
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci. , 08 September 2023
Sec. Crop and Product Physiology
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1253178
This article is part of the Research Topic Evaluation of Preharvest and Postharvest Factors on Forage Crop Quality, Physiology, and Ensiling Characteristics View all 11 articles
 Zhumei Du1,2
Zhumei Du1,2 Fuyu Yang3
Fuyu Yang3 Jiachen Fang4
Jiachen Fang4 Seishi Yamasaki2
Seishi Yamasaki2 Tetsuji Oya2
Tetsuji Oya2 Damiao Nguluve5
Damiao Nguluve5 Hajime Kumagai6
Hajime Kumagai6 Yimin Cai2*
Yimin Cai2*As the global population increases and the economy grows rapidly, the demand for livestock products such as meat, egg and milk continue to increase. The shortage of feed in livestock production is a worldwide problem restricting the development of the animal industry. Natural woody plants are widely distributed and have a huge biomass yield. The fresh leaves and branches of some woody plants are rich in nutrients such as proteins, amino acids, vitamins and minerals and can be used to produce storage feed such as silage for livestock. Therefore, the development and utilization of natural woody plants for clean fermented feed is important for the sustainable production of livestock product. This paper presents a comprehensive review of the research progress, current status and development prospects of forageable natural woody plant feed resources. The nutritional composition and uses of natural woody plants, the main factors affecting the fermentation of woody plant silage and the interaction mechanism between microbial co-occurrence network and secondary metabolite are reviewed. Various preparation technologies for clean fermentation of woody plant silage were summarized comprehensively, which provided a sustainable production mode for improving the production efficiency of livestock and producing high-quality livestock product. Therefore, woody plants play an increasingly important role as a potential natural feed resource in alleviating feed shortage and promoting sustainable development of livestock product.
● A review of recent advances in the renewable use of natural woody plant.
● Natural woody plant produces clean feed to alleviate feed shortage.
● Fermented feed of woody plant produces livestock product with added value.
● Woody plant contributes to the sustainable production of livestock product.
With the rapid development of the global economy, per capita consumption levels and demand for livestock products such as meat, eggs and milk are increasing (Komarek et al., 2021). Along with the development of livestock farming, the demand for quality forage is also increasing (Keeling et al., 2019). Currently, the main factor affecting livestock production is the insufficient supply of livestock forage, which in many countries is derived from feed crops, grasses, crop by-products and cereals (Cai et al., 2020a; Cai et al., 2021). With increasing global population and decreasing per capita arable land, traditional forage production methods cannot meet the demand for livestock feeding, leading to feed shortages and affecting the sustainable production of livestock products worldwide (Du et al., 2021b). Therefore, there is an urgent need to develop new feed resources, such as nutrient-rich natural woody plant resources, to meet the challenges posed by the rapid development of the livestock industry (Owen-Smith and Cooper, 1987; Vandermeulen et al., 2017).
The major woody plant available for feed worldwide are mulberry [Morus alba (L.)], moringa [Moringa oleifera (L.)], gliricidia [Gliricidia sepium (Jacq.)], leucaena [Kunth ex Walp. leucocephala (L.) de Wit], and paper mulberry [Broussonetia papyrifera (L.)]. These woody plants are deciduous trees or shrubs that are highly adaptable, widely distributed, drought tolerant and thrive in infertile soil, and can grow in a wide range of soil pH conditions, with high growth rates, high biomass yields, and low planting production costs (Anwar et al., 2007; He et al., 2019; Du et al., 2021a; Du et al., 2022c). In addition, the fresh branches and leaves of woody plants have a high crude protein (CP) content and are rich in various nutrients, such as bioactive components, amino acids, vitamins and macro minerals (Phesatcha and Wanapat, 2016; Oliveira et al., 2018; Wen et al., 2018; He et al., 2020; Du et al., 2021b).
Fresh branches and leaves of woody plants usually have a high moisture content, and the use of hay production methods not only increases the lignification of woody plant branches and leaves but also tends to cause leaf abscission during the drying process, resulting in a significant loss of nutrients (Du et al., 2021a). This suggests that hay processing is not suitable for the preparation of woody plant feeds. Silage, which is a fermented feed prepared from fresh forage for long-term storage, is considered a key technology for clean feed production of woody plant (Du et al., 2022a). In order to effectively utilize natural biomass resources, such as woody plant resources, to solve the problem of feed shortage and to improve the production capacity of livestock, this paper provides a comprehensive overview of the chemical composition and uses of feedable natural woody plants, the main factors affecting silage fermentation, the interactions between microbial co-occurrence networks and secondary metabolites, the regulatory mechanisms of silage fermentation, and the production of high-quality livestock products, with a view to providing important research information and technological support for the realization of the sustainable development of the animal husbandry.
Forageable woody plants are characterized by their diversity and versatility, high biomass and rich nutrient content, making them suitable for feeding ruminants (Figure 1). Mulberry belongs to the family Moraceae and is native to north-central China. It is widely cultivated in China, Korea, Japan, Mongolia, India, Vietnam, Russia and other central Asian countries, as well as some European countries (Madhav and Carolyn, 2012). Moringa belongs to the family Moraceae and is found in the tropics of Africa and Asia, and is cultivated in Guizhou, Guangdong and Taiwan in China (Çelekli et al., 2019; Pagano et al., 2020). Gliricidia belongs to the family Leguminosae and is native to the tropical dry forests of Mexico and central America. In addition to its native range, it grows in many tropical and subtropical regions, including the Caribbean, northern parts of south America, central Africa, parts of India and southeast Asia, northern and central America, and central Africa (De Carvalho et al., 2017; Oliveira et al., 2018). Leucaena also belongs to the family Leguminosae, is native to southern Mexico and northern central America (Belize and Guatemala), and is cultivated in tropical regions (Rengsirikul et al., 2011). The paper mulberry belongs to the family Moraceae and is native to southwest China, but is now widely distributed throughout China, other Asian countries, mainland Europe and the Pacific islands (Peñailillo et al., 2016).
Woody plants have multiple uses (Figure 2), mainly as edible and medicinal products, but also as feed, compost, bioenergy and fiber products (Jitjaicham and Kusuktham, 2016; Wen et al., 2018; Hao et al., 2020; Ajayo et al., 2022). Because the fresh branches and leaves of woody plants are rich in nutrients and functional components, they can be used as a potential feed resource with additional value (Du et al., 2021b). In addition, woody plants provide economic benefits to farmers by reducing feed costs and increasing the productivity of livestock (Franzel et al., 2014). As shown in Table 1, all woody plants can be used as medicinal plants and as a source of feed for cattle, sheep, pigs and poultry. Some of them are used as raw materials for food, rabbit feed, bioenergy, biogas and green manure. In addition, paper mulberry and mulberry are used as a raw material for paper and fibre products, and mulberry leaves are an important feed for silkworm.
Some woody plants are rich in biofunctional components, such as polyphenols, carotenoids, alkaloids, terpenoids and sulphur-containing compounds, which have potent effects, including enhanced free radical scavenging and powerful reducing abilities. They also have anti-oxidant, anti-cancer, anti-inflammatory, hepatoprotective, hypotensive, anti-diabetic and hypolipidemic properties, and constitute therefore potential drugs for treating various diseases in humans and animals (Gopalakrishnan et al., 2016; Abd Rani et al., 2018). Gliricidia contains small amounts of coumarin, which can be used as a spice, but is generally not suitable for consumption (Lim, 2014). The leaves of woody plants, such as paper mulberry, mulberry, gliricidia and moringa, are rich in amino acids, fatty acids, vitamin E and beta-carotene, and their calcium and magnesium concentrations are higher than those of many vegetables. Therefore, they are used in many developing countries as leafy greens that provide plant-based protein and play a role in reducing hunger and combating malnutrition (Pakade et al., 2013). The leaves and seeds of woody plants can be eaten raw, cooked or added to food in the form of a dried powder. They are an ingredient in hot pots, teas, edible spices, beverages and yoghurt, and are therefore a popular health food in some Asian countries (Leone et al., 2015). The fruits of some woody plants contain large amounts of soluble sugars (Han et al., 2016), which are usually shed at maturity and decay on the ground, resulting in the loss of these resources. Using the fruits of these woody plants as a feedstock, biotechnology has been successfully developed to produce ethanol from their free sugars (Ajayo et al., 2022). The tree trunks of the paper mulberry and mulberry are rich in various chemical constituents, including cellulose, hemicellulose, lignin, waxes and gums, which are widely used in the production of paper and fibre products (Jitjaicham and Kusuktham, 2016). In addition, the leaves of mulberry, gliricidia and leucaena can be used as raw materials for biogas production and green leaf fertilizer (Tambone et al., 2020).
Woody plants are 90−99% organic matter (OM) and consist of 17−27% CP, 3−5% ether extract (EE), 11−21% true protein (TP) and 53−71% total digestible nutrient (TDN), with these ranges being generally higher than in forage crops and grasses (Table 2). The energy content and macro mineral (e.g. calcium, phosphorus, magnesium and potassium) concentrations in woody plant are also higher than in forage. Because woody plants are often utilized as fresh branches and leaves, their fibre and lignin contents are slightly different to those of forage. Woody plants contain the forage components required by livestock and are a high protein feed source. As a result, woody plants are referred to as “woody alfalfa” and their nutritional value is comparable to that of alfalfa (Zhang et al., 2019).
Generally, forage crops and grasses grow well in the summer or tropical rainy season, but in the winter or dry season, cold and dry climatic conditions prevent forage crops from growing, resulting in the demand for feed from livestock far exceeding the production of feed (FAO, 2009). Therefore, woody plants have great potential for development as a feed resource for ruminant livestock, to make up for the shortage of feed. Woody plants are also harvested in large quantities in the summer and early autumn of temperate climates or in the tropical rainy season, and proper preparation and storage techniques after harvesting can effectively increase the self-sufficiency of local feed and the efficiency of livestock production (Su and Chen, 2020). Silage is a common traditional technique for the preparation and storage of forage crops and grasses and is an important means by which woody plants can be used effectively for feed production (Du et al., 2021b). The application of silage fermentation technology for the preparation of woody plant silage can avoid the loss of nutrients and enable long-term storage, thus solving the problem of livestock production during winter or drought seasons when feed is in short supply (Tao et al., 2020).
