- 1Centre for Law and Genetics, Law Faculty, University of Tasmania, Hobart, TAS, Australia
- 2Sydney Health Law, Law Faculty, University of Sydney, Sydney, NSW, Australia
Public participation, transparency and accountability are three of the pillars of good governance. These pillars become particularly important for innovative, personalised health technologies, because of the tendency of these technologies to raise distinct scientific, ethical, legal and social issues. Genome editing is perhaps the most personal of all innovative health technologies, involving precise modifications to an individual’s genome. This article focuses on the adequacy of current requirements for public participation, transparency and accountability in the governance of the market authorisation for genome edited products. Although clinical trials for genome edited products are only just underway, lessons can be drawn from the marketing approvals pathways for related gene therapy products. This article provides a broad overview of the regulatory pathways that have been adopted by the US Food and Drugs Administration, the European Medicines Authority, and the Australian Therapeutic Goods Administration for reviewing gene therapy products for marketing approval. This analysis focuses on the extent to which public participation processes and transparency and accountability of review pathways are incorporated into marketing approval policy and practice. Following this review, the article proposes the application of Sheila Jasanoff’s “technologies of humility” as a foundation for meaningfully incorporating these pillars of good governance into regulatory processes for the review of products of genome editing. We conclude by articulating clear mechanisms for operationalising technologies of humility in the context of public participation, transparency and accountability, providing a blueprint for future policy development.
Introduction
Public participation is increasingly expected as a core pillar of good governance, along with transparency and accountability, in such diverse contexts as international development assistance (Carothers and Brechenmacher, 2014), human rights (United Nations, 2007), and genome editing (Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing, 2021a). Appropriate attention to these three pillars of good governance should lead to higher levels of public trust and confidence, both in the subject matter being governed and in the governance regime itself. In the context of new technologies, particularly those with uncertain risks and benefits, calls for greater public participation, in one form or another, have become de rigeur.
The numerous examinations of the complex policy issues associated with human genome editing are a case in point, routinely ending with a call for some form of public engagement, as demonstrated in a report by the International Commission on the Clinical Use of Human Germline Genome Editing (International Commission on the Clinical Use of Human Germline Genome Editing, 2020). A 2017 Report by the US-based National Academies of Sciences, Engineering, and Medicine similarly recognised the need for public participation in the context of genome editing, calling for meaningful public input into the policy-making process. The Report emphasises that public involvement must go further than mere information sharing (National Academies of Sciences, Engineering, and Medicine, 2017, 167). Rather, public involvement should be extended to embrace more active forms of consultation and participation in policy setting and development.
More recently, the World Health Organisation (WHO) Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing handed down a set of three reports on the governance of genome editing: Governance Framework, Recommendations and Position Paper (Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing, 2021a; Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing, 2021b; Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing, 2021c). The Committee was established in 2018, with the purpose of providing advice and recommendations on appropriate institutional, national, regional and global governance mechanisms for human genome editing.
Two recommendations are particularly pertinent. Recommendation 2 calls for the establishment of a global genome editing clinical trials registry. The Recommendations Report states that making information on clinical trials involving human genome editing publicly accessible reflects the values and principles of openness, transparency, honesty and accountability (Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing, 2021c, 8). Adoption of this recommendation would thus be an important step in embedding transparency and accountability into the governance of genome editing. Recommendation 7 recognises the critical importance of education, engagement and empowerment. However, this recommendation does not provide the same concrete guidance in how to embed public participation in the governance of genome editing as Recommendation 2 did for transparency and accountability. Rather, the Recommendations Report states that “it would be counter-productive to be too prescriptive on how to pursue education, engagement and empowerment activities” (Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing, 2021c, 17).
Beyond these exhortations for greater public involvement, little or no guidance has been provided on how to actually engage with members of the public, at what stage and to what end. More specifically, the extent to which regulatory decision-makers considering applications for marketing approvals of new genome editing products should incorporate public involvement is unclear. This is the case whether we are talking about involvement in policy development or more direct participation in the approval process. This article explores the latter, focusing on what best practice public involvement might look like within the specific context of market authorisation for health-related genome editing products.
Market authorisation of the clinical products of genome editing provides a relevant case study for a number of reasons, including: the speed with which the technology has been adopted across the healthcare sector; the relative ease of use of genome editing tools such as Clustered Regularly Interspersed Short Palindromic Repeats (CRISPR); the currently uncertain risks and benefits of healthcare-related genome editing products; the additional normative dimensions relating to heritable genomic changes, including potential inter-generational effects; and the fact that genome editing is often directed towards rare diseases, that by definition will have a smaller evidence base on which regulatory decisions can be made.
In this article we begin with an examination of the current state of genome editing, and provide a review of literature on the core concepts of public participation, transparency and accountability. We then consider the application of these pillars of governance in several jurisdictions, notably the United States, the European Union, and Australia. Specifically, we analyse whether they can be said to be evident in decision-making in relation to gene therapies, which provide some guidance as to the regulatory approach that is likely to be adopted in relation to genome editing technologies. Finally, we discuss the notion of good governance in light of Sheila Jasanoff’s work on technologies of humility (Jasanoff, 2003; Jasanoff, 2012), before presenting some initial ideas as to how we might optimise public trust in genome editing regulation moving forward.
Why Genome Editing?
The term “genome editing” embraces a number of important technological breakthroughs which have emerged in the past decade (Gaj et al., 2013). The adaptation of naturally occurring CRISPR and CRISPR-associated (Cas) systems in bacteria for use in mammalian cells is particularly notable (Mei et al., 2016). CRISPR technology is widely seen as being as transformative in the laboratory as the polymerase chain reaction (PCR) was in the 1980s. PCR facilitates rapid multiplication of DNA strands, and is used widely in modern genomic analysis, in both research and diagnosis. Interestingly, though, PCR is currently either being replaced by or combined with CRISPR technology, particularly in COVID-19 diagnosis (Palaz et al., 2021). In much the same way that PCR was rapidly adopted in the 1980s, it seems that practically every genomics laboratory now has one or more members of the team who is skilled in the use of CRISPR technology. Although only just entering the clinical trial phase, CRISPR-Cas systems and other genome editing technologies have potential clinical application in the treatment of cancer, metabolic disorders, viral diseases, and a large range of other diseases (Li et al., 2020).
Genome editing will clearly be beneficial if it facilitates safe and effective treatment of otherwise untreatable or difficult to treat diseases (Maeder and Gersbach, 2016). The 2017 report by the US National Academy of Sciences and the National Academy of Medicine mentioned in the introduction to this article endorsed the clinical application of genome editing, noting further that the regulatory requirements for assessment of genome editing clinical trials are similar to those for other medical therapies (National Academies of Sciences, Engineering, and Medicine, 2017).
While this appraisal of the apparent ease with which clinical translation of non-heritable genome editing can be assessed within existing regulatory frameworks is encouraging, it understates the challenges involved in navigating the path from laboratory to clinic (Nicol et al., 2017). There is a pressing need to dissect and critically analyze the relevance and adequacy of current regulatory oversight for safely translating genome editing technology into the clinic. This is important because, while insufficient oversight can undermine patient safety, thereby resulting in unnecessary morbidity and mortality, undue regulatory burdens can impede innovation and associated health and economic benefits.
Countries with well-developed health systems have a range of processes for reviewing and approving clinical applications of emerging technologies, generally linked to authority to supply an unapproved medical product or to seek approval for marketing of new medical products (Isasi et al., 2016). One difficulty in the present context, however, is that novel, disruptive therapeutics like genome editing often involve considerable uncertainty about the risks and potential benefits of their use. In particular, the evidence base concerning long-term outcomes tends to be limited. Given the normative nature of risks and benefits, this creates a regulatory pathway that is challenging for regulatory agencies to navigate relying on expert judgement alone (Eckstein, 2015).
Assessments of clinical applications of genome editing present an opportunity to engage a wider range of stakeholders, especially patients and patient groups, in determining the acceptable thresholds for risk and benefits. Ideally, this means not only considering what magnitude of risks are acceptable, but discussing which outcomes are taken into consideration, what counts as harm or benefit, and to whom. More explicit articulation of these risk and benefit tradeoffs, together with the provision of more opportunities for public deliberation about them, makes for a more open, and more democratically and socially robust mode of governance for new technologies.
