
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 05 June 2023
Sec. Plant Pathogen Interactions
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1130793
This article is part of the Research TopicDetection, characterization, and management of plant pathogensView all 29 articles
 Maria Teresa Valente1‡
Maria Teresa Valente1‡ Laura Orzali1‡
Laura Orzali1‡ Giuliano Manetti1,2
Giuliano Manetti1,2 Francesco Magnanimi1,3
Francesco Magnanimi1,3 Antonio Matere1
Antonio Matere1 Valentino Bergamaschi1,2
Valentino Bergamaschi1,2 Alessandro Grottoli1
Alessandro Grottoli1 Sara Bechini1
Sara Bechini1 Luca Riccioni1†
Luca Riccioni1† Maria Aragona1*
Maria Aragona1*Common bunt of durum wheat (DW), Triticum turgidum L. ssp. durum (Desf.) Husn., is caused by the two closely related fungal species belonging to Tilletia genus (Tilletiales, Exobasidiomycetes, Ustilaginomycotina): Tilletia laevis Kühn (syn. T. foetida (Wallr.) Liro.) and T. caries (DC) Tul. (syn. T. tritici (Bjerk.) G. Winter). This is one of the most devastating diseases in wheat growing areas worldwide, causing considerable yield loss and reduction of wheat grains and flour quality. For these reasons, a fast, specific, sensitive, and cost-effective method for an early diagnosis of common bunt in wheat seedlings is urgent. Several molecular and serological methods were developed for diagnosis of common bunt in wheat seedlings but at late phenological stages (inflorescence) or based on conventional PCR amplification, with low sensitivity. In this study, a TaqMan Real Time PCR-based assay was developed for rapid diagnosis and quantification of T. laevis in young wheat seedlings, before tillering stage. This method, along with phenotypic analysis, was used to study conditions favoring pathogen infection and to evaluate the effectiveness of clove oil-based seed dressing in controlling the disease. The overall results showed that: i) the Real Time PCR assay was able to quantify T. laevis in young wheat seedlings after seed dressing by clove oil in different formulations, greatly reducing times of analysis. It showed high sensitivity, detecting up to 10 fg of pathogen DNA, specificity and robustness, allowing to directly analyze crude plant extracts and representing a useful tool to speed up the tests of genetic breeding for disease resistance; ii) temperature was a critical point for disease development when using wheat seeds contaminated by T. laevis spores; iii) at least one of the clove oil-based formulations tested was able to efficiently control wheat common bunt, suggesting that clove oil dressing could represent a promising tool for managing the disease, especially in sustainable farming.
Common wheat (Triticum aestivum L.) and durum wheat (Triticum turgidum L.) decay, also known as “wheat bunt”, is a fungal disease that has plagued wheat crops for centuries and is caused by Tilletia genus, including different species that both locally and systemically infect wheat crops. There are two main forms of this disease: “common bunt”, caused by T. laevis Kühn (synonym T. foetida) and T. caries Bjerk (synonym T. tritici), and “dwarf bunt” of wheat, caused by T. controversa Kühn. The distinction between these two diseases was unknown until dwarf wheat caries was identified in 1935 in North America (Young, 1935). To complete the wheat-Tilletia scenario it is necessary to consider the partial decay of the grain “karnal bunt” caused by T. indica (Mitra, 1931), currently absent in Europe and subject to quarantine regulations. In Italy are mainly present T. laevis (80%) and T. tritici (9%) which are very similar from both the etiological and morphological point of view: the two species differ only in shape and height of teliospore cell wall ornamentation (Vánky, 2012). However, morphological variants of exospores with intermediate characteristics between T. laevis and T. tritici have been observed (Flor, 1933; Gassner, 1938; Bremer et al., 1952; Holton, 1954). Those variables probably represent natural hybrids between the two species (Holton, 1944). Tilletia species causing common bunt can hybridize, not just with each other, but also with those causing dwarf caries, making taxonomy even more complicated (Holton, 1954; Holton and Kendrick, 1956; Jayawardena et al., 2019). To our knowledge, several DNA-based methods have attempted to distinguish Tilletia species, but none have been able to separate T. caries and T. laevis (Mulholland and Mc Ewan, 2000; Zouhar et al., 2010; Pieczul et al., 2018; Forster et al., 2022). Phylogenetic studies also failed to distinguish these two species and more recently some authors have suggested that T. caries and T. laevis could be considered as two morphotypes of the same species (Sedaghatjoo et al., 2021; Sedaghatjoo et al., 2022; Forster et al., 2022).
The fungal infection occurs below the soil surface immediately after seed germination. The teliospores, either in the seed or directly in the soil, germinate, producing infectious hyphae which penetrate the coleoptile. The hyphae initially enter in both resistant and susceptible cultivars, but in the former they do not reach the apical meristem, where they must arrive before the elongation of the internodes so that the disease can develop (Hansen, 1958; Swinburne, 1963). At seed maturity, the caryopsis content is totally composed of a huge mass of spores, called sorum or false caryopsis (Figure 1), which breaks down during the harvest, infecting healthy kernels and releasing teliospores onto the soil. Teliospore germination is stimulated by light, and they can remain viable for more than 20 years if stored in a dry atmosphere at room temperature, while, in the soil under natural field conditions, they remain viable for about two years (Woolman and Humphrey, 1924).

Figure 1 Wheat kernels infected by T. laevis. The dark mass of spores is called sorum or false caryopsis.
Since the second half of 1900’s wheat decay was the cause of substantial losses: the incidence was often higher than 70% and sometimes the whole harvest was destroyed (Cramer, 1967; Jones and Clifford, 1978; El-Naimi et al., 2000). As result of intensive use of chemical seed treatments, together with the use of resistant cultivars, the incidence dropped to less than 1% in Europe. However, the worldwide trading of wheat seeds is causing a new resurgence of this disease, also due to organic farming expansion which promotes the reduction of chemical in favor of biological treatments (Johnsson, 1991; Yarham, 1993) and the return to cultivation of ancient grains, more susceptible to wheat common bunt. It is also important to highlight that the disease incidence is affected by various factors, such as sowing depth, soil moisture, soil temperature, photoperiod, and altitude.
In this scenario, pathogen detection and targeted treatments are of fundamental importance. The easiest way to evaluate the common bunt infection is the sori detection (dark mass of powdery teliospores and host tissue), which are not visible until the ear ripening, occurring late in this host-pathogen interaction. Alternative approaches for direct pathogen detection from infected plants are few and mainly based on the conventional polymerase chain reaction (PCR) techniques (Josefsen and Christiansen, 2002; Eibel et al., 2005). A widely used technique for the disease prevention is seed treatment with authorized products, which have been regulated by phytosanitary product legislation (Reg. CE No. 1107/2009). This technique can be carried out with synthetical chemical products, such as fungicides and/or insecticides that can effectively control the wheat diseases. However, the European Union has been moving towards the replacement of chemicals in favor of substances, such as essential oils (Wilcoxson and Saari, 1996), with a lower environmental impact (Common European Agricultural Policy 2014–2020, CAP, implementing Regulation EU, 2015/408). Essential oils are rich in secondary metabolites, such as alkaloids, phenols, and terpenes, with bio-stimulating activity. These compounds are used by the plants themselves for multiple functions, including abiotic and biotic stress responses, and therefore as a defense mechanism against infections of fungi, bacteria, or insects. The effectiveness of essential oils in plant disease control is well documented (Isman, 2000; Nguefack et al., 2008; Marinelli et al., 2012; Arshad et al., 2014; Sturchio et al., 2014). The potential use in agriculture is further encouraged by their low or no toxicity against vertebrates, easy extractability and eco-compatibility, biodegradability (Zygadlo and Grosso, 1995) and the lack of persistence in soil and water (Misra and Pavlostathis, 1997; Isman, 2000; Isman and Machial, 2006). Indeed, recent studies (Riccioni and Orzali, 2011; Sharma et al., 2017) have shown that essential oils can reduce growth of some pathogenic fungi, and their potential applications in seed treatment are still being studied and developed (Moumni et al., 2021; Kesraoui et al., 2022). To our knowledge, no data are reported in literature on wheat seed treatment with clove essential oil against common bunt under field conditions.
The present work has the following main aims: i) development of a quantitative, early, fast, and low-cost method based on TaqMan Real Time PCR for the highly sensitive detection and quantification of T. laevis in young wheat plants, before tillering stage; ii) elucidation of the role of temperature for seed infection success; iii) quantitative evaluation of clove oil-based treatments effectiveness against T. laevis, by the molecular method developed. The overall results showed that at least one clove oil-based formulation was able to efficiently control wheat common bunt.
Pure clove oil stocks and two clove oil-based experimental formulations, named Bioxeda A and Bioxeda B, were provided by Cedax Italia s.r.l. (Forlì, Italy). The two formulations contained micro-encapsulated and nano-encapsulated clove oil, respectively, both at the concentration of 20% (v/v). Two T. durum cultivars susceptible to T. laevis infection were used: Grifoni 235 and Svevo (gently provided by CREA-CI, Research Centre for Cereal and Industrial Crops). These two cultivars showed the same susceptibility to T. laevis infection in preliminary trials carried out by CREA-CI (personal communication). Naturally infected seeds of cultivar Grifoni were used for progressive sowings performed in 2019 and 2020 while, for seed treatments trials, Grifoni and Svevo healthy seeds were artificially inoculated and used in 2020 and in 2022, respectively. Tilletia laevis teliospores used for inoculations were collected from sori of Grifoni naturally infected kernels. Their vitality and virulence were evaluated before inoculation. Artificial inoculations for seed dressing tests were performed following Dumalasová and Bartoš (2008) with dry teliospores: briefly, healthy kernels were placed in plastic bags, together with the teliospores, at the dose of 1g of inoculum for 1Kg of seeds resulting in ca 3×104 spores per seed. The envelope was sealed, and the contents manually shaken for 5 minutes allowing the spores to enter in contact with kernel surface, remaining attached to them. The presence of spores was verified by visually checking the kernels under a stereo microscope.
