
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 02 February 2023
Sec. Plant Abiotic Stress
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1123080
This article is part of the Research TopicSalinity and Drought Stress in Plants: Understanding Physiological, Biochemical and Molecular ResponsesView all 19 articles
 Abdul Ghaffar1*
Abdul Ghaffar1* Nadeem Hussain1*
Nadeem Hussain1* Rahaf Ajaj2
Rahaf Ajaj2 Suzan Marwan Shahin3
Suzan Marwan Shahin3 Hussan Bano4
Hussan Bano4 Muhammad Javed1,5
Muhammad Javed1,5 Ayesha Khalid1
Ayesha Khalid1 Memoona Yasmin1
Memoona Yasmin1 Kausar Hussain Shah1
Kausar Hussain Shah1 Muhammad Zaheer1
Muhammad Zaheer1 Muhammad Iqbal1,6
Muhammad Iqbal1,6 Zafar Ullah Zafar1
Zafar Ullah Zafar1 Habib-ur-Rehman Athar1
Habib-ur-Rehman Athar1The rapid increase in population growth under changing climatic conditions causes drought stress, threatening world food security. The identification of physiological and biochemical traits acting as yield-limiting factors in diverse germplasm is pre-requisite for genetic improvement under water-deficit conditions. The major aim of the present study was the identification of drought-tolerant wheat cultivars with a novel source of drought tolerance from local wheat germplasm. The study was conducted to screen 40 local wheat cultivars against drought stress at different growth stages. Barani-83, Blue Silver, Pak-81, and Pasban-90 containing shoot and root fresh weight >60% of control and shoot and root dry weight >80% and 70% of control, respectively, P (% of control >80 in shoot and >88 in root), K+ (>85% of control), and quantum yield of PSII > 90% of control under polyethylene glycol (PEG)-induced drought stress at seedling stage can be considered as tolerant, while more reduction in these parameters make FSD-08, Lasani-08, Punjab-96, and Sahar-06 as drought-sensitive cultivars. FSD-08 and Lasani-08 could not maintain growth and yield due to protoplasmic dehydration, decreased turgidity, cell enlargement, and cell division due to drought treatment at adult growth stage. Stability of leaf chlorophyll content (<20% decrease) reflects photosynthetic efficiency of tolerant cultivars, while ~30 µmol/g fwt concentration of proline, 100%–200% increase in free amino acids, and ~50% increase in accumulation of soluble sugars were associated with maintaining leaf water status by osmotic adjustment. Raw OJIP chlorophyll fluorescence curves revealed a decrease in fluorescence at O, J, I, and P steps in sensitive genotypes FSD-08 and Lasani-08, showing greater damage to photosynthetic machinery and greater decrease in JIP test parameters, performance index (PIABS), maximum quantum yield (Fv/Fm) associated with increase in Vj, absorption (ABS/RC), and dissipation per reaction center (DIo/RC) while a decrease in electron transport per reaction center (ETo/RC). During the present study, differential modifications in morpho-physiological, biochemical, and photosynthetic attributes that alleviate the damaging effects of drought stress in locally grown wheat cultivars were analyzed. Selected tolerant cultivars could be explored in various breeding programs to produce new wheat genotypes with adaptive traits to withstand water stress.
Global wheat production is forecast at 783.8 million tons in 2022 (FAOSTAT, 2022). Global human population is expected to exceed 9 billion by 2050, requiring at least 60% increase in wheat yield (Sekher, 2022). The drought puts 22 million people at risk of starvation by September 2022, and it triggered global economic losses about 124 billion US $ from 1998 to 2017, according to a report from the United Nations World Food Programme (WFP and UNICEF, 2022). The percentage of vegetation affected by drought has more than doubled in the last 40 years, and it has affected more people worldwide than any other natural hazard. Up to 26% of the usable area of the earth is subjected to drought and approximately 12 million hectares (Mha) of land is lost each year due to drought worldwide (FAO et al., 2022). Out of the total 79.6 Mha land of Pakistan, 62 Mha is vulnerable to drought stress while about 27% of land under cultivation is facing drought stress (FAOSTAT, 2022). Drought resistance in crop plants includes avoidance and tolerance. In recent years, different strategies have been adopted to minimize the challenges associated with drought, such as supplemental irrigation, modern dry land farming, mulching, artificial precipitation, ground water recharge, and use of compatible and drought-tolerant genotypes. Breeding is one of the most efficient options to overcome drought stress through the development of new genotypes adapted to drought (Blum, 2016). Different morpho-physiological traits of wheat that are hampered under drought stress include plant height, leaf area, chlorophyll content, stomatal conductance, relative water content, osmotic potential, water potential, and photosynthesis, so the mechanism of damage can be understood by studying these attributes under drought stress (Ahmad et al., 2022; Chen et al., 2022a; Peršić et al., 2022). Reduced uptake of N, P, and K+ was observed in plants under drought stress. Low N uptake under dry conditions affects leaf water relations, chlorophyll fluorescence, and other photosynthetic attributes restricting growth and yield (Sallam et al., 2019). Drought stress increases concentration of soluble sugars, proline, and anti-oxidant enzymes activities to combat reactive oxygen species (ROS) (Tefera et al., 2021). Reduction in protein content under drought stress may be due to protein hydrolysis or oxidative inhibition of protein synthesis (Ozturk et al., 2021). However, Halder et al. (2022) described that drought stress caused the accumulation of osmoprotectant proteins (dehydrins) in drought-tolerant wheat cultivars to protect plant cellular structures from drought-induced damages. These proteins are highly hydrophilic and impart drought stress tolerance by enhancing the water retention capacity, elevating chlorophyll content, maintaining photosynthetic machinery, activating ROS detoxification, and promoting the accumulation of compatible solutes (Riyazuddin et al., 2022)
Plants cannot utilize sufficient light energy under drought stress, so electron transport chain is disturbed, leading to the production of ROS, which damage thylakoid membranes (Zhu et al., 2021). The structural and functional activity of PSII can be evaluated by fast chlorophyll a fluorescence technique to screen drought-tolerant and drought-sensitive varieties of a particular plant species (Kalaji et al., 2018; Bashir et al., 2021; Li and Kim, 2021). Fluorescence transients from Fo (minimal fluorescence when all reaction centers are open) to Fm (maximum fluorescence when all reaction centers are closed) are called OJIP curves, which can be analyzed by JIP test and used to evaluate plant responses against drought stress (Tsimilli-Michael, 2020). The O–J phase tells about the PSII subunits connectivity and reduction in the acceptor side of PSII, J–I represents reduction in PQ pool, while the reduction in acceptor side of PSI is explained by the I–P phase (Hussain et al., 2022). Drought stress disturbs electron transfer at acceptor and donor sides of PSII, damages OEC activity, affects energetic connectivity among PSII subunits, and reduces electron transfer capacity and redox state of PSI (Zhou et al., 2019).
