- 1International Genome Center, Jiangsu University, Zhenjiang, China
- 2School of Life Sciences, Jiangsu University, Zhenjiang, China
- 3Chinese Education Ministry’s Key Laboratory of Western Resources and Modern Biotechnology, Key Laboratory of Biotechnology Shaanxi Province, College of Life Sciences, Northwest University, Xi’an, Shaanxi, China
- 4College of Life Sciences, Nanjing University, Nanjing, Jiangsu, China
- 5Department of Plant Protection, College of Agriculture and Natural Resources, Razi University, Kermanshah, Iran
- 6Department of Plant and Microbial Biology, University of California, Berkeley, Berkeley, CA, United States
Plant Elicitor Peptides (Peps) induce plant immune responses and inhibit root growth through their receptors PEPR1 and PEPR2, two receptor-like kinases. In our study, we found a previously unknown function of Peps that enhance root hair growth in a PEPRs-independent manner. When we characterized the expression patterns of PROPEP genes, we found several gene promoters of PROPEP gene family were particularly active in root hairs. Furthermore, we observed that PROPEP2 is vital for root hair development, as disruption of PROPEP2 gene led to a significant reduction in root hair density and length. We also discovered that PROPEP2 regulates root hair formation via the modulation of CPC and GL2 expression, thereby influencing the cell-fate determination of root hairs. Additionally, calcium signaling appeared to be involved in PROPEP2/Pep2-induced root hair growth. These findings shed light on the function of Peps in root hair development.
1 Introduction
Plants have developed highly conserved innate immune systems to protect themselves from external pathogens. These pathogens contain specific molecular patterns called PAMPs that are recognized by cell surface receptors in plants and result in PTI (pattern-triggered immunity) (Boller and Felix, 2009; Macho and Zipfel, 2014). Additionally, plants have the capability to release specific molecules termed damage- or danger-associated molecular patterns (DAMPs) in response to pathogen attacks or injuries, and these molecules also play a regulatory role in plant immunity (Endo et al., 2014; Macho and Zipfel, 2014). In Arabidopsis, a well-documented example of DAMPs is the family of plant elicitor peptides (Peps), which originate from the C-terminal regions of precursor proteins known as PROPEPs (Huffaker et al., 2006; Huffaker et al., 2013). Arabidopsis genome harbors eight PROPEPs, and they are responsible for generating eight small Pep peptides in response to pathogen invasion and physical injury (Huffaker et al., 2006; Bartels et al., 2013; Huffaker et al., 2013; Bartels and Boller, 2015; Klauser et al., 2015). Peps are recognized by a pair of closely related receptors, PEPR1 and PEPR2, which subsequently initiate downstream signaling events (Yamaguchi et al., 2006; Yamaguchi et al., 2010). These events include the elevation of cytosolic Ca2+ levels, the generation of reactive oxygen species, the expression of defense-related genes, the formation of calluses, lignin deposition, and inhibition of root growth (Millet et al., 2010; Bartels et al., 2013; Beck et al., 2014; Ma et al., 2014; Jing et al., 2019; Jing et al., 2020; Jing et al., 2023).
Root hairs are specialized tubular structures that develop from root epidermal cells. The dynamic adjustments in root hair growth, length, density, and morphology have a significant impact on the root’s surface area that determines the efficiency of nutrient and water uptake by plants, interactions between plants and microorganisms, and the stability of plant anchorage (Grierson et al., 2014). In some plant species, such as rice, all epidermal cells can differentiate into root hairs in a random manner (Kim et al., 2006; Kim and Dolan, 2011; Tominaga-Wada et al., 2013). In other species, such as Arabidopsis, only specific short epidermal cells have the potential to become root hairs.
In the well-established model of root hair development in Arabidopsis, the fate of root hair cells is determined by the position of epidermal cells (Datta et al., 2011; Grierson et al., 2014). Epidermal cells located exclusively outside of two underlying cortical cells are designated to differentiate into root hairs. Conversely, those with only one underlying cortical cell become non-hair cells (Datta et al., 2011). This cell fate determination is regulated by multiple transcription factors (TFs). Notably, the TRANSPARENT TESTA GLABRA (TTG), GLABRA3 (GL3), ENHANCER OF GLABRA3 (EGL3), and WEREWOLF (WER) TFs are expressed in non-hair cells, forming the WER-GL3/EGL3-TTG complex (Galway et al., 1994; Di Cristina et al., 1996; Masucci et al., 1996; Bernhardt et al., 2003; Schiefelbein, 2003). This complex plays a positive role in regulating GLABRA2 (GL2), a central TF responsible for inhibiting root hair formation in non-hair cells (Di Cristina et al., 1996; Masucci et al., 1996; Datta et al., 2011). In contrast, the CAPRICE (CPC) and TRIPTYCHON (TRY) TFs promote the formation of root hairs by suppressing GL2 expression (Schiefelbein, 2003; Grierson et al., 2014).
In our endeavor to unravel the signaling pathway of Peps in plants, our previous work highlighted Pep1’s role in stimulating root hair development when externally applied (Jing et al., 2019). In the present investigation, we provide evidence that both exogenous Peps and the overexpression of endogenous PROPEPs consistently promote the growth of root hairs. PROPEP2 emerges as a critical regulator of root development, as the disruption of PROPEP2 results in a significant reduction in both root hair density and length. Furthermore, we delve into the mechanism by which PROPEP2 influences the determination of root hair cell fate through the modulation of CPC and GL2 expression. Simultaneously, PROPEP2 orchestrates calcium oscillations within root hair cells, directing the course of root hair development. These findings unveil a novel signaling pathway initiated by Pep/PROPEPs governing Arabidopsis root hair development.
2 Results
2.1 Regulation of root hair development by plant elicitor peptides
In previous investigations, we documented the immunomodulatory effects of Peps and their role in inhibiting root growth in Arabidopsis (Zheng et al., 2018; Jing et al., 2019; Shen et al., 2020). Additionally, seedlings treated with synthetic Pep1 or Pep2 caused intriguing root hair (RH)-related phenotypes. This led us to hypothesize that Peps might play a pivotal role in root hair development and growth. To scrutinize the impact of Peps on RH growth, we treated wild-type Col-0 (WT) seedlings with various synthetic Peps (Pep1-8) at 10 nM. Remarkably, all exogenous Peps significantly increased both the density and length of RH compared to controls (Figure 1). Notably, Pep1 and Pep2 nearly doubled both the number and length of RH (Figure 1). Furthermore, we engineered transgenic lines overexpressing each Pep precursor gene (PROPEPs) driven by the 35S promoter (Supplementary Figures 1–8). These overexpressed PROPEP lines exhibited higher RH density and longer RHs compared to the wild-type seedlings, mirroring the effects of exogenous Peps (Figure 2; Supplementary Figures 1–8). These results collectively suggested that both exogenous and endogenous Peps consistently promoted RH growth.
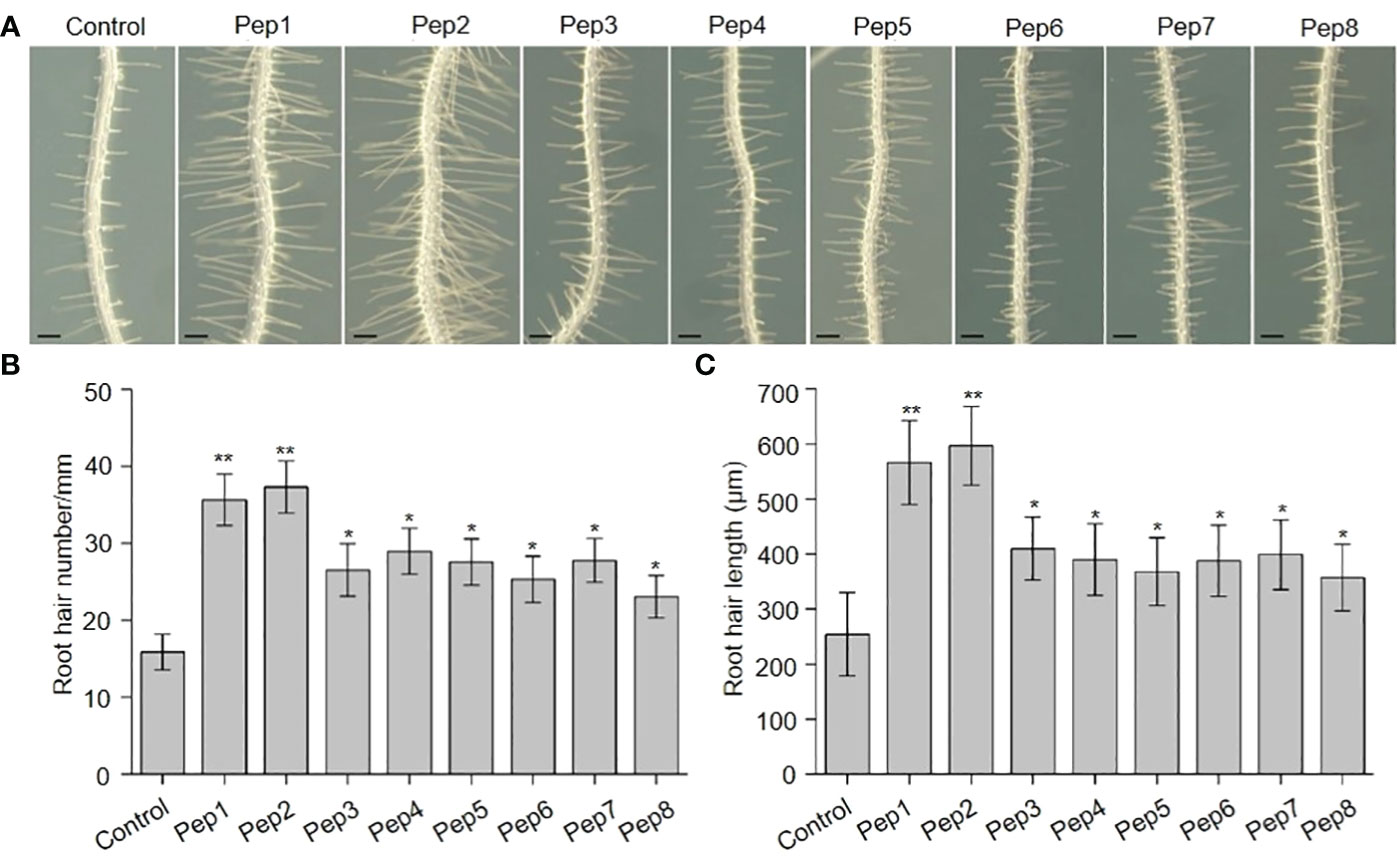
Figure 1 The effects of Peps on root hair development. (A) The growth phenotype of wild type root under Pep1-Pep8 treatment. Four-day-old WT plants were transplanted on half-strength Murashige and Skoog (MS) agar medium supplemented with or without 10 nM Pep1 to Pep8 for 48 h. Bars = 200 μm. (B, C) Statistics of the root hair number (B) and root hair length (C) as in (A). Data are means ± SD (n = 15 roots per treatment). Asterisks in (B, C) indicate statistically significant differences compared with the untreated control. (Tukey’s test; *p < 0.05, **p < 0.01).
