- Department of Plant Science and Technology, Chung-Ang University, Anseong, Republic of Korea
Acidovorax citrulli (Ac) is a causal agent of watermelon bacterial fruit blotch (BFB) disease. Because resistance cultivars/lines have not yet been developed, it is imperative to elucidate Ac’s virulence factors and their mechanisms to develop resistant cultivars/lines in different crops, including watermelon. The glucose-6-phosphate isomerase (GPI) is a reversible enzyme in both glycolysis and gluconeogenesis pathways in living organisms. However, the functions of GPI are not characterized in Ac. In this study, we determined the roles of GpiAc (GPI in Ac) by proteomic and phenotypic analyses of the mutant lacking GPI. The mutant displayed significantly reduced virulence to watermelon in two different virulence assays. The mutant’s growth patterns were comparable to the wild-type strain in rich medium and M9 with glucose but not with fructose. The comparative proteome analysis markedly identified proteins related to virulence, motility, and cell wall/membrane/envelope. In the mutant, biofilm formation and twitching halo production were reduced. We further demonstrated that the mutant was less tolerant to osmotic stress and lysozyme treatment than the wild-type strain. Interestingly, the tolerance to alkali conditions was remarkably enhanced in the mutant. These results reveal that GpiAc is involved not only in virulence and glycolysis/gluconeogenesis but also in biofilm formation, twitching motility, and tolerance to diverse external stresses suggesting the pleiotropic roles of GpiAc in Ac. Our study provides fundamental and valuable information on the functions of previously uncharacterized glucose 6-phosphate isomerase and its virulence mechanism in Ac.
Introduction
Acidovorax citrulli (Ac), formerly known as A. avenae subsp. citrulli is a Gram-negative, rod-shaped, aerobic, and seed-borne bacterium. It causes bacterial fruit blotch (BFB) disease in cucurbit crops, including watermelon worldwide (Schaad et al., 1978; Willems et al., 1992; Burdman and Walcott, 2012). Ac infection in watermelon occurs at any growth stage and in all plant tissues (Rahimi-Midani and Choi, 2020). At the initial growth stage, water-soaked lesions and wilt symptoms are observed on the cotyledons and seedlings, respectively (Burdman et al., 2005). When Ac infects the watermelon fruit, water-soaked lesions occur on the surface of the fruit, the lesions are turned black, and necrosis is observed in the internal parts of the fruit (Latin and Hopkins, 1995). Ac strains are classified into two groups (Zivanovic and Walcott, 2017); Group I is generally virulent to non-watermelon cucurbits, including melon, while Group II causes severe disease in watermelon. BFB disease poses a major threat to cucurbit crop production and industry worldwide (Shrestha et al., 2013; Islam et al., 2020). Despite the economic significance of the disease, the strategies to effectively manage BFB have not been elucidated in different crop species. In addition, watermelon cultivars resistant to the group II strains have not been developed yet. Therefore, it is essential to elucidate the virulence factors of Ac group I strain and its related mechanisms to design a strategy to control the disease.
Various virulence factors/mechanisms of Ac have been characterized. A previous study reported that Ac mutants with impaired twitching motility and biofilm formation were less virulent than the wild-type strain (Bahar et al., 2009). Type 4 pili, mediating twitching motility, are known to be involved in surface adhesion and virulence (Craig et al., 2004; Bahar et al., 2009; Yang et al., 2023). Biofilm formation also affects tolerance to osmotic, thermal stresses, and pH (Luo et al., 2015). In addition, type II secretion system, type III secretion system, and quorum sensing have been identified as major virulence factors (Wang et al., 2016; Zivanovic and Walcott, 2017; Zhang et al., 2018; Kan et al., 2023). It is also reported that the ferric uptake regulator, YggS family pyridoxal phosphate-dependent enzymes, pyridoxal phosphate-dependent aminotransferases, glycerol-3-phosphate dehydrogenases, and 2, 3-bisphosphoglycerate-dependent phosphoglycerate mutases, and the nitrogen regulator are associated with virulence in Ac (Liu et al., 2019; Kim et al., 2021; Lee et al., 2021; Lee et al., 2022b; Wang et al., 2022; Heo et al., 2023; Liu et al., 2023). However, these well-characterized factors/mechanisms involved are insufficient to comprehensively understand the virulence mechanisms of Ac. Therefore, further studies are required to elucidate the Ac’s virulence factors and related mechanisms in detail.
Glucose-6-phosphate isomerase (GPI), also known as phosphoglucose isomerase (PGI), is one of the key enzymes in both eukaryotic and prokaryotic organisms (Achari et al., 1981). It facilitates the reversible transformation of glucose-6-phosphate into fructose-6-phosphate within a complex network of carbon metabolic pathways, encompassing glycolysis/gluconeogenesis, pentose sugar, an amino sugar, and nucleotide sugar metabolisms (Achari et al., 1981; Zhang et al., 2015; Kim et al., 2020b). GPI is mostly regarded as a moonlighting protein due to its multifunctional role in diverse cellular activities, including sugar interconversion, serine proteinase inhibition, and mediation of differentiation (Jeffery et al., 2000; Angira et al., 2020). It is also associated with cell wall biosynthesis, contributing to virulence in Aspergillus fumigatus (Zhou et al., 2022). In Xanthomonas campestris pv. citri, a causal agent of citrus canker, GPI is essential for pathogenicity (Tung and Kuo, 1999). It is also reported that cryptococcal phosphoglucose isomerase is required for virulence factor production, cell wall integrity, and stress resistance (Zhang et al., 2015). Although these studies implicate that GPI is closely associated with bacterial virulence, sugar metabolism, and cell wall functions, the functions of GPI in Ac have not been documented to date.
This study reports the putative glucose-6-phosphate isomerase (GpiAc) functions in the group II Ac strain KACC17005 of Ac. To characterize the roles of GpiAc, the virulence and growth ability of gpiAc:Tn5, a gpiAc-defective mutant, were compared to the wild-type stains. To postulate the cellular mechanisms associated with GpiAc virulence, a label-free, shot-gun comparative proteomic analysis was conducted. To further confirm the pleiotropic functions of GpiAc, we investigated its involvement in biofilm formation, twitching halo, and tolerance to osmotic and alkali stresses. Our observation revealed that GpiAc is involved in diverse cellular mechanisms and virulence in Ac.
Materials and methods
Bacterial strains and growth conditions
The completely sequenced Ac group II strain KACC17005 was used as a wild-type strain (Park et al., 2017). Ac strains were grown in TSB (Tryptic Soy Broth Soybean-Casein Digested, 30g/L) and M9 (47.7 mM Na2HPO4·7H2O, 22 mM KH2PO4, 8.6 mM NaCl, 18.7 mM NH4Cl, 2 mM MgSO4, 0.1 mM CaCl2, 20 ml of 20% glucose in 1L) at 28°C. For gene cloning and plasmid construct formation, the DH5ɑ strain was used. The EC100D strain was used to identify the Tn5-inserted gene. E. coli strains were grown in LB medium (Luria Bertani; 1% tryptone, 0.5% yeast extract, and 1% NaCl) at 37°C. For selection, media was supplemented with appropriate antibiotics at the final concentrations: rifampicin, 50 μg/ml; kanamycin, 50 μg/ml; gentamycin, 10 μg/ml; and ampicillin, 100 μg/ml.
Selection of gpiAc:Tn5 and generation of GpiAc complemented strain
To select the knockout mutant, the Tn5-insertional mutant library generated by EZ-Tn5™<R6Kγori/KAN-2> Insertion Kit (Lucigen, Middleton, WI, USA) was screened as described previously (Lee et al., 2022b; Heo et al., 2023). After selecting a virulence-deficient mutant, the gene interrupted by Tn5 was identified using the manufacturer’s protocol. We observed that the identified gene encodes a putative glucose 6-phosphate isomerase (Accession No. ATG97016); therefore, the mutant was named gpiAc:Tn5. To generate a construct for strain complementation, an open reading frame of gpiAc gene was amplified using gpiAc-specific primers (Forward primer: 5`-gtcgagatgacgatgcccgcccgcgt-3` and reverse primer: 5`-aagcttcagtggtggtggtggtggtggcgacggccctgggcgagccgcgccat-3`). The amplicon was ligated into pGEM-T easy vector (Promega, Madison, WI, USA), producing pGEM-gpiAc plasmid. After confirming the clone by Sanger sequencing, the plasmid was digested with HindIII and BamHI, and the digested DNA fragment was recloned into the pBBR1-MCS5 vector, which is a broad host range vector containing LacZ promoter (Kovach et al., 1995), creating pMCS5-GpiAc plasmid. Next, the plasmid was introduced into gpiAc:Tn5 by electroporation (Bio-Rad), generating the complemented strain, gpiAc:Tn5(GpiAc). The complemented strain was confirmed by PCR using gpiAc-specific primers. To remove the side effects caused by the pBBR1-MCS5, the vector was also introduced into Ac and gpiAc:Tn5, generating Ac(EV) and gpiAc:Tn5(EV), respectively. Bacterial strains and plasmids used in this study are shown in Supplementary Table 1.
