- 1School of Ecology and environment, Tibet University, Lhasa, Tibet, China
- 2Institute of Fisheries Science, Tibet Academy of Agricultural and Animal Husbandry Sciences, Lhasa, Tibet, China
- 3School of Pharmacy, Fudan University, Shanghai, China
- 4Wolfson College, Oxford University, Oxford, United Kingdom
- 5Institute of Biomedical and Environmental Science & Technology, School of Life Sciences, University of Bedfordshire, Luton, United Kingdom
- 6School of Life Sciences, Shanxi University, Taiyuan, China
Ephedra is an important plant in Chinese medicine; however, there are few reports on two species of Ephedra which are distributed at high altitudes from 3000 to 5200 meters. We collected a total of 84 individuals representing five Ephedra gerardiana and nine Ephedra saxatilis populations respectively located from 3158 to 5200 meters altitude, and determined the relative content of 213 metabolites using UHPLC-MS/MS (Ultra-High-Performance Liquid Chromatography-tandem mass spectrometry). 37 Chemical compositions were annotated using the KEGG (Kyoto Encyclopaedia of Genes and Genomes) database. From the top five significant enrichments in metabolic KEGG pathway analysis, we found a total of 166 compounds belonging to phenylpropanoids, 123 flavonoids, 67 metabolites carried by ABC transporters, and 61 in purine metabolism. We identified the top 8 altitude-related compounds in two species. Ephedrine and pseudoephedrine were found to be associated with altitude in both E. saxatilis and E. gerardiana. To verify which environmental factors influenced the metabolic content, the soil moisture and temperature of each population site were collected, and quantitative analysis of ephedrine and pseudoephedrine was performed using UHPLC-MS (Ultra-High-Performance liquid chromatography-tandem mass spectrometry). After detection, soil moisture ranged from 0.074 to 0.177 mm3/mm3, and temperature ranged from 9.7°C to 23.9°C. The content of ephedrine ranged from (0.84 ± 0.49)% to (2.01 ± 0.41)% in E. saxatilis, which was positively correlated with soil moisture; the content of pseudoephedrine ranged from (0.72 ± 0.45)% to (1.11 ± 0.57)% and was negatively correlated with soil moisture. In contrast to these results, in E. gerardiana, the content of ephedrine and pseudoephedrine was negatively correlated with soil moisture. Furthermore, the trends of alkaloid contents in two kinds of Ephedra were similar when the temperature was lower than 17°C even if the sum was various. With the increase in soil moisture and temperature, the total alkaloid content of E. saxatilis was higher than that of E. gerardiana. When the soil moisture was lower, the alkaloid content of the two Ephedra species was higher. These results provide useful data for the future separation of new compounds, and for seed homogeneous growth to determine artificial breeding of Ephedra located at high altitudes.
1 Introduction
Ephedra is an important resource in Chinese medicine (Abourashed et al., 2003), used to treat respiratory diseases. The rich medicinal contents of Ephedra have become one of the important Chinese medicinal materials for import and export (Hayashi et al., 2019). Almost all Ephedra plants contain ephedrine and pseudoephedrine. Pseudoephedrine has antipyretic and analgesic effects. It can be used to relieve the symptoms of a cold. Ephedrine is an adrenergic receptor agonist and is commonly used in subarachnoid anesthesia (Minami et al., 2020). As its central nervous system stimulatory effects, ephedrine is the main substance in the quality control of drugs containing Ephedra (Kondo et al., 1999; Minami et al., 2020). Ephedrine and pseudoephedrine are isomers with the same formula C10H15NO, and they are members of phenethylamine alkaloids (Tian et al., 2022).
About 300 chemical compounds, including alkaloids, volatile oils, flavonoids, polysaccharides, simple phenylpropanins, condensed tannins, organic acids, and sterols have been found in these plants, using traditional separation methods, such as alcohol extraction, water extraction and other methods for extraction, UPLC (High Performance Liquid Chromatography) and other methods for separation (Tian et al., 2022). There are still many components in Ephedra that have not been identified, so the identification of Ephedra by conventional means is time-consuming and labor-consuming, and the method of metabolomics can effectively identify and shorten the identification time (Hong et al., 2011). Most evaluation studies have been limited to pharmacodynamic chemical compounds of wild Ephedra plants (Zhang et al., 2018; Tian et al., 2022), and there are few reports on chemical compositions in two kinds of Ephedra distributed at altitudes over 3000 m, namely, E.saxatilis and E. gerardiana, which are used as Tibetan medicinal plants. We therefore explored the question of whether the metabolite levels of Ephedra change under the influence of altitude. It is generally accepted that the extreme environment at high altitudes is an important factor affecting the medicinal substances of plants (Zare-Maivan et al., 2014). Most research focuses on the evolutionary origin of this plant species (Wang and Zheng, 2010; Puebla et al., 2017). Most species of Ephedra are erect or sprawling shrubs and grow at a wide range of altitude areas, from near sea level to 5000 m (Thompson, 1912; Thompson, 1918; Carlquist, 1988). Qin et al. (2013) studied the differentiation and phylogeographic history of Ephedra plants in southern Tibet. Most of the Ephedra species have low intraspecific variation and lack a strong phylogeographic structure, possibly due in part to clonal reproduction and relatively recent origin. Ephedra is one of the plants with strong drought and/or cold tolerance in arid and semi-arid areas of Tibet (Wu et al., 2016; González-Juárez et al., 2020), and the coverage rate of Ephedra populations in high-altitude areas of Tibet is lower than that in other areas of China due to perennial water shortage and drought (Wu et al., 2016). Therefore, the physiological and biochemical changes of Ephedra with changes of in altitude have not been reported. This study aims to explain the response mechanism of Ephedra to altitude gradient by metabolic level.
There are a total of 6 species of Ephedra found in Tibet, among which E. saxatilis is endemic to Tibet, and E. gerardiana is widely distributed on the Tibetan plateau over 4000 m altitude (Qin et al., 2013). These two species are typical gymnosperms of high altitudes. Due to extreme environmental stress (Strong ultraviolet light, large temperature difference between day and night, and extreme drought) at the high altitude of the Tibetan plateau, these two species are limited for exploitation and utilization (Hong et al., 2011). With the increase of altitude, environmental factors also affect the plant growth, which also provides ideas for the potential metabolite changes of Ephedra at high altitudes with drought and low temperature.
However, the chemical composition of both species and how their contents change with altitude and different environments is still unknown. Since ephedrine and pseudoephedrine are the main effective components, the content of these two substance were chosen to reveal the above problem. It is important to determine whether their contents in Ephedra vary with altitude and whether soil moisture and temperature affect the contents. In this study, metabolite and alkaloid contents of 14 different altitude populations of E. saxatilis and E. gerardiana were analyzed, and the effects of soil moisture and temperature at different altitudes on the chemical composition and alkaloid contents of ephedrine were analyzed. This will provide an important basis for the artificial cultivation of Ephedra.
