
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 02 March 2023
Sec. Functional Plant Ecology
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1143442
This article is part of the Research TopicPlant Responses to Environmental Stresses Based on Physiological and Functional EcologyView all 31 articles
 Yahui Zhang1
Yahui Zhang1 Shubin Xie1
Shubin Xie1 Xiaoting Wang1
Xiaoting Wang1 Muhammad Adnan Akram1,2
Muhammad Adnan Akram1,2 Weigang Hu1
Weigang Hu1 Longwei Dong1
Longwei Dong1 Ying Sun1
Ying Sun1 Hailing Li1
Hailing Li1 Abraham Allan Degen3
Abraham Allan Degen3 Junlan Xiong1*
Junlan Xiong1* Jinzhi Ran1*
Jinzhi Ran1* Jianming Deng1
Jianming Deng1Determining response patterns of plant leaf elements to environmental variables would be beneficial in understanding plant adaptive strategies and in predicting ecosystem biogeochemistry processes. Despite the vital role of microelements in life chemistry and ecosystem functioning, little is known about how plant microelement concentrations, especially their bioconcentration factors (BCFs, the ratio of plant to soil concentration of elements), respond to large-scale environmental gradients, such as aridity, soil properties and anthropogenic activities, in drylands. The aim of the present study was to fill this important gap. We determined leaf microelement BCFs by measuring the concentrations of Mn, Fe, Ni, Cu and Zn in soils from 33 sites and leaves of 111 plants from 67 species across the drylands of China. Leaf microelement concentrations were maintained within normal ranges to satisfy the basic requirements of plants, even in nutrient-poor soil. Aridity, soil organic carbon (SOC) and electrical conductivity (EC) had positive effects, while soil pH had a negative effect on leaf microelement concentrations. Except for Fe, aridity affected leaf microelement BCFs negatively and indirectly by increasing soil pH and SOC. Anthropogenic activities and soil clay contents had relatively weak impacts on both leaf microelement concentrations and BCFs. Moreover, leaf microelement concentrations and BCFs shifted with thresholds at 0.89 for aridity and 7.9 and 8.9 for soil pH. Woody plants were positive indicator species and herbaceous plants were mainly negative indicator species of leaf microelement concentrations and BCFs for aridity and soil pH. Our results suggest that increased aridity limits the absorption of microelements by plant leaves and enhances leaf microelement concentrations. The identification of indicator species for the response of plant microelements to aridity and key soil characteristics revealed that woody species in drylands were more tolerant to environmental changes than herbaceous species.
All 14 essential elements including 6 macroelements and 8 microelements, are important for plant ontogenetic processes such as growth, maintenance and reproduction (Marschner, 2012; Luo et al., 2016). Although there are numerous studies on the variation in concentrations of both plant and soil macroelements and on their impacts on plant growth and functions (Delgado-Baquerizo et al., 2013; Jiao et al., 2016; Li et al., 2022), little is known on how the concentrations of plant and soil microelements respond concomitantly to an environmental gradient.
Microelements play vital roles in plant ontogenetic growth and functions by influencing enzyme activities of biochemical reactions in organisms (Huang et al., 2020). For example, more than 35 enzymes, which are involved in different physiological functions and redox reactions, are activated by manganese (Mn) (Kabata-Pendias and Szteke, 2015). As a redox active metal, iron (Fe) is essential for many biological processes, including photosynthesis, mitochondrial respiration, nitrogen assimilation, and hormone biosynthesis (Marschner, 2012). Nickel (Ni) is needed for many prokaryotic enzymes and is involved in nitrogen (N) metabolism, while copper (Cu) is indispensable for photosynthesis, carbon (C) and N metabolism, oxidative stress protection and cell wall synthesis (Hänsch and Mendel, 2009). Zinc (Zn) is an essential component of enzymes and is involved in protein synthesis, energy production, and maintenance of the structural integrity of bio-membranes (Marschner, 2012). Ni, Cu and Zn are heavy metals in the soil (Qin et al., 2021), and either excessive or deficient contents can constrain plant growth, and physiological and metabolic processes (Mohamed et al., 2003; Sardar et al., 2013).
Recent studies have focused on the bioconcentration factor (BCF) of elements in plants to determine the capacity of plants to absorb metallic elements (Lin et al., 2016; Bonanno et al., 2017; Thanh-Nho et al., 2019; Zhang et al., 2019; Akram et al., 2021). The capacity of plants to absorb metallic elements is dependent not only on the plants, but also on soil temperature, texture, cation exchange capacity, organic matter, and the concentrations of available elements (Gupta et al., 2019). Furthermore, aridity can also affect the soil available elements in drylands indirectly through its effects on soil pH, organic matter and clay content (Moreno-Jiménez et al., 2019). Increasing aridity due to climate change, therefore, can have a strong influence on global biogeochemical cycles and ecosystems (Jiao et al., 2016), but its effect on the concentration and BCF of metallic elements in plants remains unclear. Additionally, anthropogenic activities related to agriculture and industry, and climate change are major drivers of global environmental change (Delgado-Baquerizo et al., 2016), which further modifies nutrient element cycling in an ecosystem. However, little is yet known on the effects of aridity and anthropogenic activities on the absorption of metallic elements by plants. It was reported that anthropogenic activities can have a positive impact on the atmospheric deposition of potassium (K), while aridity can have a negative effect on K absorption by plants in drylands (Sardans and Peñuelas, 2015).
Aridity has increased in the past decades in many drylands (Huang et al., 2016), which is of concern, as drylands are fragile and sensitive to climate change and human activities (Hu et al., 2021). Previous studies examined the effects of water, salinity and temperature stress on plants in drylands (Perri et al., 2018; Peguero-Pina et al., 2020). However, metallic elements, including microelements, in soil are usually very rich to cause stress on plant ontogenetic growth and function in drylands because of the serious soil salinization. Therefore, an understanding of microelement cycling would be beneficial in maintaining and improving the plant community structure and ecosystem functions of the terrestrial biosphere under global change (Cai et al., 2017).
Site-specific environmental factors can influence concentrations and thresholds of biological tolerance of plant nutrient elements (Ren et al., 2019). Determining the variations in leaf microelement concentrations and BCFs along environmental gradients enables the calculations of ecological thresholds, and, thereby, to understand abrupt shifts in ecological responses of microelements (Groffman et al., 2006). Generally, there is a shift in dominant plant species from herbaceous to desert shrub species with increasing aridity in dryland ecosystems (Yao et al., 2021b). Herbaceous plant species, with faster growth rates, typically grow in nutrient-rich environments, and display less tolerance to environmental stresses than woody plant species (Rebele, 2013). Woody species, with slower growth rates, tend to grow in nutrient-poor environments, and display a higher tolerance to environmental stresses than herbaceous species (Sun et al., 2020). Consequently, in a harsh environment, Xiong et al. (2021) found that species with weak stress tolerance can serve as negative indicators, while stress-tolerant species can serve as positive indicators of leaf C, N and P concentrations along an increasing environmental stress gradient. Nevertheless, it remains unclear whether leaf microelement concentrations follow the same pattern in drylands.
We selected 33 sites and 111 plant samples from 67 species across the drylands of northern China to determine the response of plant leaf microelement (Mn, Fe, Ni, Cu and Zn) concentrations and BCFs to environmental gradients. Based on previous studies, we hypothesized that: (1) leaf microelement concentrations are maintained within relatively normal ranges to satisfy the basic requirements of plants to survive and reproduce; (2) leaf microelement concentrations and BCFs are mediated by aridity, human impact index (HII), soil physical properties and soil chemical contents; (3) environmental gradients cause abrupt shifts in the response of plant element concentrations to the ecological environment; that is, there are thresholds for plant leaf microelement concentrations and BCFs along major environmental gradients; and (4) as adaptation strategies to environmental stress differ among plant taxa (Xiong et al., 2021), woody species could serve as positive indicators and herbaceous species as negative indicators for leaf microelement concentrations and BCFs along aridity and soil pH gradients; whereas, herbaceous species could serve as positive indicators and woody species as negative indicators along SOC gradients.
Thirty-three sites were selected across Xinjiang, Inner Mongolia, Gansu, and Qinghai provinces in northern China (Figure 1). The region extends between 33.67°N to 50.70°N and 76.38°E to 121.68°E, with elevations ranging between 194 and 3567 m above sea level (Figure 1). Mean annual precipitation ranges between 35 and 474 mm, mean annual temperature ranges between -3.8 and 12.1 °C, and aridity [1 – aridity index, where aridity index = precipitation/potential evapotranspiration (Delgado-Baquerizo et al., 2018)] ranges between 0.28 and 0.98. The main vegetation types in the sample sites included desert, desert grassland, typical grassland and alpine grassland. There were 14 types of soil, including cambisols, chemozems and calcisols.
