
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 06 August 2024
Sec. Functional and Applied Plant Genomics
Volume 15 - 2024 | https://doi.org/10.3389/fpls.2024.1393402
 Huaifeng Wang1,2†
Huaifeng Wang1,2† Haixing Yang1†
Haixing Yang1† Xuena Yu1
Xuena Yu1 Yongdong Xie3
Yongdong Xie3 Yu Bai1
Yu Bai1 Qiya Dai1
Qiya Dai1 Le Liang1
Le Liang1 Wen Tang1
Wen Tang1 Mao Yong2
Mao Yong2 Luzhou Wang2
Luzhou Wang2 Zhi Huang1
Zhi Huang1 Bo Sun1
Bo Sun1 Huanxiu Li1
Huanxiu Li1 Yi Tang1*
Yi Tang1*Introduction: Allium is important vegetables and seasonings in China, Tibet is rich in unique resources of the genus Allium, but lacks development and utilization.
Methods: We compared the biological features and comprehensively evaluating the quality of twelve germplasm resources of the genus Allium collected from Tibet.
Results: The results revealed that nine germplasm resources were bolting and bloom normally except for SC015, SC019, and SC048, all twelve germplasm resources were able to vegetative growth. The individual differences in moisture, soluble sugar, and protein content among the twelve germplasm resources were relatively small, with pyruvic acid content ranging from 0.11 to 1.12 mg/g and a large variation coefficient. A total of 8 categories and 97 volatile compounds were detected in twelve germplasm resources, the majority possessed the highest proportions of aldehydes and organosulfur compounds, but there were certain differences between the different Allium species. Additionally, 11 to 16 types of free amino acids were present in all germplasm resources, proline exhibited the highest content. The total content of essential and non-essential amino acids in SC009 was the highest. Carbon (C) accounted for the largest proportion of all elements, and the contents of other mineral elements varied greatly among the different plants.
Conclusion: In conclusion, combined with biological performance and comprehensive evaluation of quality, SC009 is the excellent germplasm resource suitable for growth and capable of reproduction with good quality. These results improved the exploitation and utilization of the genus Allium in Tibet, as well as provided germplasm resources for high-quality breeding of the genus Allium.
Allium L. is a perennial herb and belonging to the Amaryllidaceae family in the order Asparagales of monocotyledonous plants according to APG III (The Angiosperm Phylogeny Group, 2009) and APG IV (The Angiosperm Phylogeny Group, 2016). As the largest genera in the monocotyledon, the genus Allium contains 973 species and mainly distributed in tropical, temperate and semi-arid regions of the Northern Hemisphere (Sharifi-Rad et al., 2016; Hauenschild et al., 2017; Yan et al., 2023). Garlic (A. sativum), onion (Allium cepa), chive (A. schoenoprasum), shallot (A. hirtifolium), scallion (A. chinense), and leek (Allium ampeloprasum) are the most common and popular Allium members (Jin et al., 2018; Yan et al., 2023). These plants of genus Allium show rich biological activities including antimicrobial, antibacterial, antifungal, antiviral, antiparasitic, antioxidant, antitumor, immunoregulatory, and anti-inflammatory (Yan et al., 2023). For many years, they have been used as edible vegetable and medicinal to treat certain diseases such as fever, influenza, diabetes, arthritis, headache, hemorrhage, asthma, atherosclerosis, inflammatory diseases and other pathological condition (Lee et al., 2014; Marefati et al., 2021; Zolfaghari et al., 2021). Interestingly, it has recently been noted that these vegetables showed extensive potentials against the risk of cancer (Chu et al., 2017). The study on human leukocytes shows that onion extracts have strong protective effects on the DNA molecule and strong antiproliferative activity on human cancer cell lines, especially glioblastoma cells (Fredotović et al., 2017). The water leek extract at the concentration of 50 μg/mL suppresses the growth of MCF-7 cells human breast cancer cell more than 50% after incubation for three days (Zamri and Hamid, 2019). These functions, bioactivities, and health benefits are largely associated with the diverse bioactive components, including steroidal saponins, anthocyanins, gallic, ferulic acids, quercetin, alkaloids, and tannins (Asemani et al., 2019; Marefati et al., 2021). Furthermore, more and more studies suggest that much medical uses of the Allium species might be due to the presence of organosulfur compounds (OSCs), like ajoene, diallyl disulfide (DADS), diallyl trisulfide (DATS), polysulfanes, sulfoxides including allicin (S-allyll-L-cysteine sulfoxides), isoallicin (S-L-acryll-Lcysteine sulfoxides), methiin (S-methyl-L-cysteine sulfoxides) and propiin (S-propyl-L-cysteine sulfoxides) (Putnik et al., 2019; Subramanian et al., 2020). These organosulfur constituents not only possessed significant pharmacological activity, especially anticancer properties, but also give a sharp and tang odors and pungent spicy taste of Allium plants (Scherer et al., 2009; Alam et al., 2023). Because of abundant edible and medicinal value of Allium plants, they have been used as edible vegetable, medicinal and spice plant and widely cultivated worldwide (Scherer et al., 2009; Asemani et al., 2019; Bastaki et al., 2021).
At present, more than 141 million tons of crops of the genus Allium were produced worldwide (Yang et al., 2023), it has become the seventh largest vegetable that is cultivated and consumed throughout the world (Poojary et al., 2017; Fredotović and Puizina, 2019). The Qinghai-Tibet Plateau (QTP) is the highest and largest plateau in the world with an average elevation of greater than 4,000 m (Guo et al., 2022; Wang et al., 2023). Due to its vast territory, complex landforms, large differences in altitude, diverse climate, and ecological types, ecological diversity is extremely abundant with approximately 111 species of Allium genus growing on or surrounding the QTP (Hauenschild et al., 2017). Located in the core of the Qinghai-Tibet Plateau, Tibet is rich in unique resources of genus Allium, 32 species of wild resources have been discovered, including A. atrosanguineum, A. hookeri, A. przewalskianum, A. fasciculatum, and A. sikkimense Baker (Xie et al., 2019; Guo et al., 2022).
After a long-term scientific investigation, we collected a variety of cultivated and wild germplasm resources of the genus Allium in Tibet, but most of them have not been effectively developed and utilized. By comparing phenological phase, morphological characteristics, including plant height and fresh weight, leave number and size, pseudostem size, flowering stalk size, bud size, and comprehensively evaluating the basic nutrient quality and organosulfur compounds (OSCs), free amino acids, and mineral contents, we were intended to screen for the germplasm resource of the genus Allium that were suitable for growth and capable of reproduction with good quality. Our study not only effectively improves the exploration, development, and utilization of the resources of the genus Allium in Tibet, but also provides germplasm resources for high quality breeding of the genus Allium.
Twelve germplasm resources of the genus Allium were collected from different regions of Tibet and uniformly planted in the nursery of the Tibetan Academy of Agricultural and Animal Husbandry Sciences. Detailed information of material number, name, type, and origin was provided in Table 1.
The test area is located in the Modern Agricultural Research and Development Base of Sichuan Agricultural University, Qiquan Town, Chongzhou City, Sichuan Province (N 30°33 ‘, E 103°39 ‘), with an altitude of 560 m above sea level. It exhibits a subtropical humid monsoon climate with an average annual temperature of 16°C, an average annual sunshine duration of 1,161.5 h, an average annual precipitation of 1,015.2 mm, and a frost-free period of 283 days a year. Snowfall is rare. The ground in the test area is flat, yellow loam with pH 6.76, organic matter content 31.68 g/kg, total nitrogen content 3.53 g/kg, total phosphorus content 0.32 g/kg, total potassium content 17.41 g/kg.
In March 2021 and March 2022, twelve germplasm resources of the genus Allium were transplanted to the experimental base, and the material planting was arranged according to random block method. The area of each experiment block was approximately 1.2 m2, and 30 plants of the same germplasm resources in each experiment block were planted with row and line spacing of 20 cm respectively, three repeated experiment blocks for per material, and consistent cultivation and management conditions of all experiment blocks. At different growth stages, ten plants in each experiment block were selected to observe phenological phases of each material in the year 2021 and 2022, and investigate quantitative characters of each material in the year 2022. Before bolting phase of each material in the year 2022, three representative plants were selected from each block, whole fresh plant was used to determine the moisture, the 2nd or 3rd mature leave from top to bottom was selected to determine the basic nutritional quality including soluble sugar, soluble protein, and pyruvic acid content, 17 kinds of free amino acids, the dried leave samples were used to determine mineral element content including carbon (C), nitrogen (N), potassium (K), sulfur (S), potassium (K), iron (Fe), manganese (Mn), zinc (Zn), and copper (Cu) content. Each material was repeated three times.
From March 2021 to October 2022, field observations of the phenological period were conducted on twelve germplasm resources of the genus Allium over two years. Ten plants from each material were labeled the dates of bolting, initial bloom, full bloom, final bloom, and seed maturity. The phenological phases described as follows. Bolting phase: the flower stem extends from the leaf sheath until the flower flocs break into buds; Initial bloom phase: the buds of 30% plants rupture in the plot; Full bloom phase: 90% of the plants bloom in the plot; Final bloom phase: 90% of the plants end of flowering. Seed maturity phase: 90% of the plant seeds mature (International Plant Genetic Resources Institute, 2001).
