
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 18 December 2018
Sec. Schizophrenia
Volume 9 - 2018 | https://doi.org/10.3389/fpsyt.2018.00707
This article is part of the Research Topic Lifestyle Psychiatry View all 19 articles
 Jeroen Deenik1,2*
Jeroen Deenik1,2* Diederik E. Tenback1
Diederik E. Tenback1 Harold F. van Driel1
Harold F. van Driel1 Erwin C. P. M. Tak3
Erwin C. P. M. Tak3 Ingrid J. M. Hendriksen4
Ingrid J. M. Hendriksen4 Peter N. van Harten1,2
Peter N. van Harten1,2Besides having an unhealthy lifestyle contributing to premature mortality, inpatients with severe mental illness (SMI) use high dosages of medication. Previous research has shown improved health after lifestyle improvements in SMI. In addition, we aimed to retrospectively study whether a multidisciplinary lifestyle enhancing treatment (MULTI) was associated with changes in medication use after 18 months, as compared with patients that continued treatment as usual (TAU) and explored mediation by a change in physical activity. We conducted an observational study within a cohort of inpatients with SMI, who received MULTI (N = 65) or continued TAU (N = 49). Data on their somatic and psychotropic medications were collected, converted into defined daily dose (DDD), and analyzed using linear multilevel regression, correcting for baseline value and differences between groups in age, diagnosis, and illness severity. Compared with TAU, the DDD for psychotropic medication significantly decreased with MULTI (B = −0.55, P = 0.02). Changes in total activity did not mediate this association, suggesting that multiple components of MULTI contributed. Corrected between-group analyses for subgroups of medication were not possible due to lack of power and skewed distributions. Within-group data showed a decreased proportion of users as well as median DDD in both groups for almost all medications. In addition to previously reported health improvements after 18 months of MULTI, we observed a significant decrease in dose of psychotropic medication in MULTI compared to TAU. This first study evaluating a wide range of medications indicates a possible effect of lifestyle improvements on medication use in inpatients with SMI. Findings need to be confirmed in future controlled studies, however.
In patients with severe mental illness (SMI), a high prevalence of metabolic risk factors (1–3) contribute to their poor cardiovascular health, largely contributing to a reduced life expectancy of at least 7–20 years compared to the general population (4–6). Besides a sedentary lifestyle (7–10), side effects of psychotropic medication (such as weight gain, dyslipidemia, diabetes mellitus, and direct and indirect harm to the vascular system) are associated with these cardiovascular health issues (11–14). Polypharmacy, higher dosages, and longer-term use are associated with higher risk for most of these side effects and somatic diseases (11, 12, 15). A study in people diagnosed with schizophrenia showed that both no exposure as well as high exposure to antipsychotics were associated with higher cardiovascular mortality than either low or moderate exposure (16). Unfortunately, polypharmacy is very prevalent among people with SMI (15, 17) and the dosage of antipsychotic medication has only increased during the last decades for long-term hospitalized patients (18, 19). Alongside this psychotropic medication, patients use antihypertensive, lipid-lowering, antihyperglycemic and other additional drugs if cardiovascular health issues (whether or not caused by psychotropic medication) are too severe (20–24).
Nowadays, there is more focus on the lowest effective dose, on the avoidance of inappropriate polypharmacy to minimize the side effect of antipsychotics (25–28), and on supporting a healthy lifestyle (29–32). Despite an increased number of studies showing benefits of lifestyle interventions in people with SMI, there is a lack of evidence to support the long-term effectiveness of such interventions in hospitalized patients (33, 34). Moreover, to the best of our knowledge, studies evaluating lifestyle interventions in inpatients did not analyze changes in medication use. Recently, improved physical activity and metabolic health were shown after 18 months in this group of patients receiving a multidisciplinary lifestyle enhancing treatment for inpatients with SMI (MULTI) (35). MULTI was pragmatically implemented in a group of inpatients whose psychotropic medication was already critically reviewed prior to its implementation; hence, dose reduction was not a specific goal. The treatment focused on decreasing sedentary behavior, increasing physical activity, and improving dietary habits in the context of daily treatment.
Because the (side) effects of medications are associated with cardiometabolic health issues, we hypothesized that we might observe changes in medication use after lifestyle changes, in addition to improvements in physical health. Therefore, the present study aimed to retrospectively study whether 18 months of MULTI was associated with changes in medication use, as compared with patients that continued treatment as usual (TAU) and to explore whether changes were mediated by increased physical activity as an essential component of MULTI.
This observational cohort study was conducted at wards for long-term inpatient mental healthcare in a psychiatric hospital of GGz Centraal (The Netherlands). The current study is part of a comprehensive evaluation of MULTI, a treatment that was implemented pragmatically in February 2014 in three wards. MULTI was developed because of the identified need to address the comorbidity (e.g., sleep apnea and cardiovascular morbidity) in this population, in part due to a lack of physical activity, obesity, and dietary risks). The implementation of MULTI (described in the next paragraph) was conducted by psychiatrists, nurses and team leaders in collaboration with activity coordinators and a dietitian. During a previous MULTI study (35), data of baseline (Aug.–Dec. 2013) and one follow-up (Aug.–Dec. 2015) were collected from 114 patients (65 received MULTI and 49 continued TAU). Data on types and dose of medication were extracted from electronic patient records and compared between patients receiving MULTI and those receiving TAU. Because of the observational nature of this study, whereby MULTI was already implemented pragmatically in three wards before the start of this study, no randomization took place. Therefore, we analyzed potential differences between groups at baseline and, if significant, corrected for these differences in analyses as potential confounders. The study protocol was approved by the Medical Ethical Committee of the Isala Academy (case 14.0678). All subjects gave written informed consent in accordance with the Declaration of Helsinki.
