
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry , 22 December 2017
Sec. Psychopathology
Volume 8 - 2017 | https://doi.org/10.3389/fpsyt.2017.00289
This article is part of the Research Topic Binge Drinking in the Adolescent and Young Brain, volume I View all 22 articles
Alcohol use, particularly binge drinking (BD), is a major public health concern among adolescents. Recent national data show that the gender gap in alcohol use is lessening, and BD among girls is rising. Considering the increase in BD among adolescent girls, as well as females’ increased risk of experiencing more severe biopsychosocial negative effects and consequences from BD, the current review sought to examine gender differences in risk factors for BD. The review highlights gender differences in (1) developmental-related neurobiological vulnerability to BD, (2) psychiatric comorbidity and risk phenotypes for BD, and (3) social-related risk factors for BD among adolescents, as well as considerations for BD prevention and intervention. Most of the information gleaned thus far has come from preclinical research. However, it is expected that, with recent advances in clinical imaging technology, neurobiological effects observed in lower mammals will be confirmed in humans and vice versa. A synthesis of the literature highlights that males and females experience unique neurobiological paths of development, and although there is debate regarding the specific nature of these differences, literature suggests that these differences in turn influence gender differences in psychiatric comorbidity and risk for BD. For one, girls are more susceptible to stress, depression, and other internalizing behaviors and, in turn, these symptoms contribute to their risk for BD. On the other hand, males, given gender differences across the lifespan as well as gender differences in development, are driven by an externalizing phenotype for risk of BD, in part, due to unique paths of neurobiological development that occur across adolescence. With respect to social domains, although social and peer influences are important for both adolescent males and females, there are gender differences. For example, girls may be more sensitive to pressure from peers to fit in and impress others, while male gender role stereotypes regarding BD may be more of a risk factor for boys. Given these unique differences in male and female risk for BD, further research exploring risk factors, as well as tailoring intervention and prevention, is necessary. Although recent research has tailored substance use intervention to target males and females, more literature on gender considerations in treatment for prevention and intervention of BD in particular is warranted.
Binge drinking (BD) is a major public health concern, and adolescents are particularly vulnerable to the biological and social consequences of BD compared to adults (1). Internationally, BD is more prevalent among adolescents aged 15–19 compared to all other adults aged 25 and older (2–6). For example, recent United States national data estimates that 17.7% of high school students (7) and 39% of college students (8) reported BD in the past month, with college students often consuming at least two to three times the definition of BD (9). Rates of BD in Europe and Australia are typically higher than in the U.S. For example, one study of 36 European countries found that 39% of 15- and 16-year-olds reported BD in the past month (10). More importantly, it is well established that an early onset of alcohol use is a strong predictor of future alcohol dependence (11, 12). Significantly, about half of individuals meeting life-time diagnostic criteria for an alcohol use disorder (AUD) do so by the age of 21, with two-thirds meeting criteria by the age of 25 (13–21).
While estimates have traditionally shown higher rates of BD in males, recent national data show that the gender gap in BD is lessening, with a concomitant increase in rates of alcohol use and BD among girls and women (17). In fact, some studies have found that girls are drinking as much, if not more, than their male peers, and girls are also initiating alcohol use earlier and engaging in more binge-like alcohol drinking, while these changes have not been seen among boys in recent decades (7, 17, 22–24). Due to these increasing rates of alcohol initiation and problems among girls, some efforts have been made to create gender-informed interventions and preventions in order to better target adolescent girls (24, 25).
It is also well known that girls are more vulnerable to the negative consequences from alcohol use and BD compared to boys. Across the lifespan, females are more likely to experience alcohol-related health problems at lower drinking rates compared to males, and are also more likely to experience more severe negative alcohol-related health and psychosocial consequences compared to males (26–29). In addition to vulnerability in adolescence, there are also important gender differences in the impact of adolescent BD on later functioning in adulthood. Notably, females are more likely to experience a more rapid and severe progression from BD to addiction, a phenomenon known as “telescoping” (26). Moreover, while boys who stop abusing alcohol after adolescence are similar to men without any history of alcohol abuse (30), girls who stop abusing alcohol after adolescence continue to differ from women without a history of alcohol abuse in areas of illegal drug use, antisocial behavior, and mental health problems (31). Although prevalence rates of AUD are lower in women compared to men, women with AUD are more likely to experience more negative alcohol-related consequences (31).
In the present review, we will first review some of the literature on gender differences in neurobiological risk factors that predispose an adolescent or emerging adult to engage in BD, given developmental differences between males and females (32, 33). We will also review gender differences in alcohol sensitivity as well as differences in reward neurocircuitry and neurobiological processes in learning and memory that explain differences in risk for BD and response to BD. We will then review some of the literature on gender differences in psychiatric comorbidity among adolescents and emerging adults and the association between this comorbidity and BD. This is especially relevant since 60% of substance-using adolescents have a comorbid psychiatric diagnosis (34). Next, we will review the role of gender in social/peer influences during adolescence and emerging adulthood and how this may influence binge-drinking behavior. Lastly, we summarize findings from existing prevention and intervention research on adolescent and emerging adult BD and important gender considerations in prevention and intervention.
An extensive literature search was conducted using MEDLINE/PubMed and Academic Search Premier to identify peer-reviewed publications on adolescent and emerging adult BD published since 2000. There are various definitions for BD across the literature (1, 2, 35) and, thus, we included the literature that defined BD broadly as consuming a large alcohol quantity per drinking occasion (as defined by the WHO, NIAAA, and SAMHSA; 1). For instance, the NIAAA defines BD as consuming at least 4 or 5 (women or men, respectively) drinks in approximately 2 h and achieving a blood alcohol concentration (BAC) of at least 80 mg% (4). In general, all of these definitions include intoxication as a hallmark sign. Thus, we considered literature that defined BD by any of these definitions. We first conducted a broad search using terms for (1) BD, (2) adolescence or emerging adult, and (3) gender/sex to identify all articles that highlighted gender differences in BD. Articles that (1) did not focus on adolescents or emerging adults (age range 13–24); (2) did not consider gender/sex; and (3) did not pertain to BD as defined by either the NIAAA, WHO, or SAMHSA (as described earlier) were excluded. Furthermore, only articles that pertained to risk factors for BD, and not effects or consequences of BD, were selected. We reviewed only articles that reported on gender differences and pertained to (1) social influences, (2) neurobiological and biological aspects of BD risk, (3) psychiatric or mental health symptoms and BD risk, and (4) intervention and prevention for BD (see Figure 1). Annotated bibliographic searches of relevant review articles and/or books were also conducted.
Figure 1. Literature search results. Studies included in this count were specific to all criteria. Given limited studies, discussion also includes relevant studies that pertain to alcohol use and substance use more generally.
Figure 1 presents results from the literature search. The initial search yielded a number of studies that focused on gender differences in adolescent BD regarding differential effects and consequences of BD across males and females [see Ref. (36–39) for reviews]. Furthermore, a number of studies reported on BD prevention and intervention, but few focused on gender differences in BD treatment. Therefore, in the sections that follow, we report on identified literature but also incorporate findings from other studies related to problem alcohol use in order to inform potential gender differences in these areas.
Adolescence is a crucial stage of development during which addiction becomes a prominent public health concern (40–46). In the following section, we review literature on adolescents’ unique vulnerability to BD. We first summarize evidence for the role of alcohol sensitivity and reward neurocircuitry in BD during adolescence and highlight gender differences in these processes. We then review the role of adolescent neurobehavioral development in BD as well as important gender differences in development that differentially influence males’ and females’ risk for BD (see Table 1 for overview of studies and findings).
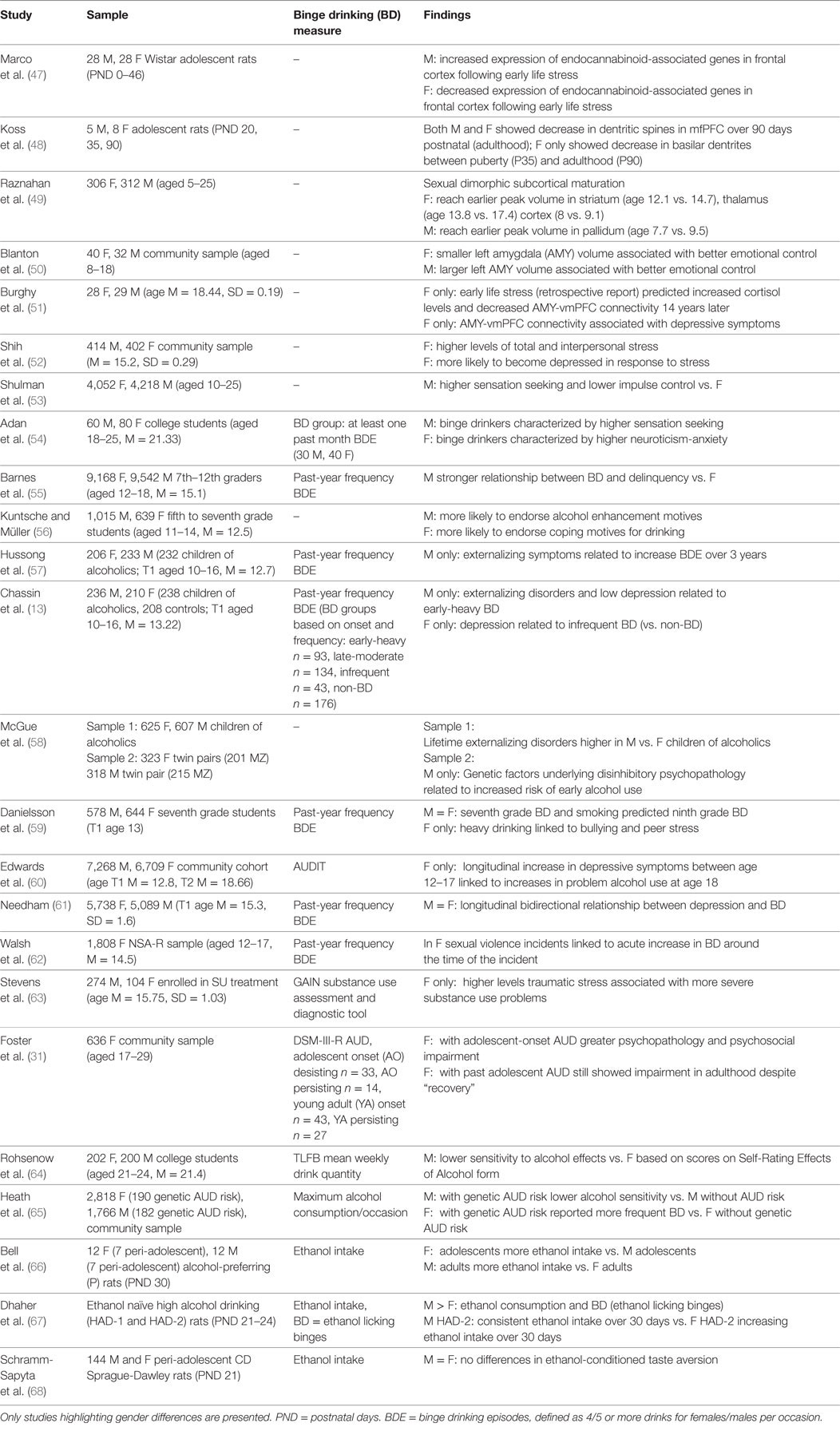
Table 1. Overview of studies on gender differences in alcohol sensitivity, neurobiological development, and risk for internalizing and externalizing disorders.