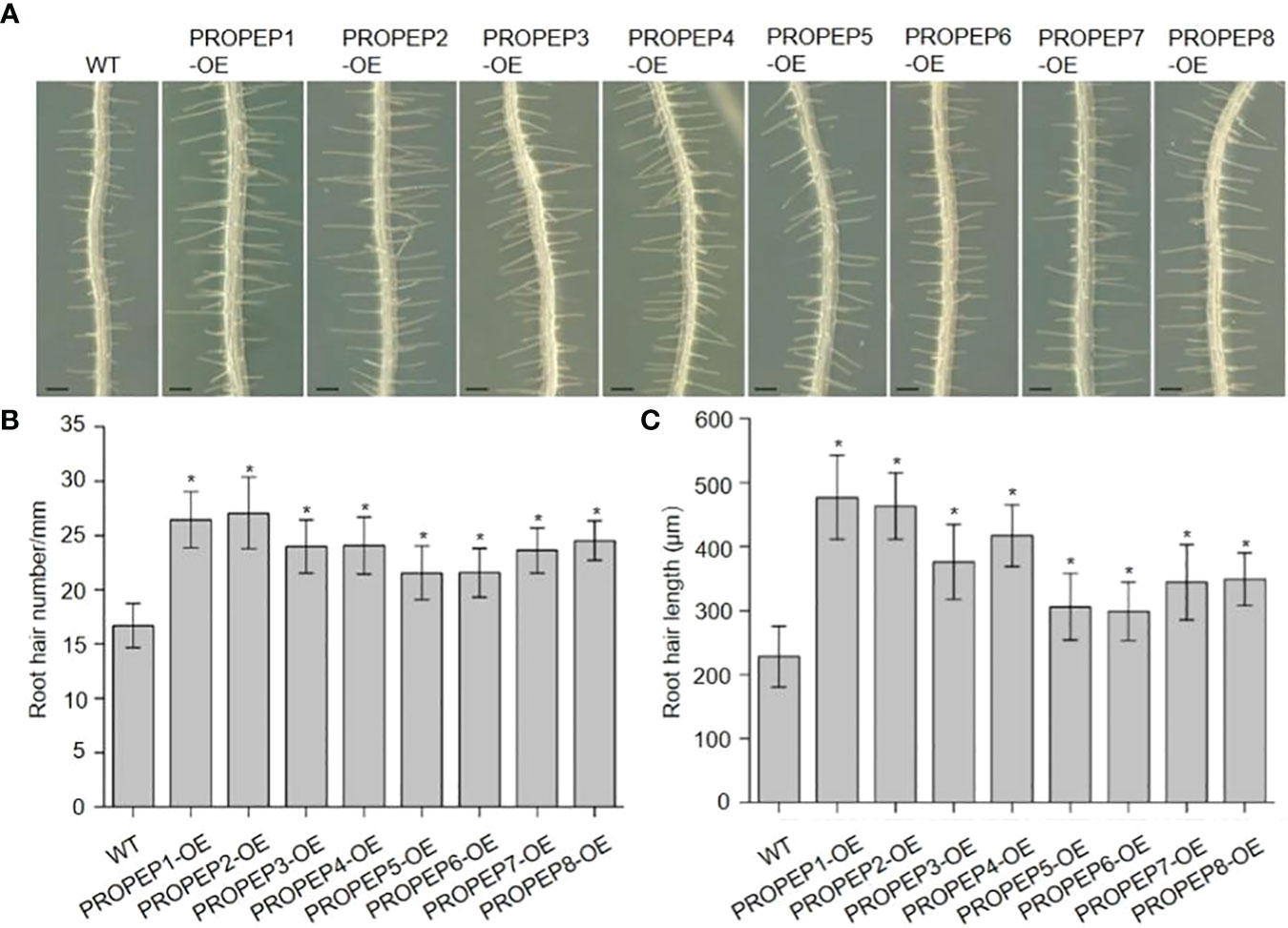
Figure 2 Over-expression of PROPEPs promote the root hair development. (A) The growth phenotype of root hair in wild type (WT)and wild type plants overexpressing PROPEPs (PROPEP1-OE to PROPEP8-OE). Four-day-old plants were transplanted on half-strength Murashige and Skoog (MS) agar medium for 48 h. Bars = 200 μm. (B, C) Statistics of the root hair number (B) and root hair length (C) as in (A). Data are means ± SD (n = 15 roots per treatment). Asterisks in (B, C) indicate statistically significant differences compared with the WT plants. (Tukey’s test; *p < 0.05).
To elucidate the expression patterns of PROPEP genes to identify those naturally expressed in the RH, we generated transgenic lines with putative PROPEP promoters fused to a β-glucuronidase (GUS) gene reporter. GUS staining of these transgenic seedlings revealed extensive expression of all PROPEPs in both shoots and systems (Figure 3A). In root tissues, GUS activity in proPROPEP3/4/5/7/8:GUS seedlings was primarily localized to vascular tissue, while the promoters of PROPEP1/2/6 exhibited activity throughout the entire root (Figure 3B). Notably, the promoters of PROPEP1/2/6 showed strong activity in root hair cells, implying their involvement in RH processes (Figure 3C). Additionally, we observed inducibility of PROPEP promoters by Peps, with GUS activity significantly enhanced in proPROPEP1/2/6:GUS plants upon exposure to Peps (Supplementary Figure 9).
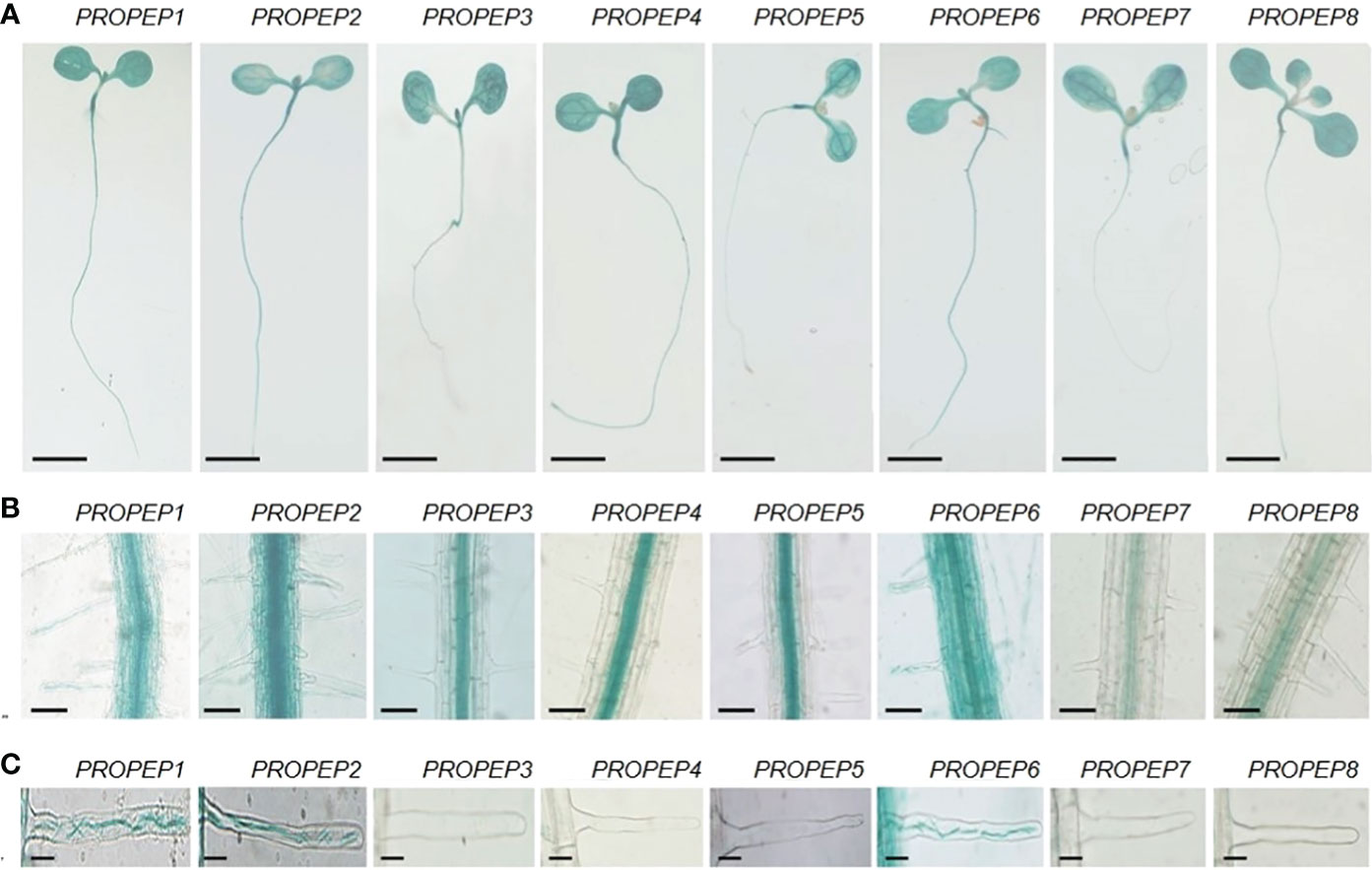
Figure 3 The tissue localization of PROPEPs. (A) Histochemical staining of GUS activity in 7-d-old transgenic plants harboring proPROPEP1:GUS (PROPEP1) to proPROPEP8:GUS (PROPEP8). Bars =2 mm. (B) The GUS activity in roots of transgenic plants PROPEP1 to PROPEP8. Bars =100 μm. (C) The GUS activity in root hairs of transgenic plants PROPEP1 to PROPEP8. Bars =25 μm.
