
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 15 May 2018
Sec. Mood Disorders
Volume 9 - 2018 | https://doi.org/10.3389/fpsyt.2018.00195
 Zhexue Xu1,2†
Zhexue Xu1,2† Shu Zhang3†
Shu Zhang3† Liyuan Huang1
Liyuan Huang1 Xiaolei Zhu4
Xiaolei Zhu4 Qing Zhao5
Qing Zhao5 Yawei Zeng6
Yawei Zeng6 Dongfeng Zhou7
Dongfeng Zhou7 Di Wang8
Di Wang8 Hironori Kuga4,9
Hironori Kuga4,9 Atsushi Kamiya4*
Atsushi Kamiya4* Miao Qu1,2*
Miao Qu1,2*Identification of biological markers for defining subtypes of major depressive disorder (MDD) is critical for better understanding MDD pathophysiology and finding effective treatment intervention. The “Yin and Yang” theory is a fundamental concept of traditional Chinese Medicine (TCM). The theory differentiates MDD patients into two subtypes, Yin and Yang, based on their somatic symptoms, which had empirically been used for the delivery of effective treatment in East Asia. Nonetheless, neural processes underlying Yin and Yang types in MDD are poorly understood. In this study, we aim to provide physiological evidence using functional magnetic resonance imaging (fMRI) to identify altered resting-state brain activity associated with Yin and Yang types in drug-naïve MDD patients. The Yin type and Yang type MDD patients showed increased amplitude of low-frequency fluctuation (ALFF) in different cortical brain areas in the parietal, temporal, and frontal lobe, compared to matched healthy controls. Differential ALFF is also observed in several cortical areas in frontal lobe and insula between Yin and Yang type group. Of note, although ALFF is increased in the inferior parietal lobe in both Yin and Yang type group, inferior parietal lobe-centered functional connectivity (FC) is increased in Yang type, but is decreased in Ying type, compared with matched healthy controls. These results suggest that differential resting-state brain activity and functional connectivity in Yin and Yang types may contribute to biological measures for better stratification of heterogeneous MDD patients.
Major depressive disorder (MDD) is a prevalent psychiatric condition associated with increased mortality and healthcare resource utilization, imposing a serious economic burden on our society (1, 2). MDD is defined based on clinical manifestation utilized in current gold standard diagnostic classification systems such as Statistical Manual of Mental Disorders (DSM) and International Statistical Classification of Diseases and Related Health Problems (ICD) (3, 4). Although these categorical frameworks contribute to the advancement of biological understanding and patient care, the pathophysiology of MDD remains elusive. Moreover, many MDD patients exhibit treatment-resistance to current antidepressants, potentially due to its heterogeneous nature with multiple etiologies (5).
Considering these issues, many studies attempt to identify biological and physiological markers to differentiate subtypes of MDD, which may be more responsive to specific treatment approaches (6–10). For instance, Raison and colleagues reported that infliximab, a tumor necrosis factor (TNF) antagonist, ameliorates depressive symptoms in a subpopulation of MDD patients who have high level of C-reactive protein (CRP) in plasma, but not in those with low levels of CRP (6). Drysdale et al. have recently demonstrated that different patterns of altered neural connectivity involved in limbic and frontostriatal regions can classify MDD patients into four physiological subtypes, so-called biotypes (8). Interestingly, these biotypes predict effectiveness of treatment with transcranial magnetic stimulation.
Traditional Chinese Medicine (TCM), developed a few thousand years ago, approaches diagnosis and treatment differently from Western medicine (11, 12). For example, patients are classified into two subgroups based on their somatic symptoms according to the “Yin and Yang” theory, the fundamental concept of the TCM. The “Yin and Yang” theory has historically been documented in the TCM book, entitled Huangdi Neijing, which was written over two thousand years ago in China (13). Yin patients are characterized by an intolerance to cold with a preference to warm environments and hot beverages and food, whereas Yang patients are characterized by an intolerance to heat with a preference to cool environments, cold beverages, and food. Although current diagnostic criteria, such as DSM-5 and ICD-10, do not consider somatic symptoms for diagnosis of MDD, the contrastive Yin and Yang phenotypes might be useful for identifying biotypes of MDD. Nonetheless, physiological neural processes underlying Yin and Yang types in MDD remain poorly explored.
The aim of this exploratory study is to provide physiological evidence, using functional magnetic resonance imaging (fMRI), to identify altered resting-state brain activity associated with Yin and Yang types in drug-naïve MDD patients. Previous studies using functional connectivity (FC) method of resting-sate fMRI demonstrated altered inter- and intral-regional brain connectivity, including local functional connectivity in the medial prefrontal cortex and frontoparietal hypoconnectivity in MDD brains (14, 15). As proposed in Drysdale's work (8), differential brain function at resting-state may be a useful physiological marker to identify specific subpopulation of MDD patients. Thus, we hypothesize that resting-state brain activity and FC in MDD patients with Yin type are altered when compared to those with Yang type. To test this hypothesis, we examined resting-state functional activities across the entire brain in MDD patients in both Yin and Yang groups as well as matched healthy controls.
