- 1Consiglio per la ricerca in agricoltura e l’analisi dell’economia agraria (CREA), Centro di ricerca Olivicoltura, Frutticoltura e Agrumicoltura, Spoleto, Italy
- 2Dipartimento di Scienze Agrarie, Alimentari e Ambientali, Università Politecnica delle Marche, Ancona, Italy
- 3Dipartimento di Scienze Agrarie, Alimentari e Ambientali, Università degli Studi di Perugia, Perugia, Italy
The olive (Olea europaea L.) is the most cultivated tree crop in the Mediterranean and among the most cultivated tree crops worldwide. Olive yield is obtained by the product of fruit number and fruit size; therefore, understanding fruit development, in terms of both number and size, is commercially and scientifically relevant. This article reviews the literature on fruit development, from the flower to the mature fruit, considering factors that affect both fruit size and number. The review focuses on olive but includes literature on other species when relevant. The review brings the different factors affecting different phases of fruit development, addressed separately in the literature, under a single frame of interpretation. It is concluded that the different mechanisms regulating the different phases of fruit development, from pistil abortion to fruit set and fruit size, can be considered as different aspects of the same overall strategy, that is, adjusting fruit load to the available resources while striving to achieve the genetically determined fruit size target and the male and female fitness targets.
1 Introduction
The olive (Olea europaea L.) tree is the most cultivated tree crop in the Mediterranean, and one of the most cultivated trees worldwide, covering approximately 10 M hectares in 2021 (FAO, 2023). Olive yield is given by the number of fruits produced and their size; therefore, both fruit number and size are relevant aspects, both commercially and scientifically. In olive, the fruit develops directly from the carpel in the flower (King, 1938), and thus, fruit size is obtained through ovary development, before, during, and after flowering. Fruit number is also related to flower development, via flower number and fruit set. Therefore, this review considers fruit development from bloom to ripe fruit, including and integrating both fruit size and fruit number.
2 Factors affecting fruit size
Fruit size results from the interaction of environmental factors and the fruit growth potential, which is genetically determined. The olive fruit is a drupe: a fruit in which the mesocarp and endocarp tissues represent the major proportion of the fruit (King, 1938). In olive, fruit size is genetically controlled (Padula et al., 2008), differing many folds among different cultivars (Barranco, 1999). In general, fruit size differences within and among cultivars can be explained by different cell numbers, cell sizes, and/or intercellular spaces (Bertin et al., 2002; Corelli-Grappadelli and Lakso, 2004). In olive, genotype differences in fruit size are mostly due to cell number (Rapoport et al., 2004; Rosati et al., 2011a), as in many other species, such as tomato (Bohner and Bangerth, 1988), peach (Scorzal et al., 1991; Quilot and Génard, 2008), barley (Tuberosa et al., 1992), strawberry (Cheng and Breen, 1992), avocado (Cowan et al., 1997), melon (Higashi et al., 1999), banana (Jullien et al., 2001), tomato (Bertin et al., 2003), and persimmon (Hamada et al., 2008). Nonetheless, from the ovary to the fruit, the fruit grows mostly via increased cell size, rather than via increased cell number, as found in olive (Rapoport et al., 2004; Hammami et al., 2011) and, more in general, in fleshy fruits (Coombe, 1976). Accordingly, when considering the whole volume of the ovary/fruit, cell number increases approximately 8–40 times in the mature olive, compared with the ovary, while cell size increases approximately 100–300 times (Rallo and Rapoport, 2001; Rapoport et al., 2004; Rosati et al., 2009; Hammami et al., 2011; Rosati et al., 2012). This is related to the fact that cell division stops early during fruit formation (approximately 6 weeks after bloom), while cell expansion continues longer, especially in the mesocarp (Rallo and Rapoport, 2001). At times, however, fruit size differences are also related, at least in part, to cell size differences, as found in peach (Yamaguchi et al., 2002) and in apple, at least between wild relatives and cultivated varieties (Harada et al., 2005). A negative correlation between cell size and cell number is often found in mature fruits, suggesting resource competition among cells (Tsukaya, 2006). In olive, this correlation was weak across cultivars with different fruit sizes, although a boundary line analysis revealed that the maximal cell size achievable can be limited by high cell numbers, even though the size achieved is usually smaller and thus not limited by cell number (Rosati et al., 2011a).
Within a genotype, fruit size is affected by environmental (or exogenous) conditions affecting either cell size, cell number, or both (Denne, 1960; Bergh, 1985; Costagli et al., 2003; Gucci et al., 2009). These include nitrogen availability (Fernandez-Escobar et al., 2008), water availability (Costagli et al., 2003; Gucci et al., 2009), and solar radiation (Trentacoste et al., 2017). Irrigation, for instance, has been found to increase fruit size in olive mostly via increasing cell size (Costagli et al., 2003; Gucci et al., 2009). However, it has been hypothesized that water stress decreases fruit size by reducing cell number, when applied during early fruit development, and by reducing cell size, when applied later (Beede and Goldhamer, 1994; Orgaz and Fereres, 1999). In apple, heavy thinning before bloom increases fruit size by increasing both cell number and their size (Denne, 1960), although in the year following an abundant crop, fruit size is reduced via reduced cell number, and the reduction already occurs in the flower primordia in the previous autumn (Bergh, 1985). In olive fruits with multiple seeds, fruit size depends also on seed number, as found in Hojiblanca (Cuevas and Oller, 2000). Altogether, these results suggest that when resources per flower/fruit are increased (or decreased) at a time when the fruit tissues are still cellularizing (i.e., before, during, and up to sometime after bloom), then fruit size will be increased via increased cell number. When resources per fruit are varied after the completion of cellularization (as with irrigation treatments imposed in the summer after cellularization is complete), then fruit size differences can be achieved only by variations in cell size. When resources are varied during a period extending both before and after cellularization, both cell number and cell size will likely be affected. Further analysis of environmental factors affecting fruit size is not considered in this review, which mainly focuses on endogenous factors, particularly on genetic differences and their mechanisms.
2.1 Contribution of mesocarp and endocarp to ovary and fruit size
Despite evidence that both endocarp and mesocarp explain the differences in fruit size, in many fruit species (McGarry et al., 2001; Yamaguchi et al., 2004; Olmstead et al, 2007), including olive (Hammami et al., 2011; Rosati et al., 2012), on most species there is no information on whether this occurs also in the ovaries. In olive, both ovary endocarp and mesocarp volumes are strongly correlated with ovary volume, both across and within cultivars differing in fruit size (Rosati et al., 2012), implying a strong proportionality in tissue size. Even the size of locules is closely correlated with both tissues and with the ovary as a whole (Rosati et al., 2012). There is proportionality also between the ovary and the other flower parts, both within the same tree (Cuevas and Polito, 2004) and among cultivars (Rosati et al., 2009).
2.2 Relations between fruit and ovary tissues
In olive, mature fruit size is correlated to ovary size at bloom across cultivars differing in fruit size (Rosati et al., 2009), as occurs in other species (Lai et al., 1990; Lawes et al., 1990; Scorzal et al., 1991; Cruz-Castillo et al., 1991; Cheng and Breen, 1992; Nesbitt and Tanksley, 2001; Handley and Dill, 2003). In olive, this correlation holds also for both the endocarp and mesocarp although the relationships differ (i.e., different slopes), the relative increase being greater for the mesocarp (Rosati et al., 2012). This relative increase, from bloom to ripe fruit, represents the relative growth (RG) of the tissue: the final tissue volume (or mass) in the fruit per unit of initial volume (or mass) in the ovary. In terms of volume, RG varies across cultivars, from approximately 400 to 1,000 mm3 mm−3 for the endocarp and from approximately 1,700 to 7,000 mm3 mm−3 for the mesocarp (Rosati et al., 2012). RG for the whole fruit has intermediate values (Rapoport et al., 2004: Rosati et al., 2009). The greater RG for the mesocarp is related to its longer growth time compared with the endocarp (Hammami et al., 2011; Rapoport and Moreno-Alías, 2017).
2.3 Tissue relative growth vs. initial cell size in the ovary
Across tissues (mesocarp and endocarp) and cultivars, RG is negatively and exponentially correlated with the initial cell size of that tissue and cultivar in the ovary (Rosati et al., 2012). This is the case also when including data from a tetraploid cultivar with much bigger cells in the mesocarp and endocarp tissues in the ovary (Rosati et al., 2020). Therefore, the size of the cells in the ovary tissues appears to indicate how far that tissue is in its growth process: bigger cells indicate that less growth remains to occur. In fact, from bloom to harvest, tissue size increases continuously (Sinnott, 1942; Jullien et al., 2001; Bertin, 2005; Harada et al., 2005; Hammami et al., 2011), and daughter cells, before dividing again, grow to a bigger size than did their mother cell (Sinnott, 1942). This implies that cell size can be used as an indication of the stage of tissue development.
