- 1 Department of Psychiatry and Neurobehavioral Sciences, University of Virginia, Charlottesville, VA, USA
- 2 Division of Pharmacotherapies and Medical Consequences of Drug Abuse, National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD, USA
- 3 Division of Biostatistics, Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA, USA
- 4 Department of Public Health Sciences, University of Virginia, Charlottesville, VA, USA
- 5 Friends Research Institute, Baltimore, MD, USA
Early-onset methamphetamine use increases the lifetime prevalence of methamphetamine dependence. An earlier onset of methamphetamine use leads to greater damage to the terminal ends of serotonin neurons, more reduction in serotonin transporter (5-HTT) density, and an increased propensity toward further methamphetamine use. Because the 5-HTT-linked polymorphic region (5′-HTTLPR) within the promoter region of the 5-HTT gene leads to differential expression of the 5-HTT, we examined, for the first time, whether there is a differential association between the long (L) and short (S) alleles of the 5′-HTTLPR and the age of first methamphetamine use (AMU). The study included 120 methamphetamine-dependent adults of European descent. Diagnosis of methamphetamine dependence and AMU were collected using structured questionnaires, and the 5′-HTTLPR genotypes were determined using the polymerase chain reaction–restriction fragment length polymorphism method. Statistical analysis with the general linear model detected a significant interactive effect of 5′-HTTLPR genotypes (SS vs. L-carriers) and gender, associated with AMU (F = 3.99; p = 0.048). Further analysis of 5′-HTTLPR effects on AMU in males and females separately showed that the SS genotype compared with L-carriers had about two times greater risk of an earlier onset of methamphetamine use in men (hazard ratio = 1.839; 95% confidence interval = 1.042–3.246; p = 0.036) but not in women. Together, our findings in this preliminary study suggest a greater risk for earlier onset methamphetamine use associated with the SS genotype of the 5′-HTTLPR among methamphetamine-dependent Caucasian males.
Introduction
Methamphetamine is a highly addictive central nervous system stimulant, and both current and recently abstinent chronic methamphetamine-dependent individuals can develop irreversible structural and neurochemical changes in the brain with long-lasting cognitive and motor deficits (Seiden and Ricaurte, 1987; Chang et al., 2002; Thompson et al., 2004).
Methamphetamine dependence is on the rise in the United States and other parts of the world (Winslow et al., 2007). According to surveys funded by the National Institute on Drug Abuse (NIDA) in 2005, 10.4 million Americans aged 12 years and older and 4.5% of 12th graders had used methamphetamine at least once in their lifetime (NIDA, 2006). Increased production and spread of methamphetamine use to other parts of the country from its traditional endemic areas in the West and Midwest have raised additional concern about the increasing prevalence of methamphetamine addiction (Ehlers et al., 2007; Johnson et al., 2008).
Several studies conducted in various countries, and with different ethnic populations, have reported an increased prevalence of adult methamphetamine dependence when the onset of methamphetamine use occurred in adolescence (Nordahl et al., 2003). The progression from first-time drug use to the development of dependence does, however, depend upon the interplay of both genetic and environmental factors (Goldman et al., 2005; McGue et al., 2006); therefore, not all adolescents who experiment with methamphetamine progress to methamphetamine dependence as adults (Fowler et al., 2007).
The importance of genetic factors has been highlighted by Ehlers et al. (2007), who showed, in a relatively homogenous population of Native Americans in Southwest California, that the liability toward the initiation of stimulant use is highly heritable at an estimated rate of 38%. Understanding the nature of the genetic factors that increase the risk of stimulant initiation can aid in appropriate screening and early intervention for those who are environmentally vulnerable to methamphetamine exposure, and can facilitate the development of medications targeted toward the treatment of those who become dependent.
Serotonin (5-HT) neurons are tonic inhibitors of dopamine neurons in the central nervous system (Johnson, 2000). Therefore, the degradation of 5-HT neurons can lead to a rise in extracellular dopamine levels (Nordahl et al., 2003), which in turn increases the individual’s behavioral propensity toward further methamphetamine use (Volkow and Li, 2004).
Of the mechanisms that control synaptic 5-HT function, perhaps the most compelling relates to the functional state of the pre-synaptic 5-HT transporter (5-HTT). The 5-HTT is responsible for removing 5-HT from the synaptic cleft (Lesch et al., 2002). Indeed, up to 60% of neuronal 5-HT function is gated by the 5-HTT. Synaptic clearance of 5-HT is determined by the number of 5-HTTs expressed at the pre-synaptic surface and the affinity of 5-HTTs to 5-HT (Beckman and Quick, 1998).
The 5-HTT gene is found at the SLC6A4 locus on chromosome 17q11.1-q12, and its 5′-regulatory promoter region contains a functional polymorphism known as the 5-HTT-linked polymorphic region (5′-HTTLPR) (Heils et al., 1996, 1997). This polymorphism is an insertion/deletion mutation in which the long (L) variant has 44 bp that are absent in the short (S) variant. The S-allelic variant of the 5′-HTTLPR is associated with reduced transcription rates in both lymphoblasts and in vitro cultured cells and, consequently, with decreased 5-HT turnover (Lesch et al., 1996; Hranilovic et al., 2004; Javors et al., 2005). In the general population, S-carriers, compared with the LL genotype, are associated with reduced 5-HT uptake into human platelets (Greenberg et al., 1999) and lymphoblasts (Lesch et al., 1996) and reduced [123I]2 beta-carboxymethoxy-3 beta-(4-iodophenyl)tropane (β-CIT) binding in human raphe nuclei (Heinz et al., 2000). Hence, healthy individuals with the SS genotype have reduced uptake and, presumably, greater intrasynaptic 5-HT levels and 5-HT neurotransmission compared with L-carriers (Heils et al., 1996; Lesch et al., 1996).
