- 1Department of Psychiatry and Psychotherapy, University Hospital Leipzig, Leipzig, Germany
- 2Leipzig University Medical Center, IFB Adiposity Diseases, Leipzig, Germany
- 3Department of Psychological Medicine, King's College London, London, United Kingdom
- 4Institute of Laboratory Medicine, University Hospital Munich, LMU Munich, Munich, Germany
Background: The personality trait neuroticism has been implicated in a poor response to stress, may relate to increased concentrations of cytokines and the development of depression. Inflammatory mechanisms may also be associated with the onset, severity and symptoms of depression. Both are related to poor antidepressant treatment outcome. Therefore, mediators of inflammation may bridge the relationship between neuroticism and depression.
Methods: To disentangle these interrelationships, the associations between neuroticism (according to NEO-PIR-N), depressive symptoms (BDI-II scores) and serum levels of hsCRP, TNF-α, IFN-γ, IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, GM-CSF were investigated in a group of 212 participants, consisting of 37 depressed and 175 non-depressed subjects. A mediation model was used to investigate whether the impact of neuroticism on depressive symptoms may be mediated by cytokines.
Results: Regression analyses revealed that IFN-γ, IL-5, and IL-12-levels, but none of the anti-inflammatory cytokines, were associated with the overall neuroticism score and several of the cytokines were related to the different facets of neuroticism. TNF-α, IFN-γ, IL-5, IL-12, and IL-13 were further related to the severity of depressive symptoms, as well as the somatic-affective and the cognitive dimensions of depression. Pro-inflammatory IFN-γ, IL-5 and IL-12 were identified as mediators of the positive prediction of depression severity by the degree of neuroticism.
Conclusions: The current findings demonstrate that conditions related to long-term stress, such as depression and high neuroticism, are related to an up-regulation of inflammatory agents. Neuroticism may increase stress perception and, thus, increase the production of pro-inflammatory messenger molecules which are involved in the development of depression. This evidence may contribute to future anti-inflammatory interventions, particularly in subjects with high neuroticism who are at risk for developing depression. Furthermore, depressed patients with high neuroticism and cytokine levels may require early escalations in the intensity of treatment, along with additional therapeutic elements to increase the rate of treatment success.
Introduction
Neuroticism, as one of the Big Five higher-order personality traits, represents the tendency to experience negative emotions, such as anxiety and anger, and to have an increased perception of stress, as well as the inability to relieve the self from and to cope with stress (1). Acute and chronic stress have been shown to significantly influence cytokine production in human and animal studies (2, 3). In accord, higher neuroticism as well as stress-related disorders, such as post-traumatic stress disorder (PTSD), have been found to be associated with higher levels of pro-inflammatory agents (4, 5). However, investigations on neuroticism have been limited to a few inflammatory parameters: for example, C-reactive protein (CRP) or interleukin (IL)-6, for which the relationship with neuroticism could not be confirmed (6). Importantly, neuroticism is considered a risk factor for the development and onset of major depression and certain subtypes of depression (7–9). However, the biological mechanisms for this relationship are not yet well understood.
Alongside neuroticism, a body of evidence supports the involvement of low-grade inflammation in the pathogenesis of depressive disorders (10). Accordingly, cross-sectional studies have demonstrated an association between pro-inflammatory markers and the presence, course and treatment outcome of major depression (11–14). Inconsistent results have been observed in the relationship between pro-inflammatory cytokines and the severity of depressive symptoms, as measured in cohorts of patients with major depression or physical illnesses concomitant with depressive syndromes, and in population-based studies (15–22). Additionally, anti-inflammatory cytokines have gained little attention in these investigations (15, 23). The relationship between the symptoms and severity of depression and cytokine regulation should be interpreted with caution given that the majority of investigations were limited to a few parameters, in particular CRP and IL-6, and relevant confounders, such as the distribution of sexes, have not always been taken into account sufficiently.
Given that only a small number of mediators of inflammation have yet been investigated with regards to neuroticism, results on inflammation and depressive symptomatology are inconclusive and the biological pathways for the relationship between neuroticism and depression are unidentified. Therefore, the aim of this study was firstly to investigate the hypothesis that pro- and anti-inflammatory markers are associated with the severity of depressive symptoms and the personality trait neuroticism. Secondly, we aimed to explore if cytokines mediate the relationship between depressive symptoms and neuroticism.