Basic Research
The fact that binge ethanol drinking occurs mostly in adolescents and emerging adults is due, at least in part, to the fact that individuals are affected bi-phasically by ethanol in an age-dependent manner (66–69). More specifically, adolescents, compared to adults, show greater sensitivity to lower doses of alcohol, which are perceived as positive and rewarding (e.g., behavioral and autonomic activation), and lower sensitivity to higher doses of alcohol, which are perceived as aversive (e.g., motor ataxia) (44, 45, 70–74). It is believed that this bi-phasic sensitivity in turn not only increases adolescents’ risk for BD but also puts adolescent binge drinkers at increased risk for developing alcohol dependence later in adulthood.
One system largely involved in alcohol sensitivity is the mesocorticolimbic system, which is also the reward neurocircuity system (see Figure 2 for explanation of mesocorticolimbic system). Importantly, binge-like alcohol use leads to increases in mesolimbic dopamine and glutamate, which are associated with the development of alcohol dependence (75, 76). For example, animal studies have shown that adolescent binge-like alcohol exposure results in increased ethanol intake and preference later in adulthood as well as a prolonged ethanol-induced increase in mesocorticolimbic dopamine and tolerance to ethanol-induced increases in mesocorticolimbic glutamate during adulthood (77). This finding of altered dopaminergic activity in the adult mesocorticolimbic reward neurocircuit following adolescent binge-like ethanol exposure has been replicated many times (78–80). Furthermore, adolescents also show less sensitivity to withdrawal symptoms following BD, which through negative reinforcement may exacerbate binge-like behavior (81, 82). For example, there is evidence that adolescent binge ethanol exposure followed by protracted withdrawal resulted in a lower ethanol-withdrawal-associated decrease in mesocorticolimbic dopamine than that observed in similarly treated adult rats (83).
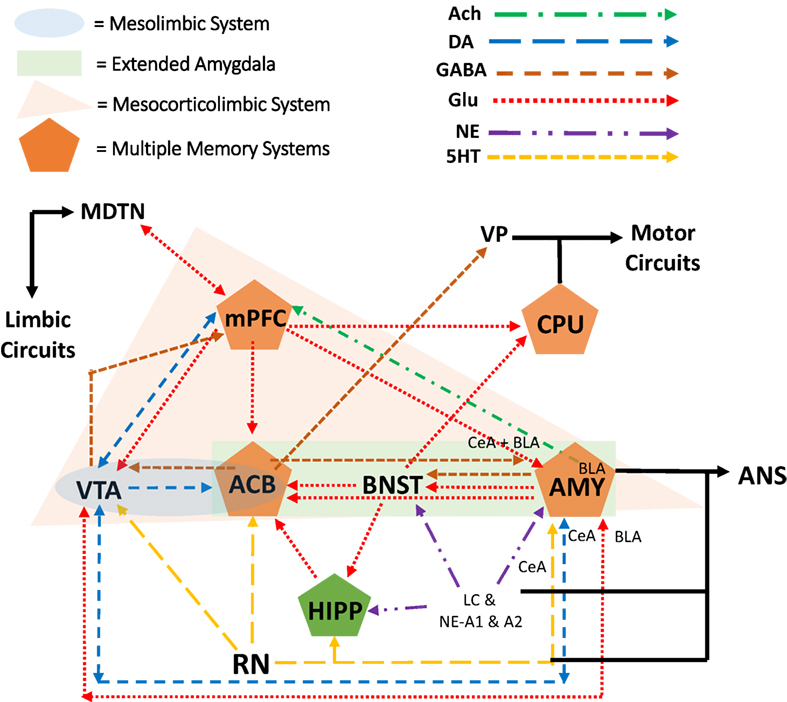
Figure 2. The mesocorticolimbic dopamine reward neurocircuitry mediates orientation toward and acquisition of rewards (e.g., alcohol). At the core of the system are dopamine projections from the ventral tegmental area (VTA), of the midbrain/mesencephalon, to the nucleus accumbens (ACB; i.e., ventral striatum of the limbic circuit). As part of the reward neurocircuit, the nucleus ACB receives dopaminergic projections from the VTA and mediates the intoxicating and euphoric effects of ethanol as well as conditioning of these rewarding effects (i.e., learning and memory). The extended amygdala (AMY) includes nuclei of the AMY, the bed nucleus of the stria terminalis (BNST), and the nucleus ACB shell. The prefrontal cortex (PFC) has reciprocal projections with all of these brain regions while integrating this information with that of other brain regions as well (84, 85). Within the PFC, the medial portions are considered part of the limbic circuit (mPFC). This limbic circuit is associated with the Papez circuit that has been modified to include other brain regions as well (86). Essentially, (a) nuclei of the AMY receive sensory input from the periphery while sending input to the peripheral autonomic nervous system (ANS), (b) the AMY sends and receives information, in part through the stria terminalis, from the septum and hypothalamus, (c) the septum sends and receives information, in part through the fornix, from the hippocampus (HIPP), (d) the HIPP, in turn, sends projections to the hypothalamic mammillary bodies via the fornix, (e) the mammillary bodies, in turn, project to the anterior thalamus and mediodorsal thalamic nucleus, which (f) project to the cingulate gyrus and medial PFC (mPFC), and which (g) project back to the entorhinal cortex and HIPP [for recent discussions on the relationship with addiction see Ref. (70, 87–90); Pariyadath et al. (89); Renteria et al. (90)].
With respect to gender differences, there is evidence for diff-erences in mesocorticolimbic activity, which may lead to differences in binge-like alcohol use. For example, stimulant-induced increases in nucleus accumbens dopamine are lower in female rodents compared with their male counterparts (91–93). Clinically, men show a greater mesolimbic dopamine response than women (27, 94), which in part demonstrates males’ greater sensitivity to the rewarding effects of alcohol. The caudate nucleus, also called the dorsal striatum (caudate–putamen or CPU in rodents, Figure 2), mediates habit learning and perseverative behavior, both of which characterize loss-of-control drinking. Estradiol in the dorsal lateral striatum (lateral portions of the caudate nucleus) mediates, in part, stimulant-induced behavioral responses as well as escalation and reinstatement of drug taking behavior (27). These estradiol effects on stimulant-induced and -taking behavior were seen in ovariectomized female rats, but not male rats (91, 95). Importantly, progesterone treatment can reduce these estrogenic effects in female rats as well as reducing stimulant intake in women, but not men (96–98).
In addition to sensitivity and reward, the mesocorticolimbic system is also involved in learning and memory, which are dynamic processes that influence BD. Animal studies have shown unique sex differences in the neurobiological processes of learning and memory. In a study examining the acquisition of an operant response for sucrose, it was found that both adult and adolescent female rats acquired the response quicker than their male counterparts (99). Moreover, these authors reported that after 1 week of training, adolescent female rats responded more than adolescent male rats, whereas adult female and male rats did not differ in their number of responses or reinforcers. Exercise has been shown to decrease ethanol intake during adolescence and appears to have a greater beneficial effect in adult women vs. men (100, 101). In addition, exercise has been shown to facilitate adult neurogenesis in the parahippocampal region of the brain, which has also been implicated in enhanced learning and is disrupted by drugs of abuse including alcohol (102, 103). Thus, it is interesting to note that voluntary exercise during adolescence reduces ethanol intake and preference to a greater extent in female vs. male high ethanol-consuming C57BL/6J mice (104). This is particularly relevant since the hippocampus (HIPP) is vulnerable to ethanol-associated damage, with evidence that adolescents may be more sensitive to this effect than adults.
As noted above, estradiol activity in the lateral caudate nucleus mediates stimulant-induced and -taking behavior, which can be disrupted by progesterone treatment (27, 91). This is important since the caudate nucleus mediates habit formation and is implicated in later stages of the addiction/dependence cycle. Within the multiple memory systems and mesocorticolimbic reward neurocircuitry, endocannabinoid activity modulates emotion and anxiety as well as learning and memory [see Ref. (105) for their roles in addiction]. Given a role for early life stress in vulnerability for addiction and associated behaviors (discussed later), it is noteworthy that the maternal deprivation model of this disorder leads to increased expression of several endocannabinoid-associated genes in the frontal cortex, but not the HIPP, of male rats; whereas the opposite is seen in female rats (47). Early life stress also affects neuroimmune activity, with this effect implicated in adolescent addiction vulnerability (106, 107). Thus, it is noteworthy that during adolescence female rats display greater microglial activation than their male counterparts, suggesting a more adaptive immune system in females during adolescence (107).
Clinical Research
While much of the research on alcohol sensitivity, reward, and learning has utilized animal models, evidence for adolescents’ greater sensitivity to alcohol has also been shown in human studies. For example, one study examined college seniors over 4 years and found that hangover insensitivity was significantly correlated with intoxication insensitivity and future alcohol problems, even after controlling for demographic variables (64). With respect to gender differences, experimental studies have shown that that males will drink more alcohol when available and also reach higher BAC’s compared to females (108). In humans, adolescent females appear to be more sensitive to the negative effects of alcohol and experience them at lower doses (65), while males may be more sensitive to the rewarding effects. These differences in effects emerge around the time of puberty and, thus, it is hypothesized that hormone-related changes across males and females are in part responsible (108). While this may be a protective factor for adolescent females (109), they are also more likely to progress more rapidly to addiction than males, due to “telescoping” (110). Still, research on gender differences in risk for BD to dependence trajectories specifically is lacking. As we will discuss next, what may be more important are gender differences in neurobiological-related development that may differentially influence trajectories of risk for BD among males and females.
In addition to differences in alcohol sensitivity, reward circuitry, and neurobiological processes of learning and memory, there are also gender differences in development that may differentially influence males’ and females’ risk for BD. For example, females undergo many neurobiological changes earlier than males, and this is in part related to the earlier onset of puberty in females (111, 112). According to the dual systems model, although the striatum matures more quickly than the prefrontal cortex (PFC) in females, it is also suggested that females undergo more extensive maturation in the PFC compared to the striatum in both humans (49, 113) and animals (48, 114). This sex-specific trajectory highlights how females develop greater levels of inhibitory control and lower peak levels of sensation seeking compared to males (53).
In addition to gender differences related to inhibitory control and sensation seeking, the triadic model hypothesizes that there are also gender differences in the development of the amygdala (AMY) as well as differences in connectivity between the PFC and AMY, which influence emotional control (115). In particular, the triadic model posits that development in the AMY and connectivity between the AMY and the PFC may have a greater influence on emotional functioning in females compared to males (33, 50, 51). While, to date, few studies have longitudinally examined the effects of these preclinically assessed neurobiological processes on later risk for BD, we do know that emotion regulation, inhibitory control, and sensation seeking have been linked to BD (54). Thus, these unique neurobiological trajectories in development may manifest as different risk paths to BD among males and females. In the next section, we review literature on the link between psychiatric issues and BD, in particular highlighting distinct risk phenotypes across adolescent males and females.