A total of 13 quantitative characters including plant height, tiller number, leaves number per plant, fresh weight per plant, leave length, leave width, pseudostem length, pseudostem diameter, flowering stalk length, flowering stalk diameter, bud length, bud width, and ball-flower diameter were investigated, ten plants were investigated for each germplasm resource and repeated three times. Besides, bud length and width were investigated before initial bloom phase, flowering stalk length and diameter, ball-flower diameter were investigated at full bloom phase, and other quantitative characters were investigated before bolting phase of each germplasm resource of the genus Allium. Plant height, leaf length, leaf width pseudostem length, flowering stalk length, bud length, and bud width were measured by millimeter scales, and plant height was determined from pseudostem base to uppermost part of plant, the 2nd or 3rd mature leave from top to bottom was selected to determine leaf length and leaf width. Pseudostem base, flowering stalk base, and the widest part of the bud was selected to measure pseudostem diameter, flowering stalk diameter, and ball-flower diameter using a vernier calipers. A balance was used to determine the fresh weight per plant (Hirata et al., 2016).
Volatile compounds were determined using headspace solid-phase microextraction (HS-SPME) and gas chromatography/mass spectrometry (GC-MS). Clean and fresh leave samples (1 g) were transferred into a 15 mL headspace sample vial with a seal. Meanwhile, the extraction head was aged at 270°C at the inlet for 1h, then inserted into the top space of the sample bottle, and finally extracted in a 45°C water bath for 30 min. The extraction head was then inserted into the predesigned GC-MS (5979-6890N, Agilent Technologies Inc., Palo Alto, USA) injection port to thermally desorb volatiles in non-shunt mode. The determination conditions for GC-MS included a chromatographic column (DB-5MS: 30 m×250 μm×0.5 μm), an injection port temperature of 260°C, carrier gas (He), and a flow rate of 1.0 mL/min. For the programmed temperature rise, the temperature was maintained at 50°C for 2 minutes, increased to 150°C at 5°C/min, and finally increased to 250°C at 10°C/min, and then hold for 5 min. The injection volume was 1 μL. The spectral conditions included a bombardment ion source, and the ion source temperature was 230°C. The transmission line temperature was 280°C, and the electron energy was 70eV (Gao et al., 2021). The NIST Standards and Technology standard spectrum library was used to match the compounds, and the content of each component was calculated using the area normalization method.
Fresh overground part including pseudostem and leave was measured for fresh weight (Fw), and then dried at 80°C to constant weight to measure the dry weight (Dw). The calculation equation was as follows.
Frozen leave samples (0.5 g) were put into a test tube and added 5 ml distilled water, water bath at 85°C for 30 min, the supernatant was collected, and this step was repeated twice. Then the supernatant collected twice was filled to a volume of 10 ml with distilled water. The soluble sugar content was determined with the sulfuric acid anthrone method at a wavelength of 620 nm (Lin et al., 2013).
Frozen leave samples (1.0 g) were ground up in a mortar with liquid nitrogen, 3 ml of phosphate-buffered solution (pH 7.0) was added to the mortar. Then the extract was centrifuged at 13,000×g for 15 min at 4 °C, and 0.1 ml of the supernatant was combined with 4.9 ml of Coomassie brilliant blue G-250 solution (0.1 g L−1). After 2 min, the soluble protein content was determined at a wavelength of 595 nm (Lin et al., 2013).
Frozen leave samples (0.5 g) were ground with 10 mL trichloroacetic acid solution (8%) in an ice bath. After standing for 30 min, the mixture was centrifuged at 4000×g at 4°C for 10 min. Then 1 ml supernatant was transferred to another 10 ml test tube and added a mixed solution containing the following ingredients: 2 mL trichloroacetic acid (8%), 1 ml 2,4-dinitrophenylhydrazine (0.1%), and 5 ml NaOH (1.5 mol L-1). Then, the solution was shook for 10 min, and the pyruvic acid was determined at 520 nm (Gao et al., 2021).
The free amino acid content in the leave samples was determined using automatic amino acid analyzer (L-8900, Hitachi Co. Ltd., Tokyo, Japan) according to a previous report (He et al., 2018).
Preparation of standard solutions: 17 amino acid standards solutions containing Valine (Val), Methionine (Met), Isoleucine (Ile), Leucine (Leu), Phenylalanine (Phe), Histidine (His), Lysine (Lys), Threonine (Thr), Aspartate (Asp), Serine (Ser), Glutamate (Glu), Proline (Pro), Glycine (Gly), Alanine (Ala), Tyrosine (Tyr), Arginine (Arg), and Cystine (Cys) (>99%, Sigma, USA) was precisely diluted to 0, 1, 5, 10, 50 and 100 μmol/ml with 0.1 mol L-1 hydrochloric acid solution as calibration line.
Sample processing: 1 g of leave power was accurately weighed and placed in hydrolysis tubes, added 10 mL hydrochloric acid solution (6 mol/L), froze the samples for 5 min, vacuum-filled the samples with nitrogen gas three times, and finally sealed the samples in a nitrogen-filled state. After a sealed hydrolysis tube was placed in a 110°C constant temperature oven for hydrolysis for 22 hours, the hydrolysis solution was filtered into a 50 mL volumetric flask. The hydrolysis tube was rinsed multiple times and transferred to a volumetric flask at constant volume. After mixing, 1 mL of the filtrate was acquired and then concentrated and dried at 40°C. The residue was dissolved in 2 mL of water and condensed until it evaporated to dryness. A 2 mL aliquot of pH 2.2 sodium citrate buffer was added for dissolution. After shaking mixed, the sample was passed through a 0.22 μm filter membrane into the injection bottle.
Sample detection: the ninhydrin derivative of proline was monitored at 440 nm, and other amino acids were monitored at 570 nm. The contents of detected amino acids were quantified by comparing their peak areas with those of authentic standards.
Detection conditions: deamination column: 4.6mm×40 mm, #2650. Separation column: 4.6mm×60mm (packed with Hitachi custom ion exchange resin #2622, 3 μm), lithium type. Sample injection volume: 20 μL. Separation buffer: MCI Buffer L-8500-PF Kit (Mitsubishi Chemical Corporation). Flow rate: 0.35 mL min−1. Column temperature: 30-70°C. Reaction column: 4.6mm×40 mm, built-in inert silicon carbide particles. Reaction agent: Ninhydrin coloring solution kit for HITACHI. Reaction temperature: 135°C. Ninhydrin flow rate: 0.30 mL min-1.
Dried leave samples were crushed and sieved through a 100-mesh sieve. The total C content was determined using the potassium dichromate volumetric method (Yang and Yang, 2020). 0.03 g crushed leaf sample was weighed and placed in a test tube with. A standard solution of 0.8 mol L-1 potassium dichromate (5 mL) was added, followed by injection with 5 mL of concentrated sulfuric acid, after soaked for 24h, the digested solution was boiled at 245°C for 5min in a retort furnace. Then the mixture was quantified to 50 mL after cooling, and transferred to a 150 mL triangle bottle. Three drops of o-phenanthroline indicator were added to the bottle, and the mixture was titrated with a 0.2 mol L-1 FeSO4 standard solution. The end point occurred when the solution color changed from green to brownish red. The total carbon content of the sample was measured by titration volume.
Total N content in leave sample was assessed by the Kjeldahl method (El-Nakhel et al., 2020). 1.0 g of crushed leaf sample was placed in a glass tube with 7 mL of sulfuric acid (96%), 5 mL of hydrogen peroxide (30%) and 7 g of catalyzer containing selenium, potassium sulphate and copper oxide were added, then the tubes were placed for digestion in a heating plate for 30 min at 420 °C. Then the digested solution was distilled with the addition of 50 mL of 32% sodium hydroxide. Finally, the titration was conducted with sulfuric acid (0.1 mol L-1) in the presence of a colored indicator containing methyl red and bromocresol green, and the titration volume was used to calculate total nitrogen content. which was converted to g of nitrogen per kg of dry weight.
Crushed leaf sample (0.5 g) was accurately weighed and placed into a microwave digestion inner tank, followed by the addition of 10 mL of HNO3: HClO4 (4:1, V/V) mixed liquid, and the sample was digested overnight. After cooling, it was removed and degassed using ultrasound for 3 min, washed into a 50 mL volumetric flask, filtered through a 0.22 μm aqueous membrane (Gao et al., 2022). The sulfur (S), potassium (K), iron (Fe), manganese (Mn), zinc (Zn), and copper (Cu) contents were determined using an inductively coupled plasma-optical emission spectrometer (ICP-OES, iCAP-7400, Thermo Scientific, USA).
The (mean), standard deviation (SD), and coefficient of variation (CV) were calculated using Microsoft Excel 2017. IBM SPSS Statistics 21.0 software was used to analyze variance for all quantitative characters and biochemical traits using one-way ANOVA. Means were compared using Duncan multiple comparison at 5% significance levels. Origin 2021b was used for drawing column charts and finger-print, and the clustering and correlation analyses with an equidistant Pearson similarity analysis. TBtools was used to generate a heat map. Principal component analysis (PCA) was performed for factor analysis using SPSS.
There were significant differences in bolting and flowering times among the different germplasm resources. With the exception of SC015, SC019, and SC048 that were unable to bolt and bloom, the bolting and seed harvesting times of other germplasm resources were predominantly concentrated from May to August. SC002 exhibited the earliest bolting and flowering. SC004 possessed the earliest seed harvest time and shortest growth period with only 34 days from bolting to seed harvest. SC022 exhibited the latest bolting, flowering, and seed harvest times and the longest growth period with 73 days from bolting to seed harvest (Table 2).
