- 1Department of Biological Sciences, College of Science, King Faisal University, Al-Ahsa, Saudi Arabia
- 2Medicinal and Aromatic Plants Department, Desert Research Center El-Mataria, Cairo, Egypt
- 3Chemistry Department, Faculty of Science, Alexandria University, Alexandria, Egypt
- 4Department of Chemistry, College of Science, King Faisal University, Al-Ahsa, Saudi Arabia
- 5Biochemistry Division, Department of Chemistry, Faculty of Science, Tanta University, Tanta, Egypt
- 6Botany and Microbiology Department, Faculty of Science, Alexandria University, Alexandria, Egypt
This study tends to reach some objectives of the sustainable development goals, which call for responsible consumption and production and climate action. Long-term global food security is affected by drought and the optimal use of water in agricultural areas under climate change scenarios. Our approach aims to amend soil for cultivation under drought stress and improve plant growth to contribute to food security. In this context, a biochar was prepared from peanut shell and thoroughly examined as a soil enhancer for broad bean cultivation during drought stress. The produced biochar exhibited 0.307 g cm−3 bulk density, 9.6 cmol kg−1 cation exchange capacity, −15.5 mV zeta potential, and an average diameter of 21.86 nm. Surprisingly, the application of biochar increased soil water holding capacity and organic matter by 66% and 220%, respectively. Moreover, its application under drought improved plant growth as indicated by stem height, leaf area index, pod number/plant, pod weight, protein level, chlorophyll content, nutrient levels in leaves, and reduced lipid peroxidation and electrolyte leakage. The principal component and factorial analysis of the current study demonstrated correlations between the physiological response of faba bean plants and soil physiochemical parameters after the application of peanut shell-derived biochar. This study presents promising nano biochar that could be an effective sustainable practice for disposing residual materials.
1 Introduction
Crop yields are being negatively impacted by climate change and will continue to do so. On average, it is projected that crop yields have decreased by 70% since 1982 as a result of climate change, with very few agricultural areas being undisturbed (Food and Agriculture Organization of the United Nations). Drought has been known as the primary cause of the decline in agricultural output, which is predicted to increase in the face of global climate change. Globally, more than 34% of the loss in crop and livestock production is due to drought stress. Drought causes a series of physiological, biochemical, and molecular changes in plants. It negatively affects plants through disrupting nutrient uptake, damaging organelle structures, inducing the decomposition of chlorophyll, inhibiting photosynthetic activity, and suppressing leaf growth (Farooq et al., 2008; Osakabe et al., 2014; Fahad et al., 2017). Under drought stress, reactive oxygen species (ROS) are increasingly produced, causing oxidative stress (OS) and accelerating the aging process (Rizwan et al., 2015). Consequently, overcoming drought is a difficult issue for achieving global food security. To counteract drought stress, organic amendments including composts, digestates, manures, and biochar are added to the soil to increase its water holding capacity (WHC). Many strategies have been used to alleviate drought stress in plants such as exogenous application of β-aminobutyric acid (Abid et al., 2020), gibberellic acid and bee honey boosts (Rady et al., 2021), as well as plant growth-promoting bacteria (PGPB) (Harkhani and Sharma, 2024), melatonin treatment (Amiri et al., 2024), seed priming with selenium (Se) (Raza et al., 2024), and biochar amendments (Gullap et al., 2024).
Biochar is a carbon-rich material produced by the thermal decomposition of biomass under limited oxygen conditions (Ali et al., 2017; Kumar et al., 2020). Biochar can be produced from different types of biomass, including agricultural wastes, wood residues, food wastes, animal manure, and sludge (Wijitkosum, 2022). Previous studies have reported that biochar amendments improve the physiochemical characteristics of soil such as nutrient availability, WHC, pH, and cation exchange capacity (CEC) as well as the mitigation of climate change through carbon sequestration and reduction of greenhouse gases (Roberts et al., 2010; Rafael et al., 2019; Rollon et al., 2020). In addition, biochar is applied in the remediation of contaminated soil and wastewater treatment due to its ability to absorb undesirable contaminants (Sattar et al., 2019; Yang et al., 2019; Zhu et al., 2020; Afzal et al., 2024). It has been shown that the structure of biochar exhibits a notable degree of stability when evaluated from the point of view of microbial and chemical degradation. This stability sets biochar apart from other organic compounds and can offer long-term advantages to soil (Agegnehu et al., 2015). Previous research has demonstrated that biochar application improved plant growth and yield of several plants under drought stress, including tomato (Akhtar et al., 2014), okra (Batool et al., 2015), maize (Haider et al., 2015), grape (Genesio et al., 2015), rapeseed (Lalay et al., 2022), and soybean (Gullap et al., 2024). Applying biochar has been shown to improve nutrient intake, soil microbial activity, photosynthetic rate (Diamadopoulos, 2019), and the plant’s defense mechanisms against drought by increasing the activity of antioxidant enzymes. This reduces the damage that drought causes to the photosynthetic machinery (Lyu et al., 2016). The vital role of biochar in improving soil WHC is a key factor in enhancing plant growth and yield during drought stress (Foster et al., 2016; Licht and Smith, 2017). Notably, because of its nano size, nano biochar is applied to increase crop yield and improve soil fertility (Shafiq et al., 2023). Previous studies have shown that applying nano biochar increased plant growth and yield in a number of drought stressed plants such as broccoli (Azadifar et al., 2023), wheat (Raza et al., 2023), and spinach (Rasheed et al., 2024).
Faba bean (Vicia faba L.) is a major food in the diets of inhabitants of the Middle East, the Mediterranean region, and China because of the high nutritional value of its seeds (Chaieb et al., 2011; De Cillis et al., 2019). It is a significant source of protein-rich food, and its nutritional value is superior to that of peas or other grain legumes (Crépon et al., 2010). It has a crucial role in improving the fertility of the soil by its rotation cultivation with cereal crops due to its ability to fix atmospheric nitrogen, thereby rendering them excellent colonizers of low-N environments (Abid et al., 2020). It is one of the most significant food legume crops cultivated in Egypt. For the year 2020, FAO statistics indicated that the areas cultivated with faba beans in Egypt decreased from 110,100 to 32,532 ha during the past 50 years and the national self-sufficiency rate also decreased from 70% to 26.9%. The reduction in faba bean yield could be related to changing environmental conditions (Quarshie et al., 2021; Abo-Hegazy and Darwish, 2022). According to Abid et al (Abid et al., 2020), drought is one of the main environmental factors that can significantly restrict the growth and productivity of faba bean plants. Martínez et al. (2007) reported that faba bean requires more water and is more susceptible to drought compared to other legume crops. Accordingly, drought stress can cause a significant decline in faba bean productivity, thus impacting agriculture and sustainable development in Egypt.
The current study was designed to evaluate the effect of application of the derived biochar as a soil amendment on growth, photosynthetic pigments, mineral nutrition, protein pattern, lipid peroxidation, and electrolyte leakage (EL) on faba bean plants under drought stress. Additionally, this study aimed to identify an alternative method for disposing peanut shells considered agricultural waste, which copes with the sustainable development goals declared by the United Nations (Farooq et al., 2008; Ali et al., 2017; Abid et al., 2020; Kumar et al., 2020). To the best of our knowledge, it is the first time to prepare biochar in the nano scale from peanut shell.
2 Materials and methods
2.1 Synthesis of nano biochar
Peanut was purchased from the local market. Peanut shells were washed with water in order to remove dust and mud impurities and then air-dried. Afterwards, the shells were ground well using a mechanical blender. A certain amount of the peanut shells was put in a closed reactor system and then heated to 450°C for 1.5 h. The obtained product is biochar (Jiang et al., 2022).
2.2 Physical and chemical characterization of the synthesized biochar
2.2.1 Production of nano biochar
The given equation was applied to estimate the production rate:
2.2.2 Electrical conductivity and pH
The electrical conductivity (EC) measurement was performed using a conductometer (WTW-LF-91, England), and the pH was recorded by means of a glass electrode pH meter (WTW model 512).
2.2.3 The bulk and trapped density
The bulk density (BD) for peanut shell and its produced biochar was calculated according to Wang and Kinsella’s method (Wang and Kinsella, 1976), whereas a graduated cylinder was filled with a known quantity of powdered samples that were dried and weighed. The cylinder was then tapped for a few minutes to compact the sample, the volume of the sample was verified in milliliters, and BD was calculated using the given equation:
The tapped density was estimated in the same way, but by tapping the cylinder containing the powder for a fixed number, then applying the given equation:
2.2.4 Surface area measurement
Sears’ method (Sears, 1956) was applied to assess the surface area of the biochar rapidly. Accordingly, 1.5 g of the biochar was agitated in 100 mL of diluted HCl at pH 3. Sodium chloride (NaCl, 30 g) was added and stirred, and the volume was completed to 150 mL using deionized water. Titration was carried out with 0.1 N NaOH to increase the pH up to 9 and the volume (V) is verified. The surface area was estimated using the equation:
2.2.5 Cation exchange capacity
To deduce the CEC of the biochar, the ammonium acetate displacement method (Gaskin et al., 2008) was carried out. Deionized water (20 mL) was used to leach 0.20 g of biochar five times. The leachates were then collected together, then the biochar was again leached with 20 mL of 1.0 M sodium acetate (pH 7) five times and collected together as exchangeable base cations (potassium, calcium, and magnesium). Ethanol was used to wash the biochar samples from any excessive sodium. Finally, 20 mL of 1 M ammonium acetate (pH 7) was applied to displace the sodium ions on the synthesized biochar. The CEC of the biochar was investigated from the sodium displaced by ammonium ion. The contents of sodium ions in the solution were detected by JENWAY PFPF7 flame photometry.
2.2.6 FT-IR spectra
FT-IR spectra of the peanut shell and its biochar were recorded using a Perkin-Elmer FT-IR 1650 Spectrophotometer ranging from 4,000 to 500 cm−1 at 25°C from KBr pellets of thickness 3 mm.
2.2.7 Zeta potential and size distribution
The biochar surface charges were determined by Zetasizer (Nano ZS Malvern).
2.2.8 Surface morphology studies
The morphologies of nanoparticles were displayed by a JEOL JSM-IT200 Scanning Electron Microscope (SEM) (Tokyo, Japan) at the Electron Microscopy Unit of the Faculty of Science, Alexandria University, Alexandria, Egypt. Sonication of the sample in deionized water for 5 min was carried out, deposited onto carbon-coated copper mesh, and dried before examination. A SEM along with an energy dispersive x-ray spectroscope (EDX) were operated to examine the elemental composition of biochar.
2.3 Experimental conditions
The experiment was conducted at the greenhouse of the Faculty of Science, Alexandria University under a complete randomized design. Three seeds were planted in each pot on 15 November until the second half of March under the following environmental conditions: temperature ranged from 18 to 26°C, relative humidity ranged from 50% to 60%, and average daylight from 10 to 14 hours/day. The experimental design consisted of the following treatments: B0 = no biochar, B1 = 2% nano biochar (w/w), B2 = 4% nano biochar (w/w), where 80% pot WHC was treated as control (C), whereas 50% pot WHC was considered as drought stress (D).
2.4 Soil analysis
Samples of control soil and soil amendment with 2% or 4% nano biochar were spread over sheets of paper in the air until they are dry, thoroughly mixed, ground well, passed through a 2-mm sieve to remove gravel and debris, and then packed in plastic bags. The sieved soil samples were analyzed for some of their physical and chemical properties according to Blume et al (Blume et al., 1982). EC and soil pH were measured in a 1:5 soil water extract using an EC meter (WTW-LF-91, England) and a glass electrode pH meter (WTW model 512). Texture and particle size distribution (PSD) for surface samples were determined using a hydrometer and sieves (Klute, 1986). Organic matter content (OM %) was quantified by using the potassium dichromatic oxidation titration technique according to the Walkley and Black method (Walkley and Black, 1934). Soil water holding capacity (SWHC) was measured by the gravimetric method according to Klute (1986). Sodium adsorption ratio (SAR) was calculated according to the following formula as described by Lesch and Suarez (2009):
Nitrogen (N) was assessed by the Kjeldahl method. Bicarbonates (HCO3−) were appraised by the titration method. Phosphorus (P) was digested using perchloric and sulfuric acids and then was analyzed using the molybdenum antimony blue colorimetric method (Olsen and Dean, 1965). Available potassium (K), magnesium (Mg), calcium (Ca), and some micronutrients were determined by using a flame photometer and an atomic absorption spectrophotometer according to Allen et al (Allen et al., 1974). Total calcium carbonate content (CaCO3%) was determined using the pressure calcimeter method (Blume et al., 1982).
