
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 06 October 2023
Sec. Functional Plant Ecology
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1270304
This article is part of the Research Topic Vegetation-based Degradation and Restoration on the Alpine Grasslands of the Tibetan Plateau View all 15 articles
Introduction: Scientific grazing management is of great significance for the ecological health and sustainable use of alpine meadows.
Methods: To explore appropriate management methods of alpine grasslands of the Qinghai-Tibet Plateau degraded by Achnatherum inebrians (Hance) Keng ex Tzvele presence, we studied the effects of different grazing systems on the A. inebrians population, grassland vegetation community traits, soil characteristics and soil microbial community structure for cold- season grazing plus supplementary feeding pasture (CSF) and four-season open public pasture (FOP) in Tianzhu County, Gansu Province.
Results: Compared with FOP, the CSF site showed significantly inhibited reproduction of A. inebrians, especially the crown width, seed yield and number of reproductive branches per plant were as high as 50%, significantly increased the aboveground biomass of edible forage and soil water content by 57% and 43–55%, better soil nutrients, and significantly reduced soil bulk density by 10– 29%. Different grazing systems affected the composition and diversity of soil microbial communities, with a greater effect on fungi than on bacterial flora. The most abundant phyla of bacteria and fungi were Proteobacteria and Ascomycota for CSF (by 30–38% and 24–28%) and for FOP (by 67–70% and 68–73%), and the relative abundance and species of bacterial and fungal genera were greater for CSF than FOP. The α-diversity indexes of fungi were improved, and the β-diversity of fungi was significant difference between CSF and FOP. However, the grazing utilization time was prolonged in FOP, which reduced the diversity and abundance of soil bacteria and increased soil spatial heterogeneity. The use of A. inebrians-type degraded grassland in the cold season, and as a winter supplementary feeding and resting ground, could effectively inhibit expansion of A. inebrians, promote edible forage growth, enhance grassland productivity and community stability, and improve soil structure.
Discussion: The results guide healthy and sustainable utilization of A. inebrians-type degraded grassland in the Qinghai-Tibet Plateau.
As a unique geographical unit of the earth, the Qinghai-Tibet Plateau is an important ecological security barrier in northwest China, with a wealth of habitat types and biological species (Fan and Fang, 2022). It has a profound impact on the surrounding area, China and the world. Alpine meadows are the most widely distributed grassland type in the Qinghai-Tibet Plateau (Niu et al., 2019), and play an important role in maintaining the ecosystem balance, including for animal husbandry development, climate regulation and water conservation (Ma et al., 2022). For decades, grazing was the dominant use in alpine meadows of the Qinghai-Tibet Plateau. However, these grasslands have also experienced spread of poisonous weeds following desertification and salinization because of the dual influence of natural and human factors, including climate change and overgrazing (Wei, 2013). This has reduced species diversity and edible forage yield, restricted the development of grassland animal husbandry and seriously threatened the livestock–soil–grassland ecosystem balance and productivity (Guo et al., 2017; Chai et al., 2019). Therefore, it is very important to study the impact of grazing on poisonous weed-type degraded grassland ecosystems in Qinghai-Tibet Plateau alpine meadows.
Achnatherum inebrians (Hance) Keng ex Tzvele is a perennial grass in the family Gramineae that is becoming increasingly widespread in northwest China, including in Gansu, Xinjiang, Qinghai, Tibet and Inner Mongolia (Liu et al., 2021; Liang et al., 2023). Relevant studies showed that the harmful area was 3 × 105 hm2 in Gansu, accounting for 17.08% of the poisonous weed degraded area in the province (Guo et al., 2017). The distribution area of A. inebrians reached 5.3 × 105 hm2 in Xinjiang’s natural grassland, with coverage of up to 85% in some areas (Sun et al., 2014), and its distribution area reached 5.7 × 105 hm2 in Qinghai. In recent years, the distribution area and coverage of A. inebrians tended to expand in different regions, with serious economic losses to animal husbandry and also leading to grassland ecological imbalance. A. inebrians is the host and mutualist symbiont of the Epichloë endophyte, with a carrier rate of up to 100%. Epichloë contains high levels of ergot alkaloids (i.e., ergopeptine and ergovaline), which make it poisonous (Miles et al., 1996), and accidental feeding and starvation of juvenile or foreign livestock have caused serious losses for animal production (Yang et al., 2015). Moreover, the Epichloë endophyte can enhance biotic and abiotic resistance (Moon et al., 2007; Zhang et al., 2012). Li et al. (1996) showed that livestock that mistakenly ingest A. inebrians can quickly identify and avoid further feeding on it, so that it has a greater advantage in interspecific competition within grassland communities and continues to spread in degraded grassland, causing serious harm.
Current research on A. inebrians mainly focuses on control (Jin et al., 2017), characteristics (Li, 2005), seed germination (Chen et al., 2021), toxic ingredients (Liu et al., 2022) and Epichloë endophyte resistance (Zhang et al., 2012) in the A. inebrians population. Research on prevention and restoration of A. inebrians-type degraded grassland focuses on responses of A. inebrians population traits, edible forage yield and grassland community diversity to fencing, mowing, manual digging and sowing excellent forage (Li et al., 1996; Mcdaniel and Sterling, 2007; Jing et al., 2014; Yang et al., 2015). However, Sun et al. (2020) showed that long-term fencing provided no ecological and economic benefits; believed that mowing and manual digging were only suitable for A. inebrians grasslands with small distribution area and high growth density, and sowing was a seemingly simple but complex improvement measure. Therefore, grazing is the most important and direct utilization and management method affecting grassland biodiversity and ecosystem function, and seasonal grazing is widely used for managing degraded grassland (Zhang et al., 2023). However, there is a lack of research on the response of poisonous weeds of grasslands to grazing systems in the Qinghai-Tibet Plateau (Wei, 2013), and the comprehensive effects of different grazing systems on grassland communities, microorganisms and soil require further study.
In this study, our main objective was to study how vegetation community traits and soil characteristics of A. inebrians-type degraded grassland under long-term different grazing systems, explore the relationship between soil microbial community structure, soil and vegetation traits in A. inebrians-type grassland, and provide a theoretical basis for the restoration and health of poisonous weed-degraded alpine meadow ecosystems. More specifically we hypothesized that: (i) grassland vegetation community traits with grazing systems vary, (ii) soil physicochemical characteristics and microbial community composition change with grazing systems, (iii) the vegetation–soil–microbe are interrelated, (iv) cold-season grazing plus supplementary feeding pasture can effectively regulate A. inebrians-type degraded grassland.
A vegetation community survey and soil sampling for this study were conducted on two separate grasslands, situated in the alpine meadow eastern edge of the Qinghai-Tibet Plateau in the administrative region of Tianzhu County, Gansu Province, China (37°11′N, 102°46′E). According to the guidance of local grassland ecological experts and herders, the A. inebrians-type degraded grassland was selected as the research area, which was open public grassland with basically the same vegetation as 35 years ago, with A. inebrians as an absolute dominant species. In 1993, it was divided into two areas: (i) one, rested in summer, had always been grazed by Tibetan sheep + yak and as a supplementary feeding site in winter, and had been fenced; and (ii) the other was still used as an open public pasture, with the same livestock grazing all year round. The distance was 20 m between cold-season grazing plus supplementary feeding pasture and four-season open public pasture, so the physical environment was similar (soil substrate and topography were the same). The altitude is 2910 m, annual mean temperature is −0.1°C, with monthly mean temperature ranging from −18.3°C in January to 12.7°C in July, and average annual accumulated temperature of 1380°C. The average annual precipitation is 416 mm, mainly concentrated in July–September and mostly terrain rain. There are only cold and warm seasons in the year, with the cold season in October–May, the warm season in June–September and the plant growth period generally April–September. The two grasslands are termed cold-season grazing plus supplementary feeding pasture (CSF) and four-season open public pasture (FOP).
During 15–18 August 2022, nine quadrats (1 m × 1 m) with representative and uniformly distributed vegetation were selected from each pasture for the vegetation community survey and soil sample collection. On September, 50 A. inebrians plants were selected from each pasture to calculate their population characteristics.
Soil samples were collected using a soil drilling core (d = 3.5 cm) divided into 0–10, 10–20 and 20–30 cm, with nine replicates in each quadrat; nine drills from each layer were mixed in bags and taken back to the laboratory. Each sample was divided into two subsamples and allocated to the following measurements: (i) soil nutrients and enzyme activity (air-dried soil) and (ii) soil microbial sequencing, for which 5–8 g of soil was dispensed into sterilized centrifuge tubes, stored on dry ice and brought back to the laboratory and stored at −80°C. Soil bulk density was sampled by a ring knife (100 cm3 volume) and an aluminum box dried in advance was used to determine soil water content.
The coverage of each species and the total coverage of the quadrat were measured by the needle-punch method. Ten plants of each species in each quadrat were randomly measured for their natural height. The aboveground biomass of each species was removed by the cutting method in each quadrat, put into an envelope, brought back to the laboratory, dried at 105°C for 30 min, then oven-dried at 65°C to constant weight and weighed. A sampling quadrat was tossed randomly 50 times into the plot and the frequency of each species recorded in each quadrat (Ren, 1998).
The crown size of A. inebrians was determined using a steel tape measure, and then a single plant was harvested and dried it at 65°C to constant weight and weighed. Another 30 clusters of A. inebrians were randomly selected to count the number of reproductive branches, and the ears were removed, put into an envelope; then their length was measured, they were threshed by hand and the weight of 100 grains was determined by 0.001 g electronic balance. Thirty reproductive branches were randomly selected and their spikelets were counted; 30 spikelets were randomly selected to count the florets and seeds per spikelet.
The soil bulk density, soil water content, total nitrogen, total phosphorus, available nitrogen, available phosphorus and soil organic matter were determined by ring knife method, drying method, Kjeldahl nitrogen method, molybdenum antimony anti-colorimetric method, alkaline diffusion method, NaHCO3 leach-colorimetry method and external heating of K2Cr2O7; and total potassium and available potassium were determined using a flame photometer (Model 2655−00, Digital Flame Analyzer, Cole-Parmer Instrument Company, Chicago, IL, USA), respectively (Bao, 2000). The urease, alkaline phosphatase, sucrase and catalase activities in soil were determined using sodium phenolate-sodium hypochlorite colorimetry, benzene disodium phosphate colorimetry, 3,5-dinitrosalicylic acid colorimetry and potassium permanganate titration, respectively (Guan, 1986). Soil pH was measured using a soil pH meter (PB-10, Sartorius, Göttingen, Germany). Soil samples were sent to Weikemeng Technology Co., Ltd. for Illumina MiSeq sequencing.
The Richness, Shannon–Wiener, Simpson diversity and Pielou evenness indexes were used to describe the species diversity of L and S, using the following formulas (Ma, 1994).
where C′ is relative coverage, H′ is relative height, F′ is relative frequency, B′ is relative biomass (Lindsey, 1956), N is the total number of species in a quadrat and Pi is the importance value of species in the quadrat.
The soil microbial community used the Illumina NovaSeq platform for two-ended sequencing. The amplicon sequencing data (16S rRNA and ITS) were quality controlled, denoised and spliced to generate operational taxonomic unit, taxonomic annotation, species screening, basic statistics, significant difference comparison, α-diversity analysis, β-diversity analysis and correlation analysis using the DADA2 plugin in Qiime2 software. The species annotation information was obtained by comparing with the database [16S rRNA default Greengenes Database (version 13_8), ITS default United database (version 8.2)] The Shannon and Simpson indexes were used to assess the species diversity of samples, the Chao1 index was used to reflect species richness, and the Kruskal–Wallis method was used to determine whether there was a significant difference in an α-diversity index between the groups. Principal coordinate analysis of β-diversity was based on the weighted UniFrac distance calculation, and the significance of β-diversity was determined using Permutational multivariate analysis of variance. Besides, the closer the distance between the dots of different colors, the more similar the species composition and structure, and the closer the distance between the dots of the same color, indicating that the samples are better clustered in the group. The correlations between environmental factors and the relative abundance of soil microbiota at the phylum level were analyzed using Redundancy analysis, and the significance of the ranking axis feature values used the Monte Carlo permutation test.
