- 1Department of Plant Breeding and Genetics, Punjab Agricultural University, Ludhiana, ;India
- 2Nuclear Institute for Agriculture and Biology (NIAB), Faisalabad, ;Pakistan
- 3Pakistan Academy of Sciences, Islamabad, ;Pakistan
- 4Faculty of Engineering and Natural Sciences, Sabanci University Orta, Istanbul, ;Türkiye
- 5International Maize and Wheat Improvement Center (CIMMYT), Texcoco, ;Mexico
Inherently low concentrations of zinc (Zn), iron (Fe), iodine (I), and selenium (Se) in wheat (Triticum aestivum L.) grains represent a major cause of micronutrient malnutrition (hidden hunger) in human populations. Genetic biofortification represents a highly useful solution to this problem. However, genetic biofortification alone may not achieve desirable concentrations of micronutrients for human nutrition due to several soil- and plant-related factors. This study investigated the response of genetically biofortified high-Zn wheat genotypes to soil-applied Zn and foliarly applied Zn, I, and Se in India and Pakistan. The effect of soil-applied Zn (at the rate of 50 kg ha−1 as ZnSO4·7H2O) and foliar-applied Zn (0.5% ZnSO4·7H2O), I (0.04% KIO3), Se (0.001% Na2SeO4), and a foliar cocktail (F-CT: combination of the above foliar solutions) on the grain concentrations of Zn, I, Se, and Fe of high-Zn wheat genotypes was investigated in field experiments over 2 years. The predominantly grown local wheat cultivars in both countries were also included as check cultivars. Wheat grain yield was not influenced by the micronutrient treatments at all field locations, except one location in Pakistan where F-CT resulted in increased grain yield. Foliar-applied Zn, I, and Se each significantly enhanced the grain concentration of the respective micronutrients. Combined application of these micronutrients was almost equally effective in enhancing grain Zn, I, and Se, but with a slight reduction in grain yield. Foliar-applied Zn, Zn+I, and F-CT also enhanced grain Fe. In India, high-Zn genotypes exhibited a minor grain yield penalty as compared with the local cultivar, whereas in Pakistan, high-Zn wheat genotypes could not produce grain yield higher than the local cultivar. The study demonstrates that there is a synergism between genetic and agronomic biofortification in enrichment of grains with micronutrients. Foliar Zn spray to Zn-biofortified genotypes provided additional increments in grain Zn of more than 15 mg kg−1. Thus, combining agronomic and genetic strategies will raise grain Zn over 50 mg kg−1. A combination of fertilization practice with plant breeding is strongly recommended to maximize accumulation of micronutrients in food crops and to make significant progress toward resolving the hidden hunger problem in human populations.
1 Introduction
Deficiencies of mineral micronutrients such as zinc (Zn), iron (Fe), iodine (I), and selenium (Se) in humans are widespread globally, affecting approximately 50% of the world’s population. In many areas of the world, multi-micronutrient deficiencies are also observed, resulting in various health complications (Welch et al., 2013; Bailey et al., 2015; Harding et al., 2018; Stevens et al., 2022; FAO et al., 2023). The root cause of this type of malnutrition in the developing world is the low density of micronutrients in staple food crops, especially cereals including wheat, maize, and rice, mainly due to the low plant availability of these micronutrients in cultivated soils.
For instance, Zn deficiency in wheat is a widespread mineral nutrient disorder in India and Pakistan because the soils in these countries are alkaline and calcareous, with low organic matter and low moisture (Rashid, 2005; Cakmak, 2009; Zou et al., 2012; Ram et al., 2015). Soil Zn deficiency not only hampers crop productivity but also results in the production of low-Zn wheat, which leads to Zn malnutrition and the consequent health hazards in humans and animals (Rashid, 2005; Cakmak, 2008; Zou et al., 2012, 2019; Morton et al., 2023). In India and Pakistan, which are among the countries with the highest prevalence of hidden hunger, wheat is a major agricultural crop cultivated on over 38.0 Mha of land and represents the predominant staple cereal, with annual production of over 110 million metric tons in India and 26 million metric tons in Pakistan (Poole et al., 2021). Singh (2010) and Hussain et al. (2012) reported widespread low levels of Zn in grains, which is affecting a large segment of resource-poor families in India and Pakistan. Similarly, Kapil and Jain (2011) reported prevalence of Zn deficiency in 43.8% population of Uttar Pradesh (Northern region), Karnataka (Southern region), Orissa (Eastern region), Gujarat (Western region), and Madhya Pradesh (Central region) in India. The target populations living in rural areas consume mainly wholemeal or high extraction rate flours which, compared with “white flours” produced with a lower extraction rate, contain more Zn-rich grain components. Wheat flour currently contributes to more than 40% of Pakistan’s daily caloric intake, with per capita wheat consumption averaging around 124 kg per year, one of the highest in the world (USDA, 2014). Use of whole wheat meal in food products ensures the retention of more Zn during milling, and thus making it available to consumers because Zn is mainly concentrated in the outer aleurone layer and embryo and extremely low in the endosperm (white flour) (Cakmak et al., 2010).
Very recently, after analyzing 27 million soil tests and large human health survey studies including over one million people in India, Morton et al. (2023) reported that there is a close link between soil Zn deficiency and childhood stunting and highlighted the relevance of agronomic biofortification to minimize Zn deficiency-related health problems in India. In Pakistan, approximately 40% of the population, mostly resource-poor women and children, suffer from Zn malnutrition (NNS, 2019). Iron deficiency anemia is also a common health disorder in Pakistan; it affects 49% of mothers and 29% of children (NNS, 2019). Overall, over 1 billion of the world population are at the risk of Zn malnutrition due to inadequate dietary intake of Zn (Kumssa et al., 2015; Chen et al., 2017; Stevens et al., 2022). Intensive cropping of high-yield potential crop genotypes has aggravated the depletion of soil Zn leading to low Zn density in edible grains. Although, historically, wheat was considered less sensitive to Zn deficiency and contained high Zn concentrations in grains (Rashid and Fox, 1992; Fan et al., 2008), significant increases in wheat grain yield and grain Zn concentration have been observed with Zn fertilization in low-Zn soils in many countries of the world including India and Pakistan (Cakmak, 2008; Zou et al., 2012; Ram et al., 2015; Zou et al., 2019). So, there is a high potential for Zn enrichment of cereal grains by Zn fertilization to contribute to the alleviation of Zn deficiency problem in human populations. Iodine deficiency is another predominant public health problem around the world, persisting despite large nationwide and worldwide efforts, including the implementation of universal salt iodization and food iodine fortification programs over the past 30 years (Zimmermann and Andersson, 2012; Hatch-McChesney and Lieberman, 2022). Iodine deficiency represents a significant risk for intellectual disability and thyroid cancer (Panth et al., 2019; Smyth, 2022). A survey on worldwide I status has listed Pakistan as a country severely affected by I deficiency, with a considerable population having insufficient I intake, due to low I in soils and consequently low I in the produced food (Zia et al., 2015; Passarelli et al., 2024). Zia et al. (2015) reported very low I status in soil-plant systems in Pakistan and indicated that the origin of widespread I deficiency in human population is reduced dietary intake. Iodine-enriched cereal grains are also one of the options for curing I deficiency disorders (IDDs) in the Indian subcontinent. Several reports are available showing that soil and foliar application of I-containing fertilizers is highly effective in increasing I concentrations of the edible parts of food crops (Smoleń et al., 2016; Cakmak et al., 2017; Gonzali et al., 2017; Lyons, 2018; Budke et al., 2020). Among the treatments, foliar spray of I-containing fertilizers such as KIO3 has been shown to be very effective in improving the grain I concentration of cereal crops (Zou et al., 2019; Prom-u-thai et al., 2020; Cakmak et al., 2020)
Selenium (Se), being a constituent of selenoproteins, is another essential mineral micronutrient for humans. Selenium has a number of critical physiological functions in mammalian systems including the healthy functioning of the immune system, anti-inflammatory effects, preventive effects on various types of cancers, and antioxidative impacts under stressful conditions (Rayman, 2000; 2012; Lyons et al., 2005; Sun et al., 2023). It is estimated that approximately one billion people worldwide are affected from Se deficiency (Lyons et al., 2005; Jones et al., 2017). Very low levels of Se in staple food crops and consequently low dietary intake of Se are known to be the major reasons behind the widespread Se deficiency in human populations (Broadley et al., 2006; Rayman, 2008; Hurst et al., 2013). This justifies greater attention being given to the enrichment of food crops with Se, for example, by agronomic biofortification. There are well-documented examples demonstrating significant effects of soil or foliar applied Se fertilizers in enriching food crops with Se (Broadley et al., 2006; Schiavon et al., 2020; Prom-u-thai et al., 2020; Cakmak et al., 2020; Daud et al., 2024).
Deficiencies of all these micronutrients in humans and animals is exacerbated by the high-yielding Green Revolution cereal varieties, which often produce less micronutrient-dense grains than previously grown cultivars (Fan et al., 2008; Dobermann et al., 2022). Therefore, biofortification of staple crops, like wheat, to achieve higher micronutrient concentrations in grains represents an important humanitarian challenge. Development of wheat varieties with higher grain micronutrient density through plant breeding approaches is widely considered as a promising and cost-effective approach for eliminating micronutrient malnutrition worldwide (Welch and Graham, 2004; Bouis and Saltzman, 2017). Significant progress has been made in the targeted development and deployment of biofortified wheat with enhanced Zn concentrations, reaching more than two million households with biofortified wheat varieties in South Asia (Govindan et al., 2019). This effort has involved transferring high-Zn genes from selected landraces, spelt wheat and emmer wheat-derived synthetic wheat, to increase grain Zn levels of high-yielding wheat varieties by 30%–40% while preserving their agronomic competitiveness across different geographies (Guzmán et al., 2014). The identification of new genetic sources of high Zn was critical due to the limited variation and low absolute content of this micronutrient in the grain of hexaploid elite wheat lines. This targeted breeding for improved Zn content led to the release of more than 20 Zn-biofortified wheat varieties in target countries. All these varieties exhibit yields, which are at least as high as the conventional varieties released in the same regions, and an average increase of 8 ppm–10 ppm (25%–40%) in Zn level in the grain (Govindan et al., 2022). CIMMYT-derived Zn-biofortified wheat varieties are already in farmers’ fields, occupying nearly 2 million hectares and have been the subject of nutrition studies that demonstrate a reduction in child morbidity associated with their consumption (Sazawal et al., 2018).
Biofortified wheat has several potential advantages as a delivery vehicle for essential micronutrients in developing countries. In India and Pakistan, CIMMYT-derived wheat varieties, which are rapidly and widely adopted in both the countries, are excellent delivery vehicles for high Zn wheat, and the self-pollinated, true-breeding nature of the species means that the trait is likely to be stable for several generations of on-farm seed production. A few biofortified wheat genotypes developed at the International Maize and Wheat Improvement Center (CIMMYT) under the HarvestPlus project with high Zn concentrations were commercialized in India and Pakistan. Those biofortified wheat cultivars including PBW-1-Zn in India, and Zincol-2016, Akbar-2019, and Nawab-2021 in Pakistan have been recommended for general cultivation. These genotypes were developed from crosses between high-yielding parents and genetic resources containing high Zn (Govindan et al., 2012).
It is, however, important to highlight that genetic biofortification alone, as in the case of wheat, may not achieve desirable concentrations of micronutrients for human nutrition if the cultivated soils have several physical and chemical limitations reducing phytoavailability and root uptake of micronutrients such as low soil moisture, low organic matter, extreme soil pH values, and reduced root colonization with arbuscular mycorrhizal fungi as shown for Zn (Cakmak, 2008; Yazici et al., 2021). Accordingly, Zn concentrations in grains of the Zn-biofortified wheat varieties are still not sufficiently high to meet required human dietary intake for Zn. Limited genetic variation within the germplasm for the targeted micronutrients and decreases in yield potential associated with genetic improvements in grain concentrations represent with limitations (Cakmak, 2008; Hussain et al., 2012; Rashid et al., 2023; Ishfaq et al., 2023). More importantly, to our knowledge no example of multimicronutrient-biofortified variety of any crop exists. These observations also indicate that there is a high need for further enhancement of grain Zn density and to biofortify the Zn-biofortified wheat cultivars with other micronutrients, including I and Se. Considering the successful examples described above, agronomic biofortification represents an excellent strategy for further enhancements of Zn as well as other micronutrients in grains of genetically biofortified genotypes. This study investigated the response of HarvestPlus-biofortified high-Zn wheat genotypes to soil applied Zn and foliarly applied Zn, I, and Se in India and Pakistan under field conditions.
2 Materials and methods
2.1 Field locations and soils of the experiment
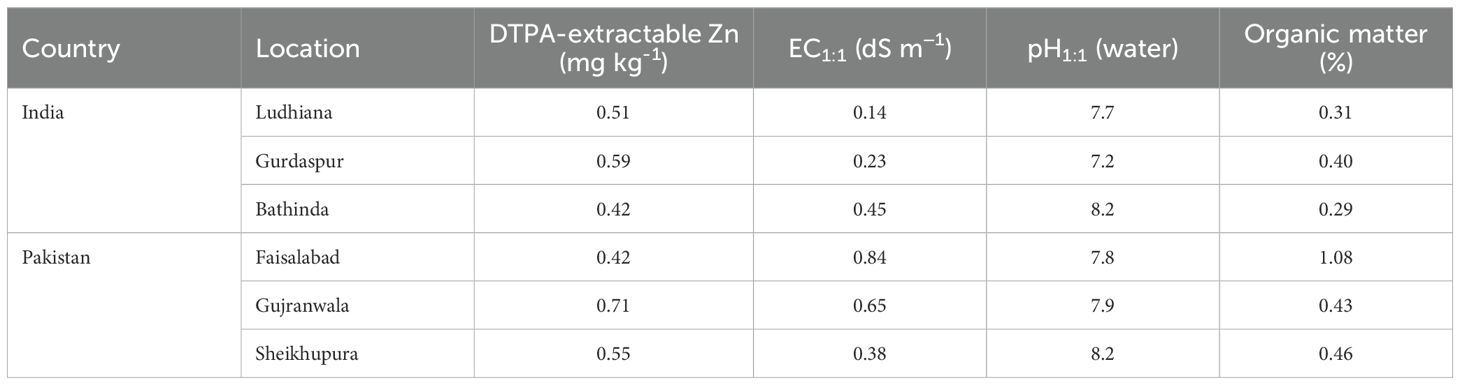
Field experiments were conducted at three locations in India, viz., Ludhiana (30.9010° N, 75.8573° E), Gurdaspur (32.0414° N, 75.4031° E), and Bathinda (30.2110° N, 74.9455° E), during 2015–2016 and 2016–2017, in Pakistan at Faisalabad (31.4504° N, 73.1350° E) during 2015–2016 and 2016–2017, and in Gujranwala (32.1877° N, 74.1945° E) and Sheikhupura (31.7167° N, 73.9850° E) during 2016–2017. Some salient characteristics of the experimental field soils are presented in Table 1. Soils of all field sites in both countries were low in DTPA-extractable Zn (0.42 mg kg−1–0.59 mg kg−1), except for Gujranwala in Pakistan in 2016–2017 where the soil contained 0.71 mg Zn kg−1. Fertilizer nutrients applied (per hectare) to each treatment were 150 kg N, 17.5 kg P, and 25 kg K in India and 120 kg N, 34.8 kg P, and 41.5 kg K in Pakistan. In India, the crop was sown during 7–28 November and harvested during 17–29 April in both years. In Pakistan, the sowing and harvesting dates were during 21–28 November and 20 April–04 May, respectively.
