
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 23 April 2018
Sec. Addictive Disorders
Volume 9 - 2018 | https://doi.org/10.3389/fpsyt.2018.00150
Although binge drinking is on the rise in women of reproductive age and during pregnancy, the consequences in the offspring, in particular the inheritance of alcohol-related mood disturbances and alcohol abuse vulnerability, are still poorly investigated. In this study, we modeled both Habitual- and Binge Alcohol Drinking (HAD and BAD) in female rats by employing a two-bottle choice paradigm, with 20% alcohol and water. The exposure started 12 weeks before pregnancy and continued during gestation and lactation. The consequences induced by the two alcohol drinking patterns in female rats were assessed before conception in terms of behavioral reactivity, anxiety- and depressive-like behavior. Afterwards, from adolescence to young-adulthood, male offspring was assessed for behavioral phenotype and alcohol abuse vulnerability. At pre-conceptional time BAD female rats showed higher mean alcohol intake and preference than HAD group; differences in drinking trajectories were attenuated during pregnancy and lactation. Pre-conceptional BAD induced a prevalent depressive/anhedonic-like behavior in female rats, rather than an increase in anxiety-like behavior, as observed in HAD rats. In the adolescent offspring, peri-gestational BAD did not affect behavioral reactivity in the open field and anxiety-like behavior in the elevated plus maze. Rather, BAD dams offspring displayed higher despair-behavior and lower social interaction with respect to control- and HAD dams progeny. Notably, only binge drinking exposure increased offspring vulnerability to alcohol abuse and relapse following forced abstinence. This is the first report showing that binge-like alcohol consumption from pre-conceptional until weaning induces relevant consequences in the affective phenotype of both the mothers and the offspring, and that such effects include heightened alcohol abuse vulnerability in the offspring. These findings highlight the need for more incisive public education campaigns about detrimental consequences of peri-gestational alcohol exposure.
Despite the increased awareness about the teratogenic effect of in utero exposure to psychotropic drugs [1–7], prenatal alcohol exposure represents one of the most common early brain insults [8–10]. Globally, about 10% of women consume alcohol during pregnancy [11], between 5 and 10% admit binge drinking incidents [12] and between 1 and 2% deliver a child with fetal alcohol spectrum disorders (FASD), whose lifelong consequences can range from minor to severe disabilities [13]. Notably, binge drinking (i.e., four to five or more drinks per occasion) prior to pregnancy represents a relevant predictor of perinatal alcohol exposure that might put the development of the offspring in jeopardy [14].
Although several studies have investigated the clinical consequences of alcohol abuse as well as the incidence of FASD, very few is known about the impact of binge alcohol drinking (BAD) on the affective and emotional pattern of females of reproductive age [15].
This is an important issue since modifications in the affective and emotional response of the mothers, as a consequence of long-term alcohol intake, can be inherited by the progeny [16, 17].
Rodent models of alcohol exposure, which can mimic both habitual- and binge human drinking patterns, allow the investigation of the epigenetic mechanisms that underlie dysfunctional emotional and affective behavior, as well as heritable stress-related phenotypes [18–23]. In this regard, well-established behavioral paradigms are essential tools to search specific endophenotypes and provide cues to investigate the neuropathology of mood disturbances. For instance, the exploratory behavior of novel and challenging environments such as the open field and the elevated plus maze, can provide a reliable readout of unconditioned anxiety-like behavior [24–26]; in addition, the animal unconditioned preference for natural rewards, such as sweet solution [27] or social interaction toward a conspecific [28], can be altered in anhedonic-like states, such as depression; lastly, despair behavior in the forced swim test is a largely employed measure of depression-like behavior and has been traditionally used to detect antidepressant properties of drugs [29].
Thus, the first aim of this research was to model pre-conceptional habitual- and binge-like alcohol consumption in female rats, in order to investigate the consequences on their emotional and affective behavior. We employed a long-term, two-bottle choice paradigm with continuous or intermittent access to 20% alcohol and water, and tested the animals for: drinking trajectories, behavioral reactivity, reward sensitivity, anxiety- and depression-like behavior.
It has been reported that mothers who drink before pregnancy often indulge in alcohol consumption during pregnancy and while breastfeeding, transferring alcohol to infants through the maternal milk [30]. Thus, in the present study, female rats continued habitual and binge-like alcohol self-administration along pregnancy and lactation. Notably, the duration of pre- and postnatal alcohol treatment allows to model human fetal alcohol exposure along the whole gestational period, since the first two postnatal weeks in rodents approximately correspond to the last gestational trimester in humans [31, 32].
In spite of the well-established association between in utero alcohol exposure and psychiatric risk in the progeny, experience suggests that mental health practitioners infrequently identify FASD before adolescence [33]. The Seattle longitudinal study revealed that 21-year young adults whose mothers reported a binge pattern of alcohol use, displayed significantly higher overall levels of psychiatric symptoms, than those exposed to low or moderate levels of alcohol in utero [34]. Adolescents' most substantial deficits, according to the Vineland Adaptive Behavior Scale [35] lied in the socialization domain, reflecting both social anxiety and social avoidance. A similar prospective study highlighted the association between maternal alcohol use during pregnancy and offspring early drinking, suggesting genetic inheritance of alcohol abuse behavior [36–38].
Based on this evidence, we investigated the occurrence of specific variations in the affective and emotional phenotypes of the offspring during adolescence, as a consequence of the perinatal exposure to the two distinct alcohol drinking paradigms. The offspring was tested for social interaction, which explores social reward and embarrassment that may occur in novel social environments [39, 40]. Eventually, the occurrence of a vulnerability to alcohol abuse was investigated by exposing the adolescent offspring to the two-bottle choice paradigm until early adulthood.
To our knowledge, this is the first report on the affective and emotional consequences of long-term binge- alcohol drinking by voluntary self-administration in female rats, and the inheritance of mood disturbances and alcohol vulnerability in the adolescent progeny.
This evidence strengthens the translational value of binge-like alcohol self-administration by the two-bottle choice procedure, in search of mechanisms of multigenerational inheritance of alcohol detrimental consequences [41].
Adult female nulliparous Wistar rats (200–220 g, Harlan, Udine, Italy) were housed individually in standard rat cages (40 × 60 cm, 20 cm in height), with ad libitum access to water and food, in a temperature- (22 ± 2°C) and humidity- (55 ± 5%) controlled room, on 12 h inverted light/dark cycle (08:00–20:00). Rats were gently handled for 3 min per day for a week before the experimental procedures, when they were randomly assigned to one of the three experimental groups, according to the self-administration paradigm: habitual alcohol drinking (HAD), BAD, water drinking controls (CTR).
At the end of the behavioral assessments, each female rat was housed with a breeder male rat.
On gestational day 1 (GD1; i.e., the day when pregnancy was confirmed) eight female rats randomly selected from each experimental group and housed in standard maternity cages, filled with wood shavings. Alcohol consumption was not measured when the male rat was present in the cage [42]. Female rats underwent the self-administration procedure throughout pregnancy, accordingly to the respective two-bottle choice paradigm. Dams were inspected twice daily for delivery until the day of parturition, considered as postnatal day 0 (PND 0); dams and litters were kept in a nursery (24 ± 2°C) and not separated until weaning, in order to avoid confounding effects due to cross-fostering procedure [43–45] and model the human condition.
One male rat from each alcohol drinking- and control litter was randomly chosen to undergo behavioral testing, beginning on PND 23, so that experimental groups of rat offspring were: perinatally habitual alcohol-exposed rats (p-HAD, n = 8), perinatally binge alcohol-exposed rats (p-BAD, n = 8), perinatally water-exposed controls (p-CTR, n = 8).
All the experiments were approved by the Committee for the Protection and Use of Animals of the University of Palermo, in accordance with the current Italian legislation on animal experimentation (D.L. 116/92) and the European directives (2010/63/EU).
Female rats underwent home cage two-bottle “alcohol vs. water” choice regimen, according to the experimental group assigned. Indeed, HAD rats were given 24 h free choice between one bottle of alcohol (20% v/v) and one of tap water, 7 days per week, except during the behavioral tests (weeks 8–11) when they were given 5 drinking sessions per week; BAD rats were given 24 h alcohol (20% v/v) access during 3 sessions per week, i.e., on Monday, Wednesday, and Friday, while they received 2 bottles of tap water on the intervening days. CTR rats received two bottles of water. Overall, HAD rats underwent 76 pre-conceptional-, 21 gestational, and 21 post-gestational drinking sessions. On the other hand, BAD rats had 36 pre-conceptional-, 9 gestational, and 9 post-gestational drinking sessions.
