- 1Medical University of South Carolina, Charleston, SC, United States
- 2Ralph H. Johnson VA Medical Center, Charleston, SC, United States
Background: Despite advances in behavioral and pharmacotherapy interventions, substance use disorders (SUDs) are frequently refractory to treatment. Glutamatergic dysregulation has received increasing attention as one common neuropathology across multiple substances of abuse. Ketamine is a potent N-methyl-D-aspartate (NMDA) glutamatergic receptor antagonist which has been found to be effective in the treatment of severe depression. Here we review the literature on the efficacy of ketamine in the treatment of SUDs.
Methods: A systematic review of the PubMed, Scopus, and ClinicalTrials.gov databases was undertaken to identify completed and ongoing human studies of the effectiveness of ketamine in the treatment of SUDs between January 1997 and January 2018.
Results and conclusion: Seven completed studies were identified. Two studies focused on alcohol use disorder, two focused on cocaine use disorder, and three focused on opioid use disorder. Both cocaine studies found improvements in craving, motivation, and decreased cocaine use rates, although studies were limited by small sample sizes, a homogeneous population and short follow-up. Studies of alcohol and opioid use disorders found improvement in abstinence rates in the ketamine group, with significant between-group effects noted for up to two years following a single infusion, although these were not placebo-controlled trials. These results suggest that ketamine may facilitate abstinence across multiple substances of abuse and warrants broader investigation in addiction treatment. We conclude with an overview of the six ongoing studies of ketamine in the treatment of alcohol, cocaine, cannabis, and opioid use disorders and discuss future directions in this emerging area of research.
Introduction
Alcohol and illicit drug use is an escalating and complex global public health burden. In 2010, the global prevalence of alcohol and illicit drug use disorders were 9.6 and 10.9% respectively (1). Mortality rates have risen to epidemic proportions in some countries due to increasing prevalence of opioid use. For example, the United States, which accounts for 25% of global overdose mortality, has experienced an 88% increase in opioid overdose deaths each year from 2013 to 2016 (2, 3). Substance use disorders (SUDs) include cognitive, behavioral, and physiological symptoms. Hallmark signs of SUDs include impaired control, cravings, social impairment, risky use, and withdrawal symptoms. Withdrawal from heavy, prolonged alcohol use can result in life-threatening seizures and autonomic instability in addition to hallucinations, severe agitation, and anxiety. Physiologic response to opioid withdrawal can also be severe, and includes nausea, emesis, diarrhea, myalgias, intractable lacrimation and rhinorrhea, fevers, dysphoria and insomnia. Fear of these withdrawal symptoms is frequently cited as a barrier to treatment and reason for relapse (4).
Despite the high prevalence and substantial societal burden of SUDs, effective pharmacotherapy options are limited. FDA-approved medications for alcohol use disorder include naltrexone (an opioid receptor antagonist) and acamprosate (a GABA-agonist) which have been shown in meta-analyses to modestly reduce rates of return to heavy drinking (5), and disulfiram and nalmefene (approved in the European Union although not FDA-approved in the U.S.) have shown overall Hedge's g effect sizes of 0.58 and 0.33, respectively (6, 7). Treatment options for opioid dependence include full opioid agonists (methadone), partial agonists (buprenorphine) and antagonists (naltrexone). For cannabis and stimulant use disorders, there are no FDA-approved treatments (8). With limited treatment options, a myriad of non-FDA approved medications (e.g., gabapentin, clonidine, bupropion) are tried as standalone pharmacotherapies and in conjunction with behavioral interventions.
Glutamatergic dysregulation in the prefrontal cortex and mesolimbic regions (including the amygdala and the nucleus accumbens) has been implicated in addiction pathology across multiple substances of abuse (9). Similarly, depression has been shown to have aberrant glutamate signaling (10–12). Ketamine is a potent, non-competitive NMDA receptor antagonist which has been widely used in conjunction with general anesthesia following FDA approval in the U.S. in 1970. More recently, ketamine has been shown in two meta-analyses to induce ultra-rapid remission of severe depression and suicidal ideation using sub-anesthetic dosages (13–15). This anti-depressant effect is hypothesized to result from improved prefrontal cortex glutamate homeostasis (16). These changes ultimately produce synaptic improvements such as structurally increased spine density at synaptic proteins (17). These effects may improve ability to learn new behaviors (18) and may be beneficial in the treatment of SUDs. Our overall objective is to provide a review of the recent literature on the efficacy of ketamine in the treatment of SUDs.
