- 1International Faculty of Applied Technology, Yibin University, Yibin, Sichuan, China
- 2College of Agriculture, Forestry, and Food Engineering, Yibin University, Yibin, Sichuan, China
- 3Department of Genetics, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 4Key Laboratory for Green and Advanced Civil Engineering Materials and Application Technology of Hunan Province, College of Civil Engineering, Hunan University, Changsha, China
- 5Department of Microbiology, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 6Agriculture Biochemistry Department, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
With a focus on plant tolerance to environmental challenges, nanotechnology has emerged as a potent instrument for assisting crops and boosting agricultural production in the face of a growing worldwide population. Nanoparticles (NPs) and plant systems may interact molecularly to change stress response, growth, and development. NPs may feed nutrients to plants, prevent plant diseases and pathogens, and detect and monitor trace components in soil by absorbing their signals. More excellent knowledge of the processes of NPs that help plants survive various stressors would aid in creating more long-term strategies to combat these challenges. Despite the many studies on NPs’ use in agriculture, we reviewed the various types of NPs and their anticipated molecular and metabolic effects upon entering plant cells. In addition, we discussed different applications of NPs against all environmental stresses. Lastly, we introduced agricultural NPs’ risks, difficulties, and prospects.
1 Introduction
Plants are exposed to a range of environmental stressors that have the potential to diminish and restrict the yield of crops (El-Sappah and Rather, 2022; Ihtisham et al., 2023). Plants encounter two distinct categories of environmental challenges: abiotic stress and biotic stress (El-Sappah and Rather, 2022). The global loss of essential crop plants is attributed to abiotic stressors such as radiation, salinity, floods, drought, temperature extremes, heavy metals (HMs), and other factors (Umar et al., 2021). Conversely, biological stressors encompass the presence of pathogens such as fungi, bacteria, oomycetes, nematodes, and herbivores (Bhatla et al., 2018). Crop production losses can be attributed to biotic and abiotic stressors (Gull et al., 2019). The overproduction of reactive oxygen species (ROS) is identified as a significant factor contributing to crop losses resulting from abiotic stressors (El-Sappah et al., 2022b; Li et al., 2023b). In recent decades, significant endeavors have been undertaken to enhance agricultural productivity by extensively employing chemicals that have enduring and profound impacts on the environment and human wellbeing. Consequently, innovative technology is necessary to nourish the global population while minimizing environmental damage (Gill and Garg, 2014; Tudi et al., 2021).
The field of nanotechnology is stimulating and undergoing significant advancements, leading to many breakthroughs (Ratner and Ratner, 2003; Abbas et al., 2022b; Naveed et al., 2022b). Nanotechnology has the potential to offer viable solutions to agricultural challenges and contribute to the attainment of a sustainable and secure future for the agricultural sector (Pandey, 2018). In recent years, nanotechnology has garnered significant attention due to its wide range of applications in medical, drug delivery, energy, poultry production, and agri-food industries (Ashraf et al., 2021). According to Khan and Upadhyaya (2019), nanoparticles (NPs) are diminutive substances with dimensions spanning from 1 to 100 nm. Unlike larger-sized entities, NPs possess unique and varied physicochemical characteristics (Hsiao and Huang, 2011). The high surface area-to-volume ratio, high adsorption efficacy, and higher connecting and working efficiencies of NPs can be attributed to their small size (Gil et al., 2010). NPs may help plants resist stress by improving nutrient uptake by assisting plants in absorbing and transporting minerals and nutrients (Singh et al., 2024), improving photosynthetic efficiency (Faizan et al., 2021), regulating hormone balance, particularly auxins and gibberellins, scavenging ROS (Tripathi et al., 2022), which can harm plants, and improving stress-responsive gene expression by boosting the expression of genes that help plants react (Abideen et al., 2022).
The current review comprehensively summarizes the advances in applying nanobiotechnology in agriculture, especially the potential of biosynthesized NPs to relieve abiotic and biotic problems in crop production.
2 Properties of nanoparticles
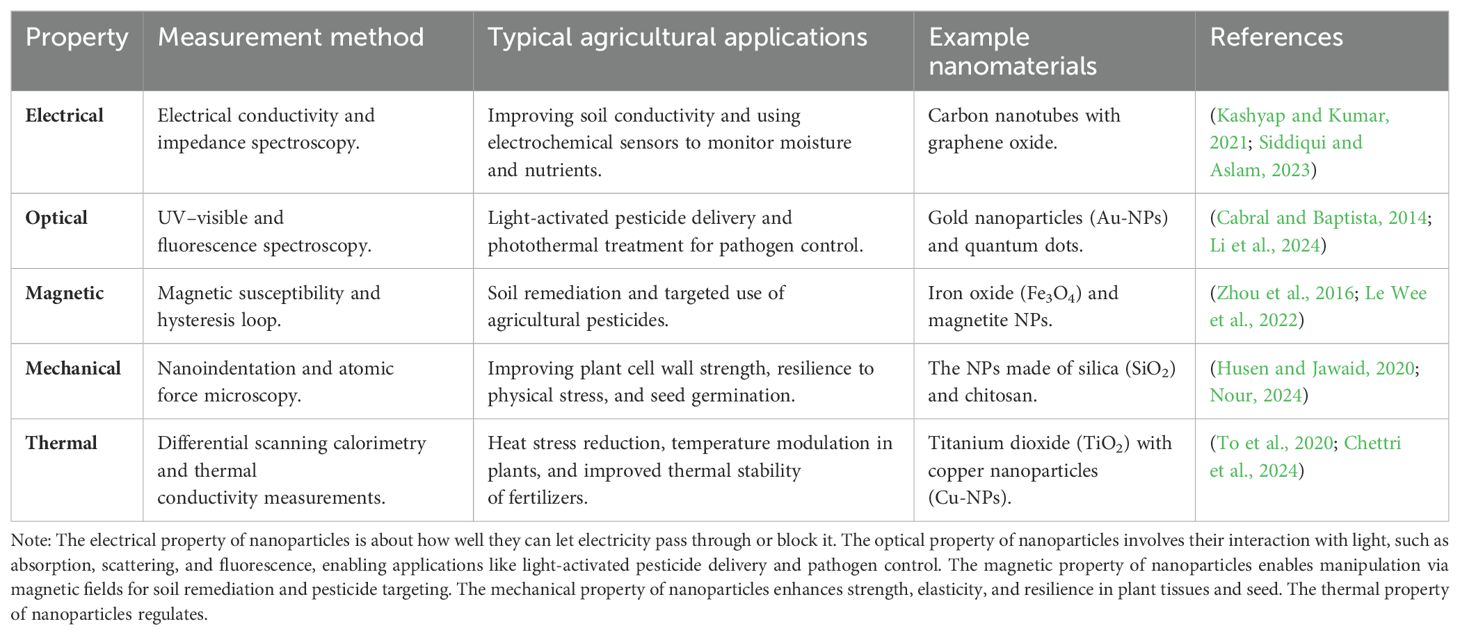
NPs have been classified into four categories: electrical and optical, magnetic, mechanical, and thermal (Khan et al., 2019), as shown in Table 1.
NPs have a higher degree of interdependence between their electrical and optical properties. NPs composed of noble metals exhibit optical properties that depend on their size and possess a distinct ultraviolet–visible (UV–Vis) extinction band absent in the bulk metal spectrum (Gautam et al., 2021). The phenomenon known as localized surface plasma resonance (LSPR) arises when the frequency of incident photons remains constant while the conduction electrons are collectively excited (Singh, 2017). The LSPR excitation induces absorption at specific wavelengths due to resonance with a significantly high molar excitation coefficient (Kochuveedu and Kim, 2014). LSPR improves NPs’ capacity to interact with light, which may be employed realistically to enhance plant health, disease management, and precision agricultural approaches.
The magnetic properties of these NPs have garnered significant attention from researchers in various domains, including heterogeneous and homogeneous catalysis, biomedicine, magnetic fluids, data storage, magnetic resonance imaging, and environmental remediation, such as water decontamination (Ali et al., 2021). According to existing scholarly works, NPs exhibit optimal performance within the critical size range of 10–20 nm (Reiss and Hütten, 2005). The magnetic characteristics of NPs were more dominant at a tiny scale, rendering these particles highly desirable and valuable in a wide range of applications (Cardoso et al., 2018). NPs exhibit magnetic properties due to their non-uniform electronic dispersion (Karunakaran et al., 2018). The qualities mentioned are influenced by the synthetic process, which can be achieved in many ways, such as solvothermal (Navas et al., 2020), co-precipitation, micro-emulsion, thermal breakdown, and flame spray synthesis (Dippong et al., 2021).
Conversely, NPs’ unique mechanical characteristics allow researchers to explore innovative applications in diverse critical domains, including tribology, surface engineering, nanofabrication, and nanomanufacturing (Khan et al., 2019). Diverse mechanical metrics like elastic modulus, hardness, stress and strain, adhesion, and friction can be examined to ascertain the precise mechanical characteristics of NPs (Wu et al., 2020). Various factors, including surface coating, coagulation, and lubrication, influence NPs’ mechanical properties (Balmert and Little, 2012; Pownraj and Valan Arasu, 2021). There are distinct mechanical properties between NPs and titles and their bulk materials. In a lubricated or greased contact, the stiffness contrast between the NPs and the external surface determines whether the NPs are indented into the plan surface or distorted when the pressure at contact is sufficiently high (Madkour and Madkour, 2019). These crucial data may provide insights into the performance of the NPs in a contact scenario. It is of utmost importance to exercise control over the mechanical properties of NPs and their interactions with various surface types to enhance surface quality and remove more material (Khan et al., 2019).
A comprehensive comprehension of the essential mechanical characteristics of NPs, including their elastic modulus and hardness, movement law, friction and interfacial adhesion, and size dependence, is typically required to achieve successful results in these areas (Mehmood et al., 2023). Metal NPs are widely recognized for their superior thermal conductivities compared to solid fluids. At room temperature, the thermal conductivity of copper is approximately 700 times greater than water’s and nearly 3,000 times greater than engine oil’s (Choi and Eastman, 1995). Thermal conductivity is stronger in oxides such as alumina (Al2O3) than in water (Senthilraja et al., 2015). It is anticipated that fluids containing suspended solid particles will exhibit considerably greater thermal conductivities than current heat transfer fluids (Choi and Eastman, 1995). Nanofluids are generated through the dispersion of solid particles at nanometric wavelengths within liquid mediums, such as water, ethylene glycol, or oils (Taylor et al., 2013). The properties of nanofluids are anticipated to surpass those of traditional heat transfer fluids and fluids containing particles at the tiny level (Das and Stephen, 2009). Because of heat transmission at the particle’s surface, particles with a substantial total surface area are favored. The enhancement of suspension stability is also attributed to the significant total surface area (Timofeeva et al., 2009). Another study demonstrated that nanofluids containing Al2O3 or CuO-NPs (copper oxide nanoparticles) in ethylene (H₂C=CH₂) or water had increased thermal conductivity (Mohamad et al., 2018).
3 Plants’ molecular and metabolic responses to nanoparticle exposure
Assessing the absorption, dispersion, and toxicity of NP exposure in plants requires understanding the nature of NP interactions with plants. According to Dietz and Herth (2011), NPs’ enormous surface area, minuscule size, and innate catalytic reactivity are the leading causes of their chemical and mechanical interactions with biological systems, such as plants.
It is commonly known that NPs alter biological architecture in various complex and little-understood ways. The effects of environmental stress on plants have been evaluated using multiple biochemical markers, including metabolite composition, membrane integrity, and enzyme activity (Singh et al., 2020). A physiological study indicates that smaller NPs, like plasmodesmata, can flow through the symplast, but larger NPs congregate in the apoplastic region (Banerjee et al., 2019; Wang et al., 2023), as seen in Figure 1. The most commonly reported mechanisms of NP toxicity in plants are the release of toxic metal ions (Dietz and Herth, 2011), increased production of ROS leading to oxidative stress (OS) (Asli and Neumann, 2009; Dietz and Herth, 2011), and mechanical damage or clogging of pores caused by cell surface coating (Pashkow et al., 2008).
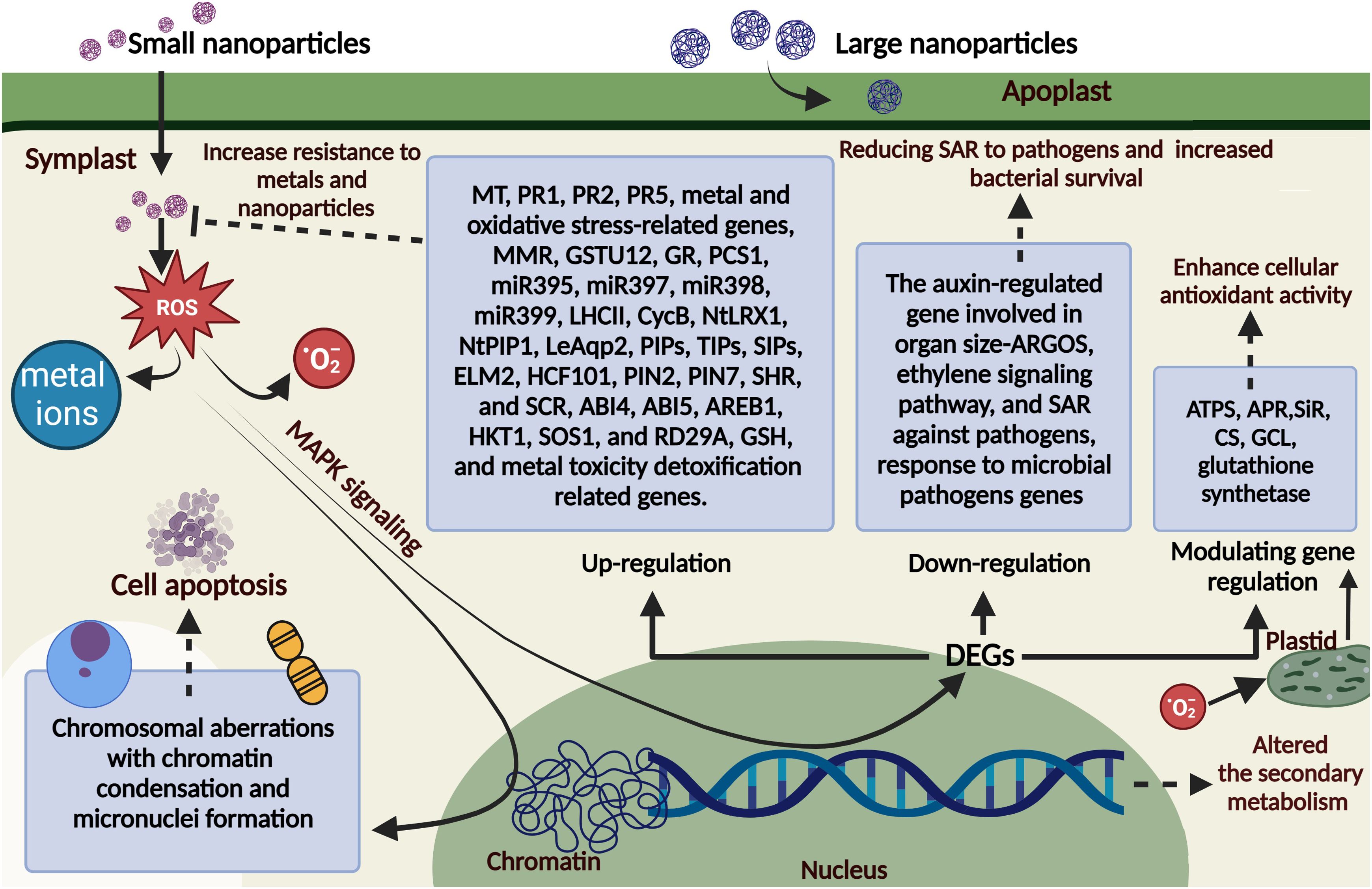
Figure 1. The plant cell’s molecular response to NP exposure. Small and large nanoparticles infiltrate plant cells, triggering a process termed MAPK signaling, leading to reactive oxygen species (ROS) generation. Numerous genes have been identified as overexpressed in response to NPs, including metallothionein (MT), pathogenesis-related genes (PRs: PR1, PR2, and PR5), DNA mismatch repair (MMR) genes, proliferating cell nuclear antigen (PCNA), glutathione S-transferase (GSTU12), glutathione reductase (GR), and phytochelatin synthase (PCS1); specific microRNAs (miR395, miR397, miR398, and miR399); the light-harvesting complex II (LHCII) b gene; and various abiotic stress-related genes, including those involved in oxidative stress, salinity, water management, sulfur metabolism, glutathione (GSH) biosynthesis, metal toxicity detoxification, cell division (CycB), cell wall extension (NtLRX1), water transport (aquaporin gene NtPIP1), aquaporin gene (LeAqp2), plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), small and essential intrinsic proteins (SIPs), and tolerance to cadmium sulfide quantum dots (MYB-containing gene ELM2), as well as genes implicated in high chlorophyll photosynthesis. Additional genes associated with hormonal stimuli and plant defense systems [e.g., the ethylene signaling pathway, systemic acquired resistance to pathogens, and the auxin-regulated gene linked to organ size (ARGOS)] were downregulated after NP treatment. It diminishes the expression of genes associated with microbial infection defense, enhancing bacterial colonization and survival while decreasing self-protection against pathogens. Additional genes affected by NP exposure are glutathione synthetase (GS), 3′-phosphoadenosine 5′-phosphosulfate reductase (APR), sulfite reductase (SiR), cysteine synthetase (CS), glutamate-cysteine ligase (GCL), and ATP sulfurylase (ATPS). This figure was created using BioRender.
OS is one of the most widely observed stressors caused by NP exposure at the cellular level (Horie and Tabei, 2021). It is widely described as an imbalance between antioxidant activity and oxidant production (Pisoschi and Pop, 2015). Furthermore, OS can be induced by elevated ROS levels at the expense of antioxidants. ROS, such as peroxynitrite (ONOO−), nitric oxide (NO), hydroxyl radical (∙OH), hydrogen peroxide (H2O2), and superoxide radical (O2∙−), are typically produced as by-products of biochemical reactions like neutrophil-mediated phagocytosis, enzymatic metabolism of cytochrome P450, and mitochondrial respiration (Cuzzocrea, 2006). ROS attacks nucleic acids, proteins, lipids, and most essential biomolecules, activating the NADPH-like system, impairing the electron transport chain (ETC), depolarizing the mitochondrial membrane, and damaging the mitochondrial structure (Egbuna et al., 2021). OS is a significant drawback of NP use because it can generate oxidants and promote ROS production due to the relative stability of free radical intermediates on particles’ reactive surfaces, NP-induced cellular response, or NP functionalization’s redox-active groups, which can interfere with cellular uptake (Manke et al., 2013). Such imbalances caused by NPs, whether directly or indirectly, may have serious consequences, including cytotoxicity (Saifi et al., 2018). The ROS produced by NPs may harm genetic materials, including DNA crosslinking, strand breaking, and genetic mutations (Rim et al., 2013). NPs may also boost ROS generation by stimulating inflammatory cells like neutrophils (Yang et al., 2019).