Public Participation, Transparency and Accountability in Regulatory Decision-Making
Public participation, transparency and accountability may occur throughout the regulatory pathway. Collectively, they can play an important role in policy development and policy agenda setting, but there are also calls for these principles to be applied to the regulatory decision-making process itself (Joss, 1999). This will require decision makers to include considerations extending beyond scientific assessment alone (Taylor, 2021). To fully understand how public participation, transparency and accountability may be utilised in regulatory decision-making generally, and decision-making about genome editing products specifically, it is first necessary to explore the key features of each of these aspects of public involvement.
Why Public Participation?
Public participation involves the “direct participation by non-governmental actors in decision making” (Mostert, 2003, 180). Public participation is widely seen as crucial in advancing the three key cornerstones of democracy: effectiveness, legitimacy and social justice (Fung, 2015). Interest in public participation first gained traction in the 1960s (Arnstein, 1969; Quick and Bryson, 2016). In following years, public participation was incorporated, in one form or another, into various aspects of government decision-making. According to Quick and Bryson, it had become routine and professionalised by the early 2000s (Quick and Bryson, 2016).
The ways in which public participation is incorporated in regulatory policy setting and decision-making continue to be many and varied. Rowe and Frewer have compiled a non-exhaustive list of no fewer than 100 public participation mechanisms which they categorise into three broad types: public communication, public consultation and public participation proper (Rowe and Frewer, 2005). At the most basic level, public communication involves the transmission of information from the regulator to members of the public and from members of the public to the regulator in a process initiated by the regulator (Rowe and Frewer, 2005). This process is aimed at gaining information on public viewpoints, but it falls short of true public participation. Public participation involves an active process of information exchange, discussion and consensus building, through which more meaningful public input is incorporated into regulatory policy setting and decision-making (Rowe and Frewer, 2005). Public consultation sits between the two, offering greater opportunities for constructive conversations than public communication, but not going as far as true participation.
Meaningful public participation clearly requires more than public communication and consultation. Beyond this, however, there is not a great deal of guidance about what best practice public participation might actually entail. While there is growing support for the more active models of public participation described by Rowe and Frewer (2005), these entail associated trade-offs in terms of viability of implementation. Deliberative democracy scholars have developed models that require deep engagement with members of the public, for example through deliberative “minipublics” (Fung, 2006). For deliberative democracy scholars, then, conventional public meetings are not adequate forms of public participation (Fung, 2015). While the various forms of citizen deliberation may be feasible in the deep and broad context of regulatory policy setting, it is difficult to see how this form of deliberative engagement could be incorporated into more routine regulatory decision-making. To require deliberative engagement for every decision about whether to approve a new drug, for example, would presumably slow the decision-making process to such an extent that it would compromise patient welfare. How, then, might public participation operate in the context of regulatory approvals for new drugs in a manner that is timely but remains legitimate, as compared with tokenistic?
Some lessons in this regard may be drawn from regulatory decision-making in the environmental and land use contexts, for which public participation has been recognised as an essential component of regulatory decision-making. In water management, for example, its crucial role is recognised in various international instruments (Mostert, 2003, 179). It has gained similar traction in the context of planning law. Over the years, however, it appears to have lost its legitimacy. Robert Stokes describes it as having become somewhat of a “sacred cow,” a matter of form not substance (Stokes, 2012). Erik Mostert similarly expresses concern that public participation has become nothing more than a “bureaucratic exercise” (Mostert, 2003, 194). There is a risk that if public participation becomes a simple box ticking exercise, rather than fostering public trust, greater levels of public mistrust could result (Innes and Booher, 2004). Public trust will only result if public participation is authentic.
The public meeting laws that are a prominent feature of government decision-making in the US illustrate this point (Piotrowski and Borry, 2010; Roeder, 2013). The Federal Advisory Committee Act 1972 (FACA) and the Government in the Sunshine Act 1976 collectively require openness of meetings and minutes of meetings of federal agencies and advisory committees. As a consequence, meetings of regulatory authorities such as the Food and Drugs Administration (FDA) are required to be held in public. However, authors such as Rebecca Long and Thomas Beierle comment that, together with other factors, the actuality is that these openness requirements “may chill participation by raising barriers to members of the public who might otherwise participate,” for example, through less formal consultation mechanisms (Long and Beierle, 1999, 11). As such, although open public meetings might be well intentioned as a strategy for increasing public participation, there is some uncertainty about their true value and associated costs.
Ultimately, the question of what constitutes legitimacy in public participation will depend on the context and goals of the participatory exercises. Relevant considerations include the quality of the exchanges; the inclusiveness of engagement with members of the public; and the effectiveness of that engagement, being the degree to which engagement meaningfully influences the regulatory position that is eventually adopted (Quick and Bryson, 2016). It thus becomes clear that the normative rationale for public participation must be properly articulated at the outset.
Why Transparency?
Transparency can be thought of as one aspect of the process of public participation. Public participation will be a meaningless exercise if it is not underpinned by a commitment to transparency and accountability. However, transparency also extends beyond this. Transparency in the context of governmental decision-making has been described as “that which “shines through” or “shows through” from an agency to its viewers,” translating as “the ability to view the agency’s inside, to see across the border separating the public from the agency’s internal decisions” (Carpenter, 2017). For those who seek to gain access to governmental information, transparency will depend on such factors as the availability of information, the information’s accessibility, and the manner in which the information may be used to support decision-making processes (Turilli and Floridi, 2009).
Transparency has been demarcated into two kinds of openness: transparency in process and transparency in rationale (Mansbridge, 2009; Licht et al., 2014). Under this delineation, transparency in rationale refers to “information on the substance of the decision and of the facts and reasons on which it was based.” In contrast, transparency in process refers to “information on actions such as deliberations, negotiations, and votes that took place among and between the decision makers during the decision-making process and were thus directly fed into the decision” (Licht et al., 2014, 113).
Transparency has been promoted as bringing a number of benefits for governmental decision-making. One such benefit is normative in nature: that is, the general belief that public institutions should be open and transparent, rather than closed and secretive (Licht et al., 2014). As articulated by former Commissioner of the US Food and Drug Administration (FDA) Donald Kennedy, “government decisions, particularly regulatory decisions, should be based on publicly available information” because “people affected by government decisions have a right to know the basis on which they are made” (Sharfstein et al., 2017).
Other benefits are instrumental in nature: that is, they can improve the process of governing. For one, transparency can promote accountability and prevent arbitrary decision-making based on the availability of a clear set of rules against which members of the public can assess government decisions (Hood, 2006). In addition, transparent government processes—and the facts and reasons considered as a part of those processes—can improve the legitimacy of governmental decision-making by helping members of the public understand the reasons for a decision and any countervailing arguments (Licht et al., 2014). This provides a basis upon which members of the public can judge, and make comment on, the fairness of those procedures, potentially improving the decision-making process further (Licht et al., 2014). Transparency in process can assuage any concerns that governmental decisions might reflect a narrow special interest—for example, of a drug manufacturer—rather than a broader public interest (Carpenter, 2017).
A further instrumental benefit of transparency when it comes to drug regulatory agencies stems from the link between these agencies and medical innovation. Matthew Herder provides the example of a manufacturer considering whether to explore the use of a drug for an expanded indication. That manufacturer would clearly benefit from knowing that a drug regulator had already considered the use of that drug for the expanded indication, and had advised against it (Herder, 2014b).
However, transparency also can require certain trade-offs when it comes to governmental decision-making, including potentially negative repercussions for governmental effectiveness, trust, and accountability. For drug regulatory agencies, measures to increase transparency also must take into account the legitimate protections that medical product manufacturers may seek for proprietary information (Califf, 2017).
In terms of effectiveness, transparency has some notable benefits (e.g., limiting corruption) but “excessive” transparency or the “wrong kind” of transparency might disrupt organisational functioning. As Jane Mansbridge has noted, some negotiations are best conducted behind closed doors, without concerns about how each word said might play out in public (Mansbridge, 2009). This points in favour of transparency in rationale (the facts and reasons on which decisions are based) rather than transparency in process (e.g., making all Committee meetings and transcripts public).
Similarly, many posit a positive relationship between transparency, trust and perceptions of decision-making legitimacy (For example, Carpenter, 2017; Licht et al., 2014). However, negative effects also can result, depending on the information that is disclosed, the way that information is shared, and the availability of avenues to independently assess the veracity of information disclosed. In her 2002 Reith lecture, philosopher Onora O’Neill articulated the limits of simply making information available as a means of promoting trust. Instead of focusing on destroying secrecy, O’Neill stressed the need to “limit the deception and deliberate misinformation that undermine relations of trust.” Although some strategies for increasing transparency may reduce deception, others may “produce a flood of unsorted information and misinformation that provides little but confusion” without an equal capacity for the information to be sorted and assessed by institutions and individuals who themselves are trusted. In sum, rather than focus on transparency, O’Neill pushes us to consider making ways to actively check one another’s claims (O’Neill, 2002).
