- 1Cancer Prevention and Control Program, University of South Carolina, Columbia, SC, United States
- 2Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, Columbia, SC, United States
- 3Department of Community Nutrition, Faculty of Nutrition Sciences and Food Technology, National Nutrition and Food Technology Research Institute (WHO Collaborating Center), Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Nutrition and Food Sciences Department, American University of Beirut, Beirut, Lebanon
Background: The relation between dietary inflammation and risk of depression has not been widely explored. We examined the association between the inflammatory effect of the diet and the odds of depression among Iranian female adolescents.
Methods: Using a stratified cluster sampling technique, 300 female adolescents aged 15–18 years were recruited from schools in Tehran between years 2014–2015. Depression was assessed using the Depression, Anxiety and Stress Scale (DASS)- a 21-point scale. The dietary inflammatory index (DII®) was used to evaluate the inflammatory potential of the diet. Dietary intake was assessed using a validated food frequency questionnaire. In addition to descriptive statistics, multivariable linear and logistic regression were used to calculate confounder-adjusted beta estimates and odds ratios.
Results: In total, 88 females (30%) had at least a moderate level of depressive symptoms (DASS > 6). Females with the most pro-inflammatory diet had higher DASS depression score (β = 1.67; 95% CI = 0.03, 3.31) and were at 3.96 (95% CI = 1.12, 13.97) times higher odds of having at least moderate depressive symptoms, compared to females with the least anti-inflammatory diets.
Conclusion: These data suggest that Iranian adolescent females eating a pro-inflammatory diet, as indicated by higher DII scores, had greater odds of having at least moderate depressive symptoms.
Introduction
Depression is expected to become the world's second leading disease burden, after cardiovascular disease, by 2020 according the World Health Organization (1). Women are more than twice as likely to be diagnosed with depression compared with men (2). In Iran, major depression is the most prevalent mood disorder, with a prevalence rate of 2.98% among the entire population and 4.38% among women (3). The peak prevalence is between 25 and 44 years of age; however, recent data indicate that depression is occurring at younger ages (4, 5). Several metabolic and inflammatory processes, such as reduced insulin sensitivity, elevations in plasma homocysteine levels and, perhaps more importantly, increased production of pro-inflammatory cytokines and endothelial dysfunction, seem to be the major factors responsible for the depression (3, 4). Proinflammatory cytokines like c-reactive protein and interleukin-6 act by reducing brain monoamine levels, activating neuroendocrine responses, promoting excitotoxicity (increased glutamate levels), and impairing brain plasticity modulate mood behavior which can result in depression (6). Various dietary components have different effects on inflammation (7–9). A prudent dietary pattern high in fish, yogurt, pulses, rice, fruit, vegetables, and pasta has been shown to be associated with lower concentrations of intermediary inflammatory markers (10).
Various studies have been conducted to evaluate dietary exposures in relation to depression (11–15). In general, prospective cohort studies have shown that dietary patterns rich in anti-inflammatory components such as fruits, vegetables, olive oil, and legumes may be protective against depression (16–19). By contrast, increased risk has been observed with “pro-inflammatory” dietary patterns rich in saturated fat, omega 6 fatty acids, and refined carbohydrates (20, 21). A literature-derived, population-based dietary inflammatory index (DII) was developed to assess the inflammatory potential of an individual's diet (22). DII has previously been shown to be associated with various inflammatory markers in different populations (23–28) including among Iranians (29, 30). With respect to health outcomes, DII was significantly associated with different health outcomes ranging from cardiovascular diseases (31–33), cancer (34–37), overall and disease-specific mortality (38–42) to various mental health disorders (43–47). Previously, DII has been shown to be associated with depression in various studies conducted in Western population (48–53) but no study has been conducted in Iran whose dietary habits and culture are very different from Western populations. Our aim was to assess the association between the inflammatory potential of diet of adolescent Iranian females, as indicated by higher DII scores, and dimensions of depression as measured by Depression Anxiety Stress Scale-21 (DASS-21) (54).
Methods and Materials
Study Population
This cross-sectional study of 300 adolescents females aged 15–18 years was carried out in Tehran (capital of Iran) from 2014 to 2015. Participants were randomly chosen by stratified cluster sampling. We first stratified the high schools based on socioeconomic status of the districts (low, intermediate, and high). Then we randomly selected 8 high schools from each stratum. Finally, subjects were chosen from a registration list (in each selected high school) by simple random sampling to fulfill the sample size requirement (n = 300).We did not include participants who reported major depression and anxiety disorder, using of any anti-depressant or sedative medication and who were pursuing a distinct diet, because there is a high probability that people with these disorders and taking antidepressant or sedative medications would have made lifestyle changes like adopting a healthier diet that may bias the results. The study protocol was approved by the research council of the Research Institute for Nutrition and Food Sciences, Shahid Beheshti University of Medical Sciences. All subjects gave written informed consent in accordance with the Declaration of Helsinki (approval number is 054577).
Assessment of Dietary Intake
Food consumption was based on a reliable and valid Food Frequency Questionnaire (FFQ) consisting of 168 food items with standard serving sizes typically used in Iran (55). FFQs were collected by specifically trained professional interviewers through private face-to-face interviews. Participants reported their daily, weekly, monthly or yearly of intake frequency for each food item. Daily frequencies for each item were computed. Then, by applying the manual for household measures the daily grams of food intake were calculated (56).