Patients with MDD were recruited from the outpatient clinic of The Third Affiliated Hospital of Beijing University of Chinese Medicine, Peking University Sixth Hospital, and Beijing Anding Hospital. Healthy controls were recruited from community-based advertising through flyers posted at hospital and university campuses. All participant-involved study activities were approved by the Medical Research Ethics Committee of The Third Affiliated Hospital of Beijing University of Chinese Medicine. All participants were recruited from October 2015 to December 2016. Exclusion criteria for MDD patient group and healthy controls were: (1) left-handedness; (2) age <18 years and >45 years; (3) any neurological illness; (4) history of any other major psychiatric disease; (5) any diagnosed physical disease; (6) any brain white matter changes detected by T2-weigheted magnetic resonance images; (7) metallic foreign bodies, pacemakers, metallic dentures, or amalgam fillings; (8) females in a post-menopausal and menopausal condition. MDD patients also met the following additional inclusion criteria: (1) diagnosis of moderate depression; (2) 17-item Hamilton Depression Rating scale (HAM-D) scores ≥17 and < 24 and Young Mania Rating scale (YMRS) scores < 5; (3) no diagnosis for any other mental disorders according to DSM-IV criteria; (4) medication-free for at least 2 weeks. The additional inclusion criteria for healthy controls was HAM-D score < 7. To control study variance, MDD subjects were matched individually to one healthy control subject for gender, age, education years, and IQ level.
All participants underwent structured clinical interview and were diagnosed by two well-trained senior psychiatrists according to the criteria defined by the DSM-IV. Clinical symptoms were measured on the same day as the fMRI measurement. HAM-D (16) and YMRS (17) were used to assess psychiatric symptoms. Three well-experienced physician scientists classified patients in Yin and Yang types based on somatic symptoms that occurred no <2 weeks before recruitment and the results of Yin and Yang questionnaires (Supplementary Table 1). The Yang group feels hot or has hot flashes, sweats during daily activities, and prefers cool environments and cold beverages and food. The Yin group feels cold or has cold flashes, does not sweat even during strenuous exercise, and prefers warm environments and hot beverages and food (18). We also confirmed that matched healthy control subjects had no somatic symptoms. The Yin-Yang Questionnaire was established by our research group, consisting of three psychiatrists and three physician scientists of traditional Chinese medicine. The reliability of this questionnaire was initially tested in 30 Yang and 30 Yin depression patients (Cronbach's Alpha was 0.861, and 0.823, respectively). In this questionnaire, we weight patients' subjective symptoms as primary scores, objective physical signs as secondary scores, and use a set of 5 levels to evaluate the degree of symptoms. In order to exclude the comorbidity of Yin and Yang types, we also set cut-off points of Yin and Yang.
T1-weighted and resting-state fMRI data were acquired using a 3T Siemens Trio scanner (Magnetom Allegra, Siemens, Erlangen, Germany) in the Beijing PLA 306 Hospital. A high-resolution image covering the whole brain was acquired with the following scan parameters: Repetition Time (TR)/Echo Time (TE) = 2,300/3.0 ms, flip angle = 90°, Field of view (FOV) = 240 × 256 mm2, voxel size = 1.0 × 1.0 × 1.0 mm3, 176 sagittal slices, duration = 6 min 06 s. Resting-state functional imaging scans contain 180 functional volumes, using a T2-weigted Echo Planar Imaging sequence with the following parameters: TR/TE = 2,000/30 ms, flip angle = 90°, acquisition matrix = 64 × 64, in-plane resolution 3.0 × 3.0 mm2, FOV = 210 × 210 mm2, axial slices = 32, thickness/gap = 4/0 mm, the total sequence time is 6 min 06 s. During the scanning process, patients were monitored to ensure that they remained awake. Immediately following the scans, a simple inquiry was conducted to exclude any sleeping periods as well as any uncomfortableness felt by the participant during the examination.
Image preprocessing and statistical analyses were performed using the Data Processing Assistant for Resting-state fMRI (DPARSF, http://www.restfmri.net/forum/DPARSF) toolkits 20 and SPM8 software (SPM8, http://www.fil.ion.ucl.ac.uk/spm/). All images were drafted by BrainNet Viewer toolkit (19).
Data pre-processing was performed by standard procedures in DPARSF toolkits. Images were pre-processed according to the following steps: the original DICOM data were converted to the NifTI format; the first 10 images were discarded to allow for instrumental stabilization of the initial signal; the images were slice-timed and realigned to correct for head motions; Patient's and healthy control's data were excluded if movement in the translational or rotational planes exceeded 3 mm or 3° and 1 mm or 1°, respectively; the images were normalized based on the Montreal Neurological Institute (MNI) Space with Smoothing Method (Full Width at Half Maximum, FWHM 6 mm); resting-state fMRI data were processed with linear detrending and 0.01–0.08 Hz band-pass filtering.
Using Resting-State fMRI Data Analysis Toolkit (REST) 1.6 software (http://restfmri.net/forum/REST) (20), linear tendency of the data after pre-processing (space smoothing was completed) was removed by linear regression. After pre-processing, the time series for each voxel was first filtered (band-pass, 0.01–0.08 Hz) to remove the effects of very low-frequency drift and high-frequency noise, and then converted to the frequency domain using a Fast Fourier Transform (FFT). The square root of the power spectrum was computed. This averaged square root was termed ALFF at the given voxel (21). In the following FC analysis, brain regions that showed significant ALFF changes were set as seeds to examine whole-brain functional connectivity across all brain regions.