2 Experimental procedures
2.1 Plant material and environment data collection
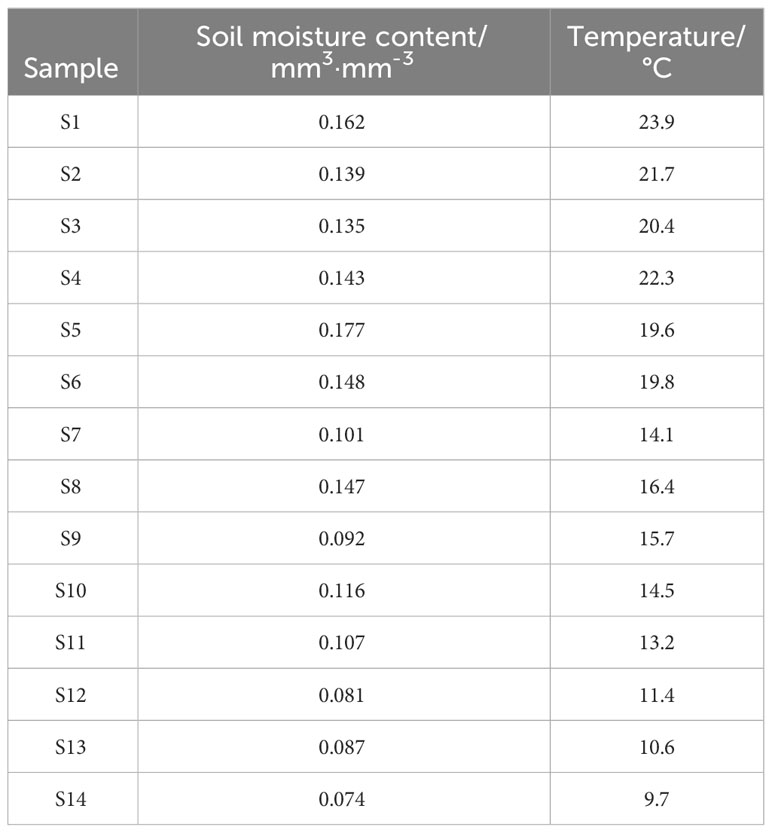
A total of 84 individuals were collected between June and July 2017 and 2018, representing five E. gerardiana populations, and nine E. saxatilis populations, respectively located from 3158 to 5200 meters altitude on the Tibet plateau (Figure 1; Table S1). Six samples were selected from each community, and a total of 84 samples were tested. The species of Ephedra were identified by Professor La Qiong, Tibet University. The soil moisture and temperature were measured automatically by inserting a soil measuring thermometer into the soil at 5 cm. The age of the Ephedra plants in the growing area was unknown, but the size of the above-ground part of the selected Ephedra specimens was the same (about 10-20 cm in diameter and 10-20 cm in height), and the overall shape of the above-ground part was close to the dome. For metabolic profiling, six above-ground plant individuals in each population were collected at the flowering period without regard to plant sex and age. To analyze the correlation between metabolite content and environmental factors, meteorological factors including air temperature, soil moisture, atmospheric pressure, and rainfall (Table S2) were collected from World Weather online (https://www.cma.gov.cn/). Soil moisture ranged from 0.074 mm3·mm-3 to 0.17 mm3·mm-3 (Table 1), and temperatures ranged from 9.7°C to 23.9°C (Table 1). The altitude, longitude, and latitude were measured using GPS (Global Positioning System). All Ephedra were stored in the refrigerator at -20°C after picking. The samples were freeze-dried after transfer to the laboratory. Freeze-drying condition: (600pa, -20°C).
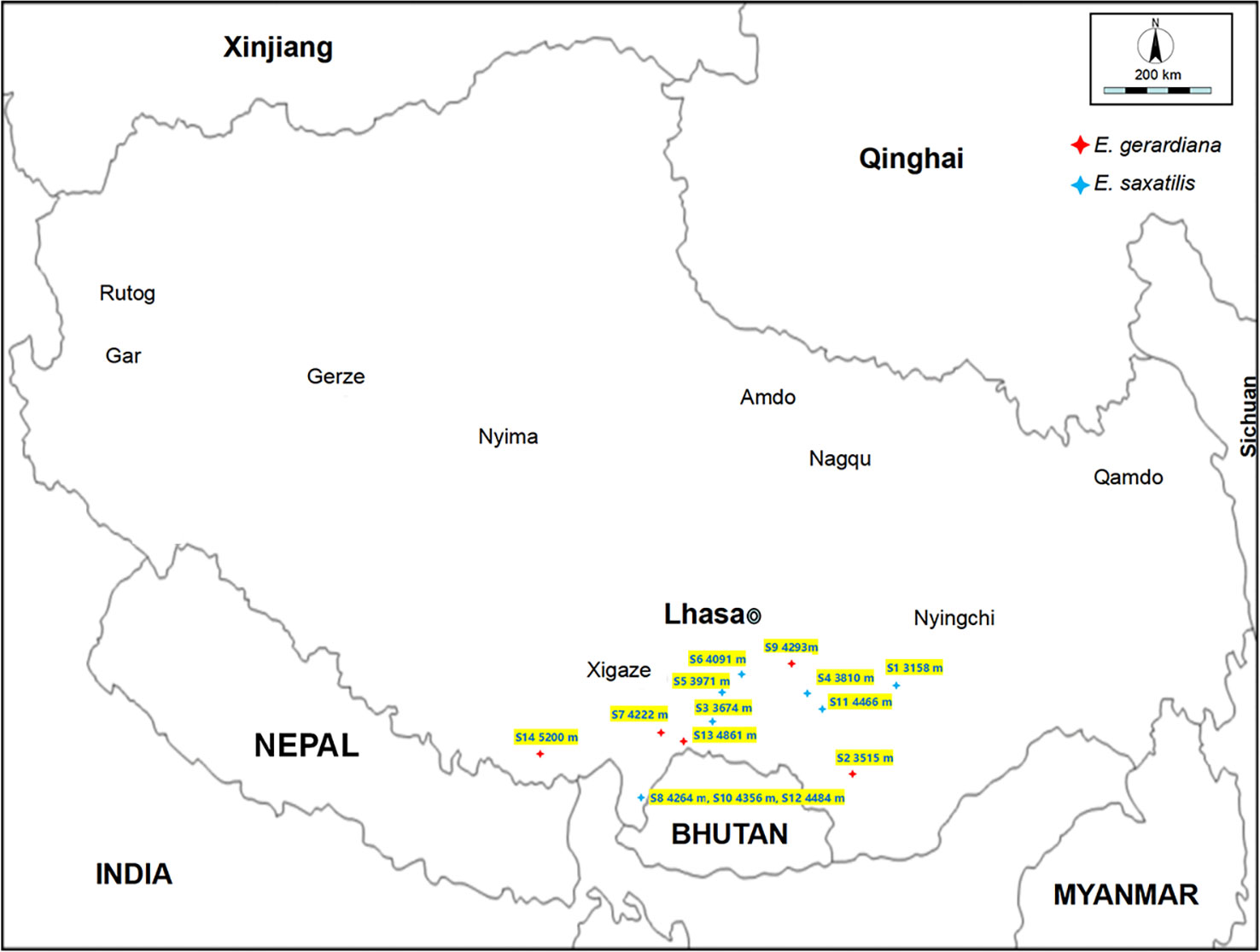
Figure 1 Map of altitude of the sample collection area. Ephedra collection site altitude and related information only represents the environmental information at that time.
2.2 Metabolite profiling and metabolomics
2.2.1 UHPLC-MSn method
Fifty milligrams of the dried, crushed, stems of Ephedra were subjected to 1.0 mL methanol-water (70%, v/v) with adding 20 μL 2-chloro-L-phenylalanine as internal standard, then was ultrasonically extracted for 30 minutes. The liquid was kept at -20°C for 20 min. After centrifuging for 10 min at 14000 × rpm (5000 × g) at 4°C, all Ephedra samples were mixed and prepared into QC, preparation conditions, and individual sample conditions, and one QC sample was inserted into every 10 analysis samples during instrumental analysis to examine the repeatability of the entire analysis process. (Blank injection: 1.0 mL methanol-water (70%, v/v) with adding 20 μL 2-chloro-L-phenylalanine as internal standard) (Ren et al., 2022).
200μL extracts were analyzed by LuYan at Fudan University using a Thermo Scientific Dionex Ultimate 3000 UHPLC system with an LTQ Velos Pro Linear Ion Trap Mass Spectrometer (Thermo Fisher Scientific, USA). The analytical conditions were as follows; UHPLC: column, ACE Excel C18 (100 x 3.0mm; Column pore: 3 mm; Particle size: 1.5 μm; Manufacturer: Waters); solvent A, ultra-pure water (Millipore, MA, USA) with 0.1% formic acid, and solvent B, acetonitrile. The gradient program (time(min), B) was as follows, (0.00, 5%); (5.00,10%); (10.00, 10%); (30,25%); (46,55%); (48,100%); (52,100%); (53,5%) and 5min for column equilibration before each injection. The column oven was set to 30°C; the flow rate was 0.3mL min-1, and the injection volume was 5μL. The detection wavelength was 254 nm by DAD (Diode Array Detector). One QC sample was injected between 10 samples for testing stability and repeatability.
2.2.2 MS parameters
The mass spectrometer was operated in both ESI positive and negative modes. The linear Ion Trap (LTQ) scan was acquired as a full MS1 scan followed by a data-dependent scan with a mass range of m/z 110–2000. The ESI source operation parameters were as follows: heater temperature, 350°C, capillary voltage, 35V; ion spray Voltage Floating, 3.5 KV; sheath gas flow rate, 40 psi; aux gas flow rate, 10 psi. MRM: Full MS resolution was 70000, and MS/MS resolution was 17500. Data acquisition was performed with the Data Dependent Acquisition (DDA) mode. The detection was carried out over a mass range of 70-1050 m/z. The data collection and analysis were performed using Thermo Xcalibur 2.2 software.
