- 1College of Pharmacy, Hebei University of Chinese Medicine, Shijiazhuang, Hebei, China
- 2Traditional Chinese Medicine Processing Technology Inheritance Base of the State Administration of Traditional Chinese Medicine, College of Pharmacy, Hebei University of Chinese Medicine, Shijiazhuang, Hebei, China
Bombyx batryticatus is derived from the dried larva of Bombyx mori Linnaeus infected by Beauveria bassiana (Bals.) Vuillant. Raw Bombyx batryticatus should be stir-fried before oral administration due to its irritation to the gastrointestinal tract. Nevertheless, it is still an arduous task to uncover the intrinsic mechanism of Bombyx batryticatus processing. In this study, we collected two types of Bombyx batryticatus, one being stir-fried and the other serving as a control. Then, an informative approach, which integrated matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) with chemometrics analysis, was established to screen processing-associated markers and reveal in situ spatial distribution patterns of protein-related metabolites. After optimization of experimental conditions, 21 ions were initially detected from Bombyx batryticatus, including amino acids and peptides. In addition, 15 differential markers were screened by orthogonal projection to potential structure discriminant analysis (OPLS-DA), which were localized and visualized in the transverse section of Bombyx batryticatus by MSI. Eventually, it can be demonstrated that the stir-frying process reduces toxicity while potentially boosting specific biological activities of Bombyx batryticatus. In summary, the established strategy could not only clarify the chemical transformation of protein-related metabolites from Bombyx batryticatus before and after frying with wheat bran, but also reveal the significance of Chinese medicine processing technology.
Introduction
Bombyx batryticatus is a famous Chinese medicine, which belongs to the dry bodies of the 4th and the 5th instar larvae of Bombyx mori Linnaeus infected (or artificially inoculated) with Beauveria bassiana (Bals.) Vuillant (Chinese Pharmacopoeia Commission, 2020). Bombyx batryticatus has a long history of clinical application in Chinese medicine, which was first recorded in the Classic of Shennong Materia. The main efficacy of Bombyx batryticatus is resolving phlegm and dispersing blood stasis, dispelling wind and settling shock. It is mainly used in the treatment of liver wind and phlegm, convulsion, infantile shock, tetanus, sore throat, and rubella pruritus (Wang et al., 2021a). The first-class Bombyx batryticatus has a hard texture, thick strips, white color and bright cross-section (Hu, 2017). Beauveria Batryticatus contains various chemical components, such as protein, amino acid, ammonium oxalate, enzymes, nucleoside bases, trace elements, etc. (Bai et al., 2018). The Bombyx mori feeds mainly on leaves of mulberry (Morus alba Linn.) throughout their life, absorbing, accumulating and metabolizing a variety of chemicals within their bodies. After invading the Bombyx mori, Beauveria bassiana proceed to multiply and grow based on the larva as a resource of nutrients, which was essentially a fermentation process by the fungus. Furthermore, the original components can be transformed into new components with higher activity in the form of hydroxylation, redox reaction, sulfonation, and hydrolysis of epoxide 4-O-methylglucoside (Xing et al., 2019). Accordingly, the bioactivities of Bombyx batryticatus are essentially derived from the co-action of Bombyx mori, mulberry leaves and Beauveria bassiana (Figure 1).
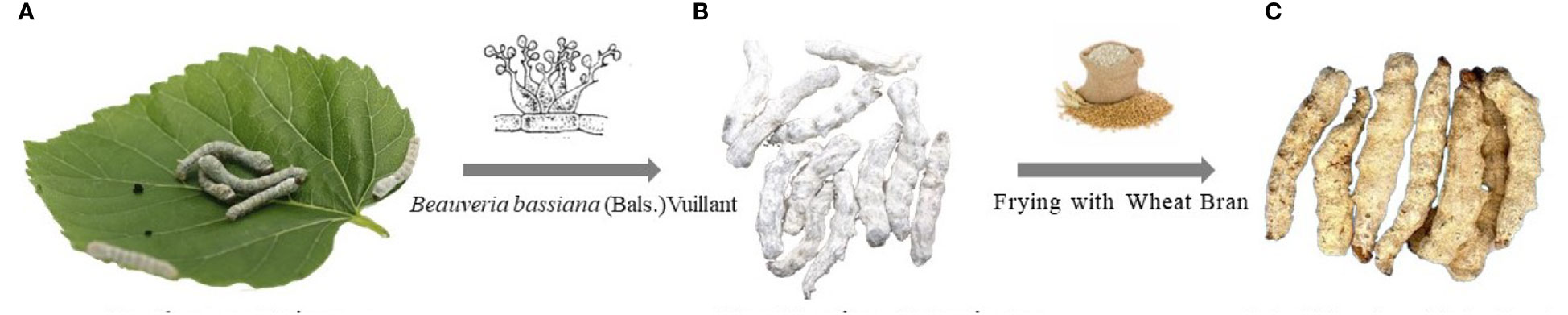
Figure 1 The formation of raw and fried Bombyx batryticatus. (A) Bombyx mori Linnaeus and mulberry leaves. (B) Raw Bombyx batryticatus. (C) Fried Bombyx batryticatus.
The processing of Chinese medicine is a unique pharmaceutical technology in China, which refers to the manufacturing process of stir-frying, roasting, carbonizing, steaming, etc., aiming to enhance efficacy and reduce toxicity (Li et al., 2020). There are two types of Bombyx batryticatus widely used in clinical applications, namely raw and fried forms. Bombyx batryticatus research has always focused on small molecular compounds with little protein-related metabolites involved in the past. For example, Wang et al. (2022) established a high performance liquid chromatography (HPLC) fingerprint combined with chemical pattern recognition technology to evaluate the quality of Bombyx batryticatus. A liquid chromatography-tandem mass spectrometry (LC-MS/MS) technology was used to qualitatively analyze the chemical constituents in water-soluble substances of Bombyx batryticatus (Wang et al., 2021b). Although several attempts have been made to elucidate the active metabolites in Bombyx batryticatus, the intrinsic transformation mechanisms of protein-related metabolites, which account for the largest proportion of endogenous components, from Bombyx batryticatus before and after frying are still unclear. Chinese medicine is a complex mixture with hundreds of chemical components. the active ingredients can be easily altered or lost, and the spatial distribution of various components in tissues is also severely damaged during processing, such as extraction, purification and enrichment, making it impossible to achieve in situ analysis of active ingredients (Wang et al., 2016). In recent years, matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) has become an imaging technique for studying the composition and distribution of molecules or ions at the tissue or cellular level (Li et al., 2020), which is characterized with high sensitivity, high spatial resolution and high laser scanning speed (Zemaitis et al., 2022). Shu et al. (2010) performed MSI to confirm that ginsenosides were located more in the cortex and periderm than in the medulla of a lateral root of Panax ginseng. Li et al. (2021a) used MALDI-MSI combined with multiple matrixes to analyze spatial metabolomes on root sections of the two Paeonia species. Li et al. (2014) showed the distribution of major tissue-specific metabolites in the rhizome of Glycyrrhiza glabra (licorice) at cellular level by atmospheric pressure MALDI-MSI. However, this technique has not been applied to study the distribution of chemical components in tissues during processing Bombyx batryticatus.
In the present work, a mass spectrometry-based profiling and imaging strategy was established to reveal the chemical transformation of protein-related metabolites from Bombyx batryticatus before and after frying with wheat bran. To begin with, the chemical profiling of peptides and amino acids from raw and fried Bombyx batryticatus was achieved by identifying characteristic ion fragments and matching our in-built database. Subsequently, multivariate statistical analysis was applied to screen processing-related markers. Finally, MALDI-MSI was performed to testify the markers discovered and visualize their chemical transformation trajectories.
Materials and methods
Instruments and reagents
Instruments used in this study included Bruker Rapiflex MALDI-TOF/TOF mass spectrometer equipped with the FlexAnalysis data processing workstation (Bruker, Karlsruhe, Germany), GET-Sprayer (І) (HIT Co., Ltd., Beijing, China), Indium Tin Oxide (ITO) conductive slides (diagonal resistance ~40 Ω, Bruker, Karlsruhe, Germany), vacuum desiccator (Shanghai Yueyang Electronic Technology Co., Ltd., Shanghai, China), and cryomicrotome (Leica CM1950, Nussloch, Germany).