Participants' demographic information, including age, gender, education level and IQ, as well as was compared between Yin and Yang MDD groups and their matched health control groups using two samples t-tests. Chi-square tests were applied to detect gender related differences. The statistical significance level was set at p < 0.05. All statistical tests were performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Pearson correlation coefficients were calculated to assess the relationship between ALFF of regions of interest and HAM-D total scores or its sub-factors. We did not apply corrections for multiple tests in this Pearson correlation coefficients because the analyses were considered exploratory in nature. The fMRI data were analyzed by technologists who were blind to the diagnosis of the participants, in the National Key Laboratory of Cognitive Neuroscience and Learning of Beijing Normal University. One-sample t-test was computed for significant brain activation in whole brain in every subject using REST 1.6 software (Threshold p < 0.05). Voxel-wise group comparisons were detected with two-sample t-test (AlphaSim correction p < 0.01; continuous voxels >71). REST 1.6 software Viewer was applied to identify the precise anatomical position in the brain with statistical significance on the corresponding MNI coordinate. Voxel-wise FC analyses revealed the Pearson correlation coefficients between the seeds and the rest of the whole brain areas. FC values were transformed into z-values by using Fisher r-to-z transformation. Two-sample t-tests were used to disclose the group differences in the functional connectivity (AlphaSim correction p < 0.01; continuous voxels >71).
MDD patients (n = 12 Yin and n = 12 Yang group) and matched healthy controls (n = 12 for Yin and Yang MDD group, respectively) participated in this study. The demographic information for these four groups was shown in Table 1. There were no statistical differences in age, gender, years of education, and IQ between Yin and Yang types MDD patient and healthy control groups. The Yin and Yang types MDD patients scored higher on the Yin and Yang questionnaires compared to their matched healthy controls (Yin: 41.08 ± 3.58 vs. 2.50 ± 1.38, p < 0.01; Yang: 39.58 ± 3.45 vs. 1.92 ± 1.73, p < 0.01). As shown in Table 2, the Yin and Yang type MDD patient groups had higher HAM-D scores compared to those of their healthy controls (Yin: 23.67 ± 2.57 vs. 0.92 ± 0.90; Yang: 20.58 ± 2.02 vs. 0.83 ± 0.84, p < 0.01). The HAM-D score of Yin type MDD patients was higher than that of Yang type MDD patients (23.67 ± 2.57 vs. 20.58 ± 2.02, p < 0.01). In the HAM-D factors, cognitive disturbance was severe in Yin type MDD patients compared with those in Yang type MDD patients (6.83 ± 1.85 vs. 5.92 ± 0.99, p < 0.05), whereas there was no difference in psychomotor retardation and dyssomnia between groups.
Intergroup differences of results from ALFF analysis were shown in Table 3. Compared to the healthy control group, MDD patients group showed increased ALFF in left inferior parietal, extending to supramarginal and angular gyri (−45, −51, 57. BA40/7) and decreased ALFF in left lingual gyrus (−27, −93, −18. BA18) (Figure 1A). Increased ALFF in Yin type MDD patients compared to their healthy controls was only observed in left superior parietal gyrus (−21, −72, 42. BA7/19) (Figure 1B). An increase in ALFF were found in Yang type MDD patient group compared to their healthy controls in the right superior frontal gyrus, extending to medial (6, 60, 3. BA10/9/32), the right middle temporal gyrus (54, −27, −9. BA21), the right extra-nuclear (33, 3, −12. BA13), the right supramarginal gyrus (60, −24, 36. BA2/3/1), the left inferior parietal, extending to supramarginal and angular gyri (−39, −57, 57. BA40/7), and the left insula (−36, 6, −6. BA13/47) (Figure 1C).
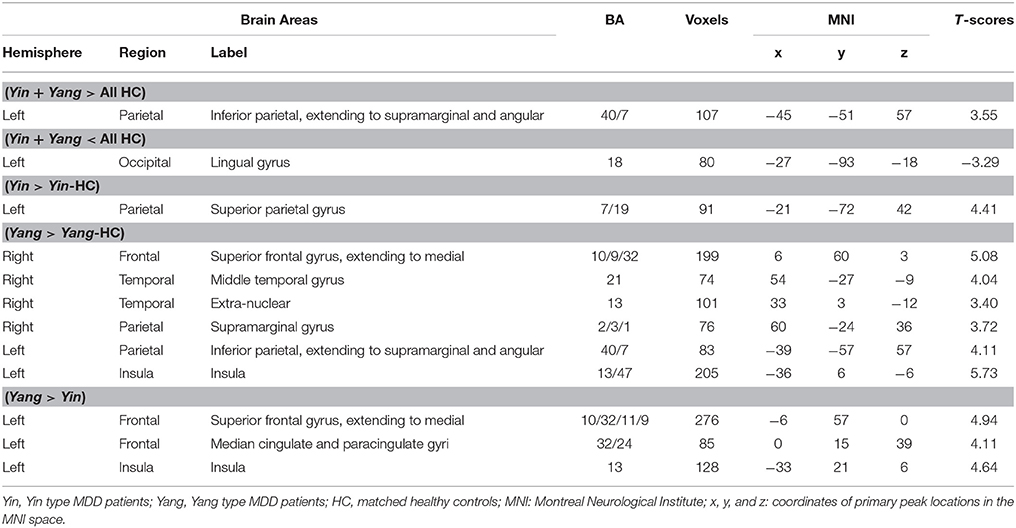
Table 3. The comparison of regional brain activity in Yin and Yang type MDD patients and matched controls (AlphaSim-corrected, p < 0.01).