All statistical analyses of vegetation community and soil physicochemical indexes were performed using SPSS software (version 19.0), including Shapiro–Wilk normal distribution assessment and independent sample t-test (p< 0.05). The measurement results are expressed as mean ± standard deviation. The data were plotted using Origin 2021.
The aboveground biomass, coverage and height of A. inebrians for CSF were 52.73%, 94.32% and 19.00% lower than those of FOP, respectively (p< 0.05); the aboveground biomass and height of edible forage in CSF were 56.82% and 123.35% higher than those of FOP, respectively (p< 0.05), and there was no significant difference in the sum of the coverage of each edible forage between CSF and FOP (p > 0.05).
The vegetation community diversity differed between the two pastures: the Simpson and Pielou indexes for CSF were 12.86% and 26.03% higher than those of FOP, respectively (p< 0.05), but there were no significant differences in Richness and Shannon–Wiener indexes between CSF and FOP (p > 0.05) (Table 1).
The length of the long and minor axes, dry weight and crown width of A. inebrians were 36.28%, 35.76%, 36.13% and 59.26% lower for CSF compared to FOP, respectively. The reproduction of A. inebrians was significantly lower for CSF than FOP, especially the seed yield and number of reproductive branches per plant were as high as 52% (p< 0.05) (Table 2).
Soil pH and bulk density tended to increase with greater soil depth in both pastures. However, soil water content, nutrients and organic matter tended to decrease (Figures 1, 2). Compared with FOP, CSF had significantly lower bulk density (by 29.46% and 10.53%) and greater soil water content (by 43.54% and 55.10%) for 0–10 and 20–30 cm, respectively (p< 0.05). Besides, CSF had significantly greater total nitrogen and total phosphorus in 0–10 cm (by 39.52% and 5.02%, respectively) and in 20–30 cm (by 33.59% and 45.23%), and had significantly greater total phosphorus and total potassium by 11.16% and 2.06% in 10–20 cm, respectively (p< 0.05). Compared with FOP, CSF had significantly greater soil organic matter, available nitrogen, available phosphorus and available potassium in 0–10 cm (by 29.67%, 28.45%, 68.42% and 23.77%, respectively) and 10–20 cm (by 10.68%, 11.25%, 93.75% and 31.20%, respectively). There were no significant differences for pH in the three soil layers, for bulk density, soil water content, total nitrogen and total phosphorus in 10–20 cm, for soil organic matter in 20–30 cm (p > 0.05).
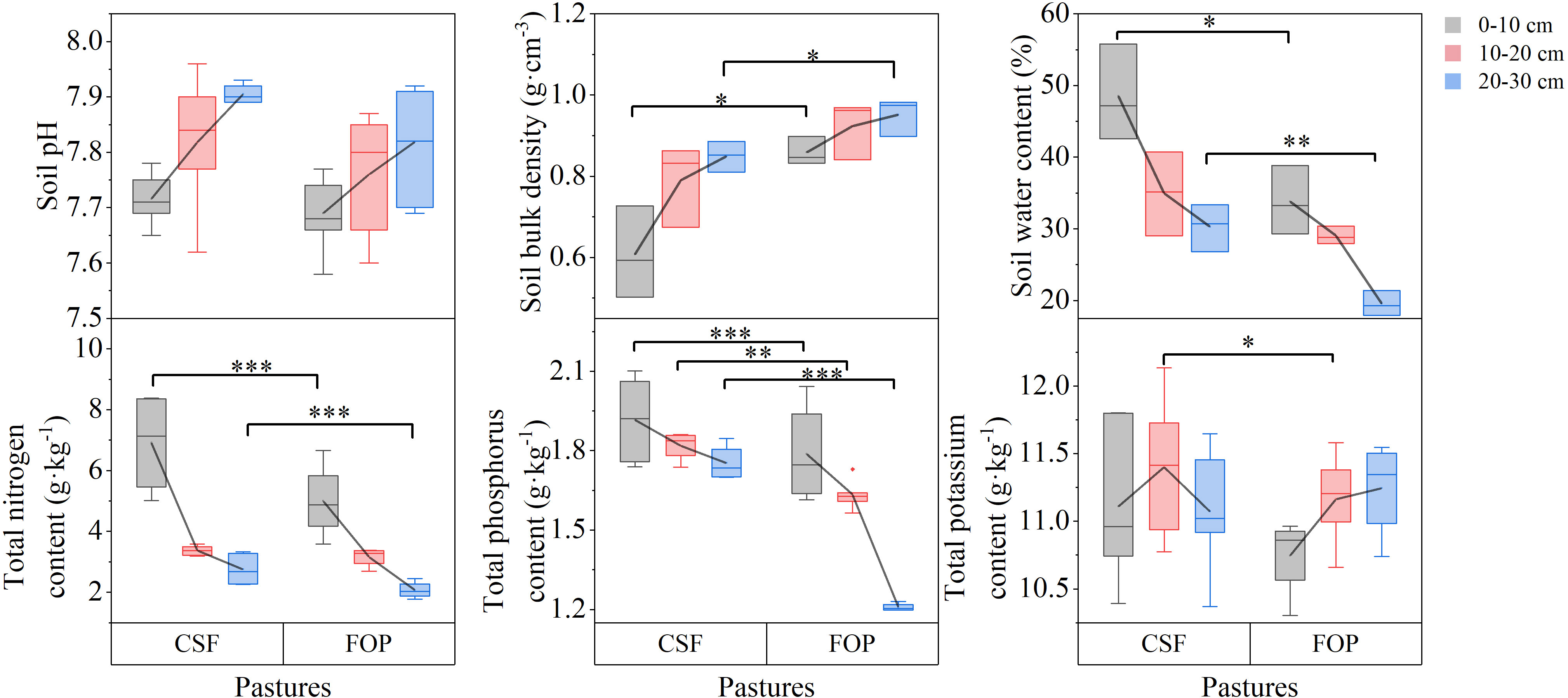
Figure 1 Soil physicochemical characteristics and total nutrients of different pastures. CSF and FOP are cold-season grazing plus supplementary feeding pasture and four-season open public pasture, respectively. *, ** and *** Significant at the 0.05, 0.01 and 0.001 probability level.
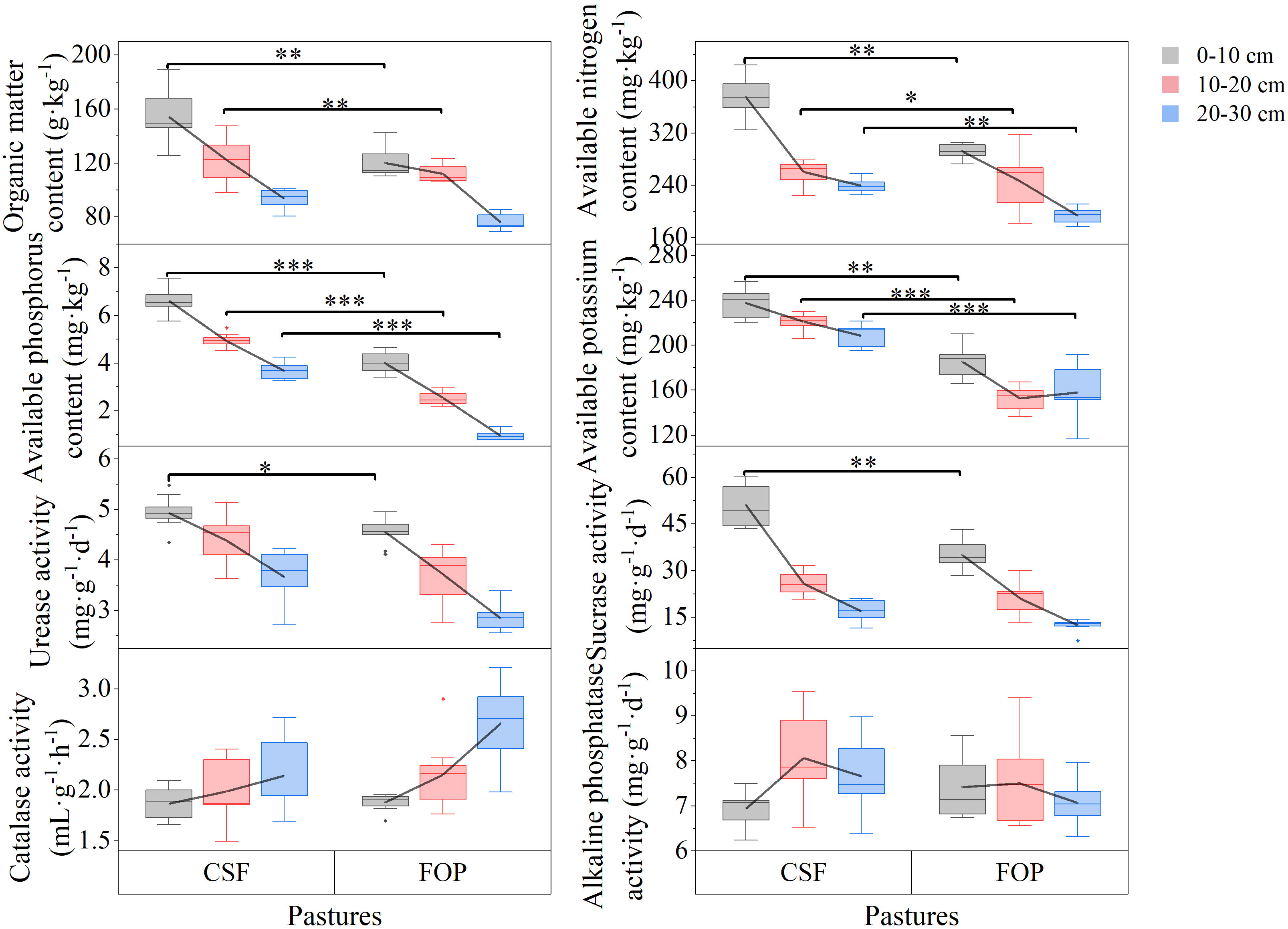
Figure 2 Soil available nutrients and enzyme activities of different pastures. CSF and FOP are cold-season grazing plus supplementary feeding pasture and four-season open public pasture, respectively. *, ** and *** Significant at the 0.05, 0.01 and 0.001 probability level.
The urease and sucrose activities tended to decrease with greater soil depth, which was opposite to the trend of catalase activity; and alkaline phosphatase activity tended to initially increase and then decrease in both pastures (Figure 2). Compared with FOP, CSF had significantly greater urease and sucrose activities by 8.31% and 45.71% only in 0–10 cm, respectively (p< 0.05). There were no significant differences in activities of the four enzymes in the other soil layers (p > 0.05).