The fact that a single regression between RG and initial cell size fits both tissues suggests that both mesocarp and endocarp have a similar relationship between cell size and timing of tissue development. In other words, the larger cells of the endocarp than of the mesocarp at bloom suggest that the endocarp is at a more advanced growth stage, with more differentiated cells, and thus, its remaining growth from bloom to cessation of tissue growth (RG) is less. As already mentioned, the endocarp ceases growth earlier than the mesocarp and has a lower RG. This hypothesis is plausible since in the endocarp vital functions (e.g., fertilization) are required soon after anthesis, and thus, its cells need to be more differentiated. The mesocarp cells, instead, do not perform particular functions at anthesis and only need to prepare for their potential growth, thus not needing to achieve advanced differentiation in this period. However, even within the mesocarp, by 4 weeks after anthesis and up to fruit maturity, there is a gradient in cell size, with outside cells remaining smaller (and rounder) than inside ones (Rallo and Rapoport, 2001), suggesting that the centrifugal gradient of cell differentiations is not only between tissues (the endocarp having bigger and more differentiated cells than the mesocarp) but also within tissues.
2.4 Fruit tissue size dependence on ovary tissue cell number
In the previous sections, it has been described how, in olive, cultivar differences in fruit (and fruit tissue) size are related to ovary (and ovary tissue) size. In turn, differences in ovary and fruit size are related to cell number, for both the endocarp and the mesocarp (Rapoport et al., 2004; Rosati et al., 2011a). Therefore, for both these fruit portions, tissue size in the fruit correlates also with tissue cell number in the ovary (Rosati et al., 2012). This occurs also in other species such as apple (Denne, 1960; Harada et al., 2005), peach (Scorzal et al., 1991), kiwi (Cruz-Castillo et al., 1991), strawberry (Cheng and Breen, 1992), tomato (Bohner and Bangerth, 1988; Nesbitt and Tanksley, 2001), saskatoon (McGarry et al., 2001), and persimmon (Hamada et al., 2008), where fruit size and cell number in some fruit tissue correlate with the cell number of the corresponding tissue in the ovary. In most fruit species, this correlation has been studied for one tissue, but not for both the endocarp and the mesocarp. In olive, both the endocarp and the mesocarp provide important contributions to the fruit, despite differing in their growth patterns and timing (Hammami et al., 2011; Rapoport and Moreno-Alías, 2017), and despite showing different characteristics in the ovary, the endocarp having approximately half the number of cells of approximately twice the size than the mesocarp (Rosati et al., 2011a). Notwithstanding these differences, fruit tissue size strongly correlates to ovary tissue cell number, with a similar quantitative relationship for both tissues (Rosati et al., 2012; Rosati et al., 2020), which implies that the endocarp and mesocarp produce a similar mass in the fruit, for each cell in the ovary, despite having different cell sizes and numbers at anthesis. This could derive from a similar cell division rate for the two tissues, while the smaller cells of the mesocarp allow for a more extended period of cell expansion (explaining the longer growth of the mesocarp). This would lead to both tissues having a similar cell size in the ripe fruit. A similar cell division rate, combined with a similar final cell size, would lead to a similar final number of cells in the fruit, of similar size, for every ovary cell, notwithstanding initial cell size differences in the ovary. Accordingly, in young cucurbit ovaries, cell division rate is very similar for all tissues, despite different cell sizes and differentiation level, the inner tissues having bogger cells than the outer tissues (Sinnott, 1942), as also found in olive. Unfortunately, no studies report the cell size in the olive endocarp, probably because of the eventual lignification of this tissue, which makes measurements difficult.
2.5 Cell number rather than tissue mass determines sink strength
As mentioned in the previous sections, in olive, fruit endocarp and mesocarp size at harvest correlates with tissue cell number in the ovary, with a single quantitative relationship across tissues and genotypes (Rosati et al., 2012; Rosati et al., 2020), suggesting that the growth potential and sink strength of both tissues are functions of their cell number. In some species, like melon (Higashi et al., 1999), pear (Zhang et al., 2006), and cherry (Olmstead et al, 2007), genetic differences in fruit size and cell number arise from different extents of cell division after antheses, instead of before. Nonetheless, fruit size still correlates with fruit cell number. This suggests that cell number is a valid indicator of sink size in fruits (Ho, 1988, Ho, 1992; Bertin, 2005; Génard et al., 2007) although cell number is not the only factor affecting sink strength (Gillaspy et al., 1993; Marcelis et al, 1998). This agrees also with the suggestion that cell division before bloom determines potential fruit size (Ho, 1996), even if an important amount of cell division continues after bloom (Scorzal et al., 1991), as is the case in olive (Hammami et al., 2011). In fact, ovary cell number before anthesis is considered a critical determinant of the sink strength of the developing fruit and is genetically controlled (Coombe, 1976). However, within tissues, cell size is similar across olive cultivars, in the ovary (Rosati et al., 2011a) as well as in the fruit (Rapoport et al., 2004), and thus, cell number correlates closely with tissue size. Therefore, the correlation between tissue cell number in the ovary and tissue size in the fruit results in correlations also between tissue size in the ovary and tissue size in the fruit although, in this case, not with a single quantitative relationship but with different regressions between tissues (Rosati et al., 2012). Thus, a causal relationship between tissue growth and tissue mass cannot be excluded.
Sink strength is the product of sink size and sink activity (Warren Wilson, 1972). This is often interpreted as the product of sink mass and relative growth rate (RGR) (Marcelis et al., 1998). However, RGR decreases exponentially during fruit development (Bangerth and Ho, 1984; Marcelis, 1992; Grossman and DeJong, 1995), and there is no mechanistic explanation for this decrease. An alternative approach is that of Ho, 1988; Ho, 1992 and Génard et al. (2007), who suggest that cell number represents sink size while the cell growth rate potential represents sink activity. The first approach (i.e., sink strength = fruit mass × RGR) is more practical since fruit mass is more easily measured than cell number. For this reason, it has been extensively used for fruit growth modeling (e.g., Grossman and DeJong, 1994). Such models, however, are not mechanistic but only phenomenological, and there is no evidence of a causal relationship between mass and sink size; on the contrary, there is evidence that there is no causal link (Marcelis, 1996). Starck and Ubysz (1974) reasoned that growth rate rather than organ size determines the partitioning of dry matter into an organ. In fact, in other models, sink size is represented by cell number and sink activity by cell growth rate (Génard et al., 2007; Fanwoua et al., 2013).
While both approaches work, empirically, it remains to be determined whether fruit (and tissue) growth is determined by tissue size or cell number. As discussed above, the fact that, at least in olive, tissue size in the fruit correlates with cell number in the ovary with a single relationship across tissues, while separate relationships occur between tissue size in the fruit and tissue size in the ovary, suggests that cell number rather than tissue mass drives tissue growth (Rosati et al., 2012). Further evidence for this stems from the observation that fruit growth is initially exponential, then virtually linear, both in olive and in other species, like apple, where this pattern has been defined as an expolinear growth model (Lakso et al., 1995). The exponential phase of the growth overlaps roughly with the cell division stage, while the linear growth occurs later when cell division ceases or slows down considerably. In fact, even differences in growth rate between different crop load treatments were related to differences in fruit cell number (Lakso et al., 1995), implying that the fruit growth rate per cell is constant. These observations suggest that fruit growth rate is proportional to cell number during the whole growth period, increasing while cell number increases, then remaining constant when cell number is constant. Further evidence that cell number (and not organ mass) drives fruit growth was obtained by using the diploid olive cultivar Leccino and its tetraploid genotype (Rosati et al., 2020). The tetraploid genotype was obtained by mutagenesis (Rugini et al., 2016) and has bigger ovaries, but the bigger size results from larger cells in approximately equal numbers (Caporali et al., 2014), unlike usually found across cultivars, where cell number explains different ovary sizes (Rosati et al., 2011a). The larger ovaries of the tetraploid grew at a similar absolute rate (thus lower RGR) and reached a similar final size as the diploid cultivar (instead of a proportionally greater size), breaking the rule that final tissue mass in the fruit correlates with initial tissue mass in the ovary (Rosati et al., 2020). However, the final tissue mass in the fruit remained correlated to initial cell number in the ovary, strongly supporting the hypothesis that it is the cell number and not the tissue mass that is causally linked to tissue growth.
2.6 Further considerations about the dependence of fruit growth on cell number
The hypothesis that fruit growth is causally related to cell number is supported by the fact that, in many species, such as tomato (Nesbitt and Tanksley, 2001) and many others (reviewed in Guo and Simmons, 2011), fruit size is affected by genes (such as fw2.2), controlling cell division before bloom. Similar genes have been found also in olive (Cirilli et al., 2012; Cirilli et al., 2013). These genes act generally across species and organs (Guo et al., 2010). Therefore, in olive, fruit growth appears to be controlled by genes regulating cell number in the ovary tissues before bloom. In fact, considering that all flower parts (petals and stamens) also vary in size with the ovary (Rosati et al., 2009), it seems that these genes act on all flower parts and tissues.