Anthropometric Measurement
Body weight was assessed to the nearest 0.1 kilogram by using digital scales (Seca 881® Germany) while participants were in light clothes with bare feet. Height was evaluated by using a stradiometer in the standing position and was recorded to the nearest 0.1 cm. Body Mass Index (BMI) was computed as weight divided by height squared (kg/m2).
Socio-Demographic Information
Characteristics including age (years), ethnicity (Fars,Tork, Gilak, others), father/mother job (Unemployed, grade 3 (e.g., laborers), grade 2 (e.g., clerks), grade 1 (e.g., managers and higher), father/mother education (<diploma, diploma, university education), marital status of parents (married, unmarried), salary (USD), chronic disease (yes/no) diet supplement (yes, no), and smoking status (never, previous, current) were collected by a general questionnaire for all subjects.
Physical Activity Assessment
Physical activity was assessed using a valid self-reported questionnaire (57) that has been used previously in a sample of Iranian women and demonstrated consistent outcomes (58). Participants were asked to check the activities in which they had participated during the last year. From these reports, the total time spent in particular activities were summed and mean durations were calculated. Total physical activity was expressed as metabolic equivalent-hours per day (Mets/d).
Other Variables
Further information on body image was collected by using 28-items of eating disorder examination questionnaire (EDE-Q-28) (59). The Persian version of EDE-Q-28 which was used in this study (60).
Dietary Inflammatory Index (DII®)
The development and validation of the DII are described in detail elsewhere (22). Briefly, developing the DII involved reviewing and scoring nearly 2,000 scientific articles representing cell culture and laboratory animal experiments, and a variety of human studies on diet and six inflammatory markers (i.e., CRP, interleukin (IL)-1b, IL-4, IL-6, IL-10, tumor necrosis factor (TNF)-α). Developing the DII also entailed creation of a world standard database that involved obtaining 11 data sets from around the world to which individuals' intakes of 45 food parameters (consisting of nutrients, spices and whole foods) on which the DII is based, could then be compared.
FFQ-derived dietary data were used to calculate DII scores for all participants. Dietary data were first linked to the previously described regionally representative world database that provided a robust estimate of a mean and standard deviation for each parameter (22). These then became the multipliers to express an individual's exposure relative to the “standard global mean” as a z-score. This score was computed by subtracting the “standard global mean” from the amount reported and dividing this value by the “global standard deviation” of the world population as represented by the 11 data sets used for comparative purposes. To minimize the effect of “right skewing,” this value was then converted to a centered proportion score.
For each individual food parameter, this score was multiplied by the respective food parameter effect score, derived from the literature review, in order to obtain a food parameter-specific DII score (22). All of the food parameter-specific DII scores were then summed to create the overall DII score for each participant in the study, DII = b1*n1+b2*n2……….b31*n31, where b refers to the literature-derived inflammatory effects score for each of the evaluable food parameters and n refers to the food parameter-specific centered percentiles, which were derived from the FFQ-derived dietary data.
For the current study, data on 31 of the 45 DII food parameters could be derived from the FFQ and were thus used for DII calculation. These include: Pro-inflammatory components (energy, carbohydrate, protein, fat, saturated fat, iron, cholesterol, trans-fat, vitamin B12) and anti-inflammatory components (alcohol, fiber, mono-unsaturated fat, poly-unsaturated fat, omega-3, omega-6, niacin, thiamin, riboflavin, magnesium, zinc, vitamin A, vitamin C, vitamin E, vitamin D, vitamin B6, folic acid, beta-carotene, tea, turmeric, garlic, and onions).
Psychological Assessment of Depression, Anxiety and Stress
The Persian version of Depression, Anxiety, Stress Scale-21 (DASS-21) which was introduced by Lovibond and Lovibond (61) has been used to determine the level of depression, anxiety and stress in our sample population. This questionnaire has three subscales and each of them consists of seven items. The score of each subscale is attained by adding the scores of relevant questions. The Persian version of the DASS-21 was found to be a reliable and valid tool to examine the level of depression, anxiety and stress among Iranian adolescents (62).
Statistical Analysis
Study participants' characteristics were described according to two parameters: (1) reporting of at least depressive symptoms and (2) tertiles of the DII. Comparisons were carried out using t-tests and ANOVA for continuous variables and chi-square test for categorical variables. Multiple linear and logistic regression analysis were then used to calculate adjusted beta estimates and odds ratios (ORs) and 95% confidence intervals (CIs) for both DASS-21 as continuous and as categorical variable (DASS-21 > 9) in relation to DII in 2 separate models. Model I adjusted for total energy intake and age, model 2 additionally adjusted for physical activity, marital status, income, smoking, BMI, and presence of chronic disease.
Results
Table 1 describes distribution of characteristics across categories of DASS-21 scores. Females with at least moderate level of depressive symptoms had lower level physical activity and greater dietary supplement use and were either past/current smokers. Table 2 shows distribution of characteristics across tertiles of DII. Females in tertile 3 had higher BMI compared to females in tertile 1. Table 3 describes the results for depression as a continuous score and as a dichotomous outcome where the scores were categorized on having at least a moderate level of depression symptoms (DASS > 9). Significant associations were observed for both types of outcomes for both models. Results are described for the full model, females in the third tertile had significantly higher depression scores (β = 1.67, 95% C.I. 0.04, 3.31); odds of having at least moderate depressive symptoms (DASS-21 > 9) (OR = 3.96, 95% CI 1.12, 13.97) compared to females in tertile 1 (Table 3).
