- 1Department of Psychiatry, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan
- 2Student Counseling Center, National Defense Medical Center, Taipei, Taiwan
- 3Department of Medical Research, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 4Taiwanese Injury Prevention and Safety Promotion Association, Taipei, Taiwan
- 5School of Public Health, National Defense Medical Center, Taipei, Taiwan
- 6Department of Psychiatry, Tri-Service General Hospital, Song-Shan Branch, National Defense Medical Center, Taipei, Taiwan
- 7Institute of Bioinformatics and Systems Biology, National Chiao Tung University, Hsin-Chu, Taiwan
- 8Department of Nursing, Tri-Service General Hospital, School of Nursing, National Defense Medical Center, Taipei, Taiwan
- 9Department of Nursing, Kang-Ning University (Taipei Campus), Taipei, Taiwan
- 10Division of Chest and Critical Medicine, Department of Medicine, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan
- 11Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei, Taiwan
- 12Graduate Institute of Life Sciences, National Defense Medical Center, Taipei, Taiwan
Background/objective: Allergic diseases, such as bronchial asthma, allergic rhinitis, atopic dermatitis, and psychiatric disorders, are major health issues. There have been reports that allergic diseases were associated with depression or anxiety disorders. This study aimed to investigate the association between these allergic diseases and the risk of developing overall psychiatric disorders in patients from Taiwan.
Methods: This cohort study used the database of the Taiwan National Health Insurance Program. A total of 186,588 enrolled patients, with 46,647 study subjects who had suffered from allergic diseases, and 139,941 controls matched for sex and age, from the Longitudinal Health Insurance Dataset of 2000–2015, were selected from a sub-dataset of the National Health Insurance Research Database. Fine and Gray’s competing risk model analysis was used to explore the hazard ratio (HR), and 95% confidence interval, for the risk of allergic diseases being associated with the risk of developing psychiatric disorders during the 15 years of follow-up.
Results: Of the study subjects, 5,038 (10.8%) developed psychiatric disorders when compared to 9,376 (6.7%) in the control group, with significant difference (p < 0.001). Fine and Gray’s competing risk model analysis revealed that the adjusted HR was 1.659 (95% CI = 1.602–1.717, p < 0.001). In this study, we found that the groups of atopic dermatitis alone and the allergic rhinitis + atopic dermatitis were associated with a lower risk of psychiatric disorders, but all the other four groups, such as bronchial asthma alone, allergic rhinitis alone, bronchial asthma + allergic rhinitis, bronchial asthma + atopic dermatitis, and the combination of all these three allergic diseases, were associated with a higher risk of psychiatric disorders.
Conclusion: Allergic diseases are therefore associated with a 1.66-fold increased hazard of psychiatric disorders in Taiwan.
Introduction
Child and adolescent allergic diseases, such as bronchial asthma, allergic rhinitis, and atopic dermatitis, are common and have made an impact on the patients’ physical health (1–3). Furthermore, previous studies have shown the association between asthma and anxiety (4), depressive, bipolar, and overall mood disorders (5, 6), attention-deficit hyperactivity disorder (ADHD) (7, 8), schizophrenia (9), and even dementia (10). Some studies have also demonstrated the associations between allergic rhinitis and depressive disorder (11, 12) or bipolar disorder (13). The association between atopic dermatitis and depressive and anxiety disorders has also been reported (14). However, another study found that allergic diseases such as rhinitis or urticarial are associated with a lower risk of schizophrenia (15). Therefore, a study is needed for the overall consideration of the association between these three common allergic diseases and important psychiatric disorders.
Psychiatric, or mental, disorders are defined as clinically significant behavioral or psychological syndromes, which are associated with present distress, disability, or an increased risk of suffering death, pain, or disability, and subsequent behavioral, psychological, or biological dysfunctions (16, 17). Previous studies have found that some psychiatric disorders are associated with several inflammatory diseases (18, 19), such as multiple sclerosis (20, 21), fibromyalgia (22, 23), or inflammatory bowel diseases (24). Psychological stressors related to these diseases might also contribute to both psychiatric and physical morbidity (25, 26).
Recent studies have been increasing on the interactions between allergy-related inflammatory or immunological factors and psychiatric disorders, such as depression (27), anxiety (28), bipolar disorder, and schizophrenia (29). However, further study is still needed to clarify the mechanisms underlying the association between allergic diseases and psychiatric disorders. Nevertheless, the association between allergic diseases and psychiatric disorders has not, as yet, been studied. Therefore, a nationwide, population-based study is necessary for the association between allergic diseases and the risk of psychiatric disorders for the clinicians who actually care for these patients.
Materials and Methods
Data Sources
The National Health Insurance (NHI) Program was launched in Taiwan in 1995, and as of June 2009, included contracts with 97% of the medical providers with approximately 23 million beneficiaries or more than 99% of the entire population (30, 31). The National Health Insurance Research Database (NHIRD), which contains all the claims data of the beneficiaries, uses the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes to record diagnoses (32). The details of the program have been documented in previous studies (22, 33–42).
A subset of the NHIRD, Longitudinal Health Insurance Database of a two million randomized sampled population in 2000–2015, was used to study the association between allergic diseases and the risk of psychiatric disorders. The present study used the NHIRD to identify patients with the diagnosis of allergic diseases, based on the ICD-9-CM codes, such as bronchial asthma (ICD-9-CM: 493.x), allergic rhinitis (ICD-9 CM code 477.X), and atopic dermatitis (ICD-9-CM code 691.X) during this period. The Institutional Review Board of the Tri-Service General Hospital approved this study and waived the need for individual written informed consent (IRB No. 2-105-05-040 and IRB No. 2-105-05-082).
Study Design and Sampled Participants
This study was of a population-based, matched-cohort design. Patients with newly diagnosed bronchial asthma, allergic rhinitis, or atopic dermatitis were selected from the Longitudinal Health Insurance Database from January 1, 2000, to December 31, 2015. The patients with asthma, allergic rhinitis, or atopic dermatitis before 2000 were excluded. This method could be viewed as a wash-out period to make sure all the allergic diseases were recent onset with references from other studies for the association between allergic diseases and psychiatric morbidity, using the NHIRD (6, 7, 11, 15). This wash out period method could avoid the carry-over bias from exposure to the pre-existing allergic diseases. In addition, the patients diagnosed with dementia, depressive disorders, anxiety disorders, eating disorders, bipolar disorders, sleep disorders, and psychotic disorders, before 2000, or before their first visit for asthma, allergic rhinitis, or atopic dermatitis were also excluded. Each enrolled patient was required to have made at least three outpatient visits within the 1-year study period for allergic diseases according to these ICD-9-CM codes. A total of the patients who were enrolled, including 46,647 subjects with allergic diseases and 139,941 controls without allergic diseases, were matched for age, sex, and index date (Figure 1).