Genes regulating cell division before bloom might not control fruit size in other species where fruit size differences across cultivars result from different post-bloom duration of cell division, rather than from differences in ovary size and cell number at bloom, as in melon (Higashi et al., 1999), pear (Zhang et al., 2006), and cherry (Olmstead et al, 2007). In such species, fruit size might be controlled by genes that regulate the duration of cell division after anthesis. Similarly, fruit size might not correlate with ovary size in species where the fruit does not develop directly from the ovary but derives from secondary tissues that cellularize after bloom, as in cereals (Benincasa et al., 2017; Benincasa et al., 2022). The expression of genes regulating cell division and thus fruit size is still subject to the availability of resources, as shown in tomato (Baldet et al., 2006). Inadequate nutrition induces proportional sterility by increasing ovary abortion or even failure to flower (Marcucci, 1950).
3 Factors affecting flower and fruit number
Olive trees have abundant flower production but low fruit set: typically, only 1%–3% of flowers turn into a fruit (Hartmann, 1950). Fruit set depends on flower quality, including pistil abortion, and on factors occurring both before and after flowering. This review considers the effects of ovary size and cell number (and the consequent sink strength and competition ability) on pistil abortion and fruit set. Evolutionary reasons for abundant flowering and low fruit set in this species will also be considered.
3.1 Fruit size and pistil abortion
Pistil or ovary abortion is an expression indicating the presence of flowers with absent or only partly formed ovaries, thus ovaries that are unable to develop into fruits. Such flowers are called staminate flowers and have only the male organs fully developed and functional. Normal flowers, which are the majority in olive, have both female and male organs fully developed and functional and are called hermaphrodite flowers. In olive, pistil abortion varies largely with cultivar and year, but also among trees for the same cultivar and within a single tree, among branches and shoots, as well as among and within inflorescences (Morettini, 1939; Bottari, 1951; Badr and Hartmann, 1971; Fabbri et al., 2004; Martin and Sibbett, 2005). Pistil abortion appears to occur at an early time (30–40 days before anthesis) during flower development (Pirotta and De Pergola, 1913; Uriu, 1959; Cuevas et al., 1999; Reale et al., 2006).
In olive, pistil abortion results mostly from resource competition since resources are insufficient for all flowers to develop, given the abundant flowering. This competition starts early, affecting both ovary abortion and fruit set (Hartmann, 1950; Uriu, 1959; Cuevas et al., 1994; Perica et al., 2001; Levin and Lavee, 2005). Conditions that decrease available resources or increase competition among flowers and fruits usually increase pistil abortion and decrease fruit set. These conditions include N deficiency, water stress (Melis, 1923; Brooks, 1948; García Gálvez, 2005; Rapoport et al., 2012), heat stress (Benlloch-González et al., 2018), and insufficient photosynthetically active radiation (Bottari, 1951; Dimassi et al., 1999). They also include foliar diseases and low leaf-to-bud ratio (Petri, 1920; Morettini, 1951; Uriu, 1953; Uriu, 1959; Fernandez-Escobar et al., 2008), abundant flowering (Reale et al., 2006) or inflorescence position in the canopy that are unfavorable (Cuevas and Polito, 2004; Seifi et al., 2008), and adverse weather and high previous-year yield (Rallo et al., 1981; Rapoport and Rallo, 1991; Cuevas et al., 1994; Lavee, 1996). The fact that aborted flowers do not contain starch also suggests a link between trophic levels and ovary abortion (Reale et al., 2009). Additionally, when the distal half of the inflorescences are removed, ovary abortion decreases drastically in the remaining flowers (Seifi et al., 2008).
Ovary abortion is also known to vary across cultivars (Campbell, 1911; Morettini, 1939; Morettini, 1951; Magherini, 1971; Lavee, 1996; Lavee et al., 2002). In olive, Rosati et al. (2011b) suggested that even the genetic component of pistil abortion may be explained in terms of competition. In fact, larger-fruited cultivars often have greater rates of ovary abortion (Morettini, 1939; Magherini, 1971; Acebedo et al, 2000; Rosati et al., 2011b) but also larger ovaries and flowers (Rosati et al., 2009). This implies greater use of resources per flower and greater sink strength (i.e., greater competition ability) of individual flowers/ovaries, related to their greater cell number (Rosati et al., 2012; Rosati et al., 2020). As discussed above, greater competition ability leads to greater abortion.
3.2 Fruit set and fruit size
As already mentioned, in olive, the fruit set is low (Hartmann, 1950). It is tempting to assume that greater yields could be achieved by increasing fruit set. However, when the flower number is experimentally reduced, the fruit set increases, resulting in a similar fruit load (Suarez et al., 1984; Rallo and Fernandez-Escobar, 1985; Lavee et al., 1996; Lavee et al., 1999). This suggests a tendency of the tree to set a fixed mass of fruit, independent of the number of flowers. Previous authors refer to this fixed mass as the tree fruiting potential (Lavee et al., 1996), which reflects source availability. When the fruit set reaches such potential, the rest of the flowers will drop. Accordingly, poor nutrition decreases proportionally the plant’s fertility, completely blocking flower formation in extreme cases (Marcucci, 1950). Heat stress also decreases fruit set (Benlloch-González et al., 2018). In olive, competition for resources among flowers has been extensively reported (Suarez et al., 1984; Rapoport and Rallo, 1991; Cuevas et al., 1994; Cuevas et al., 1995; Lavee et al., 1999; Seifi et al., 2008; Rosati et al., 2010, Rosati et al. 2011b). Rugini and Pannelli (1993) found that fruit set increases when competition between flowers and shoots is relieved by mechanically or chemically slowing down shoot growth, further supporting the hypothesis that fruit set is limited by resource availability.
Rosati et al. (2010) found that large-fruited cultivars, which have larger ovaries and flowers, have a lower fruit set so that the total fruit mass is similar. This suggests that there is compensation across cultivars between fruit size and number. Similar results had been found by Rallo and Fernandez-Escobar (1985). Thus, genetic differences in fruit set also appear to be explainable in terms of competition for resources among ovaries/flowers/fruits of different sizes, that is, in large-fruited cultivars, larger ovaries have more cells and thus greater sink strength, therefore increasing competition and decreasing fruit set. This also explains why cultivars with small fruits, like Arbosana and Arbequina for instance, set several fruits per inflorescence, whereas cultivars with large fruits produce typically only one fruit, of bigger size, per inflorescence. It could be argued that small-fruited cultivars might produce small fruits as a result of higher fruit set and the consequent increase in source competition among fruits. This is unlikely the case because the fruit is already smaller at and before anthesis (i.e., smaller ovary), which is before the fruit set occurs. Additionally, when competition between fruits is reduced by thinning, fruit size increases only marginally (compared with possible differences among cultivars) and does not eliminate cultivar differences (Rosati et al., 2010), suggesting that these are genetically predetermined.
The final fruit size within a cultivar is still subject to the availability of resources, varying up to two-fold within cultivars. However, fruit size is much more variable among cultivars (Barranco, 1999), up to six-fold (Rosati et al., 2009). Therefore, regulating fruit load across cultivars with extremely different fruit sizes necessitates a greater compensation mechanism than the regulation of fruit size within cultivar: this mechanism seems to be the regulation of fruit set (i.e., higher fruit set in small-fruited cultivars).
3.3 Andromonoecy, redundant flowering, and fitness
As discussed above, the olive flowers redundantly, relative to its potential yield and fruit set, which is particularly low. We already discussed how pistil abortion increases at increasing resource competition since abortion allows resource-saving, matching the number of ovaries to the available resources (Primack and Lloyd, 1980; Bertin, 1982; Stephenson and Bertin, 1983). Plants that abort part of the ovaries in flowers that would otherwise be hermaphrodite, like the olive, are called andromonoecious. There are approximately 4,000 andromonoecious species (Yampolsky and Yampolsky, 1922; Richards, 1986; Miller and Diggle, 2003). In evolutionary terms, andromonoecy is considered an intermediate step toward dioecy, allowing to modulate resource allocation to the female function, in response to resource availability (Lloyd, 1980; Solomon, 1985; Sutherland, 1986; Diggle, 1994; Miller and Diggle, 2003). Andromonoecy allows to save resources that would be wasted in surplus ovaries, without affecting the plant’s number of flowers: this maintains the plant’s male function and fitness (Primack and Lloyd, 1980; Bertin, 1982; Stephenson and Bertin, 1983; Vallejo-Marín and Rausher, 2007). Pistil-aborted flowers have less mass (between 19% and 41% less) than hermaphrodite flowers in olive because ovaries and petals are smaller while stamens maintain their size and pollen production (Cuevas and Polito, 2004), implying resource-saving with andromonoecy.