O’Neill’s reservations about the role of transparency in promoting trust have been echoed by others, who have stressed the need for the disclosure of information to be associated with an explanation about how the information has been produced. This includes how the information has been collected, correlated, and interpreted (Turilli and Floridi, 2009), as well as credible mechanisms for holding agencies accountable for decisions made on the basis of the information (Licht et al., 2014).
The downsides of transparency articulated by O’Neill and others highlight the importance of accepting disclosure as only one part of the transparency puzzle. To achieve true normative and instrumental benefits, transparency must be linked to broader changes in decision-making processes. Matthew Herder suggests two such pathways. First, requirements for reasons for decisions should prompt regulatory agencies to more carefully weigh a course of action’s pros and cons before coming to a decision. In this way, transparency in rationale should act as a form of internal quality improvement for agencies (Herder, 2014b). Transparency in rationale could further support the operation of drug regulatory agencies as “ideal social arbiters.” Annette Rid and David Wendler coined this term to address situations in which an agency lacks concrete guidance on how to make a decision, particularly as regards the risks and benefits of research (Rid and Wendler, 2011). Requirements for ideal social arbiters include: careful consideration of risks and potential benefits for all affected parties; fair consideration to everyone’s claims; and the treatment of like cases alike (Rid and Wendler, 2011). Each of these criteria can benefit from transparency within an agency.
Secondly, transparency of regulatory reasons as well as the information amassed to support those reasons allows independent scrutiny by “critically engaged research communities” (Herder, 2014b). Independent assessment of “the full complexity, contingency, and contested nature of regulatory decision-making” can promote regulatory legitimacy (Herder, 2014b), leading to an avenue of active checks and balances consistent with Onora O’Neill’s articulation of the pathway from transparency to trust (O’Neill, 2002).
Why Accountability?
Accountability is a conditional value where an actor is required to provide an explanation and justification for their behaviour. Accountability may also require the actor to provide forms of response or redress for breaching norms of conduct. In the context of health regulation, “public accountability” means that the justification must be made generally, or to specific publics, usually in ways that are transparent and involve the participation of community members. The functional value of accountability lies in its capacity to raise the consciousness of actors, encouraging them to reflect on whether their behaviours correspond with the norms under which they operate. Whatever its formulation, “accountability in one or another forms is increasingly seen as an independent criterion for evaluating scientific research and its technological applications, supplementing more traditional concerns with safety, efficacy, and economic efficiency” (Jasanoff, 2012, 169).
Accountability has two main dimensions:
1) a relational dimension where questions arise as to which of us should be accountable and to whom are we accountable? and
2) a procedural dimension that examines question of for what we are accountable (for eg what type of normative breach? what types of harms?) and how are we to be made accountable? (for eg, in what fora will the person have to give their account and what powers does that fora have to order restitution or reparations for poor behaviour?)
One cannot speak of holding someone to account unless there is a set of norms which can be used to judge the actor’s behaviour. The concept of accountability therefore assumes that there are a set of agreed norms of conduct against which the particular actor’s behaviour may be measured. This also requires that there must be a community or public in which the norms are generated and held. For example, in claims of scientific misconduct, a scientist will have to justify their work to other members of the scientific community. That community holds tightly to norms of conduct, data integrity and repeatable findings and the scientist must be prepared to provide a proper account of how the results in a publication were achieved. In the absence of such an account, they face sanctions like retraction of an article or loss of research income.
Similarly, in a case where a doctor is facing allegations of misconduct for providing unconventional treatments, the doctor will be held accountable for that behaviour by comparing the treatments with reasonable professional practices from the same field. The doctor must justify their departure from those standard practices before a medical board or tribunal and failure to do so may result in their practice being restricted or prevention from practising altogether. It is also worth noting that the choice of public to which a person is held to account is a political and social one, and the interplay of these forces may deem an actor to be accountable to several publics at the same time, or to none.
Accountability is therefore susceptible to failure in all its dimensions. From the relational dimension, it may fail when there is no authority to whom actors must provide an account, for example no regulator, or no license authority. In the procedural aspect, failure may arise from a lack of a forum in which to require actors to provide an account, or from a lack of agreed standards or norms within a public. And failure may also occur in the battle between publics as to which of them can set standards of behaviours and hold particular actors to account (for example, if a doctor seriously injures a patient, when should the doctor be held criminally responsible for injuring a patient, responsible in tort law and/or professionally disciplined?).
In the drug regulatory context, this raises questions as to whom a regulator is responsible when issuing (or failing to issue) an approval, the standards on which this responsibility should be based, and how such obligations link back to expectations of transparency and public participation.
Lessons From Current Regulatory Practices
Lessons From Product Approval Processes Generally
The products of genome editing designed for use in humans will require approval by national regulatory agencies before they can be made available clinically. These agencies include the Food and Drug Administration (FDA) in the US, the European Medicines Agency (EMA) and the Australian Therapeutic Goods Administration (TGA). Cognisant of calls on governmental agencies to adopt “good governance” principles of transparency, accountability and public participation, each of these agencies has committed to disclosure of their regulatory decisions at various stages of the process of market authorisation (Papathanasiou et al., 2016; Califf, 2017; Sharfstein et al., 2017). Specific mechanisms for achieving these ends range from simply requiring registration of clinical trials and summary results, to publication of reasoning behind regulatory decision-making (Herder, 2014b).
Notably, the EMA and TGA both have a stated commitment to disclosure of information leading to rejection of applications as well as approvals (Papathanasiou et al., 2016). Since 1995, European Public Assessment Reports (EPARs) published by the EMA have provided public access to a range of information, including most relevantly, assessment and product information reports for all medicines, whether approved or refused (European Medicines Agency, 2018b). The Australian TGA has implemented a system modelled on the EU system, and produces similar information in the form of Australian Public Assessment Reports (AusPARs) (Australian Government Department of Health Therapeutic Goods Administration, 2021a). AusPARs have been published since 2009 for prescription medicine applications (including biologicals). These systems resulted from commitments on the part of the EMA and TGA to increase transparency, although given the confidential nature of some information provided during clinical trials, commercially sensitive information is redacted (Papathanasiou et al., 2016). AusPARs are produced by the TGA for “major submissions” relating to prescription medicine applications for new chemical and biological entities. They are described by the TGA as “…an important part of the transparency of the TGA’s decision-making processes” (Australian Government Department of Health Therapeutic Goods Administration, 2021b).
In the US, the FDA has been less proactive in sharing data relating to clinical trial decision-making and outcomes. While there are clear requirements for industry submission of data relating to clinical trial protocols and results (DeVito et al., 2020), the FDA does not currently disclose its own analyses pertaining to regulatory assessments. Currently, FDA analyses are only released on an individual basis pursuant to freedom of information requests. Although “applicable” clinical trials are required to be registered on a publicly available registry, such as clinicaltrials.gov, information published by the FDA about clinical trials and approvals is limited to study design, administrative information and summary trial results. Further information is disclosed in the case of some medicines where the FDA considers it necessary to establish and consult advisory committees. In this instance, the meetings of these advisory committees are public and the minutes published (Sharfstein et al., 2017). There have been calls for greater sharing by sponsors and investigators of clinical trials data (Committee on Strategies for Responsible Sharing of Clinical Trial Data, 2015), but also ongoing calls for increased disclosure of information held by the FDA, including clinical study reports, clinical trial data, and FDA analyses in relation to both approved and rejected applications (Sharfstein et al., 2017). Restrictions on access to information are justified by the FDA on the basis of confidentiality. Yet although the non-disclosure of confidential, commercial data is mandated by Congress, the FDA has considerable flexibility to interpret what falls within the confines of non-disclosable, confidential data (Kapczynski and Kim, 2017).
Notwithstanding calls for increased or comprehensive disclosure by the FDA of clinical trial data and regulatory assessments, which would bring it into line with the EU and Australian systems, there is a strong argument that increased transparency alone is insufficient. While it undoubtedly reflects transparency in the rationale for individual decisions, it fails to exhibit transparency in the process of decision-making. It also neglects to incorporate public participation in grounding decisions to approve or reject applications. Publishing clinical trial data alone does not equate to effective public participation, because it permits no feedback from trial participants or other relevant parties. In accountability terms, there are issues with the procedural dimension of applicable standards and with the question of whom decision-makers should have to account to.
