
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 02 February 2018
Sec. Psychopathology
Volume 9 - 2018 | https://doi.org/10.3389/fpsyt.2018.00016
This article is part of the Research TopicCognitive Enhancement in Psychiatric DisordersView all 29 articles
 Shenghong Pu1*
Shenghong Pu1* Kazuyuki Nakagome2
Kazuyuki Nakagome2 Masashi Itakura1
Masashi Itakura1 Hiroaki Ohtachi1
Hiroaki Ohtachi1 Masaaki Iwata1
Masaaki Iwata1 Izumi Nagata3
Izumi Nagata3 Koichi Kaneko1
Koichi Kaneko1
Although prior studies identified a relationship between cognitive insight and subjective quality of life (QOL) in patients with schizophrenia, the brain regions mediating this relationship remain unknown. Recent studies have shown that the ventrolateral prefrontal cortex may be particularly important for cognitive insight in individuals with schizophrenia. Here, we examined whether frontotemporal function mediates the relationship between cognitive insight and QOL in 64 participants, including 32 patients with schizophrenia and 32 healthy controls. Cognitive insight was measured using the Beck Cognitive Insight Scale (BCIS), while participants’ subjective QOL was assessed using the Medical Outcomes Study 36-item Short-form Health Survey. Frontotemporal function was evaluated during a verbal fluency task using multichannel near-infrared spectroscopy. Consistent with previous findings, we found that frontotemporal function was impaired in patients with schizophrenia. Interestingly, our data also revealed that the right ventrolateral PFC and the right anterior part of the temporal cortex significantly mediated the relationship between the self-reflectiveness (SR) subscale of the BCIS and subjective QOL. These findings suggest that cognitive insight, particularly SR, is associated with subjective QOL in patients with schizophrenia via right frontotemporal function. The findings of this study provide important insight into a QOL model of schizophrenia, which may guide the development of cost-effective interventions that target frontotemporal function in patients with schizophrenia.
Previous research has demonstrated that individuals with schizophrenia tend to show a lack of insight that affects their symptom, psychosocial functioning, and treatment outcomes (1, 2). However, in 2004, the definition of insight was extended to include the cognitive processes that are involved in patients’ re-evaluation of their anomalous experiences and misinterpretations (3, 4). Beck and colleagues (3, 5) referred to this as cognitive insight, and further identified two underlying components, namely self-reflectiveness (SR) and self-certainty (SC). To examine these factors, the Beck Cognitive Insight Scale (BCIS) was developed (3), which revealed that when the scores for SR are low or when those for SC are high, then an individual’s overall cognitive insight ability is likely impaired.
Since the development of the BCIS, many studies have evaluated the relationship between the BCIS scores and delusions (3, 5, 6), while others have examined the relationship between the BCIS scores and anxiety (7), depression (8), negative symptoms (9, 10), and functional outcome (11–13). However, recently, interest in research on the neural correlates of cognitive insight in patients with schizophrenia has been increasing (14). Studies on this topic suggest that the ventrolateral prefrontal cortex (VLPFC) may be particularly important for cognitive insight in individuals with schizophrenia (14). For instance, higher SR has been linked to increased neural activation in the right ventrolateral PFC of individuals with schizophrenia (15). Moreover, in a previous near-infrared spectroscopy (NIRS) study performed in our laboratory, we found that SR modulated right ventrolateral PFC and right temporal functions during verbal fluency task (VFT) in people with schizophrenia (16). Interestingly, we also revealed that the ventrolateral PFC and other PFC regions played a significant role in the subjective quality of life (QOL) of individuals with schizophrenia (17, 18). These findings suggest that ventrolateral PFC function may mediate the relationship between cognitive insight and subjective QOL in schizophrenia. However, this hypothesis has not been fully examined.
Near-infrared spectroscopy is a comparably new neuroimaging technique that has received increasing attention in the field of neuroscience and psychiatry. NIRS is a non-invasive, high time resolution (0.1 s) functional optical technique revealing the spatiotemporal characteristics of brain functioning by using near-infrared light (19, 20). In contrast to other neuroimaging methodologies such as functional magnetic resonance imaging (fMRI) and electroencephalography (EEG), NIRS can be measured under a more restraint-free environment that is especially suitable for psychiatric patients. This has made it feasible to perform NIRS in real-world clinical settings (21). In NIRS, typical cortical activation represents not only decreased concentration of deoxy-hemoglobin (deoxy-Hb), which is considered the main source of blood oxygenation level-dependent (BOLD) contrast increase in fMRI, but also a relatively larger increase in oxy-hemoglobin concentration (oxy-Hb) (21). NIRS measurement during a VFT was recently approved by the Ministry of Health, Labor, and Welfare of Japan as an advanced medical technology for the aid of differential diagnosis of depressive state psychiatric illnesses and has been frequently applied in clinical settings in Japan (21). In addition, several reports suggest that the mean oxy-Hb changes induced by a VFT in patients with schizophrenia are significantly decreased compared with those observed in controls (22–26).
Cognitive insight has been found to predict positive gains in individuals undergoing psychotherapy for psychosis, and improvements in cognitive insight have been correlated with improvements in delusional beliefs (12, 27). However, better cognitive insight has also been linked to negative outcomes (8, 28, 29). Weintraub and Weisman de Mamani (30) recently suggested that cognitive insight might have a similarly equivocal relationship with subjective QOL in a subclinical sample, although the exact nature of the relationship between cognitive insight and subjective QOL for patients with schizophrenia has yet to be determined.
To examine these issues, we concurrently assessed frontotemporal function, cognitive insight, and subjective QOL in patients with schizophrenia. Our three hypotheses were as follows: (1) relative to healthy controls, patients with schizophrenia would have detectable abnormalities in VFT-related frontotemporal function; (2) cognitive insight would be related to the observed frontotemporal (specifically the right) function and subjective QOL; and (3) right frontotemporal function would mediate the relationship between cognitive insight and subjective QOL.
This study was approved by the Ethics Committee of Tottori University Faculty of Medicine (approval No. 885), and the investigation was conducted in accordance with the latest version of the Declaration of Helsinki. Written informed consent was obtained from each participant after the study procedures had been explained.