In olive, cultivars with large fruits have greater pistil abortion, but the number of flowers is similar to that of small-fruited cultivars (Rosati et al., 2010; Rosati et al., 2011b); thus, the male function (and fitness) remains probably unaffected. This explains why only the pistil is aborted and not the whole flower, despite the fact that aborting the whole flower would save much more resources, given that the flower is several times bigger than the ovary alone (Cuevas and Polito, 2004; Rosati et al., 2009). Aborting the whole flower would reduce the male fitness, while aborting only the ovary maintains the male function and thus the male fitness. Figure 1 illustrates and summarizes the male and female fitness in the olive. Maintaining the male fitness also explains why the flower production is so excessive (i.e., low fruit set) compared with the fruiting potential of the tree: pollen production is less expensive than producing fruits and seeds while still assuring the male fitness. This makes it convenient to produce more (male) flowers than fruits. Morgan (1993) demonstrates that plants maximize their fitness when they produce many more flowers than fruits, and the optimal flower/fruit ratio is higher for andromonoecious and monoecious species compared with dioecious species. The optimal ratio increases further for anemophilous species, like the olive.
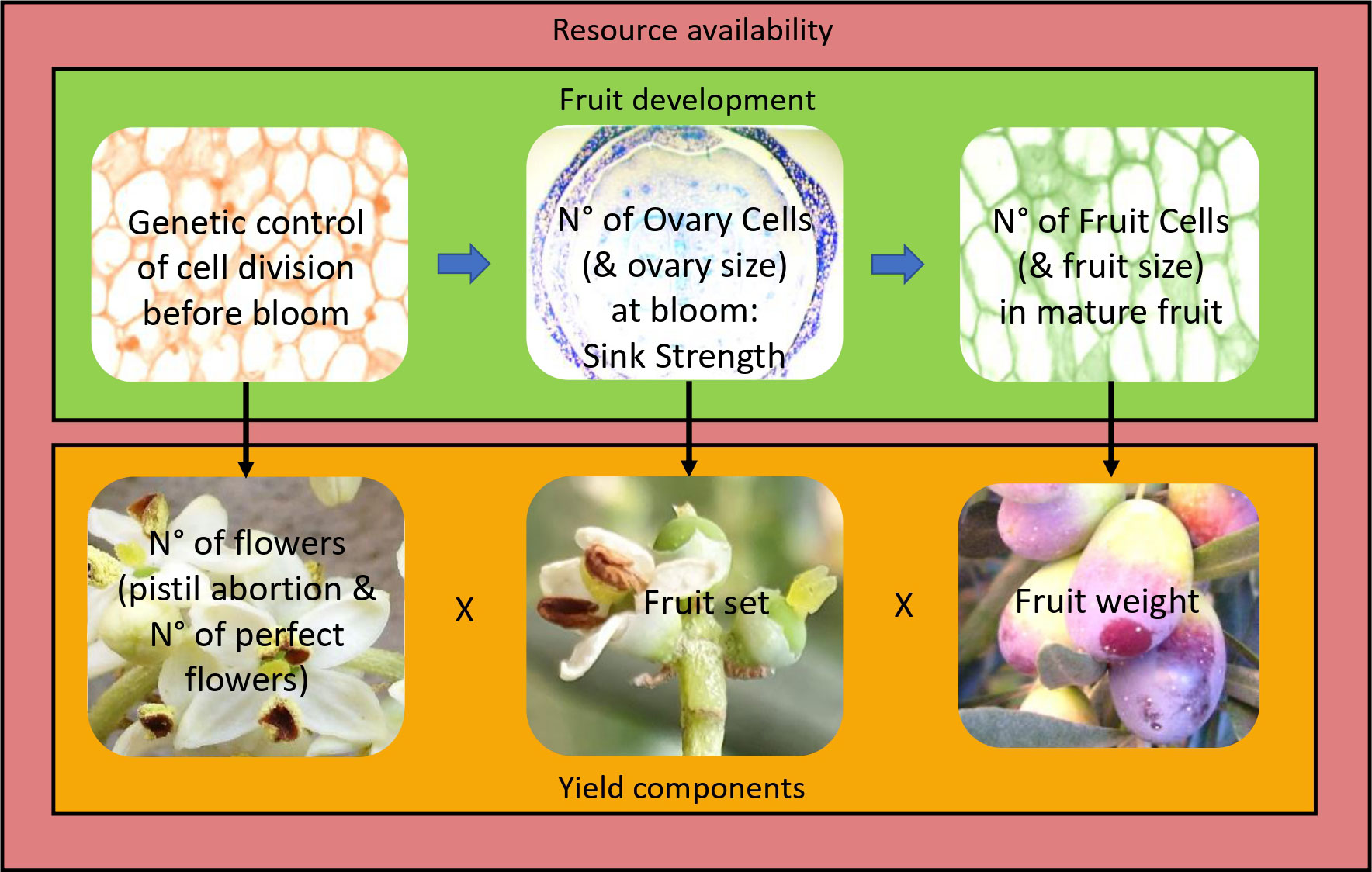
Figure 1 Proposed model of fruit development and yield formation. Cell division in the forming ovary (and other flower parts) is under genetic control. This determines cell number (and tissue size) and thus sink strength in the ovary at bloom. This in turn determines cell number in the fruit and potential fruit size. Yield is the product of the number of flowers (considering pistil abortion and thus number of perfect flowers) × fruit set × average fruit weight. All phases of fruit development and all yield components are modulated by resource availability (outside box), which is a function of the agronomic conditions (e.g., light interception, water and nutrient availability, biotic and abiotic stresses) and endogenous factors (e.g., alternate bearing, source–sink relationships, competition between vegetative growth and reproduction). Increasing resource availability will increase cell number and/or size and thus organ (flower/ovary/fruit) size at all stages of fruit development, as well as flower number, percent of perfect flowers, fruit set, and fruit weight. However, for a given resource budget, genetically larger flowers/ovaries/fruits, made up of more cells, will increase organ sink strength and competition. Thus, larger pre-anthesis flowers/ovaries will increase pistil abortion, and larger flowers/ovaries at bloom will decrease fruit set, in a compensatory manner between organ size and number, so that yield is virtually unaffected by organ size differences and mainly affected by the fruiting potential of the tree, as determined by resource availability. Arrows indicate how a fruit’s developmental stage affects the following stage (horizontal arrows) or a yield component (vertical arrows).
Therefore, the apparently redundant bloom in olive, while representing a waste of resources from an agronomical perspective, is useful to the tree in evolutionary terms, increasing the fitness of the plant. It might be desirable, therefore, to breed olive cultivars with reduced flowering (reduced male fitness), allowing the plant to save resources, which can then be invested in fruit set and development. In fact, the cost of flowering is significant in olive: inflorescence development consumes resources at a rate similar to that of fruit development (Famiani et al., 2019). Indeed, thinning up to 50% of inflorescences has resulted in increased overall fruit load per shoot (Lavee et al., 1999): this might be attributable to resource-saving for flowering. Preventing the formation of 95% of the inflorescences, rather than thinning 50% of them after their formation, could potentially save more resources and increase fruit load further.
4 Conclusions
From the reviewed literature, it may be concluded that, across olive cultivars of different potential fruit sizes, potential fruit growth is controlled by the ovary tissue characteristics at anthesis. Variations in ovary size, across and within cultivars, arise from parallel variations in the size of all tissues (i.e., endocarp and mesocarp). Understanding how sink strength and fruit (and tissue) growth are determined is of great importance, both scientifically and agronomically, and it is indispensable for developing mechanistic fruit growth models. From the literature quoted in this review, it may be concluded that, at least in olive, although fruit tissue size in the mature fruit generally correlates with ovary tissue size (with separate correlations for endocarp and mesocarp), tissue cell number is more likely to be the functional determinant of fruit and tissue growth. Therefore, fruit tissue size in olive appears to be controlled by genes regulating cell division before anthesis. The tissue RG, from anthesis to the fruit, is closely related to the size of the cells in the ovary, across both cultivars and tissues: this suggests that cell size in the ovary is indicative of tissue growth stage.
Resource competition among developing flowers and fruits appears to play a continuous and fundamental role in adjusting fruit load to the available resources, during the whole fruit development. Pistil abortion first, then fruit set, fruit drop, and finally fruit size appear to be different and sequential mechanisms of this same strategy. Also, the genetic component (i.e., greater pistil abortion and lower fruit set in cultivars with larger ovary/flower/fruit size) can be explained with the competition hypothesis, based on the different sink strength and energy costs for the development of ovaries/flowers/fruits of different sizes. Figure 2 illustrates and summarizes the interaction between resource availability, fruit development, and yield components. It can be concluded that, in the absence of dramatic events that might compromise fruit set and/or development (e.g., pollination problems, extreme drought, inadequate nutrition), the olive tree appears to regulate fruit load according to its yield potential, regardless of the number of flowers produced. Hence, correlations between the flowering amount (or the amount of pollen in the air) and yield (Moriondo et al., 2001; Fornaciari et al., 2002; Galán et al., 2004) do not entail a causal relationship. They just indicate that the phenomena are correlated: both flowering and yield levels reflect resource availability and the yield potential of the tree. The most effective way to increase yield, again in the absence of dramatic events that compromise fruit set and/or development, appears to be improving the tree’s ecophysiological status (i.e., improving nutrition and avoiding biotic and abiotic stresses), thus increasing its fruiting potential. The apparently redundant flowering in olive (i.e., low fruit set), although wasteful agronomically, serves the purpose of increasing the male fitness, which is an advantage in evolutionary terms. Selecting for reduced male fitness (i.e., reduced flowering) might allow to reallocate the saved resources to female fitness, possibly increasing yield somewhat.