2.5 Growth and yield parameters
For each treatment, plant height was measured, and leaf area was estimated using the Milford et al. (1985) equation. Yield parameters, including pod number/plant (PN/P), grain number/pod (GN/P), and weight of 100 seeds, were recorded at the maturity stage. Results are presented as the mean of three replications in each treatment.
2.6 Determination of chlorophyll a and b and total chlorophyll
The photosynthetic pigments’ total chlorophyl (TCh), chlorophyll a (Cha), and chlorophyll b (Chb) were measured spectrophotometrically at 647 and 664 nm following the N,N-dimethylformamide (DMF) method as described by Inskeep and Bloom (1985). The following formula and extinction coefficients were utilized to determine the photosynthetic pigments:
The chlorophyll stability index (CSI %) was calculated according to the following formula as described by Sivasubramaniawn (1992):
2.7 Protein assay and SDS-PAGE
Protein concentration was estimated as described by Bradford (1976) using bovine serum albumin (BSA) as the standard. Protein electrophoresis was carried out using Laemmli’s SDS-PAGE technique (Laemmli, 1970). The molecular weight of proteins was analyzed with gel image analysis software (TotalLab V.1.0.0.1).
2.8 Analysis of nutrient levels in leaves
Oven-dried broad leaves from different treatments were digested according to the methods of Jones and Case (1990). The Kjeldahl method was used to estimate the total nitrogen. Sulfur was examined according to the method of Okalebo et al (Okalebo et al., 2002). Mg, Ca, Cu, Fe, Mn, and Zn were measured using an atomic absorption spectrophotometer (Perkin-Elmer 100B) following Isaac (1980). A spectrophotometer (T80+, PG Instruments Limited, Leics, United Kingdom) was used to measure the amount of phosphorus using the vanadate–molybdate technique at 660 nm (Chapman and Pratt, 1962). A Perkin-Elmer Flame photometer was used to measure potassium and sodium (Blume et al., 1982).
2.9 Determination of lipid peroxidation
The level of lipid peroxidation was measured according to the thiobarbituric acid (TBA) test, which determines the malondialdehyde (MDA) content as the product of the lipid peroxidation reaction. MDA concentration was measured using a spectrophotometer and calculated by applying its extinction coefficient 155 mM−1 cm−1 (Heath and Packer, 1968).
2.10 Electrolyte leakage
Plant samples were immersed in deionized water and then placed in a water bath held at a constant temperature of 32°C. After 2 h, the initial EC of the medium (EC1) was recorded. The samples were then autoclaved at 120°C for 20 min, and after cooling, the final EC (EC2) was measured. The percentage of EL was calculated using the formula: EL% = (EC1/EC2) × 100 (Dionisio-Sese and Tobita, 1998).
2.11 Data analysis
Analysis of variance (ANOVA) was performed using IBM SPSS v.27.0 software to evaluate the interaction between drought and nano biochar application. The principal component analysis (PCA) was performed using Sigma Plot program V.15, correlograms were carried out using R software V.4.3.1 (Team, R.C, 2013), and factorial analysis was performed by using Design-Expert V. 13.
3 Results
3.1 Characterization of the prepared nano biochar
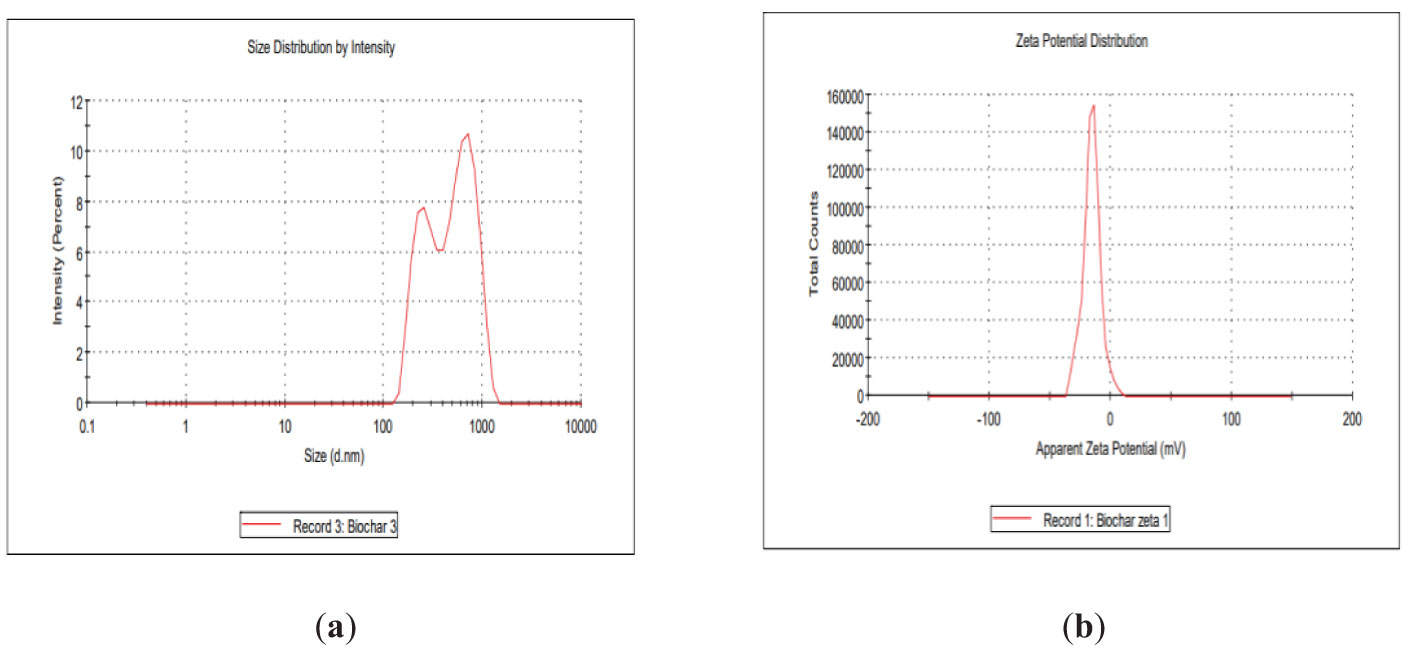
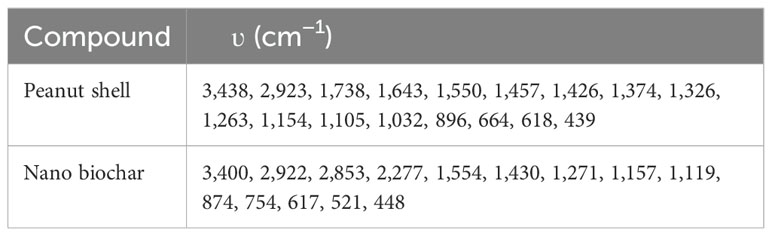
The rate of nano biochar production was 32%. The EC of the prepared nano biochar was found to be 3.40 dS m−1. The measured pH value of the nano biochar was 7.4. The BDs of the peanut shells and the derived nano biochar were 0.212 and 0.307, whereas their trapped densities were 0.226 and 0.422, respectively. The values of the bulk and trapped density for peanut shells were less dense than their nano biochar. The observed experimental CEC value of the prepared nano biochar was 9.6 cmol kg−1. The surface area of the prepared nano biochar was 208.2 m2 g−1. The PSD of fine, medium, and coarse nano biochar was measured (Figure 1A) and a Z average size of 606.2 nm was reported, whereas the zeta potential value was −15.5 mV (Figure 1B), suggesting negative charges on the nano biochar. The analysis of the FTIR spectrum of the peanut shell and nano biochar is presented in Figure 2, Table 1.
The analysis of FTIR spectrum of the peanut shell and nano biochar is presented in Table 1, Figure 2.
The particles’ average diameter of the prepared biochar was investigated from SEM images and selected randomly (Figure 3). The average diameter of the peanut shell-derived nano biochar was 21.86 ± 1.44 nm, and it was displayed as well-separated spherical nanoparticles.
The variability in the major elements of peanut shell and its prepared nano biochar was examined by EDX (Figure 4). Elements such as C, O, Na, K, Ca, Mg, and Si have been detected through EDX analysis. Compared to the peanut shell, the C proportion in the prepared biochar was found to be increased, whereas O tended to decrease. The C constituent was found to be greater with an atomic weight of 86.85% in biochar prepared at 450°C, which confirmed the presence of organic matter.
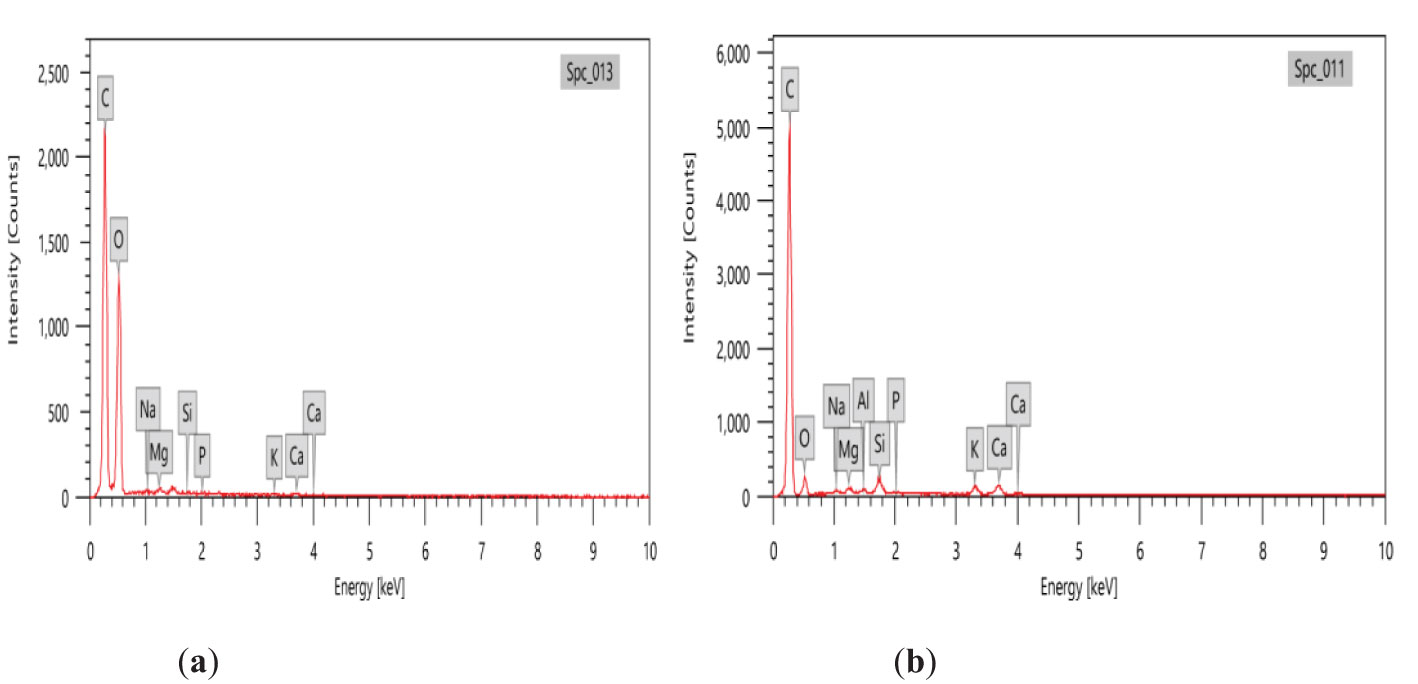
Figure 4. Energy-dispersive x-ray (EDX) analysis of (A) peanut shell and (B) the prepared nano biochar.
