- College of Life Science, Capital Normal University, Beijing, China
Globally, the species of Amanita are key components of ectomycorrhizal ecosystems. Some of them are widely known as poisonous or edible fungi. Although many new Amanita species from China have been described, the species diversity of Yanshan Mountains remains unknown. We here describe three new species, namely, A. borealis sp. nov. (Sect. Amanita), A. brunneola sp. nov. (Sect. Caesareae), and A. yanshanensis sp. nov. (Sect. Validae), based on morphological observations and molecular phylogenetic analyses. In addition, nine known species, namely, A. caesareoides (Sect. Caesareae), A. chiui (Sect. Vaginatae), A. muscaria (Sect. Amanita), A. oberwinklerana (Sect. Roanokenses), A. ovalispora (Sect. Vaginatae), A. subglobosa (Sect. Amanita), A. subjunquillea (Sect. phalloideae), A. vaginata var. vaginata (Sect. Vaginatae), and A. virosa (Sect. phalloideae), were reported from Yanshan Mountains for the first time. Our results emphasize that China has a high diversity of Amanita species and that additional studies are required to understand the exact species number. These findings play a crucial role in Amanita toxin research and ecological conservation. This study investigated the areas where Amanita species-related research is lacking. The study also attempted to better understand Amanita distribution and thus contribute to related research. This study enriches the species diversity of Amanita in Yanshan Mountains and offers additional data supporting the macrofungal systematics, toxin research, and diversity and ecological studies of Amanita in future studies.
1 Introduction
Amanita Pers., the largest genus of the family Amanitaceae E.-J. Gilbert, was established by Persoon (Persoon, 1797). It is an almost cosmopolitan genus comprising approximately 650 accepted species (Yang et al., 2004; Tulloss, 2005; Yang, 2005; Menolli et al., 2009; Yang, 2015; Kim et al., 2013a, b; Cai et al., 2014; Ariyawansa et al., 2015; Cho et al., 2015; Cai et al., 2016; Cui et al., 2018; Yang et al., 2018; Fraiture et al., 2019; Mighell et al., 2019; Mighell et al., 2021; Su et al., 2022).
Most Amanita species are ectomycorrhizal fungi of ecological importance, and more than 10 plant families are known to be symbiotically associated with Amanita (Beeli et al., 1935; Reid, 1980; Pegler and Shah-Smith, 1997; Wood, 1997; Yang, 1997; Yang, 2005; Davison et al., 2017; Cui et al., 2018). However, some Amanita species may be saprotrophic (e.g., A. pruittii A. H. Sm. ex Tulloss) (Cui et al., 2018).
Some species are commonly known as edible fungi, such as A. caesarea (Scop.) Pers., A. sinensis Zhu L. Yang, and A. yuaniana Zhu L. Yang et al. In addition, some Amanita species are poisonous, including A. subjunquillea S. Imai, A. virosa Bertill., and A. tenuifolia (Murrill) Murrill et al. (Yang, 2005). In China, deaths caused by consuming poisonous Amanita species are common (Li et al., 2020; Li et al., 2021a; Li et al., 2021b).
Based on the traditional morphological and anatomical characteristics, and the molecular phylogeny evidence, the classification of Amanita has also undergone many changes. Corner and Bas (1962) and Bas (1969) considered observing the natural characteristics of Amanita species in the wild important. They applied microscopic characteristics to taxonomy and split the species into two subgenera and six sections. Many mycologists have accepted this taxonomic method as a great historical advance in the Amanita classification (Jenkins, 1977; Hongo, 1982; Jenkins, 1986; Mao, 1990; Pegler and Shah-Smith, 1997). However, the classification of its subgenera remains disputed (Moser, 1967; Garcin, 1984; Singer, 1986). Subsequently, the systematic research on Amanita is gradually deepening with the development and advancement of molecular systematics. A recent comprehensive phylogenetic study introduced the latest classification system for Amanita. According to this system, Amanita was divided into 3 subgenera and 11 sections (Cui et al., 2018). This system is followed by other mycologists (Kumar et al., 2021; Suwannarach et al., 2022; Huang et al., 2023).
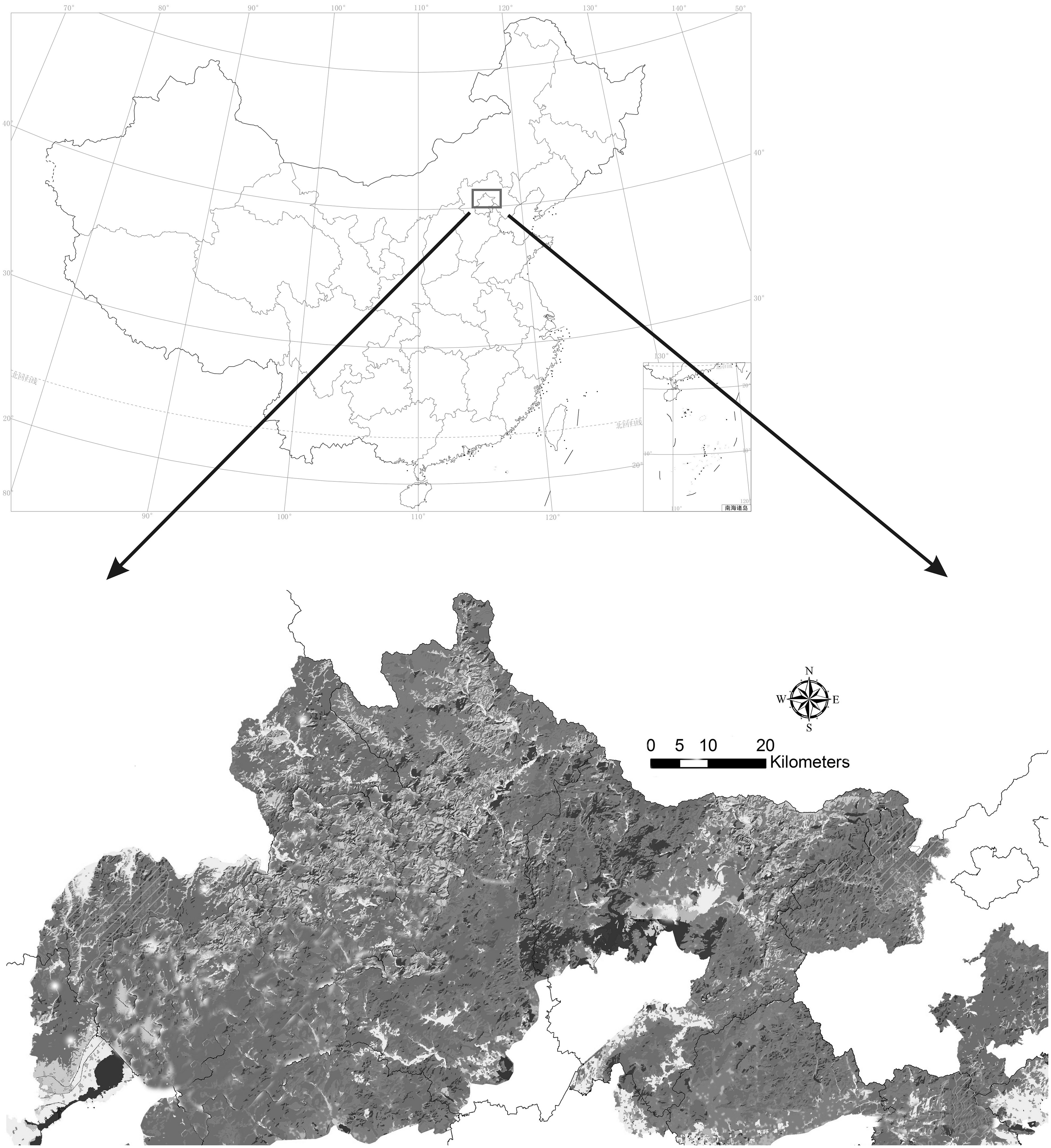
The Yanshan Mountains (115°–119°47′E, 39°40′–41°20′N) is located in northern China and has a high plant diversity (Figure 1). The main forest types on these mountains are deciduous broad-leaved forests and mixed coniferous and broad-leaved forests. The original dominant ectomycorrhizal plants included Quercus mongolica Fisch. ex Ledeb., Betula platyphylla Suk., Abies nephrolepis (Trautv.) Maxim., Populus tomentosa Carrière, and Pinus tabuliformis Carr. (Wang et al., 2021). The Yanshan Mountains region has a warm–temperate continental monsoon climate with an annual precipitation of 350–700 mm. The peak of precipitation occurs in June–August. The altitude of these mountains ranges from 200 to 2,200 m (Zhou et al., 2022a; Zhou et al., 2022b; Zhou et al., 2022c). Some past records of Amanita species in this area are available (Yang, 2004; Chen et al., 2006; Zhang et al., 2017; Cui et al., 2018; Wu et al., 2020). However, information available on Amanita species on these mountains is incomplete.
Taxonomic research has never been considered a popular study, but it can be considered the basis for understanding the world and research in related professional fields and can only be studied and applied if we figure out what the species really is. Especially think of Amanita, which are a very attractive taxa of macrofungi. It plays an important role in toxin research and ecological conservation. In the present study, 36 fresh Amanita specimens were collected from Yanshan Mountains. There were 20 herbarium specimens were loaned from the Herbarium Mycologicum Academiae Sinicae (HMAS, Institute of Microbiology, Chinese Academy of Sciences) for further research. On the basis of morphological examination and inference of phylogeny, three new species and nine known species were reported herein. The study aimed to determine the taxonomic status and phylogenetic position of Amanita species represented by these specimens so as to establish a comprehensive database of macrofungal diversity in Yanshan Mountains, especially Amanita species, and to use this database as a basis for future studies on macrofungal systematics, fungal toxin, diversity, and ecology in this region.
2 Materials and methods
2.1 Sample collection and morphological analyses
The specimen collection area is shown in Figure 1 (Figure and data provided by the Chinese Academy of Environmental Sciences). The specimens were collected during 2019–2022 from Yanshan Mountains and photographed in the field. Macroscopic features of fresh specimens such as colors and odors were noted. Color codes and designations were assigned after referring to the website ColorHexa (https://www.colourhexa.com). The specimens were dried using a Dorrex dryer at 50°C for approximately 12 h and deposited in the Herbarium of the College of Life Science, Capital Normal University, Beijing, China (BJTC). Other 20 herbarium specimens from the research area were obtained from the HMAS.
To observe microscopic characters, thin sections of the dried material were mounted in 3% KOH or sterilized water. Then, the materials were stained with 1% Congo red to increase the visibility of the structures. Microscopic features (e.g., basidiospores, pileipellis, and volval remnants) were observed and measured under a light microscope (Olympus DP71, Tokyo, Japan). Basidiospore measurements of new species are presented as (a)b − c(d). Among them, b − c, a, and d represent a minimum of 90% of the measured values, minimum extreme values, and maximum extreme values, respectively. Q represents the length/width ratio of the basidiospores, and Qm is the average Q values of all basidiospores measured (Bas, 1969). Qm values ± sample standard deviations are provided. The descriptive terms are in accordance with Cui et al. (2018).
2.2 DNA extraction, and PCR amplification and sequencing
DNA was extracted using the M5 Plant Genomic DNA Kit (Mei5 Biotechnology, Co., Ltd., Beijing, China). The extracted DNA was solubilized in 1× TE buffer/sterile water and stored at −20°C for further use. The following primer sets were used for amplification: nrITS1f/nrITS4 (White et al., 1990; Gardes and Bruns, 1993) for the nuclear ribosomal DNA internal transcribed spacer (nrITS rDNA) region, LR5/LR0R (Vilgalys and Hester, 1990) for the large subunit nuclear rDNA (nrLSU rDNA) region, rpb2-6f/rpb2-7r (Cai et al., 2014) for the second largest subunit of the RNA polymerase II (rpb 2) region, Am-b-tubulin F/Am-b-tubulin R (Cai et al., 2014) for the beta-tubulin (β-tubulin) region, and EF1-983F/EF1-1567R (Rehner and Buckley, 2005) for the translation elongation factor 1-α (tef1-α) region. PCRs were performed in a reaction volume of 25 μL. The obtained DNA was subjected to Sanger dideoxy sequencing (Sangon Biotechnology, Co., Ltd, Shanghai, China). The new sequences obtained in this study were deposited in GenBank (https://www.ncbi.nlm.nih.gov). Table 1 lists the accession numbers of the sequences used for phylogenetic analysis.
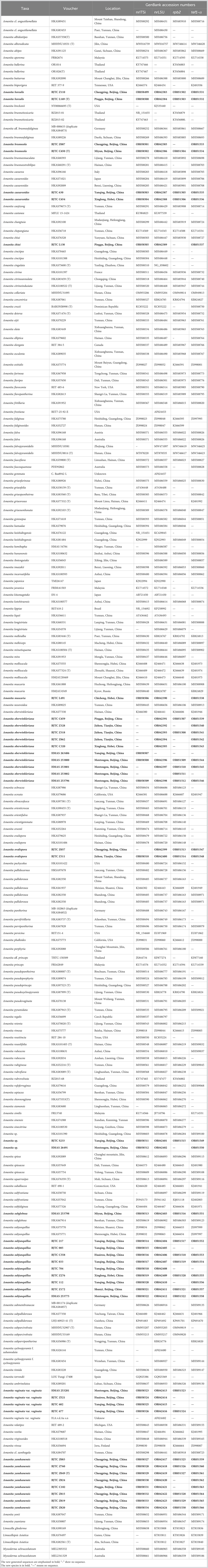
Table 1 Information of sequences used in the nrITS-nrLSU-rpb2-tef1-α phylogenetic analysis in this study.
2.3 Molecular data analyses and species delimitation
The nrITS-nrLSU-rpb2-tef1-α multi-locus dataset included 216 ingroup samples. These samples were used to infer the phylogenetic status of our Amanita specimens at the phylloclade level. The nrLSU dataset included 204 ingroup samples. These samples were used to analyze the subsection in which the species were located. Furthermore, nrITS was used to infer phylogenetic relationships between new and known Amanita species, as GenBank contains a large amount of nrITS sequence data for this genus. These nrITS sequences of new species were divided into different datasets, given that these sequences are too variable to obtain reliable genus-wide comparisons. Based on previous study findings and the GenBank database of the National Center for Biotechnology Information, reference sequences of all Amanita species in the dataset were selected for phylogenetic analysis (Cui et al., 2018; Su et al., 2022, Table 1). Limacella glioderma (Fr.) Maire (HKAS 90169), Limacellopsis asiatica Zhu L. Yang, Q. Cai & Y.Y. Cui (HKAS 76497 and 82561), Myxoderma ochraceoluteum (P.D. Orton) Zhu L. Yang, Q. Cai & Y.Y. Cui (MEL 2305332), and M. ochraceoluteum (MEL2341329) as outgroup refers to Cui et al. (2018).
All sequences were compared using MAFFT v.6 (Katoh and Toh, 2010) and trimmed automatically using Gblocks 0.91b (http://phylogeny.lirmm.fr/phylo_cgi/one_task.cgi?task_type=gblocks) (Dereeper et al., 2008). Bayesian inference (BI) analysis was performed using MrBayes v.3.1.2 (Ronquist and Huelsenbeck, 2003), and maximum likelihood (MI) analysis gene trees were estimated using RAxML 7.4.2 Black Box (Stamatakis, 2006; Stamatakis et al., 2008).
The BI analysis was performed using a Markov chain Monte Carlo (MCMC) algorithm (Rannala and Yang, 1996) and MrBayes 3.1.2 (Ronquist and Huelsenbeck, 2003) based on the best substitution model determined by MrModeltest 2.3 (Nylander, 2004), GTR + I + G for nrITS, nrLSU, rpb2, and tef-1α. Two MCMC chains were run from random trees for 10,000,000 generations, stopping when the average standard deviations of split frequencies were less than 0.01. Trees were stored for each 1,000 generations. The first 25% of the trees were excluded as the burn-in stage for each analysis. Branches with significant Bayesian posterior probability (BPP) values were then estimated in the resulting trees (Posada and Crandall, 1998). The ML analysis was performed using a GTR + GAMMA + I locus replacement model (Guindon et al., 2010). The branch support was obtained using the bootstrapping (BS) method of 1,000 replications (Hillis and Bull, 1993). Branches with a bootstrap (BS) support of ≥50% and BPP of ≥0.95 were considered significant (Hillis and Bull, 1993).