Plants use different mechanisms to combat drought stress such as completing life cycle before severe water stress (drought escape), conserving water by closing stomata and reducing leaf area (drought avoidance), and by osmotic adjustment (drought tolerance) (Qayyum et al., 2021). During osmotic adjustment, plants accumulate various organic and inorganic solutes (sugars, polyols, amino acids, etc.) under water-deficit conditions to maintain water status of plants and thus turgor potential to sustain photosynthesis (Zhang et al., 2022). There is little progress in developing drought-tolerant wheat cultivars due to limited knowledge about the mechanism of drought tolerance in crops, dramatic change in some physiological parameters in plants under drought stress, and genotype and environment interaction. It is suggested that information from morphological, physiological, biochemical, gene expression, genetic studies, and breeding must be integrated to understand complexity of drought tolerance in crops. Screening of available germplasm against drought stress is an important strategy in plants to cope up with the harmful effects of water deficit (Tefera et al., 2021). It is hypothesized that a novel source of drought tolerance is present in new local germplasm collection of wheat, which can be used for developing drought-tolerant wheat cultivars through selective breeding. However, pre-breeding requires a detailed characterization of germplasm. The current study was aimed to assess genotypic variability in physiological and biochemical traits in new local germplasm collection of wheat, particularly those that are related to photosystem-II activity. The secondary objective of the study was to draw the relationship between studied traits and degree of drought tolerance. The selected cultivars having traits with high heritability and positive correlation with grain yield may be used as donors in breeding programs.
The study was conducted at Botanic Gardens, Bahauddin Zakariya University, Multan (30°15 N and 71°30 E). Germplasm of 40 wheat cultivars collected from Ayub Agriculture Research Institute (AARI) Faisalabad and Regional Agricultural Research Institute (RARI) Bahawalpur, Pakistan, was surface sterilized with 5% sodium hypochlorite solution for 5 min to avoid any fungal infection and washed thoroughly with distilled water. A total of 40 bowls of 24 × 24 × 10 cm (length × width × depth) size obtained from G.M. Scientific Store, Multan, Pakistan, were taken and divided into two groups of 20 bowls each (Supplementary Figure S1). Seeds of two varieties (10 of each) were sown in each bowl in two separate rows. One group of plants (control) was filled with Hoagland nutrient solution (Hoagland and Arnon, 1950) having 1-in. thick layer of plastic microbeads (inert polyethylene), while the treatment block was given 10% PEG-8000 solution in Hoagland solution to induce drought stress. The plants were harvested after 14 days of germination, and various parameters including shoot and root biomass (fresh and dry) and N, P, and K+ contents were measured.
The selected drought-tolerant (Barani-83, Blue silver, Pak-81, and Pasban-90) and drought-sensitive (FSD-08, Lasani-08, Punjab-96, and Sahar-06) cultivars were further grown till adult stage, and differences in morpho-physiological and yield responses between tolerant and sensitive cultivars were explored under water stress conditions. A total of 80 plastic pots each with a diameter of 28 cm were taken. Eight seeds of each variety were sown in a pot filled with 8 kg fertile and well-textured garden soil of 18% field capacity and 36% soil saturation. Ten days after sowing, four equidistantly placed and uniformly sized plants were left in each pot after thinning. The experiment was arranged in completely randomized design (CRD) with eight cultivars, two treatments (control and drought), and five replicates. Initially, all the pots were irrigated with tap water, but after 2 weeks of germination, half of the plants were subjected to drought stress by withholding water (pre-anthesis drought cycle) till wilting state becomes evident, while the other group of plants were irrigated continuously with tap water. At this stage, different morpho-physiological and biochemical parameters were measured. Two of the four plants were harvested, and the experiment continued till the end, and finally, yield attributes were determined.
Plant length was calculated by using a measuring tape from the soil surface to the tip of the longest spike. Flag leaf area was determined by the Muller (1991) formula, which is given as: flag leaf area = maximum length × maximum breadth × 0.74. Total chlorophyll content (SPAD) was estimated by using a portable chlorophyll meter SPAD 502 (Minolta, chlorophyll meter, SPAD-502, Japan) as proposed by Del Pozo et al. (2016). The middle part of the third mature leaf of each plant was used to measure the quantum yield (QY) of PSII by using handheld FluorPen (FP-100 MX-LM, Photon System Instruments, Czech Republic). A weak measuring light was used to measure Fo, and then, a saturated pulse of 3,000 µmol m−2 s−1 was applied to measure Fm. QY of PSII was calculated using the formula: QY = Fv/Fm = (Fm – Fo)/Fm.
