- 1Cassava Research Program, National Root Crops Research Institute (NRCRI), Umudike, Nigeria
- 2West Africa Centre for Crop Improvement (WACCI), University of Ghana, Accra, Ghana
- 3Biotechnology Centre, College of Basic and Applied Sciences, University of Ghana, Accra, Ghana
- 4Department of Plant Breeding and Genetics, Cornell University, Ithaca, NY, United States
Cassava productivity is constrained by low soil nitrogen, which is predominant in most cassava-growing regions in the tropics and subtropical agroecology. Improving the low nitrogen tolerance of cassava has become an important breeding objective. The current study aimed to develop cassava varieties with improved nitrogen use efficiency by identifying genomic regions and candidate genes linked to nitrogen use efficiency in cassava. A genome-wide association study (GWAS) was performed using the Genome Association and Prediction Integrated Tool (GAPIT). A panel of 265 diverse cassava genotypes was phenotyped for 10 physiological and agronomic traits under optimum and low-nitrogen regimes. Whole-genome genotyping of these cassava cloneswas performed using the Diversity Arrays Technology (DArTseq) sequencing platform. A total of 68,814 single nucleotide polymorphisms (SNPs) were identified, which were spread across the entire 18 chromosomes of the cassava genome, of which 52 SNPs at various densities were found to be associated with nitrogen use efficiency in cassava and other yield-related traits. The putative genes identified through GWAS, especially those with significant associated SNP markers for NUE and related traits have the potential, if deployed appropriately, to develop cassava varieties with improved nitrogen use efficiency, which would translate to a reduction in the economic and environmental cost of cassava production.
Introduction
Cassava (Manihot esculenta Crantz) is among the most produced commodity crops in Nigeria, and its demand is on the rise, especially now that it is considered as an alternative to wheat. Meeting the demand of the growing population in the region calls for urgent attention, as the yield trend (production per hectare) is still low (Poverty Oxford and Human Development Initiative, 2017; FAO, 2018). This low yield is usually linked to a decline in soil fertility and limited resources for inputs such as fertilizers. However, there is a growing concern that the booming agro-allied industries, which rely heavily on cassava as a raw material, may adversely affect the continued availability of cassava-based products to Nigerian families. Soil fertility decline, caused by high rainfall, erosion, leaching, overgrazing, bush burning, and biodiversity loss, etc., is a major constraint faced by farmers in savanna zones of the world. Soil nutrient depletion, especially nitrogen, together with biotic and abiotic stresses, are the major reasons for the low productivity of cassava in sub-Saharan Africa. Nitrogen deficiency affects stay-green ability, photosynthetic rate, translocation of organic compounds from source to sink, root bulking, and root yield (De Souza et al., 2017). Nitrogen is an important nutrient element required by plants to attain their genetic yield potential. Recently, crop land around the world has received 119.41 million tons of nitrogen fertilizer to produce the desired yield (FAO, 2018).
According to the International Fertilizer Association (IFA, 2022), the demand for nitrogen fertilizer is expected to increase by 1.1% annually by 2021. As urea constitutes most of the newly added nitrogen capacity, the demand for urea is expected to grow at a faster rate. Unfortunately, the widespread use of nitrogen fertilizers has had detrimental impacts on the ecosystem, including pollution and ecological imbalances (Miller and Cramer, 2004). There are three key components of nitrogen absorption by plants: uptake, assimilation, and remobilization (Han et al., 2016). Several genetic and physiological mechanisms govern these processes. The efficiency of nitrogen utilization is defined as the capacity of the cassava genotype to grow, develop, and reproduce in the presence of nitrogen nutritional deficits in the soil (Fritsche-Neto et al., 2010). The product of nitrogen uptake efficiency (NUpE) and nitrogen utilization efficiency (NUtE) is nitrogen use efficiency (NUE) (Good et al., 2004). Reports have shown that increased NUE has a significant positive correlation with crop biomass and yield (Stanley et al., 2006; Wu et al., 2016), suggesting that selection based on NUE can improve cassava productivity. Kant et al. (2011) also claimed that a 1% improvement in NUE may result in annual savings of $1.1 billion.
However, in the genetic improvement of nitrogen use efficiency, the first step is to determine the amount of genetic variability within the plant population. To isolate the contribution of different nitrogen regimes or levels from genetics and other environmental factors to cassava root yield, it is necessary to evaluate the plant population under low and optimum nitrogen conditions. However, there are other factors that will come into play apart from genetics, such as radiation use efficiency (De Souza et al., 2017), the interplay between macro- and micronutrients, and the interaction between water availability and nitrogen uptake by plants (Mei et al., 2015). Genome-wide association studies (GWAS) are an effective method for discovering new genes and validating suspected genes involved in complex phenotypic characteristics (Altshuler et al., 2008). GWAS have been used to map the key genes for different cassava characteristics. Among the parameters that have been studied include the genetic makeup of cassava mosaic disease (Wolfe et al., 2016), beta-carotene content, dry matter content, shoot weight, fresh root yield, starch yield, starch fraction amylose content, and dry matter content (De Oliveira et al., 2012). This work aims to contribute to the development of cassava varieties with improved nitrogen use efficiency, which will translate to a reduction in the economic and environmental costs of cassava production. The main objective of this study was to identify SNP marker loci that are strongly associated with fresh root yield and other secondary characteristics under low and optimal N regimes. Other objectives included: (i) identifying candidate genes linked to nitrogen utilization efficiency and other related traits, (ii) identifying the best performing test clones under optimum and low nitrogen regimes, and (iii) assessing the population structure among the clones.
Materials and methods
Study sites, germplasm, and field trials
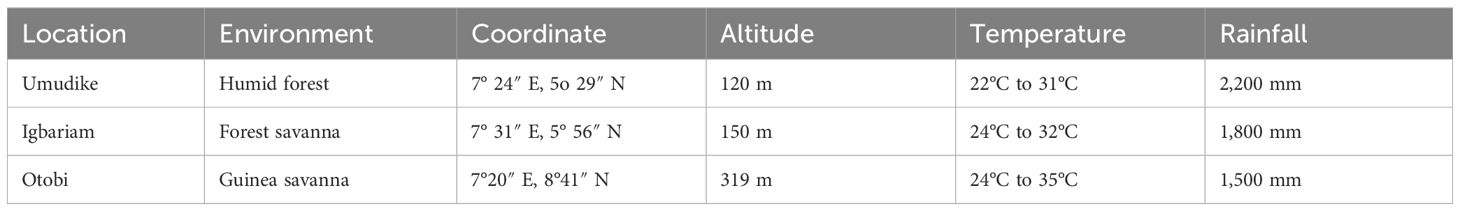
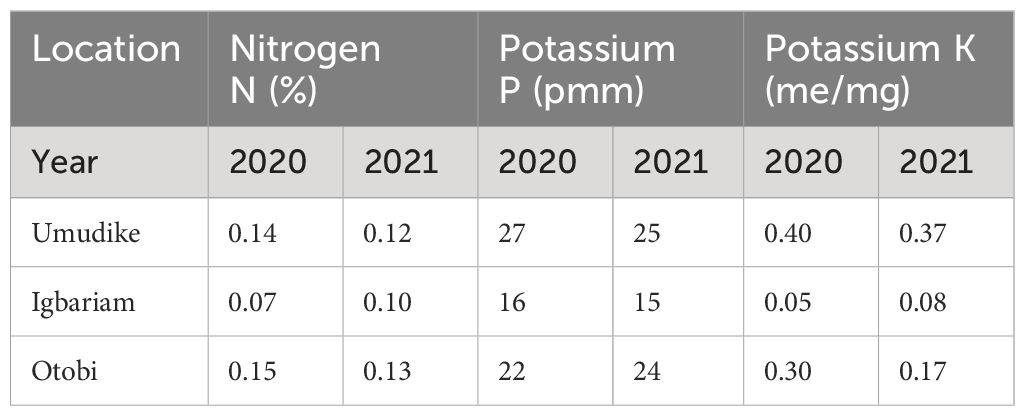
The studies were conducted in three major cassava-growing regions in Nigeria with distinct climatic attributes (Table 1). Before land preparation, composite auger soil samples were taken from the experimental sites at soil depths ranging from 0 cm to 20 cm in both years across locations. The soil samples were air-dried, crushed, thoroughly mixed, and passed through a 2-mm sieve before subjecting the soils to routine soil analysis as described by Udo and Ogunwale (1978) for the determination of nitrogen, phosphorus, and potassium (N.P.K). The data derived from soil analysis are presented in Table 2.
A panel of 260 diverse cassava genotypes and five (5) checks was used in these studies. The clones were NEXTGEN cassava materials from the International Centro Internacional de Agricultura Tropical (CIAT), the International Institute for Tropical Agriculture (IITA), and the National Root Crops Research Institute (NRCRI) Umudike Nigeria. The clones were evaluated during the 2020–2021 and 2021–2022 planting seasons in three environments (Umudike, Igbariam, and Otobi) in Nigeria, using an augmented block design. A weather logger was installed across the environment to collect climate data. Plot sizes of 1 m × 0.8 m, were adopted giving a total plant population of 12,000 plants per hectare. The optimum and low-nitrogen regimes were separated by a 0.5 m spacing distance.
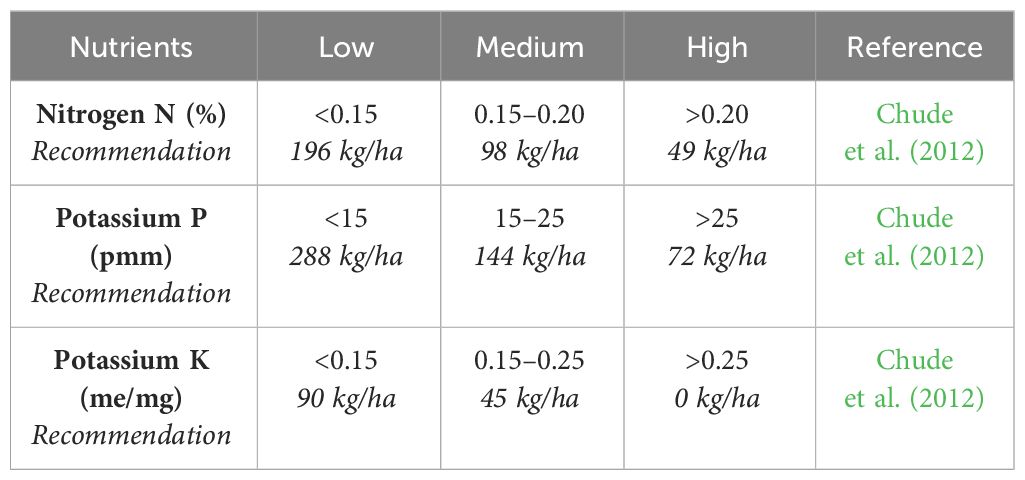
Soil fertility classes and cassava N.P.K recommendations for each class in Nigeria, as described by Chude et al. (2012), were used as a guide in fertilizer application (Table 3).
Phenotyping
An association panel of 265 cassava genotypes was phenotyped for the following qualitative and quantitative traits: plant vigor, stay green ability, leaf retention, photosynthetic active radiation, leaf area index, chlorophyll and leaf nitrogen, fresh root yield, specific gravity, dry matter content, dry root yield harvest index, and starch content using IITA standard operation practice and NEXTGEN cassava breeding ontology.
Data analyses
Using the lme4 (Linear Mixed-Effects Models utilizing “Eigen” and “S4” package implemented in R software), three experimental years’ phenotypic data were initially examined independently and then pooled across years.
Analyses of variance within and across environments for each population under each management condition were conducted using the R program integrated within the META-R software (Alvarado et al., 2015). Using the linear mixed model, the following variance components were determined,
where was the phenotypic performance of the ith genotype at the jth environment in the ith unreplicated genotypes nested within the kth replicated checks, µ was population mean, gi was the genetic effect of the ith genotype, lj was the effect of the jth environment, was the interaction of ith genotypes and the jth environment, was the effect of the ith unreplicated genotypes nested within the kth replicated checks, was the residual.
Environment and genotype were classified as random effects, whereas checks nested in the blocks were treated as fixed. The ratio of genotypic to phenotypic variance was used to estimate broad-sense heritability on an entry-mean basis. Moreover, BLUP was calculated for all attributes of each clone across all environments and management.
Estimation of nitrogen use efficiency
Most root and tuber crops (RTCs), including potatoes, have been studied for their nitrogen use efficiency (NUE) (Sharifi et al., 2007; Zebarth et al., 2008). However, the definition of NUE and the units employed in its calculation vary among studies (Tiwari et al., 2018). However, spanning across numerous crops, nitrogen use efficiency (NUE) is defined as:
Yet, each crop will define “yield” differently. The “yield” in relation to potatoes, cassava, and other root and tubers can include root wet weight, root dry weight, or whole plant dry weight (Tiwari et al., 2018). Each of these categories has limitations: plant weight and dry weight do not accurately represent edible or marketable yield, whereas tuber wet weight varies with environmental influences.
