
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 08 August 2023
Sec. Plant Pathogen Interactions
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1230968
This article is part of the Research Topic Novel Approaches for Sustainable Crop Yield and Management of Plant-Parasitic Nematodes View all 7 articles
Considered one of the most devastating plant parasitic nematodes worldwide, Meloidogyne spp. (commonly known as the root-knot nematodes (RKNs)) are obligate sedentary endoparasites that establish in the roots, causing hyperplasia and hypertrophy of surrounding cells, triggering the formation of galls. These galls will affect root development and physiology, leading to substantial yield losses. During 2017–2022, an extensive survey of Meloidogyne species was undertaken in Portugal (mainland and islands). A total of 1,071 samples were collected by the National Plant Protection Organization (DGAV) and private farmers from different regions of the country and were analysed at the Laboratory of Nematology (NemaINIAV). Samples in which the presence of Meloidogyne sp. was detected were used to perform bioassays to obtain females and juveniles for further studies. Since the accurate identification of RKNs is an important aspect of crop management, morphological and biochemical characterisation was performed. The most common morphological features were observed, showing consistency with previous descriptions of the genus. The biochemical identification using the esterase (EST) phenotype revealed the phenotypes of Meloidogyne arenaria, M enterolobi, M. hispanica, M. hapla, M. incognita, M javanica, and M. luci. Meloidogyne incognita and M. javanica were found to be the most prevalent species in the different regions followed by M. arenaria and M. hapla. This is the first distribution report performed in Portugal on RKNs, contributing to the development of management strategies and to updated information on the status of these pests in Europe.
Agriculture is the practice of cultivating natural resources to sustain human life and generate profits. In the EU, agricultural production is a big business, contributing EUR 217 billion towards the EU’s overall gross domestic product (GDP) in 2022, and it is expected to grow due to the increase in global trade caused by the growing population. European countries contribute to the total output value of the EU’s agricultural industry, being more than half (56.9%) coming from France, Germany, Italy, and Spain. Portugal contributes less than 5% (Eurostat, 2023).
In territorial, social, and economic terms, agriculture in Portugal has great importance for the whole country but particularly to rural areas concerning sustainable development. It is also well positioned in the European and world markets due to its climate, biodiversity, innovation, and ability to present differentiated and safe products. According to data from Instituto Nacional de Estatística’s economic accounts, it appears that in 2018, vegetable and horticultural products represented 17% of the national agricultural production, of which fresh vegetables represent 50% of production (GPP, 2020; INE, 2022). According to PORDATA, agriculture and forestry have an essential role in preserving the environment and landscapes in Portugal; together, they cover 75.3% of the land. Moreover, approximately 26.2% of the agricultural area corresponds to arable land, which is divided into perennial crops (21.7%), pastures (51.7%), and family farming (0.4%).
Portugal is divided into seven regions (North, Metropolitan area–Lisbon, Centre, Alentejo, Algarve, Madeira, and Azores), which have different regional specialisations because of the considerable diversity of natural and economic–social conditions (Avillez, 2015; Freire and Lains, 2017). The regions of Alentejo and Azores have the highest significance in the national agriculture production, representing 8.6% and 6.8% of GDP, respectively (Marques, 2015; GPP, 2020).
Plant parasitic nematodes (PPNs) are regarded as one of the most important soil-borne pests, accounting for USD 175 billion per year in yield losses worldwide (Bernard et al, 2017). One of the oldest and most economically important PPNs are the root-knot nematodes (RKNs), Meloidogyne spp., which are considered serious pests for agricultural production, causing annual losses of USD 157 billion globally (Abad et al., 2008). This genus comprises more than 100 species (Subbotin et al, 2021); species Meloidogyne arenaria (Neal, 1889) Chitwood, 1949, Meloidogyne hapla Chitwood, 1949, Meloidogyne incognita (Kofoid and White, 1919) Chitwood, 1949, and Meloidogyne javanica (Trub, 1885) Chitwood, 1949, are known as the most important due to their widespread distribution and broad host range (Jones et al., 2013). In Portugal, so far, only 10 species have been reported (Table 1).
Root-knot nematodes infect at the elongation zone and then move to the root tips to invade the vascular cylinder and form a feeding site, called a giant cell. At the same time, the neighbouring cells start to divide to form the typical gall or root-knot, affecting the development of the root system and causing significant yield losses (Jena and Rao, 1973; Norton and Niblack, 1991; Kyndt et al., 2014).
Most RKN species have high plasticity, enabling their establishment in different geographical areas and colonisation of different hosts. Moreover, projections by the intergovernmental panel for climate change indicate that the elevated temperature and moisture may result in an increasing rate of infection, development, and reproduction, causing shifts in their abundance and geographic distribution (Mbow et al., 2019).
Considering the impact that the RKNs have on agricultural production, species identification is essential to define sustainable management strategies. Morphological RKN identification is a valuable tool with low cost and accuracy depending on the number of characteristics and specimens evaluated. Furthermore, the biochemical electrophoretic analysis of non-specific esterase (EST), along with several molecular methods, such as internal transcribed spacer–polymerase chain reaction–restriction fragment length polymorphism (ITS-PCR-RFLP), sequence characterized amplified region (SCAR) markers, real-time PCR, and loop-mediated isothermal amplification (LAMP), have proved to be useful in the differentiation of economically important species of Meloidogyne.
Currently, in Portugal, there is a lack of detailed information on the root-knot nematode geographical distribution and species occurrence. Therefore, this study aimed to assess the presence and incidence of the RKNs in Portugal, thus contributing to knowledge about the wide dissemination of these nematodes and designing and implementing effective management practices.
From 2017 to 2022, soil and root samples were collected by inspectors of the National Plant Protection Organization (DGAV-Portugal) and by private farmers from the different regions of Portugal (Figure 1; Table 2). Samples with a volume of 1,500 mL of soil/ha were collected from the rhizosphere at approximately 15–20-cm depth for horticultural crops and 90 cm for trees. At least 100 subsamples/ha were harvested in a rectangular mesh, not less than 5 m wide and no more than 20 m long between sampling points, covering the entire field. Samples were stored in polyethylene bags and individually coded. Geographical locations at district and county levels as well as the crops installed in these fields were accessed only after the result analysis.
Carrot, potato, and tomato were the main crops surveyed; however, samples from other crops such as broccoli, cabbage, chard, courgette, cucumber, orange tree, spinach, strawberry, and sweet potato were included.