Fresh weight of the shoot and root was noted with the help of electric balance (BSM-220.4, Hanchen, China) after separating the shoot from the root; then, plants were dried in an electric oven (UN-110, Memmert, Germany) at 75°C for 72 h, and dry biomass was noted.
The second leaf from the top was cut from the main tiller to measure the water potential of the leaf (Ψw) at early in the morning using a Scholander-type pressure chamber (Arimad-2, Japan) as described by Scholander et al. (1964). The same leaf was kept in a freezer at −20°C for a week and thawed by a glass rod to extract cell sap, which was used for measuring osmotic potential (Ψs) by an osmo-meter (Vapro, 5520, USA). Turgor potential (Ψp) was calculated from the difference in Ψw and Ψs by the formula as described by Nobel (1991), i.e., Ψp = Ψw − Ψs. Fresh leaf samples were weighed (Fw) and dipped in water in the dark for 24 h so that they become turgid, and their turgid weight (Tw) was noted. These leaves were then dried in an oven at 80°C for 48 h, and the dried weight (Dw) was noted. The formula that was used to determine relative water content as described by Jones and Turner (1978) is RWC (%) = [(Fw − Tw)/(Fw − Dw)] × 100.
Fresh leaf sample (0.25 g) was ground in 3% sulfo-salicylic acid (5 ml) in a chilled pestle and mortar. The homogenate was filtered, and 2 ml was taken in a test tube in which 2 ml acid ninhydrin solution (1.25 g ninhydrin+20 ml of 6M orthophosphric acid+30 ml glacial acetic acid) and 2 ml glacial acetic acid were added and heated in a boiling water bath for 1 h at 100°C, and the reaction was terminated by cooling in ice bath. Four milliliters of toluene was added in the test tube, vortexed for 15–20 s, and allowed to stand, and then, the upper layer (chromophore) was taken. Absorbance was recorded at 520 nm using a spectrophotometer, and the concentration of proline was determined with the help of a standard curve (Bates et al., 1973). Proteins were calculated using bovine serum albumin (BSA) calibration curve on fresh weight basis (Bradford, 1976), while the concentration of total free amino acids was measured following the Hamilton et al. (1943) protocol. Total soluble sugars were estimated by standard methods as proposed by Yemm and Willis (1954). In 0.2 g dried ground leaf sample taken in a test tube, 10 ml of 80% ethyl alcohol was added and shaken overnight. The supernatant was taken in plastic bottles after three to four times washing with water and then made the volume of each sample up to 50 ml with distilled water. A 0.3 ml extract was put in a test tube and 3 ml of anthrone solution added and heated for 10 min in a water bath and cooled, and absorbance was recorded at 625 nm by a UV spectrophotometer.
The digestion mixture (Se, 0.42g; Li2SO4.2H2O2, 14g dissolved in 350 ml of H2O2 and 420 ml conc. H2SO4) was used for plant tissue digestion. A total of 100 mg of dried ground plant material was digested in 2 ml of digestion mixture, adding HClO4 (0.5 ml) on a hot plate. The digested mixture was diluted to 50 ml with distilled water and filtered, and K+ was measured by a flame photometer (Jenway, PFP-7) following the method of Allen et al. (1985). P contents were determined by a spectrophotometer (Jenway, 6850, UK), measuring absorption at 470 nm (Jackson, 1958). Leaf N contents were estimated according to Kjeldhal method (Bremner, 1965). Leaf lipid peroxidation can be estimated by measuring MDA content, adopting thCakmak and Horst (1991) method.
The number of tillers per plant, number of spikelet per spike, and number of grains per spike were counted; spike length was measured by a measuring tape, while 100 grain weight and grain yield per plant were measured by using electric balance.
Chlorophyll a fluorescence was measured from the middle part of the third mature leaf of each plant (40 min after sunset to ensure dark adaptation of leaves and then exposed to strong actinic light of 3,000 μmol m−2 s−1), with a handheld chlorophyll fluorescence meter (FluorPen FP-100 MX-LM, Photon System Instruments, Czech Republic) from 20 μs to 2 s. The data were recorded based on literature available on websites of chlorophyll fluorescence meter manufacturers using computer software FlourPen v 1.0.4.0. According to Strasser et al. (2004), the JIP test parameters used to measure energy distribution, flux ratios, and performance index include ABS/RC, TRo/RC, ETo/RC, and DIo/RC (absorption, trapped energy, electron transport, and dissipation energy flux per reaction center) and Fo, Fj, Fi, and Fm (minimum fluorescence, fluorescence intensity at J phase, fluorescence intensity at I phase, and maximum fluorescence). Fv, Vj, and Vi represent variable fluorescence, relative variable fluorescence at phase J, and relative variable fluorescence at phase I of the fluorescence transient curve. Fm/Fo = electron transport rate through PSII, Fv/Fo = ratio of photochemical to non-photochemical quantum efficiency, Fv/Fm = maximum quantum yield of primary photochemistry, while PIABS is the photosynthetic performance index on absorption basis. ΦPSII shows the effective quantum yield of PSII photochemistry, ΦDo explains maximum quantum yield of non-photochemical de-excitation, ΦEo represents quantum yield of electron transport, and ψo = ETo/TRo explains the yield of electron transport per trapped excitation.
