- 1College of Life Science and Engineering, Henan University of Urban Construction, Pingdingshan, China
- 2Zhengzhou Research Base, State Key Laboratory of Cotton Biology, School of Agricultural Sciences, Zhengzhou University, Zhengzhou, China
- 3State Key Laboratory of Cotton Biology, Institute of Cotton Research, Chinese Academy of Agricultural Sciences, Anyang, China
Cotton is widely grown in many countries around the world due to the huge economic value of the total natural fiber. Verticillium wilt, caused by the soil-borne pathogen Verticillium dahliae, is the most devastating disease that led to extensive yield losses and fiber quality reduction in cotton crops. Developing resistant cotton varieties through genetic engineering is an effective, economical, and durable strategy to control Verticillium wilt. However, there are few resistance gene resources in the currently planted cotton varieties, which has brought great challenges and difficulties for breeding through genetic engineering. Further revealing the molecular mechanism between V. dahliae and cotton interaction is crucial to discovering genes related to disease resistance. In this review, we elaborated on the pathogenic mechanism of V. dahliae and the resistance mechanism of cotton to Verticillium wilt. V. dahliae has evolved complex mechanisms to achieve pathogenicity in cotton, mainly including five aspects: (1) germination and growth of microsclerotia; (2) infection and successful colonization; (3) adaptation to the nutrient-deficient environment and competition of nutrients; (4) suppression and manipulation of cotton immune responses; (5) rapid reproduction and secretion of toxins. Cotton has evolved multiple physiological and biochemical responses to cope with V. dahliae infection, including modification of tissue structures, accumulation of antifungal substances, homeostasis of reactive oxygen species (ROS), induction of Ca2+ signaling, the mitogen-activated protein kinase (MAPK) cascades, hormone signaling, and PAMPs/effectors-triggered immune response (PTI/ETI). This review will provide an important reference for the breeding of new cotton germplasm resistant to Verticillium wilt through genetic engineering.
1 Introduction
Cotton is an extremely important economic crop in the world, as it contributes about 35% of total nature fiber for the textile industry and also serves as one of the sources of edible oil and livestock feed (Man et al., 2022). Cotton is cultivated in more than 80 countries, of which approximately 30 regard cotton as a commercially leading crop (Abdelraheem et al., 2019). Data from the U.S. Department of Agriculture show that the total global cotton production is 25.343 million tons in 2022–2023 (Meyer and Dew, 2023). China was the largest raw cotton producer, followed by India, USA, Brazil, and Pakistan producing 6.1, 5.99, 3.06, 2.83, and 0.98 million tons, respectively (Figure 1A). The cotton genera (Gossypium spp.) include 45 diploid species (2n = 2x = 26) and seven tetraploid species (2n = 4x = 52). The appearance morphology and fiber characteristics of different cotton genera are quite different, including variable leaf shapes, different fiber characteristics, and diverse plant architectures ranging from wild perennial small trees and shrubs to cultivated annual herbaceous plants (Huang et al., 2021a). Two diploid species Gossypium herbaceum (Levant or Arabian cotton) and Gossypium arboreum (Desi cotton) and two allotetraploid species Gossypium barbadense (Sea Island cotton) and Gossypium hirsutum (Upland cotton) are cultivated globally (Egan and Stiller, 2022). Among them, G. hirsutum is the most widespread and encompasses 95% of global cotton production and is also the main target of cotton breeding (Baran et al., 2022; Yang et al., 2022).
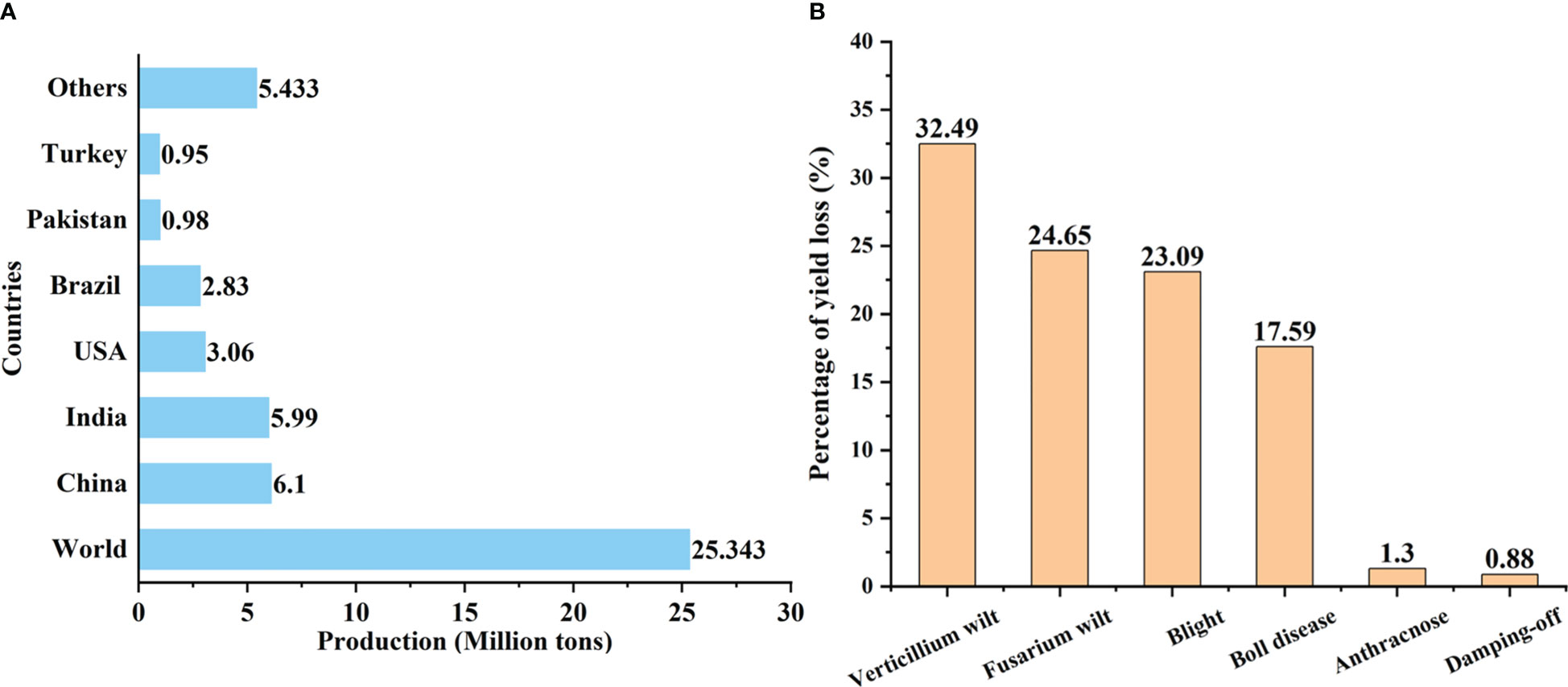
Figure 1 (A) Cotton production in major cotton-producing countries in 2022–2023. (B) Percentage of yield loss due to the major cotton diseases in China in 2021 (The data were obtained from the Agricultural Technology Extension Service Center of the Ministry of Agriculture and Rural Affairs of China).
Being exposed to various environmental cues, a significant decrease in yield and fiber quality is caused by various negative factors such as drought, salinity, temperature stress, pests, nematodes, bacteria, viruses, and fungi (Shaban et al., 2018; Kamburova et al., 2022). Especially, Verticillium wilt, the cancer of cotton crops, is the most devastating disease because of its widespread distribution and strong pathogenicity under favorable conditions. In 2021, the cotton losses in China caused by Verticillium wilt accounted for 32.49% of the total losses caused by different diseases (Figure 1B). The average loss recovery rates for five years of Verticillium wilt were much lower than that of other cotton diseases (except cotton boll disease) and pests (Kun and Shuo, 2020). In recent years, Verticillium wilt has become increasingly serious due to climatic variation, long-term monoculture, and frequent introduction of new cotton varieties/hybrids in various countries and regions in the world (Ranga et al., 2020).
In the future, in response to the exploding population of the world and global climate deterioration, the demand for food, fresh water, fiber, and bioenergy of humans will increase significantly (Maryum et al., 2022). Developing resistant cotton varieties through genetic engineering is an effective, economical, and durable strategy to control Verticillium wilt, which is critical to maintaining world agricultural production. The detailed elucidation of the molecular mechanism of V. dahliae-cotton interaction will help in discovering genes related to disease resistance. Over the past five years, many reviews have summarized the molecular mechanisms of V. dahliae-cotton interaction. Zhang et al. have elaborated on the molecular mechanism of microsclerotia development and systemic infection of V. dahliae (Zhang et al., 2022e). The molecular mechanism of cotton resistance to Verticillium wilt has also been revealed (Song et al., 2020; Man et al., 2022). However, the detailed mechanism by which V. dahliae successfully colonizes the host plant and causes the symptoms of Verticillium wilt in cotton still needs to be further elucidated. At the same time, the molecular mechanism of cotton resistance to Verticillium wilt also needs a comprehensive and detailed elaboration. Therefore, we comprehensively summarized the pathogenic mechanism of V. dahliae and the resistance mechanism of cotton to Verticillium wilt in this review. In particular, we provide additional detail on how V. dahliae adapts to nutrient-deficient environments of host plants, manipulates host immunity, and causes Verticillium wilt symptoms. In addition, we elaborated the molecular mechanism of cotton resistance to Verticillium wilt through several aspects to facilitate readers to systematically understand the molecular mechanism of cotton disease resistance.
2 Verticillium wilt
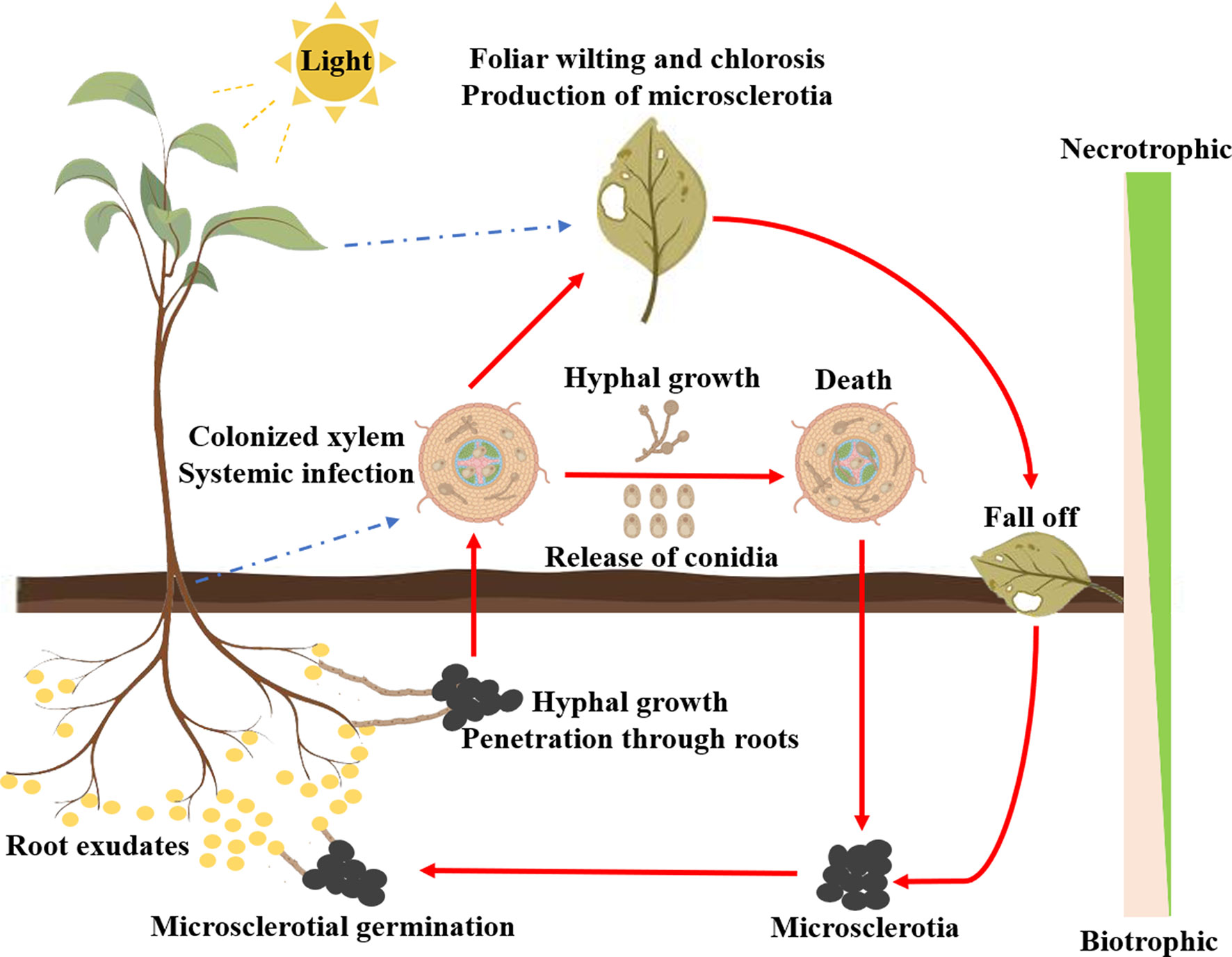
Verticillium wilt is among the most devastating plant diseases infecting a broad range of herbaceous annuals and woody perennials, such as cotton, potato, tomato, okra, eggplant, lettuce, spinach, alfalfa, watermelon, strawberry, oilseed rape, sunflower, olive, maple, and smoke-tree (Fradin and Thomma, 2006; Dhar et al., 2020; Wu et al., 2022). V. dahliae is the leading cause of Verticillium wilt and its resting body microsclerotia can survive for up to 14 years in the absence of a host or under adverse conditions (Short et al., 2015). Upon sensing the signal from the root exudates of the host plants, the microsclerotia germinate into hyphae and infect through the root tips, lateral roots, or wounds of the host plants (Fradin and Thomma, 2006). After reaching the xylem, the hyphae spread systemically along the vascular system. During the infection of the host plants, V. dahliae sequentially undergoes the biotrophic and necrotrophic stages (Lo Presti et al., 2015). In the biotrophic stage, V. dahliae draws its nutrients from the host plants for the production of conidia and systemic infection. A large number of hyphae and conidia colonize the xylem resulting in foliar wilting and chlorosis and even death of the host plants (de Sain and Rep, 2015; Zhang et al., 2022b). With the death of the host plants, V. dahliae enters the necrotrophic phase and eventually forms microsclerotia to ensure long-term survival (Figure 2).
Many environmental factors can affect the incidence of cotton Verticillium wilt, such as the number of microsclerotia in the soil and the diseased plant residues, photoperiod, light intensity, temperature, humidity, irrigation methods, and cultivation techniques (Karademir et al., 2012; Shaban et al., 2018; Wang et al., 2023). The symptoms of cotton infected by V. dahliae are yellowing and necrotic leaves, browning of vessels, and wilting. Under the favorable temperature and humidity, the V. dahliae in infected cotton will produce white spores to promote the spread of Verticillium wilt, resulting in the rapid death of the cotton (Fradin and Thomma, 2006). V. dahliae can also penetrate the bolls and seeds, and the infected seeds are conducive to the further spread of V. dahliae. The micronaire and span length of the fiber was seriously reduced in infected cotton (Zhang et al., 2012).