The cohort consisted of patients with SMI who had been hospitalized for at least 1 year. Patients were included in the MULTI study if they had not received any other intervention related to lifestyle within 18 months since the start of MULTI and if baseline accelerometer data was available. Exclusion criterion was a lack of data after 18 months due to being either discharged or deceased. Patients included for follow-up were dropped out for further analyses if they had insufficient accelerometer data (see Physical Activity below) or refused the repeated accelerometer measurement.
The purpose of MULTI was a holistic lifestyle change with a focus on decreasing sedentary behavior, increasing physical activity, and improving dietary habits. Improving the daily structure formed the base of the treatment, by starting each day with getting up on time, having three joint meals per day, and an active day program consisting of sports-related activities (e.g., walking, running, yoga, biking, indoor team sports), work-related activities (e.g., gardening and helping out with daily jobs), psycho-education (e.g., about side effects, dietary habits), and skills training (e.g., making a grocery list, shopping, cooking). Also, existing policies were reviewed critically—e.g., the use of personal transport within walking distance around the hospital area for every patient was reduced to its use for immobile patients and in case of extreme weather only. Because of heterogeneity in illness severity and different capabilities and interests, the content and intensity of the day-to-day program were tailored to the particular ward and individual patients by the nurses and specific disciplines (see below). Therefore, the actual frequency, intensity, kind of activities and format (e.g., group or alone) could vary between patients and wards. However, it was intended that all patients were doing some of the activities in the morning and afternoon, to prevent prolonged periods lying in bed or sitting at the ward. Also, the participation of nurses in the day-to-day program was an essential element, which contributed to the culture change within the institution and in the provision of support to patients. MULTI was based on a “change from within-principle” developed by current staff (psychiatrists, nurses, and team leader in collaboration with activity coordinators and a dietitian), working with regular context and resources in daily routine care. It was supervised and disseminated per ward by the head practitioner (a psychiatrist) as an innovative treatment method. Nurses received support from the psychiatrists (psycho-education), activity coordinators, and the dietitian. Adherence to and compliance with the treatment was discussed in the weekly multidisciplinary consultation. If a patient could not get along in the day-to-day program (e.g., getting out of bed or attending selected activities), it was agreed upon that specific action was to be taken to physically activate a particular patient, using extra individual motivational interviewing by their mentor (one of the nurses) or psychiatrist, who were trained in this, and by consulting an activity coordinator or dietitian if needed.
Patients who received TAU continued their treatment, which mainly concerned pharmacological treatment and a less structured day program and did not include any supported lifestyle interventions or adjustments.
Medication use was classified by type of medication in the main groups and by converting doses to daily defined doses (DDD) according to the Anatomical Therapeutic Chemical (ATC) Classification System of the World Health Organization (36). Somatic medications included medications for the alimentary tract and metabolism (A), blood and blood-forming organs (B), cardiovascular system (C), and respiratory system (R). Psychotropic medications included the medications for the nervous system (N). Due to the differences in specific purpose, efficacy, and side effects of psycholeptics (N05), we distinguished antipsychotics (N05A) from anxiolytics, hypnotics, and sedatives (N05B & N05C). Likewise, we distinguished the category antipsychotics into three groups: (i) typical and (ii) atypical antipsychotics (occurring in different subgroups) and (iii) lithium (N05AN). For this study, we only used fixed prescriptions (i.e., not including medication to be given as needed). Physical activity.
To test for potential mediation by a change in physical activity, we used accelerometer data (ActiGraph GT3X+) that was collected within the MULTI study. Detailed procedures and settings used in the baseline and follow-up measurement were described elsewhere (9, 35). Accelerometers were worn on the right hip; wear time of ≥6 h/day for ≥3 days was used as the criterion for sufficient measurement, and the same timeframe (9:00 a.m−10:00 p.m) was used for each dataset to be able to compare individual data. These were analyzed using the ActiGraph (ActiGraph Corp., Pensacola FL, USA) software ActiLife 6.8.0 and calculated into average total activity counts per hour (TAC/h) as a continuous and detailed outcome variable, where more counts indicate a higher level of activity. The GT3X+ showed a high inter- and intra-instrumental reliability and validity in healthy adults. Inter-instrument reliability in free-living conditions (hip worn) was high for overall activity (ICC = 0.97 and a 95% limit of agreement of ±81.3 counts per minute for vector magnitude) (37, 38) and moderate-to-vigorous activity (ICC = 0.99) (39). The GT3X+ showed acceptable agreement between step counts observed in a laboratory setting (ICC = 0.61–0.99) and free-living situations (ICC = 0.90) (40).
Data analyses were performed using SPSS 22.0 and interpreted at a two-tailed significance level of p < 0.05. Differences in patient and disease characteristics between patients who received MULTI and those receiving TAU were analyzed using independent t-test and chi-square statistics. Continuous variables were examined for linearity, normality, and homogeneity as assumptions for linear analysis by comparing means with medians and analyzing frequency histograms, normality plots, and plots of residuals vs. predicted values. If variables were not distributed linearly toward the dependent variables, they were added as tertiles in the analysis, with the first tertile as the reference category. If data was not distributed normally, analyses were performed using non-parametric tests.