The dominant bacterial phyla were Proteobacteria, Actinobacteriota and Acidobacteriota, but their relative abundances varied. The highest relative abundance was for Proteobacteria in 0–10, 10–20 and 20–30 cm, which was 37.78%, 30.16% and 32.99% in CSF, and 27.67%, 25.20% and 24.00% in FOP, respectively – all were higher for CSF than FOP. This was followed by Actinobacteriotaiota, with relative abundances of 16.87%, 24.33% and 26.91% in 0–10, 10–20 and 20–30 cm for CSF, respectively, and 22.68%, 28.96% and 31.67% for FOP. A total of 25 genera were detected in CSF and FOP, and nearly 30% of the bacterial taxa could not be classified clearly into any genus (i.e., designated unclassified). Sphingomonas was the dominant genera in 0–10 cm in CSF and FOP, with relative abundances of 3.91% and 3.94%, respectively, 67_14 and Ralstonia, Pseudomonas and 67_14 were the dominant genera in 10–20 and 20–30 cm, which was 4.51% and 6.04%, and 6.48% and 4.88%, respectively (Figure 3).
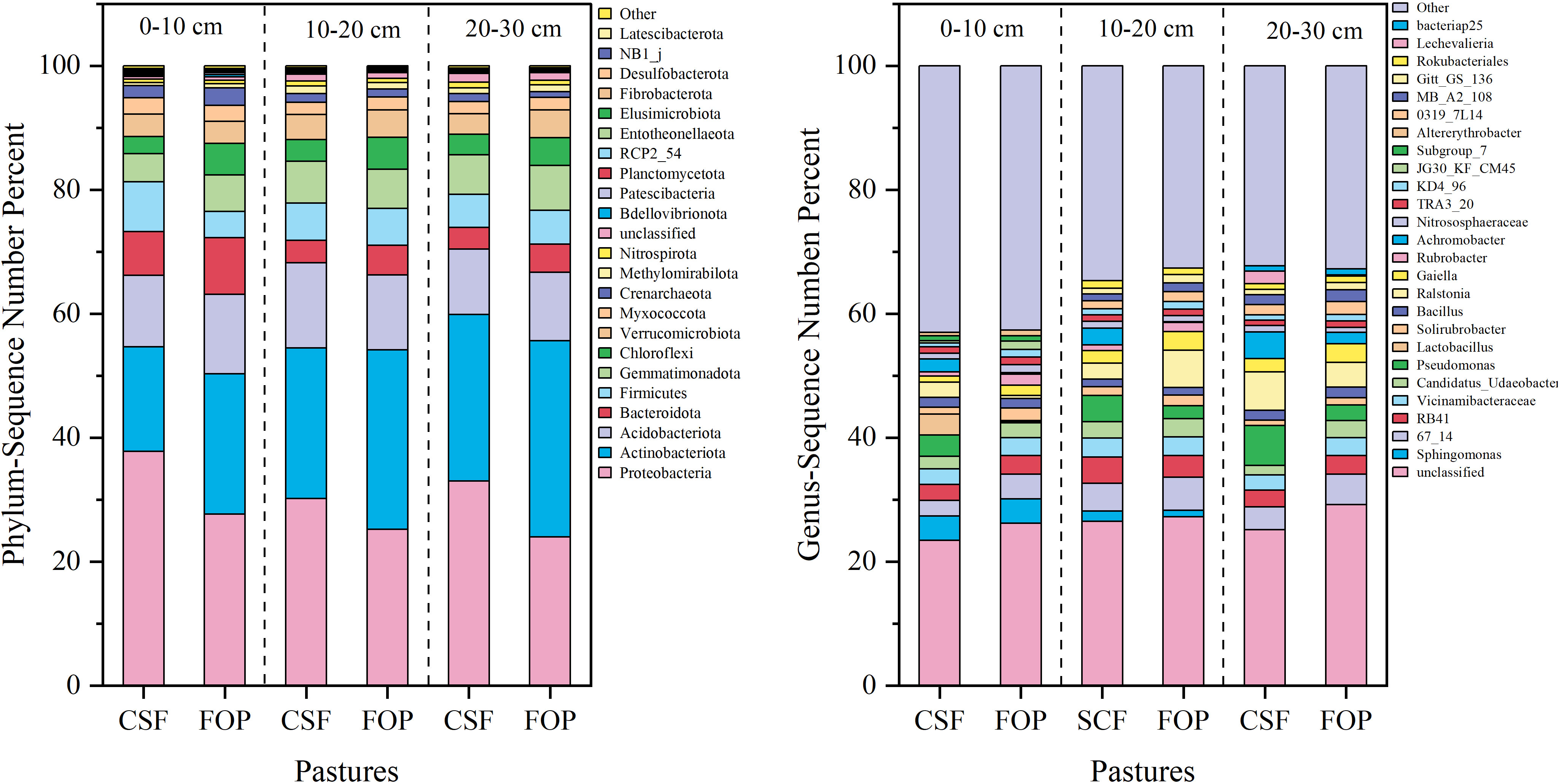
Figure 3 Relative species abundance of soil bacteria at phylum and genus levels in different pastures. CSF and FOP are cold-season grazing plus supplementary feeding pasture and four-season open public pasture, respectively.
The dominant fungal phyla were Ascomycota, Mortierellomycota and Basidiomycota, and their relative abundances varied. The highest relative abundance was for Ascomycota in 0–10, 10–20 and 20–30 cm, with 70.38%, 66.93% and 68.95% in CSF, respectively, and 73.03%, 68.42% and 70.1% in FOP – all were lower in CSF than FOP. This was followed by Mortierellomycota, with relative abundances of 16.20%, 16.59% and 14.68% for CSF, respectively, and 14.83%, 15.42% and 12.49% for FOP. A total of 32 genera were detected in CSF and FOP, with nearly 35% of the fungal taxa not able to be classified clearly into any genus (i.e., unclassified). Mortierella was the dominant genus in 0–10, 10–20 and 20–30 cm for CSF with 15.00%, 11.23% and 13.11%, respectively, and 13.95%, 13.98% and 9.61% for FOP (Figure 4).
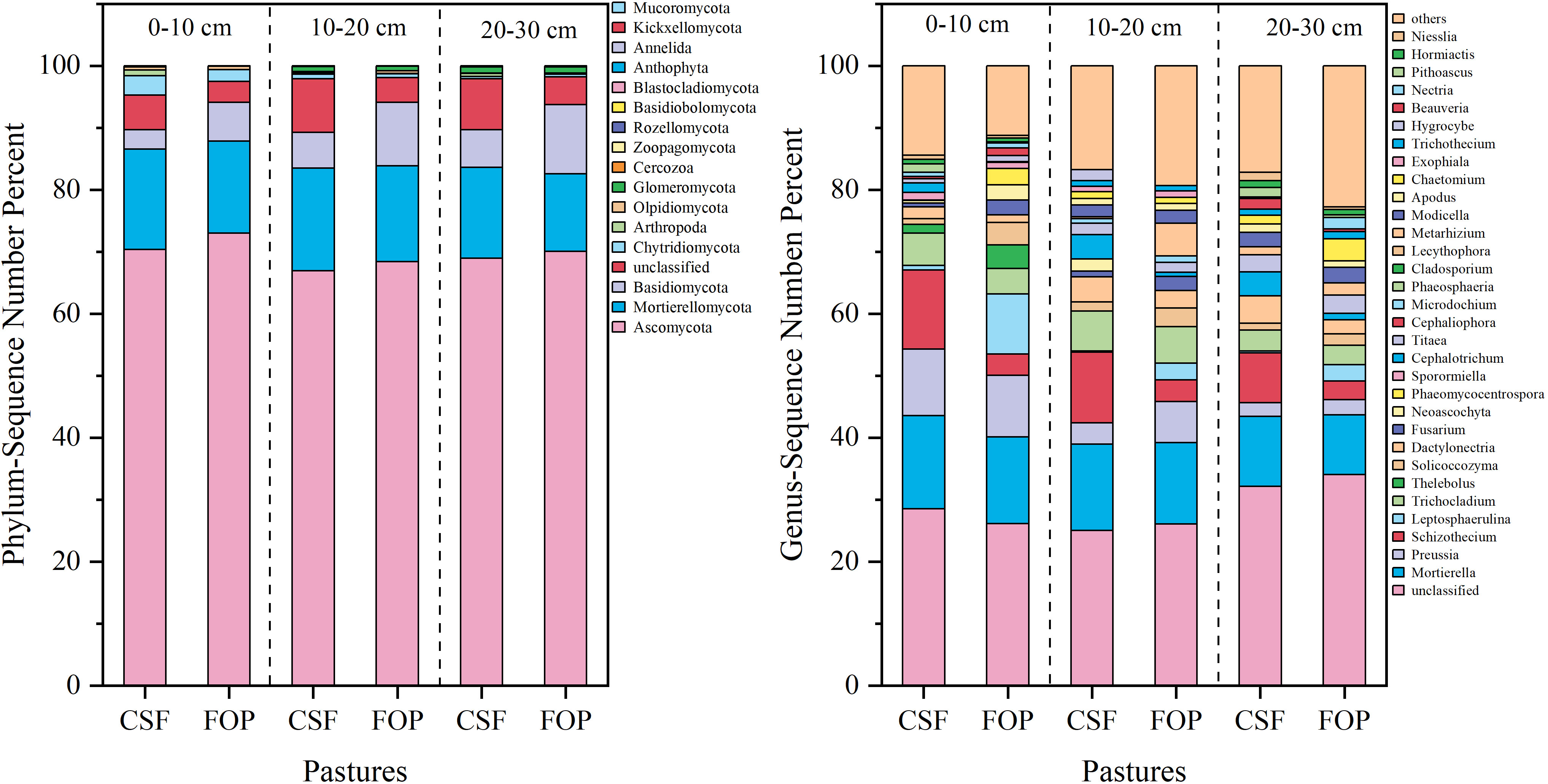
Figure 4 Relative species abundance of soil fungi at phylum and genus levels in different pastures. CSF and FOP are cold-season grazing plus supplementary feeding pasture and four-season open public pasture, respectively.
Bacterial α-diversity was overwhelmingly dominant in CSF and FOP, with the Shannon and Chao1 indexes significantly higher than those for fungi (p< 0.05). In the bacterial community, the Simpson index for FOP was significantly higher than for CSF in 0–10 cm by 1.41% (p< 0.05). In the fungal community, the Shannon, Simpson and Chao1 indexes for CSF were significantly higher than for FOP in 0–10 cm (p< 0.05), being 6.13%, 1.5% and 12.85% higher, respectively. There was no significant differences for α-diversity indexes in the other soil layers between CSF and FOP (p > 0.05). In addition, the α-diversity indexes were higher for FOP compared to CSF in the three layers of soil for bacteria, and for the Simpson index in 10–20 cm for fungi. The α-diversity indexes were higher for fungi in 0–10 and 20–30 cm of CSF compared to FOP (Figure 5).