3 Pathogenic mechanism of V. dahliae
The pathogenic molecular mechanism of V. dahliae is relatively sophisticated and controlled by multiple signaling pathways. The main mechanism that causes symptoms of Verticillium wilt is vascular occlusion and the production of toxins. As V. dahliae penetrates the xylem vessels, the massive mycelium and plant defense-driven structures and biomacromolecules produced by parenchyma cells block the vessels, interfering with the transportation of water and nutrients in plants (Zhang et al., 2022b). The water imbalance occurs in the host plants and causes wilting and yellowing of leaves and even death (Song et al., 2020). In the toxin hypothesis, the toxins produced by V. dahliae have been shown to act on the cell walls, plasma membrane, microfilaments, microtubules, and other intracellular components, leading to cytotoxicity, rapid destruction of cell walls, and disordered host defense responses (Chen et al., 2016a; Zhao et al., 2020; Zhang et al., 2022b). The growth and development and successful colonization of V. dahliae are the prerequisites for the occurrence of Verticillium wilt. In brief, both the germination and growth of microsclerotia, attachment and infection to the host roots, degrading the host cell walls, adapting to the nutrient-deficient environment and competing for nutrients, manipulating and evading host immunity, and the secretion of toxins affect the development of Verticillium wilt.
3.1 Development and germination of microsclerotia
The key steps of V. dahliae infection include germination of microsclerotia, hyphae penetrating the root epidermis, invading hyphae extending in the intercellular space of the root cortex, colonization of hyphae in vessels, production of the conidia to promote the vertical systemic reproduction, and the free passage of the hyphae through the intertracheary pits to cross adjacent xylem vessels to achieve horizontal colonization (Zhao et al., 2014; Tian and Kong, 2022; Zhang et al., 2022b). As the long-term resting structures, germination of microsclerotia is an important step in the occurrence of Verticillium wilt, which is affected by the air, temperature, humidity, soil organic matter content, and pH (Yang et al., 2004; Shaban et al., 2018). The G protein receptors, Ca2+, small GTPases, and cAMP were involved in the germination and development of microsclerotia (Luo et al., 2019). VdPbs2 (mitogen-activated protein kinase kinase), VdSkn7 (two-component stress response regulator), VdOCH1 (α-1,6-mannosyltransferase), and VdAda1 (Ada1 subunit) positively regulated the formation of microsclerotia, and the deletion of those genes reduced the pathogenicity of V. dahliae (Tian et al., 2016; Tang et al., 2017; Zhang et al., 2019b; Geng et al., 2022). However, some studies suggested that the production of microsclerotia is negatively correlated with the pathogenicity of V. dahliae. The deletion mutant strains of V. dahliae with ΔVdPKAC1 (cAMP-dependent protein kinase A), ΔVGB (G protein β subunit), ΔVdMsn2 (C2H2 transcription factor), or ΔVdPLP (patatin-like phospholipase) have increased production of microsclerotia but reduced the pathogenicity to host plants (Tzima et al., 2012; Tian et al., 2017; Qi et al., 2018).
3.2 Colonization in the roots of host plants
Before penetration of the roots of host plants, the secretome of V. dahliae exerts toxicity to suppress the growth of antagonistic bacteria in the rhizosphere environment to ensure V. dahliae survival and eventual colonization (Snelders et al., 2020; Snelders et al., 2021). To colonize the host plants, V. dahliae needs to successfully adhere to and penetrate the root of the host plants. The hyphae of V. dahliae surrounding the roots tightly adhere to the root epidermis and then form the hyphopodium at the infection site, which develops into penetration pegs piercing the root epidermis and cortical cells to further infection (Zhang et al., 2022b; Zhang et al., 2022e).
A variety of genes are involved in the infection process of V. dahliae in host plants. The deletion strains with VdBre1 (encoding a ubiquitin ligase) showed dramatically reduced penetration ability and nonpathogenic symptoms in cotton (Wang et al., 2021c). The nuclear transcription factor Som1 was crucial for adhesion and penetration to the roots, while the nuclear transcription factor Vta3 was required in the colonization of root surfaces (Bui et al., 2019). As a positive regulator of hyphopodium formation, VdSte11, a homolog gene of mitogen-activated protein kinase kinase kinase, was critical for penetrating host plants (Yu et al., 2019). The cellophane surface-induced gene VdCSIN1 regulated the formation of hyphopodium via the cAMP-mediated signal pathway to promote the colonization of the host plants (Sun et al., 2019). The sterol C-8 isomerase VdERG2 played a crucial role in the growth and penetration of mycelium on cellophane and knockout of the VdERG2 gene impaired the pathogenicity of V. dahliae (Lv et al., 2022a). While the osmosensor VdSho1 regulated the ability to penetrate the plant through the MAPK pathway (Li et al., 2019b). The velvet protein Vel1 was required for the formation and distribution of conidia in the xylem and for controlling the form of hyphae during the first phases of plant colonization (Höfer et al., 2021).
Penetration pegs are an important tool for V. dahliae to penetrate the host roots. The plasma membrane-co-located proteins VdNoxB (catalytic subunit of membrane-bound NADPH oxidase) and VdPls1 (tetraspanin) mediated ROS production, which activated VdCrz1 (calcineurin-responsive zinc finger transcription factor) signaling through Ca2+ elevation in hyphopodia to regulate the formation of penetration pegs (Zhao et al., 2016). The penetration pegs further develop into the hyphal neck, which separates the hyphopodium from the invasive hyphae and forms a fungal-host interface to facilitate the delivery and secretion of small secreted proteins (Jin et al., 2021). The cytoskeleton protein VdSep5 was critical to the septin-ring-organized hyphal neck, while the vesicular trafficking factors VdSec22 and VdSyn8 and the exocyst subunit VdExo70 positively regulated the delivery of the secreted proteins to the hyphal neck. The virulence of VdΔsep5, VdΔsec22, VdΔsyn8, and VdΔexo70 mutants was significantly reduced to cotton roots (Zhou et al., 2017).
3.3 Degradation of the plant cell walls
The plant cell walls, consisting predominantly of cellulose, hemicelluloses (especially xylan), pectin, lignin, and minor structural proteins, are a dynamic structure that plays an important role in preventing the invasion of pathogens (Mielke and Gasperini, 2019; Ishida and Noutoshi, 2022). The cell walls degrading enzymes produced by the pathogens are essential for the colonization of the host plants. Analysis of the genome sequence of V. dahliae suggested that there are a large number of cell wall degrading enzymes, including pectinase, xylanase, cellulase, and protease (Chen et al., 2016a). The sucrose nonfermented protein kinase gene VdSNF1 and the specific secreted protein gene VdSSP1 positively regulated the activities of cell walls degrading enzymes and were essential for the virulence of V. dahliae on host plants (Tzima et al., 2011; Liu et al., 2013). Besides, pathogenesis-related genes VdPR1 and VdPR3 affected the pathogenicity of V. dahliae by regulating cellulase activity (Zhang et al., 2015; Zhang et al., 2016b). The polygalacturonase VdPG1 and the xylanase VdXyn4 digested pectin and xylan respectively in the cell walls to enhance the pathogenicity of V. dahliae to cotton (Liu et al., 2017a; Wang et al., 2021a). Further, the transcription factor VdFTF1 and N-ethylmaleimide-sensitive factor attachment protein receptors VdSec22 and VdSso1 regulated the vesicle trafficking and translocation of pectinases, cellulases, and xylanases (Wang et al., 2018a; Zhang et al., 2018).
3.4 Adapting to the nutrient-deficient environment and competing for the nutrients
After penetration to the roots, the invasive hyphae of V. dahliae enter the xylem vessels from the intercellular space of the root cortex and rapidly reproduce and invade the vascular bundles. In responding to plant defense responses, V. dahliae must adapt to the nutrient-deficient intracellular environment and compete with the host for its nutrients. The glutamate-rich protein VdGARP1 sensed the infertile conditions to promote the transformation of V. dahliae from a saprophytic state to microsclerotia for long-term survival (Gao et al., 2010). VdAsp1, encoding an inositol polyphosphate kinase, regulated the transition of invasive hyphae from vegetative growth to asexual reproduction to adapt to the nutrient-deficient environment (Tian et al., 2022). The bZIP transcription factor VdAtf1 participated in virulence via the regulation of inorganic nitrogen utilization in V. dahliae (Tang et al., 2020b). As participants in the acquisition of thiamine, VdThit, VdThi4, and VdThi20 were required for the pathogenicity of V. dahliae to host plants (Hoppenau et al., 2014; Qi et al., 2016; Qin et al., 2020). Besides, two M35 family metalloproteinases VdM35-1 and VdASPF2 were involved in the utilization of carbon sources (Lv et al., 2022b). The ferric reductase FreB of V. dahliae reduced environmental ferric iron to bioavailable ferrous iron to obtain iron from plant cells and maintained its pathogenicity (Rehman et al., 2018). While VdHapX, a bZIP transcription factor, played a crucial role in iron homeostasis in response to iron-deficient and iron-excess conditions and was involved in the full virulence in V. dahliae (Wang et al., 2018b). Under the iron-deficient environment of the xylem, Asp−type small cysteine-rich secretory proteins VdSCPs sequestered ferric iron that further aggravated the deficiency of ferric iron in the xylem, thereby reducing the disease resistance of host plants (Wang et al., 2022a).
3.5 Manipulation and suppression of the immune responses of host plants
To successfully colonize and rapidly infect host plants, V. dahliae employs complex molecular mechanisms to manipulate and suppress the immune responses of host plants. Two superoxide dismutases VdSOD1 and VdSOD5 were nonessential for the normal vegetative growth of V. dahliae, but regulated the detoxification of both extracellular ROS generated from the host and intracellular ROS produced by the normal metabolism of V. dahliae (Tian et al., 2021a; Tian et al., 2021b). Besides, Chr2g00380 (cytochrome P450 monooxygenases), VdDpb4 (histone-fold protein of the ISW2 chromatin remodeling complex), and three transcription factors VdAtf1, VdYap1, and VdSkn7 were involved in responding to ROS stress produced by the host plants (Tang et al., 2020a; Wang et al., 2020c; Zhang et al., 2022c). During the infection, the nonribosomal peptide synthetase VdNPS suppressed the expression of PR genes, production of ROS, and SA-mediated signaling of host plants to enhance the pathogenicity of V. dahliae (Luo et al., 2020). While the Alt a 1 family protein PevD1 from V. dahliae inhibited the antifungal activity of the pathogenesis-related protein GhPR5 to overcome the host defense system (Zhang et al., 2019c). The polysaccharide deacetylase VdPDA1 enhanced the deacetylation of chitin oligosaccharides, leading to impaired ability of host plants to recognize chitin oligosaccharides, thereby inhibiting the host plant immune response (Gao et al., 2019). As a candidate effector, VdCE11 contributed to pathogenicity in cotton and Arabidopsis by enhancing the accumulation and activity of the aspartic proteases, which were negative regulators of immunity from cotton and Arabidopsis (Li et al., 2023).
The small RNAs (sRNAs) can deliver between filamentous pathogens and host plants to trigger transkingdom RNA silencing or RNA interference (RNAi) in recipient cells, thereby altering plant defenses and pathogen virulence (Huang et al., 2019; Zhao et al., 2021). The small RNA VdrsR-1 modulated the floral transition of host plants and prolonged the vegetative growth of host plants thereby favoring the propagation of V. dahliae (Zhang et al., 2022a). The secretory silencing repressor VdSSR1 from V. dahliae can translocate to the plant nucleus and inhibite the nucleocytoplasmic shuttling of sRNA. VdSSR1 increased the virulence of V. dahliae in plants by suppressing the accumulation of mobile plant miRNAs in fungal cells to prevent subsequent transkingdom silencing of virulence genes (Zhu et al., 2022a).
3.6 Leading to necrosis, wilting, and defoliation
Although some studies have shown that the crude extracts of V. dahliae can cause the collapse of microfilaments and microtubules in plant cells, the physiological and biochemical mechanism leading to wilting and defoliation of the host plants is still unclear. Previous studies have shown that the necrosis- and ethylene-inducing-like protein VdNLP caused foliar necrosis in host plants (Wang et al., 2004). The proposed mechanism was that VdNLP interacted with glycosylinositol phosphorylceramide (GIPC) sphingolipids to form complexes with terminal monomeric hexose moieties of GIPCs that insert into the plant plasma membrane (Lenarčič et al., 2017). Now, VdNEP (VdNLP1) was suggested as a sensitive molecular marker to distinguish the defoliating and nondefoliating V. dahliae strains (Triantafyllopoulou et al., 2022). A cytochrome P450 monooxygenase VdCYP1 regulated at least 14 kinds of secondary metabolites syntheses in V. dahliae and among them, sulfacetamide could induce necrosis and wilting symptoms in cotton (Zhang et al., 2016a). As a homologous protein of N-acylphosphatidylethanolamine-hydrolyzing phospholipase D, VdDf7 was involved in the generation of N-lauroylethanolamines (NAEs). Excessive synthesis of NAEs in V. dahliae induced the overexpression of fatty acid amide hydrolase and disrupted NAEs metabolism in cotton, finally causing defoliation by altering sensitivity to abscisic acid (Zhang et al., 2019a). While the elicitor PevD1 can target the NAC transcription factor ORE1 in Arabidopsis or cotton to manipulate ethylene biosynthesis that triggered V. dahliae-induced leaf senescence (Zhang et al., 2021).
4 Molecular mechanisms of cotton resistance to Verticillium wilt
In response to V. dahliae infection, various physiological and biochemical characteristics in cotton will change accordingly to ensure the plants survive under such stressful conditions. Both the physiological and biochemical resistance is mediated by the complex molecular mechanism. In recent years, with the rapid development of molecular biotechnology, breakthroughs have been made in the study of the molecular mechanism of the interaction between V. dahliae and cotton. The molecular mechanisms of cotton resistance to Verticillium wilt mainly include the following aspects, such as modification of tissue structures, accumulation of antifungal substances, homeostasis of ROS, secretion of oxidoreductase and hydrolase, production of receptor-like proteins and kinases, regulation of transcription factors, activation of hormone signaling, and induction of hypersensitive response (HR) and the development of systemic acquired resistance (SAR) (Figure 3) (Song et al., 2020; Wu et al., 2022). Different defense signals are regulated by different resistance-related genes, and multiple defense signals are often intertwined into a complex signaling network.