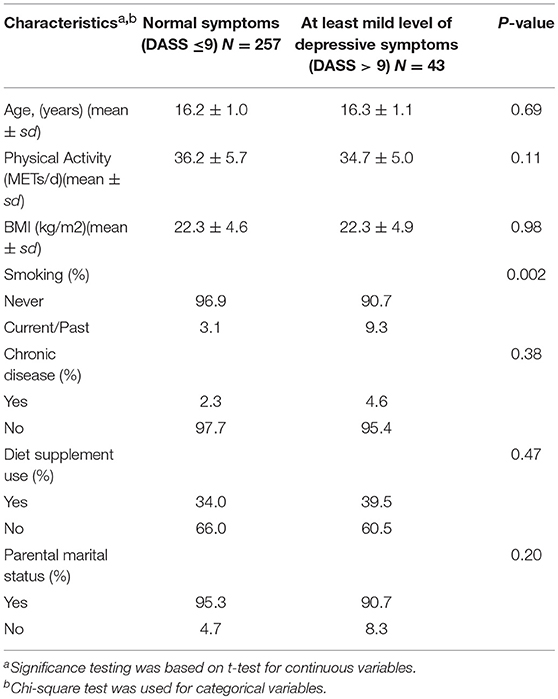
Table 1. Characteristics of participants according to different categories of depressive symptoms, Study of Diet-Inflammation and Depression in Iranian Adolescent Females, 2014 to 2015.
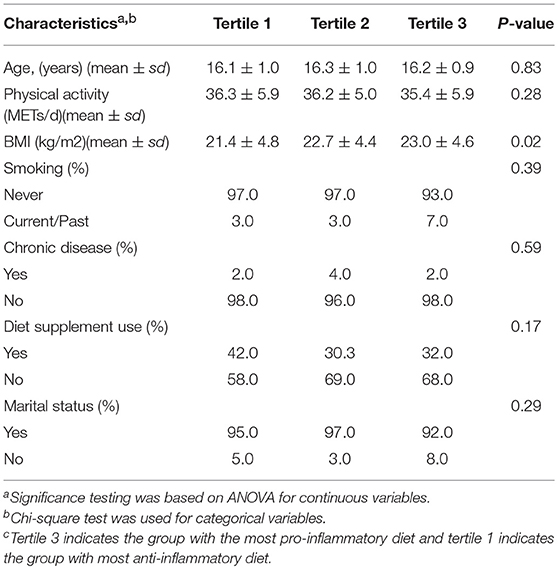
Table 2. Participant characteristics by tertiles of dietary inflammatory index (DII)c, Study of Diet-Inflammation and Depression in Iranian Adolescent Females, 2014 the 2015.
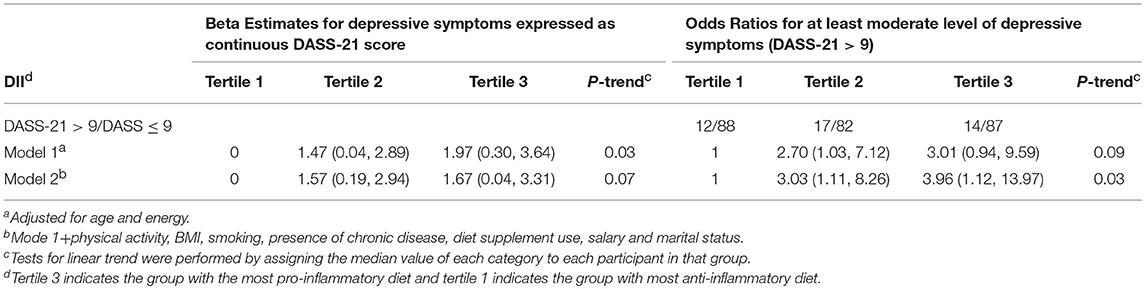
Table 3. Beta estimates and odds ratios and confidence intervals for the association between DII as tertiles and depressive symptoms, Study of Diet-Inflammation and Depression in Iranian Adolescent Females, 2014 to 2015.
Discussion
In this study we report a significant positive association between increasing inflammatory potential of diet and depressive symptoms among adolescent females in Iran. To date, this is the first study to examine this association in adolescent females. A case-control study conducted in Iran showed that a healthy dietary pattern is protective against major depressive disorders, while no such association was observed with the unhealthy dietary patterns. The healthy pattern was characterized by high intakes of fish, poultry, low fat dairy, high fat dairy, coffee, fruits, and nuts, fruit juices, vegetables, legumes, and olives, and low intakes of refined grains, fats, and soft drinks. The unhealthy dietary patterns, on the other hand, were characterized by high intakes of processed meats, red meat, tea, fried potatoes, whole grains, refined grains, snacks, cookies, oils, sugar, and soft drinks (63). Previous reports suggest that men are more likely to have mental health problems than women (64). The current work shows an association between inflammatory potential of diet and depressive symptoms, among the female adolescents. While some of the earlier studies also reported associations between dietary inflammation potential and risk of depression among women; these were female-only cohorts where inflammatory potential of diet was determined by two different methods (inflammatory dietary pattern and DII) (14, 53). Results from the Nurses' Health Study revealed a 30–40% increased risk of depression, among women in the highest quintile compared to women in the lowest quintile of inflammatory dietary pattern (14). Twelve-years' follow-up of middle-aged women in Australia (n = 6,438) identified a 20% lower risk of depression among those whose diets were in the highest DII quartile compared to those in the lowest DII quartile (53).
