- 1Neuropsychiatry Laboratory, IRCCS Santa Lucia Foundation, Rome, Italy
- 2Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy
- 3Museo Storico della Fisica e Centro Studi e Ricerche Enrico Fermi, Rome, Italy
- 4Menninger Department of Psychiatry and Behavioral Sciences, Beth K. and Stuart C. Yudofsky Division of Neuropsychiatry, Baylor College of Medicine, Houston, TX, USA
Third-generation neuroimaging research has been enriched by advances in magnetic resonance spectroscopy (MRS) measuring the concentration of important neurotrasmitters, such as the inhibitory amino acid GABA. Here, we performed a systematic mini-review on brain MRS studies measuring GABA concentration in patients affected by schizophrenia (SZ), bipolar disorder (BD), and major depressive disorder (MDD). We wondered whether multimodal investigations could overcome intrinsic technical limits of MRS giving a broader view of mental disorders pathogenesis. In SZ, unimodal studies gave mixed results, as increased, decreased, or unaltered GABA levels were reported depending on region, disease phase, and treatment. Conversely, multimodal results showed reduced level of glutamate, but not of GABA, in patients mirrored by in vitro biochemical findings revealing hippocampal reduction in glutamate signaling in SZ, and no deficits in GABA synthesis. Moreover, a mouse model confirmed the unique pathological characteristic of glutamate function in SZ. Unimodal studies in BD revealed again, inconsistent results, while no multimodal investigations including MRS on GABA exist. In MDD, unimodal studies could not differentiate patients from controls nor characterize high-risk subjects and remitted patients. However, a multimodal study combining functional magnetic resonance imaging and MRS revealed that cingulate cortex activity is related to glutamate, N-acetylaspartate levels and anhedonia in patients, and to GABA concentration in healthy subjects, improving the distinction between MDD and physiology. Overall, our results show that unimodal studies do not indicate GABA as a biomarker for the psychiatric disorders considered. Conversely, multimodal studies can widen the understanding of the link between psychopathology, genetics, neuroanatomy, and functional–biochemical brain activity in mental disorders. Although scarce, multimodal approaches seem promising for moving from GABA MRS unimodal-descriptive to causal level, and for integrating GABA results into a more comprehensive interpretation of mental disorder pathophysiology.
Introduction
An imbalance between excitation and inhibition in brain neuronal transmission has been hypothesized as one of the molecular mechanisms responsible for psychiatric disorders (1–5). In this context, multimodal studies coupling the continuous technical progresses in neuroimaging to methods for measuring neurotramsitter concentrations may represent a turning point for in vivo evidence of postmortem (6–8) and animal model (9–12) results. Moreover, the chance to link psychopathology, genetics, neuroanatomy, and functional–biochemical brain activity may take psychiatric research to the causal understanding of patients’ illness.
The support given by newly developed improvements in well known technologies, such as proton magnetic resonance spectroscopy (MRS) (13–15), has been fundamental to encourage in vivo research on gamma-aminobutyric acid (GABA) in brain physiology and pathology (16–18). GABA is the primary inhibitory neurotransmitter in the mammalian central nervous system. Theories on its dysfunction in schizophrenia (SZ) assume that alterations in the neural circuitry involving GABA have a role in the mechanisms of the disorder and associated cognitive deficits (19–21). The role of GABA dysfunction in different psychiatric disorders such as bipolar disorder (BD), or major depressive disorder (MDD) is also established (3, 22, 23).
Magnetic resonance spectroscopy is the election technique to non-invasively measure in vivo GABA concentration in selected brain regions (18, 24). However, direct interpretation of MRS results is limited by intrinsic features of the technique. In particular, acquisition of GABA signal is restricted to large (e.g., 3×3×3 cm3) single voxels, since multi-voxel spectroscopy usually measures metabolites with longer T2 relaxation, such as N-acetylaspartate (NAA), choline (Cho), and creatine (Cr). This results in a broad between-studies heterogeneity in the anatomical region investigated. Moreover, MRS can only detect total concentration of neurochemicals and cannot distinguish between separate functional pools, thus impeding conclusions on neurotransmitters availability.
In this context, multimodal approaches, combining MRS with other complementary techniques, would lead to a solid and comprehensive interpretation of neurochemical underpinnings of brain pathologies. As a case in point, multimodal MRS and functional magnetic resonance imaging (fMRI) would help in depicting the neurochemical and functional pathological mechanisms responsible for complex disorders. The support from electrobiological measurements such as electroencephalography (EEG) or magnetoencephalography (MEG), measuring the oscillatory activity in brain neuronal ensembles, could be fundamental in interpreting results on GABA concentration since the latter has been shown to be positively correlated with stimulus specific neuronal oscillations (25–27). Similarly, findings from in vitro tissue biochemistry, animal models, and genetics could provide data at higher spatial resolution and further mechanistic insights into the interpretation of GABA concentration (28).
On the basis of these considerations, we reviewed research articles focusing on GABA as measured by MRS in SZ and mood disorders (i.e., BD and MDD). In particular, we analyzed whether studies combining different approaches could overcome the technical limits intrinsic to MRS and give a broader view of the mechanisms involved into mental disorders.