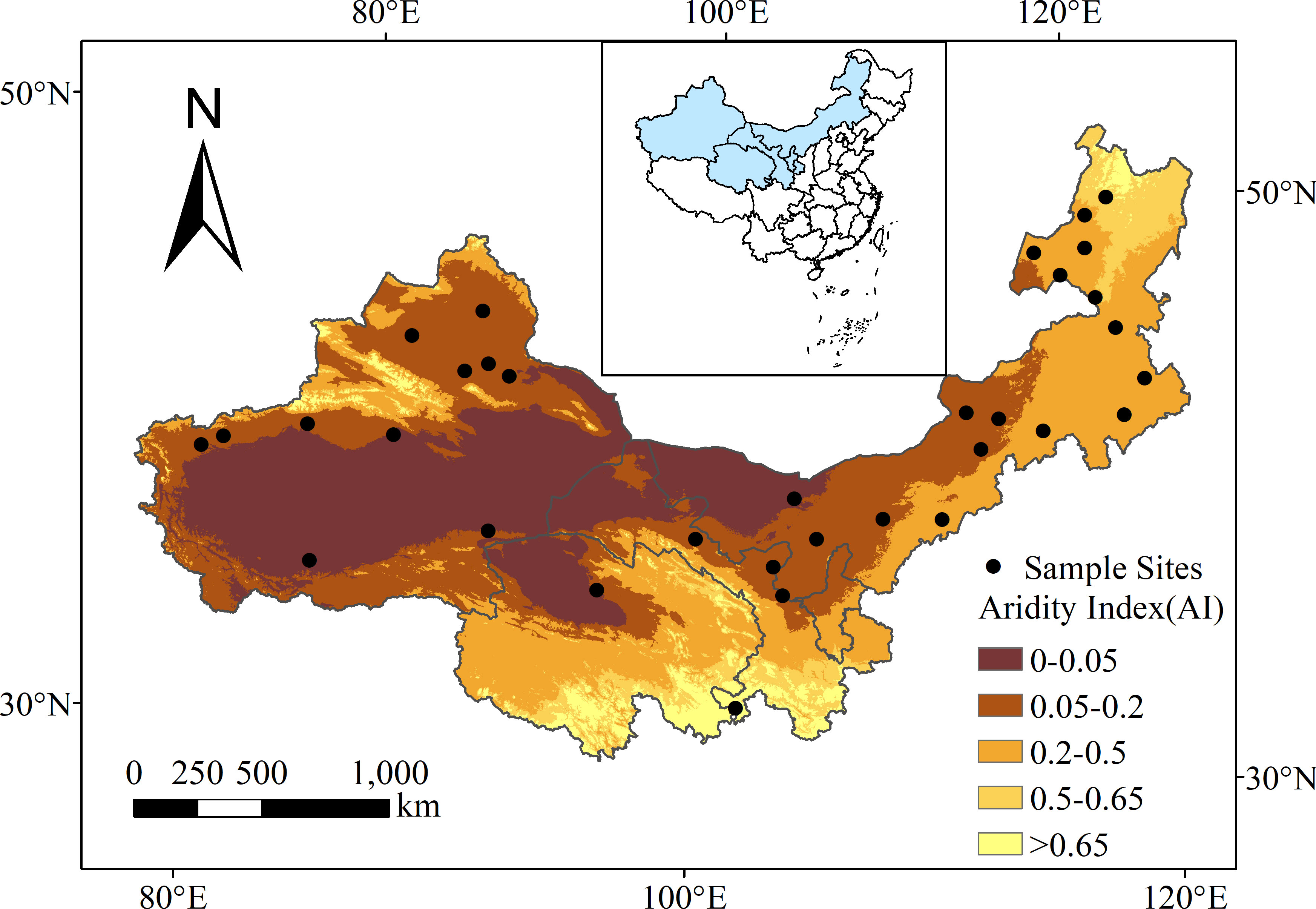
Figure 1 Distribution of the 33 sampling sites in drylands of northern China. The background map is aridity index (AI; generated by ArcGIS 10.3). Inset map shows China with the study area in blue.
Leaves and soils were sampled during the growing seasons (June to September) from 2013 to 2017, using standard protocols described by Deng et al. (2006) and Chen et al. (2019). At least three representative and relatively homogeneous vegetation quadrats, each 30 m × 30 m, were selected randomly at each site. Dominant plant species were identified in each quadrat, and at least 5 mature individuals of each species were collected. In total, there were 67 species, belonging to 57 genera and 25 families. Five core soil samples at a depth of 0-20 cm were collected randomly in non-vegetated areas (bare land) and under the canopy of dominant plant species in each quadrat. The five soil samples of each type were combined.
An in-situ portable global positioning system recorded the coordinates of each site. Aridity data were extracted from the WorldClim 2.0 database (https://www.worldclim.org). Human influence index (HII), ranging from 0 to 100 (0 is the minimum and 100 the maximum HII value) was obtained from Last of the Wild (v2) (Wildlife Conservation Society (WCS) and Center for International Earth Science Information Network (CIESIN) at Columbia University, 2005). Soil clay content was acquired from WISE30sec database (Batjes, 2016).
Soil samples were air-dried, and stones, roots, leaves and debris were removed. After homogenization, the soil was sieved through a 2 mm nylon mesh for measurements of soil variables. For the determination of soil Mn, Fe, Ni, Cu and Zn contents, a portion of the soil was ground in an agate mortar, sieved through a 0.074-mm nylon mesh, and stored in a desiccator. Intact leaves were collected from the dominant plant species, rinsed three times with double-distilled water to remove dust and soil, oven-dried at 60 °C for 72 h to constant weight, ground to a fine powder, and kept in a desiccator before measurements.
Soil pH and soil electrical conductivity (EC) were determined by the soil: distilled water method using a ratio of 1:2.5 and 1:5, with a pH meter (Sartorius PB-10, Göttingen, Germany) and EC meter (DDSJ-318, Yantai Stark Instrument, Yantai, China), respectively. Soil organic carbon (SOC) concentration was determined by the wet oxidation method: 0.5 g soil was digested with 5 mL K2Cr2O7 and 5 mL concentrated H2SO4 at 180 °C for 30 min, and then titrated with 0.5 M FeSO4 till endpoint.
To determine concentrations of Mn, Fe, Ni, Cu and Zn in soil and plant samples, different digestion procedures were used. For soil samples, an ultrapure mixture of HNO3 (6 mL) + HF (2 mL) + HCl (2 mL) was used, and for plant samples, an ultrapure mixture of HNO3 (6 mL) + H2O2 (2 mL) was used. Each sample (0.0500 g) was solubilized in a 50 mL Teflon tube, sealed, placed in a high efficiency anti-corrosive tube sheath with polytetrafluoroethylene (PTFE) coating, and digested at 192 °C for 1.5 h. The solution was evaporated to dryness, driven away by HNO3 acid, diluted with Milli-Q water to 50 g, and stored at 4 °C for further analysis. The concentrations of elements in the soil and plant samples were determined by inductively coupled plasma mass spectrometry (VG PQ ExCell; Thermo Elemental). The concentrations of the microelements are expressed as molar mass per unit dry mass (mol/kg).
Bioconcentration factor (BCF), the capacity of the plant to accumulate microelements in the leaf from soil (Pavel et al., 2014; Yang et al., 2015), was calculated as follows:
where Cleaf and Csoil represent the concentrations of the same microelement in the leaf and soil, respectively.
To determine the influence of phylogeny on the accumulation of microelements by dryland plants, K-statistics were used to detect the phylogenetic signals (K-values) in relation to the leaf microelement concentrations and BCFs. K-statistics are based on the ‘Brownian motion’ model to estimate the relationship between the total variation and the random expectation of the variance of the traits (Blomberg et al., 2003). When K > 1, the trait is controlled mainly by phylogeny, as the phylogenetic signal of the trait is stronger than the Brownian motion model; however, when K < 1, the trait is influenced mainly by environmental factors. When using phylogenetic signals to measure functional traits, species in phylogenetic trees are usually arranged randomly 999 times, and a K-value is calculated each time. If the observed value is greater than the K-value of the null model (α < 0.05), then the phylogenetic signal of the trait is significant; however, if the observed value is lesser than the K-value of the null model (α > 0.05), then the phylogenetic signal is weak (Blomberg et al., 2003). The concentrations and BCFs of microelements were treated as continuous traits. Species mean values were used and analyses used ‘ape’ (Paradis et al., 2004) and ‘picante’ (Kembel et al., 2010) packages in R.
The data were tested for normality and homoscedasticity, and, where necessary, were log10-transformed before analysis. Subsequently, we used a variance inflation factor (VIF) to test for multi-collinearity, and accepted variables with a VIF < 10 (Montgomery et al., 2012).
To identify the main driving factors of the leaf microelement concentrations and BCFs, regression models using climate (aridity), human influence impact (HII) and soil variables (pH, EC, SOC and soil microelement concentrations) were generated based on Akaike Information Criterion (AIC), with leaf microelement concentrations and BCFs as response variables by the all-subset regression analysis. Best models were selected according to the lowest value of AIC, and the standardized coefficient of each factor was used to compare the relative importance of each in explaining the variations in the leaf microelement concentrations and BCFs. For both the leaf microelement concentrations and BCFs, aridity, HII, soil clay content, EC, SOC and soil pH were reserved (Supplementary Tables 2, 3; Supplementary Figures 2, 3). The analyses used the ‘leaps’ package in R (Lumley and Miller, 2020).
Structural equation models (SEMs) determined the relative importance of variables reserved by the all-subset regression for the leaf microelement concentrations and BCFs. The rationality of a causal model was examined, which was based on priori information on the relationships among the variables, the direct and indirect effects that one variable may have on another were partitioned, and the strengths of the multiple effects were estimated (Grace, 2006). Before modelling, the bivariate relationships between all variables were tested for linearity. The BCFs of Ni, Cu and Zn in leaves were affected curvilinearly by aridity, and these relationships were well described by a second-order polynomial (Supplementary Figure 4). To include polynomial relationships, the square of aridity was introduced into the model using a composite variable approach for the BCFs of leaf microelements. Then, priori models were established that were based on the known effects and relationships among leaf microelement concentrations and BCFs and were considered as factors (Supplementary Figures 5, 6). To test the priori model, the χ2-test (the model has a good fit when 0 ≤ χ2 ≤ 2 and 0.05 < P ≤ 1.00) and the root mean square error of approximation (RMSEA; the model has a good fit when 0 ≤ RMSEA ≤ 0.05 and 0.10 < P ≤ 1.00) were used. Since some variables were not distributed normally, the fit of the model was confirmed using the Bollen–Stine bootstrap test (the model has a good fit when 0.10 < bootstrap P ≤ 1.00) (Delgado-Baquerizo et al., 2013). The priori models provided a good fit to the data. To identify the dominant drivers for leaf microelement concentrations and BCFs, the direct and indirect effects of each variable affecting leaf microelement concentrations and BCFs and the total effect (summing the direct and indirect effects) for each variable were calculated. All SEM analyses used AMOS 21.0 (Amos Development Corporation, IBM SPSS, Chicago, IL, USA).
The functional threshold and indicator species of the leaf microelement concentrations and BCFs along environmental gradients were detected using threshold indicator taxa analysis (TITAN; Baker and King, 2010) with R package ‘TITAN2’. Indicator values (IndVal) of leaf microelement concentrations and BCF were first calculated for each plant species, which were obtained from the product of the relative concentrations and BCFs of microelements between groups and the frequency of occurrence of species within the group. The IndVal was used to identify the points of change in microelements and BCFs of species along the environmental gradient. The middle value of each two environmental gradients was then used as a candidate change point, and the gradients were iteratively divided into two groups. The environmental gradient corresponding to the maximum value of IndVal for each group on either side of the candidate change point was considered to be the threshold value for the species. The z-score was obtained by normalizing the IndVal score according to the mean and standard deviation, and, thus, the z-score distinguishes between negative (z-) and positive (z+) responses of each species to the environmental gradient (Baker and King, 2010). The cumulative values of z- and z+ scores for a species within a community assessed the negative and positive responses of the community to environmental gradients, respectively. The value of the environmental gradient corresponding to the maximum value is considered the threshold for the community (Baker and King, 2010). Species with less than three samples were not considered. Taxa leaf microelement concentrations and BCFs were log10(x+1)-transformed (Xiong et al., 2021).
