- 1Faculty of Engineering, Huanghe Science and Technology University, Zhengzhou, China
- 2Institute of Water Resources and Rural Water Conservancy, Henan Provincial Water Conservancy Research Institute, Zhengzhou, China
- 3Vegetable station, Northwest Land and Resources Research Center, Shaanxi Normal University, Xi’an, China
- 4Hydraulic Research Laboratory, Yellow River Hydrologic Survey Planning and Design Co., Ltd., Zhengzhou, China
- 5Agricultural Technology Extension Center of Xi’an City, Xi’an, Shaanxi, China
- 6Key Laboratory of Vegetation Ecology, Ministry of Education, Northeast Normal University, Changchun, China
- 7State Environmental Protection Key Laboratory of Wetland Ecology and Vegetation Restoration, School of Environment, Northeast Normal University, Changchun, China
Introduction: The change in rhizosphere soil bacterial community and root system under new water-saving device is not clear.
Methods: A completely randomized experimental design was used to explore the effects of different micropore group spacing (L1: 30 cm micropore group spacing, L2: 50 cm micropore group spacing) and capillary arrangement density (C1: one pipe for one row, C2: one pipe for two rows, C3: one pipe for three rows) on tomato rhizosphere soil bacteria community, roots and tomato yield under MSPF. The bacteria in tomato rhizosphere soil were sequenced by 16S rRNA gene amplicon metagenomic sequencing technology, the interaction of bacterial community, root system and yield in tomato rhizosphere soil was quantitatively described based on regression analysis.
Results: Results showed that L1 was not only beneficial to the development of tomato root morphology, but also promoted the ACE index of tomato soil bacterial community structure and the abundance of nitrogen and phosphorus metabolism functional genes. The yield and crop water use efficiency (WUE) of spring tomato and autumn tomato in L1 were about 14.15% and 11.27%, 12.64% and 10.35% higher than those in L2. With the decrease of capillary arrangement density, the diversity of bacterial community structure in tomato rhizosphere soil decreased, and the abundance of nitrogen and phosphorus metabolism functional genes of soil bacteria also decreased. The small abundance of soil bacterial functional genes limited the absorption of soil nutrients by tomato roots and roots morphological development. The yield and crop water use efficiency of spring and autumn tomato in C2 were significantly higher than those in C3 about 34.76% and 15.23%, 31.94% and 13.91%, respectively. The positive interaction between soil bacterial community and root morphological development of tomato was promoted by the capillary layout measures of MSPF.
Discussion: The L1C2 treatment had a stable bacterial community structure and good root morphological development, which positively promoted the increase of tomato yield. The interaction between soil microorganisms and roots of tomato was regulated by optimizing the layout measures of MSPF to provide data support for water-saving and yield-increasing of tomato in Northwest China.
1 Introduction
Soil microorganisms are the main components of terrestrial ecosystems, which plays a key role in the decomposition of organic matter, nutrient cycling and the degradation of harmful substances, and also play an important role in facility planting agricultural ecosystems (Zhu et al., 2019; Ma et al., 2023). Soil bacteria are the main component of soil microorganisms (Wang et al., 2017), which have high diversity, high abundance and complete functions (Li and Ma, 2018; Xu et al., 2022; Zhu et al., 2022). Soil bacteria are mainly involved in the formation of humus and the mineralization of organic matter, which is essential for regulating soil enzyme activity, soil nutrient cycle and crop root morphological development (Özbolat et al., 2023; Zheng et al., 2023). Previous studies have found that soil bacterial diversity and heterogeneity are often used to characterize soil fertility and predict ecological environment risks (Guo et al., 2018). Soil bacteria are significantly affected by physical and chemical properties such as soil water, heat and nutrients, plant growth, and other microorganisms (Huang et al., 2018; Zhang et al., 2018). Therefore, the study of soil bacterial community is of great significance for measuring regional soil productive potential and sustainable development.
Facility Planting agriculture has the characteristics of fast crop growth and high demand for soil water and fertilizer (Nie et al., 2022; Zhao et al., 2022). Measuring the level of soil water and fertilizer has become a hot topic in current research. The nutrients needed for plant growth mainly come from the soil, in which the mineralization of soil nutrients by microorganisms and the absorption of nutrients by plant roots are important links in the nutrient cycle (Jafari et al., 2018; Morio et al., 2022). Previous studies have found that soil nitrogen-fixing bacteria can convert nitrogen in the air into nitrogen sources, promote plant root morphological development to support plant needs, and thus reduce plant nutritional stress (Wang J. et al., 2022). Plant roots can make the plant-soil feedback direction develop in a positive direction. In response to soil drought, plant fine roots can grow into aggregates and open microsites, where oxygen stimulates microbial activity and N release. Root exudates and litter produced by root growth provide energy sources for the growth of soil microorganisms (Veresoglou et al., 2022; Xiao et al., 2023). Therefore, it is urgent to explore the relationship between soil microorganisms and plant roots for the efficient utilization of land resources.
Redistribution of soil moisture, heat and air is closely related to field irrigation management, which directly or indirectly affects the development of soil microbial community and plant root morphological development (Feng et al., 2023; Vera et al., 2023). In order to improve the stability of soil microbial community structure in plant root zone and promote root morphological development, researchers have proposed methods such as microbial inoculation and growth regulator addition (Lewis et al., 2020; Zhou et al., 2023). However, there are many problems in the implementation of the above methods, such as high investment cost and environmental pollution (Boddington and Dodd, 1999; Zhao et al., 2017). At the same time, other researchers have found that it is feasible to optimize soil microbial communities and root morphological development in crop root zones by adjusting crop irrigation management methods, such as changes in field layout measures of tomato drip irrigation that can change soil microbial communities and yield (Sun et al., 2021; Wang et al., 2017). Proper field layout of drip irrigation pipe can promote the morphological development of tomato root system (Wang et al., 2020a; Li et al., 2023). The arrangement of drip irrigation under plastic film indirectly affects the morphological development of crop root system and soil microbial community (Wang et al., 2016; Ye et al., 2016). Therefore, exploring the effects of irrigation management measures on crop soil microorganisms and crop roots has guiding significance for elucidating the relationship between soil microbial community and root system in facility planting agriculture and guiding the water-saving, yield-increasing and quality-improving of facility planting agricultural crops.
At present, the mechanism of the change of crop soil bacterial community and root system regulated by the new water-saving technology of facility planting agriculture under MSPF (Zhang et al., 2020a) is not clear. At the same time, the interaction relationship between soil bacterial community-root system-yield of tomato in greenhouse under MSPF lacks qualitative and quantitative description. Therefore, in this study, greenhouse tomato was used as the research object to explore the response of rhizosphere soil bacteria community, root system and yield of greenhouse tomato under MSPF to the different micropore group spacing and capillary arrangement density of micro-sprinkler pipe. The purpose of this paper is to adjust the interaction between tomato roots system and soil microorganisms by optimizing the layout measures of MSPF through greenhouse experiment and mathematical analysis, and to provide data support for the prediction of soil production potential of facility planting agriculture and the water-saving and yield-increasing of tomato.
2 Materials and methods
2.1 Experimental site and management
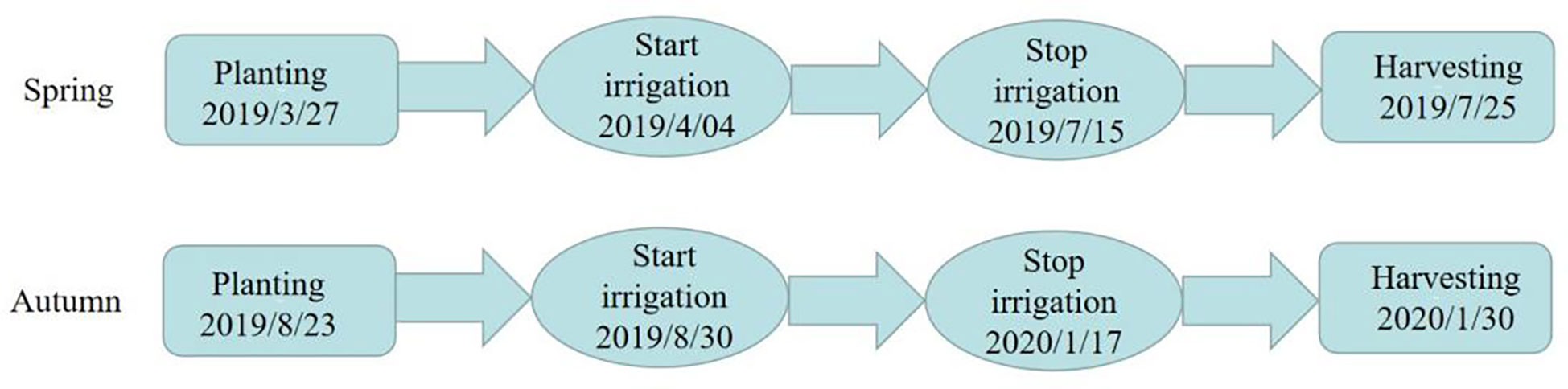
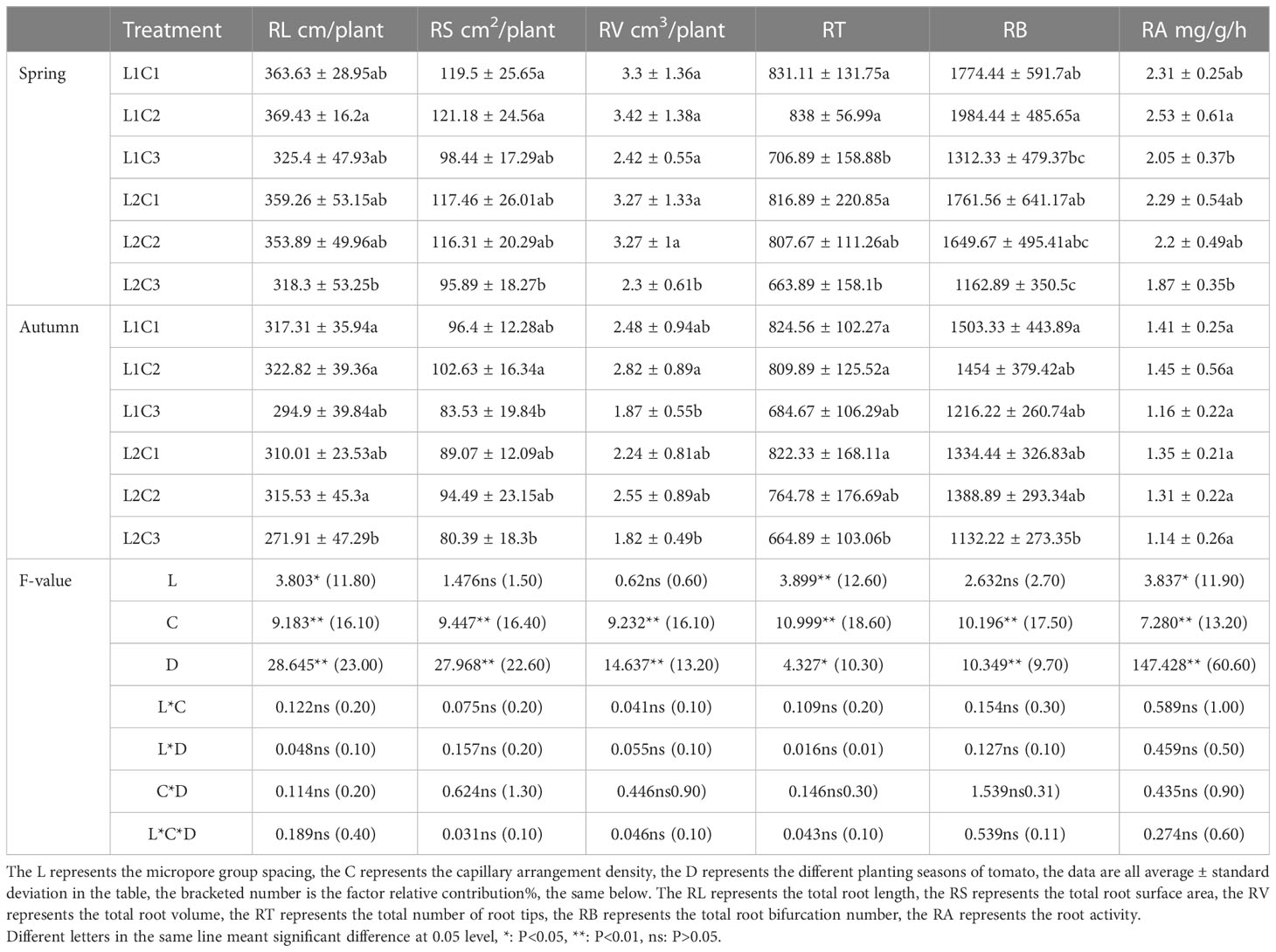
The experiment was carried out in the greenhouse of Xi ‘an Modern Agricultural Science and Technology Exhibition Center (108°52’E, 34°03’N) in Shaanxi Province from March 23, 2019 to January 30, 2020. The tested tomato variety was ‘Jingfan 401’ (Jingyan Yinong Seed Industry Technology Co., Ltd., Beijing·China). The tomato adopts the ridge planting structure mode, in which the row spacing is 50.00 cm and the plant spacing is 40.00 cm. The length of the experimental plot is 3.40 m, and the spacing between the plots is 4.00 m. The planting, irrigation and harvest time of spring tomato and autumn tomato are shown in Figure 1. Meteorological data and irrigation record of tomato growth period are shown in Figure 2.