Animal studies have shown that genetic alterations that affect 5-HT levels during embryonic development alter the formation of barrels in the somatosensory cortex (i.e., post-central gyrus) (Cases et al., 1996; Vitalis et al., 1998; Persico et al., 2001; Xu et al., 2004). The somatosensory cortex is innervated by thalamocortical circuits (Nordquist and Oreland, 2010). These circuits, although originally glutaminergic, express 5-HT during embryonic development through the 5-HTT and, therefore, exhibit 5-HT uptake, vesicular storage, and release from nerve terminals (Ausó et al., 2001). 5-HT uptake rather than release appears critical to the development of proper branching arbors at their terminal ends. It is, therefore, tempting for us to speculate that the S compared with the L allele, being associated with less efficient transcription of the 5-HT gene and, therefore, reduced uptake (Lesch et al., 1996; Hranilovic et al., 2004), would be associated with less development of the thalamocortical afferents that serve the somatosensory cortex. In humans, the somatosensory cortex is associated with the appreciation of touch, which can have emotional correlates. Perhaps more directly, thalamocortical circuits from the nucleus ventralis anterior also innervate the anterior cingulate, an important site for the expression of the drive to use stimulants (Goldstein et al., 2009; Fineberg et al., 2010). Hence, the genetic effect of alterations in 5-HTT function during embryonic development might exert a morphogenic influence that predisposes to stimulant-taking behavior.
Evidence from another line of research supports the finding that morphometric and functional changes in the limbic system (for a review, see Dawes and Johnson, 2004), particularly the amygdala, an important site for emotional learning and the conditioning of impulsivity and response to fear (Paton et al., 2006), can be associated with alterations in 5′-HTTLPR function. Healthy individuals with the S allele of the 5′-HTTLPR appear to have a reduced volume of the amygdala as well as the anterior cingulate (Pezawas et al., 2005) and, behaviorally, a reduced ability to process emotion appropriately. Pacheco et al. (2009), using magnetic resonance imaging, also showed a decreased white matter connection via the uncinate fasciculus between the amygdala and the pre-frontal cortex in S variants. Furthermore, this effect of the 5′-HTTLPR genotype might differ by sex and race (Williams et al., 2003). Indeed, individuals with the S allele have been associated with reduced 5-HT turnover and 5-hydroxyindoleacetic acid levels in men but not in women. Consistently, the S allele has been associated with greater impulsivity in Caucasian men (Walderhaug et al., 2010). This is important because Semple et al. (2005) reported that heightened impulsivity, a trait that is associated with allelic variation at the 5′-HTTLPR (Walderhaug et al., 2010), predisposes to the initiation of methamphetamine use (Munafò et al., 2003; Semple et al., 2005), although it has been debated whether it is a cause or a consequence of drug use. Taken together, these data would lead to the speculation that individuals with the S compared with the L allele of the 5′-HTTLPR might be more prone to using stimulants, especially early in life, with a greater likelihood in Caucasian men.
The 5-HT system is important for the pharmacotoxic effects of methamphetamine since its use is associated with damage to 5-HT neurons by degrading their terminal ends (Ricaurte et al., 1980; Krasnova and Cadet, 2009). Methamphetamine intake also inhibits 5-HTT protein expression, particularly in the orbitofrontal cortex, even though there also is damage to dopaminergic neurons (Kish et al., 2009) and a decrease in vesicular monoamine transporter function (Brown et al., 2000). Consistently, human brain imaging studies also have shown that chronic methamphetamine users can exhibit significantly reduced 5-HTT densities, an indication of terminal neuronal damage, in different brain regions (Sekine et al., 2006). Because these reductions in 5-HTT density occur in a time- and concentration-dependent manner, individuals with the earliest onset and greatest use of methamphetamine can be expected to experience the most impairment of 5-HT function. We speculate that this would lead to a feed-forward effect whereby those with the S allele are more prone to impulsivity and an earlier onset of methamphetamine use, the prolonged use of methamphetamine results in greater 5-HT damage, and “normalizing” 5-HT neurotransmission is achieved only by taking more methamphetamine.
The present study examined whether allelic variation of the 5′-HTTLPR was associated with age of onset of methamphetamine use in Caucasian men and women.