Virulence assay
Citrullus lanatus var. vulgaris line SBA provided by Partner Seed Company (Gimje, Korea) was used to test the virulence of Ac strains. Two different virulence assays were performed as follows. The germinated-seed inoculation method was first carried out, as reported previously (Lee et al., 2022b). Briefly, the germinated seeds were incubated in 10 mM MgCl2 containing approximately 106 colony-forming units (CFUs)/mL of Ac strains for one hour at 22°C. Ten seeds were used per strain. The infected seeds were placed in an environmentally controlled room with 25 ± 1°C, 16/8 day/night photoperiod, and approximately 70% humidity (relative). The disease severity was measured for 7 days on 0~2 scales (Lee et al., 2021). This assay was conducted at least four times with ten biological replicates per strain.
The leaf infiltration was performed following a previously established protocol (Kim et al., 2020a). Four true leaves stages were infiltrated using needless syringes. The final concentration of inoculum was approximately 105 CFU/mL. Bacterial suspensions were infiltrated through the underside of the second true leaves. Two discs were obtained using cork borers (0.4 cm in diameter) and ground. The ground samples were serially diluted, dotted onto TSA plates, and viable colonies were counted. These measurements were carried out in two-day intervals for 8 days after inoculation. At least four independent experiments were carried out with three biological replicates per strain.
Growth assay
Ac strains were adjusted to an OD600nm of 0.3 (approximately 108 CFU/mL), diluted to 105 CFU/mL with TSB media, and incubated at 28°C in a shaking incubator for 84 hours. The growth of each strain was measured every 12 hours. Three independent experiments with three biological replicates were conducted. In the M9 medium, 0.4% glucose or fructose was used. The bacterial suspension was placed in an M9 medium containing each carbon source. The final bacterial concentration was an OD600nm of 0.05 and the growth was determined every 24 hours for 10 days at 28°C in a shaking incubator. Each strain with three biological replicates was used, and three independent experiments were carried out.
Proteomics analysis
A label-free proteomic analysis was carried out using previously established protocols (Lee et al., 2022a; Heo et al., 2023). Ac and gpiAc:Tn5 strains with three biological replicates (6 samples) were used for the comparative analysis. Concisely, Ac strains incubated in TSB were harvested at an OD600nm of 0.6. The harvested bacterial cells were resuspended into the lysis buffer (6M guanidine HCl, 10 mM dithiothreitol, 50 mM Tris-HCl pH 7.8). The cell suspension was disrupted by sonication, and the concentration of total soluble proteins was measured using a BCA kit (Thermo Fisher Scientific, Rockford, IL, USA). The samples were digested by trypsin to generate peptides. For the liquid chromatography followed by tandem mass spectrometry analysis (LC-MS/MS), one μg of the samples was analyzed by split-free nano-LC (EASY-nLC II; Thermo Fisher Scientific, Bremen, Germany) linked to the LTQ Velos Pro instrument (Thermo Fisher Scientific). The samples were separated in the column with MAGIF C18AQ 200A (Michrom BioResources, Auburn, CA, USA). Six data-dependent MS/MS scans were conducted to obtain the full MS spectra. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al., 2022) partner repository with the dataset identifier PXD042560.
The previously established method was followed for the identification and quantification of the proteins/peptides (Lee et al., 2022b). The LC-MS/MS-generated raw data were analyzed by Thermo proteome discoverer (ver. 1.3.0.399) with SEQUEST search algorithm. The genome information of the group II strain KACC17005 (Accession No. CP023687) was used to search for spectra information. The target-decoy strategy was used to improve credibility in this study (Elias and Gygi, 2007). The identified proteins were imported again into Scaffold 4 (Proteomic software, Portland, OR, USA) for the comparative proteome analysis. The peptide spectra match (PSM) (Choi et al., 2008) was used for comparative values. PSM values from individual proteins were normalized against the total PSMs in the sample. The mean value from the three biological replicates was used for identifying differentially abundant proteins (more than 2-fold) between Ac and gpiAc:Tn5. A student’s t-test (P<0.05) was conducted for statistical analysis. The differentially abundant proteins were categorized using a cluster of orthologous groups (COG) analysis (Tatusov et al., 2000).
Biofilm formation assay
Biofilm formation assay was carried out as described previously (Lee et al., 2022b). Briefly, Ac strains grown in TSA were resuspended to an OD600nm of 0.3 (approximately 108 CFU/mL) and diluted to the final concentration (105 CFU/mL) with TSB. Next, 190 μl of the suspension was placed into the 96-well polyvinyl chloride plate and incubated for two days at 28°C. The bacterial cells forming biofilm were stained with the crystal violet solution (0.1%) and dissolved in 95% ethanol. Biofilm formation was determined using the Spectramax 190 microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 590nm. Twenty biological replicates of each strain were used in at least three independent experiments.
Twitching halo assay
The twitching halo motility assay was performed following the previous study (Lee et al., 2022b). Bacterial cells were resuspended in water to an OD600nm of 0.3 (approximately 108 CFU/mL) and diluted to adjust the concentration to 106 CFU/mL. The five μl bacterial suspension was dotted on the semi-solid (0.5% agar) TSA plates. Dotted samples were incubated at 28°C for two days. The colony size and twitching halo diameter were measured using the stereoscopic microscope LEICA M205 C (LEICA Wetzlar, Germany). Three replicates of each strain were used in the assay, and at least four independent experiments were carried out.
Stress tolerance assay
For the alkali and osmotic stress tolerance assay, bacterial cells were adjusted to an OD600nm of 0.3 (approximately 108 CFU/mL) and serially diluted 10-fold from 100 to 105 with water. The diluted samples were dotted onto TSA containing sodium chloride (NaCl, 1.5% or 2%) or TSA (pH 9 and 10). The untreated TSA was used as a control. For lysozyme treatment, bacterial cells were adjusted to an OD600nm of 0.3. Each strain was treated with 6 μg/mL of lysozyme for two hours in TSB. Untreated TSB was used as a control. After the treatment, the bacterial suspension was serially diluted and dotted onto TSA. The survivability of each condition was established using the ratio of viable cell numbers from the treated samples to untreated samples. At least three independent experiments were conducted with the three biological replicates for each stress condition.
Statistical analysis
The statistical significance of quantitative data was analyzed by student’s t-test and one-way analysis of variance with Turkey HSDab using SPSS 12.0K (Chicago, IL, USA). A p-value (< 0.05) indicated a statistically significant difference.
Results
GpiAc is indispensable for virulence of Ac
We identified a virulence-deficient mutant during the screen of a Tn5-insertional mutant library using the group II strain KACC17005. A putative glucose 6-phosphate isomerase in Ac (GpiAc) (Accession No. ATG97016) gene was observed to be disrupted by Tn5 in the virulence-deficient mutant. To examine whether GpiAc contributes to virulence, two virulence assays, germinated-seed inoculation and leaf infiltration, were conducted in three strains; Ac(EV), gpiAc:Tn5(EV), and gpiAc:Tn5(GpiAc). Ac(EV) and gpiAc:Tn5(EV) are the wild-type and gpiAc null mutant strains containing the empty vector, respectively. gpiAc:Tn5(GpiAc) is the mutant carrying the open reading frame of the gpiAc gene on the pBBR1-MCS5 vector. In the germinated-seed inoculation assay, severe disease symptoms were observed in Ac(EV), which continued to increase up to a disease index value of 2 at 7 days after inoculation (DAI) (Figures 1A, B). On the other hand, no symptoms were found in gpiAc:Tn5(EV), and the disease index remained at 0 at 7 DAI. The virulence of the complemented stains, gpiAc:Tn5(GpiAc), was restored, and its disease index value reached 2 at 7 DAI, demonstrating no positional effect by Tn5 insertion. The outcomes of the leaf infiltration were comparable to the findings obtained from the germinated-seed inoculation. In Ac(EV) and gpiAc:Tn5(GpiAc), typical disease symptoms, such as dark leaves and wilting, were confirmed (Figure 1C). However, the leaf infiltrated by gpiAc:Tn5(EV) showed no symptoms. Further, we evaluated the bacterial growth in watermelon leaves infected with three strains (Figure 1D). The growth of gpiAc:Tn5(EV) was significantly lower than that of Ac(EV) and gpiAc:Tn5(GpiAc) at 2, 4, 6, and 8 DAI. The gpiAc:Tn5(EV) population was comparable to gpiAc:Tn5(GpiAc). These results demonstrated that GpiAc is indispensable for the virulence of Ac.