To determine the subcellular localization of each Pep, we fused PROPEPs with the Green Fluorescent Protein gene (PROPEP-GFP) and transiently expressed them in Arabidopsis protoplasts. Despite the expected cytosolic localization based on function and the absence of a signal peptide, we unexpectedly observed cytosol-localized GFP signals exclusively in cells expressing PROPEP3-GFP or PROPEP5-GFP (Figure 4A). In contrast, PROPEP1, PROPEP2, PROPEP6, PROPEP7, and PROPEP8 were found to target the tonoplast, while the GFP signal of PROPEP4 overlapped with chloroplast fluorescence (Figure 4A). To validate the tonoplast localization of PROPEP1 and PROPEP2, which have been extensively studied, we conducted lipophilic FM4-64 staining associated with plasma membrane. The GFP and FM4-64 fluorescence signals did not overlap (Figure 4B), confirming that GFP-PROPEP signals was not localized to the PM but the tonoplast.
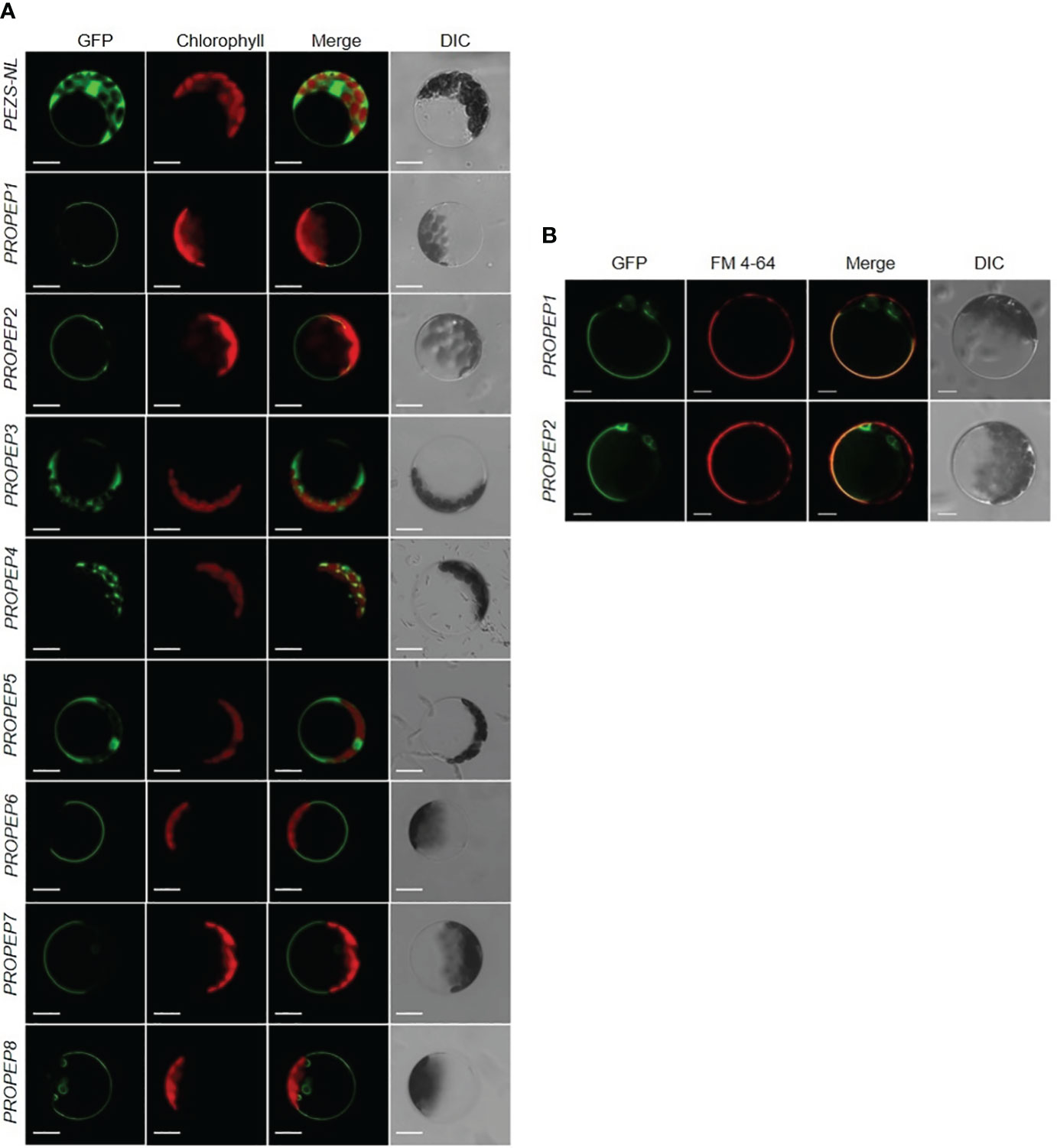
Figure 4 The subcellular localization assay of PROPEPs. (A) Arabidopsis mesophyll protoplasts were transiently transformed with a PEZS-NL vector expressed GFP signaling as the control. The coding sequence without the stop codon of PROPEP1 to PROPEP8 were cloned into pEZS-NL vector and transiently transformed into Arabidopsis mesophyll protoplasts. Columns from left to right show GFP signals (GFP), chlorophyll autofluorescence (Chlorophyll), merged images of GFP and chlorophyll (Merge), and bright-field differential interference contrast (DIC). Bars =5 μm. (B) FM 4-64 signaling was co-expressed with PROPEP1-GFP and PROPEP2-GFP in Arabidopsis protoplasts. The PROPEP1 and PROPEP2 fused GFP protein were transiently transformed into Arabidopsis mesophyll protoplasts and stained with 1 μM FM4-64 for 15 s before photographed. Columns from left to right show GFP signals (GFP), FM 4-64 fluorescence signals (FM 4-64), merged images of GFP and FM 4-64 (Merge), and bright-field differential interference contrast (DIC). Bars =5 μm.
2.2 Disruption of PROPEP2 suppresses root hair development
To further investigate the role of the PROPEP family in root hair growth, we isolated transfer DNA (T-DNA) insertional mutants for each gene, aiming to assess their RH phenotypes. Unexpectedly, all of mutants failed to yield detectable T-DNA insertions, except for propep2. Notably, propep2 (SALK_206498) contained a T-DNA insertion within the intron of PROPEP2. RT-PCR analyses demonstrated the absence of a full-length PROPEP2 transcript in propep2 (Figures 5A, B), confirming its status as a knockout mutant. Consequently, the propep2 mutant exhibited a significant reduction in both RH density and length compared to wild-type (WT) seedlings (Figures 5C–E).