According to Kratzke and Palta (1985), tuber-specific root structures may also be responsible for tubers water intake, and water stress can cause changes in root water content (Levy et al., 2013). To eliminate any confounding variables, gain insight into the phenotypic determinants of yield, and be consistent with current work in the field, root dry matter was employed in this study as the numerator for NUE (Getahun, 2017).
The two components of nitrogen use efficiency (NUE), nitrogen utilization efficiency (NUtE), and nitrogen uptake efficiency (NUpE) are typically modeled as a product (Moll et al., 1982; Bock, 1984). The relationships between NUE, NutE, and NUpE are typically represented as
Applied N is the standard denominator of NUE studies in root and tuber crops, such as potatoes (Tiwari et al., 2018) and cassava (Liang et al., 2020). In the present study, the assessment of 265 cassava genotypes showed significant differences in nitrogen use efficiency under different N treatments. A mixed model-derived best linear unbiased prediction selection index was used to evaluate the performance of the clones under optimum and low nitrogen regimes. Due to the number of clones used, NUE was categorized into five different levels: very high, high, moderate, low, and very low NUE.
Genotyping and DArTseq SNP filtering
The genomic DNA of 265 diverse cassava genotypes was extracted from fully expanded cassava leaves using the CTAB method as described by Huang et al. (2000). The DNA concentration and purity were checked using a NanoDrop 2000 spectrophotometer (ND—2000 V3.5, NanoDrop Technologies, Inc.) before it was shipped to the Diversity Arrays Technology (DArT) Pty. Ltd., Australia, (http://www.diversityarrays.com/dart-mapsequences) for whole-genome sequencing using the DArTseq platform.
On the platform created by Cruz et al. (2013), whole-genome genotyping of 265 cassava clones was performed utilizing 68, 814 DArT markers. A consensus DArT map was used to infer the marker order and position, which was then used to integrate the markers into a linkage map. With a repeatability index of 0.93, the mean polymorphic information content was 0.16 and ranged from 0.0 to 0.50.
Based on Mwadzingeni et al. (2017), DArTseq SNP-generated markers were filtered by imputation to exclude problematic SNPs with >5% of missing data. The 68, 814 silico DArT markers distributed across 18 chromosomes were used to genotype every individual. After imputing the missing values, 63,362 DArTseq markers were used in the analysis.
Linkage disequilibrium and population structure
For the 265 cassava clones, the alleles per locus, allele frequency, and anticipated heterozygosity were estimated to quantify the genetic variation. The genetic distances of the genotypes were estimated. In accordance with Pritchard et al. (2000), the population structure was created with the aid of the Bayesian clustering approach in the structure version 2.3 programs, and genetic clusters were generated using the adjacent joining approach. Based on 63,362 DArTseq-derived SNP markers dispersed throughout the cassava genome, the population structure was determined using 10,000 burn-in iterations and 10,000 Markov Chain Monte Carlo (MCMC).
To increase the number of subpopulations present in the clones and decrease the possibility of erroneous correlations, the K-value was chosen between 1 and 10 (Gupta et al., 2014). The K-value with the highest likelihood served as the benchmark to establish an appropriate population size for the dataset. After imputation, DArTseq markers were coded as binary values, with 1 denoting presence and 0 denoting absence.
In accordance with Lipka et al. (2012), linkage disequilibrium analysis was performed using the GAPIT function of R software (). Of the 68,814 polymorphic markers, 63,362 had sites that could be used to determine linkage disequilibrium. For each pair of loci, the squared allele frequency correlations R2 at p-values of 0.001 were used to evaluate significant linkage disequilibrium.
Association mapping
To identify the marker characteristic relationships in the population under low and optimal nitrogen regimes, 63,362 DArTseq-generated SNP markers were employed. Following a mixed linear model (MLM) approach that considers both kinship and population structure, association mapping was carried out on traits related to yield and nitrogen use efficiency, such as plant vigor, leaf retention, leaf nitrogen and chlorophyll, stay green ability, dry root yield, fresh root yield, harvest index, and dry matter content (Lipka et al., 2012; Gupta et al., 2014). We determined whether DArT markers were significant at a critical value of 1%. This method is more accurate and less likely to produce spurious marker-trait connections (MTAs) (Gupta et al., 2014).
Results
Phenotyping variation across genotypes in two nitrogen regimes
The majority of the traits studied had a normal distribution, as shown by the phenotypic association of the diverse panel averaged across the six different environments (Figures 1, 2). A few traits were slightly skewed towards the tail. A normally distributed random variable has a continuous bell-shaped distribution that is symmetrical about the mean. If a variable with a normal distribution is standardized, the result will be a variable with mean (µ) = 0 and standard deviation (σ) = 1. Typically, a normal distribution is determined by its mean and variance.
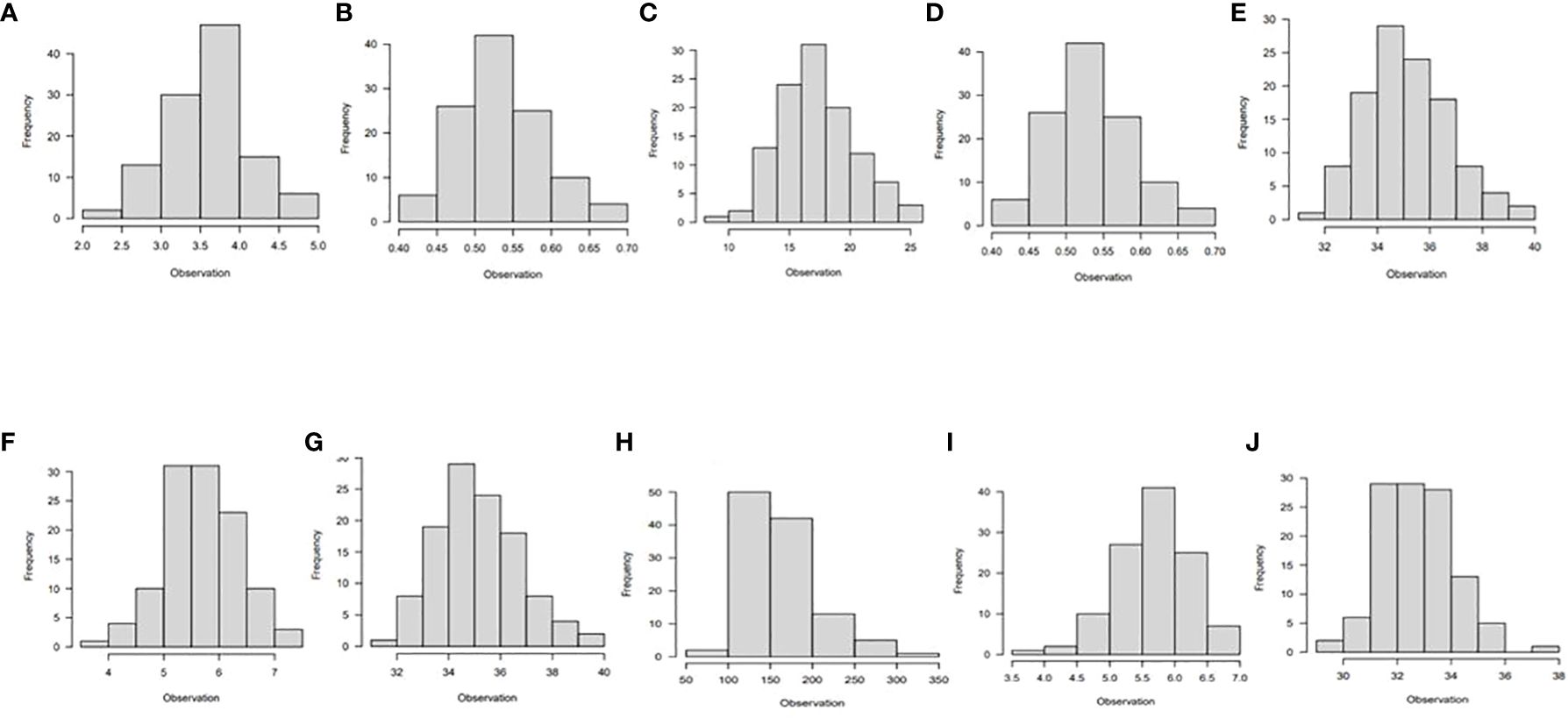
Figure 1 Histogram showing phenotypic distribution of the following traits; (A) vigor, (B) leaf nitrogen, (C) stay-green ability, (D) leaf retention, (E) chlorophyll, (F) fresh root yield, (G) dry root yield, (H) dry matter content, (I) harvest index, and (J) nitrogen use efficiency for 265 cassava genotypes evaluated under optimum nitrogen regime in six environments in Nigeria.
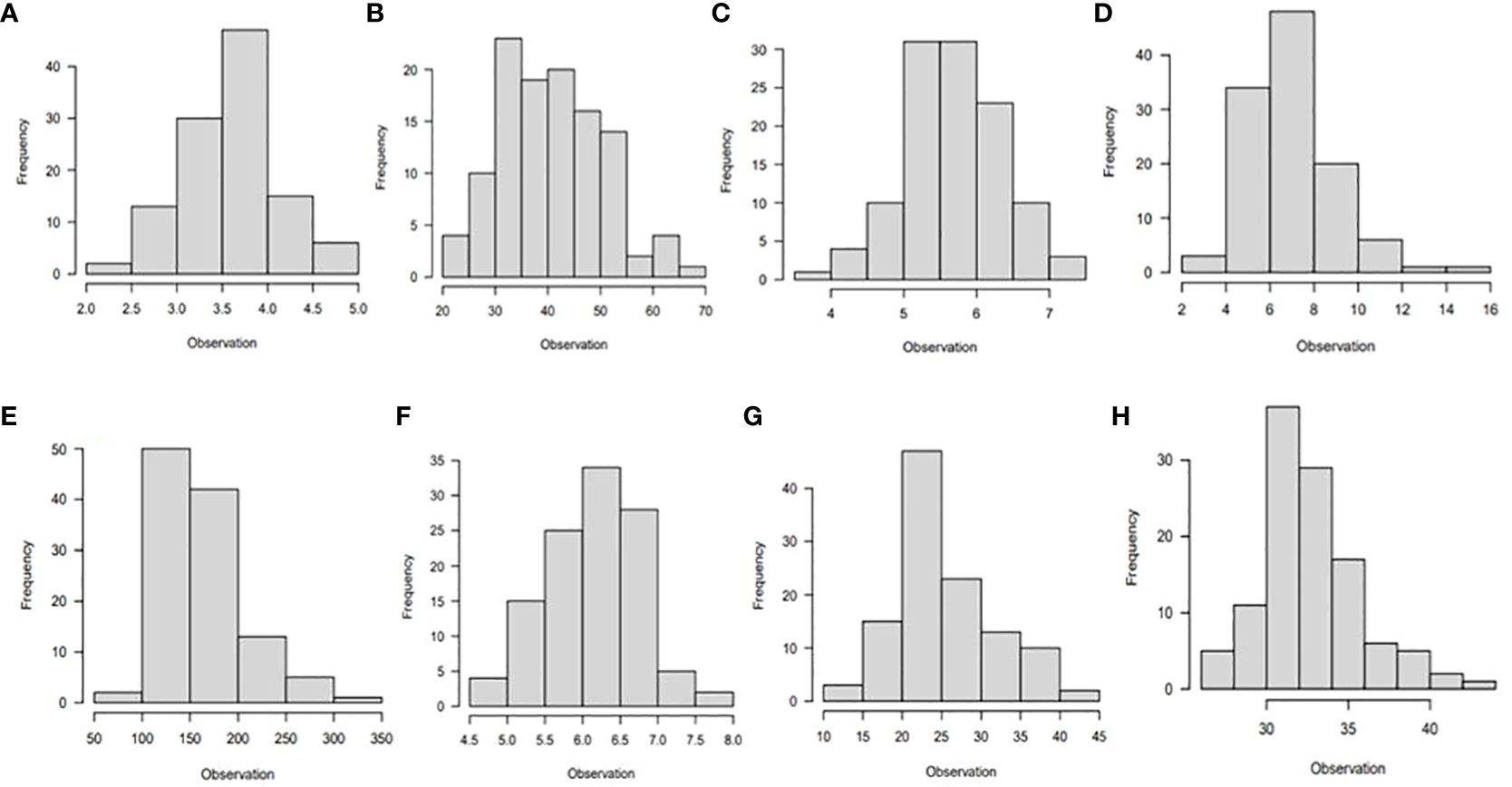
Figure 2 Histogram showing phenotypic distribution of the following traits: (A) leaf nitrogen, (B) leaf retention, (C) chlorophyll, (D) fresh root yield, (E) dry root yield, (F) dry matter content, (G) harvest index, and (H) nitrogen use efficiency for 265 cassava genotypes evaluated under low nitrogen regime in six environments in Nigeria.