Much research has been done using models of algae, monocotyledonous plants, and dicotyledonous plants to examine the effects of various NP types on secondary metabolite precursors (Selvakesavan et al., 2023). Research on the impact of NPs on the pentose phosphate pathway, glycolysis, tricarboxylic acid cycle, and the roles played by organic acids and carbohydrates in these processes has been conducted extensively (Majumdar et al., 2019). Defense mechanisms trigger many metabolic pathways and chemicals in reaction and their supplemental roles as chelators and osmoprotectors (Singh et al., 2023). In addition, silver (Ag), copper-oxide (CuO), copper (II) hydroxide Cu(OH)2, cadmium oxide (CdO), cerium dioxide (CeO2), graphene-based, tungsten disulfide (WS2), and fullerols (C60) changed the fatty acid and lipid contents of Arabidopsis thaliana, Cucumis sativus, Zea mays, Hordeum vulgare, and Phaseolus vulgaris (Al-Khayri et al., 2023a). Amino acid metabolism provides a vital link between primary and secondary metabolites. Major precursors in the biosynthesis of these compounds include glucosinolates (e.g., methionine, leucine, isoleucine, phenylalanine, and tryptophan), phenylpropanoids [e.g., Ag-, CuO-, and Cu(OH)2-NPs, as well as Ag+ and Cu2+ ions), and alkaloids (e.g., arginine, lysine, ornithine, phenylalanine, proline, tryptophan, and tyrosine) (Barros and Dixon, 2020; Jan et al., 2021). Zinc oxide (ZnO), C60, graphene NPs, and tissues from Z. mays, A. thaliana, Z. sativus, and T. aestivum all encouraged the synthesis of additional amino acids (Hu and Zhou, 2014; Zhao et al., 2019; Chen et al., 2021; Li et al., 2021).
Many studies have examined how NPs affect plants’ secondary metabolism (Marslin et al., 2017). Cucumber, maize, and wheat exposed to Ag, pepper exposed to silicone dioxide (SiO2) or ferric oxide (Fe2O3), and Arabidopsis treated with CuO-NPs after foliar spray of Cu(OH)2 were all discovered to have shikimate and phenylpropanoid pathway products (Zhao et al., 2017; Soria et al., 2019; Feng et al., 2021; Kalisz et al., 2021). Cu(OH)2, CeO2, and soil application of CuO and CdO treatments decreased lettuce, spinach, cucumber, and barley phenylpropanoids (Večeřová et al., 2016; Zhao et al., 2016; Huang et al., 2019; Zhang et al., 2019b). Low CeO2-NPs induced metabolic reprogramming in P. vulgaris roots and leaves by affecting flavonoids and phenolic compounds (Majumdar et al., 2014). Gallic and benzoic acid concentrations in sativus increased, whereas hydroxycinnamic acid derivative concentrations decreased in response to carbon (C)- and CuO-NPs (Hong et al., 2016; Selvakesavan et al., 2023). When Solanum lycopersicum was treated with multiwalled carbon nanotubes (MWCTs), it produced fewer flavonoids and more anthocyanins (Mcgehee et al., 2017). Within the Hypericum perforatum cells, the production of phenylpropanoids was impacted by metal and metal oxide NPs (Kruszka et al., 2022). Ag, Au, Cu, and Pd metal NPs decreased the amounts of flavonoids and hydroxycinnamic acid derivatives in cells while increasing the accumulation of xanthone, prenylated xanthone, and benzophenone (Selvakesavan et al., 2023).
On the other hand, the treatment with CuO-NPs increased the amount of flavonoids in biomass (Selvakesavan et al., 2023). NPs have changed the metabolism of alkaloids, a family of chemicals with great biological importance as defensive metabolites (Salehi et al., 2018). (S)-corytuberine, laudanosine, and precursors of naphthyl isoquinoline alkaloids were found to be decreased in P. vulgaris, whereas demecolcine, caconine, and tropinone were found to be increased following foliar application of CeO2-NPs (Salehi et al., 2018). Hyoscyamine and scopolamine following ZnO-NP exposure, and taxane and tropane alkaloids after Ag- NP exposure all accumulated (Asl et al., 2019). Methocotype synthase (MWCT) in S. lycopersicum and graphene oxide quantum dots (GOQDs) in Chlorella vulgaris Beijerinck were shown to decrease the production of isoquinoline alkaloids (McGehee et al., 2017; Kang et al., 2019). Under biotic and abiotic stress conditions, A. thaliana produces the camalexin of indole phytoalexin (Kruszka et al., 2020). As a consequence of applying Ag-NPs, this substance was collected (Kruszka et al., 2020).
Understanding the molecular effects of nanomaterials is critical to evaluating possible routes for the effects observed in plants (Ma et al., 2015). RNA-sequencing (RNA-seq) transcriptome analysis is a solid tool to determine cellular responses compared to other omics techniques owing to the unequaled resolution of entire transcripts (Xiong et al., 2021). Transcriptome analysis revealed that A. thaliana treated with PVPAg-NPs had increased tryptophan metabolism, a precursor to camalexin (Zhang et al., 2019a). NPs also change the expression of genes in plants and microbes. Different kinds of NPs have different impacts on gene expression after exposure. Our defense hypotheses about plant-responsive genes that showed high expression under NP exposure come from the exposure of various plants such as Arabidopsis, tobacco, barley, maize, and soybean to different NPs such as silver NPs (Ag-NPs), titanium dioxide NPs (TiO2-NPs), zinc oxide NPs (ZnO-NPs), carbon nanotubes (CNTs), graphene oxide (GO), aluminum oxide NPs (Al2O3), cerium oxide (CeO2), and indium oxide (In2O3) NPs (Zhang et al., 2006; Dubos et al., 2010; Ze et al., 2011; Burklew et al., 2012; Chu et al., 2012; Dimkpa et al., 2012; Khodakovskaya et al., 2012; Landa et al., 2012; Kaveh et al., 2013; Yruela, 2013; Frazier et al., 2014; Gopalakrishnan Nair and Chung, 2014; Marmiroli et al., 2014; Nair and Chung, 2014; Wang et al., 2014; Chen et al., 2015; García-Sánchez et al., 2015; Thiruvengadam et al., 2015).
To summarize, NPs cause chromosomal anomalies such as chromatin condensation and micronuclei formation, disruption of cell division, and DNA damage, all of which lead to programmed cell death (PCD)/apoptosis (Shen et al., 2010; Panda et al., 2011; Thiruvengadam et al., 2015). They also damage chromosomes and the cell cycle (Sudhakar et al., 2001).
4 Application of nanoparticles in plant biotic resistance
Plant health and disease control have benefited from developing several nanotechnology applications. For plant protection, multiplexed bioassays are one of the biological and non-biological uses for NPs. Hazarika et al. (2022) state that managing diseases in agriculture necessitates the employment of nano-pesticides, nano-bactericides, nano-fungicides, and nano-insecticides, along with their carefully monitored distribution. It also covers using nanosensors, nanobarcodes, and nanotubes for diagnostic purposes. Antimicrobial biomolecules and NPs can potentially eliminate harmful microorganisms, including viruses, bacteria, fungi, and yeast, in many settings, as shown in Figure 2 (Abdelgawwad et al., 2020; Munir et al., 2023).
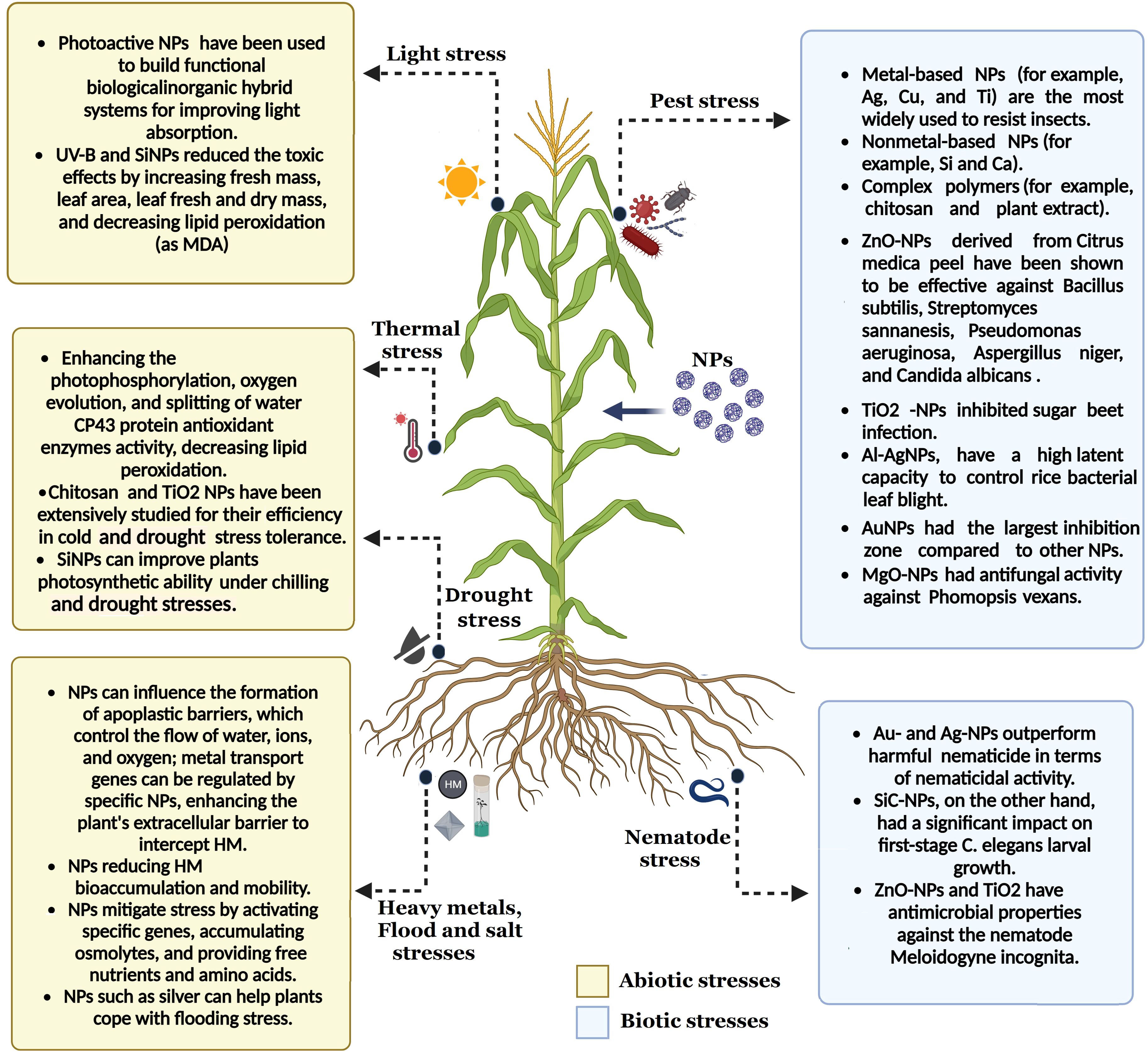
Figure 2. The key functions of nanoparticles in reducing the impact of different environmental stressors. Distinct effects of NPs on plant resistance have been shown, with blue representing biotic stress and yellow representing abiotic stress. This figure has been made using BioRender.
4.1 Insect infections
Insect pests represent substantial hazards to agricultural productivity, and the increased use of chemical pesticides has prompted concerns about environmental and health consequences (Nie et al., 2023). NPs have emerged as a promising option for pest control in agriculture because of their unique features, including large surface area, tiny size, and improved reactivity (Mittal et al., 2020). NPs may harm insects in various ways, causing them to perish or be unable to operate (Hazafa et al., 2021). Metal NPs, such as Ag-NPs and TiO₂-NPs, may kill insects by damaging cell structures, causing stress due to excess oxygen, and disrupting average body functioning (Saranya et al., 2020). Ag-NPs effectively control aphids (Aphis gossypii), whereas TiO₂-NPs and Ag-NPs significantly affect Spodoptera litura larvae (Jafir et al., 2021). NPs are more harmful than regular insecticides due to their small size and massive surface area, which allows them to penetrate deeper into insect bodies (Jafir et al., 2023). Carbon-based NPs, such as CNTs and polymer-based NPs, have shown promise for pest management (Yadav and Yadav, 2018). One advantage is that they may degrade organically, which benefits the environment. These NPs are generally designed to target specific pests, causing minimal damage to other organisms (Athanassiou et al., 2018).
Although NPs offer many benefits in controlling pests, there are still challenges to overcome, such as making them work better, using them more innovatively, and understanding how they affect the environment over time. More research is needed to fully realize the potential of NPs in pest control, ensuring they provide effective, sustainable, and environmentally friendly solutions for farming.
4.2 Fungus infection
Over 19,000 distinct types of fungi have been connected to plant diseases in agriculture worldwide (Jain et al., 2019). On the tissues of living and dead plants, they may lay dormant but alive until the conditions are right for their multiplication (Van Alfen, 2014). Certain fungi may multiply in the tissues of their hosts (Garrett, 1950). Fungus spores may be easily dispersed by soil, water, wind, and other invertebrates, including insects (Malloch and Blackwell, 1992). They might contaminate a whole crop in this way (Payne, 1998). Conversely, certain fungi benefit the host plant and could even help it grow (Mohammadi et al., 2011). One example of a mutualistic relationship is between mycorrhizae’s root systems and host plants (Johnson et al., 1997). Numerous plant diseases, including damping-off, root rot, mildew, dieback, coiled, scab, gall, blight, leaf spot, rust, and wilt, can be caused by pathogenic fungi (Jain et al., 2019). Nanotechnology is one of several methods used to mitigate the adverse effects of fungi infection in plants (Kutawa et al., 2021).
Metal NPs can be used in plant cultivation as fungicides or growth promoters (Hoang et al., 2022). The effects of Ag- and Cu-NPs on powdery mildew-affected leaves as well as on spontaneous ectomycorrhizal colonization in Quercus robur seedlings were documented by Olchowik et al. (2017). A significant decrease in the expansion of mycelial development was seen in spores treated with Ag-NPs (Li et al., 2022). As agricultural NPs are less harmful to people and animals, they are used far more often in plant disease control than store-bought fungicides (Malandrakis et al., 2019). Moreover, proteins, DNA, lipids, and other macromolecules can be harmed by the highly reactive hydroxyl radicals that Cu-containing fungicides can create (Demidchik, 2015). Banik and Pérez-de-Luque (2017) employed Cu-NPs to treat several plant diseases caused by Rhizobium spp., Oomycetes, Trichoderma harzianum, bacteria, and fungus. Alternaria alternata, Phytophthora syringae, and P. cinnamomi have all been found to grow less when Cu-NPs are mixed with non-nano Cu-like copper oxychloride (COC) (Munir et al., 2023). Cu-NPs may be advantageous to the agroecosystem as it has been demonstrated that they do not affect Rhizobium spp. or T. harzianum. In agricultural and food applications, ZnO-NPs can also be utilized as bactericides and fungicides (Banik and Pérez-de-Luque, 2017). ROS were produced by ZnO-NPs, damaging plant cells and triggering the plant’s defense system while improving plant growth and development (Sharma et al., 2019).
Regarding microbicidal activity, these NPs performed better than zinc particles in bulk (Siddiqi et al., 2018). Because they were tiny and had a high surface-to-volume ratio, they had good interaction with microorganisms (Ingle et al., 2014). Khan et al. (2021a) established the antibacterial and antifungal properties of Ag-NPs by utilizing them against Fusarium avenaceum, Fusarium graminearum, Fusarium coloratum, Erwinia sp., and Pseudomonas aeruginosa. Abdelmalek and Salaheldin (2016) assert that Ag-NPs have fungicidal action against A. alternata, Alternaria citri, and Penicillium digitatum. Ag-NPs are thought to possess antifungal properties against Rhizoctonia solani, Botrytis cinerea, Macrophomina phaseolina, A. alternata, S. sclerotiorum, and Curvularia lunata (Mansoor et al., 2021).
Additionally, Ag-NPs have antifungal properties against Bipolaris sorokiniana and Magnaporthe grisea (Rajeshkumar, 2019). Divya et al. (2017) report that chitosan-NPs have fungicidal efficacy against A. alternata, Macrophomia phaseolina, and Streptococcus pneumoniae. In addition, chitosan-NPs can be used as a fungicidal agent against Aspergillus niger and F. solani (Al-Sheikh and Yehia, 2016). On the other hand, several studies that produced Au-NPs also documented their effectiveness against various plant diseases as antifungals (Osonga et al., 2020).
Furthermore, it has been demonstrated that CuO-NPs have antifungal action against the following: Magnaporthe oryzae, P. digitatum, Sclerotium rolfsii, R. solani, Colletotrichum musae, and B. cinerea (Huang et al., 2015). CuO- and Cu2O-NPs have a fungicidal impact on Phytophthora infestans, as Giannousi et al. (2013) show. Evidence shows that a broad spectrum of fungi can be affected by the antifungal characteristics of different metal oxide NPs, such as Si-NPs, MgO-NPs, ZnO-NPs, and TiO2-NPs (Slavin and Bach, 2022). Sharma et al. (2016) provided evidence of MgO-NPs’ antifungal efficacy against Phomopsis vexans. Derbalah et al. (2018) state that Alternaria solani can benefit from silica NPs’ antifungal properties.
Additionally, Park et al. (2006) reported that M. grisea, R. solani, Pseudomonas syringae, Xanthomonas compestris, Pythium ultimum, and Colletotrichum gloeosporioides were among the bacteria impacted by the antifungal effect of Si/Ag-NPs. According to Jamdagni et al. (2018), ZnO-NPs also performed well against Penicillium expansum, F. oxysporum, B. cinerea, A. niger, and A. alternata. Furthermore, ZnO-NPs showed strong antifungal efficacy against Aspergillus fumigates, as demonstrated by Shinde (2015). ZnO-NPs have significant antifungal activity against Aspergillus nidulans, Aspergillus flavus, Rhizopus stolonifera, and T. harzianum (Gunalan et al., 2012). Dimkpa et al. (2013) reported that ZnO-NPs are efficient antifungally against F. graminearum. Furthermore, Hamza et al. (2016) found that TiO2-NPs have fungicidal properties against Cercospora beticola. On the other hand, the chemically and ecologically synthesized Ag-NPs displayed different antifungal activity (Tyagi et al., 2020).
4.3 Bacterial and viral infection
Bacteria are ubiquitous and can harm fungi, plants, and animals (Frey-Klett et al., 2011). In a bacterial cell, plasmids’ mobile genetic material outside the chromosome can transport crucial virulence or biological regulatory components (Partridge et al., 2018). Prophages, or bacteriophage DNA incorporated into the genome, may also be found in bacteria (Brüssow et al., 2004). Most bacteria proliferate by binary fission, which often entails the simultaneous duplication of extrachromosomal elements and chromosomal DNA (Baron, 1996). Conversely, viruses are non-cellular infectious agents limited to replicating within living cells (Koonin and Starokadomskyy, 2016). All species, including bacteria, plants, mammals, and archaea, are susceptible to viral infection (Kheyrodin et al., 2022). They can either replicate actively and control the host’s biosynthetic processes or integrate into the host’s genome and stay dormant as a provirus (Nagy and Pogany, 2012). A latent infection may arise from the suppression of transcription of the viral gene.
