- Postharvest Research Division, National Institute of Horticultural and Herbal Science, Wanju, Republic of Korea
Introduction: Ethylene response factors (ERFs) play a critical role in regulating hormone interactions that affect the shelf life of tomatoes. Understanding their regulation during storage and distribution can be highly beneficial.
Methods: This study examined the effects of treatment with ethylene (ET), brassinosteroid (BR), auxin (AUX), and gibberellin (GA) on fruit ripening and the expression of 18 ripening-associated ERFs in tomato stored at 20°C (room temperature) for 10 d or 4°C (cold storage) for 14 d followed by 2 d at 20°C (retailer conditions).
Results: The results showed that ripening was accelerated by ET and BR but was delayed by AUX and GA at room temperature. Cold storage delayed ripening in all groups, with ET and GA treatments showing the highest and lowest a* values, respectively. The effects of hormone treatment were consistent with room temperature when the fruits were transferred from cold storage to retail conditions. At room temperature, ERFs responsive to ET (ERF.B1, B2, B6, E2, and F1) and BR (ERF.E5, F2, and F3) were inhibited by AUX. ET-induced genes (ERF.C1, E1, F4, and H7) could be co-regulated by other hormones at cold storage. When the fruits were transferred from cold storage to retailer conditions, ERFs responsive to ET and BR were inhibited by GA. Additionally, ET-responsive ERFs could be inhibited by BR at room temperature, whereas ET could inhibit BR-responsive ERFs at retailer conditions. The same ERFs that were regulated by ET at room temperature were instead regulated by BR under retailer conditions, and vice versa.
Discussion: These findings can help provide a better understanding of the complex hormone interactions regulating the postharvest physiology of tomato and in maintaining its quality and shelf life during storage and distribution.
1 Introduction
Tomato (Solanum lycopersicum L.) is one of the most widely consumed crops worldwide. However, its quality and shelf life is significantly affected by storage and distribution conditions (Jung et al., 2019). The interplay between phytohormones is crucial in regulating various physiological aspects of the fruit in response to different environmental factors (Kumar et al., 2014). Therefore, understanding the hormonal interactions that occur during tomato storage and distribution is essential to control and optimize the physiology and biochemistry of the fruit, ensuring its safety and quality.
Ethylene response factors (ERFs) are plant-specific transcription factors (TFs) that belong to the superfamily of Apetala 2/ethylene response factors (AP2/ERFs). They are characterized by the presence of the AP2/ERF DNA-binding domain. ERFs act downstream of the ET signaling pathway to mediate ethylene (ET) responses by regulating ET-responsive genes (Liu et al., 2016; Xie et al., 2016). The tomato genome contains 77 ERFs that are differentially expressed during ripening (Liu et al., 2016). Various aspects of fruit ripening, such as fruit color, softening, flavor, and aroma, are regulated by these TFs (Li et al., 2016; Li et al., 2019; Xie X. et al., 2016; Li et al., 2017; Tucker et al., 2017). ERFs are involved in a complex network of hormone cross-talk during fruit development and plant responses to environmental factors, which involves interactions between ET and other hormones such as auxin (AUX), gibberellin (GA), brassinosteroid (BR), and abscisic acid. For instance, ERF.B3 and D7 were responsive to both ET and AUX and integrate these two signaling pathways via regulation of AUX signaling components (Liu et al., 2014; Gambhir et al., 2022). Lorenzo et al. (2003) found that ERF1 expression can be rapidly activated by either ET or jasmonate and that this activation can be synergistic when both hormones are present. ERF6 controls leaf growth under water-limiting conditions by fine-tuning the ET and GA/DELLA signaling pathways (Dubois et al., 2013). The CaERF116 and MsERF8 genes are induced by GA treatment (Chen et al., 2012; Deokar et al., 2015). As ERFs play an important role in integrating the signaling pathways of different hormones, understanding the regulation of ERFs by various hormones is essential to unravel the complex network of hormonal signaling pathways. However, the hormonal regulation of ERFs in tomatoes under different storage conditions is not well documented.
In this study, we characterized the regulation of ERFs by ET, BR, AUX, or GA in tomatoes stored at different conditions, including room temperature, cold storage, and retailer conditions.
2 Materials and methods
2.1 Cis-element analysis
The promoter sequences (2000-bp upstream of the translation start codon of ERFs) were obtained from the EnsemblPlants database (https://plants.ensembl.org/). All cis-acting elements were assessed by PlantCARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 1 June 2022) (Lescot et al., 2002).
2.2 Plant materials and treatments
Cherry tomato (S. lycopersicum L. “Betatini”) fruits at mature-green stages were harvested during summer in Jungyeum, South Korea. Disease-free and intact fruits were sterilized with 2% sodium hypochlorite solution and washed with tap water twice. Following air drying at room temperature and removal of the pedicels, the fruits were divided into four groups and then treated with ET, BR, AUX, or GA. For ET and AUX treatments, the fruits were dipped in 1 mM ethephon solution (Inbio Corp., Jecheon, South Korea) and 0.45 mM 2,4-dichlorophenoxyacetic acid (Sigma-Aldrich, St. Louis, MO, USA), respectively, under a vacuum at 30 kPa for 5 min. For the BR treatment, the fruits were immersed in 6 μM brassinolide solution (Cayman Chemical Company, MI, USA) for 15 min. For the GA treatment, the fruits were dipped in a 0.5 mM GA3 solution (prepared in ethanol/distilled water [1:1000, v/v] containing 0.1% [v/v] Tween-20; Sigma-Aldrich) for 15 min. The fruit dipped with distilled water for 15 min was used as the control. Following the treatments, the fruits were kept in the dark at 20 ± 2°C (room temperature) with 90 ± 5% relativity humidity (RH) for 10 d or 4°C (cold storage) for 14 d followed by 2 d at 20 ± 2°C (retailer conditions).
2.3 Fruit color evaluation
Fifteen fruits were sampled per treatment to assess the fruit color. Skin color was monitored using a color difference meter (CR-400; Konica Minolta, Japan) and was reported based on Hunter’s scale: light (L*), red (a*), and yellow (b*).
2.4 Quantitative real-time polymerase chain reaction
qRT-PCR was performed on a CFX96 TouchTM Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA) as described by Park et al. (2018). The transcripts were amplified using the iQTM SYBR Green Supermix (Bio-Rad Laboratories) with specific primers (Table S1). qRT-PCR was performed under the following conditions: 95°C for 30 s, followed by 40 cycles of 95°C for 10 s and 55–58°C for 40 s. The relative gene expression level was calculated using the 2-ΔΔCt method (Schmittgen and Livak, 2008) and normalized using the expression levels of the housekeeping gene actin (solyc11g005330). qRT-PCR analysis was performed using at least three biological replicates and two technical replicates.
2.5 Statistical analyses
Values are presented as the mean ± standard error. Samples were subjected to analysis of variance, and significant differences were determined using Duncan’s multiple range test. All analyses were conducted using SAS v.9.2 (SAS Institute, Cary, NC, USA). The ERF expression data and fruit color (a* values) were normalized, scaled, and used for the hierarchical clustering analysis and pattern correlation analysis (Pearson correlation coefficient) in the MetaboAnalyst 3.0 software (www.metaboanalyst.ca).