Materials and Methods
Subjects
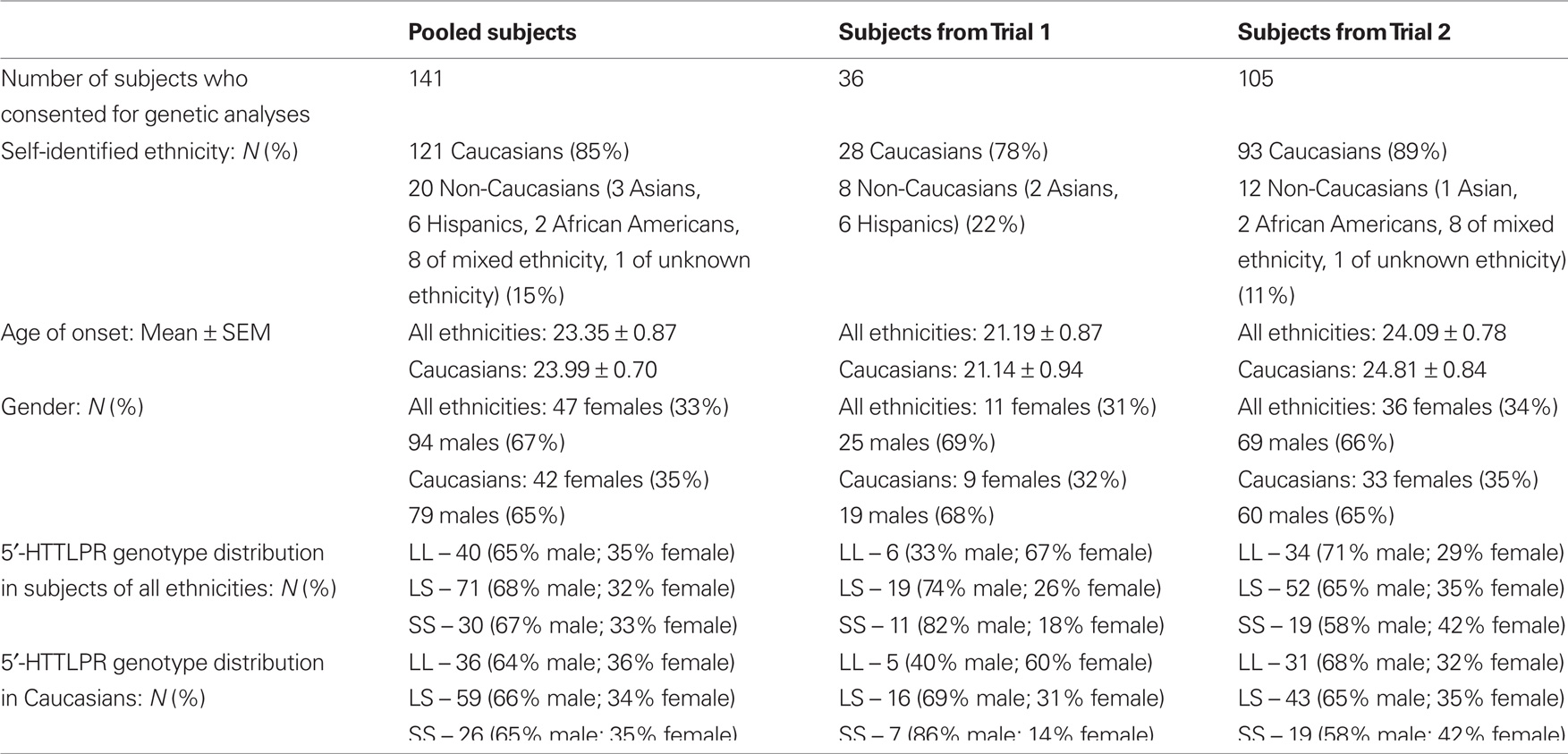
One hundred forty-one subjects, who were enrolled in two multi-site clinical trials (Trials 1 and 2) for the treatment of methamphetamine dependence, and who consented to participate in genetic analyses, were included in this study. Of the 141 subjects, 36 were from Trial 1 (Johnson et al., 2008) and 105 were from Trial 2 (data not yet published). All subjects were at least 18 years of age and diagnosed as methamphetamine dependent by Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria (American Psychiatric Association, 1994). The enrollment requirements were similar for both trials. The enrolled subjects were required to have at least one methamphetamine-positive urine specimen during the 2-week baseline period. They were in good physical health as determined by physical and laboratory examinations (i.e., hematological assessment, biochemistry, and urinalysis). Exclusion criteria were current dependence on any psychoactive substance (as defined by DSM-IV criteria) besides methamphetamine, nicotine, or marijuana, or physiological dependence on alcohol or a sedative–hypnotic, e.g., a benzodiazepine requiring medical detoxification. We also excluded individuals with current diagnoses of anxiety, affective, or psychotic disorders. We did not study individuals who: were mandated by the courts to be treated for methamphetamine dependence, were pregnant or not using an acceptable form of contraception (i.e., oral contraceptive, hormonal or surgical implant, sterilization, or spermicide and barrier), were taking psychotropic medication, were using opiate substitutes within 2 months of enrollment, were asthmatic, or had AIDS.
The following clinical sites participated in each trial: Trial 1: The University of Texas Health Science Center at San Antonio, TX, USA; Iowa Lutheran Hospital, Des Moines, IA, USA; South Bay Treatment Center, San Diego, CA, USA, and the University of Missouri-Kansas City, MO, USA. Trial 1 was coordinated by the Integrated Substance Abuse Programs at the University of California, Los Angeles, CA, USA. Study subjects were recruited between August 2002 and July 2003 by newspaper, television, or radio advertisements. Trial 2: University of Virginia, Charlottesville, VA, USA; University of California, Los Angeles, CA, USA; START Research and Treatment, Kansas City, MO, USA; University of Hawaii, Honolulu, HI, USA; South Bay Treatment Center, San Diego, CA, USA; Iowa Lutheran Hospital, Des Moines, IA, USA; Matrix Institute, West Los Angeles, CA, USA, and Salt Lake City Health Care System, Department of Veterans Affairs, Salt Lake City, UT, USA. Both trials were approved by the appropriate participating Institutional Review Boards.