Adolescence is a vulnerable period for developing psychiatric issues (116), in part due to developmental-related brain changes that occur during adolescence (117, 118). The link between psychiatric disorders and substance use is also well established, and it is estimated that up to 60% of adolescents with substance use disorders also meet criteria for another psychiatric disorder (119). Furthermore, the sex-specific neurobiological changes that occur during adolescent development put males and females at differential risk for internalizing and externalizing disorders. For example, gender differences in the development of the AMY as well as connections between the AMY and PFC may increase females’ vulnerability to anxiety and depression (51), while males’ higher peak levels of sensation seeking and slower development of impulse control leaves them more vulnerable to externalizing symptoms (53). Furthermore, gender differences in neurobiological development that occur during adolescence also lead to gender differences in vulnerability to stress and differences in how males and females respond to stress (120). In animal models, protracted stress leads to depressive-like behaviors in females but not males (121). In humans, interpersonal stress is more closely linked to cortisol stress response and internalizing symptoms in female compared to male adolescents (52). Taken together, this highlights how these differences in development and in turn risk for psychiatric issues may beget unique BD risk profiles for males and females.
The link between externalizing symptoms (including behavioral disinhibition, impulsivity, sensation seeking, and defiant behaviors) and substance use has been well documented in the literature (21, 122). As discussed previously, males consistently exhibit higher levels of sensation seeking and behavioral disinhibition throughout development, while females show greater inhibitory control (53). Thus, this externalizing risk phenotype for substance use appears to be more prominent in adolescent boys compared to girls (21). For example, in a recent study of college students, male binge drinkers were characterized by their higher scores on impulsivity and sensation seeking compared to non-BD males, and this pattern was not seen in females (54). Another study also found stronger associations between delinquency and BD in males only (55). Adolescent males are also more likely to report drinking for positive reinforcing effects as well as sensation and risk seeking (26, 56).
There is also evidence that environmental and genetic origins underlying associations between externalizing symptoms and substance use differ by gender. For example, one study found externalizing symptoms mediated the relationship between problematic alcohol use and parental alcoholism in males, but not in females (57). In another study of children of alcoholics, early BD was related to externalizing disorders in boys, but not in girls (13). In another similar study examining children of alcoholics, genetic factors associated with disinhibition and externalizing symptoms were predictive of early drinking for boys only; for girls in the study, environmental risk factors were more closely linked to alcohol initiation (58). Thus, given adolescent males’ higher levels of sensation seeking and lower inhibitory control, and evidence for males’ unique vulnerability to genetic factors underlying the link between externalizing symptoms and BD, it is not surprising that this externalizing risk phenotype for BD and other problem alcohol use is more prominent in males.
Internalizing symptoms, including depression and anxiety, have also been linked to BD (54). As explained above, there are important gender differences in risk for developing internalizing disorders that occur with pubertal development, with girls being twice as likely to develop anxiety and depression compared to boys (117). In addition to the higher rates of internalizing disorders in females, females are also more vulnerable to stress compared to boys (120). Even in animal studies, adolescent female rats exhibited depressive-like behavior following stress, while male rats did not experience depressive-like symptoms (52).
As such, in contrast to males, females’ substance use risk profile is better characterized by internalizing symptoms, such as anxiety, depression, stress vulnerability, and other negative mood symptoms. For example, among a study of college students, female binge drinkers were characterized by higher scores on neuroticism-anxiety compared to non-BD females, while male binge drinkers were better categorized by traits related to sensation seeking and impulse control (54). In addition to females’ heightened vulnerability to stress, females are even more likely to engage in BD in response to stress (123). For example, one Swedish study found that peer bullying and other risk factors had a greater effect on drinking in females than in males (59). Similarly, adolescent girls who abuse alcohol are more likely to have experienced a high level of stressful life events and exhibit post-traumatic stress symptoms, but this is not seen in boys (26).
Females’ higher vulnerability for internalizing disorders also increases their risk for addiction, in part, due to self-medicating tendencies (28). For example, one study found that among females only, greater increases in depression symptoms were also linked to greater increases in problem alcohol use and BD over time (60). Similarly, other studies have shown longitudinal bidirectional relationships between BD and depressive symptoms across adolescence that are particularly strong for females (61). Moreover, among females who began drinking in adolescence, those who continued drinking in adulthood showed high levels of depression during adolescence relative to those who stopped abusing alcohol (31). In addition to females’ greater vulnerability to stress and internalizing symptoms, females may also be more prone to BD following trauma.
Exposure to potentially traumatic events—such as physical assault or abuse, sexual assault or abuse, and witnessed violence in the home or community—is common in adolescence, with approximately two-thirds of youth reporting exposure to one or more events (124, 125). Trauma exposure has been linked to increased risk of BD and problematic alcohol use, with evidence indicating higher rates of BD among adolescents exposed to childhood maltreatment (126) and greater risk for problematic alcohol use among adolescents exposed to assault and other forms of violence (127). In addition, adolescents exposed to multiple types of victimization are more likely to experience more alcohol abuse (128) than peers who experience fewer victimization types. Hazardous drinking can also increase risk for future trauma and victimization (129, 130). BD also can co-occur with traumatic experiences, in particular, sexual victimization (i.e., drug/alcohol-facilitated and incapacitated sexual assault).
While both male and female adolescents experience sexual victimization, adolescent girls are at heightened vulnerability to sexual assault (131). A recent study of adolescent girls aged 12–17 found that girls who reported drug/alcohol-facilitated and incapacitated sexual assault were more likely to report past-year alcohol abuse than girls with other types of assault or no assault (132). Similarly, sexual victimization predicted acute increases in BD in a national sample of adolescent girls, although victimization did not predict overall escalation of BD over time (62).
Evidence suggests that girls may also be more likely to engage in BD and experience more negative psychological sequelae as a result of trauma experience compared to boys (63). There is evidence that child abuse and neglect predicts later problem drinking for girls, but not boys (133), and that girls (but not boys) who start abusing alcohol during adolescence are more likely to have experienced early traumatic stress (31). Taken together, in addition to females’ increased vulnerability to stress and increased likelihood of BD in response to acute stress, females are also more vulnerable to binge drink in response to more prolonged stress as a result of trauma.
Taken together, this recent research suggests that male and female adolescents exhibit unique BD risk phenotypes: while boys exhibit the traditional externalizing risk phenotype, girls’ risk phenotype is characterized by “internalizing” symptoms, such as high stress reactivity, and the presence of mood disorders and internalizing symptoms (54). These gender differences are related to gender differences in adolescent neural development and are also consistent with findings that adolescent males drink to enhance positive mood states while females drink to avoid negative mood states (134). These findings also highlight the importance of considering trauma in BD, and furthermore, gender differences in males and females’ vulnerability to BD following trauma and stress. Given girls’ increased vulnerability to stress and higher stress reactivity, it is not surprising that the link between trauma exposure and BD is particularly salient in girls.
From a social and environmental perspective, across many cultures, adolescence is considered a period of self-exploration and experimentation when individuals start to gain more independence and autonomy from adult caregivers (Table 2). First, this increased autonomy—in combination with developmental-related changes in reward-seeking and decision-making (43–45, 112)—puts adolescents in a vulnerable position of experimentation with less supervision which can result in risky behaviors, such as BD. Second, peer relationships become more important and social influences are prominent during adolescence (135), and are also a key risk factor for alcohol use (136). A number of social-related influences, including social norms, peer pressure, and peer affiliation, have all been shown to influence BD and other alcohol use behaviors (137–139).
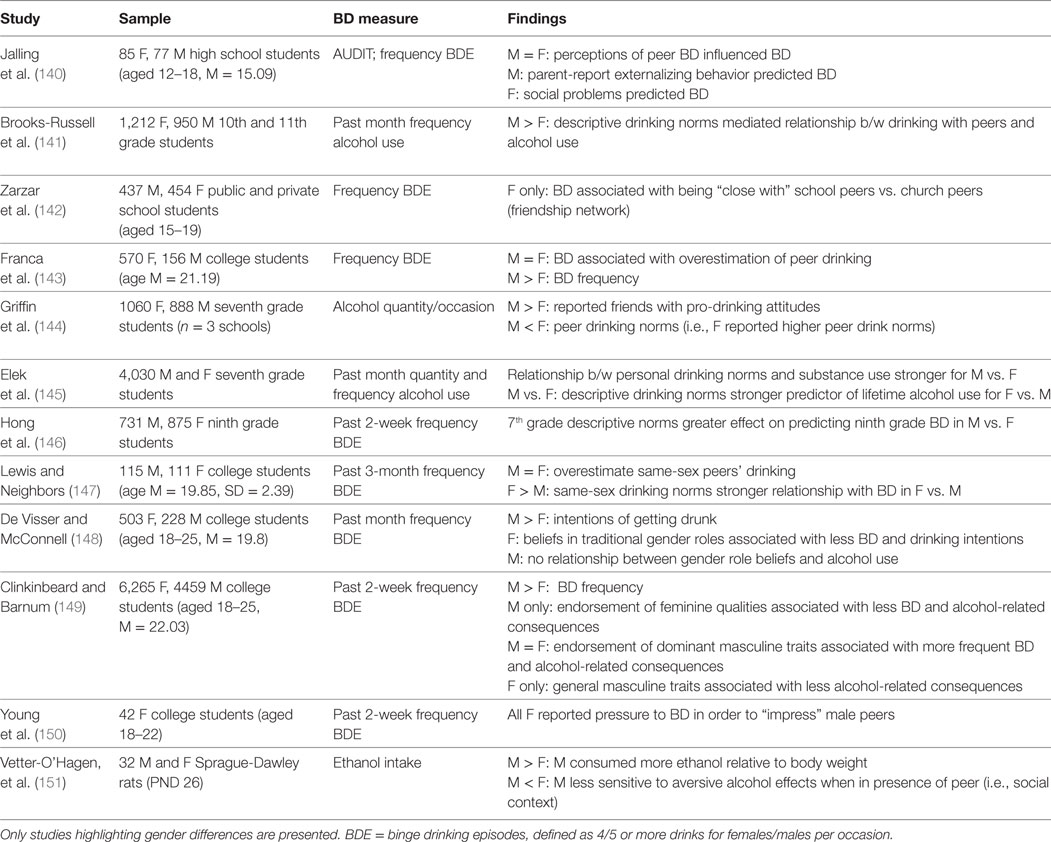
Table 2. Overview of studies highlighting gender differences in social influences on binge drinking (BD) among adolescents.
With respect to gender differences on the impact of social influence on behavior, there is even a link between sex-specific brain development and social behavior (109). For one, there is some evidence that girls are more sensitive and vulnerable to social influences, such as peer pressure and peer affiliation compared to boys (24). Association with other drinking peers is particularly influential on BD (140), and some studies have found that drinking peers are a greater risk for BD among females compared to males (59, 141). For example, one study of Brazilian high school students found that peer affiliation was more closely related to BD for girls compared to boys; more specifically, girls who reported being closer to school-based friends vs. family or church friends were more likely to binge drink, and this relationship was not seen among males (142). Furthermore, some studies have shown that adolescent girls are more likely to report drinking in order to obtain peer approval compared to boys (24).