Alcoholic solution (20% v/v) was daily prepared by diluting alcohol 96° (Carlo Erba Reagenti, Italy) with tap water. Plastic bottles (120 ml; metal cap 0.8 mm diameter hole, Tecniplast, Italy) were filled every day with 100 ml of freshly prepared solution, in alternative left-right position, to avoid side preference, and presented at lights off. Alcohol- and water intake were measured 1 h after lights off and the day after, immediately before lights-off, by weighing the bottles. Possible, fluid spillage was monitored by using multiple bottles filled with water and alcohol 20%, allocated in empty cages interspersed in the racks [46]. During the paradigm, rats were daily monitored for body weight. For each drinking session, alcohol consumption (g/kg) and preference % over total fluid consumption were measured. Mean alcohol consumption, at 24 and at 1 h after alcohol presentation, was then calculated considering the total number of drinking sessions in each group during pre-conceptional time, gestation, and lactation.
Starting from week 8 of the two-bottle choice paradigm, female rats underwent a battery of behavioral assessments that started following 12 h of acute abstinence, during the weekend, and included open field, elevated plus maze, and forced swim tests (Figure 1). Saccharin preference was employed to as a measure of sensitivity to natural rewards, rather the social interaction test, in order to avoid confounding effects due to altered behavioral reactivity as a result of alcohol abstinence.
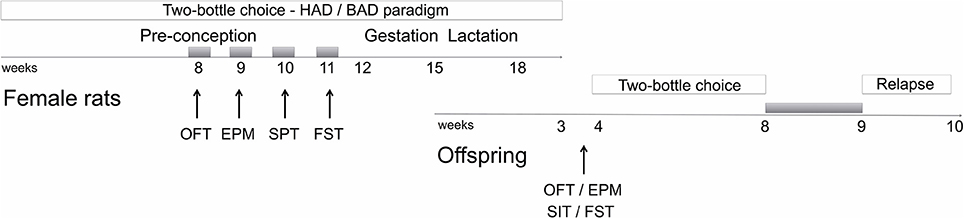
Figure 1. Schematic overview of the experimental design. Gray boxes refer to alcohol deprivation phases. For details, see section Materials and Methods. HAD, Habitual alcohol drinking rats; BAD, Binge alcohol drinking rats; OFT, open field test; EPM, elevated plus maze test; SPT, saccharin preference test; FST, forced swim test; SIT, social interaction test.
Male offspring were tested in the open field, elevated plus maze, social interaction, and forced swim test starting from PND 23. Afterwards, they were also tested for alcohol vulnerability, starting from PND 32.
On the test days, the animals were acclimatized for 60 min before the experimental session. The experiments were carried out in a soundproof room between 9:00 and 14:00. Animal performance was recorded and monitored in an adjacent room. The equipment was thoroughly cleaned before animal's entry to ensure that rat's behavior was not affected by the detection of another rat's scent.
Behavioral reactivity in a novel open field arena was measured in a Plexiglas square box (44 cm long, 44 cm wide, and 20 cm high) by employing an automatic video-tracking system (Any Maze, Ugo Basile, Italy), in a mean light (100 lx) illuminated chamber. Each experimental session lasted 5 min [25]. The test produces a qualitative mapping of the motor pattern, measuring total distance traveled (TDT), as a measure of locomotor activity, number of transitions (NTC) from peripheral to central squares of the arena, and amount of time spent on the central areas (ATC) as measures of explorative behavior.
Unconditioned anxiety-like behavior was assessed in the elevated plus maze (EPM). EPM apparatus consisted of a plus-shaped platform from dark-gray PVC elevated to a height of 70 cm above the floor. Two of the opposing arms (50 × 10 cm) were closed by 40 cm-high side end-walls (closed arms), whereas the two other arms had no walls (open arms). The arms were oriented perpendicular to each other and connected by an open central area (10 × 10 cm). At the beginning of each session, rats were placed in the center of the maze facing one of the open arms. Time spent on each arm and number of entries were recorded by using ANY MAZE Video Tracking System (Ugo Basile, Italy) along the 5 min test duration. An entry was scored when all the four paws entered into each single arm. The percentage of time spent on the open arms/total time spent on open and closed arms, and of number of entries in the open arms/number of entries in the open and closed arms were analyzed, as they constituted the primary indices of trait anxiety-like behavior in rodents. Information on general activity was obtained by measuring closed arm and total entries [26].
Anhedonia was assessed using the saccharin preference test [27].
Briefly, during the habituation phase, each cage was supplied with two drinking bottles filled with water to avoid place preference. On the next day, a bottle of water was replaced by one containing 0.2% (w/v) saccharin solution (Sigma-Aldrich, Italy). Saccharin- and water intake were measured by weighing the bottles 24 h later. The location of the two bottles was randomly alternated to avoid location preference bias. Saccharin preference was calculated as percentage of the volume of the saccharin solution consumed divided by the total fluid volume (water plus saccharin).
Porsolt test was conducted as already described in Plescia et al. [47]. Briefly, each rat was placed individually in a glass cylinder (40 cm high, 18 cm inside diameter) containing ≈5 l of clean water. Water temperature was maintained at 22–23°C. Rats were forced to swim for 15 min (pre-test). Animals were then allowed to return to their home cages. On the next day, each rat was placed into the water and forced to swim for 5 min (test). The session was videotaped and the duration of immobility, swimming and climbing was recorded (sec). The rat was considered as immobile when it stopped struggling and remained floating in the water, keeping its head above the water. Climbing was defined by active attempts to climb the walls of the cylinder to escape.
Social reward was measured by the social interaction test, adapted from Christoffel et al. [48]. The offspring was placed into a novel arena equipped with a small animal cage (10 × 10 cm) at one end. Rat movements were monitored for 5 min in the absence of a social stimulus into the cage (used to determine baseline exploratory behavior), followed by 5 min in the presence of a caged conspecific male rat, matched for age. Partners had not been socially isolated prior to testing and were unfamiliar with both the apparatus and the experimental animal with which they were paired for testing. We measured duration of time spent in the interaction zone, including 2 cm2 surrounding the small animal cage, by using Anymaze software (Ugo Basile, Italy). Social interaction was calculated as a ratio between the time spent on the interaction zone when the stimulus rat was present, over the time spent when the stimulus rat was absent.
Male offspring underwent home cage two-bottle “alcohol vs. water” choice regimen, and were given 24 h free choice between one bottle of alcohol (10% v/v) and one of tap water, 7 days per week, for 4 weeks, followed by a relapse-like week, after deprivation. Alcoholic solution (10% v/v) was daily prepared by diluting alcohol 96° (Carlo Erba Reagenti, Italy) with tap water. Plastic bottles (120 ml; metal cap 0.8 mm diameter hole, Tecniplast, Italy) were refilled with 100 ml fresh solution every day, and presented at lights off in alternative left-right position, to avoid side preference. Alcohol- and water intake were measured by weighing the bottles. Possible fluid spillage was monitored by using multiple bottles filled with water and alcohol 10%, positioned in empty cages interspersed in the cage racks. During the paradigm, rats were daily monitored for body weight.
The analysis included results from rat dams whose pups were assessed along different phases of the experimental design. The differences in mean alcohol consumption and preference of female rats were analyzed by Student's t-test or Mann–Whitney test, when appropriate. The analysis of drinking trajectories of female rats, starting from the last 3 weeks of pre-conceptional time to the end of the drinking paradigm, was performed by two-way ANOVA for repeated measures (RM 2-way ANOVA), followed by Bonferroni post-hoc test, on both mean alcohol consumption per week, including “alcohol drinking pattern” as the between-subject factor and “week” as the within-subject factor, and mean weekly alcohol consumption during pre-conceptional time, gestation and lactation, including “alcohol drinking pattern” as the between-subject factor and “period” as the repeated measure factor. Data analysis on behavioral experiments was performed by one-way ANOVA, including “treatment” as the between subjects factor. Data analysis on alcohol vulnerability experiments was performed by RM 2-way ANOVA, including “maternal alcohol drinking pattern” as the between-subject factor and “week” as the within-subject factor. Bonferroni post-hoc test was employed, when necessary. Kruskal–Wallis test, followed by Dunn's multiple comparison test, was employed for the analysis of alcohol deprivation effect. Data are reported as mean ± SEM. Statistical significance was set at p < 0.05 (α = 0.05).
Female rats had habitual—continuous—or binge—intermittent—access to the “two-bottle choice” paradigm during 12 weeks, before mating, during pregnancy and lactation. Fluid spillage, constantly monitored by using multiple bottles filled with water and alcohol 20%, allocated in empty cages interspersed in the racks, was neither relevant nor systematic. Measures of alcohol intake along the paradigm are reported in Table 1. As expected pre-conceptional time BAD paradigm induced higher means alcohol consumption and preference, at both time points considered, 1 and 24 h after alcohol presentation, with respect to HAD. After fecundation, as well as during post-partum days, BAD rats increase in mean alcohol consumption and preference was attenuated with respect to HAD rats.
