
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 23 March 2023
Sec. Plant Abiotic Stress
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1151722
This article is part of the Research Topic The Potential Role of Melatonin in the Regulation of Abiotic Stress in Plants View all 10 articles
 Faisal Zulfiqar1*
Faisal Zulfiqar1* Anam Moosa2*
Anam Moosa2* Anastasios Darras3
Anastasios Darras3 Muhammad Nafees1
Muhammad Nafees1 Antonio Ferrante4
Antonio Ferrante4 Kadambot H. M. Siddique5*
Kadambot H. M. Siddique5*Introduction: Melatonin (MLT) is a bioactive molecule involved in the physiological functioning of plants. Reports related to preharvest applications of melatonin on the postharvest performance of cut flowers are not available in the literature.
Materials & methods: This study evaluated the effects of different concentrations of exogenous MLT [0 mM (MT0), 0.5 mM (MT1), 0.7 mM (MT2), 1 mM (MT3)] applied preharvest on the physiological characteristics and postharvest performance of cut tuberose, a globally demanded cut flower.
Results & discussion: The results revealed that all treatments increased postharvest vase life by up to 4 d. The MT1, MT2, and MT3 treatments increased total soluble proteins (TSP) by 25%, 41%, and 17%, soluble sugars (SS) by 21%, 36%, and 33%, an+d postharvest catalase (CAT) activity by 52%, 66%, and 70%, respectively. Malondialdehyde (MDA) and hydrogen peroxide (H2O2) decreased in all preharvest treatments by up to 23% and 56%, respectively. Proline concentration decreased in all treatments, particularly MT3 (38%). These findings suggest that preharvest MLT treatment is a promising strategy for improving the postharvest quality of cut tuberose.
Tuberose (Asparagaceae) is a herbaceous perennial tropical and subtropical ornamental geophyte native to Mexico. Its tubular, sweet-scented white flowers are economically important as cut flowers, fragrance, and aromatic oil. The inflorescence of tuberose has a spike ranging from 90–120 cm, arranged in single or paired, waxy, highly fragrant flowers (Dole and Wilkins, 2005) that can be harvested for commercial purposes or used in landscape designs.
The vase life of cut flowers is a critical quality influencing profit margins for growers in national and international markets (Zulfiqar and Ashraf, 2022). After harvest, the metabolic activities of flowers remain active in cells, performing crucial processes using stored substrates in the tissues (Jhanji et al., 2023). Enhanced vase life and delayed senescence can be attained by maintaining carbohydrate levels and water absorption and ameliorating oxidative stress produced by excessive reactive oxygen species (ROS) generation (Zulfiqar and Ashraf, 2022).
Techniques for delaying the senescence of cut flowers can significantly increase their market potential since postharvest longevity is a crucial factor for cut flower value (Olsen et al., 2015). Various postharvest treatments using growth regulators, sugars, signaling molecules, and biostimulants can inhibit postharvest senescence and increase vase life (Zulfiqar et al., 2020). However, few studies have focused on preharvest applications of these substances to enhance the postharvest performance of cut flowers. Recently, preharvest applications of biostimulants and potassium enhanced postharvest performance of gladiolus and statice cut flowers by altering postharvest physiological conditions and mitigating oxidative stress during senescence (Zulfiqar et al., 2020; Khandan-Mirkohi et al., 2021; Zulfiqar and Ashraf, 2022).
Melatonin (MLT; N-acetyl-5-methoxytrytamine) is a multi-regulatory pleiotropic molecule involved in various physiological and cellular functions in response to biotic and abiotic stresses (Zhang et al., 2018; Arnao et al., 2022). Due to its antioxidant impact, MLT can prevent oxidative stress in plants (Altaf et al., 2021a; Altaf et al., 2021b; Altaf et al., 2022). Treatments with MLT extended the postharvest quality and shelf life of various horticultural products (Luo et al., 2020; Wang et al., 2020; Lin et al., 2022) by regulating gene expression and inducing antioxidant enzyme production (Zheng et al., 2019; Aghdam et al., 2021). However, MLT application for improving floricultural products is in its infancy. A recent study on cut carnations (Dianthus caryophyllus L) evaluated different MLT concentrations (0.01, 0.1, and 1 mM) added to the vase solution (Lezoul et al., 2022). The optimum concentration (0.1 mM) decreased senescence and increased vase life by up to 10 d compared to the untreated controls. The authors found that postharvest MLT treatments improved water relations, lowered metabolic rate, and maintained membrane stability due to antioxidant activity (Lezoul et al., 2022).
To date, no studies have investigated the effects of preharvest MLT applications on growth, vase life traits, and oxidative stress-related characteristics in tuberose plants. Therefore, we hypothesized that exogenous MLT improves postharvest performance and delays senescence in cut tuberose. We assessed the effect of different concentrations of foliar MLT applications on the growth and ornamental traits of tuberose plants and the association between photosynthesis and postharvest flower longevity and enzyme activities that reduce oxidative stress during senescence.
An outdoor pot trial was established in the summer of 2022 at the Floriculture Research Area of the Islamia University of Bahawalpur, Pakistan (lat. 29° 23’ 44.5956’’ N, long.71° 41’ 0.0024” E). The desert region of Bahawalpur is in the subtropical zone, associated with hot summers (March–August) and mild winters (December–February). The physio-chemical traits of the experimental soil were: sandy clay loam (sand 45%, silt 24%, clay 31%), pH 7.4, 2.78 dSm–1 electrical conductivity, and 4.04 cmolc kg−1 cation exchange capacity. Soil nutrients were: nitrogen (N), 79 g kg–1 soil; phosphorus (P), 9.03 g kg–1 soil; potassium (K), 152.54 g kg–1 soil. The soil was air-dried, ground, and sieved (2 mm pore size) ahead of filling 3 L earthen pots (19 cm and 13 cm top and base diameters, respectively).