Sum scores of changes in DDDs of somatic and psychotropic medications were distributed normally, after excluding two outliers (z < −3) from the DDD of somatic medication. We used linear multilevel regression to evaluate changes in these outcomes between MULTI and TAU, whereby possible clustering of data within wards was taken into account using a two-level structure, with the wards as the first level and the patients as the second. The change scores of the outcome variables were regressed on the treatment variable and adjusted for the baseline value to prevent potential regression to the mean (crude, model 1). We added patient and disease characteristics that significantly differed between patients receiving MULTI and TAU as covariates in models 2 and 3, respectively. We were not able to properly run corrected between-group analyses on subgroups of medication due to skewed distributions and insufficient power for non-parametric between-group analyses. Therefore, we analyzed the change in DDDs within subgroups of medication separately within MULTI and TAU, using Wilcoxon signed-rank tests.
Additionally, final significant models were analyzed for possible mediation by a change in total activity counts per hour (TAC/h), using the PROCESS tool in SPSS (41) (Figure 1). The mediation effect was calculated as the product of those coefficients (ab, not shown within the model) and was considered significant if the bias-corrected and accelerated (BCa) bootstrapped confidence intervals did not include zero.
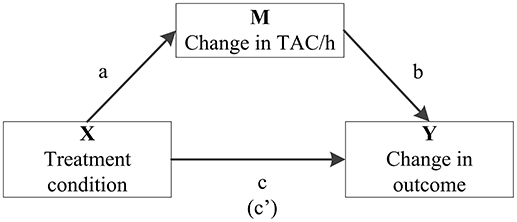
Figure 1. Model summarizing how the role of change in average total activity counts per hour (TAC/h) as a potential mediator (M) in the association between treatment condition (X) and significant change in medication use (Y) is quantified in mediation analysis. It includes the associations between treatment condition and TAC/h (a), TAC/h and the particular outcome variable (b) and treatment condition on the particular outcome variable (c). The coefficient between the latter two, controlling for change in TAC/h, is in parentheses (c′).
Table 1 shows the baseline characteristics of patients receiving MULTI and TAU. None of the included 114 patients dropped out. On average, patients receiving MULTI were younger (M = −6.45 years, 95% CI: −10.27 to −2.64), had a higher baseline illness severity (M = 0.63, 95% CI: 0.17 to 1.08) and were more frequently diagnosed with schizophrenia or other psychotic disorders (X2 = 21.98, p < 0.001) than patients receiving TAU.
Table 2 shows the number of patients using ≥1 medicine within the relevant groups at baseline and follow-up, including their median DDD and the result of analyzing dose changes within both groups. In both MULTI and TAU, there were fewer patients using somatic medication after 18 months, and their median DDD significantly decreased. The same was observed in the totals for psychotropic medication. Table 3 shows the linear regression estimating the effect of MULTI on the change in DDD sum scores for both somatic and psychotropic medication. After adjusting for baseline sum scores, age, diagnosis, and baseline illness severity, the association with the change in the dose of psychotropic medication remained significant in favor of MULTI. This association was not mediated by a change in TAC/h, as no indirect effect was found (ab = 0.06, 95% BCa CI: −0.01–0.26).
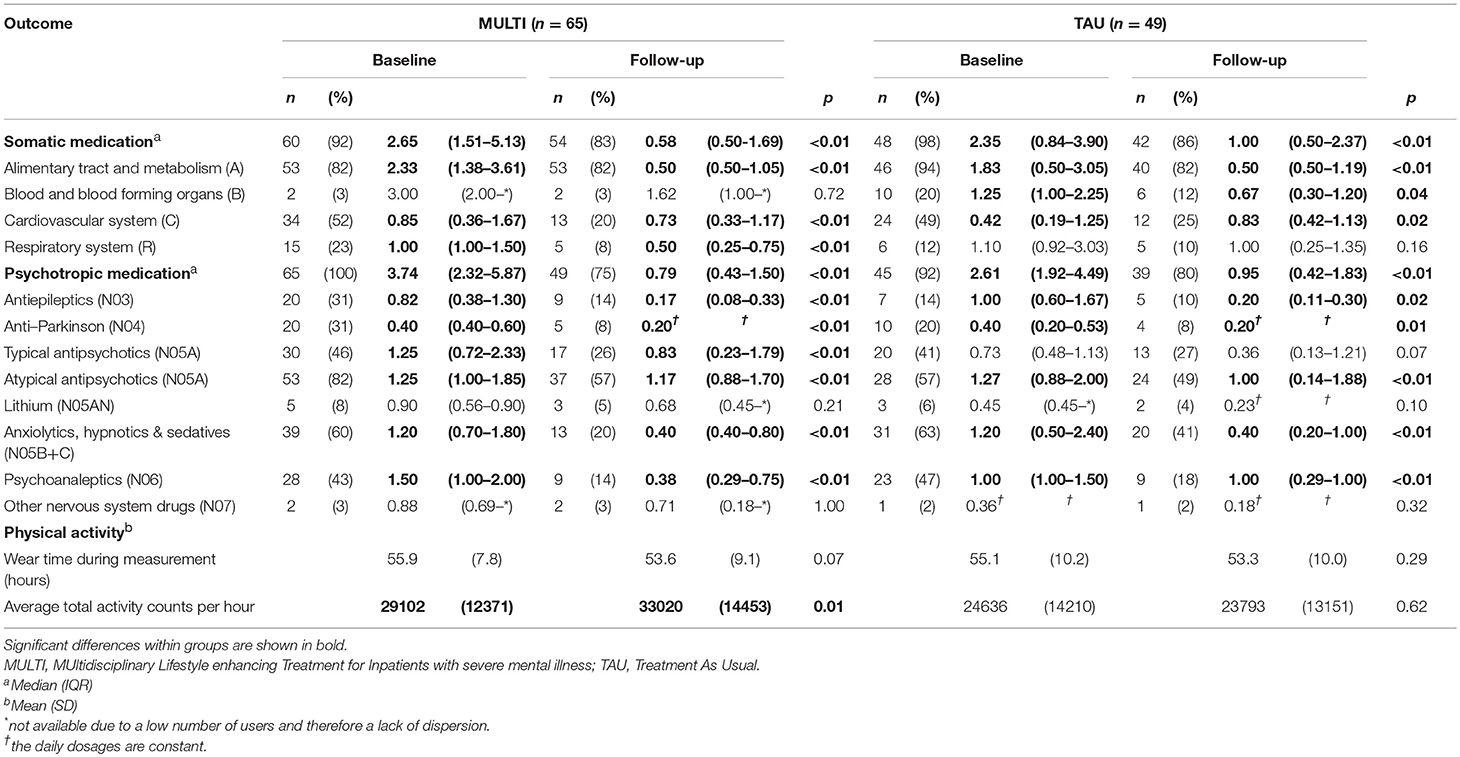
Table 2. Number of patients using at least one medicine within main groups of medication at baseline and follow-up, their median daily defined dose, physical activity scores and the results of analyzing changes in dose and physical activity within both MULTI and TAU (N = 114).
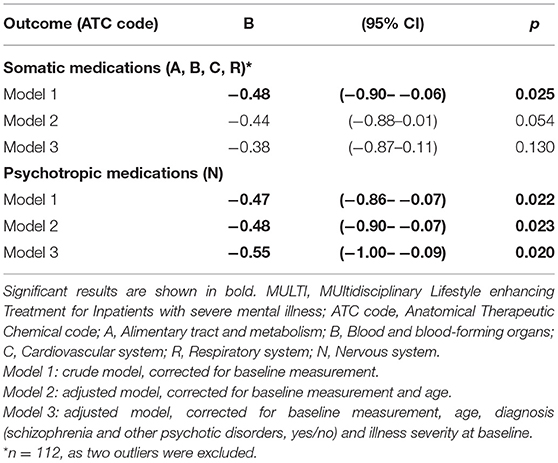
Table 3. Linear regression estimating treatment effects of MULTI compared to treatment as usual on mean daily defined dose (N = 114).