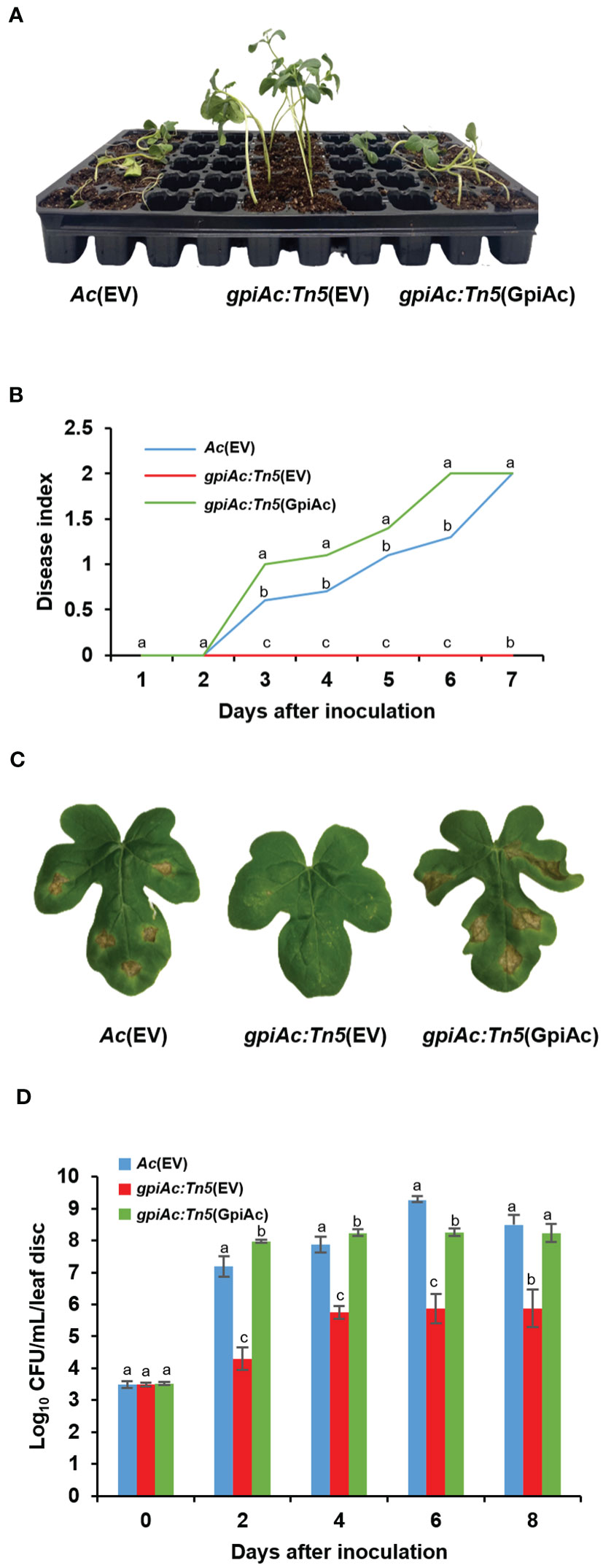
Figure 1 Virulence assay for Ac(EV), gpiAc:Tn5(EV), and gpiAc:Tn5(GpiAc). (A) The photograph was captured seven days after inoculation from the germinated seed inoculation. (B) Disease index variation during seven days in the seed-germinated inoculation. The disease index: [(numbers of plants with no symptoms) × 0 + (numbers of spotted plants) × 1 + (numbers of wilted plants) × 2]/Total (numbers of plants). (C) The photographs were captured 8 days after inoculation from the leaf infiltration. (D) The living bacterial population in infiltrated leaves for 8 days using the colony counting method. All strains were inoculated into the leaves of a two-week-old watermelon using the syringe without a needle. Different letters on the error bars (standard errors of means) represent the statistical difference by ANOVA (p<0.05) with Turkey HSDab. At least four independent experiments were carried out and displayed similar patterns.
GpiAc is not associated with multiplication in Ac
The bacterial growth and virulence of gpiAc:Tn5(EV) were decreased in both virulence assays compared with those of Ac(EV) and gpiAc:Tn5(GpiAc) (Figure 1). To determine whether the reduction in growth and virulence in watermelon was due to the difference in the multiplication of the mutant, we investigated the bacterial growth curves of three strains, Ac(EV), gpiAc:Tn5(EV), and gpiAc:Tn5(GpiAc), in TSB (the nutrient-rich condition). All three strains showed similar growth patterns (Figure 2A). Ac strains reached the log phase 12 hours after incubation and the stationary phase 24 hours after incubation. There was no statistically significant difference in the growth curves during the observation period among the three strains.
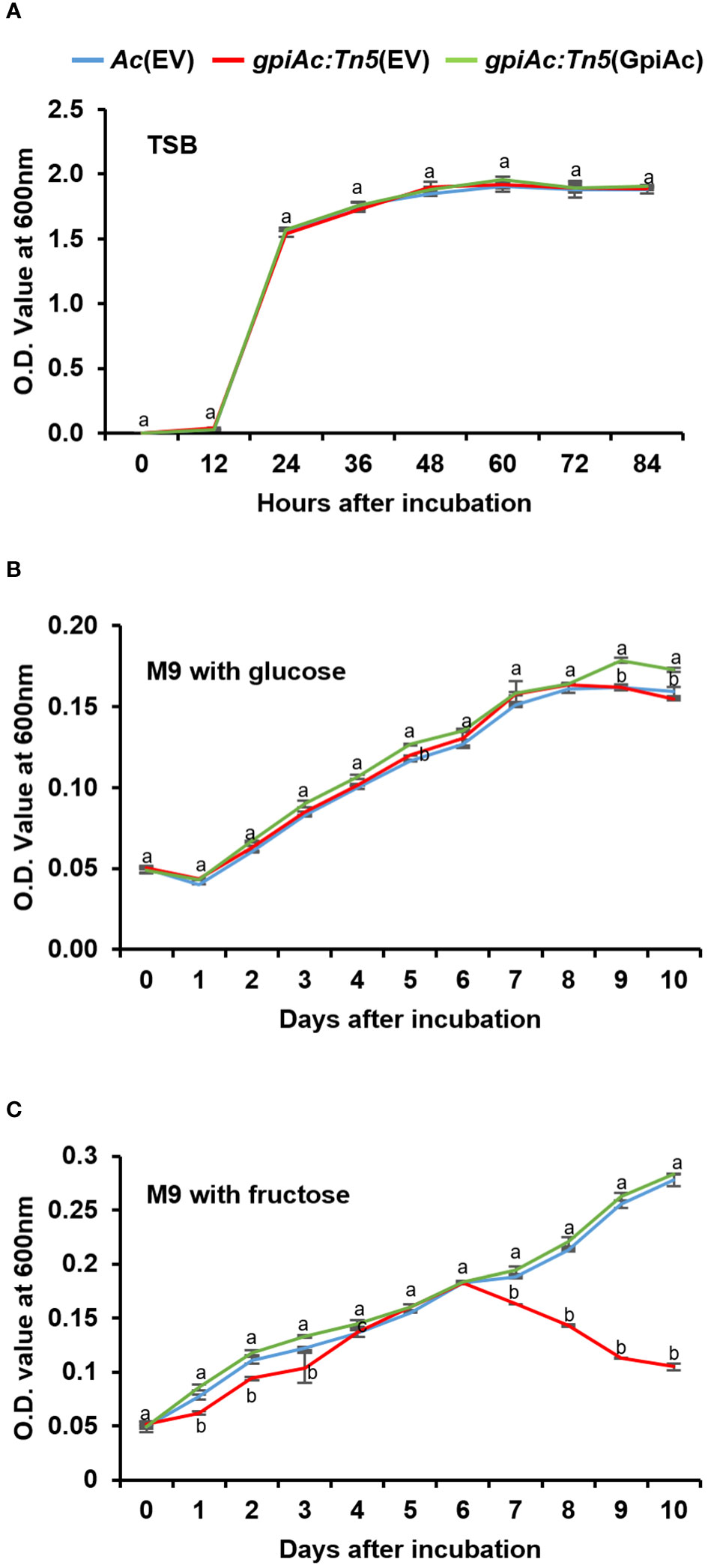
Figure 2 The bacterial growth curve in rich and minimal media with sole carbon source. Bacterial growth measurement in (A) TSB, M9 minimal supplemented with 0.4% (final concentration) of (B) glucose and (C) fructose as a sole carbon source. The population of bacterial strains was measured at OD600nm using a UV spectrophotometer with (A) 12h intervals and (B, C) 24h intervals. Different letters indicate statistically significant differences by ANOVA (p<0.05) with Turkey HSDab. At least three independent experiments were conducted, showing similar patterns.
GpiAc is involved in fructose utilization in Ac
Since GpiAc catalyzes the conversion of glucose-6-phosphate to fructose-6-phosphate, we sought to determine whether GpiAc affects carbon source utilization by examining growth differences in M9 minimal medium supplemented with glucose or fructose for 10 days. The growth of the three strains was comparable in the M9 medium containing glucose (Figure 2B). Interestingly, the growth pattern of gpiAc:Tn5(EV) differed from the wild-type and complemented strains in the M9 medium supplemented with fructose (Figure 2C). All three strains showed similar growth patterns until 6 DAI. However, the growth of gpiAc:Tn5(EV) was significantly reduced from 7 DAI to the end of the incubation period. These results indicate that GpiAc is involved in fructose utilization in Ac.