Methods
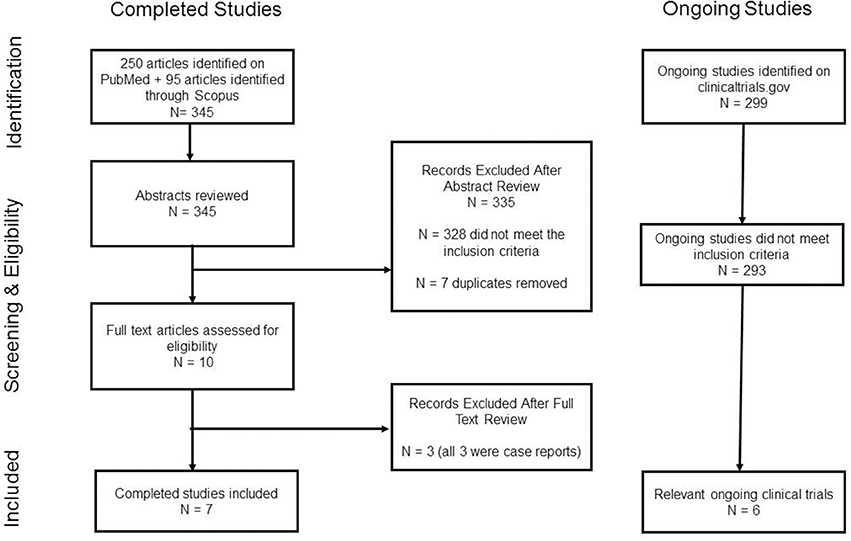
A comprehensive search in the PubMed/MEDLINE, Scopus and clinicaltrials.gov databases from 1 January 1996 to 1 January 2018 was conducted. PubMed was searched using the following MESH search terms: “Substance-Related Disorders” and “Ketamine/therapeutic use.” The Scopus database was searched using the term “ketamine” in conjunction with the following title keywords: “cocaine,” “alcohol,” “cannabis,” “marijuana,” “amphetamine,” “methamphetamine,” “tobacco,” “nicotine,” “heroin,” and “opi.*” Results from both databases were filtered to include only human studies with full text articles available in English. Case reports were excluded. “Ketamine” was the term used in the clinicaltrial.gov database search of active studies. The inclusion criteria were as follows: studies must have evaluated the efficacy of ketamine (with or without a behavioral intervention) in humans for the treatment of a SUD or the treatment of withdrawal symptoms from a substance of abuse. See Figure 1 for detailed study methodology characteristics.
Results
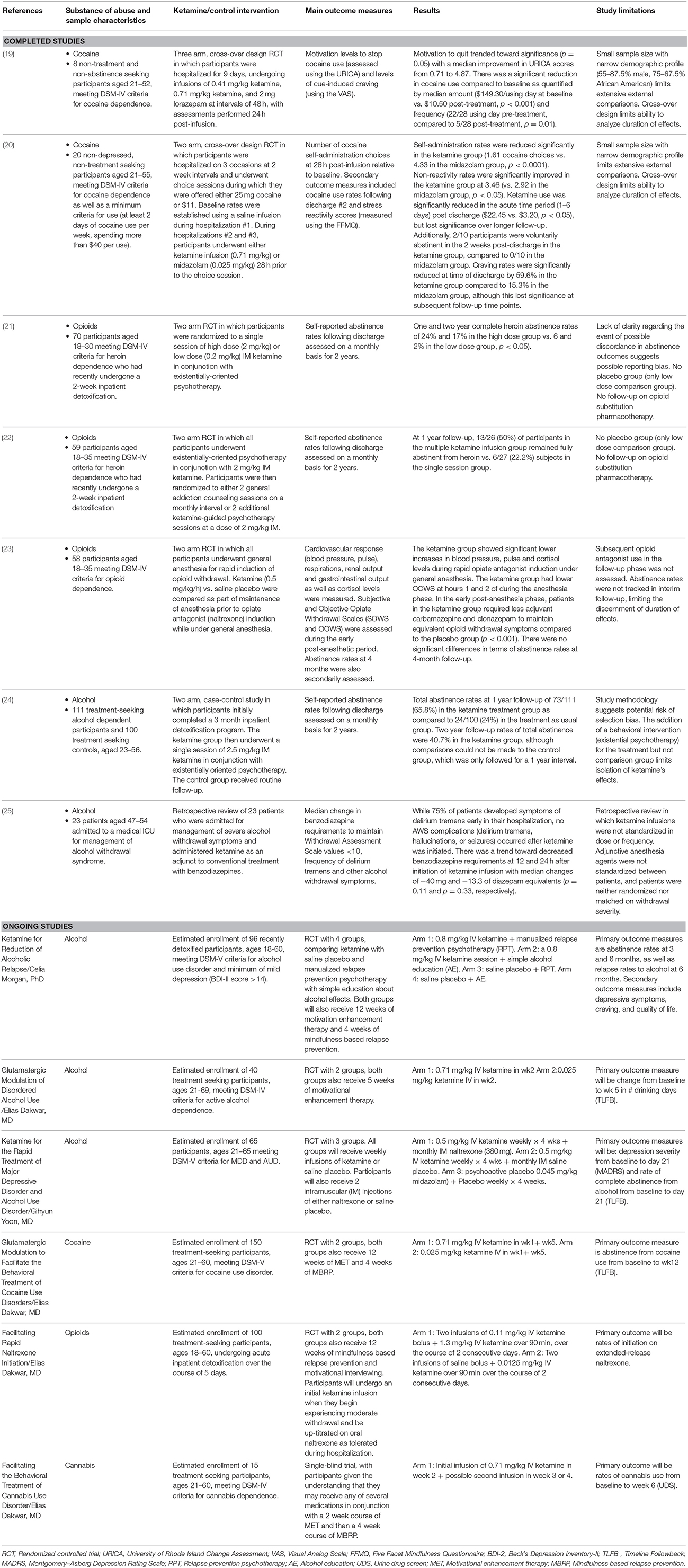
After evaluating for the inclusion criteria and for duplicates, we identified seven completed relevant clinical studies. Six ongoing relevant clinical trials were also identified. Substances of abuse studied included cocaine, alcohol, and opioids. No human studies were found that evaluated the efficacy of ketamine in the treatment of tobacco or stimulant use disorders other than cocaine. The included studies are summarized in Table 1.
Effects on Cocaine Use