Population structure and kingship matrix
The population structure analyses revealed a delta K probability value for five clusters of 265 cassava clones (Figure 3A), which suggests that the population could be structured into five clusters, as represented by the red, green, and yellow colors (Figure 3B). The 265 diverse cassava genotypes in the population formed a clear population structure and were assembled into two clusters and five subclusters (Figures 3A, B). The various colored segments represent the percentage of membership of each genotype in the corresponding clusters. With 27.5% of the population, Cluster 2 had the highest membership, while Cluster 5 had the lowest, at only 12% while the other groups averaged around 15%, Clusters 4 and 5 had the largest level of heterozygosity, resulting in a mean distance within group of 0.61. Principal component analysis (PCA) was used to display information on where the diverse cassava accessions used in this study are from geographically as well as how they are clustered (Figure 3C).
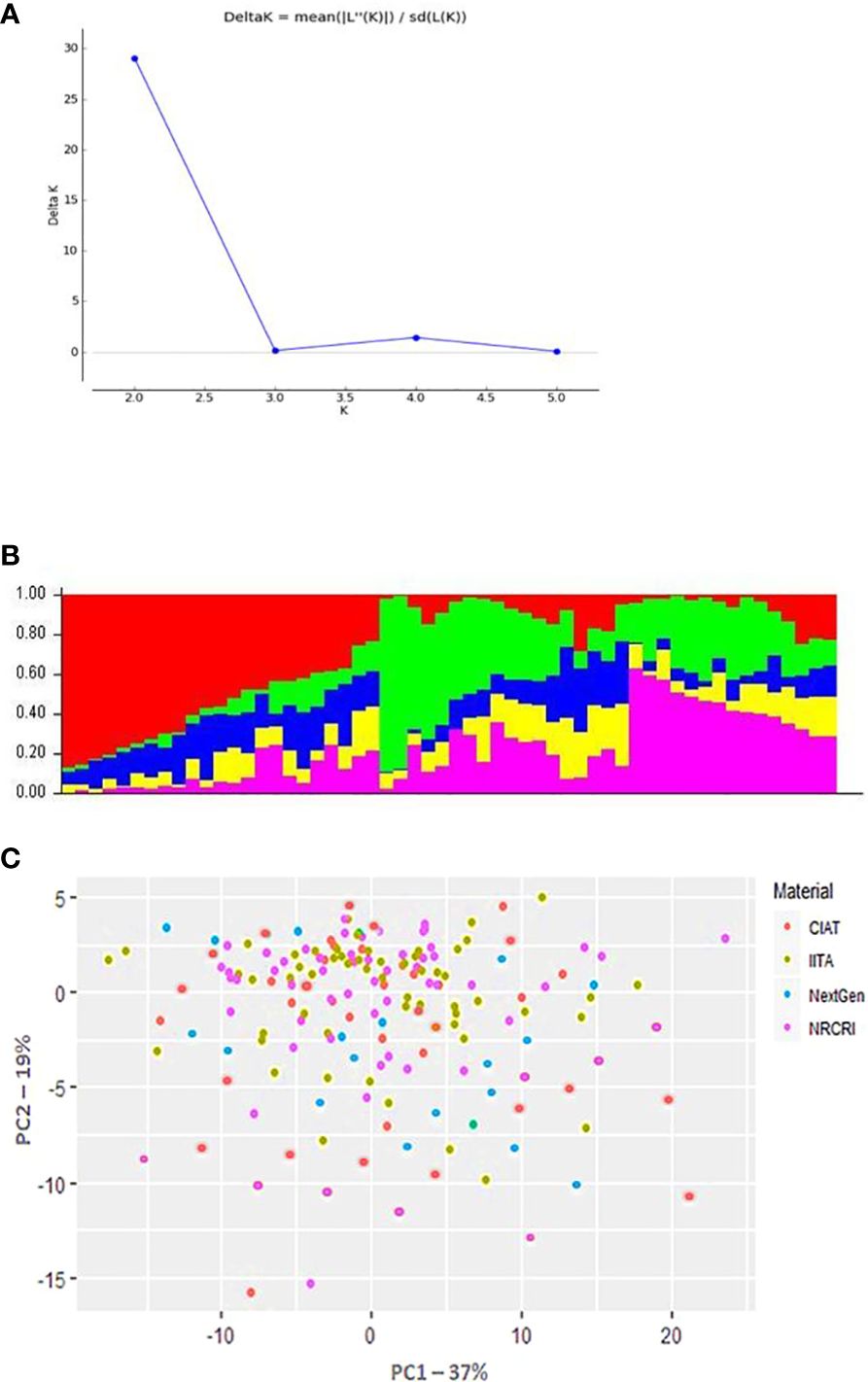
Figure 3 Population structure of 265 cassava genotypes based on 16,362 DArTseq-derived SNP markers. (A) Delta K probability values for the five clusters. (B) Five clusters, represented by red, green, and yellow. (C) Principal Component Analysis (PCA) Plot of PCI against PC2 illustrating population structure in the cassava (Manihot esculent) diversity panel genotyped with the SNP markers. The CIAT, IITA, Next-Gen, and NRCRI origins of M. esculenta are represented by the red, green, blue, and purple circles, respectively.
The clusters’ average fixation index (Fst) ranged from 0.01 to 0.88. The degree of kinship between the tested clones was a significant factor influencing the GWAS. A kinship value of 0.5 was present in approximately 99% of the pairwise kinship comparisons among the 265 genotypes, demonstrating the unrelatedness of the clones in the population utilized for GWAS. Low levels of relatedness were also seen in the kinship heatmap (Figure 4) produced by the vanRanden algorithm in the association panel of the “GAPIT” basic scenario. Low levels of relatedness among the examined clones were further confirmed using a heatmap, where the count of kinship values peaked at zero. In addition, the average genetic distance among the 84,255 pairwise comparisons was 0.3115, with genetic distances ranging from 0.004 to 0.3390. The degree of genetic variation in this study association panel may be shown by the fraction of pairwise comparisons with values higher than 0.3 (14.95%) and with values higher than 0.2 (more than 99%).
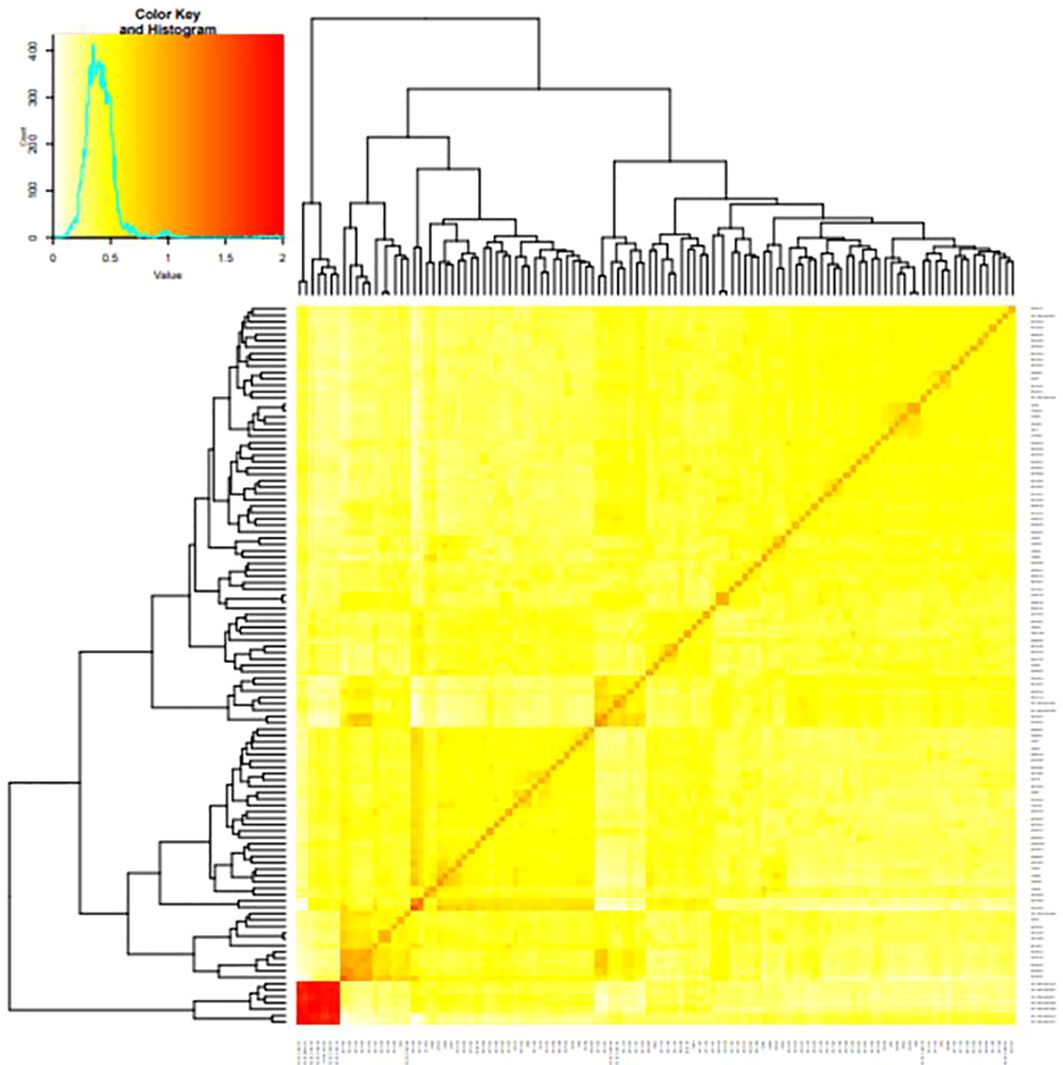
Figure 4 A heat map of the kinship matrix was created to indicate the relationship between the 68,814 SNP markers used in the current study.
Linkage disequilibrium and genetic distance among genotypes
LDs were determined on sliding windows with 100 nearby genetic markers (Figure 5A). Each dot indicates a pair of distances between two markers on the window and the squared correlation coefficient. The average of the ten nearby markers is represented by the red line (Figure 5B). Using linkage disequilibrium analysis, 10,164 loci pairings within a physical range of up to 162,831 bp were found. Only 9.02% of the locus pairs had R2 values between 0 and 1, and only 0.43% were completely out of equilibrium. Linkage disequilibrium decreased between distant pairs of markers, and there was a negative correlation between LD and physical distance (r = −0.14). (Figure 5C).
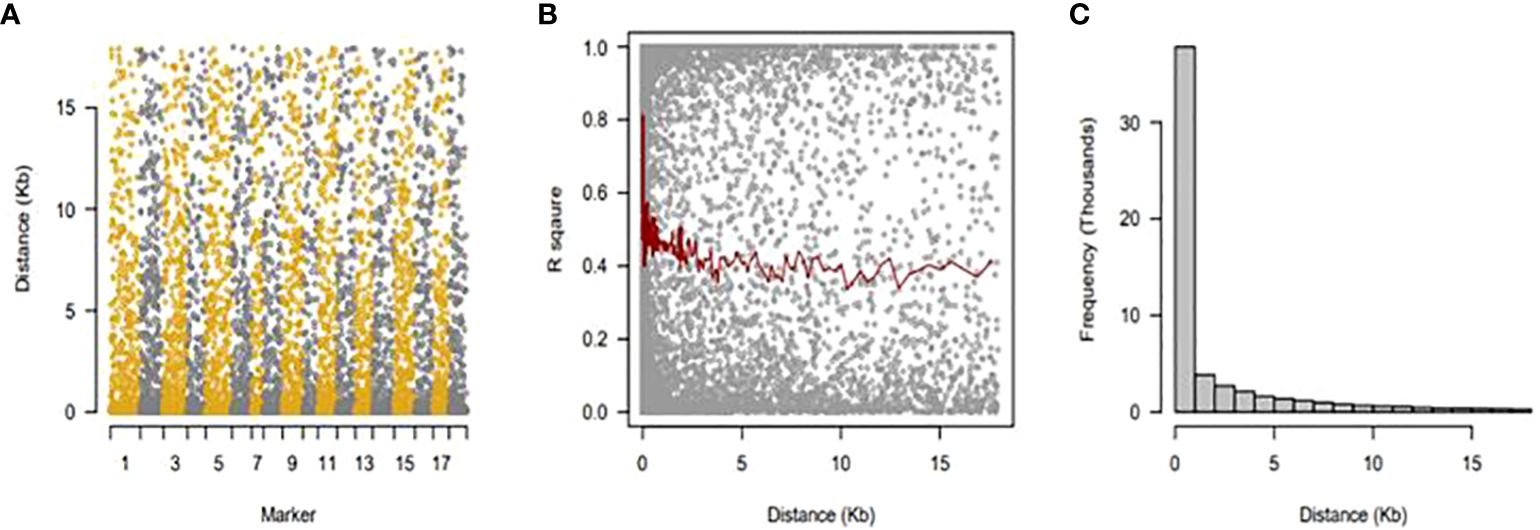
Figure 5 Linkage disequilibrium (LD), decay over distance. (A) Sliding windows with 100 nearby genetic markers. (B) The average of 10 nearby markers is represented by a red line. (C) There was a negative correlation between LD and physical distance (r = −0.14).
