
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 06 March 2025
Sec. Crop and Product Physiology
Volume 16 - 2025 | https://doi.org/10.3389/fpls.2025.1488576
Adequate irrigation with low-quality water, aligned with the specific water requirements of crops, will be critical for the future establishment of cereal crops on marginally fertile soils. This approach is essential to support global food security. To identify suitable cereal species and genotypes for these challenging conditions with the aim of optimizing yield and resilience, three different cereal species were tested under sandy soil conditions at the experimental fields of ICBA (Dubai, UAE). The experimental design employed a factorial combination split-plot arrangement including five primary factors: crop species (barley, triticale and finger millet), genotypes (3 in barley, 3 in triticale and 2 in finger millet), salinity levels (2 and 10 dS m-1), irrigation levels (100%, 150%, and 200% ETo), and planting densities (30 and 50 cm of spacing between rows). Agronomic parameters (e.g. plant height, grain yield, total plant dry weight and harvest index) and physiological parameters [Normalized Difference Vegetation Index (NDVI) readings, together with nitrogen and carbon concentration isotopic composition, chlorophyll, flavonoids, and anthocyanins concentrations in flag leaves and the Nitrogen Balance Index (NBI)] exhibited distinct genotypic responses across the species investigated. Regarding grain yield, salt stress did not impact barley and finger millet, whereas triticale experienced a reduction of nearly one third of its yield. Increased irrigation led to higher grain yields only in barley, while increased planting density significantly improved grain yield across all species examined demonstrating its potential as a simple agronomic intervention. Physiological responses highlighted reduced nitrogen isotope composition under both salt stress and higher planting density in all species. Nevertheless, the response to irrigation varied among species exhibiting significant negative correlations with aerial plant dry matter. In contrast, carbon isotope composition did not display a clear pattern in any of the species studied under different agronomic treatments. These results underscore the importance of selecting salt and drought tolerant species and optimizing planting density to maximize productivity on marginal soils. Future research should focus on refining irrigation strategies and identification of high-performing genotypes to improve cereal cultivation in arid regions, contributing to global food security.
Projections regarding climate change in forthcoming decades portend rises in global temperatures, alongside the increase in frequency and duration of drought periods across various regions. These climatic shifts, coupled with the growing need to implement supplemental irrigation as a strategy to mitigate the adverse effects of climate change in arid regions, are expected to accelerate the reliance on brackish water as an alternative irrigation source. However, this reliance is expected to impede plant growth and reduce agricultural productivity (Minhas et al., 2020; Devkota et al., 2022).
In arid and semi-arid regions, water scarcity and the progressive salinization of irrigation water represent critical constraints on plant productivity (Chamekh et al., 2016). Drought stress induces a wide range of morphological, physiological, biochemical, and molecular changes in both below-ground and above-ground tissues of cereal crops. For example, water deficit can lead to a reduced photosynthetic area and accelerated leaf senescence during the late grain-filling stage, which consequently affects crop yield (Ben Mariem et al., 2021; Toulotte et al., 2022). Accurate irrigation scheduling and precise measurement of crop water requirements are indispensable for effective water management in agriculture. The application of irrigation water based on reference evapotranspiration (ETo) is critical for the conservation of water resources. Adequate fulfillment of crop water requirements would result in enhanced growth and increased yield (Alotaibi et al., 2023; Bashir et al., 2023). Despite these measures, water scarcity in arid regions compels the use of low-quality water sources with high NaCl concentrations as the sole alternative for crop irrigation. Several studies have demonstrated the feasibility of cultivating cereal grains such as barley, triticale, and finger millet in arid and semi-arid regions using brackish water (Hammami et al., 2016; Mukami et al., 2020; Kankarla et al., 2020). Nevertheless, salt stress adversely affects the overall performance of cereal crops by promoting whole-plant senescence and reducing the remobilization and transfer of pre-stored assimilates from vegetative tissues to grains. This results in a decreased grain filling rate, reduced grain weight, and consequently a marked decline in yield (Shahbaz and Ashraf, 2013; Kumar et al., 2022). The levels of salt tolerance vary among cereal species and even among cultivars. Previous studies indicate that barley demonstrates relatively high salinity tolerance, while finger millet and triticale exhibit moderate tolerance to saline conditions (Zeeshan et al., 2020; Mbinda and Mukami, 2021; Mohammadi Alagoz et al., 2023).
Planting density is a critical agronomic practice that significantly influences grain yield and a range of other key agronomic traits in crops (Postma et al., 2021; Cao et al., 2022). Previous research has indicated that the optimal planting density is crop-specific varying according to the species and environmental conditions (Wilke et al., 2021; Niharika et al., 2021; Mohamed, 2023). Furthermore, different cultivars exhibit distinct responses to planting density in terms of productivity and resource use efficiency (Ghazvineh et al., 2020; Zheng et al., 2021). Enhancing planting density and optimizing light utilization represent pivotal strategies for achieving higher crop yields (Zhang et al., 2021; Ming et al., 2017). Nonetheless, augmenting planting density concurrently intensifies intra-specific competition for essential resources such as light, nutrients, and, particularly in arid and semiarid regions, water (MacLaren et al., 2023).
Phenotype can be defined as the set of observable characteristics of an organism, which arise from the complex interplay among its genotype, the environment, and crop management practices (Yang et al., 2020; Großkinsky et al., 2023). The relative contributions of genotype by environment and management interactions contribute to the phenotypic complexity of traits such as yield (Araus et al., 2018, 2023). In modern agriculture, the non-invasive assessment of plant traits such as crop growth, potential photosynthetic capacity, and water status is gaining increasing importance for optimizing crop performance and resource use efficiency (Yang et al., 2017; Vargas et al., 2020).
Incorporating remote sensing methodologies alongside targeted laboratory techniques, such as the analysis of stable isotope signatures, presents a promising avenue for enhancing the predictive efficacy of phenotyping processes under arid and semi-arid conditions (Gracia-Romero et al., 2019; Rezzouk et al., 2020a). Stable carbon and nitrogen isotope compositions in plants offer valuable, time-integrated indicators of their physiological responses and interactions with abiotic and biotic environmental factors (Dong et al., 2022; Rezzouk et al., 2022). Evidence from various studies indicates that abiotic stresses, such as salinity and drought, can result in either an increase or a decrease in the carbon and nitrogen isotope composition of cereal grains (Lopes et al., 2004; Lopes and Araus, 2006; Yousfi et al., 2009, 2012). This isotopic response varies on the plant`s photosynthetic pathway, differing between C3 crops, such as barley and triticale, and C4 crops, such as finger millet (Aranibar et al., 2008; Murphy and Bowman, 2009). The carbon isotope composition (δ13C) serves as a temporally integrated indicator for elucidating the interplay between intracellular and atmospheric carbon dioxide (CO2) ratios (Ci/Ca) attained by the plant throughout the photosynthetic process. The Ci/Ca ratio reflects the equilibrium between stomatal conductance and the photosynthetic activity of the plant (Wallace et al., 2013; Vernooij et al., 2021). The total carbon content in crops represents the assimilation of atmospheric CO2 by plant chloroplasts during the pivotal process of carbon fixation. Similarly, the nitrogen isotope composition (δ15N), in conjunction with the total nitrogen (N) content in plant biomass, serves as a dual indicator elucidating the influence of growing conditions on the intricate nitrogen metabolism of the plant organism (Vicente et al., 2019; Rezzouk et al., 2020b). Nevertheless, the precise mechanisms and functional roles underlying these processes remain incompletely understood (Pritchard and Guy, 2005; Coque et al., 2006).
Cereals constitute a significant proportion of global plant-derived food production and represent a predominant category among harvested crops. Barley is generally used as both food and fodder, whereas triticale and finger millet are widely used as animal feed (Luo et al., 2019; Pour-Aboughadareh et al., 2021; Hussain et al., 2023). In arid and desert regions, where fodder scarcity represents significant challenges, these crops may represent a strategic alternative. Nevertheless, the ongoing impacts of climate change have heightened the influence of abiotic stressors, consequently exacerbating yield attenuation and potentially compromising global food security. Within this paradigm, improvement of agricultural practices and enhancing crop adaptability to water scarcity and rising salinity levels in soils and irrigation water assume pivotal significance (Wang et al., 2018). A comprehensive review of the existing literature reveals a plethora of studies focused on investigating the individual impacts of water deficit, salinity, planting density, and varietal differences on yield performance and physiological responses in barley, finger millet, and triticale cultivation (Soleymani and Shahrajabian, 2011; Hasanuzzaman et al., 2019; Zeeshan et al., 2020; Mukami et al., 2019, 2020; Amulya Manasa and Umesha, 2022; Mohammadi Alagoz et al., 2023; He et al., 2024). Nevertheless, there is a notable gap in the literature regarding the combined effects of these agricultural practices and abiotic stressors on these crops, especially in arid regions. The usual situation under field conditions is the appearance of simultaneous stress, rather than the effect of a single stress. Our research hypothesis proposes that the combined influence of these factors may result in a greater impact on crop productivity and physiological performance compared to the direct effect of each factor independently. Consequently, the primary objective of this study was to ascertain the maximum attainable biomass and grain productivity as well as to discern the key physiological attributes, across various cultivars of barley, finger millet, and triticale. This was achieved by evaluating their performance under diverse management conditions, which incorporated the interactions between different water salinities, irrigation levels, planting densities and multiple genotypes per species.
The experiment was conducted at the field facilities of the International Center for Biosaline Agriculture (ICBA) in Dubai, United Arab Emirates (25°05′49′′ N, 55°23′25′′E). Meteorological data, comprising temperature values and precipitation measurements, were systematically collected from the Dubai International Airport meteorological station (https://meteostat.net/en/station/41194?t=2019-08-01/2019-10-31) and are showed in Supplementary Figure S1. During the experimental period, mean daily temperature values ranged from 15.6°C to 29.7°C, while the mean daily rainfall ranged from 0 to 11.7 mm. The soil characteristics at ICBA’s experimental fields were predominantly sandy, with a fine sand composition of 98%, minimal silt (1%), and clay content (1%). The soils were calcareous, with calcium carbonate equivalents ranging from 50 to 60%, highly porous (45% porosity), and moderately alkaline, with a pH of 8.22. Organic matter content was notably low, measuring less than 0.5%. The soil exhibited a saturation percentage of 26, indicative of a high drainage capacity, and an electrical conductivity of the saturated extract (ECe) of 1.2 dS m-1. According to the American Soil Taxonomy system (Soil Survey Staff, 2010), the soil type was classified as Typic Torripsamments, characterized by its carbonate-rich nature and hyperthermic conditions (Shahid et al., 2009). To meet crop nutrient requirements, organic fertilizer (N 1.5%, K 1.65%, Na 1.22%, pH 7.7, C/N ratio 16.5, organic matter 41% and moisture content 1.64%) was applied at a rate of 30 t ha-1, incorporated into the soil. Sowing was performed manually on November 29 and 30, 2016. During the subsequent two weeks, drip irrigation using fresh water with an electrical conductivity of 1 dS m-1, was applied to ensure unimpeded germination processes, as recommended by Koyro and Eisa (2008). Following this establishment phase, the salt and irrigation treatments were initiated and maintained throughout the crop cultivation cycle.
The experimental design employed a factorial combination split-plot arrangement with three replications per treatment, including five primary factors: crop species, genotypes, salinity levels, irrigation levels, and planting densities. Salinity and irrigation manipulations were administered via the Supervisory Control and Data Acquisition (SCADA) system. Salinity levels were categorized into two conditions: S1 denoting low electrical conductivity (EC) levels ranging from 1-2 dS m-1, and S2 corresponding to high EC levels ranging from 8-10 dS m-1. Irrigation levels were divided into three categories: I1 supplying 100% of the reference evapotranspiration (ET0), I2 supplying 150% ET0, and I3 supplying 200% ET0. Planting densities were specified as D1, with 30 cm row spacing, and D2 with 50 cm row spacing. Within the barley genotype category, three accessions were evaluated: C1 (N2-35), C2 (N2-4), and C3 (IPA7). Similarly, three triticale accessions were examined: C1 (PI388678), C2 (PI4295152), and C3, a variety originating from Jordan and sourced from Syria. Additionally, two finger millet genotypes were included in the study: C1 and C2, both representing local cultivars from Yemen. The selection of these genotypes was based on the germplasm resources accessible in ICBA and cultivated in the region.
The plot dimensions were set at 2 × 2 m, with a plant-to-plant distance of 25 cm and inter-row spacing set at either 30 or 50 cm, depending on the planting density treatments. Drip irrigation was employed throughout the experimental period, with drippers positioned at 25 cm intervals. The discharge rate from each dripper was calibrated to 4 L h-1 per plant. The irrigation period varied based on climatic conditions and the crop development stage, ranging from the full tillering to the dough-making stage. Manual weeding practices were implemented throughout the entire crop cycle as required, without the use of herbicidal agents. To mitigate grain losses during the grain filling period, a net with a mesh size of approximately 15 × 15 mm2 was installed to prevent the entry of small birds.
Yield and biomass parameters were evaluated using five randomly selected plants harvested from the central row of each plot upon reaching grain physiological maturity (third week of March). Plant height was recorded using a ruler, while the number of stems and spikes were counted directly. After threshing the plants, grain yield was evaluated. The determination of dry weight for stems, spikes and total plant biomass, entailed an initial sun-drying period of two days, followed by a subsequent drying in a forced-air oven at 80°C for 48 h. The harvest index (HI) was calculated as the ratio of grain yield to total plant dry weight.
Physiological measurements and sampling for the three crops species were conducted during the period between heading and early anthesis.
