
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 27 March 2024
Sec. Functional and Applied Plant Genomics
Volume 15 - 2024 | https://doi.org/10.3389/fpls.2024.1384246
This article is part of the Research Topic Crop Abiotic Stress: Advances in Germplasm/Gene Discovery and Utilization View all 18 articles
Introduction: Salt tolerance during seed germination is an important trait for direct seeding and low-cost rice production. Nevertheless, it is still not clear how seed germination under salt stress is regulated genetically.
Methods: In this study, genome-wide association studies (GWAS) were performed to decipher the genetic basis of seed germination under salt stress using 541 rice varieties collected worldwide.
Results and discussion: Three quantitative trait loci (QTLs) were identified including qGRG3-1 on chromosome 3, qGRG3-2 on chromosome 5, and qGRG4 on chromosome 4. Assessment of candidate genes in these loci for their responses to salt stress identified a TATA modulatory factor (OsTMF) in qGRG3-2. The expression of OsTMF was up-regulated in both roots and shoots after exposure to salt stress, and OsTMF knockout mutants exhibited delayed seed germination under salt stress. Haplotype analysis showed that rice varieties carrying OsTMF-Hap2 displayed elevated salt tolerance during seed germination. These results provide important knowledge and resources to improve rice seed germination under salt stress in the future.
Salinity is a major abiotic stress threatening global food production. High salt concentrations in soil cause osmotic stress, ionic toxicity and nutrient deficiency, and hamper plant growth and crop productivity (Hu et al., 2012; Yang and Guo, 2018). For rice production, about 30% agricultural land in the world suffers from high salinity, representing nearly ten thousand hectares in China alone (Takehisa et al., 2004; He and Luo, 2021).
Efforts have been made in the past decades to decipher the mechanisms underlying salt sensitivity in rice through genetic studies. These studies allowed us to identify multiple quantitative trait loci (QTLs) for salt tolerance, although only few genes have been cloned and functionally characterized. Shoot K+ Concentration 1 (SKC1) was identified from a F2 population generated by crossing the salt-tolerant (ST) indica variety ‘Nona Bokra’ with the salt-sensitive (SS) japonica variety ‘Koshihikari’. SKC1 encodes a high-affinity K+ transporter that maintains K+/Na+ equilibrium under salt stress (Lin et al., 2004; Ren et al., 2005). A zinc-finger transcription factor, Drought and Salt Tolerance (DST), regulates salt and drought responses by modulating reactive oxygen species (ROS) homeostasis and stomatal movements (Huang et al., 2009). Hitomebore Salt Tolerant 1 (HST1) was identified as a negative regulator of salt tolerance, and the introduction of a non-functional hst1 allele to a cultivated variety through backcrosses resulted in improved salt tolerance (Takagi et al., 2015).
With the tremendous progresses made in whole-genome sequencing, genome-wide association studies (GWAS) become a powerful tool to elucidate molecular machinery underlying traits regulated by multiple genes, such as salt tolerance (Wang et al., 2005). In Arabidopsis, GWAS was employed to identify genetic components regulating root architecture under salt stress, yielding Cytochrome P450 family 79 subfamily B2 (CYP79B2) as a positive regulator, and High-Affinity K+ Transporter 1 (HKT1) as a negative regulator (Julkowska et al., 2017). Zhang et al. (2019) identified ZmHAK4 that encodes a Na+ transporter as a Na+ level regulator in maize. In wheat, a QTL in a 1.5 Mb genomic region on chromosome 7B was identified, in which three putative K+ transporters (TaHKT8-B, TaHKT9-B and TaHKT10-B) were located. Lines overexpressing TaHKT9-B and varieties carrying the In‐1077 haplotype of TaHKT9-B displayed higher salt sensitivity due to lower K+ accumulation in shoots (Du et al., 2024). Recently, two genes were identified via GWAS, a transcription factor OsWRKY53 that regulates water contents in shoots, and a mitogen-activated protein kinase kinase 10.2 (OsMKK10.2) that is implicated in seedling survival under salt stress (Yu et al., 2023).
Seed germination is a critical developmental stage for plants to start a new phase of life. Under salt stress, seed germination in most crop species is compromised, manifested primarily by lower germination rates and longer germination time (Shi et al., 2017; Hasseb et al., 2022; Yang et al., 2023). Increased osmotic pressures induced by salt stress may affect water absorption, starch mobilization and catabolism of storage products during seed germination (Xu et al., 2017; Yan et al., 2022; Xiong et al., 2024). Enzymatic and antioxidant activities, hormone levels and ion homeostasis are also affected by salt stress, which may consequently impact both seed germination and seedling establishment (Munns and Tester, 2008; Yang and Guo, 2018; He et al., 2019).
In recent years, direct seeding is increasingly used in many rice-growing areas because of lower labor input, which demands uniform seed germination and high seedling vigor, especially under stress conditions (Farooq et al., 2011). A QTL for seed germination and seedling establishment under salt stress, Seedling Establishment 3 (qSE3), was identified using substitution lines generated by introgressions of chromosomal segments from a japonica variety ‘Jiucaiqing’ (with low seedling establishment under salt stress) into an indica variety ‘IR26’ (with high seedling establishment under salt stress). High-resolution mapping revealed that High-Affinity K+ Transporter 21 (OsHAK21) is likely to be the candidate gene in qSE3, which confers higher salt tolerance by decreasing ROS levels in abscisic acid (ABA)-dependent manner (He et al., 2019). Beyond this, very little is known about the genetic machinery underlying rice seed germination under salt stress.
The aim of this study is to characterize the genetic basis of rice seed germination under salt stress conditions, with the long-term goal to improve seed germination for direct seeding in saline land. We conducted GWAS to dissect the process using a panel of 541 rice varieties collected from different regions of the world. Three QTLs, qGRG3-1 on chromosome 3, qGRG3-2 on chromosome 5 and qGRG4 on chromosome 4, were detected under treatments of two concentrations of NaCl. OsTMF was showed to be the candidate gene in qGRG3-2 as its expression was highly responsive to salt stress, and ostmf mutants exhibited delayed seed germination under salt stress.
A total of 541 accessions of rice (Oryza sativa L.) from major rice-growing areas worldwide, consisting of 328 indica, 162 japonica, 18 admix, 17 aus, and 16 basmati varieties (Supplementary Table S1), were obtained from the China Rice Data Center and the 3,010 Rice Genomes Project (Wang et al., 2018). All varieties were planted, and seeds were harvested in Lingshui, Hainan Province, China. A single nucleotide polymorphism (SNP) dataset was downloaded from the Rice SNP-Seek database (https://snpseek.irri.org/) (Mansueto et al., 2016). Non-sequenced varieties were sequenced on an Illumina HiSeq 2000 Sequencing Platform (Illumina Inc., San Diego, CA, USA), with an average sequencing depth of approximately 10×.
Fully mature seeds were harvested in field right after the grains turned to yellow, and dried in a 37°C oven for 7 days to break dormancy. Fifty rice varieties (Supplementary Table S2) were randomly chosen for germination test to determine the optimal salt stress conditions using 0.3%, 0.4%, 0.5%, 0.6%, 0.7%, 0.8%, 0.9% and 1.0% NaCl (w/v) dissolved in deionized water. Accordingly, the concentrations of 0.3% and 0.5% were subsequently used for GWAS. For each variety, 90 seeds were divided into three replicates and placed onto two layers of filter paper soaked with either 8 mL of deionized water, or 0.3% or 0.5% NaCl solutions, in 6-cm petri dishes without seal. All solutions were refreshed every two days. These dishes were cultured in an incubator at 28°C under a 12 h light/12 h dark photoperiod for 7 days. Germinated seeds, with the criterion of root lengths longer than the seed length, and shoot lengths longer than half of the seed length (Shi et al., 2017), were counted to calculate their germination rates on the 3rd day (GR3), germination rates on the 4th day (GR4), and germination indexes in 7 days (GI), with the calculation formula of GR3 = accumulated number of germinated seeds on the 3rd day/the total number of seeds used × 100%; GR4 = accumulated number of germinated seeds on the 4th day/the total number of seeds used × 100%; GI = ∑(Gt/Tt), where Gt is the number of germinated seeds on day t and Tt is the time in days corresponding to Gt (Shi et al., 2017). To obtain germination rate grades on the 3rd (GRG3) and the 4th day (GRG4), and germination index grades (GIG), the ratios of GR3, GR4 or GI for salt treatment to those in the control condition were calculated, and then assigned to germination grades from 1 to 11 (Supplementary Table S3). For seed germination in saline soil, pots with the size of 7 cm × 7 cm × 10 cm were filled with soil, and immersed in either deionized water or 0.5% NaCl solution for 24 hours to ensure saturation. Thirty seeds were sown into each pot and covered with a thin layer of soil. Subsequently, these pots were placed in an incubator at 28°C under 12 h light/12 h dark, watered with either deionized water or 0.5% NaCl solution in every two days. The germinated rates and shoot lengths were measured on the 10th day after sowing.