Nematodes were extracted from a 500-mL subsample using the Oostenbrink dish technique according to protocol PM 7/119 (1) (Standard Protocol PM 7/119 (1), 2013). The suspensions were observed under a stereomicroscope (Nikon SMZ1500, Tokyo, Japan), and suspected specimens of Meloidogyne sp. were observed using a bright-field light microscope (Olympus BX-51, Hamburg, Germany) for confirmation. Roots were also examined for gall presence.
Morphological characterisation was performed using second-stage juveniles (J2) and males individually placed in a drop of water on a glass slide and gently heat killed. Nematodes were observed using a bright-field light microscope (Olympus BX-51, Hamburg, Germany) and photographed with a digital camera (Leica MC190 HD, Wetzlar, Germany). The features observed for characterisation were stylet, excretory pore, tail, hyaline tail terminus, and spicule. Additionally, perineal patterns of mature females were cut in 45% lactic acid and permanently mounted in glycerin (Hartman and Sasser, 1985).
Bioassays were carried out by planting tomato plants cv. Oxheart in the remaining soil or inoculating them using egg masses and maintaining them in a quarantine greenhouse for 2 months to obtain material for further studies. From infected tomato roots, young egg-laying females were handpicked and transferred to micro-haematocrit capillary tubes (one female per tube) with 5 µL of extraction buffer (20% sucrose v/v and 1% Triton X-100 v/v). Maceration of the females was performed with a pestle, frozen, and stored at −20°C until use. After centrifugation, the protein extracts were separated by polyacrylamide gel electrophoresis (PAGE) on thin-slab 7% separating polyacrylamide gels in a Mini-Protean II (BioRad Laboratories, Hercules, CA, USA) according to Esbenshade (1985) and Pais et al. (1986). The gels were stained for EST activity with the substrate α-naphthyl acetate. Protein extracts of Meloidogyne javanica (Treub, 1885) isolate were included in the gel as a reference.
To evaluate the frequency and abundance of the different species of Meloidogyne in Portugal, multiple proportion tests were performed using the software R (https://www.r-project.org). For one of the tests, only samples identified to species level were used. The hypothesis tests were performed with a significance level α = 0.05.
Meloidogyne sp.-positive detections maps were made using the ArcMap 10.6 software (ESRI, USA): CAOP2017_PORTUGAL and CAOPP2017_DISTRITOS shapefiles (DGT, 2017) for continental detections, CAOP2019_Madeira shapefiles (DGT, 2019a) for Madeira Island detections, and CAOP2019_Açores (Grupo Oriental), CAOP2019_Açores (Grupo Central), and CAOP2019_Açores (Grupo Occidental) shapefiles for Azores Island detections (DGT, 2019b; DGT, 2019c; DGT, 2019d).
During the period 2017–2022, a total of 1,071 samples were collected and analysed from the seven regions of Portugal. Root-knot nematodes were detected in 243 samples distributed along the country (mainland and islands) and corresponding to 22.7% of the total (Figure 2). Among the positive detections, Azores Island contributed with 37% (90 samples), followed by the region of Alentejo at 22.7% (55 samples) and the Centre at 18.5% (45 samples) (Table 3).
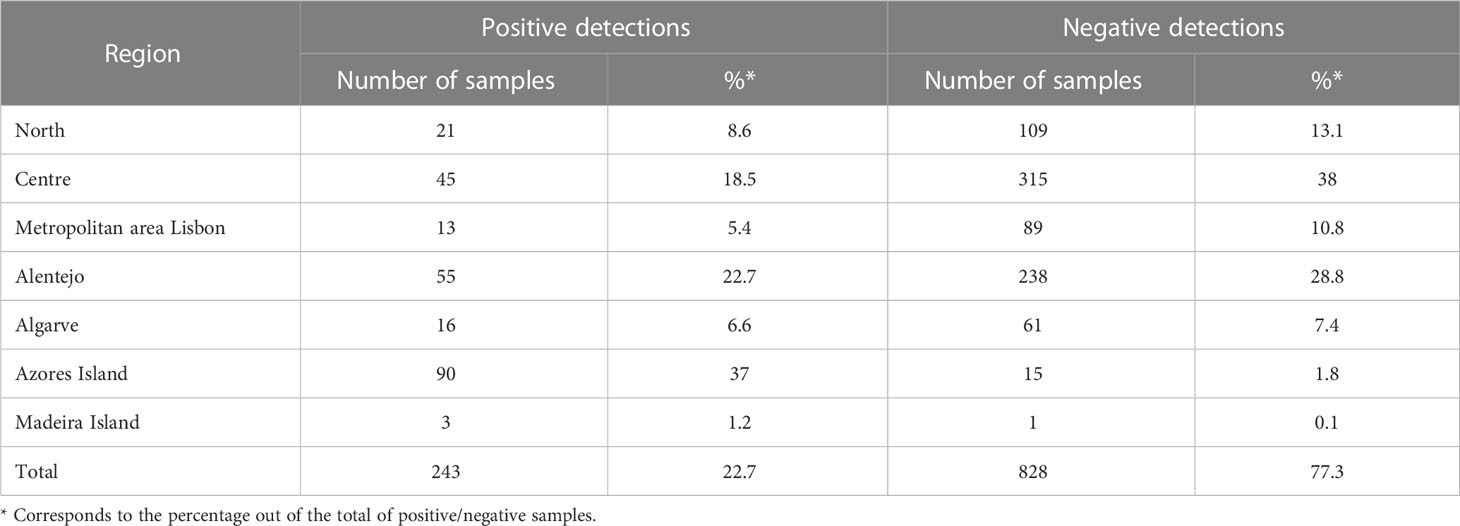
Table 3 Positive and negative detections of Meloidogyne in the seven Portuguese regions between 2017 and 2022 (absolute values and %).
The statistical analysis confirmed that the abundance of Meloidogyne is not equal in all regions, and comparison tests at a significance level of 5% showed that the region of Azores has a significantly higher abundance than the rest of the regions.
Morphological characterisation of second-stage juveniles recovered from soil was performed on 10 specimens. Nematodes were vermiform, slender, and annulated. The head region was slightly set off from the body. The stylet was delicate, narrow, and sharply pointed, with small knobs. The excretory pore was distinct. The tail was conoid with a hyaline terminus distinctive in most species. Males were vermiform, bluntly rounded posteriorly and with an anterior end narrowing. The head region was smooth, not set off from the body. The stylet was robust, with a straight cone, pointed and widen gradually to the posterior end. Knobs were rounded merging gradually into the shaft. The tail was short and round. Spicules were long and curved (Figure 3), agreeing with previous descriptions from Eisenback (1985) and Jepson (1987). Some specimens presented vesicle-like structures around the lumen of the juvenile metacorpus characteristic of Meloidogyne naasi and Meloidogyne sasseri. Therefore, to determine the identity of these specimens, as stated by Karssen (1996), morphometrics of second-stage juveniles (body, tail, and hyaline tail terminus length) were carried out, confirming the presence of M. naasi.