2.2 Experimental treatments
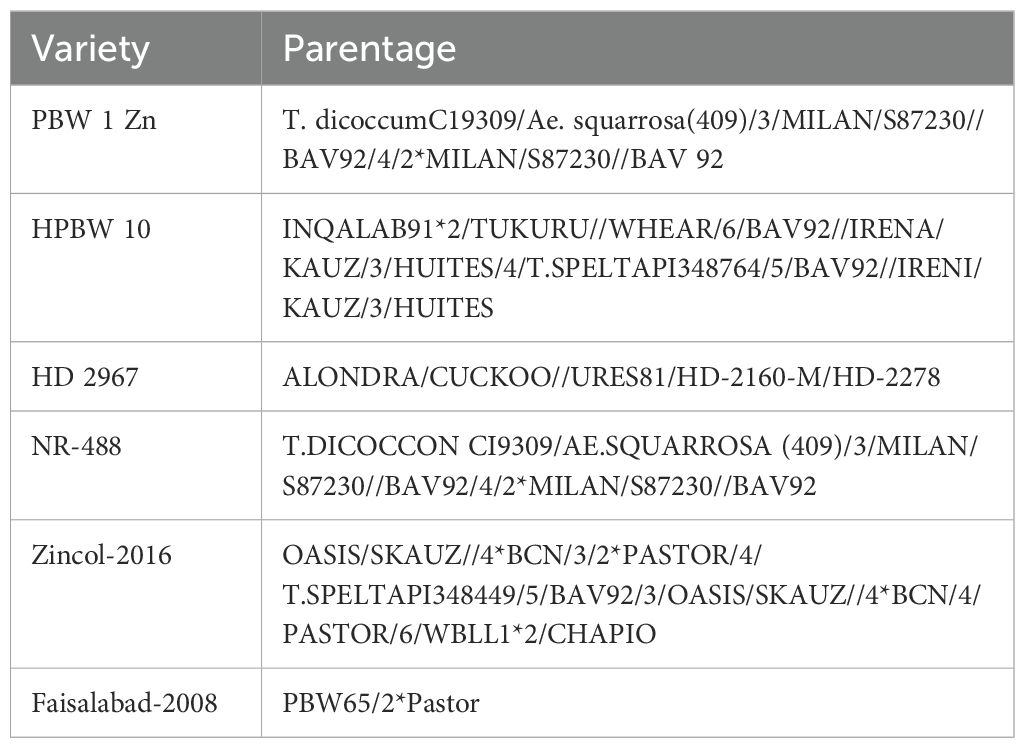
The field experiment evaluated the following five micronutrient treatments: (i) local control (LC, i.e., soil application of recommended rates of N, P, and K only); (ii) LC + soil Zn (S-Zn, i.e., 50 kg ZnSO4·7H2O ha−1); (iii) LC + foliar Zn (F-Zn, i.e., 0.5% solution of ZnSO4·7H2O); (iv) LC + foliar I (F-I, i.e., 0.04% solution of KIO3); and (v) foliar cocktail (F-CT, i.e., a combined solution of 0.5% ZnSO4·7H2O + 0.04% KIO3 + 0.001% Na2SeO4). All spray solutions contained 0.05% detergent powder (Surf) as surfactant. Three wheat genotypes were used in the experiments in each country: CIMMYT-derived PBW-1-Zn, HPBW-10, and locally well-adapted cv. HD-2967 in India and CIMMYT-derived Zincol-2016, NR-488, and locally well-adapted cv. Faisalabad-2008 in Pakistan. The parentages of the genotypes are given in Table 2. The experiment was conducted in split plot design with four replicates, by allocating micronutrient treatments in main plots and wheat genotypes in subplots. The size of each experimental subplot was 10 m2. Wheat was sown using a specialized hand sowing two-tined drill fitted with a seed metering device, adjusted to a seed sowing rate of 100 kg ha−1. Soil Zn was broadcast applied just prior to crop sowing, and all foliar treatments were applied twice, i.e., at ear initiation and early grain milk stage. Foliar sprays, at the rate of 500 L per hectare, were applied very late in the afternoon or under cloudy day to minimize rapid evaporation of the sprayed solution as described by Zou et al. (2019).
2.3 Grain yield
At maturity, the crop was harvested from each plot, avoiding border rows. After allowing a few days to dry in the sun, the crop was threshed and grain yield was recorded. Grain yield was adjusted to account for 13% moisture content. Wheat grains were sampled from each plot for analysis of Zn, Fe, I, and Se.
2.4 Grain analysis for micronutrients
The grain samples collected were first washed with tap water and then with deionized water to remove the adhering soil dusts and other possible contaminants. The washed samples were dried at approximately 40°C in a forced-draft oven to a constant weight. For analysis of Zn, Fe, and Se, dried grains were digested with nitric acid (HNO3, Suprapure 65%) in a microwave-accelerated reaction system (MARS 6, CEM Corp., USA) and diluted to 100 mL. For measuring the I concentration, the grains (250 ± 10 mg) were extracted in tetramethyl-ammonium hydroxide (TMAH; 20 mL 1.25%) at 90°C using a closed-vessel microwave reaction system (MARS 6, CEM Corp., USA), as described by Cakmak et al. (2017). The cooled extracts were centrifuged for 20 min at 5,000 rpm, diluted to 1:1 by volume with double-deionized water (4 μS cm−1 at 25°C), and filtered in capped plastic vials. The measurement of Zn and Fe concentrations in the acid digests was made by inductively coupled plasma optical emission spectroscopy (ICP-OES; Vista-PRO Axial; Varian Pty Ltd; Mulgrave, Australia) and of I and Se concentrations by inductively coupled plasma mass spectrometry (ICP-MS; 7700 Series, Agilent Technologies, USA).
2.5 Statistical analysis
The data were statistically analyzed following two-way analysis of variance (ANOVA), with variety and soil/foliar micronutrient treatment as the two factors, and means were compared by protected least significance difference (protected LSD) test at a confidence level of 95%. The statistical analyses were performed using computer-based SAS software (SAS 8.0, USA).
3 Results
3.1 Grain yield
Wheat grain yield was not affected by any of the micronutrient treatments in both years at all field locations, except at Faisalabad in Pakistan during 2015–2016 (Table 3). During 2015–2016 in Faisalabad, Pakistan, foliar cocktail (F-CT) application resulted in significantly higher grain yield than the local control (LC). Averaged across soil/foliar treatments, except at Gurdaspur in 2015–2016, the grain yield of wheat genotypes in India did not differ from each other at all field locations, whereas in Pakistan, grain yield differed significantly among the wheat genotypes during both the years. At Gurdaspur (India) in 2015–2016, no grain yield of the biofortified high-Zn genotypes could surpass the yield of Indian check variety cv. HD 2967. In Pakistan during 2015–2016, averaged across all micronutrient treatments, maximum grain yield was recorded for the HarvestPlus wheat line NR-488 (3.52 Mg ha−1), which was significantly higher than Zincol-2016 and Faisalabad-2008 at Faisalabad. During 2016–2017, the check variety cv. Faisalabad-2008 in Pakistan had the highest grain yield across all experimental sites, followed by HarvestPlus-genotype Zincol-2016 except at the Faisalabad location in 2015–2016.
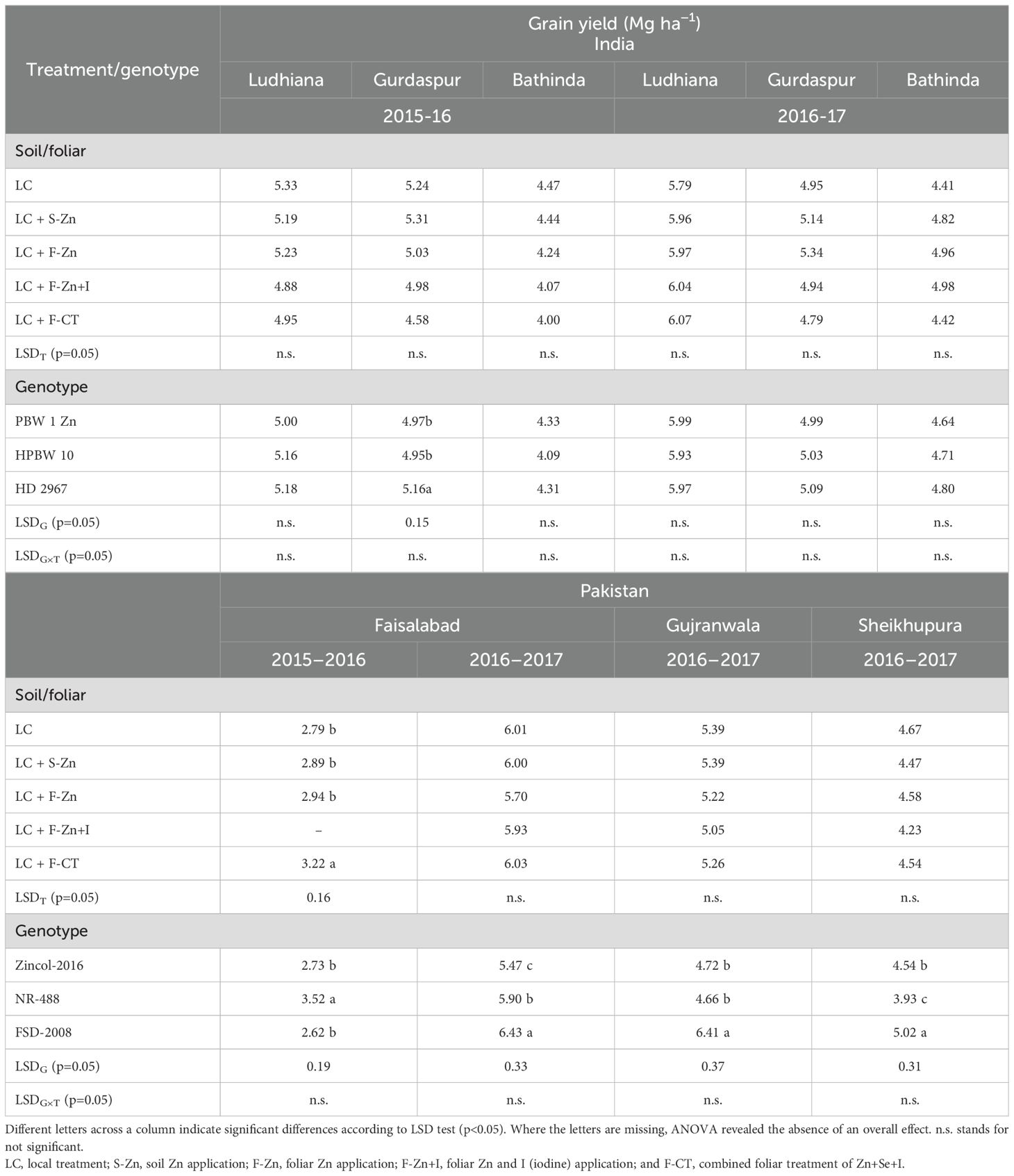
Table 3. Effect of micronutrient fertilizer treatments on grain yield of different wheat genotypes grown at various locations in India and Pakistan.
Based on the pooled means across years and locations, significant reductions of 3.9% and 6.9% in grain yield were recorded in India with foliar Zn+I (F-Zn+I) and F-CT treatments, respectively (Table 4). Soil application of Zn (S-Zn) resulted in the highest grain yield, which was 2.5% higher than with local control treatment. In contrast, based on the pooled means of 2016–2017 across locations in Pakistan, the grain yield was non-significantly affected by F-Zn+I and F-CT treatments, with decreases of only 2.1% and 1.1%, respectively, as compared with the local control (LC). Based on the pooled analysis, the local check variety of India, i.e., HD 2967, produced the highest grain yield. The grain yield of HarvestPlus-genotype PBW-1-Zn was similar to HD 2967, but HPBW 10 had the lowest grain yield. In Pakistan, Zincol-2016 and NR-488 produced 17.5% and 18.4% less grain yield than the local check variety Faisalabad-2008. Based on the pooled analysis across all field locations in India and Pakistan in 2016–2017, interaction between genotypes and treatments was non-significant. A slight reduction in grain yield, i.e., 3.1% with F-CT and 4.8% with F-Zn+I, was recorded across the genotypes and countries.