The same quantitative characteristics exhibited significant differences among the different germplasm resources of the genus Allium, thus indicating high morphological diversity. The standard deviations of most quantitative traits were less than 10%, thus indicating high uniformity within the same species. The differences in plant height, leaf length, and pseudostem length among the different species were relatively small with low variation coefficient of 22.73%, 29.75%, and 33.91%, respectively. As the germplasms SC015, SC019, and SC048 were unable to bolt and bloom, the flowering stalk length, flowering stalk diameter, bud length, bud width, and ball-flower diameter were calculated to be zero. Therefore, the CV values of these five quantitative characteristics were greater than 67%. SC015 and SC048 possessed great advantages in terms of plant weight, and both were greater than 50 g. The CV of plant height was the highest and reached 135% (Figure 1; Supplementary Table S1).
The moisture content of all twelve germplasm resources of the genus Allium was higher than 80%, but the difference among the various plants was very small with a CV of only 2.12%. With the exception of SC009, there were no significant differences in moisture content among the other 11 germplasm resources (Figure 2A). The protein content in SC022 was the highest, but there was no significant difference compared to that of SC002, SC007, or SC009. The protein content of the different materials was relatively stable, and the maximum and minimum values did not exceed 25% of the average value (Figure 2B). The soluble sugar content was highest in SC012 and was significantly higher than that in the other materials, and it was the lowest in SC037. However, there was no significant difference compared to that of SC002, SC004, SC007, SC009, SC019, and SC048. The value was 21.39% lower than the average value (Figure 2C). The difference in pyruvic acid among the different germplasm resources was the largest with a CV of 64.89%. The highest content was in SC007 and was 10-fold higher than that of SC020 (Figure 2D).
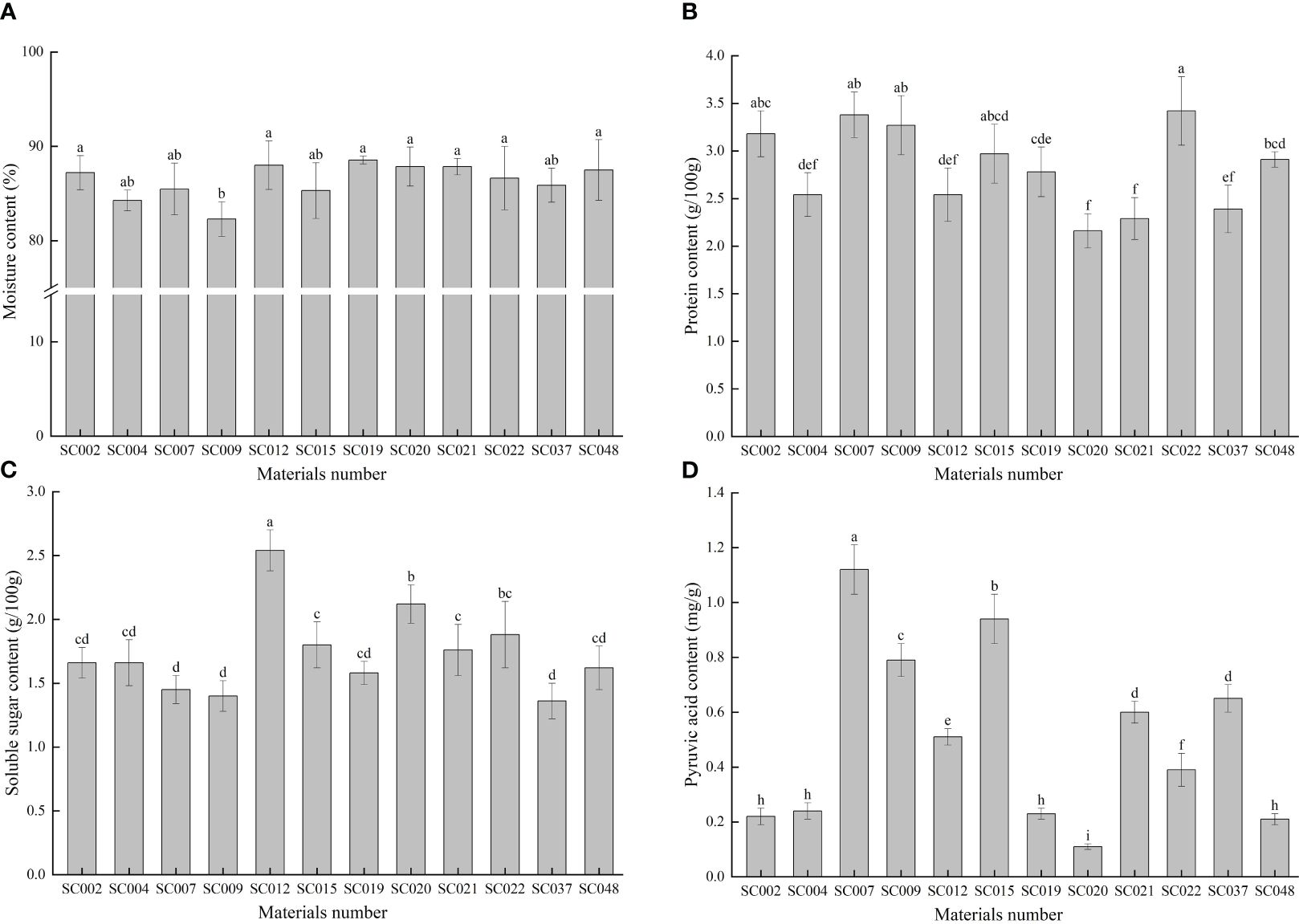
Figure 2 Comparison of basic nutritional qualities of twelve germplasm resources of the genus Allium. (A) Moisture content; (B) Protein content; (C) Soluble sugar content; (D) Pyruvic acid content.
The volatile compounds in the leaves of twelve germplasm resources of the genus Allium were identified and analyzed using SPME-GC-MS. Based on parameters such as peak retention time and peak area, volatile compound maps of twelve germplasm resources were established, and the peak emergence time of all germplasm resources was concentrated between the 10th and 16th minutes (Figure 3). Eight types containing 97 kinds of volatile compounds were identified in the twelve germplasm resources, including 18 aldehydes, 33 sulfur-containing compounds, 10 ketones, 7 alcohols, 7 terpenoids, 9 alkanes, 4 furans, and 10 other compounds. Among them, crotonaldehyde, 2-hexenal, styrene, and 2-methyltetrahydrofuran were common compounds in the twelve germplasm resources with relative contents ranging from 9.06% to 48.45%. Acetaldehyde, propanal, 2-methyl-2-butenal, n-hexanal, 2-methyl-2-pentenal, benzaldehyde, nonanal, and 2,5-dimethylthiophene were common compounds in most plants (8 or more). These compounds were predominantly volatile aldehydes with a relative content ranging from 3.72% to 44.84% (Supplementary Table S2). A heat map was created based on the relative contents of volatile compounds. In general, the content of aldehyde compounds such as acetaldehyde, propanol, 2-methyl-2-butenal, and 2-methyl-2-pentenal in the six germplasm resources of A. przewalskianum (SC002, SC004, SC007, SC009, SC019, and SC037) was relatively high. The content of organic sulfur compounds such as dimethyl disulfide, 1,3-dithiane, and dimethyl trisulfide in the four germplasm resources of A. hookeri Thwaites and A. tuberosum Rottl. ex Spreng (SC012, SC020, SC021, and SC022) was higher than that in the other plants (Figure 3B).
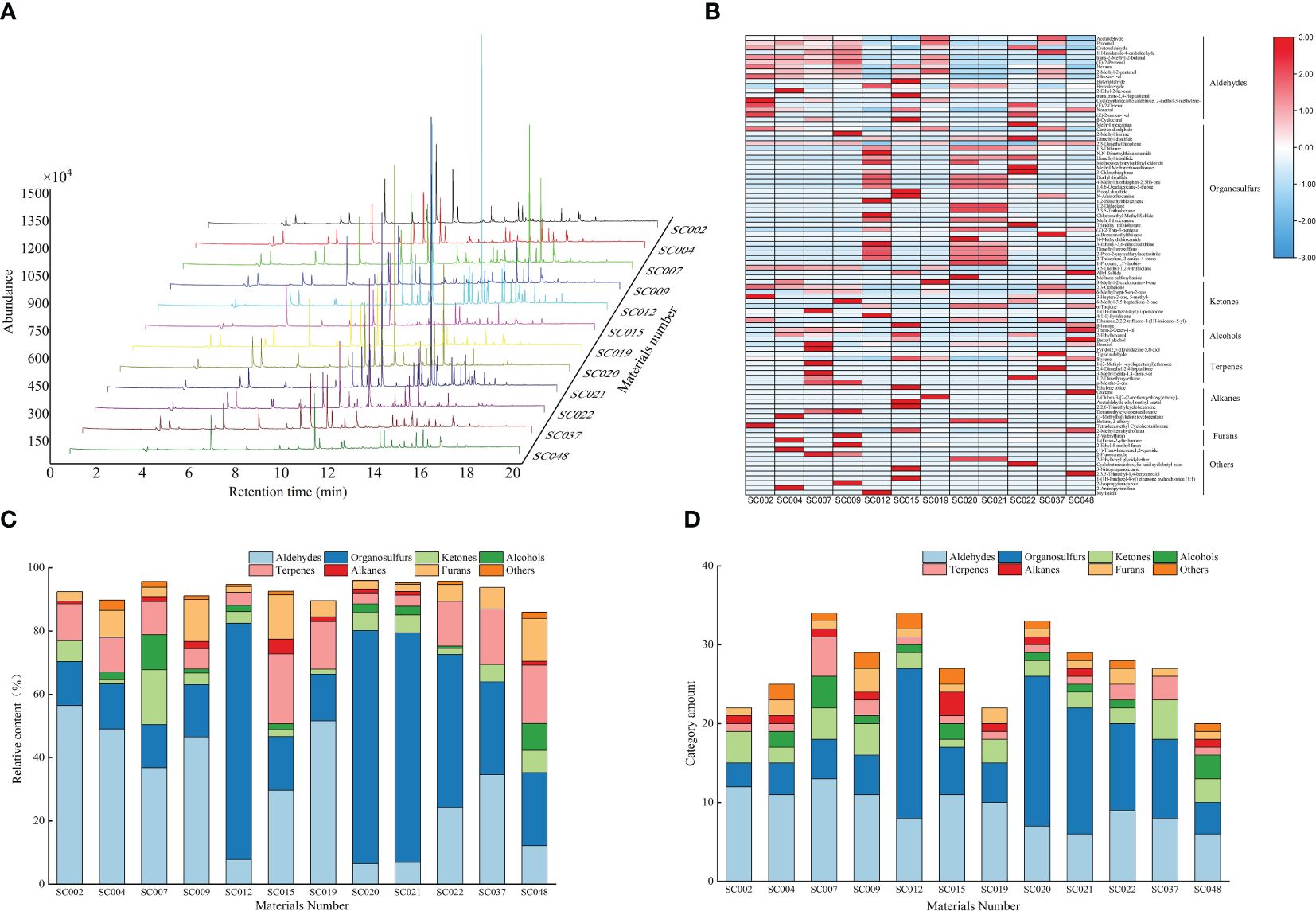
Figure 3 The volatile compounds in the twelve germplasm resources of the genus Allium as assessed by SPME-GC–MS. (A) Difference of the area of peaks for volatiles compounds; (B) Heat map of volatile compounds; (C) Relative content of different categories of volatile compounds; (D) Amount of each category of volatile compounds.