Two published studies have evaluated the efficacy of ketamine for cocaine use disorder. Dakwar et al. (19) conducted a three-arm crossover trial which evaluated the effects of 0.41 and 0.71 mg/kg IV ketamine compared to an active placebo control (2 mg lorazepam) in eight non-treatment seeking cocaine dependent participants. Low dose ketamine always preceded the high dose and infusions were spaced at 48 h intervals. Primary outcome measures were changes in pre-infusion and 24-h post-infusion levels of motivation to quit cocaine as assessed by the University of Rhode Island Change Assessment (URICA) questionnaire and self-reported cocaine cravings on the Visual Analog Scale (VAS). VAS measurements were taken every 3 min during a 15-min cocaine cue exposure task, with a total score range of 0–600 mm. Within-subject statistical comparisons were made to baseline scores to assess order effects. Following the first infusion (either 0.41 mg/kg ketamine or 2 mg lorazepam), they found that ketamine increased motivation to quit cocaine over lorazepam (median score of 0.15 vs. 3.6, p = 0.012) and reduced cocaine craving on the VAS by a mean of 168 mm (a 60% change, p = 0.012). The post-lorazepam URICA scores were significantly improved when ketamine was received first (median change from baseline: 1.1 vs. 3.28, p = 0.047), indicative of a persistent effect of 0.41 mg/kg ketamine at 48 h; this may explain the lack of significant change in URICA and VAS scores following the 0.71 mg/kg ketamine infusion. Although there was no placebo comparison, there was a significant reduction in frequency (22 days of use/28 days at baseline vs. 5/28 days at 4 week follow-up, p = 0.012) and amount of cocaine use in the follow-up period ($149.30/use day at baseline vs. $10.50/use day at 4 week follow-up, p < 0.001).
