
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry, 21 September 2016
Sec. Addictive Disorders
Volume 7 - 2016 | https://doi.org/10.3389/fpsyt.2016.00160
This article is part of the Research TopicMemory systems of the addicted brain: the underestimated role of drug-induced cognitive biases in addiction and its treatmentView all 14 articles
Although smoking prevalence has declined in recent years, certain subpopulations continue to smoke at disproportionately high rates and show resistance to cessation treatments. Individuals showing cognitive and affective impairments, including emotional distress and deficits in attention, memory, and inhibitory control, particularly in the context of psychiatric conditions, such as attention-deficit hyperactivity disorder, schizophrenia, and mood disorders, are at higher risk for tobacco addiction. Nicotine has been shown to improve cognitive and emotional processing in some conditions, including during tobacco abstinence. Self-medication of cognitive deficits or negative affect has been proposed to underlie high rates of tobacco smoking among people with psychiatric disorders. However, pre-existing cognitive and mood disorders may also influence the development and maintenance of nicotine dependence, by biasing nicotine-induced alterations in information processing and associative learning, decision-making, and inhibitory control. Here, we discuss the potential forms of contribution of cognitive and affective deficits to nicotine addiction-related processes, by reviewing major clinical and preclinical studies investigating either the procognitive and therapeutic action of nicotine or the putative primary role of cognitive and emotional impairments in addiction-like features.
Smoking tobacco remains the most preventable cause of morbidity and mortality worldwide. Nicotine is the main psychoactive component of tobacco responsible for its addictive properties and modifies the function of the brain via its interaction with the nicotinic acetylcholine receptors (nAChRs) (1, 2). Drug addiction is a complex psychiatric disorder, and there are individual differences in the vulnerability to develop this pathology that can be conceptualized at different levels interacting with each other, such as environmental, genetic, and psychological contributions. Only a percentage of individuals starting to smoke tobacco eventually develop an addiction (3). In particular, there is a high prevalence of smoking in patients with psychiatric disorders. However, it has been difficult to define in clinical studies the nature of the causal interactions between these pathologies. The psychological and neural processes that underlie addiction have been shown to overlap with those that support cognitive and emotional functions. One critical question is to which extent psychiatric conditions may pre-date smoking or develop after chronic exposure to nicotine. One of the main limitations to resolve this issue is the difficulty to conduct longitudinal prospective studies in humans and to control for co-use of multiple substances in patient cohorts. As a consequence, preclinical research has increasingly aimed at identifying distinctive endophenotypes that may predispose individuals to nicotine addiction-like processes and/or that are influenced by nicotine exposure. Animal models can never encompass entirely the complexity of the psychological processes underlying behavior related to addiction and other psychiatric conditions in humans with full face and construct validities. Yet, they provide a valuable tool to precisely control the environmental (and genetic) context, the conditions of drug delivery, and to determine whether beforehand drug consumption influences the risk to develop specific endophenotypes or whether pre-existing endophenotypes confer vulnerability to addiction, through the implementation of longitudinal studies. They also allow detailed investigations of the distinct stages of addiction that may be connected to some endophenotypes to varying extents. In fact, the defining criteria of addiction are still a matter of debate, and this pathology exhibits complex dynamics with different stages, from the initiation and maintenance of drug taking to a switch toward a loss of control over drug intake, compulsive drug taking and seeking, i.e., despite negative consequences, together with high rate of relapse after abstinence (4–8). With the use of experimental models of distinct addiction-like behaviors in addition to epidemiological and neurocognitive studies in human subjects, specific behavioral endophenotypes of presumed genetic origin have been identified as significant risk factors for drug addiction according to different modalities. Understanding the causal relationship between nicotine addiction and psychiatric disorders may significantly contribute to the treatment of comorbid psychiatric conditions and smoking. This review will describe and discuss both clinical and preclinical studies that brought significant insight in that matter.
Vulnerability to addiction varies across individuals. Thus, although many people experiment with drugs of abuse, most do not develop drug addiction as defined by diagnostic criteria for substance-use disorder (9). Individual differences in vulnerability to abuse are thought to exist before the first drug experience and clinical evidence suggests that these differences reflect both genetic and environmental determinants, including social influences, as well as their interaction [see Ref. (10) for review]. Cigarette smoking is the leading preventable cause of death in the Western world (11) with a prevalence considerably higher in individuals with psychiatric diagnosis. In this part of the review, we will examine non-exhaustively the relationships described in clinical studies between smoking behavior, personality traits, and psychiatric disorders, such as impulsivity, novelty/sensation seeking, attention-deficit hyperactivity disorder (ADHD), depression, and anxiety disorders (see Table 1).
Impulsivity is a heritable and multifaceted psychiatric construct defined by the tendency to engage in inappropriate, premature, poorly planned, and unduly risky actions without adequate forethought about the potential consequences of this behavior (50–53). It has been associated with drug addiction, including tobacco smoking (54).
Current theories differentiate between motor and cognitive aspects of impulsive behavior. Motor impulsivity reflects a failure in motor inhibition leading to impulsive actions and can be assessed by the ability to exert volitional control over a response that has already been initiated or rendered prominent with extensive training. This type of impulsivity can be notably measured in the “stop-signal reaction time task,” in which subjects are trained to respond as quickly as possible but must inhibit their response when a stop signal is presented, or in a go/no go task (54). While several studies linked deficits in this type of impulsivity with alcohol (55), cocaine (56, 57), and methamphetamine (58) addiction, the data about tobacco addiction are less clear. Thus, tobacco smoking has been shown to decrease inhibitory control in a stop-signal task, where an increased number of errors during the stop signal and increased stop latencies were observed (59). But, another study reported no baseline differences between smokers and non-smokers in the same task (60). In addition, an increase in failure in response inhibition in both stop signal and go/no go tasks was observed after nicotine deprivation in tobacco smokers (61, 62), suggesting that nicotine withdrawal induces deficits in inhibitory control. Interestingly, a recent longitudinal prospective study showed that alterations in neural correlates of response inhibition in adolescents increase the risk for subsequent regular cigarette smoking (15), suggesting that functional brain correlates of response inhibition can be used as a marker of risk for tobacco addiction.
Cognitive aspects of impulsivity include response inhibition, delay discounting, and reward/punishment-based decision-making skills and represent the cognitive processes that regulate impulse control (54, 63–65). The delay discounting describes the tendency to discount the value of a reward as a function of the length of delay to its delivery. Higher delay discounting rates have been associated with cigarette smoking. Thus, current smokers tended to discount future monetary reinforcers more than ex-smokers and non-smokers (66), suggesting that smoking increases cognitive impulsivity in this task and that this effect is reversible. Another study confirmed the increased delay discounting in smokers but found no differences in discounting rates for either money or cigarettes between light and heavy smokers (67), a result confirmed in a recent report (68).
Interestingly, performances in delay discounting at age 10 were shown to predict the initiation of smoking behavior in adolescents at age 14 (12). Also, delay-discounting rate has been identified as a strong prognostic indicator of smoking relapse (13), suggesting that cognitive impulsivity can be a risk factor for subsequent tobacco smoking. Trait impulsivity has also been positively associated with the subjective rewarding effects of nicotine (14) as well as explicit expectancies about nicotine reward (16). A longitudinal study using a sample of college men and women showed that trait impulsivity predicts subsequent smoking initiation (17).
Novelty or sensation seeking can be defined as a heritable tendency to seek out varied, novel, complex, and intense sensations and emotional experiences and to show enhanced behavioral responses to novel situations (69–73). It is one of the most critical individual difference factors predicting drug use among humans (74, 75). Novelty seeking is typically measured in humans by using questionnaires such as the Tridimensional Personality Questionnaire (76), the Zuckerman Sensation Seeking Scale, or the Cloninger’s Temperament and Character Inventory (77). This personality trait was shown to predict tobacco use during adolescence (75, 78) and the early onset of smoking in adolescents (79, 80). In line with this, a study of longitudinal smoking patterns in adolescents found that individuals with high novelty seeking were significantly more likely to become regular smokers than never smokers (18). In addition, novelty seeking was increased in heavy smokers (81) and was positively associated with sensitivity to the initial reinforcing effect of acute nicotine under controlled laboratory conditions (14, 82). A longitudinal study also showed that sensation seeking in college men and women predicts the initiation of smoking and its continuation 20 years later (17). Finally, high levels of novelty seeking have been negatively correlated with smoking-cessation success, with reduced odds of cessation compliance and outcomes (19, 20).
Thus, novelty seeking seems to predict tobacco addiction, but more studies are needed in order to determine the effect of tobacco exposure on this personality trait.
One should nevertheless bear in mind that, although the association between some personality traits and drug addiction is frequently observed, there are no structured and established pre-addictive personalities. Some dissociable personality profiles, including impulsiveness and novelty seeking, may rather be considered as vulnerability factors and facilitate some aspects of the addiction process.
Attention-deficit hyperactivity disorder is a developmental disorder characterized by hyperactivity, high impulsivity, and an inability to sustain directed attention (83). ADHD affects approximately 6.5–8.4% of children and between 1.9 and 6% of adults (84–86). Evidence suggests that ADHD is a predisposition factor for tobacco smoking. For example, ADHD predicted future smoking (21) and adolescents with ADHD were more likely to experiment with cigarettes and become smokers (22). In addition, ADHD symptoms during childhood, particularly hyperactivity/impulsivity, predicted later nicotine dependence in adulthood (87). ADHD status in childhood was also shown to predict time to relapse to smoking after controlling for gender, history of depression, and baseline smoking variables (23). Smokers with ADHD present an earlier onset of regular smoking, have a higher frequency of smoking behavior, show greater withdrawal symptoms, are more willing to work harder for cigarette puffs, and exhibit a higher level of nicotine dependence than smokers without ADHD (24–29, 88, 89). In addition, there is an increase of ADHD symptoms during periods of abstinence in smokers that was associated with an increased risk of relapse (90). This suggests that the increased withdrawal symptoms observed in ADHD patients negatively affect the success of quitting tobacco smoking. Since ADHD is a neurodevelopmental disorder, there are no data on the influence of tobacco smoking on the emergence of ADHD. However, smoking during pregnancy has previously been strongly associated with the risk of ADHD in offspring (91–95) suggesting a direct causality. However, these studies did not rule out the potential influence of unmeasured familial factors (96, 97), and the association no longer holds in recent studies that used different designs accounting for these factors (97–99). This suggests that maternal smoking during pregnancy reflects a genetic predisposition rather than a causal risk factor for ADHD in offspring. Individuals with ADHD may also be more susceptible to the negative effects of smoking. Thus, smokers exhibited a greater increase in attention deficits over the years than their never-smoking twins (100), suggesting that smoking can worsen attention problems.
In conclusion, there is a complex relationship between ADHD and smoking with ADHD contributing to smoking, but smoking may also contribute to the development of attention deficits.
Depression is characterized by depressed mood, anhedonia, vegetative symptoms, and impaired psychosocial functioning. Cigarette smoking and depression both account for significant morbidity, mortality, and economic burden. Depression is overrepresented among adult smokers and contributes to lower smoking-cessation rates and cigarette smoking is overrepresented in adult smokers prone to depression (101, 102). Longitudinal studies are useful to determine if depressive states can influence tobacco smoking. Thus, a 21-year longitudinal study found an association between major depression (MD) and smoking, with a 19% increase in the average daily smoking rate and a 75% increase in the odds of being nicotine dependent from mid-adolescence to young adulthood (30) in people with MD episode. In addition, adolescents with a history of MD had 50% more risk to progress to daily smoking and were significantly less likely to quit by age 25 compared with controls (31). These results suggest a strong influence of MD on the likelihood to develop tobacco addiction, but several studies suggested that less severe depressive symptoms are also a risk factor for tobacco dependence. For example, depression symptoms at mid-adolescence predicted smoking progression across mid-to-late adolescence (103). Adolescents with higher depressive symptoms were more likely to start smoking (34) and to progress to regular smoking compared with adolescents with lower depressive symptoms (35–37). Another longitudinal study found that depressive symptoms in early adolescence predict faster increases in smoking behavior (104).
In addition, depression seems to have a negative influence on smoking cessation since history of MD reduced the odds of short- and long-term smoking abstinence (32, 33). An increase in negative mood in the early stages of treatment for tobacco dependence was predictive of failure to quit smoking or smoking relapse (105, 106).
These data clearly indicate that depression is a risk factor for tobacco addiction, but other studies also support the opposite, i.e., that smoking influences the development of depression. Thus, cigarette smoking during adolescence was shown to predict the development of depressive symptoms (107–111) and an increased time of smoking dependency has been correlated with increased risk of depression. This suggests that the vulnerability for depression increases with higher rates of smoking (110).
In addition, quitting smoking has been associated with a significant decrease in depression compared with continued smoking (112), supporting the hypothesis that smoking might be the cause for mental health problems and not necessarily the inverse.
In conclusion, despite the fact that some of these studies failed to identify a reciprocal relationship between tobacco addiction and depression (30, 37, 108), the relationship seems to be bidirectional (113). As described earlier, tobacco dependence predicts the development of depressive symptoms and MD, while a history of MD predicts the onset of daily smoking and progression to tobacco dependence. This conclusion is supported by a meta-analysis of 15 longitudinal studies in adolescents that reported evidence for a bidirectional relationship, with a larger effect of depression status on smoking likelihood than the effect of smoking on depression (114).
Anxiety disorders, such as panic disorders, phobias, generalized anxiety disorder, and posttraumatic stress disorder (PTSD), are among the most common mental disorders (115, 116). A strong relationship between anxiety disorders and tobacco smoking has been established in humans. Indeed, while tobacco smoking rates significantly declined from 2004 to 2011 in people without psychiatric illness, this is not the case in people with anxiety disorders (117). Along this line, patients with anxiety disorders had significantly higher smoking rates than a control population (38, 39), and anxiety disorders were significantly more prevalent in people diagnosed with nicotine dependence than in a non-dependent population (118). In addition, patients with social anxiety or generalized anxiety disorders exhibited more severe nicotine dependence at baseline and smokers with a lifetime history of anxiety disorder were resistant to pharmacotherapy for abstinence (40).
PTSD is one of the most common anxiety disorders that can develop in humans after an exposure to one or more traumatic events, with a lifetime prevalence of approximately 8% in the general population (119). Smoking initiation and daily smoking rates were shown to increase after trauma (120, 121), and the presence of PTSD symptoms, such as hyperarousal and emotional numbing, is a predictor of tobacco dependence (43–46). Taken together, these data suggest that anxiety disorders are risk factors for the development of tobacco addiction, but prior smoking has also been found to be associated with increased risk to develop PTSD after a trauma or panic disorder (122, 123). In addition, smoking or smoke exposure in early life increased the likelihood of developing an anxiety disorder later in life (124, 125).
Finally, anxiety disorders have also been associated with greater difficulties for quitting tobacco smoking since smokers with lifetime anxiety disorder have significantly lower rates of abstinence and report more severe withdrawal symptoms than control smokers (41, 42, 126, 127). PTSD patients also exhibited lower rates of quitting, shorter times to first smoking relapse after quitting (38, 47, 48) and experienced worsened nicotine withdrawal symptoms compared with a non-PTSD population (49). However, as for depression, anxiety and stress were shown to be decreased in abstinent subjects by follow-up studies (112). This suggests that the assumption of beneficial effects of nicotine on anxiety and mood, which probably contributes to the maintenance of smoking in populations with mental health problems, should be more drastically challenged to motivate quitting.
Thus, the relationship between anxiety disorders and tobacco addiction is probably bidirectional, a conclusion supported by several additional studies (120, 128–130).
Schizophrenia is a chronic disabling disorder characterized by positive symptoms (hallucinations and delusions), negative symptoms (blunted affect, alogia, reduced sociability, and anhedonia), and persistent cognitive deficits (memory, concentration, and learning). It affects approximately 1% of the population (131). Cigarette smoking is highly prevalent in persons with schizophrenia and schizoaffective disorder since it ranges from 45 to 88%, compared with <20% in the general population (132). Individuals with schizophrenia smoke more cigarettes per day, are more nicotine dependent, and also have more difficulties in quitting smoking than smokers with no history of mental health problems (38), leading to high mortality due to tobacco-related illnesses (39). Interestingly, smokers with schizophrenia have higher plasma and urine levels of nicotine, even when matched for the number of cigarettes smoked per day and other indices of nicotine dependence (133–135). This is not due to a difference in nicotine metabolism (136) but rather to the manner in which cigarettes are smoked by schizophrenic patients. Indeed, schizophrenic patients take significantly more puffs, have shorter inter puff intervals, and larger total cigarette puff volumes compared with matched healthy control smokers (137). Smokers with schizophrenia also exhibited a higher intensity of demand and greater consumption and expenditure in a cigarette purchase task, suggesting a higher incentive value of cigarettes in smokers with schizophrenia (138).