3 Results
3.1 Phylogenetic analyses
The nrLSU dataset contained 209 sequences, including 42 newly obtained sequences. The length of the aligned dataset was 790 bp long. The nrLSU phylogenetic analysis results revealed that our specimens belonged to six sections under Amanita, namely, sections Amanita Pers., Caesareae Singer, Roanokenses Singer, Phalloideae Quél., Validae Quél, and Vaginatae Quél (Figure S1), and were divided into 12 clades. Subsequently, the nrITS-nrLSU-rpb2-tef1-α multi-locus phylogenetic analysis was performed to infer the phylogenetic status of our Amanita specimens at the phylloclade level (Figure 2). The combined nrITS-nrLSU-rpb2-tef1-α dataset had 726 sequences, including 143 newly obtained sequences in this study. The aligned dataset was 2,150 bp long including alignment gaps (245 bp for nrITS, 790 bp for nrLSU, 660 bp for rpb2, and 365 bp for tef1-α). Results of the nrITS-nrLSU-rpb2-tef1-α and nrLSU phylogenetic analyses revealed that the subgenera and sections proposed by Cui et al. (2018) were strongly supported with significant BPP values and ML bootstrap (MLB).
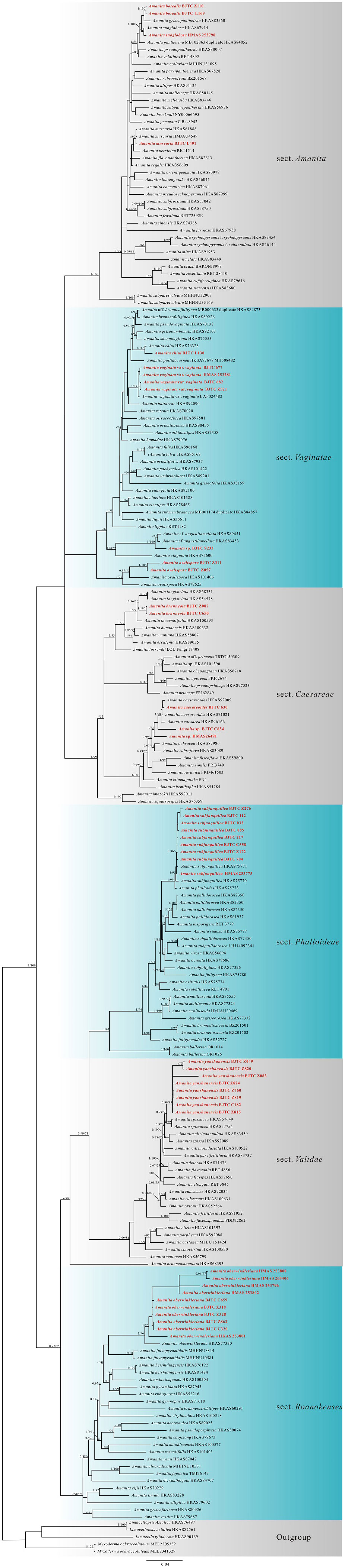
Figure 2 The nrITS-nrLSU-rpb2-tef1-α multi-locus phylogenetic tree obtained from the Bayesian analysis.
The nrITS-nrLSU-rpb2-tef1-α multi-locus phylogenetic analysis showed that our specimens were clustered into 14 clades. Moreover, they formed three distinct and strongly supported new branches, which were nested in sections Amanita, Caesareae, and Validae, respectively. These three new lineages were as follows: two specimens (BJTC Z110 and BJTC L169) formed one clade (BPP = 1.00, MLB = 100%) and were closely related to A. griseopantherina Yang-Yang Cui, Qing Cai & Zhu L. Yang, A. pantherina (DC.) Krombh., and A. subglobosa Zhu L. Yang on Figure 2. The two specimens (BJTC Z087 and BJTC C650) clustered into a branch with a high support (BPP = 1.00, MLB = 100%), which further clustered into a clade containing A. longistriata S. Imai., with moderate support. Eight specimens (BJTC Z049, BJTC Z820, BJTC Z083, BJTC Z824, BJTC Z760, BJTC Z819, BJTC C182, and BJTC Z815) were clustered together, forming a completely supported clade (BPP = 0.99, MLB = 99%). The new branches clustered with A. spissacea S. Imai and formed a sister clade in the phylogenetic tree. The nrLSU phylogenetic analysis revealed topologies similar to those of the multi-locus phylogenetic tree, and the specimens also formed three new lineages. Therefore, based on the results of phylogenetic and morphological analyses, these new clades were identified as three new species herein.
In addition, through morphological and phylogenetic analyses, we identified nine known Amanita species collected from Yanshan Mountains, northern China, including A. caesareoides Lj. N. Vassiljeva, A. chiui Yang-Yang Cui, Qing Cai & Zhu L. Yang, A. muscaria (L.: Fr.) Lam., A. oberwinkleriana Zhu L. Yang & Yoshim. Doi, A. ovalispora Boedijn, A. virosa Bertillon, A. subglobosa Zhu L. Yang, A. subjunquillea S. Imai, and A. vaginata var. vaginata (Bull.) Lam. Because we could not obtain the sequence information of the herbarium specimens (HMAS 40501 and HMAS 40503) of A. virosa, we could only identify them through morphological observation.
Although the specimen BJTC S233 was clustered with A. cf. angustilamellata (HKAS 89451 and HKAS 83453) in the nrITS-nrLSU-rpb2-tef1-α and nrLSU phylogenetic analyses, additional specimens are required to elucidate its phylogenetic position and morphological characters. The specimens BJTC C654 and HMAS 26491 probably represented undescribed species. However, they could not be described in the present study because of the poor condition of their basidiomata, inadequate number of specimens, and uncertain phylogenetic position in the nrITS-nrLSU-rpb2-tef1-α phylogenetic analysis. Therefore, we only temporarily termed them as Amanita sp.
The numbers above the branches represent strong support (BPP ≥0.95 and/or MLB ≥50%). Red font represents t the location of the newly acquired sequences. Table 1 presents the accession numbers of sequence information used.
3.2 Taxonomy
Based on our phylogenetic and morphological data, three new species and nine known species of Amanita from Yanshan Mountains are described below.
3.2.1 Amanita borealis H. Zhou & C. L. Hou, sp. nov.

Figure 3 Fresh basidiomata of new species of Amanita in this study. (A, B) Amanita borealis sp. nov. (type, BJTC L169); (C) Amanita brunneola sp. nov. (type, BJTC C650); (D) Amanita brunneola sp. nov. (BJTC Z087); (E, F) Amanita yanshanensis sp. nov. (type, BJTC Z049). Bars: (A–E) = 2 cm.
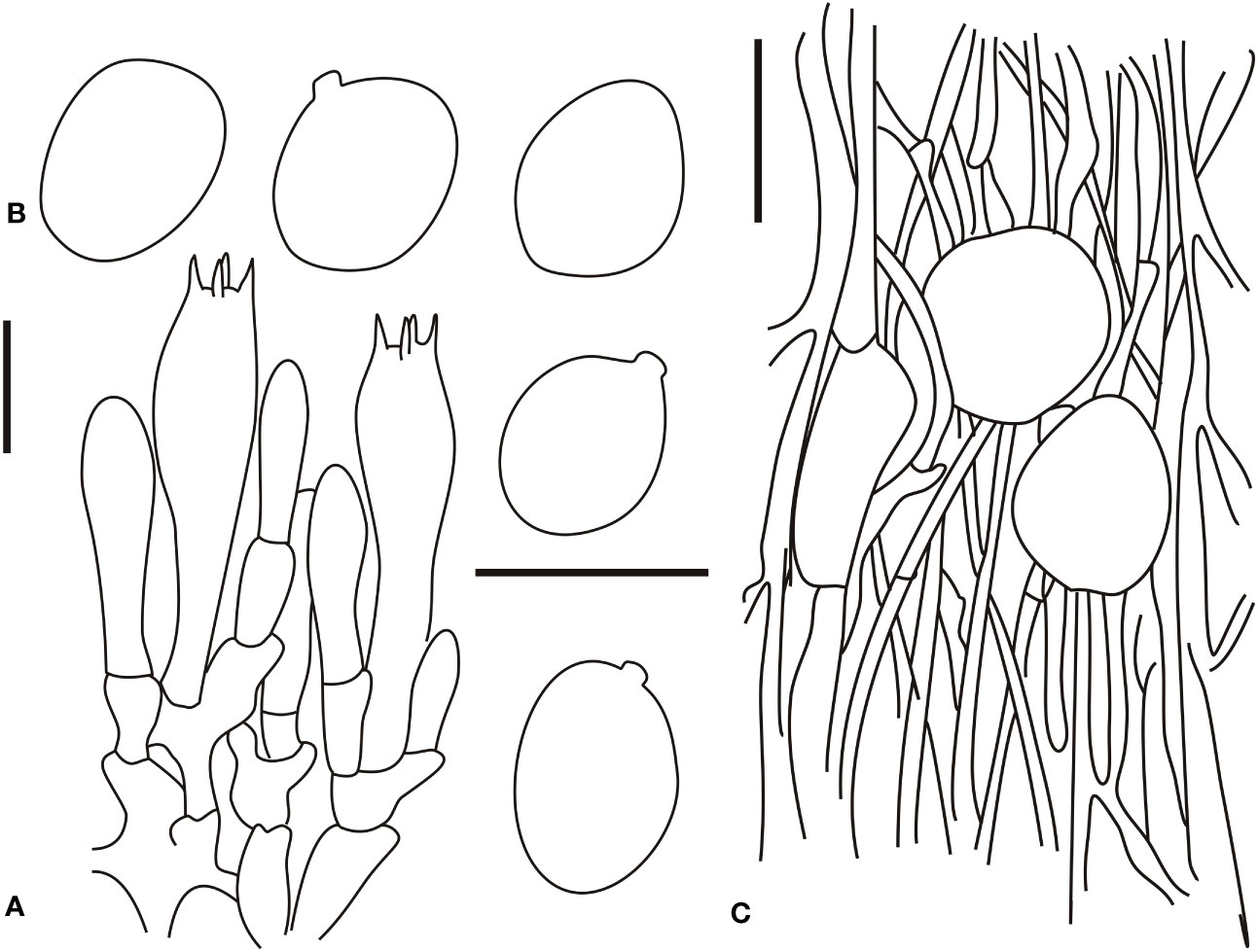
Figure 4 Microscopic characters of Amanita borealis (type, BJTC L169). (A). Hymenium and subhymenium; (B). Basidiospores; (C). Longitudinal section of volval remnants on pileus. Bars: (A, B) = 10 μm, (C) = 40 μm.
MycoBank: MB 847659
Etymology: The specific epithet “borealis” means “northern,” referring to the native occurrence of this species in the Northern China.
Type: CHINA. Beijing, Pinggu district, Dongniujiaoyu village, 40.323599 N, 117.156964 E, alt. 477 m, 19 Aug., 2020, coll. G.Q.C., C.L.H. and Y.T.Z. (L169/BJTC L169).
Basidiomata small- to medium-sized. Pileus 2–6 cm in diam., plano-convex to applanate, lacking an obvious depression or umbo at the center; surface brownish (#915b25), brown (#7d4e20) to dark brown (#402810), darker at the center, pyramidal, subverrucose to subconical, dirty white (#f2f2f2) to white (#ffffff) volval remnants on the pileus (ca. 2–8-mm diam.), densely arranged over the disk; margin slightly striate (ca. 0.05–0.1 R), non-appendiculate; trama white (#ffffff), unchanging. Free, crowded, white (#ffffff) lamellae; truncated, plentiful lamellulae. Stipe 5–10 cm long, 0.4–1.1 cm wide at the apex, subcylindric or slightly attenuate upward, surface white (#ffffff), with silk luster, glabrous or covered with concolorous, floccose squamules; white (#ffffff) to yellowish (#ffffe7) context; basal bulb subglobose to fusiform (1.5–2.5-cm diam.), white (#ffffff) to yellowish (#ffffe7); floccose volval remnants on the stipe base, arranged in belts on the lower part of the stipe, and often forms a collar-like or shortly limbate volva on limit between the stipe and basal bulb, white (#ffffff) to yellowish (#ffffe7). Annulus apical, subapical to fugacious, thick, with white (#ffffff) and silk luster on the upper surface. Spore print not observed. Odor indistinct.
Bilateral lamellar trama, 20–60-μm-wide mediostratum, composed of abundant subfusiform, ellipsoid to clavate inflated cells (20–80 × 12–50 μm); abundant, 2–9-μm-wide filamentous hyphae; scarce vascular hyphae. Lateral stratum is composed of abundant subfusiform to ellipsoid inflated cells (20–45 × 6–25 μm), diverging at an angle of ca. 30°–45° to the mediostratum; abundant, 3–8-μm-wide filamentous hyphae. A 30–60-μm-thick subhymenium, with two to three layers of ovoid, subglobose, fusiform, ellipsoid, or irregular cells (10–30 × 8–20 μm). Basidia (30–50 × 7.5–14 μm), slenderly clavate, four-spored, with clamps, hyaline; 3–5-μm-long sterigmata; basidiospores [60/2/2] (8.0–)8.7–9.6(–11.5) × (6.0–)6.5–8.2(–8.5) μm, Q = (1.10–)1.22–1.45(–1.51), Qm = 1.31 ± 0.10, broadly ellipsoid to ellipsoid, thin-walled, hyaline, pale yellow, smooth, small apiculus, inamyloid; sterile lamellar edge, composed of subglobose to ellipsoid or sphaeropedunculate inflated cells (15–40 × 10–30 μm), single and terminal or in chains of 2–3, thin-walled, hyaline; abundant, 3–9-μm-wide filamentous hyphae, irregularly arranged or ± running parallel to the lamellar edge. Pileipellis 50–200 μm thick, gelatinized upper layer (30–75 μm thick), composed of radially, thin-walled, colorless, 2–8-μm-wide filamentous hyphae; lower layer (40–100 μm thick) composed of radially and compactly arranged, colorless, 2–8-μm-wide filamentous hyphae; scarce vascular hyphae. Volval remnants on the pileus are composed of somewhat vertically to irregularly arranged elements: 3–7-μm-wide scarce to scattered, subcolorless, thin-walled, branching, anastomosing filamentous hyphae; globose, subglobose, fusiform to ellipsoid, sometimes irregular, inflated cells (30–70 × 10–55 μm) that are nearly colorless, slightly thick-walled, and terminal or in chains of 2–3; scarce vascular hyphae. Volval remnants on the stipe base similar to that on the pileus, but with more abundant filamentous hyphae and fairly abundant vascular hyphae. Longitudinally acrophysalidic stipe trama; acrophysalides (50–300 × 15–50 μm); scattered to abundant, 3–15-μm-wide filamentous hyphae. Annulus is composed of radially arranged elements: abundant, subglobose, fusiform to ellipsoid inflated cells (20–40 × 10–30 μm) that were hyaline and thin-walled; abundant, 2–7-μm-wide filamentous hyphae that were hyaline and thin-walled. Clamps present in all parts of basidioma.
Habitat and distribution: This species is scattered in the broad-leaved forests of Q. mongolica Fisch. Ex Ledeb. Basidioma occurs in summer and autumn.
Additional specimens examined: CHINA. Beijing, Changping district, Beitaizi, 40.272906 N, 116.420298 E, alt. 149 m, 15 Aug., 2019, coll. H.Z., X.Y.S. and Y.T.Z. (ZH110/BJTC Z110).