In sum, there is presently very little meaningful public involvement evident in approval decisions. Public participation though consultation processes is frequently utilised in setting the boundaries of regulatory frameworks. But public participation in specific regulatory decision-making when it comes to marketing authorisation for new therapeutic products is lacking. Given the normative nature of decisions surrounding the approval of new therapeutic products, particularly those involving new technologies such as genome editing, some context for decisions beyond scientific reasoning would promote more meaningful decision-making.
Lessons From Gene Therapy Market Authorisations
Beyond the public participation embedded in product approval processes more generally, it is helpful to consider the specific regulatory pathways likely to apply to products of genome editing. At present, the best comparator is the regulation of gene therapy products given that genome editing is likely to be regulated in a similar manner. This raises the question of what role the three pillars of public participation, transparency and accountability play in this context?
Although regulatory authorities have had limited opportunity to consider and approve gene therapy products,1 there are a number of projects in the pipeline that look set to test the capacity of regulatory authorities in coming years, as the number of gene therapies in the development pipeline increases (O’sullivan et al., 2019; Horgan et al., 2020). Because gene therapies often treat rare diseases, they are not usually assessed through submission of data from large scale clinical trials. Rather, sponsors frequently rely on trials that involve small numbers of patients and the development of novel clinical endpoints (High, 2020). While traditional clinical trial pathways govern personalised medicine therapies, patients are likely to have to rely on lobbying for individual access to medicines, through pathways for compassionate use (Australian Council of Learned Academies, 2018). These pathways rely less on evidence and more on responding to desperation on the part of patients (Lewis et al., 2017). All of these distinctions have relevance for the manner in which public participation, transparency and accountability feed into approval processes.
The European Union
Under the EU regulatory scheme, biologic products are brought within the scope of the medicinal products scheme (Medicinal Products Directive 2001/83/EC and Regulation (EC) No 726/2004), by the Advanced Therapy Medicinal Products Regulation 2007 (ATMP Regulation). Products will fall within the ATMP scheme if cells or tissues have been “engineered.” This requires them to have been subject to “substantial manipulation” in order to achieve particular biological, physiological or structural properties, or that was intended to achieve a function differing from their original function (Art 2(c) ATMP Regulation).
ATMPs may be developed in line with the traditional therapeutic development pathways. The EMA’s Committee for Advanced Therapies (CAT) provides specialised scientific advice on advanced therapy applications, as well as general scientific advice (European Medicines Agency, 2018a. Aside from conventional development routes, CAT provides a certification procedure for ATMPS being developed by small and medium-sized enterprises (SMEs). This procedure certifies compliance with the standards for issuing a marketing authorisation on the basis of available “quality and non-clinical data” and is available to provide an incentive for SMEs to develop ATMPs. In some cases, products in development may also be made available to groups of patients with unmet need, under very strict conditions, through the compassionate use pathway.
There is limited evidence of EMA consideration of patient perspectives in risk-benefit analyses conducted during conventional therapeutic processes, but increasingly there have been calls to elevate patient input into decision-making by Health Technology Assessment (HTA) bodies (Coulter et al., 2008; Sarri et al., 2021), and some moves by the EMA to involve patients in decision-making (Mühlbacher et al., 2016). There is some scope for those developing innovative medicines to apply to follow alternative pathways for approval, some of which provide greater opportunity for early patient dialogue and consultation in relation to regulatory decisions than conventional approval pathways. For example, scientific advice may be sought in some cases where innovative technologies are being developed and it is appropriate to deviate from traditional development pathways. (Nicotera et al., 2019). Protocol assistance is the term given to advice provided by the EMA in the development of orphan medicines for rare diseases. Accelerated assessment is available for priority medicines in areas of unmet clinical need whereby support for development is provided by the EMA (European Medicines Agency, 2018c). All of these processes provide a forum for more systematically recording and incorporating the opinions and experiences of patient representatives from an early stage, and iteratively engaging with patients (among other stakeholders). They are likely to better take into account the ‘trade-offs ‘weighed by patients, which can differ to those taken account of by regulators (Mühlbacher et al., 2016).
The EMA has also adopted an Adaptive Pathways approach, (European Medicines Agency 2018a) described as “a prospectively planned, iterative approach to bringing medicines to market” (European Medicines Agency, 2016). The premise behind the scheme is that testing of particular therapeutic products will be directed toward carefully defined groups of patients with ‘high medical need’ who are likely to benefit from the treatment, rather than gathering data through conventional routes (European Medicines Agency, 2018a). The process for approval may occur in stages and involve limited patient populations. It may also incorporate evidence gathered through discussions between sponsors, regulators and other relevant parties, including patient representatives whose participation and input is encouraged (European Medicines Agency, 2016). To be effective, these discussions generally need to take place prior to phase II trials (Nicotera et al., 2019). A pilot project initiated in 2014 was reported to have provided a successful pathway for a number of products fitting the application criteria, which enabled contributions to be made by patient group representatives among others (European Medicines Agency, 2016).
Australia
The application of the Australian TG Act to the products of gene therapy and genome editing is complex, with legislative differentiations based on whether products are made with versus from human cells or tissues, and whether use is in vivo or ex vivo. (Nicol and Eckstein, 2019). The TGA has advised that gene therapy products are regulated as medicines under the Therapeutic Goods Act 1989 (Cth) (TG Act) where gene therapy is performed in vivo. Where performed ex vivo, gene therapy will be regulated as a biological (Smith, 2019). Genome editing is likely to be regulated in a similar manner. Although both medicines and biologicals will require review by the TGA in order to be listed on the Australian Register of Therapeutic Goods, the process for review will be subject to some differences (e.g., the reviewing advisory committee).
The TGA’s approval processes closely mirror those under the EU scheme. While traditional regulatory pathways are the norm, several special access schemes exist under the TG Act (sections 18(1), 32CA (2) and 41HA; regulation 12A of the Therapeutic Goods Regulations) which access to unapproved therapies may be sought. At the time of writing, the TGA has approved just three gene therapy products, one in vivo and two ex vivo. Kymriah was approved in December 2018, Luxturna in August 2020 and Zolgensma in February 2021. Because Kymriah is delivered in vivo, it was assessed through the TGA’s medicines pathway. Luxturna and Zolgensma were assessed via the biologicals pathway.
In the standard product approval processes for both medicines and biologicals, there is little evidence that the TGA currently takes into account patient preferences, transparency and pillars of accountability in regulatory decision-making. TGA reviews are conducted in private, and are not open to public input (Eckstein, 2015). Expertise is made available to the TGA through relevant advisory committees (e.g., the Advisory Committee on Biologicals), however—with the exception of one consumer representative—committee members are medical researchers and clinicians (Australian Government Department of Health Therapeutic Goods Administration, 2020). With respect to transparency, AusPARs are prepared by the TGA and made available on its website for “applications where the significance to the public is considered to be high,” including for submissions that have been approved, withdrawn, or rejected in the application process (Australian Government Department of Health Therapeutic Goods Administration, 2021a). AusPARs for the gene therapy products mentioned above indicate close reliance by the TGA on European and US trial results and regulatory outcomes, (Australian Government Department of Health Therapeutic Goods Administration, 2021b), which is unsurprising given the innovative nature of these therapies.
The need for public input in taking genome editing and other breakthrough technologies into the clinic has been recognised, but the challenges in doing so and in achieving meaningful outcomes at critical stages of the regulatory process have been acknowledged (Australian Council of Learned Academies, 2018). Few public engagement mechanisms have been tested in Australia to date (Australian Council of Learned Academies, 2018).
Special access scheme pathways provide another potential opportunity for public participation. Category A applies to terminally ill patients, who may be supplied with unapproved drugs by their medical provider based on notification only to the TGA where death is likely to be imminent. Category C allows medical providers to apply to the TGA to supply specific unauthorised goods to patients or groups of patients on an ongoing basis where products have an established history of use, making it inapplicable at present to products of gene therapy or genome editing (Australian Government Department of Health Therapeutic Goods Administration).
In addition to clinical trials, perhaps the most relevant category is Category B, which may be relied upon by patients with non-serious or life-threatening illnesses and an unmet clinical need. These patients may by supplied with unapproved goods, subject to TGA approval. Because Category B approvals require a higher threshold than Category A approvals, it is difficult to predict whether it would be reached for gene therapy or genome editing products, even where patients are prepared to accept the risks of therapy. The requisite tests of “seriousness” and “unmet need” are likely to present interpretational difficulties (Von Tigerstrom, 2015).