In a related follow-up study of 20 non-treatment seeking cocaine dependent participants, Dakwar et al. (20) conducted a cross-over design, inpatient laboratory paradigm trial evaluating the efficacy of a single infusion of 0.71 mg/kg ketamine with 0.025 mg/kg midazolam as the active control. During a 6-day hospitalization, subjects participated in “choice sessions” (during which they could elect to self-administer 25 mg cocaine or receive $11). Rates of cocaine self-administration were reduced by 66% relative to pre-infusion baseline choice rates with no significant pre-/post- infusion self-administration differences noted in the control group (p < 0.0001).
Effects on Opioid Use Disorder and Opioid Withdrawal
Two published studies have evaluated the efficacy of ketamine for opioid use disorder. Krupitsky et al. (21) conducted a randomized controlled trial of 70 heroin-dependent participants in which they compared the efficacy of high dose ketamine (2 mg/kg IM) vs. low dose ketamine (0.2 mg/kg IM) in conjunction with psychotherapy. Primary outcome measures were heroin abstinence rates (assessed by self-report, collateral information, physical examination of skin, and urine drug screen) at 1, 3, 6, 12, 18, and 24 months. Abstinence rates at 1 month approached 85% in the 2 mg/kg group (compared to 55% in the 0.2 mg/kg group, p < 0.01) and were 24% at 1 year in the 2 mg/kg group (compared to 6% in the 0.2 mg/kg group, p < 0.05). Craving was also notably reduced in the high vs. low groups, with an enduring decline craving noted pre-/post- infusion in the high dose group on the VAS (baseline mean of 29.24 mm, 7.7 mm at 1 month, and 5.4 mm at 3 month follow-up, p < 0.001). In a follow-up study, Krupitsky et al. (22) evaluated the efficacy of single vs. repeated sessions of ketamine-assisted psychotherapy in increasing abstinence from heroin. Participants were randomized to a 1 or 3 sessions of ketamine (2 mg/kg IM, given at 1 month intervals). They found that 50% of subjects receiving multiple ketamine treatments were abstinent at 1 year follow-up, compared to 22% of single-session treatments (p < 0.05). They also noted significantly greater reductions in heroin craving in the repeated treatment group as compared to the single treatment group.
Jovaiša et al. (23) conducted a randomized controlled trial in which participants were given either saline placebo infusion or 0.5 mg/kg/h of IV ketamine prior to rapid opiate antagonist induction under general anesthesia. Their results showed that ketamine could suppress physiologic response to opiate withdrawal. Mean arterial pressure, heart rate, and serum cortisol were significantly lower in the ketamine group during opiate antagonist induction under anesthesia. There were no significant group differences at 4 months on their secondary outcome measures of aftercare treatment retention, abstinence rates, self-reported health, or social/family life improvements, although both the placebo and ketamine groups were also started on opioid antagonist treatment. This lack of group differences may be related to initial opioid antagonist treatment in both groups or to administration of ketamine while the participants were unconscious.
Effects on Alcohol Use Disorder and Alcohol Withdrawal Symptoms
Krupitsky et al. (24) evaluated two cohorts with alcohol use disorder that underwent inpatient detoxification and residential treatment. Following the 3-month residential treatment, the group compared the abstinence rates of 111 participants who volunteered to undergo a ketamine-assisted psychotherapy session with 100 subjects who completed only follow-up as usual. They found that 1-year abstinence rates were 65.8% in the ketamine-treated group compared to 24% in the follow-up as usual comparison group (p < 0.01). However, the study was not randomized and is limited by the lack of a control group. Wong et al. (25) completed a retrospective review of 23 patients who were hospitalized for management of severe alcohol withdrawal symptoms and who were administered ketamine as an adjunct to conventional treatment with benzodiazepines. They found a trend (p = 0.11) toward reduced benzodiazepine requirements at 12 and 24 h post-ketamine initiation with medians of −40 and −13.3 mg of diazepam equivalents.