2.2.3 Annotation
After the mass spectrometry detection was completed, qualitative analysis of the metabolite data was analyzed. The raw data of LC/MS was preprocessed for peak picking (Thermo Fisher Scientific™), after date transmission, and alignment using progenesis QI (Thermo) software. The internal standard peaks, as well as any known false positive peaks, including noise, column bleed, and derivatized reagent peaks were removed and the raw signal was smoothed to remove fluctuations. The MS/MS of metabolite peak matching was proposed by the MassFragment™ application manager (Waters Corporation, Milford, USA) and identified using biochemical databases, including KEGG, the Human Metabolome Database (HMDB), and METLIN. The response intensity of the sample mass spectrum peaks was normalized to reduce the errors caused by sample preparation and instrument instability, and the variables of relative standard deviation (RSD)>30% of QC samples were removed using a Majorbio cloud platform (https://cloud.majorbio.com) (Ren et al., 2022).
2.2.4 Preparation of samples and quantitative analysis of ephedrine and pseudoephedrine content annotation
Extraction of ephedrine alkaloids and analysis was performed as described by He et al. (2019) and Zhu et al. (2009) with some modifications. Two hundred grams of Ephedra powder was weighed and extracted with 20ml pure methanol containing 1.44% phosphoric acid solution (1:1). After boiling for 2-3 min, it was ultrasonically treated for 30 min. Following centrifugation at 5000 r/min (1800 × g) for 10 min, the supernatant was absorbed and filtered through a microporous membrane (Microporous filter membrane washed with ultra-pure water) with a 0.22 µm pore size for analysis of ephedrine and pseudoephedrine contents using HPLC. The analytical conditions were as follows, HPLC: column, XTERRA® henyl (4.6 mm×250 mm, 5µm); solvent system, methanol (A): 0.092% phosphoric acid aqueous solution added with 0.04% triethylamine and 0.02% dibutylamine (B) (1.5:98.5,v/v); the flow rate was 0.5ml/min-1; column temperature: 30°C; injection volume: 10μl; the detection wavelengths were 210 nm(Chinese pharmacopeia, Ch.P2020 (Chinese pharmacopeia, Ch.P2020). Under these conditions, standard curves were constructed with different concentrations of standards of ephedrine and pseudoephedrine (purchased from https://www.zhzyw.com/).
Note: (total absolute amount of alkaloids/total relative amount of alkaloids) × relative amount parameter = sample alkaloids content.
2.3 Statistical analysis
Limma software in the R statistical package (http://www.r-project.org) was used for screening. Differential metabolites among groups were summarized and mapped into their biochemical pathways through metabolic enrichment and pathway analysis based on a database search (KEGG, http://www.genome.jp/kegg/). The data were analyzed through the free online platform of the majorbio cloud platform (cloud.majorbio.com). Ephedrine and pseudoephedrine statistical analyses were performed with SPSS 13.0 (SPSS Inc., Chicago, Illinois, US). The data were analyzed using a two-way analysis of variance (ANOVA) with treatment and time as factors. The means were separated by Duncan’s multiple range test, and differences at P < 0.05 were considered to be significant.
3 Results
3.1 Metabolic profiling of Ephedra, enrichment pathways, and the relationship contents of metabolism with altitude
We determined the relative content of 213 distinct metabolites (Table S3) using Ultra High Pressure Liquid Chromatography-tandem mass spectrometry (UHPLC-MS). 37 chemicals were annotated by the KEGG database (Figure S1A). 199 metabolites were annotated by the HMDB database, including prenol lipids (22.68%), Glycerophospholipids (20.62%), steroids and steroid derivatives (14.43%), fatty acyls (8.76%), carboxylic acids and derivatives (4.64%) (Figure S1B). From the top five significant enrichments of metabolic KEGG pathway analysis, we found a total of 166 compounds belonging to phenylpropanoids, 123 compounds of flavonoids, 67 metabolites carried by ABC transporters, and 61 of purine metabolism (Figure S2A).
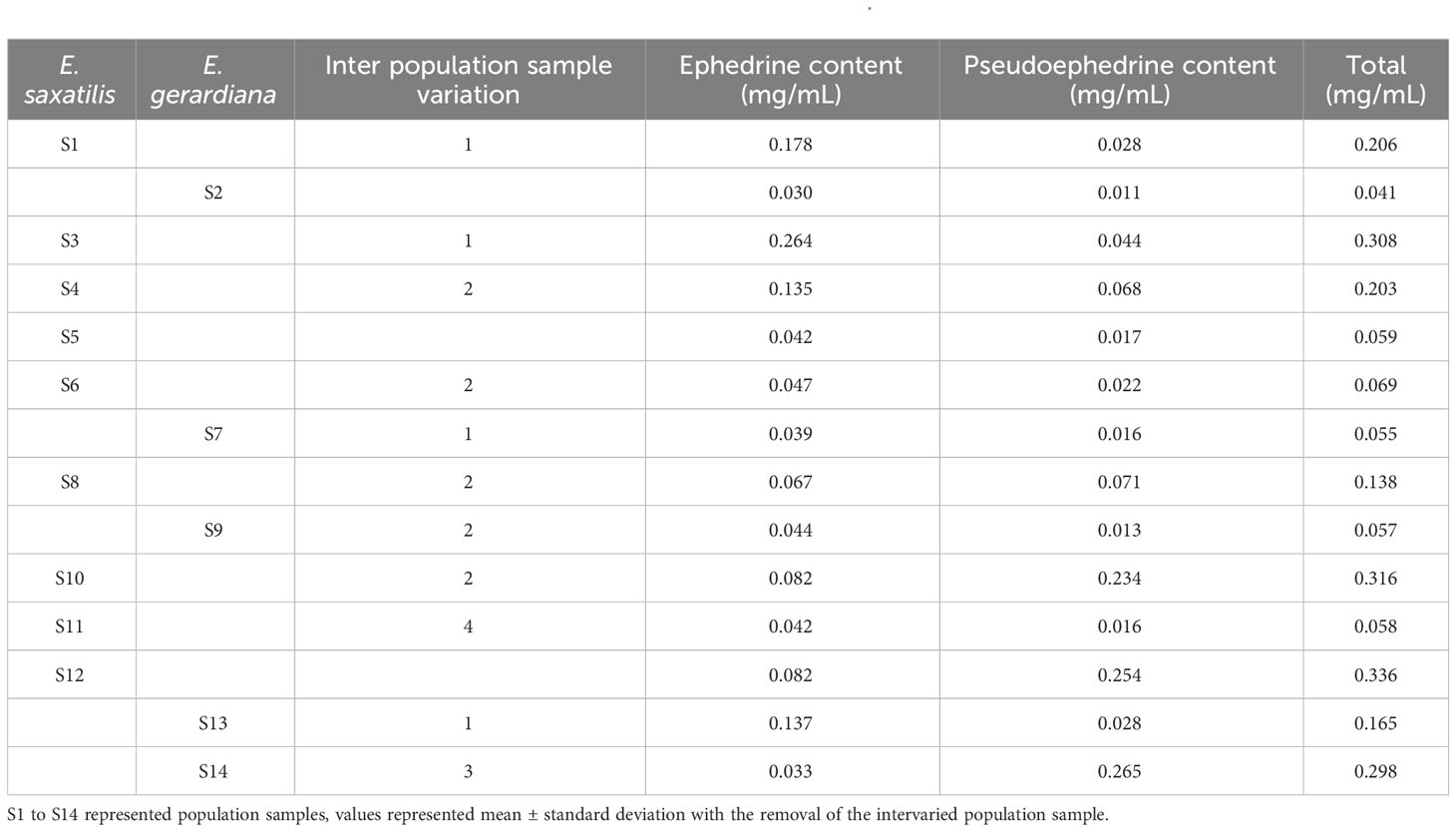
The relationships between relative content of metabolism and altitude showed that eight compounds in E. saxatilis, including butylbis amine, dihydrokaempferol, ferulic acid, pseudoephedrine, ethyl-N-propylamine, ephedrine, palatinitol and L-epicatechol, had significant variations with altitude (Figure 2A) and eight compounds in E.gerardiana, including L-epicatechol, pseudoephedrine, sinapic acid, salicylic acid, ethyl-N-propylamine, dihydrokaempferol, butylbis amine, and ephedrine, had significant correlation with altitude (Figure 2B).