Estimation of nitrogen use efficiency in cassava using BLUP selection index under contrasting N regime
Under optimum N treatment, the BLUP selection index identified two genotypes, NR110178 and AR1410, which were very high in nitrogen utilization and most efficient, while four genotypes, IITA-TMS-IBA950211, NR100297, COB511, and IITA-IBA-MM961751, were observed to have high nitrogen use efficient test clones, and six genotypes, CR8A22, IITA-IBA010169, NR090127, AR144, NR110213, and COB1163, were identified as moderately in utilizing N efficiently. It is important to note that the clones identified as very high, high, and moderate outperformed all the checks used in this study. Low NUE test clones were equally identified, including NR100070, TMEB693, NR110181, NR100252, CW45036, NR100106, NR090176, and NR060169 (Figure 6).
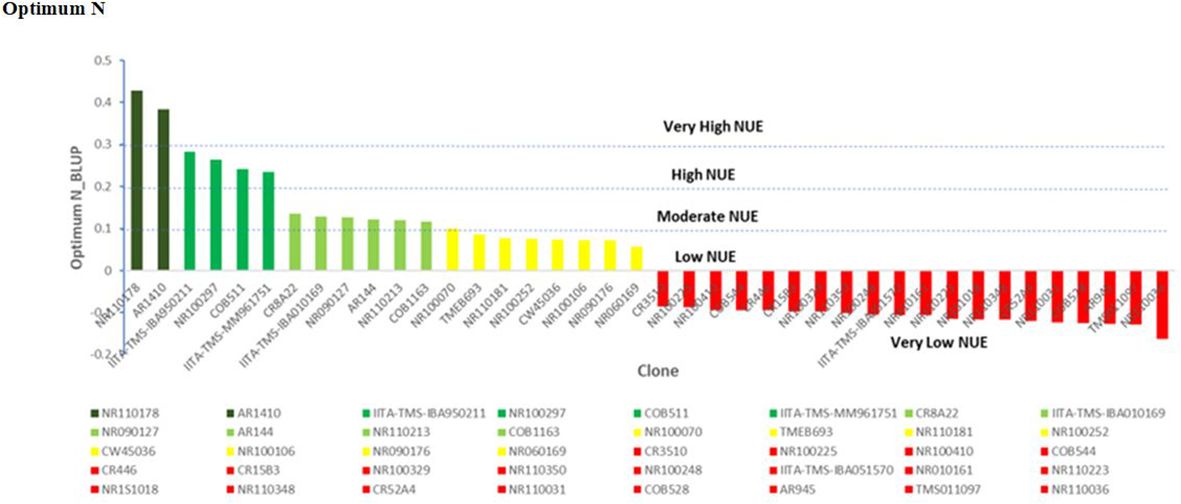
Figure 6 Nitrogen use efficiency (NUE), by genotype and optimum N treatment. Efficiency values are inherently unitless but are based on mass. NUE, units of tuber dry matter produced per unit of nitrogen applied to the field.
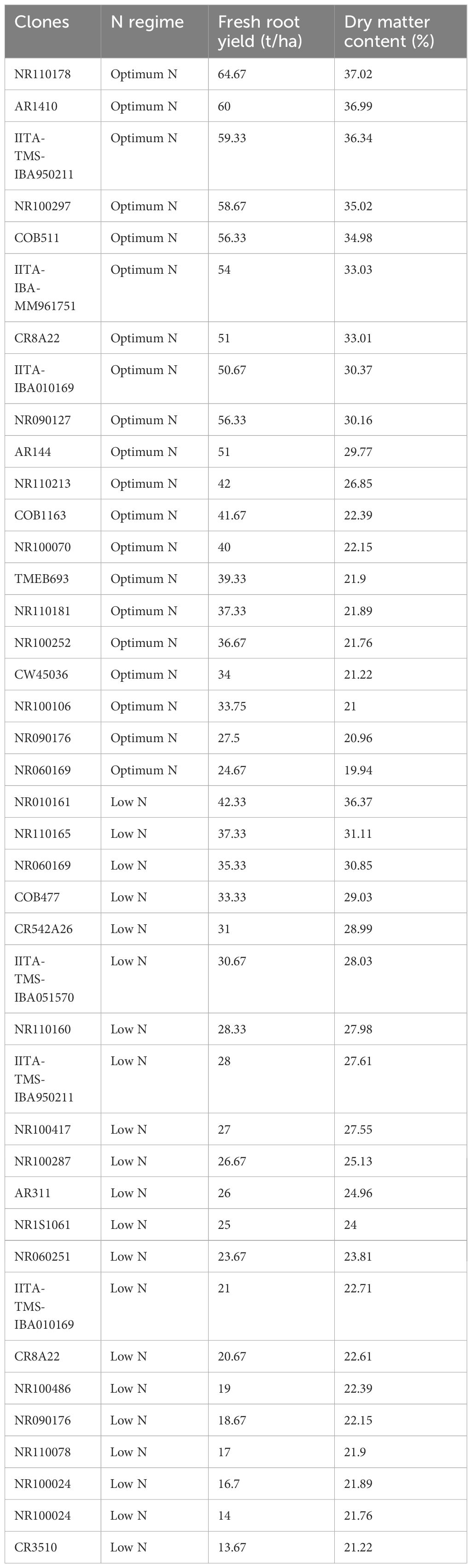
Under high-N conditions, nitrogen stress was not a factor, since the optimum nitrogen regime made available an exogenous excess of nitrogen; therefore, the genotypes that captured the most nitrogen from their surrounding soil environment were the most N-efficient. Similarly, under the low nitrogen treatment, the test clones were classified into four groups: high, moderate, low, and very low NUE. One genotype (NR010161) showed a high response (substantial yield under low nitrogen conditions) and could be described as having high nitrogen use efficiency. Six genotypes, NR110165, NR060169, COB477, CR542A26, IITA-TMS-IBA051570, and NR110160, were classified as having moderate NUE, whereas IITA-TMS-IBA950211, NR100417, NR100287, AR311, NR1S1061, NR060251, IITA-TMS-IBA010169, CR8A22, NR100486, NR090176, NR110078, NR100024, and CR3510 were classified as having low nitrogen use efficiency (Figure 7). The identified genotypes with high and moderate nitrogen use efficiency (NUE) also had high dry matter content, which is an important quality attribute required by consumers, processors, and industries (Table 4).
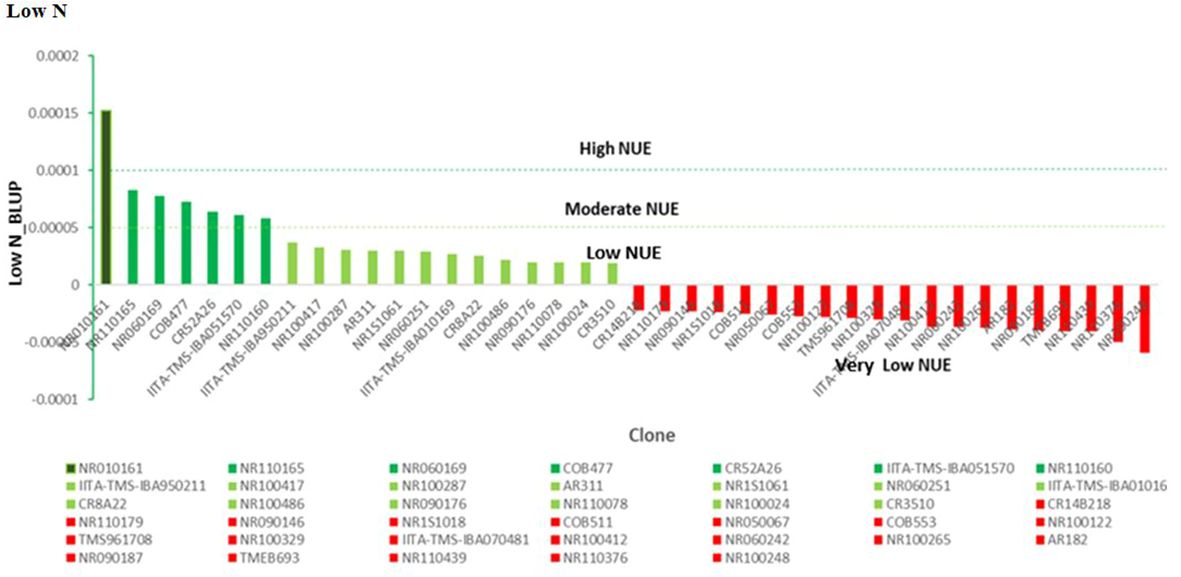
Figure 7 Nitrogen use efficiency (NUE), according to genotype and low-N treatment. Efficiency values are inherently unitless but based on mass. NUE, units of tuber dry matter produced per unit of nitrogen applied to the field.
SNP marker-trait associations under optimum and low nitrogen regimes
Ten nitrogen use efficiency and yield-related traits (plant vigor, leaf nitrogen content, stay-green ability, leaf retention, chlorophyll, fresh root yield, dry matter content, harvest index, dry root yield, and nitrogen use efficiency) were subjected to GWAS using 68,814 DArTseq-derived SNP markers. Of 68,814 SNP markers, 17 and 35 SNPs with positive associations were detected under low and optimum N regimes, respectively, at a Bonferroni significance level of 5% (Tables 5, 6; Figures 8, 9).
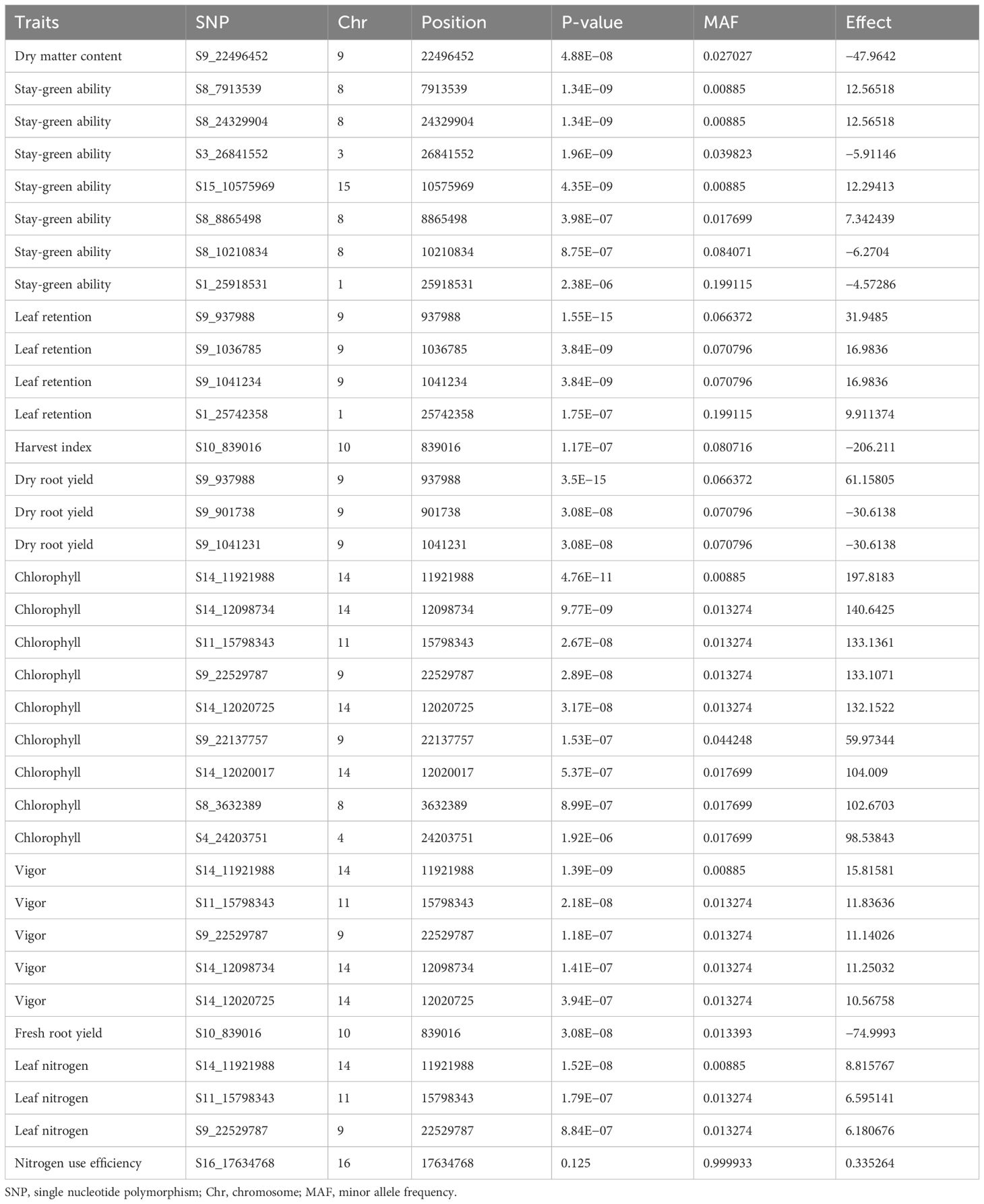
Table 5 Summary of SNPs with genome-wide association significance for nitrogen use efficiency and other yield related traits in cassava evaluated under optimum nitrogen regime.