This study is unique because for the first time this association has been examined in a Middle-Eastern population whose dietary habits and culture is very different from the Western population where this relationship has previously been examined (48–53). Traditional Iranian diet consist of food like cooked rice, mixed pilaf, rice, and stew, rice, high protein dishes and stuffed vegetables (65).
In agreement with our findings, the results of the only other study that examined the association between inflammatory potential of diet and depression showed that the hazards ratio for participants in the highest quintile of the DII (strongly pro-inflammatory) was 1.47 (95% confidence interval (CI): 1.17–1.85) compared with those in the bottom quintile, with a significant dose-response relationship (p for trend = 0.01) (66).
The DII, thus far, has been shown to be associated with several inflammatory markers and various chronic inflammation related outcomes. For instance, higher DII scores were positively associated with various inflammatory markers, including C-reactive protein (67, 68), interleukin-6 (69, 70), and homocysteine (69). Additionally, the DII has been shown to be associated with bone mineral density among postmenopausal women in Iran (70), two colorectal cancer case-control studies in Spain and Italy (71, 75) and in a cohort study in women in the USA (37, 71), esophageal cancer (72–74), breast cancer (75), pancreatic cancer (76), prostate cancer (77, 78), cardiovascular diseases (79, 80) and biomarker of aging (81).
The direct association between the DII and the odds of depression observed in this study could be explained by the fact many of the anti-inflammatory components of the index, namely zinc, omega 3 fatty acids, and coffee, have been shown to be negatively associated with the risk of depression (13). On the other hand, pro-inflammatory components of the index, such as energy and carbohydrate, have been linked to a higher risk of depression. For instance, results from a large cohort study conducted in the USA showed that frequent consumption of sweetened beverages, especially diet drinks, increases the risk of depression among older adults, whereas coffee consumption lowered the risk (11). Various studies have been conducted examining the association between dietary pattern and depression. In a systematic review conducted on 21 studies high intakes of fruit, vegetables, fish, and whole grains may be associated with a reduced depression risk (82). There is substantial evidence linking inflammation and depression (83–86).
Despite its strengths, our study had some limitations, which should also be considered in interpreting the results. First, the validity and reliability of FFQ has not been established in the context measuring dimensions of depression measured using DASS-21. Second, we could not directly infer causality due to the cross-sectional nature of the study design. Other limitations include small sample size, and the possibility of recall bias. Fourth, in this study, data were available on 31 of the 45 food parameters; absence of information on the remaining food parameters can be considered as a limitation. Additionally, validity and reliability of FFQ has not been established in the context measuring dimensions of depression measured using DASS-21. Another limitation is the inability to evaluate the history of stressful life events in the last 12 months. Self-reported dietary methods like the FFQ is subjected to bias of under reporting and implausible values for energy intake, the inability to measure this bias and adjust for it in the analyses is a limitation.
In conclusion, female adolescents with a pro-inflammatory diet have greater odds of having at least a moderate level of depressive symptoms. So, promoting diets with a higher concentration of anti-inflammatory foods, such as vegetables and fruits, at younger age may be protective against the development of depression. However, further studies analyzing the link between diet, inflammation and depression are warranted among both men and women to further elucidate the role of diet in the development of depression and other mental disorders.
Author Contributions
NS calculated the DII, ran the analyses and also wrote the first draft of the manuscript. Upon receiving comments from the co-authors he also made changes to the manuscript and finalized it. JRH, AN, BB, FN, and BR reviewed and provided important input to the paper.
Funding
NS and JRH were supported by grant number R44DK103377 from the United States National Institute of Diabetes and Digestive and Kidney Diseases.
Conflict of Interest Statement
JRH owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. NS is an employee of CHI.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past co-authorship with the authors NS, JRH.
References
1. World Health Organization. Mental Health - Disorders Management 2011, Available online at: from: http://www.who.int/mental_health/management/depression/definition/en/. 2011 (Accessed May 07, 2015)
2. Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. (1994) 115:424–43.
3. Mohammadi MR, Davidian H, Noorbala AA, Malekafzali H, Naghavi HR, Pouretemad HR, et al. An epidemiological survey of psychiatric disorders in Iran. Clin Pract Epidemiol Ment Health (2005) 1:16. doi: 10.1186/1745-0179-1-16
4. Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord. (1993) 29:85–96.
5. Zisook S, Rush AJ, Albala A, Alpert J, Balasubramani GK, Fava M, et al. Factors that differentiate early vs. later onset of major depression disorder. Psychiatry Res. (2004) 129:127–40. doi: 10.1016/j.psychres.2004.07.004
6. Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann NY Acad Sci. (2018) doi: 10.1111/nyas.13712. [Epub ahead of print].