Healthy, uniform tuberose bulbs of cv. Single (21–23 mm diameter) were acquired from a local supplier in Lahore, Pakistan. One tuberose bulb was planted per pot. There were ten replicate bulbs for each of the four treatments and four replications (total 160 plants). Basal N, P, and K fertilizers (6 g pot–1) were applied manually using 46% urea, 50% muriate of potash (Fauji Fertilizer Company Limited, Pakistan), and 18% single super phosphate (Safi Chemicals and Fertilizer (PVT) Limited, Multan, Pakistan). Second and third applications of these fertilizers at 25 d and 40 d after planting. The experiment had a completely randomized design with four treatments: (1) distilled water used as the control (MT0), (2) 0.05 mM MLT (MT1), (3) 0.07 mM MLT (MT2), and (4) 1 mM MLT (MT3). The treatments were applied in the middle of the growing cycle and 5 d before inflorescence harvest by manually spraying the leaves until run-off. Before each foliar spray application, the top of each pot was concealed with polyethylene sheeting to avoid contamination. Watering was done manually every four days until harvest.
Leaf gas exchange traits [net CO2 assimilation (As), and transpiration (E)] were measured at the onset of the flowering bud occurrence stage between 7.00 am and 8.00 am on three fully expanded leaf blades using an infrared gas analyzer (LI-COR 6400, LI-COR, Lincoln, NE, USA) at 400 μmol m−2 s−1 CO2 and flow rate of 300 μmol m−2 s−1 on eight plants per treatment. At the same time, chlorophyll SPAD values were recorded on the lower, middle, and tip parts of four fully expanded tuberose plant leaves.
At the initiation of the inflorescence maturity stage, uniform size and quality inflorescences were cut manually using a sterilized knife in the early morning (7:00 am to 8:00 am), placed vertically in a bucket half-filled with distilled water and transferred to the laboratory within 30 min. In laboratory, the inflorescences were re-cut at 85 cm length under running distilled water to revert vascular system blockage and air emboli. Individual inflorescences were placed into 200 mL glass vases containing deionized distilled water. The vases were covered with aluminum foil to reduce vase water evaporation and placed on laboratory bench at 26 ± 3°C, 65 ± 3% relative humidity, 12 h light period provided by white fluorescent lamps, and 12 h dark period. Ten cut inflorescences per treatment were kept for vase life determination. Vase life expiration was calculated as the number of days from harvest until the flower petals wilted and lost their visual aesthetic by color change and/or loss of turgidity. Data were documented daily for 15 d.
The SS content (g kg–1) in 0.5 g tuberose leaves was measured 5 d before inflorescence harvest following the methodology of Frohlich and Kutscherah (1995), with absorbance measured at 620 nm. The TSP content (g kg–1; fresh weight basis) of tuberose leaves was determined following the methodology of Bradford (1976).
Cut flower florets (0.5 g) of the inflorescence were taken on day 5 postharvest to assess H2O2 and MDA contents following the methodologies of Patterson et al. (1984) and Hodges et al. (1999), respectively.
Fresh floret samples (0.5 g) were collected from the inflorescence on day 5 postharvest to assess SOD and CAT activities in stored supernatant following the methodologies of van Rossum et al. (1997) and Chance and Maehly (1955), respectively.
The ninhydrin-oriented method was used to measure leaf proline concentration (Bates et al., 1973), with absorbance read at 520 nm.
Experiments were conducted in a CRD with MLT treatments as the only factor and were replicated four times. Data were subjected to one-way ANOVA using SPSS v. 21 (SPSS Inc., Chicago, IL, USA). Comparisons between treatment means were carried out using the LSD multiple range test at P = 0.05. Linear regression analysis (y = ax+b) was performed in Sigmaplot 10 (Systat software Inc. USA) to highlight the dynamic trends in MLT effectiveness.
MLT did not affect leaf gas exchange and chlorophyll content. All differences between treatment means were not significant at P = 0.05 (Figure 1). Slight increases in As and E values were recorded compared to the untreated control plants. For example, plants treated with MT2 had As value of 4.28 μmol m-2. sec, whereas the control plants had 3.03 μmol m-2. sec (Figure 1A). Likewise, E values of the MT2 treated plants averaged at 0.97 mmol m-2. sec, whereas the control plants averaged at 0.68 mmol m-2. sec (Figure 1B). SPAD values of the MT2 treated plants reached 14.99, whereas the untreated control plants 12.41 (Figure 1C).