Most plant viruses are single-stranded and DNA-containing retroviruses and single-stranded or double-stranded RNA viruses (Shafiq et al., 2020). Ag, Cu, ZnO, and TiO2 are among the metal NPs whose antibacterial and antiviral qualities have been the subject of much research (Padmavathi and Anuradha, 2022). In addition to having inhibitory solid qualities, Ag-NPs have a wide range of antibacterial activities (Chen et al., 2020; Naveed et al., 2022a; Hayat et al., 2023). Ag-NPs have antibacterial activities against Escherichia coli and Bacillus subtilis (Shehzad et al., 2018), Staphylococcus aureus, and Klebsiella pneumoniae (Hussein et al., 2019; Waseem et al., 2023). Additionally, Ag-NPs prevented the growth of three dangerous food-borne bacteria: Pseudomonas aeruginosa, E. coli, and B. subtilis (Mohanta et al., 2017). Ag-NPs and an Ag-chitosan composite, according to Shahryari et al. (2020), suppressed the growth of P. syringae bacteria. According to Dang et al. (2019), Au-NPs were also demonstrated to possess bactericidal characteristics concerning E. coli. A range of harmful bacteria were shown to be susceptible to the bactericidal effects of distinct metal oxide NPs, such as MgO-NPs against Ralstonia solanacearum (Sharma et al., 2016), Cu composites against the bacterium Xanthomonas euvesicatoria (Fan et al., 2021), and ZnO-NPs against E. coli and solanacearum (Imada et al., 2016; Attar and Yapaoz, 2018).
Plants treated with ZnO-NPs both before and during the bacterial inoculation were able to stop the Pantonea ananatis bacterium from proliferating throughout the maize crop (Shahid et al., 2021). ZnO-NPs were also demonstrated to efficiently reduce the bacterial blight diseases that pea plants contracted from P. syringae and M. incognita (Kashyap, 2022). Furthermore, ZnO-NP additions to the soil increased rhizospheric microbial diversity, stimulated antioxidant response and plant growth in tomato plants, and reduced the occurrence of R. solanacearum-caused diseases (Jiang et al., 2021). ZnO-NPs derived from Matricaria chamomilla flower extract were found to be bactericidal against R. solanacearum and to reduce bacterial wilt disease in tomato plants (Khan et al., 2021b). The same ZnO-NPs derived from Citrus medica peel are effective against B. subtilis, Streptomyces sannanesis, P. aeruginosa, A. niger, and Candida albicans (Keerthana et al., 2021). Furthermore, biogenic ZnO-NPs derived from Trichoderma reesei, T. harzianum, and co-culture, as well as the Paenibacillus polymyxa strain Sx3, were used to inhibit the growth of Xanthomonas oryzae (Shobha et al., 2020). Furthermore, several studies discovered that TiO2-NPs inhibited sugar beet infection (cause: P. syringae pv. aptata), apple scab disease (cause: Venturia inaequalis), Fusarium wilt diseases in tomato and potato plants (cause: F. solani), and bacterial blight on geranium and leaf spot on poinsettia (cause: F. solani) (Hamza et al., 2016). Rice bacterial leaf blight was shown to be significantly inhibited by AgNPs, especially Al-Ag-NPs, as revealed by Tian et al. (2022).
4.4 Nematode infection
Nematode infestations adversely affect plant growth and vitality in the majority of crops (El-Sappah et al., 2022a). Large feeding cells are produced by the parasites’ infection of plant roots, which reduces plant nutrition and water uptake (El-Sappah et al., 2019). This can cause plants to wilt and stunt, which makes them more vulnerable to diseases and significantly lowers their yield. Of the more than 100 species of nematodes that have been discovered, root-knot nematodes (RKNs), also called Meloidogyne spp., are the most harmful (El-Sappah et al., 2019), with the annual global cost of RKNs being $100 billion (Khan, 2015). Hussain et al. (2016) claim that because of their fast rate of reproduction and broad host range, RKNs are challenging to regulate (Hussain et al., 2016). Some conventional means of controlling nematodes include crop rotation, chemical nematodes, cultivating resistant plant varieties, management of resistant varieties, and managing fallow land (Sivasubramaniam et al., 2020).
To manage significant plant-parasitic nematodes, nemacids are utilized despite their severe toxicity and environmental concerns (Kiriga, 2018). A multi-site mode of action has been demonstrated for NPs, making them efficient nematicides against various parasitic plant worms (Abdollahdokht et al., 2022). Comparing Au- and Ag-NPs to synthetic and hazardous nematicides, Thakur and Shirkot (2017) claim that the former have better nematicidal activity. As promising findings in treating plant diseases caused by RKNs like M. incognita are being obtained, the utilization of easily accessible materials for nanotechnology is increasing, according to Sharon et al. (2010). The number of deaths from M. incognita J2 increased after Si-NPs were applied because the SiC-NPs significantly altered the first phase of C. elegans larval development (Al Banna et al., 2018). According to Thounaojam et al. (2021), ZnO-NPs have antibacterial activity against nematodes, bacteria, and fungi, including M. incognita. TiO2-NPs, on the other hand, can be used to treat nematodes and viruses (Almoneafy et al., 2023). M. incognita in tomato plants was susceptible to the nematicidal effects of TiO2 (Ardakani, 2013).
5 Application of nanoparticles in abiotic stresses
Among the most thoroughly researched effective NPs include nanoscale crystalline powders (Fe, Co, and Cu), metal-oxide NPs (Fe2O3-NPs, TiO2-NPs, ZnO-NPs, SiO2-NPs, CuO-NPs, and CaCO3-NPs), fullerols, metal-based NPs (Ag-NPs and Au-NPs), and carbon nanotubes (CNTs) (Singh et al., 2021). Because of NPs’ high surface energy and high surface/volume ratio, which enhance their heightened metabolic activity and responsiveness, plants undergo a range of consequences (Juárez-Maldonado et al., 2019). NPs swiftly agitate plants through their molecular processes (Ahmad et al., 2020). Furthermore, NMs have two functions: first, they trigger OS, which triggers plants’ antioxidant defense system to guard against ROS (Figure 2) (González‐García et al., 2021).
5.1 Drought stress
As they grow and develop, plants face various environmental problems in both natural and agricultural situations (El-Sappah and Rather, 2022). Drought is one of the most detrimental environmental conditions to plant production (Seleiman et al., 2021; El-Sappah and Rather, 2022). Approximately 80%–95% of the fresh biomass in a plant is made up of water, which is crucial for several physiological functions, such as metabolism, growth, and development (Abbasi and Abbasi, 2010; Brodersen et al., 2019). Because of this, some believe that drought is the primary environmental stressor for different plants, especially in locations that are prone to drought (Anjum et al., 2011; Diatta et al., 2020), that it poses the greatest danger to future global food security, and that it has historically caused large-scale famines. Several NPs can be used to modify drought stress; research has demonstrated that silica NPs can increase plants’ resistance to drought (Chandrashekar et al., 2023). Ashkavand et al. (2015) found that silica NPs enhanced seedling development and physiological parameters in hawthorns under drought stress. Similarly, Triticum aestivum showed improvements in starch and gluten levels and higher growth and yield in response to dryness and wit (Li et al., 2023d). This modification has happened because TiO2 can promote seed germination and seedling growth (Shafea et al., 2017). During droughts, TiO2 also boosts biomass in plants, preserves relative water content (RWC), and promotes antioxidant enzymes (Ostadi et al., 2022). By regulating the amount of proline and promoting proline production, jute seedlings treated with hydroxyapatite NPs demonstrated enhanced drought resistance (Das et al., 2016).
Drought stress negatively affected B. napus while significantly impeding and delaying the growth of corn seedlings; yet, application of yttrium-doped Fe2O3-NPs improved photosynthetic apparatus, resulting in higher levels of carotenoid and chlorophyll (Palmqvist et al., 2017). By using seed reserves more quickly, ZnO in G. max increased seed germination percent and dry weight due to increased gibberellin activity (Sedghi et al., 2013). Similarly, Fe2O3 improved drought resistance by altering glucose metabolism and stomatal mobility (Sarraf et al., 2022). Micro ZnO has been shown in studies on maize to slow down the degradation of photosynthetic pigment, speeding up stomatal movement and photosynthesis (Chandrashekar et al., 2023). Necessary enzymes, including phosphoglucoisomerase, cytoplasmic invertase, and UDP glucose pyrophosphorylase, were modified to improve starch and sucrose production and drought stress performance (Chen et al., 2018). Zinc oxide (ZnO) might be employed as a nano agent to lessen the harmful impacts of drought stress (Jafir et al., 2024). Van Nguyen et al. (2021) report that CuO-NPs in maize favorably impact the pigment system and the ROS scavenging mechanism. It has a favorable effect on yield and aids in supplement absorption and stress resistance. Another study by Li et al. (2023b) found that foliar application of Zn-NPs, rather than ZnSO4, is beneficial in increasing turnip plant growth and yield under drought stress.
5.2 Thermal stress
From a physical standpoint, heat and cold are in the same temperature range (El-Sappah and Rather, 2022). However, in order to maintain their survival and the success of their reproduction, organisms respond to dramatically varied temperature regimes on a biological level (Acevedo-Whitehouse and Duffus, 2009). Sessile plants are subject to daily variations in temperature and seasonal variations brought on by climate change, as they cannot seek shelter (Leisner et al., 2023). According to Penfield (2008), temperature signals are a crucial factor in making decisions about the life history, including when to blossom or germinate and how long to keep seeds dormant. On the other hand, temperature cues can cause plants to begin tolerance or escape mechanisms at any stage of their life cycle (Dai Vu et al., 2019; De Smet et al., 2021; Zhu et al., 2021). Specifically, plants can acquire two tolerance mechanisms in response to almost fatal temperatures: freezing tolerance and heat stress tolerance (Hincha and Zuther, 2014; Ritonga and Chen, 2020; Haider et al., 2022). While insufficient temperature conditions might lead to optimum performance, small temperature changes within the physiological range typically result in (growth) acclimation responses (Quint et al., 2016; Casal and Balasubramanian, 2019). Hasanuzzaman et al. (2013) state that high ROS levels cause OS, which harms growth, development, and yield. As a result, plants adapt morphologically and biochemically to withstand heat stress (Bhardwaj et al., 2023).
Plants respond to this abiotic stress by triggering signaling pathways that produce osmolytes, which regulate the osmotic pressure in cells to preserve turgidity and other secondary metabolites that alter the antioxidant system (Arif et al., 2020). Increased photophosphorylation, oxygen evolution, and splitting of the water CP43 protein (Pradhan et al., 2013), nitrogen metabolism (Pradhan et al., 2014), photosynthetic capacity (Younis et al., 2020), antioxidant enzyme activity, decreased lipid peroxidation (Hassan et al., 2021a), and restoration of ultrastructural distortions of chloroplasts and the nucleus (Younis et al., 2020) are indications of the beneficial effects of NPs on plants under temperature stress. Extensive research has been conducted on the ability of TiO2-NPs and chitosan to withstand cold stress (Singh and Husen, 2019; Pramanik et al., 2023). Ti-NPs are helpful in cold-stressed chickpea plants for enhancing photosynthetic activity, electrolyte leakage, and membrane damage through transcriptional regulation (Singh and Husen, 2019).
The antioxidative system and transcription factors involved in the chilling response may be used by rice plants when ZnO-NPs are applied foliarly (Mirakhorli et al., 2021). Increased photosynthetic capacity in sugarcane plants under cooling stress may also result from Si-NPs (Hassan et al., 2021b). Se-NPs are effective in mitigating adverse effects, including membrane damage, reduced pollen germination, and lower crop yields, when used to strengthen the antioxidant defense system in sorghum plants subjected to high temperatures (Djanaguiraman et al., 2018). Ag-NPs encouraged morphological growth in wheat plants, protecting them against heat stress (Iqbal et al., 2019a). Zinc NPs have been shown to reduce lipid peroxidation and increase the synthesis of antioxidant enzymes, hence improving wheat’s resistance to heat stress (Hassan et al., 2018). Tomato leaves have foliar coatings of NPs that activate when temperatures rise over specific thresholds, shielding the plants from heat stress. Si-NPs could potentially be helpful in lowering heat stress (Kim et al., 2017).
5.3 Heavy metal stress
HM stress is one of the current negative factors affecting agricultural output (El- Sappah et al., 2021; El-Sappah et al., 2023). HM contamination has risen globally due to human activities, including industrialization and urbanization (Abbas et al., 2022a; Li et al., 2023a). Additionally, aggravating crop plant HM stress is the increasing use of chemical pesticides and fertilizers in agriculture (El-Sappah et al., 2024). HMs, such as Ag, Pb, Cd, Ni, Co, Cr, and Hg, can harm plants (Rashid et al., 2023). HMs can persist in the soil for long since they do not biodegrade (Priya et al., 2023). The mobility and availability of HMs are regulated by the rhizosphere, which has a varied root microbiome that enhances soil fertility and is heavily influenced by these substances, soil type, and biogeochemical processes (such as mineralization, precipitation, adsorption, and protonation) (Aguirre-Becerra et al., 2022). Several strategies have been put out to lessen the harmful consequences of abiotic stress (Hossain et al., 2021).
The capacity of NPs to immobilize metal ions by chemical reduction, oxidation, or absorption is another essential feature that makes them useful for soil remediation in many different nations (Rajput et al., 2022). Artificial neural patches, also known as NPs, can affect some plant processes, including the creation of apoplastic barriers that regulate the movement of water, ions, and oxygen; the regulation of metal transport genes by particular NPs that fortify the plant’s extracellular barrier against HMs; the chelation of organic acids accumulated in the cell walls of roots and leaves with HM to lessen the harm that HM stress causes to plants; and, lastly, the activation defense system. Many studies have been conducted on using NPs to reduce HM stress (Zhou et al., 2020). The HMs from the soil can be changed and absorbed by adding NPs, which lowers their mobility and bioaccumulation (Zhou et al., 2020). The amount of Cd metal available in the soil has decreased after Fe3O4-NP treatment (Wang et al., 2020). Hydroxyapatite NPs maintain soil pH and lessen the harmful effects of metals in the soil by releasing phosphate ions (Cui et al., 2018).
Furthermore, NPs stimulate the apoplast barriers to form, which reduces the concentration of HMs in the root. Additionally, plants with certain NPs that might impede HM translocation by building complexes with them can have their metal transporter genes altered to redirect HMs (Wang et al., 2021). By promoting the synthesis of organic acids, Si-NPs have lessened the harm that HM stress has produced (Cui et al., 2017; Wu et al., 2017).
5.4 Salt stress
An osmoregulation strategy is utilized to counteract OS brought on by the generation of ROS, which exacerbates cytotoxicity and nutritional imbalance brought on by an excessive rise in sodium (Na+) and chloride (Cl) (Das and Roychoudhury, 2014; Ihtisham et al., 2023). When organic molecules, including sugars, glycine betaine, amino acids, polyols, and quaternary ammonium compounds, are absorbed by a plant, their osmotic potential is reduced during the process of osmoregulation (Ghosh et al., 2021b). Another crucial tactic to counteract the ROS impact and activate the enzymatic machinery is ion homeostasis, which involves increasing the concentration of K+ and decreasing the concentration of Na+ in the cell (Tripathy and Oelmüller, 2012). By boosting osmolytes, activating specific genes, and supplying free nutrients and amino acids, NPs reduce stress (Mittal et al., 2020; Zia-ur-Rehman et al., 2023). The rate of plant transpiration, water usage efficiency (WUE), enzyme carbonic anhydrase activity, and Cucurbita pepo’s protective response to salt stress were all enhanced by SiO2-NP treatment (Siddiqui et al., 2014). Inside the ETC, TiO2 (anatase) blocks linolenic acid and modifies photoreduction activity (Mingyu et al., 2008).
According to a study, using a foliar spray of Abelmoschus esculentus ZnO enhances photosynthetic efficiency and enzymatic machinery to lessen the adverse impacts of salt stress (Alabdallah and Alzahrani, 2020). Raising photosystem II’s efficiency improved photosynthesis and positively affected plant development. In addition, it lessens membrane damage and aids in maintaining RWC (Alabdallah and Alzahrani, 2020). Similarly, foliar spraying ZnO and Si to mango seedlings improved growth circumstances by increasing carbon assimilation and nutrient absorption (Elsheery et al., 2020). Numerous studies on the application of SiO2 have verified the enhanced vegetative growth, increased thickness of the epicuticular wax layer, accumulation of proline, and altered expression of genes related to salt stress in S. lycopersicum, strawberries, and Ocimum basilicum, among other plants (Almutairi, 2016; Avestan et al., 2019; Oprica et al., 2021; Gou et al., 2023). According to reports, one well-known nanomaterial that may be utilized as a possible nano-agent to lessen salt stress is Ag-NPs. Ag-NPs caused T. aestivum to accumulate more POD, proline, and sugar, and a rise in germination (Mohamed et al., 2017).
To enhance photosynthetic carbon absorption, boost proteins and amino acids during the reproductive stage, and confer resistance to salt stress, CeO, CNTs, and graphene NPs were applied to Gossypium and Catharanthus roseus (McGehee et al., 2019; An et al., 2020). ZnO increased the germination of cumin seeds and enhanced the salt tolerance of lupine plants by lowering malondialdehyde (MDA) and Na+ levels. By maintaining appropriate osmoregulation, reducing MDA and Na+ levels, and strengthening photosynthetic system activity, n-ZnO was able to mitigate the detrimental effects of NaCl (Abdel Latef et al., 2017). According to Zulfiqar et al. (2019), NPs such as SiO2-NPs, Cu-NPs, Fe-NPs, Mn-NPs, C-NPs, Ti-NPs, Ce-NPs, and K-NPs were helpful in reducing the detrimental effects of salt stress in a variety of plants. El-Sharkawy et al. (2017) and Jafir et al. (2022) found that applying K-NPs topically, lowering electrolyte leakage, and raising proline and antioxidant-enzyme content, such as catalase, might all help enhance salt tolerance by foliar treatment. It has been demonstrated that cerium oxide NPs may improve mineral absorption and modify root cells in Brassica napus, hence boosting photosynthetic activity (Al-Khayri et al., 2023b). More and more research points to the possibility that plants might employ NPs to significantly reduce the detrimental effects of salt stress.
