
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci., 26 April 2024
Sec. Crop and Product Physiology
Volume 15 - 2024 | https://doi.org/10.3389/fpls.2024.1368692
This article is part of the Research TopicEnhancing Berry Fruit Quality: Unraveling the Influence of Preharvest and Postharvest FactorsView all 8 articles
In recent years, the ethylene-mediated ripening and softening of non-climacteric fruits have been widely mentioned. In this paper, recent research into the ethylene-mediated ripening and softening of non-climacteric fruits is summarized, including the involvement of ethylene biosynthesis and signal transduction. In addition, detailed studies on how ethylene interacts with other hormones to regulate the ripening and softening of non-climacteric fruits are also reviewed. These findings reveal that many regulators of ethylene biosynthesis and signal transduction are linked with the ripening and softening of non-climacteric fruits. Meanwhile, the perspectives of future research on the regulation of ethylene in non-climacteric fruit are also proposed. The overview of the progress of ethylene on the ripening and softening of non-climacteric fruit will aid in the identification and characterization of key genes associated with ethylene perception and signal transduction during non-climacteric fruit ripening and softening.
Ethylene (C2H4), one of the most important phytohormones, is crucial to the entire plant growth and development process, including inducing seed germination, inhibiting stem and root elongation, and promoting fruit ripening, abscission, and senescence (Liu et al., 2015). Ethylene also serves as a key mediator of biotic and abiotic stress responses in plants (Schaller, 2012; Dubois et al., 2018), which has made this phytohormone a research focus in recent years. Numerous studies have demonstrated that ethylene is a key regulator of changes in fruit color, texture, aroma, flavor, and nutritional compounds during ripening (Pech et al., 2012). Based on physiological characteristics such as respiratory rate, ethylene release, and response to exogenous ethylene, fruits can be categorized into two basic types: climacteric and non-climacteric. Climacteric fruits exhibit obvious respiratory peaks before the initial maturation stage, and ethylene release increases accordingly. In contrast, ethylene release during the ripening process of non-climacteric fruits is significantly lower than that of climacteric fruits, and the peak in ethylene release is absent (Chervin et al., 2004; Giovannoni, 2004; Paul et al., 2012; Cherian et al., 2014). Numerous studies concluded that ethylene played a crucial role in regulating the ripening and softening of not only climacteric fruits but also non-climacteric fruits (Chervin et al., 2004; Villarreal et al., 2010; Sun et al., 2013).
Non-climacteric fruits express many ethylene biosynthesis genes as well as a series of ethylene signaling components, such as ethylene receptors (ETRs), the negative regulator constitutive triple response (CTR1), and the transduction factors ethylene insensitive 2 (EIN2) and ethylene insensitive 3 (EIN3), EIN3-like (EIL), and ethylene-responsive factor (ERF) (Osorio et al., 2012). Expression of these important signal transduction elements is upregulated to varying degrees during the ripening and softening processes of non-climacteric fruits, as observed in strawberry (Trainotti et al., 2005; Sun et al., 2013; Qian et al., 2016), grape (Chervin and Deluc, 2010; Muñoz-Robredo et al., 2013; Qian et al., 2016; Ye et al., 2017), orange (Katz et al., 2004; Distefano et al., 2009; Wu et al., 2016; Kashyap and Banu, 2019), loquat (Alós et al., 2017), cherry (Ren et al., 2011; Xanthopoulou et al., 2022), and watermelon (Karakurt and Huber, 2008; Karakurt et al., 2014). These findings indicate that ethylene indeed plays a critical role in regulating the ripening of non-climacteric fruits. In this review, we summarized the existing literature regarding ethylene biosynthesis, ethylene physiology, and ethylene signaling in non-climacteric fruit. This review is aimed at helping researchers understand the regulatory mechanisms underlying the ripening and softening of non-climacteric fruits.
Generally, non-climacteric fruits have been classified as a totally separate group from climacteric fruits, characterized by the absence of a typical climacteric ripening pattern. However, comparative genomic studies carried out in climacteric and non-climacteric fruit models suggest that the expression of ethylene biosynthesis- and signaling pathway-related components is common to both climacteric and non-climacteric fruits (Bernales et al., 2019). In this paper, the ethylene biosynthesis pathway (Figure 1), the ethylene signal transduction pathway (Figure 2), and the functional members of these pathways during the ripening and softening processes of representative non-climacteric fruit models (Table 1) are summarized. As shown in Figure 1, the involvement of the ethylene biosynthesis pathway during the early ripening period has been confirmed in many reports (Bleecker and Kende, 2000; Glick, 2003; Paul, 2015). Ethylene is produced from the bio-precursor methionine (Met) and is further synthesized into S-adenosyl methionine (SAM) via SAM synthetase (Bürstenbinder et al., 2007). Next, SAM is converted into 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC synthase (ACS) via the cleavage of 5′-methylthioadenosine (MTA). ACS, the key rate-limiting enzyme in this pathway, belongs to the pyridoxal-5′-phosphate (PLP)-dependent aminotransferase family and thus requires vitamin B6 as a co-factor (Pattyn et al., 2021), and application of the aminoethoxyvinylglycine (AVG), an inhibitor of ACS activity, could significantly inhibit the evolution of ethylene (Mullins et al., 2010). Afterward, a series of reactions catalyze the conversion of MTA into Met via the Yang or Met cycle (Bürstenbinder et al., 2007). Meanwhile, ACC produces ethylene under the catalysis of ACC oxidase (ACO), thus activating downstream ethylene signaling components and responses (Yoon and Kieber, 2013).
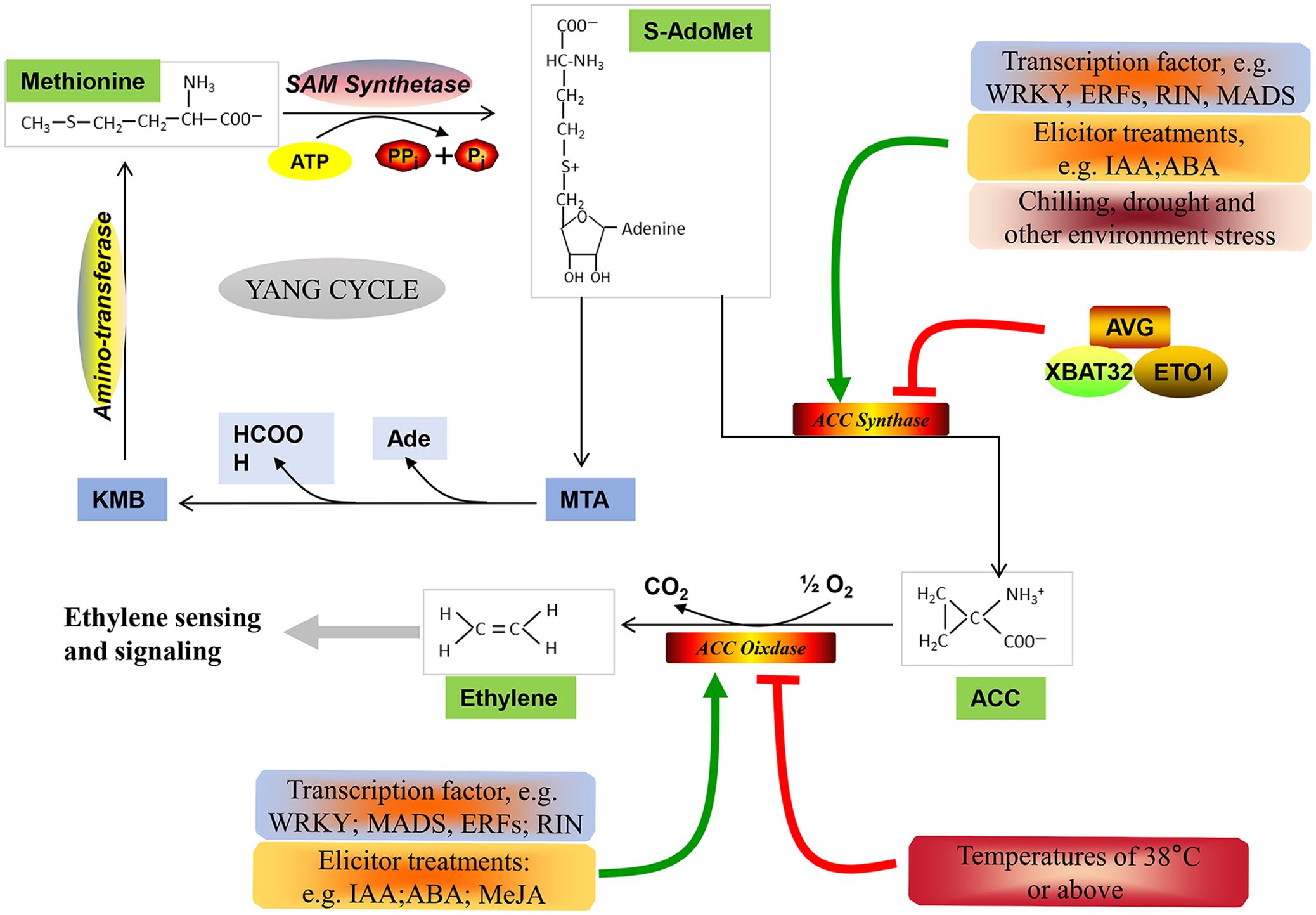
Figure 1 Ethylene biosynthetic pathway. Methionine serves as the precursor of ethylene, and it is catalyzed by SAM synthetase to format S-AdoMet (SAM) at the expense of one molecule of ATP per molecule (Bürstenbinder et al., 2007). Subsequently, SAM is metabolized into 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC synthase (ACS) (Pattyn et al., 2021). Additionally, SAM can be diverted to methylthioadenosine (MTA), which can be recycled back to methionine via α-keto-γ-methylthio-butyric acid (KMB) through the Yang cycle (Bürstenbinder et al., 2007). Finally, the catalytic action of ACC oxidase (ACO) converts ACC into ethylene, initiating downstream ethylene sensing and signaling (Yoon and Kieber, 2013). Furthermore, various transcription factors, ubiquitin ligases, regulators, and environmental stresses are involved in regulating the expression of ACS and ACO expression (Lyzenga and Stone, 2012; Pattyn et al., 2021).
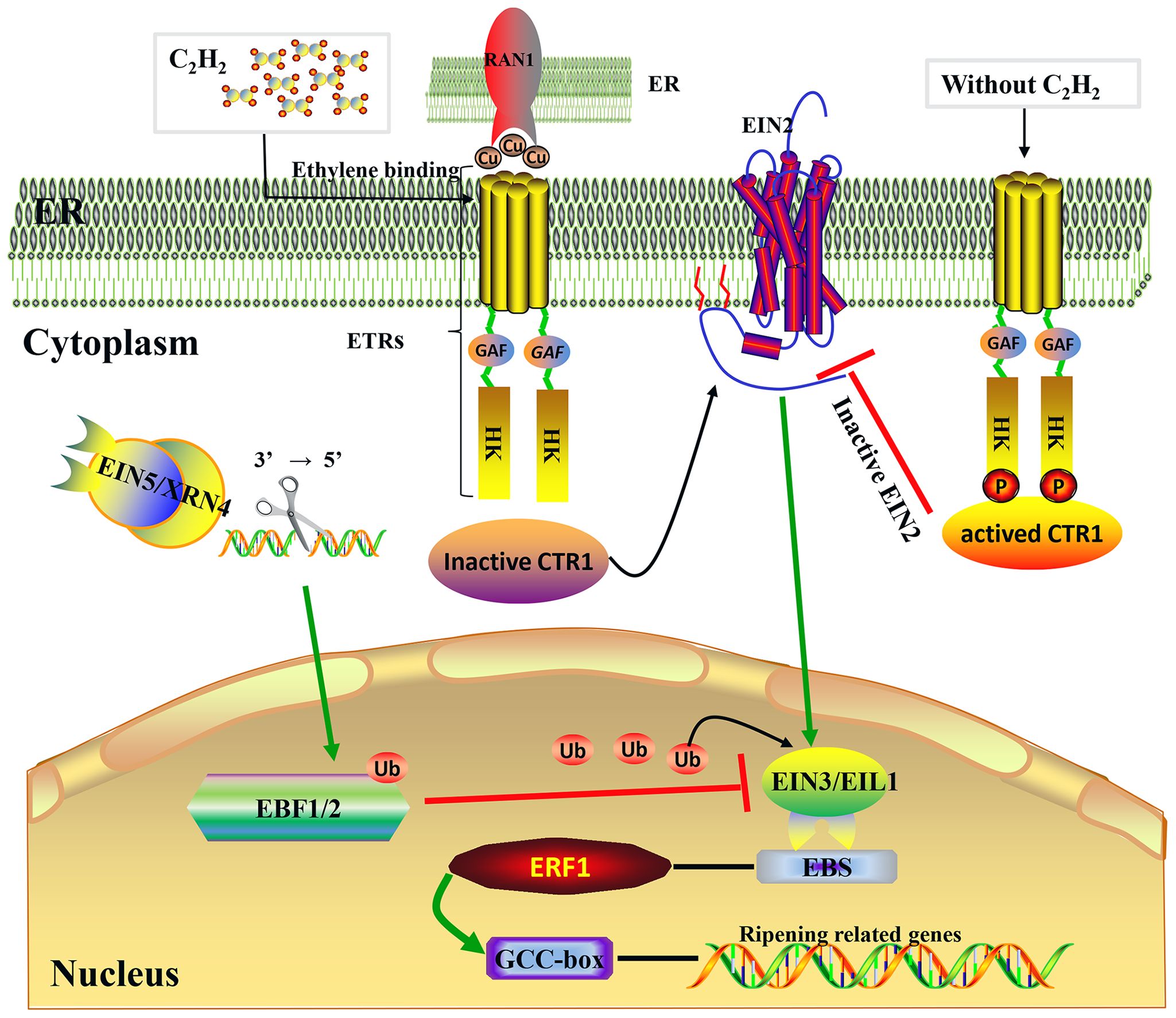
Figure 2 Ethylene signaling transduction pathway. Model of the ethylene signal transduction. Under the mediation of RAN1, which transports copper to ethylene receptors, the presence of ethylene causes the loss of phosphorylation (P) of ethylene receptors (ETRs) at the membrane level (ER) (Binder et al., 2010); following this, the receptor–CTR1 complex is inactivated, the delivery of phosphate groups from CTR1 to EIN2 becomes incapable, and then EIN2 is cleaved and partly enters the nucleus to activate EIN3/EIL1 (Wen et al., 2012). Subsequently, EIN3/EIL1 binds to a conserved motif known as the EIN3 binding site (EBS), which is present within the promoters of ERF1, and this ultimately activates ERF1, which binds to the GCC box in the promoters of many ethylene-inducible, ripening-related genes (Fujimoto et al., 2000). Protein degradation of EIN3/EIL1 is regulated by EBF1/2 via the ubiquitin/26S proteasome pathway, and EIN5/XRN4 5′–3′ exoribonuclease mediated control of EBF1/2 mRNA levels (Potuschak et al., 2003; Olmedo et al., 2006).