3 Results
3.1 Effect of hormone treatments on fruit ripening
To explore the hormonal regulation of ERFs, we first analyzed the 2000 bp region upstream of the translation start codon of the 18 genes and identified the cis-elements related to ET, AUX, and GA (Figure 1). All the ERFs contained ET-related cis-elements. Additionally, all the genes contained cis-elements related to more than one hormone (Figure 1). Then, tomatoes at the mature-green stage were treated with ET, BR, AUX, or GA to determine the hormonal effect on ripening progression and the expression of ERFs during storage at room temperature for 10 d and/or cold storage for 14 d followed by 2 d at retailer conditions. The effect of hormones on fruit color was observed on day 3 at room temperature (Figure 2A). ET and BR treatments accelerated fruit reddening, with consistently higher a* (redness, Hunter scale) values than those of the control for 3 d at room temperature (Figure 2B). The AUX- and GA-treated fruits showed delayed color transition, as evidenced by consistently lower a* values than those of the control, ET-, and BR-treated fruits throughout storage at room temperature (Figures 2A, B). During cold storage, ripening was delayed in all treatment groups, and a visible color break was observed on day 14. ET-treated fruits showed the highest a* values, while GA-treated fruits showed the lowest values. BR- and AUX-treated fruits had values lower than those of the control fruits (Figures 2C, D). Under retailer conditions, the ripening process was accelerated in all treatment groups. However, ET- and GA-treated fruits still had the highest and lowest a* values, respectively. AUX-treated fruits had a* values in between those of control and GA-treated fruits, while BR-treated fruits did not significantly differ from control fruits (Figures 2C, D). Additionally, fruits treated with AUX and GA tended to have higher L* values than those of ET and BR-treated fruits on days 3–7 at room temperature and under retailer conditions (Figures S1A, C). However, there was no specific trend in the changes of the b* value between control and hormone-treated fruits at both storage conditions (Figures S1B, D).
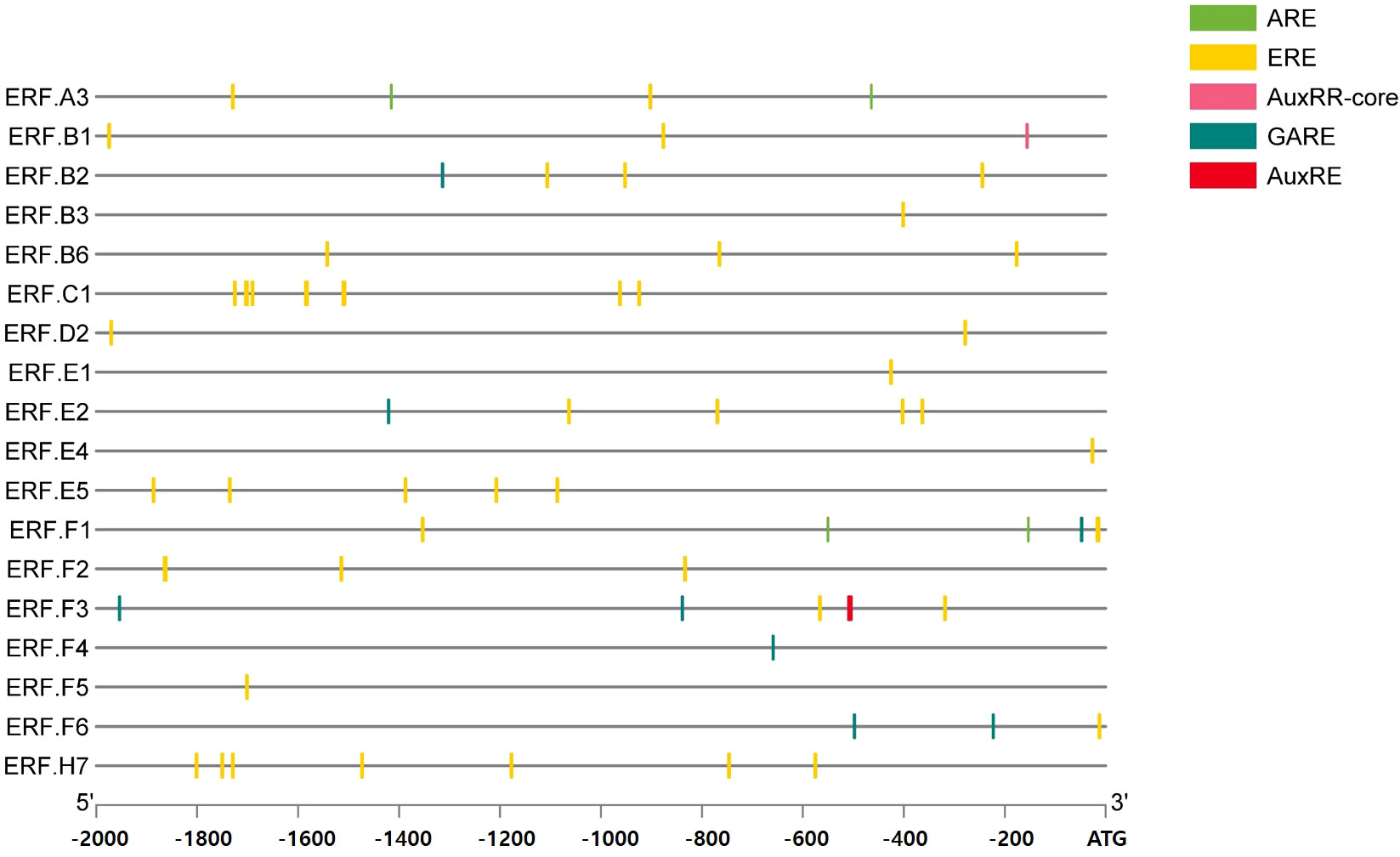
Figure 1 Promoter analysis for the presence of cis-regulatory elements. A total of 18 ethylene response factors (ERFs) were analyzed for the presence of cis-regulatory elements in the 2000 bp region upstream of the translation start codon. ARE, auxin response element; AUXRR-core, cis-acting regulatory element involved in auxin response; ERE, ethylene response element; GARE, gibberellin responsive element.