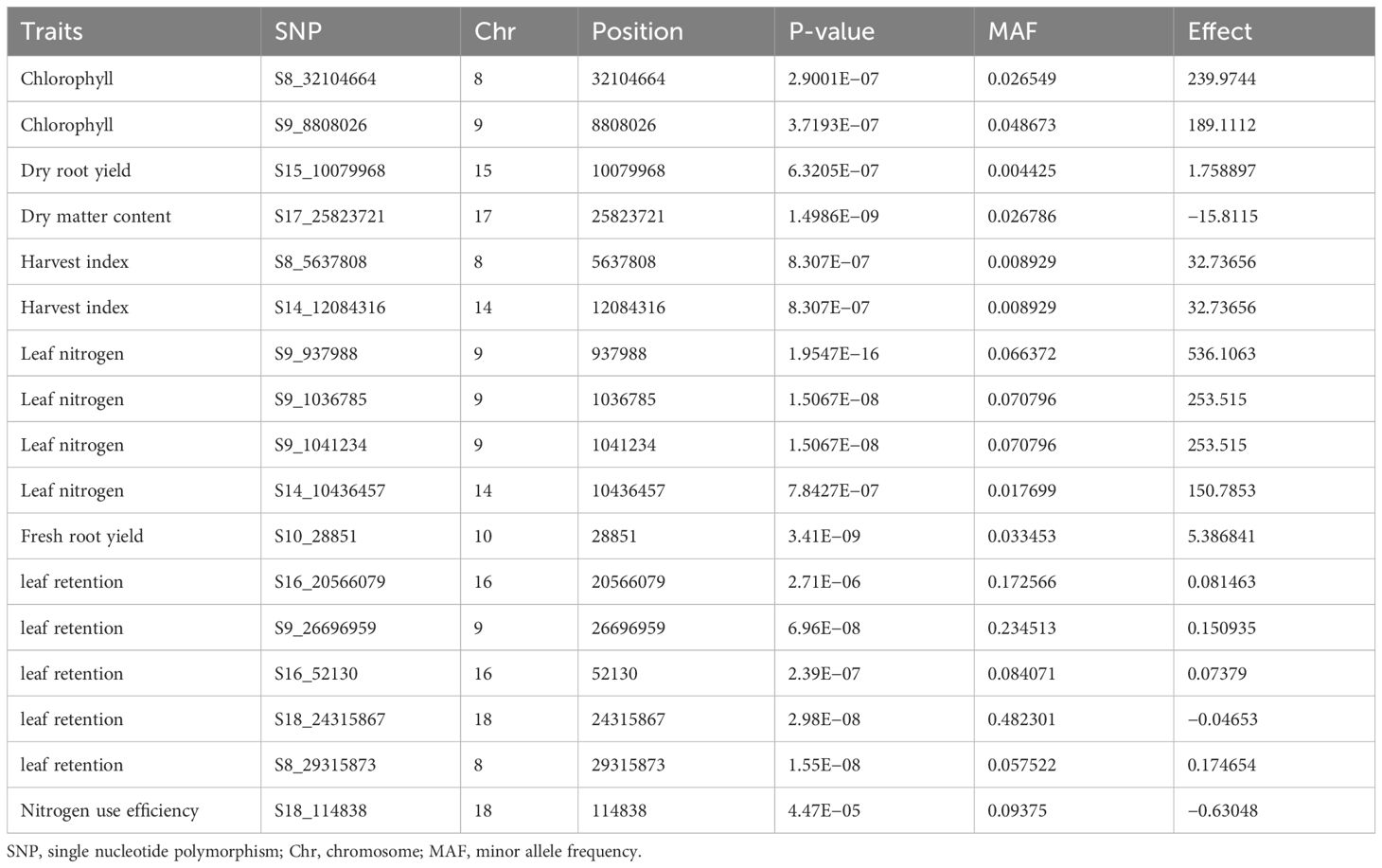
Table 6 Summary of SNPs with genome-wide association significance for nitrogen use efficiency and other yield related traits in cassava evaluated under low nitrogen regime.
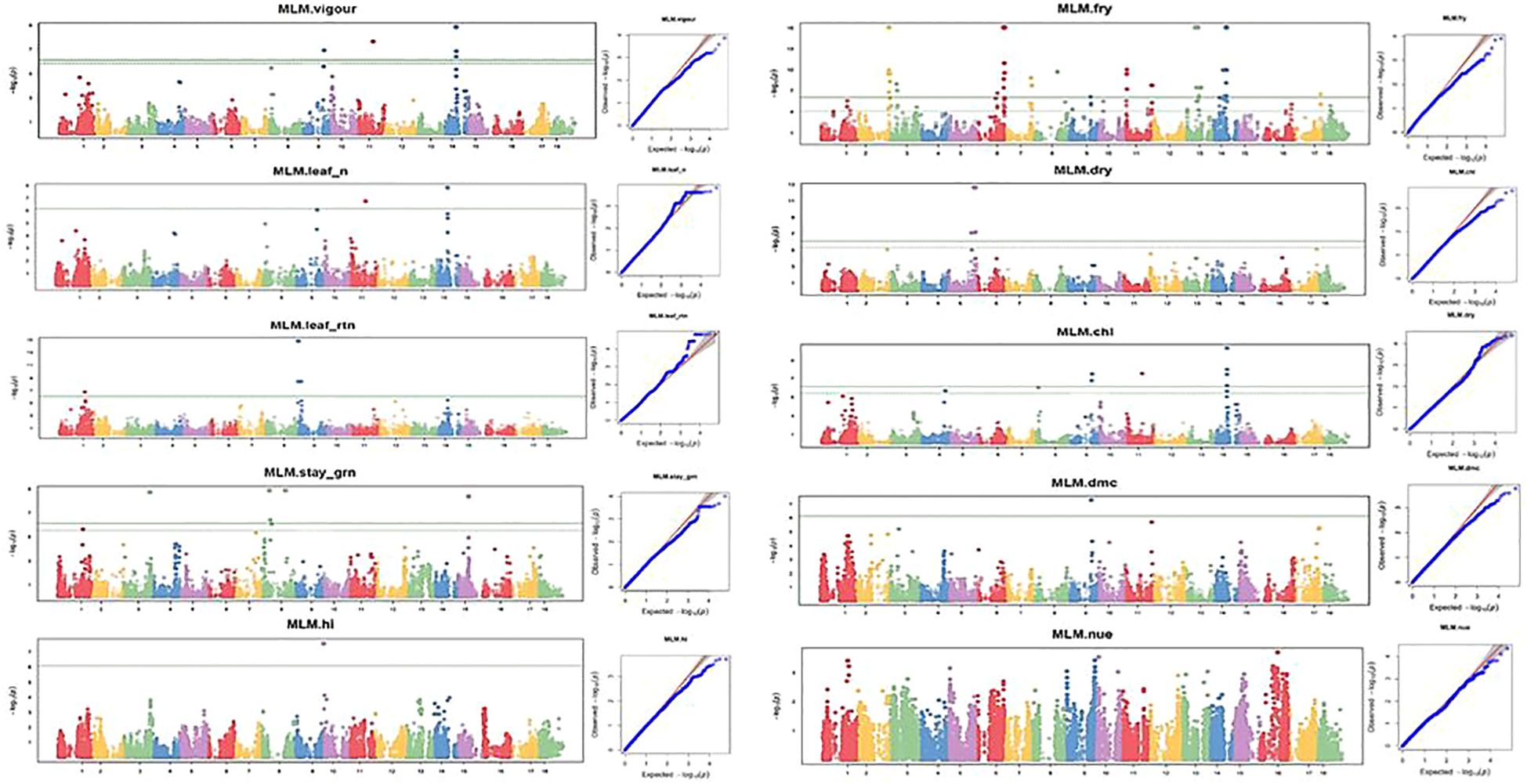
Figure 8 Manhattan and QQ plots for nitrogen use efficiency, fresh root yield, and other related traits evaluated under the optimal nitrogen regime. The horizontal lines in the Manhattan plots show the threshold p-value at the Bonferroni cutoff point of 0.05.
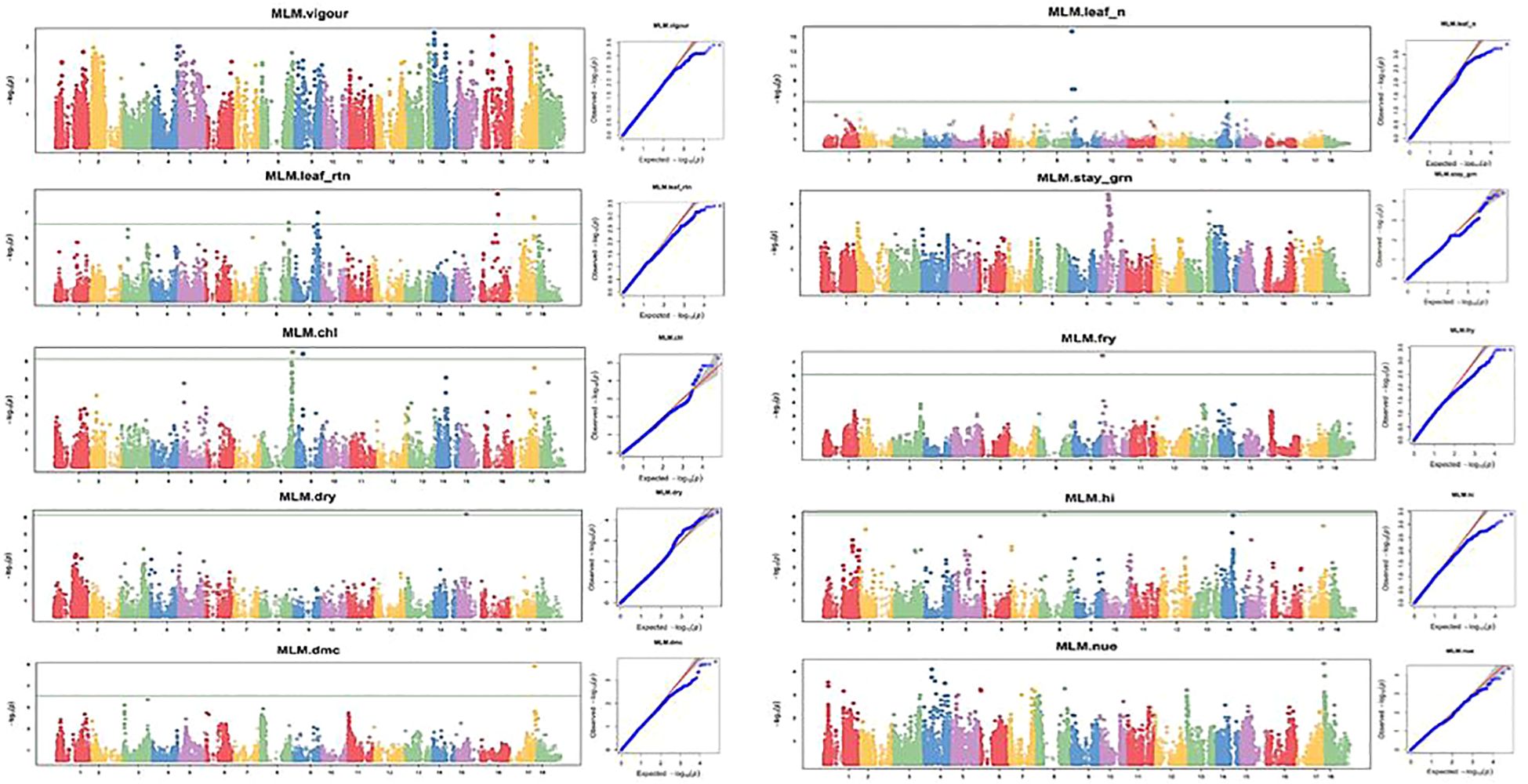
Figure 9 Manhattan and QQ plots for nitrogen use efficiency, fresh root yield, and other related traits evaluated under a low-nitrogen regime. The horizontal lines at Manhattan plots show the threshold p-value at Bonferroni cutoff point of 0.05.
GWAS analysis for NUE at both optimum and low nitrogen regimes revealed non-marker trait associations (MTAs) for the total number of SNPs deployed in this study, but revealed significant SNPs at GWAS thresholds of −log(P) = 3 and 4 for optimum and low nitrogen regimes, respectively. However, the association results for the other nine traits under the optimum nitrogen regime were found at a P-value<0.001, showing putative gene loci for each trait. Manhattan plots for vigor, leaf nitrogen content, leaf retention, dry matter content, chlorophyll, dry root yield, stay-green ability, fresh root yield, and harvest index were obtained from GWAS to explain the positive associations (−log10 (p-value) >6) (Figures 8, 9). Association results for traits under the low nitrogen regime revealed marker trait associations (MTAs) for all the traits under study, except for plant vigor and stay-green ability, although significant SNPs were detected at a GWAS threshold of −log(P) = 3 and 4 for vigor and stay green ability, respectively.