Methods
Participants
The presented data were collected as part of the “OBDEP” research project (Obesity and Depression: pathogenetic role of sleep and wakefulness regulation, motor activity level and neurochemical aspects). Three-hundred and four participants were initially recruited from the outpatient clinic of the Integrated Research and Treatment Center (IFB) for Adiposity Diseases Leipzig, from the Department of Psychiatry and Psychotherapy at the University Hospital Leipzig and via advertisements (intranet, internet, local newspapers). Evaluation of inclusion and exclusion criteria for the study was performed in two stages, as previously reported (24). First, potential participants took part in a telephone screening interview, which involved collecting socio-demographic data, assessing the presence of physical illnesses and completing a checklist of the Structured Clinical Interview for DSM-IV [SCID-I; (25)]. Following this, potentially eligible participants were invited to the study center, where exclusion criteria were assessed in more detail. In cases of positive SCID-screening or known diagnosis of depression, the SCID-I was performed in full. Exclusion criteria were DSM-IV Axis 1 disorders for the non-depressed participants and DSM-IV Axis 1 disorders other than a major depression for the depressed subjects. For all subjects, further exclusion criteria were acute or chronic infections (according to clinical investigation and/or CRP ≥ 10 mg/l), current medication treatment with a non-steroidal anti-inflammatory drug (NSAID), the presence of current and/or past neurological disorders, and a history of head injury with loss of consciousness exceeding 1 h. Assessments for current and past history of health problems and current medication were performed using standardized questionnaires. Only data sets of participants who completed both the Beck Depression Inventory II [BDI-II; (26)] and the Revised NEO Personality Inventory [NEO-PIR-N; (27)] were included in the statistical analyses presented herewith, resulting in a total sample of 212 participants, including 175 non-depressed and 37 depressed subjects. All participants were aged 18–70 years. Written informed consent was obtained from all participants. The study was approved by Leipzig University Ethics Committee (#015-10-18012009).
Assessments
Depressive symptoms were assessed using the Beck Depression Inventory II [BDI-II; (26)]. Along with a total sum score, sub-scale scores for a somatic-affective factor and a cognitive factor were calculated (28). The somatic-affective factor was calculated by summation of scores on items 4, 11-13, and 15-21 and the cognitive factor by summation of scores on items 1-3, 5-9, and 14. To assess neuroticism, the neuroticism domain (consisting of six dimensions: anxiety, hostility, depression, self-consciousness, stress vulnerability, impulsiveness) of the Revised NEO Personality Inventory [NEO-PIR-N; (27)] was used.
Cytokine Measurement
After blood drawing, serum probes were immediately centrifuged at 3,000 rpm for 10 min. The supernatant was aliquoted and stored in non-absorbing polypropylene tubes of 300 μl. Probes were shock-frozen in liquid nitrogen and stored in freezers at −80°C until required. Cytokines were measured using the Bio-Plex Pro human cytokine Th1/Th2 immunoassay from Bio Rad, Germany, a 96-well kit that uses coupled magnetic beads and detection antibodies. This multiplex assay detects IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, GM-CSF, IFN-γ, and TNF-α. The intraassay coefficient of variation (CV) for cytokines was between 1.6 and 3.8%. High sensitivity (hs)-CRP was measured using a turbidimetric assay on an AU 5800 analyzer, Beckman Coulter, Germany. For hs-CRP intraassay CV was 4.1%.
Statistics
CRP and the nine pro- and anti-inflammatory cytokines were log-transformed in order to obtain approximately normally distributed variables. We investigated the association between depressive symptoms and neuroticism with the log-transformed serum concentrations of CRP and the cytokines by linear regression analyses.
Multiple regression analyses were performed to determine the relationship between the dependent variable (i.e., depressive symptoms according to BDI-scores) and each of the log-transformed CRP and cytokine values. Regression analyses were controlled for confounding variables known to impact cytokine levels: age, sex, smoking status, BMI and time of blood drawing. Sum values of the physical illnesses and medication listed in Table 1 were further included as control variables ranging from 0 (“none”) to the maximum of 4. Levels of significance were adjusted for multiple testing using the False Discovery Rate [FDR; according to (29)] for BDI-II, NEO-PIR-N and the respective cytokines separately. For all other analyses, the level of significance was set at p < 0.05. Mediation analyses were performed for those cytokines significantly associated with sum scores of both BDI-II and NEO-PIR-N. For the mediation analyses, residuals were calculated for logTNF-α, logIFN-γ, logIL-5, logIL-12, logIL-13, BDI-II sum scores and NEO-PIR-N sum scores through listwise-regression analyses in order to exclude significant confounders. Covariates included into the analyses were: “age,” “sex,” “BMI,” “smoking status,” “time of blood sampling,” “sum of physical illnesses,” and “sum of medication.” After excluding multicollinearity between the parameters, mediation analyses were performed with the freely available SPSS macro “sobel” (http://www.processmacro.org/download.html), which includes bootstrapping and Sobel test (30). All further analyses were performed with SPSS Version 24 (IBM Corporation; Armonk, NY, USA).