Social norms regarding drinking, or rather, individuals’ perceptions of peers and others’ BD, also influence one’s own drinking behavior. Descriptive norms refer to beliefs about the prevalence of BD among peers while injunctive norms pertain to the perceived social pressure to conform and engage in BD with other peers (137). Social drinking norms are largely dependent on cultural context, and although the majority of studies have examined social drinking norms using US college samples (137), there are some studies that have examined this phenomenon in other areas across Europe (18, 143, 152, 153). There is a pattern across findings that boys are more likely to endorse more permissive or pro-drinking norms (injunctive norms) and perceive higher prevalence rates of BD (descriptive norms) compared to girls (137, 144). However, findings are mixed as to the influence of social norms on actual BD, with some evidence that girls are more influenced by social norms compared to boys (145), and other evidence that social norms are more influential on boys’ BD (146). Thus, further research regarding differential influences of peer norms on BD is warranted.
Along the same lines as drinking norms, gender norms and gender stereotypes are also important to consider in BD (147, 154, 155). Across cultures, there is a double standard for drinking, such that among males, BD is considered more socially acceptable and masculine, while females are often more likely to be judged negatively for BD, as it is seen as less feminine (148, 156). Thus in this way, gender stereotypes may reinforce and perpetuate BD for males (148, 156), while for females, the negative outlook of BD may be a protective factor against BD (149, 156). Still, another study of college females found that females who engaged in more frequent BD did so as a means to feel more equal to their male peers and as a way to impress their male peers (150). Thus, this largely depends on one’s identification with gender roles as well as their motives for BD.
Animal literature has also shown sex differences in adolescent social drinking behavior. Among a study of adolescent Sprague-Dawley rats, adolescent males consumed more ethanol than females when they were in the presence of other peers, and furthermore, males were less sensitive to ethanol’s aversive properties when in the presence of a peer (151). Similarly, in another study of adolescent rats, males consumed more ethanol when in social situations compared to when alone, while females consumed more ethanol when alone; however, there were differential effects across females based on social anxiety-like behavior (157). Female rats with high levels of social anxiety-like behavior had higher ethanol intake in social vs. isolated situations. These findings from animal models (44, 70, 78, 158) suggest that social situations and influences may be more influential on males’ BD behavior. Taken together, these differences in social influences are likely to influence drinking behavior and, therefore, should be addressed in prevention and intervention, and in fact recent research has focused on gender-specific interventions for girls that are based on social learning theory, which is discussed below (see section below; see also sections for sex differences in neurobiological processes of learning and memory).
While there is extensive literature on treating problem alcohol use and AUDs in adults and adolescents, less research has focused on the importance of treating BD in adolescents. One issue is that due to a lack of discrepancy in the literature over BD vs. other alcohol-related problems, treatment literature often does not differentiate target populations, which is important since binge drinkers are a unique typology (1). Psychosocial interventions are recommended as the first-line treatment for alcohol and substance use disorders more generally (34), and cognitive-behavioral skills training and motivational enhancement therapy are the recommended evidence-based strategies [as the most promising evidence-based strategies to target problem drinking (159)]. A few recent literature reviews have summarized existing evidence of the effectiveness of randomized controlled trials of these treatments for binge and other problem drinking for adolescents and college students [see Ref. (160–164) for reviews]. Briefly, interventions that incorporate skills-building, motivational, and personalized normative feedback components have been successful in reducing BD and other problem alcohol use. One limitation noted in these literature reviews and based on the present literature search is the lack of studies’ reports of findings across gender. Thus, in the next sections, we highlight gender considerations in BD intervention and prevention based on findings from the literature discussed previously and other important findings for gender considerations in substance use treatment more broadly (see Table 3 for overview of studies and findings).
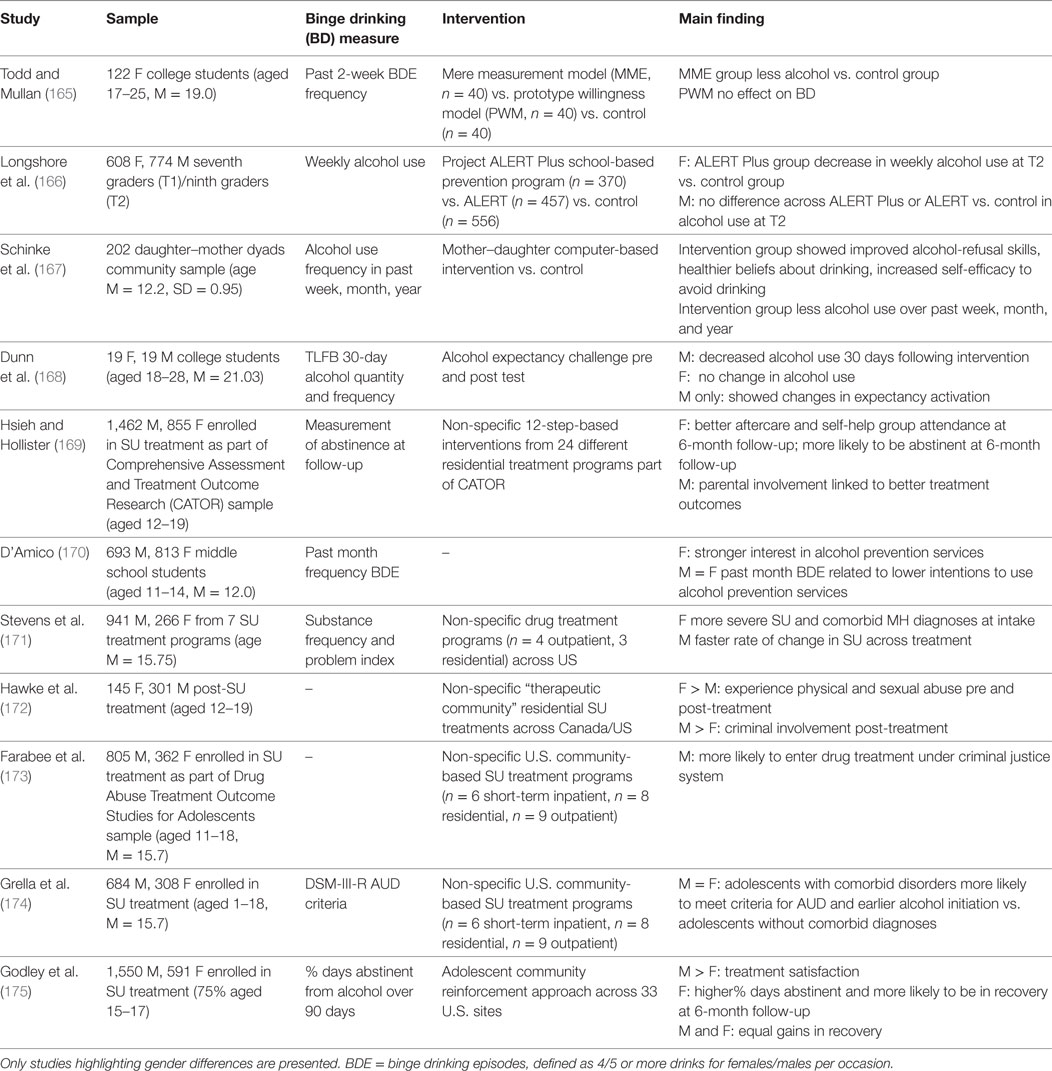
Table 3. Overview of studies highlighting gender differences in substance use prevention and intervention.
Despite advances in tailoring treatments to address comorbid psychiatric and substance use issues (34), less research has focused on developing gender-specific treatment approaches or identifying gender differences in evidence-based treatments for substance use (176, 177). Over the past few decades, there have been efforts to develop gender-specific treatment programs and focus on issues among adult women; however, less research has focused on adolescent girls in particular (177, 178). Furthermore, many studies do not assess for or report on gender differences in treatment effectiveness (177, 178), and as noted previously, few studies focus intervention for BD specifically. Thus, in the following section, we highlight findings from literature on adolescent substance use treatment more broadly and discuss the potential utility of these findings to inform treatment considerations for BD.
There have been some substance use programs developed to target female adolescents in particular, and these gender-specific programs have been based on social learning and behavior theories (165, 177), which is consistent with the previous discussion that adolescent girls may be more vulnerable to social influences on BD (24). For example, programs focused on social skills training, including teaching assertiveness skills and refusal skills to combat peer pressure, how to develop positive peer networks, and challenge perceptions of the prevalence of alcohol use among peers (e.g., “everyone’s doing it”), are most effective in reducing adolescent girls’ problem alcohol use (166, 177). Furthermore, small group settings may be particularly beneficial for girls, as girls may benefit more from sharing experiences and expressing opinions with others (179). In addition, given girls’ proneness to internalizing symptoms and heightened sensitivity to stress, programs focusing on teaching coping skills and stress and tension reduction techniques may be particularly beneficial (167). For example, the coping skills component of CBT-based treatments are likely particularly beneficial for girls as this may help them to learn healthy and adaptive coping skills to manage stress, negative mood, and other internalizing symptoms that trigger BD.
For boys, given their externalizing risk phenotype, they may benefit more from contingency management techniques that reinforce and reward prosocial behaviors as well as expectancy challenge techniques that challenge their beliefs about the positive effects of drinking (168). Furthermore, the personalized feedback component of MET may be particularly beneficial for boys, given that adolescent boys may be more likely to be “in competition” with or trying to keep up with male peers, given that heavy drinking is seen as more socially acceptable for males and sometimes encouraged, such as in college settings (147). Adolescent boys are more prone to overestimate their peers’ drinking and, thus, challenging these perceptions, such as using personalized feedback, could influence males’ behavior (155). In addition, although gender differences in medication treatment effectiveness among adolescents are unknown (180), among adults, men have better treatment outcomes to pharmacologic treatment for alcohol use than women (181–183). Thus, if these gender differences are similar in adolescents, adolescent males may particularly benefit from pharmacological treatment compared to females.
With respect to gender and parental involvement in treatment, findings are mixed, with some evidence showing more effectiveness of parent involvement in treatment for girls (167) and others showing more effectiveness in boys (169). Among adolescents in residential treatment for substance use, parental involvement in treatment had a significant effect on abstinence at 6-month post-treatment status among boys only; however, treatment characteristics were unknown (169). It may be that the type of parental involvement and family support targeted in treatment should be gender-specific. For example, addressing discipline and rewarding and reinforcing prosocial behaviors may be important for boys given their externalizing risk profile, while for girls, better communication and emotional understanding and support might better target their internalizing risk profile.