The adult experimental plan was a two-factor factorial completely randomized design with eight cultivars, two treatments, and five replications. Data were analyzed by two-way analysis of variance (ANOVA) using the computer program CoStat (Version 6.303, USA). Means ± SE were compared with least significant difference (LSD) test at 5% level of significance by Snedecor and Cochran (1989). Graphical representation of data was performed by MS Excel software.
All the wheat cultivars showed significant (p ≤ 0.001) reduction in shoot and root fresh and dry biomass and K+ and P accumulation under water-deficit conditions. The extent of reduction in biomass and K+ and P accumulation in the leaves and roots varied considerably in all wheat cultivars. The percentages of control values of shoot and root fresh and dry weight were maximum in four cultivars (Barani-83, Blue silver, Pak-81, and Pasban-90), while FSD-08, Lasani-08, Punjab-96, and Sahar-06 showed minimum percent of control values of shoot and root fresh and dry weight due to PEG-induced water stress (Figures 1A-D). Shoot and root K+ concentration calculated on dry weight basis decreased under drought stress. Maximum percent of control or minimum decrease was observed in cultivar Barani-83 (97% of control of shoot and 83% of control of root), followed by Blue Silver, Pak-13, Pak-81, and Pasban-90. However, a maximum decrease or minimum percent of control was observed in FSD-08 (67% of control of the shoot and 65% of control of the root) followed by Lasani-08, Punjab-96, and Sahar-06 under drought stress conditions (Figures 2A, B). Shoot and root P concentration decreased in all the cultivars under drought stress. A maximum decrease was observed in FSD-08 (54% of control of shoot and 56% of control of root) and then come varieties Lasani-08 (56% of control of the shoot and root) followed by Punjab-96 and Sahar-06 under drought stress conditions. A minimum decrease was observed in Barani-83 with 85% and 89% of control of the shoot and root, respectively, followed by Blue Silver, Pak-81, and Pasban-90 under water scarce conditions (Figures 2C, D).
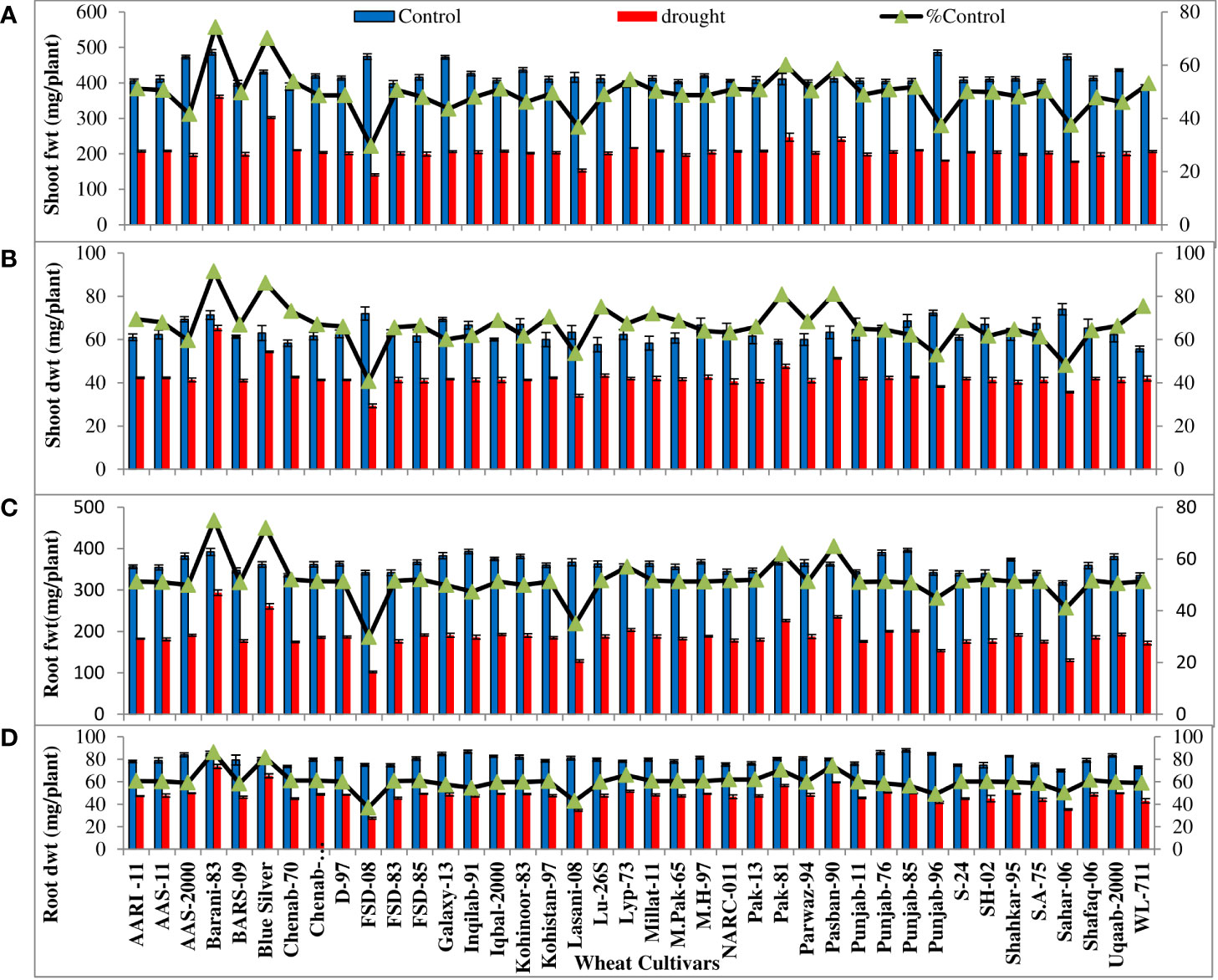
Figure 1 Shoot and root fresh and dry weights (mg/plant) of wheat seedlings, when plants of 40 local wheat cultivars were grown under normal or PEG-induced drought stress for 2 weeks. Means (± SE; n=5) are presented on the primary vertical axis, while % of the control values are presented on the secondary vertical axis. (A) Shoot fwt, (B) shoot dwt, (C) root fwt, and (D) root dwt.