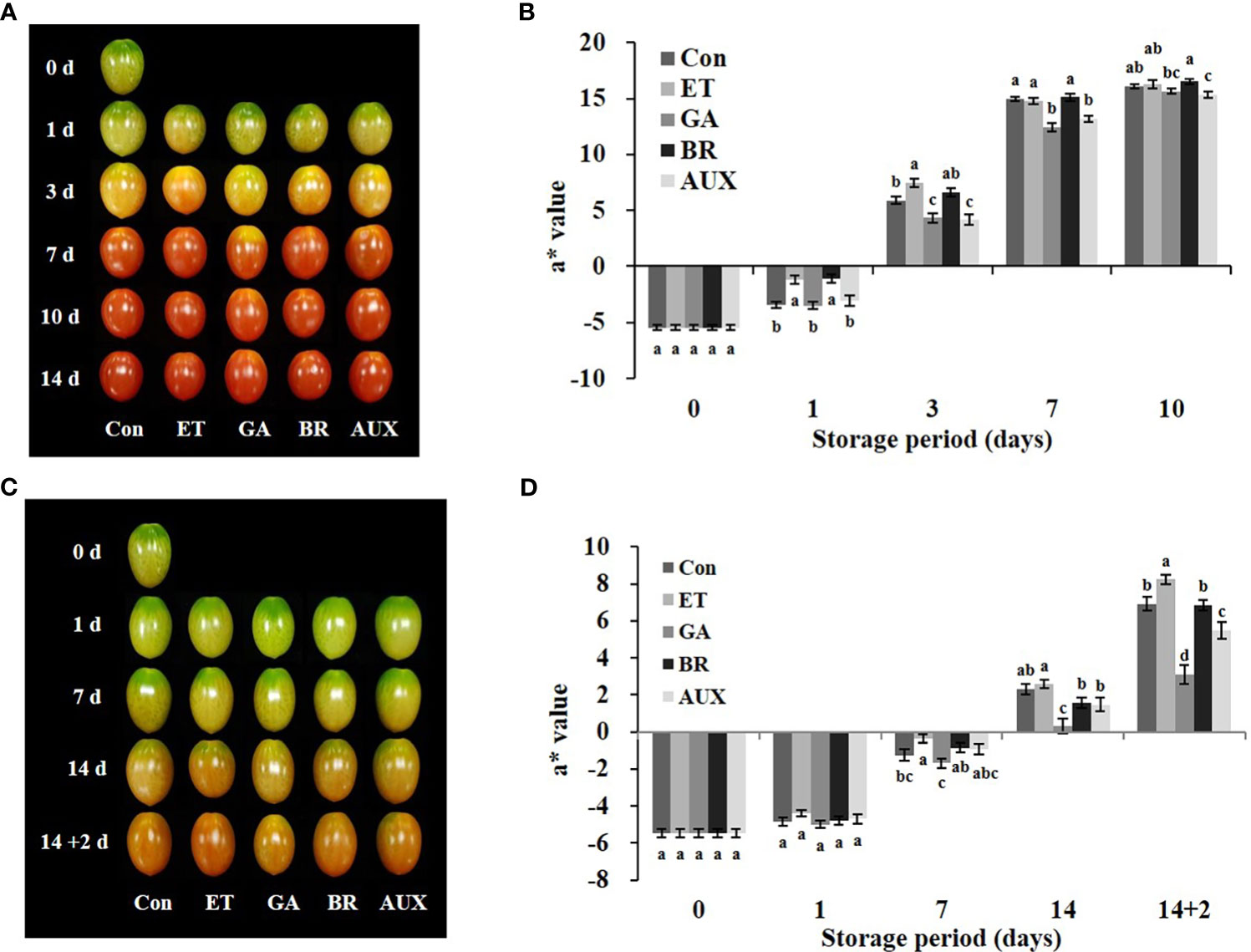
Figure 2 Effect of hormone treatments on tomato fruit ripening at different storage conditions. Changes in (A) color and (B) a* values in tomatoes stored at 20 ± 2°C (room temperature) for 10 d. Changes in (C) color and (D) a* values in tomatoes stored at 4°C (cold storage) for 14 d followed by 2 d at 20 ± 2°C (retailer conditions). Error bars represent standard error, and different letters on the graphs represent significant differences between the control and hormone treatments (P < 0.05). Con, control; ET, ethylene; AUX, auxin; BR, brassinosteroid; GA, gibberellin.
3.2 Hormonal response of ERFs at room temperature
On day 1 at room temperature, ET treatment led to the expression of ERF.B2, B6, E2, and F1, while BR treatment increased the expression of ERF.E5, F2, and F3. However, AUX treatment reduced the expression of both ET- and BR-responsive ERFs, including ERF.C1, which was induced by all hormone treatments (Figure 3). BR was found to inhibit ET-responsive ERFs, except ERF.E2. GA treatment had no significant effect on most ET- and BR-responsive genes, except that it suppressed the ET-responsive ERF.B2. The expression of ERF.B3 was enhanced by treatment with all hormones except AUX, and ERF.F4, F5, and D2 were specifically reduced by AUX and BR treatments. Furthermore, ERF.E4 was downregulated upon treatment with all hormones, except ET (Figure 3).
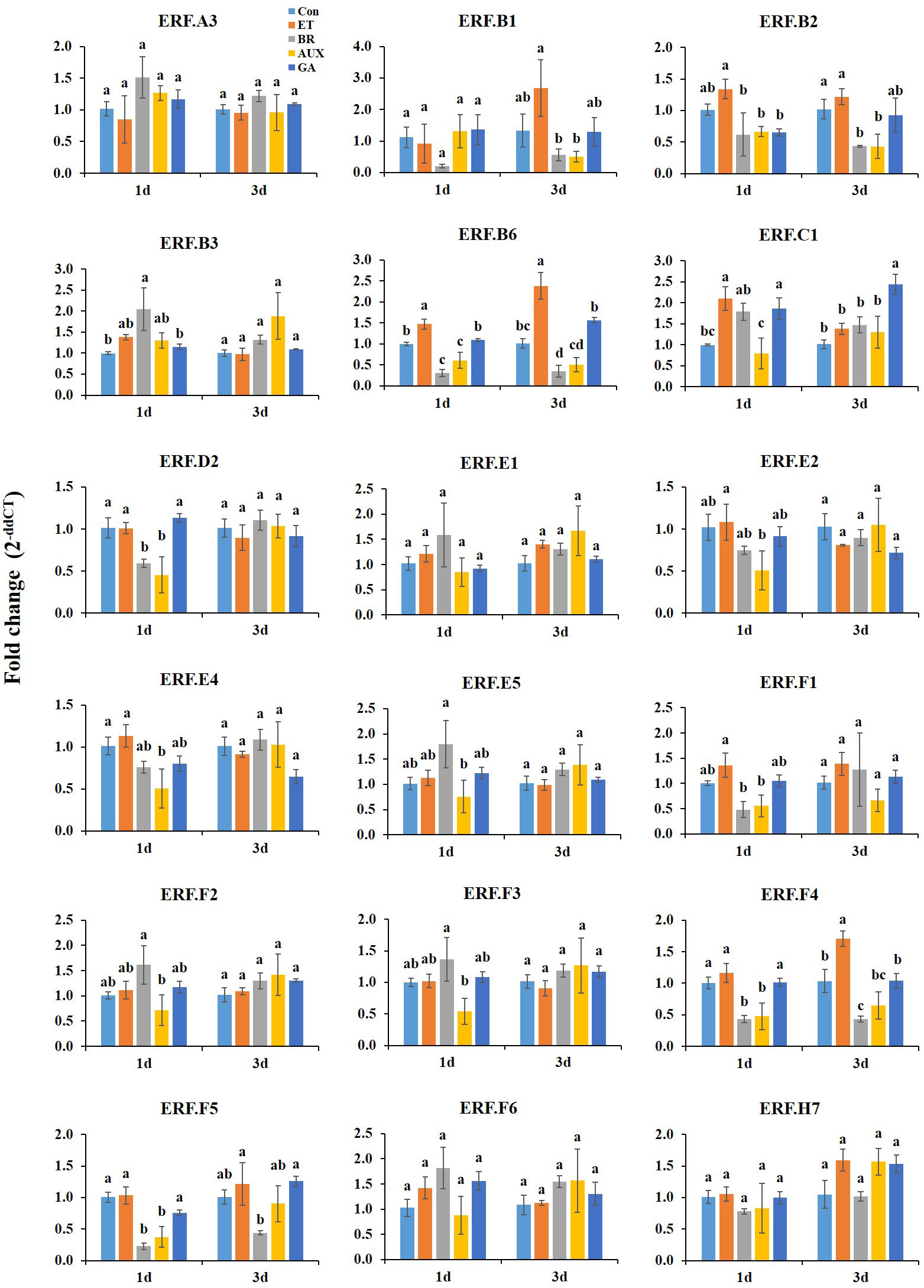
Figure 3 Effect of hormone treatments on the expression of ERFs in tomatoes during storage at room temperature (20 ± 2°C) for 10 d. Error bars represent standard error, and different letters on the graphs represent significant differences between the hormone treatments (P < 0.05). Con, control; ET, ethylene; AUX, auxin; BR, brassinosteroid; GA, gibberellin.