The United States
US regulation of the products of gene therapy is undertaken pursuant to the Code of Federal Regulations (Title 21CFR), enacted under the Federal Food, Drug and Cosmetic Act (FD&C Act) The agency overseeing the regulation of biological products is the Center for Biological Evaluation and Research (CBER). Because gene therapy involves “more than minimal manipulation” of cells, tissues and cellular and tissue-based products (HCT/Ps), it is regulated under the biologics regulatory system comprising the Public Health Service Act, § 351 and the Human Cells, Tissues and Cellular and Tissue-based Products Regulation (21 CFR Part 127). For cells or non-structural tissues, “minimal manipulation” means processing that does not alter biological characteristics. “Minimal manipulation” in respect of structural tissue means processing that does not alter the original characteristics relating to reconstruction, repair or replacement.
In addition to clinical review by the FDA, gene therapies have previously been subject to review by the NIH Recombinant DNA Advisory Committee (RAC). This dual oversight system was concluded in 2018 when primary oversight was handed to the FDA, removing duplicative effort and reflecting the fact that gene therapy is now viewed as having no greater risk than other fields (Collins and Gottlieb, 2018). The RAC will function as an advisory board on emerging biotechnologies going forward (Collins and Gottlieb, 2018). The FDA has also published a number of guidance documents for developers of gene therapy products (Center for Biologics Evaluation and Research, 2021.
A number of accelerated approval procedures are available for gene therapy products under the US scheme. Section 506(g) of the FD&C Act provides a procedure to designate a regenerative medicine therapy as a “regenerative advanced therapy” (RMAT) if it meets certain criteria (Food and Drug Administration, 2019). This provides a sponsor with the capacity to undertake accelerated procedures that are available, including fast track designation, breakthrough therapy designation, RMAT designation, accelerated approval and priority review designation. Specifically, the treatment must be to treat a serious disease or condition or address an unmet clinical need. Depending on the designation given, data demonstrating efficacy may be derived from surrogate or other clinically significant endpoints permitting consideration of preliminary clinical evidence (Food and Drug Administration, 2019). Novel approaches to clinical data collection are encouraged, as is the obtaining of input from patient communities. This direction is not new—indeed it is in line with general FDA guidance focused on the incorporation of patient input into risk-benefit analysis (Food and Drug Administration, 2020). The more general FDA guidance provides recommendations as to how patient preferences and experiences may effectively inform risk-benefit assessments in conducting clinical studies. A further necessary step is building capacity amongst researchers to effectively capture qualitative data on patient preferences, and amongst regulators to successfully evaluate this study data (Johnson and Zhou, 2016).
Further initiatives to increase the weight of patient preferences in decision-making include a program by the FDA which ran a series of workshops between 2013 and 2018 focused around particular diseases, in which input of patient advocates was a key component (Center for Drug Evaluation and Research, 2021b). The input of many thousands of patients was captured in a series of 25 patient input reports. The initiative was a core component of the Center for Drug Evaluation and Research’s (CDER) Patient-Focused Drug Development Initiative, a “systematic approach to help ensure that patients” experiences, perspectives, needs, and priorities are captured and meaningfully incorporated into drug development and evaluation’ (Center for Drug Evaluation and Research, 2021a).
Growing patient participation in decisions relating to drug development appears to be improving transparency and accountability by drug developers (Wicks et al., 2011; Lowe et al., 2016). Increasingly there are moves by the FDA to incorporate patient experience into risk-benefit analyses in relation to particular applications before the FDA. However, this expansion in normative consideration of patient perspectives has not consistently resulted in incorporation of patient views at the regulatory evaluation stage, particularly for therapeutic products progressing through conventional approval pathways. There is real scope with products aimed at rare conditions (and in which clinical evidence is often lacking), to address this imbalance.
A patient involvement pilot for orphan drugs in Canada provides a useful starting point for investigating the potential methods through which patient involvement in the medicines evaluation process (Klein et al., 2016). Data detailing patients’ views was generated through questionnaires designed to elicit qualitative information on patients’ quality of life, experience with existing therapies, unmet medical needs, and level of risk patients were prepared to tolerate. This information is sometimes gathered through the clinical trials process, but that process is inevitably concentrated on gathering of quantitative evidence. Certainly, there is scope for increased consideration of patient preferences and experiences.
Lessons for Genome Editing
Taking patient views and experience into account is by no means an easy exercise. Genome editing is a case in point: there is no clear methodology in weighing the risks and benefits of passing on heritable traits that may ensue from genome editing. Being cognisant of the scientific evidence as to risks involved remains paramount and must be communicated to patients in order to ground patient opinion in objective fact. Dialogue must be two-way, direct, and incorporate the views of broad societal interests rather than select groups (Sturgis, 2014).
Several decisions of the FDA illustrate the point that patient participation must be approached with caution, and that it must be tempered with scientific evidence in drawing the boundaries of public participation and ensuring the safety of all approved therapies and medicines. Approval through the accelerated approval process of the Duchene Muscular Dystrophy drug Eteplirsen went against the recommendations of FDA staff and an advisory committee, and proceeded on the basis of a twelve-person trial, and testimony by patients, families and advocates (Schwartz, 2017). While the approval was positively received by patients, FDA staff expressed concern that scientific evidence (or rather the fact that scientific evidence was lacking) was not prioritised (Railroading at the FDA, 2016).
In 2021, the FDA granted marketing approval to Biogen for its Alzheimer’s drug Aduhelm (generic name aducanumab), using the accelerated approval pathway and surrogate endpoints: namely a reduction in amyloid-beta plaque. (Mullard, 2021). Aduhelm marks the first drug approved that targets the underlying cause of Alzheimer’s rather than just the symptoms. Further clinical trials will now be required to gain final approval, but in the meantime, a select group of patients will be prescribed the drug at a cost of US$56,000 per year (Armstrong, 2021). This is despite the available clinical trial data revealing some not-negligible side effects in a significant number of patients and ongoing questions about the drug’s efficacy (Mullard, 2021). At least one factor in the drug’s approval appears to have been lobbying by the Alzheimer’s Association, with the association’s CEO stating that “Clearly, this is not a cure, and it is a marginal difference for people, but a marginal difference can make a real difference for people who have only the devastation of Alzheimer’s to look to” (Feuerstein et al., 2021).
As with Eteplirsen, the advisory committee considering the drug recommended against approval. It was unanimous in its rejection, and the FDA’s decision has resulted in the resignation of a committee member (Joseph, 2021). In this respect it runs counter to previous FDA practice, whereby approvals and advisory committee recommendations strongly align (Zhang et al., 2019). The Aduhelm decision, in particular, has divided the research community, with many claiming it has the potential to stymie research, erode public trust in regulatory agencies, and cause real harm to patients using the drug for no clear benefit (Mullard, 2021) It raises the question as to whether the pendulum has swung too far in favour of incorporating normative views of risks and benefits—including benefits based on untested surrogate endpoints—at the expense of scientific evidence.
Future Strategies to Better Incorporate Public Participation, Transparency and Accountability in Market Authorisation of Genome Editing Products
Although the preceding discussion highlights some attempts to better facilitate public participation, transparency and accountability in public health regulatory decision-making, such steps have been piecemeal and, in some cases, controversial. Disclosure has formed a key component of the transparency arsenal of many regulatory agencies. The adequacy of such disclosures in satisfying the commitment to transparency has been subject to criticism (Kapczynski and Kim, 2017; Sharfstein et al., 2017) and appears to have limitations in practical uptake. Going beyond disclosure of information, there are increasing calls for these agencies to be more democratic in their decision-making generally, and to move away from an exclusive focus on the technical benefit-risk calculation (Jasanoff et al., 2015), towards “a more participatory, public model of drug regulation” (Herder, 2014a, S131). However, these must be balanced with ongoing respect for scientific evidence. Sheila Jasanoff’s pioneering concept of “technologies of humility” provides one such opportunity.