5.5 Flood stress
By modifying root cells and enhancing mineral absorption, cerium oxide NPs have been shown to help boost photosynthetic activity in B. napus (Al-Khayri et al., 2023b). Research suggests that treating plants with NPs may significantly reduce the detrimental effects of salt stress and modulate their responses (Zulfiqar et al., 2019). According to reports, plants stressed by flooding can gain from exposure to NPs. Ag-NPs controlled proteins, glycolysis, amino acid synthesis, and wax production in soybean plants to reduce flooding stress and promote plant development (Mustafa et al., 2015; 2016). The influence of Ag-NPs on the synthesis of the cytotoxic marker glyoxalase II 3 was one of the most noteworthy findings of proteomics. Plant tolerance to floods is increased when Ag-NPs are combined with potassium nitrate (KNO3) and nicotinic acid (Hashimoto et al., 2020). Soybeans’ resistance to flood stress was markedly enhanced by an additional Al2O3 metal NPs (Mustafa and Komatsu, 2016).
Additionally, because S-adenosyl-l-methionine-dependent methyltransferases and enolase are implicated, soybeans subjected to Al2O3-NPs undergo recovery (Yasmeen et al., 2016). Additionally, the use of NPs accelerates the kinetics of flooding stress recovery. NP size affects flood tolerance more than NP amount and kind, according to Mustafa and Komatsu (2016) research. When plants were exposed to flood conditions, three distinct sizes of Al2O3-NPs caused various biochemical reactions. Isobutane dehydrogenase’s catalytic activity was enhanced by applying Al2O3-NPs; however, under flood circumstances, Al2O3-NPs caused the synthesis of ribosomal proteins, and the mitochondria’s membrane permeability was increased by large concentrations of Al2O3-NPs (Mustafa and Komatsu, 2016).
5.6 Light stress
Plant chloroplasts use sunlight to convert carbon dioxide and water into organic matter and oxygen (Alberts, 2017). Light is an essential environmental factor for photosynthesis since it fluctuates in time and place concerning its spectral quality and light intensity (Shafiq et al., 2021). Light may be a significant abiotic stressor for plants when illumination conditions are unsuitable for their growth (Fiorucci and Fankhauser, 2017). Variable light levels can reduce photosynthetic efficiency, cause photodamage in high light, and prevent plant development in low light. When plants experience light stress, especially severe light stress, they experience photoinhibition, which results in an imbalanced energy distribution between photosystem I (PSI) and photosystem II (PSII) and a precipitous drop in photosynthetic efficiency (Walters, 2005).
Plants have developed defense mechanisms against light stress, which include the production of anthocyanins, movement of chloroplasts, generation and scavenging of chloroplastic ROS, opening and closing of stomata, and coordination of responses through systemic signaling (Shi et al., 2022). Because of their vulnerability to damage, the molecular devices that perform photosynthesis known as photosynthetic apparatuses have evolved fast responses to light stress (Shi et al., 2022). The complex states and structures of proteins linked to the thylakoid membrane are altered as one such response (Pottosin and Shabala, 2016). Plants have evolved defense strategies in response to light stress, which include the production of anthocyanins, movement of chloroplasts, stomata opening and closing, generation and scavenging of chloroplastic ROS, and coordination of responses through systemic signaling. Because of their vulnerability to harm, the molecular devices that perform photosynthesis, called photosynthetic apparatuses, have evolved rapid responses to light stress.
According to Pottosin and Shabala (2016), one such reaction is altering proteins’ complex states and structures attached to the thylakoid membrane. Increased NP concentrations may harm the photosynthetic machinery, inhibit Rubisco activity, produce toxic ROS, or interfere with CO2 reduction or photosynthetic electron transport (Singh et al., 2015). For these reasons, research is ongoing to determine the ideal dosages for maximizing photosynthesis (Li et al., 2023c).
Furthermore, most studies evaluate the effects of NPs on photosynthesis in stressful circumstances (like heat waves, droughts, or trace metals) or how to enhance this biological activity under regular lighting (Nayeri et al., 2023). Although the measurement’s primary focus is the energy conversion efficiency, light is not often used as a stressor in studies (e.g., high or low light intensities, harmful wavelengths, or inadequate photoperiods) (Zhang et al., 2020). Si-NPs were given to hydroponically grown wheat seedlings after exposure to UV-B light (Ruttkay-Nedecky et al., 2017; Tripathi et al., 2017).
These substances reduced the harmful effects by lowering lipid peroxidation (as MDA), raising fresh mass, leaf area, and fresh and dry leaf mass (Rizwan et al., 2019). UV-B significantly decreased total chlorophyll, and these reductions were present in Si-NPs (Tripathi et al., 2017). Additionally, fewer and milder formazan spots were observed in the leaves, suggesting a preventive function against OS caused by UV-B-induced H2O2 production. Furthermore, the loss of plant leaf thickness was compensated by less internal leaf damage from deformed palisade and mesophyll layers (Tripathi et al., 2017).
6 Hazard, challenges, and prospective
Because of their unique characteristics and inventive features, NPs have been widely used in various industries, including catalysts, semiconductors, environmental energy, medication delivery, and cosmetics (Nel et al., 2006). Scholars are now considering the challenges, issues, and consequences of NPs’ environmental impact because of their widespread and uncontrolled use (Gottschalk et al., 2015; Tolaymat et al., 2015). NPs improve plants’ ability to adapt to stress by altering critical metabolic pathways, boosting antioxidant defense systems, and improving processes that detoxify free radicals (Jalil and Ansari, 2019). Specific plant components such as seeds, flowers, fruits, stems, bark, leaves, peels, and roots have generated a broad spectrum of NPs, including iron, gold, platinum, palladium, silver, zinc, selenium, copper, and others (Ishak et al., 2019; Zhao et al., 2024). In stressed settings, these NPs have been demonstrated to rewire plant physio-biochemical processes (Kumari et al., 2022).
Moreover, a range of NPs have been shown to have a substantial impact on how plants react to environmental stressors such as heat stress, salinity, drought, and HM toxicity (Venkatachalam et al., 2017; Iqbal et al., 2019b; Sreelekshmi et al., 2020). Moreover, it has been shown that microorganisms, including viruses, bacteria, actinomycetes, and fungi, are all equally capable of creating NPs, which have a wide range of usefulness in easing different environmental restrictions (Ghosh et al., 2021a). Most studies involving nanomaterials in agriculture are conducted in labs or on a small scale, and these materials are still in the early phases of development (Iavicoli et al., 2017). As a result, little is known about the advantages of various NPs as well as the potential risks to the public’s health and the environment if they are used in specific applications (Kumah et al., 2023). Determining the effectiveness of these substances when added to the soil and encouraging greener practices and the sustainability of agricultural systems is thus of particular importance (Gupta et al., 2023). It is typically possible that metallic NPs have incredibly beneficial impacts on germination (Santás-Miguel et al., 2023). Regretfully, most tests are carried out under carefully monitored circumstances in laboratories. Owing to their small size, NPs present several environmental risks, such as ease of transport and dispersion, the potential to be ecotoxic, persistent in the environment, and capable of bioconcentrating or bioaccumulating in higher organisms (Ray et al., 2009). There is also a chance that these processes may be reversible.
Additional studies are needed to determine the toxicity and ecotoxicity of various NPs on land and aquatic creatures in the food chain because of these potential environmental hazards. Therefore, research on risk assessment is necessary to understand the possible impacts on non-target species and their significance for agricultural productivity and healthy soils (Chauhan and Gill, 2023). More investigation is essential to comprehend how NPs interact with cellular components and, therefore, their function in biochemical reactions through OS pathways, as they can operate more quickly than larger-sized NPs. The public’s health is in danger from ingesting contaminated food and drink, skin contact, inhalation, or exposure to NPs through polluted air (Handy and Shaw, 2007). Thus far, genotoxicity, pulmonary illnesses, OS, and lipid peroxidation have been reported as consequences.
DNA mutations have been reported to cause cell harm in other, more significant cases (Bhabra et al., 2009). Therefore, one must take caution while using NPs until the safety parameters required for proper usage are established. After the environmental and public health effects are understood, nations, accountable institutions, or groups can create the necessary laws and regulations for using these NPs in agriculture.
7 Conclusion
Numerous environmental influences on plants limit and reduce agricultural crop yields. Plants are exposed to two environmental stressors: biotic and abiotic stress. Nanotechnology is one of the many approaches to tackle these pressures. In the face of a burgeoning global population, agricultural nanotechnology has become a powerful tool for aiding crops and increasing agricultural output. It also helps plants adapt to environmental conditions. The molecular effects of NPs on diverse plant species are not the same. For example, certain plants are more vulnerable to the ROS mode generation by NPs, while others undergo metabolic changes and differential expression of defense-related genes. The usage of NPs provides benefits; however, there are also downsides for public and plant health. Plants are vulnerable to various molecular effects caused by NPs, which begin with the activation of the ROS mode, progress to metabolic changes, and conclude in the differential expression of specific defense genes. Despite their many applications, NPs are detrimental to human and plant health.
Author contributions
XZ: Conceptualization, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. AE-S: Conceptualization, Writing – original draft, Writing – review & editing. AK: Writing – review & editing. QH: Writing – review & editing. AA: Writing – review & editing. ME: Writing – review & editing. MI: Writing – review & editing. ME-M: Writing – review & editing. SS: Writing – review & editing. WT: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Supported by Solid-state Fermentation Resource Utilization Key Laboratory of Sichuan Province (No.2022GTYY05 and 2015GTY009) and the Natural Science Foundation of Sichuan Province (No. 2023NSFSC1265).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NPs, nanoparticles; Au-NPs, gold nanoparticles; PSI, photosystem I; PSII, photosystem II; Si-NPs, silica nanoparticles; Fe3O4, iron oxide; SiO2, silica; Cu-NPs, copper nanoparticles; Ag-NPs, silver nanoparticles; TiO2-NPs, titanium dioxide nanoparticles; ZnO-NPs, zinc oxide nanoparticles; CNTs, carbon nanotubes; GO, graphene oxide; Al2O3-NPs, aluminum oxide nanoparticles; CeO2-NPs, cerium oxide nanoparticles; In2O3-NPs, indium oxide nanoparticles; CuO-NPs, copper oxide nanoparticles; ethylene, CH2=CH2; LSPR, localized surface plasma resonance; OS, oxidative stress; WS2, tungsten disulfide; ROS, reactive oxygen species; GOQD, graphene oxide quantum dot; PCD, programmed cell death; UDP, uridine diphosphate; MDA, malondialdehyde.
References
Abbas, M., Li, Y., Elbaiomy, R. G., Yan, K., Ragauskas, A. J., Yadav, V., et al. (2022a). Genome-Wide Analysis and Expression Profiling of SlHsp70 Gene Family in Solanum lycopersicum Revealed Higher Expression of SlHsp70-11 in Roots under Cd2+ Stress. FBL 27, 186. doi: 10.31083/j.fbl2706186
Abbas, M., Yan, K., Li, J., Zafar, S., Hasnain, Z., Aslam, N., et al. (2022b). Agri-nanotechnology and tree nanobionics: Augmentation in crop yield, biosafety, and biomass accumulation. Front. bioengineering Biotechnol. 10, 853045. doi: 10.3389/fbioe.2022.853045
Abbasi, T., Abbasi, S. (2010). Biomass energy and the environmental impacts associated with its production and utilization. Renewable Sustain. Energy Rev. 14, 919–937. doi: 10.1016/j.rser.2009.11.006
Abdelgawwad, M. R., Mahmutović, E., Al Farraj, D. A., Elshikh, M. S. (2020). In silico prediction of silver nitrate nanoparticles and Nitrate Reductase A (NAR A) interaction in the treatment of infectious disease causing clinical strains of E. coli. J. Infection Public Health 13, 1580–1585. doi: 10.1016/j.jiph.2020.08.004
Abdel Latef, A., Alhmad, A. ,. M. F., Abdelfattah, K. E. (2017). The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 36, 60–70. doi: 10.1007/s00344-016-9618-x
Abdelmalek, G., Salaheldin, T. (2016). Silver nanoparticles as a potent fungicide for citrus phytopathogenic fungi. J. Nanomedicine Res. 3, 00065. doi: 10.15406/jnmr.2016.03.00065
Abdollahdokht, D., Gao, Y., Faramarz, S., Poustforoosh, A., Abbasi, M., Asadikaram, G., et al. (2022). Conventional agrochemicals towards nano-biopesticides: An overview on recent advances. Chem. Biol. Technol. Agric. 9, 1–19. doi: 10.1186/s40538-021-00281-0
Abideen, Z., Hanif, M., Munir, N., Nielsen, B. L. (2022). Impact of nanomaterials on the regulation of gene expression and metabolomics of plants under salt stress. Plants 11, 691. doi: 10.3390/plants11050691
Acevedo-Whitehouse, K., Duffus, A. L. (2009). Effects of environmental change on wildlife health. Philos. Trans. R Soc. Lond B Biol. Sci. 364, 3429–3438. doi: 10.1098/rstb.2009.0128
Aguirre-Becerra, H., Feregrino-Perez, A. A., Esquivel, K., Perez-Garcia, C. E., Vazquez-Hernandez, M. C., Mariana-Alvarado, A. (2022). Nanomaterials as an alternative to increase plant resistance to abiotic stresses. Front. Plant Sci. 13, 1023636. doi: 10.3389/fpls.2022.1023636
Ahmad, J., Qamar, S., Kausar, N., Qureshi, M. I. (2020). Nanoparticles: the magic bullets in mitigating drought stress in plants. Nanobiotechnology Agriculture: Approach Towards Sustainability, 145–161. doi: 10.1007/978-3-030-39978-8_8
Alabdallah, N. M., Alzahrani, H. S. (2020). The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi J. Biol. Sci. 27, 3132–3137. doi: 10.1016/j.sjbs.2020.08.005
Al Banna, L., Salem, N., Ghrair, A., Habash, S. (2018). Impact of silicon carbide nanoparticles on hatching and survival of soil nematodes Caenorhabditis elegans and Meloidogyne incognita. Appl. Ecol. Environ. Res. 16, 2651–2662. doi: 10.15666/aeer/1603_26512662
Ali, A., Shah, T., Ullah, R., Zhou, P., Guo, M., Ovais, M., et al. (2021). Review on recent progress in magnetic nanoparticles: Synthesis, characterization, and diverse applications. Front. Chem. 9, 629054. doi: 10.3389/fchem.2021.629054
Al-Khayri, J. M., Alnaddaf, L. M., Jain, S. M. (2023a). Nanomaterial interactions with plant cellular mechanisms and macromolecules and agricultural implications (Switzerland: Springer Nature).
Al-Khayri, J. M., Rashmi, R., Surya Ulhas, R., Sudheer, W. N., Banadka, A., Nagella, P., et al. (2023b). The role of nanoparticles in response of plants to abiotic stress at physiological, biochemical, and molecular levels. Plants (Basel) 12, 292. doi: 10.3390/plants12020292
Almoneafy, A. A., Algam, S. A., Alhammadi, A. S., Moustafa-Farag, M., Moghalles, M. A. (2023). “Role of fungi-mediated nanoparticles in mitigation of biotic and abiotic stresses in plants,” in Fungal cell factories for sustainable nanomaterials productions and agricultural applications (Elsevier), 601–633.
Almutairi, Z. M. (2016). Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (‘Solanum lycopersicum’L.) seedlings under salt stress. Plant Omics 9, 106–114. doi: 10.3316/informit.888088806058398
Al-Sheikh, H., Yehia, R. (2016). In vitro antifungal efficacy of Aspergillus Niger ATCC 9642 chitosan-AgNPs composite against post-harvest disease of citrus fruits. Appl. Biochem. Microbiol. 52, 413–420. doi: 10.1134/S0003683816040177
An, J., Hu, P., Li, F., Wu, H., Shen, Y., White, J. C., et al. (2020). Emerging investigator series: molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ. Science: Nano 7, 2214–2228. doi: 10.1039/D0EN00387E
Anjum, S. A., Xie, X., Wang, L. C., Saleem, M. F., Man, C., Lei, W. (2011). Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 6, 2026–2032. doi: 10.5897/AJAR10.027
Ardakani, A. S. (2013). Toxicity of silver, titanium and silicon nanoparticles on the root-knot nematode, Meloidogyne incognita, and growth parameters of tomato. Nematology 15, 671–677. doi: 10.1163/15685411-00002710
Arif, I., Batool, M., Schenk, P. M. (2020). Plant microbiome engineering: expected benefits for improved crop growth and resilience. Trends Biotechnol. 38, 1385–1396. doi: 10.1016/j.tibtech.2020.04.015
Ashkavand, P., Tabari, M., Zarafshar, M., Tomásková, I., Struve, D. (2015). Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. Leśne Prace Badawcze 76, 350–359. doi: 10.1515/frp-2015-0034
Ashraf, S. A., Siddiqui, A. J., Abd Elmoneim, O. E., Khan, M. I., Patel, M., Alreshidi, M., et al. (2021). Innovations in nanoscience for the sustainable development of food and agriculture with implications on health and environment. Sci. Total Environ. 768, 144990. doi: 10.1016/j.scitotenv.2021.144990
Asl, K. R., Hosseini, B., Sharafi, A., Palazon, J. (2019). Influence of nano-zinc oxide on tropane alkaloid production, h6h gene transcription and antioxidant enzyme activity in Hyoscyamus reticulatus L. hairy roots. Eng. Life Sci. 19, 73–89. doi: 10.1002/elsc.201800087
Asli, S., Neumann, P. M. (2009). Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 32, 577–584. doi: 10.1111/j.1365-3040.2009.01952.x
Athanassiou, C., Kavallieratos, N., Benelli, G., Losic, D., Usha Rani, P., Desneux, N. (2018). Nanoparticles for pest control: current status and future perspectives. J. Pest Sci. 91, 1–15. doi: 10.1007/s10340-017-0898-0
Attar, A., Yapaoz, M. A. (2018). Biomimetic synthesis, characterization and antibacterial efficacy of ZnO and Au nanoparticles using eChinacea flower extract precursor. Materials Res. Express 5, 055403. doi: 10.1088/2053-1591/aac05f
Avestan, S., Ghasemnezhad, M., Esfahani, M., Byrt, C. S. (2019). Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 9, 246. doi: 10.3390/agronomy9050246
Balmert, S. C., Little, S. R. (2012). Biomimetic delivery with micro-and nanoparticles. Advanced materials 24, 3757–3778. doi: 10.1002/adma.201200224
Banerjee, K., Pramanik, P., Maity, A., Joshi, D., Wani, S., Krishnan, P. (2019). “Methods of using nanomaterials to plant systems and their delivery to plants (Mode of entry, uptake, translocation, accumulation, biotransformation and barriers),” in Advances in phytonanotechnology (Elsevier), 123–152.
Banik, S., Pérez-De-Luque, A. (2017). In vitro effects of copper nanoparticles on plant pathogens, beneficial microbes and crop plants. Spanish J. Agric. Res. 15, e1005–e1005. doi: 10.5424/sjar/2017152-10305
Baron, S., Fons, M, Albrecht, T (1996). Viral Pathogenesis. In: Baron, S. editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston. Chapter 45. Available from: https://www.ncbi.nlm.