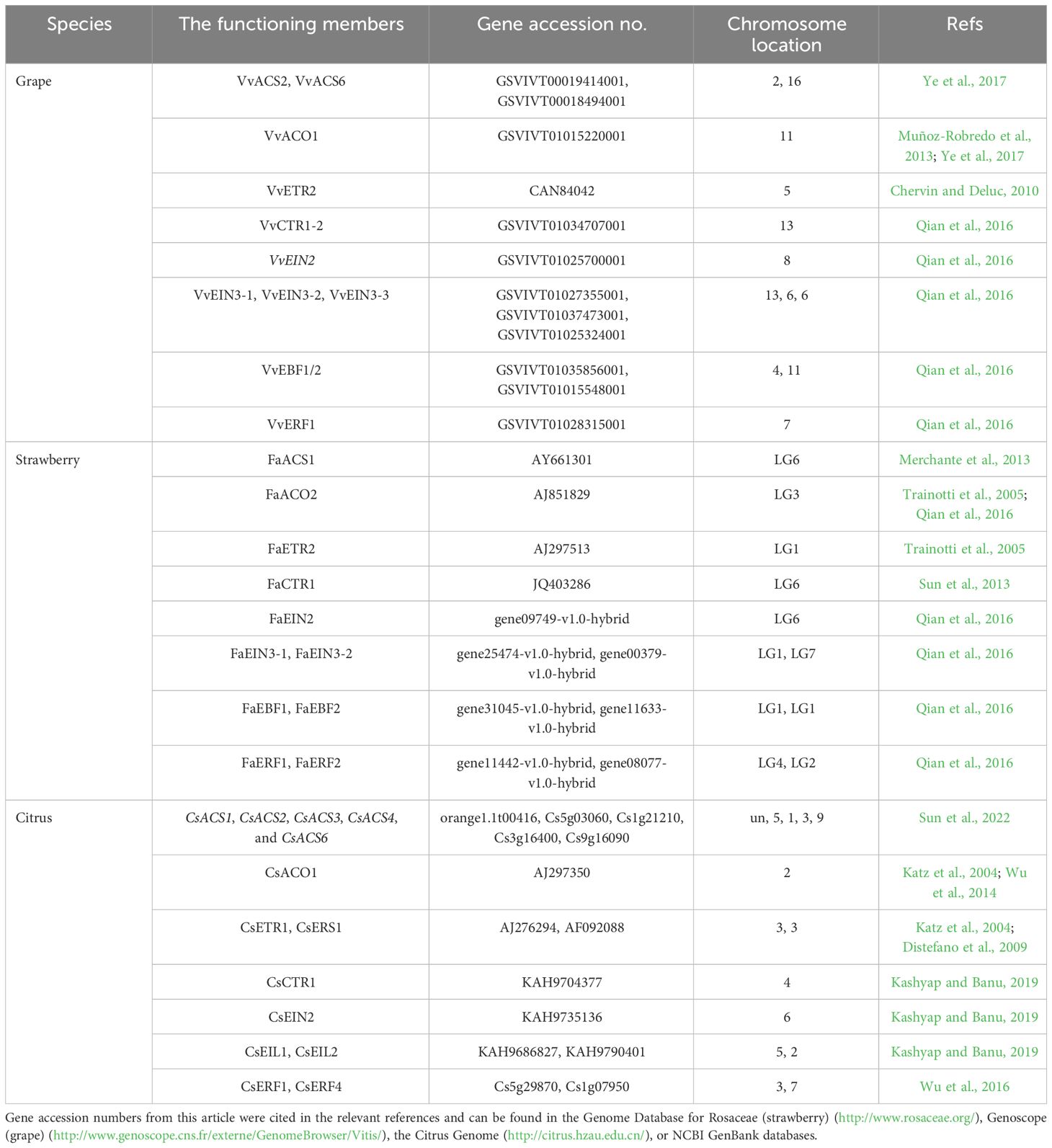
Table 1 The functioning members of ethylene biosynthesis and signal transduction in ripening and softening processes for the representative non-climacteric fruit models.
Throughout the process of fruit development, autocatalytic ethylene production is correlated with increased ACC content as well as increased activities of ACS and ACO. ACS and ACO are encoded by multigene families. In tomatoes, fruit ripening and ethylene production were strongly repressed in transgenic tomato fruits harboring the hpRNAi-ACO construct and an antisense inhibitor of ACS (Alexander and Grierson, 2002; Behboodian et al., 2012). This indicates that both ACS and ACO are important for controlling ethylene production in climacteric fruit. Although ethylene levels are relatively low in non-climacteric fruits during ripening and softening, some physiological and genetic studies have revealed that ethylene still plays an important role in this process (Paul et al., 2012; Zhou et al., 2016; Fenn and Giovannoni, 2021; Ma et al., 2021c). Meanwhile, the function of the ACS and ACO genes has also been investigated.
ACS belongs to a large multigene family, and at least 9, 3, and 18 ACS genes have been separately cloned from grapes, strawberries, and oranges, respectively (Merchante et al., 2013; Qian et al., 2016; Sun et al., 2022). As shown in Table 1, VvACS2 and VvACS6 play important roles in ethylene biosynthesis in grapes (Ye et al., 2017). In strawberries, three members of the ACS family all showed high expression levels during the green-fruit stage (Qian et al., 2016). Furthermore, increases in FaACS1 expression occur alongside accelerated ethylene biosynthesis, indicating that FaACS1 may be the key ACS gene responsible for regulating ethylene biosynthesis in strawberries (Merchante et al., 2013). In sweet oranges, both CsACS1 and CsACS2 may regulate ethylene biosynthesis during the early coloring stage, whereas CsACS3, CsACS4, and CsACS6 are upregulated during ripening. Each of these genes plays important roles in maintaining stable, low-level ethylene biosynthesis, which is required for ripening (Sun et al., 2022).
Among ACO genes, VvACO1 expression peaks before and after the véraison stage in grapes, while VvACO2 and VvACO3 expressions are observed only after the véraison stage (Muñoz-Robredo et al., 2013). Because there is a slight increase in ethylene production around véraison (Xu et al., 2018), VvACO1 likely plays a critical role in ethylene biosynthesis in grapes (Ye et al., 2017). In strawberries, the expression levels of FaACO1 and FaACO3 continue to rise from the green-fruit stage to the white-fruit stage, followed by a gradual reduction to their lowest levels during fruit ripening and coloring. However, FaACO2 reaches its lowest expression level during the white-fruit stage and then gradually increases during the ripening stage, resulting in substantially increased ethylene biosynthesis just prior to maturation (Trainotti et al., 2005; Qian et al., 2016). In citrus fruits, CsACO1 appears to be highly expressed during the system II-like stage, in which an autocatalytic burst of ethylene production accompanies the fruit ripening process (Katz et al., 2004; Wu et al., 2014).
Additionally, the expression of ACO and ACS genes is regulated by multiple transcription factors, including MADS-box, ERF, NAC, and WRKY (Liu and Zhang, 2004; Zhijin et al., 2009; Qin et al., 2012; Xiao et al., 2013; Kou et al., 2016), and the RING E3 ligases, XBAT32 and ETO1, also mediate the proteasomal degradation of ACS proteins in the regulation of ethylene production (Lyzenga and Stone, 2012). Moreover, biotic stressors such as pathogenic bacteria and phytohormones, as well as abiotic stressors such as drought, cold injury, and high temperature, also affect the expression of ACO and ACS genes (Tsuchisaka and Theologis, 2004). However, research on the transcription factor and ubiquitin ligase-mediated regulation of ACO and ACS genes in non-climacteric fruit is currently rare, and we suggest that this subject deserves more attention.
In recent years, molecular genetic studies on the model plant Arabidopsis thaliana have established the signal transduction pathway underlying the plant response to ethylene. This response occurs when receptors bind ethylene and then send a signal along a linear pathway that comprises MAPK and transcriptional cascades: C2H4 → ETRs → CTR1→ EIN2→ EIN3/EIL1→ ERF1→ downstream genes. As shown in Figure 2, the same transduction mechanism is found in both non-climacteric and climacteric fruits (Bernales et al., 2019).
ETRs are upstream elements and play a negative regulatory role in the entire ethylene signal transduction pathway. In the absence of ethylene, ETRs activate the downstream raf-like serine/threonine kinase CTR1, thus inhibiting the expression of relevant ethylene-inducible genes. However, in the presence of ethylene, ethylene molecules bind to ETRs located at the endoplasmic reticulum membrane, resulting in the dephosphorylation of ETRs. This leads to the inactivation of the ETR–CTR1 complex and the relief of inhibitory activity on ethylene-inducible genes (Tieman et al., 2000). ETR proteins are encoded by various genes, which differ in their structures and expression levels during fruit development, and both mono-deletion and co-deletion of members may induce constituent ethylene responses (Tieman et al., 2000; Cancel and Larsen, 2002; Hall and Bleecker, 2003). At present, three ETR genes (FaETR1, FaERS1, and FaETR2) have been cloned from strawberries, which exhibit differential expression patterns during the fruit ripening process. Specifically, the expression of FaETR1 and FaERS1 rises significantly during the ripening stage, while the expression level of FaETR2 peaks during the white-fruit stage and remains high during the ripening stage. As shown in Table 1, FaETR2 may play an important role in the process of ethylene-mediated fruit ripening (Trainotti et al., 2005). In grapes, at least six ETRs have been identified. Among these, VvETR1 through VvETR4 exhibit peak expression before and during the véraison stage, followed by a gradual decline during the ripening process (Chervin and Deluc, 2010; Qian et al., 2016). The transcript abundance of VvETR2 shows a transient peak at the inception of berry ripening, coinciding with an internal ethylene peak preceding color change (Chervin and Deluc, 2010). By contrast, the transcriptional levels of VvERS1 and VvEIN4 gradually rise throughout the late ripening stage until complete maturation (Chervin and Deluc, 2010). Therefore, VvETR2 may be the key ethylene receptor responsible for grape berry ripening. In oranges, CsETR1 and CsERS1 exhibit peak transcriptional levels before and after the degreening stage, indicative of their important roles in fruit degreening and ripening (Katz et al., 2004; Distefano et al., 2009). Additionally, the interaction between ethylene and receptor proteins requires the assistance of copper ions, facilitated by the copper transporter RAN1. Mutants lacking RAN1 cannot bind ethylene due to their inability to transport copper ions (Binder et al., 2010). However, there is limited research on the RAN1 gene in non-climacteric fruit.
CTR1 is a downstream component of ETRs in the ethylene signaling pathway. ctr1 mutants exhibit sustained ethylene responses, indicating that CTR1 functions as a negative regulatory element in the ethylene signaling pathway (Huang et al., 2003). In grapes, two CTR1 family genes, VvCTR1-1 and VvCTR1-2, have been identified. VvCTR1-1 expression remains relatively stable throughout grape berry development, while VvCTR1-2 expression increases consistently during fruit development and peaks during the véraison stage, followed by a gradual decrease to minimal levels (Qian et al., 2016). Only one FaCTR1 gene has been identified in strawberries, with peak expression also occurring during the coloring phase (Sun et al., 2013; Qian et al., 2016). Downregulation of FaCTR1 transcription inhibits the softening and coloring of strawberries, suggesting that FaCTR1 plays an important role in the process of ethylene-mediated ripening (Sun et al., 2013). In citrus pulp, the CsCTR1 gene is upregulated throughout development until harvest (Kashyap and Banu, 2019). Furthermore, several lines of evidence indicate that ethylene can modulate the level of ethylene receptor/CTR1 signaling complexes through transcriptional induction (Shakeel et al., 2015). However, there have been limited studies exploring the relationship between ethylene and the level of ethylene receptor/CTR1 signaling complexes in non-climacteric fruit. Additional research is needed to further investigate ethylene-mediated transcriptional and post-transcriptional regulation of CTR1 and ethylene receptor members in these fruits.