Canopy reflectance measurements were conducted utilizing a GreenSeeker hand-held Optical Sensor (Ntech Industries, Inc., Ukiah, CA, USA). This sensor is specifically designed to determine the Normalized Difference Vegetation Index (NDVI) by employing its proprietary light source. The NDVI calculation is based on the spectral reflectance measurements obtained at red (660 nm) and near-infrared (770 nm) wavelengths following the equation reported by Tucker and Sellers (1986). The NDVI is computed using the following Equation 1:
where NIR and VIS denote the near infrared and visible red wavelengths, respectively.
The GreenSeeker device recorded a range of 10-15 NDVI counts per plot. These counts were subsequently averaged within each plot, resulting in a singular value representative of the vegetation therein. Measurement acquisition occurred near solar noon, between 11 a.m. and 1 p.m. Data collection occurred on January 19 and February 1, 2017 (referred to as NDV1 and NDVI2, respectively) to evaluate the potential differences between two different crop phenological stages of the experimental study.
Leaf pigment concentrations were assessed in the flag leaf of the three species using a portable leaf-clip sensor (Dualex, Dualex Force-A, Orsay, France). The Dualex sensor enables non-destructive determinations of chlorophyll (Chl, in µg cm-²), flavonoid (Fla, dimensionless index), and anthocyanin (Anth, dimensionless index) concentration, leveraging chlorophyll fluorescence excitation spectra (Cerovic et al., 2012). Additionally, this sensor computes the nitrogen balance index (NBI), representing the Chl/Flav ratio in relation to nitrogen and carbon allocation dynamics (Cerovic et al., 2015). Following the experimental protocol, ten recently fully expanded (i.e., non-senescent) leaves were chosen from the central rows of each plot. Measurements were conducted on the adaxial surface of the leaves, with data acquisition taking place on January 19, 2017.
The leaves used for pigment content quantification underwent successive washing cycles with both tap and distilled water. They were then desiccated in an oven set at 60°C for a duration of two days. Once dried, the leaves were finely ground into an uniform powder. A subsample of the dried leaf powder was used to determine total carbon and nitrogen concentrations, as well as the stable isotopic signatures of carbon (13C/12C ratio) and nitrogen (15N/14N ratio). These analyses were conducted at the Scientific Facilities of the University of Barcelona. Approximately 1 mg of subsample was weighed into tin capsules and the analyses were carried out through an elemental analyzer (Flash 1112 EA; ThermoFinnigan, Schwerte, Germany) integrated with an isotope ratio mass spectrometer (Delta C IRMS, ThermoFinnigan), which operated in continuous flow mode. The 13C/12C ratios (R) of leaf material were represented in δ notation (Coplen, 2008), denoted in per mil (‰), with the sample denoting the leaf plant material and the standard representing Pee Dee Belemnite (PDB) calcium carbonate. International isotope secondary standards with established 13C/12C ratios (IAEA CH7 3, polyethylene foil; IAEA CH6, sucrose; USGS 40, l-glutamic acid) were employed, ensuring an analytical precision of 0.1‰. The identical δ notation convention was applied for expressing the 15N/14N ratio, with the standard referencing N2 in air (Coplen, 2008). For nitrogen, international isotope secondary standards (IAEA N1, IAEAN2, IAEANO3, and USGS40) were utilized, maintaining a precision of 0.3‰. The nitrogen and carbon content in leaves were expressed as percentages (%).
The carbon and nitrogen isotopic compositions, denoted as δ13C and δ15N respectively, were expressed utilizing the following notation according to Coplen (2008) (Equation 2).
where δ13C and δ15N represents the ratios of isotopes 13C/12C and 15N/14N in the sample, respectively, both expressed in ‰. Meanwhile, R standard denotes the molar abundance ratio of the secondary standard calibrated against the primary standard.
Analysis of variance (ANOVA) was conducted using the statistical software Statgraphics Centurion XVI (Statpoint Technologies, Inc. Warrenton, VA, USA) to scrutinize the impacts of the following factors across diverse species: genotypes, irrigation treatments, salt treatments, planting density, and their respective interactions. Means were compared based on the Least Significant Difference (LSD) test at the 5% probability level. Additionally, a bivariate correlation analysis was carried out using the same software to compute Pearson correlation coefficients among the analytic traits.
In evaluating agronomic parameters in relation to genotype variability, there were clear differences among the examined barley cultivars. Notably, the barley cultivar C1 (N2-35) showed superior performance, recording the highest values for spike dry weight and total plant dry weight. In contrast, barley C2 (N2-4) exhibited the highest harvest index among the genotypes assessed. Under conditions of increasing saline concentrations, no significant changes in the agronomic parameters of barley plants were detected. Nevertheless, an analysis of irrigation rates revealed that the supply of the lowest irrigation rate, (I100), resulted in reduced both grain yield and harvest index. Furthermore, the evaluation of planting density indicated that higher planting densities were generally associated with improvements across agronomic parameters, except for the harvest index, which showed a notable reduction (Table 1).
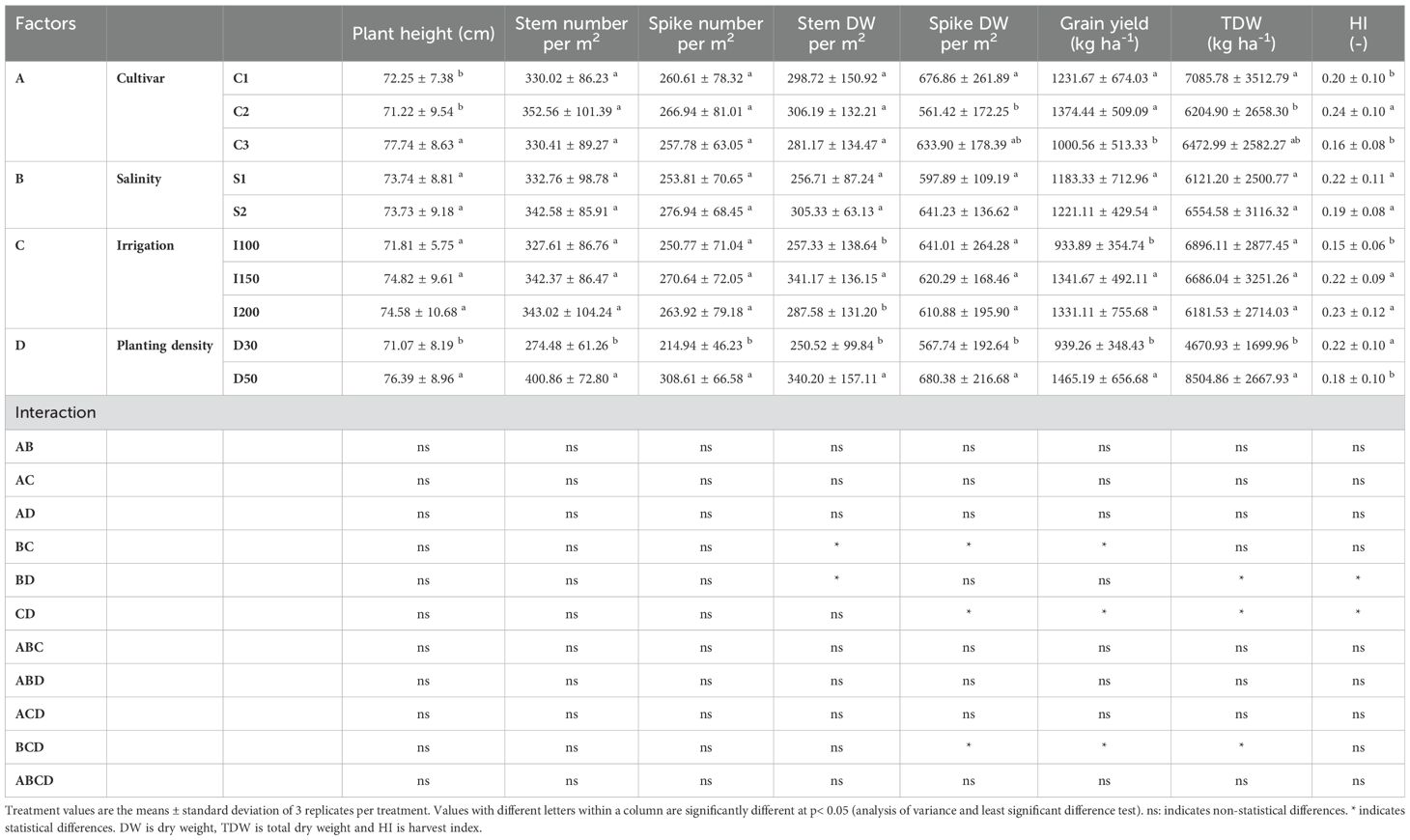
Table 1. Effects of cultivar (C1:; C2:; C3): salinity levels [S1: low electrical conductivity (EC) (1-2 dS m-1), S2: high EC (8-10 dS m-1)], irrigation levels (I1: 100% ET0, I2: 150% ET0 and I3: 200% ET0), and planting density (D1: 30 cm and D2: 50 cm of space between rows) on biomass and yield parameters in barley plants over the experimental period.
In evaluating physiological parameters across different barley genotypes, there were no clear differences, except for barley cv. C3 (IPA-7), which exhibited the highest NDVI1 readings. Under increasing salinity levels, discernible trends in physiological parameters were noted. Specifically, NDVI (1 and 2) readings and δ15N exhibited a decreasing trend. Conversely, salinity was positively correlated with increased N, δ13C, chlorophyll (Chl) and anthocyanin (Anth) concentration, and the nitrogen balance index (NBI). The impact of irrigation rates reported that the lowest irrigation rate (I100) resulted in increased N, δ13C, Chl concentration, and NBI. In contrast, the highest irrigation rate (I200) was associated with elevated NDVI (1 and 2) readings but resulted in a reduction in C concentration. An exploration of planting density revealed multifaceted effects on physiological parameters. Specifically, higher planting densities increased NDVI (1 and 2) readings, N and Chl concentration, and NBI. Nevertheless, δ15N showed an opposite trend, with its levels decreasing as planting density increased (Table 2).
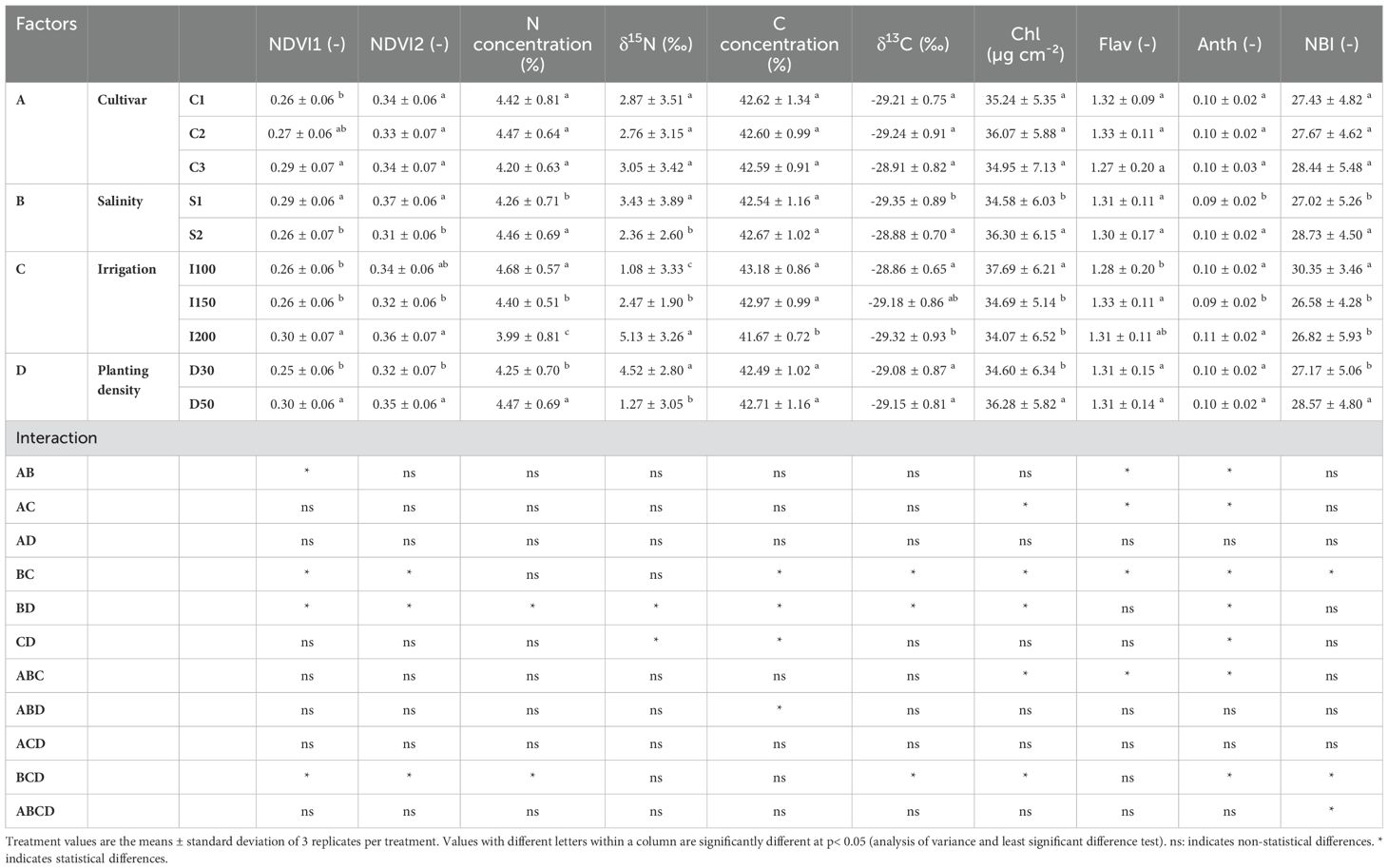
Table 2. Effects of cultivar (C1:; C2:; C3): salinity levels [S1: low electrical conductivity (EC) (1-2 dS m-1), S2: high EC (8-10 dS m-1)], irrigation levels (I1: 100% ET0, I2: 150% ET0 and I3: 200% ET0), and planting density (D1: 30 cm and D2: 50 cm of space between rows) on physiological parameters in barley plants over the experimental period.