Barros, J., Dixon, R. A. (2020). Plant phenylalanine/tyrosine ammonia-lyases. Trends Plant Sci. 25, 66–79. doi: 10.1016/j.tplants.2019.09.011
Bhabra, G., Sood, A., Fisher, B., Cartwright, L., Saunders, M., Evans, W. H., et al. (2009). Nanoparticles can cause DNA damage across a cellular barrier. Nat. nanotechnology 4, 876–883. doi: 10.1038/nnano.2009.313
Bhardwaj, R., Lone, J. K., Pandey, R., Mondal, N., Dhandapani, R., Meena, S. K., et al. (2023). Insights into morphological and physio-biochemical adaptive responses in mungbean (Vigna radiata L.) under heat stress. Front. Genet. 14, 1206451. doi: 10.3389/fgene.2023.1206451
Bhatla, S. C., Lal, A., M., A., Kathpalia, R., Sisodia, R., Shakya, R. (2018). Biotic stress. Plant Physiology Dev. Metab., 1029–1095. doi: 10.1007/978-981-13-2023-1_32
Brodersen, C. R., Roddy, A. B., Wason, J. W., Mcelrone, A. J. (2019). Functional status of xylem through time. Annu. Rev. Plant Biol. 70, 407–433. doi: 10.1146/annurev-arplant-050718-100455
Brüssow, H., Canchaya, C., Hardt, W. D. (2004). Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68, 560–602. doi: 10.1128/MMBR.68.3.560-602.2004
Burklew, C. E., Ashlock, J., Winfrey, W. B., Zhang, B. (2012). Effects of aluminum oxide nanoparticles on the growth, development, and microRNA expression of tobacco (Nicotiana tabacum). PloS One 7, e34783. doi: 10.1371/journal.pone.0034783
Cabral, R. M., Baptista, P. V. (2014). Anti-cancer precision theranostics: a focus on multifunctional gold nanoparticles. Expert Rev. Mol. diagnostics 14, 1041–1052. doi: 10.1586/14737159.2014.965683
Cardoso, V. F., Francesko, A., Ribeiro, C., Bañobre-López, M., Martins, P., Lanceros-Mendez, S. (2018). Advances in magnetic nanoparticles for biomedical applications. Advanced healthcare materials 7, 1700845. doi: 10.1002/adhm.201700845
Casal, J. J., Balasubramanian, S. (2019). Thermomorphogenesis. Annu. Rev. Plant Biol. 70, 321–346. doi: 10.1146/annurev-arplant-050718-095919
Chandrashekar, H. K., Singh, G., Kaniyassery, A., Thorat, S. A., Nayak, R., Murali, T. S., et al. (2023). Nanoparticle-mediated amelioration of drought stress in plants: a systematic review. 3 Biotech. 13, 336. doi: 10.1007/s13205-023-03751-4
Chauhan, N. S., Gill, S. S. (2023). The impact of nanoparticles on agriculture and soil. (Elsevier).
Chen, B., Jiang, Y., Zhao, M., Wang, W., Chu, Z., Huo, R., et al. (2020). Ag nanoparticles decorated hybrid microspheres for superior antibacterial properties. Materials Lett. 262, 127057. doi: 10.1016/j.matlet.2019.127057
Chen, J., Liu, B., Yang, Z., Qu, J., Xun, H., Dou, R., et al. (2018). Phenotypic, transcriptional, physiological and metabolic responses to carbon nanodot exposure in Arabidopsis thaliana (L.). Environ. Science: Nano 5, 2672–2685. doi: 10.1039/C8EN00674A
Chen, Z., Niu, J., Guo, Z., Sui, X., Xu, N., Kareem, H. A., et al. (2021). Integrating transcriptome and physiological analyses to elucidate the essential biological mechanisms of graphene phytotoxicity of alfalfa (Medicago sativa L.). Ecotoxicology Environ. Saf. 220, 112348. doi: 10.1016/j.ecoenv.2021.112348
Chen, C., Unrine, J. M., Judy, J. D., Lewis, R. W., Guo, J., Mcnear, D. H., Jr., et al. (2015). Toxicogenomic responses of the model legume Medicago truncatula to aged biosolids containing a mixture of nanomaterials (TiO2, Ag, and ZnO) from a pilot wastewater treatment plant. Environ. Sci. Technol. 49, 8759–8768. doi: 10.1021/acs.est.5b01211
Chettri, D., Verma, A. K., Verma, A. K. (2024). Nanotechnology as an emerging innovation: sources, application in a sustainable agriculture and environmental analysis. BioNanoScience 14, 1–19. doi: 10.1007/s12668-024-01526-6
Choi, S. U., Eastman, J. A. (1995). Enhancing thermal conductivity of fluids with nanoparticles (Argonne, IL (United States: Argonne National Lab.(ANL).
Chu, H., Kim, H.-J., Kim, J. S., Kim, M.-S., Yoon, B.-D., Park, H.-J., et al. (2012). A nanosized Ag–silica hybrid complex prepared by γ-irradiation activates the defense response in Arabidopsis. Radiat. Phys. Chem. 81, 180–184. doi: 10.1016/j.radphyschem.2011.10.004
Cui, J., Liu, T., Li, F., Yi, J., Liu, C., Yu, H. (2017). Silica nanoparticles alleviate cadmium toxicity in rice cells: mechanisms and size effects. Environ. pollut. 228, 363–369. doi: 10.1016/j.envpol.2017.05.014
Cui, H., Shi, Y., Zhou, J., Chu, H., Cang, L., Zhou, D. (2018). Effect of different grain sizes of hydroxyapatite on soil heavy metal bioavailability and microbial community composition. Agriculture Ecosyst. Environ. 267, 165–173. doi: 10.1016/j.agee.2018.08.017
Cuzzocrea, S. (2006). Role of nitric oxide and reactive oxygen species in arthritis. Curr. Pharm. design 12, 3551–3570. doi: 10.2174/138161206778343082
Dai Vu, L., Gevaert, K., De Smet, I. (2019). Feeling the heat: searching for plant thermosensors. Trends Plant Sci. 24, 210–219. doi: 10.1016/j.tplants.2018.11.004
Dang, F., Chen, Y.-Z., Huang, Y.-N., Hintelmann, H., Si, Y.-B., Zhou, D.-M. (2019). Discerning the sources of silver nanoparticle in a terrestrial food chain by stable isotope tracer technique. Environ. Sci. Technol. 53, 3802–3810. doi: 10.1021/acs.est.8b06135
Das, A., Ray, R., Mandal, N., Chakrabarti, K. (2016). An analysis of transcripts and enzyme profiles in drought stressed jute (Corchorus capsularis) and rice (Oryza sativa) seedlings treated with CaCl 2, hydroxyapatite nano-particle and β-amino butyric acid. Plant Growth Regul. 79, 401–412. doi: 10.1007/s10725-015-0144-9
Das, K., Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2, 53. doi: 10.3389/fenvs.2014.00053
Das, S. K., Stephen, U. (2009). A review of heat transfer in nanofluids. Adv. heat transfer 41, 81–197. doi: 10.1016/S0065-2717(08)41002-X
Demidchik, V. (2015). Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ. Exp. Bot. 109, 212–228. doi: 10.1016/j.envexpbot.2014.06.021
Derbalah, A., Shenashen, M., Hamza, A., Mohamed, A., El Safty, S. (2018). Antifungal activity of fabricated mesoporous silica nanoparticles against early blight of tomato. Egyptian Journal of Basic and Applied Sciences. 5.
De Smet, I., Quint, M., Van Zanten, M. (2021). High and low temperature signalling and response. J. Exp. Bot. 72, 7339–7344 doi: 10.1093/jxb/erab447
Diatta, A. A., Fike, J. H., Battaglia, M. L., Galbraith, J. M., Baig, M. B. (2020). Effects of biochar on soil fertility and crop productivity in arid regions: a review. Arabian J. Geosciences 13, 1–17. doi: 10.1007/s12517-020-05586-2
Dietz, K.-J., Herth, S. (2011). Plant nanotoxicology. Trends Plant Sci. 16, 582–589. doi: 10.1016/j.tplants.2011.08.003
Dimkpa, C. O., Latta, D. E., Mclean, J. E., Britt, D. W., Boyanov, M. I., Anderson, A. J. (2013). Fate of CuO and ZnO nano-and microparticles in the plant environment. Environ. Sci. Technol. 47, 4734–4742. doi: 10.1021/es304736y
Dimkpa, C. O., Mclean, J. E., Latta, D. E., Manangón, E., Britt, D. W., Johnson, W. P., et al. (2012). CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanoparticle Res. 14, 1–15. doi: 10.1007/s11051-012-1125-9
Dippong, T., Levei, E. A., Cadar, O. (2021). Recent advances in synthesis and applications of MFe2O4 (M= Co, Cu, Mn, Ni, Zn) nanoparticles. Nanomaterials 11, 1560. doi: 10.3390/nano11061560
Divya, K., Vijayan, S., George, T. K., Jisha, M. (2017). Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers polymers 18, 221–230. doi: 10.1007/s12221-017-6690-1
Djanaguiraman, M., Belliraj, N., Bossmann, S. H., Prasad, P. V. (2018). High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS omega 3, 2479–2491. doi: 10.1021/acsomega.7b01934
Dubos, C., Stracke, R., Grotewold, E., Weisshaar, B., Martin, C., Lepiniec, L. (2010). MYB transcription factors in Arabidopsis. Trends Plant Sci. 15, 573–581. doi: 10.1016/j.tplants.2010.06.005
Egbuna, C., Parmar, V. K., Jeevanandam, J., Ezzat, S. M., Patrick-Iwuanyanwu, K. C., Adetunji, C. O., et al. (2021). Toxicity of nanoparticles in biomedical application: nanotoxicology. J. Toxicol. 2021, 9954443. doi: 10.1155/2021/9954443
El-Sappah, A. H., Abbas, M., Rather, S. A., Wani, S. H., Soaud, N., Noor, Z., et al. (2023). Genome-wide identification and expression analysis of metal tolerance protein (MTP) gene family in soybean (Glycine max) under heavy metal stress. Mol. Biol. Rep. 50, 2975 –2990. doi: 10.1007/s11033-022-08100-x
El- Sappah, A. H., Elrys, A. S., Desoky, E.-S. M., Zhao, X., Bingwen, W., El-Sappah, H. H., et al. (2021). Comprehensive genome wide identification and expression analysis of MTP gene family in tomato (Solanum lycopersicum) under multiple heavy metal stress. Saudi J. Biol. Sci. 28, 6946–6956. doi: 10.1016/j.sjbs.2021.07.073
El-Sappah, A. H., Islam., M. M., El-Awady, H. H., Yan, S., Qi, S., Liu, J., et al. (2019). Tomato natural resistance genes in controlling the root-knot nematode. Genes 10, 925. doi: 10.3390/genes10110925
El-Sappah, A., Islam, M., Rather, S., Li, J., Yan, K., Xianming, Z., et al. (2022a). Identification of novelroot-knot nematode (Meloidogyne incognita) resistant tomato genotypes. JAPS: J. Anim. Plant Sci. 32, 99–113. doi: 10.36899/JAPS.2022.1.0407
El-Sappah, A. H., Rather, S. A. (2022). Genomics approaches to study abiotic stress tolerance in plants. Plant abiotic Stress Physiol. 2, 25–46. doi: 10.5772/57399
El-Sappah, A. H., Rather, S. A., Wani, S. H., Elrys, A. S., Bilal, M., Huang, Q., et al. (2022b). Heat stress-mediated constraints in maize (Zea mays) production: challenges and solutions. Front. Plant Sci. 13, 879366. doi: 10.3389/fpls.2022.879366
El-Sappah, A. H., Zhu, Y., Huang, Q., Chen, B., Soaud, S. A., Abd Elhamid, M. A., et al. (2024). Plants’ molecular behavior to heavy metals: from criticality to toxicity. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1423625
El-Sharkawy, M. S., El-Beshsbeshy, T. R., Mahmoud, E. K., Abdelkader, N. I., Al-Shal, R. M., Missaoui, A. M. (2017). Response of alfalfa under salt stress to the application of potassium sulfate nanoparticles. Am. J. Plant Sci. 8, 1751–1773. doi: 10.4236/ajps.2017.88120
Elsheery, N. I., Helaly, M. N., El-Hoseiny, H. M., Alam-Eldein, S. M. (2020). Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 10, 558. doi: 10.3390/agronomy10040558
Faizan, M., Bhat, J. A., Hessini, K., Yu, F., Ahmad, P. (2021). Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxicology Environ. Saf. 220, 112401. doi: 10.1016/j.ecoenv.2021.112401
Fan, Q., Liao, Y.-Y., Kunwar, S., Da Silva, S., Young, M., Santra, S., et al. (2021). Antibacterial effect of copper composites against Xanthomonas euvesicatoria. Crop Prot. 139, 105366. doi: 10.1016/j.cropro.2020.105366
Feng, L., Xu, N., Qu, Q., Zhang, Z., Ke, M., Lu, T., et al. (2021). Synergetic toxicity of silver nanoparticle and glyphosate on wheat (Triticum aestivum L.). Sci. Total Environ. 797, 149200. doi: 10.1016/j.scitotenv.2021.149200
Fiorucci, A.-S., Fankhauser, C. (2017). Plant strategies for enhancing access to sunlight. Curr. Biol. 27, R931–R940. doi: 10.1016/j.cub.2017.05.085
Frazier, T. P., Burklew, C. E., Zhang, B. (2014). Titanium dioxide nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana tabacum). Funct. Integr. Genomics 14, 75–83. doi: 10.1007/s10142-013-0341-4
Frey-Klett, P., Burlinson, P., Deveau, A., Barret, M., Tarkka, M., Sarniguet, A. (2011). Bacterial-fungal interactions: hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol. Mol. Biol. Rev. 75, 583–609. doi: 10.1128/MMBR.00020-11
García-Sánchez, S., Bernales, I., Cristobal, S. (2015). Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genomics 16, 1–17. doi: 10.1186/s12864-015-1530-4
Garrett, S. (1950). Ecology of the root inhabiting fungi. Biol. Rev. 25, 220–254. doi: 10.1111/j.1469-185X.1950.tb00591.x
Gautam, A., Komal, P., Gautam, P., Sharma, A., Kumar, N., Jung, J. P. (2021). Recent trends in noble metal nanoparticles for colorimetric chemical sensing and micro-electronic packaging applications. Metals 11, 329. doi: 10.3390/met11020329
Ghosh, S., Ahmad, R., Zeyaullah, M., Khare, S. K. (2021a). Microbial nano-factories: synthesis and biomedical applications. Front. Chem. 9, 626834. doi: 10.3389/fchem.2021.626834
Ghosh, U. K., Islam, M. N., Siddiqui, M. N., Khan, M. (2021b). Understanding the roles of osmolytes for acclimatizing plants to changing environment: a review of potential mechanism. Plant Signal Behav. 16, 1913306. doi: 10.1080/15592324.2021.1913306
Giannousi, K., Avramidis, I., Dendrinou-Samara, C. (2013). Synthesis, characterization and evaluation of copper based nanoparticles as agrochemicals against Phytophthora infestans. RSC Advances 3, 21743.
Gil, P. R., Hühn, D., Del Mercato, L. L., Sasse, D., Parak, W. J. (2010). Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds. Pharmacol. Res. 62, 115–125. doi: 10.1016/j.phrs.2010.01.009
Gill, H. K., Garg, H. (2014). Pesticide: environmental impacts and management strategies. Pesticides-toxic aspects 8, 10.5772. doi: 10.5772/57399
González-García, Y., González-Moscoso, M., Hernández-Hernández, H., Méndez-López, A., Juárez-Maldonado, A. (2021). Induction of stress tolerance in crops by applying nanomaterials. Nanotechnology Plant Growth Promotion Protection: Recent Adv. Impacts, 129–169. doi: 10.1002/9781119745884
Gopalakrishnan Nair, P. M., Chung, I.-M. (2014). Cell cycle and mismatch repair genes as potential biomarkers in Arabidopsis thaliana seedlings exposed to silver nanoparticles. Bull. Environ. contamination Toxicol. 92, 719–725. doi: 10.1007/s00128-014-1254-1
Gottschalk, F., Lassen, C., Kjoelholt, J., Christensen, F., Nowack, B. (2015). Modeling flows and concentrations of nine engineered nanomaterials in the Danish environment. Int. J. Environ. Res. Public Health 12, 5581–5602. doi: 10.3390/ijerph120505581
Gou, C., Huang, Q., Rady, M. M., Wang, L., Ihtisham, M., El-Awady, H. H., et al. (2023). Integrative application of silicon and/or proline improves Sweet corn (Zea mays L. saccharata) production and antioxidant defense system under salt stress condition. Sci. Rep. 13, 18315. doi: 10.1038/s41598-023-45003-8
Gull, A., Lone, A. A., Wani, N. U. I. (2019). Biotic and abiotic stresses in plants. Abiotic biotic Stress Plants, 1–19. doi: 10.5772/intechopen.85832
Gunalan, S., Sivaraj, R., Rajendran, V. (2012). Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Natural Science: Materials Int. 22, 693–700. doi: 10.1016/j.pnsc.2012.11.015
Gupta, A., Rayeen, F., Mishra, R., Tripathi, M., Pathak, N. (2023). Nanotechnology applications in sustainable agriculture: An emerging eco-friendly approach. Plant Nano Biol. 4, 100033. doi: 10.1016/j.plana.2023.100033
Haider, S., Iqbal, J., Naseer, S., Shaukat, M., Abbasi, B. A., Yaseen, T., et al. (2022). Unfolding molecular switches in plant heat stress resistance: A comprehensive review. Plant Cell Rep. 41, 775–798. doi: 10.1007/s00299-021-02754-w
Hamza, A., El-Mogazy, S., Derbalah, A. (2016). Fenton reagent and titanium dioxide nanoparticles as antifungal agents to control leaf spot of sugar beet under field conditions. J. Plant Prot. Res. 56, 270–278. doi: 10.1515/jppr-2016-0040
Handy, R. D., Shaw, B. J. (2007). Toxic effects of nanoparticles and nanomaterials: implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc. 9, 125–144. doi: 10.1080/13698570701306807
Hasanuzzaman, M., Nahar, K., Alam, M. M., Roychowdhury, R., Fujita, M. (2013). Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 14, 9643–9684. doi: 10.3390/ijms14059643
Hashimoto, T., Mustafa, G., Nishiuchi, T., Komatsu, S. (2020). Comparative analysis of the effect of inorganic and organic chemicals with silver nanoparticles on soybean under flooding stress. Int. J. Mol. Sci. 21, 1300. doi: 10.3390/ijms21041300
Hassan, H., Alatawi, A., Abdulmajeed, A., Emam, M., Khattab, H. (2021b). Roles of Si and SiNPs in improving thermotolerance of wheat photosynthetic machinery via upregulation of PsbH, PsbB and PsbD genes encoding PSII core proteins. Horticulturae 7, 16. doi: 10.3390/horticulturae7020016
Hassan, F., Ali, E., Gaber, A., Fetouh, M., Mazrou, R. (2021a). Chitosan nanoparticles effectively combat salinity stress by enhancing antioxidant activity and alkaloid biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 162, 291–300. doi: 10.1016/j.plaphy.2021.03.004
Hassan, N. S., Salah El Din, T. A., Hendawey, M. H., Borai, I. H., Mahdi, A. A. (2018). Magnetite and zinc oxide nanoparticles alleviated heat stress in wheat plants. Curr. nanomaterials 3, 32–43. doi: 10.2174/2405461503666180619160923
Hayat, P., Khan, I., Rehman, A., Jamil, T., Hayat, A., Rehman, M. U., et al. (2023). Myogenesis and analysis of antimicrobial potential of silver nanoparticles (AgNPs) against pathogenic bacteria. Molecules 28, 637. doi: 10.3390/molecules28020637
Hazafa, A., Murad, M., Masood, M. U., Bilal, S., Khan, M. N., Farooq, Q., et al. (2021). “Nano-biopesticides as an emerging technology for pest management,” in Insecticides-impact and benefits of its use for humanity (IntechOpen).