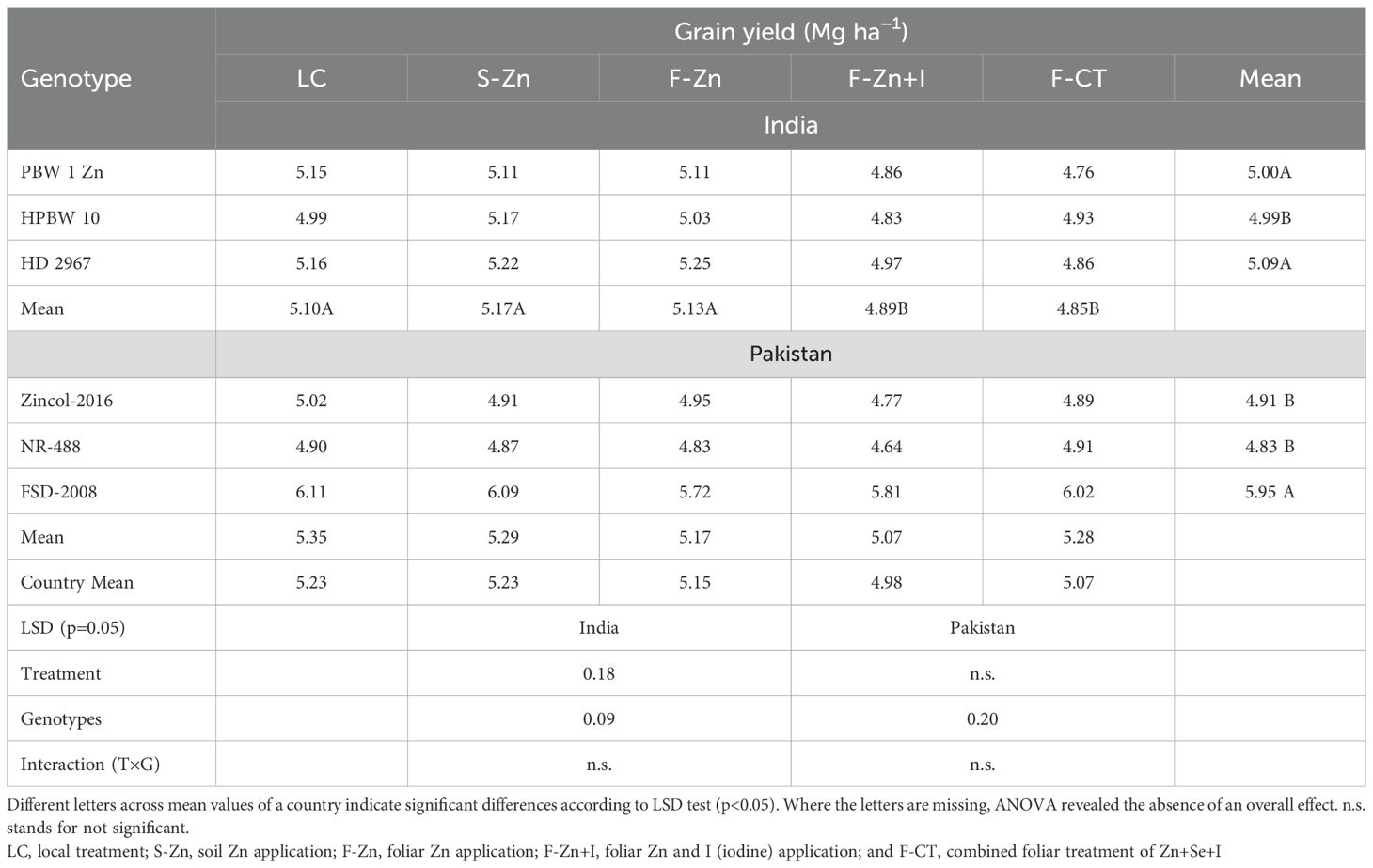
Table 4. Pooled means of grain yield across field locations and years in India and Pakistan in 2016–2017.
3.2 Grain zinc concentration
The zinc concentration in wheat grains differed significantly among the micronutrient treatments at all field locations during both years in India and Pakistan, except at Bathinda in India during 2016–2017 where it varied non-significantly (Table 5). In India during both years, the grain Zn concentration was consistently highest with the application of F-CT at all field locations; however, all the foliar Zn treatments (F-Zn, F-Zn+I, and F-CT) were statistically at par with each other at Ludhiana. At Gurdaspur during 2015–2016, the grain Zn concentration with F-CT treatment was more than double of the recorded value with local control treatment. In India, at Gurdaspur and Bathinda during 2015–2016 and at Bathinda during 2016–2017, soil application of Zn did not improve the grain Zn concentration as compared with control. In Pakistan during 2015–2016, F-Zn treatment resulted in the highest grain Zn concentration at Faisalabad (51.7 mg kg−1), which was significantly higher than the Zn concentrations obtained with local control and S-Zn treatments. Foliar application of cocktail (F-CT) also resulted in higher grain Zn concentration than local control and S-Zn treatments. At the same field site in Pakistan, during 2016–2017, application of F-Zn and F-Zn+I resulted in significantly higher grain Zn concentrations than the local control as well as S-Zn and F-CT treatments. Except for Faisalabad during 2015–2016, soil Zn application did not improve grain Zn concentration as compared with local control.
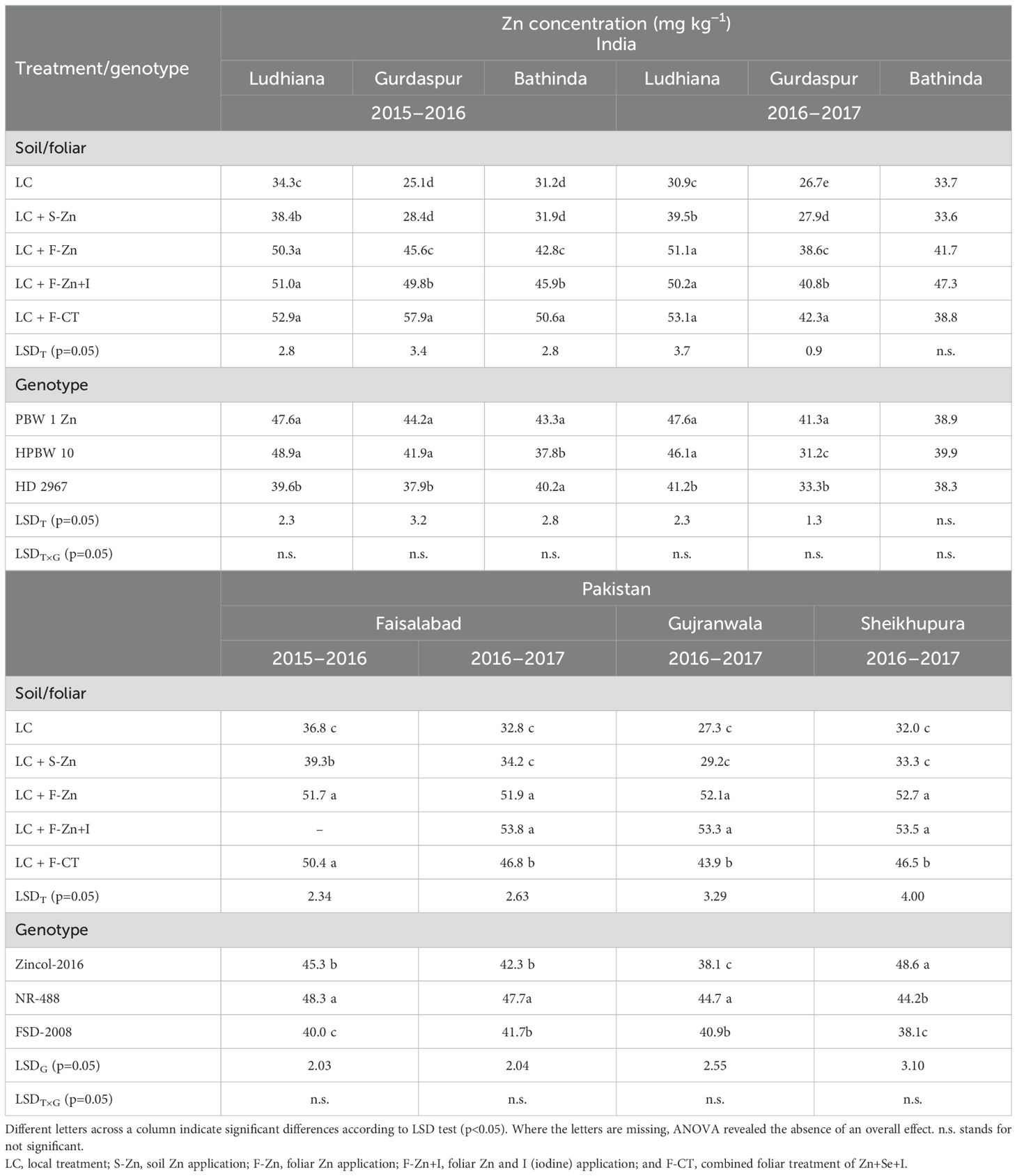
Table 5. Effect of micronutrient treatments on grain Zn concentrations of different wheat genotypes grown at various locations in India and Pakistan.
In India, the studied wheat genotypes differed significantly with respect to grain Zn concentration at all locations, except at Bathinda in 2016–2017. The maximum grain Zn concentration was generally recorded in the HarvestPlus-biofortified genotype PBW-1-Zn, which was significantly higher than local check variety HD 2967 at all the locations, except at Bathinda where the Zn concentration was similar in both the varieties. Among wheat genotypes studied in Pakistan, the highest grain Zn concentration was found in the HarvestPlus-biofortified genotype NR-488 at Faisalabad during 2015–16 and at Faisalabad and Gujranwala during 2016–2017, and it was significantly higher than Zincol-2016 and the local check Faisalabad-2008. In Sheikhupura during 2016–2017, Zincol-16 had the highest grain Zn concentration among the genotypes tested.
Based on the pooled analysis across locations and years in India, F-CT resulted in the highest grain Zn concentration (49.3 mg kg−1), which was statistically at par with F-Zn+I (Table 6). Compared with Zn concentrations recorded with local control treatment, F-Zn resulted in 48.5% higher Zn concentration, F-Zn+I in 56.8% higher concentration, and F-CT in 62.7% higher Zn concentration. In Pakistan, on average, the highest increase in grain Zn concentration (43%) was recorded with F-Zn+I and the lowest increase was observed with soil Zn application. The grain Zn concentration found with F-Zn+I was similar to that with F-Zn, but significantly higher than the rest of the treatments.
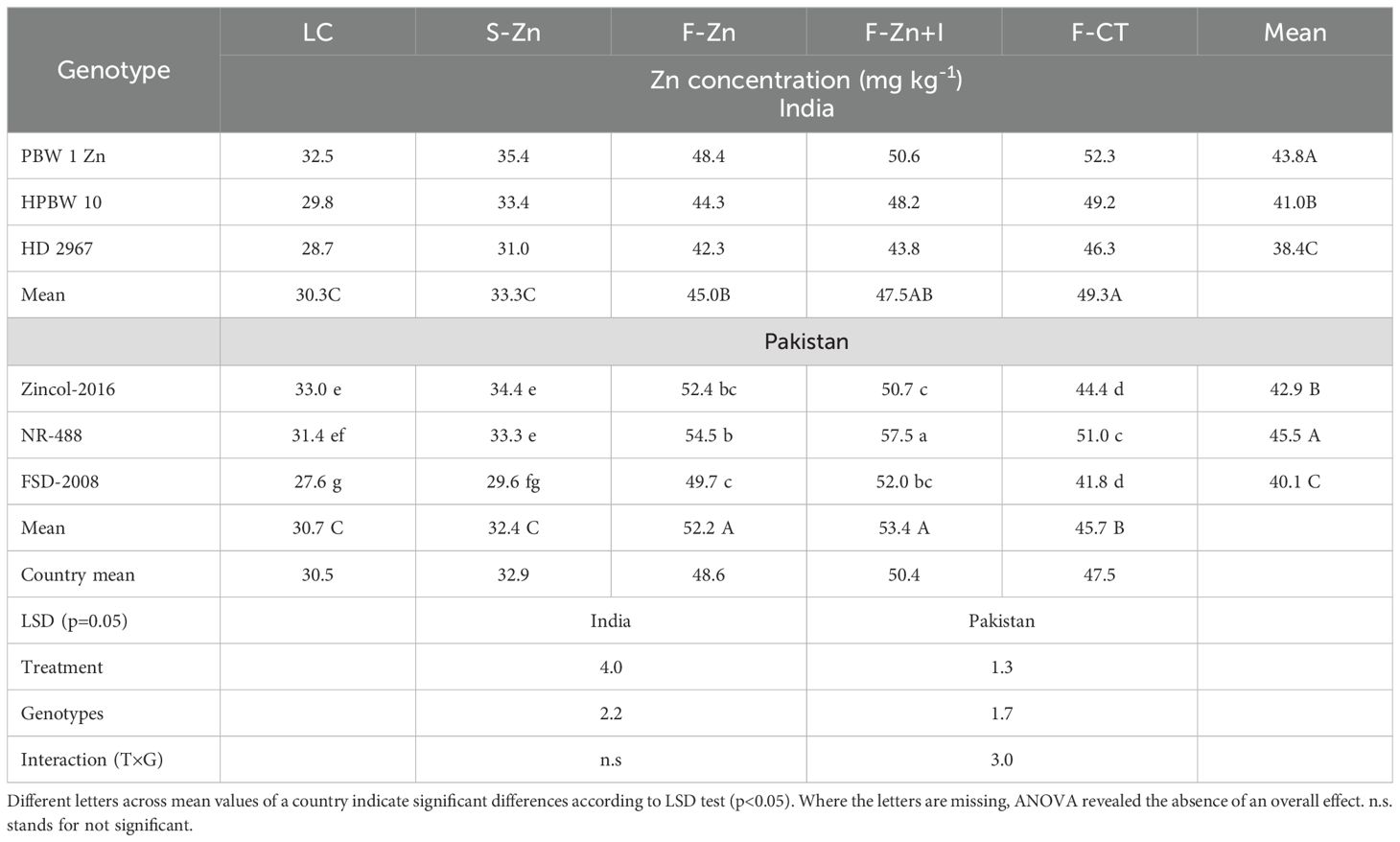
Table 6. Pooled means of grain zinc concentration in wheat genotypes across locations in India and Pakistan in 2016–2017.