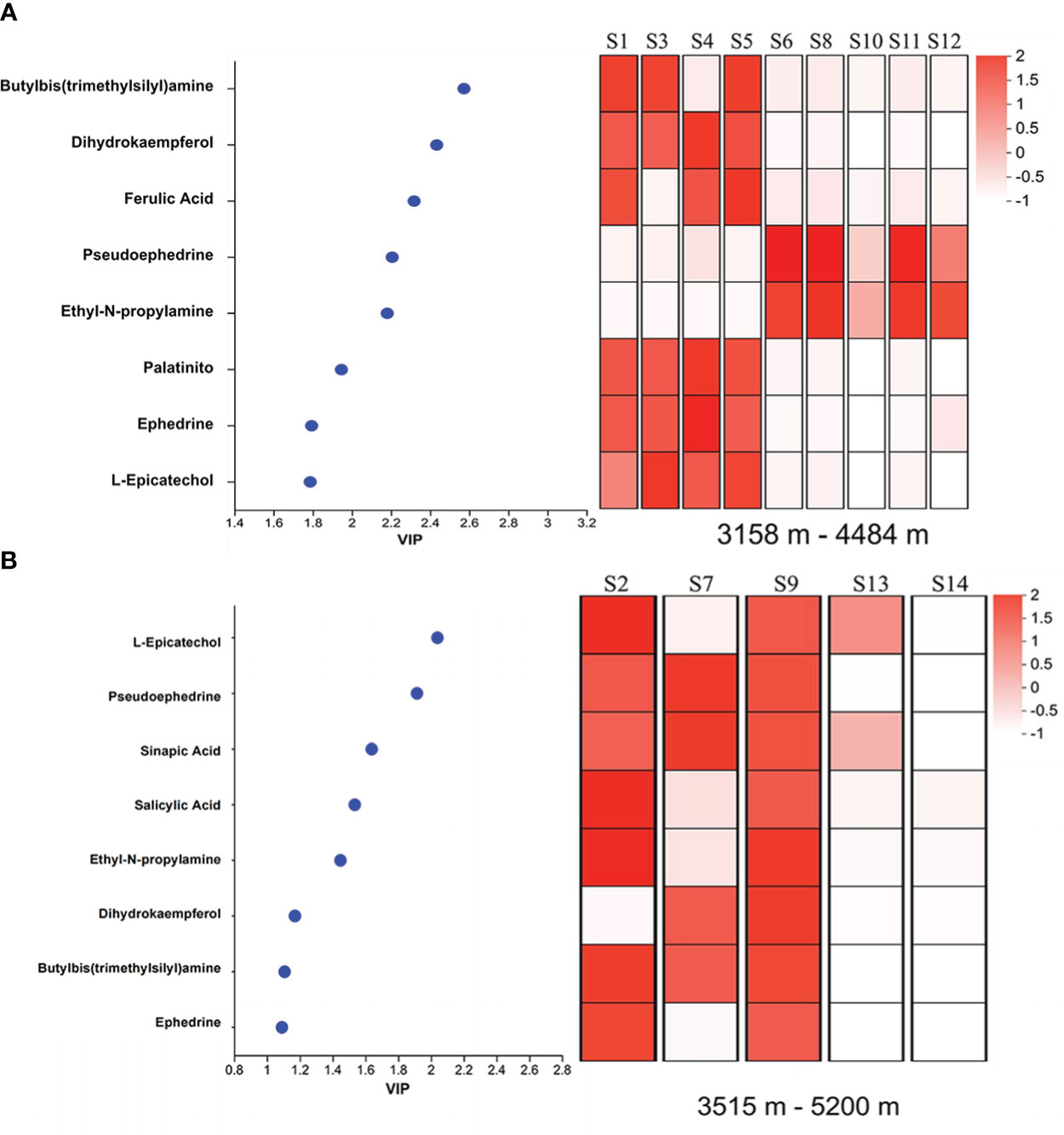
Figure 2 Correlation analyses of metabolites belonging to the phenylpropanoids pathway with altitude. (A) shows E. saxatilis and (B) shows E. gerardiana. VIP, Variable Independent Parameters. Each grid in the figure represents the correlation between samples, and each row represents a metabolite. The colors in the figure indicate the relative expression of metabolites in this set of samples. For the specific trend of expression, refer to the number under the color bar at the top right. Different colors indicate the relative magnitude of the correlation coefficient between the samples. The larger the VIP value, the more significant the difference between the samples. The overall hierarchical clustering diagram in the figure normalized the enrichment values and clustered them, with red representing high metabolic enrichment and white representing low metabolic enrichment; Color from red to white, enrichment degree from large to small. The default VIP value > 1 (P < 0.05), and the larger the VIP value, the more significant the direct difference between metabolites.
3.2 Composition and divination of various ephedrine contents in samples
We used UHPLC for the determination of ephedrine and pseudoephedrine. Correlation analysis was performed according to environmental factors. Among 84 individuals of 14 population samples, the content of ephedrine and pseudoephedrine in the 6 replicated samples varied. The percentage contents of ephedrine, pseudoephedrine, and total alkaloids in each sample community are shown in Table 2. The content of ephedrine and pseudoephedrine in E. saxatilis ranged from 0.264 mg/mL to 0.042 mg/mL, and from 0.254 mg/mL to 0.017 mg/mL, respectively, and in E. gerardiana ranged from 0.137 mg/mL to 0.030 mg/mL, and from 0.265 mg/mL to 0.011 mg/mL, respectively. The total content of these two compounds ranged from 0.336 mg/mL to 0.058 mg/mL in E. saxatilis, and E. gerardiana ranging from 0.298 mg/mL to 0.041 mg/mL.
3.3 Variation of the sum of ephedrine and pseudoephedrine in E. saxatilis and E. gerardiana with soil temperature and moisture
The metabolome studies showed that the contents of ephedrine and pseudoephedrine in these two species were correlated with altitude. To verify which factors influenced the content of the major compounds of Ephedra species, the local climate factors and soil moisture were collected. The correlation of the content of ephedrine and pseudoephedrine with soil moisture was analyzed. The results showed that with an increase in soil moisture and temperature, the sum of ephedrine and pseudoephedrine in E. saxatilis first decreased, then increased again, and repeatedly decreased, increased, and finally decreased. In contrast, the contents of E. gerardiana decreased with the increase of soil moisture and temperature (Figure 3).
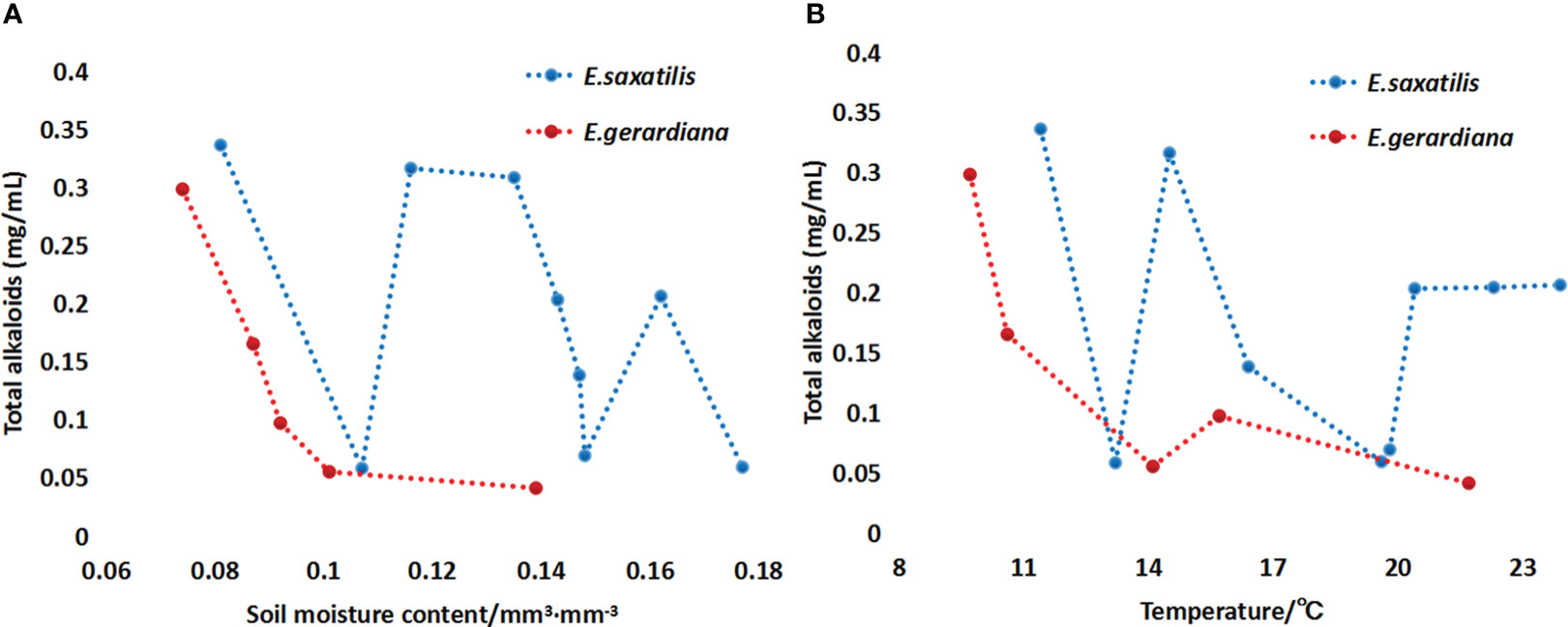
Figure 3 Variation the sum of ephedrine and pseudoephedrine in E. saxatilis and E. gerardiana with soil mositure (A) and temperature (B). Total alkaloid on the vertical is the sum of ephedrine and pseudoephedrine content. The 9 blue dots represent samples collected from 9 populations of Ephedra in E. saxatilis. The content of ephedrine and pseudoephedrine in 6 individuals from each population was determined, respectively, and the average value is shown by a dot. Similarly, the five red dots represent the sum of ephedrine and pseudoephedrine in the five E. gerardiana populations. The vertical line on figure in the histograms indicates the SD.