The following reagents were used in this study: methanol and acetonitrile (mass spectrometry pure, Merck, Darmstadt, Germany), optimum cutting temperature (OCT) embedding agent (Leica, Nussloch, Germany), and trifluoroacetic acid (TFA) (Sigma, St. Louis, MO, USA). Water was purified by a Milli-Q filtration system (Millipore, Billerica, MA, USA). A total of 26 compounds representing five different classes of bioactive components (e.g., amino acids, peptides, nucleosides, flavonoids, and others) were procured from the National Institutes of Food and Drug Control (Beijing, China) and used as reference compounds (Supplementary Table 1).
Sample preparation
Both raw Bombyx batryticatus (RBB) and fried Bombyx batryticatus (FBB) were purchased from Hebei Chenghai Tang Chinese Medicine Co., Shijiazhuang, Hebei Province, China. Morphological identification of Bombyx batryticatus was carried out by Professor Yu-Guang Zheng at the Processing Technology Innovation Center of Traditional Chinese Medicine (TCM), Shijiazhuang, Hebei Province. After the matrix application, three samples from the two types of Bombyx batryticatus were selected, and three biological replicates of each sample were performed. Bombyx batryticatus was placed in a freezing microtome at -25°C for 30 min and fixed to the microtome tray with OCT embedding agent. Frozen tissue sections of 20 μm in thickness were transferred to ITO conductive glass slides and vacuumed in a vacuum desiccator for 20 min.
Matrix and standard solution preparation
200mg control powder of 2-mercaptobenzothiazole (2-MBT) was weighed and dissolved in 10 ml of methanol-water (7:3) solution, followed by the addition of 0.1% trifluoroacetic acid (TFA) dropwise to promote ionization, and finally prepared into a matrix solution with a mass concentration of 20 mg/ml. The control solution of standard substances was prepared at 1.0 mg/ml. Then, 1 μl of the control solution was added dropwise to the Bruker ground steel target plate and dried in vacuum for 20 minutes until the sample spot was dry. After that, the matrix solution (1 μl) was added dropwise to cover the sample spot and dried in vacuum again until the solvent evaporated, followed by MALDI-MS analysis.
Matrix application
Vacuum-dried Bombyx batryticatus tissue sections were fixed in a matrix sprayer and matrix spraying was performed with 2-MBT. The spray program was performed for 8 cycles with a matrix flow rate of 0.075 mL/min, nozzle temperature at 60°C, nozzle travel speed of 800 mm/min, nozzle travel row spacing of 3 mm, and nozzle-sample row spacing of 3 cm. Tissue sections were prepared for the MALDI mass spectrometry analysis after spraying.
MALDI-TOF-MSI
Tissue sections of sprayed matrix were placed in a Bruker Rapiflex MALDI-TOF/TOF type mass spectrometer (Bruker, Karlsruhe, Germany) equipped with a smartbeamTM 3D laser for mass spectrometry imaging analysis. The wavelength was 355 nm at a sampling rate of 2.5 GS/s. MALDI mass spectrometry imaging was performed in a positive ion detection mode with a mass-to-charge ratio (m/z) ranging from 100 to 1500 and a spatial resolution of 100 μm for scanning samples.
MALDI-TOF-MS/MS
Peak values of precursor ions were obtained in the positive ionization and reflectron modes. Tandem mass spectrometry was performed in a LIFT mode with a mass tolerance of 0.05%. Precursor ions were accelerated at 7.5 kV under optimized ion-selective gating. The acceleration voltage of the LIFT unit was set to 19 kV. All mass spectra were analyzed with FlexAnalysis software 3.4 (https://softwaretopic.informer.com/). Default settings were used for baseline correction and smoothing.
Data analysis
For the mass spectrometry analysis, FlexAnalysis 3.4 software of Bruker (https://softwaretopic.informer.com/) was used for initial mass spectrum observation and processing. These extracted mass spectra were baseline subtracted, normalized, and recalibrated based on the total ion count (TIC) in the observed mass range. Ion screening was performed based on accurate mass-to-charge ratios from the literature and databases of METLIN (https://metlin.scripps.edu/) and HMDB (https://hmdb.ca). Three ion addition forms, i.e., [M+H]+, [M+Na]+, and [M+K]+, were used to perform database search. Data sets were tabulated and imported into SIMCA 14 software (Umetrics, Malmo, Sweden) for multivariate statistical analyses, including principal component analysis (PCA), orthogonal projection to potential structure discriminant analysis (OPLS-DA) and hierarchical cluster analysis (HCA), to distinguish samples with different quality characteristics and identify potential quality-related markers. In order to further confirm the differential metabolites identified above, a Student’s t-test analysis was conducted, which will be illustrated in the form of statistical tables (Supplementary Table 2).
Results
Chemical composition profile of Bombyx batryticatus before and after frying with wheat bran
In order to comprehensively, systematically and accurately identify the various types of active ingredients in raw Bombyx batryticatus and fried Bombyx batryticatus, we analyzed the characteristic ion fragmentation information of the standards, inferring the fragmentation patterns and suitable ionization conditions applicable to the ions of different components. Finally, the positive mode was adopted in the ranges of m/z 100-400 and m/z 400-800 for higher intensities and clearer fragmentation patterns to identify the ionic structure of different components. The representative overall average mass spectra are shown in Figure 2. There were 9 amino acid peaks and 12 peptide peaks identified from the transverse sections of Bombyx batryticatus by MS/MS fragmentation patterns of reference standards in conjunction with the precise mass-to-charge ratios, isotopic peaks, data from various literature and other databases (Supplementary Figure 1). We detected three main ionic adduct forms, i.e., [M+H]+, [M+Na]+, and [M+K]+. Most amino acids had all been identified as [M+H]+ form ions, while a large number of peptides were revealed to be sodium or potassium adducts (Table 1). Notably, owing to the extreme degradation and transformation of certain active ingredients in high-temperature environments, the contents of most active ingredients in fried Bombyx batryticatus, with the exception of some specific ingredients, were drastically reduced, although the varieties of compounds contained were essentially identical.
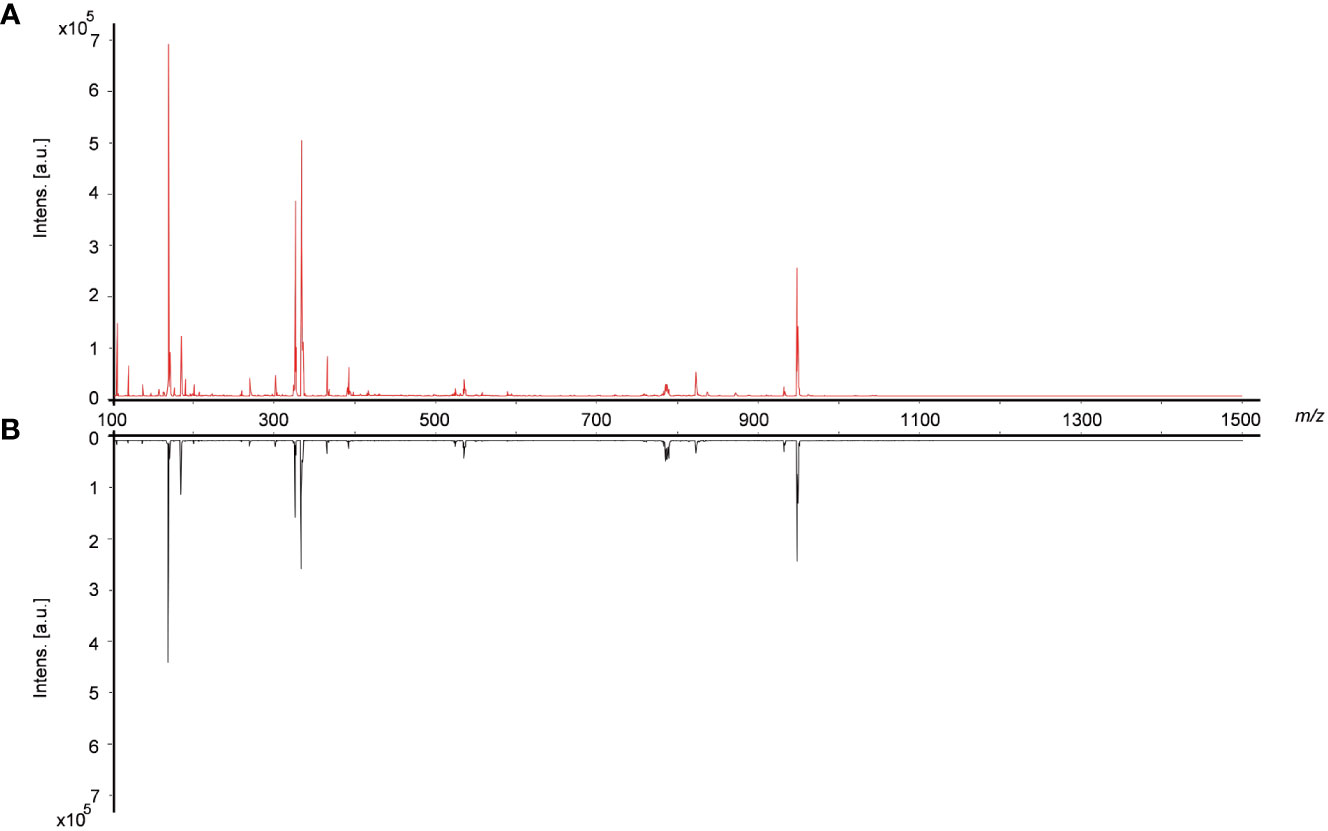
Figure 2 Typical overall average mass spectra obtained from cross sections of raw and fried Bombyx batryticatus by MALDI-TOF-MS in a positive mode. (A) Raw Bombyx batryticatus (red). (B) Fried Bombyx batryticatus (black).