7. de Mello VD, Schwab U, Kolehmainen M, Koenig W, Siloaho M, Poutanen K, et al. A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: the Sysdimet study. Diabetologia (2011) 54:2755–67. doi: 10.1007/s00125-011-2285-3
8. Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. (2011) 8:2868–75. doi: 10.1111/j.1743-6109.2011.02417.x
9. Luciano M, Mottus R, Starr JM, McNeill G, Jia X, Craig LC, et al. Depressive symptoms and diet: their effects on prospective inflammation levels in the elderly. Brain Behav Immun. (2012) 26:717–20. doi: 10.1016/j.bbi.2011.10.007
10. Wood AD, Strachan AA, Thies F, Aucott LS, Reid DM, Hardcastle AC, et al. Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Br J Nutr. (2014) 112:1341–52. doi: 10.1017/S0007114514001962
11. Guo XG, Park Y, Freedman ND, Sinha R, Hollenbeck AR, Blair A, et al. Sweetened beverages, coffee, and tea and depression risk among older us adults. PLoS ONE (2014) 9:e94715. doi: 10.1371/journal.pone.0094715
12. Hegarty BD, Parker GB. Marine omega-3 fatty acids and mood disorders–linking the sea and the soul. ‘Food for Thought' I. Acta Psychiatr Scand. (2011) 124:42–51. doi: 10.1111/j.1600-0447.2011.01703.x
13. Liperoti R, Landi F, Fusco O, Bernabei R, Onder G. Omega-3 polyunsaturated fatty acids and depression: a review of the evidence. Curr Pharm Design. (2009) 15:4165–72. doi: 10.2174/138161209789909683
14. Lucas M, Chocano-Bedoya P, Shulze MB, Mirzaei F, O'Reilly EJ, Okereke OI, et al. Inflammatory dietary pattern and risk of depression among women. Brain Behav Immun. (2014) 36:46–53. doi: 10.1016/j.bbi.2013.09.014
15. Mihrshahi S, Dobson AJ, Mishra GD. Fruit and vegetable consumption and prevalence and incidence of depressive symptoms in mid-age women: results from the Australian longitudinal study on women's health. Eur J Clin Nutr. (2015) 69:585–91. doi: 10.1038/ejcn.2014.222
16. Jacka FN, Kremer PJ, Berk M, de Silva-Sanigorski AM, Moodie M, Leslie ER, et al. A prospective study of diet quality and mental health in adolescents. PLoS ONE (2011) 6:e24805. doi: 10.1371/journal.pone.0024805
17. Jacka FN, Rothon C, Taylor S, Berk M, Stansfeld SA. Diet quality and mental health problems in adolescents from East London: a prospective study. Soc Psychiatry Psychiatric Epidemiol. (2013) 48:1297–306. doi: 10.1007/s00127-012-0623-5
18. Rienks J, Dobson AJ, Mishra GD. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: results from a large community-based prospective study. Eur J Clin Nutr. (2013) 67:75–82. doi: 10.1038/ejcn.2012.193.
19. Sanchez-Villegas A, Delgado-Rodriguez M, Alonso A, Schlatter J, Lahortiga F, Serra Majem L, et al. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch General Psychiatry (2009) 66:1090–8. doi: 10.1001/archgenpsychiatry.2009.129
20. Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry (2009) 195:408–13. doi: 10.1192/bjp.bp.108.058925
21. Le Port A, Gueguen A, Kesse-Guyot E, Melchior M, Lemogne C, Nabi H, et al. Association between dietary patterns and depressive symptoms over time: a 10-year follow-up study of the GAZEL cohort. PLoS ONE (2012) 7:e51593. doi: 10.1371/journal.pone.0051593.
22. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
23. Shivappa N, Wirth MD, Murphy EA, Hurley TG, Hebert JR. Association between the Dietary Inflammatory Index (DII) and urinary enterolignans and C-reactive protein from the National Health and Nutrition Examination Survey-2003-2008. Eur J Nutr. (2018) doi: 10.1007/s00394-018-1690-5. [Epub ahead of print].