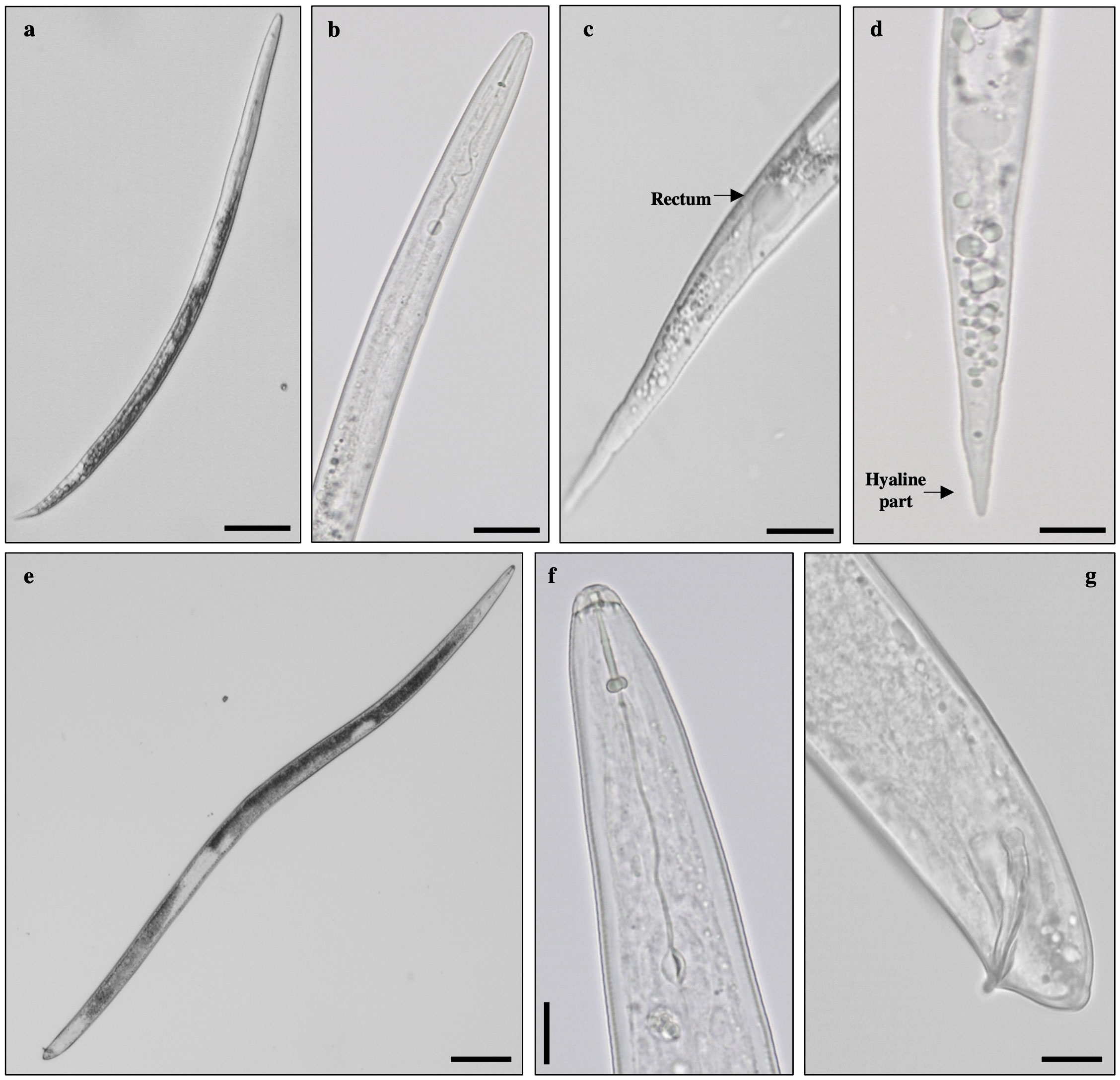
Figure 3 Morphological traits observed in specimens of Meloidogyne sp. Second-stage juvenile: (A) whole specimen, (B) anterior region, (C) tail region–rectum, (D) tail region–hyaline part. Male: (E) whole specimen, (F) head region, and (G) spicule. Bar = 20 µm.
Females were elongated, ovoid, or pear-shaped. The perineal pattern comprised the vulva-anus area, tail terminus, phasmids, lateral lines, and surronding cuticule striae (Karssen et al., 2013). Some variability was observed among the perineal patterns. The shape was ovoid to rounded in the species Meloidogyne arenaria, M. incognita, M. javanica, and M. enterolobii while oval to squarish in species M. luci and M. hispanica. Distinctive lateral lines were present in M. javanica and M. arenaria whereas absent or weakly demarcated in M. incognita, M. luci, and M. enterolobii (Figure 4).