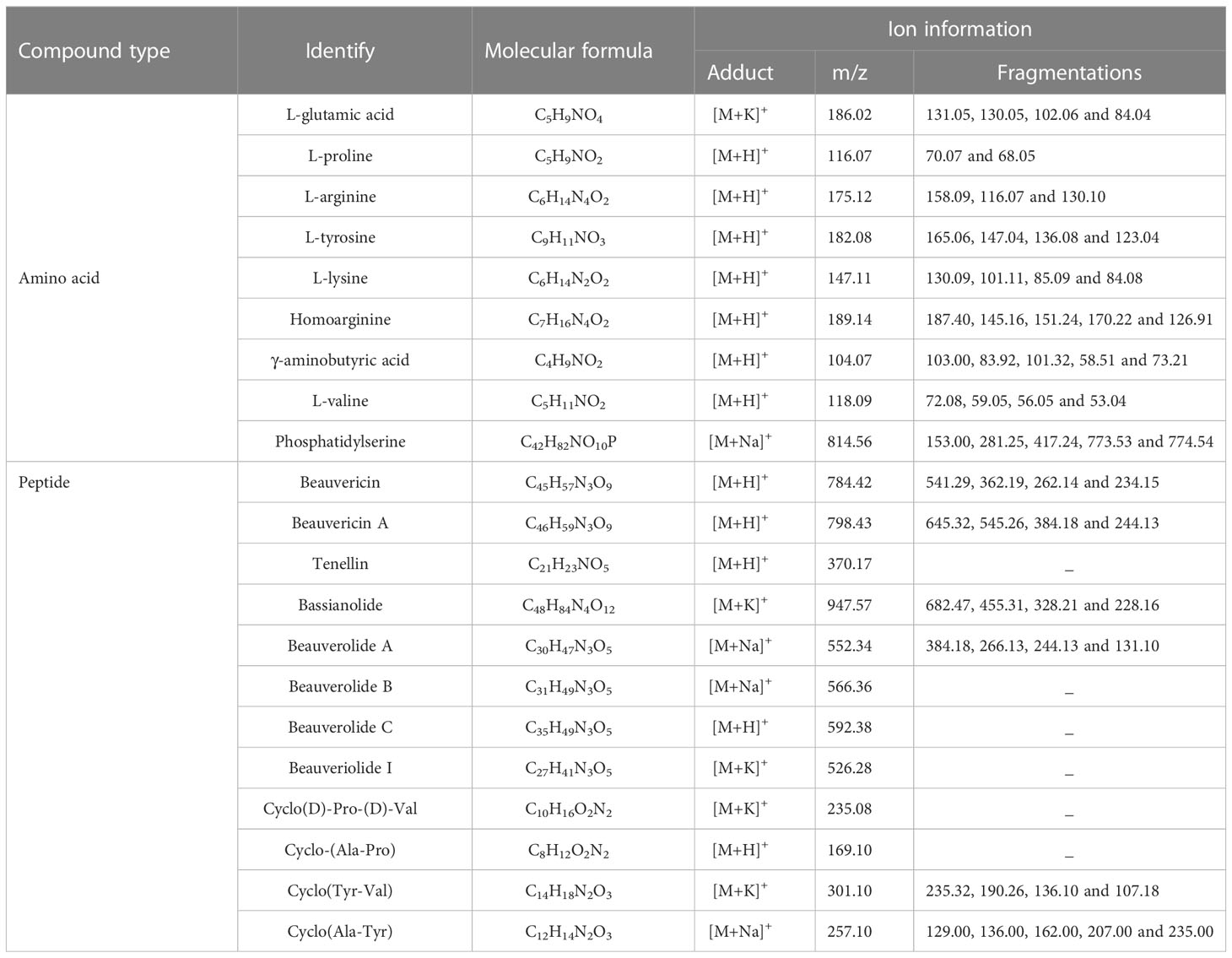
Table 1 Amino acids and peptides detected in Bombyx batryticatus by MALDI-TOF-MSI and confirmed by MALDI-TOF-MS/MS.
Multivariate statistical analysis of protein-related metabolites in raw Bombyx batryticatus and fried Bombyx batryticatus
The raw profile data of endogenous compounds detected were preprocessed including data filtering, area normalization, peak extraction and deconvolution via Bruker FlexAnalysis 3.4 software. After the standardization processing, the processed data were loaded into SIMCA-P 14 for further multivariate statistical analysis. Various samples of raw Bombyx batryticatus and fried Bombyx batryticatus were clearly separated into two unique clusters in the principal component analysis (PCA) score plots, indicating substantial distinction between the two treatment groups (Figure 3A). The R2X (0.848) and Q2 (0.691) values of the established PCA model demonstrated its superior fitness and prediction.