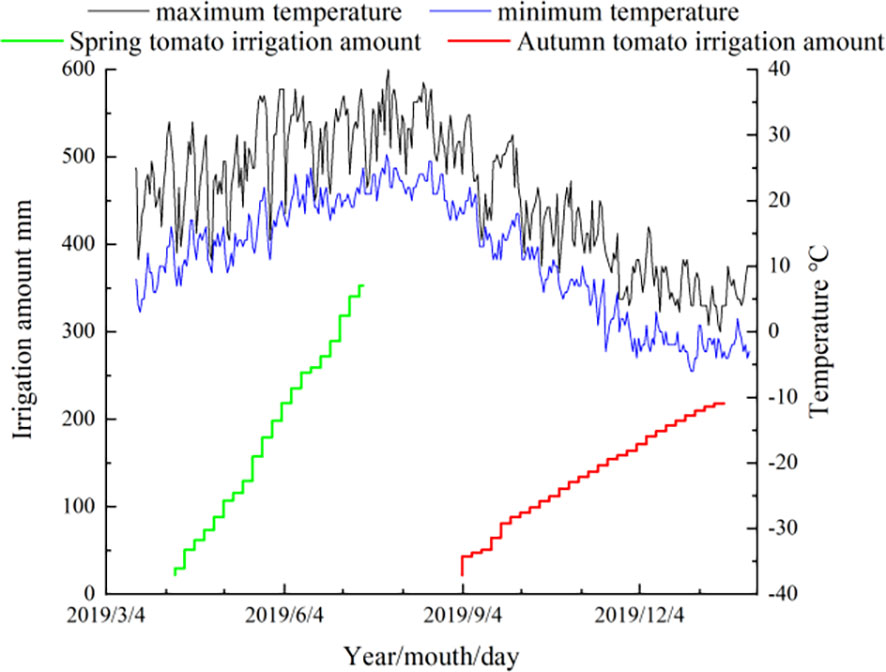
Figure 2 Meteorological data and irrigation record of tomato growth period. Diameter of micropore is d=0.8mm; The internal spacing of the micropores group was I =0.4cm; The Angle of micropores is =68°; The micropore group spacing is L.
In this experiment, the irrigation amount was controlled on the basis of the cumulative evaporation from a 20-cm diameter standard pan (Epan, DY.AM3, Weifang Dayu Hydrology Technology Co., Ltd., Shandong, China) following Zhang (Zhang et al., 2020a). The evaporation amount was measured at 08:00 am every 5 d. The irrigation amount was evaluated after the measurement. The irrigation quota was calculated according to Formula (1), and the irrigation times and amounts were recorded (see Figure 2).
Epan represents the evaporation within the interval of two irrigation, based on the cumulative evaporation from a 20 cm diameter pan (mm); A represents the capillary control area (mm) and kcp represents the crop- pan coefficient. In this paper, adopting adequate irrigation mode, the crop-pan coefficient of is 1.0 (Zhang et al., 2020a).
2.2 Experimental design
In this experiment, two factors of micropore group spacing and capillary arrangement density were set.
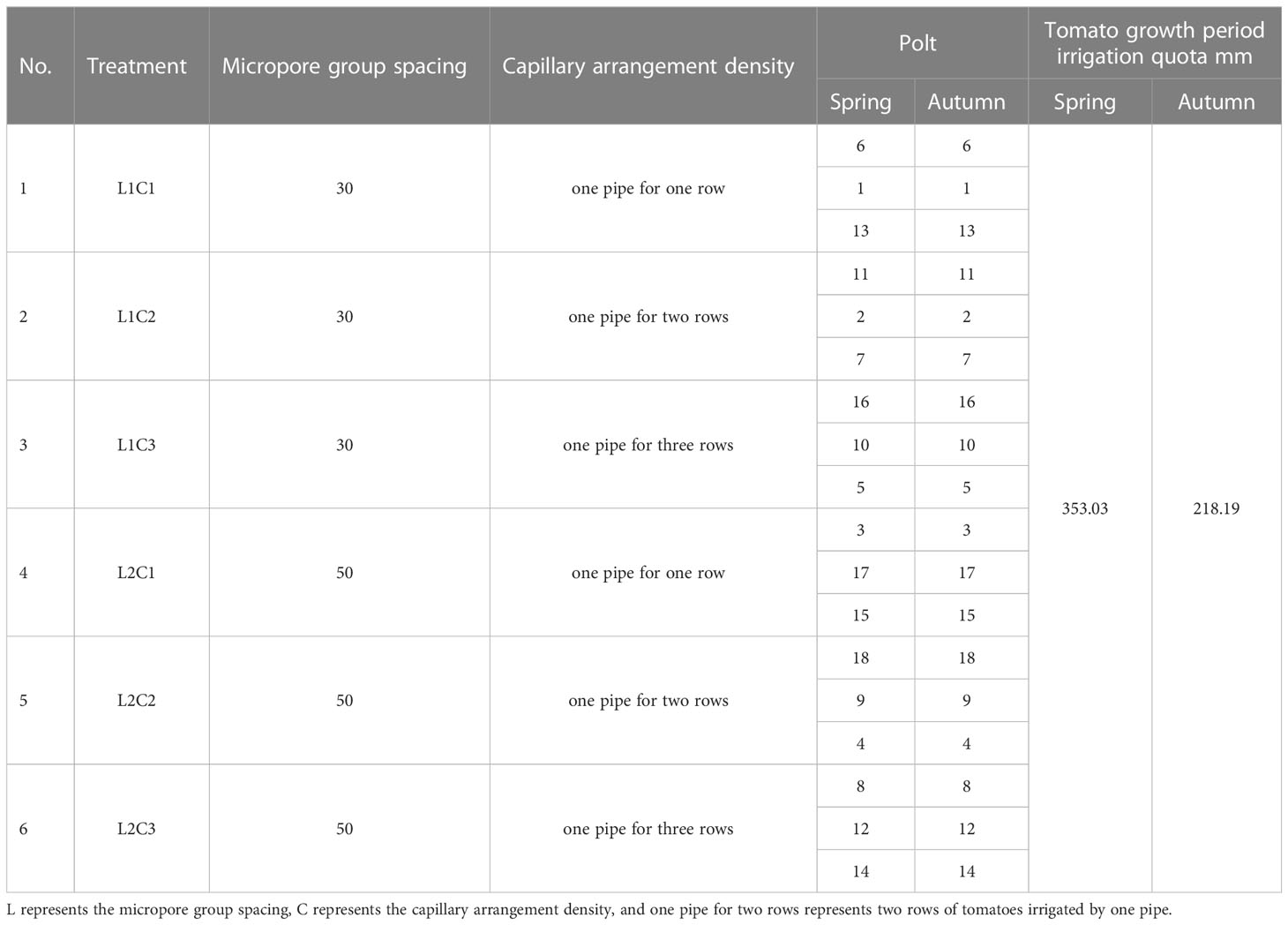
Micropore group spacing (L, see Figure 3) sets 2 levels (30, 50 cm). The capillary arrangement density (C, see Figure 4) sets 3 levels ((one pipe for one row, one row of tomatoes irrigated by one pipe, C1), (one pipe for two rows, two rows of tomatoes irrigated by one pipe, C2), (one pipe for three rows, three rows of tomatoes irrigated by one pipe, C3)).
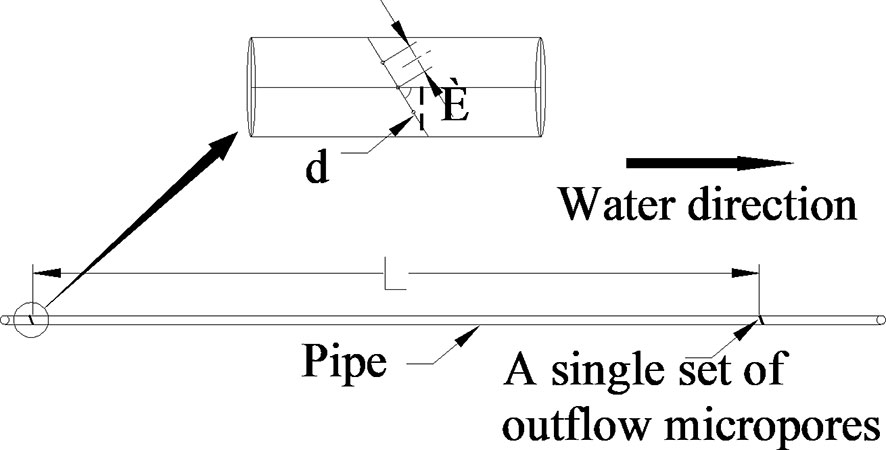
Figure 3 Micropores group (inside) spacing structure parameters (Zhang et al., 2020b). Diameter of micropore is d=0.8mm; The internal spacing of the micropores group was I =0.4cm; The Angle of micropores is =68º; The micropore group spacing is L.
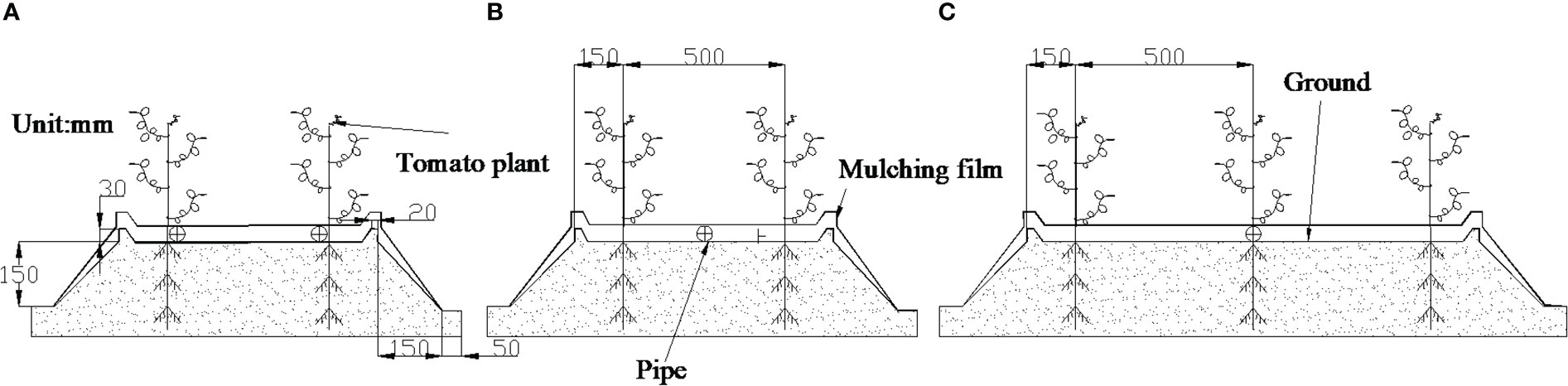
Figure 4 Capillary arrangement. (Zhang et al., 2020b). (A) represents one pipe for one row; (B) represents one pipe for two rows; (C) represents one pipe for three rows.
This study consisted of 6 treatments (Table 1), each treatment was repeated 3 times, a total of 18 experimental plots. The irrigation water in this experiment comes from the groundwater in the region. The head of the water source is connected in series with a 120-mesh sieve filter. The principle of diversion is used to ensure that the working pressure of the control system is constant, and the complete random test is used to arrange the test fields. Each experimental field was irrigated separately and controlled by a spherical valve.
2.3 Measurements and computational methods
2.3.1 Collection and determination of soil bacterial
1) Soil samples were collected from rhizosphere soil of spring tomato and autumn tomato after 72 days of planting. The soil shaking method was used to extract (Taking tomato plants as the center, the cylindrical soil with tomato roots at a radius of 20 cm and a depth of 5-25 cm was excavated, and the loose soil combined with tomato roots was shaken off. The soil closely combined with tomato roots in greenhouse was gently brushed with a soft brush after sterilization as the rhizosphere soil of tomato in greenhouse). Three rhizosphere soil samples were randomly taken from each experimental plot, and the samples were transported to the laboratory on ice. The samples were brought back to the room and the fresh soil plant residues were removed. Three soil samples in the plot were fully stirred and evenly mixed as soil samples for sequencing in the experimental plot. The soil samples were collected and quickly frozen in liquid nitrogen. The soil samples were stored in a -80°C refrigerator and sent to Shanghai Meiji Biomedical Technology Co., Ltd. (Shanghai, China) to determine soil bacterial community. The main analysis steps of soil bacterial community determination are divided into three parts:
A. DNA extraction and PCR amplification
Total DNA was extracted from tomato rhizosphere soil using the E.Z.N.A. ® soil kit (Omega Bio-tek, Norcross, GA, USA), which reliably and quickly separates high-quality genomic DNA from various soil samples (up to 1 g of soil can be processed in 60 min). After the quality of DNA extraction was detected by 1% agarose gel electrophoresis, the V3-V4 variable region was amplified by PCR with 338 F (5 ‘ -ACTCCTACGGGAGGGAGCAGCAG-3 ‘) and 806 R (5 ‘ -GGACTACHVGGGTWTCTAAT-3 ‘) primers. The amplification procedure was: 95 °C pre-denaturation 3 min, 27 cycles (95 °C denaturation 30 s, 55 °C annealing 30 s, 72 °C extension 30 s). Finally, extension at 72 °C for 10 min (PCR instrument: ABI GeneAmp ® 9700).
