- 1Psychology, School of Medicine, University of Tasmania, Hobart, TAS, Australia
- 2Psychiatry, School of Medicine, University of Tasmania, Hobart, TAS, Australia
- 3Menzies Institute for Medical Research, Tasmania, Hobart, TAS, Australia
Background: The ketogenic diet (KD) has been used in treatment-resistant epilepsy since the 1920s. It has been researched in a variety of neurological conditions in both animal models and human trials. The aim of this review is to clarify the potential role of KD in psychiatry.
Methods: Narrative review of electronic databases PubMED, PsychINFO, and Scopus.
Results: The search yielded 15 studies that related the use of KD in mental disorders including anxiety, depression, bipolar disorder, schizophrenia, autism spectrum disorder (ASD), and attention deficit hyperactivity disorder (ADHD). These studies comprised nine animal models, four case studies, and two open-label studies in humans. In anxiety, exogenous ketone supplementation reduced anxiety-related behaviors in a rat model. In depression, KD significantly reduced depression-like behaviors in rat and mice models in two controlled studies. In bipolar disorder, one case study reported a reduction in symptomatology, while a second case study reported no improvement. In schizophrenia, an open-label study in female patients (n = 10) reported reduced symptoms after 2 weeks of KD, a single case study reported no improvement. In a brief report, 3 weeks of KD in a mouse model normalized pathological behaviors. In ASD, an open-label study in children (n = 30) reported no significant improvement; one case study reported a pronounced and sustained response to KD. In ASD, in four controlled animal studies, KD significantly reduced ASD-related behaviors in mice and rats. In ADHD, in one controlled trial of KD in dogs with comorbid epilepsy, both conditions significantly improved.
Conclusion: Despite its long history in neurology, the role of KD in mental disorders is unclear. Half of the published studies are based on animal models of mental disorders with limited generalizability to the analog conditions in humans. The review lists some major limitations including the lack of measuring ketone levels in four studies and the issue of compliance to the rigid diet in humans. Currently, there is insufficient evidence for the use of KD in mental disorders, and it is not a recommended treatment option. Future research should include long-term, prospective, randomized, placebo-controlled crossover dietary trials to examine the effect of KD in various mental disorders.
Introduction
The ketogenic diet (KD) has a long-standing place in neurology and has been used for treatment-resistant epilepsy since the 1920s (1). KD consists of a rigidly controlled high-fat, low-protein, and low-carbohydrate diet usually with a 4:1 lipid:non-lipid ratio (fat to protein and carbohydrate ratio) (2). Woodyatt noted that in a normal person in a state of starvation or eating a diet containing low carbohydrate and a high percentage of fat, the ketones acetone, acetoacetate, and beta-hydroxybutyric acid increase (3), and the absence of glucose serves as alternative fuels for the body. KD has been proven an effective treatment in difficult-to-control seizures with its use primarily in children with epilepsy (4, 5), particularly those with epileptic encephalopathies whereby epileptic activity may contribute to severe neurological and cognitive impairments (6). The finding that KD is beneficial for epilepsy was supported by a systematic review (7), meta-analysis (8), and a Cochrane review (9). KD and related diets have been proven useful in pharmacoresistant childhood epilepsy (10).
The mechanism by which KD acts is not clearly understood. However, among the many hypotheses advanced, elevation of brain acetone may account for the efficacy of the diet in epilepsy as it has proven anticonvulsant effects (11). In a variation of the diet, the medium-chain triglyceride (MCT) KD increases plasma levels of decanoic acid, which in vivo has been shown to be anticonvulsant; although the precise mechanism remains unclear (12). In young and adult rats, KD increases concentrations of kynurenic acid (KYNA) in the hippocampus and striatum but not the cortex (13). Elevated levels of KYNA in the cerebrospinal fluid have been demonstrated in patients with schizophrenia (14) and bipolar disorder (15). Pharmacological manipulation of kynurenines is a potential treatment strategy for psychiatric disorders (16).
Currently, there are no international protocols guiding the implementation of the diet, rather dietary recommendations are based on individual treating physician’s advice. Consequently, there exists a need for more standardized protocols for management recommendations for clinical and research use (17). In 2006, a group of 26 pediatric epileptologists and dieticians was convened to create a consensus statement regarding the clinical management of KD. They specified the following absolute contraindications to commencing KD “carnitine deficiency (primary), carnitine palmitoyltransferase (CPT) I or II deficiency, carnitine translocase deficiency, beta-oxidation deficiencies including medium-chain acyl dehydrogenase deficiency (MCAD), long-chain acyl dehydrogenase deficiency (LCAD), short-chain acyl dehydrogenase deficiency (SCAD), long-chain 3-hydroxyacl-CoA deficiency, medium-chain 3-hydroxyacl-CoA deficiency, pyruvate carboxylase deficiency and porphyria. Relative contraindications of KD include the following: inability to maintain adequate nutrition, surgical focus identified by neuroimaging and video EEG monitoring, and parent or caregiver non-compliance” (18). The possible risks of KD must be weighted against its potential value for seizure control or its other benefits (19).
Ketogenic diet has been assessed in a variety of neurological conditions other than epilepsy in both animal models and human trials. In an animal model of amyotrophic lateral sclerosis, SOD1-G93A transgenic mice were fed KD. It was shown that KD led to significant alterations in the clinical manifestation of the disease, specifically a higher motor neuron count in the lumbar spinal cord and preserved motor function (20). KD has also been trialed in rats following controlled cortical impact injury, a model for brain trauma, showing that the diet improves both cognitive and motor functioning (21). In an animal model of multiple sclerosis, the effects of KD on memory impairments and inflammation expressed by experimental autoimmune encephalomyelitis were examined. In mice, it was demonstrated that brain inflammation was associated with impaired spatial learning and memory function, and the administration of KD exerted protective effects against these. The proposed mode of action was through attenuation of the immune response and increased oxidative stress observed in the mice (22).
In humans, KD has been trialed in a number of neurological conditions. In a randomized, double-blind, placebo-controlled, parallel group study in Alzheimer’s disease, an oral ketogenic compound AC-1202 was tested on 152 patients. Regular medications were continued throughout the study. Daily dosing of AC-1202 significantly elevated the levels of beta-hydroxybutyrate 2 h after administration. After 45 and 90 days, patients treated with AC-1202 had significant improvements on the ADAS-Cog scale (23). In a small study of seven patients with Parkinson’s disease, five adhered to KD for 28 days (24). Scores on the Unified Parkinson’s Disease Rating Scale improved in all five as did symptoms such as resting tremor, freezing, balance, gait, mood, and energy levels. These results should be interpreted with caution due to the small sample size, subjective ratings, and the lack of a control group to exclude a placebo effect. The modified Atkins diet (a high-fat, low-carbohydrate diet), which creates a ketotic state was trialed in adolescent patients with chronic daily headaches (25). Due to difficulties adhering to the diet, the study was terminated prematurely. Three participants reported an improvement in headache severity and quality of life; however, they still required pharmacotherapy to manage their condition. In a comprehensive review of KD in diverse neurological conditions, Stafstrom and Rho concluded that there are rich opportunities for further investigation of KD in both the laboratory and clinical practice (26).
