
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 18 July 2023
Sec. Plant Bioinformatics
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1214907
This article is part of the Research TopicOmics and Bioinformatics Approaches to Decipher Plant-Microbe InteractionsView all 5 articles
Aflatoxin (AF) contamination, caused by Aspergillus flavus, compromises the food safety and marketability of commodities, such as maize, cotton, peanuts, and tree nuts. Multigenic inheritance of AF resistance impedes conventional introgression of resistance traits into high-yielding commercial maize varieties. Several AF resistance-associated quantitative trait loci (QTLs) and markers have been reported from multiple biparental mapping and genome-wide association studies (GWAS) in maize. However, QTLs with large confidence intervals (CI) explaining inconsistent phenotypic variance limit their use in marker-assisted selection. Meta-analysis of published QTLs can identify significant meta-QTLs (MQTLs) with a narrower CI for reliable identification of genes and linked markers for AF resistance. Using 276 out of 356 reported QTLs controlling resistance to A. flavus infection and AF contamination in maize, we identified 58 MQTLs on all 10 chromosomes with a 66.5% reduction in the average CI. Similarly, a meta-analysis of maize genes differentially expressed in response to (a)biotic stresses from the to-date published literature identified 591 genes putatively responding to only A. flavus infection, of which 14 were significantly differentially expressed (−1.0 ≤ Log2Fc ≥ 1.0; p ≤ 0.05). Eight MQTLs were validated by their colocalization with 14 A. flavus resistance-associated SNPs identified from GWAS in maize. A total of 15 genes were physically close between the MQTL intervals and SNPs. Assessment of 12 MQTL-linked SSR markers identified three markers that could discriminate 14 and eight cultivars with resistance and susceptible responses, respectively. A comprehensive meta-analysis of QTLs and differentially expressed genes led to the identification of genes and makers for their potential application in marker-assisted breeding of A. flavus-resistant maize varieties.
Aflatoxin (AF) contamination in food and feed crops such as maize, peanuts, cottonseed, and tree nuts, caused by Aspergillus flavus, continues to be a persistent problem that compromises food safety and marketability worldwide, especially in developing countries. AF are potent carcinogens, and their contamination in food is one of the major causes of liver cancer. AF levels are strictly monitored in food and feed by the Food and Drug Administration (FDA) and European agencies where American food products are imported. Strict enforcement of action levels for AF in over 120 countries places an adverse burden on growers and food processors because of significant economic losses resulting from the decreased value of contaminated commodities (Bedre et al., 2015). Estimates reveal direct annual crop revenue losses in the US in the tens of millions of dollars, and the losses are exacerbated to hundreds of millions of dollars during severe drought years.
Maize is a major food and feed crop grown worldwide and is highly susceptible to A. flavus infection. Abiotic stressors such as drought significantly increase AF contamination in maize (Kebede et al., 2012; Fountain et al., 2014). Computer model-based prediction projects that alterations in environmental conditions due to climate change could lead to a significant increase in AF contamination in maize resulting in an estimated annual loss of $50 million to $1.7 billion to the US maize industry (Mitchell et al., 2016). In 2013, economic losses in the US resulting from A. flavus alone in maize were estimated to be $686.6 million (Mitchell et al., 2016). Total costs attributable to AF contamination are much higher when factors such as sampling and testing, destruction and disposal, and human and animal health effects are accounted for.
The most efficient and practical approach to reducing pre-harvest AF contamination in maize is the development of resistant lines. There is a dire need for AF-resistant maize germplasms that will also possess resistance to other mycotoxins such as fumonisin and tolerance to abiotic stresses such as drought. Three types of A. flavus resistance mechanisms, such as in vitro seed colonization, preharvest aflatoxin contamination, and aflatoxin production/accumulation, have been reported in different genetic backgrounds of crops, yet there is no report of a single genotype possessing all three resistance mechanisms (Pandey et al., 2019). Natural resistance to AF contamination in maize is a complex multigenic trait, and therefore, it is difficult and slow to introgress AF resistance traits into agronomically superior commercial varieties. Genomics-enabled marker-assisted breeding (MAB) can facilitate the development of AF-resistant maize germplasm suitable for a specific ecogeographic system. A primary requisite for MAB is the identification and validation of genes and linked diagnostic molecular markers associated with AF resistance. A large number of QTLs and markers associated with AF resistance in maize have been reported from multiple biparental mapping and genome-wide association studies (GWAS) (Soni et al., 2020). Resistance to aflatoxin accumulation explained by an individual QTL has not been more than 20% (Warburton and Williams, 2014), although a QTL explaining up to 41% variance was reported by Mideros et al. (2014). Yet, molecular breeding for the development of AF resistance in maize remains to be realized. This is because most of the markers identified for AF resistance in maize emanate from biparental QTL mapping, which is strongly influenced by experimental design, the genetic background of parents, the type and size of mapping population, the growing environment, the choice and density of markers, and the statistical methods used for analysis (Zhao et al., 2018). Moreover, QTLs detected using different mapping populations with diverse genetic backgrounds under different environmental conditions with often low-resolution genotyping and inconsistent phenotyping explain low and varying phenotypic variance with low logarithm of odds (LOD) scores, which create uncertainty for their introgression by MAB (Price, 2006). The efficacy of the QTLs in MAB is further negated by undesirable epistatic and modifier effects of different genetic backgrounds, which are important for QTL stability (Swamy et al., 2011). Also, the lack of fine mapping of these QTLs with large confidence intervals (CI) hinders the application of these overlapping genomic regions in breeding programs (Zhao et al., 2018).
An alternative approach to fine mapping is to leverage previously identified QTLs from multiple studies through meta-QTL (MQTL) analysis. MQTL analysis is a fast-emerging, effective computational technique that precisely combines previously reported QTLs to identify true QTLs with refined positions by reducing the CI of overlapping QTLs on a reconstructed consensus map (Goffinet and Gerber, 2000; Veyrieras et al., 2007; Sosnowski et al., 2012). Meta-analysis sequentially combines QTLs at a 95% CI reported from different studies where the Akaike Information Criterion (AIC)-based model simulation is performed to determine the number of actual QTLs for the trait of interest (Veyrieras et al., 2007). The MQTLs with clustering of a high number of initial QTLs with the smallest CI and a consistent, major effect on target trait(s) allow the identification of promising trait-linked markers that can be effectively utilized in MAB programs upon their validation in a set of germplasm accessions (Soriano and Alvaro, 2019). Moreover, the identification of accurate locations of the MQTLs facilitates mining the of candidate genes from the available physical map. Functional analysis of these candidate genes can narrow down to the actual gene(s) that will have a direct or indirect effect on the desired traits.
Several studies have identified QTLs for various traits for mining the candidate genes in different crop plants, including maize for traits such as flowering time (Chardon et al., 2004; Wang et al., 2016), popping traits (Kaur et al., 2021), grain moisture content and dehydration (Xiang et al., 2012; Wang et al., 2022), ear rot resistance (Xiang et al., 2012), and abiotic stress tolerance (Zhao et al., 2018; Liu et al., 2019; Sheoran et al., 2022). These studies focused on meta-trait (more than one trait), which are functions of several component traits, and therefore such analysis is not able to conclusively describe the genetic and molecular mechanisms underlying the component traits (Liu et al., 2020). Until now, there have been only two MQTL studies reported in maize for AF and Aspergillus ear rot resistance (Xiang et al., 2010; Mideros et al., 2014).
Similarly, several transcriptome studies have been conducted in maize to identify differentially expressed genes with potential roles in the resistance response in maize-A. flavus interaction (Luo et al., 2011; Kelley et al., 2012; Dolezal et al., 2014; Shu et al., 2017; Han et al., 2020; Musungu et al., 2020; Liu et al., 2021). However, a meta-analysis of the transcriptome data is lacking in maize. In the present study, a comprehensive meta-analysis of previously published QTLs and genes with putative roles in AF resistance response in maize was conducted to identify candidate causal QTLs/genes and linked markers.
Published literature on QTL mapping for aflatoxin accumulation in maize was curated through database searches using the keywords, viz., QTL, metaQTL, maize, Aspergillus flavus, and aflatoxin, using Web of Science (http://apps.webofknowledge.com) and Google search engines. Altogether, 16 reports on separate experiments based on different mapping populations were available on QTL analysis of the AF resistance traits in maize. These QTL studies were scanned for methods of QTL mapping, parents, type and size of mapping population and molecular markers, genetic map, QTL position, peak marker of QTL, marker/confidence interval (CI), LOD score of QTLs, and phenotypic variance explained (PVE or R2) by the QTLs. A LOD score of 3.0 was uniformly assumed for experiments that did not provide the LOD value for the QTLs. For studies that did not provide CI for QTLs, the 95% CI was calculated as described by Darvasi and Soller (1997) and Guo et al. (2006). Most of the studies reported QTLs from experiments involving individual environments (locations), years, and overall based on data from multiple environments/years. Therefore, each QTL reported for each year and location in the studies was treated as an independent QTL. Of these QTL reports, 11 published a genetic map and analyzed their data using composite interval mapping (CIM) and/or multiple interval mapping (MIP) to report QTLs with information on key parameters such as QTL peak and flanking markers, LOD score, and PVE, which were used to perform the meta-analysis (Supplementary Table S1).
Similarly, literature available on the gene expression changes in response to several abiotic and biotic stresses in maize was collected following a thorough search for all stress-related studies from both microarray and RNA-seq data available in the NCBI Gene Expression Omnibus (Luo et al., 2008; Luo et al., 2011; Kelley et al., 2012; Shu et al., 2017; Kebede et al., 2018; Han et al., 2020; Musungu et al., 2020; Liu et al., 2021) (Supplementary Table S2). All the genes and their relative fold change in expression values were collected and/or deduced using signal intensity, FPKM, or TPM values using methods described earlier (Baisakh et al., 2012; Bedre et al., 2015).
First, a consensus genetic map was developed using the B73 reference genetic map available in MaizeGDB (https://www.maizegdb.org/data_center/map). The genetic maps and QTL files from published data, as well as the reference map, were uploaded in BioMercator v4.2.3 (De Oliveira et al., 2014), following an InfoMap analysis (Veyrieras et al., 2007) to confirm that all genetic maps shared at least one marker among them and the reference map. The consensus map was created by ConsMap, which calculates the goodness-of-fit value of the maps for each chromosome (Veyrieras et al., 2007; Truntzler et al., 2010). If a common marker was not found between the individual genetic map and the consensus map, a third map was used as a cross-reference based on the marker positions and order.
All QTLs with LOD score, R2 value, flanking marker positions, and CI from published studies were projected on the consensus genetic map using the QTLProj command, which utilized a simple scaling rule between the original QTL marker interval and the corresponding interval on the consensus map (Veyrieras et al., 2007). The new CI of the projected QTLs was approximated with a Gaussian distribution encompassing the most probable QTL position. Two initial QTLs that did not meet a minimal distance ratio of 0.25 and the p-value of homogeneity of flanking markers between the original map and the consensus map at 0.5 were not projected.
Meta-analysis of independent QTLs obtained from different mapping populations, locations/environments, and years was performed using the consensus map with QTL projections using the Meta-analysis command to predict the MQTLs (representative consensus regions). Meta-analysis was performed in two steps. First, QTLs on each chromosome were clustered, assuming their normal distribution around the true location. All possible QTL combinations were tested with maximum clusters (Kmax) set to 15, and the one with the maximum likelihood was selected. The QTL model on each chromosome was selected using the AIC or corrected AIC (AICc, when the QTL sample size was less than six), which returned the number of MQTLs that represent the most significant regions. Next, MQTLs were generated from QTL projection on a consensus map based on the best model with the lowest AIC. Furthermore, the position and 95% CI of the MQTLs were calculated, and the flanking markers for MQTLs were retrieved.
A local database was created with the genes retrieved from the gene expression omnibus that are differentially regulated in maize under various biotic and abiotic stresses (unpublished data). Based on the gene ID comparison, a sub-set of genes with expression changes unique to AF resistance was identified.