On day 3 at room temperature, ET treatment induced the expression of ERF.B1, B6, F4, and F5 up to 2.5-fold, and these ERFs were downregulated by both AUX and BR treatments. ET-responsive ERFs were not affected by GA treatment, except ERF.F5, which was induced by GA treatment. Moreover, ERF.C1 transcription was exclusively increased in GA-treated fruits. The level of ERF.B2 was reduced in all fruits except those treated with ET. Hormone treatments had no significant effect on ERF.B1 (day 1), ERF.E2, F1, E5, F2, F3, B3, D2, and E4 (day 3), and ERF.A3, E1, F6, and H7 (days 1 and 3) (Figure 3).
3.3 Hormonal response of ERFs in cold storage
On the first day of cold storage, ET treatment increased the expression of ERF. C1, D2, E1, F4, and H7, while GA treatment also increased the expression of these genes except ERF.D2. AUX treatment decreased the expression of ERF.D2 and C1 and moderately increased the expression of ERF.E1, F4, and H7. BR treatment had varied effects, as it could trigger ERF.F4 while decreasing ERF.C1 and H7. The expression of ERF.F1 was found to be most responsive to BR treatment but was reduced in other hormone treatment groups. ERF.B3, E5, and F5 showed the highest level of responsiveness to AUX treatment, while ERF.E5 was exclusively expressed in AUX-treated fruits. The transcript levels of ERF.B3 and F5 were considerably decreased in other hormone-treated fruits. Multiple hormones led to a decrease in ERF.A3, B6, and E4. However, on the first day of cold storage, the hormone treatments did not have a significant effect on the expression of ERF.B1, B2, E2, F2, F3, and F6 (Figure 4).
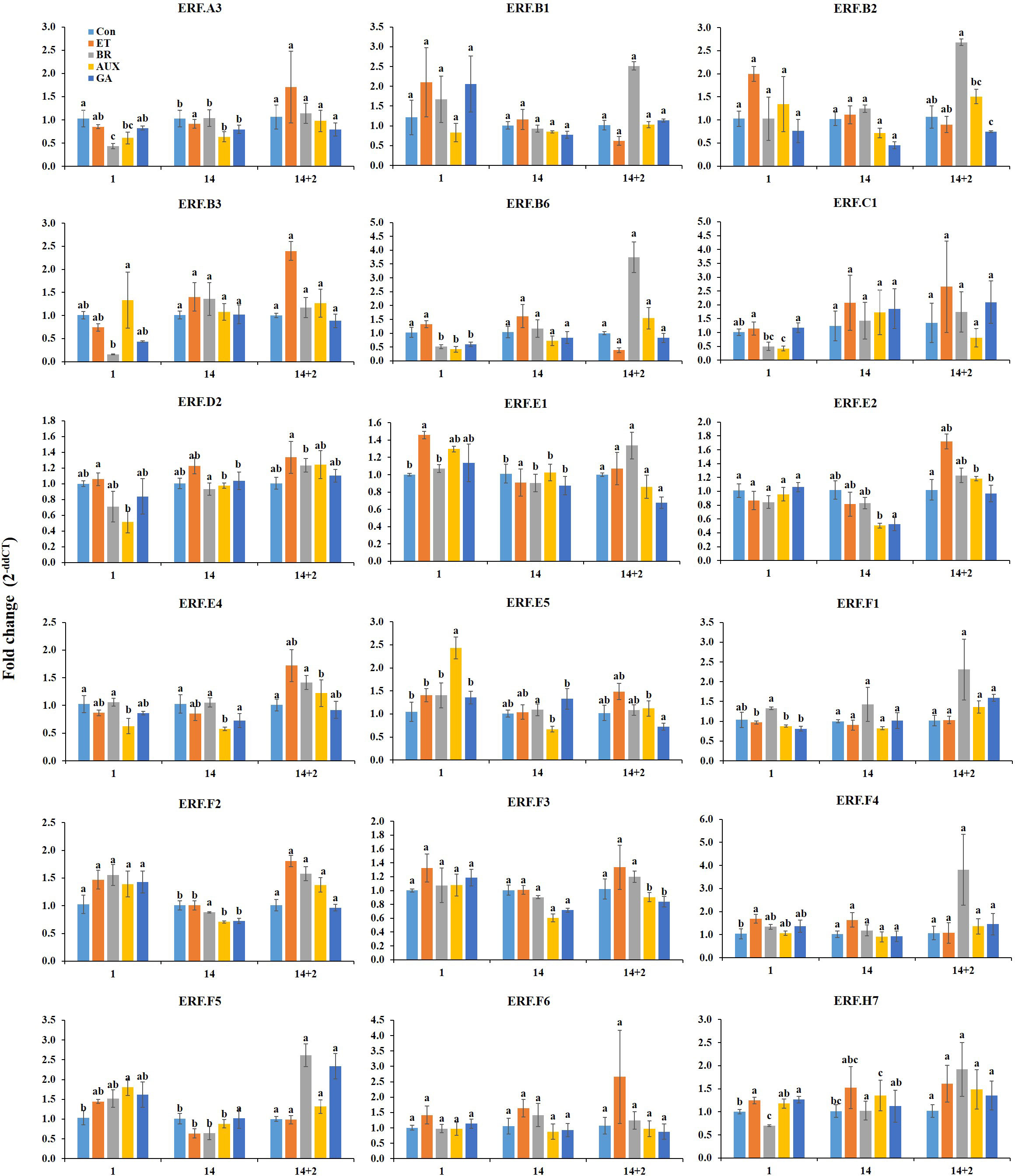
Figure 4 Effect of hormone treatments on the expression of ERFs in tomatoes during storage at 4°C (cold storage) for 14 d followed by 2 d at 20 ± 2°C (retailer conditions). Error bars represent standard error, and different letters on the graphs represent significant differences between the hormone treatments (P < 0.05). Con, control; ET, ethylene; AUX, auxin; BR, brassinosteroid; GA, gibberellin.
On day 14 at cold storage, the hormone treatments had a significant impact on six ERFs (ERF.B2, D2, E2, E4, E5, and F3). ERF.D2 and B2 were most responsive to ET and BR treatments, respectively. BR treatment was found to suppress ERF.D2, while ET treatment had no effect on ERF.B2. In contrast, AUX and GA treatments could reduce the expression of ERF.B2 but not D2. The response of ERF.E4 and E5 to AUX and GA treatments was the opposite, while ET and BR treatments had little effect on these genes. Additionally, ERF.E2 and F3 expression was found to be suppressed in AUX- and GA-treated fruits. ERF. A3, B1, B3, B6, C1, E1, F4, F1, F2, F5, F6, and H7 were not significantly affected by hormone treatments after 14 d (Figure 4).
3.4 Hormonal response of ERFs at retailer conditions
Two days after transferring the fruits from cold storage to retailer conditions, twelve ERF genes were found to be significantly affected by hormone treatments. Interestingly, all these genes were most responsive to either ET or BR treatments. Specifically, ERF.B3, E2, E4, E5, and F2 were highly responsive to ET treatment, with ERF.B3 and E2 were exclusively activated in ET-treated fruits. However, the activity of ERF.E5 and F2 could be reduced by GA treatment, while BR and AUX treatments showed moderate activation. BR treatment resulted in an increased expression of ERF.B1, B2, B6, F1, F4, F5, and E1. Notably, ET treatment was found to decrease the expression levels of some of the BR-responsive genes (ERF.B1, B2, and B6). AUX treatment had an inductive effect on some of the ET-responsive (ERF.F2) and BR-responsive (ERF.B2, B6, F1, and F4) genes, while its suppressive effect was limited to ERF.E1. Meanwhile, GA treatment was found to reduce the expression of ET-responsive (ERF.E4 and F2) and BR-responsive genes (ERF.B2 and E1). However, GA treatment increased the expression of the ET-responsive gene ERF.E4 and BR-responsive genes ERF.F1, F4, and F5. The expression of ERF.A3, F3, C1, D2, F6, and H7 were not significantly affected by treatment with any of the hormones (Figure 4).
