
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 06 February 2025
Sec. Plant Symbiotic Interactions
Volume 15 - 2024 | https://doi.org/10.3389/fpls.2024.1486607
This article is part of the Research TopicPathogen Suppression by Plant-Associated MicrobiotaView all 7 articles
 Rasha M. Elmeihy1†
Rasha M. Elmeihy1† Omar A. Hewedy2*†
Omar A. Hewedy2*† Maryam S. Alhumaidi3
Maryam S. Alhumaidi3 Khadijah A. Altammar3
Khadijah A. Altammar3 Eman O. Hassan4
Eman O. Hassan4 Samah A. El-Debaiky5
Samah A. El-Debaiky5Plant diseases caused by fungal pathogens are responsible for severe damage to strategic crops worldwide. Late wilt disease (LWD) is a vascular disease that occurs late in maize development. Harpophora maydis, the causative agent of maize LWD, is responsible for significant economic losses in Egypt. Therefore, the aim of this study was to control LWD of maize using an alternative approach to reduce the use of chemical pesticides. A combination of Trichoderma viride, a fungal biocontrol agent, and Azospirillum brasilense, a bacterial endophytic plant growth promoter, was applied in vitro and in planta. T. viride showed high mycoparasitic potential against H. maydis via various antagonistic activities, including the production of lytic enzymes, secondary metabolites, volatile compounds, and siderophores. A. brasilense and T. viride filtrates were also shown to suppress H. maydis growth, in addition to their ability to produce gibberellic and indole acetic acids. A significant change in the metabolites secreted by T. viride was observed using GC/MS in the presence of H. maydis. A field experiment was conducted on susceptible and resistant hybrids of maize to evaluate the antagonistic activity of T. viride combined with A. brasilense on LWD incidence as well as plant growth promotion under field conditions. The data revealed a significant decrease in both disease incidence and severity in maize plants treated with T. viride and/or A. brasilense. Further, there was a noticeable increase in all plant growth and yield parameters. An anatomical examination of the control and inoculated maize roots was also reflective of plant responses under biotic stress. Taken together, the obtained results provide successful eco-friendly management strategies against LWD in maize.
The future development of sustainable agriculture is one of the backbones of the national economy in Egypt. In 2015, the Egyptian Government initiated a national project to reclaim 1.5 million acres to increase agricultural production of the strategic crops (i.e., wheat (Triticum aestivum L.), rice (Oryza sativa), and maize (corn, Zea mays L.)) (Moghazy and Kaluarachchi, 2020). Maize is one of the most stable foods and cereal crops in the world and is the third leading cereal crop after rice and wheat in cultivated area and productivity (Chinaru Nwosu et al., 2015; He et al., 2024). In addition, maize is an economically important crop in Africa, which is severely affected by many fungal pathogens (Veenstra et al., 2019; Benjamin et al., 2024). Late wilt disease (LWD) or black bundle disease (the causal agent of the maize LWD) is a severe vascular disease of maize caused by Harpophora maydis fungus, which is implicated in the PFSR complex. H. maydis is a soil- and seed-borne fungus related to the root-infecting species (Samra et al., 1962; Gams, 2000; Singh et al., 2020). The general symptoms include rapid and visible wilting of maize plants before tasseling, which continues until maturity. Moreover, the leaves between the veins change to a pale green before the whole leaf rolls. This disease phenotype gradually progresses from lower to upper leaves. Some plants develop yellow, purple, or dark brown streaks that appear on the lower stem, which then dry up and become shrunken. Subsequently, vascular bundles in the stalk turn reddish-brown, and internodes become discolored (Drori et al., 2013; Degani and Dor, 2021; Degani, 2022). This disease has been designated as “late wilt” because of the delayed appearance of initial symptoms until flowering, with no cobs in severe cases and undeveloped seeds (Samra et al., 1962; Payak et al., 1970; Gams, 2000; Molinero-Ruiz et al., 2010). Furthermore, the dormant sclerotia of this phytopathogenic fungus remains in the soil for many years, where it continues to colonize and infect maize roots (Drori et al., 2013). Ultimately, the fungus causes seed rot and delayed seedling emergence (Payak et al., 1970). The disease is considered the most severe threat to commercial maize production in Egypt (El-Naggarr et al., 2015). The first case of LWD disease was identified and reported in Egypt in 1961–1962, which affected 70% of the susceptible varieties (Samra et al., 1963; Johal et al., 2004) and gradually reported in other maize-growing countries such as Portugal and Spain (Ortiz-Bustos et al., 2015). H. maydis has recently become a significant problem in Egypt due to transmission by seeds and survival as sclerotia on corn debris. Importantly, infected seeds, crop residues, high temperature, and low humidity are the main factors affecting the distribution and development of LWD in maize. Numerous attempts have been made to reduce LWD development with integrated disease management strategies. These include the introduction of new agricultural practices, biological control strategies, physical interventions (e.g., solar heating), and chemical fungicides to protect susceptible maize varieties (Tej et al., 2018; Degani et al., 2018; Degani and Dor, 2021). However, excessive chemical fungicides (e.g., Azoxystrobin) negatively impact global health and sustainable food production. Consequently, biological control strategies have gained increased importance as an alternative environmentally friendly approach for LWD control. Interestingly, diverse beneficial microbes (i.e., Bacillus subtilis, Pseudomonas koreensis, and Trichoderma species) were applied as an alternative method to control LWD (Elshahawy and El-Sayed, 2018; Ghazy and El-Nahrawy, 2021). Trichoderma (Hypocreales) fungus is widely regarded as the most common fungal biocontrol agent for plant health management, including for ubiquitous species localized in diverse habitats (Nakkeeran et al., 2021; Woo et al., 2023). Trichoderma viride, T. harzianum, T. atroviride, T. virens, T. hamatum, and T. longibrachiatum have been developed as promising biological control agents due to their significant antagonistic potential (Jiang et al., 2011; Błaszczyk et al., 2016; Ghasemi et al., 2020; Cai and Druzhinina, 2021; (Dutta et al., 2023; Contreras-Cornejo et al., 2024; Guzmán-Guzmán et al., 2024; Santoyo et al., 2024)). H. maydis, like most fungi controlled by Trichoderma spp. have cell walls that contain chitin as a structural backbone and laminarin (ß-1, 3-glucan) as a filling material (Ulhoa and Peberdy, 1991). Trichoderma can penetrate fungal cell walls and grow extensively within mycelium by destroying their cell walls. This mechanism of action shows that the fungus produces chitinase and ß-1,3 glucanase enzymes (Chen et al., 2016; Ghasemi et al., 2019). T. viride can antagonistically affect plant-pathogenic fungi and nematodes, as well as improve crop resistance and promote plant growth via bioactive substances (Druzhinina et al., 2018; Kubicek et al., 2019; Abdelaziz et al., 2023). Trichoderma can produce hundreds of antimicrobial secondary metabolites, including trichomycin, gelatinomycin, chlorotrichomycin, and antibacterial peptides (Maruyama et al., 2020; Tamizi et al., 2022). These secondary metabolites can act as antibacterial agents and promote plant growth (Nawrocka et al., 2023). Importantly, there is a lack of data regarding the antagonistic activity of Trichoderma against LWD. The antifungal and anti-mycotic activities of Trichoderma viride and Trichoderma harzianum against different pathogenic fungal strains have previously been evaluated in vitro using a dual culture assay (Yogalakshmi et al., 2021; Hossain and Sultana, 2024). T. viride was shown to have an effective, potent activity for suppressing the mycelial growth of diverse pathogens, including Curvularia lunata, Exserohilum rostratum, Fusarium chlamydosporum, Fusarium incarnatum, Fusarium proliferatum, and Macrophomina phaseolina (Yassin et al., 2021). Moreover, eight Trichoderma isolates were tested as biocontrol agents against M. maydis. T. longibrachiatum and T. asperelloides showed high mycoparasitic activity against the pathogen by producing soluble metabolites that inhibit or kill the maize pathogen (Degani and Dor, 2021). Azospirillum is a type of Rhizobacteria and an associative nitrogen fixer (diazotroph) that comprises seven species, i.e., A. amazonense, A. brasilense, A. doebereinnerae, A. halopraeferens, A. irakense, A. largimobile, and A. lipoferum. A. brasilense is an aerobic bacterium that exhibits the main characteristics that define plant growth-promoting, such as nitrogen fixation and siderophore production (Galindo et al., 2020). It has been reported that Azospirillum strains have the capability to produce different phytohormones, including indole acetic acid (IAA), cytokinins, gibberellins, and other compounds, such as polyamines and amino acids. Notably, the inoculation of Azospirillum brasilense represents a potentially efficient method to improve plant development (Mehnaz, 2014; Fukami et al., 2018; Cassán et al., 2020; Rabani et al., 2023; Gureeva and Gureev, 2023). The attachment of Azospirillum to the roots is considered the first necessary step for the colonization of the host plants, which mainly colonizes the root surface (Steenhoudt and Vanderleyden, 2000; Pedraza et al., 2020; Yadav et al., 2024). Azospirillum species are able to colonize hundreds of plant species and improve their growth, development, and productivity, such as maize (Fukami et al., 2016; Cardozo et al., 2022). Interestingly, Azospirillum brasilense Sp7 and a bio-control fungus (Trichoderma harzianum Rifai 1295-22), were evaluated for their single and combined effects on dry bean (Phaseolus vulgaris) and wheat (Triticum aestivum L.) grown in soil (Öğüt et al., 2005). A field experiment was carried out to evaluate the feasibility of inoculating rice seedlings with biofertilizers (Azospirillum and Trichoderma) to reduce the use of chemical inorganic nitrogen (N) fertilizer on rice (Khan, 2018). Hence, this study aimed to use Trichoderma viride as a biocontrol agent to control LWD of maize plants caused by H. maydis-infected maize in the presence of Azospirillum, as a plant-growth promoter already known for its ability to associate with cereal crops such as maize.
This study was divided into two parts; the first was conducted in vitro, while the second utilized microbial application in the field (Summer, 2020).
T. viride strain T27 (accession number MH908510) was isolated and identified in a previous study (Hewedy et al., 2020b), as presented in (Figures 1A–C). H. maydis (isolate C5) was kindly supplied by the Department of Plant Pathology, Faculty of Agriculture, Benha University, Egypt. All fungi were propagated on potato dextrose agar (PDA; HIMEDIA Co.) at 28°C for five days and then maintained at 4°C until further testing.
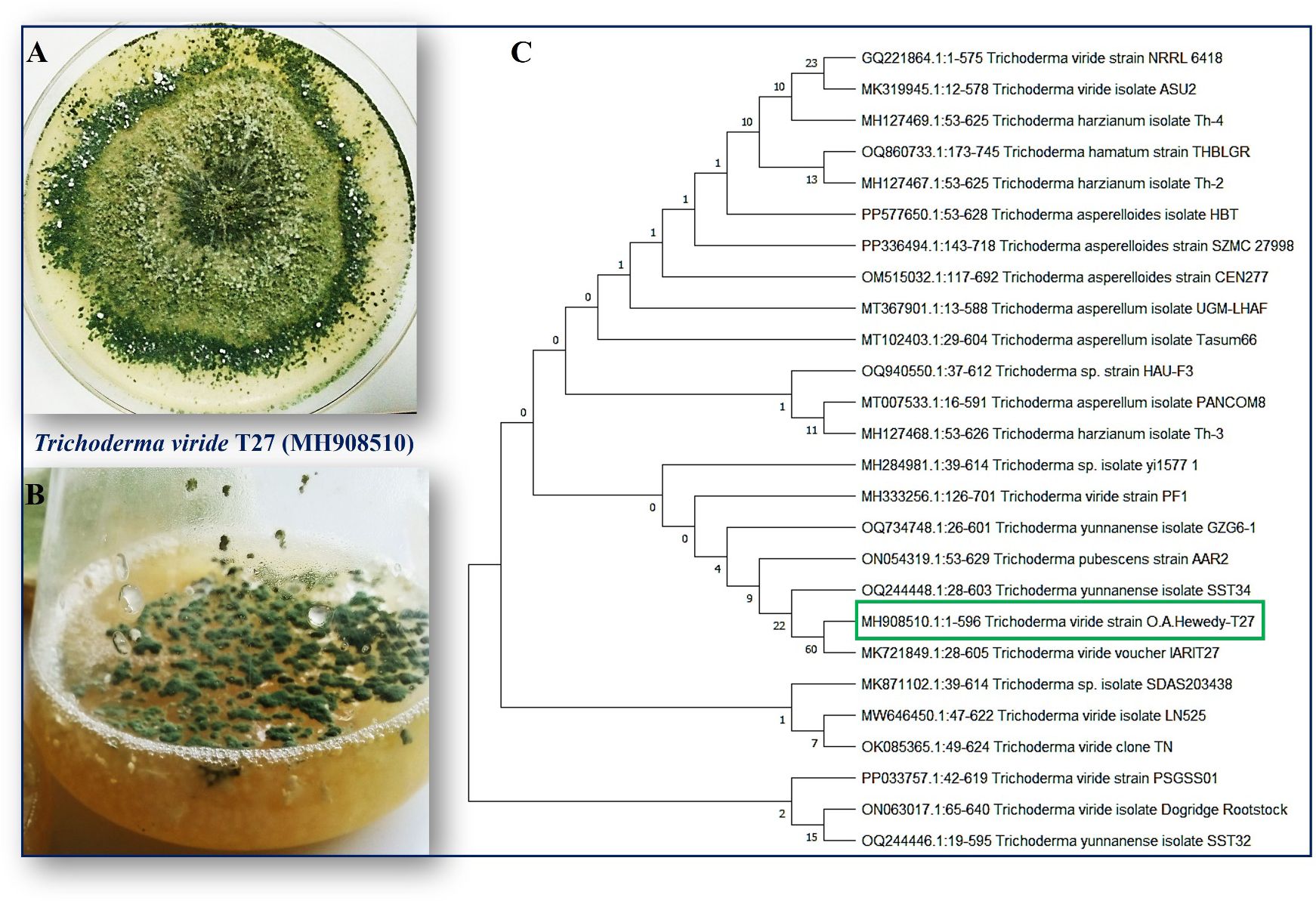
Figure 1. (A) Colonies of endophytic fungi T. viride grown on potato dextrose agar (PDA) media under photoperiod conditions at 28°C for five days show a ring around the original inoculum. (B) T. viride grown on liquid media potato dextrose broth (PDB) under photoperiod conditions at 150 rpm. (C) A phylogenetic tree was inferred through a maximum-likelihood analysis of aligned rDNA internal transcribed spacers ITS (ITS4 and ITS5) sequences from different Trichoderma isolates using MEGA11.0. T. viride T27 (MH908510) was identified based on the maximum likelihood model and their closest matches, followed by the GenBank accession number. The numbers above the branches indicate the number of times the group consisting of the species to the right of that fork occurred among the tree out of 100 trees. The boxed species indicate the phylogenetic position of Trichoderma viride (T27) compared with other fungal strains deposited on the GenBank.
A. brasilense (strain MC12) was obtained from the Department of Agricultural Microbiology, Faculty of Agriculture, Benha University, Egypt, and cultured on nutrient broth medium (HIMEDIA Co. M002, 13.0 g in 1000 mL ddH2O) at 28°C ± 2°C for four days before storage at 4°C until subsequent testing.
The antagonistic activity of Trichoderma (T27, MH908510) was evaluated in vitro against H. maydis using the dual culture technique at 28°C ± 2°C (Pan and Bhagat, 2008). The pathogen inhibition percentage (IP) against mycelial growth was calculated according to the following formula (Hajieghrari et al., 2008):
where, C = radial growth in control (pathogen only), T = radial growth in treatment.
Next, the hyphal interaction in the contact area was examined and photographed using a light microscope equipped with a USB camera at a magnification power of 800× (OPTIKA C-B5 5.1 Megapixel CMOS USB 2.0 Camera, Microscope Company, USA).
The antifungal activity of the culture filtrate of T27 against H. maydis was tested in vitro as described by (Elshahawy and El-Sayed, 2018). Trichoderma T27 was grown for ten days at 28°C ± 2°C with agitation (150 rpm) in 100 mL Erlenmeyer flasks containing 50 mL of sterilized PDB. Next, the mycelial growth was removed by filtration using filter paper (Whatman filter paper #1, WHA1001090) and centrifuged at 5000 rpm with slight modifications. Subsequently, 5 mL of Trichoderma filtrate was added to 45 mL of PDB medium to make a final concentration of filtrate (10% v/v). This was performed in triplicate, and a negative control was prepared using 5 mL sterile distilled water instead of fungal filtrate (Rahman et al., 2023).
The amended flasks were then inoculated with a five-day-old (5 mm) disk of H. maydis and incubated at 28°C with shaking at 150 rpm for nine days. All flasks were weighed at zero time and after 3, 6, and 9 days of inoculation. The reduction of H. maydis growth was measured according to the following equation:
where, Wc = weight of control flask, Wt = weight of treatment flask at the same time as control.