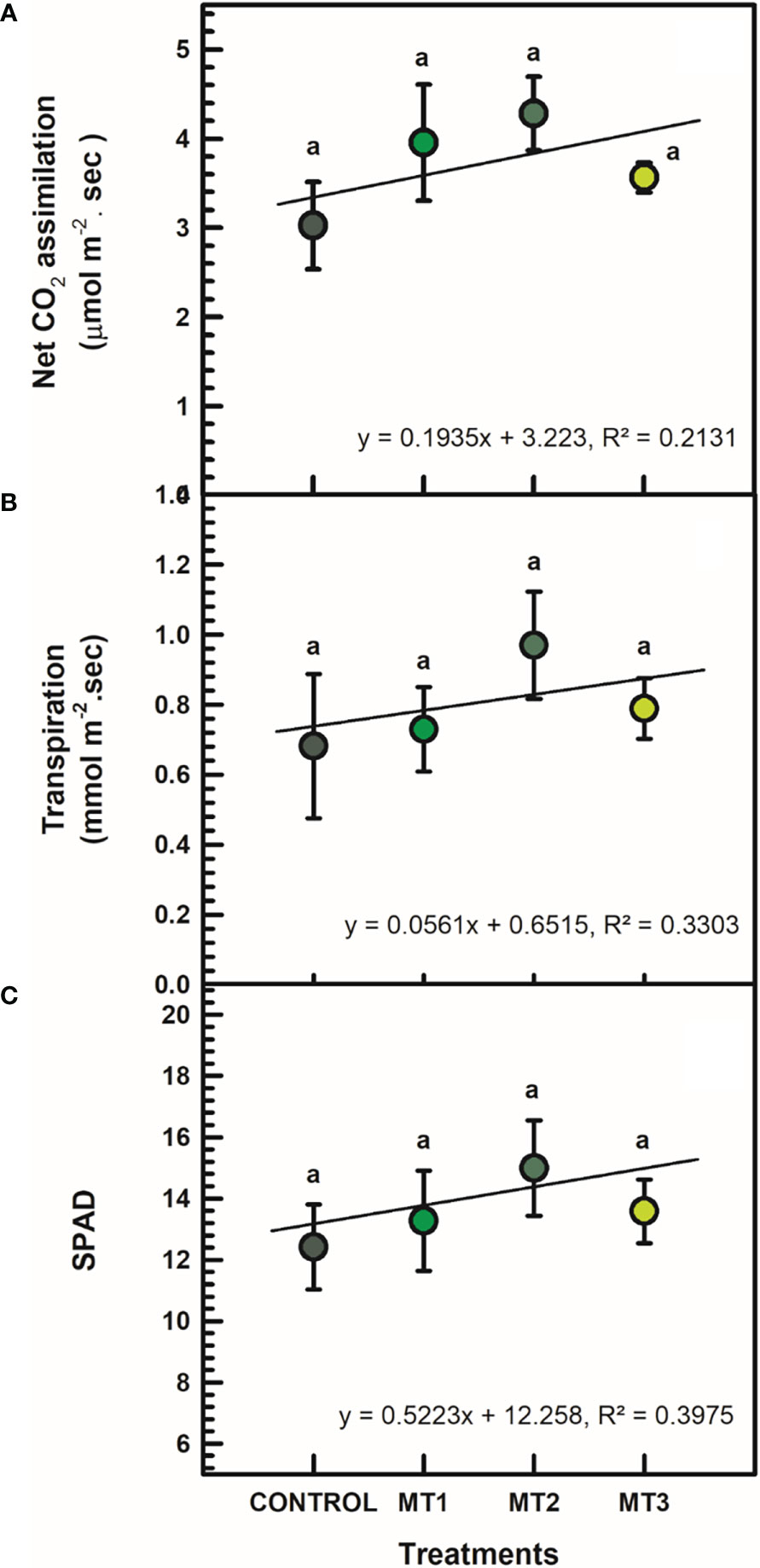
Figure 1 Net CO2 assimilation (A; μmol m-2 sec), transpiration (B; mmol m-2 sec), and SPAD (C) of tuberose plants treated with preharvest foliar applications of distilled water (control; MT0) and three concentrations of melatonin (MLT): 0.05 mM (MT1), 0.07 mM (MT2), and 1 mM (MT3). Data are means ± SE (n = 40). Different letters above the bars indicate significant differences according to the LSD test at P = 0.05.
Vase life increased only in MT3 treated plants (Figure 2). Plants treated with MT3 produced inflorescences with the longest VL of 9.6 d (increase by 41%), compared to the 5.6 d recorded for the control inflorescences (Figure 2).
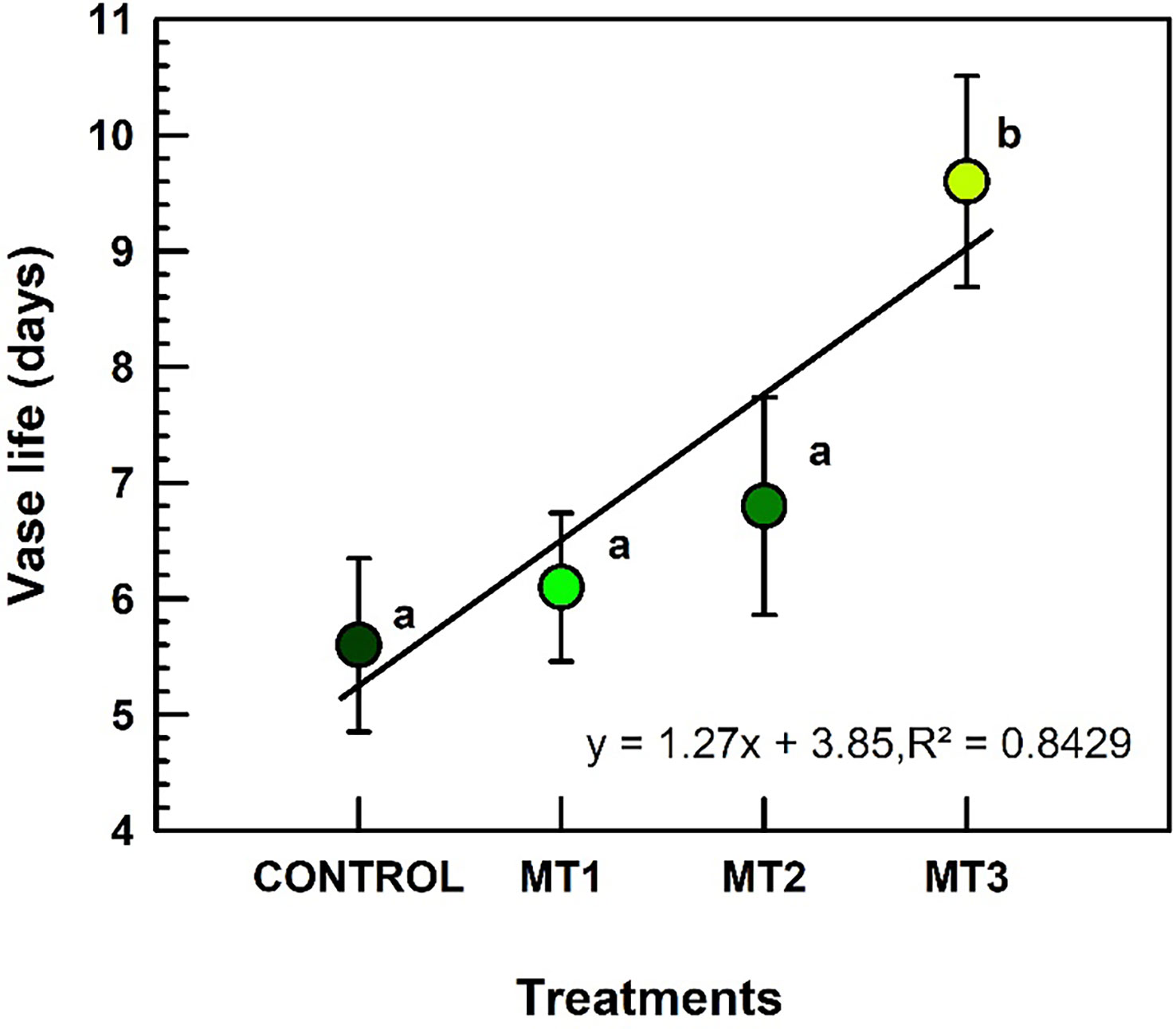
Figure 2 Vase life (d) of tuberose flowers harvested from the plants treated with preharvest foliar applications of distilled water (control; MT0) and three concentrations of melatonin (MLT): 0.05 mM (MT1), 0.07 mM (MT2), and 1 mM (MT3). Data are means ± SE (n = 40). Different letters above the bars indicate significant differences according to the LSD test at P = 0.05.
Plants treated with MT2 showed a significant increase in TSP and SS (Figures 3A, B). MT2 treated plants had a TSP mean value of 0.42 g kg-1, whereas the untreated controls averaged at 0.33 g kg-1 (Figure 3A). That was an average increase of 41%. Likewise, MT2 treated plants had a SS mean value of 4.59 g kg-1, whereas the untreated controls averaged at 3.26 g kg-1 (Figure 3B). This increase was by up to 41% between the MT2-treated and the untreated plants. Additionally, the proline content in MT2-treated plants was significantly reduced by up to 78%, compared to the controls (Figure 3C). The MT2-treated plants had a proline content of 22.4 μmol g-1 FW, whereas the untreated control plants averaged at 39.8 μmol g-1 FW (Figure 3C).