The therapeutic advantage of KD has been replicated in animal models of neurological illnesses, and the purported underlying mechanisms include those which improve mitochondrial function (27). Molecular, biochemical, and physiological studies tend to support the assumption that cellular energy status is a determinant for multiple disorders (28). Aberrant energy production has been associated with cancer (29), heart failure (30), aging (31), and neurological conditions such as epilepsy (32) and Alzheimer’s disease (33). The precise pathways by which energy disruption is related to these and other disorders are unknown. There are also strong indications of metabolic pathways involving energy production in the pathophysiology of some mental disorders including bipolar disorder, depression, schizophrenia (34) autism spectrum disorder (ASD) (35), and potentially attention deficit hyperactivity disorder (ADHD) (36). There is also a recognized comorbidity between epilepsy and mental disorders (37), which might indicate some commonality of mechanisms.
Given the degree of interest in KD and neurological conditions, the aim of this narrative review is to examine the effect of the diet in mental disorders. The literature searched in anxiety, depression, bipolar disorder, schizophrenia, ASD, and ADHD.
Method
A comprehensive search of the electronic databases PubMed, PsychINFO, and Scopus for peer-reviewed articles published in English was conducted in the last week of November 2016 and updated in January 2017. Search terms were “bipolar disorder” “manic depress*” “depress*” “schizophren*” “autism” “ASD” “attention deficit hyperactivity disorder” “ADHD” “obsessive compulsive disorder” “OCD” “anxiety” “anxi*” “psychiatry” “mental disorder*” (group 1) AND “ketogenic diet” “ketosis” “ketogenesis” “ketone bodies” “high fat low carbohydrate” “diet” “acetone” “acetoacetic acid” “beta-hydroxybutyric acid” “acetyl-coA” “ketonemia” “ketonuria” “fatty acid metabolism” “hyperketonemia” “fasting” “nutritional ketosis” “acidotic” (group 2). These terms were combined as follows: group 1 AND group 2. In addition, a hand-search of the reference lists of published articles was also conducted, and articles were assessed for their suitability in the review. An initial search was conducted using all the search terms listed above, and abstracts were reviewed by author Emmanuelle C. S. Bostock. Full text publications were retrieved for those that addressed the subject matter.
Results
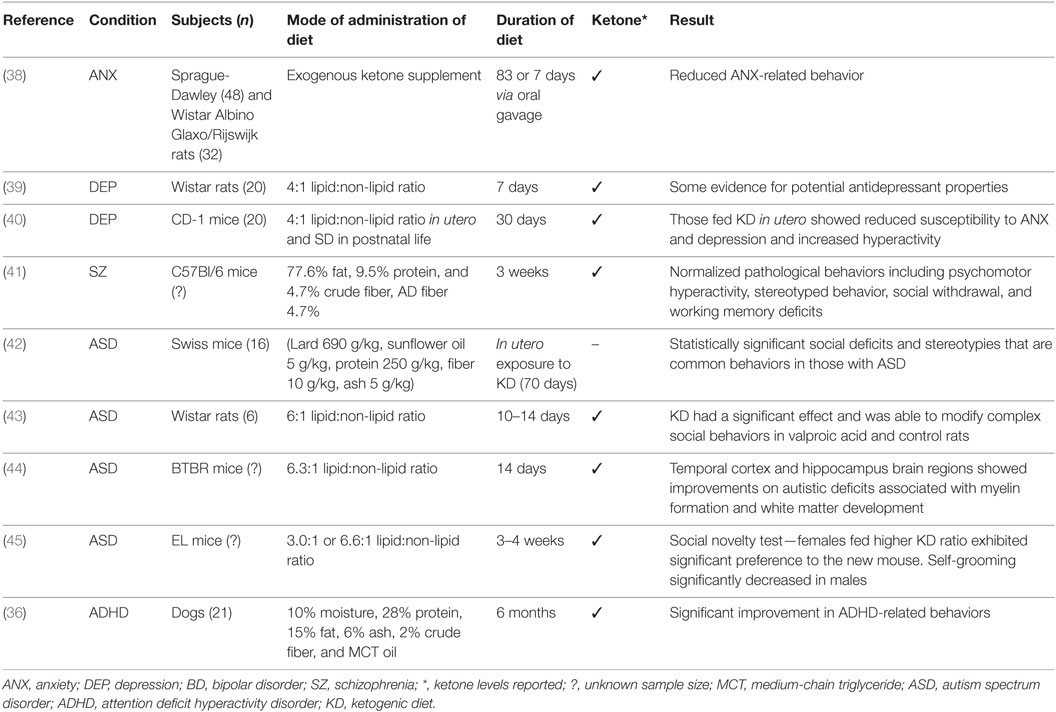
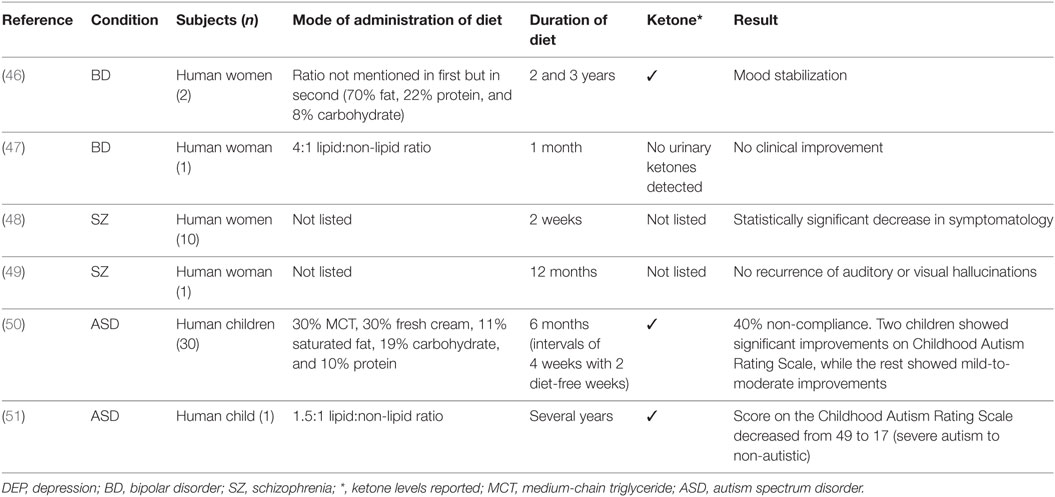
The results are discussed by mental disorders examining animal and human studies including case reports and studies of patient groups. The search yielded 15 studies that examined KD in mental disorders, specifically anxiety, depression, bipolar disorder, schizophrenia, autism, and, ADHD. These studies included nine animal models, and in humans four case studies and two uncontrolled trials. A summary of results by animal models and human studies are presented in Tables 1 and 2, respectively.
Anxiety
Anxiety is a common mental disorder affecting 18.1% of the population in the United States (52). In humans, functional magnetic resonance imaging indicates that anxiety is associated with activation in the ventromedial prefrontal cortex and hippocampal regions of the brain (53). Symptoms of anxiety and disorders are more frequent in patients with epilepsy with one recent study reporting a lifetime incidence of 22.8% as opposed to 11.2% in people without epilepsy (54).