The synergistic effect of culture filtrates of T27 and A. brasilense was also studied. Briefly, 5 mL of bacterial culture (A. brasilense) was transferred to 100 mL Erlenmeyer flasks containing 50 mL of Dobereiner’s broth (DB) medium and incubated at 28°C ± 2°C for four days. The microbial cells were then removed from each culture by filtration through filter paper (Whatman no. 1) and then centrifugation (centrifuge Tube GKF, China) at 5,000 rpm for 10 min to obtain cell-free filtrate for further experiments (Abdulkareem et al., 2014).
Subsequently, 5 mL of bacterial filtrate was mixed separately with 45 mL of PDB. In comparison, 2.5 mL of bacterial filtrate, 2.5 mL of the T27 filtrate, and 45 mL of PDB were prepared for a final concentration of 10% (v/v). Sterilized DB medium and distilled water served as the negative control. Next, control and treatment flasks were inoculated separately with 5 mm diameter mycelial discs of H. maydis and incubated at 28°C ± 2°C with shaking at 150 rpm. The weight of each flask was measured at zero time and after 3, 6, and 9 days of inoculation. The reduction in H. maydis growth was calculated as described in the equation above. All treatments and controls were performed in triplicate.
Mono- and dual cultures of T. viride T27 alone and Trichoderma in the presence of H. maydis C5 were tested to produce bioactive secondary metabolites. T. viride (T27) was grown separately or in combination on a PDB medium at optimum temperature for 20 days. Cultures were then filtered through filter papers (Whatman no. 1.), and equal volumes of filtrate and hexane (1:1 v/v) were mixed gently to extract the metabolites. Subsequently, hexane was evaporated using a rotary evaporator with a rotor speed of 120 rpm at 400 °C until the precipitate was formed. The precipitate was re-suspended in acetone for further characterization by GC/MS (GCMS-QP2010 Plus ultra), as previously described by (Bhardwaj and Kumar, 2017).
Ammonia (NH3) and hydrogen cyanide (HCN) produced from T27 culture filtrate were estimated spectroscopically according to previously described methods. Briefly, a fresh culture of T27 was prepared in a test tube containing 10 mL of peptone water and incubated for five days at 28°C ± 2°C. After incubation, 1 mL of the culture was transferred to Eppendorf and combined with 50 μL of Nessler’s reagent, which was prepared by mixing 2 g KI in 5 mL of H2O. Next, 3 g of HgI2 was added, and the resulting solution was made up to 20 mL. Finally, 40 g of KOH (30%) was added to provide the alkaline base. A color change from a clear solution to a faint yellow indicated the presence of a small amount of ammonia, while a deep yellow or brown color was indicative of higher ammonia content. The color change was measured using a spectrophotometer (Sco. Tech, SP UV-19) at 450 nm. A standardized curve was generated by titrating ammonium sulfate from 0.1 – 5 μmol/mL (Cappuccino and Sherman, 1992; Reetha et al., 2014; Abdenaceur et al., 2022).
Next, 10 mL of T27 was inoculated in a 100 mL Erlenmeyer flask containing King’s B broth medium amended with 4.4 g/L glycine to detect HCN as previously described (El-Rahman et al., 2019). Non-inoculated flasks were used as negative controls, and all treatments and controls were performed in triplicate. Sterilized filter paper strips dipped in picrate solution (0.5% picric acid in 2% sodium carbonate) were attached to the neck of the flasks. Each flask was then plugged and sealed off with Parafilm and incubated with shaking at 140 rpm for four days at 28 °C ± 2°C. A change in the color of the filter paper strips from yellow to light brown, brown, or brick red was recorded as a weak (+), moderate (++), or strong (+++) reaction, respectively. A lack of color change was recorded as a negative (−) reaction. Moreover, the color intensity was detected and measured by spectrophotometry at 625 nm by dipping the filter paper strips into 10 mL of distilled water to elute the produced color. Likewise, qualitative and quantitative assessment of siderophores using chrome azurol S (CAS) reagent was also performed (Payne, 1994; Dutta et al., 2015).
For the qualitative evaluation, T27 was grown on CAS agar plates at 28°C for five days. Siderophore production was detected when the color of the medium changed to orange color. The CAS-shuttle assay was used for quantitative detection of siderophores. A total of 0.5 mL of supernatant was obtained from the filtration of broth cultures of the tested T27, which was mixed with an equal volume of CAS reagent. Siderophores were analyzed by spectrophotometry at 630 nm, where uninoculated broth medium was used as control, and the proportion of siderophore units was calculated as a percentage according to the following formula:
where, Ac = absorbance of the control, As = absorbance of the sample.
Chitinase (3.2.1.14) activity was qualitatively estimated using the Lukewarm agar medium, amended with bromocresol purple for colored zone formation. The constituents of the medium were as follows: MgSO4.7H2O, 0.3 g/L; (NH4)2SO4, 3; KH2PO4, 2 g/L; citric acid monohydrate,1 g/L; agar, 15 g/L; colloidal chitin, 4.5 g/L; bromocresol purple, 0.15 g/L; and 200 μL Tween-80, pH 4.7 (Sigma-Aldrich, USA). Solidified medium plates were inoculated with T27, incubated at 28°C ± 2°C for seven days, and observed for colored zone formation (Agrawal and Kotasthane, 2012). Moreover, for quantitative assessment of chitinase, T27 was inoculated in Lukewarm broth medium without bromocresol purple and incubated in a shaker at 28°C with shaking at 150 rpm for five days. After the incubation period, the culture was centrifuged at 5000 rpm for 15 min, and chitinolytic activity was quantitatively assayed in culture filtrate by measuring the released reducing sugars from colloidal chitin. Briefly, 0.3 mL of 1 M sodium acetate buffer (pH 4.6) and 0.2 mL of colloidal chitin were transferred to a test tube containing 1 mL of culture filtrate, then incubated at 40°C for 20 h. After incubation, the mixture was centrifuged at 10,000 rpm for 5 min. Next, 0.75 mL of the tested mixture was combined with 0.25 mL of DNS solution (i.e., 1.0 g of 3,5 dinitro salicylic acid in 20 mL 2 M NaOH, to which 30 g of sodium potassium tartrate was slowly added before dilution to a final volume of 100 mL using distilled water) were mixed in test tubes and heated at 100°C for 5 min. After cooling, the absorbance was detected at 582 nm using a spectrophotometer (Sco. Tech, SP UV-19) (Miller, 1959). A standard N-acetyl-glucosamine (NAGA) curve was used to calculate chitinolytic activity using the concentration of released NAGA as the readout. Both amylase and cellulase activities were estimated in crude culture filtrate of T27. Starch broth and carboxy methyl cellulose media were used to estimate amylase and cellulase. The dinitrosalicylic acid method was applied for both enzymes to measure the released amounts of glucose by spectrophotometry at 575 nm using a glucose standard curve as previously described (Miller, 1959).
Indeed, most endophytic microbes, either fungi or bacteria, live in association with the roots of many plants. The ability of Trichoderma T27 and A. brasilense to produce indole acetic acid (IAA) and gibberellic acid (GA3) in vitro was assessed using Salkowski’s and Folin–Ciocalteu (FC) reagents as previously described (Crozier et al., 1988; Pastrana et al., 1995; Perrig et al., 2007; Spaepen et al., 2008; Zhang et al., 2013; Nieto-Jacobo et al., 2017; Abdenaceur et al., 2022). T27 and A. brasilense cultures were grown for seven days on Czapek-dox broth and nutrient broth media supplemented with L-tryptophan (1 mg/L), respectively. After the incubation period, the microbial growth was removed by filtration. Next, 20 mL of each culture filtrate was centrifuged at 3000 rpm for 5 minutes. IAA production was then tested by adding 2 mL of the filtrate to 2 mL of Salkowski reagent (0.5 M ferric chloride (FeCl3) and 35% perchloric acid (HClO4)) and allowed to stand for 15 minutes. A color change to pink (measured at 535 nm) was indicative of a positive result. Additionally, for the GA3 assay, 1 mL of microbial supernatant was combined and boiled with 1 mL of the reagent, 1 mL of concentrated HCl, and 3 mL dH2O for 5 min in a water bath. Finally, after cooling to room temperature, the produced color change from green to blue was measured at 750 nm using a spectrophotometer (Sco. Tech, SP UV-19).
Mycoparasitism activity was studied as previously described (Bhat, 2017; Mukherjee et al., 2022) with minor modifications. Briefly, 15 mL of PDA was poured into a 90 mm petri dish and allowed to solidify. Then, 5 mm discs of T. viride (T27) and H. maydis C5 were inoculated at opposite points on the edges of PDA agar and then incubated for 5–7 days at 28°C, allowing the two fungi to grow toward each other. Upon interaction, a small portion was carefully separated without destroying the interacting mycelia and transferred to a clean glass slide. The interfering mycelia were then examined under a light microscope (800×) with a USB camera (OPTIKA C-B5 5.1 Megapixel CMOS USB 2.0 Camera, Microscope Company, USA).
The results of the in vitro studies on maize plants (Zea mays L.) were applied in the field during the growing season (Summer 2020). This experiment was carried out at the Faculty of Agriculture, Benha University, Egypt. The current study applied a randomized complete block design in triplicate. Each experimental plot (21 m2) was split into six rows. The in planta and field treatments were designed as follows: T1 as the uninfected control; T2 as the infected control by the pathogen H. maydis; T3: bioagent (T. viride T27 only) inoculated plants; T4: plants inoculated with a combination of (T27 + A. brasilense); T5: plants infected with a combination of (T27 + H. maydis); and T6: the last treatment which consisted of the inoculated plants with a combination of (T27 + A. brasilense + H. maydis). The grains of two yellow solitary hybrid cultivars of maize varied in their susceptibility to LWD and were purchased from Pioneer Company, Egypt (https://www.pioneer.com/landing), (Supplementary Figure S1). The first hybrid (11N30) was registered as a tolerant cultivar, while the second (3062) was considered susceptible. This experiment evaluated the influence of T. viride T27 and A. brasilense on the plant growth criteria and incidence of LWD caused by H. maydis. The soil was clay loam comprised of 1.52% organic matter (pH 8.2).
Next, we tested antagonistic microorganisms following inoculation. Briefly, T. viride (27) was prepared on PDB medium at 28 °C ± 2°C with shaking (150 rpm) for seven days before it was homogenized and mixed with soil one week before cultivation (excluding the treatment of the control rows). Then, T. virdie homogenized culture containing 106 Spore/ml was added at the rate of 100 ml/Jura (10 cm and depth 10 cm). Extra doses of T. viride suspension were added to the plants three times during the growing season at a rate of 50 mL/plant. Further, cell suspensions of A. brasilense MC12 were grown on DB medium and incubated at 28°C ± 2°C for four days. Then, maize seeds were soaked for 30 min in a mixture of the cell suspension (108 colony forming unit (cfu) mL−1) and 10% Arabic gum as an adhesive agent before cultivation. An excessive dose of A. brasilense inoculum was added to the soil rhizosphere near each plant three times during the growing season at a rate of 50 mL/plant.
Maize seeds were sown at a 20 cm distance between plants. After 21 days of emergence, plants were manually thinned to one plant/Jura. Plants were irrigated and chemically fertilized with a nitrogen (N; 140 kg N2 as ammonium sulfate), phosphate (P; 200 kg P2O5 as calcium superphosphate), and potassium (K; 50 kg K2O as potassium sulfate) mix as recommended by the Ministry of Agriculture and Land Reclamation of Egypt in two equal doses at vegetative and flowering stages. The dose of N was reduced to half in treatments with A. brasilense.
H. maydis was cultured on PDB at 28 °C ± 2°C with shaking (150 rpm) for seven days, then used to infect maize plants as previously described (Shekhar and Kumar, 2012) with some modifications. A sterilized thin syringe was filled with homogenized H. maydis inoculum under sterilized conditions. Subsequently, the outer surface of the plants (40 days old) was sterilized with 70% (v/v) ethyl alcohol, and the pathogen was injected into the second lower internodes above the soil level. In addition, the infected plants were apparently observed with late wilt symptoms at 20–25 days after infection. Each experimental plot (21 m2) was split into six rows; Maize seeds were sown at a 20 cm distance between plants. After 21 days of emergence, plants were manually thinned to one plant/Jura.
Disease assessment was performed periodically as disease incidence and severity after 60, 80, and 120 days of sowing (or after 20, 40, and 80 days of infestation). Disease incidence and severity were periodically recorded by examining the stems and leaves of 10 randomly selected infected plants after 60, 80, and 120 days of sowing (or after 20, 40, and 80 days of infection).
Ten plants of each replicate were randomly selected and labeled with all treatments to determine disease severity. A previously published scoring scale, which is divided into six grades as follows, was used for estimating LWD severity (El-Naggarr et al., 2015):
0: No symptoms on stalk and leaves.
1: Dark green longitudinal streaks appear with healthy-appearing leaves on the first basal internode.
2: Shrinking appears on the first internode; dark green longitudinal streaks extend to the second internode, and a slight yellowing occurs on the lower leaves.
3: Shrinking extends to the second and third internodes while a few lower leaves appear slightly dry.
4: Shrinking overcomes most internodes, twists the first internode, and dries most leaves.
5: All stalk internodes and leaves are dried, and the plant has died.
Mature maize plants were used to determine all growth parameters such as plant height (cm), number of leaves, fresh weight/plant (g), dry weight/plant (g), cone length (cm), cone diameter (cm), cone weight (g), number of rows/cones, number of grains/rows, weight of seeds/cone, weight of cones/15 plant (g) and weight of seeds/15 plant (g).
Eighty-day-old leaves were collected to extract and measure photosynthetic pigments (Nornal, 1982; Zhao et al., 2003). Different photosynthetic pigments (i.e., chlorophyll A, chlorophyll B, and carotenoids) in the leaf tissues were detected by extracting 1 g of leaf sample in 10 mL 80% (v/v) acetone for 2 mins before filtration through Whatman no. 1 filter paper, and the volume was then made up to 100 mL. Next, the optical density (OD) was measured at 660, 644, and 440 nm, and the quantity of each pigment was calculated as mg/L according to the equations:
After 80 days of cultivation, 2.0 g of fresh maize leaves were collected and grinded using a mortar and pestle in washed and dried sand with 4 mL of 0.1 M sodium phosphate buffer (pH 7) under sterilized conditions. The samples were then filtered and centrifuged at 3000 rpm for 20 mins, and the supernatant was taken to measure oxidative enzyme activity. All measurements and assays were performed in triplicate. Polyphenol oxidase (PPO, 1.10.3.1) activity was estimated (Lippolis et al., 2008). In a clean test tube, 0.2 mL of crude enzyme extract was mixed with 1 mL of 0.2 M sodium phosphate buffer (pH 7) and 1 mL of 1 mM catechol. Then, the final volume was adjusted to 6 mL with distilled water. The reaction mixture was incubated at 30°C for 30 min, and the absorbance was detected at 495 nm (Matta and Dimond, 1963). Catalase (CAT, 1.11.1.6) activity was evaluated according to published methods (Shim et al., 2003). In a reaction mixture of 0.5 mL 0.2 M sodium phosphate buffer (pH 7.6) and 0.3 mL of 0.5% (v/v) hydrogen peroxide (H2O2), the enzyme extract (0.4 mL) was added, and the final volume was completed to 3 mL with distilled water. The decrease in H2O2 absorbance at 240 nm was monitored, and CAT activity was calculated as µmol/min/g of fresh weight. Peroxidase (PO, 1.11.1.7) activity was measured by mixing 0.3 mL enzyme extract with a reaction mixture containing 0.5 mL 0.1 M potassium phosphate buffer (pH 7), 0.3 mL 0.05 M pyrogallol, 0.1 mL 1% (v/v) H2O2 and completed to 3 mL with distilled water. H2SO4 (5% (v/v)) was used to terminate the reaction after incubation for 15 min at 25°C. Subsequently, the change in absorbance at 470 nm was monitored, and PO activity was calculated as µmol/min/g of fresh weight (Allam and Hollis, 1972). Next, phenylalanine ammonia-lyase (PAL) activity was measured by combining 3.8 mL of sodium borate buffer (pH 8.8) with 1 mL of 0.33% (w/v) L-phenyl alanine and 0.2 mL of crude enzyme. After incubation at 40°C for 15 mins, the mixture was left to cool at room temperature, and the absorbance was measured at 290 nm (Cheng and Breen, 1991). Chitinase (Chit, 3.2.1.14) activity was measured as previously described (Mauch et al., 1988) by measuring absorbance at 540 nm and reported as mM N-acetyl glucose amine released/g of fresh weight/60 mins.