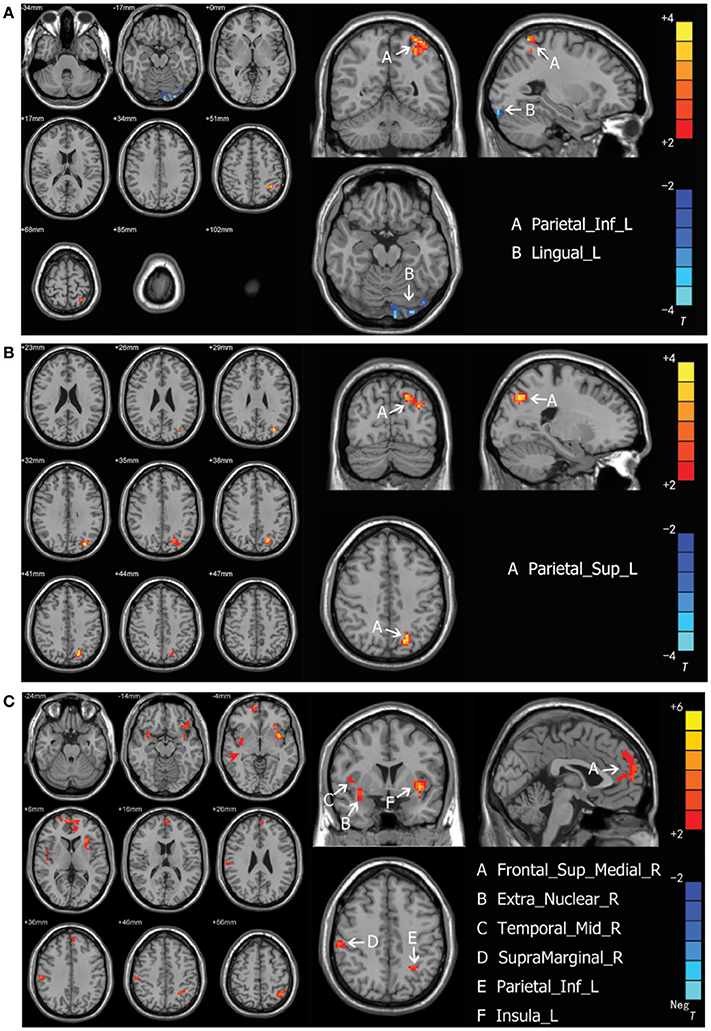
Figure 1. (A) Brain activation regions of rs-fMRI using Amplitude of Low-Frequency Fluctuation (ALFF) method in depression patients compared with the entire normal control cohort. A Parietal_Inf_L; left inferior parietal extending to supramarginal and angular gyri, B Lingual_L; left lingual gyrus. (B) Brain activation regions of rs-fMRI using Amplitude of Low-Frequency Fluctuation (ALFF) method in Yin type compared with matched healthy controls. A Parietal_Sup_L; left superior parietal gyrus. (C) Brain activation regions of rs-fMRI using Amplitude of Low-Frequency Fluctuation (ALFF) method in Yang type compared with matched healthy controls. A Frontal_Sup_Medial_R; right superior frontal gyrus, extending to medial, B Extra_Nuclear_R; right extra-nuclear, C Temporal_Mid_R; right middle temporal gyrus; D SupraMarginal_R; right supramarginal gyrus, E Parietal_Inf_L; left inferior parietal, extending to supramarginal and angular gyri, F Insula_L; left insula.
In the MDD patients group, increased ALFF was observed in Yang type group compared to Yin type group in the left superior frontal gyrus, extending to medial (−6, 57, 0. BA10/32/11/9), the left median cingulate and paracingulate gyri (0, 15, 39. BA32/24), and the left insula (−33, 21, 6. BA13) (Figure 2A). There was no significant correlation between ALFF values and HAM-D scores in either Yin and Yang type of MDD patients. Nonetheless, we found that the ALFF value of the right extra-nuclear in the Yang type group was positively correlated with cognitive disturbance (r = 0.6773, 0.95 CI: 0.169–0.901; p = 0.016), and was negatively correlated with insomnia (r = 0.6269, 0.95 CI: −0.883 to −0.083; p = 0.029) in sub-factor analysis (Figure 2B). We also analyzed the correlation between the Yin and Yang scores and ALFF of brain regions. Although we observed a negative correlation between Yang scores and left insula (r = −0.577 p = 0.050), there is no significant correlation after FDR correction (p = 0.3). We also found no significant correlation of Yin score and ALFF.
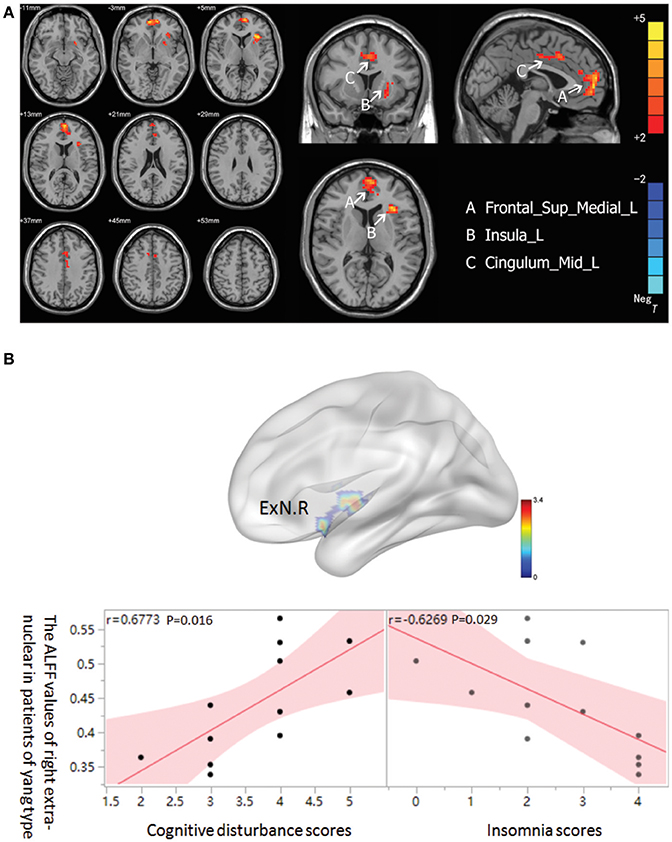
Figure 2. (A) Brain activation regions of rs-fMRI using Amplitude of Low-Frequency Fluctuation (ALFF) method in Yang type compared with Yin type. A Frontal_Sup_Medial_R; left superior frontal gyrus, extending to medial, B Insula_L; left insula, C Cingulum_Mid_L; left median cingulated and paracingulate gyri. (B) The correlation between ALFF of right extra-nuclear and two sub-factors of HAMD in Yang type MDD patients. The graph indicates that the ALFF of right extra-nuclear (ExN.R) had positive correlation with cognitive disturbance and negative correlation with insomnia.