Genes, along with their available functional annotation, linked to each MQTL within their confidence intervals were retrieved using the genome version in Biomercator 4.2.3, which was built based on the ZmB73_5b genome. Genes common between MQTL intervals and differentially regulated under A. flavus (Musungu et al., 2020) were queried against the putative unique genes expressed by A. flavus infection to identify candidate genes within MQTLs responsive exclusively to A. flavus.
AgriGO v2.0 (Tian et al., 2017) was used to perform the gene ontology (GO) enrichment analysis for the genes common between MQTLs-linked genes and A. flavus-responsive genes to examine the GO terms overrepresented that describe gene products in biological processes, molecular functions, and cellular components. Enrichment for each GO slim term was queried in the gene list to identify related gene entries and GO terms at a hypergeometric significance threshold (FDR < 0.05). Kyoto Encyclopedia of Genes and Genomes KEGG pathway enrichment analysis of the genes was performed using the KEGG mapper (https://www.genome.jp/kegg/mapper/).
Expression of the unique A. flavus-responsive genes in MQTL regions was assessed using the Log2-fold change values in inoculated maize tissues relative to uninoculated control reported in the RNA-seq data of Musungu et al. (2020). In addition, the expression of these genes under abiotic stresses such as drought, salt, and the heat was analyzed using qTeller, a comparative RNA-seq expression platform in maizeGDB, which compares expression across multiple data sources in a user-provided gene list. Heat maps with hierarchical clustering of the genes based on their log2 fold-change expression values were built for visualization using the R package pheatmap.
The log2 fold-change values of significantly differentially expressed genes (p ≤ 0.05) common between MQTLs and Musungu et al. (2020) were used for co-expression network analysis using the R package WGCNA. Clusters (modules) of highly correlated genes (nodes) and intramodular hub genes were identified using the eigen values (Langfelder and Horvath, 2008). Briefly, the soft threshold = 9, according to the criterion of scale-free topology module, was selected to create an adjacency matrix, which was transformed into a topological overlap measure (TOM) matrix to estimate its connectivity of the network, and the weighted adjacency matrix of genes was hierarchically clustered based on dissimilarity among genes. The minimal gene number of 30 and a threshold cut-off of 0.25 were set to identify significant modules. In each intramodular connectivity, the genes with KM value >0.8 were declared as hub genes.
A two-way validation was performed for the MQTL identified in the study. The MQTLs and the markers associated with A. flavus resistance in maize from five reported GWAS (Farfan et al., 2015; Warburton et al., 2015; Zhang et al., 2016; Han et al., 2020; Bertagna et al., 2021) were compared based on the physical positions to ascertain their proximity. Also, the consensus map of maize was used to locate SSR markers flanking each MQTL and their genomic positions. Primers for 12 SSR markers closest to the MQTLs that colocalized with GWAS SNPs (except MQTL2.4 and MQTL8.2) were custom synthesized at Integrated DNA Technologies Inc. (www.idtdna.com). Based on the reports on the resistance response of maize genotypes to A. flavus (Busboom and White, 2004; Brooks et al., 2005; Menkir et al., 2006; Bello, 2007; Alwala et al., 2008; Warburton et al., 2009; Warburton et al., 2011; Willcox et al., 2013; Williams et al., 2015; Brown et al., 2016; Smith et al., 2019; Womack et al., 2020; Castano-Duque et al., 2021; Ogunola et al., 2021), 14 resistant and eight susceptible maize genotypes were selected for MQTL/marker validation.
Maize seeds were germinated in moist filter paper inside an incubator at 28°C. Leaf tissues from 1-week-old seedlings were used for total genomic DNA extraction by a modified CTAB protocol and the DNA quality and quantity were assessed on a 1% agarose gel and nanodrop as described earlier (Khan et al., 2013). Polymerase chain reaction (PCR) was performed using 50 ng genomic DNA following the thermal profile: one cycle of denaturation at 95°C for 5 min, 35 cycles of 95°C for 45 s, 58°C for 45 s, and 72°C for 1 min, followed by a final extension at 72°C for 5 min, and the PCR amplicons were resolved on a 12% polyacrylamide gel using the method described in Khan et al. (2013).
A total of 356 QTLs controlling A. flavus resistance and/or AF accumulation were reported from 17 studies, with the highest number of QTLs (76) on chromosome 1 and the lowest (13) on chromosome 9 (Supplementary Table S1). The average PVE by the QTLs was 7.56%, with a range from 0.004% to 53%. Only 11 studies reporting 278 QTLs that provided adequate relevant information on linkage maps and QTL parameters were used for map projections and subsequent MQTL analysis. The remaining six experiments lacked either genetic maps or molecular markers delimiting the QTLs that were not found in the final integrated linkage map for their use in the downstream investigation. The integrated linkage maps associated with A. flavus resistance were assembled from 11 published individual linkage maps and the reference map of maize variety B73 (https://www.maizegdb.org/data_center/map). The integrated linkage map contained 960 markers with a total length of 2,372.9 cM and an average marker density of 2.47 markers/cM (Supplementary Table S3).
The consensus genetic map developed using the integrated linkage map on the B73 reference map with shared markers and the goodness-of-fit value of the genetic maps for each chromosome containing the markers linked to the QTLs from published data consisted of a total of 5,023 loci that included 2,968 SNPs and 2,055 SSR markers (Supplementary Table S4; Supplementary Figure S1). The consensus map was highly saturated, covering a total length of 2,555.10 cM with an average genetic distance of 0.51 cM between the adjacent markers. Comparison of the consensus map with the physical map obtained from the B73 RefGen_V5b reference genome showed very high collinearity in SSR marker order with 94.8% correlation (Supplementary Table S4), which suggested that the consensus map was perfectly ideal for QTL projection. Therefore, 276 out of 278 QTLs, considered out of 356 initial QTLs, were successfully projected on the map.
The meta-analysis of 276 projected QTLs identified a total of 58 MQTLs distributed over all 10 maize chromosomes, with chromosome 1 having the highest number (10), while chromosome 9 registered only two MQTLs (Table 1; Figures 1A, B). On average, each MQTL accounted for 4.8 original QTLs, with six MQTLs (MQTL2.2, MQTL2.6, MQTL4.3, MQTL5.3, MQTL8.4, and MQTL 10.6) spanning two QTLs, whereas MQTL 1.1 covered 16 original QTLs.
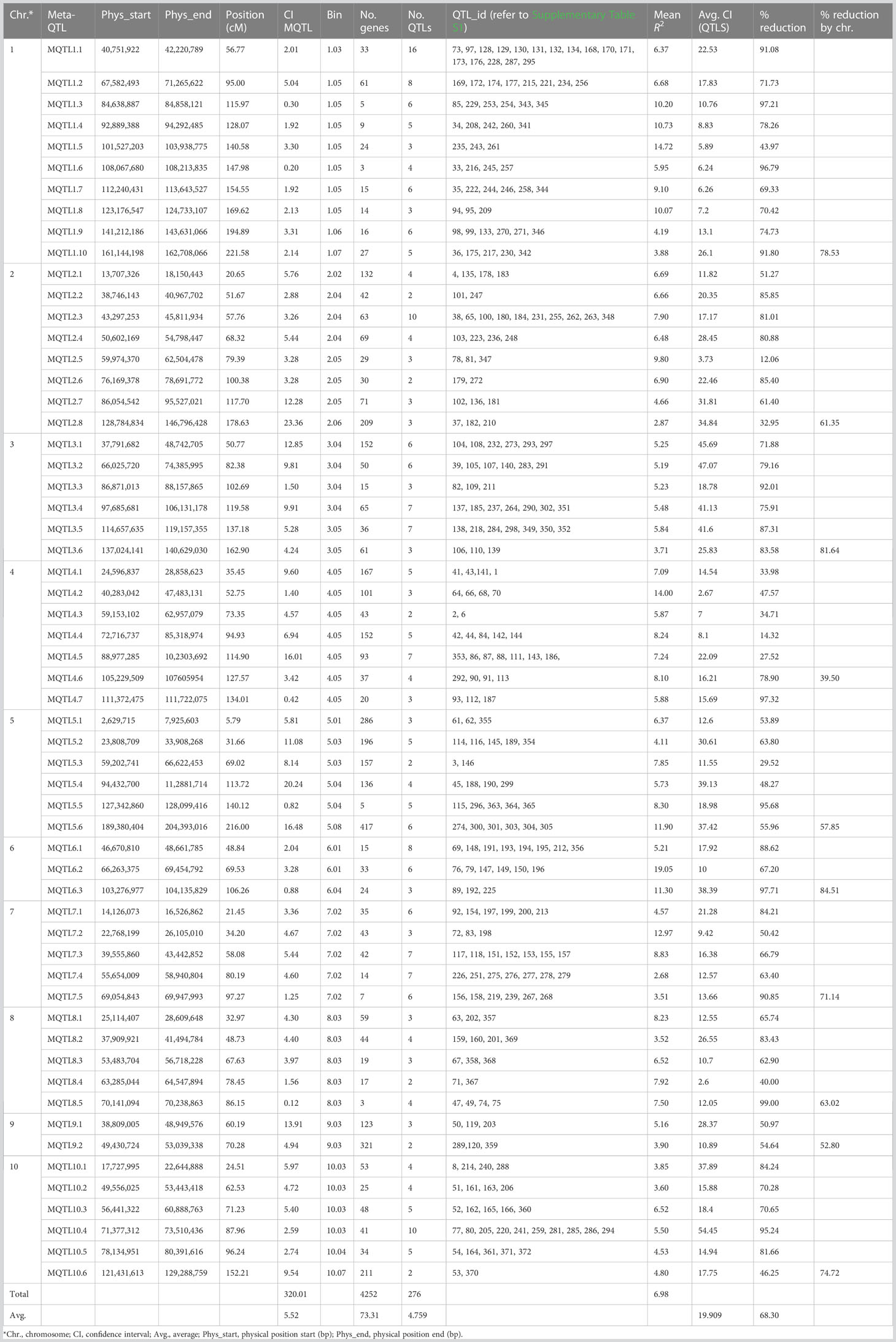
Table 1 Detail information on the 58 MQTLs generated with the 276 initial QTLs associated with Aspergillus flavus resistance in maize.