To set-up the early diagnostic assay, based on Real Time PCR, artificially inoculated wheat seedlings were sampled at the stage immediately previous the tillering (from 20 to 40 days from seedling emergence; Feekes growth stage 2.0 – beginning of tillering; Miller, 1992). DNA was extracted from 10 single plants as described by Li et al. (2017). Briefly, 0.5-1 cm of the basal shoot was cut from the seedlings, quickly sterilized in sodium hypochlorite 2% (v/v), rinsed with sterile water and homogenized in 40 µL of lysis buffer (0.5% casein in 10 mM KOH) using a micropestel. Subsequently, the sample was incubated at 95°C for 5 minutes and cooled on ice for 5 minutes, finally, it was centrifuged at 13000 g for 1 minute. The supernatant was recovered into a new tube and the obtained crude extract was used as template for amplification. A TaqMan Real Time PCR assay was carried out using the primers Tri-DL-For (5’-ATTGCCGTACTTCTCTTC-3’), Tri-DL-Rev (5’-GTAGTCTTGTGTTTGGATAATAG-3’) and the dual-labelled probe (5’Cy5-AGAGGTCGGCTCTAATCCCATCA-BHQ2-3’), targeting a specific sequence in the ITS1 region of T. laevis and other close related species (T. caries, T. controversa, T. fusca, T. bromi, T. goloskokovii). The assay was performed following Tan et al. (2009), modified as described below: the reaction mixture was carried out in 25 μL consisted of 1 × ImmoBuffer (Bioline, London), 5 mM MgCl2, 0.2 mg/mL BSA, 200 mM of each deoxynucleotides, dATP, dTTP, dCTP and dTTP, 1U Immolase DNA polymerase (Bioline) and 200, 400 and 900 nM of each of the dual-labelled probes, the forward and the reverse primers, respectively. The reactions were performed on a CFX96 Real Time PCR system (Bio-Rad Laboratories Inc., CA, USA). The thermal cycling parameters included an initial cycle of 95°C for 10 minutes, followed by 40 cycles of 94°C for 15 seconds, 65°C for 60 seconds with the annealing temperature decreased by 1°C/cycle for 6 cycles to 60°C. To evaluate the presence of PCR inhibitory effects and to verify the amplification efficiency, serial dilutions of purified T. laevis DNA, from 10 pg to 1 fg per reaction, spiked with a fixed amount (2 μl) of plant crude extract from Tilletia-free wheat plants, were amplified.
To identify the optimal conditions (photoperiod, temperature, rainfall) for T. laevis infection, wheat seeds were artificially inoculated as previously described, and seedlings were grown both outdoor, in pot progressive sowings, and in growth chamber. The term “progressive sowing” refers to a series of repeated sowings deferred at predetermined time points, with the aim of covering the entire period of wheat sowing. For this purpose, five progressive sowings at regular intervals of about two weeks from November to January were carried out for two consecutive years. Each sowing was structured in two pots (pot A and pot B) with 50 inoculated seeds each, located outdoor close to weather station (Vantage Pro2, Davis Instrument, USA) recording temperature, rainfall, and relative humidity.
The time to obtain 50% of seedling emergence (T50) was calculated according to Farooq et al. (2005). For each sowing the emerged plants were randomly sampled from both pots. Half of them were used for molecular assay and half for phenotypic evaluation of the presence/absence of the disease symptoms in plants grown to maturity (Feekes growth stage 11.4; Miller, 1992). In the molecular assay a plant was considered infected if produced a positive signal in the Real Time PCR, whereas in the phenotypic evaluation if at least one sorum was present. The disease incidence was calculated by the formula: (Number of infected plants/Total number of analyzed plants) × 100.
From sowing to sampling date, meteorological data (average temperature, minimum temperature, maximum temperature) were recorded every hour; for each day, the average, lowest and highest temperature values were calculated and collected for each day. Days in which the temperature falls within one of these ranges were summed: low-range included temperatures between –5°C and 5°C (range 1), mid-range included temperatures between 5°C and 15°C (range 2) and high-range included temperatures above 15°C (range 3).
To better understand the ideal pedo-climatic conditions that allow fungal infection, Svevo wheat seeds were artificially inoculated as reported above and planted in pots in a growth chamber (KW Apparecchi Scientifici, Siena, Italy). Pedo-climatic conditions were set according to Zscheile jr (1966) and Swinburne (1963): low soil temperatures after sowing (5-10°C), seed vernalization, short photoperiod early in ontogeny, soil humidity between field capacity and drying point and deep sowing at 4-7 cm. Two main sowing conditions were chosen: 1) after seed vernalization; 2) direct sowing, without seed vernalization. For each condition 60 seeds were sowed at 5 cm depth in the soil, in two different pots (30 seeds/pot) kept at 5-10°C up to third-leaf stage. For all the four pots, a 9-hour-light photoperiod was set after seedling emergence. Seed vernalization was carried out before sowing by allowing the seeds to germinate at 27°C for 48 hours (blotter test) and then leaving them at 4°C for 2 weeks. For T. laevis in planta detection, 25 seedlings per pot were harvested at the third-leaf stage and analysis was performed as reported in 2.2 paragraph.
The treatments were applied to inoculated seeds by seed submersion or seed spraying, as detailed in Table 1. Seed submersion was carried out by soaking the seeds in the clove oil emulsion (with the addition of 0.05% pinolene as emulsifier) or in the diluted formulations with gentle agitation for 10 minutes; after that they were completely dried on a sterile blotting paper at room temperature in a laminar flow cabinet. Spray treatments were performed with two consecutive applications of the diluted formulations or essential clove oil emulsion with the addition of 0.05% pinolene using a Rotostat (Marline General Engineers Limited, England) seed dressing/coating machine. Pinolene acted as a film-forming compound for improving oil distribution and persistence. Copper sulphate (15.2%) Cutril® (CU) (Serbios s.r.l., Badia Polesine, RO, Italy) was applied by spray in the second season as a further control treatment allowed in organic agriculture (later coded as CU). Sterile water was applied by submersion and used as control to evaluate the washing effect of the seed submersion treatments without active compounds (mechanical effect). The seeds inoculated with T. laevis spores as described in 2.1 but not treated (NT), were used as positive control. Not inoculated and not treated seeds were used as negative control. Phytotoxicity of the different treatments was evaluated as the effect on seed germination and seedling emergence by counting the number of established seedlings at the beginning of the secondary tillers arising (Feekes growth stage 2.0; Miller, 1992). The whole experiment was carried out for two growing-crop-productive seasons with cultivar Grifoni in 2020 and Svevo in 2022. For each treatment, three replicated pots, each consisting of 30 and 45 seeds for season 2020 and 2022, respectively, were sowed and placed outdoor with a completely randomized design (CRD). After 14 days from sowing, the number of germinated plants was recorded. Plant sampling was performed at the stage preceding the tillering (from 20 to 40 days from seedling emergence; Feekes growth stage 2.0 – beginning of tillering; Miller, 1992) by cutting plants at the culm base and collecting from each pot at least 5 bulks of 5 plants each; the bulks were then processed for DNA extraction following the protocol described in 2.5 paragraph.
For seed dressing evaluation, DNA was extracted from plants sampled in the pre-tillering stage (about one month after sowing). From each seedling, a fragment of 4-5 cm was taken from the culm base. Bulks of 5 seedlings each were homogenized in liquid nitrogen; 150-200 mg of powder was lysed in 700 µL of lysis buffer (2% CTAB; 0.2 M Tris HCl pH 7.5; 0.05 M EDTA pH 8; 2 M NaCl) and incubated at 65°C for 10 min. After adding 70 µL of NaAc (3 M, pH 5.2), the samples were kept in ice for 5 minutes and centrifuged at 13.000 g per 10 minutes at 4°C; the supernatant was recovered and treated with RNAse. DNA was purified with 1 volume of chloroform:isoamyl alcohol 24:1 (Sigma-Aldrich, USA) and precipitated with ½ volume of isopropanol. The pellet was resuspended in 200 µL of milliQ water. Finally, DNA was quantified by a Qubit dsDNA HS assay on Qubit 2 fluorometer (Life Technologies™, Invitrogen, USA). A duplex Real Time PCR was set up, using a 18S universal target for fungi and plants (Ioos et al., 2009) as endogenous calibrator to normalize the T. laevis qPCR signal to the plant qPCR signal. The reaction was carried out in 20 µL volume, containing SensiMix™ II Probe (Bioline, Taunton, Massachusetts, USA), 0.2 mg/mL BSA, 900 and 400 nM of Tri-DL-For and Tri-DL-Rev primers respectively, 200 nM T. laevis probe, 100 nM each 18S primers (18S uni-F, 5’-GCAAGGCTGAAACTTAAAGGAA-3’;18S uni-R, 5’- CCACCACCCATAGAATCAAGA-3’) and 18S probe (5’-JOE-ACGGAAGGGCACCACCAGGAGT-BHQ1-3’) and 10 ng of plant DNA. The thermocycling conditions used were the same applied for the rapid molecular assay. The pathogen biomass was quantified as the ratio of the amplification of T. laevis ITS1 relative to the 18S target, which was calculated as 2−ΔCt (Livak and Schmittgen, 2001). To verify the correlation between the number of infected plants and the relative quantification values, 5 bulks with known amounts of infected plants were analyzed.
Tilletia infection in progressive sowing assay was evaluated by molecular and phenotypical analysis. The correlation coefficient between the two methods was calculated by Pearson correlation analysis using R software v4.2.2 (R Core Team, 2022) and the package ggpubr v0.5.0 (Kassambara, 2022). A homogeneity test, by a chi-square analysis, was carried out to compare the infection frequencies between the two pots (A and B) both for molecular and phenotypical results.
The meteorological data analysis was performed using a Principal Component Analysis (PCA) biplot, to determine the correlation between temperature and infection. The PCA was performed with R software v4.2.2 (R Core Team, 2022) by using the package factoextra v1.0.7 (Kassambara and Mundt, 2020).
The seed treatments data obtained by Real Time PCR were normalized as described in 2.5 paragraph. Data were then subjected to the analysis of variance (ANOVA) and LSD (least significant difference) test for multiple comparison at p = 0.05. To display and compare the results obtained in the two seasons, relative quantification data were balanced to the mean value of the NT control and the proportion to it of the other treatment mean values was calculated: (T/NT) × 100 with T= relative quantification data mean of the treated samples, NT= relative quantification data mean of the not treated samples. The correlation coefficient between the 2020 and 2022 seasons was calculated by Pearson correlation as described above.
For oil phytotoxicity evaluation, the average percentages of established seedlings of 2020 and 2022 seasons were combined after homogeneity evaluation by a chi-square test (data not shown). They were arcsin transformed before ANOVA, followed by Duncan’s test (p=0.01), however, mean values were back transformed into percentages for making data reading easier.
A Real Time PCR-based method for the early diagnosis of common bunt in wheat seedling has been developed. The qPCR conditions set up in this work showed no inhibition using the crude extract DNA and no differences in the amplification performance between the two extraction methods: the amplification standard curves for both extracts showed an optimal efficiency, a high coefficient of determination (R2>0.99) and the same sensitivity, detecting up to 10 fg of target DNA (Supplementary Figure 1).
The progressive sowings were evaluated together with infection percentages obtained from molecular and phenotypic assay. The disease incidence values in pot A and pot B were combined after homogeneity evaluation by a chi-square test (Supplementary Table 1). In the season 2019/20, the first three sowings (01-03), gave back no bunt infection both in molecular and phenotypic analysis. In the following sowings the infection increased, and the highest values were obtained in the fourth sowing (82% for molecular and 90% for phenotypic analysis), then decreasing by the fifth sowing (22% for molecular and 2.7% for phenotypic analysis). In the season 2020/2021 T. laevis infection was recorded for all the sowings; only by phenotypic analysis for sowing n. 07, and only by molecular analysis for sowing n. 10. The highest infection values were found in sowing n. 06 (20% from molecular and 53% from phenotypic analysis) and n. 08 (48% from molecular and 41.4% from phenotypic analysis) (Table 2).