Commentary: A. borealis is well circumscribed by its brownish to dark-brown pileus densely covered with pyramidal, subverrucose to subconical, floccose volval remnants on the stipe base arranged in incomplete belts, and broadly ellipsoid to ellipsoid basidiospores (8.0–11.5 × 6.0–8.5 μm). Furthermore, it is found in association with Fagaceae (Quercus mongolica) trees.
A. borealis belongs to section Amanita and is closely related to A. griseopantherina, A. pantherine, and A. subglobosa on the multi-locus and nrITS phylogenetic trees (Figures 2, S2). In addition, A. borealis, A. subglobosa, and A. pantherine have similar morphologies. A. pantherine is a species originally described from Europe but is not found in China. It has a relatively lower annulus, narrower basidiospores, and no clamps (Cui et al., 2018). From the morphological viewpoint, A. subglobosa can be separated from A. borealis based on its relatively darker-colored pileus, usually with the presence of clamps and wider basidiospores (8.5–12.0 × 7.0–9.5 μm) (Yang, 1997; Yang, 2005; Yang, 2015; Cui et al., 2018).
3.2.2 Amanita brunneola H. Zhou & C. L. Hou, sp. nov.
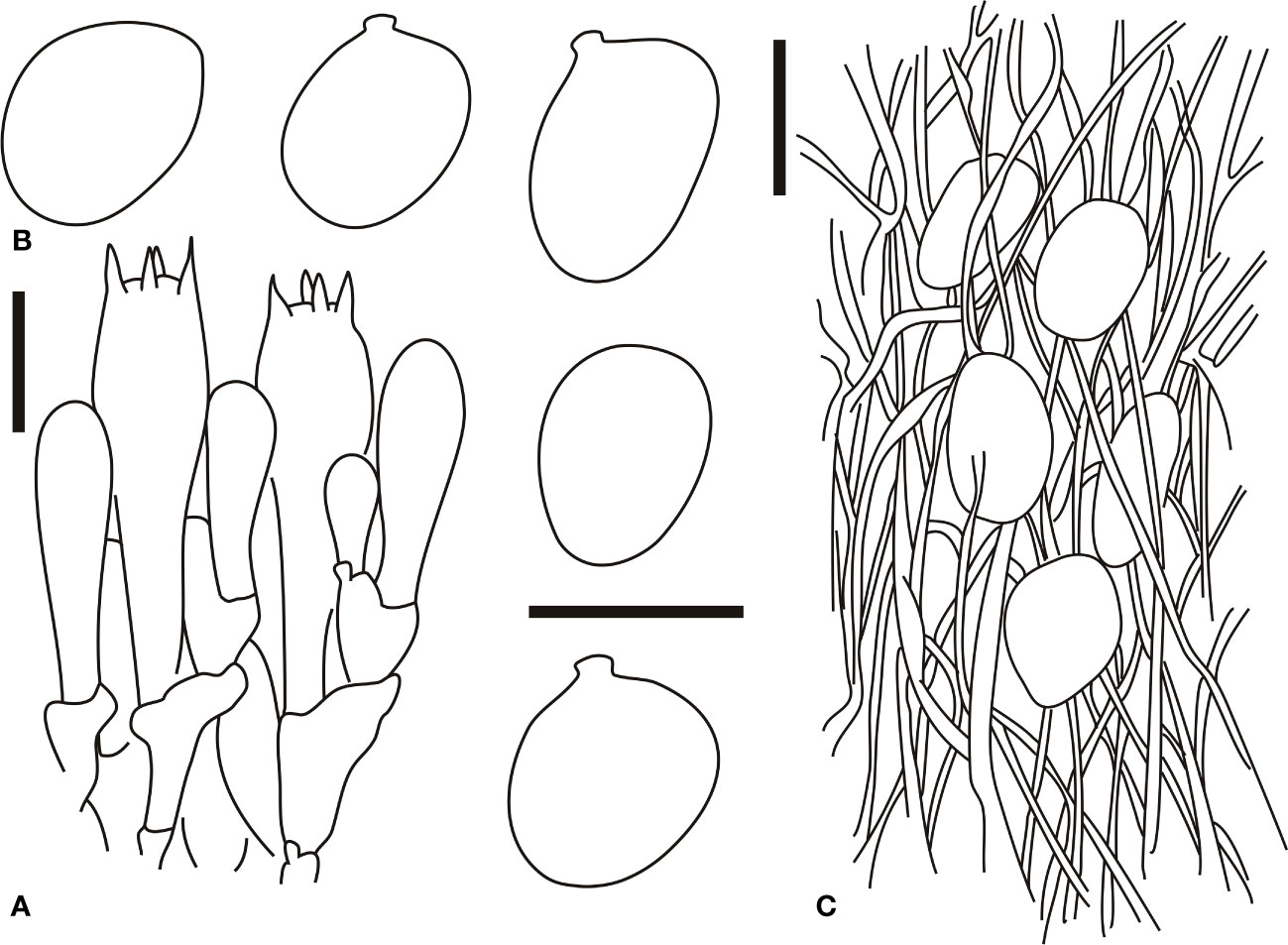
Figure 5 Microscopic characters of Amanita brunneola (type, BJTC C650). (A). Hymenium and subhymenium; (B). Basidiospores; (C). Longitudinal section of outer part of volval remnants on stipe base. Bars: (A, B) = 10 μm, (C) = 40 μm.
MycoBank: MB 847660
Etymology: The epithet “brunneola” refers to the brown tone of pileus.
Type: CHINA. Beijing, Miyun district, Heilongtan, 40.560617 N, 116.782229 E, alt. 260 m, 27 Aug., 2020, coll. G.Q.C., C.L.H. and Y.T.Z. (C650/BJTC C650).
Basidiomata small- to medium-sized. Pileus 3–7 cm in diam., convex to applanate, with an umbo at the center, brown tone (#808080) to dark orange (#a5682a) over the disk; verrucose to subconical, dirty white (#f2f2f2) to white (#ffffff) volval remnants on the pileus; margin slightly striate (ca. 0.25–0.3 R), non-appendiculate; trama white (#ffffff), unchanging. Free, crowded, white (#ffffff) to cream (#fffdd0) lamellae; truncated, plentiful lamellulae. Stipe 8–15 cm long, 0.5–1.5 cm wide at the apex, subcylindric or slightly tapering upward, with the apex slightly expanded, white (#ffffff) to pale grayish (#a6a6a6), glabrous above the annulus, and densely covered with gray (#a1a1a1) to dark gray (#888888) squamules under the annulus; white (#ffffff) context, hollow in the center; absence of basal bulb; volva saccate (ca. 2 × 2.5 cm) membranous, both surfaces white (#ffffff) to dirty white (#f2f2f2). Annulus subapical, pendant from attachment ca. 2 cm below the apex of the stipe, membranous, pale grayish upper surface (#808080), brownish gray lower surface (#a5682a). Spore print not observed. Odor indistinct.
Bilateral lamellar trama, 20–60-μm-wide mediostratum, composed of abundant subfusiform, ellipsoid to clavate inflated cells (35–80 × 10–40 μm); abundant, 4–12-μm-wide filamentous hyphae; scarce vascular hyphae. Lateral stratum is composed of abundant subfusiform to ellipsoid inflated cells (25–55 × 9–15 μm), diverging at an angle of ca. 30–45° to the mediostratum; abundant, 2–6-μm-wide filamentous hyphae. A 30–55-μm-thick subhymenium, with two to three layers of subglobose to ellipsoid or irregular cells (10–20 × 8–20 μm). Basidia (Figure 5A) 35–50 × 8.5–15 μm, slenderly clavate, four-spored, with clamps, hyaline; 2–5-μm-long sterigmata; basidiospores [60/2/2] (9.5–)9.9–11(–12) × (7.5–)8.5–9.2(–9.5) μm, Q = (1.12–)1.22–1.42(–1.53), Qm = 1.34 ± 0.12, mostly ellipsoid, occasionally broadly ellipsoid, thin-walled, hyaline, smooth, occasionally with a small apiculus, inamyloid; sterile lamellar edge, composed of subglobose to ellipsoid or sphaeropedunculate inflated cells (10–40 × 10–30 μm), single and terminal or in chains of 2–3, thin-walled, hyaline; abundant, 2–5-μm-wide filamentous hyphae, irregularly arranged or ± running parallel to the lamellar edge. Pileipellis 80–150 μm thick, slightly gelatinized upper layer (20–50-μm thick), composed of radially arranged, thin-walled, colorless, 2–5-μm-wide filamentous hyphae; lower layer (40–90-μm thick) composed of radially arranged, colorless to brownish (#a53f2a), 2–8-μm-wide filamentous hyphae; scarce vascular hyphae. Volval remnants on the pileus are composed of inflated cells up to 20–40 × 15–30 μm and abundant filamentous hyphae, subglobose, ovoid to ellipsoid, clavate or sphaeropedunculate, with hyaline to yellowish (#ffff9a) vacuolar pigments; septa with clamps; inner part of volval remnants on the pileus often with conspicuous vascular hyphae. Volval remnants on the stipe base are similar to those on the pileus, but with more abundant filamentous hyphae and fairly abundant vascular hyphae. Longitudinally acrophysalidic stipe trama; acrophysalides (35–280 × 15–50 μm); scattered to abundant, 3–13-μm-wide filamentous hyphae. Annulus is composed of radially arranged elements: scarce, ellipsoid to cylindrical, colorless, thin-walled inflated cells (30–80 × 10–20 μm); very abundant to dominant, 2–8 μm wide, colorless, thin-walled filamentous hyphae; scarce vascular hyphae. Clamps are present in all parts of basidioma.
Habitat and distribution: This species is scattered in the broad-leaved forests of Castanea mollissima Blume and Carpinus turczaninowii Hance. Basidioma occurs in summer and autumn.
Additional specimens examined: CHINA. Beijing, Changping district, Dayangshan Mountains National Forest Park, 40.30799 N, 116.424929 E, alt. 260 m, 14 Aug., 2019, coll. H.Z., X.Y.S.and Y.T.Z. (ZH087/BJTC Z087).
Commentary: A. brunneola is well circumscribed by its brownish to dark orange pileus, with an umbo at the center, covered with verrucose to subconical, densely covered with gray to dark gray squamules under the annulus, and mostly ellipsoid, occasionally broadly ellipsoid basidiospores (9.5–12 × 7.5–9.5 μm). Furthermore, it is found in association with Fagaceae and Betulaceae trees.
A. brunneola belongs to section Caesareae and is closely related to A. longistriata, A. fense M. Mu & L.P. Tang, and A. incarnatifolia Zhu L. Yang on the multi-locus and nrITS phylogenetic trees (Figures 2, S3). A. brunneola can be distinguished from A. fense and A. longistriata on the basis of its longer striations (0.3–0.5 R) on the pileal margin, white to cream lamellae, and a white stipe. Basidiospores of A. brunneola (10.0–13.0 × 8.0–11.0 μm) are longer than those of A. longistriata, whereas they are shorter than those of A. fense (Imai, 1938; Gilbert, 1940; Gilbert, 1941; Hongo, 1959; Yang and Doi, 1999; Yang, 2005; Yang, 2015; Cui et al., 2018; Wu et al., 2021).
3.2.3 Amanita caesareoides Lj. N. Vassiljeva, Notul. syst. Sect. cryptog. Inst. bot. Acad. Sci. U. S. S. R. 6: 199 (1950)

Figure 6 Fresh basidiomata of known species of Amanita in this study. (A, B) A caesareoides (BJTC C630); (C, D) A chiui (BJTC L130); (E, F) A muscaria (BJTC L491); (G) oberwinkleriana (BJTC C320); (H, I) A oberwinkleriana (BJTC C659); (J, K) A ovalispora (BJTC Z311); (L) A subjunquillea (BJTC 085); (M) A subjunquillea (BJTC 704); (N) A vaginata var. vaginata (BJTC 677); (O) A vaginata var. vaginata (BJTC 682). Bars: (A–O) = 2 cm.
Basidiomata small- to medium-sized. Pileus 5–10 cm in diam., applanate, often umbonate at the center, orange-red (#ffa500) to orange (#ffb733); absence of volval remnants on the pileus; margin striate (0.3–0.5 R), non-appendiculate. Cream (#fffdd0) to yellowish (#ffff4d) lamellae; truncated lamellulae. Stipe 8–18 cm long, 0.7–2 cm wide, yellowish (#ffff4d) to orange-red (#ffa500), with its surface covered with snakeskin-shaped, orange (#ffb733) squamules; absence of basal bulb; volval remnants on the stipe base saccate. Annulus subapical, orange-red (#ffa500) to orange (#ffb733). Spore print not observed. Odor indistinct.
Basidia (33–45 × 8–12 μm), clavate, four-spored. Basidiospores [60/4/2] (7.0–)7.5–9.5(–10.5) × (6.0–)6.5–8.2(–8.5) μm, Q = (1.06–)1.12–1.34(–1.40), Qm = 1.23 ± 0.06, broadly ellipsoid, rarely ellipsoid or subglobose, inamyloid. Clamps are present in all parts of basidioma.
Distribution: This species is known to be found in northeastern China (Yang, 2015; Cui et al., 2018), India (Bhatt et al., 2003; Bhatt et al., 2017), Japan (Imazeki and Hongo, 1987; Endo et al., 2016), Republic of Korea (Cho et al., 2015), and Russian Far East (Vassiljeva, 1950).
Habitat and distribution: It is present individually or is scattered in the broad-leaved forests of C. viminea Lindley and P. davidiana Dode. Basidioma occurs in summer and autumn.
Specimens examined: CHINA. Beijing, Yanqing district, Yudushan Mountains, 40.550099 N, 115.875254 E, alt. 963 m, 20 Aug., 2018, coll. C.L. Hou, H. Zhou and J.Q. Li (630/BJTC 630).
Commentary: A. caesareoides was first described from the Russian Far East by Vassiljeva (1950). It was subsequently reported from China, India, Japan, and Republic of Korea (Imazeki and Hongo, 1987; Bhatt et al., 2003; Cho et al., 2015; Yang, 2015; Endo et al., 2016; Bhatt et al., 2017). The morphological description of our specimen is consistent with that provided by Vassiljeva (1950). In our multi-locus phylogenetic analysis, the specimen BJTC 630 clustered with A. caesareoides (HKAS 92009 and HKAS 71021), forming a completely supported clade (BPP = 1.00, MLB = 88%) (Figure 2). Based on these characters, we described our specimen BJTC 630 as A. caesareoides. Detailed descriptions, line drawings, and images of A. caesareoides can be found in Yang (2015).
3.2.4 Amanita chiui Yang-Yang Cui, Qing Cai & Zhu L. Yang, Fungal Diversity 91: 77 (2018)
Basidiomata small- to medium-sized. Pileus 3–8 cm in diam., often dark gray (#a9a9a9), brown (#a5682a) to brownish (#d18e4a); volval remnants on the pileus mostly absent or occasionally retained as small, white (#ffffff) patches; margin striate (0.25–0.3 R), non-appendiculate; trama white (#ffffff), unchanging. Free, white (#ffffff) to cream (#fffdd0) lamellae. Stipe 6–10 cm long, 0.5–1.5 cm wide at the apex, white (#ffffff), dirty white (#fcfcfc), brownish (#a5682a) to brown (#cc8236). Annulus absent. Spore print not observed. Odor indistinct.
Basidia (45–65 × 14–17 μm), clavate, four-spored; 4–6-μm-long sterigmata; basal septa lacking clamps. Basidiospores [30/2/1] (9.0–)9.5–12.0(–12.5) × (8.0–)9.0–11.0(–11.5) μm, Q = (1.00–)1.05–1.25(–1.30), Qm = 1.15 ± 0.06, subglobose to broadly ellipsoid, inamyloid, colorless, thin-walled, smooth; small apiculus. Volval remnants on the stipe base are composed of longitudinally arranged elements: abundant to very abundant, branching, anastomosing filamentous hyphae. Clamps are absent in all parts of basidioma.