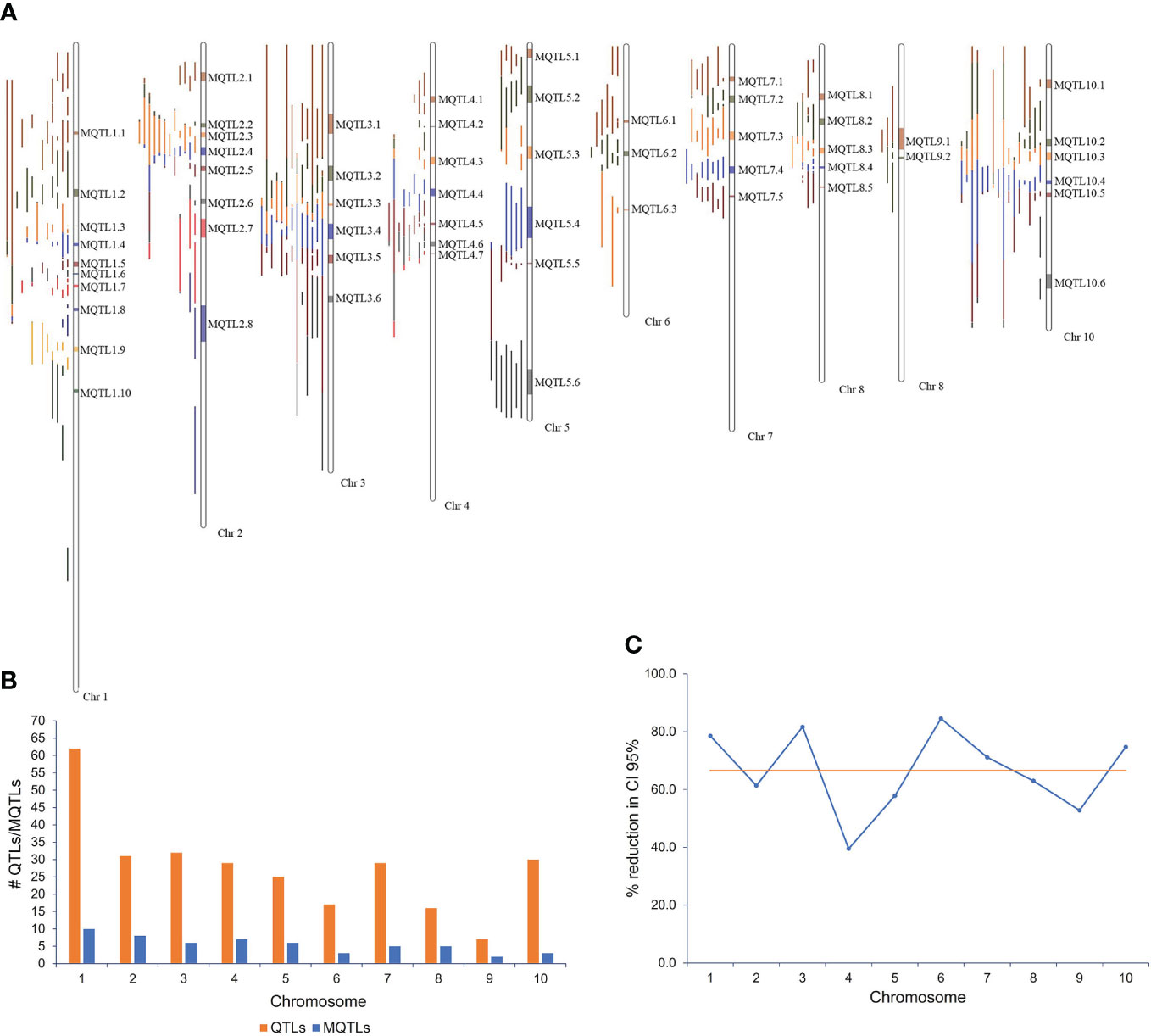
Figure 1 MQTLs associated with resistance to Aspergillus flavus and aflatoxin accumulation in maize. (A) Fifty-eight MQTLs distributed over 10 maize chromosomes; (B) chromosomal distribution of QTLs and MQTLs; (C) reduction (%) of confidence interval (CI) of MQTLs relative to the original QTLs.
The CI of individual MQTL regions was narrower than the mean CI of the original QTLs for that region. The average CI of the MQTLs was 5.52 cM in comparison with 19.91 cM for the original QTLs (Table 1). There was a reduction of 68.3% in the average CI of the MQTLs over the mean CI of the original QTLs, with a range from 12.1% (for MQTL2.5 at 79.39 cM) to 99.0% for MQTL8.5 at 86.15 cM (Table 1). The average reduction in CI from the initial QTLs per chromosome was 66.5%, with the lowest (39.5%) reduction on chromosome 4 and the highest (84.6%) on chromosome 6 (Figure 1C). The genetic and physical lengths of MQTLs ranged from 0.12 cM and 0.098 Mb (MQTL8.5) to 23.36 cM and 18.01 Mb (MQTL2.8), respectively. Five MQTLs (MQTL1.3, 1.6, 5.5, 7.5, and 8.5) had a physical interval of less than 1 Mb. Bins 4.05 and 1.05 harbored the maximum number (seven) of MQTLs, followed by five MQTLs in bin 8.03. The MQTLs explained from 2.9% (MQTL2.8) up to 19.1% (MQTL6.2) of the variance for A. flavus resistance. There was no significant difference between the average PVE (7.0%) of the MQTLs (Table 1) and the average PVE of the original QTLs (7.5%) (Supplementary Table S1).
A total of 4,252 genes were identified in the 58 MQTL intervals based on the maize genome ZmB73_5b built in Biomercator 4.2.3 (Table 1; Supplementary Table S5). On average, 73 genes were identified in the MQTL regions, with the lowest (three) in MQTL8.5 and the highest (429) in MQTL9.2. The number of genes linked to the MQTLs varied from 72 (chromosome 6) to 1,197 (chromosome 5).
The biological roles of the genes in MQTL regions were ascertained by GO enrichment analysis, which showed that the highest number of genes significantly enriched in biological processes belonged to the GO term establishment of localization (217), closely followed by 215 transport mechanism and 188 in response to chemical stimulus (Supplementary Table S6; Figures 2). Significantly overrepresented GO terms associated with molecular function were related to genes involved in cation/ion binding (371), metal ion binding activity (369), transition metal ion binding (287), and oxidoreductase activity (201). For the cellular component, a significantly higher number of candidate genes were mainly related to the cell and cell part (1,018 genes), membrane (499 genes), and organelle (301 genes). KEGG analysis revealed that the MQTL genes belonged to 30 significant pathways, of which metabolic pathways and biosynthesis of secondary metabolites were the two most enriched pathways with 1,646 and 926 candidate genes, respectively (Supplementary Table S7; Figure 3).
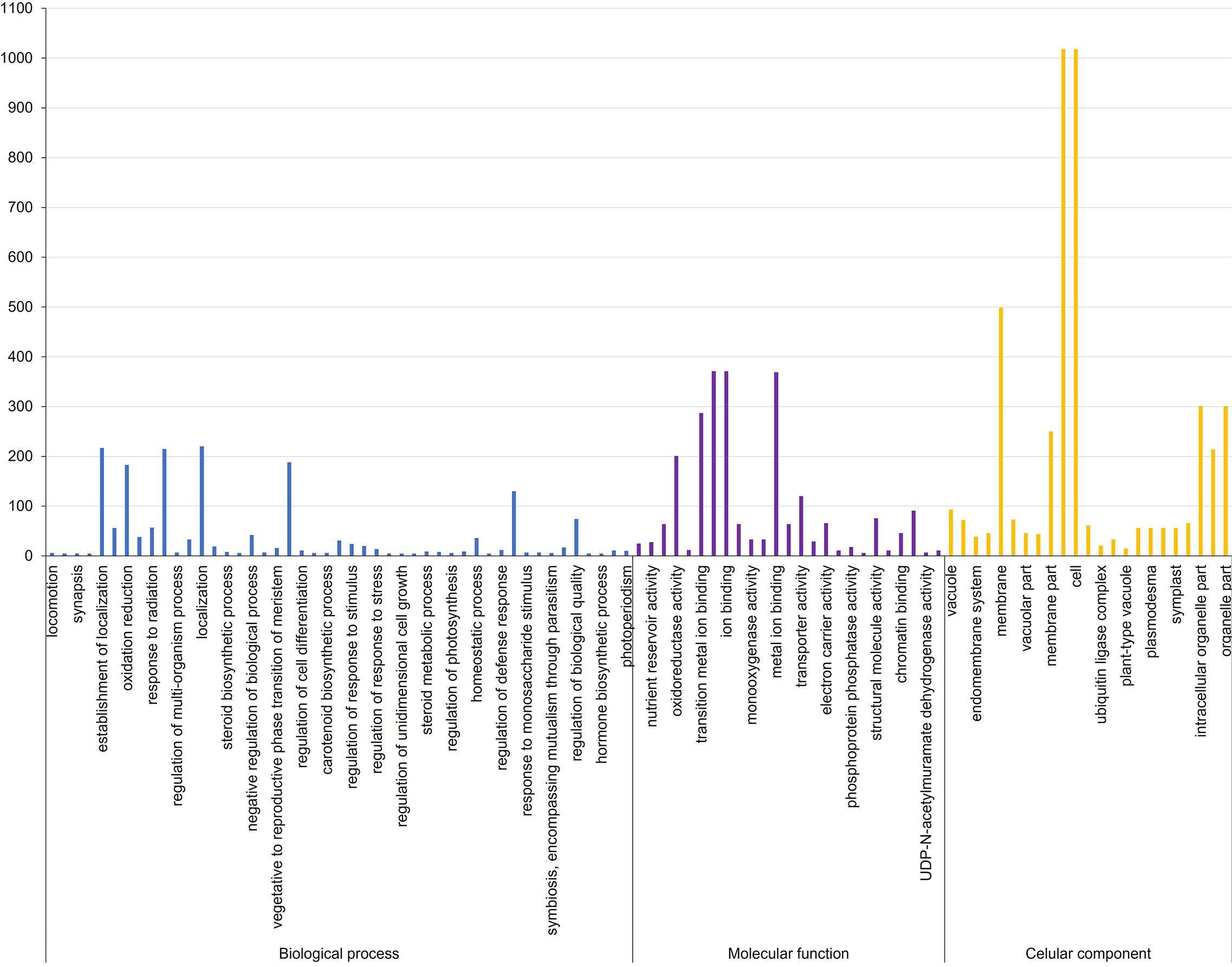
Figure 2 Gene ontology terms associated with biological processes, molecular function, and cellular component in Aspergillus flavus resistance associated 58 MQTL intervals in maize.
Gene co-expression network analysis of the genes in MQTLs regions revealed that genes in four modules, such as Turquoise (445 genes), Blue (221 genes), Brown (71 genes), and Yellow (23 genes) were significantly co-expressed in maize–A. flavus system (Supplementary Table S8) with Kme values more than 0.8. In the Turquois module, out of 12 candidate hub genes with significantly high interaction (threshold >0.5), eight had functional annotations: Zm00001d003488 (UDP-glycosyltransferase 85A7), Zm00001d024444 (aldehyde dehydrogenase 26), Zm00001d017532 (RING-H2 finger protein ATL71), Zm00001d025776 (cell division control protein 48 homolog B), Zm00001d049374 (DUF295 domain-containing protein), Zm00001d025651 (fasciclin-like arabinogalactan protein 7), Zm00001d030855 (alpha zein z1D_4), and Zm00001d025672 (autophagy10) with 954, 375, 348, 306, 285, 178, 134, and 60 interactions, respectively. On the other hand, only one gene Zm00001d000004 coding for cysteine-rich PDZ-binding protein passed the threshold with 36 interactions in Blue module (Supplementary Table S8).
Matching analysis of the 4,252 candidate genes in MQTL regions with previously reported A. flavus-responsive genes (Musungu et al., 2020) identified 3,384 common genes. Comparison of the subsets of genes expressed in response to various abiotic and biotic stresses, including A. flavus infection (Musungu et al., 2020), in the database of both RNA-seq and microarray experiments showed that 591 genes (256 from RNA-seq and 335 from microarray) were induced only in response to A. flavus (Supplementary Table S5). Of these 591 genes, 50 showed significantly differential expressions at −1.0 ≤ Log2FC ≥ 1.0 (p ≤ 0.05) at different stages of response to A. flavus (Musungu et al., 2020; Supplementary Table S5). Furthermore, querying these 591 genes against the 3,384 genes identified 43 genes as candidates differentially uniquely expressed upon A. flavus infection. These 43 genes with significantly differential expressions at different stages of A. flavus infection (Supplementary Figure S2) were distributed over all chromosomes except chromosome 6, with the maximum number (14) in chromosome 5 and only one on chromosomes 1 and 8 (Supplementary Table S9).
These 43 genes were also queried against the gene list in qTeller, a comparative maize RNA-seq expression platform. Interestingly, 29 out of 43 genes showed expression induction or repression under abiotic stresses such as drought and/or salt (Supplementary Table S10; Supplementary Figure S3). Therefore, the remaining 14 genes were considered putative A. flavus-specific unique genes with no reported expression under stresses other than A. flavus. Eight out of the 14 genes did not have any assigned functional annotation. Two genes, Zm00001d030855 and Zm00001d003677, encode endosperm-specific proteins alpha zein and opaque endosperm 11, respectively. The remaining four genes, one each, coded for fasciclin-like arabinogalactan protein (Zm00001d025672), the mediator of RNA polymerase II transcription subunit 26b (Zm00001d040506), carboxylesterase (Zm00001d050270), and UDP-glycosyl transferase (Zm00001d013246) (Supplementary Table S11, S12).