Figure 3 Total soluble protein (TSP) (A; g kg-1), soluble sugars (SS) (B; g kg-1), and proline contents (C; mmol g-1 FW) in leaves of tuberose plants treated with preharvest foliar applications of distilled water (control; MT0) and three concentrations of melatonin (MLT): 0.05 mM (MT1), 0.07 mM (MT2), and 1 mM (MT3). Data are means ± SE (n = 40). Different letters above the bars indicate significant differences according to the LSD test at P = 0.05.
H2O2 and MDA contents were generally reduced by MLT treatments (Figure 4). H2O2 was significantly reduced in MT3 plants by up to 56% (Figure 4A). MT3-treated plants had a H2O2 content of 12 mmol kg-1, whereas the untreated control had 18.8 mmol kg-1. Furthermore, the MT2-treated plants showed a significantly reduced MDA content by 23% compared to the controls (Figure 4B). MT2-treated plants showed an average of 107.6 mmol kg-1 MDA, whereas the untreated control 132.3 mmol kg-1.
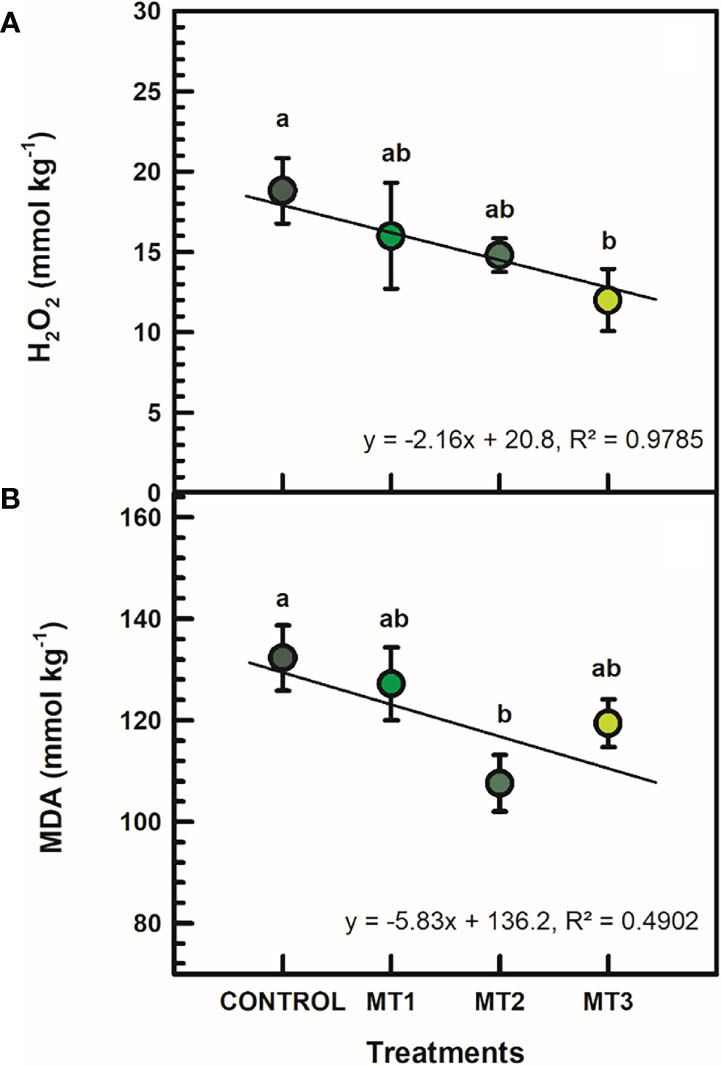
Figure 4 Hydrogen peroxide (H2O2) (A; mmol kg-1) and malondialdehyde (MDA) (B; mmol kg-1) contents in flowers of tuberose plants treated with preharvest foliar applications of distilled water (control; MT0) and three concentrations of melatonin (MLT): 0.05 mM (MT1), 0.07 mM (MT2), and 1 mM (MT3). Data are means ± SE (n = 40). Different letters above the bars indicate significant differences according to the LSD test at P = 0.05.