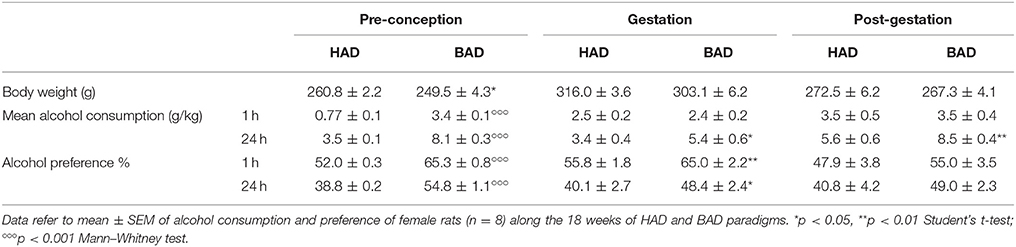
Table 1. Alcohol drinking pattern in habitual alcohol drinking (HAD) rats and binge alcohol drinking (BAD) rats at pre-conceptional, gestational, and post-gestational time.
In order to have a more detailed insight into drinking trajectories, mean alcohol intake of HAD and BAD rats, from the last three pre-conceptional weeks throughout peri-gestational time was analyzed. The RM 2-way ANOVA on mean alcohol consumption during the 9 weeks considered showed a significant effect of alcohol drinking pattern [F(1, 14) = 71.07, p < 0.0001], weeks [F(8, 112) = 7.465, p < 0.0001], and their interaction [F(8, 112) = 3.453, p = 0.0014]. In particular, BAD rats displayed increased alcohol consumption with respect to HAD rats on week 10, 11, and 12 of pre-conceptional period (t = 6.091, p < 0.001; t = 4.099, p = 0.0007; t = 5.354, p < 0.001); during gestational week 3 (t = 2.877, p = 0.0424) and post-gestational weeks 1 and 3 (t = 3.015, p = 0.0279; t = 4.788, p < 0.001).
Moreover, when we analyzed the effects of the three periods considered, the RM 2-way ANOVA showed a significant effect of period [F(2, 4) = 51.53, p = 0.0014], alcohol drinking pattern [F(1, 2) = 45.05, p = 0.0215], and their interaction [F(2, 4) = 21.33, p = 0.0073]. After fecundation, BAD rats decreased their alcohol consumption with respect to their pre-conceptional levels (t = 9.70, p = 0.0020). During post-partum days, the drinking trajectories of both groups increased with respect to gestational period (HAD: t = 4.688, p = 0.0282; BAD: t = 9.715, p = 0.0019), and reproduced a similar pattern as on pre-conceptional time (Figure 2).
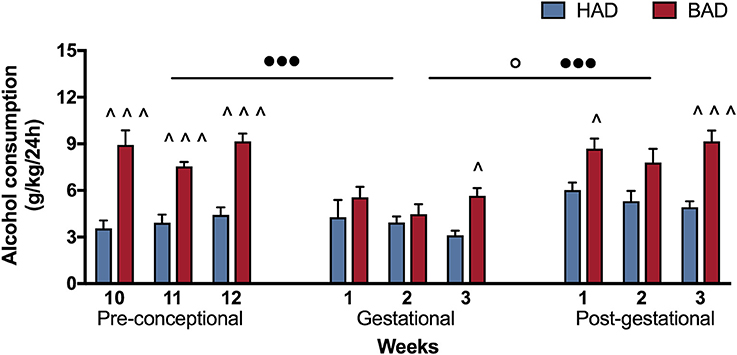
Figure 2. Female rat alcohol drinking trajectories. Binge alcohol drinking (BAD) rats showed higher alcohol consumption than habitual alcohol drinking (HAD) rats during the last three pre-conceptional weeks, gestation and post-gestation. During gestation, BAD rats displayed a significant decrease in alcohol consumption with respect to the last three pre-gestational weeks. During post-partum days, drinking consumption of both groups increased with respect to gestational period, reproducing similar trajectories as recorded on pre-conceptional time. Each bar represents the mean ± SEM of n = 8 rats. ∧p < 0.05, ∧∧∧p < 0.001 vs. HAD rats; ◦p < 0.05 within HAD group; •••p < 0.001 within BAD group.
At the end of week 8, female rats' performance in the open field was assessed as a measure of locomotor activity and behavioral reactivity in a novel environment, following 12 h alcohol deprivation. Results from a one-way ANOVA showed no significant differences in locomotor activity, in terms of TDT, among the three experimental groups [F(2, 21) = 0.1032, p = 0.9024; Figure 3A]. Statistical analysis indicated significant differences in NTC [F(2, 21) = 3.895, p = 0.0364], and ATC [F(2, 21) = 4.516, p = 0.0234]. In particular, Bonferroni post-hoc test highlighted a significant decrease in both NTC (t = 2.721, p < 0.05) and ATC (t = 3.005, p < 0.05) in HAD- with respect to CTR rats (Figures 3B,C).
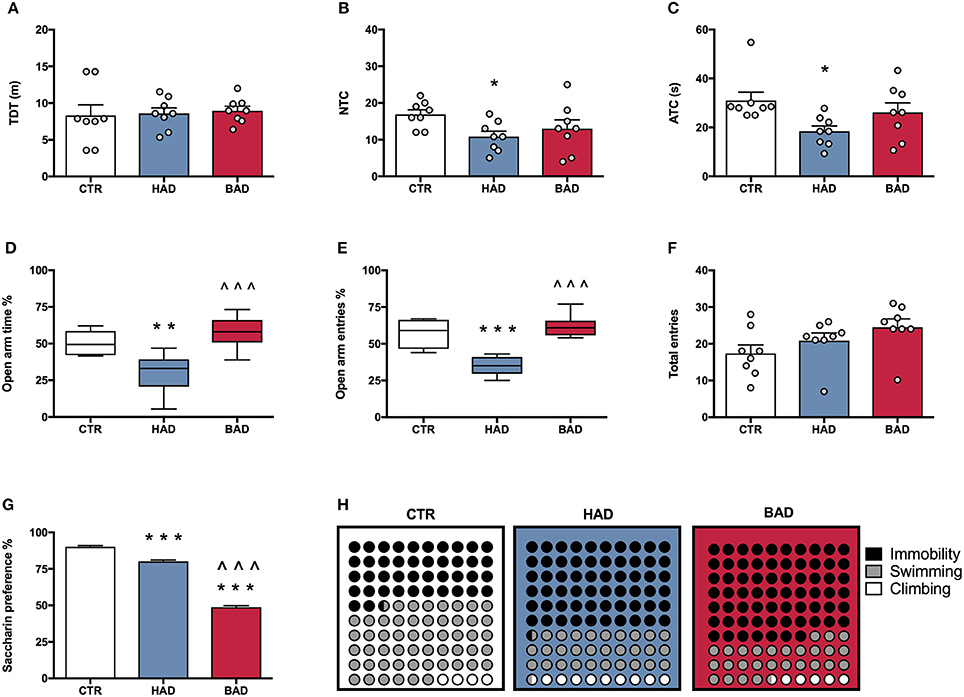
Figure 3. Female rat pre-conceptional phenotype. Habitual (HAD) and binge (BAD) alcohol drinking patterns affect behavioral reactivity, anxiety-like behavior, reward sensitivity, and depression-like behavior. Open field test—(A) total distance traveled (TDT); (B) number of transitions in central areas (NTC); and (C) amount of time spent on the central areas of the arena (ATC). Elevated plus maze test—(D) percentage of time spent on the open arm; (E) percentage of entries in the open arm; (F) total entries. (G) Saccharin preference; (H) Forced swim test, where the parts-of-whole−10 × 10 dot—plots represent normalized mean values from immobility time (black); swimming time (gray); climbing time (white) in the three experimental groups. Each bar represents the mean ± SEM of n = 8 rats. Each box-and-whisker plot represents the median (horizontal line in the box), 25–75% (box) and min-to-max (whiskers) values of n = 8 rats. *p < 0.05, **p < 0.01; ***p < 0.001 vs. CTR rats; ∧∧∧p < 0.001 vs. HAD rats.
At the end of week 9, female rats were tested in the EPM to evaluate anxiety-like behavior at 12 h alcohol deprivation. One-way ANOVA, performed on data from the percentage of time spent on open arm/time on open and closed arms, showed significant differences among the experimental groups [F(2, 21) = 14.42, p = 0.0001]. In particular, Bonferroni post-hoc test indicated a significant lower preference for the open arms in HAD rats, compared to CTR (t = 3.959, p < 0.01), while BAD rats spent more time in the open arms than HAD group (t = 5.121, p < 0.001; Figure 3D). When the percentage of open arm entries out of total entries was analyzed, one-way ANOVA showed significant differences among the three experimental groups [F(2, 21) = 26.13, p < 0.0001]. In particular, Bonferroni post-hoc analysis showed a significant decrease in HAD rats, with respect to CTR (t = 5.604, p < 0.001), and an increase in BAD-, with respect to HAD rats (t = 6.758, p < 0.001; Figure 3E). No significant differences among the three experimental groups in number of total entries were observed [F(2, 21) = 2.579, p = 0.0997; Figure 3F).