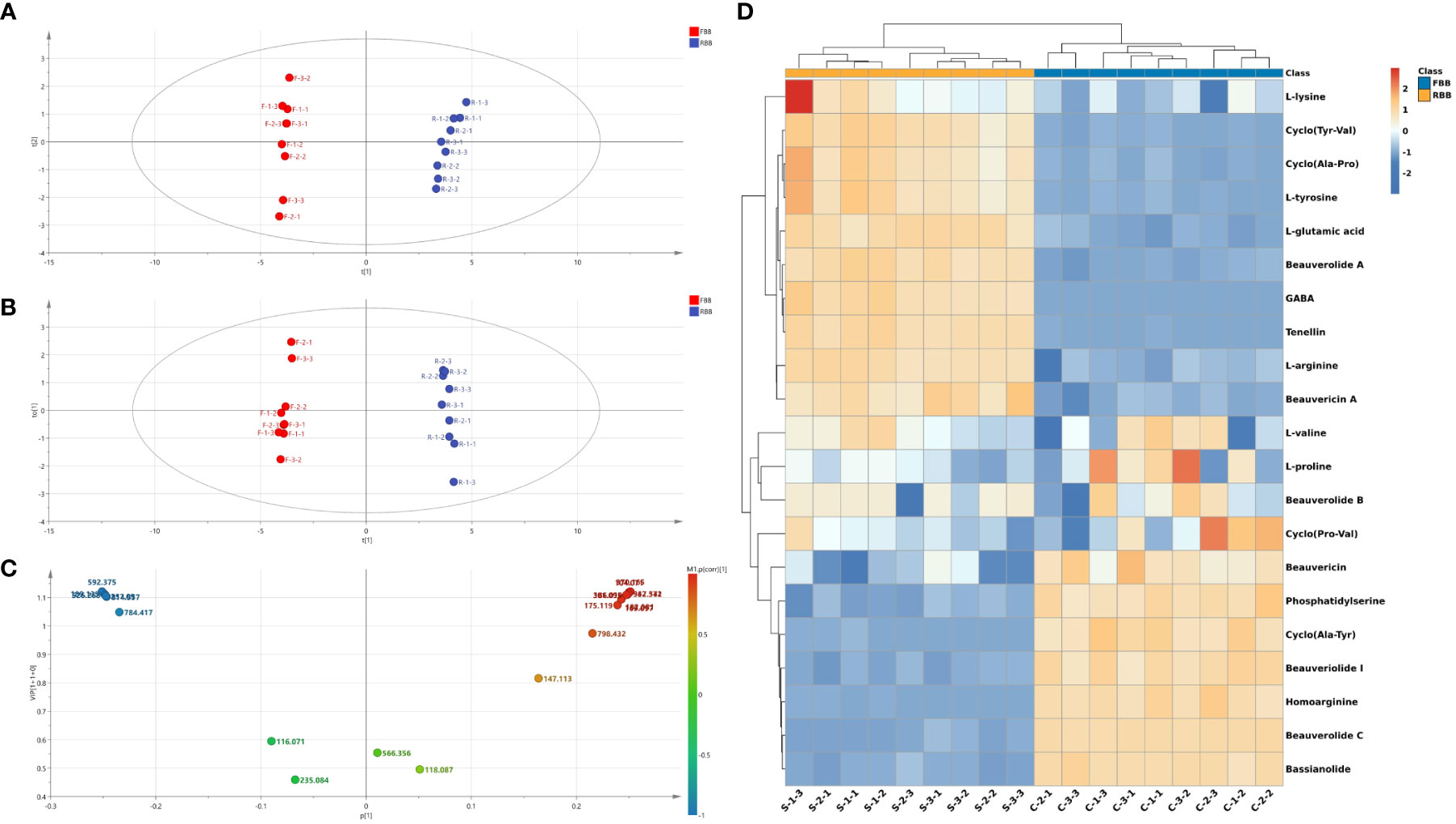
Figure 3 Multivariate statistical analysis of MALDI-TOF-MS data from raw and fried Bombyx batryticatus samples. (A) PCA score plot. (B) OPLS-DA score plot. (C) VIP diagram. (D) HCA heatmap.
OPLS-DA was used to detect characteristic components that cause the differences in the product quality (Gennebäck et al., 2013). The OPLS-DA model clearly distinguished samples of raw Bombyx batryticatus and fried Bombyx batryticatus (Figure 3B). The cumulative statistic R2X (0.768), model interpretation rate parameter R2Y (0.993), and prediction ability parameter Q2 (0.985) of the fitted model were all higher than 0.5, indicating that the established model is stable and reliable, and the prediction ability is strong. In addition, OPLS-DA model verification (permutation test n=200) was conducted, resulting in R2 of 0.278 and Q2 of -0.639 (Supplementary Figure 2). The values of R2 and Q2 were higher than those on the left side, and Q2 intersected with the Y-axis on the negative half-axis, indicating that the model is reliable without any overfitting phenomenon.
Variable importance projection (VIP) can reflect the degree of contribution of ions to the model classification. Therefore, differential marker components of amino acids and peptides from raw Bombyx batryticatus and fried Bombyx batryticatus were screened with VIP > 1 as a criterion for differential substances. The results for VIP > 1 included [homoarginine+H]+ (m/z: 189.14, VIP=1.16), [beauverolide C acid+H]+ (m/z: 592.38, VIP=1.16), [cyclo(Tyr-Val)+K]+ (m/z: 301.10, VIP=1.16), [γ-aminobutyric acid+H]+ (m/z: 104.07, VIP=1.16), [bassianolide+K]+ (m/z: 947.57, VIP=1.16), [L-tyrosine+H]+ (m/z:182.08, VIP=1.15), [phosphatidylserine+Na]+ (m/z: 814.56, VIP=1.15), [cyclo-(Ala-Pro)+H]+ (m/z: 169.10, VIP=1.15), [beauverolide A+Na]+ (m/z: 552.34, VIP=1.11), [tenellin+H]+ (m/z: 370.17, VIP=1.10), [cyclo(Ala-Tyr)+Na]+ (m/z: 257.10, VIP=1.10), [L-arginine+H]+ (m/z: 175.12, VIP=1.08), [beauveriolide I+K]+ (m/z: 526.28, VIP=1.08), [L-glutamic acid+K]+ (m/z: 186.02, VIP=1.05), and [beauvericin+H]+ (m/z: 784.42, VIP=1.05) (Figure 3C). These ions were the major markers of raw Bombyx batryticatus and fried Bombyx batryticatus.
To reveal metabolic changes between raw Bombyx batryticatus and fried Bombyx batryticatus, a total of 21 protein-related metabolites were visualized in hierarchical clustering analysis (HCA). All data mean values are centralized before analysis, clustering by Euclidean rank and measuring distance by mean link. As shown in Figure 3D, each small rectangle in the heat map represents a differentiator, and the content of each metabolite is represented by a standardized range, with darker colors indicating higher metabolite levels. The cluster analysis plot of Bombyx batryticatus samples obviously indicated a division into two sub-groups, a result consistent with the previous PCA and OPLS-DA analyses.
Spatial distribution of characteristic protein-related metabolites in raw Bombyx batryticatus and fried Bombyx batryticatus
MALDI-MSI is an advanced technique to unravel the spatiotemporal variations of the metabolic network of endogenous active ingredients in various tissues and layers of Bombyx batryticatus during the stir-frying process. To further investigate the spatial localization of the diverse metabolic pathways of the bioactive constituents in Bombyx batryticatus, the distribution of signature metabolites of proteins, i.e., peptides and amino acids, was explored in the various tissues. Histologically, the anatomical structure of Bombyx batryticatus was separated into silk gland, digestive tract, mesenchyme and integument from inside to outside (Figure 4).
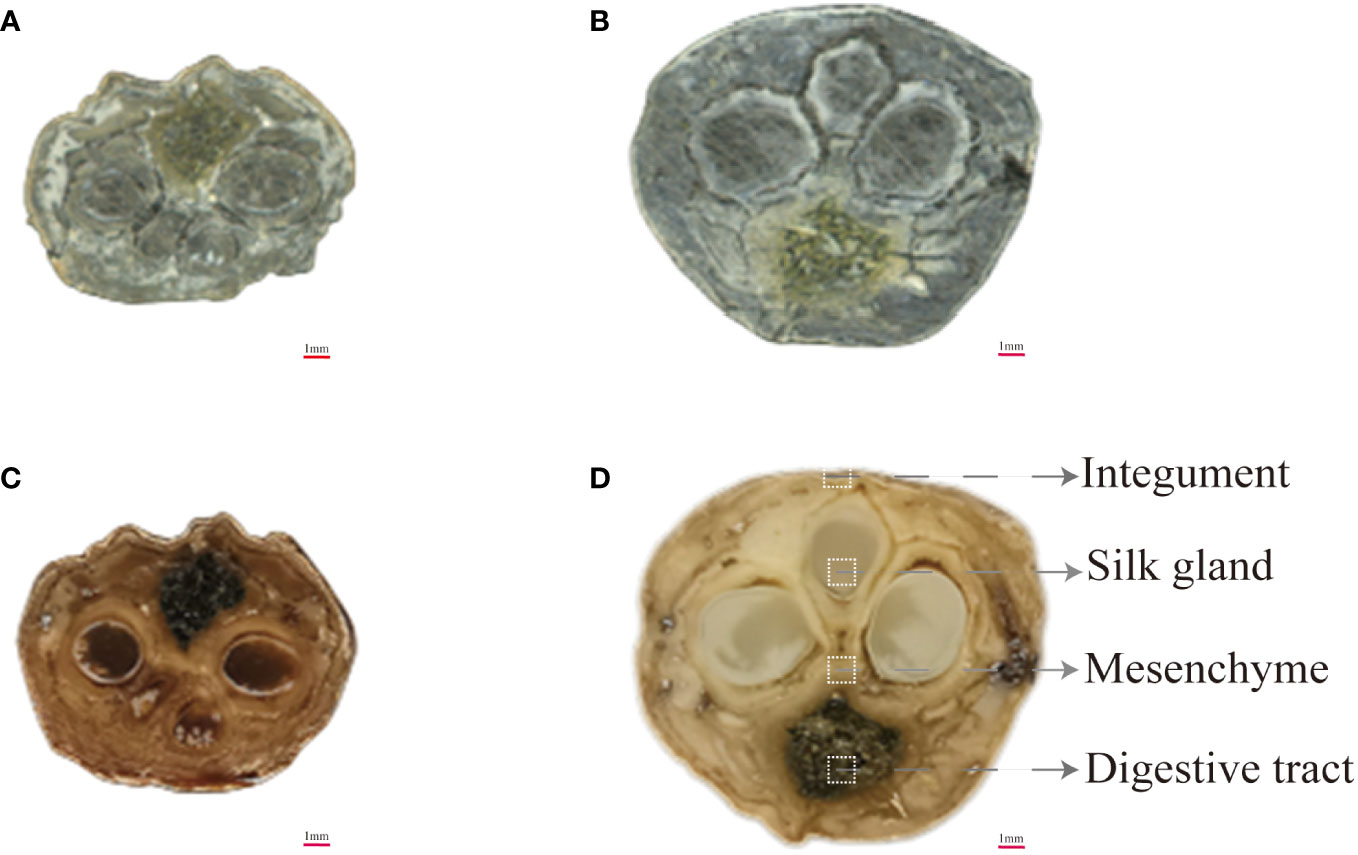
Figure 4 Cross-sectional optical images of raw and fried Bombyx batryticatus. (A, B) RBB. (C, D) FBB.