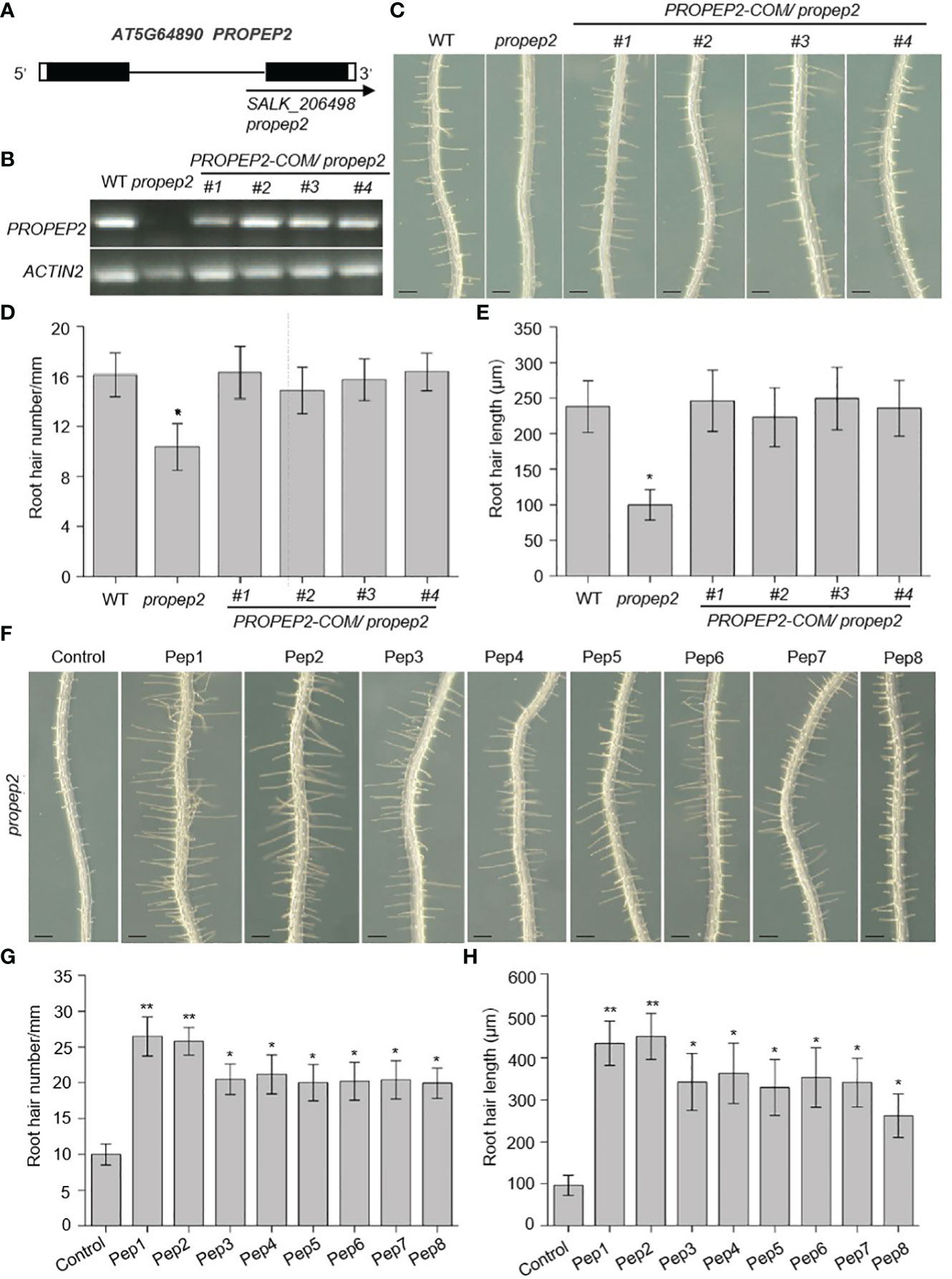
Figure 5 PROPEP2 regulates the root hair development. (A) Schematic map of T-DNA insertion location of propep2 mutant. Black boxes, lines, and arrow represent exons, introns, and the position of the T-DNA insertion, respectively. The while boxes indicate the 5’or 3’UTRs. (B) RT-PCR analysis of the transcriptional level of PROPEP2 in WT, propep2 mutant and four independent complementation lines transformed with PROPEP2 genomic DNA into propep2 mutant (PROPEP2-COM/propep2). Actin2 was used as internal standards. (C) The growth phenotype of root hairs in wild type (WT), propep2 mutant and four PROPEP2-COM/propep2 complementation lines. Four-day-old WT plants were transplanted on half-strength Murashige and Skoog (MS) agar medium for 48 h. Bars = 200 μm. (D, E) Statistics of the root hair number (D) and root hair length (E) as in (C). Data are means ± SD (n = 15 roots per treatment). (F) The growth phenotype of root hairs in propep2 mutant under Pep1-Pep8 treatment. Four-day-old plants were transplanted on half-strength Murashige and Skoog (MS) agar medium supplemented with or without 10 nM Pep1 to Pep8 for 48 h. Bars = 200 μm. (G, H) Statistics of the root hair number (G) and root hair length (H) as in (F). Data are means ± SD (n = 15 roots per treatment). Asterisks in (D, E, G, H) indicate statistically significant differences compared with the control. (Tukey’s test; *p < 0.05, **p < 0.01).
To corroborate that the observed RH phenotype in the propep2 mutant was indeed caused by the T-DNA insertion, we generated complementation lines by introducing a genomic fragment of PROPEP2 into the mutant. Remarkably, transgenic expression of PROPEP2 in the mutant led to the restoration of PROPEP2 transcript levels to a comparable level as in WT plants in four independent PROPEP2-COM lines (Figure 5B), fully rescuing the RH defect (Figures 5C–E). The Pep2 peptide was released from its precursor protein PROPEP2, the disruption of PROPEP2 could not synthesis the Pep2 peptide anymore, we further used the exogenous Pep2 peptide to analyzed the root hair formation in propep2 mutant. As shown in Figures 5F–H and Supplementary Figure 10, supplementing the propep2 mutant with synthesized Pep2 also resulted in the recovery of RH growth (Figures 5F–H; Supplementary Figure 10). These findings provide compelling evidence that PROPEP2 plays an indispensable role in RH growth in Arabidopsis. Moreover, other Peps, in addition to Pep2, also restored RH growth in the propep2 mutant, suggesting that other PROPEPs (such as PROPEP1 and PROPEP6) with expression in RH may also regulate RH growth (Figures 5F–H).
2.3 PROPEP2 relies on CPC and GL2 in regulating root hair formation
As Peps affect both the density and length of root hairs, we aimed to investigate whether PROPEPs/Peps work together with other components known to have a role in determining RH cell fate. We focused on examining the expression patterns of GL2 and CPC in relation to Pep2 treatment. The GL2 gene encodes a homeodomain-leucine zipper protein primarily expressed in non-hair cells, suppressing hair cell differentiation (Di Cristina et al., 1996; Masucci et al., 1996). On the other hand, CPC encodes a small protein containing a MYB-like DNA-binding domain, lacking a transcription activation domain, and it acts as a negative transcription regulator of GL2, indirectly promoting hair cell differentiation (Wada et al., 1997, 2002; Schellmann et al., 2002; Kirik et al., 2004). Initially, we assessed whether Pep2 impacts the expression levels of CPC and GL2 using real-time RT-PCR. Following Pep2 treatment, CPC transcripts increased significantly, while GL2 expression sharply decreased (Figure 6A). We next examined whether the expression pattern of CPC and GL2 was altered in the propep2 mutant. Interestingly, CPC mRNA levels decreased, whereas GL2 expression increased significantly, opposite to the data from Pep2 treated samples (Figures 6B, C), suggesting that PROPEP2 modulates RH growth, at least in part, by regulating CPC and GL2 expression levels. Furthermore, all PROPEP-overexpressing (OE) lines showed increased CPC expression and decreased GL2 mRNA levels (Supplementary Figures 11A, B), further supporting the notion that PROPEPs regulate the CPC and GL2 expression.
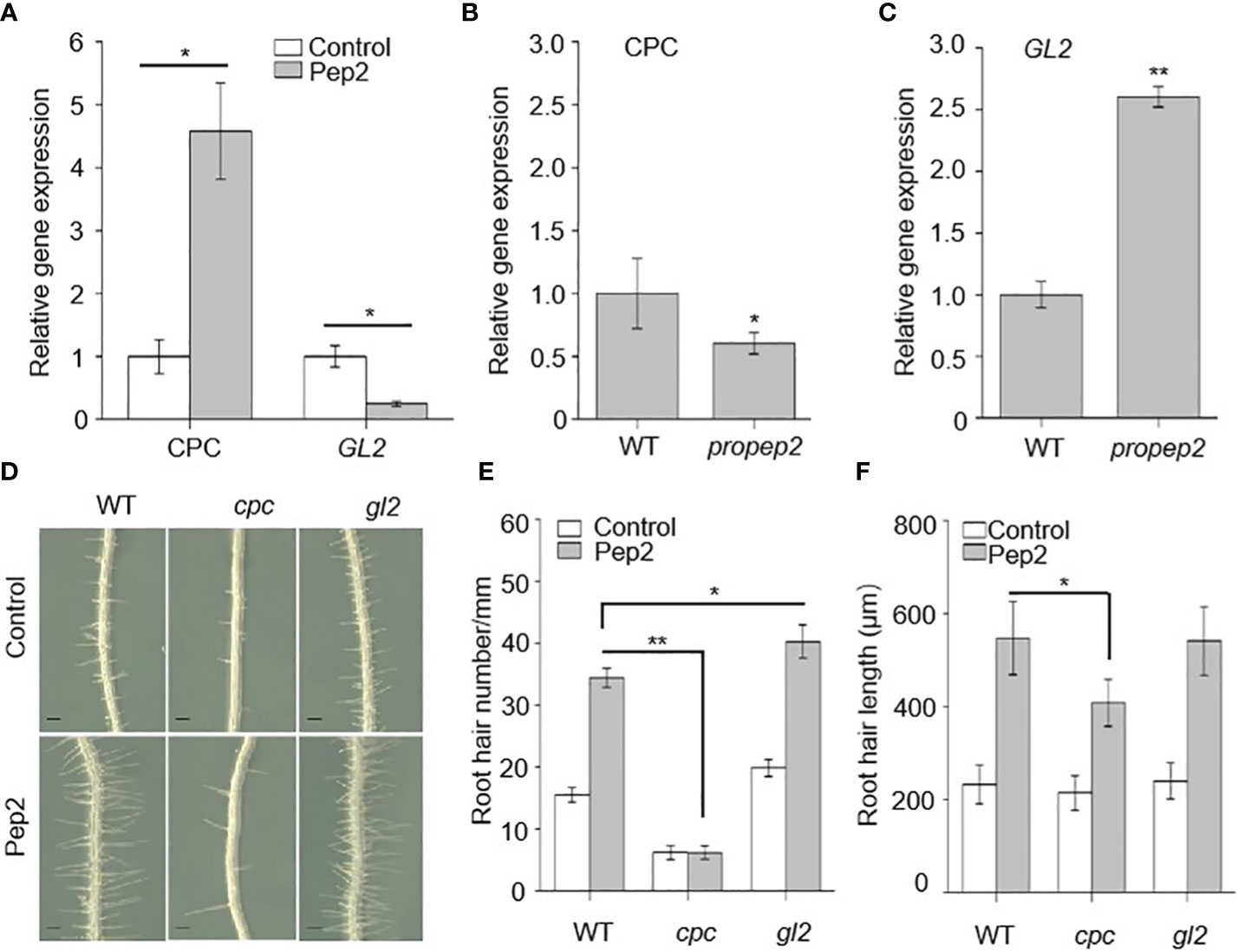
Figure 6 CPC and GL2 mediates the PROPEPs signals in root hair formation. (A) qRT-PCR analysis of CPC and GL2 mRNA levels in wild type (WT) roots treated with 10 nM Pep2 for 24 h. The expression level of the untreated control (0 h) was set to 1.0, and Pep2 treatment levels were normalized to the control level. Data are means ± SD (n =3 individual reactions). (B, C) qRT-PCR analysis of CPC (B) and GL2 (C) mRNA levels in 6-d-old WT and propep2 roots. The expression level in WT root was set to 1.0. Data are means ± SD (n =3 individual reactions). (D) The growth phenotype of root hairs in wild type (WT), cpc and gl2 mutant. Four-day-old plants were transplanted on half-strength Murashige and Skoog (MS) agar medium supplemented with or without 10 nm Pep2 for 48h. Bars = 200 μm. (E, F) Statistics of the root hair number (E) and root hair length (F) as in (D). Data are means ± SD (n = 15 roots per treatment). Asterisks in (A–C, E, F) indicate statistically significant differences compared with the control. (Tukey’s test; *p < 0.05, **p < 0.01).