Methods
To investigate recent MRS studies evaluating GABA level in the brain, we performed a systematic literature search on PubMed, PsycNET (including PsycINFO, PsycBOOKS, PsycCRITIQUES, PsycARTICLES, and PsycEXTRA databases), and Scopus database till November 2015 using the keywords “GABA” AND “spectroscopy” AND any of the following terms: “schizophrenia,” “bipolar disorder,” “major depressive disorder.” The reference list of identified articles and review papers was also hand searched to obtain additional articles. Inclusion criteria for studies selection were (1) English language, (2) articles published in peer-reviewed journals after 2000, (3) original research article (comments, letters to editors and review articles were excluded), (4a) inclusion of patients diagnosed with the specific neuropsychiatric disorder of interest according to ICD or DSM criteria or (4b) inclusion of high risk (HR) subjects, (5) inclusion of at least 10 patients, (6) comparison between patients and healthy controls (HC), (7) performance of MRS using a magnetic field of at least 3 T (to have a good signal-to-noise ratio and to resolve GABA peak from those of other more concentrated molecular compounds, e.g., NAA or Cr).
In the search for SZ studies, 72 papers were initially identified. Among them, 11 were not original researches (9 reviews, 1 comment, and 1 letter), 2 studies did not consider HC and 9 did not include SZ patients, 22 papers did not include humans (e.g., studies on animal models and in vitro measurements), 9 studies measured the unresolved glutamate + glutamine (Glx) with GABA contamination peak as a proxy of GABA concentration, 1 study included less than 10 patients and 6 studies were published before 2000. At the end of the selection process, 12 studies on SZ fulfilled the inclusion criteria.
In the search for BD studies, 21 papers were screened, but we excluded 7 reviews, 3 studies not performing in vivo MRS on humans, 1 on healthy men only, 1 not measuring GABA, 3 studies considering Glx, and 1 including less than10 patients. Only five studies survived the selection process for BD.
At last, 53 studies were initially identified for MDD, but only 11 studies were eligible for the review, and 42 were excluded (6 studies without a control group, 5 not focusing on MDD patients, 6 not using in vivo MRS on humans, 11 reviews, 1 comment, 5 measuring Glx, 4 considering less than 10 patients, 3 not in English, and 1 published before 2000).
Results
Schizophrenia
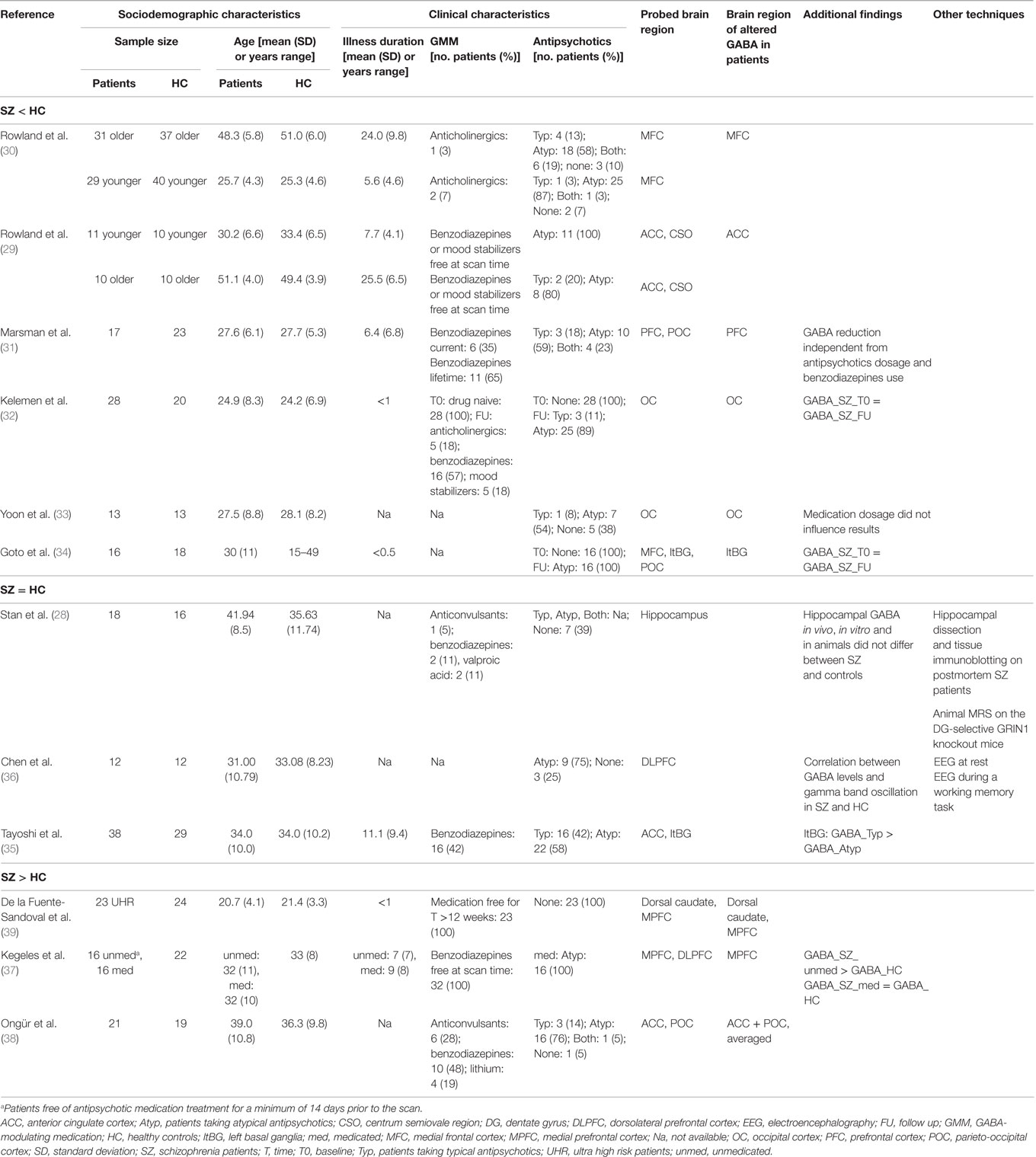
GABA MRS results in SZ are very scattered, since GABA concentration was found reduced, increased, or unaltered in patients (see Table 1). Such heterogeneity is mostly due to the different methodological approaches used, as studies vary in terms of patients’ clinical characteristics, brain region under investigation, and aims of the studies. Indeed, while most authors evaluated the diagnosis effect on GABA concentration, others considered the effect of age, of antipsychotics, and the role of GABA in different illness phases.