Proteomic analysis
We ascertained that GpiAc is indispensable for virulence and the growth in M9 with fructose. A previous study revealed that BdpmAc is associated with fructose metabolism as well as other phenotypes, including biofilm formation and tolerance to osmotic stress (Lee et al., 2022b). Therefore, to postulate the biological mechanisms affected by GpiAc, comparative proteomic analysis based on the label-free shotgun approach was performed using Ac and gpiAc:Tn5 strains. In the three biological replicates of the wild-type strain, 1105, 1108, and 1093 proteins were identified from 59582, 59503, and 59560 peptide spectra matches (PSMs), respectively (Supplementary Table 2). In the gpiAc:Tn5 strain, 1161, 1149, and 1139 proteins were detected from 57989, 58005, and 58007 PSMs, respectively. Among the proteins, 1046 and 1069 proteins were commonly found in the three biological replicates of Ac and gpiAc:Tn5 strains, respectively. The comparative analysis of commonly identified proteins showed that 53 and 80 proteins were uniquely detected, and 5 and 9 proteins were more abundant (over 2-fold) in Ac and gpiAc:Tn5, respectively (Figure 3A). The differentially abundant proteins were categorized by cluster of orthologous groups (COG) analysis (Figure 3B, Supplementary Tables 3, 4). Proteins belonging to group I (Lipid metabolism), M (Cell wall/membrane/envelope biogenesis), N (Cell motility), and V (Defense mechanisms) were markedly abundant in the wild-type strain. Proteins categorized in group G (Carbohydrate metabolism and transport), H (Coenzyme metabolism), L (Replication), O (Post-translational modification, protein turnover, chaperone functions), P (Inorganic ion transport and metabolism), and Q (Secondary structure) were significantly detected in the mutant. Specifically, the abundance of virulence-related proteins was altered, including phospholipase, two-component system response regulators, DNA-binding response regulators, and bifunctional diguanylate cyclase/phosphodiesterases. In addition, proteins related to bacterial motility were identified, such as flagella basal body protein FliL and three methyl-accepting chemotaxis proteins. Moreover, diverse proteins (TonB-dependent receptor, protein TolA, UDP-3-O-[3-hydroxymyristoyl] N-acetylglucosamine deacetylase, N-acetyltransferase, and transglycosylase) involved in cell wall/membrane function, were detected in our study. The comparative proteomic analysis reveals that GpiAc is involved in virulence, bacterial motility, and cell wall/membrane functions.
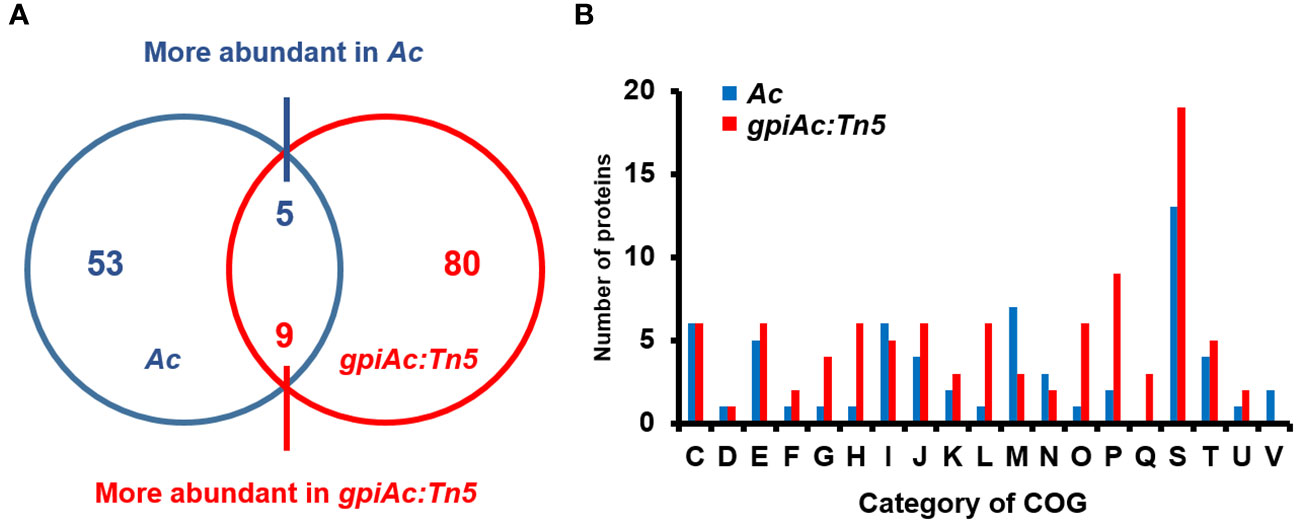
Figure 3 Comparative proteomic analysis of Ac and gpiAc:Tn5. (A) Venn diagram displaying the number of the differentially (over two-fold) abundant proteins from the proteomic analysis. (B) Fifty-three and 80 proteins were uniquely identified, and 5 and 9 proteins were more abundant in Ac and gpiAc:Tn5 strains, respectively. (B) A cluster orthologous group (COG) analysis of proteins from the comparative analysis. C, Energy production and conversion; D, Cell cycle control and mitosis; E, Amino acid metabolism and transport; F, Nucleotide metabolism and transport; G, Carbohydrate metabolism and transport; H, Coenzyme metabolism; I, Lipid metabolism; J, Translation; K, Transcription; L, Replication and repair; M, Cell wall/membrane/envelop biogenesis; N, Cell motility; O, Post-translational modification, protein turnover, chaperone functions; P, Inorganic ion transport and metabolism; Q, Secondary structure; S, Function unknown; T, Signal transduction; U, Intracellular trafficking and secretion; V, Defense mechanisms.
gpiAc:Tn5 reduces biofilm formation
Plant pathogenic bacteria form biofilms for protection against external or environmental stresses, which is an important pathogenic factor (Davey and O’Toole, 2000). Our comparative proteomics analysis suggests that GpiAc may affect the cell wall/membrane functions required for biofilm formation. A previous study reported that glucose-6-phosphate, a putative substrate for GpiAc, is involved in biofilm formation in Pseudomonas aeruginosa (Park et al., 2021). Therefore, we evaluated the ability of biofilm formation in Ac(EV), gpiAc:Tn5(EV), and gpiAc:Tn5(GpiAc) using the 96-well PVC plate assay. We observed that the biofilm formation ability of gpiAc:Tn5(EV) was significantly decreased compared to Ac(EV) (Figure 4). The complemented strains, gpiAc:Tn5(GpiAc), restored the biofilm formation function to Ac(EV) level. These results highlight that GpiAc is associated with biofilm formation.
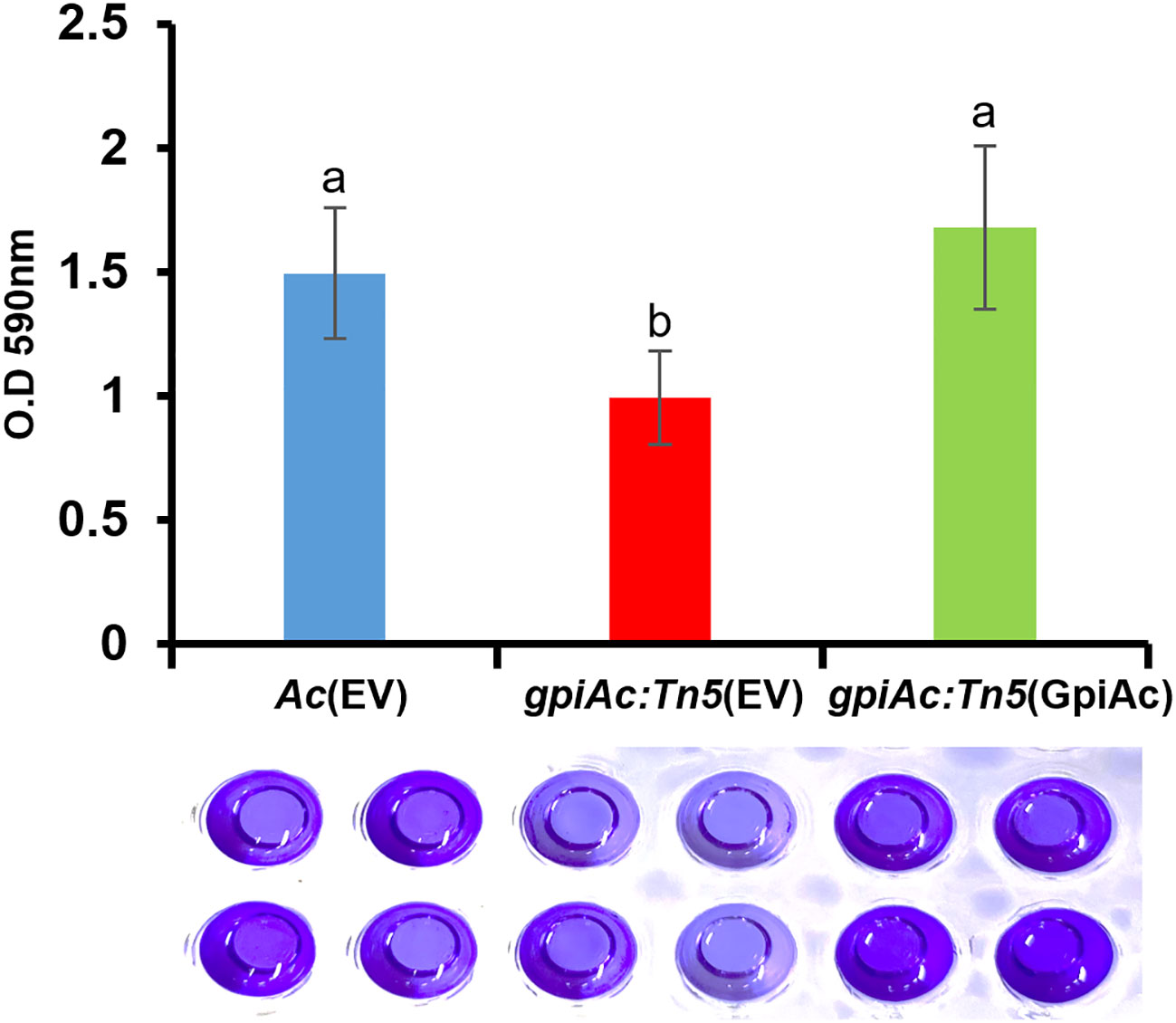
Figure 4 Biofilm formation from Ac(EV), gpiAc:Tn5(EV), and gpiAc:Tn5(GpiAc). Ac strains were incubated in TSB on polyvinyl chloride 96-well plates, and biofilm formation was quantified after two days of incubation. The amount of biofilm formed by three strains. Different letters on error bars represent the statistical difference by ANOVA (p<0.05) with Turkey HSDab. A photograph of biofilm stained with 0.1% crystal violet and eluted with 95% ethanol was captured. Three independent experiments with twenty biological replicates were carried out, and all experiments exhibited similar patterns.