B. Illumina Miseq sequencing
The bacterial 16S rDNA V3-V4 region was selected, and the NA samples were sequenced using the Illumina Miseq PE300 high-throughput sequencing platform (Shanghai Meiji Biomedical Technology Co., Ltd.). The bacterial 16 S rDNA V3-V4 amplification primers were 338 F (5 ‘ -ACTCCTACGGGGAGGCAGCAG-3 ‘) and 806 R (5 ‘ -GGACTACNNGGGTATCTAAT-3 ‘). The PCR products were recovered using 2% agarose gel, purified and eluted for detection. PCR reaction system (total system was 25 μL): 12.5 μL KAPA 2G Robust Hot Start Ready Mix, 1 μL Forward Primer (5 μmol/L), 1 μL Reverse Primer (5 μmol/L), 5 μL DNA (the total amount of DNA added was 30 ng), and finally 5.5 μL dd H2O was added to make up to 25 μL. Reaction parameters: 95 °C for 5 min; 95 °C denaturation 45 s, 55 °C annealing 50 s, 72 °C extension 45 s, 28 cycles; 72 °C for 10 min. The original sequence was uploaded to the figshare database (https://figshare.com/articles/dataset/The_Layout_Measures_of_Micro-sprinkler_Irrigation_under_Plastic_Film_Regulate_Tomato_Soil_Bacterial_Community_and_Root_System/21818610).
C. Sequencing data processing
The original data obtained by Miseq sequencing were optimized after splicing and quality control. After distinguishing the samples, OTU (Operational taxonomic unit) cluster analysis and species taxonomy analysis were performed. The OUT similarity was set to 97%. The OTU was subjected to diversity index analysis and statistical analysis of community structure at each classification level, and then a series of in-depth statistical and visual analysis such as multivariate analysis and difference significance test of sample community composition and phylogenetic information were completed. The ACE index of soil bacterial (ACE), CHAO index of soil bacterial (CHAO), COVERAGE index of soil bacterial (COVERAGE), SHANNON index of soil bacterial (SHANNON), SIMPSON index of soil bacterial (SIMPSON), SOBS index of soil bacterial (SOBS), Species Veen diagram analysis and species composition analysis can be obtained by direct analysis of Shanghai Meiji Biological Cloud platform (https://login.majorbio.com/login).
2) Based on the results of soil bacterial community determination, and referring to the Kyoto Encyclopedia of Genes and Genomes and related references (Wang et al., 2020b), the functional genes related to soil bacterial nitrogen fixation, nitrification, denitrification and phosphorus metabolism functional gene abundance were obtained. The functional genes related to soil bacterial nitrogen metabolism are the sum of the abundance of soil bacterial nitrogen fixation, nitrification and denitrification functional genes.
2.3.2 Root system
Three tomato plants were randomly selected from each plot to dig a soil volume with a depth of about 0.4 m and a diameter of 0.2 m centered on the plant at 76 and 78 days after planting spring tomato and autumn tomato, respectively. The samples were placed in a 150 mesh sieve to rinse the roots. The roots were scanned by Epson Perfection V700 scanner to obtain the TIF diagram. Finally, the TIF diagram was processed by WinRHIZO Pro software to obtain the total root length, total number of root tips and bifurcation number of greenhouse tomatoes. The root activity of tomato was determined by triphenyltetrazolium chloride method (Li et al., 2020).
2.3.3 Yield and water use efficiency
Four tomato plants were randomly selected, and the mature fruit mass of four tomatoes was weighed by electronic scale with precision of 0.01 g, and the yield per hectare was converted.
Time-domain reflectometry soil moisture sensor (TRIME-PICO-IPH, IMKO, Inc., Ettlingen, Germany) was used to measure the soil volume moisture content at different layers of soil (0–10, 10–20, 20–30, 30–40, 40–50, 50–60, 60–70, and 70–80 cm, respectively). It was measured once before and after each growth period. Water consumption (ETa )and crop water use efficiency (WUE) were calculated formulas (2) and (3), respectively (Zhang et al., 2020a):
represents crop water consumption during growth period (mm); I represents the irrigation quota of crop growth period (mm); H represents the depth of the wetting layer with plan (H = 0.8 m); θt1 and θt2 represent 80-cm average soil volumetric water contents at times t1 and t2 cm3/cm3), respectively.
Y indicates crop grain yield (t/hm2).
2.4 Data analysis
The interaction of bacterial community, root system and yield in tomato rhizosphere soil was quantitatively described based on regression analysis. The significant difference was analyzed by F test of SPSS22.0 (IBM Crop., Armonk, New York, NY, USA), and the significant level was set to P<0.05. The picture was drawn by OriginPro2019 (Origin Lab Corporation, Northampton, MA, USA). Excel 2016 (Microsoft Excel, Microsoft, Washington, USA) was used for regression analysis.
2.5 Abbreviations
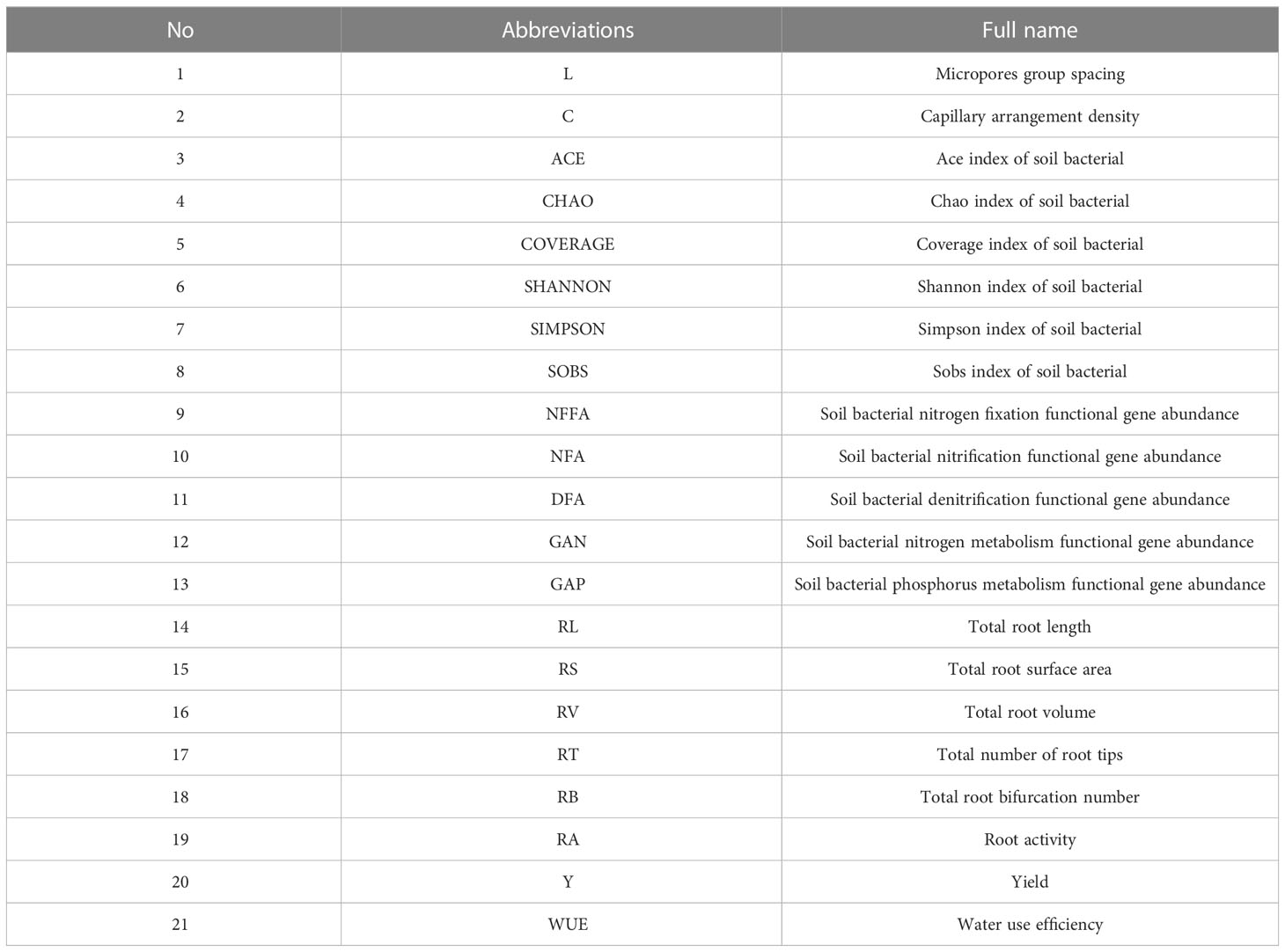
The abbreviations of this article are explained in Table 2.
3 Results
3.1 Effects of different treatments on soil bacterial community of greenhouse tomato
3.1.1 Diversity of soil bacterial community structure
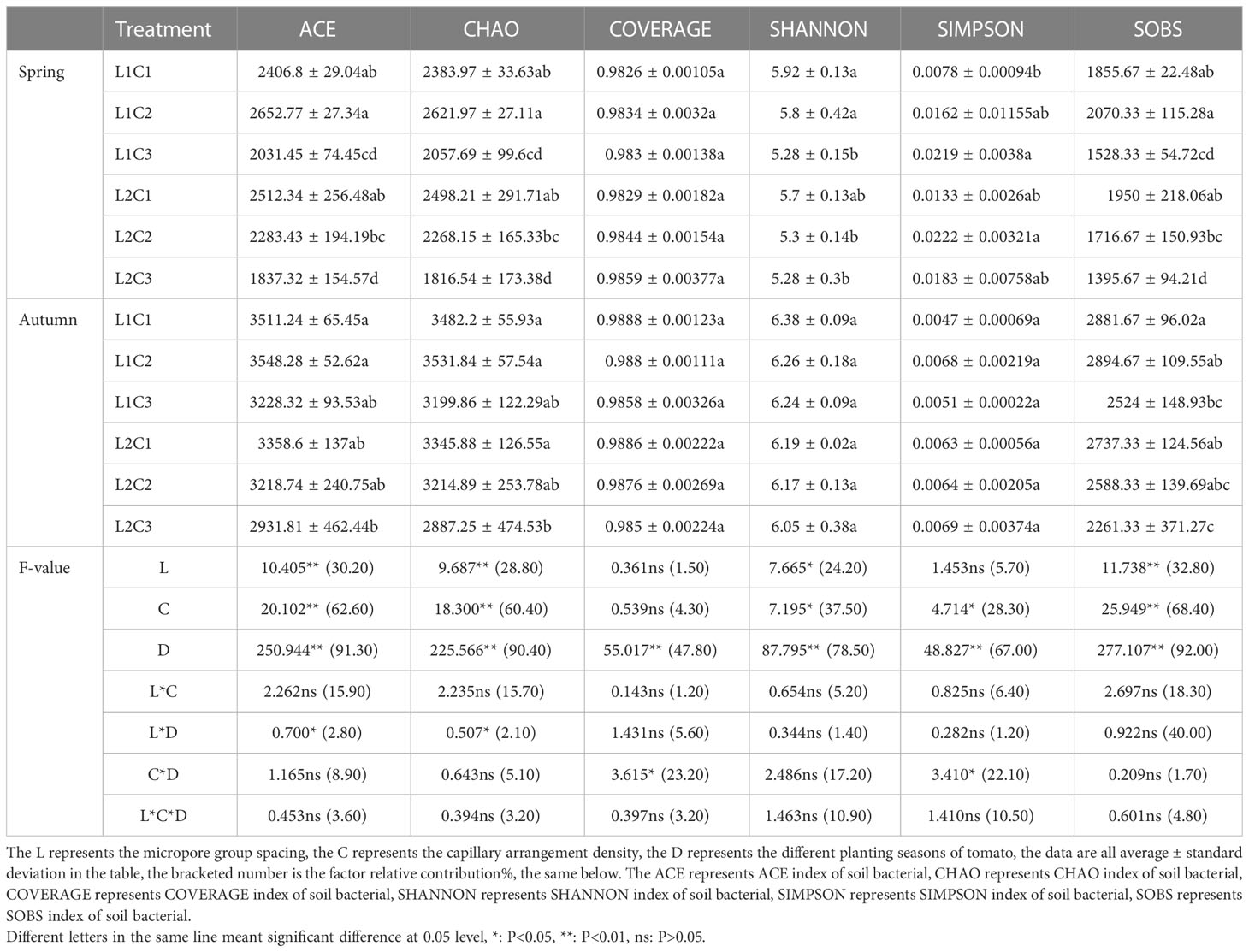
It can be seen from the dilution curve of Figure 5 that the amount of sequencing data in each treatment is sufficient. It can be seen from Table 3 that the micropore group spacing (L) had a significant effect on the ACE index of soil bacterial (ACE), CHAO index of soil bacterial (CHAO), SHANNON index of soil bacterial (SHANNON) and SOBS index of soil bacterial (SOBS) of spring tomato and autumn tomato. The relative contribution of L to ACE, CHAO, SHANNON, and SOBS of tomato was 30.20%, 28.80%, 24.20% and 32.80%, respectively. The capillary arrangement density (C) had a significant effect on the ACE, CHAO, SHANNON, SIMPSON index of soil bacterial (SIMPSON) and SOBS of spring tomato and autumn tomato. The relative contribution of C to ACE, CHAO, SHANNON, SIMPSON and SOBS of tomato was 62.60%, 60.40%, 37.50%, 28.30% and 38.4%, respectively. The different planting seasons (D) had a significant effect on the ACE, CHAO, COVERAGE index of soil bacterial (COVERAGE), SHANNON, SIMPSON and SOBS of spring tomato and autumn tomato. The relative contribution of D to ACE, CHAO, COVERAGE, SHANNON, SIMPSON and SOBS of tomato was 62.60%, 60.40%, 37.50%, 28.30% and 38.4%, respectively.