The most reported result (i.e., replicated in six studies) is that GABA concentration is reduced in SZ patients with respect to HC (29–34). Specifically, GABA was reduced in medial frontal cortex (MFC) (29, 30) and occipital cortex (OC) (32, 33), and the result was modulated by age in MFC (29, 30) and not affected by current medication type or dosage in OC (32, 33). The observed reduction in MFC GABA level in old SZ subjects as compared to age-matched HC suggests that GABA concentration decreases as age increases in patients and not in controls (29, 30). The independence from medication dosage in the OC (33) was further extended to the basal ganglia (34) suggesting that GABA reduction in these areas is driven by the disorder, being observable also in first-episode patients (32), and not an effect of treatment. A reduced GABA level in prefrontal areas of SZ patients was described only performing MRS at very high (7 T) magnetic field (31). Conversely, three studies (34–36) failed to find alterations in GABA level in SZ with respect to HC in any of the considered regions. Among them, one study found that patients taking only typical antipsychotics had higher GABA concentration than those taking only atypical antipsychotics (35). The other two studies failing to find GABA alterations in SZ, probed the hippocampus and the dorsolateral prefrontal cortex (DLPFC) (28, 36). These studies are of particular interest since they combined MRS with different experimental techniques. In particular, one correlated GABA levels in DLPFC to gamma oscillations, as measured by EEG during a working memory task, and found that both baseline and working memory-induced gamma oscillations were strongly dependent on GABA levels either in patients and controls (36). Within a data rich experimental design, the second multimodal study integrated in vivo MRS measurements of hippocampal GABA (and glutamate) concentration in patients with in vitro tissue biochemistry (sampling postmortem human hippocampal tissue) and MRS on a mouse model recapitulating symptoms of SZ (dentate gyrus-selective knockout of the GRIN1 gene, encoding a critical unit of N-methyl-d-aspartate receptors) (28). Looking at in vivo MRS results, authors found no global difference in GABA level between SZ and HC both in humans and animals, while they found decreased glutamate in SZ. Looking at in vitro results, authors found reduced level of GluN1 protein, a marker of the glutamatergic system, in SZ, but no alterations with respect to HC in the level of GAD67, the main enzyme in the GABAergic system. The combination of such findings provides evidence that the excitatory, but not the inhibitory, system within the hippocampus is implicated in SZ pathogenesis.
Finally, three studies found increased GABA concentration in SZ with respect to HC. One of them compared unmedicated SZ and patients medicated only with atypical antipsychotics to HC (37). Authors showed increased prefrontal GABA in unmedicated SZ patients with respect to both medicated and HC samples. Such results partially confirmed those presented in a previous research in which, averaging GABA concentration in anterior cingulate cortex (ACC) and parieto-occipital cortex (POC), authors found increased GABA in chronic SZ (38). More recently, an increased GABA concentration in dorsal caudate area and medial prefrontal cortex has been observed also considering ultra HR patients free from GABA modulating medications (GMM) (i.e., benzodiazepines, mood stabilizers, or antidepressants) and antipsychotics (39).