Mean concentrations of the five microelements in leaves and soils varied greatly. Microelement concentrations ranged from 3.75E-5 mol/kg for Ni to 0.026 mol/kg for Fe in leaves, and from 2.99E-4 mol/kg for Cu to 0.51 mol/kg for Fe in soil. Leaf microelement BCFs ranged from 0.05 for Fe to 0.65 for Zn (Table 1; Supplementary Figure 1). There were significant positive relationships between soil microelement concentrations, as well as between leaf microelement concentrations and BCFs. Leaf microelement concentrations were correlated weakly with their respective soil microelement concentrations (Figure 2).
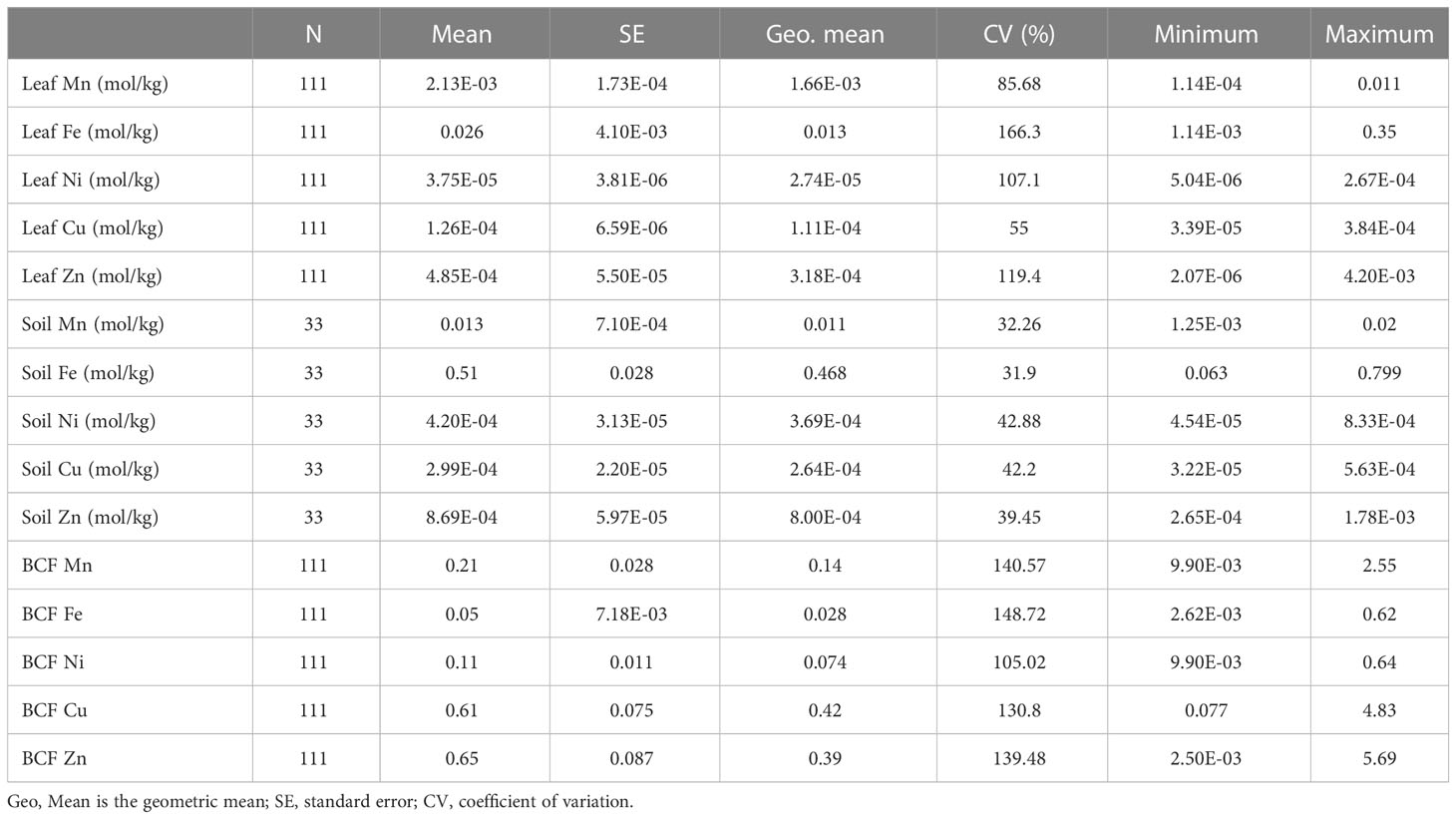
Table 1 Variations of leaf and soil total microelement concentrations and leaf microelement bioconcentration factors (BCFs).
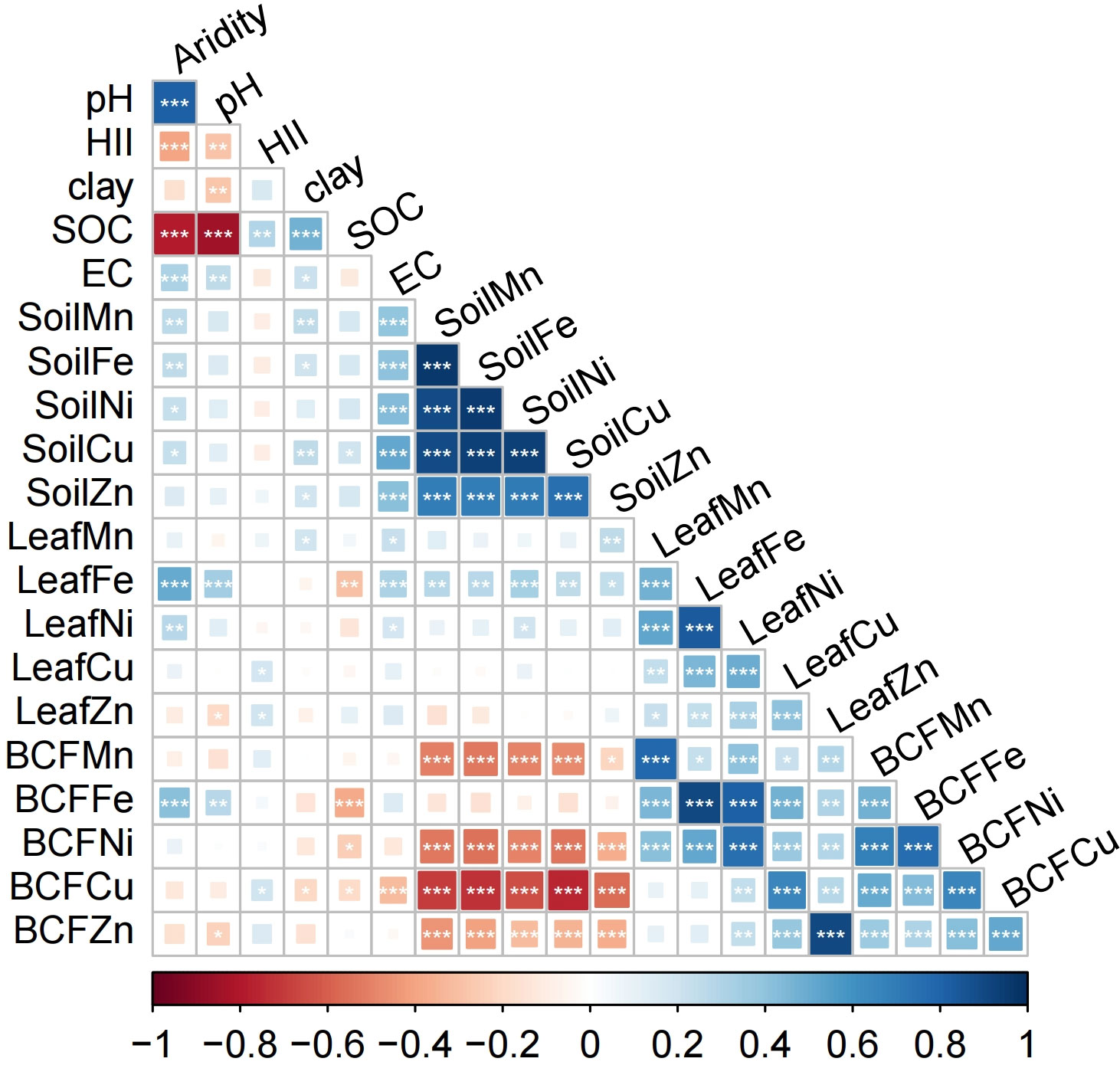
Figure 2 Pearson’s correlation matrix for aridity, HII, soil properties, leaf microelement concentrations and bioconcentration factors (BCFs). HII, human impact index; SOC, soil organic carbon; EC, soil electrical conductivity. Blue squares are positive correlations and red squares are negative correlations. *P< 0.05, **P< 0.01, ***P< 0.001.
Leaf microelement concentrations and BCFs of angiosperm species were combined to calculate the Blomberg’s K-value (Supplementary Table 1). The K-values were less than 1 and not significant, indicating that none of the traits revealed significant phylogenetic signals. Consequently, the influence of phylogeny was not considered in subsequent analyses on the microelement concentrations and BCFs.
The SEM model explained 13.1%, 37.3%, 13.3%, 10.7% and 18.4% of the variance in the concentrations of Mn, Fe, Ni, Cu and Zn in leaves, respectively (Figure 3). Aridity affected the concentrations of leaf Mn, Fe and Ni positively (Figures 3A–C), while soil pH affected leaf Mn and Zn negatively (Figures 3A, E). Concentrations of leaf Fe, Cu and Zn were affected positively by HII (Figures 3B, D, E), and Fe and Zn concentrations were affected positively by EC (Figures 3B, E). The SEM explained 20.0%, 28.2%, 24.9%, 49.2% and 21.7% of the variance in the BCFs of Mn, Fe, Ni, Cu and Zn in leaves, respectively (Supplementary Figure 7). BCFs of Mn, Ni, Cu and Zn were affected negatively by soil pH and SOC (Supplementary Figures 7A, C–E), while the BCF of Fe was only affected negatively by soil pH (Supplementary Figure 7B). Aridity had negative effects on the BCFs of leaf Ni and Cu (Supplementary Figures 7C, D), but a positive effect on the BCF of leaf Fe (Supplementary Figure 7B).