The participants included 32 patients with schizophrenia who were clinically stable enough to undergo the assessments and 32 age- and gender-matched healthy controls (Table 1). All the patients were receiving second-generation antipsychotic medication during the study, and their chlorpromazine-equivalent daily doses were calculated and are shown in Table 1 (monotherapy/two drugs therapy: 28/4; 10 aripiprazole, 8 blonanserin, 8 olanzapine, 3 risperidone, 3 perospirone, 2 paliperidone, 2 quetiapine). The patients were recruited from the outpatient population of Tottori University Hospital and were diagnosed by the same experienced psychiatrists (Masaaki Iwata and Koichi Kaneko) using the criteria specified in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (31). On the day of the NIRS experiment, patients’ psychiatric symptoms were evaluated by the same psychiatrists (Masaaki Iwata and Koichi Kaneko) using the Positive and Negative Syndrome Scale (32).
All participants were right-handed according to the Edinburgh Handedness Inventory (33). The participants in this study partially overlapped with those in our previous studies (16–18), but were not identical.
Cognitive insight was assessed with the BCIS (3, 10), a 15-item self-report inventory. The BCIS consisted of the following two components: SR and SC. The former includes items measuring objectivity, reflectiveness, and openness to feedback, whereas the latter measures the certainty about one’s own beliefs or judgment. The BCIS was administered to only the patients.
All patients completed a self-assessment of QOL using the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36) (34, 35). The SF-36 is scored such that 8 scale scores are given: physical functioning, role physical, bodily pain, general health perceptions, vitality, social functioning, role emotional, and mental health. The SF-36 Scoring Manual (36) does not provide support to calculate a single measure of health-related QOL, such as an “SF-36 Total/Global/Overall Score.” Therefore, we only adopted the subscale scores, which were transformed to make a minimum and maximum possible score of between 0 and 100, where higher scores indicate better health and well-being.
The 52-channel NIRS machine measures relative changes in oxy-hemoglobin (oxy-Hb) and deoxy-hemoglobin (deoxy-Hb) using two wavelengths (695 and 830 nm) of infrared light based on the modified Beer–Lambert law (37, 38). The arrangement of the source-detector probes enabled us to measure the Hb values from both the PFC and temporal regions(39, 40).
The task procedure used in this study was similar to that described by Takizawa et al. (24) in that Hb changes were measured during a letter version of the VFT. When performing the task, the participant sat on a comfortable chair and was instructed to minimize movements, such as head movements, jaw clenching, and eye blinking, to avoid producing artifacts during the NIRS measurements. The 160-s block-design VFT was divided into the following three periods: 30-s pre-task period, 60-s task period, and 70-s post-task period. The total number of correct words generated during the VFT was adopted as a measure of task performance (16).
The 160-s block-design VFT contains three different time periods: a 30-s pre-task period, a 60-s task period, and a 70-s post-task period (Figure 1). For the pre- and post-task baseline periods, the subjects were instructed to consecutively repeat the five Japanese vowels (“a,” “i,” “u,” “e,” and “o”) aloud. As readout from NIRS, the contrast between the verbal fluency condition and the vocalization condition was used to increase specificity for the verbal fluency canceling out the vocalization effect. During the task period, they were instructed to generate as many Japanese words beginning with a designated syllable as possible. The three sets of initial syllables (A; /to/, /se/, /o/, B; /a/, /ki/, /ha/, C; /na/, /i/, /ta/) were presented in counterbalanced order among the subjects and each syllable changed every 20 s during the 60-s task.
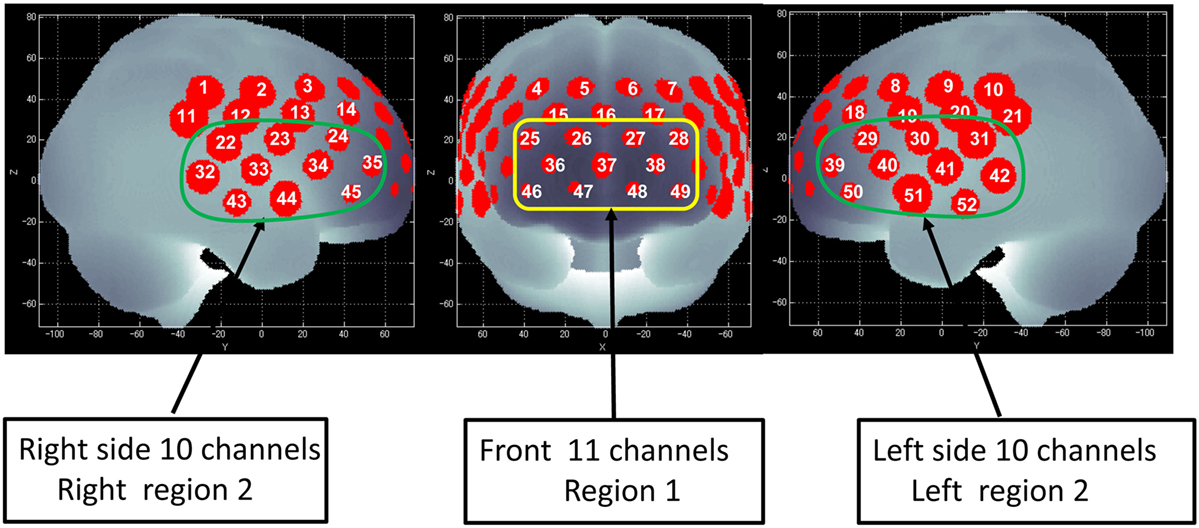
Figure 1. Channel positions on the brain surface. Right region 2 consists of the right 10 channels (22–24, 32–35, and 43–45) and is located approximately on the right ventrolateral prefrontal cortex (VLPFC) and anterior part of the temporal cortex (aTC) region. Left region 2 consists of the left 10 channels (29–31, 39–42, and 50–52) and is located approximately on the left VLPFC and aTC region. Region 1 consists of the center 11 channels (25–28, 36–38, and 46–49) and is located approximately on the dorsolateral prefrontal cortex (DLPFC) and frontopolar cortex (FPC) region (45).
The sampling frequency was 10 Hz. To examine VFT task-related activation, data were analyzed using the “integral mode” installed on the NIRS machine, in which the pre-task baseline was calculated as the mean over the 10-s period immediately prior to the task period, and the post-task baseline was calculated as the mean over the 5-s period that followed after the 50-s post-task period. Linear fitting was applied to the data recorded between these two baselines. A moving-average method, using a 5-s window, was applied to remove any short-term motion artifacts. In addition, we rejected noise related to body-movement artifacts (no signal, high frequency, and low frequency) using the algorithm published by Takizawa et al. (21).