3.4 Variation trend of ephedrine and pseudoephedrine in E. saxatilis and E. gerardiana with soil moisture
The contents of ephedrine and pseudoephedrine were higher in E. saxatilis than in E. gerardiana. To reveal the influence of soil moisture on the content of these two alkaloids, a correlation analysis was performed. The results showed that the content of ephedrine in E. saxatilis was not correlated with soil moisture, while the content of pseudoephedrine was negatively correlated with soil moisture. In contrast to the results above, in E. gerardiana, the content of ephedrine was negatively correlated with soil moisture, while the content of pseudoephedrine was also negatively correlated with moisture (Figure 4).
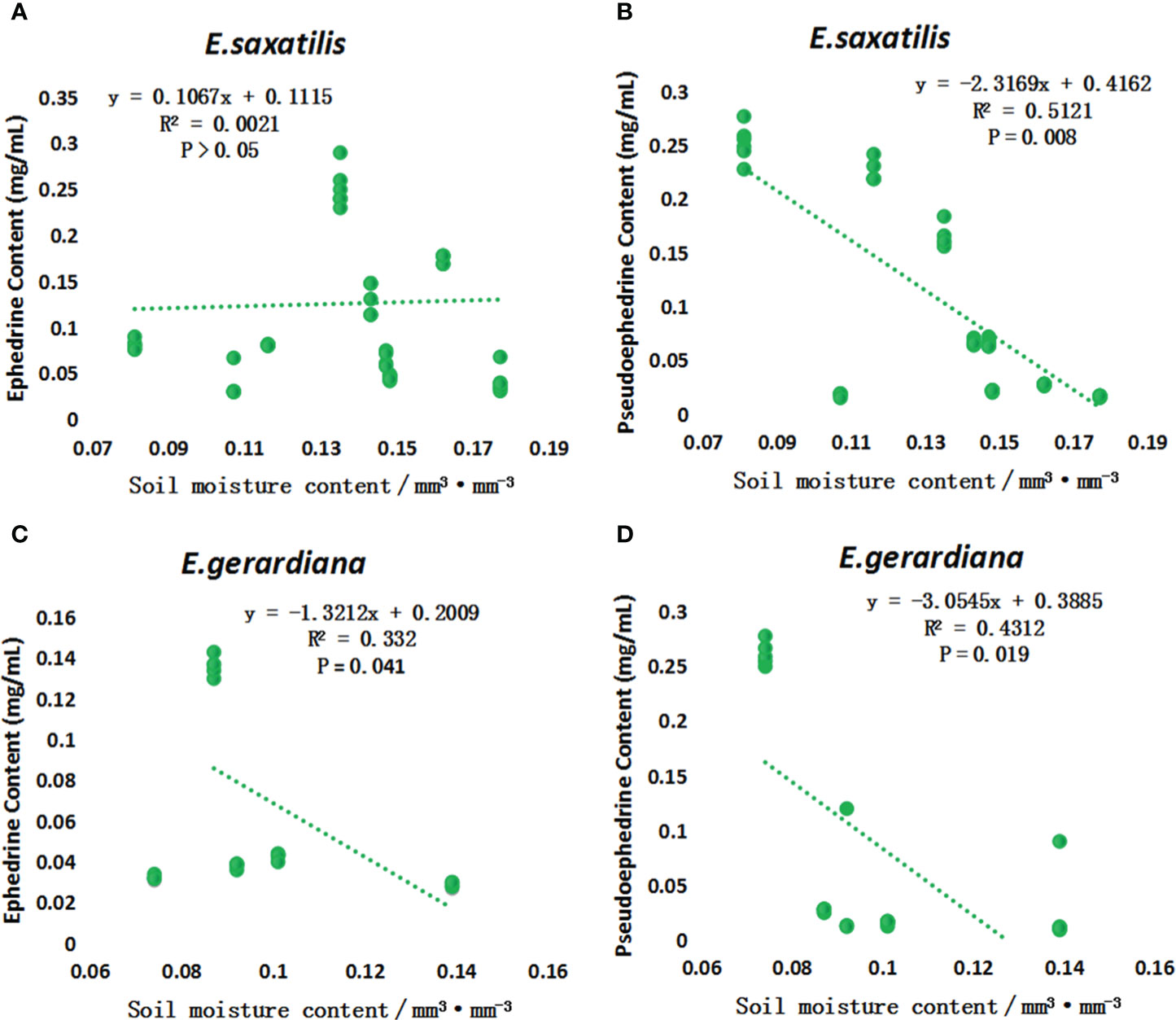
Figure 4 The variation of ephedrine and pseudoephedrine content in E. saxatilis and E. gerardiana with soil moisture. Variation of ephedrine in E. saxatilis with soil moisture (A), Variation of pseudoephedrine in E. saxatilis with soil moisture (B). Variation of ephedrine in E. gerardiana with soil moisture (C), Variation of pseudoephedrine in E. gerardiana with soil moisture (D). The value of each point represents the mean value.
3.5 Variation trend of ephedrine and pseudoephedrine in E. saxatilis and E. gerardiana with temperature
The correlation of the content of ephedrine and pseudoephedrine with temperature was analyzed. The results showed that ephedrine showed a significant upward trend with temperature in E. saxatilis (P < 0.05) (Figure 5A). Ephedrine showed a significant decreasing trend with temperature in E. gerardiana (P < 0.05) (Figure 5C). Pseudoephedrine showed a decreasing trend in both species of ephedrine, the decrease in temperature was significant only in E. saxatilis (P < 0.05) (Figure 5B). Only the trend relationship in Figure 5D is not significant.