The results of analyses of changes in subgroups of medication are shown in Table 2. For almost all somatic medications, a reduction in the percentage of users was shown in both MULTI and TAU, the largest being in the subgroup regarding the cardiovascular system. The DDD concerning the alimentary tract and metabolism significantly decreased within both groups. For MULTI and TAU this concerned significantly decreased doses of drugs for acid-related disorders (A02; z = −4.22, p < 0.001), constipation (A06; z = −4.68, p < 0.001), and diabetes (A10; z = −2.97, p = 0.003). For TAU, decreases in this main group were also reflected in dose reductions in drugs for acid-related disorders (A02; z = −4.0, p < 0.001) and constipation (A06; z = −4.11, p < 0.001), but not for diabetes. Although the median dose of cardiovascular system drugs increased within TAU, the percentage of users decreased. A significant decrease in dose was observed in beta-blocking agents in both MULTI (C07; z = −4.00, p < 0.001) and TAU (C07; z = −3.54, p < 0.001). Patients receiving MULTI also used a lower dose for disorders of the respiratory system, as shown by a significant decrease of medication for obstructive airway diseases (R03; z = −2.56, p = 0.01), while TAU showed a decreased use of medication of blood and blood-forming organs, as reflected by lower use of antithrombotic agents (B01; z = −2.06, p = 0.04).
For psychotropic medication, a reduction in the percentage of users was shown for almost all subgroups as well, the highest decrease being 40% in users of anxiolytics, hypnotics, and sedatives within MULTI. Both groups showed significant reductions in dose of medications such as antiepileptics, anti-Parkinson drugs, atypical antipsychotics, anxiolytics, hypnotics and sedatives, and psychoanaleptics. In contrast to TAU, MULTI also showed a decrease in the dose of typical antipsychotics.
In the current study, we observed a significant dose reduction in psychotropic medications after 18 months of MULTI, compared to TAU. Although the dose reduction in somatic medication was in favor of MULTI too, it was not significant. When these main groups were split into subgroups, there was a lack of power for corrected between-group analyses. However, the first steps in gaining more detailed insight into dose reductions by using within-group analyses showed interesting leads, for example, in the reduction of medication for diabetes and the respiratory system, which is in line with previously reported physical health improvements within MULTI (35). It also showed clearly the remarkable dose reductions in TAU. It is not likely that this is a time effect, as there was no change of policy in the organization regarding the dosage of medication during these 18 months. The reductions might, however, be a disruptive effect caused by a change in medical staff during the 18 months of this study. The psychiatrist responsible for the majority of the patients within TAU retired and was replaced by a younger colleague, who critically reviewed medication policies. Nevertheless, such a critical review had already been done on the wards receiving MULTI prior to its implementation, so dose reduction was not a specific goal within MULTI. Therefore, the significant decrease in the dose of psychotropic medication is even more striking. One explanation could be that this may be caused partly by decreased psychotic symptoms, but earlier results of the MULTI study showed no significant changes in these symptoms, as compared with TAU, after 18 months (35). Another explanation may be that the psychiatrists reduced the dose of medication based on observed improvements in psychosocial functioning in people who received MULTI (42). It is, however, challenging to clarify specific mechanisms with regard to dose reduction, as there are many variables involved (e.g., interactions between different medications; changes in and preferences of the staff; etc.).