The classification and statistics of volatile compounds in all germplasm resources of the genus Allium revealed that the total content of volatile compounds in all germplasm resources exceeded 80%, and the composition of volatile compounds was consistent. However, the contents were significantly different. With the exception of SC015 and SC048 that are two scallion plants, the volatile compounds in the other germplasm resources were primarily aldehydes and organosulfur, and the total relative contents of these two categories exceeded 50%. SC007 and SC012 possessed the most abundant volatile compounds (34 types), whereas SC048 possessed the least number of volatile compounds (20 types). There were 12 aldehyde compounds in SC002 with a relative content of 56.47% that accounted for the largest proportion, whereas seven aldehyde compounds in SC020 accounted for only 6.54%. There were 19 OSCs in SC012 and SC020 with contents of 74.68% and 73.59%, respectively, and these were the highest among all the plants. There were four ketone and four terpenoid compounds in SC007 with relative contents of 17.31% and 11.07%, respectively, and both of these were the highest. The relative content and types of alkane compounds were relatively low in all germplasm resources with a maximum of only 4.69% in SC015, and the three germplasm resources did not contain such compounds. A total of one to three types of furan compounds were detected in the different germplasm resources, and the highest relative content of 13.46% was observed in SC048 (Figures 3C, D).
Sixteen free amino acids were detected in twelve germplasm resources of the genus Allium, including 8 essential amino acids (EAA) identified as Val, Met, Ile, Leu, Phe, His, Lys, and, Thr, and 8 non-essential amino acids (NEAA) identified as Asp, Ser, Glu, Pro, Gly, Ala, Tyr, Arg. In terms of EAA, the highest content was Lys was 0.18g/100 g in SC009, and this was significantly higher than that in other plants and 38.46% higher than the average value. Next was Leu that exhibited the highest content in both SC037 and SC048. Met content was the lowest among EAA with an average value of only 0.02 g/100 g, and Met was not detected in SC015 and SC048. There was little difference in the content of Val, Ile, Phe, Lys, and Thr among different germplasm resources, and the CV values were all less than 25%; As Met and His were detected in some germplasm resources, the CV values of these two EAA exceeded 55%. The total EAA content varied slightly among the different plants with a CV of 19.24%. In SC002, the total EAA content was the lowest (37.5% lower than the average), and it was the highest in SC009 at 41.67% higher than the average (Table 3). Pro content was significantly higher than that of the other NEAA, and the highest content was 1.61g/100 g in SC009. The Ser, Gly, and Tyr contents were relatively low with average values less than 0.1 g/100 g. The differences in Tyr and Arg content among the different germplasm resources were relatively small with a CV below 22%, and the difference in Ala content was the largest with a CV exceeding 100% (Table 4).
Mineral elements were detected in the above-ground portions of twelve germplasm resources of the genus Allium. Among the macroelements, the C content in all germplasm resources were relatively high and ranged from 33.69 to 41.97 g/100 g. The difference between the different germplasm resources was small with the highest content in SC009. The only significantly different amount was that in SC002. The N content was the highest in SC002, but there was no significant difference compared to the content in SC002, SC007, and SC009 that was 57.34% higher than the lowest N content in SC020. The K content in SC004, SC019, and SC048 was significantly higher than that in the other eight germplasm resources with the exception of SC009. The K content in SC015 was the lowest with only 43.62% in SC048, and this was significantly lower than that of the other germplasm resources. The maximum difference in S content between different germplasm resources was only detected in SC002, SC004, SC007, SC020, SC021, and SC047, where the highest content was observed in SC002. This was not significantly different compared to that in SC037. No P content was detected in any of the plants, and therefore, the data are not listed (Figure 4A). In terms of microelements, SC048 possessed the highest Fe content, and this was followed by SC004 and SC019 and was significantly higher than that in the other germplasm resources. The Mn content was the highest in SC021, but there was no significant difference compared to the content in SC048 that was 2.78-fold higher than that of SC015. The Cu content in SC019 and SC048 was significantly higher than that in the other germplasm resources, whereas the Cu content in SC037 was the lowest at only 35.55% of the average value. The Zn content in SC021 and SC048 was significantly higher than that in the other germplasm resources, but due to the absence of Zn detection in SC002, SC004, SC009, SC015, and SC037, the Zn content varied the most among the twelve germplasm resources with a CV of up to 92.20% (Figure 4B).
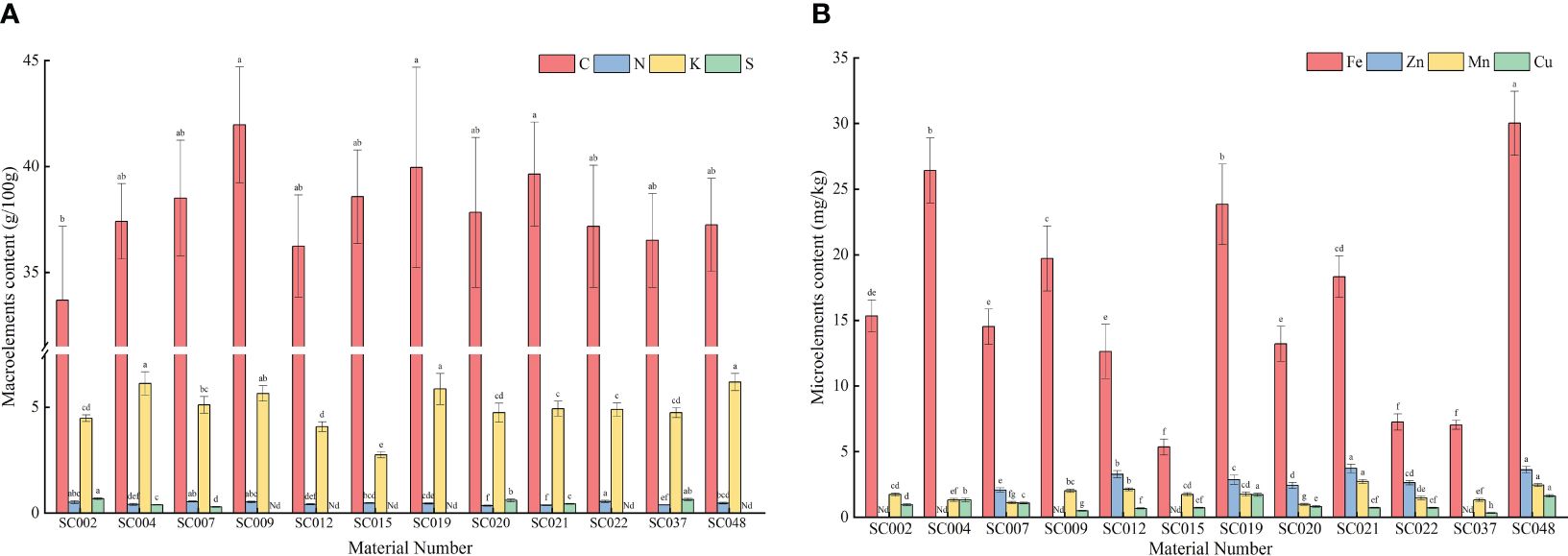
Figure 4 Contents of mineral elements in twelve germplasm resources of the genus Allium. (A) Macroelements content; (B) Microelements content. Note: “Nd” in the figure indicates that it is not detected.
Correlation analysis of 15 quality indices, including NEAA, EAA, protein, soluble sugar, pyruvic acid, moisture, Fe, Zn, Mn, Cu, S, N, K, and C content in different germplasm resources of the genus Allium, revealed a highly significant positive correlation between EAA and C, protein and N, Zn and moisture, and Fe and Cu and K content (P<0.01) with correlation coefficients of 0.72, 1.00, 0.72, 0.76, and 0.83, respectively. There was a significant negative correlation between organosulfur and Fe, NEAA, soluble sugar, EAA, and S contents (Figure 5).