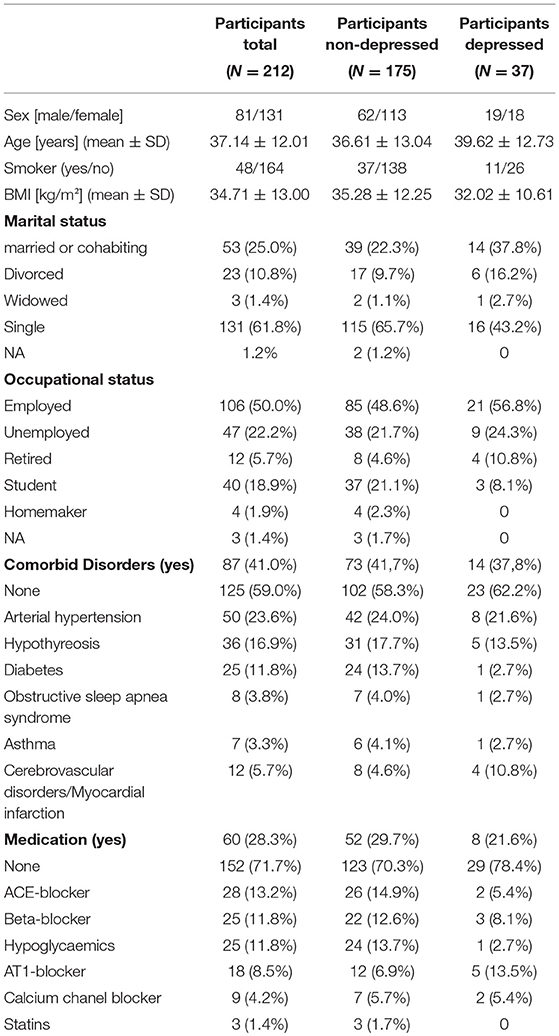
Table 1. Sociodemographic details for the total sample, and for participants with and without a diagnosis of major depression, separately.
Results
Two hundred twelve participants, consisting of 37 depressed and 175 non-depressed subjects, were included in the final analyses. Demographic data, including medication status and the presence of physical illnesses, are presented in Table 1. Serum concentrations of CRP and cytokines, depression symptoms and neuroticism scores are shown in Table 2.
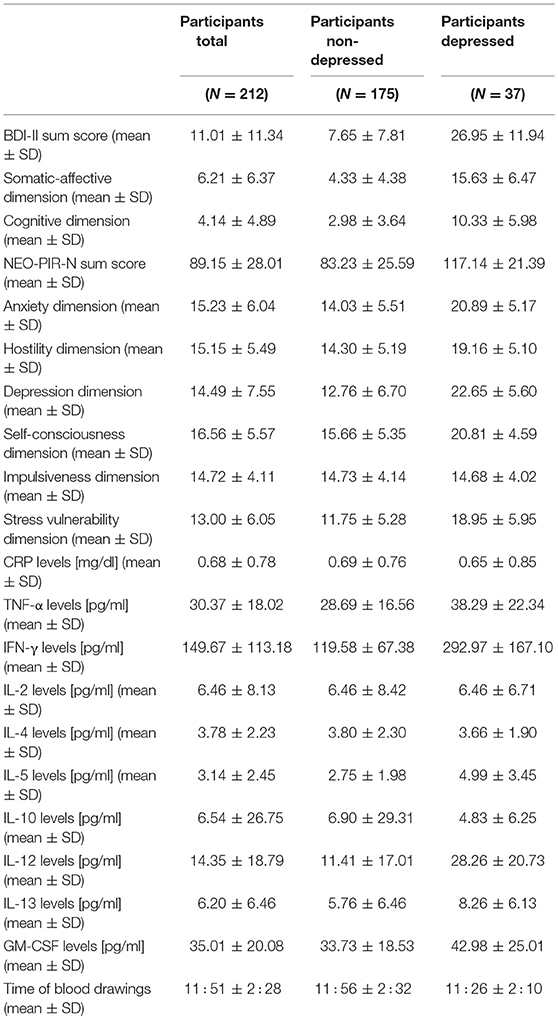
Table 2. Sum total and dimension scores for depression and neuroticism, and concentrations of markers of inflammation in the total sample, and for participants with and without a diagnosis of major depression, separately.
CRP, Cytokines and Depression Symptoms
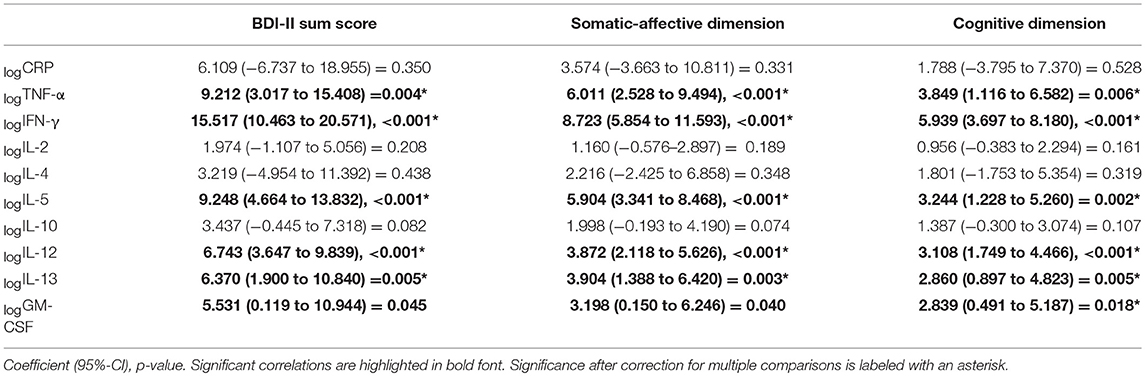
Linear regression analyses showed a significant increase in the severity of depressive symptoms associated with higher logTNF-α, logIFN-γ, logIL-5, logIL-12, and logIL13 (Table 3). The BDI-II sum score increased by a coefficient of up to 15.5 for each standard deviation of logIFN-γ, followed by 9.2 for each standard deviation of logTNF-α, by a coefficient of 9.2 for logIL-5, 6.7 for logIL-12 and 6.4 for logIL-13. Scores on the somatic-affective and cognitive dimensions of the BDI-II were significantly associated with logTNF-α, logIFN-γ, logIL-5, logIL-12, logIL13 and logGM-CSF. No significant association between the sum scores, the dimensions of depression and the cytokine levels were observed for logIL-2, logIL-4 or logIL-10 and also logCRP.