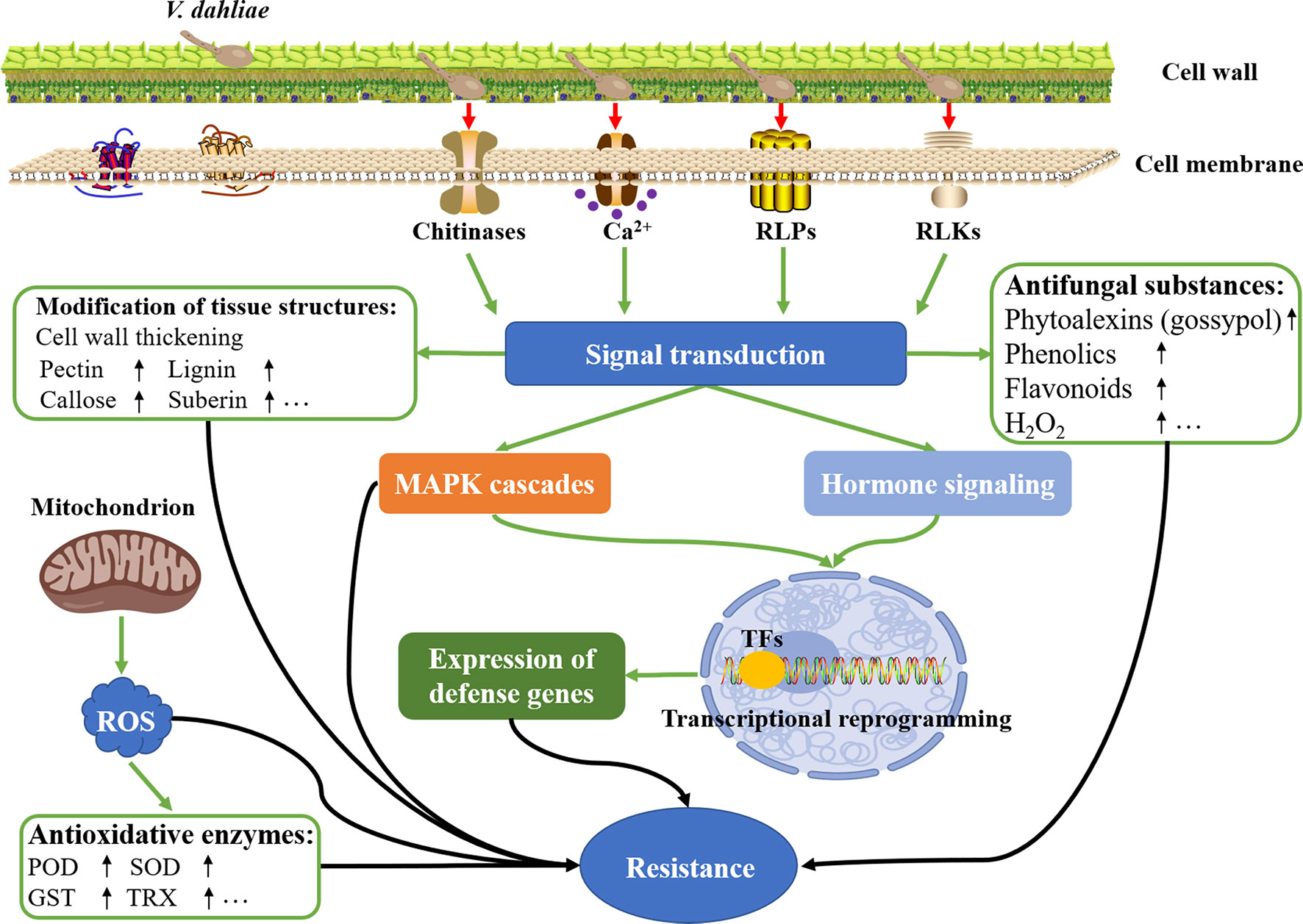
Figure 3 Molecular mechanisms of cotton resistance to V. dahliae infection. Upon V. dahliae infection, a series of physiological and biochemical responses regulated by the sophisticated molecular mechanism was activated, thereby initiating the resistance to Verticillium wilt in cotton plants.
4.1 Modification of tissue structures
To protect cell walls from being degraded by V. dahliae, cotton will modify the cell walls or inhibit the cell wall degrading enzymes from V. dahliae by defense-related protein. As the main component of the cell walls, pectin is involved in the plant defense responses against V. dahliae. The polygalacturonase VdPG1 could digest the pectin of cell walls, while the polygalacturonase-inhibiting protein GhPGIP1 inhibited the activity of VdPG1 to ensure the integrity of the cell walls and enhanced the resistance of cotton to V. dahliae (Liu et al., 2017a; Liu et al., 2017b). Pectin methylesterase GhPME2/GhPME31 catalyzed the demethylation of pectin, resulting in pectin being more easily hydrolyzed by VdPG1. The pectin methylesterase inhibitor GhPMEI3 inhibited the activity of GhPME2/GhPME31 and enhanced the degree of methyl esterification of pectin thereby protecting the cell walls from degradation (Liu et al., 2018). Moreover, the long non-coding RNA lncRNA7 and its regulating gene GbPMEI13 positively regulated the accumulation of high-methyl esterified pectin in cell walls and enhanced cotton resistance to V. dahliae (Zhang et al., 2022d). Chang et al. verified that the galactosyltransferase gene GhRFS6 positively regulated cotton resistance to Verticillium wilt through involvement in pectin and cell wall synthesis (Chang et al., 2023). Proteomic analyses on xylem sap suggested that the stem mechanical strength and accumulation of cell wall-related proteins significantly enhanced in the resistant cotton varieties after V. dahliae infection, but not in the susceptible varieties (Yadav et al., 2020).
During the infection of V. dahliae, the deposition of lignin, callose, and suberin occur rapidly to limit or delay the invasion of V. dahliae. The lignin and callose deposition increased in resistant and susceptible cotton varieties after V. dahliae infection, but the increase was more pronounced in resistant cotton varieties (Xu et al., 2011; Zhang et al., 2017). It has been reported that GhBOP1 (BLADE-ON-PETIOLE1), the laccase GhLAC15, the ribosomal protein L18A GhARPL18A-6, the cinnamyl alcohol dehydrogenases GhCADs, the nonspecific lipid transfer protein GhnsLTPsA10, and transcription factors GbERF1-like, GhWRKY1-like, and GhODO1 enhanced the cotton resistance to V. dahliae through functioning as the positive regulators of lignin biosynthesis (Guo et al., 2016; Zhang et al., 2019d; Zhang et al., 2019e; Zhang et al., 2019; Chen et al., 2021a; Hu et al., 2021; Li et al., 2022; Zhu et al., 2022b). Suberin is deposited in the cell walls of root endodermis, outer cortex, periderm, and other marginal tissues, forming another physical barrier to resist the invasion of pathogens (Xin and Herburger, 2021). GbCYP86A1-1, a cytochrome P450 fatty acid ω‐hydroxylase, was involved in the formation of suberin and positively regulated the resistance of cotton and Arabidopsis to V. dahliae (Wang et al., 2020a). Overexpression of the pathogenesis-related protein gene GbPR10.5D1 in cotton enhanced the resistance to V. dahliae, accompanied by the activation of genes involved in suberin biosynthesis (Guo et al., 2022).
4.2 Accumulation of antifungal substances
The antifungal substances including phytoalexins, phenolics, and flavonoids of cotton accumulate to inhibit the spore germination and germ tube elongation of V. dahliae. As the toxic phytoalexins, the deoxyhemigossypol (dHG), hemigossypol (HG), desoxy-6-methyoxyhemigossypol (dMHG), and 6-methoxyhemigossypol (MHG) played a vital role to kill the conidia and mycelia as well as inhibited the sporulation of V. dahliae (Wagner et al., 2015). Silencing two major melatonin biosynthesis genes GhSNAT1 (serotonin N-acetyltransferase) and GhCOMT (caffeic acid O-methyltransferase) compromised cotton resistance to V. dahliae accompanied by reduced gossypol levels (Li et al., 2019a). Phenolics such as caffeic acid and ferulic acid significantly inhibit the growth of V. dahliae colony, while the flavonoids such as naringenin, quercetin, and dihydroquercetin could inhibit the growth of V. dahliae mycelia (Hu et al., 2018a; Xiong et al., 2021a). Compared with the control cotton variety S78, the spontaneous mutant cotton with red coloration (S156) has increased resistance to V. dahliae due to the increased flavonoid content and gene expressions of flavonoid biosynthesis (Long et al., 2019). Luo et al. found that the biosynthetic pathway of flavonoids was activated under phosphate-deficient conditions, thereby enhancing the resistance of cotton to V. dahliae (Luo et al., 2021). Moreover, GhWRKY41 positively regulated the cotton resistance to V. dahliae by enhancing the accumulation of flavonoids (Xiao et al., 2023).
4.3 PAMP- and effector-triggered immunity
To defend against pathogen invasion, plants can employ the innate immune system to sense specific molecules and initiate subsequent resistance responses. The first tier is based on the cell surface pattern recognition receptors (PRRs) to recognize pathogen/microbe-associated molecular patterns (PAMPs/MAMPs), resulting in PAMPs-triggered immune response (PTI) (Ramirez-Prado et al., 2018). A growing amount of PAMPs/MAMPs has been identified, such as bacterial flagellin, lipopolysaccharides, elongation factor Tu, peptidoglycan, fungal chitin, xylanase, and oligogalacturonides derived from the plants (Zhu et al., 2022c). Successfully invaded pathogens have evolved to generate diverse effector proteins to inhibit PTI. While plants can perceive effectors from pathogens through transmembrane or intracellular receptors (R proteins) to initiate the second tier of defense, which is called effector-triggered immunity (ETI) (Ramirez-Prado et al., 2018). Activation of PTI or ETI triggers numerous overlapping cell signaling events, including Ca2+ fluxes, production of ROS, MAPK cascades, transcriptional reprogramming, and hormone biosynthesis (Köster et al., 2022).
As a typical PAMP, chitin released from fungal cell walls by plant chitinases can trigger the plant immune responses (Ramirez-Prado et al., 2018). The infection of V. dahliae triggered the secretion of chitinases to degrade the fungal cell wall and the cotton chitinases 23, 28, 32, and 47 have been shown to positively regulate resistance to Verticillium wilt (Xu et al., 2016; Han et al., 2019). To suppress the chitin signaling pathway, the serine protease VdSSEP1 secreted from V. dahliae could hydrolyze chitinase 28. While the cysteine-rich repeat protein CRR1 protected chitinase 28 from cleavage by VdSSEP1. Thus, silencing either chitinase 28 or CRR1 in cotton reduced resistance to Verticillium wilt, whereas overexpression of CRR1 in cotton enhanced its resistance (Han et al., 2019).
Receptor-like proteins (RLPs) and receptor-like kinases (RLKs) are important PRRs with distinct extracellular domains for the perception of different ligands (He et al., 2018c). As the leucine-rich repeat receptor-like protein (eLRR-RLP), Ve1 is responsible for resistance to Verticillium spp in tomato, Arabidopsis, tobacco, and cotton (Fradin et al., 2011; Song et al., 2018). The Ve homologous genes GbVe1, GbaVd1, GbaVd2, Gbvdr3, Gbvdr5, and Gbvdr6 also conferred resistance to Verticillium wilt in Arabidopsis and cotton (Li et al., 2015; Chen et al., 2016b; Chen et al., 2017; Yang et al., 2017). Another large class of receptor-like proteins is nucleotide-binding site-leucine rich repeat (NBS-LRR) proteins that contain a central NBS and a C-terminal LRR domain. According to the diversity of N-terminal structures, NBS-LRR proteins are further divided into CC-NBS-LRR (CNL) and TIR-NBS-LRR (TNL) families. The CNL genes GbRVd and GbCNL130 promote resistance to Verticillium wilt by activating the SA signaling pathway and strong accumulation of ROS (Yang et al., 2016; Li et al., 2021). While another CNL gene GbaNA1 mediated resistance to V. dahliae by activating the production of ROS and activation of the Eth signaling pathway (Abreu et al., 2018). Furthermore, the transfer of TNL gene GhDSC1 to dsc1 A. thaliana mutant conferred resistance to V. dahliae and this resistance was coupled with ROS accumulation and activation of the JA signaling pathway (Li et al., 2019d).
As another important PRRs, RLKs are divided into more than 21 subfamilies according to the extracellular ligand binding domain, including leucine-rich repeats (LRRs), lectin, lysin motif (LysM), cysteine-rich receptor-like kinases (CRKs), and wall-associated kinases (WAKs) (Feng et al., 2021). Many pieces of literature have confirmed that receptor-like kinases are involved in the defense response of host plants to V. dahliae. The defense-related RLK GbSOBIR1 could phosphorylate GbbHLH171 and play a critical role in cotton resistance to V. dahliae (Zhou et al., 2019). While the lysin-motif receptor kinases Lyk1, Lyk2, Lyk7, Lyp1, and LysMe3 played important roles in chitin perception and positively regulate cotton resistance to V. dahliae through chitin signaling (Gu et al., 2017; Xu et al., 2017). Two cysteine-rich receptor-like kinases CRK5 and CRK22 played positive regulators in defense responses to V. dahliae toxins in Arabidopsis plants by mediating the MPK3/6-WRKY70-TGA2/6-NPR1/3/4-SA signaling pathways (Zhao et al., 2022). In addition, the WAKs also positively regulated cotton response to V. dahliae infection (Wang et al., 2020b; Feng et al., 2021; Yang et al., 2021).
4.4 Homeostasis of ROS
ROS plays a crucial role in the initial stages of abiotic and biotic stress sensing and contributes to the establishment of subsequent defenses, such as the reinforcement of cell wall structures, hormonal signaling, HR, and SAR (Qi et al., 2017; Mittler et al., 2022). Plant NADPH oxidases (NOXs), also known as respiratory burst oxidase homologs (rbohs), play a predominant role in the metabolic network of ROS. GbRboh5/18 and GhRbohD have been reported to activate ROS production and enhance cotton resistance to Verticillium wilt (Chang et al., 2020; Huang et al., 2021b). However, excessive ROS impairs many cellular functions by altering the structure and function of multiple proteins and causing oxidative damage to DNA, RNA, and membrane lipids (Phua et al., 2021; Mittler et al., 2022). The antioxidative enzymes of plants can scavenge ROS and maintain homeostasis of ROS, including superoxide dismutase (SOD), peroxidase (peroxidase, POD), glutathione S-transferase (glutathione S-transferase, GST), glutathione peroxidase (GPX), and thioredoxin (TRX) (Shaban et al., 2018; Phua et al., 2021). GST, POD, and SOD were involved in the homeostasis of ROS during V. dahliae infection so that cotton can prevent itself from being damaged due to excessive accumulation of ROS when initiating defense responses (Li et al., 2019c; Li et al., 2019e; Pei et al., 2019). Moreover, the thioredoxin GbNRX1 regulated the rapid balancing of redox to maintain the homeostasis of apoplastic ROS after infection of V. dahliae, which was important for the apoplastic immune response of cotton (Li et al., 2016). Besides, the microRNA miR398b mediated the cleavage of mRNAs of genes that function in ROS homeostasis and caused excessive ROS accumulation, thereby reducing the resistance to V. dahliae in miR398b-overexpressing cotton (Miao et al., 2022). As an important antioxidant, anthocyanins also have an effect to maintain the homeostasis of ROS. The silencing of the anthocyanin synthase gene GbANS reduced the anthocyanin content in cotton, resulting in excessive accumulation of H2O2 and attenuating cotton resistance to V. dahliae (Long et al., 2018).
4.5 Ca2+ signaling
The Ca2+ signaling is an important messenger of the plant immune system and builds an extremely complicated network of many interconnected nodes, transferring external or internal danger signals to activate multiple defense responses (Jiang and Ding, 2023). The MYB transcription factor GhMYB108 positively regulated cotton resistance to V. dahliae by interacting with the major Ca2+ sensor GhCML11 (calmodulin-like protein). Further analysis found that Ca2+ was beneficial for GhCML11 to enhance the transcriptional activity of GhMYB108, indicating that Ca2+ played an important role in the defense response against V. dahliae mediated by GhMYB108-GhCML11 (Cheng et al., 2016). Recently, Sun et al. demonstrated that PAMPs activated the expression of the calcium sensor TOUCH 3 (TCH3, also named CML12), which interfered with the auto-inhibitory region of calcium-dependent protein kinases5 (CPK5) and promoted CPK5-mediated phosphorylation of CAM-BINDING PROTEIN 60-LIKE G (CBP60g). The phosphorylation of CBP60g enhanced its transcription factor activity and positively regulated the defense against V. dahliae (Sun et al., 2022). In addition, the accumulation of Ca2+ induced by V. dahliae promoted the acetylation of the calmodulin GhCaM7, thereby activating JA and ROS defense signaling pathways and changing cell osmotic potential to enhance cotton resistance to Verticillium wilt (Zhang et al., 2023a).