Compared with L2, the ACE, CHAO, SHANNON, and SOBS of spring tomato and autumn tomato treated with L1 were higher. With the decrease of C, the ACE, CHAO, SHANNON, SIMPSON and SOBS of soil bacterial with spring tomato and autumn tomato showed a decreasing trend. The diversity of soil bacterial community structure in spring tomato was lower than that in autumn tomato.
3.1.2 Soil bacterial community structure species composition
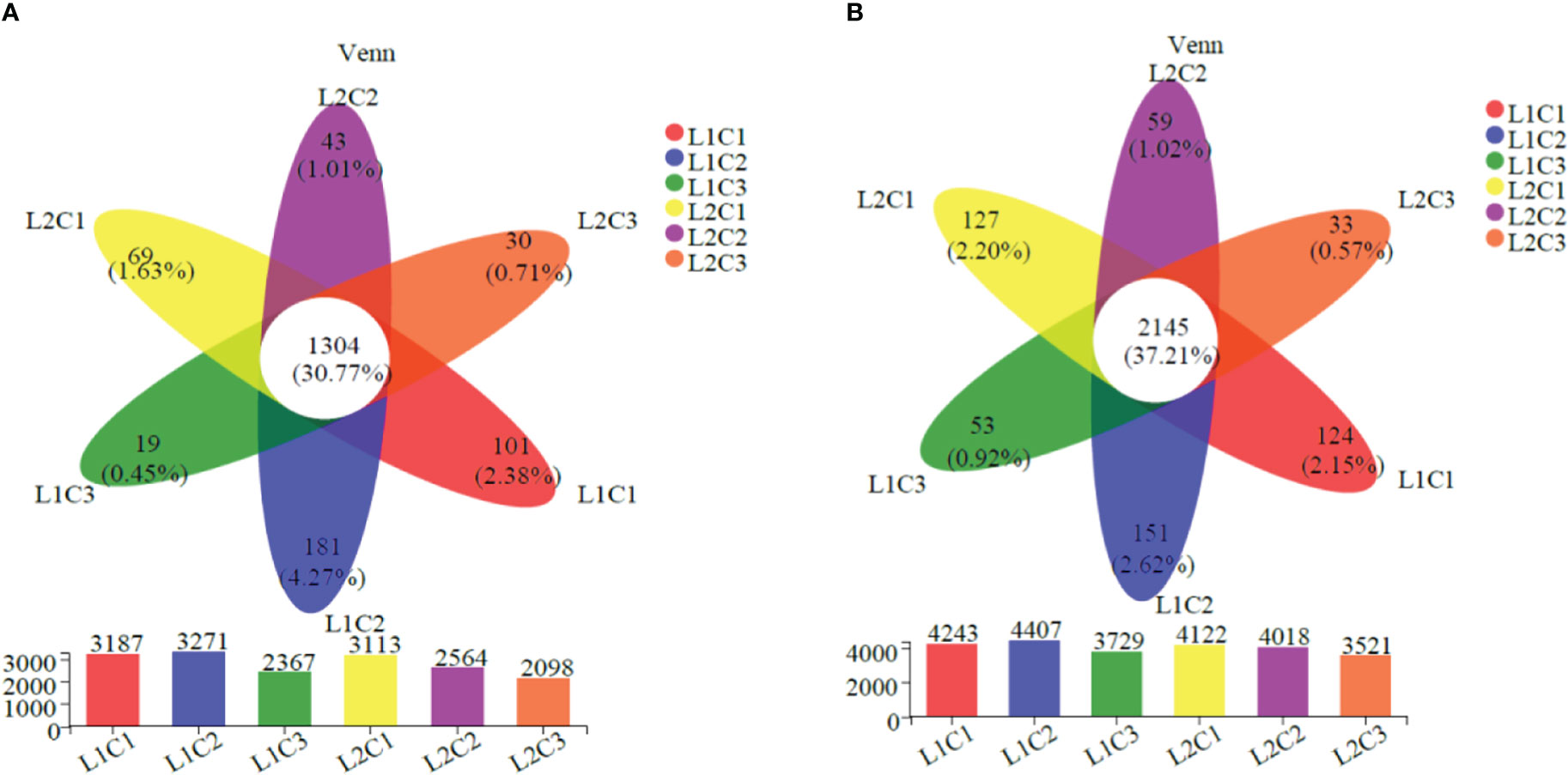
As can be seen from Figure 6, there are 1304 and 2145 identical OTUs in the soil bacteria of spring tomato and autumn tomato in the six treatments, accounting for 30.77% and 37.21% of the total OTUs. Single factor significant analysis showed that the total number of soil bacteria in spring tomato and autumn tomato treated with L1C2 was the highest at the OTUs classification level (3271 and 4407). Compared with L2, the total number of soil bacteria in spring tomato and autumn tomato treated with L1 increased by 13.50% and 6.16% at the OTUs classification level. With the decrease of C, the total number of soil bacteria in spring tomato and autumn tomato decreased at the OTUs classification level. Compared with C3, the total number of soil bacteria in C2 spring tomato and autumn tomato increased by about 30.68% and 16.21% at the OTUs classification level.
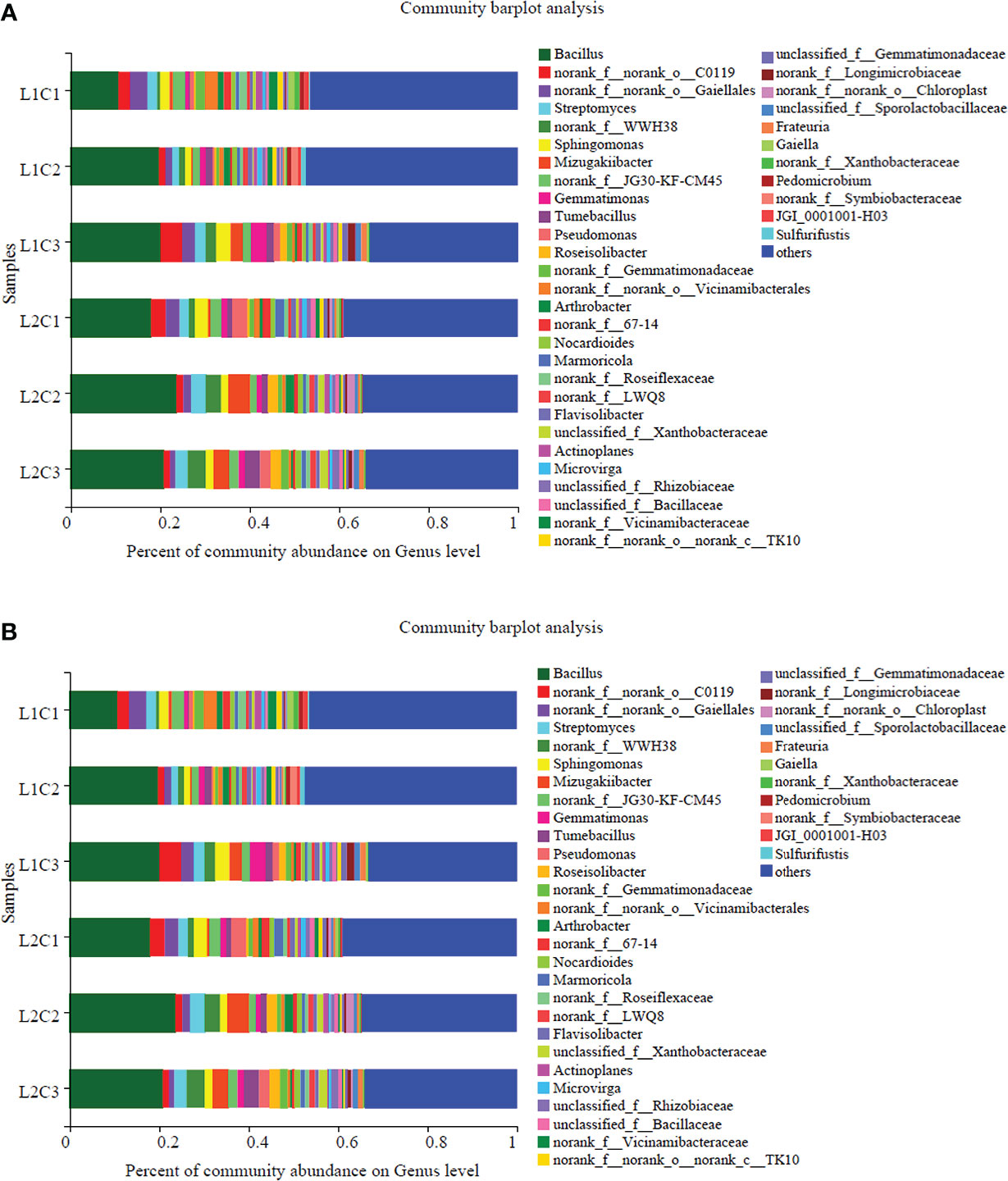
From Figure 7, it can be seen that the dominant bacterial populations in spring tomato soil at the genus level of soil bacteria mainly include Bacillus (10.71%-23.68%), Streptomyces (1.44%-3.17%), Sphingomonas (1.40%-3.21%); the soil of autumn tomato mainly includes Sphingomonas (6.07%-10.68%), Bacillus (2.55%-6.85%) and Gemmatimonas (1.75%-2.65%). Bacillus is a common genus of spring tomato and autumn tomato soil. With the decrease of C, the abundance of Bacillus in spring and autumn tomato increased first and then decreased. Compared with L2, the abundance of Bacillus with spring and autumnin L1 treatment increased by 23.50% and 76.17% at genus level.