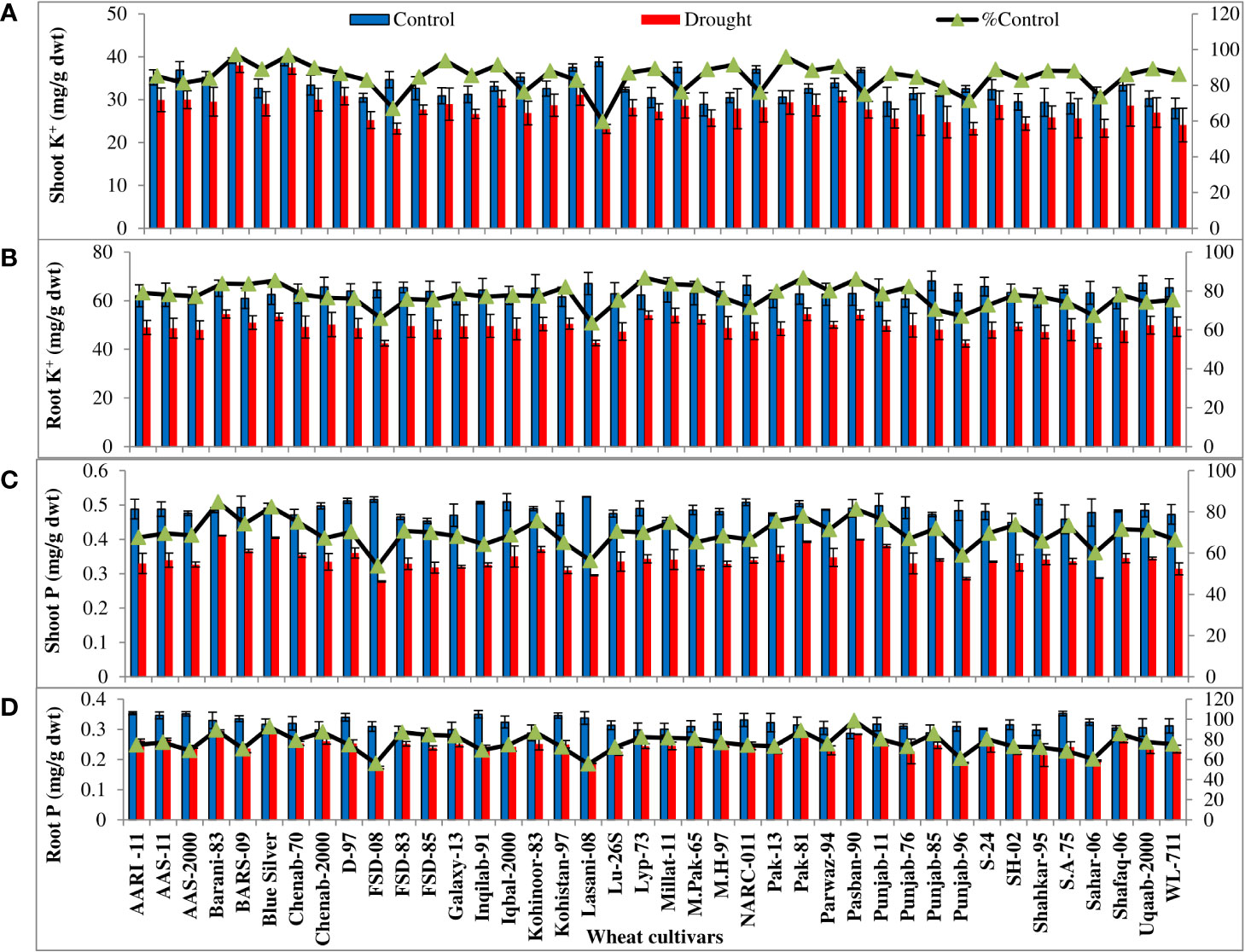
Figure 2 Shoot and root K+ and P accumulation in wheat seedlings when plants of 40 local wheat cultivars were grown under normal or PEG-induced drought stress for 2 weeks. Means (± SE; n=5) are presented on the primary vertical axis, while percent of the control values is presented on the secondary vertical axis. (A) Shoot K+, (B) root K+, (C) shoot P, and (D) root P.
Drought stress reduced shoot and root fresh and dry biomass significantly (p ≤ 0.001) in all wheat cultivars (Table 1) examined in the present study. Less decrease was observed in fresh and dry biomass of the shoot and root in Barani-83 than Blue Silver, Pak-81, and Pasban-90; however, maximum decrease in FSD-08 followed by Lasani-08, Punjab-96, and Sahar-06 was found under drought stress (Figures 3A-D). ANOVA of the data showed significant (p ≤ 0.001) decrease in plant height in all wheat cultivars under drought stress (Table 1). Differences among cultivars were also significant with respect to plant height; a lesser decrease (16%) in plant height in Pasban-90, while greater decrease in Pak-81 (17%), Blue Silver (20%), and Barani-83 (24%), respectively, was observed, whereas Sahar-06 showed maximum decrease (48%) in plant height, followed by Punjab-96 (37%), Lasani-08 (32%), and FSD-08 (28%), respectively, under water stress conditions (Figure 3E). Although the cultivars differed significantly based on flag leaf area, total chlorophyll contents, and quantum yield of PSII under drought stress (Table 1), the reduction in flag leaf area, total chlorophyll contents, and quantum yield of PSII was 85%, 35%, and 18%, respectively, in FSD-08; 81%, 28%, and 14%, respectively, in Lasani-08; and only 63%, 10%, and 4% in Barani-83; while 70%, 14%, and 6%, respectively, in Blue Silver was noted under the influence of water stress. The varieties Pak-81, Pasban-90, Punjab-96, and Sahar-06 show intermediate reduction in flag leaf area, total chlorophyll contents, and quantum yield under drought stress (Figures 3F-H).