SOD and CAT activities were significantly induced by MLT only in certain cases (Figure 5). SOD was significantly increased by up to 54% in the MT2-treated plants (Figure 5A). SOD in MT2-treated plants was 0.21 units mg-1 protein and 0.01 units mg-1 protein in the untreated control plants (Figure 5A). CAT activity was increase by up to 70% in the MT3-treated plants (Figure 5B). CAT in MT3-treated plants was 32.5 units mg-1 protein and 9.6 units mg-1 protein in the untreated controls (Figure 5B).
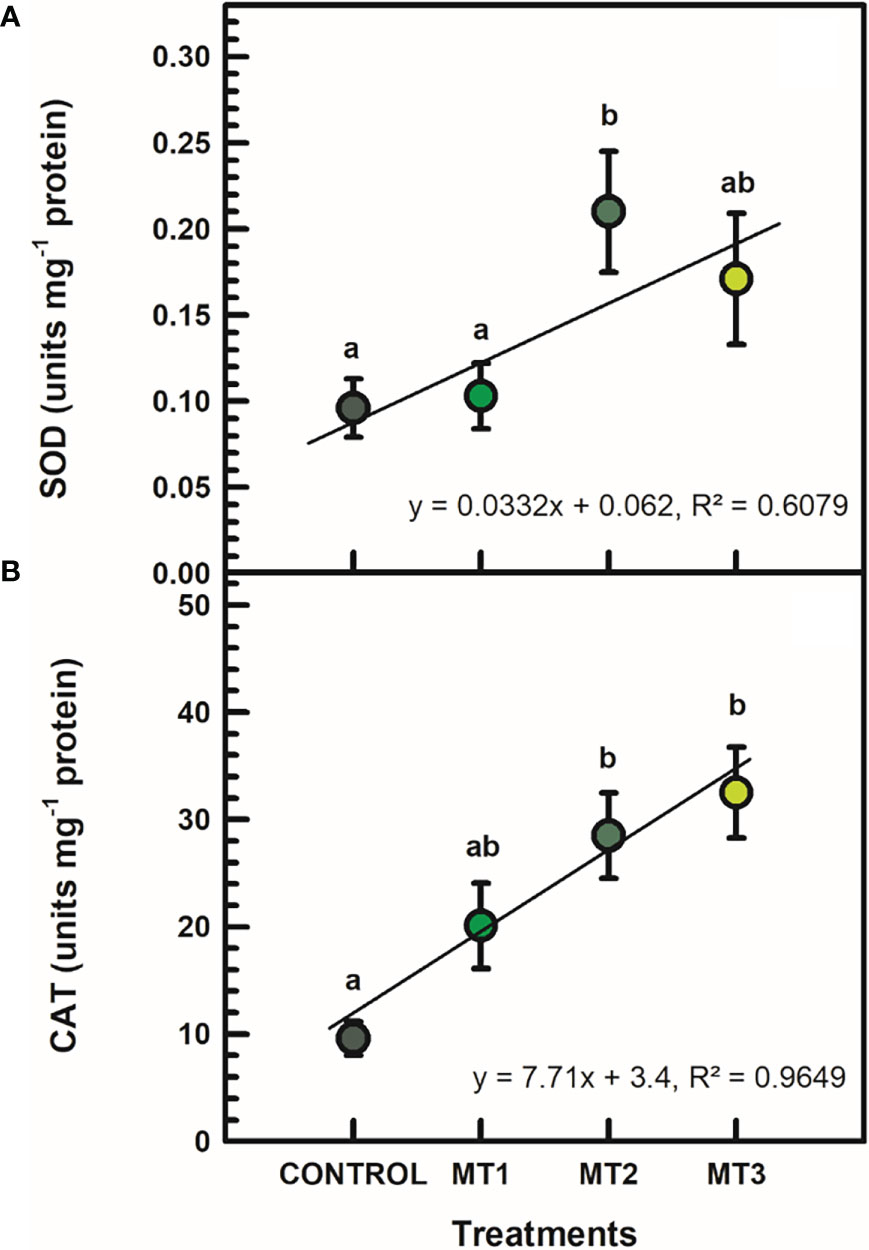
Figure 5 Superoxide dismutase (SOD) (A; units mg-1 protein) and catalase (CAT) (B; units mg-1 protein)activities in flowers of tuberose plants treated with preharvest foliar applications of distilled water (control; MT0) and three concentrations of melatonin (MLT): 0.05 mM (MT1), 0.07 mM (MT2), and 1 mM (MT3). Data are means ± SE (n = 40). Different letters above the bars indicate significant differences according to the LSD test at P = 0.05.
Numerous studies have discovered that endogenous MLT content is involved in floral senescence. However, among different flower species, MLT levels appear to decline during development, particularly at later phases of senescence (Murch et al., 2009; Zhao et al., 2017). In the current study, preharvest MLT treatments enhanced physiological traits and had an anti-senescent effect on tuberose cut flowers, elongating vase life, particularly in MT3. The increased vase life was associated with improved biochemical characteristics, as the MLT treatments controlled antioxidant defenses postharvest for longer than the control.