We defined each measuring area between pairs of source-detector probes as “channel.” It is supposed that the NIRS machine, in which the source-detector spacing is 3.0 cm, measures points at 2–3 cm depth from the scalp, i.e., the surface of the cerebral cortex (41–43). The probes of the NIRS machine were fixed with thermoplastic 3 × 11 shells, with the lowest probes positioned along the Fp1–Fp2 line according to the international 10–20 system used in EEG. The 52 measuring areas are labeled ch1–ch52 from the right-posterior to the left-anterior.
Of the 52 NIRS channels, region 1 included channels 25–28, 36–38, and 46–49. The right side of region 2 included channels 22–24, 32–35, and 43–45, while the left side of region 2 included channels 29–31, 39–42, and 50–52 (Figure 1). The NIRS signal of region 1 consisted of the signals from channels located approximately in the dorsolateral PFC and frontopolar cortex (FPC) [dorsolateral prefrontal cortex (DLPFC)/FPC; i.e., the superior and middle frontal gyrus]. Region 2 consisted of signals from channels located approximately in the ventrolateral PFC and the anterior part of the temporal cortex (VLPFC/aTC) (21, 44, 45).
Statistical analyses were performed using SPSS Statistics 24.0 (IBM Corporation, Armonk, NY, USA). Hemodynamic responses during the VFT in region 1 and left and right region 2 were assessed by the “integral value” of Hb changes. We used the integral value of the oxy-Hb (as opposed to deoxy-Hb) changes that occurred during the VFT, as an index of cortical activity, because oxy-Hb better reflects this activity and is better correlated with fMRI BOLD signals (46–48) compared with deoxy-Hb.
First, the integral value of the oxy-Hb changes that occurred during the task period were compared between the participant groups using Student’s t-tests [with a Bonferroni-corrected alpha level of 0.0167 (0.05/3)]. When there was a significant between-group difference in the performance level, we performed additional analyses of covariance using the performance level as a covariate to the integral value of oxy-Hb changes. Next, Pearson’s product–moment correlation coefficients adopting the false discovery rate (FDR) method were calculated to test the correlations between the integral value of oxy-Hb changes in each region of interest (ROI; region 1 and left and right region 2) and the BCIS subscale scores (SR and SC) and SF-36 subscale scores.
A mediation analysis was performed using Hayes’ PROCESS macro, a regression-based path analysis technique (49). Using an ordinary least-squares framework, PROCESS estimates the direct and indirect effects in mediator models. To test the mediation hypotheses, PROCESS uses bootstrapping to construct confidence intervals (CIs) for indirect effects through repeated sampling of the data set. Findings are based on 5,000 bias-corrected bootstrapped samples. In the event that 0 does not lie within the 95% CI for the bootstrapped results for indirect effects, we can conclude that the indirect effect is significantly different from 0 and that mediation is demonstrated (50).
The participants’ demographic data are shown in Table 1. Patients with schizophrenia had a significantly lower performance level on the VFT (number of words generated) compared with healthy controls (t = 2.496, p = 0.015).
We used NIRS to evaluate our first hypothesis that patients with schizophrenia would have abnormalities in frontotemporal regions. The overall mean oxy-Hb change waveforms for the 52 channels and three ROIs in each group are shown in Figure 2. Compared with healthy controls, patients with schizophrenia exhibited significantly smaller integral values of oxy-Hb changes (region 1: t = 4.177, p < 0.001; left region: t = 4.923, p < 0.001; right region 2: t = 4.027, p < 0.001). The between-group differences in the integral values of oxy-Hb changes remained significant after correcting for the performance level in the three ROIs (region 1: F = 14.907, p < 0.001; left region: F = 21.261, p < 0.001; right region 2: F = 14.327, p < 0.001), according to analyses of covariance using performance on the VFT as a covariate to the integral values of oxy-Hb changes.