Alpha zein (Zm00001d030855) did not exhibit a specific expression pattern although it showed higher upregulation at most stages except at S3 and S7 where it showed downregulation by -0.12 and -0.32-fold, respectively, and at S3, S9, S16, and S18 there was no significant change in its expression (Supplementary Table S13; Figure 4). The expression was especially higher at late stages of infection. On the other hand, Zm00001d049476 for alpha-zein 19 kDa A-1 showed upregulation at all stages.
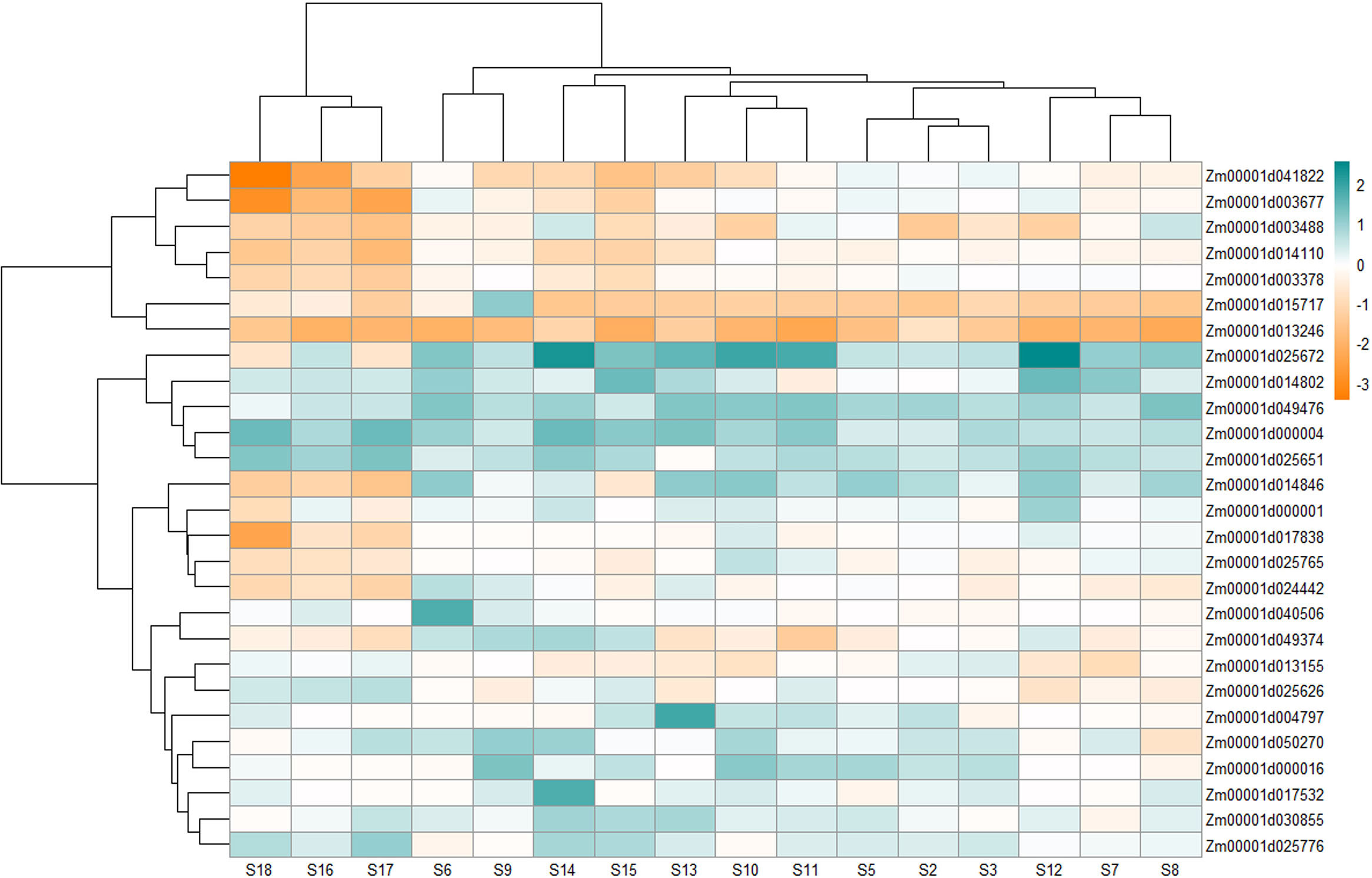
Figure 4 Heatmap showing the differential expression of 27 (out of 29) genes that include 14 genes uniquely expressed under Aspergillus flavus infection and 15 genes that were physically close between MQTL intervals and SNPs from published GWA studies. The expression values at different stages were retrieved from Musungu et al. (2020).
Expression of the Opaque 11 gene (Zm00001d003677), on the other hand, was repressed at most stages except for slight upregulation in S2, S5, S9, and S12. Zm00001d025672, coding for fasciclin-like arabinogalactan protein, was overexpressed with time postinfection, with its highest at S12 and S14, and then showed downregulation (S17 and S18). A putative mediator of RNA polymerase II transcription subunit 26b (Zm00001d040506) remained unchanged or maintained low upregulation with its highest at S5 and slight downregulation at S8. Zm00001d050270 (carboxylesterase) showed moderately high upregulation at all stages, with its highest at S9; however, it was also downregulated at S8. The transcript accumulation of UDP-glycosyltransferase (Zm00001d013246) was repressed at all stages postinoculation of the fungus (Figure 4).
A total of 272 MTAs were identified from five GWAS related to A. flavus resistance-related traits in maize (six—Farfan et al., 2015; 222—Warburton et al., 2015; 25—Zhang et al., 2016; 11—Han et al., 2020; and eight—Bertagna et al., 2021) (Supplementary Table S12). Comparative analysis of the MTAs with MQTLs based on their physical intervals identified eight MQTLs (two on chromosome 2, one each on chromosomes 4 and 5, and three on chromosome 10) that colocalized with 14 MTAs, all from three GWAS (Table 2). Of 14 MTAs, 11 were from one GWAS (Warburton et al., 2015) that collocated with MQTL2.1, MQTL2.2, MQTL9.1, MQTL10.1, MQTL10.4, and MQTL0.6, whereas two MTAs (Bertagna et al., 2021) and one MTA (Farfan et al., 2015) co-located in the MQTL5.6 region. A total of 40 genes with functional divergence were identified within 10 kb of the GWAS-identified SNPs in the matching MQTL regions (Table 2). In total, 23 genes showed differential expression at various stages of infection by Aspergillus flavus (Supplementary Figure S4). Comparing these 40 GWAS genes with the 43 putative unique candidate genes in MQTL intervals showed that eight genes from GWAS were physically close (≤4 Mb) to seven genes in MQTL intervals (Supplementary Table S13).
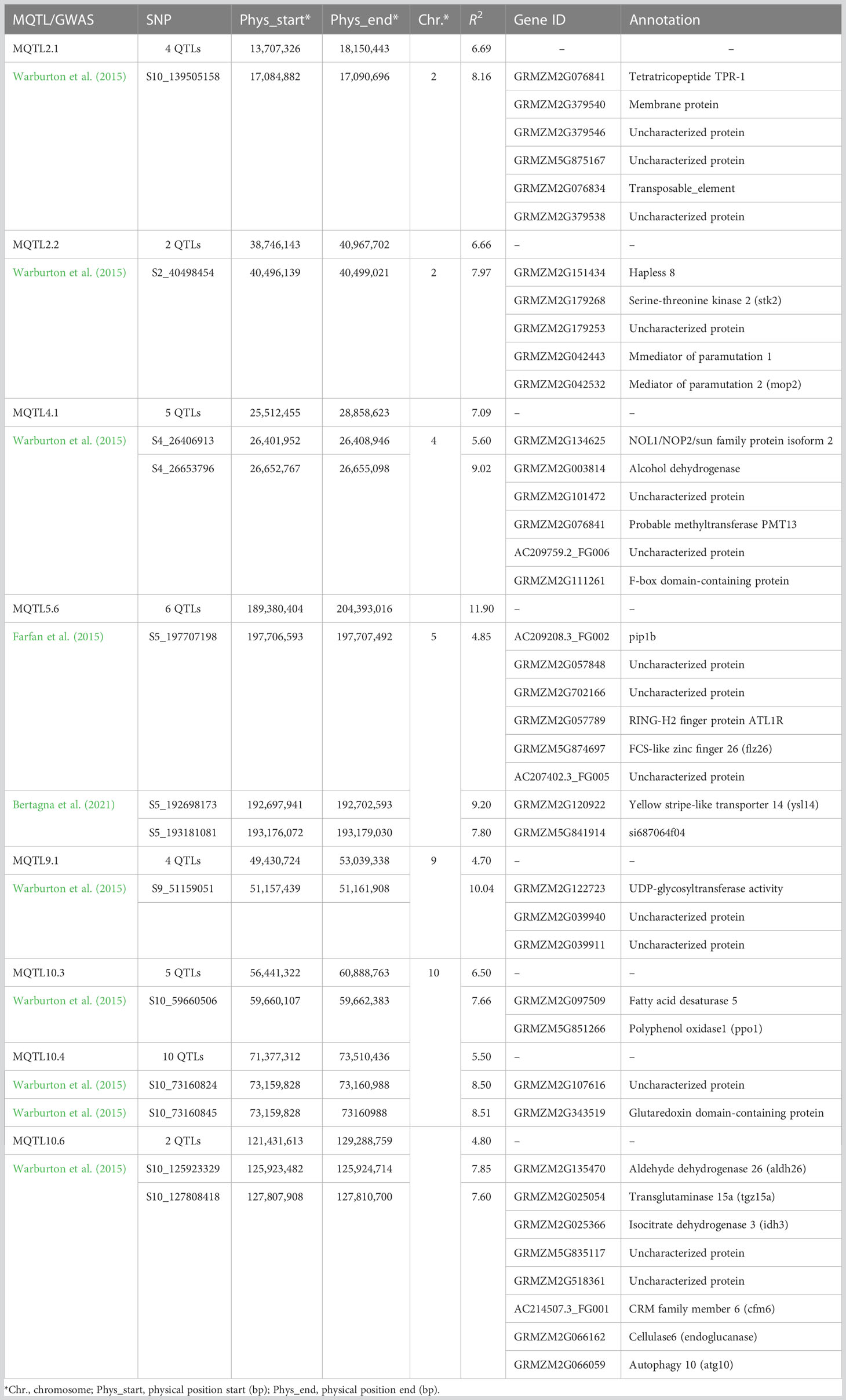
Table 2 Forty genes within 10 kb of the SNPs associated with Aspergillus flavus resistance were identified from five genome-wide association studies.
In total, 12 SSR markers closely linked to MQTLs (Supplementary Table S14) were tested with 22 maize lines with known reactions to A. flavus. Among these, three markers, umc1555, umc1757, and umc1817 linked to MQTL2.4, MQTL4.1, and MQTL8.2 clearly discriminated between 14 and eight maize genotypes with known A. flavus resistance and susceptible phenotypes, respectively (Figure 5).