Comparative anatomical characteristics of the maize roots between treated and non-treated plants (control) were examined approximately 150 days after sowing (Johanson, 1940). Briefly, root cross-sections were taken from the primary roots and examined for histological changes. Maize primary roots were collected from each plant during the flowering stage and fixed in FAA (5 mL formalin, 5 mL glacial acetic acid, and 90 mL 70% (v/v) ethyl alcohol). Next, the samples were prepared for analysis with some modifications according to (Sass, 1951). Briefly, the samples were washed in 50% (v/v) ethyl alcohol, dehydrated in serial dilutions of ethyl alcohol (70, 90, 95, and 100% (v/v)), incubated in xylene, embedded in paraffin wax with a melting point of 60°C–63°C, sectioned to 12 μm sections, double-stained with fast green and safranin, cleared in xylene, and finally, mounted in Canada balsam. Next, root cross-sections were examined for histological changes. The prepared sections were examined, counted, and measured under a light microscope with an optical camera (magnification power is 100×).
All experiments were conducted using factorial and a completely randomized design with three replicates for each experiment, considering the corn variety (S&R) and the different treatments as factors A and B. A two-way analysis of variance (ANOVA) was performed for all parameters using GraphPad Prism Software version 9.3.1 (GraphPad Software, USA) and SPSS v.28. All error bars shown represent the range of data points. Means were compared using Duncan multiple range test DMRT at a 95% significance level (p ≤ 0.05) (Duncan, 1955; Gomez, 1984).
The fungus, Trichoderma viride is one such biocontrol agent, mainly used for the control of various fungal pathogens. T. viride appears to be a bit granular on PDA, with green conidia distributed throughout. The cultures are typically fast growing at 28–30°C on PDA media (Figures 1A, B). A molecular phylogenetic tree based on rDNA internal transcribed spacers (ITS) identifies T. viride T27 (MH908510) by the maximum Likelihood Model of MEGA11.0 (Figure 1C). T. viride T27 (MH908510) was tested using a dual culture assay for its antagonistic activity against H. maydis in vitro (Figure 2A). The results of this assay indicated that T. viride grew faster than H. maydis and occupied the whole plate. (Figure 2A) showed the overgrowth of T27 upon the mycelia of H. maydis, where the inhibition percentage (IP) was 68.33%. Using microscopy, we also assessed the interaction area between two fungi for mycoparasitism activity. Results showed that T. viride approached H. maydis, began attaching to it, and ultimately penetrated its cell wall (Figure 2B). Subsequently, T. viride formed structures such as haustoria and appressorium that absorb nutrients from cells of the pathogenic fungus, which finally led to cell denaturation and lysis. Specifically, cell wall degrading enzymes accumulate inside the pathogenic fungal cell and cause progressive degradation of its cell wall until the fungal cell walls are entirely decomposed (Figures 2C-G). This may be due to the action of lytic enzymes such as chitinase and other secreted secondary metabolites by T27(Figure 3A).
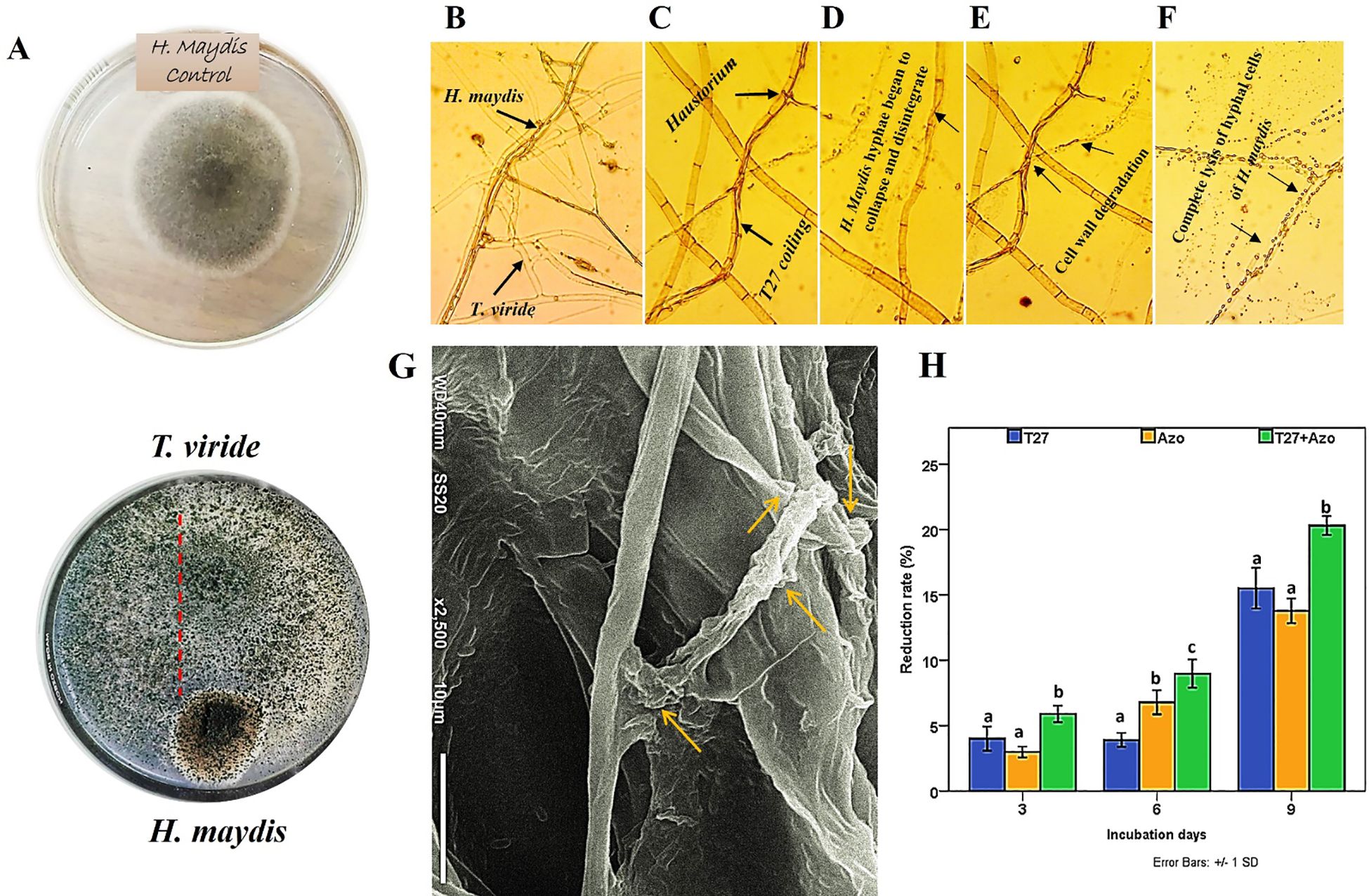
Figure 2. Dual culture assay and light microscopy show the interactions between the mycoparasite T. viride (T27) and the fungal plant pathogen H. maydis. (A) The left panel shows the growth of the pathogen and antagonist co-cultured in Petri dishes on a PDA medium. (B, C) The middle panel shows microscopic observations of the contact zones of the pathogen and antagonist through an initial interaction of both fungal hyphae between T. viride (T27) and H. maydis, where the former penetrates mycelial cells of the latter by haustoria and coiling. (D-F) The black arrow points to the degradation of hyphal cell walls of H. maydis due to the secretion of degrading enzymes by T. viride (T27) following the complete lysis of hyphal cells of H. maydis due to mycoparasitic attack; Magnification (800×). (G) Scanning electron microscopy (SEM) shows the hyphal interactions between T. viride and H. maydis via penetrating and coiling structures of T. viride hyphae around H. maydis hyphae (yellow arrows). The SEM picture was taken in the region where both fungi have contact, Scale bar-10 µm. (H) The effect of culture filtrates of T. viride (Blue) and A. brasilense (Orange), either separately or in combination (Green), on the growth rate of H. maydis at (3,6 and 9) incubation days. The letters above the histograms represent unique statistical groups based on ordinary one-way ANOVA (P value<0.05).
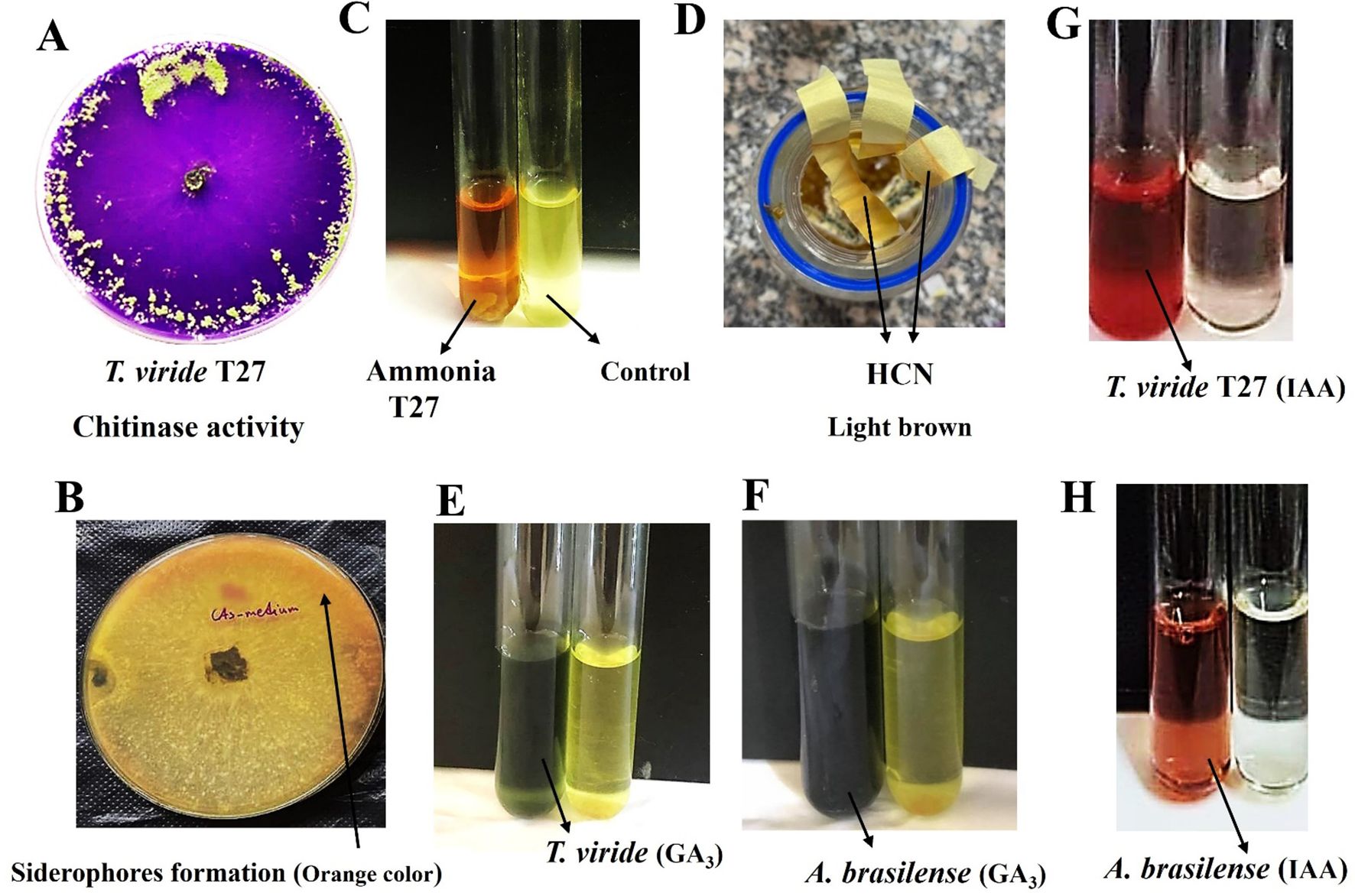
Figure 3. (A) Positive chitinase activity of T. viride (T27) on Lukewarm agar medium and bromocresol purple at 28°C ± 2°C for seven days. (B) Siderophores formation in T. viride was observed as a yellow-orange halo around the fungal inoculum using CAS medium (Chrome Azurol Sulfonate). (C) Yellow color (left) due to ammonia production by T. viride (T27) compared with the control (right). (D) A light brown color on filter paper strips indicates Hydrogen Cyanide (HCN) formation by T. viride (T27) using King’s B broth medium amended with glycine to detect HCN. (E, F) Green color (left tube) indicative of GA3 formation by T. viride (T27; E panel) and A brasilense (F panel). (G, H) IAA production (Salkowski’s reagent) of T. viride (T27; G panel) and A brasilense (H panel), both are left tubes.
The inhibitory nature of secreted T. viride metabolites was tested, and cell-free filtrate of 7-day-old T. viride was examined to verify its activity against the innate growth of the phytopathogenic fungus, H. maydis, during three incubation periods (3, 6, and 9 days). Furthermore, the combination of T. viride and A. brasilense cell filtrates on H. maydis growth was also compared. Here, we found that the cell-free filtrate of T. viride T27 was more effective than A. brasilense in suppressing H. maydis growth during all incubation periods, with the exception of day 6. The reduction rate gradually increased from three days until it reached its maximum after nine days of incubation. In addition, the combination of the cell-free filtrates of A. brasilense and T. viride significantly suppressed the growth of H. maydis compared to the separate mono-treatments (Figure 2H).
The production of secondary antifungal metabolites was evaluated in a single culture of T. viride and its co-culture with H. maydis using GC/MS. The similarity of the metabolic profiles in both single and mixed cultures is shown in (Table 1). Results indicated that 17 bioactive compounds were detected in the single culture of T27. Conversely, the interaction of H. maydis and T. viride stimulates the production of 10 new bioactive compounds compared to the individual culture of T27. These include cis-1,4-Cyclohexanediamine, N-methyl; 10-Undecen-1-al,2-methyl-; 7-Hexadecenal, (Z)-; 9,12-Octadecadienoyl chloride, (Z, Z)-; 2,5-Octadecadiynoic acid, methyl ester; Cyclobarbital (1); Cyclobarbital (5); Cyclobarbital (6), and Cyclobarbital (7). We also found that five compounds were inhibited in the mixed culture compared to the single culture, namely 1,2-15,16-Diepoxyhexadecane; Cyclobarbital (2); Cyclobarbital (3); Cyclobarbital (4), and Cyclobarbital (8). Otherwise, 12 compounds were detected in various proportions in single and mixed cultures. The analyzed metabolites included organic acids, aromatics, fatty acids, alcohols, esters, and hydrocarbons.
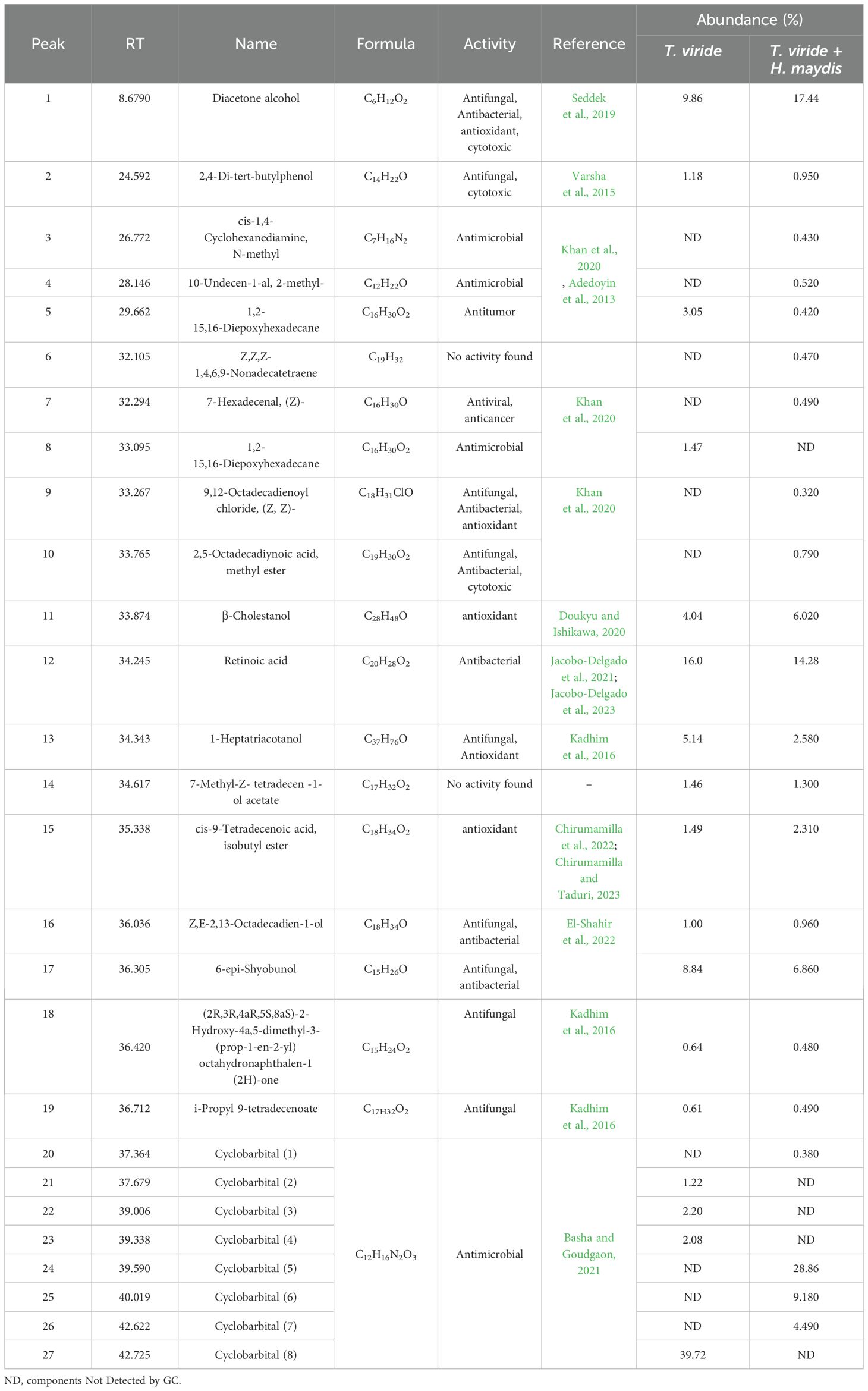
Table 1. GC-mass analysis of the secreted secondary metabolites by an individual culture of T. viride (T27) and mixed culture of T. viride (T27) and H. maydis.