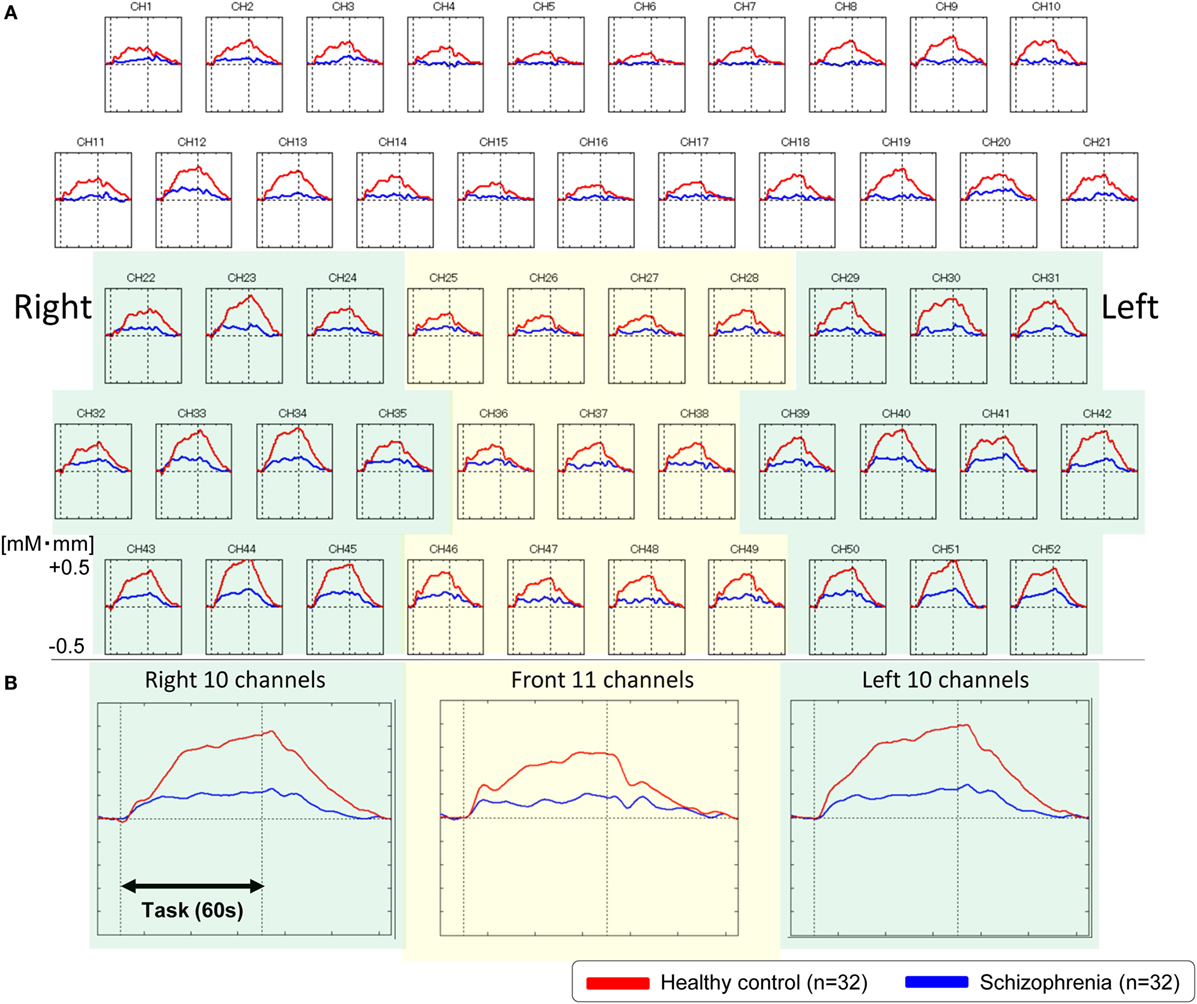
Figure 2. Waveforms of oxygenated-hemoglobin (oxy-Hb) concentration changes during the VFT. (A) The upper figures show the overall average waveforms of concentrations of oxy-Hb changes during the VFT in patients with schizophrenia (blue) and healthy controls (red). (B) In the lower three figures, the right figure shows the average waveforms of oxy-Hb changes for the 10 channels in the right VLPFC/aTC region, the center figure shows the average waveforms of oxy-Hb changes for the 11 channels in the frontal pole region, and the left figure shows the average waveforms of oxy-Hb changes for the 10 channels in the left VLPFC/aTC region. Abbreviations: VLPFC, ventrolateral prefrontal cortex; aTC, anterior part of the temporal cortex; VFT, verbal fluency task.
Next, we examined whether cognitive insight was related to frontotemporal function and subjective QOL in patients with schizophrenia. To test this hypothesis, we extracted the integral values of oxy-Hb changes from the three ROIs (region 1 and left and right region 2), where patients with schizophrenia showed reduced VFT-related neural activity compared with healthy controls, and conducted Pearson’s correlations with the cognitive insight and subjective QOL scores (Table 2).
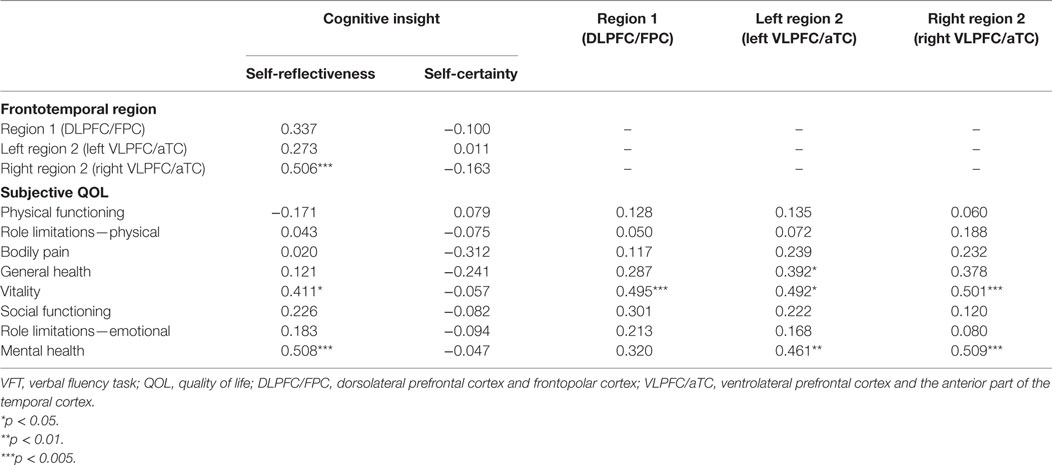
Table 2. Correlations between the VFT-related hemodynamic responses and measures of cognitive insight and subjective QOL in patients with schizophrenia.
In patients with schizophrenia, better cognitive insight, according to the SR subscale of the BCIS was related to a higher subjective QOL (vitality: r = 0.411, FDR-corrected p = 0.013; mental health: r = 0.508, FDR-corrected p = 0.006) and to increased right region 2 VFT-related activity (right VLPFC/aTC; r = 0.506, FDR-corrected p = 0.017). A higher subjective QOL was also related to increased VFT-related activity in region 1 (DLPFC/FPC; vitality: r = 0.495, FDR-corrected p = 0.006), left region 2 (left VLPFC/aTC; vitality: r = 0.492, FDR-corrected p = 0.006; mental health: r = 0.461, FDR-corrected p = 0.013), and right region 2 (right VLPFC/aTC; vitality: r = 0.501, FDR-corrected p = 0.013; mental health: r = 0.509, FDR-corrected p = 0.006), but neither region 1 (r = 0.273, FDR-corrected p > 0.50) nor left region 2 (r = 0.337, FDR-corrected p > 0.05) were related to SR.
However, there were no significant correlations between the SC and other subscale of QOL or all ROIs VFT-related activity (FDR-corrected p > 0.05).
Finally, we determined whether right frontotemporal function mediated the relationship between cognitive insight and subjective QOL. Because the vitality and mental health subscales were found to relate to both right region 2 (right VLPFC/aTC) VFT-related activity and our measure of cognitive insight (SR), to test our third hypothesis that right VLPFC/aTC function mediates the relationship between cognitive insight and subjective QOL, we entered right VLPFC/aTC VFT-related activity, SR, and vitality or mental health scores into a single mediator model. For the mediation analysis to be justified, the predictor, mediator, and outcome variables must all be inter-related (51, 52). Indeed, all four paths were in the predicted direction (Figure 3). Cognitive insight had a positive effect on subjective QOL (vitality: β = 1.456, p = 0.029; mental health: β = 1.757, p = 0.007) and right region 2 VFT-related activity (β = 7.920, p < 0.001), and right region 2 VFT-related activity had a positive effect on subjective QOL (vitality: β = 0.089, p = 0.034; mental health: β = 0.075, p = 0.062). Bootstrap analysis of the indirect effect revealed bias-corrected CIs excluding 0 (vitality: β = 0.71, SE = 0.39, 95% CI = 0.071–1.59; mental health: β = 0.59, SE = 0.33, 95% CI = 0.03–1.37). Importantly, the direct effect of cognitive insight on subjective QOL, after controlling for right VLPFC/aTC function, was no longer significant (vitality: β = 0.75, 95% CI = −0.730 to 2.229; mental health: β = 1.17, 95% CI = −0.259 to 2.589), indicating that right VLPFC/aTC function fully mediated the relationship between cognitive insight in the SR domain and subjective QOL.