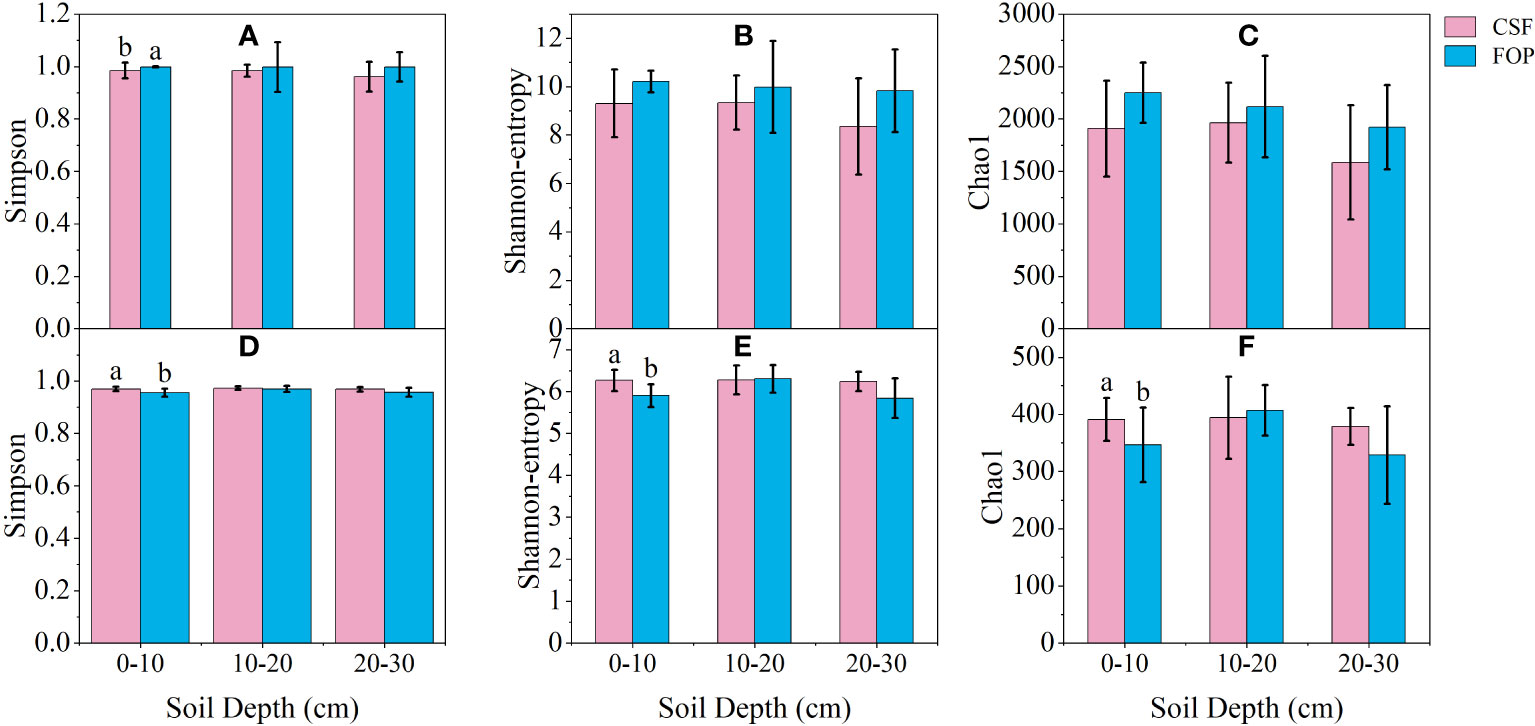
Figure 5 The α-diversity indexes of soil bacterial (A-C) and fungal (D-F) communities in different pastures. CSF and FOP are cold-season grazing plus supplementary feeding pasture and four-season open public pasture, respectively. Different letters indicate significant variation at the level of p=0.05.
The Bray–Curtis Permutational multivariate analysis of variance showed a significant difference in 0–10 cm for bacteria (p< 0.05), but no significant difference in deeper soil between the two pastures (p > 0.05). The fungal communities significantly differed in the three soil layers between CSF and FOP (p< 0.05) (Figure 6; Table 3).
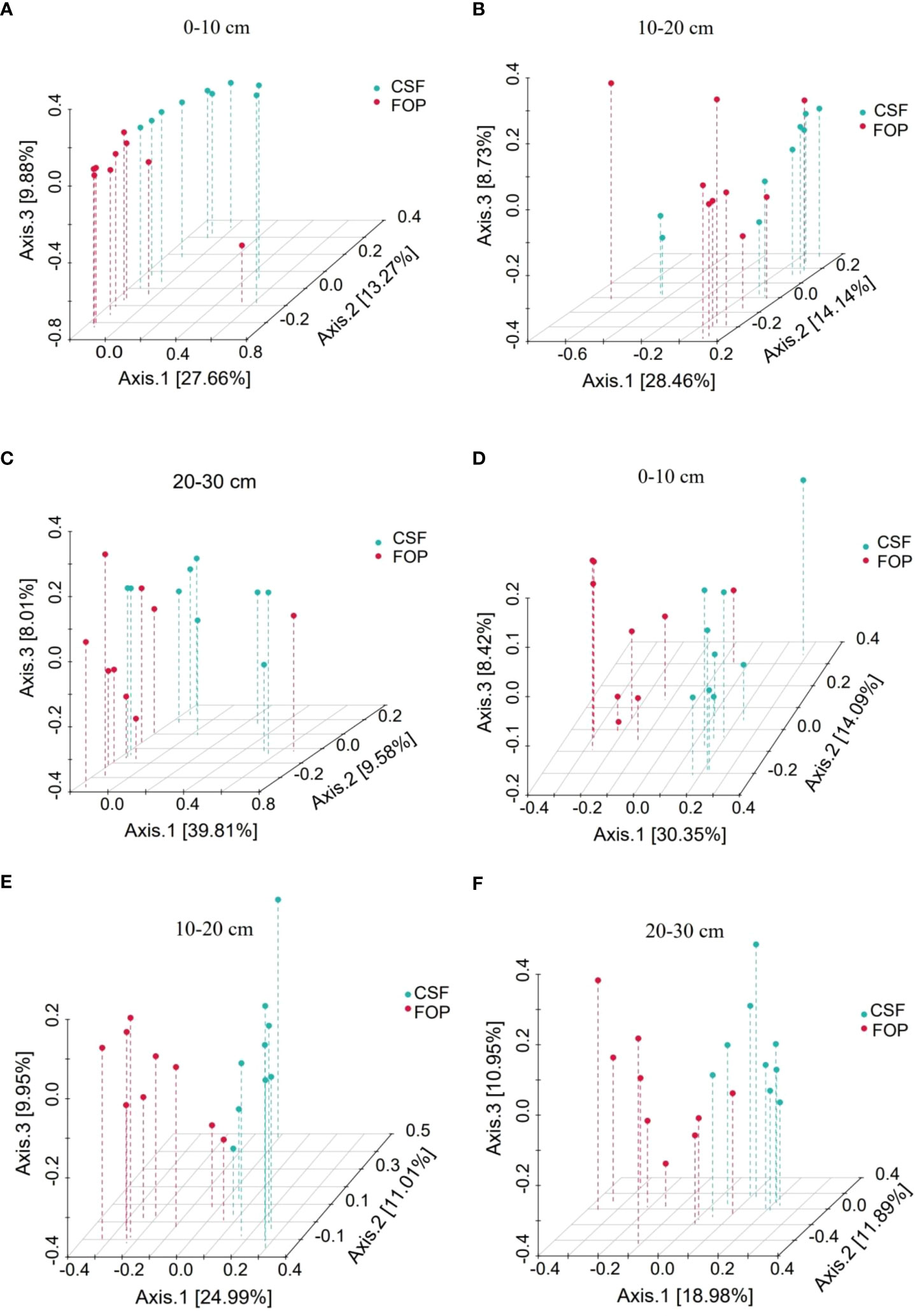
Figure 6 PCoA of β-diversity of soil bacterial (A–C) and fungal (D–F) communities in different pastures. CSF and FOP are cold-season grazing plus supplementary feeding pasture and four-season open public pasture, respectively. – This applies in other tables and figures below.
Vegetation communities and soil physicochemical indexes were screened using Principal component analysis (Figure 7). The vegetation and soil factors were screened by PC1 load values greater than 0.28 and 0.30: the important vegetation factors were aboveground biomass, coverage crown width, number of reproductive branches and seed yield of A. inebrians. The important soil factors were total nitrogen, total phosphorus, soil organic matter, and soil available nutrients in 0–10 cm; soil organic matter, available phosphorus and available potassium in 10–20 cm; and total nitrogen, total phosphorus and soil available nutrients in 20–30 cm.
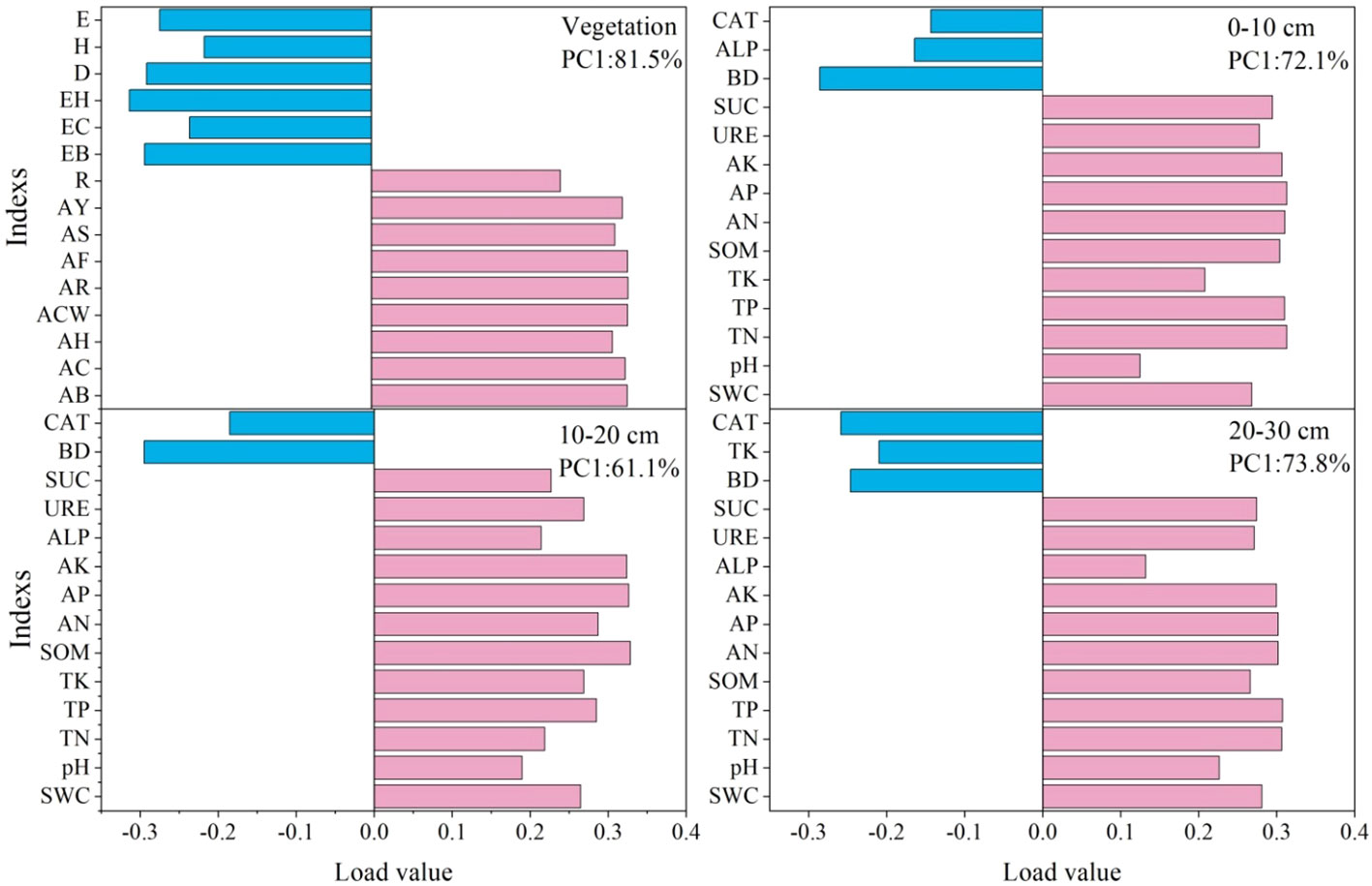
Figure 7 Vegetation community traits and soil characteristics of PC1 (Principal component analysis) axis load value. AB, AC, AH, ACW, AF, AR, AS, AY represent aboveground biomass, coverage, height, crown width, number of florets per spikelet, number of reproductive branches, spikelet per reproductive branches, seed yield of A. inebrians in turn; EB, EC, EH represent aboveground biomass, coverage and average height of edible forage in turn. E, Pielou evenness; H, Shannon–Wiener; D, Simpson diversity; BD, SWC, TN, TP, TK, SOM, AN, AP, AK, ALP, URE, SUC and CAT represent soil bulk density, water content, total nitrogen, total phosphorus, total potassium, soil organic matter, available nitrogen, available phosphorus, available potassium, alkaline phosphatase activity, urease activity, sucrase activity and catalase activity in turn. – This applies in other tables and figures below.