Despite some minor differences between the two different analyses, the Pearson correlation coefficient showed a strong correlation between them, with a coefficient R of 0.9 (Figure 2).
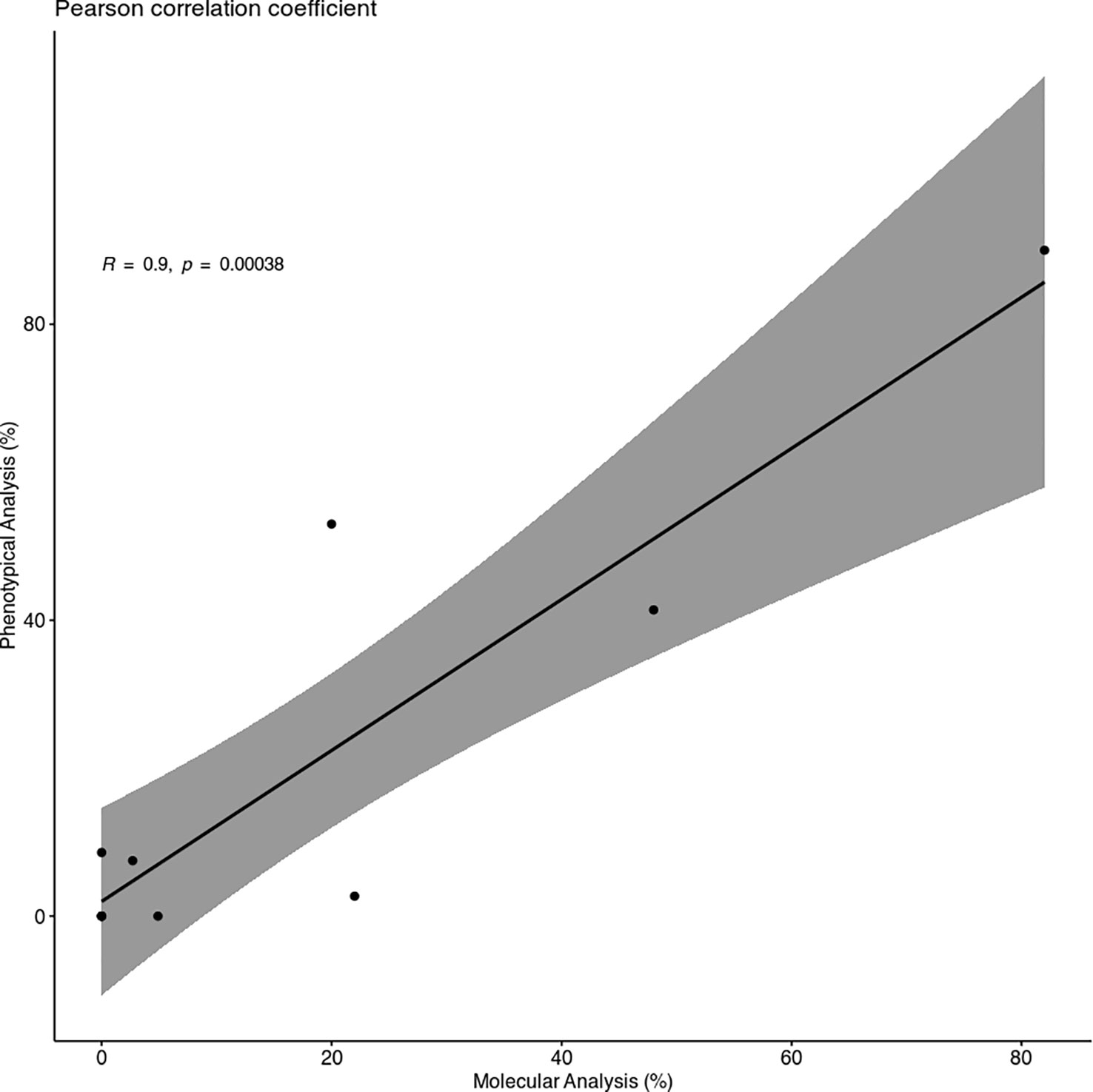
Figure 2 Scatter Plot of the Pearson correlation coefficient. The plot shows a strong and positive correlation between the infection percentages of Molecular (MA) and Phenotypical Analysis (PA) (R = 0.9) and a p < 0.01.
With the aim of understanding the optimal in vivo conditions for T. laevis infection, the meteorological conditions during the growth period from sowing to the second true leaf stage (about 30 days) were analyzed: this period represents the limit phase within which the seedlings are considered susceptible to infection to Tilletia spp. (Gaudet et al., 2007).
To assess and discriminate if specific temperature ranges play a role in the infection process, data were analyzed by using the PCA biplot (Data in Supplementary Table 2). This method geometrically analyzes information in multivariate data. When the variable positions are close, there is a high correlation, moreover, the acute angle between each pair of variables indicates a positive correlation, while the obtuse angle indicates a negative correlation (Jolliffe and Cadima, 2016).
The disease incidence data, from both molecular and phenotypical analyses (MA and PA), T50 and all the variables indicating the number of the days with lower temperature (AVG.T1, LT1 and TH2) were well negatively correlated with the variables indicating the number of the days with higher temperature (AVG.T3, LT2 and HT3) (Figure 3). The negative correlations between the number of the days with higher and lower temperature, together with the distribution of the sowings point on the PC1, suggest a significant role of the temperature in the infection process. As shown in Figure 3, the sowings dates were well separated on the PCA axis 1 (59.5% of variance) in two groups: presence or absence of infection. Moreover, the greater the days with low temperatures, the higher the molecular and phenotypical infection rates. In addition, T50 had a positive correlation with higher number of days at lower temperatures and higher infection rates. The percentages reported on the axis referred to the variability explained by the principal component. There was no correlation between all variables and number of days with an average temperature ranging from 5 to 15°C (AVG.T2); no day was recorded with the maximum temperature below 5°C (TH1) (Figure 3).
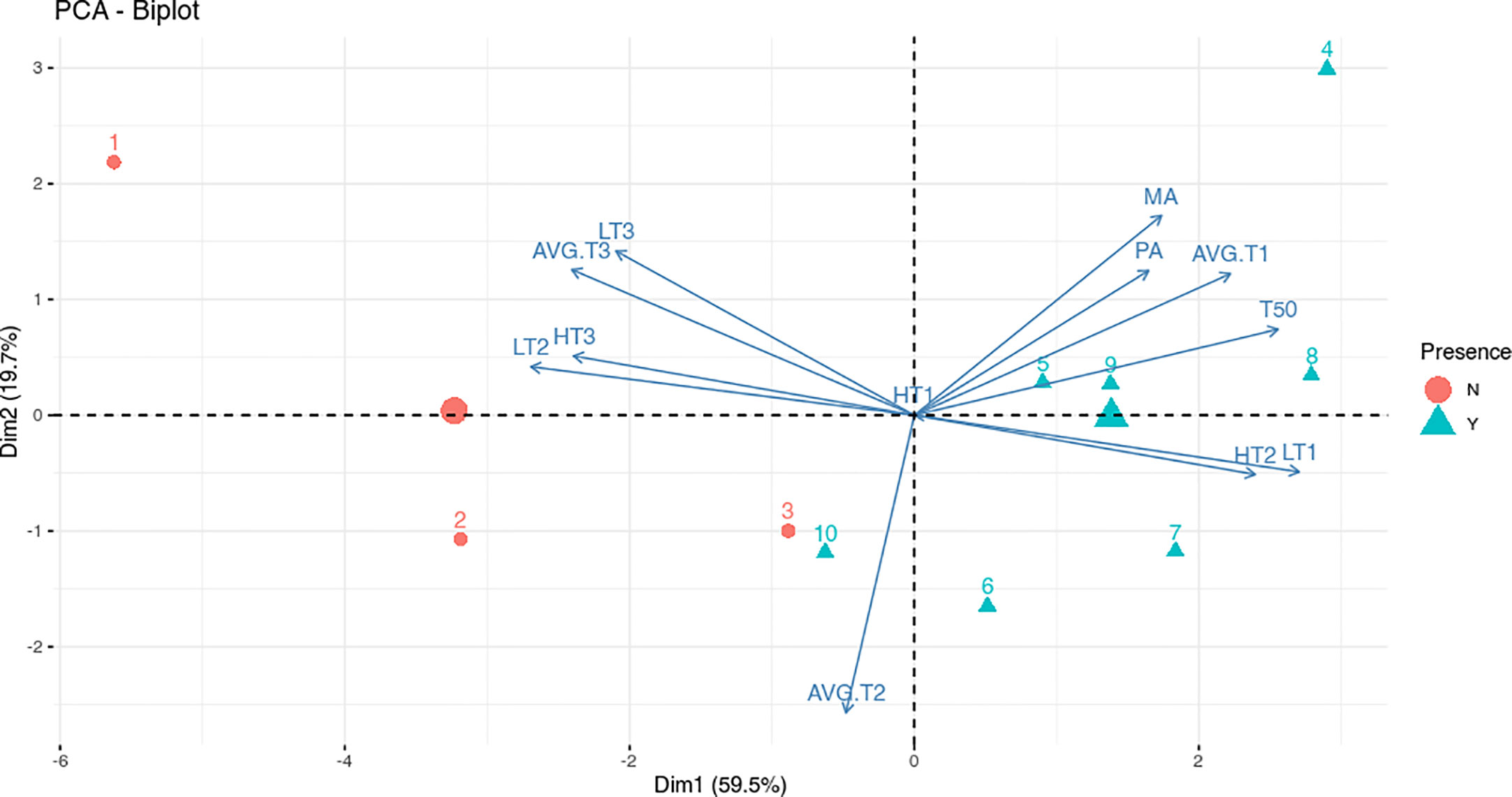
Figure 3 Biplot for the principal component analysis (PCA) of meteorological data and infection analyses. Red dots indicate sowings that did not show disease (N) while blue triangles indicate sowings that showed disease (Y). The numbers indicate sowings while the red dot and the blue triangle with no number indicate the group centroid. The arrows indicate the contribution of variables to the two axes of the PCA: number of days at Highest Temperature (HT), Lowest Temperature (LT) and Average Temperature (AVG.T); Molecular Analysis (MA) and Phenotypical Analysis (PA) percentages and median germination time (T50). The temperature ranges considered are indicated on arrows variables with 1, 2 or 3 (1: -5/5°C; 2: 5/15°C; 3:15/25°C).
Wheat is a winter cereal and T. laevis needs low temperatures to infect this crop, so we tested the in vivo fungal infection capability in the typical winter temperature range of 5-10°C. The main goal of the experiment performed in growth chamber was to achieve a high level of seedling infection also in controlled conditions, in order to be able to replicate the method for seed dressing and sowing tests. In both seed vernalization and direct sowing, T. laevis infection rates were comparable (approx. 70%), suggesting that seed vernalization did not affect the early infection. The infection rates obtained were in agreement with those reported in literature (Wilcoxson and Saari, 1996).