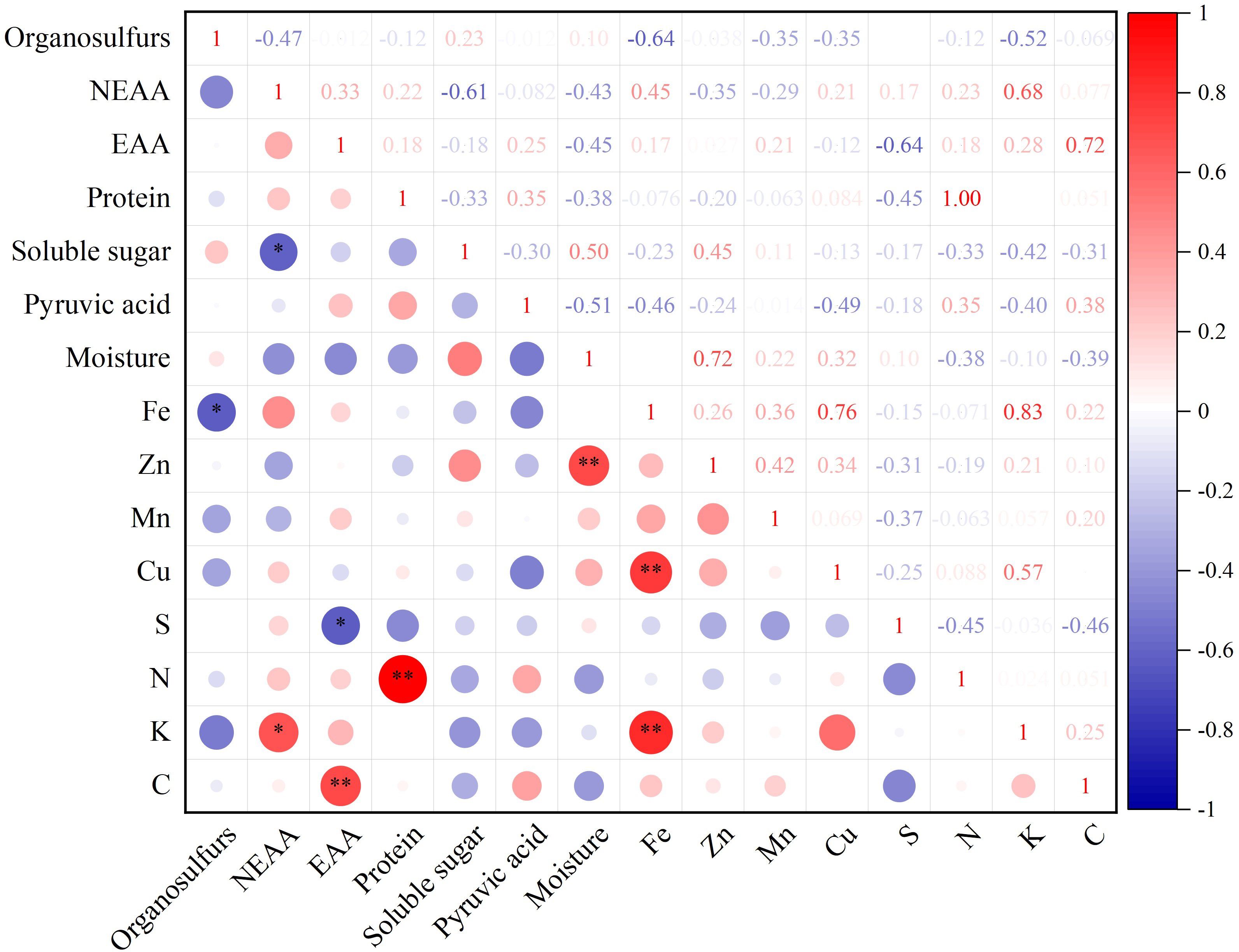
Figure 5 Quality correlation analysis of twelve germplasm resources of the genus Allium. * Correlation is significant at the 0.05 level, ** Correlation is significant at the 0.01 level.
Principal component analysis was conducted on 15 quality indicators of twelve germplasm resources of the genus Allium, and 5 principal components with eigenvalues of greater than 1 were extracted. The cumulative contribution of the 5 principal components was 85.37% (Table 5). According to the loading matrix, principal component 1 was jointly influenced by six indices that included NEAA, EAA, K, soluble sugar, and moisture. Among them, NEAA, EAA, and K possessed the highest positive loadings, whereas soluble sugars possessed the highest negative loadings. Based on the correlation analysis, NEAAs were positively correlated with K and negatively correlated with soluble sugar content. Therefore, principal component 1 mainly represented the amino acid composition factors of the twelve germplasm resources of the genus Allium. Principal component 2 was primarily influenced by Fe, Zn, Cu, K, and pyruvic acid content with pyruvic acid as a negative loading control, whereas principal component 3 was predominantly influenced by S, Zn, and Cu, with S exhibiting the highest absolute loading value. Therefore, principal components 2 and 3 primarily represent the essential element composition factors of the 12 Allium sources. The representative indices of principal component 4 were protein and N, and correlation analysis revealed a significant positive correlation between protein and N content. Therefore, principal component 4 represented the basic nutritional quality components that were primarily composed of proteins. The loading value of organic sulfur in principal component 5 was the highest, thus indicating that principal component 5 mainly reflected the sulfur-containing volatile components of germplasm resources of the genus Allium (Table 6).
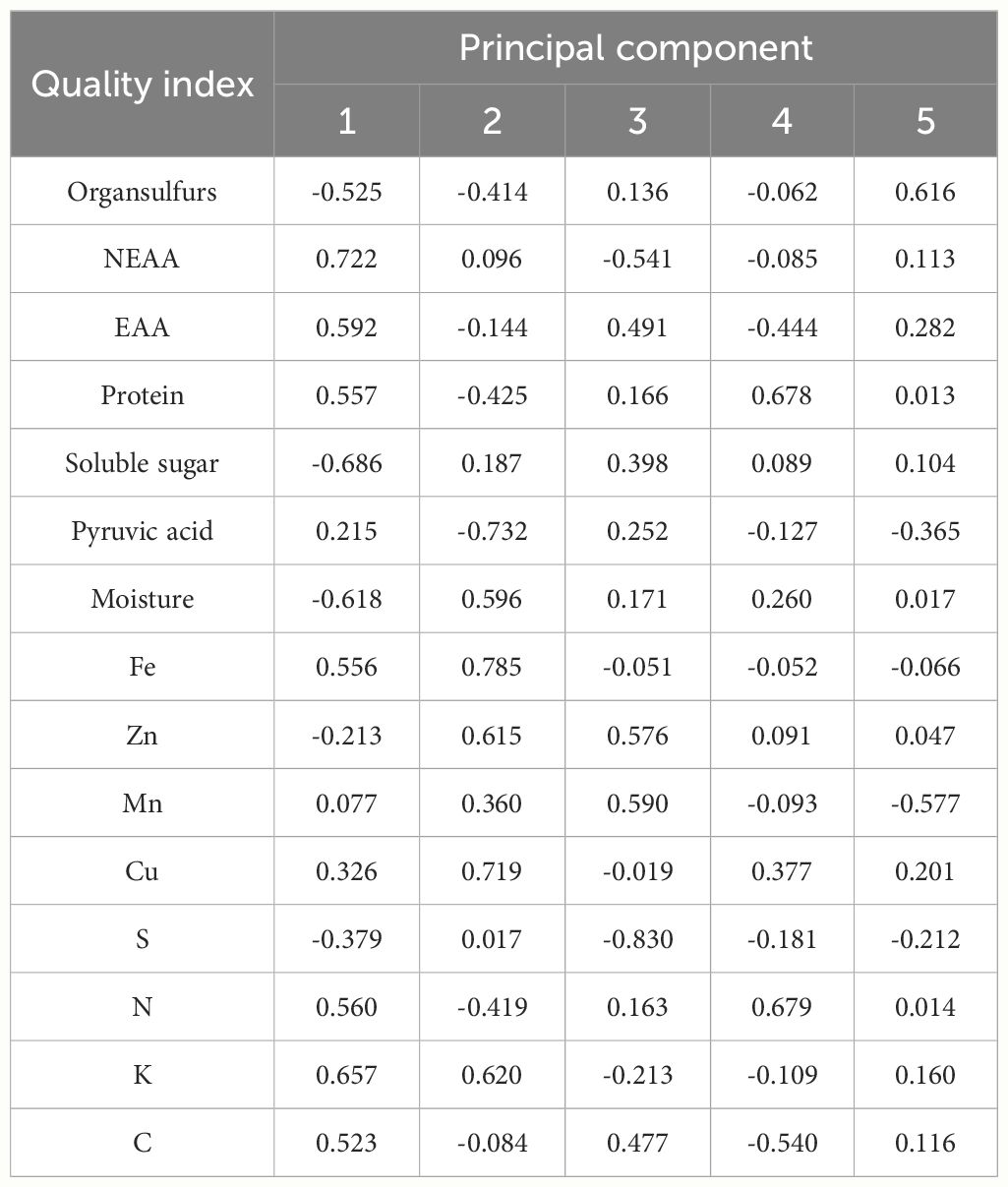
Table 6 Principal component loading matrix of quality of twelve germplasm resources of the genus Allium .
A comprehensive quality evaluation of twelve germplasm resources of the genus Allium was conducted.
.