Distribution: This species is known to be found in northwestern and southwestern China (Cui et al., 2018).
Habitat and distribution: It is present individually or scattered in the broad-leaved forests of Castanea mollissima Blume, with basidioma occurring in summer and autumn.
Specimens examined: CHINA. Beijing, Pinggu district, Sizuolou, 40.272187 N, 117.135187 E, elev. 223 m, 16 August 2019, coll. C.L. Hou, G.Q. Cheng and R.T. Zhang (L130/BJTC L130).
Commentary: Generally, A. chiui is characterized by a dark gray, brown to brownish pileus; a white to dirty white stipe that is often densely covered with brownish squamules; and subglobose to broadly ellipsoid basidiospores (10.0–12.5 × 9.0–11.0 μm) (Cui et al., 2018). Our multi-locus phylogenetic analysis revealed that the specimen BJTC L130 clustered together with A. chiui (HKAS 77330, type), forming a completely supported clade (pp = 1.00, MLB = 100%). The nrLSU phylogenetic analysis exhibited topologies similar to those of the multi-locus phylogenetic tree (Figure S1). Based on these characters and phylogenetic analysis results, the specimen BJTC L130 was described as A. chiui. Detailed descriptions, line drawings, and images of A. caesareoides can be found in Cui et al. (2018).
3.2.5 Amanita muscaria (L.: Fr.) Lam., Encycl. Méth. Bot. (Paris) 1(1): 111 (1783)
Basidiomata small- to large-sized. Pileus 5–15 cm in diam., orange-red (#ffa500); pyramidal, conical, verrucose to felted, white (#fffff), sometimes yellowish (#ffffd8), removable volval remnants on the pileus; margin striate (up to 0.2 R), non-appendiculate; trama white (#ffffff), unchanging. White lamellae (#fffff), truncated lamellulae. Stipe 7–16 cm long, 0.5–2.0 cm wide at the apex., white (#fffff), covered with white (#fffff) fibrils; white (#fffff) context, unchanging; ovoid, fusiform to subglobose basal bulb (1–4-cm diam.); verrucose to conical, white (#fffff) to yellowish (#ffffd8) volval remnants on the stipe base, sometimes arranged in incomplete rings. Subapical to submedian annulus. Spore print not observed. Odor indistinct.
Basidia (40–60 × 12–15 μm), clavate, four-spored. Basidiospores [30/1/1] (9–)9.5–10.8(–12.5) × (7–)7.3–8.5(–9.0) μm, Q = (1.2–)1.24–1.45(–1.47), Qm = 1.32 ± 0.06, broadly ellipsoid to ellipsoid, inamyloid. Volval remnants on the pileus are composed of vertically arranged elements: scattered filamentous hyphae; very abundant inflated cells. The structure of volval remnants on the stipe base is similar to that of volval remnants on the pileus, but with irregularly arranged elements. Clamps are present in all parts of basidioma.
Distribution: This species is known to be found in Asia (Imai, 1933; Imai, 1938; Kumar et al., 1990; Yang, 2005; Geml et al., 2006; Geml et al., 2008; Yang, 2015), Europe (Neville and Poumarat, 2004), North America (Geml et al., 2006; Geml et al., 2008), and northeastern and northwestern areas of China (Cui et al., 2018).
Habitat and distribution: It is present individually or is scattered in the broad-leaved forests of B. platyphylla Suk. Basidioma occurs in summer and autumn.
Specimens examined: CHINA. Hebei Province, Chicheng County, Dahaituo Mountains National Nature Reserve, 40.563828 N, 115.798698 E, elev. 1640 m, 22 August 2018, coll. C.L. Hou, J.Q. Li and G.Q. Cheng (L491/BJTC L491).
Commentary: A. muscaria was described from Europe and represents the type species of Amanita (Gilbert, 1940; Gilbert, 1941; Jenkins and Petersen, 1976; Galli, 2001; Neville and Poumarat, 2004; Yang, 2005; Yang, 2015; Cui et al., 2018). Yang and Oberwinkler (1999) conducted a detailed study on A. muscaria basidiomal development and anatomy. The morphological description of our specimen is consistent with that given by Cui et al. (2018). In our multi-locus analysis (Figure 2), A. muscaria was found to be closely related to A. persicina (D.T. Jenkins) Tulloss & Gem. These results are consistent with those of Cui et al. (2018). Based on these characters, our specimen BJTC L491 was described as A. muscaria. Detailed descriptions of A. muscaria can be found in Cui et al. (2018).
3.2.6 Amanita oberwinkleriana Zhu L. Yang & Yoshim. Doi, Bull. Natn. Sci. Mus., Tokyo, Ser. B 25 (3): 120 (1999)
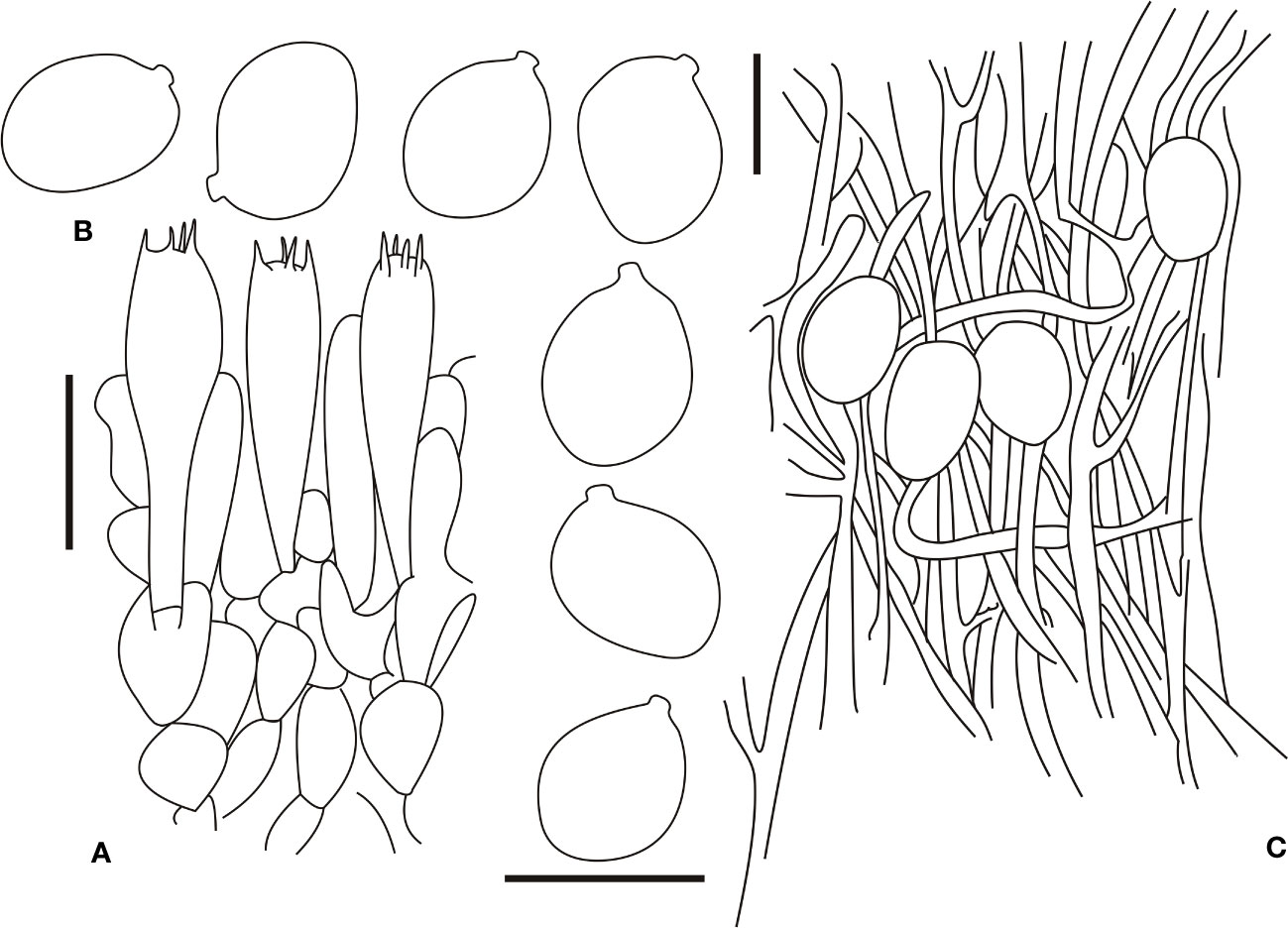
Figure 7 Microscopic characters of Amanita oberwinkleriana (BJTC C320). (A). Hymenium and subhymenium; (B). Basidiospores; (C). Longitudinal section of outer part of volval remnants on stipe base. Bars: (A, B) = 10 μm, (C) = 40 μm.
Basidiomata small- to medium-sized. Pileus 3–8 cm in diam., applanate, no slightly depressed at the center, surface gray-white (#e6e6e6) to white (#ffffff), large, gray-white (#e6e6e6) volval remnants on the pileus (ca. 2–4 (–6)-mm diam.), densely arranged over the disk; margin slightly striate (ca. 0.05–0.15 R), non-appendiculate. Free, white (#ffffff), unchanging, somewhat crowded lamellae; attenuate, plentiful lamellulae. Stipe 8–15 cm long, 0.5–2.0 cm wide at the apex, subcylindric or slightly tapering upward, with the apex slightly expanded, gray-white (#e6e6e6) to white (#ffffff), with minute concolorous squamules; grayish (#e6e6e6), unchanging context; subglobose basal bulb (1.0–2.0-cm diam.), dirty white (#f2f2f2), with upper part covered with verrucose, dirty grayish (#e6e6e6) to dirty white (#f2f2f2) volval remnants arranged in irregular concentric rings. Annulus absent. Spore print not observed. Odor indistinct.
Basidia (28–45 × 8–13 μm), slenderly clavate, four-spored, with clamps, hyaline to pale yellow (#ffffd8); 5–6-μm-long sterigmata, clamped basal septa; basidiospores [80/4/3] (6–)7.0–10(–10.5) × (5–)5.5–6.8(7.5) μm, Q = (1.25–)1.35–1.58(–1.62), Qm = 1.41 ± 0.11, mostly ellipsoid, occasionally broadly ellipsoid, thin-walled, hyaline, pale yellow, smooth, with a small to medium-large apiculus, amyloid. Volval remnants on the stipe base are similar to those on the pileus, but with more abundant filamentous hyphae and fairly abundant vascular hyphae. Clamps are present in all parts of basidioma.
Distribution: This species is known to be present in central, eastern, southern, and southwestern China (Yang and Li, 2001; Yang, 2005; Yang, 2015; Cui et al., 2018); India (Bhatt et al., 2003); Japan (Yang and Doi, 1999); and Republic of Korea (Kim et al., 2013a).
Habitat and distribution: It is present individually or is scattered in the coniferous forests and mixed coniferous and broad-leaved forests of Pinus tabuliformis Carr., Q. mongolica Fisch. ex Ledeb., and P. davidiana Dode. Basidioma occurs in summer and autumn.
Specimens examined: CHINA. Beijing, Hebei Province, Xinglong country, Babaziling village, 40.310326 N, 117.585117 E, alt. 878 m, 22 Aug., 2020, coll. G.Q. Cheng., C.L. Hou.and Y.T. Zhang (C320/BJTC C320); CHINA. Tianjin, Jizhou district, JiulongShan Mountains National Forest Park, 40.152701 N, 117.508008 E, alt. 300 m, 18 Aug., 2019, coll. H. Zhou, X.Y. Shen and Y.T. Zhou (ZH328/BJTC Z328); CHINA. Beijing, Pinggu district, Dongxinzhuang village, 40.560714 N, 116.779865 E, alt. 286 m, 27 Aug., 2020, coll. G.Q. Cheng., C.L. Hou and Y.T. Zhang (C659/BJTC C659); CHINA. Tianjin, Jizhou district, JiulongShan Mountains National Forest Park, 40.151969 N, 117.509612 E, alt. 226 m, 18 Aug., 2019, coll. H. Zhou, X.Y. Shen and Y.T. Zhou (ZH318/BJTC Z318); CHINA. Beijing, Huairou district, Baiquanshan, 40.496637 N, 116.649129 E, alt. 268 m, 17 Aug., 2020, coll. H. Zhou, X.Y. Shen and Y.T. Zhou (ZH862/BJTC Z862); CHINA. Beijing, Yanqing district, Songshan Mountains National Nature Reserve, 40.27 N, 115.57 E, elev. unknown, 11 July 2011, coll. unknown (HMAS 263406); CHINA. Beijing, Mentougou district, Donglingshan Mountains, 40.22 N, 116.50 E, elev. unknown, 29 July., 2013, coll. W.L. Lu (HMAS 253800); CHINA. Beijing, Mentougou district, Donglingshan Mountains, 40.22 N, 116.50 E, elev. unknown, 29 July., 2013, coll. W.L. Lu (HMAS 253801); CHINA. Beijing, Mentougou district, Donglingshan Mountains, 40.22 N, 116.50 E, elev. unknown, 29 July., 2013, coll. W.L. Lu (HMAS 253802); CHINA. Beijing, Mentougou district, Yunmengshan Mountains, 40.22 N, 116.50 E, elev. unknown, 29 July., 2013, coll. T.Z Wei (HMAS 253796).
Commentary: Yang and Doi (1999) described A. oberwinkleriana from Japan, and it was subsequently reported from China, India, and Republic of Korea (Yang and Li, 2001; Bhatt et al., 2003; Yang, 2005; Kim et al., 2013a; Yang, 2015; Cui et al., 2018). The morphological description of our specimen is consistent with that provided by Yang and Doi (1999). In our multi-locus phylogenetic analysis, 10 specimens clustered together with A. oberwinkleriana (HKAS 77330), forming a completely supported clade (BPP = 1.00, MLB = 96%). Based on these characters, our specimens were described as A. oberwinkleriana. Of note, the samples from the herbarium specimens (HMAS) exhibited poor sequence quality because of a long time. The multi-locus and nrLSU phylogenetic trees revealed that the branches of these herbarium specimens were longer (Figures 2, S1).