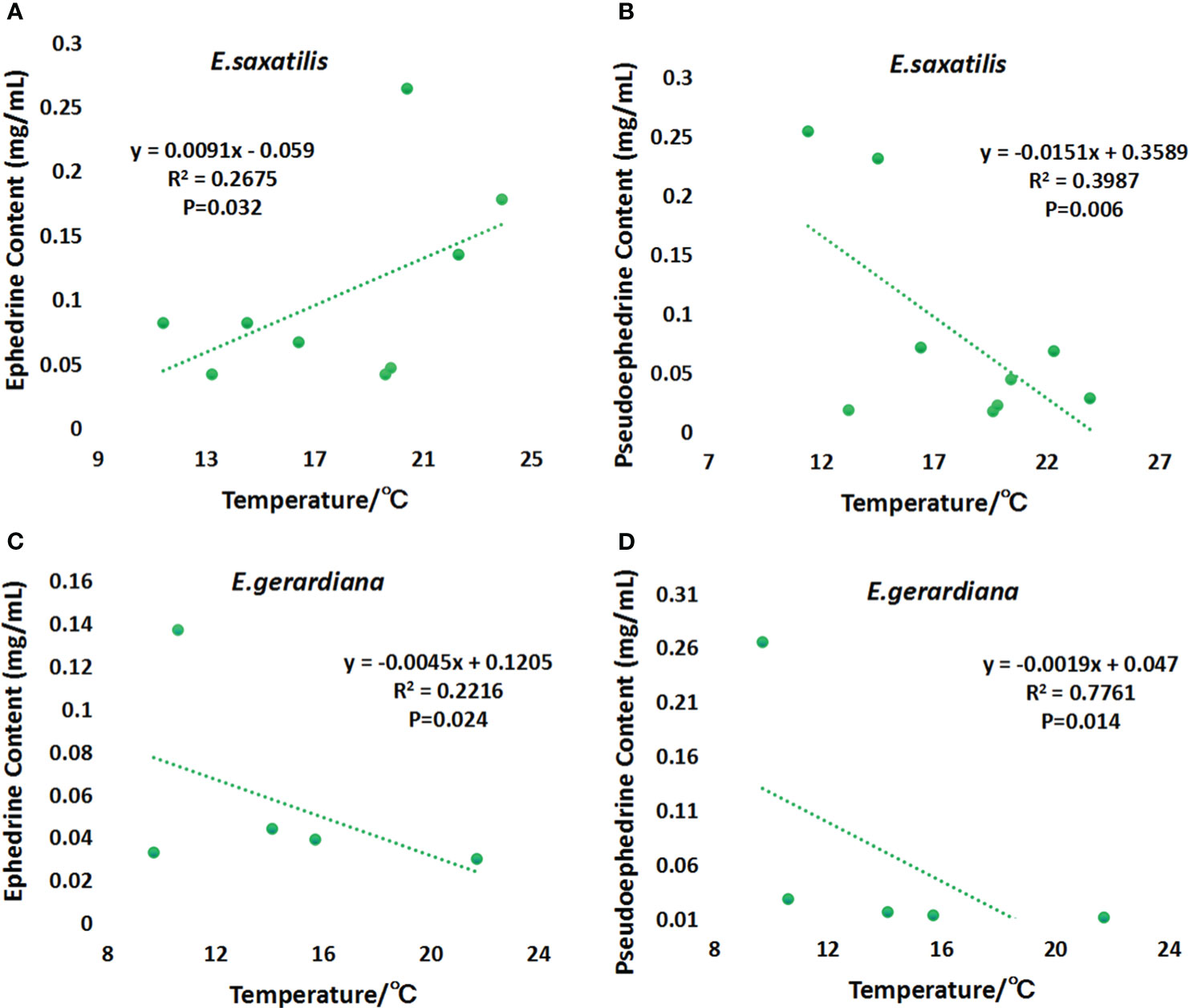
Figure 5 Variation of ephedrine and pseudoephedrine with soil temperature. Variation of ephedrine in E. saxatilis with temperature (A), Variation of pseudoephedrine in E. saxatilis with temperature (B). Variation of ephedrine in E. gerardiana with temperature (C), Variation of pseudoephedrine in E. gerardiana with temperature (D). The value of each point represents the mean value.
4 Discussion
4.1 Collection environment
The spatial differentiation of the Qinghai-Tibet Plateau ecosystem is mainly determined by the high altitude (Xie et al., 2019). Temperature and precipitation decrease in the northwest, with a warm and humid climate in the southeast and a cold and dry climate in the northwest (Chang, 1981; Chang, 1983). The diverse environmental states at different altitudes lead to different characteristics in vegetation ecosystems. Even for plants in different locations in the same region, there are differences of soil moisture and temperature (Minami et al., 2021). In addition, Tibet’s water reserves are also affected by global warming, and water resources are becoming more abundant (Chu et al., 2018; Fu et al., 2019). The shifts in temperature and soil moisture cause the same plant to produce divergent metabolism (Xiao et al., 2014). Considering the species of E. saxatilis and E. gerardiana distributed at altitudes from 3,200 m to 5,200 m, the habitat in which they grow varies from humid to arid. Therefore, it can be inferred that their metabolites, including ephedrine and pseudoephedrine, will change with altitude. To reveal which metabolites of Ephedra are associated with altitude, the samples were collected from different altitudes, and analyzed the correlation metabolism with soil moisture and temperature.
4.2 Ephedrine alkaloid in relation to soil temperature and moisture
In this study, 213 compounds with annotated information were found. However, due to the limitations of the metabolome methods, there are still many compounds that have been reported in the literature, that were not detected in this study. Another reason for these undetected compounds may due to that the plant of E. saxatilis and E. gerardiana in this study is quite different from other Ephedra species that have been reported. There are still many compounds that have not been annotated, which will be our next research direction. Different temperatures can lead to changes in plant genes, as well as changes in plant metabolites. Some plants’ adaptation to temperature is regulated by internal metabolites. For example, 5-Hydroxybuspirone tends to increase with increasing temperature (Ito et al., 2022). Drastic temperature fluctuations caused by climate change adversely affect plant growth and threaten crop productivity. Uncovering metabolites in the plant immune system that are themselves protected from temperature stress is not only a key fundamental question, but also crucial for agricultural sustainability and food security. Ergosterol peroxide can enhance the drought-resistant ability of plants (Ding and Yang, 2022). Ito et al. (2022) found that poor fruit set and growth were caused by temperature stress in tomato cultivation. Low-temperature stress in spring is an abiotic stress limiting the growth and productivity of winter wheat (Li et al., 2022). The photosynthetic characteristics and tolerance of peony decreased at high temperatures, damaging the photosynthetic capacity of peony leaves and their photosynthetic mechanism (Ji et al., 2022). As shown in Figure 3 of this study, with the increase in soil temperature, the sum of ephedrine and pseudoephedrine content fluctuated in E. saxatilis, such as an initial decrease, and then an increase, followed by a decrease again, while in E. gerardiana it decreased with soil temperature. This means that the influence of soil temperature on the total ephedrine content for these two kinds of Ephedra was different. As shown in Figure 5, in E. saxatilis, the relative content of ephedrine increased with soil temperature increasing(s (P < 0.05) (Figure 5A), while the content of pseudoephedrine decreased (P < 0.05) (Figure 5B). The relative content of ephedrine and pseudoephedrine deceased with soil temperature (Figures 5C, D). From this result, we suggest that the increase of temperature accelerates the synthesis of ephedrine, while the synthesis of pseudoephedrine decreases in E. saxatilis. However, it was not fit for E. gerardiana to synthesize these two alkaloids in response to higher soil temperature at high altitudes. It would be ideal to increase the sample size to explain this conclusion for E. gerardiana.
The above research provides us with ideas to enhance ephedrine content. In the further cultivation of large quantities of Ephedra, it would be important to regulate the temperature to ensure the stable content of ephedrine.
Under the stress of drought, high temperature, low temperature, salt and alkali, and toxic pollutants, important substances in plants will accumulate a large amount in cells, which makes these organisms show amazing adaptability to harsh environments (Stracke et al., 2020). Soil moisture is the main factor in plant growth, vegetation restoration, and plant ecology (Sheffer et al., 2020). As shown in Figure 3, soil moisture influenced the ephedrine and pseudoephedrine content; the content of ephedrine in E. saxatilis increased while the content of pseudoephedrine decreased. In this study, the results showed that with increasing soil moisture and temperature, the sum of ephedrine and pseudoephedrine content fluctuated in E. saxatilis, while in E. gerardiana it decreased with soil temperature. In contrast, the contents in E. gerardiana decreased with an increase in soil moisture and temperature. Similar results have been reported on E. gerardiana, Minami et al. (2022). This provides a reference for the changes of alkaloids of the two Ephedra species in this study. Changes of chemical compositions in plants with soil moisture have also been found in other plants, but the trends were different, such as the contents of two alkaloids, galanthamine and lycorine of Lycoris aurea (L. aurea) were increased due to drought stress (Quan and Liang, 2017; Liang et al., 2020). Plant growth and metabolism are affected by temperature, where the differences among species play an important role. Different plants produce different metabolites in response to soil temperature. For example, in carrots, the higher the temperature, the lower the sucrose level (Abreha et al., 2021). The mechanism for variations of metabolisms due to the genotype and the genetic variations of the plant, such as potato, was stressed by water deficit at different altitudes. Metabolic change was due to morphology, and physiological change under drought stress, such as reported in Chickpea (Gökmen and Ceyhan, 2015) and Sorghum (Abreha et al., 2021). According to this research and reported in other plants, we demonstrated that the content of alkaloid changes in Ephedra possibly caused by changes in environmental factors, such as soil moisture and temperature. It can be found that the different species of Ephedra response to altitude was different, which may be because of their adaptation metabolism different.