In a recent animal model study of anxiety in male rats, two methods of administration of exogenous ketone supplement were applied (38). In the chronic administration condition, 48 male Sprague-Dawley (SPD) rats were fed for 83 days with either a standard diet (n = 9) or standard diet plus one of four ketone supplementation conditions. In the sub-chronic intragastric gavage bolus condition, 39 SPD rats were fed with standard diet and gavaged daily with water (control, n = 11) or 1 of 3 levels of ketone supplementation for 7 days; this was repeated with 32 Wistar Albino Glaxo/Rijswijk rats receiving a half-dose of supplementation. In both modes of supplementation, beta-hydroxybutyrate was significantly elevated indicating ketosis. All treatment conditions resulted in reduced anxiety as assessed by behavior on the elevated plus maze. The dependent variables of less entries and time spent in closed arms, more entries and time spent in open arms, more distance traveled in open arms, and delayed entry to closed arm were used as an analog of anxiety in humans. The authors hypothesized that the mode of action was through the glutamatergic and/or GABAergic and purinergic systems.
Depression
In a recent review, a number of studies suggested that depression is associated with an increased risk of epilepsy (55). The effectiveness of conventional antidepressant therapies is frequently examined in animals. In rodents, to test current levels of depression, a methodology known as the Porsolt forced swim test is often employed (56) and has been used in testing the effectiveness of new antidepressant drugs (57). In the two-part swim test, animals are first placed in a container from which they cannot escape. When they then stop trying and immobility ensues, a state of behavioral despair is shown. Second, to assess the effects of antidepressants, the time spent immobile is used as a dependent variable, and reductions are interpreted for significance (56). To examine the antidepressant properties of KD, 20 Wistar rats given the diet (4:1 lipid:non-lipid ratio) were compared to 20 fed a standard diet (39). It was found that rats on KD spent less time immobile than control rats thus providing some evidence for potential antidepressant effects of the diet. The diet duration was 7 days, and levels of beta-hydroxybutyrate were measured.
Brain morphology and behavior of CD-1 mice exposed to KD (4:1 lipid:non-lipid ratio) for 30 days in utero and fed a standard diet in postnatal life were examined (40). Adult mice that were fed the diet in utero showed reduced susceptibility to anxiety and depression and exhibited elevated physical activity when compared with control mice fed a standard diet in utero. Morphological differences included cerebellar volumetric enlargement by 4.8%, a hypothalamic reduction by 1.39%, and a corpus callosum reduction by 4.77%, as computed relative to total brain volume.
While animal models pave the way for future research in humans, the conclusions that may be made are limited. The mechanism by which KD acts in animal models of depression is unknown; however, in children with epilepsy, KD resulted in significant alterations in levels of serotonin and dopamine neurotransmitters (58), both of which are implicated in anxiety and depression. To the best of our knowledge, there are no studies examining the effects of KD in depressed humans.
Bipolar Disorder
A diagnosis of bipolar disorder type I requires an episode of mania, which consists of “a distinct period of abnormally and persistently elevated, expansive or irritable mood, lasting at least 1 week (or any duration if hospitalization is necessary)” (59). A diagnosis of bipolar disorder type II requires at least one episode of hypomania. In a study of nutrition and exercise behavior, when compared to patients with schizophrenia or healthy controls, it was found that patients with bipolar disorder were more likely to report risk factors for poor nutrition including difficulty obtaining or cooking food (60). Treatments for bipolar disorder typically include an antipsychotic and a mood stabilizer, and many patients are treated with adjunct anticonvulsants.
In a case study of two women with bipolar disorder type II, the patients maintained ketosis for an extended period of 2 and 3 years, respectively. The women reported subjective mood stabilization, which exceeded that of medication as well as an overall improvement in their condition that they related to ketosis (measured in the urine). Both women tolerated the diet well with few or no side effects reported (46). The ratio of KD was not mentioned in the first case, but in the second it was estimated to be around 70% fat, 22% protein, and 8% carbohydrates.
In a separate case study, a woman with treatment-resistant bipolar disorder was placed on KD (4:1 lipid:non-lipid ratio) and showed no clinical improvement (47). It should be noted that no urinary ketones were detected, the type of bipolar disorder was not listed (type I or type II), and treatment duration limited to 1 month.
These studies illustrate that careful attention should be paid to the intricacies of the diet (such as measuring ketones and calculating macronutrient ratios) to fully examine its efficacy in bipolar disorder, as well as the need for larger well-designed placebo-controlled studies in this area. The mechanism by which KD may be effective in bipolar disorder is based on the hypothesis that acidosis achieved through ketosis reduces intracellular sodium and calcium, both of which are elevated in the disorder (47). Mood stabilizers reduce intracellular sodium in an activity-dependent manner within the context of KD; this is hypothesized as being achieved through the acidification of the blood (46).
Schizophrenia
Schizophrenia is associated with high levels of morbidity. The precise pathophysiology of the disorder is unknown, and current pharmacological treatment options are limited (61). Animal models of schizophrenia fit into four induction methods including developmental, drug-induced, lesional, or genetic manipulation (62). In a recent drug-induced (MK-801, dizocilpine) animal model of schizophrenia in C57BL/6 mice, it was demonstrated that 3 weeks of KD (77.6% fat, 9.5% protein, and 4.7% crude fiber, AD fiber 4.7%) normalized pathological behaviors (41). These included psychomotor hyperactivity, stereotyped behavior, social withdrawal, and working memory deficits, which reflect the positive, negative, and cognitive symptoms of the disorder. Weight loss was an observed side effect. Elevated levels of the ketone beta-hydroxybutyrate and decreased glucose levels indicated that metabolic adaptation had occurred.
In a 1965 study, the effect of KD was tested in 10 female patients with schizophrenia. All participants were reported to have a poor prognosis and were not treatment responsive at the time. Concurrent therapies remained throughout the duration of the diet including pharmacotherapy and electroconvulsive therapy. The Beckomberga Rating Scale was administered to patients three times during the diet period (2 days, 2 weeks, and 1 week after discontinuation), there was a statistically significant decrease in symptomatology after 2 weeks of established KD (48). This was, however, a small, poorly controlled study, and in addition, the lipid:non-lipid ratios were not detailed, and it was not stated whether ketone levels were measured throughout the study. A further consideration is that the study was conducted in 1965 before the advent of atypical antipsychotics and their metabolic side effects.
In a case study of a 70-year-old overweight woman with a diagnosis of schizophrenia, KD was initiated by her treating physician (49). The patient remained on KD for 12 months and reportedly had no recurrences of auditory or visual hallucinations, and the patient lost weight. The patient reported eating mainly lean proteins and low-carbohydrate vegetables (the lipid:non-lipid ratio was not listed), ketosis was not confirmed and perhaps not established due to the lack of dietary fats listed; therefore, this case report is of indeterminate value.
Some studies suggest that abnormal glucose and energy metabolism may underlie the pathophysiology of schizophrenia, which may provide some potential pointers into the hypothesized mode of action of KD in the disorder (63, 64). Others have noted that abnormal glucose metabolism may occur secondary to antipsychotic medications alongside significant treatment side effects such as weight gain, hyperglycemia, and diabetes (65). The high metabolic risk associated with schizophrenia is due to genetic and environmental factors (66).