Bipolar Disorder
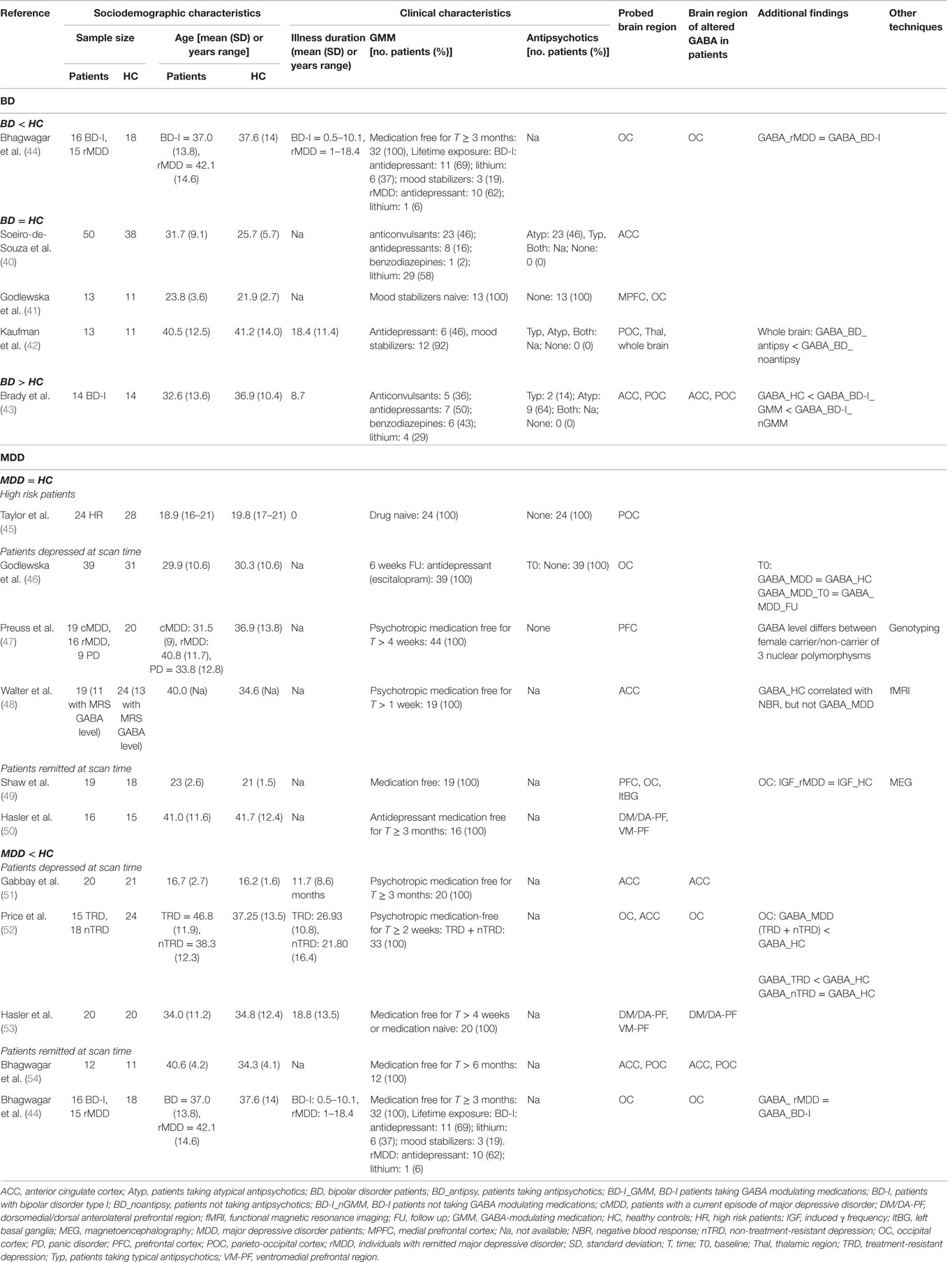
Among the few studies using MRS to measure GABA concentration in BD, three reported no difference between patients and HC (40–42). However, papers contributing to such evidence are very heterogeneous in terms of localization of MRS voxel, clinical characteristics of BD samples, and GMM (see Table 2), which were scarcely considered in the analyses. Their effect was specifically taken into account in a study indicating an increased GABA in BD as a whole, with respect to HC. However, within the patients group, there was a reduction of GABA in those taking GMM (43). To clarify the impact of medication dosage and lifetime exposure on GABA concentration, some authors considered only drug free patients (for at least 3 months before MR scan) who however, had lifetime exposure to lithium, antidepressants, or mood stabilizers (44). Results indicated decreased GABA level in recovered unmedicated BD patients with respect to HC.
No study using multimodal techniques has been published so far on BD patients.