The cumulative contribution rates of the first and second axes were 45.34%, 45.63% and 46.67% in 0–10, 10–20 and 20–30cm, respectively (Figure 8) . The most abundant phyla were Proteobacteria, Actinobacteriota, Acidobacteriota and Bacteroidota, in 0–10 cm, which had large positive correlations with A. inebrians reproductive capacity; and Proteobacteria and Bacteroidota had high positive correlations with soil available nutrients. Proteobacteria, Acidobacteriota and Actinobacteriota were the most abundant phyla in 10–20 cm, which were influenced by available potassium, soil organic matter and crown width of A. inebrians, respectively. Proteobacteria and Actinobacteriota were the most abundant in 20–30 cm, with positive correlations with available nitrogen and seed yield of A. inebrians. Soil bacteria were most strongly correlated with the A. inebrians reproductive capacity and soil organic matter in 0–10 cm of CSF and FOP. In 10–20 cm, soil bacteria were strongly correlated with soil organic matter, available phosphorus and potassium in CSF, and seed yield, number of florets per spikelet, coverage and number of reproductive branches of A. inebrians in FOP. Soil bacteria were also highly positively correlated with crown width, number of reproductive branches, number of florets per spikelet of A. inebrians in 20–30 cm of FOP, but the dispersion of CSF sample points was high. Combined with a Monte Carlo permutation test, soil bacteria were significantly correlated with available nitrogen only in 20–30 cm (p< 0.05), and had no significant correlations with each factor in the remaining two layers (p > 0.05) (Table 4).
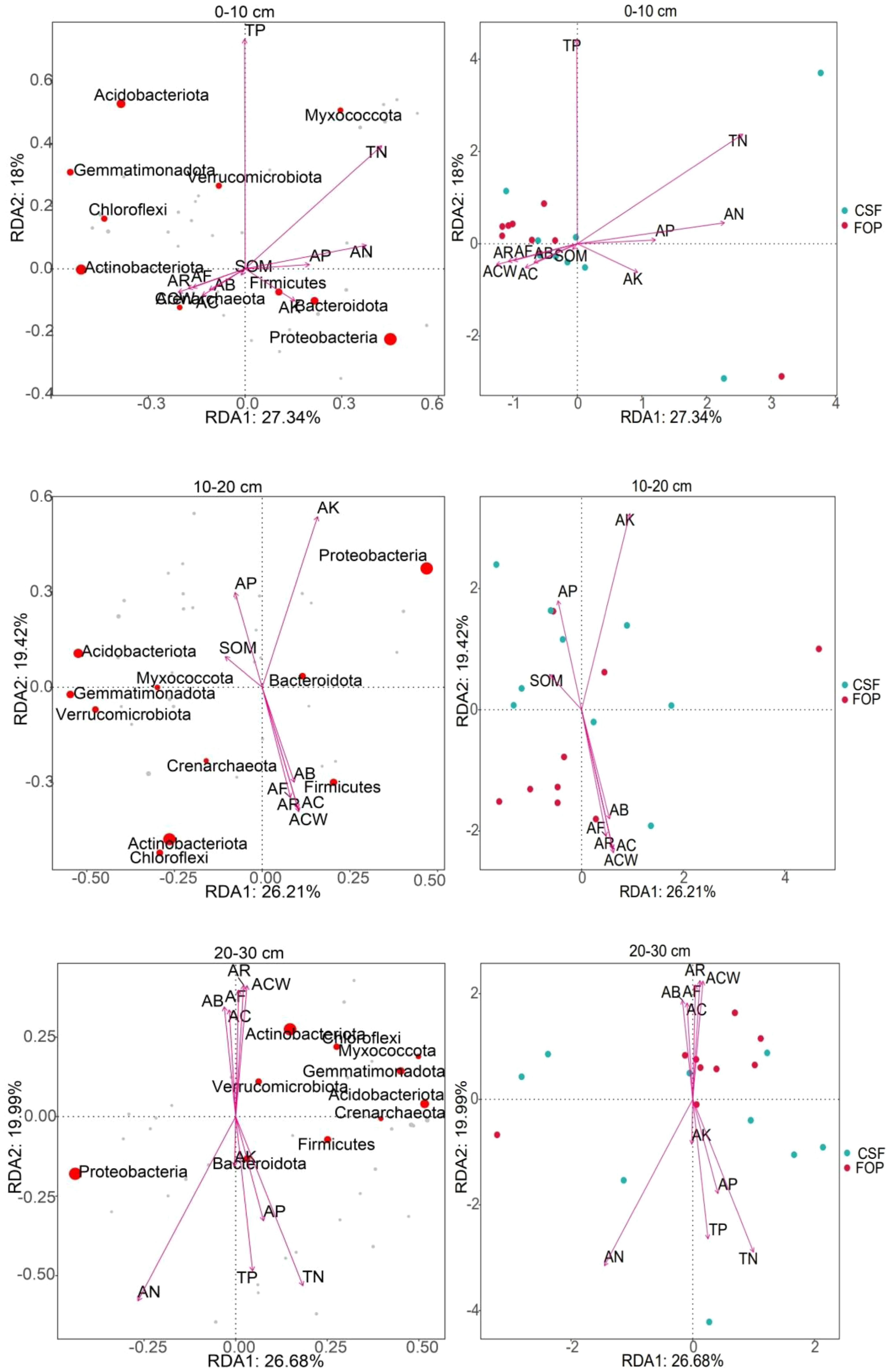
Figure 8 RDA of soil bacterial communities at the phylum level and environmental factors in different pastures.
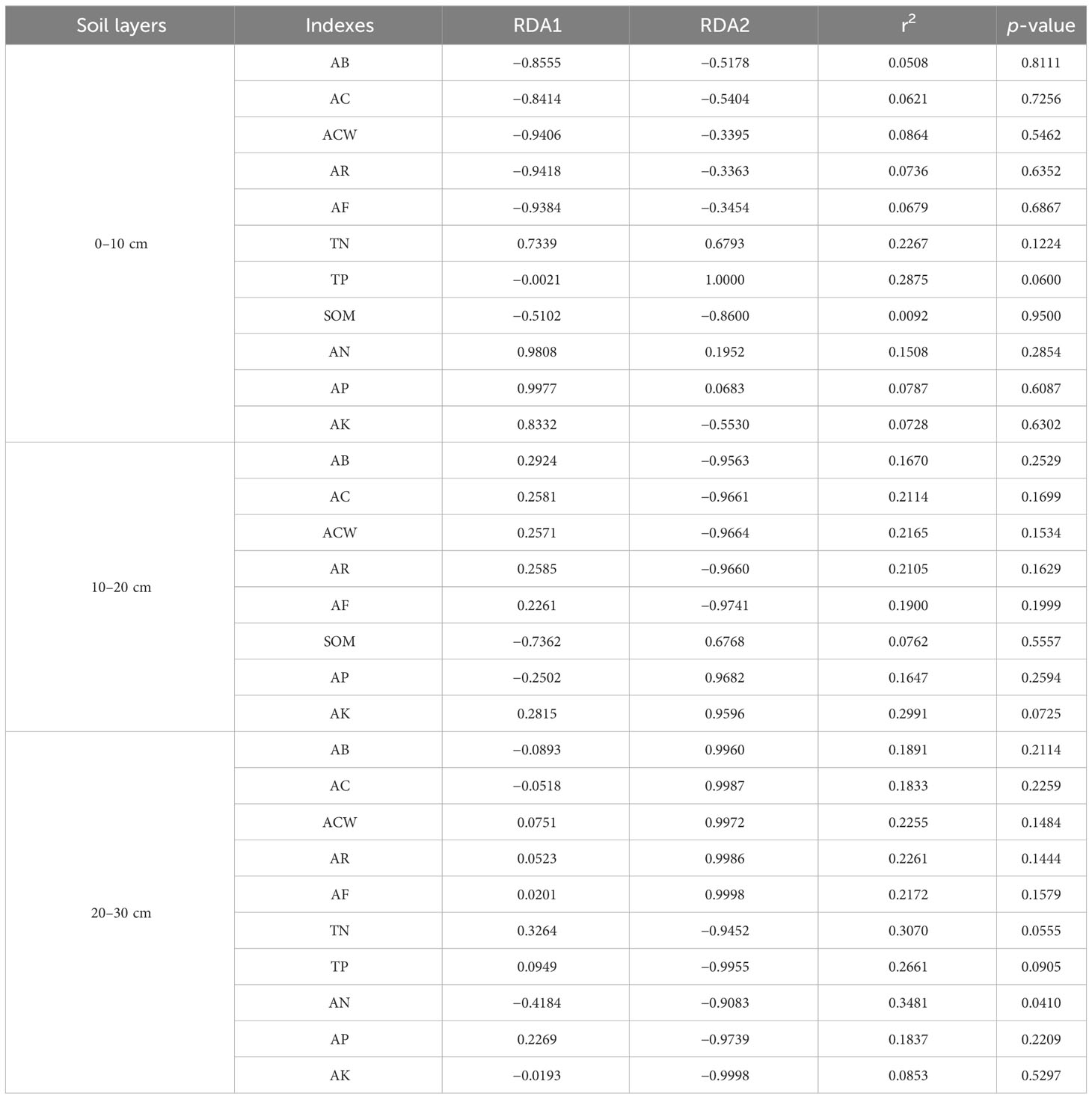
Table 4 Monte Carlo permutation test between soil bacteria, vegetation and soil physicochemical properties.
The cumulative contribution rates of first and second axes were 51.58%, 54.61% and 48.64% in 0–10, 10–20 and 20–30 cm, respectively (Figure 9). The most abundant phyla were Ascomycota and Mortierellomycota in 0–10 cm, both with high positive correlations with number of reproductive branches of A. inebrians and available potassium. Ascomycota and Mortierellomycota were the most abundant phyla in 10–20 cm, and Ascomycota was positively correlated with the A. inebrians population, and Mortierellomycota was strongly positive correlations with soil organic matter, available phosphorus and potassium. The fungal phyla with high abundance in 20–30 cm was consistent with abundances in shallower soil but were not highly correlated with each factor. Soil fungi were strongly correlated with soil chemical properties in CSF, and A. inebrians population traits in FOP. Sample point dispersion was high in 20–30 cm of CSF and FOP. Combined with the Monte Carlo permutation test, for 0–10 cm, fungi were significantly correlated with all factors except total phosphorus (p< 0.05), and very significantly correlated with aboveground biomass, coverage, crown width, number of reproductive branches and number of florets per spikelet of A. inebrians, soil organic matter and available nitrogen (p< 0.01); for 10–20 cm there were very significant correlations of fungi with all factors (p< 0.01). There were no significant correlations in 20–30 cm (p > 0.05) (Table 5).