Autism Spectrum Disorder
Features of patients with ASD include compromised social interaction and communication (67). It is estimated that between 5 and 40% of patients with autism will develop epilepsy (68), and while most patients will respond to pharmacotherapy, in one study, 34% of 170 patients had medically refractory epilepsy (69). The precise pathogenesis of ASD remains unknown, but genetic and environmental factors have been known to contribute to its onset. One such factor is exposure to valproic acid (VPA) in utero, which is associated with a 12% incidence of ASD in children (70) and is used as an animal model of induction of ASD (42).
Using the animal model of autism induced by prenatal exposure to VPA in mice, the effects of KD were examined. Pregnant Swiss mice received a single intraperitoneal injection of 600 mg/kg of VPA (n = 26) or saline (n = 18) on gestational day 11. At day 21, 16 VPA treated and 16 control mice were used. Half of each group was fed KD (lard 690 g/kg, sunflower oil 5 g/kg, protein 250 g/kg, fiber 10 g/kg, ash 5 g/kg), while the other received a standard diet. Ketone levels were not measured. After 70 days on KD, a statistically significant result was found in mice with VPA in behaviors such as social deficits and stereotypies that are common behaviors in those with ASD (42). It is also believed that mitochondrial dysfunction may play a role in the onset of ASD (35). Ahn et al. (43) aimed to determine if KD could reverse the social deficits and mitochondrial dysfunction seen in a prenatal VPA animal model of autism using Wistar rats. On postnatal day 21, rats were placed on either KD (6:1 lipid:non-lipid ratio) or standard diet for 10–14 days. Beta-hydroxybutyrate was measured. KD had a significant effect and was able to modify complex social behaviors in VPA and control rats and mitochondrial respiration (43).
Another animal model of autism using the inbred BTBR mouse strain that exhibits three core features of autism, including reduced sociability, communication, and increased repetitive behavior, was studied (71). In another study, 33 genes were differentially expressed in the temporal cortex and 48 in the hippocampus suggesting deficits in the stress response and in neuronal signaling and communication in BTBR mice. After 14 days on KD (6.3:1 lipid:non-lipid ratio), both brain regions showed improvements on autistic deficits associated with myelin formation and white matter development (44). One study has found that in BTBR mice, KD reduces total gut microbial and compositional remodeling of the mouse microbiome providing a potential explanation as to its efficacy in this model (72).
In an animal model with behavioral characteristics of ASD and comorbid epilepsy in male and female EL mice, the effect of KD was assessed (45). Testing occurred at 8–9 weeks postpartum following 3–4 weeks of dietary treatment. Animals were fed either a standard diet or one of two KDs (3.0:1 or 6.6:1 lipid:non-lipid ratio). KD raised ketones in all groups, but the higher fat ratio deepened ketosis. Both KDs significantly increased sociability, time spent in the chamber with another mouse, in females and males. Social novelty, preference for a newly introduced mouse was higher in females fed the higher KD ratio. The test of repetitive behavior (self-grooming) was significantly decreased in males but was non-significant in females. This study provides some intriguing results regarding the effects of sex and KD in a mouse model of ASD and idiopathic epilepsy.
The role of KD in ASD has been examined in a pilot study of 30 children (50). The diet (30% of energy as MCT oil, 30% fresh cream, 11% as saturated fat, 19% carbohydrates, and 10% as protein) was administered for 6 months with intervals of 4 weeks with 2 diet-free weeks. Of the total sample, 40% did not comply or did not tolerate the diet. Urinary ketones were measured. In the remaining sample, two children showed significant improvements on the Childhood Autism Rating Scale, while the rest showed mild-to-moderate improvements. As observed in patients with epilepsy, after the termination of KD the benefits persisted, which raise intriguing questions regarding the effects of plasticity.
In a case study of a child with autism and epilepsy, following standard treatment non-response, the individual was placed on KD (1.5:1 lipid:non-lipid ratio) with adjunct anticonvulsant therapy (51). The patient was in ketosis. After initiation of the diet several benefits ensued including the resolution of morbid obesity and the improvement of cognitive and behavioral features of the disorder. After several years on the diet, the patient’s score on the Childhood Autism Rating Scale decreased from 49 to 17, a change from a rating of severe autism to non-autistic, and IQ increased by 70 points. Fourteen months following the initiation of the diet the patient was also seizure free.
The suggested mechanisms of action of KD in ASD include that it may reduce pain sensitivity through the reduction of glucose and may have anti-inflammatory properties as it reduces swelling and plasma extravasation (42). In a systematic review of KD in ASD it was concluded that the limited number of reports of improvements after treatment with the diet is not sufficient to attest to the practicability of KD as a treatment for the disorder (73).
Attention Deficit Hyperactivity Disorder
Attention deficit hyperactivity disorder is characterized by a lack of behavioral inhibition and by neuropsychological deficits in four areas, including working memory, self-regulation of affect–motivation–arousal, internalization of speech, and behavioral analysis and synthesis (74). ADHD is the most commonly occurring mental disorder in children and adolescents with epilepsy occurring in 16 (29.1%) of 78 patients (75). Children with ADHD have a high frequency of epileptiform discharges as observed by EEG (76). In a prospective study of children with epilepsy (n = 34) on KD it was found that after 1 year on the diet there was a statistically significant improvement of attention and social functioning (77).
There is little evidence examining ADHD and KD, but a 6-month prospective, randomized, double-blinded, placebo-controlled, crossover dietary trial compared the effects of KD (10% moisture, 28% protein, 15% fat, 6% ash, 2% crude fiber, and MCT oil) or a standard diet on behavior in 21 dogs with comorbid ADHD and idiopathic epilepsy (36). It was hypothesized that there were three specific behaviors related to ADHD in dogs including excitability, chasing, and trainability. ADHD in dogs is manifested as inattention and excitability/impulsivity, which have been likened to the disorder in humans (78). When compared with the standard diet, KD resulted in a significant improvement in ADHD-related behaviors. Serum beta-hydroxybutyrate was measured. The mechanisms of behavioral improvements during KD remain unknown. The authors postulated that alterations of energy metabolism in the brain may contribute to behavioral changes. Research into humans with ADHD and KD is lacking.
Discussion
In neurology, KD is an established treatment option for treatment-resistant epilepsy with evidence from a range of studies including controlled trials. By contrast, KD research in humans with mental disorders, though extending over a 50-year period, has received little attention with few studies other than case reports, small sample size open studies, and no controlled trials. Animal studies have been more systematic, investigating mechanisms as well as outcomes on putative disease analogs in rodents and canines, the latter including randomized controlled trials of KD.
With respect to mechanisms, the pathophysiology of the mental disorders covered in this review is not clearly understood, though impaired metabolism due to mitochondrial dysfunction has been identified as an important substrate (34). This is congruent with findings in neurological conditions, Stafstrom and Rho concluding that energy metabolism changes induced by KD in neurological conditions suggest a final common pathway implicating mitochondrial function (26). KD may also influence neuronal plasticity by modifying neural circuits and cellular properties to normalize function (26). Mitochondrial dysfunction may be relevant in some mental disorders including schizophrenia, ASD, and ADHD, whereas the improvements seen in anxiety, depression, and bipolar disorder may be related to alterations of neurotransmitters.