We also examined mutant plants for cpc and gl2. Consistent with previous research, cpc mutant seedlings had sparse root hairs, while gl2 mutants had more root hairs than WT plants (Masucci et al., 1996; Wada et al., 2002). The expression of PROPEP2 in cpc and gl2 mutant did not show significant differences compared with this in WT root (Supplementary Figure 11C). After Pep2 treatment, the increase in root hair density induced by Pep2 was compromised in cpc mutants, although Pep2-triggered root hair elongation persisted (Figures 6D–F), suggesting that CPC acts downstream of Pep2 signal to regulates the root hair formation. However, the root hair density in gl2 mutant was further increased after Pep2 treatment, which displays significant difference compared with this in WT root (Figures 6D–F).
2.4 Calcium signaling may be involved in PROPEP2-mediated root hair growth
Root hair growth is a finely tuned process in plants, regulated by a multitude of factors such as reactive oxygen species (ROS), cytoskeletal dynamics, and calcium signaling (Dunand et al., 2007; Pei et al., 2012; Zhang et al., 2016; Tan et al., 2019). Among these factors, the role of calcium, especially at the root hair tip, is critical for the elongation of these tubular cells (Takeda et al., 2008; Tan et al., 2019). To examine the link between Pep2 action and Ca signaling, we first observed that a reduction in calcium levels within the growth medium had an inhibitory effect on both the initiation and elongation of root hairs in wild-type (WT) seedlings (Figures 7A–C). Strikingly, the propep2 mutant exhibited a complete absence of root hairs under conditions of reduced calcium availability (Figures 7A–C). Subsequently, we introduced the calcium-specific chelator EGTA into the growth medium. As the EGTA concentration increased, both root hair density and length in WT roots progressively decreased, ultimately leading to a complete absence of root hairs when EGTA concentrations reached 500 mM (Supplementary Figure 12). Concurrently, the effectiveness of Pep2 in promoting root hair growth diminished with the introduction of EGTA. Similarly, the propep2 mutant encountered significant challenges in root hair development when exposed to EGTA concentrations exceeding 50 mM. However, the supplementation of Pep2 partially reinstated root hair growth in the mutant, although to a lesser extent than observed in WT seedlings (Supplementary Figure 12). These findings implied the pivotal role of external calcium availability in facilitating Pep2-induced root hair growth.
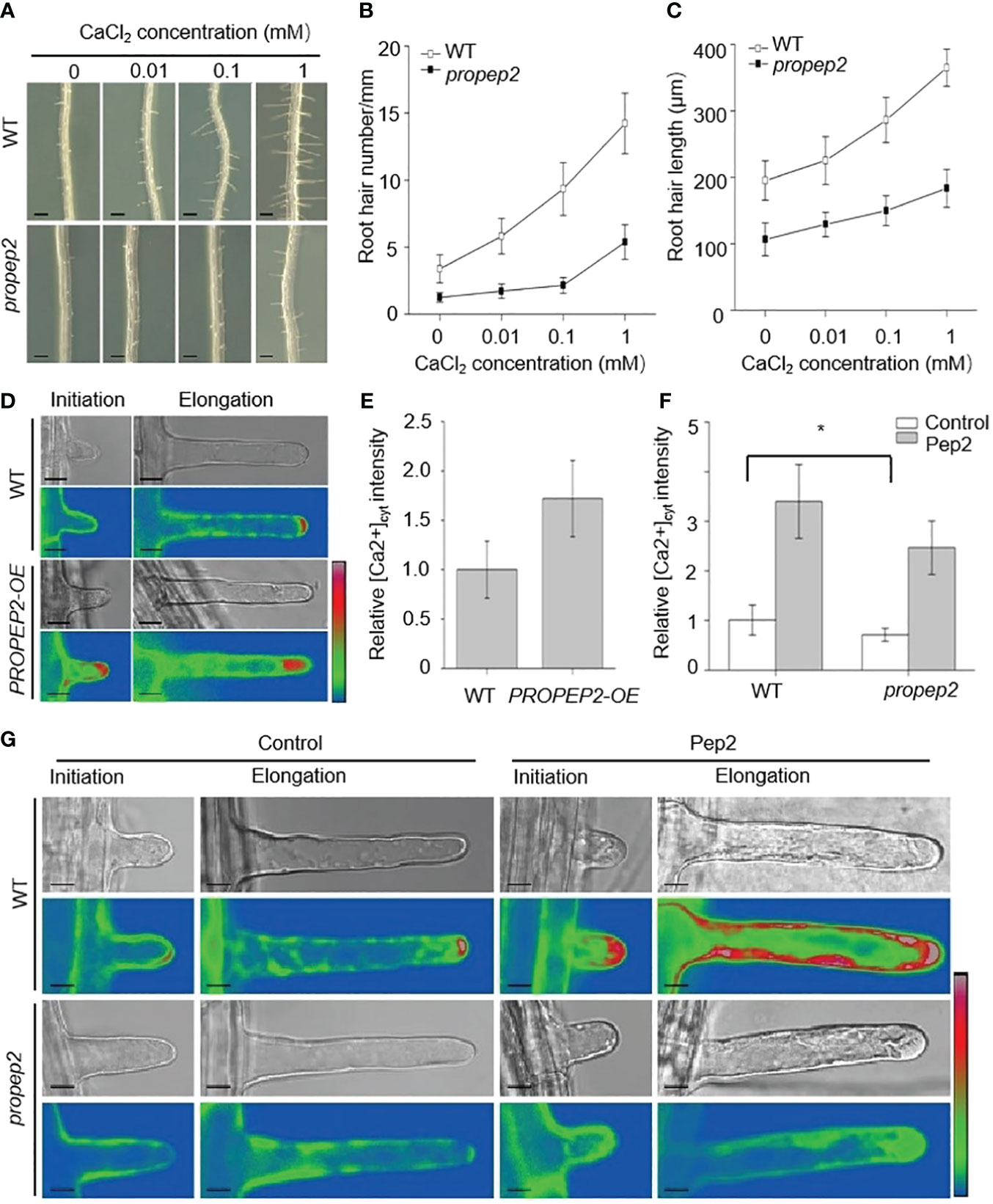
Figure 7 PROPEP2 mediates the root hair development dependent on Ca2+ concentrations changes. (A) The growth phenotype of root hairs in wild type (WT) and propep2 mutant under CaCl2 treatment. Four-day-old plants were transplanted on half-strength Murashige and Skoog (MS) agar medium supplemented with or without 0.01, 0.1 and 1mM CaCl2 for 48 h. Bars = 200 μm. (B, C) Statistics of the root hair number (B) and root hair length (C) as in (A). Data are means ± SD (n = 15 roots per treatment). (D) Imaging of Ca2+ fluorescence signals in the initiation and elongation root hairs. The 6-day-old wild type (WT) and PROPEP2-overexpression line (PROPEP2-OE) expressing the genetically encoded intracellular Ca2+ indicator GCaMP6s were used. Bars = 10 μm. (E) Quantitative analysis of cytosolic Ca2+ signals in the elongation root hair as in (D), Relative fluorescence was normalized against that in WT root hairs (1.0). Data are mean ± SD; (n = 35 root hairs of 10 roots per treatment). (F) Quantitative analysis of cytosolic Ca2+ signals in the elongation root hairs of WT and propep2 plants, 6-day-old plants were transplanted on half-strength Murashige and Skoog (MS) agar medium supplemented with or without 10 nm Pep2 for 6 h. Relative fluorescence was normalized against that in WT root hairs without Pep2 treatment (1.0). Data are mean ± SD; (n = 35 root hairs of 10 roots per treatment). (G) Imaging of Ca2+ fluorescence signals in the initiation and elongation root hairs of WT and propep2 plants. Six-day-old WT and propep2 plants expressing GCaMP6s were transplanted on half-strength Murashige and Skoog (MS) agar medium supplemented with or without 10 nm Pep2 for 6 h. Bars = 10 μm. A pseudocolor scale bar for relative cytosolic Ca2+ level calibration in (D, G) is shown on the right. Asterisks in (F) indicate statistically significant differences compared with the untreated control. (Tukey’s test; *p < 0.05).
To delve deeper into the calcium dynamics occurring within root hairs, we introduced a fluorescent protein-based [Ca2+] cytosol sensor, GCaMP6 (Gao et al., 2023). The GCaMP6 was expressed in WT plant under the control of the UBQ10 promoter (Gao et al., 2023). To generate PROPEP2-OE/GCaMP6 plants, we utilized the homozygous pUBQ10:GCaMP6/WT plant as the background and introduced the PROPEP2-OE construct. To generate pUBQ10:GCaMP6/propep2 plants, we utilized the homozygous pUBQ10:GCaMP6/WT plant as the background and hybridized the pUBQ10:GCaMP6/WT with propep2 mutant. As a result, the expression levels of GCaMP6 in various plant lines should be comparable. In PROPEP2-OE seedlings, the root hair tips exhibited significantly enhanced [Ca2+] cytosol signals when compared to WT plants, both during the initiation and elongation stages of root hair growth (Figures 7D–F). Additionally, we generated 35S:PROPEP2-mRFP transgenic plants, revealing extensive presence of PROPEP2 proteins in the root, including root hairs (Supplementary Figure 13). The [Ca2+] cytosol signals in these plants were notably stronger than in WT, and these signals closely overlapped with PROPEP2-mRFP signals, emphasizing the correlation between PROPEP2 expression and elevated [Ca2+] cytosol levels (Supplementary Figure 13). Similarly, the application of Pep2 induced a substantial increase in [Ca2+] cytosol levels, not only at the root hair tip but also throughout the entire root hair cell (Figure 7G; Supplementary Figure 14). These calcium dynamics were also observed with other Peps, mirroring the response to Pep2 (Supplementary Figure 14). In stark contrast, in the propep2 mutant, there was an absence of discernible Ca2+ accumulation at the root hair tip (Figure 7G; Supplementary Figure 15). Moreover, the PROPEP2 mutation resulted in a reduced response of Ca2+ elevation in root hairs to Pep2 compared to that in WT plants (Figure 7G; Supplementary Figure 15). We speculate that Pep2 intersects with Ca signaling to regulate root hair growth.