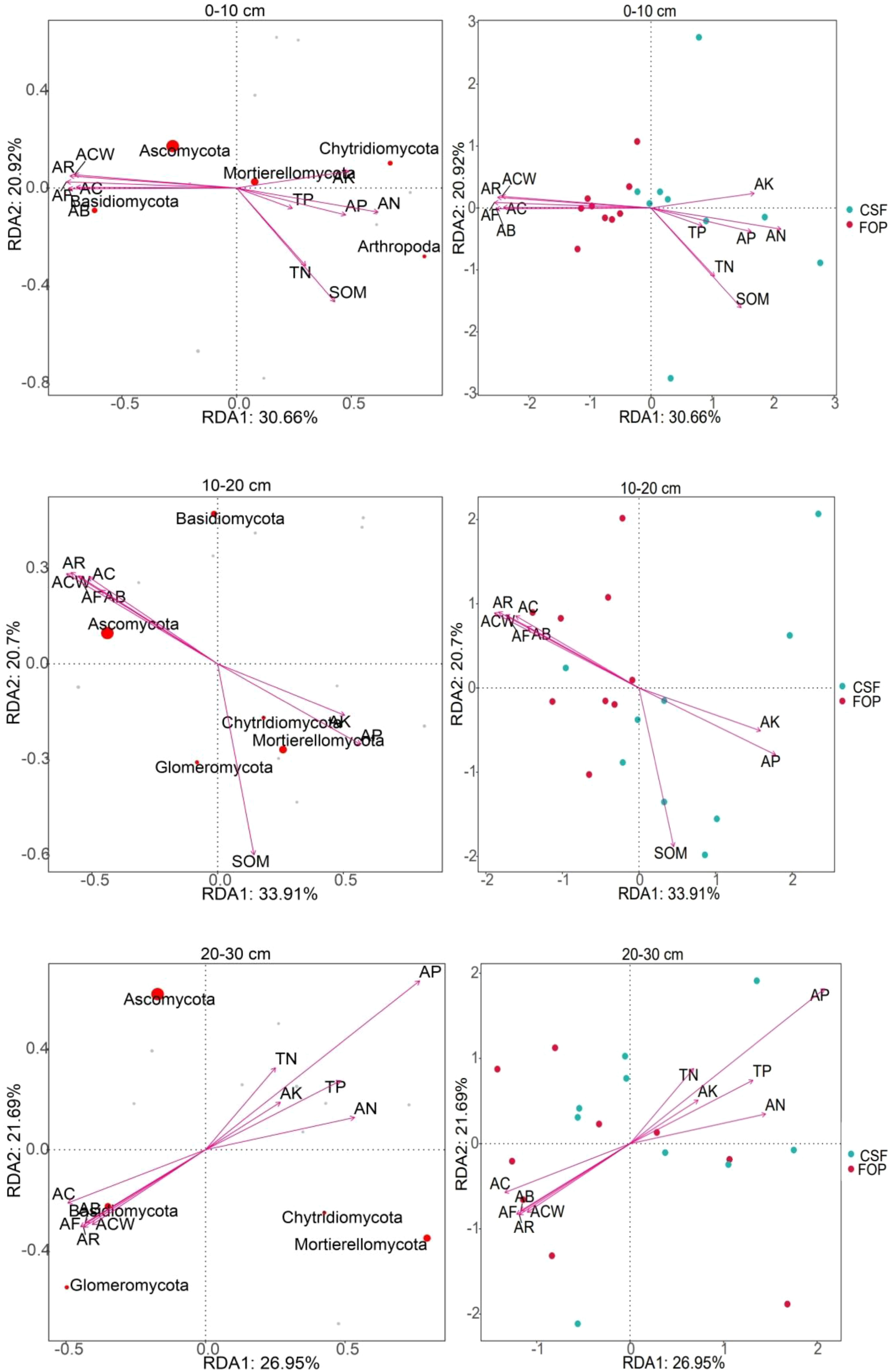
Figure 9 RDA of soil fungal communities at the phylum level and environmental factors in different pastures.
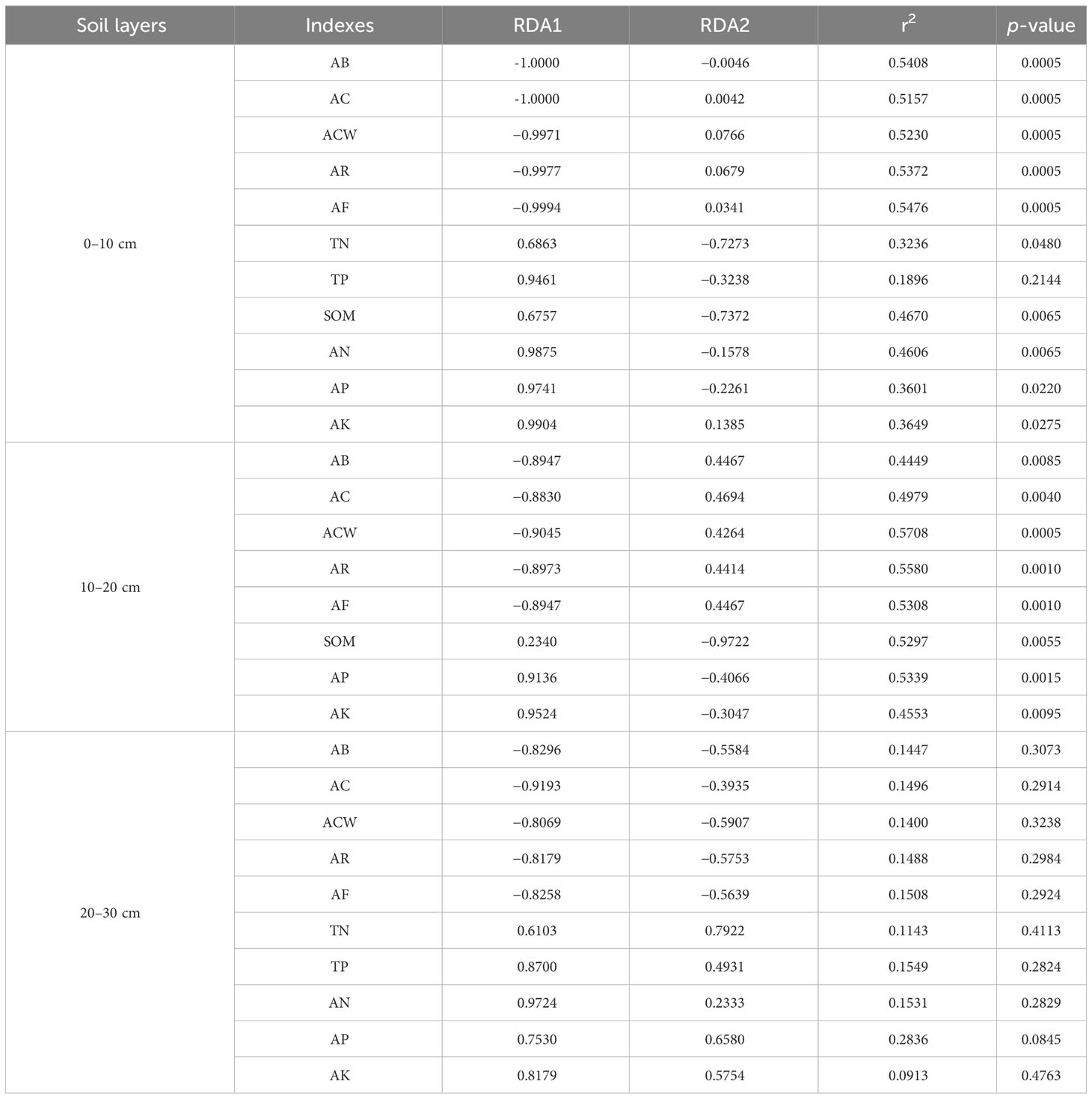
Table 5 Monte Carlo permutation test between soil fungi, vegetation and soil physicochemical properties.
The ecological restoration of poisonous weed-type degraded grasslands is not only reflected in the poisonous weeds themselves but also in other plants and environmental changes in grassland communities. This study focused on A. inebrians, edible forage and community diversity, and attempted to study the vegetation community’s changes in different pastures from multiple perspectives. The aboveground biomass and height of edible forage in the CSF were significantly better than in the FOP, while A. inebrians performed the opposite, and had the strongest inhibition of its coverage. Wang (1962) studied the “camping circle” of sheep in Tianzhu grassland in Gansu Province and found that the grass yield and the proportion of Gramineae with excellent quality were increased significantly compared with outside the circle, and poisonous and pest grasses decreased significantly, consistent with our results. The reasons were that resting from grazing during the pasture rejuvenation period protected the plant’s underground organs from being trampled by livestock, and resting from grazing during the pasture vigorous growth period prevented livestock feeding on plants with good palatability, and so these plants had stronger community competitiveness than plants with poor palatability (Yao, 2019). Thus, resting from grazing provided more abundant grass resources for grazing in the cold season, and was conducive to the vegetation renewal of plants with bud bank as their vegetative propagation mode of potential population. Zhang et al. (2018) showed that moderate grazing affected community structure and interspecific relationships, and that the competitiveness of general dominant species was inhibited by grazing, and the coexistence of dominant species and grazing-tolerant species improved grassland productivity and stability.
A. inebrians is the dominant grass species in degraded alpine meadows, the study of its response to different grazing systems plays an important role in the sustainable use and conservation of species diversity in A. inebrians-type degraded grassland (Zhang L. et al., 2019). Our studies showed that the A. inebrians developed adaptive strategies for different grazing systems, and the population characteristics, seed yield and composition factors had significant differences. The selective feeding of livestock can regulate the competition between liking and not liking forage, inhibit the forage-hating and enhance the forage-loving, and then affect the community structure and function and grassland health (Hou and Yang, 2006). Continuous grazing throughout the seasons, without giving the grassland a chance to recuperate, and frequent gnawing of livestock reduces the biomass of edible forage, affects its material energy accumulation and so affects its growth, development and reproduction, and this gave A. inebrians an advantage in competition with excellent plant species and so its cluster diameter increased, effective photosynthetic area was larger, and more energy accumulated, which could be used for the production of more constituent factors. Studies in the Qinghai-Tibet Plateau also showed that grazing intensity exceeded the threshold, and the adaptability of A. inebrians to grazing environment was stronger than that of Stipa purpurea (Zhang et al., 2022). However, moderate trampling of livestock can stimulate and enhance the tillering ability of Gramineae in CSF, the density and coverage are bound to increase, and the ecological conditions in the grassland also change, and the growth of A. inebrians is inhibited with the weakening of light, and it will even be expelled from the grass for a long time. In addition, there are few studies on field seed yield in A. inebrians, so it cannot be compared with other literature.
As an important part of community structure, grassland plant community diversity plays a vital role in maintaining grassland ecosystem stability and productivity (Song et al., 2018). In this study, the Pielou index was higher for CSF and the Simpson index higher for FOP, indicating that the grassland community composition included fewer weeds, excellent forage grew well and was evenly distributed for CSF, while more weeds invaded FOP. This is likely because Gramineae and Leguminosae with good palatability were eaten first in FOP, which provided a better microenvironment and growth opportunities for some small non-dominant plant species, especially weeds. In addition, frequent grazing provided greater opportunities for livestock hair and hooves to carry and transfer external plant propagules (e.g., seeds and reproductive organs). Studies have also shown that continuous grazing leads to soil compactness, nutrient loss, inhibits compensatory growth of edible pasture, increases the proportion of inferior pasture, and decreases the diversity of community species, while reasonable grazing weakens the growth of dominant species in the community, provides survival opportunities for inferior species, increases community diversity, and then increases system productivity, which is not completely consistent with the our results (Zhang et al., 2022). Therefore, ecosystem function is closely linked to its biodiversity, but biodiversity does not necessarily provide the grazing services provided by ecosystems (Msadek, 2021).