GWAS analysis for the optimum nitrogen regime using multiple loci mixed linear model as described by Segura et al. (2012) identified 5, 3, 7, 4, 9, 1, 3, 1, 1, and 1 QTL that were significantly associated with variation in plant vigor, leaf nitrogen, stay-green ability, leaf retention, chlorophyll, dry matter content, fresh root yield, dry root yield, harvest index, and nitrogen use efficiency, respectively. In the low nitrogen regime, GWAS analysis using a multi-linear mixed model identified four, five, two, one, one, one, two, and one significant SNPs found to be linked with variation in leaf nitrogen, leaf retention, chlorophyll, fresh root yield, dry root yield, dry matter content, harvest index, and nitrogen use efficiency, respectively (Figure 9).
A quantile–quantile (Q–Q) plot was constructed for each trait under the high- and low-nitrogen regimes to validate their normal distribution (Figures 8, 9). For the optimum nitrogen regime, variation in plant vigor was found to be associated with three loci, one on Chromosome 9, one on chromosome 11, and three on chromosome 14, at a 5% Bonferroni correction. The most significant locus was observed on chromosome 14 and was tagged with marker S14_11921988 (P = 1.39E−09) (Table 5). Significant SNPs distributions for leaf nitrogen across chromosomes were identified in two loci on Chromosomes 11 and 14 under both low and optimum nitrogen regimes, highlighting the fact that these two markers contributed to the proportion of phenotypic variance.
Variation in stay-green ability in the optimum nitrogen regime was found to be associated with seven loci: one on Chromosome 1, one on Chromosome 3, four on Chromosome 8, and one on Chromosome 15. No significant SNP was above the threshold level in the low nitrogen regime, probably due to nitrogen deficiency in this environment. GWA analysis for leaf retention was significant for both optimum and low nitrogen regimes on Chromosome 9, which accounted for the majority of the variation observed in the traits, S9_937988 (P = 1.55E−1) and S9_26696959 (P = 6.96E−08), respectively. Similarly, Chromosome 8 had an SNP detected under low and optimum nitrogen regimes, such as S8_3632389 (P = 8.99E−07) for optimum N and S8_32104664 (P = 2.9001E−07) for the low N regime. In the current GWA analysis, a high number of significant SNPs were identified for fresh root yield under the optimum nitrogen regime.
Thirty-one loci of which seven were on Chromosome 2, six on Chromosome 6, two on Chromosome 7, four on Chromosome 11, five on Chromosome 13, and one each on Chromosomes 8 and 9. However, a single marker on chromosome 10 was found to be significantly associated with fresh root yield under low nitrogen regime. GWA analysis of dry root yield identified loci on Chromosomes 4 and 5 under the optimum nitrogen regime, but one locus on Chromosome 15.
Two SNP markers, one on Chromosome 9 and the other on Chromosome 17, for optimum and low nitrogen regimes (Figures 8, 9), were found to be linked to dry matter content. These significant loci were tagged by SNP S9_22496452 (P = 4.88E−08) and S17_25823721 (P = 1.4986E−09), respectively. Surprisingly, no significant SNP was identified for both optimum and low N regimes for nitrogen use efficiency (NUE) as a trait but revealed top peaks close to the threshold level, signifying loci harboring candidate genes coding for the variability observed for nitrogen use efficiency among SNPs. Marker availability for different traits is fundamental to simultaneously improving two or more traits. In this study, SNPs common to the majority of traits were identified under both low and optimum nitrogen conditions.
Putative candidate genes
The position of the highly significant SNP markers was explored by subjecting them to fine mapping and a BLAST search on the National Center for Biotechnology Information (NCBI) Genome Viewer v6.0, to annotate genomic regions and detect nearby putative candidate genes associated with nitrogen use efficiency. Putative genes within the significant SNP region were searched for due regard to the significant SNPs position flanking the right and left. Using the databases of the European Molecular Biology Laboratory-European Bioinformatics Institute (EMBL-EBI) and Universal Protein Resource (UniProt), the functions of genes linked to the identified SNPs were discovered. Genomic regions harboring most of the SNPs found to be significant with high peaks were further probed to isolate genes with putative functions using the National Center for Biotechnology Information (NCBI genome information for reference and representative genome assembly M. esculenta_v8). In total, 52 putative coding genes were identified that were associated with significant SNPs for both optimum and low nitrogen regimes (Tables 5, 6). These candidate genes have been found to be involved in abiotic stress tolerance in plants, including nutrient deprivation.
The candidate gene LOC110620977 MANES_08G064200v8 coding for stay-green ability under optimum N and annotated as V-type proton ATPase subunit H was found within SNP S8_8865498 on Chromosome 8, which is associated with an ATP-driven enzyme that uses the major active transport of H+ to convert ATP hydrolysis energy into electrochemical potential differences of protons across various biological membranes.
A putative gene (LOC110600224 MANES_14G137400) encoding the ubiquitin-like domain-containing protein CIP73 was found to be very close to the significant SNPs identified for chlorophyll under the low-N regime on Chromosome 14. A potential candidate gene (LOC110600354 MANES_14G130700) encoding for NADH-cytochrome b5 (CB5s) reductase-like protein in leaf nitrogen revealed that hormone signaling regulators or plant stress response, catalytic enzymes such as hydroxylases, transporters, P450 monooxygenases, desaturases, reductases, and others all engage strongly with CB5s. Such diverse interactions suggest that plant CB5s may perform roles other than those of simple electron carriers in regulating enzymatic reactions and metabolic processes.
Multiple sequence alignment
The Clustal Omega website of the European Molecular Biology Laboratory for European Bioinformatics Institute (EMBL-EBI), a genomic website, was used for multiple alignments of putative candidate genes to plot the phylogenetic tree analysis. This tool was used to identify inherent relationships and common patterns between genes. It shows the sequence alignment of three or more biological sequences, usually DNA, RNA, or proteins. The alignments were generated and analyzed using computational algorithms. It uses seeded guide tree techniques to generate alignments between three or more sequences. In the present study, the alignment of divergent protein sequences was computed by assigning individual weights to each sequence in a partial alignment in order to downweight near-duplicate sequences and up-weight the most divergent sequences (Figure 10). Similarly, amino acid substitution matrices vary at different alignment stages, according to the divergence of the sequences to be aligned. Neighbor-joining tree analysis explained how the associated candidate genes were biologically linked. The results showed six and four clusters of gene association for the optimum and low-nitrogen regimes, respectively. Most of the genes shared common associations and linkages.
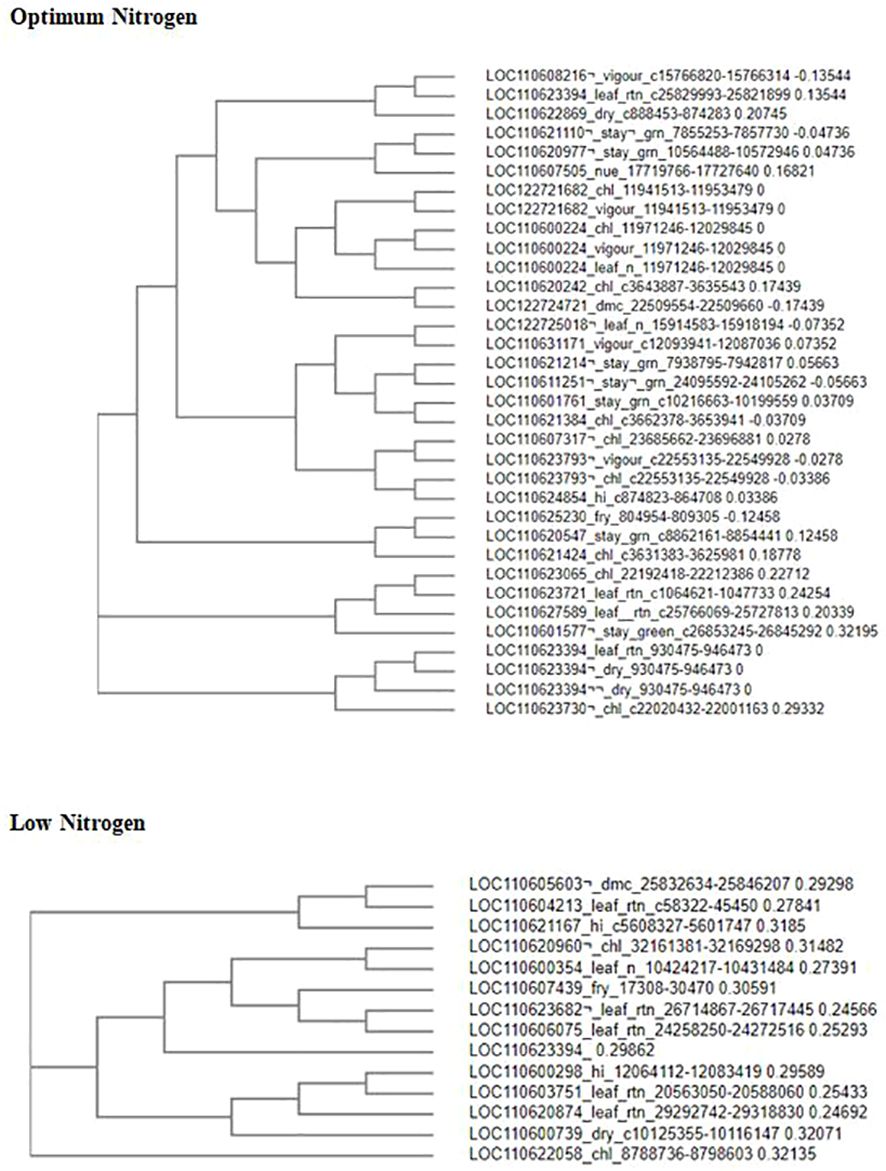
Figure 10 Phylogenetic tree showing the distribution and relatedness of protein coding genes for nitrogen use efficiency and other related traits in cassava: vigor, leaf retention, stay green, leaf nitrogen, chlorophyll, fresh root yield, dry matter content, dry, and harvest index, Sequences were aligned with cluster omega in multiple sequence alignment.
Discussion
This study showed that all types of efficiency were higher under the low-N regime, as utilization was the major driver of yield in a low-N environment. This concurs with earlier studies on potatoes and other crops (Hewitt, 1963; Novoa and Loomis, 1981). The N level determines the relative importance of two NUE components. However, NUE in maize seems to be relatively constant across N levels (Gallais and Coque, 2005). However, discrepancies in NUE under low-N and high-N regimes have been observed in other crops, including winter wheat (Triticum aestivum L.) and spinach (Biemond, 1995; Gaju et al., 2011).
The names “high-NUE genotypes” and “low-NUE genotypes,” according to a number of studies, refer to genotypes that produce high yields at levels of high and low N, respectively (Ladha et al., 2005). Some experts define high-NUE genotypes as those with the ability to produce significant yields while surviving in low-N environments (He et al., 2014; Quan et al., 2017). The two benefits of screening for high-NUE genotypes at high N levels are their high fertilizer tolerance and facilitation of high yields under high N conditions. Not all genotypes with high NUEs were found as a result of selection in high-N environments. Therefore, low-nitrogen conditions should be used for selection in breeding operations.
A total of 68,814 SNPs at various densities were distributed along the 18 chromosomes, of which 52 SNPs were positively associated with nitrogen use efficiency in cassava and other yield-related traits. The average polymorphic information content (PIC) value (0.60) obtained in the present study, is much higher than that reported by Udoh et al. (2017), as well as that reported in several other studies (Simko et al., 2012; Zhang et al., 2016; Adewale et al., 2020). This revealed the informativeness and high polymorphism of the SNP markers used in this study.
The population structure was estimated to elucidate individual relatedness in association studies, which is a prerequisite for eliminating or reducing false associations (Yu and Buckler, 2006). The analysis revealed the delta K probability value of the five clusters, which is indicative of the diversity of the cassava clones used in the study. The genotypes formed a clear population structure and were grouped into two clusters and five subclusters (Figures 3A, B), and the various colored segments estimated the proportion of each genotype’s membership in the respective clusters.
Quantile–quantile (Q–Q) plot was used to determine model fitness for the association analysis, plotting the observed versus expected P-values under the null hypothesis that there is association existing between an SNP and the phenotype. This has become imperative because GWAS has been widely deployed to find genetic variations that are statistically related to a particular attribute by examining hundreds of thousands of them across numerous genomes. However, complex genetic architecture and polygenic traits, on the other hand, can produce false positive and indirect associations in GWAS. The results revealed that for all traits studied, the majority of the Q–Q plot points were aligned on the diagonal line, suggesting that population structure and relative kinship-based spurious allelic correlations were significantly decreased. This finding is consistent with that of a previous study by Adewale et al. (2020).
The identification of major alleles and protein-coding genes directly associated with nitrogen use efficiency and other yield-related traits in cassava is imperative for the development of cassava genotypes that would respond to low-nitrogen soils through marker-assisted breeding. These GWA results provide insights into the genetic basis of NUE and other yield-related traits in cassava. A total of 52 significant SNP markers were associated with vigor, leaf nitrogen, stay green ability, leaf retention, dry matter content chlorophyll, fresh root yield, dry root yield, and harvest index at the threshold of –log(P) = 6.