The general linear model (GLM) with the first three principal components matrix calculated from genotypes was employed using Tassel 5.2.54 software (Bradbury et al., 2007) to perform GWAS for seed germination under salt stress. Across the whole panel of varieties, 302,900 high-quality SNPs were used for GWAS after removing SNPs with missing rates of more than 20% and minor allele frequencies of less than 5% (Ju et al., 2022; Mei et al., 2022). To identify significant SNPs, the Bonferroni correction method was used to calculate suggestive significance thresholds of associations, resulting in a p value of 1.65 × 10-7 (0.05/N, with N = number of SNPs) (Shi et al., 2017). The qqman package in R3.4.1 software was used to generate Manhattan plots (Turner, 2014). Functional annotations for genes within genomic regions of interest were obtained in reference to the Nipponbare genome IRGSP 1.0 (Kawahara et al., 2013). Haplotype analyses were performed using SNPs in the coding sequences, with each haplotype possessing at least twenty rice varieties (Mei et al., 2022).
Candidate genes were investigated by quantitative real-time PCR (qRT-PCR) using RNA extracted from shoots and roots of 7-day-old Zhonghua11 (a japonica rice variety, ZH11) seedlings grown under 0.3% or 0.5% NaCl, or control (deionized water) conditions. To examine expression levels of candidate genes in varieties with different germination grades, shoots from 7-day-old seedlings of ST (L_363, L_417 and L_505, with GRG3 and GRG4 ≤ 2) and SS varieties (L_065, L_274 and L_352, with GRG3 and GRG4 ≥9) grown under 0.5% NaCl or control conditions were collected and analyzed by qRT-PCR. To examine expression patterns of OsTMF in time-course salt stress treatments, germinated seeds under 0.5% NaCl or control conditions for 1, 2, or 3 days, and seedlings grown in Hoagland solution treated with or without 0.5% NaCl for 1, 3, or 7 days, were sampled. Total RNA was extracted using TRIzol™ reagent (Thermo Fisher, USA), cDNA was synthesized using the PrimeScript™ RT reagent kit (Takara, Japan), and qRT-PCR was conducted using TB Green® Premix Ex Taq™ II (Takara, Japan) on LightCycler® 96 (Roche Life Science, Switzerland). Primers for qRT-PCR were designed using Primer3 (https://primer3.ut.ee) based on coding sequences of genes (Supplementary Table S4). OsActin1 was used as an internal control for normalization, and the 2−△△CT method was used to calculate relative expression levels.
Clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated nuclease 9 (CRISPR/Cas9) constructs were made according to Cheng et al. (2021), using the guide sequences for candidate genes designed by the web tool CRISPR-GE (http://cbi.hzau.edu.cn/cgi-bin/CRISPR) (Xie et al., 2017; Supplementary Table S5), and transformed into ZH11 via Agrobacterium-mediated transformation (Nishimura et al., 2007). Sanger sequencing (Sangon Biotech, Beijing) was performed to identify homozygous mutants. PCR products amplified with HPT-F (5′-TGCCGTCAACCAAGCTCTGA-3′) and HPT-R (5′-GCTTCGATGTAGGAGGGCGT-3′) primers were used to select transgene-free lines.
Correlation analyses and significant differences were assessed between pairs of evaluated traits using SAS software (Shi et al., 2017). Differences between varieties, subpopulations, treatments, or haplotypes were examined using a one-way ANOVA method based on a significance level of 0.01. Figures were generated using GraphPad Prism 8.0 software (https://www.graphpad.com).
To define optimal conditions for assessing rice seed germination under salt stress, seeds of 50 varieties randomly selected from 541 varieties collected from different regions of the world (Supplementary Table S2) were germinated in either deionized water (control) or different concentrations of NaCl solutions (see Materials and Methods). Well-germinated seeds, according to the criterion reported (Shi et al., 2017) were counted every day over a 7-day period. GRG3, GRG4 and GIG were calculated, with germination grade 1 for the highest, and grade 11 for the lowest salt tolerances (see Materials and Methods). Results showed that, under 0.3% and 0.5% NaCl treatments, all three traits were consistently distributed (Supplementary Figure S1), therefore, these two treatments were used for subsequently experiments.
To examine variations in seed germinations under salt stress in 541 rice varieties, seeds of these varieties were placed on plates with water, 0.3% or 0.5% NaCl, and germinations were counted daily over a 7-day period. Variations of seed germinations under salt stress were observed in different varieties (Supplementary Table S1). As showed for two representative varieties, L_541 with lower salt tolerance, and L_022 with higher salt tolerance in germination, numbers of germinated seeds differed on the 3rd and the 4th day, with more germinated seeds for the ST variety (Figure 1; above dash lines). All three traits, GRG3, GRG4 and GIG, showed large variations (Figure 2), with the highest coefficient of variation observed for GRG4 (73.53%) under 0.3% NaCl condition, followed by GRG4 under 0.5% NaCl (57.31%), and GRG3 under 0.3% NaCl (53.43%) (Table 1). Phenotypic variations in subpopulations were also examined, and results showed that, under treatments of either 0.3% or 0.5% NaCl, indica varieties displayed lower GRG3 (Supplementary Figure S2A) and GRG4 (Supplementary Figure S2B) when compared with other 4 subpopulations that showed no significant differences, suggesting a higher salt tolerance of indica varieties. To be noted, no significant difference was observed for GIG among these subpopulations (Supplementary Figure S2C). In addition, on average, GRG3 was higher than GRG4 under both 0.3% and 0.5% NaCl (Table 1), suggesting that the germination of rice seeds on the 3rd day was more vulnerable to salt stress than that on the 4th day. Further analyses revealed that GRG3 and GRG4 had moderately positive correlations at these two NaCl concentrations (Supplementary Table S6).