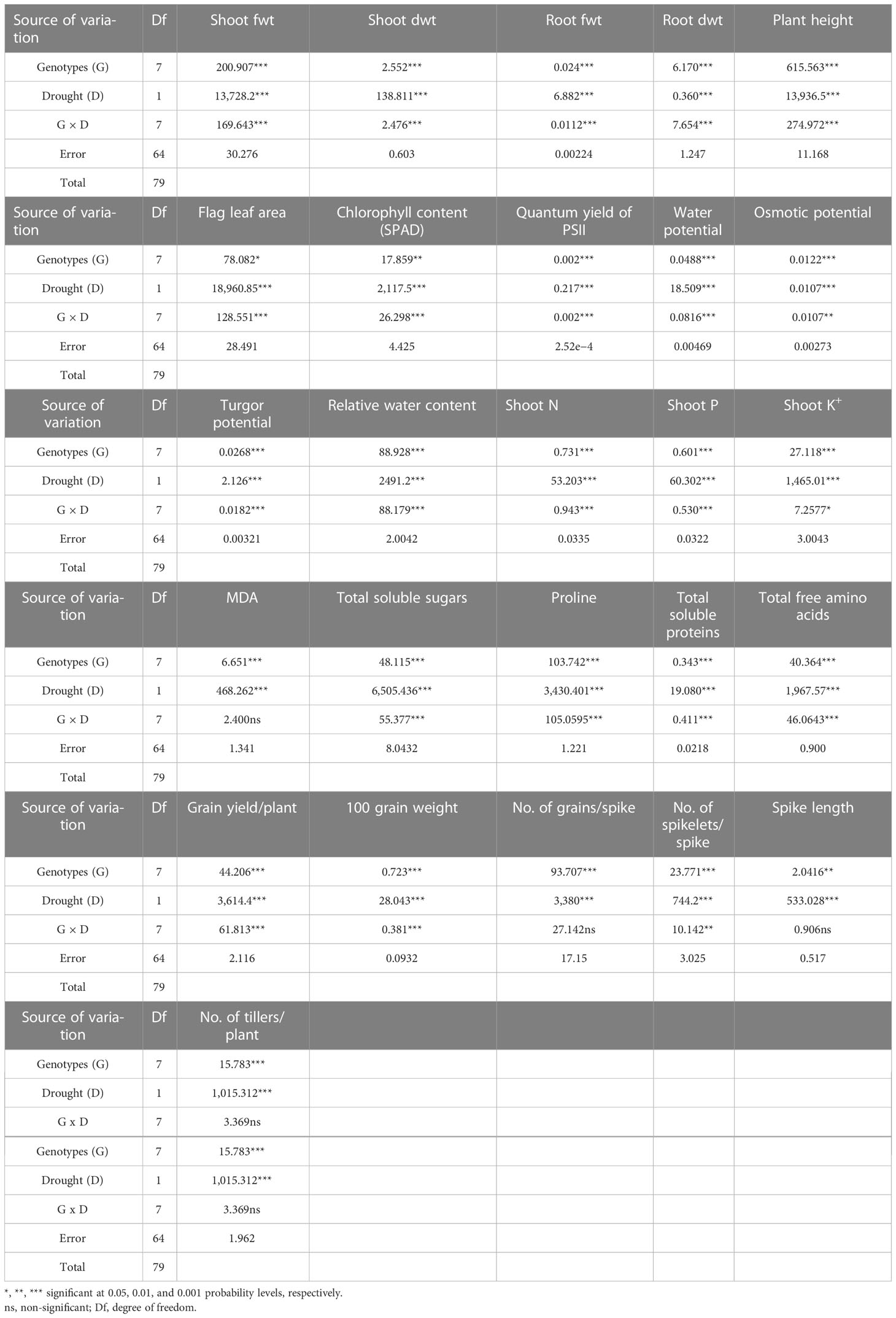
Table 1 Mean squares from ANOVA of the data for growth parameters, water relation parameters, mineral nutrients, MDA, organic osmolytes, and yield attributes of eight wheat (Triticum aestivum L.) cultivars subjected to drought stress at adult growth stage.