Ongoing Studies
Six ongoing studies were identified through clinical trials.gov that are evaluating the use of ketamine in the treatment of SUDs (see Table 1). Three of these studies are focused on alcohol use disorder, and the other three are focused on cocaine, opioid, and cannabis use disorders.
The first randomized controlled trial evaluating the efficacy of ketamine in alcohol use disorder is in progress (NCT02649231 led by Celia Morgan, Ph.D.). This trial of 96 participants will compare ketamine with saline placebo and manualized relapse prevention psychotherapy with simple education about alcohol effects. Primary outcome measures include abstinence rates at 3 and 6 months, and relapse rates at 6 months. This study will evaluate the efficacy of a 0.8 mg/kg ketamine infusion in maintenance of abstinence from alcohol. By studying the effects on depression as a secondary outcome, this study will add to the intriguing findings that a positive family history of alcohol use disorder is associated with greater duration of anti-depressant effect (13). The inclusion of simple alcohol education and manualized relapse prevention psychotherapy arms will also assess whether the effects of ketamine are enhanced with cognitive behavioral psychotherapy.
A related randomized controlled trial of 40 subjects (NCT02539511 led by Elias Dakwar, MD) will evaluate the efficacy of a single infusion of ketamine (0.71 mg/kg) in conjunction with a 5 week course of motivational enhancement psychotherapy in reduction in alcohol use. Primary outcome measures include change in self-reported alcohol use rates at 5 weeks vs. baseline. These results will provide complementary information as to the efficacy of ketamine in conjunction with a standardized psychotherapy.
A third study of 65 subjects with alcohol use disorder and major depressive disorder (NCT02461927 led by Gihyn Yoon, MD) will evaluate the effects of ketamine in 3 treatment arms (0.5 mg/kg ketamine + 380 mg IM naltrexone vs. 0.5 mg/kg ketamine alone vs. placebo). The ketamine treatments will consist of once weekly infusions of IV ketamine for a total of 4 weeks and two injections of naltrexone or saline placebo spaced 1 month apart. Primary outcome measures include change in depression severity as measured by the Montgomery-Asberg Depression Rating Scale and rate of complete abstinence from alcohol as measured on the Time Line Follow Back at 4 week follow-up. This trial will inform questions regarding the utility of weekly infusion sessions as well as combination pharmacotherapy with naltrexone.
In a follow-up study to his initial investigations of ketamine in cocaine use disorder, Elias Dakwar, MD is leading a randomized, placebo-controlled trial (NCT03344419) of 150 subjects to evaluate the efficacy of 2 ketamine infusions (0.71 mg/kg active dose at a 1 month interval) on abstinence rates at baseline and following 12 weeks of adjunctive psychotherapy. This study will expand on the prior findings that ketamine can increase ability to achieve and maintain abstinence from cocaine and further evaluate the duration of efficacy in cocaine abstinence.
A related study (NCT03345173 led by Elias Dakwar, MD) will evaluate the use of ketamine in the acute detoxification of 100 opioid users to facilitate transition to extended release naltrexone injections. Participants will initially be hospitalized for up to 5 days for detoxification followed by naltrexone initiation (with ketamine infusions of 0.11 mg/kg in a 2-min bolus followed by 1.3 mg/kg over 90 min on 2 sequential days when they begin experiencing moderate withdrawal symptoms). Subjects will then complete 12 weeks of motivational enhancement therapy and mindfulness based relapse prevention psychotherapy. Primary outcome measure will be rates of successful initiation on extended release naltrexone.
A 15 subject, proof-of-concept study (NCT02946489 led by Elias Dakwar, MD) will investigate the use of 0.71 mg/kg ketamine in cannabis use disorder. This randomized controlled trial is the first to explore the effects of ketamine on cannabis abstinence rates. This study will recruit treatment seeking individuals and will also include motivational enhancement therapy and mindfulness based relapse prevention psychotherapy components. Abstinence rates will be assessed at baseline and at 6 week follow-up.