Hazarika, A., Yadav, M., Yadav, D. K., Yadav, H. S. (2022). An overview of the role of nanoparticles in sustainable agriculture. Biocatalysis Agric. Biotechnol. 43, 102399. doi: 10.1016/j.bcab.2022.102399
Hincha, D. K., Zuther, E. (2014). Introduction: plant cold acclimation and freezing tolerance. Plant Cold Acclimation: Methods Protoc. 1166, 1–6. doi: 10.1007/978-1-4939-0844-8
Hoang, A., Cong, H., Shukanov, V., Karytsko, L., Poljanskaja, S., Melnikava, E., et al. (2022). Evaluation of metal nano-particles as growth promoters and fungi inhibitors for cereal crops. Chem. Biol. Technol. Agric. 9, 12. doi: 10.1186/s40538-021-00277-w
Hong, J., Wang, L., Sun, Y., Zhao, L., Niu, G., Tan, W., et al. (2016). Foliar applied nanoscale and microscale CeO2 and CuO alter cucumber (Cucumis sativus) fruit quality. Sci. Total Environ. 563, 904–911. doi: 10.1016/j.scitotenv.2015.08.029
Horie, M., Tabei, Y. (2021). Role of oxidative stress in nanoparticles toxicity. Free Radical Res. 55, 331–342. doi: 10.1080/10715762.2020.1859108
Hossain, A., Skalicky, M., Brestic, M., Maitra, S., Ashraful Alam, M., Syed, M. A., et al. (2021). Consequences and mitigation strategies of abiotic stresses in wheat (Triticum aestivum L.) under the changing climate. Agronomy 11, 241. doi: 10.3390/agronomy11020241
Hsiao, I.-L., Huang, Y.-J. (2011). Effects of various physicochemical characteristics on the toxicities of ZnO and TiO2 nanoparticles toward human lung epithelial cells. Sci. total Environ. 409, 1219–1228. doi: 10.1016/j.scitotenv.2010.12.033
Hu, X., Zhou, Q. (2014). Novel hydrated graphene ribbon unexpectedly promotes aged seed germination and root differentiation. Sci. Rep. 4, 3782. doi: 10.1038/srep03782
Huang, Y., Adeleye, A. S., Zhao, L., Minakova, A. S., Anumol, T., Keller, A. A. (2019). Antioxidant response of cucumber (Cucumis sativus) exposed to nano copper pesticide: Quantitative determination via LC-MS/MS. Food Chem. 270, 47–52. doi: 10.1016/j.foodchem.2018.07.069
Huang, S., Wang, L., Liu, L., Hou, Y., Li, L. (2015). Nanotechnology in agriculture, livestock, and aquaculture in China. A review. Agron. Sustain. Dev. 35, 369–400. doi: 10.1007/s13593-014-0274-x
Hussain, M. A., Mukhtar, T., Kayani, M. Z. (2016). Reproduction of Meloidogyne incognita on resistant and susceptible okra cultivars. Pakistan J. Agric. Sci. 53, 371–375. doi: 10.21162/PAKJAS/16.4175
Hussein, M. M., Gad, E., Ahmed, M. M., Arisha, A. H., Mahdy, H. F., Swelum, A.-A., et al. (2019). Amelioration of titanium dioxide nanoparticle reprotoxicity by the antioxidants morin and rutin. Environ. Sci. pollut. Res. 26, 29074–29084. doi: 10.1007/s11356-019-06091-0
Iavicoli, I., Leso, V., Beezhold, D. H., Shvedova, A. A. (2017). Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 329, 96–111. doi: 10.1016/j.taap.2017.05.025
Ihtisham, M., Hasanuzzaman, M., El-Sappah, A. H., Zaman, F., Khan, N., Raza, A., et al. (2023). Primary plant nutrients modulate the reactive oxygen species metabolism and mitigate the impact of cold stress in overseeded perennial ryegrass. Front. Plant Sci. 14, 1149832. doi: 10.3389/fpls.2023.1149832
Imada, K., Sakai, S., Kajihara, H., Tanaka, S., Ito, S. (2016). Magnesium oxide nanoparticles induce systemic resistance in tomato against bacterial wilt disease. Plant Pathol. 65, 551–560. doi: 10.1111/ppa.2016.65.issue-4
Ingle, A. P., Duran, N., Rai, M. (2014). Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: a review. Appl. Microbiol. Biotechnol. 98, 1001–1009. doi: 10.1007/s00253-013-5422-8
Iqbal, S., Fakhar-E-Alam, M., Akbar, F., Shafiq, M., Atif, M., Amin, N., et al. (2019b). Application of silver oxide nanoparticles for the treatment of cancer. J. Mol. Structure 1189, 203–209. doi: 10.1016/j.molstruc.2019.04.041
Iqbal, M., Raja, N. I., Mashwani, Z.-U.-R., Hussain, M., Ejaz, M., Yasmeen, F. (2019a). Effect of silver nanoparticles on growth of wheat under heat stress. Iranian J. Sci. Technology Trans. A: Sci. 43, 387–395. doi: 10.1007/s40995-017-0417-4
Ishak, N. M., Kamarudin, S., Timmiati, S. (2019). Green synthesis of metal and metal oxide nanoparticles via plant extracts: an overview. Materials Res. Express 6, 112004. doi: 10.1088/2053-1591/ab4458
Jafir, M., Ahmad, J. N., Arif, M. J., Ali, S., Ahmad, S. J. N. (2021). Characterization of Ocimum basilicum synthesized silver nanoparticles and its relative toxicity to some insecticides against tobacco cutworm, Spodoptera litura Feb. (Lepidoptera; Noctuidae). Ecotoxicology Environ. Saf. 218, 112278. doi: 10.1016/j.ecoenv.2021.112278
Jafir, M., Ayub, M. A., Ur Rehman, M. Z. (2022). “Chapter 9 - Nanosilicon-mediated salt stress tolerance in plants,” in Silicon and nano-silicon in environmental stress management and crop quality improvement. Eds. Etesami, H., Saeedi, A. H. Al, Ramady, H.E.-., Fujita, M., Pessarakli, M., Anwar Hossain, M. (Academic Press), 105–119.
Jafir, M., Irfan, M., Zia-Ur-Rehman, M., Hafeez, F., Ahmad, J. N., Sabir, M. A., et al. (2023). The global trend of nanomaterial usage to control the important agricultural arthropod pests: A comprehensive review. Plant Stress 10, 100208. doi: 10.1016/j.stress.2023.100208
Jafir, M., Khan, A., Ahmad, A., Hussain, K., Ur Rehman, M. Z., Nazeer Ahmad, S. J., et al. (2024). Zinc nanoparticles for enhancing plant tolerance to abiotic stress: A bibliometric analysis and review. J. Soil Sci. Plant Nutr. 24, 1704–1719. doi: 10.1007/s42729-024-01733-w
Jain, A., Sarsaiya, S., Wu, Q., Lu, Y., Shi, J. (2019). A review of plant leaf fungal diseases and its environment speciation. Bioengineered 10, 409–424. doi: 10.1080/21655979.2019.1649520
Jalil, S. U., Ansari, M. I. (2019). Nanoparticles and abiotic stress tolerance in plants: synthesis, action, and signaling mechanisms. Plant Signaling molecules, 549–561. doi: 10.1016/B978-0-12-816451-8.00034-4
Jamdagni, P., Khatri, P., Rana, J.-S. (2018). Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud University-Science 30, 168–175. doi: 10.1016/j.jksus.2016.10.002
Jan, R., Asaf, S., Numan, M., Lubna, Kim, K.-M. (2021). Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 11, 968. doi: 10.3390/agronomy11050968
Jiang, H., Lv, L., Ahmed, T., Jin, S., Shahid, M., Noman, M., et al. (2021). Effect of the nanoparticle exposures on the tomato bacterial wilt disease control by modulating the rhizosphere bacterial community. Int. J. Mol. Sci. 23, 414. doi: 10.3390/ijms23010414
Johnson, N. C., Graham, J. H., Smith, F. A. (1997). Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol. 135, 575–585. doi: 10.1046/j.1469-8137.1997.00729.x
Juárez-Maldonado, A., Ortega-Ortíz, H., Morales-Díaz, A. B., González-Morales, S., Morelos-Moreno, Á., Cabrera-De La Fuente, M., et al. (2019). Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 20, 162. doi: 10.3390/ijms20010162
Kalisz, A., Húska, D., Jurkow, R., Dvořák, M., Klejdus, B., Caruso, G., et al. (2021). Nanoparticles of cerium, iron, and silicon oxides change the metabolism of phenols and flavonoids in butterhead lettuce and sweet pepper seedlings. Environ. Science: Nano 8, 1945–1959. doi: 10.1039/D1EN00262G
Kang, W., Li, X., Sun, A., Yu, F., Hu, X. (2019). Study of the persistence of the phytotoxicity induced by graphene oxide quantum dots and of the specific molecular mechanisms by integrating omics and regular analyses. Environ. Sci. Technol. 53, 3791–3801. doi: 10.1021/acs.est.8b06023
Karunakaran, G., Jagathambal, M., Van Minh, N., Kolesnikov, E., Kuznetsov, D. (2018). Green synthesis of NiFe 2 O 4 spinel-structured nanoparticles using Hydrangea paniculata flower extract with excellent magnetic property. JOM 70, 1337–1343. doi: 10.1007/s11837-018-2871-7
Kashyap, B., Kumar, R. (2021). Sensing methodologies in agriculture for soil moisture and nutrient monitoring. IEEE Access 9, 14095–14121. doi: 10.1109/Access.6287639
Kashyap, B. (2022). Effect of zinc oxide nanoparticles and rhizobium leguminosarum on growth, photosynthetic pigments and blight disease complex of pea. Gesunde Pflanzen 74, 1–12.
Kaveh, R., Li, Y.-S., Ranjbar, S., Tehrani, R., Brueck, C. L., Van Aken, B. (2013). Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 47, 10637–10644. doi: 10.1021/es402209w
Keerthana, M., Arivarasu, L., Kumar, S. R., Thangavelu, L. (2021). Anti-diabetic and cytotoxic effect of zinc oxide nanoparticles synthesised using boerhaavia diffusa. J. Pharm. Res. Int. 33, 371–379. doi: 10.9734/jpri/2021/v33i62A35611
Khan, M. R. (2015). Nematode diseases of crops in India. Recent Adv. diagnosis Manage. Plant Dis., 183–224. doi: 10.1007/978-81-322-2571-3_16
Khan, M., Khan, A. U., Bogdanchikova, N., Garibo, D. (2021a). Antibacterial and Antifungal Studies of Biosynthesized Silver Nanoparticles against Plant Parasitic Nematode Meloidogyne incognita, Plant Pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules 26, 2462. doi: 10.3390/molecules26092462
Khan, I., Saeed, K., Khan, I. (2019). Nanoparticles: Properties, applications and toxicities. Arabian J. Chem. 12, 908–931. doi: 10.1016/j.arabjc.2017.05.011
Khan, R., Tang, Y., Naz, I., Alam, S. S., Wang, W., Ahmad, M., et al. (2021b). Management of Ralstonia solanacearum in Tomato Using ZnO Nanoparticles Synthesized Through Matricaria chamomilla. Plant Dis. 105, 3224–3230. doi: 10.1094/PDIS-08-20-1763-RE
Khan, Z., Upadhyaya, H. (2019). Impact of nanoparticles on abiotic stress responses in plants: an overview. Nanomaterials plants algae microorganisms, 305–322. doi: 10.1016/B978-0-12-811488-9.00015-9
Kheyrodin, H., Jami, R., Rehman, F. U. (2022). Cellular structure and molecular functions of plants, animals, bacteria, and viruses. Cellular Mol. Biomed. Rep. 2, 33–41. doi: 10.55705/cmbr.2022.330941.1021
Khodakovskaya, M. V., De Silva, K., Biris, A. S., Dervishi, E., Villagarcia, H. (2012). Carbon nanotubes induce growth enhancement of tobacco cells. ACS nano 6, 2128–2135. doi: 10.1021/nn204643g
Kim, Y.-H., Khan, A. L., Waqas, M., Lee, I.-J. (2017). Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: a review. Front. Plant Sci. 8, 510. doi: 10.3389/fpls.2017.00510
Kiriga, A. W. (2018). Occurrence of Plant Parasitic Nematodes in Commercial Pineapple Fields and Effect of Biocontrol agents on Meloidogyne species in Kenya (Kenyatta University).
Kochuveedu, S. T., Kim, D. H. (2014). Surface plasmon resonance mediated photoluminescence properties of nanostructured multicomponent fluorophore systems. Nanoscale 6, 4966–4984. doi: 10.1039/C4NR00241E
Koonin, E. V., Starokadomskyy, P. (2016). Are viruses alive? The replicator paradigm sheds decisive light on an old but misguided question. Stud. history philosophy Sci. Part C: Stud. history philosophy Biol. Biomed. Sci. 59, 125–134. doi: 10.1016/j.shpsc
Kruszka, D., Sawikowska, A., Selvakesavan, R. K., Krajewski, P., Kachlicki, P., Franklin, G. (2020). Silver nanoparticles affect phenolic and phytoalexin composition of Arabidopsis thaliana. Sci. Total Environ. 716, 135361. doi: 10.1016/j.scitotenv.2019.135361
Kruszka, D., Selvakesavan, R. K., Kachlicki, P., Franklin, G. (2022). Untargeted metabolomics analysis reveals the elicitation of important secondary metabolites upon treatment with various metal and metal oxide nanoparticles in Hypericum perforatum L. cell suspension cultures. Ind. Crops Products 178, 114561. doi: 10.1016/j.indcrop.2022.114561
Kumah, E. A., Fopa, R. D., Harati, S., Boadu, P., Zohoori, F. V., Pak, T. (2023). Human and environmental impacts of nanoparticles: a scoping review of the current literature. BMC Public Health 23, 1059. doi: 10.1186/s12889-023-15958-4
Kumari, P., Srivastava, A., Sharma, R. K., Saini, A., Sharma, D., Tawale, J. S., et al. (2022). Facile synthesis and tailoring the structural and photoluminescence properties of ZnO nanoparticles via annealing in air atmosphere. Materials Today Commun. 32, 103845. doi: 10.1016/j.mtcomm.2022.103845
Kutawa, A. B., Ahmad, K., Ali, A., Hussein, M. Z., Abdul Wahab, M. A., Adamu, A., et al. (2021). Trends in nanotechnology and its potentialities to control plant pathogenic fungi: A review. Biology 10, 881. doi: 10.3390/biology10090881
Landa, P., Vankova, R., Andrlova, J., Hodek, J., Marsik, P., Storchova, H., et al. (2012). Nanoparticle-specific changes in Arabidopsis thaliana gene expression after exposure to ZnO, TiO2, and fullerene soot. J. hazardous materials 241, 55–62. doi: 10.1016/j.jhazmat.2012.08.059
Leisner, C. P., Potnis, N., Sanz-Saez, A. (2023). Crosstalk and trade-offs: Plant responses to climate change-associated abiotic and biotic stresses. Plant Cell Environ. 46, 2946–2963. doi: 10.1111/pce.v46.10
Le Wee, J., Law, M. C., Chan, Y. S., Choy, S. Y., Tiong, A. N. T. (2022). The potential of Fe-based magnetic nanomaterials for the agriculture sector. ChemistrySelect 7, e202104603. doi: 10.1002/slct.202104603
Li, J., Abbas, M., Desoky, E.-S. M., Zafar, S., Soaud, S. A., Hussain, S. S., et al. (2023a). Analysis of metal tolerance protein (MTP) family in sunflower (Helianthus annus L.) and role of HaMTP10 as Cadmium antiporter under moringa seed extract. Ind. Crops Products 202, 117023. doi: 10.1016/j.indcrop.2023.117023
Li, Y., Guo, C., Yuan, J., Yang, X., Wu, M., Wu, L., et al. (2024). Recent advances and prospects of persistent luminescent materials in public health applications. Chem. Eng. J. 150424, 150424. doi: 10.1016/j.cej.2024.150424
Li, R., He, Y., Chen, J., Zheng, S., Zhuang, C. (2023c). Research progress in improving photosynthetic efficiency. Int. J. Mol. Sci. 24, 9286. doi: 10.3390/ijms24119286
Li, S., Liu, J., Wang, Y., Gao, Y., Zhang, Z., Xu, J., et al. (2021). Comparative physiological and metabolomic analyses revealed that foliar spraying with zinc oxide and silica nanoparticles modulates metabolite profiles in cucumber (Cucumis sativus L.). Food Energy Secur. 10, e269. doi: 10.1002/fes3.v10.1
Li, L., Pan, H., Deng, L., Qian, G., Wang, Z., Li, W., et al. (2022). The antifungal activity and mechanism of silver nanoparticles against four pathogens causing kiwifruit post-harvest rot. Front. Microbiol. 13, 988633. doi: 10.3389/fmicb.2022.988633
Li, Y., Xi, K., Liu, X., Han, S., Han, X., Li, G., et al. (2023d). Silica nanoparticles promote wheat growth by mediating hormones and sugar metabolism. J. Nanobiotechnology 21, 2. doi: 10.1186/s12951-022-01753-7
Li, J., Zafar, S., Javaid, A., Perveen, S., Hasnain, Z., Ihtisham, M., et al. (2023b). Zinc Nanoparticles (ZnNPs): High-Fidelity Amelioration in Turnip (Brassica rapa L.) Production under Drought Stress. Sustainability 15, 6512. doi: 10.3390/su15086512
Ma, C., White, J. C., Dhankher, O. P., Xing, B. (2015). Metal-based nanotoxicity and detoxification pathways in higher plants. Environ. Sci. Technol. 49, 7109–7122. doi: 10.1021/acs.est.5b00685
Madkour, L. H., Madkour, L. H. (2019). Properties of nanostructured materials (NSMs) and physicochemical properties of (NPs). Nanoelectronic Materials: Fundamentals Appl. 116, 479–564. doi: 10.1007/978-3-030-21621-4
Majumdar, S., Pagano, L., Wohlschlegel, J. A., Villani, M., Zappettini, A., White, J. C., et al. (2019). Proteomic, gene and metabolite characterization reveal the uptake and toxicity mechanisms of cadmium sulfide quantum dots in soybean plants. Environ. Science: Nano 6, 3010–3026. doi: 10.1039/C9EN00599D
Majumdar, S., Peralta-Videa, J. R., Bandyopadhyay, S., Castillo-Michel, H., Hernandez-Viezcas, J.-A., Sahi, S., et al. (2014). Exposure of cerium oxide nanoparticles to kidney bean shows disturbance in the plant defense mechanisms. J. hazardous materials 278, 279–287. doi: 10.1016/j.jhazmat.2014.06.009
Malandrakis, A. A., Kavroulakis, N., Chrysikopoulos, C. V. (2019). Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Sci. Total Environ. 670, 292–299. doi: 10.1016/j.scitotenv.2019.03.210
Malloch, D., Blackwell, M. (1992). Dispersal of fungal diaspores. Fungal community: its Organ. role ecosystem, 147–171.