Our findings are somewhat in line with the only other study known to us that evaluated medication use after a lifestyle focused program in patients with SMI (43). In that naturalistic study, Højlund et al. found a decrease in the use of antipsychotics in patients, although they found no specific association between these changes and the degree of participation in the intervention they delivered. However, their reported proportions of patients who used psychotropic co-medication (N categories except for N05) showed little change at follow-up, which is not in line with our observations. Differences may be caused by a different population, as their study was conducted in outpatients with a substantially shorter history of illness, who were relatively young and of whom only a minority used psychotropic co-medication. Also, the absence of a comparison group without lifestyle support makes it difficult to compare results. Indicating decreases in medication use after lifestyle improvement is however very relevant for clinical practice. The ideal situation would be to have medication that addresses the symptoms of psychosis with minimal side effects. However, due to a lack of fundamental innovation in psychopharmacology during recent decades, we still have to work with antipsychotics with a range of motor, metabolic, and cognitive side effects (28, 44). Moreover, as there is still an overall favorable benefit-to-risk ratio for the use of antipsychotics (45), addressing lifestyle is of importance to help minimize such side effects. Besides previously reported improvements in physical health (35), current results show that an integrated multidisciplinary approach is associated with a decrease in the dose of medication. Thereby, it potentially reduces (the risk for) those side effects, which are partly dose-dependent (11, 46). Additionally, because many but not all side effects are reversible, it suggests that lifestyle improvements could contribute to the prevention of irreversible effects, e.g., motor side effects. The reductions found are also promising from the patient's point of view, as side effects and negative attitudes toward medication use are associated with non-adherence, distress, and life impact (47–50).
Our explorative mediation analysis showed that the increase in physical activity observed in patients receiving MULTI after 18 months (35) did not mediate the association between MULTI and change in psychotropic medication use in this same period of time. This indicates that the decrease of psychotropic mediation was not a result of just increasing physical activity. We hypothesize that, with an organizational culture change as a base, multiple components that complement physical activity contribute to improvements, including focus on dietary habits, psycho-education, personal tailoring, and support by peers and qualified participating staff, in line with recent studies advocating the use of such elements (29, 30, 51–53). Such a holistic approach, in which patients are encouraged to do any activities instead of none (i.e., decreasing sedentary behavior), may be more feasible and beneficial in the longer term for inpatients with SMI. While previous studies have shown that structured exercise is feasible in those with SMI in general (31), sedentary behavior (as distinct from physical inactivity) is highly prevalent and associated with cardiometabolic risks that are largely independent of time spent in structured exercise (7, 8). Thus, controlled trials are needed to examine further the feasibility and effects of interventions targeting sedentary behavior.
Limitations in this study mainly arise from the naturalistic and observational design. In this design, we were not able to randomize patients (e.g., the TAU wards treated fewer patients with psychotic disorders) and control for treatment settings in advance (e.g., the continuity of psychiatrists). In addition, although dose reduction was not a specific goal of MULTI, psychiatrists were not blinded for patients' treatment condition (i.e., receiving MULTI or not), which might have affected observed dose reductions. Although this observational study seems the first indicating a possible effect of lifestyle improvements on medication use in inpatients with SMI, future well-designed trials are needed to study the efficacy of lifestyle changes on medication use. Regarding the participation of patients in MULTI, we have no specific data on (the degree of) their adherence. It was intended that all patients were doing some activities in the morning and afternoon, tailored to the particular ward and patients' abilities and interests. However, data on adherence to these individualized day-to-day programs including more detailed data on participation (e.g., frequency and intensity of activities) would make it possible to study the association between the (degree of) adherence and treatment outcomes. Also, our analyses depended on the availability of data within the cohort. Nevertheless, by using multilevel analyses adjusting for differences between MULTI and TAU, we aimed for robust results. However, more data will be needed to have sufficient power for adequate between-group analyses within subgroups of medication, whereby we could gain more detailed insight into specific reductions as indicated by within-group analyses (e.g., drugs for diabetes or obstructive airway diseases). This would provide the opportunity to control for possible differences between groups such as age, which can affect the prescribed dosages of medication (54–56). Furthermore, we used a simple mediation model to explore the influence of a change in physical activity, as an essential element of MULTI, on the association between MULTI and change in medication use. However, the main limitation of this is that by measuring all variables within the mediation analyses nearly simultaneously, we assumed that the association between change in total activity and change in medication use between our pre (T1) and post measurement (T2) would be the same as we would have measured between T2 and a (theoretical) third time point. To study if physical activity mediates associations between lifestyle interventions and medication use, in general, it would be the best to measure physical activity as a middle time-point to study the effect of a change in physical activity over time. Finally, we did not include pro necessitate medication (i.e., to be given as needed) because we cannot specify if and to what extent patients took these medications. The strength of this study is that it is the first study to report detailed dose-specific data on changes in both somatic and psychotropic medication use in inpatients with SMI after lifestyle changes. Despite the limitations, first steps were taken to analyze associations between lifestyle improvements and medication use in these patients, who are suffering from severe physical health problems and receive high dosages of medication. The naturalistic setting of the study improves the generalizability of the results and meets the need for observational studies to supplement randomized controlled trials in order to improve the external validity, such that clinicians treating patients in real-world settings have relevant evidence on which to base their clinical decisions (57). Regarding the impact of critically reviewing medication use that was most likely observed in TAU, it is a strength in favor of MULTI that medication was already critically reviewed on these wards before its implementation, so dose reduction was not a specific goal within MULTI. This suggests that lifestyle improvement has added value with regard to decreasing the dose of medication in inpatients with SMI. Above all, this strengthens the fact that we compared it with TAU. Otherwise, we would only have observed the improvements in MULTI without any context. Moreover, by analyzing possible mediation by total activity, we took a first step to gaining more insight into the contribution of different elements of MULTI to the observed improvements. Finally, by including baseline measures in this analysis, our mediation analysis is mathematically in line with a covariance approach, which was the recommended method for mediation analyses (58, 59).