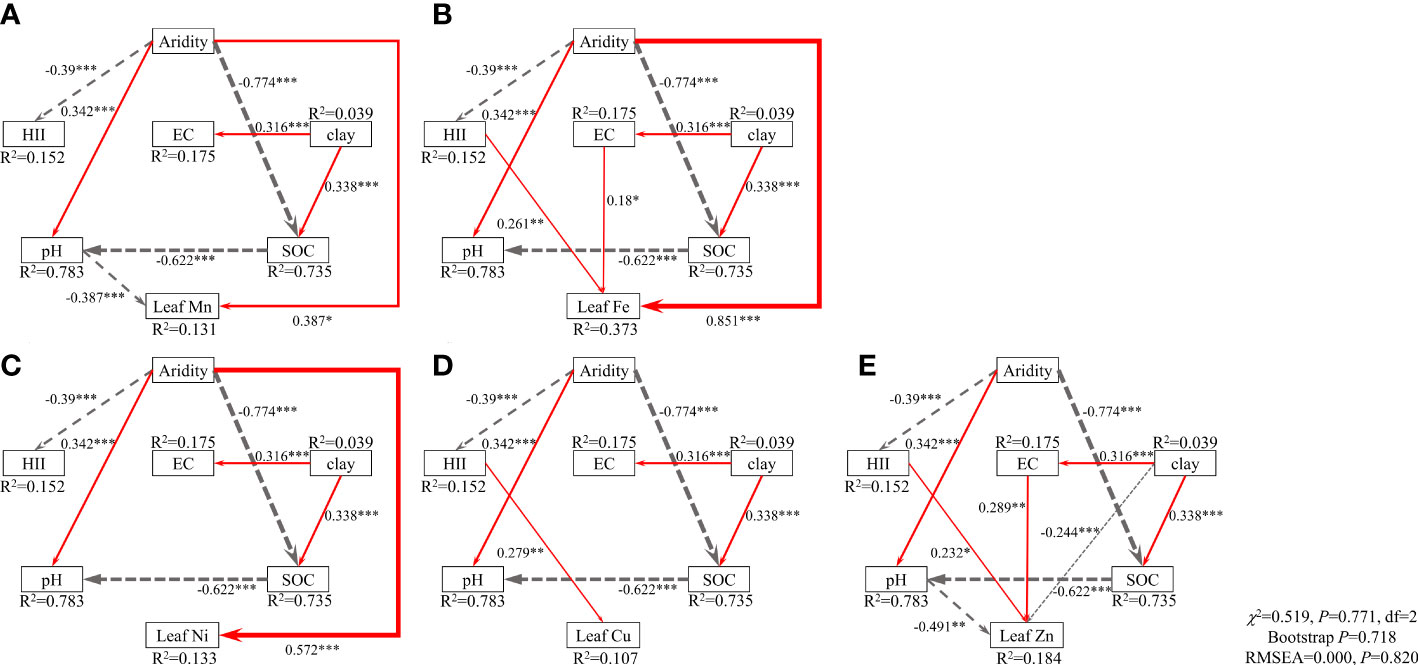
Figure 3 Effects of aridity, human impact index (HII), soil clay content, pH, electrical conductivity (EC) and organic carbon (SOC) on the concentrations of leaf Mn (A), Fe (B), Ni (C), Cu (D) and Zn (E). Numbers adjacent to arrows are standardized path coefficients (analogous to relative regression weights) and indicative of the effect size of the relationship. Continuous, red arrows indicate positive relationships, and dashed, grey arrows indicate negative relationships. The width of the arrows is proportional to the strength of path coefficients. R2 denotes the proportion of variance explained. Goodness-of-fit statistics for each model are shown in the lower right corner (df, degrees of freedom; RMSEA, root mean squared error of approximation), and all five of them are same. The priori model was refined by removing paths with non-significant relationships (see the priori model in Supplementary Figure 5). *P< 0.05, **P< 0.01, ***P< 0.001.
The direct and indirect drivers of the microelement concentrations and BCFs in leaves were identified by SEM (Figure 4; Supplementary Figure 8). Aridity had the greatest direct positive effect and soil pH had the greatest direct negative effect on leaf microelement concentrations. SOC and EC were strong drivers of leaf microelement concentrations (Figure 4). Aridity had a direct, slight, negative effect on BCF of leaf Mn, a strong, positive effect on the BCF of leaf Fe, and an indirect, negative effect on BCFs of leaf Ni, Cu and Zn. Soil pH and SOC had strong, direct, negative effects on BCFs of leaf Mn, Fe, Ni, Cu and Zn (Supplementary Figure 8). HII and clay content had minor effects on both leaf microelement concentrations and BCFs.
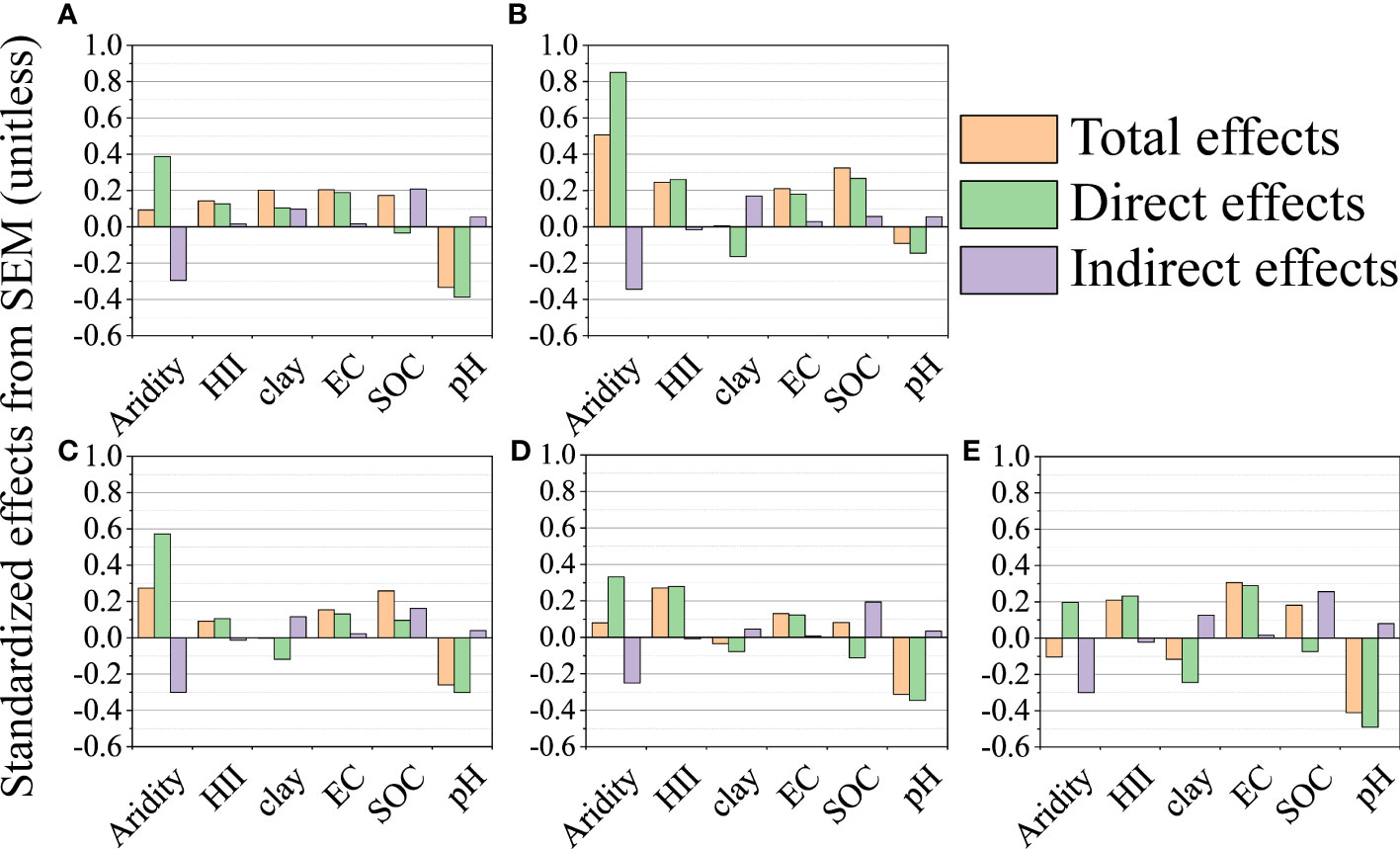
Figure 4 Total, direct and indirect effects based on the structural equation model (SEM) for the concentrations of leaf Mn (A), Fe (B), Ni (C), Cu (D) and Zn (E). Standardized total effects (direct plus indirect effects), and direct and indirect effects of aridity, human impact index (HII), soil clay content, pH, electrical conductivity (EC) and organic carbon (SOC) on the concentrations of leaf Mn, Fe, Ni, Cu and Zn. The yellow column is the total effect, the green column is the direct effect, and the purple column is the indirect effect.
Environmental thresholds of microelement concentrations and BCFs were determined in leaves by cumulating z− and z+ change points. Aridity, soil pH and SOC had the strongest impacts on leaf microelement concentrations and BCFs, and, therefore, the thresholds and indicator species along aridity, soil pH and SOC gradients were assessed further. Based on TITAN, both leaf microelement concentrations and BCFs did not differ significantly along the SOC gradient, so they are not presented. There was an obvious peak for the leaf microelement concentrations and BCFs along the aridity gradient for both sum (z-) and sum (z+) at 0.89 (Supplementary Figure 9), and along the soil pH gradient at 7.9 and 8.9 (Figure 5).