Various mechanisms were studied to understand the biological antifungal activity of T. viride strain T27 against H. maydis by producing certain volatile compounds such as hydrogen cyanide, ammonia, and siderophores. A color change to orange at the bottom of the T27 culture plates on CAS agar medium was considered an indicator of siderophores formation, where the concentration was 0.765% (Supplementary Table S1, Figure 3B). Moreover, Ammonia was detected using a colorimetric assay (i.e., color change to yellow) and was quantified at 3.12 mg/L (Supplementary Table S1, Figure 3C). Further, a color change to light brown in filter paper strips indicated moderate (++) HCN production, which was then quantified by measuring the OD (0.208; Supplementary Table S1, Figure 3D).
Besides the production of the bioactive compounds, three cell wall degrading enzymes (cellulase, chitinase, and amylase) were considered in this study. Qualitative analysis of chitinase activity by T. viride strain T27 was observed by conversion of Lukewarm agar medium to a violet color (Figure 3A). We observed superior production of chitinase rather than cellulase and amylase in T27 (2.206, 0.121, and 0.493 mg/mL, respectively; Supplementary Table S1). Conversely, the plant growth promotion activity of T27 was compared to that of A. brasilense through their ability to produce the plant growth hormones IAA and GA3. Here, A. brasilense produced considerable amounts of GA3 compared to T27, where concentrations were 46.9 and 30.8 mg/L, respectively (Figures 3E, F; Supplementary Table S1). In contrast, we found that T27 produced IAA in higher quantities than A. brasilense, where concentrations were 33.2 and 15.3 mg/L, respectively (Figures 3G, H; Supplementary Table S1). Taken together, we confirmed that T. viride produces all estimated antifungal compounds besides two growth promotors.
Both microbial application and LWD progression were evaluated via a field trial, which designed to assess the efficacy of the biocontrol agent, T. viride, and the bacterial strain, A. brasilense, in controlling the maize LWD. Generally, no disease symptoms were recorded in uninfected plants (T1, T3, T4).
However, the infection of maize plants with H. maydis resulted in disease symptoms at varying severities with or without any treatment. In contrast, uninfected control plants (+ve; T1) appeared healthy without any disease symptoms (Figure 4A), compared with the infected control plants (–ve; T2), which showed rapid wilting of the near ground leaves and gradually lost their color and appearance of yellow streaks on the leaves (Figure 4B) and the lower internodes (Figures 4C–E).

Figure 4. (A) Appearance of healthy (+ve) control maize plant and (B) LWD symptoms on the unhealthy (-ve) control maize plant. (C-E) Yellow streaks on diseased maize plant leaves.
Characteristically, the disease symptoms first appeared in the lower part of the plant and then spread to the upper parts over time. Data in (Figures 5A–C) shows two types of disease progression in the form of disease severity (DS) and disease incidence (DI) in the treated hybrid maize plants. Plants treated with Trichoderma T27 and A. brasilense separately in T3 and T4 treatments appeared healthy and vigorous (Figures 5A–C). Additionally, promising results were observed in the T6 treatment, which showed a high suppressing effect on DS and DI, indicating the synergistic activity between Trichoderma T27 and A. brasilense under pathogen stress. Meanwhile, treatment of infected plants with only T27 in T4 treatment showed moderate suppression of DS and DI. DS and DI data were higher in the susceptible hybrid than in the resistant plants under the same treatments. The DS and DI increased throughout the tested time intervals till they reached their maximum after 120 days of infection in the susceptible hybrid (Figures 5A–F).
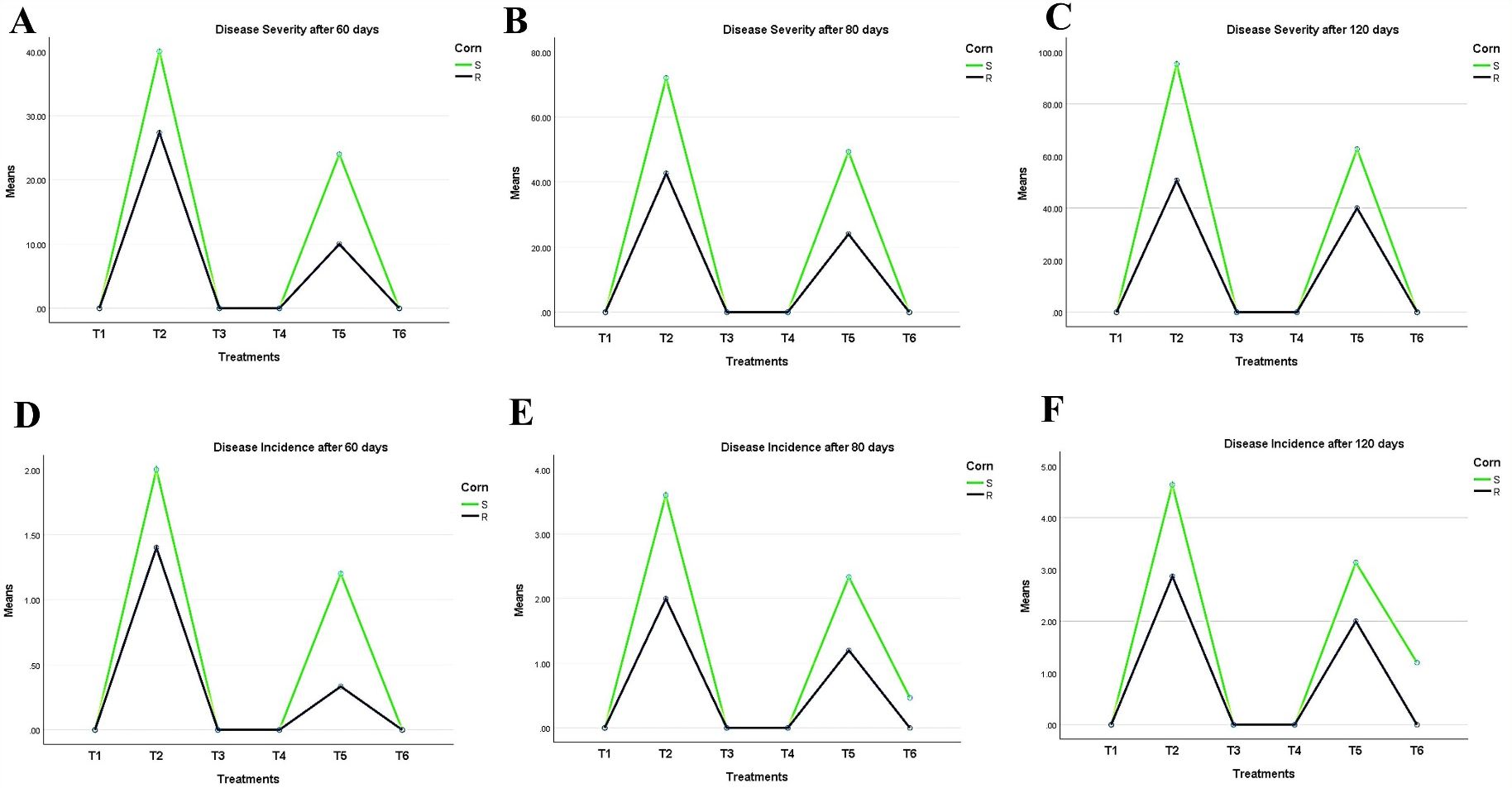
Figure 5. Periodic observation of LWD progression on maize hybrids after 60, 80, and 120 days of sowing shows (A–C) disease severity and (D–F) incidence. T1: Plant control (no microbes), T2: Fungal plant pathogen H. maydis, T3: T. viride, T4: T. viride + A. brasilense, T5: T. viride + H. maydis, T6: T. viride + A. brasilense + H. maydis.
In conclusion, during the experimental trial, no symptoms were recorded in plants infected with Azospirillum and Trichoderma. Results in (Figure 6) were obtained from the two-way ANOVA analysis for all maize plants, which assessed parameters following inoculation with H. maydis, treatment with T. viride and/or A. brasilense, and their combined interactions. These data indicated that H. maydis significantly influences the measured parameters of both hybrids of maize plants (Figure 6). In addition, the weight of seeds per plant decreased by 42.8% and 32.5% for susceptible and resistant cultivars, respectively. Conversely, grain yield/plant in the treatments (T5 & T6) for the susceptible hybrid increased by 44.3% and 67%, respectively, compared to the control plants in the T2 treatment. In addition, the increment in grain yield/plant for the tolerant cultivar increased by 16.9% and 55.9% for the same treatments (Figure 6D). Moreover, this affects the cone criteria (i.e., length, weight, diameter, number of rows/cones, and number of grains/rows), of which the lowest values were recorded in the combined treatments (Figure 6E, Figure 7). Notably, vegetative and cone parameters were highest in T4 plants (i.e., plants treated with T27 and A. brasilense without H. maydis).
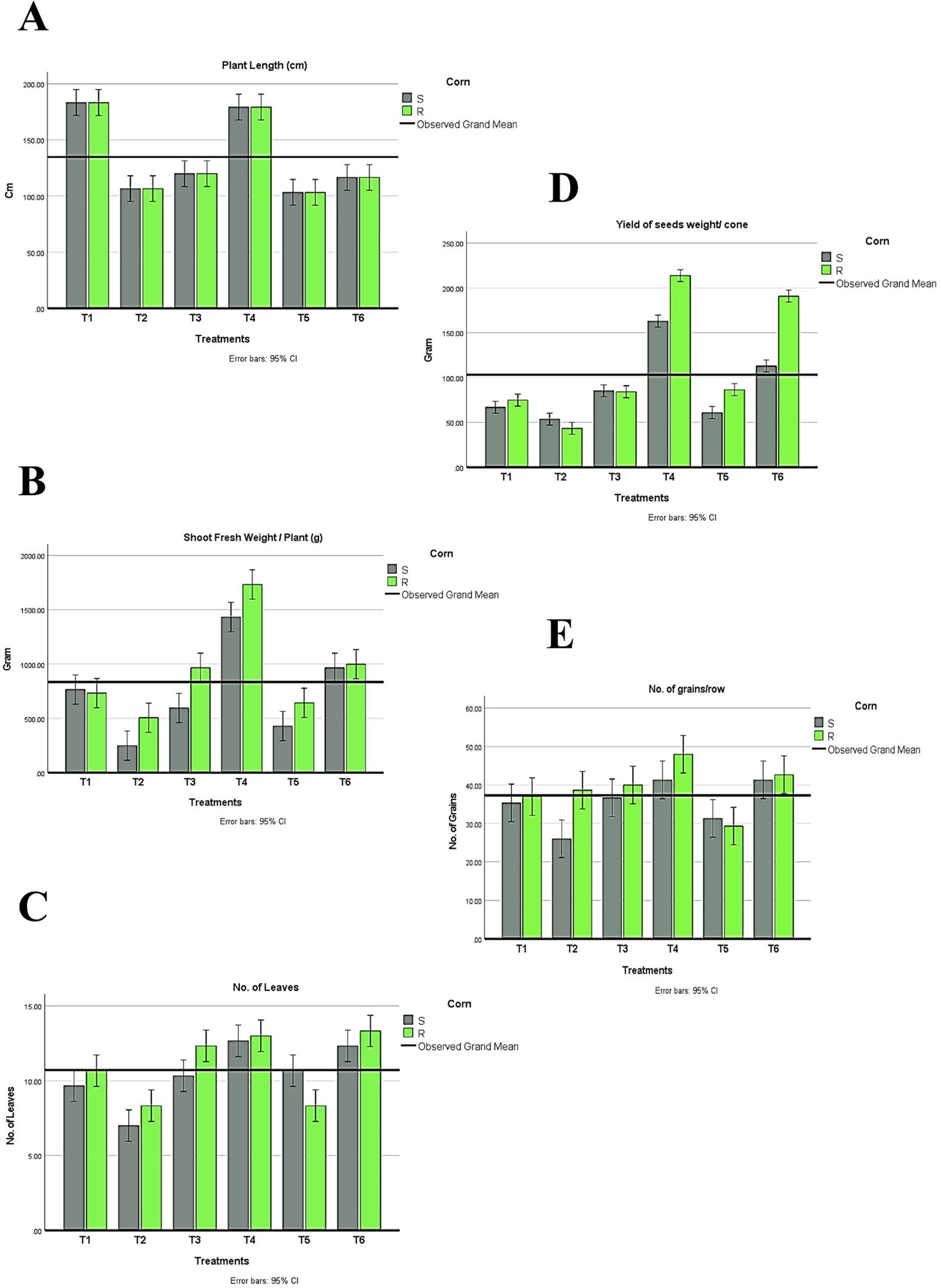
Figure 6. Changes in plant development under different treatments (T1-T6) of both maize hybrids showing (A) Plant length, (B) Shoot fresh weight, (C) Number of leaves, and (D) Yield of seed weight/cone and (E) Number of grains/rows. S: susceptible. R: resistant. T1: Plant control (no microbes), T2: Fungal plant pathogen H. maydis, T3: T. viride, T4: T. viride + A. brasilense, T5: T. viride + H. maydis, T6: T. viride + A. brasilense + H. maydis. The letters above the histograms represent unique statistical groups based on ordinary one-way ANOVA (P value<0.05).