4.6 MAPK cascades
MAPK cascades are highly conserved in eukaryotes and widely used to amplify and translate environmental and developmental signals into complex physiological and biochemical reactions. The rapid activation of MAPK cascades typically relays and amplifies PTI/ETI-induced downstream signals, such as transcriptional recombination and hormone signaling (Zhang and Zhang, 2022). The typical MAPK cascades are composed of MAPKs (MPKs), MAPK Kinases (MAPKKs/MKKs/MEKs), and MAPK Kinase Kinases (MAPKKKs/MEKKs). Previous studies have shown that silencing of GhMKK2, GhMKK4, GhMKK6, GhMKK9, GhMPK9, GhMPK13, and GhMPK25 compromised cotton resistance to the infection by V. dahliae, whereas silencing of GhMKK10 increased resistance to V. dahliae (Gao et al., 2011; Zhang et al., 2014; Meng et al., 2018). Moreover, the phosphorylation of the MPK homolog GhNTF6 activated ROS production, callose deposition, and the JA signaling pathway to increase the resistance to V. dahliae infection (Zhou et al., 2022). While the overexpression of GbMPK3 in cotton activated the SA signaling transduction but reduced resistance to V. dahliae (Long et al., 2020).
4.7 Hormone signaling
Upon pathogen attack, phytohormone signaling is the primary perception of plants and then the subsequent defense signaling network was activated or repressed, including the elicitation of PR genes, the reinforcement of cell wall, production of phytoalexins, induction of SAR, ROS, and MAPK cascades (Dhar et al., 2020; Billah et al., 2021). Salicylic acid (SA), jasmonic acid (JA), ethylene (Eth), auxin (AUX), cytokinin (CTK), gibberellic acid (GA), abscisic acid (ABA), brassinosteroids (BR), and strigolactones (SLs) have been documented in plant defense responses (Shaban et al., 2018; Dhar et al., 2020). These plant hormones form complex signaling networks that integrate environmental cues and respond to diverse pathogens. There is a complex synergy and antagonism between different hormone signals in response to the invasion of diverse pathogens. While SA and JA are the main players against V. dahliae, and their roles are well-established (Table 1).
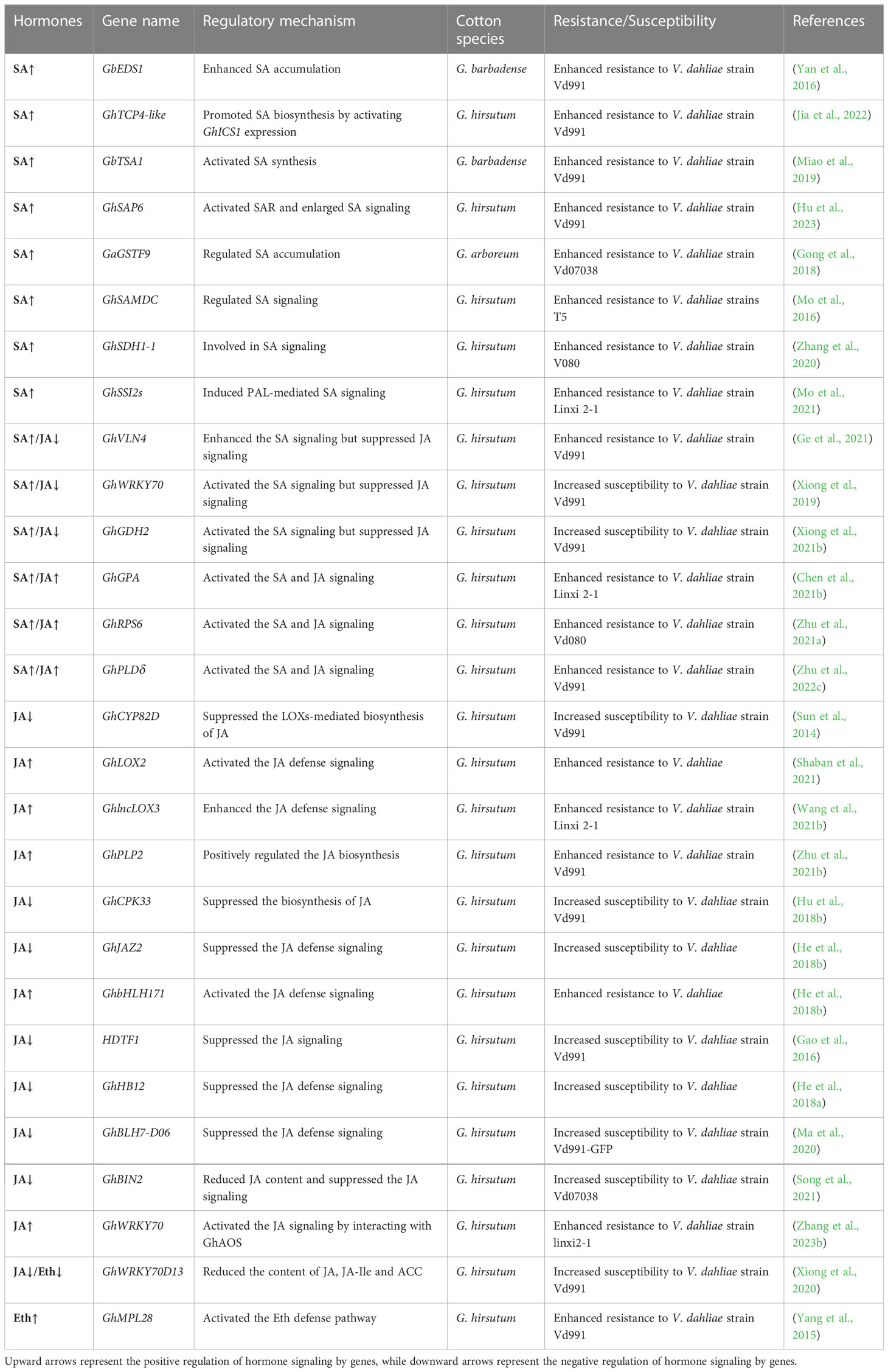
Table 1 Genes implicated in the SA, JA, and Eth defense signaling in response to V. dahliae infection.
4.7.1 SA signaling in resistance to Verticillium wilt
SA, one of the major defense-related hormones, plays an important role in the activation of the expression of PR genes and establishment of the long-lasting and broad-spectrum disease resistance SAR (Shaban et al., 2018). Although SA has been implicated in defense responses against a wide range of biotrophic and hemibiotrophic pathogens, it plays an auxiliary role against necrotrophic pathogens (Dhar et al., 2020). As a hemibiotrophic pathogen, V. dahliae behaves as a biotrophic pathogen during the early stages of infection but it switches to a necrotrophic lifestyle during the later infectious stages. Thus, the SA signaling pathway was required to confer resistance against V. dahliae.
The enhanced disease susceptibility 1 (EDS1) gene is a core genetic component in SA-mediated defense responses and is critical for resistance against biotrophic and hemibiotrophic pathogens (Chen et al., 2021c). Silencing of GbEDS1 in cotton significantly decreased the accumulation of SA and enhanced the susceptibility of cotton to V. dahliae (Yan et al., 2016). In plants, the enzymatic reactions for the synthesis of salicylic acid are mainly mediated by phenylalanine lyase (PAL) and isochorismate synthase (ICS). The teosinte branched1/Cincinnata/proliferating cell factor (TCP) transcription factor GhTCP4-like interacted with GhNPR1 to promote GhICS1 expression, leading to accumulation of SA, which was sensed by NPR1 to increase cotton resistance against V. dahliae (Jia et al., 2022). As a key player in the elicitation of SA biosynthesis, indole enhances the expression of genes involved in SA defense signaling pathways. Knock-down of GbTSA1 (tryptophan synthase α) and GbTSB1 (tryptophan synthase β) enhanced the accumulation of indole, thereby activating SA biosynthesis and defense signaling pathways and improving cotton resistance to V. dahliae (Miao et al., 2019). Moreover, the miR530-GhSAP6 module in cotton leaves responded remotely to V. dahliae infection from roots via SAR, and then enlarged SA signaling at locations farther from the injection sites, leading to enhanced resistance of cotton plants to V. dahliae (Hu et al., 2023).
Moreover, the complex metabolic pathways of plant secondary products often intersect with SA signaling. Gong et al. confirmed that glutathione S-transferase GaGSTF9 positively regulated cotton resistance to V. dahliae by maintaining the low-level accumulation of ROS and then affecting SA content (Gong et al., 2018). The S-adenosylmethionine decarboxylase gene GhSAMDC encoded key rate-limiting enzymes of spermine biosynthesis, and the constitutive overexpression of GhSAMDC in Arabidopsis improved resistance against V. dahliae through activating SA signaling (Mo et al., 2016). Additionally, the silencing of succinate dehydrogenase gene GhSDH1-1 in cotton led to decreased resistance to V. dahliae because of the severe damage to the SA-signaling pathway (Zhang et al., 2020). The soluble fatty acid desaturase stearoyl-ACP desaturase regulates the desaturation of fatty acids and produces the monounsaturated fatty acid oleic acid (18:1) in the plastids. Suppressing the expression of GhSSI2s (encoding stearoyl-ACP desaturases) reduced the 18:1 level in cotton and autoactivated PAL-mediated SA defense response, thereby enhancing cotton resistance to V. dahliae (Mo et al., 2021).
Studies have reported the antagonism interaction between SA and JA/Eth signaling pathways. Overexpression of the actin cytoskeleton gene GhVLN4 in Arabidopsis enhanced resistance to V. dahliae by enhancing the SA defense signaling but suppressing JA defense signaling (Ge et al., 2021). The silence of GhWRKY70 or GhGDH2 (encoding glutamate dehydrogenase) led to the suppression of the SA signaling pathway and initiation of the JA signaling pathway, which consequently enhanced the cotton resistance against V. dahliae (Xiong et al., 2019; Xiong et al., 2021b). Interestingly, SA and JA signaling pathways are not always antagonistic to each other but also have mutual synergy. The G-protein α-subunit GhGPA positively regulated the resistance of cotton and Arabidopsis plants to V. dahliae by activating both SA and JA signaling pathways (Chen et al., 2021b). Knockdown of the ribosomal protein gene GhRPS6 resulted in decreased SA and JA content and suppressed a series of defensive responses in cotton (Zhu et al., 2021a). The phospholipase GhPLDδ played a positive role in the tolerance to Verticillium wilt through the activation of JA and SA signaling pathways (Zhu et al., 2022c).
4.7.2 JA signaling in resistance to Verticillium wilt
Jasmonates (JAs) is the collective name for JA and its derivatives and belongs to the family of oxylipin compounds, which were produced through oxidation and further conversions of polyunsaturated fatty acids by lipoxygenases (LOXs) and α-LOXs (Cook et al., 2021). JAs play crucial roles in plant responses to biotic and abiotic stresses, especially in defense responses against herbivores, insect pests, wounding, and pathogens (Wasternack and Strnad, 2016; Ghorbel et al., 2021). The JA signaling pathway has been extensively studied in the interaction between V. dahliae and cotton. The cytochrome P450 CYP82D (SSN) competed for fatty acids with LOXs and suppressed the LOXs-mediated JA biosynthetic pathway in cotton, thereby weakening the resistance to V. dahliae (Sun et al., 2014). Knockdown of GhLOX2 and GhlncLOX3 suppressed the expression of JA-related genes and increased cotton susceptibility to V. dahliae (Shaban et al., 2021; Wang et al., 2021b). GhPLP2, encoding the patatin-like protein, regulated the fatty acid metabolism pools for JA biosynthesis and activated the JA signaling pathway, thereby enhancing the resistance of cotton and Arabidopsis plants to V. dahliae (Zhu et al., 2021b). The calcium-dependent protein kinase GhCPK33 phosphorylated the 12-oxophytodienoate reductase GhOPR3, leading to destabilization of GhOPR3, which limited the biosynthesis of JA and decreased cotton resistance to V. dahliae (Hu et al., 2018b). Jasmonate-ZIM-domain protein GhJAZ2 inhibited the activity of GhbHLH171, a positive regulator of the JA signaling pathway, resulting in attenuated resistance of GhJAZ2-overexpressed cotton to V. dahliae (He et al., 2018b).
Many studies have explored the roles of transcription factors involved in the defense system of cotton by modulating JA signaling. As a homeodomain transcription factor, HDTF1 negatively regulated cotton resistance to V. dahliae by inactivating the JA-mediated signaling and JA accumulation, but not affecting SA signaling (Gao et al., 2016). Overexpression of the homeodomain-leucine zipper (HD-ZIP) transcription factor GhHB12 increased the susceptibility of the cotton to V. dahliae, which was associated with the repression of genes expression in JA defense signaling (He et al., 2018a). Silencing of the BEL1-like transcription factor GhBLH7-D06 enhanced the tolerance of cotton to Verticillium wilt, which was mainly attributed to the activation of lignin biosynthesis and JA defense signaling pathways (Ma et al., 2020). Moreover, the knock-down of the GSK3-like kinase gene BIN2 (brassinosteroid insensitive 2) significantly enhanced the resistance of Arabidopsis and cotton to V. dahliae by regulating the endogenous content of JA and the expression of JA-responsive marker genes (Song et al., 2021). Recently, Zhang et al. identified a new WRKY70 (highly homologous to GhWRKY70D02 sequence), which activated the JA defense signaling pathway by interacting with GhAOS, a key enzyme in the biosynthesis of JA, to enhance the tolerance of cotton to Verticillium wilt (Zhang et al., 2023b).
4.7.3 Other hormone signaling in resistance to Verticillium wilt
The ET and JA signaling pathways usually cooperate to defend against V. dahliae infection. Silencing of GhWRKY70D13 led to the increased accumulation of JA, JA-Ile, and ET synthesis precursor ACC and enhanced resistance of cotton to V. dahliae (Xiong et al., 2020). By enhancing the transcriptional activity of the ethylene response factor GhERF6, the defense-related major latex protein GhMPL28 activated the ET defense pathway to enhance cotton resistance to V. dahliae (Yang et al., 2015). However, there are studies indicating that ET seems to promote the development of Verticillium wilt symptoms. Pantelides et al. confirmed that the perception of ethylene via ethylene receptor ETR1 was required in Arabidopsis infection by V. dahliae (Pantelides et al., 2010). Overexpression of AtCTR1 (a negative regulator of ethylene signaling) in cotton led to reduced sensitivity to ethylene but increased resistance to V. dahliae (Wang et al., 2022b). The elicitor PevD1 manipulated ethylene biosynthesis in Arabidopsis and cotton and triggered V. dahliae-induced leaf senescence (Zhang et al., 2021). Collectively, these results indicated that ET plays a dual role in resistance as well as the development of wilt symptoms.
In the hormone defense signaling network of plants against V. dahliae, there are often complex relationships between SA/JA and other hormones. During the infection of V. dahliae, the Aux/IAA protein GhIAA43 was a negative regulator of cotton immune response and played a major role in the connection between SA defense signaling and auxin signaling in cotton plants (Su et al., 2022). The APETALA2/ETHYLENE RESPONSIVE FACTOR gene GhTINY2 positively regulated the resistance of cotton and Arabidopsis plants to V. dahliae. Further studies determined that GhTINY2 fine-tuned the trade-off between immunity and growth by indirectly linking the WRKY51-mediated SA signaling and BZR1-IAA19-regulated BR signaling (Xiao et al., 2021). The carotenoid cleavage dioxygenases GbCCD7 and GbCCD8b positively regulated the accumulation of strigolactone and consequently activated the JA and ABA signaling. The positive feedback loop of ABA and the negative feedback loop of JA could regulate the homeostasis of strigolactone and maintain the balance among these three hormones, thereby improving the tolerance of cotton to Verticillium wilt (Yi et al., 2023).