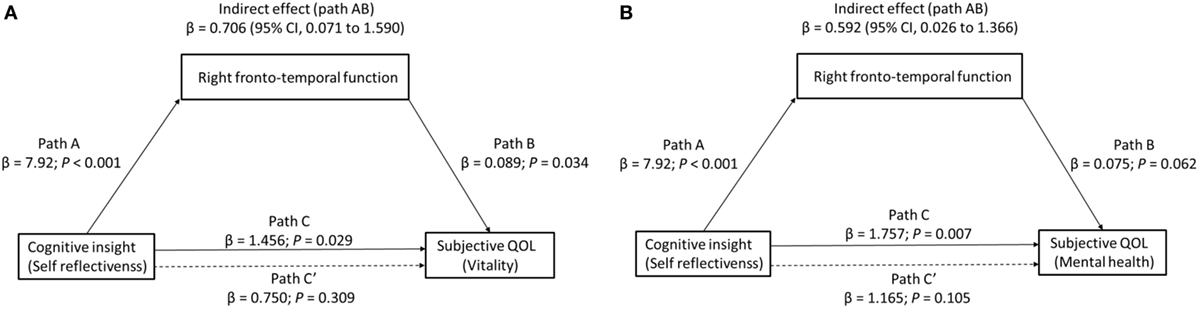
Figure 3. The effect of cognitive insight on subjective quality of life (QOL) through right frontotemporal function. Path C represents the variance in the level of cognitive insight that is associated with the subjective QOL ((A) vitality or (B) mental health) level, and Path C′ represents the association between the level of cognitive insight and subjective QOL after taking into account right frontotemporal function as a mediator. When right frontotemporal function was included in the model, the direct effect of cognitive insight (self-reflectiveness) on subjective QOL (dashed line) (left: vitality, right: mental health) was no longer significant, indicating a fully mediated effect. Path AB is the mediation effect and is significant at p < 0.05 based on confidence intervals from bias-corrected bootstrapping of 5,000 samples.
This study had three main findings. First, we identified regions within our selected frontotemporal area (all ROIs) where participants with schizophrenia had reduced hemodynamic responses compared with healthy controls. This finding is consistent with those of prior studies (16, 22–26) and provides further evidence that patients with schizophrenia exhibit abnormalities in frontotemporal regions. Second, the level of cognitive insight as well as the level of subjective QOL were related to neural activity in the right VLPFC/aTC regions, demonstrating a relationship between the neurobiological characteristics of schizophrenia and cognitive insight and subjective QOL. Third, VFT-related neural activity in the right VLPFC/aTC regions fully mediated the relationship between cognitive insight and subjective QOL, indicating that the disease-related level of cognitive insight may affect the subjective QOL through abnormalities in frontotemporal function.
Collectively, our findings have implications for understanding the specific role the cognitive insight level (particularly SR) plays in the level of subjective QOL in individuals with schizophrenia. Recent functional (fMRI and NIRS) (16, 53) and structural MRI (54) studies highlighted the relationship between SR and the right ventrolateral PFC. SR is defined as the ability to simultaneously consider various types of information, perspectives, and alternative hypotheses to generate judgments about the self, and this ability utilizes verbal working memory and decision-making processes (16, 54). High levels of SR may encourage patients to doubt their distorted and unrealistic perceptions or thoughts, leading them to have a more-objective attitude toward their illness (55). For instance, Phalen et al. (2) (p. 840) stated that “while engaged in treatment, those patients with higher cognitive insight may be better able to incorporate the feedback of mental health professionals and consider alternative ways of thinking” (56). Trials employing cognitive behavior therapy for psychosis support this theory, as they have consistently found that better cognitive insight is predictive of better responses to psychosocial treatments (2, 27). Moreover, in decision-making, the right ventrolateral PFC plays a role generating alternative perspectives in tasks requiring individuals to respond to a problem that has various potential answers (57, 58). In the context of SR, one’s willingness to admit fallibility and corrigibility and to recognize dysfunctional reasoning may in part depend on the controlled retrieval of information from memory, which is mediated by the ventrolateral PFC (15, 53). In our prior NIRS studies, we identified a relationship between the ventrolateral PFC and other PFC areas and subjective QOL in patients with schizophrenia (17, 18). Here, we found that the right VLPFC/aTC fully mediated the relationship between SR and subjective QOL, implying that this region is critical for both functions. This findings confirm our prior findings, while the results from the mediation analysis additionally demonstrate that right ventrolateral PFC function is one of the mechanisms underlying the relationship between cognitive insight (particularly SR) and subjective QOL.
Although cognitive insight is commonly considered an important factor in schizophrenia (2, 59), how cognitive insight relates to broader outcomes like QOL remains unknown. A well-replicated pattern in schizophrenia research is that higher levels of cognitive insight are often associated with better outcomes [e.g., Ref. (30, 60)]. Our findings imply that higher SR levels may be generally associated with better subjective QOL. However, this association is not necessarily consistent. Some lines of evidence suggest that superior cognitive insight is related to positive outcomes for patents with psychotic disorders (8, 12, 27), while others imply that it is associated with negative outcomes (8, 28, 29). One possible explanation for these equivocal findings is that the effect of cognitive insight on QOL depends on the presence of other variables. Recently, Phalen et al. (2) found that SR had an unmoderated positive relationship with QOL and that the effect of SC on QOL was moderated by symptom severity. The authors suggested that cognitive insight is related to QOL, but that different aspects of cognitive insight may relate to QOL in different ways (2). According to their view, it is likely that symptom severity moderates the effects of cognitive insight on QOL because the flexible perspective shifting abilities associated with better cognitive insight may differ in patients with varying levels of symptom severity (2). For patients whose symptoms are severe and obvious to others, higher SC may serve as a protective factor against the social stigma that may harm the QOL (2). On the other hand, SR may generally be associated with better QOL as noted above. However, the meta-analysis by Palmer et al. (8) supports the alternative view of Kim et al. (4) that SR is negatively associated with the level of subjective QOL. Thus, further studies are needed to clarify the relationship between SR and QOL. Overall, our findings suggest that cognitive insight (SR) is related to subjective QOL; it is possible that SR contributes to improved subjective QOL (vitality, mental health) via the neural activity in the right VLPFC/aTC. Additional studies are necessary to explore other possible mediating and moderating factors and to evaluate the effects that various therapeutic interventions may have on the relationship between cognitive insight and QOL.