Silage is a stored feed made from fresh forage crops, grass and crop by-products, and is prepared by microbial fermentation under anaerobic conditions (Muck et al., 2018). Silage is widely used in many countries to make up for the lack of animal feed during the winter or in dry seasons. The fermentation of silage is influenced by various conditions, of which moisture, water-soluble carbohydrate (WSC), buffer energy and the epiphytic microbial community of the material are important factors affecting the fermentation process (McDonald et al., 1991). The appropriate moisture content for silage fermentation is 60−70%. Within this range lactic acid fermentation is promoted and the proliferation of spoilage microorganisms is inhibited. If the silage moisture content is too high, it tends to lead to butyric acid fermentation, dominated by clostridia, which decomposes proteins to produce ammonia nitrogen (NH3-N), thus reducing the fermentation quality of the silage. If the silage moisture content is too low, lactic acid bacteria (LAB) will be affected by the water activity and their growth will be inhibited, preventing them from producing large amounts of lactic acid to lower the pH of the silage. In addition, low moisture levels result in residual air not being effectively removed from the raw material, thus providing better conditions for harmful microorganisms such as aerobic bacteria and mold to survive, leading to poor quality fermentation (Du et al., 2022a). As shown in Table 3, the moisture content of fresh branches and leaves of woody plants can be as high as 70−80%, which is outside the ideal moisture range for silage fermentation. It is therefore necessary to make moisture adjustments when preparing woody plant silage. To make high quality silage, the material also needs to be above 6% WSC in dry matter (DM) and 105 LAB colony-forming unit per gram (cfu/g) in fresh matter (FM). As shown in Table 3, both the WSC content and the LAB count of woody plants were below the theoretical threshold. Moreover, microorganisms harmful to silage fermentation, such as aerobic and coliform bacteria, were present at higher levels than the LAB count. These harmful microorganisms can compete with LAB for nutrients during ensiling and will affect the fermentation quality of silage. The lactic acid buffer capacity (LBC) is also an important factor affecting silage fermentation. Du et al. (2022c) reported that the LBC of woody plants is similar to that of leguminous grasses, generally above 700 mEq/kg of DM. The LBC strength of the silage material is closely related to the mineral composition, as shown in Table 2 above. Woody plants are usually rich in mineral components such as K+, Ca2+ and Mg2+, and these cations neutralize the lactic acid and other organic acids produced by silage fermentation and inhibit the reduction of pH. This allows harmful microorganisms to grow and decompose proteins and produce NH3-N, resulting in low-quality silage fermentation (Cai et al., 2021). The chemical composition of woody plants in terms of moisture, protein, fiber and minerals varies at different growth stages and can directly affect the fermentation quality of silage (Du et al., 2021b; Du et al., 2022c). In addition, the growth stage and mixing ration of woody plant branches and leaves not only have an important influence on the fermentation quality of silage, but also on the digestibility and productivity of livestock (Anadón et al., 2014).
Woody plants in cultivation or in the native state are usually harvested by harvesters or by hand and then used for silage preparation. In recent years, woody plants have been grown in greenhouses by tissue culture and mechanically harvested after cultivation at a growth height of about 1−1.5 m. They can be harvested 4−5 times a year in tropical or subtropical regions. The fresh branches and leaves of harvested woody plants can have a moisture content of up to 80%. When they are prepared and stored as hay the leaves will fall off, causing the nutrients to be lost during the drying and storage process. Furthermore, the drying process increases the lignin content and reduces the nutritional value and palatability of woody plants to livestock (Du et al., 2021b). Silage fermentation is therefore a good way of preserving the nutrients of woody plants and ensuring a year-round supply of feed for livestock through storage (Du et al., 2021b). Woody plant silage is prepared in the same way as forage crops and grasses, i.e. the woody plants are harvested at a suitable growth stage and the material is cut directly into 1−2 cm. To make good-quality silage, agricultural by-products such as rice bran, wheat bran and corn bran are usually added at 10-15% to regulate the moisture and then packed into silos or drums, sealed and covered for a period of time (Du et al., 2021c). Silage prepared in this way is usually well fermented and can store the nutrients of the silage for a long time.
To investigate the natural fermentation characteristics of woody plant silage, fresh branches and leaves of woody plants were used as the raw material for silage preparation using a drumcan silo. Naturally fermented woody plant silage typically has a high pH, butyric acid and NH3-N content and a low lactic acid content (Figure 3). This is due to the high moisture content in the woody plant and the competitive use of WSC by the harmful epiphytic microorganisms, which prevents LAB from producing enough lactic acid to lower the pH during silage fermentation. This leads to butyric acid fermentation and the degradation of proteins to produce more NH3-N (Cai et al., 1999).
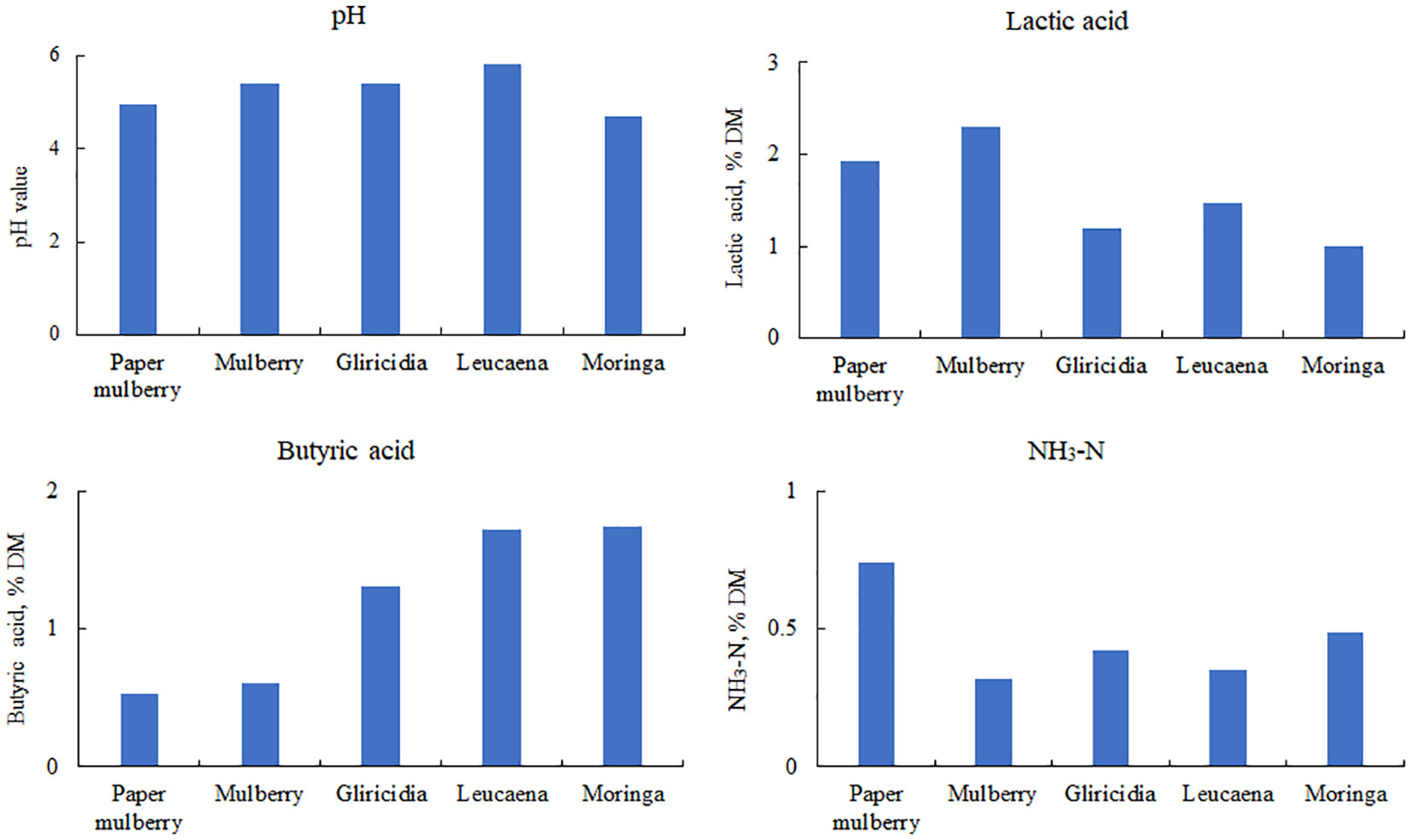
Figure 3 Fermentation characteristics of woody plant silage. DM, dry matter; NH3-N, ammonia nitrogen. Data cited from the following references: paper mulberry from Du et al., 2022b; Mulberry from Zhang et al., 2020; Gliricidia and leucaena from Du et al., 2022c; Moringa from He et al., 2020.
To verify the natural fermentation characteristics of woody plant silage, a comprehensive analysis of the dynamic changes in microbial diversity and community structure during woody plant silage fermentation was conducted using the PacBio single molecule real-time (SMRT) sequencing technology. The Venn diagrams in Figures 4A, B clearly show the dynamics of the common and special microbial communities of woody plants before and after silage fermentation. Before ensiling, high moisture content woody plants under aerobic conditions are more suitable for aerobic microbial growth and, therefore, display high operational taxonomic unit (OTU) numbers and a rich microbial diversity. After ensiling, the double stress of the anaerobic and acidic environment created as fermentation progresses leads to the rapid death of some Gram-negative bacteria with thin cell walls. In the final stage of fermentation, Gram-positive bacteria that can adapt to an anaerobic and acidic environment, such as LAB, become the dominant microbial community and carry out lactic acid fermentation, lowering the pH and inhibiting the growth of harmful microorganisms, resulting in a significant decrease in the number and microbial diversity of OTUs (Méndez-García et al., 2015).