5 Conclusions and perspectives
Since there is a conserved co-evolutionary relationship between plants and pathogens in nature, revealing the pathogenic mechanism of pathogens and the resistance mechanism of host plants will help to improve plant disease resistance. Elucidating the molecular mechanisms of V. dahliae-cotton interaction is a key step for the durable and efficient control of cotton Verticillium wilt. However, only a few key molecular mechanisms have been well elucidated. With the release of the genome sequence of V. dahliae and cotton, scientific researchers have focused on the molecular mechanisms of V. dahliae-cotton interaction, giving us a deep understanding of the complex mechanisms. In this review, we provide a broader picture of the new insights into the interaction between V. dahliae and cotton. V. dahliae have evolved a variety of approaches to infect cotton, while cotton has developed various defense mechanisms to cope with the threat of Verticillium wilt.
In V. dahliae, the molecular mechanism of pathogenicity that has been revealed so far can be roughly divided into five aspects: (1) germination and growth of microsclerotia; (2) infection and successful colonization; (3) adaptation to the nutrient-deficient environment of cotton and competing with cotton for its nutrients; (4) suppression and manipulation of cotton immune response; (5) leading to wilting and defoliation of cotton through rapid reproduction of V. dahliae and secretion of toxins. To deal with V. dahliae, cotton has evolved a complex defense system, mainly including modification of tissue structures, accumulation of antifungal substances, homeostasis of ROS, induction of Ca2+ signaling, the MAPK cascades, hormone signaling, and PTI/ETI. As V. dahliae is a hemibiotrophic pathogen generally it is perceived that the defense mechanism of cotton against V. dahliae is relatively complex. There are differences in the defense signals dominated by different resistance-related genes and multiple defense signals intersect into a complex signal network.
The pathogenic mechanism of V. dahliae and the resistance mechanism of cotton were comprehensively analyzed in this review, providing the target gene resources for effective control of cotton Verticillium wilt. With the development of multi-omics integrative analyses and molecular biology technology, it is possible to mine more key genes in the interaction between cotton and V. dahliae, which will be contributed to obtaining cotton varieties that are resistant to Verticillium wilt through genetic engineering and breeding technology.
Author contributions
YuZ: project administration, writing – original draft, writing – review and editing. MZ: visualization. TL: investigation. LW: visualization. CL: supervision. DL: writing – review and editing. HZ: supervision. YaZ: writing – review and editing. LL: supervision. XG: investigation, supervision. BL: funding acquisition, writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Henan Province Science and Technology Research Project (Grant No. 222102110001) and the Henan Province Key R&D Special Project (Grant No. 221111110900).
Acknowledgments
Thanks to Professor Yuxia Hou and Associate Professor Ping Wang from the College of Science, China Agricultural University for their suggestions on this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelraheem, A., Esmaeili, N., O’Connell, M., Zhang, J. (2019). Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Products 130, 118–129. doi: 10.1016/j.indcrop.2018.12.070
Abreu, F. R. M., Dedicova, B., Vianello, R. P., Lanna, A. C., de Oliveira, J. A. V., Vieira, A. F., et al. (2018). Overexpression of a phospholipase (OsPLDα1) for drought tolerance in upland rice (Oryza sativa l.). Protoplasma 255 (6), 1751–1761. doi: 10.1007/s00709-018-1265-6
Baran, N., Shimira, F., Nyirahabimana, F. (2022). “Marker-assisted selection and traits of interest in cotton breeding,” in Natural and engineering sciences (Boston: Gece Publishing), 99–120.
Billah, M., Li, F., Yang, Z. (2021). Regulatory network of cotton genes in response to salt, drought and wilt diseases (Verticillium and fusarium): progress and perspective. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.759245
Bui, T. T., Harting, R., Braus-Stromeyer, S. A., Tran, V. T., Leonard, M., Höfer, A., et al. (2019). Verticillium dahliae transcription factors Som1 and Vta3 control microsclerotia formation and sequential steps of plant root penetration and colonisation to induce disease. New Phytol. 221 (4), 2138–2159. doi: 10.1111/nph.15514
Chang, Y., Li, B., Shi, Q., Geng, R., Geng, S., Liu, J., et al. (2020). Comprehensive analysis of respiratory burst oxidase homologs (Rboh) gene family and function of GbRboh5/18 on verticillium wilt resistance in Gossypium barbadense. Front. Genet. 11. doi: 10.3389/fgene.2020.00788
Chang, B., Zhao, L., Feng, Z., Wei, F., Zhang, Y., Zhang, Y., et al. (2023). Galactosyltransferase GhRFS6 interacting with GhOPR9 involved in defense against verticillium wilt in cotton. Plant Sci. 328, 111582–111598. doi: 10.1016/j.plantsci.2022.111582
Chen, T., Kan, J., Yang, Y., Ling, X., Chang, Y., Zhang, B. (2016b). A ve homologous gene from Gossypium barbadense, Gbvdr3, enhances the defense response against Verticillium dahliae. Plant Physiol. Biochem. 98, 101–111. doi: 10.1016/j.plaphy.2015.11.015
Chen, J., Li, N., Ma, X., Gupta, V. K., Zhang, D., Li, T., et al. (2017). The ectopic overexpression of the cotton Ve1 and Ve2-homolog sequences leads to resistance response to verticillium wilt in arabidopsis. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00844
Chen, H., Li, M., Qi, G., Zhao, M., Liu, L., Zhang, J., et al. (2021c). Two interacting transcriptional coactivators cooperatively control plant immune responses. Sci. Adv. 7 (45), eabl7173–eabl7190. doi: 10.1126/sciadv.abl7173
Chen, J. Y., Xiao, H. L., Gui, Y. J., Zhang, D. D., Li, L., Bao, Y. M., et al. (2016a). Characterization of the Verticillium dahliae exoproteome involves in pathogenicity from cotton-containing medium. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.01709
Chen, B., Zhang, Y., Sun, Z., Liu, Z., Zhang, D., Yang, J., et al. (2021a). Tissue-specific expression of GhnsLTPs identified via GWAS sophisticatedly coordinates disease and insect resistance by regulating metabolic flux redirection in cotton. Plant J. 107 (3), 831–846. doi: 10.1111/tpj.15349
Chen, B., Zhang, Y., Yang, J., Zhang, M., Ma, Q., Wang, X., et al. (2021b). The G-protein α subunit GhGPA positively regulates Gossypium hirsutum resistance to Verticillium dahliae via induction of SA and JA signaling pathways and ROS accumulation. Crop J. 9 (4), 823–833. doi: 10.1016/j.cj.2020.09.008
Cheng, H. Q., Han, L. B., Yang, C. L., Wu, X. M., Zhong, N. Q., Wu, J. H., et al. (2016). The cotton MYB108 forms a positive feedback regulation loop with CML11 and participates in the defense response against Verticillium dahliae infection. J. Exp. Bot. 67 (6), 1935–1950. doi: 10.1093/jxb/erw016
Cook, R., Lupette, J., Benning, C. (2021). The role of chloroplast membrane lipid metabolism in plant environmental responses. Cells 10 (3), 706–726. doi: 10.3390/cells10030706
de Sain, M., Rep, M. (2015). The role of pathogen-secreted proteins in fungal vascular wilt diseases. Int. J. Mol. Sci. 16 (10), 23970–23993. doi: 10.3390/ijms161023970
Dhar, N., Chen, J. Y., Subbarao, K. V., Klosterman, S. J. (2020). Hormone signaling and its interplay with development and defense responses in Verticillium-plant interactions. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.584997
Egan, L. M., Stiller, W. N. (2022). The past, present, and future of host plant resistance in cotton: An Australian perspective. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.895877
Feng, H. J., Li, C., Zhou, J. L., Yuan, Y., Feng, Z. L., Shi, Y. Q., et al. (2021). A cotton WAKL protein interacted with a DnaJ protein and was involved in defense against Verticillium dahliae. Int. J. Biol. Macromolecules 167, 633–643. doi: 10.1016/j.ijbiomac.2020.11.191
Fradin, E. F., Abd-El-Haliem, A., Masini, L., van den Berg, G. C., Joosten, M. H., Thomma, B. P. (2011). Interfamily transfer of tomato Ve1 mediates Verticillium resistance in arabidopsis. Plant Physiol. 156 (4), 2255–2265. doi: 10.1104/pp.111.180067
Fradin, E. F., Thomma, B. P. H. J. (2006). Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 7 (2), 71–86. doi: 10.1111/j.1364-3703.2006.00323.x
Gao, W., Long, L., Xu, L., Lindsey, K., Zhang, X., Zhu, L. (2016). Suppression of the homeobox gene HDTF1 enhances resistance to Verticillium dahliae and Botrytis cinerea in cotton. J. Integr. Plant Biol. 58 (5), 503–513. doi: 10.1111/jipb.12432
Gao, X., Wheeler, T., Li, Z., Kenerley, C. M., He, P., Shan, L. (2011). Silencing GhNDR1 and GhMKK2 compromises cotton resistance to verticillium wilt. Plant J. 66 (2), 293–305. doi: 10.1111/j.1365-313X.2011.04491.x
Gao, F., Zhang, B. S., Zhao, J. H., Huang, J. F., Jia, P. S., Wang, S., et al. (2019). Deacetylation of chitin oligomers increases virulence in soil-borne fungal pathogens. Nat. Plants 5 (11), 1167–1176. doi: 10.1038/s41477-019-0527-4
Gao, F., Zhou, B. J., Li, G. Y., Jia, P. S., Li, H., Zhao, Y. L., et al. (2010). A glutamic acid-rich protein identified in Verticillium dahliae from an insertional mutagenesis affects microsclerotial formation and pathogenicity. PloS One 5 (12), e15319–e15330. doi: 10.1371/journal.pone.0015319
Ge, D., Pan, T., Zhang, P., Wang, L., Zhang, J., Zhang, Z., et al. (2021). GhVLN4 is involved in multiple stress responses and required for resistance to verticillium wilt. Plant Sci. 302, 110629–110643. doi: 10.1016/j.plantsci.2020.110629
Geng, Q., Li, H., Wang, D., Sheng, R. C., Zhu, H., Klosterman, S. J., et al. (2022). The Verticillium dahliae spt-Ada-Gcn5 acetyltransferase complex subunit Ada1 is essential for conidia and microsclerotia production and contributes to virulence. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.852571
Ghorbel, M., Brini, F., Sharma, A., Landi, M. (2021). Role of jasmonic acid in plants: the molecular point of view. Plant Cell Rep. 40 (8), 1471–1494. doi: 10.1007/s00299-021-02687-4
Gong, Q., Yang, Z., Chen, E., Sun, G., He, S., Butt, H. I., et al. (2018). A phi-class glutathione s-transferase gene for verticillium wilt resistance in Gossypium arboreum identified in a genome-wide association study. Plant Cell Physiol. 59 (2), 275–289. doi: 10.1093/pcp/pcx180
Gu, Z., Liu, T., Ding, B., Li, F., Wang, Q., Qian, S., et al. (2017). Two lysin-motif receptor kinases, gh-LYK1 and gh-LYK2, contribute to resistance against verticillium wilt in upland cotton. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.02133
Guo, J., Cao, P., Yuan, L., Xia, G., Zhang, H., Li, J., et al. (2022). Revealing the contribution of GbPR10.5D1 to resistance against Verticillium dahliae and its regulation for structural defense and immune signaling. Plant Genome 15 (4), e20271–e20293. doi: 10.1002/tpg2.20271
Guo, W., Jin, L., Miao, Y., He, X., Hu, Q., Guo, K., et al. (2016). An ethylene response-related factor, GbERF1-like, from Gossypium barbadense improves resistance to Verticillium dahliae via activating lignin synthesis. Plant Mol. Biol. 91 (3), 305–318. doi: 10.1007/s11103-016-0467-6
Han, L. B., Li, Y. B., Wang, F. X., Wang, W. Y., Liu, J., Wu, J. H., et al. (2019). The cotton apoplastic protein CRR1 stabilizes chitinase 28 to facilitate defense against the fungal pathogen Verticillium dahliae. Plant Cell 31 (2), 520–536. doi: 10.1105/tpc.18.00390
He, X., Wang, T., Zhu, W., Wang, Y., Zhu, L. (2018a). GhHB12, a HD-ZIP I transcription factor, negatively regulates the cotton resistance to Verticillium dahliae. Int. J. Mol. Sci. 19 (12), 3997–4010. doi: 10.3390/ijms19123997