Manke, A., Wang, L., Rojanasakul, Y. (2013). Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed. Res. Int. 2013, 942916. doi: 10.1155/2013/942916
Mansoor, S., Zahoor, I., Baba, T. R., Padder, S. A., Bhat, Z., Koul, A. M., et al. (2021). Fabrication of silver nanoparticles against fungal pathogens. Front. Nanotechnology 3, 679358. doi: 10.3389/fnano.2021.679358
Marmiroli, M., Pagano, L., Savo Sardaro, M. L., Villani, M., Marmiroli, N. (2014). Genome-wide approach in Arabidopsis thaliana to assess the toxicity of cadmium sulfide quantum dots. Environ. Sci. Technol. 48, 5902–5909. doi: 10.1021/es404958r
Marslin, G., Sheeba, C. J., Franklin, G. (2017). Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 8, 832. doi: 10.3389/fpls.2017.00832
Mcgehee, D. L., Alimohammadi, M., Khodakovskaya, M. V. (2019). Carbon-based nanomaterials as stimulators of production of pharmaceutically active alkaloids in cell culture of Catharanthus roseus. Nanotechnology 30, 275102. doi: 10.1088/1361-6528/ab1286
Mcgehee, D. L., Lahiani, M. H., Irin, F., Green, M. J., Khodakovskaya, M. V. (2017). Multiwalled carbon nanotubes dramatically affect the fruit metabolome of exposed tomato plants. ACS Appl. materials interfaces 9, 32430–32435. doi: 10.1021/acsami.7b10511
Mehmood, U., Yasmeen, S., Nazar, R., Tiwari, S. K. (2023). “Metallic nanoparticles: status and prospect,” in Nanoparticles reinforced metal nanocomposites: mechanical performance and durability. (Springer), 127–159.
Mingyu, S., Chao, L., Chunxiang, Q., Lei, Z., Liang, C., Hao, H., et al. (2008). Nano-anatase relieves the inhibition of electron transport caused by linolenic acid in chloroplasts of spinach. Biol. Trace element Res. 122, 73–81. doi: 10.1007/s12011-007-8055-x
Mirakhorli, T., Ardebili, Z. O., Ladan-Moghadam, A., Danaee, E. (2021). Bulk and nanoparticles of zinc oxide exerted their beneficial effects by conferring modifications in transcription factors, histone deacetylase, carbon and nitrogen assimilation, antioxidant biomarkers, and secondary metabolism in soybean. PloS One 16, e0256905. doi: 10.1371/journal.pone.0256905
Mittal, D., Kaur, G., Singh, P., Yadav, K., Ali, S. A. (2020). Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnology 2, 579954. doi: 10.3389/fnano.2020.579954
Mohamad, A. T., Kaur, J., Sidik, N., Rahman, S. (2018). Nanoparticles: A review on their synthesis, characterization and physicochemical properties for energy technology industry. J. Advanced Res. Fluid Mechanics Thermal Sci. 46, 1–10.
Mohamed, A. K. S., Qayyum, M. F., Abdel-Hadi, A. M., Rehman, R. A., Ali, S., Rizwan, M. (2017). Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agron. Soil Sci. 63, 1736–1747. doi: 10.1080/03650340.2017.1300256
Mohammadi, K., Khalesro, S., Sohrabi, Y., Heidari, G. (2011). A review: beneficial effects of the mycorrhizal fungi for plant growth. J. Appl. Environ. Biol. Sci. 1, 310–319.
Mohanta, Y. K., Panda, S. K., Jayabalan, R., Sharma, N., Bastia, A. K., Mohanta, T. K. (2017). Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of erythrina suberosa (Roxb.). Front. Mol. Biosci. 4. doi: 10.3389/fmolb.2017.00014
Munir, N., Gulzar, W., Abideen, Z., Hancook, J. T., El-Keblawy, A., Radicetti, E. (2023). Nanotechnology improves disease resistance in plants for food security: Applications and challenges. Biocatalysis Agric. Biotechnol. 102781, 102781. doi: 10.1016/j.bcab.2023.102781
Mustafa, G., Komatsu, S. (2016). Insights into the response of soybean mitochondrial proteins to various sizes of aluminum oxide nanoparticles under flooding stress. J. Proteome Res. 15, 4464–4475. doi: 10.1021/acs.jproteome.6b00572
Mustafa, G., Sakata, K., Komatsu, S. (2015). Proteomic analysis of flooded soybean root exposed to aluminum oxide nanoparticles. J. Proteomics 128, 280–297. doi: 10.1016/j.jprot.2015.08.010
Mustafa, G., Sakata, K., Komatsu, S. (2016). Proteomic analysis of soybean root exposed to varying sizes of silver nanoparticles under flooding stress. J. Proteomics 148, 113–125. doi: 10.1016/j.jprot.2016.07.027
Nagy, P. D., Pogany, J. (2012). The dependence of viral RNA replication on co-opted host factors. Nat. Rev. Microbiol. 10, 137–149. doi: 10.1038/nrmicro2692
Nair, P. M. G., Chung, I. M. (2014). Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ. Sci. pollut. Res. 21, 8858–8869. doi: 10.1007/s11356-014-2822-y
Navas, D., Ibañez, A., González, I., Palma, J. L., Dreyse, P. (2020). Controlled dispersion of ZnO nanoparticles produced by basic precipitation in solvothermal processes. Heliyon 6, e05821. doi: 10.1016/j.heliyon.2020.e05821
Naveed, M., Batool, H., Rehman, S. U., Javed, A., Makhdoom, S. I., Aziz, T., et al. (2022a). Characterization and evaluation of the antioxidant, antidiabetic, anti-inflammatory, and cytotoxic activities of silver nanoparticles synthesized using Brachychiton populneus leaf extract. Processes 10, 1521. doi: 10.3390/pr10081521
Naveed, M., Bukhari, B., Aziz, T., Zaib, S., Mansoor, M. A., Khan, A. A., et al. (2022b). Green synthesis of silver nanoparticles using the plant extract of Acer oblongifolium and study of its antibacterial and antiproliferative activity via mathematical approaches. Molecules 27, 4226. doi: 10.3390/molecules27134226
Nayeri, S., Dolatyari, M., Mouladoost, N., Nayeri, S., Zarghami, A., Mirtagioglu, H., et al. (2023). Ag/ZnO core-shell NPs boost photosynthesis and growth rate in wheat seedlings under simulated full sun spectrum. Sci. Rep. 13, 14385. doi: 10.1038/s41598-023-41575-7
Nel, A., Xia, T., Madler, L., Li, N. (2006). Toxic potential of materials at the nanolevel. science 311, 622–627. doi: 10.1126/science.1114397
Nie, D., Li, J., Xie, Q., Ai, L., Zhu, C., Wu, Y., et al. (2023). Nanoparticles: A potential and effective method to control insect-borne diseases. Bioinorganic Chem. Appl. 2023, 5898160. doi: 10.1155/2023/5898160
Nour, A. A. (2024). Impact of nano and micro analysis studies on the sustainability of products and components [Master's Thesis, the American University in Cairo]. AUC Knowledge Fountain. Available at: https://fount.aucegypt.edu/etds/2351.
Olchowik, J., Bzdyk, R. M., Studnicki, M., Bederska-Błaszczyk, M., Urban, A., Aleksandrowicz-Trzcińska, M. (2017). The effect of silver and copper nanoparticles on the condition of english oak (Quercus robur L.) seedlings in a container nursery experiment. Forests 8, 310. doi: 10.3390/f8090310
Oprica, L., Grigore, M.-N., Bara, I., Vochita, G. (2021). “Salinity and siO 2 impact on growth and biochemical responses of basil (Ocimum basilicum L.) seedlings,” 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania. 1–4.
Osonga, F. J., Akgul, A., Yazgan, I., Akgul, A., Eshun, G. B., Sakhaee, L., et al. (2020). Size and shape-dependent antimicrobial activities of silver and gold nanoparticles: A model study as potential fungicides. Molecules 25, 2682. doi: 10.3390/molecules25112682
Ostadi, A., Javanmard, A., Amani Machiani, M., Sadeghpour, A., Maggi, F., Nouraein, M., et al. (2022). Co-application of tiO2 nanoparticles and arbuscular mycorrhizal fungi improves essential oil quantity and quality of sage (Salvia officinalis L.) in drought stress conditions. Plants 11, 1659. doi: 10.3390/plants11131659
Padmavathi, S., Anuradha, C. S. (2022). NANOTECHNOLOGY IN PLANT DISEASE MANAGEMENT-AN OVERVIEW. J. Advanced Sci. Res. 13, 01–06. doi: 10.55218/JASR.2022131001
Palmqvist, N. M., Seisenbaeva, G. A., Svedlindh, P., Kessler, V. G. (2017). Maghemite nanoparticles acts as nanozymes, improving growth and abiotic stress tolerance in Brassica napus. Nanoscale Res. Lett. 12, 1–9. doi: 10.1186/s11671-017-2404-2
Panda, K. K., Achary, V. M. M., Krishnaveni, R., Padhi, B. K., Sarangi, S. N., Sahu, S. N., et al. (2011). In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. Vitro 25, 1097–1105. doi: 10.1016/j.tiv.2011.03.008
Pandey, G. (2018). Challenges and future prospects of agri-nanotechnology for sustainable agriculture in India. Environ. Technol. Innovation 11, 299–307. doi: 10.1016/j.eti.2018.06.012
Park, H. J., Kim, S. H., Kim, H. J., Choi, S. H. (2006). A new composition of nanosized silica-silver for control of various plant diseases. Plant Pathol. J. 22, 295–302. doi: 10.5423/PPJ.2006.22.3.295
Partridge, S. R., Kwong, S. M., Firth, N., Jensen, S. O. (2018). Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 31, e00088-17. doi: 10.1128/CMR.00088-17
Pashkow, F. J., Watumull, D. G., Campbell, C. L. (2008). Astaxanthin: a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 101, S58–S68. doi: 10.1016/j.amjcard.2008.02.010
Payne, G. A. (1998). Process of contamination by aflatoxin-producing fungi and their impact on crops. Mycotoxins Agric. Food Saf. 9, 279–306.
Penfield, S. (2008). Temperature perception and signal transduction in plants. New Phytol. 179, 615–628. doi: 10.1111/j.1469-8137.2008.02478.x
Pisoschi, A. M., Pop, A. (2015). The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. medicinal Chem. 97, 55–74. doi: 10.1016/j.ejmech.2015.04.040
Pottosin, I., Shabala, S. (2016). Transport across chloroplast membranes: optimizing photosynthesis for adverse environmental conditions. Mol. Plant 9, 356–370. doi: 10.1016/j.molp.2015.10.006
Pownraj, C., Valan Arasu, A. (2021). Effect of dispersing single and hybrid nanoparticles on tribological, thermo-physical, and stability characteristics of lubricants: a review. J. Thermal Anal. Calorimetry 143, 1773–1809. doi: 10.1007/s10973-020-09837-y
Pradhan, S., Patra, P., Das, S., Chandra, S., Mitra, S., Dey, K. K., et al. (2013). Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: a detailed molecular, biochemical, and biophysical study. Environ. Sci. Technol. 47, 13122–13131. doi: 10.1021/es402659t
Pradhan, S., Patra, P., Mitra, S., Dey, K. K., Jain, S., Sarkar, S., et al. (2014). Manganese nanoparticles: impact on non-nodulated plant as a potent enhancer in nitrogen metabolism and toxicity study both in vivo and in vitro. J. Agric. Food Chem. 62, 8777–8785. doi: 10.1021/jf502716c
Pramanik, B., Sar, P., Bharti, R., Gupta, R., Purkayastha, S., Sinha, S., et al. (2023). Multifactorial role of nanoparticles in alleviating environmental stresses for sustainable crop production and protection. Plant Physiol. Biochem. 107831, 107831. doi: 10.1016/j.plaphy.2023.107831
Priya, A. K., Muruganandam, M., Ali, S. S., Kornaros, M. (2023). Clean-up of heavy metals from contaminated soil by phytoremediation: A multidisciplinary and eco-friendly approach. Toxics 11, 422. doi: 10.3390/toxics11050422
Quint, M., Delker, C., Franklin, K. A., Wigge, P. A., Halliday, K. J., Van Zanten, M. (2016). Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2, 1–9. doi: 10.1038/nplants.2015.190
Rajeshkumar, S. (2019). “Antifungal impact of nanoparticles against different plant pathogenic fungi,” in Nanomaterials in plants, algae and microorganisms (Elsevier), 197–217.
Rajput, V. D., Minkina, T., Upadhyay, S. K., Kumari, A., Ranjan, A., Mandzhieva, S., et al. (2022). Nanotechnology in the restoration of polluted soil. Nanomaterials (Basel) 12, 769. doi: 10.3390/nano12050769
Rashid, A., Schutte, B. J., Ulery, A., Deyholos, M. K., Sanogo, S., Lehnhoff, E. A., et al. (2023). Heavy metal contamination in agricultural soil: environmental pollutants affecting crop health. Agronomy 13, 1521. doi: 10.3390/agronomy13061521
Ratner, M. A., Ratner, D. (2003). Nanotechnology: A gentle introduction to the next big idea. (Prentice Hall Professional).
Ray, P. C., Yu, H., Fu, P. P. (2009). Toxicity and environmental risks of nanomaterials: challenges and future needs. J. Environ. Sci. Health C Environ. Carcinog Ecotoxicol Rev. 27, 1–35. doi: 10.1080/10590500802708267
Reiss, G., Hütten, A. (2005). Applications beyond data storage. Nat. materials 4, 725–726. doi: 10.1038/nmat1494
Rim, K.-T., Song, S.-W., Kim, H.-Y. (2013). Oxidative DNA damage from nanoparticle exposure and its application to workers’ health: a literature review. Saf. Health at work 4, 177–186. doi: 10.1016/j.shaw.2013.07.006
Ritonga, F. N., Chen, S. (2020). Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 9, 560. doi: 10.3390/plants9050560
Rizwan, M., Ali, S., Ur Rehman, M. Z., Malik, S., Adrees, M., Qayyum, M. F., et al. (2019). Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta physiologiae plantarum 41, 1–12. doi: 10.1007/s11738-019-2828-7
Ruttkay-Nedecky, B., Krystofova, O., Nejdl, L., Adam, V. (2017). Nanoparticles based on essential metals and their phytotoxicity. J. Nanobiotechnology 15, 33. doi: 10.1186/s12951-017-0268-3
Saifi, M. A., Khan, W., Godugu, C. (2018). Cytotoxicity of nanomaterials: Using nanotoxicology to address the safety concerns of nanoparticles. Pharm. nanotechnology 6, 3–16. doi: 10.2174/2211738505666171023152928
Salehi, H., Chehregani, A., Lucini, L., Majd, A., Gholami, M. (2018). Morphological, proteomic and metabolomic insight into the effect of cerium dioxide nanoparticles to Phaseolus vulgaris L. under soil or foliar application. Sci. Total Environ. 616, 1540–1551. doi: 10.1016/j.scitotenv.2017.10.159
Santás-Miguel, V., Arias-Estévez, M., Rodríguez-Seijo, A., Arenas-Lago, D. (2023). Use of metal nanoparticles in agriculture. A review on the effects on plant germination. Environ. Pollut. 334, 122222. doi: 10.1016/j.envpol.2023.122222
Saranya, S., Selvi, A., Babujanarthanam, R., Rajasekar, A., Madhavan, J. (2020). Insecticidal activity of nanoparticles and mechanism of action. Model. organisms to study Biol. activities toxicity nanoparticles, 243–266. doi: 10.1007/978-981-15-1702-0_12
Sarraf, M., Vishwakarma, K., Kumar, V., Arif, N., Das, S., Johnson, R., et al. (2022). Metal/metalloid-based nanomaterials for plant abiotic stress tolerance: An overview of the mechanisms. Plants 11, 316. doi: 10.3390/plants11030316
Sedghi, M., Hadi, M., Toluie, S. G. (2013). Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Ann. West Univ Timisoara Ser. Biol. 16, 73.
Seleiman, M. F., Al-Suhaibani, N., Ali, N., Akmal, M., Alotaibi, M., Refay, Y., et al. (2021). Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10, 259. doi: 10.3390/plants10020259
Selvakesavan, R. K., Kruszka, D., Shakya, P., Mondal, D., Franklin, G. (2023). Impact of nanomaterials on plant secondary metabolism. Nanomaterial Interact. Plant Cell. Mech. Macromolecules Agric. Implications, 133–170. doi: 10.1007/978-3-031-20878
Senthilraja, S., Vijayakumar, K., Gangadevi, R. (2015). A comparative study on thermal conductivity of Al2O3/water, CuO/water and Al2O3–CuO/water nanofluids. Digest J. Nanomaterials Biostructures 10, 1449–1458.
Shafea, A. A., Dawood, M. F., Zidan, M. A. (2017). Wheat seedlings traits as affected by soaking at titanium dioxide nanoparticles. Environment Earth Ecol. 1, 102-111. doi: 10.24051/eee/68607
Shafiq, I., Hussain, S., Raza, M. A., Iqbal, N., Asghar, M. A., Raza, A., et al. (2021). Crop photosynthetic response to light quality and light intensity. J. Integr. Agric. 20, 4–23. doi: 10.1016/S2095-3119(20)63227-0
Shafiq, M., Qurashi, F., Mushtaq, S., Hussain, M., Hameed, A., Haider, M. S. (2020). “DNA plant viruses: biochemistry, replication, and molecular genetics,” in Applied plant virology (Elsevier), 169–182.