The analysis of correlations across all barley genotypes revealed significant positive correlations in stem number/spike number (r = 0.81), NDVI reading 1/NDVI reading 2 (r = 0.80), and total plant dry weight/spike dry weight (r = 0.75). Conversely, the strongest negative correlation was observed between N concentration and δ15N with a correlation coefficient of r = -0.76 (Figure 1). A more detailed analysis for each genotype is provided in the Supplementary Material (Supplementary Figures S2–S4).

Figure 1. Correlation between agronomic and physiological parameters in barley. GY, grain yield; TDW, total plant dry weight; HI, harvest index; PH, plant height; StN, stem number; SpN, spike number; StDW, stem dry weight; SpDW, spike dry weight; NDVI1, greenseeker reading 1; NDVI2, greenseeker reading 2; N, total nitrogen; δ15N, stable nitrogen isotope composition; C, total carbon; δ13C, stable carbon isotope composition; Chl, Chlorophyll; Flav, flavonoid; Anth, anthocyanin; NBI, Nitrogen Balance Index. Pearson correlation coefficient (r) is represented using a color gradient, where deep red corresponds to r=1, white indicates r=0, and deep blue represents r=-1.
In the evaluation of agronomic parameters within the context of genotype variability, significant findings were observed, particularly in triticale cv. C2 (PI429152), which showed the lowest grain yield and harvest index among the genotypes studied. Under increasing saline concentrations, a discernible pattern emerged where only stem dry weight increased, while other agronomic parameters declined. The analysis of irrigation rates revealed different patterns in the agronomic performance of triticale plants. Notably, the lowest irrigation rate (I100) was associated with reductions in spike dry weight and harvest index. In contrast, the medium irrigation rate (I150) resulted in heightened spike number, stem dry weight, total plant dry weight, and grain yield. Furthermore, an exploration of planting density elucidated notable trends in agronomic parameters. Higher planting densities were linked to increases in all parameters studied. However, this increase in performance was accompanied by a decline in harvest index (Table 3).
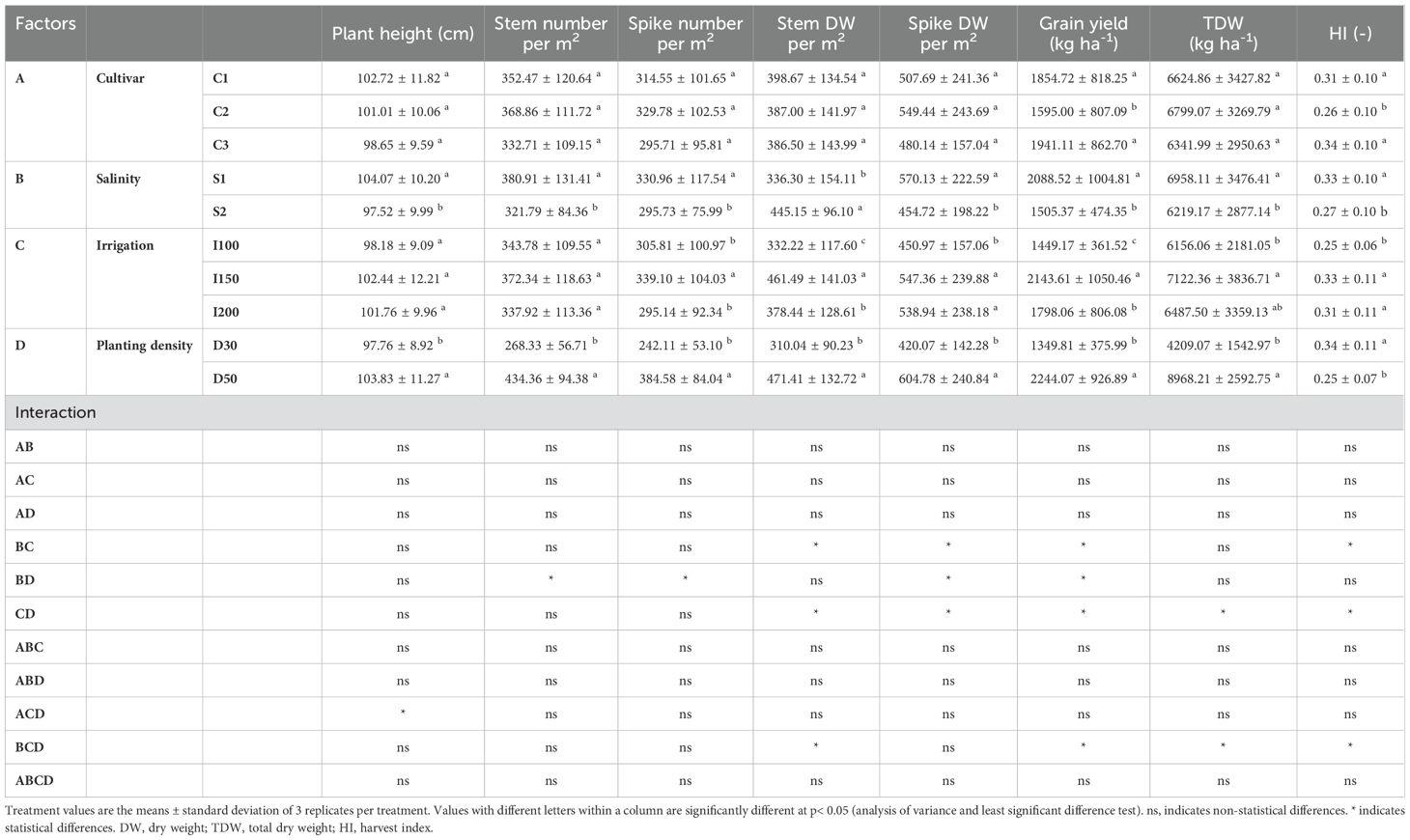
Table 3. Effects of cultivar (C1:; C2:; C3): salinity levels [S1: low electrical conductivity (EC) (1-2 dS m-1), S2: high EC (8-10 dS m-1)], irrigation levels (I1: 100% ET0, I2: 150% ET0 and I3: 200% ET0), and planting density (D1: 30 cm and D2: 50 cm of space between rows) on biomass and yield parameters in triticale plants over the experimental period.
The assessment of physiological parameters among various triticale cultivars reported clear differences, indicative of genotype-specific physiological attributes. For instance, triticale cv. C1 (PI388678) displayed the highest flavonoid concentration among the examined cultivars. Conversely, triticale Jordan, originating from Syria (C3) showed the lowest NDVI (1 and 2) readings and the highest anthocyanin concentration. Upon exposure to higher salinity levels, clear trends emerged in physiological parameters, highlighting the effects of salinity stress. Specifically, NDVI (1 and 2) readings, and δ15N exhibited a decreasing trend, indicative of salinity-induced stress. In contrast, N and C concentrations increased under saline conditions. Analysis of irrigation rates revealed differential impacts on physiological parameters. The lowest irrigation rate (I100) was linked with reduced NDVI 1 readings, coupled with elevated N and C concentration, δ13C, and nitrogen balance index (NBI) values. In contrast, the highest irrigation rate (I200) resulted in elevated flavonoids and anthocyanin concentrations. Furthermore, an examination of planting density highlighted differential trends in physiological parameters. Higher planting densities were associated with increased NDVI (1 and 2) readings, N and C concentrations. However, this increase in density was accompanied by a decrease in chlorophyll concentration (Chl) and δ15N (Table 4).
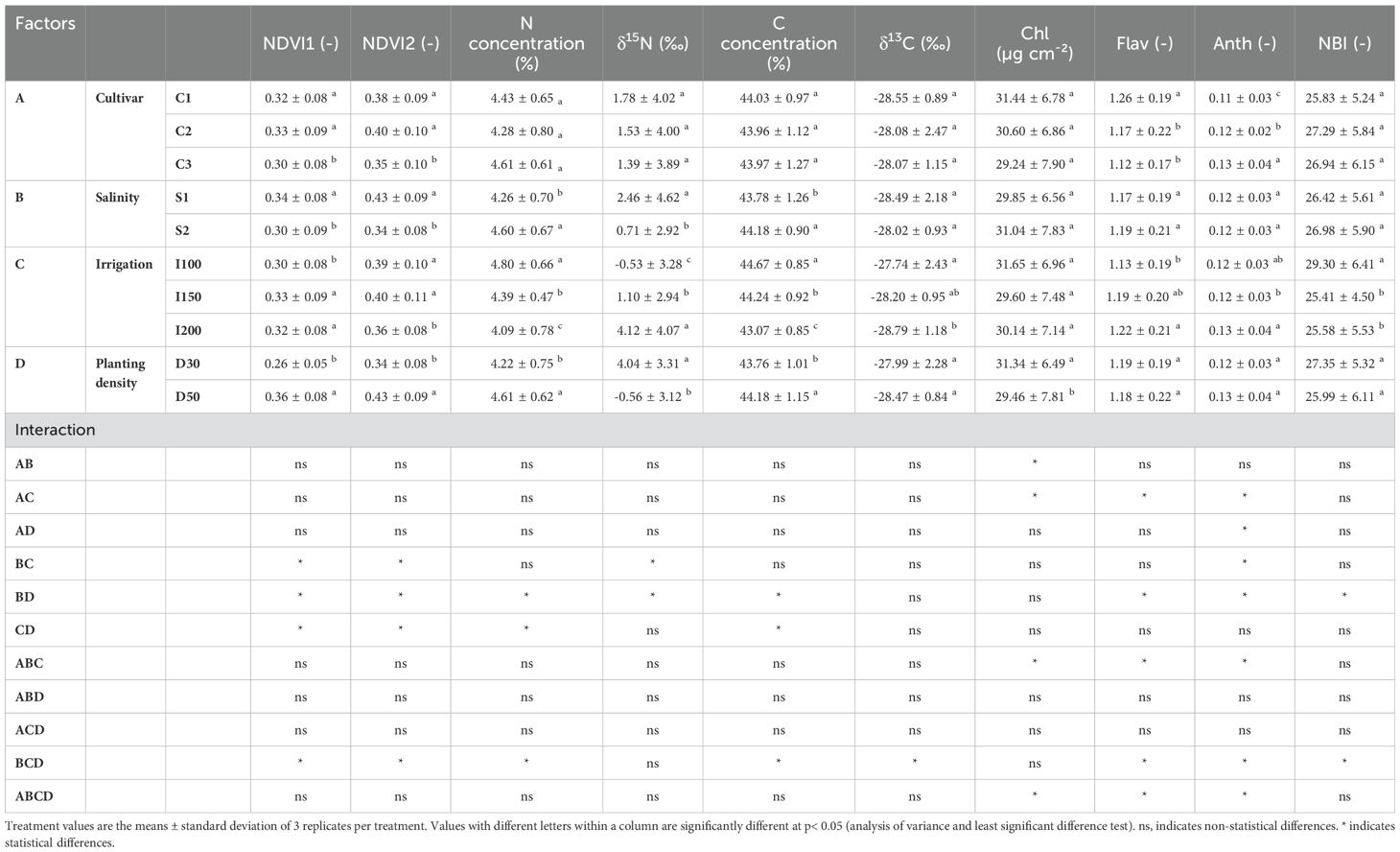
Table 4. Effects of cultivar (C1:; C2:; C3): salinity levels [S1: low electrical conductivity (EC) (1-2 dS m-1), S2: high EC (8-10 dS m-1)], irrigation levels (I1: 100% ET0, I2: 150% ET0 and I3: 200% ET0), and planting density (D1: 30 cm and D2: 50 cm of space between rows) on physiological parameters in triticale plants over the experimental period.
The analysis of correlations across all triticale genotypes revealed significant positive correlations in total plant dry weight/harvest index (r = 1), grain yield/NDVI reading 1 (r = 1), and spike number/stem number (r = 0.97). Conversely, the strongest negative correlation was observed between C concentration/δ13C with a correlation coefficient of r = -0.76 (Figure 2). A more detailed analysis for each genotype is provided in the Supplementary Material (Supplementary Figures S5–S7).