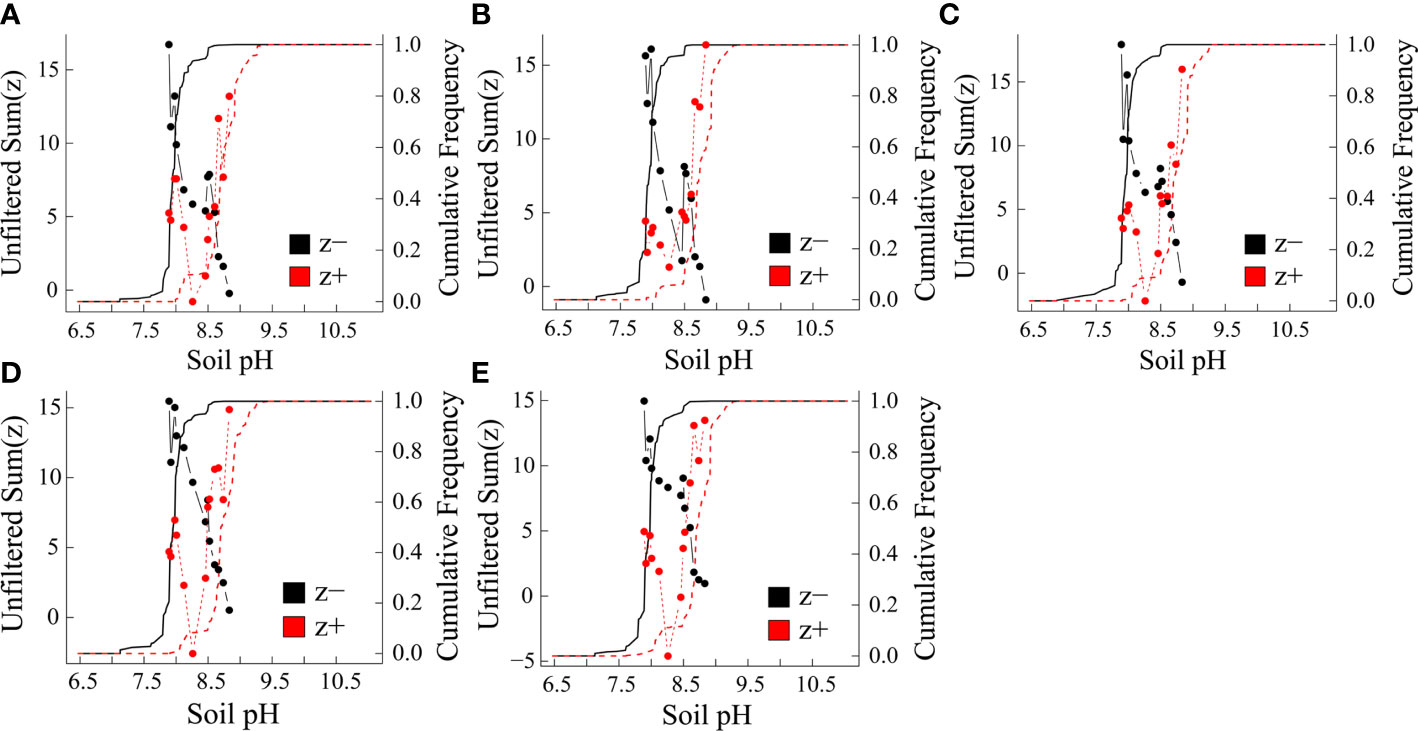
Figure 5 Leaf microelement concentrations and bioconcentration factors (BCFs) in response to soil pH. (A) Mn (B) Fe (C) Ni (D) Cu and (E) Zn. Black and red symbols present accumulative negative (sum z-) and positive (sum z+) responses, respectively. Vertical dashed lines indicate pH gradient thresholds corresponding to maximal sum (z).
For the aridity gradient, z− of leaf microelement concentrations and BCFs were the woody species Artemisia halodendron and the herbaceous species Stipa capillata and Corispermum mongolicum, and the z+ for the woody species Reaumuria soongorica (Supplementary Figure 10). For soil pH, all z− were herbaceous species (Stipa capillata and Corispermum mongolicum), and all z+ were woody species (Haloxylon ammodendron, Salsola abrotanoides and Reaumuria soongorica) (Figure 6).
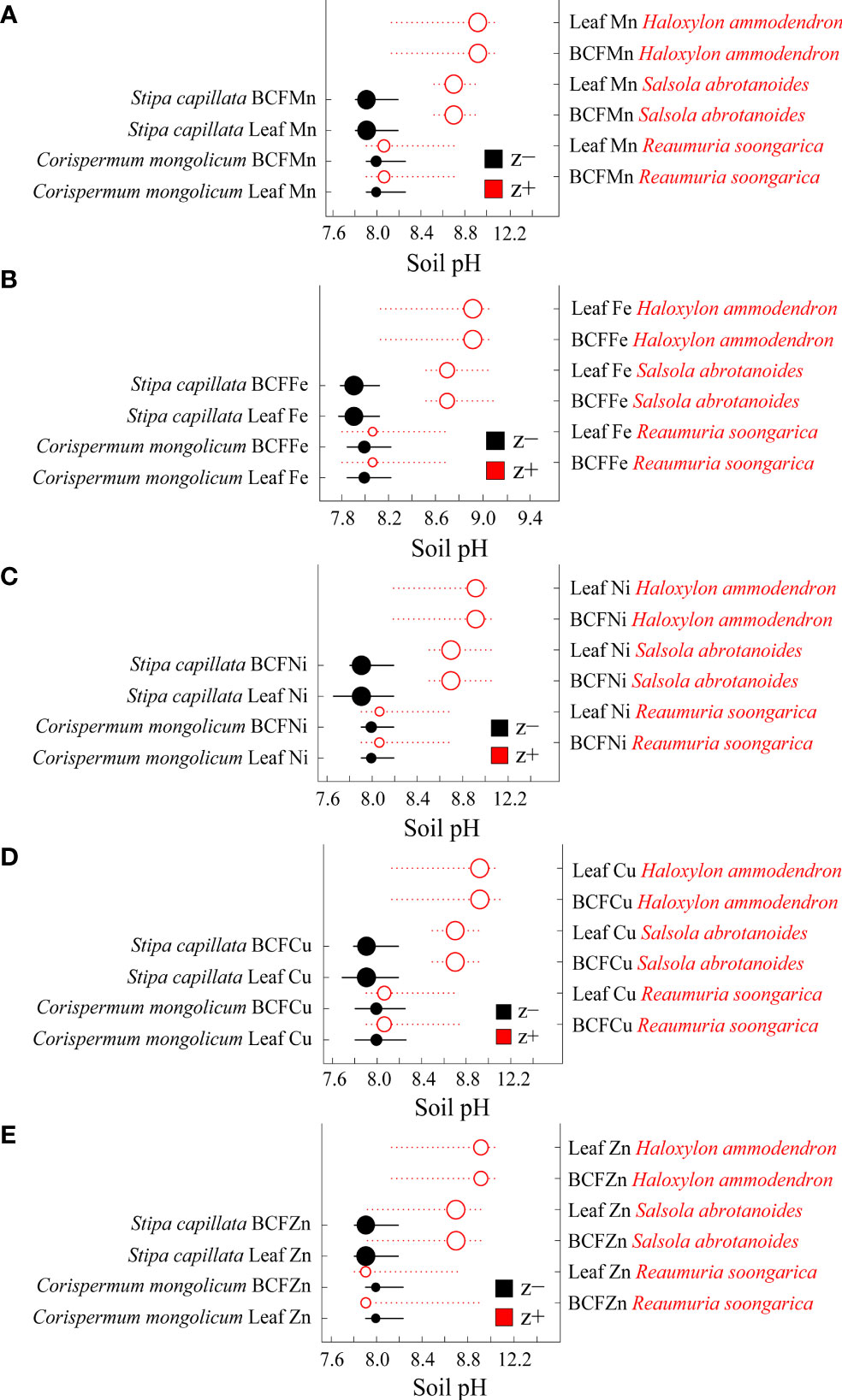
Figure 6 Threshold Indicator Taxa Analysis (TITAN) of leaf microelement concentrations and bioconcentration factors (BCFs) in response to soil pH. (A) Mn and the BCF of Mn, (B) Fe and the BCF of Fe, (C) Ni and the BCF of Ni, (D) Cu and the BCF of Cu and (E) Zn and the BCF of Zn. Black and red symbols represent accumulative negative (sum z-) and positive (sum z+) responses, respectively. Symbol size is in proportion to z scores (magnitude of response). Horizontal lines represent the 95% bootstrap confidence intervals. Woody and herbaceous species are shown in red and in black fonts, respectively.
In the present study, the concentrations of leaf and soil Mn, Fe, Ni, Cu and Zn and the leaf BCFs of these elements were determined in the drylands of northern China. Leaf microelement concentrations were within the normal range of mature leaf microelements [CNEMC (China National Environmental Monitoring Centre), 1990], which supported our first hypothesis. The concentration of leaf Mn was similar to leaf Fe concentration, but was slightly greater than concentrations in leaves in Chinese terrestrial plants (Han et al., 2011), while Mn, Ni, Cu and Zn concentrations in leaves were similar to leaf Fe concentration, but were slightly greater than concentrations in leaves in forests of eastern China (Zhao et al., 2016). Leaf Mn, Fe, Cu and Zn concentrations were similar to those reported in the dominant herbaceous plant species in the Alxa Desert (He et al., 2016).
The soil total microelement concentrations in the present study were generally below the national Chinese levels [CNEMC (China National Environmental Monitoring Centre), 1990]. Concentrations of Mn, Ni, Cu and Zn were on average lesser and of leaf Fe concentration was greater than concentrations of these elements in six land use types in southeast China (Ren et al., 2019). In addition, soil Fe, Cu and Zn concentrations were similar to the soil Mn concentration, but was lesser than concentrations in the Taklamakan Desert (Zhang et al., 2022), while soil Mn and Zn concentrations were greater and of soil Fe and Zn concentrations were similar to soil concentrations in the Alxa Desert (He et al., 2016).
Significant correlations emerged among soil total microelement concentrations, probably because of the strong sorption capacity of microelement oxides to other microelements. For example, microelements in soil can be adsorbed and co-precipitated with Mn/Fe oxides, which leads to increased correlations among soil microelement concentrations (Suda and Makino, 2016). There were also significant positive relationships among the leaf microelement concentrations and BCFs. This occurrence might be due, at least in part, to the positive synergistic interactions among leaf microelements (Kabata-Pendias, 2011). The weak relationships between leaf and soil microelement concentrations may be the result of the dilution effect of plants in water-limited ecosystems. Increasing aridity may result in a greater element uptake by the plant than needed, making the plant less dependent on soil elements (Luo et al., 2016).