EIN2 is located downstream of CTR1 on the endoplasmic reticulum membrane and serves as a positive regulator of the ethylene signaling pathway. ein2 mutants exhibit total ethylene insensitivity. Moreover, the C-terminal end of EIN2 is thought to participate in signaling output, and ectopic expression of this domain alone can partially activate ethylene responses (Wen et al., 2012). The stability of the EIN2 protein is regulated by two F-box proteins: ETP1 and ETP2 (Qiao et al., 2009). The EIN2 gene, similar to that in A. thaliana, has also been cloned in grapes and tomatoes. As shown in Table 1, VvEIN2 expression is minimal during the early developmental period in grapes, rises rapidly during early véraison, and then gradually declines during ripening. In contrast, in strawberries, FaEIN2 expression is stable throughout the entire developmental process (Qian et al., 2016). In citrus fruits, CsEIN2 expression remains high until harvest (Kashyap and Banu, 2019). However, further studies should be conducted to evaluate the molecular biological function of the EIN2 genes in the maturation of non-climacteric fruits.
The ethylene signaling pathway downstream of EIN2 is mediated by the EIN3 gene family, including EIN3 and EIN3-like 1 (EIL1), which act as transcription factors regulating gene expression in cell nuclei. In A. thaliana, ein3 mutants exhibit severely limited ethylene responses, indicating that EIN3 and EIL1 play key roles in ethylene signal transduction (Dolgikh et al., 2019). In grapes, three EIN3/EIL1 gene family members exhibited the same expression pattern during the developmental process. Specifically, their expressions are significantly upregulated 2 weeks before and after the véraison stage and reach the lowest level during the véraison stage (Qian et al., 2016). This is consistent with previous research (Chervin and Deluc, 2010). To date, two EIN3 members (FaEIN3-1 and FaEIN3-2) have been identified in strawberries, both of which exhibit similar expression patterns to those in grapes. That is, their expression levels are significantly upregulated before and after the coloring stage and decline to their lowest levels during the coloring stage (Qian et al., 2016). These results suggest that the EIN3 family plays an important regulatory role in controlling anthocyanin synthesis and maturation-related changes in non-climacteric fruits. Similar results were reported in sweet orange. As shown in Table 1, the transcript abundance of CsEIL1 and CsEIL2 increased during fruit enlargement and ripening, suggesting that they may play important roles during the ripening of citrus fruits (Kashyap and Banu, 2019). Through the 26S ubiquitin/proteasome degradation pathway, the EBF1 and EBF2 proteins regulate the stability of the EIN3 and EIL1 proteins (Potuschak et al., 2003). The degradation process takes effect through EIN5/XRN4 with 3′−5′ exonuclease activity, thereby mediating the RNA degradation pathway of these two F-box genes (Olmedo et al., 2006). The expression levels of FaEBF1 and FaEBF2 gradually increase during the early development of strawberry fruits but then decline as the fruits ripen. In grapes, the expression of VvEBF1/2 increases during the expansion stage but significantly decreases during the véraison and ripening stages (Qian et al., 2016). Because EBF1/EBF2 accelerates the degradation of EIN3, the downregulated transcriptional levels of EBF1/EBF2 during the véraison and ripening stages further suggest that the EIN3 family may potentially play a positive regulatory role in the ripening process of non-climacteric fruits. However, this hypothesis requires further verification.
The ERF family consists of regulatory factors downstream of the ethylene signaling pathway. These factors can be induced by ethylene signaling and either activate or inhibit the transduction and expression of downstream ethylene-responsive genes via binding of their ERF binding domains to GCC-box (AGCCGCC motifs) cis-elements (Fujimoto et al., 2000). Numerous studies have indicated that ERF transcription factors are involved in the coloring, ripening, and softening processes of climacteric fruits, including kiwifruit (Zhang et al., 2016), banana (Xiao et al., 2013), pear (Wu et al., 2020; Cheng et al., 2022), and tomato (Liu et al., 2016). However, information about the regulation of ERFs in the development- and ripening-related processes of non-climacteric fruits remains scarce. In grapes, VvERF1 maintains minimal expression throughout the development process until the late stage of maturation, at which point it is significantly upregulated. However, in strawberries, FaERF1 and FaERF2 both exhibit high expression during the early development stage, while their expression gradually declines during the ripening stage (Qian et al., 2016). Similarly, in citrus fruits, genome-wide identification of transcription factors indicates that ERF1 and ERF4 may be important regulators of the late-ripening trait (Wu et al., 2016). Therefore, it is important to investigate the regulatory function of ERF transcription factors, as well as to identify the relevant interacting factors, as they may be linked to ripening and softening in non-climacteric fruits.
In general, climacteric fruits exhibit a dramatic increase in the rate of respiration and ethylene production during the process of ripening. For example, in tomato fruits, ethylene production begins to increase at the turning stage and reaches its peak at the pink stage (Figure 3A) (Zhang et al., 2009). By contrast, in non-climacteric fruit, the increase in respiration and ethylene production is limited to a certain extent (Paul et al., 2012). However, recent research has highlighted the important role of ethylene in non-climacteric fruits. For example, in strawberries, although ethylene synthesis is low, the first rapid emission of ethylene occurs in green fruit, and this emission extends to the degreening stage (20 days after anthesis). Additionally, a second slower emission of ethylene occurs following the white stage (23 days after anthesis) (Figure 3B), suggesting that ethylene production is likely a key component of the ripening process in strawberries.
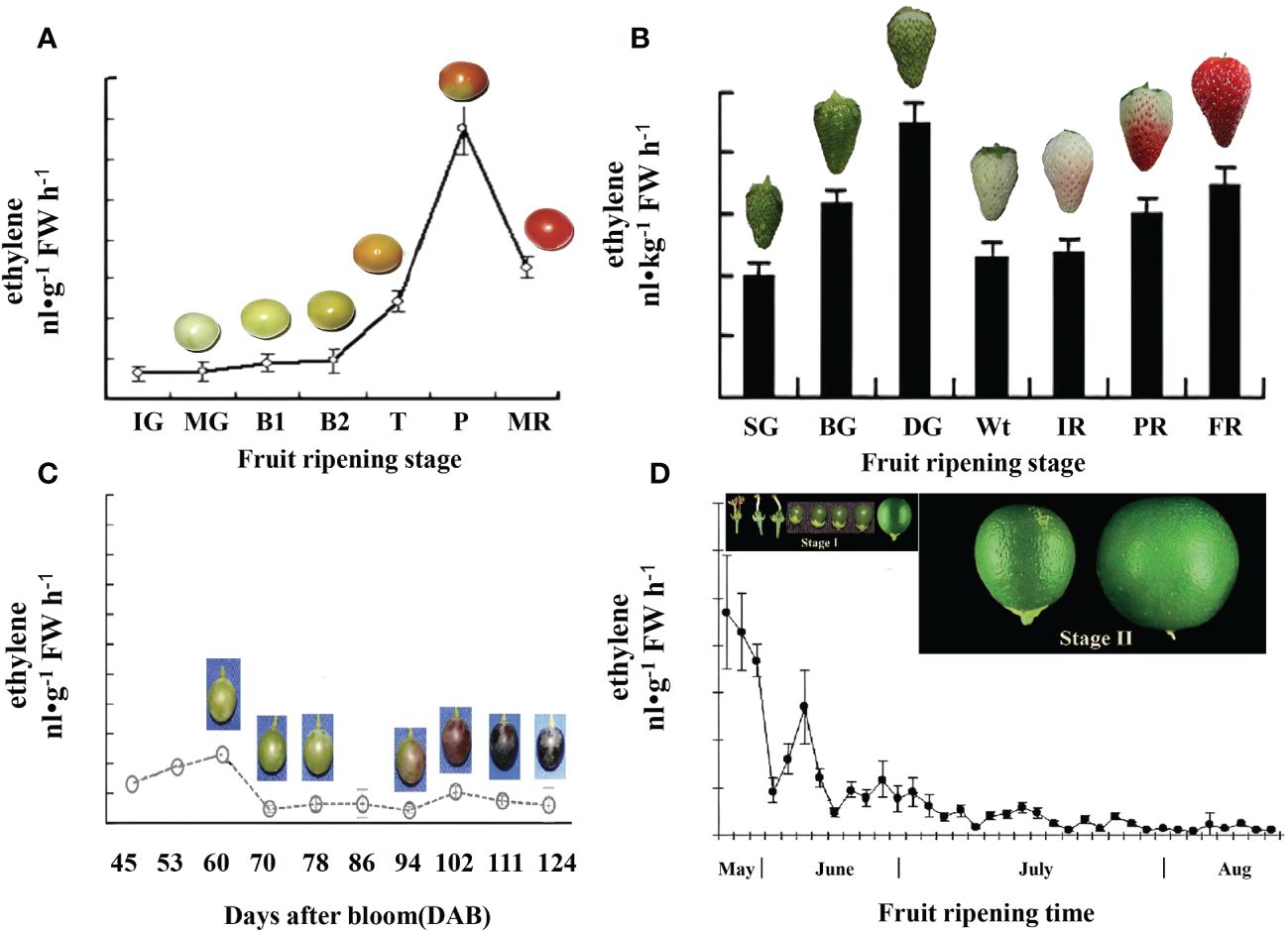
Figure 3 Comparative ethylene evolution in representative climacteric and non-climacteric fruit models. (A) Ethylene evolution in the development of the tomato from the immature green to the red ripe stage (Zhang et al., 2009). IG, immature green (20 days after anthesis); MG, mature green (40 days after anthesis); B1, breaker (44 days after anthesis); B2, breaker (45 days after anthesis); T, turning (47 days after anthesis); P, pink (50 days after anthesis); MR, mature red (53 days after anthesis). (B) Ethylene evolution in the development of ‘Camarosa’ strawberry fruit during seven stages (Sun et al., 2013), SG (small green), BG (big green), DG (degreening), Wt (white), IR (initially red), PR (partially red), and FR (fully red), which occurred for 7, 15, 20, 23, 27, 31, and 35 days, respectively, after anthesis. (C) Ethylene evolution in the development of ‘Moldova’ grape fruit during contentious growth points of berry ripening (Xu et al., 2018). (D) Ethylene evolution in the development of attached ‘Valencia’ orange fruit during two growth stages (Katz et al., 2004). Stage I, the cell division stage, starts immediately after fruit set and lasts for approximately 90 days after full bloom (DAFB). Stage II, the cell expansion stage, during which fruit growth continues, mostly by cell expansion, extends until 150–180 DAFB.
Notably, exogenous ethylene affects many important quality attributes in strawberries, including firmness (Jiang et al., 2001; Villarreal et al., 2009, 2016; Elmi et al., 2017), anthocyanin accumulation, phenylalanine ammonia-lyase (PAL) activity (Villarreal et al., 2009; Merchante et al., 2013; Sun et al., 2013), organic acid content (Merchante et al., 2013; Elmi et al., 2017), sugar content (Villarreal et al., 2016; Tosetti et al., 2020), phenolic accumulation (Villarreal et al., 2010; Lopes et al., 2015), and the expression of volatile-related genes (Merchante et al., 2013). In addition, some studies also report that ethephon affects fruit diameter and anthocyanin content, and these effects are dependent on the fruit developmental stage at which the treatment is applied. Ethephon treatment at the green stage of fruits results in a larger diameter, while ethephon treatment at the pink stage results in a smaller diameter. Meanwhile, only treatment of white fruits with ethephon increases the anthocyanin content (Reis et al., 2020). Similarly, another study also reports that ethylene treatment at the green stage of strawberry fruits can delay the accumulation of anthocyanins, as well as downregulate the key anthocyanin biosynthesis genes FcANS and FcUFGT (Figueroa et al., 2021). Conversely, the use of 1-methyl cyclopropane (1-MCP) at the white stage can significantly inhibit fruit ripening and coloring (Villarreal et al., 2009). Moreover, exogenous ethylene treatment can stimulate the upregulation of the ethylene receptor genes FaETR1 and FaERS1, the ethylene-response factor gene FaERF2, and the ethylene biosynthesis gene FaACO1 (Trainotti et al., 2005; Lopes et al., 2015). Meanwhile, the negative regulatory factor FaCTR1 also plays an important role in the maturation of strawberry fruits. Constructing an interfering vector to downregulate FaCTR1 results in the inhibition of strawberry coloring and softening, as well as the facilitation of ethylene biosynthesis (Sun et al., 2013). Thus, it appears that the ethylene signal transduction pathway plays a highly important role in the ripening process of strawberries.