Thus, schizophrenia appears to be a strong risk factor for tobacco addiction, and individuals with schizophrenia may sustain smoking because of its higher reinforcing effect and to remedy certain symptoms of the disorder (139). Further research is now needed to look at the alternative possibility that tobacco smoking may confer vulnerability to the development of schizophrenia.
As described in the first part of this review, several clinical studies have linked tobacco addiction with impulsivity, novelty seeking, attention, mood disorders, ADHD, and schizophrenia. But, an investigation of the effects of nicotine on these personality traits and psychiatric disorder-associated phenotypes is important to better understand these relationships (see Table 2).
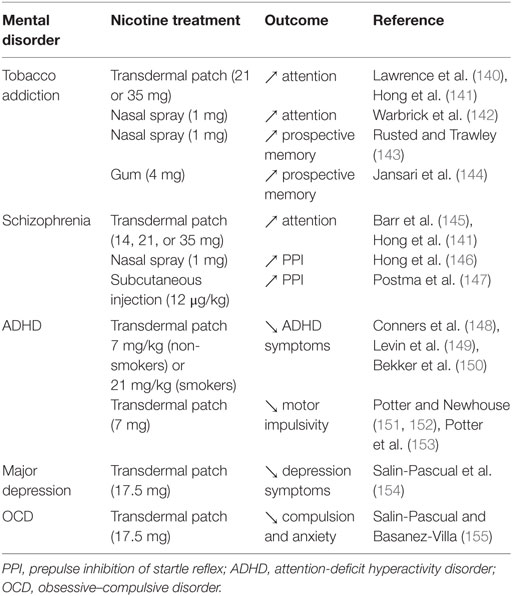
Table 2. Effects of nicotine administration on mental disorder-related processes in clinical studies.
In addition to its abuse liability, nicotine can also enhance cognitive functions, including attention and memory (156). Thus, nicotine and other nAChR ligands have been proposed as potential therapeutics for the treatment of cognitive deficits in pathologies, such as schizophrenia, ADHD, and Alzheimer’s disease (157, 158). However, chronic cigarette smoking has also been associated with decreased cognitive performance in middle age (159, 160) and increased risk for cognitive decline and dementia later in life (161).
Few studies have investigated the impact of nicotine on attention in humans. For example, transdermal nicotine improved the performance in a rapid visual information-processing task (140, 141) and nicotine exposure trough nasal spray decreased the reaction times in a visual oddball task in smokers (142), suggesting an increase in sustained attention induced by acute nicotine in smokers. Transdermal nicotine also significantly improved attention in both schizophrenic patients and controls (145) and visual attentional performance in mildly deprived smokers (162, 163). These studies clearly indicate that nicotine has a pro-attentional effect in humans. Along this line, there is evidence to suggest that nicotine may be useful in treating the symptoms of ADHD. Thus, positive effects of nicotine have been reported on attention, concentration, and other ADHD symptoms among adults with ADHD (22, 148, 149, 164, 165), indicating that ADHD patients may smoke as a form of self-medication.
Some studies further suggest a promnesic effect of smoking. Thus, abstinent smokers exhibited more impairment in visuospatial working memory (VSWM) compared with current smokers (166), and overnight smoking abstinence in schizophrenic patients’ impaired VSWM performance, an effect reversed by reinstatement of cigarette smoking. The effect of smoking reinstatement was blocked by the non-selective nAChR antagonist mecamylamine (167), indicating that the procognitive effect of tobacco smoking in VSWM tasks is through nAChR activation in patients with schizophrenia. Nicotine administration via gum, patch, or injection also improved short-term memory recall in non-smokers (168–170). Interestingly, the effect of nicotine on memory seems to be dependent on baseline performance. Thus, Niemegeers et al. showed that the effect of subchronic nicotine (1 or 2 mg trough oromucosal spray three times daily for 3 days) was dependent on baseline performance in working and visual memory in young and elderly healthy subjects (171). Subjects with lower baseline performance benefited from nicotine administration, while subjects with higher baseline performance performed worse after nicotine administration. This suggests that subjects with lower cognitive performance, irrespective of age, may benefit from nicotine.
There have been few publications on the effect of nicotine on executive functions, and it is difficult to draw conclusions due to the heterogeneity of the procedures and results. For example, nicotine (1 mg through nasal spray) improved prospective memory in minimally deprived (2 h) smokers and non-smokers when the subjects were able to devote resources to that task, but impaired the performance when they completed a concurrent auditory monitoring task (143). Nicotine (2 mg gum) has been shown to improve performance in complex flight simulation tasks, which involve high cognitive load, in non-smoking pilots, but had no effect on the executive function aspect of attention in never smokers (2 and 4 mg gums) (172).
In a study investigating the effect of nicotine on the performance of male non-smokers with high or low attentiveness on the Wisconsin Card Sorting Test (WCST), nicotine administration (7 mg patch) in the high attentiveness group impaired the performance (173). This suggests a deleterious effect of nicotine on strategic planning, set-shifting, and mental flexibility in this sub-population. Finally, in a study using a virtual reality paradigm that assesses multiple cognitive constructs simultaneously (144), nicotine improved the overall performance, time-based prospective memory, and event-based prospective memory in minimally (2 h) deprived smokers (4 mg nicotine gum), but not in never smokers (2 mg nicotine gum). At the same time, action-based prospective memory was enhanced in both groups.
Thus, nicotine seems capable to improve, impair, or have no effect on executive functions depending on the task, the dose of nicotine or the target population, highlighting the need for new studies to obtain a clearer picture on that issue.
Several studies show that cigarette smoking impairs decision-making processes assessed through different neurocognitive tasks (174–177). However, these studies do not discriminate the effects of nicotine alone from the effects other psychoactive compounds found in tobacco smoke. Further studies are needed for providing clear information about the consequences of chronic nicotine exposure on decision-making.
Deficits in pre-attentive sensory information processing, characterized by the inability to filter out or gate sensory information, are thought to contribute to the higher order cognitive deficits observed in schizophrenia. This includes attention, working memory, verbal learning and memory, decision-making, and executive functioning (178, 179). One measure of sensory processing is the P50 suppression that measures the inhibition of electroencephalic cortical response to the second auditory stimulus presented 50 ms after the first. Patients with schizophrenia fail to suppress the response to the second auditory stimulus reflecting gating deficits (180). Several studies have shown that nicotine can improve P50 suppression. Thus, cigarette smoking improved P50 suppression in abstinent smokers with schizophrenia (181), and nicotine gum improved P50 suppression in non-smoking subjects with impaired gating or healthy controls (182–184).
Another measure of sensory information processing is the prepulse inhibition (PPI) of startle reflex that reflects the inhibition of a blinking reflex to a loud startling stimulus presented after a weak prepulse stimulus. This gating mechanism is also impaired in patients with schizophrenia (185) and nicotine (administered via nasal spray or subcutaneous injection) improved PPI in smokers and non-smokers with schizophrenia or in healthy subjects (146, 147). In addition, PPI of satiated smokers with schizophrenia is comparable to PPI of smokers without schizophrenia (186). Taken together, these data suggest that nicotine can improve sensory information processing and those patients with schizophrenia may smoke in part to alleviate their deficit in sensory gating.
Very few studies have investigated the effect of nicotine on impulsivity in humans. A positive correlation between levels of nicotine exposure and discounting of delayed monetary reinforcers has been observed in chronic smokers but not in ex-smokers (187, 188), suggesting that nicotine administration trough smoking increases cognitive impulsivity, an effect that is reversible. However, a positive effect of nicotine on the Stop Signal Reaction Time measure of the Stop Signal Task has been observed in adolescent and young non-smoking adults with ADHD, and in a control population (151–153), indicating that nicotine can reduce motor impulsivity. Thus, nicotine appears to have a differential effect on these two types of impulsivity, but more studies are needed to conclude.
We did not find additional clinical data on the effects of nicotine on cognitive impulsivity or on novelty seeking, highlighting the need for such investigations.
Self-medication is one of the possible explanations for the impact of depression on cigarette smoking since nicotine reduces negative affect and can have antidepressant effects (189). This theory is supported by the fact that patients with MD increased their smoking behavior when they experienced depressive symptoms (190). In addition, several clinical studies reported that nicotine administration through transdermal patches reduced symptoms of depression, even in non-smoking depressed patients (154, 191) and relieved self-reported depression in regular smokers (150).
Interestingly, chronic administration of low levels of nicotine, as delivered by the nicotine patch, is thought to desensitize, rather than activate, nAChRs (192, 193), suggesting that the therapeutic effect of nicotine on depression may be mediated by inactivation of nAChRs. This is supported by the fact that mecamylamine, a non-selective antagonist at heteromeric nicotinic receptors, decreased depression-like symptoms in patients with Tourette’s disorder (194–197) and enhanced the effects of a selective serotonin reuptake inhibitor (SSRI) in depressed subjects (198).
In conclusion, nicotine can relieve some symptoms of depression, potentially via desensitization of nAChRs thus supporting the self-medication hypothesis, which may nevertheless not be the only valid one.
Several studies have shown a positive association between symptom severity in PTSD patients and their desire to smoke in order to reduce negative affect (129, 199–201). Other studies also suggested that this association was mediated by the expectancy that smoking would reduce negative affect (202) and that patients with PTSD smoked and relapsed to smoking in response to negative affect and trauma (48, 203). This suggests that people with PTSD smoke to relieve negative affect and anxiety as a form of self-medication, an hypothesis supported by the fact that PTSD symptoms are reduced by nicotine intake (43–46) and by the anxiolytic effect of nicotine patches in non-smokers with obsessive–compulsive disorders (155). Thus, people with anxiety disorders may smoke to alleviate their symptoms, but more clinical studies on the effect of nicotine on anxiety are needed to support this conclusion.
Some psychological constructs, in particular, have been repeatedly associated with vulnerability to addiction, e.g., sensation seeking, impulsivity, and anxiety (6, 7, 204, 205). To date, the majority of preclinical animal research on individual differences in the response to drugs of abuse has mostly focused on cocaine. Additional work is now needed for nicotine, although some interesting data have nevertheless been generated as detailed in the following paragraphs (see Table 3). In this review, we will strictly focus on behaviors reflecting processes that directly contribute to the addiction cycle, such as those related to (i) drug rewarding properties (e.g., conditioned place preference (CPP), acquisition of self-administration), (ii) later stages of self-administration (e.g., increasing fixed ratios), (iii) motivation for the drug (e.g., progressive ratio schedules of reinforcement), (iv) persistence of drug seeking (e.g., extinction of self-administration), (v) relapse, and (vi) withdrawal syndrome during abstinence.
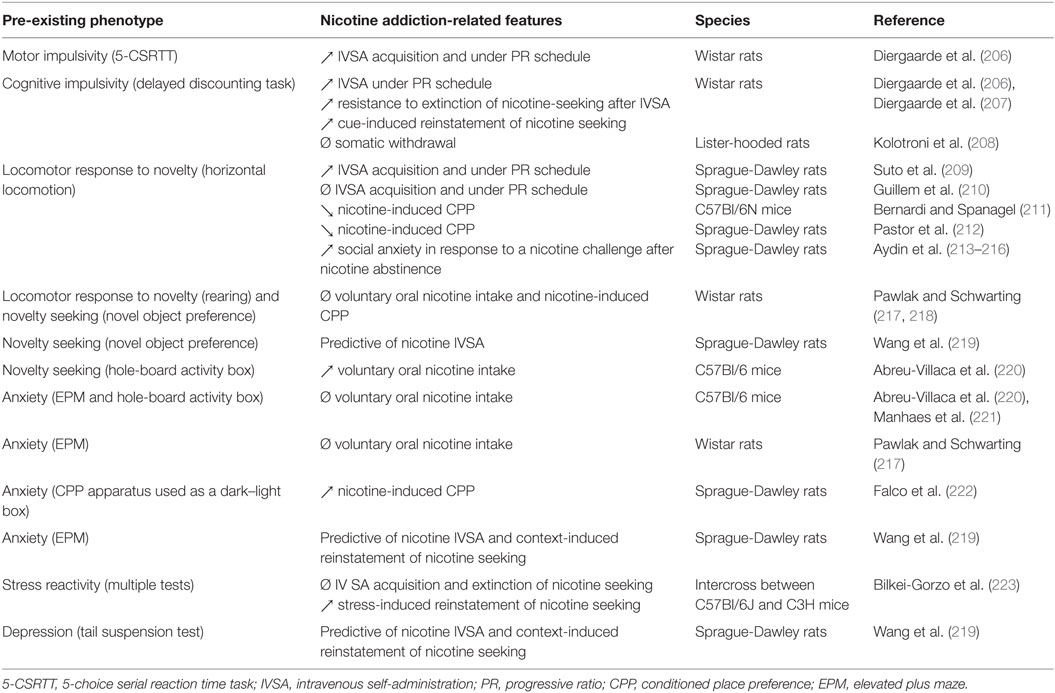
Table 3. Association between pre-existing endophenotypes and nicotine addiction-related features in animal studies.
High impulsivity has been associated with a wide range of neuropsychiatric disorders, including ADHD (224), mood disorders (225), and also drug addiction (64, 226, 227). Findings in trait-impulsive laboratory animals suggest that high impulsivity represents a vulnerability factor for addiction to several classes of drugs including cocaine (228–230), alcohol (231), and nicotine (53, 206). One plausible hypothesis is that high impulsivity results from a dysfunction of the frontal cortex and that this pre-existing dysfunction may facilitate the progressive incapacity of the frontal cortex to suppress maladaptive responses that develop following repeated exposure to a drug (232). Alternatively, drug intake may normalize excessive impulsivity in some individuals and may therefore represent a form of self-medication (53). As described earlier, impulsivity encompasses a complex array of behavioral processes, which can be categorized through at least two major components: motor/action impulsivity (motor disinhibition) and cognitive/choice impulsivity (impulsive decision-making). Several procedures have been developed to provide objective measures of impulsivity in animals, including delay-discounting tasks and the 5-choice serial reaction time task, an analog of the human continuous performance task (233, 234).
Very few preclinical studies have examined the putative link between pre-existing manifestations of impulsivity and nicotine addiction-like behaviors. Yet, one comprehensive study has shown that poor impulse control influences the motivational properties of nicotine and of nicotine-associated cues on a self-administration procedure in rats, and that sub-dimensions of impulsivity predict vulnerability to distinct stages of nicotine-seeking behavior (206). The authors found that high motor impulsivity on a 5-choice serial reaction time task predicts both enhanced self-administration of nicotine during the acquisition and increased motivation for nicotine under progressive ratio of reinforcement. At the same time, high choice impulsivity on a delayed reward task was mostly predictive of both increased resistance to extinction of nicotine-seeking and increased cue-induced relapse of nicotine seeking after extinction. High-impulsive choice was also associated with higher motivation for nicotine when ratios of response requirement are increased, an observation that was confirmed by these authors in the second study (207). In contrast, high- and low-impulsive rats selected on a delay discounting task appear to show similar somatic withdrawal syndrome intensity after chronic exposure to low dose of nicotine (208). These data suggest that the two sub-dimensions of impulsivity influence both distinct and overlapping processes through the dynamics of addiction development in vulnerable individuals.
The second behavioral factor strongly linked to addiction including smoking is the novelty/sensation seeking trait (7, 205, 235). Like impulsivity, novelty/sensation seeking represents a multifaceted behavioral construct and can be divided into a number of dimensions. Several tasks have been developed in animal models to assess responses to novelty.
The primary animal model of sensation seeking is measured as an enhanced locomotor activity in a novel and inescapable environment (236, 237). As for impulsivity, only a small number of preclinical studies have examined the relationship between pre-existing high locomotor response to novelty and nicotine addiction-like behaviors. Consistent with what was reported for other psychostimulants (237), one study found that high locomotor responding to a novel environment predicted the propensity to self-administer nicotine under both fixed and progressive ratios of reinforcement in rats (209). However, such an association was not observed in a more recent study where rats screened as high and low responders to novelty displayed similar levels of nicotine self-administration, although high responders were more prone to self-administer nicotine when it was delivered concomitantly with IMAOs (210). In contrast, a study reported that mice showing low basal locomotor activity manifested nicotine-induced CPP, while mice exhibiting high basal locomotor activity did not (211). However, in this study, the mice had previously been exposed to nicotine for prior experimental testing, which might have influenced subsequent nicotine rewarding effects (238). Consistently, other authors showed that rats classified as low responders according to their locomotor response to novelty following an injection of nicotine, showed nicotine-induced CPP after a long- but not short-term conditioning procedure, while rats classified as high responders did not show CPP under any condition (212). Also, rats selected as high locomotor responders to novelty showed enhanced social anxiety-like behavior during abstinence after repeated nicotine exposure (213–216).