Ephedrine and pseudoephedrine have been the main substances used for the quality identification standards for Ephedra. Other compounds belonging to phenylpropanoid pathways, such as palatinitol, Ethyl-N-propylamine, Butylbis (trimethylsilyl) amine, etc., were identified in this research, many of which have been effective in relaxing bronchial smooth muscle, and inhibit diplococcus pneumoniae (Li et al., 2012). There were also many other pharmacoactive substances in Ephedra, such as catechin, epicatechin, catechol, tetramethylpyrazine, etc., that have been reported (Tian et al., 2022). However, there are still many difficulties in determining their efficiency, because it needs to isolate individual substances for pharmacodynamic evaluation. Our analyzed compounds provide ideas for screening pharmacodynamic substances and discovering new compounds.
4.3 Comparison of ephedrine content in different Ephedra species and quality evaluation
The variation of ephedrine and pseudoephedrine content in Ephedra growing in different environments is very important for the selection of suitable soil characteristics. (Minami et al., 2022). According to the Chinese Pharmacopoeia (ChP, 2020), the daily dose of Ephedra is 2-10 g. The total amount of ephedrine hydrochloride and pseudoephedrine hydrochloride in ephedrine should not be less than 0.8% in terms of alkaloid composition. Hong et al. (2011) systematically compared the content of ephedrine hydrochloride in E. equisetin (34 samples collected from 5 provinces in China), E. intermedia (23 samples collected from 4 provinces in China), and E. inica (7 samples collected from 1 province in China). It was found that the ephedrine alkaloid in E. equisetin was between 0.32% and 2.34%, so the quality of 76% of samples of this species meets the standard of the ChP, 2020. Furthermore, 78% of the samples of E. inica (the content of ephedrine alkaloid:0.41%-3.40%), and 100% of the samples of E. intermedia (the content of ephedrine alkaloid:1.43%-2.97%) met the quality standard of the ChP, 2020. It was reported that the total alkaloid content of E. gerardiana collected from three areas in Tibet met the quality standard of the Japanese Pharmacopoeia XVII (JPXVII) (Minami et al. (2022). Whereas, the quality standard for the content in the ChP (2020) is 0.1% higher than in JPXVII. In this research, the content of alkaloids in E. saxatilis was between 1.86% and 2.73%, including ephedrine and pseudoephedrine content ranged from 0.84% to 1.87%, and from 0.7% to1.1%, respectively. Similar to its results, the content of alkaloid in E. gerardiana was between 1.75% and 2.08%, including ephedrine and pseudoephedrine content ranging from 1.14%-to 1.84%, and from 0.24% to 0.61%, respectively. Besides, the content of ephedrine and pseudoephedrine in E. saxatilis and E. gerardiana also met the quality standard of the ChP, 2020. In comparison the content of ephedrine, there are little variations between Ephedra species, although without any statistical analysis. However, high altitude with water deficit may be more beneficial for Ephedra to produce more ephedrine.
The collected samples in this research were mainly distributed in southern Tibet, where exist differences in plant distribution, environment, and plant age. Therefore, we should further research by seeds for homogeneous growth to determine the content of Ephedra under different temperatures and moisture stress.
5 Conclusion
Ephedra is widely distributed in Tibet, and is used for Tibetan medicine, such as “Wu Wei Gan Lu Yu”. However, its shoots are particularly short, which is not conducive to the collection and development of medicinal materials. Ephedra saxatilis and E. gerardiana are the main species of Ephedra distributed gradient with altitudes above 3200 meters, in Tibet. Therefore, we selected these two species to determine the metabolism under variations in soil temperature and moisture. From the result, by correlation analysis for environmental factors with ephedrine and pseudoephedrine alkaloids, we found that the content of ephedrine alkaloids varied with temperature and soil moisture change. With the increase in soil moisture and temperature, the total alkaloid content of E. saxatilis was higher than that of E. gerardiana.
The ephedrine content in E. saxatilis ranged from 0.84% to 2.01%, the pseudoephedrine content ranged from 0.72%-1.11%, and the total alkaloid content ranged from 1.86% to 2.73%, while the soil moisture ranged from 0.081mm3/mm3 to 0.177mm3/mm3, and soil temperature ranged from 11.4°C to 23.9°C. In the same way, the ephedrine content in E. gerardiana ranged from 1.14% to 1.84%, pseudoephedrine content ranged from 0.24 mm3/mm3 to 0.61 mm3/mm3, and total alkaloid content ranged from 1.75% to 2.08%, when the soil moisture ranged from 0.074 mm3/mm3 to 0.139 mm3/mm3, temperature ranged from 9.1°C to 21.7°C. Nevertheless, soil moisture and soil temperature affect the content ephedrine and pseudoephedrine variously. With the increase in soil moisture and temperature, the total alkaloid content of E. saxatilis was higher than that of E. gerardiana. When the soil moisture was lower, the alkaloid content of the two Ephedra species was higher. In conclusion, high altitude with water deficit may be more beneficial for Ephedra to produce more alkaloids.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
ML and JD designed this experiment and wrote this paper. WH collected sample and data. YL and ZX participated in data analysis. MC participated writing this paper. All authors contributed to the article and approved the submitted version.
Funding
Our work was partly financially supported by the National Natural Science Foundation of China (32060087).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1236145/full#supplementary-material
References
Abourashed, E. A., El-Alfy, A. T., Khan, I. A., Walker, L. (2003). Ephedra in perspective – a current review. Phytother. Res. 17, 703–712. doi: 10.1002/ptr.1337
Abreha, K. B., Enyew, M., Carlsson, A. S., Vetukuri, R. R., Feyissa, T., Motlhaodi, T., et al. (2021). Sorghum in dryland: morphological, physiological, and molecular responses of sorghum under drought stress. Planta 255, 20. doi: 10.1007/s00425-021-03799-7
Carlquist, S. (1988). Near-vessellessness in Ephedra and its significance. Am. J. Bot. 75, 598–601. doi: 10.1002/j.1537-2197.1988.tb13480.x
Chang, D. H. S. (1981). The vegetation zonation of the Tibetan Plateau. Mt. Res. Dev. 1, 29–48. doi: 10.2307/3672945
Chang, D. H. S. (1983). The Tibetan Plateau in relation to the vegetation of China. Ann. Mo. Bot. Gard. 70, 564–570. doi: 10.2307/2992087
Chinese Pharmacopoeia Commission (2020). Chinese pharmacopoeia (I) (Beijing: China Medical Science Press).
Chu, H., Wei, J., Li, J., Li, T. (2018). Investigation of the relationship between runoff and atmospheric oscillations, sea surface temperature, and local-scale climate variables in the Yellow River headwaters region. Hydrol. Process. 32, 1434–1448. doi: 10.1002/hyp.11502
Ding, Y., Yang, S. (2022). Surviving and thriving: how plants perceive and respond to temperature stress. Dev. Cell 57, 947–958. doi: 10.1016/j.devcel.2022.03.010
Fu, J. T., Hu, X. S., Li, X. L., Yu, D. M., Liu, Y. B., Yang, Y. Q., et al. (2019). Influences of soil moisture and salt content on loess shear strength in the Xining Basin, northeastern Qinghai-Tibet Plateau. J. Mt. Sci. 16, 1184–1197. doi: 10.1007/s11629-018-5206-9
Gökmen, E., Ceyhan, E. (2015). Effects of drought stress on growth parameters, enzyme activities and proline content in chickpea genotypes. Bangladesh J. Bot. 44, 177–183. doi: 10.3329/bjb.v44i2.38505
González-Juárez, D. E., Escobedo-Moratilla, A., Flores, J., Hidalgo-Figueroa, S., Martínez-Tagüeña, N., Morales-Jiménez, J., et al. (2020). A review of the Ephedra genus: distribution, ecology, ethnobotany, phytochemistry and pharmacological properties. Molecules 25, 3283. doi: 10.3390/molecules25143283
Hayashi, H., Shukurova, M., Oikawa, S., Ohta, M., Fujii, I., Nasyrova, F., et al. (2019). Field survey of Ephedra plants in Central Asia (1). Characterization of Ephedra equisetina, Ephedra intermedia, and their putative hybrids collected in the Zaravshan Mountains of Tajikistan. Biol. Pharm. Bull. 42, 552–560. doi: 10.1248/bpb.b18-00289
He, W., De, J., Cairang, N., Duojie, R. (2019). Determination of ephedrine content in Ephedra and it's application on species identification. Plateau Sci. Res. 3, 78–84. doi: 10.16249/j.cnki.2096-4617.2019.04.011
Hong, H., Chen, H. B., Yang, D. H., Shang, M. Y., Wang, X., Cai, S. Q., et al. (2011). Comparison of contents of five ephedrine alkaloids in three official origins of Ephedra herb in China by high-performance liquid chromatography. J. Nat. Med. 65, 623–628. doi: 10.1007/s11418-011-0528-8
Ito, H., Kanayama, Y., Shibuya, T., Mohammed, S. A., Nishiyama, M., Kato, K. (2022). Effect of short-term temperature stress on fruit set and the expression of an auxin reporter gene and auxin synthesis genes in tomato. Sci. Hortic. 300, 111039. doi: 10.1016/j.scienta.2022.111039
Ji, W., Luo, H., Song, Y., Hong, E., Li, Z., Lin, B., et al. (2022). Changes in photosynthetic characteristics of Paeonia suffruticosa under high temperature stress. Agronomy 12, 1203. doi: 10.3390/agronomy12051203
Kondo, N., Mikage, M., Idaka, K. (1999). Medico-botanical studies of Ephedra plants from the Himalayan region, part III: causative factors of variation of alkaloid content in herbal stems. Nat. Med. 53, 194–200.