There are also important gender differences in treatment seeking. In 2008, only 30% of adolescents who sought substance use treatment were girls (35); however, one study found that girls reported higher intentions to seek treatment for alcohol-related problems (170). One reason for this could be related to treatment referrals. Among adolescents, many substance use treatment referrals come from the juvenile justice system (171). Importantly, boys are more likely to get referred for substance use treatment due to a legal issue (171, 172) or to enter treatment under criminal justice supervision (171, 173). Thus, girls are often not identified as early as boys for needing treatment since the criminal justice system is more likely to identify boys. Girls may be more likely to get referred for treatment or identified from another issue in which BD may be secondary. For example, females seeking substance use treatment in general are twice as likely to be diagnosed with depression (174). Still, girls entering treatment have more severe alcohol problems and higher rates of mental health problems, sexual abuse (171), general health problems (63) and family-related stress (169), while males have more school and legal problems [see Ref. (175) for discussion]. Therefore, girls may also have more severe problems before being identified for treatment which could be detrimental to treatment success. In addition, while not studied in adolescents, among adults, women are less likely to seek treatment due to social stigma, and thus, girls may be less likely to seek treatment due to social stigma as well (28). Due to these differences, further work may need to be done to train and educate health care providers to more effectively screen for and identify BD and other substance use problems in adolescents (184).
There have been mixed findings on treatment outcomes for alcohol and substance use treatment among adolescents. There is some evidence that boys were more likely to become non-drinkers compared to girls following non-specific alcohol use treatment (185), but another study found that girls were more likely to become non-drinkers compared to boys (171). However, one limitation is that these results are based on non-specific treatment across multiple treatment sites (171), thus limiting understanding of specific factors influencing results. One study using data from multiple treatment sites implementing adolescent community reinforcement approach (175) showed similar change rates in substance use problems across boys and girls in treatment but unique course of treatment. Specifically, boys showed quicker improvement in mental health symptoms while girls had more abstinent days from alcohol and were more likely to be in recovery at 6-month follow-up (175). There is also evidence that girls are more likely to utilize social resources and attend after-care and self-help groups such as Alcoholics Anonymous (186), which may lead to better long-term treatment outcomes (169). One consistent finding is that across all adolescents, peer affiliation, school engagement, and parental supervision influence successful treatment in changing adolescents in treatment from binge drinkers to non-binge drinkers (175). Taken together, these mixed findings emphasize the need for further research to determine treatment components that contribute to potential gender differences in outcomes (24, 25). Furthermore, these findings do not address BD in particular, and thus, it is unknown whether these considerations also apply in BD treatment.
Based on the literature review, it is clear that adolescents are a unique, vulnerable population at risk for BD, and that there are important gender differences to consider in treatment. While literature on risk factors and consequences of BD in particular has increased, there is still a gap in the literature on unique considerations in prevention and intervention techniques for BD, as well as in how to effectively target unique differences in psychiatric comorbidity and risks across girls and boy in treatment.
The review sought to highlight gender differences in risk for BD, focusing on gender differences in (1) adolescent neurobiological development, (2) psychiatric symptoms and the relationship between psychiatric disorders and BD, and (3) social-related risk factors in BD, as well as considerations of these gender differences in BD prevention and intervention. The literature highlights unique vulnerabilities for BD among girls and boys. Developmentally, there are unique risks among boys and girls in relation to BD due to differences in rates of neurobiological changes as well as gender differences in alcohol sensitivity that influence risk for BD. Furthermore, many of these sex-specific neurobiological changes that occur during adolescence also influence differential risk for psychiatric issues among males and females which also influence risk for BD. Notably, while males may be more drawn to BD due to higher levels of sensation seeking and lower inhibitory control, females may be more prone to BD due to their heightened stress reactivity and vulnerability to internalizing symptoms. With respect to social development in adolescence, while development of peer relationships is important for both girls and boys during this developmental period, adolescent girls in particular may be more vulnerable to BD due to social influences. For boys, while peer influence may not be as strong, boys may be at greater risk for BD due to the social gender role norms that it is more socially acceptable and even can be rewarding for boys to drink in excess. These social norms may in turn actually serve as a protective factor in girls as BD does not necessarily align with the feminine stereotype.
These differential risk factors in turn provide important considerations for targeting BD intervention and prevention for females and males. Females may benefit from intervention and prevention that focuses on coping skills training and stress reduction, while males may benefit more from impulse control training and engagement in prosocial activities that fulfill the need for sensation seeking. Regarding social risk factors, while both male and female adolescents would benefit from social skills training, challenging social norms may be more effective for boys while assertiveness skills may be more effective for girls in preventing BD.
This systematic review highlights two important areas that are in need of further consideration in the literature. The first area is in regard to the necessity of further research on gender-specific risk factors for BD in order to better target at-risk adolescents and also inform prevention for BD. Extensive literature has identified gender differences in the effects of BD on biopsychosocial functioning in adolescents; however, less research has identified risk factors for BD.
There is also extensive literature on theories of adolescent neurobiological development that explain adolescents’ heightened risk for engaging in risk-taking and substance use more generally; however, literature on risk for BD vs. other substance use is lacking. Given evidence that BD is a unique alcohol use typology, more research understanding different mechanisms in the risk process for BD vs. other problem alcohol use vs. other substance use is warranted. Furthermore, given that BD is a hazardous, yet prevalent, developmental phenomenon, more research is needed to better target adolescents that are at risk of developing more severe alcohol use or substance use problems. For example, literature has highlighted the phenomenon of telescoping in women; however, more research on adolescent females and BD is needed.
The second area is the necessity of further research on gender differences in treating and preventing BD among adolescents. For one, many of the randomized controlled trials of BD interventions have focused on college populations, which are a unique group. More research on other adolescent samples, such as younger adolescents, as well as non-college older adolescents, is needed. More importantly, few studies report treatment effects by gender, thus, it is unknown whether there are gender differences in the effectiveness of BD treatment or whether there are gender differences in treatment course or outcomes. Given the increase in BD among adolescent females, as well as the more deleterious effects of alcohol on females, more research in this area is warranted.
AD: manuscript concept, writing content across all sections, editing all sections, and references. RB: writing content for introduction, biological developmental risk section, and editing content. ZA: writing content for trauma and binge drinking section, and intervention section. LH: manuscript concept, writing content for biological developmental risk section, editing content across all sections.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported in part by grant AA013522 awarded to RB by the National Institute on Alcoholism and Alcohol Abuse and by grant K23DA038257 awarded to ZA by the National Institute on Drug Abuse.
1. Rolland B, Naassila M. Binge drinking: current diagnostic and therapeutic issues. CNS Drugs (2017) 31(3):181–6. doi:10.1007/s40263-017-0413-4
2. Courtney KE, Polich J. Binge drinking in young adults: data, definitions, and determinants. Psychol Bull (2009) 135(1):142. doi:10.1037/a0014414
3. Marczinski CA, Grant EC, Grant VJ. Binge Drinking in Adolescents and College Students. New York: Nova Science (2009).
4. Martinic M, Measham F, editors. Swimming with Crocodiles: The Culture of Extreme Drinking. New York: Routledge (2008).
5. Plant M, Plant M. Binge Britain: Alcohol and the National Response. New York: Oxford University Press (2006).
6. World Health Organization. Management of Substance Abuse Unit. Global Status Report on Alcohol and Health, 2014. Luxemberg: World Health Organization (2014).
7. Substance Abuse and Mental Health Services Administration. Substance Abuse Treatment: Addressing the Specific Needs of Women. Knowledge Application Program (Keys) for Clinicians. Rockville: U.S. Department of Health and Human Services (2014).
8. Grunbaum JA, Kann L, Kinchen S, Ross J, Hawkins J, Lowry R, et al. Youth risk behavior surveillance – United States, 2003. MMWR Surveill Summ (2004) 53(2):1–96.
9. White AM, Kraus CL, Swartzwelder HS. Many college freshmen drink at levels far beyond the binge threshold. Alcohol Clin Exp Res (2006) 30(6):1006–10. doi:10.1111/j.1530-0277.2006.00122.x
10. Hibell B, Guttormsson U, Ahlström S, Balakireva O, Bjarnason T, Kokkevi A, et al. The 2011 ESPAD Report: Substance Use among Students in 36 European Countries. Stockholm: ESPAD (2012).
11. Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse (1997) 9:103–10. doi:10.1016/S0899-3289(97)90009-2
12. Rossow I, Kuntsche E. Early onset of drinking and risk of heavy drinking in young adulthood – a 13-year prospective study. Alcohol Clin Exp Res (2013) 37(s1):E297–304. doi:10.1111/j.1530-0277.2012.01924.x
13. Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: predictors and substance abuse outcomes. J Consult Clin Psychol (2002) 70(1):67. doi:10.1037/0022-006X.70.1.67
14. Dawson DA, Grant BF, Stinson FS, Chou PS. Another look at heavy episodic drinking and alcohol use disorders among college and noncollege youth. J Stud Alcohol (2004) 65(4):477–88. doi:10.15288/jsa.2004.65.47
15. Gmel G, Kuntsche E, Rehm J. Risky single-occasion drinking: bingeing is not bingeing. Addiction (2011) 106(6):1037–45. doi:10.1111/j.1360-0443.2010.03167.x
16. Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med (2006) 160(7):739–46. doi:10.1001/archpedi.160.7.739
17. Johnston LD, O’Malley PM, Bachman JG. National Survey Results on Drug Use from the Monitoring the Future Study. Bethesda: National Institute on Drug Abuse (1999).
18. Kuntsche E, Rehm J, Gmel G. Characteristics of binge drinkers in Europe. Soc Sci Med (2004) 59(1):113–27. doi:10.1016/j.socscimed.2003.10.009
19. National Institute on Alcohol and Alcohol Abuse. Underage Drinking. Rockville: NIAAA Fact Sheet (2012).
20. Viner RM, Taylor B. Adult outcomes of binge drinking in adolescence: findings from a UK national birth cohort. J Epidemiol Community Health (2007) 61(10):902–7. doi:10.1136/jech.2005.038117
21. Zucker RA. Anticipating problem alcohol use developmentally from childhood into middle adulthood: what have we learned? Addiction (2008) 103(s1):100–8. doi:10.1111/j.1360-0443.2008.02179.x
22. Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend (2008) 93(1):21–9. doi:10.1016/j.drugalcdep.2007.08.017
23. Keyes KM, Martins SS, Blanco C, Hasin DS. Telescoping and gender differences in alcohol dependence: new evidence from two national surveys. Am J Psychiatry (2010) 167(8):969–76. doi:10.1176/appi.ajp.2009.09081161
24. Amaro H, Blake SM, Schwartz PM, Flinchbaugh LJ. Developing theory-based substance abuse prevention programs for young adolescent girls. J Early Adolesc (2001) 21(3):256–93. doi:10.1177/0272431601021003002
25. Grella CE. From generic to gender-responsive treatment: changes in social policies, treatment services, and outcomes of women in substance abuse treatment. J Psychoactive Drugs (2008) 40(sup5):327–43. doi:10.1080/02791072.2008.10400661
26. Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ (2012) 3(1):14. doi:10.1186/2042-6410-3-14
27. Becker JB, Koob GF. Sex differences in animal models: focus on addiction. Pharmacol Rev (2016) 68(2):242. doi:10.1124/pr.115.011163
28. Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev (2004) 24(8):981–1010. doi:10.1016/j.cpr.2004.08.003
29. Nolen-Hoeksema S, Hilt L. Possible contributors to the gender differences in alcohol use and problems. J Gen Psychol (2006) 133(4):357–74. doi:10.3200/GENP.133.4.357-374
30. Hicks BM, Iacono WG, McGue M. Consequences of an adolescent onset and persistent course of alcohol dependence in men: adolescent risk factors and adult outcomes. Alcohol Clin Exp Res (2010) 34(5):819–33. doi:10.1111/j.1530-0277.2010.01154.x
31. Foster KT, Hicks BM, Iacono WG, McGue M. Alcohol use disorder in women: risks and consequences of an adolescent onset and persistent course. Psychol Addict Behav (2014) 28(2):322. doi:10.1037/a0035488
32. Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev (2011) 35(8):1687–703. doi:10.1016/j.neubiorev.2011.04.013
33. Hammerslag LR, Gulley JM. Sex differences in behavior and neural development and their role in adolescent vulnerability to substance use. Behav Brain Res (2016) 298:15–26. doi:10.1016/j.bbr.2015.04.008
34. Robinson ZD, Riggs PD. Co-occurring psychiatric and substance use disorders. Child Adolesc Psychiatr Clin (2016) 25(4):713–22. doi:10.1016/j.chc.2016.05.005
35. US Department of Health and Human Services. NIAAA Council Approves Definition of Binge Drinking. (Vol. 3). Rockville: NIAAA Newsletter (2004).