The two subscales that showed a positive association with oxy-Hb changes, vitality and mental health, were closely related to mental aspects of QOL, which was similar to the motivation/energy subscale showing a positive relationship with left frontal and temporal gray matter volume in Ubukata et al. (61). Interestingly, executive functioning, a cognitive process involved in VFT, has been reported to show a positive relationship with different aspects of subjective QOL from those found in this study, which are self-evaluation of side-effects and symptoms (62, 63). These findings suggest a possibility that oxy-Hb changes elicited by VFT may reflect the motivation and positive engagement of the task rather than cognitive ability per se (18).
Our findings need to be interpreted within the context of the study limitations. First, multichannel NIRS has limited spatial resolution compared with fMRI (~1 mm). However, a recent MRI and NIRS combination study, which used a method for the probabilistic registration of NIRS data onto the Montreal Neurological Institute coordinate space, suggested that the errors of spatial estimation, expressed as SDs, were approximately 10 mm (40, 64). Second, the relationship among cognitive insight, QOL in the mental aspects and right frontotemporal activities was observed only in patients with schizophrenia, and therefore care must be taken that it cannot be applied generally to other populations.
In conclusion, this study demonstrated that the level of cognitive insight (especially SR) affected the subjective QOL level in patients with schizophrenia owing to abnormalities in VFT-related frontotemporal function. These findings improve our understanding of how the cognitive insight indicators of schizophrenia are related to the clinical and behavioral presentations of the illness.
This study was approved by the ethics committee of the Faculty of Medicine of Tottori University (approval No. 885) and the investigation was conducted in accordance with the latest version of the Declaration of Helsinki. Written informed consent was obtained from each participant after the study procedures had been explained.
SP, KN, and KK designed the study; SP acquired and analyzed the data; SP and KN wrote the first draft of the article; SP, KN, MSI, HO, MKI, IN, and KK contributed to the interpretation of the results and the writing of the manuscript. All authors have approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank all the participants in this study. The authors also thank the Hitachi Medical Corporation for providing technical advice.
This study was supported by the following: Intramural Research Grant for Neurological and Psychiatric Disorders of the NCNP (National Center of Neurology and Psychiatry) (23-10 and 26-3 to KK); Takeda Science Foundation (to SP); Suzuken Memorial Foundation (to SP); JSPS KAKENHI Grant Number JP15K09866 (to KK) and JP17K10330 (to SP).
1. Lysaker PH, Vohs J, Hillis JD, Kukla M, Popolo R, Salvatore G, et al. Poor insight into schizophrenia: contributing factors, consequences and emerging treatment approaches. Expert Rev Neurother (2013) 13:785–93. doi:10.1586/14737175.2013.811150
2. Phalen PL, Viswanadhan K, Lysaker PH, Warman DM. The relationship between cognitive insight and quality of life in schizophrenia spectrum disorders: symptom severity as potential moderator. Psychiatry Res (2015) 230:839–45. doi:10.1016/j.psychres.2015.10.014
3. Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res (2004) 68(2–3):319–29. doi:10.1016/S0920-9964(03)00189-0
4. Kim JH, Lee S, Han AY, Kim K, Lee J. Relationship between cognitive insight and subjective quality of life in outpatients with schizophrenia. Neuropsychiatr Dis Treat (2015) 11:2041–8. doi:10.2147/NDT.S90143
5. Beck AT, Warman DM. Cognitive insight: theory and assessment. 2nd ed. In: Amador X, David A, editors. Insight and Psychosis: Awareness of Illness in Schizophrenia and Related Disorders. New York: Oxford University Press (2004). p. 79–87.
6. Engh JA, Friis S, Birkenaes AB, Jonsdottir H, Klungsoyr O, Ringen PA, et al. Delusions are associated with poor cognitive insight in schizophrenia. Schizophr Bull (2010) 36:830–5. doi:10.1093/schbul/sbn193
7. Colis MJ, Steer RA, Beck AT. Cognitive insight in inpatients with psychotic, bipolar, and major depressive disorders. J Psychopathol Behav Assess (2006) 28:242–9. doi:10.1007/s10862-005-9012-7
8. Palmer EC, Gilleen J, David AS. The relationship between cognitive insight and depression in psychosis and schizophrenia: a review and meta-analysis. Schizophr Res (2015) 166:261–8. doi:10.1016/j.schres.2015.05.032
9. Bora E, Erkan A, Kayahan B, Veznedaroglu B. Cognitive insight and acute psychosis in schizophrenia. Psychiatry Clin Neurosci (2007) 61:634–9. doi:10.1111/j.1440-1819.2007.01731.x
10. Uchida T, Matsumoto K, Kikuchi A, Miyakoshi T, Ito F, Ueno T, et al. Psychometric properties of the Japanese version of the Beck Cognitive Insight Scale: relation of cognitive insight to clinical insight. Psychiatry Clin Neurosci (2009) 63:291–7. doi:10.1111/j.1440-1819.2009.01946.x
11. Favrod J, Zimmermann G, Raffard S, Pomini V, Khazaal Y. The Beck Cognitive Insight Scale in outpatients with psychotic disorders: further evidence from a French-speaking sample. Can J Psychiatry (2008) 53:783–7. doi:10.1177/070674370805301111
12. Riggs SE, Grant PM, Perivoliotis D, Beck AT. Assessment of cognitive insight: a qualitative review. Schizophr Bull (2012) 38:338–50. doi:10.1093/schbul/sbq085
13. O’Connor JA, Wiffen B, Diforti M, Ferraro L, Joseph C, Kolliakou A, et al. Neuropsychological, clinical and cognitive insight predictors of outcome in a first episode psychosis study. Schizophr Res (2013) 149:70–6. doi:10.1016/j.schres.2013.06.005
14. Kuang C, Buchy L, Barbato M, Makowski C, Macmaster FP, Bray S, et al. A pilot study of cognitive insight and structural covariance in first-episode psychosis. Schizophr Res (2017) 179:91–6. doi:10.1016/j.schres.2016.09.036
15. Buchy L, Hawco C, Joober R, Malla A, Lepage M. Cognitive insight in first-episode schizophrenia: further evidence for a role of the ventrolateral prefrontal cortex. Schizophr Res (2015) 166:65–8. doi:10.1016/j.schres.2015.05.009
16. Pu S, Nakagome K, Yamada T, Itakura M, Satake T, Ishida H, et al. Association between cognitive insight and prefrontal function during a cognitive task in schizophrenia: a multichannel near-infrared spectroscopy study. Schizophr Res (2013) 150:81–7. doi:10.1016/j.schres.2013.07.048
17. Pu S, Nakagome K, Yamada T, Yokoyama K, Itakura M, Satake T, et al. Association between subjective well-being and prefrontal function during a cognitive task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr Res (2013) 149:180–5. doi:10.1016/j.schres.2013.06.036
18. Pu S, Nakagome K, Yamada T, Itakura M, Satake T, Ishida H, et al. Association between prefrontal hemodynamic responses during a cognitive task and subjective quality of life in schizophrenia. Schizophr Res (2014) 152:319–21. doi:10.1016/j.schres.2013.11.008
19. Boas DA, Dale AM, Franceschini MA. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage (2004) 23(Suppl 1):S275–88.