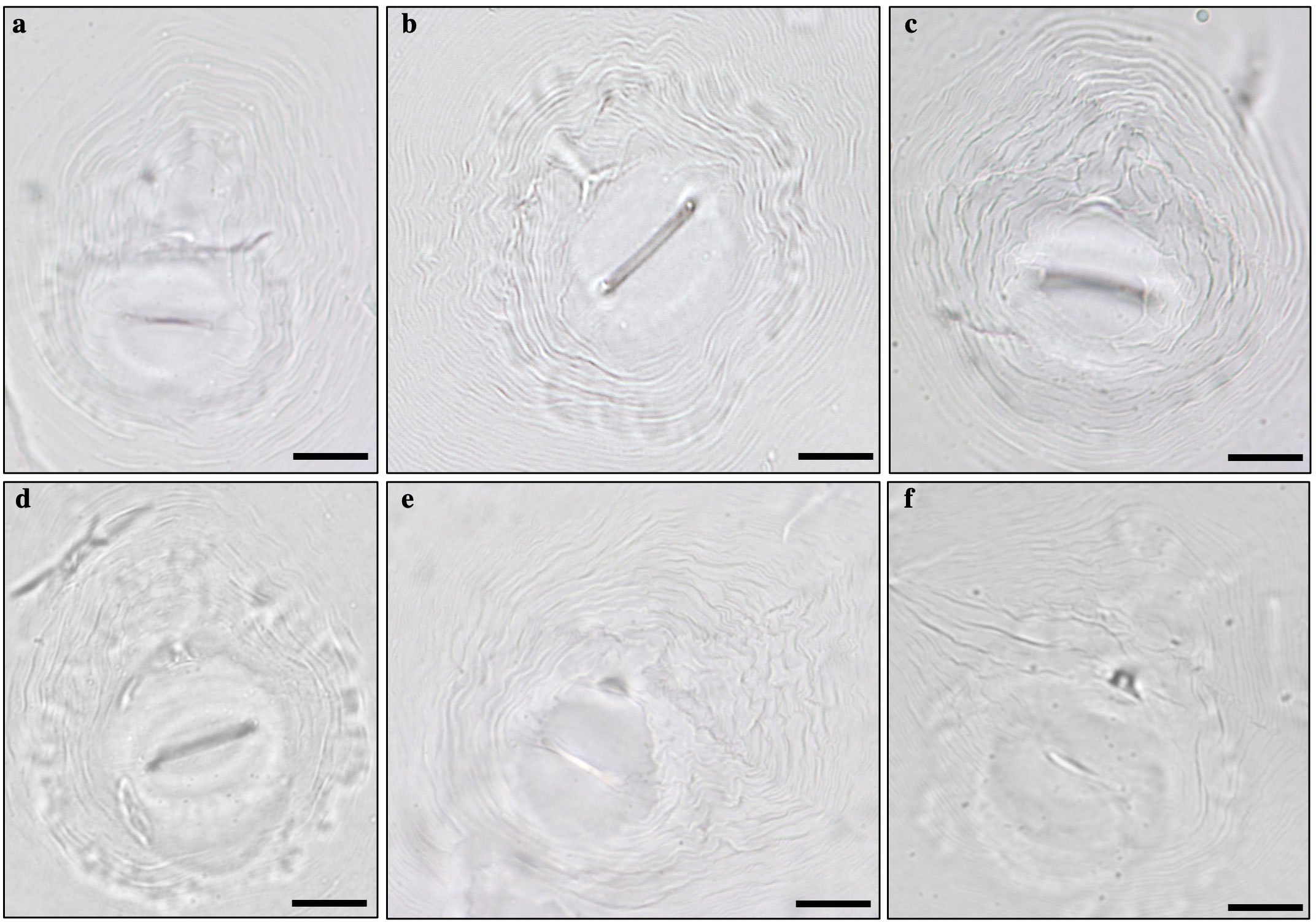
Figure 4 Perineal patterns observed in specimens of Meloidogyne sp.: (A) Meloidogyne arenaria, (B) Meloidogyne incognita, (C) Meloidogyne javanica, (D) Meloidogyne enterolobii, (E) Meloidogyne luci, and (F) Meloidogyne hispanica. Bar = 20 µm.
Enzyme phenotype analyses allowed us to identify some of the species present within the positive samples. Despite many attempts, it was only possible to reach the species identification of 51% of the positive samples (123). For the remaining 49%, we can only confirm the presence of individuals of the genus Meloidogyne. Seven different phenotypes were identified corresponding to the following species of root-knot nematodes: Meloidogyne arenaria–A2 phenotype, M. enterolobii–En5 phenotype, M. hapla–H1 phenotype, M. hispanica–Hi3 phenotype, M. incognita–I2 phenotype, M. javanica–J3 phenotype, and M. luci–L3 phenotype (Figure 5).
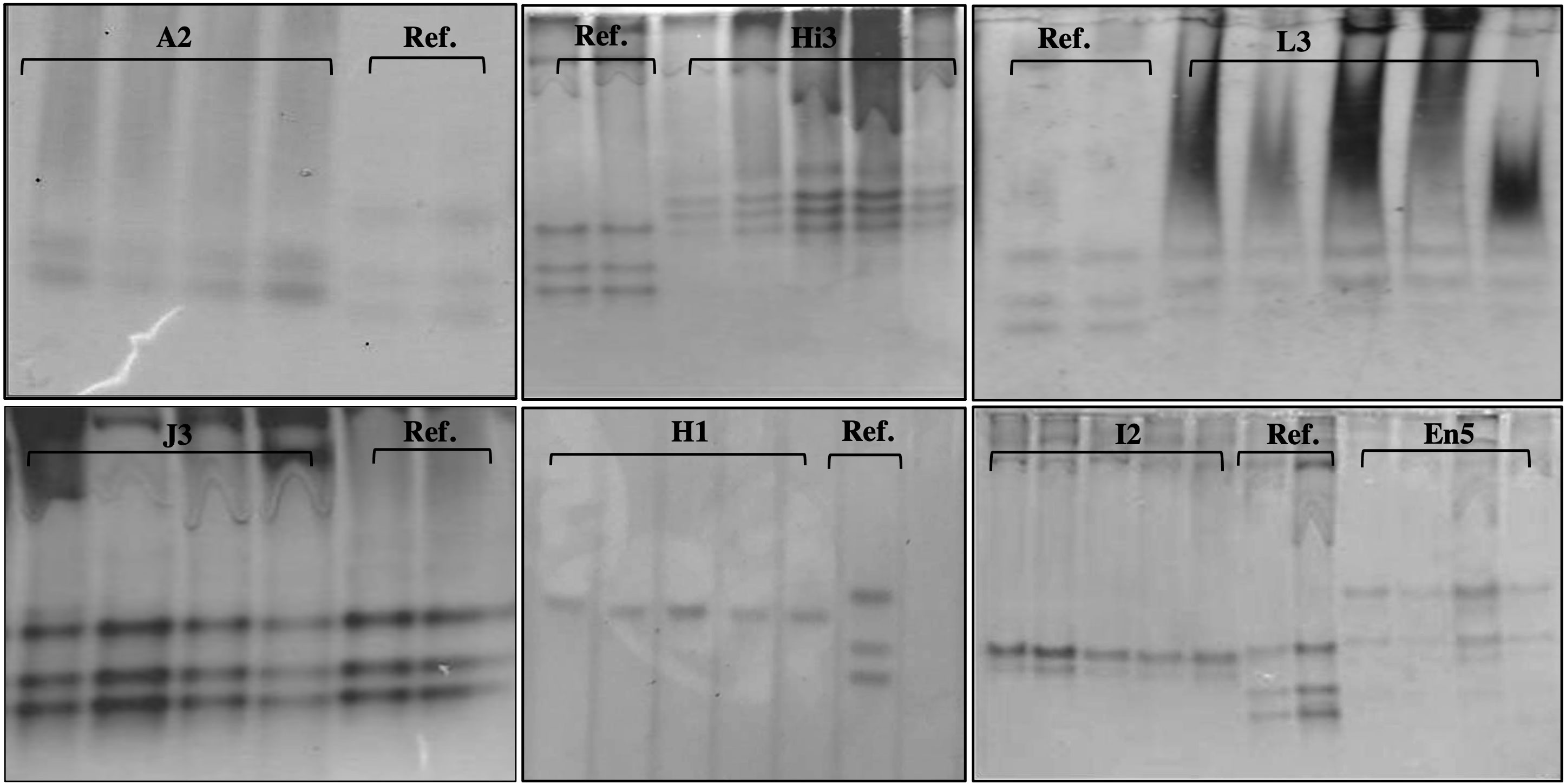
Figure 5 Esterase phenotypes of protein homogenates from one egg-laying female of Meloidogyne species: Meloidogyne arenaria (A2), Meloidogyne hispanica (Hi3), Meloidogyne luci (L3), Meloidogyne javanica (J3), Meloidogyne hapla (H1), Meloidogyne incognita (I2), Meloidogyne enterolobii (En5), and reference (J3).