Photosynthesis is the primary process for harnessing light energy to manufacture carbohydrates, and is closely linked to plant growth. The MLT treatments enhanced photosynthetic activity in tuberose, evident in the leaf gas exchange properties (Figure 1A), and in line with similar studies on cotton (Khattak et al., 2022) and tomato (Altaf et al., 2022). In contrast, Zhao et al. (2021) reported that MLT treatments did not affect leaf gas exchange in maize (Zea mays) under normal conditions. Furthermore, MLT has a protective impact on chlorophyll (Campos et al., 2019; Li et al., 2021; Altaf et al., 2022). The MLT-treated tuberose plants had more chlorophyll than control plants (Figure 1C), showing that exogenous MLT inhibits photosynthetic machinery damage. An increased photosynthetic capability provides plants with more energy, allowing them to withstand stressors like postharvest stress (Fan et al., 2015).
This study is the first to investigate the effect of preharvest MLT on the vase life of cut tuberose flowers. The prolonged vase life with preharvest MLT is likely related to enhanced photosynthesis, soluble sugars and antixodant defense system. In addition, the increased protein content and antioxidant activity with preharvest MLT may have reduced the oxidative damage in tuberose tissues, extending the vase life. At the highest dose (MT3), this cost-effective preharvest treatment could benefit cut flower sellers, prolonging tuberose vase life. Lezoul et al. (2022) reported that MLT improved the vase life of carnation due to its antioxidative potential. Our results showed improved vase life, which also shows that MLT mitigates oxidative stress, as reported by Mazrou et al. (2022).
The water balance in petals is crucial for extending the vase life of cut flowers. Numerous investigations have revealed a strong connection between water balance and the capacity of cut flowers for osmotic adjustment (Hou et al., 2018; Zheng and Guo, 2019; Lu et al., 2020). In plants, the levels of osmolytes, such as soluble proteins, soluble sugars, and proline, are closely linked with the ability to modify osmotic pressure. The data increasingly indicates that exogenous substances could improve water balance by controlling osmolyte concentrations (Shan and Zhao, 2015). Shan and Zhao (2015) demonstrated that lanthanum improved water balance in Lilium longiflorum cut flowers by increasing soluble protein, soluble sugar, and proline contents, further enhancing the relative water content of the petals and extending vase life. We found that MLT increased soluble protein and sugar contents in tuberose leaves, consistent with Xing et al. (2021) for chrysanthemum seedlings. Proline, an osmotic adjustment chemical, in addition to the cell’s antioxidant system, participates in the defense mechanism against adverse situations (Zulfiqar and Ashraf, 2022). Proline is a non-polar amino acid renowned for its numerous and significant roles in plant metabolism, particularly in response to biotic and abiotic stresses (Zulfiqar and Ashraf, 2022). Under stress, proline functions as a signaling molecule, compatible osmolyte, non-enzymatic antioxidant, molecular chaperone, and energy provider (Szepesi and Szőllősi, 2018). Studies have shown that proline content plays a vital role in the postharvest performance of cut flowers (Aghdam et al., 2019; Sukpitak and Seraypheap, 2023; Zeng et al., 2023). For example, sucrose application to rose cut flowers prolonged vase life, compared to non-treated cut flowers, which was associated with proline content (Zeng et al., 2023).
In the current study, the MLT treatments decreased MDA content, reflecting a decrease in lipid peroxidation in cut flowers, hence maintaining membrane integrity. Other studies have shown that MLT reduces lipid peroxidation while preserving the membrane stability index (Hassan et al., 2020; Zulfiqar and Ashraf, 2022).
Antioxidant enzymes such as SOD and CAT are the most important defense enzymes for ROS detoxification in plant tissues (Hasanuzzaman et al., 2020; Zulfiqar and Ashraf, 2021). The MLT treatments, especially at 0.07 and 1 mM, increased SOD and CAT activities in tuberose cut flowers. Horticultural commodities, including cut flowers, have increased antioxidant enzyme activity, reducing lipid peroxidation and H2O2 concentration during their postharvest (Lezoul et al., 2022; Mazrou et al., 2022). Several studies have demonstrated that MLT treatments increased antioxidant enzyme activities (e.g., SOD and CAT) in horticultural produce (Sharafi et al., 2021; Mazrou et al., 2022). In the current study, the improved antioxidant activities with MLT were related to decreased oxidative stress, as indicated by the reductions in H2O2 and MDA.