3.5 Correlation analysis of ERF expression and fruit color
To investigate the correlation between fruit color and ERF expression in hormone-treated tomatoes, hierarchical clustering and pattern correlation analysis were performed by combining the gene expression data with a* values. The heat map of hierarchical clustering analysis showed that the profiles of all treatment groups, except that of AUX-treated fruits, were closely correlated on days 1 and 3 at room temperature (Figure 5A). The a* values were grouped with ERF.F3, F6, E5, F2, B3, E1, and A3, and these genes, except ERF.F6, E1, and A3, were most responsive to BR treatment (Figure 5A). In cold-stored fruits, a distinct separation was observed between the profiles of ET treatment and those of other treatments. ERF.B1, F4, F6, F2, F3, and B2 were clustered with a* values, and their profiles in ET-treated fruits were significantly different from those in fruits treated with other hormones (Figure 5B). ERF.E5, F5, B3, E1, and H7 were closely clustered, and their expression was induced by AUX (Figure 5B). When fruits were transferred from cold storage to retailer conditions, a distinct separation was observed between the profiles of ET- and BR-treated fruits and other hormone-treated fruits (Figure 5C). The a* values were found to be correlated with several ERFs, including ERF.E1, F3, H7, D2, E4, and F2, which were most responsive to ET and/or BR (Figure 5C).
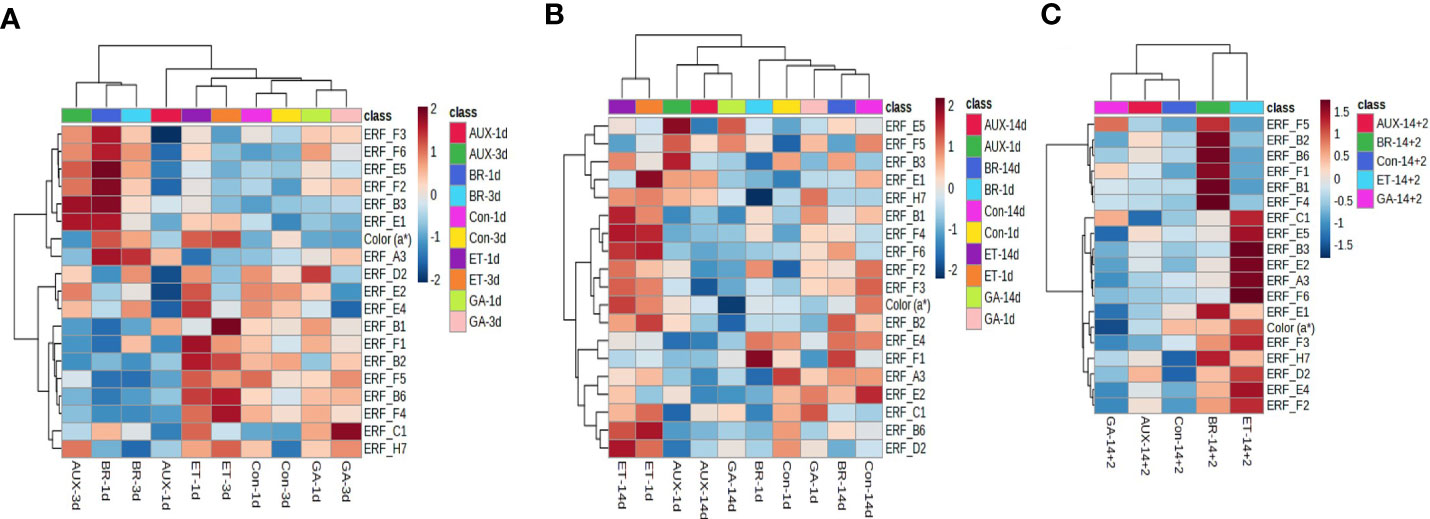
Figure 5 Correlation analysis of ERFs with fruit color in hormone-treated tomatoes at different storage temperatures. Heat map of hierarchical clustering analysis of fruits stored at (A) room temperature (20 ± 2°C) for 10 d and (B) 4°C (cold storage) for 14 d, (C) followed by 2 d at 20 ± 2°C (retailer conditions). Con, control; ET, ethylene; AUX, auxin; BR, brassinosteroid; GA, gibberellin.
Pattern analysis between a* values and gene expression revealed that certain ERFs (ERF.B2, B6, and E4) were positively associated with the a* values in fruits stored at room temperature, whereas other ERFs (ERF.F5 and E2) were negatively associated (Figure 6A). When stored in cold temperatures, a* values were positively correlated with ERF.A3, B2, B6, E4, and F4, but negatively correlated with ERF. B3, E5, and F1 (Figure 6B). Under retailer conditions, most ERFs were positively associated with color development, which suggests that ripening accelerated and quality changes occurred after cold storage regardless of hormone treatment (Figure 6C).
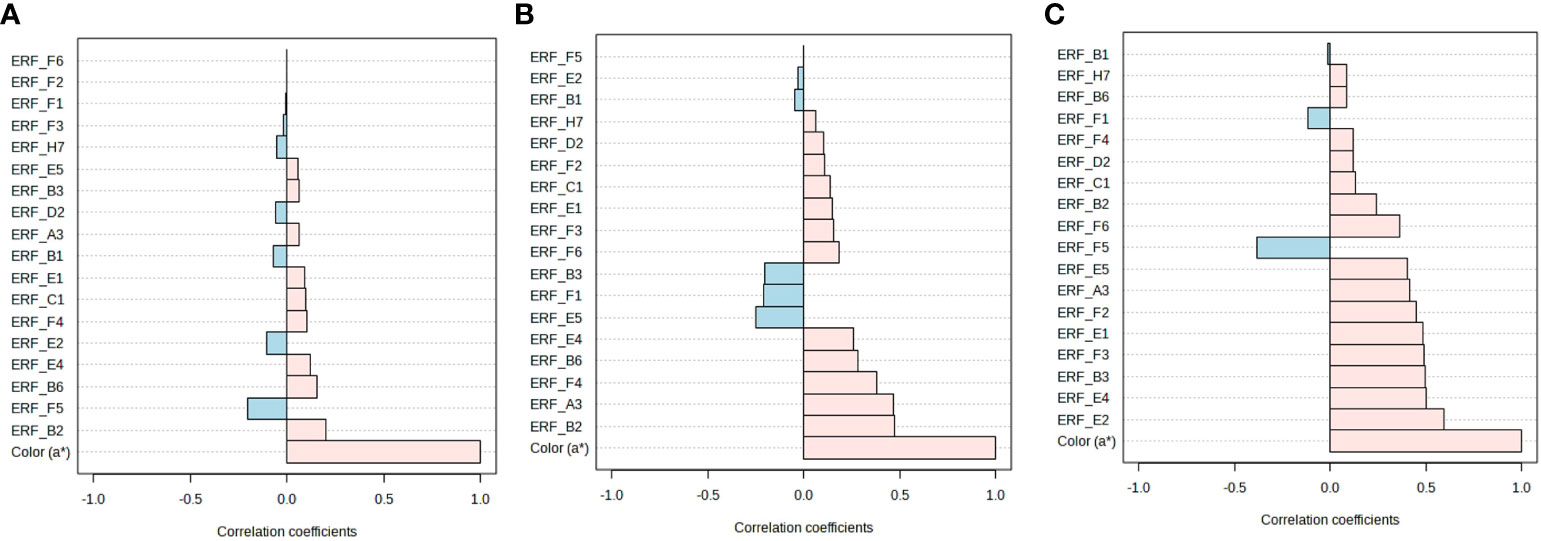
Figure 6 Pattern analysis of ERFs and fruit color in hormone-treated tomatoes at different storage temperatures. (A) Room temperature (20 ± 2°C) for 10 d. (B) 4°C (cold storage) for 14 d, (C) followed by 2 d at 20 ± 2°C (retailer conditions). Con, control; ET, ethylene; AUX, auxin; BR, brassinosteroid; GA, gibberellin.