Unipolar Major Depressive Disorder
Studies investigating unipolar MDD patients showed either no difference in GABA concentration between patients and HC (45–50), either a reduction of GABA in MDD (44, 51–54). A decreased GABA level has been observed mainly in patients depressed at scan time (51–53), but some authors found a reduction also in remitted patients (44, 54). One study comparing GABA level between HR subjects (i.e., having a family history of parental depression) and a control group without a family history of depression described negative results (45). Among studies failing to find an alteration of GABA in MDD, one combined genotyping with MRS in order to test the effect of common variants of the tryptophan hydroxylase isoform 2 (TPH2) gene, modulating serotonergic neurotransmission and brain circuits for emotion and adaptation, on GABA concentration in the prefrontal cortex (PFC) (47). Authors found a significant association between increased GABA concentration in the PFC and the allele frequencies of three TPH2 SNPs in female subjects, independently from diagnosis. Along with MRS, another research focused on remitted, formerly severe MDD patients and HC using MEG to measure the induced gamma oscillation frequency (IGF), a reliable surrogate marker of postsynaptic GABA function, in the OC (49). Authors found that MDD have normal IGF and GABA concentration in the OC. In a further multimodal investigation, MRS quantifying GABA, glutamate, and NAA concentrations was combined with fMRI measuring blood oxygenation level-dependent (BOLD) response to emotional stimuli in the pregenual ACC, part of the default mode network, related to anhedonia (48). MRS results showed no alteration in metabolites concentration in MDD patients, while fMRI indicated that negative BOLD responses, as well as glutamate and N-acetylaspartate concentrations, correlated with emotional intensity ratings, an anhedonia surrogate, in MDD but not in HC. Differently, negative BOLD responses in HC correlated with GABA. The fact that GABA concentration could not differentiate between MDD patients and HC together with the absence of GABA modulating effects on anhedonia were interpreted as secondary outcomes consequent to a primary deficit in glutamatergic metabolism, which may lead to a distortion of the excitation–inhibition balance and cause anhedonic depression.
Discussion
The involvement of GABA abnormalities in the mechanisms of psychiatric disorders is strongly debated. In particular, recent developments in MRS sequences allow discriminating the peak of GABA from those of more concentrated metabolites in the brain, thus permitting its measurement. However, despite postmortem evidence and preclinical studies highlighting GABAergic abnormalities in patients with mental disorders, the connection between these abnormalities and categorical/diagnostic or dimensional/symptomatic characteristics is still unclear. In this framework, we reviewed the body of evidence on GABA concentration, as measured by MRS in localized brain regions of SZ, BD, and MDD patients, particularly highlighting results obtained by multimodal methods and multiple experimental techniques.
Although this topic is under continuous development, some conclusions can be drawn from the present results.
Schizophrenia
First, the reduction of GABA level in SZ (the most frequent reported result) seems to occur in specific brain areas (frontal, occipital, and basal ganglia) and in old age, being probably a mixed effect of chronicity, lifetime exposure (more than current type or dosage) to antipsychotics, and GMM, particularly benzodiazepines (17). The latter is known to allosterically increase GABAA receptor activation, but available experimental techniques are still too coarse to detect circuit-specific perturbations in GABA levels as induced by benzodiazepines (or other medications modulating neuronal transmission), and results are not concordant. From our review, a slight majority of authors failed to find a link between GABA level and medications. Such heterogeneous results might be reconciled performing technically more precise experiments (e.g., MRS at ultra high magnetic field) and enrolling HR subjects in their preclinical stage or drug naive patients to be followed longitudinally.
The second interesting conclusion derived from multimodal studies on SZ is that GABA concentration alone cannot be considered a biomarker for this disorder, while a potential perturbation in the balance between excitation and inhibition, measurable through glutamate/GABA ratio, needs to be more deeply investigated in SZ (28). The latter should be the target for studies aimed at clarifying mechanisms and/or novel therapeutic strategies.
Bipolar Disorder
Unfortunately, GABA cannot be considered a biomarker of BD yet. Indeed, the only study including young and drug naive patients failed to find differences with respect to HC (41). From the other few studies, it appears that both current and lifetime exposure to GMM tend to reduce GABA level in BD patients, especially in the OC (43, 44). However, heterogeneity of patients’ clinical characteristics, illness phase at scan time, number of previous manic/depressive episodes, and eventual action of the complex mixtures of GMM (not only benzodiazepines but also antidepressants, lithium, mood stabilizers, etc.) justify the need to start multimodal researches focused on more homogeneous clinical subsamples.
Major Depressive Disorder
Research on neurotransmission in MDD is truly promising and intriguing in the hunt for innovative approaches to prevention. Understanding whether eventual changes in GABA reflect an underlying trait vulnerability to depression, or can be considered “scars” of depressive episodes or treatment effects, may have implications for preventative strategies in HR subjects (55). The only study measuring GABA concentration with MRS in subjects at risk of depression did not find differences in the parieto-occipital cortex with respect to subjects not at risk, indicating that, at the actual level of accuracy, GABA level in such brain region cannot be considered an endophenotype for depression (45). Moreover, the study including genotyping showed that GABA concentration in PFC is associated with allele frequencies of three polymorphisms linked to anxiety only in women, independently from the diagnosis (47). This result reinforces the notion that GABA levels are not a marker of MDD (at least in the POC and PFC). The other two multimodal studies associating MRS with fMRI (48) and MEG (49) failed to find differences in GABA concentration in diffuse brain regions between MDD and HC. However, the classification of studies in terms of patients state (i.e., depressed/remitted) at scan time (see Table 2) allows us to support the idea that GABA level identifies the state of being ill, and is not a trait marker for diagnosis, since physiological concentration has been described in the majority of studies including MDD patients during the remission phase (44, 49, 50, 53, 54). Conversely, a primary deficit in glutamatergic metabolism may cause aberrant neuronal activations patterns in regions specifically relevant for the expression of anhedonic behavior in MDD.