In addition to the sensation seeking trait that is modeled as high locomotor reactivity to novel environments, novelty seeking has been proposed to reflect a distinct dimension of sensation seeking that would differentially contribute to the vulnerability to develop addiction (239, 240). The terms sensation seeking and novelty seeking are often used in an exchangeable way throughout the literature, though. In animal studies, novelty seeking per se is modeled by a high propensity to visit a novel object or environment in a free choice procedure, the so-called novelty preference. Very few studies have attempted to identify the predictive value of novelty seeking to the appetence for nicotine. Interestingly, it has been shown that rats, screened as high novelty seekers as measured by their preference for a novel object in a procedure where they could freely explore either a novel or a familiar object, were also characterized as high locomotor responders to novelty as measured by the number of rears they displayed in an open-field (217). However, high novelty seeker rats did not show differences compared with rats screened as low novelty seekers when subsequently tested for oral nicotine consumption. In another study, the same authors also observed no enhanced nicotine-induced CPP in rats with high rearing activity, although it is difficult to conclude since they did not observe nicotine CPP in any of the rat subpopulations tested in this study (218). Using multiple regression analysis, other authors reported that novelty seeking measured as exploration of a novel object predicted nicotine self-administration in female, but not in male, rats (219). Another animal model of novelty seeking based on the number of head-dips in the hole-board apparatus has been used (241). Mice preselected for high novelty seeking in this test showed a marked increase for oral nicotine intake over time, while mice with low novelty seeking did not (220). However, mice showing high head-dip behavior in the hole-board task and that had been exposed to nicotine during gestation and suckling tended to consume less nicotine when tested during adolescence (242). In contrast, the same study showed that mice similarly exposed to nicotine and showing high rearing or high general locomotor behavior in the hole-board displayed increased oral nicotine intake.
Taken together, these data suggest that additional work is clearly needed to conclusively acknowledge whether high response to novelty/high novelty seeking represents a significant risk factor for nicotine addiction and, if so, for which specific features of this disorder. Novelty seeking measured as high novelty preference, but not high novelty-induced locomotor activity, has notably been shown to predict the compulsive use of cocaine in rats, a hallmark feature of addiction (243). The existence of a similar causal association has not been investigated for nicotine, partly because behaviors reflecting loss of control over nicotine intake and compulsive nicotine taking and seeking have not been accurately modeled so far. The recent development of increasingly reliable models may open new paths for such longitudinal investigations (244–247).
There is a high prevalence of tobacco smoking in subjects with mood or anxiety disorders (235, 248–250). It has been proposed that individuals may use drugs including nicotine as a coping strategy to self-regulate affective distress states (251–253). Drug users may self-medicate for affective distress existing before the initiation of drug use and also to alleviate mood and anxiety distress that are part of the withdrawal syndrome resulting from abstinence (254). Alternative explanations for the strong association between smoking and mood and anxiety disorders are also to be considered, notably since repeated use of nicotine significantly impacts anxiety and mood processing. Below, we review the preclinical studies that assessed whether the manifestation of such disorders beforehand may predict the future response to nicotine.
In preclinical studies, anxiety is usually assessed using procedures that exploit the emotional conflict occurring between the innate strong tendency to explore novel environments and the natural fear of open and/or brightly lit spaces. In particular, the elevated plus maze (EPM) is commonly used with anxiety measured as the preference of animals for closed versus open arms (255). High anxiety in this task predicts several features of cocaine and alcohol, but not heroin, addiction (7). Adolescent mice with high anxiety in this test showed similar levels of oral nicotine intake as mice with low anxiety in a free choice procedure (221). However, during a withdrawal period after 2 weeks of exposure to nicotine through their drinking bottles, adolescent mice with high anxiety consumed less nicotine than mice with low anxiety when tested in a free choice procedure (221). The same group further showed no differences in oral consumption of nicotine in a free choice procedure between adolescent mice with high and low anxiety classified according to their percentage of center squares crossed in a hole-board activity box (220). Another study also reported no association between prior behavioral measurements on the EPM and oral nicotine consumption in rats (217). In contrast, a study in adolescent rats reported that individuals with high anxiety measured as the time spent in the white versus the black chamber of a biased CPP apparatus manifested subsequent nicotine-induced CPP while individuals with low anxiety did not (222). Furthermore, in a comprehensive study assessing several risk factors for nicotine self-administration in a social context in rats, multiple regression analysis found that anxiety measures on the EPM were a predictor of nicotine intake in males, but not in females, while measures of depression on the tail suspension test were predictors of nicotine intake in both males and females (219). In males, both depression- and anxiety-related measures also predicted context-induced nicotine reinstatement. Interestingly, mice generated from the intercross of high (C57BL/6J) and low (C3H/J) emotional mouse strains and classified as “high stress reactive” according to their scores in an elevated zero maze, light–dark box, startle response, and forced swim tests, showed higher vulnerability to relapse but not to initiation or maintenance of nicotine self-administration compared with low and average stress reactive animals (223).
In addition to data regarding the causal link between inter-individual differences in anxiety- and depression-like behaviors and appetence for nicotine, it was demonstrated that acute stressor exposure through a single episode of intermittent footshock administered 24 h before the start of place conditioning dose-dependently facilitated acquisition of CPP to nicotine in adolescent rats (256). Prenatal stress in rats also increased nicotine reinforcing properties in a CPP procedure and anxiety withdrawal symptom at the cessation of nicotine exposure (257, 258). Finally, chronic mild stress, considered as a model of depression, which was delivered prior to nicotine exposure was found to exacerbate nicotine withdrawal syndrome in rats (259).
Although these data are heterogeneous, they suggest that anxiety and mood disorders may represent a significant predictor of nicotine addiction and may notably influence the vulnerability to relapse after abstinence, depending on the sex and the age of the individual.
In addition to alleviating stress, anxiety, and improving mood, nicotine has the ability to enhance cognition. Nicotine use has also been proposed as a self-treatment for cognitive deficits that are encountered in numerous psychiatric diseases strongly represented in smoker populations such as schizophrenia or ADHD (260). As for other aspects of the comorbidity between smoking and psychiatric conditions, one fundamental pending question is whether cognitive deficits are of premorbid origin or develop after long-term exposure to nicotine and subsequent withdrawal. Animal models have proven to be useful tools for helping to resolve these issues with the possibility for well-controlled longitudinal studies to be conducted. Nevertheless, while many studies have looked at the effects of nicotine on cognitive processes, there is a great lack of preclinical studies investigating the relationship between inter-individual differences in cognitive functions, such as baseline impairments in attention, learning, and memory functions, and addiction-like behaviors, especially with regard to nicotine. One study provided evidence for a causal link between prior cognitive deficits and behavioral response to nicotine, by looking at individual differences in baseline PPI of acoustic startle reflex and subsequent nicotine-induced locomotor effects including locomotor sensitization. Disruption in the PPI is a model of cognitive impairment in schizophrenia and reveals deficits in the sensorimotor gating system which is critical for the integration of sensory and cognitive information processing and execution of appropriate motor responses. The authors showed that the acute effect of nicotine on locomotion was higher in rats classified as high-inhibitory, while a locomotor sensitization after repeated exposure to nicotine developed only in low-inhibitory rats (261). Another study reported that neonatal ventral hippocampal lesions that produced post-adolescent onset, pharmacological, neurobiological, and cognitive features of schizophrenia, such as spatial learning and working memory deficits, increased nicotine self-administration and nicotine seeking during extinction in adult rats (262). Furthermore, spontaneously hypertensive rats, considered as the most valid animal model of ADHD and that display symptoms of inattentiveness, impulsivity, and hyperactivity, show enhanced nicotine self-administration (263) and CPP (264). It has also been shown that social interaction phenotypes are predictor of nicotine self-administration and nicotine seeking in rats, although it is difficult to conclude about which cognitive functions – if any – were implicated in such a causal association (219).
Taken together, these data suggest that different behavioral factors may preferentially contribute to some of the many dimensions of the addiction cycle. Combinations of some predisposing behavioral traits may result in specific vulnerability profiles predicting higher risk for starting nicotine use or shifting toward nicotine abuse, or for relapse during abstinence. For instance, outbred rats classified as high locomotor responders to novelty show decreased anxiety as compared with low responders (265). Also, as mentioned earlier, a study based on a dimensional analysis approach within a single and large population of rats reported that high locomotor reactivity to novelty predicts the propensity to self-administer cocaine, while high novelty seeking in a free choice procedure predicts the transition to compulsive cocaine seeking (243). Additional studies measuring the inter-individual vulnerability for different personality traits and addiction-like phenotypes in the same population of animals may significantly improve our understanding of vulnerability to nicotine addiction.
In addition to a possible influence of pre-existing impulsivity on later development of drug abuse, psychostimulant abuse may itself lead to the increased impulsivity often observed in chronic drug abusers, including nicotine, and, thereby, help to develop and maintain addiction (see Table 4) (348).
Animal studies on the effects of nicotine on inhibitory control have mostly focused on motor impulsivity using attentional tasks. Acute nicotine exposure consistently increased premature responding on serial reaction time- (266–272) and go/no-go-tasks in rats (273). These effects appear to be long-lasting, although data about chronic exposure to nicotine on motor impulsivity are fewer and less consistent (268, 271, 274, 276). One recent study in mice demonstrated that chronic oral, but not acute, injections of nicotine attenuated phencyclidine-induced increases in motor impulsivity (349). Increased motor impulsivity was further reported in rats after prenatal exposure to nicotine, while cognitive impulsivity was not affected (350, 351). In adolescent, but not post-adolescent rats, repeated exposure to nicotine increased impulsive action but not impulsive choice (275).
Few animal studies have focused on the consequences of nicotine exposure on cognitive impulsivity using delay-discounting tasks, and the data are more heterogeneous. Acute injections of nicotine dose-dependently increased impulsive choice in rats, while repeated injections of nicotine also increased impulsive choice, but to the same extent regardless of the dose (277). After nicotine treatment cessation, impulsive choice remained enhanced for a long period before gradually returning to baseline, suggesting that chronic nicotine exposure can produce long-lasting although reversible alterations in inhibitory control. Acute exposure to nicotine increased both impulsive action in a go/no go task and impulsive choice in a delayed reward task in rats, with greater sensitivity of impulsive choice to nicotine (273). Both acute and subchronic injections of nicotine increased impulsive choice in rats in a procedure where the delayed reward was made preferable by decreasing the probability rather than the magnitude of the immediate reward (278). In contrast, a study reported decreased impulsive choice in rats after acute nicotine, and this effect was abolished after repeated nicotine injections (279). Finally, in rats with high cognitive impulsivity, chronic nicotine exposure and nicotine withdrawal had no effect on impulsive choice, while chronic nicotine exposure increased impulsive choice in low-impulsive rats, with no effects on animals with intermediate impulsivity levels (352). Nicotine may result in varying effects on choice processing, depending on key parameters such as basal levels of impulsivity, reinforcement amount, or delay (e.g., adjusting versus fixed delay), and genetic background of rats.
The effects of acute nicotine exposure on anxiety-like behavior is highly dependent on the task, dose, timing of testing, sex, strain, age, and basal anxiety levels of the animals (353, 354). In the EPM, acute or subchronic systemic nicotine was found anxiolytic in some studies (280, 285, 293), anxiogenic at both low and high doses in others (288, 289, 292, 294), or to have no effects (288), in rats. Inconclusive data have also been obtained in mice, with anxiolytic effects at low doses and anxiogenic effects at high doses of nicotine in C57BL/6J, CD1, and BALB/C mice (283, 284, 286, 287), and anxiogenic effects with an intermediate dose with anxiolytic action when given subchronically in Swiss mice (290, 291). In the social interaction test, it is also generally found that low doses of nicotine induce anxiolytic effects, while high doses are anxiogenic (281). However, a study reported that acute nicotine injections performed 5 min before testing induced anxiogenic effects, whereas nicotine injections using the same dose but performed 30 min before the task elicited anxiolytic effects (282). Nicotine reduced stress-induced hyperthermia (355).
Interestingly, a tolerance to nicotine’s effects on anxiety may develop over time. Chronic exposure to nicotine was found to have no longer effects on anxiety or to induce anxiolytic effects to which tolerance also develops eventually in the EPM and the social interaction test, in rats and mice (289–291, 293, 299, 300). The consequences of chronic nicotine exposure also depend on several factors such as sex or basal levels of anxiety. For instance, mice that overexpress the R isoform of acetylcholinesterase exhibit increased anxiety that is normalized by chronic forced nicotine consumption (356). Chronic nicotine treatment also reversed affective deficits produced by chronic mild stress (357). Yet, increased anxiety was also observed in the EPM and the light–dark box after chronic nicotine consumption (296–298). One study reported increased anxiety in the social interaction test in rats after nicotine self-administration, which may appear contradictory to the self-medication hypothesis (295).
Increased anxiety is consistently observed when testing is performed during nicotine withdrawal in the EPM, light–dark box, or social interaction test (221, 282, 295, 358–361) and is reduced by nicotine injection (289). Nicotine withdrawal also increased sensitivity to stressors in the light-enhanced startle paradigm (362).
These studies suggest that nicotine effects on anxiety are dependent on various factors such as the source of anxiety, baseline levels, and genetic background of the individuals. Nicotine may be used to self-medicate anxiety-related distress associated with abstinence or in people with predisposing phenotypes, while it may have opposite effects on anxiety in other individuals or under different conditions. In the latter case, smoking behavior might be sustained by the belief that nicotine consumption will alleviate the anxiety that was essentially induced by smoking itself in the first place, while long-term smoking cessation would actually be much more beneficial for reversing such anxiety-related problems.
The effects of nicotine on fear conditioning in rodents are clearer than those on anxiety-like behavior (363). Studies have consistently reported enhanced hippocampus-dependent fear conditioning in mice after acute nicotine exposure (302–305, 307), while there is no effect on hippocampus-independent fear conditioning or on general freezing behavior (301, 302). Acute nicotine was further shown to impair contextual safety discrimination in a safety learning paradigm (310). A tolerance to these effects seems to develop under chronic nicotine exposure in mice and rats, while nicotine withdrawal altered fear conditioning (306, 308, 309, 353, 363). Furthermore, a study showed that nicotine had differential effects on extinction of fear conditioning depending on when it was administered, during training and/or during extinction, and on the context during extinction (364), suggesting that nicotine may strengthen contextual fear memories and interfere with extinction. Chronic nicotine administration 2 weeks prior to the training impaired subsequent cued – but enhanced contextual – fear extinction (365). Studies on fear conditioning extinction are particularly relevant in the context of the self-medication for emotional distress hypothesis of nicotine abuse. Further investigation will hopefully be carried along this line in the future.
Numerous studies showed antidepressant-like effects of nicotine in rat and mouse models, such as in learned helplessness (316) and forced swim tests (311–314, 317). However, some authors have observed decreased depression-like phenotypes in response to nicotine only in rat strains that display enhanced basal levels of depressive features, with contradictory effects depending on the post-injection time of the testing (311, 315, 318). As for anxiety, factors including age, sex, and genetic background may also influence the action of nicotine on mood. One study notably demonstrated that while acute nicotine decreased depression-like behavior in adult Sprague-Dawley rats, it had no effect in adolescent rats (285). There is also evidence for decreased depression-like phenotypes following chronic nicotine exposure (312, 316). Furthermore, chronic administration of nicotine results in an enhanced response to classical antidepressants (314, 366) and reverses anhedonia induced by chronic stress (367). Acute and chronic exposure to nicotine also had antidepressant effects in environmentally induced rat models of depression (357, 368, 369). Interestingly, chronic oral nicotine intake or repeated nicotine injections diminished depressive symptoms more than transcranial magnetic stimulation (369). However, one study found no depression-like phenotypes in response to chronic nicotine in the tail suspension task in male and female rats, whatever the dose of nicotine tested (300). By contrast, nicotine withdrawal is clearly associated with enhanced depression-like behaviors, including elevated reward thresholds (370) in rats. At early stages of withdrawal, mice exhibited a depression-like profile similar to that observed following a chronic stress regimen (367). Acute administration of the antidepressant fluoxetine reversed nicotine withdrawal-induced intra-cranial self-stimulation threshold elevations when coadministered with a 5-HT1A receptor antagonist (371).
Overall, there is evidence supporting the self-medication hypothesis for anxiety and depressive-like symptoms, including those resulting from nicotine exposure cessation. Subsequent nicotine-seeking relapse may be driven by negative reinforcement mechanisms that anticipate such affective distress (260). However, nicotine-elicited improvements of anxiety and mood appear to strongly depend on several conditions. Nicotine can also deteriorate affective states in some conditions, an important fact that may paradoxically contribute to smoking maintenance and should be taken in account to provide appropriate smoking cessation help.
Accumulating evidence suggests that cognitive enhancement may contribute to nicotine addiction through different modalities. Research using experimental animals has provided a better understanding of the effects of nicotine on cognitive processes.