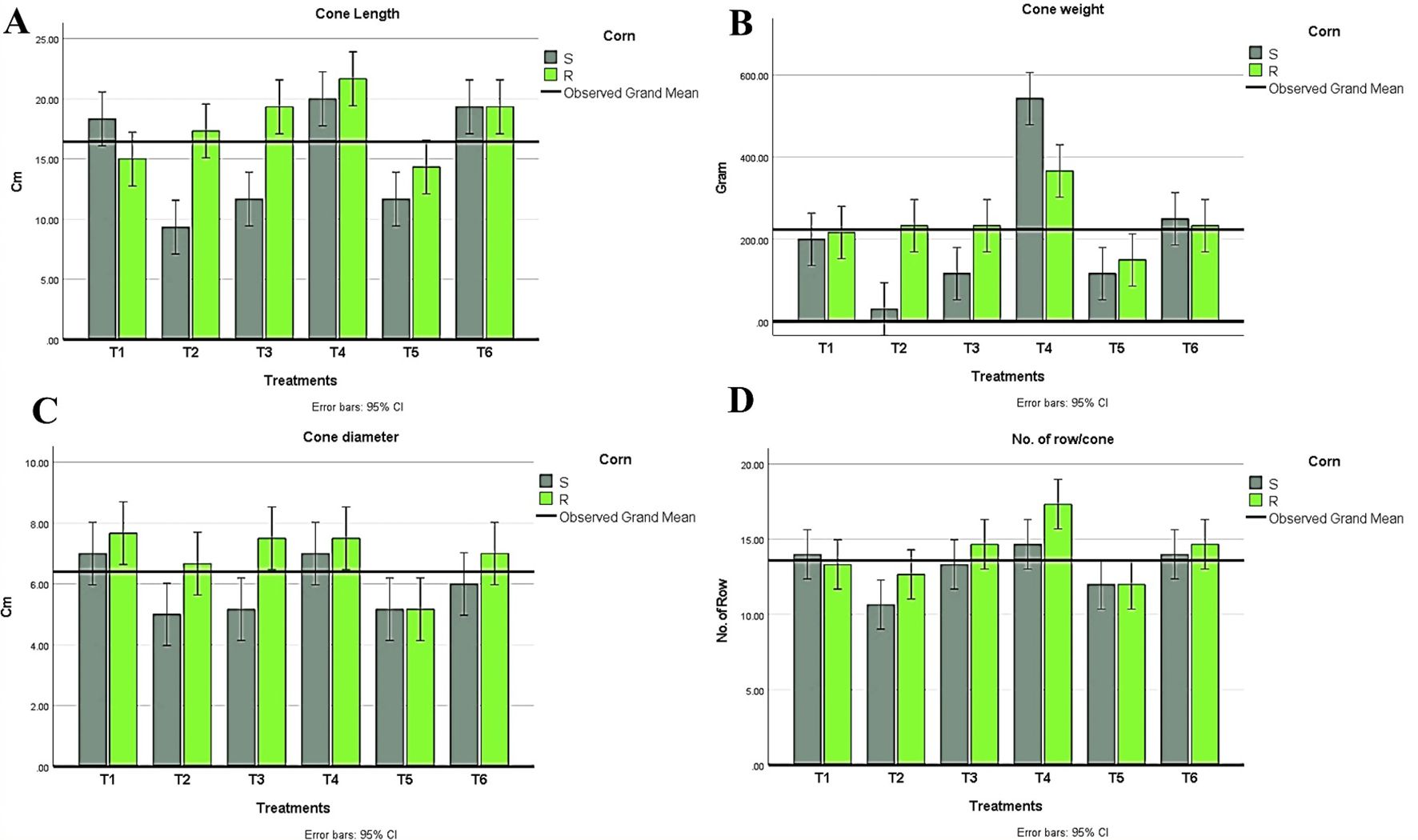
Figure 7. Measurements of cone parameters in all treatment conditions in both maize hybrids showing (A) Cone length, (B) Cone weight, (C) Cone diameter, (D) Number of rows/cones. T1: Plant control (no microbes), T2: Fungal plant pathogen H maydis, T3: T. viride, T4: T. viride + A brasilense, T5: T. viride + H maydis, T6: T. viride + A brasilense + H maydis. The letters above the histograms represent unique statistical groups based on ordinary one-way ANOVA (P value<0.05).
In conclusion, inoculated maize plants with Trichoderma alone reduced both DI and DS compared to plants without inoculation.
Regarding the indices of plant development, plant height, the number of leaves, shoot fresh weight, and shoot dry weight were recorded. Interestingly, all treatments recorded higher values than plants infected with H. maydis only (Figure 6). This trend was confirmed in both susceptible and resistant hybrids. As a result of maize plant infestation with H. maydis, all cone traits (length, weight, diameter, number of rows/cones, number of grains/row, and seeds weight/cone (g)) were significantly affected and gave lower values in both susceptible and resistant hybrids compared to uninfected plants (Figures 7A–D). In addition, the weight of seeds per plant decreased by 42.8% and 32.5% for susceptible and resistant cultivars, respectively. On the contrary, the inoculation of the LWD infected-susceptible hybrid with T. viride only (T5) or with A. brasilense (T6) caused an increase in weight of seeds per cone compared to the LWD-infected plants (T2) by 44.3% and 67%, respectively. Moreover, the weight of seeds per cone of tolerant cultivar increased by 16.9% and 55.9% in inoculated plants with T. viride only (T5) or with A. brasilense (T6), respectively, compared to plants infected with H. maydis only (T2) (Figure 6D).
A significant difference was observed among hybrids regarding photosynthetic pigments for the foliar pigments index. The photosynthetic pigment content in maize leaves was detected in all treatments, where chlorophyll values were higher in the resistant hybrid, while chlorophyll b and carotenoid contents were higher in the susceptible hybrid (Figures 8A–C). Moreover, a significant decrease in chlorophyll b and carotenoids was recorded in the infected plant with H. maydis (T2). This trend was the opposite for chlorophyll a, which was higher in infected fungal plants compared to the control. The chlorophyll content was recorded at higher values in infected control plants than in uninfected control plants. However, we observed a significant increase in chlorophyll b and carotenoid content in T4 plants for both hybrids. These data suggest that the inoculation of maize with a mixture of A. brasilense and T. viride in the absence of H. maydis caused a significant increase in chlorophyll b and carotenoid content compared to control plants in both hybrids (Figure 8D). Additionally, results showed that carotenoids increased in maize leaves inoculated with Trichoderma and Azospirillum in the presence or absence of H. maydis but decreased in maize plants infected with H. maydis only (Figure 8C).
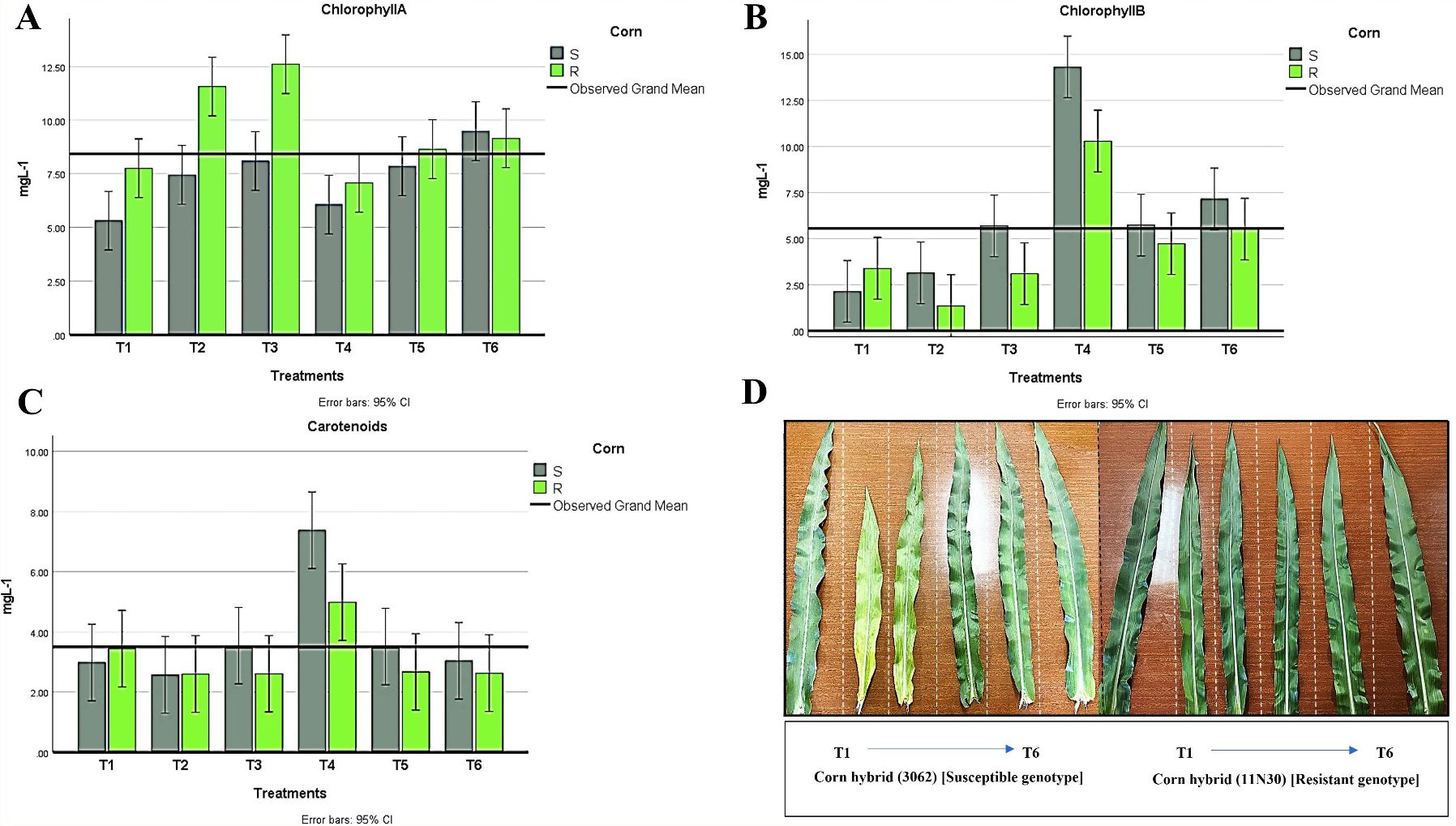
Figure 8. Changes in photosynthetic pigments in all treatment conditions for both maize hybrids showing (A) chlorophyll A, (B) chlorophyll B, and (C) Carotenoids. (D) Leaves have different color appearances in various susceptible and resistant hybrid treatments. T1: Plant control (no microbes), T2: Fungal plant pathogen H maydis, T3: T. viride, T4: T. viride + A brasilense, T5: T. viride + H maydis, T6: T. viride + A brasilense + H maydis. The letters above the histograms represent unique statistical groups based on ordinary one-way ANOVA (P value<0.05).
Regarding oxidative enzymes, we showed that chitinase and CAT activities were significantly higher in control plants infected with H. maydis (Supplementary Table S2). However, no significant differences were observed in PO activity among various treatments. PPO activity was higher in T4 and T6 plants than in T5 plants. These results showed the impact of Azospirillum treatment on pathogen suppression compared with the Trichoderma alone and control with or without the pathogen. Furthermore, PPO and POD activity was higher in resistant hybrids than in susceptible plants.
Infection of maize roots with H. mayids decreased whole root thickness by 40.9% and 34.5% compared to control plants in susceptible and resistant hybrids, respectively. Notably, the combination of T. viride and A. brasilense enhanced the root thickness in the presence or absence of H. mayidism. We also noted that the diameter of the phloem and pith and the endodermis thickness was thinner in H. mayids-infected roots than in other treatments, and the same trend was observed in both hybrids. Moreover, we also found that susceptible hybrids increased the number and thickness of their xylem vessels compared to resistant hybrids. The highest degradation area of the root cortex was observed in H. maydis-infected plants in both hybrids. We also noted changes in the cortex area with plant response to fungal infection. Lateral root (LR) development was also observed in plants infected with T. viride only or H. maydis in a suspectable hybrid. Conversely, the recorded number and thickness of aerenchyma (i.e., air pockets forming in the cortex cell layers that may aid in gas exchange) was lower in H. maydis-infected plants (Supplementary Table S3; Figure 9). Taken together, we consider T. viride to be a potentially promising approach as a safe and effective natural fungicide compared to other chemical fungicides due to its potent suppression of LWD in maize.
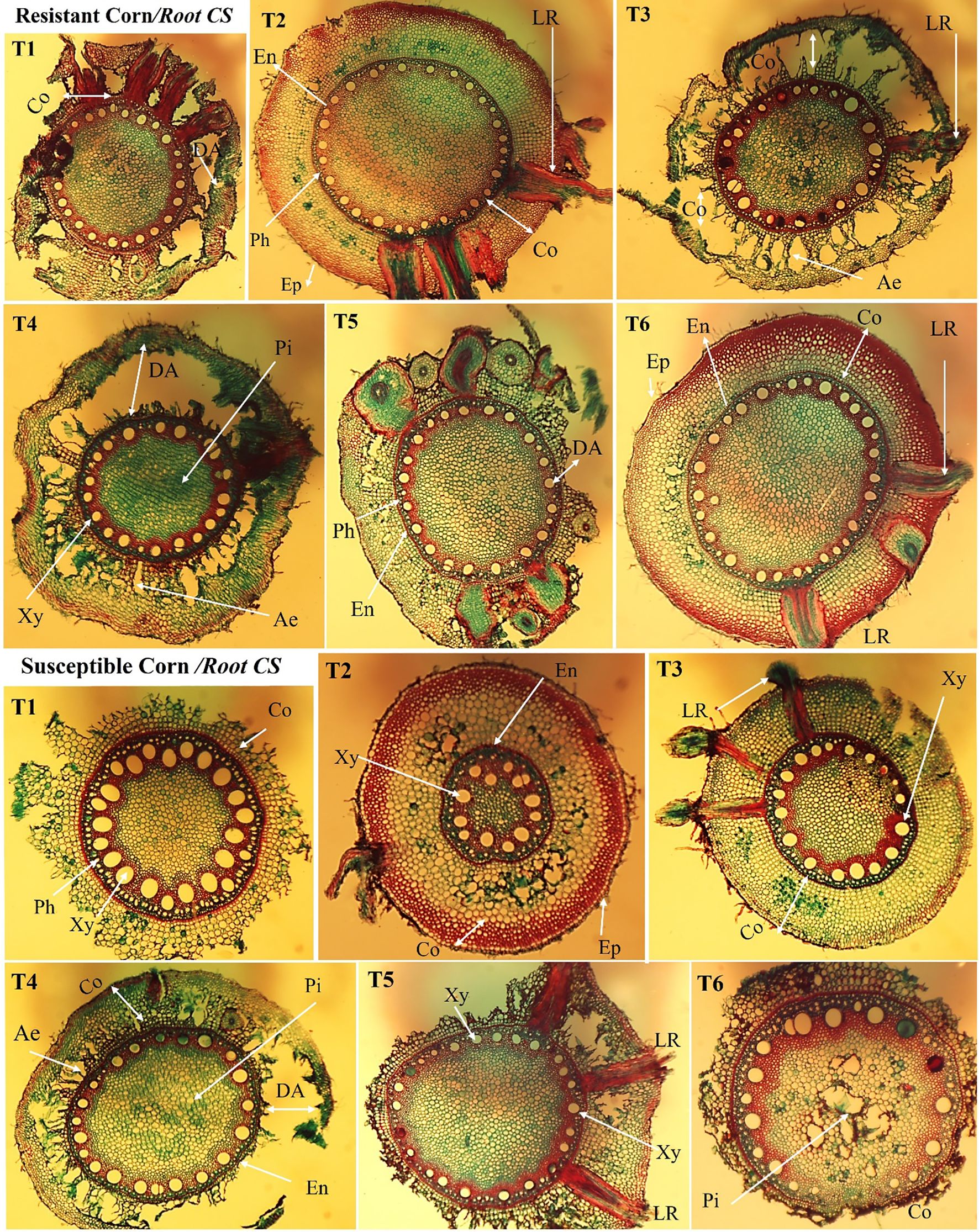
Figure 9. Anatomical study of the cross-section of cultivated maize root under different treatments (T1–T6). The root cross-section of an inoculated plant between two maize hybrids shows different root measurements in the maize epidermis, endodermis, phloem, and xylem for the resistant and susceptible hybrids. LR: lateral roots, Ep: epidermis, Co: cortex, DA: degradation area, En: endodermis, Xy: xylem, Ae: aerenchyma, Pi: pith, and Ph: phloem; Magnification (100×). T1: Plant control (no microbes), T2: Fungal plant pathogen H. maydis, T3: T. viride, T4: T. viride + A. brasilense, T5: T. viride + H. maydis, T6: T. viride + A. brasilense + H. maydis.