Publicly available Genome Viewer v6.0 (NCBI) was used for annotation of candidate genes surrounding or adjacent to the significant SNP markers. Some candidate genes play an important role in plant growth, hormonal signalling, and abiotic stress tolerance, including drought, salinity, and nutrient deficiency. A putative gene (LOC110600224 MANES_14G137400) encoding the ubiquitin-like domain-containing protein CIP73 (Table 7), was found to be very close to the significant SNPs identified for chlorophyll under the low nitrogen regime on chromosome 14. This candidate gene plays an important role in growth, hormonal signalling, and abiotic stress tolerance in plants, such as salinity, heat stress, drought, and nutrient deprivation (Sharma et al., 2016). Similarly, under optimum nitrogen regime, a candidate gene (LOC110607439 MANES_10G007200) with the gene function protein reticulate-related 6, chloroplast (Table 8), associated with proteins of the reticulate family functionally link photoperiodic growth, amino acid homeostasis, and reactive oxygen species metabolism during Arabidopsis leaf growth (Perez-Perez, 2013). According to Xiao-Hong et al. (2014), to maintain an electrochemical H+-gradient across the tonoplast, stimulating Na+ sequestration into the central vacuole, and improving salt stress tolerance in plants, V-type proton ATPase subunit H is essential. To further understand the potential roles of these putative genes in cassava, more research is required, which has not been thoroughly researched in cassava. The putative genes identified through GWAS in this study, particularly those with significant associated markers for the traits under consideration, can serve as crucial guides for the creation of functional markers. By utilizing marker-assisted selection, these gene-based functional markers may enhance the nitrogen utilization efficiency and other yield-related parameters in cassava.
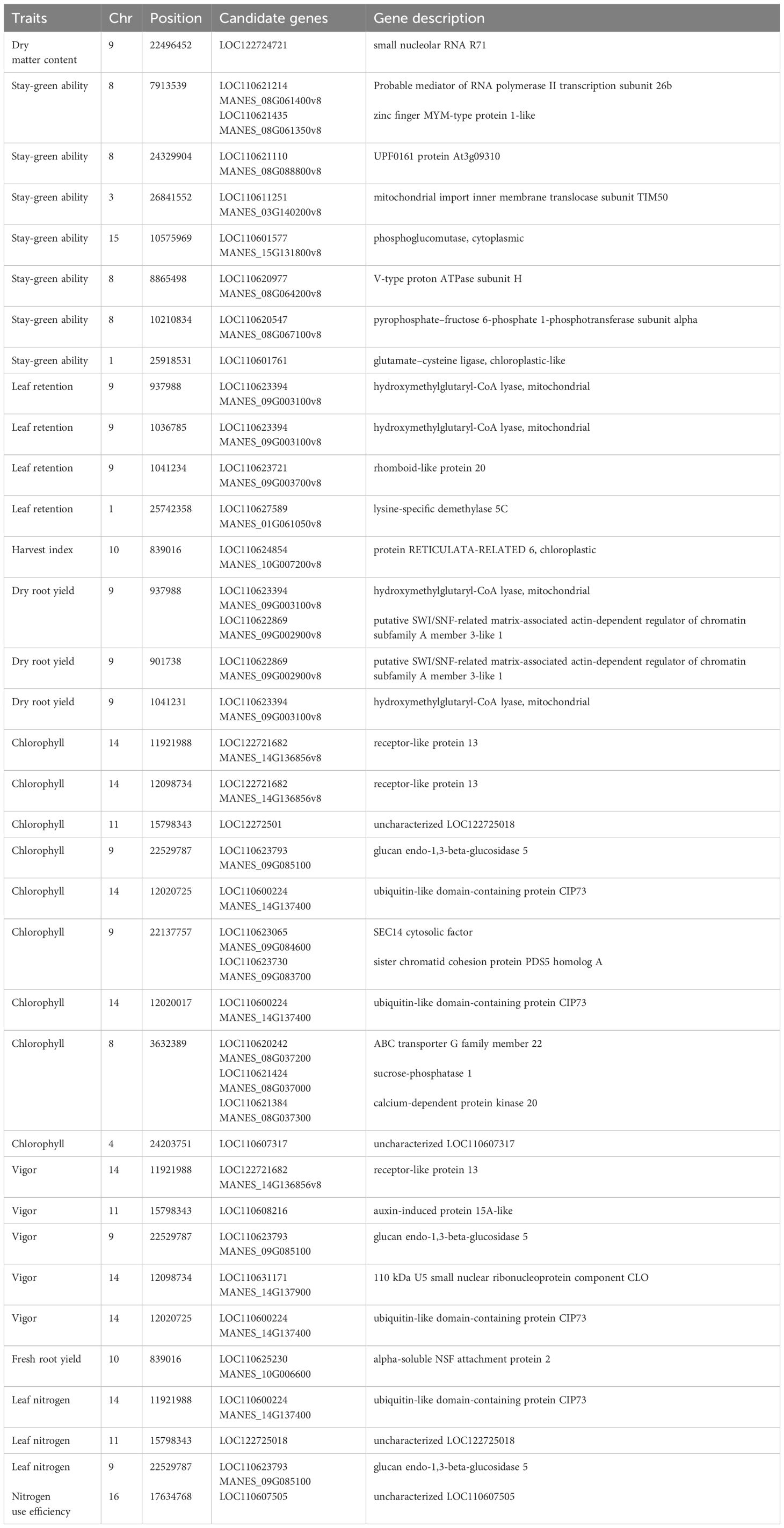
Table 7 Candidate genes co-located with significant SNPs associated with nitrogen use efficiency and other related traits in cassava evaluated under optimum nitrogen regime.
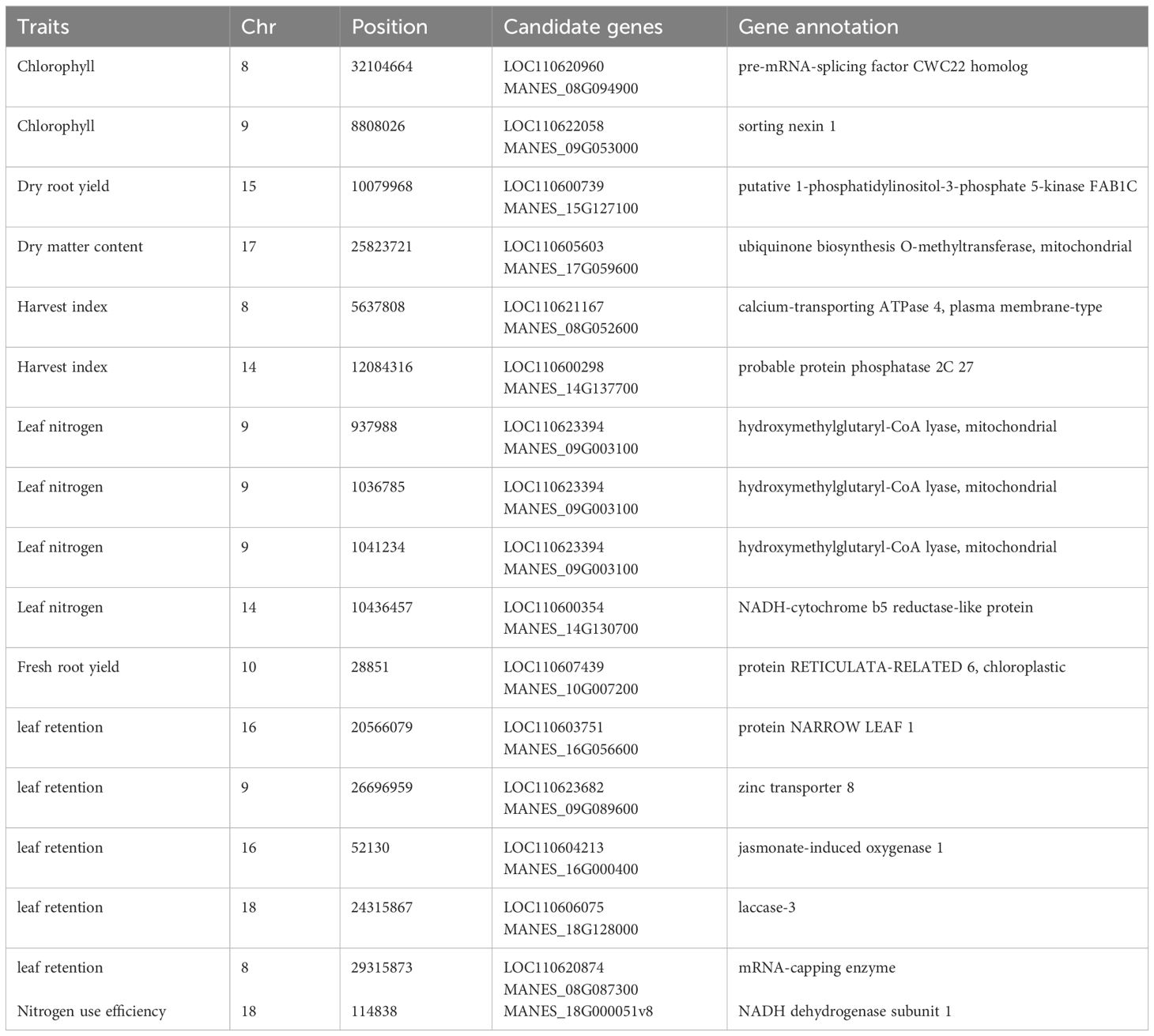
Table 8 Candidate genes co-located with significant SNPs associated with nitrogen use efficiency and other related traits in cassava evaluated under low-nitrogen regime.
Conclusion
This study is one of the few on nitrogen use efficiency in cassava using a GWAS approach to identify candidate genes associated with NUE and other yield-related traits. A mixed-model derived best linear unbiased prediction selection index was used to estimate nitrogen use efficient genotypes in the optimum and low-nitrogen regimes. Under optimum nitrogen treatment, two genotypes, NR110178 and AR1410, were very high in nitrogen utilization and were the most efficient, while four genotypes, IITA-TMS-IBA950211, NR100297, COB511, and IITA-IBA-MM961751, were observed to have high nitrogen use efficiency. However, under the low nitrogen treatment, one genotype (NR010161) responded very well to the N stress condition and could be described as having high nitrogen use efficiency. Six genotypes were classified as having moderate NUE: NR110165, NR060169, COB477, CR542A26, IITA-TMS-IBA051570, and NR110160. These genotypes identified under low-N conditions are promising genotypes that can be ascribed to being very efficient in N use. A total of 68,814 SNPs were at various densities found scattered along 18 chromosomes, of which 52 were identified as being associated with nitrogen use efficiency in cassava and other yield-related traits. These putative genes identified through GWAS, especially those with significantly associated SNP markers for NUE and related traits, have the potential, if deployed appropriately, to improve nitrogen use efficiency in cassava varieties.
Finally, some of the putative genes LOC122725018 and LOC110607317 were discovered on Chromosomes 11and 4. For chlorophyll, LOC122725018 found in Chromosome 11 for leaf nitrogen, and LOC110607505 in Chromosome 16 for nitrogen use efficiency under the optimum N regime are uncharacterized genes and have not been reported elsewhere. These are new discoveries; therefore, they significantly contribute to knowledge.
Data availability statement
The original contributions presented in the study are included in the article/supplementary files, further inquiries can be directed to the corresponding author/s.
Author contributions
JM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DD: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing. SA: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. DN: Investigation, Methodology, Writing – review & editing. JO: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Validation, Writing – review & editing. PT: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. CE: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors thank the UK’s Foreign, Commonwealth and Development Office (FCDO) and Bill and Melinda Gates Foundation (Grant INV-007637, http://www.gatesfoundation.org) for their financial support.
Acknowledgments
The authors are thankful to the National Root Crops Research Institute (NRCRI), Umudike, for providing funding and experimental sites for field trials. We sincerely appreciate the West Africa Centre for Crop Improvement (WACCI) for their great input that made this work better. The authors also acknowledge the huge support received from the NEXTGEN Cassava Breeding Project for providing the field staff and germplasm used in this study. The authors also acknowledge the contribution of Dr. Anoh, A. O, for guiding the field trials of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adewale, S. A., Badu-Apraku, B., Akinwale, R. O., Paterne, A. A., Gedil, M., Garcia-Oliveira, A. L. (2020). Genome-wide association study of striga resistance in early maturing white tropical maize inbred lines. BMC Plant Biol. 20, 203. doi: 10.1186/s12870-020-02360-0
Altshuler, D., Daly, M. J., Lander, E. S. (2008). Genetic mapping in human disease. Science 322, 881–888. doi: 10.1126/science.1156409
Alvarado, G., López, M., Vargas, M., Pacheco, Á., Rodríguez, F., Burgueño, J. (2015). META-R (Multi Environment Trail Analysis with R for Windows) Version 5.0 (Mexico: International Maize and Wheat Improvement Center (CIMMYT).