20. Strangman G, Boas DA, Sutton JP. Non-invasive neuroimaging using near-infrared light. Biol Psychiatry (2002) 52:679–93. doi:10.1016/S0006-3223(02)01550-0
21. Takizawa R, Fukuda M, Kawasaki S, Kasai K, Mimura M, Pu S, et al. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage (2014) 85:498–507. doi:10.1016/j.neuroimage.2013.05.126
22. Suto T, Fukuda M, Ito M, Uehara T, Mikuni M. Multichannel near-infrared spectroscopy in depression and schizophrenia: cognitive brain activation study. Biol Psychiatry (2004) 55:501–11. doi:10.1016/j.biopsych.2003.09.008
23. Ehlis A-C, Herrmann MJ, Plichta MM, Fallgatter AJ. Cortical activation during two verbal fluency tasks in schizophrenic patients and healthy controls as assessed by multi-channel near-infrared spectroscopy. Psychiatry Res (2007) 156:1–13. doi:10.1016/j.pscychresns.2006.11.007
24. Takizawa R, Kasai K, Kawakubo Y, Marumo K, Kawasaki S, Yamasue H, et al. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr Res (2008) 99:250–62. doi:10.1016/j.schres.2007.10.025
25. Chou P-H, Koike S, Nishimura Y, Satomura Y, Kinoshita A, Takizawa R, et al. Similar age-related decline in cortical activity over frontotemporal regions in schizophrenia: a multichannel near-infrared spectroscopy study. Schizophr Bull (2015) 41:268–79. doi:10.1093/schbul/sbu086
26. Quan W, Wu T, Li Z, Wang Y, Dong W, Lv B. Reduced prefrontal activation during a verbal fluency task in Chinese-speaking patients with schizophrenia as measured by near-infrared spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry (2015) 58:51–8. doi:10.1016/j.pnpbp.2014.12.005
27. Premkumar P, Peters ER, Fannon D, Anilkumar AP, Kuipers E, Kumari V. Coping styles predict responsiveness to cognitive behaviour therapy in psychosis. Psychiatry Res (2011) 187:354–62. doi:10.1016/j.psychres.2010.12.029
28. Granholm E, Mcquaid JR, Mcclure FS, Auslander LA, Perivoliotis D, Pedrelli P, et al. A randomized, controlled trial of cognitive behavioral social skills training for middle-aged and older outpatients with chronic schizophrenia. Am J Psychiatry (2005) 162:520–9. doi:10.1176/appi.ajp.162.3.520
29. Mak WW, Wu CF. Cognitive insight and causal attribution in the development of self-stigma among individuals with schizophrenia. Psychiatr Serv (2006) 57:1800–2. doi:10.1176/ps.2006.57.12.1800
30. Weintraub MJ, Weisman de Mamani A. Effects of sub-clinical psychosis and cognitive insight on psychological well-being: a structural equation model. Psychiatry Res (2015) 226:149–55. doi:10.1016/j.psychres.2014.12.039
31. American Psychiatric Association. DSM-IV® Sourcebook. New York: American Psychiatric Association (1994).
32. Kay SR, Fiszbein A, Opfer LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull (1987) 13:261. doi:10.1093/schbul/13.2.261
33. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia (1971) 9:97–113. doi:10.1016/0028-3932(71)90067-4
34. Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care (1992) 30(6):473–83. doi:10.1097/00005650-199206000-00002
35. Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 health survey for use in Japan. J Clin Epidemiol (1998) 51:1037–44. doi:10.1016/S0895-4356(98)00096-1
36. Saris-Baglama RN, Dewey CJ, Chisholm GB, Plumb E, King J, Rasicot P, et al. QualityMetric Health Outcomes™ Scoring Software 4.0. (Vol. 2010). Lincoln, RI: QualityMetric Incorporated (2010). 138 p.