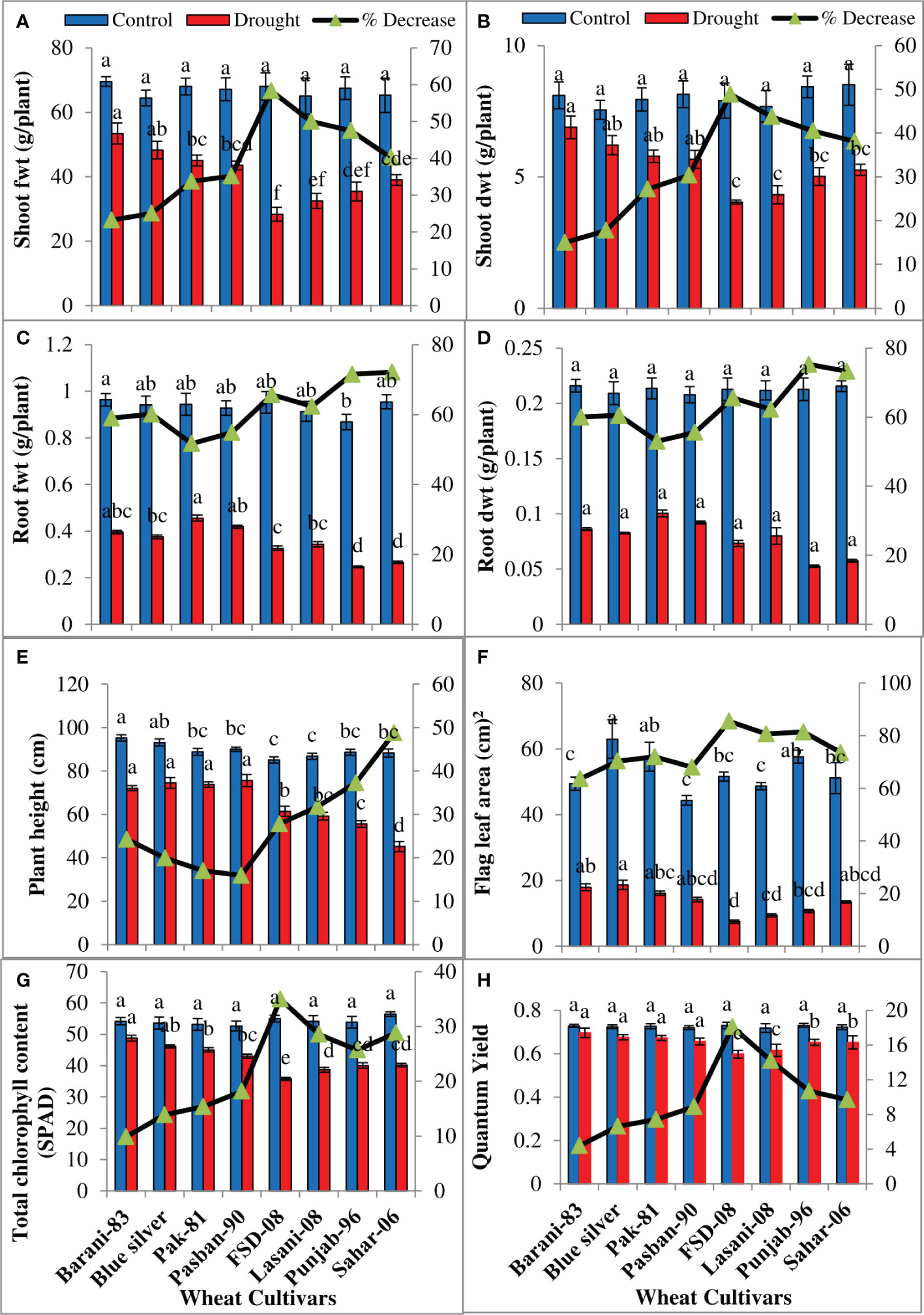
Figure 3 Growth attributes of selected wheat cultivars subjected to control or drought stress at adult stage. Means (± SE; n=5) are presented on the primary vertical axis, while percent decrease value is presented on the secondary vertical axis. Different letters represent significant differences among cultivars at p < 0.05 according to LSD test. (A) Shoot fresh weight, (B) shoot dry weight, (C) root fresh weight, (D) root dry weight, (E) plant height, (F) flag leaf area, (G) total chlorophyll content (SPAD), and (H) quantum yield of PSII.
ANOVA of the data explained significant (p ≤ 0.001) decrease in all water relation parameters in all wheat cultivars on exposure to drought stress (Table 1). Drought stress reduced water potential (Ψw) in all wheat cultivars; however, a minimum decrease (87%) in Barani-83 while more in Blue Silver, Pak-81, and Pasban-90 was noted due to the effect of drought stress. A maximum decline in Ψw in FSD-08 (210%) followed by Lasani-08, Punjab-96, and Sahar-06 under drought conditions was observed (Figure 4A). As far as osmotic potential (Ψs) is concerned, Barani-83 showed a minimum decrease (39%) followed by Blue Silver, Pak-81, and Pasban-90, which shows more decrease in the respective order; however, genotype FSD-08 showed maximum decrease (53%), followed by Lasani-08, Punjab-96, and Sahar-06, showing more decrease, respectively, under drought stress conditions (Figure 4B). Drought stress caused minimum reduction (24%) in turgor potential (Ψp) in Barani-83 while maximum (55%) in cv. FSD-08 (Figure 4C). Reduction in relative water content (RWC) due to drought stress was only 3% in Barani-83 followed by Blue Silver, Pak-81, and Pasban-90 with 7%, 10%, and 15% decrease, respectively, as compared to control. A maximum decrease (24%) in RWC was seen in FSD-08 followed by Lasani-08, Punjab-96, and Sahar-06 with 20%, 16%, and 15% decrease, respectively, due to drought stress (Figure 4D).