Duplex qPCR showed both very good efficiency and coefficient of determination (R2>0.99), and a limit of detection (LOD) of 10 fg of T. laevis DNA per reaction (Figure 4). Relative quantification, showing the amount of target amplicon (T. laevis) normalized to the total DNA (18S), correlated efficiently with the amounts of target DNA. Normalized amplifications corresponded to an exponentially increasing trend of about an order of magnitude (Figure 5A), and the best fit curve, correlating the data of normalized expression signal to the known amounts of T. laevis DNA, showed a good linear correlation (R2 = 0.99) (Figure 5B). Furthermore, the presence of the 18S endogenous calibrator guaranteed greater reliability in the quantification, representing an internal amplification control, and allowing the precise quantification of total DNA used as template in the reaction.
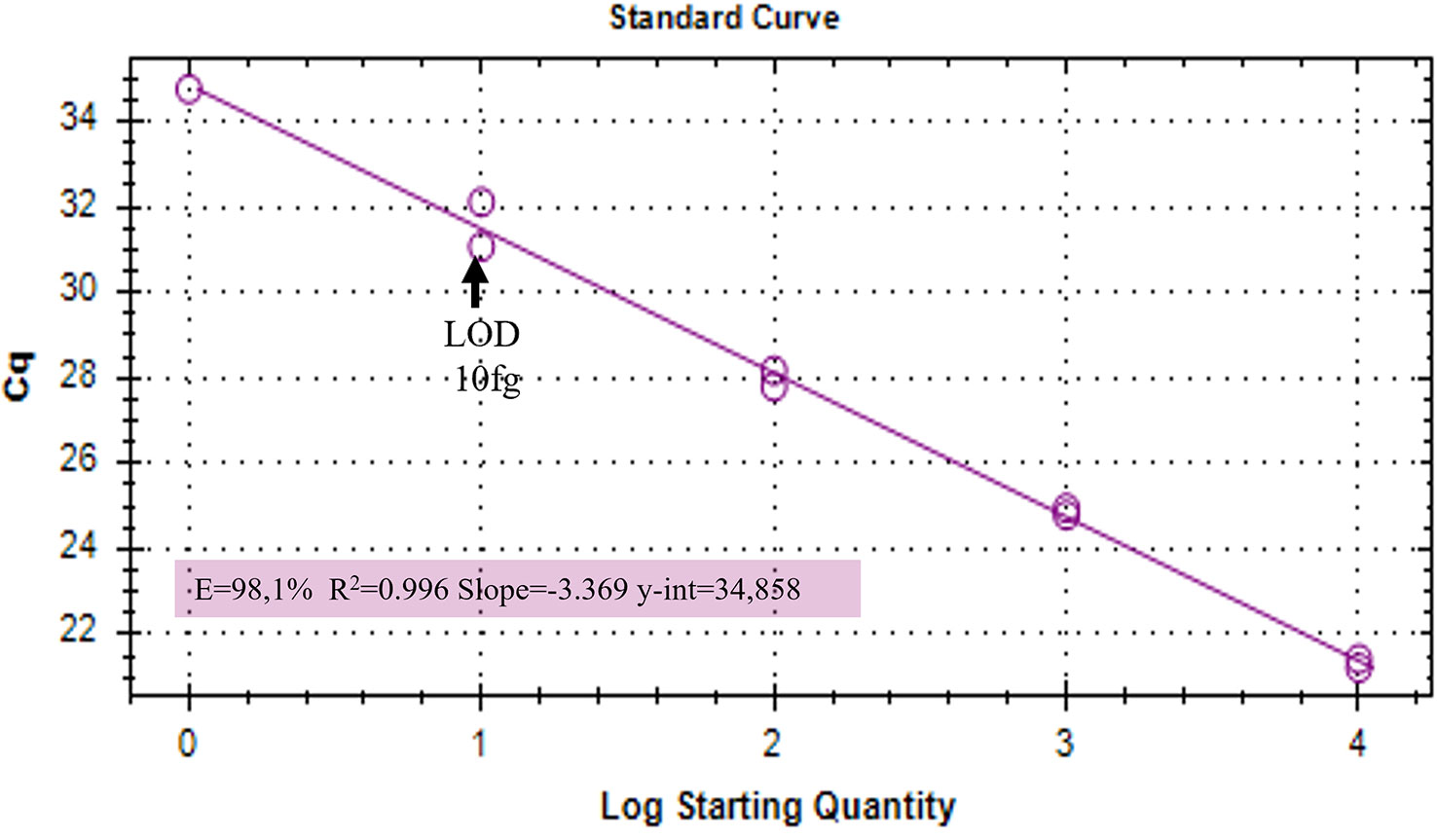
Figure 4 Amplifications of serial dilutions of T. laevis DNA starting from 10000 fg. LOD, Limit Of Detection.
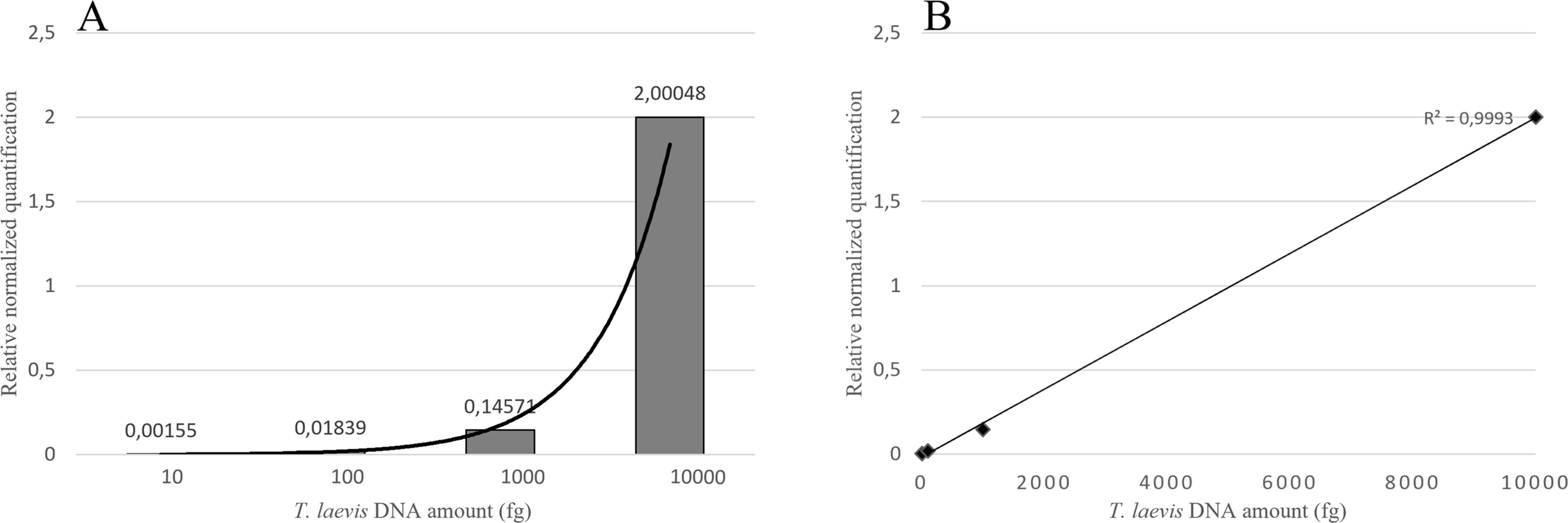
Figure 5 Target normalized quantification of serial dilution of T. laevis DNA (A) and best fit correlation between the relative quantification values and the known amounts of target DNA (B).
The correlation between relative quantification and disease incidence was verified by analyzing bulks with a known number of infected plants (R2 = 0.93; Figure 6). To compare the infection success between treatment assays in seasons 2020 and 2022, the equation resulting from the interpolation line of the aforesaid data was used to obtain an approximate percentage of infected plants in the untreated control tests. In 2020 the average infection of NT plants resulted to be about 50%, versus 20% in 2022.
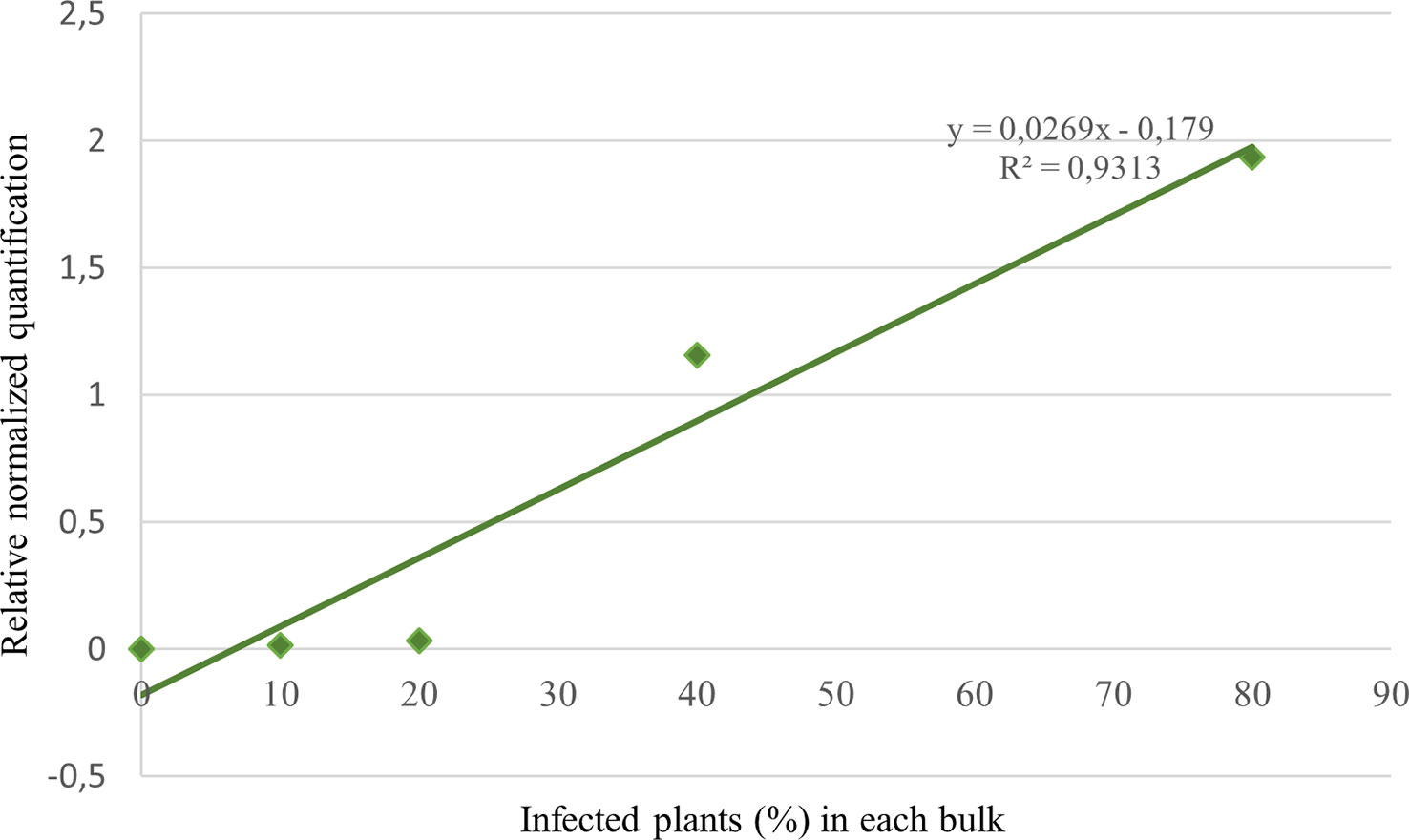
Figure 6 Target normalized quantification of 5 bulks consisting in 10 wheat seedlings each with a known number of infected plants (0, 1, 2, 4, 8).