CRP, Cytokines and Neuroticism
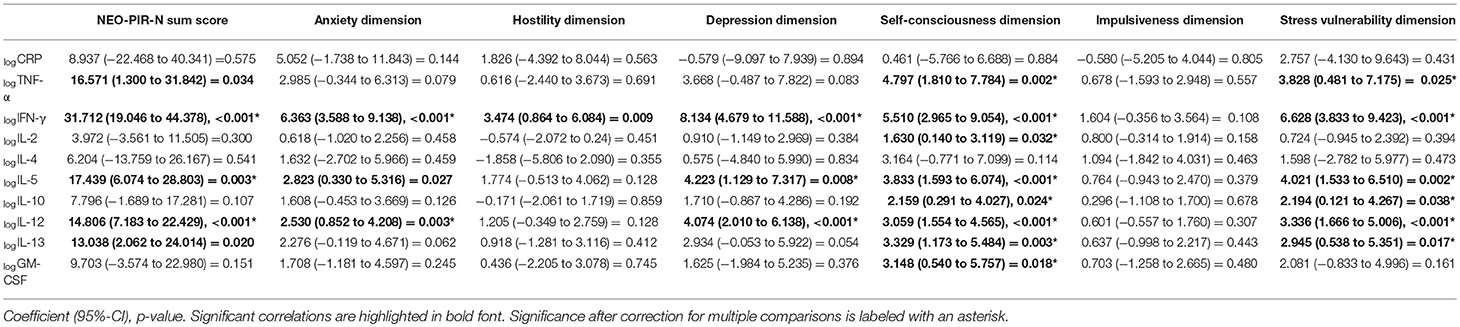
Linear regression analyses between the pro-and anti-inflammatory markers, the six different dimensions of neuroticism and the sum score of neuroticism showed that a significant increase in the magnitude of neuroticism (NEO PI-R-N sum score) was dependent on higher logIFN-γ, logIL-5 and logIL-12 after correcting for multiple testing (Table 4). The increase in the anxiety dimension was predicted by logIFN-γ and logIL-12 levels. The depression dimension was found to significantly depend on logIFN-γ, logIL-5, and logIL-12. The self-consciousness dimension depended significantly on all parameters except logCRP and logIL4. The stress vulnerability dimension depended on logTNF-α, logIFN-γ, logIL-5, logIL-10, logIL-12, and logIL-13. The hostility dimension and the impulsiveness dimension were not associated with any of the cytokines.
Relationship Between Neuroticism, Depressive Symptoms and Inflammatory Markers
In a first step, the residuals of the BDI-II scores were predicted by the residuals of the NEO-scores. In a second step, the residuals of the cytokine levels (logTNF-α, logIFN-γ, logIL-5, logIL-12, logIL-13) were predicted by the residuals of the NEO-scores. In a third step, the residuals of the BDI-II-scores were predicted by the residuals of the cytokine levels and the NEO-scores. The results of the regression analyses are presented in Table 5
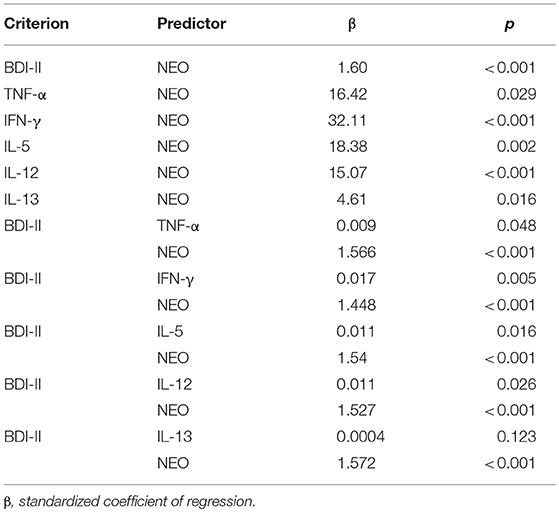
Table 5. Mediation analyses Regression analyses between dimensions of depression, neuroticism and the inflammatory agents.
The bootstrapping analysis with m = 5,000 estimates revealed a significant indirect effect of the residuals of the NEO-scores on the residuals of the BDI-scores through the residuals of logIFN-γ (95% CI 0.0097, 0.0462), logIL-5 (95% CI 0.0027, 0.0230) and logIL-12 (95% CI 0.0013, 0.0291) -residuals. As an example, the standardized coefficients of regression for logIFN-γ are depicted in Figure 1. The Sobel-Z-test revealed a significant effect for the residuals through logIFN-γ (Z = 2.88, p = 0.004) that was trending for logIL-5 (Z = 1.89, p = 0.059) and IL-12 (Z = 1.91, p = 0.056).
