
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Plant Sci., 05 September 2023
Sec. Plant Systems and Synthetic Biology
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1260454
This article is part of the Research TopicMetabolic Engineering of Valuable Compounds in Photosynthetic OrganismsView all 11 articles
 Zhi-Yan Du1*
Zhi-Yan Du1* Wajid Waheed Bhat2
Wajid Waheed Bhat2 Guoyin Kai3
Guoyin Kai3 Inna Khozin-Goldberg4
Inna Khozin-Goldberg4 Xiao-Hong Yu5
Xiao-Hong Yu5 Agnieszka Zienkiewicz6
Agnieszka Zienkiewicz6 Krzysztof Zienkiewicz6
Krzysztof Zienkiewicz6Editorial on the Research Topic
Metabolic engineering of valuable compounds in photosynthetic organisms
Photosynthetic organisms, including plants and algae, possess a remarkable ability to harness carbon dioxide and solar energy, enabling them to produce a vast array of complex compounds such as phenolic acids (Zhou et al., 2021), terpenes (Miller et al., 2020), unsaturated fatty acids (Kokabi et al., 2020; Gan et al., 2022), and other lipid products (Zienkiewicz and Zienkiewicz, 2020). This inherent capability positions them as highly promising platforms for the sustainable production of valuable biomolecules. While the industrial application of photosynthetic organisms in synthetic biology is not as advanced as that of model heterotrophs or mammalian systems, their significance as primary contributors to global biomass can be further developed. In fact, they are increasingly emerging as key players in the booming field of synthetic bioproducts, driven by advancements in genome editing tools and other innovative technologies. As we explore and exploit the potential of photosynthetic organisms, we open up exciting possibilities for the production of environmentally friendly and renewable biomaterials that can address pressing societal and ecological challenges.
This Research Topic includes eight original research and two review articles, with a special focus on the metabolic engineering of valuable biomaterials in plants and algae. Taparia et al., developed modular CRISPR/Cas9 constructs for the model diatom Phaeodactylum tricornutum that allow the multiplexed targeting and creation of marker-free genome-edited lines. The system was used to knock out StLDP, the gene encoding Stramenopile-type lipid droplet protein essential for lipid droplet biogenesis (Figure 1). Mellor et al. expressed human P450s in tobacco chloroplasts to produce indican, suggesting a strategy for producing high-value chemicals or drug metabolites in photosynthetic organisms (Figure 1). Another research ariticle investigated the biosynthesis of isoprenoids in poplar, and revealed that the 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) and 1-deoxy-D-xylulose5-phosphate reductoisomerase (DXR) play important roles in regulating the genes in methylerythritol phosphate (MEP) and mevalonic acid (MVA) pathways and isoprenoids made from the MEP and MVA pathways (Figure 1) (Movahedi et al.). Li et al. produced carotenoids in rice (Oryza sativa) endosperm by overexpressing rice GOLDEN2-LIKE (OsGLK) transcription factor and OsGLK with three other carotenogenic genes, tHMG1 (truncated Saccharomyces cerevisiae 3-hydroxy-3-methylglutaryl-CoA reductase), ZmPSY1 (Zea mays L. phytoene synthase), and PaCrtI (Pantoea ananatis phytoene desaturase), to improve the nutritional composition of rice (Figure 1). Another research article in rice developed models by multilevel mathematical modeling using the data from rice lines with genome modification in MVA pathways, providing tools that can help prioritize metabolic engineering strategies for specific metabolic goals through exogenous pathways (Figure 1) (Basallo et al.). In perennial herbs, Wang et al. identified physiological/biochemical indicators, such as enzyme activities of glutamine synthetase (GS), glutamate synthase (GLS), glutamate dehydrogenase (GDH), peroxidase (POD), and catalase (CAT), were related to biomass accumulation in Salvia miltiorrhiza (Figure 1); Su et al. characterized the β-glucosidase in Platycodon grandifloras, which can convert glycosylated platycoside E to Platycodin D in vitro (Figure 1); Jin et al. identified an MYB transcription factor OvMYBPA2 in Onobrychis viciifolia by transcriptome analyses and confirmed its function in the regulation of proanthocyanidins in transgenic Medicago sativa (Figure 1). Strand and Walker reviewed bioengineering from an energetics perspective using photosynthetic organisms for bioproducts of interest (Figure 1). Another review article discussed the recent progress in engineering fatty acids and storage lipids in various plant species and tissues and summarized an inventory of specific lipogenic factors for plant lipid products (Figure 1) (Cai et al.).