Among all wheat genotypes in India, the genotype PBW-1-Zn exhibited the highest grain Zn concentration, which was statistically higher than the grain Zn concentration of HPBW 10 and HD 2967 genotypes. PBW-1-Zn had 14.1% higher Zn and HPBW 10 had 7.5% higher grain Zn concentrations, compared with the grain Zn concentration of the local check cultivar (i.e., HD 2967) (p=0.05). Similarly, HarvestPlus-developed high-Zn genotypes NR-488 and Zincol-2016 had significantly higher grain Zn than the local check cv. Faisalabad-2008 in Pakistan. The effect of the Zn treatments on grain Zn concentration was cultivar-specific in Pakistan. In the HarvestPlus-biofortified genotype NR-488, the enhancement in grain Zn concentration was maximum with F-Zn+I application and was significantly higher than that recorded for the HarvestPlus-biofortified genotype Zincol-2016 and local check cv. Faisalabad-2008 under the same treatment. Enhancement in grain Zn concentration with foliar Zn, as F-Zn or F-Zn+I, was higher in the HarvestPlus-biofortified genotypes than the local check variety. The measured grain Zn concentrations were close to each other for the F-Zn treatment (i.e., 48.6 mg kg−1), F-Zn+I (i.e., 50.4 mg kg−1), and F-CT (i.e., 47.5 mg kg−1) in the mean data from both countries.
3.3 Grain iron concentration
Although Fe was not applied, there was a significant variation in grain Fe concentrations among the experimental treatments at all field locations in India, except at Bathinda during 2016–2017 (Table 7). The highest grain Fe concentration was recorded with the F-CT treatment at all field locations during 2015–2016 and at Ludhiana during 2016–2017. At Gurdaspur, the grain Fe concentrations with the three foliar treatments (F-CT, F-Zn, and F-Zn+I) were statistically similar, and all were significantly higher than the control and S-Zn treatments. The grain Fe concentration also differed significantly among wheat genotypes across field locations and years, except at Gurdaspur (2015–2016) and Bathinda (2016–2017) locations in India. During 2015–2016, at Ludhiana the genotype PBW-1-Zn had significantly higher grain Fe concentration compared with rest of the genotypes. However, at Bathinda (India), the grain Fe concentration of genotype PBW-1-Zn was significantly higher compared with genotype HPBW 10, but was similar to HD 2967. During 2016–2017 at Ludhiana (India), the grain Fe concentrations in PBW-1-Zn (30.0 mg kg−1) and HPBW 10 were statistically similar, and higher than that of HD 2967. At Gurdaspur in India during 2016–2017, the grain Fe concentration in PBW-1-Zn (33.8 mg kg−1) was higher than the other two genotypes.
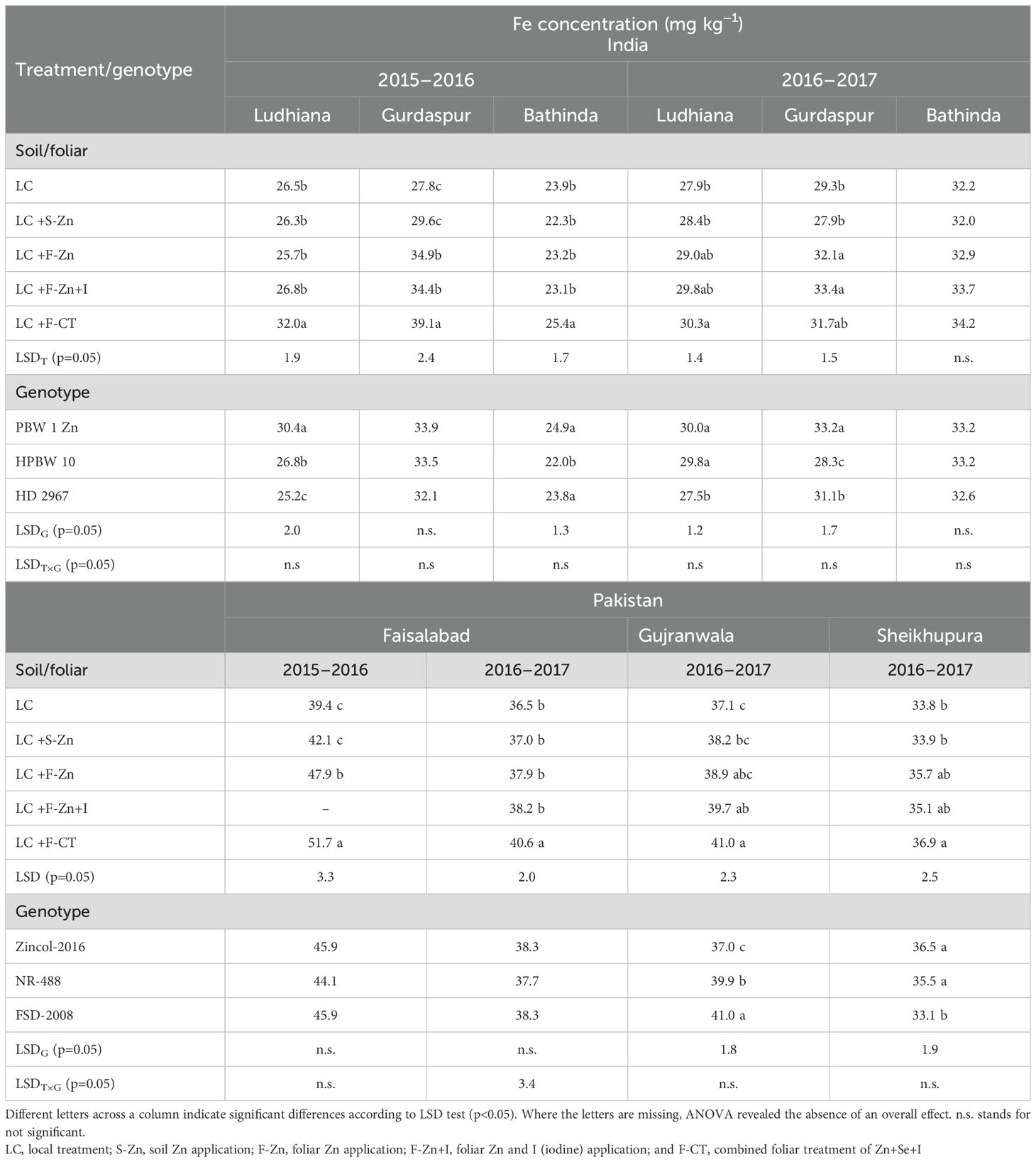
Table 7. Effect of micronutrient treatments on grain Fe concentrations of various wheat genotypes grown at different locations of India and Pakistan.
In Pakistan, F-CT consistently resulted in the highest grain Fe concentration among the treatments; however, the concentration values differed non-significantly with the other foliar treatments (F-Zn and F-Zn+I) at Gujranwala and Sheikhupura during 2016–2017 (Table 7). At Faisalabad during both the years, the grain Fe concentration differed non-significantly among the tested genotypes. During 2016–2017, the grain Fe concentrations of HarvestPlus high-Zn wheat genotypes (Zincol-2016 and NR-488) were statistically lower than standard check Faisalabad-2008 at Gujranwala, whereas a reverse was found true at Sheikhupura.
Pooled means of grain Fe concentrations revealed that F-CT and F-Zn+I treatments in India and F-CT, F-Zn+I, and F-Zn treatments in Pakistan significantly enhanced the grain Fe concentration (Table 8). In Pakistan, F-CT treatment resulted in the highest (19%) increase in grain Fe concentration than the local control treatment. Pooled analysis of grain Fe concentrations also revealed that all genotypes in India contained similar grain Fe concentrations. In Pakistan, genotype NR-488 had the highest grain Fe concentration with F-CT than other soil/foliar treatments. F-CT recorded 3.9 mg kg−1 higher grain Fe than the local control treatment.
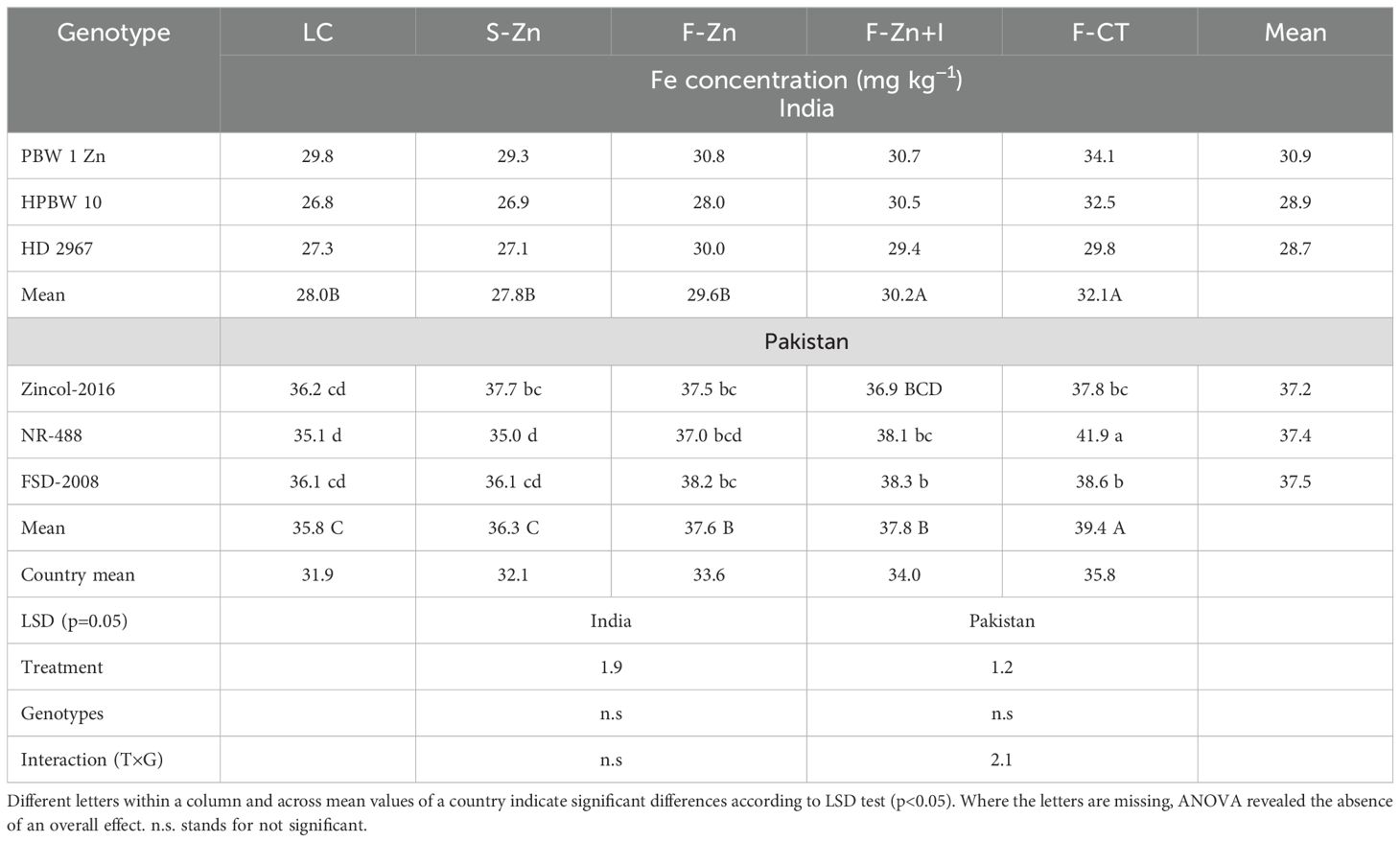
Table 8. Pooled means of grain Fe concentration in wheat grains across locations during both years in India and Pakistan in 2016–2017.
3.4 Grain iodine
The wheat grain I concentration substantially increased with all micronutrient treatments containing I, in both the countries during both the years (Table 9). As expected, the lowest grain I concentrations were found with the control treatment (LC). The F-CT and/or F-Zn+I treatments resulted in significantly higher grain I concentration than the local control treatment at all field locations during both the years. During 2016–2017, at Gurdaspur and Bathinda in India and at Sheikhupura in Pakistan, grain I concentrations with F-CT and F-Zn+I were statistically at par with each other.
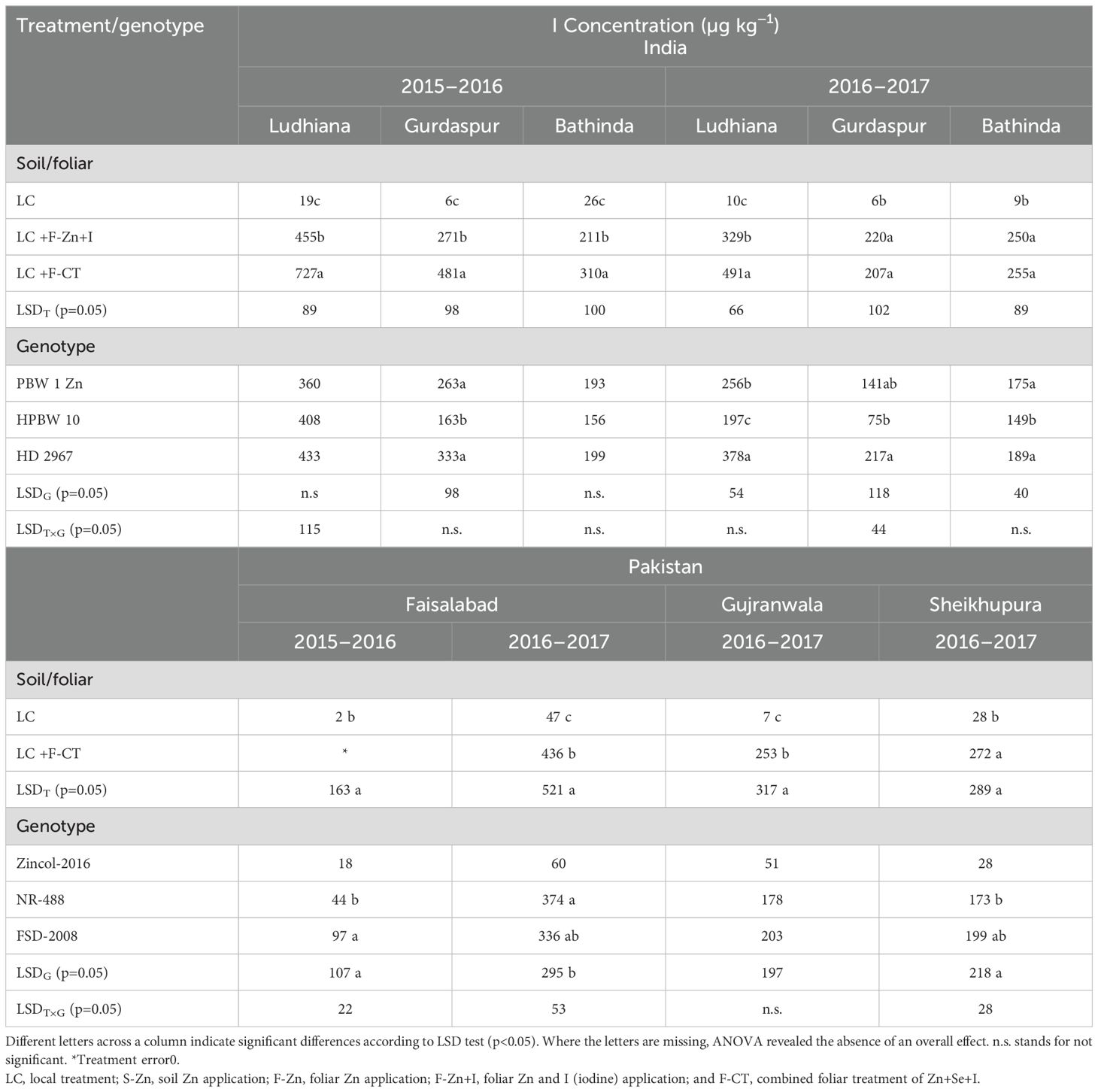
Table 9. Effect of micronutrient treatments on grain I concentration of various wheat genotypes grown at different locations of India and Pakistan.