3.2 Soil bacterial nitrogen metabolism functional gene abundance and soil bacterial phosphorus metabolism functional gene abundance
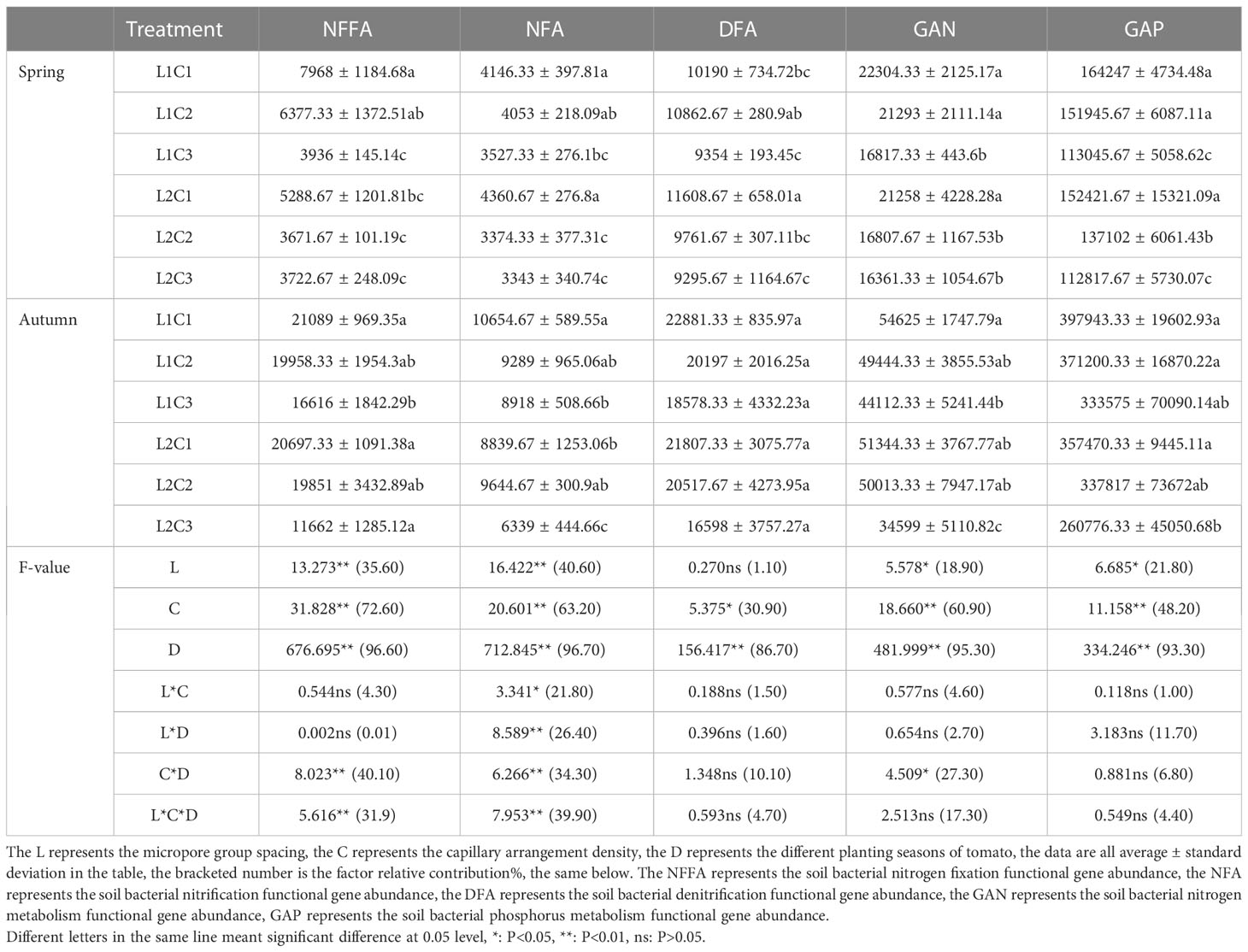
From Table 4, it can be seen that the L has a significant effect on the soil bacterial nitrogen metabolism functional gene abundance (GAN) and soil bacterial phosphorus metabolism functional gene abundance (GAP). The contribution of L to the GAN and GAP in tomato were 18.90%, 21.80%, respectively. The C had a significant effect on the soil bacterial nitrogen fixation functional gene abundance (NFFA), soil bacterial nitrification functional gene abundance (NFA), soil bacterial nitrification functional gene abundance (DFA), GAN and GAP. The relative contribution of capillary arrangement density to the NFFA, NFA, GAN and GAP in tomato soil were 72.60%, 63.20%, 30.90%, 60.90% and 48.20%, respectively. The D had a significant effect on the NFFA, NFA, DFA, GAN and GAP. The relative contribution of D to the NFFA, NFA, DFA, GAN and GAP in tomato soil were 96.60%, 96.70%, 86.70%, 95.30% and 93.30%, respectively. The GAN and GAP in L1C2 treatment were not significantly lower than that in L1C1 and L2C1, but significantly higher than that in L1C3, L2C2 and L2C3 treatments, indicating that L1C2 treatment had higher nitrogen and phosphorus metabolism functional gene abundance, which could promote soil nitrogen and phosphorus cycle transformation.
Compared with L2, the NFFA, NFA, soil bacterial denitrification functional gene abundance (DFA), GAN and GAP in spring tomato and autumn tomato soil treated with L1 increased by 44.14% and 10.44%, 5.85% and 16.27%, -0.85% and 4.64%, 11.00% and 8.99%, 6.69% and 15.34%, respectively. With the decrease of C, the NFFA, NFA, DFA, GAN and GAP in spring tomato and autumn tomato soil increased first and then decreased. The NFFA, NFA, DFA, GAN and GAP in C2 of spring tomato and autumn tomato soil was significantly higher than that in C3 about 31.21% and 40.78%, 8.11% and 24.10%, 10.59% and 15.74%, 14.83% and 26.36%, 27.97% and 19.29%, respectively. The abundance of soil bacterial nitrogen metabolism and phosphorus metabolism functional genes in spring tomato was lower than that in autumn tomato.
3.3 Effects of different treatments on tomato roots in greenhouse
It can be seen from Table 5 that the L had significant effects on total root length (RL), total number of root tips (RT) and root activity (RA) of tomato, in which the relative contributions of L to RL, RT and RA of tomato are 11.80%, 12.60% and 11.90%, respectively. The C had a significant effect on RL, total root surface area (RS), total root volume (RV), RT, total root bifurcation number (RB) and RA of tomato (P ≤ 0.05). The relative contribution of C to RL, RS, RV, RT, RB and RA of tomato was 16.10%, 16.40%, 16.10%, 18.60%, 17.50% and 13.20%, respectively. The D had a significant effect on RL, RS, RV, RT, RB and RA of spring tomato and autumn tomato (P ≤ 0.05). The relative contribution of D to RL, RS, RV, RT, RB and RA of tomato was 23.00%, 22.60%, 13.20%, 10.30%, 9.70% and 13.20%, respectively.
Compared with L2, the RL, RS, RV, RT, RB and RA of spring tomato and autumn tomato in L1 increased by 2.62% and 4.19%, 2.87% and 7.05%, 3.45% and 8.49%, 3.83% and 2.98%, 10.87% and 8.25%, 8.31% and 5.94%, respectively. With the decrease of C, the RL, RS, RV, RT, RB and RA of spring tomato and autumn tomato increased first and then decreased. The RL, RS, RV, RT, RB and RA of spring tomato and autumn tomato in C2 treatment were about 1.06% and 1.76%, 1.22% and 6.28%, 1.76% and 14.07%, 0.14% and 4.39%, 2.77% and 0.18%, 2.67% and 1.04% higher than those in C1 treatment. It was also higher than C3 treatment by about 12.37% and 12.62%, 22.21% and 20.25%, 41.73% and 45.63%, 20.05% and 16.68%, 46.82% and 21.05%, 20.42% and 20.23%, respectively. The root morphological development of spring tomato was better than that of autumn tomato.
3.4 Effects of different treatments on yield and water use efficiency of greenhouse tomato
It can be seen from Figure 8 that the L and C have significant effects on yield (Y) and water use efficiency (WUE) of spring and autumn tomatoes. Compared with L2, the Y and WUE of spring tomato and autumn tomato in L1 treatment increased by 14.15% and 11.27%, 12.64% and 10.35%, respectively. With the decrease of C, the Y and WUE of spring tomato and autumn tomato showed an increasing trend. The Y and WUE of C2 spring tomato and autumn tomato were significantly higher than those of C3 by about 34.76% and 15.23%, 31.94% and 13.91%, respectively.
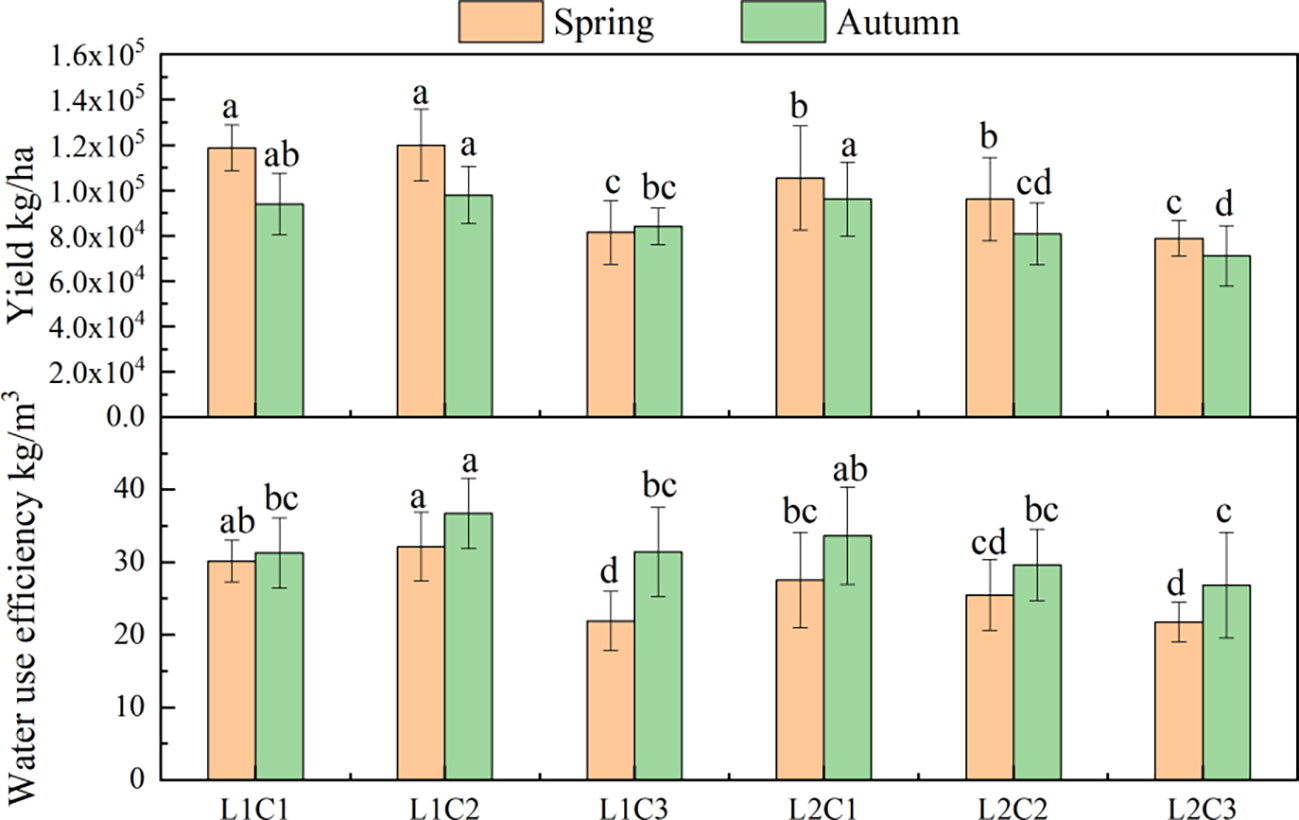
Figure 8 Yield and water use efficiency of tomato. The data are all average±standard deviation in the figure, different letters in the same column meanS significant difference at 0.05 level, the same as blow.
3.5 Correlation of tomato soil bacterial community, root system and yield
Based on Pearson’s two-tailed test, the indexes with better correlation among soil bacterial community, root system, and yield were screened out. Correlation analysis (Figure 9) showed that ACE, GAN, GAP RL, RT and RA were positively correlated with tomato yield, indicating that there was a positive interaction between soil bacterial community and root system. A stable bacterial community and good root morphological development positively promoted the increase of tomato yield. It was also found that the ACE, GAN and GAP had the highest positive correlation with the RT in the root index (0.758 and 0.870, 0.704 and 0.875, 0.756 and 0.853). In order to further quantitatively describe how the soil bacterial community affects tomato roots and thus affects tomato yield, we selected RT, which has the highest correlation with ACE, GAN and GAP, as a bridge. The relationship between ACE, GAN, GAP and RT, and the relationship between RT and yield were quantitatively described by regression analysis, respectively. The analysis results are shown in Figure 9.
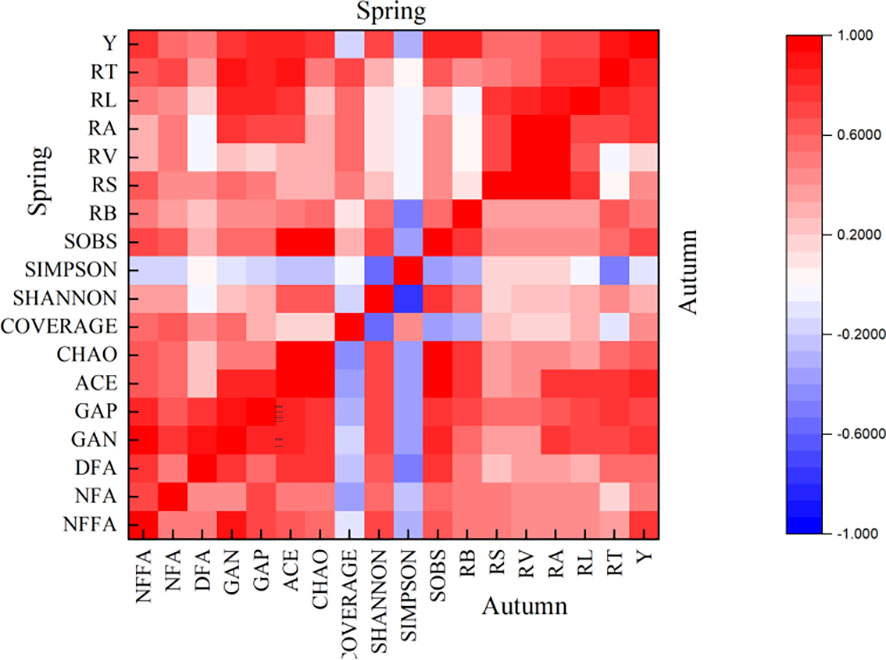
Figure 9 Correlation between tomato soil bacterial community, root system and yield. Spring represents the correlation between the indicators of spring tomato; Autumn represents the correlation between the indicators of autumn tomato. The NFFA represents the soil bacterial nitrogen fixation functional gene abundance, the NFA represents the soil bacterial nitrification functional gene abundance, the DFA represents the soil bacterial denitrification functional gene abundance, the GAN represents the soil bacterial nitrogen metabolism functional gene abundance, GAP represents the soil bacterial phosphorus metabolism functional gene abundance. The ACE represents ACE index of soil bacterial, CHAO represents CHAO index of soil bacterial, COVERAGE represents COVERAGE index of soil bacterial, SHANNON represents SHANNON index of soil bacterial, SIMPSON represents SIMPSON index of soil bacterial, SOBS represents SOBS index of soil bacterial. The RL represents the total root length, the RS represents the total root surface area, the RV represents the total root volume, the RT represents the total number of root tips, the RB represents the total root bifurcation number, the RA represents the root activity. The Y represents the yield.