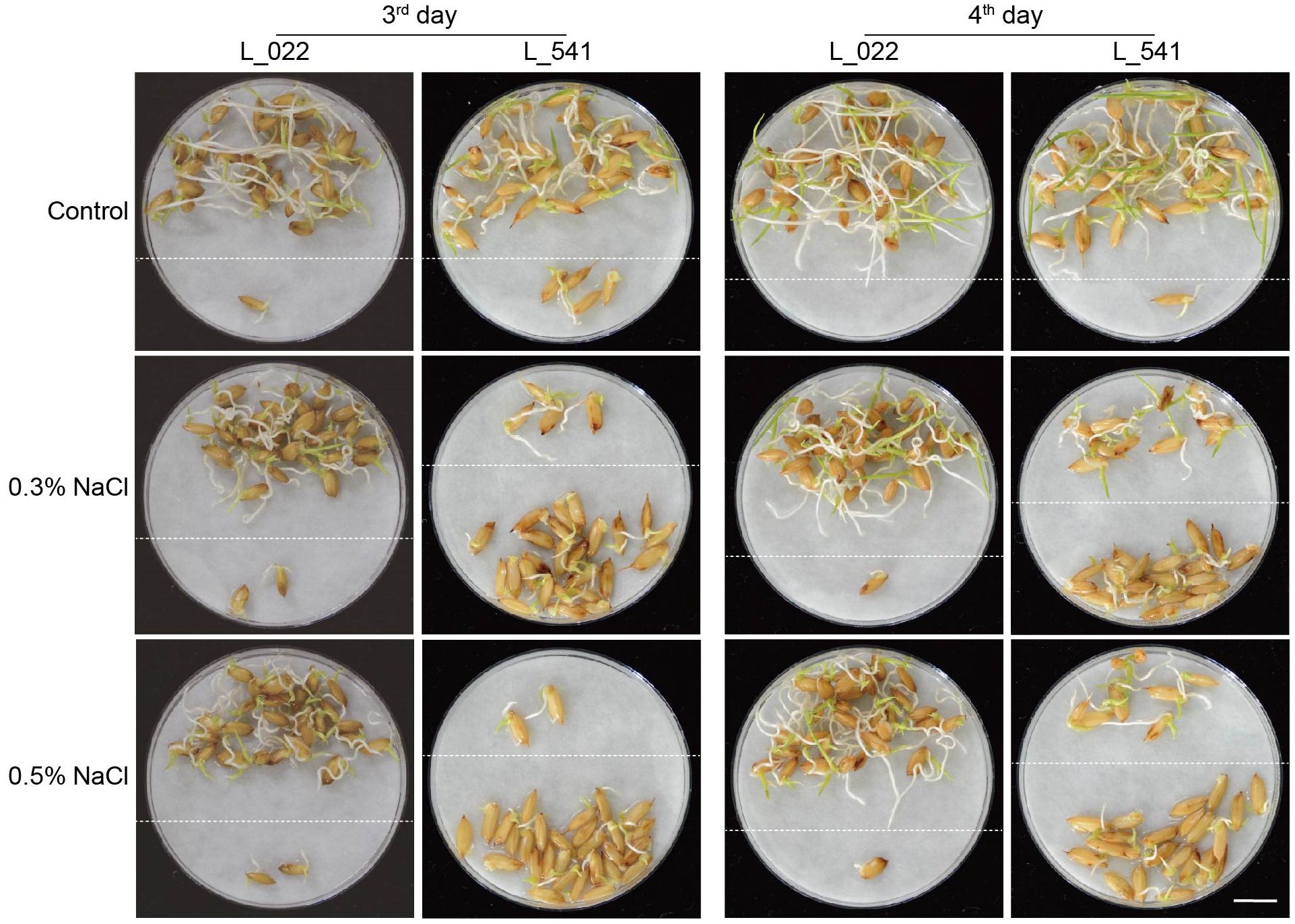
Figure 1 Differences in seed germination under salt stress in two representative rice varieties. Representative photos of rice seed germinations under salt stress, on the 3rd and 4th days for the salt-tolerant (ST) variety L_022 and the salt-sensitive (SS) variety L_541. Seeds were placed on filter paper soaked with deionized water (control), 0.3% or 0.5% NaCl for the indicated numbers of days. Germinated seeds, with root length longer than seed length and shoot length longer than half of the seed length, were repositioned above dashed lines. Scale bar, 1 cm.
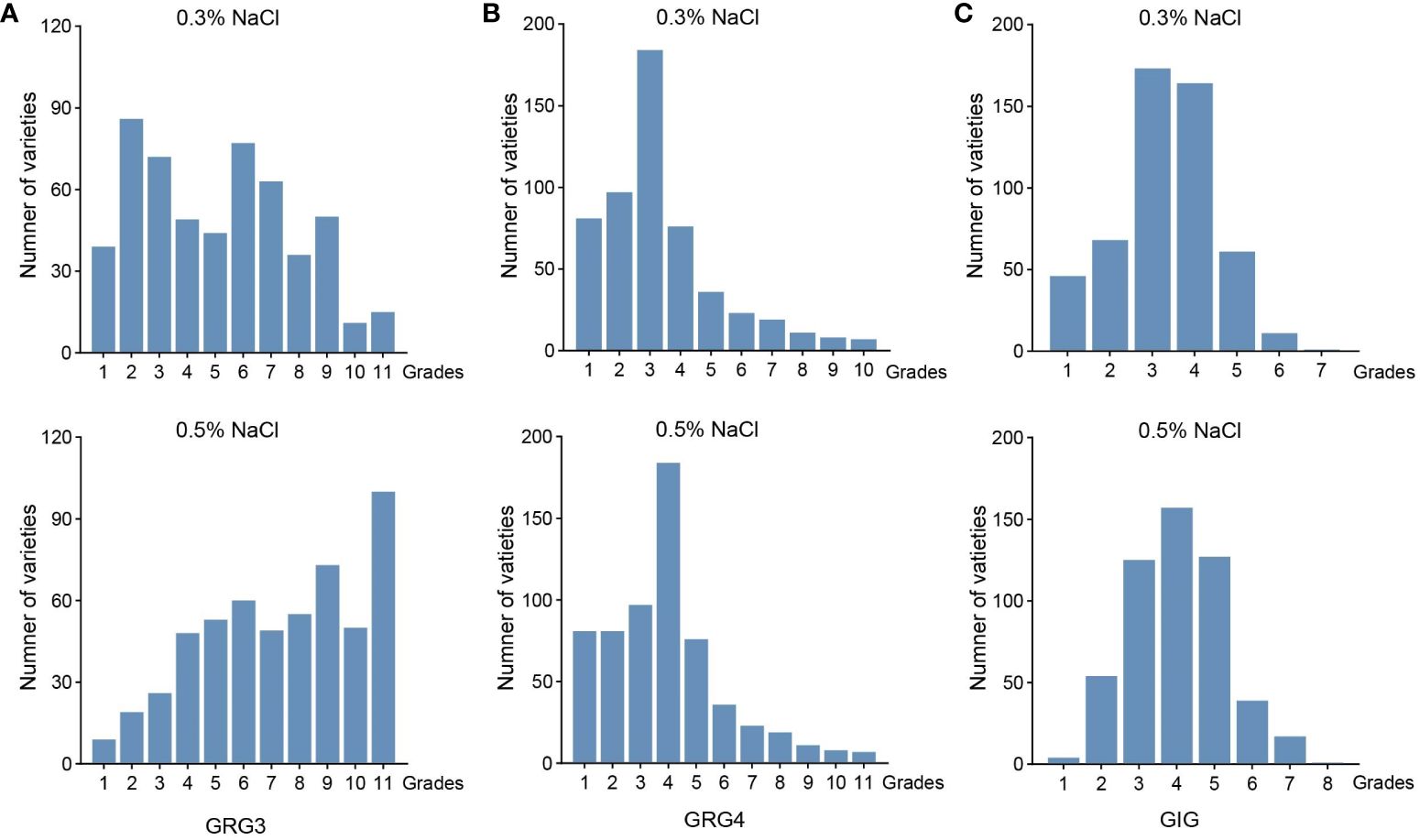
Figure 2 Phenotypic variations of 541 rice varieties in seed germinations under salt stress. (A) Germination rate grades on the 3rd day (GRG3) under the 0.3% (top) and 0.5% NaCl treatments (bottom). (B) Germination rate grades on the 4th day (GRG4) under the 0.3% (top) and 0.5% NaCl treatment (bottom). (C) Germination index grades (GIG) under the 0.3% (top) and 0.5% NaCl treatments (bottom).
To dissect the genetic basis of rice seed germination under salt stress, GWAS was conducted based on the GLM model using 302,900 SNPs from public database and in-house re-sequencing project. These SNPs were evenly distributed across all 12 chromosomes, with a density of about 800 SNPs/Mb (Supplementary Table S7). The significance threshold for GWAS was set to 6.78 (−log10(0.05/N), N = effective SNP number) after Bonferroni correction. Manhattan plots were generated, showing that 1,494 SNPs were significantly correlated with rice seed germination under salt stress, in particular, GRG3 (Figure 3A; Supplementary Table S8) and GRG4 (Figure 3B; Supplementary Table S8). These SNPs explained 4.54% to 10.06% of phenotypic variations. No SNP association was identified for GIG (Figure 3C).