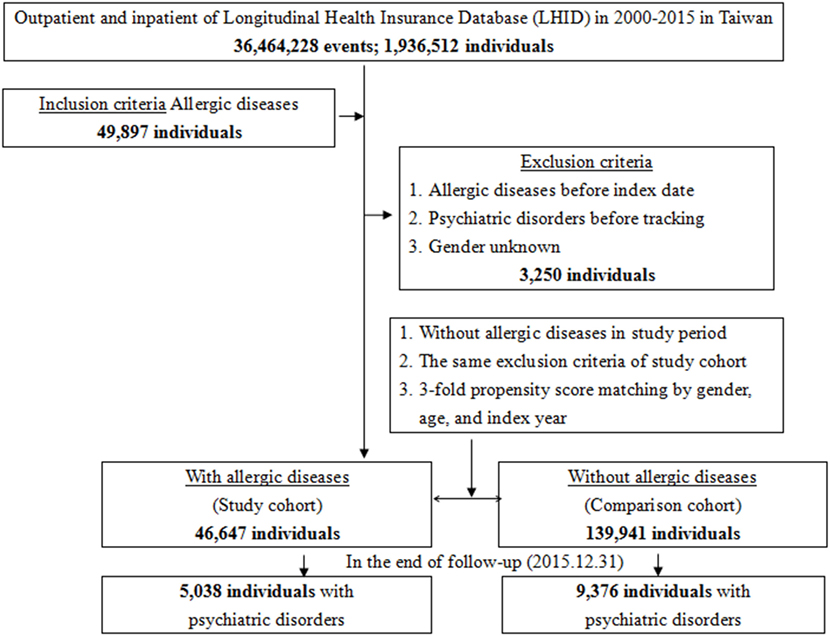
Figure 1. Flowchart of the study sample selection from the National Health Insurance Research Database in Taiwan.
Covariates
The covariates included sex, age groups (<20, 20–49, ≥50 years), geographical area of residence (north, center, south, and east of Taiwan), urbanization level of residence (levels 1–4), and monthly income (in New Taiwan Dollars; <18,000, 18,000–34,999, and ≥35,000). The urbanization level of residence was defined according to the population and various indicators of the level of development. Level 1 was defined as a population of >1,250,000, and a specific designation as political, economic, cultural, and metropolitan development. Level 2 was defined as a population between 500,000 and 1,249,999 and as playing an important role in the politics, economy, and culture. Urbanization levels 3 and 4 were defined as a population between 149,999 and 499,999, and <149,999, respectively (43).
Comorbidities
Comorbidities were assessed using the Charlson Comorbidity Index (CCI), which categorizes the comorbidities using the ICD-9-CM codes, scores each comorbidity category (44–49), and combines all scores to calculate a single comorbidity score. A score of zero indicates that no comorbidities were found, and higher scores indicate higher comorbidity burdens (31).
Major Outcome
All of the study participants were followed from the index date until the onset of dementia (ICD-9-CM codes: 290.0, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.41, 290.42, 290.43, 290.8, 290.9, and 331.0), anxiety disorders (ICD-9-CM 300), depressive disorders (ICD-9-CM 296.2–296.3, 300.4, and 311), eating disorders (anorexia nervosa 307.1, bulimia nervosa 307.51, and other disorders of eating 307.59), bipolar disorders (ICD-9-CM 296.0, and 296.4–296.8), sleep disorders (ICD-9-CM 307.4 and 780.5), and psychotic disorders (ICD-9-CM 295 and 297–298), withdrawal from the NHI program, or the end of 2015. In addition, each psychiatric diagnosis was required to have made at least three outpatient visits within the 1-year study period for psychiatric disorders according to these ICD-9-CM codes.
Statistical Analyses
All statistical analyses were performed using the SPSS for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). χ2 and t tests were used to evaluate the distributions of the categorical and continuous variables, respectively, with a Fisher’s exact examination. Fine and Gray’s competing risk analysis was used to determine the risk of psychiatric disorders since death can act as a competing risk factor for psychiatric disorders (40, 50, 51). The results were presented as hazard ratio (HR) with a 95% confidence interval (CI). Differences in the risk of psychiatric disorders between the study and control groups were estimated using the Kaplan–Meier method with the log-rank test. A two-tailed p value < 0.05 was considered to indicate the statistical significance.
Results
Baseline Characteristics of the Study Population
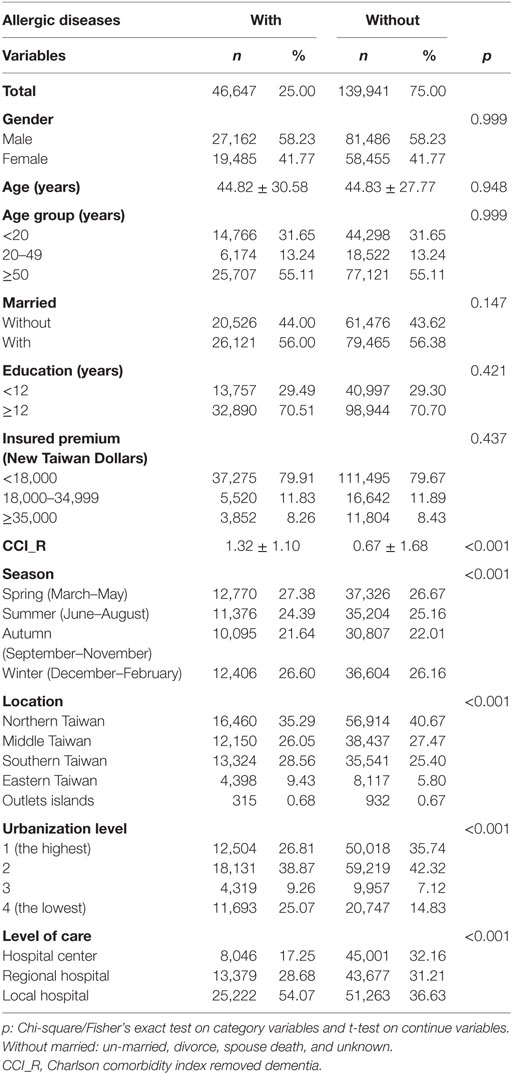
The baseline characteristics of the study population are depicted in Table 1. There were 46,647 subjects in the allergic diseases group and 139,941 non-allergic diseases in the control group, with a similar distribution of sex, age, marital status, education years, and monthly insured premiums. The mean CCR (SD) for the subjects was 1.32 (1.10) and 0.67 (1.68) for the allergic diseases and control group, respectively. The subjects had more medical visits in the spring and winter, with residence in the south, east, and offshore islands of Taiwan, living in levels 3 and 4 in urbanization, or seeking help in local hospitals than the control group (p < 0.001 for all). In the subjects with allergic diseases, 40,405 have asthma, 1,809, allergic rhinitis, and 4,433, atopic dermatitis.
Allergic Diseases Associated With Psychiatric Disorders
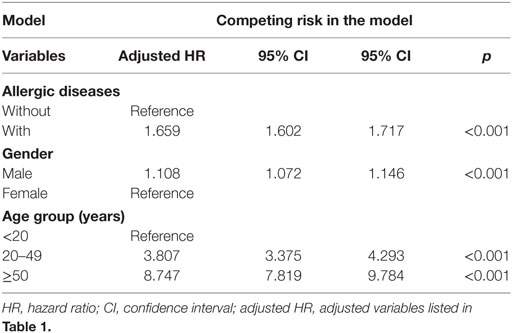
Of the study subjects, 5,038 (10.8%) developed psychiatric disorders when compared to 9,376 (6.7%) in the control group, in the 15-year follow-up. We have also examined the risk of psychiatric disorders associated with allergic diseases. After adjusting for age, sex, CCI scores, and all the covariates, the Fine and Gray’s survival analysis revealed that the adjusted HR for psychiatric disorders was 1.659 for the subjects (95% CI = 1.602–1.717, p < 0.001) when compared with the control group. With reference to the female subjects, the male subjects were associated with a higher risk of psychiatric disorders. With reference to the younger group (aged < 20 years), older age groups (aged 20–49 years and aged ≥ 50 years) were associated with a higher risk of psychiatric disorders (p < 0.001) (Table 2). Figure 2 shows the Kaplan–Meier analysis for the cumulative incidence of psychiatric disorders in the study subjects and the control groups (log-rank test, p < 0.001).