Nicotine administration has been shown to improve learning and memory (157, 319, 321, 329, 331, 372, 373). Single injections of nicotine notably improved working memory in rodents (157, 320). Acute nicotine administration also enhanced acquisition, consolidation, and restitution of the information in an object recognition task in rats (319). Yet, it was reported that acute nicotine did not improve acquisition in the water maze in group housed mice and even impaired performances in this task in individually housed mice (322). Importantly, many preclinical studies show that the efficacy of nicotine on memory does not diminish with chronic administration. For instances, chronic nicotine exposure improves memory performances in rats (323–326) or memory consolidation in mice (332). Nevertheless, some studies found no effects of chronic administration on memory function. Notably, chronic nicotine in NMRI male mice did not significantly change performance in the water maze (333). Age may be a significant factor influencing the action of nicotine on memory. A study reported that nicotine improved the acquisition of a serial pattern learning task in young but not old Fisher 344 rats, while no effects were found on reference memory in either group (330). Chronic nicotine administration also failed to improve working memory in old rats (328). Yet, other studies obtained contrasting data with improvements of memory in response to nicotine in senescence-accelerated mice (374) and aged rats (327). Nicotine also alleviated memory deficits induced by chemical or pharmacological agents (375, 376), and brain lesions (377, 378). By contrast, nicotine withdrawal resulted in learning and memory impairments including in contextual fear conditioning (306, 379, 380).
Although these data suggest primary mnemonic effects of nicotine, there has been much debate as to whether beneficial effects of nicotine in tasks of learning and memory may be secondary to effects on attentional functions. A first study reported that small doses of nicotine reversed deficits in 5-CSRTT accuracy in basal forebrain lesioned rats, but not in non-lesioned animals (381). Nevertheless, other studies showed improvements in 5-CSRTT response accuracy following acute (266, 334, 338, 339) and chronic (271, 274, 340, 341) exposure to nicotine, although these effects may be strain dependent (337). Nicotine also induced improvements in choice accuracy in a two-choice stimulus detection task (335, 336). As observed for learning and memory, nicotine reversed attentional impairments caused by brain or pharmacologically induced lesions (325, 381, 382). Nicotine withdrawal was shown to impair choice accuracy, to increase omission errors in the 5-CSRTT (271, 383), and to impair PPI of acoustic startle in mice (384), although contrasting results were found with another stain of mice (385).
Apart from learning, memory, and attention functions, very few studies have focused on the consequences of nicotine exposure on executive functions in animals. Some studies have evaluated the effects of nicotine on measures of cognitive flexibility. Deficits in cognitive flexibility may contribute to drug addiction as the inability to change a response to stimuli previously associated with a drug stimulus or reward (386). Acute nicotine injections impaired decision-making, and this effect was associated with deficits in behavioral flexibility measured as perseverating responding in rats (342). The same authors reported that chronic neonatal nicotine did not impair decision-making in rats (343). Yet, chronic exposure to a high, but not low, dose of nicotine impaired response reversal learning in mice (344, 345). In contrast, other authors (346, 347) reported that acute and repeated nicotine administration improved attentional set-shifting in rats.
The studies related across this review strongly support the idea that inter-individual differences in cognitive and affective processing both preceding and resulting from repeated exposure to nicotine contribute to nicotine addiction. There is growing evidence that nicotine addiction arises from the combined interactions of various processes underlying cognition and emotion with nicotine exposure according to several modalities.
First, human studies, but mostly preclinical investigations, clearly indicate that nicotine can have direct facilitator effects on cognitive processing and alleviate negative affective states, supporting the hypothesis of tobacco smoking as a form of self-medication. This seems to be particularly the case for memory and attention deficits, as well as anxiety and depression-like phenotypes. Reversal of such cognitive and affective deficits by nicotine is even clearer for withdrawal-associated phenotypes. Tobacco smoking may thus also be maintained as a form of self-medication in individuals who show moderate cognitive or affective impairments and who are not diagnosed with a particular psychiatric condition. However, despite demonstrable nicotine-induced improvements of affective states and cognitive deficits, this is only indirect evidence supporting the self-medication hypothesis, which should not be considered as the only plausible explanation for high rates of smoking behavior in psychiatric populations. One should also emphasize the fact that chronic exposure to nicotine can also impair anxiety and mood in some conditions, to help attenuate hesitations in smoking-cessation attempts. Second, pre-existing phenotypes, such as high impulsivity and sensation seeking, appear to influence the appetence for nicotine according to most studies and may drive the propensity for initiating and pursuing smoking behavior. However, additional preclinical longitudinal studies need to be performed for resolving this issue, particularly to investigate the relationship between predisposing phenotypes and behavioral models that still need to be developed to truly capture addiction-like features such as habitual and compulsive nicotine taking and seeking. Last but not least, numerous studies reviewed here show that nicotine can trigger “pro-addiction” phenotypes such as impulsivity and deficits in cognitive flexibility. Nicotine-induced enhancements of learning, memory, and attention may also promote the shift toward nicotine addiction by facilitating the associations between smoking and contextual cues that underlie habitual drug use, craving, and relapse.
The great heterogeneity regarding the effects of nicotine observed across the different studies that we reviewed further suggests that the underlying reasons for smoking may vary across individuals, according to their pre-existing differences in genetics, life experiences, tobacco history, or personality traits.
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Dr. Uwe Maskos for editing and improving the use of English in the manuscript.
1. Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci (2010) 11:389–401. doi:10.1038/nrn2849
2. Dome P, Lazary J, Kalapos MP, Rihmer Z. Smoking, nicotine and neuropsychiatric disorders. Neurosci Biobehav Rev (2010) 34:295–342. doi:10.1016/j.neubiorev.2009.07.013
3. Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the national comorbidity survey. Exp Clin Psychopharmacol (1994) 2:244–68. doi:10.1037/1064-1297.2.3.244
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association (2013).
5. Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology (2014) 39:254–62. doi:10.1038/npp.2013.261
6. Belin-Rauscent A, Fouyssac M, Bonci A, Belin D. How preclinical models evolved to resemble the diagnostic criteria of drug addiction. Biol Psychiatry (2015) 79(1):39–46. doi:10.1016/j.biopsych.2015.01.004
7. Belin D, Belin-Rauscent A, Everitt BJ, Dalley JW. In search of predictive endophenotypes in addiction: insights from preclinical research. Genes Brain Behav (2015) 15(1):74–88. doi:10.1111/gbb.12265
8. Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol (2015) 67:23–50. doi:10.1146/annurev-psych-122414-033457
9. Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction (2006) 101(Suppl 1):23–30. doi:10.1111/j.1360-0443.2006.01586.x
10. Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev (2013) 65:255–90. doi:10.1124/pr.111.005124
11. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA (2004) 291:1238–45. doi:10.1001/jama.291.10.1238
12. Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend (2009) 103:99–106. doi:10.1016/j.drugalcdep.2008.12.019
13. Sheffer CE, Christensen DR, Landes R, Carter LP, Jackson L, Bickel WK. Delay discounting rates: a strong prognostic indicator of smoking relapse. Addict Behav (2014) 39:1682–9. doi:10.1016/j.addbeh.2014.04.019
14. Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, et al. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology (Berl) (2008) 200:529–44. doi:10.1007/s00213-008-1231-7
15. Anokhin AP, Golosheykin S. Neural correlates of response inhibition in adolescents prospectively predict regular tobacco smoking. Dev Neuropsychol (2016) 41:22–37. doi:10.1080/87565641.2016.1195833
16. Doran N, McChargue D, Cohen L. Impulsivity and the reinforcing value of cigarette smoking. Addict Behav (2007) 32:90–8. doi:10.1016/j.addbeh.2006.03.023
17. Lipkus IM, Barefoot JC, Williams RB, Siegler IC. Personality measures as predictors of smoking initiation and cessation in the UNC Alumni Heart Study. Health Psychol (1994) 13:149–55. doi:10.1037/0278-6133.13.2.149
18. Audrain-McGovern J, Rodriguez D, Tercyak KP, Cuevas J, Rodgers K, Patterson F. Identifying and characterizing adolescent smoking trajectories. Cancer Epidemiol Biomarkers Prev (2004) 13:2023–34.
19. Kahler CW, Spillane NS, Metrik J, Leventhal AM, Monti PM. Sensation seeking as a predictor of treatment compliance and smoking cessation treatment outcomes in heavy social drinkers. Pharmacol Biochem Behav (2009) 93:285–90. doi:10.1016/j.pbb.2009.01.003
20. Batra A, Collins SE, Schroter M, Eck S, Torchalla I, Buchkremer G. A cluster-randomized effectiveness trial of smoking cessation modified for at-risk smoker subgroups. J Subst Abuse Treat (2010) 38:128–40. doi:10.1016/j.jsat.2009.08.003
21. Fuemmeler BF, Kollins SH, McClernon FJ. Attention deficit hyperactivity disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood. J Pediatr Psychol (2007) 32:1203–13. doi:10.1093/jpepsy/jsm051
22. Tercyak KP, Lerman C, Audrain J. Association of attention-deficit/hyperactivity disorder symptoms with levels of cigarette smoking in a community sample of adolescents. J Am Acad Child Adolesc Psychiatry (2002) 41:799–805. doi:10.1097/00004583-200207000-00011
23. Humfleet GL, Prochaska JJ, Mengis M, Cullen J, Munoz R, Reus V, et al. Preliminary evidence of the association between the history of childhood attention-deficit/hyperactivity disorder and smoking treatment failure. Nicotine Tob Res (2005) 7:453–60. doi:10.1080/14622200500125310
24. Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil (1998) 31:533–44. doi:10.1177/002221949803100603
25. Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry (2005) 62:1142–7. doi:10.1001/archpsyc.62.10.1142
26. Pomerleau CS, Downey KK, Snedecor SM, Mehringer AM, Marks JL, Pomerleau OF. Smoking patterns and abstinence effects in smokers with no ADHD, childhood ADHD, and adult ADHD symptomatology. Addict Behav (2003) 28:1149–57. doi:10.1016/S0306-4603(02)00223-X
27. McClernon FJ, Van Voorhees EE, English J, Hallyburton M, Holdaway A, Kollins SH. Smoking withdrawal symptoms are more severe among smokers with ADHD and independent of ADHD symptom change: results from a 12-day contingency-managed abstinence trial. Nicotine Tob Res (2011) 13:784–92. doi:10.1093/ntr/ntr073
28. Kollins SH, English JS, Roley ME, O’Brien B, Blair J, Lane SD, et al. Effects of smoking abstinence on smoking-reinforced responding, withdrawal, and cognition in adults with and without attention deficit hyperactivity disorder. Psychopharmacology (Berl) (2013) 227:19–30. doi:10.1007/s00213-012-2937-0
29. Wilens TE, Vitulano M, Upadhyaya H, Adamson J, Sawtelle R, Utzinger L, et al. Cigarette smoking associated with attention deficit hyperactivity disorder. J Pediatr (2008) 153:414–9. doi:10.1016/j.jpeds.2008.04.030
30. Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychol Med (2003) 33:1357–67. doi:10.1017/S0033291703008596
31. Rohde P, Lewinsohn PM, Brown RA, Gau JM, Kahler CW. Psychiatric disorders, familial factors and cigarette smoking: I. Associations with smoking initiation. Nicotine Tob Res (2003) 5:85–98. doi:10.1080/1462220031000070507
32. Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, et al. Smoking, smoking cessation, and major depression. JAMA (1990) 264:1546–9. doi:10.1001/jama.264.12.1546
33. Hitsman B, Papandonatos GD, McChargue DE, DeMott A, Herrera MJ, Spring B, et al. Past major depression and smoking cessation outcome: a systematic review and meta-analysis update. Addiction (2013) 108:294–306. doi:10.1111/add.12009
34. Escobedo LG, Reddy M, Giovino GA. The relationship between depressive symptoms and cigarette smoking in US adolescents. Addiction (1998) 93:433–40. doi:10.1046/j.1360-0443.1998.93343311.x
35. Killen JD, Robinson TN, Haydel KF, Hayward C, Wilson DM, Hammer LD, et al. Prospective study of risk factors for the initiation of cigarette smoking. J Consult Clin Psychol (1997) 65:1011–6. doi:10.1037/0022-006X.65.6.1011
36. Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G. Depression, anxiety, and smoking initiation: a prospective study over 3 years. Am J Public Health (1998) 88:1518–22. doi:10.2105/AJPH.88.10.1518
37. Wang MQ, Fitzhugh EC, Green BL, Turner LW, Eddy JM, Westerfield RC. Prospective social-psychological factors of adolescent smoking progression. J Adolesc Health (1999) 24:2–9. doi:10.1016/S1054-139X(98)00080-9
38. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA (2000) 284:2606–10. doi:10.1001/jama.284.20.2606
39. Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health Report. Nicotine Tob Res (2008) 10:1691–715. doi:10.1080/14622200802443569
40. Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction (2011) 106:418–27. doi:10.1111/j.1360-0443.2010.03173.x
41. Piper ME, Smith SS, Schlam TR, Fleming MF, Bittrich AA, Brown JL, et al. Psychiatric disorders in smokers seeking treatment for tobacco dependence: relations with tobacco dependence and cessation. J Consult Clin Psychol (2010) 78:13–23. doi:10.1037/a0018065
42. Weinberger AH, Desai RA, McKee SA. Nicotine withdrawal in U.S. smokers with current mood, anxiety, alcohol use, and substance use disorders. Drug Alcohol Depend (2010) 108:7–12. doi:10.1016/j.drugalcdep.2009.11.004
43. Beckham JC, Feldman ME, Vrana SR, Mozley SL, Erkanli A, Clancy CP, et al. Immediate antecedents of cigarette smoking in smokers with and without posttraumatic stress disorder: a preliminary study. Exp Clin Psychopharmacol (2005) 13:219–28. doi:10.1037/1064-1297.13.3.219
44. Thorndike FP, Wernicke R, Pearlman MY, Haaga DA. Nicotine dependence, PTSD symptoms, and depression proneness among male and female smokers. Addict Behav (2006) 31:223–31. doi:10.1016/j.addbeh.2005.04.023
45. Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and post-traumatic stress: a critical review of the empirical literature. Clin Psychol Rev (2007) 27:14–45. doi:10.1016/j.cpr.2006.08.004
46. Greenberg JB, Ameringer KJ, Trujillo MA, Sun P, Sussman S, Brightman M, et al. Associations between posttraumatic stress disorder symptom clusters and cigarette smoking. Psychol Addict Behav (2012) 26:89–98. doi:10.1037/a0024328
47. Hapke U, Schumann A, Rumpf HJ, John U, Konerding U, Meyer C. Association of smoking and nicotine dependence with trauma and posttraumatic stress disorder in a general population sample. J Nerv Ment Dis (2005) 193:843–6. doi:10.1097/01.nmd.0000188964.83476.e0
48. Beckham JC, Calhoun PS, Dennis MF, Wilson SM, Dedert EA. Predictors of lapse in first week of smoking abstinence in PTSD and non-PTSD smokers. Nicotine Tob Res (2013) 15:1122–9. doi:10.1093/ntr/nts252
49. Dedert EA, Calhoun PS, Harper LA, Dutton CE, McClernon FJ, Beckham JC. Smoking withdrawal in smokers with and without posttraumatic stress disorder. Nicotine Tob Res (2012) 14:372–6. doi:10.1093/ntr/ntr142
50. Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) (1999) 146:348–61. doi:10.1007/PL00005481
51. de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol (2009) 14:22–31. doi:10.1111/j.1369-1600.2008.00129.x
52. Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, de Wit H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcohol Clin Exp Res (2010) 34:1334–45. doi:10.1111/j.1530-0277.2010.01217.x
53. Jupp B, Caprioli D, Dalley JW. Highly impulsive rats: modelling an endophenotype to determine the neurobiological, genetic and environmental mechanisms of addiction. Dis Model Mech (2013) 6:302–11. doi:10.1242/dmm.010934
54. Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron (2011) 69:680–94. doi:10.1016/j.neuron.2011.01.020