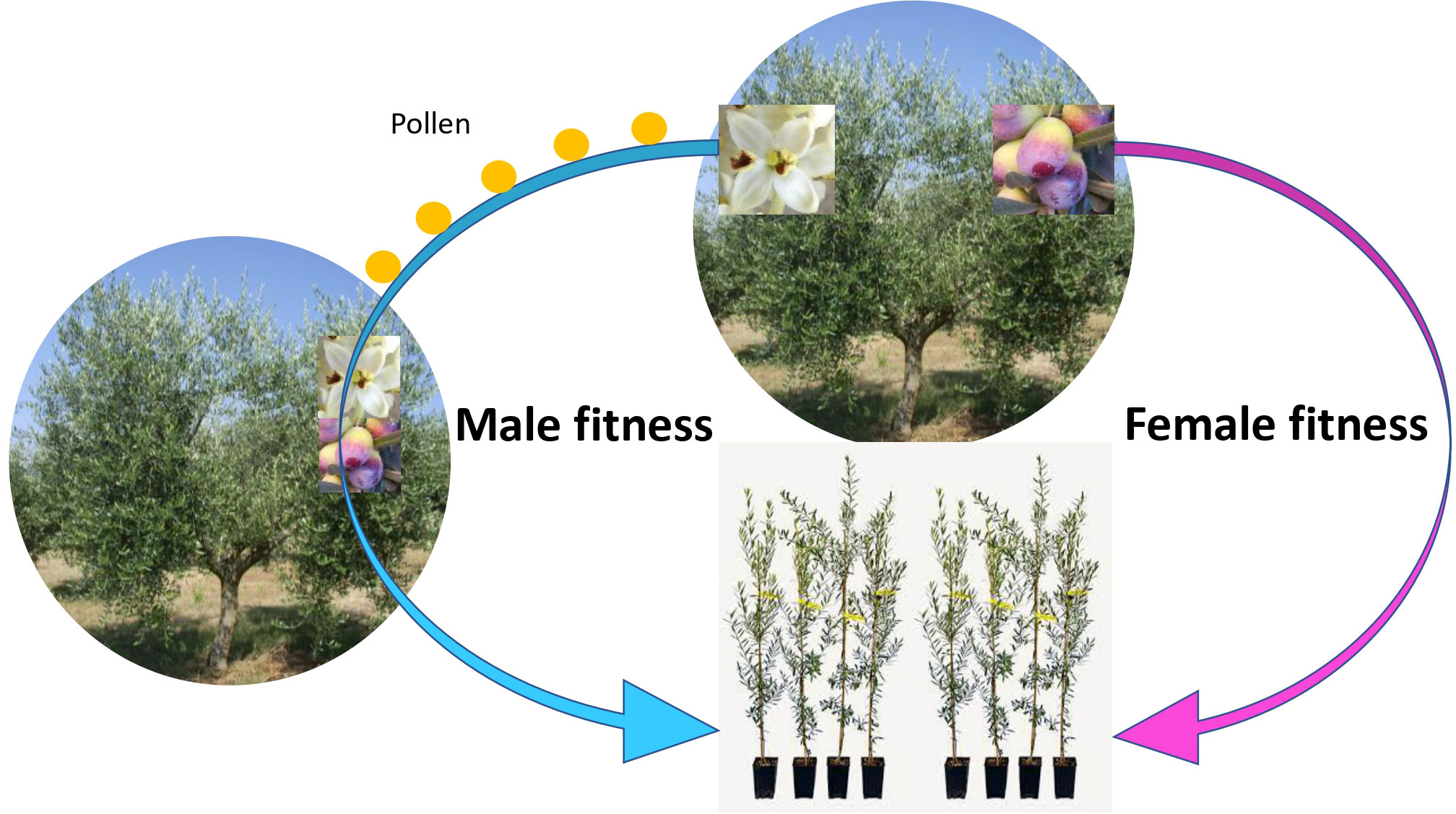
Figure 2 Schematic representation of male and female fitness. The tree invests in fruits to increase its female fitness (producing offsprings with 50% of the genome from the mother plant). However, producing pollen is just as effective at producing offsprings with 50% of the genome of the father plant, via pollinizing other trees. In fact, the biomass and resource investment per offspring might be smaller with the male fitness pattern. Redundant flowering (i.e., low fruit set) in olive, while being agronomically inefficient, is efficient in terms of fitness, thus in evolutionary terms.
Author contributions
AR, FF: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing. EML: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acebedo, M. M., Cañete, M. L., Cuevas, J. (2000). Processes affecting fruit distribution and its quality in the canopy of olive trees. Adv. Hortic. Sci. 14, 169–175. doi: 10.1400/14060
Badr, S. A., Hartmann, H. T. (1971). Effect of diurnally fluctuating vs. constant temperatures on flower induction and sex expression in olive (Olea europaea L.). Physiol. Plant 24, 40–45. doi: 10.1111/j.1399-3054.1971.tb06712.x
Baldet, P., Hernould, M., Laporte, F., Mounet, F., Just, D., Mouras, A., et al. (2006). The expression of cell proliferation-related genes in early developing flowers is affected by a fruit load reduction in tomato plants. J. Exp. Bot. 57, 961–970. doi: 10.1093/jxb/erj082
Bangerth, F., Ho, L. C. (1984). Fruit position and fruit set sequence in a truss as factors determining final size of tomato fruits. Ann. Bot. 53, 315–320. doi: 10.1093/oxfordjournals.aob.a086695
Barranco, D. (1999). “ariedades y patrones,” in El cultivo del olivo. Eds. Barranco, D., Fernàndez-Escobar, R., Rallo, L. (Madrid: Ed. Mundi-Prensa), 63–89.
Beede, R. H., Goldhamer, D. A. (1994). “Olive irrigation management,” in Olive production, manual. Eds. Ferguson, L., Sibbett, G., Martin, G. (Oakland: University of Californian), 61–68.
Benincasa, P., Reale, L., Cerri, M., Tedeschini, E., Tosti, G., Falcinelli, B., et al. (2022). Nitrogen fertilization levels and timing affect the plasticity of yield components in bread wheat (Triticum aestivum L.). Field Crops Res. 289, 108734. doi: 10.1016/j.fcr.2022.108734
Benincasa, P., Reale, L., Tedeschini, E., Ferri, V., Cerri, M., Ghitarrini, S., et al. (2017). The relationship between grain and ovary size in wheat: An analysis of contrasting grain weight cultivars under diffierent growing conditions. Field Crop Res. 210, 175–182. doi: 10.1016/j.fcr.2017.05.019
Benlloch-González, M., Sánchez-Lucas, R., Benlloch, M., Fernández-Escobar, R. (2018). An approach to global warming effects on flowering and fruit set of olive trees growing under field conditions. Sci. Hortic. 240, 405–410. doi: 10.1016/j.scienta.2018.06.054
Bergh, O. (1985). Effect of the previous crop on cortical cell number of Malus domestica cv. “Starking Delicious” apple flower primordia, flowers and fruit. South Afric. J. Plant Soil 2, 191–196. doi: 10.520/AJA02571862_56
Bertin, N. (2005). Analysis of the tomato fruit growth response to temperature and plant fruit load in relation to cell division, cell expansion and DNA endoreduplication. Ann. Bot. 95, 439–447. doi: 10.1093/aob/mci042
Bertin, N., Borel, C., Brunel, B., Cheniclet, C., Causse, M. (2003). Do genetic make-up and growth manipulation affect tomato fruit size by cell number, or cell size and DNA endoreduplication. Ann. Bot. 92, 415–424. doi: 10.1093/aob/mcg146
Bertin, N., Gautier, H., Roche, C. (2002). Number of cells in tomato fruit depending on fruit position and source-sink balance during plant development. Plant Growth Regul. 36, 105–112. doi: 10.1023/A:1015075821976
Bohner, J., Bangerth, F. (1988). Cell number, cell size and hormone levels in semi-isogenic mutants of Lycopersicon pimpinellifolium differing in fruit size. Physiol. Plant 72, 316–320. doi: 10.1111/j.1399-3054.1988.tb05839.x
Bottari, V. (1951). ). Quattro anni di infruttuose osservazioni sull’aborto dell’ovario nel fiore dell’olivo. Ann. Sper. Agr. 5, 359–376.
Brooks, R. M. (1948). Seasonal incidence of perfect and staminate olive flowers. Proc. Am. Soc Hortic. Sci. 52, 213–218.