4 Discussion
In this study, we explored the hormonal regulation of ERFs and their impact on tomato fruit ripening during postharvest storage. Fruit ripening is a crucial process that affects the quality and shelf life of fruits. Our results showed that all ERFs contained ET-related cis-elements, indicating their potential regulation by ET (Figure 1). Furthermore, all ERFs contained cis-elements related to more than one hormone, indicating their potential regulation by multiple phytohormones (Figure 1). This finding is consistent with previous studies showing that plant hormones interact with each other to regulate plant growth and development (Wang and Irving, 2011).
4.1 Hormone treatments differentially affected tomato fruit ripening
The effect of ET, BR, AUX, and GA treatment on the ripening process and ERF expression was evaluated during storage at room temperature, cold temperature, and retailer conditions. The ripening process was characterized by measuring fruit color. At room temperature, the ripening process of the fruits was influenced by hormone treatment, being accelerated by ET and BR and delayed by AUX and GA. This was reflected in the fruit color as shown in Figure 2A and was consistent with previous research (Dostal and Leopold, 1967; Li J. et al., 2017; Malka and Park, 2022). While the a* values of control and ET- and BR-treated fruits were not significantly different after 7 d at room temperature, AUX- and GA-treated fruits consistently showed lower values throughout the storage period (Figure 2B). Of note, low storage temperatures inhibit fruit ripening (Vincent et al., 2020). Thus, during cold storage, the ripening process of fruits in all treatment groups was delayed (Figure 2C). This was evidenced by a delayed color break on day 14, with the highest and lowest a* values observed in ET- and GA-treated fruits, respectively (Figure 2D). When the fruits were transferred from cold storage to retailer conditions, the ripening process was accelerated in all treatment groups. Nonetheless, the effects of the hormone treatments remained largely consistent with those observed at room temperature (Figures 2C, D).
4.2 ERFs responsive ET and BR are inhibited by AUX at room temperature
The fruit quality of tomatoes rapidly changed at room temperature. ET is considered to be the primary regulator of fruit ripening, with BRs and AUX acting as modulators of ET signaling and biosynthesis (Kumar et al., 2014). In this study, ET treatment induced the expression of ERF.B1, B2, B6, E2, and F1 in fruits stored at room temperature. Meanwhile, the transcript levels of ERF.E5, F2, and F3 were increased by BR (Figure 3). Strikingly, the ERFs that were responsive to ET and BR treatment were suppressed in AUX-treated tomatoes concomitant with its inhibitory effect on ripening (Figure 3). The interaction between ET and AUX is well-established in various physiological processes of plant growth and development. In tomato fruit ripening, ERF.B3 and D7 play a critical role in integrating ET and AUX signaling (Liu et al., 2014; Gambhir et al., 2022). Furthermore, some of the ET-responsive ERFs were suppressed in BR-treated fruits (Figure 3), suggesting that they may be involved in a feedback regulation between ET and BR signaling. Additionally, ET-responsive ERF.B2 and B6, along with AUX-suppressive ERF.E4, were positively correlated with color development (Figure 6A). ERF.E4 has been shown to participate in ripening and carotenoid accumulation by integrating both ET-dependent and ET-independent regulatory activities, thereby enabling precise signal output modulation (Lee et al., 2012). These results suggest that the ET- and BR-responsive ERFs identified in this study may play crucial roles in the interplay between ET, BR, and AUX during the transition to ripening in tomato at normal temperature.
4.3 ERFs responsive to ET are co-regulated by BR, AUX, and GA under cold conditions
Low temperatures can significantly affect ET biosynthesis and signaling pathways in tomato fruit, which can result in delayed ripening (Kumar et al., 2014). During cold storage, the ERFs that were most responsive to ET and BR at room temperature remained largely unaffected by these hormones, potentially contributing to the observed ripening inhibition (Figure 4). While several ERFs responded specifically to ET during cold storage, they can also be co-regulated by other hormones, and the specific effect of each hormone may vary depending on the ERF (Figure 4). This suggests that these hormones may interact with the ET signaling pathway to regulate cold acclimation at low temperature.
Several studies highlighted the role of ERFs in the regulation of cold stress. For instance, ERF105 has been found to be critical in freezing tolerance by operating in conjunction with CBF-regulon in Arabidopsis (Bolt et al., 2017). ERF108 and ERF9 from Poncirus trifoliata have been found to positively regulate cold tolerance by activating the raffinose biosynthesis gene and glutathione S-transferase gene, respectively (Khan et al., 2021; Zhang et al., 2022). Additionally, genes most responsive to BR (ERF.B2) and AUX (ERF.B3 and F5) were reported to be involved in stress regulation (Tournier et al., 2003; Chen et al., 2008; Pan et al., 2012; Klay et al., 2014). Interestingly, the expression of AUX-responsive ERF.B3 and E5 were also negatively correlated with a* values (Figure 6C). These results suggest that these ERFs are involved in complex hormone interactions that govern cold response and ripening in cold storage.
4.4 ERFs responsive to ET and BR are inhibited by GA at retailer conditions
When fruits were transferred from cold storage to retailer conditions, the majority of these genes were activated by ET or BR (Figure 4). For example, ERF.B3, E5, and F5 regulated by AUX on day 1 at cold storage were upregulated in ET- or BR-treated fruits at retailer conditions (Figure 4). ERF.B3 responds to both ET and AUX, mediating salt and cold stress response in addition to its role in the regulation of ripening in tomato (Klay et al., 2014; Liu et al., 2014). Moreover, ERF.E2 was found to be responsive to ET at both room temperature and retailer conditions but was downregulated by all the hormones during cold storage (Figures 3, 4). ERF.E2 integrates multiple signaling pathways that are responsive to biotic and abiotic stresses, including ET (Zhang et al., 2004). This suggests that the ERFs responsive to ET or BR have a dual function: cold response at low temperatures and ripening progression at retailer conditions. They may also be involved in the control of chilling injury. ERF.E1, which was most responsive to ET on day 1 during cold storage, was induced by BR at retailer conditions. ERF.E1 has been found to be responsive to chilling injury in tomato (Bai et al., 2021).
Interestingly, certain ERFs most responsive to ET at room temperature were instead regulated by BR at retailer conditions; similarly, ERFs regulated by BR at room temperature were found to be most responsive to ET at retailer conditions (Figures 3, 4). This indicates that although both room temperature and retailer conditions favored ripening, the hormonal regulation of ERFs was specific and depended on the storage conditions. However, ERF.E2 was regulated by ET at both room temperature and retailer conditions (Figures 3, 4). Furthermore, ERF.F5 was negatively correlated with a* values at both room temperature and retailer conditions (Figures 6A, C), suggesting that these genes may play a significant role in the ripening and color development of tomatoes. Moreover, the inhibitory effect of AUX on ET- and BR-regulated ERFs, which was observed at room temperature, was largely absent under retailer conditions (Figure 4). However, GA had the ability to inhibit particular ERFs that were responsive to ET (ERF.E5 and F2) and BR (ERF.B2 and E1) (Figure 4). Furthermore, GA could co-regulate some ET- or BR-responsive genes at both room temperature and retailer conditions, suggesting complex interactions between these hormones in the harvested tomatoes.