Shahid, M., Ameen, F., Maheshwari, H. S., Ahmed, B., Alnadhari, S., Khan, M. S. (2021). Colonization of Vigna radiata by a halotolerant bacterium Kosakonia sacchari improves the ionic balance, stressor metabolites, antioxidant status and yield under NaCl stress. Appl. Soil Ecol. 158, 103809. doi: 10.1016/j.apsoil.2020.103809
Shahryari, F., Rabiei, Z., Sadighian, S. (2020). Antibacterial activity of synthesized silver nanoparticles by sumac aqueous extract and silver-chitosan nanocomposite against Pseudomonas syringae pv. syringae. J. Plant Pathol. 102, 469–475. doi: 10.1007/s42161-019-00478-1
Sharma, N., Jandaik, S., Kumar, S., Chitkara, M., Sandhu, I. S. (2016). Synthesis, characterisation and antimicrobial activity of manganese-and iron-doped zinc oxide nanoparticles. J. Exp. Nanoscience 11, 54–71. doi: 10.1080/17458080.2015.1025302
Sharma, S., Singh, V. K., Kumar, A., Mallubhotla, S. (2019). Effect of nanoparticles on oxidative damage and antioxidant defense system in plants. Mol. Plant Abiotic Stress: Biol. Biotechnol., 315–333. doi: 10.1002/9781119463665.ch17
Sharon, M., Choudhary, A. K., Kumar, R. (2010). Nanotechnology in agricultural diseases and food safety. J. Phytology 2, 83–92.
Shehzad, N., Tahir, M., Johari, K., Murugesan, T., Hussain, M. (2018). A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Utilization 26, 98–122. doi: 10.1016/j.jcou.2018.04.026
Shen, C. X., Zhang, Q. F., Li, J., Bi, F. C., Yao, N. (2010). Induction of programmed cell death in Arabidopsis and rice by single-wall carbon nanotubes. Am. J. Bot. 97, 1602–1609. doi: 10.3732/ajb.1000073
Shi, Y., Ke, X., Yang, X., Liu, Y., Hou, X. (2022). Plants response to light stress. J. Genet. Genomics. 49, 737-747. doi: 10.1016/j.jgg.2022.04.017
Shinde, S. S. (2015). Antimicrobial activity of ZnO nanoparticles against pathogenic bacteria and fungi. Sci. Med. Cent. 3, 1033.
Shobha, B., Lakshmeesha, T. R., Ansari, M. A., Almatroudi, A., Alzohairy, M. A., Basavaraju, S., et al. (2020). Mycosynthesis of ZnO nanoparticles using Trichoderma spp. isolated from rhizosphere soils and its synergistic antibacterial effect against Xanthomonas oryzae pv. oryzae. J. Fungi 6, 181. doi: 10.3390/jof6030181
Siddiqi, K. S., Ur Rahman, A., Tajuddin, N., Husen, A. (2018). Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 13, 1–13. doi: 10.1186/s11671-018-2532-3
Siddiqui, M. H., Al-Whaibi, M. H., Faisal, M., Al Sahli, A. A. (2014). Nano-silicon dioxide mitigates the adverse effects of salt stress on. Environ. Toxicol. Chem. 33, 2429–2437. doi: 10.1002/etc.v33.11
Siddiqui, M. S., Aslam, M. (2023). Three-dimensional conductometric network based on reduced graphene oxide for soil pH sensors. ACS Appl. Nano Materials 6, 17376–17386. doi: 10.1021/acsanm.3c02033
Singh, P. (2017). LSPR biosensing: recent advances and approaches. Rev. Plasmonics 2016, 211–238. doi: 10.1007/978-3-319-48081-7_10
Singh, A. K., Dhanapal, S., Yadav, B. S. (2020). The dynamic responses of plant physiology and metabolism during environmental stress progression. Mol. Biol. Rep. 47, 1459–1470. doi: 10.1007/s11033-019-05198-4
Singh, S., Husen, A. (2019). Role of nanomaterials in the mitigation of abiotic stress in plants. Nanomaterials Plant potential, 441–471. doi: 10.1007/978-3-030-05569
Singh, S., Parihar, P., Singh, R., Singh, V. P., Prasad, S. M. (2015). Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 6, 1143. doi: 10.3389/fpls.2015.01143
Singh, A., Rajput, V. D., Sharma, R., Ghazaryan, K., Minkina, T. (2023). Salinity stress and nanoparticles: Insights into antioxidative enzymatic resistance, signaling, and defense mechanisms. Environ. Res. 116585, 116585. doi: 10.1016/j.envres.2023.116585
Singh, A., Sharma, A., Singh, O., Rajput, V. D., Movsesyan, H. S., Minkina, T., et al. (2024). In-depth exploration of nanoparticles for enhanced nutrient use efficiency and abiotic stresses management: present insights and future horizons. Plant Stress 100576, 100576. doi: 10.1016/j.stress.2024.100576
Singh, A., Tiwari, S., Pandey, J., Lata, C., Singh, I. K. (2021). Role of nanoparticles in crop improvement and abiotic stress management. J. Biotechnol. 337, 57–70. doi: 10.1016/j.jbiotec.2021.06.022
Sivasubramaniam, N., Hariharan, G., Zakeel, M. C. M. (2020). Sustainable management of plant-parasitic nematodes: an overview from conventional practices to modern techniques. Manage. phytonematodes: Recent Adv. Future challenges, 353–399. doi: 10.1007/978-981-15
Slavin, Y. N., Bach, H. (2022). Mechanisms of antifungal properties of metal nanoparticles. Nanomaterials (Basel) 12, 4470. doi: 10.3390/nano12244470
Soria, N. G. C., Bisson, M. A., Atilla-Gokcumen, G. E., Aga, D. S. (2019). High-resolution mass spectrometry-based metabolomics reveal the disruption of jasmonic pathway in Arabidopsis thaliana upon copper oxide nanoparticle exposure. Sci. Total Environ. 693, 133443. doi: 10.1016/j.scitotenv.2019.07.249
Sreelekshmi, P., Lakshmi, S. A., Babu, G., Devika, V., Rajeev, N., Sadanandan, S. (2020). Peptide dendrimer stabilized gold nanoparticles as sensors. Materials Today: Proc. 26, 382–386. doi: 10.1016/j.matpr.2019.12.060
Sudhakar, R., Kn, N. G., Venu, G. (2001). Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia 66, 235–239. doi: 10.1508/cytologia.66.235
Taylor, R., Coulombe, S., Otanicar, T., Phelan, P., Gunawan, A., Lv, W., et al. (2013). Small particles, big impacts: A review of the diverse applications of nanofluids. J. Appl. Phys. 113, 011301. doi: 10.1063/1.4754271
Thakur, R., Shirkot, P. (2017). Potential of biogold nanoparticles to control plant pathogenic nematodes. J. Bioanal BioMed. 9, 220–222. doi: 10.4172/1948-593X.1000182
Thiruvengadam, M., Gurunathan, S., Chung, I.-M. (2015). Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma 252, 1031–1046. doi: 10.1007/s00709-014-0738-5
Thounaojam, T. C., Meetei, T. T., Devi, Y. B., Panda, S. K., Upadhyaya, H. (2021). Zinc oxide nanoparticles (ZnO-NPs): a promising nanoparticle in renovating plant science. Acta Physiologiae Plantarum 43, 1–21. doi: 10.1007/s11738-021-03307-0
Tian, Y., Luo, J., Wang, H., Zaki, H. E., Yu, S., Wang, X., et al. (2022). Bioinspired green synthesis of silver nanoparticles using three plant extracts and their antibacterial activity against rice bacterial leaf blight pathogen xanthomonas oryzae pv. oryzae. Plants 11, 2892. doi: 10.3390/plants11212892
Timofeeva, E. V., Routbort, J. L., Singh, D. (2009). Particle shape effects on thermophysical properties of alumina nanofluids. J. Appl. Phys. 106, 106. doi: 10.1063/1.3155999
To, K. C., Ben-Jaber, S., Parkin, I. P. (2020). Recent developments in the field of explosive trace detection. ACS nano 14, 10804–10833. doi: 10.1021/acsnano.0c01579
Tolaymat, T., El Badawy, A., Sequeira, R., Genaidy, A. (2015). An integrated science-based methodology to assess potential risks and implications of engineered nanomaterials. J. Hazardous Materials 298, 270–281. doi: 10.1016/j.jhazmat.2015.04.019
Tripathi, D., Singh, M., Pandey-Rai, S. (2022). Crosstalk of nanoparticles and phytohormones regulate plant growth and metabolism under abiotic and biotic stress. Plant Stress 6, 100107. doi: 10.1016/j.stress.2022.100107
Tripathi, D. K., Singh, S., Singh, V. P., Prasad, S. M., Dubey, N. K., Chauhan, D. K. (2017). Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 110, 70–81. doi: 10.1016/j.plaphy.2016.06.026
Tripathy, B. C., Oelmüller, R. (2012). Reactive oxygen species generation and signaling in plants. Plant Signal Behav. 7, 1621–1633. doi: 10.4161/psb.22455
Tudi, M., Daniel Ruan, H., Wang, L., Lyu, J., Sadler, R., Connell, D., et al. (2021). Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 18, 1112. doi: 10.3390/ijerph18031112
Tyagi, P. K., Mishra, R., Khan, F., Gupta, D., Gola, D. (2020). Antifungal effects of silver nanoparticles against various plant pathogenic fungi and its safety evaluation on Drosophila melanogaster. Biointerface Res. Appl. Chem. 10, 6587–6596. doi: 10.33263/BRIAC
Umar, O. B., Ranti, L. A., Abdulbaki, A. S., Bola, A., Abdulhamid, A., Biola, M., et al. (2021). Stresses in plants: Biotic and abiotic. Curr. Trends Wheat Res., 140-143. doi: 10.5772/intechopen.100501
Van Nguyen, D., Nguyen, H. M., Le, N. T., Nguyen, K. H., Nguyen, H. T., Le, H. M., et al. (2021). Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J. Plant Growth Regul., 1–12. doi: 10.1007/s00344-021-10301
Večeřová, K., Večeřa, Z., Dočekal, B., Oravec, M., Pompeiano, A., Tříska, J., et al. (2016). Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles. Environ. pollut. 218, 207–218. doi: 10.1016/j.envpol.2016.05.013
Venkatachalam, P., Kayalvizhi, T., Udayabanu, J., Benelli, G., Geetha, N. (2017). Enhanced antibacterial and cytotoxic activity of phytochemical loaded-silver nanoparticles using Curculigo orchioides leaf extracts with different extraction techniques. J. Cluster Sci. 28, 607–619. doi: 10.1007/s10876-016-1141-5
Walters, R. G. (2005). Towards an understanding of photosynthetic acclimation. J. Exp. Bot. 56, 435–447. doi: 10.1093/jxb/eri060
Wang, Y., Liu, Y., Zhan, W., Zheng, K., Lian, M., Zhang, C., et al. (2020). Long-term stabilization of Cd in agricultural soil using mercapto-functionalized nano-silica (MPTS/nano-silica): A three-year field study. Ecotoxicology Environ. Saf. 197, 110600. doi: 10.1016/j.ecoenv.2020.110600
Wang, K., Wang, Y., Wan, Y., Mi, Z., Wang, Q., Wang, Q., et al. (2021). The fate of arsenic in rice plants (Oryza sativa L.): Influence of different forms of selenium. Chemosphere 264, 128417. doi: 10.1016/j.chemosphere.2020.128417
Wang, X., Xie, H., Wang, P., Yin, H. (2023). Nanoparticles in plants: uptake, transport and physiological activity in leaf and root. Materials 16, 3097. doi: 10.3390/ma16083097
Wang, Q., Zhao, S., Zhao, Y., Rui, Q., Wang, D. (2014). Toxicity and translocation of graphene oxide in Arabidopsis plants under stress conditions. RSC Adv. 4, 60891–60901. doi: 10.1039/C4RA10621K
Waseem, M., Naveed, M., Rehman, S. U., Makhdoom, S. I., Aziz, T., Alharbi, M., et al. (2023). Molecular characterization of spa, hld, fmhA, and l ukD Genes and computational modeling the multidrug resistance of staphylococcus species through Callindra Harrisii silver nanoparticles. ACS omega 8, 20920–20936. doi: 10.1021/acsomega.3c01597
Wu, Q., Miao, W.-S., Zhang, Y.-D., Gao, H.-J., Hui, D. (2020). Mechanical properties of nanomaterials: A review. Nanotechnology Rev. 9, 259–273. doi: 10.1515/ntrev-2020-0021
Wu, H., Tito, N., Giraldo, J. P. (2017). Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS nano 11, 11283–11297. doi: 10.1021/acsnano.7b05723
Xiong, T., Zhang, S., Kang, Z., Zhang, T., Li, S. (2021). Dose-dependent physiological and transcriptomic responses of lettuce (Lactuca sativa L.) to copper oxide nanoparticles—Insights into the phytotoxicity mechanisms. Int. J. Mol. Sci. 22, 3688. doi: 10.3390/ijms22073688
Yadav, A., Yadav, K. (2018). Nanoparticle-based plant disease management: tools for sustainable agriculture. Nanobiotechnology Appl. Plant Prot., 29–61. doi: 10.1007/978-3-319-91161
Yang, H., Marion, T. N., Liu, Y., Zhang, L., Cao, X., Hu, H., et al. (2019). Nanomaterial exposure induced neutrophil extracellular traps: a new target in inflammation and innate immunity. J. Immunol. Res. 2019, 3560180. doi: 10.1155/2019/3560180
Yasmeen, F., Raja, N. I., Mustafa, G., Sakata, K., Komatsu, S. (2016). Quantitative proteomic analysis of post-flooding recovery in soybean root exposed to aluminum oxide nanoparticles. J. Proteomics 143, 136–150. doi: 10.1016/j.jprot.2016.03.014
Younis, A., Khattab, H., Emam, M. (2020). Impacts of silicon and silicon nanoparticles on leaf ultrastructure and TaPIP1 and TaNIP2 gene expressions in heat stressed wheat seedlings. Biol. plantarum 64, 343-352. doi: 10.32615/bp.2020.030
Yruela, I. (2013). Transition metals in plant photosynthesis. Metallomics 5, 1090–1109. doi: 10.1039/c3mt00086a
Ze, Y., Liu, C., Wang, L., Hong, M., Hong, F. (2011). The regulation of TiO 2 nanoparticles on the expression of light-harvesting complex II and photosynthesis of chloroplasts of Arabidopsis thaliana. Biol. Trace element Res. 143, 1131–1141. doi: 10.1007/s12011-010-8901-0
Zhang, X., Bian, Z., Yuan, X., Chen, X., Lu, C. (2020). A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 99, 203–216. doi: 10.1016/j.tifs.2020.02.031
Zhang, C. L., Jiang, H. S., Gu, S. P., Zhou, X. H., Lu, Z. W., Kang, X. H., et al. (2019a). Combination analysis of the physiology and transcriptome provides insights into the mechanism of silver nanoparticles phytotoxicity. Environ. pollut. 252, 1539–1549. doi: 10.1016/j.envpol.2019.06.032
Zhang, H., Lu, L., Zhao, X., Zhao, S., Gu, X., Du, W., et al. (2019b). Metabolomics reveals the “invisible” responses of spinach plants exposed to CeO2 nanoparticles. Environ. Sci. Technol. 53, 6007–6017. doi: 10.1021/acs.est.9b00593
Zhang, B., Pan, X., Cobb, G. P., Anderson, T. A. (2006). Plant microRNA: a small regulatory molecule with big impact. Dev. Biol. 289, 3–16. doi: 10.1016/j.ydbio.2005.10.036
Zhao, L., Huang, Y., Keller, A. A. (2017). Comparative metabolic response between cucumber (Cucumis sativus) and corn (Zea mays) to a Cu (OH) 2 nanopesticide. J. Agric. Food Chem. 66, 6628–6636. doi: 10.1021/acs.jafc.7b01306
Zhao, L., Ortiz, C., Adeleye, A. S., Hu, Q., Zhou, H., Huang, Y., et al. (2016). Metabolomics to detect response of lettuce (Lactuca sativa) to Cu (OH) 2 nanopesticides: oxidative stress response and detoxification mechanisms. Environ. Sci. Technol. 50, 9697–9707. doi: 10.1021/acs.est.6b02763
Zhao, X., Zhang, X., Wang, L., Huang, Q., Dai, H., Liu, L., et al. (2024). Foliar application of iron impacts flavonoid glycosylation and promotes flavonoid metabolism in coloured rice. Food Chem. 444, 138454. doi: 10.1016/j.foodchem.2024.138454
Zhao, L., Zhang, H., Wang, J., Tian, L., Li, F., Liu, S., et al. (2019). C60 fullerols enhance copper toxicity and alter the leaf metabolite and protein profile in cucumber. Environ. Sci. Technol. 53, 2171–2180. doi: 10.1021/acs.est.8b06758
Zhou, P., Adeel, M., Shakoor, N., Guo, M., Hao, Y., Azeem, I., et al. (2020). Application of nanoparticles alleviates heavy metals stress and promotes plant growth: an overview. Nanomaterials (Basel) 11, 26. doi: 10.3390/nano11010026
Zhou, Q., Li, J., Wang, M., Zhao, D. (2016). Iron-based magnetic nanomaterials and their environmental applications. Crit. Rev. Environ. Sci. Technol. 46, 783–826. doi: 10.1080/10643389.2016.1160815
Zhu, T., Fonseca De Lima, C. F., De Smet, I. (2021). The heat is on: how crop growth, development, and yield respond to high temperature. J. Exp. Bot. 72, 7359–7373. doi: 10.1093/jxb/erab308
Zia-Ur-Rehman, M., Anayatullah, S., Irfan, E., Hussain, S. M., Rizwan, M., Sohail, M. I., et al. (2023). Nanoparticles assisted regulation of oxidative stress and antioxidant enzyme system in plants under salt stress: A review. Chemosphere 314, 137649. doi: 10.1016/j.chemosphere.2022.137649
Keywords: abiotic stress, biotic stress, secondary metabolic response, molecular response, and nanotechnology
Citation: Zhou X, El-Sappah AH, Khaskhoussi A, Huang Q, Atif AM, Elhamid MAA, Ihtisham M, El-Maati MFA, Soaud SA and Tahri W (2025) Nanoparticles: a promising tool against environmental stress in plants. Front. Plant Sci. 15:1509047. doi: 10.3389/fpls.2024.1509047
Received: 10 October 2024; Accepted: 16 December 2024;
Published: 27 January 2025.
Edited by:
Babar Iqbal, Jiangsu University, ChinaReviewed by:
Yogesh K. Ahlawat, Chandigarh University, IndiaMuhammad Asim, Tianjin University, China
Muhammad Jafir, Anhui University, China
Copyright © 2025 Zhou, El-Sappah, Khaskhoussi, Huang, Atif, Elhamid, Ihtisham, El-Maati, Soaud and Tahri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Walid Tahri, d3RhaHJpOTY3QGdtYWlsLmNvbQ==; MjAyMDA5MDEwNUB5aWJpbnUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Xu Zhou1†
Xu Zhou1† Ahmed H. El-Sappah
Ahmed H. El-Sappah Qiulan Huang
Qiulan Huang Muhammad Ihtisham
Muhammad Ihtisham Walid Tahri
Walid Tahri