At the end of week 10, female rats underwent 12 h of alcohol deprivation and were subjected to saccharin preference test, in order to evaluate their response to a natural reward during abstinence. Results from one-way ANOVA showed significant differences among the three experimental groups [F(2, 21) = 581.0, p < 0.001]. In details, Bonferroni post-hoc test highlighted a significant decrease in saccharin preference in both HAD-(t = 7.961, p < 0.001) and BAD rats (t = 32.71, p < 0.001), with respect to CTR. Moreover, BAD rats displayed a significant decrease in saccharin preference when compared to HAD rats (t = 24.74, p < 0.001; Figure 3G).
The experimental groups were evaluated for depressive-like behavior in the Porsolt test at the end of week 11. After 12 h of alcohol abstinence, rats were exposed to the pretest and, at 36 h abstinence, rats underwent the 5-min Test session, when immobility, swimming, and climbing time were recorded. One-way ANOVA showed a significant effect of treatment on immobility [F(2, 21) = 30.92, p < 0.0001]. Bonferroni post-hoc test highlighted a significant increase both in HAD (t = 6.441, p < 0.001) and in BAD rats (t = 7.127, p < 0.001) with respect to CTR. In line with these results, a significant effect of treatment was observed on swimming time [F(2, 21) = 15.36, p < 0.001], with a significant decrease in both HAD- (t = 4.375, p < 0.001) and BAD-rats (t = 4.019, p < 0.01), when compared to controls. Furthermore, when the parameter climbing was analyzed, significant differences were observed among groups [F(2, 21) = 10.87, p = 0.0006]: HAD rats displayed a significant increase in climbing with respect to CTR (t = 4.491, p < 0.001), and BAD-rats (t = 3.331, p < 0.01). Figure 3H shows the composite behavioral pattern of the three experimental groups.
Offspring's performance in the open field was assessed as a measure of locomotor activity and behavioral reactivity in a novel environment. One-way ANOVA showed significant effects of maternal alcohol drinking pattern on locomotor activity, in terms of TDT, among the three experimental groups [F(2, 21) = 4.915, p = 0.0177]; indeed, p-HAD offspring showed decreased locomotor activity, with respect to both p-CTR (t = 2.776, p < 0.05) and p-BAD offspring (t = 2.65, p < 0.05; Figure 4A). Moreover, significant differences in NTC were observed [F(2, 21) = 14.8, p < 0.001], with a decrease in p-HAD when compared to p-CTR (t = 5.396, p < 0.001) and p-BAD offspring (t = 3.293, p < 0.05).
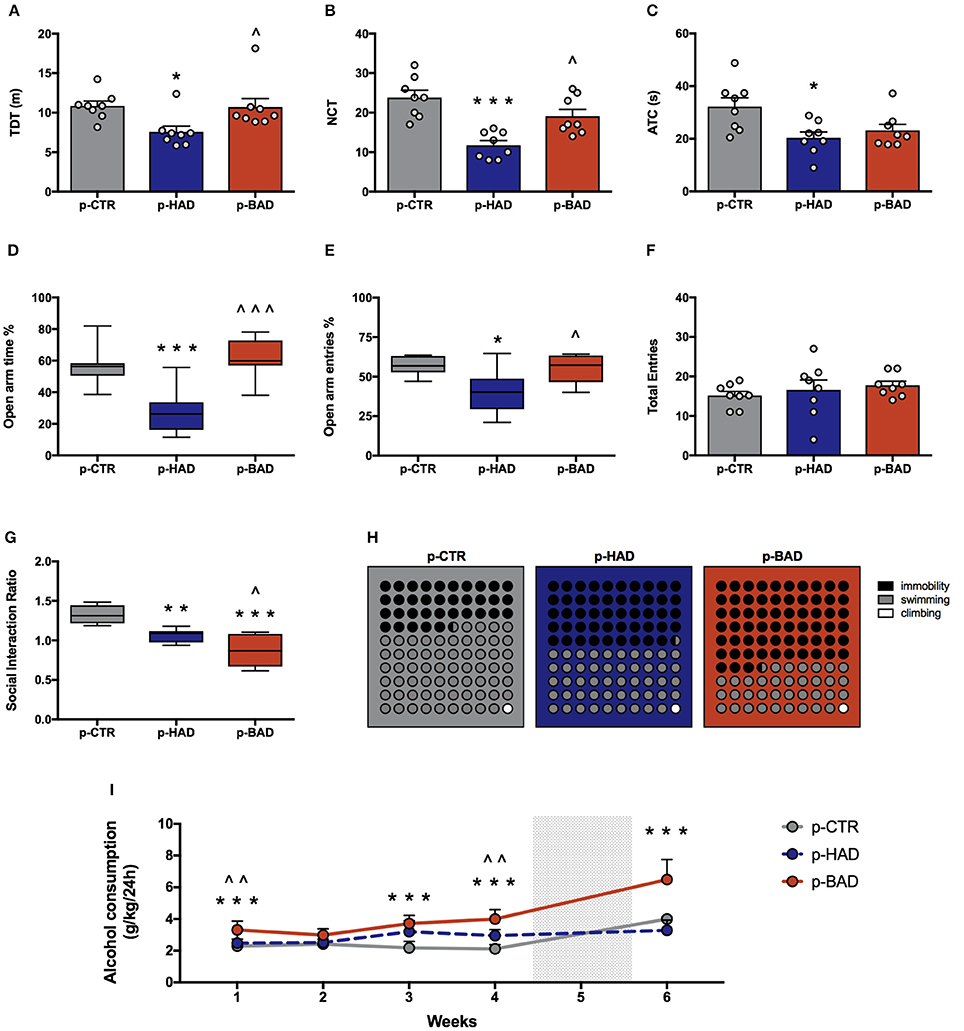
Figure 4. Offspring phenotype and alcohol vulnerability. Pre-and post-gestational habitual (p-HAD) and binge (p-BAD) alcohol drinking affect offspring behavioral reactivity, anxiety-like behavior, social reward sensitivity, and depression-like behavior. Open field test—(A) total distance traveled (TDT); (B) number of transitions in central areas (NTC); and (C) amount of time spent on the central areas of the arena (ATC). Elevated plus maze test—(D) percentage of time spent on the open arm; (E) percentage of entries in the open arm; (F) total entries. (G) Social interaction test; (H) Forced swim test, where the parts-of-whole−10 × 10 dot—plots represent normalized mean values from immobility time (black); swimming time (gray); climbing time (white) in the three experimental groups. (I) Vulnerability to alcohol abuse is measured in the two bottle choice paradigm during drinking induction (weeks 1–4) and relapse following forced abstinence (week 6). Each bar and each dot represents the mean ± SEM of n = 8 rats. Each box-and-whisker plot represents the median (horizontal line in the box), 25–75% (box) and min-to-max (whiskers) values of n = 8 rats. *p < 0.05, **p < 0.01; ***p < 0.001 vs. p-CTR rats; ∧p < 0.05; ∧∧p < 0.01; ∧∧∧p < 0.001 vs. p-HAD rats.
Analysis of ATC data also showed significant differences [F(2, 21) = 5.441, p = 0.0125]. In particular, Bonferroni post-hoc test highlighted a significant decrease in p-HAD offspring with respect to p-CTR rats (t = 3.155, p < 0.05; Figures 4B,C).
Animals were tested in the EPM to evaluate anxiety-like behavior. One-way ANOVA, performed on data from the percentage of time spent on open arm/time on open and closed arms, showed significant differences among the experimental groups [F(2, 21) = 16.05, p < 0.0001]. In particular, Bonferroni post-hoc test indicated a significant lower preference for the open arms in p-HAD offspring, compared to p-CTR (t = 4.466, p < 0.001), while p-BAD offspring spent more time in the open arms than p-HAD ones (t = 5.253, p < 0.001; Figure 4D). When the percentage of open arm entries out of total entries was analyzed, one-way ANOVA showed significant differences among the three experimental groups [F(2, 21) = 6.559, p = 0.0061]. In particular, Bonferroni post-hoc analysis showed a significant decrease in p-HAD offspring, with respect to p-CTR (t = 3.284, p < 0.05), and an increase in p-BAD, with respect to p-HAD offspring (t = 2.965, p < 0.05; Figure 4E). No significant differences among the three experimental groups in number of total entries were observed [F(2, 21) = 0.6072, p = 0.5542; Figure 4F].