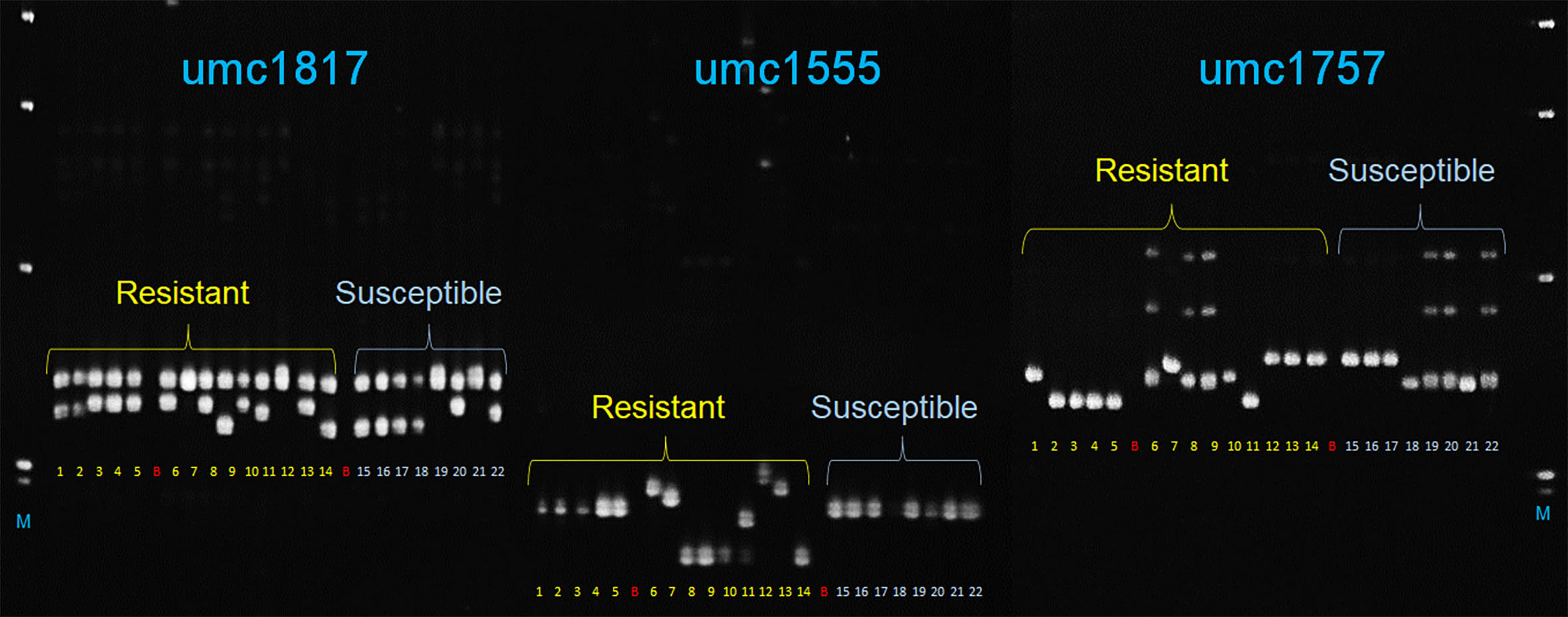
Figure 5 Polyacrylamide gel image showing amplicons generated by three SSR markers (umc1817, umc1555, and umc1757) linked to MQTL8.2, MQTL2.4, and MQTL4.1, respectively, with 22 maize genotypes that included 14 resistant (samples 1–14) and eight susceptible (15–22) maize cultivars. 1–12: TZAR102, TZAR103, TZAR104, TZAR105, TZAR106, MP715, MP10-127, MP04:127, MP494, MP717, MP317, MP313E, MP718, MP719, PHW79, VA35, TI73, SA212M, HI-11, A188, B104, B73; B, blank; M, 1-kb plus DNA size marker (Invitrogen Inc.).
Aspergillus flavus resistance is a quantitative trait, controlled by both genetic and environmental determinants, which makes breeding aflatoxin-resistant maize variety challenging. Aflatoxin resistance is a trait with low heritability (21% for A. flavus infection; Busboom and White, 2004), although H2 up to 63% (Mideros et al., 2014) and 74% (Maupin et al., 2003) have been reported. Several studies have identified QTLs for A. flavus resistance in maize (Paul et al., 2003; Widstrom et al., 2003; Busboom and White, 2004; Brooks et al., 2005; Alwala et al., 2008; Warburton et al., 2009; Mayfield et al., 2011; Warburton et al., 2011; Willcox et al., 2013; Mideros et al., 2014; Yin et al., 2014; Dhakal et al., 2016; Zhang et al., 2016). Except for Zhang et al. (2016), where the authors combined linkage-based QTL mapping with GWAS to resolve a major QTL for A. flavus to identify several candidate genes for resistance, constitutive, robust, and large-effect QTLs over multiple environments and across populations that are critical for deployment of markers linked to the QTLs in genomics-assisted breeding for aflatoxin resistance are lacking. Hence, consensus genomic regions that include more than one QTL surrogated by a single marker are important to incorporate one or a few alleles from the constituent QTL to improve A. flavus resistance. Thus far, only two studies have reported MQTLs using QTL studies on A. flavus resistance traits identified until 2011 (Xiang et al., 2010; Mideros et al., 2014).
We undertook a combinatorial approach of meta-analysis of QTLs reported until 2022 and gene expression data to pinpoint candidate genes (and markers) associated with A. flavus resistance. To identify consensus genomic regions with stable expression, we performed a meta-analysis with all 10 maize chromosomes that harbored at least six QTLs, so the possibility of poor performance of the model because of over-parameterization was ruled out (Courtois et al., 2009). The QTL dataset that we used for meta-analysis was not representative of all individual QTL mapping studies reported for A. flavus resistance in maize due to the lack of sufficient information and/or heterogeneity of the data needed for the MQTL analysis tool. A high-density linkage map is key to identifying stable MQTLs and selecting candidate genes for functional characterization or marker development for use in marker-assisted breeding. Consensus genetic maps created in earlier QTL meta-analysis studies (Truntzler et al., 2010; Mideros et al., 2014) rejected the existence of the same genetic map for all mapping populations. However, using markers related to the position of QTLs and (re)ordering of the markers on some genetic maps based on both genetic and physical positions, we developed an integrated high-density consensus linkage map with 5,023 markers and 2,555 cM long, much higher than 1,803 markers reported earlier (Mideros et al., 2014). High collinearity between the consensus map and the physical map obtained from the reference map B73 RefGen_v5 observed in our study was in congruence with the study of Akohoue and Miedaner (2022), which led us to project 276 out of 278 QTLs in the consensus map. Most of the MQTLs reported by Mideros et al. (2014) were identified in our 58 MQTLs over all 10 chromosomes, whereas 62 MQTLs were reported by Mideros et al. (2014) on eight chromosomes with no MQTL on chromosomes 9 and 10. The disparity in the number was due in part to some single original QTLs reported as MQTLs by Mideros et al. (2014).
As expected in a meta-analysis of QTLs, the CI (95%) of the MQTLs in our study was 3.2-fold narrower with an average of 6.3 cM (range 0.12 cM to 23.36 cM) in comparison with 19.8 cM of the initial QTLs. Previous studies in maize have shown comparable reductions of MQTL 95% CI, such as 1.4- to 36.4-fold (Akohoue and Miedaner, 2022), 3.8-fold (range 0.02 to 29.87 cM; Sheoran et al., 2022), 32% to 91% (Wang et al., 2022), and 1.89 to 14.27 cM (Kaur et al., 2021). Mideros et al. (2014) reported 8.1 cM as the average 95% CI of MQTLs, whereas three MQTLs had a 95% CI of less than 2 cM. However, we found six MQTLs, MQTL1.3, MQTL1.6, MQTL5.5, MQTL6.3, MQTL7.5, and MQTL8.5 with 95% CI <1 cM and narrower physical interval (<1 Mbp) and shorter genetic distance with at least three QTLs in those regions.
MQTLs are the target genomic regions for introgression via marker-assisted breeding programs. Thus, constant validation of identified MQTLs is needed for their effective utilization in molecular breeding. In this study, the correlations of MQTLs with MTAs obtained from GWAS were established, and the positions of MQTLs were compared with 272 MTAs associated with A. flavus resistance in five GWAS. Eight (MQTL2.1, MQTL2.2, MQTL4.1, MQTL5.6, MQTL9.1, MQTL10.3, MQTL10.4, and MQTL10.6) of 58 MQTLs matched MTAs from three GWAS (Farfan et al., 2015; Warburton et al., 2015; Bertagna et al., 2021), suggesting that the effects of these genomic regions on A. flavus resistance traits with moderate influence from the genetic background. Except for MQTL5.6, these MQTLs were different than the nine MQTLs distributed on chromosomes 1, 4, 5, 6, 7, and 8 (MQTL1.3, MQTL1.4, MQTL1.5, MQT1.8, MQTL4.3, MQTL5.6, MQTL6.2, MQTL6.3, and MQTL7.2) that were considered significant for their association with A. flavus resistance based on the R2 ≥ 10 criterion. This result suggests that PVE should not be the only focus while selecting genomic regions of interest for their further validation and marker development, especially for multigenic traits such as resistance to A. flavus and aflatoxin accumulation.
Validation of the MQTLs was also performed using 12 microsatellite markers linked to a few selected MQTLs that were either close to the SNPs from the GWAS studies, with an average R2 ≥ 10 or with 95% CI of less than 1 cM. The three markers, umc1555, umc1757, and umc1817, closest to MQTL2.4, MQTL4.1, and MQTL8.2, respectively, were able to distinguish the A. flavus-resistant and susceptible genotypes. While umc1757 at bin 4.05 of chromosome 4 collocated with S4_26406913 at ~ and S4_26653796 at ~1.1 Mb (Warburton et al., 2015), umc1555 linked to MQTL2.4 at bin 2.04 on chromosome 2 was ~10 Mb distant from S2_40498454 (Warburton et al., 2015) that was close to MQTL2.2 within the same bin. These three markers have great potential for their use in marker-assisted selection for A. flavus resistance, although only umc1555 and umc1757 with their discriminatory power for contrasting disease response phenotypes can be considered for the development of potential diagnostic markers. Sequences around these markers and additional sequence-based markers must be identified from large, diverse maize genotypes with known A. flavus resistance response to develop haplotype-specific markers for use in breeding applications.
Identification of MQTLs allowed us to efficiently search for candidate genes with potential involvement in maize–A. flavus interaction leading to aflatoxin toxin accumulation. A thorough comparative search of the 4,252 candidate genes in MQTL intervals with our in-house compilation of the maize transcriptome dataset identified 43 genes that were significantly differentially expressed only following A. flavus infection (Musungu et al., 2020). However, further interrogation of these genes against the maize gene expression database qTeller at maizeGDB showed that 14 genes were not induced/repressed under any other stress. Concurrently, 40 genes involved in various biological processes were identified as linked to the SNP markers from GWAS that collocated with eight MQTL intervals. Of the 15 genes that were close to each other between GWAS and MQTL region genes within ≤4 Mb interval, 13 had functional annotation. Therefore, these 29 genes (27 genes with functional annotation) were considered significant for their possible implications in A. flavus resistance. Specifically, the 13 genes out of 27 (12 in the Turquoise module and one in the Blue module) with a significant number of interactions with other genes in the network can be important candidates for further validation.
Polyphenol oxidase (PPO) such as Zm00001eb413300 plays an important role in plant defense mechanisms against biotic stresses. PPO could modify proteins by alkylating different compounds which reduces the bioavailability of proteins and prevents the digestion/absorption of nutrients in fungi (Zhang and Sun, 2021). Cysteine-rich proteins (CRPs) are involved in binding to known receptors in plants (Huang W et al., 2014). Although the signaling mechanisms and protein interactions are largely unknown, most characterized genes function as short-range intercellular signals during plant defense against pathogens (Marshall et al., 2011). A pathogenesis-related protein belonging to cysteine-rich secretory protein responded to A. flavus infection in maize (Hawkins et al., 2018). In cotton, a cysteine-rich kinase was differentially regulated in both pericarp and seed tissues following A. flavus infection (Bedre et al., 2015). The gene Zm00001eb249940 linked to MQTL5.6 was a plasma membrane intrinsic protein (PIP). PIPs are highly hydrophobic aquaporin proteins with six membrane-spanning domains that play important roles in channels that facilitate the passage of water, small solutes, and possibly other moieties through the membrane and confer abiotic stress tolerance in plants. Although the pathways involving their role in plant defense are not completely understood, the transport of H2O2, produced in response to pathogen attack by aquaporins suggests their functions in plant defense (Dynowski et al., 2008). Downregulation of some members of PIPs in soybean leaves and citrus plants upon Pseudomonas syringae and Candidatus liberibacter infection highlight their correlation with the disease development (Zou et al., 2005; Martins et al., 2015). A plasma membrane-associated protein was differentially expressed in maize upon infection by A. flavus (Dhakal et al., 2017).