3 Discussion
Previous research has primarily focused on the immunomodulatory effects of Peps and their role in inhibiting overall root growth and have contributed significantly to our understanding of the functions of Peps in plant defense mechanisms. In this study, we demonstrate the multifaceted effects of Peps on root hair growth, unveil the expression patterns and subcellular localization of PROPEPs, and highlight the pivotal role of PROPEP2 in this process. Additionally, we reveal the involvement of the CPC-GL2 module and calcium signature as downstream targets of PROPEPs/Peps in root hair differentiation, initiation, and elongation.
The development of plant root hairs is intricately regulated by a range of phytohormones, including auxins, ethylene, abscisic acid, and jasmonic acid. Phytohormones, notably auxins, primarily exert their influence on root hair development by promoting key processes such as root hair initiation, tip elongation, and the elongation of fully developed root hairs (Bruex et al., 2012; Lee and Cho, 2013; Zhang et al., 2016; Rymen et al., 2017). In addition to the pivotal role played by plant hormones in governing root hair development, recent scientific investigations have unveiled the significant involvement of hormone-like substances, specifically small peptides, in regulating various aspects of root hair development (Takahashi et al., 2019; Hsiao and Yamada, 2021). For instance, within plant cells, a class of small peptides known as Rapid Alkalinization Factors (RALFs) can be recognized by receptor-like kinases located in the plant cell membrane, such as FERONIA (FER) (Haruta et al., 2014). This recognition event triggers the formation of a protein complex involving FER and an intracellular receptor-like kinase called RIPK (Du et al., 2016). Together, they orchestrate the regulation of cytoplasmic alkalinization in root epidermal cells, consequently impacting the initiation of root hairs (Du et al., 2016). Another peptide, namely CLV3/ESR-related peptide 14 (CLE14), has been observed to enhance the expression of CPC, leading to the suppression of GL2 transcription levels. This, in turn, promotes cell differentiation into hair cells, ultimately driving root hair development (Hayashi et al., 2018). In our study, we observed distinct tissue-specific expression patterns of PROPEPs, and their proteins exhibited varying subcellular localization. Notably, the introduction of exogenous synthetic Peps (Pep1-8) or the utilization of transgenic PROPEPs-OE lines led to a significant enhancement in both root hair density and length in seedlings. These results imply that Pep/PROPEPs play a pivotal role throughout all stages of root hair development, and the subcellular localization of Pep may not be directly correlated with its function in regulating root hair growth. Notably, propep2 mutant seedlings with impaired root hair growth displayed elevated GL2 levels but reduced CPC expression compared to WT seedlings. In contrast, both the transgenic PROPEP-OE lines and seedlings treated with Pep2 exhibited higher CPC expression but lower GL2 levels. These observations imply that, akin to CLE14 (Hayashi et al., 2018), Pep/PROPEPs promote the differentiation of root hair cells by modulating the CPC-GL2 regulatory module.
The polarization and growth of cells, such as pollen tubes and root hairs, have been demonstrated to coincide with highly organized and polarized cytoplasmic contents (Rosen et al., 1964; Emons, 1987). Calcium, among other factors, plays a crucial role in activating proteins and enzymes that contribute to the organization of cytoskeletal elements and membrane structures necessary for the development and maintenance of cell polarity (Bush, 1995). A localized gradient of cytoplasmic free Ca2+ toward the growing apex has been observed in growing root hairs and pollen tubes, and the intensity of this gradient correlates with the growth rate of these cells (Pierson et al., 1996; Felle and Hepler, 1997; Wymer et al., 1997). In line with this, the deprivation of calcium in the medium resulted in the inhibition of root hair growth in both propep2 and WT plants. Conversely, either overexpression of PROPEP2 or supplementation of Pep2 significantly enhanced the tip calcium gradient of root hairs (Figure 7). Exploring the calcium channels/transporters or calcium signature elements expressed in root hairs would be intriguing for further elucidating the crosstalk between calcium oscillation and Peps-triggered root hair growth.
Regarding the perception of Peps, it has been established that PEPR1 and PEPR2 serve as the principal receptors responsible for transmitting the Pep signal and triggering corresponding responses, albeit with varying affinities for different Peps. Notably, the mutation of PROPEP2 resulted in stunted root hair growth in plants. In contrast to the propep2 mutant, neither pepr1, pepr2, nor the double mutant pepr1 pepr2 displayed any discernible root hair deficit phenotype (Supplementary Figure 16). However, it is worth highlighting that exogenous application of Pep2 failed to stimulate root hair growth in pepr1 pepr2, underscoring the exclusive role of PEPR1/2 as the receptors for perceiving exogenous Peps (Supplementary Figure 16). Recent research introduced sucrose-induced receptor kinase 1 (SIRK1) as a novel receptor for Pep7, orchestrating sucrose-mediated water flux regulation and lateral root development (Wang et al., 2022). Consequently, we posit the existence of an unidentified perception system within the cell, which may facilitate the sensing of Peps and subsequently regulate root hair growth. In light of this, unraveling the signaling pathways downstream of Peps and PROPEPs becomes imperative for a holistic comprehension of root hair development. The identification of novel receptors and components participating in these pathways promises valuable insights into the mechanisms by which Peps govern root hair fate and growth.
4 Materials and methods
4.1 Plant materials and growth conditions
Arabidopsis (Arabidopsis thaliana) mutant lines propep2 (SALK_206498), cpc (Wada et al., 2002), gl2 (Wang et al., 2010) and transgenic line pUBQ10:GCaMP6s (Gao et al., 2023) were described previously. Homozygous mutant plants were identified by RT-PCR or DNA sequencing using primers described in Supplementary Table 1. The seedlings were grown on half-strength Murashige and Skoog (MS) medium, containing 1% sucrose and 0.8% phytogel (Sigma-Aldrich, St. Louis, MO, USA) in the growth chamber. The growth conditions had 90 μmol/m2/s light intensity with a 16 h light/8 h dark photoperiod at 22°C.
4.2 Peptide synthesis
The peptides used in this study were synthesized by GL Biochem. The sequences (from the N terminus to the C terminus) were as follows:
Pep1, ATKVKAKQRGKEKVSSGRPGQHN;
Pep2, DNKAKSKKRDKEKPSSGRPGQTNSVPNAAIQVYKED;
Pep3, EIKARGKNKTKPTPSSGKGGKHN;
Pep4, GLPGKKNVLKKSRESSGKPGGTNKKPF;
Pep5, SLNVMRKGIRKQPVSSGKRGGVNDYDM;
Pep6, ITAVLRRRPRPPPYSSGRPGQNN;
Pep7, VSGNVAARKGKQQTSSGKGGGTN;
Pep8, GGVIVKSKKAARELPSSGKPGRRN;
4.3 Plasmid constructions and plant transformation
To produce transgenic proPROPEP1:GUS to proPROPEP8:GUS lines, the promoter regions upstream of the start codons of PROPEP1 (1640-bp), PROPEP2 (765-bp), PROPEP3 (1437-bp), PROPEP4 (1197-bp), PROPEP5 (798-bp), PROPEP6 (1707-bp), PROPEP7 (1170-bp), PROPEP8 (678-bp) were amplified and cloned into the pCAMBIA1300-GUS binary vector. For the transgenic PROPEP1 to PROPEP8 over-expression lines (termed as PROPEP1-OE to PROPEP8-OE), the coding sequence (CDS) without the stop codon of PROPEP1 to PROPEP8 were cloned into pEZS-NL to generate 35S-PROPEPs-GFP constructs and then cloned into the pART27 binary vector. The constructs were transformed into Agrobacterium tumefaciens strain GV3101 and further transformed into wild type plants using the floral-dip method (Clough and Bent, 1998). For construction of the genetic PROPEP2-OE harboring GCaMP6s, the CDS without the stop codon of PROPEP2 was cloned into pEZS-NL to fuse with mRFP. The 35S-PROPEPs-mRFP construct was cloned into pART27 binary vector and then transformed into pUBQ10:GCaMP6s plants through GV3101 infection of floral-dip.
For construction of the genetic PROPEP2 complementary lines (termed as PROPEP2-COM/propep2), the full-length genomic DNA of the PROPEP2 fragment (a 1557-bp fragment containing a 590-bp promoter and a 967-bp genomic region from translation initiation codon to 3’ UTR domain) was amplified from the genomic DNA of wild-type seedlings and cloned into the binary vector pCAMBIA-1300. Then, the recombinant plasmid was transformed into the Agrobacterium tumefaciens strain GV3101 and further transformed into the propep2 mutant using the floral-dip method. The primers used to produce the constructs are listed in Supplementary Table 1.
4.4 Root hairs growth analyze
The root hairs growth was analyzed as previously described (Tan et al., 2019) with modifications. In brief, 4-day-old seedlings were transferred onto half-strength MS agar medium supplemented with different treatment conditions, and the plates were placed vertically in growth room for another 48h. The roots were covered by cover glass to push the angle of root hairs parallel to the surface of solid medium. Roots were photographed under an SZX16 microscope (Olympus). The 2 mm root hair distribution zone, which located 0.5 cm far away from the root tip was counted by using Image J software to analyze the root hairs length and root hair number. No less than 15 roots were analyzed for each treatment, three independent repetitions were performed.