24. Shivappa N, Schneider A, Hebert JR, Koenig W, Peters A, Thorand B. Association between dietary inflammatory index, and cause-specific mortality in the MONICA/KORA Augsburg Cohort Study. Eur J Public Health (2018) 28:167–72. doi: 10.1093/eurpub/ckx060
25. Wirth MD, Shivappa N, Davis L, Hurley TG, Ortaglia A, Drayton R, et al. Construct validation of the dietary inflammatory index among African Americans. J Nutr Health Aging (2017) 21:487–91. doi: 10.1007/s12603-016-0775-1
26. Julia C, Assmann KE, Shivappa N, Hebert JR, Wirth MD, Hercberg S, et al. Long-term associations between inflammatory dietary scores in relation to long-term C-reactive protein status measured 12 years later: findings from the Supplementation en Vitamines et Mineraux Antioxydants (SU.VI.MAX) cohort. Br J Nutr. (2017) 117:306–14. doi: 10.1017/S0007114517000034
27. Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr. (2015) 113:665–71. doi: 10.1017/S000711451400395X
28. Boden S, Wennberg M, Van Guelpen B, Johansson I, Lindahl B, Andersson J, et al. Dietary inflammatory index and risk of first myocardial infarction; a prospective population-based study. Nutr J. (2017) 16:21. doi: 10.1186/s12937-017-0243-8
29. Vahid F, Shivappa N, Faghfoori Z, Khodabakhshi A, Zayeri F, Hebert JR, et al. Validation of a Dietary Inflammatory Index (DII) and Association with Risk of Gastric Cancer: a Case-Control Study. Asian Pac J Cancer Prev. (2018) 19:1471–7. doi: 10.22034/APJCP.2018.19.6.1471
30. Vahid F, Shivappa N, Hekmatdoost A, Hebert JR, Davoodi SH, Sadeghi M. Association between Maternal Dietary Inflammatory Index (DII) and abortion in Iranian women and validation of DII with serum concentration of inflammatory factors: case-control study. Appl Physiol Nutr Metab. (2017) 42:511–6. doi: 10.1139/apnm-2016-0274
31. Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, et al. Dietary inflammatory index and cardiovascular risk and mortality-a meta-analysis. Nutrients (2018) 10:E200. doi: 10.3390/nu10020200
32. Hodge AM, Bassett JK, Dugue PA, Shivappa N, Hebert JR, Milne RL, et al. Dietary inflammatory index or Mediterranean diet score as risk factors for total and cardiovascular mortality. Nutr Metab Cardiovasc Dis. (2018) 28:461–9. doi: 10.1016/j.numecd.2018.01.010
33. O'Neil A, Shivappa N, Jacka FN, Kotowicz MA, Kibbey K, Hebert JR, et al. Pro-inflammatory dietary intake as a risk factor for CVD in men: a 5-year longitudinal study. Br J Nutr. (2015) 114:2074–82. doi: 10.1017/S0007114515003815
34. Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, et al. Dietary inflammatory index and colorectal cancer risk-a meta-analysis. Nutrients (2017) 9:E1043. doi: 10.3390/nu9091043
35. Niclis C, Pou SA, Shivappa N, Hebert JR, Steck SE, Diaz MDP. Proinflammatory dietary intake is associated with increased risk of colorectal cancer: results of a case-control study in argentina using a multilevel modeling approach. Nutr Cancer (2018) 70:61–8. doi: 10.1080/01635581.2018.1397710
36. Shivappa N, Hebert JR, Steck SE, Hofseth LJ, Shehadah I, Bani-Hani KE, et al. Dietary inflammatory index and odds of colorectal cancer in a case-control study from Jordan. Appl Physiol Nutr Metab. (2017) 42:744–9. doi: 10.1139/apnm-2017-0035
37. Shivappa N, Prizment AE, Blair CK, Jacobs DR, Jr., Steck SE, Hebert JR. Dietary inflammatory index and risk of colorectal cancer in the Iowa women's health study. Cancer Epidemiol Biomark Prevent. (2014) 23:2383–92. doi: 10.1158/1055-9965.EPI-14-0537
38. Park YM, Choi MK, Lee SS, Shivappa N, Han K, Steck SE, et al. Dietary inflammatory potential and risk of mortality in metabolically healthy and unhealthy phenotypes among overweight and obese adults. Clin Nutr. (2018) doi: 10.1016/j.clnu.2018.04.002. [Epub ahead of print].
39. Bondonno NP, Lewis JR, Blekkenhorst LC, Shivappa N, Woodman RJ, Bondonno CP, et al. Dietary inflammatory index in relation to sub-clinical atherosclerosis and atherosclerotic vascular disease mortality in older women. Br J Nutr. (2017) 117:1577–86. doi: 10.1017/S0007114517001520
40. Shivappa N, Steck SE, Hussey JR, Ma Y, Hebert JR. Inflammatory potential of diet and all-cause, cardiovascular, and cancer mortality in National Health and Nutrition Examination Survey III Study. Eur J Nutr. (2017) 56:683–92. doi: 10.1007/s00394-015-1112-x
41. Shivappa N, Harris H, Wolk A, Hebert JR. Association between inflammatory potential of diet and mortality among women in the Swedish Mammography Cohort. Eur J Nutr. (2016) 55:1891–900. doi: 10.1007/s00394-015-1005-z
42. Shivappa N, Blair CK, Prizment AE, Jacobs DR Jr, Steck SE, Hebert JR. Association between inflammatory potential of diet and mortality in the Iowa Women's Health study. Eur J Nutr. (2016) 55:1491–502. doi: 10.1007/s00394-015-0967-1
43. Shin D, Kwon SC, Kim MH, Lee KW, Choi SY, Shivappa N, et al. Inflammatory potential of diet is associated with cognitive function in an older adult Korean population. Nutrition (2018) 55–6:56–62. doi: 10.1016/j.nut.2018.02.026
44. Hayden KM, Beavers DP, Steck SE, Hebert JR, Tabung FK, Shivappa N, et al. The association between an inflammatory diet and global cognitive function and incident dementia in older women: the Women's Health Initiative Memory Study. Alzheimers Dement. (2017) 13:1187–96. doi: 10.1016/j.jalz.2017.04.004
45. Kesse-Guyot E, Assmann KE, Andreeva VA, Touvier M, Neufcourt L, Shivappa N, et al. Long-term association between the dietary inflammatory index and cognitive functioning: findings from the SU.VI.MAX study. Eur J Nutr. (2017) 56:1647–55. doi: 10.1007/s00394-016-1211-3
46. Frith E, Shivappa N, Mann JR, Hebert JR, Wirth MD, Loprinzi PD. Dietary inflammatory index and memory function: population-based national sample of elderly Americans. Br J Nutr. (2018) 119:552–8. doi: 10.1017/S0007114517003804
47. Shivappa N, Hebert JR, Rashidkhani B. Association between inflammatory potential of diet and stress levels in adolescent women in Iran. Arch Iran Med. (2017) 20:108–12.