Caporali, S., Hammami, S. B. M., Moreno-Alìas, I., Rapoport, H. F., Chiancone, B., Germanà, M. A., et al. (2014). Effects of tetraploidy on olive floral and fruit biology. Sci. Hortic. 179, 198–203. doi: 10.1016/j.scienta.2014.09.012
Cheng, G. W., Breen, P. J. (1992). Cell count and size in relation to fruit size among strawberry cultivars. J. Am. Soc Hortic. Sci. 117, 946–950. doi: 10.21273/JASHS.117.6.946
Cirilli, M., Caporali, S., Frioni, E., Paoletti, A., Zega, A., Latini, G., et al. (2013). “I geni OeCNR regolano la dimensione della drupa di olivo sin dalle prime fasi dello sviluppo dell’ovario?",” in Riassunti dei lavori, X giornate Scientifiche SOI, Padova, 25-27 giugno 2013, vol. 12 . Eds. Bonghi, C., Sambo, P. (Firenze: Società di Ortoflrofrutticoltura Italiana (SOI)) Acta Italus Hortus), 88, ISBN: ISBN: 978-88-905628-3-9.
Cirilli, M., Muleo, R., Caporali, S., Frioni, E., Paoletti, A., Rapoport, H. F., et al. (2012). Olive fruit size is regulated early in the flower (Italy: Proceedings of the 56th Italian Society of Agricultural Genetics Annual Congress).
Coombe, B. G. (1976). The development of fleshy fruits. Ann. Rev. Plant Physiol. 27, 207–228. doi: 10.1146/annurev.pp.27.060176.001231
Corelli-Grappadelli, L., Lakso, A. (2004). Fruit development in deciduous tree crops as affected by physiological factors and environmental conditions. Acta Hortic. 636, 425–441. doi: 10.17660/ActaHortic.2004.636.52
Costagli, G., Gucci, R., Rapoport, H. F. (2003). Growth and development of olive fruits (cv. ‘Frantoio’ under irrigated and rainfed conditions. J. Hortic. Sci. Biotech. 78, 119–124. doi: 10.1080/14620316.2003.11511577
Cowan, A. K., Moore-Gordon, C. S., Bertling, I., Wolstenholme, B. N. (1997). Metabolic control of avocado fruit growth (isoprenoid growth regulators and the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase). Plant Physiol. 114, 511–518. doi: 10.1104/pp.114.2.511
Cruz-Castillo, J. G., Lawes, G. S., Woolley, D. J. (1991). The influence of the time of anthesis, seed factors, and the application of a growth regulator mixture on the growth of kiwifruit. Acta Hortic. 297, 475–480. doi: 10.17660/ActaHortic.1992.297.62
Cuevas, J., Oller, R. (2000). Olive seed set and its impact on seed and fruit weight. Acta Hortic. 586, 485–488. doi: 10.17660/ActaHortic.2002.586.100
Cuevas, J., Pinney, K., Polito, V. S. (1999). Flower differentiation, pistil development and pistil abortion in olive. Acta Hortic. 474, 293–296. doi: 10.17660/ActaHortic.1999.474.59
Cuevas, J., Polito, V. S. (2004). The role of staminate flowers in the breeding system of Olea europaea (Oleaceae): an andromonoecious, wind-pollinated Taxon. Ann. Bot. 93, 547–553. doi: 10.1093/aob/mch079
Cuevas, J., Rallo, L., Rapoport, H. F. (1994). ). Crop load effects on floral quality in olive. Sci. Hortic. 59, 123–130. doi: 10.1016/0304-4238(94)90079-5
Cuevas, J., Rallo, L., Rapoport, H. F. (1995). Relationships among reproductive processes and fruitlets abscission in 'Arbequina' olive. Adv. Hortic. Sci. 9, 92–96.
Denne, M. P. (1960). The growth of apple fruitlets, and the effect of early thinning on fruit development. Ann. Bot. 24, 397–406. doi: 10.1093/oxfordjournals.aob.a083713
Diggle, P. K. (1994). The expression of andromonoecy in Solanum hirtum (Solanaceae): phenotypic plasticity and ontogenetic contingency. Am. J. Bot. 81, 1354–1365. doi: 10.1002/j.1537-2197.1994.tb11457.x
Dimassi, K., Therios, I., Balatsos, A. (1999). The blooming period and self-fruitfulness in twelve Greek and three foreign olive cultivars. Acta Hortic. 474, 275–278. doi: 10.17660/ActaHortic.1999.474.55
Fabbri, A., Bartolini, G., Lambardi, M., Kailis, S. G. (2004). Olive propagation manual. Collingwood (Victoria: Landlinks Press).
Famiani, F., Farinelli, D., Gardi, T., Rosati, A. (2019). The cost of flowering in olive (Olea europaea L.). Sci. Hortic. 252, 268–273. doi: 10.1016/j.scienta.2019.03.008
Fanwoua, J., de Visser, P. H. B., Heuvelink, E., Yin, X., Struik, P. C., Marcelis, L. F. M. (2013). A dynamic model of tomato fruit growth integrating cell division, cell growth and endoreduplication. Funct. Plant Biol. 40, 1098–1114. doi: 10.1071/FP13007
FAO (2023) ) faostat, FAO statistics division. Available at: http://faostat3.fao.org (Accessed 30 May 2023).
Fernandez-Escobar, R., Ortiz-Urquiza, A., Prado, M., Rapoport, H. F. (2008). Nitrogen status influence on olive tree flower quality and ovule longevity. Environ. Exp. Bot. 64, 113–119. doi: 10.1016/j.envexpbot.2008.04.007
Fornaciari, M., Pieroni, L., Orlandi, F., Romano, B. (2002). A new approach to consider the pollen variable in forecasting yield models. Econ. Bot. 56, 66–72. doi: 10.1663/0013-0001(2002)056[0066:ANATCT]2.0.CO;2
Galán, C., Vázquez, L., García-Mozo, H., Domínguez, E. (2004). ). Forecasting olive (Olea europaea) crop yield based on pollen emission. Field Crops Res. 86, 43–51. doi: 10.1016/S0378-4290(03)00170-9
García Gálvez, G. (2005). Calidad de flor del olivo en relación al régimen de riego y a la salinidad (Trabajo Professional Fin de Carrera, E.T.S.I.A.M. Spain: University of Cordoba).
Génard, M., Bertin, N., Borel, C., Bussières, P., Gautier, H., Habib, R., et al. (2007). Towards a virtual fruit focusing on quality: modelling features and potential uses. J. Exp. Bot. 58, 917–928. doi: 10.1093/jxb/erl287
Gillaspy, G., Ben-David, H., Gruissem, W. (1993). Fruits: a developmental perspective. Plant Cell 5, 1439–1451. doi: 10.1105/tpc.5.10.1439
Grossman, Y. L., DeJong, T. M. (1994). Peach: a simulation model of reproductive and vegetative growth in peach trees. Tree Physiol. 14, 329–345. doi: 10.1093/treephys/14.4.329
Grossman, Y. L., DeJong, T. M. (1995). Maximum fruit growth potential following resource limitation during peach growth. Ann. Bot. 75, 561–567. doi: 10.1006/anbo.1995.1059
Gucci, R., Lodolini, E. M., Rapoport, H. F. (2009). Water deficit-induced changes in mesocarp cellular processes and the relationship between mesocarp and endocarp during olive fruit development. Tree Physiol. 29, 1575–1585. doi: 10.1093/treephys/tpp086
Guo, M., Rupe, M. A., Dieter, J. A., Zou, J., Spielbauer, D., Duncan, K. E., et al. (2010). Cell number regulator1 affects plant and organ size in maize: Implications for crop yield enhancement and heterosis. Plant Cell 22, 1057–1073. doi: 10.1105/tpc.109.073676
Guo, M., Simmons, C. R. (2011). Cell number counts –The fw2.2 and CNR genes and implications for controlling plant fruit and organ size. Plant Sci. 181, 1–7. doi: 10.1016/j.plantsci.2011.03.010
Hamada, K., Hasegawa, K., Kitajima, A., Ogata, T. (2008). The relationship between fruit size and cell division and enlargement in cultivated and wild persimmons. J. Hortic. Sci. Biotechn. 83, 218–222. doi: 10.1080/14620316.2008.11512372
Hammami, S. B. M., Manrique, T., Rapoport, H. F. (2011). Cultivar based fruit size in olive depends on different tissue and cellular processes throughout growth. Sci. Hortic. 130, 445–451. doi: 10.1016/j.scienta.2011.07.018
Handley, D. T., Dill, J. F. (2003). Vegetative and floral characteristics of six strawberry cultivars associated with fruit size, yield and susceptibility to tarnished plant bug injury. Acta Hortic. 626, 161–167. doi: 10.17660/ActaHortic.2003.626.21
Harada, T., Kurahashi, W., Yanai, M., Wakasa, Y., Satoh, T. (2005). Involvement of cell proliferation and cell enlargement in increasing the fruit size of Malus species. Sci. Hortic. 105, 447–456. doi: 10.1016/j.scienta.2005.02.006
Hartmann, H. T. (1950). The effect of girdling on flower type, fruit set, and yields in the olive. Proc. Am. Soc Hortic. Sci. 56, 217–226.