He, Y., Zhou, J., Shan, L., Meng, X. (2018c). Plant cell surface receptor-mediated signaling - a common theme amid diversity. J. Cell Sci. 131 (2), jcs209353. doi: 10.1242/jcs.209353
He, X., Zhu, L., Wassan, G. M., Wang, Y., Miao, Y., Shaban, M., et al. (2018b). GhJAZ2 attenuates cotton resistance to biotic stresses via the inhibition of the transcriptional activity of GhbHLH171. Mol. Plant Pathol. 19 (4), 896–908. doi: 10.1111/mpp.12575
Höfer, A. M., Harting, R., Aßmann, N. F., Gerke, J., Schmitt, K., Starke, J., et al. (2021). The velvet protein Vel1 controls initial plant root colonization and conidia formation for xylem distribution in verticillium wilt. PloS Genet. 17 (3), e1009434–e1009469. doi: 10.1371/journal.pgen.1009434
Hoppenau, C. E., Tran, V.-T., Kusch, H., Aßhauer, K. P., Landesfeind, M., Meinicke, P., et al. (2014). Verticillium dahliae VdTHI4, involved in thiazole biosynthesis, stress response and DNA repair functions, is required for vascular disease induction in tomato. Environ. Exp. Bot. 108, 14–22. doi: 10.1016/j.envexpbot.2013.12.015
Hu, Q., Min, L., Yang, X., Jin, S., Zhang, L., Li, Y., et al. (2018a). Laccase GhLac1 modulates broad-spectrum biotic stress tolerance via manipulating phenylpropanoid pathway and jasmonic acid synthesis. Plant Physiol. 176 (2), 1808–1823. doi: 10.1104/pp.17.01628
Hu, G., Wang, B., Jia, P., Wu, P., Lu, C., Xu, Y., et al. (2023). The cotton miR530-SAP6 module activated by systemic acquired resistance mediates plant defense against Verticillium dahliae. Plant Sci. 330, 111647–111659. doi: 10.1016/j.plantsci.2023.111647
Hu, Q., Xiao, S. H., Wang, X. R., Ao, C. W., Zhang, X. L., Zhu, L. F. (2021). GhWRKY1-like enhances cotton resistance to Verticillium dahliae via an increase in defense-induced lignification and s monolignol content. Plant Sci. 305, 110833–110843. doi: 10.1016/j.plantsci.2021.110833
Hu, Q., Zhu, L., Zhang, X., Guan, Q., Xiao, S., Min, L., et al. (2018b). GhCPK33 negatively regulates defense against Verticillium dahliae by phosphorylating GhOPR3. Plant Physiol. 178 (2), 876–889. doi: 10.1104/pp.18.00737
Huang, G., Huang, J.-Q., Chen, X.-Y., Zhu, Y.-X. (2021a). Recent advances and future perspectives in cotton research. Annu. Rev. Plant Biol. 72 (1), 437–462. doi: 10.1146/annurev-arplant-080720-113241
Huang, C. Y., Wang, H., Hu, P., Hamby, R., Jin, H. (2019). Small RNAs - big players in plant-microbe interactions. Cell Host Microbe 26 (2), 173–182. doi: 10.1016/j.chom.2019.07.021
Huang, W., Zhang, Y., Zhou, J., Wei, F., Feng, Z., Zhao, L., et al. (2021b). The respiratory burst oxidase homolog protein d (GhRbohD) positively regulates the cotton resistance to Verticillium dahliae. Int. J. Mol. Sci. 22 (23), 13041–13057. doi: 10.3390/ijms222313041
Ishida, K., Noutoshi, Y. (2022). The function of the plant cell wall in plant-microbe interactions. Plant Physiol. Biochem. 192, 273–284. doi: 10.1016/j.plaphy.2022.10.015
Jia, P., Tang, Y., Hu, G., Quan, Y., Chen, A., Zhong, N., et al. (2022). Cotton miR319b-targeted TCP4-like enhances plant defense against Verticillium dahliae by activating GhICS1 transcription expression. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.870882
Jiang, Y., Ding, P. (2023). Calcium signaling in plant immunity: a spatiotemporally controlled symphony. Trends Plant Sci. 28 (1), 74–89. doi: 10.1016/j.tplants.2022.11.001
Jin, L. R., Huang, W., Yang, N. N., Yin, H. C., Wan, P. (2021). Research progress on the pathogenesis and pathogenic related genes of Verticillium dahliae. J. Anhui Agric. Sci. 49 (10), 15–19. doi: 10.3969/j.issn.0517-6611.2021.10.005
Kamburova, V., Salakhutdinov, I., Abdurakhmonov, I. (2022) Cotton breeding in the view of abiotic and biotic stresses: challenges and perspectives Cotton doi: 10.5772/intechopen.104761
Karademir, E., Karademir, Ç., Ekinci, R., Baran, B., Sagir, A. (2012). Effect of Verticillium dahliae kleb. on cotton yield and fiber technological properties. Int. J. Plant Production 6 (4), 387–407. doi: 10.1111/j.1365-3180.2012.00940.x
Köster, P., DeFalco, T. A., Zipfel, C. (2022). Ca2+ signals in plant immunity. EMBO J. 41 (12), e110741–e110759. doi: 10.15252/embj.2022110741
Kun, M., Shuo, Y. (2020). Analysis of the occurrence and control of cotton main diseases and pests in China in recent years. Cotton Sci. 42 (3), 13–19. doi: 10.3969/j.issn.2095-3143.2020.03.002
Lenarčič, T., Albert, I., Böhm, H., Hodnik, V., Pirc, K., Zavec, A. B., et al. (2017). Eudicot plant-specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science 358 (6369), 1431–1434. doi: 10.1126/science.aan6874
Li, Z. K., Chen, B., Li, X. X., Wang, J. P., Zhang, Y., Wang, X. F., et al. (2019e). A newly identified cluster of glutathione s-transferase genes provides verticillium wilt resistance in cotton. Plant J. 98 (2), 213–227. doi: 10.1111/tpj.14206
Li, Y. B., Han, L. B., Wang, H. Y., Zhang, J., Sun, S. T., Feng, D. Q., et al. (2016). The thioredoxin GbNRX1 plays a crucial role in homeostasis of apoplastic reactive oxygen species in response to Verticillium dahliae infection in cotton. Plant Physiol. 170 (4), 2392–2406. doi: 10.1104/pp.15.01930
Li, C., He, Q. L., Zhang, F., Yu, J. W., Li, C., Zhao, T. L., et al. (2019a). Melatonin enhances cotton immunity to verticillium wilt via manipulating lignin and gossypol biosynthesis. Plant J. 100 (4), 784–800. doi: 10.1111/tpj.14477
Li, S., Huang, M., Di, Q., Ji, T., Wang, X., Wei, M., et al. (2015). The functions of a cucumber phospholipase d alpha gene (CsPLDα) in growth and tolerance to hyperosmotic stress. Plant Physiol. Biochem. 97, 175–186. doi: 10.1016/j.plaphy.2015.10.006
Li, C., Qin, J., Huang, Y., Shang, W., Chen, J., Klosterman, S. J., et al. (2023). Verticillium dahliae effector VdCE11 contributes to virulence by promoting accumulation and activity of the aspartic protease GhAP1 from cotton. Microbiol. Spectr. 11 (1), e0354722. doi: 10.1128/spectrum.03547-22
Li, P. T., Rashid, M. H. O., Chen, T. T., Lu, Q. W., Ge, Q., Gong, W. K., et al. (2019c). Transcriptomic and biochemical analysis of upland cotton (Gossypium hirsutum) and a chromosome segment substitution line from G. hirsutum × G. barbadense in response to Verticillium dahliae infection. BMC Plant Biol. 19 (1), 19–43. doi: 10.1186/s12870-018-1619-4
Li, T. G., Wang, B. L., Yin, C. M., Zhang, D. D., Wang, D., Song, J., et al. (2019d). The Gossypium hirsutum TIR-NBS-LRR gene GhDSC1 mediates resistance against verticillium wilt. Mol. Plant Pathol. 20 (6), 857–876. doi: 10.1111/mpp.12797
Li, T., Zhang, Q., Jiang, X., Li, R., Dhar, N. (2021). Cotton CC-NBS-LRR gene GbCNL130 confers resistance to verticillium wilt across different species. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.695691
Li, H., Zhang, S., Zhao, Y., Zhao, X., Xie, W., Guo, Y., et al. (2022). Identification and characterization of cinnamyl alcohol dehydrogenase encoding genes involved in lignin biosynthesis and resistance to Verticillium dahliae in upland cotton (Gossypium hirsutum l.). Fronts Plant Sci. 13. doi: 10.3389/fpls.2022.840397
Li, J. J., Zhou, L., Yin, C. M., Zhang, D. D., Klosterman, S. J., Wang, B. L., et al. (2019b). The Verticillium dahliae Sho1-MAPK pathway regulates melanin biosynthesis and is required for cotton infection. Environ. Microbiol. 21 (12), 4852–4874. doi: 10.1111/1462-2920.14846
Liu, S. Y., Chen, J. Y., Wang, J. L., Li, L., Xiao, H. L., Adam, S. M., et al. (2013). Molecular characterization and functional analysis of a specific secreted protein from highly virulent defoliating Verticillium dahliae. Gene 529 (2), 307–316. doi: 10.1016/j.gene.2013.06.089
Liu, N., Ma, X., Sun, Y., Hou, Y., Zhang, X., Li, F. (2017a). Necrotizing activity of Verticillium dahliae and Fusarium oxysporum f. sp. vasinfectum endopolygalacturonases in cotton. Plant Dis. 101 (7), 1128–1138. doi: 10.1094/pdis-05-16-0657-re
Liu, N., Sun, Y., Pei, Y., Zhang, X., Wang, P., Li, X., et al. (2018). A pectin methylesterase inhibitor enhances resistance to verticillium wilt. Plant Physiol. 176 (3), 2202–2220. doi: 10.1104/pp.17.01399
Liu, N., Zhang, X., Sun, Y., Wang, P., Li, X., Pei, Y., et al. (2017b). Molecular evidence for the involvement of a polygalacturonase-inhibiting protein, GhPGIP1, in enhanced resistance to verticillium and fusarium wilts in cotton. Sci. Rep. 7, 39840–39858. doi: 10.1038/srep39840
Long, L., Liu, J., Gao, Y., Xu, F.-C., Zhao, J.-R., Li, B., et al. (2019). Flavonoid accumulation in spontaneous cotton mutant results in red coloration and enhanced disease resistance. Plant Physiol. Biochem. 143, 40–49. doi: 10.1016/j.plaphy.2019.08.021
Long, L., Xu, F. C., Zhao, J. R., Li, B., Xu, L., Gao, W. (2020). GbMPK3 overexpression increases cotton sensitivity to Verticillium dahliae by regulating salicylic acid signaling. Plant Sci. 292, 110374–110383. doi: 10.1016/j.plantsci.2019.110374
Long, L., Zhao, J.-R., Xu, F.-C., Yang, W.-W., Liao, P., Gao, Y., et al. (2018). Silencing of GbANS reduces cotton resistance to Verticillium dahliae through decreased ROS scavenging during the pathogen invasion process. Plant Cell Tissue Organ Culture (PCTOC) 135 (2), 213–221. doi: 10.1007/s11240-018-1457-y
Lo Presti, L., Lanver, D., Schweizer, G., Tanaka, S., Liang, L., Tollot, M., et al. (2015). Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 66, 513–545. doi: 10.1146/annurev-arplant-043014-114623
Luo, X., Li, Z., Xiao, S., Ye, Z., Nie, X., Zhang, X., et al. (2021). Phosphate deficiency enhances cotton resistance to Verticillium dahliae through activating jasmonic acid biosynthesis and phenylpropanoid pathway. Plant Sci. 302, 110724–110734. doi: 10.1016/j.plantsci.2020.110724
Luo, X., Tian, T., Tan, X., Zheng, Y., Xie, C., Xu, Y., et al. (2020). VdNPS, a nonribosomal peptide synthetase, is involved in regulating virulence in Verticillium dahliae. Phytopathology 110 (8), 1398–1409. doi: 10.1094/phyto-02-20-0031-r
Luo, X., Xie, C., Dong, J., Yang, X. (2019). Comparative transcriptome analysis reveals regulatory networks and key genes of microsclerotia formation in the cotton vascular wilt pathogen. Fungal Genet. Biol. 126, 25–36. doi: 10.1016/j.fgb.2019.01.009
Lv, J., Liu, S., Zhang, X., Zhao, L., Zhang, T., Zhang, Z., et al. (2022a). VdERG2 was involved in ergosterol biosynthesis, nutritional differentiation and virulence of Verticillium dahliae. Curr. Genet. 69 (1), 25–40. doi: 10.1007/s00294-022-01257-9
Lv, J., Zhou, J., Chang, B., Zhang, Y., Feng, Z., Wei, F., et al. (2022b). Two metalloproteases VdM35-1 and VdASPF2 from Verticillium dahliae are required for fungal pathogenicity, stress adaptation, and activating immune response of host. Microbiol. Spectr. 10 (6), e0247722. doi: 10.1128/spectrum.02477-22
Ma, Q., Wang, N., Ma, L., Lu, J., Wang, H., Wang, C., et al. (2020). The cotton BEL1-like transcription factor GhBLH7-D06 negatively regulates the defense response against Verticillium dahliae. Int. J. Mol. Sci. 21 (19), 7126–7143. doi: 10.3390/ijms21197126
Man, M., Zhu, Y., Liu, L., Luo, L., Han, X., Qiu, L., et al. (2022). Defense mechanisms of cotton fusarium and verticillium wilt and comparison of pathogenic response in cotton and humans. Int. J. Mol. Sci. 23 (20), 12217–12239. doi: 10.3390/ijms232012217
Maryum, Z., Luqman, T., Nadeem, S., Khan, S., Wang, B., Ditta, A., et al. (2022). An overview of salinity stress, mechanism of salinity tolerance and strategies for its management in cotton. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.907937
Meng, J., Gao, H., Zhai, W., Shi, J., Zhang, M., Zhang, W., et al. (2018). Subtle regulation of cotton resistance to verticillium wilt mediated by MAPKK family members. Plant Sci. 272, 235–242. doi: 10.1016/j.plantsci.2018.05.003
Meyer, L., Dew, T. (2023) Cotton and wool outlook tables. Available at: https://www.ers.usda.gov/publications/pub-details/?pubid=105597.