Conclusion
Complex and multimodal researches looking at GABA in psychiatric populations are still a minority. Our review shows that fMRI, in vitro biochemistry, genotyping, EEG, and MEG have been combined to MRS, and each of them adds a piece to the puzzle depicting the role of GABA abnormalities in psychiatric disorders. Indeed, fMRI can differentiate neural response patterns induced by stimulation (56), in vitro biochemistry allows higher resolution spatial information and correlations between MRS results and biochemical activity of the brain, while genotyping can elucidate the genetic correlates of GABAergic transmission. Furthermore, as EEG reflects voltage changes resulting from the synchronous firing of groups of neurons (57), and MEG describes the effects of synchronous postsynaptic activity (58), when combined with MRS they allow the in vivo investigation of GABA effect on neuronal transmission. Thus, from studies using a multimodal approach, it appears that GABA level alone may not be the best biomarker for the psychiatric disorders here considered. However, it is a promising parameter, particularly for the stratification of patients in more homogeneous subtypes sharing specific biological features. The possibility to reduce heterogeneity in psychiatric patients is fundamental both in research (giving the opportunity to gain new insight in the underlying pathophysiology of different mental disorders) and in clinical practice (allowing the prescription of effective and tailored medical treatments).
Conversely, although still scarce, the so-called third-generation paradigms will be the turning point of neuroimaging research on neurotransmission in general, and on GABA dysfunctions in particular. The effort spent in the design and realization of multimodal studies, as well as multicentre ones to include larger samples, would then be rewarded by the strong translational impact of such researches. This approach would support clinicians in the design of preventative interventions with defined, expected outcomes for specific types of psychiatric patients making “precision medicine” a more realistic medical model. The precise medicine is the final end.
Author Contributions
CCh and GS conceived the paper and performed literature search. CCh, FeP, FaP, and GS wrote the paper. All authors critically reviewed the manuscript and agreed on its final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was supported by the FILAS-RU-2014-1092 (PAMINA) grant of Regione Lazio.
References
1. Harrison PJ. GABA circuitry, cells and molecular regulation in schizophrenia: life in the graveyard. Schizophr Res (2015) 167:108–10. doi: 10.1016/j.schres.2015.02.003
2. Egerton A, Stone JM. The glutamate hypothesis of schizophrenia: neuroimaging and drug development. Curr Pharm Biotechnol (2012) 13:1500–12. doi:10.2174/138920112800784961
3. Bustillo JR. Use of proton magnetic resonance spectroscopy in the treatment of psychiatric disorders: a critical update. Dialogues Clin Neurosci (2013) 15:329–37.
4. Leuchter AF, Hunter AM, Krantz DE, Cook IA. Rhythms and blues: modulation of oscillatory synchrony and the mechanism of action of antidepressant treatments. Ann N Y Acad Sci (2015) 1344:78–91. doi:10.1111/nyas.12742
5. Cohen SM, Tsien RW, Goff DC, Halassa MM. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res (2015) 167:98–107. doi:10.1016/j.schres.2014.12.026
6. Taylor SF, Tso IF. GABA abnormalities in schizophrenia: a methodological review of in vivo studies. Schizophr Res (2015) 167:84–90. doi:10.1016/j.schres.2014.10.011
7. Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci (2005) 6:312–24. doi:10.1038/nrn1648
8. Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause? Biochem Pharmacol (2004) 68:1507–14. doi:10.1016/j.bcp.2004.07.034
9. Napolitano A, Shah K, Schubert MI, Porkess V, Fone KCF, Auer DP. In vivo neurometabolic profiling to characterize the effects of social isolation and ketamine-induced NMDA antagonism: a rodent study at 7.0 T. Schizophr Bull (2014) 40:566–74. doi:10.1093/schbul/sbt067
10. Puhl MD, Mintzopoulos D, Jensen JE, Gillis TE, Konopaske GT, Kaufman MJ, et al. In vivo magnetic resonance studies reveal neuroanatomical and neurochemical abnormalities in the serine racemase knockout mouse model of schizophrenia. Neurobiol Dis (2014) 73C:269–74. doi:10.1016/j.nbd.2014.10.009
11. Xu S, Gullapalli RP, Frost DO. Olanzapine antipsychotic treatment of adolescent rats causes long term changes in glutamate and GABA levels in the nucleus accumbens. Schizophr Res (2015) 161:452–7. doi:10.1016/j.schres.2014.10.034