Plant-derived fatty acids (FA) have been shown to regulate A. flavus colonization in the seeds by controlling pathogen development and mycotoxin production (Upchurch, 2008). Zm00001eb413200 codes for fatty acid desaturase 5 (FAD5), which is a key regulator of FA desaturation, and FADs modulate the activation of defense signaling pathways in plants, leading to PR gene expression involved in plant disease resistance (Kachroo et al., 2001).
The gene Zm00001d003378, a mediator of paramutation 1 (mop1), codes for RNA-directed RNA polymerase, which controls paramutation, the directed, heritable alteration of the expression of one allele at multiple loci (Alleman et al., 2006). The differential regulation of this gene in maize postinfection with A. flavus indicates that RdRP-mediated epigenetic changes via DNA methylation or small RNA regulation could play an important role in disease resistance responses. Poly(A)-specific ribonuclease (PARN) influences the poly(A) status of cytoplasmic mRNA in most eukaryotes. PARN might regulate the efficient translation of mRNAs that control cytosolic Ca2+ elevation, leading to plant responses to pathogenic fungi (Johnson et al., 2018). Plants are known to regulate defense mechanisms using polyadenylation controlled by PARN (Yang et al., 2014). The modulation of expression of maize PARN gene (Zm00001d025651) in response to A. flavus implies its involvement in alternative splicing of other genes involved in resistance response. Pathogen infection triggers dramatic transcriptome reprogramming, leading to a shift in plant growth and development and an immune response. During this rapid process, the mediator plays an important role in fine-tuning gene-specific and pathway-specific transcriptional reprogramming by acting as a coregulator bridge between gene-specific regulatory proteins and basal RNA polymerase II transcription machinery (Richter et al., 2022). The role of maize mediator of RNA polymerase II transcription subunit 26b (Zm00001d040506) in A. flavus has not been established, but the upregulation of its expression in S6, S9, and S16 stages (Musungu et al., 2020) suggests its possible involvement in resistance reactions against the pathogen in maize. A ring finger protein (Zm00001eb250960) was linked to the MQTL5.6, contained six QTLs, and was located near S5_19770198 (Farfan et al., 2015). Expression of ring/zinc finger proteins was upregulated in resistant maize cultivar MP715 (Dhakal et al., 2017) and cotton upon A. flavus inoculation (Bedre et al., 2015).
Cell wall-modifying genes such as UDP-glycosyltransferase (Zm00001eb382140) have been shown to be regulated by A. flavus infection in cotton (Bedre et al., 2015). Carboxylesterase (CXE) is an enzyme that catalyzes carboxylic ester and water into alcohol and carboxylate. CXEs in plants have been implicated in defense mechanisms. For example, a tobacco CXE suppresses the accumulation of tobacco mosaic virus accumulation (Guo and Wong, 2020). In maize, the CXE SOBER1 (Zm00001d050270) may regulate HR-mediated plant defense against A. flavus by possibly hydrolyzing a lipid or precursor required for HR induction, as was observed in Arabidopsis, where SOBER1 resulted in low phosphatidic acid accumulation in response to bacterial effector AvrBsT, causing suppression of plant immunity (Kirik and Mudgett, 2009). Cell division control protein 48 (CDC48) is an evolutionarily conserved major chaperone-like protein component of ubiquitin-dependent protein degradation pathways. In plants, CDC48 most likely contributes to protein degradation through the ubiquitin-proteasome system (Bègue et al., 2019), suggesting its role in the plant’s defense mechanism. CDC48 caused an upregulation of the expression of the NB-LRR gene SNC1 and downregulation of the ubiquitin E3 ligase CPR1, leading to plant immunity against pathogens in Arabidopsis and tobacco (Cheng et al., 2011; Huang Y. et al., 2014), which clearly suggests the involvement of CDC48 in maize (Zm00001d025776) in resistance response under A. flavus attack.
Plants accumulate several reactive molecules, including aldehydes, which play dual roles in plant–pathogen interactions. Aldehydes can kill pathogens directly or act as secondary defense signaling molecules for activating durable host resistance against invading pathogens (Norvienyeku et al., 2017). At the same time, aldehydes are toxic to plant cells, and thus expression of aldehyde dehydrogenase can help scavenge excess pathogen-induced reactive aldehydes, contributing to disease resistance. Aldehyde dehydrogenase (ALDH) is a member of a group of evolutionarily conserved polymorphic enzymes that promote stress tolerance in plants (Zhu et al., 2014). In cotton, ADH was induced in the pericarp and seed tissues of cotton in response to A. flavus infection (Bedre et al., 2015). The gene Zm00001eb425740 codes for atg10, a member of the autophagy gene family. Most cytosolic proteins and organelle materials are sequestered and transported to the lysosome or vacuole for degradation via autophagy (Xie and Klionsky, 2007). Expression of the ATG genes is induced by the oxidative stress caused during necrotrophic fungal pathogen attack in plants (Lai et al., 2011) and thus could play an important role in the regulation of immunity-related programmed cell death or hypersensitive reactions in response to A. flavus resistance in maize. Patel and Dinesh-Kumar (2008) demonstrated that Arabidopsis plants with antisense suppression of the ATG6 gene showed that RPM1 triggered a limited hypersensitive reaction in response to Pseudomonas syringae pv. tomato DC3000.
Zm00001d030855 and Zm00001d049476, both coding for alpha Zein proteins, showed mostly upregulation in their expression patterns in maize. Alpha Zein 19C2 precursor (P1B10) was also highly expressed after A. flavus infection in maize (Dhakal et al., 2017). The gene Zm00001d003677 for Opaque11 is considered the central hub of the regulatory network for maize endosperm development and nutrient metabolism (Feng et al., 2018). Novel insights are being proposed for plant storage proteins as antimicrobial proteins (De Souza Cândido et al., 2011), yet the role of storage proteins such as zein and Opaque 11 in plant defense mechanisms has not been demonstrated. They possibly regulate the A. flavus infection by modulating the amino acid (especially lysine) profile of the seeds.
The gene Zm00001d049374 in MQTL4.1 codes for a protein with the DUF295 domain. These plant proteins also contain an F-box domain. F-box domain-containing proteins are well established for their roles in regulating cell death and plant defense in response to pathogen responses (Den Burg et al., 2008). An F-box protein was downregulated in maize PRms RNAi lines, which suggested its possible function in PR proteins-mediated resistance response (Majumdar et al., 2017). The involvement of F-box genes in A. flavus was demonstrated by the upregulation of F-box3 in peanuts during the late stages of infection by the fungus (Bhatnagar-Mathur et al., 2021).
Understanding the genetic basis and molecular mechanisms underlying A. flavus resistance is critical to developing maize varieties with improved resistance against factors affecting aflatoxin accumulation. Using a comprehensive meta-analysis of QTLs and transcriptome data, we have provided evidence of the presence of important MQTLs of possible significance in A. flavus resistance in maize bins 2.04, 4.05, and 8.03. Furthermore, the meta-analysis helped delimit the MQTLs to physical intervals of less than 1 Mb. Our results suggest that, although A. flavus resistance is a trait with a high genotype by environmental interaction effect, recurrent selection involving markers linked to the significant MQTLs as discussed above could lead to the accumulation of resistance loci that reduce A. flavus infection, colonization, and subsequent aflatoxin accumulation. A detailed omics study and functional validation of the identified putative A. flavus-specific candidate genes through genome editing or genetic engineering tools will enhance our understanding of maize—A. flavus interaction. Further characterization of identified MQTLs in this study to develop haplotype-specific markers and subsequent marker-assisted introgression of important MQTLs may significantly strengthen the breeding efforts for developing A. flavus-resistant maize cultivars.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
NB - conceptualization, data curation and analysis, funding acquisition, investigation, original manuscript draft preparation. ES - investigation, data curation and analysis, review of manuscript draft. AP - data curation and analysis. KR - planning, funding acquisition, experimental materials, review of manuscript draft. All authors approved the final manuscript.
This study was funded by a grant from the USDA-ARS NACA #58-6054-0-010.
EAS acknowledges the CAPES, Brazil for funding his visiting researcher scholarship at the Louisiana State University Agricultural Center.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1214907/full#supplementary-material
Akohoue, F., Miedaner, T. (2022). Meta-analysis and co-expression analysis revealed stable QTL and candidate genes conferring resistances to Fusarium and Gibberella ear rots while reducing mycotoxin contamination in maize. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1050891
Alleman, M., Sidorenko, L., McGinnis, K., Seshadri, V., Dorweiler, E. J., White, J., et al. (2006). An RNA-dependent RNA polymerase is required for paramutation in maize. Nature 442, 295–298. doi: 10.1038/nature04884
Alwala, S., Kimbeng, C. A., Williams, W. P., Kang, M. S. (2008). Molecular markers associated with resistance to Aspergillus flavus in maize grain: QTL and discriminant analyses. J. New Seeds. 9, 1–18. doi: 10.1080/15228860701879067
Baisakh, N., RamanaRao, M. V., Rajasekaran, K., Subudhi, P., Galbraith, D., Jonda, J., et al. (2012). Enhanced salt stress tolerance of rice plants expressing a vacuolar h+-ATPase subunit c1 (SaVHAc1) gene from a halophyte grass Spartina alterniflora löisel. Plant Biotechnol. J. 10, 453–464. doi: 10.1111/j.1467-7652.2012.00678.x
Bedre, R., Rajasekaran, K., Mangu, V. R., Sanchez Timm, L. E., Bhatnagar, D., Baisakh, N. (2015). Genome-wide transcriptome analysis of cotton (Gossypium hirsutum l.) identifies candidate gene signatures in response to aflatoxin producing fungus Aspergillus flavus. PloS One 10, e0138025. doi: 10.1371/journal.pone.0138025
Bègue, H., Mounier, A., Rosnoblet, C., Wendehenne, D. (2019). Toward the understanding of the role of CDC48, a major component of the protein quality control, in plant immunity. Plant Sci. 279, 34–44. doi: 10.1016/j.plantsci.2018.10.029
Bello, H. T. (2007). Phenotypic and genotypic evaluation of generations and recombinant inbred lines for response to aflatoxin (Texas A&M University, College Station, TX).
Bertagna, F. A. B., Kuki, M. C., Zeni Neto, H., Tessmann, D. J., Pinto, R. J. B., Scapim, C. A., et al. (2021). Association mapping and pathway analysis of ear rot disease caused by Aspergillus flavus in a panel of tropical maize germplasm. Crop Sci. 61, 4128–4138. doi: 10.1002/csc2.20629
Bhatnagar-Mathur, P., Yogendra, K., Parankusam, S., Sanivarapu, H., Prasad, K., Lingampali, S. B., et al. (2021). Comparative proteomics provide insights on the basis of resistance to Aspergillus flavus infection and aflatoxin production in peanut (Arachis hypogea l.). J. Plant Interact. 16, 494–509. doi: 10.1080/17429145.2021.1995058
Brooks, T. D., Williams, W. P., Windham, G. L., Willcox, M. C., Abbas, H. K. (2005). Quantitative trait loci contributing resistance to aflatoxin accumulation in the maize inbred Mp313E. Crop Sci. 45, 171–174. doi: 10.2135/cropsci2005.0171a
Brown, R. L., Williams, W. P., Windham, G. L., Menkir, A., Chen, Z. Y. (2016). Evaluation of African-bred maize germplasm lines for resistance to aflatoxin accumulation. Agronomy 6 (2), 24. doi: 10.3390/agronomy6020024
Busboom, K. N., White, D. G. (2004). Inheritance of resistance to aflatoxin production and Aspergillus ear rot of mize from the cross of inbreds B73 and Oh516. Phytopathology 94, 1107–1115. doi: 10.1094/PHYTO.2004.94.10.1107
Castano-Duque, L., Gilbert, M. K., Mack, B. M., Lebar, M. D., Carter-Wientjes, C. H., Sickler, C. M., et al. (2021). Flavonoids modulate the accumulation of toxins from Aspergillus flavus in maize kernels. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.761446
Chardon, F., Virlon, B., Moreau, L., Falque, M., Joets, J., Decousset, L., et al. (2004). Genetic architecture of flowering time in maize as inferred from quantitative trait loci meta-analysis and synteny conservation with the rice genome. Genetics 168, 2169–2185. doi: 10.1534/genetics.104.032375
Cheng, Y. T., Li, Y., Huang, S., Huang, Y., Dong, X., Zhang, Y., et al. (2011). Stability of plant immune-receptor resistance proteins is controlled by SKP1-CULLIN1-F-box (SCF)-mediated protein degradation. Proc. Natl. Acad. Sci. U.S.A. 108, 14694–14699. doi: 10.1073/pnas.110568510
Courtois, B., Ahmadi, N., Khowaja, F., Price, A. H., Rami, J. F., Frouin, J., et al. (2009). Rice root genetic architecture: meta-analysis from a drought QTL database. Rice 2, 115–128. doi: 10.1007/s12284-009-9028-9
Darvasi, A., Soller, M. (1997). A simple method to calculate resolving power and confidence interval of QTL map location. Behav. Genet. 27, 125–132. doi: 10.1023/A:1025685324830
Den Burg, H. A., Tsitsigiannis, D. I., Rowland, O., Lo, J., Rallapalli, G., MacLean, D., et al. (2008). The f-box protein ACRE189/ACIF1 regulates cell death and defense responses activated during pathogen recognition in tobacco and tomato. Plant Cell. 20, 696–719. doi: 10.1105/tpc.107.056978
De Oliveira, Y., Sosnowski, O., Charcosset, A., Joets, J. (2014). “BioMercator 4: a complete framework to integrate QTL, meta-QTL, and genome annotation,” in European Conference on Computational Biology (Nicod,Strasbourg, France: Le Centre Pour La Communication Scientifique Directe - HAL - Institut.).