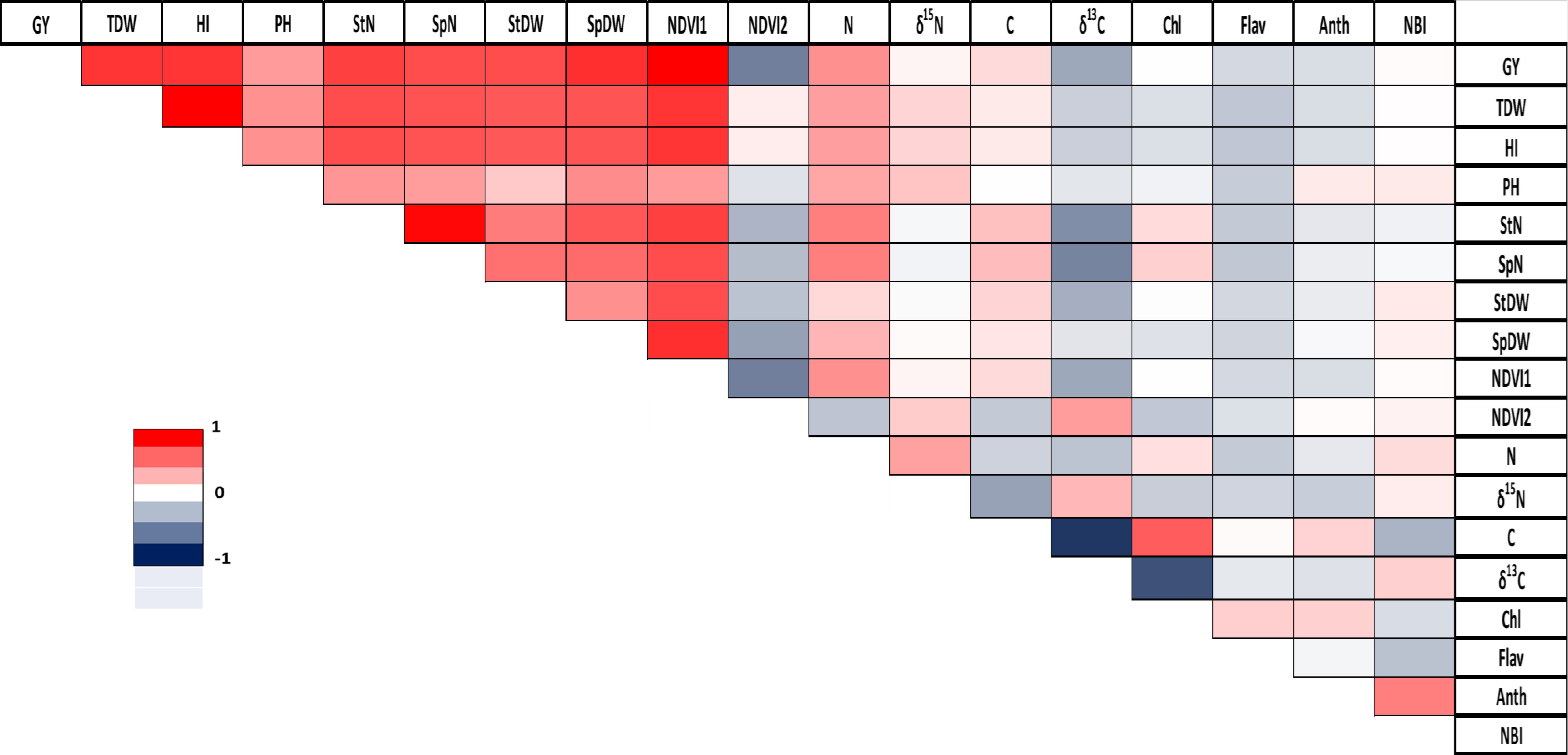
Figure 2. Correlation between agronomic and physiological parameters in triticale. GY, grain yield; TDW, total plant dry weight; HI, harvest index; PH, plant height; StN, stem number; SpN, spike number; StDW, stem dry weight; SpDW, spike dry weight; NDVI1, greenseeker reading 1; NDVI2, greenseeker reading 2; N, total nitrogen; δ15N, stable nitrogen isotope composition; C, total carbon; δ13C, stable carbon isotope composition; Chl, Chlorophyll; Flav, flavonoid; Anth, anthocyanin; NBI, Nitrogen Balance Index. Pearson correlation coefficient (r) is represented using a color gradient, where deep red corresponds to r=1, white indicates r=0, and deep blue represents r=-1.
The evaluation of agronomic parameters across distinct finger millet cultivars did not reveal significant differences in the parameters assessed. However, under increasing salinity levels, a consistent trend emerged wherein plant height, spike dry weight, and harvest index decreased, while stem dry weight increased. Analysis of irrigation rates revealed that the lowest irrigation rate (I100) resulted in the highest spike dry weight; however, it was also associated with the lowest values for both stem dry weight and harvest index. Furthermore, an exploration of planting density indicated a general augmentation across all parameters studied, except for the harvest index, which decreased with higher planting density (Table 5).
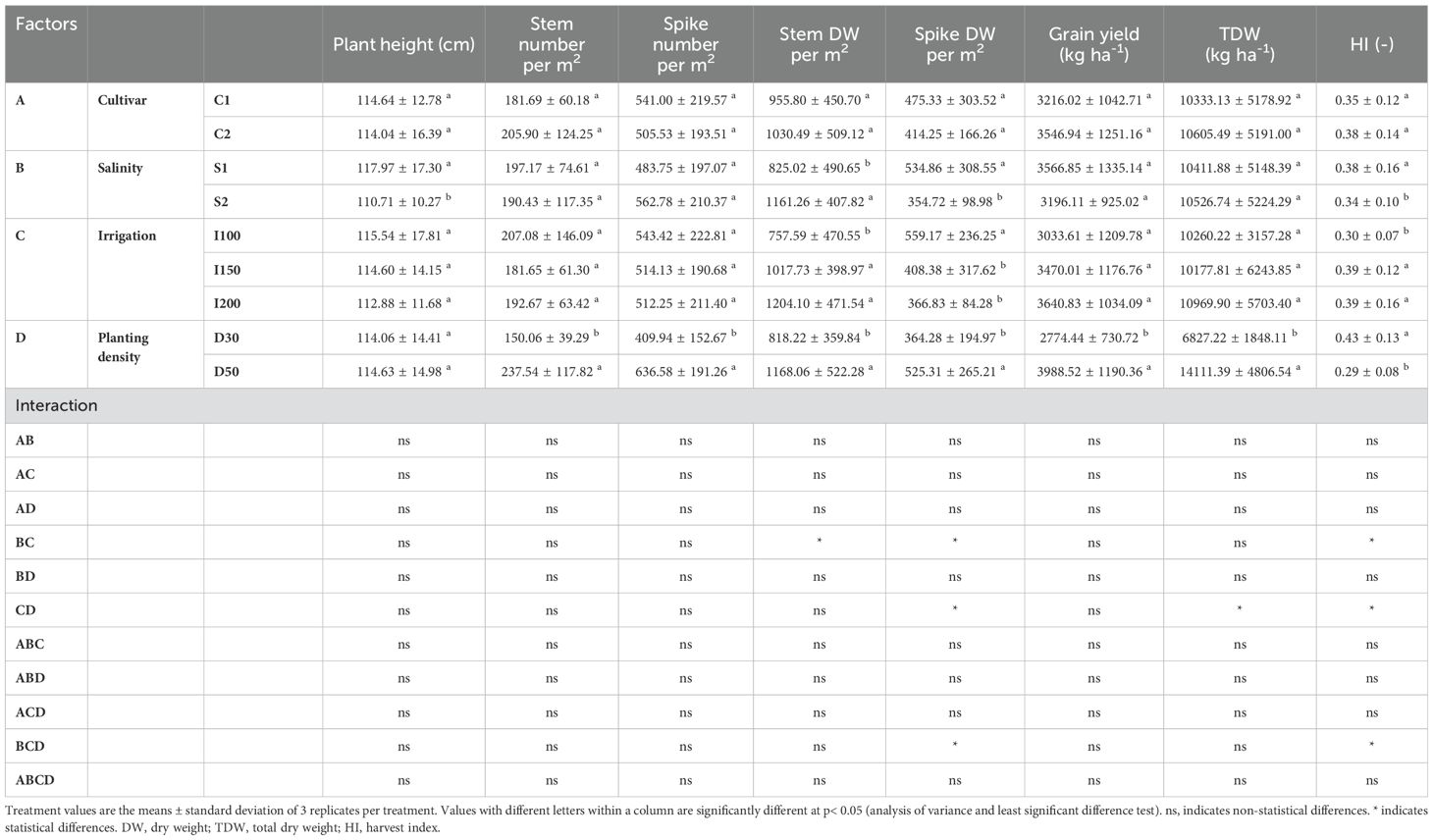
Table 5. Effects of cultivar (C1:; C2): salinity levels [S1: low electrical conductivity (EC) (1-2 dS m-1), S2: high EC (8-10 dS m-1)], irrigation levels (I1: 100% ET0, I2: 150% ET0 and I3: 200% ET0), and planting density (D1: 30 cm and D2: 50 cm of space between rows) on biomass and yield parameters in finger millet plants over the experimental period.
The assessment of physiological parameters across different genotypes of finger millet revealed distinct genotype-specific differences, highlighting the unique physiological attributes of each cultivar. Notably, finger millet cultivar 2 exhibited the highest concentrations of chlorophyll (Chl) and flavonoids (Flav), while showed the lowest levels of anthocyanins (Anth) among the two tested cultivars. As salinity levels increased, NDVI (1 and 2) readings, and δ15N showed a significant reduction, while leaf nitrogen concentration increased. Analysis of irrigation regimes revealed different patterns on physiological parameters. The lowest irrigation rate (I100) was associated with elevated N concentration, Chl concentration, Flav concentration, and nitrogen balance index (NBI). On the other hand, the highest irrigation rate (I200) led to decreased NBI values and increased δ15N and Anth concentrations. Furthermore, an examination of planting density elucidated clear trends in physiological parameters. Higher planting densities were associated with increased NDVI 1 readings, while concurrently resulting in decreased δ15N (Table 6).
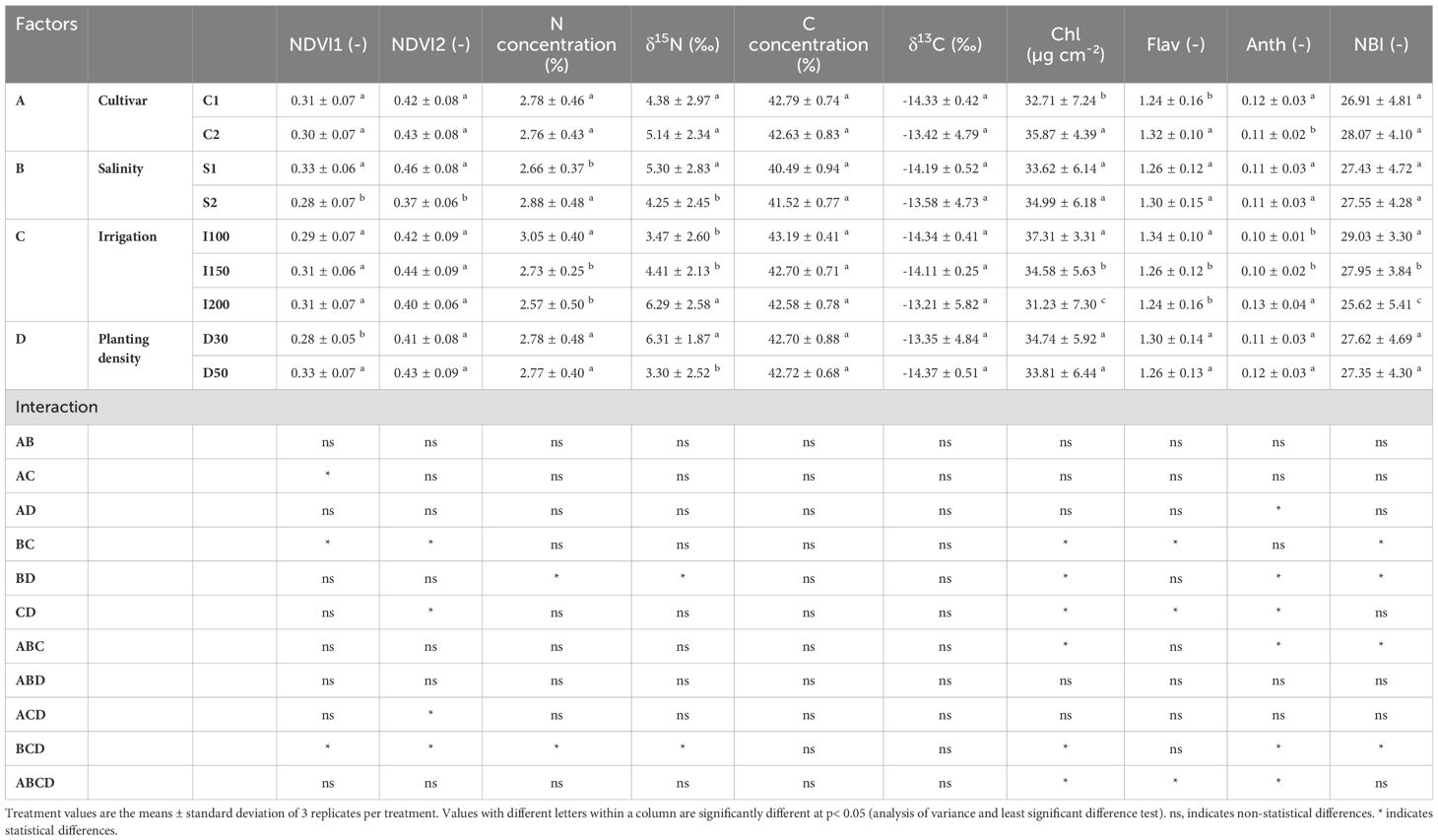
Table 6. Effects of cultivar (C1:; C2): salinity levels [S1: low electrical conductivity (EC) (1-2 dS m-1), S2: high EC (8-10 dS m-1)], irrigation levels (I1: 100% ET0, I2: 150% ET0 and I3: 200% ET0), and planting density (D1: 30 cm and D2: 50 cm of space between rows) on physiological parameters in finger millet plants over the experimental period.