The 0.07 and 1 mM preharvest MLT treatments were the most effective in delaying the senescence of tuberose cut flowers by improving leaf gas exchange, and TSP and soluble sugar contents and decreasing proline, H2O2, and MDA contents. Furthermore, these treatments increased SOD and CAT activities, decreasing oxidative stress. Preharvest MLT treatments at specific concentrations could prolong the vase life of tuberose cut inflorescences.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
FZ and AM designed and executed the experiments. All authors contributed on the writing, editing and revision of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aghdam, M. S., Jannatizadeh, A., Nojadeh, M. S., Ebrahimzadeh, A. (2019). Exogenous melatonin ameliorates chilling injury in cut anthurium flowers during low temperature storage. Postharvest Biol. Technol. 148, 184–191. doi: 10.1016/j.postharvbio.2018.11.008
Aghdam, M. S., Mukherjee, S., Flores, F. B., Arnao, M. B., Luo, Z., Corpas, F. J. (2021). Functions of melatonin during postharvest of horticultural crops. Plant Cell Physiol. 63 (12), 1764–1786. doi: 10.1016/j.tifs.2021.07.034
Altaf, M. A., Shahid, R., Ren, M. X., Altaf, M. M., Khan, L. U., Shahid, S., et al. (2021a). Melatonin alleviates salt damage in tomato seedling: a root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci. Hortic. 285, 110145. doi: 10.1016/j.scienta.2021.110145
Altaf, M. A., Shahid, R., Ren, M. X., Mora-Poblete, F., Arnao, M. B., Naz, S., et al. (2021b). Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant 172 (2), 820–846. doi: 10.1111/ppl.13262
Altaf, M. A., Shahid, R., Ren, M.-X., Naz, S., Altaf, M. M., Khan, L. U., et al. (2022). Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants. 11 (2), 309. doi: 10.3390/antiox11020309
Arnao, M. B., Cano, A., Hernández-Ruiz, J. (2022). Phytomelatonin: An unexpected molecule with amazing performances in plants. J. Exp. Bot. 73, 5779–5800. doi: 10.1093/jxb/erac009
Bates, L. S., Waldren, R. P., Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil. 39, 205–207. doi: 10.1007/BF00018060
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Campos, C. N., Avila, R. G., de Souza, K. R. D., Azevedo, L. M., Alves, J. D. (2019). Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica l. plants. agric. Water Manage. 211, 37–47. doi: 10.1016/j.agwat.2018.09.025
Chance, B., Maehly, A. C. (1955). Assay of catalases and peroxidases. Methods Enzymol. 2, 764–385 775. doi: 10.1002/9780470110171.ch14
Dole, J., Wilkins, H. (2005). Floriculture: Principles and species, prentice-hall, upper saddle river, NJ, USA Pearson, NJ, USA.
Fan, H. M., Li, T., Sun, X., Sun, X. Z., Zheng, C. S. (2015). Effects of humic acid derived from sediments on the postharvest vase life extension in cut chrysanthemum flowers. Postharvest Biol. Technol. 101, 82–87. doi: 10.1016/j.postharvbio.2014.09.019
Frohlich, M., Kutscherah, U. (1995). Changes in soluble sugars and proteins during.Development of rye coleoptiles. J. Plant Physiol. 146, 121–125. doi: 10.1016/S0176-1617(11)81977-2
Hasanuzzaman, M., Bhuyan, M. B., Zulfiqar, F., Raza, A., Moshin, S. M., Mahmud, J. A., et al. (2020). Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 9 (8), 681. doi: 10.3390/antiox9080681
Hassan, F., Ali, E., Mazrou, R. (2020). Involvement of ethylene synthetic inhibitors in regulating the senescence of cut carnations through membrane integrity maintenance. J. Hortic. Res. 28, 39–48. doi: 10.2478/johr-2020-0010
Hodges, D. M., DeLong, J. M., Forney, C. F., Prange, R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 207, 604–611. doi: 10.1007/s004250050524
Hou, K., Bao, D., Shan, C. (2018). Cerium improves the vase life of lilium longiflorum cut flowers through ascorbate-glutathione cycle and osmoregulation in the petals. Sci. Hortic. 227, 142–145. doi: 10.1016/j.scienta.2017.09.040
Jhanji, S., Kaur, G., Kaur, R., Dhatt, U. K. (2023). Physiological and biochemical changes during flower development and senescence in chrysanthemum and gladiolus. Acta Physiol. Plant 45 (1), 1–13. doi: 10.1007/s11738-022-03486-4
Khandan-Mirkohi, A., Pirgazi, R., Taheri, M. R., Ajdanian, L., Babaei, M., Jozay, M., et al. (2021). Effects of salicylic acid and humic material preharvest treatments on postharvest physiological properties of statice cut flowers. Sci. Hortic. 283, 110009. doi: 10.1016/j.scienta.2021.110009
Khattak, W. A., He, J., Abdalmegeed, D., Hu, W., Wang, Y., Zhou, Z. (2022). Foliar melatonin stimulates cotton boll distribution characteristics by modifying leaf sugar metabolism and antioxidant activities during drought conditions. Physiol. Plant 174 (1), e13526. doi: 10.1111/ppl.13526
Lezoul, N. E. H., Serrano, M., Ruiz-Aracil, M. C., Belkadi, M., Castillo, S., Valero, D., et al. (2022). Melatonin as a new postharvest treatment for increasing cut carnation (Dianthus caryophyllus l.) vase life. Postharvest Biol. Technol. 184, 111759. doi: 10.1016/j.postharvbio.2021.111759
Li, Z., Su, X. Y., Chen, Y. L., Fan, X. C., He, L. Z., Guo, J. M., et al. (2021). Melatonin improves drought resistance in maize seedlings by enhancing the antioxidant system and regulating abscisic acid metabolism to maintain stomatal opening under PEG-induced drought. J. Plant Biol. 64, 299–312. doi: 10.1007/s12374-021-09297-3
Lin, Y., Zhan, L., Shao, P., Sun, P. (2022). Phase-change materials and exogenous melatonin treatment alleviated postharvest senescence of Agaricus bisporus by inhibiting browning and maintaining cell membrane integrity. Postharvest Biol. Technol. 192, 112009. doi: 10.1016/j.postharvbio.2022.112009