The results from FC analysis, indicating intergroup differences, are shown in Table 4. The significantly changed ALFF in Yin and Yang types compared with matched healthy controls were taken as seeds. In Yin type patients, negative FC was observed between left superior parietal gyrus and left inferior parietal, extending to supramarginal and angular gyri (−48, −60, 42. BA40; Figure 3A). In Yang type patients, the left inferior parietal, extending to supramarginal and angular gyri showed positive FC with left supramarginal gyrus (−57, −24, 36. BA2/40), whereas right extra-nuclear and right middle temporal gyrus showed negative FC with right lingual gyrus (24, −78, −6. BA17) and right fusiform gyrus (30, −51, −6. BA19), respectively (Figure 3B).
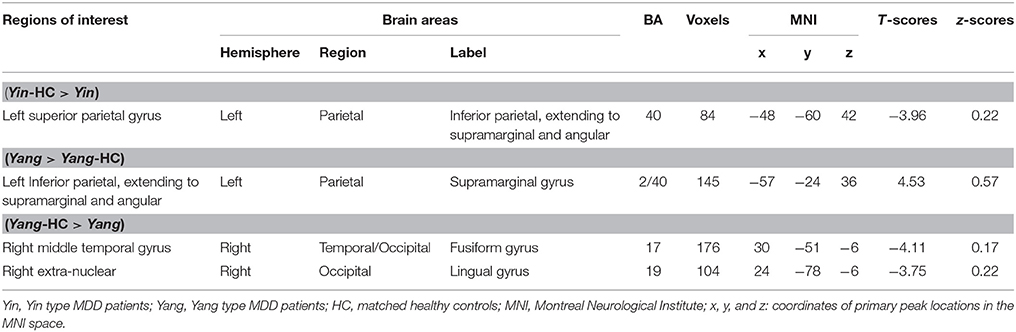
Table 4. The comparison of functional connectivity in Yin and Yang type MDD patients with matched controls (AlphaSim-corrected, p < 0.01).
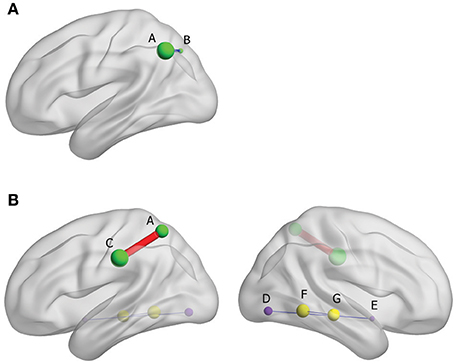
Figure 3. (A) Brain functional connectivity of rs-fMRI in Yin type compared with healthy controls; size of balls represent the t-scores of every brain regions; blue thin edge represents the negative FC between the different regions; A: left inferior parietal, extending to supramarginal and angular gyri, B: left superior parietal gyrus. (B) Brain functional connectivity of rs-fMRI in Yang type compared with healthy controls; size of balls represents the t-scores of every brain regions; red bold edge represents the positive FC between the different regions, conversely, blue thin edges represent the negative FC; same colored ball represents connecting brain region; A: left inferior parietal, extending to supramarginal and angular gyri, C: left supramarginal gyrus, D: right extra-nuclear, E: right lingual gyurs, F: right middle temporal gyrus, G: Fusiform gyrus.
The recent meta-analysis of resting-state fMRI studies demonstrated altered neural connectivity in several brain regions in MDD patients (15). These include hypoconnectivity of frontoparietal systems and dorsal attention network, which are critical brain regions for regulating mood, emotion, and attention (22–25). Another meta-analysis of resting-state fMRI studies showed altered local functional connectivity in the medial prefrontal cortex in drug-naïve MDD patients (14). Consistent with previous studies (26–29), we found increased resting-state brain activity in left inferior parietal, extending to supramarginal gyrus, and decreased activation in left lingual gyrus in MDD patients compared to the healthy control group. We also observed increased activation in angular gyrus, whereas the previous study reported decreased activity in this area in patients with MDD (30). Note that considering heterogeneous etiology and symptomatology of MDD, we focused on drug-naïve MDD patients with moderate symptoms in the present study. Thus, the discrepancy between these results may be explained by different criteria of patient recruitment.