Trichoderma viride MH908510 (T27) had significant antagonistic activity against H. maydis in both solid and liquid media. T. viride is a fast-growing and highly sporulating strain that overgrew and covered the entire surface of the medium plate during the first three days of incubation, thereby limiting the growth of H. maydis (Hariharan et al., 2022; Arif et al., 2023). Furthermore, mycoparasitism is another behavior of numerous Trichoderma strains that enables them to attack plant pathogens (Druzhinina et al., 2011; Hewedy et al., 2020a; Woo et al., 2023; Kumari et al., 2024). We confirmed that the antifungal activity of T27 against H. maydis by the production of volatile compounds (NH3 and HCN) as well as siderophores (Figures 3B–D) (Saadaoui et al., 2023). Alternatively, HCN might inhibit H. maydis growth by inhibiting cytochrome C oxidase and blocking the respiratory electron transport chain (Walia et al., 2021; Lahlali et al., 2022; Bhadrecha et al., 2023). Several studies have reported that NH3 might have had a fungistatic role in inhibiting conidial germination and inducing endoplasmic reticulum stress, which might suppress protein synthesis (Avalos et al., 2020; Liu et al., 2021). Likewise, NH3 could induce oxidative stress and/or cell apoptosis in pathogenic fungi tissues (Missall et al., 2004; Görg et al., 2019; Liu et al., 2021). Moreover, HCN can be converted into NH4 by the cyanide dioxygenase system (Luque-Almagro et al., 2018), which promotes plant growth. Thus, biocontrol agents can inhibit the growth of pathogenic fungi through their ability to form biofilms and reduce pathogenic root colonization, as well as siderophore production (Howell et al., 1988; Singh et al., 2019). A. brasilense (diazotrophic bacteria) is widely applied in the cultivation of cereal crops, especially maize, wheat, and rice, as a nitrogen fixer, rock minerals solubilizer, and plant growth promoter, and it also has a significant role as an antimicrobial agent (Bashan and De-Bashan, 2010; Galindo et al., 2022). Moreover, T27 stimulated plant growth by producing GA3 and IAA, which are implicated in phytonutrient availability. Thus, the combination of T27 and A. brasilense supplies various pathways for phytonutrient availability and enhancement of the plant defense system toward H. mayids (Olanrewaju et al., 2017; Cui et al., 2024). The GC/MS data (Table 1) showed that the T. viride culture had more diacetone alcohols in the co-culture than in the T. viride culture alone under biotic stress. This confirms that the T. viride filtrate was a good suppressor for H. mayids. These results were aligned with previous reports indicating that ketones and alcohol are cytotoxic, in addition to their inhibitory effect on pathogenic fungi by delaying their conidial growth (Vinale et al., 2008; Seddek et al., 2019). Cis-1,4-Cyclohexanediamine, N-methyl; 10-Undecen-1-al, 2-methyl-; 1,2-15,16-Diepoxyhexadecane and 7-Hexadecenal, (Z)- were identified as significant components in the co-culture, which suggests that T. viride was able to produce these compounds in the presence of H. mayids, concluding that these compounds have antimicrobial activity (Kadhim et al., 2016; Li et al., 2022). Additionally, others have also detected isopropyl e-9-tetradecenoate among 31 bioactive compounds found in Candid albicans culture (Brighenti et al., 2017), which we also detected in both cultures in this work. Furthermore, 6-epi-shyobunol and cis-13-Octadecenoic acid have previously been reported as antifungal compounds (El-Shahir et al., 2022). LWD is typified by the rapid wilting of maize leaves, which firstly become faint green before they entirely lose color and dry with inward rolling from the edges. Lastly, the whole plant becomes dry with yellow-brown discoloration of the vascular bundles, followed by the appearance of red-brown stripes advancing up to the fifth internode or further up (Degani and Cernica, 2014; Ortiz-Bustos et al., 2016; Degani and Dor, 2021). Therefore, a field experiment was designed to develop an alternative biological control strategy for LWD in maize. T. viride was used as a biocontrol agent, and A. brasilense was used as a plant growth promoter to minimize both disease severity and incidence. In addition, a highly significant suppression was observed when maize plants were inoculated with T. viride combined with A. brasilense. Similarly, previous studies have also reported the potential of this combination as a biocontrol agent (Karthika and Vanangamudi, 2013; Khalil and Shimaa, 2020). These microbes protect maize plants against LWD via hydrolytic enzymes that inhibit the growth of the pathogenic fungi, as well as their ability to colonize plant roots (Degani, 2021; Degani and Dor, 2021). By tracking the effects of H. maydis on physiological changes in maize plants, we found that the production of photosynthetic pigments was a remarkable indicator of the plant’s response to biotic stress (Marín-Ortiz et al., 2020; Jha and Mohamed, 2023). Due to this fungal infection, the noticeable decrease in photosynthetic pigments in H. maydis-infected maize disrupts the enzymes responsible for pigment production, thus reducing their production rate and increasing their degradation rate (Horst et al., 2008; Matei et al., 2018). In both maize hybrids, the formed cone parameters (length, weight, diameter, the number of rows/cones, the number of grains/row) were significantly decreased as a result of plant infection with H. maydis, and these findings were consistent with published work (Degani and Cernica, 2014; Ortiz-Bustos et al., 2016). In addition, we also found that the fungal infection negatively affected the photosynthetic pigments as well as caused a decrease in the efficiency of the photosynthesis process due to the change in leaf anatomy under stress conditions such as leaf pruning, chlorosis and reduction of leaf area (Drori et al., 2013; Sunitha et al., 2020). Indeed, auxin and cytokinin enhanced the function of the root system, which increased water and nutrient availability to other parts of the plant, especially the leaves, thus enhancing photosynthetic pigment production (Werner et al., 2010; Bielach et al., 2017). Furthermore, the combined treatment of maize plants with T27 and A. brasilense modified the plant cell structure and physio-biochemical reactions, resulting in the synthesis of proteins and enzymes associated with different pigment stabilities and protection of carotenoids from oxidation (Pal et al., 2021). Since the bacterium Azospirillum fixes atmospheric nitrogen in the form of ammonia, which is considered a precursor of glutamate formation, it is consequently used in the synthesis of chlorophyll in most plants (Costa et al., 2015; Fukami et al., 2018). Interestingly, our results showed a significant difference between maize hybrids regarding photosynthetic pigments, possibly due to genetic variability for disease susceptibility (Campos et al., 2021). Previous studies have shown the production of oxidative enzymes in stressed plants. PO and CAT were formed to remove the accumulated reactive oxygen species (ROS), such as H2O2, produced due to pathogen invasion (Feng et al., 2022) (Supplementary Table S2). Additionally, PO is required for synthesizing phenolic compounds, re-building the plant cell wall at infection sites, and synthesizing ethylene (Magbanua et al., 2007; Terna et al., 2022). In addition, our results were agreed by (Pereyra et al., 2010), who reported that the oxidative enzymes increased in Azospirillum-treated plants because this bacterium activated the plant antioxidant system and increased the activity of antioxidant enzymes, which scavenged or reduced ROS in the maize plants and provided better growth conditions. Considering our results, PAL has a regulatory role in defense mechanisms against fungal pathogen attacks because it plays an essential role in the biosynthesis of phenolics. Accordingly, the high PAL activity is associated with the accumulation of phenolic compounds in plant tissues (Aoun, 2017; Jiang et al., 2019). Furthermore, Trichoderma can alter several physiological operations, including transpiration, stomatal conductance, water use efficiency, nutrient uptake, and balancing the phytohormones changes, as well as improving their capacity to suppress fungal diseases (Swain and Mukherjee, 2020; Yu et al., 2021). Maize plants secret massive amounts of secondary metabolites as root exudates that protect plants from pathogens, and some act as botanical fungicides (Elshahawy and Khattab, 2022). These metabolites damage the fungal cell walls and membranes, inhibiting spore germination, mycelial development, germ tube elongation, sporulation, and synthesizing enzymes, DNA, and proteins (Yoon et al., 2013). Accordingly, the development of cone traits in both hybrids (length, weight, diameter, the number of rows/cones, the number of grains/row) was significantly decreased as a result of plant infection with H. maydis, and this was consistent (Sunitha et al., 2020). In contrast, the cone traits were enhanced in maize plants inoculated with T. viride and A. brasilense, compared to previous reports for the inoculation of maize plants with Trichoderma and A. brasilense (Akladious and Abbas, 2012; Galindo et al., 2022), which enhanced cereal growth parameters like root and shoot dry weight, root and shoot fresh weight, leaf number, plant height, root, and shoot length. Following the histological characterization of the maize roots, a reduction in root tissue diameters was observed in infected plants as a defense mechanism to slow the spread of pathogens to other plant parts. In contrast, the number, diameter, and thickness of xylem vessels were higher in H. maids-infected plants than in different treatments (Figure 9). Plants with wider xylem vessels were more susceptible to diseases than those with narrower xylem diameters (Pouzoulet et al., 2017). Pathogenic fungi degradation of the root cortex could result from the activity of the synthesized cell wall degrading enzymes to promote tissue invasion and colonization (Terna et al., 2022). The interaction between associated microbes with roots, whether beneficial or pathogenic, and their phenotypes is vital to their ability to avoid diseases (Lynch, 2019). H. maydis infected maize plants led to an increase in whole root thickness, cortex area degradation, and pith thickness for resistant maize hybrids in comparison to plants inoculated with A. brasilense and/or T. viride, which decreased cortex thickness due to their beneficial effects on inhibiting the pathogenic fungus and restoring the healing of the roots (Figure 9). Furthermore, the inoculation of maize plants with T. viride and A. brasilense reduced the cortex area, enhancing the root’s ability to reduce respiration and promote deeper roots, enhancing plant growth and grain yield (Chimungu et al., 2015; Chaudhary et al., 2022). Therefore, T. viride strain T27 is vital for genetically protecting cereal crops against several pathogenic fungi. However, further experiments are needed to determine the effectiveness of these endophytic microbes under different field conditions. Ultimately, T. viride and A. brasilense are multifunctional allies for maize growth under biotic stress.
Indeed, host-pathogen interaction is a highly dynamic process between phytopathogenic microbes and their host plants. Thus, in this study, we sought to improve and establish an environmentally friendly and consistent method to control the development of phytopathogenic disease. The combination of T. viride and A. brasilense in protecting maize crops infected with H. maydis, was studied in vitro and the field. T. viride strain T27 significantly displayed antagonistic activities against H. maydis using different mechanisms. Notably, lytic enzymes produced by T. viride T27 and the bioactive secondary metabolitesplayed a vital role in suppressing the pathogen,. Maize plants treated with T27 and A. brasilense alone or in combination showed remarkable potential for suppressing LWD and promoting plant growth. These findings suggest that corn seed coating with beneficial fungi and/or bacterial endophytes supports mutualistic colonization. Additionally, de novo transcriptome assembly, functional annotation, and expression profiling of the root system inoculated with T. viride are necessary.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
RE: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft. OAH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MA: Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Software, Validation, Writing – review & editing. KA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Resources, Software, Writing – review & editing. EH: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft. SE-D: Data curation, Formal analysis, Funding acquisition, Investigation, Software, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1486607/full#supplementary-material
Abdelaziz, A. M., Sharaf, M. H., Hashem, A. H., Al-Askar, A. A., Marey, S. A., Mohamed, F. A., et al. (2023). Biocontrol of Fusarium wilt disease in pepper plant by plant growth promoting Penicillium expansum and Trichoderma harzianum. Notulae Bot. Hort. Agrobot. Cluj-Napoca 51, 1–23. doi: 10.15835/NBHA51313302
Abdenaceur, R., Farida, B. T., Mourad, D., Rima, H., Zahia, O., Fatma, S. H. (2022). Effective biofertilizer Trichoderma spp. isolates with enzymatic activity and metabolites enhancing plant growth. Int. Microbiol. 25, 817–829. doi: 10.1007/s10123-022-00263-8
Abdulkareem, M., Aboud, H. M., Saood, H. M., Shibly, M. K. (2014). Antagonistic activity of some plant growth rhizobacteria to Fusarium graminearum. Int. J. Phytopathol. 3, 49–54. doi: 10.33687/phytopath.003.01.0660
Adedoyin, B. J., Okeniyi, S. O., Garba, S., Salihu, L. (2013). Cytotoxicity, antioxidant and antimicrobial activities of essential oil extracted from Euphorbia heterophylla plant. Topclass J. Herb. Med. 2, 84–89.
Agrawal, T., Kotasthane, A. S. (2012). Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chhattisgarh in Central India. SpringerPlus 1, 1–10. doi: 10.1186/2193-1801-1-73
Akladious, S. A., Abbas, S. M. (2012). Application of Trichoderma harziunum T22 as a biofertilizer supporting maize growth. Afr. J. Biotechnol. 11, 8672–8683. doi: 10.5897/AJB11.4323
Allam, A. I., Hollis, J. P. (1972). Former Graduate Student and Professor of Plant Pathology, respectively, Department of Plant Pathology, Louisiana State University and A&M College, Baton Rouge 70803 (USA). Research supported through National Science Foundation Grant GB-29633X. Phytopathology 62, 634–639. doi: 10.1094/Phyto-62-634
Aoun, M. (2017). Host defense mechanisms during fungal pathogenesis and how these are overcome in susceptible plants: A review. Int. J. Bot. 13, 82–102. doi: 10.3923/ijb.2017.82.102
Arif, S., Munis, M. F. H., Liaquat, F., Gulzar, S., Haroon, U., Zhao, L., et al. (2023). Trichoderma viride establishes biodefense against clubroot (Plasmodiophora brassicae) and fosters plant growth via colonizing root hairs in pak choi (Brassica campestris spp. chinesnsis). Biol. Control 183, 105265. doi: 10.1016/j.biocontrol.2023.105265
Avalos, M., Garbeva, P., Raaijmakers, J. M., van Wezel, G. P. (2020). Production of ammonia as a low-cost and long-distance antibiotic strategy by Streptomyces species. ISME J. 14, 569–583. doi: 10.1038/s41396-019-0537-2
Basha, J., Goudgaon, N. M. (2021). A comprehensive review on pyrimidine analogs-versatile scaffold with medicinal and biological potential. J. Mol. Struct. 1246, 131168. doi: 10.1016/j.molstruc.2021.131168
Bashan, Y., De-Bashan, L. E. (2010). How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. Adv. Agron. 108, 77–136. doi: 10.1016/S0065-2113(10)08002-8
Benjamin, J., Oyedokun, D. O., Oziegbe, E. V., Oni, J., Ogundare, E. B., Ujah, G. O., et al. (2024). Cereal production in Africa: the threat of current plant pathogens in changing climate-a review. Discover Agric. 2, 33. doi: 10.1007/s44279-024-00040-3
Bhadrecha, P., Singh, S., Dwibedi, V. (2023). [amp]]lsquo;A plant’s major strength in rhizosphere’: the plant growth promoting rhizobacteria. Arch. Microbiol. 205, 165. doi: 10.1007/s00203-023-03502-2
Bhardwaj, N. R., Kumar, J. (2017). Characterization of volatile secondary metabolites from Trichoderma asperellum. J. Appl. Natural Sci. 9, 954–959. doi: 10.31018/jans.v9i2.1303
Bhat, K. A. (2017). A new agar plate assisted slide culture technique to study mycoparasitism of Trichoderma sp. on Rhizoctonia solani and Fusarium oxysporium. Int. J. Curr. Microbiol. Appl. Sci. 6, 3176–3180. doi: 10.20546/ijcmas.2017.608.378
Bielach, A., Hrtyan, M., Tognetti, V. B. (2017). Plants under stress: involvement of auxin and cytokinin. Int. J. Mol. Sci. 18, 1427. doi: 10.3390/ijms18071427
Błaszczyk, L., Strakowska, J., Chełkowski, J., Gąbka-Buszek, A., Kaczmarek, J. (2016). Trichoderma species occurring on wood with decay symptoms in mountain forests in Central Europe: genetic and enzymatic characterization. J. Appl. Genet. 57, 397–407. doi: 10.1007/s13353-015-0326-1
Brighenti, F. L., Salvador, M. J., Gontijo, A. V. L., Delbem, A. C. B., Delbem, Á.C.B., Soares, C. P., et al. (2017). Plant extracts: initial screening, identification of bioactive compounds and effect against Candida albicans biofilms. Future Microbiol. 12, 15–27. doi: 10.2217/fmb-2016-0094
Cai, F., Druzhinina, I. S. (2021). In honor of John Bissett: authoritative guidelines on molecular identification of Trichoderma. Fungal Diversity 107, 1–69. doi: 10.1007/s13225-020-00464-4
Campos, L. J. M., de Almeida, R. E. M., da Silva, D. D., Cota, L. V., Naoe, A. M. L., Peluzio, J. M., et al. (2021). Physiological and biophysical alterations in maize plants caused by Colletotrichum graminicola infection verified by OJIP study. Trop. Plant Pathol. 46, 674–683. doi: 10.1007/s40858-021-00465-x
Cappuccino, J. C., Sherman, N. (1992). “Negative staining,” in Microbiology: a laboratory manual. Eds. Cappuccino, J. C., Sherman, N. (Benjamin/Cummings PubCo, Redwood Cit), 125–179.
Cardozo, P., Di Palma, A., Martin, S., Cerliani, C., Esposito, G., Reinoso, H., et al. (2022). Improvement of maize yield by foliar application of azospirillum brasilense az39. J. Plant Growth Regul. 41, 1032–1040. doi: 10.1007/s00344-021-10356-9
Cassán, F., Coniglio, A., López, G., Molina, R., Nievas, S., de Carlan, C. L. N., et al. (2020). Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol. Fertil. Soils 56, 461–479. doi: 10.1007/s00374-020-01463-y
Chaudhary, P., Agri, U., Chaudhary, A., Kumar, A., Kumar, G. (2022). Endophytes and their potential in biotic stress management and crop production. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.933017
Chen, J. L., Sun, S. Z., Miao, C. P., Wu, K., Chen, Y. W., Xu, L. H., et al. (2016). Endophytic Trichoderma gamsii YIM PH30019: a promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng. J. Ginseng Res. 40, 315–324. doi: 10.1016/j.jgr.2015.09.006
Cheng, G. W., Breen, P. J. (1991). Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J. Am. Soc. Horticult. Sci. 116, 865–869. doi: 10.21273/JASHS.116.5.865
Chimungu, J. G., Maliro, M. F., Nalivata, P. C., Kanyama-Phiri, G., Brown, K. M., Lynch, J. P. (2015). Utility of root cortical aerenchyma under water limited conditions in tropical maize (Zea mays L.). Field Crops Res. 171, 86–98. doi: 10.1016/j.fcr.2014.10.009
Chinaru Nwosu, L., Olukayode Adedire, C., Oludele Ogunwolu, E. (2015). Screening for new sources of resistance to Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) infestation in stored maize genotypes. J. Crop Prot. 4, 277–290.