48. Shivappa N, Hebert JR, Veronese N, Caruso MG, Notarnicola M, Maggi S, et al. The relationship between the dietary inflammatory index (DII((R))) and incident depressive symptoms: a longitudinal cohort study. J Affect Disord. (2018) 235:39–44. doi: 10.1016/j.jad.2018.04.014
49. Phillips CM, Shivappa N, Hebert JR, Perry IJ. Dietary inflammatory index and mental health: A cross-sectional analysis of the relationship with depressive symptoms, anxiety and well-being in adults. Clin Nutr. (2017) doi: 10.1016/j.clnu.2017.08.029. [Epub ahead of print].
50. Wirth MD, Shivappa N, Burch JB, Hurley TG, Hebert JR. The dietary inflammatory index, shift work, and depression: results from NHANES. Health Psychol. (2017) 36:760–9. doi: 10.1037/hea0000514
51. Adjibade M, Andreeva VA, Lemogne C, Touvier M, Shivappa N, Hebert JR, et al. The inflammatory potential of the diet is associated with depressive symptoms in different subgroups of the general population. J Nutr. (2017) 147:879–87. doi: 10.3945/jn.116.245167
52. Akbaraly T, Kerlau C, Wyart M, Chevallier N, Ndiaye L, Shivappa N, et al. Dietary inflammatory index and recurrence of depressive symptoms: results from the Whitehall II Study. Clin Psychol Sci. (2016) 4:1125–34. doi: 10.1177/2167702616645777
53. Shivappa N, Schoenaker DA, Hebert JR, Mishra GD. Association between inflammatory potential of diet and risk of depression in middle-aged women: the Australian Longitudinal Study on Women's Health. Br J Nutr. (2016) 116:1077–86. doi: 10.1017/S0007114516002853
54. Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. (2005) 44(Pt 2):227–39. doi: 10.1348/014466505X29657
55. Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study J Epidemiol. (2010) 20:150–8. doi: 10.2188/jea.JE20090083
56. Ghaffarpour M, Houshiar-Rad A, Kianfar H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran: Nashre Olume Keshavarzy (1999) 7:213.
57. Aadahl M, Jorgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. (2003) 35:1196–202. doi: 10.1249/01.MSS.0000074446.02192.14
58. Rezazadeh A, Rashidkhani B, Omidvar N. Association of major dietary patterns with socioeconomic and lifestyle factors of adult women living in Tehran, Iran. Nutrition (2010) 26:337–41. doi: 10.1016/j.nut.2009.06.019
59. Fairburn CG, Beglin SJ. Eating Disorder Examination Questionnaire (EDE-Q 6.0). In: C. G. Fairburn editors. Cognitive Behavior Therapy and Eating Disorders. New York, NY: Guilford Press (2008). pp. 309–13.
60. Mahmoodi M, Moloodi R, Ghaderi A. persian version of eating disorder examination questionnaire and clinical impairment assessment: norms and psychometric properties for undergraduate women. Iran J Psychiatry (2016) 11:67–74.
61. Lovibond P. Manual for the depression anxiety stress Scales. Sydney: Sydney Psychology edition (1995).
62. Bayani AA. Reliability and preliminary evidence of validity of a Farsi version of the depression anxiety stress scales. Percept Mot Skills (2010) 111:107–14. doi: 10.2466/08.13.PMS.111.4.107-114
63. Rashidkhani B, Pourghassem Gargari B, Ranjbar F, Zareiy S, Kargarnovin Z. Dietary patterns and anthropometric indices among Iranian women with major depressive disorder. Psychiatry Res. (2013) 210:115–20. doi: 10.1016/j.psychres.2013.05.022
64. Albert PR. Why is depression more prevalent in women? J Psychiatry Neurosci. (2015) 40:219–21. doi: 10.1503/jpn.150205
65. Karizaki VM. Ethnic and traditional Iranian rice-based foods. J Ethnic Foods (2016) 3:124–34. doi: 10.1016/j.jef.2016.05.002
66. Sanchez-Villegas A, Ruiz-Canela M, de la Fuente-Arrillaga C, Gea A, Shivappa N, Hebert JR, et al. Dietary inflammatory index, cardiometabolic conditions and depression in the Seguimiento Universidad de Navarra cohort study. Br J Nutr. (2015) 114:1471–9. doi: 10.1017/S0007114515003074
67. Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. (2014) 17:1825–33. doi: 10.1017/S1368980013002565
68. Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med. (2014) 56:986–9. doi: 10.1097/JOM.0000000000000213
69. Ruiz-Canela M, Zazpe I, Shivappa N, Hebert JR, Sanchez-Tainta A, Corella D, et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvencion con DIeta MEDiterranea) trial. Br J Nutr. (2015) 113:984–95. doi: 10.1017/S0007114514004401
70. Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy (2015) 45:177–83. doi: 10.1111/cea.12323