After obtaining written informed consent, and prior to the subjects’ enrollment in the clinical trial, we determined psychiatric diagnosis using the Structured Clinical Interview for DSM-IV (First et al., 1994) and age of onset of methamphetamine use using the Addiction Severity Index-Lite (Cacciola et al., 2007). Other structured measures were collected at enrollment and at scheduled intervals during the clinical trial, as reported elsewhere (Johnson et al., 2008).
Collection of Blood Samples for Genotyping
Approximately 10 ml of blood was drawn from each subject to obtain white blood cells for the determination of 5′-HTTLPR genotypes.
Genotyping
DNA was extracted using a Gentra Puregene® kit (QIAGEN Inc., Valencia, CA, USA). Fifty nanograms of genomic DNA was polymerase chain reaction amplified for the 5′-HTTLPR 44-bp promoter-region repeat polymorphism using the primers 5′-TCCTCCG CTTTGGCGCCTCTTCC-3′ (forward) and 5′-TGGGG GTTGCAGGGGAGATCCTG-3′ (reverse) in a 20-μl final volume with 2.5 U of BIOLASE™ DNA polymerase (Bioline, London, UK), 1× NH4 reaction buffer, 0.5 mM MgCl2, 0.8 mM deoxynucleotide triphosphates, dimethyl sulfoxide, and 100 nM of each primer. The thermal cycling included: initial denaturation at 95°C for 15 min; 45 cycles of 94°C for 30 s, 65.5°C for 90 s, and 72°C for 1 min; a final extension of 72°C for 10 min, and a terminal hold at 4°C. The alleles for the 5′-HTTLPR were separated by gel electrophoresis using 3% agarose (Cambrex, Rockland, ME, USA) and visualized by an ethidium bromide/ultraviolet detection system.
Statistical Analysis
Considering that the majority of participants in both trials were of European descent, as well as the potential confounding effects of ethnicity on the association analysis, only participants of European descent were included in the statistical analyses that are reported in this study. We employed a general linear regression model (GLM) to analyze 5′-HTTLPR genotype associations with the age of first methamphetamine use (AMU) under the additive, dominant, and recessive genetic models. As several previous studies have shown a gender difference in both methamphetamine use and methamphetamine-induced neuropathology (Brecht et al., 2004; Scott et al., 2007; Dluzen et al., 2010), we included gender in all genetic models to test for the main effects of gender and genotype as well as the interactive gender-by-genotype effect. Next, based on the GLM analysis results, we used the Cox proportional-hazards model to assess the relative risk of an earlier onset of methamphetamine use for the SS genotype compared with L-carriers of the 5′-HTTLPR in males and females separately. Additionally, we obtained Kaplan–Meier estimates of the probability of not yet starting methamphetamine use as a function of age (years) for SS and L-carrier males and females. A log-rank test was used to compare the shapes of these age of onset curves. All analyses were performed using SAS statistical software (version 9.1; SAS Institute Inc., Cary, NC, USA).
Results
Table 1 shows the demographic information for the subjects from the pooled sample and each trial. DNA samples from 141 methamphetamine-dependent subjects aged between 18 and 60 years were genotyped in this study.
The 5′-HTTLPR genotype distribution in the pooled Caucasian sample did not deviate from Hardy–Weinberg equilibrium expectations. As shown in Table 2, there was a significant effect of gender and 5′-HTTLPR genotypes on AMU when the SS genotype group was compared with L-carriers. In Caucasian males, the SS homozygotes showed a significantly higher risk of an earlier onset of methamphetamine use compared with L-carriers (hazard ratio = 1.839; 95% confidence interval [CI] = 1.042–3.246; p = 0.036), while the difference between SS and L-carrier female subjects was not significant (hazard ratio = 0.719; 95% CI = 0.329–1.573; p = 0.409). The comparisons for the differences among the three genotypic groups or between the LL genotype and S-carriers did not show a significant risk effect on AMU, suggesting a dominant effect of the L allele over the S allele.
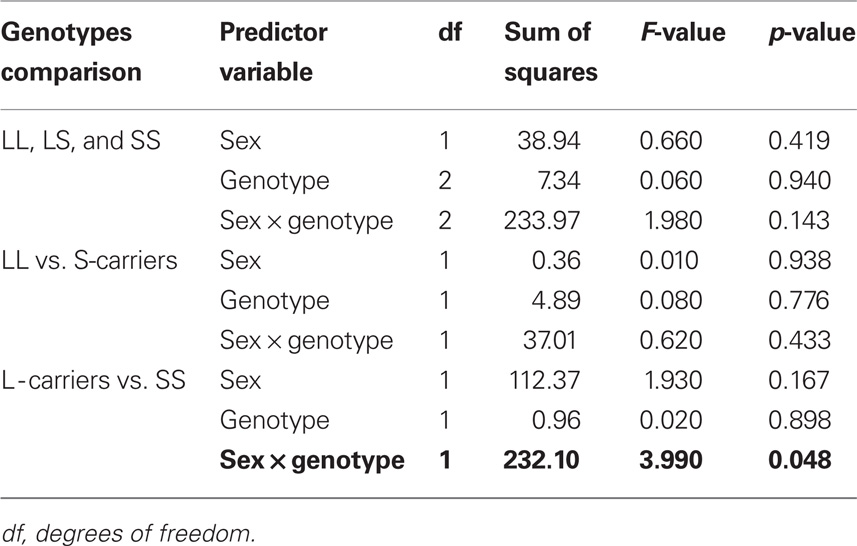
Table 2 Results of general linear model for the 5′-HTTLPR genotypic associations with age of onset of methamphetamine use.