Among all the compounds, the contents of peptides and amino acids accounted for the highest proportion, which existed in a variety of forms. Figure 5 illustrates the spatial distribution features of beauvericin (m/z 784.42) and bassianolide (m/z 947.57), two types of ring-shaped polypeptides, which were detected as the [M+H]+ and [M+K]+ ionic modes. Although they were both cyclic multi-peptide analogs, their distribution patterns were remarkably divergent. The former was more plentiful in the peripheral zone of the silk gland and the latter was more abundant in the epidermis layer of integument. Beauverolide was a large class of cyclic tricarboxylic acid peptide analogs with a variety of subtypes, such as beauverolide A, beauverolide C and beauverolide I. As determined from the ion signal distribution graphs in Figure 5, the peripheral tissue of the silk gland, mesenchyme and the digestive tract were the dominant concentration region for beauverolide A in the form of sodium adducts (m/z 552.34), beauverolide C in the form of proton adducts (m/z 592.38) and beauverolide I in the form of potassium adducts (m/z 526.28). It was evident from Figure 5 that the cyclic dipeptides cyclo(Ala-Tyr) (m/z 257.10), cyclo(Tyr-Val) (m/z 301.10) and cyclo(Ala-Pro) (m/z 169.10) were distributed in the silk gland, digestive tract and mesenchyme region primarily in the form of sodium, potassium and proton adducts, respectively. Notably, the distribution pattern of the proton adducts of tenellin (m/z 370.17) was accumulated in the area corresponding to almost all mesenchyme layers of Bombyx batryticatus. As a consequence of high-temperature stir-frying, the corresponding distribution areas of some certain cyclic dipeptides, such as cyclo(Tyr-Val), cyclo(Ala-Pro), beauverolide A, and tenellin, were reduced to a certain extent, accompanied by a dramatic decrease in the intensity of the detected ion signals. On the contrary, it was noteworthy that the abundance and distribution range of cyclo(Ala-Tyr), beauvericin, bassianolide, beauverolide C, and beauverolide I significantly increased in their respective regions (Table 2), which might imply that the latter was more thermally stable.
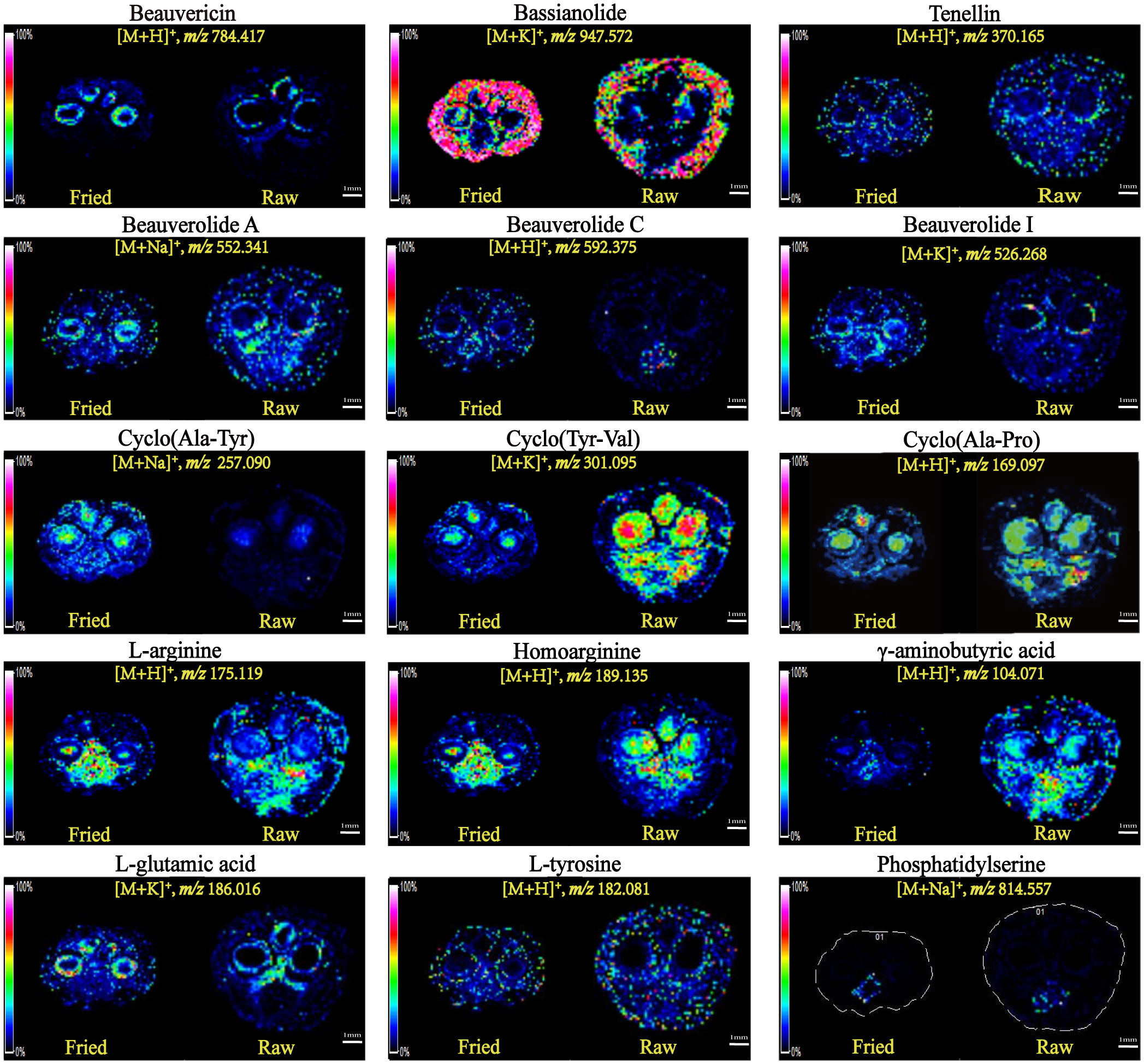
Figure 5 Mass spectrometry images of the ions of representative protein-related metabolites in the tissue sections of raw and fried Bombyx batryticatus.