37. Pu S, Nakagome K, Yamada T, Yokoyama K, Matsumura H, Mitani H, et al. The relationship between the prefrontal activation during a verbal fluency task and stress-coping style in major depressive disorder: a near-infrared spectroscopy study. J Psychiatr Res (2012) 46(11):1427–34. doi:10.1016/j.jpsychires.2012.08.001
38. Yamashita Y, Maki A, Ito Y, Watanabe E, Mayanagi Y, Koizumi H. Noninvasive near-infrared topography of human brain activity using intensity modulation spectroscopy. Opt Eng (1996) 35:1046–9. doi:10.1117/1.600721
39. Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage (2004) 21:99–111. doi:10.1016/j.neuroimage.2003.08.026
40. Tsuzuki D, Jurcak V, Singh AK, Okamoto M, Watanabe E, Dan I. Virtual spatial registration of stand-alone fNIRS data to MNI space. Neuroimage (2007) 34:1506–18. doi:10.1016/j.neuroimage.2006.10.043
41. Hock C, Villringer K, Müller-Spahn F, Wenzel R, Heekeren H, Schuh-Hofer S, et al. Decrease in parietal cerebral hemoglobin oxygenation during performance of a verbal fluency task in patients with Alzheimer’s disease monitored by means of near-infrared spectroscopy (NIRS) – correlation with simultaneous rCBF-PET measurements. Brain Res (1997) 755:293–303. doi:10.1016/S0006-8993(97)00122-4
42. Okada E, Delpy DT. Near-infrared light propagation in an adult head model. II. Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal. Appl Opt (2003) 42:2915–22. doi:10.1364/AO.42.002915
43. Toronov V, Webb A, Choi JH, Wolf M, Michalos A, Gratton E, et al. Investigation of human brain hemodynamics by simultaneous near-infrared spectroscopy and functional magnetic resonance imaging. Med Phys (2001) 28:521–7. doi:10.1118/1.1354627
44. Ohtani T, Nishimura Y, Takahashi K, Ikeda-Sugita R, Okada N, Okazaki Y. Association between longitudinal changes in prefrontal hemodynamic responses and social adaptation in patients with bipolar disorder and major depressive disorder. J Affect Disord (2015) 176:78–86. doi:10.1016/j.jad.2015.01.042
45. Pu S, Nakagome K, Itakura M, Iwata M, Nagata I, Kaneko K. Association of fronto-temporal function with cognitive ability in schizophrenia. Sci Rep (2017) 7:42858. doi:10.1038/srep42858
46. Hoshi Y, Kobayashi N, Tamura M. Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol (2001) 90:1657–62. doi:10.1152/jappl.2001.90.5.1657
47. Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage (2002) 17:719–31. doi:10.1006/nimg.2002.1227
48. Sato H, Yahata N, Funane T, Takizawa R, Katura T, Atsumori H, et al. A NIRS-fMRI investigation of prefrontal cortex activity during a working memory task. Neuroimage (2013) 83:158–73. doi:10.1016/j.neuroimage.2013.06.043
49. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guilford Press (2013).
50. Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods (2004) 36:717–31. doi:10.3758/BF03206553
52. Tully LM, Lincoln SH, Liyanage-Don N, Hooker CI. Impaired cognitive control mediates the relationship between cortical thickness of the superior frontal gyrus and role functioning in schizophrenia. Schizophr Res (2014) 152(2–3):358–64. doi:10.1016/j.schres.2013.12.005
53. Buchy L, Hawco C, Bodnar M, Izadi S, Dell’Elce J, Messina K, et al. Functional magnetic resonance imaging study of external source memory and its relation to cognitive insight in non-clinical subjects. Psychiatry Clin Neurosci (2014) 68:683–91. doi:10.1111/pcn.12177
54. Orfei MD, Piras F, Macci E, Caltagirone C, Spalletta G. The neuroanatomical correlates of cognitive insight in schizophrenia. Soc Cogn Affect Neurosci (2013) 8:418–23. doi:10.1093/scan/nss016
55. Orfei MD, Piras F, Banaj N, Di Lorenzo G, Siracusano A, Caltagirone C, et al. Unrealistic self-overconfidence in schizophrenia is associated with left presubiculum atrophy and impaired episodic memory. Cortex (2017) 86:132–9. doi:10.1016/j.cortex.2016.10.017
56. De Vos AE, Pijnenborg GH, Aleman A, Van Der Meer L. Implicit and explicit self-related processing in relation to insight in patients with schizophrenia. Cogn Neuropsychiatry (2015) 20:311–29. doi:10.1080/13546805.2015.1040151
57. Goel V, Vartanian O. Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set-shift problems. Cereb Cortex (2005) 15(8):1170–7. doi:10.1093/cercor/bhh217
58. Vartanian O, Goel V. Task constraints modulate activation in right ventral lateral prefrontal cortex. Neuroimage (2005) 27:927–33. doi:10.1016/j.neuroimage.2005.05.016
59. Warman DM, Lysaker PH, Martin JM. Cognitive insight and psychotic disorder: the impact of active delusions. Schizophr Res (2007) 90:325–33. doi:10.1016/j.schres.2006.09.011
60. Sim K, Chan YH, Chua TH, Mahendran R, Chong SA, McGorry P. Physical comorbidity, insight, quality of life and global functioning in first episode schizophrenia: a 24-month, longitudinal outcome study. Schizophr Res (2006) 88(1–3):82–9. doi:10.1016/j.schres.2006.07.004
61. Ubukata S, Miyata J, Yoshizumi M, Uwatoko T, Hirao K, Fujiwara H, et al. Regional gray matter reduction correlates with subjective quality of life in schizophrenia. J Psychiatr Res (2013) 47(4):548–54. doi:10.1016/j.jpsychires.2013.01.002
62. Hwang SS, Lee JY, Cho SJ, Lee DW, Kim YS, Jung HY. The model of the relationships among the predictors of quality of life in chronic stage of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2009) 33(7):1113–8. doi:10.1016/j.pnpbp.2009.06.006
63. Tomida K, Takahashi N, Saito S, Maeno N, Iwamoto K, Yoshida K, et al. Relationship of psychopathological symptoms and cognitive function to subjective quality of life in patients with chronic schizophrenia. Psychiatry Clin Neurosci (2010) 64(1):62–9. doi:10.1111/j.1440-1819.2009.02033.x
Keywords: cognitive insight, near-infrared spectroscopy, verbal fluency task, frontotemporal function, quality of life, schizophrenia
Citation: Pu S, Nakagome K, Itakura M, Ohtachi H, Iwata M, Nagata I and Kaneko K (2018) Right Frontotemporal Cortex Mediates the Relationship between Cognitive Insight and Subjective Quality of Life in Patients with Schizophrenia. Front. Psychiatry 9:16. doi: 10.3389/fpsyt.2018.00016
Received: 22 October 2017; Accepted: 17 January 2018;
Published: 02 February 2018
Edited by:
Kenji Hashimoto, Chiba University, JapanReviewed by:
Jonathan K. Wynn, University of California, Los Angeles, United StatesCopyright: © 2018 Pu, Nakagome, Itakura, Ohtachi, Iwata, Nagata and Kaneko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shenghong Pu, pshh0517@yahoo.co.jp
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.