Grassland soil is an important basic factor in the grassland ecosystem, and grassland vegetation change is closely related to changes in grassland soil fertility and activity, which affect each other (Merbold et al., 2014). Our study showed that soil pH increased with deeper soil, because plant roots were mostly concentrated in the 0–20 cm soil layer, so microorganisms were enriched in rhizosphere soil, and the respiration of root and rhizosphere microorganisms produces CO2 that, combined with rhizosphere water, resulted in lower pH in the surface soil. The soil water content was enhanced and soil bulk density was reduced in CSF, because CSF was rested from grazing in the warm season; grass resources were abundant when grazing in October, grassland vegetation coverage was enhanced and livestock feeding time was shortened. When coupled with the low temperature in the cold season, bedding time was prolonged and walking time was shortened, which reduced the damage of livestock hooves to the turf and the soil water dispersion loss (Wang, 2017).
In terms of soil nutrients, we found that the content of soil total and available nutrients was low in FOP, because the amount of livestock gnawing and trampling rose with the increased grazing intensity, which decreases grassland primary productivity, existing stock of grassland plants and litter decomposition, thereby reducing soil nutrient content (Li, 2022). The non-growing season supplementary feeding and bedding scatter manure layer about 2 cm on the surface in CSF, and when the temperature increases in spring and the snow melts, it has favorable conditions, and the manure layer can decompose to produce a large amount of humus, which is conducive to the formation of soil aggregate structure and microbial activity, thereby improving soil physicochemical properties and fertility. As we all know, sheep’s urine is rich in nitrogen fertilizer, which creates favorable nutritional conditions for the growth and development of Gramineae with high feed value, this is consistent with the view that grazing livestock trampling, and return of manure and urine had positive effects on soil nutrient improvement (Wang et al., 2008). So the response of soil physicochemical factors to grazing is uncertain. In addition, Sun et al. (2016) found that grassland degradation presented synchronous degradation of aboveground communities and soil, consistent with our conclusions.
Soil enzyme activity is related to microbial metabolic processes and biochemical cycles of nutrients. In this study, the alkaline phosphatase, urease, and sucrase activities were high in CSF, consistent with the results of Qin et al. (2014) showing that increasing grassland degradation in the Qilian Mountains could gradually reduce soil enzyme activities. The reasons are that soil enzymes are mainly derived from soil microorganisms, plant root exudates and animal and plant residues, continuous grazing in four seasons intensifies grassland degradation, affects plant root aggregation and lowers the amount and activity of soil enzymes (Zhang et al., 2015). Especially entering the grass withering period, the bedding time was shortened, the feeding and walking time was prolonged to eat food-loving plants, and there was even damaged or exposured of the hypocotyl or plumule of seedlings in the cold season. However, urease and sucrose activities in CSF were significantly higher than in FOP, because the high aboveground biomass increased the plant roots in the 0–10 cm soil layer, coupled with less soil compaction and good permeability, and so enhanced soil nutrient utilization, microbial activity and reproductive capacity (Poeplau, 2016). The catalase activity is involved in conversion of soil matter and energy, its activity can characterize the strength of soil biological oxidation process and it showed no significant change in CSF and FOP.
Soil microbes play an important role in grassland ecosystems. Grazing affects changes in the composition of soil microbial communities that may have direct or lasting effects on ecosystems (Jing et al., 2022). The bacteria with the highest relative abundance in CSF and FOP is Proteobacteria at the phyla level, showing CSF higher than FOP. Since Proteobacteria is the most common phylum in the world, and includes important soil bacteria, with its metabolic activity the most important bacterial activity in soil (Iino et al., 2010), which plays an important role in soil improvement, can degrade waste in the soil, not only can increase the soil nitrogen content, but also reflect the soil quality, so CSF treatment can improve the soil quality of of poisonous weed-type degraded grassland (Lin, 2017). The dominant genera in CSF and FOP were Sphingomonas and 67_14 for bacteria, and Mortierella for fungi. However, their relative abundances varied in the different pastures, which was partly consistent with the results from the degraded grasslands of the Qinghai-Tibet Plateau (Wang et al., 2022). This indicated that although the effects of seasonal grazing on soil microbial community composition differed, dominant microbiota were the same. Basidiomycota and Ascomycota predominate in environments with high soil lignin content and are some of the main decomposers of lignified vegetation debris (Araujo et al., 2017), which may be one important reason for Basidiomycota and Ascomycota becoming the dominant fungal phyla. Finally, there were characteristic microorganisms for CSF and FOP, indicating that grazing in different seasons had different effects on soil microbial species.
Soil microbial diversity is a key indicator reflecting the soil’s ecological characteristics. We found that the bacterial α-diversity was higher than that of fungi in CSF and FOP, indicating bacterial dominance in the soil, which may be due to the trampling of livestock made the soil more compact, the aeration decreased, the number of gas-repellent bacteria increased slightly, and the bacteria’s ability to adapt to poor environments led to the occurrence of this phenomenon (Yin et al., 2020; Wang et al., 2022). The α-diversity of bacteria and fungi differed only in shallow soil, likely due to the gnawing of livestock allocating more assimilated carbon to roots (Hou and Yang, 2006), while plant roots were mostly concentrated in shallow soil, which stimulates growth and activity of rhizosphere heterotrophic microorganisms. Besides, FOP increased grazing disturbance, had a strong spatial heterogeneity in the sample communities, and the sample information was more dispersed, resulting in greater differences in the abundance and diversity of soil microorganisms within the group, while soil samples had lower heterogeneity in CSF. The Principal coordinate analysis of β-diversity showed significant differences in fungal communities between the two pastures, possibly related to plant species composition differences, species composition variation of grassland vegetation may cause changes of litter and root exudate components, and soil fungal community structure is indirectly affected available substrate (Yin et al., 2020). Studies have also shown that differences in plant diversity mainly affect bacterial rather than fungal β-diversity (Prober et al., 2014).
Different grazing management of poisonous weed-type degraded grasslands cause successional changes in grassland vegetation community structure in alpine meadows, which then lead to soil characteristics changes, resulting in changes soil microbial community structure and diversity and finally feeding back to degraded grassland vegetation communities (Li, 2022), making the relationships among them more complex.
In this study, there were significant differences in plant community composition, soil physical properties, nutrients and organic matter. These factors were the main drivers of changes in the diversity and composition of soil bacterial and fungal flora, and there were differences in their correlations. Variations in plant species affect soil microbial communities by altering the number and diversity of soil rhizosphere exudates, the increase of aboveground biomass of grassland vegetation significantly affects the amount of litter input to the soil, increases the nutrient content of the surface soil, and is of great significance to its dynamic change (Zhang C. et al., 2019; Wang et al., 2022). And soil nutrient content can affect the population and distribution of soil microorganisms, soil microbial habitat change is directly related to changes in soil physical properties (Li, 2022). In addition, soil bacteria and soil organic matter had a high correlation, Milchunas and Lauenroth (1993) also found that grazing affects soil microbiota abundance by changing the amount of organic matter returned to soil. Soil fungi had a high positive correlation with the A. inebrians population, which may be related to the fact that A. inebrians itself is the host and mutualist symbiont of the Epichloë endophyte. Therefore, the grazing system plays an important role in changing the vegetation community’s composition and soil characteristics, and causing changes in the soil microbial community’s structure and composition (Turatsinze et al., 2021).
The effects of environmental factors on soil microorganisms are complex, and these factors also have complex interactions. When these factors are combined with other factors, such as precipitation, temperature and human disturbance, the interactions among them are more variable. From the perspective of vegetation–soil–microorganism interrelationships, the influence of different grazing systems on poisonous weed-type degraded grassland was studied, and appropriate grazing management strategies were proposed, which will be a vital part of future alpine grassland degradation research.
In the cold-season grazing plus supplementary feeding pasture, the aboveground biomass, coverage and reproduction of Achnatherum inebrians (Hance) Keng ex Tzvele were significantly inhibited, and the coverage and aboveground biomass of edible forage were significantly increased. And long-term utilization plus supplementary feeding in cold season had positive effects on grassland communities Simpson and Pielou indexes, soil water content, total nitrogen, total phosphorus and available nutrients. Moderate grazing in the cold season affected community structure and interspecific relationships, and dominant species were inhibited, providing opportunities for the growth and development of inferior species. The non-growing season supplementary feeding and bedding help soil agglomerate structure formation and microbial activity, improve soil traits, and increase soil fertility. Soil microbial communities were also affected by grazing systems, with a higher abundance of bacterial dominant species but the lower abundance of fungal dominant species in the cold-season plus supplementary feeding pasture. And only soil fungal community diversity differed significantly between the two pastures. Different grazing systems have a greater impact on vegetation and soil characteristics than microorganisms, and the response of soil microorganisms to grazing systems may be time-lagging and inconsistent, and bacterial and fungal communities respond differently. Therefore, grazing and supplementary feeding in the cold season treatment can effectively inhibit the growth of A. inebrians, restore edible pasture, and improve soil nutrients, which is particularly important for the A. inebrians-type degraded grassland ecosystem.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: BioProject, PRJNA1013135, https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1013135.
YC: investigation, data curation, methodology, writing – original draft, writing – review & editing. CX: investigation. KM: investigation. QH: supervision, writing – review & editing. XY: supervision, funding acquisition, writing – review & editing.
This work was supported by the Grassland Ecological Restoration Management Science and Technology Support Project of Gansu Province (2021GSLY).
We thank the Key Laboratory of the Ministry of Education of the Grassland Ecosystem of Gansu Agricultural University for providing experimental equipment. We also would like to thank Yuanyuan Liu, Caiyan Yang and Na Xu for their help in sampling.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Araujo, A., Bezerra, W., Santos, V., Nune, L., Lyra, M., Figueiredo, M., et al. (2017). Fungal diversity in soils across a gradient of preserved Brazilian Cerrado. J. Microbiol. 55 (4), 273–279. doi: 10.1007/s12275-017-6350-6
Chai, J., Yu, X., Xu, C., Xiao, H., Zhang, J., Yang, H., et al. (2019). Effects of yak and Tibetan sheep trampling on soil properties in the northeastern Qinghai Tibetan Plateau. Appl. Soil Ecol. 144, 147–154. doi: 10.1016/j.apsoil.2019.0.017
Chen, Y., Su, K., Li, C., White, J. (2021). Interactive effects of Epichloë endophyte, dormancy-breaking treatments and geographic origin on seed germination of Achnatherum inebrians. Microorg 9 (11), 2183. doi: 10.3390/microorganisms9112183
Fan, Y., Fang, C. (2022). Measuring Qinghai-Tibet plateau’s sustainability. Sustain. Cities Soc 85, 104058. doi: 10.1016/j.scs.2022.104058
Guo, Y., Zhang, R., Sun, D., Zhao, S., You, Y., Lu, H., et al. (2017). Hazards, prevention, control and comprehensive utilization of poisonous weeds in natural grassland in Gansu Province. Acta Agrestia Sin. 25 (02), 243–256.
Hou, F., Yang, Z. (2006). Effects of grazing of livestock on grassland. Acta Ecol. Sin. 01, 244–264. doi: 10.3321/j.issn:1000-0933.2006.01.031
Iino, T., Mori, K., Uchino, Y., Nakagawa, T., Harayama, S., Suzuki, K. (2010). Ignavibacterium album gen. nov., sp. nov., a moderately thermophilic anaerobic bacterium isolated from microbial mats at a terrestrial hot spring and proposal of Ignavibacteria classis nov., for a novel lineage at the periphery of green sulfur bacteria. Int. J. Syst. Evol. Microbiol. 60, 1376–1382. doi: 10.1099/ijs.0.012484-0
Jin, G., Zhu, X., Wei, X., Sun, Z., Tang, L., Zou, L. (2017). Comparison of the prevention and control effects of different methods on Achnatherum inebrians. Acta Agrestia Sin. 25 (03), 625–632.