Dhakal, R., Windham, G. L., Williams, W. P., Subudhi, P. K.. (2016). Quantitative trait loci (QTL) for reducing aflatoxin accumulation in corn. Mol. Breed 36, 164. doi: 10.1007/s11032-016-0590-8
De Souza Cândido, E., Pinto, M. F. S., Pelegrini, P. B., Lima, T. B., Silva, O. N., Pogue, R., et al. (2011). Plant storage proteins with antimicrobial activity: novel insights into plant defense mechanisms. FASEB J. 25, 3290–3305. doi: 10.1096/fj.11-184291
Dhakal, R., Chai, C., Karan, R., Windham, G. L., Williams, W. P., Subudhi, P. K. (2017). Expression profiling coupled with in-silico mapping identifies candidate genes for reducing aflatoxin accumulation in maize. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00503
Dolezal, A. L., Shu, X., OBrian, G. R., Neilson, D. M., Woloshuk, C. P., Boston, R. S., et al. (2014). Aspergillus flavus infection induces transcriptional and physical changes in developing maize kernels. Front. Microbiol. 5. doi: 10.3389/fmicb.2014.00384
Dynowski, M., Schaaf, G., Loque, D., Moran, O., Ludewig, U. (2008). Plant plasma membrane water channels conduct the signaling molecule H2O2. Biochem. J. 414, 53–61. doi: 10.1042/BJ20080287
Farfan, I. D., de la Fuente, G. N., Murray, S. C., Isakeit, T., Huang, P., Warburton, M. L., et al. (2015). Genome wide association study for drought, aflatoxin resistance, and important agronomic traits of maize hybrids in the sub-tropics. PloS One 10, e0117737. doi: 10.1371/journal.pone.0117737
Feng, F., Qi, W., Lv, Y., Yan, S., Xu, L., Yang, W., et al. (2018). OPAQUE11 is a central hub of the regulatory network for maize endosperm development and nutrient metabolism. Plant Cell 30, 375–396. doi: 10.1105/tpc.17.00616
Fountain, J. C., Scully, B. T., Ni, X., Kemerait, R. C., Lee, R. D., Chen, Z. Y., et al. (2014). Environmental influences on maize-aspergillus flavus interactions and aflatoxin production. Front. Microbiol. 5. doi: 10.3389/fmicb.2014.00040
Goffinet, B., Gerber, S. (2000). Quantitative trait loci: a meta-analysis. Genetics 155 (1), 463–473. doi: 10.1093/genetics/155.1.463
Guo, B., Sleper, D. A., Lu, P., Shannon, J. G., Nguyen, H. T., Arelli, P. R. (2006). QTLs associated with resistance to soybean cyst nematode in soybean: meta-analysis of QTL locations. Crop Sci. 46, 202–208. doi: 10.2135/cropsci2005.04-0036
Guo, S., Wong, S. M. (2020). A conserved carboxylesterase inhibits tobacco mosaic virus (TMV) accumulation in Nicotiana benthamiana plants. Viruses 12, 195. doi: 10.3390/v12020195
Han, G., Li, C., Xiang, F., Zhao, Q., Zhao, Y., Cai, R., et al. (2020). Genome-wide association study leads to novel genetic insights into resistance to Aspergillus flavus in maize kernels. BMC Plant Biol. 20, 206. doi: 10.1186/s12870-020-02404-5
Hawkins, L. K., Warburton, M. L., Tang, J. D., Tomashek, J., Alves Oliveira, D., Ogunola, O. F., et al. (2018). Survey of candidate genes for maize resistance to infection by Aspergillus flavus and/or aflatoxin contamination Toxins 10, 61. doi: 10.3390/toxins10020061
Huang, W. J., Liu, H. K., McCormick, S., et al. (2014). Tomato pistil factor STIG1 promotes in vivo pollen tube growth by binding to phosphatidylinositol 3-phosphate and the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell. 26, 2505–2523. doi: 10.1105/tpc.114.123281
Huang, Y., Minaker, S., Roth, C., Huang, S., Hieter, P., Lipka, V., et al. (2014). An E4 ligase facilitates polyubiquitination of plant immune receptor resistance proteins in arabidopsis. Plant Cell. 26, 485–496. doi: 10.1105/tpc.113.119057
Johnson, J. M., Thürich, J., Petutschnig, E. K., Altschmied, L., Meichsner, D., Sherameti, I., et al. (2018). A Poly(A) ribonuclease controls the cellotriose-based interaction between Piriformospora indica and its host arabidopsis. Plant Physiol. 176, 2496–2514. doi: 10.1104/pp.17.01423
Kachroo, P., Shanklin, J., Shah, J., Whittle, E. J., Klessig, D. F. (2001). A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc. Natl. Acad. Sci. U.S.A. 98, 9448–9453. doi: 10.1073/pnas.151258398
Kaur, S., Rakshit, S., Choudhary, M., Das, A. K., Kumar, R. R. (2021). Meta-analysis of QTLs associated with popping traits in maize (Zea mays l.). PloS One 16, e0256389. doi: 10.1371/journal.pone.0256389
Kebede, H., Abbas, H. K., Fisher, D. K., Bellaloui, N. (2012). Relationship between aflatoxin contamination and physiological responses of maize plants under drought and heat stress. Toxins 4, 1385–1403. doi: 10.3390/toxins4111385
Kebede, A. Z., Johnston, A., Schneiderman, D., Bosnich, W., Harris, J. D. (2018). Transcriptome profiling of two maize inbreds with distinct responses to Gibberella ear rot disease to identify candidate resistance genes. BMC Genomics 19, 131. doi: 10.1186/s12864-018-4513-4
Kelley, R. Y., Williams, W. P., Mylroie, J. E., Boykin, D. L., Harper, J. W., Windham, G. L., et al. (2012). Identification of maize genes associated with host plant resistance or susceptibility to Aspergillus flavus infection and aflatoxin accumulation. PloS One 7, e36892. doi: 10.1371/journal.pone.0036892
Khan, N., Bedre, R., Parco, A., Bernaola, L., Hale, A., Kimbeng, C., et al. (2013). Identification of cold responsive genes in sugar/energycane for their use in genetic diversity analysis and future functional marker development. Plant Sci. 211, 122–131. doi: 10.1016/j.plantsci.2013.07.001
Kirik, A., Mudgett, M. B. (2009). SOBER1 phospholipase activity suppresses phosphatidic acid accumulation and plant immunity in response to bacterial effector AvrBsT. Proc. Natl. Acad. Sci. U.S.A. 106, 20532–20537. doi: 10.1073/pnas.0903859106
Lai, Z., Wang, F., Zheng, Z., Fan, B., Chen, Z. (2011). A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J. 66, 953–968. doi: 10.1111/j.1365-313X.2011.04553.x
Langfelder, P., Horvath, S. (2008). WGCNA: an r package for weighted correlation network analysis. BMC Bioinf. 9, 559. doi: 10.1186/1471-2105-9-559
Liu, H., Mullan, D., Zhang, C., Zhao, S., Li, X., Zhang, A., et al. (2020). Major genomic regions responsible for wheat yield and its components as revealed by meta-QTL and genotype–phenotype association analyses. Planta 252, 1–22. doi: 10.1007/s00425-020-03466-3
Liu, H., Wu, H., Wang, Y., Wang, H., Chen, S., Yin, Z. (2021). Comparative transcriptome profiling and co-expression network analysis uncover the key genes associated withearly-stage resistance to aspergillus flavus in maize. BMC Plant Biol. 21, 216. doi: 10.1186/s12870-021-02983-x
Liu, S., Zenda, T., Wang, X., Liu, G., Jin, H., Yang, Y., et al. (2019). Comprehensive meta-analysis of maize QTLs associated with grain yield, flowering date and plant height under drought conditions. J. Agric. Sci. 11, 1–19. doi: 10.5539/jas.v11n8p1
Luo, M., Brown, R. L., Chen, Z. Y., Menkir, A., Yu, J., Bhatnagar, D. (2011). Transcriptional profiles uncover aspergillus flavus-induced resistance in maize kernels. Toxins 3, 766–786. doi: 10.3390/toxins3070766
Luo, M., Liu, J., Lee, R. D., Guo, B. Z. (2008). Characterization of gene expression profiles in developing kernels of maize (Zea mays) inbred Tex6. Plant Breed. 127, 569–578. doi: 10.1111/j.1439-0523.2008.01538.x
Majumdar, R., Rajasekaran, K., Sickler, C., Lebar, M., Musungu, B. M., Fakhoury, A. M., et al. (2017). The pathogenesis-related maize seed (PRms) gene plays a role in resistance to Aspergillus flavus infection and aflatoxin contamination. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01758
Marshall, E., Costa, L. M., Gutierrez-Marcos, J. (2011). Cysteine-rich peptides (CRPs) mediate diverse aspects of cell-cell communication in plant reproduction and development". J. Exp. Bot. 62, 1677–1686. doi: 10.1093/jxb/err002
Martins, C. D. P. S., Pedrosa, A. M., Du, D., Goncalves, L. P., Yu, Q., Gmitter, F. G., et al. (2015). Genome-wide characterization and expression analysis of major intrinsic proteins during abiotic and biotic stresses in sweet orange (Citrus sinensis l. osb.). PloS One 10, e0138786. doi: 10.1371/journal.pone.0138786
Maupin, L. M., Clements, M. J., White, D. G. (2003). Evaluation of the MI82 maize line as a source of resistance to aflatoxin in grain and use of BGYF as a selection tool. Plant Dis. 87, 1059–1066. doi: 10.1094/PDIS.2003.87.9.1059
Mayfield, K. L., Murray, S. C., Rooney, W. L., Isakeit, T., Odvody, G. A. (2011). Confirmation of QTL reducing aflatoxin in maize testcrosses. Crop Sci. 51, 2489–2498. doi: 10.2135/cropsci2011.02.0112
Menkir, A., Kling, J. G., Badu-Apraku, B., Ibikunle, O. (2006). Registration of 26 tropical maize germplasm lines with resistance to Striga hermonthica. Crop Sci. 46, 1007. doi: 10.2135/cropsci2005.0143
Mideros, S. X., Warburton, M. L., Jamann, T. M., Windham, G. L., Williams, W. P., Nelson, R. J. (2014). Quantitative trait loci influencing mycotoxin contamination of maize: analysis by linkage mapping, characterization of near-isogenic lines, and meta-analysis. Crop Sci. 54, 127–142. doi: 10.2135/cropsci2013.04.0249
Mitchell, N. J., Bowers, E., Hurburgh, C., Wu, F. (2016). Potential economic losses to the US maize industry from aflatoxin contamination. Food Add. Cont. Part A. 33, 540–550. doi: 10.1080/19440049.2016.1138545
Musungu, B., Bhatnagar, D., Quiniou, S., Brown, R. L., Payne, G. A., O’Brian, G., et al. (2020). Use of dual RNA-seq for systems biology analysis of Zea mays and Aspergillus flavus interaction. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.00853