Li, J., Lei, F., Zhang, Y., Wang, X., Zhang, Q. (2012). Research progress on the chemical constituents and pharmacological activities of Ephedra. Mod. Chin. Med. 14, 21–27. doi: 10.3969/j.issn.1673-4890.2012.07.006
Li, C., Yang, J., Zhu, M., Ding, J., Zhu, X., Zhou, G., et al. (2022). Urea amendment alleviated morphological and physiological damages and yield loss of winter wheat subjected to low temperature stress at jointing stage. Plant Growth Regul. 98, 589–598. doi: 10.1007/s10725-022-00881-2
Liang, J., Quan, M., She, C., He, A., Xiang, X., Cao, F. (2020). Effects of drought stress on growth, photosynthesis and alkaloid accumulation of Lycoris aurea. Pak. J. Bot. 52, 1137–1142. doi: 10.30848/PJB2020-4(15
Minami, M., Mori, T., Honda, Y., Ueno, K., Murakami, T., Ajioka, Y., et al. (2020). Physical and chemical characteristics of soils in Ephedra gerardiana and E. pachyclada habitats of Kali Gandaki Valley in Central Nepal. J. Nat. Med. 74, 825–833. doi: 10.1007/s11418-020-01413-w
Minami, M., Mori, T., Honda, Y., Ueno, K., Murakami, T., Matsunaka, T., et al. (2022). Relationship between ephedrine alkaloid profile in Ephedra gerardiana and soil characteristics of glacial landforms in southeastern Tibetan Plateau, China. J. Nat. Med. 76, 703–714. doi: 10.1007/s11418-022-01628-z
Minami, M., Taichi, F., Honda, Y., Ueno, K., Shinozaki, J., Itoh, S., et al. (2021). Environmental and soil characteristics in Ephedra habitats of Uzbekistan. J. Nat. Med. 75, 246–258. doi: 10.1007/s11418-020-01460-3
Puebla, G. G., Iglesias, A., Gómez, M. A., Prámparo, M. B. (2017). Fossil record of Ephedra in the lower cretaceous (Aptian), Argentina. J. Plant Res. 130, 975–988. doi: 10.1007/s10265-017-0953-1
Qin, A. L., Wang, M. M., Cun, Y. Z., Yang, F. S., Wang, S. S., Ran, J. H., et al. (2013). Phylogeographic evidence for a link of species divergence of Ephedra in the Qinghai-Tibetan Plateau and adjacent regions to the Miocene Asian aridification. PloS One 8, e56243. doi: 10.1371/journal.pone.0056243
Quan, M., Liang, J. (2017). The influences of four types of soil on the growth, physiological and biochemical characteristics of Lycoris aurea (L’ Her.) Herb. Sci. Rep. 7, 43284. doi: 10.1038/srep43284
Ren, Y., Yu, G., Shi, C., Liu, L., Guo, Q., Han, C., et al. (2022). Majorbio Cloud: a one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 1, e12. doi: 10.1002/imt2.12
Sheffer, E., Cooper, A., Perevolotsky, A., Moshe, Y., Osem, Y. (2020). Consequences of pine colonization in dry oak woodlands: effects on water stress. Eur. J. For. Res. 139, 817–828. doi: 10.1007/s10342-020-01287-3
Stracke, C., Meyer Benjamin, H., Hagemann, A., Jo, E., Lee, A., Albers, S. V., et al. (2020). Salt stress response of Sulfolobus acidocaldarius involves complex trehalose metabolism utilizing a novel trehalose-6-phosphate synthase (TPS)/trehalose-6-phosphate phosphatase (TPP) pathway. Appl. Environ. Microbiol. 86, e01565–e01520. doi: 10.1128/AEM.01565-20
Thompson, W. P. (1912). The anatomy and relationships of the Gnetales: I. the genus Ephedra. Ann. Bot. 26, 1077–1104. doi: 10.1093/oxfordjournals.aob.a089432
Thompson, W. P. (1918). Independent evolution of vessels in Gnetales and Angiosperms. Bot. Gaz. 65, 83–90. doi: 10.1086/332191
Tian, N., Yang, X., Zhu, Y., Zeng, X., Yuan, J., Yang, J., et al. (2022). Mahuang (herbaceous stem of Ephedra spp.): chemistry, pharmacodynamics, and pharmacokinetics. China J. Chin. Mater. Med. 47, 3409–3424. doi: 10.19540/j.cnki.cjcmm.20220425.601
Wang, X., Zheng, S. L. (2010). Whole fossil plants of Ephedra and their implications on the morphology, ecology and evolution of Ephedraceae (Gnetales). Chin. Sci. Bull. 55, 1511–1519. doi: 10.1007/s11434-010-3069-8
Wu, H., Ma, Z., Wang, M. M., Qin, A. L., Ran, J. H., Wang, X. Q. (2016). A high frequency of allopolyploid speciation in the gymnospermous genus Ephedra and its possible association with some biological and ecological features. Mol. Ecol. 25, 1192–1210. doi: 10.1111/mec.13538
Xiao, D., Zhao, P., Wang, Y., Tian, Q., Zhou, X. (2014). Millennial-scale phase relationship between North Atlantic deep-level temperature and Qinghai-Tibet Plateau temperature and its evolution since the Last Interglaciation. Chin. Sci. Bull. 59, 75–81. doi: 10.1007/s11434-013-0028-1
Xie, Y. L., Yu, Q. H., You, Y. H., Zhang, Z. Q., Gou, T. T. (2019). The changing process and trend of ground temperature around tower foundations of Qinghai-Tibet Power Transmission line. Sci. Cold Arid Reg. 11, 13–20. doi: 10.3724/SP.J.1226.2019.00013
Zare-Maivan, H., Khajehzadeh, M. H., Ghanati, F., Sharifi, M. (2014). Changes of enzymes activity and production of secondary metabolites of Artemisia aucheri in different altitudes and its relation to adaptation. J. Chem. Health Risks 4, 57–66. doi: 10.22034/jchr.2018.544076
Zhang, B. M., Wang, Z. B., Xin, P., Wang, Q. H., Bu, H., Kuang, H. X. (2018). Phytochemistry and pharmacology of genus Ephedra. Chin. J. Nat. Med. 16, 811–828. doi: 10.3724/SP.J.1009.2018.00811
Keywords: Ephedra, pseudoephedrine, ephedrine, metabolomics, altitude, environment
Citation: Lu M, He W, Xu Z, Lu Y, Crabbe MJC and De J (2023) The effect of high altitude on ephedrine content and metabolic variations in two species of Ephedra. Front. Plant Sci. 14:1236145. doi: 10.3389/fpls.2023.1236145
Received: 07 June 2023; Accepted: 19 September 2023;
Published: 16 October 2023.
Edited by:
Zhentian Lei, University of Missouri, United StatesReviewed by:
Clayton Kranawetter, University of Missouri, United StatesAnil Bhatia, University of California, Riverside, United States
Copyright © 2023 Lu, He, Xu, Lu, Crabbe and De. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji De, ZGVreWkxOTgxQHV0aWJldC5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
§These authors have contributed equally to this work and share senior authorship
‖These authors have contributed equally to this work and share last authorship
 Mengnan Lu1†
Mengnan Lu1† Yan Lu
Yan Lu M. James C. Crabbe
M. James C. Crabbe Ji De
Ji De