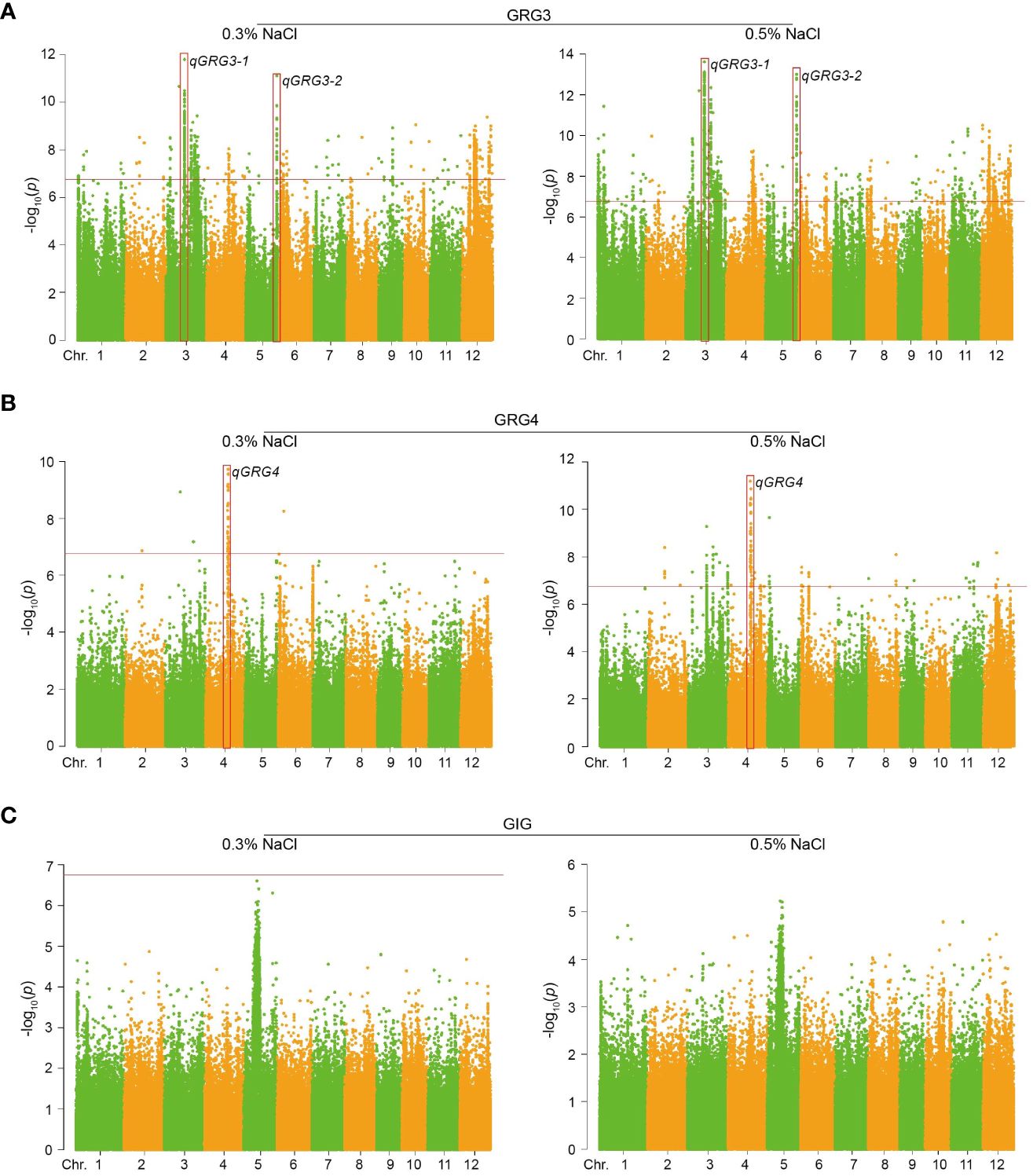
Figure 3 Manhattan plots for GWAS of rice seed germinations under salt stress. (A) Manhattan plots of GRG3 under 0.3% (left) or 0.5% NaCl treatments (right). (B) Manhattan plots of GRG4 under 0.3% (left) or 0.5% NaCl treatments (right). (C) Manhattan plots of GIG under 0.3% (left) or 0.5% NaCl treatments (right). Red boxes show genomic regions with clustered SNPs above the significance thresholds (−log10(p); indicated by horizontal red lines).
To identify putative genomic regions associated with salt tolerance, we screened for co-located and clustered SNPs detected under both 0.3% and 0.5% NaCl treatments. Three QTLs, qGRG3-1 (chr.3; 16,666,490 − 17,013,993) and qGRG3-2 (chr.5; 27,858,716 − 28,081,822) associated with GRG3 (Figure 3A), and qGRG4 (chr.4; 19,891,019 − 20,069,206) associated with GRG4 (Figure 3B), were identified. Notably, the qGRG4 locus contained a high-affinity potassium transporter OsHAK1, harboring a significant SNP (S04_19891019, −log10(p) = 7.19 under 0.3% and −log10(p) = 7.58 under 0.5% NaCl). Since OsHAK1 is known to regulate K+/Na+ homeostasis in rice seedlings under salt stress (Chen et al., 2015), our subsequent work was focused on two other loci, qGRG3-1 and qGRG3-2.
To identify candidate genes that are responsive to salt stress in qGRG3-1 and qGRG3-2 loci, the roots and shoots of 7-day-old ZH11 seedlings grown under 0.3% or 0.5% NaCl treatments were collected, and expression analyses were performed using qRT-PCR to analyze expressions of genes located in these two loci. No consistent responses to salt stress were observed in any of the 19 genes in the qGRG3-1 locus (Figure 4A). In contrast, 8 of 21 genes examined in the qGRG3-2 locus showed responsive expressions under the salt stress (Figure 4B). In particular, LOC_Os05g48620, LOC_Os05g48730, and LOC_Os05g48900 were up-regulated in roots and shoots of seedlings exposed to either 0.3% or 0.5% NaCl, while expressions of LOC_Os05g48650 and LOC_Os05g48670 were only up-regulated under 0.5% NaCl treatment (Figure 4B). Three other genes, LOC_Os05g48600, LOC_Os05g48760 and LOC_Os05g48930, were down-regulated in roots upon exposure to 0.3% or 0.5% NaCl (Figure 4B, right). We selected these genes as candidates for further analysis.
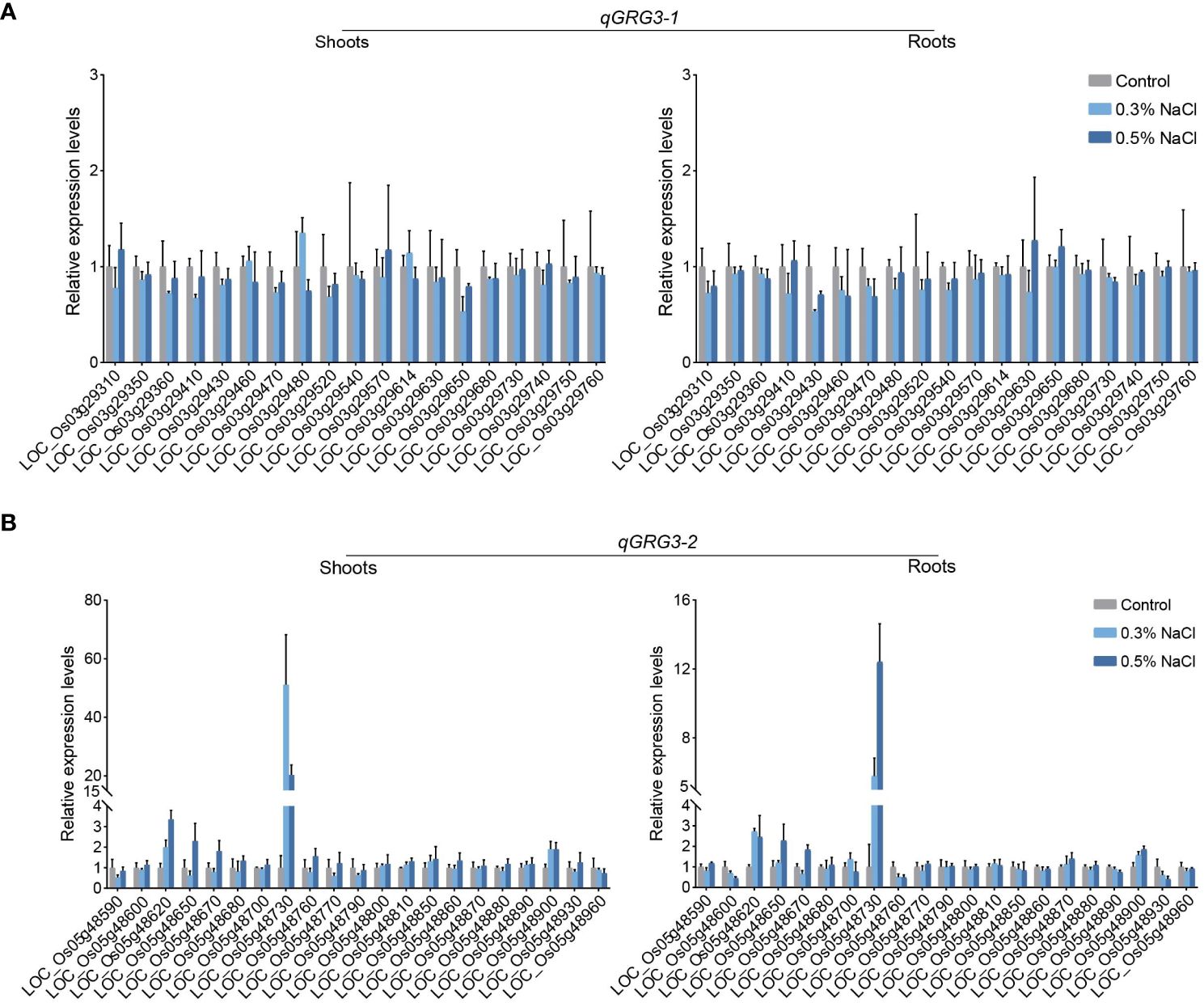
Figure 4 Relative expressions of genes in qGRG3-1 and qGRG3-2 loci under the salt stress. qRT-PCR analyses of genes in qGRG3-1 (A) and qGRG3-2 (B) loci in shoots (left) and roots (right) excised from 7-day-old ZH11 seedlings germinated under control (deionized water), 0.3% NaCl or 0.5% NaCl conditions. Data are shown as means ± SD from three biological replicates.