Chirumamilla, P., Taduri, S. (2023). Assessment of in vitro anti-inflammatory, antioxidant and antidiabetic activities of Solanum khasianum Clarke. Vegetos 36, 575–582. doi: 10.1007/s42535-022-00410-6
Chirumamilla, P., Vankudoth, S., Dharavath, S. B., Dasari, R., Taduri, S. (2022). In vitro anti-inflammatory activity of green synthesized silver nanoparticles and leaf methanolic extract of Solanum khasianum ClarkeProc. Natl. Acad. Sci. India Sect. B. Biol. Sci. 10, 1–7. doi: 10.1007/s40011-021-01337-9
Contreras-Cornejo, H. A., Schmoll, M., Esquivel-Ayala, B. A., González-Esquivel, C. E., Rocha-Ramírez, V., Larsen, J. (2024). Mechanisms for plant growth promotion activated by Trichoderma in natural and managed terrestrial ecosystem. Microbiol. Res. 281, 127621. doi: 10.1016/j.micres.2024.127621
Costa, R. R. G. F., Quirino, G. D. S. F., Naves, D. C. D. F., Santos, C. B., Rocha, A. F. D. S. (2015). Efficiency of inoculant with Azospirillum brasilense on the growth and yield of second-harvest maize. Pesquisa Agropecuária Trop. 45, 304–311. doi: 10.1590/1983-40632015v4534593
Crozier, A., Arruda, P., Jasmim, J. M., Monteiro, A. M., Sandberg, G. (1988). Analysis of indole-3-acetic acid and related indoles in culture medium from Azospirillum lipoferum and Azospirillum brasilense. Appl. Environ. Microbiol. 54, 2833–2837. doi: 10.1128/aem.54.11.2833-2837.1988
Cui, Q., Beiyuan, J., Chen, Y., Li, M., Qiu, T., Zhao, S., et al. (2024). Synergistic enhancement of plant growth and cadmium stress defense by Azospirillum brasilense and plant heme: Modulating the growth–defense relationship. Sci. Total Environ. 946, 174503. doi: 10.1016/j.scitotenv.2024.174503
Degani, O. (2021). Control strategies to cope with late wilt of maize. Pathogens 11, 13. doi: 10.3390/pathogens11010013
Degani, O. (2022). Control strategies to cope with late wilt of maize. Pathogens 11 (1), 13. doi: 10.3390/pathogens11010013
Degani, O., Cernica, G. (2014). Diagnosis and control of Harpophora maydis, the cause of late wilt in maize. Adv. Microbiol. 4, 94–105. doi: 10.4236/aim.2014.42014
Degani, O., Dor, S. (2021). Trichoderma biological control to protect sensitive maize hybrids against late wilt disease in the field. J. Fungi 7, 315. doi: 10.3390/jof7040315
Degani, O., Dor, S., Movshowitz, D., Fraidman, E., Rabinovitz, O., Graph, S. (2018). Effective chemical protection against the maize late wilt causal agent, Harpophora maydis, in the field. PloS One 13, e0208353. doi: 10.1371/journal.pone.0208353
Doukyu, N., Ishikawa, M. (2020). Cholesterol oxidase from Rhodococcus erythropolis with high specificity toward β-cholestanol and pytosterols. PloS One 15, e0241126. doi: 10.1371/journal.pone.0241126
Drori, R., Sharon, A., Goldberg, D., Rabinovitz, O., Levy, M., Degani, O. (2013). Molecular diagnosis for Harpophora maydis, the cause of maize late wilt in Israel. Phytopathol. Mediterr. 52 (1), 16–29.
Druzhinina, I. S., Chenthamara, K., Zhang, J., Atanasova, L., Yang, D., Miao, Y., et al. (2018). Massive lateral transfer of genes encoding plant cell wall-degrading enzymes to the mycoparasitic fungus Trichoderma from its plant-associated hosts. PloS Genet. 14, e1007322. doi: 10.1371/journal.pgen.1007322
Druzhinina, I. S., Seidl-Seiboth, V., Herrera-Estrella, A., Horwitz, B. A., Kenerley, C. M., Monte, E., et al. (2011). Trichoderma: the genomics of opportunistic success. Nat. Rev. Microbiol. 9, 749–759. doi: 10.1038/nrmicro2637
Dutta, J., Handique, P. J., Thakur, D. (2015). Assessment of culturable tea rhizobacteria isolated from tea estates of Assam, India for growth promotion in commercial tea cultivars. Front. Microbiol. 6. doi: 10.3389/fmicb.2015.01252
Dutta, P., Mahanta, M., Singh, S. B., Thakuria, D., Deb, L., Kumari, A., et al. (2023). Molecular interaction between plants and Trichoderma species against soil-borne plant pathogens. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1145715
El-Naggarr, A. A. A., Sabryr, A. M., Yassin, M. A. (2015). Impact of late wilt disease caused by Harpophora maydis on maize yield. J. Biol. Chem. Environ. Sci. 10, 577–595.
El-Rahman, A., Shaheen, H. A., El-Aziz, A., Rabab, M., Ibrahim, D. S. (2019). Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egypt. J. Biol. Pest Control 29, 1–11. doi: 10.1186/s41938-019-0143-7
Elshahawy, I. E., El-Sayed, A. E. K. B. (2018). Maximizing the efficacy of Trichoderma to control Cephalosporium maydis, causing maize late wilt disease, using freshwater microalgae extracts. Egypt. J. Biol. Pest Control 28, 1–11. doi: 10.1186/s41938-018-0052-1
Elshahawy, I. E., Khattab, A. E. N. A. (2022). Endophyte Chaetomium globosum improves the growth of maize plants and induces their resistance to late wilt disease. J. Plant Dis. Prot. 129, 1125–1144. doi: 10.1007/s41348-022-00626-3
El-Shahir, A. A., El-Wakil, D. A., Abdel Latef, A. A. H., Youssef, N. H. (2022). Bioactive compounds and antifungal activity of leaves and fruits methanolic extracts of ziziphus spina-christi L. Plants 11, 746. doi: 10.3390/plants11060746
Feng, L., Sun, J., Jiang, Y., Duan, X. (2022). Role of reactive oxygen species against pathogens in relation to postharvest disease of papaya fruit. Horticulturae 8, 205. doi: 10.3390/horticulturae8030205
Fukami, J., Cerezini, P., Hungria, M. (2018). Azospirillum: benefits that go far beyond biological nitrogen fixation. Amb. Express 8, 73. doi: 10.1186/s13568-018-0608-1
Fukami, J., Nogueira, M. A., Araujo, R. S., Hungria, M. (2016). Accessing inoculation methods of maize and wheat with Azospirillum brasilense. AMB express 6, 1–13. doi: 10.1186/s13568-015-0171-y
Galindo, F. S., Filho, M. C. M. T., Buzetti, S., Rodrigues, W. L., Fernandes, G. C., Boleta, E. H. M., et al. (2020). Influence of Azospirillum brasilense associated with silicon and nitrogen fertilization on macronutrient contents in corn. Open Agric. 5, 126–137. doi: 10.1515/opag-2020-0013
Galindo, F. S., Pagliari, P. H., Fernandes, G. C., Rodrigues, W. L., Boleta, E. H. M., Jalal, A., et al. (2022). Improving sustainable field-grown wheat production with Azospirillum brasilense under tropical conditions: a potential tool for improving nitrogen management. Front. Environ. Sci. 10. doi: 10.3389/fenvs.2022.821628
Gams, W. (2000). Phialophora and some similar morphologically little-differentiated anamorphs of divergent ascomycetes. Stud. Mycol. 45, 187–199.
Ghasemi, S., Safaie, N., Shahbazi, S., Shams-Bakhsh, M., Askari, H. (2019). Enhancement of lytic enzymes activity and antagonistic traits of Trichoderma harzianum using γ-radiation induced mutation. J. Agric. Sci. Technol. 21, 1035–1048.
Ghasemi, S., Safaie, N., Shahbazi, S., Shams-Bakhsh, M., Askari, H. (2020). The role of cell wall degrading enzymes in antagonistic traits of Trichoderma virens against Rhizoctonia solani. Iran. J. Biotechnol. 18, e2333. doi: 10.30498/IJB.2020.2333
Ghazy, N., El-Nahrawy, S. (2021). Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Arch. Microbiol. 203, 1195–1209. doi: 10.1007/s00203-020-02113-5
Gomez, K. A. (1984). Statistical procedures for agricultural research (John NewYork: Wiley and Sons).
Görg, B., Karababa, A., Schütz, E., Paluschinski, M., Schrimpf, A., Shafigullina, A., et al. (2019). O-GlcNAcylation-dependent upregulation of HO1 triggers ammonia-induced oxidative stress and senescence in hepatic encephalopathy. J. Hepatol. 71, 930–941. doi: 10.1016/j.jhep.2019.06.020
Gureeva, M. V., Gureev, A. P. (2023). Molecular mechanisms determining the role of bacteria from the genus azospirillum in plant adaptation to damaging environmental factors. Int. J. Mol. Sci. 24, 9122. doi: 10.3390/ijms24119122
Guzmán-Guzmán, P., Valencia-Cantero, E., Santoyo, G. (2024). Plant growth-promoting bacteria potentiate antifungal and plant-beneficial responses of Trichoderma atroviride by upregulating its effector functions. PloS One 19, e0301139. doi: 10.1371/journal.pone.0301139
Hajieghrari, B., Torabi-Giglou, M., Mohammadi, M. R., Davari, M. (2008). Biological potantial of some Iranian Trichoderma isolates in the control of soil borne plant pathogenic fungi. Afr. J. Biotechnol. 7 (8), 967–972.
Hariharan, G., Rifnas, L. M., Prasannath, K. (2022). “Role of Trichoderma spp. in biocontrol of plant diseases,” in Microbial Biocontrol: Food Security and Post Harvest Management, vol. 2. (Springer International Publishing, Cham), 39–78.
He, B., Pan, S., Zhao, J., Zou, X., Liu, X., Wu, S. (2024). Maize improvement based on modern breeding strategies. Prog. Perspect. ACS Agric. Sci. Technol. 4, 274–282. doi: 10.1021/acsagscitech.3c00427
Hewedy, O. A., Abdel Lateif, K. S., Seleiman, M. F., Shami, A., Albarakaty, F. M., M. El-Meihy, R. (2020a). Phylogenetic diversity of Trichoderma strains and their antagonistic potential against soil-borne pathogens under stress conditions. Biology 9, 189. doi: 10.3390/biology9080189
Hewedy, O. A., El-Zanaty, A. M., Fahmi, A. I. (2020b). Screening and identification of novel cellulolytic Trichoderma species from Egyptian habitats. BioTechnologia. J. Biotechnol. Comput. Biol. Bionanotechnol. 101. doi: 10.5114/bta.2020.96413
Horst, R. J., Engelsdorf, T., Sonnewald, U., Voll, L. M. (2008). Infection of maize leaves with Ustilago maydis prevents establishment of C4 photosynthesis. J. Plant Physiol. 165, 19–28. doi: 10.1016/j.jplph.2007.05.008
Hossain, M. M., Sultana, F. (2024). Genetics of trichoderma-plant-pathogen interactions. Microbial. Genet. doi: 10.1201/9781003328933
Howell, C. R., Beier, R. C., Stipanovic, R. D. (1988). Production of ammonia by Enterobacter cloacae and its possible role in the biological control of Pythium preemergence damping-off by the bacterium. Phytopathology 78.8, 1075–1078. doi: 10.1094/Phyto-78-1075
Jacobo-Delgado, Y. M., Torres-Juarez, F., Rodríguez-Carlos, A., Santos-Mena, A., Enciso-Moreno, J. E., Rivas-antiago, C., et al. (2021). Retinoic acid induces antimicrobial peptides and cytokines leading to Mycobacterium tuberculosis elimination in airway epithelial cells. Peptides 142. doi: 10.1016/j.peptides.2021.170580
Jacobo-Delgado, Y. M., Rodríguez-Carlos, A., Serrano, C. J., Rivas-Santiago, B. (2023). Mycobacterium tuberculosis cell-wall and antimicrobial peptides: a mission impossible? Front. Immunol. 14. doi: 10.3389/fimmu.2023.1194923
Jha, Y., Mohamed, H. I. (2023). Enhancement of disease resistance, growth potential, and biochemical markers in maize plants by inoculation with plant growth-promoting bacteria under biotic stress. J. Plant Pathol. 105, 729–748. doi: 10.1007/s42161-023-01338-9
Jiang, X., Geng, A., He, N., Li, Q. (2011). New isolate of Trichoderma viride strain for enhanced cellulolytic enzyme complex production. J. Biosc. Bioeng. 111, 121–127. doi: 10.1016/j.jbiosc.2010.09.004
Jiang, S., Han, S., He, D., Cao, G., Fang, K., Xiao, X., et al. (2019). The accumulation of phenolic compounds and increased activities of related enzymes contribute to early defense against walnut blight. Physiol. Mol. Plant Pathol. 108, 101433. doi: 10.1016/j.pmpp.2019.101433
Johal, L., Huber, D. M., Martyn, R. (2004). Late wilt of corn (maize) pathway analysis: Intentional introduction of Cephalosporium maydis. Pathways Analysis for the Introduction to the US of Plant Pathogens of Economic Importance
Johanson, D. V. (1940). Plant microtechnique (New York and London: New York and London McGrow-hill Book Co. Inc), 27–154.
Kadhim, M. J., Mohammed, G. J., Hussein, H. (2016). Analysis of bioactive metabolites from Candida albicans using (GC-MS) and evaluation of antibacterial activity. Int. J. Pharm. Clin. Res. 8, 655–670.
Karthika, C., Vanangamudi, K. (2013). Biopriming of maize hybrid COH (M) 5 seed with liquid biofertilizers for enhanced germination and vigour. Afr. J. Agric. Res. 8, 3310–3317.
Khalil, M., Shimaa, H. F. A. (2020). Use of biological control and bio-fertilization against Fusarium wilt disease and its effect on growth characteristics and tomato productivity. Current Research in Environmental & Applied Mycology. J. Fungal Biol. 10, 71–84.
Khan, H. I. (2018). Appraisal of biofertilizers in rice: To supplement inorganic chemical fertilizer. Rice Sci. 25, 357–362. doi: 10.1016/j.rsci.2018.10.006
Khan, R. A. A., Najeeb, S., Hussain, S., Xie, B., Li, Y. (2020). Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 8, 817. doi: 10.3390/microorganisms8060817
Kubicek, C. P., Steindorff, A. S., Chenthamara, K., Manganiello, G., Henrissat, B., Zhang, J., et al. (2019). Evolution and comparative genomics of the most common Trichoderma species. BMC Genomics 20, 1–24. doi: 10.1186/s12864-019-5680-7
Kumari, R., Kumar, V., Arukha, A. P., Rabbee, M. F., Ameen, F., Koul, B. (2024). Screening of the Biocontrol Efficacy of Potent Trichoderma Strains against Fusarium oxysporum f. sp. ciceri and Scelrotium rolfsii Causing Wilt and Collar Rot in Chickpea. Microorganisms 12, 1280. doi: 10.3390/microorganisms12071280
Lahlali, R., Ezrari, S., Radouane, N., Kenfaoui, J., Esmaeel, Q., El Hamss, H., et al. (2022). Biological control of plant pathogens: A global perspective. Microorganisms 10, 596. doi: 10.3390/microorganisms10030596
Li, H., Yang, J., Zhang, X., Xu, X., Song, F., Li, H. (2022). Biocontrol of Candida albicans by antagonistic microorganisms and bioactive compounds. Antibiotics 11, 1238. doi: 10.3390/microorganisms10030596
Lippolis, V., Pascale, M., Maragos, C. M., Visconti, A. (2008). Improvement of detection sensitivity of T-2 and HT-2 toxins using different fluorescent labeling reagents by high-performance liquid chromatography. Talanta 74, 1476–1483. doi: 10.1016/j.talanta.2007.09.024
Liu, T., Long, X., Zhou, J. P., Tian, D. W., Yang, Y. H., Zou, C. G., et al. (2021). Fungistatic mechanism of ammonia against nematode-trapping fungus Arthrobotrys oligospora, and strategy for this fungus to survive ammonia. Msystems 6, 10–1128. doi: 10.1128/msystems.00879-21
Luque-Almagro, V. M., Cabello, P., Sáez, L. P., Olaya-Abril, A., Moreno-Vivián, C., Roldán, M. D. (2018). Exploring anaerobic environments for cyanide and cyano-derivatives microbial degradation. Appl. Microbiol. Biotechnol. 102, 1067–1074. doi: 10.1007/s00253-017-8678-6
Lynch, J. P. (2019). Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytol. 223, 548–564. doi: 10.1111/nph.15738
Magbanua, Z. V., De Moraes, C. M., Brooks, T. D., Williams, W. P., Luthe, D. S. (2007). Is catalase activity one of the factors associated with maize resistance to Aspergillus flavus? Mol. Plant-Microbe Interact. 20, 697–706. doi: 10.1094/MPMI-20-6-0697
Marín-Ortiz, J. C., Gutierrez-Toro, N., Botero-Fernández, V., Hoyos-Carvajal, L. M. (2020). Linking physiological parameters with visible/near-infrared leaf reflectance in the incubation period of vascular wilt disease. Saudi J. Biol. Sci. 27, 88–99. doi: 10.1016/j.sjbs.2019.05.007
Maruyama, C. R., Bilesky-José, N., de Lima, R., Fraceto, L. F. (2020). Encapsulation of Trichoderma harzianum preserves enzymatic activity and enhances the potential for biological control. Front. Bioeng. Biotechnol. 8. doi: 10.3389/fbioe.2020.00225
Matei, A., Ernst, C., Günl, M., Thiele, B., Altmüller, J., Walbot, V., et al. (2018). How to make a tumour: cell type specific dissection of Ustilago maydis-induced tumour development in maize leaves. New Phytol. 217, 1681–1695. doi: 10.1111/nph.14960
Matta, A., Dimond, A. E. (1963). Symptoms of Fusarium wilt in relation to quantity of fungus and enzyme activity in Tomato stems. Phytopathology 53, 574–578.