gpiAc:Tn5 reduces twitching motility
Like other bacteria, Ac possesses two types of motilities; pili- and flagella-dependent, while the group II strains, including KACC17005, can only show pili-dependent motility in in vitro conditions (Bahar et al., 2010). In addition, diverse proteins associated with bacterial movement were identified in the COG classification. Therefore, the twitching motility, which is pili-dependent motility, was examined in the TSA plate with 0.5% agar, and the colony and twitching halos sizes were measured from the three strains (Figure 5). The colony size of gpiAc:Tn5(EV) was not different from those of Ac(EV) and gpiAc:Tn5(GpiAc). On the other hand, the twitching halo size of gpiAc:Tn5(EV) was significantly reduced (0.93 cm) compared to that (1.5 cm) of the wild-type strain. The production of the twitching halo was reestablished (1.7 cm) in gpiAc:Tn5(GpiAc). There was no statistical difference in the sizes of gpiAc:Tn5(GpiAc) and Ac(EV). These results indicate that GpiAc is associated with twitching motility in Ac.
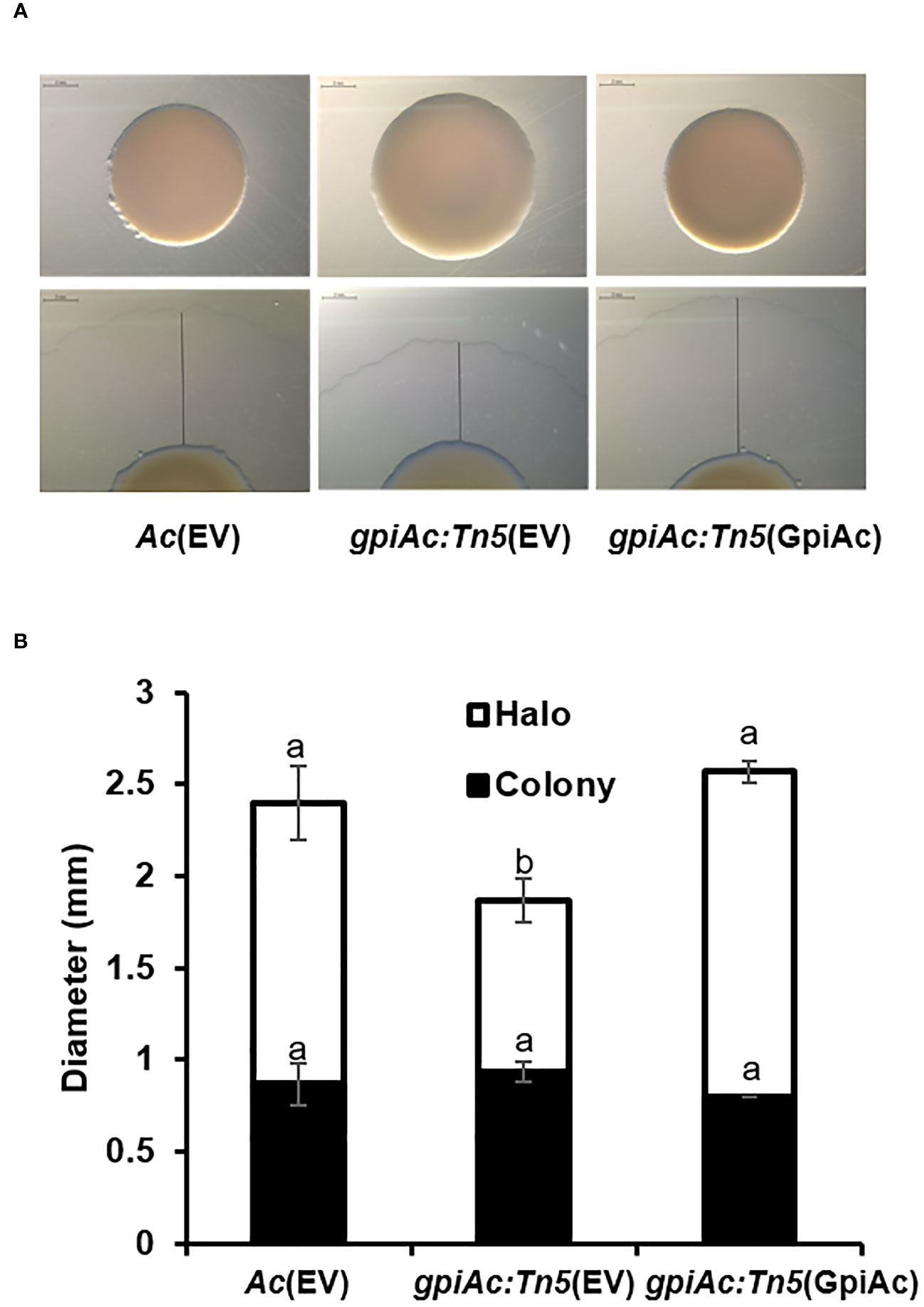
Figure 5 Twitching halo production in Ac(EV), gpiAc:Tn5(EV), and gpiAc:Tn5(GpiAc). Five μL of bacterial suspension (108 CFU/mL) was dotted onto TSA medium containing 0.5% agar and incubated for two days. (A) Images from twitching motility assay. The black line indicates the halo size. The scale bar is 2 mm. (B) Measurement of the sizes from the colony and twitching halo. The black and white bars indicate the diameter of the colony and twitching halo, respectively. The error bars indicate standard deviations. Different letters indicate statistically significant differences by ANOVA (p<0.05) with Turkey HSDab. Four independent experiments were conducted, all of which showed similar patterns.
GpiAc is involved in stress tolerance
The comparative proteomic analysis identified several proteins related to the cell wall/membrane. Therefore, we further investigated the involvement of GpiAc in membrane/wall integrity and tolerance to various stresses in Ac(EV), gpiAc:Tn5(EV), and gpiAc:Tn5(GpiAc). Firstly, tolerance to osmotic stress was evaluated using NaCl (Figures 6A, B). The three strains were exposed to TSA media supplemented with 1.5% and 2% NaCl. gpiAc:Tn5(EV) showed survivability of 0.003% and 0.0003% in 1.5% and 2% NaCl, respectively, which was dramatically reduced compared to the survivability of 21.8% and 0.7% of Ac(EV). In addition, gpiAc:Tn5(GpiAc) showed survivability similar to wild-type stains (8% and 1%). Next, Ac strains were incubated in TSB with 0.6 μg/mL lysozyme for 2 hours (Figure 6C). The results were similar to the osmotic stress assay. The survivability in gpiAc:Tn5(EV) was significantly reduced with lysozyme treatment. Precisely, Ac(EV), gpiAc:Tn5(EV), and gpiAc:Tn5(GpiAc) showed 109%, 44%, and 141% survivability, respectively. On the other hand, in the alkali condition, the mutant exhibited opposite survivability patterns to other stresses. In the pH 9 condition, the survivability of gpiAc:Tn5(EV) (118.9%) was significantly enhanced compared to that of Ac(EV) (77.8%) (Figure 6D). gpiAc:Tn5(GpiAc) displayed comparable survivability (73.3%) to the wild-type strain. The survivability patterns of strains incubated at pH 10 were similar to those at pH 9 (Figure 6E). However, in the acidic condition (pH 5), the patterns of the three strains were similar (data not shown). Taken together, these results indicate that GpiAc is associated with environmental stress tolerance.