3.2.7 Amanita ovalispora Boedijn, Sydowia 5(3-6): 320 (1951)
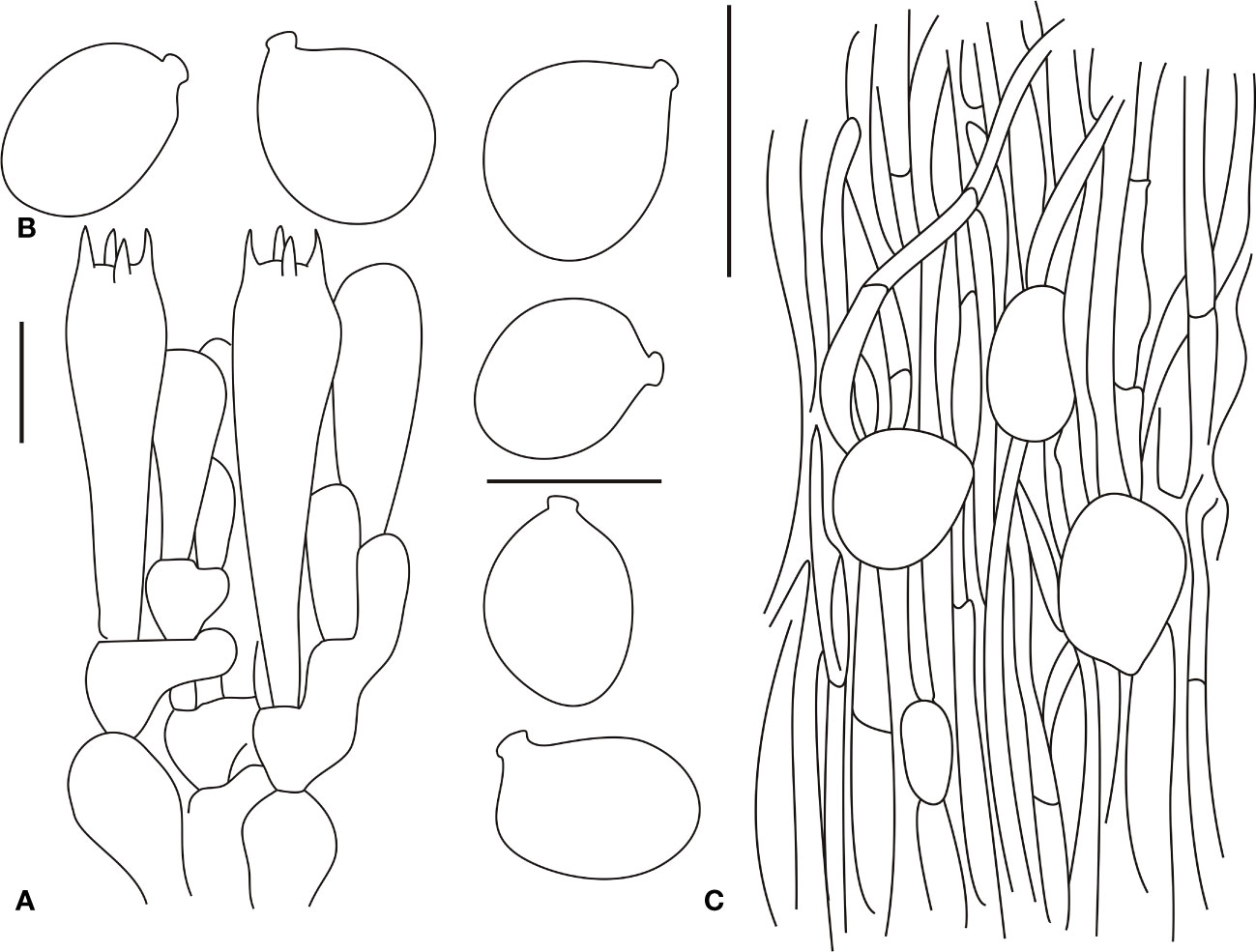
Figure 8 Microscopic characters of Amanita ovalispora. (BJTC Z311). (A). Hymenium and subhymenium; (B). Basidiospores; (C). Longitudinal section of outer part of volval remnants on stipe base. Bars: (A, B) = 10 μm, (C) = 40 μm.
Basidiomata small- to medium-sized. Pileus 3–6 cm in diam., plano-convex to applanate, distinctly umbonate at the center, gray-brown (#75615a) to gray (#808080), absence of volval remnants on the pileus, margin striate (0.2–0.5 R), non-appendiculate; trama white (#ffffff), unchanging. Free, crowded, white (#ffffff) lamellae; white (#ffffff) lamellar edge; truncated, plentiful lamellulae. Stipe 8–16 cm long, 0.5–1.5 cm wide at the apex, subcylindric or slightly tapering upward, with the apex slightly expanded, white (#ffffff) to dirty white (#f2f2f2), glabrous or covered with minute, concolorous fibrils; white (#ffffff), hollow in the center context; absence of basal bulb; volva saccate, membranous, both white (#ffffff) surfaces. Annulus absent. Spore print not observed. Odor indistinct.
Basidia (30–55 × 8–14 μm), clavate, four-spored, basal septa lacking clamps, hyaline; 4–6-μm-long sterigmata; basidiospores [60/2/2] (8–)9.5–11.8(–12.5) × (7–)7.3–9.1(–10.2) μm, Q = (1.15–)1.23–1.49(–1.52), Qm = 1.34 ± 0.10, globose to subglobose, thin-walled, hyaline, smooth, with a small apiculus, inamyloid. Volval remnants on the stipe are composed of longitudinally arranged elements: very abundant filamentous hyphae. Outer surface of the volval remnants on the stipe base is similar to the structure of the outer part, but with more abundant filamentous hyphae; gelatinized inner surface with structure similar to that of the inner part. Clamps are absent in all parts of basidioma.
Distribution: This species is known to be present in southern and southwestern China (Yang, 1997; Yang, 2005; Yang, 2015; Cui et al., 2018), and Indonesia (Boedijn, 1951).
Habitat and distribution: It is present individually or scattered in the coniferous forests and mixed coniferous and broad-leaved forests of Pinus tabuliformis Carr. and Juglans mandshurica Maxim., with basidioma occurring in summer and autumn.
Specimens examined: CHINA. Tianjin, Jizhou district, JiulongShan National Forest Park, 40.147837 N, 117.510365 E, alt. 174 m, 18 Aug., 2019, coll. H. Zhou, X.Y. Shen and Y.T. Zhang (ZH311/BJTC Z311); CHINA. Beijing, Changping district, Tibiyinshan, 40.317323 N, 116.321293 E, alt. 353 m, 14 Aug., 2019, coll. H H. Zhou, X.Y. Shen and Y.T. Zhang (ZH057/BJTC Z057).
Commentary: Boedijn (1951) was first described A. ovalispora from Indonesia. Subsequently, Yang (1997) examined the holotype, and described their basidiospores. According to Cui et al. (2018), no sequence of A. ovalispora is available from its type locality to delimit this species accurately. The morphological description of our specimens is consistent with that provided by Boedijn (1951). In our multi-locus phylogenetic analysis, two specimens clustered together with A. oberwinklerana (HKAS 79625 and HKAS 101406), forming a completely supported clade (BPP = 1.00, MLB = 96%) (Figure 2). The nrLSU phylogenetic analysis exhibited topologies similar to those of the multi-locus phylogenetic tree (Figure S1). According to these characters, our specimens were described as A. ovalispora.
3.2.8 Amanita subglobosa Zhu L. Yang, Bibl. Mycol. 170: 18 (1997)
Basidiomata small- to medium-sized. Pileus 4–8 cm in diam., brownish (#a5682a) to dark brown (#68421a); pyramidal to verrucose, white (#ffffff) to yellowish (#ffff9a), removable volval remnants on the pileus; margin striate (0.1–0.4 R), non-appendiculate; trama white (#ffffff), unchanging. White (#ffffff) to cream (#fffeea) lamellae, truncated lamellulae. Stipe 5–15 cm long, 0.5–2 cm wide, white (#ffffff) to dirty white (#f2f2f2); white (#ffffff), unchanging context; subglobose basal bulb (1.5–3.5-cm diam.), with its upper part covered with conical to pulverulent, yellowish (#ffff9a) to brownish (#a5682a) volval remnants, often forming a collar between the limit of the stipe and basal bulb. Subapical to submedian, white (#ffffff), persistent annulus. Spore print not observed. Odor indistinct.
Basidia (35–55 × 10–15 μm), clavate, four-spored. Basidiospores [60/2/2] (7.0–)8.3–11.0 (–13.0) × (6.0–)6.5–9.5(–11.5) μm, Q = (1.05–) 1.13–1.40(–1.60), Qm = 1.29 ± 0.08, broadly ellipsoid to ellipsoid, inamyloid. Volval remnants on the pileus are composed of vertically arranged elements: fairly abundant filamentous hyphae; abundant inflated cells. Clamps are present in all parts of basidioma.
Distribution: This species is known to be present in central, northeastern, and southwestern China (Yang, 1997; Yang, 2005; Yang, 2015; Cui et al., 2018); India (Semwal et al., 2007); Republic of Korea (Kim et al., 2013b); and Thailand (Sanmee et al., 2008).
Habitat and distribution: It is present solitary or is scattered in the pine, broad-leaved, or mixed forests of Fagaceae and Pinaceae trees. Basidioma occurs in summer and autumn (Cui et al., 2018).
Specimens examined: CHINA. Beijing, Mentougou district, Baihuashan Mountains, 39.52 N, 115.36 E, elev. unknown, 22 Aug., 1964, coll. Y.C. Zong and Q.T. Tao (HMAS 34658); CHINA. Beijing, Mentougou district, Tanzhe Temple, 39.54 N, 116.01 E, elev. unknown, 22 Aug., 1965, coll. Q.M. Ma (HMAS 253798).
Commentary: Yang (1997) first described A. subglobosa from China. Later, it was reported from India, Republic of Korea, and Thailand (Semwal et al., 2007; Sanmee et al., 2008; Kim et al., 2013b). The morphological description of our specimen is consistent with that provided by Yang (1997). Our multi-locus phylogenetic analysis revealed that a specimen HMAS 253798 clustered together with A. subglobosa (HKAS 67914), forming a completely supported clade (BPP = 1.00, MLB = 100%) (Figure 2). The nrLSU phylogenetic analysis revealed topologies similar to those of the multi-locus phylogenetic tree (Figure S1). Based on these characters, we described our specimen as A. subglobosa. In addition, DNA sequences from another herbarium specimens (HMAS 34658) could not be generated. However, we also examined the morphology of these specimens, and the results proved that these specimens were A. subglobosa.
3.2.9 Amanita subjunquillea S. Imai, Bot Mag (Tokyo) 47: 424 (1933)
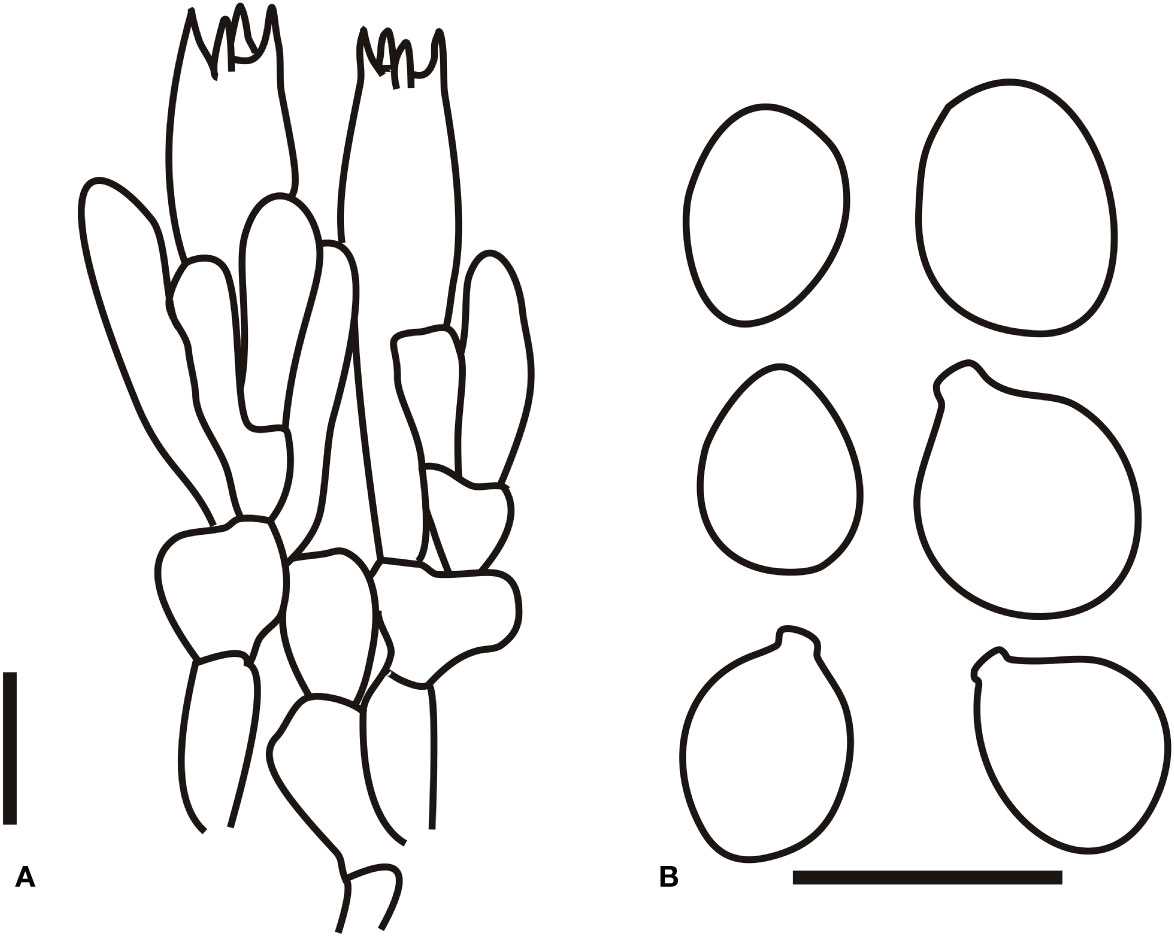
Figure 9 Microscopic characters of Amanita subjunquillea (BJTC 217). (A). Hymenium and subhymenium; (B). Basidiospores. Bars: (A, B) = 10 μm.
Synonym: Amanita subjunquillea var. alba Zhu L. Yang, Bibl. Mycol. 170: 174 (1997)
Basidiomata small- to medium-sized. Pileus 3–6 cm in diam., globose when young, hemispherical when expanding, later plano-convex to applanate, surface pale yellow (#ffff00) to light yellow (#ffffa1), becoming paler toward the margin; absence of volval remnants on the pileus. Free, white (#ffffff) to cream (#fffdd0), plentiful lamellae. Stipe 4–10 cm long, 0.8–2 cm wide at the apex, subcylindric or slightly tapering upward, with the apex slightly expanded, surface pale yellow (#ffff00) to light yellow (#ffffa1), with silk luster, upper part often covered with pale yellow (#ffff00) floccose to farinose squamules, lower part often covered with white (#ffffff) to pale yellow (#ffff00) floccose squamules; white (#ffffff) to pale yellow (#ffff00) context; marginate, dirty white (#fcfcfc) to grayish (#cccccc) basal bulb (1–2-cm diam.), with upper edge shortly limbate, dirty white (#fcfcfc) to grayish (#cccccc) volval remnants. Annulus absent. Spore print not observed. Odor indistinct.
Basidia (25–55 × 6.5–12 μm), slenderly clavate, four-spored, with clamps, hyaline; 3–5-μm-long sterigmata; basidiospores [60/4/4] (6–)6.5–8.4(–9) × (4–)4.1–6.6(–7.5) μm, Q = (1.02)1.18–1.36(1.51), Qm = 1.34 ± 0.20, mostly ellipsoid, occasionally spherical, thin-walled, hyaline, smooth, with a small apiculus, weakly amyloid. Volval remnants on the pileus are composed of irregularly to subvertically arranged elements: fairly abundant, branching, anastomosing filamentous hyphae; very abundant, globose, subglobose to ellipsoid inflated cells. Clamps are present in all parts of basidioma.
Distribution: This species is known to be from central, northern, northeastern, northwestern, and southwestern China (Yang, 1997; Yang, 2005; Yang, 2015; Cai et al., 2016; Cui et al., 2018); India (Bhatt et al., 2003; Bhatt et al., 2007); Japan (Imai, 1933; Imai, 1938; Yang and Doi, 1999; Imazeki et al., 2011); and Republic of Korea (Kim et al., 2013a; Cho et al., 2015).
Habitat and distribution: It is present individually or is scattered in coniferous forests and mixed coniferous and broad-leaved forests of Pinus tabuliformis Carr., J. mandshurica Maxim, and P. davidiana Dode., with basidioma occurring in summer and autumn.