The host status of crops of economic importance and the host range of Meloidogyne spp. are issues of major concern in integrated nematode management recommendations. Currently, in Portugal, this information is very incomplete. In this survey, RKN species were found parasitising 22 different plant hosts, mostly horticultural crops in open fields and also grasses, ornamentals, and fruit trees (Table 4). So far, many of these crops have not been reported in Portugal as hosts of Meloidogyne. Therefore, to our knowledge, this is the first report of species of Meloidogyne parasitising aubergine, broccoli, carrot, chard, courgette, orange tree, okra, pepper, and strawberry in Portugal’s mainland.
The statistical analysis regarding the different species of RKNs showed that M. incognita and M. javanica presence was significantly different (p-value ≤2e−16) from the rest of the species, indicating that the frequency of occurrence in fields across the country is high. The less frequent species found in the fields were M. enterolobii and M. naasi with a p-value ≤0.77.
From the total samples identified to species level, M. incognita is the predominant species in the country (mainland and islands), as it was found in 71 samples (57.8%) and six of the seven regions surveyed. Following in prevalence, M. javanica was identified in 20 samples (16.2%) and present in five regions. Meloidogyne arenaria and M. hapla were detected in 10 samples (8.1%) and 12 samples (9.7%), respectively, and were present in four regions. Species of the least frequent occurrence “minor species” such as M. enterolobii, M. hispanica, M. luci, and M. naasi, were also detected, corresponding to 8.2% of the total. The region with the highest diversity of species is the centre region (seven species) followed by the Azores Island (five species), Alentejo, Algarve (four species), and North (three species) (Figures 6A–D).
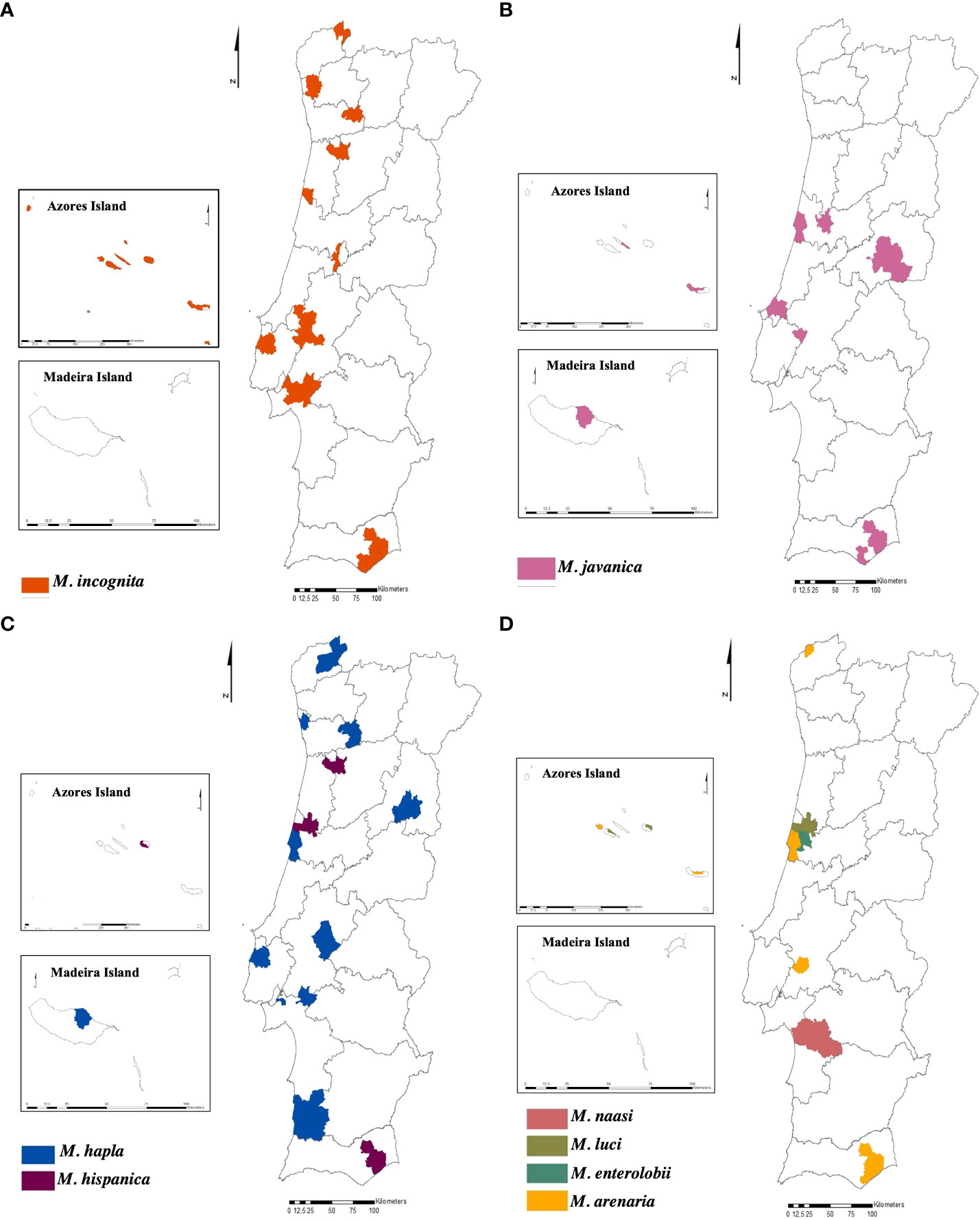
Figure 6 Distribution of the species of Meloidogyne sp. in Portugal (mainland and islands). (A) Meloidogyne incognita. (B) Meloidogyne javanica. (C) Meloidogyne hapla and Meloidogyne hispanica. (D) Meloidogyne naasi, Meloidogyne luci, Meloidogyne enterolobii, and Meloidogyne arenaria.