From Figure 10, it can be seen that the ACE and RT showed a quadratic parabolic curve relationship, in which the determination coefficient R2>0.6015, indicating that the ACE in the regression model can explain 60.15% of the change of tomato RT. The GAN and GAP also showed a quadratic parabolic curve relationship with the RT of tomato. The determination coefficient R2 of the regression equation between the GAN and the RT of tomato was higher than that of the determination coefficient R2 of the regression equation between the GAP and the RT of tomato, indicating that the GAN had a greater impact on the RT of tomato. The relationship between the RT of tomato and the yield was a quadratic parabolic curve, and the coefficient of determination R2>0.6461, indicating that the RT of tomato in the regression model could explain 64.61% of the change in yield of tomato. The RT of tomato could be used to estimate the yield, and the tomato production potential in this area could be indirectly evaluated by soil bacterial community.
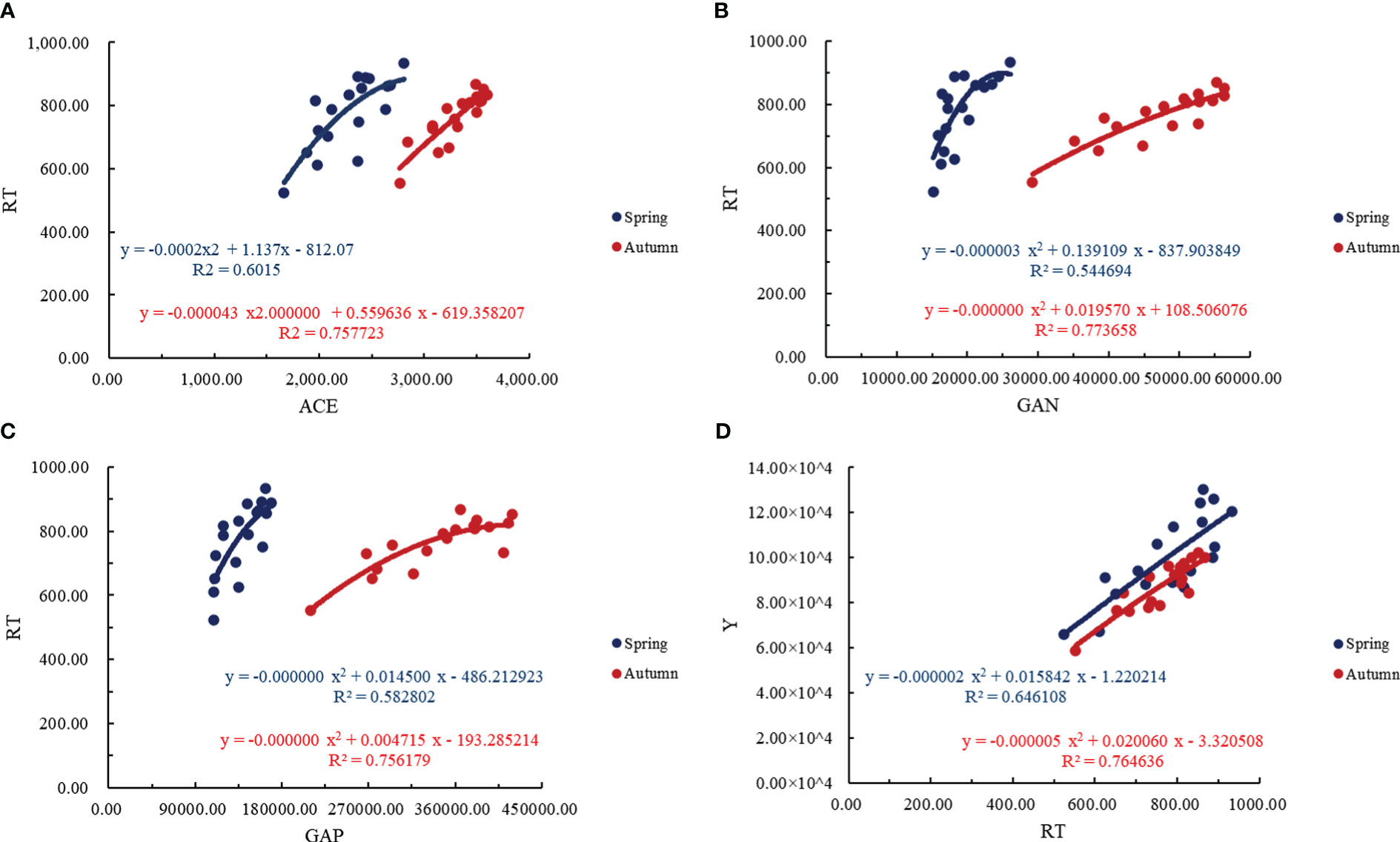
Figure 10 Quantitative analysis of tomato soil bacterial community, root system and yield in greenhouse. (A) represents the regression analysis of ACE index of soil bacterial and total number of root tips, (B) represents the regression analysis of soil bacterial nitrogen metabolism functional gene abundance and total number of root tips, (C) represents the regression analysis of soil bacterial phosphorus metabolism functional gene abundance and total number of root tips, (D) represents the regression analysis of yield and total number of root tips. The ACE represents ACE index of soil bacterial, the GAN represents the soil bacterial nitrogen metabolism functional gene abundance, GAP represents the soil bacterial phosphorus metabolism functional gene abundance, the RT represents the total number of root tips, the Y represents the yield.
4 Discussion
4.1 The layout measures regulate the soil bacterial community of greenhouse tomato
Previous studies have shown that under drought stress, plants will reduce the supply of carbon sources for soil bacteria, and sustainably reduce the diversity and richness of bacterial community structure (de Vries et al., 2018; Zeng et al., 2019). It was found that the diversity of bacterial community structure in tomato soil treated with L1 was higher than that of L2. It may be due to the high soil volumetric water content and strong soil aeration in the tillage layer with a micropore group spacing of 30 cm. The average soil volumetric water content in the 0-40 cm soil layer of spring tomato and autumn tomato irrigated by L1 treatment was about 2.18% and 3.61% higher than that of L2, and the soil water filling porosity was only 0.92 and 0.93 times that of L2. The higher water vapor environment of the soil can reduce the limitation of drought stress and hypoxia stress on soil bacterial community (Hartmann and Six, 2022; Tang et al., 2022). This conclusion is consistent with the study of Qu (Qu et al., 2022) and Alekhina (Alekhina et al., 2001) that drought stress reduces the diversity of soil bacterial community structure. The L1 had higher soil GAN of tomato than L2. It may be due to the fact that the L1 has higher soil volume moisture content and strong soil ventilation, increase root exudates and root nitrogenase activity and increase the process of above-ground carbon assimilation of tomato. Further increase the transport of carbohydrates to the underground part of tomato (root), higher root morphological development can provide more living substrates for soil bacteria (Pathania et al., 2020; Tiziani et al., 2022; Wang et al., 2023). At the same time, the rhizobia in tomato soil were greatly affected by soil moisture, which easily affected the identification and infection of host plants, and finally promoted the abundance of functional genes of soil bacterial nitrogen metabolism under L1 treatment irrigation (Mantovani et al., 2014).
The C can change the composition of microbial community by changing the wet/dry conditions of soil, thus changing the mineralization rate of soil nutrients and affecting the absorption capacity of crop roots to nutrients (Wang Z. et al., 2022; Wang et al., 2021). This study found that the diversity of bacterial community structure in tomato rhizosphere soil of C2 treatment was significantly higher than that of C3 treatment. It may be because the soil water availability per unit irrigated plough layer of C2 treatment is significantly higher than that of C3 by 6.67% and 6.69%, respectively. Stress effects of lower soil moisture increase on soil microorganisms (Alekhina et al., 2001). At the same time, the irrigation control area of C3 is larger, the irrigation amount in the plot is larger, and the local soil moisture experience is longer and higher, which is easy to increase the effect of low oxygen stress on soil microorganisms. The uneven distribution of soil moisture per unit area of C3 promotes the soil organisms in the rhizosphere of tomato under low water and low oxygen stress. Because of the existence of the filtration mechanism of the living environment, some bacteria in the soil are eliminated (Xu et al., 2020; Yang et al., 2020). The conclusion of this study is consistent with the conclusion of Wang (Wang, 2017) that the capillary density of one tube and two rows of drip irrigation promotes the diversity of microbial community structure in greenhouse tomato rhizosphere soil, and it is also consistent with the conclusion that the excessive number of dry-wet cycles of Jiao (Jiao et al., 2022) reduces the diversity of soil bacterial structure. The study also found that the abundance of nitrogen and phosphorus metabolism functional genes in C2 treated tomato rhizosphere soil was significantly higher than that in C3. It may be due to the decrease of soil litter concentration and carbon decomposition under C3, the substrate concentration of soil nitrogen and phosphorus bacteria decreased, and then decreased the abundance of nitrogen and phosphorus metabolism genes of soil bacteria (Jin et al., 2013; Zhang J. et al., 2022; Ullah et al., 2023).
4.2 The layout measures regulate the root system of greenhouse tomato
The effect of C change of MSPF on soil wetted body is similar to that of dripper spacing change (Chen et al., 2010; Zhang M. et al., 2022). There is a phenomenon that wetting fronts intersect between two adjacent groups of micropores on the pipe. The larger the distance is, the smaller the ratio of horizontal migration distance to vertical migration distance of soil moisture wetting front is, which is not conducive to the overall deep migration of soil moisture between two groups of micropores on the capillary. The volumetric water content and irrigation uniformity of plough layer per unit area will decrease (Ould Mohamed El-Hafedh et al., 2001; Sun and Wang, 2007; Del Vigo et al., 2020). The average soil volumetric water content in 0-40 cm soil layer of spring tomato and autumn tomato in L1 was 2.18% and 3.61% higher than that in L2. The higher soil volumetric water content in the plough layer was beneficial to the development of crop root morphology (Abdelhafeez et al., 1975; Silveira et al., 2020). This may be one of the reasons why the root morphological development and root activity of spring tomato and autumn tomato under L1 were higher than those under L2. The conclusion of this study is consistent with Zhang (Zhang S. et al., 2022) who found that the uniformity of soil nutrient distribution of drip irrigation 30 cm dripper spacing is higher than that of 50 cm dripper spacing, which is conducive to the development of apple root morphology.
This study found that too high or too low capillary density was not conducive to tomato root morphological development. It may be due to the increase of the outflow cross section per unit area in the test area when the C is too high (C1), which is easy to be saturated in shallow soil, form water accumulation layer on the surface, restrict the exchange of soil air and external gas, produce hypoxia stress and restrict root growth (Aixia et al., 2022; Junhao et al., 2022). When the C was too low (C3), the outflow cross section per unit area in the experimental plot decreased, the soil moisture distribution per unit area was uneven, the deep leakage increased, which led to the decrease of soil moisture in tomato tillage layer. The average soil volumetric water content in the cultivated layer (0-40 cm) of spring tomato and autumn tomato treated with C2 was significantly higher than that of C3 by about 6.67% and 6.69%, respectively. Drought stress limits root morphological development (Enciso et al., 2007; Samoy-Pascual et al., 2022; Fan et al., 2023). The conclusion of this study is consistent with the conclusion of Liu’s (Liu et al., 2019) study that too high or too low drip pipe spacing is not conducive to the development of alfalfa root morphology under drip irrigation.
4.3 Layout measures to regulate bacterial community and root interaction in tomato soil
Previous studies have found that the difference in soil water distribution caused by irrigation can significantly affect soil bacterial community, crop root morphological development, and increase or decrease soil carbon, nitrogen and phosphorus metabolic activity (Wang et al., 2021; Wang J. et al., 2022). It was found that there was a significant positive correlation between the diversity of bacterial community structure soil, the abundance of soil nitrogen and phosphorus metabolic genes in tomato rhizosphere and the RA, RL and RT of tomato roots. It may be that the RA, RL and RT determine the distribution and structure of tomato root system in soil. The high values of these indexes can improve the ability of plant roots to obtain soil moisture and nutrients, to colonize roots and enhance and enhance the strength of root-microorganism interaction. On the contrary, the diversity and abundance of soil bacterial community structure are high, which is conducive to the mineralization of soil organic matter, more soil nutrients are absorbed and utilized by plants, and the development of tomato root morphology is promoted (Leng et al., 2022; Andrade et al., 2023). It is consistent with the conclusion that Wang (Wang et al., 2017) drip irrigation crop root length is positively correlated with soil bacterial community structure diversity and yield, which is consistent with the conclusion that Nazir (Nazir et al., 2021) drip irrigation rape root activity, length and yield are positively correlated.