Based on these results, we propose a model in which ERFs responsive to ET (ERF.B2, B6, E2, and F1) and BR (ERF.E5, F2, and F3) regulate fruit transition to ripening and its associated changes in harvested tomatoes at room temperature (Figure 7). ERFs, such as B3, E1, and F5, participate in intricate hormone interactions contributing to both cold acclimation and ripening inhibition in cold storage. Upon transfer of fruits from cold storage to retailer conditions, ET and BR may induce several ERFs to recover from chilling injuries and progress in ripening. AUX and GA can fine-tune the transition to ripening in tomatoes at room temperature and after being transferred from cold storage to retailer conditions, respectively (Figure 7).
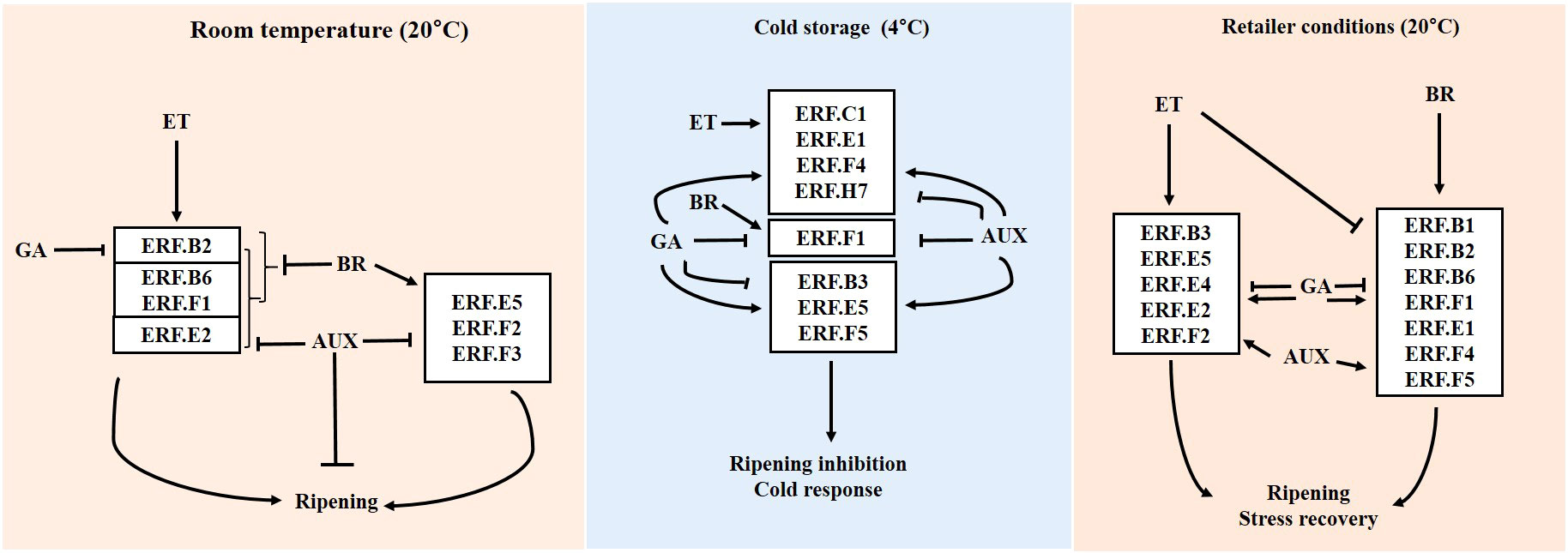
Figure 7 ERF regulation by hormones is affected by storage temperature. Solid and blunt arrows represent positive and negative regulation, respectively. Con, control; ET, ethylene; AUX, auxin; BR, brassinosteroid; GA, gibberellin.
4.5 Conclusion
In summary, we identified ERFs responsive to specific hormones at different storage temperatures. ERFs responsive to ET and BR were inhibited by AUX at room temperature and by GA at retailer conditions. Some ERFs may be involved in cold response at low temperatures and ripening progression and stress recovery after transfer from cold storage to retailer conditions. Overall, this study provides valuable insights into the complex hormonal regulation of ERFs associated with tomato postharvest ripening. These findings will be useful for further understanding dynamic hormonal interactions and for developing strategies to improve fruit quality and shelf life through regulation of hormone signaling pathways and ERF expression.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
M-HP: conceptualization and supervision. H-JY: execution. SM: execution and writing–original draft preparation, and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Cooperative Research Program for Agriculture, Science, and Technology (Project No. PJ01502903) in the Rural Development Administration of the Republic of Korea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1197776/full#supplementary-material
Supplementary Table 1 | List of primers.
Supplementary Figure 1 | Effect of hormone treatments on changes in (A) L* and (B) b* values in tomatoes stored at 20 ± 2°C (room temperature) for 10 d. Changes in (C) L* and (D) b* values in tomatoes stored at 4°C (cold storage) for 14 d followed by 2 d at 20 ± 2°C (retailer conditions). Error bars represent standard error. Con, control; ET, ethylene; AUX, auxin; BR, brassinosteroid; GA, gibberellin.