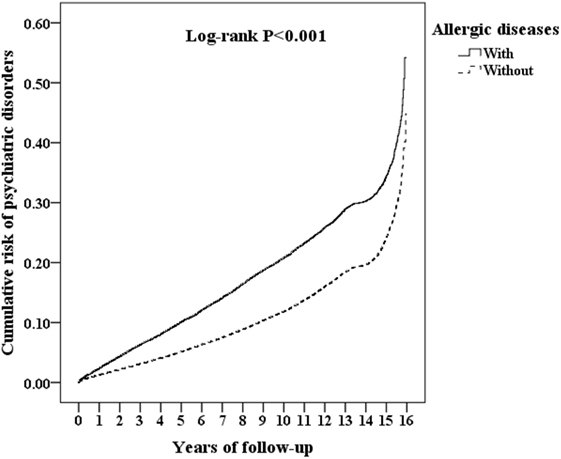
Figure 2. Kaplan–Meier for the cumulative risk of psychiatric disorders stratified by allergic diseases with the log-rank test.
Furthermore, each single increased score of CCI was associated with a 2% increased risk of psychiatric disorders, and study subjects who sought medical help in the summer (adjusted HR 0.914, p < 0.001), autumn (adjusted HR 0.748, p < 0.001), and winter (adjusted HR 0.921, p < 0.001) were associated with a lower risk of psychiatric disorders (data not shown). Study subjects who live in urbanization level 3 were associated with a lower risk of psychiatric disorders than those in level 4 (adjusted HR 0.817, p < 0.001). Study subjects who sought medical help in the medical centers (adjusted HR 0.504, p < 0.001) and regional hospitals (adjusted HR 0.635, p < 0.001) were associated with a lower risk of psychiatric disorders than those in the local hospitals (data not shown).
Association Between Different Allergic Diseases and Risk of Psychiatric Disorders
Table 3 reveals that the association between each group of allergic diseases and the individual psychiatric disorders using Fine and Gray’s competing risk model. The subjects with atopic dermatitis were associated with a decreased risk of overall and individual psychiatric disorders.
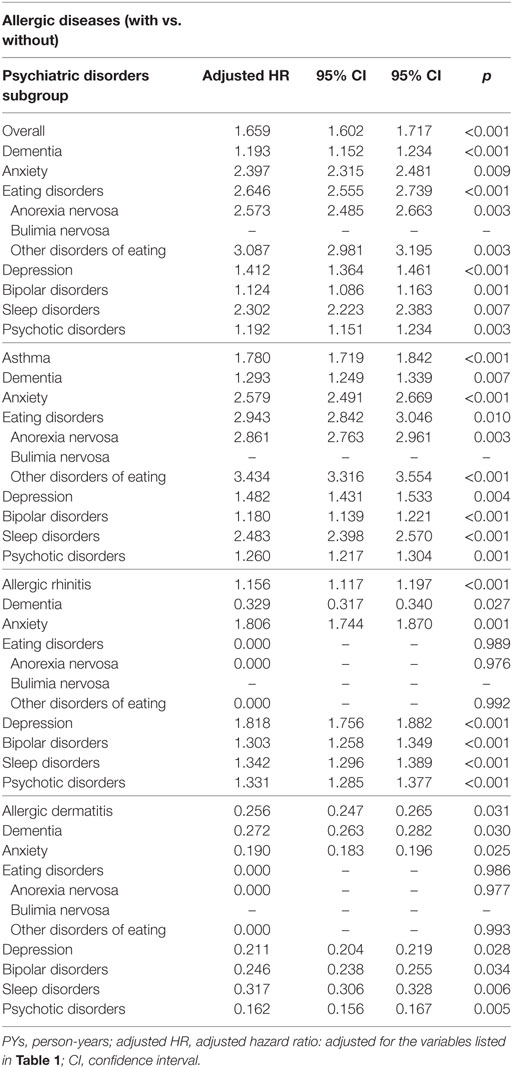
Table 3. Factors of psychiatric disorders subgroup stratified by allergic diseases subgroup by using Fine and Gray’s competing risk model.
In addition, the patients with allergic diseases who live in the middle (adjusted HR: 1.118, p < 0.001), southern (adjusted HR: 1.055, p = 0.003), and eastern Taiwan (adjusted HR: 1.596, p < 0.001) showed slightly higher risk of psychiatric disorders, with reference of those who live in northern Taiwan. The patients who live in area of urbanization level 3 (adjusted HR: 0.763, p < 0.001) showed lower risk of psychiatric disorders, with reference of those who live in the urbanization level 4. The patients who sought for medical help in the hospital centers (adjusted HR: 0.504, p < 0.001) and regional hospitals (adjusted HR: 0.635, p < 0.001) showed lower risk of psychiatric disorders with the reference of local hospitals (Data not shown).
Table 4 reveals the adjusted HR of affective disorders (adjusted HR: 1.385, p = 0.001, in which depression with adjusted HR: 1.412, p = 0.001, and bipolar disorders with adjusted HR: 1.124, p = 0.001), anxiety disorders (adjusted HR: 2.397, p = 0.009), psychotic disorders (adjusted HR: 1.192 p = 0.003), and dementia (adjusted HR: 1.193, p < 0.001) in patients with allergic diseases, when compared to the patients those without allergic diseases.
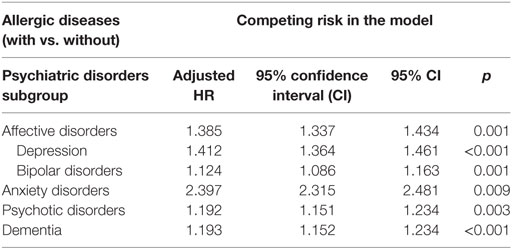
Table 4. Hazard ratio (HR) of affective, anxiety, psychotic disorders, and dementia in patients with vs. without allergic diseases.
We divided the subjects with allergic diseases into six groups: bronchial asthma alone, allergic rhinitis alone, atopic dermatitis alone, bronchial asthma + allergic rhinitis, bronchial asthma + atopic dermatitis, allergic rhinitis + atopic dermatitis, and a combination of all these three allergic diseases. In this study, we found that the groups of atopic dermatitis alone and allergic rhinitis + atopic dermatitis were associated with a lower risk of psychiatric disorders, but the other four groups were associated with a higher risk of psychiatric disorders (Table 5).
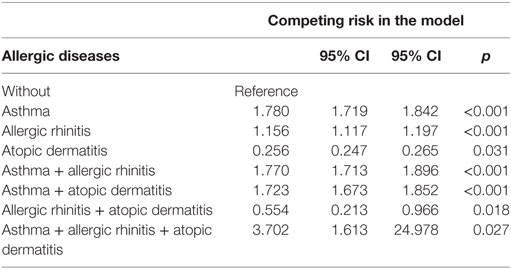
Table 5. Factors of psychiatric disorders by using Cox regression and Fine and Gray’s competing risk model.
Risk of Psychiatric Disorders Stratified by Covariates
We analyzed the data by the stratification of the factors such as sex, age, marital status, education levels, urbanization level, geographic areas of residence, seasons of medical visits, monthly insured premiums, and levels of care from medical services providers. We found that the subjects, who were either male or female, married or not married, and in different educational years, urbanization levels, residence areas, seasons of visits, insured premiums, and levels of care, were associated with an increased risk of psychiatric disorders. In the different age groups, subjects aged < 20 years were associated with a lower risk of psychiatric disorder, while subjects in other age groups (≥20 years) were associated with a higher risk (Table 6).