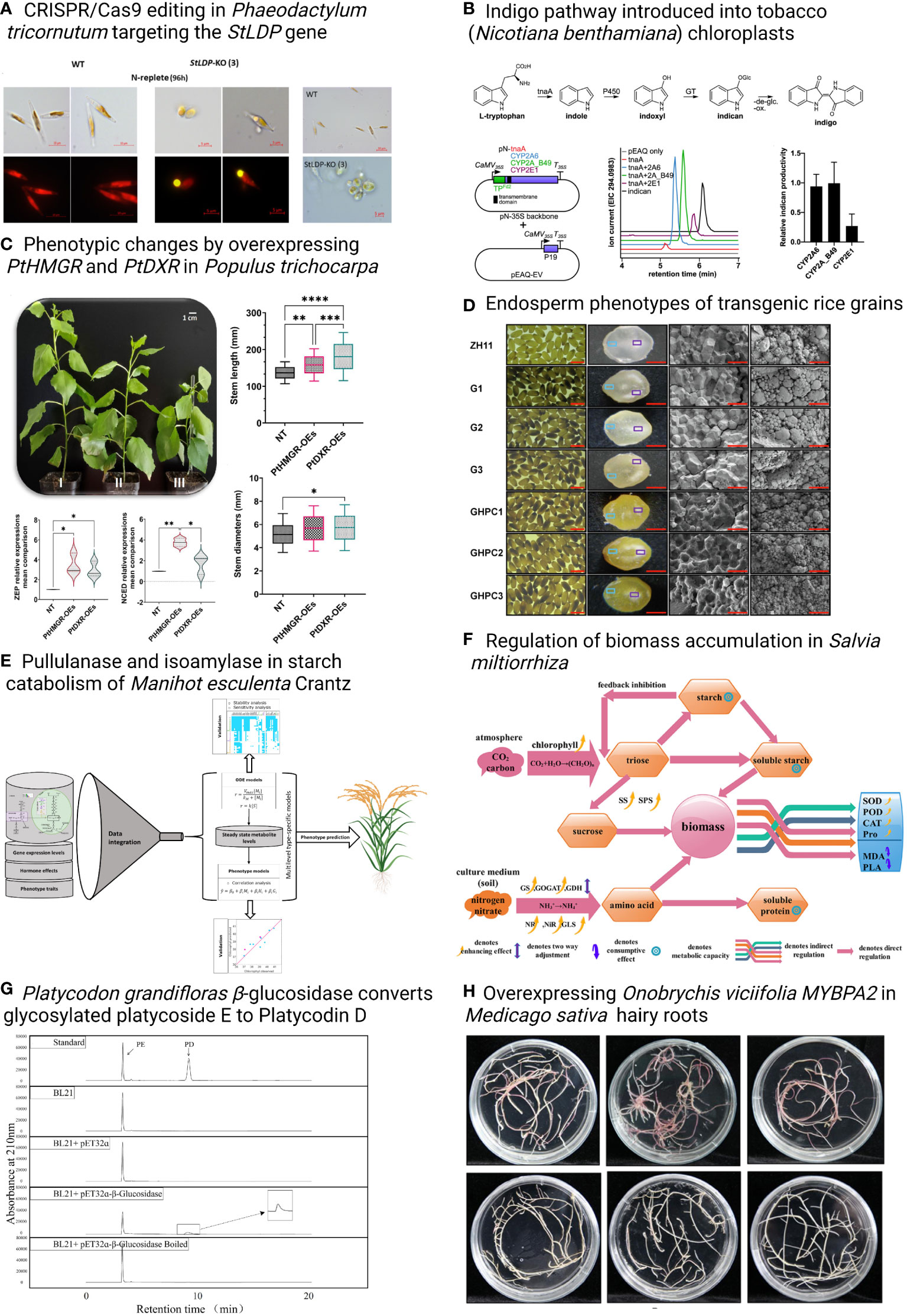
Figure 1 Overview of the original research articles in this Synthetic Biology Research Topic. Asterisks indicate statistically significant differences, *p <0.05, **p <0.01, ***p <0.001, ****p <0.0001
Z-YD: Funding acquisition, Resources, Visualization, Writing – original draft, Writing – review & editing. WB: Writing – review & editing. GK: Writing – review & editing. IK-G: Writing – review & editing. X-HY: Writing – review & editing. AZ: Writing – review & editing. KZ: Writing – review & editing.
Research of the Topic Editors is supported by the funds from NSF 2121410 (-YD), the DOE Office of Science, Office of BER, DE-SC0021369 (X-HY).
The editors would like to thank all reviewers who evaluated manuscripts and contributors to this Research Topic.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Gan, L., Park, K., Chai, J., Updike, E. M., Kim, H., Voshall, A., et al. (2022). Divergent evolution of extreme production of variant plant monounsaturated fatty acids. Proc. Natl. Acad. Sci. U.S.A. 119, e2201160119. doi: 10.1073/pnas.2201160119
Kokabi, K., Gorelova, O., Zorin, B., Didi-Cohen, S., Itkin, M., Malitsky, S., et al. (2020). Lipidome remodeling and autophagic respose in the arachidonic-acid-rich microalga lobosphaera incisa under nitrogen and phosphorous deprivation. Front. Plant Sci. 11, 614846. doi: 10.3389/fpls.2020.614846
Miller, G. P., Bhat, W. W., Lanier, E. R., Johnson, S. R., Mathieu, D. T., Hamberger, B. (2020). The biosynthesis of the anti-microbial diterpenoid leubethanol in Leucophyllum frutescens proceeds via an all- cis prenyl intermediate. Plant J. 104, 693–705. doi: 10.1111/tpj.14957
Zhou, W., Li, S., Maoz, I., Wang, Q., Xu, M., Feng, Y., et al. (2021). SmJRB1 positively regulates the accumulation of phenolic acid in Salvia miltiorrhiza. Ind. Crops Prod 164, 113417. doi: 10.1016/j.indcrop.2021.113417
Keywords: metabolic engineering, synthetic biology, plants and algae, natural products, secondary metabolites, genetic modification, breeding
Citation: Du Z-Y, Bhat WW, Kai G, Khozin-Goldberg I, Yu X-H, Zienkiewicz A and Zienkiewicz K (2023) Editorial: Metabolic engineering of valuable compounds in photosynthetic organisms. Front. Plant Sci. 14:1260454. doi: 10.3389/fpls.2023.1260454
Received: 17 July 2023; Accepted: 22 August 2023;
Published: 05 September 2023.
Edited and Reviewed by:
Mark Blyth, University of East Anglia, United KingdomCopyright © 2023 Du, Bhat, Kai, Khozin-Goldberg, Yu, Zienkiewicz and Zienkiewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Yan Du, ZHV6QGhhd2FpaS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.