Specimens examined: CHINA. Beijing, Yanqing district, Songshan Mountains National Nature Reserve, 40.596637 N, 115.849129 E, alt. 648 m, 17 Sep., 2017, coll. C.L. Hou, H. Zhou and J.Q. Li (217/BJTC 217); CHINA. Beijing, Yanqing district, Songshan Mountains National Nature Reserve, 40.316637 N, 115.459129 E, alt. 1102 m, 17 Sep., 2017, coll. C.L. Hou, H. Zhou and J.Q. Li (085/BJTC 085); CHINA. Beijing, Yanqing district, Songshan Mountains National Nature Reserve, 40.517899 N, 115.820799 E, alt. 906 m, 20 Aug., 2018, coll. C.L. Hou, H. Zhou and J.Q. Li (704/BJTC 704); CHINA. Beijing, Yanqing district, Songshan Mountains National Nature Reserve, 40.30024 N, 115.499129 E, alt. 860 m, 17 Sep., 2017, coll. C.L. Hou, H. Zhou and J.Q. Li (033/BJTC 033); CHINA. Beijing, Mentougou district, Donglingshan Mountains, 40.22 N, 116.50 E, elev. unknown, coll. unknown (HMAS 253775); CHINA. Beijing, Yanqing district, Songshan Mountains National Nature Reserve, 40.31 N, 115.45 E, alt. 1216 m, 16 Sep., 2017, coll. C.L. Hou, H. Zhou and J.Q. Li (112/BJTC 112); CHINA. Beijing, Hebei Province, Xinglong country, Changgou, 40.205334 N, 117.625724 E, alt. 878 m, 18 Aug., 2019, coll. G.Q. Cheng., C.L. Hou.and H. Zhou (ZH276/BJTC Z276); CHINA. Beijing, Huairou district, Sunzhazi Village 40.943482 N, 116.507391 E, alt. 789 m, 25 Aug., 2020, coll. C.L. Hou, G.Q. Cheng and Y.T. Zhang (C558/BJTC C558); CHINA. Beijing, Shunyi district, Mulin Town, 40.235317 N, 116.819856 E, alt. 45 m, 16 Aug., 2019, coll. G.Q. Cheng., C.L. Hou.and H. Zhou (ZH172/BJTC Z172).
Commentary: Imai (1933) first described A. subjunquillea from Japan. Later, it was reported from China, India, and Republic of Korea (Yang, 1997; Bhatt et al., 2003; Yang, 2005; Bhatt et al., 2007; Kim et al., 2013a; Cho et al., 2015; Yang, 2015; Cai et al., 2016; Cui et al., 2018). This species is deadly poisonous (Imazeki et al., 1988; Kawase et al., 1992; Chen et al., 2016; Tang et al., 2016). The morphological description of our specimen is consistent with that provided by Imai (1933). In our multi-locus phylogenetic analysis, nine specimens clustered together with A. oberwinklerana (HKAS 75771, HKAS 75770, and HKAS 75773), forming a completely supported clade (BPP = 1.00, MLB = 99%) (Figure 2). The nrLSU phylogenetic analysis showed topologies similar to those of the multi-locus phylogenetic tree (Figure S1). Based on these characters, our specimens were described as A. subjunquillea.
3.2.10 Amanita vaginata var. vaginata (Bull.) Lam., Encycl. Méth. Bot. (Paris) 1(1): 109 (1783)
Basionym: Agaricus vaginatus Bull., Herb. Fr. (Paris) 3: tab. 98 (1783) [1782-83].
Basidiomata small- to medium-sized. Pileus 3–7 cm in diam., somewhat umbonate, grayish (#a6a6a6) to gray (#b3b3b3); volval remnants on the pileus absent or retained as white (#ffffff) patches; margin striate (0.1–0.3 R), non-appendiculate. White (#ffffff) lamellae; truncated lamellulae. Stipe 5–10 cm long, 0.5–2.0 cm wide at the apex., white (#ffffff) to dirty white (#fcfcfc), glabrous or covered with fibrous, grayish (#a6a6a6) to gray (#b3b3b3) fibrils; absence of basal bulb; volval remnants on the stipe base saccate, outer surface white (#ffffff) to dirty white (#fcfcfc), inner surface white (#ffffff). Annulus absent. Spore print not observed. Odor indistinct.
Basidia (48–60 × 13–18 μm), clavate, four-spored. Basidiospores [60/3/3] (9.2–) 9.5–11.2 (–13.0) × (8.0–) 9.0–10.9 (–13.0) μm, Q = (1.0–)1.02–1.08 (–1.10), Qm = 1.01 ± 0.03, globose to subglobose, inamyloid. Clamps are absent in all parts of basidioma.
Distribution: This species is known to be from Asia (Imai, 1933; Imai, 1938; Tulloss et al., 2001; Yang, 2005; Imazeki et al., 2011; Kim et al., 2013b; Cui et al., 2018), North America (Coker, 1917; Thiers, 1982; Jenkins, 1986; Tulloss et al., 1995), and Europe (Horak, 1968; Consiglio, 2000).
Habitat and distribution: It is present individually or is scattered in broad-leaved forests of Fagaceae. Basidioma occurs in summer and autumn.
Specimens examined: CHINA. Beijing, Yanqing district, Songshan Mountains National Nature Reserve, 40.512712 N, 115.817818 E, elev. 555 m, 26 August 2018, coll. C.L. Hou, J.Q. Li and H. Zhou (682/BJTC 682); CHINA. Beijing, Yanqing district, Songshan Mountains National Nature Reserve, 40.512154 N, 115.817489 E, elev. 901 m, 26 August 2018, coll. C.L. Hou, J.Q. Li and H. Zhou (677/BJTC 677); CHINA. Beijing, Huairou district, Shangdian, 40.927121 N, 116.697837 E, elev. 681 m, 19 August 2019, coll. H. Zhou, X.Y. Shen and R.T. Zhang (ZH521/BJTC Z521); CHINA. Beijing, Mentougou district, Donglingshan Mountains, 39.55 N, 116.24 E, elev. unknown, coll. unknown (HMAS 253281); CHINA. Beijing, Miyun district, 40.22 N, 116.5 E, elev. unknown, August 1998, coll. X.L. Mao (HMAS 78411); CHINA. Beijing, Mentougou district, Donglingshan Mountains, 39.55 N, 116.24 E, elev. unknown, 19 August 1998, coll. H.A. Wen and S.X. Sun (HMAS 75237).
Commentary: A. vaginata var. vaginata is characterized by a gray pileus with striations at the margin, a white stipe lacking an annulus, and globose to subglobose basidiospores (9.0–13.0 (–14.0) μm) (Lange, 1935; Huijsman, 1959; Bas, 1967; Horak, 1968; Jenkins, 1986; Breitenbach and Kränzlin, 1995; Tulloss et al., 1995). In our multi-locus phylogenetic analysis, four specimens clustered together with A. vaginata var. vaginata (LAF 024482, type), forming a completely supported clade (BPP = 1.00, MLB = 99%) (Figure 2). The nrLSU phylogenetic analysis displayed topologies similar to those of the multi-locus phylogenetic tree (Figure S1). Based on these characters and results of phylogenetic analyses, our specimens were described as A. vaginata var. vaginata. Detailed descriptions, line drawings, and images of A. vaginata var. vaginata are available in Yang (2015). Unfortunately, DNA sequences could not be generated from two herbarium specimens (HMAS 78411 and HMAS 75273). However, on examining the morphology of these specimens, we confirmed that these specimens were A. vaginata var. vaginata.
3.2.11 Amanita virosa Bertillon, Dict. Encycl. Sci. Médic.: 497 (1866)
Replaced synonym: Agaricus virosus Fr., Epicr. syst. mycol. (Upsaliae): 3 (1838) [1836-1838]; Agaricus virosus Sowerby, Col. fig. Engl. Fung. Mushr. (London) 3: tab. 407 (1809).
Basidiomata small- to medium-sized. Pileus 5–10 cm in diam., umbonate at the center, white (#ffffff), often cream (#fffdd0) at the center; absence of volval remnants on the pileus; margin non-striate, non-appendiculate; white (#ffffff), unchanging trama. White (#ffffff) lamellae; attenuate lamellulae. Stipe 8–13 cm long, 0.7–2.0 cm wide, white (#ffffff), covered with concolorous squamules; white (#ffffff), unchanging context; Subapical, white (#ffffff), persistent annulus. Spore print not observed. Odor not observed.
Basidia (30–50 × 10–12 μm), clavate, four-spored. Basidiospores [60/2/2] (7.5–)8.0–11.0(–12.0) × (7.0–)8.0–10.0(–11.0) μm, Q = (1.00–)1.05–1.15 (–1.21), Qm = 1.05 ± 0.05, globose to subglobose, amyloid. Volval remnants on the stipe base are composed of longitudinally to irregularly arranged elements: very abundant to nearly dominant filamentous hyphae; scarce to scattered inflated cells. Clamps are absent in all parts of basidioma.
Distribution: This species is known to be present in Europe (Konrad and Maublanc, 1924; Chiusa, 2000; Contu, 2000; Neville and Poumarat, 2004) and East Asia (Imai, 1933; Imai, 1938; Zhang et al., 2010; Imazeki et al., 2011; Li et al., 2015; Yang, 2015; Cai et al., 2016; Cui et al., 2018).
Habitat and distribution: It is present solitary or is scattered on soil in the subtropical to temperate forests of Fagaceae and Pinaceae. Basidioma occurs in summer and autumn (Cui et al., 2018).
Specimens examined: CHINA. Beijing, Miyun district, 40.22 N, 116.50 E, elev. unknown, 4 August 1987, coll. Y.C. Zong (HMAS 40501); CHINA. Beijing, Miyun district, 40.22 N, 116.50 E, elev. unknown, 29 August 1987, coll. Y.C. Zong (HMAS 40530).
Commentary: A. virosa is widely distributed across Europe and temperate to subtropical Asia (Contu 2000; Chiusa, 2000; Neville and Poumarat, 2004; Zhang et al., 2010; Li et al., 2015; Yang, 2015; Cai et al., 2016; Cui et al., 2018). The morphological description of our specimen is consistent with that provided by Cui et al. (2018). Unfortunately, we could not obtain sequence data from any of the herbarium specimens (HMAS 40501 and HMAS 40503). We also examined the morphology of these specimens, and the results proved that these specimens were A. virosa.
3.2.12 Amanita yanshanensis H. Zhou & C. L. Hou, sp. nov.
Figure 10 Microscopic characters of Amanita yanshanensis (type, BJTC Z049). (A). Hymenium and subhymenium; (B). Basidiospores; (C). Longitudinal section of outer part of volval remnants on stipe base; (D). longitudinal section of inner part of volval remnants on stipe base. Bars: (A, B) = 10 μm, (C, D) = 40 μm.
MycoBank: MB 847661
Etymology: The epithet “yanshanensis” refers to the locality where the type specimen was collected.
Type: CHINA. Beijing, Changping district, Yanshou temple, 40.373152 N, 116.322892 E, alt. 268 m, 14 Aug., 2019, coll. H.Z., X.Y.S.and Y.T.Z. (ZH049/BJTC Z049).
Basidiomata small- to medium-sized. Pileus 4–8 cm diam., sub-hemisphere when young, hemispherical when expanding, later convex, plano-convex to applanate, with an umbo at the center; surface gray-white (#e6e6e6) when young, then gray-white (#e6e6e6) to dark grayish red (#755a5a), large, dark grayish red (#755a5a) to very dark (mostly black) red (#1f1818), or very dark grayish red (#4a3939) volval remnants on the pileus (ca. 2–6 mm diam.), densely arranged over the disk; margin slightly striate (ca. 0.05–0.2 R), non-appendiculate; trama white (#ffffff), unchanging. Free, white (#ffffff), unchanging, somewhat crowded lamellae; attenuate, plentiful lamellulae. Stipe 7–12 cm long, 0.5–1.5 cm wide at the apex, subcylindric or slightly attenuate upward, surface white (#ffffff) to gray-white (#e6e6e6), with silk luster, upper part often covered with gray-white (#e6e6e6) to gray-brown (#755a5a) floccose to farinose squamules, lower part often covered with gray-brown (#755a5a) to dark-brown (#1f1818) verrucose, floccose squamules; white (#ffffff) context; subglobose to clubbed, dirty white (#f2f2f2) to white (#ffffff) basal bulb (1–2 cm diam.); floccose to felted, dark grayish red (#755a5a) to dark (mostly black) red (#1f1818) volval remnants, arranged irregularly or in incomplete belts or rings on the stipe base. Apical, subapical to fugacious, thin annulus, with cream color (#fffeea) and silk luster on the upper surface, a matte cream-colored (#fffeea) lower surface with floccose to farinose warts. Spore print not observed. Odor indistinct.
Bilateral lamellar trama. Mediostratum 20–50 μm wide, composed of abundant ellipsoid to clavate inflated cells (45–60 × 20–40 μm); abundant, 2–6-μm-wide filamentous hyphae; scarce vascular hyphae. Lateral stratum is composed of abundant subfusiform to ellipsoid inflated cells (20–40 × 5–15 μm), diverging at an angle of ca. 30–45° to the mediostratum; abundant, 2–5-μm-wide filamentous hyphae. A 30–55-μm-thick subhymenium, with two to three layers of subglobose to ellipsoid or irregular cells (8–15 × 5–10 μm). Basidia (25–40 × 6–12 μm), slenderly clavate, four-spored, with clamps, hyaline to pale yellow (#ffffd8); 5–6-μm-long sterigmata; basidiospores [120/5/4] 6.0–8.0(–9.0) × 4.0–5.5(–6.0) μm, Q = 1.24–1.45(–1.57), Qm = 1.40 ± 0.12, mostly ellipsoid, occasionally broadly ellipsoid, thin-walled, hyaline, pale yellow, smooth, occasionally with a small to medium-large apiculus, amyloid; sterile lamellar edge, composed of subglobose to ellipsoid inflated cells (20–40 × 10–30 μm), single and terminal or in chains of 2–3, thin-walled, hyaline; abundant, 2–5-μm-wide filamentous hyphae, irregularly arranged or ± running parallel to the lamellar edge. Pileipellis 100–200-μm thick, gelatinized upper layer (25–100-μm thick), composed of subradially to somewhat interwoven, thin-walled, colorless, 2–7-μm-wide filamentous hyphae; lower layer (70–100-μm thick) composed of radially and compactly arranged, pale yellow (#ffffd8), 4–8 (–10)-μm-wide filamentous hyphae; scarce vascular hyphae. Volval remnants on the pileus are composed of inflated cells and filamentous hyphae, more or less arranged in erect chains; abundant to dominant, subglobose, ovoid to ellipsoid, clavate or sphaeropedunculate inflated cells (up to 30 × 25 μm), with hyaline to pale yellow (#ffffd8) vacuolar pigments; septa with clamps; inner part of volval remnants on the pileus often with conspicuous vascular hyphae. Volval remnants on the stipe base are similar to those on the pileus, but with more abundant inflated cells. Stipe trama composed of longitudinally arranged, long clavate terminal cells (50–300 × 15–40 μm); scattered to abundant, 5–10-μm-wide filamentous hyphae; scarce vascular hyphae. Annulus is composed of radially arranged elements: abundant, subglobose, fusiform to ellipsoid, hyaline, thin-walled inflated cells (15–25 × 8–15 μm); abundant, hyaline, thin-walled, 2–5-μm-wide filamentous hyphae. Clamps are present in all parts of basidioma.
Habitat and distribution: This species is scattered in the broad-leaved forests of Castanea mollissima Blume., with basidioma occurring in summer and autumn.
Additional specimens examined: CHINA. Beijing, Changping district, Yanshou Temple, 40.368272 N, 116.321008 E, alt. 234 m, 17 Aug., 2020, coll. H. Zhou, X.Y. Shen and X.B. Huang (ZH820/BJTC Z820); CHINA. Beijing, Changping district, Yanshou temple, 40.368309 N, 116.321011 E, alt. 227 m, 17 Aug., 2020, coll. H. Zhou, X.Y. Shen and X.B. Huang (ZH819/BJTC Z819); CHINA. Beijing, Changping district, Yanshou temple, 40.368654 N, 116.322574 E, alt. 216 m, 17 Aug., 2020, coll. H. Zhou, X.Y. Shen and X.B. Huang (ZH815/BJTC Z815); CHINA. Beijing, Changping district, Yanshou temple, 40.371961 N, 116.321044 E, alt. 245 m, 17 Aug., 2020, coll. H. Zhou, X.Y. Shen and X.B. Huang (ZH824/BJTC Z824); CHINA. Beijing, Changping district, DaYangShan Mountains National Forest Park, 40.308138 N, 116.42437 E, alt. 245 m, 25 July, 2020, coll. H. Zhou, Y.T. Zhang.and X.B. Huang (ZH760/BJTC Z760); CHINA. Beijing, Changping district, DaYangShan Mountains National Forest Park, 40.308003 N, 116.425251 E, alt. 265 m, 14 Aug., 2019, coll. H. Zhou, X.Y. Shen and Y.T. Zhang (ZH083/BJTC Z083); CHINA. Beijing, Pinggu district, Dongxinzhuang village, 40.294008 N, 117.051816 E, alt. 198 m, 19 Aug., 2020, coll. G.Q. Cheng, C.L. Hou.and Y.T. Zhang (C182/BJTC C182); CHINA. Beijing, Changping district, Yanshou temple, 40.373152 N, 116.322892 E, alt. 268 m, 14 Aug., 2019, coll. H. Zhou, X.Y. Shen and X.B. Huang (ZH049/BJTC Z049).