One other possible mediator of the beneficial effects of KD in mental disorders is the effect on sleep. In a study of 18 children with treatment-resistant epilepsy, after 3 months of KD sleep was reported to be enhanced with a pattern of significant reduction in total night sleep, preservation of slow-wave sleep, increased rapid eye movement (REM) sleep, and decrease in sleep stage 2 (79). The mechanisms by which KD affects sleep is unclear (80), and more studies are necessary to confirm reports that certain dietary patterns and foods improve sleep (81).
Sleep problems and mental disorders are codependent conditions that exacerbate each other and lead to impaired quality of life and increased disability (82). Impairments of sleep are a widespread feature of mental disorders. Anxious patients have been found to have significantly less sleep period time, total sleep time, percentage stage REM and percent stage 4 sleep, shorter latency to stage REM, and greater percent stage 1 sleep than healthy controls (83). REM sleep abnormalities including shortening of REM latency, lengthening of the duration of the first REM period, and heightening of REM density are found in patients with depression (84). In patients with inter-episode bipolar disorder, shorter sleep onset latency and increased REM density has been observed (85). A decrease of REM sleep latency in schizophrenia has been described (86). Individuals with ASD have prolonged sleep latency, more frequent nocturnal awakenings, lower sleep efficiency, increased duration of NREM stage 1 sleep, and decreased deeper stages of NREM sleep (87). In ADHD, disturbed sleep architecture has been described including shorter REM latencies, reduced REM sleep, and increased delta sleep percentage (88). It should also be noted that sleep deprivation can precipitate mania in bipolar disorder and seizures in epilepsy (89) and can be used as a treatment for depression (90). The specific effects of KD on these mental disorder-related sleep symptoms has not been studied in detail, but interactions are likely and may be possible mediators of a therapeutic effect.
In epilepsy, KD acts differently to antiepileptic drugs (AED) in seizure prevention. While AED act directly on ion channels and synaptic processes, KD acts through intermediary metabolic pathways (91). Chang et al. showed that an MCT (palm oil and coconut oil) diet, a variation of KD, reduces seizures in children via inhibition on AMPA receptors (12, 92, 93). The questions posed by the literature indicate that the mechanism of action is still unknown, and there may be many potential pathways involved. The mechanism of action appears different from AED and therefore probably psychiatric drugs also, which opens potential avenues for treatment in a manner that may supplement conventional pharmacological treatment approaches. The exact mechanism of action of KD is unclear, and for detailed discussion, see Rogawski et al. (91). Thus, present knowledge indicates that KD exerts its effects on seizure control by mechanisms different from conventional AED and therefore, in psychiatry, this may also be the case although as yet unproven.
There are a number of reasons why the effectiveness of KD in mental disorders remains unproven. In addition to the low number of human studies, the quality of the studies has some significant limitations. Sample sizes are small, there is no control for placebo effects, and the establishment of ketosis is generally lacking with no confirmatory measurement of ketones in three human studies. There are also significant limitations associated with the diet itself including the detailed regimen, unpalatable food choices, side effects, and duration of diet required. There are also no enforced standards as to what constitutes KD in humans with variable lipid:non-lipid ratios reported. KD monotherapy is used in animal models of mental disorders but remains unexamined in human studies. Ten adult patients with epilepsy followed KD monotherapy, and it was concluded that it may be feasible, well tolerated, and an effective long-term alternative (94).
To comply with KD, patients who may be acutely unwell are required to measure food portions to ensure that the macronutrient targets associated with the diet are met, and they may find it difficult to adhere to such a demanding diet (47). This is particularly so for patients with mental disorders where symptoms such as impulsivity in mania, apathy, and reduced appetite in depression, food cravings, and binge eating associated with antipsychotic medications may variously interfere with compliance with KD (95). A mitigating factor to the outcomes in children with epilepsy may be that the diet is typically administered in a hospital setting initially and subsequently, by caregivers.
El-Mallakh and Paskitti have outlined the adverse consequences of KD including constipation, menstrual irregularities, elevated serum cholesterol and triglycerides, hypoproteinemia, hemolytic anemia, elevated liver enzymes, and gall stones (96). Kidney stones have been noted to occur in 1 of 20 children on the diet (97). In a period of almost 2 years, prospective monitoring of 52 children with pediatric epilepsy was conducted. Ten percent of children experienced serious adverse events associated with the diet 1 month after initiation (98). This included presacral and periorbital edema, developmental impairment, and unwanted weight loss in an infant, renal tubular acidosis, viral gastroenteritis, abnormal liver function, and thrombocytopenia. It should be noted that all patients were being treated with concomitant VPA. It was reported in a retrospective study of 158 children with intractable epilepsy that, in 80% emesis, food refusal and hypoglycemia occurred (99).
By definition, KD is confirmed by the production of ketones measured in the blood or urine. In the reviewed literature covering KD in mental disorders, four studies did not report ketone levels, which severely limit comparability across studies and the ability to invoke any consistent mechanism. One study compared whether measuring serum beta-hydroxybutyrate or urinary ketones was superior to monitor KD (100). In humans, it was found that beta-hydroxybutyrate correlated more strongly with a reduction in seizures than urinary ketones; therefore, future studies should measure ketones in the blood. Another issue is that the lipid:non-lipid ratios used were different (see Tables 1 and 2). In a study that compared the efficacy and tolerability of the 3:1 versus the 4:1 lipid:non-lipid ratios, the latter was shown to have a higher seizure-free outcome (2).
One issue when interpreting the results is the levels of evidence in the evidence-based hierarchy. Animal models of mental disorders are considered valuable preclinical tools to investigate the neurobiological basis of a disorder (62). While this may be true, they are nonetheless subject to a number of limitations. One such limitation is the issue of validity, and their use is based on the assumption that humans and animals share basic neurobiological mechanisms associated with the complex behaviors that mimic mental disorders in animals (101).
Another difficulty posed to practitioners is that there are currently no international protocols guiding the administration of the diet; this is something that may be established from future research into KD. There was only one case study that detailed what the participant, diagnosed with schizophrenia, ate, and it was not established whether this individual was in ketosis. In the various studies in humans, outcomes were assessed following dietary durations that varied from 7 days to 2 years.
Further research into the neural correlates of KD is needed to help explain the mechanisms by which it acts. Some suggestions regarding methodologies, provided by Fusar-Poli are elaborated below. Changes in glucose metabolism seen in KD could be examined using positron emission tomography fluorodeoxyglucose. To observe the neural correlates of KD, a combination of electrophysiological measures including EEG and magnetoencephalogram and fMRI/PET to combine the high temporal resolution of the former with the high spatial resolution of the latter may be used (102).
In the neurological literature, a single study, in Alzheimer’s disease, used a synthesized ketogenic compound AC-1202 rather than a KD. AC-1202 is an MCT composed of glycerine and caprylic acid (23). It is not yet clear what role ketogenic pharmacotherapy options might play alongside or as a substitute for KD.
While these animal studies are placing research into KD on a firm footing and identifying some promising leads, on balance the evidence in humans is insufficient to form an opinion as to the efficacy or lack thereof of this intervention in the mental disorders reported. Further basic research to clarify the specifics of dietary manipulation or supplementation required to produce optimum ketosis in specific models is an obvious intermediate step toward studying the effectiveness of the diet in human mental disorders using conventional phases of research including open-label studies and randomized controlled trials.