Higashi, K., Hosoya, K., Ezura, H. (1999). Histological analysis of fruit development between two melon (Cucumis melo L. reticulatus) genotypes setting a different size of fruit. J. Exp. Bot. 50, 1593–1597. doi: 10.1093/jxb/50.339.1593
Ho, L. C. (1988). Metabolism and compartmentation of imported sugars in sink organs in relation to sink strength. Ann. Rev. Plant Physiol. Plant Mol. Biol. 39, 355–378. doi: 10.1146/annurev.pp.39.060188.002035
Ho, L. C. (1992). “Fruit growth and sink strength,” in Fruit and seed production: aspects of development, environmental physiology and ecology, vol. 47 . Eds. Marshall, C., Grace, J. (Cambridge, UK: Cambridge University Press), 101–124.
Ho, L. C. (1996). Tomato," in Photoassimilate distribution in plant and crops. Eds. Zamki, E., Shaffer, A. A. (New York: Marcel Dekker, Inc), 709–728.
Jullien, A., Munier-Jolain, N. G., Malèzieux, E., Chillet, M., Ney, B. (2001). Effect of pulp cell number and assimilate availability on dry matter accumulation rate in a banana fruit [Musa sp. AAA group ‘‘Grande Naine’’ (Cavendish subgroup). Ann. Bot. 88, 321–330. doi: 10.1006/anbo.2001.1464
King, J. R. (1938). Morphological development of the fruit of the olive. Hilgardia 11, 435–458. doi: 10.3733/hilg.v11n08p435
Lai, R., Woolley, D. J., Lawes, G. S. (1990). The effect of inter-fruit competition, type of fruiting lateral and time of anthesis on fruit growth of kiwifruit (Actinidia deliciosa). J. Hortic. Sci. 65, 87–96. doi: 10.1080/00221589.1990.11516034
Lakso, A. N., Corelli Grappadelli, L., Barnard, J., Goffinet, M. C. (1995). An expolinear model of the growth pattern of the apple fruit. J. Hortic. Sci. 70, 389–394. doi: 10.1080/14620316.1995.11515308
Lavee, S. (1996). “Biology and physiology of the olive,” in World olive encyclopedia (Barcelona, Spain: Plaza & Janés Editorial), 59–110. IOOC International Olive Oil Council.
Lavee, S., Rallo, L., Rapoport, H. F., Troncoso, A. (1996). The floral biology of the olive: effect of flower number, type and distribution on fruitset. Sci. Hortic. 66, 149–158. doi: 10.1016/S0304-4238(96)00941-7
Lavee, S., Rallo, L., Rapoport, H. F., Troncoso, A. (1999). The floral biology of the olive: II. The effect of inflorescence load and distribution per shoot on fruit set and load. Sci. Hortic. 82, 181–192. doi: 10.1016/S0304-4238(99)00057-6
Lavee, S., Taryan, J., Levin, J., Haskal, A. (2002). The significance of crosspollination for various olive cultivars under irrigated intensive growing conditions. Olivae 91, 25–36.
Lawes, G. S., Woolley, D. J., Lai, R. (1990). Seeds and other factors affecting fruit size in kiwifruit. Acta Hortic. 282, 257–264. doi: 10.17660/ActaHortic.1990.282.33
Levin, A. G., Lavee, S. (2005). The influence of girdling on flower type, number, inflorescence density, fruit set, and yields in three different olive cultivars (Barnea, Picual, and Souri). Aust. J. Agric. Res. 56, 827–831. doi: 10.1071/AR04094
Lloyd, D. G. (1980). Sexual strategies in plants: I. An hypothesis of serial adjustment of maternal investment during one reproductive session. New Phytol. 86, 69–79. doi: 10.1111/j.1469-8137.1980.tb00780.x
Magherini, R. (1971). Osservazioni sull'aborto dell'ovario nell'olivo. L’Agricoltura Italiana 71, 291–301.
Marcelis, L. F. M. (1992). Non-destructive measurements and growth analysis of the cucumber fruit. J. Hortic. Sci. 67, 457–464. doi: 10.1080/00221589.1992.11516271
Marcelis, L. F. M. (1996). Sink strenght as a determinant of dry matter partitioning in the whole plant. J. Exp. Bot. 47, 1281–1291. doi: 10.1093/jxb/47.Special_Issue.1281
Marcelis, L. F. M., Heuvelink, E., Goudriaan, J. (1998). Modelling biomass production and yield of horticultural crops: a review. Sci. Hortic. 74, 83–111. doi: 10.1016/S0304-4238(98)00083-1
Marcucci, G. B. (1950). “Cultivation of the olive. Recent contributions to, knowledge on the floral biology of the olive. A chronological account of the results of some investigations,” in 13th congres international oleicult, vol. 3. (Madrid: Actas Oleicult), 341–351.
Martin, G. C., Sibbett, G. S. (2005). “Botany of the olive,” in Olive production manual. Eds. Sibbett, G. S., Ferguson, L., Coviello, J. L., Lindstrand, M. (Oakland, California: University of California, Agriculture and Natural Resources), 15–19.
McGarry, R., Ozga, J. A., Reinecke, D. M. (2001). Differences in fruit development among large- and small-fruited cultivars of saskatoon (Amelanchier alnifolia). J. Am. Soc Hortic. Sci. 126, 381–385. doi: 10.21273/JASHS.126.4.381
Melis, A. (1923). Cause di aborto nel gineceo del fiore dell’olivo. Staz. Sperim. Agric. Ital. 56, 302–312.
Miller, J. S., Diggle, P. K. (2003). Diversification of andromonoecy in Solanum section Lasiocarpa (Solanaceae): the roles of phenotypic plasticity and architecture. Am. J. Bot. 90, 707–715. doi: 10.3732/ajb.90.5.707
Morettini, A. (1951). ). Ulteriore contributo allo studio dell’aborto dell’ovario nel fiore dell’olivo. Ann. Sperim. Agr. 5, 309–329.
Morgan, M. (1993). Fruit to flower ratio and trade-offs in size and number. Evol. Ecol. 7, 219–232. doi: 10.1007/BF01237740
Moriondo, M., Orlandini, S., De Nuntiis, P., Mandrioli, P. (2001). ). Effect of agrometeorological parameters on the phenology of pollen emission and production of olive trees (Olea europea L.). Aerobiologia 17, 225–232. doi: 10.1023/A:1011893411266
Nesbitt, T. C., Tanksley, S. D. (2001). fw2.2 directly affects the size of developing tomato fruit, with secondary effects on fruit number and photosynthate distribution. Plant Physiol. 127, 575–583. doi: 10.1104/pp.010087
Olmstead, J. W., Iezzoni, A. F., Whiting, M. D. (2007). Genotypic differences in sweet cherry fruit size are primarily a function of cell number. J. Am. Soc Hortic. Sci. 132, 697–703. doi: 10.21273/JASHS.132.5.697
Orgaz, F., Fereres, E. (1999). “Riego,” in El cultivo del olivo. Eds. Barranco, D., Fernández-Escobar, R., Rallo, L. (Madrid, Barcelona, Spain: Mundi-Prensa), 269–288.
Padula, G., Giordani, E., Bellini, E., Rosati, A., Pandolfi, S., Paoletti, A., et al. (2008). Field evaluation of new olive (Olea europaea L.) selections and effects of genotype and environment on productivity and fruit characteristics. Adv. Hortic. Sci. 22, 87–94. doi: 10.1400/94381
Perica, S., Brown, P. H., Connell, J. H., Nyomora, A. M. S., Dordas, C., Hu, H., et al. (2001). Foliar boron application improves flower fertility and fruit set of olive. HortScience 36, 714–716. doi: 10.21273/HORTSCI.36.4.714
Petri, L. (1920). Sulle cause di arresto di sviluppo dell’ovario nel fiore dell’olivo. Ren. Accad. Naz. Lincei 29, 472–477.
Primack, R. B., Lloyd, D. G. (1980). Andromonoecy in the New Zealand montane shrub manuka, Leptospermum scoparium (Myrtaceae). Am. J. Bot. 67, 361–368. doi: 10.1002/j.1537-2197.1980.tb07661.x
Quilot, B., Génard, M. (2008). Is competition between mesocarp cells of peach fruits affected by the percentage of wild species (Prunus davidiana) genome? J. Plant Res. 121, 55–63. doi: 10.1007/s10265-007-0125-9
Rallo, L., Fernandez-Escobar, R. (1985). Influence of cultivar and flower thinning within the inflorescence on competition among olive fruit. J. Am. Soc Hortic. Sci. 110, 303–308. doi: 10.21273/JASHS.110.2.303
Rallo, L., Martin, G. C., Lavee, S. (1981). Relationship between abnormal embryo sac development and fruitfulness in olive. J. Am. Soc Hortic. Sci. 106, 813–817. doi: 10.21273/JASHS.106.6.813
Rallo, P., Rapoport, H. (2001). Early growth and development of the olive fruit mesocarp. J. Hortic. Sci. Biotechn. 76, 408–412. doi: 10.1080/14620316.2001.11511385
Rapoport, H. F., Hammami, S. B. M., Martins, P., Pérez-Priego, O., Orgaz, F. (2012). Influence of water deficits at different times during olive tree inflorescence and flower development. Environ. Exp. Bot. 77, 227–233. doi: 10.1016/j.envexpbot.2011.11.021
Rapoport, H. F., Manrique, T., Gucci, R. (2004). Cell division and expansion in the olive fruit. Acta Hortic. 636, 461–465. doi: 10.17660/ActaHortic.2004.636.56
Rapoport, H. F., Moreno-Alías, I. (2017). “Botanica y morfologia,” in El cultivo del olivo. Eds. Barranco, D., Fernández-Escobar, R., Rallo, L. (Madrid, Spain: Mundi-Prensa), 35–64.