Discussion and Future Directions
Collectively, these studies suggest that ketamine may improve the ability to establish and maintain abstinence in SUDs. Improvement in cravings, motivation to quit, and self-administration have been shown in cocaine use disorder (19, 20, 26). Significant long-term improvements in complete abstinence from alcohol and heroin have been demonstrated with ketamine following extended inpatient treatment (21, 22, 24), and ketamine reduced physiological response during opioid withdrawal (23). However, these preliminary studies have several important limitations. The findings in the cocaine trials are limited by small sample sizes, narrow demographic sectors, and limited follow-up windows (19, 20). Additionally, both the heroin and alcohol use disorder studies by Krupitsky et al. (21), Krupitsky et al. (22), and Jovaiša et al. (23) utilized a low dose ketamine comparison group rather than a true placebo control and did not control for adjunctive pharmacotherapy in the follow-up.
A number of important questions also remain. It is unclear to what extent baseline motivation, desire to quit, or duration of prior abstinence influences the effectiveness of ketamine in achieving and maintaining abstinence. The heroin and alcohol dependent populations in Russia studied (21, 22, 24) were treatment seeking and had completed 3 months of residential inpatient treatment prior to ketamine infusions; this is markedly different from the non-treatment seeking cocaine studies (19, 20). It is of note however, that 20% of the non-treatment, non-abstinence seeking cocaine trial participants (19) were voluntarily abstinent following the single ketamine infusion (compared to 0% of the midazolam control group). While the abstinence improvements in heroin use noted at 1 and 2 year follow-up are promising (21, 22), their unique demographic, genetic, and socioeconomic characteristics may contribute to these results. Potential gender differences are also an important aspect to consider in future trial design and analysis.
The effects of ketamine on withdrawal states are particularly important to further investigate. Alcohol and benzodiazepine withdrawal can result in life-threatening medical sequela, and the severe physiologic response to opioid withdrawal may deterrent to initial treatment. The opioid withdrawal study (23) showed physiologic suppression of opiate withdrawal. While the effects of ketamine on opioid withdrawal independent from its use in conjunction with general anesthesia have not been systematically studied, several case reports have utilized ketamine in conscious patients with improvement in their opioid withdrawal symptoms (27–29). The results from the ongoing naltrexone induction study should provide some insight as to whether ketamine improves opioid withdrawal symptoms independent from rapid opioid induction under general anesthesia. Future studies should assess and report on concurrent use of FDA-approved treatments for opioid use disorder (both at baseline and in the follow-up phase). Prospective trials are also needed to give further information about ketamine's efficacy in alcohol withdrawal.
The utility of behavioral interventions as adjuvants to ketamine pharmacotherapy in addictions treatment is understudied. The ongoing trial led by Morgan and colleagues will evaluate the addition of psychotherapy to ketamine infusions. However, additional research is needed to examine behavioral interventions which may be synergistic with ketamine pharmacotherapy and help enhance long-term treatment outcomes.
At sub-anesthetic dosing, ketamine produces mild dissociative psychoactive effects (30, 31). While these psychotomimetic characteristics may increase abuse liability (32), more recent studies in both depression and substance abuse populations suggest that the therapeutic events of ketamine may be mediated by participant perception of these psychoactive effects (26, 30, 31). Future studies should include assessment of the psychoactive effects of ketamine to further evaluate whether perceptual experience mediates therapeutic benefit.
Finally, future ketamine trials should include evaluation of optimal dose and frequency schedules. The majority of the studies have utilized prior depression trial dosages of 0.5–0.8 mg/kg IV ketamine, although a few studies utilized doses of 2–2.5 mg/kg IM. Intranasal dosing (which is currently under evaluation for the treatment of depression) could also widely expand the availability of ketamine treatment. Further characterization in other substances of abuse (such as nicotine, amphetamines, and the ongoing cannabis trial) may also provide important insights as to the overall efficacy of ketamine in the treatment of SUDs. In summary, the most pressing public health question is whether ketamine (in single or multiple dose treatments) can significantly reduce addiction morbidity and mortality. Further studies are urgently needed.
Author Contributions
JJ designed the strategy for the present review, searched for the references, read the manuscripts, and drafted the manuscript. CM drafted content for the manuscript introduction and figure. RM, KB, and SB provided content and editorial oversight. All authors discussed the results, reviewed the manuscript, and helped with the final writing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by NIDA grants R25 DA020537 (SB and KB) and K02 DA039229 (SB), as well as NIDA grant DA020537-12 (McGinty).