Previous studies have found that there is an interactive relationship between tomato root morphological development and soil microorganisms (Wang J. et al., 2022). In this study, the root morphological development of L1C2 treatment is better, which may be one of the reasons why the soil bacterial community of L1C2 treatment is superior to the other five treatments. In addition, we found that the RT was the key factor of tomato soil bacteria and tomato root interaction and played an important role in enhancing the positive interaction between soil bacteria and roots (Figure 10). The L1C2 significantly increased the RT of tomato, thus promoting the benign interaction between bacteria and roots in tomato soil. The effect of the layout measures of MSPF on the interaction between soil bacteria community and roots in tomato will inevitably affect the tomato yield. In this study, we found that the RT and ACE had the greatest influence on tomato yield under the regulation of MSPF. It may be that the layout measures of MSPF directly affects the RT and soil bacteria, and indirectly affects the interaction between tomato root system and soil bacteria through soil moisture, and regulates tomato yield directly or indirectly. In this study, the soil moisture distribution in the root zone of tomato treated with L1C2 was uniform, and the RT and ACE were significantly increased, which promoted the tomato yield under L1C2 better than other treatments.
5 Conclusion
By exploring the layout measures of MSPF to regulate the soil bacterial community and root morphological development of tomato in greenhouse, it was found that the diversity and abundance of soil bacterial community structure of spring tomato and autumn tomato in L1 were higher than those of L2. Among them, the total number of OTUs classification of soil bacteria in spring tomato and autumn tomato with L1 was higher than that of L2 by about 13.50% and 6.16%, respectively, and the ACE in spring tomato and autumn tomato with L1 was higher than that of L2 about 6.90% and 8.19%, respectively. The GAN and GAP in spring tomato and autumn tomato soil with L1 was higher than that of L2 about 11.00% and 8.99%, 6.69% and 15.34%, respectively. As a result, the yield and water use efficiency of spring tomato and autumn tomato treated with L1 were higher than those of L2 by about 14.15% and 11.27%, 12.64% and 10.35%, respectively. With the decrease of C, the diversity of soil bacterial community structure decreased significantly. Among them, the ACE and total number of OTUs classification of soil bacteria with C2 was significantly higher than that of C3 about 27.59% and 9.85%, 30.68% and 16.21%, respectively. The GAN and GAP decreased with the decrease of C. For example, the GAN and GAP in soil of spring tomato and autumn tomato with C2 was significantly higher than that of C3 about 14.83% and 26.36%, 27.97% and 19.29%, respectively. Lower soil bacterial community structure diversity and soil bacterial nitrogen and phosphorus metabolism functional gene abundance reduced greenhouse tomato soil nitrogen and phosphorus cycle. As a result, the yield and water use efficiency of spring and autumn tomatoes with C2 were significantly higher than those of C3 by 34.76% and 15.23%, 31.94% and 13.91%, respectively. Pearson two-tailed test and regression analysis showed that there was a positive interaction between soil bacterial community and root morphological development. The relationship between soil bacterial community and RT was a quadratic curve, and the relationship between RT and yield was also quadratic curve, indicating that the RT of tomato could be used to estimate the yield, and the tomato production potential in this area could be indirectly evaluated by soil bacterial community. This study provides a reference for regulating tomato root system and soil microbial interaction and increasing tomato yield by optimizing the layout measures of MSPF.
Data availability statement
The data presented in the study are deposited in the figshare repository, https://figshare.com/articles/dataset/The_Layout_Measures_of_Micro-sprinkler_Irrigation_under_Plastic_Film_Regulate_Tomato_Soil_Bacterial_Community_and_Root_System/21818610.
Author contributions
MZ wrote and revised the manuscript. YL participated in the experiments. NX, HY, FG, and JL collected the materials and analyzed the data. YL, ZZ and MZ conceived and designed the research. All authors contributed to the article and approved the submitted version.
Funding
This work is supported jointly by Natural Science Foundation of China (No. 41807041), Ninth batch of key disciplines in Henan Province—Mechanical Design, Manufacturing and Automation (JG [2018] No.119), Key Research and Development Program of Shaanxi (No. 2022NY-191), Funda-mental Research Funds for the Central Universities (GK202103129), and the Program of Introducing Talents of Discipline to Universities (B16011).
Conflict of interest
Author FG was employed by Yellow River Hydrologic Survey Planning and Design Co.,Ltd. Author JL was employed by Agricultural Technology Extension Center of Xi’an City.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelhafeez, A. T., Harssema, H., Verkerk, K. (1975). Effects of air temperature, soil temperature and soil moisture on growth and development of tomato itself and grafted on its own and egg-plant rootstock. Scientia Hortic. 3, 65–73. doi: 10.1016/0304-4238(75)90035-7
Aixia, R., Weifeng, Z., Anwar, S., Wen, L., Pengcheng, D., Ruixuan, H., et al. (2022). Effects of tillage and seasonal variation of rainfall on soil water content and root growth distribution of winter wheat under rainfed conditions of the loess plateau, China. Agric. Water Manage. 268, 107533. doi: 10.1016/j.agwat.2022.107533
Alekhina, L. K., Dobrovol'Skaya, T. G., Pochatkova, T. N., Zvyagintsev, D. G. (2001). Evaluation of bacterial diversity in soil microcosms at different moisture contents. Microbiology 70, 731–737. doi: 10.1023/A:1013100218465
Andrade, G. V. S., Rodrigues, F. A., Nadal, M. C., Da Silva Dambroz, C. M., Martins, A. D., Rodrigues, V. A., et al. (2023). Plant-endophytic bacteria interactions associated with root and leaf microbiomes of cattleya walkeriana and their effect on plant growth. Scientia Hortic. 309, 111656. doi: 10.1016/j.scienta.2022.111656
Boddington, C. L., Dodd, J. C. (1999). Evidence that differences in phosphate metabolism in mycorrhizas formed by species of glomus and gigaspora might be related to their life-cycle strategies. New Phytol. 142, 531–538. doi: 10.1046/j.1469-8137.1999.00422.x
Chen, R., Wang, Q., Yang, Y. (2010). Numerical analysis of layout parameters and reasonable design of grape drip irrigation system for stony soil in xinjiang uighur autonomous region. Trans. Chin. Soc. Agric. Eng. (Transactions CSAE) 26, 40–46. doi: 10.3969/j.issn.1002-6819.2010.12.007
Del Vigo, Á., Zubelzu, S., Juana, L. (2020). Numerical routine for soil water dynamics from trickle irrigation. Appl. Math. Model. 83, 371–385. doi: 10.1016/j.apm.2020.01.058
de Vries, F. T., Griffiths, R. I., Bailey, M., Craig, H., Girlanda, M., Gweon, H. S., et al. (2018). Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 9, 3033–3033. doi: 10.1038/s41467-018-05516-7
Enciso, J., Jifon, J., Wiedenfeld, B. (2007). Subsurface drip irrigation of onions: Effects of drip tape emitter spacing on yield and quality. Agric. Water Manage. 92, 126–130. doi: 10.1016/j.agwat.2007.05.017
Fan, J., Wu, X., Yu, Y., Zuo, Q., Shi, J., Halpern, M., et al. (2023). Characterizing root-water-uptake of wheat under elevated CO2 concentration. Agric. Water Manage. 275, 108005. doi: 10.1016/j.agwat.2022.108005
Feng, S., Ding, W., Shi, C., Zhu, X., Hu, T., Ru, Z. (2023). Optimizing the spatial distribution of roots by supplemental irrigation to improve grain yield and water use efficiency of wheat in the north China plain. Agric. Water Manage. 275, 107989. doi: 10.1016/j.agwat.2022.107989
Guo, Y., Chen, X., Wu, Y., Zhang, L., Cheng, J., Wei, G., et al. (2018). Natural revegetation of a semiarid habitat alters taxonomic and functional diversity of soil microbial communities. Sci. Total Environ. 635, 598–606. doi: 10.1016/j.scitotenv.2018.04.171
Hartmann, M., Six, J. (2022). Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environment. 4, 4–18. doi: 10.1038/s43017-022-00366-w
Huang, Y., Huang, M., Chai, L., Zhao, Y. (2018). Drivers of the spatial patterns of soil microbial communities in arid and semi-arid regions. Ecol. Environ. Sci. 27, 191–198. doi: CNKI:SUN:TRYJ.0.2018-01-026
Jafari, M., Yari, M., Ghabooli, M., Sepehri, M., Ghasemi, E., Jonker, A. (2018). Inoculation and co-inoculation of alfalfa seedlings with root growth promoting microorganisms ( piriformospora indica , glomus intraradices and sinorhizobium meliloti ) affect molecular structures, nutrient profiles and availability of hay for ruminants. Anim. Nutr. 4, 90–99. doi: 10.1016/j.aninu.2017.08.008
Jiao, P., Xiao, H., Li, Z., Yang, L., And Zheng, P. (2022). Drying-rewetting cycles reduce bacterial diversity and carbon loss in soil on the loess plateau of China. Pedosphere 9, 1262–1273. doi: 10.1016/j.pedsph.2022.09.002
Jin, V. L., Haney, R. L., Fay, P. A., Polley, H. W. (2013). Soil type and moisture regime control microbial c and n mineralization in grassland soils more than atmospheric CO2-induced changes in litter quality. Soil Biol. Biochem. 58, 172–180. doi: 10.1016/j.soilbio.2012.11.024
Junhao, C., Pengpeng, C., Xiaodong, G., Qifang, Z., Yunjie, F., Xiaobo, G., et al. (2022). Effects of plastic film residue and emitter flow rate on soil water infiltration and redistribution under different initial moisture content and dry bulk density. Sci. Total Environ. 807, 151381. doi: 10.1016/j.scitotenv.2021.151381
Leng, Z., Wu, Y., Li, J., Nie, Z., Jia, H., Yan, C., et al. (2022). Phenolic root exudates enhance avicennia marina tolerance to cadmium under the mediation of functional bacteria in mangrove sediments. Mar. pollut. Bull. 185, 114227. doi: 10.1016/j.marpolbul.2022.114227
Lewis, W. H., Tahon, G., Geesink, P., Sousa, D. Z., Ettema, T. J. G. (2020). Innovations to culturing the uncultured microbial majority. Nat. Rev. Microbiol 19 (4), 225–240.
Li, D., Ma, K. (2018). PICRUSt-based predicted metagenomic analysis of treeline soil bacteria on mount Dongling, Beijing. Acta Ecologica Sin. 38, 2180–2186. doi: 10.5846/stxb201703130423
Li, Y., Niu, W., Zhang, M., Wang, J., Zhang, Z. (2020). Artificial soil aeration increases soil bacterial diversity and tomato root performance under greenhouse conditions. Land Degradation Dev. 31, 1443–1461. doi: 10.1002/ldr.3560
Li, X., Yang, J., Jia, H., Lv, Q., Sha, R., Yao, D., et al. (2023). Impact of fruit tree hole storage brick treatment on the growth of grape seedlings and water transport in the root zone under root restriction and subsurface drip irrigation. Scientia Hortic. 308, 111552. doi: 10.1016/j.scienta.2022.111552
Liu, Y., Jiao, X., Chen, J., Wang, S. F. (2019). Effect of drip-line distance of shallow buried drip irrigation on the growth of alfalfa. J. Water Resour. Water Eng. 30, 255–260. doi: CNKI:SUN:XBSZ.0.2019-05-038
Ma, W., Du, W., Gu, K., Xu, M., Yin, Y., Sun, Y., et al. (2023). Elevated CO2 exacerbates effects of TiO2 nanoparticles on rice (Oryza sativa l.) leaf transcriptome and soil bacteria. Sci. Total Environ. 857, 159689.