In India, the highest grain I concentration was recorded in local check genotype HD 2967 which was significantly higher than the HarvestPlus-biofortified genotypes PBW-1-Zn and HPBW 10 in Gurdaspur during 2015–2016 and at all locations during 2016–2017. HarvestPlus wheat genotype NR-488 and local check variety Faisalabad-2008 had similar grain I concentrations across the locations. At Faisalabad during 2015–2016, the genotype NR-488 and local check cv. Faisalabad-2008 contained substantially higher I concentrations in grains than Zincol-2016.
Overall, among the treatments, the highest grain I concentration across all field locations in both countries was found with the F-CT treatment (Table 10). All experimental treatments markedly increased the grain I concentration, compared with the local control, at all field locations; however, the magnitude of increase was cultivar- and location-specific (Table 9). Among the genotypes studied, the highest I concentration was recorded with F-CT treatment in the genotypes PBW-1- Zn in India and Zincol-2016 in Pakistan, which was significantly higher than I concentration in HPBW 10 (India) and NR-488 (Pakistan), respectively.
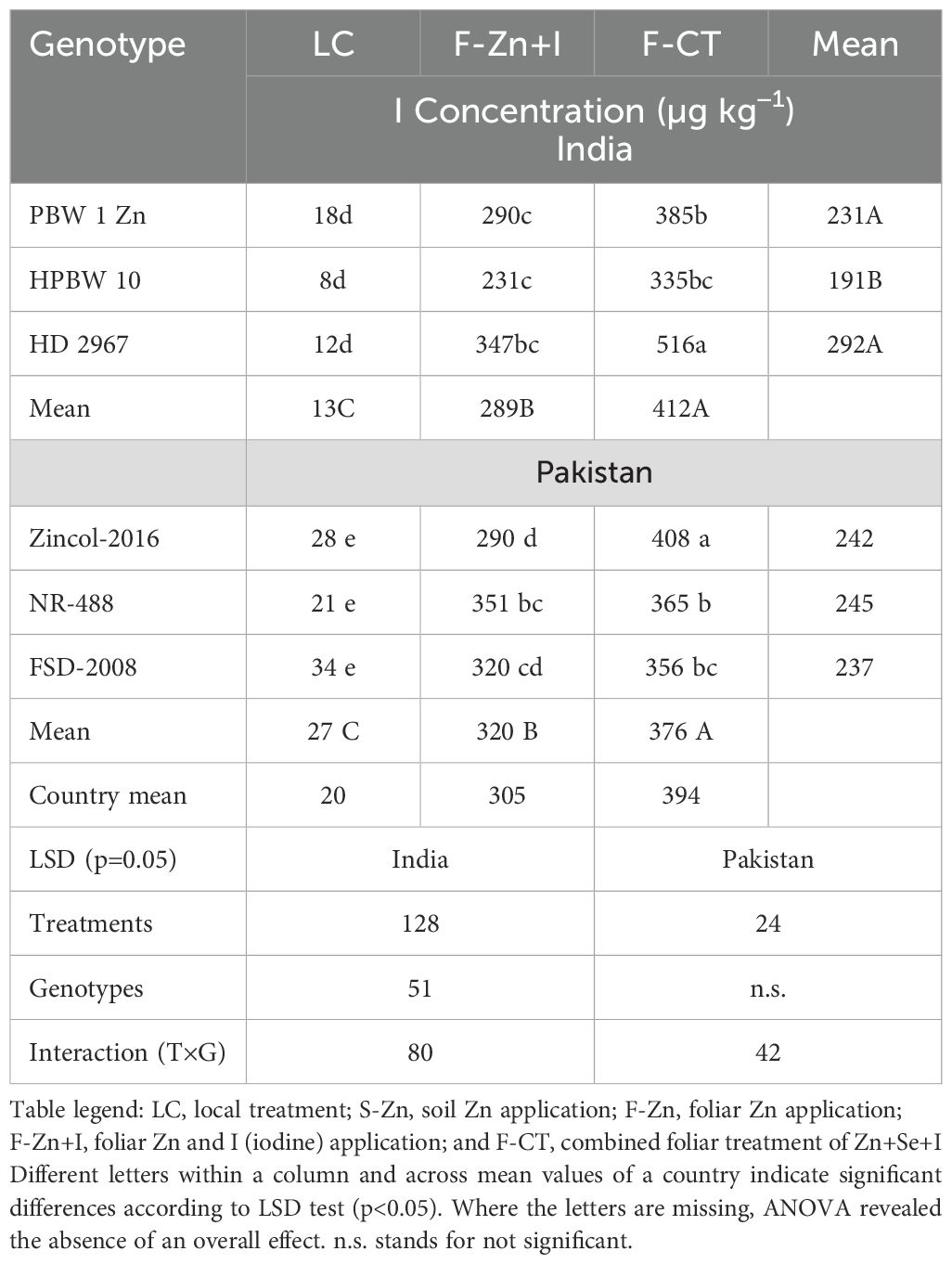
Table 10. Pooled means of wheat grain I concentration across locations and years in India and in Pakistan in 2016–2017.
3.5 Grain selenium
Grain Se concentrations of wheat genotypes increased significantly with the F-CT treatment at all field locations both in India and in Pakistan (Table 11). During 2015–2016, wheat genotypes did not differ significantly in grain Se concentration in India. However, during 2016–2017, genotype PBW-1-Zn and HPBW 10 had significantly higher grain Se concentrations than the local check variety HD 2967. During 2015–2016 at Faisalabad, the local check cv. Faisalabad-2008 exhibited the highest Se whereas during 2016–2017, the genotypes differed non-significantly at this location. At Gujranwala, genotypes Zincol-2016 and NR-488 had higher grain Se concentrations than the local check cv. Faisalabad-2008, whereas NR-488 had the highest grain Se concentration among the genotypes. On average, the F-CT increased Se concentration in wheat grains by 106% in India and 123% in Pakistan (Table 12). Genotype response to Se micronutrient was similar in India, but F-CT treatment increased the Se concentration in grains of genotype NR-488 higher than the rest of the genotypes in Pakistan. Overall, F-CT showed 112.8% higher grain Se than local control treatment across India and Pakistan.
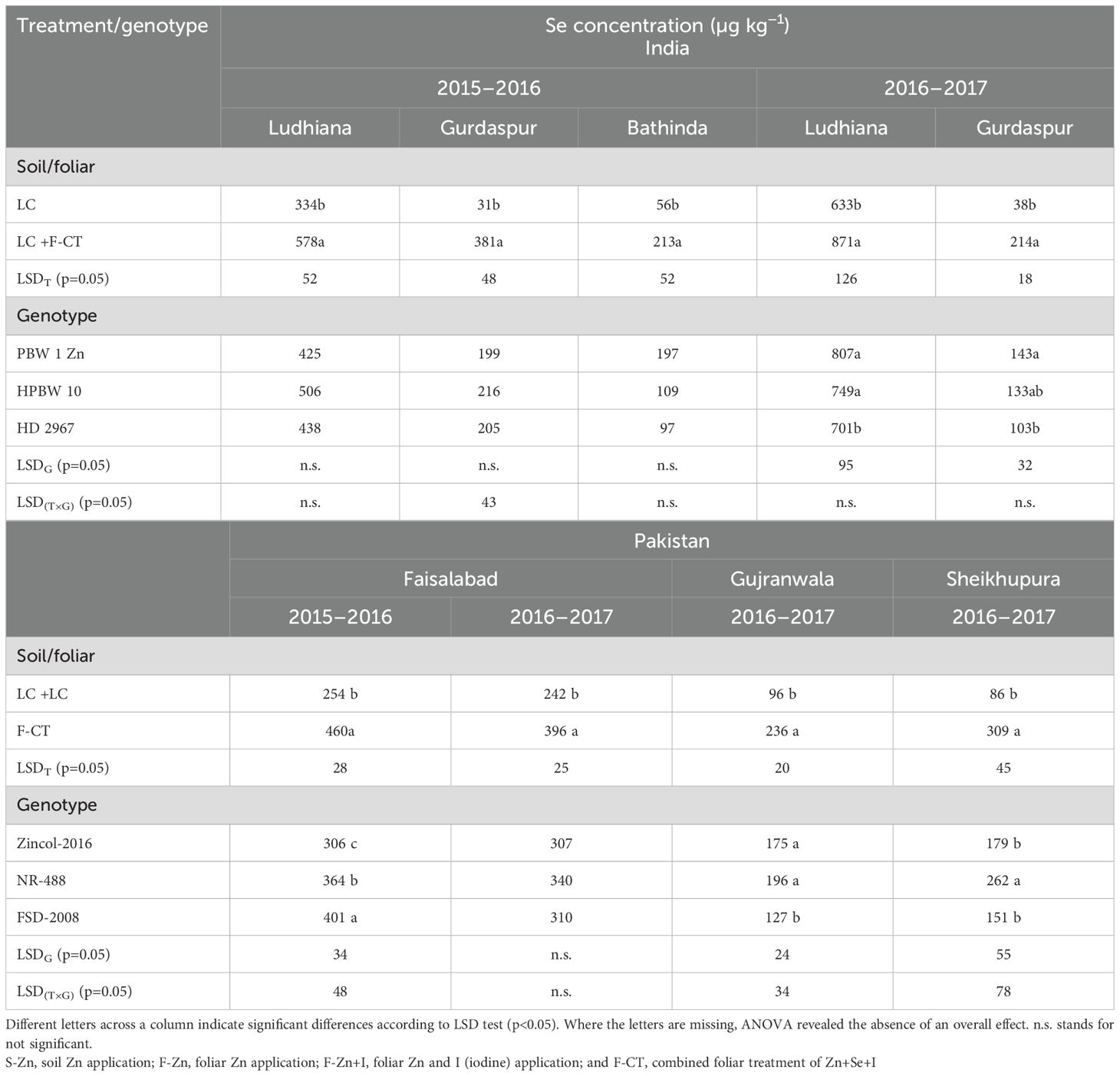
Table 11. Effect of micronutrient treatments on grain Se concentrations of various wheat genotypes grown at different locations of India and Pakistan.
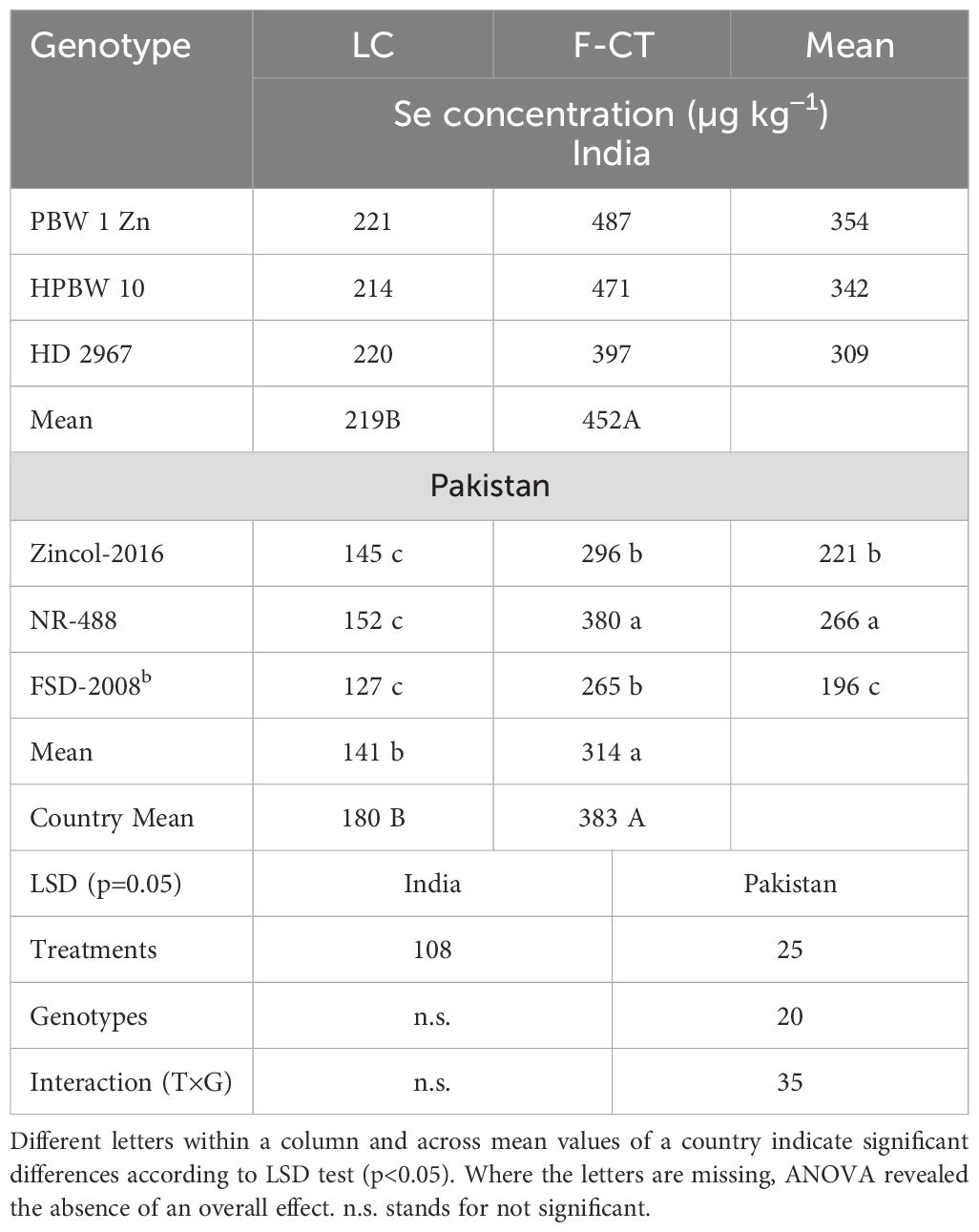
Table 12. Pooled means of wheat grain Se concentration across locations and years in India and Pakistan in 2016-17.