Grape is a non-climacteric fruit that does not exhibit a typical respiratory peak and whose maturation apparently does not require ethylene. However, recent research has shown that ethylene plays a critical role in the ripening of grape berries. Grapes exhibit a slight increase in ethylene production around véraison, as shown in Figure 3C (Xu et al., 2018). It has been confirmed that the content of endogenous ethylene increases significantly before the véraison stage. During this period, the grape berries expand rapidly, anthocyanin is constantly accumulated, and pulp acidity is reduced (Chervin et al., 2004). Meanwhile, the contents of aroma compounds such as terpinols, ethyl alcohols, and esters increase significantly (Bellincontro et al., 2006).
Exogenous ethylene upregulates the transcription of xyloglucan endotransferase (XET) and aquaporin (AQUA) genes, thus altering fruit texture and promoting softening (Chervin et al., 2008). Exogenous ethylene also facilitates the early abscission of grape berries (Bessis et al., 2000), promotes anthocyanin accumulation, and induces expression of the anthocyanin synthesis-related genes CHS, F3H, and UFGT (El-Kereamy et al., 2003). Further research has shown that exogenous ethylene induces the expression of anthocyanin regulatory genes, and this is caused by direct action of the ethylene signal transduction pathway on the UFGT and MYBA1 promoters to directly regulate gene expression (Tira-Umphon et al., 2007). Exogenous ethylene also facilitates the production of endogenous ethylene and the expression of VvACO genes (Muñoz-Robredo et al., 2013), as well as promotes the expression of VvETR2 and VvCTR1 (Chervin and Deluc, 2010). On the contrary, the ethylene inhibitor 1-MCP can significantly suppress ethylene biosynthesis and the rise in respiratory rate, reduce anthocyanin accumulation, inhibit fruit expansion and the reduction of acidity (Chervin et al., 2004), and suppress the expression of sugar transporters (e.g., SUC11 and SUC12) and ethanol dehydrogenases (Tesniere et al., 2004; Chervin et al., 2006). 1-MCP treatment also markedly affects grape storage quality by decreasing respiration and ethylene production, reducing rachis browning and chlorophyll degradation, and maintaining higher anthocyanin content and lower ester content (Silva et al., 2013; Wang et al., 2019). These studies indicate that ethylene regulates fruit coloring, the formation of quality- and flavor-associated substances, and the visual and nutritional quality of grapes during storage.
Ethylene biosynthesis genes and key genes in the ethylene signaling pathway exhibit different expression levels during different grape developmental stages. The highest ACC oxidase transcript abundance is observed immediately before véraison, which suggests that peak ethylene production occurs before véraison (Deluc et al., 2007; Pilati et al., 2007). VviERF045 expression gradually increases before véraison and peaks during the ripening stage (Leida et al., 2016). The transcript abundance of ERF6 transcription factors is significantly affected by ripening and correlated with the transcript abundance of terpene synthases and lipoxygenases involved in flavor formation (Cramer et al., 2014). In addition, both VvACO4 and VvEIL3 regulate ethylene synthesis and fruit ripening, and over-expression of VvACO4 and VvEIL3 shows a significant ethylene production and accelerates fruit ripening compared to control fruits (Wang et al., 2022). These findings suggest that the ethylene signaling pathway plays an important role in the ripening process of grape berries.
Citrus is also a non-climacteric fruit and lacks an ethylene-induced respiratory peak and climacteric rise in ethylene production (Chen et al., 2018). However, a previous study showed that citrus fruits exhibit “pseudoclimacteric” behavior and that young citrus fruitlets attached to the tree produce high levels of ethylene, which decrease dramatically toward the end of stage I and thereafter (Figure 3D). Moreover, citrus exhibits high sensitivity to ethylene at stage I, and exogenous ethylene treatment advances and increases ethylene production as in climacteric fruits (Katz et al., 2004). Previous studies suggest that ethylene biosynthesis can be divided into two types: system I and system II. System I ethylene biosynthesis mainly occurs before the maturation of climacteric fruits and throughout the ripening stage of non-climacteric fruits, with a small amount of ethylene synthesized. In contrast, system II ethylene biosynthesis mainly takes place during the ripening stage of climacteric fruits. Orange development may involve two ethylene biosynthesis pathways: system I-like and system II-like (Katz et al., 2004). CsACS2 plays a significant role in system I-like ethylene production, while CsACS1 is involved in system II-like ethylene production (Katz et al., 2004; Kashyap and Banu, 2019).
To improve skin color in early season or early harvest cultivars, ethylene degreening treatment is widely used to promote chlorophyll degradation and carotenoid accumulation (Zhou et al., 2010; Fauziah and Bintoro, 2021). The increased accumulation of carotenoid pigments in citrus fruits observed following ethylene or ethephon treatment is due to the induction of carotenoid biosynthesis genes (Rodrigo and Zacarias, 2007; Matsumoto et al., 2009; Huang et al., 2021). In addition, the repression of β-carotene hydroxylase genes is significantly increased by ethylene or ethephon, thereby leading to the preferential accumulation of β-carotene and β-cryptoxanthin (both of which contribute to orange coloration) (Zhou et al., 2010). Exposure to ethylene can also stimulate various adaptation and metabolic processes, which can impact fruit internal and nutritional quality (Mayuoni et al., 2011), thereby regulating citrus fruit ripening (Katz et al., 2004; Ding et al., 2015; Li et al., 2019). Moreover, ethylene can promote the accumulation of aroma substances in orange fruit (Sharon-Asa et al., 2003), while 1-MCP delays coloring and inhibits ripening (Cai et al., 2006). These findings indicate that citrus fruit ripening is mediated by ethylene.
Loquat, litchi, sweet cherry, longan, ananas, blueberry, and watermelon are all defined as non-climacteric fruits. Ethylene production increases slightly during the coloring stage in loquat fruits, and ethylene is also essential for peel coloration and carotenoid biosynthesis (Alós et al., 2019). Treating loquats with exogenous ethylene during the véraison or post-harvest stage significantly promotes the expression of the ACO1 gene and the biosynthesis of endogenous ethylene (Alós et al., 2017). Conversely, 1-MCP treatment inhibits the expression of ethylene biosynthesis genes, delays the ripening process, and reduces the activities of lipoxidase (LOX) and peroxidase (POD) in post-harvest loquat fruits (Liguori et al., 2015; Alós et al., 2017). Additionally, 1-MCP suppresses the accumulation of reactive oxygen species and slows the oxidation of phenols, thus inhibiting fruit rot (Cai et al., 2006). In peppers and jujubes, recent studies have shown that ethylene plays an important role in ripening and post-harvest storage. In these species, the expressions of certain ACS genes, including CaACS1, CaACS2, ZjACS2, ZjACS3, ZjACS5, and ZjACS7, were altered alongside ethylene production during fruit ripening (Aizat et al., 2013; Zhang et al., 2018). In sweet cherries, ethylene biosynthesis increases significantly during ripening, and ethylene can increase the respiration rate (Gong et al., 2002). Meanwhile, 1-MCP treatment inhibits the rotting of post-harvest cherries (Mozetič et al., 2006) by decreasing the respiratory rate and enhancing the activity of superoxide dismutase (SOD) (Sharma et al., 2010). 1-MCP also delays the reduction of POD and catalase (CAT) activities and reduces the malondialdehyde (MDA) content (Yang et al., 2011). Additionally, high-concentration exogenous ethylene treatment can facilitate chlorophyll degradation and anthocyanin biosynthesis in litchi fruits (Wang et al., 2007), while 1-MCP treatment suppresses the browning of post-harvest litchi and ananas fruits, maintaining their quality (Selvarajah et al., 2001; Sivakumar and Korsten, 2010). Although raspberries are non-climacteric fruits, increased ethylene production and respiration rate were detected at the white-fruit stage and continued to increase until maturity. Treatment of raspberries with 1-MCP at the white stage delayed loss of firmness, suggesting that softening may be partially regulated by ethylene in raspberries (Fuentes et al., 2015). Blueberries are also known as non-climacteric fruits, although ethylene is reported to be involved in their ripening process (Watanabe et al., 2021). Ethylene absorbent treatment can reduce weight loss and decay, as well as prevent the loss of total phenolic content and maintain firmness, in blueberries (Wang et al., 2018). Furthermore, pre-treatment with 1-MCP prevents berry softening and cell wall degradation in blueberries (Ortiz et al., 2018). Longan fruits are also non-climacteric and exhibit minimal changes in soluble solids content (SSC) and titratable acidity (TA) after harvest (Jiang et al., 2002). However, there has been an increase in ethylene production associated with post-harvest decay for longan fruits stored at 20°C (Wall et al., 2011), and the ethylene-responsive factor-like gene DlERF1 has been reported to regulate senescence-associated gene expression in longan fruits (Kuang et al., 2012). In watermelons, the expressions of two ACS isoforms and two ACO isoforms were significantly upregulated during ripening, indicating that ethylene biosynthesis genes could potentially play important roles in the ripening process of watermelon flesh (Zhou et al., 2016). Watermelons are also sensitive to exogenous ethylene, which can promote maturation (Mao et al., 2004) and induce the appearance of the water-soaking phenomenon by inducing the expression of softening-related genes (Karakurt and Huber, 2004). Furthermore, exogenous ethylene treatment of fresh-cut watermelons can increase the respiration rate and decrease fruit quality (Saftner et al., 2007). These findings suggest that the development and ripening of non-climacteric fruits may be at least partially regulated by ethylene.
Several other phytohormones may also contribute to regulating the ripening process in non-climacteric fruits, as shown in Figure 4. Recent reports have revealed that abscisic acid (ABA) participates in the regulation of non-climacteric fruit ripening, including in grapes (Jia et al., 2017), strawberries (Jia et al., 2011), citrus (Rodrigo et al., 2006; Wang et al., 2016), and watermelons (Wang et al., 2017). There are many examples of hormone crosstalk in plant growth regulation. For example, ethylene and ABA interact to induce flowering in Pharbitis nil (Arc et al., 2013), mediate the effects of soil compaction on shoot growth (Hussain et al., 2000), induce hyponastic growth in A. thaliana (Benschop et al., 2007), and regulate climacteric fruit ripening (Qiao et al., 2021). These studies have shown that the relationship between ABA and ethylene is antagonistic. Generally, the ripening of climacteric fruits is controlled by ethylene, while non-climacteric fruit ripening is mainly regulated by ABA (Bai et al., 2021). However, several studies have reported that ethylene also participates in non-climacteric fruit ripening by interacting with ABA. In post-harvest strawberries, ethylene facilitates ABA accumulation in receptacle tissue (Tosetti et al., 2020). In grapes, functional interaction and synergism between ABA and ethylene at the onset of ripening have been observed. Endogenous ethylene induces the transcription of VvNCED1 and the synthesis of ABA, and both ethylene and ABA are likely to be important and required to initiate the process of berry ripening (Sun et al., 2010). ABA treatment can stimulate ethylene production in strawberries (Jiang and Joyce, 2003). Thus, the interaction of ABA with ethylene appears to play a vital role in non-climacteric fruit ripening. Similar results from the effects of jasmonates (JAs) in the non-climacteric fruit ripening process have also been reported. JAs have been found to be involved in fruit ripening and to upregulate the phenylpropanoid pathway in strawberries (Concha et al., 2013; Delgado et al., 2018; Garrido-Bigotes et al., 2018) and grapes (Liu et al., 2011). Moreover, JA-activated fruit ripening is possibly associated with the stimulation of ethylene biosynthesis via an increase in ACO and ACS activities (Mukkun and Singh, 2009). These results suggest that JA is involved in strawberry fruit ripening in an ethylene-dependent manner.