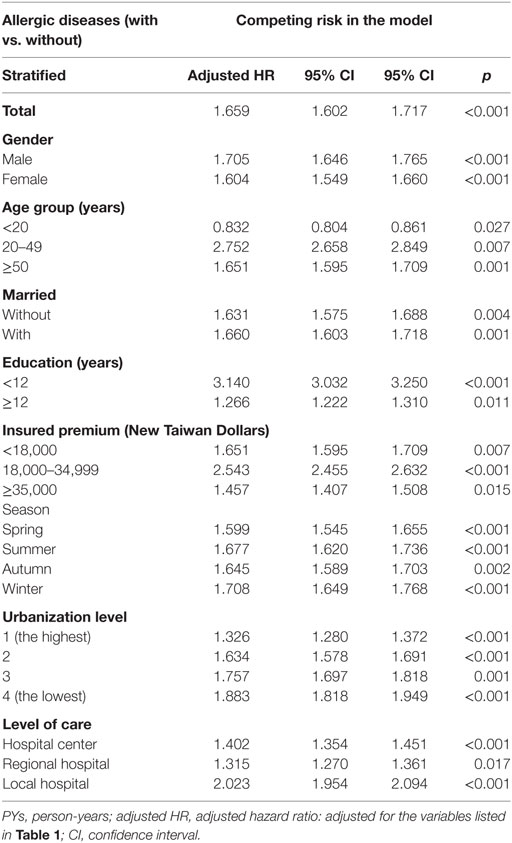
Table 6. Factors of psychiatric disorders stratified by variables listed in the table by using Fine and Gray’s competing risk model.
HR Analysis of Psychiatric Disorders in Patients With Different Medications for the Treatment of Asthma
The different medications of treatment durations more than 30 days for the treatment of bronchial asthma were grouped into four classes by the number of subjects who had used these medications: oral prednisolone, inhaled steroids, beta-agonist, aminophylline, leukotriene receptor antagonists, and anti-IgE antibody (omalizumab). Oral prednisolone usage was associated with a lower risk of developing psychiatric disorders in bronchial asthma patients. Besides, usage of beta-agonist, aminophylline, and leukotriene receptor antagonists was also associated with a lower risk of developing psychiatric disorders while adjusted with all the covariates and comorbidities, either with or without prednisolone usage (Table 7).
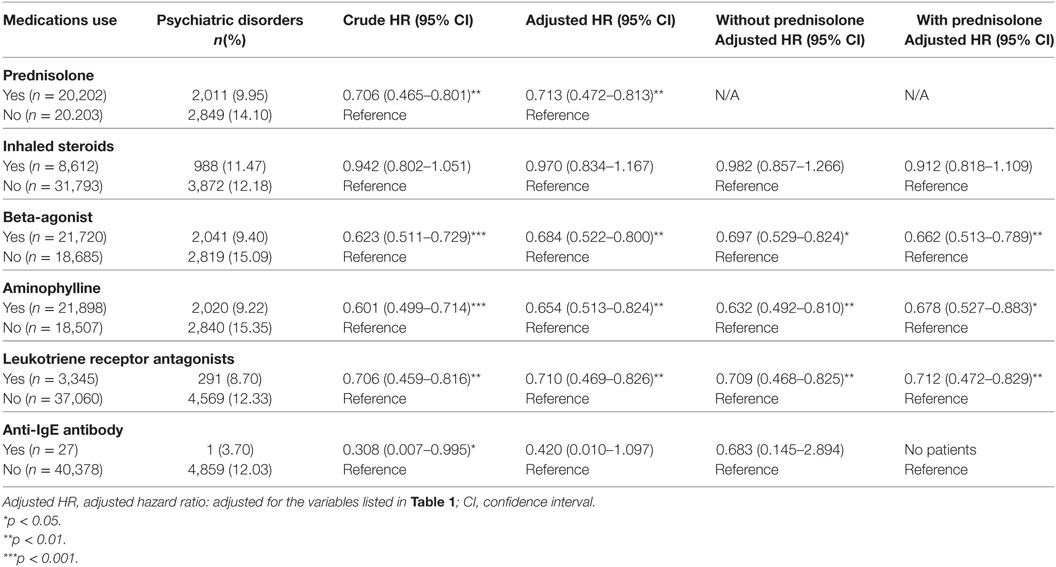
Table 7. HRs of patients with psychiatric disorders by medication use compared with patients with oral prednisolone use.
Discussion
In this study, we examined the association between the overall allergic diseases and the risk of psychiatric disorders. After adjusting the covariates, the adjusted HR was 1.659 for the subjects (95% CI = 1.602–1.717, p < 0.001) when compared with the control group. In other words, the adult patients with allergic diseases had a 1.66-fold increased risk of developing psychiatric disorders. The Kaplan–Meier analysis revealed that the study subjects had a significantly higher 15-year psychiatric disorders-free survival rate than the controls.
Bronchial asthma and allergic rhinitis were associated with the risk of overall psychiatric disorders, but atopic dermatitis was associated with a lower risk of psychiatric disorders. Bronchial asthma was associated with the risk of individual psychiatric disorders, such as dementia, anxiety disorders, depressive disorders, eating disorders, bipolar disorders, sleep disorders, and psychotic disorders, but allergic rhinitis was associated with a lower risk of dementia and an increased risk of other psychiatric disorders. However, the increasing number of allergic diseases was associated with an increased risk of psychiatric disorders. In comparison to the previous studies for individual allergic diseases in several psychiatric disorders, such as anxiety disorders, depressive disorders, bipolar disorders, schizophrenia, and ADHD (4, 6–8, 10–15, 43), this is the first study on the topic of the association of allergic diseases and the broader spectrum of psychiatric disorders. Atopic dermatitis seems to be an exception in these allergic diseases, by being associated with a lower risk of psychiatric disorders, even though previous reports found that atopic dermatitis are associated with ADHD and autism spectrum disorders (52–55). Further study is needed to clarify the association between atopic dermatitis and the risk of psychiatric disorders.
The results of this study showed the association between overall allergic diseases and the risk of psychiatric disorders, which echoed the findings of other studies on the association between the overall allergic or atopic diseases and the risk of psychiatric disorders: for example, children with eczema, asthma, or hay fever had more emotional, conduct, and hyperactivity problems in a cross-sectional study in Denmark (56), and atopic diseases might be associated with an elevated risk of developing depression in a birth cohort study in Finland (57). Several studies have reported the associations between bronchial asthma or other atopic diseases and psychiatric disorders, such as affective disorders, anxiety disorders, schizophrenia, substance-related disorders, autism-spectrum disorders, or ADHDs (9, 58–62). Some researchers have also reported the associations between atopic dermatitis and attention-deficit/hyperactivity disorder, or speech disorders in childhood (63), and the association between allergic rhinitis and the risk of suicides and depression (64). Furthermore, parental exposure to occupational asthmagens might also be associated with children autism-spectrum disorders (65). Previous studies have also reported the associations between other immune or inflammation-related diseases, such as periodontitis (66), gluten-related illnesses (67), amyotrophiclateral sclerosis (68), psoriasis (69), food or other allergies (70), and idiopathic environmental intolerance (71) and the risk of psychiatric disorders. Conversely, psychiatric disorders might also affect the immune systems (72). In this study, the overall allergic diseases were associated with affective disorders, anxiety disorders, psychotic disorders, and dementia, respectively. The interplay between allergic diseases and psychiatric disorders, therefore, warrant further study.