Tilletia laevis quantification in seedlings deriving from dressed seeds was performed by TaqMan Real Time PCR. Data were analyzed considering the two growing seasons separately (Figures 7, 8). The seed treatments were generally able to control bunt infection. In season 2020, significant differences were observed between the treatments performed by submersion (BioxA, BioxB and CO) and NT control. Among the spray treatments, only Bioxeda A gave results significatively different from the NT control, while the other two (Clove oil spray and Bioxeda B spray), together with water treatment, were comparable to NT control. Water treatment has produced a reduction of infection too, probably due to the water washing effect on the teliospores present on the kernel surface (Figure 7). Negative control (not treated and not inoculated) always gave negative results (data not shown).
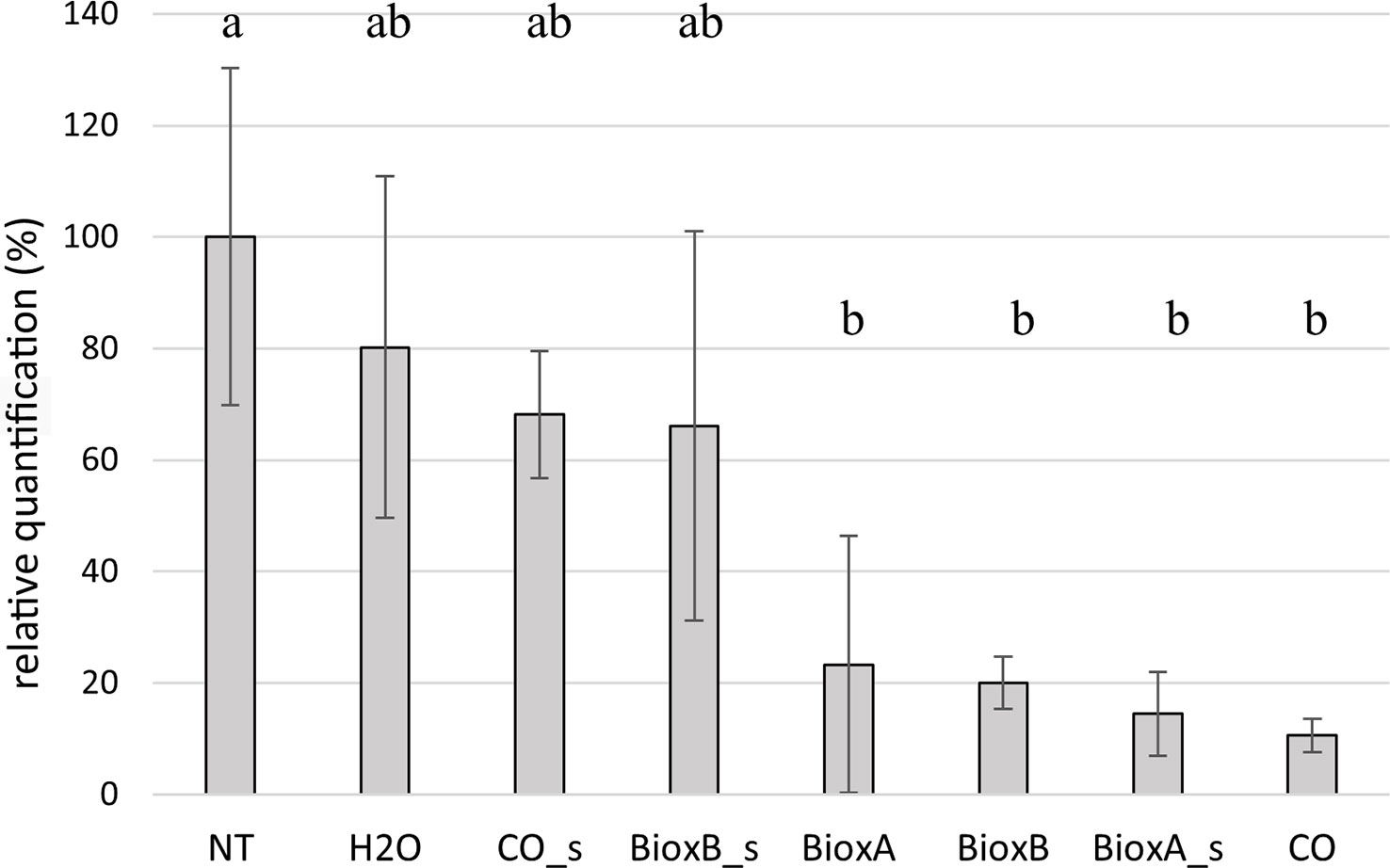
Figure 7 Barplot of season 2020. Relative quantification, expressed as percentage referring to NT as 100%, is reported on the y-axe. The values obtained from the different treatments are the mean of three replications (± standard error). Significant differences (p = 0.01) found using the LDS test for multiple comparison are indicated as letters a–b; the same letter means no statistical significance. NT, Not treated; H2O, water; CO, clove oil; BioxB, Bioxeda B; BioxA, Bioxeda A; s, spray treatment.
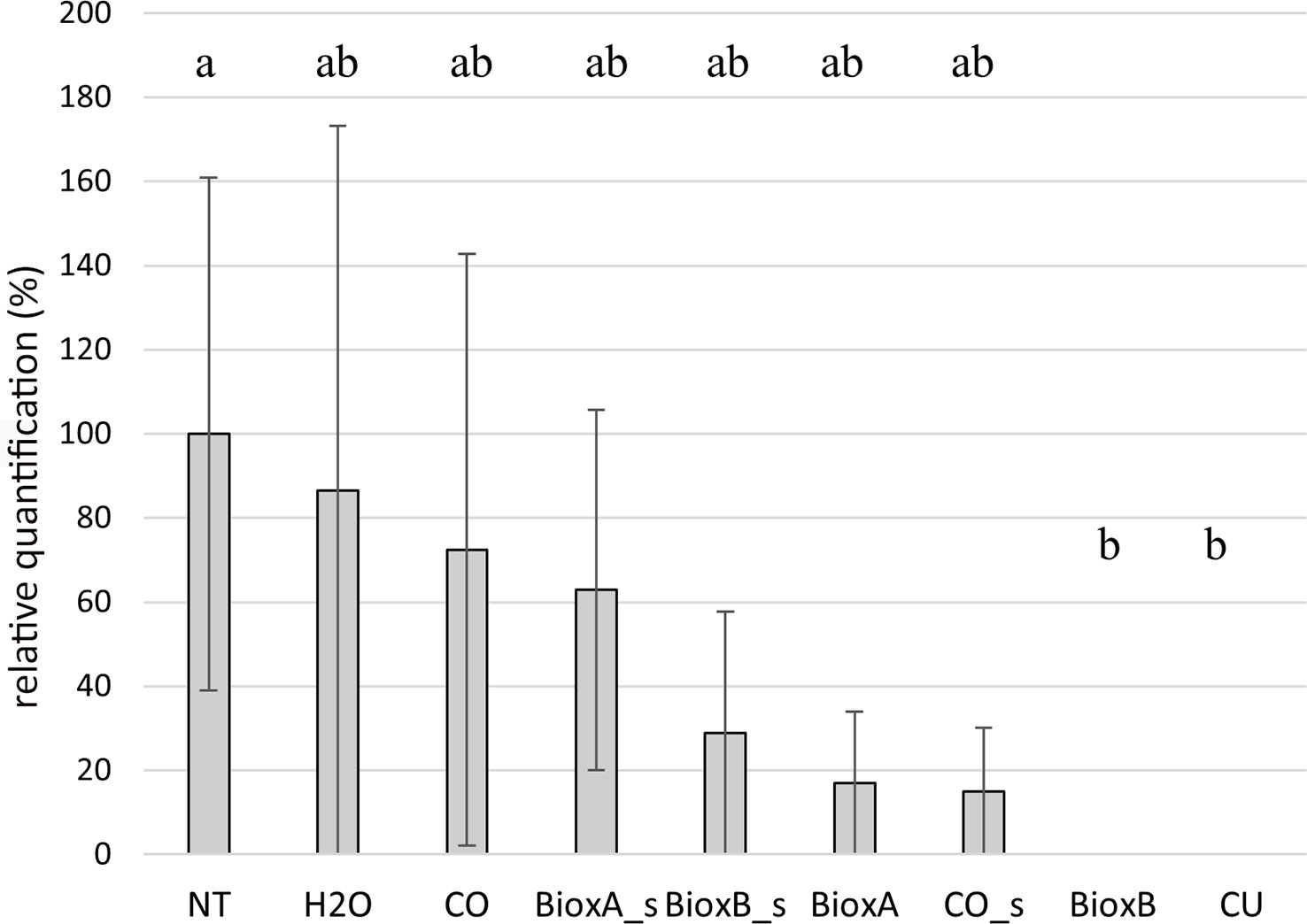
Figure 8 Barplot of season 2022. Relative quantification, expressed as percentage referring to NT as 100%, is reported on the y-axe. The values obtained from the different treatments are the mean of three replications (± standard error). Significant differences (p = 0.01) found using the LSD test for multiple comparison are indicated as letters a–b; the same letter means no statistical significance. NT, Not treated; H2O, water; CO, clove oil; BioxB, Bioxeda B; BioxA, Bioxeda A; s, spray treatment; CU, Cutril®.
In season 2022, the Bioxeda B submersion treatment showed the best results as no T. laevis infection was recorded, as well as with the chemical treatment (CU) used as control. The other treatments showed a lower infection rate compared to NT control, suggesting a protective (or just washing) effect against the pathogen, however no significant differences were found (Figure 8). In this season, expression data of the whole experiment, including the NT control, were lower than in season 2020, and no correlation was found between the average infection rates of the tested oil treatments of 2020 and 2022 seasons (R2 = 0.09; Supplementary Figure 2).
The phytotoxicity of the different treatments used for seed dressing was evaluated as effect on seed germination and seedling emergence, assessing the number of established seedlings before the secondary tillers arising. Data collected from the two seasons were merged and analyzed together: significant differences were found only for clove oil treatment by immersion (CO), compared to NT control (Table 3). This treatment showed a toxic effect on wheat seeds significantly reducing germinability and seedling emergence. A significant correlation was found from the analysis of 2020 and 2022 phytotoxicity data (R2 = 0.94; Supplementary Figure 3), indicating the reproducibility in the different seasons.