3.2 Soil analysis
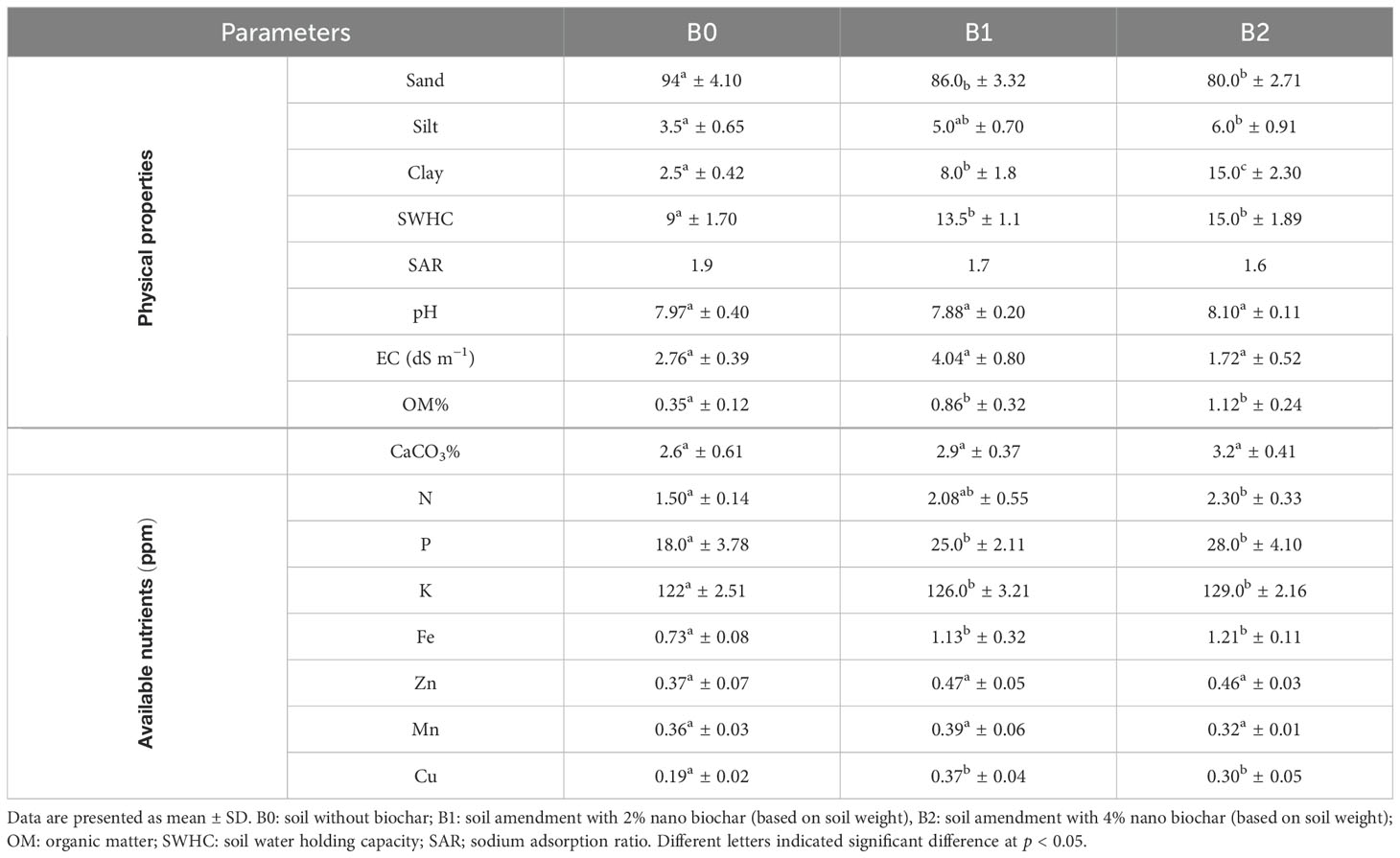
Soil physiochemical properties were significantly affected by the application of nano biochar as shown in Table 2. In contrast to control (no biochar) soil, the addition of 2% (w/w) and 4% (w/w) nano biochar led to a 145% and 220% increase of organic matter (OM), respectively. Moreover, nano biochar amendments significantly improved SWHC by 44.4% and 66% in B1 and B2 treatments, respectively, as compared to control soil. However, SAR decreased significantly in response to nano biochar application. The available levels of N, P, K, and Cu increased by 0.8, 10, 7, and 0.19 ppm by the application of nano biochar (B2), respectively, as compared to control soil, whereas there was no significant change in the available levels of both Zn and Mn upon nano biochar application.
3.3 Effect of nano biochar and drought on growth and yield parameters
Drought stress caused a significant reduction in growth and yield parameters (Figure 5). Compared to control plants, drought significantly reduced shoot height, leaf area, pod number/plant, and pod weight by 45%, 55%, 73%, and 116%, respectively. A significant improvement in the aforementioned parameters was noticed by 54%, 63%, 66%, and 33% compared to drought stressed plants when nano biochar was applied at a rate of 4% (w/w).
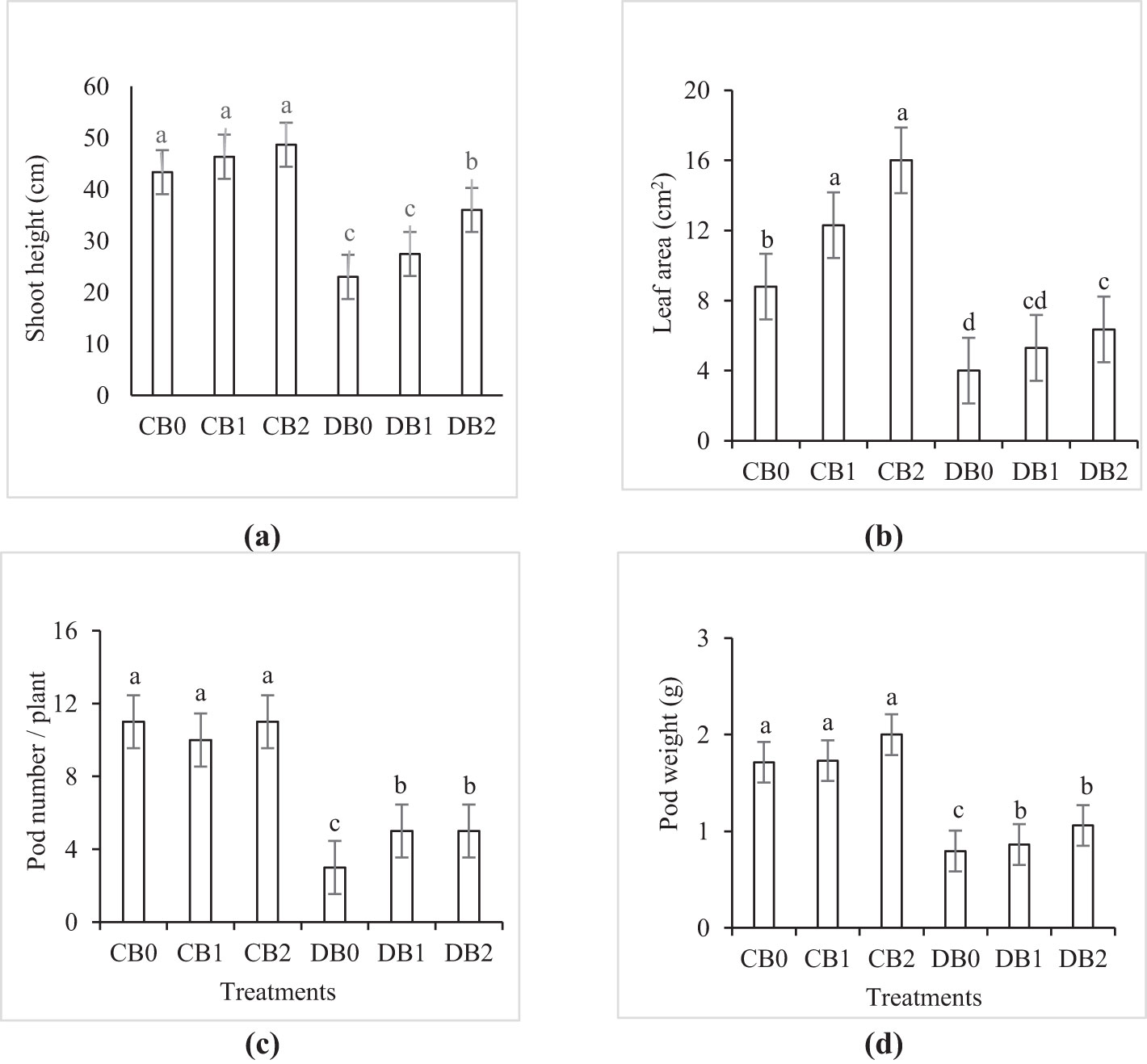
Figure 5. Effect of nano biochar application and drought stress on growth and yield parameters of faba bean. (A) Shoot height; (B) leaf area; (C) pod number/plant; (D) pod weight. CB0: control − zero biochar application; CB1: control + 2% nano biochar (w/w); CB2: control + 4% nano biochar (w/w); DBO: drought + zero biochar application; DB1: drought + 2% nano biochar (w/w); DB2: drought + 4% nano biochar (w/w). Different letters on bars indicated significant difference at p < 0.05.
3.4 Effect of nano biochar and drought on photosynthetic pigments
The effect of drought and nano biochar levels on Cha, Chb, and TCh is presented in Figure 6. A significant decrease in TCh content by 40% was observed under drought stress compared to control treatment. Nano biochar application at a rate of 4% (w/w) substantially improved the TCh content by 42% in case of DB2 treatment compared to DB0 treatment. The chlorophyll stability index (CSI) significantly increased from 60% in DB0 to 80% in DB2.
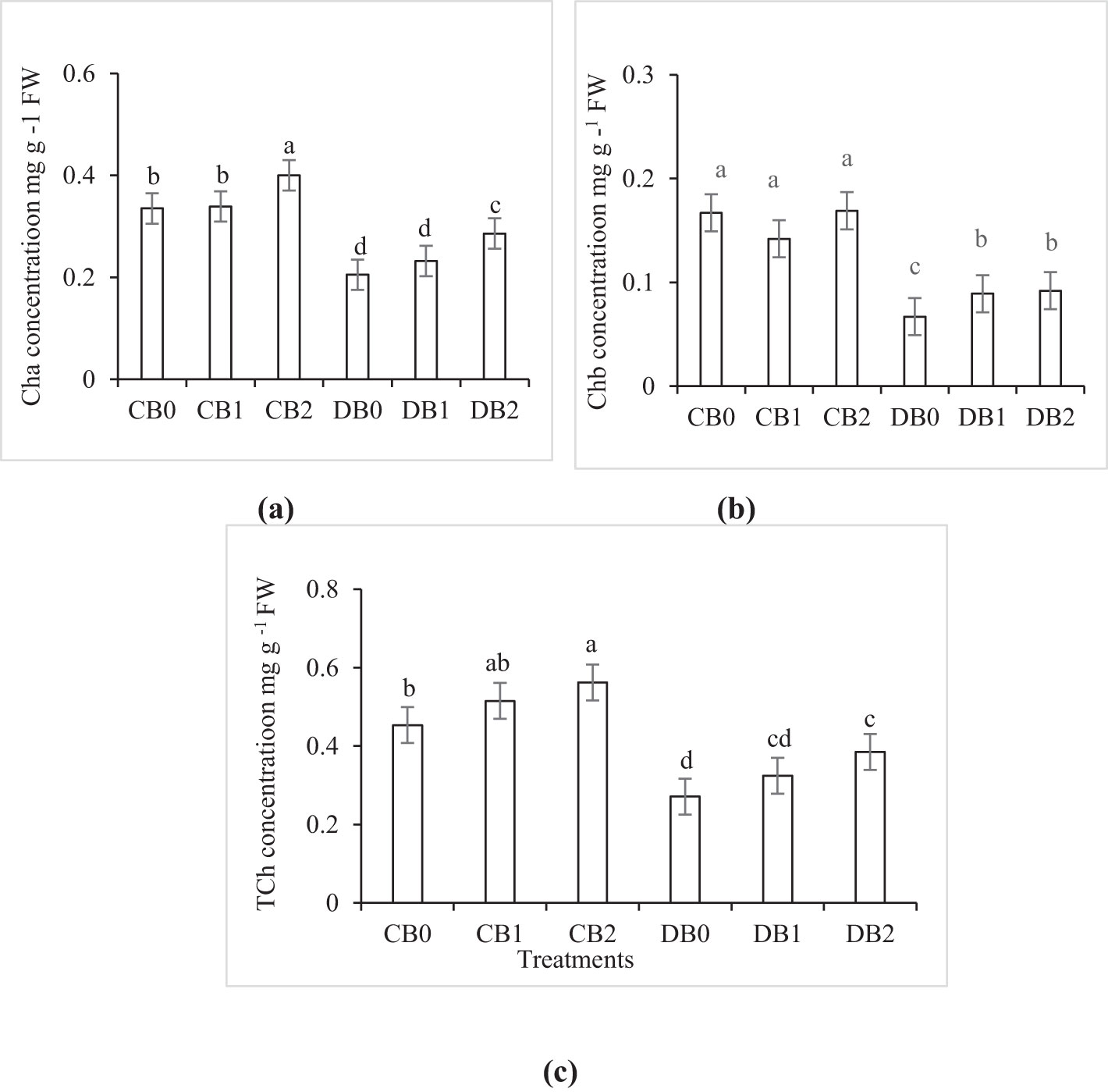
Figure 6. Effect of nano biochar application and drought stress on photosynthetic pigments (A) chlorophyll a (Cha); (B) chlorophyll b (Chb); and (C) total chlorophyll (TCh) of faba bean. CB0: control − zero biochar application, CB1: control + 2% biochar (w/w); CB2: control + 4% nano biochar (w/w); DBO: drought + zero biochar application; DB1: drought + 2% nano biochar (w/w); DB2: drought + 4% nano biochar 4% (w/w). Different letters on bars indicated significant difference at p < 0.05.