References
1. Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet (2013) 382:1575–86. doi: 10.1016/S0140-6736(13)61611-6
2. Hedden SL. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration, Department of Heath and Human Services (2015).
3. Merz F. United Nations Office on Drugs and Crime: World Drug Report 2017. SIRIUS-Zeitschrift für Strategische Analysen (2017).
4. Hedegaard H, Warner M, Miniño AM. Drug Overdose Deaths in the United States, 1999–2015. NCHS Data Brief. Hyattsville, MD: National Center for Health Statistics (2017).
5. Notley C, Blyth A, Maskrey V, Pinto H, Holland R. Exploring the concepts of abstinence and recovery through the experiences of long-term opiate substitution clients. Subst Abus. (2015) 36:232–9. doi: 10.1080/08897077.2014.941085
6. Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA (2014) 311:1889–900. doi: 10.1001/jama.2014.3628
7. Skinner MD, Lahmek P, Pham H, Aubin HJ. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis. PLoS ONE (2014) 9:e87366. doi: 10.1371/journal.pone.0087366
8. Mann K, Torup L, Sørensen P, Gual A, Swift R, Walker B, et al. Nalmefene for the management of alcohol dependence: review on its pharmacology, mechanism of action and meta-analysis on its clinical efficacy. Eur Neuropsychopharmacol. (2016) 26:1941–9. doi: 10.1016/j.euroneuro.2016.10.008
9. McLellan AT, Lewis DC, O'brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA (2000) 284:1689–95. doi: 10.1001/jama.284.13.1689
10. Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. (2008) 75:218–65. doi: 10.1016/j.bcp.2007.06.039
11. Abdallah CG, Averill CL, Salas R, Averill LA, Baldwin PR, Krystal JH, et al. Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol Psychiatry (2017) 2:566–74. doi: 10.1016/j.bpsc.2017.04.006
12. Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov. (2017) 16:472–86. doi: 10.1038/nrd.2017.16
13. Niciu MJ, Luckenbaugh DA, Ionescu DF, Richards EM, Vande Voort JL, Ballard ED, et al. Ketamine's antidepressant efficacy is extended for at least four weeks in subjects with a family history of an alcohol use disorder. Int J Neuropsychopharmacol. (2014) 18:pyu039. doi: 10.1093/ijnp/pyu039
14. Han Y, Chen J, Zou D, Zheng P, Li Q, Wang H, et al. Efficacy of ketamine in the rapid treatment of major depressive disorder: a meta-analysis of randomized, double-blind, placebo-controlled studies. Neuropsychiatr. Dis. Treat. (2016) 12:2859. doi: 10.2147/NDT.S117146
15. Bartoli F, Riboldi I, Crocamo C, Di Brita C, Clerici M, Carrà G. Ketamine as a rapid-acting agent for suicidal ideation: a meta-analysis. Neurosci Biobehav Rev. (2017) 77:232–6. doi: 10.1016/j.neubiorev.2017.03.010
16. Strasburger SE, Bhimani PM, Kaabe JH, Krysiak JT, Nanchanatt DL, Nguyen TN, et al. What is the mechanism of Ketamine's rapid-onset antidepressant effect? A concise overview of the surprisingly large number of possibilities. J Clin Pharm Ther. (2017) 42:147–54. doi: 10.1111/jcpt.12497
17. Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Phil Trans R Soc B (2012) 367:2475–84. doi: 10.1098/rstb.2011.0357
18. Naughton M, Clarke G, Olivia FO, Cryan JF, Dinan TG. A review of ketamine in affective disorders: current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action. J Affect Disord. (2014) 156:24–35. doi: 10.1016/j.jad.2013.11.014
19. Dakwar E, Levin F, Foltin RW, Nunes EV, Hart CL. The effects of subanesthetic ketamine infusions on motivation to quit and cue-induced craving in cocaine-dependent research volunteers. Biol Psychiatry (2014) 76:40–6. doi: 10.1016/j.biopsych.2013.08.009
20. Dakwar E, Hart CL, Levin FR, Nunes EV, Foltin RW. Cocaine self-administration disrupted by the N-methyl-D-aspartate receptor antagonist ketamine: a randomized, crossover trial. Mol Psychiatry (2017) 22:76–81. doi: 10.1038/mp.2016.39
21. Krupitsky E, Burakov A, Romanova T, Dunaevsky I, Strassman R, Grinenko A. Ketamine psychotherapy for heroin addiction: immediate effects and two-year follow-up. J Subst Abus Treat. (2002) 23:273–83. doi: 10.1016/S0740-5472(02)00275-1
22. Krupitsky EM, Burakov AM, Dunaevsky IV, Romanova TN, Slavina TY, Grinenko AY. Single versus repeated sessions of ketamine-assisted psychotherapy for people with heroin dependence. J Psychoact Drugs (2007) 39:13–9. doi: 10.1080/02791072.2007.10399860
23. Jovaiša T, Laurinenas G, Vosylius S, Šipylaite J, Badaras R, Ivaškevičius J. Effects of ketamine on precipitated opiate withdrawal. Medicina (2006) 42:625–34.