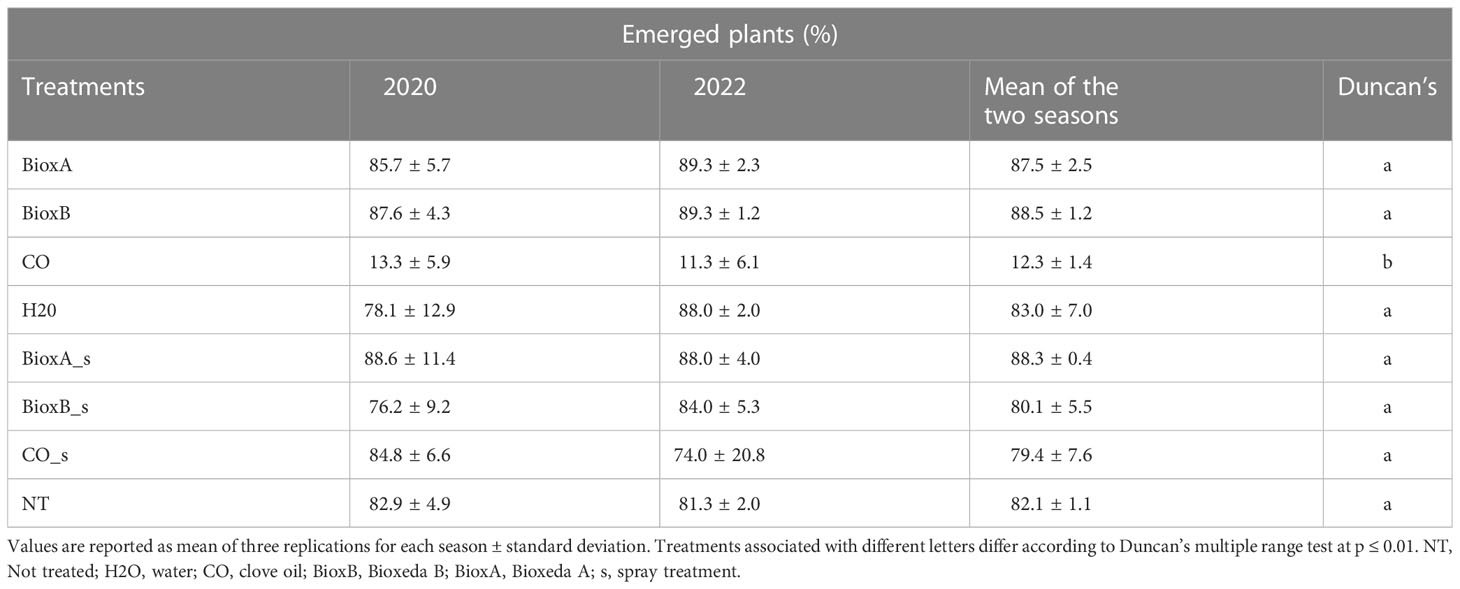
Table 3 In pot seedling emergence at the beginning of the secondary tillers arising (Feekes growth stage 2.0) for treatments phytotoxicity evaluation.
The TaqMan Real Time PCR represents the assay of choice in diagnosis of plant diseases, compared to conventional PCR and, in this work, it was confirmed its high degree of sensitivity, specificity and reproducibility. The development of a molecular method based on Real Time PCR for the early diagnosis of common bunt in wheat seedling, which to date and to our knowledge is not available, was an important goal of this work. The excellent sensitivity, detecting up to 10 fg of pathogen DNA, the specificity and robustness of the assay, thanks to which it was possible to directly analyze the crude plant extract without lowering the performance levels, guaranteed the effectiveness of the method. Furthermore, the possibility of working on a crude extract, without the need to purify the DNA with the use of commercial kits or more expensive protocols in terms of time and costs, guaranteed an easy, fast and cheap application. The comparable results obtained from molecular and phenotypic analyses showed the efficacy of this method and it could represent a useful tool especially in breeding tests for genetic resistance or in seed treatments evaluation trials, where the waiting periods for results are rather long: 8-9 months for phenotypic symptom analysis compared to 1-2 months required for molecular analysis. Previous works have developed molecular and serological methods for diagnosis of common bunt in wheat seedlings but at later phenological stages, starting from the inflorescence, or based on conventional PCR amplification, with a very low sensitivity (Josefsen and Christiansen, 2002; Eibel et al., 2005). Real Time PCR allows to precisely quantify fungal biomass and therefore can be used in the assessment of plant infection, evaluating the degree of susceptibility/tolerance (Wessling and Panstruga, 2012; Anderson and McDowell, 2015; He et al., 2020).
In this study, the developed duplex Real Time PCR assay, combined with an optimized DNA extraction protocol, has proved to be an effective tool for accurate quantification of the target pathogen in plant material and it was successfully used for evaluating the effectiveness of clove oil-based seed dressing in controlling the disease. The method was able to quantify T. laevis in young wheat seedlings after seed dressing with clove oil in different formulations and ways of application, significantly reducing the time of analysis. Correlation between the amount of T. laevis DNA and the percentage of infected plants, with relative quantification, showed the accuracy of the method.
To set up the most favorable conditions for wheat seed infection by T. laevis, we evaluated the influence of temperature for the success of infection process, for subsequently testing the efficacy of seed dressing by different clove oil-based formulations. In literature, different works reported a positive correlation between the frequency of common bunt infected spikes caused by seedborne Tilletia teliospores and low temperatures. For example, Johnsson (1992) reported that the common bunt infection of winter wheat in field experiments was correlated with climate data, but only for the critical period of 1-11 days after sowing. The infection was most severe when the average temperature during this period was 6-7°C. In alignment with Johnsson (1992) and Koprivica et al. (2009), in this work the critical role of temperature as daily average, maximum and minimum temperatures, in the infection process has been confirmed in the progressive sowing assay. PCA analysis showed that both molecular and phenotypical infection were well correlated with the number of days with an average temperature between -5 and 5°C, while there was a negative correlation with the number of days with an average temperature between 15 and 25°C. These correlations confirmed the important role of low temperature in T. laevis infection process. The sowings were well separated into two groups: presence or absence of infection, so the low temperature lasting for more than 7-10 days could be related to the disease, but probably other factors, such as wheat cultivar susceptibility and external factors could repress or enhance the infection. Another interesting point concerns the influence of temperature during the germination period for fungal tissue infection. Koprivica et al. (2009) showed that the lower the temperature, the longer the wheat germination time, giving the pathogen more time to reach the apical meristem tissue. In this work, the clove oil was tested on durum wheat as seed treatment against T. laevis during two seasons, by using different compositions (pure oil and as main component of two Bioxeda experimental formulations) and two different types of seed dressings (submersion and spray). Since no correlation was found between the 2020 and 2022 treatments (average infection frequency of the untreated plants in 2022 experiments was lower than in 2020), the seed treatment data were analyzed and discussed separately for the two seasons. This low degree of infection did not allow the evaluation of treatments effect compared to season 2022 where the differences among NT and treatments were more pronounced. In the season 2020 all the submersion treatments, regardless of the product/formulation used, showed an evident positive effect significantly reducing Tilletia plant infection, as well as the spray treatment with Bioxeda A. In season 2022, the best results were performed by the Bioxeda B submersion treatment, as no T. laevis infection was recorded.
In previous works, essential clove oil has been shown to be effective against various fungal and bacterial pathogens in vitro (Rabadia et al., 2011; Duduk et al., 2015; Orzali et al., 2020), but few data have been published on its efficacy in vivo and even fewer under field conditions. An in vivo laboratory study showed that Fusarium spp. infection on wheat seeds was reduced after soaking the kernels in clove essential oil solutions at various doses (Grzanka et al., 2021). Rice seeds immersion treatment with clove essential oil was found to reduce by 50% the disease incidence caused by the bacterium Burkholderia glumae (Sari et al., 2022). Field pea seed treatments (spray and submersion) with clove oil-based formulations were tested for efficacy against artificially inoculated Ascochyta blight fungi under field conditions, although variability between years was found (Riccioni et al., 2019). The clove oil, applied as soil treatment in F. oxysporum f. sp. lycopersici infected soil, showed a very strong inhibitory effect causing significant reduction of wilt disease in tomato plants (Sharma et al., 2017). Clove oil was also tested as a post-harvest treatment: maize seeds fumigation with clove oil for at least 24 h was found to protect the seeds against Aspergillus flavus during storage (Boukaew et al., 2017); green mold (Penicillium digitatum) growth and symptomatology in navel oranges was controlled by clove oil applied on post-harvested fruits as microemulsions or in vapor phase (He et al., 2016). To our knowledge, no data are available in literature on wheat seed treatment with clove essential oil against common bunt under field conditions.
In conventional agriculture, wheat common bunt disease can be well controlled by chemical fungicide seed treatment, but in sustainable farming these fungicides are no longer allowed, and reduction of synthetic pesticides, whenever possible, has been mandatory in EU since 2014 (Romanazzi et al., 2022). To date, there are two clove oil-based products with fungicidal activity, already available in the market and allowed for post-harvest fruit treatments, but none is registered for wheat common bunt control (http://www.fitosanitari.salute.gov.it/fitosanitariws_new/FitosanitariServlet). Previous studies on alternative and eco-friendly control methods of common bunt have mainly focused on different seed treatments, e.g. with plant extracts from Cannabis sativa, Eucalyptus globulus, Thuja sinensis and Datura stramonium, cereal flour, milk powder and other organic compounds, hot water, hot air treatments and antagonistic bacteria and fungi, achieving good results in controlling the disease (Singh et al., 1979; Borgen et al., 1995; El-Naimi et al., 2000; Shams-Allah et al., 2016). Oil application on seeds deserves deep attention, as different treatments may give different results. When considering submersion treatment, it is important to take in account also the eventual phytotoxicity, which depends not only on the oil, but also on the dose, the timing of application and the crop (Somda et al., 2007; Orzali et al., 2014). Since no information about clove oil phytotoxicity on durum wheat seeds is available, the 0.3% clove oil dose tested in this work, was chosen from data obtained from other crops (Riccioni et al., 2016; Orzali et al., 2020). For example, it was reported that for tomato seeds the 0.3% clove oil submersion treatment for 10 minutes did not affect germination while the 0.4% concentration had negative effects, even if in that case the germination reduction was quite exiguous, around 10% (Orzali et al., 2020). The fumigation treatment of maize seeds with 0.5% clove essential oil had negative effects on seed germination, stem length and root length (Boukaew et al., 2017). In the current research, the 0.3% clove oil concentration was found to be toxic for durum wheat seeds by submersion application, as the number of emerged plants was significantly lower than the untreated plants, with more than 80% reduction in germination. On the other hand, spray treatments at the same concentration did not show any phytotoxic effects.