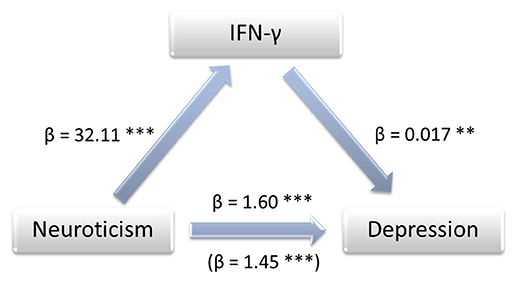
Figure 1. Mediation model showing the impact of IFN-γ on the association between neuroticism and depression. Annotations: β, standardized coefficient of regression, **p < 0.01, ***p < 0.001.
Discussion
The current findings on the relationship between both pro- and anti-inflammatory markers and characteristics of depression and neuroticism confirmed our hypothesis of a positive association between inflammatory agents and the degree of depression and neuroticism. The results further revealed that markers of inflammation may be significant mediators for the positive relationship between neuroticism and depressive symptoms.
After correcting for multiple testing and controlling for potential confounding variables, the analyses revealed that depressive symptoms were association with the cytokines TNF-α, IFN-γ, IL-5, IL-12, and IL-13. These results expand on the range of cytokines previously investigated. They also add to existing findings suggesting that a pro-inflammatory state is related to depressive symptoms in the general population as well as in cohorts of depressed subjects [e.g., (15–17, 31)]. Extending on previous research that focussed on pro-inflammatory markers, our analyses could not demonstrate an affection of the degree of depressive mood and cognition by the anti-inflammatory mediators IL-4 and IL-10. Though anti-inflammatory cytokines, have been found to be elevated in major depression and in response to pro-depressive agents (24, 32, 33), the degree of depression could previously not be statistically explained by IL-10 or IL-4 (24, 31). However, the sample sizes here and in previous studies may, in part, account for the negative results for which small effect sizes were to be expected (34). Results of a decreased IL-4-responsiveness of microglia as well as the inhibition of IL-10-signaling leading to depression-like behavior in animal models of depression demonstrate the need for further investigations on the role of anti-inflammatory agents in depression (35, 36).
The present results do not support the involvement of CRP as a relevant mediator once relevant covariates were accounted for (13, 15). In line with this, most of the mechanisms involved in the inflammation-depression -relationship have been described for TNF-α and IFN-γ, but not CRP, including the stimulation of the indolamine-2,3-dioxygenase (IDO) (10), the relationship with the psychopathology of depression (37–39) and the potential as an antidepressant drug target (14, 40). As for CRP, the differences in results between correlation and regression analyses (41) and the variability in results across other studies, showing no relationship between cytokines and depressive symptoms (20, 24), illustrates the need to account for potential confounders. Studies not including essential confounding parameters, such as BMI, smoking habits and inflammation-modulating drugs, should therefore be considered with caution.
Overall neuroticism and also several dimensions of neuroticism, in particular the self-consciousness and the stress vulnerability dimensions, were related to multiple cytokines, such as IFN-γ and TNF-α; however no such relationship was observed for the anti-inflammatory IL-4 and IL-10. These associations highlight the relationship between personality traits leading to long-term impairments in stress response and the regulation of mediators of inflammation. There is little data to which we can compare our findings; however, for CRP, the absence of associations are in line with a recent meta-analysis showing CRP and IL-6 not to be related to neuroticism (6). To our knowledge, this is the first study in the English language to examine the relationship between neuroticism and the anti-inflammatory cytokines IL-4 and IL-10, which warrants replication. For TNF-α, the findings from the limited number of previous studies conducted are consistent with ours; for example, in patients with hepatitis C, TNF-α correlated with scores of neuroticism after therapy (4). Hypothetically, the finding of up-regulation of markers of inflammation in neuroticism may explain the increased incidence of a wide range of physical illnesses in people vulnerable to neuroticism (42). Also, neuroticism may be associated with increased cytokine levels due to its relationship with a higher prevalence of obesity and smoking habits, factors known to up-regulate pro-inflammatory cytokines (9, 43, 44).
With regards to our findings of a relationship between neuroticism and depressive symptoms, neuroticism has repeatedly been shown to be associated with or to be a risk factor for the development of depressive disorders (7–9). To the best of our knowledge, this is the first time that inflammatory markers have been identified as a relevant mediator of this association. One possible patho-biological pathway for this link could be hypothalamic-pituitary-adrenal (HPA) axis activity: chronic stress induces the upregulation of HPA axis activity as well as the synthesis of cytokines (45–47). Some cytokines, for example IFN-γ, in turn stimulate the HPA axis (48). Of note, HPA axis activity has been found to be altered both in major depression and neuroticism (45). Other identified links between neuroticism and depression include a decreased expression of brain-derived neurotrophic factor (BDNF), as well as the activity of the anterior cingulate cortex [ACC; (45, 46)], for which an involvement of cytokines has been described (11, 49). Regarding the integration of this relationship into antidepressant treatment strategies, it should be of note that both inflammation (13, 18) and neuroticism (50, 51) impact on the treatment outcome of depression. Therefore, depressed patients with high neuroticism may require more specialized clinical efforts (50). In addition, the determination of inflammatory mediators as well as factors associated with increased cytokine levels and neuroticism, such as obesity and smoking should be taken into account (9, 19, 43, 44). Psychotherapeutic interventions may exert anti-depressant effects via modulation of neuroticism (52), however the question as to whether this is related to observed reductions of inflammation (53) is yet unknown.