Lu, N., Wu, L., Shi, M. (2020). Selenium enhances the vase life of Lilium longiflorum cut flower by regulating postharvest physiological characteristics. Sci. Hortic. 264, 109172. doi: 10.1016/j.scienta.2019.109172
Luo, S., Hu, H., Wang, Y., Zhou, H., Li, P. (2020). The role of melatonin in alleviating the postharvest browning of lotus seeds through energy metabolism and membrane lipid metabolism. Postharvest Biol. Technol. 167, 111243. doi: 10.1016/j.postharvbio.2020.111243
Mazrou, R. M., Hassan, S., Yang, M., Hassan, F. A. (2022). Melatonin preserves the postharvest quality of cut roses through enhancing the antioxidant system. Plants. 11 (20), 2713. doi: 10.3390/plants11202713
Murch, S. J., Alan, A. R., Cao, J., Saxena, P. K. (2009). Melatonin and serotonin in flowers and fruits of Datura metel l. J. Pineal Res. 47 (3), 277–283. doi: 10.1111/j.1600-079X.2009.00711.x
Olsen, A., Lütken, H., Hegelund, J. N., Müller, R. (2015). Ethylene resistance in flowering ornamental plants–improvements and future perspectives. Hortic. Res. 2, 15038. doi: 10.1038/hortres.2015.38
Patterson, B. D., MacRae, E. A., Ferguson, I. B. (1984). Estimation of hydrogen peroxide in plant extracts using titanium (IV). Analytical Biochem. 139, 487–492. doi: 10.1016/0003-2697(84)90039-3
Shan, C., Zhao, X. (2015). Lanthanum delays the senescence of Lilium longiflorum cut flowers by improving antioxidant defense system and water retaining capacity. Sci. Hortic. 197, 516–520. doi: 10.1016/j.scienta.2015.10.012
Sharafi, Y., Jannatizadeh, A., Fard, J. R., Aghdam, M. S. (2021). Melatonin treatment delays senescence and improves antioxidant potential of sweet cherry fruits during cold storage. Sci. Hortic. 288, 110304. doi: 10.1016/j.scienta.2021.110304
Sukpitak, C., Seraypheap, K. (2023). Postharvest transient water deficit limits longevity of cut dendrobium ‘Khao sanan’orchid. Sci. Hortic. 309, 111637. doi: 10.1016/j.scienta.2022.111637
Szepesi, Á., Szőllősi, R. (2018). Mechanism of proline biosynthesis and role of proline metabolism enzymes under environmental stress in plants. in: Plant metabolites and regulation under environmental stress (USA: Academic Press), 337–353.
van Rossum, M. W. P. C., Alberda, M., van der Plas, L. H. W. (1997). Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci. 130, 207–216. doi: 10.1016/S0168-9452(97)00215-X
Wang, S. Y., Shi, X. C., Wang, R., Wang, H. L., Liu, F., Laborda, P. (2020). Melatonin in fruit production and postharvest preservation: A review. Food Chem. 320, 126642. doi: 10.1016/j.foodchem.2020.126642
Xing, X., Ding, Y., Jin, J., Song, A., Chen, S., Chen, F., et al. (2021). Physiological and transcripts analyses reveal the mechanism by which melatonin alleviates heat stress in chrysanthemum seedlings. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.673236
Zeng, F., Xu, S., Geng, X., Hu, C., Zheng, F. (2023). Sucrose+ 8-HQC improves the postharvest quality of lily and rose cut flowers by regulating ROS-scavenging systems and ethylene release. Sci. Hortic. 308, 111550. doi: 10.1016/j.scienta.2022.111550
Zhang, Y., Huber, D. J., Hu, M., Jiang, G., Gao, Z., Xu, X., et al. (2018). Delay of postharvest browning in litchi fruit by melatonin via the enhancing of antioxidative processes and oxidation repair. J. Agric. Food Chem. 66, 7475–7484. doi: 10.1021/acs.jafc.8b01922
Zhao, C., Guo, H., Wang, J., Wang, Y., Zhang, R. (2021). Melatonin enhances drought tolerance by regulating leaf stomatal behavior, carbon and nitrogen metabolism, and related gene expression in maize plants. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.779382
Zhao, D., Wang, R., Meng, J., Zhiyuan, Li, Wu, Y., Tao, J. (2017). Ameliorative effects of melatonin on dark-induced leaf senescence in gardenia (Gardenia jasminoides ellis): leaf morphology, anatomy, physiology and transcriptome. Sci. Rep. 7, 10423. doi: 10.1038/s41598-017-10799-9
Zheng, M., Guo, Y. (2019). Effects of neodymium on the vase life and physiological characteristics of Lilium casa blanca petals. Sci. Hortic. 256, 108553. doi: 10.1016/j.scienta.2019.108553
Zheng, H., Liu, W., Liu, S., Liu, C., Zheng, L. (2019). Effects of melatonin treatment on the enzymatic browning and nutritional quality of fresh-cut pear fruit. Food Chem. 299, 125111–125116. doi: 10.1016/j.foodchem.2019.125116
Zulfiqar, F., Ashraf, M. (2021). Bioregulators: Unlocking their potential role in regulation of the plant oxidative defense system. Plant Mol. Biol. 105, 11–41. doi: 10.1007/s11103-020-01077-w
Zulfiqar, F., Ashraf, M. (2022). Proline alleviates abiotic stress induced oxidative stress in plants. J. Plant Growth Regul., 1–23. doi: 10.1007/s00344-022-10839-3
Keywords: oxidative stress, postharvest, ornamental cut flower, soluble proteins, soluble sugars, antioxidants
Citation: Zulfiqar F, Moosa A, Darras A, Nafees M, Ferrante A and Siddique KHM (2023) Preharvest melatonin foliar treatments enhance postharvest longevity of cut tuberose via altering physio-biochemical traits. Front. Plant Sci. 14:1151722. doi: 10.3389/fpls.2023.1151722
Received: 26 January 2023; Accepted: 09 March 2023;
Published: 23 March 2023.
Edited by:
Vijay Gahlaut, Chandigarh University, IndiaReviewed by:
Sanaullah Jalil, Zhejiang University, ChinaCopyright © 2023 Zulfiqar, Moosa, Darras, Nafees, Ferrante and Siddique. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faisal Zulfiqar, Y2guZmFpc2FsLnp1bGZpcWFyQGdtYWlsLmNvbQ==; Anam Moosa, YW5hbW1vb3NhMUBvdXRsb29rLmNvbQ==; Kadambot H. M Siddique, a2FkYW1ib3Quc2lkZGlxdWVAdXdhLmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.