To the best of our knowledge, this is the first study demonstrating biological differences in brain function associated with Yin and Yang types characterized by somatic symptoms. The “Yin and Yang” theory that had originally been developed in the ancient China, is utilized for patient treatment in TCM (31–34). Western modern science has recently begun to explore biological significance of “Yin and Yang” types. For instance, the Yin and Yang types are associated with genetic mutations in epidermal growth factor receptor (EGFR) in patients with non-small cell lung cancer (18). We observed increased resting-state brain activity in Yang type patients in the left superior frontal gyrus, extending to medial, the left median cingulate and paracingulate gyri, and the left insula compared to Yin type group. The resting-state activity of these brain regions was also increased in Yang type patients compared to their healthy controls, but not in Yin type patients. In addition to mood and emotion, these regions are critical for cognitive processing (35–37), thus their differential activity may contribute to better cognitive performance in Yang Type, compared with Yin type. We also found that the ALFF value of the right extra-nuclear in the Yang type group was positively correlated with cognitive disturbance, and was negatively correlated with insomnia. Interestingly, the extra-nuclear is a part of the ventral affective processing system, including subcortical areas such as the hippocampus, amygdala, and thalamus. Importantly, previous studies reported the importance of these subcortical areas in the control of cognitive processing and sleep regulation (38–43). While ALFF in the inferior parietal lobe is increased in both Yin and Yang type group, we found different FC patterns between Yin and Yang type group in this brain area. Inferior parietal lobe-centered FC is increased in Yang type, but is decreased in Ying type, compared with matched healthy controls, suggesting that differential FC may be useful in physiologically differentiating Yin and Yang type MDD patients.
Somatic symptoms may, in part, be mediated by imbalance between sympathetic nervous system (SNS) and parasympathetic nervous system (PNS) (44, 45). As mentioned above, specific brain areas differentially activated in Yin and Yang types are involved in emotional processing which may affect sympathetic and parasympathetic neuronal activities (46, 47). Notably, several neuroimaging studies suggest that insular cortex plays a role for thermoregulatory mechanisms to exogenous thermal stimulation (48–50). To delineate differential brain function in Yin and Yang types, it would be of great interest to further examine neural mechanisms underlying the impact of imbalance of SNS and PNS on somatic symptoms.
There are several limitations of this study. First, as we recruited only moderate MDD patients, selection bias should be considered. Mild and severe MDD patients should be examined by the same approach to determine if the altered neural activity that we observed is a state-dependent endophenotype. Second, given that the number of participants was small, the statistical power for assessing brain activity is limited. Future research, with recruitment of a larger sample size, is needed to determine if resting-state brain activity can differentiate Yin and Yang types in MDD. It is also important to examine whether clinical manifestation using Yin and Yang” theory may differentiate brain function in other depressive conditions. Third, although three well-experienced physician scientists classified patients in Yin and Yang types based on somatic symptoms, establishing a standardized clinical assessment tool of Yin and Yan types is necessary for future studies. Nevertheless, this line of research may contribute to the understanding of the neural basis of Yin and Yang types, which may provide a step toward evidence-based application of “Yin and Yang” theory in modern Western medicine.
The “Yin and Yang” theory has long time been utilized for patient treatment in the field of TCM. However, lack of evidence of the underlying biological mechanisms hampers its use in modern Western medicine. Our study revealed potential differential resting-state brain activation in Yin and Yang types in drug-naïve MDD patients. Understanding the neural mechanism underlying somatic symptoms may contribute to biological measure for better stratification of heterogeneous MDD patients, which might improve treatment approaches.
This study was carried out in accordance with the recommendations of the Ethical Committee at the Third Affiliated Hospital of Beijing University of Chinese Medicine. The protocol (KTPJ-BZYSY-2015-13) was approved by the Ethical Committee at the Third Affiliated Hospital of Beijing University of Chinese Medicine. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
ZX and SZ: participated in the design of the study, conducted the analyses, and wrote the manuscript; SZ: collected the clinical information and performed the TCM syndrome assessment; LH and XZ: helped with the design and coordination of the study and wrote the manuscript; YZ: participated in fMRI data collection; DZ and DW: helped collected depression patients; QZ, HK, and AK: contributed to interpretation of the data and drafting the manuscript; MQ: conceived and coordinated the design of the study, and wrote the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank their study participants; Zhanjun Zhang and Ting Xu for their assistance in fMRI data analysis; and Aisa Moreno-Megui and Li Gao for critical reading of the manuscript. This work was supported by the National Natural Science Foundation of China (Grant No. 81573905), Beijing University of Chinese Medicine (Grant No 2015-JYB-XS199), and the National Institutes of Health funding [P50MH094268 (AK), DA-041208 (AK), and AT008547 (AK)].
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2018.00195/full#supplementary-material
1. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA (2003) 289:3095–105. doi: 10.1001/jama.289.23.3095
2. Gilman SE, Sucha E, Kingsbury M, Horton NJ, Murphy JM, Colman I. Depression and mortality in a longitudinal study: 1952-2011. CMAJ (2017) 189:E1304–10. doi: 10.1503/cmaj.170125
3. Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5th Edn. Washington, DC: American Psychiatric Publishing (2013).