3.5 Effect of nano biochar and drought on protein content
Drought stress significantly reduced protein content in faba leaves, whereas the nano biochar amendment caused a significant increase in protein content (Figure 7). The effect of drought and nano biochar application on polypeptide pattern of faba leaves is presented in Supplementary Figure S2. A polypeptide band with a molecular mass of 96 kDa completely disappeared in drought stressed leaves. In addition, the intensity of three bands, with a molecular mass of 72, 26, and 20 kDa, decreased in drought stressed leaves in comparison to control leaves.
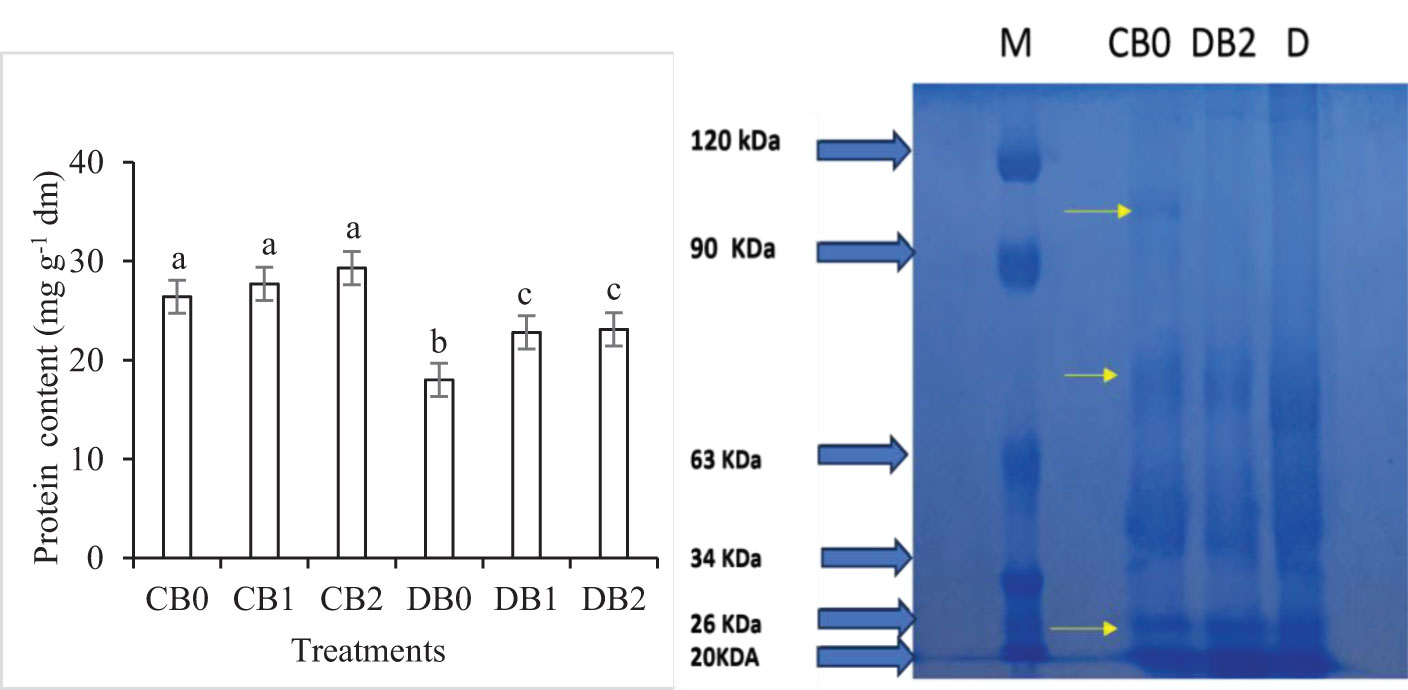
Figure 7. Effect of nano biochar application and drought stress on protein level and protein pattern of faba leaves. (A) Total protein; (B) Protein pattern; M: Marker; CB0: control − zero biochar application; DBO: drought + zero biochar application; DB2: drought + 4% nano biochar (w/w). Different letters on bars indicated significant difference at p < 0.05.
3.6 Effect of nano biochar and drought on nutrients in leaves
The effect of drought and nano biochar level on faba leaves’ nutrient content is shown in Supplementary Figure S3. Drought stress negatively affected the nutrient content of faba leaves. However, nano biochar amendment mitigates the negative effect of drought stress on faba leaves’ nutrient content (Figure 8). Maximum N (4.7), K (2.8), Ca (2.4), and Mg (0.68) content (mg g−1 dm) were recorded in B2 treatment, while minimum values of 2.5, 1.9, 1.53, and 0.311 (mg g−1 dm), respectively, were recorded in the drought stressed plants without nano biochar application. However, P content did not exhibit any difference between different treatments.
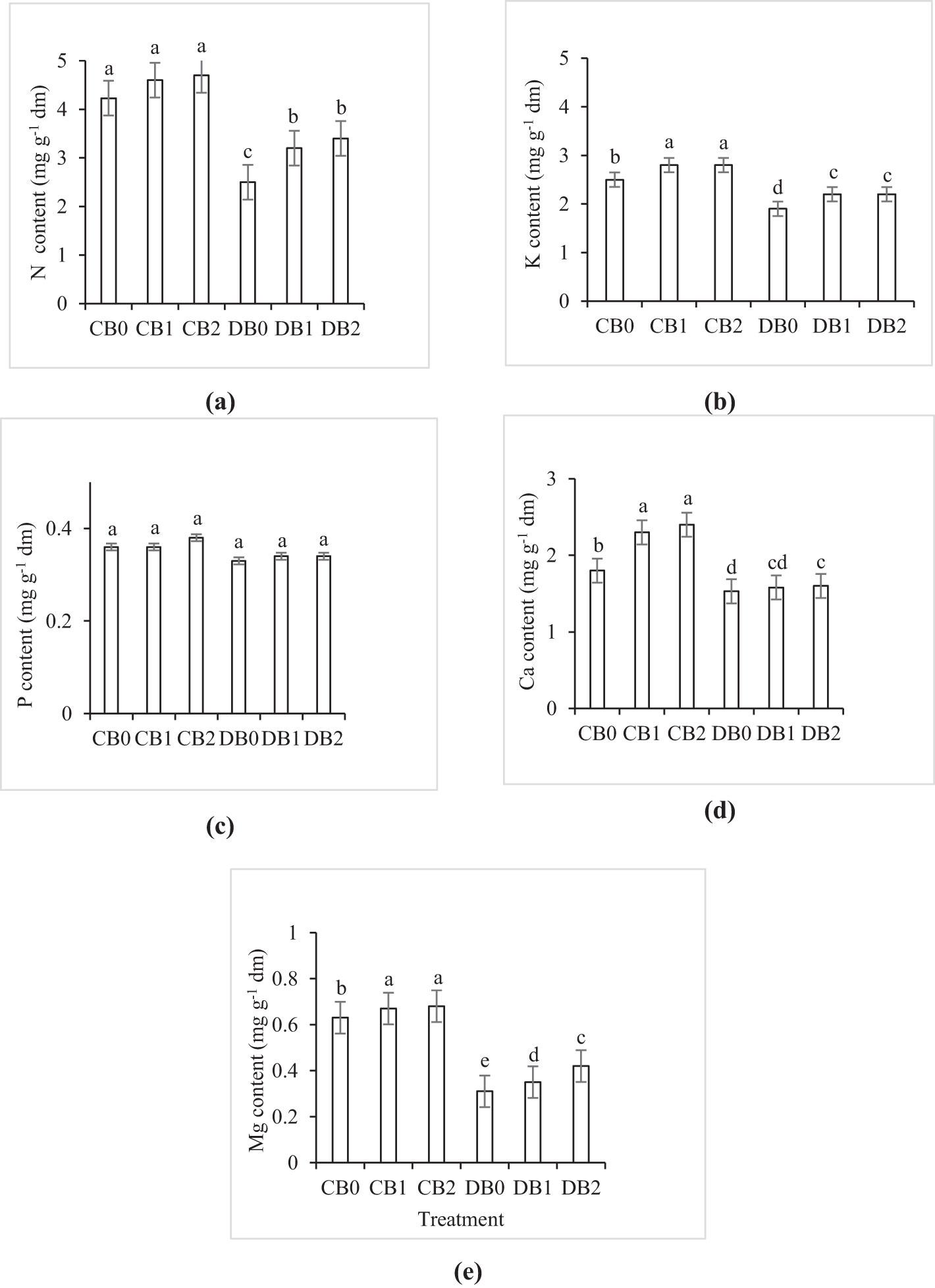
Figure 8. Effect of nano biochar application and drought stress on leaf elemental analysis of faba bean. (A) N; (B) K; (C) P; (D) Ca, and (E) Mg CB0: control − zero biochar application; CB1: control + 2% nano biochar (w/w); CB2: control + 4% nano biochar (w/w); DBO: drought + zero biochar application; DB1: drought + 2% nano biochar (w/w); DB2: drought + 4% nano biochar (w/w). Different letters on bars indicated significant difference at p < 0.05.
3.7 Effect of nano biochar and drought on lipid peroxidation and electrolyte leakage
Data presented in Figure 9 showed that MDA, a marker of lipid peroxidation, and EL were markedly increased under drought stress. Maximum values of MDA (6 µmol g−1 FW) and EL (74%) were recorded in DB0 treatment, whereas minimum values were detected in CB1 and CB2. Application of nano biochar resulted in a significant decrease in MDA and EL in drought stressed faba leaves.
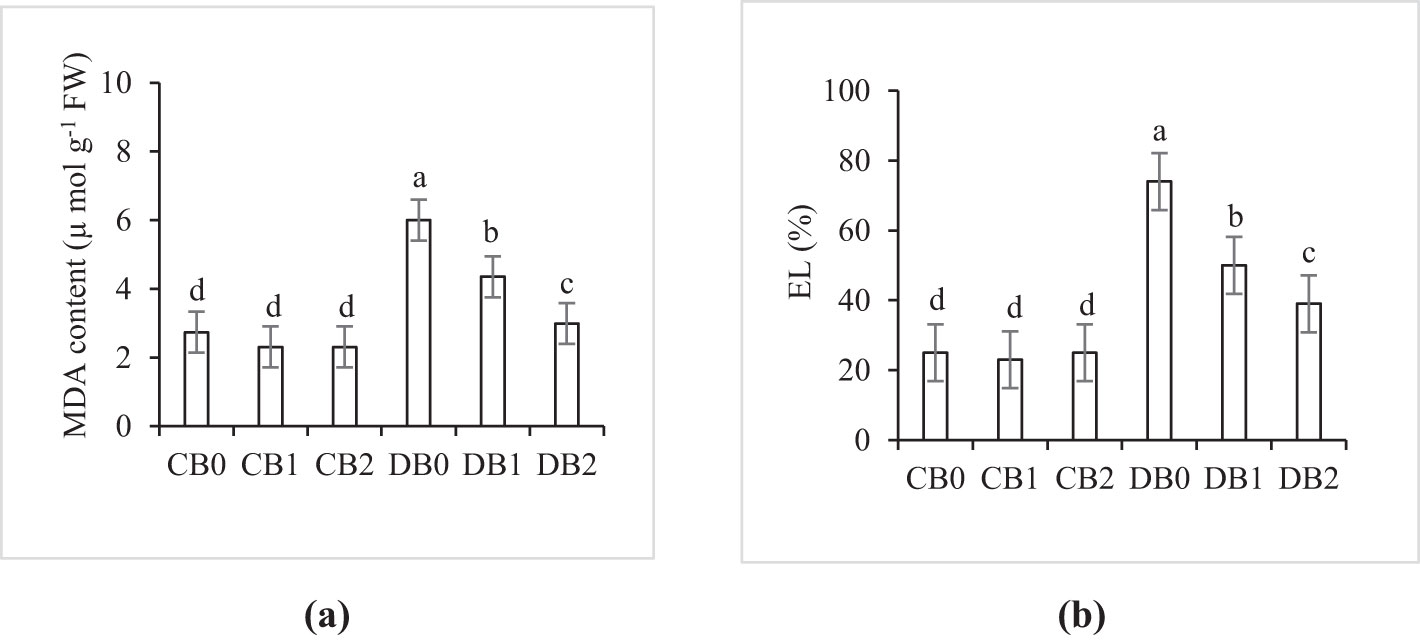
Figure 9. Effect of nano biochar application and drought stress on (A) lipid peroxidation (MDA content) and (B) electrolyte leakage (EL) of faba bean. CB0: control − zero biochar application, CB1: control + 2% nano biochar (w/w); CB2: control + 4% nano biochar (w/w); DBO: drought + zero biochar application; DB1: drought + 2% nano biochar (w/w); DB2: drought + 4% nano biochar (w/w). Different letters on bars indicated significant difference at p < 0.05.