4 Discussion
4.1 Grain yield
Micronutrient malnutrition in developing countries can be alleviated by cultivation of micronutrient-biofortified staple foods. In the present study, 2.6% higher grain yield in India with soil-applied Zn than with local control treatment (Table 3) is attributed to a positive impact of Zn fertilization on the wheat crop when soils are marginally supplied with Zn, as shown before (Ram et al., 2015; Cakmak, 2009; Zou et al., 2012). According to several earlier studies, wheat grain yield can also be increased by foliar application of Zn as well, but most commonly only in soils having very low plant available Zn, such as soils having DTPA-Zn ≤ 0.2 mg kg−1 (Graham et al., 1992; Cakmak et al., 1999; Cakmak, 2009; Ram et al., 2015). A slight reduction in wheat grain yield with F-Zn+I or F-CT treatments, across different field locations, might have occurred, possibly due to I toxicity to wheat. Although I is not an essential nutrient for crop plants, it might be beneficial to plant growth, depending on the soil and environmental conditions (Kiferle et al., 2021; Ma et al., 2023). The use of I in nutrient solution in soilless culture (equivalent to 0.13 mg L−1) produced higher biomass of leafy vegetables, such as Chinese cabbage, spinach, and lettuce (Zhu et al., 2003; Dai et al., 2004; Blasco et al., 2013). The source of I for foliar spays is also quite important. A higher sensitivity of plants to high doses of soil-applied KI, compared with soil-applied KIO3, was observed in rice (Kato et al., 2013), lettuce (Lawson et al., 2015), and strawberry (Li et al., 2017), and KIO3 is considered a better source of I for foliar feeding (Cakmak et al., 2017).
The reason for the lower yield performance of the HarvestPlus-developed wheat genotypes compared with the local check variety observed at Gurdaspur in India during 2015–16 could not be determined. One explanation could be related to a possible lower resistance of the tested HarvestPlus wheat lines to leaf rust in this region. Due to its higher humidity and proximity to the Shiwalik Hills, Gurdaspur, is considered as a hot spot for wheat leaf rust. At Bathinda in India, the similar performance of all wheat genotypes might be due to a lack of leaf rust incidence, because this field location had less relative humidity. In Pakistan, in addition to lower yield potential of the HarvestPlus-developed high-Zn wheat genotypes, compared with local check cv. Faisalabad-2008, the HarvestPlus-developed wheat lines (in particular NR-488) were observed to be susceptible to fungal disease loose smut at all three field locations (data not shown).
4.2 Grain zinc
Generally, soil application of Zn (S-Zn treatment) resulted in either no or minimal increases in grain Zn concentrations of the tested genotypes in India and Pakistan. Marked increases in grain Zn concentration were, however, recorded with the foliar application of F-Zn, F-Zn+I, and F-CT treatments across all field locations in both the countries (Tables 5, 6). A higher grain Zn concentration might result from more effective translocation of foliar-applied Zn to grains, than the soil-applied Zn. Similar effects of foliar Zn feeding on wheat and rice grain Zn were also reported in earlier field studies across the world (Zou et al., 2012; Ram et al., 2015; Prom-u-thai et al., 2020; Stangoulis and Knez, 2022). Zinc exhibits at least a moderate phloem mobility in plants, so that sufficient foliar supply of Zn at appropriate crop growth stages could significantly increase the grain Zn concentration in crop plants to the desired levels (Erenoglu et al., 2011; Gupta et al., 2016; Cakmak and Kutman, 2018; Kutman, 2023). In wheat and rice, it has been shown that significant increases in grain Zn by foliar Zn application occur if Zn spray takes place at a later, rather than an earlier, growth stage (i.e., during grain-filling period). Maintaining a high pool of available Zn during the seed-filling period, for example through foliar Zn spray, is, therefore, critical to achieving successful enrichments of grains with Zn (Cakmak and Kutman, 2018)
We did not observe an antagonism for micronutrient accumulation in wheat grains when micronutrients are applied together (i.e., F-Zn+I and F-CT). However, check cv. Faisalabad-2008 and HarvestPlus-developed high Zn cv. Zincol-2016 in Pakistan had statistically similar grain Zn concentrations under foliar Zn treatments (F-Zn, F-Zn+I, and F-CT), which reached higher levels than the target value. This highlights the importance of foliar Zn fertilization and demonstrates that the target value of grain concentration could be achieved in the normal wheat cultivar by foliar Zn fertilization. This is of particular importance in the case of growing wheat in cadmium (Cd)- and lead (Pb)-contaminated soils wherein HarvestPlus-developed cv. Zincol-2016 had been reported to accumulate more Cd/Pb in grains than the check cv. Faisalabad-2008 (Hussain et al., 2019). However, the experiment conducted by Hussain et al. (2019) was a pot experiment in which an extremely high Cd application rate was used, raising Cd levels well beyond those typically found in any cultivated soil. When both genotypes were grown in a normal field soil, both genotypes had low and comparable Cd concentrations.
In India, the grain Zn concentration of HarvestPlus-genotype PBW-1-Zn was 6.4 mg kg−1 higher than the local check cv. HD 2967. In Pakistan, grains of all HarvestPlus-developed genotypes contained higher grain Zn than local check cv. Faisalabad-2008. This was likely due to the inherent capacity of the HarvestPlus wheat genotypes to accumulate more Zn in grains than the local Pakistan check genotype. Govindan et al. (2012) reported that a number of genotypes developed under HarvestPlus breeding programs are available with required target of Zn concentrations over 40 mg kg−1, indicating that it is possible to develop Zn-biofortified varieties with better agronomic traits and grain yield. They also found a positive correlation between Zn and Fe concentration in wheat grains, which suggested a good scope for simultaneous enrichment of both the micronutrients in this staple cereal.
Khokhar et al. (2018) observed that inherent wheat grain Zn concentration variability in different genotypes was between 24.9 mg kg−1 to 34.8 mg kg−1. The variability in Zn concentration of wheat grains observed in our studies is consistent with the earlier observations of Zhao et al. (2009) who reported that the grain Zn concentration in 150 bread wheat genotypes varied from 14 mg kg−1 to 35 mg kg−1. Similarly, Morgounov et al. (2007) recorded a wide range of grain Zn concentrations (i.e., 20 mg kg−1–39 mg kg−1) among 66 wheat genotypes selected from Central Asian Breeding Programs, and grown in Kazakhstan, Kyrgyzstan, and Tajikistan. Compared with the respective check cultivars, increases in grain Zn with F-Zn treatment were higher in both HarvestPlus-developed wheat genotypes in India, but only in NR-488 in Pakistan, indicating synergism between the genetic and agronomic biofortification strategies.
HarvestPlus-developed Zn-biofortified wheat cultivars could significantly contribute to enhancing daily Zn intake requirements. For example, in a very recent study, Lowe et al. (2021) reported that based on an average flour consumption of 224 g day−1 for 4 weeks, Zincol-2016 provided an additional daily Zn intake of between 3.0 mg and 6.0 mg for white and whole grain flour, respectively. This resulted in a significant increase, a mean difference of 41.5 μg L−1, in plasma Zn concentration. Regular consumption of Zincol-2016 flour increased the daily Zn intake of women of reproductive age by 30%–60%. In Pakistan, the HarvestPlus program also released a new and more promising wheat cultivar Akbar-2019, characterized by much higher grain Zn concentration, high grain yield, and better yield stability (Virk et al., 2021; Wani et al., 2022; and see also www.harvestplus.org/countries/pakistan). It would be interesting to include Akbar-2019 in future agronomic biofortification programs to study its response to soil and foliar application of micronutrients.
4.3 Grain iron
Among the applied treatments, the highest grain Fe concentration was recorded with the application of F-CT across all field locations during both years, revealing that foliar cocktail containing Zn, I, and Se fertilizers was effective in enriching wheat grains with Fe as well (Tables 7, 8). Pooled analysis of the data revealed that F-CT treatment led to 19.0% higher grain Fe concentration in India and 16.2% higher Fe concentration in Pakistan, than the respective local control treatments. Even F-Zn and F-Zn+I treatments also resulted in significantly higher grain Fe concentrations. In earlier foliar Zn feeding experiments, Zn application had also shown significant positive effects on grain Fe concentrations in bran and embryo parts of wheat grain, which were not related to any Fe contamination of grain samples through soil dusts or particles (Kutman et al., 2011; Li et al., 2016; Zou et al., 2019). This might have resulted from foliar-applied Zn-induced increase in Fe translocation from shoots to grains. Previously, Kutman et al. (2011) suggested that marked increases in grain Zn associated with foliar Zn spray probably induced production of Zn binding compounds in grain, which possibly act as a sink for Fe translocation and deposition in the grains. It is known that the gene GPC-1 affecting grain protein concentration simultaneously improves Zn and Fe concentrations in grain (Distelfeld et al., 2007). Strongly positive correlations between Zn and Fe in cereal grains have been repeatedly reported in the previous studies (Cakmak et al., 2004; Peleg et al., 2008; Gomez-Becerra et al., 2010; Swamy et al., 2016; Stangoulis and Knez, 2022). These findings indicate that there are probably common genetic and physiological factors affecting gran deposition of Zn and Fe in wheat.
4.4 Grain iodine
Iodine is an essentially required micronutrient for humans, and the incidence of widespread I deficiency disorders (IDDs) has been closely studied for decades (Hatch-McChesney and Lieberman, 2022). Use of iodized common salt is an effective strategy to combat I malnutrition. However, many social and scientific factors, such as reduced use of salt because of its adverse effects on health, lack of monitoring of I content in the salts, and stability of I in the iodized salts, have led to a failure of this strategy in effectively mitigating I deficiency in human populations (Diosady et al., 1998; Hatch-McChesney and Lieberman, 2022; Nista et al., 2022). In this scenario, the provision of I-enriched staple cereals, such as wheat, appears to be a more effective and sustainable strategy to address I malnutrition.
In the present study, foliar I-application led to substantial increases in grain I concentration (Tables 9, 10). In Pakistan, F-CT treatment led to a 1,420-fold increase in grain I concentration. HarvestPlus-developed genotype PBW-1-Zn and cv. HD 2967 in India and genotype NR-488 and cv. Faisalabad-2008 in Pakistan accumulated significantly higher grain I concentrations compared with the rest of the genotypes in both the countries. Similar marked increases in the I concentrations of food crops through application of I fertilizers were also shown in rice (Prom-u-thai et al., 2020; Naeem et al., 2022), maize (Xue et al., 2023), and fruits of tomato (Landini et al., 2011; Kiferle et al., 2013) and pepper (Li et al., 2017). Very likely, these substantial enrichments were the consequence of effective translocation of foliar-applied I from wheat leaves to the grains. However, it could also be partially related to a direct contamination of florets and seeds through late season I sprays (Cakmak et al., 2017). In lettuce plants, foliar-sprayed I was effectively translocated to plant roots (Smoleń et al., 2014).
4.5 Grain selenium
Selenium is a further critical micronutrient that is essential for humans (Rayman, 2012; Lyons et al., 2005; Jones et al., 2017). There are also some published data indicating importance of Se for crop plants (Ma et al., 2023). In our studies, F-CT treatment enhanced the Se concentration in wheat grains by almost twofold (Tables 11, 12). Thus, similar to Zn enrichment of wheat grains, foliar-applied Se enhanced its accumulation in wheat grains. Slight variations in Se accumulation were observed in different wheat genotypes, but, overall, Se enrichment was almost uniform among the tested wheat genotypes. Similar significant increases in grain Se concentrations have been shown in various staple food crops (Curtin et al., 2006; Chilimba et al., 2012; Mao et al., 2014; Prom-u-thai et al., 2020).
It is interesting to note that in contrast to Zn and Fe (Cakmak et al., 2010), Se is more or less evenly distributed within the grain fractions (Lyons et al., 2005). Zinc and Fe are predominantly localized in the aleurone and embryo parts of wheat grains (Cakmak et al., 2010). However, Rodrigo et al. (2014) showed that only 15% of total grain Se was found in the bran (including seed coat and aleurone) and germ whereas the remaining part of Se was found in white flour, which is the most widely consumed grain part. Also, Lyons et al. (2005) showed that up to 95% of the total Se in wheat grain is present in white flour, meaning that those consuming primarily white flour can also benefit from Se biofortification of wheat grain.
Mao et al. (2014) observed similar enrichments of food grains with Se, Zn, and I when these micronutrients were applied singly or simultaneously as a cocktail. Thus, they advocated that agronomic biofortification of various food crops with Zn, Se, and I can be an effective approach to enrich their edible parts with these micronutrients; however, the effectiveness of biofortification varies with crop species, fertilizer form, and application method of micronutrients.