12. Bustillo J, Galloway MP, Ghoddoussi F, Bolognani F, Perrone-Bizzozero N. Medial-frontal cortex hypometabolism in chronic phencyclidine exposed rats assessed by high resolution magic angle spin 11.7 T proton magnetic resonance spectroscopy. Neurochem Int (2012) 61:128–31. doi:10.1016/j.neuint.2012.04.003
13. Puts NAJ, Edden RAE. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc (2012) 60:29–41. doi:10.1016/j.pnmrs.2011.06.001
14. Mullins PG, McGonigle DJ, O’Gorman RL, Puts NAJ, Vidyasagar R, Evans CJ, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage (2014) 86:43–52. doi:10.1016/j.neuroimage.2012.12.004
15. Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging (2014) 40:1445–52. doi:10.1002/jmri.24478
16. Ende G. Proton magnetic resonance spectroscopy: relevance of glutamate and GABA to neuropsychology. Neuropsychol Rev (2015) 25:315–25. doi:10.1007/s11065-015-9295-8
17. Wijtenburg SA, Yang S, Fischer BA, Rowland LM. In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neurosci Biobehav Rev (2015) 51:276–95. doi:10.1016/j.neubiorev.2015.01.007
18. Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Curr Top Behav Neurosci (2012) 11:199–251. doi:10.1007/7854_2011_197
19. Lin C-Y, Tsai GE, Lane H-Y. Assessing and treating cognitive impairment in schizophrenia: current and future. Curr Pharm Des (2014) 20:5127–38. doi:10.2174/1381612819666140110120015
20. Liu S-K, Fitzgerald PB, Daigle M, Chen R, Daskalakis ZJ. The relationship between cortical inhibition, antipsychotic treatment, and the symptoms of schizophrenia. Biol Psychiatry (2009) 65:503–9. doi:10.1016/j.biopsych.2008.09.012
21. Tamminga CA. The neurobiology of cognition in schizophrenia. J Clin Psychiatry (2006) 67(Suppl 9):9–13; discussion 36–42. doi:10.4088/JCP.0906e11
22. Jembrek MJ, Vlainic J. GABA receptors: pharmacological potential and pitfalls. Curr Pharm Des (2015) 21:4943–59. doi:10.2174/1381612821666150914121624
23. Northoff G, Sibille E. Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol Psychiatry (2014) 19:966–77. doi:10.1038/mp.2014.68
24. Buonocore MH, Maddock RJ. Magnetic resonance spectroscopy of the brain: a review of physical principles and technical methods. Rev Neurosci (2015) 26:609–32. doi:10.1515/revneuro-2015-0010
25. Williams S, Boksa P. Gamma oscillations and schizophrenia. J Psychiatry Neurosci (2010) 35:75–7. doi:10.1503/jpn.100021
26. Duncan NW, Wiebking C, Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans – a review of multimodal imaging studies. Neurosci Biobehav Rev (2014) 47:36–52. doi:10.1016/j.neubiorev.2014.07.016
27. Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA (2009) 106:8356–61. doi:10.1073/pnas.0900728106
28. Stan AD, Ghose S, Zhao C, Hulsey K, Mihalakos P, Yanagi M, et al. Magnetic resonance spectroscopy and tissue protein concentrations together suggest lower glutamate signaling in dentate gyrus in schizophrenia. Mol Psychiatry (2015) 20:433–9. doi:10.1038/mp.2014.54
29. Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull (2013) 39:1096–104. doi:10.1093/schbul/sbs092
30. Rowland LM, Krause BW, Wijtenburg SA, McMahon RP, Chiappelli J, Nugent KL, et al. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol Psychiatry (2016) 21:198–204. doi:10.1038/mp.2015.34
31. Marsman A, Mandl RCW, Klomp DWJ, Bohlken MM, Boer VO, Andreychenko A, et al. GABA and glutamate in schizophrenia: a 7 T 1H-MRS study. NeuroImage Clin (2014) 6:398–407. doi:10.1016/j.nicl.2014.10.005
32. Kelemen O, Kiss I, Benedek G, Kéri S. Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: the potential impact of GABA concentration in the visual cortex. Prog Neuropsychopharmacol Biol Psychiatry (2013) 47:13–9. doi:10.1016/j.pnpbp.2013.07.024
33. Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci (2010) 30:3777–81. doi:10.1523/JNEUROSCI.6158-09.2010
34. Goto N, Yoshimura R, Kakeda S, Moriya J, Hori H, Hayashi K, et al. No alterations of brain GABA after 6 months of treatment with atypical antipsychotic drugs in early-stage first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2010) 34:1480–3. doi:10.1016/j.pnpbp.2010.08.007
35. Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, et al. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res (2010) 117:83–91. doi:10.1016/j.schres.2009.11.011
36. Chen C-MA, Stanford AD, Mao X, Abi-Dargham A, Shungu DC, Lisanby SH, et al. GABA level, gamma oscillation, and working memory performance in schizophrenia. NeuroImage Clin (2014) 4:531–9. doi:10.1016/j.nicl.2014.03.007
37. Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry (2012) 69:449–59. doi:10.1001/archgenpsychiatry.2011.1519
38. Ongür D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry (2010) 68:667–70. doi:10.1016/j.biopsych.2010.05.016