The analysis of correlations across all finger millet genotypes reveals significant positive correlations in Chl/NBI (r = 0.83) and density/total plant dry weight (r = 0.72). Conversely, the strongest negative correlation was observed between Chl/Anth with a correlation coefficient of r = -0.88 (Figure 3). A more detailed analysis for each genotype is provided in the Supplementary Material (Supplementary Figures S8, S9).
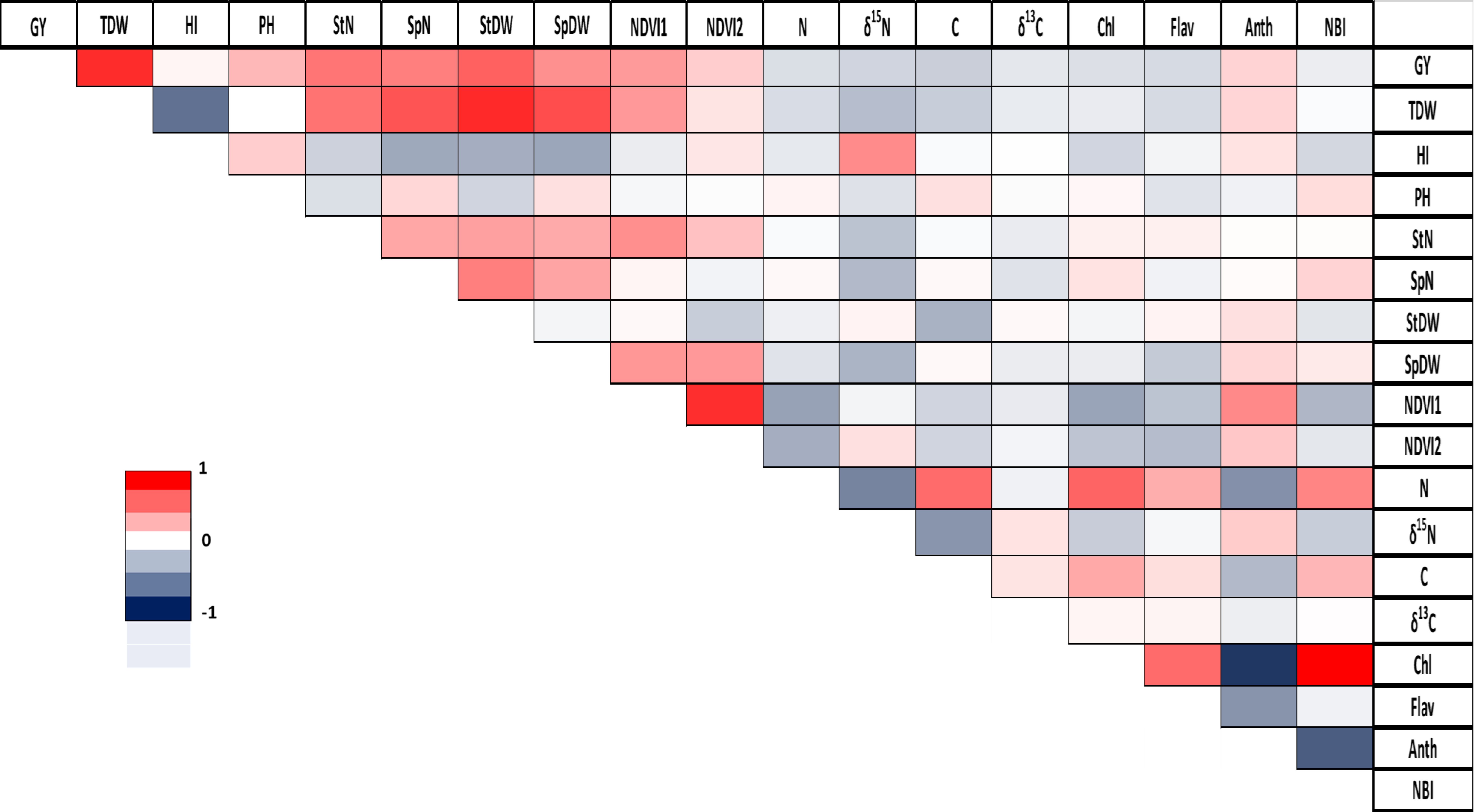
Figure 3. Correlation between agronomic and physiological parameters in finger millet. GY, grain yield; TDW, total plant dry weight; HI, harvest index; PH, plant height; StN, stem number; SpN, spike number; StDW, stem dry weight; SpDW, spike dry weight; NDVI1, greenseeker reading 1; NDVI2, greenseeker reading 2; N, total nitrogen; δ15N, stable nitrogen isotope composition; C, total carbon; δ13C, stable carbon isotope composition; Chl, Chlorophyll; Flav, flavonoid; Anth, anthocyanin; and NBI, Nitrogen Balance Index. Pearson correlation coefficient (r) is represented using a color gradient, where deep red corresponds to r=1, white indicates r=0, and deep blue represents r=-1.
The analysis of the correlation between total plant dry weight (TDW) and grain yield (GY) revealed a strong positive relationship across all species examined, with triticale displaying the highest correlation value (r = 0.74). Furthermore, the study identified a negative correlation between nitrogen isotope composition and total plant dry weight in both barley and triticale. Conversely, no significant correlation was observed between carbon isotope composition and total plant dry weight across the species studied (Figure 4).
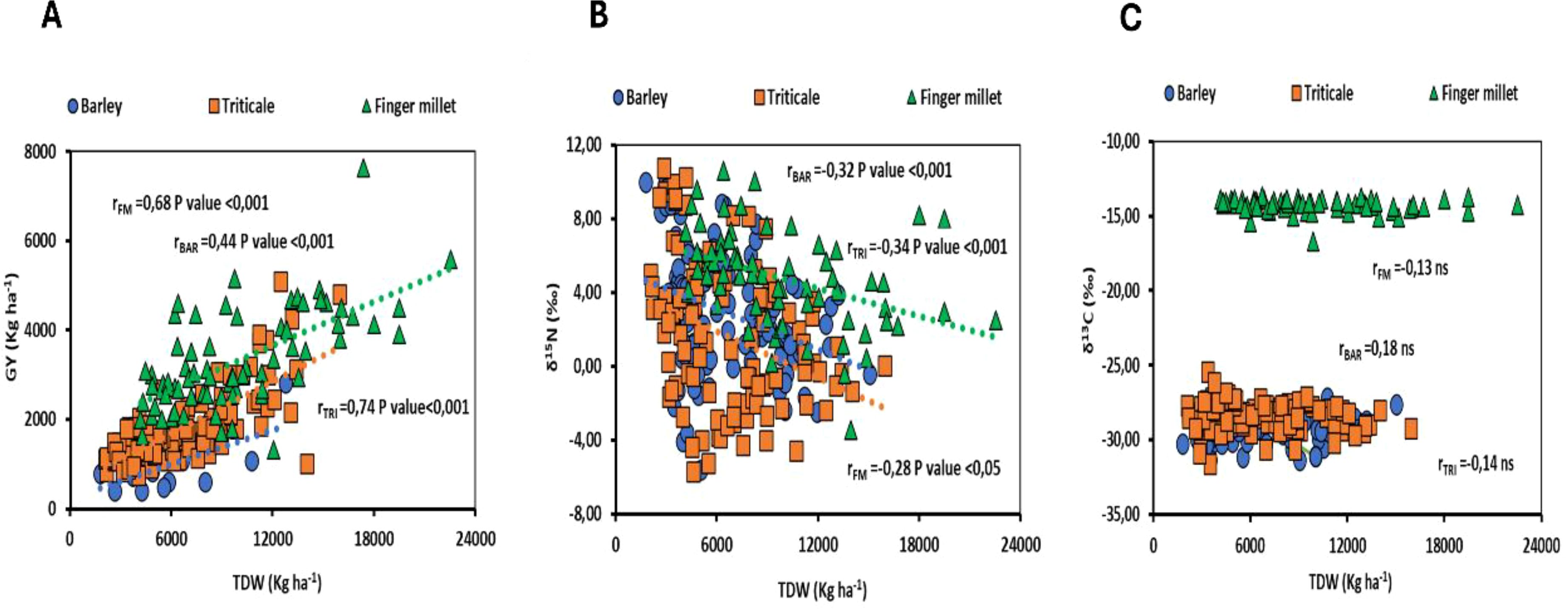
Figure 4. (A) Relationship between grain yield (GY) and total plant dry weight (TDW), (B) Relationship between nitrogen isotope composition (δ15N) and total plant dry weight (TDW) and (C) Relationship between carbon isotope composition (δ13C) and total plant dry weight (TDW). The figures presented encompass data from all genotypes, analyzed across varying levels of salinity, irrigation, and planting density within the studied species. Statistical significance at P<0.001, P<0.01, P<0.05 and ns (non-statistically significant).
There were clear genotypic effects on agronomic traits evaluated in barley plants. Among the cultivars evaluated, N2-35 demonstrated superior agronomic performance, particularly in terms of grain yield (GY) and total plant dry weight (TDW). In our experiment, increasing salinity levels did not affect any of the agronomic parameters assessed in barley. Contrarily, other researchers have reported a significant decline in several agronomic parameters such as the number of spikes per m2 and grain yield under saline conditions (Hessini et al., 2015). Such discrepancies can likely be attributed to variations in the electrical conductivity of irrigation water used and the drainage capacities of the soil in which the experiments were conducted. In contrast, water deficit in our study resulted in a notable reduction in grain yield. Although barley is considered one of the most resilient cereal crops under moderate water deficit conditions (Robredo et al., 2007; Sanchez-Díaz et al., 2002), water stress has been shown to significantly reduce yield (Bahadur et al., 2013; Shrief and Abd-El-Mohsen, 2014; Pardo et al., 2022). It is important to note that, beyond water availability, other factors such as vapor pressure deficit (VPD), temperature and radiation can also affect yield performance in barley and other cereal crops (Van Ittersum et al., 2013; Dreccer et al., 2018). Both water deficit and salt stress impose detrimental effects on grain yield by reducing photosynthesis, restricting cell growth, limiting leaf expansion, and decreasing transpiration (Sabagh et al., 2019). In our experiment, heightened planting densities were associated with an overall improvement across agronomic parameters, consistent with findings from previous studies (Soleymani et al., 2011). Nevertheless, contrasting trends have been reported in other studies (Soleymani and Shahrajabian, 2011; Ertekin, 2022), highlighting the complex interplay of factors influencing barley performance under varying environmental and management conditions.
From a physiological perspective, the growth of barley plants under saline conditions resulted in increased δ¹³C values, consistent with findings previously reported by Araus et al. (2021). This enrichment in δ¹³C is likely associated with stomatal closure, which reduces the ratio of intercellular to atmospheric CO2 concentration (Ci/Ca) (Munns and Tester, 2008). Furthermore, this phenomenon can be directly associated with the activity of Rubisco, the primary enzyme in carbon fixation, which exhibits altered discrimination against 13C under conditions of reduced CO2 availability (Abdulbaki et al., 2022). In addition, the observed increases in total chlorophyll and NBI may be attributed to the effects of salinity, which induces the development of thicker or more compact leaves. This response, however, may be interpreted as a negative reaction to stress, as similarly observed in quinoa plants by Rezzouk et al. (2020b). Anthocyanin levels also increased under salt stress, consistent with the findings reported by Mansour (2023). Moreover, the reduction in nitrogen isotope composition values observed under saline conditions may be attributed to the well-documented antagonism between chloride and nitrate (Corrado et al., 2020). This reduction in nitrogen concentration, which can adversely impact crop yield due to its critical role in plant growth and development can be mitigated with the application of nitrogenous fertilizers.
Barley plants exposed to the lowest irrigation levels exhibited reduced NDVI readings (1 and 2) alongside an increase in carbon concentration. The observed increase in carbon (C) concentration under water stress conditions in our experiment aligns with the findings reported by Rezzouk et al. (2022). The reduction in NDVI readings under water stress is likely due to its negative impact on green aboveground biomass (AB), as previously documented by Aparicio et al. (2000, 2004). The assessment of aboveground biomass (AB) is crucial for monitoring crop growth, as it can indicate the effects of various stresses on crop development (Araus et al., 2008; Elazab et al., 2015).
The observed increases in NDVI readings, chlorophyll concentration, nitrogen concentration, and Nitrogen Balance Index (NBI) in response to higher planting densities can be interpreted as a compensatory mechanism to mitigate the effects of reduced light distribution within the crop canopy (Ming et al., 2017). This reduction in light interception likely stimulates the accumulation of photosynthetic pigments, enabling the plants to maintain optimal photosynthetic efficiency suboptimal light conditions. As a result, this adaptation is reflected in the intensified green coloration of the leaves and an elevated nitrogen content (Paradiso and Proietti, 2022). The strongest positive correlations between agronomic parameters, such as stem number/spike number as well as total plant dry weight/spike dry weight, are expected, as these traits are intrinsically linked to the successful progression of various growth stages in barley plants.
There were no significant genotypic effects on most of the agronomic traits assessed in triticale cultivars. Increasing electrical conductivity in the irrigation water resulted in a marked decline in agronomic parameters observed in triticale except for stem dry weight. These findings align with the established moderate tolerance of this species to salt stress and are consistent with previous research on its salinity responses (Karim et al., 1993; Mohammadi Alagoz et al., 2023). Triticale’s reduced performance under salinity stress arises from both physiological constraints (osmotic imbalance, ion toxicity, oxidative stress) and genetic limitations (weaker expression of salt-tolerance genes, incomplete inheritance of rye’s resilience) (Blum, 2014). The development of triticale cultivars with improved salt tolerance has the potential to significantly enhance this trait, thereby contributing to increasing yield. The highest agronomic performance was recorded in triticale plants subjected to an intermediate irrigation rate. This observation is consistent with prior literature, which highlights the positive impact of increased irrigation, particularly under arid conditions, on triticale agronomic performance (Yusuf et al., 2023). In addition, higher planting densities resulted in improvements across all assessed agronomic parameters, supporting findings from previous experiments on triticale under varying planting densities (Giunta and Motzo, 2004; Mendoza-Elos et al., 2011). However, it is important to note that these results may be influenced by season, cultivar, and site-specific factors. Enhanced agronomic performance under higher planting densities is beneficial, as it indicates that closely spaced plants have a better performance using available resources such as water and nutrients, moreover than incoming radiation, therefore maximizing land use efficiency.