Desert plants rely on internal nutrient cycling and require mineral elements within specific ranges for optimal growth and functions (Sardans et al., 2015; Huang et al., 2018). These plants possess a certain degree of stoichiometric flexibility to respond to the stress of deficient water and soil nutrients in drylands (He et al., 2016). The homeostasis strategy of plants to maintain normal nutrient ranges enables their growth even under nutrient stress (Sterner and Elser, 2002).
The all-subset regression analysis and structural equation modeling indicated that leaf microelement concentrations and BCFs were regulated by aridity, HII, and soil physical properties, which partially supported our second hypothesis. Among these variables, aridity, soil pH and SOC were the strongest drivers of both leaf microelement concentrations and BCFs. The large direct positive effect of aridity on leaf Mn, Fe, Ni, Cu and Zn concentrations could be attributed to the low concentration of soil microelements compared with the background value. Due to concentration effects and regulation of plant growth equilibrium, nutrient concentrations do not decrease when plants grow slower under drought, even with reduced nutrient absorption (Sardans and Peñuelas, 2004). Furthermore, interactions between shrubs and herbs may result in different responses to drought. In more xeric sites, the deep roots of shrubs could mitigate drought stress through hydraulic and nutrient lift effects, and, thus, increase nutrient availability in the topsoil for neighboring herbs. This promotes a greater uptake of soil nutrients by herbaceous plants, resulting in increased nutrient concentrations (Wang et al., 2019).
The current study provided empirical evidence that aridity reduces BCFs of leaf Mn, Ni, Cu and Zn, but increases the BCF of Fe. How can these patterns be explained? Drought can reduce element uptake of plants by reducing mineralization or decreasing the diffusion and flow rates of soil elements (He and Dijkstra, 2014). However, with aridity-induced water deficit and high soil temperature, ferrihydrite (Fe (OH)2) and goethite (α-FeOOH) are converted to haematite (α-Fe2O3), that is, a form of Fe that is more recalcitrant and less mobile in soils (Moreno-Jiménez et al., 2019), resulting in reduced availability of soil Fe. With a deficiency in soil Fe, dicotyledonous and non-gramineous plants and gramineous plants use two different strategies to chelate Fe ions from the soil. Dicotyledonous and non-gramineous plants obtain Fe ions by acid-solubilizing and reducing Fe3+ from the rhizosphere and then absorbing Fe2+ into the cells through a high-affinity Fe transport system (strategy I). In contrast, gramineous plants obtain Fe ions by absorbing Fe3+ from soil on the basis of chelation (strategy II) (Marschner, 2012).
Among the soil properties that affect the uptake of plant elements, soil pH plays the most important role in determining element speciation, metal surface solubility and migration, and, ultimately, metallic element bioavailability (Zeng et al., 2011; Fijałkowski et al., 2012). The pH mediates acid-base equilibrium directly, and affects the complexation of metal cations and ion exchange in soil (Janoš et al., 2010). Microelements are generally easiest available to plants in a pH range of 5 to 7, and less so outside this optimal range (McCauley et al., 2009). The pH in the present study was greater than 7 in most areas. A high soil pH causes metals to precipitate as hydroxides and combine preferentially with ionized functional groups of solid soil components (Sposito, 2008; Hamid et al., 2020). A decrease in metal solubility reduces the content of available metallic elements in the soil, restricts plant absorption of elements (Sacristán et al., 2015), and, thereby, reduces the leaf microelement concentrations and BCFs.
SOC also affected soil metal availability and mobility, as organic matter: (1) provides organic chemicals to soil solutions, which are used as chelates to increase the utilization of metals by plants; and (2) retains more metals by adsorption or forming stable complexes with humus, resulting in an increase in total metal content and decrease in metal availability in soil (Zeng et al., 2011; Niesiobedzka, 2012). Humus can fix metals on solid materials in an arid soil environment (Chen et al., 2016), which could partially explain the negative correlation of leaf BCFs and SOC in the current study. In addition, organic matter has high selectivity for cations of microelements (Kwiatkowska-Malina, 2018), and most of the transition cations (Pb, Cu, Cr, Ni, Co, Zn, Al, Fe and Mn) are adsorbed to a greater extent than alkali metal cations (Violante et al., 2010). As a result, organic matter retains more microelements and limits the absorption of microelements by plants (BCFs).
Interestingly, the effect of EC (an approximate representation of soil salinity) was stronger on leaf microelement concentrations than on the BCFs. A possible mechanism for the stronger direct and positive effects of EC on leaf microelement concentrations is that salinity causes an increase in reactive oxygen species (ROS), with deleterious effects on cell metabolism. However, ROS is usually detoxified by superoxide dismutases (SODs) containing Mn, Fe, Cu or Zn metals for survival in saline soils (Slater et al., 2008; Patel et al., 2009). Additionally, the relatively weak direct and positive effects of anthropogenic activities on leaf microelement concentrations indicate that these activities increase the metallic elements released into soil, water and atmosphere and, thus, enhance leaves absorbing and accumulating microelements (Kabata-Pendias, 2011). The small effect of clay content on both the leaf microelement concentrations and BCFs may be due to the coarse texture and poor development of dryland soils (Dregne, 1976), which minimize soil weathering and the formation of active sites on minerals, thereby, reducing their influence on metal availability (Moreno-Jiménez et al., 2019).
Soil pH and SOC (as also reported by Hu et al., 2020) and aridity were key environmental variables in the absorption and accumulation of plant elements in the present study. We then determined ecological thresholds and indicator species for the leaf microelement concentrations and BCFs along gradients of these variables. In support of our third hypothesis, there were abrupt shifts of leaf microelement concentrations and BCFs along the aridity and soil pH gradients, with a key tipping point for aridity at 0.89, and key tipping points for soil pH at 7.9 and 8.9. However, there was no obvious tipping point along the SOC gradient. Supporting our fourth hypothesis, indicator species for the soil pH gradient were clearly separated between herbaceous and woody species: leaf microelement concentrations and BCFs of woody species (Haloxylon ammodendron, Salsola abrotanoides and Reaumuria soongorica) and herbaceous species (Stipa capillata and Corispermum mongolicum) were positive and negative indicators, respectively. Along the aridity gradient, Reaumuria soongorica was a positive indicator, while Artemisia halodendron, Stipa capillata and Corispermum mongolicum were negative indicators.
Soil pH, aridity and EC were correlated positively, which indicates that areas with high aridity also had high soil alkalinity and salinity. To cope with drought, desert woody plants tend to allocate more photosynthate to roots than shoots. This results in higher root: shoot ratios and enables the plant to absorb more water and minerals (Chen et al., 2019). Moreover, desert plant species have other distinct adaptive strategies for survival (Akram et al., 2020; Yao et al., 2021a). For example, Haloxylon ammodendron developed water storage tissue in assimilating branches to increase water retention and water absorption capabilities and, thus, to better tolerate drought (Gong et al., 2019). Crystal cells in water storage tissue not only alter the osmotic pressure of the cells, but also accumulate excess salt to avoid salt toxicity (Grigore and Toma, 2021). Reaumuria soongorica possesses salt glands on the leaf surface, which secrete excess ions, regulate ion balance and maintain osmotic pressure stable to minimize drought and salt-alkaline stress (He et al., 2019). Consequently, shrub species can serve as positive indicators of aridity and soil pH.
The herbaceous plant species Stipa capillata is widespread in Eurasia and is distributed in montane steppe where only it can be dominant (Li et al., 2020). Corispermum mongolicum is a typical sandy species distributed widely in deserts, and acts mainly as a pioneering species in sandy arid environments (Wang et al., 2009). Herbaceous plant species are sensitive to soil biota (Kulmatiski et al., 2008), and, in comparison to woody plant species, tend to grow faster but have higher nutrient requirements (Poorter, 1989; Gong et al., 2020). These species act primarily as positive indicators for soil metallic elements and nutrients, and as negative indicator for aridity and soil pH (Xiong et al., 2021). In addition, some herbaceous plant species have inherent characteristics that cause them to exhibit a weaker ability to resist stress than woody plant species. For example, Corispermum mongolicum acts as a mesophyte, and has a thin leaf cuticle and poorly developed xeromorphic structures, which results in a high transpiration rate, a low water holding capacity (Wang et al., 2004), and poor resistance to water deficits.
Our results demonstrated that leaf microelement concentrations can be maintained within the normal ranges to satisfy growth requirements, regardless of their concentrations in soil (homeostasis), which supports our first hypothesis. Supporting our second hypothesis, leaf microelement concentrations and BCFs were regulated by aridity, HII and soil properties, where aridity, soil pH and SOC were the main drivers of both the microelement concentrations and BCFs of leaves, while EC was also important for leaf micronutrient concentrations. In support of our third hypothesis, there were shifts in leaf microelement concentrations and BCFs at an aridity of 0.89 and soil pHs of 7.9 and 8.9. These results suggest that leaf microelement concentration and BCF thresholds, combined with local aridity and soil pH, can be used as predictors of plant responses to future climate change. Finally, as hypothesized, woody species, with a strong tolerance of arid and alkaline environments, were positive indicators of leaf microelement concentrations and BCFs, while herbaceous species, with a lesser tolerance of arid and alkaline environments, were mainly negative indicators along aridity and soil pH gradients. The identification of indicator species for leaf microelement concentrations and BCFs along environmental gradients can be beneficial in understanding species-specific biogeochemical processes and adaptation strategies of dryland plants.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
JR, JX and JD designed this study. Field observational data were obtained by all authors. All authors carried out the laboratory measurements and data analyses. YZ, JR, JX and JD wrote the first draft of the manuscript and AD edited the final version. All authors contributed critically to the drafts and gave final approval for publication. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the Innovation Base Project of Gansu Province (20190323), the Top Leading Talents in Gansu Province to JD, the National Natural Science Foundation of China (32225032, 32001192, 31322010), the National Scientific and Technological Program on Basic Resources Investigation (2019FY102002), Biodiversity Survey, Fundamental Research Funds for Central Universities (lzujbky-2020-kb45, lzujbky-2021-sp19), and Hubei Provincial Natural Science Foundation of China (2019CFB273).