Jing, Y., Bai, M., Xu, C., Wang, L., Yang, H., Jiang, J., et al. (2022). Advancing the spring rest-grazing time until the critical period when soil thaws promotes soil recovery and bacterial diversity in alpine meadows. Ecol. Indic. 139, 108929. doi: 10.1016/j.ecolind.2022.108929
Jing, Y., Yu, X., Xu, C., Jin, Y., Chen, L., Zhang, J., et al. (2014). Effects of mowing frequency on Achnatherum inebrians in the alpine meadow of Tianzhu. Grassl. Turf. 34 (04), 47–51. doi: 10.3969/j.issn.1009-5500.2014.04.009
Li, C. (2005). Biological and ecological characteristics of Achnatherum inebrians/Neotyphodium endophyte symbiont (Lanzhou: Lanzhou University).
Li, H. (2022). Effects of grazing on plant and soil microbial communities in mountain meadows on the northern slopes of Tianshan Mountain (Xinjiang: Xinjiang Agricultural University).
Li, X., Ren, J., Feng, K., Lei, T., A, Y., Zhang, X., et al. (1996). Study on ecological control of Achnatherum inebrians. Acta Pratac. Sin. 6 (2), 14–17.
Liang, J., Gao, G., Zhong, R., Liu, B., Christensen, M., Ju, Y., et al. (2023). Effect of Epichloë gansuensis endophyte on seed-borne microbes and seed metabolites in Achnatherum inebrians. Microbiol. Spectrum 11 (2), e01350–e01322. doi: 10.1128/spectrum.01350-22
Lin, Y. (2017). The Effect of Soil Microbial Community Structure on Natural Grassland Land-use Types in Bashang (Hebei: Hebei University).
Lindsey, A. (1956). Sampling methods and community attributes in forest ecology. For. Sci. 2 (4), 287–296. doi: 10.1093/forestscience/2.4.287
Liu, J., Chen, Z., White, J., Chen, T., Shi, Q., Jin, Y., et al. (2022). Ergot alkaloid and endogenous hormones quantities and relationship in Epichloë endophyte: Drunken Horse grass is affected by altitude. J. Plant Growth Regul. 42, 1979–1990. doi: 10.1007/s00344-022-10675-5
Liu, Y., Hou, W., Jin, J., Christensen, M., Gu, L., Cheng, C., et al. (2021). Epichloë gansuensis increases the tolerance of Achnatherum inebrians to low-P stress by modulating amino acids metabolism and phosphorus utilization efficiency. J. Fungi 7 (5), 390. doi: 10.3390/jof7050390
Ma, K. (1994). Method for measuring the diversity of biological communities (Beijing: Beijing Science and Technology Press).
Ma, K., Xu, C., Yu, X., Liu, Y., Yang, H., Wei, K., et al. (2022). Rest grazing start from the critical period of soil thawing and optimizes plant community characteristics and grassland grazing capacity in alpine meadows. Ecol. Eng. 183, 106763. doi: 10.1016/j.ecoleng.2022.106763
Mcdaniel, K., Sterling, T. (2007). Herbicidal control of locoweed (Wallingford:Poisonous plants: global research and solutions), 353–358.
Merbold, L., Eugster, W., Stieger, J., Zahniser, M., Nelson, D., Buchmann, N. (2014). Greenhouse gas budget (CO2, CH4 and N2O) of intensively managed grassland following restoration. Global Change Biol. 20 (6), 1913–1928. doi: 10.1111/gcb.12518
Milchunas, D., Lauenroth, W. (1993). Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol. Monogr. 63 (4), 327–366. doi: 10.2307/2937150
Miles, C., Lane, G., Menna, M., Garthwaite, I., Harris, P. (1996). High Levels of ergonovine and lysergic acid amide in toxic Achnatherum inebrians accompany infection by an Acremonium-like endophytic fungus. J. Agric. Food Chem. 44, 1285–1290. doi: 10.1021/jf950410k
Moon, C., Guillaumin, J., Ravel, C., Li, C., Schardl, C. (2007). New Neotyphodium endophyte species from the grass tribes Stipeae and Meliceae. Mycologia 99 (06), 895–905. doi: 10.1080/15572536.2007.11832521
Msadek, M. (2021). Community diversity, functional traits and adaptation of stipa tenacissima l. under different grazing regimes in north African arid montane rangeland. Afr. J. Range Forage Sci. 38 (1), 122–129. doi: 10.2989/10220119.2020.1845796
Niu, Y., Zhu, Yang, S., Ma, S., Zhou, J., Chu, B., et al. (2019). Overgrazing leads to soil cracking that later triggers the severe degradation of alpine meadows on the Tibetan Plateau. Land. Degrad. Dev. 30 (10), 1243–1257. doi: 10.1002/ldr.3312
Poeplau, C. (2016). Estimating root: shoot ratio and soil carbon inputs in temperate grasslands with the RothC model. Plant Soil. 407 (1-2), 293–305. doi: 10.1007/s11104-016-3017-8
Prober, S., Leff, J., Bates, S., Borer, E., Firn, J., Harpole, W., et al. (2014). Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 18, 85–95. doi: 10.1111/ele.12381
Qin, J., Zhang, Y., Zhao, Y., Wang, Z., Li, C., Gao, H. (2014). Soil physicochemical properties and variations of nutrients and enzyme activity in the degrading grasslands in the upper reaches of the Heihe River, Qilian Mountains. J. Glaciol. Geocryol. 36 (02), 335–346. doi: 10.7522/j.issn.1000-0240.2014.0041
Song, M., Liu, L., Chen, J., Zhang, X. (2018). Biology, multi-function and optimized management in grassland ecosystem. Ecol. Environ. Sci. 27 (6), 1179–1188. doi: 10.16258/j.cnki.1674-5906.2018.06.025
Sun, Z., An, S., Li, P., Jin, M., Wei, H., Ma, J. (2014). Primarily studies on seed germination characteristics of Achnatherum inebriants (Hance) Keng. Seed 33 (01), 10–13+18. doi: 10.16590/j.cnki.1001-4705.2014.01.044
Sun, J., Liu, M., Fu, B., David, K., Zhao, W., Liu, G., et al. (2020). Reconsidering the efficiency of grazing exclusion using fences on the Tibetan Plateau. Sci. Bull. 65 (16), 1405–1414. doi: 10.1016/j.scib.2020.04.035
Sun, L., Liu, Y., Wu, G., Wei, X. (2016). The relationships between community biomass and soil nutrients in the northern Tibet degradation grassland. Pratac. Sci. 33 (6), 1062–1069. doi: 10.11829/j.issn.1001-0629.2015-0544
Turatsinze, A., Kang, B., Zhu, T., Hou, F., Bowatte, S. (2021). Soil bacterial and fungal composition and diversity responses to seasonal deer grazing in a Subalpine Meadow. Diversity 13 (2), 84. doi: 10.3390/D13020084
Wang, Q. (1962). A preliminary study on the effect of “camping circles” of Tianzhu pasture flocks on pasture productivity. J. Gansu Norm. Univ. Nat. Sci. 3, 1–7. doi: 10.16783/j.cnki.nwnuz.1962.03.001
Wang, X. (2017). The influence of different grazing management systems on livestock behavior (Inner Mongolia: Inner Mongolia University).
Wang, C., Long, R., Wang, Q., Cao, G., Shi, J., Du, Y. (2008). Response of plant diversity and productivity to soil resources changing under grazing disturbance on an alpine meadow. Acta Ecol. Sin. 9, 4144–4152. doi: 10.3321/j.issn:1000-0933.2008.09.011
Wang, L., Yu, X., Xu, C., Jing, Y., Song, M. (2022). Grazing by Tibetan sheep enhances soil bacterial and fungal diversity in cold season pastures of Alpine Meadows on the Northern Qinghai-Tibetan Plateau. J. Soil Sci. Plant Nutr. 22, 2434–2456. doi: 10.1007/s42729-022-00819-7
Wei, B. (2013). Effects of grassland management measures on poisonous plants in alpine meadow (Lanzhou: Lanzhou University).
Yang, H., Song, Y., Sun, Z., Jin, G., An, S., Shi, Z., et al. (2015). Effects of different reseeding patterns on population characteristics of Achnatherum inebrians and diversity of grassland community. Guizhou Agric. Sci. 43 (10), 67–71. doi: 10.3969/j.issn.1001-3601.2015.10.017
Yao, X. (2019). Studies on Response Mechanism of Plant Community and Livestock to Grazing Pressure in an Alpine Meadow (Gansu: Gansu Agricultural University).
Yin, Y., Li, S., Ma, Y. (2020). Effects of replanting on soil fungal community characteristics in degraded alpine meadow. Acta Pratac. Sin. 28 (6), 1791–1797. doi: 10.11733/j.issn.1007-0435.2020.06.036
Zhang, X., Li, C., Nan, Z. (2012). Effects of cadmium stress on seed germination and seedling growth of Elymus dahuricus infected with the Neotyphodium endophyte. China Life Sci. 55 (09), 793–799. doi: 10.1007/s11427-012-4359-y
Zhang, X., Chang, S., Jia, Q., Li, D., An, Y., Hu, A. (2022). Response of interspecific competition between Stipa purpurea and Achnatherum inebrians to different grazing intensities in an alpine steppe. Pratac. Sci. 39 (6), 1225–1234. doi: 10.11829/j.issn.1001-0629.2021-0462
Zhang, Y., Chen, L., Chen, X., Tan, M., Duan, Z., Wu, Z., et al. (2015). Response of soil enzyme activity to long-term restoration of desertified land. Catena 133, 64–70. doi: 10.1016/j.catena.2015.04.012
Zhang, C., Dong, Q., Chu, H., Shi, J., Li, S., Wang, Y., et al. (2018). Grassland community composition response to grazing intensity under different grazing regimes. Rangel. Ecol. Manag. 71 (2), 196–204. doi: 10.1016/j.rama.2017.09.007
Zhang, L., Pang, R., Xu, X., Song, M., Ouyang, H. (2019). Three Tibetan grassland plant species tend to partition niches with limited plasticity in nitrogen use. Plant Soil 441 (1), 601–611. doi: 10.1007/s11104-019-04148-0
Zhang, C., Wang, J., Liu, G., Song, Z., Fang, L. (2019). Impact of soil leachate on microbial biomass and diversity affected by plant diversity. Plant Soil. 439 (1), 505–523. doi: 10.1007/s11104-019-04032-x
Keywords: grazing system, Achnatherum inebrians, plant communities, soil microbial communities, alpine meadows
Citation: Chen Y, Xu C, Ma K, Hou Q and Yu X (2023) Responses of community traits and soil characteristics of Achnatherum inebrians-type degraded grassland to grazing systems in alpine meadows on the Qinghai-Tibet Plateau. Front. Plant Sci. 14:1270304. doi: 10.3389/fpls.2023.1270304
Received: 31 July 2023; Accepted: 14 September 2023;
Published: 06 October 2023.
Edited by:
Sergio Rossi, Université du Québec à Chicoutimi, CanadaReviewed by:
Yan Ruirui, Chinese Academy of Agricultural Sciences (CAAS), ChinaCopyright © 2023 Chen, Xu, Ma, Hou and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojun Yu, eXV4akBnc2F1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.