Using the Kaplan–Meier method, we found that males with the SS genotype, compared with their L-carrier counterparts, became dependent on methamphetamine significantly earlier (log-rank, all p values = 0.027; Figure 1A). We, however, found no significant difference in AMU between the SS and L-carrier genotypic groups among females (p = 0.390; Figure 1B).
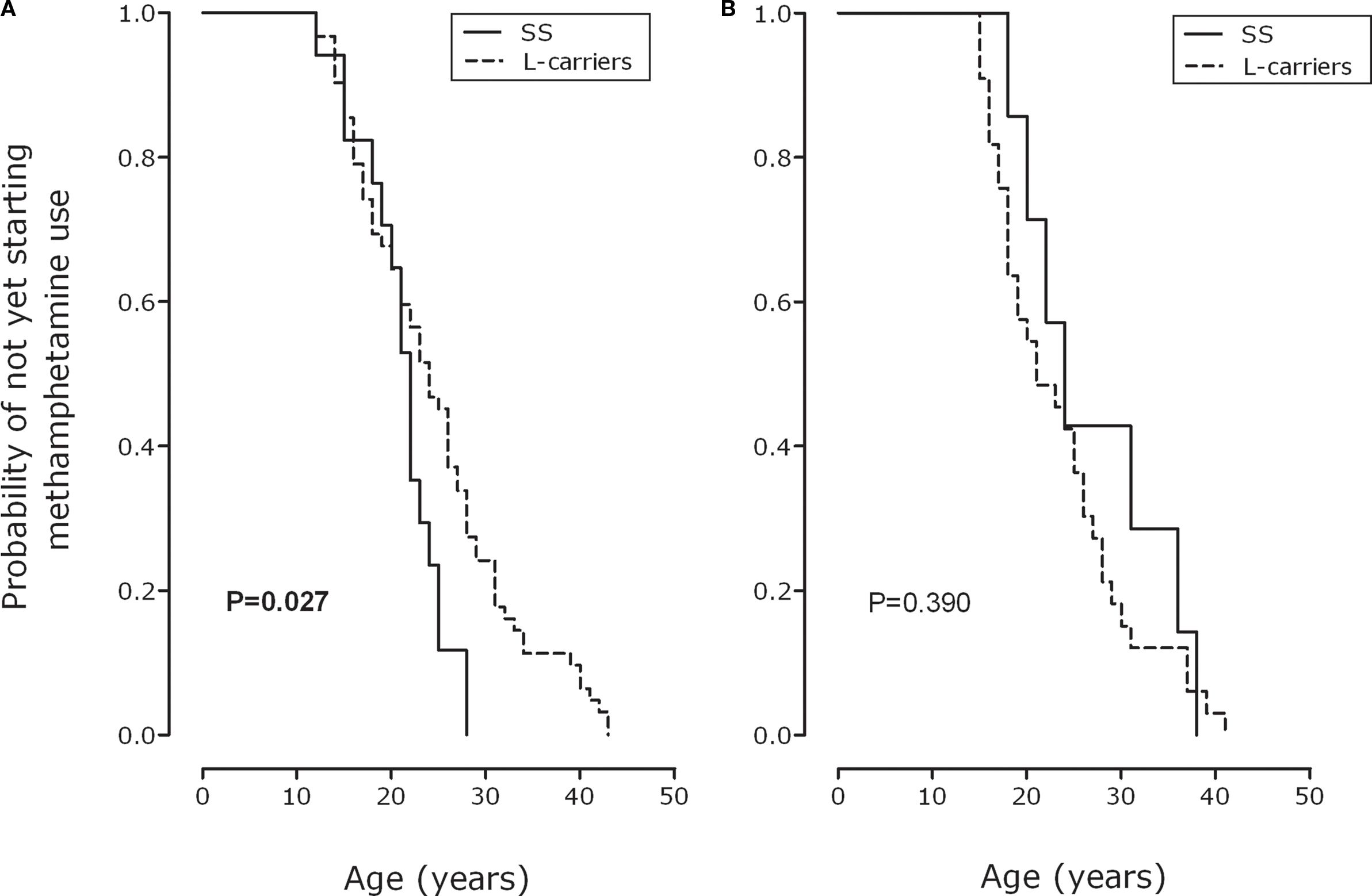
Figure 1 Probability of not yet starting methamphetamine use as a function of age for SS and L-carriers of the serotonin transporter-linked polymorphic region in males (A) and females (B).
Discussion
Our results suggest that among methamphetamine-dependent Caucasian males, possession of the SS genotype in the 5′-HTTLPR, compared with their L-carrier counterparts, was associated with about a two times greater risk of having had an earlier onset of methamphetamine use.
Interestingly, in this study, the SS genotype was associated with an early onset of methamphetamine use in men. Indeed, this is explainable as results from animal studies have constantly demonstrated a protective role of estrogen on methamphetamine-induced 5-HT and dopamine depletion; male mice and ovariectomized female mice were shown to have significantly depleted 5-HT and dopamine levels compared with female mice (Yu and Liao, 2000). It is, therefore, tempting to speculate that methamphetamine use in males might be associated with alterations in intrasynaptic 5-HT levels due to polymorphic differences at the 5′-HTTLPR, with SS subjects having an earlier onset of methamphetamine use than L-carriers. These results also are consistent with the finding of greater impulsivity in male Caucasians with the S allele of the 5′-HTTLPR (Walderhaug et al., 2010).