Plant parasitic nematodes represent a risk to agricultural production worldwide. Once a field is infested, it is difficult to eradicate them. Instead, the goal is to keep nematode densities low and reduce crop damage. RKNs are among the most widely distributed pests causing economically important damage to a great range of crops. Due to their importance and given the current concerns regarding climate change and food security, the main challenge is to find management strategies that can be efficient and sustainable to control them since the current practices are not enough; therefore, species identification is essential.
Although traditionally morphology is used for RKN identification, currently, it represents a challenge due to the variability between individuals, the indistinctive differences among them, and the increase in the number of species (Eisenback, 1985; Hirschmann and Volume, 1985; KarssenVan Aelst, 2001). Hence, it is of primary importance to have specialised and well-trained researchers to minimise the level of inaccuracy.
Furthermore, the effectiveness of the non-specific EST phenotype as the more stable and quicker method to identify Meloidogyne spp. has been demonstrated in many studies, showing to be highly polymorphic and able to detect different EST phenotypes of a single female (Esbenshade and Triantaphyllou, 1985; Carneiro et al., 2000). Nonetheless, its main disadvantage is that requires females in a specific developmental stage (Hunt and Handoo, 2009).
Among the tropical species found in this study, M. incognita, M. arenaria, and M. javanica are probably the most widely distributed and economically important species of plant parasitic nematodes, so much that in some areas of the world, galls on roots are considered normal. The species M. luci is included in the European and Mediterranean Plant Protection (EPPO) alert list and M. enterolobii in the A2 List of pests recommended for regulation as quarantine pests (EPPO, 2017a). Their habitats in general terms are the tropical and subtropical regions; however, they have also been found in temperate zones overwintering in mild winters. Meanwhile, M. hapla occurred mainly in temperate regions, being able to survive in temperatures below 0°C, though there is no evidence of its inability to survive in hot temperatures. Based on the above, the presence of these species in Portugal in a wide variety of hosts and climates is not an unusual event; on the contrary, it is an expected fact since the temperature increase is contributing to the geographical expansion not only of these major species but also of species of minor or restricted occurrence.
Management of RKNs is difficult due to the complexity of the soil environment (Norton and Schmitt, 1978). Biological, cultural, and chemical methods are some of the strategies that have reduced the risk of damage by many nematode species (Hague and Gowen, 1987; Heald, 1987; Kerry, 1987; Halbrendt and La Mondia, 2004; Starr and Roberts, 2004). However, all these techniques have associated challenges (Abd-Elgawad, 2022a). Synthetic nematicides were a commonly used strategy; nevertheless, some active substances have been strictly regulated or banned from the market owing to adverse environmental and health impacts, reducing the number of alternatives for control.
Cultural methods also appear to control to some degree RKNs; however, the extensive host range that includes nearly every horticultural, fruit, and ornamental crop poses severe constraints. Similarly, many bacterial and fungal agents as well as chemical compounds have been described for Meloidogyne spp. as a potential strategy to be included in integrated pest management programs, among which some have not yet been tested in the field and others have not provided consistent results (Faria et al., 2022; Pires et al., 2022). Resistant cultivars have also shown some efficacy on RKN control, but some species are able to overcome that resistance and the cultivars are not always commercially available (Abd-Elgawad, 2022b). A combination of microbial strategies using both bacterial and fungal agents with other cultural control practices or host resistance poses an alternative that can be used as a multidisciplinary approach to improve the management strategies for RKNs.
Finally, extensive surveys had not been performed in Portugal, and so, the results presented here confirm other reports on the widespread distribution of Meloidogyne, its high frequency of occurrence, and its potential as a problem for agricultural production. This assessment included crops of economic importance that are grown, intensively favouring the survival and rapid build-up of nematode populations in the soil. This fact and the ability of RKNs to be transmitted by soil, agricultural machinery, infected plants, and running water explain the presence of a high number of species in a wide diversity of hosts. The information here presented regarding the species of Meloidogyne found in the country will help farmers and technicians in the development and establishment of efficient and sustainable practices and policymakers in the provision of phytosanitary measures and monitoring programmes to prevent the introduction and spread of these pests of concern in Europe.
This study shows the high occurrence and frequency of RKNs in Portugal, confirming the widespread distribution of these nematodes. Moreover, the detection of a great variety of species of Meloidogyne in different regions around the country evidenced that there is a northward movement of pests caused by trade activity and climate changes. Due to this fact, the identification of Meloidogyne species is of great importance for the development of appropriate management practices for its control.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Conceptualisation: LR. Research and data analysis: LR, MC, MI, and FN. Writing—original draft preparation: LR. Writing—review and editing: LR, MC, CS, FN, and MI. Resources: MI. All authors contributed to the article and approved the submitted version.
This research was supported by the “Fundação para a Ciência e a Tecnologia” (FCT, Portugal) and the European Social Funds, through the “Programa Operacional Regional Centro”, under the Ph.D. fellowship 2020.05541.BD and through the R&D Unit, UIDB/04551/2020 (GREEN-IT—Bioresources for Sustainability).
The authors would like to thank the technicians of the Laboratory of Nematology INIAV—Nema-INIAV, Margarida Fontes, Nídia Laureano, Marina Cardoso e Lourdes Silva, and the Laboratory of Biochemistry and Molecular Genetics. A special thanks to DGAV (the National Plant Protection Authority) for the information to support this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abad, P., Gouzy, J., Aury, J. M., Castagnone-Sereno, P., Danchin, E. G., Deleury, E., et al. (2008). Genome sequence of the metazoan plant parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 26(8), 909–915. doi: 10.1038/nbt.1482
Abd-Elgawad, M. M. M. (2022a). Exploiting plant–phytonematode interactions to upgrade safe and effective nematode control. Life 12, 1916–1933. doi: 10.3390/life12111916
Abd-Elgawad, M. M. M. (2022b). Understanding molecular plant-nematode interactions to develop alternative approaches for nematode control. Plants 11 16, 2141. doi: 10.3390/plants11162141
Abrantes, I. M. O., de A. Santos, M. S. (1991). Meloidogyne lusitanica n. sp. (Nematoda: Meloidogynidae), a Root-knot Nematode Parasitizing Olive Tree (Olea europaea L.). J. Nematol. 23(2), 210–224.