We examined expressions of these eight candidate genes in three ST (L_363, L_417, and L_505) and three SS varieties (L_065, L_274, and L_352), selected as described in Materials and Methods. Results showed that LOC_Os05g48730 (Supplementary Figure S3A) and LOC_Os05g48930 (Supplementary Figure S3B) exhibited higher levels of expression in ST varieties than those in SS varieties, under both the control and salt stress conditions. Three other genes, LOC_Os05g48620 (Supplementary Figure S3C), LOC_Os05g48670 (Supplementary Figure S3D) and LOC_Os05g48760 (Supplementary Figure S3E), showed elevated expressions under salt stress in ST varieties, but not in SS varieties. For LOC_Os05g48600, elevated expressions were observed in L_363 (ST) and L_417 (ST) under salt stress, but no significantly changed expressions in L_505 (ST), and three SS varieties (L_065, L_274 and L_352; Supplementary Figure S3F). For LOC_Os05g48650 and LOC_Os05g48900, although no consistent pattern was observed between ST and SS varieties, elevated expressions were detected for LOC_Os05g48650 under salt stress in all varieties except L_065 (Supplementary Figure S3G), whereas no salt-induced expressions were observed for LOC_Os05g48900 in neither ST nor SS varieties (Supplementary Figure S3H).
To further characterize the eight candidate genes in qGRG3-2 locus, knockout mutants were generated using CRISPR/Cas9-mediated gene editing in ZH11. For each gene, at least two independent homozygous transgene-free lines with frameshifts in translation were identified in either T1 or T2 generations, confirmed by PCR amplifications and sequencing (Supplementary Table S5). We tested the germination rates of mature seeds from mutant lines under the control, 0.3% and 0.5% NaCl treatments. Among lines assayed, ostmf-1 and ostmf-2 (Figures 5A, B), carrying mutations in LOC_Os05g48620 that encodes OsTMF (a rice homolog of human TATA modulatory factor, TMF), exhibited delayed germination under 0.3% NaCl treatments when compared to ZH11 (Figures 5C, D). OsTMF was previously reported to attenuate cold tolerance in rice by modulating expressions of cell wall biosynthesis-related genes (Xu et al., 2020). With the increased NaCl concentration (0.5%), further delays of germination were observed (Figures 5C (below dashed lines), E).
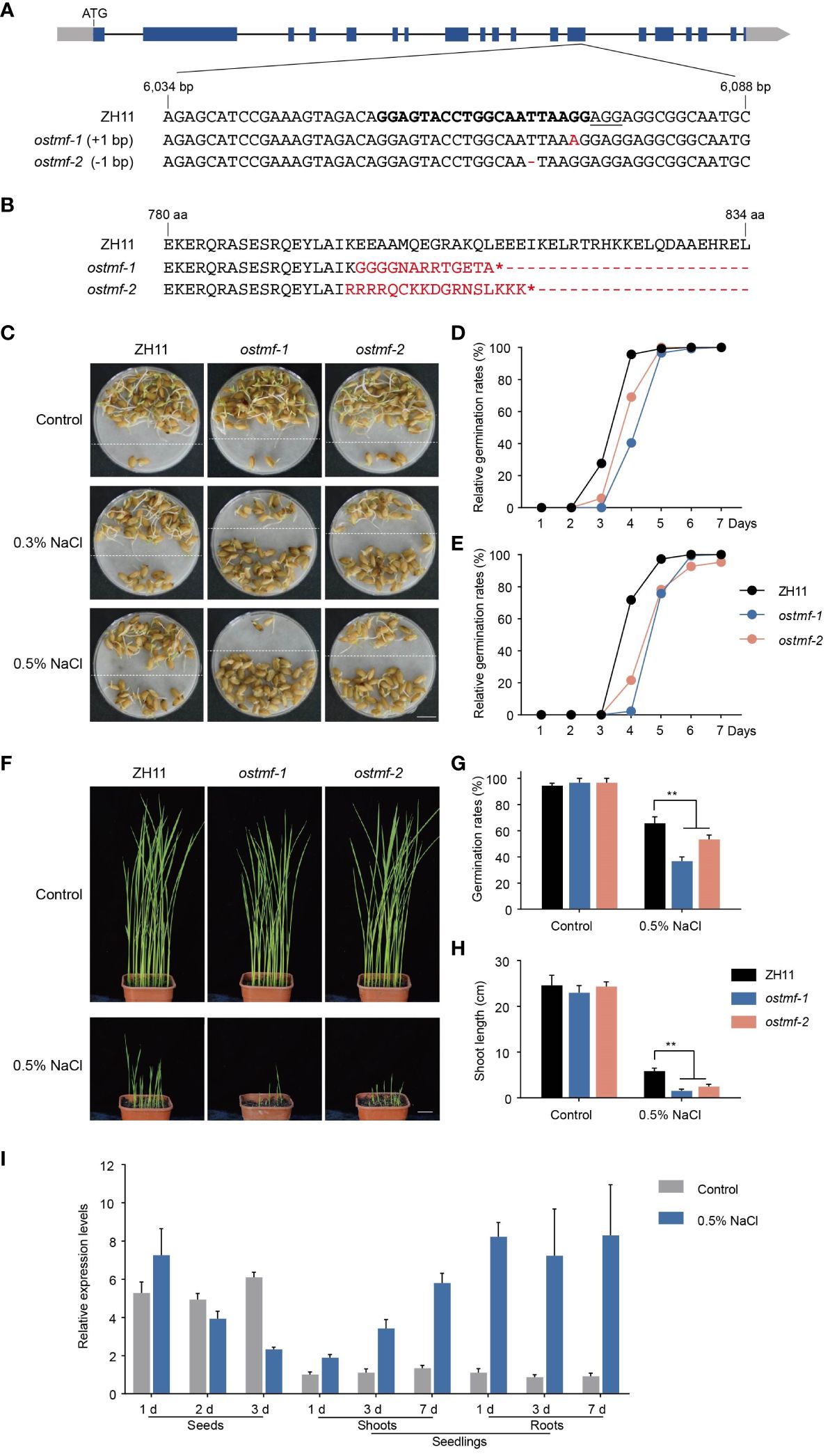
Figure 5 Mutations of OsTMF led to delayed seed germinations under salt stress. (A) A diagram of the OsTMF gene model (top), and partial sequence of OsTMF containing the target sites (in bold) for CRISPR/Cas9-based gene editing in ZH11, and the sequences of ostmf-1 and ostmf-2 mutants. Blue rectangles represent exons, black lines represent introns, and gray rectangles represent untranslated regions. The protospacer adjacent motif (PAM) is underlined, and mutations are in red. (B) Partial peptide sequences of OsTMF from ZH11, ostmf-1 and ostmf-2. Red, altered sequences in ostmf-1 and ostmf-2; asterisks, stop codon. (C) Representative photos for seed germinations of ZH11 and ostmf mutants under the control (deionized water), 0.3% or 0.5% NaCl treatments for 4 days. Germinated seeds were repositioned above the dashed lines. Scale bar, 1 cm. (D, E) Relative germination rates of ZH11 and ostmf mutants in different time points under 0.3% (D) and 0.5% (E) NaCl treatments. (F) Ten-day-old seedlings of ZH11 and ostmf mutants, germinated in soil-filled pots immersed in deionized water (the upper panel) or 0.5% NaCl (the lower panel). Scale bar, 2 cm. (G) Germination rates on the 10th day of ZH11 and ostmf mutants. germinated in soil-filled pots immersed in deionized water or 0.5% NaCl. **, p < 0.05. (H) Shoot length of seedlings of 10-day-old ZH11 and ostmf mutants germinated in soil-filled pots immersed in deionized water or 0.5% NaCl **, p < 0.05. (I) Expressions of OsTMF in seeds (treated with deionized water or 0.5% NaCl for 1, 2 or 3 days) and seedlings (treated with Hoagland solution or Hoagland solution containing 0.5% NaCl for 1, 3 or 7 days).