3.8 Principal component analysis
The PCA of correlation between the physiological response of the plants and soil parameters affected by nano biochar application is presented in Figure 7. The PCA showed that the first and the second dimension with eigenvalues explained the cumulative variance (100%) of the total variation in control (Figure 10A) and under drought stress (Figure 10B). The biplot graph from the PCA showed several significant correlations, including a negative correlation between SAR and the measured physiological parameters.
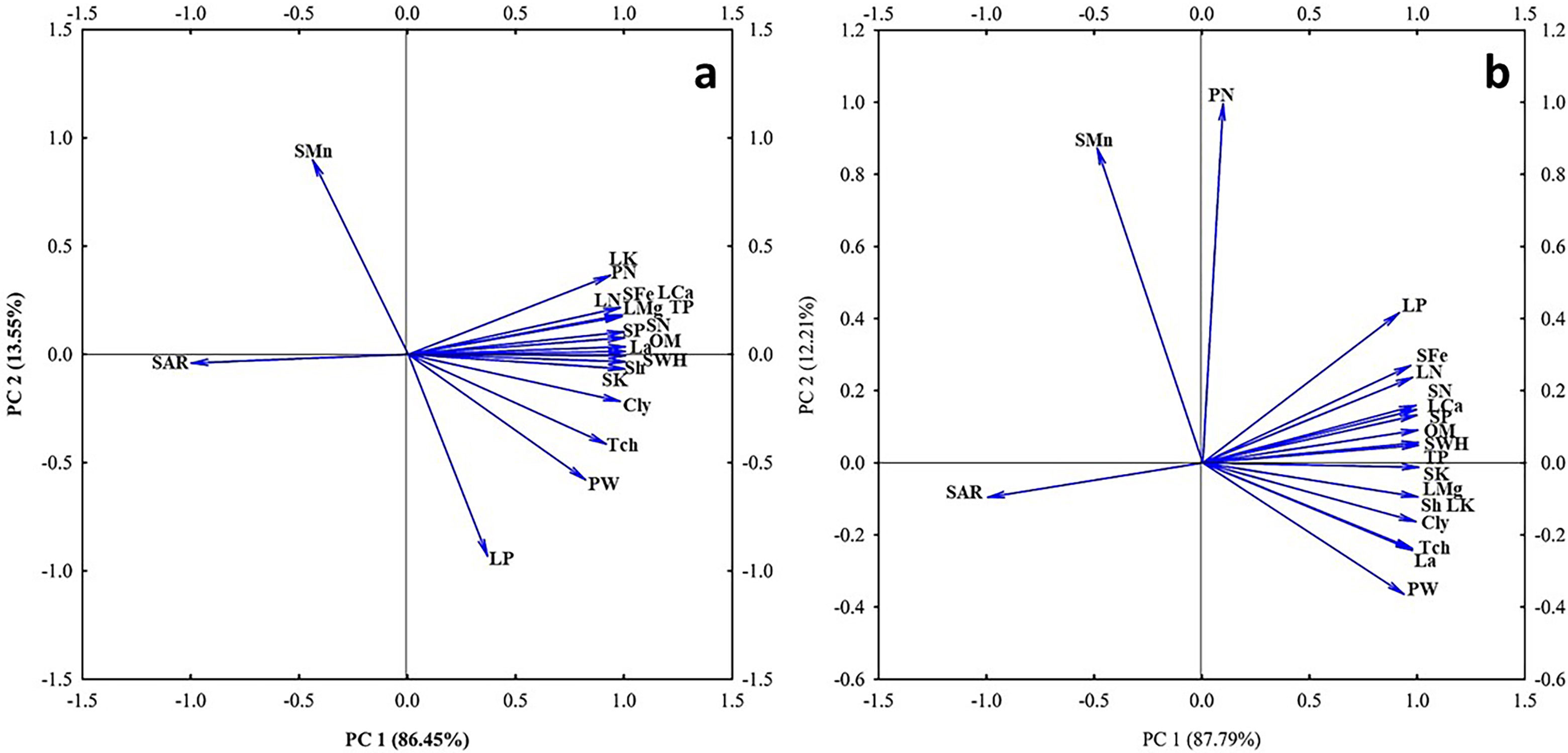
Figure 10. Biplot for the principal component analysis (PCA) describing correlation between the physiological response of the plants and soil parameters affected by nano biochar application. The angles between the vectors derived from the middle point of biplots exhibit positive or negative interactions of studied parameters. Biplot explains correlations between clay % (Cly), organic matter % (OM), soil water holding capacity (SWHC), sodium adsorption (SAR), soil N (SN), soil P (SP), soil K (SK), Soil Fe (SFe), soil Mn (SMn), shoot height (Sh), leaf area (La), total protein (total chlorophyll),total chlorophyll (TCh), pod weight (PW), pod number (PN), leaf N (LN), leaf P (LP), leaf Mg (LMg), and leaf Ca (LCa) under (A) control or (B) drought stress.
Correlograms based on the correlation coefficients between the physiological response of the plants and soil parameters affected by nano biochar application under control or drought stress are shown in Figures 11A, B, respectively. It is apparent from the correlograms that the clay % (Cly), organic matter % (OM), and SWHC are strongly positively correlated with shoot height (Sh), leaf area (La), total protein (total chlorophyll), total chlorophyll (TCh), pod weight (PW), leaf N (LN), leaf P (LP), leaf Mg (LMg), leaf K (LK), and leaf Ca (LCa).
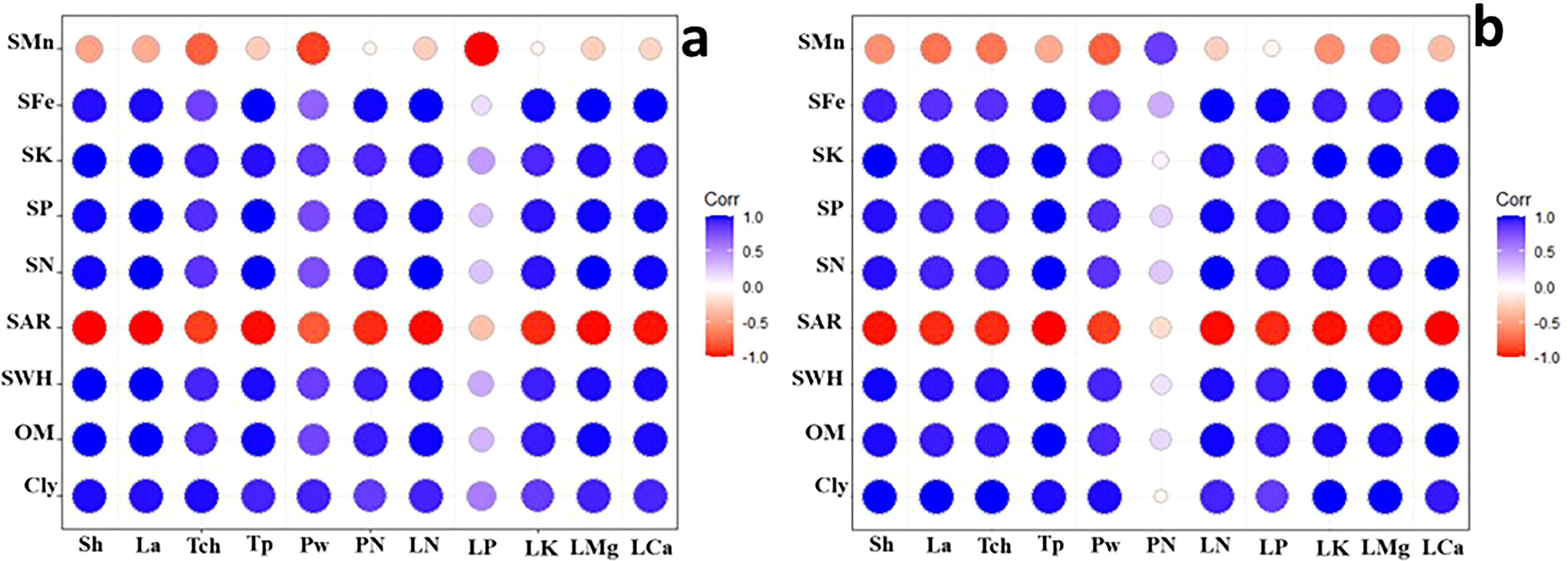
Figure 11. Correlogram based on the correlation coefficients between the physiological response of the plants and soil parameters affected by nano biochar application, under (A) control or (B) drought stress. Red color with −1 indicates a strong negative correlation, whereas white color with 0 means that there is no association between the two variables; however, blue color with 1 indicates a strong positive correlation. Correlation coefficients are proportional to color intensity.
3.9 Factorial analysis
Figure 12 shows the three-dimensional response surface plot of K, Ca, Mg, and P affected by SWHC and SAR under measured (4%) and estimated (6% and 8%) nano biochar application. Increasing SWHC and decreasing SAR result in increasing the K, Ca, Mg, and P content to the maximum level.
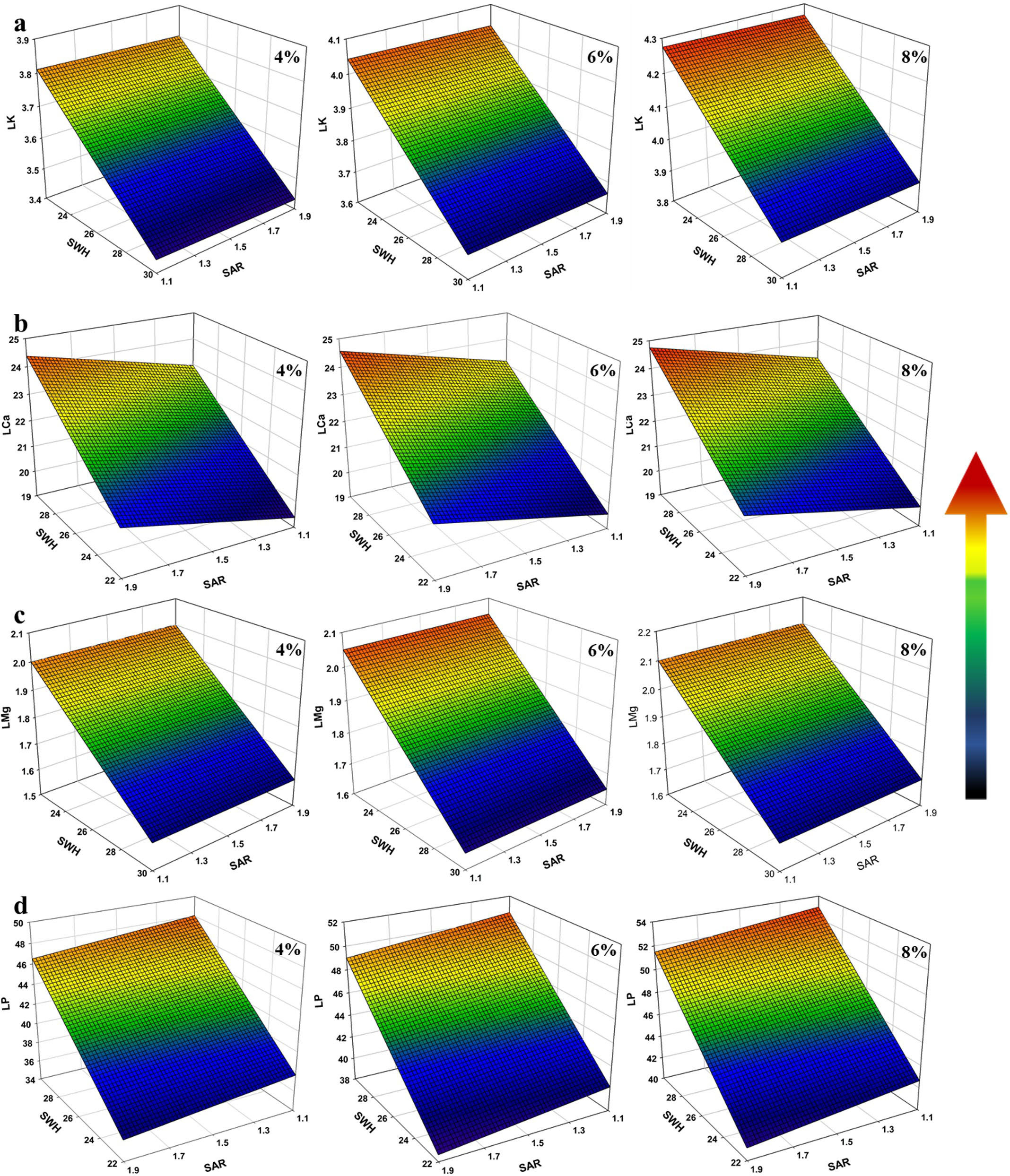
Figure 12. Response surface plot of (A) leaf K, (B) leaf Ca, (C) leaf Mg, and (D) leaf P as a function of soil water holding capacity (SWH) and sodium adsorption ratio (SAR) under measured (4%) and estimated (6% and 8%) nano biochar application.