Miao, Y., Chen, K., Deng, J., Zhang, L., Wang, W., Kong, J., et al. (2022). miR398b negatively regulates cotton immune responses to Verticillium dahliae via multiple targets. Crop J. 10 (4), 1026–1036. doi: 10.1016/j.cj.2021.12.010
Miao, Y., Xu, L., He, X., Zhang, L., Shaban, M., Zhang, X., et al. (2019). Suppression of tryptophan synthase activates cotton immunity by triggering cell death via promoting SA synthesis. Plant J. 98 (2), 329–345. doi: 10.1111/tpj.14222
Mielke, S., Gasperini, D. (2019). Interplay between plant cell walls and jasmonate production. Plant Cell Physiol. 60 (12), 2629–2637. doi: 10.1093/pcp/pcz119
Mittler, R., Zandalinas, S. I., Fichman, Y., Van Breusegem, F. (2022). Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 23 (10), 663–679. doi: 10.1038/s41580-022-00499-2
Mo, H. J., Sun, Y. X., Zhu, X. L., Wang, X. F., Zhang, Y., Yang, J., et al. (2016). Cotton s-adenosylmethionine decarboxylase-mediated spermine biosynthesis is required for salicylic acid- and leucine-correlated signaling in the defense response to Verticillium dahliae. Planta 243 (4), 1023–1039. doi: 10.1007/s00425-015-2463-5
Mo, S., Zhang, Y., Wang, X., Yang, J., Sun, Z., Zhang, D., et al. (2021). Cotton GhSSI2 isoforms from the stearoyl acyl carrier protein fatty acid desaturase family regulate verticillium wilt resistance. Mol. Plant Pathol. 22 (9), 1041–1056. doi: 10.1111/mpp.13093
Pantelides, I. S., Tjamos, S. E., Paplomatas, E. J. (2010). Ethylene perception via ETR1 is required in arabidopsis infection by Verticillium dahliae. Mol. Plant Pathol. 11 (2), 191–202. doi: 10.1111/j.1364-3703.2009.00592.x
Pei, Y., Li, X., Zhu, Y., Ge, X., Sun, Y., Liu, N., et al. (2019). GhABP19, a novel germin-like protein from Gossypium hirsutum, plays an important role in the regulation of resistance to verticillium and fusarium wilt pathogens. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00583
Phua, S. Y., De Smet, B., Remacle, C., Chan, K. X., Van Breusegem, F. (2021). Reactive oxygen species and organellar signaling. J. Exp. Bot. 72 (16), 5807–5824. doi: 10.1093/jxb/erab218
Qi, X., Li, X., Guo, H., Guo, N., Cheng, H. (2018). VdPLP, a patatin-like phospholipase in Verticillium dahliae, is involved in cell wall integrity and required for pathogenicity. Genes 9 (3), 162–179. doi: 10.3390/genes9030162
Qi, X., Su, X., Guo, H., Qi, J., Cheng, H. (2016). VdThit, a thiamine transport protein, is required for pathogenicity of the vascular pathogen Verticillium dahliae. Mol. Plant-Microbe Interact. 29 (7), 545–559. doi: 10.1094/mpmi-03-16-0057-r
Qi, J., Wang, J., Gong, Z., Zhou, J. M. (2017). Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol. 38, 92–100. doi: 10.1016/j.pbi.2017.04.022
Qin, T., Hao, W., Sun, R., Li, Y., Wang, Y., Wei, C., et al. (2020). Verticillium dahliae VdTHI20, involved in pyrimidine biosynthesis, is required for DNA repair functions and pathogenicity. Int. J. Biol. Sci. 21 (4), 1378–1397. doi: 10.3390/ijms21041378
Ramirez-Prado, J. S., Abulfaraj, A. A., Rayapuram, N., Benhamed, M., Hirt, H. (2018). Plant immunity: from signaling to epigenetic control of defense. Trends Plant Sci. 23 (9), 833–844. doi: 10.1016/j.tplants.2018.06.004
Ranga, A., Kak, V., Darvhankar, M. (2020). Genetic and molecular research of resistance to wilt in cotton: a concise review. Int. J. Curr. Microbiol. Appl. Sci. 9, 2410–2422. doi: 10.20546/ijcmas.2020.906.296
Rehman, L., Su, X., Li, X., Qi, X., Guo, H., Cheng, H. (2018). FreB is involved in the ferric metabolism and multiple pathogenicity-related traits of Verticillium dahliae. Curr. Genet. 64 (3), 645–659. doi: 10.1007/s00294-017-0780-x
Shaban, M., Khan, A. H., Noor, E., Malik, W., Ali, H. M. W., Shehzad, M., et al. (2021). A 13-lipoxygenase, GhLOX2, positively regulates cotton tolerance against Verticillium dahliae through JA-mediated pathway. Gene 796-797, 145797–145805. doi: 10.1016/j.gene.2021.145797
Shaban, M., Miao, Y., Ullah, A., Khan, A. Q., Menghwar, H., Khan, A. H., et al. (2018). Physiological and molecular mechanism of defense in cotton against Verticillium dahliae. Plant Physiol. Biochem. 125, 193–204. doi: 10.1016/j.plaphy.2018.02.011
Short, D. P., Sandoya, G., Vallad, G. E., Koike, S. T., Xiao, C. L., Wu, B. M., et al. (2015). Dynamics of Verticillium species microsclerotia in field soils in response to fumigation, cropping patterns, and flooding. Phytopathology 105 (5), 638–645. doi: 10.1094/phyto-09-14-0259-r
Snelders, N. C., Petti, G. C., van den Berg, G. C. M., Seidl, M. F., Thomma, B. (2021). An ancient antimicrobial protein co-opted by a fungal plant pathogen for in planta mycobiome manipulation. Proc. Natl. Acad. Sci. United States America 118 (49), e2110968118. doi: 10.1073/pnas.2110968118
Snelders, N. C., Rovenich, H., Petti, G. C., Rocafort, M., van den Berg, G. C. M., Vorholt, J. A., et al. (2020). Microbiome manipulation by a soil-borne fungal plant pathogen using effector proteins. Nat. Plants 6 (11), 1365–1374. doi: 10.1038/s41477-020-00799-5
Song, R., Li, J., Xie, C., Jian, W., Yang, X. (2020). An overview of the molecular genetics of plant resistance to the verticillium wilt pathogen Verticillium dahliae. Int. J. Mol. Sci. 21 (3), 1120–1136. doi: 10.3390/ijms21031120
Song, Y., Liu, L., Wang, Y., Valkenburg, D. J., Zhang, X., Zhu, L., et al. (2018). Transfer of tomato immune receptor Ve1 confers Ave1-dependent Verticillium resistance in tobacco and cotton. Plant Biotechnol. J. 16 (2), 638–648. doi: 10.1111/pbi.12804
Song, Y., Zhai, Y., Li, L., Yang, Z., Ge, X., Yang, Z., et al. (2021). BIN2 negatively regulates plant defense against Verticillium dahliae in arabidopsis and cotton. Plant Biotechnol. J. 19 (10), 2097–2112. doi: 10.1111/pbi.13640
Su, Y., Wang, G., Huang, Z., Hu, L., Fu, T., Wang, X. (2022). Silencing GhIAA43, a member of cotton AUX/IAA genes, enhances wilt resistance via activation of salicylic acid-mediated defenses. Plant Sci. 314, 111126–111136. doi: 10.1016/j.plantsci.2021.111126
Sun, L., Qin, J., Rong, W., Ni, H., Guo, H. S., Zhang, J. (2019). Cellophane surface-induced gene, VdCSIN1, regulates hyphopodium formation and pathogenesis via cAMP-mediated signalling in Verticillium dahliae. Mol. Plant Pathol. 20 (3), 323–333. doi: 10.1111/mpp.12756
Sun, L., Qin, J., Wu, X., Zhang, J., Zhang, J. (2022). TOUCH 3 and CALMODULIN 1/4/6 cooperate with calcium-dependent protein kinases to trigger calcium-dependent activation of CAM-BINDING PROTEIN 60-LIKE G and regulate fungal resistance in plants. Plant Cell 34 (10), 4088–4104. doi: 10.1093/plcell/koac209
Sun, L., Zhu, L., Xu, L., Yuan, D., Min, L., Zhang, X. (2014). Cotton cytochrome P450 CYP82D regulates systemic cell death by modulating the octadecanoid pathway. Nat. Communication 5, 5372–5384. doi: 10.1038/ncomms6372
Tang, C., Jin, X., Klosterman, S. J., Wang, Y. (2020a). Convergent and distinctive functions of transcription factors VdYap1, VdAtf1, and VdSkn7 in the regulation of nitrosative stress resistance, microsclerotia formation, and virulence in Verticillium dahliae. Mol. Plant Pathol. 21 (11), 1451–1466. doi: 10.1111/mpp.12988
Tang, C., Li, T., Klosterman, S. J., Tian, C., Wang, Y. (2020b). The bZIP transcription factor VdAtf1 regulates virulence by mediating nitrogen metabolism in Verticillium dahliae. New Phytol. 226 (5), 1461–1479. doi: 10.1111/nph.16481
Tang, C., Xiong, D., Fang, Y., Tian, C., Wang, Y. (2017). The two-component response regulator VdSkn7 plays key roles in microsclerotial development, stress resistance and virulence of Verticillium dahliae. Fungal Genet. And Biol. 108, 26–35. doi: 10.1016/j.fgb.2017.09.002
Tian, L., Huang, C. M., Zhang, D. D., Ran, L. I., Chen, J. Y., Sun, W. X., et al. (2021a). Extracellular superoxide dismutase VdSOD5 is required for virulence in Verticillium dahliae. J. Integr. Agric. 20 (7), 1858–1870. doi: 10.1016/S2095-3119(20)63353-6
Tian, J., Kong, Z. (2022). Live-cell imaging elaborating epidermal invasion and vascular proliferation/colonization strategy of Verticillium dahliae in host plants. Mol. Plant Pathol. 23 (6), 895–900. doi: 10.1111/mpp.13212
Tian, L., Li, J., Huang, C., Zhang, D., Xu, Y., Yang, X., et al. (2021b). Cu/Zn superoxide dismutase (VdSOD1) mediates reactive oxygen species detoxification and modulates virulence in Verticillium dahliae. Mol. Plant Pathol. 22 (9), 1092–1108. doi: 10.1111/mpp.13099
Tian, J., Pu, M., Chen, B., Wang, G., Li, C., Zhang, X., et al. (2022). Verticillium dahliae Asp1 regulates the transition from vegetative growth to asexual reproduction by modulating microtubule dynamic organization. Environ. Microbiol. 25 (3), 738–750. doi: 10.1111/1462-2920.16320
Tian, L., Wang, Y., Yu, J., Xiong, D., Zhao, H., Tian, C. (2016). The mitogen-activated protein kinase kinase VdPbs2 of Verticillium dahliae regulates microsclerotia formation, stress response, and plant infection. Front. In Microbiol. 7. doi: 10.3389/fmicb.2016.01532
Tian, L., Yu, J., Wang, Y., Tian, C. (2017). The C2H2 transcription factor VdMsn2 controls hyphal growth, microsclerotia formation, and virulence of Verticillium dahliae. Fungal Biol. 121 (12), 1001–1010. doi: 10.1016/j.funbio.2017.08.005
Triantafyllopoulou, A., Tzanaki, A., Balomenou, O. I., Jiménez-Díaz, R. M., Tzima, A., Paplomatas, E. (2022). Development of a robust, VdNEP gene-based molecular marker to differentiate between pathotypes of Verticillium dahliae. Plant Pathol. 71 (6), 1404–1416. doi: 10.1111/ppa.13559
Tzima, A. K., Paplomatas, E. J., Rauyaree, P., Ospina-Giraldo, M. D., Kang, S. (2011). VdSNF1, the sucrose nonfermenting protein kinase gene of Verticillium dahliae, is required for virulence and expression of genes involved in cell-wall degradation. Mol. Plant-Microbe Interact. 24 (1), 129–142. doi: 10.1094/mpmi-09-09-0217
Tzima, A. K., Paplomatas, E. J., Tsitsigiannis, D. I., Kang, S. (2012). The G protein β subunit controls virulence and multiple growth- and development-related traits in Verticillium dahliae. Fungal Genet. Biol. 49 (4), 271–283. doi: 10.1016/j.fgb.2012.02.005
Wagner, T. A., Liu, J., Puckhaber, L. S., Bell, A. A., Williams, H., Stipanovic, R. D. (2015). RNAi construct of a cytochrome P450 gene CYP82D109 blocks an early step in the biosynthesis of hemigossypolone and gossypol in transgenic cotton plants. Phytochemistry 115, 59–69. doi: 10.1016/j.phytochem.2015.02.016
Wang, J. Y., Cai, Y., Gou, J. Y., Mao, Y. B., Xu, Y. H., Jiang, W. H., et al. (2004). VdNEP, an elicitor from Verticillium dahliae, induces cotton plant wilting. Appl. Environ. Microbiol. 70 (8), 4989–4995. doi: 10.1128/AEM.70.8.4989-4995.2004
Wang, D., Chen, J. Y., Song, J., Li, J. J., Klosterman, S. J., Li, R., et al. (2021a). Cytotoxic function of xylanase VdXyn4 in the plant vascular wilt pathogen Verticillium dahliae. Plant Physiol. 187 (1), 409–429. doi: 10.1093/plphys/kiab274
Wang, H., Chen, B., Tian, J., Kong, Z. (2021c). Verticillium dahliae VdBre1 is required for cotton infection by modulating lipid metabolism and secondary metabolites. Environ. Microbiol. 23 (4), 1991–2003. doi: 10.1111/1462-2920.15319
Wang, Y., Deng, C., Tian, L., Xiong, D., Tian, C., Klosterman, S. J. (2018b). The transcription factor VdHapX controls iron homeostasis and is crucial for virulence in the vascular pathogen Verticillium dahliae. mSphere 3 (5), e00400–e00418. doi: 10.1128/mSphere.00400-18
Wang, T., Shaban, M., Shi, J., Wang, W., Liu, S., Nie, X., et al. (2022b). Attenuation of ethylene signaling increases cotton resistance to a defoliating strain of Verticillium dahliae. Crop J. 11 (1), 89–98. doi: 10.1016/j.cj.2022.05.008
Wang, J., Tian, L., Zhang, D. D., Short, D. P. G., Zhou, L., Song, S. S., et al. (2018a). SNARE-encoding genes VdSec22 and VdSso1 mediate protein secretion required for full virulence in Verticillium dahliae. Mol. Plant-Microbe Interact. 31 (6), 651–664. doi: 10.1094/mpmi-12-17-0289-r
Wang, G. M., Wang, X. F., Zhang, Y., Yang, J., Li, Z. K., Wu, L. Z., et al. (2021b). Dynamic characteristics and functional analysis provide new insights into long non-coding RNA responsive to Verticillium dahliae infection in Gossypium hirsutum. BMC Plant Biol. 21 (1), 68–81. doi: 10.1186/s12870-021-02835-8
Wang, S., Wu, X. M., Liu, C. H., Shang, J. Y., Gao, F., Guo, H. S. (2020c). Verticillium dahliae chromatin remodeling facilitates the DNA damage repair in response to plant ROS stress. PloS Pathog. 16 (4), e1008481–e1008503. doi: 10.1371/journal.ppat.1008481
Wang, G., Xu, J., Li, L., Guo, Z., Si, Q., Zhu, G., et al. (2020a). GbCYP86A1-1 from Gossypium barbadense positively regulates defence against Verticillium dahliae by cell wall modification and activation of immune pathways. Plant Biotechnol. J. 18 (1), 222–238. doi: 10.1111/pbi.13190
Wang, D., Zhang, D. D., Song, J., Li, J. J., Wang, J., Li, R., et al. (2022a). Verticillium dahliae CFEM proteins manipulate host immunity and differentially contribute to virulence. BMC Biol. 20 (1), 55–76. doi: 10.1186/s12915-022-01254-x
Wang, Y., Zhao, J., Chen, Q., Zheng, K., Deng, X., Gao, W., et al. (2023). Quantitative trait locus mapping and identification of candidate genes for resistance to verticillium wilt in four recombinant inbred line populations of Gossypium hirsutum. Plant Sci. 327, 111562–111572. doi: 10.1016/j.plantsci.2022.111562
Wang, P., Zhou, L., Jamieson, P., Zhang, L., Zhao, Z. X., Babilonia, K., et al. (2020b). The cotton wall-associated kinase GhWAK7A mediates responses to fungal wilt pathogens by complexing with the chitin sensory receptors. Plant Cell 32 (12), 3978–4001. doi: 10.1105/tpc.19.00950
Wasternack, C., Strnad, M. (2016). Jasmonate signaling in plant stress responses and development-active and inactive compounds. New Biotechnol. 33 (5 Pt B), 604–613. doi: 10.1016/j.nbt.2015.11.001
Wu, M., Li, Q., Xia, G., Zhang, Y., Wang, F. (2022). New insights into defense responses against Verticillium dahliae infection revealed by a quantitative proteomic analysis in Arabidopsis thaliana. Funct. Plant Biol. 49 (11), 980–994. doi: 10.1071/fp22006
Xiao, S., Hu, Q., Zhang, X., Si, H., Liu, S., Chen, L., et al. (2021). Orchestration of plant development and defense by indirect crosstalk of salicylic acid and brassinosteorid signaling via transcription factor GhTINY2. J. Exp. Bot. 72 (13), 4721–4743. doi: 10.1093/jxb/erab186
Xiao, S., Ming, Y., Hu, Q., Ye, Z., Si, H., Liu, S., et al. (2023). GhWRKY41 forms a positive feedback regulation loop and increases cotton defense response against Verticillium dahliae by regulating phenylpropanoid metabolism. Plant Biotechnol. J. doi: 10.1111/pbi.14008