36. Carbia C, Cadaveira F, Caamaño-Isorna F, Holguín SR, Corral M. Binge drinking trajectory and decision-making during late adolescence: gender and developmental differences. Front Psychol (2017) 8:783. doi:10.3389/fpsyg.2017.00783
37. Montgomery C, Fisk JE, Murphy PN, Ryland I, Hilton J. The effects of heavy social drinking on executive function: a systematic review and meta-analytic study of existing literature and new empirical findings. Hum Psychopharmacol Clin Exp (2012) 27(2):187–99. doi:10.1002/hup.1268
38. López-Caneda E, Holguín SR, Corral M, Doallo S, Cadaveira F. Evolution of the binge drinking pattern in college students: neurophysiological correlates. Alcohol (2014) 48(5):407–18. doi:10.1016/j.alcohol.2014.01.009
39. Petit G, Maurage P, Kornreich C, Verbanck P, Campanella S. Binge drinking in adolescents: a review of neurophysiological and neuroimaging research. Alcohol Alcohol (2013) 49(2):198–206. doi:10.1093/alcalc/agt172
40. Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav (2007) 86(2):189–99. doi:10.1016/j.pbb.2006.12.001
41. Essau CA, editor. Adolescent Addiction: Epidemiology, Assessment, and Treatment. Oxford: Academic Press (2008).
42. Masten AS, Faden VB, Zucker RA, Spear LP. Underage drinking: a developmental framework. Pediatrics (2008) 121(Suppl 4):S235–51. doi:10.1542/peds.2007-2243A
43. Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev (2000) 24(4):417–63. doi:10.1016/S0149-7634(00)00014-2
45. Spear LP. Adolescent neurodevelopment. J Adolesc Health (2013) 52(2):S7–13. doi:10.1016/j.jadohealth.2012.05.006
46. Sussman S, Arnett JJ. Emerging adulthood: developmental period facilitative of the addictions. Eval Health Prof (2014) 37(2):147–55. doi:10.1177/0163278714521812
47. Marco EM, Echeverry-Alzate V, López-Moreno JA, Giné E, Peñasco S, Viveros MP. Consequences of early life stress on the expression of endocannabinoid-related genes in the rat brain. Behav Pharmacol (2014) 25(5–6):547–56. doi:10.1097/FBP.0000000000000068
48. Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse (2014) 68(2):61–72. doi:10.1002/syn.21716
49. Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci U S A (2014) 111(4):1592–7. doi:10.1073/pnas.1316911111
50. Blanton RE, Chaplin TM, Sinha R. Sex differences in the correlation of emotional control and amygdala volumes in adolescents. Neuroreport (2010) 21(14):953. doi:10.1097/WNR.0b013e32833e7866
51. Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci (2012) 15(12):1736–41. doi:10.1038/nn.3257
52. Shih JH, Eberhart NK, Hammen CL, Brennan PA. Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. J Clin Child Adolesc Psychol (2006) 35(1):103–15. doi:10.1207/s15374424jccp3501_9
53. Shulman EP, Harden KP, Chein JM, Steinberg L. Sex differences in the developmental trajectories of impulse control and sensation-seeking from early adolescence to early adulthood. J Youth Adolesc (2015) 44(1):1–7. doi:10.1007/s10964-014-0116-9
54. Adan A, Navarro JF, Forero DA. Personality profile of binge drinking in university students is modulated by sex. A study using the alternative five factor model. Drug Alcohol Depend (2016) 165:120–5. doi:10.1016/j.drugalcdep.2016.05.015
55. Barnes GM, Welte JW, Hoffman JH. Relationship of alcohol use to delinquency and illicit drug use in adolescents: gender, age, and racial/ethnic differences. J Drug Issues (2002) 32(1):153–78. doi:10.1177/002204260203200107
56. Kuntsche E, Müller S. Why do young people start drinking? Motives for first-time alcohol consumption and links to risky drinking in early adolescence. Eur Addict Res (2012) 18(1):34–9. doi:10.1159/000333036
57. Hussong AM, Curran PJ, Chassin L. Pathways of risk for accelerated heavy alcohol use among adolescent children of alcoholic parents. J Abnorm Child Psychol (1998) 26(6):453–66. doi:10.1023/A:1022699701996
58. McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res (2001) 25(8):1166–73. doi:10.1111/j.1530-0277.2001.tb02331.x/full
59. Danielsson AK, Romelsjö A, Tengström A. Heavy episodic drinking in early adolescence: gender-specific risk and protective factors. Subst Use Misuse (2011) 46(5):633–43. doi:10.3109/10826084.2010.528120
60. Edwards AC, Joinson C, Dick DM, Kendler KS, Macleod J, Munafò M, et al. The association between depressive symptoms from early to late adolescence and later use and harmful use of alcohol. Eur Child Adolesc Psychiatry (2014) 23(12):1219–30. doi:10.1007/s00787-014-0600-5
61. Needham BL. Gender differences in trajectories of depressive symptomatology and substance use during the transition from adolescence to young adulthood. Soc Sci Med (2007) 65(6):1166–79. doi:10.1016/j.socscimed.2007.04.037
62. Walsh K, Danielson CK, McCauley J, Hanson RF, Smith DW, Resnick HS, et al. Longitudinal trajectories of posttraumatic stress disorder symptoms and binge drinking among adolescent girls: the role of sexual victimization. J Adolesc Health (2012) 50(1):54–9. doi:10.1016/j.jadohealth.2011.05.017
63. Stevens SJ, Murphy BS, McKnight K. Traumatic stress and gender differences in relationship to substance abuse, mental health, physical health, and HIV risk behavior in a sample of adolescents enrolled in drug treatment. Child Maltreat (2003) 8(1):46–57. doi:10.1177/1077559502239611
64. Rohsenow DJ, Howland J, Winter M, Bliss CA, Littlefield CA, Heeren TC, et al. Hangover sensitivity after controlled alcohol administration as predictor of post-college drinking. J Abnorm Psychol (2012) 121(1):270. doi:10.1037/a0024706
65. Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med (1999) 29(5):1069–81. doi:10.1017/S0033291799008909
66. Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, et al. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav (2006) 83(1):35–46. doi:10.1016/j.pbb.2005.12.004
67. Dhaher R, McConnell KK, Rodd ZA, McBride WJ, Bell RL. Daily patterns of ethanol drinking in adolescent and adult, male and female, high alcohol drinking (HAD) replicate lines of rats. Pharmacol Biochem Behav (2012) 102(4):540–8. doi:10.1016/j.pbb.2012.04.017
68. Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn CM. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology (2014) 231(8):1831–9. doi:10.1007/s00213-013-3319-y
69. Brabant C, Guarnieri DJ, Quertemont E. Stimulant and motivational effects of alcohol: lessons from rodent and primate models. Pharmacol Biochem Behav (2014) 122:37–52. doi:10.1016/j.pbb.2014.03.006
70. Bell RL, Franklin KM, Hauser S, Engleman EA. Next stop dependence-binge drinking on the road to alcoholism: preclinical findings on its neurobiology from rat animal models. Binge Eating and Binge Drinking: Psychological, Social and Medical Implications. New York: Nova Science Publishers, Inc (2013). p. 1–60.
71. Novier A, Diaz-Granados JL, Matthews DB. Alcohol use across the lifespan: an analysis of adolescent and aged rodents and humans. Pharmacol Biochem Behav (2015) 133:65–82. doi:10.1016/j.pbb.2015.03.015
72. Novier A, Van Skike CE, Chin VS, Diaz-Granados JL, Matthews DB. Low and moderate doses of acute ethanol do not impair spatial cognition but facilitate accelerating rotarod performance in adolescent and adult rats. Neurosci Lett (2012) 512(1):38–42. doi:10.1016/j.neulet.2012.01.059
73. Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev (2014) 45:1–8. doi:10.1016/j.neubiorev.2014.04.012
74. Squeglia LM, Boissoneault J, Van Skike CE, Nixon SJ, Matthews DB. Age-related effects of alcohol from adolescent, adult, and aged populations using human and animal models. Alcohol Clin Exp Res (2014) 38(10):2509–16. doi:10.1111/acer.12531
75. Nestler EJ. From neurobiology to treatment: progress against addiction. Nat Neurosci (2002) 5:1076–9. doi:10.1038/nn945
76. Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev (2016) 68(3):816–71. doi:10.1124/pr.116.012484
77. Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem (2009) 108(4):920–31. doi:10.1111/j.1471-4159.2008.05835.x
78. Maldonado-Devincci AM, Badanich KA, Kirstein CL. Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry. Alcohol (2010) 44(1):57–66. doi:10.1016/j.alcohol.2009.09.035
79. Sahr AE, Thielen RJ, Lumeng L, Li TK, McBride WJ. Long-lasting alterations of the mesolimbic dopamine system after periadolescent ethanol drinking by alcohol-preferring rats. Alcohol Clin Exp Res (2004) 28(5):702–11. doi:10.1097/01.ALC.0000125344.79677.1C
80. Shnitko TA, Spear LP, Robinson DL. Adolescent binge-like alcohol alters sensitivity to acute alcohol effects on dopamine release in the nucleus accumbens of adult rats. Psychopharmacology (2016) 233(3):361–71. doi:10.1007/s00213-015-4106-8
81. Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav (2003) 75(2):411–8. doi:10.1016/S0091-3057(03)00134-5
82. Doremus-Fitzwater TL, Spear LP. Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res (2007) 31(9):1516–27. doi:10.1111/j.1530-0277.2007.00457.x
83. Zandy SL, Matthews DB, Tokunaga S, Miller AD, Blaha CD, Mittleman G. Reduced dopamine release in the nucleus accumbens core of adult rats following adolescent binge alcohol exposure: age and dose-dependent analysis. Psychopharmacology (2015) 232(4):777–84. doi:10.1007/s00213-014-3712-1
84. Popoli M, Diamond D, Sanacora G, editors. Synaptic Stress and Pathogenesis of Neuropsychiatric Disorders. New York, NY: Springer (2014).