In summary, in addition to previously reported improvements in physical activity and metabolic health after 18 months of MULTI, we observed a significant decrease in dose of psychotropic medication as compared with TAU. We encourage further longitudinal controlled research to gain more insight into the relationship between lifestyle changes and possible dose reductions to improve health outcomes in inpatients with SMI.
Data are available from the corresponding author on reasonable request and with permission of GGz Centraal.
JD: obtaining funding, study design, retrieving, processing and analyzing data, drafting the manuscript; DT: study design, supporting retrieving, processing and analyzing data; HvD: retrieving, processing and supporting analyzing data; IH: supporting analyzing data; DT and PvH: supporting grant applications; All authors critically revised the manuscript and approved the final version.
This study was supported by an unrestricted grant from Stichting tot Steun VCVGZ. The authors declare that they have no competing interests.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to acknowledge, with thanks, all patients and staff of GGz Centraal who have made efforts to collect the data on which this study was based.
1. Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry (2015) 14:339–47. doi: 10.1002/wps.20252
2. Stubbs B, Vancampfort D, De Hert M, Mitchell AJ. The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: a systematic review and comparative meta-analysis. Acta Psychiatr Scand. (2015) 132:144–57. doi: 10.1111/acps.12439
3. Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry (2017) 16:163–80. doi: 10.1002/wps.20420
4. Piotrowski P, Gondek TM, Krolicka-Deregowska A, Misiak B, Adamowski T, Kiejna A. Causes of mortality in schizophrenia: An updated review of European studies. Psychiatr Danub. (2017) 29:108–20.
5. Hjorthøj C, Sturup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry (2017) 4:295–301. doi: 10.1016/s2215-0366(17)30078-0
6. Tanskanen A, Tiihonen J, Taipale H. Mortality in schizophrenia: 30-year nationwide follow-up study. Acta Psychiatr Scand. 138:492–99. (2018). doi: 10.1111/acps.12913
7. Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary Time and Its Association With Risk for Disease incidence, mortality, and hospitalization in adults a systematic review and meta-analysis. Ann Intern Med. (2015) 162:123–32. doi: 10.7326/m14-1651
8. Brocklebank LA, Falconer CL, Page AS, Perry R, Cooper AR. Accelerometer-measured sedentary time and cardiometabolic biomarkers: a systematic review. Prev Med. (2015) 76:92–102. doi: 10.1016/j.ypmed.2015.04.013
9. Kruisdijk F, Deenik J, Tenback D, Tak E, Beekman A, van Harten P, et al. Accelerometer-measured sedentary behaviour and physical activity of inpatients with severe mental illness. Psychiatry Res. (2017) 254:67–74. doi: 10.1016/j.psychres.2017.04.035
10. Stubbs B, Firth J, Berry A, Schuch FB, Rosenbaum S, Gaughran F, et al. How much physical activity do people with schizophrenia engage in? A systematic review, comparative meta-analysis and meta-regression. Schizophr Res. (2016) 176:431–40. doi: 10.1016/j.schres.2016.05.017
11. Correll CU, Detraux J, De Lepeleire J, De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry (2015) 14:119–36. doi: 10.1002/wps.20204
12. Kahl KG, Westhoff-Bleck M, Kruger THC. Effects of psychopharmacological treatment with antipsychotic drugs on the vascular system. Vascul Pharmacol. (2017). 100:20–5. doi: 10.1016/j.vph.2017.09.001
13. Solmi M, Murru A, Pacchiarotti I, Undurraga J, Veronese N, Fornaro M, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. (2017) 13:757–77. doi: 10.2147/tcrm.s117321
14. Verhaegen AA, Van Gaal LF. Drug-induced obesity and its metabolic consequences: a review with a focus on mechanisms and possible therapeutic options. J Endocrinol Invest. (2017) 40:1165–74. doi: 10.1007/s40618-017-0719-6
15. Westaway K, Sluggett JK, Alderman C, Procter N, Roughead E. Prevalence of multiple antipsychotic use and associated adverse effects in Australians with mental illness. Int J Evid Based Health. (2016) 14:104–12. doi: 10.1097/xeb.0000000000000082
16. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, Bjorkenstam C, Suvisaari J, Alexanderson K, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. (2015) 41:656–63. doi: 10.1093/schbul/sbu164
17. Brett J, Daniels B, Karanges EA, Buckley NA, Schneider C, Nassir A, et al. Psychotropic polypharmacy in Australia, 2006 to 2015: a descriptive cohort study. Br J Clin Pharmacol. (2017) 83:2581–8. doi: 10.1111/bcp.13369