The underlying mechanism of the association between allergic diseases and psychiatric disorders remains unclear. The “orchestration” of the pro-inflammatory cytokines plays an important role in the pathogenesis of allergy-related diseases, such as asthma (73). Reports have shown that cytokines such as interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), IL-10, and monocyte chemoattractant protein-1/CCL2 might be well associated with depressive, bipolar, or anxiety disorders (74, 75). Reports also show that dementia is related to peripheral pro-inflammatory factors released by periodontal inflammatory mediators such as C-reactive protein, IL-6, haptoglobin, TNF-α, and fibrinogen (76–78). Furthermore, some studies have also found that inflammation play an important role in eating disorders (79, 80) and sleep disorders (81–83).
Additionally, we have included several psychosocial factors in the analysis, such as marital status, educational level, monthly insured premiums, urbanization level, and residence. In comparison to the allergy-disease subjects who sought medical help in the local hospitals, study subjects who received medical help from medical centers or regional hospitals were associated with a lower risk of psychiatric disorders. These findings suggest that disadvantageous socioeconomic factors might well contribute to the risk of psychiatric disorders.
We found that the study subjects of those aged < 20 years had a lower risk in developing psychiatric disorders. We therefore hypothesize that the reason for a lower risk is that the maximal follow-up time is 15 years, which might not have, as yet, reached the age of onset of the most major psychiatric disorders for the patients of allergic diseases (84). Further study is needed to clarify the association between age and the risk of psychiatric disorders in the patients with allergic diseases.
Furthermore, we found that oral prednisolone usage was associated with a lower risk of developing psychiatric disorders in bronchial asthma patients. Moreover, the usage of beta-agonist, aminophylline, and leukotriene receptor antagonists was also associated with a lower risk of developing psychiatric disorders, with or without prednisolone usage. The mechanisms and effects of these medications on the risk of psychiatric disorders in bronchial asthma patients merit further study.
Limitations
The present study has several limitations that warrant consideration. First, similar to previous studies using the NHIRD on allergic diseases as aforementioned, not all the data were recorded in the NHIRD, and we were unable to evaluate the severity, weakness severity, laboratory parameters, or lung function examinations in asthma patients. Second, other factors, such as genetic, psychosocial, and environmental factors, were not included in the dataset. Third, for patients who have to pay for their own for over-the-counter drugs for the allergic diseases, their self-medications would not have been included in the NHIRD. There is no study about the rates of self-medications for these allergic diseases. However, due to the high coverage of medical providers (97%) and beneficiaries (more than 99%) of the NHI system in Taiwan, most of the people would ask for help from the NHI-contracted hospital or clinics for their allergic diseases.
Strength of This Study
One of the primary strengths of this study is the use of ICD-9 codes, and a number of studies have demonstrated the accuracy and validity of several diagnoses in the NHIRD, including DM (85, 86), cancer (87–89), myocardial infarction (85, 90, 91), and central nervous system diseases, such as Tourette syndrome (92), and stroke (85, 93–95), outcomes (89), mortality (85, 96), or comorbidity (89, 96). In a wide spectrum of conditions, some studies have also demonstrated the concordance between Taiwan’s National Health Survey and the NHIRD on various diagnoses (97), medication usage (97), and health system utilizations (97, 98). Correspondingly, the long-term observation period from 2000 to 2015 allowed for more credibility, when compared with other similar studies, to propose physical mechanisms and plausible hypotheses. Finally, and most importantly, we have tried to explain the mutual biological and psychological mechanism between the allergic diseases and the psychiatric disorders.
Conclusion
We have evaluated the risk of psychiatric disorders in association with the allergic diseases such as asthma, allergic rhinitis, and atopic dermatitis in Taiwan’s population, using the representative population-based data. We have demonstrated that the patients with allergic diseases were at a significantly higher risk of psychiatric disorders than the control group. Further studies are therefore needed for patients with allergic diseases not only to prevent its clinical exacerbation but also to decrease the possibility of developing psychiatric disorders. We sincerely hope that this study will provide the necessary information for an earlier intervention for patients with allergic diseases.
Ethics Statement
The Institutional Review Board of the Tri-Service General Hospital approved this study and waived the need for individual written informed consent (IRB No. 2-105-05-040 and IRB No. 2-105-05-082).
Author Contributions
N-ST, H-AC, and W-CC conceived, planned, and conducted this study. C-HC and W-SC contributed to the data analysis and interpretation. Y-CK, C-CC, H-WY, Y-CC, and S-Y contributed to this data interpretation. N-ST wrote the draft. All the authors approved this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was supported in part by the Tri-Service General Hospital Research Foundation (TSGH-C106-002, TSGH-C106-106, and TSGH-C107-004). These funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
1. Hong S, Son DK, Lim WR, Kim SH, Kim H, Yum HY, et al. The prevalence of atopic dermatitis, asthma, and allergic rhinitis and the comorbidity of allergic diseases in children. Environ Health Toxicol (2012) 27:e2012006. doi:10.5620/eht.2012.27.e2012006
2. Grize L, Gassner M, Wuthrich B, Bringolf-Isler B, Takken-Sahli K, Sennhauser FH, et al. Trends in prevalence of asthma, allergic rhinitis and atopic dermatitis in 5–7-year old Swiss children from 1992 to 2001. Allergy (2006) 61(5):556–62. doi:10.1111/j.1398-9995.2006.01030.x
3. Yan DC, Ou LS, Tsai TL, Wu WF, Huang JL. Prevalence and severity of symptoms of asthma, rhinitis, and eczema in 13- to 14-year-old children in Taipei, Taiwan. Ann Allergy Asthma Immunol (2005) 95(6):579–85. doi:10.1016/S1081-1206(10)61022-8
4. Lee YC, Lee CT, Lai YR, Chen VC, Stewart R. Association of asthma and anxiety: a nationwide population-based study in Taiwan. J Affect Disord (2016) 189:98–105. doi:10.1016/j.jad.2015.09.040
5. Lin TC, Lee CT, Lai TJ, Lee CT, Lee KY, Chen VC, et al. Association of asthma and bipolar disorder: a nationwide population-based study in Taiwan. J Affect Disord (2014) 168:30–6. doi:10.1016/j.jad.2014.06.033
6. Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, et al. Higher risk of developing major depression and bipolar disorder in later life among adolescents with asthma: a nationwide prospective study. J Psychiatr Res (2014) 49:25–30. doi:10.1016/j.jpsychires.2013.10.015
7. Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, et al. Asthma and attention-deficit/hyperactivity disorder: a nationwide population-based prospective cohort study. J Child Psychol Psychiatry (2013) 54(11):1208–14. doi:10.1111/jcpp.12087
8. Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, et al. Attention deficit hyperactivity disorder, tic disorder, and allergy: is there a link? A nationwide population-based study. J Child Psychol Psychiatry (2013) 54(5):545–51. doi:10.1111/jcpp.12018
9. Wang WC, Lu ML, Chen VC, Ng MH, Huang KY, Hsieh MH, et al. Asthma, corticosteroid use and schizophrenia: a nationwide population-based study in Taiwan. PLoS One (2017) 12(3):e0173063. doi:10.1371/journal.pone.0173063
10. Chen MH, Li CT, Tsai CF, Lin WC, Chang WH, Chen TJ, et al. Risk of dementia among patients with asthma: a nationwide longitudinal study. J Am Med Dir Assoc (2014) 15(10):763–7. doi:10.1016/j.jamda.2014.06.003
11. Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, et al. Allergic rhinitis in adolescence increases the risk of depression in later life: a nationwide population-based prospective cohort study. J Affect Disord (2013) 145(1):49–53. doi:10.1016/j.jad.2012.07.011
12. Hsu CL, Wang TC, Shen TC, Huang YJ, Lin CL, Sung FC. Risk of depression in patients with chronic rhinosinusitis: a nationwide population-based retrospective cohort study. J Affect Disord (2016) 206:294–9. doi:10.1016/j.jad.2016.09.001
13. Chen MH, Lan WH, Hsu JW, Huang KL, Chen YS, Li CT, et al. Risk of bipolar disorder among adolescents with allergic rhinitis: a nationwide longitudinal study. J Psychosom Res (2015) 79(6):533–6. doi:10.1016/j.jpsychores.2015.08.009
14. Cheng CM, Hsu JW, Huang KL, Bai YM, Su TP, Li CT, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord (2015) 178:60–5. doi:10.1016/j.jad.2015.02.025
15. Chen YH, Lee HC, Lin HC. Prevalence and risk of atopic disorders among schizophrenia patients: a nationwide population based study. Schizophr Res (2009) 108(1–3):191–6. doi:10.1016/j.schres.2008.12.021
16. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. USA: American Psychiatric Association (1994).