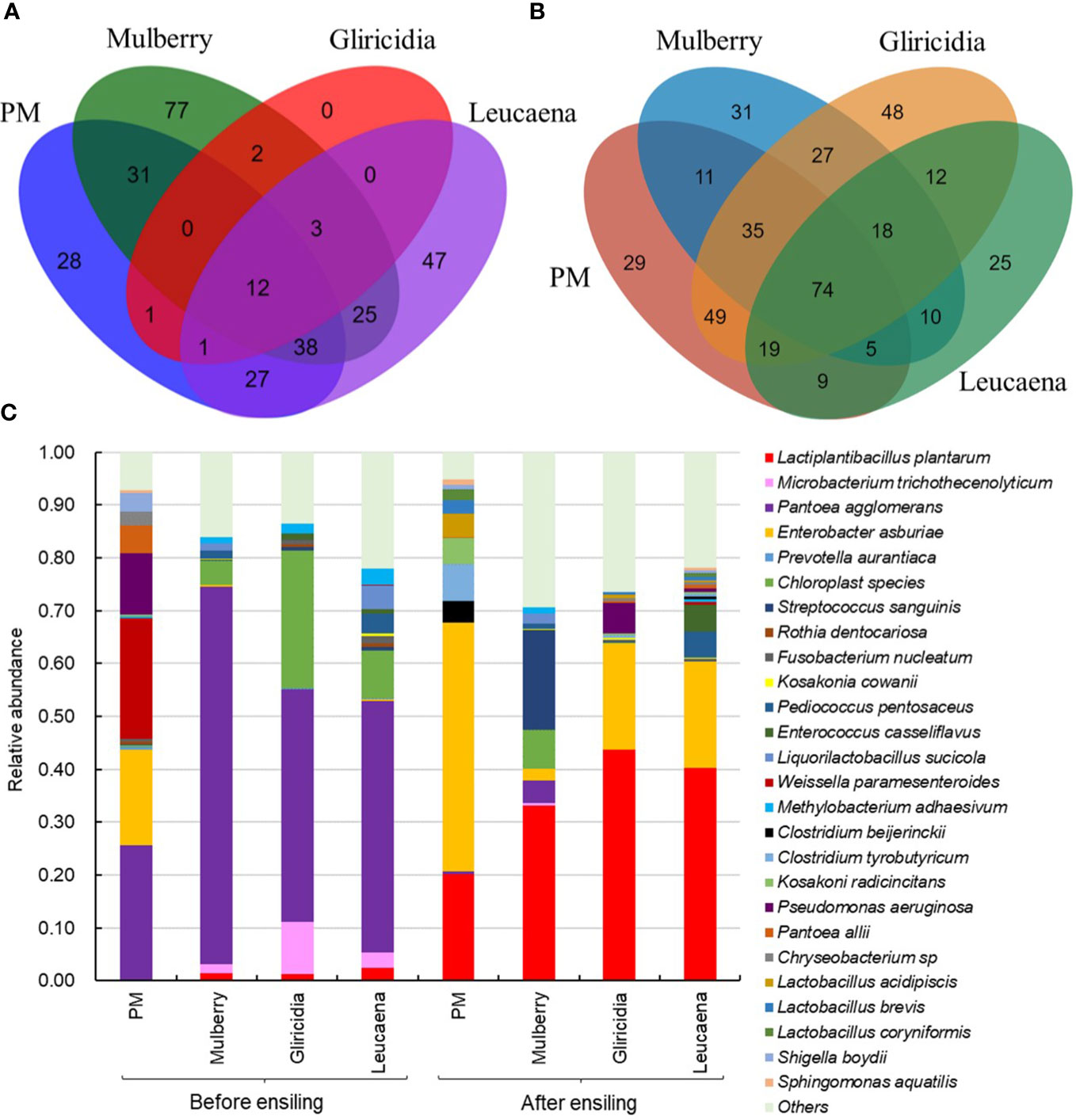
Figure 4 Microbial diversity and community structure of woody plant. (A) Venn diagram depicting unique or shared bacterial OTU of 97% sequence identity before ensiling; (B) Venn diagram after ensiling; (C) Relative abundances of the bacterial communities at the species levels. PM, paper mulberry. Data cited from the following references: Paper mulberry from Du et al., 2022b; Mulberry from Zhang et al., 2020; Gliricidia and leucaena from Du et al., 2022c.
Among the microorganisms epiphytic to the fresh branches and leaves of woody plants, the relative abundance of LAB beneficial to silage fermentation is low, while the relative abundance of Gram-negative bacteria harmful to silage fermentation, such as Pantoea, Enterobacter and Pseudomonas, is high (Figure 4C). Pantoea agglomerans, the dominant bacterial community of woody plants, is a parthenogenic anaerobic Gram-negative pathogenic bacteria that is usually found on the surface of plants and is suitable for growth in neutral environments (Jacek et al., 2016). This kind of bacteria can compete with LAB for nutrients in the early stages of silage fermentation, breaking down glucose or other sugars and producing acids (Li and Nishino, 2013). Enterobacter constitute an aerobic or facultative anaerobic Gram-negative bacteria with a wide distribution and a large host range, which can be parasitic or symbiotic, epiphytic, saprophytic on humans, animals and plants, and can also survive in soil or water. If grown on plants, it can easily lead to blight (Si et al., 2023). Pseudomonas is a common aerobic or facultative anaerobic pathogen that prefers to live in moist environments, usually on soil, water, air and plants. It is designated as low pathogenic but is highly resistant to medication (Roberson and Firestone, 1992).
During ensiling, LAB can proliferate and use the WSC in the plant material to produce lactic acid and lower the pH. The anaerobic acidic environment that forms can play an important role in inhibiting the growth of harmful bacteria in the silage (Cai et al., 2020a). Figure 4C also confirms a significant increase in the relative abundance of LAB in woody plant silage, with Lactiplantibacillus plantarum becoming the main dominant species. This species of bacteria is able to respond rapidly to the dual stress of anaerobic and acidic ensiling environments, to carry out lactic acid fermentation and improve the fermentation quality of the silage. In addition, Enterobacter and Citrobacter have a proportional relative abundance in naturally fermented woody plant silage. Citrobacter constitute Gram-negative bacteria that utilize citrate as a sole source of carbon and may be associated with the production of citric acid aroma components in woody plant silage (Janda et al., 1994). This species of bacteria has a low acid tolerance and therefore has a low relative abundance in acidic silage environments. Enterobacter constitute some harmful silage bacteria that break down proteins in the early stages of silage fermentation, causing protein deamination and decarboxylation, which produce toxic compounds such as amines and branching fatty acids, resulting in foul-smelling silage (Ávila and Carvalho, 2020). This not only reduces the nutritional value of the silage and the palatability to the animal but can also have an impact on the hygiene and safety of the feed. Therefore, in terms of microbial community dynamics, naturally fermented woody plant silage does not reach a suitable level of quality and it is necessary to regulate the microbial community structure of the silage fermentation and promote lactic acid fermentation.
To address the factors that hinder the fermentation of high-quality silage (i.e. high moisture content, strong LBC, low LAB count and low WSC content) in woody plants, rice bran and wheat bran have been added to adjust the moisture content and increase the fermentation substrate (Du et al., 2021a). Microbial additives, such as LAB, and cellulolytic enzymes have also been applied to improve the microbial community and increase the WSC content (Du et al., 2021b). In addition, hays including Napier grass and rice straw have been used to optimize the silage fermentation process of woody plants (Du et al., 2022a). The PacBio SMRT sequencing technology was applied to conduct an in-depth study of microbial diversity, co-occurrence microbial networks, metabolic pathways, final metabolites and the fermentation regulation mechanism of woody plant silage.
The moisture content of the rice bran and wheat bran used in the study was less than 10%. When mixed with woody plants at a rate of 10−30% of the FM, the moisture was adjusted to exactly 60−70% (Du et al., 2021a), which is the ideal moisture range for silage preparation. Woody plants have a similar epiphytic microbial community structure to forage crops and grasses, i.e. they have a higher relative abundance of harmful microorganisms and a lower relative abundance of LAB in the fresh material. The relative abundance of epiphytic L. plantarum from the woody plant material was below the detection level, but increased significantly after ensiling (Figure 5A). Thus, the aerobic environment created by woody plants prior to ensiling was not beneficial for the competitive growth of LAB, while aerobic microorganisms were at an advantage. Figure 5B shows that the relative abundance of clostridia in the mixed silage of woody plants and wheat bran was below the detection level, but the mixed silage of woody plants with rice bran increased the relative abundance of clostridia. Wheat bran effectively regulated the moisture and increased the fermentation substrate of woody plants, and the anaerobic conditions created by silage fermentation accelerated the succession of LAB, which became the dominant community and inhibited the proliferation of clostridia. The silage prepared with 30% wheat bran and 70% woody plants had the best microbial community structure. The addition of rice bran also served to adjust the moisture and fermentation substrate, but rice bran can sometimes be enriched with Clostridium spp., which is a strictly anaerobic Gram-positive bacteria whose spores tolerate the acidic and anaerobic environmental conditions of silage (Li et al., 2020). Clostridia breaks down sugars, organic acids and proteins during the silage process, producing butyric acid, NH3-N, carbon dioxide (CO2) and hydrogen (H2), thereby reducing the fermentation quality of the silage (Cassir et al., 2016). In addition, some proportion of clostridia is pathogenic and can be harmful to the health of livestock (Uzal et al., 2018). The microbial community dynamics in Figure 5C also confirmed that L. plantarum and Clostridium typhimurium were the two dominant bacteria in woody plant silage mixed with rice bran and wheat bran. There were significant differences in the relative abundance of these two species compared to other silage bacterial communities. Therefore, compared to rice bran, wheat bran is a more suitable exogenous addition to the silage fermentation of woody plants, not only to regulate the fermentation conditions but also to improve the microbial community structure, thus promoting woody plant silage fermentation.
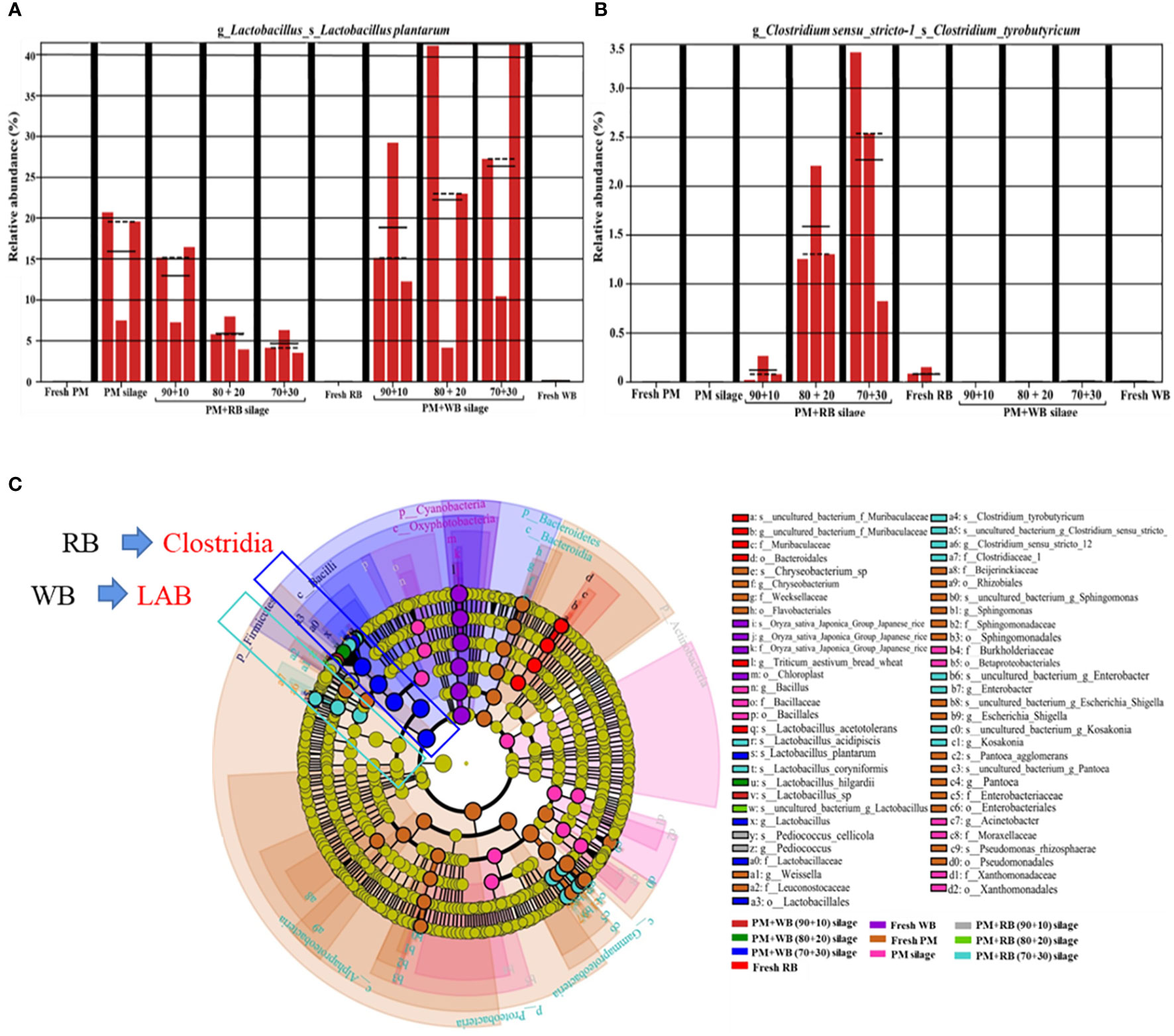
Figure 5 The community dynamics of lactic acid bacteria and clostridia in PM prepared with RB and WB before and after ensiling. (A) metaStat analysis of Lactobacillus plantarum; (B) metaStat analysis of Clostridium tyrobutyricum; (C) cladogram comparison of bacterial community. PM, paper mulberry; RB, rice bran; WB, wheat bran. The figure cited from Du et al., 2021a.