It is useful here to reflect upon Jasanoff’s dual concepts of regulatory technologies of hubris and technologies of humility (Jasanoff, 2003, 2012). Jasanoff defines technologies (or regulatory methods) of hubris by reference to traditional regulatory cultures of the 20th century that focussed on predictive methods and risk assessments and that targeted the facilitation of “management and control, even in areas of high uncertainty” (Jasanoff, 2012, 178). The focus of these regulatory techniques is to allow the public to feel confident in decision-making through reliance on scientific expertise, but the cost of using such regulatory techniques is that they downplay the limitations of expertise, and stifle public participation and policy review. Given their narrow focus on scientific expertise, technologies of hubris are also ill-equipped to deal with challenges that arise from outside of their cultural frames.
As a response, Jasanoff has proposed the adoption of regulatory technologies of humility to complement technologies of hubris. This requires positively including the consequences of uncertainty in the policy frame; making the normative and cultural assumptions of scientific expertise explicit, and acknowledging the plurality of values that exists concerning emerging health innovations. She argues that the development of technologies of humility should have a framework that incorporates four focal points, these being “framing, vulnerability, distribution, and learning” which underscore the normative questions “what is the purpose; who will be hurt; who benefits; and how can we know?” (Jasanoff, 2003, 240):
1) Framing: Frames are “interpretive schemata and ordering devices that are needed by policy-makers to structure the reality of a policy issue” (Dekker, 2017, 129). Frames set values and rank them in order of importance. They create a structure around what is problematic and they also provide suggested solutions. But frames can also exclude values, factors and experience which may later prove to invaluable. Techniques should therefore be adopted to make sure that frames are tested and revisited and revised in systematic and iterative ways.
2) Vulnerability: Traditional risk-based assessments lack an understanding of the social determinates of risk but even recent attempts to include socio-economic determinates operate at a population level, with no methods for listening to or understanding individual differences. Jasanoff argues that individuals need more meaningful ways to participate. She states that “through participation in the analysis of their vulnerability, ordinary citizens may regain their status as active subjects, rather than remain undifferentiated objects in yet another expert discourse.” (Jasanoff, 2012, 180).
3) Distribution: It would be rare for ethical issues of distribution to be included in policies for the approval of health innovations. Policies should include consideration for how new technologies will be distributed including the kinds of disparities and realignments that may occur when new technology is introduced into populations.
4) Learning: Jasanoff argues that while policies often include lessons for learning from errors, mistakes and failures, there is a tendency to assume there is one set of factors which explain failure, and they should rather be designed to take into account “Ways to design avenues through which societies can collectively reflect on the ambiguity of their experiences, and to assess the strengths and weaknesses of alternative explanations.” (Jasanoff, 2012, 181).
What might models that bring technologies of humility into future discourse and regulatory action in the genome editing sphere encapsulate? In Table 1, we provide some preliminary thoughts as to how considerations that might better incorporate technologies of humility could be captured to support the three pillars of public participation, transparency and accountability. While further work is needed to comprehensively assess these proposed mechanisms, we can use the lessons learnt from recent regulatory setbacks to inform normative regulatory decision-making in genome editing.
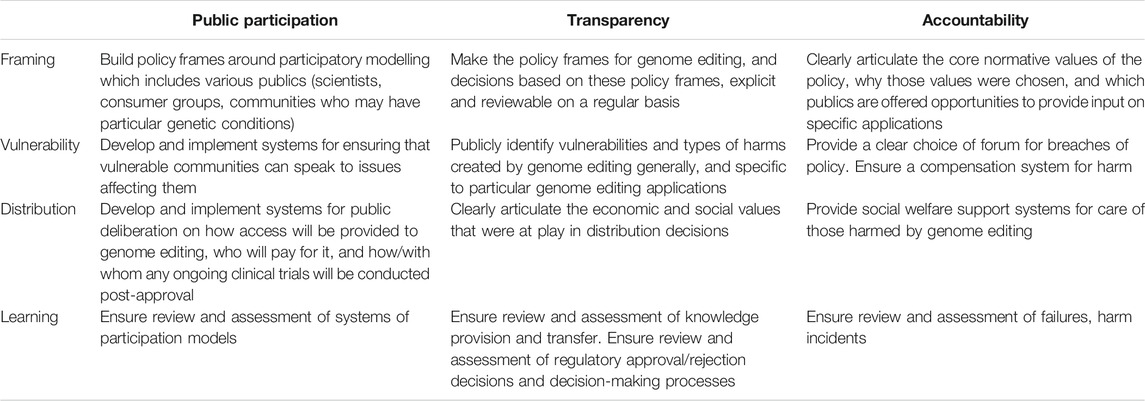
TABLE 1. Options for incorporating technologies of humility into marketing authorisations for genome editing products.
Jasanoff herself recognised the modesty of the focal points identified as a “starting point” for engaging in deeper social discourse on the ethical and political implications of emerging technologies (Jasanoff, 2003). The relatively superficial evaluation of these focal points undertaken here highlights the range of considerations that are often overlooked in a traditional, hubristic model of regulation. In the interests of encouraging public trust and engagement, and injecting social considerations pertaining to risk analysis into governance, the development of models for regulating genome editing should be approached systematically, ethically, inclusively and with caution.
Conclusion
In this article, we have shown that both policy makers and regulators charged with responsibility for market approvals for new healthcare products support adoption of public participation, transparency and accountability in their policy-making and decision-making. However, the rhetorical force of these statements is difficult to translate into concrete actions. The path to true adoption of public participation, transparency and accountability in regulatory policy-making and decision-making for genome editing products is strewn with boulders. On the one hand, slavish adoption of these principles could reduce the weight given to scientific evidence. On the other hand, formalisation of these principles could result in them being applied in a tokenistic fashion. New models are urgently needed, particularly given the speed with which genome editing is being adopted in the laboratory and promising new genome editing product leads are emerging. We have proposed that one such model, Jasanoff’s technology of humility, is worthy of consideration. There will doubtless be others. In this article, our aim to contribute to the start of a deeper conversation about these vitally important issues.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
All authors have contributed equally to the conceptualisation of this article. JN wrote the first draft of the substantive analysis of regulatory approvals and gene therapy market approvals. All authors contributed equally to first drafts of the rest of the article. All authors contributed equally to further drafts of the article, including the final submitted version.
Funding
This research was supported by a grant from the Australian Research Council, DP180101262.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1As of the date of writing, 14 gene therapy products have been approved and marketed in the EU, 20 in the US, and just three in Australia.
References
Armstrong, R. (2021). FDA Approves Controversial Alzheimer’s Drug from Biogen. EPM Magazine. Available at: https://www.epmmagazine.com/api/content/f437944e-c9d0-11eb-9ec9-1244d5f7c7c6/(Accessed July 23, 2021).
Arnstein, S. R. (1969). A Ladder of Citizen Participation. J. Am. Inst. Planners. 35, 216–224. doi:10.1080/01944366908977225
Australian Council of Learned Academies (2018). The Future of Precision Medicine in Australia. Published by the Australian Council of Learned Academies, Melbourne, Australia. Available at: https://acola.org/hs2-precision-medicine-australia/ (Accessed September 29, 2021).
Australian Government Department of Health Therapeutic Goods Administration (2021b). Accessing Unapproved Products. Therapeutic Goods Administration (TGA). Available at: https://www.tga.gov.au/accessing-unapproved-products (Accessed July 26, 2021).
Australian Government Department of Health Therapeutic Goods Administration (2020). Advisory Committee on Biologicals (ACB). Therapeutic Goods Administration (TGA). Available at: https://www.tga.gov.au/committee/advisory-committee-biologicals-acb (Accessed July 23, 2021).
Australian Government Department of Health Therapeutic Goods Administration (2021a). Australian Public Assessment Report (AusPAR) Guidance. Therapeutic Goods Administration (TGA). Available at: https://www.tga.gov.au/australian-public-assessment-report-auspar-guidance (Accessed July 23, 2021).
Califf, R. M. (2017). Transparency at the U.S. Food and Drug Administration. J. L. Med. Ethics. 45, 24–28. doi:10.1177/1073110517750616
Carothers, T., and Brechenmacher, S. (2014). Accountability, Transparency, Participation, and Inclusion: A New Development Consensus? Carnegie Endowment International Peace. Available at: https://www.jstor.org/stable/resrep12957 (Accessed July 26, 2021).
Carpenter, D. (2017). FDA Transparency in an Inescapably Political World. J. L. Med. Ethics. 45, 29–32. doi:10.1177/1073110517750617
Center for Biologics Evaluation and Research (2021). Cellular & Gene Therapy Guidances. FDA. Available at: https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/cellular-gene-therapy-guidances (Accessed July 23, 2021).