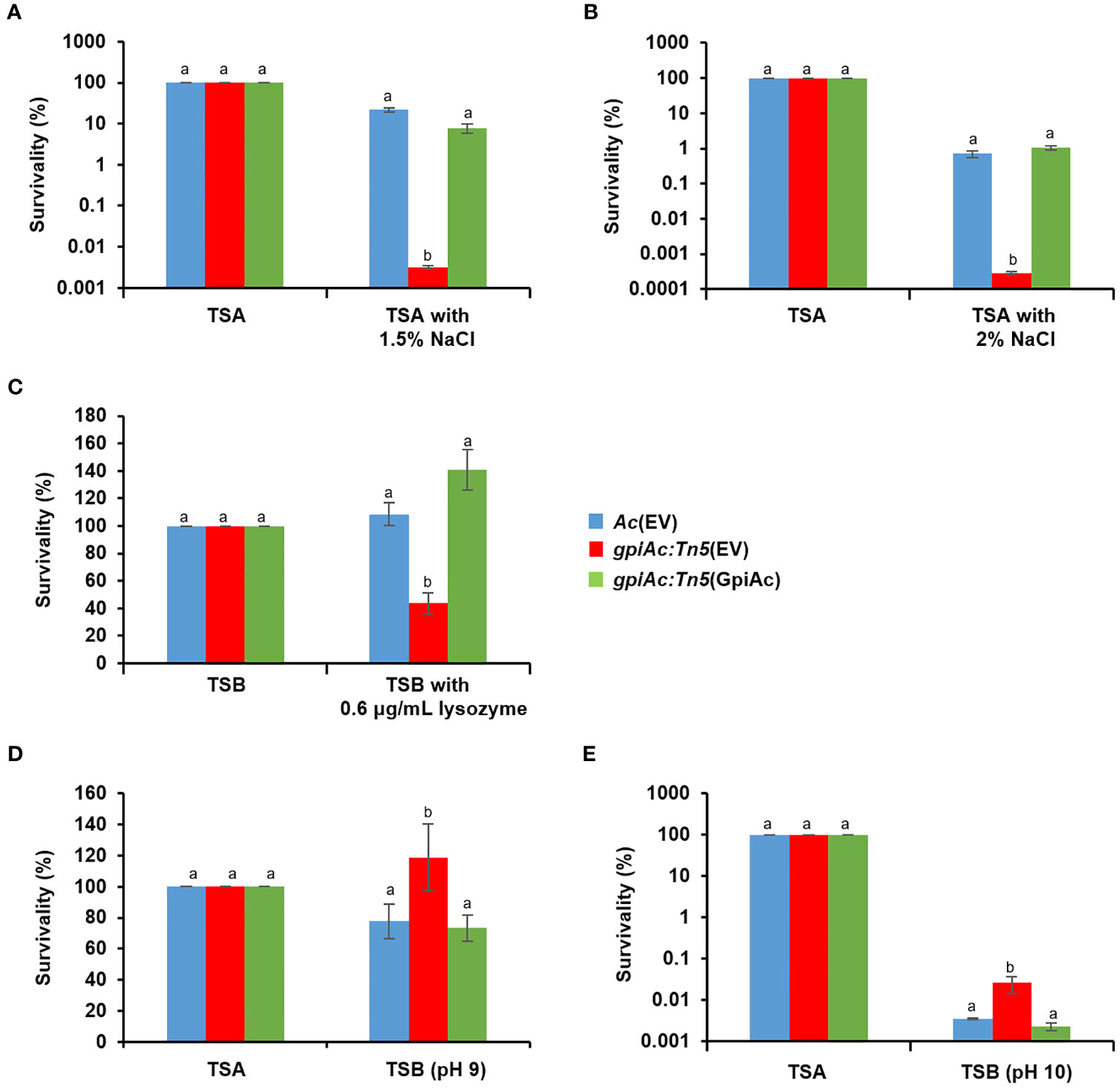
Figure 6 Tolerance assay for osmotic stress, lysozyme, and pH treatment. Tolerance was determined in the rich media (TSA or TSB) in the presence of (A) 1.5% NaCl, (B) 2% NaCl, (C) 0.6 μg/mL lysozyme, and (D) pH 9, and (E) pH 10 treatment. The rich media without stress factors were used as a negative control. The numbers of viable cell numbers were established by the colony counting method. The survivability was calculated based on the ratio of the viable cell numbers from the stress conditions to the control. Different letters indicate statistically significant differences by ANOVA (p<0.05) with Turkey HSDab. At least three independent experiments were carried out exhibiting similar patterns.
Discussion
Previously, it has been shown that glucose-6-phosphate isomerases (GPIs) are involved in bacterial virulence in the Gram-negative bacterium X. oryzae pv. oryzicola and the Gram-positive bacterium, Cryptococcus neoformans (Zhang et al., 2015; Guo et al., 2017). In addition, GPIs-related mechanisms, glycolysis and gluconeogenesis, are also crucial for virulence in various plant and animal pathogenic bacteria, including Staphylococcus aureus, Erwinia amylovora, Mycobacterium marinum, and X. axonopodis pv. glycines (Vitko et al., 2015; Tong et al., 2016; Klee et al., 2019; Guo et al., 2020). Consistent with these observations, we demonstrate that GpiAc is indispensable for virulence in Ac using germinated seed inoculation and leaf infiltration assays. Notably, the growth pattern of gpiAc:Tn5(EV) in TSB was not different from Ac(EV). These results indicate that the reduced virulence in gpiAc:Tn5(EV) is not due to bacterial multiplication. In addition, both Ac(EV) and gpiAc:Tn5(EV) display similar patterns in M9 containing glucose, pointing out that gpiAc:Tn5(EV) is not an auxotroph, and GpiAc is not related to the production of primary metabolites.
GPI is a reversible and essential enzyme in glycolysis/gluconeogenesis. Glucose-6-phosphate and fructose-6-phosphate are substrates for GPI in glycolysis and gluconeogenesis pathways, respectively (Hers and Hue, 1983). Interestingly, gpiAc:Tn5(EV) could grow in an M9 medium supplemented with glucose. However, any GPI-homologs except GpiAc were not annotated in the KACC17005 genome (Park et al., 2017), suggesting an alternative pathway for glucose utilization in this strain. The Entner-Doudoroff pathway has been reported as another glucose catabolism pathway in gram-negative bacteria (Kersters and De Ley, 1968; Conway, 1992). Specifically, two enzymes, phosphogluconate dehydratase and keto-deoxy-phosphogluconate aldolase, are reported to be crucial for the Entner-Doudoroff pathway (Conway, 1992). The strain KACC17005 contains two genes, ATG92716 and ATG92717, encoding a putative phosphogluconate dehydratase and a putative keto-deoxy-phosphogluconate aldolase, respectively (Park et al., 2017). Therefore, it is postulated that gpiAc:Tn5(EV) likely employs the Entner-Doudoroff pathway and can use glucose as the sole carbon source in the M9 medium. However, it is not completely ruled out that the strain KACC17005 carries genes for GPI function. In contrast to the glucose metabolism pathways, an alternative fructose metabolism pathway has not been documented in bacteria. Our results, in which gpiAc:Tn5(EV) did not grow well in M9 supplemented with fructose, also indicate no alternative fructose pathway in Ac. Altogether, we speculated that glycolysis can proceed without GpiAc, but gluconeogenesis is attenuated in the mutant.
In addition to the interconversion of sugar, GPI is associated with diverse functions as a neurotrophic factor, an autocrine motility factor, and a differentiation/maturation mediator in the eukaryotic organism (Sriram et al., 2005). However, these activities are not conserved in all organisms. In Cryptococcus neoformans, the GPI mutant exhibits increased sensitivity to cell wall-damaging agents and osmotic stresses (Zhang et al., 2015). Moreover, GPI is required for extracellular polysaccharide (EPS) biosynthesis, cell motility, and full virulence of X. oryzae pv. oryzicola in rice (Guo et al., 2017). In agreement with these previous reports, our comparative proteomic analysis implicates that GpiAc is associated with various biological mechanisms. Further, phenotypic observation substantiates the involvement of GpiAc in diverse functions, indicating that GpiAc shows a pleiotropic effect.
Bacterial cell wall/membrane/envelope are essential for biofilm formation as well as response to environmental stresses (Hews et al., 2019; Viljoen et al., 2020). The proteomic analysis identified diverse proteins associated with cell wall/membrane/envelope biosynthesis and its related mechanisms. Among these proteins, two alpha/beta hydrolases (ATG94531, ATG95002) were uniquely detected in gpiAc:Tn5. A previous study reported that the overexpression of alpha/beta hydrolase led to the reduction of biofilm formation and pathogenicity in Pseudomonas fluorescens and Pectobacterium carotovorum, respectively (Mei et al., 2010). In our study, biofilm formation was also reduced in gpiAc:Tn5(EV). In addition, biofilms are known as a major virulence factor of bacteria (Davey and O'Toole, 2000). Therefore, the function of GpiAc is associated with biofilm formation, contributing to virulence in Ac. Furthermore, we also demonstrated that the mutant lacking GpiAc exhibited reduced tolerance to osmotic stress and lysozyme treatment in the rich condition, in which the mutant showed similar growth patterns with the wild-type strain. In addition to the osmotic stresses, pH condition is also crucial for bacterial membrane homeostasis (Krulwich et al., 2011). The optimal pH condition for Ac is around neutral (pH), but the bacterium can grow in pH 5 ~ 9 (Luo et al., 2015). Our study showed that the tolerance to alkali conditions in the mutant was enhanced. It suggests that membrane integrity is affected by the absence of GpiAc in the mutant, resulting in the alteration of tolerance to alkali conditions. These results indicate that GipAc is related to cell wall/membrane integrity in Ac. Although the specific molecular mechanisms underlying the pleiotropic roles of GpiAc have to be elucidated, it is clear that GpiAc affects various phenotypes along with virulence.