4.5 RT-PCR and qRT-PCR analysis
Total RNA in roots were extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s protocol. Two μg RNA was used to synthesis the cDNA by using M-MLV Reverse Transcriptase (Promega, Madison, WI, USA). Real time qRT-PCR analysis was performed using the FastStart Universal SYBR Green mastermix (Roche Diagnostics, Hong Kong) on a CFX Connect Real Time System (Bio-Rad, Berkeley, CA, USA) using Actin2 as internal standards. All individual reactions were performed in triplicate. The primers used are listed in Supplementary Table.
4.6 Histochemical GUS analysis
GUS activity was detected by histochemical staining of tissues as previously described (Liu et al., 2015). Briefly, T2 transgenic seedlings were incubated in GUS staining solution (2 mM 5-bromo-4-chloro-3-indolyl-b-D-glucuronide, 1 mM K3Fe(CN)6, 1 mM K4Fe(CN)6·3H2O, 10 mM Na2EDTA, 0.1% Triton X-100, and 50 mM Na3PO4, pH 7.0) at 37°C for 6 h. After the tissue with 75% (vol/vol) ethanol was sufficiently decolorized to remove chlorophyll, individual representative plant tissues, the roots and root hairs were photographed under a microscope (Olympus, SZX16) equipped with a camera.
4.7 Subcellular localization assays in planta
Subcellular localization assays were performed as previously described (Mao et al., 2014) with slight modifications. Briefly, 4-week-old Arabidopsis rosette leaves were digested by Cellulase R-10 and Macerozyme R-10 (Yakult Pharmaceutical) to prepare the mesophyll protoplasts. The protoplasts were resuspended with suspension solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 2 mM 4-Morpholineethanesulfonic acid (MES) adjusted to pH 5.7 with KOH) and further transfected with 20 μg recombinant plasmid DNA (PROPEP1 to PROPEP8-pEZS-NL-GFP) by using polyethylene glycol-mediated transformation protocol (Sheen, 2001). The transformed protoplasts were incubated in the dark at 23°C for 16 h before confocal imaging analysis. Imaging was performed on an LSM-710 argon/krypton laser scanning confocal microscope (Zeiss) with a 63 × objective. FM 4-64 excitation at 514 nm and emission at 600-700 nm. GFP signals were excited at 488 nm wavelength and collected emission between 495 and 550 nm. Z-stack images were collected with 1 μm steps and the scan speed was 8 s/scan.
4.8 Root hairs calcium imaging
For Peps-induced root hairs [Ca2+]cytosol signals assays, the 6-day-old seedlings expressing GCaMP6s were supplemented with or without Peps for 6 h, the root hairs harboring GCaMP6s were monitored by a LSM-710 confocal microscope with a 20 × objective. The interval of data acquisition was 10 seconds, the Z-stack images were acquired from top to bottom of the cells with 1 μm steps and the scan speed was 6 s/scan. The excitation wavelengths for [Ca2+]cytosol fluorescence signals was 488 nm. To quantitatively analyze fluorescence intensity, confocal images were captured under strictly identical acquisition parameters, which included laser power, photomultiplier settings, offset, zoom factor, and resolution, across all experimental root samples. The fluorescence intensity was analyzed by Image J software.
4.9 Statistical analysis
For all experiments, three independent repetitions were performed. One way ANOVA Tukey’s test was used for statistical analysis. Asterisks in the figures denote significant differences as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. FZ: Data curation, Writing – original draft, Writing – review & editing. KL: Data curation, Writing – review & editing. FS: Data curation, Writing – review & editing. CS: Investigation, Writing – review & editing. XYZ: Investigation, Writing – review & editing. MX: Writing – original draft, Writing – review & editing. AF: Writing – original draft, Writing – review & editing. JC: Conceptualization, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing. XJZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing. SL: Conceptualization, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. RS: Data curation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Nation Nature Science Foundation of China (31900223 to XJZ, 32000201 to JC, and 32200258 to YJ), Basic Research Program of Shaanxi Province (22JHQ061 to XJZ), the Natural Science Foundation of Jiangsu Province (BK20211319 to JC), Qinchuangyuan Recruited High-level Innovation and Entrepreneurship Talents Project of Science and Technology Department of Shaanxi Province (QCYRCXM-2022-223 to XJZ), and the China Postdoctoral Science Foundation (2020M673626XB to YJ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1336129/full#supplementary-material
References
Bartels, S., Boller, T. (2015). Quo vadis, Pep? Plant elicitor peptides at the crossroads of immunity, stress, and development. J. Exp. Bot. 66, 5183–5193. doi: 10.1093/jxb/erv180
Bartels, S., Lori, M., Mbengue, M., van Verk, M., Klauser, D., Hander, T., et al. (2013). The family of Peps and their precursors in Arabidopsis: Differential expression and localization but similar induction of pattern-triggered immune responses. J. Exp. Bot. 64, 5309–5321. doi: 10.1093/jxb/ert330
Beck, M., Wyrsch, I., Strutt, J., Wimalasekera, R., Webb, A., Boller, T., et al. (2014). Expression patterns of flagellin sensing 2 map to bacterial entry sites in plant shoots and roots. J. Exp. Bot. 65, 6487–6498. doi: 10.1093/jxb/eru366
Bernhardt, C., Lee, M. M., Gonzalez, A., Zhang, F., Lloyd, A., Schiefelbein, J. (2003). The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 130, 6431–6439. doi: 10.1242/dev.00880
Boller, T., Felix, G. (2009). A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. doi: 10.1146/annurev.arplant.57.032905.105346
Bruex, A., Kainkaryam, R. M., Wieckowski, Y., Kang, Y. H., Bernhardt, C., Xia, Y., et al. (2012). A gene regulatory network for root epidermis cell differentiation in Arabidopsis. PloS Genet. 8, e1002446. doi: 10.1371/journal.pgen.1002446
Bush, D. (1995). Calcium regulation in plant cells and its role in signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 95–122. doi: 10.1146/annurev.pp.46.060195.000523
Clough, S., Bent, A. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. doi: 10.1046/j.1365-313x.1998.00343.x
Datta, S., Kim, C., Pernas, M., Pires, N., Hélène, P., Tam, T., et al. (2011). Root hairs: development, growth and evolution at the plant-soil interface. Plant Soil. 346, 1–14. doi: 10.1007/s11104-011-0845-4
Di Cristina, M., Sessa, G., Dolan, L., Linstead, P., Baima, S., Ruberti, I., et al. (1996). The Arabidopsis Athb-10 (GLABRA2) is an HD-Zip protein required for regulation of root hair development. Plant J. 10, 393–402. doi: 10.1046/j.1365-313X.1996.10030393.x
Du, C., Li, X., Chen, J., Chen, W., Li, B., Li, C., et al. (2016). Receptor kinase complex transmits RALF peptide signal to inhibit root growth in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 113 (51), 8326–8334.
Dunand, C., Crèvecoeur, M., Penel, C. (2007). Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: Possible interaction with peroxidases. New Phytol. 174, 332–341. doi: 10.1111/j.1469-8137.2007.01995.x
Emons, A. (1987). The cytoskeleton and secretory vesicles in root hairs of Equisetum and Limnobium and cytoplasmic streaming in root hairs of Equisetum. Ann. Bot. 60, 625–632. doi: 10.1093/oxfordjournals.aob.a087492
Endo, S., Betsuyaku, S., Fukuda, H. (2014). Endogenous peptide ligand-receptor systems for diverse signaling networks in plants. Curr. Opin. Plant Biol. 21, 140–146. doi: 10.1016/j.pbi.2014.07.011
Felle, H., Hepler, P. (1997). The cytosolic Ca2+-concentration gradient of Sinapis alba root hairs as revealed by Ca2+-selective microelectrode tests and fura-dextran ratio imaging. Plant Physiol. 114, 39–45. doi: 10.1104/pp.114.1.39
Galway, M., Masucci, J., Lloyd, A., Walbot, V., Davis, R., Schiefelbein, J. (1994). The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev. Biol. 166, 740–754. doi: 10.1006/dbio.1994.1352
Gao, Q., Wang, C., Xi, Y., Shao, Q., Hou, C., Li, L., et al. (2023). RALF signaling pathway activates MLO calcium channels to maintain pollen tube integrity. Cell Res. 33, 71–79. doi: 10.1038/s41422-022-00754-3
Grierson, C., Nielsen, E., Ketelaarc, T., Schiefelbein, J. (2014). Root hairs. Arabidopsis Book. doi: 10.1199/tab.0172
Haruta, M., Sabat, G., Stecker, K., Minkoff, B., Sussman, M. (2014). A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343, 408–411. doi: 10.1126/science.1244454
Hayashi, N., Tetsumura, T., Sawa, S., Wada, T., Tominaga-Wada, R. (2018). CLE14 peptide signaling in Arabidopsis root hair cell fate determination. Plant Biotechnol. 35, 17–22. doi: 10.5511/plantbiotechnology.18.0122a
Hsiao, Y., Yamada, M. (2021). The roles of peptide hormones and their receptors during plant root development. Genes 12, 22. doi: 10.3390/genes12010022
Huffaker, A., Pearce, G., Ryan, C. (2006). An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl. Acad. Sci. U.S.A. 103, 10098–10103. doi: 10.1073/pnas.0603727103
Huffaker, A., Pearce, G., Veyrat, N., Erb, M., Turlings, T., Sartor, R., et al. (2013). Plant elicitor peptides are conserved signals regulating direct and indirect antiherbivore defense. Proc. Natl. Acad. Sci. U.S.A. 110, 5707–5712. doi: 10.1073/pnas.1214668110
Jing, Y., Shen, N., Zheng, X., Fu, A., Zhao, F., Lan, W., et al. (2020). Danger-associated peptide regulates root immune responses and root growth by affecting ROS formation in Arabidopsis. Int. J. Mol. Sci. 21, 4590. doi: 10.3390/ijms21134590
Jing, Y., Zheng, X., Zhang, D., Shen, N., Wang, Y., Yang, L., et al. (2019). Danger-associated peptides interact with PIN-dependent local auxin distribution to inhibit root growth in Arabidopsis. Plant Cell 31, 1767–1787. doi: 10.1105/tpc.18.00757
Jing, Y., Zou, X., Sun, C., Qin, X., Zheng, X. (2023). Danger-associate Peptide regulates root immunity in Arabidopsis. Biochem. Biophys. Res. Commun. 663, 163–170. doi: 10.1016/j.bbrc.2023.04.091
Kim, C., Dolan, L. (2011). Root hair development involves asymmetric cell division in Brachypodium distachyon and symmetric division in Oryza sativa. New Phytol. 192 (3), 601–610.