4 Discussion
Peanut shell is composed of cellulose, hemicellulose, and lignin, and carbon, hydrogen, oxygen and nitrogen elements are their main contents. Generally, the surface of biochar contains plenty of oxygen-containing functional groups such as (–COOH), (–OH), (–COH), (–CONH), and lactonic groups, in addition to nitrogen-containing ones including (–NH2) (Yaashikaa et al., 2020). In the current study, the obtained EC value of the prepared nano biochar (3.40 dS m−1) is in agreement with previously stated values of Xu et al (Xu et al., 2015). The EC value is mostly related to the presence of K+, Ca2+, and Mg2+ (Usman et al., 2015). The obtained pH value of the prepared biochar was 7.4, even though synthesized biochar is mostly alkaline in nature, and variation greatly relies on the feedstock type and pyrolysis conditions (Novak et al., 2009). As shown in the study of Zhang et al (Zhang et al., 2017), low temperatures (<500°C) had a larger impact on biochar pH than high temperatures. The values of the bulk and tapped density for peanut shell were less dense than the prepared biochar, a finding that is in agreement with Wang et al (Wang et al., 2019).
The analysis of the FTIR spectrum of the peanut shell sample shows a broad band at 3,448 cm−1, which corresponds to the mixed stretching vibration absorption bands of alcoholic (–OH) and amino (–NH) groups (Dolatkhah et al., 2019; Sattar et al., 2019). The intensity of this peak decreased and showed a considerable shift to 3,400 cm−1 in the spectrum of the nano biochar, indicating that the conversion of the shell to nano biochar decreased the quantity of hydroxyl groups (Yuan and Xu, 2011). The aliphatic C–H stretching mode appeared at 2,923 cm−1 in both spectra and can be nominated to –CH2 stretching. The peaks at wavenumber (ʋ) 1,738 and 1,643 cm−1 in the spectrum of peanut shell were ascribed to the asymmetric and symmetric stretching of the carboxyl group (COOH) and/or the –COO stretching of amino acids. These two peaks disappeared in the spectrum of the prepared biochar, suggesting the decrease of –COO- content and confirming ignition loss of –COO- under oxygen environment deficiency and a pyrolysis temperature of 450°C. Moreover, thermal cracking occurs and produces volatile matter that improves the amount of aromatic surface functional groups through dehydrogenation of carbohydrates followed by a condensation process (Niazi et al., 2018). Moreover, the peak at 1,030 cm−1 in the peanut shell spectrum representing the vibration of the carboxylic group also disappeared in the biochar spectrum. The peak observed at ca. 1,550 cm−1 in both spectra may be attributed to the ether group (Dolatkhah et al., 2019), while the peak at 1,457 cm−1 corresponds to aromatic C=C and/or C=C–C vibrations or may be indicative of lignin and aromatic carbon. The peak at 1,426 cm−1 in case of peanut shell corresponds to the in-plane bending of carbonyl (–COH), which shifted to 1,430 cm−1 in its biochar (Yuan and Xu, 2011). The observed peaks ranging from 1,200 to 1,150 cm−1 may correspond to the C–O stretching of alcohols, carboxylic acids, esters, and ethers. The spectrum of peanut shell showed a peak at 1,154 cm−1 that may be ascribed to C–O–C symmetric stretching in ester groups of hemicellulose and cellulose. This peak slightly shifted to 1,157 cm−1 in the biochar spectrum. The C–O–C symmetric stretching in aliphatic groups of peanut shell appeared at 1,032 cm−1 (Jiang et al., 2022). The peak that appeared at 754 cm−1 is ascribed to the aromatic C–H out-of-plane stretching (Sattar et al., 2019).
The present study revealed that nano biochar application significantly improved soil physiochemical characteristics. These findings are in line with previous studies (Roberts et al., 2010; Rollon et al., 2020), which showed that applying biochar to agricultural land can improve the chemical and physical properties of the soil, working as an enhancer. SWHC is a primary indicator for soil quality, plant productivity, and microbial activity. The current study seems to be consistent with other studies of Basso et al. (2013) and Wang et al (Wang et al., 2019), which found that biochar amendment resulted in a significant increase in SWHC. A possible explanation for this finding is the polar interaction between OH and C–O–H functional groups on the surface of biochar and water (Lebrun et al., 2022). Furthermore, biochar might indirectly improve water retention through the addition of organic matter (Głąb et al., 2016; Jačka et al., 2018). The particle size of biochar has a vital role in regulating the storage water in soils because it has a great effect on both intrapores and interpores (Liu et al., 2017). When the biochar is added to the soil, the intrapores can hold more water and enhance water retention. Soil pore size distribution can be altered by adding biochar to soils, which affects both the movement and storage of water. The particles of the biochar can fill the big pore spaces that exist in rough grained soils, thus enhancing retention and decreasing the water flow rate (Blanco-Canqui, 2017). In this work, the average diameter of the synthesized nano biochar particles was 21.86 nm. As reported in the literature, a small biochar particle size greatly affects soil water retention compared to a large particle size, because it has a large surface area that can fit easily into the sandy soil’s large pores. Moreover, it can greatly increase meso-porosity even at lower application rates (Edeh and Mašek, 2022). In contrast to these studies, Hardie et al. (2014) and (Jeffery et al. (2015) demonstrated that biochar amendment did not show any significant improvement in soil water retention.
In this work, drought negatively affected the growth, yield, and protein content of faba bean. These results are consistent with many previous studies, which recorded that drought has a devastating effect on plant growth and yield (Farooq et al., 2008; Osakabe et al., 2014; Fahad et al., 2017). However, the application of nano biochar under drought stress resulted in a significant increase in growth, yield, and protein content of faba bean plants. The present findings seem to be consistent with other research that found that applying biochar increased the growth and yield of a number of drought-stressed plants such as tomato (Akhtar et al., 2014), maize (Haider et al., 2015), and okra (Batool et al., 2015). In addition, Sammar Raza (Raza et al., 2023) reported that application of nano biochar improved wheat growth under drought stress. This beneficial role of biochar in alleviating the harmful effects of drought stress in faba bean plants may result from its ability to increase SWHC and organic matter and decrease SAR. Abed Hussein et al. (2022) stated that the addition of nano biochar to the soil resulted in a significant increase in the level of organic matter. In the current study, there is a strong positive correlation that appeared in the correlogram between organic matter % (OM), SWHC, and physiological responses of faba plants (Figure 8). These findings further support the idea of Foster et al. (2016) who stated that the vital role of biochar in improving SWHC is a key factor in enhancing plant growth and yield during drought stress. In addition, Govindasamy et al. (2023) reported that a management’s practice effect on SWHC, whether positive or negative, could directly indicate its possible impact on plant yield.
The application of nano biochar resulted in a significant increase in the levels of available N, P, K, Zn, and Cu. The present findings seem to be consistent with other research, which found that the addition of biochar to soil may improve its nutrient availability (Diamadopoulos, 2019; Edeh and Mašek, 2022). A possible explanation for this enhanced nutrient availability might be the large specific surface and small particle size of the nano biochar that enables it to couple easily with nutrients to become a high-efficiency fertilizer (Guo et al., 2013). Moreover, the low BD of the prepared nano biochar allows it to keep the nutrients and water content, thus minimizing the hardening of soil [application of nano biochar increased SWHC (by 66%), N (by 53%), P (by 55%), and K (by 7%)]. The observed surface area of the prepared nano biochar in the current study (208.2 m2 g−1) represented the impact of the utilized pyrolysis temperature on carbonization. During pyrolysis, the release of volatile matter produced from celluloses and hemicelluloses can enhance the formation of vascular bundle structure in the biochar and thus expand the specific surface area of the biochar. The release of O and H bearing functional groups of phenolic (–OH), alkyl (–CH2), aromatic (–CO), and ester C=O groups could result in the high surface area value, which forms a high sorption capacity to keep soil moisture and nutrients, provides a refuge for beneficial soil microorganisms, minimizes the hardening of soil, and enhances the availability of nutrients (Liang et al., 2006; Agegnehu et al., 2015). In line with this, nano biochar amendment boosted the mineral content of N, K, Ca, and Mg in faba bean leaves under drought stress. In contrast, Brtnicky et al. (2021) and Zhao et al. (2019) found that biochar application may decrease nutrient availability because of its high sorption capacity.
In the current study, the total chlorophyll content of faba bean decreased significantly under drought stress. Similarly, Kiani et al. (2020) reported that water stress disturbs photosynthetic apparatus and decreases the synthesis of photosynthetic pigments. One of the defense strategies the plant uses to lower the quantity of energy received by the leaf during a drought is the loss of photosynthetic pigments (Liu et al., 2017). The significant increase in total chlorophyll content of faba bean in response to nano biochar application of the current study corroborates the ideas of (Azadifar et al. (2023) who stated that nano biochar amendment increased chlorophyll content in broccoli under deficit irrigation. This could be associated with the improvement in mineral content resulting from biochar application (Abideen et al., 2020).
Drought leads to the excessive formation of ROS, which has high affinity to react with nucleic acids, lipids, and proteins, leading to malfunctioning of these macromolecules and accelerating the aging process (Kocsy et al., 2013; Rizwan et al., 2015). In the current study, drought stress elevated MDA and EL %, whereas nano biochar amendment resulted in a significant decrease in the aforementioned parameters. These results, consistent with those of Lyu et al. (2016) and Gharred et al (Gharred et al., 2022), suggest that biochar application under drought stress led to a reduction in the level of MDA. Recently, Rasheed et al. (2024) reported that application of nano biochar enhanced membrane stability and reduced EL in spinach under conditions of drought and salt stress. This could be attributed to the improvement in the WHC and nutrient availability of the soil, which helps in drought amelioration.
The use of nano biochar in soil amelioration depends on several factors, such as the method of preparation and modification of biochar (Yang et al., 2019), type of feedstock (Wijitkosum, 2022), and rate of application (Yeboah et al., 2016). Applying too much biochar can disturb the nutrient availability in soil (Jiang et al., 2022). Surprisingly, factorial analysis of this study indicates that no significant differences were found in the levels of N, K, Mg, and Ca in faba bean plants when biochar was applied at rates of 6% and 8% (expected rates) compared to 4% (measured), as demonstrated in the three-dimensional response surface plot (Figure 9). Hence, the application of biochar as a soil improver is less expensive and more environmentally sustainable.