Cell motility requires energy from carbon metabolism (Mitchell and Kogure, 2006). Previously, it was reported that genes encoding catalytic enzymes in the carbohydrate catabolic pathway are required for cell motility in plant pathogens (Guo et al., 2017). The comparative proteomic analysis identified cell motility-related proteins, including TolA protein (ATG93209), which was more abundant in the wild-type strain. Inactivation of TolA in E. coli resulted in a complete loss of motility as well as pathogenic attenuation (Morgan et al., 2014). In the nutrient-rich condition (TSB), the growth of gpiAc:Tn5(EV) was not impaired because the medium contained enough carbon sources for the survival and multiplication of the mutant. Surprisingly, the twitching halo size produced by gpiAc:Tn5(EV) was significantly reduced in the same nutrient conditions. These results also suggest that GpiAc possesses diverse roles in virulence as well as other phenotypic mechanisms in Ac.
In summary, we demonstrated that GpiAc, a putative glucose 6-phosphate isomerase, is not only involved in glycolysis/gluconeogenesis but also exerts a broad pleiotropic effect on bacterial virulence, biofilm formation, motility, and tolerance to various external stresses. Our study provides new insights into the functions of a glucose 6-phosphate isomerase exhibiting pleiotropic effects in Ac. Particularly, GpiAc could be used as a potential target to interfere with bacterial virulence by anti-virulence reagents to reduce the occurrence of BFB disease in watermelon.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
LH: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft. YH: Investigation, Methodology, Writing – review & editing. YC: Investigation, Methodology, Writing – review & editing. JC: Investigation, Methodology, Writing – review & editing. JL: Investigation, Methodology, Writing – review & editing. SH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2020R1A2C1013040), Republic of Korea. This research was also supported by the Chung-Ang University Graduate Research Scholarship in 2023 (to YH).
Acknowledgments
We thank J. Kim for technical help at the BT research facility center, Chung-Ang University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1275438/full#supplementary-material
References
Achari, A., Marshall, S. E., Muirhead, H., Palmieri, R. H., Noltmann, E. A. (1981). Glucose-6-phosphate isomerase. Philos. Trans. R. Soc Lond. B Biol. Sci. Philos. T R Soc. B 293, 145–157. doi: 10.1098/rstb.1981.0068
Angira, D., Natarajan, N., Dedania, S. R., Patel, D. H., Thiruvenkatam, V. (2020). Characterization of P. aeruginosa glucose 6-phosphate isomerase: a functional insight via in-vitro activity study. Curr. Top. Med. Chem. 20, 2651–2661. doi: 10.2174/1568026620666200820153751
Bahar, O., de la Fuente, L., Burdman, S. (2010). Assessing adhesion, biofilm formation and motility of Acidovorax citrulli using microfluidic flow chambers. FEMS Microbiol. Lett. 312, 33–39. doi: 10.1111/j.1574-6968.2010.02094.x
Bahar, O., Goffer, T., Burdman, S. (2009). Type IV pili are required for virulence, twitching motility, and biofilm formation of Acidovorax avenae subsp citrulli. Mol. Plant-Microbe Interact. 22, 909–920. doi: 10.1094/MPMI-22-8-0909
Burdman, S., Kots, N., Kritzman, G., Kopelowitz, J. (2005). Molecular, physiological, and host-range characterization of Acidovorax avenae subsp citrulli isolates from watermelon and melon in Israel. Plant Dis. 89, 1339–1347. doi: 10.1094/PD-89-1339
Burdman, S., Walcott, R. (2012). Acidovorax citrulli: generating basic and applied knowledge to tackle a global threat to the cucurbit industry. Mol. Plant Pathol. 13, 805–815. doi: 10.1111/j.1364-3703.2012.00810.x
Choi, H., Fermin, D., Nesvizhskii, A. I. (2008). Significance analysis of spectral count data in label-free shotgun proteomics. Mol. Cell Proteomics 7, 2373–2385. doi: 10.1074/mcp.M800203-MCP200
Conway, T. (1992). The Entner-Doudoroff pathway - history, physiology and molecular-biology. FEMS Microbiol. Lett. 103, 1–28. doi: 10.1111/j.1574-6968.1992.tb05822.x
Craig, L., Pique, M. E., Tainer, J. A. (2004). Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2, 363–378. doi: 10.1038/nrmicro885
Davey, M. E., O'Toole, G. A. (2000). Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64, 847–867. doi: 10.1128/MMBR.64.4.847-867.2000
Elias, J. E., Gygi, S. P. (2007). Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214. doi: 10.1038/nmeth1019
Guo, W., Gao, J., Wang, H. J., Su, R. Y., Sun, C. Y., Gao, S. H., et al. (2020). Phosphoglycerate kinase is involved in carbohydrate utilization, extracellular polysaccharide biosynthesis, and cell motility of Xanthomonas axonopodis pv. glycines independent of Clp. Front. Microbiol. 11, 91. doi: 10.3389/fmicb.2020.00091
Guo, W., Zou, L., Ji, Z., Cai, L., Chen, G. (2017). Glucose 6-phosphate isomerase (Pgi) is required for extracellular polysaccharide biosynthesis, DSF signals production and full virulence of Xanthomonas oryzae pv. oryzicola in rice. Physiol. Mol. Plant Pathol. 100, 209–219. doi: 10.1016/j.pmpp.2017.10.010
Heo, L., Cho, Y., Choi, J., Lee, J., Han, Y., Han, S. W. (2023). Proteomic and phenotypic analyses of a putative YggS family pyridoxal phosphate-dependent enzyme in Acidovorax citrulli. Plant Pathol. J. 39, 235–244. doi: 10.5423/PPJ.OA.03.2023.0050
Hers, H. G., Hue, L. (1983). Gluconeogenesis and related aspects of glycolysis. Annu. Rev. Biochem. 52, 617–653. doi: 10.1146/annurev.bi.52.070183.003153
Hews, C. L., Cho, T., Rowley, G., Raivio, T. L. (2019). Maintaining integrity under stress: envelope stress response regulation of pathogenesis in Gram-negative bacteria. Front. Cell. Infect. Microbiol. 9, 313. doi: 10.3389/fcimb.2019.00313
Islam, R., Hossain, M. R., Kim, H. T., Nath, U. K., Abuyusuf, M., Jung, H. J., et al. (2020). Molecular characterization of Acidovorax citrulli strain NIHHS15-280 causing bacterial fruit blotch disease in Korea and screening of resistance sources in melon. Horticulture Environ. Biotechnol. 61, 115–126. doi: 10.1007/s13580-019-00190-0
Jeffery, C. J., Bahnson, B. J., Chien, W., Ringe, D., Petsko, A. (2000). Crystal structure of rabbit phosphoglucose isomerase, a glycolytic enzyme that moonlights as neuroleukin, autocrine motility factor, and differentiation mediator. Biochemistry 39, 955–964. doi: 10.1021/bi991604m
Kan, Y., Zhang, Y., Lin, W., Dong, T. (2023). Differential plant cell responses to Acidovorax citrulli T3SS and T6SS reveal an effective strategy for controlling plant-associated pathogens. mBio 14, e0045923. doi: 10.1128/mbio.00459-23
Kersters, K., De Ley, J. (1968). The occurrence of the Entner-Doudoroff pathway in bacteria. Antonie Van Leeuwenhoek 34, 393–408. doi: 10.1007/BF02046462
Kim, S. J., Kim, Y. S., Yeom, S. J. (2020b). Phosphate sugar isomerases and their potential for rare sugar bioconversion. J. Microbiol. 58, 725–733. doi: 10.1007/s12275-020-0226-x
Kim, M., Lee, J., Heo, L., Han, S. W. (2020a). Putative bifunctional chorismate mutase/prephenate dehydratase contributes to the virulence of Acidovorax citrulli. Front. Plant Sci. 11, 569552. doi: 10.3389/fpls.2020.569552
Kim, M., Lee, J., Heo, L., Lee, S. J., Han, S. W. (2021). Proteomic and phenotypic analyses of a putative glycerol-3-phosphate dehydrogenase required for virulence in Acidovorax citrulli. Plant Pathol. J. 37, 36–46. doi: 10.5423/PPJ.OA.12.2020.0221
Klee, S. M., Sinn, J. P., Finley, M., Allman, E. L., Smith, P. B., Aimufua, O., et al. (2019). Erwinia amylovora auxotrophic mutant exometabolomics and virulence on apples. Appl. Environ. Microbiol. 85, e00935–e00919. doi: 10.1128/AEM.00935-19
Kovach, M. E., Elzer, P. H., Hill, D. S., Robertson, G. T., Farris, M. A., Roop, R. M., et al. (1995). Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166, 175–176. doi: 10.1016/0378-1119(95)00584-1
Krulwich, T. A., Sachs, G., Padan, E. (2011). Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 9, 330–343. doi: 10.1038/nrmicro2549