17. Stein DJ, Phillips KA, Bolton D, Fulford KW, Sadler JZ, Kendler KS. What is a mental/psychiatric disorder? From DSM-IV to DSM-V. Psychol Med (2010) 40(11):1759–65. doi:10.1017/S0033291709992261
18. Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, Patten SB, et al. Rising incidence of psychiatric disorders before diagnosis of immune-mediated inflammatory disease. Epidemiol Psychiatr Sci (2017):1–10. doi:10.1017/S2045796017000579
19. Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, Patten SB, et al. Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. J Psychosom Res (2017) 101:17–23. doi:10.1016/j.jpsychores.2017.07.015
20. Marrie RA, Reingold S, Cohen J, Stuve O, Trojano M, Sorensen PS, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler (2015) 21(3):305–17. doi:10.1177/1352458514564490
21. Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. The burden of mental comorbidity in multiple sclerosis: frequent, underdiagnosed, and undertreated. Mult Scler (2009) 15(3):385–92. doi:10.1177/1352458508099477
22. Chao PC, Chien WC, Chung CH, Chu CW, Yeh CB, Huang SY, et al. Cognitive enhancers associated with decreased risk of injury in patients with dementia: a nationwide cohort study in Taiwan. J Investig Med (2017) 66(3):684–92. doi:10.1136/jim-2017-000595
23. Cunningham NR, Tran ST, Lynch-Jordan AM, Ting TV, Sil S, Strotman D, et al. Psychiatric disorders in young adults diagnosed with juvenile fibromyalgia in Adolescence. J Rheumatol (2015) 42(12):2427–33. doi:10.3899/jrheum.141369
24. Chen YT, Su JS, Tseng CW, Chen CC, Lin CL, Kao CH. Inflammatory bowel disease on the risk of acute pancreatitis: a population-based cohort study. J Gastroenterol Hepatol (2016) 31(4):782–7. doi:10.1111/jgh.13171
25. Suarez AL, Feramisco JD, Koo J, Steinhoff M. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Derm Venereol (2012) 92(1):7–15. doi:10.2340/00015555-1188
26. Sgambato D, Miranda A, Ranaldo R, Federico A, Romano M. The role of stress in inflammatory bowel diseases. Curr Pharm Des (2017) 23(27):3997–4002. doi:10.2174/1381612823666170228123357
27. Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, et al. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS One (2013) 8(3):e58488. doi:10.1371/journal.pone.0058488
28. Trueba AF, Ritz T, Trueba G. The role of the microbiome in the relationship of asthma and affective disorders. Adv Exp Med Biol (2016) 874:263–88. doi:10.1007/978-3-319-20215-0_13
29. Altamura AC, Buoli M, Pozzoli S. Role of immunological factors in the pathophysiology and diagnosis of bipolar disorder: comparison with schizophrenia. Psychiatry Clin Neurosci (2014) 68(1):21–36. doi:10.1111/pcn.12089
30. Ho Chan W. Taiwan’s healthcare report 2010. EPMA J (2010) 1(4):563–85. doi:10.1007/s13167-010-0056-8
31. Needham DM, Scales DC, Laupacis A, Pronovost PJ. A systematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care (2005) 20(1):12–9. doi:10.1016/j.jcrc.2004.09.007
32. Chinese Hospital Association. ICD-9-CM English-Chinese Dictionary. Taipei, Taiwan: Chinese Hospital Association Press (2000).
33. Huang HL, Ho SY, Li CH, Chu FY, Ciou LP, Lee HC, et al. Bronchial asthma is associated with increased risk of chronic kidney disease. BMC Pulm Med (2014) 14:80. doi:10.1186/1471-2466-14-80
34. Tzeng NS, Hsu YH, Ho SY, Kuo YC, Lee HC, Yin YJ, et al. Is schizophrenia associated with an increased risk of chronic kidney disease? A nationwide matched-cohort study. BMJ Open (2015) 5(1):e006777. doi:10.1136/bmjopen-2014-006777
35. Tang YJ, Ho SY, Chu FY, Chen HA, Yin YJ, Lee HC, et al. Is zolpidem associated with increased risk of fractures in the elderly with sleep disorders? A nationwide case cross-over study in Taiwan. PLoS One (2015) 10(12):e0146030. doi:10.1371/journal.pone.0146030
36. Yang CW, Tzeng NS, Yin YJ, Li CH, Chen HA, Chiu SH, et al. Angiotensin receptor blockers decrease the risk of major adverse cardiovascular events in patients with end-stage renal disease on maintenance dialysis: a nationwide matched-cohort study. PLoS One (2015) 10(10):e0140633. doi:10.1371/journal.pone.0140633
37. Tzeng NS, Chung CH, Yeh CB, Huang RY, Yuh DY, Huang SY, et al. Are chronic periodontitis and gingivitis associated with dementia? A nationwide, retrospective, matched-cohort study in Taiwan. Neuroepidemiology (2016) 47(2):82–93. doi:10.1159/000449166
38. Tzeng NS, Chang HA, Chung CH, Lin FH, Yeh CB, Huang SY, et al. Risk of psychiatric disorders in Guillain-Barre syndrome: a nationwide, population-based, cohort study. J Neurol Sci (2017) 381:88–94. doi:10.1016/j.jns.2017.08.022
39. Tzeng NS, Chung CH, Lin FH, Huang CF, Yeh CB, Huang SY, et al. Magnesium oxide use and reduced risk of dementia: a retrospective, nationwide cohort study in Taiwan. Curr Med Res Opin (2017) 34(1):163–9. doi:10.1080/03007995.2017.1385449
40. Tzeng NS, Chung CH, Lin FH, Yeh CB, Huang SY, Lu RB, et al. Headaches and risk of dementia. Am J Med Sci (2017) 353(3):197–206. doi:10.1016/j.amjms.2016.12.014
41. Chien WC, Chung CH, Lin FH, Chang HA, Kao YC, Tzeng NS. Is weight control surgery associated with increased risk of newly onset psychiatric disorders? A population-based, matched cohort study in Taiwan. J Med Sci (2017) 37(4):137–49. doi:10.4103/jmedsci.jmedsci_94_16
42. Tzeng NS, Chung CH, Lin FH, Yeh CB, Huang SY, Lu RB, et al. Risk of dementia in adults with ADHD: a nationwide, population-based cohort study in Taiwan. J Atten Disord (2017). doi:10.1177/1087054717714057
43. Chang CY, Chen WL, Liou YF, Ke CC, Lee HC, Huang HL, et al. Increased risk of major depression in the three years following a femoral neck fracture – a national population-based follow-up study. PLoS One (2014) 9(3):e89867. doi:10.1371/journal.pone.0089867
44. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide. Neuroepidemiology (2009) 32(2):150–63. doi:10.1159/000184748
45. van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol (2014) 10(8):469–82. doi:10.1038/nrneurol.2014.121
46. Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome: surveillance and cost of treatment strategies – authors’ reply. Lancet (2017) 389(10066):253–4. doi:10.1016/S0140-6736(17)30047-8
47. Group SG-BST. Randomised trial of plasma exchange, intravenous immuno-globulin, and combined treatments in Guillain-Barre syndrome. Plasma exchange. Lancet (1997) 349(9047):225–30. doi:10.1016/S0140-6736(96)09095-2