55. Noel X, Van der Linden M, d’Acremont M, Bechara A, Dan B, Hanak C, et al. Alcohol cues increase cognitive impulsivity in individuals with alcoholism. Psychopharmacology (Berl) (2007) 192:291–8. doi:10.1007/s00213-006-0695-6
56. Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend (2002) 66:265–73. doi:10.1016/S0376-8716(01)00206-X
57. Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci (2004) 24:11017–22. doi:10.1523/JNEUROSCI.3321-04.2004
58. Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend (2005) 79:273–7. doi:10.1016/j.drugalcdep.2005.02.002
59. Austin AJ, Duka T, Rusted J, Jackson A. Effect of varenicline on aspects of inhibitory control in smokers. Psychopharmacology (Berl) (2014) 231:3771–85. doi:10.1007/s00213-014-3512-7
60. Logemann HN, Bocker KB, Deschamps PK, Kemner C, Kenemans JL. Differences between nicotine-abstinent smokers and non-smokers in terms of visuospatial attention and inhibition before and after single-blind nicotine administration. Neuroscience (2014) 277:375–82. doi:10.1016/j.neuroscience.2014.07.016
61. Harrison EL, Coppola S, McKee SA. Nicotine deprivation and trait impulsivity affect smokers’ performance on cognitive tasks of inhibition and attention. Exp Clin Psychopharmacol (2009) 17:91–8. doi:10.1037/a0015657
62. Tsaur S, Strasser AA, Souprountchouk V, Evans GC, Ashare RL. Time dependency of craving and response inhibition during nicotine abstinence. Addict Res Theory (2015) 23:205–12. doi:10.3109/16066359.2014.953940
63. Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol (1995) 51:768–74. doi:10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1
64. Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev (2008) 32:777–810. doi:10.1016/j.neubiorev.2007.11.003
65. Cyders MA, Coskunpinar A. Measurement of constructs using self-report and behavioral lab tasks: is there overlap in nomothetic span and construct representation for impulsivity? Clin Psychol Rev (2011) 31:965–82. doi:10.1016/j.cpr.2011.06.001
66. Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) (1999) 146:447–54. doi:10.1007/PL00005490
67. Johnson MW, Bickel WK, Baker F. Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers. Exp Clin Psychopharmacol (2007) 15:187–94. doi:10.1037/1064-1297.15.2.187
68. Carim-Todd L, Mitchell SH, Oken BS. Impulsivity and stress response in nondependent smokers (tobacco chippers) in comparison to heavy smokers and nonsmokers. Nicotine Tob Res (2015) 18(5):547–56. doi:10.1093/ntr/ntv210
69. Zuckerman M, Bone RN, Neary R, Mangelsdorff D, Brustman B. What is the sensation seeker? Personality trait and experience correlates of the Sensation-Seeking Scales. J Consult Clin Psychol (1972) 39:308–21. doi:10.1037/h0033398
70. Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry (1987) 44:573–88. doi:10.1001/archpsyc.1987.01800180093014
71. Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry (1993) 50:975–90. doi:10.1001/archpsyc.1993.01820240059008
72. Stallings MC, Hewitt JK, Cloninger CR, Heath AC, Eaves LJ. Genetic and environmental structure of the Tridimensional Personality Questionnaire: three or four temperament dimensions? J Pers Soc Psychol (1996) 70:127–40. doi:10.1037/0022-3514.70.1.127
73. Peritogiannis V. Sensation/novelty seeking in psychotic disorders: a review of the literature. World J Psychiatry (2015) 5:79–87. doi:10.5498/wjp.v5.i1.79
74. Kosten TA, Ball SA, Rounsaville BJ. A sibling study of sensation seeking and opiate addiction. J Nerv Ment Dis (1994) 182:284–9. doi:10.1097/00005053-199405000-00006
75. Wills TA, Windle M, Cleary SD. Temperament and novelty seeking in adolescent substance use: convergence of dimensions of temperament with constructs from Cloninger’s theory. J Pers Soc Psychol (1998) 74:387–406. doi:10.1037/0022-3514.74.2.387
76. Cloninger CR, Przybeck TR, Svrakic DM. The tridimensional personality questionnaire: U.S. normative data. Psychol Rep (1991) 69:1047–57. doi:10.2466/pr0.1991.69.3.1047
77. Zuckerman M, Cloninger CR. Relationships between Cloninger’s, Zuckerman’s, and Eysenck’s dimensions of personality. Pers Individ Dif (1996) 21:283–5. doi:10.1016/0191-8869(96)00042-6
78. Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger’s theory. J Subst Abuse (1994) 6:1–20. doi:10.1016/S0899-3289(94)90039-6
79. Masse LC, Tremblay RE. Behavior of boys in kindergarten and the onset of substance use during adolescence. Arch Gen Psychiatry (1997) 54:62–8. doi:10.1001/archpsyc.1997.01830130068014
80. Sargent JD, Tanski S, Stoolmiller M, Hanewinkel R. Using sensation seeking to target adolescents for substance use interventions. Addiction (2010) 105:506–14. doi:10.1111/j.1360-0443.2009.02782.x
81. Etter JF, Pelissolo A, Pomerleau C, De Saint-Hilaire Z. Associations between smoking and heritable temperament traits. Nicotine Tob Res (2003) 5:401–9. doi:10.1080/1462220031000094240
82. Perkins KA, Gerlach D, Broge M, Grobe JE, Wilson A. Greater sensitivity to subjective effects of nicotine in nonsmokers high in sensation seeking. Exp Clin Psychopharmacol (2000) 8:462–71. doi:10.1037/1064-1297.8.4.462
83. Barkley RA. Issues in the diagnosis of attention-deficit/hyperactivity disorder in children. Brain Dev (2003) 25:77–83. doi:10.1016/S0387-7604(02)00152-3
84. Barbaresi WJ, Katusic SK, Colligan RC, Pankratz VS, Weaver AL, Weber KJ, et al. How common is attention-deficit/hyperactivity disorder? Incidence in a population-based birth cohort in Rochester, Minn. Arch Pediatr Adolesc Med (2002) 156:217–24. doi:10.1001/archpedi.156.3.217
85. Barbaresi W, Katusic S, Colligan R, Weaver A, Pankratz V, Mrazek D, et al. How common is attention-deficit/hyperactivity disorder? Towards resolution of the controversy: results from a population-based study. Acta Paediatr Suppl (2004) 93:55–9. doi:10.1111/j.1651-2227.2004.tb03058.x
86. Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry (2006) 163:716–23. doi:10.1176/ajp.2006.163.4.716
87. Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry (2007) 64:1145–52. doi:10.1001/archpsyc.64.10.1145
88. Hartsough CS, Lambert NM. Pattern and progression of drug use among hyperactives and controls: a prospective short-term longitudinal study. J Child Psychol Psychiatry (1987) 28:543–53. doi:10.1111/j.1469-7610.1987.tb00222.x
89. Biederman J, Monuteaux MC, Mick E, Wilens TE, Fontanella JA, Poetzl KM, et al. Is cigarette smoking a gateway to alcohol and illicit drug use disorders? A study of youths with and without attention deficit hyperactivity disorder. Biol Psychiatry (2006) 59:258–64. doi:10.1016/j.biopsych.2005.07.009
90. Rukstalis M, Jepson C, Patterson F, Lerman C. Increases in hyperactive-impulsive symptoms predict relapse among smokers in nicotine replacement therapy. J Subst Abuse Treat (2005) 28:297–304. doi:10.1016/j.jsat.2005.02.002
91. Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry (2003) 160:1028–40. doi:10.1176/appi.ajp.160.6.1028
92. Langley K, Rice F, van den Bree MB, Thapar A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva Pediatr (2005) 57:359–71.
93. Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J Child Psychol Psychiatry (2005) 46:246–54. doi:10.1111/j.1469-7610.2004.00359.x
94. Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr (2007) 96:1269–74. doi:10.1111/j.1651-2227.2007.00430.x
95. Motlagh MG, Sukhodolsky DG, Landeros-Weisenberger A, Katsovich L, Thompson N, Scahill L, et al. Adverse effects of heavy prenatal maternal smoking on attentional control in children with ADHD. J Atten Disord (2011) 15:593–603. doi:10.1177/1087054710374576
96. Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev Neuropsychol (2009) 34:1–36. doi:10.1080/87565640802564366
97. Thapar A, Rice F, Hay D, Boivin J, Langley K, van den Bree M, et al. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biol Psychiatry (2009) 66:722–7. doi:10.1016/j.biopsych.2009.05.032
98. Skoglund C, Chen Q, D’Onofrio BM, Lichtenstein P, Larsson H. Familial confounding of the association between maternal smoking during pregnancy and ADHD in offspring. J Child Psychol Psychiatry (2014) 55:61–8. doi:10.1111/jcpp.12124
99. Obel C, Zhu JL, Olsen J, Breining S, Li J, Gronborg TK, et al. The risk of attention deficit hyperactivity disorder in children exposed to maternal smoking during pregnancy – a reexamination using a sibling design. J Child Psychol Psychiatry (2015) 57(4):532–7. doi:10.1111/jcpp.12478
100. Treur JL, Willemsen G, Bartels M, Geels LM, van Beek JH, Huppertz C, et al. Smoking during adolescence as a risk factor for attention problems. Biol Psychiatry (2015) 78:656–63. doi:10.1016/j.biopsych.2014.06.019
101. Kutlu MG, Parikh V, Gould TJ. Nicotine addiction and psychiatric disorders. Int Rev Neurobiol (2015) 124:171–208. doi:10.1016/bs.irn.2015.08.004
102. Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ (2015) 351:h4065. doi:10.1136/bmj.h4065
103. Audrain-McGovern J, Rodriguez D, Kassel JD. Adolescent smoking and depression: evidence for self-medication and peer smoking mediation. Addiction (2009) 104:1743–56. doi:10.1111/j.1360-0443.2009.02617.x
104. Hooshmand S, Willoughby T, Good M. Does the direction of effects in the association between depressive symptoms and health-risk behaviors differ by behavior? A longitudinal study across the high school years. J Adolesc Health (2012) 50:140–7. doi:10.1016/j.jadohealth.2011.05.016
105. Berlin I, Covey LS. Pre-cessation depressive mood predicts failure to quit smoking: the role of coping and personality traits. Addiction (2006) 101:1814–21. doi:10.1111/j.1360-0443.2006.01616.x
106. Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd-Richardson E, Niaura R, et al. Impact of bupropion and cognitive-behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine Tob Res (2009) 11:1142–53. doi:10.1093/ntr/ntp111
107. Choi WS, Patten CA, Gillin JC, Kaplan RM, Pierce JP. Cigarette smoking predicts development of depressive symptoms among U.S. adolescents. Ann Behav Med (1997) 19:42–50. doi:10.1007/BF02883426
108. Wu LT, Anthony JC. Tobacco smoking and depressed mood in late childhood and early adolescence. Am J Public Health (1999) 89:1837–40. doi:10.2105/AJPH.89.12.1837
109. Goodman E, Capitman J. Depressive symptoms and cigarette smoking among teens. Pediatrics (2000) 106:748–55. doi:10.1542/peds.106.4.748
110. Klungsoyr O, Nygard JF, Sorensen T, Sandanger I. Cigarette smoking and incidence of first depressive episode: an 11-year, population-based follow-up study. Am J Epidemiol (2006) 163:421–32. doi:10.1093/aje/kwj058
111. Steuber TL, Danner F. Adolescent smoking and depression: which comes first? Addict Behav (2006) 31:133–6. doi:10.1016/j.addbeh.2005.04.010
112. Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ (2014) 348:g1151. doi:10.1136/bmj.g1151
113. Ischaki E, Gratziou C. Smoking and depression: is smoking cessation effective? Ther Adv Respir Dis (2009) 3:31–8. doi:10.1177/1753465809102662
114. Chaiton MO, Cohen JE, O’Loughlin J, Rehm J. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC Public Health (2009) 9:356. doi:10.1186/1471-2458-9-356
115. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry (2005) 62:593–602. doi:10.1001/archpsyc.62.6.593
116. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry (2005) 62:617–27. doi:10.1001/archpsyc.62.6.617
117. Cook BL, Wayne GF, Kafali EN, Liu Z, Shu C, Flores M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA (2014) 311:172–82. doi:10.1001/jama.2013.284985
118. Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry (2004) 61:1107–15. doi:10.1001/archpsyc.61.11.1107
119. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry (1995) 52:1048–60. doi:10.1001/archpsyc.1995.03950240066012
120. Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry (2003) 60:289–94. doi:10.1001/archpsyc.60.3.289
121. Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biol Psychiatry (2004) 55:69–76. doi:10.1016/S0006-3223(03)00317-2
122. Goodwin RD, Lewinsohn PM, Seeley JR. Cigarette smoking and panic attacks among young adults in the community: the role of parental smoking and anxiety disorders. Biol Psychiatry (2005) 58:686–93. doi:10.1016/j.biopsych.2005.04.042
123. Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, et al. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch Gen Psychiatry (2005) 62:1258–65. doi:10.1001/archpsyc.62.11.1258
124. Moylan S, Jacka FN, Pasco JA, Berk M. Cigarette smoking, nicotine dependence and anxiety disorders: a systematic review of population-based, epidemiological studies. BMC Med (2012) 10:123. doi:10.1186/1741-7015-10-123
125. Jiang F, Li S, Pan L, Zhang N, Jia C. Association of anxiety disorders with the risk of smoking behaviors: a meta-analysis of prospective observational studies. Drug Alcohol Depend (2014) 145:69–76. doi:10.1016/j.drugalcdep.2014.10.022
126. Siru R, Hulse GK, Tait RJ. Assessing motivation to quit smoking in people with mental illness: a review. Addiction (2009) 104:719–33. doi:10.1111/j.1360-0443.2009.02545.x
127. Zehe JM, Colder CR, Read JP, Wieczorek WF, Lengua LJ. Social and generalized anxiety symptoms and alcohol and cigarette use in early adolescence: the moderating role of perceived peer norms. Addict Behav (2013) 38:1931–9. doi:10.1016/j.addbeh.2012.11.013
128. Breslau N, Novak SP, Kessler RC. Daily smoking and the subsequent onset of psychiatric disorders. Psychol Med (2004) 34:323–33. doi:10.1017/S0033291703008869
129. Feldner MT, Babson KA, Zvolensky MJ, Vujanovic AA, Lewis SF, Gibson LE, et al. Posttraumatic stress symptoms and smoking to reduce negative affect: an investigation of trauma-exposed daily smokers. Addict Behav (2007) 32:214–27. doi:10.1016/j.addbeh.2006.03.032
130. Fu SS, McFall M, Saxon AJ, Beckham JC, Carmody TP, Baker DG, et al. Post-traumatic stress disorder and smoking: a systematic review. Nicotine Tob Res (2007) 9:1071–84. doi:10.1080/14622200701488418
131. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res (2008) 102:1–18. doi:10.1016/j.schres.2008.04.011
132. Morisano D, Bacher I, Audrain-McGovern J, George TP. Mechanisms underlying the comorbidity of tobacco use in mental health and addictive disorders. Can J Psychiatry (2009) 54:356–67.
133. Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry (1997) 42:1–5. doi:10.1016/S0006-3223(96)00302-2
134. Weinberger AH, Sacco KA, Creeden CL, Vessicchio JC, Jatlow PI, George TP. Effects of acute abstinence, reinstatement, and mecamylamine on biochemical and behavioral measures of cigarette smoking in schizophrenia. Schizophr Res (2007) 91:217–25. doi:10.1016/j.schres.2006.12.007
135. Williams JM, Gandhi KK, Lu SE, Kumar S, Shen J, Foulds J, et al. Higher nicotine levels in schizophrenia compared with controls after smoking a single cigarette. Nicotine Tob Res (2010) 12:855–9. doi:10.1093/ntr/ntq102
136. Williams JM, Ziedonis DM, Abanyie F, Steinberg ML, Foulds J, Benowitz NL. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophr Res (2005) 79:323–35. doi:10.1016/j.schres.2005.04.016
137. Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alcohol Depend (2005) 80:259–65. doi:10.1016/j.drugalcdep.2005.04.002
138. MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology (Berl) (2011) 216:91–9. doi:10.1007/s00213-011-2185-8
139. Winterer G. Why do patients with schizophrenia smoke? Curr Opin Psychiatry (2010) 23:112–9. doi:10.1097/YCO.0b013e3283366643
140. Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron (2002) 36:539–48. doi:10.1016/S0896-6273(02)01004-8
141. Hong LE, Schroeder M, Ross TJ, Buchholz B, Salmeron BJ, Wonodi I, et al. Nicotine enhances but does not normalize visual sustained attention and the associated brain network in schizophrenia. Schizophr Bull (2011) 37:416–25. doi:10.1093/schbul/sbp089
142. Warbrick T, Mobascher A, Brinkmeyer J, Musso F, Stoecker T, Shah NJ, et al. Nicotine effects on brain function during a visual oddball task: a comparison between conventional and EEG-informed fMRI analysis. J Cogn Neurosci (2012) 24:1682–94. doi:10.1162/jocn_a_00236
143. Rusted JM, Trawley S. Comparable effects of nicotine in smokers and nonsmokers on a prospective memory task. Neuropsychopharmacology (2006) 31:1545–9. doi:10.1038/sj.npp.1300965
144. Jansari AS, Froggatt D, Edginton T, Dawkins L. Investigating the impact of nicotine on executive functions using a novel virtual reality assessment. Addiction (2013) 108:977–84. doi:10.1111/add.12082
145. Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology (2008) 33:480–90. doi:10.1038/sj.npp.1301423
146. Hong LE, Wonodi I, Lewis J, Thaker GK. Nicotine effect on prepulse inhibition and prepulse facilitation in schizophrenia patients. Neuropsychopharmacology (2008) 33:2167–74. doi:10.1038/sj.npp.1301601
147. Postma P, Gray JA, Sharma T, Geyer M, Mehrotra R, Das M, et al. A behavioural and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacology (Berl) (2006) 184:589–99. doi:10.1007/s00213-006-0307-5
148. Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD). Psychopharmacol Bull (1996) 32:67–73.
149. Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) (1996) 123:55–63. doi:10.1007/BF02246281
150. Bekker EM, Bocker KB, Van Hunsel F, van den Berg MC, Kenemans JL. Acute effects of nicotine on attention and response inhibition. Pharmacol Biochem Behav (2005) 82:539–48. doi:10.1016/j.pbb.2005.10.009
151. Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) (2004) 176:182–94. doi:10.1007/s00213-004-1874-y
152. Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav (2008) 88:407–17. doi:10.1016/j.pbb.2007.09.014
153. Potter AS, Bucci DJ, Newhouse PA. Manipulation of nicotinic acetylcholine receptors differentially affects behavioral inhibition in human subjects with and without disordered baseline impulsivity. Psychopharmacology (Berl) (2012) 220:331–40. doi:10.1007/s00213-011-2476-0
154. Salin-Pascual RJ, Rosas M, Jimenez-Genchi A, Rivera-Meza BL, Delgado-Parra V. Antidepressant effect of transdermal nicotine patches in nonsmoking patients with major depression. J Clin Psychiatry (1996) 57:387–9.
155. Salin-Pascual RJ, Basanez-Villa E. Changes in compulsion and anxiety symptoms with nicotine transdermal patches in non-smoking obsessive-compulsive disorder patients. Rev Invest Clin (2003) 55:650–4.
156. Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) (2010) 210:453–69. doi:10.1007/s00213-010-1848-1
157. Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) (2006) 184:523–39. doi:10.1007/s00213-005-0164-7
158. D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology (2012) 62:1564–73. doi:10.1016/j.neuropharm.2011.01.044
159. Kalmijn S, van Boxtel MP, Verschuren MW, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. Am J Epidemiol (2002) 156:936–44. doi:10.1093/aje/kwf135
160. Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am J Public Health (2003) 93:994–8. doi:10.2105/AJPH.93.6.994
161. Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol (2007) 166:367–78. doi:10.1093/aje/kwm116
162. Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology (2001) 25:313–9. doi:10.1016/S0893-133X(01)00257-3
163. Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci (2007) 27:3477–89. doi:10.1523/JNEUROSCI.5129-06.2007
164. Burke JD, Loeber R, Lahey BB. Which aspects of ADHD are associated with tobacco use in early adolescence? J Child Psychol Psychiatry (2001) 42:493–502. doi:10.1111/1469-7610.00743
165. Lerman C, Audrain J, Tercyak K, Hawk LW Jr, Bush A, Crystal-Mansour S, et al. Attention-Deficit Hyperactivity Disorder (ADHD) symptoms and smoking patterns among participants in a smoking-cessation program. Nicotine Tob Res (2001) 3:353–9. doi:10.1080/14622200110072156
166. George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, et al. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology (2002) 26:75–85. doi:10.1016/S0893-133X(01)00296-2
167. Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry (2005) 62:649–59. doi:10.1001/archpsyc.62.6.649
168. Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology (Berl) (1996) 127:31–8. doi:10.1007/BF02805972
169. Grobe JE, Perkins KA, Goettler-Good J, Wilson A. Importance of environmental distractors in the effects of nicotine on short-term memory. Exp Clin Psychopharmacol (1998) 6:209–16. doi:10.1037/1064-1297.6.2.209
170. Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, et al. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry (2005) 58:143–50. doi:10.1016/j.biopsych.2005.03.028
171. Niemegeers P, Dumont GJ, Quisenaerts C, Morrens M, Boonzaier J, Fransen E, et al. The effects of nicotine on cognition are dependent on baseline performance. Eur Neuropsychopharmacol (2014) 24:1015–23. doi:10.1016/j.euroneuro.2014.03.011
172. Kleykamp BA, Jennings JM, Blank MD, Eissenberg T. The effects of nicotine on attention and working memory in never-smokers. Psychol Addict Behav (2005) 19:433–8. doi:10.1037/0893-164X.19.4.433
173. Poltavski DV, Petros T. Effects of transdermal nicotine on attention in adult non-smokers with and without attentional deficits. Physiol Behav (2006) 87:614–24. doi:10.1016/j.physbeh.2005.12.011
174. Lejuez CW, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, et al. The Balloon Analogue Risk Task (BART) differentiates smokers and nonsmokers. Exp Clin Psychopharmacol (2003) 11:26–33. doi:10.1037/1064-1297.11.1.26
175. Galvan A, Schonberg T, Mumford J, Kohno M, Poldrack RA, London ED. Greater risk sensitivity of dorsolateral prefrontal cortex in young smokers than in nonsmokers. Psychopharmacology (Berl) (2013) 229:345–55. doi:10.1007/s00213-013-3113-x
176. Briggs Z, O’Connor M, Jollans EK, O’Halloran L, Dymond S, Whelan R. Flexible emotion-based decision-making behavior varies in current and former smokers. Addict Behav (2015) 45:269–75. doi:10.1016/j.addbeh.2015.02.011
177. Wei Z, Yang N, Liu Y, Yang L, Wang Y, Han L, et al. Resting-state functional connectivity between the dorsal anterior cingulate cortex and thalamus is associated with risky decision-making in nicotine addicts. Sci Rep (2016) 6:21778. doi:10.1038/srep21778
178. Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophr Bull (1993) 19:233–59. doi:10.1093/schbul/19.2.233
179. Andreasen NC. Schizophrenia: the fundamental questions. Brain Res Brain Res Rev (2000) 31:106–12. doi:10.1016/S0165-0173(99)00027-2
180. Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry (1982) 17:639–54.
181. Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry (1993) 150:1856–61. doi:10.1176/ajp.150.12.1856
182. Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry (1992) 32:607–16. doi:10.1016/0006-3223(92)90073-9
183. Knott V, Millar A, Fisher D, Albert P. Effects of nicotine on the amplitude and gating of the auditory P50 and its influence by dopamine D2 receptor gene polymorphism. Neuroscience (2010) 166:145–56. doi:10.1016/j.neuroscience.2009.11.053
184. Millar A, Smith D, Choueiry J, Fisher D, Albert P, Knott V. The moderating role of the dopamine transporter 1 gene on P50 sensory gating and its modulation by nicotine. Neuroscience (2011) 180:148–56. doi:10.1016/j.neuroscience.2011.02.008
185. Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry (1992) 49:206–15. doi:10.1001/archpsyc.1992.01820030038005
186. Woznica AA, Sacco KA, George TP. Prepulse inhibition deficits in schizophrenia are modified by smoking status. Schizophr Res (2009) 112:86–90. doi:10.1016/j.schres.2009.04.016
187. Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav Processes (2004) 65:35–42. doi:10.1016/S0376-6357(03)00109-8
188. Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology (Berl) (2005) 182:508–15. doi:10.1007/s00213-005-0110-8
189. Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology (1998) 18:135–74. doi:10.1016/S0893-133X(97)00113-9
190. Schleicher HE, Harris KJ, Catley D, Nazir N. The role of depression and negative affect regulation expectancies in tobacco smoking among college students. J Am Coll Health (2009) 57:507–12. doi:10.3200/JACH.57.5.507-512
191. Salin-Pascual RJ, de la Fuente JR, Galicia-Polo L, Drucker-Colin R. Effects of transderman nicotine on mood and sleep in nonsmoking major depressed patients. Psychopharmacology (Berl) (1995) 121:476–9. doi:10.1007/BF02246496
192. Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature (1997) 390:401–4. doi:10.1038/37120
193. Reitstetter R, Lukas RJ, Gruener R. Dependence of nicotinic acetylcholine receptor recovery from desensitization on the duration of agonist exposure. J Pharmacol Exp Ther (1999) 289:656–60.
194. Shytle RD, Silver AA, Sanberg PR. Comorbid bipolar disorder in Tourette’s syndrome responds to the nicotinic receptor antagonist mecamylamine (Inversine). Biol Psychiatry (2000) 48:1028–31. doi:10.1016/S0006-3223(00)00945-8
195. Silver AA, Shytle RD, Sanberg PR. Mecamylamine in Tourette’s syndrome: a two-year retrospective case study. J Child Adolesc Psychopharmacol (2000) 10:59–68. doi:10.1089/cap.2000.10.59
196. Silver AA, Shytle RD, Sheehan KH, Sheehan DV, Ramos A, Sanberg PR. Multicenter, double-blind, placebo-controlled study of mecamylamine monotherapy for Tourette’s disorder. J Am Acad Child Adolesc Psychiatry (2001) 40:1103–10. doi:10.1097/00004583-200109000-00020
197. Shytle RD, Silver AA, Sheehan KH, Sheehan DV, Sanberg PR. Neuronal nicotinic receptor inhibition for treating mood disorders: preliminary controlled evidence with mecamylamine. Depress Anxiety (2002) 16:89–92. doi:10.1002/da.10035
198. George TP, Sacco KA, Vessicchio JC, Weinberger AH, Shytle RD. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: a preliminary study. J Clin Psychopharmacol (2008) 28:340–4. doi:10.1097/JCP.0b013e318172b49e
199. Beckham JC, Kirby AC, Feldman ME, Hertzberg MA, Moore SD, Crawford AL, et al. Prevalence and correlates of heavy smoking in Vietnam veterans with chronic posttraumatic stress disorder. Addict Behav (1997) 22:637–47. doi:10.1016/S0306-4603(96)00071-8
200. Calhoun PS, Bosworth HB, Siegler IC, Bastian LA. The relationship between hostility and behavioral risk factors for poor health in women veterans. Prev Med (2001) 33:552–7. doi:10.1006/pmed.2001.0921
201. Marshall EC, Zvolensky MJ, Vujanovic AA, Gibson LE, Gregor K, Bernstein A. Evaluation of smoking characteristics among community-recruited daily smokers with and without posttraumatic stress disorder and panic psychopathology. J Anxiety Disord (2008) 22:1214–26. doi:10.1016/j.janxdis.2008.01.003
202. Carmody TP, McFall M, Saxon AJ, Malte CA, Chow B, Joseph AM, et al. Smoking outcome expectancies in military veteran smokers with posttraumatic stress disorder. Nicotine Tob Res (2012) 14:919–26. doi:10.1093/ntr/ntr304
203. Dedert EA, Dennis PA, Swinkels CM, Calhoun PS, Dennis MF, Beckham JC. Ecological momentary assessment of posttraumatic stress disorder symptoms during a smoking quit attempt. Nicotine Tob Res (2014) 16:430–6. doi:10.1093/ntr/ntt167
204. Jupp B, Dalley JW. Behavioral endophenotypes of drug addiction: etiological insights from neuroimaging studies. Neuropharmacology (2014) 76 Pt B:487–97. doi:10.1016/j.neuropharm.2013.05.041
205. Falco AM, Bevins RA. Individual differences in the behavioral effects of nicotine: a review of the preclinical animal literature. Pharmacol Biochem Behav (2015) 138:80–90. doi:10.1016/j.pbb.2015.09.017
206. Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, et al. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry (2008) 63:301–8. doi:10.1016/j.biopsych.2007.07.011
207. Diergaarde L, van Mourik Y, Pattij T, Schoffelmeer AN, De Vries TJ. Poor impulse control predicts inelastic demand for nicotine but not alcohol in rats. Addict Biol (2012) 17:576–87. doi:10.1111/j.1369-1600.2011.00376.x
208. Kolokotroni KZ, Rodgers RJ, Harrison AA. Trait differences in response to chronic nicotine and nicotine withdrawal in rats. Psychopharmacology (Berl) (2014) 231:567–80. doi:10.1007/s00213-013-3270-y
209. Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology (Berl) (2001) 158:175–80. doi:10.1007/s002130100867
210. Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci (2005) 25:8593–600. doi:10.1523/JNEUROSCI.2139-05.2005
211. Bernardi RE, Spanagel R. Basal activity level in mice predicts the initial and sensitized locomotor response to nicotine only in high responders. Behav Brain Res (2014) 264:143–50. doi:10.1016/j.bbr.2014.01.046
212. Pastor V, Andres ME, Bernabeu RO. The effect of previous exposure to nicotine on nicotine place preference. Psychopharmacology (Berl) (2013) 226:551–60. doi:10.1007/s00213-012-2928-1
213. Aydin C, Oztan O, Isgor C. Vulnerability to nicotine abstinence-related social anxiety-like behavior: molecular correlates in neuropeptide Y, Y2 receptor and corticotropin releasing factor. Neurosci Lett (2011) 490:220–5. doi:10.1016/j.neulet.2010.12.056
214. Aydin C, Oztan O, Isgor C. Long-term effects of juvenile nicotine exposure on abstinence-related social anxiety-like behavior and amygdalar cannabinoid receptor 1 (CB1R) mRNA expression in the novelty-seeking phenotype. Behav Brain Res (2012) 228:236–9. doi:10.1016/j.bbr.2011.11.015
215. Aydin C, Oztan O, Isgor C. Nicotine-induced anxiety-like behavior in a rat model of the novelty-seeking phenotype is associated with long-lasting neuropeptidergic and neuroplastic adaptations in the amygdala: effects of the cannabinoid receptor 1 antagonist AM251. Neuropharmacology (2012) 63:1335–45. doi:10.1016/j.neuropharm.2012.08.016
216. Aydin C, Oztan O, Isgor C. Hippocampal Y2 receptor-mediated mossy fiber plasticity is implicated in nicotine abstinence-related social anxiety-like behavior in an outbred rat model of the novelty-seeking phenotype. Pharmacol Biochem Behav (2014) 125:48–54. doi:10.1016/j.pbb.2014.08.004
217. Pawlak CR, Schwarting RK. Object preference and nicotine consumption in rats with high vs. low rearing activity in a novel open field. Pharmacol Biochem Behav (2002) 73:679–87. doi:10.1016/S0091-3057(02)00852-3
218. Pawlak CR, Schwarting RK. Repeated nicotine treatment in rats with high versus low rearing activity: analyses of behavioural sensitisation and place preference. Psychopharmacology (Berl) (2005) 178:440–50. doi:10.1007/s00213-004-2024-2
219. Wang T, Han W, Wang B, Jiang Q, Solberg-Woods LC, Palmer AA, et al. Propensity for social interaction predicts nicotine-reinforced behaviors in outbred rats. Genes Brain Behav (2014) 13:202–12. doi:10.1111/gbb.12112
220. Abreu-Villaca Y, Queiroz-Gomes Fdo E, Dal Monte AP, Filgueiras CC, Manhaes AC. Individual differences in novelty-seeking behavior but not in anxiety response to a new environment can predict nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res (2006) 167:175–82. doi:10.1016/j.bbr.2005.09.003
221. Manhaes AC, Guthierrez MC, Filgueiras CC, Abreu-Villaca Y. Anxiety-like behavior during nicotine withdrawal predict subsequent nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res (2008) 193:216–24. doi:10.1016/j.bbr.2008.05.018
222. Falco AM, McDonald CG, Smith RF. Anxiety status affects nicotine- and baclofen-induced locomotor activity, anxiety, and single-trial conditioned place preference in male adolescent rats. Dev Psychobiol (2014) 56:1352–64. doi:10.1002/dev.21217
223. Bilkei-Gorzo A, Racz I, Michel K, Darvas M, Maldonado R, Zimmer A. A common genetic predisposition to stress sensitivity and stress-induced nicotine craving. Biol Psychiatry (2008) 63:164–71. doi:10.1016/j.biopsych.2007.02.010
224. Gomez R, Corr PJ. ADHD and personality: a meta-analytic review. Clin Psychol Rev (2014) 34:376–88. doi:10.1016/j.cpr.2014.05.002
225. Lombardo LE, Bearden CE, Barrett J, Brumbaugh MS, Pittman B, Frangou S, et al. Trait impulsivity as an endophenotype for bipolar I disorder. Bipolar Disord (2012) 14:565–70. doi:10.1111/j.1399-5618.2012.01035.x
226. Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci (2005) 8:1450–7. doi:10.1038/nn1583
227. Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry (2010) 68:770–3. doi:10.1016/j.biopsych.2010.06.015
228. Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science (2007) 315:1267–70. doi:10.1126/science.1137073
229. Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science (2008) 320:1352–5. doi:10.1126/science.1158136
230. Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry (2009) 65:851–6. doi:10.1016/j.biopsych.2008.12.008
231. Radwanska K, Kaczmarek L. Characterization of an alcohol addiction-prone phenotype in mice. Addict Biol (2012) 17:601–12. doi:10.1111/j.1369-1600.2011.00394.x
232. Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav (2009) 93:237–47. doi:10.1016/j.pbb.2009.04.018
233. Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) (2002) 163:362–80. doi:10.1007/s00213-002-1154-7
234. Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol (2011) 164:1301–21. doi:10.1111/j.1476-5381.2011.01323.x
235. Batra A, Collins SE, Torchalla I, Schroter M, Buchkremer G. Multidimensional smoker profiles and their prediction of smoking following a pharmacobehavioral intervention. J Subst Abuse Treat (2008) 35:41–52. doi:10.1016/j.jsat.2007.08.006
236. Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science (1989) 245:1511–3. doi:10.1126/science.2781295
237. Blanchard MM, Mendelsohn D, Stamp JA. The HR/LR model: further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev (2009) 33:1145–54. doi:10.1016/j.neubiorev.2009.05.009