To examine the germinations of ostmf-1 and ostmf-2 in saline soil, we sowed their seeds in pots, filled with soil, and soaked in deionized water or 0.5% NaCl. On the 10th day, no evident phenotypic difference was observed in ostmf-1 and ostmf-2 under the control conditions, when compared to ZH11 (Figure 5F). However, in soil soaked with 0.5% NaCl, both ostmf-1 and ostmf-2 showed lower germination rates (Figure 5G) and shorter shoot lengths (Figure 5H).
To investigate the expression of OsTMF in response to salt stress, we conducted qRT-PCR analyses in seeds and seedlings using RNAs collected at different days after growing in 0.5% NaCl and deionized water. Results showed that the OsTMF expression was increased in seeds after growing in 0.5% NaCl solution for 1 day, and then decreased gradually, whereas it maintained stable in those seeds grown in water (Figure 5I). In the shoots and roots of seedlings, salt stress enhanced OsTMF expressions from day 1 to 7, whereas in the control conditions, OsTMF expression remained at stable and low levels throughout the time course. These findings suggest that OsTMF may positively regulate seed germination under salt stress.
To examine the relationship between OsTMF haplotypes and seed germination under salt stress, haplotype analysis was conducted in 541 rice varieties. In the coding region of OsTMF, six SNPs were detected (Figure 6A), with which we defined four major haplotypes, based on their presences in at least 20 varieties. We observed that Hap1, Hap2, and Hap4 are predominant present in the indica subpopulation, while Hap3 is predominant in the japonica subpopulation (Figures 6A, B). GRG3 of varieties harboring Hap2 were 3.710 and 5.794 under 0.3% and 0.5% NaCl treatments, respectively, which were lower than those varieties carrying Hap1, Hap3, or Hap4 (Figure 6C), indicating that Hap2 is more tolerant to salt stress. These results together suggest that Hap2 is a potential haplotype to be used for improving rice seed germination under salt stress.

Figure 6 Haplotype analyses in OsTMF. (A) Genomic variations in OsTMF coding region, showing the presence of 4 haplotypes (Hap) in 541 rice varieties. (B) Frequencies of 4 OsTMF haplotypes in different rice subpopulations. (C) Comparison of GRG3 under 0.3% (left) or 0.5% NaCl (right) in 4 OsTMF haplotypes. Lowercase letters indicate significant differences based on Duncan’s multiple range post-hoc test (p < 0.05).
Efficient seed germination under salt stress is a crucial prerequisite for seedling establishment and subsequent crop production in saline land, and is a quantitative trait regulated by multiple genes (Shi et al., 2017; Li et al., 2022). In this study, we conducted GWAS to investigate the genetic basis of seed germination under salt stress utilizing a set of 541 rice varieties collected worldwide. We identified three QTLs, qGRG3-1, qGRG3-2 and qGRG4, which are significantly associated with seed germination under salt stress, imposed by using 0.3% or 0.5% NaCl. Detailed analyses showed that the most likely candidate gene underlying qGRG3-2 is OsTMF. Under salt stress, the expressions of OsTMF were up-regulated, and ostmf mutants exhibited delayed seed germinations when compared to the wild type.
In general, rice seed germination is defined visually by the emergence of the radicle penetrating through the hull, which has been used to study seed dormancy and germination (Jung et al., 2019; Prasad et al., 2022; Jin et al., 2023). However, under salt stress, rice seeds exhibit not only delayed germination, but also compromised growths of radicle and coleoptile, leading to lower seedling viability. Therefore, a more adequate criterion to define seed germination under salt stress is based on a combination of two measures: the root length longer than the seed length, and the shoot length longer than half of the seed length (Shi et al., 2017). To accurately measure seed germination in such a manner, we did the germination experiments on water- or NaCl solution-immersed filter paper in petri dishes (with a cover, but without seal), and refreshed the solutions in every two days. After counting germinated seeds, germination rate and germination index were calculated to evaluate seed germination status under salt stress (Shi et al., 2017; Ju et al., 2022; Li et al., 2022; Mei et al., 2022; Zhan et al., 2023). In this study, we established germination grades (1 to 11) using the GR3, GR4 and GI ratios of salt stress condition to the control condition. Under such a criterion, all varieties showed continuous and extensive phenotypic variations, suggesting that they are suitable for GWAS analysis.
Although GWASs have been performed in rice to decipher seed germination under salt stress (Shi et al., 2017; Yu et al., 2018; Islam et al., 2022; Ju et al., 2022; Li et al., 2022), no genes have been identified so far. In this study, three QTLs, two for GRG3 (qGRG3-1 and qGRG3-2) and one for GRG4 (qGRG4), were consistently detected under 0.3% and 0.5% NaCl treatments. In the qGRG4 locus, OsHAK1, a well-characterized high-affinity K+ transporter that promotes rice seedling growth under salt stress, is present (Chen et al., 2015, 2018). The OsHAK1 gene was also reported before in the q4.8 locus (Zhang et al., 2023), and is associated with relative germination rate on the 3rd day under saline-alkali stress. In addition, other HAK genes such as OsHAK12 (Zhang et al., 2021), OsHAK16 (Feng et al., 2019) and OsHAK21 (He et al., 2019; Song et al., 2021) are implicated in salt tolerance in rice. Among them, OsHAK21 may play a role in seed germination under salt stress (He et al., 2019). Therefore, in experiments followed, we did not pay much attention to the genomic region of qGRG4. qGRG3-1 overlaps with the reported 16.66−17.02 Mbp region on chromosome 3, which is associated with shoot fresh weight under salt stress (Yu et al., 2023). qGRG3-2 overlaps with the reported q5.11 locus that is associated with the relative germination rate under saline-alkali stress (Zhang et al., 2023). However, no salt tolerance-related gene has been reported in these regions.