After calculating the eigenvector coefficients of each principal component, the score function of 1-5 principal components was constructed as follows:
y1=–0.26X1+0.36X2+0.30X3+0.28X4–0.34X5+0.11X6–0.31X7+0.28X8–0.11X9+0.04X10+0.16X11–0.19X12+0.28X13+0.33X14+0.26X15
y2=–0.22X1+0.05X2–0.08X3–0.23X4+0.10X5–0.039X6+0.32X7+0.42X8+0.33X9+0.19X10 +0.39X11+0.01X12–0.22X13+0.33X14–0.04X15
y3 = 0.08X1–0.34X2+0.31X3+0.11X4+0.25X5+0.16X6+0.11X7–0.03X8+0.36X9+0.37X10–0.01X11–0.52X12+0.10X13–0.13X14+0.30X15
y4=–0.05X1–0.06X2–0.34X3+0.52X4+0.07X5–0.10X6+0.20X7–0.04X8+0.07X9–0.07X10 +0.29X11–0.14X12+0.52X13–0.08X14–0.41X15
y5 = 0.59X1+0.11X2+0.27X3+0.01X4+0.10X5–0.35X6+0.02X7–0.06X8+0.05X9–0.56X10 +0.19X11–0.20X12+0.01X13+0.15X14+0.11X15
where y1, y2, y3, y4, and y5 are the eigenvector weights of one to five principal components, and X1, X2,…, and X15 are the normalized values of organosulfur, NEAA, EAA, protein, soluble sugar, pyruvate, water, Fe, Zn, Mn, Cu, S, N, K, and C contents for each Allium plant. Comprehensive quality score of Allium plants,
That is, y=0.31y1 + 0.27y2 + 0.20y3 + 0.13y4 + 0.08y5, where t1, t2, t3, t4, and t5 were the eigenvalues of the 1 to 5 principal components, respectively (Han et al., 2022). The comprehensive scores from highest to lowest were SC048, SC019, SC009, SC004, SC007, SC022, SC012, SC021, SC002, SC020, SC015, and SC037 (Table 7).
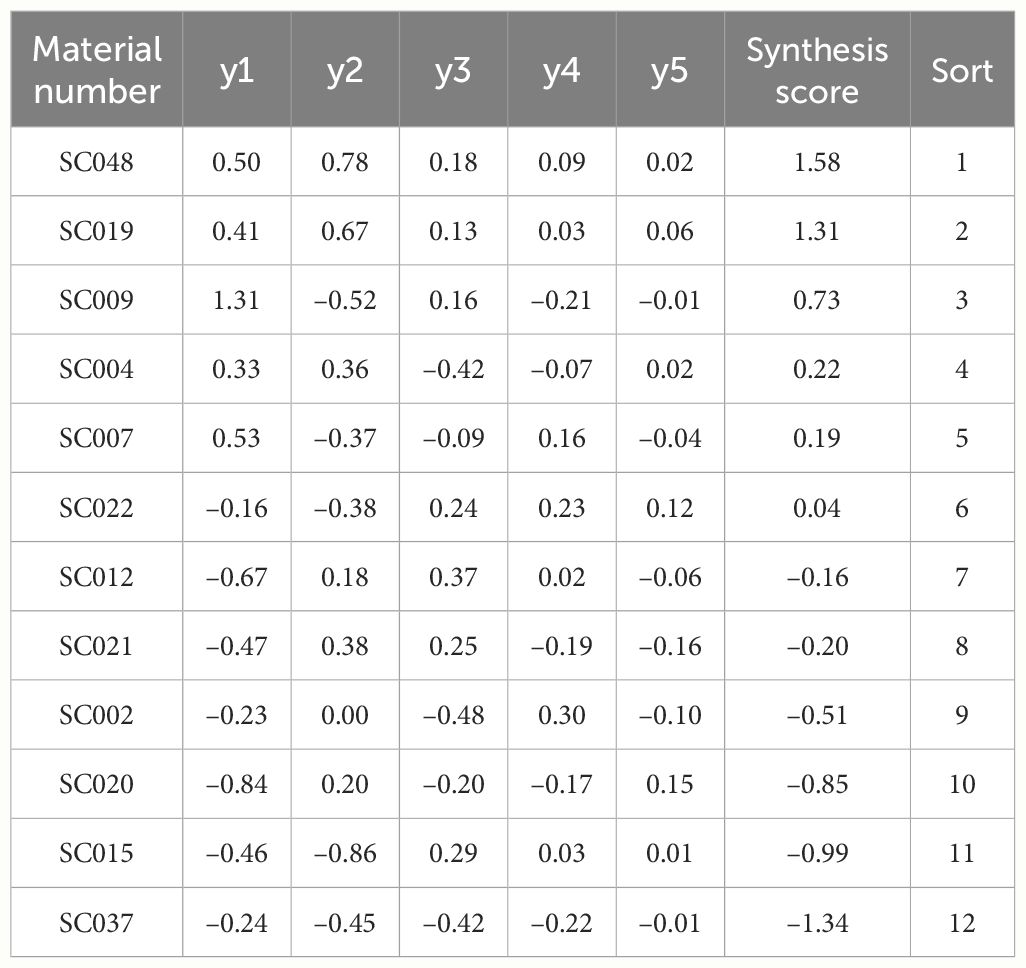
Table 7 Score and sorting of principal component factors of quality of twelve germplasm resources of the genus Allium.
Cluster analysis was conducted on 15 quality indices of the twelve germplasm resources of the genus Allium, and a cluster tree chart was established. At a Euclidean distance of 20, the twelve germplasm resources could be divided into three major categories. The first category consisted of eight materials, including SC048, SC019, SC009, SC004, SC007, SC022, SC012, and SC021, that ranked 1-8 comprehensively and accounted for 66.67%. The second category consisted of three materials (SC002, SC020, and SC015) with a comprehensive ranking of 9-11 that accounted for 25%. SC037 with a comprehensive ranking of 12 was grouped separately into the third category and accounted for 8.33% (Figure 6).
The phenotype is determined by the genotype and living environment, and quantitative characteristics are easily influenced by the external environment (Langstroff et al., 2022). During plant growth, the phenological phase reflects the comprehensive impact of climate on the plant and is an important indicator of if the plant can adapt to the region and grow normally (Stuble et al., 2021). Through two consecutive years of observation of the phenological period of twelve germplasm resources of the genus Allium from Tibet, it was observed that three twelve germplasm resources were unable to normally bolt and flower, likely due to the observation that some plants of the genus Allium collected from Tibet grew under severe agroclimatic conditions such as harsh, cold winters, and high altitudes and thus evolved to be non-bolting. The bolting, flowering, and seed harvest phase of the other nine plants remained highly consistent within species, whereas there were significant differences between species, and this is consistent with the results for wild plants of the genus Allium in Inner Mongolia (Yang et al., 2013). We investigated the quantitative characteristics of twelve germplasm resources of the genus Allium and observed that single plant weight, flowering stalk length, and flowering stalk diameter were the most diverse; however, the variation coefficient of plant height was the smallest, and which was determined by their genotype.
Allium species are revered worldwide as spices with sharp and tang odors and pungent spicy taste (Singh et al., 2018). Pyruvic acid is closely related to the pungent taste of the genus Allium and can be used to determine the pungency of the genus Allium (Gîtin et al., 2014). In this study, there was a significant variation in pyruvic acid content among different germplasm resources of the genus Allium, thus result to the significant differences in spicy taste. Besides, volatile compounds are important factors that affect the flavor of the genus Allium. Different volatile compounds cause different flavor sensations. Eight types of volatile compounds were identified in the twelve germplasm resources included aldehydes, organosulfur compounds, ketones, alcohols, terpenoids, alkanes, and furans. The majority of germplasm resources possessed the highest proportions of aldehydes and organosulfur compounds, but there were certain differences between the different Allium species. These results are in line with the previous findings that the volatile compounds identified in the genus Allium primarily include sulfides, aldehydes, ketones, alcohols, hydrocarbons, furans, and heterocyclic compounds (Yin et al., 2019; Xie et al., 2022; Liu et al., 2022a). OSC determines the unique aroma and taste of the genus Allium, making them a potential symbol of the genus Allium, but the type and content of OSC vary depending on the species of Allium plants, and this results in clearly distinguish in flavors and fragrances (Baky et al., 2022). In this study, different degrees of OSCs were detected in all twelve germplasm resources of the genus Allium, and which was the most diverse compound among all volatiles. The dimethyl trisulfide content accounted for the largest proportion in the species of Allium tuberosum Rottl. ex Spreng and Allium hookeri Thwaites. Aldehyde was primarily volatile compounds, secondly OSCs in Allium przewalskianum species, which exhibited intense the aroma of green grass, and 3,5-diethyl-1,2,4-trichlorothecyclopentane and 2,5-dimethylthiophene was primary and unique component of OSCs. Similar results were obtained in wild plants of the genus Allium in the Qinghai-Tibet Plateau (Guan et al., 2020), leeks (Yabuki et al., 2010; Zhang et al., 2012) and Allium przewalskianum, but differently, the primary and unique component of OSCs in Allium przewalskianum are methylpropyl sulfide and dipropyl sulfide (Lang et al., 2018).
Plants of the genus Allium are rich in amino acids that play important roles in nutrition, medicine, and sensory value (He et al., 2018). Except for Cys, a total of 16 free amino acids including eight EAA and eight NEAA were detected in twelve germplasm resources examined in this study. Similarly, Liu et al. (2022b) also reported that Chinese chive contains almost Cys, thus indicating that leek and A. przewalskianum are extremely lack of Cys. Studies have demonstrated that different amino acids cause different flavors in plants of the genus Allium. Glu and Asp provide umami flavor, whereas Ser, Gly, Ala, Pro, and Gln contribute to a sweet taste (Abdelrahman et al., 2020). The twelve germplasm resources examined in this study exhibited significant interspecies differences in amino acid composition and content, the biggest disparity in the total amino acid content was nearly three-fold, and this led to significant flavor differences among the different plants of the genus Allium in this study. And almost all germplasm resources possessed the highest content of Pro, and followed by Glu. This is similar to the results of the highest content of Glu in wild Leek in Tibet (Wang et al., 2017). It is speculated that Pro is related to plant resistance. To adapt to cold and high-altitude environments, these plants of the genus Allium in Tibet have evolved strong resistance to abiotic stress.
