. doi: 10.1016/j.acthis.2004.07.008
82. Meng XZ, Lao LX, Shen XY, Berman BM, Ren K, Wei PK, et al. Electroacupuncture inhibits spinal interleukin-17A to alleviate inflammatory pain in a rat model. Open Pain J. (2013) 6:183–9. doi: 10.2174/1876386320130624001
Keywords: acupuncture, glutamate, neuropsychiatric disorders, Alzheimer's disease, depression, anxiety, schizophrenia
Citation: Tu C-H, MacDonald I and Chen Y-H (2019) The Effects of Acupuncture on Glutamatergic Neurotransmission in Depression, Anxiety, Schizophrenia, and Alzheimer's Disease: A Review of the Literature. Front. Psychiatry 10:14. doi: 10.3389/fpsyt.2019.00014
Received: 01 September 2018; Accepted: 10 January 2019;
Published: 12 February 2019.
Edited by:
Kenji Hashimoto, Chiba University, JapanReviewed by:
Marek Schwendt, University of Florida, United StatesLi-Hong Kong, Hubei University of Chinese Medicine, China
Tuya Bao, Beijing University of Chinese Medicine, China
Copyright © 2019 Tu, MacDonald and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Hung Chen, eWlodW5nY2hlbkBtYWlsLmNtdS5lZHUudHc=
 Cheng-Hao Tu
Cheng-Hao Tu Iona MacDonald1
Iona MacDonald1 Yi-Hung Chen
Yi-Hung Chen