This study had four limitations. First, this study was only a preliminary analysis with a modest sample size that did not provide sufficient statistical power to examine ethnic populations other than Caucasians. Large-scale studies are, therefore, needed to replicate and extend our findings. Second, because of the cross-sectional nature of the study, we were unable to assess how genetic variation in the 5′-HTTLPR interacted with the progression of methamphetamine use over time. Third, our cohort was composed of methamphetamine-dependent individuals who were seeking treatment. Since treatment seekers can vary in pathophysiology from those in the community, often being more motivated and generally healthier, we do not know whether our findings can be generalized to the entire population of those who are using methamphetamine. Fourth, the cohort for this genetic study was not drawn from the general population, but rather from a subpopulation of treatment-seeking, methamphetamine-dependent individuals; thus, the absolute risk of an early onset of methamphetamine use among those in the community who possess the SS genotype of the 5′-HTTLPR cannot be determined. Because this is the first study to examine whether methamphetamine-dependent individuals who vary in genotype in the 5′-HTTLPR differ in the age of onset of methamphetamine use, there are no studies against which we can directly compare our results. Interestingly, however, these data might have some parallels in research in the alcohol field, where a meta-analysis of 17 published studies indicated an S-allele association with the early onset of alcoholism (Feinn et al., 2005) whilst only a few other studies revealed an association of alcoholism with the L allele (Dawes et al., 2009; Laucht et al., 2009).
In summary, our findings provide the first preliminary evidence of a gender-based genetic vulnerability, and that possession of the SS genotype of the 5′-HTTLPR might confer increased predisposition toward early-onset methamphetamine use in Caucasian males.
Conflict of Interest Statement
B. A. Johnson has served as a consultant to Johnson & Johnson (Ortho-McNeil Janssen Scientific Affairs, LLC), Transcept Pharmaceuticals, Inc., D&A Pharma, Organon, ADial Corporation, Psychological Education Publishing Company (PEPCo LLC), and Eli Lilly and Company. M. D. Li serves as a scientific advisor to ADial Corporation.
Acknowledgments
We are grateful to the National Institute on Drug Abuse, which provided funding for this study through contracts N01DA-9-8101 (awarded to The University of Texas Health Science Center at San Antonio) and N01DA-0-8804 (awarded to the University of California, Los Angeles). We also thank Robert H. Cormier, Jr., B.A., for his assistance with manuscript preparation.
References
American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th Edn. Washington, DC: American Psychiatric Association.
Ausó, E., Cases, O., Fouquet, C., Camacho, M., García-Velasco, J. V., Gaspar, P., and Berbel, P. (2001). Protracted expression of serotonin transporter and altered thalamocortical projections in the barrelfield of hypothyroid rats. Eur. J. Neurosci. 14, 1968–1980.
Beckman, M. L., and Quick, M. W. (1998). Neurotransmitter transporters: regulators of function and functional regulation. J. Membr. Biol. 164, 1–10.
Brecht, M.-L., O’Brien, A., von Mayrhauser, C., and Anglin, M. D. (2004). Methamphetamine use behaviors and gender differences. Addict. Behav. 29, 89–106.
Brown, J. M., Hanson, G. R., and Fleckenstein, A. E. (2000). Methamphetamine rapidly decreases vesicular dopamine uptake. J. Neurochem. 74, 2221–2223.
Cacciola, J. S., Alterman, A. I., McLellan, A. T., Lin, Y.-T., and Lynch, K. G. (2007). Initial evidence for the reliability and validity of a ‘Lite’ version of the Addiction Severity Index. Drug Alcohol Depend. 87, 297–302.
Cases, O., Vitalis, T., Seif, I., De Maeyer, E., Sotelo, C., and Gaspar, P. (1996). Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron 16, 297–307.
Chang, L., Ernst, T., Speck, O., Patel, H., DeSilva, M., Leonido-Yee, M., and Miller, E. N. (2002). Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res. 114, 65–79.
Dawes, M. A., and Johnson, B. A. (2004). Pharmacotherapeutic trials in adolescent alcohol use disorders: opportunities and challenges. Alcohol Alcohol. 39, 166–177.
Dawes, M. A., Roache, J. D., Javors, M. A., Bergeson, S. E., Richard, D. M., Mathias, C. W., Ait-Daoud, N., Dougherty, D. M., and Johnson, B. A. (2009). Drinking histories in alcohol-use-disordered youth: preliminary findings on relationships to platelet serotonin transporter expression with genotypes of the serotonin transporter. J. Stud. Alcohol Drugs 70, 899–907.
Dluzen, D. E., McDermott, J. L., and Darvesh, A. S. (2010). Relationships among gender, age, time, and temperature in methamphetamine-induced striatal dopaminergic neurotoxicity. Neuroscience 167, 985–993.
Ehlers, C. L., Wall, T. L., Corey, L., Lau, P., Gilder, D. A., and Wilhelmsen, K. (2007). Heritability of illicit drug use and transition to dependence in Southwest California Indians. Psychiatr. Genet. 17, 171–176.