18. Bakker PR, de Groot IW, van Os J, van Harten PN. Long-Stay psychiatric patients: a prospective study revealing persistent Antipsychotic-Induced movement disorder. PLoS ONE (2011) 6:e25588. doi: 10.1371/journal.pone.0025588
19. Mentzel CL, Bakker PR, Van Os J, Drukker M, Matroos GE, Hoek HW, et al. Effect of Antipsychotic type and dose changes on tardive dyskinesia and parkinsonism severity in patients with a serious mental illness: the curacao extrapyramidal syndromes study XII. J Clin Psychiatry (2017) 78:E279–85. doi: 10.4088/JCP.16m11049
20. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. (2016) 37:2999–3058. doi: 10.1093/eurheartj/ehw272
21. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and Management of the Metabolic Syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation (2005) 112:2735–52. doi: 10.1161/circulationaha.105.169404
22. Maayan L, Correll CU. Management of antipsychotic-related weight gain. Expert Rev Neurother. (2010) 10:1175–200. doi: 10.1586/ern.10.85
23. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. (2013) 31:1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc
24. Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. (2013) 34:3035–87. doi: 10.1093/eurheartj/eht108
25. Barnes TR. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. (2011) 25:567–620. doi: 10.1177/0269881110391123
26. Bhugra D, Howes O. Handbook for Psychiatric Trainees. London: Royal College of Psychiatrists (2008).
27. Emsley R. On discontinuing treatment in schizophrenia: a clinical conundrum. NPJ Schizophr. (2017) 3:4. doi: 10.1038/s41537-016-0004-2
28. Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull. (2015) 114:169–79. doi: 10.1093/bmb/ldv017
29. Teasdale SB, Ward PB, Rosenbaum S, Samaras K, Stubbs B. Solving a weighty problem: systematic review and meta-analysis of nutrition interventions in severe mental illness. Br J Psychiatry (2017) 210:110–8. doi: 10.1192/bjp.bp.115.177139
30. Ward MC, White DT, Druss BG. A meta-review of lifestyle interventions for cardiovascular risk factors in the general medical population: lessons for individuals with serious mental illness. J Clin Psychiatry (2015) 76:e477–86. doi: 10.4088/JCP.13r08657
31. Stubbs B, Vancampfort D, Hallgren M, Firth J, Veronese N, Solmi M, et al. EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur Psychiatry (2018) 54:124–44. doi: 10.1016/j.eurpsy.2018.07.004
32. Liu NH, Daumit GL, Dua T, Aquila R, Charlson F, Cuijpers P, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry (2017) 16:30–40. doi: 10.1002/wps.20384
33. Levitt GA, Shinault K, Patterson S, Otaluka O. Weight Gain in Psychiatric Inpatients: Are Interventions Making a Positive Impact? Prim Care Companion CNS Disord. (2017) 19:17m02111. doi: 10.4088/PCC.17m02111
34. Looijmans A, Stiekema A, Bruggeman R, van der Meer L, Stolk RP, Schoevers RA, et al. Changing the obesogenic environment to improve cardiometabolic health in residential patients with a severe mental Illness: ELIPS, a randomized controlled trial. Br J Psychiatry (2017) 211:296–303. doi: 10.1186/s12888-014-0293-9
35. Deenik J, Tenback DE, Tak ECPM, Rutters F, Hendriksen IJM, van Harten PN. Changes in physical and psychiatric health after a multidisciplinary lifestyle enhancing treatment for inpatients with severe mental illness: the MULTI study I. Schizophr Res. (2018). doi: 10.1016/j.schres.2018.07.033. [Epub ahead of print].
36. WHO Collaborating Centre for Drug Statistics Methodology. ATC classification index with DDDs, 2017. Oslo: World Health Organisation (2016).
37. Ozemek C, Kirschner MM, Wilkerson BS, Byun W, Kaminsky LA. Intermonitor reliability of the GT3X+ accelerometer at hip, wrist and ankle sites during activities of daily living. Physiol Meas. (2014) 35:129–38. doi: 10.1088/0967-3334/35/2/12
38. Aadland E, Ylvisaker E. Reliability of the actigraph GT3X+ accelerometer in adults under free-living conditions. PLoS ONE (2015) 10:e0134606. doi: 10.1371/journal.pone.0134606
39. Jarrett H, Fitzgerald L, Routen AC. Interinstrument reliability of the ActiGraph GT3X+ambulatory activity monitor during free-living conditions in adults. J Phys Activity Health (2015) 12:382–7. doi: 10.1123/jpah.2013-0070
40. Lee JA, Williams SM, Brown DD, Laurson KR. Concurrent validation of the Actigraph gt3x+, Polar Active accelerometer, Omron HJ-720 and Yamax Digiwalker SW-701 pedometer step counts in lab-based and free-living settings. J Sports Sci. (2015) 33:991–1000. doi: 10.1080/02640414.2014.981848
41. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis, Second Edition: A Regression-Based Approach. New York, NY: Guilford Publications (2018).