71. Zamora-Ros R, Shivappa N, Steck SE, Canzian F, Landi S, Alonso MH, et al. Dietary inflammatory index and inflammatory gene interactions in relation to colorectal cancer risk in the Bellvitge colorectal cancer case-control study. Genes Nutr. (2015) 10:447. doi: 10.1007/s12263-014-0447-x
72. Shivappa N, Hebert JR, Rashidkhani B. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case-control study from Iran. Nutr Cancer (2015) 23:1–7. doi: 10.1080/01635581.2015.1082108
73. Lu Y, Shivappa N, Lin Y, Lagergren J, Hébert J. Diet-related inflammation and oesophageal cancer by histological type: a nationwide case–control study in Sweden. Eur J Nutr. (2015) 55:1683–94. doi: 10.1007/s00394-015-0987-x
74. Shivappa N, Zucchetto A, Serraino D, Rossi M, La Vecchia C, Hébert J. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case–control study from Italy. Cancer Causes Control (2015) 26:1439–47 doi: 10.1007/s10552-015-0636-y
75. Shivappa N, Sandin S, Lof M, Hebert JR, Adami HO, Weiderpass E. Prospective study of dietary inflammatory index and risk of breast cancer in Swedish women. Br J Cancer (2015) 113:1099–103 doi: 10.1038/bjc.2015.304
76. Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hébert JR. Dietary inflammatory index and risk of pancreatic cancer in an Italian case–control study. Br J Nutr. (2015) 113:292–8. doi: 10.1017/S0007114514003626
77. Shivappa N, Bosetti C, Zucchetto A, Montella M, Serraino D, La Vecchia C, et al. Association between dietary inflammatory index and prostate cancer among Italian men. Br J Nutr. (2015) 113:278–83. doi: 10.1017/S0007114514003572
78. Shivappa N, Jackson MD, Bennett F, Hébert JR. Increased Dietary Inflammatory Index (DII) is associated with increased risk of prostate cancer in Jamaican men. Nutr Cancer (2015) 67:941–8. doi: 10.1080/01635581.2015.1062117
79. Garcia-Arellano A, Ramallal R, Ruiz-Canela M, Salas-Salvadó J, Corella D, Shivappa N, et al. Dietary inflammatory index and incidence of cardiovascular disease in the PREDIMED study. Nutrients (2015) 7:4124–38. doi: 10.3390/nu7064124
80. Ramallal R, Toledo E, Martínez-González MA, Hernández-Hernández A, García-Arellano A, Shivappa N, et al. Dietary inflammatory index and incidence of cardiovascular disease in the SUN cohort. PLoS ONE (2015) 10:e0135221. doi: 10.1371/journal.pone.0135221
81. García-Calzón S, Zalba G, Ruiz-Canela M, Shivappa N, Hébert JR, Martínez JA, et al. Dietary inflammatory index and telomere length in subjects with a high cardiovascular disease risk from the PREDIMED-NAVARRA study: cross-sectional and longitudinal analyses over 5 y. Am J Clin Nutr. (2015) 102:897–904 doi: 10.3945/ajcn.115.116863
82. Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. (2014) 99:181–97. doi: 10.3945/ajcn.113.069880
83. Chocano-Bedoya PO, Mirzaei F, O'Reilly EJ, Lucas M, Okereke OI, Hu FB, et al. C-reactive protein, interleukin-6, soluble tumor necrosis factor alpha receptor 2 and incident clinical depression. J Affect Disord. (2014) 163:25–32. doi: 10.1016/j.jad.2014.03.023
84. Hood KK, Lawrence JM, Anderson A, Bell R, Dabelea D, Daniels S, et al. Metabolic and inflammatory links to depression in youth with diabetes. Diabetes Care (2012) 35:2443–6. doi: 10.2337/dc11-2329
85. Doyle TA, de Groot M, Harris T, Schwartz F, Strotmeyer ES, Johnson KC, et al. Diabetes, depressive symptoms, and inflammation in older adults: results from the health, aging, and body composition study. J Psychosomat Res. (2013) 75:419–24. doi: 10.1016/j.jpsychores.2013.08.006
Keywords: dietary inflammatory index, diet, inflammation, depression, Iran
Citation: Shivappa N, Hebert JR, Neshatbini Tehrani A, Bayzai B, Naja F and Rashidkhani B (2018) A Pro-Inflammatory Diet Is Associated With an Increased Odds of Depression Symptoms Among Iranian Female Adolescents: A Cross-Sectional Study. Front. Psychiatry 9:400. doi: 10.3389/fpsyt.2018.00400
Received: 26 May 2018; Accepted: 09 August 2018;
Published: 29 August 2018.
Edited by:
Joseph Firth, Western Sydney University, AustraliaReviewed by:
Rebekah Carney, Greater Manchester Mental Health NHS Foundation Trust, United KingdomScott B. Teasdale, University of New South Wales, Australia
Copyright © 2018 Shivappa, Hebert, Neshatbini Tehrani, Bayzai, Naja and Rashidkhani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bahram Rashidkhani, cmFzaGlka2hhbmlAeWFob28uY29t; Yl9yYXNoaWRraGFuaUBzYm11LmFjLmly
 Nitin Shivappa
Nitin Shivappa James R. Hebert1,2
James R. Hebert1,2