Biemond, H. (1995). Effects of nitrogen on development and growth of the leaves of vegetables. 3. Appearance and expansion growth of leaves of spinach. Netherlands J. Agric. Sci. 43, 247–260. doi: 10.18174/njas.v43i2.580
Bock, B. R. (1984). “Efficient use of nitrogen in cropping systems,” in Nitrogen in crop production. Ed. Hauck, R. D. (ASA, CSSA, and SSSA, Madison, WI), 273–294.
Chude, V. O., Olayiwola, S. O., Daudu, C., Ekeoma, A. (2012). Fertilizer use and management practices for Crops in Nigeria. 4th edition (Abuja: Federal Fertilizer Department, Federal Ministry of Agriculture and Rural Development), 224.
Cruz, A. A., Hiskia, A., Kaloudis, T., Chernoff, N., Hill, D., G. Antoniou, M., et al. (2013). A review on cylindrospermopsin: The global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environmental Science: Processes & Impacts 15(11), 1979–2003. doi: 10.1039/C3EM00353A
De Oliveira, E. J., de Resende, M. D. V., da Silva Santos, V., Ferreira, C. F., Oliveira, G. A. F., da Silva, M. S., et al. (2012). Genome-wide selection in cassava. Euphytica 187, 263–276. doi: 10.1007/s10681-012-0722-0
De Souza, A. P., Massenburg, L. N., Jaiswal, D., Cheng, S., Shekar, R., Long, S. P. (2017). Rooting for cassava: insights into photosynthesis and associated physiology as a route to improve yield potential. New Phytol. 213, 50–65. doi: 10.1111/nph.14250
FAO (2018) Production; Cassava; all Countries; 1961–2016 (Food and Agriculture Organization of the United Nations). Available online at: http://www.fao.org/faostat/en/#data/QC (Accessed 12 January 2018).
Fritsche-Neto, R., Miranda, G. V., DeLima, R. O., Souza, L. V., Silva, J. (2010). Inheritance of characters associated with efficiency of use of phosphorus in corn. Braz. Agric. Res. 45, 465–471. doi: 10.1590/S0100-204X2010000500005
Gaju, O., Allard, V., Martre, P., Snape, J. W., Heumez, E., Le Gouis, J., et al. (2011). Identification of traits to improve the nitrogen-use efficiency of wheat genotypes. Field Crops Res. 123, 139–152. doi: 10.1016/j.fcr.2011.05.010
Gallais, A., Coque, M. (2005). Genetic variation and selection for nitrogen use efficiency in maize: A synthesis. Maydica 50, 531–547.
Getahun, B. B. (2017). Genetic diversity of potato for nitrogen use effi-ciency under low input conditions in Ethiopia (Doctoral dissertation, Graduate School of Experimental Plant Sciences (Wageningen, the Netherlands: Wageningen Uni-versity).
Good, A. G., Shrawat, A. K., Muench, D. G. (2004). Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 9, 597–605. doi: 10.1016/j.tplants.2004.10.008
Gupta, P. K., Kulwal, P. L., Jaiswal, V. (2014). Association mapping in crop plants. Adv. Genet. 85, 109–147. doi: 10.1016/B978-0-12-800271-1.00002-0
Han, M., Wong, J., Su, T., Beatty, P. H., Good, A. G. (2016). Identification of nitrogen use efficiency genes in barley: searching for QTLs controlling complex physiological traits. Front. Plant Sci. 7, 1587. doi: 10.3389/fpls.2016.01587
He, C., Liu, C., Liu, Q., Gao, X., Li, N., Zhang, J., et al. (2014). Over-expression of glutamine synthetase genes Gln1-3/Gln1-4 improved nitrogen assimilation and maize yields. Maydica 59, 250–256.
Hewitt, E. J. (1963). “Chapter 2: The essential nutrient elements, requirements and interaction in plants,” in Plant physiology, A Treatise, Vol. 3, Inorganic nutrition of plants. Ed. Steward, F. C. (Academic Press, New York), 137–360.
Huang, X., Zeller, F. J., Hsam, S. L., Wenzel, G. (2000). Chromosomal location of AFLP markers in common wheat utilizing nulli-tetrasomic stocks. Genome 43, 298–305. doi: 10.1139/g99-118
IFA (2022) Five key fertilizer market dynamics of 2022. Available online at: www.fertilizer.org/news/five-fertilizer-market-dynamics-that-tell-the-story-of-2022/.
Kant, S., Bi, Y. M., Rothstein, S. J. (2011). Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 62, 1499–1509. doi: 10.1093/jxb/erq297
Kratzke, M. G., Palta, J. P. (1985). Evidence for the existence of functional roots on potato tubers and stolons: Significance in water transport to the tuber. Am. Potato J. 62, 227–236. doi: 10.1007/BF02852802
Ladha, J. K., Pathak, H. P., Krupnik, T. J., Six, J., Kessel, C. V. (2005). Efficiency of fertilizer nitrogen in cereal production: retrospect and prospect. Adv. Agron. 87, 86–156. doi: 10.1016/S0065-2113(05)87003-8
Levy, D., Coleman, W. K., Veilleux, R. E. (2013). Adaptation of potato to water shortage: irrigation management and enhancement of tolerance to drought and salinity. Am. J. Potato Res. 90, 186–206. doi: 10.1007/s12230-012-9291-y
Liang, X., Zhang, T., Lu, X., Ellsworth, D. S., BassiriRad, H., You, C., et al. (2020). Global response patterns of plant photosynthesis to addition: A meta-analysis. Global Change Biol. 26, 3585–3600. doi: 10.1111/gcb.15071
Lipka, A. E., Tian, F., Wang, Q., Peiffer, J., Li, M., Bradbury, P. J., et al. (2012). GAPIT: genome association and prediction integrated tool. Bioinformatics 28, 2397–2399. doi: 10.1093/bioinformatics/bts444
Mei, H., Okamoto, M., Beatty, P. H., Rothstein, S. J., Good, A. G. (2015). The genetics of nitrogen use efficiency in crop plants. Annu. Rev. Genet. 49, 269–289. doi: 10.1146/annurev-genet-112414-055037
Miller, A. J., Cramer, M. D. (2004). Root nitrogen acquisition and assimilation. Plant Soil 274, 1–36. doi: 10.1007/s11104-004-0965-1
Moll, R., Kamprath, E., Jackson, W. (1982). Analysis and interpretation of factors which contribute to efciency of nitrogen utilization 1. Agron. J. 74, 562–564. doi: 10.2134/agronj1982.00021962007400030037x
Mwadzingeni, L., Shimelis, H., Rees, D. J. G., Tsilo, T. J. (2017). Genome-wide association analysis of agronomic traits in wheat under drought-stressed and non-stressed conditions. PloS One 12, e0171692. doi: 10.1371/journal.pone.0171692
Novoa, R., Loomis, R. S. (1981). Nitrogen and plant production. Plant Soil 58, 177–204. doi: 10.1007/BF02180053
Perez-Perez, J. M., Esteve-Bruna, D., Gonzalez-Bayon, R., Kangasjarvi, S., Caldana, C., Hannah, M. A., et al. (2013). Functional Redundancy and Divergence within the Arabidopsis RETICULATA-RELATED Gene Family. Plant Physiology 162(2), 589–603. doi: 10.1104/pp.113.217323
Poverty Oxford and Human Development Initiative (2017). “Nigeria Country Briefing,” The Oxford Poverty and Human Development Initiative. (Multidimensional Poverty Index Data Bank. OPHI, University of Oxford). Available at: www.ophi.org.uk/multidimensional-poverty-index/mpi-country-briefings/.
Pritchard, J. K., Stephens, M., Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics 155, 945–959. doi: 10.1093/genetics/155.2.945
Quan, X., Zeng, J., Han, Z., Zhang, G. (2017). Ionomic and physiological responses to low nitrogen stress in Tibetan wild and cultivated barley. Plant Physiol. Biochem. 111, 257–265. doi: 10.1016/j.plaphy.2016.12.008
Segura, V., Vilhjálmsson, B. J., Platt, A., Korte, A., Seren, Ü., Long, Q., et al. (2012). An efficient multi-locus mixed-model approach for genome-wide association studies in structured populations. Nat. Genet. 44, 825–830. doi: 10.1038/ng.2314
Sharifi, M., Zebarth, B. J., Coleman, W. (2007). Screening for nitrogen-use efficiency in potato with a recirculating hydroponic system. Commun. Soil Sci. Plant Anal. 38, 359–370. doi: 10.1080/00103620601172357
Sharma, B., Joshi, D., Yadav, P. K., Gupta, A. K., Bhatt, T. K. (2016). Role of ubiquitin-Mediated degradation system in plant biology. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00806
Simko, I., Eujayl, I., van Hintum, T. J. L. (2012). Empirical evaluation of DArT, SNP, and SSR marker systems for genotyping, clustering, and assigning sugar beet hybrid varieties into populations. Plant Sci. 184, 54–62. doi: 10.1016/j.plantsci.2011.12.009
Stanley, O. P. B., Samonte, W. L. T., Medley, J. C., Pinson, S. R. M., Mcchund, A. M., Joreno, S. (2006). Nitrogen utilization efficiency: Relationship with grain yield, grain protein and yield related traits in rice (Oryza sativa L.). Agron. J. 98, 168–176. doi: 10.2134/agronj2005.0180
Tiwari, J. K., Plett, D., Garnett, T., Chakrabarti, S. K., Singh, R. K. (2018). Integrated genomics, physiology and breeding approaches forimproving nitrogen use efficiency in potato: Translating knowledgefrom other crops. Funct. Plant Biol. 45, 587–605. doi: 10.1071/FP17303
Udo, E. J., Ogunwale, J. A. (1978). Laboratory Manual for the Analysis of Soils, Plants and water Samples (Dept. of Agronomy University of Ibadan), 45.
Udoh, L. I., Gedil, M., Parkes, E. Y., Kulakow, P., Adesoye, A., Nwuba, C., et al. (2017). Candidate gene sequencing and validation of SNP markers linked to carotenoid content in cassava (Manihot esculenta Crantz). Mol. Breed. 37, 123. doi: 10.1007/s11032-017-0718-5
Wolfe, M. D., Rabbi, I. Y., Egesi, C., Hamblin, M., Kawuki, R., Kulakow, P., et al. (2016). Genome-wide association and prediction reveals genetic architecture of cassava mosaic disease resistance and prospects for rapid genetic improvement. Plant Genome 10 (3), plantgenome2017.03.0015. doi: 10.3835/plantgenome2017.03.0015
Wu, L., Yuan, S., Huang, L., Sun, F., Zhu, G., Li, G., et al. (2016). Physiological mechanisms underlying the high-grain yield and high-nitrogen use efficiency of elite rice varieties under a low rate of nitrogen application in China. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01024
Xiao-Hong, Z., Bo Li, Y. H., Liang, C., Dong, M. (2014). The wheat E subunit of V-type H+-ATPase is involved in the plant response to osmotic stress. Int. J. Mol. Sci. 15, 16196–16210. doi: 10.3390/ijms150916196
Yu, J., Buckler, E. S. (2006). Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol. 17, 155–160. doi: 10.1016/j.copbio.2006.02.003
Zebarth, B. J., Tarn, T. R., de Jong, H., Murphy, A. (2008). Nitrogen use efficiency characteristics of andigena and diploid potato selections. Am. J. Potato Res. 85, 210–218. doi: 10.1007/s12230-008-9014-6
Zhang, X., Zhang, H., Li, L., Lan, H., Ren, Z., Liu, D., et al. (2016). Characterizing the population structure and genetic diversity of maize breeding germplasm in Southwest China using genome-wide SNP markers. BMC Genomics 17, 697. doi: 10.1186/s12864-016-3041-3
Keywords: cassava, genome-wide association studies, nitrogen use efficiency, SNP, marker
Citation: Mbe JO, Dzidzienyo DK, Abah SP, Njoku DN, Onyeka J, Tongoona P and Egesi C (2024) Novel SNP markers and other stress-related genomic regions associated with nitrogen use efficiency in cassava. Front. Plant Sci. 15:1376520. doi: 10.3389/fpls.2024.1376520
Received: 25 January 2024; Accepted: 15 March 2024;
Published: 04 April 2024.
Edited by:
Francis Chuks Ogbonnaya, Grains Research and Development Corporation, AustraliaReviewed by:
Bradley Colin Campbell, The University of Queensland, AustraliaSumit Jangra, University of Florida, United States
Copyright © 2024 Mbe, Dzidzienyo, Abah, Njoku, Onyeka, Tongoona and Egesi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Kwadjo Dzidzienyo, ZGR6aWR6aWVueW9Ad2FjY2kudWcuZWR1Lmdo
 Joseph Okpani Mbe
Joseph Okpani Mbe Daniel Kwadjo Dzidzienyo2,3*
Daniel Kwadjo Dzidzienyo2,3* Simon Peter Abah
Simon Peter Abah Damian Ndubuisi Njoku
Damian Ndubuisi Njoku Joseph Onyeka
Joseph Onyeka Pangirayi Tongoona
Pangirayi Tongoona Chiedozie Egesi
Chiedozie Egesi