Center for Drug Evaluation and Research (2021a). CDER Patient-Focused Drug Development. FDA. Available at: https://www.fda.gov/drugs/development-approval-process-drugs/cder-patient-focused-drug-development (Accessed July 23, 2021).
Center for Drug Evaluation and Research (2021b). FDA-led Patient-Focused Drug Development (PFDD) Public Meetings. FDA. Available at: https://www.fda.gov/industry/prescription-drug-user-fee-amendments/fda-led-patient-focused-drug-development-pfdd-public-meetings (Accessed July 23, 2021).
Collins, F. S., and Gottlieb, S. (2018). The Next Phase of Human Gene-Therapy Oversight. N. Engl. J. Med. 379, 1393–1395. doi:10.1056/NEJMp1810628
Committee on Strategies for Responsible Sharing of Clinical Trial Data (2015). Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk. doi:10.17226/18998[Accessed July 23, 2021].
Dekker, R. (2017). Frame Ambiguity in Policy Controversies: Critical Frame Analysis of Migrant Integration Policies in Antwerp and Rotterdam. Crit. Pol. Stud. 11, 127–145. doi:10.1080/19460171.2016.1147365
DeVito, N. J., Bacon, S., and Goldacre, B. (2020). Compliance with Legal Requirement to Report Clinical Trial Results on ClinicalTrials.Gov: a Cohort Study. The Lancet. 395, 361–369. doi:10.1016/S0140-6736(19)33220-9
Eckstein, P. P. (2015). Aufgaben zur Zusammenhangsanalyse. Macquarie L.J. 15, 65–79. doi:10.1007/978-3-658-10339-2_7
European Medicines Agency (2018a). Adaptive Pathways. European Medicines Agency. Available at: https://www.ema.europa.eu/en/human-regulatory/research-development/adaptive-pathways (Accessed July 23, 2021).
European Medicines Agency (2018b). European Public Assessment Reports: Background and Context. Available at: https://www.ema.europa.eu/en/medicines/what-we-publish-when/european-public-assessment-reports-background-context (Accessed July 23, 2021).
European Medicines Agency (2016). Guidance for Companies Considering the Adaptive Pathways Approach. Available at: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guidance-companies-considering-adaptive-pathways-approach_en.pdf (Accessed July 23, 2021).
European Medicines Agency (2018c). Support for Early Access. European Medicines Agency. Available at: https://www.ema.europa.eu/en/human-regulatory/overview/support-early-access (Accessed July 23, 2021).
Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing (2021a). Human Genome Editing: a Framework for Governance. Available at: https://www.who.int/publications/i/item/9789240030060 (Accessed July 26, 2021).
Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing (2021b). Human Genome Editing: Position Paper. Available at: https://www.who.int/publications/i/item/9789240030404 (Accessed July 26, 2021).
Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing (2021c). Human Genome Editing: Recommendations. Available at: https://www.who.int/publications/i/item/9789240030381 (Accessed July 26, 2021).
Feuerstein, A., Herper, M., and Garde, D. (2021). How Biogen Used an FDA Back Channel to Win Alzheimer’s Drug Approval Stat+. Available at: https://www.statnews.com/2021/06/29/biogen-fda-alzheimers-drug-approval-aduhelm-project-onyx/(Accessed July 23, 2021).
Food and Drug Administration (2019). Expedited Programs for Regenerative Medicine Therapies for Serious Conditions, Guidance for Industry. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expedited-programs-regenerative-medicine-therapies-serious-conditions (Accessed July 23, 2021).
Food and Drug Administration (2020). Patient-Focused Drug Development: Collecting Comprehensive and Representative Input, Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. Available at: https://www.fda.gov/media/139088/download (Accessed July 23, 2021).
Fung, A. (2015). Putting the Public Back into Governance: The Challenges of Citizen Participation and its Future. Public Admin Rev. 75, 513–522. doi:10.1111/puar.12361
Fung, A. (2006). Varieties of Participation in Complex Governance. Public Adm. Rev. 66, 66–75. doi:10.1111/j.1540-6210.2006.00667.x
Gaj, T., Gersbach, C. A., and Barbas, C. F. (2013). ZFN, TALEN, and CRISPR/Cas-based Methods for Genome Engineering. Trends Biotechnol. 31, 397–405. doi:10.1016/j.tibtech.2013.04.004
Herder, M. (2014a). Denaturalizing Transparency in Drug Regulation. Mcgill J.L. Health. 8, S57–S144.
Herder, M. (2014b). Toward a Jurisprudence of Drug Regulation. J. L. Med. Ethics. 42, 244–262. doi:10.1111/jlme.12139
High, K. A. (2020). Turning Genes into Medicines-What Have We Learned from Gene Therapy Drug Development in the Past Decade? Nat. Commun. 11, 5821. doi:10.1038/s41467-020-19507-0
Hood, C. (2006). “Transparency in Historical Perspective,” in In Transparency: The Key To Better Governance? Editors C. Hood, and D. Heald. Oxford: Oxford University Press. doi:10.5871/bacad/9780197263839.003.0001
Horgan, D., Metspalu, A., Ouillade, M.-C., Athanasiou, D., Pasi, J., Adjali, O., et al. (2020). Propelling Healthcare with Advanced Therapy Medicinal Products: A Policy Discussion. BMH 5, 1–23. doi:10.1159/000511678
Innes, J. E., and Booher, D. E. (2004). Reframing Public Participation: Strategies for the 21st century. Plann. Theor. Pract. 5, 419–436. doi:10.1080/1464935042000293170
International Commission on the and Clinical Use of Human Germline Genome Editing (2020). Heritable Human Genome Editing. Available at: https://www.nap.edu/read/25665/chapter/1 (Accessed July 23, 2021).
Isasi, R., Rahimzadeh, V., and Charlebois, K. (2016). Uncertainty and Innovation: Understanding the Role of Cell-Based Manufacturing Facilities in Shaping Regulatory and Commercialization Environments. Appl. Translational Genomics. 11, 27–39. doi:10.1016/j.atg.2016.11.001
Jasanoff, S., Hurlbut, J., and Saha, K. (2015). Crispr Democracy: Gene Editing and the Need for Inclusive Deliberation. Issues Sci. Technol. 32, 25–32.
Jasanoff, S. (2003). Technologies of Humility: Citizen Participation in Governing Science. Minerva: A Review Of Science. Learn. Pol. 41, 223. doi:10.1023/A:1025557512320
Johnson, F. R., and Zhou, M. (2016). Patient Preferences in Regulatory Benefit-Risk Assessments: A US Perspective. Value in Health. 19, 741–745. doi:10.1016/j.jval.2016.04.008
Joseph, A. (2021). Member of FDA’s Expert Panel Resigns over Alzheimer’s Therapy Approval. STAT. Available at:https://www.statnews.com/2021/06/08/fda-expert-panel-resigns-alzheimers-approval/(Accessed July 23, 2021).
Joss, S. (1999). Public Participation in Science and Technology Policy- and Decision-Making — Ephemeral Phenomenon or Lasting Change? Sci. Public Pol. 26, 290–293. doi:10.3152/147154399781782338
Kapczynski, A., and Kim, J. (2017). Clinical Trial Transparency: The FDA Should and Can Do More. J. L. Med Ethics 45, 33–38. doi:10.1177/1073110517750618
Klein, A. V., Hardy, S., Lim, R., and Marshall, D. A. (2016). Regulatory Decision Making in Canada—Exploring New Frontiers in Patient Involvement. Value in Health 19, 730–733. doi:10.1016/j.jval.2016.03.1855
Lewis, J. R. R., Kerridge, I., and Lipworth, W. (2017). Use of Real-World Data for the Research, Development, and Evaluation of Oncology Precision Medicines. JCO Precision Oncol., 1, 1–11. doi:10.1200/PO.17.00157
Li, H., Yang, Y., Hong, W., Huang, M., Wu, M., and Zhao, X. (2020). Applications of Genome Editing Technology in the Targeted Therapy of Human Diseases: Mechanisms, Advances and Prospects. Sig Transduct Target. Ther. 5, 1–23. doi:10.1038/s41392-019-0089-y
Licht, J. D. F., Naurin, D., Esaiasson, P., and Gilljam, M. (2014). When Does Transparency Generate Legitimacy? Experimenting on a Context-Bound Relationship. Governance 27, 111–134. doi:10.1111/gove.12021
Long, R., and Beierle, T. C. (1999). The Federal Advisory Committee Act and Public Participation in Environmental Policy. Available at: https://ageconsearch.umn.edu/record/10817 (Accessed July 23, 2021).