Mauch, F., Mauch-Mani, B., Boller, T. (1988). Antifungal hydrolases in pea tissue: II. Inhibition of fungal growth by combinations of chitinase and β-1, 3-glucanase. Plant Physiol. 88, 936–942. doi: 10.1104/pp.88.3.936
Mehnaz, S. (2014). “Azospirillum: a biofertilizer for every crop,” in Plant microbes symbiosis: Applied facets (Springer India, New Delhi), 297–314. doi: 10.1007/978-81-322-2068-8_15
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428. doi: 10.1021/ac60147a030
Missall, T. A., Lodge, J. K., McEwen, J. E. (2004). Mechanisms of resistance to oxidative and nitrosative stress: implications for fungal survival in mammalian hosts. Eukar. Cell 3, 835–846. doi: 10.1128/ec.3.4.835-846.2004
Moghazy, N. H., Kaluarachchi, J. J. (2020). Sustainable agriculture development in the western desert of Egypt: A case study on crop production, profit, and uncertainty in the Siwa region. Sustainability 12, 6568. doi: 10.3390/su12166568
Molinero-Ruiz, M., Melero-Vara, J. M., Mateos, A. (2010). Cephalosporium maydis, the cause of late wilt in maize, a pathogen new to Portugal and Spain. Plant Dis. 94, 379–379. doi: 10.1094/PDIS-94-3-0379A
Mukherjee, P. K., Mendoza-Mendoza, A., Zeilinger, S., Horwitz, B. A. (2022). Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol. Rev. 39, 15–13. doi: 10.1016/j.fbr.2021.11.004
Nawrocka, J., Szymczak, K., Skwarek-Fadecka, M., Małolepsza, U. (2023). Toward the Analysis of Volatile Organic Compounds from Tomato Plants (Solanum lycopersicum L.) Treated with Trichoderma virens or/and Botrytis cinerea. Cells 12, 1271. doi: 10.3390/cells12091271
Nieto-Jacobo, M. F., Steyaert, J. M., Salazar-Badillo, F. B., Nguyen, D. V., Rostás, M., Braithwaite, M., et al. (2017). Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00102
Nornal, R. (1982). Formulae for determination of chlorophyllous pigments extracted with N, N-Dimethylamide. Plant Physiol. doi: 10.1104/pp.69.6.1376
Öğüt, M., Akdağ, C., Düzdemir, O., Sakin, M. A. (2005). Single and double inoculation with Azospirillum/Trichoderma: the effects on dry bean and wheat. Biol. Fertil. Soils 41, 262–272. doi: 10.1007/s00374-004-0818-3
Olanrewaju, O. S., Glick, B. R., Babalola, O. O. (2017). Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 33, 1–16. doi: 10.1007/s11274-017-2364-9
Ortiz-Bustos, C. M., García-Carneros, A. B., Molinero-Ruiz, L. (2015). The late wilt of corn (Zea mays L.) caused by Cephalosporium maydis and other fungi associated at the Iberian Peninsula. Summa Phytopathologica 41 (2), 107–114. doi: 10.1590/0100-5405/1998
Ortiz-Bustos, C. M., Testi, L., García-Carneros, A. B., Molinero-Ruiz, L. (2016). Geographic distribution and aggressiveness of Harpophora maydis in the Iberian peninsula, and thermal detection of maize late wilt. Eur. J. Plant Pathol. 144, 383–397. doi: 10.1007/s10658-015-0775-8
Pal, G., Kumar, K., Verma, A., Verma, S. K. (2021). “Application of bacterial biostimulants in promoting growth and disease prevention in crop plants,” in Biostimulants for Crops from Seed Germination to Plant Development (Cambridge, Massachusetts: Academic Press), 393–410.
Pan, S., Bhagat, S. (2008). Characterization of antagonistic potential of Trichoderma spp. against some soil borne plant pathogens. J. Biol. Control 22, 43–49.
Pastrana, L. M., Gonzalez, M., Pintado, J., Murado, M. A. (1995). Interactions affecting gibberellic acid production in solid-state culture: a factorial study. Enzyme Microbial. Technol. 17, 784–790. doi: 10.1016/0141-0229(94)00024-L
Payak, M., Lal, S., Renfro, B. (1970). Cephalosporium maydis-a new threat to Maize in India. Indian Phytopathol. 23, 562–569.
Payne, S. M. (1994). 25] Detection, isolation, and characterization of siderophores. Methods Enzymol. 235, 329–344. doi: 10.1016/0076-6879(94)35151-1
Pedraza, R. O., Filippone, M. P., Fontana, C., Salazar, S. M., Ramírez-Mata, A., Sierra-Cacho, D., et al. (2020). “Azospirillum,” in Beneficial microbes in agro-ecology (Cambridge, Massachusetts: Academic Press), 73–105. doi: 10.1016/B978-0-12-823414-3.00006-X
Pereyra, C. M., Ramella, N. A., Pereyra, M. A., Barassi, C. A., Creus, C. M. (2010). Changes in cucumber hypocotyl cell wall dynamics caused by Azospirillum brasilense inoculation. Plant Physiol. Biochem. 48, 62–69. doi: 10.1016/j.plaphy.2009.10.001
Perrig, D., Boiero, M. L., Masciarelli, O. A., Penna, C., Ruiz, O. A., Cassán, F. D., et al. (2007). Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl. Microbiol. Biotechnol. 75, 1143–1150. doi: 10.1007/s00253-007-0909-9
Pouzoulet, J., Scudiero, E., Schiavon, M., Rolshausen, P. E. (2017). Xylem vessel diameter affects the compartmentalization of the vascular pathogen Phaeomoniella chlamydospora in grapevine. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01442
Rabani, M. S., Hameed, I., Gupta, M. K., Wani, B. A., Fayaz, M., Hussain, H., et al. (2023). “Introduction of biofertilizers in agriculture with emphasis on nitrogen fixers and phosphate solubilizers,” in Microbiomes for the Management of Agricultural Sustainability (Springer Nature Switzerland, Cham), 71–93. doi: 10.1007/978-3-031-32967-8_4
Rahman, M., Borah, S. M., Borah, P. K., Bora, P., Sarmah, B. K., Lal, M. K., et al. (2023). Deciphering the antimicrobial activity of multifaceted rhizospheric biocontrol agents of solanaceous crops viz., Trichoderma harzianum MC2, and Trichoderma harzianum NBG. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1141506
Reetha, A. K., Pavani, S. L., Mohan, S. (2014). Hydrogen cyanide production ability by bacterial antagonist and their antibiotics inhibition potential on Macrophomina phaseolina (Tassi.) Goid. Int. J. Curr. Microbiol. Appl. Sci. 3, 172–178.
Saadaoui, M., Faize, M., Bonhomme, L., Benyoussef, N. O., Kharrat, M., Chaar, H., et al. (2023). Assessment of Tunisian trichoderma isolates on wheat seed germination, seedling growth and fusarium seedling blight suppression. Microorganisms 11, 1512. doi: 10.3390/microorganisms11061512
Samra, A., Sabet, K., Hingorani, M. (1962). A new wilt disease of Maize in Egypt. Plant Dis. Rep. 46.
Samra, A., Sabet, K., Hingorani, M. (1963). Late wilt disease of maize caused by Cephalosporium maydis. Phytopathology 53.
Santoyo, G., Orozco-Mosqueda, M. D. C., Afridi, M. S., Mitra, D., Valencia-Cantero, E., Macías-Rodríguez, L. (2024). Trichoderma and Bacillus multifunctional allies for plant growth and health in saline soils: recent advances and future challenges. Front. Microbiol. 15, 1423980. doi: 10.3389/fmicb.2024.1423980
Sass, J. E. (1951). Response of meristems of seedlings to benzene hexachloride used as a seed protectant. Science 114, 466–466. doi: 10.1126/science.114.2966.466.a
Seddek, N. H., Fawzy, M. A., El-Said, W. A., Ahmed, M. M. R. (2019). Evaluation of antimicrobial, antioxidant and cytotoxic activities and characterization of bioactive substances from freshwater blue-green algae. Global NEST J. 21, 329–337.
Shekhar, M., Kumar, S. (2012). Inoculation methods and disease rating scales for maize diseases, Directorate of maize research. Indian Council Agric. Res.
Shim, I. S., Momose, Y., Yamamoto, A., Kim, D. W., Usui, K. (2003). Inhibition of catalase activity by oxidative stress and its relationship to salicylic acid accumulation in plants. Plant Growth Regul. 39, 285–292. doi: 10.1023/A:1022861312375
Singh, M., Singh, D., Gupta, A., Pandey, K. D., Singh, P. K., Kumar, A. (2019). “Plant growth promoting rhizobacteria: application in biofertilizers and biocontrol of phytopathogens,” in PGPR amelioration in sustainable agriculture (Sawston, Cambridge: Woodhead Publishing), 41–66.
Singh, A. K., Singh, V. B., Srivastava, J. N., Singh, S. K., Gupta, A. (2020). “Diseases of Maize Crops and Their Integrated Management,” in Diseases of Field Crops Diagnosis and Management (New Jersey and Canada: Apple Academic Press), 105–140.
Spaepen, S., Dobbelaere, S., Croonenborghs, A., Vanderleyden, J. (2008). Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant Soil 312, 15–23. doi: 10.1007/s11104-008-9560-1
Steenhoudt, O., Vanderleyden, J. (2000). Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 24, 487–506. doi: 10.1111/j.1574-6976.2000.tb00552.x
Sunitha, N. C., Gangappa, E., Gowda, R. V., Ramesh, S., Biradar, S., Swamy, D., et al. (2020). Assessment of impact of late wilt caused by Harpophora maydis (Samra, Sabet and Hing) on grain yield and its attributing traits in maize (Zea mays L.);. Mysore J. Agric. Sci. 54, 30–36.
Swain, H., Mukherjee, A. K. (2020). Host–pathogen–trichoderma interaction. Trichoderma: Host Pathogen Interact. Appl. 149–165.
Tamizi, A. A., Mat-Amin, N., Weaver, J. A., Olumakaiye, R. T., Akbar, M. A., Jin, S., et al. (2022). Genome sequencing and analysis of Trichoderma (Hypocreaceae) isolates exhibiting antagonistic activity against the papaya dieback pathogen, Erwinia mallotivora. J. Fungi 8, 246. doi: 10.3390/jof8030246
Tej, R., Rodríguez-Mallol, C., Rodríguez-Arcos, R., Karray-Bouraoui, N., Molinero-Ruiz, L. (2018). Inhibitory effect of Lycium europaeum extracts on phytopathogenic soil-borne fungi and the reduction of late wilt in maize. European. J. Plant Pathol. 152, 249–265. doi: 10.1007/s10658-018-1469-9
Terna, T. P., Mohamed Nor, N. M. I., Zakaria, L. (2022). Histopathology of corn plants infected by endophytic fungi. Biology 11, 641. doi: 10.3390/biology11050641
Ulhoa, C. J., Peberdy, J. F. (1991). Regulation of chitinase synthesis in Trichoderma harzianum. Microbiology 137, 2163–2169. doi: 10.1099/00221287-137-9-2163
Varsha, K. K., Devendra, L., Shilpa, G., Priya, S., Pandey, A., Nampoothiri, K. M. (2015). 2, 4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int. J. Food Microbiol. 211, 44–50. doi: 10.1016/j.ijfoodmicro.2015.06.025
Veenstra, A., Moola, N., Wighard, S., Korsman, J., Christensen, S. A., Rafudeen, M. S., et al. (2019). Kauralexins and zealexins accumulate in sub-tropical maize lines and play a role in seedling resistance to Fusarium verticillioides. Eur. J. Plant Pathol. 153, 223–237. doi: 10.1007/s10658-018-1557-x
Vinale, F., Sivasithamparam, K., Ghisalberti, E. L., Marra, R., Barbetti, M. J., Li, H., et al. (2008). A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 72, 80–86. doi: 10.1016/j.pmpp.2008.05.005
Walia, A., Putatunda, C., Sharma, R., Sharma, S., Thakur, A. (2021). “Biocontrol: a sustainable agricultural solution for management of plant diseases,” in Microbial biotechnology in crop protection (Springer Singapore, Singapore), 1–54.
Werner, T., Nehnevajova, E., Köllmer, I., Novák, O., Strnad, M., Krämer, U., et al. (2010). Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 22, 3905–3920. doi: 10.1105/tpc.109.072694
Woo, S. L., Hermosa, R., Lorito, M., Monte, E. (2023). Trichoderma: a multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 21, 312–326. doi: 10.1038/s41579-022-00819-5
Yadav, D., Dasgupta, M. D., Dey, A. (2024). “Mechanisms and applications of nitrogen fixing Azotobacter and Azospirillum in horticultural crops,” in Bio-Inoculants in Horticultural Crops (Sawston, Cambridge: Woodhead Publishing), 139–154. doi: 10.1016/B978-0-323-96005-2.00005-2
Yassin, M. T., Mostafa, A. A. F., Al-Askar, A. A. (2021). In vitro antagonistic activity of Trichoderma harzianum and T. viride strains compared to carbendazim fungicide against the fungal phytopathogens of Sorghum bicolor (L.) Moench. Egypt. J. Biol. Pest Control 31, 1–9. doi: 10.1186/s41938-021-00463-w
Yogalakshmi, S., Thiruvudainambi, S., Kalpana, K., Vendan, R. T. (2021). Antifungal activity of Trichoderma atroviride against Fusarium oxysporum. f. sp. lycopersici causing wilt disease of tomato. J. Hortic. Sci. 16, 241–250.
Yoon, M. Y., Cha, B., Kim, J. C. (2013). Recent trends in studies on botanical fungicides in agriculture. Plant Pathol. J. 29, 1. doi: 10.5423/PPJ.RW.05.2012.0072
Yu, Z., Wang, Z., Zhang, Y., Wang, Y., Liu, Z. (2021). Biocontrol and growth-promoting effect of Trichoderma asperellum TaspHu1 isolate from Juglans mandshurica rhizosphere soil. Microbiol. Res. 242, 126596. doi: 10.1016/j.micres.2020.126596
Zhang, F., Yuan, J., Yang, X., Cui, Y., Chen, L., Ran, W., et al. (2013). Putative Trichoderma harzianum mutant promotes cucumber growth by enhanced production of indole acetic acid and plant colonization. Plant Soil 368, 433–444. doi: 10.1007/s11104-012-1519-6
Keywords: Trichoderma viride, Azospirillum brasilense, Harpophora maydis, secondary metabolites, maize, late wilt
Citation: Elmeihy RM, Hewedy OA, Alhumaidi MS, Altammar KA, Hassan EO and El-Debaiky SA (2025) Co-inoculation of Trichoderma viride with Azospirillum brasilense could suppress the development of Harpophora maydis-infected maize in Egypt. Front. Plant Sci. 15:1486607. doi: 10.3389/fpls.2024.1486607
Received: 26 August 2024; Accepted: 26 December 2024;
Published: 06 February 2025.
Edited by:
Grace Armijo, Agriaquaculture Nutritional Genomic Center, ChileReviewed by:
Mariela I. Monteoliva, National Institute of Agricultural Technology (INTA), ArgentinaCopyright © 2025 Elmeihy, Hewedy, Alhumaidi, Altammar, Hassan and El-Debaiky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Omar A. Hewedy, aGV3ZWR5Lm9tYXJAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.