4. Sampogna G. ICD-11 - Draft diagnostic guidelines for mental disorders: a report for WPA Membership. Psychiatr Pol. (2017) 51:397–406. doi: 10.12740/PP/73721
5. Lieblich SM, Castle DJ, Pantelis C, Hopwood M, Young AH, Everall IP. High heterogeneity and low reliability in the diagnosis of major depression will impair the development of new drugs. BJPsych Open (2015) 1:e5–7. doi: 10.1192/bjpo.bp.115.000786
6. Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry (2013) 70:31–41. doi: 10.1001/2013.jamapsychiatry.4
7. Lasserre AM, Glaus J, Vandeleur CL, Marques-Vidal P, Vaucher J, Bastardot F, et al. Depression with atypical features and increase in obesity, body mass index, waist circumference, and fat mass: a prospective, population-based study. JAMA Psychiatry (2014) 71:880–888. doi: 10.1001/jamapsychiatry.2014.411
8. Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. (2017) 23:28–38. doi: 10.1038/nm.4246
9. Kageyama Y, Kasahara T, Nakamura T, Hattori K, Deguchi Y, Tani M, et al. Plasma nervonic acid is a potential biomarker for major depressive disorder: a pilot study. Int J Neuropsychopharmacol. (2017) 21:207–15. doi: 10.1093/ijnp/pyx089
10. Milaneschi Y, Lamers F, Peyrot WJ, Baune BT, Breen G, Dehghan A, et al. Genetic association of major depression with atypical features and obesity-related immunometabolic dysregulations. JAMA Psychiatry (2017) 74:1214–25. doi: 10.1001/jamapsychiatry.2017.3016
11. Normile D. Asian medicine. The new face of traditional Chinese medicine. Science (2003) 299:188–90. doi: 10.1126/science.299.5604.188
12. Tang JL, Liu BY, Ma KW. Traditional Chinese medicine. Lancet (2008) 372:1938–40. doi: 10.1016/S0140-6736(08)61354-9.
13. Ye X, Dong MH. A review on different English versions of an ancient classic of Chinese medicine: Huang Di Nei Jing. J Integr Med. (2017) 15:11–8. doi: 10.1016/S2095-4964(17)60310-8
14. Iwabuchi SJ, Krishnadas R, Li C, Auer DP, Radua J, Palaniyappan L. Localized connectivity in depression: a meta-analysis of resting state functional imaging studies. Neurosci Biobehav Rev. (2015) 51:77–86. doi: 10.1016/j.neubiorev.2015.01.006
15. Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry (2015) 72:603–11. doi: 10.1001/jamapsychiatry.2015.0071
16. Boessen R, Groenwold RH, Knol MJ, Grobbee DE, Roes KC. Comparing HAMD(17) and HAMD subscales on their ability to differentiate active treatment from placebo in randomized controlled trials. J Affect Disord. (2013) 145:363–9. doi: 10.1016/j.jad.2012.08.026
17. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry (1978) 133:429–35. doi: 10.1192/bjp.133.5.429
18. Zhu YJ, Zhang HB, Liu LR, Liu YH, Zhang FL, Bai JP, et al. Yin-Cold or Yang-Heat syndrome type of traditional chinese medicine was associated with the epidermal growth factor receptor gene status in non-small cell lung cancer patients: confirmation of a TCM concept. Evid Based Complement Alternat Med. (2017) 2017:7063859. doi: 10.1155/2017/7063859
19. Liu X, Wang Y, Liu H, Liu Z, Zhou W. [Diffusion tensor imaging and resting state functional magnetic resonance imaging on young patients with major depressive disorder]. Zhong Nan Da Xue Xue Bao Yi Xue Ban (2010) 35:25–31. doi: 10.3969/j.issn.1672-7347.2010.01.004
20. Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE (2011) 6:e25031. doi: 10.1371/journal.pone.0025031
21. Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. (2007) 29:83–91. doi: 10.1016/j.braindev.2006.07.002
22. Sicong L, Xianxian K, Zhenlan J, Ling L. The causal interaction within attention networks and emotion network: a fMRI study. Conf Proc IEEE Eng Med Biol Soc. (2014) 2014:2388–91. doi: 10.1109/EMBC.2014.6944102
23. Wessing I, Rehbein MA, Romer G, Achtergarde S, Dobel C, Zwitserlood P, et al. Cognitive emotion regulation in children: reappraisal of emotional faces modulates neural source activity in a frontoparietal network. Dev Cogn Neurosci. (2015) 13:1–10. doi: 10.1016/j.dcn.2015.01.012
24. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. (2002) 3:201–15. doi: 10.1038/nrn755
25. Scolari M, Seidl-Rathkopf KN, Kastner S. Functions of the human frontoparietal attention network: evidence from neuroimaging. Curr Opin Behav Sci. (2015) 1:32–9. doi: 10.1016/j.cobeha.2014.08.003
26. Kenny ER, O'Brien JT, Cousins DA, Richardson J, Thomas AJ, Firbank MJ, et al. Functional connectivity in late-life depression using resting-state functional magnetic resonance imaging. Am J Geriatr Psychiatry (2010) 18:643–51. doi: 10.1097/JGP.0b013e3181cabd0e
27. Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J Affect Disord. (2011) 130:66–74. doi: 10.1016/j.jad.2010.09.032
28. Liu CH, Ma X, Song LP, Tang LR, Jing B, Zhang Y, et al. Alteration of spontaneous neuronal activity within the salience network in partially remitted depression. Brain Res. (2015) 1599:93–102. doi: 10.1016/j.brainres.2014.12.040