In addition, the fermentation characteristics of woody plant silage were explored by applying additions such as LAB inoculant and cellulolytic enzymes (Du et al., 2021b). Microbial additives can cause a significant decrease in microbial diversity in woody plant silage and the microbial community rapidly completes a dynamic succession process from Gram-negative to Gram-positive bacteria. In the anaerobic and acidic environment created by silage fermentation, Gram-negative bacteria such as Enterobacter asburiae die off rapidly, while LAB respond rapidly to the anaerobic conditions of silage and become the dominant community, dominating the fermentation process. Thus, microbial additives influence silage fermentation by improving the microbial community. In addition, the use of locally available low-cost crop straw and hay mixed with woody plants for silage making is a viable option for improving the silage fermentation quality. The results showed that the addition of Napier grass and rice straw allowed L. plantarum and Lactococcus cellulosus to act synergistically with each other, enabling LAB rapidly to become the dominant community for woody plant silage, and that the addition of 10−30% hay was effective in improving the fermentation quality of woody plant silage.
The metabolites produced by silage microorganisms during fermentation have a strong influence on the fermentation quality, flavor and aerobic spoilage of silage (Ávila and Carvalho, 2020). Silage fermentation forms a microbial co-occurrence network system, which includes a complex process of dynamic succession of the microbial community and changes in their metabolites, which vary greatly from the different microorganisms through their metabolic pathways (Du et al., 2022c). The LAB produce metabolites, such as organic acids, ethanol, 1,2 propylene glycol and biogenic amines during silage fermentation, some of which play an important role in inhibiting the growth of harmful bacteria and improving aerobic stability (Guo et al., 2018). The LAB usually use WSC to produce lactic acid and lower the pH, which can improve the fermentation quality of silage (Okoye et al., 2022). In contrast, Enterobater species can ferment glucose to produce succinic acid, lactic acid, acetic acid, formic acid and ethanol, as well as producing CO2 and H2 gas, and increasing the DM and energy loss (Thompson et al., 2008). In addition, some metabolites such as lactic and acetic acid reduce the pH and inhibit the growth of aerobic bacteria and molds, which can improve the fermentation quality and aerobic stability of silage (Cai et al., 2020a). Thus, the microbial community structure and metabolites interact and influence the fermentation quality of silage.
The abundance levels of harmful microorganisms such as Enterobacter spp. and Clostridium tyrobutyricum were positively correlated, which may be related to the low-quality fermentation of the silage (Figure 6A). As silage fermentation progressed, L. plantarum rapidly formed the dominant community, which in turn replaced the dominant population of the harmful bacteria P. agglomerans in the early stages of fermentation.
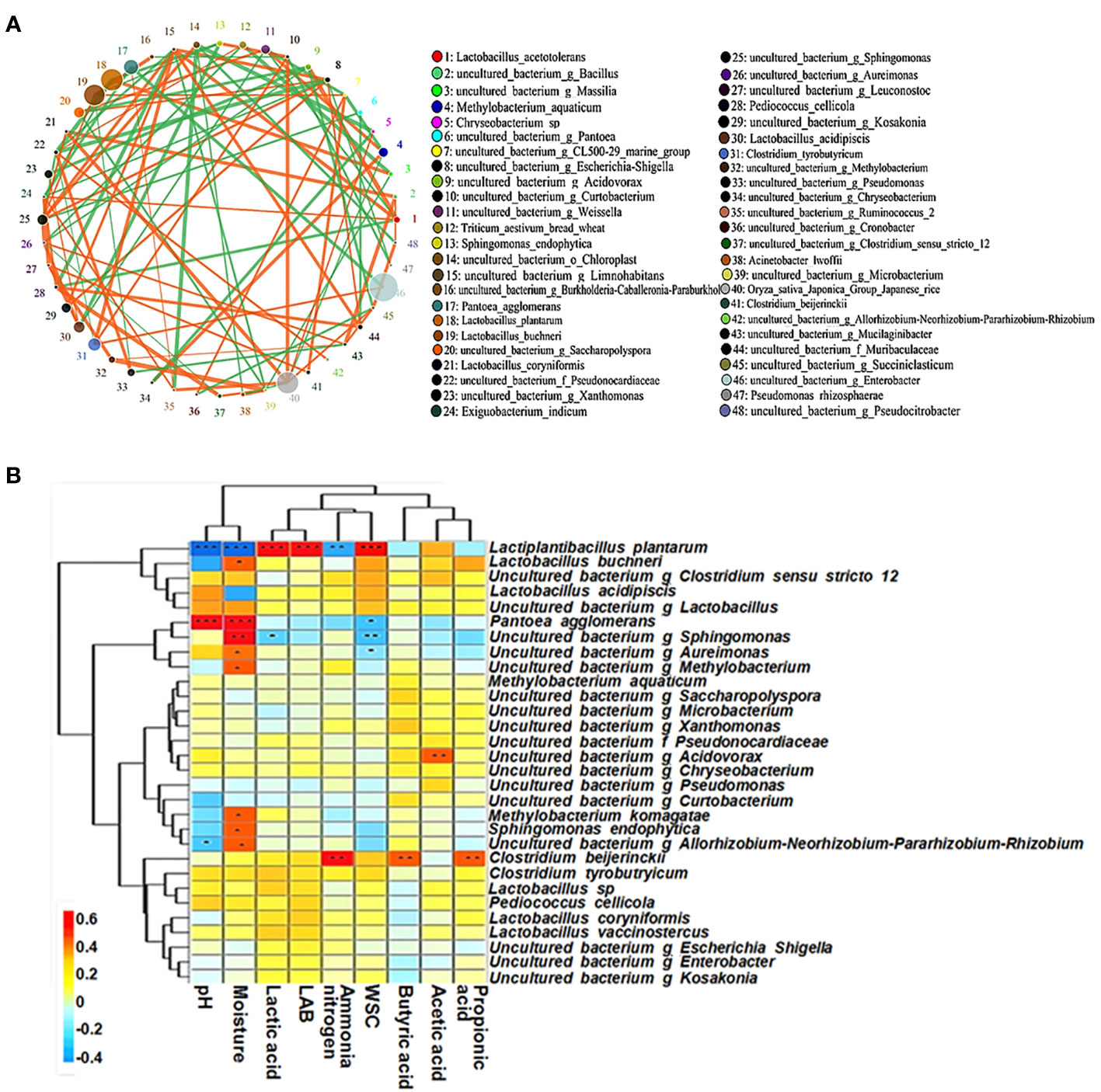
Figure 6 The microbial co-occurrence network (A) and correlation analyses between bacterial community and terminal fermentation product (B) at species level in paper mulberry silage. The figure cited from Du et al., 2021a.
A positive correlation was found between the moisture content and P. agglomerans, Sphingomonas spp., Aureimonas spp. and Methylobacterium spp. and between WSC and LAB (Figure 6B). This suggests that a high moisture content silage environment encourages the proliferation of these harmful bacteria and that WSC can promote the growth of LAB. In addition, LAB produce lactic acid during ensiling, which inhibits the growth of these harmful bacteria, thereby lowering pH and reducing the production of NH3-N. Clostridium beijerinckii form a nutrient-competitive interrelationship with LAB, i.e. C. beijerinckii undergo butyric acid fermentation, which promotes the production of propionic and butyric acids and hinders the proliferation of LAB. Acidovorax spp. are aerobic or facultative anaerobic Gram-negative bacteria that can use residual oxygen to produce acetic acid in the early stages of silage fermentation (Du et al., 2021a). Therefore, Gram-positive and Gram-negative bacteria (e.g. L. plantarum) form a mutually constraining opposition during silage fermentation; their community structure and metabolites interact with each other and influence the fermentation quality of the silage.
To explore the fermentation regulation mechanism of woody plant silage, non-targeted metabolomics techniques were used to study the mechanisms of interaction between the co-occurrence microbial networks and secondary metabolites of woody plant silage prepared with bran (Du et al., 2022a). A Spearman correlation analysis of the main bacterial community and metabolites of silage showed that the aromatic compound-like metabolites of silage were positively correlated with LAB, such as L. plantarum and Weissella paramesenteroides, and negatively correlated with other bacterial community members, such as C. tyrobutyricum and E. asburiae (Figure 7). Among the aromatic compounds, citric acid and L-malic acid are key intermediates in the tricarboxylic acid cycle metabolic pathway, which is produced by most LAB. Citric acid is formed by the carboxylation of acetyl coenzyme A and oxaloacetate in the tricarboxylic acid cycle and is involved in the metabolism of sugars, fats and proteins (Ke et al., 2017). During silage fermentation, citric acid can play a role in lowering silage pH and inhibiting the activity of undesirable fermentation fungi, such as yeasts and moulds. In addition, LAB in the silage fermentation process can metabolize citric acid to produce diacetyl and acetic acids, and other substances with flavor, which can improve the flavor of silage and the palatability to livestock. When animals are fed silage containing citric acid the proliferation of pathogens can be reduced and the production of toxic metabolites can be inhibited, which can improve the stress capacity of livestock. L-malic acid is an important natural organic acid with antioxidant properties that regulates silage pH and promotes the growth of LAB (Wang et al., 2009). During silage fermentation, LAB with the function of saccharifying starch can use starch directly for fermentation, thus producing L-malic acid, which will play a role in improving the silage fermentation quality. In addition, LAB can use D-(+)-cellobiose as a substrate for energy production, accelerating growth and inhibiting the proliferation of yeast, mold, clostridia and enterobacter. These findings suggest that silage microbiomics are closely linked to metabolomics. The microbial community structure can influence the types of final and secondary metabolites, which in turn are important factors influencing the fermentation quality of silage.