We thank Guazhou Desert Ecosystem Field Observation Research Station and Core Facility of College of Ecology, Lanzhou University, for their help.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1143442/full#supplementary-material
Akram, M. A., Wahid, A., Abrar, M., Manan, A., Naeem, S., Zahid, M. A., et al. (2021). Comparative study of six maize (Zea mays L.) cultivars concerning cadmium uptake, partitioning and tolerance. Appl. Ecol. Environ. Res. 19, 2305–2331. doi: 10.15666/aeer/1903_23052331
Akram, M. A., Wang, X., Hu, W., Xiong, J., Zhang, Y., Deng, Y., et al. (2020). Convergent variations in the leaf traits of desert plants. Plants 9, 990. doi: 10.3390/plants9080990
Baker, M. E., King, R. S. (2010). A new method for detecting and interpreting biodiversity and ecological community thresholds. Methods Ecol. Evol. 1, 25–37. doi: 10.1111/j.2041-210X.2009.00007.x
Batjes, N. H. (2016). Harmonized soil property values for broad-scale modelling (WISE30sec) with estimates of global soil carbon stocks. Geoderma 269, 61–68. doi: 10.1016/j.geoderma.2016.01.034
Blomberg, S. P., Garland, T., Jr., Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x
Bonanno, G., Borg, J. A., Di, M. V. (2017). Levels of heavy metals in wetland and marine vascular plants and their biomonitoring potential: A comparative assessment. Sci. Total Environ. 576, 796–806. doi: 10.1016/j.scitotenv.2016.10.171
Cai, J., Weiner, J., Wang, R., Luo, W., Zhang, Y., Liu, H., et al. (2017). Effects of nitrogen and water addition on trace element stoichiometry in five grassland species. J. Plant Res. 130, 659–668. doi: 10.1007/s10265-017-0928-2
Chen, R., Ran, J., Huang, H., Dong, L., Sun, Y., Ji, M., et al. (2019). Life history strategies drive size-dependent biomass allocation patterns of dryland ephemerals and shrubs. Ecosphere 10, e02709. doi: 10.1002/ecs2.2709
Chen, H., Yuan, X., Li, T., Hu, S., Ji, J., Wang, C. (2016). Characteristics of heavy metal transfer and their influencing factors in different soil-crop systems of the industrialization region, China. Ecotox. Environ. Safe. 126, 193–201. doi: 10.1016/j.ecoenv.2015.12.042
CNEMC (China National Environmental Monitoring Centre) (1990). Background values of soil elements in China (Beijing: Environmental Science Press).
Delgado-Baquerizo, M., Eldridge, D. J., Maestre, F. T., Ochoa, V., Gozalo, B., Reich, P. B., et al. (2018). Aridity decouples C: N: P stoichiometry across multiple trophic levels in terrestrial ecosystems. Ecosystems 21, 459–468. doi: 10.1007/s10021-017-0161-9
Delgado-Baquerizo, M., Maestre, F. T., Gallardo, A., Bowker, M. A., Wallenstein, M. D., Quero, J. L., et al. (2013). Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 502, 672–676. doi: 10.1038/nature12670
Delgado-Baquerizo, M., Maestre, F. T., Gallardo, A., Eldridge, D. J., Soliveres, S., Bowker, M. A., et al. (2016). Human impacts and aridity differentially alter soil n availability in drylands worldwide. Glob. Ecol. Biogeogr. 25, 36–45. doi: 10.1111/geb.12382
Deng, J., Wang, G., Morris, E. C., Wei, X., Li, D., Chen, B., et al. (2006). Plant mass-density relationship along a moisture gradient in north–west China. J. Ecol. 94, 953–958. doi: 10.1111/j.1365-2745.2006.01141.x
Fijałkowski, K., Kacprzak, M., Grobelak, A., Placek, A. (2012). The influence of selected soil parameters on the mobility of heavy metals in soils. Inżynieria i Ochrona środowiska 15, 81–92.
Gong, Y., Ling, H., Chen, Y., Cao, J., Guo, Z., Lv, G. (2020). N:P stoichiometric changes via species turnover in arid versus saline desert environments. Ecol. Evol. 10, 6636–6645. doi: 10.1002/ece3.6395
Gong, X., Lü, G., He, X., Sarkar, B., Yang, X. (2019). High air humidity causes atmospheric water absorption via assimilating branches in the deep-rooted tree Haloxylon ammodendron in an arid desert region of northwest China. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00573
Grace, J. B. (2006). Structural equation modeling and natural systems (New York: Cambridge University Press). doi: 10.1017/CBO9780511617799
Grigore, M. N., Toma, C. (2021). Morphological and anatomical adaptations of halophytes: A review, handbook of halophytes: From molecules to ecosystems towards biosaline agriculture (Cham: Springer). doi: 10.1007/978-3-030-17854-3_37-1
Groffman, P. M., Baron, J. S., Blett, T., Gold, A. J., Goodman, I., Gunderson, L. H., et al. (2006). Ecological thresholds: The key to successful environmental management or an important concept with no practical application? Ecosystems 9, 1–13. doi: 10.1007/s10021-003-0142-z
Gupta, N., Yadav, K. K., Vinit, K., Kumar, S., Chadd, R. P., Kumar, A. (2019). Trace elements in soil-vegetables interface: Translocation, bioaccumulation, toxicity and amelioration - a review. Sci. Total Environ. 651, 2927–2942. doi: 10.1016/j.scitotenv.2018.10.047
Hamid, Y., Tang, L., Hussain, B., Usman, M., Lin, Q., Rashid, M. S., et al. (2020). Organic soil additives for the remediation of cadmium contaminated soils and their impact on the soil-plant system: A review. Sci. Total Environ. 707:136121. doi: 10.1016/j.scitotenv.2019.136121
Han, W., Fang, J., Reich, P. B., Woodward, F. I., Wang, Z. (2011). Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 14, 788–796. doi: 10.1111/j.1461-0248.2011.01641.x
Hänsch, R., Mendel, R. R. (2009). Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 12, 259–266. doi: 10.1016/j.pbi.2009.05.006
He, F., Bao, A., Wang, S., Jin, H. (2019). NaCl Stimulates growth and alleviates drought stress in the salt-secreting xerophyte Reaumuria soongorica. Environ. Exp. Bot. 162, 433–443. doi: 10.1016/j.envexpbot.2019.03.014
He, M., Dijkstra, F.A. (2014). Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 204, 924–931. doi: 10.1111/nph.12952
He, M., Dijkstra, F. A., Zhang, K., Tan, H., Zhao, Y., Li, X. (2016). Influence of life form, taxonomy, climate, and soil properties on shoot and root concentrations of 11 elements in herbaceous plants in a temperate desert. Plant Soil 398, 339–350. doi: 10.1007/s11104-015-2669-0
Hu, W., Ran, J., Dong, L., Du, Q., Ji, M., Yao, S., et al. (2021). Aridity-driven shift in biodiversity–soil multifunctionality relationships. Nat. Commun. 12, 1–15. doi: 10.1038/s41467-021-25641-0
Hu, B., Xue, J., Zhou, Y., Shao, S., Fu, Z., Li, Y., et al. (2020). Modelling bioaccumulation of heavy metals in soil-crop ecosystems and identifying its controlling factors using machine learning. Environ. pollut. 262, 114308. doi: 10.1016/j.envpol.2020.114308
Huang, H., Ran, J., Ji, M., Wang, Z., Dong, L., Hu, W., et al. (2020). Water content quantitatively affects metabolic rates over the course of plant ontogeny. New Phytol. 228, 1524–1534. doi: 10.1111/nph.16808
Huang, G., Su, Y., Mu, X., Li, Y. (2018). Foliar nutrient resorption responses of three life-form plants to water and nitrogen additions in a temperate desert. Plant Soil 424, 479–489. doi: 10.1007/s11104-017-3551-z
Huang, J., Yu, H., Guan, X., Wang, G., Guo, R. (2016). Accelerated dryland expansion under climate change. Nat. Clim. Change 6, 166–171. doi: 10.1038/nclimate2837
Janoš, P., Vávrová, J., Herzogová, L., Pilařová, V. (2010). Effects of inorganic and organic amendments on the mobility (leachability) of heavy metals in contaminated soil: A sequential extraction study. Geoderma 159, 335–341. doi: 10.1016/j.geoderma.2010.08.009
Jiao, F., Shi, X., Han, F., Yuan, Z. (2016). Increasing aridity, temperature and soil pH induce soil C-N-P imbalance in grasslands. Sci. Rep. 6, 19601. doi: 10.1038/srep19601
Kabata-Pendias, A. (2011). Trace elements in soils and plants. 4th ed. (Boca Raton: CRC press). doi: 10.1201/b10158
Kabata-Pendias, A., Szteke, B. (2015). Trace elements in abiotic and biotic environments (Boca Raton: CRC Rress). doi: 10.1201/b18198
Kembel, S. W., Cowan, P. D., Helmus, M. R., Cornwell, W. K., Morlon, H., Ackerly, D. D., et al. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. doi: 10.1093/bioinformatics/btq166
Kulmatiski, A., Beard, K. H., Stevens, J. R., Cobbold, S. M. (2008). Plant–soil feedbacks: A meta-analytical review. Ecol. Lett. 11, 980–992. doi: 10.1111/j.1461-0248.2008.01209.x
Kwiatkowska-Malina, J. (2018). Functions of organic matter in polluted soils: The effect of organic amendments on phytoavailability of heavy metals. Appl. Soil Ecol. 123, 542–545. doi: 10.1016/j.apsoil.2017.06.021
Li, L., Chen, J., Han, X., Zhang, W., Shao, C. (2020). Grassland ecosystems of China: A synthesis and resume (Singapore: Springer). doi: 10.1007/978-981-15-3421-8
Li, L., Liu, L., Yu, Z., Peñuelas, J., Sardans, J., Chen, Q., et al. (2022). Carbon, nitrogen and phosphorus stoichiometry in natural and plantation forests in China. Forests 13, 755. doi: 10.3390/f13050755
Lin, H., Sun, T., Xue, S., Jiang, X. (2016). Heavy metal spatial variation, bioaccumulation, and risk assessment of Zostera japonica habitat in the yellow river estuary, China. Sci. Total Environ. 541, 435–443. doi: 10.1016/j.scitotenv.2015.09.050
Lumley, T., Miller, A. (2020) Leaps: regression subset selection. R package version. Available at: http://CRAN.R-project.org/package=leaps.