29. Yamamura T, Okamoto Y, Okada G, Takaishi Y, Takamura M, Mantani A, et al. Association of thalamic hyperactivity with treatment-resistant depression and poor response in early treatment for major depression: a resting-state fMRI study using fractional amplitude of low-frequency fluctuations. Transl Psychiatry (2016) 6:e754. doi: 10.1038/tp.2016.18
30. Bench CJ, Frackowiak RS, Dolan RJ. Changes in regional cerebral blood flow on recovery from depression. Psychol Med. (1995) 25:247–61. doi: 10.1017/S0033291700036151
31. Lu CC, Jan YM, Li TC, Hsieh CL. Electroacupuncture induces differential effects between Yin and Yang: a study using cutaneous blood flow and temperature recordings of the hand's dorsum and palm. Am J Chin Med. (2009) 37:639–45. doi: 10.1142/S0192415X09007120
32. Liu P, Wang P, Tian D, Liu J, Chen G, Liu S. Study on traditional Chinese medicine theory of lung being connected with large intestine. J Tradit Chin Med. (2012) 32:482–7. doi: 10.1016/S0254-6272(13)60059-X
33. Fu H, Qiu Y, Xia M, Wei F, He H, Yang L. Spleen-yang-deficiency patients with polycystic ovary syndrome have higher levels of visfatin. J Tradit Chin Med. (2014) 34:42–7. doi: 10.1016/S0254-6272(14)60052-2
34. Fu C, Zhang NL, Chen BX, Chen ZR, Jin XL, Guo RJ, et al. Identification and classification of traditional Chinese medicine syndrome types among senior patients with vascular mild cognitive impairment using latent tree analysis. J Integr Med. (2017) 15:186–200. doi: 10.1016/S2095-4964(17)60335-2
35. Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, et al. The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport (2002) 13:2023–6. doi: 10.1097/00001756-200211150-00006
36. Krolak-Salmon P, Henaff MA, Isnard J, Tallon-Baudry C, Guenot M, Vighetto A, et al. An attention modulated response to disgust in human ventral anterior insula. Ann Neurol. (2003) 53:446–53. doi: 10.1002/ana.10502
37. Lemche E, Surguladze SA, Brammer MJ, Phillips ML, Sierra M, David AS, et al. Dissociable brain correlates for depression, anxiety, dissociation, and somatization in depersonalization-derealization disorder. CNS Spectr. (2016) 21:35–42. doi: 10.1017/S1092852913000588
39. Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron (2004) 44:109–20. doi: 10.1016/j.neuron.2004.08.028
40. Huang Z, Liang P, Jia X, Zhan S, Li N, Ding Y, et al. Abnormal amygdala connectivity in patients with primary insomnia: evidence from resting state fMRI. Eur J Radiol. (2012) 81:1288–95. doi: 10.1016/j.ejrad.2011.03.029
41. Zhou B, Liu Y, Zhang Z, An N, Yao H, Wang P, et al. Impaired functional connectivity of the thalamus in Alzheimer's disease and mild cognitive impairment: a resting-state fMRI study. Curr Alzheimer Res. (2013) 10:754–66. doi: 10.2174/15672050113109990146
42. Zheng LJ, Yang GF, Zhang XY, Wang YF, Liu Y, Zheng G, et al. Altered amygdala and hippocampus effective connectivity in mild cognitive impairment patients with depression: a resting-state functional MR imaging study with granger causality analysis. Oncotarget (2017) 8:25021–31. doi: 10.18632/oncotarget.15335
43. Leerssen J, Wassing R, Ramautar JR, Stoffers D, Lakbila-Kamal O, Perrier J, et al. (2018). Increased hippocampal-prefrontal functional connectivity in insomnia. Neurobiol Learn Mem. doi: 10.1016/j.nlm.2018.02.006. [Epub ahead of print].
44. Quick C, Kliem A, Berger S, Hocke M, Tancer M, Juckel G, et al. Gastric dysmotility in major depression. Prog. Neuropsychopharmacol Biol Psychiatry (2010) 34:92–7. doi: 10.1016/j.pnpbp.2009.10.003
45. Pollatos O, Herbert BM, Wankner S, Dietel A, Wachsmuth C, Henningsen P, et al. Autonomic imbalance is associated with reduced facial recognition in somatoform disorders. J Psychosom Res. (2011) 71:232–9. doi: 10.1016/j.jpsychores.2011.03.012
46. Stifter CA, Dollar JM, Cipriano EA. Temperament and emotion regulation: the role of autonomic nervous system reactivity. Dev Psychobiol. (2011) 53:266–79. doi: 10.1002/dev.20519
48. Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. (2000) 3:184–90. doi: 10.1038/72131
49. Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. (2002) 3:655–66. doi: 10.1038/nrn894
Keywords: major depressive disorder, somatic symptoms, resting-state fMRI, Yin-Yang theory, traditional Chinese medicine
Citation: Xu Z, Zhang S, Huang L, Zhu X, Zhao Q, Zeng Y, Zhou D, Wang D, Kuga H, Kamiya A and Qu M (2018) Altered Resting-State Brain Activities in Drug-Naïve Major Depressive Disorder Assessed by fMRI: Associations With Somatic Symptoms Defined by Yin-Yang Theory of the Traditional Chinese Medicine. Front. Psychiatry 9:195. doi: 10.3389/fpsyt.2018.00195
Received: 27 February 2018; Accepted: 25 April 2018;
Published: 15 May 2018.
Edited by:
Iria Grande, Institut de Neurociències, Universitat Barcelona, SpainReviewed by:
Jiliang Fang, Guang'anmen Hospital, China Academy of Chinese medical Science, ChinaCopyright © 2018 Xu, Zhang, Huang, Zhu, Zhao, Zeng, Zhou, Wang, Kuga, Kamiya and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miao Qu, cXVtaWFvdGNtQDEyNi5jb20=
Atsushi Kamiya, YWthbWl5YTFAamhtaS5lZHU=
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.