5 Conclusion
This study revealed that foliar applications of Zn, I, and Se at earing and early milk development growth stages are effective in enriching wheat grains with these micronutrients as well as with Fe. Agronomically biofortified wheat grains containing high concentrations of these micronutrients could contribute to the fight against a host of health problems in populations suffering with micronutrient malnutrition. Cereal-based foods made from biofortified grains obtained through the application of micronutrient fertilizers will contain nutritionally significant amounts of micronutrients with high levels of bioaccessibility, demonstrating an effective transfer of Zn, Se, and I from the field to the grain and, ultimately, to the end product. The biofortified wheat genotypes in India were able to compete for grain yield with local check variety HD 2967; however, Zincol-2016 and NR-488 gave lower yields than local check variety Faisalabad-2008. However, in Pakistan, the newly released Akbar-2019 appears to be a much more promising Zn-biofortified wheat with higher Zn and higher yield than well-known local wheat varieties (Ahmad et al., 2019). As shown with high-Zn genotypes PBW-1-Zn in India and NR-488 in Pakistan, foliar Zn spray to Zn-biofortified genotypes provides additional increments in grain Zn, of more than 15 mg per kg grain. Thus, combining agronomic and genetic strategies will raise grain Zn concentrations to over 50 mg kg−1. This demonstrates the potential of combining plant genetic- and fertilizer-based approaches to create additive and synergistic impacts for the accumulation of wheat grain Zn, at desirable amounts for human nutrition.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
IC: Writing – original draft, Writing – review & editing. HR: Writing – original draft, Writing – review & editing. AN: Writing – review & editing. AR: Writing – review & editing. CK: Writing – review & editing. MYA: Writing – review & editing. SM: Writing – review & editing. MA: Writing – review & editing. GM: Writing – review & editing. AY: Writing – review & editing. YT: Writing – review & editing. VG: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the HarvestPlus Program (www.harvestplus.org) and the sponsors of the HarvestPlus Zinc Fertilizer Project Phase-III (www.harvestzinc.org).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, J., Anwar, J., Owais, M., Tanveer, M. H., Javed, A., Nadeem, M., et al. (2020). Akbar-2019: a new high yielding and rust resistant bread wheat variety for irrigated areas of Punjab, Pakistan. J. Agricul. Res. 58(4), 221–227.
Bailey, R. L., West, K. P., Jr., Black, R. E. (2015). The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab 66, 22–33. doi: 10.1159/000371618
Blasco, B., Leyva, R., Romero, L., Ruiz, J. M. (2013). Iodine effects on phenolic metabolism in lettuce plants under salt stress. J. Agric. Food Chem 61, 2591–2596. doi: 10.1021/jf303917n
Bouis, H. E., Saltzman, A. (2017). Improving nutrition through biofortification: a review of evidence from HarvestPlus 2003 through 2016. Glob. Food Sec 12, 49–58. doi: 10.1016/j.gfs.2017.01.009
Broadley, M. R., White, P. J., Bryson, R. J., Meacham, M. C., Bowen, H. C., Johnson, S. E., et al. (2006). Biofortification of UK food crops with selenium. Proc. Nutr. Soc 65, 169–181. doi: 10.1079/pns2006490
Budke, C., Mühling, K. H., Daum, D. (2020). Iodine uptake and translocation in apple trees grown under protected cultivation. J. Plant Nutr. Soil Sci 183, 468–481. doi: 10.1002/jpln.202000099
Cakmak, I. (2008). Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302, 1–17. doi: 10.1007/s11104-007-9466-3
Cakmak, I. (2009). Enrichment of fertilizers with zinc: An excellent investment for humanity and crop production in India. J. Trace Elem. Med. Biol 23, 281–289. doi: 10.1016/j.jtemb.2009.05.002
Cakmak, I., Kalayci, M., Ekiz, H., Braun, H. J., Yilmaz, A. (1999). Zinc deficiency as an actual problem in plant and human nutrition in Turkey: A NATO-Science for Stability Project. Field Crops Res 60, 175–188. doi: 10.1016/S0378-4290(98)00139-7
Cakmak, I., Kalayci, M., Kaya, Y., Torun, A. A., Aydin, N., Wang, Y., et al. (2010). Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem 58, 9092–9102. doi: 10.1021/jf101197h
Cakmak, I., Kutman, U.Á. (2018). Agronomic biofortification of cereals with zinc: a review. Eur. J. Soil Sci 69, 172–180. doi: 10.1111/ejss.12437
Cakmak, I., Marzorati, M., Van den Abbeele, P., Hora, K., Holwerda, H. T., Yazici, M. A., et al. (2020). Fate and bioaccessibility of iodine in food prepared from agronomically biofortified wheat and rice and impact of cofertilization with zinc and selenium. J. Agric. Food Chem 68, 1525–1535. doi: 10.1021/acs.jafc.9b05912
Cakmak, I., Prom-u-thai, C., Guiherme, L. R. G., Rashid, A., Hora, K. H., Yazici, A., et al. (2017). Iodine biofortification of wheat, rice and maize through fertilizer strategy. Plant Soil 418, 319–335. doi: 10.1007/s11104-017-3295-9
Cakmak, I., Torun, A., Millet, E., Feldman, M., Fahima, T., Korol, A. B., et al. (2004). Triticum dicoccoides: an important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci. Plant Nutr 50, 1047–1054. doi: 10.1080/00380768.2004.10408573
Chen, X. P., Zhang, Y. Q., Tong, Y. P., Xue, Y. F., Liu, D. Y., Zhang, W., et al. (2017). Harvesting more grain zinc of wheat for human health. Sci. Rep 7, 7016. doi: 10.1038/s41598-017-07484-2
Chilimba, A. D. C., Young, S. D., Black, C. R., Meacham, M. C., Lammel, J., Broadley, M. R. (2012). Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crop Res 125, 118–128. doi: 10.1016/j.fcr.2011.08.014
Curtin, D., Hanson, R., Lindley, T. N., Butler, R. C. (2006). Selenium concentration in wheat (Triticum aestivum) grain as influenced by method, rate, and timing of sodium selenate application. New Z. J. Crop Hortic. Sci 34, 329–339. doi: 10.1080/01140671.2006.9514423
Dai, J. L., Zhu, Y. G., Zhang, M., Huang, Y. Z. (2004). Selecting iodine-enriched vegetables and the residual effect of iodate application to soil. Biol. Trace Elem. Res 101, 265–276. doi: 10.1385/BTER:101:3:265
Daud, M. F. B., Rempelos, L., Cakmak, I., Leifert, C., Bilsborrow, P. (2024). Increasing grain selenium concentration via genetic and agronomic innovations. Plant Soil 494, 477–496. doi: 10.1007/s11104-023-06293-z
Diosady, L. L., Alberti, J. O., Venkatesh Mannar, M. G., FitzGerald, S. (1998). Stability of iodine in iodized salt used for correction of iodine-deficiency disorders, II. Food Nutr. Bull 19, 240–250. doi: 10.1177/156482659801900306
Distelfeld, A., Cakmak, I., Peleg, Z., Ozturk, L., Yazici, A. M., Budak, H., et al. (2007). Multiple QTL-effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations. Physiol. Plant 129, 635–643. doi: 10.1111/j.1399-3054.2006.00841.x
Dobermann, A., Bruulsema, T., Cakmak, I., Gerard, B., Majumdar, K., McLaughlin, M., et al. (2022). Responsible plant nutrition: A new paradigm to support food system transformation. Glob. Food Secur 33, 100636. doi: 10.1016/j.gfs.2022.100636
Erenoglu, E. B., Kutman, U. B., Ceylan, Y., Yildiz, B., Cakmak, I. (2011). Improved nitrogen nutrition enhances root uptake, root-to-shoot translocation and remobilization of zinc (65Zn) in wheat. New Phytol 189, 438–448. doi: 10.1111/j.1469-8137.2010.03488.x
Fan, M. S., Zhao, F. J., Fairweather-Tait, S. J., Poulton, P. R., Dunham, S. J., McGrath, S. P. (2008). Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol 22, 315–324. doi: 10.1016/j.jtemb.2008.07.002
FAO, IFAD, UNICEF, WFP, WHO (2023). “The State of Food Security and Nutrition in the World 2023,” in Urbanization, agrifood systems transformation and healthy diets across the rural–urban continuum (FAO, Rome). doi: 10.4060/cc3017en
Gomez-Becerra, H. F., Erdem, H., Yazici, A., Tutus, Y., Torun, B., Ozturk, L., et al. (2010). Grain concentrations of protein and mineral nutrients in a large collection of spelt wheat grown under different environments. J. Cereal Sci 52, 342–349. doi: 10.1016/j.jcs.2010.05.003
Gonzali, S., Kiferle, C., Perata, P. (2017). Iodine biofortification of crops: agronomic biofortification, metabolic engineering and iodine bioavailability. Curr. Opin. Biotechnol 44, 16–26. doi: 10.1016/j.copbio.2016.10.004
Govindan, V., Atanda, S., Singh, R. P., Huerta-Espino, J., Crespo-Herrera, L. A., Juliana, P., et al. (2022). Breeding increases grain yield, zinc, and iron, supporting enhanced wheat biofortification. Crop Sci 62, 1912–1925. doi: 10.1002/csc2.20759
Govindan, V., Herrera, L. C., Guzman, C., Huerta, J., Payne, T., Singh, R. P. (2019). Assessing genetic diversity to breed competitive biofortified wheat with enhanced grain Zn and Fe concentrations. Front. Plant Sci 9. doi: 10.3389/fpls.2018.01971
Govindan, V., Singh, R. P., Huerta-Espinoa, J., Pena, R. J., Arun, B., Singh, M. A., et al. (2012). Performance of biofortified spring wheat genotypes in target environments for grain zinc and iron concentrations. Field Crops Res 137, 261–267. doi: 10.1016/j.fcr.2012.07.018
Graham, R. D., Ascher, J. S., Hynes, S. C. (1992). Selecting zinc-efficient cereal genotypes for soils of low zinc status. Plant Soil 146, 241–250. doi: 10.1007/BF00012018
Gupta, N., Ram, H., Kumar, B. (2016). Mechanism of zinc absorption in plants: uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol 15, 89–109. doi: 10.1007/s11157-016-9390-1
Guzmán, C., Medina-Larqué, A. S., Velu, G., González-Santoyo, H., Singh, R. P., Huerta-Espino, J., et al. (2014). Use of wheat genetic resources to develop biofortified wheat with enhanced grain zinc and iron concentrations and desirable processing quality. J. Cereal Sci 60, 617–622. doi: 10.1016/j.jcs.2014.07.006
Harding, K. L., Aguayo, V. M., Webb, P. (2018). Hidden hunger in South Asia: a review of recent trends and persistent challenges. Public Health Nutr 21, 785–795. doi: 10.1017/S1368980017003202
Hatch-McChesney, A., Lieberman, H. R. (2022). Iodine and iodine deficiency: A comprehensive review of a re-emerging issue. . Nutrients 14, 3474. doi: 10.3390/nu14173474
Hurst, R., Siyame, E. W. P., Young, S. D., Chilimba, A. D. C., Joy, E. J. M., Black, C. R., et al. (2013). Soil-type influences human selenium status and underlies widespread se deficiency risks in Malawi. Sci. Rep 3, 1425. doi: 10.1038/srep01425
Hussain, S., Khan, A. M., Rengel, Z. (2019). Zinc-biofortified wheat accumulates more cadmium in grains than standard wheat when grown on cadmium-contaminated soil regardless of soil and foliar zinc application. Sci. Total Environ 654, 402–408. doi: 10.1016/j.scitotenv.2018.11.097
Hussain, S., Maqsood, M., Miller, L. (2012). Bioavailable zinc in grains of bread wheat varieties of Pakistan. Cereal Res. Commun 40, 62–73. doi: 10.1556/CRC.2011.003
Ishfaq, M., Wang, Y., Xu, J., Hassan, M. U., Yuan, H., Liu, L., et al. (2023). Improvement of nutritional quality of food crops with fertilizer: a global meta-analysis. Agron. Sustain. Dev 43, 74. doi: 10.1007/s13593-023-00923-7
Jones, G. D., Droz, B., Greve, P., Gottschalk, P., Poffet, D., McGrath, S. P., et al. (2017). Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci 114, 2848–2853. doi: 10.1073/pnas.1611576114
Kapil, U., Jain, K. (2011). Magnitude of zinc deficiency amongst under five children in India. Indian J. Pediatr 78, 1069–1072. doi: 10.1007/s12098-011-0379-z
Kato, S., Wachi, T., Yoshihira, K., Nakagawa, T., Ishikawa, A., Takagi, D., et al. (2013). Rice (Oryza sativa L.) roots have iodate reduction activity in response to iodine. Front. Plant Sci 4. doi: 10.3389/fpls.2013.00227
Khokhar, J. S., Sindhu, S., Tyagi, B. S., Singh, G., Wilson, L., King, I. P., et al. (2018). Variation in grain Zn concentration, and the grain ionome, in field-grown Indian wheat. PloS One 13, e0192026. doi: 10.1371/journal.pone.0192026
Kiferle, C., Gonzali, S., Holwerda, H. T., Ibaceta, R. R., Perata, P. (2013). Tomato fruits: a good target for iodine biofortification. Front. Plant Sci 4. doi: 10.3389/fpls.2013.00205
Kiferle, C., Martinelli, M., Salzano, A. M., Gonzali, S., Beltrami, S., Salvadori, P. A., et al. (2021). Evidences for a nutritional role of iodine in plants. Front. Plant Sci 12. doi: 10.3389/fpls.2021.616868
Kumssa, D. B., Joy, E. J. M., Ander, E. L., Watts, M. J., Young, S. D., Walker, S., et al. (2015). Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci. Rep 5, 10974. doi: 10.1038/srep10974
Kutman, U. B. (2023). “Mineral nutrition and crop quality,” in Marschner’s Mineral Nutrition of Plants, 4th edn. Eds. Rengel, Z., Cakmak, I., White, P. J. (Academic Press, San Diego, CA, USA), 419–444. doi: 10.1016/B978-0-12-819773-8.00020-4
Kutman, U. B., Yildiz, B., Cakmak, I. (2011). Improved nitrogen status enhances zinc and iron concentrations both in the whole grain and the endosperm fraction of wheat. J. Cereal Sci 53, 118–125. doi: 10.1016/j.jcs.2010.10.006
Landini, M., Gonzali, S., Perata, P. (2011). Iodine biofortification in tomato. J. Plant Nutr. Soil Sci 174, 480–486. doi: 10.1002/jpln.201000395
Lawson, P. G., Daum, D., Czauderna, R., Meuser, H., Härtling, J. W. (2015). Soil versus foliar iodine fertilization as a biofortification strategy for field-grown vegetables. Front. Plant Sci 6. doi: 10.3389/fpls.2015.00450
Li, R., Liu, H. P., Hong, C. L., Dai, Z. X., Liu, J. W., Zhou, J., et al. (2017). Iodide and iodate effects on the growth and fruit quality of strawberry. J. Sci. Food Agric 97, 230–235. doi: 10.1002/jsfa.7719
Li, M., Wang, S., Tian, X., Li, S., Chen, Y., Jia., Z., et al. (2016). Zinc and iron concentrations in grain milling fractions through combined foliar applications of Zn and macronutrients. Field Crops Res 187, 135–141. doi: 10.1016/j.fcr.2015.12.018
Lowe, N. M., Zaman, M., Khan, M. J., Brazier, A. K., Shahzad, B., Ullah, U., et al. (2021). Biofortified wheat increases dietary zinc intake: A randomised controlled efficacy study of Zincol-2016 in rural Pakistan. Front. Nutr 8. doi: 10.3389/fnut.2021.809783
Lyons, G. (2018). Biofortification of cereals with foliar selenium and iodine could reduce hypothyroidism. Front. Plant Sci 9. doi: 10.3389/fpls.2018.00730
Lyons, G. H., Genc, Y., Stangoulis, J. C. R., Palmer, L. T., Graham, R. D. (2005). Selenium distribution in wheat grain, and the effect of postharvest processing on wheat selenium content. Biol. Trace Element Res 103, 155–168. doi: 10.1385/BTER:103:2:155
Ma, J. F., Zhao, F. J., Rengel, Z., Cakmak, I. (2023). “Beneficial elements,” in Marschner’s Mineral Nutrition of Plants, 4th edn. Ed. Rengel, Z., Cakmak, I., White, P. J. (Academic Press, San Diego, CA, USA), 283–238. doi: 10.1016/B978-0-12-819773-8.00012-5
Mao, H., Wang, J., Wang, Z., Zan, Y., Lyons, Zou, C. (2014). Using agronomic biofortification to boost zinc, selenium, and iodine concentrations of food crops grown on the loess plateau in China. J. Soil Sci. Plant Nutt 14, 459–470. doi: 10.4067/S0718-95162014005000036
Morgounov, A., Gómez -Becerra, H. F., Abugalieva, A., Dzhunusova, M., Yessimbekova, M., Muminjanov, H. (2007). Iron and zinc grain density in common wheat grown in Central Asia. Euphytica 155, 193–203. doi: 10.1007/s10681-006-9321-2
Morton, C. M., Pullabhotla, H., Bevis, L., Lobell, D. B. (2023). Soil micronutrients linked to human health in India. Sci. Rep 13, 13591. doi: 10.1038/s41598-023-39084-8
Naeem, A., Aslam, M., Ahmad, M., Asif, M., Yazici, M. A., Cakmak, I., et al. (2022). Biofortification of diverse basmati rice cultivars with iodine, selenium, and zinc by individual and cocktail spray of micronutrients. Agronomy 12, 49. doi: 10.3390/agronomy12010049
Nista, F., Bagnasco, M., Gatto, F., Albertelli, M., Vera, L., Boschetti, M., et al. (2022). The effect of sodium restriction on iodine prophylaxis: A review. J. Endocrinol. Investig 45, 1121–1138. doi: 10.1007/s40618-022-01749-y
NNS (2019). National Nutrition Survey 2018: Key Findings Report (Islamabad: Ministry of National Health Services, Regulation and Coordination, Government of Pakistan), 52.