Lowe, M. M., Blaser, D. A., Cone, L., Arcona, S., Ko, J., Sasane, R., et al. (2016). Increasing Patient Involvement in Drug Development. Value in Health 19, 869–878. doi:10.1016/j.jval.2016.04.009
Maeder, M. L., and Gersbach, C. A. (2016). Genome-editing Technologies for Gene and Cell Therapy. Mol. Ther. 24, 430–446. doi:10.1038/mt.2016.10
Mansbridge, J. (2009). A “Selection Model” of Political Representation*. J. Polit. Philos. 17, 369–398. doi:10.1111/j.1467-9760.2009.00337.x
Mei, Y., Wang, Y., Chen, H., Sun, Z. S., and Ju, X.-D. (2016). Recent Progress in CRISPR/Cas9 Technology. J. Genet. Genomics 43, 63–75. doi:10.1016/j.jgg.2016.01.001
Mostert, E. (2003). The challenge of Public Participation. Water Policy 5, 179–197. doi:10.2166/wp.2003.0011
Mühlbacher, A. C., Juhnke, C., Beyer, A. R., and Garner, S. (2016). Patient-Focused Benefit-Risk Analysis to Inform Regulatory Decisions: The European Union Perspective. Value in Health 19, 734–740. doi:10.1016/j.jval.2016.04.006
Mullard, A. (2021). Landmark Alzheimer’s Drug Approval Confounds Research Community. Nature 594, 309–310. doi:10.1038/d41586-021-01546-2
National Academies of Sciences, Engineering, and Medicine (2017). Human Genome Editing: Science, Ethics, and Governance. Washington, DC: The National Academies Press. doi:10.17226/24623
Nicol, D., and Eckstein, L. (2019). Gene Editing Clinical Trials Could Slip through Australian Regulatory Cracks. J. L. Med. 27, 274–283.
Nicol, D., Eckstein, L., Morrison, M., Sherkow, J. S., Otlowski, M., Whitton, T., et al. (2017). Key Challenges in Bringing CRISPR-Mediated Somatic Cell Therapy into the Clinic. Genome Med. 9, 85. doi:10.1186/s13073-017-0475-4
Nicotera, G., Sferrazza, G., Serafino, A., and Pierimarchi, P. (2019). The Iterative Development of Medicines through the European Medicine Agency’s Adaptive Pathway Approach. Front. Med. 6. doi:10.3389/fmed.2019.00148
O’Neill, O. (2002). A Question of Trust: Trust and Transparency. Available at: https://www.bbc.co.uk/programmes/p00gpzcz (Accessed September 29, 2021)
O’sullivan, G. M., Velickovic, Z. M., Keir, M. W., Macpherson, J. L., and Rasko, J. E. J. (2019). Cell and Gene Therapy Manufacturing Capabilities in Australia and New Zealand. Cytotherapy 21, 1258–1273. doi:10.1016/j.jcyt.2019.10.010
Palaz, F., Kalkan, A. K., Tozluyurt, A., and Ozsoz, M. (2021). CRISPR-based Tools: Alternative Methods for the Diagnosis of COVID-19. Clin. Biochem. 89, 1–13. doi:10.1016/j.clinbiochem.2020.12.011
Papathanasiou, P., Brassart, L., Blake, P., Hart, A., Whitbread, L., Pembrey, R., et al. (2016). Transparency in Drug Regulation: Public Assessment Reports in Europe and Australia. Drug Discov. Today 21, 1806–1813. doi:10.1016/j.drudis.2016.06.025
Piotrowski, S. J., and Borry, E. (2010). An Analytic Framework for Open Meetings and Transparency. Public Adm. Manage. 15, 138–176.
Quick, K. S., and Bryson, J. M. (2016). “Public Participation,” in Handbook On Theories Of Governance. Editors C. Ansell, and J. Torfing (North Hampton, MA: Edward Elgar Publishing), 158–169.
Rid, A., and Wendler, D. (2011). A Framework for Risk-Benefit Evaluations in Biomedical Research. Kennedy Inst. Ethics J. 21, 141–179. doi:10.1353/ken.2011.0007
Roeder, C. B. (2013). Transparency Trumps Technology: Reconciling Open Meeting Laws with Modern Technology Note. Wm. Mary L. Rev. 55, 2287–2316.
Rowe, G., and Frewer, L. J. (2005). A Typology of Public Engagement Mechanisms. Sci. Technol. Hum. Values 30, 251–290. doi:10.1177/0162243904271724
Sarri, G., Freitag, A., Szegvari, B., Mountian, I., Brixner, D., Bertelsen, N., et al. (2021). The Role of Patient Experience in the Value Assessment of Complex Technologies – Do HTA Bodies Need to Reconsider How Value Is Assessed? Health Policy 125, 593–601. doi:10.1016/j.healthpol.2021.03.006
Schwartz, J. L. (2017). Real-World Evidence, Public Participation, and the FDA. Hastings Cent. Rep. 47, 7–8. doi:10.1002/hast.779
Sharfstein, J. M., Miller, J. D., Davis, A. L., Ross, J. S., McCarthy, M. E., Smith, B., et al. (2017). Blueprint for Transparency at the U.S. Food and Drug Administration: Recommendations to Advance the Development of Safe and Effective Medical Products. J. L. Med Ethics 45, 7–23. doi:10.1177/1073110517750615
Smith, G. (2019). Regulation, Ethics and Reimbursement of Novel Biological Therapies in Australia – an Update. Available at: https://www.tga.gov.au/sites/default/files/presentation-regulation-ethics-and-reimbursement-novel-biological-therapies-australia-update.pdf (Accessed July 23, 2021).
Stokes, R. (2012). Defining the Ideology of Public Participation: “Democracy”, “Devolution”, “Deliberation”, “Dispute Resolution” and a New System for Identifying Public Participation in Planning Law. Macquarie J. Int. Comp. Environ. L. 8, 1–20. doi:10.3316/informit.391108622724494
Sturgis, P. (2014). On the Limits of Public Engagement for the Governance of Emerging Technologies. Public Underst Sci. 23, 38–42. doi:10.1177/0963662512468657
Taylor, L. (2021). Public Actors without Public Values: Legitimacy, Domination and the Regulation of the Technology Sector. Philos. Technol. doi:10.1007/s13347-020-00441-4
Turilli, M., and Floridi, L. (2009). The Ethics of Information Transparency. Ethics Inf. Technol. 11, 105–112. doi:10.1007/s10676-009-9187-9
United Nations (2007). Good Governance Practices for the Protection of Human Rights. Available at: https://www.ohchr.org/Documents/Publications/GoodGovernance.pdf (Accessed July 26, 2021).
Von Tigerstrom, B. (2015). Revising the Regulation of Stem Cell-Based Therapies: Critical Assessment of Potential Models. Food Drug L. J 70, 315.
Wicks, P., Vaughan, T. E., Massagli, M. P., and Heywood, J. (2011). Accelerated Clinical Discovery Using Self-Reported Patient Data Collected Online and a Patient-Matching Algorithm. Nat. Biotechnol. 29(5), 411-414 doi:10.1038/nbt.1837
World Health Organisation. Regional Office for Europe, Health Evidence Network, European Observatory on Health Systems and Policies, Coulter, A., Parsons, S., and Ashkam, J. (2008). Where Are the Patients in Decision-Making about Their Own Care?
Keywords: genome editing, public participation, transparency, accountability, marketing approvals, gene therapy
Citation: Nielsen J, Eckstein L, Nicol D and Stewart C (2021) Integrating Public Participation, Transparency and Accountability Into Governance of Marketing Authorisation for Genome Editing Products. Front. Polit. Sci. 3:747838. doi: 10.3389/fpos.2021.747838
Received: 26 July 2021; Accepted: 23 September 2021;
Published: 15 October 2021.
Edited by:
Alberto Asquer, SOAS University of London, United KingdomReviewed by:
Francesco Mureddu, The Lisbon Council for Economic Competitiveness and Social Renewal, BelgiumKatrien Steenmans, Coventry University, United Kingdom
Copyright © 2021 Nielsen, Eckstein, Nicol and Stewart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jane Nielsen, SmFuZS5uaWVsc2VuQHV0YXMuZWR1LmF1
 Jane Nielsen
Jane Nielsen Lisa Eckstein
Lisa Eckstein Dianne Nicol
Dianne Nicol Cameron Stewart2
Cameron Stewart2