Xin, A., Herburger, K. (2021). Precursor biosynthesis regulation of lignin, suberin and cutin. Protoplasma 258 (6), 1171–1178. doi: 10.1007/s00709-021-01676-4
Xiong, X., Sun, S., Li, Y., Zhang, X., Sun, J., Xue, F. (2019). The cotton WRKY transcription factor GhWRKY70 negatively regulates the defense response against Verticillium dahliae. Crop J. 7 (3), 393–402. doi: 10.1016/j.cj.2018.10.005
Xiong, X. P., Sun, S. C., Zhang, X. Y., Li, Y. J., Liu, F., Zhu, Q. H., et al. (2020). GhWRKY70D13 regulates resistance to Verticillium dahliae in cotton through the ethylene and jasmonic acid signaling pathways. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00069
Xiong, X. P., Sun, S. C., Zhu, Q. H., Zhang, X. Y., Li, Y. J., Liu, F., et al. (2021a). The cotton lignin biosynthetic gene Gh4CL30 regulates lignification and phenolic content and contributes to verticillium wilt resistance. Mol. Plant Microbe Interact. 34 (3), 240–254. doi: 10.1094/mpmi-03-20-0071-r
Xiong, X. P., Sun, S. C., Zhu, Q. H., Zhang, X. Y., Liu, F., Li, Y. J., et al. (2021b). Transcriptome analysis and RNA interference reveal GhGDH2 regulating cotton resistance to verticillium wilt by JA and SA signaling pathways. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.654676
Xu, J., Wang, G., Wang, J., Li, Y., Tian, L., Wang, X., et al. (2017). The lysin motif-containing proteins, Lyp1, Lyk7 and LysMe3, play important roles in chitin perception and defense against Verticillium dahliae in cotton. BMC Plant Biol. 17 (1), 148–166. doi: 10.1186/s12870-017-1096-1
Xu, J., Xu, X., Tian, L., Wang, G., Zhang, X., Wang, X., et al. (2016). Discovery and identification of candidate genes from the chitinase gene family for Verticillium dahliae resistance in cotton. Sci. Rep. 6, 29022–29044. doi: 10.1038/srep29022
Xu, L., Zhu, L. F., Tu, L. L., Liu, L. L., Yuan, D. J., Jin, L., et al. (2011). Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-seq-dependent transcriptional analysis and histochemistry. J. Exp. Bot. 62 (15), 5607–5621. doi: 10.1093/jxb/err245
Yadav, V., Wang, Z. Y., Wei, C. H., Amo, A., Ahmed, B., Yang, X. Z., et al. (2020). Phenylpropanoid pathway engineering: an emerging approach towards plant defense. Pathogens 9 (4), 312–337. doi: 10.3390/pathogens9040312
Yan, Z., Xingfen, W., Wei, R., Jun, Y., Zhiying, M. (2016). Island cotton enhanced disease susceptibility 1 gene encoding a lipase-like protein plays a crucial role in response to Verticillium dahliae by regulating the SA level and H2O2 accumulation. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01830
Yang, Y., Chen, T., Ling, X., Ma, Z. (2017). Gbvdr6, a gene encoding a receptor-like protein of cotton (Gossypium barbadense), confers resistance to verticillium wilt in arabidopsis and upland cotton. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.02272
Yang, Z., Gao, C., Zhang, Y., Yan, Q., Hu, W., Yang, L., et al. (2022). Recent progression and future perspectives in cotton genomic breeding. J. Integr. Plant Biol. 65 (2), 548–649. doi: 10.1111/jipb.13388
Yang, C. L., Liang, S., Wang, H. Y., Han, L. B., Wang, F. X., Cheng, H. Q., et al. (2015). Cotton major latex protein 28 functions as a positive regulator of the ethylene responsive factor 6 in defense against Verticillium dahliae. Mol. Plant 8 (3), 399–411. doi: 10.1016/j.molp.2014.11.023
Yang, J., Ma, Q., Zhang, Y., Wang, X., Zhang, G., Ma, Z. (2016). Molecular cloning and functional analysis of GbRVd, a gene in Gossypium barbadense that plays an important role in conferring resistance to verticillium wilt. Gene 575 (2 Pt 3), 687–694. doi: 10.1016/j.gene.2015.09.046
Yang, J. R., Shang, H. S., Gao, L. Q. (2004). The effect of soil habitat factors on survival of microsclerotia of Verticillium dahliae of cotton. Acta Phytopathol. Sin. 34 (2), 180–183. doi: 10.3321/j.issn:0412-0914.2004.02.014
Yang, J., Xie, M., Wang, X., Wang, G., Zhang, Y., Li, Z., et al. (2021). Identification of cell wall-associated kinases as important regulators involved in Gossypium hirsutum resistance to Verticillium dahliae. BMC Plant Biol. 21 (1), 220–236. doi: 10.1186/s12870-021-02992-w
Yi, F., An, G., Song, A., Cheng, K., Liu, J., Wang, C., et al. (2023). Strigolactones positively regulate verticillium wilt resistance in cotton via crosstalk with other hormones. Plant Physiol. doi: 10.1093/plphys/kiad053
Yu, J., Li, T., Tian, L., Tang, C., Klosterman, S. J., Tian, C., et al. (2019). Two Verticillium dahliae MAPKKKs, VdSsk2 and VdSte11, have distinct roles in pathogenicity, microsclerotial formation, and stress adaptation. mSphere 4 (4), e00426–e00419. doi: 10.1128/mSphere.00426-19
Zhang, D. D., Dai, X. F., Klosterman, S. J., Subbarao, K. V., Chen, J. Y. (2022b). The secretome of Verticillium dahliae in collusion with plant defence responses modulates verticillium wilt symptoms. Biol. Rev. Cambridge Philos. Soc. 97 (5), 1810–1822. doi: 10.1111/brv.12863
Zhang, S., Dong, L., Zhang, X., Fu, X., Zhao, L., Wu, L., et al. (2023b). The transcription factor GhWRKY70 from Gossypium hirsutum enhances resistance to verticillium wilt via the jasmonic acid pathway. BMC Plant Biol. 23 (1), 141–156. doi: 10.1186/s12870-023-04141-x
Zhang, X., Feng, Z., Zhao, L., Liu, S., Wei, F., Shi, Y., et al. (2020). Succinate dehydrogenase SDH1-1 positively regulates cotton resistance to Verticillium dahliae through a salicylic acid pathway. J. Cotton Res. 3 (1), 12–24. doi: 10.1186/s42397-020-00052-6
Zhang, Y., Gao, Y., Liang, Y., Dong, Y., Yang, X., Qiu, D. (2019c). Verticillium dahliae PevD1, an alt a 1-like protein, targets cotton PR5-like protein and promotes fungal infection. J. Exp. Bot. 70 (2), 613–626. doi: 10.1093/jxb/ery351
Zhang, Y., Gao, Y., Wang, H. L., Kan, C., Li, Z., Yang, X., et al. (2021). Verticillium dahliae secretory effector PevD1 induces leaf senescence by promoting ORE1-mediated ethylene biosynthesis. Mol. Plant 14 (11), 1901–1917. doi: 10.1016/j.molp.2021.07.014
Zhang, W. Q., Gui, Y. J., Short, D. P. G., Li, T. G., Zhang, D. D., Zhou, L., et al. (2018). Verticillium dahliae transcription factor VdFTF1 regulates the expression of multiple secreted virulence factors and is required for full virulence in cotton. Mol. Plant Pathol. 19 (4), 841–857. doi: 10.1111/mpp.12569
Zhang, Y., Jin, Y., Gong, Q., Li, Z., Zhao, L., Han, X., et al. (2019d). Mechanismal analysis of resistance to Verticillium dahliae in upland cotton conferred by overexpression of RPL18A-6 (Ribosomal protein L18A-6). Ind. Crops Products 141, 111742–111753. doi: 10.1016/j.indcrop.2019.111742
Zhang, J., Jin, X., Wang, Y., Zhang, B., Liu, T. (2022c). A cytochrome P450 monooxygenase in nondefoliating strain of Verticillium dahliae manipulates virulence via scavenging reactive oxygen species. Phytopathology 112 (8), 1723–1729. doi: 10.1094/phyto-08-21-0318-r
Zhang, Y. L., Li, Z. F., Feng, Z. L., Feng, H. J., Shi, Y. Q., Zhao, L. H., et al. (2016b). Functional analysis of the pathogenicity-related gene VdPR1 in the vascular wilt fungus Verticillium dahliae. PloS One 11 (11), e0166000. doi: 10.1371/journal.pone.0166000
Zhang, Y. L., Li, Z. F., Feng, Z. L., Feng, H. J., Zhao, L. H., Shi, Y. Q., et al. (2015). Isolation and functional analysis of the pathogenicity-related gene VdPR3 from Verticillium dahliae on cotton. Curr. Genet. 61 (4), 555–566. doi: 10.1007/s00294-015-0476-z
Zhang, B. S., Li, Y. C., Guo, H. S., Zhao, J. H. (2022a). Verticillium dahliae secretes small RNA to target host mir157d and retard plant floral transition during infection. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.847086
Zhang, L., Liu, J., Cheng, J., Sun, Q., Zhang, Y., Liu, J., et al. (2022d). Cotton long non-coding RNAs regulate cell wall defense genes and strengthen resistance to verticillium wilt. Plant Physiol. 189 (1), 264–284. doi: 10.1093/plphys/kiac041
Zhang, J., Sanogo, S., Flynn, R., Baral, J. B., Bajaj, S., Hughs, S. E., et al. (2012). Germplasm evaluation and transfer of verticillium wilt resistance from pima (Gossypium barbadense) to upland cotton (G. hirsutum). Euphytica 2), 187. doi: 10.1007/s10681-011-0549-0
Zhang, D. D., Wang, X. Y., Chen, J. Y., Kong, Z. Q., Gui, Y. J., Li, N. Y., et al. (2016a). Identification and characterization of a pathogenicity-related gene VdCYP1 from Verticillium dahliae. Sci. Rep. 6, 27979–27991. doi: 10.1038/srep27979
Zhang, Z. N., Wang, P., Luo, X. L., Yang, C. L., Tang, Y., Wang, Z. A., et al.(2019). Cotton plant defence against a fungal pathogen is enhanced by expanding BLADE-ON-PETIOLE1 expression beyond lateral-organ boundaries. Commun. Biol. 2), 238–253. doi: 10.1038/s42003-019-0468-5
Zhang, Y., Wang, X. F., Rong, W., Yang, J., Li, Z. K., Wu, L. Q., et al. (2017). Histochemical analyses reveal that stronger intrinsic defenses in Gossypium barbadense than in G. hirsutum are associated with resistance to Verticillium dahliae. Mol. Plant-Microbe Interact. 30 (12), 984–996. doi: 10.1094/mpmi-03-17-0067-r
Zhang, D. D., Wang, J., Wang, D., Kong, Z. Q., Zhou, L., Zhang, G. Y., et al. (2019a). Population genomics demystifies the defoliation phenotype in the plant pathogen Verticillium dahliae. New Phytol. 222 (2), 1012–1029. doi: 10.1111/nph.15672
Zhang, X., Wang, L., Xu, X., Cai, C., Guo, W. (2014). Genome-wide identification of mitogen-activated protein kinase gene family in Gossypium raimondii and the function of their corresponding orthologs in tetraploid cultivated cotton. BMC Plant Biol. 14, 345–362. doi: 10.1186/s12870-014-0345-9
Zhang, Y., Wu, L. Z., Wang, X. F., Chen, B., Zhao, J., Cui, J., et al. (2019e). The cotton laccase gene GhLAC15 enhances verticillium wilt resistance via an increase in defence-induced lignification and lignin components in the cell walls of plants. Mol. Plant Pathol. 20 (3), 309–322. doi: 10.1111/mpp.12755
Zhang, L., Wu, Y., Yu, Y., Zhang, Y., Wei, F., Zhu, Q. H., et al. (2023a). Acetylation of GhCaM7 enhances cotton resistance to Verticillium dahliae. Plant J. doi: 10.1111/tpj.16200
Zhang, M., Zhang, S. (2022). Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 64 (2), 301–341. doi: 10.1111/jipb.13215
Zhang, J., Zhang, Y., Yang, J., Kang, L., Elo, R. A., Zhou, H., et al. (2019b). The α-1,6-mannosyltransferase VdOCH1 plays a major role in microsclerotium formation and virulence in the soil-borne pathogen Verticillium dahliae. Fungal Biol. 123 (7), 539–546. doi: 10.1016/j.funbio.2019.05.007
Zhang, Y., Zhou, J., Zhao, L., Feng, Z., Wei, F., Bai, H., et al. (2022e). A review of the pathogenicity mechanism of Verticillium dahliae in cotton. J. Cotton Res. 5 (1), 3–16. doi: 10.1186/s42397-021-00111-6
Zhao, J., Chen, Q., Zhou, S., Sun, Y., Li, X., Li, Y. (2020). H2Bub1 regulates rbohD-dependent hydrogen peroxide signal pathway in the defense responses to Verticillium dahliae toxins. Plant Physiol. 182 (1), 640–657. doi: 10.1104/pp.19.00913
Zhao, J., Sun, Y., Li, X., Li, Y. (2022). CYSTEINE-RICH RECEPTOR-LIKE KINASE5 (CRK5) and CRK22 regulate the response to Verticillium dahliae toxins. Plant Physiol. 190 (1), 714–731. doi: 10.1093/plphys/kiac277
Zhao, J. H., Zhang, T., Liu, Q. Y., Guo, H. S. (2021). Trans-kingdom RNAs and their fates in recipient cells: advances, utilization, and perspectives. Plant Commun. 2 (2), 100167–100176. doi: 10.1016/j.xplc.2021.100167
Zhao, P., Zhao, Y. L., Jin, Y., Zhang, T., Guo, H. S. (2014). Colonization process of Arabidopsis thaliana roots by a green fluorescent protein-tagged isolate of Verticillium dahliae. Protein Cell 5 (2), 94–98. doi: 10.1007/s13238-013-0009-9
Zhao, Y. L., Zhou, T. T., Guo, H. S. (2016). Hyphopodium-specific VdNoxB/VdPls1-dependent ROS-Ca2+ signaling is required for plant infection by Verticillium dahliae. PloS Pathog. 12 (7), e1005793–e1005816. doi: 10.1371/journal.ppat.1005793
Zhou, Y., Sun, L., Wassan, G. M., He, X., Shaban, M., Zhang, L., et al. (2019). GbSOBIR1 confers verticillium wilt resistance by phosphorylating the transcriptional factor GbbHLH171 in Gossypium barbadense. Plant Biotechnol. J. 17 (1), 152–163. doi: 10.1111/pbi.12954
Zhou, J., Wu, Y., Zhang, X., Zhao, L., Feng, Z., Wei, F., et al. (2022). MPK homolog GhNTF6 was involved in cotton against verticillium wilt by interacted with VdEPG1. Int. J. Biol. Macromolecules 195, 456–465. doi: 10.1016/j.ijbiomac.2021.12.037
Zhou, T. T., Zhao, Y. L., Guo, H. S. (2017). Secretory proteins are delivered to the septin-organized penetration interface during root infection by Verticillium dahliae. PloS Pathog. 13 (3), e1006275–e1006275. doi: 10.1371/journal.ppat.1006275
Zhu, Y. T., Hu, X. Q., Wang, P., Gao, L. Y., Pei, Y. K., Ge, Z. Y., et al. (2021b). GhPLP2 positively regulates cotton resistance to verticillium wilt by modulating fatty acid accumulation and jasmonic acid signaling pathway. Front. Plant Sci. 12 (2458). doi: 10.3389/fpls.2021.749630
Zhu, Y., Hu, X., Wang, P., Wang, H., Ge, X., Li, F., et al. (2022b). GhODO1, an R2R3-type MYB transcription factor, positively regulates cotton resistance to Verticillium dahliae via the lignin biosynthesis and jasmonic acid signaling pathway. Int. J. Biol. Macromolecules 201, 580–591. doi: 10.1016/j.ijbiomac.2022.01.120
Zhu, Y., Hu, X., Wang, P., Wang, H., Ge, X., Li, F., et al. (2022c). The phospholipase d gene GhPLDδ confers resistance to Verticillium dahliae and improves tolerance to salt stress. Plant Sci. 321, 111322–111336. doi: 10.1016/j.plantsci.2022.111322
Zhu, C., Liu, J. H., Zhao, J. H., Liu, T., Chen, Y. Y., Wang, C. H., et al. (2022a). A fungal effector suppresses the nuclear export of AGO1-miRNA complex to promote infection in plants. Proc. Natl. Acad. Sci. United States America 119 (12), e2114583119. doi: 10.1073/pnas.2114583119
Keywords: cotton, Verticillium wilt, Verticillium dahliae, pathogenic mechanism, resistance mechanism
Citation: Zhu Y, Zhao M, Li T, Wang L, Liao C, Liu D, Zhang H, Zhao Y, Liu L, Ge X and Li B (2023) Interactions between Verticillium dahliae and cotton: pathogenic mechanism and cotton resistance mechanism to Verticillium wilt. Front. Plant Sci. 14:1174281. doi: 10.3389/fpls.2023.1174281
Received: 26 February 2023; Accepted: 28 March 2023;
Published: 21 April 2023.
Edited by:
Tika Adhikari, North Carolina State University, United StatesReviewed by:
Tingli Liu, Nanjing Xiaozhuang University, ChinaKunal Singh, Institute of Himalayan Bioresource Technology (CSIR), India
Copyright © 2023 Zhu, Zhao, Li, Wang, Liao, Liu, Zhang, Zhao, Liu, Ge and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yutao Zhu, enl0MTc0OTRAMTYzLmNvbQ==; Bingbing Li, bGliaW5nYmluZ0BobmNqLmVkdS5jbg==
 Yutao Zhu
Yutao Zhu Mei Zhao1
Mei Zhao1 Chunli Liao
Chunli Liao Huamin Zhang
Huamin Zhang Yanpeng Zhao
Yanpeng Zhao Xiaoyang Ge
Xiaoyang Ge