In presence of T. laevis seed inoculum, our data showed that seed treatment is therefore recommended to control this pathogen. Use of clove essential oil (by submersion and spray application) showed an interesting potential in the control of wheat common bunt disease, albeit with fluctuating efficacies related to environmental conditions and pathogen inoculum pressure. In fact, it turned out that it is difficult to predict the outcome of infection, which is linked to climatic conditions. These results were very promising because, if antifungal efficacy of clove oil dressing will be further confirmed, it could represent a useful, effective, natural, and ecological alternative to chemical pesticides in wheat common bunt control. Concerning the two application methods, the spray treatment requires lower formulation volumes, allowing the use of lower amounts of active ingredients in favor of an economically sustainable industrial process. On the other hand, specific devices and equipment are required and a lower reproducibility was observed in this kind of application. The submersion process demonstrated a more reliable effectiveness over the years, but it is anyway considered cumbersome and time-consuming when treating large amounts of seeds on a large scale, due to a larger volume of liquid and subsequent drying (Afzal et al., 2020). Phytotoxicity is also an important aspect to consider when treating seed by dipping at concentrations greater than 0.3%. Both submersion and spray methods can be considered as valid tools for the application of clove oil (preferably in formulated form) and the choice of application may depend on the advantages and disadvantages that should be evaluated at industrial/farm level.
In conclusion, the developed diagnostic method proved to be a fast, specific, sensitive, and cost-effective tool for early diagnosis of common bunt in wheat seedling. Thanks to these features, it could also be useful in early screening for resistance, taking advantage of the possibility of assessing the occurrence of infection even at early stages without waiting for the symptoms of the ear to fully ripen stages. This is the first report on the effectiveness of wheat seed dressing by natural oil-based treatments for controlling common bunt disease. Further studies are needed with experimental trials in open field to compare and validate the results obtained in this first pot trial-based research. Finally, in future works the search of other natural oils for controlling T. laevis with efficacy and low phytotoxicity, is highly desirable and necessary.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conceptualization, LR and MA. Molecular analysis, MV, GM, FM, VB and SB. Statistical analysis, AG, GM and LO. Infection tests and treatments assay, LO, FM, VB, GM and AM. Writing - original draft preparation, MA, MV, LO and AG with contributions from GM, FM, SB, VB and AM. Writing - review and editing, MA, MV, LO and AG. All authors contributed to the article and approved the submitted version.
This work was supported by the project “Riduzione input di origine extra-aziendale per la Difesa delle coltivazioni BIOlogiche mediante approccio agroecologico (DIBIO)”, funded by Ministry of Agriculture, and carried out within the “EUPHRESCO Basic substances as an environmentally friendly alternative to synthetic pesticides for plant protection (BasicS)” project (Objective 2020-C-353).
We wish to thank Anita Haegi and Alessandro Infantino for their suggestions and the revision of the final manuscript.
This paper is dedicated to the memory of Luca Riccioni, who conceived and started this work with dedication and passion.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1130793/full#supplementary-material
Supplementary Figure 1 | Amplification performance of rapid molecular assay.
Supplementary Figure 2 | Scatter Plot of Pearson correlation coefficient between the T. laevis relative quantification of tested oil treatments in 2020 and 2022 seasons.
Supplementary Figure 3 | Scatter Plot of Pearson correlation coefficient between phytotoxicity frequencies for each treatment in 2020 and 2022 seasons.
Supplementary Table 1 | Chi-square analysis comparing the infection ratios between pot A and pot B for each sowing and diagnostic assay.
Supplementary Table 2 | molecular and phenotypical analysis, meteorological and germination mean time data used for statistical analysis.
Afzal, I., Javed, T., Amirkhani, M., Taylor, A. G. (2020). Modern seed technology: seed coating delivery systems for enhancing seed and crop performance. Agriculture 10 (11), 526. doi: 10.3390/agriculture10110526
Anderson, R. G., McDowell, J. M. (2015). A PCR assay for the quantification of growth of the oomycete pathogen Hyaloperonospora arabidopsidis in Arabidopsis thaliana. Mol. Plant Pathol. 16, 893–898. doi: 10.1111/mpp.12247
Arshad, Z., Hanif, M. A., Qadri, R. W. K., Khan, M. (2014). Role of essential oils in plant diseases protection: a review. IJCBS. 6, 11–17.
Borgen, A., Kristensen, L., Kølster, P. (1995). “Control of common bunt (Tilletia caries) without use of pesticides,” in 12th Danish Plant Protection Conference, Boras, Sweden. 5, 149–158.
Boukaew, S., Prasertsan, P., Sattayasamitsathit, S. (2017). Evaluation of antifungal activity of essential oils against aflatoxigenic Aspergillus flavus and their allelopathic activity from fumigation to protect maize seeds during storage. Ind. Crops Products 97, 558–566. doi: 10.1016/j.indcrop.2017.01.005
Bremer, H., Karel, G., Biyikoglu, K., Goskel, N., Petrak, F. (1952). Beiträge zur kenntnis der parasitischen pilze der turkei Vol. 17 (Revue de la Faculté des Sciences de l'Université d'Istanbul. Ser B, Istambul. Turkey) 17, 161–181.
Cramer, H. H. (1967). Plant protection and world crop production (Byer. Pflanzenschutz Germany: Leverkusen).
Duduk, N., Markovic, T., Vasic, M., Duduk, B., Vico, I., Obradovic, A. (2015). Antifungal activity of three essential oils against Colletotrichum acutatum, the causal agent of strawberry anthracnose. J. Essent. Oil-Bear. Plants 18 (3), 529–537. doi: 10.1080/0972060X.2015.1004120
Dumalasová, V., Bartoš, P. (2008). Effect of inoculum doses on common bunt infection on wheat caused by Tilletia tritici and T. laevis. Czech J. Genet. Plant Breed. 44, 73–77. doi: 10.17221/2/2008-CJGPB
Eibel, P., Wolf, G. A., Koch, E. (2005). Detection of Tilletia caries, causal agent of common bunt of wheat, by ELISA and PCR. J. Phytopathol. 153, 297–306. doi: 10.1111/j.1439-0434.2005.00973.x
El-Naimi, M., Toubia-Rahme, H., Mamluk, O. F. (2000). Organic seed-treatment as a substitute for chemical seed-treatment to control common bunt of wheat. Eur. J. Plant Pathol. 106, 433–437. doi: 10.1023/A:1008785021771
Farooq, M., Basra, S. M. A., Ahmad, N., Hafeez, K. (2005). Thermal hardening: a new seed vigor enhancement tool in rice. J. Integr. Plant Biol. 47, 187–193. doi: 10.1111/j.1744-7909.2005.00031.x
Flor, H. H. (1933). Studies on physiologic specialization in Tilletia tritici and t. levis in the pacific Northwest. J. Agric. Res. 47, 193–213.
Forster, M. K., Sedaghatjoo, S., Maier, W., Killermann, B., Niessen, L. (2022). Discrimination of Tilletia controversa from the T. caries / T. laevis complex by MALDI-TOF MS analysis of teliospores. Appl. Microbiol. Biotechnol. 106, 1257–1278. doi: 10.1007/s00253-021-11757-2
Gassner, G. (1938). Untersuchungen uber keimgeschwindigkeit und infektionsvermögen verschiedener stamme von Tilletia foetens und Tilletia tritici. J. Phytopathol. 11, 489–516.
Gaudet, D. A., Lu, Z. X., Leggett, F., Puchalski, B., Laroche, A. (2007). Compatible and incompatible interactions in wheat involving the bt-10 gene for resistance to Tilletia tritici, the common bunt pathogen. Phytopathol. 97, 1397–1405. doi: 10.1094/PHYTO-97-11-1397
Grzanka, M., Sobiech, Ł., Danielewicz, J., Horoszkiewicz-Janka, J., Skrzypczak, G., Sawinska, Z., et al. (2021). Impact of essential oils on the development of pathogens of the fusarium genus and germination parameters of selected crops. Open Chem. 19 (1), 884–893. doi: 10.1515/chem-2021-0079
Hansen, F. (1958). Anatomical investigations on the penetration and extension of Tilletia species in cereals in relation to the stage of development of the host plant. J. Phytopathol. 34, 169–208. doi: 10.1111/j.1439-0434.1959.tb01811.x
He, S., Chen, H., Wei, Y., An, T., Liu, S. (2020). Development of a DNA-based real-time PCR assay for the quantification of Colletotrichum camelliae growth in tea (Camellia sinensis). Plant Methods 16, 17. doi: 10.1186/s13007-020-00564-x
He, S., Ren, X., Lu, Y., Zhang, Y., Wang, Y., Sun, L. (2016). Microemulsification of clove essential oil improves its in vitro and in vivo control of Penicillium digitatum. Food Control 65, 106–111. doi: 10.1016/j.foodcont.2016.01.020
Holton, C. S. (1944). Inheritance of chlamydospore and sorus characters in species and race hybrids of Tilletia caries and t. foetida. Phytopathol. 34, 586–592.
Holton, C. S. (1954). Genetic phenomena in the smut fungi as related to the dynamics of the species. Phytopathol. 44, 352–355.
Holton, C. S., Kendrick, E. L. (1956). Problems on the delimitation of species of tilletia occurring on wheat Vol. 24 (Washington. U.S.A: Research Studies of the State College of Washington). 24, 318–325.
Ioos, R., Fourrier, C., Iancu, G., Gordon, T. R. (2009). Sensitive detection of Fusarium circinatum in pine seed by combining an enrichment procedure with a real time PCR using dual-labeled probe chemistry. Phytopathology 99, 582–590. doi: 10.1094/PHYTO-99-5-0582
Isman, M. B. (2000). Plant essential oils for pest and disease management. Crop Prot 19, 603–608. doi: 10.1016/S0261-2194(00)00079-X
Isman, M. B., Machial, C. M. (2006). “Pesticides based on plant essential oils: from traditional practice to commercialization,” in Naturally occurring bioactive compounds. advances in phytomedicine, vol. 3 . Eds. Rai, M., Carpinella, M. C. (Amsterdam: Elsevier), 29–44. doi: 10.1016/S1572-557X(06)03002-9
Jayawardena, R. S., Hyde, K. D., Jeewon, R., Ghobad-Nejhad, M., Wanasinghe, D. N., Liu, N. G., et al. (2019). One stop shop II: taxonomic update with molecular phylogeny for important phytopathogenic genera: 26–50. Fungal Divers. 94, 41–129. doi: 10.1007/s13225-019-00418-5
Johnsson, L. (1991). Survey of common bunt (Tilletia caries (DC) tul.) in winter wheat during the period 1967-1987 in Sweden. JPDP. 98, 67–72.
Johnsson, L. (1992). Climate factors influencing attack of common bunt (Tilletia caries (DC) tul.) in winter wheat in 1940-1988 in Sweden. Z. für Pflanzenkrankheiten und Pflanzenschutz / JPDP. 99, 21–28.
Jolliffe, I. T., Cadima, J. (2016). Principal component analysis: a review and recent developments. Philos. Trans. R. Soc A-Math. Phys. Eng. Sci. Philos. Trans. A Math Phys. Eng. Sci. 374, 20150202. doi: 10.1098/rsta.2015.0202
Jones, G. D., Clifford, B. C. (1978). Cereal diseases: their pathology and control (Ipswich: BASF United Kingdom Limited).