Offspring was subjected to the social interaction test in order to evaluate their response to social reward. Results from one-way ANOVA showed significant effects of maternal alcohol drinking pattern among the three experimental groups [F(2, 21) = 21.51, p < 0.001]. In details, Bonferroni post-hoc test highlighted a significant decrease in social interaction in p-BAD, which showed the lowest values (t = 2.871, p < 0.05), and p-HAD offspring, with respect to p-CTR (t = 6.542, p < 0.001; t = 3.671, p < 0.01; Figure 4G).
The experimental groups were evaluated for depressive-like behavior in the Porsolt test. Offspring underwent a 5-min test session 24 h after the pretest session, when immobility, swimming, and climbing time were recorded. One-way ANOVA showed a significant effect of treatment on immobility [F(2, 21) = 30.08, p < 0.0001]. Bonferroni post-hoc test highlighted a significant increase both in p-HAD- (t = 3.978, p < 0.01) and in p-BAD offspring (t = 7.756, p < 0.001) with respect to p-CTR. Moreover, p-BAD offspring displayed a significant increase in immobility time when compared to p-HAD offspring (t = 3.778, p < 0.01).
In agreement with these results, a significant effect of treatment was observed on swimming time [F(2, 21) = 34.54, p < 0.001], with a significant decrease both in p-HAD- (t = 4.241, p < 0.01) and in p-BAD offspring (t = 8.311, p < 0.001), when compared to p-CTR. Again, p-BAD offspring displayed a significant decrease in swimming time when compared to p-HAD offspring (t = 4.069, p = 0.0017).
When the parameter climbing was analyzed, no significant differences were observed [F(2, 21) = 0.391, p = 0.6809]. Figure 4H shows the composite behavioral pattern of the three experimental groups.
Offspring had access to the “two-bottle choice” paradigm from PND 32 for 4 weeks, followed by a relapse-like week, after deprivation in order to test them for alcohol deprivation effect. RM 2-way ANOVA on mean alcohol intake (g/kg) along the first 4 weeks of the paradigm showed a significant effect of maternal alcohol drinking pattern [F(2, 21) = 36.73, p < 0.0001], weeks [F(3, 63) = 4.856, p = 0.0042], and their interaction [F(6, 63) = 3.825, p = 0.0026]. In particular, offspring from BAD dams showed higher alcohol intake with respect to p-CTR on week 1, 3, and 4 (t = 4.091, p = 0.0003; t = 6.164, p < 0.0001; t = 7.548, p < 0.0001), and with respect to p-HAD offspring on week 1 and 4 (t = 3.325, p = 0.0039; t = 4.192, p = 0.0002). Moreover, p-HAD offspring showed increased alcohol intake with respect to p-CTR rats on week 3 and 4 (t = 4.096, p = 0.0003; t = 3.356, p = 0.0036). When alcohol deprivation effect was measured as mean alcohol intake (g/kg) during the relapse-like time window, following a week of alcohol abstinence, Kruskal–Wallis test showed a significant effect of maternal alcohol drinking pattern (p < 0.001). In details, Dunn's multiple comparison test showed an increase in alcohol consumption in p-BAD- when compared with p-HAD-offspring (p < 0.01) and p-CTR ones (p = 0.0295; Figure 4I).
The present study aimed at evaluating the consequences of pre-conceptional long-term binge-like alcohol self-administration on different behavioral dimensions in female rats; moreover, we prolonged alcohol exposure during pregnancy and lactation in order to evaluate the occurrence of relevant effects on adolescent male offspring's phenotype, including the inheritance of alcohol abuse. Our results show that the experimental model of BAD provides a robust opportunity to explore the discrete consequences of this worldwide spread, excessive alcohol drinking habit, and highlight the occurrence of the inheritance of mood- and reward-related disturbances in the offspring.
Indeed, we modeled binge drinking in female rats by using a two-bottle choice paradigm with water and intermittent access to 20% alcohol, over 12 weeks. In order to explore discrete pattern-related effects, a continuous/habitual alcohol-drinking group was included, as positive control. In accordance with previous reports in both male and female rats [21, 46, 49–51], the intermittent access to alcohol reliably models excessive binge-like alcohol consumption. Indeed, BAD rats showed a significantly higher alcohol intake than HAD rats [52]: within the first hour of alcohol access they drank intoxicating amounts of alcohol [46], in accordance with the definition of binge drinking [53]. The “limited-access” model appropriately recreates the binging patterns observed in some human alcoholics [53–55] and, importantly, the voluntary self-administration prevents rats from undergoing stressful procedures that may contribute to the alteration of the subsequent behavioral outcomes. Indeed, once rats acquired a steady drinking behavior, a characterization of the affective phenotype was carried out on alcohol deprivation days.
Our data show that alcohol-drinking pattern is a determiner in the discrete modifications of the emotional and affective homeostasis in female rats here observed. Indeed, whether in the open field both HAD- and BAD female rats did not differ from CTR in terms of locomotor activity, HAD rats showed a significant avoidance of the central zone of the arena, while BAD rats did not differ from controls. This finding was confirmed by the results of the elevated-plus maze, where only HAD rats displayed increased anxiety-like behavior. Notably, the intermittent pattern of alcohol consumption is not associated to overt typical signs of alcohol withdrawal: mainly, rats trained in the intermittent model display cognitive deficits rather than increased anxiety-like behavior during acute abstinence [56, 57]. However, no significant differences appeared in the number of total arm entries, ruling out any impairment in locomotor activity [58].
To further analyze the consequences of binge-like alcohol consumption, anhedonia, and depression-like behavior were assessed. Anhedonia, i.e., the decreased experience of pleasure in response to nondrug reward [59], is a key symptom of depressive disorders, commonly described in subjects who are long-term abstinent from drugs of abuse: the development of a negative effect, such as anhedonia associated to dysphoria, is a relevant vulnerability factor for relapse (DSM-V, [60, 61]). In the saccharin preference test we evaluated animals' preference between regular water and a sweet solution, which represents a highly rewarding natural stimulus for rodents. Interestingly, whether habitual alcohol exposure induced a lower preference for the natural reward with respect to CTR, the most anhedonic response was observed in BAD rats which showed significant lower preference for saccharin than HAD rats. The reduction in the affective tone was confirmed by the results of the forced swim test, where BAD rats showed longer immobility score, when compared both to CTR and HAD rats, suggesting the occurrence of “despair-like behavior” as a consequence of the binge-like drinking exposure. On the other hand, HAD rats displayed a less defined picture, in that they showed a lower immobility time with respect to BAD rats and an increase in climbing, which suggests the expression of lower behavioral despair [62].
Overall, binge-like alcohol drinking seems to be responsible for a depressive-oriented phenotype, when animals are tested on abstinence days, which includes anhedonia- and despair-, whereas HAD rather induces an anxiety-oriented behavior. The repeated cycles of alcohol exposure and withdrawal typical of binge-like drinking may lead to enduring aberrant plasticity in strategic brain circuitries that drive a transition from positive to negative reinforcement-based alcohol seeking, and abstinence-induced affective disturbances [57, 63–74]. Supplementary and in depth evidence on the subject are available on seminal reviews [75].
Notably, after fecundation, alcohol consumption was evidently modified and differences in drinking trajectories were attenuated during pregnancy and lactation. This observation is in agreement with data showing that in the general female population the reproductive state is able to modulate alcohol intake and preference [76]. In accordance with our previous reports (51), during post-partum days, the drinking patterns reproduced similar trajectories as recorded on pre-conceptional time. The following aim of this research was the investigation of the consequences of perinatal alcohol exposure on the behavioral phenotype of the offspring. Few studies have addressed the multigenerational effects of maternal binge drinking; among them, a recent research demonstrated that maternal binge drinking induces a subtle range of cognitive defects, mainly referred to working memory deficits, associated with persistent neuroinflammation and myelin damage [77]. The present research widens the extent of the detrimental consequences of maternal binge drinking to the affective dimension: indeed, maternal binge-like alcohol drinking resulted in the inheritance of a depression-like phenotype in the offspring, when tested in adolescence. In details, whereas p-HAD offspring showed anxiety-like behavior in the open field and in the EPM, a dramatic decrease in social reward sensitivity in the social interaction, and a higher despair-like behavior in the FST characterized p-BAD progeny as adolescents. As a matter of fact, p-HAD progeny displayed the same alterations in coping with inescapable stress as their mothers, and a higher social interaction ratio than p-BAD offspring but lower than p-CTR.