Panth, P., Guerin, G., DiMarco, N. M. (2019). A review of iodine status of women of reproductive age in the USA. Biol. Trace Elem Res 188, 208–220. doi: 10.1007/s12011-018-1606-5
Passarelli, S., Free, C. M., Shepon, A., Beal, T., Batis, C., Golden, C. D. (2024). Global estimation of dietary micronutrient inadequacies: a modelling analysis. The Lancet Global Health 12(10), e1590–e1599.
Peleg, Z., Saranga, Y., Yazici, A., Fahima, T., Ozturk, L., Cakmak, I. (2008). Grain zinc, iron and protein concentrations and zinc-efficiency in wild emmer wheat under contrasting irrigation regimes. . Plant Soil 306, 57–67. doi: 10.1007/s11104-007-9417-z
Poole, N., Donovan, J., Erenstein, O. (2021). Continuing cereals research for sustainable health and well-being. Inter. J. Agric. Sustain 20, 693–704. doi: 10.1080/14735903.2021.1975437
Prom-u-thai, C., Rashid, A., Ram, H., Zou, C., Guilherme, L. R. G., Corguinha, A. P. B., et al. (2020). Simultaneous biofortification of rice with zinc, iodine, iron and selenium through foliar treatment of a micronutrient cocktail in five countries. Front. Plant Sci 11. doi: 10.3389/fpls.2020.589835
Ram, H., Sohu, V. S., Cakmak, I., Singh, K., Buttar, G. S., Sodhi, G. P. S., et al. (2015). Agronomic fortification of rice and wheat grains with zinc for nutritional security. Curr. Sci 109, 1171–1176. doi: 10.18520/v109/i6/1171-1176
Rashid, A. (2005). Establishment and management of micronutrient deficiencies in Pakistan: a review. Soil Environ 24, 1–22.
Rashid, A., Fox, R. L. (1992). Evaluating the internal zinc requirements of grain crops by seed analysis. . Agron. J 84, 469–474. doi: 10.2134/agronj1992.00021962008400030022x
Rashid, A., Zia, M. H., Ahmad, W. (2023). Micronutrient fertilizer use in Pakistan: Historical perspective and 4R nutrient stewardship (Boca Raton: CRC Press). doi: 10.1201/9781003314226
Rayman, M. P. (2000). The importance of selenium to human health. Lancet 356, 233–241. doi: 10.1016/S0140-6736(00)02490-9
Rayman, M. P. (2008). Food-chain selenium and human health: emphasis on intake. Br. J. Nutr 100, 254–268. doi: 10.1017/S0007114508939830
Rayman, M. P. (2012). Selenium and human health. Lancet 379, 1256–1268. doi: 10.1016/S0140-6736(11)61452-9
Rodrigo, S., Santamaría, O., Poblaciones, M. J. (2014). Selenium application timing: Influence in wheat grain and flour selenium accumulation under Mediterranean conditions. J. Agric. Sci 6, 23–30. doi: 10.5539/jas.v6n3p23
Sazawal, S., Dhingra, U., Dhingra, P., Dutta, A., Deb, S., Kumar, J., et al. (2018). Efficacy of high zinc biofortified wheat in improvement of micronutrient status, and prevention of morbidity among preschool children and women - a double masked, randomized, controlled trial. Nutr. J 17, 86. doi: 10.1186/s12937-018-0391-5
Schiavon, M., Nardi, S., dalla Vecchia, F., Ertani, A. (2020). Selenium biofortification in the 21st century: status and challenges for healthy human nutrition. Plant Soil 453, 245–270. doi: 10.1007/s11104-020-04635-9
Singh, M. V. (2010). Detrimental effect of zinc deficiency on crops productivity and human health. In First Global Conference on Biofortification, HarvestPlus, Washington, USA.
Smoleń, S., Kowalska, I., Czernicka, M., Halka, M., Keska, K., Sady, W. (2016). Iodine and selenium biofortification with additional application of salicylic acid affects yield, selected molecular parameters and chemical composition of lettuce plants (Lactuca sativa L. var. capitata). Front. Plant Sci 7. doi: 10.3389/fpls.2016.01553
Smoleń, S., Kowalska, I., Sady, W. (2014). Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. . Sci. Hortic 166, 9–16. doi: 10.1016/j.scienta.2013.11.011
Smyth, P. P. A. (2022). Narrative review: iodine— thyroidal and extrathyroidal actions. Ann. Thyroid 7, 4. doi: 10.21037/aot-21-28
Stangoulis, J. C., Knez, M. (2022). Biofortification of major crop plants with iron and zinc- achievements and future directions. Plant Soil 474, 57–76. doi: 10.1007/s11104-022-05330-7
Stevens, G. A., Beal, T., Mbuya, M. N. N., Luo, H., Neufeld, L. M., Addo, O. Y., et al. (2022). Micronutrient deficiencies among preschool aged children and women of reproductive age worldwide: a pooled analysis of individual level data from population-representative surveys. Lancet Glob Health 10, e1590–e1599. doi: 10.1016/S2214-109X(22)00367-9
Sun, Y., Wang, Z., Gong, P., Yao, W., Ba, Q., Wang, H. (2023). Review on the health-promoting effect of adequate selenium status. Front. Nutr 10. doi: 10.3389/fnut.2023.1136458
Swamy, B. P. M., Rahman, M. A., Inabangan-Asilo, M. A., Amparado, A., Manito, C., Chadha-Mohanty, P., et al. (2016). Advances in breeding for high grain zinc in rice. Rice 9, 49. doi: 10.1186/s12284-016-0122-5
USDA (2014). Grain and Feed 2014. Global Agricultural Information Network, Foreign Agricultural Services, U.S. Department of Agriculture, Washington DC, USA.
Virk, P. S., Andersson, M. S., Arcos, J., Govindaraj, M., Pfeiffer, W. H. (2021). Transition from targeted breeding to mainstreaming of biofortification traits in crop improvement programs. Front. Plant Sci 12. doi: 10.3389/fpls.2021.703990
Wani, S. H., Gaikwad, K., Razzaq, A., Samantara, K., Kumar, M., Govindan, V. (2022). Improving zinc and iron biofortification in wheat through genomics approaches. . Mol. Biol. Rep 49, 8007–8023. doi: 10.1007/s11033-022-07326-z
Welch, R. M., Graham, R. D. (2004). Breeding for micronutrients in staple food crops from a human nutrition perspective. . J. Exp. Bot 55, 353–364. doi: 10.1093/jxb/erh064
Welch, R. M., Graham, R. D., Cakmak, I. (2013). “Linking agricultural production practices to improving human nutrition and health, expert paper written for ICN2,” in Second International Conference on Nutrition Preparatory Technical Meeting, Rome, Italy, 13-15 November. Available online at: http://www.fao.org/3/a–as574e.pdf.
Xue, Y. F., Li, X. J., Yan, W., Miao, Q., Zhang, C. Y., Huang, M., et al. (2023). Biofortification of different maize cultivars with zinc, iron and selenium by foliar fertilizer applications. Front. Plant Sci 14. doi: 10.3389/fpls.2023.1144514
Yazici, M. A., Asif, M., Tutus, Y., Ortas, I., Ozturk, L., Lambers, H., et al. (2021). Reduced root mycorrhizal colonization as affected by phosphorus fertilization is responsible for high cadmium accumulation in wheat. Plant and Soil 468, 19–35. doi: 10.1007/s11104-021-05041-5
Zhao, F. J., Su, Y. H., Dunham, S. J., Rakszegi, M., Bedo, Z., McGrath, S. P. (2009). Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J. Cereal Sci 49, 290–295. doi: 10.1016/j.jcs.2008.11.007
Zhu, Y. G., Huang, Y. Z., Hu, Y., Liu, Y. X. (2003). Iodine uptake by spinach (Spinacia Oleracea L.) plants grown in solution culture: effects of iodine species and solution concentrations. Environ. Int 29, 33–37. doi: 10.1016/S0160-4120(02)00129-0
Zia, M. H., Watts, M. J., Gardner, A., Chenery, S. R. (2015). Iodine status of soils, grain crops, and irrigation waters in Pakistan. Environ. Earth Sci 73, 7995–8008. doi: 10.1007/s12665-014-3952-8
Zimmermann, M. B., Andersson, M. (2012). Assessment of iodine nutrition in populations: past, present, and future. Nutr. Rev 70, 553–570. doi: 10.1111/j.1753-4887.2012.00528.x
Zou, C. Q., Du, Y. F., Rashid, A., Ram, H., Savasli, E., Pieterse, P., et al. (2019). Simultaneous biofortification of wheat with zinc, iodine, selenium, and iron through foliar treatment of a micronutrient cocktail in six countries. J. Agric. Food Chem 67, 8096–8106. doi: 10.1021/acs.jafc.9b01829
Keywords: agronomic biofortification, wheat, genotypes, micronutrients, hidden hunger
Citation: Ram H, Naeem A, Rashid A, Kaur C, Ashraf MY, Malik SS, Aslam M, Mavi GS, Tutus Y, Yazici MA, Govindan V and Cakmak I (2024) Agronomic biofortification of genetically biofortified wheat genotypes with zinc, selenium, iodine, and iron under field conditions. Front. Plant Sci. 15:1455901. doi: 10.3389/fpls.2024.1455901
Received: 27 June 2024; Accepted: 14 November 2024;
Published: 06 December 2024.
Edited by:
Mohd. Kamran Khan, Selçuk University, TürkiyeReviewed by:
Umit Baris Kutman, Gebze Technical University, TürkiyeHiroshi Masuda, Akita Prefectural University, Japan
Copyright © 2024 Ram, Naeem, Rashid, Kaur, Ashraf, Malik, Aslam, Mavi, Tutus, Yazici, Govindan and Cakmak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ismail Cakmak, Y2FrbWFrQHNhYmFuY2l1bml2LmVkdQ==
 Hari Ram1
Hari Ram1 Asif Naeem
Asif Naeem Abdul Rashid
Abdul Rashid Muhammad Y. Ashraf
Muhammad Y. Ashraf Muhammad Aslam
Muhammad Aslam Velu Govindan
Velu Govindan Ismail Cakmak
Ismail Cakmak