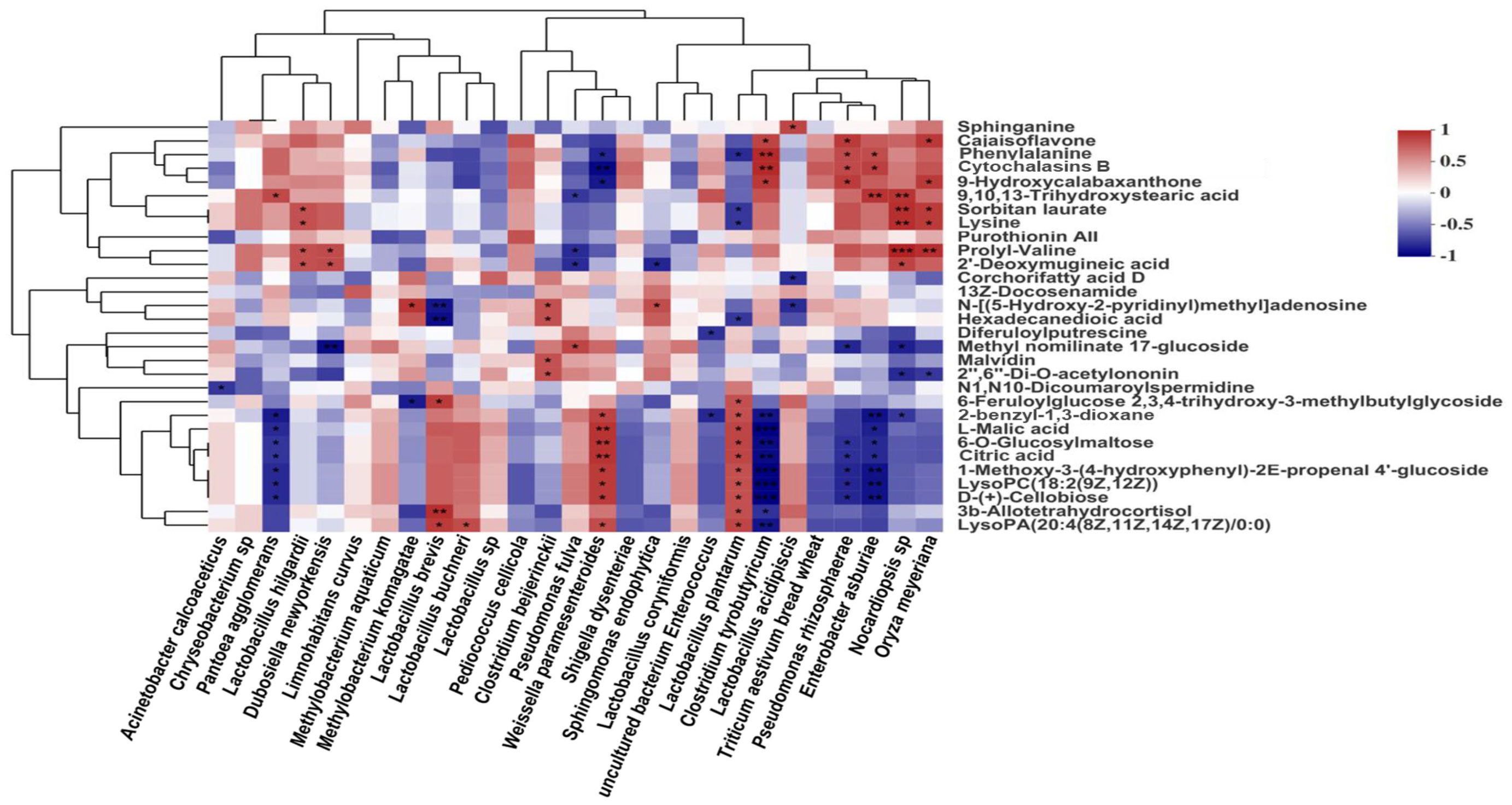
Figure 7 Correlations between microbial community and metabolites in silage. Microbial community, main bacterial species; metabolites, differentially presented metabolites. The figure cited from Du et al., 2022b.
To popularize the use of woody feed resources in livestock production, feeding experiments have been conducted using a variety of livestock, including cattle, sheep and poultry.
In experiments with fed cattle, Holstein cows fed total mixed fermentation (TMR) formulated with 10−15% paper mulberry instead of maize silage, alfalfa hay and oat hay had a similar DM intake and milk production to those fed with conventional TMR. However, the addition of paper mulberry to TMR diets significantly increased the serum levels of immunoglobulin A (IgA), immunoglobulin (IgG), catalase and superoxide dismutase and the total antioxidant capacity, but decreased the levels of 8-hydroxy-2′-deoxyguanosine. In addition, paper mulberry TMR can cause a significant decrease in the milk somatic cell count, enhance the immune and antioxidant function of cows and increase the polyunsaturated fatty acid content of milk (Si et al., 2018). The fresh branches and leaves of mulberry are rich in flavonoids and feeding them to weaned Holstein calves promotes the average daily gain and feed efficiency (Kong et al., 2019). Gliricidia leaves and elephant ear pods are rich in tannins and saponins. When a mixture of the two was fed to beef cattle, it increased the digestible CP intake, inhibited methanogenic bacteria in the rumen and reduced methane emissions (Isabel et al., 2019). Leucaena silage improves the DM intake, feed digestibility and rumen end fermentation products in dairy cows and reduces methane production (Giang et al., 2016). A TMR including moringa improves the milk fat content and levels of secondary metabolites, regulates rumen fermentation and inhibits methane production by dairy cattle (Dong et al., 2019).
In sheep feeding trials, replacing some of the soybean meal and corn stover with paper mulberry silage reduced total volatile fatty acids in the rumen, and increased DM intake and average daily gain of lambs (Xiong et al., 2021). When some of the hay and concentrate in the TMR was replaced by mulberry leaves, there was no significant difference in the digestibility of DM, CP, and crude fibre in sheep compared to the conventional TMR (Kandylis et al., 2009). In addition, silage prepared from a mixture of gliricidia with cassava improves growth performance, digestibility, feeding behavior and the carcass characteristics of lambs, and increases the yield of key commercial meats, such as loin and ham, as well as typical foodstuffs, such as lamb (Oliveira et al., 2018). Feed supplementation with moringaleaf extract increased milk production, reduced saturated fatty acids and increased the levels of unsaturated fatty acids and conjugated linoleic acid in goats (Kholif et al., 2018).
In poultry feeding trials, the use of woody plants, such as paper mulberry and mulberry, in place of some of the commercial feeds maintained good indicators in terms of the egg production rate, egg weight, fertilized egg hatching rate, growth performance and meat quality (Yang et al., 2020). There is great future potential for seedling production and breeding of woody plants for healthy livestock feeding with antibiotic substitutes.
To develop and utilize new woody feed resources to produce high-quality livestock products, this review provided a comprehensive overview of the composition and uses of natural woody plants that can be fed to animals, the dominant factors affecting woody plant silage fermentation, microbial succession patterns and the mechanisms of interaction between microbial co-occurrence networks and secondary metabolites. Woody plants are rich in nutrients and can be used for the preparation of fermented feeds and the production of value-added livestock products (Figure 8). This has important implications for alleviating feed shortages and promoting sustainable development of animal husbandry.
ZD and YC: Writing - original draft, Investigation. ZD, YC, and FY: Writing–original draft, Formal analysis. ZD and JF: Validation, Writing−review & editing; SY: Conceptualization, Visualization. TO, DN, and HK: Supervision, Resources. YC and FY: Resources, Writing−review & editing, Supervision, Funding acquisition. All authors contributed to the article and approved the submitted version.