Expression analysis is a powerful way to identify genes underlying salt tolerance, with either elevated (Chen et al., 2018; Feng et al., 2019) or repressed expressions under salt stress (Wang et al., 2020), or differential expressions in ST and SS varieties (Zhang et al., 2018, 2019). We examined expressions of genes located in qGRG3-1 and qGRG3-2 loci, which allowed us to identify eight candidate genes in the qGRG3-2 locus, which exhibited elevated or repressed expressions under salt stresses. Among them, five showed differential expressions between SS and ST varieties. To narrow down candidate genes in the qGRG3-2 locus, we employed CRISPR/Cas9-based gene editing to generate mutants for all these eight genes. Seed germination analyses performed in these mutants under salt stress allowed us to establish that OsTMF is the most likely candidate gene, since OsTMF knockout mutants showed delayed seed germination under salt stress. We showed further that varieties with different OsTMF haplotypes showed differences in early seed germination under salt stress. However, as the germination progressed to the seventh day, the germination rates of ostmf mutants were comparable with ZH11, suggesting a major role of OsTMF in regulating early seed germination under salt stress, not so much in seedling establishment. OsTMF has previously been reported to be a negative regulator of cold tolerance in rice (Xu et al., 2020). In another report, it was shown that OsTMF interacted with Ski-Interacting Protein a (OsSKIPa), that regulates cell vitality, and overexpression of OsSKIPa led to enhanced vegetative growth under salt stress (Hou et al., 2009). Whether OsTMF acts through OsSKIPa in seed germination under salt stress warrants further investigation.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
LL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. YM: Data curation, Writing – review & editing. HZ: Writing – review & editing. LG: Investigation, Writing – review & editing. YG: Supervision, Validation, Writing – review & editing. C-ML: Formal Analysis, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Key R&D Program of China (2020YFE0202300).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1384246/full#supplementary-material
Supplementary Figure 1 | Phenotypic variations of 50 randomly selected rice varieties for seed germination in NaCl concentrations from 0.3% to 1%. Germination rate grades on the 3rd (GRG3; A) and the 4th day (GRG4; B), and germination index grades (GIG; C) under the treatments if indicated NaCl concentrations.
Supplementary Figure 2 | Phenotypic variations for seed germinations under salt stresses among varieties in different subpopulations. (A) Germination rate grades on the 3rd day (GRG3) under 0.3% (top) and 0.5% NaCl (bottom) treatments in different subpopulations. (B) Germination rate grades on the 4th day (GRG4) under 0.3% (top) and 0.5% NaCl (bottom) conditions in different subpopulations. (C) Germination index grades (GIG) under the treatment of 0.3% (top) and 0.5% NaCl (bottom) in different subpopulations. Lowercase letters indicate significant differences based on Duncan’s multiple range post-hoc test (p < 0.05).
Supplementary Figure 3 | Expressions of candidate genes in the qGRG3-2 locus in representative salt-tolerant and salt-sensitive varieties. ST, representative salt-tolerant varieties; SS, representative salt-sensitive varieties.
Bradbury, P. J., Zhang, Z. W., Kroon, D. E., Casstevens, T. M., Ramdoss, Y., Buckler, E. S. (2007). TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635. doi: 10.1093/bioinformatics/btm308
Chen, G., Hu, Q. D., Luo, L., Yang, T. Y., Zhang, S., Hu, Y. B., et al. (2015). Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ. 38, 2747–2765. doi: 10.1111/pce.12585
Chen, G., Liu, C., Gao, Z., Zhang, Y., Zhang, A., Zhu, L., et al. (2018). Variation in the abundance of OsHAK1 transcript underlies the differential salinity tolerance of an indica and a japonica rice cultivar. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.02216
Cheng, Z. X., Sun, Y., Yang, S. H., Zhi, H., Yin, T., Ma, X. J., et al. (2021). Establishing in planta haploid inducer line by edited SiMTL in foxtail millet (Setaria italica). Plant Biotechnol. J. 19, 1089–1091. doi: 10.1111/pbi.13584
Du, L. Y., Ding, L., Huang, X. L., Tang, D. L., Chen, B., Tian, H., et al. (2024). Natural variation in a K+-preferring HKT transporter contributes to wheat shoot K+ accumulation and salt tolerance. Plant Cell Environ. 47, 540–556. doi: 10.1111/pce.14746
Farooq, M., Siddique, K., Rehman, H., Aziz, T., Lee, D., Wahid, A. (2011). Rice direct seeding: experiences, challenges and opportunities. Soil Tillage. Res. 111, 87–98. doi: 10.1016/j.still.2010.10.008
Feng, H. M., Tang, Q., Cai, J., Xu, B. C., Xu, G. H., Yu, L. (2019). Rice OsHAK16 functions in potassium uptake and translocation in shoot, maintaining potassium homeostasis and salt tolerance. Planta 250, 549–561. doi: 10.1007/s00425-019-03194-3
Hasseb, N. M., Sallam, A., Karam, M. A., Gao, L., Wang, R. R. C., Moursi, Y. S. (2022). High-LD SNP markers exhibiting pleiotropic effects on salt tolerance at germination and seedlings stages in spring wheat. Plant Mol. Biol. 108, 585–603. doi: 10.1007/s11103-022-01248-x
He, L. R., Luo, Y. H. (2021). Effects of long-term sand mixing ameliorating measures on soil salinity characteristics of saline-alkali land in northern shaanxi. IOP. Conf. Series.: Earth Environ. Sci. 781, 1–5. doi: 10.1088/1755-1315/781/2/022084
He, Y. Q., Yang, B., He, Y., Zhan, C. F., Cheng, Y. H., Zhang, J. H., et al. (2019). A quantitative trait locus, qSE3, promotes seed germination and seedling establishment under salinity stress in rice. Plant J. 97, 1089–1104. doi: 10.1111/tpj.14181
Hou, X., Xie, K. B., Yao, J. L., Qi, Z., Xiong, L. Z. (2009). A homolog of human ski-interacting protein in rice positively regulates cell viability and stress tolerance. Proc. Natl. Acad. Sci. U. S. A. 106, 6410–6415. doi: 10.1073/pnas.0901940106
Hu, S. K., Tao, H. J., Qian, Q., Guo, L. B. (2012). Genetics and molecular breeding for salt tolerance in rice. Rice Genom. Genet. 3, 39–49. doi: 10.5376/rgg.2012.03.0007
Huang, X. Y., Chao, D. Y., Gao, J. P., Zhu, M. Z., Shi, M., Lin, H. X. (2009). A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Gene. Dev. 23, 1805–1817. doi: 10.1101/gad.1812409
Islam, M. R., Naveed, S. A., Zhang, Y., Li, Z. K., Zhao, X. Q., Fiaz, S., et al. (2022). Identification of candidate genes for salinity and anaerobic tolerance at the germination stage in rice by genome wide association analyses. Front. Genet. 13. doi: 10.3389/fgene.2022.822516
Jin, X. K., Li, X. X., Xie, Z. Z., Sun, Y., Jin, L., Hu, T. Z., et al. (2023). Nuclear factor OsNF-YC5 modulates rice seed germination by regulating synergistic hormone signaling. Plant Physiol. 193, 2825–2847. doi: 10.1093/plphys/kiad499
Ju, C. Y., Ma, X. D., Han, B., Zhang, W., Zhao, Z. W., Geng, L. Y., et al. (2022). Candidate gene discovery for salt tolerance in rice (Oryza sativa L.) at the germination stage based on genome-wide association study. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1010654
Julkowska, M. M., Koevoets, I. T., Mol, S., Hoefsloot, H., Feron, R., Tester, M. A., et al. (2017). Genetic components of root architecture remodeling in response to salt stress. Plant Cell 29, 3198–3213. doi: 10.1105/tpc.16.00680