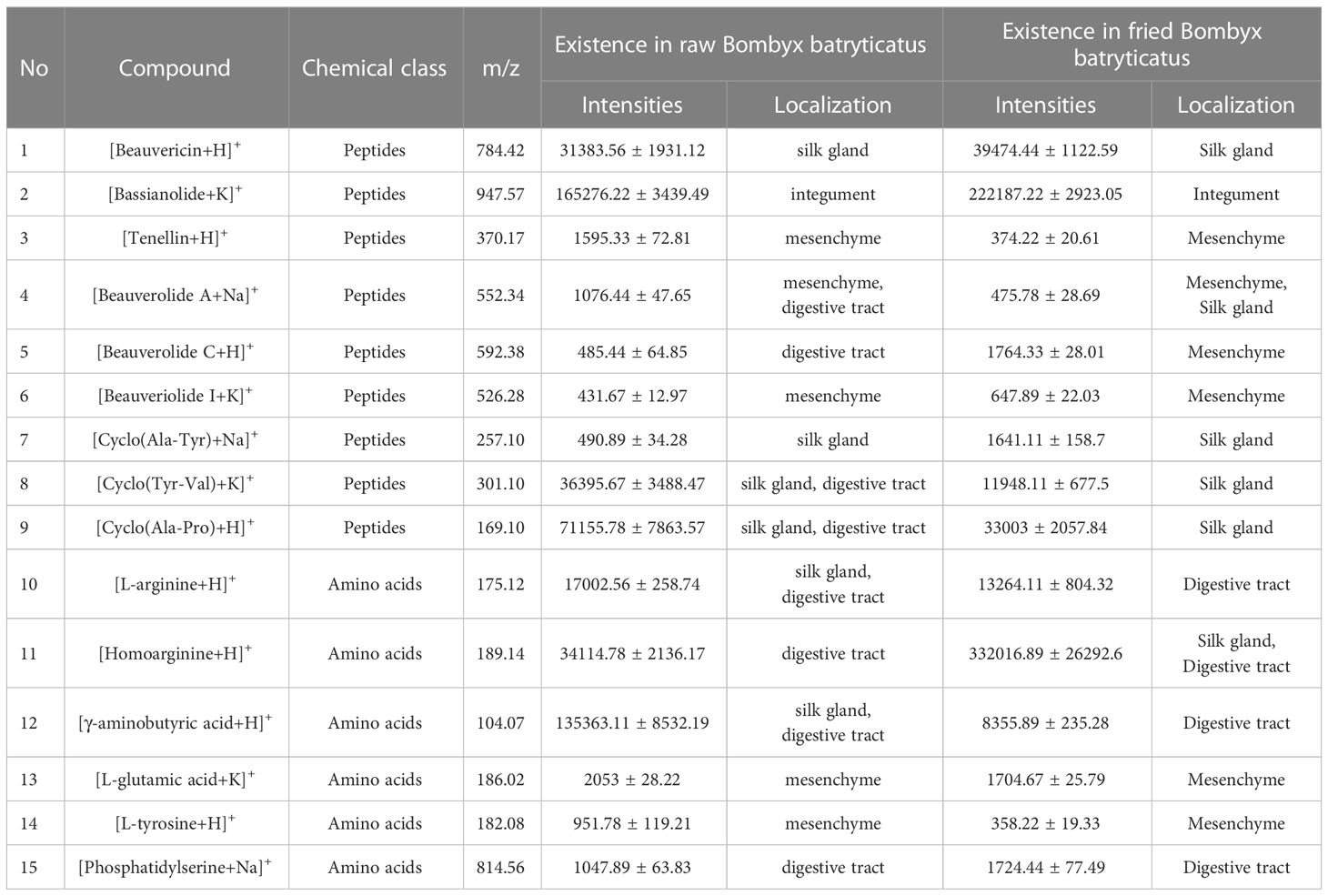
Table 2 Potential markers for the differentiation of raw Bombyx batryticatus and fried Bombyx batryticatus with their relative intensities and localization.
Amino acids, the most widely distributed chemical compounds in Bombyx batryticatus, were virtually present as proton adducts and exhibited a variable distribution in multiple areas. γ-aminobutyric acid (GABA), as a small non-protein amino acid, was an important inhibitory neurotransmitter of the central nervous system that existed widely in vertebrates, plants and microorganisms (Cifelli et al., 2021). L-arginine (m/z 175.12), homoarginine (m/z 189.14) and GABA (m/z 104.07) were more concentrated in the medial part of the silk gland and the digestive tract (Figure 5). In contrast, it was noted that a kind of amino acid was more plentiful in the peripheral region of the silk gland (Figure 5), which was ultimately characterized as L-glutamic acid (m/z 186.02), i.e., a kind of nonessential amino acid possessing the effect of treating hepatic coma. The distribution pattern of the proton adducts of L-tyrosine (m/z 182.08) was completely different from the above-mentioned localization of amino acids and accumulated at the area corresponding to almost all regions except the silk gland. Phosphatidylserine (m/z 814.56) appeared to have a clear tendency to distribute only in the central area of the digestive tract (Figure 5). Furthermore, it was noteworthy that the distribution areas of a considerable percentage of amino acids were shrunk to a large extent along with the content levels decreased significantly, whereas those of homoarginine increased substantially through the high-temperature processing in contrast (Table 2).
Discussion
By integrating factors such as tissue integrity, data accuracy and detection sensitivity, we eventually set the section thickness of the tissue at 20 μm and the temperature at -25°. Further, in the pre-experiments for the extraction of active ingredients of Bombyx batryticatus we observed that more ionic peaks could be detected in the positive mode by spraying with 2-MBT as the matrix compared to the negative mode, possibly related to the spatial structure and ionization mode of the compounds. Therefore, we determined to choose the positive mode in our experiments.
The body of the authentic Bombyx batryticatus was white, straight, stiff, crisp, etc., among which the key characters that determined the quality of Bombyx batryticatus were the traits of silk gland in the cross section of Bombyx batryticatus after fracture. The transverse section of Bombyx batryticatus with the best curative properties was described as black, full and shiny like mirrors (Hu et al, 2017). By virtue of the in situ spatial distribution of 15 signature peptides and amino acids, we found that silk gland tissue was the enrichment region for the majority of active ingredients, demonstrating the spatial localization advantage of MSI over traditional analysis techniques. In the field of Chinese medicine research and development, silk gland could be regarded as the primary region for extracting the effective components of Bombyx batryticatus, so as to improve potency and reduce side effects.
Beauvericin is a cyclic tricarboxylic acid peptide, the essentially secondary metabolite secreted by Beauveria bassiana during the growing process as one of the most crucial active ingredients of the Bombyx batryticatus (Chen et al., 2019). We detected various types of beauvericin and its derivatives, such as beauvericin A, bassianolide and beauverolide A, which had pharmacological properties including antitumor (Zhan et al., 2007; Jiang et al., 2014; Wu et al., 2017), antibacterial (Kouri et al., 2005; Mei et al., 2009), antiviral, and anticonvulsant effects (Jiang et al., 2023). Cyclodipeptide is formed by the internal cyclization of two amino acids, with 2,5-diketopiperazine or 2,5-dioxypiperazine as its parent nucleus (Wei, 2020). There were several types of cyclic dipeptides identified by MALDI-MSI, such as cyclo(Tyr-Val) and cyclo(Ala-Pro), with the bioactivities of anti-tumor, neuroprotective, immune and metabolic regulatory, and anti-inflammatory effects (Qiang and Mei, 2014). It was revealed that some certain peptides and amino acids in stir-fried Bombyx batryticatus were elevated in specific regions by MSI, which might be attributed to the local degradation effect of proteins. Notably, we observed that the apparent increase in the abundance of beauvericin and bassianolide was accompanied by dramatic descents of cyclo(Tyr-Val) and cyclo(Ala-Pro). Such a tendency of variation in the content of peptides might greatly indicate the enhancement of antitumor, antiviral and anticonvulsant effects, as well as the decrease of antioxidant, neuroprotective, immune and metabolic regulatory activities.
GABA is an important active ingredient in mulberry leaves. It was reported that the intake of certain amounts of GABA had physiological effects, such as improving the quality of sleep, reducing toxic impairment of hepatocytes, and lowering blood pressure (Nakamura et al., 2011). Arginine is involved in the ornithine cycle in the body, which facilitates the conversion of ammonia produced in the body into non-toxic urea for excretion, thereby reducing the blood ammonia concentration and correcting the acid-base balance in hepatic encephalopathy (Mangoni et al., 2019). Furthermore, homoarginine is a naturally occurring, non-protein-derived, cationic amino acid, which increases the availability of nitric oxide (NO), affecting endothelial function (Rodionov et al., 2021). Elevated levels of homoarginine could exert positive effects that were associated with cardiovascular protection, in terms of strengthening endothelial function, suppressing platelet aggregation and boosting insulin secretion (Kathrin et al., 2019). The results of our study demonstrated that significant decreases in arginine and GABA levels were accompanied by increased homoarginine content during the stir-frying process. The fried Bombyx batryticatus may not be as effective as raw Bombyx batryticatus in treating liver disease and neurotrophic effects, while it was more valuable in reducing the risk of cardiovascular disease (Kong et al., 2014; Zhao et al., 2018).