Luo, W., Sardans, J., Dijkstra, F. A., Peñuelas, J., Lü, X., Wu, H., et al. (2016). Thresholds in decoupled soil-plant elements under changing climatic conditions. Plant Soil 409, 159–173. doi: 10.1007/s11104-016-2955-5
McCauley, A., Jones, C., Jacobsen, J. (2009). “Soil pH and organic matter,” in Nutrient management module no. 8 (Bozeman: MSU Extension).
Mohamed, A. E., Rashed, M. N., Mofty, A. (2003). Assessment of essential and toxic elements in some kinds of vegetables. Ecotox. Environ. Safe. 55, 251–260. doi: 10.1016/S0147-6513(03)00026-5
Montgomery, D. C., Peck, E. A., Vining, G. G. (2012). Introduction to linear regression analysis. Fifth edn (New Jersey: John Wiley & Sons, Inc).
Moreno-Jiménez, E., Plaza, C., Saiz, H., Manzano, R., Flagmeier, M., Maestre, F. T. (2019). Aridity and reduced soil micronutrient availability in global drylands. Nat. Sustain. 2, 371–377. doi: 10.1038/s41893-019-0262-x
Niesiobedzka, K. (2012). Transfer of copper, lead and zinc in soil-grass ecosystem in aspect of soils properties, in poland. bull. Environ. Contam. Toxicol. 88, 627–633. doi: 10.1007/s00128-012-0540-z
Paradis, E., Claude, J., Strimmer, K. (2004). APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. doi: 10.1093/bioinformatics/btg412
Patel, A. D., Bhensdadia, H., Pandey, A. N. (2009). Effect of salinisation of soil on growth, water status and general nutrient accumulation in seedlings of Delonix regia (Fabaceae). Acta Ecol. Sin. 29, 109–115. doi: 10.1016/j.chnaes.2009.05.005
Pavel, P. B., Puschenreiter, M., Wenzel, W. W., Diacu, E., Barbu, C. H. (2014). Aided phytostabilization using Miscanthus sinensis × giganteus on heavy metal-contaminated soils. Sci. Total Environ. 479-480, 125–131. doi: 10.1016/j.scitotenv.2014.01.097
Peguero-Pina, J. J., Vilagrosa, A., Alonso-Forn, D., Ferrio, J. P., Sancho-Knapik, D., Gil-Pelegrín, E. (2020). Living in drylands: Functional adaptations of trees and shrubs to cope with high temperatures and water scarcity. Forests 11, 1028. doi: 10.3390/f11101028
Perri, S., Suweis, S., Entekhabi, D., Molini, A. (2018). Vegetation controls on dryland salinity. Geophys. Res. Lett. 45, 11,669–11,682. doi: 10.1029/2018GL079766
Poorter, H. (1989). “Interspecific variation in relative growth rate: On ecological causes and physiological consequences,” in Causes and consequences of variation in growth rate and productivity of higher plants. Ed. Lambers, H., Cambridge, M. L., Konings, H., Pons, T. L.. (Hague: Academic Publishing), 45–68.
Qin, G., Niu, Z., Yu, J., Li, Z., Ma, J., Xiang, P. (2021). Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 267, 129205. doi: 10.1016/j.chemosphere.2020.129205
Rebele, F. (2013). Differential succession towards woodland along a nutrient gradient. Appl. Veg. Sci. 16, 365–378. doi: 10.1111/avsc.12006
Ren, H., Zhou, Q., He, J., Hou, Y., Jiang, Y., Rodrigues, J. L., et al. (2019). Determining landscape-level drivers of variability for over fifty soil chemical elements. Sci. Total Environ. 657, 279–286. doi: 10.1016/j.scitotenv.2018.12.024
Sacristán, D., Recatalá, L., Rossel, R. A. V. (2015). Toxicity and bioaccumulation of Cu in an accumulator crop (Lactuca sativa L.) in different Australian agricultural soils. Sci. Hortic. 193, 346–352. doi: 10.1016/j.scienta.2015.06.051
Sardans, J., Janssens, I. A., Alonso, R., Veresoglou, S. D., Rillig, M. C., Sanders, T. G. M., et al. (2015). Foliar elemental composition of European forest tree species associated with evolutionary traits and present environmental and competitive conditions. Glob. Ecol. Biogeogr. 24, 240–255. doi: 10.1111/geb.12253
Sardans, J., Peñuelas, J. (2004). Increasing drought decreases phosphorus availability in an evergreen Mediterranean forest. Plant Soil 267, 367–377. doi: 10.1007/s11104-005-0172-8
Sardans, J., Peñuelas, J. (2015). Potassium: a neglected nutrient in global change. Glob. Ecol. Biogeogr. 24, 261–275. doi: 10.1111/geb.12259
Sardar, K., Ali, S., Hameed, S., Afzal, S., Fatima, S., Shakoor, M. B., et al. (2013). Heavy metals contamination and what are the impacts on living organisms. Greener J. Environ. Manag. Public Safe 2, 172–179. doi: 10.15580/GJEMPS.2013.4.060413652
Slater, A., Scott, N., Fowler, M. (2008). Plant biotechnology: The genetic manipulation of plants (New York: Oxford University Press, Inc).
Sposito, G. (2008). The chemistry of soils (New York: Oxford University Press). doi: 10.1021/es304524p
Sterner, R. W., Elser, J. J. (2002). Ecological stoichiometry: The biology of elements from molecules to the biosphere (New Jersey: Princeton University Press).
Suda, A., Makino, T. (2016). Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on arsenic and cadmium: A review. Geoderma 270, 68–75. doi: 10.1016/j.geoderma.2015.12.017
Sun, S., Jung, E., Gaviria, J., Engelbrecht, B. M. J. (2020). Drought survival is positively associated with high turgor loss points in temperate perennial grassland species. Funct. Ecol. 34, 788–798. doi: 10.1111/1365-2435.13522
Thanh-Nho, N., Marchand, C., Strady, E., Huu-Phat, N., Nhu-Trang, T. T. (2019). Bioaccumulation of some trace elements in tropical mangrove plants and snails (Can gio, Vietnam). Environ. pollut. 248, 635–645. doi: 10.1016/j.envpol.2019.02.041
Violante, A., Cozzolino, V., Perelomov, L., Caporale, A., Pigna, M. (2010). Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr. 10, 268–292. doi: 10.4067/S0718-95162010000100005
Wang, Y., He, W., Yu, F., Zhang, L., Cui, Q., Chu, Y., et al. (2009). Brushing effects on the growth and mechanical properties of Corispermum mongolicum vary with water regime. Plant Biol. 11, 694–700. doi: 10.1111/j.1438-8677.2008.00168.x
Wang, X., Lü, X., Dijkstra, F. A., Zhang, H., Wang, X., Wu, Y., et al. (2019). Changes of plant N:P stoichiometry across a 3000-km aridity transect in grasslands of northern China. Plant Soil 443, 107–119. doi: 10.1007/s11104-019-04211-w
Wang, S., Wan, C., Wang, Y., Chen, H., Zhou, Z., Fu, H., et al. (2004). The characteristics of Na+, K+ and free proline distribution in several drought-resistant plants of the Alxa desert, China. J. Arid. Environ. 56, 525–539. doi: 10.1016/S0140-1963(03)00063-6
Wildlife Conservation Society (WCS), Center for International Earth Science Information Network (CIESIN) at Columbia University (2005). Last of the wild project, version 2, 2005 (LWP-2): Global human influence index (HII) dataset (geographic) New York: NASA socioeconomic data and applications center (SEDAC). doi: 10.7927/H4M61H5F
Xiong, J., Dong, L., Lu, J., Hu, W., Gong, H., Xie, S., et al. (2021). Variation in plant carbon, nitrogen, and phosphorus contents across the drylands of China. Funct. Ecol. 36, 174–186. doi: 10.1111/1365-2435.13937
Yang, Y., Liang, Y., Ghosh, A., Song, Y., Chen, H., Tang, M. (2015). Assessment of arbuscular mycorrhizal fungi status and heavy metal accumulation characteristics of tree species in a lead-zinc mine area: Potential applications for phytoremediation. Environ. Sci. pollut. Res. 22, 13179–13193. doi: 10.1007/s11356-015-4521-8
Yao, S., Akram, M. A., Hu, W., Sun, Y., Sun, Y., Deng, Y., et al. (2021b). Effects of water and energy on plant diversity along the aridity gradient across dryland in China. Plants 10, 636. doi: 10.3390/plants10040636
Yao, G., Nie, Z., Turner, N. C., Li, F., Gao, T., Fang, X., et al. (2021a). Combined high leaf hydraulic safety and efficiency provides drought tolerance in Caragana species adapted to low mean annual precipitation. New Phytol. 229, 230–244. doi: 10.1111/nph.16845
Zeng, F., Ali, S., Zhang, H., Ouyang, Y., Qiu, B., Wu, F., et al. (2011). The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. pollut. 159, 84–91. doi: 10.1016/j.envpol.2010.09.019
Zhang, Z., Chai, X., Tariq, A., Zeng, F., Graciano, C., Li, X., et al. (2022). Coordinated patterns in the allocation, composition, and variability of multiple elements among organs of two desert shrubs under nitrogen addition and drought. J. Soil Sci. Plant Nutr. 22, 47–58. doi: 10.1007/s42729-021-00632-8
Zhang, J., Li, Y., Liu, C., Li, F., Zhu, L., Qiu, Z., et al. (2019). Concentration levels, biological enrichment capacities and potential health risk assessment of trace elements in Eichhornia crassipes from honghu lake, China. Sci. Rep. 9, 2431. doi: 10.1038/s41598-018-36511-z
Keywords: Northern China, aridity, leaf microelements, bioconcentration factor, indicator plant species
Citation: Zhang Y, Xie S, Wang X, Akram MA, Hu W, Dong L, Sun Y, Li H, Degen AA, Xiong J, Ran J and Deng J (2023) Concentrations and bioconcentration factors of leaf microelements in response to environmental gradients in drylands of China. Front. Plant Sci. 14:1143442. doi: 10.3389/fpls.2023.1143442
Received: 13 January 2023; Accepted: 20 February 2023;
Published: 02 March 2023.
Edited by:
Kaixiong Xing, Hainan Normal University, ChinaCopyright © 2023 Zhang, Xie, Wang, Akram, Hu, Dong, Sun, Li, Degen, Xiong, Ran and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinzhi Ran, cmFuanpAbHp1LmVkdS5jbg==; Junlan Xiong, eGlvbmdqbEBsenUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.