Commentary: A. yanshanensis is well circumscribed by its gray-white to dark grayish red pileus densely arranged with pyramidal, subverrucose to subconical, floccose volval remnants on the stipe base arranged irregularly or in incomplete belts or rings, and mostly ellipsoid, occasionally broadly ellipsoid basidiospores (6.0–9.0 × 4.0–6.0 μm). Furthermore, it is associated with the trees of Fagaceae (Castanea mollissima).
This species belongs to section Validae and is closely related to A. spissacea on the multi-locus phylogenetic tree (Figure 2). In the single loci phylogenetic trees of both nrITS and nrLSU, A. yanshanensis clustered into an independent clade (Figures S1, S4). Moreover, basidiomata of A. yanshanensis with a gray-brown to gray pileus are also comparable with those of A. spissacea and A. fritillaria (Sacc.) Sacc. However, A. fritillaria has a basal bulb covered with conical, blackish, dark-gray to gray-brown volval remnants and a dirty white to gray annulus (Corner and Bas, 1962; Kumar et al., 1990; Yang, 2005; Yang, 2015; Cui et al., 2018). A. spissacea has a stipe covered with grayish to brownish squamules, pulverulent to floccose, a grayish annulus, and wider basidiospores (7.0–9.5× 6.0–7.5 μm) than A. yanshanensis (Imai, 1933; Imai, 1938; Hongo, 1959; Imazeki and Hongo, 1987; Imazeki et al., 1988; Imazeki et al., 2011; Cui et al., 2018).
4 Discussion
In our study, the molecular phylogenetic analyses further supported the delineation of Amanita into two subgenera, namely, subgen. Amanita Pers. and Amanitana (E.-J. Gilbert) E.-J. Gilbert, as suggested by Cui et al. (2018) (Figures 2, S1). Based on the macroscopic morphology and the preliminary comparison of the original sequence we obtained, the sections where the specimens were located were initially known. Therefore, the sequence information of subgen. Lepidella Beauseigneur and sections Amarrendiae (Bougher & T. Lebel) Zhu L. Yang, Y.Y. Cui, Q. Cai & Ling Ping Tang, Arenariae Zhu L. Yang, Y.Y. Cui & Q. Cai, Amidella (J. E. Gilbert) Konrad & Maubl., and Strobiliformes Singer ex Q. Cai, Zhu L. Yang & Y.Y. Cui were not included in the final phylogenetic analyses. Of them, species in sections Amarrendiae and Arenariae have not been found in China. Cui et al. (2018) mentioned that, for a better understanding of the range of variation in characters, new species should be described based on several specimens, but the technology of molecular systematics used in the present study improved the accuracy of our description of species. As specified in the results, the quality of sequences of these old herbarium specimens (HMAS 283800, HMAS 253802, HMAS 263406, and HMAS 253796) identified as A. oberwinkleriana was not good, but its systematic position and combined morphology could still be somewhat helpful in specimen identification. In addition, we could only depend on morphological observations for identifying these specimens as their sequences could not be obtained because of the poor condition of their basidiomata.
In this study, three new species belonged to three sections under the subgenera Amanita and Amanitana, namely, sections Amanita (A. boreqalis), Caesareae (A. brunneola), and Validae (A. yanshanensis). Section Amanita is distinguished by basidioma with agarose; a pileus with persistent volval remnants, a pileal margin striate; truncated lamellae; presence of a basal bulb; and inamyloid basidiospores (Cui et al., 2018). Many species in this section produced neuropsychotoxins (Wieland, 1973; Yang, 2005; Chen et al., 2016). Studies have identified 27 taxa, comprising 23 species, 2 varieties, and 2 forms, in China (Cui et al., 2018; Su et al., 2022). Previous studies have reported 21 species of section Caesareae (Cui et al., 2018; Mu et al., 2021). The species belonging to section Validae were characterized by a pileal margin non-striae and non-appendiculate; annulus membranous, dominance of filamentous hyphae; volval remnants often as verrucae, warts, flocci, patches, or occasionally as short limb; amyloid basidiospores; and absence of clamps. To date, 18 taxa have been identified from this section (Cui et al., 2018).
Studies have found and recorded 169 Amanita species in China, with most of them being concentrated in the southwest, northwest, and south of China, including Yunnan, Guangdong, Heilongjiang, Liaoning, and Hunan provinces (Yang, 1994; Yang, 1997; Yang et al., 2004; Yang, 2005; Deng et al., 2014; Li and Cai, 2014; Ariyawansa et al., 2015; Li et al., 2015; Yang, 2015; Cai et al., 2016; Deng et al., 2016; Liu et al., 2017; Cui et al., 2018; Su et al., 2022). Records of Amanita species in Yanshan Mountains, northern China, are few. In this study, 12 Amanita species from Yanshan Mountains were recognized. Of them, 10 species or approximately 83% of the species were new or recorded for the first time in this area. Therefore, accelerating the discovery and description of Amanita species by using both morphological and molecular approaches in this area is necessary.
Literature review revealed that 14 taxa of Amanita were identified from Yanshan Mountains. Three species were identified by Yang (2004): A. parvipantherina Zhu L. Yang, M. Weiss & Oberw (HMKS 32350) and A. griseofolia Zhu L. Yang (HMKS 22610) collected from Tanzhe Temple in Beijing and A. subjunquillea var. alba Zhu L. Yang (HMKS 35536) collected from Beishicheng in Beijing. A. vaginata var. vaginata was collected from Donglingshan Mountains, Beijing (Cui et al., 2018). The morphology of HMKS 75237 was consistent with that of A. vaginata var. vaginata, but we could not obtain the DNA sequence from the specimen. The specimen HMAS 26491 was collected from Baihuashan Mountains, Beijing, and was originally identified as A. subglobosa Zhu L. Yang. However, the results of morphological and phylogenetic analyses conducted in the present study were inconsistent with the original identification. We tentatively named this specimen as Amanita sp. because of the poor status of the specimen and await the subsequent collection of additional specimens for further study. In addition, some Amanita species have been recorded in the literature even in the absence of any detailed information about the specimen, and so, the accuracy of the information about these species needs to be further verified by collecting specimens and obtaining molecular data. These species with only distribution records available are as follows. A. caesarea, A. inaurata Gillet, and A. yuaniana are distributed in Wulingshan Mountains, Hebei Province (Wang et al., 2005); A. flavoconia Alk., A. phalloides (Vaill. ex Fr.) Link, and A. subjunquillea are distributed in Dahaituo Mountains National Nature Reserve, Hebei Province (Wu et al., 2017); A. orientigemmata is distributed in Songshan Mountains National Nature Reserve, Beijing (Wu et al., 2020); A. verna Bull ex Lam. is distributed in Badaling Forest Park, Beijing (Zhang et al., 2017); and A. panterina (DC. ex Fr.) Schrmm is distributed in Dayangshan National Forest Park, Beijing (Chen et al., 2006). Therefore, specimens of Amanita spp. must be more extensively collected from the Yanshan Mountains region to improve the study of their biodiversity.
Amanita deserves special attention because of its unique research and popular science education value. However, because some Amanita species are similar in morphology and color, distinguishing them in the field is difficult. Moreover, many casualties have been caused through mistakenly consumed poisonous Amanita species in many places in China (Li et al., 2020; Li et al., 2021a; Li et al., 2021b). The China Center for Disease Control and Prevention reported eight incidents of mushroom poisoning in Beijing in 2020, with 23 people poisoned. Seven incidents of mushroom poisoning were reported from Hebei Province around Beijing, with 33 people poisoned. In these incidents, the main species causing poisoning were A. rimosa, A. subjunquillea, and A. oberwinkleriana (Li et al., 2021a; Li et al., 2021b). The present study is the initial report on Amanita’s biodiversity in the region of Yanshan Mountains, including northern part of Beijing, and Tianjin and Hebei provinces. Given the large area of North China and its diverse forest types, many more Amanita species may be discovered in this region in future.
5 Conclusions
In this research, 20 Amanita specimens deposited in Chinese herbaria and 36 newly collected specimens from North China were studied based on the results of morphological and phylogenetic analyses. In total, 12 phylogenetic species were found. Of them, three species were described as new species, namely A. borealis sp. nov., A. brunneola sp. nov., and A. yanshanensis sp. nov. Furthermore, nine known species were identified, namely, A. caesareoides, A. chiui, A. muscaria, A. oberwinklerana, A. ovalispora, A. subglobosa, A. subjunquillea, A. vaginata var. vaginata, and A. virosa. Our results underscore that China has a very high biodiversity of Amanita species and that additional studies are required to completely determine the exact number of species. It plays a crucial role in Amanita toxin research and ecological conservation. This study investigated the areas where Amanita species-related research is lacking. The study attempted to better understand Amanita distribution and thus contribute to related research. This study improves the knowledge regarding the species diversity of Amanita in Yanshan Mountains and provides new data for the macrofungal systematics, toxin research, and diversity and ecological studies of Amanita in subsequent studies.
Data availability statement
The datasets presented in this study can be found in online repositories (www.treebase.org, study S41229). The NCBI accession number(s) can be found in the article.
Author contributions
HZ wrote the manuscript, conducted phylogenetic analysis and morphological observations; MG conducted phylogenetic analysis and morphological observations, conducted the experiments; LZ conducted phylogenetic analysis and morphological observations conducted the experiments; HY conducted phylogenetic analysis and morphological observations; XS conducted phylogenetic analysis and morphological observations; YG conducted the experiments; CH conceived and designed the study. All authors contributed to the article and approved the submitted version.
Funding
This study was financed by the National Natural Science Foundation of China (No. 32270012) and the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, China (2019HJ2096001006).
Acknowledgments
We are grateful to Prof. Zhu-Liang Yang at Kunming Institute of Botany, Chinese Academy of Sciences, for his improvements to the manuscript. We also thank the two reviewers for their constructive criticism and suggestions to improve our work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1226794/full#supplementary-material
References
Ariyawansa, H. A., Hyde, K. D., Jayasiri, S. C., Buyck, B., Chethana, K. W. T., Dai, D. Q., et al. (2015). Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 75 (1), 27–274. doi: 10.1007/s13225-015-0346-5
Bas, C. (1967). Amanita argentea Huijsman am Teutoburger Wald gefunden. Westfäl Pilzbr 6 (7), 125–129.
Bas, C. (1969). Morphology and subdivision of Amanita and a monograph of its section Lepidella. Persoonia 5 (4), 285–579.
Beeli, M., Goossens-Fontana, M., Robyns, W. (1935). Flore iconographique des champignons du Congo, Fasc.I (Bruxelles: Office de Publicité).
Bhatt, R. P., Mehmood, T., Uniyal, P., Singh, U. (2017). Six new records of genus Amanita (Amanitaceae) from uttarakhand, india. Curr. Res. Environ. Appl. Mycol. 7 (3), 161–182. doi: 10.5943/cream/7/3/3
Bhatt, R. P., Semwal, K. C., Upadhyay, R. C. (2007). New records of section Phalloideae of the genus Amanita from Garhwal Himalaya. India. Mushroom Res. 16 (2), 61–67.
Bhatt, R. P., Tulloss, R. E., Semwal, K. C., Bhatt, V. K., Moncalvo, J. M., Stephenson, S. L. (2003). Amanitaceae reported from India. A critically annotated checklist. Mycotaxon 88, 249–270.
Breitenbach, J., Kränzlin, F. (1995). Pilze der Schweiz. Band 4. Blätterpilze, 2. Teil. Luzern (Paris: Verlag Mykologia, Lucerne).
Cai, Q., Cui, Y. Y., Yang, Z. L. (2016). Lethal amanita species in China. Mycologia 108 (5), 993–1009. doi: 10.3852/16-008
Cai, Q., Tulloss, R. E., Tang, L. P., Tolgor, B., Zhang, P., Chen, Z. H., et al. (2014). Multi-locus phylogeny of lethal Amanitas: implications for species diversity and historical biogeography. BMC Evol. Biol. 14 (1), 143. doi: 10.1186/1471-2148-14-143
Chen, J. Q., Cheng, J. H., Du, Y. Y., Wang, Q. B., Yao, Y. J. (2006). Preliminary investigation of macrofungi resource in Beijing. J. Beijing Agric. Coll. 21 (2), 39–43.
Chen, Z. H., Yang, Z. L., Bau, T., Li, T. H. (2016). Poisonous mushrooms: recognition and poisoning treatment (Beijing: Science Press).
Cho, H. J., Park, M. S., Lee, H., Oh, S., Jang, Y., Fong, J. J., et al. (2015). Four new species of Amanita in Inje County, Korea. Mycobiology 43 (4), 408–414. doi: 10.5941/MYCO.2015.43.4.408
Coker, W. C. (1917). The amanitas of the eastern united states. J. Elisha Mitchell Sci. Soc. 33, 1–88.
Consiglio, G. (2000). Contributo alla conoscenza dei macromiceti dell’Emilia-romagna. XXI. Genere amanita. Boll Gr Micol G Bres NS 43 (2), 211–232.
Contu, M. (2000). Saggio di una chiave per la determinazione delle specie del genere Amanita osservate in Sardegna. Boll Gr Micol G Bres NS 43 (2), 67–86.
Corner, E. J. H., Bas, C. (1962). The genus Amanita in Singapore and Malaya. Persoonia 2 (3), 241–304.
Cui, Y. Y., Cai, Q., Tang, L. P., Liu, J. W., Yang, Z. L. (2018). The family Amanitaceae: molecular phylogeny, higher-rank taxonomy and the species in China. Fungal Divers. 91, 5–230. doi: 10.1007/s11557-019-01506-1
Davison, E. M., Giustiniano, D., Busetti, F., Gates, G. M., Syme, K. (2017). Death cap mushrooms from southern Australia: additions to Amanita (Amanitaceae, Agaricales) section Phalloideae Clade IX. Aust. Syst. Bot. 30, 371–389. doi: 10.1071/SB17032
Deng, W. Q., Li, T. H., Hosen, M. I. (2016). Amanita rufobrunnescens, a new species of Amanita section Amidella from South China. Phytotaxa 243 (2), 147–154. doi: 10.11646/phytotaxa.243.2.4
Deng, W. Q., Li, T. H., Li, P., Yang, Z. L. (2014). A new species of Amanita section Lepidella from South China. Mycol Prog. 13 (2), 211–217. doi: 10.1007/s11557-013-0906-6
Dereeper, A., Guignon, V., Blanc, G., Audic, S., Buffet, S., Chevenet, F., et al. (2008). Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36, W465–W469. doi: 10.1093/nar/gkn180
Endo, N., Fangfuk, W., Sakuma, D., Phosri, C., Matsushita, N., Fukuda, M., et al. (2016). Taxonomic consideration of the Japanese redcap Caesar’s mushroom based on morphological and phylogenetic analyses. Mycoscience 57 (3), 200–207. doi: 10.1016/j.myc.2016.01.005
Fraiture, A., Amalfi, M., Raspé, O., Kaya, E., Akata, I., Degreef, J. (2019). Two new species of Amanita sect. Phalloideae from Africa, one of which is devoid of amatoxins and phallotoxins. MycoKeys 53, 93–125. doi: 10.3897/mycokeys.53.34560
Gardes, M., Bruns, T. D. (1993). ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 2 (2), 113–118. doi: 10.1111/j.1365-294X.1993.tb00005.x
Geml, J., Laursen, G. A., O’neil, K., Nusbaum, H. C., Taylor, D. L. (2006). Beringian origins and cryptic speciation events in the fly agaric (Amanita muscaria). Mol. Ecol. 15, 225–239. doi: 10.1111/j.1365-294X.2005.02799.x
Geml, J., Tulloss, R. E., Laursen, G. A., Sazanova, N. A., Taylor, D. L. (2008). Evidence for strong inter- and intracontinental phylogeographic structure in Amanita muscaria, a wind-dispersed ectomycorrhizal basidiomycete. Mol. Phylogenet Evol. 48, 694–701. doi: 10.1016/j.ympev.2008.04.029
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Sys. Biol. 59, 307–321. doi: 10.1093/sysbio/syq010
Hillis, D. M., Bull, J. J. (1993). An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Sys. Biol. 42, 182–192. doi: 10.1093/sysbio/42.2.182
Hongo, T. (1959). The agaricales of Japan I-(1). Mem Fac Liberal Arts Shiga Univ Nat. Sci. 9, 47–94.