39. de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, León-Ortiz P, Rodríguez-Mayoral O, Solís-Vivanco R, et al. Cortico-striatal GABAergic and glutamatergic dysregulations in subjects at ultra-high risk for psychosis investigated with proton magnetic resonance spectroscopy. Int J Neuropsychopharmacol (2015) 19:1–10. doi:10.1093/ijnp/pyv105
40. Soeiro-de-Souza MG, Henning A, Machado-Vieira R, Moreno RA, Pastorello BF, da Costa Leite C, et al. Anterior cingulate Glutamate-Glutamine cycle metabolites are altered in euthymic bipolar I disorder. Eur Neuropsychopharmacol (2015) 25:2221–9. doi:10.1016/j.euroneuro.2015.09.020
41. Godlewska BR, Yip SW, Near J, Goodwin GM, Cowen PJ. Cortical glutathione levels in young people with bipolar disorder: a pilot study using magnetic resonance spectroscopy. Psychopharmacology (2014) 231:327–32. doi:10.1007/s00213-013-3244-0
42. Kaufman RE, Ostacher MJ, Marks EH, Simon NM, Sachs GS, Jensen JE, et al. Brain GABA levels in patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry (2009) 33:427–34. doi:10.1016/j.pnpbp.2008.12.025
43. Brady RO, McCarthy JM, Prescot AP, Jensen JE, Cooper AJ, Cohen BM, et al. Brain gamma-aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar Disord (2013) 15:434–9. doi:10.1111/bdi.12074
44. Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, et al. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry (2007) 61:806–12. doi:10.1016/j.biopsych.2006.08.048
45. Taylor MJ, Mannie ZN, Norbury R, Near J, Cowen PJ. Elevated cortical glutamate in young people at increased familial risk of depression. Int J Neuropsychopharmacol (2011) 14:255–9. doi:10.1017/S1461145710001094
46. Godlewska BR, Near J, Cowen PJ. Neurochemistry of major depression: a study using magnetic resonance spectroscopy. Psychopharmacology (2015) 232:501–7. doi:10.1007/s00213-014-3687-y
47. Preuss N, Salehi B, van der Veen JW, Shen J, Drevets WC, Hodgkinson C, et al. Associations between prefrontal γ-aminobutyric acid concentration and the tryptophan hydroxylase isoform 2 gene, a panic disorder risk allele in women. Int J Neuropsychopharmacol (2013) 16:1707–17. doi:10.1017/S1461145713000254
48. Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry (2009) 66:478–86. doi:10.1001/archgenpsychiatry.2009.39
49. Shaw A, Brealy J, Richardson H, Muthukumaraswamy SD, Edden RA, John Evans C, et al. Marked reductions in visual evoked responses but not γ-aminobutyric acid concentrations or γ-band measures in remitted depression. Biol Psychiatry (2013) 73:691–8. doi:10.1016/j.biopsych.2012.09.032
50. Hasler G, Neumeister A, van der Veen JW, Tumonis T, Bain EE, Shen J, et al. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry (2005) 58:969–73. doi:10.1016/j.biopsych.2005.05.017
51. Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. Anterior cingulate cortex γ-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry (2012) 69:139–49. doi:10.1001/archgenpsychiatry.2011.131
52. Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry (2009) 65:792–800. doi:10.1016/j.biopsych.2008.10.025
53. Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry (2007) 64:193–200. doi:10.1001/archpsyc.64.2.193
54. Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, M Matthews P, et al. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol (2008) 11:255–60. doi:10.1017/S1461145707007924
55. Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol Psychiatry (2011) 16:604–19. doi:10.1038/mp.2011.23
56. Logothetis NK. What we can do and what we cannot do with fMRI. Nature (2008) 453:869–78. doi:10.1038/nature06976
57. Elul R. The genesis of the EEG. Int Rev Neurobiol (1971) 15:227–72. doi:10.1016/S0074-7742(08)60333-5
Keywords: GABA, MRS, multimodal imaging, schizophrenia, bipolar disorder, major depressive disorder
Citation: Chiapponi C, Piras F, Piras F, Caltagirone C and Spalletta G (2016) GABA System in Schizophrenia and Mood Disorders: A Mini Review on Third-Generation Imaging Studies. Front. Psychiatry 7:61. doi: 10.3389/fpsyt.2016.00061
Received: 22 December 2015; Accepted: 29 March 2016;
Published: 19 April 2016
Edited by:
Stefan Borgwardt, University of Basel, SwitzerlandReviewed by:
Gabriele Sani, Sapienza University of Rome, ItalyAgata Szulc, Medical University of Warsaw, Poland
Copyright: © 2016 Chiapponi, Piras, Piras, Caltagirone and Spalletta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Chiapponi, Yy5jaGlhcHBvbmlAaHNhbnRhbHVjaWEuaXQ=
 Chiara Chiapponi
Chiara Chiapponi Federica Piras
Federica Piras Fabrizio Piras
Fabrizio Piras Carlo Caltagirone
Carlo Caltagirone Gianfranco Spalletta
Gianfranco Spalletta