This study needs to be considered in light of several limitations: The analyses were performed in participants with a high proportion of obesity which is unlikely to represent the general population. The sample size of the group of depressed patients was too small to perform separate analysis in this group and to rule out type II errors. This may also be the case for the total number of participants and potential effects with small effect sizes, like the relationship between depressiveness and serum levels of IL-4 and IL-10.
Our results show a relationship between depressive symptoms, neuroticism and cytokine levels, but cannot provide information on the molecular mechanisms underlying this association. A longitudinal design could more clearly demonstrate whether the personality trait neuroticism contributes to the risk of developing depression. Further, our analyses highlight the importance of including several potential covariates into the statistical analyses, such as BMI, medication status, and concurrent physical illnesses, due to their potential impact on inflammation. However, a more distinct impact, e.g., of the dosage of the separate drugs or the activity and acuity of the diagnoses, remains unclear.
In conclusion, the current study included 37 depressed and 175 non-depressed subjects, finding significant associations between depressive symptoms, the degree and dimensions of neuroticism and serum levels of pro-inflammatory, but not anti-inflammatory, cytokines. Further, IFN-γ, IL-5 and IL-12 were found to be significant mediators of the effect of neuroticism on depressive symptoms. These findings support the relationship between depressive psychopathology and pro-inflammatory conditions. Neuroticism as a long-term psychological stressor may exert pro-depressive features by facilitating a persistent low-grade inflammation. Since neuroticism and inflammation are related to the course of depression, psychotherapeutic emphasis on neuroticism and pharmacological targeting of inflammation may contribute to more personalized antidepressant therapeutic interventions, helping to prevent therapeutic non-response and the development of a chronic course of illness.
Author Contributions
FS and HH designed the study. FS, CS, and HH wrote the manuscript. LH and DT conducted the chemical analyses. FS and RM performed the statistical analyses. JM, RM, BD, and UH revised the manuscript. All authors approved the final manuscript.
Funding
This study was supported by the Integrated Research and Treatment Centre for Adiposity Diseases (IFB), University of Leipzig, the Federal Ministry of Education and Research (BMBF), FKZ: 01EO1001, and the Claussen-Simon-Foundation. We acknowledge support from the German Research Foundation (DFG) and Universität Leipzig within the program of Open Access Publishing. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Abbas IS. The role of neuroticism in the maintenance of chronic baseline stress perception and negative affect. Span J Psychol. (2016) 19:E9. doi: 10.1017/sjp.2016.7
2. Wirtz PH, von Känel R. Psychological stress, inflammation, and coronary heart disease. Curr Cardiol Rep. (2017) 19:111. doi: 10.1007/s11886-017-0919-x
3. Himmerich H, Fischer J, Bauer K, Kirkby KC, Sack U, Krügel U. Stress-induced cytokine changes in rats. Eur Cytokine Netw. (2013) 24:97–103. doi: 10.1684/ecn.2013.0338
4. Pawlowski T, Radkowski M, Małyszczak K, Inglot M, Zalewska M, Jablonska J, et al. Depression and neuroticism in patients with chronic hepatitis C: correlation with peripheral blood mononuclear cells activation. J Clin Virol. (2014) 60:105–11. doi: 10.1016/j.jcv.2014.03.004
5. Hussein S, Dalton B, Willmund GD, Ibrahim MAA, Himmerich H. A systematic review of tumor necrosis factor-α in post-traumatic stress disorder: evidence from human and animal studies. Psychiatr Danub. (2017) 29:407–20. doi: 10.24869/psyd.2017.407
6. Luchetti M, Barkley JM, Stephan Y, Terracciano A, Sutin AR. Five-factor model personality traits and inflammatory markers: new data and a meta-analysis. Psychoneuroendocrinology (2014) 50:181–93. doi: 10.1016/j.psyneuen.2014.08.014
7. Rudaz DA, Vandeleur CL, Gebreab SZ, Gholam-Rezaee M, Strippoli MF, Lasserre AM, et al. Partially distinct combinations of psychological, metabolic and inflammatory risk factors are prospectively associated with the onset of the subtypes of major depressive disorder in midlife. J Affect Disord. (2017) 222:195–203. doi: 10.1016/j.jad.2017.07.016
8. Jeronimus BF, Kotov R, Riese H, Ormel J. Neuroticism's prospective association with mental disorders halves after adjustment for baseline symptoms and psychiatric history, but the adjusted association hardly decays with time: a meta-analysis on 59 longitudinal/prospective studies with 443 313 participants. Psychol Med. (2016) 46:2883–906. doi: 10.1017/S0033291716001653
9. Gale CR, Hagenaars SP, Davies G, Hill WD, Liewald DC, Cullen B, et al. Pleiotropy between neuroticism and physical and mental health: findings from 108 038 men and women in UK Biobank. Transl Psychiatry (2016) 6:e791. doi: 10.1038/tp.2016.56
10. Lichtblau N, Schmidt FM, Schumann R, Kirkby KC, Himmerich H. Cytokines as biomarkers in depressive disorder: current standing and prospects. Int Rev Psychiatry (2013) 25:592–603. doi: 10.3109/09540261.2013.813442
11. Schmidt FM, Kirkby KC, Lichtblau N. Inflammation and immune regulation as potential drug targets in antidepressant treatment. Curr Neuropharmacol. (2016) 14:674–87. doi: 10.2174/1570159X14666160115130414
12. Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. (2015) 49:206–15. doi: 10.1016/j.bbi.2015.06.001
13. Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry (2014) 171:1278–86. doi: 10.1176/appi.ajp.2014.14010094
14. Schmidt FM, Kirkby KC, Himmerich H. The TNF-alpha inhibitor etanercept as monotherapy in treatment-resistant depression - report of two cases. Psychiatr Danub. (2014) 26:288–90.