42. Deenik J, Tenback DE, Tak ECPM, Hendriksen IJM, van Harten PN. Improved quality of life and psychosocial functioning in inpatients with severe mental illness receiving a multidisciplinary lifestyle enhancing treatment. The MULTI study II. Mental Health Phys Activity (2018) 15:145–52. doi: 10.1016/j.mhpa.2018.10.004
43. Højlund M, Elliott AF, Madsen NJ, Viuff AG, Munk-Jørgensen P, Hjorth P. Changes in antipsychotics and other psychotropic drugs during a 30-month lifestyle intervention among outpatients with schizophrenia. Nordic J Psychiatry (2017) 71:598–604. doi: 10.1080/08039488.2017.1365379
44. Dimitrelis K, Shankar R. Pharmacological treatment of schizophrenia – a review of progress. Prog Neurol Psychiatry (2016) 20:28–35. doi: 10.1002/pnp.430
45. Correll CU, Rubio JM, Kane JM. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry (2018) 17:149–60. doi: 10.1002/wps.20516
46. Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet (2009) 373:31–41. doi: 10.1016/s0140-6736(08)61764-x
47. Ashoorian D, Davidson R, Rock D, Dragovic M, Clifford R. A clinical communication tool for the assessment of psychotropic medication side effects. Psychiatry Res. (2015) 230:643–57. doi: 10.1016/j.psychres.2015.10.022
48. Velligan DI, Sajatovic M, Hatch A, Kramata P, Docherty JP. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adher. (2017) 11:449–68. doi: 10.2147/ppa.s124658
49. Wykes T, Evans J, Paton C, Barnes TRE, Taylor D, Bentall R, et al. What side effects are problematic for patients prescribed antipsychotic medication? The Maudsley Side Effects (MSE) measure for antipsychotic medication. Psychol Med. (2017) 47:2369–78. doi: 10.1017/s0033291717000903
50. Hui CL, Poon VW, Ko WT, Miao HY, Chang WC, Lee EH, et al. Risk factors for antipsychotic medication non-adherence behaviors and attitudes in adult-onset psychosis. Schizophr Res. (2016) 174:144–9. doi: 10.1016/j.schres.2016.03.026
51. Long C, Rowell A, Rigg S, Livesey F, McAllister P. What is effective in promoting a healthy lifestyle in secure psychiatric settings? A review of the evidence for an integrated programme that targets modifiable health risk behaviours. J Forensic Pract. (2016) 18:204–15. doi: 10.1108/jfp-12-2015-0055
52. Roberts SH, Bailey JE. Incentives and barriers to lifestyle interventions for people with severe mental illness: a narrative synthesis of quantitative, qualitative and mixed methods studies. J Adv Nurs. (2011) 67:690–708. doi: 10.1111/j.1365-2648.2010.05546.x
53. Vancampfort D, Rosenbaum S, Schuch F, Ward PB, Richards J, Mugisha J, et al. Cardiorespiratory fitness in severe mental illness: a systematic review and meta-analysis. Sports Med. (2017) 47:343–52. doi: 10.1007/s40279-016-0574-1
54. Rej S, Beaulieu S, Segal M, Low NC, Mucsi I, Holcroft C, et al. Lithium dosing and serum concentrations across the age spectrum: from early adulthood to the tenth decade of life. Drugs Aging (2014) 31:911–6. doi: 10.1007/s40266-014-0221-1
55. de Mendonca Lima CA, Baumann P, Brawand-Amey M, Brogli C, Jacquet S, Cochard N, et al. Effect of age and gender on citalopram and desmethylcitalopram steady-state plasma concentrations in adults and elderly depressed patients. Prog Neuropsychopharmacol Biol Psychiatry (2005) 29:952–6. doi: 10.1016/j.pnpbp.2005.06.001
56. Beyth RJ, Shorr RI. Principles of drug therapy in older patients: rational drug prescribing. Clin Geriatr Med. (2002) 18:577–92. doi: 10.1016/S0749-0690(02)00017-4
57. Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials (2015) 16:495. doi: 10.1186/s13063-015-1023-4
58. Landau S, Emsley R, Dunn G. Beyond total treatment effects in randomised controlled trials: Baseline measurement of intermediate outcomes needed to reduce confounding in mediation investigations. Clin Trials (2018) 15:247–56. doi: 10.1177/1740774518760300
Keywords: medication, antipsychotics, physical activity, hospitalization, severe mental illness, schizophrenia, lifestyle, side effects
Citation: Deenik J, Tenback DE, van Driel HF, Tak ECPM, Hendriksen IJM and van Harten PN (2018) Less Medication Use in Inpatients With Severe Mental Illness Receiving a Multidisciplinary Lifestyle Enhancing Treatment. The MULTI Study III. Front. Psychiatry 9:707. doi: 10.3389/fpsyt.2018.00707
Received: 22 September 2018; Accepted: 03 December 2018;
Published: 18 December 2018.
Edited by:
Joseph Firth, Western Sydney University, AustraliaReviewed by:
Simon Rosenbaum, University of New South Wales, AustraliaCopyright © 2018 Deenik, Tenback, van Driel, Tak, Hendriksen and van Harten. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeroen Deenik, ai5kZWVuaWtAZ2d6Y2VudHJhYWwubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.