Mineral elements in vegetables are the main sources of essential elements for the human body (Loppi et al., 2021). The essential element content in twelve germplasm resources of the genus Allium basically meets the need of the human body for macro- and micro-elements. As the basic macroelement, C content was the highest, and followed by K, and Fe was the highest microelement, the contents of the other mineral elements except C varied greatly among the different materials. This is consistent with the results for garlic, onion, and leek (Czech et al., 2022; Gao et al., 2022).
Comprehensive evaluation such as correlation analysis, principal component analysis, and cluster analysis is useful method to evaluate the quality of germplasm resources (Liu et al., 2023). In the present study, through comprehensive evaluation of fifteen quality traits, it suggested that SC048 possessed advantage in comprehensive quality, and this was followed by SC019 and SC009. Similar method has been reported on pears, raspberries, and other fruits (Yazdanpour et al., 2018; Han et al., 2022). Besides, it also found some germplasm resources with high content of mineral elements, amino acids or OSCs alone, which can be further cultivated and used for quality breeding of the genus Allium.
Though observing the biological characteristics, the germplasm resources of SC015, SC019, and SC048 were unable to bolt and bloom normally, whereas the remaining nine germplasm resources performed well. The twelve germplasm resources of the genus Allium varied in basic nutritional quality, content of amino acid, OSCs and essential elements. Comprehensive evaluation of the 15 quality indices showed that SC048 possessed advantage in comprehensive quality, followed by SC019 and SC009. In briefly, combined with biological performance and comprehensive evaluation of quality, SC009 is the excellent germplasm resource that were suitable for growth and capable of reproduction with good quality. The findings of this study are practically significant for utilization and high-quality breeding of the genus Allium.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
HW: Investigation, Writing – original draft. HY: Investigation, Writing – original draft. XY: Investigation, Writing – original draft. YX: Investigation, Writing – original draft. YB: Investigation, Writing – original draft. QD: Investigation, Writing – original draft. LL: Investigation, Writing – original draft. WT: Investigation, Writing – original draft. MY: Investigation, Writing – original draft. LW: Investigation, Writing – original draft. ZH: Investigation, Writing – original draft. BS: Investigation, Writing – original draft. HL: Investigation, Writing – original draft. YT: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Science and Technology Plan Project of Tibet (XZ202101ZY0001N), Second Tibetan Plateau Scientific Expedition Program (2019QZKK0502), and Research Basic Ability Improvement Project of Young and Middle-aged Teachers of College in Guangxi (2023KY1270).
We would like to thank Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1393402/full#supplementary-material
Abdelrahman, M., Ariyanti, N. A., Sawada, Y., Tsuji, F., Hirata, S., Hang, T. T. M., et al. (2020). Metabolome-based discrimination analysis of shallot landraces and bulb onion cultivars associated with differences in the amino acid and flavonoid profiles. Molecules 25, 5300. doi: 10.3390/molecules25225300
Alam, A., Al Arif Jahan, A., Bari, M. S., Khandokar, L., Mahmud, M. H., Junaid, M., et al. (2023). Allium vegetables: Traditional uses, phytoconstituents, and beneficial effects in inflammation and cancer. Crit. Rev. Food. Sci. 63, 6580–6614. doi: 10.1080/10408398.2022.2036094
Asemani, Y., Zamani, N., Bayat, M., Amirghofran, Z. (2019). Allium vegetables for possible future of cancer treatment. Phytother. Res. 33, 3019–3039. doi: 10.1002/ptr.6490
Baky, M. H., Shamma, S. N., Khalifa, M. R., Farag, M. A. (2022). How does Allium leafy parts metabolome differ in context to edible or inedible taxa? Case study in seven Allium species as analyzed using MS-based metabolomics. Metabolites 13, 18. doi: 10.3390/metabo13010018
Bastaki, S. M. A., Ojha, S., Kalasz, H., Adeghate, E. (2021). Chemical constituents and medicinal properties of Allium species. Mol. Cell. Biochem. 476, 4301–4321. doi: 10.1007/s11010-021-04213-2
Chu, C. C., Wu, W. S., Shieh, J. P., Chu, H. L., Lee, C. P., Duh, P. D. (2017). The anti-inflammatory and vasodilating effects of three selected dietary organic sulfur compounds from Allium species. J. Funct. Biomater. 8, 5. doi: 10.3390/jfb8010005
Czech, A., Szmigielski, M., Sembratowicz, I. (2022). Nutritional value and antioxidant capacity of organic and conventional vegetables of the genus Allium. Sci. Rep-Uk. 12, 18713. doi: 10.1038/s41598-022-23497-y
El-Nakhel, C., Pannico, A., Kyriacou, M. C., Petropoulos, S. A., Giordano, M., Colla, G., et al. (2020). Dataset on the organic acids, sulphate, total nitrogen and total chlorophyll contents of two lettuce cultivars grown hydroponically using nutrient solutions of variable macrocation ratios. Data. Brief 29, 105135. doi: 10.1016/j.dib.2020.105135
Fredotović, Ž., Puizina, J. (2019). Edible Allium species: chemical composition, biological activity and health effects. Ital. J. Food. Sci. 31, 19–39. doi: 10.14674/IJFS-1221
Fredotović, Ž., Šprung, M., Soldo, B., Ljubenkov, I., Budić-Leto, I., Bilušić, T., et al. (2017). Chemical composition and biological activity of Allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) methanolic extracts. Molecules 22, 448. doi: 10.3390/molecules22030448
Gao, S., Kong, Y. W., Lv, Y., Cao, B. L., Chen, Z. J., Xu, K. (2022). Effect of different LED light quality combination on the content of vitamin C, soluble sugar, organic acids, amino acids, antioxidant capacity and mineral elements in green onion (Allium fistulosum L.). Food. Res. Int. 156, 111329. doi: 10.1016/j.foodres.2022.111329
Gao, S., Liu, X. N., Liu, Y., Cao, B. L., Chen, Z. J., Xu, K. (2021). Comparison of the effects of LED light quality combination on growth and nutrient accumulation in green onion (Allium fistulosum L.). Protoplasma 258, 753–763. doi: 10.1007/s00709-020-01593-y
Gîtin, L., Dinică, R., Neagu, C., Dumitrascu, L. (2014). Sulfur compounds identification and quantification from Allium spp. fresh leaves. J. Food. Drug Anal. 22, 425–430. doi: 10.1016/j.jfda.2014.04.002
Guan, Z. H., Wang, Z. H., Lang, J., Deqing, C. M. (2020). Study on the volatile components of 8 wild Allium L. plants from Qinghai-Tibet Plateau. J. Plant Genet. Resour. 21, 1036–1043. doi: 10.13430/j.cnki.jpgr.20190609002
Guo, C. A., Ding, X., Hu, H., Zhang, Y., Yang, H., Wang, Y. (2022). An ethnobotanical study on wild plants used by Tibetan people in Gyirong Valley, Tibet, China. J. Ethnobiol. Ethnomed. 18, 67. doi: 10.1186/s13002-022-00565-1
Han, S. J., Zhao, J. F., Liu, Y., Xi, L. Q., Liao, J. A., Liu, X. Y., et al. (2022). Effects of green manure planting mode on the quality of Korla fragrant pears (Pyrus sinkiangensis Yu). Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1027595
Hauenschild, F., Favre, A., Schnitzler, J., Michalak, I., Freiberg, M., Muellner-Riehl, A. N. (2017). Spatio-temporal evolution of Allium L. in the Qinghai-Tibet-Plateau region: Immigration and in situ radiation. Plant Diversity 39, 167–179. doi: 10.1016/j.pld.2017.05.010
He, Q., Huang, S. H., Wu, Y. H., Zhang, W. Q., Wang, F. C., Cao, J. W., et al. (2018). Comparative study on the composition of free amino acids and derivatives in the two botanical origins of an edible Chinese herb “Xiebai”, i.e., Allium chinense G. Don and Allium macrostemon Bunge species. Food. Res. Int. 106, 446–457. doi: 10.1016/j.foodres.2018.01.007
Hirata, S., Abdelrahman, M., Yamauchi, N., Shigyo, M. (2016). Diversity evaluation based on morphological, physiological and isozyme variation in genetic resources of garlic (Allium sativum L.) collected worldwide. Genes Genet. Syst. 91, 161–173. doi: 10.1266/ggs.15-00004
International Plant Genetic Resources Institute (2001). Descriptors for allium spp (Florence: Food and Agriculture Organization of the United Nations).