Feinn, R., Nellissery, M., and Kranzler, H. R. (2005). Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 133B, 79–84.
Fineberg, N. A., Potenza, M. N., Chamberlain, S. R., Berlin, H. A., Menzies, L., Bechara, A., Sahakian, B. J., Robbins, T. W., Bullmore, E. T., and Hollander, E. (2010). Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 35, 591–604.
First, M. B., Spitzer, R. L., Gibbon, M., and Williams, J. B. W. (1994). Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P, Version 2.0). New York: New York State Psychiatric Institute, Biometrics Research Department.
Fowler, T., Lifford, K., Shelton, K., Rice, F., Thapar, A., Neale, M. C., McBride, A., and van den Bree, M. B. (2007). Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction 102, 413–422.
Goldman, D., Oroszi, G., and Ducci, F. (2005). The genetics of addictions: uncovering the genes. Nat. Rev. Genet. 6, 521–532.
Goldstein, R. Z., Craig, A. D., Bechara, A., Garavan, H., Childress, A. R., Paulus, M. P., and Volkow, N. D. (2009). The neurocircuitry of impaired insight in drug addiction. Trends Cogn. Sci. 13, 372–380.
Greenberg, B. D., Tolliver, T. J., Huang, S. J., Li, Q., Bengel, D., and Murphy, D. L. (1999). Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am. J. Med. Genet. 88, 83–87.
Heils, A., Mossner, R., and Lesch, K. P. (1997). The human serotonin transporter gene polymorphism–basic research and clinical implications. J. Neural Transm. 104, 1005–1014.
Heils, A., Teufel, A., Petri, S., Stober, G., Riederer, P., Bengel, D., and Lesch, K. P. (1996). Allelic variation of human serotonin transporter gene expression. J. Neurochem. 66, 2621–2624.
Heinz, A., Jones, D. W., Mazzanti, C., Goldman, D., Ragan, P., Hommer, D., Linnoila, M., and Weinberger, D. R. (2000). A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol. Psychiatry 47, 643–649.
Hranilovic, D., Stefulj, J., Schwab, S., Borrmann-Hassenbach, M., Albus, M., Jernej, B., and Wildenauer, D. (2004). Serotonin transporter promoter and intron 2 polymorphisms: relationship between allelic variants and gene expression. Biol. Psychiatry 55, 1090–1094.
Javors, M. A., Seneviratne, C., Roache, J. D., Ait-Daoud, N., Bergeson, S. E., Walss-Bass, M. C., Akhtar, F. Z., and Johnson, B. A. (2005). Platelet serotonin uptake and paroxetine binding among allelic genotypes of the serotonin transporter in alcoholics. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 7–13.
Johnson, B. A. (2000). Serotonergic agents and alcoholism treatment: rebirth of the subtype concept – an hypothesis. Alcohol. Clin. Exp. Res. 24, 1597–1601.
Johnson, B. A., Ait-Daoud, N., Elkashef, A. M., Smith, E. V., Kahn, R., Vocci, F., Li, S.-H., Bloch, D. A., and the Methamphetamine Study Group. (2008). A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of methamphetamine dependence. Int. J. Neuropsychopharmacol. 11, 1–14.
Kish, S. J., Fitzmaurice, P. S., Boileau, I., Schmunk, G. A., Ang, L. C., Furukawa, Y., Chang, L. J., Wickham, D. J., Sherwin, A., and Tong, J. (2009). Brain serotonin transporter in human methamphetamine users. Psychopharmacology 202, 649–661.
Krasnova, I. N., and Cadet, J. L. (2009). Methamphetamine toxicity and messengers of death. Brain Res. Rev. 60, 379–407.
Laucht, M., Treutlein, J., Schmid, B., Blomeyer, D., Becker, K., Buchmann, A. F., Schmidt, M. H., Esser, G., Jennen-Steinmetz, C., Rietschel, M., Zimmerman, U. S., and Banaschewski, T. (2009). Impact of psychosocial adversity on alcohol intake in young adults: moderation by the LL genotype of the serotonin transporter polymorphism. Biol. Psychiatry 66, 102–109.
Lesch, K. P., Bengel, D., Heils, A., Sabol, S. Z., Greenberg, B. D., Petri, S., Benjamin, J., Muller, C. R., Hamer, D. H., and Murphy, D. L. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531.
Lesch, K. P., Greenberg, B. D., and Higley, J. D. (2002). “Serotonin transporter, personality, and behavior: toward dissection of gene-gene and gene-environment interaction,” in Molecular Genetics and the Human Personality, eds J. Benjamin, R. P. Ebstein, and R. H. Belmaker (Washington, DC: American Psychiatric Publishing), 109–136.
McGue, M., Iacono, W. G., and Krueger, R. (2006). The association of early adolescent problem behavior and adult psychopathology: a multivariate behavioral genetic perspective. Behav. Genet. 36, 591–602.
Munafò, M. R., Clark, T. G., Moore, L. R., Payne, E., Walton, R., and Flint, J. (2003). Genetic polymorphisms and personality in healthy adults: a systematic review and meta-analysis. Mol. Psychiatry 8, 471–484.
National Institute on Drug Abuse. (2006). NIDA InfoFacts: Methamphetamine (http://www.nida.nih.gov/Infofacts/methamphetamine.html). Washington, DC: U.S. Department of Health and Human Services.