This work was supported by the project “High-level Talents Program of Lv-Yang-Jin-Feng of Yangzhou”, “Development of Sustainable Technologies to Increase Agricultural Productivity and Improve Food Security in Africa (Food Security in Africa)”, and “National Key Research and Development Program of China (2022YFD1300900)”.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abd Rani, N. Z., Husain, K., Kumolosasi, E. (2018). Moringa genus: A review of phytochemistry and pharmacology. Front. Pharmacol. 9. doi: 10.3389/fphar.2018.00108
Ajayo, P. C., Huang, M., Zhao, L., Tian, D., Jiang, Q., Deng, S., et al. (2022). Paper mulberry fruit juice: A novel biomass resource for bioethanol production. Bioresour. Bioprocess. 9, 3. doi: 10.1186/s40643-021-00490-3
Amna, T., Hassan, M. S., Sheikh, F. A., Seo, H. C., Kim, H. C., Alotaibi, N., et al. (2021). Natural mulberry biomass fibers doped with silver as an antimicrobial textile: A new generation fabric. Text. Res. J. 91 (21-22), 2581–2587. doi: 10.1177/004051752110134
Anadón, J. D., Sala, O. E., Turner, B. L., Bennett, E. M. (2014). Effect of woody-plant encroachment on livestock production in North and South America. PNAS 111 (35), 12948–12953. doi: 10.1073/pnas.1320585111
Anwar, F., Latif, S., Ashraf, M., Gilani, A. H. (2007). Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 21 (1), 17–25. doi: 10.1002/ptr.2023
Aulanni’Am, A., Ora, K. M., Ariandini, N. A., Wuragil, D. K., Permata, F. S., Riawan, W., et al. (2021). Wound healing properties of Gliricidia sepium leaves from Indonesia and the Philippines in rats (Rattus norvegicus). Vet. World 14 (3), 820–824. doi: 10.14202/vetworld.2021.820-824
Ávila, C. L. S., Carvalho, B. F. (2020). Silage fermentation-updates focusing on the performance of micro-organisms. J. Appl. Microbiol. 128 (4), 966–984. doi: 10.1111/jam.14450
Cai, Y., Benno, Y., Ogawa, M., Kumai, S. (1999). Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy. Sci. 82 (3), 520–526. doi: 10.3168/jds.S0022-0302(99)75263-X
Cai, Y., Du, Z., Jethro, D. B., Nignan, M., Yamasaki, S. (2021). Analysis of main factors affecting silage fermentation of sorghum prepared with whole crop and stover in semiarid West Africa. Afr. J. Range For. Sci. 38 (2), 169–178. doi: 10.2989/10220119.2020.1794959
Cai, Y., Du, Z., Yamasaki, S., Nguluve, D., Tinga, B., Macome, F., et al. (2020a). Community of natural lactic acid bacteria and silage fermentation of corn stover and sugarcane tops in Africa. Asian-Austral. J. Anim. Sci. 33 (8), 1252–1264. doi: 10.5713/ajas.19.0348
Cai, Y., Du, Z., Yamasaki, S., Nguluve, D., Tinga, B., Macome, F., et al. (2020b). Influence of microbial additive on microbial populations, ensiling characteristics, and spoilage loss of delayed sealing silage of Napier grass. Asian-Austral. J. Anim. 33, 1103–1112. doi: 10.5713/ajas.19.0471
Cassir, N., Benamar, S., La Scola, B. (2016). Clostridium butyricum: From beneficial to a new emerging pathogen. Clin. Microbiol. Infect. 22 (1), 37–45. doi: 10.1016/j.cmi.2015.10.014
Çelekli, A., Al-Nuaimi, A. I., Bozkurt, H. (2019). Adsorption kinetic and isotherms of Reactive Red 120 on Moringa oleifera seed as an eco-friendly process. J. Mol. Struct. 1195, 168–178. doi: 10.1016/j.molstruc.2019.05.106
Chen, G., Shui, S., Chai, M., Wang, D., Su, Y., Wu, H., et al. (2020). Effects of paper mulberry (Broussonetia papyrifera) leaf extract on growth performance and fecal microflora of weaned piglets. BioMed. Res. Int. 2020, 6508494. doi: 10.1155/2020/6508494
Cheng, Z., Lin, C., Hwang, T., Teng, C. (2001). Broussochalcone a, a potent antioxidant and effective suppressor of inducible nitric oxide synthase in lipopolysaccharide-activated macrophages. Biochem. Pharmacol. 61 (8), 939–946. doi: 10.1016/S0006-2952(01)00543-3
Chigurupati, S., Vijayabalan, S., Selvarajan, K. K., Alhowail, A., Kauser, F. (2020). Bacterial endosymbiont inhabiting Leucaena leucocephala leaves and their antioxidant and antidiabetic potential. J. Complement. Integr. Med. 18 (2), 319–325. doi: 10.1515/jcim-2020-0203
Dana, N., Tegegne, A., Shenkoru, T. (2000). Feed intake, sperm output and seminal characteristics of Ethiopian highland sheep supplemented with different levels of leucaena (Leucaena leucocephala) leaf hay. Anim. Feed. Sci. Tech. 86 (3-4), 239–249. doi: 10.1016/S0377-8401(00)00152-8
De Carvalho, G. G. P., Rebouças, R. A., Campos, F. S., Santos, E. M., Araújo, G. G. L., Gois, G. C., et al. (2017). Intake, digestibility, performance, and feeding behavior of lambs fed diets containing silages of different tropical forage species. Anim. Feed. Sci. Tech. 228, 140–148. doi: 10.1016/j.anifeedsci.2017.04.006
Diaz, C., Grageola, F., Lemus, C., Ly, J. (2007). Studies on the cell wall digestibility in pigs fed leucaena (Leucaena leucophala (Lam.) de Wit) leaf meal. J. Anim. Vet. Adv. 6 (10), 1190–1193. doi: 10.2460/javma.231.7.1098
Ding, Y., Jiang, X., Yao, X., Zhang, H., Song, Z., He, X., et al. (2021). Effects of feeding fermented mulberry leaf powder on growth performance, slaughter performance, and meat quality in chicken broilers. Animals 11 (11), 3294. doi: 10.3390/ani11113294
Dong, Z., Ding, Z., Zhang, S., Zhang, Y., Fan, H., Yang, Y. (2017). Natural fibers from the bark of mulberry branches for textile application. Fibers. Text. East. Eur. 25 (3), 20–25. doi: 10.5604/10.5604/01.3001.0010.1684
Dong, Y., Li, P., Li, P., Chen, C. (2021). First comprehensive analysis of lysine succinylation in paper mulberry (Broussonetia papyrifera). BMC Genomics 22 (1), 255. doi: 10.1186/s12864-021-07567-5
Dong, L., Zhang, T., Diao, Q. (2019). Effect of dietary supplementation of moringa oleifera on the production performance and fecal methanogenic community of lactating dairy cows. Animals 9 (5), 262. doi: 10.3390/ani9050262
Du, Z., Lin, Y., Sun, L., Yang, F., Cai, Y. (2021a). Microbial community structure, co-occurrence network and fermentation characteristics of woody plant silage. J. Sci. Food Agr. 102 (3), 1193–1204. doi: 10.1002/jsfa.11457
Du, Z., Sun, L., Chen, C., Lin, J., Yang, F., Cai, Y. (2021b). Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim. Feed. Sci. Tech. 275, 114766. doi: 10.1016/j.anifeedsci.2020.114766
Du, Z., Sun, L., Lin, Y., Chen, C., Yang, F., Cai, Y. (2022a). Use of Napier grass and rice straw hay as exogenous additive improves microbial community and fermentation quality of paper mulberry silage. Anim. Feed. Sci. Tech. 285, 115219. doi: 10.1016/j.anifeedsci.2022.115219
Du, Z., Sun, L., Lin, Y., Yang, F., Cai, Y. (2021c). The use of PacBio SMRT technology to explore the microbial network and fermentation characteristics of woody silage prepared with exogenous carbohydrate additives. J. Appl. Microbiol. 131 (5), 2193–2211. doi: 10.1111/jam.15124
Du, Z., Sun, L., Lin, Y., Yang, F., Cai, Y. (2022b). Using PacBio SMRT sequencing technology and metabolomics to explore the microbiota-metabolome interaction related to silage fermentation of woody plant. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.857431
Du, Z., Yamasaki, S., Oya, T., Nguluve, D., Euridse, D., Tinga, B., et al. (2022c). Microbial co-occurrence network and fermentation information of natural woody-plant silage prepared with grass and crop by-product in Southern Africa. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.756209
FAO. (2009). “The state of food and agriculture,” in Livestock in the balance (Rome, Italy: Food and Agriculture Organization of the United Nations).
Franzel, S., Carsan, S., Lukuyu, B., Sinja, J., Wambugu, C. (2014). Fodder trees for improving livestock productivity and smallholder livelihoods in Africa. Curr. Opin. Env. Sust. 6, 98–103. doi: 10.1016/j.cosust.2013.11.008
Fulvia, T., Matteo, P., Floriana, B., Fabrizio, A. (2019). Moringa oleifera Lam. As an energy crop for biogas production in developing countries. Biomass Convers. Bior. 10, 1083–1089. doi: 10.1007/s13399-019-00550-x
Giang, N. T. T., Wanapat, M., Phesatcha, K., Kang, S. (2016). Level of Leucaena leucocephala silage feeding on intake, rumen fermentation, and nutrient digestibility in dairy steers. Trop. Anim. Health Pro. 48, 1057–1064. doi: 10.1007/s11250-016-1060-3
Gopalakrishnan, L., Doriya, K., Kumar, D. S. (2016). Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Well. 5 (2), 49–56. doi: 10.1016/j.fshw.2016.04.001
Grosshagauer, S., Pirkwieser, P., Kraemer, K., Somoza, V. (2021). The future of moringa foods: A food chemistry perspective. Front. Nutr. 8. doi: 10.3389/fnut.2021.751076
Guo, X. S., Ke, W. C., Ding, W. R., Ding, L. M., Xu, D. M., Wang, W. W., et al. (2018). Profiling of metabolome and bacterial community dynamics in ensiled Medicago sativa inoculated without or with Lactobacillus plantarum or Lactobacillus buchneri. Sci. Rep-UK. 8 (1), 357. doi: 10.1038/s41598-017-18348-0
Han, Q., Wu, Z., Huang, B., Sun, L., Ding, C., Yuan, S., et al. (2016). Extraction, antioxidant and antibacterial activities of Broussonetia papyrifera fruits polysaccharides. Int. J. Biol. Macromol. 92, 116–124. doi: 10.1016/j.ijbiomac.2016.06.087
Hao, Y., Huang, S., Si, J., Zhang, J., Gaowa, N., Sun, X., et al. (2020). Effects of paper mulberry silage on the milk production, apparent digestibility, antioxidant capacity, and fecal bacteria composition in Holstein dairy cows. Animals 10, 1152. doi: 10.3390/ani10071152
Hariyadi, F. H. P., Hartono, A. (2018). Biomass and carbon stock potential of Gliricidia Sepium as an alternative energy at Timor Tengah Utara Regency, East Nusa Tenggara Province, Indonesia. IOP. Conf. Series.: Earth Env. Sci. 141 (1), 12022. doi: 10.1088/1755-1315/141/1/012022
Harun, N. L. A., Alimon, A. R., Jahromi, M. F., Samsudin, A. A. (2017). Effects of feeding goats with Leucaena leucocephala and Manihot esculenta leaves supplemented diets on rumen fermentation profiles, urinary purine derivatives and rumen microbial population. J. Appl. Anim. Res. 45 (1), 409–416. doi: 10.1080/09712119.2016.1205499
He, L., Zhou, W., Wang, C., Yang, F., Chen, X., Zhang, Q. (2019). Effect of cellulase and Lactobacillus casei on ensiling characteristics, chemical composition, antioxidant activity, and digestibility of mulberry leaf silage. J. Dairy. Sci. 102 (11), 9919–9931. doi: 10.3168/jds.2019-16468
He, L., Zhou, W., Xing, Y., Pian, R., Chen, X., Zhang, Q. (2020). Improving the quality of rice straw silage with Moringa oleifera leaves and propionic acid: Fermentation, nutrition, aerobic stability and microbial communities. Bioresour. Technol. 299, 122579. doi: 10.1016/j.biortech.2019.122579
Hong, H., Jo, H. J., Kim, S. J. (2017). The physical properties of handmade Jumchi-Hanji made with Korea paper mulberry. J. Korean. Soc. Clothing. Textiles. 41 (4), 633–645. doi: 10.5850/JKSCT.2017.41.4.633
Hossam, M. E., Ahmed, E. K., Maria, C., Uchenna, Y. A. (2019). Ruminal fermentation kinetics of Moringa oleifera leaf and seed as protein feeds in dairy cow diets: In sacco degradability and protein and fiber fractions assessed by the CNCPS method. Agroforest. Syst. 94, s905–s915. doi: 10.1007/s10457-019-00456-7
Hussain, J., Reddy, P. V. V. S., Reddy, V. R. (1991). Utilisation of leucaena leaf meal by broilers. Brit. Poultry. Sci. 32 (1), 131–137. doi: 10.1080/00071669108417334
Isabel, C. M., Julian, A., Sara, V., Rolando, B., Carlos, F. A., Armín, A. B., et al. (2019). Effects of tannins and saponins contained in foliage of Gliricidia sepium and pods of Enterolobium cyclocarpum on fermentation, methane emissions and rumen microbial population in crossbred heifers. Anim. Feed. Sci. Technol. 251, 1–11. doi: 10.1016/j.anifeedsci.2019.01.011
Jacek, D., Barbara, M., Marta, K. L., Marcin, G., Janusz, M. (2016). Pantoea agglomerans: A mysterious bacterium of evil and good. Part III. Deleterious effects: Infections of humans, animals and plants. Ann. Agr. Env. Med. 23 (2), 197–205. doi: 10.5604/12321966.1203878
Janardhan, G., Gowda, S. T., Thippesh, D., Mahanthesh, B., Ramachandra, C. (2008). Effect of integrated nitrogen management practices on nutrient content of mulberry (Morus indica L.) leaves. Res. Crop 9 (2), 331–334.