Social interaction, which allows studying social attitudes in the experimental model, is experienced as a rewarding event in animals as in humans [78, 79]. In particular, whereas an age-typical inhibition of social behavior occurs in adulthood [80], social interactions are considered highly rewarding in young mammals [28]. Recent neuroimaging studies have shown that the activation of the reward system mediates the processing of social stimuli in a similar manner as nonsocial rewards and thus motivates social behavior [81]. Early experiences, such as prenatal alcohol exposure, may influence the ongoing development of reward-related circuitries (reviewed in 82, 83). The evidence that the major disruption in social behavior occurred in BAD progeny is not easy to explain. We can hypothesize that the long-term binge-like alcohol drinking affects the physiological response to natural rewarding stimuli in the mothers and that the inheritance of such disturbance may alter future social behavior in the offspring. In accordance, a longitudinal prospective study showed that pre-gestational exposure to alcohol binge drinking may be a risk factor for decreased social interaction, attention, hyperactivity in childhood [84], and is associated with substance dependence or abuse disorders vulnerability in early adulthood [34]. Consistently, our data show, for the first time, a specific vulnerability to alcohol abuse in the offspring of BAD dams. Indeed, when all groups were exposed to the two-bottle choice with alcohol solution and water, from adolescence to early adulthood, p-BAD progeny displayed a relevant increase in alcohol consumption compared to p-HAD- and CTR progeny along the paradigm, which suggests a faster acceptance and a higher preference for alcohol. Moreover, p-BAD progeny displayed a significant increase in voluntary alcohol consumption after a week of forced abstinence, resembling the so called “alcohol deprivation effect” (ADE). This phenomenon is well-established as a robust and reliable measure of the motivation to seek and consume alcohol, loss of control, and relapse in rats [85]. The evidence of ADE only in p-BAD offspring is suggestive of the occurrence of peculiar signs of vulnerability to alcohol abuse, such as compulsive alcohol-seeking and reinstatement behavior. Unfortunately, the time window for ADE assessment had to be short in order to study the phenomenon from adolescence to early adulthood. This allowed us to parallel clinical evidence of equivalent 14–25 years human subjects, along the temporal window when maturation of cortical and limbic regions is under completion [86]. A longer exposure to the drinking procedure and/or to the operant chamber will help to further investigate the reward-related responses to alcohol of p-BAD progeny, in the next future.
Overall, the idea that the progeny can epigenetically inherit aberrant functions due to parental alcohol abuse is not a novel issue. Recently, pre-conceptional alcohol intake has been reported to negatively affect offspring adult health by increasing stress hormone response to an immune challenge and altering the expression and methylation profiles of stress regulatory genes in various brain areas: this evidence suggests that pre-conceptional alcohol intake may play a role in the inheritance of stress-related diseases [87]. Moreover, clinical and epidemiological studies indicate that pre-conceptional exposure to alcohol is not only associated with the risk for alcohol abuse in adolescent and young adult humans [88, 89], but is perhaps the best predictor of later alcohol abuse [90, 91].
In conclusion, this study shows that pre-conceptional and peri-gestational habitual and binge-like alcohol consumption induce respectively an anxiety-like and a depression-like oriented phenotype in the mothers, which can be accordingly inherited by the adolescent male progeny. These preclinical findings prompt the early detection of discrete psychopathological traits that may represent predictive signs of alcohol vulnerability in the adolescents, in order to carry out rescue strategies for at risk endophenotypes [92–98]. The occurrence of similar inheritance in the female progeny is still to be ascertained. Ultimately, our findings highlight the need for more incisive public educational campaigns that can educate women of childbearing age avoiding binge—and continuous—alcohol drinking during pregnancy, in order to increase their awareness on the severe consequences they may expose their offspring, even at a later developmental epoch.
AB experimental procedures, contribution to experimental design, and writing. FP statistical analysis and graphical layout. VC, AC, GL experimental procedures, contribution to writing. CC experimental design, data interpretation, and writing. All of the authors read and approved the final manuscript.
Supported by a grant from PO.FESR 2007/2013 (grant number: R4D18+D18+0000).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Cannizzaro C, Martire M, Steardo L, Cannizzaro E, Gagliano M, Mineo A, et al. Prenatal exposure to diazepam and alprazolam, but not to zolpidem, affects behavioural stress reactivity in handling-naïve and handling-habituated adult male rat progeny. Brain Res. (2002) 953:170–80. doi: 10.1016/S0006-8993(02)03282-1
2. Cannizzaro C, Martire M, Cannizzaro E, Monastero R, Gagliano M, Mineo A, et al. Effects of 8-OH-DPAT on open field performance of young and aged rats prenatally exposed to diazepam: a tool to reveal 5-HT1A receptor function. Eur Neuropsychopharmacol. (2003) 13:209–17. doi: 10.1016/S0924-977X(03)00012-9
3. Cannizzaro C, D'Amico M, Altobelli D, Preziosi P, Martire M. Neurosteroid modulation of the presynaptic NMDA receptors regulating hippocampal noradrenaline release in normal rats and those exposed prenatally to diazepam. Neurochem Int. (2003) 43:121–7. doi: 10.1016/S0197-0186(02)00226-7
4. Cannizzaro C, Plescia F, Gagliano M, Cannizzaro G, Provenzano G, Mantia G, et al. Effects of pre- and postnatal exposure to 5-methoxytryptamine and early handling on an object-place association learning task in adolescent rat offspring. Neurosci Res. (2007) 59:74–80. doi: 10.1016/j.neures.2007.05.012
5. Cannizzaro C, Plescia F, Gagliano M, Cannizzaro G, Mantia G, La Barbera M, et al. Perinatal exposure to 5-metoxytryptamine, behavioural-stress reactivity and functional response of 5-HT1A receptors in the adolescent rat. Behav Brain Res. (2008) 186:98–106. doi: 10.1016/j.bbr.2007.07.036
6. Cannizzaro E, Martire M, Gagliano M, Plescia F, La Barbera M, Mantia G, et al. Reversal of prenatal diazepam-induced deficit in a spatial-object learning task by brief, periodic maternal separation in adult rats. Behav Brain Res. (2005) 161:320–30. doi: 10.1016/j.bbr.2005.02.022
7. Martire M, Altobelli D, Cannizzaro C, Maurizi S, Preziosi P. Prenatal diazepam exposure functionally alters the GABAA receptor that modulates [3H] Noradrenaline release from rat hippocampal synaptosomes. Dev Neurosci. (2002) 24:71–8. doi: 10.1159/000064947
8. Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol Clin Exp Res. (2002) 26:1584–91. doi: 10.1111/j.1530-0277.2002.tb02459.x
9. Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, et al. Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB). J Child Psychol Psychiatry (2009) 50:688–97. doi: 10.1111/j.1469-7610.2008.01990.x
10. Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res. (1998) 22:528–33. doi: 10.1111/j.1530-0277.1998.tb03684.x
11. Mårdby AC, Lupattelli A, Hensing G, Nordeng H. Consumption of alcohol during pregnancy-A multinational European study. Women Birth (2017) 30:e207–13. doi: 10.1016/j.wombi.2017.01.003
12. Ethen M, Ramadhani T, Scheuerle A, Canfield M, Wyszynski D, Druschel CM, et al. Alcohol consumption by women before and during pregnancy. Matern Child Health J. (2009) 13:274–85. doi: 10.1007/s10995-008-0328-2
13. Popova S, Lange S, Probst C, Gmel G, Rehm J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Glob Health (2017) 5:e290–9. doi: 10.1016/S2214-109X(17)30021-9
14. Anderson AE, Hure AJ, Forder PM, Powers J, Kay-Lambkin FJ, Loxton DJ. Risky drinking patterns are being continued into pregnancy: a prospective cohort study. PLoS ONE (2014) 9:e86171. doi: 10.1371/journal.pone.0086171
15. Bekman NM, Winward JL, Lau LL, Wagner CC, Brown SA. The impact of adolescent binge drinking and sustained abstinence on affective state. Alcohol Clin Exp Res. (2013) 37:1432–9. doi: 10.1111/acer.12096
16. Mahnke AH, Miranda RC, Homanics GE. Epigenetic mediators and consequences of excessive alcohol consumption. Alcohol (2017) 60:1–6. doi: 10.1016/j.alcohol.2017.02.357
17. Banik A, Kandilya D, Ramya S, Stünkel W, Chong YS, Dheen ST. Maternal factors that induce epigenetic changes contribute to neurological disorders in offspring. Genes (2017) 8:150. doi: 10.3390/genes8060150
18. Lam MKP, Homewood J, Taylor AJ, Mazurski EJ. Second generation effects of maternal alcohol consumption during pregnancy in rats. Prog Neuropsychopharmacol Biol. Psychiatry (2000) 24:619–31. doi: 10.1016/S0278-5846(00)00097-X
19. Cacace S, Plescia F, La Barbera M, Cannizzaro C. Evaluation of chronic alcohol self-administration by a 3-bottle choice paradigm in adult male rats. Effects on behavioural reactivity, spatial learning and reference memory. Behav Brain Res. (2011) 219:213–20. doi: 10.1016/j.bbr.2011.01.004