References
Bai, C., Fang, M., Zhai, B., Ma, L., Fu, A., Gao, L., et al. (2021). Regulations of m6A methylation on tomato fruit chilling injury. Hortic. Plant J. 7 (5), 434–442. doi: 10.1016/j.hpj.2021.05.005
Bolt, S., Zuther, E., Zintl, S., Hincha, D. K., Schmülling, T. (2017). ERF105 is a transcription factor gene of arabidopsis thaliana required for freezing tolerance and cold acclimation. Plant Cell Environ. 40 (1), 108–120. doi: 10.1111/pce.12838
Chen, G., Hu, Z., Grierson, D. (2008). Differential regulation of tomato ethylene responsive factor LeERF3b, a putative repressor, and the activator Pti4 in ripening mutants and in response to environmental stresses. J. Plant Physiol. 165 (6), 662–670. doi: 10.1016/j.jplph.2007.03.006
Chen, T., Yang, Q., Gruber, M., Kang, J., Sun, Y., Ding, W., et al. (2012). Expression of an alfalfa (Medicago sativa l.) ethylene response factor gene MsERF8 in tobacco plants enhances resistance to salinity. Mol. Biol. Rep. 39 (5), 6067–6075. doi: 10.1007/s11033-011-1421-y
Deokar, A. A., Kondawar, V., Kohli, D., Aslam, M., Jain, P. K., Karuppayil, S. M., et al. (2015). The CarERF genes in chickpea (Cicer arietinum l.) and the identification of CarERF116 as abiotic stress responsive transcription factor. Funct. Integr. Genomics 15 (1), 27–46. doi: 10.1007/s10142-014-0399-7
Dostal, H. C., Leopold, A. C. (1967). Gibberellin delays ripening of tomatoes. Science 158 (3808), 1579–1580. doi: 10.1126/science.158.3808.1579
Dubois, M., Skirycz, A., Claeys, H., Maleux, K., Dhondt, S., De Bodt, S., et al. (2013). Ethylene response Factor6 acts as a central regulator of leaf growth under water-limiting conditions in arabidopsis. Plant Physiol. 162 (1), 319–332. doi: 10.1104/pp.113.216341
Gambhir, P., Singh, V., Parida, A., Raghuvanshi, U., Kumar, R., Sharma, A. K. (2022). Ethylene response factor ERF.D7 activates auxin response factor 2 paralogs to regulate tomato fruit ripening. Plant Physiol. 190 (4), 2775–2796. doi: 10.1093/plphys/kiac441
Jung, J.-M., Shim, J.-Y., Chung, S.-O., Hwang, Y.-S., Lee, W.-H., Lee, H. (2019). Changes in quality parameters of tomatoes during storage: a review. Korean J. Agric. Sci. 46 (2), 239–256. doi: 10.7744/KJOAS.20190011
Khan, M., Hu, J., Dahro, B., Ming, R., Zhang, Y., Wang, Y., et al. (2021). ERF108 from poncirus trifoliata (L.) raf. functions in cold tolerance by modulating raffinose synthesis through transcriptional regulation of PtrRafS. Plant J. 108 (3), 705–724. doi: 10.1111/tpj.15465
Klay, I., Pirrello, J., Riahi, L., Bernadac, A., Cherif, A., Bouzayen, M., et al. (2014). Ethylene response factor sl-ERF.B.3 is responsive to abiotic stresses and mediates salt and cold stress response regulation in tomato. Sci. World J. 2014, 167681. doi: 10.1155/2014/167681
Kumar, R., Khurana, A., Sharma, A. K. (2014). Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 65 (16), 4561–4575. doi: 10.1093/jxb/eru277
Lee, J. M., Joung, J. G., McQuinn, R., Chung, M. Y., Fei, Z., Tieman, D., et al. (2012). Combined transcriptome, genetic diversity and metabolite profiling in tomato fruit reveals that the ethylene response factor SlERF6 plays an important role in ripening and carotenoid accumulation. Plant J. 70 (2), 191–204. doi: 10.1111/j.1365-313X.2011.04863.x
Lescot, M., Déhais, P., Thijs, G., Marchal, K., Moreau, Y., Van de Peer, Y., et al. (2002). PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30 (1), 325–327. doi: 10.1093/nar/30.1.325
Li, J., Tao, X., Bu, J., Ying, T., Mao, L., Luo, Z. (2017). Global transcriptome profiling analysis of ethylene-auxin interaction during tomato fruit ripening. Postharvest Biol. Technol. 130, 28–38. doi: 10.1016/j.postharvbio.2017.03.021
Li, S.-j., Xie, X.-l., Liu, S.-c., Chen, K.-s., Yin, X.-r. (2019). Auto- and mutual-regulation between two CitERFs contribute to ethylene-induced citrus fruit degreening. Food Chem. 299, 125163. doi: 10.1016/j.foodchem.2019.125163
Li, X., Xu, Y., Shen, S., Yin, X., Klee, H., Zhang, B., et al. (2017). Transcription factor CitERF71 activates the terpene synthase gene CitTPS16 involved in the synthesis of e-geraniol in sweet orange fruit. J. Exp. Bot. 68 (17), 4929–4938. doi: 10.1093/jxb/erx316
Li, S.-j., Yin, X.-r., Xie, X.-l., Allan, A. C., Ge, H., Shen, S.-l., et al. (2016). The citrus transcription factor, CitERF13, regulates citric acid accumulation via a protein-protein interaction with the vacuolar proton pump, CitVHA-c4. Sci. Rep. 6 (1), 20151. doi: 10.1038/srep20151
Liu, M., Diretto, G., Pirrello, J., Roustan, J.-P., Li, Z., Giuliano, G., et al. (2014). The chimeric repressor version of an ethylene response factor (ERF) family member, sl-ERF.B3, shows contrasting effects on tomato fruit ripening. New Phytol. 203 (1), 206–218. doi: 10.1111/nph.12771
Liu, M., Gomes, B. L., Mila, I., Purgatto, E., Peres, L. E. P., Frasse, P., et al. (2016). Comprehensive profiling of ethylene response factor expression identifies ripening-associated ERF genes and their link to key regulators of fruit ripening in tomato. Plant Physiol. 170 (3), 1732–1744. doi: 10.1104/pp.15.01859
Lorenzo, O., Piqueras, R., Sánchez-Serrano, J. J., Solano, R. (2003). ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant Defense[W]. Plant Cell 15 (1), 165–178. doi: 10.1105/tpc.007468
Malka, S. K., Park, M.-H. (2022). Fresh produce safety and quality: chlorine dioxide’s role [Review]. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.775629
Pan, Y., Seymour, G. B., Lu, C., Hu, Z., Chen, X., Chen, G. (2012). An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep. 31 (2), 349–360. doi: 10.1007/s00299-011-1170-3
Park, M.-H., Sangwanangkul, P., Choi, J.-W. (2018). Reduced chilling injury and delayed fruit ripening in tomatoes with modified atmosphere and humidity packaging. Scientia Hortic. 231, 66–72. doi: 10.1016/j.scienta.2017.12.021
Schmittgen, T. D., Livak, K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3 (6), 1101–1108. doi: 10.1038/nprot.2008.73
Tournier, B., Sanchez-Ballesta, M. T., Jones, B., Pesquet, E., Regad, F., Latché, A., et al. (2003). New members of the tomato ERF family show specific expression pattern and diverse DNA-binding capacity to the GCC box element. FEBS Lett. 550 (1-3), 149–154. doi: 10.1016/s0014-5793(03)00757-9
Tucker, G., Yin, X., Zhang, A., Wang, M., Zhu, Q., Liu, X., et al. (2017). Ethylene† and fruit softening. Food Qual. Saf. 1 (4), 253–267. doi: 10.1093/fqsafe/fyx024
Vincent, C., Mesa, T., Munné-Bosch, S. (2020). Hormonal interplay in the regulation of fruit ripening and cold acclimation in avocados. J. Plant Physiol. 251, 153225. doi: 10.1016/j.jplph.2020.153225
Wang, Y. H., Irving, H. R. (2011). Developing a model of plant hormone interactions. Plant Signal Behav. 6 (4), 494–500. doi: 10.4161/psb.6.4.14558
Xie, X.-l., Yin, X.-r., Chen, K.-s. (2016). Roles of APETALA2/Ethylene-response factors in regulation of fruit quality. Crit. Rev. Plant Sci. 35 (2), 120–130. doi: 10.1080/07352689.2016.1213119
Zhang, H., Huang, Z., Xie, B., Chen, Q., Tian, X., Zhang, X., et al. (2004). The ethylene-, jasmonate-, abscisic acid- and NaCl-responsive tomato transcription factor JERF1 modulates expression of GCC box-containing genes and salt tolerance in tobacco. Planta 220 (2), 262–270. doi: 10.1007/s00425-004-1347-x
Keywords: ethylene response factors, hormonal regulation, storage temperature, fruit quality, ripening, ethylene, auxin, gibberellin
Citation: Park M-H, Yang H-J and Malka SK (2023) Hormonal regulation of ethylene response factors in tomato during storage and distribution. Front. Plant Sci. 14:1197776. doi: 10.3389/fpls.2023.1197776
Received: 31 March 2023; Accepted: 06 June 2023;
Published: 28 June 2023.
Edited by:
Tong Chen, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Gholamreza Khaksar, Chulalongkorn University, ThailandJunfeng Guan, Hebei Academy of Agriculture and Forestry Sciences (HAAFS), China
Copyright © 2023 Park, Yang and Malka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siva Kumar Malka, malka@korea.kr
 Me-Hea Park
Me-Hea Park