Janda, J. M., Abbott, S. L., Cheung, W. K., Hanson, D. F. (1994). Biochemical identification of citrobacteria in the clinical laboratory. J. Clin. Microbiol. 32 (8), 1850–1854. doi: 10.1128/jcm.32.8.1850-1854.1994
Jitjaicham, M., Kusuktham, B. (2016). Preparation of paper mulberry fibers and possibility of cotton/paper mulberry yarns production. Ind. J. Mater. Sci. 2016, 1–6. doi: 10.1155/2016/1498967
Kagya-Agyemang, J., Takyi-Boampong, G., Adjei, M., Karikari-Bonsu, F. (2007). A note on the effect of Gliricidia sepium leaf meal on the growth performance and carcass characteristics of broiler chickens. J. Anim. Feed. Sci. 16 (1), 104–108. doi: 10.22358/jafs/66731/2007
Kandylis, K., Hadjigeorgiou, I., Harizanis, P. (2009). The nutritive value of mulberry leaves (Morus alba) as a feed supplement for sheep. Trop. Anim. Health Pro. 41 (1), 17–24. doi: 10.1007/s11250-008-9149-y
Ke, W. C., Ding, W. R., Xu, D. M., Ding, L. M., Zhang, P., Li, F. D., et al. (2017). Effects of addition of Malic or citric acids on fermentation quality and chemical characteristics of alfalfa silage. J. Dairy. Sci. 100 (11), 8958–8966. doi: 10.3168/jds.2017-12875
Keeling, L., Tunon, H., Olmos, A. G., Berg, C., Jones, M., Stuardo, L., et al. (2019). Animal welfare and the united nations sustainable development goals. Front. Vet. Sci. 6. doi: 10.3389/fvets.2019.00336
Kholif, A. E., Gouda, G. A., Anele, U. Y., Galyean, M. L. (2018). Extract of Moringa oleifera leaves improves feed utilization of lactating Nubian goats. Small. Ruminant. Res. 158, 69–75. doi: 10.1016/j.smallrumres.2017.10.014
Komarek, A. M., Dunston, S., Enahoro, D., Godfray, H., Herrero, M., Mason-D’Croz, D., et al. (2021). Income, consumer preferences, and the future of livestock-derived food demand. Global Environ. Change 70, 102343. doi: 10.1016/j.gloenvcha.2021.102343
Kong, L., Yang, C., Dong, L., Diao, Q., Si, B., Ma, J., et al. (2019). Rumen fermentation characteristics in pre- and post-weaning calves upon feeding with mulberry Leaf flavonoids and candida tropicalis individually or in combination as a supplement. Animals 9 (11), 990. doi: 10.3390/ani9110990
Leone, A., Spada, A., Battezzati, A., Schiraldi, A., Aristil, J., Bertoli, S. (2015). Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of moringa oleifera leaves: An overview. Int. J. Mol. Sci. 16 (6), 12791–12835. doi: 10.3390/ijms160612791
Li, R., Jiang, D., Zheng, M., Tian, P., Zheng, M., Xu, C. (2020). Microbial community dynamics during alfalfa silage with or without clostridial fermentation. Sci. Rep-UK. 10 (1), 17782. doi: 10.1038/s41598-020-74958-1
Li, Y., Nishino, N. (2013). Effects of ensiling fermentation and aerobic deterioration on the bacterial community in Italian ryegrass, Guinea grass, and whole-crop maize silages stored at high moisture content. Asian-Austral. J. Anim. Sci. 26, 1304–1312. doi: 10.5713/ajas.2013.13175
Lim, T. K. (2014). “Gliricidia sepium,” in Edible medicinal and non-medicinal plants (Dordrecht: Springer), 806–816.
Liu, Q., Dong, Z., Shao, T. (2018). Effect of additives on fatty acid profile of high moisture alfalfa silage during ensiling and after exposure to air. Anim. Feed. Sci. Technol. 236, 29–38. doi: 10.1016/j.anifeedsci.2017.11.022
Liu, Y., Xiao, Y., Xie, J., Peng, Y., Li, F., Chen, C., et al. (2022). Dietary supplementation with flavonoids from mulberry leaves improves growth performance and meat quality, and alters lipid metabolism of skeletal muscle in a Chinese Hybrid pig. Anim. Feed. Sci. Technol. 285, 115211. doi: 10.1016/j.anifeedsci.2022.115211
Ma, G., Chai, X., Hou, G., Zhao, F., Meng, Q. (2022). Phytochemistry, bioactivities and future prospects of mulberry leaves: A review. Food Chem. 372, 131335. doi: 10.1016/j.foodchem.2021.131335
Maasdorp, B. V., Muchenje, V., Titterton, M. (1999). Palatability and effect on dairy cow milk yield of dried fodder from the forage trees Acacia Boliviana, Calliandra calothyrsus and Leucaena leucocephala. Anim. Feed. Sci. Technol. 77 (1-2), 49–59. doi: 10.1016/S0377-8401(98)00232-6
Madhav, P. N., Carolyn, J. F. (2012). Phylogenetics of Morus (Moraceae) inferred from its and trnL-trnF sequence data. Syst. Bot. 37, 442–450. doi: 10.2307/41515134
Maqsood, M., Saeed, A. R., Sahar, A., Khan, I. M. (2022). Mulberry plant as a source of functional food with therapeutic and nutritional applications: A review. J. Food Biochem. 46 (11), e14263. doi: 10.1111/jfbc.14263
Marsetyo, Sulendre, I. W., Takdir, M., Harper, K. J., Poppi, D. P. (2021). Formulating diets based on whole cassava tuber (Manihot esculenta) and gliricidia (Gliricidia sepium) increased feed intake, liveweight gain and income over feed cost of Ongole and Bali bulls fed low quality forage in Central Sulawesi, Indonesia. Anim. Prod. Sci. 61 (8), 761–769. doi: 10.1071/AN20297
Martínez, M., Motta, W., Cervera, C., Pla, M. (2005). Feeding mulberry leaves to fattening rabbits: effects on growth, carcass characteristics and meat quality. Anim. Sci. 80 (3), 275–280. doi: 10.1079/ASC41110275
McDonald, P., Henderson, A. R., Heron, S. J. E. (1991). The biochemistry of silage. 2nd ed. (Marlow, UK: Chalcombe Publications).
Méndez-García, C., Peláez, A. I., Mesa, V., Sánchez, J., Golyshina, O. V., Ferrer, M. (2015). Microbial diversity and metabolic networks in acid mine drainage habitats. Front. Microbiol. 6. doi: 10.3389/fmicb.2015.00475
Mpairwe, D. R., Sabiiti, E. N., Mugerwa, J. S. (1998). Effect of dried Gliricidia sepium leaf supplement on feed intake, digestibility and nitrogen retention in sheep fed dried KW4 elephant grass (Pennisetum purpureum) ad libitum. Agroforest. Syst. 41 (2), 139–150. doi: 10.1023/A:1006097902270
Muck, R. E., Nadeau, E. M. G., McAllister, T. A., Contreras-Govea, F. E., Santos, M. C., Kung, L. (2018). Silage review: Recent advances and future uses of silage additives. J. Dairy. Sci. 101, 3980–4000. doi: 10.3168/jds.2017-13839
Mukumbo, F. E., Maphosa, V., Hugo, A., Nkukwana, T. T., Mabusela, T. P., Muchenje, V. (2014). Effect of Moringa oleifera leaf meal on finisher pig growth performance, meat quality, shelf life and fatty acid composition of pork. S. Afr. J. Anim. Sci. 44 (4), 388–400. doi: 10.4314/sajas.v44i4.9
Nallathambi Gunaseelan, V. (1988). Anaerobic digestion of gliricidia leaves for biogas and organic manure. Biomass 17 (1), 1–11. doi: 10.1016/0144-4565(88)90066-2
Okoye, C. O., Wang, Y., Gao, L., Wu, Y., Li, X., Sun, J., et al. (2022). The performance of lactic acid bacteria in silage production: A review of modern biotechnology for silage improvement. Microbiol. Res. 266, 127212. doi: 10.1016/j.micres.2022.127212
Oliveira, A. P. D., Bagaldo, A. R., Loures, D. R. S., Bezerra, L. R., Moraes, S. A., Yamamoto, S. M., et al. (2018). Effect of ensiling gliricidia with cassava on silage quality, growth performance, digestibility, ingestive behavior and carcass traits in lambs. Anim. Feed. Sci. Technol. 241, 198–209. doi: 10.1016/j.anifeedsci.2018.05.004
Olugbenga, D. O., Johnson, O. A., Simeon, O. A., Eyanlola, S. A., Olajumoke, T. D., Deborah, A. O. (2018). Gliricidia leaf meal and multi-enzyme in rabbits diet: Effect on performance, blood indices, serum metabolites and antioxidant status. J. Anim. Sci. Technol. 60, 24. doi: 10.1186/s40781-018-0182-8
Owen-Smith, N., Cooper, S. M. (1987). Palatability of woody plants to browsing ruminants in a South African Savanna. Ecology 2, 319–331. doi: 10.2307/1939263
Pagano, C., Perioli, L., Baiocchi, C., Bartoccini, A., Beccari, T., Blasi, F., et al. (2020). Preparation and characterization of polymeric microparticles loaded with Moringa oleifera leaf extract for exuding wound treatment. Int. J. Pharmaceut. 587, 119700. doi: 10.1016/j.ijpharm.2020.119700
Pakade, V., Cukrowska, E., Chimuka, L. (2013). Metal and flavonol contents of Moringa oleifera grown in South Africa. S. Afr. J. Sci. 109, 3–4. doi: 10.1590/sajs.2013/835
Peñailillo, J., Olivares, G., Moncada, X., Payacán, C., Chang, C., Chung, K., et al. (2016). Correction: Sex distribution of paper mulberry (Broussonetia papyrifera) in the Pacific. PloS One 11 (9), e0163188. doi: 10.1371/journal.pone.0163188
Phesatcha, K., Wanapat, M. (2016). Improvement of nutritive value and ruminal fermentation of silage by molasses and urea supplementation. Asian-. Asian-Austral. J. Anim. Sci. 29, 1136–1144. doi: 10.5713/ajas.15.0591
Phimphachanhvongsod, V., Ledin, I. (2002). Performance of growing goats fed panicum maximum and leaves of gliricidia sepium. Asian-Austral. J. Anim. Sci. 15 (11), 1585–1590. doi: 10.5713/ajas.2002.1585
Rengsirikul, K., Kanjanakuha, A., Ishii, Y., Kangvansaichol, K., Sripichitt, P., Punsuvon, V., et al. (2011). Potential forage and biomass production of newly introduced varieties of leucaena (Leucaena leucocephala (Lam.) de Wit.) in Thailand. Grassl. Sci. 57, 94–100. doi: 10.1111/j.1744-697X.2011.00213.x
Roberson, E. B., Firestone, M. K. (1992). Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl. Environ. Microb. 58 (4), 1284–1291. doi: 10.1128/aem.58.4.1284-1291.1992
Salem, H. B., Makkar, H. P. S. (2008). Defatted Moringa oleifera seed meal as a feed additive for sheep. Anim. Feed. Sci. Technol. 150 (1), 27–33. doi: 10.1016/j.anifeedsci.2008.07.007
Santos-Ricalde, R., Gutiérrez-Ruiz, E., Novelo-Ucan, W., Martinez-Romero, P., Segura-Correa, J. (2017). Effect of feed restriction on intake of Moringa oleifera and Leucaena leucocephala and growth performance of rabbits. Trop. Anim. Health Pro. 49 (8), 1685–1688. doi: 10.1007/s11250-017-1377-6
Sheng, P., He, L., Ji, S., Huang, J., Zhang, Z., Wang, D., et al. (2021). Effect of Broussonetia papyrifera L. (Paper mulberry) on growth performance, carcase traits, meat quality and immune performance in Hu ram lambs. Ital. J. Anim. Sci. 20 (1), 691–697. doi: 10.1080/1828051X.2021.1904795
Si, B., Tao, H., Zhang, X., Guo, J., Cui, K., Tu, Y., et al. (2018). Effect of Broussonetia papyrifera L. (paper mulberry) silage on dry matter intake, milk composition, antioxidant capacity and milk fatty acid profile in dairy cows. Asian-Austral. J. Anim. Sci. 31 (8), 1259–1266. doi: 10.5713/ajas.17.0847
Si, Q., Wang, Z., Liu, W., Liu, M., Ge, G., Jia, Y., et al. (2023). Influence of cellulase or Lactiplantibacillus plantarum on the ensiling performance and bacterial community in mixed silage of alfalfa and leymus chinensis. Microorganisms 11 (2), 426. doi: 10.3390/microorganisms11020426
Singh, R., Bhatia, D., Bhasin, V. (1997). Evaluation of paper mulberry (Broussonetia papyrifera) leaves in adult rabbits. Indian J. Anim. Sci. 67, 170–171.