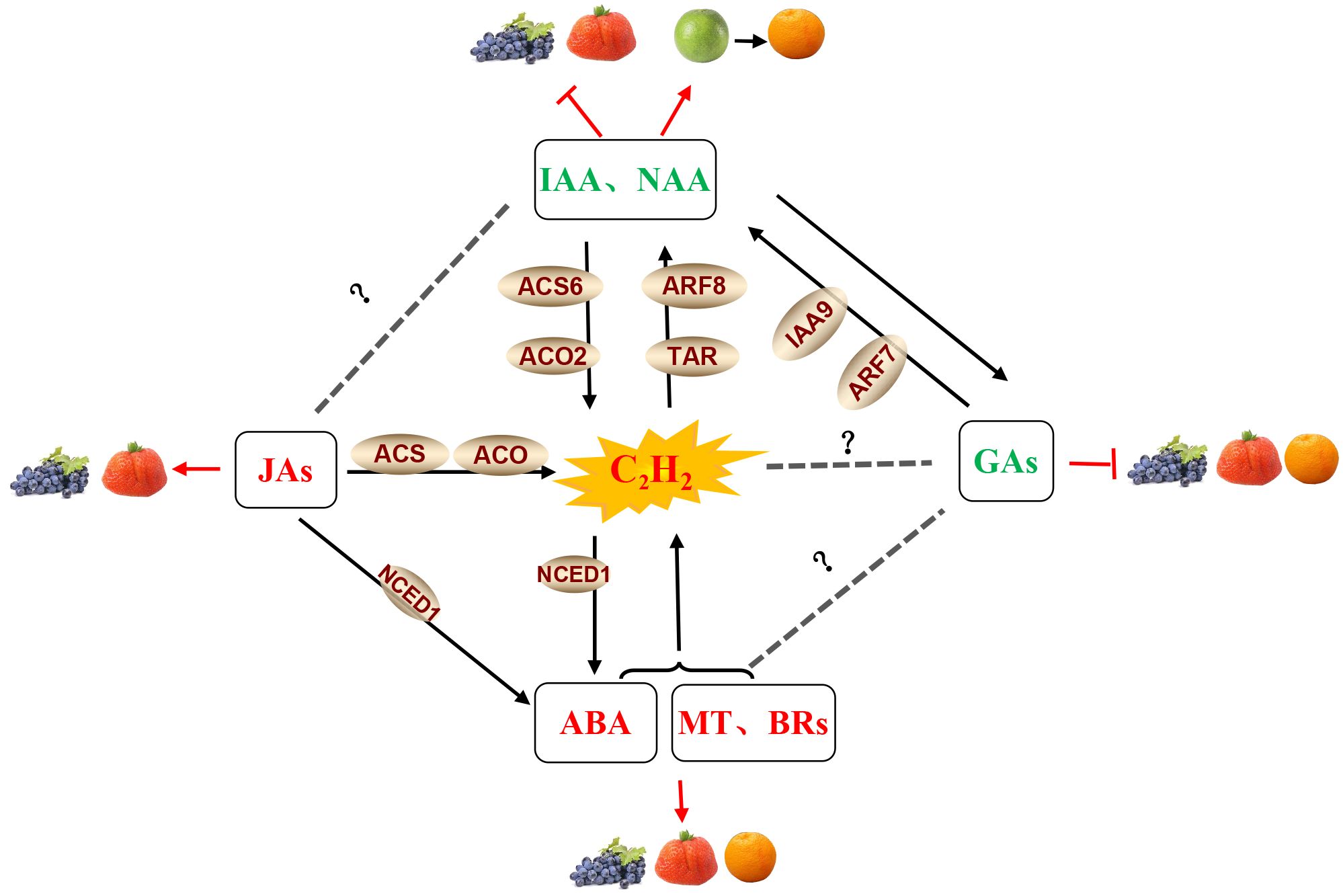
Figure 4 Phytohormone crosstalk between ethylene and other hormones in grape, strawberry, and citrus fruits. Note: The hormones and their related components involved in fruit ripening shown in the figure are abscisic acid (ABA), auxin (IAA),1-naphthaleneacetic acid (NAA), ethylene (C2H4), brassinosteroids (BRs), gibberellins (GAs), melatonin (MT) and jasmonates (JAs), tryptophan aminotransferase (TAR), auxin response factor (ARF), an auxin/indole-3-acetic acid (Aux/IAA) protein (IAA9), 9-cis-epoxycarotenoid dioxygenase 1 (NCED1), 1-aminocyclopropane-1-carboxylic acid synthase (ACS), and 1-aminocyclopropane-1-carboxylic acid oxidase (ACO). JA, ABA, BRs, and MT in the red fond have a positive effect on fruit ripening (Davies et al., 2006; Sun et al., 2010; Liu et al., 2011; Concha et al., 2013; Delgado et al., 2018; Garrido-Bigotes et al., 2018; Mansouri et al., 2021; Xia et al., 2021); Gas and IAA/NAA in the green fond had a negative effect on fruit ripening (Böttcher et al., 2011; Liu et al., 2011; Ma et al., 2021a, b; Tyagi et al., 2022); and jasmonate-activated fruit ripening is possibly associated with the stimulation of ethylene biosynthesis by an increase in ACO and ACS activities (Mukkun and Singh, 2009), while ABA, BRs, and MT can also stimulate ethylene production (Jiang and Joyce, 2003; Xu et al., 2018). C2H4 and IAA/NAA have a mutually reinforcing relationship. NAA can strongly upregulate ACS6 and ACO2 to improve ethylene biosynthesis (Ziliotto et al., 2012). In turn, the elevated concentrations of ethylene may lead to the induction of TAR expression, thus increasing the production of IAA (Böttcher et al., 2013). Ethylene also induces the transcription of VvNCED1 and the synthesis of ABA (Sun et al., 2010). The crosstalk between JAs and ABA, as well as GAs and IAA/NAA, also exists in non-climacteric fruit (Jung et al., 2014; Wang et al., 2015).
It is well known that ripening can be delayed by the application of auxins to grapes before véraison (Böttcher et al., 2011), as well as strawberries (Liu et al., 2011). Auxin and 1-naphthaleneacetic acid (NAA) can induce carotenoid accumulation in citrus fruits (Ma et al., 2021a, b). Elevated concentrations of ethylene prior to the initiation of ripening may induce the expression of the auxin biosynthesis-related gene TAR, thus increasing the production of indole-3-acetic acid (IAA) during grape berry ripening (Böttcher et al., 2013). In addition, pre-véraison NAA treatment strongly upregulated the expression of the ACS6 and ACO2 genes (Ziliotto et al., 2012), indicating the existence of crosstalk between ethylene and auxin in non-climacteric fruit (Figure 4). Meanwhile, an antagonistic relationship between auxin and gibberellic acid (GA) has also been reported. Treatment with GA at the pre-bloom stage decreases the expression of VvIAA9 and VvARF7 and partially activates auxin signaling in grapes (Jung et al., 2014). Exogenous application of the auxin analog 4-chlorophenoxyacetic acid (4-CPA) to grapes promoted the biosynthesis of GA3 (Lu et al., 2016). These results suggest interactions between GAs and auxin signaling in non-climacteric fruit (Figure 4). Additionally, GA treatment of grapes delayed sugar accumulation, acid degradation, and color development (Tyagi et al., 2022) and also delayed the color break and harvest date (Alós et al., 2006; Fujii et al., 2008). As such, the effect of GA on delayed ripening is possibly associated with the activation of auxin signaling. However, our knowledge about the interactions of gibberellins with ethylene and ABA in the ripening process needs to be deciphered. Moreover, previous studies reported that the application of brassinosteroids (BRs) and melatonin to grapes and strawberries significantly promoted fruit ripening (Davies et al., 2006; Mansouri et al., 2021; Xia et al., 2021). An antagonistic interaction between melatonin and ethylene has been reported, and melatonin application coordinates with ethylene biosynthesis to regulate grape ripening (Xu et al., 2018). Similarly, the application of brassinolides (BRs) is known to induce fruit ripening in some climacteric fruits (Zhu et al., 2015; Liu et al., 2022), and as such, it will be important to investigate the relationship between the BRs and ethylene biosynthesis in non-climacteric fruits. Meantime, further research should be conducted to clarify such multi-hormonal regulatory mechanisms in relation to the ripening and softening of non-climacteric fruits.
There have been considerable advances in our understanding of the important role of ethylene in regulating the ripening and softening of non-climacteric fruits. It appears that endogenous and exogenous ethylene mediates changes in numerous ripening- and softening-related qualities, such as color, texture, aroma, and flavor. An array of genes associated with the ethylene signaling pathway have been found to regulate the ripening and softening of non-climacteric fruits. In addition, the antagonistic interaction between multiple hormones appears to be associated with the ripening and softening of non-climacteric fruits. These findings clearly show that ethylene biosynthesis and signal transduction are regulated in a variety of ways, many of which are linked with the processes of ripening and softening, in non-climacteric fruits.
Because many of the regulators of fruit ripening and softening are shared among both climacteric and non-climacteric fruits, the comprehensive and detailed molecular regulatory mechanism for the regulation of ethylene signaling in the ripening and softening of non-climacteric fruit remains to be further explored. We suggest the following as important research priorities and questions requiring answers:
● Certain transcription factors play a role upstream of the ethylene biosynthesis pathway and participate in ethylene biosynthesis. However, little is known about the regulatory relationships between these transcription factors, including MADS-RIN, NAC, and ERFs, and the ACS and ACO genes in non-climacteric fruits.
● The structures and protein phosphorylation mechanisms of ethylene receptor genes involved in the ripening and softening of non-climacteric fruits require validation.
● With the exception of certain ethylene biosynthesis genes and ethylene receptors, the expression patterns and functions of other ripening- and softening-associated transcription factors, such as RAN1, EIN2, EIN3, and EBF, should be characterized in non-climacteric fruits.
● Ethylene exhibits complex interactions with other phytohormones, including ABA and IAA. Research should be conducted to better understand how these phytohormones jointly regulate the ripening process in non-climacteric fruits.
● Abiotic stressors, such as drought, cold injury, high temperature, and other environmental factors, play an important role in regulating the expression of ACS and ACO. However, the mechanisms underlying how these abiotic stressors regulate the ethylene biosynthesis in non-climacteric fruits remain to be explored.
● Establishing the complete transcriptional regulatory network underlying the ripening and softening of non-climacteric fruits, as well as exploring key regulatory components, will promote the development of preservation technologies for non-climacteric fruits using molecular biological approaches.
ML: Formal analysis, Writing – original draft, Data curation, Methodology. CW: Writing – review & editing. MS: Formal analysis, Methodology, Writing – original draft. HJ: Formal analysis, Methodology, Writing – original draft. TL: Data curation, Formal analysis, Writing – original draft. JW: Writing – review & editing. HC: Conceptualization, Supervision, Validation, Writing – review & editing, Formal analysis. QZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (Nos. 32272780 and 32072518).