20. Cacace S, Plescia F, Sardo P, Cannizzaro C. Alcohol preference, behavioural reactivity and cognitive functioning in female rats exposed to a three-bottle choice paradigm. Behav Brain Res. (2012) 234:11–9. doi: 10.1016/j.bbr.2012.05.018
21. Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacology (1973) 29:203–10. doi: 10.1007/BF00414034
22. Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. (2008) 32:1816–23. doi: 10.1111/j.1530-0277.2008.00753.x
23. Brancato A, Lavanco G, Cavallaro A, Plescia F, Cannizzaro C. Acetaldehyde, motivation and stress: behavioral evidence of an addictive ménage à trois. Front Behav Neurosci. (2017) 11:23. doi: 10.3389/fnbeh.2017.00023
24. Plescia F, Sardo P, Rizzo V, Cacace S, Marino RAM, Brancato A, et al. Pregnenolone sulphate enhances spatial orientation and object discrimination in adult male rats: evidence from a behavioural and electrophysiological study. Behav Brain Res. (2014) 258:193–201. doi: 10.1016/j.bbr.2013.10.026
25. Plescia F, Brancato A, Venniro M, Maniaci G, Cannizzaro E, Sutera FM, et al. Acetaldehyde self-administration by a two-bottle choice paradigm: consequences on emotional reactivity, spatial learning, and memory. Alcohol (2015) 49:139–48. doi: 10.1016/j.alcohol.2015.01.002
26. Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. (1997) 21:801–10. doi: 10.1016/S0149-7634(96)00058-9
27. Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS. Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiol Behav. (1993) 54:1215–20. doi: 10.1016/0031-9384(93)90351-F
28. van Kerkhof LW, Damsteegt R, Trezza V, Voorn P, Vanderschuren LJ. Social play behavior in adolescent rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacology (2013) 38:1899–909. doi: 10.1038/npp.2013.83
29. Slattery DA, Cryan JF. Modelling depression in animals: at the interface of reward and stress pathways. Psychopharmacology (2017) 234:1451–65. doi: 10.1007/s00213-017-4552-6
30. Haastrup MB, Pottegård A, Damkier P. Alcohol and breastfeeding. Basic Clin Pharmacol Toxicol. (2014) 114:168–73. doi: 10.1111/bcpt.12149
31. Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit Rev Clin Lab Sci. (2011) 48:19–47. doi: 10.3109/10408363.2011.580567
32. Patten AR, Fontaine CJ, Christie BR. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front Pediatr. (2014) 2:93. doi: 10.3389/fped.2014.00093
33. Lynch ME, Kable JA, Coles CD. Effects of prenatal alcohol exposure in a prospective sample of young adults: mental health, substance use, and difficulties with the legal system. Neurotoxicol Teratol. (2017) 64:50–62. doi: 10.1016/j.ntt.2017.10.001
34. Barr HM, Bookstein FL, O'Malley KD, Connor PD, Huggins JE, Streissguth AP. Binge drinking during pregnancy as a predictor of psychiatric disorders on the Structured Clinical Interview for DSM-IV in young adult offspring. Am J Psychiatry (2006) 163:1061–5. doi: 10.1176/ajp.2006.163.6.1061
35. Sparrow SS, Cicchetti DV. Diagnostic uses of the vineland adaptive behavior scales. J Pediatr Psychol. (1985) 10:215–25. doi: 10.1093/jpepsy/10.2.215
36. Alati R, Clavarino A, Najman JM, O'Callaghan M, Bor W, Mamun AA, et al. The developmental origin of adolescent alcohol use: findings from the Mater University Study of Pregnancy and its outcomes. Drug Alcohol Depend. (2008) 98:136–43. doi: 10.1016/j.drugalcdep.2008.05.011
37. Vermeulen-Smit E, Koning IM, Verdurmen JE, Van der Vorst H H, Engels RC, Vollebergh WA. The influence of paternal and maternal drinking patterns within two-partner families on the initiation and development of adolescent drinking. Addict Behav. (2012) 37:1248–56. doi: 10.1016/j.addbeh.2012.06.005
38. Doremus-Fitzwater TL, Spear LP. Reward-centricity and attenuated aversions: an adolescent phenotype emerging from studies in laboratory animals. Neurosci Biobehav Rev. (2016) 70:121–34. doi: 10.1016/j.neubiorev.2016.08.015
39. File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. (2003) 463:35–53. doi: 10.1016/S0014-2999(03)01273-1
40. Poole KL, Van Lieshout RJ, McHolm AE, Cunningham CE, Schmidt LA. Trajectories of social anxiety in children: influence of child cortisol reactivity and parental social anxiety. J Abnorm Child Psychol. (2017) doi: 10.1007/s10802-017-0385-3. [Epub ahead of print].
41. Yohn NL, Bartolomei MS, Blendy JA. Multigenerational and transgenerational inheritance of drug exposure: the effects of alcohol opiates cocaine marijuana and nicotine. Prog Biophys Mol Biol. (2015) 118:21–33. doi: 10.1016/j.pbiomolbio.2015.03.002
42. Allan AM, Chynoweth J, Tyler LA, Caldwell KK. A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm. Alcohol Clin Exp Res. (2003) 27:2009–16. doi: 10.1097/01.ALC.0000100940.95053.72
43. Santangeli O, Lehtikuja H, Palomäki E, Wigren HK, Paunio T, Porkka-Heiskanen T. Sleep and behavior in cross-fostering rats: developmental and sex aspects. Sleep (2016) 39:2211–21. doi: 10.5665/sleep.6328
44. Subramanian MG. Lactation and prolactin release in foster dams suckling prenatally ethanol exposed pups. Alcohol Clin Exp Res. (1992) 16:891–94. doi: 10.1111/j.1530-0277.1992.tb01888.x
45. Wilson JH, Kelly SJ, Wilson MA. Early postnatal alcohol exposure in rats: maternal behavior and estradiol levels. Physiol Behav. (1996) 59:287–93. doi: 10.1016/0031-9384(95)02094-2
46. Loi B, Colombo G, MacCioni P, Carai MA, Franconi F, Gessa GL. High alcohol intake in female Sardinian alcohol-preferring rats. Alcohol (2014) 48:345–51. doi: 10.1016/j.alcohol.2014.01.001
47. Plescia F, Marino RA, Cannizzaro E, Brancato A, Cannizzaro C. The role of pregnenolone sulphate in spatial orientation-acquisition and retention: an interplay between cognitive potentiation and mood regulation. Behav Process. (2013) 99:130–7. doi: 10.1016/j.beproc.2013.07.001
48. Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, et al. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat Neurosci. (2015) 18:962–4. doi: 10.1038/nn.4034
49. Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. (2007) 104:12518–23. doi: 10.1073/pnas.0705368104
50. Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol (2014) 48:243–52. doi: 10.1016/j.alcohol.2014.01.006
51. Brancato A, Plescia F, Lavanco G, Cavallaro A, Cannizzaro C. Continuous and Intermittent alcohol free-choice from pre-gestational time to lactation: focus on drinking trajectories and maternal behavior. Front Behav Neurosci. (2016) 10:31. doi: 10.3389/fnbeh.2016.00031
52. Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. (2008) 32:1714–20. doi: 10.1111/j.1530-0277.2008.00749.x
53. Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann NY Acad Sci. (2011) 1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x
54. Boehm SL II, Moore EM, Walsh CD, Gross CD, Cavelli AM, Gigante E, et al. Using drinking in the dark to model prenatal binge-like exposure to ethanol in C57BL/6J mice. Dev Psychobiol. (2008) 50:566–78. doi: 10.1002/dev.20320
55. Sprow GM, Thiele TE. The neurobiology of binge-like ethanol drinking: evidence from rodent models. Physiol Behav. (2012) 106:325–31. doi: 10.1016/j.physbeh.2011.12.026
56. Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL, et al. Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacol Biochem Behav. (2012) 100:522–9. doi: 10.1016/j.pbb.2011.10.016
57. George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, et al. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci USA. (2012) 109:18156–61. doi: 10.1073/pnas.1116523109
58. Wall PM, Messier C. Methodological and conceptual issues in the use of the elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neurosci Biobehav Rev. (2001) 25:275–86. doi: 10.1016/S0149-7634(01)00013-6
59. Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature (1998) 393:76–9. doi: 10.1038/30001
60. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Publishing (2013).
61. Maniaci G, Picone F, Dimarco T, Lipari A, Brancato A, Cannizzaro C. Psychodiagnostic assessment of pathological gamblers: a focus on personality disorders, clinical syndromes and alexithymia. Int J Ment Health Addict. (2015) 13:728–39. doi: 10.1007/s11469-015-9550-5
62. Liebsch G, Montkowski A, Holsboer F, Landgraf R. Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav Brain Res. (1998) 94:301–10. doi: 10.1016/S0166-4328(97)00198-8
63. Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, Jones SR. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend. (2015) 150:24–30. doi: 10.1016/j.drugalcdep.2015.01.019
64. Holleran KM, Winder DG. Preclinical voluntary drinking models for alcohol abstinence-induced affective disturbances in mice. Genes Brain Behav. (2017) 16:8–14. doi: 10.1111/gbb.12338
65. Plescia F, Brancato A, Marino RAM, Cannizzaro C. Acetaldehyde as a drug of abuse: insight into AM281 administration on operant-conflict paradigm in rats. Front Behav Neurosci. (2013) 7:1–9. doi: 10.3389/fnbeh.2013.00064
66. Plescia F, Brancato A, Marino RA, Vita C, Navarra M, Cannizzaro C. Effect of acetaldehyde intoxication and withdrawal on npy expression: focus on endocannabinoidergic system involvement. Front Psychiatry (2014) 5:138. doi: 10.3389/fpsyt.2014.00138
67. Plescia F, Cannizzaro E, Brancato A, Martines F, Di Naro A, Mucia M, et al. Acetaldehyde effects in the brain. Acta Med Mediterr. (2015) 31:813–7.