Abrantes, I. M. O., dos Santos, M. V., da Conceição, I. L., Santos, M. D., Vovlas, N. (2008). Root-knot and other plant-parasitic nematodes associated with fig trees in Portugal. Nema. Mediterr. 36, 131–136.
Avillez, F. (2015). A agricultura portuguesa (Lisbon, Portugal: Fundação Francisco Manuel dos Santos), ISBN: ISBN 9789898819000.
Bernard, G. C., Egnin, M., Bonsi (2017). The impact of plant-parasitic nematodes on agriculture and methods of control. IntechOpen. doi: 10.5772/intechopen.68958
Carneiro, R. M. D. G., Almeida, M. R. A., Quénéhervé, P. (2000). Enzyme phenotypes of Meloidogyne spp. isolates. Nematology 2, 645–654. doi: 10.1163/156854100509510
Da Conceição, I. L., da Cunha, M. J., Feio, G., Correia, M., dos Santos, M. C. V., Abrantes, I. M. O., et al. (2009). Root-knot nematodes, Meloidogyne spp., on potato in Portugal. Nematology 11, 311–313. doi: 10.1163/156854109X415515
DGT. (2017). Carta Administrativa Oficial de Portugal - CAOP2017 (Direção-Geral do Território). Available at: https://www.dgterritorio.gov.pt/cartografia/cartografia-tematica/caop#tab-24b1t-1 (Accessed December 2018).
DGT. (2019a). Carta Administrativa Oficial da Madeira - CAOP2019 (Direção-Geral do Território). Available at: https://www.dgterritorio.gov.pt/cartografia/cartografia-tematica/caop#tab-24b1t-1 (Accessed April 2023).
DGT. (2019b). Carta Administrativa Oficial de Açores (Grupo Oriental) - CAOP2019 (Direção-Geral do Território). Available at: https://www.dgterritorio.gov.pt/cartografia/cartografia-tematica/caop#tab-24b1t-1 (Accessed April 2023).
DGT. (2019c). Carta Administrativa Oficial de Açores (Grupo Central) - CAOP2019 (Direção-Geral do Território). Available at: https://www.dgterritorio.gov.pt/cartografia/cartografia-tematica/caop#tab-24b1t-1 (Accessed April 2023).
DGT. (2019d). Carta Administrativa Oficial de Açores (Grupo Ocidental) - CAOP2019 (Direção-Geral do Território). Available at: https://www.dgterritorio.gov.pt/cartografia/cartografia-tematica/caop#tab-24b1t-1 (Accessed April 2023).
Eisenback, J. D. (1985). “Detailed morphology and anatomy of second-stage juveniles, males, and females of the genus Meloidogyne (root-knot nematodes),” in An advanced treatise on meloidogyne, vol. Volume I . Eds. Sasser, J. N., Carter, C. C. (Raleigh, NC, USA: State University Graphics Raleigh), 47–77.
EPPO. (2017a). Alert List: Addition of Meloidogyne luci Together with M. ethiopica. Reporting Service 11. Available at: https://www.eppo.int/ACTIVITIES/plant_quarantine/alert_list (Accessed 30 March 2023).
EPPO. (2017b). Alert List: Addition of Meloidogyne luci Together with M. ethiopica. Reporting Service 05. Available at: https://www.eppo.int/ACTIVITIES/plant_quarantine/alert_list (Accessed 30 March 2023).
Esbenshade, P. R. (1985). Triantaphyllou, A.C. Use of enzyme phenotypes for identification of Meloidogyne species. J. Nematol 17, 6–20.
Esbenshade, P. R., Triantaphyllou, A. C. (1985). “Identification of major Meloidogyne species employing enzyme phenotypes as differentiating characters,” in An advanced treatise on meloidogyne, vol. Volume I . Eds. Sasser, J. N., Carter, C. C. (Raleigh, NC, USA: North Carolina State University Graphics), 135–140.
Eurostat. (2023). Performance of the agricultural sector – value of agricultural output. Available at: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Performance_of_the_agricultural_sector#Value_of_agricultural_output (Accessed June 2023).
Faria, J. M. S., Rusinque, L., Vicente, C. S. L., Inácio, M. L. (2022). Bioactivity of Monoterpene Alcohols as an Indicator of Biopesticidal Essential Oils against the Root Knot Nematode Meloidogyne ethiopica. Biol. Life Sci. Forum 16, 15. doi: 10.3390/IECHo2022-12485
Freire, D., Lains, P. (2017). An agrarian history of portuga –2000: economic development on the european frontier. Eds. Freire, D., Lains, P. (Leiden, The Netherlands: Brill).
Gabinete de planeamento and polı́ticas e administração geral (GPP). (2020). Análise setorial FRUTAS &HORTÍCOLAS. 39 Portugal.
Hague, N. G. M., Gowen, S. R. (1987). “Chemical control of nematodes,” in Principles and practice of nematode control in crops. Eds. Brown, R. H., Kerry, B. R. (Sydney: Academic Press), 131–178.
Halbrendt, J. M., La Mondia, J. A. (2004). “Crop rotation and other cultural practices,” in Nematology advances and perspectives: vol. II nematode management and utilization. Eds. Chen, Z. X., Chen, S. Y., Dickson, D. W. (UK: CAB International, Oxfordshire), 909–930.
Hartman, K. M., Sasser, J. N. (1985). “Identification of Meloidogyne species on the basis of differential host test and perineal-pattern morphology,” in An advanced treatise on meloidogyne II. Methodology, vol. Volume II . Eds. Sasser, J. N., Carter, C. C. (Raleigh, NC, USA: North Carolina State University Graphics), 69–77.
Heald, C. M. (1987). “Classical nematode management practices,” in Vistas on nematology. Eds. Veech, J. A., Dickson, D. W. (Hyatssville: MD: Society of Nematologists), 100–105.
Hirschmann, H., Volume, I. (1985). “The genus Meloidogyne morphological characters differentiating species,” in An advanced treatise on Meloidogyne. Eds. Sasser, J. N., Carter, C. C. (Raleigh, USA: Eds North Carolina State University Graphics), . 79–. 93.