We would like to thank TopEdit (www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aizat, W. M., Able, J. A., Stangoulis, J. C. R., Able, A. J. (2013). Proteomic analysis during capsicum ripening reveals differential expression of ACC oxidase isoform 4 and other candidates. Funct. Plant Biol. 40, 1115–1128. doi: 10.1071/FP12330
Alexander, L., Grierson, D. (2002). Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J. Exp. Bot. 53, 2039–2055. doi: 10.1093/jxb/erf072
Alós, E., Cercós, M., Rodrigo, M. J., Zacarías, L., Talón, M. (2006). Regulation of color break in citrus fruits. Changes in pigment profiling and gene expression induced by gibberellins and nitrate, two ripening retardants. J. Agric. Food Chem. 54, 4888–4895. doi: 10.1021/jf0606712
Alós, E., Martinez-Fuentes, A., Reig, C., Mesejo, C., Rodrigo, M. J., Agustí, M., et al. (2017). Ethylene biosynthesis and perception during ripening of loquat fruit (Eriobotrya japonica Lindl.). J. Plant Physiol. 210, 64–71. doi: 10.1016/j.jplph.2016.12.008
Alós, E., Martinez-Fuentes, A., Reig, C., Mesejo, C., Zacarías, L., Agustí, M., et al. (2019). Involvement of ethylene in color changes and carotenoid biosynthesis in loquat fruit (Eriobotrya japonica Lindl. cv. Algerie). Postharvest Biol. Technol. 149, 129–138. doi: 10.1016/j.postharvbio.2018.11.022
Arc, E., Sechet, J., Corbineau, F., Rajjou, L., Marion-Poll, A. (2013). ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front. Plant Sci. 4. doi: 10.3389/fpls.2013.00063
Bai, Q., Huang, Y., Shen, Y. (2021). The physiological and molecular mechanism of abscisic acid in regulation of fleshy fruit ripening. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.619953
Behboodian, B., Mohd Ali, Z., Ismail, I., Zainal, Z. (2012). Postharvest analysis of lowland transgenic tomato fruits harboring hpRNAi-ACO1 construct. Sci. World J. 2012, 439870. doi: 10.1100/2012/439870
Bellincontro, A., Fardelli, A., De Santis, D., Botondi, R., Mencarelli, F. (2006). Postharvest ethylene and 1-MCP treatments both affect phenols, anthocyanins, and aromatic quality of Aleatico grapes and wine. Aust. J. Grape Wine Res. 12, 141–149. doi: 10.1111/j.1755-0238.2006.tb00054.x
Benschop, J. J., Millenaar, F. F., Smeets, M. E., Van Zanten, M., Voesenek, L. A. C. J., Peeters, A. J. M. (2007). Abscisic acid antagonizes ethylene-induced hyponastic growth in Arabidopsis. Plant Physiol. 143, 1013–1023. doi: 10.1104/pp.106.092700
Bernales, M., Monsalve, L., Ayala-Raso, A., Valdenegro, M., Martínez, J. P., Travisany, D., et al. (2019). Expression of two indole-3-acetic acid (IAA)-amido synthetase (GH3) genes during fruit development of raspberry (Rubus idaeus Heritage). Sci. Hortic. (Amsterdam). 246, 168–175. doi: 10.1016/j.scienta.2018.09.077
Bessis, R., Charpentier, N., Hilt, C., Fournioux, J. C. (2000). Grapevine fruit set: Physiology of the abscission zone. Aust. J. Grape Wine Res. 6, 125–130. doi: 10.1111/j.1755-0238.2000.tb00170.x
Binder, B. M., Rodríguez, F. I., Bleecker, A. B. (2010). The copper transporter RAN1 is essential for biogenesis of ethylene receptors in Arabidopsis. J. Biol. Chem. 285, 37263–37270. doi: 10.1074/jbc.M110.170027
Bleecker, A. B., Kende, H. (2000). Ethylene: a gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 16, 1–18. doi: 10.1146/annurev.cellbio.16.1.1
Böttcher, C., Burbidge, C. A., Boss, P. K., Davies, C. (2013). Interactions between ethylene and auxin are crucial to the control of grape (Vitis vinifera L.) berry ripening. BMC Plant Biol. 13, 222. doi: 10.1186/1471-2229-13-222
Böttcher, C., Harvey, K., Forde, C. G., Boss, P. K., Davies, C. (2011). Auxin treatment of pre-veraison grape (Vitis vinifera L.) berries both delays ripening and increases the synchronicity of sugar accumulation. Aust. J. Grape Wine Res. 17, 1–8. doi: 10.1111/j.1755-0238.2010.00110.x
Bürstenbinder, K., Rzewuski, G., Wirtz, M., Hell, R., Sauteret, M. (2007). The role of methionine recycling for ethylene synthesis in Arabidopsis. T. P. J. 49, 238–249. doi: 10.1111/j.1365-313X.2006.02942.x
Cai, C., Xu, C., Li, X., Ferguson, I., Chen, K. (2006). Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol. Technol. 40, 163–169. doi: 10.1016/j.postharvbio.2005.12.009
Cancel, J. D., Larsen, P. B. (2002). Loss-of-function mutations in the ethylene receptor ETR1 cause enhanced sensitivity and exaggerated response to ethylene in Arabidopsis. Plant Physiol. 129, 1557–1567. doi: 10.1104/pp.003780
Chen, Y., Grimplet, J., David, K., Castellarin, S. D., Terol, J., Wong, D. C. J., et al. (2018). Ethylene receptors and related proteins in climacteric and non-climacteric fruits. Plant Sci. 276, 63–72. doi: 10.1016/j.plantsci.2018.07.012
Cheng, C., Liu, J., Wang, X., Wang, Y., Yuan, Y., Yang, S. (2022). PpERF/ABR1 functions as an activator to regulate PpPG expression resulting in fruit softening during storage in peach (Prunus persica). Postharvest Biol. Technol. 189, 111919. doi: 10.1016/j.postharvbio.2022.111919
Cherian, S., Figueroa, C. R., Nair, H. (2014). “Movers and shakers” in the regulation of fruit ripening: A cross-dissection of climacteric versus non-climacteric fruit. J. Exp. Bot. 65, 4705–4722. doi: 10.1093/jxb/eru280
Chervin, C., Deluc, L. (2010). Ethylene signalling receptors and transcription factors over the grape berry development: Gene expression profiling. Vitis - J. Grapevine Res. 49, 129–136. doi: 10.1016/j.ssi.2010.01.014
Chervin, C., El-Kereamy, A., Roustan, J. P., Latché, A., Lamon, J., Bouzayen, M. (2004). Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci. 167, 1301–1305. doi: 10.1016/j.plantsci.2004.06.026
Chervin, C., Terrier, N., Ageorges, A., Ribes, F., Kuapunyakoon, T. (2006). Influence of ethylene on sucrose accumulation in grape berry. Am. J. Enol. Vitic. 57, 511–513. doi: 10.5344/ajev.2006.57.4.511
Chervin, C., Tira-Umphon, A., Terrier, N., Zouine, M., Severac, D., Roustan, J. P. (2008). Stimulation of the grape berry expansion by ethylene and effects on related gene transcripts, over the ripening phase. Physiol. Plant 134, 534–546. doi: 10.1111/j.1399-3054.2008.01158.x
Concha, C. M., Figueroa, N. E., Poblete, L. A., Oñate, F. A., Schwab, W., Figueroa, C. R. (2013). Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit. Plant Physiol. Biochem. 70, 433–444. doi: 10.1016/j.plaphy.2013.06.008
Cramer, G. R., Ghan, R., Schlauch, K. A., Tillett, R. L., Heymann, H., Ferrarini, A., et al. (2014). Transcriptomic analysis of the late stages of grapevine (Vitis vinifera cv. Cabernet Sauvignon) berry ripening reveals significant induction of ethylene signaling and flavor pathways in the skin. BMC Plant Biol. 14, 1–21. doi: 10.1186/s12870-014-0370-8
Davies, C., Reid, J. B., Dry, I. B., Symons, G. M., Shavrukov, Y., Thomas, M. R. (2006). Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol. 140, 150–158. doi: 10.1104/pp.105.070706.150
Delgado, L. D., Zúñiga, P. E., Figueroa, N. E., Pastene, E., Escobar-Sepúlveda, H. F., Figueroa, P. M., et al. (2018). Application of a JA-Ile biosynthesis inhibitor to methyl jasmonate-treated strawberry fruit induces upregulation of specific MBW complex-related genes and accumulation of proanthocyanidins. Molecules 23, 1433. doi: 10.3390/molecules23061433
Deluc, L. G., Grimplet, J., Wheatley, M. D., Tillett, R. L., Quilici, D. R., Osborne, C., et al. (2007). Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genomics 8, 1–42. doi: 10.1186/1471-2164-8-429
Ding, Y., Chang, J., Ma, Q., Chen, L., Liu, S., Jin, S., et al. (2015). Network analysis of postharvest senescence process in citrus fruits revealed by transcriptomic and metabolomic profiling. Plant Physiol. 168, 357–376. doi: 10.1104/pp.114.255711
Distefano, G., Giuseppina, L. C., Caruso, M., Todaro, A., Rapisarda, P., Malfa, S. L. A., et al. (2009). Physiological and molecular analysis of the maturation process in fruits of clementine mandarin and one of its late-ripening mutants. J. Agric. Food Chem. 57, 7974–7982. doi: 10.1021/jf900710v
Dolgikh, V. A., Pukhovaya, E. M., Zemlyanskaya, E. V. (2019). Shaping ethylene response: the role of ein3/eil1 transcription factors. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01030
Dubois, M., Van den Broeck, L., Inzé, D. (2018). The pivotal role of ethylene in plant growth. Trends Plant Sci. 23, 311–323. doi: 10.1016/j.tplants.2018.01.003
El-Kereamy, A., Chervin, C., Roustan, J. P., Cheynier, V., Souquet, J. M., Moutounet, M., et al. (2003). Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiol. Plant 119, 175–182. doi: 10.1034/j.1399-3054.2003.00165.x
Elmi, F., Pradas, I., Tosetti, R., Cools, K., Terry, L. A. (2017). Effect of ethylene on postharvest strawberry fruit tissue biochemistry. Acta Hortic. 1156, 667–672. doi: 10.17660/ActaHortic.2017.1156.97
Fauziah, T. N., Bintoro, N. (2021). The effect of different concentrations and exposure durations of ethylene gas on peel colour change of citrus (Citrus nobilis) in the de-greening process. IOP Conf. Ser. Earth Environ. Sci. 828, 012041. doi: 10.1088/1755-1315/828/1/012041
Fenn, M. A., Giovannoni, J. J. (2021). Phytohormones in fruit development and maturation. Plant J. 105, 446–458. doi: 10.1111/tpj.15112
Figueroa, N. E., Gatica-Meléndez, C., Figueroa, C. R. (2021). Ethylene application at the immature stage of Fragaria chiloensis fruit represses the anthocyanin biosynthesis with a concomitant accumulation of lignin. Food Chem. 358, 129913. doi: 10.1016/j.foodchem.2021.129913
Fuentes, L., Monsalve, L., Morales-Quintana, L., Valdenegro, M., Martínez, J. P., Defilippi, B. G., et al. (2015). Differential expression of ethylene biosynthesis genes in drupelets and receptacle of raspberry (Rubus idaeus). J. Plant Physiol. 179, 100–105. doi: 10.1016/j.jplph.2015.02.005
Fujii, H., Shimada, T., Sugiyama, A., Endo, T., Nishikawa, F., Nakano, M., et al. (2008). Profiling gibberellin (GA3)-responsive genes in mature mandarin fruit using a citrus 22K oligoarray. Sci. Hortic. (Amsterdam). 116, 291–298. doi: 10.1016/j.scienta.2008.01.010
Fujimoto, S. Y., Ohta, M., Usui, A., Shinshi, H., Ohme-Takagi, M. (2000). Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box–mediated gene expression. Plant Cell 12, 393–404. doi: 10.1105/tpc.12.3.393
Garrido-Bigotes, A., Figueroa, P. M., Figueroa, C. R. (2018). Jasmonate metabolism and its relationship with abscisic acid during strawberry fruit development and ripening. J. Plant Growth Regul. 37, 101–113. doi: 10.1007/s00344-017-9710-x
Giovannoni, J. J. (2004). Genetic regulation of fruit development and ripening. Plant Cell 16, S170–S181. doi: 10.1105/tpc.019158
Glick, B. R. (2003). Transgenic plants with altered ethylene biosynthesis or perception. Biotechnol. Adv. 21, 193–210. doi: 10.1016/S0734-9750(03)00024-7
Gong, Y., Fan, X., Mattheis, J. P. (2002). Responses of “Bing” and “Rainier” sweet cherries to ethylene and 1-methylcyclopropene. J. Am. Soc Hortic. Sci. 127, 831–835. doi: 10.21273/JASHS.127.5.831
Hall, A. E., Bleecker, A. B. (2003). Analysis of combinatorial loss-of-function mutants in the Arabidopsis ethylene receptors reveals that the ers1 etr1 double mutant has severe developmental defects that are EIN2 dependent. Plant Cell 15, 2032–2041. doi: 10.1105/tpc.013060
Huang, X., Zheng, L., Xie, R. (2021). Effect of pre-harvest application of ethephon on colouration and expression of ripening related genes in citrus fruit. J. Hortic. Sci. Biotechnol. 96, 514–526. doi: 10.1080/14620316.2020.1863160
Huang, Y., Li, H., Hutchison, C. E., Laskey, J., Kieber, J. J. (2003). Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J. 33, 221–233. doi: 10.1046/j.1365-313X.2003.01620.x
Hussain, A., Black, C. R., Taylor, I. B., Roberts, J. A. (2000). Does an antagonist relationship between ABA and ethylene mediate shoot growth when tomato (Lycopersicon esculentum Mill.) plants encounter compacted soil? Plant Cell Environ. 23, 1217–1226. doi: 10.1046/j.1365-3040.2000.00639.x
Jia, H., Xie, Z., Wang, C., Shangguan, L., Qian, N., Cui, M., et al. (2017). Abscisic acid, sucrose, and auxin coordinately regulate berry ripening process of the Fujiminori grape. Funct. Integr. Genomics 17, 441–457. doi: 10.1007/s10142-017-0546-z