Author Contributions
EB derived the concept of the article from which she received supervision and expert advice in the area of psychiatry from KK and neurology from BT.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
EB’s research is supported by an Australian Postgraduate Award and the Goddard Sapin-Jaloustre Trust.
References
1. Wheless JW. History of the ketogenic diet. Epilepsia (2008) 49(s8):3–5. doi: 10.1111/j.1528-1167.2008.01821.x
2. Hee Seo J, Mock Lee Y, Soo Lee J, Chul Kang H, Dong Kim H. Efficacy and tolerability of the ketogenic diet according to lipid:nonlipid ratios – comparison of 3: 1 with 4: 1 diet. Epilepsia (2007) 48(4):801–5. doi:10.1111/j.1528-1167.2007.01025.x
3. Woodyatt R. Objects and method of diet adjustment in diabetes. Arch Intern Med (1921) 28(2):125–41. doi:10.1001/archinte.1921.00100140002001
4. Vining EP, Freeman JM, Ballaban-Gil K, Camfield CS, Camfield PR, Holmes GL, et al. A multicenter study of the efficacy of the ketogenic diet. Arch Neurol (1998) 55(11):1433–7. doi:10.1001/archneur.55.11.1433
5. Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol (2008) 7(6):500–6. doi:10.1016/S1474-4422(08)70092-9
6. McTague A, Cross JH. Treatment of epileptic encephalopathies. CNS Drugs (2013) 27(3):175–84. doi:10.1007/s40263-013-0041-6
7. Lefevre F, Aronson N. Ketogenic diet for the treatment of refractory epilepsy in children: a systematic review of efficacy. Pediatrics (2000) 105(4):e46–46. doi:10.1542/peds.105.4.e46
8. Henderson CB, Filloux FM, Alder SC, Lyon JL, Caplin DA. Efficacy of the ketogenic diet as a treatment option for epilepsy: meta-analysis. J Child Neurol (2006) 21(3):193–8. doi:10.2310/7010.2006.00044
9. Levy RG, Cooper PN, Giri P, Weston J. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev (2012) 14(3):CD001903. doi:10.1002/14651858.CD001903.pub2
10. Winesett SP, Bessone SK, Kossoff EH. The ketogenic diet in pharmacoresistant childhood epilepsy. Expert Rev Neurother (2015) 15(6):621–8. doi:10.1586/14737175.2015.1044982
11. Likhodii SS, Serbanescu I, Cortez MA, Murphy P, Snead OC III, Burnham WM. Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet. Ann Neurol (2003) 54(2):219–26. doi:10.1002/ana.10634
12. Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain (2016) 139(2):431–43. doi:10.1093/brain/awv325
13. Żarnowski T, Choragiewicz T, Tulidowicz-Bielak M, Thaler S, Rejdak R, Żarnowska I, et al. Ketogenic diet increases concentrations of kynurenic acid in discrete brain structures of young and adult rats. J Neural Transm (2012) 119(6):679–84. doi:10.1007/s00702-011-0750-2
14. Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett (2001) 313(1):96–8. doi:10.1016/S0304-3940(01)02242-X
15. Olsson SK, Samuelsson M, Jönsson EG. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J Psychiatry Neurosci (2010) 35(3):195. doi:10.1503/jpn.090180
16. Erhardt S, Olsson SK, Engberg G. Pharmacological manipulation of kynurenic acid. CNS Drugs (2009) 23(2):91–101. doi:10.2165/00023210-200923020-00001
17. Kossoff EH. International consensus statement on clinical implementation of the ketogenic diet: agreement, flexibility, and controversy. Epilepsia (2008) 49(s8):11–3. doi:10.1111/j.1528-1167.2008.01823.x
18. Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist A, Blackford R, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia (2009) 50(2):304–17. doi:10.1111/j.1528-1167.2008.01765.x
19. Kossoff E. Danger in the pipeline for the ketogenic diet? Epilepsy Curr (2014) 14(6):343–4. doi:10.5698/1535-7597-14.6.343
20. Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, et al. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci (2006) 7(1):29. doi:10.1186/1471-2202-7-29
21. Appelberg KS, Hovda DA, Prins ML. The effects of a ketogenic diet on behavioral outcome after controlled cortical impact injury in the juvenile and adult rat. J Neurotrauma (2009) 26(4):497–506. doi:10.1089/neu.2008.0664
22. Hao J, Liu R, Turner G, Shi F-D, Rho JM. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One (2012) 7(5):e35476. doi:10.1371/journal.pone.0035476
23. Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (2009) 6(1):1. doi:10.1186/1743-7075-6-31
24. Vanitallie T, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield S. Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology (2005) 64(4):728–30. doi:10.1212/01.WNL.0000152046.11390.45
25. Kossoff E, Huffman J, Turner Z, Gladstein J. Use of the modified Atkins diet for adolescents with chronic daily headache. Cephalalgia (2010) 30(8):1014–6. doi:10.1111/j.1468-2982.2009.02016.x
26. Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol (2012) 3:59. doi:10.3389/fphar.2012.00059
27. Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev (2009) 59(2):293–315. doi:10.1016/j.brainresrev.2008.09.002
28. Appanna VD, Auger C, Lemire J. Energy, the driving force behind good and ill health. Front Cell Dev Biol (2014) 2:28. doi:10.3389/fcell.2014.00028
29. Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab (2010) 7(1):1. doi:10.1186/1743-7075-7-7
30. Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation (2007) 116(4):434–48. doi:10.1161/CIRCULATIONAHA.107.702795
31. Roberts SB, Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev (2006) 86(2):651–67. doi:10.1152/physrev.00019.2005
32. Waldbaum S, Patel M. Mitochondrial dysfunction and oxidative stress: a contributing link to acquired epilepsy? J Bioenerg Biomembr (2010) 42(6):449–55. doi:10.1007/s10863-010-9320-9
33. Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol (2011) 10(2):187–98. doi:10.1016/S1474-4422(10)70277-5
34. Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction and psychiatric disorders. Neurochem Res (2009) 34(6):1021–9. doi:10.1007/s11064-008-9865-8