85. Schmidt WJ, Reith ME, editors. Dopamine and Glutamate in Psychiatric Disorders. Totowa: Humana Press (2005).
86. Nieuwenhuys R. The greater limbic system, the emotional motor system and the brain. Prog Brain Res (1996) 107:551–80. doi:10.1016/S0079-6123(08)61887-7
87. Bell RL, Hauser S, Rodd ZA, Liang T, Sari Y, McClintick J, et al. Chapter seven-A genetic animal model of alcoholism for screening medications to treat addiction. Int Rev Neurobiol (2016) 126:179–261. doi:10.1016/bs.irn.2016.02.017
89. Pariyadath V, Gowin JL, Stein EA. Resting state functional connectivity analysis for addiction medicine: from individual loci to complex networks. Prog Brain Res (2016) 224:155–73. doi:10.1016/bs.pbr.2015.07.015
90. Renteria R, Jeanes ZM, Mangieri RA, Maier EY, Kircher DM, Buske TR, et al. Chapter fourteen-using in vitro electrophysiology to screen medications: accumbal plasticity as an engram of alcohol dependence. Int Rev Neurobiol (2016) 126:441–65. doi:10.1016/bs.irn.2016.02.018
91. Cummings JA, Jagannathan L, Jackson LR, Becker JB. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend (2014) 135:22–8. doi:10.1016/j.drugalcdep.2013.09.009
92. Gillies GE, Virdee K, McArthur S, Dalley JW. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: a molecular, cellular and behavioral analysis. Neuroscience (2014) 282:69–85. doi:10.1016/j.neuroscience.2014.05.033
93. Virdee K, McArthur S, Brischoux F, Caprioli D, Ungless MA, Robbins TW, et al. Antenatal glucocorticoid treatment induces adaptations in adult midbrain dopamine neurons, which underpin sexually dimorphic behavioral resilience. Neuropsychopharmacology (2014) 39(2):339–50. doi:10.1038/npp.2013.196
94. Cosgrove KP, Wang S, Kim SJ, McGovern E, Nabulsi N, Gao H, et al. Sex differences in the brain’s dopamine signature of cigarette smoking. J Neurosci (2014) 34(50):16851–5. doi:10.1523/JNEUROSCI.3661-14.2014
95. Becker JB. Direct effect of 17β-estradiol on striatum: sex differences in dopamine release. Synapse (1990) 5(2):157–64. doi:10.1002/syn.890050211
96. Evans SM. The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans. Exp Clin Psychopharmacol (2007) 15(5):418. doi:10.1037/1064-1297.15.5.418
97. Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology (2006) 31(3):659–74. doi:10.1038/sj.npp.1300887
98. Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol (2007) 15(5):461. doi:10.1037/1064-1297.15.5.461
99. Hammerslag LR, Gulley JM. Age and sex differences in reward behavior in adolescent and adult rats. Dev Psychobiol (2014) 56(4):611–21. doi:10.1002/dev.21127
100. Korhonen T, Kujala UM, Rose RJ, Kaprio J. Physical activity in adolescence as a predictor of alcohol and illicit drug use in early adulthood: a longitudinal population-based twin study. Twin Res Hum Genet (2009) 12(3):261–8. doi:10.1375/twin.12.3.261
101. Nelson MC, Gordon-Larsen P. Physical activity and sedentary behavior patterns are associated with selected adolescent health risk behaviors. Pediatrics (2006) 117(4):1281–90. doi:10.1542/peds.2005-1692
102. Castilla-Ortega E, de Guevara-Miranda DL, Serrano A, Pavón FJ, Suárez J, de Fonseca FR, et al. The impact of cocaine on adult hippocampal neurogenesis: potential neurobiological mechanisms and contributions to maladaptive cognition in cocaine addiction disorder. Biochem Pharmacol (2017) 141:100–17. doi:10.1016/j.bcp.2017.05.003
103. Geil CR, Hayes DM, McClain JA, Liput DJ, Marshall SA, Chen KY, et al. Alcohol and adult hippocampal neurogenesis: promiscuous drug, wanton effects. Prog Neuropsychopharmacol Biol Psychiatry (2014) 54:103–13. doi:10.1016/j.pnpbp.2014.05.003
104. Gallego X, Cox RJ, Funk E, Foster RA, Ehringer MA. Voluntary exercise decreases ethanol preference and consumption in C57BL/6 adolescent mice: sex differences and hippocampal BDNF expression. Physiol Behav (2015) 138:28–36. doi:10.1016/j.physbeh.2014.10.008
105. Lee JL, Bertoglio LJ, Guimarães FS, Stevenson CW. Cannabidiol regulation of emotion and emotional memory processing: relevance for treating anxiety-related and substance abuse disorders. Br J Pharmacol (2017) 174(19):3242–56. doi:10.1111/bph.13724
106. Brenhouse HC, Schwarz JM. Immunoadolescence: neuroimmune development and adolescent behavior. Neurosci Biobehav Rev (2016) 70:288–99. doi:10.1016/j.neubiorev.2016.05.035
107. Schwarz JM, Bilbo SD. The Immune System and the Developing Brain. San Rafael, CA: Morgan & Claypool Life Sciences (2011).
108. Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol (2007) 29(1):81–95. doi:10.1016/j.ntt.2006.10.013
109. Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev (2009) 29(6):535–47. doi:10.1016/j.cpr.2009.06.003
110. Piazza NJ, Vrbka JL, Yeager RD. Telescoping of alcoholism in women alcoholics. Int J Addict (1989) 24(1):19–28. doi:10.3109/10826088909047272
111. Forbes EE, Dahl RE. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn (2010) 72(1):66–72. doi:10.1016/j.bandc.2009.10.007
112. Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol (2010) 52(3):216–24. doi:10.1002/dev.20445
113. Barkley-Levenson EE, Van Leijenhorst L, Galván A. Behavioral and neural correlates of loss aversion and risk avoidance in adolescents and adults. Dev Cogn Neurosci (2013) 3:72–83. doi:10.1016/j.dcn.2012.09.007
114. Juraska JM, Sisk CL, DonCarlos LL. Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm Behav (2013) 64(2):203–10. doi:10.1016/j.yhbeh.2013.05.010
115. Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med (2006) 36(3):299–312. doi:10.1017/S0033291705005891
116. Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci (2004) 1021(1):77–85. doi:10.1196/annals.1308.009/full
117. Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci (2008) 9(12):947–57. doi:10.1038/nrn2513
118. Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci (2008) 1124(1):111–26. doi:10.1196/annals.1440.010/full
119. Groenman AP, Janssen TW, Oosterlaan J, Strategy S. Childhood psychiatric disorders as risk factor for subsequent substance abuse: a meta-analysis. J Am Acad Child Adolesc Psychiatry (2017) 56(7):556–69. doi:10.1016/j.jaac.2017.05.004
120. Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience (2013) 249:162–71. doi:10.1016/j.neuroscience.2012.10.048
121. McCormick CM, Green MR. From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience (2013) 249:242–57. doi:10.1016/j.neuroscience.2012.08.063
122. Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol (2008) 4:325–48. doi:10.1146/annurev.clinpsy.4.022007.141157
123. Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol (2014) 35(3):303–19. doi:10.1016/j.yfrne.2014.03.008
124. Copeland WE, Keeler G, Angold A, Costello EJ. Traumatic events and posttraumatic stress in childhood. Arch Gen Psychiatry (2007) 64(5):577–84. doi:10.1001/archpsyc.64.5.577
125. Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the national survey of adolescents. J Consult Clin Psychol (2003) 71(4):692. doi:10.1037/0022-006X.71.4.692
126. Shin SH, Edwards EM, Heeren T. Child abuse and neglect: relations to adolescent binge drinking in the national longitudinal study of Adolescent Health (AddHealth) Study. Addict Behav (2009) 34(3):277–80. doi:10.1016/j.addbeh.2008.10.023
127. Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: data from a national sample. J Consult Clin Psychol (2000) 68(1):19. doi:10.1037//0022-006X.68.1.19
128. Adams ZW, Moreland A, Cohen JR, Lee RC, Hanson RF, Danielson CK, et al. Polyvictimization: latent profiles and mental health outcomes in a clinical sample of adolescents. Psychol Violence (2016) 6(1):145. doi:10.1037/a0039713
129. Abbey A. Alcohol’s role in sexual violence perpetration: theoretical explanations, existing evidence and future directions. Drug Alcohol Rev (2011) 30(5):481–9. doi:10.1111/j.1465-3362.2011.00296.x
130. Walsh K, Moreland AM, Hanson RF, Resnick HS, Saunders BE, Kilpatrick DG. Relationship violence victimization and binge drinking trajectories among a nationally representative sample of adolescents. J Adolesc (2017) 58:49–55. doi:10.1016/j.adolescence.2017.05.002
131. Fisher BS, Daigle LE, Cullen FT, Turner MG. Reporting sexual victimization to the police and others: results from a national-level study of college women. Crim Justice Behav (2003) 30(1):6–38. doi:10.1177/0093854802239161
132. McCauley JL, Conoscenti LM, Ruggiero KJ, Resnick HS, Saunders BE, Kilpatrick DG. Prevalence and correlates of drug/alcohol-facilitated and incapacitated sexual assault in a nationally representative sample of adolescent girls. J Clin Child Adolesc Psychol (2009) 38(2):295–300. doi:10.1080/15374410802698453
133. Wilson HW, Widom CS. A prospective examination of the path from child abuse and neglect to illicit drug use in middle adulthood: the potential mediating role of four risk factors. J Youth Adolesc (2009) 38(3):340–54. doi:10.1007/s10964-008-9331-6
134. Comeau N, Stewart SH, Loba P. The relations of trait anxiety, anxiety sensitivity, and sensation seeking to adolescents’ motivations for alcohol, cigarette, and marijuana use. Addict Behav (2001) 26(6):803–25. doi:10.1016/S0306-4603(01)00238-6
135. Jessor R, Jessor S. A social-psychological framework for studying drug use. NIDA Res Monogr (1980) 30:102.
136. Bates ME, Labouvie EW. Personality environment constellations and alcohol use: a process-oriented study of intraindividual change during adolescence. Psychol Addict Behav (1995) 9(1):23. doi:10.1037/0893-164X.9.1.23
137. Borsari B, Carey KB. Effects of a brief motivational intervention with college student drinkers. J Consult Clin Psychol (2000) 68(4):728. doi:10.1037/0022-006X.68.4.728
138. Donovan JE. Adolescent alcohol initiation: a review of psychosocial risk factors. J Adolesc Health (2004) 35(6):529.e7–18. doi:10.1016/j.jadohealth.2004.02.003