Latin, R. X., Hopkins, D. L. (1995). Bacterial fruit blotch of watermelon - the hypothetical exam question becomes reality. Plant Dis. 79, 761–765. doi: 10.1094/PD-79-0761
Lee, J., Choi, J., Lee, J., Cho, Y., Kang, I. J., Han, S. W. (2022a). Comparing protein expression in Erwinia amylovora strain TS3128 cultured under three sets of environmental conditions. Plant Pathol. J. 38, 410–416. doi: 10.5423/PPJ.NT.05.2022.0073
Lee, J., Heo, L., Han, S. W. (2021). Comparative proteomic analysis for a putative pyridoxal phosphate-dependent aminotransferase required for virulence in Acidovorax citrulli. Plant Pathol. J. 37, 673–680. doi: 10.5423/PPJ.NT.09.2021.0139
Lee, J., Lee, J., Cho, Y., Choi, J., Han, S. W. (2022b). A putative 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase is involved in the virulence, carbohydrate metabolism, biofilm formation, twitching halo, and osmotic tolerance in Acidovorax citrulli. Front. Plant Sci. 13, 1039420. doi: 10.3389/fpls.2022.1039420
Liu, J., Tian, Y. L., Zhao, Y. Q., Zeng, R., Chen, B. H., Hu, B. S., et al. (2019). Ferric uptake regulator (FurA) is required for Acidovorax citrulli virulence on watermelon. Phytopathology 109, 1997–2008. doi: 10.1094/PHYTO-05-19-0172-R
Liu, D., Zhao, M., Qiao, P., Li, Z., Chen, G., Guan, W., et al. (2023). ntrC contributes to nitrogen utilization, stress tolerance, and virulence in Acidovorax citrulli. Microorganisms 11, 767. doi: 10.3390/microorganisms11030767
Luo, J. Y., Qiu, W., Chen, L., Anjum, S. I., Yu, M. H., Shan, C. L., et al. (2015). Identification of pathogenicity-related genes in biofilm-defective Acidovorax citrulli by transposon Tn5 mutagenesis. Int. J. Mol. Sci. 16, 28050–28062. doi: 10.3390/ijms161226076
Mei, G. Y., Yan, X. X., Turak, A., Luo, Z. Q., Zhang, L. Q. (2010). AidH, an alpha/beta-hydrolase fold family member from an Ochrobactrum sp. strain, is a novel N-acylhomoserine lactonase. Appl. Environ. Microbiol. 76, 4933–4942. doi: 10.1128/AEM.00477-10
Mitchell, J. G., Kogure, K. (2006). Bacterial motility: links to the environment and a driving force for microbial physics. FEMS Microbiol. Ecol. 55, 3–16. doi: 10.1111/j.1574-6941.2005.00003.x
Morgan, J. K., Ortiz, J. A., Riordan, J. T. (2014). The role for TolA in enterohemorrhagic Escherichia coli pathogenesis and virulence gene transcription. Microb. Pathog. 77, 42–52. doi: 10.1016/j.micpath.2014.10.010
Park, S., Dingemans, J., Gowett, M., Sauer, K. (2021). Glucose-6-phosphate acts as an extracellular signal of SagS to modulate Pseudomonas aeruginosa c-di-GMP levels, attachment, and biofilm formation. mSphere 6, e01231–e01220. doi: 10.1128/mSphere.01231-20
Park, H.-J., Seong, H. J., Sul, W. J., Oh, C.-S., Han, S.-W. (2017). Complete genome sequence of Acidovorax citrulli strain KACC17005, a causal agent for bacterial fruit blotch on watermelon. Korean J. Microbiol. 53, 340–341. doi: 10.7845/kjm.2017.7084
Perez-Riverol, Y., Bai, J. W., Bandla, C., Garcia-Seisdedos, D., Hewapathirana, S., KamatChinathan, S., et al. (2022). The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552. doi: 10.1093/nar/gkab1038
Rahimi-Midani, A., Choi, T. J. (2020). Transport of phage in melon plants and inhibition of progression of bacterial fruit blotch. Viruses-Basel 12, 477. doi: 10.3390/v12040477
Schaad, N. W., Sowell, G., Goth, R. W., Colwell, R. R., Webb, R. E. (1978). Pseudomonas pseudoalcaligenes subsp citrulli sub-Nov. Int. J. Syst. Bacteriol. 28, 117–125. doi: 10.1099/00207713-28-1-117
Shrestha, R. K., Rosenberg, T., Makarovsky, D., Eckshtain-Levi, N., Zelinger, E., Kopelowitz, J., et al. (2013). Phenotypic variation in the plant pathogenic bacterium Acidovorax citrulli. PLoS One 8, e73189. doi: 10.1371/journal.pone.0073189
Sriram, G., Martinez, J. A., Mccabe, E. R. B., Liao, J. C., Dipple, K. M. (2005). Single-gene disorders: what role could moonlighting enzymes play? Am. J. Hum. Genet. 76, 911–924. doi: 10.1086/430799
Tatusov, R. L., Galperin, M. Y., Natale, D. A., Koonin, E. V. (2000). The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 28, 33–36. doi: 10.1093/nar/28.1.33
Tong, J., Meng, L., Wang, X., Liu, L., Lyu, L., Wang, C., et al. (2016). The FBPase encoding gene glpX is required for gluconeogenesis, bacterial proliferation and division in vivo of Mycobacterium marinum. PLoS One 11, e0156663. doi: 10.1371/journal.pone.0156663
Tung, S. Y., Kuo, T. H. (1999). Requirement for phosphoglucose isomerase of Xanthomonas campestris in pathogenesis of citrus canker. Appl. Environ. Microbiol. 65, 5564–5570. doi: 10.1128/AEM.65.12.5564-5573.1999
Viljoen, A., Foster, S. J., Fantner, G., Hobbs, J. K., Dufrene, Y. F. (2020). Scratching the surface: bacterial cell envelopes at the nanoscale. mBio 11, e03020–e03019. doi: 10.1128/mBio.03020-19
Vitko, N. P., Spahich, N. A., Richardson, A. R. (2015). Glycolytic dependency of high-level nitric oxide resistance and virulence in Staphylococcus aureus. mBio 6, 3020–3019. doi: 0.1128/mBio.00045-15
Wang, T. L., Guan, W., Huang, Q., Yang, Y. W., Yan, W. R., Sun, B. X., et al. (2016). Quorum-sensing contributes to virulence, twitching motility, seed attachment and biofilm formation in the wild type strain Aac-5 of Acidovorax citrulli. Microb. Pathog. 100, 133–140. doi: 10.1016/j.micpath.2016.08.039
Wang, Y. J., Zhao, Y. Q., Xia, L. M., Chen, L., Liao, Y. J., Chen, B. H., et al. (2022). yggS encoding pyridoxal 5 '-phosphate binding protein is required for Acidovorax citrulli virulence. Front. Microbiol. 12, 783862. doi: 10.3389/fmicb.2021.783862
Willems, A., Goor, M., Thielemans, S., Gillis, M., Kersters, K., De Ley, J. (1992). Transfer of several phytopathogenic Pseudomonas species to Acidovorax as Acidovorax avenae subsp. avenae subsp. nov., comb. nov., Acidovorax avenae subsp. citrulli, Acidovorax avenae subsp. cattleyae, and Acidovorax konjaci. Int. J. Syst. Bacteriol 42, 107–119. doi: 10.1099/00207713-42-1-107
Yang, Y., Fei, N., Ji, W., Qiao, P., Yang, L., Liu, D., et al. (2023). pilA gene contributes to virulence, motility, biofilm formation, and interspecific competition of bacteria in Acidovorax citrulli. Microorganisms 11, 1806. doi: 10.3390/microorganisms11071806
Zhang, P., Wei, D. S., Li, Z. M., Sun, Z. X., Pan, J., Zhu, X. D. (2015). Cryptococcal phosphoglucose isomerase is required for virulence factor production, cell wall integrity and stress resistance. FEMS Yeast Res. 15, fov072. doi: 10.1093/femsyr/fov072
Zhang, X. X., Zhao, M., Yan, J. P., Yang, L. L., Yang, Y. W., Guan, W., et al. (2018). Involvement of hrpX and hrpG in the virulence of Acidovorax citrulli strain Aac5, causal agent of bacterial fruit blotch in cucurbits. Front. Microbiol. 9, 507. doi: 10.3389/fmicb.2018.00507
Zhou, Y., Yan, K. Z., Qin, Q. J., Raimi, O. G., Du, C., Wang, B., et al. (2022). Phosphoglucose isomerase is important for Aspergillus fumigatus cell wall biogenesis. mBio 13, 1426–1422. doi: 10.1128/mbio.01426-22
Keywords: Acidovorax citrulli, proteomics, glucose 6-phosphate isomerase, virulence, pleiotropic effect
Citation: Heo L, Han Y, Cho Y, Choi J, Lee J and Han S-W (2023) A putative glucose 6-phosphate isomerase has pleiotropic functions on virulence and other mechanisms in Acidovorax citrulli. Front. Plant Sci. 14:1275438. doi: 10.3389/fpls.2023.1275438
Received: 10 August 2023; Accepted: 23 October 2023;
Published: 07 November 2023.
Edited by:
Hyong Woo Choi, Andong National University, Republic of KoreaReviewed by:
Rikky Rai, University of Allahabad, IndiaDixit Sharma, Central University of Himachal Pradesh, India
Mee Kyung Sang, National Institute of Agricultural Science (South Korea), Republic of Korea
Copyright © 2023 Heo, Han, Cho, Choi, Lee and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sang-Wook Han, c3doYW5AY2F1LmFjLmty
 Lynn Heo
Lynn Heo Yoobin Han
Yoobin Han Yongmin Cho
Yongmin Cho Junhyeok Choi
Junhyeok Choi Jeongwook Lee
Jeongwook Lee Sang-Wook Han
Sang-Wook Han