238. Nesil T, Yararbas G, Mola G, Kanit L, Pogun S. Previous chronic exposure eliminates the conditioning effect of nicotine in rats. Brain Res Bull (2011) 85:339–45. doi:10.1016/j.brainresbull.2011.05.011
239. Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol (2005) 13:367–75. doi:10.1037/1064-1297.13.4.367
240. Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harb Perspect Med (2012) 2(11). doi:10.1101/cshperspect.a011940
241. Redolat R, Perez-Martinez A, Carrasco MC, Mesa P. Individual differences in novelty-seeking and behavioral responses to nicotine: a review of animal studies. Curr Drug Abuse Rev (2009) 2:230–42. doi:10.2174/1874473710902030230
242. Gyekis J, Foreman JE, Anthony K, Klein LC, Vandenbergh DJ. Activity-related behaviors in the hole-board predict nicotine consumption in C57B6 mice perinatally exposed to nicotine. Behav Brain Res (2010) 206:139–42. doi:10.1016/j.bbr.2009.08.024
243. Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology (2011) 36:569–79. doi:10.1038/npp.2010.188
244. Caille S, Clemens K, Stinus L, Cador M. Modeling nicotine addiction in rats. Methods Mol Biol (2012) 829:243–56. doi:10.1007/978-1-61779-458-2_15
245. Cohen A, Koob GF, George O. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology (2012) 37:2153–60. doi:10.1038/npp.2012.67
246. Clemens KJ, Castino MR, Cornish JL, Goodchild AK, Holmes NM. Behavioral and neural substrates of habit formation in rats intravenously self-administering nicotine. Neuropsychopharmacology (2014) 39:2584–93. doi:10.1038/npp.2014.111
247. Gilpin NW, Whitaker AM, Baynes B, Abdel AY, Weil MT, George O. Nicotine vapor inhalation escalates nicotine self-administration. Addict Biol (2014) 19:587–92. doi:10.1111/adb.12021
248. Linares Scott TJ, Heil SH, Higgins ST, Badger GJ, Bernstein IM. Depressive symptoms predict smoking status among pregnant women. Addict Behav (2009) 34:705–8. doi:10.1016/j.addbeh.2009.04.003
249. Kushner MG, Menary KR, Maurer EW, Thuras P. Greater elevation in risk for nicotine dependence per pack of cigarettes smoked among those with an anxiety disorder. J Stud Alcohol Drugs (2012) 73:920–4. doi:10.15288/jsad.2012.73.920
250. Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS. Mood and anxiety regulation by nicotinic acetylcholine receptors: a potential pathway to modulate aggression and related behavioral states. Neuropharmacology (2015) 96:235–43. doi:10.1016/j.neuropharm.2014.12.028
251. Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry (1985) 142:1259–64. doi:10.1176/ajp.142.11.1259
252. Lejuez CW, Zvolensky MJ, Daughters SB, Bornovalova MA, Paulson A, Tull MT, et al. Anxiety sensitivity: a unique predictor of dropout among inner-city heroin and crack/cocaine users in residential substance use treatment. Behav Res Ther (2008) 46:811–8. doi:10.1016/j.brat.2008.03.010
253. Khantzian EJ. Addiction as a self-regulation disorder and the role of self-medication. Addiction (2013) 108:668–9. doi:10.1111/add.12004
254. Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med (2008) 121:S3–10. doi:10.1016/j.amjmed.2008.01.015
255. Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev (2005) 29:1193–205. doi:10.1016/j.neubiorev.2005.04.017
256. Brielmaier J, McDonald CG, Smith RF. Effects of acute stress on acquisition of nicotine conditioned place preference in adolescent rats: a role for corticotropin-releasing factor 1 receptors. Psychopharmacology (Berl) (2012) 219:73–82. doi:10.1007/s00213-011-2378-1
257. Said N, Lakehayli S, El Khachibi M, El Ouahli M, Nadifi S, Hakkou F, et al. Effect of prenatal stress on memory, nicotine withdrawal and 5HT1A expression in raphe nuclei of adult rats. Int J Dev Neurosci (2015) 43:92–8. doi:10.1016/j.ijdevneu.2015.04.008
258. Said N, Lakehayli S, El Khachibi M, El Ouahli M, Nadifi S, Hakkou F, et al. Prenatal stress induces vulnerability to nicotine addiction and alters D2 receptors’ expression in the nucleus accumbens in adult rats. Neuroscience (2015) 304:279–85. doi:10.1016/j.neuroscience.2015.07.029
259. Papp M, Gruca P, Lason-Tyburkiewicz M, Litwa E, Willner P. Effects of chronic mild stress on the development of drug dependence in rats. Behav Pharmacol (2014) 25:518–31. doi:10.1097/FBP.0000000000000046
260. Hall FS, Der-Avakian A, Gould TJ, Markou A, Shoaib M, Young JW. Negative affective states and cognitive impairments in nicotine dependence. Neurosci Biobehav Rev (2015) 58:168–85. doi:10.1016/j.neubiorev.2015.06.004
261. Kayir H, Goktalay G, Yavuz O, Uzbay TI. Impact of baseline prepulse inhibition on nicotine-induced locomotor sensitization in rats. Behav Brain Res (2011) 216:275–80. doi:10.1016/j.bbr.2010.08.004
262. Berg SA, Sentir AM, Cooley BS, Engleman EA, Chambers RA. Nicotine is more addictive, not more cognitively therapeutic in a neurodevelopmental model of schizophrenia produced by neonatal ventral hippocampal lesions. Addict Biol (2014) 19:1020–31. doi:10.1111/adb.12082
263. Chen H, Hiler KA, Tolley EA, Matta SG, Sharp BM. Genetic factors control nicotine self-administration in isogenic adolescent rat strains. PLoS One (2012) 7:e44234. doi:10.1371/journal.pone.0044234
264. Watterson E, Daniels CW, Watterson LR, Mazur GJ, Brackney RJ, Olive MF, et al. Nicotine-induced place conditioning and locomotor activity in an adolescent animal model of attention deficit/hyperactivity disorder (ADHD). Behav Brain Res (2015) 291:184–8. doi:10.1016/j.bbr.2015.05.031
265. Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, et al. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet (2006) 36:697–712. doi:10.1007/s10519-006-9058-7
266. Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology (Berl) (1998) 138:266–74. doi:10.1007/s002130050671
267. Stolerman IP, Mirza NR, Hahn B, Shoaib M. Nicotine in an animal model of attention. Eur J Pharmacol (2000) 393:147–54. doi:10.1016/S0014-2999(99)00886-9
268. Blondel A, Sanger DJ, Moser PC. Characterisation of the effects of nicotine in the five-choice serial reaction time task in rats: antagonist studies. Psychopharmacology (Berl) (2000) 149:293–305. doi:10.1007/s002130000378
269. Bizarro L, Patel S, Murtagh C, Stolerman IP. Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine and methylphenidate. Behav Pharmacol (2004) 15:195–206.
270. van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) (2006) 187:73–85. doi:10.1007/s00213-006-0396-1
271. Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol Biochem Behav (2007) 87:360–8. doi:10.1016/j.pbb.2007.05.009
272. Tsutsui-Kimura I, Ohmura Y, Izumi T, Yamaguchi T, Yoshida T, Yoshioka M. Nicotine provokes impulsive-like action by stimulating alpha4beta2 nicotinic acetylcholine receptors in the infralimbic, but not in the prelimbic cortex. Psychopharmacology (Berl) (2010) 209:351–9. doi:10.1007/s00213-010-1804-0
273. Kolokotroni KZ, Rodgers RJ, Harrison AA. Acute nicotine increases both impulsive choice and behavioural disinhibition in rats. Psychopharmacology (Berl) (2011) 217:455–73. doi:10.1007/s00213-011-2296-2
274. Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res (2000) 117:197–208. doi:10.1016/S0166-4328(00)00305-3
275. Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, et al. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology (2009) 34:299–306. doi:10.1038/npp.2008.96
276. Kirshenbaum AP, Jackson ER, Brown SJ, Fuchs JR, Miltner BC, Doughty AH. Nicotine-induced impulsive action: sensitization and attenuation by mecamylamine. Behav Pharmacol (2011) 22:207–21. doi:10.1097/FBP.0b013e328345ca1c
277. Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol (2005) 16:15–23. doi:10.1097/00008877-200502000-00002
278. Kelsey JE, Niraula A. Effects of acute and sub-chronic nicotine on impulsive choice in rats in a probabilistic delay-discounting task. Psychopharmacology (Berl) (2013) 227:385–92. doi:10.1007/s00213-013-2984-1
279. Anderson KG, Diller JW. Effects of acute and repeated nicotine administration on delay discounting in Lewis and Fischer 344 rats. Behav Pharmacol (2010) 21:754–64. doi:10.1097/FBP.0b013e328340a050
280. O’Neill AB, Brioni JD. Benzodiazepine receptor mediation of the anxiolytic-like effect of (-)-nicotine in mice. Pharmacol Biochem Behav (1994) 49:755–7. doi:10.1016/0091-3057(94)90097-3
281. File SE, Kenny PJ, Ouagazzal AM. Bimodal modulation by nicotine of anxiety in the social interaction test: role of the dorsal hippocampus. Behav Neurosci (1998) 112:1423–9. doi:10.1037/0735-7044.112.6.1423
282. Irvine EE, Cheeta S, File SE. Time-course of changes in the social interaction test of anxiety following acute and chronic administration of nicotine. Behav Pharmacol (1999) 10:691–7. doi:10.1097/00008877-199911000-00016
283. Balerio GN, Aso E, Maldonado R. Involvement of the opioid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology (Berl) (2005) 181:260–9. doi:10.1007/s00213-005-2238-y
284. Balerio GN, Aso E, Maldonado R. Role of the cannabinoid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology (Berl) (2006) 184:504–13. doi:10.1007/s00213-005-0251-9
285. Villegier AS, Gallager B, Heston J, Belluzzi JD, Leslie FM. Age influences the effects of nicotine and monoamine oxidase inhibition on mood-related behaviors in rats. Psychopharmacology (Berl) (2010) 208:593–601. doi:10.1007/s00213-009-1760-8
286. McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. alpha4beta2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J Neurosci (2011) 31:10891–902. doi:10.1523/JNEUROSCI.0937-11.2011
287. Varani AP, Moutinho LM, Bettler B, Balerio GN. Acute behavioural responses to nicotine and nicotine withdrawal syndrome are modified in GABA(B1) knockout mice. Neuropharmacology (2012) 63:863–72. doi:10.1016/j.neuropharm.2012.06.006
288. Ouagazzal AM, Kenny PJ, File SE. Modulation of behaviour on trials 1 and 2 in the elevated plus-maze test of anxiety after systemic and hippocampal administration of nicotine. Psychopharmacology (Berl) (1999) 144:54–60. doi:10.1007/s002130050976
289. Irvine EE, Cheeta S, File SE. Tolerance to nicotine’s effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacol Biochem Behav (2001) 68:319–25. doi:10.1016/S0091-3057(00)00449-4
290. Biala G, Kruk M. Effects of co-administration of bupropion and nicotine or d-amphetamine on the elevated plus maze test in mice. J Pharm Pharmacol (2009) 61:493–502. doi:10.1211/jpp/61.04.0012
291. Biala G, Kruk M, Budzynska B. Effects of the cannabinoid receptor ligands on anxiety-related effects of d-amphetamine and nicotine in the mouse elevated plus maze test. J Physiol Pharmacol (2009) 60:113–22.
292. Zarrindast MR, Aghamohammadi-Sereshki A, Rezayof A, Rostami P. Nicotine-induced anxiogenic-like behaviours of rats in the elevated plus-maze: possible role of NMDA receptors of the central amygdala. J Psychopharmacol (2012) 26:555–63. doi:10.1177/0269881111412094
293. Ericson M, Olausson P, Engel JA, Soderpalm B. Nicotine induces disinhibitory behavior in the rat after subchronic peripheral nicotinic acetylcholine receptor blockade. Eur J Pharmacol (2000) 397:103–11. doi:10.1016/S0014-2999(00)00191-6
294. Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav (2004) 77:21–8. doi:10.1016/j.pbb.2003.09.016
295. Irvine EE, Bagnalasta M, Marcon C, Motta C, Tessari M, File SE, et al. Nicotine self-administration and withdrawal: modulation of anxiety in the social interaction test in rats. Psychopharmacology (Berl) (2001) 153:315–20. doi:10.1007/s002130000586
296. Caldarone BJ, King SL, Picciotto MR. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Lett (2008) 439:187–91. doi:10.1016/j.neulet.2008.05.023
297. Trigo JM, Zimmer A, Maldonado R. Nicotine anxiogenic and rewarding effects are decreased in mice lacking beta-endorphin. Neuropharmacology (2009) 56:1147–53. doi:10.1016/j.neuropharm.2009.03.013
298. Bura SA, Burokas A, Martin-Garcia E, Maldonado R. Effects of chronic nicotine on food intake and anxiety-like behaviour in CB(1) knockout mice. Eur Neuropsychopharmacol (2010) 20:369–78. doi:10.1016/j.euroneuro.2010.02.003
299. Besson M, Suarez S, Cormier A, Changeux JP, Granon S. Chronic nicotine exposure has dissociable behavioural effects on control and beta2-/- mice. Behav Genet (2008) 38:503–14. doi:10.1007/s10519-008-9216-1
300. Ijomone OM, Olaibi OK, Mba C, Biose IJ, Tete SA, Nwoha PU. Chronic nicotine administration does not alter cognitive or mood associated behavioural parameters. Pathophysiology (2015) 22:57–63. doi:10.1016/j.pathophys.2014.12.004
301. Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res (1999) 102:31–9. doi:10.1016/S0166-4328(98)00157-0
302. Gould TJ. Nicotine produces a within-subject enhancement of contextual fear conditioning in C57BL/6 mice independent of sex. Integr Physiol Behav Sci (2003) 38:124–32. doi:10.1007/BF02688830
303. Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem (2003) 80:147–57. doi:10.1016/S1074-7427(03)00057-1
304. Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci (2003) 117:1276–82. doi:10.1037/0735-7044.117.6.1276
305. Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, et al. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience (2004) 129:11–24. doi:10.1016/j.neuroscience.2004.07.016
306. Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci (2005) 25:8708–13. doi:10.1523/JNEUROSCI.2853-05.2005
307. Davis JA, Porter J, Gould TJ. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett (2006) 394:202–5. doi:10.1016/j.neulet.2005.10.026
308. Portugal GS, Wilkinson DS, Kenney JW, Sullivan C, Gould TJ. Strain-dependent effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Behav Genet (2012) 42:133–50. doi:10.1007/s10519-011-9489-7
309. Szyndler J, Sienkiewicz-Jarosz H, Maciejak P, Siemiatkowski M, Rokicki D, Czlonkowska AI, et al. The anxiolytic-like effect of nicotine undergoes rapid tolerance in a model of contextual fear conditioning in rats. Pharmacol Biochem Behav (2001) 69:511–8. doi:10.1016/S0091-3057(01)00548-2
310. Kutlu MG, Oliver C, Gould TJ. The effects of acute nicotine on contextual safety discrimination. J Psychopharmacol (2014) 28:1064–70. doi:10.1177/0269881114552743
311. Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E Jr, Janowsky DS, et al. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) (1999) 142:193–9. doi:10.1007/s002130050879
312. Vazquez-Palacios G, Bonilla-Jaime H, Velazquez-Moctezuma J. Antidepressant-like effects of the acute and chronic administration of nicotine in the rat forced swimming test and its interaction with fluoxetine [correction of flouxetine]. Pharmacol Biochem Behav (2004) 78:165–9. doi:10.1016/j.pbb.2004.03.002
313. Suemaru K, Yasuda K, Cui R, Li B, Umeda K, Amano M, et al. Antidepressant-like action of nicotine in forced swimming test and brain serotonin in mice. Physiol Behav (2006) 88:545–9. doi:10.1016/j.physbeh.2006.05.007
314. Andreasen JT, Redrobe JP. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: role of strain, test and sex. Behav Pharmacol (2009) 20:286–95. doi:10.1097/FBP.0b013e32832c713e
315. Tizabi Y, Getachew B, Rezvani AH, Hauser SR, Overstreet DH. Antidepressant-like effects of nicotine and reduced nicotinic receptor binding in the Fawn-hooded rat, an animal model of co-morbid depression and alcoholism. Prog Neuropsychopharmacol Biol Psychiatry (2009) 33:398–402. doi:10.1016/j.pnpbp.2008.09.010
316. Semba J, Mataki C, Yamada S, Nankai M, Toru M. Antidepressantlike effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry (1998) 43:389–91. doi:10.1016/S0006-3223(97)00477-0
317. Djuric VJ, Dunn E, Overstreet DH, Dragomir A, Steiner M. Antidepressant effect of ingested nicotine in female rats of Flinders resistant and sensitive lines. Physiol Behav (1999) 67:533–7. doi:10.1016/S0031-9384(99)00091-8
318. Tizabi Y, Hauser SR, Tyler KY, Getachew B, Madani R, Sharma Y, et al. Effects of nicotine on depressive-like behavior and hippocampal volume of female WKY rats. Prog Neuropsychopharmacol Biol Psychiatry (2010) 34:62–9. doi:10.1016/j.pnpbp.2009.09.024
319. Puma C, Deschaux O, Molimard R, Bizot JC. Nicotine improves memory in an object recognition task in rats. Eur Neuropsychopharmacol (1999) 9:323–7. doi:10.1016/S0924-977X(99)00002-4
320. Levin ED, Kaplan S, Boardman A. Acute nicotine interactions with nicotinic and muscarinic antagonists: working and reference memory effects in the 16-arm radial maze. Behav Pharmacol (1997) 8:236–42.