48. van den Berg B, Bunschoten C, van Doorn PA, Jacobs BC. Mortality in Guillain-Barre syndrome. Neurology (2013) 80(18):1650–4. doi:10.1212/WNL.0b013e3182904fcc
49. Wong AHY, Umapathi T, Shahrizaila N, Chan YC, Kokubun N, Fong MK, et al. The value of comparing mortality of Guillain-Barré syndrome across different regions. J Neurol Sci (2014) 344(1–2):60–2. doi:10.1016/j.jns.2014.06.021
50. Marzona I, Baviera M, Vannini T, Tettamanti M, Cortesi L, Riva E, et al. Risk of dementia and death in patients with atrial fibrillation: a competing risk analysis of a population-based cohort. Int J Cardiol (2016) 220:440–4. doi:10.1016/j.ijcard.2016.06.235
51. Blanche P, Proust-Lima C, Loubere L, Berr C, Dartigues JF, Jacqmin-Gadda H. Quantifying and comparing dynamic predictive accuracy of joint models for longitudinal marker and time-to-event in presence of censoring and competing risks. Biometrics (2015) 71(1):102–13. doi:10.1111/biom.12232
52. Billeci L, Tonacci A, Tartarisco G, Ruta L, Pioggia G, Gangemi S. Association between atopic dermatitis and autism spectrum disorders: a systematic review. Am J Clin Dermatol (2015) 16(5):371–88. doi:10.1007/s40257-015-0145-5
53. Lee CY, Chen MH, Jeng MJ, Hsu JW, Tsai SJ, Bai YM, et al. Longitudinal association between early atopic dermatitis and subsequent attention-deficit or autistic disorder: a population-based case-control study. Medicine (2016) 95(39):e5005. doi:10.1097/MD.0000000000005005
54. Liao TC, Lien YT, Wang S, Huang SL, Chen CY. Comorbidity of atopic disorders with autism spectrum disorder and attention deficit/hyperactivity disorder. J Pediatr (2016) 171:248–55. doi:10.1016/j.jpeds.2015.12.063
55. Strom MA, Fishbein AB, Paller AS, Silverberg JI. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br J Dermatol (2016) 175(5):920–9. doi:10.1111/bjd.14697
56. Hammer-Helmich L, Linneberg A, Obel C, Thomsen SF, Tang Mollehave L, Glumer C. Mental health associations with eczema, asthma and hay fever in children: a cross-sectional survey. BMJ Open (2016) 6(10):e012637. doi:10.1136/bmjopen-2016-012637
57. Timonen M, Jokelainen J, Hakko H, Silvennoinen-Kassinen S, Meyer-Rochow VB, Herva A, et al. Atopy and depression: results from the Northern Finland 1966 Birth Cohort Study. Mol Psychiatry (2003) 8(8):738–44. doi:10.1038/sj.mp.4001274
58. Jonsdottir U, Lang JE. How does autism spectrum disorder affect the risk and severity of childhood asthma? Ann Allergy Asthma Immunol (2017) 118(5):570–6. doi:10.1016/j.anai.2017.02.020
59. Heck S, Al-Shobash S, Rapp D, Le DD, Omlor A, Bekhit A, et al. High probability of comorbidities in bronchial asthma in Germany. NPJ Prim Care Respir Med (2017) 27(1):28. doi:10.1038/s41533-017-0026-x
60. To T, Ryckman K, Zhu J, Williams D, Feldman LY, Larsen K, et al. Mental health services claims and adult onset asthma in Ontario, Canada. J Allergy Clin Immunol Pract (2017) 5(5):1388–93.e3. doi:10.1016/j.jaip.2017.02.016
61. Miyazaki C, Koyama M, Ota E, Swa T, Mlunde LB, Amiya RM, et al. Allergic diseases in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis. BMC Psychiatry (2017) 17(1):120. doi:10.1186/s12888-017-1281-7
62. Schans JV, Cicek R, de Vries TW, Hak E, Hoekstra PJ. Association of atopic diseases and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. Neurosci Biobehav Rev (2017) 74(Pt A):139–48. doi:10.1016/j.neubiorev.2017.01.011
63. Silverberg JI. Selected comorbidities of atopic dermatitis: atopy, neuropsychiatric, and musculoskeletal disorders. Clin Dermatol (2017) 35(4):360–6. doi:10.1016/j.clindermatol.2017.03.008
64. Amritwar AU, Lowry CA, Brenner LA, Hoisington AJ, Hamilton R, Stiller JW, et al. Mental health in allergic rhinitis: depression and suicidal behavior. Curr Treat Options Allergy (2017) 4(1):71–97. doi:10.1007/s40521-017-0110-z
65. Singer AB, Burstyn I, Thygesen M, Mortensen PB, Fallin MD, Schendel DE. Parental exposures to occupational asthmagens and risk of autism spectrum disorder in a Danish population-based case-control study. Environ Health (2017) 16(1):31. doi:10.1186/s12940-017-0230-8
66. Sperr M, Kundi M, Tursic V, Bristela M, Moritz A, Andrukhov O, et al. Prevalence of comorbidities in periodontitis patients compared to the general Austrian population. J Periodontol (2017) 89:1–13. doi:10.1902/jop.2017.170333
67. Brietzke E, Cerqueira RO, Mansur RB, McIntyre RS. Gluten related illnesses and severe mental disorders: a comprehensive review. Neurosci Biobehav Rev (2018) 84:368–75. doi:10.1016/j.neubiorev.2017.08.009
68. Longinetti E, Mariosa D, Larsson H, Ye W, Ingre C, Almqvist C, et al. Neurodegenerative and psychiatric diseases among families with amyotrophic lateral sclerosis. Neurology (2017) 89(6):578–85. doi:10.1212/WNL.0000000000004179
69. Pompili M, Innamorati M, Forte A, Erbuto D, Lamis DA, Narcisi A, et al. Psychiatric comorbidity and suicidal ideation in psoriasis, melanoma and allergic disorders. Int J Psychiatry Clin Pract (2017) 21(3):209–14. doi:10.1080/13651501.2017.1301482
70. Aberle D, Wu SE, Oklu R, Erinjeri J, Deipolyi AR. Association between allergies and psychiatric disorders in patients undergoing invasive procedures. Psychosomatics (2017) 58(5):490–5. doi:10.1016/j.psym.2017.03.015
71. Weiss EM, Singewald E, Baldus C, Hofer E, Marksteiner J, Nasrouei S, et al. Differences in psychological and somatic symptom cluster score profiles between subjects with idiopathic environmental intolerance, major depression and schizophrenia. Psychiatry Res (2017) 249:187–94. doi:10.1016/j.psychres.2016.12.057
72. Larsen JB, Iversen VC, Reitan SK. Association of psychosis, affective disorders and diseases affecting the immune system. Nord J Psychiatry (2017) 72:145–9. doi:10.1080/08039488.2017.1402952
73. Castillo EF, Zheng H, Yang XO. Orchestration of epithelial-derived cytokines and innate immune cells in allergic airway inflammation. Cytokine Growth Factor Rev (2017) 39:19–25. doi:10.1016/j.cytogfr.2017.11.004
74. Kohler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol (2017). doi:10.1007/s12035-017-0632-1
75. Rosenblat JD, McIntyre RS. Bipolar disorder and immune dysfunction: epidemiological findings, proposed pathophysiology and clinical implications. Brain Sci (2017) 7(11):E144. doi:10.3390/brainsci7110144
76. D’Aiuto F, Graziani F, Tete S, Gabriele M, Tonetti MS. Periodontitis: from local infection to systemic diseases. Int J Immunopathol Pharmacol (2005) 18(3 Suppl):1–11.