From a physiological perspective, irrigation with saline water led to reductions in NDVI readings and δ15N values. The observed decline in NDVI readings is likely associated with reduced photosynthetic efficiency under saline conditions, which induces pigment photo-oxidation (Stefanov et al., 2021). Photo-oxidation can impair the functionality of the photosynthetic machinery, thereby affecting plant performance. Additionally, salinity disrupts nitrogen uptake, assimilation, release, and internal recycling processes, contributing to the observed decrease in plant δ15N (Cernusak et al., 2009), as evidenced in our experiment. The observed increase in nitrogen and carbon concentrations in triticale plants under salt stress may be attributed to the synthesis of carbon-rich secondary compounds, such as phenolics and lignin, as well as nitrogen-rich metabolites, including amino acids (Hurtado et al., 2020). These metabolic shifts are likely adaptive responses designed to mitigate the adverse effects of soil salinity. Furthermore, the observed increase in flavonoid and anthocyanin levels in leaves under the highest irrigation rate could be associated with the protective role that these compounds play in mitigating damage to photosystem II caused by excessive water irrigation (Agati et al., 2021; Sperdouli et al., 2021).
Higher planting densities in triticale plants were associated with increased NDVI (1 and 2) readings, as well as higher %N and %C content, reflecting enhanced physiological activity and resource acquisition within densely populated crop stands (Schulze and Chapin, 1987). However, this increase in density was accompanied by a decrease in chlorophyll concentration (Chl) and δ15N, highlighting a trade-off between resource utilization and physiological performance under intensified intra-specific competition (Auer et al., 2020). The reduced δ15N values suggest that diminished access to nitrogen sources, driven by interspecific competition, resulted in decreased nitrogen availability. This limitation adversely impacted chlorophyll synthesis, thereby resulting in a decline in chlorophyll concentration.
The strong positive correlations, with coefficients approaching 1, observed between total plant dry weight and harvest index, as well as between spike number and stem number, underscore the uniformity in growth patterns among triticale plants subjected to varying agronomic practices and genotypic differences.
There were no clear genotypic effects on the agronomic traits evaluated in finger millet plants. Similar to triticale, certain agronomic parameters, such as plant height and spike dry weight, declined with increasing electrical conductivity in the irrigation water. These findings align with the results reported by Krishnamurthy et al. (2014), who evaluated the tolerance of different cultivars of finger millet under rising salinity levels in the soil solution. Notably, the lowest irrigation rate (I100) resulted in the highest spike dry weight in our experiment, aligning with the findings reported by Krishnamurthy et al. (2016) in their evaluation of a minicore collection of finger millet germplasm. Increasing planting density resulted in a clear increase across all agronomic parameters studied. However, contrasting results have been documented in other studies involving finger millets plants subjected to increased planting densities (Shinggu et al., 2009; Shinggu and Gani, 2012). Similar to triticale, the improved performance in using available resources such as water and nutrients, along with the maximization of land use efficiency, suggests that increasing planting density could be a valuable agronomic practice in arid regions.
From a physiological perspective, similar to triticale plants, finger millet plants subjected to increasing EC resulted in a reduction in NDVI readings and δ15N values. The decline in NDVI readings observed in this study is likely attributable to a reduction in photosynthetic efficiency under saline conditions, which promotes pigment photo-oxidation (Stefanov et al., 2021). Furthermore, salinity appears to disrupt key processes involved in nitrogen uptake, assimilation, release, and internal recycling, thereby contributing to the observed decrease in plant δ15N (Cernusak et al., 2009).
Finger millet plants subjected to the highest levels of irrigation exhibited decreased Nitrogen Balance Index (NBI) values, alongside increased δ15N and anthocyanin concentrations. Abiotic stresses, such as overirrigation, can disrupt nitrogen uptake, assimilation, and release processes, contributing to the observed increase in δ15N and corresponding decrease in NBI. This may be attributed to the accumulation or breakdown of free amino acids, which play a critical role in maintaining cellular homeostasis (Xu et al., 2005; Xu and Yu, 2006). Additionally, similar to plant responses under salt and drought stress, anthocyanin levels have been shown to rise in response to excessive irrigation (Chalker-Scott, 2002).
Finger millet plants cultivated at higher planting densities exhibited increased NDVI readings alongside reduced δ15N values. The increase in NDVI readings may reflect a compensatory response to reduced light distribution across the canopy, a common characteristic of crops grown under higher planting densities (Ming et al., 2017). Furthermore, the reduction in δ15N values is likely attributable to intensified competition for nutrients, particularly nitrogen, under higher planting densities, as supported by the findings of Ciampitti and Vyn (2011) and Dai et al. (2014). The δ¹3C values of finger millet were typical of a C4 species (Varalli et al., 2024), where the range of variability associated with water and salinity stresses far is lower than that of C3 plants (Farquhar et al., 1989).
Our findings revealed a strong positive correlation between grain yield (GY) and total plant dry weight (TDW) across the different species studied, which is consistent with the results reported by other researchers in cereal crops (Elazab et al., 2015; Rezzouk et al., 2022). The stable isotopic compositions of carbon and nitrogen proved to be valuable indicators for assessing crop responses to varying environmental conditions. In this context, we analyzed the relationships between these isotopic compositions and total plant dry weight across the different species studied, considering genotype-specific variations and the diverse agronomic practices employed. In our experiment, a negative correlation between nitrogen isotope composition and total plant dry weight was observed across all three species, indicating restricted nitrogen uptake under adverse conditions. This finding is consistent with expectations, as nitrogen uptake is commonly reduced under stressors such as salinity, drought, or increased planting density (Ehtaiwesh, 2022; He and Dijkstra, 2014; Zhang et al., 2016). In contrast, no significant relationship was identified between carbon isotope composition and total plant dry weight across the species studied, with correlation values generally low. Despite this, previous research has demonstrated that correlations between carbon isotope composition and total plant dry weight can be significant, with the direction of the correlation-either negative or positive-depending on the specific plant tissue sampled and the environmental conditions tested (Voltas et al., 1999). In fact, the combination of different planting densities, which strong affect total dry weight independently of the water stress, may justify the absence of such a correlation between δ¹³C and total plant dry weight. Additionally, phenology may significantly influence both total plant dry weight and carbon isotope composition, potentially accounting for observed negative relationships (Condon et al., 2004). Consequently, when investigating the relationship between δ¹³C and total plant dry weight (TDW), it is crucial to evaluate genotypes at comparable phenological stages (Richards et al., 2011).
The results of this study demonstrate that genotypic variation among barley, triticale and finger millet accessions led to distinct trends in agronomic and physiological parameters in combinatorial stress treatment in arid regions. Additionally, the differential tolerance of these cereal species to salt, drought and planting densities significantly influenced the experimental outcomes. In barley, C1 (N2-35) showed superior biomass, while C2 (N2-4) had the highest harvest index. Low irrigation reduced yield, and higher planting densities improved most agronomics parameters but reduced harvest index. Salinity had minimal agronomical effects but affected physiological traits, increasing nitrogen, δ13C, chlorophyll and anthocyanins in the two C3 crop species. For triticale, cv. C2 (PI429152) showed the lowest grain yield and harvest index. Medium irrigation enhanced growth, while planting densities increased all agronomic parameters studied. Salinity stress reduced NDVI and δ¹5N, while nitrogen and carbon concentrations increased. In finger millet, salinity reduced growth and harvest index. Low irrigation increased spike dry weight but reduced other parameters. Higher planting densities improved most traits but reduced harvest index. While this experiment has provided valuable insights into the agronomic and physiological responses of three cereal species, further research is needed to enhance total aerial biomass and grain productivity in arid environments. The current study has suffered one limitation such as the lack of seasonal replication. Nevertheless, futures studies could address these limitations, including for example multiple planting cycles or a similar plating date, but across successive years, to provide a broader understanding of how environmental factors interact with the factors already studied.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
PG-C: Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Data curation. AA-D: Formal analysis, Methodology, Writing – review & editing. MDS: Formal analysis, Methodology, Writing – review & editing. JLA: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research work was funded in part by the 2021 SGR 00688 project from AGAUR, Generalitat de Catalunya, the PID2022-138307OB-C21 project from Ministerio de Ciencia e Innovación, and the IdRA Institute from the University of Barcelona, Spain.
The authors gratefully acknowledge the International Center for Biosaline Agriculture (Dubai, UAE) staff for all their support during the field work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1488576/full#supplementary-material
Abdulbaki, A. S., Alsamadany, H., Alzahrani, Y., Olayinka, B. U. (2022). Rubisco and abiotic stresses in plants: Current assessment. Turk. J. Bot. 46, 541–552. doi: 10.55730/1300-008X.2730
Agati, G., Guidi, L., Landi, M., Tattini, M. (2021). Anthocyanins in photoprotection: knowing the actors in play to solve this complex ecophysiological issue. New Phytol. 232, 2228. doi: 10.1111/nph.v232.6
Alotaibi, M., Alhajeri, N. S., Al-Fadhli, F. M., Al Jabri, S., Gabr, M. (2023). Impact of climate change on crop irrigation requirements in arid regions. Wat. Resour. Manage. 37, 1965–1984. doi: 10.1007/s11269-023-03465-5
Amulya Manasa, N., Umesha, C. (2022). Effect of spacing and plant growth regulators on growth and yield of finger millet (Eleusine coracana L.). Int. J. Plant Soil Sci. 34, 106–111. doi: 10.9734/ijpss/2022/v34i1330981
Aparicio, N., Villegas, D., Casadesús, J., Araus, J. L., Royo, C. (2000). Spectral vegetation indices as non-destructive tools for determining durum wheat yield. Agron. J. 92, 83–91. doi: 10.2134/agronj2000.92183x
Aparicio, N., Villegas, D., Royo, C., Casadesús, J., Araus, J. L. (2004). Effect of sensor view angle on the assessment of agronomic traits by ground level hyper-spectral reflectance measurements in durum wheat under contrasting Mediterranean conditions. Int. J. Remote Sens. 25, 1131–1152. doi: 10.1080/0143116031000116967
Aranibar, J. N., Anderson, I. C., Epstein, H. E., Feral, C. J. W., Swap, R. J., Ramontsho, J., et al. (2008). Nitrogen isotope composition of soils, C3 and C4 plants along land use gradients in southern Africa. J. Arid Environ. 72, 326–337. doi: 10.1016/j.jaridenv.2007.06.007
Araus, J. L., Kefauver, S. C., Zaman-Allah, M., Olsen, M. S., Cairns, J. E. (2018). Translating high-throughput phenotyping into genetic gain. Trends Plant Sci. 23, 451–466. doi: 10.1016/j.tplants.2018.02.001
Araus, J. L., Rezzouk, F. Z., Sanchez-Bragado, R., Aparicio, N., Serret, M. D. (2023). Phenotyping genotypic performance under multistress conditions: Mediterranean wheat as a case study. Field Crops Res. 303, 109122. doi: 10.1016/j.fcr.2023.109122
Araus, J. L., Rezzouk, F. Z., Thushar, S., Shahid, M., Elouafi, I. A., Bort, J., et al. (2021). Effect of irrigation salinity and ecotype on the growth, physiological indicators and seed yield and quality of Salicornia europaea. Plant Sci. 304, 110819. doi: 10.1016/j.plantsci.2021.110819
Araus, J. L., Slafer, G. A., Royo, C., Serret, M. D. (2008). Breeding for yield potential and stress adaptation in cereals. Crit. Rev. Plant Sci. 27, 377–412. doi: 10.1080/07352680802467736
Auer, S. K., Solowey, J. R., Rajesh, S., Rezende, E. L. (2020). Energetic mechanisms for coping with changes in resource availability. Biol. Lett. 16, 20200580. doi: 10.1098/rsbl.2020.0580
Bahadur, M. M., Paul, N. K., Chowdhury, M. F. (2013). Effect of irrigation levels on growth and yield of barley (Hordeum vulgare L.). J. Sci. Tech. 6, 11.