Nordahl, T. E., Salo, R., and Leamon, M. (2003). Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J. Neuropsychiatry Clin. Neurosci. 15, 317–325.
Nordquist, N., and Oreland, L. (2010). Serotonin, genetic variability, behaviour, and psychiatric disorders – a review. Ups. J. Med. Sci. 115, 2–10.
Pacheco, J., Beevers, C. G., Benavides, C., McGeary, J., Stice, E., and Schnyer, D. M. (2009). Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. J. Neurosci. 29, 6229–6233.
Paton, J. J., Belova, M. A., Morrison, S. E., and Salzman, C. D. (2006). The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439, 865–870.
Persico, A. M., Mengual, E., Moessner, R., Hall, F. S., Revay, R. S., Sora, I., Arellano, J., DeFelipe, J., Gimenez-Amaya, J. M., Conciatori, M., Marino, R., Baldi, A., Cabib, S., Pascucci, T., Uhl, G. R., Murphy, D. L., Lesch, K. P., and Keller, F. (2001). Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J. Neurosci. 21, 6862–6873.
Pezawas, L., Meyer-Lindenberg, A., Drabant, E. M., Verchinski, B. A., Munoz, K. E., Kolachana, B. S., Egan, M. F., Mattay, V. S., Hariri, A. R., and Weinberger, D. R. (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 8, 828–834.
Ricaurte, G. A., Schuster, C. R., and Seiden, L. S. (1980). Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 193, 153–163.
Scott, J. C., Woods, S. P., Matt, G. E., Meyer, R. A., Heaton, R. K., Atkinson, J. H., and Grant, I. (2007). Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol. Rev. 17, 275–297.
Seiden, L. S., and Ricaurte, G. (1987). “Neurotoxicity of methamphetamine and related drugs,” in Psychopharmacology: The Third Generation of Progress, ed H. Y. Meltzer (New York: Raven Press), 359–366.
Sekine, Y., Ouchi, Y., Takei, N., Yoshikawa, E., Nakamura, K., Futatsubashi, M., Okada, H., Minabe, Y., Suzuki, K., Iwata, Y., Tsuchiya, K. J., Tsukada, H., Iyo, M., and Mori, N. (2006). Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch. Gen. Psychiatry 63, 90–100.
Semple, S. J., Zians, J., Grant, I., and Patterson, T. L. (2005). Impulsivity and methamphetamine use. J. Subst. Abuse Treat. 29, 85–93.
Thompson, P. M., Hayashi, K. M., Simon, S. L., Geaga, J. A., Hong, M. S., Sui, Y., Le, J. Y., Toga, A. W., Ling, W., and London, E. D. (2004). Structural abnormalities in the brains of human subjects who use methamphetamine. J. Neurosci. 24, 6028–6036.
Vitalis, T., Cases, O., Callebert, J., Launay, J. M., Price, D. J., Seif, I., and Gaspar, P. (1998). Effects of monoamine oxidase A inhibition on barrel formation in the mouse somatosensory cortex: determination of a sensitive developmental period. J. Comp. Neurol. 393, 169–184.
Volkow, N. D., and Li, T.-K. (2004). Drug addiction: the neurobiology of behaviour gone awry. Nat. Rev. Neurosci. 5, 963–970.
Walderhaug, E., Herman, A. I., Magnusson, A., Morgan, M. J., and Landrø, N. I. (2010). The short (S) allele of the serotonin transporter polymorphism and acute tryptophan depletion both increase impulsivity in men. Neurosci. Lett. 473, 208–211.
Williams, R. B., Marchuk, D. A., Gadde, K. M., Barefoot, J. C., Grichnik, K., Helms, M. J., Kuhn, C. M., Lewis, J. G., Schanberg, S. M., Stafford-Smith, M., Suarez, E. C., Clary, G. L., Svenson, I. K., and Siegler, I. C. (2003). Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology 28, 533–541.
Winslow, B. T., Voorhees, K. I., and Pehl, K. A. (2007). Methamphetamine abuse. Am. Fam. Physician 76, 1169–1174.
Xu, Y., Sari, Y., and Zhou, F. C. (2004). Selective serotonin reuptake inhibitor disrupts organization of thalamocortical somatosensory barrels during development. Brain Res. Dev. Brain Res. 150, 151–161.
Keywords: methamphetamine, age of onset, serotonin transporter, genotype, serotonin transporter-linked polymorphic region
Citation: Johnson BA, Elkashef AM, Seneviratne C, Ait-Daoud N, Kahn RC, Li S-H, Bloch DA, Holmes TH, Wang X-Q, Vocci Jr. FJ, Li MD and the Methamphetamine Study Group (2010) Association between genotype of the serotonin transporter-linked polymorphic region of the serotonin transporter gene and age of onset of methamphetamine use: a preliminary analysis. Front. Psychiatry 1:145. doi: 10.3389/fpsyt.2010.00145
Received: 15 February 2010;
Accepted: 08 October 2010;
Published online: 01 November 2010.
Edited by:
Lorenzo Leggio, Brown University, USACopyright: © 2010 Johnson, Elkashef, Seneviratne, Ait-Daoud, Kahn, Li, Bloch, Holmes, Wang, Vocci Jr., Li and the Methamphetamine Study Group. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Bankole A. Johnson, Department of Psychiatry and Neurobehavioral Sciences, University of Virginia, P.O. Box 800623, Charlottesville, VA 22908-0623, USA. e-mail: bankolejohnson@virginia.edu