Rapoport, H. F., Rallo, L. (1991). Postanthesis flower and fruit abscission in 'Manzanillo' olive. HortScience 116, 720–723. doi: 10.21273/JASHS.116.4.720
Reale, L., Sgromo, C., Bonofiglio, T., Orlandi, F., Fornaciari, M., Ferranti, F., et al. (2006). Reproductive biology of olive (Olea europaea L.) DOP Umbria cultivars. Sex Plant Reprod. 19, 151–161. doi: 10.1007/s00497-006-0032-6
Reale, L., Sgromo, C., Ederli, L., Pasqualini, S., Orlandi, F., Fornaciari, M., et al. (2009). Morphological and cytological development and starch accumulation in hermaphrodite and staminate flowers of olive (Olea europaea L.). Sex Plant Reprod. 22, 109–119. doi: 10.1007/s00497-009-0096-1
Rosati, A., Caporali, S., Hammami, S. B. M., Moreno-Alìas, I., Paoletti, A., Rapoport, H. F. (2011a). Differences in ovary size among olive (Olea europaea L.) cultivars are mainly related to cell number, not to cell size. Sci. Hortic. 130, 185–190. doi: 10.1016/j.scienta.2011.06.035
Rosati, A., Caporali, S., Hammami, S. B. M., Moreno-Alìas, I., Paoletti, A., Rapoport, H. F. (2012). Tissue size and cell number in the olive (Olea europaea) ovary determine tissue growth and partitioning in the fruit. Funct. Plant Biol. 39, 580–587. doi: 10.1071/FP12114
Rosati, A., Caporali, S., Hammami, S. B. M., Moreno-Alías, I., Rapoport, H. (2020). ). Fruit growth and sink strength in olive (Olea europaea) are related to cell number, not to tissue size. Funct. Plant Biol. 47, 1098–1104. doi: 10.1071/FP20076
Rosati, A., Caporali, S., Paoletti, A., Famiani, F. (2011b). Pistil abortion is related to ovary mass in olive (Olea europaea L.). Sci. Hortic. 127, 515–519. doi: 10.1016/j.scienta.2010.12.002
Rosati, A., Zipanćič, M., Caporali, S., Padula, G. (2009). Fruit weight is related to ovary weight in olive (Olea europaea L.). Sci. Hortic. 122, 399–403. doi: 10.1016/j.scienta.2009.05.034
Rosati, A., Zipanćič, M., Caporali, S., Paoletti, A. (2010). Fruit set is inversely related to flower and fruit weight in olive (Olea europaea L.). Sci. Hortic. 126, 200–204. doi: 10.1016/j.scienta.2010.07.010
Rugini, E., Pannelli, G. (1993). Preliminary results on increasing fruit set in olive (Olea europaea L.) by chemical and mechanical treatments. Acta Hortic. 329, 209–210. doi: 10.17660/ActaHortic.1993.329.45
Rugini, E., Silvestri, C., Ceccarelli, M., Muleo, R., Cristofori, V. (2016). Mutagenesis and biotechnology techniques as tools for selecting new stable diploid and tetraploid olive genotypes and their dwarfing agronomical characterization. HortScience 51, 799–804. doi: 10.21273/HORTSCI.51.7.799
Scorzal, R., May, L. G., Purnell, B., Upchurch, B. (1991). Differences in number and area of mesocarp cells between small-and large-fruited peach cultivars. J. Am. Soc Hortic. Sci. 116, 861–864. doi: 10.21273/JASHS.116.5.861
Seifi, E., Guerin, J., Kaiser, B., Sedgley, M. (2008). Inflorescence architecture of olive. Sci. Hortic. 116, 273–279. doi: 10.1016/j.scienta.2008.01.003
Sinnott, E. W. (1942). An analysis of the comparative rates of cell division in various parts of the developing cucurbit ovary. Am. J. Bot. 29, 317–323. doi: 10.2307/2437566
Solomon, B. P. (1985). Environmentally influenced changes in sex expression in an andromonoecious plant. Ecology 66, 1321–1332. doi: 10.2307/1939185
Starck, Z., Ubysz, L. (1974). Effect of limited supply of assimilates on the relationships between their sources and acceptors. Acta Soc Bot. Poloniae 43427–, 445. doi: 10.5586/asbp.1974.039
Stephenson, A. G., Bertin, R. I. (1983). “Male competition, female choice and sexual selection in plants,” in Pollination biology. Ed. Real, L. (London: Academic Press), 109–149.
Suarez, M. P., Fernandez-Escobar, R., Rallo, L. (1984). Competition among fruits in olive II. Influence of inflorescence or fruit thinning and cross-pollination on fruit set components and crop efficiency. Acta Hortic. 149, 131–144. doi: 10.17660/ActaHortic.1984.149.16
Sutherland, S. (1986). Floral sex ratios, fruit-set, and resource allocation in plants. Ecology 67, 991–1001. doi: 10.2307/1939822
Trentacoste, E. R., Moreno-Alías, I., Gómez-del-Campo, M., Beyá-Marshall, V., Rapoport, H. F. (2017). Olive floral development in different hedgerow positions and orientations as affected by irradiance. Sci. Hortic. 225, 226–234. doi: 10.1016/j.scienta.2017.06.029
Tsukaya, H. (2006). Mechanism of leaf-shape determination. Ann. Rev. Plant Biol. 57, 477–496. doi: 10.1146/annurev.arplant.57.032905.105320
Tuberosa, R., Sanguineti, M. C., Stefanelli, S., Quarrie, S. A. (1992). Number of endosperm cells and endosperm abscisic acid content in relation to kernel weight in four barley genotypes. Eur. J. Agron. 1, 125–132. doi: 10.1016/S1161-0301(14)80010-5
Uriu, K. (1953). Pistil abortion in the olive (Olea europaea L.) as influenced by certain physiological conditions (USA: University of California).
Uriu, K. (1959). Periods of pistil abortion in the development of the olive flower. Proc. Am. Soc Hortic. Sci. 73, 194–202.
Vallejo-Marín, M., Rausher, M. D. (2007). The role of male flowers in andromonoecious species: energetic costs and siring success in Solanum carolinense L. Evolution 61, 404–412. doi: 10.1111/j.1558-5646.2007.00031.x
Warren Wilson, J. (1972). “Control of crop processes,” in Crop processes in controlled environments. Eds. Rees, A. R., Cockshull, K. E., Hand, D. W., Hurd, R. G. (London: Academic Press), 7–30.
Yamaguchi, M., Haji, T., Miyake, M., Yaegaki, H. (2002). Varietal differences in cell division and enlargement periods during peach (Prunus persica Batsch) fruit development. J. Jap. Soc Hortic. Sci. 71, 155–163. doi: 10.2503/jjshs.71.155
Yamaguchi, M., Haji, T., Yaegaki, H. (2004). Differences in mesocarp cell number, cell length, and occurrence of gumming in fruit of Japanese apricot (Prunus mume Sieb. et Zucc.) cultivars during their development. J. Jap. Soc Hortic. Sci. 73, 200–207. doi: 10.2503/jjshs.73.200
Yampolsky, C., Yampolsky, H. (1922). Distribution of sex forms in the phanerogamic flora. Bibl. Genet. 3, 1–62.
Keywords: cell number, fruit size, blooming, fruit set, ovary, pistil abortion, sink strength, yield components
Citation: Rosati A, Lodolini EM and Famiani F (2023) From flower to fruit: fruit growth and development in olive (Olea europaea L.)—a review. Front. Plant Sci. 14:1276178. doi: 10.3389/fpls.2023.1276178
Received: 11 August 2023; Accepted: 23 October 2023;
Published: 13 November 2023.
Edited by:
Gaetano Distefano, University of Catania, ItalyReviewed by:
Valerio Cristofori, University of Tuscia, ItalyHava Rapoport, Spanish National Research Council (CSIC), Spain
Copyright © 2023 Rosati, Lodolini and Famiani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adolfo Rosati, YWRvbGZvLnJvc2F0aUBjcmVhLmdvdi5pdA==
 Adolfo Rosati
Adolfo Rosati Enrico Maria Lodolini
Enrico Maria Lodolini Franco Famiani
Franco Famiani