35. Rossignol D, Frye R. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry (2012) 17(3):290–314. doi:10.1038/mp.2010.136
36. Packer RM, Law TH, Davies E, Zanghi B, Pan Y, Volk HA. Effects of a ketogenic diet on ADHD-like behavior in dogs with idiopathic epilepsy. Epilepsy Behav (2016) 55:62–8. doi:10.1016/j.yebeh.2015.11.014
37. Tellez-Zenteno JF, Patten SB, Jetté N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia (2007) 48(12):2336–44. doi:10.1111/j.1528-1167.2007.01222.x
38. Ari C, Kovács Z, Juhasz G, Murdun C, Goldhagen CR, Koutnik AM, et al. Exogenous ketone supplements reduce anxiety-related behavior in Sprague-Dawley and Wistar Albino Glaxo/Rijswijk rats. Front Mol Neurosci (2016) 9:137. doi:10.3389/fnmol.2016.00137
39. Murphy P, Likhodii S, Nylen K, Burnham W. The antidepressant properties of the ketogenic diet. Biol Psychiatry (2004) 56(12):981–3. doi:10.1016/j.biopsych.2004.09.019
40. Sussman D, Germann J, Henkelman M. Gestational ketogenic diet programs brain structure and susceptibility to depression & anxiety in the adult mouse offspring. Brain Behav (2015) 5(2):e00300. doi:10.1002/brb3.300
41. Kraeuter AK, Loxton H, Lima BC, Rudd D, Sarnyai Z. Ketogenic diet reverses behavioral abnormalities in an acute NMDA receptor hypofunction model of schizophrenia. Schizophr Res (2015) 169(1–3):491. doi:10.1016/j.schres.2015.10.041
42. Castro K, Baronio D, Perry IS, Riesgo RDS, Gottfried C. The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutr Neurosci (2016) 19:1–8. doi:10.1080/1028415X.2015.1133029
43. Ahn Y, Narous M, Tobias R, Rho JM, Mychasiuk R. The ketogenic diet modifies social and metabolic alterations identified in the prenatal valproic acid model of autism spectrum disorder. Dev Neurosci (2014) 36(5):371–80. doi:10.1159/000362645
44. Mychasiuk R, Rho JM. Genetic modifications associated with ketogenic diet treatment in the BTBRT+ Tf/J mouse model of autism spectrum disorder. Autism Res (2016) 8:1–16. doi:10.1002/aur.1682
45. Ruskin DN, Fortin JA, Bisnauth SN, Masino SA. Ketogenic diets improve behaviors associated with autism spectrum disorder in a sex-specific manner in the EL mouse. Physiol Behav (2017) 168:138–45. doi:10.1016/j.physbeh.2016.10.023
46. Phelps JR, Siemers SV, El-Mallakh RS. The ketogenic diet for type II bipolar disorder. Neurocase (2013) 19(5):423–6. doi:10.1080/13554794.2012.690421
47. Yaroslavsky Y, Stahl Z, Belmaker R. Ketogenic diet in bipolar illness. Bipolar Disord (2002) 4(1):75–75. doi:10.1034/j.1399-5618.2002.01212.x
48. Pacheco A, Easterling W, Pryer M. A pilot study of the ketogenic diet in schizophrenia. Am J Psychiatry (1965) 121(11):1110–1. doi:10.1176/ajp.121.11.1110
49. Kraft BD, Westman EC. Schizophrenia, gluten, and low-carbohydrate, ketogenic diets: a case report and review of the literature. Nutr Metab (2009) 6(1):1. doi:10.1186/1743-7075-6-10
50. Evangeliou A, Vlachonikolis I, Mihailidou H, Spilioti M, Skarpalezou A, Makaronas N, et al. Application of a ketogenic diet in children with autistic behavior: pilot study. J Child Neurol (2003) 18(2):113–8. doi:10.1177/08830738030180020501
51. Herbert MR, Buckley JA. Autism and dietary therapy case report and review of the literature. J Child Neurol (2013) 28(8):975–82. doi:10.1177/0883073813488668
52. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry (2005) 62(6):617–27. doi:10.1001/archpsyc.62.6.593
53. Rigoli F, Ewbank M, Dalgleish T, Calder A. Threat visibility modulates the defensive brain circuit underlying fear and anxiety. Neurosci Lett (2016) 612:7–13. doi:10.1016/j.neulet.2015.11.026
54. Brandt C, Mula M. Anxiety disorders in people with epilepsy. Epilepsy Behav (2016) 59:87–91. doi:10.1016/j.yebeh.2016.03.020
55. Mula M. Depression in epilepsy. Curr Opin Neurol (2017) 30(2):180–6. doi:10.1097/WCO.0000000000000431
56. Porsolt R, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther (1977) 229(2):327–36.
57. Kroczka B, Branski P, Palucha A, Pilc A, Nowak G. Antidepressant-like properties of zinc in rodent forced swim test. Brain Res Bull (2001) 55(2):297–300. doi:10.1016/S0361-9230(01)00473-7
58. Dahlin M, Månsson J-E, Åmark P. CSF levels of dopamine and serotonin, but not norepinephrine, metabolites are influenced by the ketogenic diet in children with epilepsy. Epilepsy Res (2012) 99(1):132–8. doi:10.1016/j.eplepsyres.2011.11.003
59. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association (2013).
60. Kilbourne AM, Rofey DL, McCarthy JF, Post EP, Welsh D, Blow FC. Nutrition and exercise behavior among patients with bipolar disorder1. Bipolar Disord (2007) 9(5):443–52. doi:10.1111/j.1399-5618.2007.00386.x
61. Ripke S, Neale BM, Corvin A, Walters JT, Farh K-H, Holmans PA, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature (2014) 511(7510):421. doi:10.1038/nature13595
62. Jones C, Watson D, Fone K. Animal models of schizophrenia. Br J Pharmacol (2011) 164(4):1162–94. doi:10.1111/j.1476-5381.2011.01386.x
63. Martins-de-Souza D, Harris LW, Guest PC, Bahn S. The role of energy metabolism dysfunction and oxidative stress in schizophrenia revealed by proteomics. Antioxid Redox Signal (2011) 15(7):2067–79. doi:10.1089/ars.2010.3459
64. Harris LW, Guest PC, Wayland MT, Umrania Y, Krishnamurthy D, Rahmoune H, et al. Schizophrenia: metabolic aspects of aetiology, diagnosis and future treatment strategies. Psychoneuroendocrinology (2013) 38(6):752–66. doi:10.1016/j.psyneuen.2012.09.009
65. Dwyer DS, Bradley RJ, Kablinger AS, Freeman AM III. Glucose metabolism in relation to schizophrenia and antipsychotic drug treatment. Ann Clin Psychiatry (2001) 13(2):103–13. doi:10.3109/10401230109148955
66. Scheen A, De Hert M. Abnormal glucose metabolism in patients treated with antipsychotics. Diabetes Metab (2007) 33(3):169–75. doi:10.1016/j.diabet.2007.01.003