Soedarjo, M., Borthakur, D. (1996). Simple procedures to remove mimosine from young leaves, pods and seeds of Leucaena leucocephala used as food. Int. J. Food Sci. Tech. 31 (1), 97–103. doi: 10.1111/j.1365-2621.1996.24-321.x
Song, W. T., Zhu, F. F., Chen, K. P. (2021). The molecular mechanisms and factors affecting the feeding habits of silkworm (Lepidoptera: Bombyxidae). J. Asia-Pac. Entomol. 24 (4), 955–962. doi: 10.1016/j.aspen.2021.08.010
Srinivasulu, C., Reddy, M., Reddy, D. (1999). Effect of supplementation of Gliricidia maculata to urea-treated and untreated paddy straw in crossbred cows. Indian J. Anim. Sci. 69 (5), 357–359.
Su, B., Chen, X. (2020). Current status and potential of moringa oleifera leaf as an alternative protein source for animal feeds. Front. Vet. Sci. 7. doi: 10.3389/fvets.2020.00053
Takasaki, M., Ogura, R., Morikawa, H., Chino, S., Tsuiki, H. (2011). Preparation and properties of paper yarn from mulberry. Adv. Mat. Res. 175-176, 575–579. doi: 10.4028/www.scientific.net/AMR.175-176.575
Tambone, F., Pradella, M., Bedussi, F., Adani, F. (2020). Moringa oleifera Lam. as an energy crop for biogas production in developing countries. Biomass Convers. Bior. 10, 1083–1089. doi: 10.1007/s13399-019-00550-x
Tang, T., Bai, J., Ao, Z., Wei, Z., Hu, Y., Liu, S. (2021). Effects of dietary paper mulberry (Broussonetia papyrifera) on growth performance and muscle quality of grass carp (ctenopharyngodon idella). Animals 11 (6), 1655. doi: 10.3390/ani11061655
Tao, H., Si, B., Xu, W., Tu, Y., Diao, Q. (2020). Effect of Broussonetia papyrifera L. Silage on blood biochemical parameters, growth performance, meat amino acids and fatty acids compositions in beef cattle. Asian-Austral. J. Anim. Sci. 33, 732–741. doi: 10.5713/ajas.19.0150
Thompson, L. J., Gray, V. M., Kalala, B., Lindsay, D., Reynolds, K., von Holy, A. (2008). Biohydrogen production by Enterobacter cloacae and Citrobacter freundii in carrier induced granules. Biotechnol. Lett. 30 (2), 271–274. doi: 10.1007/s10529-007-9527-y
Tian, M., Wang, Y., Lu, A., Zhang, Q., Li, X., Zhang, N., et al. (2022). From metabolomic analysis to quality assessment and biosynthetic insight in traditional Chinese medicine: Mulberry tree as a case study. Phytochem. Anal. 33 (4), 644–653. doi: 10.1002/pca.3117
Tudsri, S., Chotchutima, S., Nakamanee, K., Kangwansaichol, K. (2019). Dual use of leucaena for bioenergy and animal feed in Thailand. Trop. Grassl-Forrajes. 7 (2), 193–199. doi: 10.17138/tgft(7)193-199
Uzal, F. A., Navarro, M. A., Li, J., Freedman, J. C., Shrestha, A., McClane, B. A. (2018). Comparative pathogenesis of enteric clostridial infections in humans and animals. Anaerobe 53, 11–20. doi: 10.1016/j.anaerobe.2018.06.002
Vandermeulen, S., Ramírez-Restrepo, C. A., Beckers, Y., Claessens, H., Bindelle, J. (2017). Agroforestry for ruminants: A review of trees and shrubs as fodder in silvopastoral temperate and tropical production systems. Anim. Prod. Sci. 58 (5), 767–777. doi: 10.1071/AN16434
Wang, C., Liu, Q., Meng, J., Yang, W., Yang, X., He, D., et al. (2009). Effects of citric acid supplementation on rumen fermentation, urinary excretion of purine derivatives and feed digestibility in steers. J. Sci. Food Agr. 89 (13), 2302–2307. doi: 10.1002/jsfa.3724
Wang, H., Ning, T., Hao, W., Zheng, M., Xu, C. (2016). Dynamics associated with prolonged ensiling and aerobic deterioration of total mixed ration silage containing whole crop corn. Asian-Austral. J. Anim. Sci. 29 (1), 62–72. doi: 10.5713/ajas.15.0319
Wang, Z., Ning, P., Hu, L., Nie, Q., Liu, Y., Zhou, Y., et al. (2020). Efficient ethanol production from paper mulberry pretreated at high solid loading in fed-nonisothermal-simultaneous saccharification and fermentation. Renew. Energ. 160, 211–219. doi: 10.1016/j.renene.2020.06.128
Wang, Y., Wu, K., Zhen, Z., Shi, Z., Yang, H., Zhang, J., et al. (2018). Combined pretreatment with NaOH and fenton of mulberry wood to enhance enzymatic digestibility for biofuels. J. Biobased. Mater. Bio. 12 (1), 65–75. doi: 10.1166/jbmb.2018.1734
Wang, Y., Zhang, Y., Li, J., Lin, J., Zhang, N., Cao, W. (2021). Biogas energy generated from livestock manure in China: Current situation and future trends. J. Environ. Manage. 297, 113324. doi: 10.1016/j.jenvman.2021.113324
Wen, P., Hu, T., Linhardt, R. J., Liao, S., Wu, H., Zou, Y. (2018). Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Tech. 83, 138–158. doi: 10.1016/j.tifs.2018.11.017
Wu, Z., Liang, C., Huang, R., Ouyang, J., Zhao, L., Bu, D. (2022). Replacing alfalfa hay with paper mulberry (Broussonetia papyrifera L.) silage in diets do not affect the production performance of the low lactating dairy cows. Anim. Feed. Sci. Technol. 294, 115477. doi: 10.1016/j.anifeedsci.2022.115477
Wu, Y., Xu, Y., Lau, A. T. Y. (2021). Anti-cancer and medicinal potentials of moringa isothiocyanate. Molecules 26 (24), 7512. doi: 10.3390/molecules26247512
Xiong, Y., Guo, C., Wang, L., Chen, F., Dong, X., Li, X., et al. (2021). Effects of paper mulberry silage on the growth performance, rumen microbiota and muscle fatty acid composition in Hu lambs. Fermentation 7 (4), 286. doi: 10.3390/fermentation7040286
Yang, S. L., Yang, R. C., Zhou, X., Yang, S. H., Luo, L. L., Zhu, Y. C., et al. (2020). Effects of feeding diets with processed Moringa oleifera stem meal on growth and laying performance, and immunological and antioxidant activities in laying ducks. Poultry. Sci. 99 (7), 3445–3451. doi: 10.1016/j.psj.2020.04.002
Zhang, Y. C., Li, D. X., Wang, X. K., Lin, Y. L., Zhang, Q., Chen, X. Y., et al. (2019). Fermentation dynamics and diversity of bacterial community in four typical woody forages. Ann. Microbiol. 69 (3), 233–240. doi: 10.1007/s13213-018-1398-z
Zhang, Y., Ni, S., Wu, R., Fu, Y., Qin, M., Willför, S., et al. (2022). Green fractionation approaches for isolation of biopolymers and the critical technical challenges. Ind. Crops. Prod. 177 (4), 114451. doi: 10.1016/j.indcrop.2021.114451
Keywords: animal product, natural biomass resource, silage fermentation, sustainable livestock production, woody plant
Citation: Du Z, Yang F, Fang J, Yamasaki S, Oya T, Nguluve D, Kumagai H and Cai Y (2023) Silage preparation and sustainable livestock production of natural woody plant. Front. Plant Sci. 14:1253178. doi: 10.3389/fpls.2023.1253178
Received: 05 July 2023; Accepted: 23 August 2023;
Published: 08 September 2023.
Edited by:
Siran Wang, Nanjing Agricultural University, ChinaReviewed by:
Mao Li, Chinese Academy of Tropical Agricultural Sciences, ChinaCopyright © 2023 Du, Yang, Fang, Yamasaki, Oya, Nguluve, Kumagai and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yimin Cai, Y2FpQGFmZnJjLmdvLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.