Josefsen, L., Christiansen, S. K. (2002). PCR as a tool for early detection and diagnosis of common bunt in wheat, caused by Tilletia tritici. Mycol. Res. 106 (11), 1287–1292. doi: 10.1017/S0953756202006603
Kassambara, A. (2022) Ggpubr: ‘ggplot2’ based publication ready plots. Available at: https://CRAN.R-project.org/package=ggpubr (Accessed December 2022).
Kassambara, A., Mundt, F. (2020) (Factoextra: Extract and Visualize the Results of Multivariate). Available at: https://CRAN.R-project.org/package=factoextra (Accessed December 2022).
Kesraoui, S., Andrés, M. F., Berrocal-Lobo, M., Soudani, S., Gonzalez-Coloma, A. (2022). Direct and indirect effects of essential oils for sustainable crop protection. Plants 11 (16), 2144. doi: 10.3390/plants11162144
Koprivica, M., Jevtić, R., Dulić-Marković, I. (2009). The influence of Tilletia spp. inoculum source and environmental conditions on the frequency of infected wheat spikes. Pestic. fitomed 24, 185–196. doi: 10.2298/PIF0903185K
Li, Y., Zhao, H., Yan, X., Li, M., Chen, P., Zhang, S. (2017). A universal method for direct PCR amplification of plant tissues. Anal. Methods 9, 1800–1805. doi: 10.1039/C6AY03156K
Livak, K. J., Schmittgen, T. D. (2001). Analysis of relative gene expression data using real time quantitative PCR and the 2(-DELTA DELTA c (T)) method. Methods. 25, 402–408. doi: 10.1006/meth.2001.1262
Marinelli, E., Orzali, L., Lotti, E., Riccioni, L. (2012). Activity of some essential oils against pathogenic seed borne fungi on legumes. Asian J. Plant Pathol. 6, 66–74. doi: 10.3923/ajppaj.2012.66.74
Miller, T. D. (1992). "Growth stages of wheat" better crops with plant food. Potash Phosphate Institute. 76, 12.
Misra, G., Pavlostathis, S. G. (1997). Biodegradation kinetics of monoterpenes in liquid and soil-slurry systems. Appl. Microbiol. Biotechnol. 47, 572–577. doi: 10.1007/s002530050975
Mitra, M. (1931). A new bunt of wheat in India. Ann. Appl. Biol. 18, 178–179. doi: 10.1111/j.1744-7348.1931.tb02294.x
Moumni, M., Allagui, M. B., Mezrioui, K., Ben Amara, H., Romanazzi, G. (2021). Evaluation of seven essential oils as seed treatments against seedborne fungal pathogens of Cucurbita maxima. Molecules 26, 2354. doi: 10.3390/molecules26082354
Mulholland, V., Mc Ewan, M. (2000). PCR-based diagnostics of Microdochium nivale and Tilletia tritici infecting winter wheat seeds. OEPP/EPPO Bull. 30, 543–547. doi: 10.1111/j.1365-2338.2000.tb00944.x
Nguefack, J., Leth, V., Dongmo, J. B. L., Torp, J., Zollo, P. H. A., Nyasse, S. (2008). Use of three essential oils as seed treatments against seed-borne fungi of rice (Oryza sativa l.). American-Eurasian J. Agric. Environ. Sci. 4, 554–560.
Orzali, L., Luison, D., Riccioni, L. (2014). Utilizzo di principi attivi di origine naturale per la concia delle sementi e per il controllo delle malattie trasmesse per seme. Dal Seme. 9, 46–48.
Orzali, L., Valente, M. T., Scala, V., Loreti, S., Pucci, N. (2020). Antibacterial activity of essential oils and Trametes versicolor extract against Clavibacter michiganensis subsp. Michiganensis and Ralstonia solanacearum for seed treatment and development of a rapid In vivo assay. Antibiotics 9, 628. doi: 10.3390/antibiotics9090628
Pieczul, K., Perek, A., Kubiak, K. (2018). Detection of Tilletia caries, Tilletia laevis and Tilletia controversa wheat grain contamination using loop-mediated isothermal DNA amplifcation (LAMP). J. Microbiol. Methods 154, 141–146. doi: 10.1016/j.mimet.2018.10.018
Rabadia, A. G., Kamat, S., Kamat, D. (2011). Antifungal activity of essential oils against fluconazole resistant fungi. Int. J. Phytomed. 3, 506–510. doi: 10.5138/ijpm.v3i4.418
R Core Team (2022) R: a language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/ (Accessed December 2022).
Riccioni, L., Orzali, L. (2011). Activity of tea tree (Melaleuca alternifolia. cheel) and thyme (Thymus vulgaris. linnaeus.) essential oils against some pathogenic seed borne fungi. J. Essent. Oil Res. 23, 43–47. doi: 10.1080/10412905.2011.9712280
Riccioni, L., Orzali, L., Pecetti, L., Lotti, E., Fabiani, A. (2016). “Seed treatment with natural products against seed-borne fungal pathogens: five years of lab test and field trials,” in Abstract 38, retrieved from ISTA Seed Symposium Abstracts, 31st ISTA Congress. (Tallinn, Estonia: Ministry of Rural Affairs, Republic of Estonia).15–17.
Riccioni, L., Orzali, L., Romani, M., Annicchiarico, P., Pecetti, L. (2019). Organic seed treatments with essential oils to control ascochyta blight in pea. Eur. J. Plant Pathol. 155, 831–840. doi: 10.1007/s10658-019-01815-x
Romanazzi, G., Orçonneau, Y., Moumni, M., Davillerd, Y., Marchand, P. A. (2022). Basic substances, a sustainable tool to complement and eventually replace synthetic pesticides in the management of pre and postharvest diseases: reviewed instructions for users. Molecules. 27, 3484. doi: 10.3390/molecules27113484
Sari, S. P., Safni, I., Lubis, L. (2022). “Seed treatments to control Burkholderia glumae on rice seeds in the screenhouse,” in IOP Conf. Ser.: Earth Environ. Sci 977, 012021. doi: 10.1088/1755-1315/977/1/012021
Sedaghatjoo, S., Forster, M. K., Niessen, L., Karlovsky, P., Killermann, B., Maier, W. (2021). Development of a loop - mediated isothermal amplifcation assay for the detection of Tilletia controversa based on genome comparison. Sci. Rep. 11, 1–13. doi: 10.1038/s41598-021-91098-2
Sedaghatjoo, S., Mishra, B., Forster, M. K., Becker, Y., Keilwagen, J., Killermann, B., et al. (2022). Comparative genomics reveals low levels of inter- and intraspecies diversity in the causal agents of dwarf and common bunt of wheat and hint at conspecificity of Tilletia caries and T. laevis. IMA Fungus. 13, 11. doi: 10.1186/s43008-022-00098-y
Shams-Allah, S. A., Al-Maaroof, E. M., Hassan, M. S. (2016). Effects of some chemical and biological agents against Tilletia tritici (Bjerk) and T. laevis (Kühn) causal agents of wheat common bunt disease. Baghdad Sci. J. 13. (2), 253–259. doi: 10.21123/bsj.2016.13.2.0253
Sharma, A., Rajendran, S., Srivastava, A., Sharma, S., Kundu, B. (2017). Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng. 123 (3), 308–313. doi: 10.1016/j.jbiosc.2016.09.011
Singh, S., Goel, L. B., Sharma, S. K., Nayar, S. K. (1979). Fungitoxicants and plant extracts in the control of hill bunt of wheat [India]. Indian Phytopathol. (India). 32, 297–299.
Somda, I., Leth, V., Sereme, P. (2007). Antifungal effect of Cymbopogon citratus, Eucalyptus camaldulensis and Azadirachta indica oil extracts on sorghum seed-borne fungi. Asian J. Plant Sci. 6, 1182–1189. doi: 10.3923/ajps.2007.1182.1189
Sturchio, E., Donnarumma, L., Annesi, T., Milano, F., Casorri, L., Masciarelli, E., et al. (2014). Essential oils: an alternative approach to management of powdery mildew diseases. Phytopathol. Mediterr. 53, 385–395. doi: 10.14601/Phytopathol_Mediterr-13607
Swinburne, T. R. (1963). Infection of wheat by Tilletia caries (DC.) TUL., the causal organism of bunt. Trans. Brit. Mycol. Soc 46, 145–156. doi: 10.1016/S0007-1536(63)80016-9
Tan, M., Ghalayini, A., Sharma, I., Yi, J., Shivas, R., Priest, M., et al. (2009). A one-tube fluorescent assay for the quarantine detection and identification of Tilletia indica and other grass bunts in wheat. Australas. Plant Pathol. 38, 101–109. doi: 10.1071/AP08088
Vánky, K. (2012). Smut fungi of the world Vol. 1458. Ed. Vánky, K. (St. Paul. Minn. APS Press, St. Paul, Minnesota. U.S.A.).
Wessling, R., Panstruga, R. (2012). Rapid quantification of plant-powdery mildew interactions by qPCR and conidiospore counts. Plant Methods 8, 35. doi: 10.1186/1746-4811-8-35
Wilcoxson, R. D., Saari, E. E. (1996). Bunt and smut diseases of wheat: concepts and methods of disease management (Mexico: D.F. CIMMYT).
Woolman, H. M., Humphrey, H. B. (1924). Summary of literature on bunt. or stinking smut. of wheat (Washington D.C.. U.S.A.: U.S. Department of Agriculture), 1–44.
Yarham, D. (1993). Soilborne spores as a source of inoculum for wheat bunt (Tilletia caries). Plant Pathol. 42, 654–656. doi: 10.1111/j.1365-3059.1993.tb01546.x
Zouhar, M., Mazáková, J., Prokinová, E., Váňová, M., Ryšánek, P. (2010). Quantifcation of Tilletia caries and Tilletia controversa mycelium in wheat apical meristem by real time PCR. Plant Protect. Sci. 46, 107–115. doi: 10.17221/50/2009-PPS
Zscheile jr, F. P. (1966). Photoperiod-Temperature-Light relationships in development of wheat and bunt. J. Phytopathol. 57, 329–374. doi: 10.1111/j.1439-0434.1966.tb02288.x
Keywords: Tilletia laevis, wheat common bunt, seed borne disease, real time PCR, seed dressing, clove oil, Triticum durum
Citation: Valente MT, Orzali L, Manetti G, Magnanimi F, Matere A, Bergamaschi V, Grottoli A, Bechini S, Riccioni L and Aragona M (2023) Rapid molecular assay for the evaluation of clove essential oil antifungal activity against wheat common bunt. Front. Plant Sci. 14:1130793. doi: 10.3389/fpls.2023.1130793
Received: 23 December 2022; Accepted: 17 May 2023;
Published: 05 June 2023.
Edited by:
Islam Hamim, Bangladesh Agricultural University, BangladeshReviewed by:
Abhay K. Pandey, North Bengal Regional R & D Center, IndiaCopyright © 2023 Valente, Orzali, Manetti, Magnanimi, Matere, Bergamaschi, Grottoli, Bechini, Riccioni and Aragona. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Aragona, bWFyaWEuYXJhZ29uYUBjcmVhLmdvdi5pdA==
†Deceased
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.