24. Krupitsky EM, Grinenko AY. Ketamine psychedelic therapy (KPT): a review of the results of ten years of research. J Psychoact Drugs (1997) 29:165–83. doi: 10.1080/02791072.1997.10400185
25. Wong A, Benedict NJ, Armahizer MJ, Kane-Gill SL. Evaluation of adjunctive ketamine to benzodiazepines for management of alcohol withdrawal syndrome. Ann Pharmacother. (2015) 49:14–9. doi: 10.1177/1060028014555859
26. Dakwar E, Nunes EV, Hart CL, Hu MC, Foltin RW, Levin FR. A sub-set of psychoactive effects may be critical to the behavioral impact of ketamine on cocaine use disorder: results from a randomized, controlled laboratory study. Neuropharmacology (2018). doi: 10.1016/j.neuropharm.2018.01.005 [Epub ahead of print].
27. Lalanne L, Nicot C, Lang JP, Bertschy G, Salvat E. Experience of the use of ketamine to manage opioid withdrawal in an addicted woman: a case report. BMC Psychiatry (2016) 16:395. doi: 10.1186/s12888-016-1112-2
28. Omoigui S, Hashmat F, Bernardo Z. Use of ketamine in ameliorating opioid withdrawal symptoms during an induction phase of buprenorphine. Open Pain J. (2011) 4:1–3. doi: 10.2174/1876386301104010001
29. Strickler EM, Schwenk ES, Cohen MJ, Viscusi ER. Use of Ketamine in a multimodal analgesia setting for rapid opioid tapering in a profoundly opioid-tolerant patient: a case report. AA Pract. (2018) 10:179–81. doi: 10.1213/XAA.0000000000000653
30. Bartoli F, Clerici M, Carrà G. Antidepressant response and dissociative effects after ketamine treatment: two sides of the same coin? J Clin Psychiatry (2017) 78:e1318. doi: 10.4088/JCP.17lr11789
31. Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. Relationship of ketamine's antidepressant and psychotomimetic effects in unipolar depression. Neuroendocrinol Lett. (2013) 34:287–93.
Keywords: ketamine, substance use disorders, addiction, glutamate, abstinence
Citation: Jones JL, Mateus CF, Malcolm RJ, Brady KT and Back SE (2018) Efficacy of Ketamine in the Treatment of Substance Use Disorders: A Systematic Review. Front. Psychiatry 9:277. doi: 10.3389/fpsyt.2018.00277
Received: 08 March 2018; Accepted: 07 June 2018;
Published: 24 July 2018.
Edited by:
Wendy J. Lynch, University of Virginia, United StatesReviewed by:
Giuseppe Carrà, Università degli Studi di Milano Bicocca, ItalyFrancisco José Eiroa-Orosa, University of Barcelona, Spain
Copyright © 2018 Jones, Mateus, Malcolm, Brady and Back. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer L. Jones, am9uamVuQG11c2MuZWR1
 Jennifer L. Jones
Jennifer L. Jones Camilo F. Mateus1
Camilo F. Mateus1 Sudie E. Back
Sudie E. Back