Jia, H. F., Chai, Y. M., Li, C. L., Lu, D., Luo, J. J., Qin, L., et al. (2011). Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 157, 188–199. doi: 10.1104/pp.111.177311
Jiang, Y., Joyce, D. C. (2003). ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 39, 171–174. doi: 10.1023/A:1022539901044
Jiang, Y., Joyce, D. C., Terry, L. A. (2001). 1-Methylcyclopropene treatment affects strawberry fruit decay. Postharvest Biol. Technol. 23, 227–232. doi: 10.1016/S0925-5214(01)00123-5
Jiang, Y. M., Zhang, Z. Q., Joyce, D. C., Ketsa, S. (2002). Postharvest biology and handling of longan fruit (Dimocarpus longan Lour.). Postharvest Biol. Technol. 26, 241–252. doi: 10.1016/S0925-5214(02)00047-9
Jung, C. J., Hur, Y. Y., Yu, H. J., Noh, J. H., Park, K. S., Lee, H. J. (2014). Gibberellin application at pre-bloom in grapevines down-regulates the expressions of VvIAA9and VvARF7, negative regulators of fruit set initiation, during parthenocarpic fruit development. PloS One 9, e95634. doi: 10.1371/journal.pone.0095634
Karakurt, Y., Huber, D. J. (2004). Ethylene-induced gene expression, enzyme activities, and water soaking in immature and ripe watermelon (Citrullus lanatus) fruit. J. Plant Physiol. 161, 381–388. doi: 10.1078/0176-1617-01221
Karakurt, Y., Huber, D. J. (2008). Cloning and characterization of differentially expressed genes in ethylene-treated watermelon fruit. Postharvest Biol. Technol. 48, 372–377. doi: 10.1016/j.postharvbio.2007.09.002
Karakurt, Y., Tonguc, M., Ünlü, H. (2014). The molecular characterization and expression analyses of ethylene receptor genes from watermelon fruit. Turk. J. Bot. 38, 1123–1131. doi: 10.3906/bot-1405-29
Kashyap, K., Banu, S. (2019). Characterizing ethylene pathway genes during the development, ripening, and postharvest response in citrus reticulata blanco fruit pulp. Turk. J. Bot. 43, 173–184. doi: 10.3906/bot-1711-45
Katz, E., Lagunes, P. M., Riov, J., Weiss, D., Goldschmidt, E. E. (2004). Molecular and physiological evidence suggests the existence of a system II-like pathway of ethylene production in non-climacteric Citrus fruit. Planta 219, 243–252. doi: 10.1007/s00425-004-1228-3
Kou, X., Liu, C., Han, L., Wang, S., Xue, Z. (2016). NAC transcription factors play an important role in ethylene biosynthesis, reception and signaling of tomato fruit ripening. Mol. Genet. Genomics 291, 1205–1217. doi: 10.1007/s00438-016-1177-0
Kuang, J. F., Chen, J. Y., Luo, M., Wu, K. Q., Sun, W., Jiang, Y. M., et al. (2012). Histone deacetylase HD2 interacts with ERF1 and is involved in longan fruit senescence. J. Exp. Bot. 63, 441–454. doi: 10.1093/jxb/err290
Leida, C., Dal Rì, A., Dalla Costa, L., Gómez, M. D., Pompili, V., Sonego, P., et al. (2016). Insights into the role of the berry-specific ethylene responsive factor vviERF045. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01793
Li, S.-j., Xie, X.-l., Liu, S.-c., Chen, K.-s., Yin, X.-r. (2019). Auto- and mutual-regulation between two CitERFs contribute to ethylene-induced citrus fruit degreening. Food Chem. 299, 125163. doi: 10.1016/j.foodchem.2019.125163
Liguori, G., Barone, E., Farina, V., Inglese, P. (2015). 1-Methylcyclopropene delays ripening and improves postharvest quality of white flesh loquat. Acta Hortic. 1092, 153–158. doi: 10.17660/ActaHortic.2015.1092.23
Liu, D., Chen, J., Lu, W. (2011). Expression and regulation of the early auxin-responsive Aux/IAA genes during strawberry fruit development. Mol. Biol. Rep. 38, 1187–1193. doi: 10.1007/s11033-010-0216-x
Liu, M., Gomes, B. L., Mila, I., Purgatto, E., Peres, L. E. P., Frasse, P., et al. (2016). Comprehensive profiling of ethylene response factor expression identifies ripening-associated ERF genes and their link to key regulators of fruit ripening in tomato. Plant Physiol. 170, 1732–1744. doi: 10.1104/pp.15.01859
Liu, M., Pirrello, J., Chervin, C., Roustan, J.-P., Bouzayen, M. (2015). Ethylene control of fruit ripening: revisiting the complex network of transcriptional regulation. Plant Physiol. 169, 2380–2390. doi: 10.1104/pp.15.01361
Liu, X., Li, D., Li, Y., Li, S., Zhao, Z. (2022). Brassinosteroids are involved in volatile compounds biosynthesis related to mdbzr1 in ‘ruixue’ (malus x domestica borkh.) fruit. Postharvest Biol. Technol. 189, 111931. doi: 10.1016/j.postharvbio.2022.111931
Liu, Y., Zhang, S. (2004). Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in arabidopsis. Plant Cell 16, 3386–3399. doi: 10.1105/tpc.104.026609
Lopes, P. Z., Fornazzari, I. M., Almeida, A. T., Galvão, C. W., Ayub, R. A. (2015). Effect of ethylene treatment on phytochemical and ethylene-related gene expression during ripening in strawberry fruit Fragaria x ananassa cv. Camino real. Genet. Mol. Res. 14, 16113–16125. doi: 10.4238/2015.December.7.23
Lu, L., Liang, J., Zhu, X., Xiao, K., Li, T., Hu, J. (2016). Auxin- and cytokinin-induced berries set in grapevine partly rely on enhanced gibberellin biosynthesis. Tree Genet. Genomes 12, 41. doi: 10.1007/s11295-016-0980-4
Lyzenga, W. J., Stone, S. L. (2012). Regulation of ethylene biosynthesis through protein degradation. Plant Signal. Behav. 7, 1438–1442. doi: 10.4161/psb.21930
Ma, G., Zhang, L., Kudaka, R., Inaba, H., Furuya, T., Kitamura, M., et al. (2021a). Exogenous application of ABA and NAA alleviates the delayed coloring caused by puffing inhibitor in citrus fruit. Cells 10, 1–13. doi: 10.3390/cells10020308
Ma, G., Zhang, L., Kudaka, R., Inaba, H., Murakami, K., Yamamoto, M., et al. (2021b). Auxin induced carotenoid accumulation in GA and PDJ-treated citrus fruit after harvest. Postharvest Biol. Technol. 181, 111676. doi: 10.1016/j.postharvbio.2021.111676
Ma, W., Xu, L., Gao, S., Lyu, X., Cao, X., Yao, Y. (2021c). Melatonin alters the secondary metabolite profile of grape berry skin by promoting VvMYB14-mediated ethylene biosynthesis. Hortic. Res. 8, 43. doi: 10.1038/s41438-021-00478-2
Mansouri, S., Sarikhani, H., Sayyari, M., Soleimani Aghdam, M. (2021). Melatonin accelerates strawberry fruit ripening by triggering GAMYB gene expression and promoting ABA accumulation. Sci. Hortic. (Amsterdam). 281, 109919. doi: 10.1016/j.scienta.2021.109919
Mao, L., Karakurt, Y., Huber, D. J. (2004). Incidence of water-soaking and phospholipid catabolism in ripe watermelon (Citrullus lanatus) fruit: Induction by ethylene and prophylactic effects of 1-methylcyclopropene. Postharvest Biol. Technol. 33, 1–9. doi: 10.1016/j.postharvbio.2003.12.007
Matsumoto, H., Ikoma, Y., Kato, M., Nakajima, N., Hasegawa, Y. (2009). Effect of postharvest temperature and ethylene on carotenoid accumulation in the flavedo and juice sacs of satsuma mandarin (Citrus unshiu Marc.) Fruit. J. Agric. Food Chem. 57, 4724–4732. doi: 10.1021/jf9005998
Mayuoni, L., Sharabi-Schwager, M., Feldmesser, E., Porat, R. (2011). Effects of ethylene degreening on the transcriptome of mandarin flesh. Postharvest Biol. Technol. 60, 75–82. doi: 10.1016/j.postharvbio.2010.11.009
Merchante, C., Vallarino, J. G., Osorio, S., Aragüez, I., Villarreal, N., Ariza, M. T., et al. (2013). Ethylene is involved in strawberry fruit ripening in an organ-specific manner. J. Exp. Bot. 64, 4421–4439. doi: 10.1093/jxb/ert257
Mozetič, B., Simčič, M., Trebše, P. (2006). Anthocyanins and hydroxycinnamic acids of Lambert Compact cherries (Prunus avium L.) after cold storage and 1-methylcyclopropene treatment. Food Chem. 97, 302–309. doi: 10.1016/j.foodchem.2005.04.018
Mukkun, L., Singh, Z. (2009). Methyl jasmonate plays a role in fruit ripening of “Pajaro” strawberry through stimulation of ethylene biosynthesis. Sci. Hortic. (Amsterdam). 123, 5–10. doi: 10.1016/j.scienta.2009.07.006
Mullins, E. D., Mccollum, T. G., Mcdonald, R. E. (2010). Ethylene: a regulator of stress-induced acc synthase activity in nonclimacteric fruit. Physiol. Plant. 107, 1–7. doi: 10.1034/j.1399-3054.1999.100101.x
Muñoz-Robredo, P., Gudenschwager, O., Chervin, C., Campos-Vargas, R., González-Agüero, M., Defilippi, B. G. (2013). Study on differential expression of 1-aminocyclopropane-1-carboxylic acid oxidase genes in table grape cv. Thompson Seedless. Postharvest Biol. Technol. 76, 163–169. doi: 10.1016/j.postharvbio.2012.10.006
Olmedo, G., Guo, H., Gregory, B. D., Nourizadeh, S. D., Aguilar-Henonin, L., Li, H., et al. (2006). ETHYLENE-INSENSITIVE5 encodes a 5′→3′ exoribonuclease required for regulation of the EIN3-targeting F-box proteins EBF1/2. Proc. Natl. Acad. Sci. U. S. A. 103, 13286–13293. doi: 10.1073/pnas.0605528103
Ortiz, C. M., Franceschinis, C., Gergoff Grozeff, E. G., Chan, H. L., Labavitch, J. M., Crisosto, C., et al. (2018). Pre-treatment with 1-methylcyclopropene alleviates methyl bromide-induced internal breakdown, softening and wall degradation in blueberry. Postharvest Biol. Technol. 146, 90–98. doi: 10.1016/j.postharvbio.2018.08.018
Osorio, S., Alba, R., Nikoloski, Z., Kochevenko, A., Giovannoni, F. J. J. (2012). Integrative comparative analyses of transcript and metabolite profiles from pepper and tomato ripening and development stages uncovers species-specific patterns of network regulatory behavior. Plant Physiol. 159, 1713–1729. doi: 10.1104/pp.112.199711
Pattyn, J., John, V. H., Poel, B. V. D. (2021). The regulation of ethylene biosynthesis: a complex multilevel control circuitry. New Phytol. 229, 770–782. doi: 10.1111/nph.16873
Paul, B. L. (2015). Mechanisms of ethylene biosynthesis and response in plants. Essays Biochem. 58, 61–70. doi: 10.1042/bse0580061
Paul, V., Pandey, R., Srivastava, G. C. (2012). The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene-An overview. J. Food Sci. Technol. 49, 1–21. doi: 10.1007/s13197-011-0293-4
Pech, J. C., Purgatto, E., Bouzayen, M., Latché, A. (2012). Ethylene and fruit ripening. Plant Horm. Ethyl. 44, 275–304. doi: 10.1002/9781118223086.ch11
Pilati, S., Perazzolli, M., Malossini, A., Cestaro, A., Demattè, L., Fontana, P., et al. (2007). Genome-wide transcriptional analysis of grapevine berry ripening reveals a set of genes similarly modulated during three seasons and the occurrence of an oxidative burst at vèraison. BMC Genomics 8, 1–22. doi: 10.1186/1471-2164-8-428
Potuschak, T., Lechner, E., Parmentier, Y., Yanagisawa, S., Grava, S., Koncz, C., et al. (2003). EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115, 679–689. doi: 10.1016/S0092-8674(03)00968-1
Qian, M., Baoju, W., Xiangpeng, L., Xin, S., Lingfei, S., Haifeng, J., et al. (2016). Comparison and verification of the genes involved in ethylene biosynthesis and signaling in apple, grape, peach, pear and strawberry. Acta Physiol. Plant 38, 1–22. doi: 10.1007/s11738-016-2067-0
Qiao, H., Chang, K. N., Yazaki, J., Ecker, J. R. (2009). Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in. Arabidopsis. Genes Dev. 23, 512–521. doi: 10.1101/gad.1765709
Qiao, H., Zhang, H., Wang, Z., Shen, Y. (2021). Fig fruit ripening is regulated by the interaction between ethylene and abscisic acid. J. Integr. Plant Biol. 63, 553–569. doi: 10.1111/jipb.13065
Qin, G., Wang, Y., Cao, B., Wang, W., Tian, S. (2012). Unraveling the regulatory network of the MADS box transcription factor RIN in fruit ripening. Plant J. 70, 243–255. doi: 10.1111/j.1365-313X.2011.04861.x
Reis, L., Forney, C. F., Jordan, M., Munro Pennell, K., Fillmore, S., Schemberger, M. O., et al. (2020). Metabolic profile of strawberry fruit ripened on the plant following treatment with an ethylene elicitor or inhibitor. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00995
Ren, J., Chen, P., Dai, S. J., Li, P., Li, Q., Ji, K., et al. (2011). Role of abscisic acid and ethylene in sweet cherry fruit maturation: molecular aspects. New Zeal. J. Crop Hortic. 39, 161–174. doi: 10.1080/01140671.2011.563424
Rodrigo, M. J., Alquezar, B., Zacarías, L. (2006). Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J. Exp. Bot. 57, 633–643. doi: 10.1093/jxb/erj048
Rodrigo, M. J., Zacarias, L. (2007). Effect of postharvest ethylene treatment on carotenoid accumulation and the expression of carotenoid biosynthetic genes in the flavedo of orange (Citrus sinensis L. Osbeck) fruit. Postharvest Biol. Technol. 43, 14–22. doi: 10.1016/j.postharvbio.2006.07.008