Bashir, R. N., Khan, F. A., Khan, A. A., Tausif, M., Abbas, M. Z., Shahid, M. M. A., et al. (2023). Intelligent optimization of reference evapotranspiration (ETo) for precision irrigation. J. Comp. Sci. 69, 102025. doi: 10.1016/j.jocs.2023.102025
Ben Mariem, S., Soba, D., Zhou, B., Loladze, I., Morales, F., Aranjuelo, I. (2021). Climate change, crop yields, and grain quality of C3 cereals: A meta-analysis of CO2, temperature, and drought effects. Plants 10, 1052. doi: 10.3390/plants10061052
Blum, A. (2014). The abiotic stress response and adaptation of triticale—A review. Cer. Res. Commun. 42, 359–375. doi: 10.1556/CRC.42.2014.3.1
Cao, Y., Zhong, Z., Wang, H., Shen, R. (2022). Leaf angle: a target of genetic improvement in cereal crops tailored for high-density planting. Plant Biotech. J. 20, 426–436. doi: 10.1111/pbi.13780
Cernusak, L. A., Winter, K., Turner, B. L. (2009). Plant δ15N correlates with the transpiration efficiency of nitrogen acquisition in tropical trees. Plant Physiol. 151, 1667–1676. doi: 10.1104/pp.109.145870
Cerovic, Z. G., Ghozlen, N. B., Milhade, C., Obert, M., Debuisson, S., Le Moigne, M. (2015). Nondestructive diagnostic test for nitrogen nutrition of grapevine (Vitis vinifera L.) based on dualex leaf-clip measurements in the field. J. Agric. Food Chem. 63, 3669–3680. doi: 10.1021/acs.jafc.5b00304
Cerovic, Z. G., Masdoumier, G., Ghozlen, N. B., Latouche, G. (2012). A new optical leaf clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant 146, 251–260. doi: 10.1111/j.1399-3054.2012.01639.x
Chalker-Scott, L. (2002). Do anthocyanins function as osmoregulators in leaf tissues? Adv. Bot. Res. 37, 103–106. doi: 10.1016/S0065-2296(02)37046-0
Chamekh, Z., Ayadi, S., Karmous, C., Trifa, Y., Amara, H., Boudabbous, K., et al. (2016). Comparative effect of salinity on growth, grain yield, water use efficiency, δ13C and δ15N of landraces and improved durum wheat varieties. Plant Sci. 251, 44–53. doi: 10.1016/j.plantsci.2016.07.005
Ciampitti, I. A., Vyn, T. J. (2011). A comprehensive study of plant density consequences on nitrogen uptake dynamics of maize plants from vegetative to reproductive stages. Field Crops Res. 121, 2–18. doi: 10.1016/j.fcr.2010.10.009
Condon, A. G., Richards, R. A., Rebetzke, G. J., Farquhar, G. D. (2004). Breeding for high water-use efficiency. J. Exp. Bot. 55, 2447–2460. doi: 10.1093/jxb/erh277
Coplen, T. B. (2008). “Explanatory Glossary of Terms used in expression of Relative Isotope ratios and gas ratios. IUPAC Provisional Recommendations. Inorganic Chemistry Division,” in Commission on Isotopic Abundances and Atomic Weights. Available online at: http://old.iupac.org/reports/provisional/abstract08/coplen310508.html/ (Accessed February 21, 2025).
Coque, M., Bertin, P., Hirel, B., Gallais, A. (2006). Genetic variation and QTLs for 15N natural abundance in a set of maize recombinant inbred lines. Field Crops Res. 97, 310–321. doi: 10.1016/j.fcr.2005.11.002
Corrado, G., Lucini, L., Miras-Moreno, B., Chiaiese, P., Colla, G., De Pascale, S., et al. (2020). Metabolic insights into the anion-anion antagonism in sweet basil: Effects of different nitrate/chloride ratios in the nutrient solution. Int. J. Mol. Sci. 21, 2482. doi: 10.3390/ijms21072482
Dai, X., Xiao, L., Jia, D., Kong, H., Wang, Y., Li, C., et al. (2014). Increased plant density of winter wheat can enhance nitrogen–uptake from deep soil. Plant Soil 384, 141–152. doi: 10.1007/s11104-014-2190-x
Devkota, K. P., Devkota, M., Rezaei, M., Oosterbaan, R. (2022). Managing salinity for sustainable agricultural production in salt-affected soils of irrigated drylands. Agric. Syst. 198, 103390. doi: 10.1016/j.agsy.2022.103390
Dong, Y., Bi, X., Wu, R., Belfield, E. J., Harberd, N. P., Christensen, B. T., et al. (2022). The potential of stable carbon and nitrogen isotope analysis of foxtail and broomcorn millets for investigating ancient farming systems. Front. Plant Sci. 13, 1018312. doi: 10.3389/fpls.2022.1018312
Dreccer, M. F., Fainges, J., Whish, J., Ogbonnaya, F. C., Sadras, V. O. (2018). Comparison of sensitive stages of wheat, barley, canola, chickpea and field pea to temperature and water stress across Australia. Agric. Meteorol. 248, 275–294. doi: 10.1016/j.agrformet.2017.10.006
Ehtaiwesh, A. F. (2022). The effect of salinity on nutrient availability and uptake in crop plants. J. Appl. Sci. 9, 55–73.
Elazab, A., Bort, J., Zhou, B., Serret, M. D., Nieto-Taladriz, M. T., Araus, J. L. (2015). The combined use of vegetation indices and stable isotopes to predict durum wheat grain yield under contrasting water conditions. Agric. Wat. Manage. 158, 196–208. doi: 10.1016/j.agwat.2015.05.003
Ertekin, I. (2022). Influence of nitrogen dose and plant density on the yield and quality properties of dual-purpose barley grown under the Mediterranean climatic conditions. J. Element. 27, 113–126. doi: 10.5601/jelem.2022.27.1.2225
Farquhar, G. D., Ehleringer, J. R., Hubick, K. T. (1989). Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503–537. doi: 10.1146/annurev.pp.40.060189.002443
Ghazvineh, S., Valadabadi, A. R., Abdolahi, A. V., Seyfzadeh, S., Zakerin, H. R. (2020). Response of durum wheat genotypes to different planting dates and plant densities under dryland conditions. J. Crop Ecophysiol. 14, 401–422. doi: 10.30495/jcep.2020.679070
Giunta, F., Motzo, R. (2004). Sowing rate and cultivar affect total biomass and grain yield of spring triticale (× Triticosecale Wittmack) grown in a Mediterranean-type environment. Field Crops Res. 87, 179–193. doi: 10.1016/j.fcr.2003.11.009
Gracia-Romero, A., Kefauver, S. C., Fernandez-Gallego, J. A., Vergara-Díaz, O., Araus, J. L. (2019). UAV and ground image-based phenotyping: a proof of concept with durum wheat. Remote Sens. 11, 1–25. doi: 10.3390/rs11101244
Großkinsky, D. K., Faure, J. D., Gibon, Y., Haslam, R. P., Usadel, B., Zanetti, F., et al. (2023). The potential of integrative phenomics to harness underutilized crops for improving stress resilience. Front. Plant Sci. 14, 1216337. doi: 10.3389/fpls.2023.1216337
Hammami, Z., Sbei, H., Kadri, K., Jmel, Z., Sahli, A., Fraj, M. B., et al. (2016). Evaluation of performance of different barley genotypes irrigated with saline water in South Tunisian Saharan conditions. Environ. Exp. Biol. 14, 15–21. doi: 10.22364/eeb.14.03
Hasanuzzaman, M., Shabala, L., Brodribb, T. J., Zhou, M., Shabala, S. (2019). Understanding physiological and morphological traits contributing to drought tolerance in barley. J. Agron. Crop Sci. 205, 129–140. doi: 10.1111/jac.2019.205.issue-2
He, M., Dijkstra, F. A. (2014). Drought effect on plant nitrogen and phosphorus: a meta-analysis. New Phytol. 204, 924–931. doi: 10.1111/nph.2014.204.issue-4
He, J., Liu, H., Ren, Y., Liu, H., Du, W. (2024). Effects of nitrogen fertilization rate and seeding density on the forage yield and quality of autumn-sown triticale in an alpine grazing area of the Qinghai-Tibetan Plateau, China. Grass Forage Sci. 79, 392–403. doi: 10.1111/gfs.12666
Hessini, K., Ferchichi, S., Ben Youssef, S., Werner, K. H., Cruz, C., Gandour, M. (2015). How does salinity duration affect growth and productivity of cultivated barley? Agron. J. 107, 174–180. doi: 10.2134/agronj14.0281
Hurtado, A. C., Chiconato, D. A., de Mello Prado, R., Junior, G. D. S. S., Viciedo, D. O., de Cássia Piccolo, M. (2020). Silicon application induces changes C: N: P stoichiometry and enhances stoichiometric homeostasis of sorghum and sunflower plants under salt stress. Saudi J. Biol. Sci. 27, 3711–3719. doi: 10.1016/j.sjbs.2020.08.017
Hussain, M. I., Al-Dakheel, A. J., Ahmed, M. (2023). “Integrated crop–livestock system case study: Prospectus for Jordan’s climate change adaptation,” in Global Agricultural Production: Resilience to Climate Change (Springer International Publishing, Cham), 565–585.
Kankarla, V., Shukla, M. K., Picchioni, G. A., VanLeeuwen, D., Schutte, B. J. (2020). Germination and emergence responses of alfalfa, triticale and quinoa irrigated with brackish groundwater and desalination concentrate. Agronomy 10, 549. doi: 10.3390/agronomy10040549
Karim, M. A., Nawata, E., Shigenaga, S. (1993). Effects of salinity and water stress on the growth, yield and physiological characteristics in hexaploid triticale. Japan. J. Trop. Agric. 37, 46–52. doi: 10.11248/jsta1957.37.46
Koyro, H. W., Eisa, S. S. (2008). Effect of salinity on composition, viability and germination of seeds of Chenopodium quinoa Willd. Plant Soil 302, 79–90. doi: 10.1007/s11104-007-9457-4
Krishnamurthy, L., Upadhyaya, H. D., Kashiwagi, J., Purushothaman, R., Dwivedi, S. L., Vadez, V. (2016). Variation in drought-tolerance components and their interrelationships in the minicore collection of finger millet germplasm. Crop Sci. 56, 1914–1926. doi: 10.2135/cropsci2016.03.0191
Krishnamurthy, L., Upadhyaya, H. D., Purushothaman, R., Gowda, C. L. L., Kashiwagi, J., Dwivedi, S. L., et al. (2014). The extent of variation in salinity tolerance of the minicore collection of finger millet (Eleusine coracana L. Gaertn.) germplasm. Plant Sci. 227, 51–59. doi: 10.1016/j.plantsci.2014.07.001
Kumar, P., Choudhary, M., Halder, T., Prakash, N. R., Singh, V., Vineeth, T. V., et al. (2022). Salinity stress tolerance and omics approaches: revisiting the progress and achievements in major cereal crops. Heredity 128, 497–518. doi: 10.1038/s41437-022-00516-2
Lopes, M., Araus, J. L. (2006). Nitrogen source and water regime effects on durum wheat photosynthesis, and stable carbon and nitrogen isotope composition. Physiol. Plant 126, 435–445. doi: 10.1111/j.1399-3054.2006.00595.x
Lopes, M., Nogués, S., Araus, J. L. (2004). Nitrogen source and water regime effects on barley photosynthesis and isotope discrimination. Funct. Plant Biol. 31, 995–1003. doi: 10.1071/FP04031
Luo, M., Zhang, Y., Chen, K., Kong, M., Song, W., Lu, B., et al. (2019). Mapping of quantitative trait loci for seedling salt tolerance in maize. Mol. Breed. 39, 1–12. doi: 10.1007/s11032-019-0974-7
MacLaren, C., Waswa, W., Aliyu, K. T., Claessens, L., Mead, A., Schöb, C., et al. (2023). Predicting intercrop competition, facilitation, and productivity from simple functional traits. Field Crops Res. 297, 108926. doi: 10.1016/j.fcr.2023.108926
Mansour, M. M. F. (2023). Anthocyanins: Biotechnological targets for enhancing crop tolerance to salinity stress. Sci. Hortic. 319, 112182. doi: 10.1016/j.scienta.2023.112182
Mbinda, W., Mukami, A. (2021). A review of recent advances and future directions in the management of salinity stress in finger millet. Front. Plant Sci. 12, 734798. doi: 10.3389/fpls.2021.734798
Mendoza-Elos, M., Cortez-Baheza, E., Rivera-Reyes, J. G., Rangel-Lucio, J. A., Andrio-Enríquez, E., Cervantes-Ortiz, F. (2011). [amp]]Eacute;poca y densidad de siembra en la producción y calidad de semilla de triticale (X Triticosecale Wittmack). Agronom. Mesoamericana 22, 309–316. doi: 10.15517/am.v22i2.11804
Ming, B., Xie, R. Z., Hou, P., Li, L. L., Wang, K. R., Li, S. K. (2017). Changes of maize planting density in China. Sci. Agric. Sin. 50, 1960–1972. doi: 10.3864/j.issn.0578-1752.2017.11.002
Minhas, P. S., Ramos, T. B., Ben-Gal, A., Pereira, L. S. (2020). Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agr. Wat. Manage. 227, 105832. doi: 10.1016/j.agwat.2019.105832
Mohamed, A. A. (2023). Impact of sowing rates and humic acid application on productivity of some triticale genotypes at East El-Quntra station. J. Desert Environ. Agric. 3, 1–17. doi: 10.21608/jdea.2023.183169.1014
Mohammadi Alagoz, S., Hadi, H., Toorchi, M., Pawłowski, T. A., Asgari Lajayer, B., Price, G. W., et al. (2023). Morpho-physiological responses and growth indices of triticale to drought and salt stresses. Sci. Rep. 13, 8896. doi: 10.1038/s41598-023-36119-y
Mukami, A., Ngetich, A., Mweu, C., Oduor, R. O., Muthangya, M., Mbinda, W. M. (2019). Differential characterization of physiological and biochemical responses during drought stress in finger millet varieties. Physiol. Mol. Biol. Plants 25, 837–846. doi: 10.1007/s12298-019-00679-z
Mukami, A., Ngetich, A., Syombua, E., Oduor, R., Mbinda, W. (2020). Varietal differences in physiological and biochemical responses to salinity stress in six finger millet plants. Physiol. Mol. Biol. Plants 26, 1569–1582. doi: 10.1007/s12298-020-00853-8
Munns, R., Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Murphy, B. P., Bowman, D. M. (2009). The carbon and nitrogen isotope composition of Australian grasses in relation to climate. Funct. Ecol. 23, 1040–1049. doi: 10.1111/j.1365-2435.2009.01576.x
Niharika, M., Vidya Sagar, G. E. C. H., Rekha, K. B., Anjaiah, T. (2021). Response of finger millet (Eleusine coracana L.) to varying levels of plant density and nitrogen. Int. J. Environ. Climate Change 11, 308–314. doi: 10.9734/ijecc/2021/v11i1130546
Paradiso, R., Proietti, S. (2022). Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 41, 742–780. doi: 10.1007/s00344-021-10337-y
Pardo, J. J., Sánchez-Virosta, A., Léllis, B. C., Domínguez, A., Martínez-Romero, A. (2022). Physiological basis to assess barley response to optimized regulated deficit irrigation for limited volumes of water (ORDIL). Agric. Wat. Manage. 274, 107917. doi: 10.1016/j.agwat.2022.107917
Postma, J. A., Hecht, V. L., Hikosaka, K., Nord, E. A., Pons, T. L., Poorter, H. (2021). Dividing the pie: A quantitative review on plant density responses. Plant Cell Environ. 44, 1072–1094. doi: 10.1111/pce.13968
Pour-Aboughadareh, A., Mehrvar, A., Sanjani, S., Amini, A., Nikkhah-Chamanabad, H., Asadi, A. (2021). Effects of salinity stress on seedling biomass, physiochemical properties, and grain yield in different breeding wheat genotypes. Acta Physiol. Plant 43, 1–14. doi: 10.1007/s11738-021-03265-7
Pritchard, E. S., Guy, R. D. (2005). Nitrogen isotope discrimination in white spruce fed with low concentrations of ammonium and nitrate. Trees – Struct. Funct. 19, 89–98. doi: 10.1007/s00468-004-0367-2
Rezzouk, F. Z., Gracia-Romero, A., Kefauver, S. C., Gutierrez, N. A., Aranjuelo, I., Serret, M. D., et al. (2020a). Remote sensing techniques and stable isotopes as phenotyping tools to assess wheat yield performance: effects of growing temperature and vernalization. Plant Sci. 295, 1–16. doi: 10.1016/j.plantsci.2019.110281
Rezzouk, F. Z., Gracia-Romero, A., Kefauver, S. C., Nieto-Taladriz, M. T., Serret, M. D., Araus, J. L. (2022). Durum wheat ideotypes in Mediterranean environments differing in water and temperature conditions. Agric. Wat. Manage. 259, 107257. doi: 10.1016/j.agwat.2021.107257
Rezzouk, F. Z., Shahid, M. A., Elouafi, I. A., Zhou, B., Araus, J. L., Serret, M. D. (2020b). Agronomic performance of irrigated quinoa in desert areas: Comparing different approaches for early assessment of salinity stress. Agric. Wat. Manag 240, 106205. doi: 10.1016/j.agwat.2020.106205
Richards, R. A., Rebetzke, G. J., Condon, A. G., Watt, M. (2011). “Breeding to improve grain yield in water limited environments: the CSIRO experience with wheat,” in Crop Stress Management and Global Climate Change. Eds. Araus, J. L., Slafer, G. A. (CABI, UK), 105–121.