15. Köhler-Forsberg O, Buttenschøn HN, Tansey KE, Maier W, Hauser J, Dernovsek MZ, et al. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav Immun. (2017) 62:344–50. doi: 10.1016/j.bbi.2017.02.020
16. Jokela M, Virtanen M, Batty GD, Kivimäki M. Inflammation and Specific Symptoms of Depression. JAMA Psychiatry (2016) 73:87–8. doi: 10.1001/jamapsychiatry.2015.1977
17. White J, Kivimäki M, Jokela M, Batty GD. Association of inflammation with specific symptoms of depression in a general population of older people: the english Longitudinal study of ageing. Brain Behav Immun. (2017) 61:27–30. doi: 10.1016/j.bbi.2016.08.012
18. Schmidt FM, Schröder T, Kirkby KC, Sander C, Suslow T, Holdt LM, et al. Pro- and anti-inflammatory cytokines, but not CRP, are inversely correlated with severity and symptoms of major depression. Psychiatry Res. (2016) 239:85–91. doi: 10.1016/j.psychres.2016.02.052
19. Schmidt FM, Weschenfelder J, Sander C, Minkwitz J, Thormann J, Chittka T, et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE (2015) 10:e0121971. doi: 10.1371/journal.pone.0121971
20. Dahl J, Ormstad H, Aass HC, Malt UF, Bendz LT, Sandvik L, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology (2014) 45:77–86. doi: 10.1016/j.psyneuen.2014.03.019
21. Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O'Brien JT. Increase in Interleukin-1beta in late-life depression. Am J Psychiatry (2005) 162:175–7. doi: 10.1176/appi.ajp.162.1.175
22. Steptoe A, Kunz-Ebrecht SR, Owen N. Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychol Med. (2003) 33:667–74. doi: 10.1017/S0033291702007250
23. Ramsey JM, Cooper JD, Bot M, Guest PC, Lamers F, Weickert CS, et al. Sex differences in serum markers of major depressive disorder in the netherlands study of depression and anxiety (NESDA). PLoS ONE (2016) 11:e0156624. doi: 10.1371/journal.pone.0156624
24. Schmidt FM, Lichtblau N, Minkwitz J, Chittka T, Thormann J, Kirkby KC, et al. Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity. J Psychiatr Res. (2014) 55:29–34. doi: 10.1016/j.jpsychires.2014.04.021
25. Wittchen HU, Zaudig M, Fydrich T. SKID Strukturiertes Klinisches Interview für DSM-IV (Achse I und II) [Structured Clinical Interview for DSM-IV (axis I and II)]. Göttingen: Hogrefe (1997).
26. Beck AT, Steer RA, Brown GK. BDI–II, Beck Depression Inventory: Manual. 2nd edn. Boston: Harcourt Brace (1996).
27. Costa PT, McCrae RR. NEO PI-R Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc (1992).
28. Huang C, Chen JH. Meta-Analysis of the factor structures of the beck depression inventory-II. Assessment (2015) 22:459–72. doi: 10.1177/1073191114548873
29. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. (1995) 57:289–300.
30. Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. (2004) 36:717–31. doi: 10.3758/BF03206553
31. Zou W, Feng R, Yang Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS ONE (2018) 13:e0197267. doi: 10.1371/journal.pone.0197267
32. Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. (2008) 18:230–3. doi: 10.1016/j.euroneuro.2007.06.004
33. Hernández ME, Mendieta D, Martínez-Fong D, Loría F, Moreno J, Estrada I, et al. Variations in circulating cytokine levels during 52 week course of treatment with SSRI for major depressive disorder. Eur Neuropsychopharmacol. (2008) 18:917–24. doi: 10.1016/j.euroneuro.2008.08.001
34. Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. (2017) 135:373–87. doi: 10.1111/acps.12698
35. Wachholz S, Knorr A, Mengert L, Plümper J, Sommer R, Juckel G, et al. Interleukin-4 is a participant in the regulation of depressive-like behavior. Behav Brain Res. (2017) 326:165–72. doi: 10.1016/j.bbr.2017.03.02
36. Laumet G, Edralin JD, Chiang AC, Dantzer R, Heijnen CJ, Kavelaars A. Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology (2018). doi: 10.1038/s41386-018-0154-1
37. Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BW. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands study of depression and anxiety (NESDA). Psychoneuroendocrinology (2013) 38:1573–85. doi: 10.1016/j.psyneuen.2013.01.002
38. Black C, Miller BJ. Meta-Analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol Psychiatry (2015) 78:28–37. doi: 10.1016/j.biopsych.2014.10.014
39. Eller T, Aluoja A, Maron E, Vasar V. Soluble interleukin-2 receptor and tumor necrosis factor levels in depressed patients in Estonia. Medicina (2009) 45:971–7. doi: 10.3390/medicina45120124
40. Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet (2006) 367:29–35 doi: 10.1016/S0140-6736(05)67763-X
41. Krogh J, Benros ME, Jørgensen MB, Vesterager L, Elfving B, Nordentoft M. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behav Immun. (2014) 35:70–6. doi: 10.1016/j.bbi.2013.08.014
42. Goodwin RD, Cox BJ, Clara I. Neuroticism and physical disorders among adults in the community: results from the National Comorbidity Survey. J Behav Med. (2006) 29:229–38. doi: 10.1007/s10865-006-9048-5
43. Sutin AR, Ferrucci L, Zonderman AB, Terracciano A. Personality and obesity across the adult life span. J Pers Soc Psychol. (2011) 101:579–92. doi: 10.1037/a0024286
44. Terracciano A, Costa PTJr. Smoking and the five-factor model of personality. Addiction (2004) 99:472–81. doi: 10.1111/j.1360-0443.2004.00687.x
45. Foster JA, MacQueen G. Neurobiological factors linking personality traits and major depression. Can J Psychiatry (2008) 53:6–13. doi: 10.1177/070674370805300103
46. Barlow DH, Ellard KK, Sauer-Zavala S, Bullis JR, Carl JR. The origins of neuroticism. Perspect Psychol Sci. (2014) 9:481–96. doi: 10.1177/1745691614544528
47. Krügel U, Fischer J, Radicke S, Sack U, Himmerich H. Antidepressant effects of TNF-α blockade in an animal model of depression. J Psychiatr Res. (2013) 47:611–6.
48. Besedovsky HO, del Rey A, Klusman I, Furukawa H, Monge Arditi G, Kabiersch A. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J. Steroid. Biochem. Mol. Biol. (1991) 40:613–8. doi: 10.1016/0960-0760(91)90284-C
49. van Velzen LS, Schmaal L, Milaneschi Y, van Tol MJ, van der Wee NJ, Veltman DJ, et al. Immunometabolic dysregulation is associated with reduced cortical thickness of the anterior cingulate cortex. Brain Behav Immun. (2017) 60:361–8. doi: 10.1016/j.bbi.2016.10.019
50. Wardenaar KJ, Conradi HJ, Bos EH, de Jonge P. Personality modulates the efficacy of treatment in patients with major depressive disorder. J Clin Psychiatry (2014) 75:e916–23. doi: 10.4088/JCP.13m08855
51. Bukh JD, Andersen PK, Kessing LV. Personality and the long-term outcome of first-episode depression: a prospective 5-year follow-up study. J Clin Psychiatry (2016) 77:e704–10. doi: 10.4088/JCP.15m09823
52. Spinhoven P, Huijbers MJ, Ormel J, Speckens AEM. Improvement of mindfulness skills during mindfulness-based cognitive therapy predicts long-term reductions of neuroticism in persons with recurrent depression in remission. J Affect Disord. (2017) 213:112–7. doi: 10.1016/j.jad.2017.02.011
Keywords: neuroticism, depression, chronic stress, cytokines, TNF-α, mediation analyses
Citation: Schmidt FM, Sander C, Minkwitz J, Mergl R, Dalton B, Holdt LM, Teupser D, Hegerl U and Himmerich H (2018) Serum Markers of Inflammation Mediate the Positive Association Between Neuroticism and Depression. Front. Psychiatry 9:609. doi: 10.3389/fpsyt.2018.00609
Received: 10 August 2018; Accepted: 30 October 2018;
Published: 20 November 2018.
Edited by:
Gianluca Serafini, Dipartimento di Neuroscienze e Organi di Senso, Ospedale San Martino (IRCCS), ItalyReviewed by:
Catherine Toben, University of Adelaide, AustraliaAtsuo Nakagawa, School of Medicine, Keio University, Japan
Copyright © 2018 Schmidt, Sander, Minkwitz, Mergl, Dalton, Holdt, Teupser, Hegerl and Himmerich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frank M. Schmidt, Zi5zY2htaWR0QG1lZGl6aW4udW5pLWxlaXB6aWcuZGU=
 Frank M. Schmidt
Frank M. Schmidt Christian Sander
Christian Sander Juliane Minkwitz2
Juliane Minkwitz2 Roland Mergl
Roland Mergl Daniel Teupser
Daniel Teupser Ulrich Hegerl
Ulrich Hegerl Hubertus Himmerich
Hubertus Himmerich