321. Levin ED, Weber E, Icenogle L. Baclofen interactions with nicotine in rats: effects on memory. Pharmacol Biochem Behav (2004) 79:343–8. doi:10.1016/j.pbb.2004.08.013
322. Moragrega I, Carrasco MC, Vicens P, Redolat R. Spatial learning in male mice with different levels of aggressiveness: effects of housing conditions and nicotine administration. Behav Brain Res (2003) 147:1–8. doi:10.1016/S0166-4328(03)00112-8
323. Levin ED, Lee C, Rose JE, Reyes A, Ellison G, Jarvik M, et al. Chronic nicotine and withdrawal effects on radial-arm maze performance in rats. Behav Neural Biol (1990) 53:269–76. doi:10.1016/0163-1047(90)90509-5
324. Levin ED, Briggs SJ, Christopher NC, Rose JE. Persistence of chronic nicotine-induced cognitive facilitation. Behav Neural Biol (1992) 58:152–8. doi:10.1016/0163-1047(92)90399-O
325. Levin ED, Christopher NC, Briggs SJ, Rose JE. Chronic nicotine reverses working memory deficits caused by lesions of the fimbria or medial basalocortical projection. Brain Res Cogn Brain Res (1993) 1:137–43. doi:10.1016/0926-6410(93)90021-V
326. Arendash GW, Sanberg PR, Sengstock GJ. Nicotine enhances the learning and memory of aged rats. Pharmacol Biochem Behav (1995) 52:517–23. doi:10.1016/0091-3057(95)00119-H
327. Socci DJ, Sanberg PR, Arendash GW. Nicotine enhances Morris water maze performance of young and aged rats. Neurobiol Aging (1995) 16:857–60. doi:10.1016/0197-4580(95)00091-R
328. Levin ED, Torry D. Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology (Berl) (1996) 123:88–97. doi:10.1007/BF02246285
329. Yilmaz O, Kanit L, Okur BE, Pogun S. Effects of nicotine on active avoidance learning in rats: sex differences. Behav Pharmacol (1997) 8:253–60.
330. Attaway CM, Compton DM, Turner MD. The effects of nicotine on learning and memory: a neuropsychological assessment in young and senescent Fischer 344 rats. Physiol Behav (1999) 67:421–31. doi:10.1016/S0031-9384(99)00081-5
331. Levin ED, Christopher NC, Weaver T, Moore J, Brucato F. Ventral hippocampal ibotenic acid lesions block chronic nicotine-induced spatial working memory improvement in rats. Brain Res Cogn Brain Res (1999) 7:405–10. doi:10.1016/S0926-6410(98)00044-5
332. Ciamei A, Aversano M, Cestari V, Castellano C. Effects of MK-801 and nicotine combinations on memory consolidation in CD1 mice. Psychopharmacology (Berl) (2001) 154:126–30. doi:10.1007/s002130000584
333. Vicens P, Carrasco MC, Redolat R. Effects of early training and nicotine treatment on the performance of male NMRI mice in the water maze. Neural Plast (2003) 10:303–17. doi:10.1155/NP.2003.303
334. Blondel A, Simon H, Sanger DJ, Moser P. The effect of repeated nicotine administration on the performance of drug-naive rats in a five-choice serial reaction time task. Behav Pharmacol (1999) 10:665–73. doi:10.1097/00008877-199911000-00013
335. Grilly DM. A verification of psychostimulant-induced improvement in sustained attention in rats: effects of d-amphetamine, nicotine, and pemoline. Exp Clin Psychopharmacol (2000) 8:14–21. doi:10.1037/1064-1297.8.1.14
336. Grilly DM, Simon BB, Levin ED. Nicotine enhances stimulus detection performance of middle- and old-aged rats: a longitudinal study. Pharmacol Biochem Behav (2000) 65:665–70. doi:10.1016/S0091-3057(99)00259-2
337. Mirza NR, Bright JL. Nicotine-induced enhancements in the five-choice serial reaction time task in rats are strain-dependent. Psychopharmacology (Berl) (2001) 154:8–12. doi:10.1007/s002130000605
338. Bizarro L, Stolerman IP. Attentional effects of nicotine and amphetamine in rats at different levels of motivation. Psychopharmacology (Berl) (2003) 170:271–7. doi:10.1007/s00213-003-1543-6
339. Quarta D, Naylor CG, Morris HV, Patel S, Genn RF, Stolerman IP. Different effects of ionotropic and metabotropic glutamate receptor antagonists on attention and the attentional properties of nicotine. Neuropharmacology (2007) 53:421–30. doi:10.1016/j.neuropharm.2007.05.023
340. Hahn B, Stolerman IP. Nicotine-induced attentional enhancement in rats: effects of chronic exposure to nicotine. Neuropsychopharmacology (2002) 27:712–22. doi:10.1016/S0893-133X(02)00348-2
341. Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) (2002) 162:129–37. doi:10.1007/s00213-002-1005-6
342. Mendez IA, Gilbert RJ, Bizon JL, Setlow B. Effects of acute administration of nicotinic and muscarinic cholinergic agonists and antagonists on performance in different cost-benefit decision making tasks in rats. Psychopharmacology (Berl) (2012) 224:489–99. doi:10.1007/s00213-012-2777-y
343. Mitchell MR, Mendez IA, Vokes CM, Damborsky JC, Winzer-Serhan UH, Setlow B. Effects of developmental nicotine exposure in rats on decision-making in adulthood. Behav Pharmacol (2012) 23:34–42. doi:10.1097/FBP.0b013e32834eb04a
344. Ortega LA, Tracy BA, Gould TJ, Parikh V. Effects of chronic low- and high-dose nicotine on cognitive flexibility in C57BL/6J mice. Behav Brain Res (2013) 238:134–45. doi:10.1016/j.bbr.2012.10.032
345. Cole RD, Poole RL, Guzman DM, Gould TJ, Parikh V. Contributions of beta2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice. Psychopharmacology (Berl) (2015) 232:1207–17. doi:10.1007/s00213-014-3754-4
346. Allison C, Shoaib M. Nicotine improves performance in an attentional set shifting task in rats. Neuropharmacology (2013) 64:314–20. doi:10.1016/j.neuropharm.2012.06.055
347. Wood C, Kohli S, Malcolm E, Allison C, Shoaib M. Subtype-selective nicotinic acetylcholine receptor agonists can improve cognitive flexibility in an attentional set shifting task. Neuropharmacology (2016) 105:106–13. doi:10.1016/j.neuropharm.2016.01.006
348. Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) (2008) 200:1–26. doi:10.1007/s00213-008-1173-0
349. Scott D, Taylor JR. Chronic nicotine attenuates phencyclidine-induced impulsivity in a mouse serial reaction time task. Behav Brain Res (2014) 259:164–73. doi:10.1016/j.bbr.2013.11.009
350. Schneider T, Ilott N, Brolese G, Bizarro L, Asherson PJ, Stolerman IP. Prenatal exposure to nicotine impairs performance of the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology (2011) 36:1114–25. doi:10.1038/npp.2010.249
351. Schneider T, Bizarro L, Asherson PJ, Stolerman IP. Hyperactivity, increased nicotine consumption and impaired performance in the five-choice serial reaction time task in adolescent rats prenatally exposed to nicotine. Psychopharmacology (Berl) (2012) 223:401–15. doi:10.1007/s00213-012-2728-7
352. Kayir H, Semenova S, Markou A. Baseline impulsive choice predicts the effects of nicotine and nicotine withdrawal on impulsivity in rats. Prog Neuropsychopharmacol Biol Psychiatry (2014) 48:6–13. doi:10.1016/j.pnpbp.2013.09.007
353. Kutlu MG, Gould TJ. Nicotine modulation of fear memories and anxiety: implications for learning and anxiety disorders. Biochem Pharmacol (2015) 97:498–511. doi:10.1016/j.bcp.2015.07.029
354. Le Foll B, Ng E, Di Ciano P, Trigo JM. Psychiatric disorders as vulnerability factors for nicotine addiction: what have we learned from animal models? Curr Top Behav Neurosci (2015) 24:155–70. doi:10.1007/978-3-319-13482-6_6
355. Vinkers CH, de Jong NM, Kalkman CJ, Westphal KG, van Oorschot R, Olivier B, et al. Stress-induced hyperthermia is reduced by rapid-acting anxiolytic drugs independent of injection stress in rats. Pharmacol Biochem Behav (2009) 93:413–8. doi:10.1016/j.pbb.2009.05.017
356. Salas R, Main A, Gangitano DA, Zimmerman G, Ben-Ari S, Soreq H, et al. Nicotine relieves anxiogenic-like behavior in mice that overexpress the read-through variant of acetylcholinesterase but not in wild-type mice. Mol Pharmacol (2008) 74:1641–8. doi:10.1124/mol.108.048454
357. Andreasen JT, Henningsen K, Bate S, Christiansen S, Wiborg O. Nicotine reverses anhedonic-like response and cognitive impairment in the rat chronic mild stress model of depression: comparison with sertraline. J Psychopharmacol (2011) 25:1134–41. doi:10.1177/0269881110391831
358. Bhattacharya SK, Chakrabarti A, Sandler M, Glover V. Rat brain monoamine oxidase A and B inhibitory (tribulin) activity during drug withdrawal anxiety. Neurosci Lett (1995) 199:103–6. doi:10.1016/0304-3940(95)12032-Y
359. Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther (2003) 307:526–34. doi:10.1124/jpet.103.054908
360. Biala G, Weglinska B. Blockade of the expression of mecamylamine-precipitated nicotine withdrawal by calcium channel antagonists. Pharmacol Res (2005) 51:483–8. doi:10.1016/j.phrs.2004.11.009
361. Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology (2008) 54:1223–32. doi:10.1016/j.neuropharm.2008.03.013
362. Jonkman S, Risbrough VB, Geyer MA, Markou A. Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacology (2008) 33:2131–8. doi:10.1038/sj.npp.1301607
363. Gould TJ, Leach PT. Cellular, molecular, and genetic substrates underlying the impact of nicotine on learning. Neurobiol Learn Mem (2014) 107:108–32. doi:10.1016/j.nlm.2013.08.004
364. Elias GA, Gulick D, Wilkinson DS, Gould TJ. Nicotine and extinction of fear conditioning. Neuroscience (2010) 165:1063–73. doi:10.1016/j.neuroscience.2009.11.022
365. Tian S, Gao J, Han L, Fu J, Li C, Li Z. Prior chronic nicotine impairs cued fear extinction but enhances contextual fear conditioning in rats. Neuroscience (2008) 153:935–43. doi:10.1016/j.neuroscience.2008.03.005
366. Andreasen JT, Nielsen EO, Redrobe JP. Chronic oral nicotine increases brain [3H]epibatidine binding and responsiveness to antidepressant drugs, but not nicotine, in the mouse forced swim test. Psychopharmacology (Berl) (2009) 205:517–28. doi:10.1007/s00213-009-1560-1
367. Hayase T. Depression-related anhedonic behaviors caused by immobilization stress: a comparison with nicotine-induced depression-like behavioral alterations and effects of nicotine and/or “antidepressant” drugs. J Toxicol Sci (2011) 36:31–41. doi:10.2131/jts.36.31
368. Vazquez-Palacios G, Bonilla-Jaime H, Velazquez-Moctezuma J. Antidepressant effects of nicotine and fluoxetine in an animal model of depression induced by neonatal treatment with clomipramine. Prog Neuropsychopharmacol Biol Psychiatry (2005) 29:39–46. doi:10.1016/j.pnpbp.2004.08.008
369. Vieyra-Reyes P, Mineur YS, Picciotto MR, Tunez I, Vidaltamayo R, Drucker-Colin R. Antidepressant-like effects of nicotine and transcranial magnetic stimulation in the olfactory bulbectomy rat model of depression. Brain Res Bull (2008) 77:13–8. doi:10.1016/j.brainresbull.2008.05.007
370. Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature (1998) 393:76–9. doi:10.1038/30001
371. Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology (2001) 25:55–71. doi:10.1016/S0893-133X(00)00237-2
372. Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry (2001) 49:258–67. doi:10.1016/S0006-3223(00)01094-5
373. Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol (2008) 38:101–21. doi:10.1007/s12035-008-8037-9
374. Meguro K, Yamaguchi S, Arai H, Nakagawa T, Doi C, Yamada M, et al. Nicotine improves cognitive disturbance in senescence-accelerated mice. Pharmacol Biochem Behav (1994) 49:769–72. doi:10.1016/0091-3057(94)90100-7
375. Woodruff-Pak DS. Mecamylamine reversal by nicotine and by a partial alpha7 nicotinic acetylcholine receptor agonist (GTS-21) in rabbits tested with delay eyeblink classical conditioning. Behav Brain Res (2003) 143:159–67. doi:10.1016/S0166-4328(03)00039-1
376. Zhou M, Suszkiw JB. Nicotine attenuates spatial learning deficits induced in the rat by perinatal lead exposure. Brain Res (2004) 999:142–7. doi:10.1016/j.brainres.2003.10.068
377. Grigoryan GA, Mitchell SN, Hodges H, Sinden JD, Gray JA. Are the cognitive-enhancing effects of nicotine in the rat with lesions to the forebrain cholinergic projection system mediated by an interaction with the noradrenergic system? Pharmacol Biochem Behav (1994) 49:511–21. doi:10.1016/0091-3057(94)90063-9
378. Hiramatsu M, Yamatsu T, Kameyama T, Nabeshima T. Effects of repeated administration of (-)-nicotine on AF64A-induced learning and memory impairment in rats. J Neural Transm (Vienna) (2002) 109:361–75. doi:10.1007/s007020200029
379. Portugal GS, Gould TJ. Nicotine withdrawal disrupts new contextual learning. Pharmacol Biochem Behav (2009) 92:117–23. doi:10.1016/j.pbb.2008.11.001
380. Raybuck JD, Gould TJ. Nicotine withdrawal-induced deficits in trace fear conditioning in C57BL/6 mice – a role for high-affinity beta2 subunit-containing nicotinic acetylcholine receptors. Eur J Neurosci (2009) 29:377–87. doi:10.1111/j.1460-9568.2008.06580.x
381. Muir JL, Everitt BJ, Robbins TW. Reversal of visual attentional dysfunction following lesions of the cholinergic basal forebrain by physostigmine and nicotine but not by the 5-HT3 receptor antagonist, ondansetron. Psychopharmacology (Berl) (1995) 118:82–92. doi:10.1007/BF02245253
382. Rezvani AH, Levin ED. Nicotine-antipsychotic drug interactions and attentional performance in female rats. Eur J Pharmacol (2004) 486:175–82. doi:10.1016/j.ejphar.2003.12.021
383. Shoaib M, Bizarro L. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology (Berl) (2005) 178:211–22. doi:10.1007/s00213-004-2004-6
384. Semenova S, Bespalov A, Markou A. Decreased prepulse inhibition during nicotine withdrawal in DBA/2J mice is reversed by nicotine self-administration. Eur J Pharmacol (2003) 472:99–110. doi:10.1016/S0014-2999(03)01904-6
385. Andre JM, Gulick D, Portugal GS, Gould TJ. Nicotine withdrawal disrupts both foreground and background contextual fear conditioning but not pre-pulse inhibition of the acoustic startle response in C57BL/6 mice. Behav Brain Res (2008) 190:174–81. doi:10.1016/j.bbr.2008.02.018
Keywords: nicotine, predisposition, psychiatric disorders, cognition, addiction, emotion
Citation: Besson M and Forget B (2016) Cognitive Dysfunction, Affective States, and Vulnerability to Nicotine Addiction: A Multifactorial Perspective. Front. Psychiatry 7:160. doi: 10.3389/fpsyt.2016.00160
Received: 25 November 2015; Accepted: 06 September 2016;
Published: 21 September 2016
Edited by:
Mark Walton, University of Oxford, UKReviewed by:
François Paille, Université de Lorraine, FranceCopyright: © 2016 Besson and Forget. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Morgane Besson, bW9yZ2FuZS5iZXNzb25AcGFzdGV1ci5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.