Jung, Y. J., Lee, H. J., Bae, S., Kim, J. H., Kim, D. H., Kim, H. K., et al. (2019). Acquisition of seed dormancy breaking in rice (Oryza sativa L.) via CRISPR/Cas9-targeted mutagenesis of OsVP1 gene. Plant Biotechnol. Rep. 13, 511–520. doi: 10.1007/s11816-019-00580-x
Kawahara, Y., Bastide, M., Hamilton, J. P., Kanamori, H., McCombie, W. R., Ouyang, S., et al. (2013). Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice (N. Y). 6, 4. doi: 10.1186/1939-8433-6-4
Li, C. J., Lu, C. S., Zou, B. L., Yang, M. M., Wu, G. L., Wang, P., et al. (2022). Genome-wide association study reveals a genetic mechanism of salt tolerance germinability in rice (Oryza sativa L.). Front. Plant Sci. 13. doi: 10.3389/fpls.2022.934515
Lin, H. X., Zhu, M. Z., Yano, M., Gao, J. P., Liang, Z. W., Su, W. A., et al. (2004). QTLs for Na+ and K+ uptake of shoot and root controlling rice salt tolerance. Theor. Appl. Genet. 108, 253–260. doi: 10.1007/s00122-003-1421-y
Mansueto, L., Fuentes, R. R., Borja, F. N., Detras, J., Abriol-Santos, J. M., Chebotarov, D., et al. (2016). Rice SNP-seek database update: new SNPs, indels, and queries. Nucleic Acids Res. 45, 1075–1081. doi: 10.1093/nar/gkw1135
Mei, S., Zhang, G. G., Jiang, J., Lu, J. B., Zhang, F. (2022). Combining genome-wide association study and gene-based haplotype analysis to identify candidate genes for alkali tolerance at the germination stage in rice. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.887239
Munns, R., Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Nishimura, A., Aichi, I., Matsuoka, M. (2007). A protocol for Agrobacterium-mediated transformation in rice. Nat. Protoc. 1, 2796–2802. doi: 10.1038/nprot.2006.469
Prasad, C. T. M., Kodde, J., Angenent, G. C., de Vos, R. C. H., Diez-Simon, C., Mumm, R., et al. (2022). Experimental rice seed aging under elevated oxygen pressure: Methodology and mechanism. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1050411
Ren, Z. H., Gao, J. P., Li, L. G., Cai, X. L., Huang, W., Chao, D. Y., et al. (2005). A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 37, 1141–1146. doi: 10.1038/ng1643
Shi, Y. Y., Gao, L. L., Wu, Z. C., Zhang, X. J., Wang, M. M., Zhang, C. S., et al. (2017). Genome-wide association study of salt tolerance at the seed germination stage in rice. BMC Plant Biol. 17, 92. doi: 10.1186/s12870-017-1044-0
Song, T. Z., Shi, Y. Y., Shen, L. K., Cao, C. J., Shen, Y., Jing, W., et al. (2021). An endoplasmic reticulum-localized cytochrome b5 regulates high-affinity K+ transport in response to salt stress in rice. Proc. Natl. Acad. Sci. U.S.A. 118, e2114347118. doi: 10.1073/pnas.2114347118
Takagi, H., Tamiru, M., Abe, A., Yoshida, K., Uemura, A., Yaegashi, H., et al. (2015). MutMap accelerates breeding of a salt-tolerant rice cultivar. Nat. Biotechnol. 33, 445–449. doi: 10.1038/nbt.3188
Takehisa, H., Shimodate, T., Fukuta, Y., Ueda, T., Yano, M., Yamaya, T., et al. (2004). Identification of quantitative trait loci for plant growth of rice in paddy field flooded with salt water. Field Crops Res. 89, 85–95. doi: 10.1016/j.fcr.2004.01.026
Turner, S. D. (2014). qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. J. Open Source Software 3, 1731. doi: 10.21105/joss.00731
Wang, W. Y., Barratt, B. J., Clayton, D. J., Todd, J. A. (2005). Genome-wide association studies: theoretical and practical concerns. Nat. Rev. Genet. 6, 109–118. doi: 10.1038/nrg1522
Wang, W. S., Mauleon, R., Hu, Z. Q., Chebotarov, D., Tai, S. S., Wu, Z. C., et al. (2018). Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557, 43–49. doi: 10.1038/s41586-018-0063-9
Wang, J., Qin, H., Zhou, S. R., Wei, P. C., Zhang, H. W., Zhou, Y., et al. (2020). The ubiquitin-binding protein OsDSK2a mediates seedling growth and salt responses by regulating gibberellin metabolism in rice. Plant Cell 32, 414–428. doi: 10.1105/tpc.19.00593
Xie, X. R., Ma, X. L., Zhu, Q. L., Zeng, D. C., Li, G. S., Liu, Y. G. (2017). CRISPR-GE: a convenient software toolkit for CRISPR-based genome editing. Mol. Plant 10, 1246–1249. doi: 10.1016/j.molp.2017.06.004
Xiong, M., Xu, J., Zhou, Z., Peng, B., Shen, Y., Shen, H., et al. (2024). Salinity inhibits seed germination and embryo growth by reducing starch mobilization efficiency in barley. Plant Direct. 8, e564. doi: 10.1002/pld3.564
Xu, E. S., Chen, M. M., He, H., Zhan, C. F., Cheng, Y. H., Zhang, H. S., et al. (2017). Proteomic analysis reveals proteins involved in seed imbibition under salt stress in rice. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.02006
Xu, Y., Hu, D., Hou, X., Shen, J. Q., Liu, J. H., Cen, X., et al. (2020). OsTMF attenuates cold tolerance by affecting cell wall properties in rice. New Phytol. 227, 498–512. doi: 10.1111/nph.16549
Yan, H., Nie, Y., Cui, K., Sun, J. (2022). Integrative transcriptome and metabolome profiles reveal common and unique pathways involved in seed initial imbibition under artificial and natural salt stresses during germination of halophyte quinoa. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.853326
Yang, Y. Q., Guo, Y. (2018). Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 60, 796–804. doi: 10.1111/jipb.12689
Yang, W., Liu, X., Yu, S. W., Liu, J. S., Jiang, L. J., Lu, X. D., et al. (2023). The maize ATP-binding cassette (ABC) transporter ZmMRPA6 confers cold and salt stress tolerance in plants. Plant Cell Rep. 43, 13. doi: 10.1007/s00299-023-03094-7
Yu, J., Zhao, W. G., Tong, W., He, Q., Yoon, M. Y., Li, F. P., et al. (2018). A genome-wide association study reveals candidate genes related to salt tolerance in rice (Oryza sativa) at the germination stage. Int. J. Mol. Sci. 19, 3145. doi: 10.3390/ijms19103145
Yu, J., Zhu, C. S., Xuan, W., An, H. Z., Tian, Y. L., Wang, B. X., et al. (2023). Genome-wide association studies identify OsWRKY53 as a key regulator of salt tolerance in rice. Nat. Commun. 14, 3550. doi: 10.1038/s41467-023-39167-0
Zhan, C. F., Zhu, P. W., Chen, Y. J., Chen, X. Y., Liu, K. X., Chen, S. S., et al. (2023). Identification of a key locus, qNL3.1, associated with seed germination under salt stress via a genome-wide association study in rice. Theor. Appl. Genet. 136, 58. doi: 10.1007/s00122-023-04252-x
Zhang, G. G., Bi, Z. Y., Jiang, J., Lu, J. B., Li, K. Y., Bai, D., et al. (2023). Genome-wide association and epistasis studies reveal the genetic basis of saline-alkali tolerance at the germination stage in rice. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1170641
Zhang, M., Cao, Y. B., Wang, Z. P., Wang, Z. Q., Shi, J. P., Liang, X. Y., et al. (2018). A retrotransposon in an HKT1 family sodium transporter causes variation of leaf Na+ exclusion and salt tolerance in maize. New Phytol. 217, 1161–1176. doi: 10.1111/nph.14882
Zhang, M., Liang, X. Y., Wang, L. M., Cao, Y. B., Song, W. B., Shi, J. P., et al. (2019). A HAK family Na+ transporter confers natural variation of salt tolerance in maize. Nat. Plants 5, 1297–1308. doi: 10.1038/s41477-019-0565-y
Keywords: rice, seed germination, salt stress, GWAS, OsTMF
Citation: Liu L, Ma Y, Zhao H, Guo L, Guo Y and Liu C-M (2024) Genome-wide association studies identified OsTMF as a gene regulating rice seed germination under salt stress. Front. Plant Sci. 15:1384246. doi: 10.3389/fpls.2024.1384246
Received: 09 February 2024; Accepted: 15 March 2024;
Published: 27 March 2024.
Edited by:
Dayong Cui, Qilu Normal University, ChinaReviewed by:
Babatope Samuel Ajayo, Sichuan Agricultural University, ChinaCopyright © 2024 Liu, Ma, Zhao, Guo, Guo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Ming Liu, Y21saXVAaWJjYXMuYWMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.