Huang, T., Su, L. J., Zeng, N. K., Lee, S. M. L., Lee, S. S., Thi, B. K., et al. (2023). Notes on amanita section validae in Hainan Island, China. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1087756
Imai, S. (1933). Studies on the agaricaceae of Japan. I: volvate agarics in Hokkaido. Bot. Mag (Tokyo) 47, 423–432. doi: 10.15281/jplantres1887.47.423
Imai, S. (1938). Studies on the agaricaceae of Hokkaido: I. J. Facul Agric. Hokkaido Imp Univ Sapporo 43 (1), 1–178.
Imazeki, R., Otani, Y., Hongo, T. (2011). Fungi of Japan (Revisied and enlarged edition) (Tokyo: Yama-kei Publishers Co.).
Jenkins, D. T. (1977). A taxonomic and nomenclatural study of the genus Amanita section Amanita for North America. Bibl Mycol 57, 3–126.
Jenkins, D. T., Petersen, R. H. (1976). A neotype specimen for Amanita muscaria. Mycologia 68 (3), 463–469. doi: 10.1080/00275514.1976.12019935
Katoh, K., Toh, H. (2010). Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 26, 1899–1900. doi: 10.1093/bioinformatics/btq224
Kawase, I., Shirakawa, H., Watanabe, M. (1992). Deaths caused by Amanita subjunquillea poisoning and the distribution of this mushroom in Hokkaido. Trans. Mycol Soc. Jpn 33, 107–110.
Kim, C. S., Jo, J. W., Kwag, Y. N., Kim, J. H., Shrestha, B., Sung, G. H., et al. (2013a). Taxonomic study of Amanita subgenus Lepidella and three unrecorded Amanita species in Korea. Mycobiology 41 (4), 183–190. doi: 10.5941/MYCO.2013.41.4.183
Kim, C. S., Jo, J. W., Kwag, Y. N., Oh, J., Shrestha, B., Sung, G. H., et al. (2013b). Four newly recorded Amanita species in Korea: Amanita sect. Amanita and sect. Vaginatae. Mycobiology 41 (3), 131–138. doi: 10.5941/MYCO.2013.41.3.131
Kumar, A., Bhatt, R., Lakhanpal, T. N. (1990). The Amanitaceae of India (Dehra Dun: Bishen Singh Mahendra Pal Singh).
Kumar, A., Mehmood, T., Atri, N. S., Sharma, Y. P. (2021). Revised and an updated checklist of the Amanitaceae from India with its specific distribution in the Indian States. Nova Hedwigia 112 (1–2), 223–240. doi: 10.1127/nova_hedwigia/2021/0617
Lange, J. E. (1935). Flora agaricina danica, vol I. Recato (Copenhagen: printed by Recato A/S Copenhagen).
Li, F., Cai, Q. (2014). Amanita heishidingensis, a new species of Amanita sect. Lepidella from China. Mycol Prog. 13 (4), 1191–1197. doi: 10.1007/s11557-014-1008-9
Li, W. W., Pires, S. M., Liu, Z. T., Liang, J. J., Wang, Y. F., Chen, W., et al. (2021b). Mushroom poisoning outbreaks China 2010-2020. China CDC Week. 3 (24), 518–522. doi: 10.46234/ccdcw2021.134
Li, H. J., Xie, J. W., Zhang, S., Zhou, Y. J., Ma, P. B., Zhou, J., et al. (2015). Amanita subpallidorosea, a new lethal fungus from China. Mycol Prog. 14 (6), 43. doi: 10.1007/s11557-015-1055-x
Li, H. J., Zhang, H. S., Zhang, Y. Z., Zhang, K. P., Zhou, J., Yin, Y., et al. (2020). Mushroom poisoning outbreaks China. 2019. China CDC Week. 2 (2), 19–24. doi: 10.46234/ccdcw2020.005
Li, H. J., Zhang, H. S., Zhang, Y. Z., Zhou, J., Yin, Y., He, Q., et al. (2021a). Mushroom poisoning outbreaks China 2020. China CDC Week. 3 (3), 41–45. doi: 10.46234/ccdcw2021.014
Liu, J. W., Cai, Q., Cui, Y. Y., Yang, Z. L. (2017). Amanita cingulata, a new annulate species of Amanita sect. Vaginatae from subtropical China. Phytotaxa 326 (1), 41–53. doi: 10.11646/phytotaxa.326.1.3
Mao, X. L. (1990). Taxonomic study on the genus Amanita from Xizang, China. Acta Mycol Sin. 9, 206–217.
Menolli, N. J., Capelari, M., Baseia, I. G. (2009). Amanita viscidolutea, a new species from Brazil with a key to Central and South American species of Amanita section Amanita. Mycologia 101 (3), 395–400. doi: 10.3852/07-079
Mighell, K. S., Henkel, T. W., Koch, R. A., Chin, M. L., Brann, M. A., Aime, M. C. (2021). Amanita in the Guineo-Congolian rainforest: epitypes and new species from the Dja Biosphere Reserve. Cameroon. Mycol. 113 (1), 168–190. doi: 10.1080/00275514.2020.1816386
Mighell, K. S., Henkel, T. W., Koch, R. A., Goss, A., Alime, M. C. (2019). New species of Amanita subgen. Lepidella from Guyana. Fungal Syst. Evol. 3, 1–12. doi: 10.3114/fuse.2019.03.01
Moser, M. (1967). “Die Röhrlinge und Blätterpilze (Agaricales),” in Kleine Kryptogamenflora, Band II b/2. Basidiomyceten II. Teil. 3. Ed. Gams, H. H. (Stuttgart/New York: Gustav Fischer Verlag).
Mu, M., Huang, H. Y., Zhang, W. H., Tang, L. P. (2021). A new species with pink lamellae of Amanita section Caesareae from China. Phytotaxa 478 (1), 141–150. doi: 10.11646/phytotaxa.478.1.10
Neville, P., Poumarat, S. (2004). Amaniteae: Amanita, Limacella & Torrendia (Italy: Edizioni Candusso, Alassio).
Nylander, J. A. A. (2004). MrModeltest 2.2. Computer software distributed by the University of Uppsala (Uppsala, Sweden: Evolutionary Biology Centre, University of Uppsala).
Pegler, D. N., Shah-Smith, D. (1997). The genus amanita (Amanitaceae, agaricales) in Zambia. Mycotaxon 61, 389–417.
Posada, D., Crandall, K. A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14, 817–818. doi: 10.1093/bioinformatics/14.9.817
Rannala, B., Yang, Z. (1996). Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J. Mol. Evol. 43, 304–311. doi: 10.1007/BF02338839
Rehner, S. A., Buckley, E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97 (1), 84–98. doi: 10.1080/15572536.2006.11832842
Reid, D. A. (1980). A monograph of the Australian species of Amanita Pers. ex Hook. (Fungi). Aust. J. Bot. Suppl. Ser. 10 (8), 1–96.
Ronquist, F., Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Sanmee, R., Tulloss, R., Lumyong, P., Dell, B., Lumyong, S. (2008). Studies on amanita (Basidiomycetes: amanitaceae) in northern Thailand. Fungal Divers. 32, 97–123.
Semwal, K. C., Tulloss, R., Bhatt, R., Stephenson, S., Upadhyay, R. (2007). New records of Amanita section Amanita from Garwhal Himalaya, India. Mycotaxon 101, 331–348.
Singer, R. (1986). The Agaricales in modern taxonomy. 4th edn (Koenigstein: Koeltz Scientific Books).
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Stamatakis, A., Hoover, P., Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML web servers. Sys. Biol. 57, 758–771. doi: 10.1080/10635150802429642
Su, Y. T., Cai, Q., Qin, W. Q., Cui, Y. Y., Chen, Z. H., Yang, Z. L. (2022). Two new species of Amanita section Amanita from Central China. Mycol Prog. 21, 78. doi: 10.1007/s11557-022-01828-7
Suwannarach, N., Kumla, J., Khuna, S., Wannathes, N., Thongklang, N., Sysouphanthong, P., et al. (2022). History of Thai mycology and resolution of taxonomy for Thai macrofungi confused with Europe and American names. Chiang Mai J. Sci. 49 (3), 654–683. doi: 10.12982/CMJS.2022.052
Tang, S. S., Zhou, Q., He, Z. M., Luo, T., Zhang, P., Cai, Q., et al. (2016). Cyclopeptide toxins of lethal amanitas: compositions, distribution and phylogenetic implication. Toxicon 120, 78–88. doi: 10.1016/j.toxicon.2016.07.018
Thiers, H. D. (1982). The agaricales (Gilled fungi) of california: Amanitaceae (Eurecka: Mad River Press).
Tulloss, R. E. (2005). Amanita-distribution in the Americas, with comparison to eastern and southern Asia and notes on spore character variation with latitude and ecology. Mycotaxon 93, 189–232.
Tulloss, R. E., Bhatt, R., Stephenson, S., Kumar, A. (1995). Studies on Amanita (Amanitaceae) in West Virginia and adjacent areas of the Mid-Appalachians. Preliminary results. Mycotaxon 56, 243–293.
Tulloss, R. E., Iqbal, S. H., Khalid, A. N., Bhatt, R. P., Bhat, V. K. (2001). Studies in Amanita (Amanitaceae) from southern asia. i. some species of pakistan’s northwest frontier province. Mycotaxon 77, 455–490.
Vassiljeva, L. N. (1950). Species novae fungorum. Notul Syst. Sect Cryptog Inst Bot. Acad. Sci. USSR 6, 188–200.
Vilgalys, R., Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Wang, Y., Huang, Z. H., Wang, J., Zhang, T., Cui, G. F. (2021). The population structure and dynamic characteristics of Phellodendron amurense in Yanshan Mountains. Acta Ecol. Sin. 47 (7), 2826–2834. doi: 10.5846/stxb202003300743
Wang, Q., Liu, H. X., Zhang, J. G., Lu, J., Liu, Y. X., Yang, L. H., et al. (2005). Investigation of wild edible and medical fungi of Wuling mountain area. J. Hebei Univ. (Natural Sci. Edition). 25 (5), 523–525.
White, T. J., Bruns, T., Lee, S., Taylor, J. (1990). Amplifcation and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: Guide to Methods Appl. 18, 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wood, A. (1997). Studies in the genus amanita (Agaricales) in Australia. Aust. Syst. Bot. 10, 723–854. doi: 10.1071/SB95049
Wu, M., Huang, H. Y., Zhang, W. H., Tang, L. P. (2021). A new species with pink lamellae of Amanita section Caesareae from China. Phytotaxa 478 (1), 141–150. doi: 10.11646/phytotaxa.478.1.10
Wu, Z. J., Song, C., Lin, T. M., Yu, Q. Y., Yu, M. F., Xing, S. H., et al. (2017). Resource and ecological distribution of microfungi in Dahaituo national nature reserve of Hebei province. Edible Fungi China 36 (4), 8–14.
Wu, J. G., Zhou, H., Hou, C. L. (2020). Diversity of poisonous macrofungi in Beijing Songshan national nature reserve. J. Capital Normal Univ. (Natural Sci. Edition) 41 (4), 52–56.
Yang, Z. L. (1994). Studies of the genus Amanita from southwestern China (I). Mycotaxon 51, 459–470.
Yang, Z. L. (2004). Two new species of Amanita (Basidiomycota) from China. Eds. Agerer, R., Piepenbring, M., Blanz, P. (Frontiers), (Eching: IHW Verlag).
Yang, Z. L., Cai, Q., Cui, Y. Y. (2018). Phylogeny, diversity and morphological evolution of Amanitaceae. Biosyst. Ecol. Ser. 34, 359–380.
Yang, Z. L., Doi, Y. (1999). A contribution to the knowledge of Amanita (Amanitaceae, Agaricales) in Japan. Bull. Natn Sci. Mus Tokyo Ser. B 25 (3), 107–130.
Yang, Z. L., Li, T. H. (2001). Notes on three white Amanitae of section Phalloideae (Amanitaceae) from China. Mycotaxon 78, 439–448.
Yang, Z. L., Oberwinkler, F. (1999). Die Fruchtköperentwicklung von Amanita muscaria (Basidiomycetes). Nova Hedwigia 68, 441–468. doi: 10.1127/nova.hedwigia/68/1999/441
Yang, Z. L., Weiß, M., Oberwinkler, F. (2004). New species of Amanita from the eastern Himalaya and adjacent regions. Mycologia 96 (3), 636–646. doi: 10.1080/15572536.2005.11832959
Zhang, P., Chen, Z. H., Xiao, B., Tolgor, B., Bao, H. Y., Yang, Z. L. (2010). Lethal amanitas of East Asia characterized by morphological and molecular data. Fungal Divers. 42 (1), 119–133. doi: 10.1007/s13225-010-0018-4
Zhang, X. R., Zhou, X., Huang, Z. H., Pu, Z., Zhang, X. L., Wang, Q. C., et al. (2017). Ecological distribution of marcofungi in Badaling Forest Park, Beijing. J. Arid Land Resour. Environ. 31 (8), 181–186. doi: 10.1016/j.ecocom.2017.02.005
Zhou, H., Cheng, G. Q., Hou, C. L. (2022b). A new species, Russula luteolamellata (Russulaceae, Russulales) from China. Phytotaxa 556 (2), 136–148. doi: 10.11646/phytotaxa.556.2.3
Zhou, H., Cheng, G. Q., Sun, X. M., Cheng, R. Y., Zhang, H. L., Dong, Y. M., et al. (2022a). Three new species of Candolleomyces (Agaricomycetes, Agaricales, Psathyrellaceae) from the Yanshan Mountains in China. MycoKeys 88, 109–121. doi: 10.3897/mycokeys.88.81437
Keywords: new taxa, poisonous fungi, ectomycorrhizal, species diversity, molecular systematics
Citation: Zhou H, Guo M, Zhuo L, Yan H, Sui X, Gao Y and Hou C (2023) Diversity and taxonomy of the genus Amanita (Amanitaceae, Agaricales) in the Yanshan Mountains, Northern China. Front. Plant Sci. 14:1226794. doi: 10.3389/fpls.2023.1226794
Received: 22 May 2023; Accepted: 23 August 2023;
Published: 14 September 2023.
Edited by:
Daniel Pinero, National Autonomous University of Mexico, MexicoReviewed by:
Jing Si, Beijing Forestry University, ChinaTolgor Bau, Jilin Agriculture University, China
Copyright © 2023 Zhou, Guo, Zhuo, Yan, Sui, Gao and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: ChengLin Hou, Y2hlbmdsaW4taG91QGNudS5lZHUuY24=
†These authors have contributed equally to this work
 Hao Zhou
Hao Zhou MeiJun Guo†
MeiJun Guo†