67. De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature (2014) 515(7526):209–15. doi:10.1038/nature13772
68. Garcia-Penas J. [Autism spectrum disorder and epilepsy: the role of ketogenic diet]. Rev Neurol (2016) 62:S73–8.
69. Sansa G, Carlson C, Doyle W, Weiner HL, Bluvstein J, Barr W, et al. Medically refractory epilepsy in autism. Epilepsia (2011) 52(6):1071–5. doi:10.1111/j.1528-1167.2011.03069.x
70. Bromley RL, Mawer GE, Briggs M, Cheyne C, Clayton-Smith J, García-Fiñana M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry (2013) 84(6):637–43. doi:10.1136/jnnp-2012-304270
71. Ruskin DN, Svedova J, Cote JL, Sandau U, Rho JM, Kawamura M Jr, et al. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS One (2013) 8(6):e65021. doi:10.1371/journal.pone.0065021
72. Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Shearer J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism (2016) 7(1):37. doi:10.1186/s13229-016-0099-3
73. Castro K, Faccioli LS, Baronio D, Gottfried C, Perry IS, dos Santos Riesgo R. Effect of a ketogenic diet on autism spectrum disorder: a systematic review. Res Autism Spectr Disord (2015) 20:31–8. doi:10.1016/j.rasd.2015.08.005
74. Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull (1997) 121(1):65. doi:10.1037/0033-2909.121.1.65
75. Thome-Souza S, Kuczynski E, Assumpção F, Rzezak P, Fuentes D, Fiore L, et al. Which factors may play a pivotal role on determining the type of psychiatric disorder in children and adolescents with epilepsy? Epilepsy Behav (2004) 5(6):988–94. doi:10.1016/j.yebeh.2004.09.001
76. Millichap JJ, Stack CV, Millichap JG. Frequency of epileptiform discharges in the sleep-deprived electroencephalogram in children evaluated for attention-deficit disorders. J Child Neurol (2010) 26(1):6–11. doi:10.1177/0883073810371228
77. Pulsifer MB, Gordon JM, Brandt J, Vining EP, Freeman JM. Effects of ketogenic diet on development and behavior: preliminary report of a prospective study. Dev Med Child Neurol (2001) 43(05):301–6. doi:10.1111/j.1469-8749.2001.tb00209.x
78. Jokinen T, Tiira K, Metsähonkala L, Seppälä E, Hielm-Björkman A, Lohi H, et al. Behavioral abnormalities in Lagotto Romagnolo dogs with a history of benign familial juvenile epilepsy: a long-term follow-up study. J Vet Intern Med (2015) 29(4):1081–7. doi:10.1111/jvim.12611
79. Hallböök T, Lundgren J, Rosén I. Ketogenic diet improves sleep quality in children with therapy-resistant epilepsy. Epilepsia (2007) 48(1):59–65. doi:10.1111/j.1528-1167.2006.00834.x
80. Hallböök T, Ji S, Maudsley S, Martin B. The effects of the ketogenic diet on behavior and cognition. Epilepsy Res (2012) 100(3):304–9. doi:10.1016/j.eplepsyres.2011.04.017
81. St-Onge MP, Mikic A, Pietrolungo CE. Effects of diet on sleep quality. Adv Nutr (2016) 7(5):938–49. doi:10.3945/an.116.012336
83. Rosa RR, Bonnet MH, Kramer M. The relationship of sleep and anxiety in anxious subjects. Biol Psychol (1983) 16(1):119–26. doi:10.1016/0301-0511(83)90058-3
84. Berger M, Riemann D. REM sleep in depression – an overview. J Sleep Res (1993) 4:211–23. doi:10.1111/j.1365-2869.1993.tb00092.x
85. Talbot LS, Hairston IS, Eidelman P, Gruber J, Harvey AG. The effect of mood on sleep onset latency and REM sleep in interepisode bipolar disorder. J Abnorm Psychol (2009) 118(3):448. doi:10.1037/a0016605
86. Gottesmann C, Gottesman I. The neurobiological characteristics of rapid eye movement (REM) sleep are candidate endophenotypes of depression, schizophrenia, mental retardation and dementia. Prog Neurobiol (2007) 81(4):237–50. doi:10.1016/j.pneurobio.2007.01.004
87. Richdale AL. Sleep problems in autism: prevalence, cause, and intervention. Dev Med Child Neurol (1999) 41(1):60–6. doi:10.1017/S0012162299000122
88. van der Heijden KB, Smits MG, Gunning WB. Sleep-related disorders in ADHD: a review. Clin Pediatr (2005) 44(3):201–10. doi:10.1177/000992280504400303
89. Bostock ECS, Kirkby KC, Garry MI, Taylor BVM. Comparison of precipitating factors for mania and partial seizures: indicative of shared pathophysiology? J Affect Disord (2015) 183:57–67. doi:10.1016/j.jad.2015.04.057
90. Dallaspezia S, Benedetti F. Sleep deprivation therapy for dsepression. Curr Top Behav Neurosci (2015) 25:483–502. doi:10.1007/7854_2014_363
91. Rogawski MA, Rho JM, Löscher W. Mechanisms of action of antiseizure drugs and the ketogenic diet. Cold Spring Harb Perspect Med (2016). doi:10.1101/cshperspect.a022780
92. Freeman J, Veggiotti P, Lanzi G, Tagliabue A, Perucca E, Institute of Neurology IRCCS C. Mondino Foundation. The ketogenic diet: from molecular mechanisms to clinical effects. Epilepsy Res (2006) 68(2):145–80. doi:10.1016/j.eplepsyres.2005.10.003
93. Rogawski MA. A fatty acid in the MCT ketogenic diet for epilepsy treatment blocks AMPA receptors. Brain (2016) 139(2):306–9. doi:10.1093/brain/awv369
94. Cervenka MC, Henry-Barron BJ, Kossoff EH. Is there a role for diet monotherapy in adult epilepsy? Epilepsy Behav Case Rep (2017) 7:6–9. doi:10.1016/j.ebcr.2016.09.005
95. Kluge M, Schuld A, Himmerich H, Dalal M, Schacht A, Wehmeier PM, et al. Clozapine and olanzapine are associated with food craving and binge eating: results from a randomized double-blind study. J Clin Psychopharmacol (2007) 27(6):662–6. doi:10.1097/jcp.0b013e31815a8872
96. El-Mallakh R, Paskitti M. The ketogenic diet may have mood-stabilizing properties. Med Hypotheses (2001) 57(6):724–6. doi:10.1054/mehy.2001.1446
97. Sampath A, Kossoff EH, Furth SL, Pyzik PL, Vining EP. Kidney stones and the ketogenic diet: risk factors and prevention. J Child Neurol (2007) 22(4):375–8. doi:10.1177/0883073807301926
98. Ballaban-Gil K, Callahan C, O’dell C, Pappo M, Moshe S, Shinnar S. Complications of the ketogenic diet. Epilepsia (1998) 39(7):744–8. doi:10.1111/j.1528-1157.1998.tb01160.x
99. Lin A, Turner Z, Doerrer SC, Stanfield A, Kossoff EH. Complications during ketogenic diet initiation: prevalence, treatment, and influence on seizure outcomes. Pediatr Neurol (2017) 68:35–9. doi:10.1016/j.pediatrneurol.2017.01.007
100. van Delft R, Lambrechts D, Verschuure P, Hulsman J, Majoie M. Blood beta-hydroxybutyrate correlates better with seizure reduction due to ketogenic diet than do ketones in the urine. Seizure (2010) 19(1):36–9. doi:10.1016/j.seizure.2009.10.009
101. Tordjman S, Drapier D, Bonnot O, Graignic R, Fortes S, Cohen D, et al. Animal models relevant to schizophrenia and autism: validity and limitations. Behav Genet (2007) 37(1):61–78. doi:10.1007/s10519-006-9120-5
Keywords: ketogenic diet, psychiatry, mental disorders, ketones, epilepsy
Citation: Bostock ECS, Kirkby KC and Taylor BVM (2017) The Current Status of the Ketogenic Diet in Psychiatry. Front. Psychiatry 8:43. doi: 10.3389/fpsyt.2017.00043
Received: 02 February 2017; Accepted: 02 March 2017;
Published: 20 March 2017
Edited by:
Roumen Kirov, Bulgarian Academy of Sciences, BulgariaReviewed by:
Christian Benedict, Uppsala University, SwedenJong Min Rho, University of Calgary, Canada
Kamila C. Grokoski, Universidade Federal do Rio Grande do Sul, Brazil
Copyright: © 2017 Bostock, Kirkby and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emmanuelle C. S. Bostock, ebostock@utas.edu.au
 Emmanuelle C. S. Bostock
Emmanuelle C. S. Bostock Kenneth C. Kirkby2
Kenneth C. Kirkby2