139. Windle M, Windle RC. Adolescent tobacco, alcohol, and drug use: current findings. Adolesc Med (1999) 10(1):153–63.
140. Jalling C, Elgán TH, Tengström A, Birgegård A. Gender-specific predictors of at-risk adolescents’ hazardous alcohol use – a cohort study. Subst Abuse Treat Prev Policy (2017) 12(1):23. doi:10.1186/s13011-017-0105-6
141. Brooks-Russell A, Simons-Morton B, Haynie D, Farhat T, Wang J. Longitudinal relationship between drinking with peers, descriptive norms, and adolescent alcohol use. Prev Sci (2014) 15(4):497–505. doi:10.1007/s11121-013-0391-9
142. Zarzar PM, Jorge KO, Oksanen T, Vale MP, Ferreira EF, Kawachi I. Association between binge drinking, type of friends and gender: a cross-sectional study among Brazilian adolescents. BMC Public Health (2012) 12(1):257. doi:10.1186/1471-2458-12-257
143. Franca LR, Dautzenberg B, Falissard B, Reynaud M. Peer substance use overestimation among French university students: a cross-sectional survey. BMC Public Health (2010) 10(1):169. doi:10.1186/1471-2458-10-169
144. Griffin KW, Scheier LM, Botvin GJ, Diaz T. Ethnic and gender differences in psychosocial risk, protection, and adolescent alcohol use. Prev Sci (2000) 1(4):199–212. doi:10.1023/A:1026599112279
145. Elek E, Miller-Day M, Hecht ML. Influences of personal, injunctive, and descriptive norms on early adolescent substance use. J Drug Issues (2006) 36(1):147–72. doi:10.1177/002204260603600107
146. Hong T, Beaudoin CE, Johnson C. A panel study of peer norms and adolescent alcohol consumption: developing strategies for communication interventions. J Health Commun (2013) 18(8):913–30. doi:10.1080/10810730.2013.768729
147. Lewis MA, Neighbors C. Gender-specific misperceptions of college student drinking norms. Psychol Addict Behav (2004) 18(4):334. doi:10.1037/0893-164X.18.4.334
148. De Visser RO, McDonnell EJ. ‘That’s ok. He’s a guy’: a mixed-methods study of gender double-standards for alcohol use. Psychol Health (2012) 27(5):618–39. doi:10.1080/08870446.2011.617444
149. Clinkinbeard SS, Barnum TC. Gendered self-concepts and drinking behavior in a national sample of emerging adults. Feminist Criminol (2017) 12(2):145–70. doi:10.1177/1557085115614391
150. Young AM, Morales M, McCabe SE, Boyd CJ, D’Arcy HA. Drinking like a guy: frequent binge drinking among undergraduate women. Subst Use Misuse (2005) 40(2):241–67. doi:10.1081/JA-200048464
151. Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol (2009) 44(6):547–54. doi:10.1093/alcalc/agp048
152. Kypri K, Langley JD. Perceived social norms and their relation to university student drinking. J Stud Alcohol (2003) 64(6):829–34. doi:10.15288/jsa.2003.64.829
153. McAlaney J, McMahon J. Normative beliefs, misperceptions, and heavy episodic drinking in a British student sample. J Stud Alcohol Drugs (2007) 68(3):385–92. doi:10.15288/jsad.2007.68.385
154. Brady J, Iwamoto DK, Grivel M, Kaya A, Clinton L. A systematic review of the salient role of feminine norms on substance use among women. Addict Behav (2016) 62:83–90. doi:10.1016/j.addbeh.2016.06.005
155. Lewis MA, Neighbors C. Social norms approaches using descriptive drinking norms education: a review of the research on personalized normative feedback. J Am Coll Health (2006) 54(4):213–8. doi:10.3200/JACH.54.4.213-218
156. Vaughan EL, Wong YJ, Middendorf KG. Gender roles and binge drinking among Latino emerging adults: a latent class regression analysis. Psychol Addict Behav (2014) 28(3):719. doi:10.1037/a0037406
157. Varlinskaya EI, Truxell EM, Spear LP. Sex differences in sensitivity to the social consequences of acute ethanol and social drinking during adolescence. Behav Brain Res (2015) 282:6–13. doi:10.1016/j.bbr.2014.12.054
158. Kuhn C. Emergence of sex differences in the development of substance use and abuse during adolescence. Pharmacol Ther (2015) 153:55–78. doi:10.1016/j.pharmthera.2015.06.003
160. Carey KB, Scott-Sheldon LA, Garey L, Elliott JC, Carey MP. Alcohol interventions for mandated college students: a meta-analytic review. J Consult Clin Psychol (2016) 84(7):619–32. doi:10.1037/a0040275
161. Becker SJ, Curry JF. Outpatient interventions for adolescent substance abuse: a quality of evidence review. J Consult Clin Psychol (2008) 76(4):531. doi:10.1037/0022-006X.76.4.531
162. O’Rourke L, Humphris G, Baldacchino A. Electronic communication based interventions for hazardous young drinkers: a systematic review. Neurosci Biobehav Rev (2016) 68:880–90. doi:10.1016/j.neubiorev.2016.07.021
163. Larimer ME, Cronce JM. Identification, prevention, and treatment revisited: individual-focused college drinking prevention strategies 1999–2006. Addict Behav (2007) 32(11):2439–68. doi:10.1016/j.addbeh.2007.05.006
164. Wachtel T, Staniford M. The effectiveness of brief interventions in the clinical setting in reducing alcohol misuse and binge drinking in adolescents: a critical review of the literature. J Clin Nurs (2010) 19(5–6):605–20. doi:10.1111/j.1365-2702.2009.03060.x
165. Todd J, Mullan B. Using the theory of planned behaviour and prototype willingness model to target binge drinking in female undergraduate university students. Addict Behav (2011) 36(10):980–6. doi:10.1016/j.addbeh.2011.05.010
166. Longshore D, Ellickson PL, McCaffrey DF, Clair PA. School-based drug prevention among at-risk adolescents: effects of ALERT plus. Health Educ Behav (2007) 34(4):651–68. doi:10.1177/1090198106294895
167. Schinke SP, Cole KC, Fang L. Gender-specific intervention to reduce underage drinking among early adolescent girls: a test of a computer-mediated, mother-daughter program. J Stud Alcohol Drugs (2009) 70(1):70–7. doi:10.15288/jsad.2009.70.70
168. Dunn ME, Lau HC, Cruz IY. Changes in activation of alcohol expectancies in memory in relation to changes in alcohol use after participation in an expectancy challenge program. Exp Clin Psychopharmacol (2000) 8(4):566. doi:10.1Q37//1064-I297.8.4.566
169. Hsieh S, Hollister CD. Examining gender differences in adolescent substance abuse behavior: comparisons and implications for treatment. J Child Adolesc Subst Abuse (2004) 13(3):53–70. doi:10.1300/J029v13n03_03
170. D’Amico EJ. Factors that impact adolescents’ intentions to utilize alcohol-related prevention services. J Behav Health Serv Res (2005) 32(3):332–40. doi:10.1007/BF02291832
171. Stevens SJ, Estrada B, Murphy BS, McKnight KM, Tims F. Gender difference in substance use, mental health, and criminal justice involvement of adolescents at treatment entry and at three, six, 12 and 30 month follow-up. J Psychoactive Drugs (2004) 36(1):13–25. doi:10.1080/02791072.2004.10399720
172. Hawke JM, Jainchill N, De Leon G. Posttreatment victimization and violence among adolescents following residential drug treatment. Child Maltreat (2003) 8(1):58–71. doi:10.1177/1077559502239609
173. Farabee D, Shen H, Hser YI, Grella CE, Anglin MD. The effect of drug treatment on criminal behavior among adolescents in DATOS-A. J Adolesc Res (2001) 16(6):679–96. doi:10.1177/0743558401166009
174. Grella CE, Hser YI, Joshi V, Rounds-Bryant J. Drug treatment outcomes for adolescents with comorbid mental and substance use disorders. J Nerv Ment Dis (2001) 189(6):384–92. doi:10.1097/00005053-200106000-00006
175. Godley SH, Hedges K, Hunter B. Gender and racial differences in treatment process and outcome among participants in the adolescent community reinforcement approach. Psychol Addict Behav (2011) 25(1):143. doi:10.1037/a0022179
176. Bernal G, Scharró-del-Río MR. Are empirically supported treatments valid for ethnic minorities? Toward an alternative approach for treatment research. Cultur Divers Ethnic Minor Psychol (2001) 7(4):328. doi:10.1037//1099-9809.7.4.328
177. Kumpfer KL, Smith P, Summerhays JF. A wakeup call to the prevention field: are prevention programs for substance use effective for girls? Subst Use Misuse (2008) 43(8–9):978–1001. doi:10.1080/10826080801914261
178. Blake SM, Amaro H, Schwartz PM, Flinchbaugh LJ. A review of substance abuse prevention interventions for young adolescent girls. J Early Adolesc (2001) 21(3):294–324. doi:10.1177/0272431601021003003
179. O’Neil AL, Lucas J. DAWN Drugs and Alcohol Women Network: Promoting a Gender Responsive Approach to Addiction. Turin, Italy: United Nations Interregional Crime and Justice Research Initiative (UNICRI) Publication (2013).
180. Dawes MA, Johnson BA. Pharmacotherapeutic trials in adolescent alcohol use disorders: opportunities and challenges. Alcohol Alcohol (2004) 39(3):166–77. doi:10.1093/alcalc/agh045
181. Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA (2005) 293(13):1617–25. doi:10.1001/jama.293.13.1617
182. Hernandez-Avila CA, Song C, Kuo L, Tennen H, Armeli S, Kranzler HR. Targeted versus daily naltrexone: secondary analysis of effects on average daily drinking. Alcohol Clin Exp Res (2006) 30(5):860–5. doi:10.1111/j.1530-0277.2006.00101.x
183. Pettinati HM, Dundon W, Lipkin C. Gender differences in response to sertraline pharmacotherapy in type A alcohol dependence. Am J Addict (2004) 13(3):236–47. doi:10.1080/10550490490459906
184. Anderson P, Bendtsen P, Spak F, Reynolds J, Drummond C, Segura L, et al. Improving the delivery of brief interventions for heavy drinking in primary health care: outcome results of the optimizing delivery of health care intervention (ODHIN) five-country cluster randomized factorial trial. Addiction (2016) 111(11):1935–45. doi:10.1111/add.13476
185. Kloos A, Weller RA, Chan R, Weller EB. Gender differences in adolescent substance abuse. Curr Psychiatry Rep (2009) 11(2):120–6. doi:10.1007/s11920-009-0019-8
Keywords: adolescence, binge drinking, gender, intervention, comorbidity, prevention
Citation: Dir AL, Bell RL, Adams ZW and Hulvershorn LA (2017) Gender Differences in Risk Factors for Adolescent Binge Drinking and Implications for Intervention and Prevention. Front. Psychiatry 8:289. doi: 10.3389/fpsyt.2017.00289
Received: 31 July 2017; Accepted: 04 December 2017;
Published: 22 December 2017
Edited by:
Eduardo López-Caneda, Universidade do Minho, PortugalReviewed by:
Ana Adan, University of Barcelona, SpainCopyright: © 2017 Dir, Bell, Adams and Hulvershorn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard L. Bell, cmliZWxsQGl1cHVpLmVkdQ==;
Leslie A. Hulvershorn, bGh1bHZlcnNAaXVwdWkuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.