Mantovani, D., Veste, M., Boldt-Burisch, K., Fritsch, S., Koning, L. A., Freese, D. (2014). Carbon allocation, nodulation, and biological nitrogen fixation of black locust (Robinia pseudoacacia l.) under soil water limitation. Ann. For. Res. 58, 1–16. doi: 10.15287/afr.2015.420
Morio, K. A., Sternowski, R. H., Brogden, K. A. (2022). Dataset of endodontic microorganisms killed at 265 nm wavelength by an ultraviolet c light emitting diode in root canals of extracted, instrumented teeth. Data Brief 40, 107750. doi: 10.1016/j.dib.2021.107750
Nazir, F., Fariduddin, Q., Hussain, A., Khan, T. A. (2021). Brassinosteroid and hydrogen peroxide improve photosynthetic machinery, stomatal movement, root morphology and cell viability and reduce Cu- triggered oxidative burst in tomato. Ecotoxicology Environ. Saf. 207, 111081. doi: 10.1016/j.ecoenv.2020.111081
Nie, K., Bai, Q., Chen, C., Zhang, M., Li, Y. (2022). DMPP and polymer-coated urea promoted growth and increased yield of greenhouse tomatoes. Horticulturae 8, 472. doi: 10.3390/horticulturae8060472
Ould Mohamed El-Hafedh, A. V., Daghari, H., Maalej, M. (2001). Analysis of several discharge rate–spacing–duration combinations in drip irrigation system. Agric. Water Manage. 52, 33–52. doi: 10.1016/S0378-3774(01)00126-3
Özbolat, O., Sánchez-Navarro, V., Zornoza, R., Egea-Cortines, M., Cuartero, J., Ros, M., et al. (2023). Long-term adoption of reduced tillage and green manure improves soil physicochemical properties and increases the abundance of beneficial bacteria in a Mediterranean rainfed almond orchard. Geoderma 429, 116218. doi: 10.1016/j.geoderma.2022.116218
Pathania, P., Bhatia, R., Khatri, M. (2020). Cross-competence and affectivity of maize rhizosphere bacteria bacillus sp. MT7 in tomato rhizosphere. Scientia Hortic. 272, 109480. doi: 10.1016/j.scienta.2020.109480
Qu, Z. M., Haojie, F., Qi, C., Yanli, L., Chengliang, L. (2022). Effects of controlled release potassium chloride application on rhizosphere bacterial community and metabolites under reduced irrigation volume. Appl. Soil Ecol. 180, 104617.
Samoy-Pascual, K., Lampayan, R. M., Remocal, A. T., Orge, R. F., Tokida, T., Mizoguchi, M. (2022). Optimizing the lateral dripline spacing of drip-irrigated aerobic rice to increase water productivity and profitability under the water-limited condition. Field Crops Res. 287, 108669. doi: 10.1016/j.fcr.2022.108669
Silveira, L. K., Pavão, G. C., Dos Santos Dias, C. T., Quaggio, J. A., Pires, R. C. D. M. (2020). Deficit irrigation effect on fruit yield, quality and water use efficiency: A long-term study on pêra-IAC sweet orange. Agric. Water Manage. 231, 106019. doi: 10.1016/j.agwat.2020.106019
Sun, A., Jiao, X., Chen, Q., Wu, A., Zheng, Y., Lin, Y., et al. (2021). Microbial communities in crop phyllosphere and root endosphere are more resistant than soil microbiota to fertilization. Soil Biol. Biochem. 153, 108113. doi: 10.1016/j.soilbio.2020.108113
Sun, H., Wang, Q. (2007). Research for soil water movement from drip irrigation iterference infiltration. J. Soil Water Conserv., 115–118.
Tang, Y., Winterfeldt, S., Brangarí, A. C., Hicks, L. C., Rousk, J. (2022). Higher resistance and resilience of bacterial growth to drought in grasslands with historically lower precipitation. Soil Biol. Biochem. 177, 108889.
Tiziani, R., Miras-Moreno, B., Malacrinò, A., Vescio, R., Lucini, L., Mimmo, T., et al. (2022). Drought, heat, and their combination impact the root exudation patterns and rhizosphere microbiome in maize roots. Environ. Exp. Bot. 203, 105071. doi: 10.1016/j.envexpbot.2022.105071
Ullah, M. R., Carrillo, Y., Dijkstra, F. A. (2023). Relative contributions of fungi and bacteria to litter decomposition under low and high soil moisture in an Australian grassland. Appl. Soil Ecol. 182, 104737. doi: 10.1016/j.apsoil.2022.104737
Vera, A., Bastida, F., Patiño-García, M., Moreno, J. L. (2023). The effects of boron-enriched water irrigation on soil microbial community are dependent on crop species. Appl. Soil Ecol. 181, 104677. doi: 10.1016/j.apsoil.2022.104677
Veresoglou, S. D., Li, G. C., Chen, J., Johnson, D. (2022). Direction of plant–soil feedback determines plant responses to drought. Global Change Biol. 28, 3995–3997. doi: 10.1111/gcb.16204
Wang, J. (2017). Effect of mulched drip irrigation on crop root-zone soil microenvironment and crop growth in plastic greenhouse (Northwest A&F University). http://cdmd.cnki.com.cn/Article/CDMD-10712-1017064536.htm.
Wang, J., Du, Y., Niu, W., Han, J., Li, Y., Yang, P. (2022). Drip irrigation mode affects tomato yield by regulating root–soil–microbe interactions. Agric. Water Manage. 260, 107188. doi: 10.1016/j.agwat.2021.107188
Wang, J., Li, Y., Niu, W. (2020a). Deficit alternate drip irrigation increased root-Soil-Plant interaction, tomato yield, and quality. Int. J. Environ. Res. Public Health 17, 781. doi: 10.3390/ijerph17030781
Wang, J., Li, Y., Niu, W. (2021). Effect of alternating drip irrigation on soil gas emissions, microbial community composition, and root–soil interactions. Agric. Water Manage. 256, 107123. doi: 10.1016/j.agwat.2021.107123
Wang, Z., Li, X., Wang, J., Qi, S., Dai, Z., Du, D. (2022). Effect of nitrogen-fixing bacteria on resource investment of the root system in an invasive clonal plant under low nutritional environment. Flora 297, 152166. doi: 10.1016/j.flora.2022.152166
Wang, X., Luo, J. F., Liu, R., Liu, X., Jiang, J. (2023). Gibberellins regulate root growth by antagonizing the jasmonate pathway in tomato plants in response to potassium deficiency. Scientia Hortic. 309, 111693. doi: 10.1016/j.scienta.2022.111693
Wang, J., Niu, W., Li, Y. (2020b). Nitrogen and phosphorus absorption and yield of tomato increased by regulating the bacterial community under greenhouse conditions via the alternate drip irrigation method. Agronomy 10, 315. doi: 10.3390/agronomy10030315
Wang, J., Niu, W., Xu, J., Li, Y. (2016). Effects of drip irrigation under plastic film on muskmelon soil environment and yield in greenhouse. Trans. Chin. Soc. Agric. Eng. (Transactions CSAE) 32 (6), 232–241. doi: 10.11975/j.issn.1002-6819.2016.06.032
Wang, J., Niu, W., Zhang, M., Li, Y. (2017). Effect of alternate partial root-zone drip irrigation on soil bacterial communities and tomato yield. Appl. Soil Ecol. 119, 250–259. doi: 10.1016/j.apsoil.2017.06.032
Xiao, W., Zhang, Q., Zhao, S., Chen, D., Gao, N., Huang, M., et al. (2023). Citric acid secretion from rice roots contributes to reduction and immobilization of Cr(VI) by driving microbial sulfur and iron cycle in paddy soil. Sci. Total Environ. 854, 158832. doi: 10.1016/j.scitotenv.2022.158832
Xu, H., Qu, Q., Li, G., Liu, G., Geissen, V., Ritsema, C. J., et al. (2022). Impact of nitrogen addition on plant-soil-enzyme c–N–P stoichiometry and microbial nutrient limitation. Soil Biol. Biochem. 170, 108714. doi: 10.1016/j.soilbio.2022.108714
Xu, X., Wang, N., Lipson, D., Sinsabaugh, R., Schimel, J., He, L., et al. (2020). Microbial macroecology: In search of mechanisms governing microbial biogeographic patterns. Global Ecol. Biogeography 29, 1870–1886. doi: 10.1111/geb.13162
Yang, Y., Liang, C., Wang, Y., Cheng, H., An, S., Chang, S. X. (2020). Soil extracellular enzyme stoichiometry reflects the shift from p- to n-limitation of microorganisms with grassland restoration. Soil Biol. Biochem. 149, 107928. doi: 10.1016/j.soilbio.2020.107928
Ye, D., Q, R., Zhang, M., And Li, H. (2016). Study of saving-irrigation regulated the soil microbial Characteristics, Soil enzyme activities and soil nutrient in the winter wheat field. Acta Agriculturae Boreali-Sinica 31, 224–231. doi: 10.7668/hbnxb.2016.01.036
Zeng, Q., An, S., Liu, Y., Wang, H., Wang, Y. (2019). Biogeography and the driving factors affecting forest soil bacteria in an arid area. Sci. Total Environ. 680, 124–131. doi: 10.1016/j.scitotenv.2019.04.184
Zhang, S., Hu, T., Chen, S., Li, H., Zhang, J. (2022). Effects of drip technical parameters and fertilization cycle on spatial and temporal distribution of nitrate nitrogenin apple root-zone soil. Agric. Res. Arid Areas 40, 79–87. doi: 10.7606/j.issn.1000-7601.2022.03.10
Zhang, M., Li, Y., Liu, J., Wang, J., Zhang, Z., Xiao, N. (2022). Changes of soil water and heat transport and yield of tomato (Solanum lycopersicum) in greenhouses with micro-sprinkler irrigation under plastic film. Agron. (Basel) 12, 664. doi: 10.3390/agronomy12030664
Zhang, M., Lu, Z., Bai, Q., Zhang, Y., Qiu, X., Qin, H., et al. (2020a). Effect of microsprinkler irrigation under plastic film on photosynthesis and fruit yield of greenhouse tomato. J. Sensors 2020, 1–14. doi: 10.1155/2020/8849419
Zhang, M., Niu, W., Bai, Q., Li, Y., Wang, J., Wang, Z., et al. (2020b). Improvement of quality and yield of greenhouse tomato (Solanum lycopersicum l.) plants by micro-sprinkler irrigation under plastic film. Appl. Ecol. Environ. Res. 18, 6905–6926. doi: 10.15666/aeer/1805_69056926
Zhang, J., Zhang, B., Liu, Y., Guo, Y., Shi, P., Wei, G. (2018). Distinct large-scale biogeographic patterns of fungal communities in bulk soil and soybean rhizosphere in China. Sci. Total Environ. 644, 791–800. doi: 10.1016/j.scitotenv.2018.07.016
Zhang, J., Zhou, J., Lambers, H., Li, Y., Li, Y., Qin, G., et al. (2022). Nitrogen and phosphorus addition exerted different influences on litter and soil carbon release in a tropical forest. Sci. Total Environ. 832, 155049. doi: 10.1016/j.scitotenv.2022.155049
Zhao, M., Li, C., Zhang, C., Han, B., Wang, X., Zhang, J., et al. (2022). Typical microplastics in field and facility agriculture dynamically affect available cadmium in different soil types through physicochemical dynamics of carbon, iron and microbes. J. Hazardous Materials 440, 129726. doi: 10.1016/j.jhazmat.2022.129726
Zhao, Q., Xing, Y., Sun, Y., Lin, X., Zhu, F. F., Long, Y. Z., et al. (2017). Effects of different soil conditioner application on coffee seedlings growth and soil enzyme activities in acidic continuous cropping soil. Chin. J. Trop. Crops 38, 1868–1873. doi: 10.3969/j.issn.1000-2561.2017.10.016
Zheng, W., Wu, Q., Rao, C., Chen, X., Wang, E., Liang, X., et al. (2023). Characteristics and interactions of soil bacteria, phytocommunity and soil properties in rocky desertification ecosystems of southwest China. CATENA 220, 106731. doi: 10.1016/j.catena.2022.106731
Zhou, Y., Ma, J., Yang, J., Lv, Z., Song, Z., Han, H. (2023). Soybean rhizosphere microorganisms alleviate Mo nanomaterials induced stress by improving soil microbial community structure. Chemosphere 310, 136784. doi: 10.1016/j.chemosphere.2022.136784
Zhu, X., Gong, W., Li, W., Bai, X., Zhang, C. (2022). Reclamation of waste coal gangue activated by stenotrophomonas maltophilia for mine soil improvement: Solubilizing behavior of bacteria on nutrient elements. J. Environ. Manage. 320, 115865. doi: 10.1016/j.jenvman.2022.115865
Keywords: rhizosphere soil, soil bacteria, root system, yield, positive interaction
Citation: Zhang M, Xiao N, Yang H, Li Y, Gao F, Li J and Zhang Z (2023) The layout measures of micro-sprinkler irrigation under plastic film regulate tomato soil bacterial community and root system. Front. Plant Sci. 14:1136439. doi: 10.3389/fpls.2023.1136439
Received: 03 January 2023; Accepted: 13 February 2023;
Published: 08 March 2023.
Edited by:
Long Yang, Shandong Agricultural University, ChinaReviewed by:
Giuseppe Di Miceli, University of Palermo, ItalyBo Zhou, China Agricultural University, China
Copyright © 2023 Zhang, Xiao, Yang, Li, Gao, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Xiao, MjAxNjA4MTU2QGhoc3R1LmVkdS5jbg==; Yuan Li, bGl5NjgxQHNubnUuZWR1LmNu
 Mingzhi Zhang
Mingzhi Zhang Na Xiao
Na Xiao Haijian Yang
Haijian Yang Yuan Li
Yuan Li Fangrong Gao4
Fangrong Gao4 Zhenxing Zhang
Zhenxing Zhang