77. Farhad SZ, Amini S, Khalilian A, Barekatain M, Mafi M, Barekatain M, et al. The effect of chronic periodontitis on serum levels of tumor necrosis factor-alpha in Alzheimer disease. Dent Res J (Isfahan) (2014) 11(5):549–52.
78. Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry (2003) 74(6):788–9. doi:10.1136/jnnp.74.6.788
79. Raevuori A, Lukkariniemi L, Suokas JT, Gissler M, Suvisaari JM, Haukka J. Increased use of antimicrobial medication in bulimia nervosa and binge-eating disorder prior to the eating disorder treatment. Int J Eat Disord (2016) 49(6):542–52. doi:10.1002/eat.22497
80. Succurro E, Segura-Garcia C, Ruffo M, Caroleo M, Rania M, Aloi M, et al. Obese patients with a binge eating disorder have an unfavorable metabolic and inflammatory profile. Medicine (2015) 94(52):e2098. doi:10.1097/MD.0000000000002098
81. Troester N, Palfner M, Schmidberger E, Olschewski H, Avian A. Sleep related breathing disorders and inflammation – the missing link? A Cohort study evaluating the interaction of inflammation and sleep related breathing disorders and effects of treatment. PLoS One (2015) 10(9):e0137594. doi:10.1371/journal.pone.0137594
82. Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, et al. Cognitive behavioral therapy and tai chi reverse cellular and genomic markers of inflammation in late-life insomnia: a randomized controlled trial. Biol Psychiatry (2015) 78(10):721–9. doi:10.1016/j.biopsych.2015.01.010
83. Irwin MR, Opp MR. Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology (2017) 42(1):129–55. doi:10.1038/npp.2016.148
84. Yin H, Xu G, Tian H, Yang G, Wardenaar KJ, Schoevers RA. The prevalence, age-of-onset and the correlates of DSM-IV psychiatric disorders in the Tianjin Mental Health Survey (TJMHS). Psychol Med (2017) 48(3):473–87. doi:10.1017/S0033291717001878
85. Cheng CL, Chien HC, Lee CH, Lin SJ, Yang YH. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in national health insurance research database in Taiwan. Int J Cardiol (2015) 201:96–101. doi:10.1016/j.ijcard.2015.07.075
86. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc (2005) 104(3):157–63.
87. Liang JA, Sun LM, Muo CH, Sung FC, Chang SN, Kao CH. The analysis of depression and subsequent cancer risk in Taiwan. Cancer Epidemiol Biomarkers Prev (2011) 20(3):473–5. doi:10.1158/1055-9965.EPI-10-1280
88. Li-Ting C, Chung-Ho C, Yi-Hsin Y, Pei-Shan H. The development and validation of oral cancer staging using administrative health data. BMC Cancer (2014) 14:380. doi:10.1186/1471-2407-14-380
89. Yang CC, Chen PC, Hsu CW, Chang SL, Lee CC. Validity of the age-adjusted charlson comorbidity index on clinical outcomes for patients with nasopharyngeal cancer post radiation treatment: a 5-year nationwide cohort study. PLoS One (2015) 10(1):e0117323. doi:10.1371/journal.pone.0117323
90. Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. Validation of acute myocardial infarction cases in the national health insurance research database in taiwan. J Epidemiol (2014) 24(6):500–7. doi:10.2188/jea.JE20140076
91. Hsieh CY, Chen CH, Li CY, Lai ML. Validating the diagnosis of acute ischemic stroke in a national health insurance claims database. J Formos Med Assoc (2015) 114(3):254–9. doi:10.1016/j.jfma.2013.09.009
92. Chou IC, Lin HC, Lin CC, Sung FC, Kao CH. Tourette syndrome and risk of depression: a population-based cohort study in Taiwan. J Dev Behav Pediatr (2013) 34(3):181–5. doi:10.1097/DBP.0b013e3182829f2b
93. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the national health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf (2011) 20(3):236–42. doi:10.1002/pds.2087
94. Sung SF, Hsieh CY, Lin HJ, Chen YW, Yang YH, Li CY. Validation of algorithms to identify stroke risk factors in patients with acute ischemic stroke, transient ischemic attack, or intracerebral hemorrhage in an administrative claims database. Int J Cardiol (2016) 215:277–82. doi:10.1016/j.ijcard.2016.04.069
95. Tseng HP, Lin FJ, Chen PT, Mou CH, Lee SP, Chang CY, et al. Derivation and validation of a discharge disposition predicting model after acute stroke. J Stroke Cerebrovasc Dis (2015) 24(6):1179–86. doi:10.1016/j.jstrokecerebrovasdis.2015.01.010
96. Yang H, Chen YH, Hsieh TF, Chuang SY, Wu MJ. Prediction of mortality in incident hemodialysis patients: a validation and comparison of CHADS2, CHA2DS2, and CCI scores. PLoS One (2016) 11(5):e0154627. doi:10.1371/journal.pone.0154627
97. Wu CS, Lai MS, Gau SS, Wang SC, Tsai HJ. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS One (2014) 9(12):e112257. doi:10.1371/journal.pone.0112257
Keywords: bronchial asthma, allergic rhinitis, atopic dermatitis, psychiatric disorders, risk factors, cohort study, Taiwan National Health Insurance Program, National Health Insurance Research Database
Citation: Tzeng N-S, Chang H-A, Chung C-H, Kao Y-C, Chang C-C, Yeh H-W, Chiang W-S, Chou Y-C, Chang S-Y and Chien W-C (2018) Increased Risk of Psychiatric Disorders in Allergic Diseases: A Nationwide, Population-Based, Cohort Study. Front. Psychiatry 9:133. doi: 10.3389/fpsyt.2018.00133
Received: 04 December 2017; Accepted: 28 March 2018;
Published: 24 April 2018
Edited by:
Drozdstoy Stoyanov Stoyanov, Plovdiv Medical University, BulgariaReviewed by:
Massimiliano Aragona, Crossing Dialogues Association, ItalyMarianna Atanassova Murdjeva, Plovdiv Medical University, Bulgaria
Copyright: © 2018 Tzeng, Chang, Chung, Kao, Chang, Yeh, Chiang, Chou, Chang and Chien. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wu-Chien Chien, Y2hpZW53dUBuZG1jdHNnaC5lZHUudHc=
 Nian-Sheng Tzeng
Nian-Sheng Tzeng Hsin-An Chang1,2
Hsin-An Chang1,2 Chi-Hsiang Chung
Chi-Hsiang Chung Chuan-Chia Chang
Chuan-Chia Chang Wu-Chien Chien
Wu-Chien Chien