In recent years, it had been reported that the consumption of raw Bombyx batryticatus could sometimes trigger adverse reactions, probably due to the entry of foreign proteins into the body, which could lead to allergy and intense multi-organ syndromes through the immune mechanism, manifesting as respiratory obstruction, circulatory symptoms and abdominal pain as well as other type I allergic reactions. The allergen was also cross-reactive with the central nervous system, resulting in metabolic disorders and dysfunction of the central nervous system (Tu and Yu, 2012). Therefore, it was confirmed that the Bombyx batryticatus should be stir-fried with wheat bran before being applied to therapeutic purposes on account of the clinical experience from ancient Chinese medicine practitioners. As a matter of fact, this empirical medical theory was not fully accepted by the western medicine. Notably, it could be demonstrated from the rigorous data obtained in our study that fried Bombyx batryticatus had the following advantages over raw Bombyx batryticatus with reference to the relevant literature.
Above all, during the process of high-temperature frying, the energy absorbed by the protein exceeded the intermolecular interaction force, leading to the destruction of the spatial conformation in protein, followed by the rupture of the peptide chain, thus partially denaturing and inactivating the protein (Lin et al., 2020). The reduction of the animal protein and peptides in the fried Bombyx batryticatus moderated the irritation of the medicine applied to the patient, which was in accordance with the principles of processing (Ma et al., 2015). In addition, due to the breakage of the peptide bond by thermal oscillation during the stir-frying process, small molecule oligopeptides could be produced for oral absorption, solving the problem of low absorption rate of natural oligopeptides and the difficulty of industrialization, and providing an experimental basis for the development and utilization of the Bombyx batryticatus resources (Li et al., 2019).
Furthermore, the interaction between the excipients and the drug existed in the container because of substantial addition of wheat bran during the process of concoction. The surface of wheat bran was loose and porous with a higher surface area, reinforcing the adsorption activity. Meanwhile, wheat bran was rich in dietary fiber possessing an anti-oxidant effect. After being fried, there was plenty of dietary fiber remaining on the surface of the Bombyx batryticatus, which inhibited the biosynthesis of fungal toxins (Zhao et al., 2010). As a consequence of Maillard reaction on the Bombyx batryticatus during the stir-frying process, the epidermis of Bombyx batryticatus became golden in color with an aromatic smell, which promoted the digestive function and improves the therapy compliance of patients when taking medicine (Hu et al., 2016; Li et al., 2021b).
Conclusion
In this study, a high-resolution MALDI-MSI technique was applied to elucidate the in situ distribution of protein-related metabolites in the cross sections of raw and fried Bombyx batryticatus. Ultimately, 15 processing-related markers were screened and visualized in combination with OPLS-DA, which may have a significant impact on the research of the holistic chemical transformation mechanism of Bombyx batryticatus before and after frying with wheat bran.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
PL and J-MW performed the experiments and preparation of the draft manuscript. H-CG, M-WZ, and Y-XS analyzed the data. HG and X-HD prepared figures and tables. Y-PY project conceptualization, conceived, and designed the experiments. Y-GZ conceived and designed the experiments, and reviewed drafts of the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the Scientific and Technological Research Projects of Colleges and Universities in Hebei Province (ZD2020111), the Innovation Team of Hebei Province Modern Agricultural Industry Technology System (HBCT2018060205 and HBCT2018060201), the S&T Program of Hebei (22326418D,19277637D, and H2022418001), and the Scientific Research Capability Improvement Project of Hebei University of Chinese Medicine (KTZ2019006 and KTY2019077).
Acknowledgments
Thanks to Professor Hongjie Li of the Institute of Crop Science, Chinese Academy of Agricultural Sciences for revising the language of the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1144556/full#supplementary-material
References
Bai, Y.-P., Zhao, Q., He, M.-X., Ye, X.-T., Zhang, X.-Z. (2018). Extensive characterization and differential analysis of endogenous peptides from bombyx batryticatus using mass spectrometric approach. J. Pharm. Biomed. Anal. 163, 78–87. doi: 10.1016/j.jpba.2018.09.033
Chen, W.-W., Hu, M.-B., Peng, W., Wu, C.-J. (2019). Research progress of effective ingredient beauvericin in the bombyx batryticatus. China Pharm. 30 (24), 3452–3456. doi: 10.6039/j.issn.1001-0408.2019.24.26
Chinese Pharmacopoeia Commission (2020). Pharmacopoeia of the people’s republic of China Vol. Volume I (Beijing: China Medical Science Press).
Cifelli, P., Di, A.-S., Alfano, V., Morano, A., De, F.-E., Aronica, E., et al. (2021). Dissecting the molecular determinants of GABA receptors current rundown, a hallmark of refractory human epilepsy. Brain Sci. 11 (4), 441. doi: 10.3390/brainsci11040441
Gennebäck, N., Malm, L., Hellman, U., Waldenström, A., Mörner, S. (2013). Using OPLS-DA to find new hypotheses in vast amounts of gene expression data - studying the progression of cardiac hypertrophy in the heart of aorta ligated rat. Gene 522 (1), 27–36. doi: 10.1016/j.gene.2013.03.018
Hu, M.-B. (2017). The processing technology standardization and quality related research of bombyx batryticatus stir-fried with bran (Chengdu: Chengdu University of Traditional Chinese Medicine).
Hu, B.-M., Liu, Y.-J., Xie, D.-S., Xiao, H., Li, Y.-C. (2016). Maillard reaction in process of bombyx batryticatus stir-fried with bran. J. Chin. Med. Mater. 39 (10), 2221–2224. doi: 10.13863/j.issn1001-4454.2016.10.017
Hu, M.-B., Yu, Z.-J., Wang, J.-L., Fan, W.-X., Liu, Y.-J., Li, J.-H., et al. (2017). Traditional uses, origins, chemistry and pharmacology of bombyx batryticatus: A review. Molecules 22 (10), 1779. doi: 10.3390/molecules22101779
Jiang, Q., Wang, L.-N., Liu, Y., Liu, Q., Yang, R., Zhang, Y.-Q. (2023). Research progress on processing history evolution, chemical constituents and pharmacological action of bombyx batryticatus. China J. Chin. Mater. Med. 1-13. doi: 10.19540/j.cnki.cjcmm.20230113.201
Jiang, X., Zhang, Z.-F., Chen, Y.-J., Cui, Z.-T., Shi, L.-G. (2014). Structural elucidation and in vitro antitumor activity of a novel oligosaccharide from bombyx batryticatus. Carbohydr. Polym. 103, 434–441. doi: 10.1016/j.carbpol.2013.12.039
Kathrin, C., Doreen, S., Natalie, L., Lucie, C., Rainer, H.-B., Maxim, A., et al. (2019). Association of asymmetric dimethylarginine and diastolic dysfunction in patients with hypertrophic cardiomyopathy. Biomolecules 9 (7), 277. doi: 10.3390/biom9070277
Kong, Y., Xu, C., He, Z.-L., Zhou, Q.-M., Wang, J.-B., Li, Z.-Y., et al. (2014). A novel peptide inhibitor of platelet aggregation from stiff silkworm, bombyx batryticatus. Peptides 53, 70–78. doi: 10.1016/j.peptides.2013.12.004