Hunt, D. J., Handoo, Z. A. (2009). “Taxonomy, identification and principal species,” in Root-knot nematodes. Eds. Perry, R. N., Moens, M., Starr, J. L. (Wallingford, UK: CAB International), 55–97.
Jena, R. N., Rao, Y. S. (1973). “Root-knot nematode resistance in rice,” in Proceedings of the second general congress breeding re-searches in asia and oceana SABRAO (New Delhi, India), 1080–1109.
Jepson, S. B. (1987). Identification of root-knot nematodes (Meloidogyne species). 1st ed (Wallingford, UK: CAB International).
Jones, J. T., Haegeman, A., Danchin, E. G. J., Gaur, H. S., Helder, J., Jones, M. G. K., et al. (2013). Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 14, 946–961. doi: 10.1111/mpp.12057
Karssen, G. (1996). On the morphology of Meloidogyne sasseri Handoo, Huettel & Morgan Golde. Nematologica 42 (2), 262–264. doi: 10.1163/004325996X00093
Karssen, G., Van Aelst, A. C. (2001). Root-knot nematode perineal pattern development: reconsideration. Nematology 32, 95–111. doi: 10.1163/156854101750236231
Karssen, G., Wesemael, W., Moens, M. (2013). “Root-knot nematodes,” in Plant nematology, 2nd ed. Eds. Perry, R. N., Moens, M. (Wallingford, UK: CABI, International), 73–108.
Kerry, B. R. (1987). “Biological control,” in Principles and practice of nematode control in crops. Eds. Brown, R. H., Kerry, B. R. (Sydney, Australia: Academic Press), 223–263.
Kyndt, T., Fernandez, D., Gheysen, G. (2014). Plant-parasitic nematode infections in rice: Molecular and cellular insights. Annu. Rev. Phytopathol. 52, 135–153. doi: 10.1146/annurev-phyto-102313-050111
Maleita, C., Esteves, I., Cardoso, J. M. S., Cunha, M. J., Carneiro, R. M. D. G., Abrantes, I. (2018). Meloidogyne luci, a new root-knot nematode parasitizing potato in Portugal. Plant Pathol. 67, 366–376. doi: 10.1111/ppa.12755
Marques, C. A. F. (2015). A brief overview of the Portuguese Agriculture – Its evolution, performance, and current situation. Informe Gepec 19 (1), 174–184. doi: 10.48075/igepec.v19i1.12570
Mbow, C., Rosenzweig, C., Barioni, L. G., Benton, T. G., Herrero, M., Krishnapillai, M., et al. (2019)“Food security,” in Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. Eds. Shukla, P. R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D. C., Zhai, P., Slade, R., Connors, S., van Diemen, R., Ferrat, M., Haughey, E., Luz, S., Neogi, S., Pathak, M., Petzold, J., Portugal Pereira, J., Vyas, P., Huntley, E., Kissick, K., Belkacemi, M., Malley, J.doi: 10.1017/9781009157988.007
Norton, D. C., Niblack, T. L. (1991). “Biology and ecology of nematodes,” in Manual of agricultural nematology. Ed. Nickle, W. R. (New York, NY, USA: CRC Press), 47–71.
Norton, D. C., Schmitt, D. P. (1978). Community analyses of plant-parasitic nematodes in the Kalsow Prairie, Iowa. J. Nematol. 10, 171–176.
Pais, C. S., Abrantes, I. M. O. (1989). Esterase and malate dehydrogenase phenotypes in portuguese populations of meloidogyne species. J. Nematol. 21 (3), 342–346.
Pais, C. S., Abrantes, I. M. O., Fernandes, M. F. M., de Santos, M. S. N. A. (1986). Tecnica de electroforese aplicada ao estudo das enzimas dos nematodes-das-galhas-radiculares, Meloidogyne spp. Ciec. Biol. Ecol. Syst. 6, 19–34.
Pires, D., Vicente, C. S. L., Menéndez, E., Faria, J. M. S., Rusinque, L., Camacho, M. J., et al. (2022). The fight against plant-parasitic nematodes: current status of bacterial and fungal biocontrol agents. Pathogens 11, 1178. doi: 10.3390/pathogens11101178
PORDATA. (2022). Explorações agrícolas e superfície. Available at: https://www.pordata.pt/portugal/superficie+agricola+utilizada+total+e+por+tipo+de+composicao-3346 (Accessed February 2023).
Rusinque, L., Nóbrega, F., Cordeiro, L., Serra, C., Inácio, M. L. (2021). First detection of meloidogyne luci (Nematoda: meloidogynidae) parasitizing potato in the azores, portugal. Plants 10, 99. doi: 10.3390/plants10010099
Santos, D., Correia, A., Abrantes, I., Maleita, C. (2019). The quarantine root knot nematode Meloidogyne enterolobii—A potential threat to Portugal and Europe. Plant Pathol. 68, 1607–1615. doi: 10.1111/ppa.13079
Standard Protocol PM 7/119 (1). (2013). Nematode extraction; EPPO bulletin 43 (Paris, France: EPPO), 471–495.
Starr, J. L., Roberts, P. A. (2004). “Resistance to plant-parasitic nematodes,” in Nematology advances and perspectives: vol. II nematode management and utilization. Eds. Chen, Z. X., Chen, S. Y., Dickson, D. W. (Oxfordshire, UK: CAB International), 879–907.
Subbotin, S. A., Palomares-Rius, J. E., Castillo, P. (2021). “Chapter 1 taxonomic history,” in Systematics of root-knot nematodes (Nematoda: meloidogynidae) (Leiden, The Netherlands: Brill). doi: 10.1163/9789004387584_002
Keywords: esterase, horticulture, Meloidogyne, management, frequency
Citation: Rusinque L, Camacho MJ, Serra C, Nóbrega F and Inácio ML (2023) Root-knot nematode assessment: species identification, distribution, and new host records in Portugal. Front. Plant Sci. 14:1230968. doi: 10.3389/fpls.2023.1230968
Received: 29 May 2023; Accepted: 11 July 2023;
Published: 08 August 2023.
Edited by:
Zafar Ahmad Handoo, United States Department of Agriculture, United StatesReviewed by:
Ashish Kumar Singh, ICAR-Vivekananda Parvatiya Krishi Anusandhan Sansthan, IndiaCopyright © 2023 Rusinque, Camacho, Serra, Nóbrega and Inácio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria L. Inácio, bHVyZGVzLmluYWNpb0Bpbmlhdi5wdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.