5 Conclusion
The current study showed that drought caused a significant reduction in growth and yield parameters of faba bean. Cost-effective and eco-friendly nano biochar is produced from peanut shells as a feedstock. The application of the synthesized nano biochar resulted in a significant increase in SWHC, organic matter content, and nutrient levels, which are primary indicators of biochar’s beneficial effects on soil. These improvements were reflected in growth, yield, and a decrease in OS, as indicated by the reduced levels of lipid peroxidation and EL in faba beans under drought stress in the presence of biochar. The principal component and factorial analysis of the present study showed the relationship between the physiological response of faba bean plants and soil parameters affected by the application of nano biochar. This study sheds light on a sustainable way of disposing peanut shells as agricultural waste, which copes with the sustainable development goals declared by the United Nations (Farooq et al., 2008; Ali et al., 2017; Abid et al., 2020; Kumar et al., 2020).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
HA: Writing – review & editing, Writing – original draft, Visualization, Investigation. AE: Writing – review & editing, Writing – original draft, Visualization. AE: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation. KF: Writing – original draft, Methodology. RA: Writing – review & editing, Methodology. MA: Writing – review & editing, Visualization, Investigation. NA: Writing – original draft, Visualization. SA: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant No. A438).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abed Hussein, B., Mahdi, A. B., Izzat, S. E., Dwijendra, N. K. A., Romero Parra, R. M., Barboza Arenas, L. A., et al. (2022). Production, structural properties nano biochar and effects nano biochar in soil: a review. Egyptian J. Chem. 65, 607–618. doi: 10.21608/ejchem.2022.131162.5772
Abid, G., Ouertani, R., Jebara, S., Boubakri, H., Muhovski, Y., Ghouili, E., et al. (2020). Alleviation of drought stress in faba bean (Vicia faba L.) by exogenous application of β-aminobutyric acid (BABA). Physiol. Mol. Biol. Plants. 26, 1173–1186. doi: 10.1007/s12298-020-00796-0
Abideen, Z., Koyro, H. W., Huchzermeyer, B., Ansari, R., Zulfiqar, F., Gul, B. (2020). Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol. 22, 259–266. doi: 10.1111/plb.13054
Abo-Hegazy, S. R., Darwish, D. S. (2022). Genotype x Environment Analysis of Egyptian Faba Bean Cultivars and their Resilience to Different Sowing Dates in Egypt. Sci. J. Agric. Sci. 4, 102–113. doi: 10.21608/sjas.2022.132734.1213
Afzal, S., Alghanem, S. M. S., Alsudays, I. M., Malik, Z., Abbasi, G. H., Ali, A., et al. (2024). Effect of biochar, zeolite and bentonite on physiological and biochemical parameters and lead and zinc uptake by maize (Zea Mays L.) plants grown in contaminated soil. J. Hazardous Materials. 469, 133927. doi: 10.1016/j.jhazmat.2024.133927
Agegnehu, G., Bass, A. M., Nelson, P. N., Muirhead, B., Wright, G., Bird, M. I. (2015). Biochar and biochar-compost as soil amendments: Effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agriculture Ecosyst. Environ. 213, 72–85. doi: 10.1016/j.agee.2015.07.027
Akhtar, S., Li, G., Andersen, M., Liu, F. (2014). Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Management. 138, 37–44. doi: 10.1016/j.agwat.2014.02.016
Ali, S., Rizwan, M., Qayyum, M. F., Ok, Y. S., Ibrahim, M., Riaz, M., et al. (2017). Biochar soil amendment on alleviation of drought and salt stress in plants: a critical review. Environ. Sci. pollut. Res. 24, 12700–12712. doi: 10.1007/s11356-017-8904-x
Allen, S. E., Grimshaw, H. M., Parkinson, J. A., Quarmby, C., Roberts, J. D. (1974). Chemical Analysis of Ecological Materials (Oxford, London: Osney Blackwell Scientific Publ.).
Amiri, H., Zamani, Z., Arnao, M. B., Ismaili, A., Gavyar and Khodayari, P. H. H. H. (2024). Optimal concentration of melatonin enhances drought stress tolerance in fenugreek. Acta Physiologiae Plantarum. 46, 15. doi: 10.1007/s11738-023-03643-3
Azadifar, A., Zare Abyaneh, H., Sarikhani, H., Mosaddeghi, M. (2023). Broccoli response to application of natural nanobiochar amendment, deficit irrigation and nitrogen fertigation. J. Soil Water Conserv. 30, 29–56. doi: 10.22069/jwsc.2023.21265.3661
Basso, A. S., Miguez, F. E., Laird, D. A., Horton, R., Westgate, M. (2013). Assessing potential of biochar for increasing water-holding capacity of sandy soils. Gcb Bioenergy 5, 132–143. doi: 10.1111/gcbb.2013.5.issue-2
Batool, A., Taj, S., Rashid, A., Khalid, A., Qadeer, S., Saleem, A. R., et al. (2015). Potential of soil amendments (Biochar and Gypsum) in increasing water use efficiency of Abelmoschus esculentus L. Moench. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00733
Blanco-Canqui, H. (2017). Biochar and soil physical properties. Soil Sci. Soc. America J. 81 (4), 687–711. doi: 10.2136/sssaj2017.01.0017
Blume, H. P., Page, A. L., Miller, R. H., Keeney, D. R. (Eds.) (19821985). “Methods of soil analysis,” in Chemical and microbiological properties, vol. 148 (American Soc. of Agronomy (Publ, Madison, Wisconsin, USA), 363–364. 2. Aufl. 1184 Sgebunden 36 Dollar. Zeitschrift für Pflanzenernährung und Bodenkunde.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Brtnicky, M., Datta, R., Holatko, J., Bielska, L., Gusiatin, Z. M., Kucerik, J., et al. (2021). A critical review of the possible adverse effects of biochar in the soil environment. Sci. total environment. 796, 148756. doi: 10.1016/j.scitotenv.2021.148756
Chaieb, N., Bouslama, M., Mars, M. (2011). Growth and yield parameters variability among faba bean (Vicia faba L.) genotypes. J. Nat. Prod. Plant Resour 1, 81–90.
Chapman, H. D., Pratt, P. F. (1962). Methods of analysis for soils, plants and waters. Soil Sci. 93, 68. doi: 10.1097/00010694-196201000-00015
Crépon, K., Marget, P., Peyronnet, C., Carrouée, B., Arese, P., Duc, G. (2010). Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crops Res. 115, 329–339. doi: 10.1016/j.fcr.2009.09.016
De Cillis, F., Leoni, B., Massaro, M., Renna, M., Santamaria, P. (2019). Yield and quality of faba bean (Vicia faba L. var. major) genotypes as a vegetable for fresh consumption: A comparison between Italian landraces and commercial varieties. Agriculture 9, 253. doi: 10.3390/agriculture9120253
Diamadopoulos, M. (2019). Positive effects of biochar and biochar-compost on maize growth and nutrient availability in two agricultural soils. Communicationin Soil Sci. Plant Analyis. 50, 512–552. doi: 10.1080/00103624.2019.1566468
Dionisio-Sese, M. L., Tobita, S. (1998). Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 135, 1–9. doi: 10.1016/S0168-9452(98)00025-9
Dolatkhah, Z., Mohammadkhani, A., Javanshir, S., Bazgir, A. (2019). Peanut shell as a green biomolecule support for anchoring Cu2O: a biocatalyst for green synthesis of 1, 2, 3-triazoles under ultrasonic irradiation. BMC Chem. 13, 1–10. doi: 10.1186/s13065-019-0612-9
Edeh, I. G., Mašek, O. (2022). The role of biochar particle size and hydrophobicity in improving soil hydraulic properties. Eur. J. Soil Science. 73, e13138. doi: 10.1111/ejss.13138
Fahad, S., Bajwa, A. A., Nazir, U., Anjum, S. A., Farooq, A., Zohaib, A., et al. (2017). Crop production under drought and heat stress: plant responses and management options. Front. Plant Sci. 8, 1147. doi: 10.3389/fpls.2017.01147
Farooq, M., Basra, S. M. A., Wahid, A., Cheema, Z. A., Cheema, M. A., Khaliq, A. (2008). Physiological role of exogenously applied glycinebetaine to improve drought tolerance in fine grain aromatic rice (Oryza sativa L.). J. Agron. Crop Sci. 194, 325–333. doi: 10.1111/j.1439-037X.2008.00323.x
Food and Agriculture Organization of the United Nations FAOSTAT. Available online at: www.fao.org/faostat/en/data/QC (Accessed 9 November 2023).
Foster, E. J., Hansen, N., Wallenstein, M. (2016). Cotrufo, M. F.Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agriculture Ecosyst. Environ. 233, 404–414. doi: 10.1016/j.agee.2016.09.029
Gaskin, J. W., Steiner, C., Harris, K., Das, K. C., Bibens, B. (2008). Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 51, 2061–2069. doi: 10.13031/2013.25409
Genesio, L., Miglietta, F., Baronti, S., Vaccari, F. P. (2015). Biochar increases vineyard productivity without affecting grape quality: Results from a four years field experiment in Tuscany. Agriculture Ecosyst. Environ. 201, 20–25. doi: 10.1016/j.agee.2014.11.021
Gharred, J., Derbali, W., Derbali, I., Badri, M., Abdelly, C., Slama, I., et al. (2022). Impact ofBiochar Application at Water Shortage on Biochemical andPhysiological Processes in Medicago ciliaris. Plants 11, 2411. doi: 10.3390/plants11182411
Głąb, T., Palmowska, J., Zaleski, T., Gondek, K. (2016). Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Geoderma 281, 11–20. doi: 10.1016/j.geoderma.2016.06.028
Govindasamy, P., Mahawer, S. K., Mowrer, J., Bagavathiannan, M., Prasad, M., Ramakrishnan, S., et al. (2023). Chandra, A.Comparison of low-cost methods for soil water holding capacity. Commun. Soil Sci. Plant Analysis. 54, 287–296. doi: 10.1080/00103624.2022.2112216
Gullap, M., Severoglu, S., Karabacak, T., Yazici, A., Ekinci, M., Turan, M., et al. (2024). Biochar derived from hazelnut shells mitigates the impact of drought stress on soybean seedlings. New Z. J. Crop Hortic. Sci. 52, 19–37. doi: 10.1080/01140671.2022.2079680
Guo, Y., Li, Y., Zhu, T., Ye, M. (2013). Effects of concentration and adsorption product on the adsorption of SO2 and NO on activated carbon. Energy Fuels 27, 360–366. doi: 10.1021/ef3016975
Haider, G., Koyro, H.-W., Azam, F., Steffens, D., Müller, C., Kammann, C. (2015). Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil 395, 141–157. doi: 10.1007/s11104-014-2294-3
Hardie, M., Clothier, B., Bound, S., Oliver, G., Close, D. (2014). Does biochar influence soil physical properties and soil water availability? Plant Soil. 376, 347–361. doi: 10.1007/s11104-013-1980-x
Harkhani, K., Sharma, A. K. (2024). Alleviation of drought stress by plant growth-promoting rhizobacteria (PGPR) in crop plants: A review. Commun. Soil Sci. Plant Anal. 55 (5), 735–758. doi: 10.1080/00103624.2023.2276265
Heath, R. L., Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. biophysics 125, 189–198. doi: 10.1016/0003-9861(68)90654-1
Inskeep, W. P., Bloom, P. R. (1985). Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80% acetone. Plant Physiol. 77, 483–485. doi: 10.1104/pp.77.2.483
Isaac, R. A. (1980). Atomic absorption methods for analysis of soil extracts and plant tissue digests. J. Assoc. Off. Analytical Chem. 63, 788–796. doi: 10.1093/jaoac/63.4.788
Jačka, L., Trakal, L., Ouředníček, P., Pohořelý, M., Šípek, V. (2018). Biochar presence in soil significantly decreased saturated hydraulic conductivity due to swelling. Soil Tillage Res. 184, 181–185. doi: 10.1016/j.still.2018.07.018
Jeffery, S., Meinders, M. B. J., Stoof, C. R., Bezemer, T. M., van de Voorde, T. F. J., Mommer, L., et al. (2015). Biochar application does not improve the soil hydrological function of a sandy soil. Geoderma 251–252, 47–54. doi: 10.1016/j.geoderma.2015.03.022