Kouri, K., Duchen, M.-R., Lemmens-Gruber, R. (2005). Effects of beauvericin on the metabolic state and ionic homeostasis of ventricular myocytes of the guinea pig. Chem. Res. Toxicol. 18, 1661–1668. doi: 10.1021/tx050096g
Li, B., Bhandari, D.-R., Janfelt, C., Römpp, A., Spengler, B. (2014). Natural products in Glycyrrhiza glabra (licorice) rhizome imaged at the cellular level by atmospheric pressure matrix-assisted laser desorption/ionization tandem mass spectrometry imaging. Plant Journal: Cell Mol. Biol. 80, 161–171. doi: 10.1111/tpj.12608
Li, B., Ge, J.-Y., Liu, W., Hu, D.-J., Li, P. (2021a). Unveiling spatial metabolome of Paeonia suffruticosa and Paeonia lactiflora roots using MALDI MS imaging. New Phytol. 231 (2), 892–902. doi: 10.1111/nph.17393
Li, J. F., Wang, Y. P., Bian, X. F., Zhang, H., Sun, J. M. (2019). Anti-parkinson activity of bombyx batryticatus in vitro before and after processing based on thermal oscillation theory of peptide Bond. Mod. Chin. Med. 9, 1229–1235. doi: 10.13313/j.issn.1673-4890.20190215001
Li, M.-R., Wang, X.-Y., Han, L.-F., Jia, L., Liu, E., Li, Z., et al. (2020). Integration of multicomponent characterization, untargeted metabolomics and mass spectrometry imaging to unveil the holistic chemical transformations and key markers associated with wine steaming of ligustri lucidi fructus. J. Chromatogr. A 1624, 461228. doi: 10.1016/j.chroma.2020.461228
Li, Y.-L., Zhu, Y.-Z., Zheng, P.-H., Qu, Z.-Y., Zhang, H., Hou, W., et al. (2021b). Potentially harmful maillard reaction products in food and herb medicines. J. Food Qual. 2021, 1–11. doi: 10.1155/2021/1798936
Lin, L., Zhang, Y.-J., Li, Y., Fu, H.-Z., Hu, J.-H., Zhou, Y., et al. (2020). Identification of signature proteins of processed bombyx batryticatus by comparative proteomic analysis. Int. J. Biol. Macromol. 153, 289–296. doi: 10.1016/j.ijbiomac.2020.03.010
Ma, L., Wang, X., Ma, L., Wang, M.-Y., Qiu, F. (2015). Scientific connotation of processing bombyx batryticatus under high temperature. China J. Chin. Mater. Med. 40 (23), 4629–4633. doi: 10.4268/cjcmm20152317
Mangoni, A. A., Rodionov, R. N., McEvoy, M., Zinellu, A., Carru, C., Sotgia, S. (2019). New horizons in arginine metabolism, ageing and chronic disease states. Age Ageing 48, 776–782. doi: 10.1093/ageing/afz083
Mei, L., Zhang, L.-X., Dai, R. (2009). An inhibition study of beauvericin on human and rat cytochrome p450 enzymes and its pharmacokinetics in rats. J. Enzyme inhibit. med. Chem. 24 (3), 753–762. doi: 10.1080/14756360802362041
Nakamura, Y., Hinoi, E., Takarada, T., Takahata, Y., Yamamoto, T., Fujita, H., et al. (2011). Positive regulation by gaba(b)r1 subunit of leptin expression through gene transactivation in adipocytes. PloS One 6 (5), e20167. doi: 10.1371/journal.pone.0020167
Qiang, W., Mei, G. (2014). Research progress on antitumor activity of fungal cyclic dipeptides and their derivatives. China Pharm. 21, 2007–2010. doi: 10.6039/j.issn.1001-0408.2014.21.30
Rodionov, R.-N., Beyer-Westendorf, J., Bode-Boger, S.-M., Eggebrecht, L., Konstantinides, S., Martens-Lobenhoffer, J., et al. (2021). Homoarginine and methylarginines independently predict long-term outcome in patients presenting with suspicion of venous thromboembolism. Sci. Rep. 11, 9569. doi: 10.1038/s41598-021-88986-y
Shu, T., Ryuzo, I., Naohiko, Y., Issey, O., Manabu, S., Mitsuro, K., et al. (2010). Mass spectrometric imaging of ginsenosides localization in Panax ginseng root. Am. J. Chin. Med. 38 (3), 485–493. doi: 10.1142/S0192415X10008007
Tu, Y. D., Yu, X. P. (2012). The clinical use of banbusa-lxilis in lung diseases and its side effects. Shanghai J. Tradit. Chin. Med. 46 (12), 64–66. doi: 10.16305/j.1007-1334.2012.12.021
Wang, S.-J., Bai, H.-R., Cai, Z.-W., Gao, D., Jiang, Y.-Y., Liu, J.-J., et al. (2016). MALDI imaging for the localization of saponins in root tissues and rapid differentiation of three Panax herbs. Electrophoresis 37 (13), 1956–1966. doi: 10.1002/elps.201600027
Wang, P.-L., Shang, E.-X., Fan, X.-S. (2021a). Effect of san’ao decoction with scorpio and bombyx batryticatus on CVA mice model via airway inflammation and regulation of TRPA1/TRPV1/TRPV5 channels. J. Ethnopharmacol. 264, 113342. doi: 10.1016/j.jep.2020.113342
Wang, M., Wan, Z.-Y., Chen, H., Zhang, D.-L., Yu, W., Li, M., et al. (2022). Study on quality evaluation of bombyx batryticatus by chemical pattern recognition combined with HPLC fingerprint. Natural Prod. Res. Dev. 34, 203–212. doi: 10.16333/j.1001-6880.2022.2.004
Wang, S.-Q., Zhang, L.-R., Chen, W., Pan, Y.-Z., Zhou, F., Su, Z.-H., et al. (2021b). A study on fingerprint and common peaks identification of water-soluble components in bombyx batryticatus. J. Clin. Med. Pract. 25 (23), 53–57. doi: 10.7619/jcmp.20213009
Wei, G.-X. (2020). Research progress of cyclic dipeptides. Contemp. Chem. Industry 49 (2), 406–409. doi: 10.13840/j.cnki.cn21-1457/tq.2020.02.038
Wu, J.-Y., Sheikho, A., Ma, H., Li, T.-C., Zhao, Y.-Q., Zhao, Y.-L., et al. (2017). Molecular mechanisms of bombyx batryticatus ethanol extract inducing gastric cancer sgc-7901 cells apoptosis. Cytotechnology 69 (6), 875–883. doi: 10.1007/s10616-017-0102-7
Xing, D.-X., Shen, G.-W., Li, Q.-, Xiao, Y., Yang, Q., Xia, Q.-Y. (2019). Quality formation mechanism of stiff silkworm, bombyx batryticatus using UPLC-Q-TOF-MS-based metabolomics. Molecules 24 (20), 3780. doi: 10.3390/molecules24203780
Zemaitis, K.-J., Veličković, D., Kew, W., Fort, K.-L., Reinhardt-Szyba, M., Pamreddy, A., et al. (2022). Enhanced spatial mapping of histone proteoforms in human kidney through MALDI-MSI by high-field UHMR-orbitrap detection. Analytical Chem. 94 (37), 12604–12613. doi: 10.1021/acs.analchem.2c01034
Zhan, J.-X., Burns, A.-M., Liu, M.-X., Faeth, S.-H., Gunatilaka, A.-A. (2007). Search for cell motility and angiogenesis inhibitors with potential anticancer activity: beauvericin and other constituents of two endophytic strains of Fusarium oxysporum. J. Natural Prod. 70 (2), 227–232. doi: 10.1021/np060394t
Zhao, Q., Hao, L. J., Ma, X. L., Yuan, W. Y., Guo, H. (2010). Study on thin layer chromatography differentiation and determination of six kinds of prepared bombyx batryticatus. Liaoning J. Tradit. Chin. Med. 12, 2421–2424. doi: 10.13192/j.ljtcm.2010.12.154.zhaoq.070
Keywords: MALDS-MSI, before and after frying, OPLS-DA, protein-related metabolites, Chinese medicine, Bombyx batryticatus
Citation: Liu P, Wang J-M, Guo H-C, Zhao M-W, Song Y-X, Guo H, Duan X-H, Yan Y-P and Zheng Y-G (2023) In situ detection and mass spectrometry imaging of protein-related metabolites in Bombyx batryticatus before and after frying with wheat bran. Front. Plant Sci. 14:1144556. doi: 10.3389/fpls.2023.1144556
Received: 14 January 2023; Accepted: 10 March 2023;
Published: 06 April 2023.
Edited by:
Yinglang Wan, Hainan University, ChinaReviewed by:
Zhi Chao, Southern Medical University, ChinaLixing Nie, National Institutes for Food and Drug Control, China
Copyright © 2023 Liu, Wang, Guo, Zhao, Song, Guo, Duan, Yan and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Ping Yan, eWFueXVwaW5nMjAwOEAxMjYuY29t; Yu-Guang Zheng, enlnMzE0QDE2My5jb20=
†These authors have contributed equally to this work
 Pai Liu1,2†
Pai Liu1,2† Jie-Min Wang
Jie-Min Wang Yu-Guang Zheng
Yu-Guang Zheng