Norvienyeku, J., Zhong, Z., Lin, L., Dang, X., Chen, M., Lin, X., et al. (2017). Methylmalonate-semialdehyde dehydrogenase mediated metabolite homeostasis essentially regulate conidiation, polarized germination and pathogenesis in Magnaporthe oryzae. Environ. Microbiol. 19, 4256–4277. doi: 10.1111/1462-2920.13888
Ogunola, O., Smith., J. S., Xu, W., Bhattramakki, D., Jeffers, D., Williams, W., et al. (2021). Characterization of a source of resistance to aflatoxin accumulation in maize. Agrosyst. Geosci Environ. 4, e20203. doi: 10.1002/agg2.20203
Pandey, M. K., Kumar, R., Pandey, A. K., Soni, P., Gangurde, S. S., Sudini, H. K., et al. (2019). Mitigating aflatoxin contamination in groundnut through a combination of genetic resistance and post-harvest management practices. Toxins 11, 315. doi: 10.3390/toxins11060315
Patel, S., Dinesh-Kumar, S. P. (2008). Arabidopsis ATG6 is required to limit the pathogen-associated cell death response. Autophagy 4, 20–27. doi: 10.4161/auto.5056
Paul, C., Naidoo, G., Forbes, A., Mikkilineni, V., White, D., Rocheford, T. (2003). Quantitative trait loci for low aflatoxin production in two related maize populations. Theor. Appl. Genet. 107, 263–270. doi: 10.1007/s00122-003-1241-0
Price, A. H. (2006). Believe it or not, QTLs are accurate! Trends Plant Sci. 11, 213–216. doi: 10.1016/j.tplants.2006.03.006
Richter, W. F., Nayak, S., Iwasa, J., Taatjes, D. J. (2022). The mediator complex as a master regulator of transcription by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 23, 732–749. doi: 10.1038/s41580-022-00498-3
Sheoran, S., Gupta, M., Kumari, S., Kumar, S., Rakshit, S. (2022). Meta-QTL analysis and candidate genes identification for various abiotic stresses in maize (Zea mays l.) and their implications in breeding programs. Mol. Breed. 42, 26. doi: 10.1007/s11032-022-01294-9
Shu, X., Livingston, D. P., III, Woloshuk, C. P., Payne, G. A. (2017). Comparative histological and transcriptional analysis of maize kernels infected with Aspergillus flavus and Fusarium verticillioides. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.02075
Smith, J. S., Williams, W. P., Windham, G. L., Xu, W., Warburton, M. L., Bhattramakki, D. (2019). Identification of quantitative trait loci contributing resistance to aflatoxin accumulation in maize inbred Mp715. Mol. Breed. 39, 91. doi: 10.1007/s11032-019-0997-0
Soni, P., Gangurde, S. S., Ortega-Beltran, A., Kumar, R., Parmar, S., Sudini, H. K., et al. (2020). Functional biology and molecular mechanisms of host-pathogen interactions for aflatoxin contamination in groundnut (Arachis hypogaea l.) and maize (Zea mays l.). Front. Plant Sci. 11. doi: 10.3389/fmicb.2020.00227
Soriano, J. M., Alvaro, F. (2019). Discovering consensus genomic regions in wheat for root-related traits by QTL meta-analysis. Sci. Rep. 9, 10537. doi: 10.1038/s41598-019-47038-2
Sosnowski, O., Charcosset, A., Joets, J. (2012). BioMercator V3: an upgrade of genetic map compilation and quantitative trait loci meta-analysis algorithms. Bioinformatics 28, 2082–2083. doi: 10.1093/bioinformatics/bts313
Swamy, B. M., Vikram, P., Dixit, S., Ahmed, H. U., Kumar, A. (2011). Meta-analysis of grain yield QTL identified during agricultural drought in grasses showed consensus. BMC Genomics 12, 319. doi: 10.1186/1471-2164-12-319
Tian, T., Liu, Y., Yan, H., You, Q., Yi, X., Du, Z., et al. (2017). agriGO v2.0: a GO analysis toolkit for the agricultural community 2017 update. Nucl. Acids Res. 45, W122–W129. doi: 10.1093/nar/gkx382
Truntzler, M., Barriere, Y., Sawkins, M. C., Lespinasse, D., Betran, J., Charcosset, A., et al. (2010). Meta-analysis of QTL involved in silage quality of maize and comparison with the position of candidate genes. Theor. Appl. Genet. 121, 1465–1482. doi: 10.1007/s00122-010-1402-x
Upchurch, R. G. (2008). Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 30, 967–977. doi: 10.1007/s10529-008-9639-z
Veyrieras, J. B., Goffinet, B., Charcosset, A. (2007). MetaQTL: a package of new computational methods for the meta-analysis of QTL mapping experiments. BMC. Bioinformatics 8, 49. doi: 10.1186/1471-2105-8-49
Wang, J., Li, H., Zhang, L., Meng, L. (2016). Users’ manual of QTL IciMapping (Mexico, D.F., Mexico: The Quantitative Genetics Group, Institute of Crop Science, Chinese Academy of Agricultural Sciences (CAAS), Beijing 100081, China, and Genetic Resources Program, International Maize and Wheat Improvement Centre (CIMMYT).
Wang, W., Ren, Z., Li, L., Du., Y., Zhou, Y., Zhang, M., et al. (2022). Meta-QTL analysis explores the key genes, especially hormone related genes, involved in the regulation of grain water content and grain dehydration rate in maize. BMC Plant Biol. 22, 346. doi: 10.1186/s12870-022-03738-y
Warburton, M. L., Brooks, T. D., Krakowsky, M. D., Shan, X., Windham, G. L., Williams, W. P. (2009). Identification and mapping of new sources of resistance to aflatoxin accumulation in maize. Crop Sci. 49, 1403–1408. doi: 10.2135/cropsci2008.12.0696
Warburton, M. L., Brooks, T. D., Windham, G. L., Williams, W. P. (2011). Identification of novel QTL contributing resistance to aflatoxin accumulation in maize. Mol. Breed. 27, 491–499. doi: 10.1007/s11032-010-9446-9
Warburton, M. L., Tang, J. D., Windham, G. L., Hawkins, L. K., Murray, S. C., Xu, W., et al. (2015). Genome-wide association mapping of Aspergillus flavus and aflatoxin accumulation resistance in maize. Crop Sci. 55, 1857–1867. doi: 10.2135/cropsci2014.06.0424
Warburton, M., Williams, W. P. (2014). Aflatoxin resistance in maize: what have we learned lately? Adv. Bot. 2014, 352831. doi: 10.1155/2014/352831
Widstrom, N. W., Butron, A., Guo, B. Z., Wilson, D. M., Snook, M. E., Cleveland, T. E., et al. (2003). Control of preharvest aflatoxin contamination in maize by pyramiding QTL involved in resistance to ear-feeding insects and invasion by aspergillus spp. Eur. J. Agro. 19, 563–572. doi: 10.1016/S1161-0301(03)00004-2
Willcox, M. C., Davis, G. L., Warburton, M. L., Widham, G. L., Abbas, H. K., Betran, J., et al. (2013). Confirming quantitative trait loci for aflatoxin resistance from Mp313E in different genetic backgrounds. Mol. Breed. 32, 15–26. doi: 10.1007/s11032-012-9821-9
Williams, W. P., Krakowsky, M. D., Scully, B. T., Brown, R. L., Menkir, A., Warburton, M. L., et al. (2015). Identifying and developing maize germplasm with resistance to accumulation of aflatoxins. World Mycotoxin J. 8, 193–209. doi: 10.3920/wmj2014.1751
Womack, E. D., Williams, W. P., Windham, G. L., Xu, W. (2020). Mapping quantitative trait loci associated with resistance to aflatoxin accumulation in maize inbred Mp719. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.00045
Xiang, K., Reid, L. M., Zhang, Z. M., Zhu, X. Y., Pan, G. T. (2012). Characterization of correlation between grain moisture and ear rot resistance in maize by QTL meta-analysis. Euphytica 183, 185–195. doi: 10.1007/s10681-011-0440-z
Xiang, K., Zhang, Z. M., Reid, L. M., Zhu, X. Y., Yuan, G. S., Pan, G. T. (2010). A meta-analysis of QTL associated with ear rot resistance in maize. Maydica 55, 281–290.
Xie, Z., Klionsky, D. J. (2007). Autophagosome formation: core machinery and adaptations. Nat. Cell Biol. 9, 1102–1109. doi: 10.1038/ncb1007-1102
Yang, S., Tang, F., Zhu, H. (2014). Alternative splicing in plant immunity. Int. J. Mol. Sci. 15, 10424–10445. doi: 10.3390/ijms150610424
Yin, Z., Wang, Y., Wu, F., Gu, X., Bian, Y., Wang, Y., et al. (2014). Quantitative trait locus mapping of resistance to Aspergillus flavus infection using a recombinant inbred line population in maize. Mol. Breed. 33, 39–49. doi: 10.1007/s11032-013-9932-y
Zhang, Y., Cui, M., Zhang, J., Zhang, L., Li, C., Kan, X., et al. (2016). Confirmation and fine mapping of a major QTL for aflatoxin resistance in maize using a combination of linkage and association mapping. Toxins 8, 258. doi: 10.3390/toxins8090258
Zhang, J., Sun, X. (2021). Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry 181, 112588. doi: 10.1016/j.phytochem.2020.112588
Zhao, X., Luo, L., Cao, Y., Liu, Y., Wu, W., Yuzhou, L., et al. (2018). Genome-wide association analysis and QTL mapping reveal the genetic control of cadmium accumulation in maize leaf. BMC Genomics 19, 91. doi: 10.1186/s12864-017-4395-x
Zhu, C., Ming, C., Zhao-Shi, X., Lian-Cheng, L., Xue-Ping, C., You-Zhi, M. (2014). Characteristics and expression patterns of the aldehyde dehydrogenase (ALDH) gene superfamily of foxtail millet (Setaria italica l.). PloS One 9, e101136. doi: 10.1371/journal.pone.0101136
Zou, J., Rodriguez-Zas, S., Aldea, M., Li, M., Zhu, J., Gonzalez, D. O., et al. (2005). Expression profiling soybean response to pseudomonas syringae reveals new defense-related genes and rapid HR-specific downregulation of photosynthesis. Mol. Plant Microb. Interact. 18, 1161–1174. doi: 10.1094/MPMI-18-1161
Keywords: aflatoxin, co-expression network, genomic region, maize, marker, Meta-QTL, microarray, RNA-seq
Citation: Baisakh N, Da Silva EA, Pradhan AK and Rajasekaran K (2023) Comprehensive meta-analysis of QTL and gene expression studies identify candidate genes associated with Aspergillus flavus resistance in maize. Front. Plant Sci. 14:1214907. doi: 10.3389/fpls.2023.1214907
Received: 30 April 2023; Accepted: 26 June 2023;
Published: 18 July 2023.
Edited by:
Renesh Bedre, Texas A&M AgriLife Research & Extension Center, Weslaco, United StatesReviewed by:
Sunil S. Gangurde, University of Georgia, United StatesCopyright © 2023 Baisakh, Da Silva, Pradhan and Rajasekaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Niranjan Baisakh, TkJhaXNha2hAYWdjZW50ZXIubHN1LmVkdQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.