Jiang, S., Yan, L., Wang, R., Li, G., Rao, P., Ju, M., et al. (2022). Recyclable nitrogen-doped biochar via low-temperature pyrolysis for enhanced lead (II) removal. Chemosphere 286, 1–8. doi: 10.1016/j.chemosphere.2021.131666
Jones, J. J.B., Case, V. W. (1990). Sampling, handling, and analyzing plant tissue samples. Soil testing Plant Anal. 3, 389–427. doi: 10.2136/sssabookser3.3ed.c15
Kiani, R., Nazeri, V., Shokrpour, M., Hano, C. (2020). Morphological, physiological, and bio-chemical impacts of different levels of long-term water deficit stress on Linum album Ky. ex Boiss. Accessions. Agronomy. 10, 1966. doi: 10.3390/agron-omy10121966
Klute, A. (1986). Water retention: laboratory methods. Methods Soil analysis: Part 1 Phys. mineralogical Methods 5, 635–662. doi: 10.2136/sssabookser5.1.2ed.c26
Kocsy, G., Tari, I., Vanková, R., Zechmann, B., Gulyás, Z., Poór, P., et al. (2013). Redox control of plant growth and development. Plant Science. 211, 77–91. doi: 10.1016/j.plantsci.2013.07.004
Kumar, M., Xiong, X., Sun, Y., Yu, I. K. M., Tsang, D. C. W., Hou, D., et al. (2020). Critical review on biochar-supported catalysts for pollutant degradation and sustainable biorefinery. Advanced Sustain. Syst. 4, 1–20. doi: 10.1002/adsu.201900149
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature 227, 680–685. doi: 10.1038/227680a0
Lalay, G., Ullah, S., Ahmed, I. (2022). Physiological and biochemical responses of Brassica napus L. @ to drought-induced stress by the application of biochar and Plant Growth Promoting Rhizobacteria. Microscopy Res. technique 85, 1267–1281. doi: 10.1002/jemt.23993
Lebrun, M., Bouček, J., Bímová, K. B., Kraus, K., Haisel, D., Kulhánek, M., et al. (2022). Biochar in manure can suppress water stress of sugar beet (Beta vulgaris) and increase sucrose content in tubers. Sci. Total Environ. 814, 152772. doi: 10.1016/j.scitotenv.2021.152772
Lesch, S. M., Suarez, D. L. (2009). A short note on calculating the adjusted SAR index. Trans. ASABE 52, 493–496. doi: 10.13031/2013.26842
Liang, B., Lehmann, J., Solomon, D., Kinyangi, J., Grossman, J., O'Neill, B., et al. (2006). Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. America J. 70, 1719–1730. doi: 10.2136/sssaj2005.0383
Licht, J., Smith, N. (2017). The influence of lignocellulose and hemicellulose biochar on photosynthesis and water use efficiency in seedlings from a Northeastern US pine-oak ecosystem. J. Sustain. Forestry 37, 25–37. doi: 10.1080/10549811.2017.1386113
Liu, Z., Dugan, B., Masiello, C. A., Gonnermann, H. M. (2017). Biochar particle size, shape, and porosity act together to influence soil water properties. PloS One 12 (6), e0179079. doi: 10.1371/journal.pone.0179079
Liu, C., Wang, Y., Pan, K., Wang, Q., Liang, J., Jin, Y., et al. (2017). The synergistic re-sponses of different photoprotective pathways in dwarf bamboo (Fargesia rufa) to drought and subsequent rewatering. Front. Plant Science. 8, 489. doi: 10.3389/fpls.2017.00489
Lyu, S., Du, G., Liu, Z., Zhao, L., Lyu, D. (2016). Effects of biochar on photosystem function and activities of protective enzymes in Pyrusus suriensis Maxim. under drought stress. Acta Physiologiae Plantarum 38, 1–10. doi: 10.1007/s11738-016-2236-1
Martínez, J.-P., Silva, H., Ledent, J.-F., Pinto, M. (2007). Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur. J. Agron. 26, 30–38. doi: 10.1016/j.eja.2006.08.003
Milford, G. F. J., Pocock, T. O., Riley, J. (1985). An analysis of leaf growth in sugar beet. Ann. Appl. Biol. 106, 173–185. doi: 10.1111/j.1744-7348.1985.tb03107.x
Niazi, N. K., Bibi, I., Shahid, M., Ok, Y. S., Burton, E. D., Wang, H., et al. (2018). Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: an integrated spectroscopic and microscopic examination. Environ. pollut. 232, 31–41. doi: 10.1016/j.envpol.2017.09.051
Novak, J., Lima, I., Xing, B., Gaskin, J., Steiner, C., Das, K., et al. (2009). Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. science. 3, 1939–2621. Available at: https://openjournals.neu.edu/aes/journal/article/view/v3art5.
Okalebo, J. R., Gathua, K. W., Woomer, P. L. (2002). Laboratory methods of soil and plant analysis: a working manual second edition. Sacred Africa Nairobi 21, 25–26.
Osakabe, Y., Osakabe, K., Shinozaki, K., Tran, L.-S. P. (2014). Response of plants to water stress. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00086
Quarshie, V. G., Abo Hegazy, S. R., Yacoub, I. H., Darwish, D. S. (2021). Performance and variation of some faba bean genotypes to broomrape infestation and environmental conditions. Plant Arch. 21, 357–364. doi: 10.51470/PLANTARCHIVES.2021.v21.S1.057
Rady, M. M., Boriek, S. H. K., Abd El-Mageed, T. A., Seif El-Yazal, M. A., Ali, E. F., Hassan, F. A. S., et al. (2021). Exogenous Gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by Rebalancing osmoprotectants, antioxidants,Nutrients, and phytohormones. Plants 10, 748. doi: 10.3390/plants10040748
Rafael, R. B. A., FernÁNdez-Marcos, M. L., Cocco, S., Ruello, M. L., Fornasier, F., Corti, G. (2019). Benefits of biochars and NPK fertilizers for soil quality and growth of cowpea (Vigna unguiculata L. Walp.) in an acid Arenosol. Pedosphere 29, 311–333. doi: 10.1016/S1002-0160(19)60805-2
Rasheed, A., Anwar, S., Shafiq, F., Khan Ashraf, S. M. (2024). Physiological and biochemical effects of biochar nanoparticles on spinach exposed to salinity and drought stresses. Environ. Sci. pollut. Res. 31, 14103–14122. doi: 10.1007/s11356-024-31953-7
Raza, M.A., Shah, A. N., Shahid, M. A., Nawaz, M., Ibrahim, M. A., Iqbal, R., et al. (2024). Seed priming with selenium improves growth and yield of quinoa plants suffering drought. Sci. Rep. 14, 886. doi: 10.1038/s41598-024-51371-6
Raza, M. A. S., Shah, A. N., Shahid, M. A., Nawaz, M., Ibrahim, M. A., Iqbal, R., et al. (2023). Nano-biochar enhances wheat crop productivity by vindicating the effects of drought: in relation to physiological and phenological stages. AliACS Omega 8, 37808–37819. doi: 10.1021/acsomega.3c01629
Rizwan, M., Ali, S., Ibrahim, M., Farid, M., Adrees, M., Bharwana, S. A., et al. (2015). Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environ. Sci. pollut. Res. 22, 15416–15431. doi: 10.1007/s11356-015-5305-x
Roberts, K. G., Gloy, B. A., Joseph, S., Scott, N. R., Lehmann, J. (2010). Life cycle assessment of biochar systems: estimating the energetic, economic, and climate change potential. Environ. Sci. Technol. 44, 827–833. doi: 10.1021/es902266r
Rollon, R. J. C., Malig-on, P. L. B., Guindang, P. R., Dolorito, R. V. (2020). Corn (Zea mays L.) growth, nutrient uptake and soil fertility improvement of strongly acidic soil applied with biochar and animal manure. J. Appl. Biol. Biotechnol. 8, 35–40. doi: 10.7324/JABB.2020.80206
Sattar, M. S., Shakoor, M. B., Ali, S., Rizwan, M., Niazi, N. K., Jilani, A. (2019). Comparative efficiency of peanut shell and peanut shell biochar for removal of arsenic from water. Environ. Sci. pollut. Res. 26, 18624–18635. doi: 10.1007/s11356-019-05185-z
Sears, G. W. (1956). Determination of specific surface area of colloidal silica by titration with sodium hydroxide. Analytical Chem. 28, 1981–1983. doi: 10.1021/ac60120a048
Shafiq, F., Anwar, S., Firdaus-e-Bareen, Zhang, L., Ashraf, M. (2023). Nano-biochar: Properties and prospects for sustainable agriculture. Land Degrad. Deve. 34, 2445–2463. doi: 10.1002/ldr.4620
Sivasubramaniawn, K. (1992). Chlorophyll stability index method for determining drought hardiness of acacia species. Nitr. Fix. Tree Res. Reports. 10, 111–112.
Team, R.C (2013). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing).
Usman, A. R. A., Abduljabbar, A., Vithanage, M., Ok, Y. S., Ahmad, M., Ahmad, M., et al. (2015). Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Analytical Appl. Pyrolysis. 115, 392–400. doi: 10.1016/j.jaap.2015.08.016
Walkley, A., Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
Wang, J. C., Kinsella, J. E. (1976). Functional properties of novel proteins: Alfalfa leaf protein. J. Food Sci. 41, 286–292. doi: 10.1111/j.1365-2621.1976.tb00602.x
Wang, D., Li, C., Parikh, S. J., Scow, K. M. (2019). Impact of biochar on water retention of two agricultural soils–A multi-scale analysis. Geoderma 340, 185–191. doi: 10.1016/j.geoderma.2019.01.012
Wijitkosum, S. (2022). Biochar derived from agricultural wastes and wood residues for sustainable agricultural and environmental applications. Int. Soil Water Conserv. Res. 10, 335–341. doi: 10.1016/j.iswcr.2021.09.006
Xu, C.-Y., Bai, S. H., Hao, Y., Rachaputi, R. C. N., Xu, Z., Wallace, H. M. (2015). Peanut shell biochar improves soil properties and peanut kernel quality on a red Ferrosol. J. Soils Sediments 15, 2220–2231. doi: 10.1007/s11368-015-1242-z
Yaashikaa, P. R., Kumar, P. S., Varjani, S., Saravanan, A. (2020). A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 28, e00570. doi: 10.1016/j.btre.2020.e00570
Yang, X., Zhang, S., Ju, M., Liu, L. (2019). Preparation and modification of biochar materials and their application in soil remediation. Appl. Sci. 9, 1–25. doi: 10.3390/app9071365
Yeboah, E., ASamoah, G., Kofi, B., Abunyewa, A. A. (2016). Effect of biochar type and rate of application on maize yield indices and water use efficiency on an Ultisol in Ghana. Energy Procedia. 93, 14–18. doi: 10.1016/j.egypro.2016.07.143
Yuan, J. H., Xu, R. K. (2011). The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manage. 27, 110–115. doi: 10.1111/j.1475-2743.2010.00317.x
Zhang, H., Chen, C., Gray, E. M., Boyd, S. E. (2017). Effect of feedstock and pyrolysis temperature on properties of biochar governing end use efficacy. Biomass Bioenergy 105, 136–146. doi: 10.1016/j.biombioe.2017.06.024
Zhao, J., Shen, X.-J., Domene, X., Alcañiz, J.-M., Liao, X., Palet, C. (2019). Comparison of biochars derived from different types of feedstock and their potential for heavy metal removal in multiple-metal solutions. Scitific. Rep. 9, 9869. doi: 10.1038/s41598-019-46234-4
Keywords: broad bean, nanoparticles, green chemistry, biochar, drought
Citation: Abd El-Moaty HI, El-Dissouky A, Elhusseiny AF, Farag KM, Abu-Khudir R, Alkuwayti MA, Al Abdulsalam NK and Abdel Rahman SM (2024) Low-cost nano biochar: a sustainable approach for drought stress mitigation in faba bean (Vicia faba L.). Front. Plant Sci. 15:1438893. doi: 10.3389/fpls.2024.1438893
Received: 26 May 2024; Accepted: 15 October 2024;
Published: 12 November 2024.
Edited by:
Karl H. Hasenstein, University of Louisiana at Lafayette, United StatesReviewed by:
Shamsul Haque, Charles Sturt University, AustraliaSurendra Kumar Meena, Indian Grassland and Fodder Research Institute (ICAR), India
Copyright © 2024 Abd El-Moaty, El-Dissouky, Elhusseiny, Farag, Abu-Khudir, Alkuwayti, Al Abdulsalam and Abdel Rahman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heba Ibrahim Abd El-Moaty, aGl0b3JrZXlAa2Z1LmVkdS5zYQ==
 Heba Ibrahim Abd El-Moaty
Heba Ibrahim Abd El-Moaty Ali El-Dissouky
Ali El-Dissouky Amel F. Elhusseiny3
Amel F. Elhusseiny3 Rasha Abu-Khudir
Rasha Abu-Khudir