68. Brancato A, Plescia F, Marino RA, Maniaci G, Navarra M, Cannizzaro C. Involvement of dopamine D2 receptors in addictive-like behaviour for acetaldehyde. PLoS ONE (2014) 9:e99454. doi: 10.1371/journal.pone.0099454
69. Brancato A, Cannizzaro C. Mothering under the influence: how perinatal drugs of abuse alter the mother-infant interaction. Rev Neurosci. (2018). 29:283–94. doi: 10.1515/revneuro-2017-0052
70. Cannizzaro C, La Barbera M Plescia, F Cacace, S Tringali G. Ethanol modulates corticotropin releasing hormone release from the rat hypothalamus: does acetaldehyde play a role? Alcohol Clin Exp Res. (2010) 34:588–93. doi: 10.1111/j.1530-0277.2009.01127.x
71. Cacace S, Plescia F, Barberi I, Cannizzaro C. Acetaldehyde oral self-administration: evidence from the operant-conflict paradigm. Alcohol Clin Exp Res. (2012) 36:1278–87. doi: 10.1111/j.1530-0277.2011.01725.x
72. Carletti F, Ferraro G, Rizzo V, Cannizzaro C, Sardo P. Antiepileptic effect of dimethyl sulfoxide in a rat model of temporal lobe epilepsy. Neurosci Lett. (2013) 546:31–5. doi: 10.1016/j.neulet.2013.04.031
73. Rizzo V, Carletti F, Gambino G, Schiera G, Cannizzaro C, Ferraro G, et al. Role of CB2 receptors and cGMP pathway on the cannabinoid-dependent antiepileptic effects in an in vivo model of partial epilepsy. Epilepsy Res. (2014) 108:1711–8. doi: 10.1016/j.eplepsyres.2014.10.001
74. Castelli V, Brancato A, Cavallaro A, Lavanco G, Cannizzaro C. Homer2 and alcohol: a mutual interaction. Front Psychiatry (2017) 8:268. doi: 10.3389/fpsyt.2017.00268
75. Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry (2013) 4:72. doi: 10.3389/fpsyt.2013.00072
76. Little RE, Schultz FA, Mandell W. Drinking during pregnancy. J Stud Alcohol. (1976) 37:375–9. doi: 10.15288/jsa.1976.37.375
77. Cantacorps L, Alfonso-Loeches S, Moscoso-Castro M, Cuitavi J, Gracia-Rubio I, López-Arnau R, et al. Maternal alcohol binge drinking induces persistent neuroinflammation associated with myelin damage and behavioural dysfunctions in offspring mice. Neuropharmacology (2017) 123:368–84. doi: 10.1016/j.neuropharm.2017.05.034
78. Marquardt K, Brigman JL. The impact of prenatal alcohol exposure on social, cognitive and affective behavioral domains: insights from rodent models. Alcohol (2016) 51:1–15. doi: 10.1016/j.alcohol.2015.12.002
79. Gold MS, Blum K, Febo M, Baron D, Modestino EJ, Elman I, et al. Molecular role of dopamine in anhedonia linked to reward deficiency syndrome (RDS) and anti- reward systems. Front Biosci. (2018) 10:309–25. doi: 10.2741/s518
80. Varlinskaya EI, Truxell E, Spear LP. Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol (2014) 48:433–44. doi: 10.1016/j.alcohol.2014.01.012
81. Goerlich KS, Votinov M, Lammertz SE, Winkler L, Spreckelmeyer KN, Habel U, et al. Effects of alexithymia and empathy on the neural processing of social and monetary rewards. Brain Struct Funct. (2017) 222:2235–50. doi: 10.1007/s00429-016-1339-1
82. Buwalda B, Geerdink M, Vidal J, Koolhaas JM. Social behavior and social stress in adolescence: a focus on animal models. Neurosci Biobehav Rev. (2011) 35:1713–21. doi: 10.1016/j.neubiorev.2010.10.004
83. McCormick CM, Hodges TE, Simone JJ. Peer pressures: social instability stress in adolescence and social deficits in adulthood in a rodent model. Dev Cogn Neurosci. (2015) 11:2–11. doi: 10.1016/j.dcn.2014.04.002
84. Clarke ME, Gibbard WB. Overview of fetal alcohol spectrum disorders for mental health professionals. Can Child Adolesc Psychiatry Rev.(2003) 12:57–63.
85. Martin-Fardon R, Weiss F. Modeling relapse in animals. Curr Top Behav Neurosci. (2013) 13:403–32. doi: 10.1007/978-3-642-28720-6_202
86. Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. (2013) 9:449–461. doi: 10.2147/NDT.S39776
87. Jabbar S, Chastain LG, Gangisetty O, Cabrera MA, Sochacki K, Sarkar DK. Preconception alcohol increases offspring vulnerability to stress. Neuropsychopharmacology (2016) 41:2782–93. doi: 10.1038/npp.2016.92
88. Alati R, Al Mamun A, Williams GM, O'Callaghan M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Arch Gen Psychiatry (2006) 63:1009–016. doi: 10.1001/archpsyc.63.9.1009
89. Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol (1998) 59:533–43. doi: 10.15288/jsa.1998.59.533
90. Streissguth AP, Bookstein FL, Barr HM, Press S, Sampson PD. A fetal alcohol behavior scale. Alcoholism (1998) 22:325–33. doi: 10.1111/j.1530-0277.1998.tb03656.x
91. Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res. (1998) 22:914–20. doi: 10.1111/j.1530-0277.1998.tb03889.x
92. Clementi ME, Pani G, Sampaolese B, Tringali G. Punicalagin reduces H2O2-induced cytotoxicity and apoptosis in PC12 cells by modulating the levels of reactive oxygen species. Nutr Neurosci. (2017). doi: 10.1080/1028415X.2017.1306935. [Epub ahead of print].
93. Greco MC, Capuano A, Navarra P, Tringali G. Lacosamide inhibits calcitonin gene-related peptide production and release at trigeminal level in the rat. Eur J Pain (2016) 20:959–66. doi: 10.1002/ejp.820
94. Greco MC, Navarra P, Tringali G. The analgesic agent tapentadol inhibits calcitonin gene-related peptide release from isolated rat brainstem via a serotonergic mechanism. Life Sci. (2016) 145:161–5. doi: 10.1016/j.lfs.2015.12.032
95. Greco MC, Lisi L, Currò D, Navarra P, Tringali G. Tapentadol inhibits calcitonin gene-related peptide release from rat brainstem in vitro. Peptides (2014) 56:8–13. doi: 10.1016/j.peptides.2014.03.009
96. Tringali G, Greco MC, Capuano A, Guerriero G, Currò D, Navarra P. Flupirtine inhibits calcitonin-gene related peptide release from rat brainstem in vitro. Neurosci Lett. (2012) 506:332–5. doi: 10.1016/j.neulet.2011.11.039
97. Tringali G, Greco MC, Lisi L, Pozzoli G, Navarra P. Cortistatin modulates the expression and release of corticotrophin releasing hormone in rat brain. Comparison with somatostatin and octreotide. Peptides (2012) 34:353–9. doi: 10.1016/j.peptides.2012.02.004
Keywords: alcohol, binge drinking, female, perinatal, adolescence, abuse vulnerability
Citation: Brancato A, Castelli V, Cavallaro A, Lavanco G, Plescia F and Cannizzaro C (2018) Pre-conceptional and Peri-Gestational Maternal Binge Alcohol Drinking Produces Inheritance of Mood Disturbances and Alcohol Vulnerability in the Adolescent Offspring. Front. Psychiatry 9:150. doi: 10.3389/fpsyt.2018.00150
Received: 25 January 2018; Accepted: 04 April 2018;
Published: 23 April 2018.
Edited by:
Lorenzo Leggio, National Institutes of Health (NIH), United StatesReviewed by:
Hansi Pathak, Hannover Medical School, GermanyCopyright © 2018 Brancato, Castelli, Cavallaro, Lavanco, Plescia and Cannizzaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carla Cannizzaro, Y2FybGEuY2Fubml6emFyb0B1bmlwYS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.