Saftner, R., Luo, Y., McEvoy, J., Abbott, J. A., Vinyard, B. (2007). Quality characteristics of fresh-cut watermelon slices from non-treated and 1-methylcyclopropene- and/or ethylene-treated whole fruit. Postharvest Biol. Technol. 44, 71–79. doi: 10.1016/j.postharvbio.2006.11.002
Schaller, G. E. (2012). Ethylene and the regulation of plant development. BMC Biol. 10, 9–11. doi: 10.1186/1741-7007-10-9
Selvarajah, S., Bauchot, A. D., John, P. (2001). Internal browning in cold-stored pineapples is suppressed by a postharvest application of 1-methylcyclopropene. Postharvest Biol. Technol. 23, 167–170. doi: 10.1016/S0925-5214(01)00099-0
Shakeel, S. N., Gao, Z., Amir, M., Chen, Y., Rai, M. I., Haq, N. U., et al. (2015). Ethylene regulates levels of ethylene receptor/CTR1 signaling complexes in arabidopsis thaliana. J. Biol. Chem. 290, 12415–12424. doi: 10.1074/jbc.M115.652503
Sharma, M., Jacob, J. K., Subramanian, J., Paliyath, G. (2010). Hexanal and 1-MCP treatments for enhancing the shelf life and quality of sweet cherry (Prunus avium L.). Sci. Hortic. (Amsterdam). 125, 239–247. doi: 10.1016/j.scienta.2010.03.020
Sharon-Asa, L., Shalit, M., Frydman, A., Bar, E., Holland, D., Or, E., et al. (2003). Citrus fruit flavor and aroma biosynthesis: Isolation, functional characterization, and developmental regulation of Cstps1, a key gene in the production of the sesquiterpene aroma compound valencene. Plant J. 36, 664–674. doi: 10.1046/j.1365-313X.2003.01910.x
Silva, R. S., Silva, S. M., Rocha, A., Dantas, R. L., Schunemann, A. P. P., Pereira, W. E. (2013). Influence of 1-MCP on berry drop and quality of “Isabel” grape. Acta Hortic. 1012, 509–514. doi: 10.17660/ActaHortic.2013.1012.67
Sivakumar, D., Korsten, L. (2010). Fruit quality and physiological responses of litchi cultivar McLean’s Red to 1-methylcyclopropene pre-treatment and controlled atmosphere storage conditions. LWT - Food Sci. Technol. 43, 942–948. doi: 10.1016/j.lwt.2010.02.001
Sun, J.-H., Luo, J.-J., Tian, L., Li, C.-L., Xing, Y., Shen, Y.-Y. (2013). New evidence for the role of ethylene in strawberry fruit ripening. J. Plant Growth Regul. 32, 461–470. doi: 10.1007/s00344-012-9312-6
Sun, L., Nasrullah, Ke, F., Nie, Z., Xu, J., Huang, X., et al. (2022). Genome-wide identification and transcript analysis during fruit ripening of ACS gene family in sweet orange (Citrus sinensis). Sci. Hortic. (Amsterdam). 294, 110786. doi: 10.1016/j.scienta.2021.110786
Sun, L., Zhang, M., Ren, J., Qi, J., Zhang, G., Leng, P. (2010). Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biol. 10, 257. doi: 10.1186/1471-2229-10-257
Tesniere, C., Pradal, M., El-Kereamy, A., Torregrosa, L., Chatelet, P., Roustan, J. P., et al. (2004). Involvement of ethylene signalling in a non-climacteric fruit: New elements regarding the regulation of ADH expression in grapevine. J. Exp. Bot. 55, 2235–2240. doi: 10.1093/jxb/erh244
Tieman, D. M., Taylor, M. G., Ciardi, J. A., Klee, H. J. (2000). The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc. Natl. Acad. Sci. 97, 5663–5668. doi: 10.1073/pnas.090550597
Tira-Umphon, A., Roustan, J. P., Chervin, C. (2007). The stimulation by ethylene of the UDP glucose-flavonoid 3-O-glucosyltransferase (UFGT) in grape tissues is independent from the MybA transcription factors. Vitis - J. Grapevine Res. 46, 210–211. doi: 10.5073/VITIS.2007.46.210-211
Tosetti, R., Elmi, F., Pradas, I., Cools, K., Terry, L. A. (2020). Continuous exposure to ethylene differentially affects senescence in receptacle and achene tissues in strawberry fruit. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00174
Trainotti, L., Pavanello, A., Casadoro, G. (2005). Different ethylene receptors show an increased expression during the ripening of strawberries: Does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? J. Exp. Bot. 56, 2037–2046. doi: 10.1093/jxb/eri202
Tsuchisaka, A., Theologis, A. (2004). Heterodimeric interactions among the 1-amino-cyclopropane-1-carboxylate synthase polypeptides encoded by the Arabidopsis gene family. Proc. Natl. Acad. Sci. U.S.A. 101, 2275–2280. doi: 10.1073/pnas.0308515101
Tyagi, K., Maoz, I., Lapidot, O., Kochanek, B., Butnaro, Y., Shlisel, M., et al. (2022). Effects of gibberellin and cytokinin on phenolic and volatile composition of Sangiovese grapes. Sci. Hortic. (Amsterdam). 295, 110860. doi: 10.1016/j.scienta.2021.110860
Villarreal, N. M., Bustamante, C. A., Civello, P. M., Martínez, G. A. (2010). Effect of ethylene and 1-mcp treatments on strawberry fruit ripening. J. Sci. Food Agric. 90, 683–689. doi: 10.1002/jsfa.3868
Villarreal, N. M., Marina, M., Nardi, C. F., Civello, P. M., Martínez, G. A. (2016). Novel insights of ethylene role in strawberry cell wall metabolism. Plant Sci. 252, 1–11. doi: 10.1016/j.plantsci.2016.06.018
Villarreal, N. M., Martínez, G. A., Civello, P. M. (2009). Influence of plant growth regulators on polygalacturonase expression in strawberry fruit. Plant Sci. 176, 749–757. doi: 10.1016/j.plantsci.2009.02.019
Wall, M. M., Nishijima, K. A., Keith, L. M. (2011). Influence of packaging on quality retention of longans (dimocarpus longan) under constant and fluctuating postharvest temperatures. Hortscience 46, 917–923. doi: 10.21273/HORTSCI.46.6.917
Wang, H., Huang, H., Huang, X. (2007). Differential effects of abscisic acid and ethylene on the fruit maturation of Litchi chinensis Sonn. Plant Growth Regul. 52, 189–198. doi: 10.1007/s10725-007-9189-8
Wang, L., Luo, Z., Li, J., Yang, M., Yan, J., Lu, H., et al. (2019). Morphological and quality characterization of grape berry and rachis in response to postharvest 1-methylcyclopropene and elevated oxygen and carbon dioxide atmospheres. Postharvest Biol. Technol. 153, 107–117. doi: 10.1016/j.postharvbio.2019.04.001
Wang, Y., Guo, S., Tian, S., Zhang, J., Ren, Y., Sun, H., et al. (2017). Abscisic acid pathway involved in the regulation of watermelon fruit ripening and quality trait evolution. PloS One 12, 1–20. doi: 10.1371/journal.pone.0179944
Wang, S. S., Takahashi, H., Saito, T., Okawa, K., Ohara, H., Shishido, M., et al. (2015). Jasmonate application influences endogenous abscisic acid, jasmonic acid and aroma volatiles in grapes infected by a pathogen (glomerella cingulata). Sci. Hortic. 192, 166–172. doi: 10.1016/j.scienta.2015.06.001
Wang, X., Yin, W., Wu, J., Chai, L., Yi, H. (2016). Effects of exogenous abscisic acid on the expression of citrus fruit ripening-related genes and fruit ripening. Sci. Hortic. (Amsterdam). 201, 175–183. doi: 10.1016/j.scienta.2015.12.024
Wang, P. P., Yu, A. S., Ji, X. L., Mu, Q., Haider, M. S., Wei, R. N., et al. (2022). Transcriptome and metabolite integrated analysis reveals that exogenous ethylene controls berry ripening processes in grapevine. Food Res. Int. 155, 111084. doi: 10.1016/j.foodres.2022.111084
Wang, S., Zhou, Q., Zhou, X., Wei, B., Ji, S. (2018). The effect of ethylene absorbent treatment on the softening of blueberry fruit. Food Chem. 246, 286–294. doi: 10.1016/j.foodchem.2017.11.004
Watanabe, M., Goto, R., Murakami, M., Komori, S., Suzuki, A. (2021). Interaction between ethylene and abscisic acid and maturation in highbush blueberry. Horticult. J. 90, 14–21. doi: 10.2503/hortj.UTD-210
Wen, X., Zhang, C., Ji, Y., Zhao, Q., He, W., An, F., et al. (2012). Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res. 22, 1613–1616. doi: 10.1038/cr.2012.145
Wu, J., Fu, L., Yi, H. (2016). Genome-wide identification of the transcription factors involved in citrus fruit ripening from the transcriptomes of a late-ripening sweet orange mutant and its wild type. PloS One 11, e0154330. doi: 10.1371/journal.pone.0154330
Wu, T., Liu, H. T., Zhao, G. P., Song, J. X., Wang, X. L., Yang, C. Q., et al. (2020). Jasmonate and ethylene-regulated ethylene response factor 22 promotes lanolin-induced anthocyanin biosynthesis in ‘zaosu’ pear (Pyrus bretschneideri Rehd.) fruit. Biomolecules 10, 1–16. doi: 10.3390/biom10020278
Wu, J., Xu, Z., Zhang, Y., Chai, L., Yi, H., Deng, X. (2014). An integrative analysis of the transcriptome and proteome of the pulp of a spontaneous late-ripening sweet orange mutant and its wild type improves our understanding of fruit ripening in citrus. J. Exp. Bot. 6), 1651–1671. doi: 10.1093/jxb/eru044
Xanthopoulou, A., Moysiadis, T., Bazakos, C., Karagiannis, E., Karamichali, I., Stamatakis, G., et al. (2022). The perennial fruit tree proteogenomics atlas: a spatial map of the sweet cherry proteome and transcriptome. T. P. J. 109, 1319–1336. doi: 10.1111/tpj.15612
Xia, H., Shen, Y., Deng, H., Wang, J., Lin, L., Deng, Q., et al. (2021). Melatonin application improves berry coloration, sucrose synthesis, and nutrient absorption in ‘Summer Black’ grape. Food Chem. 356, 129713. doi: 10.1016/j.foodchem.2021.129713
Xiao, Y. Y., Chen, J. Y., Kuang, J. F., Shan, W., Xie, H., Jiang, Y. M., et al. (2013). Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. J. Exp. Bot. 64, 2499–2510. doi: 10.1093/jxb/ert108
Xu, L., Yue, Q., Xiang, G., Bian, F., Yao, Y. (2018). Melatonin promotes ripening of grape berry via increasing the levels of ABA, H2O2, and particularly ethylene. Hortic. Res. 5, 41. doi: 10.1038/s41438-018-0045-y
Yang, Q., Wang, L., Li, F., Ma, J., Zhang, Z. (2011). Impact of 1-MCP on postharvest quality of sweet cherry during cold storage. Front. Agric. China 5, 631–636. doi: 10.1007/s11703-011-1110-6
Ye, X., Zheng, X., Zhai, D., Song, W., Tan, B., Li, J., et al. (2017). Expression patterns of ACS and ACO gene families and ethylene production in rachis and berry of grapes. HortScience 52, 413–422. doi: 10.21273/HORTSCI11050-16
Yoon, G. M., Kieber, J. J. (2013). 1-Aminocyclopropane-1-carboxylic acid as a signalling molecule in plants. AoB Plants 5, plt017. doi: 10.1093/aobpla/plt017
Zhang, A.-d., Hu, X., Kuang, S., Ge, H., Yin, X.-r., Chen, K.-s. (2016). Isolation, classification and transcription profiles of the Ethylene Response Factors (ERFs) in ripening kiwifruit. Sci. Hortic. (Amsterdam). 199, 209–215. doi: 10.1016/j.scienta.2015.12.055
Zhang, Z., Huang, J., Li, X. (2018). Transcript analyses of ethylene pathway genes during ripening of Chinese jujube fruit. J. Plant Physiol. 224–225, 1–10. doi: 10.1016/j.jplph.2018.03.004
Zhang, M., Yuan, B., Leng, P. (2009). The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 60, 1579–1588. doi: 10.1093/jxb/erp026
Zhijin, Z., Haiwen, Z., Ruidan, Q., Xue-Chen, W., Rongfeng, H. (2009). Transcriptional regulation of the ethylene response factor leerf2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol. 150, 365–377. doi: 10.1104/pp.109.135830
Zhou, M., Guo, S., Zhang, J., Zhang, H., Li, C., Tang, X., et al. (2016). Comparative dynamics of ethylene production and expression of the ACS and ACO genes in normal-ripening and non-ripening watermelon fruits. Acta Physiol. Plant 38, 228. doi: 10.1007/s11738-016-2248-x
Zhou, J. Y., Sun, C.-D., Zhang, L. L., Dai, X., Xu, C. J., Chen, K. S. (2010). Preferential accumulation of orange-colored carotenoids in Ponkan (Citrus reticulata) fruit peel following postharvest application of ethylene or ethephon. Sci. Hortic. (Amsterdam). 126, 229–235. doi: 10.1016/j.scienta.2010.07.019
Zhu, T., Tan, W. R., Deng, X. G., Zheng, T., Zhang, D. W., Lin, H. H. (2015). Effects of brassinosteroids on quality attributes and ethylene synthesis in postharvest tomato fruit. Postharvest Biol. Technol. 100, 196–204. doi: 10.1016/j.postharvbio.2014.09.016
Keywords: non-climacteric fruit, ripening, softening, ethylene biosynthesis, ethylene signal transduction
Citation: Liu M, Wang C, Ji H, Sun M, Liu T, Wang J, Cao H and Zhu Q (2024) Ethylene biosynthesis and signal transduction during ripening and softening in non-climacteric fruits: an overview. Front. Plant Sci. 15:1368692. doi: 10.3389/fpls.2024.1368692
Received: 11 January 2024; Accepted: 08 April 2024;
Published: 26 April 2024.
Edited by:
Hansheng Gong, Ludong University, ChinaReviewed by:
Hua Huang, Independent Researcher, Guangzhou, ChinaCopyright © 2024 Liu, Wang, Ji, Sun, Liu, Wang, Cao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Cao, aHVpNTIzMkAxNjMuY29t; Qinggang Zhu, cWluZ2dhbmd6aHVAbndhZnUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.