Kim, D., Lee, S., Choi, S., Won, S., Heo, Y., Cho, M., et al. (2006). Functional conservation of a root hair cell-specific cis-element in angiosperms with different root hair distribution patterns. Plant Cell. 18, 2958–2970. doi: 10.1105/tpc.106.045229
Kirik, V., Simon, M., Huelskamp, M., Schiefelbein, J. (2004). The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Dev. Biol. 268, 506–513. doi: 10.1016/j.ydbio.2003.12.037
Klauser, D., Desurmont, G., Glauser, G., Vallat, A., Flury, P., Boller, T., et al. (2015). The Arabidopsis Pep-PEPR system is induced by herbivore feeding and contributes to JA-mediated plant defence against herbivory. J. Exp. Bot. 66, 5327–5336. doi: 10.1093/jxb/erv250
Lee, R., Cho, H. (2013). Auxin, the organizer of the hormonal/environmental signals for root hair growth. Front. Plant Sci. 4, 448. doi: 10.3389/fpls.2013.00448
Liu, J., Yang, L., Luan, M., Wang, Y., Zhang, C., Zhang, B., et al. (2015). A vacuolar phosphate transporter essential for phosphate homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 112, E6571–E6578. doi: 10.1073/pnas.1514598112
Ma, C., Guo, J., Kang, Y., Doman, K., Bryan, A. C., Tax, F., et al. (2014). AtPEPTIDE RECEPTOR2 mediates the AtPEPTIDE1-induced cytosolic Ca2+ rise, which is required for the suppression of glutamine dumper gene expression in Arabidopsis roots. J. Integr. Plant Biol. 56, 684–694. doi: 10.1111/jipb.12171
Macho, A., Zipfel, C. (2014). Plant PRRs and the activation of innate immune signaling. Mol. Cell. 54, 263–272. doi: 10.1016/j.molcel.2014.03.028
Mao, D., Chen, J., Tian, L., Liu, Z., Yang, L., Tang, R., et al. (2014). Arabidopsis transporter MGT6 mediates magnesium uptake and is required for growth under magnesium limitation. Plant Cell. 26, 2234–2248. doi: 10.1105/tpc.114.124628
Masucci, J., Rerie, W., Foreman, D., Zhang, M., Galway, M., Marks, M., et al. (1996). The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development 122, 1253–1260. doi: 10.1242/dev.122.4.1253
Millet, Y., Danna, C., Clay, N., Songnuan, W., Simon, M., Werck-Reichhart, D., et al. (2010). Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell. 22, 973–990. doi: 10.1105/tpc.109.069658
Pei, W., Du, F., Zhang, Y., He, T., Ren, H. (2012). Control of the actin cytoskeleton in root hair development. Plant Sci. 187, 10–18. doi: 10.1016/j.plantsci.2012.01.008
Pierson, E., Miller, D., Callaham, D., van Aken, J., Hackett, G., Hepler, P. (1996). Tip-localized calcium entry fluctuates during pollen tube growth. Dev. Biol. 174, 160–173. doi: 10.1006/dbio.1996.0060
Rosen, W., Gawlik, S., Dashek, W., Siegesmund, K. (1964). Fine structure and cytochemistry of Lilium pollen tubes. Am. J. Bot. 51, 61–71. doi: 10.1002/j.1537-2197.1964.tb06601.x
Rymen, B., Kawamura, A., Sabine, S., Breuer, C., Iwase, A. (2017). ABA suppresses root hair growth via the OBP4 transcriptional regulator. Plant Physiol. 173, 1750–1762. doi: 10.1104/pp.16.01945
Schellmann, S., Schnittger, A., Kirik, V., Wada, T., Okada, K., Beermann, A., et al. (2002). TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J. 21, 5036–5046. doi: 10.1093/emboj/cdf524
Schiefelbein, J. (2003). Cell-fate specification in the epidermis: a common patterning mechanism in the root and shoot. Curr. Opin. Plant Biol. 6, 74–78. doi: 10.1016/S136952660200002X
Sheen, J. (2001). Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol. 127, 1466–1475. doi: 10.1104/pp.010820
Shen, N., Jing, Y., Tu, G., Fu, A., Lan, W. (2020). Danger-associated peptide regulates root growth by promoting protons extrusion in an AHA2-dependent manner in arabidopsis. Int. J. Mol. Sci. 21, 7963. doi: 10.3390/ijms21217963
Takahashi, F., Hanada, K., Kondo, T., Shinozaki, K. (2019). Hormone-like peptides and small coding genes in plant stress signaling and development. Curr. Opin. Plant Biol. 51, 88–95. doi: 10.1016/j.pbi.2019.05.011
Takeda, S., Gapper, C., Kaya, H., Bell, E., Kuchitsu, K., Dolan, L. (2008). Local positive feedback regulation determines cell shape in root hair cells. Science 319, 1241–1244. doi: 10.1126/science.1152505
Tan, Y., Yang, Y., Zhang, A., Fei, C., Gu, L., Sun, S., et al. (2019). Three CNGC family members, CNGC5, CNGC6, and CNGC9, are required for constitutive growth of arabidopsis root hairs as ca2+-permeable channels. Plant Commun. 1, 100001. doi: 10.1016/j.xplc.2019.100001
Tominaga-Wada, R., Nukumizu, Y., Sato, S., Wada, T. (2013). Control of plant trichome and root-hair development by a tomato (Solanum lycopersicum) R3 MYB transcription factor. PloS One 8, e54019. doi: 10.1371/journal.pone.0054019
Wada, T., Kurata, T., Tominaga, R., Koshino-Kimura, Y., Tachibana, T., Goto, K., et al. (2002). Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development 129, 5409–5419. doi: 10.1242/dev.00111
Wada, T., Tachibana, T., Shimura, Y., Okada, K. (1997). Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277, 1113–1116. doi: 10.1126/science.277.5329.1113
Wang, J., Xi, L., Wu, X., Konig, S., Rohr, L., Neumann, T., et al. (2022). PEP7 acts as a peptide ligand for the receptor kinase SIRK1 to regulate aquaporin-mediated water influx and lateral root growth. Mol. Plant 15, 1615–1631. doi: 10.1016/j.molp.2022.09.016
Wang, S., Barron, C., Schiefelbein, J., Chen, J. (2010). Distinct relationships between GLABRA2 and single-repeat R3 MYB transcription factors in the regulation of trichome and root hair patterning in Arabidopsis. New Phytol. 185, 387–400. doi: 10.1111/j.1469-8137.2009.03067.x
Wymer, C., Bibikova, T. N., Gilroy, S. (1997). Cytoplasmic free calcium distribution during the development of root hairs of Arabidopsis thaliana. Plant J. 12, 427–439. doi: 10.1046/j.1365-313X.1997.12020427.x
Yamaguchi, Y., Huffaker, A., Bryan, A., Tax, F., Ryan, C. (2010). PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell. 22, 508–522. doi: 10.1105/tpc.109.068874
Yamaguchi, Y., Pearce, G., Ryan, C. (2006). The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc. Natl. Acad. Sci. U.S.A. 10, 10104–10109. doi: 10.1073/pnas.0603729103
Zhang, S., Huang, L., Yan, A., Liu, Y., Liu, B., Yu, C., et al. (2016). Multiple phytohormones promote root hair elongation by regulating a similar set of genes in the root epidermis in Arabidopsis. J. Exp. Bot. 67, 6363–6372. doi: 10.1093/jxb/erw400
Keywords: PROPEP, root hair growth, Ca signaling, regulatory mechanism, plant elicitor peptide (Pep)
Citation: Jing Y, Zhao F, Lai K, Sun F, Sun C, Zou X, Xu M, Fu A, Sharifi R, Chen J, Zheng X and Luan S (2024) Plant elicitor Peptides regulate root hair development in Arabidopsis. Front. Plant Sci. 15:1336129. doi: 10.3389/fpls.2024.1336129
Received: 10 November 2023; Accepted: 02 February 2024;
Published: 15 February 2024.
Edited by:
Hirotomo Takatsuka, Kanazawa University, JapanReviewed by:
Yanping Jing, Beijing Forestry University, ChinaRui Malhó, University of Lisbon, Portugal
Copyright © 2024 Jing, Zhao, Lai, Sun, Sun, Zou, Xu, Fu, Sharifi, Chen, Zheng and Luan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Chen, amlhbmNoZW5AdWpzLmVkdS5jbg==; Xiaojiang Zheng, eGp6aGVuZ0Bud3UuZWR1LmNu; Sheng Luan, c2x1YW5AYmVya2VsZXkuZWR1
 Yanping Jing
Yanping Jing Fugeng Zhao
Fugeng Zhao Ke Lai3
Ke Lai3 Min Xu
Min Xu Aigen Fu
Aigen Fu Rouhallah Sharifi
Rouhallah Sharifi Jian Chen
Jian Chen Xiaojiang Zheng
Xiaojiang Zheng Sheng Luan
Sheng Luan