Jin, F. Y., Xie, D. F., Zhou, S. D., He, X. J. (2018). Characterization of the complete chloroplast genome of Allium prattii. Mitochondrial. Dna. B. 3, 153–154. doi: 10.1080/23802359.2018.1436994
Lang, J., Wang, L. Z., Guan, Z. H., Deqing, C. M., Wang, Z. H. (2018). The development and utilization status and nutritional quality analysis of flower & leaf of Allium przewalskianum Regel. J. Plant Genet. Resour. 19, 96–102. doi: 10.13430/j.cnki.jpgr.2018.01.011
Langstroff, A., Heuermann, M. C., Stahl, A., Junker, A. (2022). Opportunities and limits of controlled-environment plant phenotyping for climate response traits. Theor. Appl. Genet. 135, 1–16. doi: 10.1007/s00122-021-03892-1
Lee, W. S., Yi, S. M., Yun, J. W., Jung, J. H., Kim, D. H., Kim, H. J., et al. (2014). Comparative study on the composition of free amino acids and derivatives in the two botanical origins of an edible Chinese herb “Xiebai”, i.e., Allium chinense G. Don and Allium macrostemon Bunge species. J. Cancer. Prev. 19, 14–22. doi: 10.15430/JCP.2014.19.1.14
Lin, K. H., Huang, M. Y., Huang, W. D., Hsu, M. H., Yang, Z. W., Yang, C. M. (2013). The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic-Amsterdam. 150, 86–91. doi: 10.1016/j.scienta.2012.10.002
Liu, N., Hu, M. M., Liang, H., Tong, J., Xie, L., Wang, B. J., et al. (2022b). Physiological, transcriptomic, and metabolic analyses reveal that mild salinity improves the growth, nutrition, and flavor properties of hydroponic Chinese chive (Allium tuberosum Rottler ex Spr). Front. Nutr. 9. doi: 10.3389/fnut.2022.1000271
Liu, C., Li, H. L., Ren, A. H., Chen, G. Y., Ye, W. J., Wu, Y. X., et al. (2023). A comparison of the mineral element content of 70 different varieties of pear fruit (Pyrus ussuriensis) in China. Peerj 11, e15328. doi: 10.7717/peerj.15328
Liu, G. M., Wang, Y. Q., Hu, L. P., He, H. J. (2022a). Characterization of the volatile vompounds of onion with different fresh-cut styles and storage temperatures. Foods 11, 3829. doi: 10.3390/foods11233829
Loppi, S., Fedeli, R., Canali, G., Guarnieri, M., Biagiotti, S., Vannini, A. (2021). Comparison of the mineral and nutraceutical profiles of elephant garlic (Allium ampeloprasum L.) grown in organic and conventional fields of Valdichiana, a traditional cultivation area of Tuscany, Italy. Biology-Basel 10, 1058. doi: 10.3390/biology10101058
Marefati, N., Ghorani, V., Shakeri, F., Boskabady, M., Kianian, F., Rezaee, R., Boskabady, M. (2021). A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. H. Pharm. Biol. 59, 287–302. doi: 10.1080/13880209.2021.1874028
Poojary, M. M., Putnik, P., Kovačević, D. B., Francisco, J. B., Lorenzo, J. M., Dias, D. A., et al. (2017). Stability and extraction of bioactive sulfur compounds from Allium genus processed by traditional and innovative technologies. J. Food. Compos. Anal. 61, 28–39. doi: 10.1016/j.jfca.2017.04.007
Putnik, P., Gabrić, D., Roohinejad, S., Barba, F. J., Granato, D., Mallikarjunan, K., et al. (2019). An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food. Chem. 276, 680–691. doi: 10.1016/j.foodchem.2018.10.068
Scherer, C. C., Jacob, M., Dicato, M. (2009). Potential role of organic sulfur compounds from Allium species in cancer prevention and therapy. Phytochem. Rev. 8, 349–368. doi: 10.1007/s11101-009-9122-z
Sharifi-Rad, J., Mnayer, D., Tabanelli, G., Stojanović-Radić, Z. Z., Sharifi-Rad, M., Yousaf, Z., et al. (2016). Plants of the genus Allium as antibacterial agents: From tradition to pharmacy. Cell. Mol. Biol. 62, 57–68. doi: 10.14715/cmb/2016.62.9.10
Singh, V., Chauhan, G., Krishan, P., Shri, R. (2018). Allium schoenoprasum L.: a review of phytochemistry, pharmacology and future directions. Nat. Prod. Res. 32, 2202–2216. doi: 10.1080/14786419.2017.1367783
Stuble, K. L., Bennion, L. D., Kuebbing, S. E. (2021). Plant phenological responses to experimental warming-A synthesis. Global. Change. Biol. 27, 4110–4124. doi: 10.1111/gcb.15685
Subramanian, M. S., Nandagopal, M. S. G., Amin, N. S., Thilakavathy, K., Joseph, N. (2020). Prevailing knowledge on the bioavailability and biological activities of sulphur compounds from Alliums: A potential drug candidate. Molecules 25, 4111. doi: 10.3390/molecules25184111
The Angiosperm Phylogeny Group (2009). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc 161, 105–121. doi: 10.1111/(ISSN)1095-8339
The Angiosperm Phylogeny Group (2016). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc 181, 1–20. doi: 10.1111/boj.2016.181.issue-1
Wang, Z. H., Deqing, C. M., Guan, Z. H., Chen, S. C. (2017). Flavor substances and nutritional ingredients of wild Allium hookeri Thwaites in Tibetan. J. Northwest. A&F. Univ. 45, 153–160,167. doi: 10.13207/j.cnki.jnwafu.2017.05.021
Wang, G. Y., Zhou, N., Chen, Q., Yang, Y., Yang, Y. P., Duan, Y. W. (2023). Gradual genome size evolution and polyploidy in Allium from the Qinghai-Tibetan Plateau. Ann. Bot-London. 131, 109–122. doi: 10.1093/aob/mcab155
Xie, B. J., Wu, Q., Wei, S. H., Li, H. Y., Wei, J. M., Hanif, M., et al. (2022). Optimization of headspace solid-phase micro-extraction conditions (HS-SPME) and identification of major volatile aroma-active compounds in Chinese chive (Allium tuberosum Rottler). Molecules 27, 2425. doi: 10.3390/molecules27082425
Xie, C., Xie, D. F., Zhong, Y., Guo, X. L., Liu, Q., Zhou, S. D., et al. (2019). The effect of Hengduan Mountains Region (HMR) uplift to environmental changes in the HMR and its eastern adjacent area: Tracing the evolutionary history of Allium section Sikkimensia (Amaryllidaceae). Mol. Phylogenet. Evol. 130, 380–396. doi: 10.1016/j.ympev.2018.09.011
Xu, Y., Kou, J. M., Zhang, Q., Tan, S. D., Zhu, L. C., Geng, Z. H., et al. (2023). Visual detection of water content range of seabuckthorn fruit based on transfer deep learning. Foods 12, 550. doi: 10.3390/foods12030550
Yabuki, Y., Mukaida, Y., Saito, Y., Oshima, K., Takahashi, T., Muroi, E., et al. (2010). Characterisation of volatile sulphur-containing compounds generated in crushed leaves of Chinese chive (Allium tuberosum Rottler). Food. Chem. 120, 343–348. doi: 10.1016/j.foodchem.2009.11.028
Yan, J. K., Zhu, J., Liu, Y. J., Chen, X., Wang, W. H., Zhang, H. N., et al. (2023). Recent advances in research on Allium plants: functional ingredients, physiological activities, and applications in agricultural and food sciences. Crit. Rev. Food. Sci. 63, 8107–8135. doi: 10.1080/10408398.2022.2056132
Yang, Z. R., Liu, J. W., Hao, L. Z., Zhang, F. L., Zhao, Q. Y. (2013). Research on flowering phonology and nutrition of blossom of four wild vegetables of Allium in Inner Mongolia. North. Hortic. 08), 1–4. doi: CNKI:SUN:BFYY.0.2013-08-002
Yang, L. J., Yang, K. J. (2020). Biological function of Klebsiella variicola and its effect on the rhizosphere soil of maize seedlings. Peerj 8, e9894. doi: 10.7717/peerj.9894
Yang, P. T., Yuan, Y., Yan, C., Jia, Y., You, Q., Da, L., et al. (2023). AlliumDB: a central portal for comparative and functional genomics in Allium. Hortic. Res-England. 11, uhad285. doi: 10.1093/hr/uhad285
Yazdanpour, F., Khadivi, A., Khah, A. E. (2018). Phenotypic characterization of black raspberry toselect the promising genotypes. Sci. Hortic-Amsterdam. 235, 95–105. doi: 10.1016/j.scienta.2018.02.071
Yin, C. M., Fan, X. Z., Fan, Z., Shi, D. F., Yao, F., Gao, H. (2019). Comparison of non-volatile and volatile flavor compounds in six Pleurotus mushrooms. J. Sci. Food. Agr. 99, 1691–1699. doi: 10.1002/jsfa.9358
Zamri, N., Hamid, H. A. (2019). Comparative study of onion (Allium cepa) and leek (Allium ampeloprasum): identification of organosulphur compounds by UPLC-QTOF/MS and anticancer effect on MCF-7 cells. Plant Food. Hum. Nutr. 74, 525–530. doi: 10.1007/s11130-019-00770-6
Zhang, S. L., Du, Z., Li, G. K. (2012). Graphene-supported zinc oxide solid-phase microextraction coating with enhanced selectivity and sensitivity for the determination of sulfur volatiles in Allium species. J. Chromatogr. A. 1260, 1–8. doi: 10.1016/j.chroma.2012.08.045
Keywords: phenological period, quantitative characters, organosulfur compounds, amino acid, principal component analysis
Citation: Wang H, Yang H, Yu X, Xie Y, Bai Y, Dai Q, Liang L, Tang W, Yong M, Wang L, Huang Z, Sun B, Li H and Tang Y (2024) Biological features and quality comprehensive analysis of twelve germplasm resources of the genus Allium from Tibet. Front. Plant Sci. 15:1393402. doi: 10.3389/fpls.2024.1393402
Received: 29 February 2024; Accepted: 22 July 2024;
Published: 06 August 2024.
Edited by:
Laura De Gara, Università Campus Bio-Medico di Roma, ItalyReviewed by:
Miroslawa Chwil, University of Life Sciences of Lublin, PolandCopyright © 2024 Wang, Yang, Yu, Xie, Bai, Dai, Liang, Tang, Yong, Wang, Huang, Sun, Li and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Tang, dGFuZ3lpQHNpY2F1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.