Robredo, A., Perez-Lopez, U., de la Maza, H. S., Gonzalez-Moro, B., Lacuesta, M., Mena-Petite, A., et al. (2007). Elevated CO2 alleviates the impact of drought on barley improving water status by lowering stomatal conductance and delaying its effects on photosynthesis. Environ. Exp. Bot. 59, 252–263. doi: 10.1016/j.envexpbot.2006.01.001
Sabagh, A. E., Hossain, A., Islam, M. S., Barutcular, C., Hussain, S., Hasanuzzaman, M., et al. (2019). Drought and salinity stresses in barley: consequences and mitigation strategies. Aust. J. Crop Sci. 13, 810–820. doi: 10.21475/ajcs
Sanchez-Díaz, M., García, J. L., Antolín, M. C., Araus, J. L. (2002). Effects of soil drought and atmospheric humidity on yield, gas exchange, and stable carbon isotope composition of barley. Photosynthetica 40, 415–421. doi: 10.1023/A:1022683210334
Schulze, E. D., Chapin, F. S., III (1987). “Plant specialization to environments of different resource availability,” in Potentials and limitations of ecosystem analysis (Springer Berlin Heidelberg, Berlin, Heidelberg), 120–148.
Shahbaz, M., Ashraf, M. (2013). Improving salinity tolerance in cereals. Crit. Rev. Plant Sci. 32, 237–249. doi: 10.1080/07352689.2013.758544
Shahid, S. A., Dakheel, A. J., Mufti, K. A., Shabbir, G. (2009). Automated in-situ soil salinity logging in irrigated agriculture. Eur. J. Sci. Res. 26, 288–297.
Shinggu, C. P., Dadari, S. A., Shebayan, J. A. Y., Adekpe, D. I., Mahadi, M. A., Mukhtar, A., et al. (2009). Influence of spacing and seed rate on weed suppression in finger millet (Eleucine carocana gaertn). Middle-East J. Sci. Res. 4, 267–270.
Shinggu, C. P., Gani, M. (2012). Effects of planting methods, sowing dates and spacing on weed and the productivity of finger millet (Eleusine corocana (L.) Gaertn) in the northern Guinea-savanna of Nigeria. Global J. Bio-Sci. Biotechnol. 1, 160–162.
Shrief, S. A., Abd-El-Mohsen, A. A. (2014). Effect of different irrigation regimes on grain and protein yields and water use efficiency of barley. Scientia 8, 140–147. doi: 10.15192/PSCP.SA.2014.4.3.140147
Soil Survey Staff (2010). Keys to Soil Taxonomy. 11th ed (Washington, DC: Government Printing Office). USDA-NRCS, US.
Soleymani, A., Shahrajabian, M. H. (2011). Influence of planting date and plant density on grain and biological yields of barley cultivars. Res. Crops 12, 698–700.
Soleymani, A., Shahrajabian, M. H., Naranjani, L. (2011). Determination of the suitable planting date and plant density for different cultivars of barley (Hordeum vulgare L.) in Fars. Afr. J. Plant Sci. 5, 284–286.
Sperdouli, I., Moustaka, J., Ouzounidou, G., Moustakas, M. (2021). Leaf age-dependent photosystem II photochemistry and oxidative stress responses to drought stress in Arabidopsis thaliana are modulated by flavonoid accumulation. Molecules 26, 4157. doi: 10.3390/molecules26144157
Stefanov, M. A., Rashkov, G. D., Yotsova, E. K., Borisova, P. B., Dobrikova, A. G., Apostolova, E. L. (2021). Different sensitivity levels of the photosynthetic apparatus in Zea mays L. and Sorghum bicolor L. under salt stress. Plants 10, 1469. doi: 10.3390/plants10071469
Toulotte, J. M., Pantazopoulou, C. K., Sanclemente, M. A., Voesenek, L. A., Sasidharan, R. (2022). Water stress resilient cereal crops: Lessons from wild relatives. J. Integ. Plant Biol. 64, 412–430. doi: 10.1111/jipb.13222
Tucker, C. J., Sellers, P. J. (1986). Satellite remote sensing of primary production. Int. J. Remote Sens. 7, 1395–1416. doi: 10.1080/01431168608948944
Van Ittersum, M. K., Cassman, K. G., Grassini, P., Wolf, J., Tittonell, P., Hochman, Z. (2013). Yield gap analysis with local to global relevance-a review. Field Crop Res. 143, 4–17. doi: 10.1016/j.fcr.2012.09.009
Varalli, A., Beldados, A., d'Agostini, F., Mvimi, M., D'Andrea, C., Lancelotti, C. (2024). Isotopic analysis of modern sorghum and finger millet from different altitudes in Ethiopia: implications for ancient farming practices. Front. Environ. Archaeol. 3, 1473056. doi: 10.3389/fearc.2024.1473056
Vargas, J. Q., Bendig, J., Mac Arthur, A., Burkart, A., Julitta, T., Maseyk, K., et al. (2020). Unmanned aerial systems (UAS)-based methods for solar induced chlorophyll fluorescence (SIF) retrieval with non-imaging spectrometers: state of the art. Remote Sens. 12, 1624. doi: 10.3390/rs12101624
Vernooij, R., Dusek, U., Popa, M. E., Yao, P., Shaikat, A., Qiu, C., et al. (2021). Stable carbon isotopic composition of biomass burning emissions-implications for estimating the contribution of C3 and C4 plants. Atmos. Chem. Phys. Disc. 2021, 1–35. doi: 10.5194/acp-2021-897
Vicente, R., Vergara-Díaz, O., Kerfal, S., López, A., Melichar, J., Bort, J., et al. (2019). Identification of traits associated with barley yield performance using contrasting nitrogen fertilizations and genotypes. Plant Sci. 282, 83–94. doi: 10.1016/j.plantsci.2018.10.002
Voltas, J., Romagosa, I., Lafarga, A., Armesto, A. P., Sombrero, A., Araus, J. L. (1999). Genotype by environment interaction for grain yield and carbon isotope discrimination of barley in Mediterranean Spain. Aust. J. Agric. Res. 50, 1263–1271. doi: 10.1071/AR98137
Wallace, M., Jones, G., Charles, M., Fraser, R., Halstead, P., Heaton, T. H., et al. (2013). Stable carbon isotope analysis as a direct means of inferring crop water status and water management practices. World Archae. 45, 388–409. doi: 10.1080/00438243.2013.821671
Wang, J., Vanga, S. K., Saxena, R., Orsat, V., Raghavan, V. (2018). Effect of climate change on the yield of cereal crops: A review. Climate 6, 41. doi: 10.3390/cli6020041
Wilke, N., Siegmann, B., Postma, J. A., Muller, O., Krieger, V., Pude, R., et al. (2021). Assessment of plant density for barley and wheat using UAV multispectral imagery for high-throughput field phenotyping. Comp. Elect. Agric. 189, 106380. doi: 10.1016/j.compag.2021.106380
Xu, Z. Z., Yu, Z. W. (2006). Nitrogen metabolism in flag leaf and grain of wheat in response to irrigation regimes. J. Plant Nutr. Soil Sci. 169, 118–126. doi: 10.1002/jpln.200420418
Xu, Z. Z., Yu, Z. W., Wang, D., Zhang, Y. L. (2005). Nitrogen accumulation and translocation for winter wheat under different irrigation regimes. J. Agron. Crop Sci. 191, 439–449. doi: 10.1111/j.1439-037X.2005.00178.x
Yang, W., Feng, H., Zhang, X., Zhang, J., Doonan, J. H., Batchelor, W. D., et al. (2020). Crop phenomics and high-throughput phenotyping: past decades, current challenges, and future perspectives. Mol. Plant 13, 187–214. doi: 10.1016/j.molp.2020.01.008
Yang, G., Liu, J., Zhao, C., Li, Z., Huang, Y., Yu, H., et al. (2017). Unmanned aerial vehicle remote sensing for field-based crop phenotyping: current status and perspectives. Front. Plant Sci. 8, 1111. doi: 10.3389/fpls.2017.01111
Yousfi, S., Serret, M. D., Araus, J. L. (2009). Shoot δ15N gives a better indication than ion concentration or Δ13C of genotypic differences in the response of durum wheat to salinity. Funct. Plant Biol. 36, 144–155. doi: 10.1071/FP08135
Yousfi, S., Serret, M. D., Marquez, A. J., Voltas, J., Araus, J. L. (2012). Combined use of δ13C, δ18O and δ15N tracks nitrogen metabolism and genotypic adaptation of durum wheat to salinity and water deficit. New Phytol. 194, 230–244. doi: 10.1111/j.1469-8137.2011.04036.x
Yusuf, N. B., Fagam, A. S., Shuaibu, Y. M., Bala, R. A. (2023). Growth and grain yield of triticale as influenced by irrigation intervals, nitrogen rates and weeding regimes. Nig. J. Agric. Agric. Technol. 3, 10–20.
Zeeshan, M., Lu, M., Sehar, S., Holford, P., Wu, F. (2020). Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy 10, 127. doi: 10.3390/agronomy10010127
Zhang, J., Dong, S., Dai, X., Wu, T., Wang, X., Bai, H., et al. (2016). Combined effect of plant density and nitrogen input on grain yield, nitrogen uptake and utilization of winter wheat. Vegetos- Int. J. Plant Res. 29, 2. doi: 10.5958/2229-4473.2016.00023.9
Zhang, Y., Xu, Z., Li, J., Wang, R. (2021). Optimum planting density improves resource use efficiency and yield stability of rainfed maize in semiarid climate. Front. Plant Sci. 12, 752606. doi: 10.3389/fpls.2021.752606
Keywords: carbon isotope composition, finger millet, Hordeum vulgare, nitrogen isotope composition, triticale, yield
Citation: García-Caparros P, Al-Dakheel AJ, Serret MD and Araus JL (2025) Optimization of cereal productivity and physiological performance under desert conditions: varying irrigation, salinity and planting density levels. Front. Plant Sci. 16:1488576. doi: 10.3389/fpls.2025.1488576
Received: 30 August 2024; Accepted: 12 February 2025;
Published: 06 March 2025.
Edited by:
Lorenzo Barbanti, University of Bologna, ItalyReviewed by:
Ali Baghdadi, University of Saskatchewan, CanadaCopyright © 2025 García-Caparros, Al-Dakheel, Serret and Araus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pedro García-Caparros, cGVkcm9nYXIxMjNAaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.