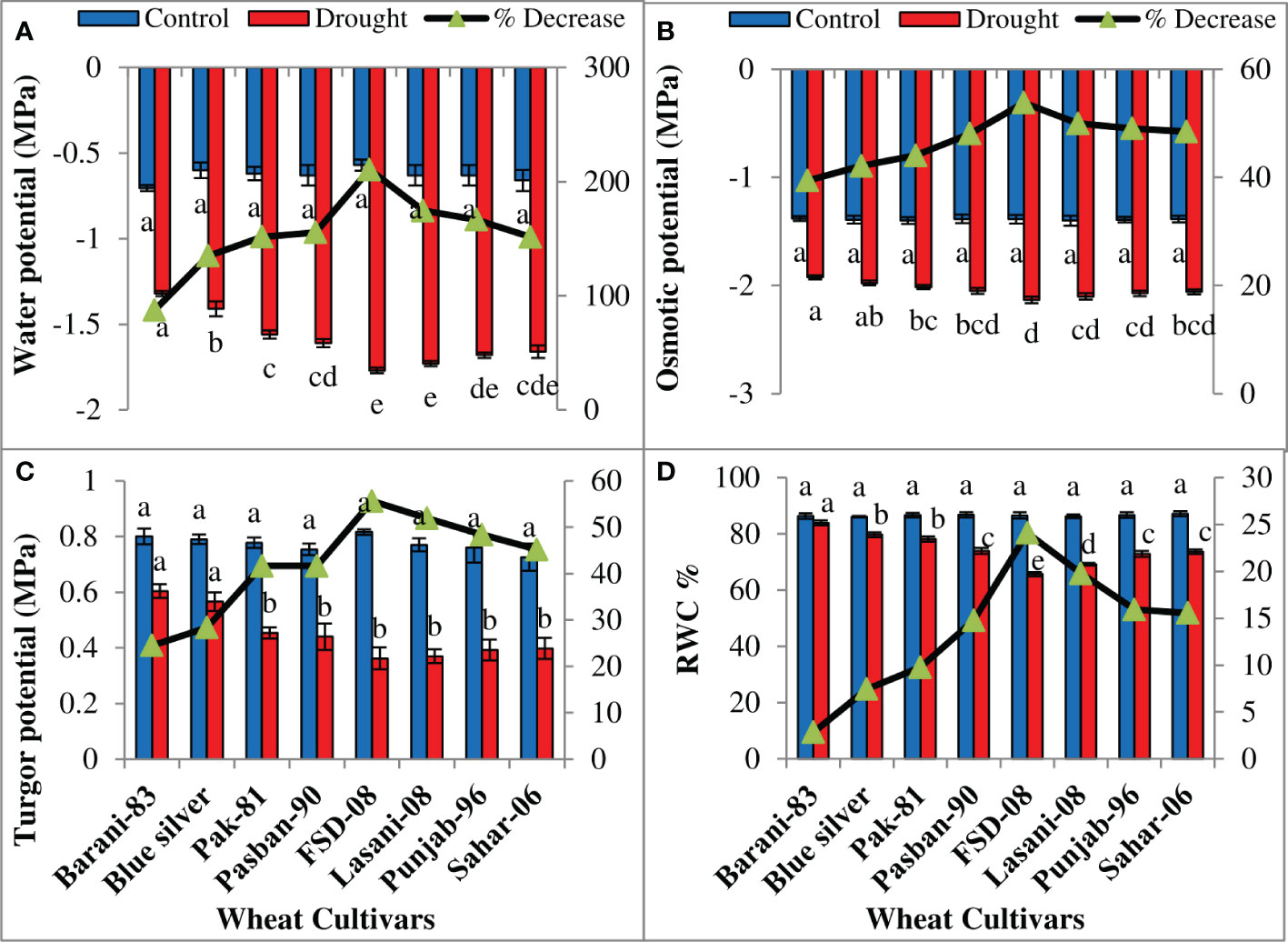
Figure 4 Water relations attributes of selected wheat cultivars subjected to control or drought stress at adult stage. Means (± SE; n=5) are presented on the primary vertical axis, while percent decrease value is presented on the secondary vertical axis. Different letters represent significant differences among cultivars at p < 0.05 according to LSD test. (A) Water potential, (B) osmotic potential, (C) turgor potential, and (D) % relative water content.
The percentage of N, P, and K+ of the shoot was significantly (p ≤ 0.001) reduced among the wheat cultivars under drought conditions (Table 1). The cultivars differed significantly in accumulation of mineral nutrients in leaves; a slightly less decrease in N in Barani-83 (18%), Blue Silver (28%), Pak-81(34%), and Pasban-90 (38%) was observed, while a decreasing order of N was 62%, 59%, 53%, and 46% in FSD-08, Lasani-08, Punjab-96, and Sahar-06 cultivars, respectively (Figure 5A). The percentage decrease in P was least (21%) in Barani-83 followed by Blue Silver (30%), Pak-81 (29%), and Pasban-90 (33%). The cv. FSD-08 retained the least P contents with 50% decrease, while Lasani-08, Punjab-96, and Sahar-06 cultivars exhibited 48%, 47%, and 41% decrease, respectively, under drought stress conditions and may be considered as drought-sensitive cultivars (Figure 5B). Maximum K+ contents under drought conditions were retained by cv. Barani-83 and showed least reduction (20%), while Blue Silver, Pak-81, and Pasban-90 showed 28%, 38%, and 39% decrease, respectively. On the other hand, the maximum percent decrease was shown by FSD-08, i.e., 68% followed by Lasani-08, Punjab-96, and Sahar-06 varieties with 59%, 56%, and 47% decrease, respectively, due to water stress (Figure 5C). MDA contents represent a measure of lipid peroxidation by ROS. A significant increase in MDA concentration (p ≤ 0.001) was observed in all wheat cultivars under the influence of drought stress (Table 1). Drought stress caused less damage to plasma membranes in Barani-83, Blue Silver, Pak-81, and Pasban-90, i.e., less lipid peroxidation as compared to FSD-08, Lasani-08, Punjab-96, and Sahar-06 wheat cultivars, which exhibit a significant increase in MDA contents (Figure 5D).
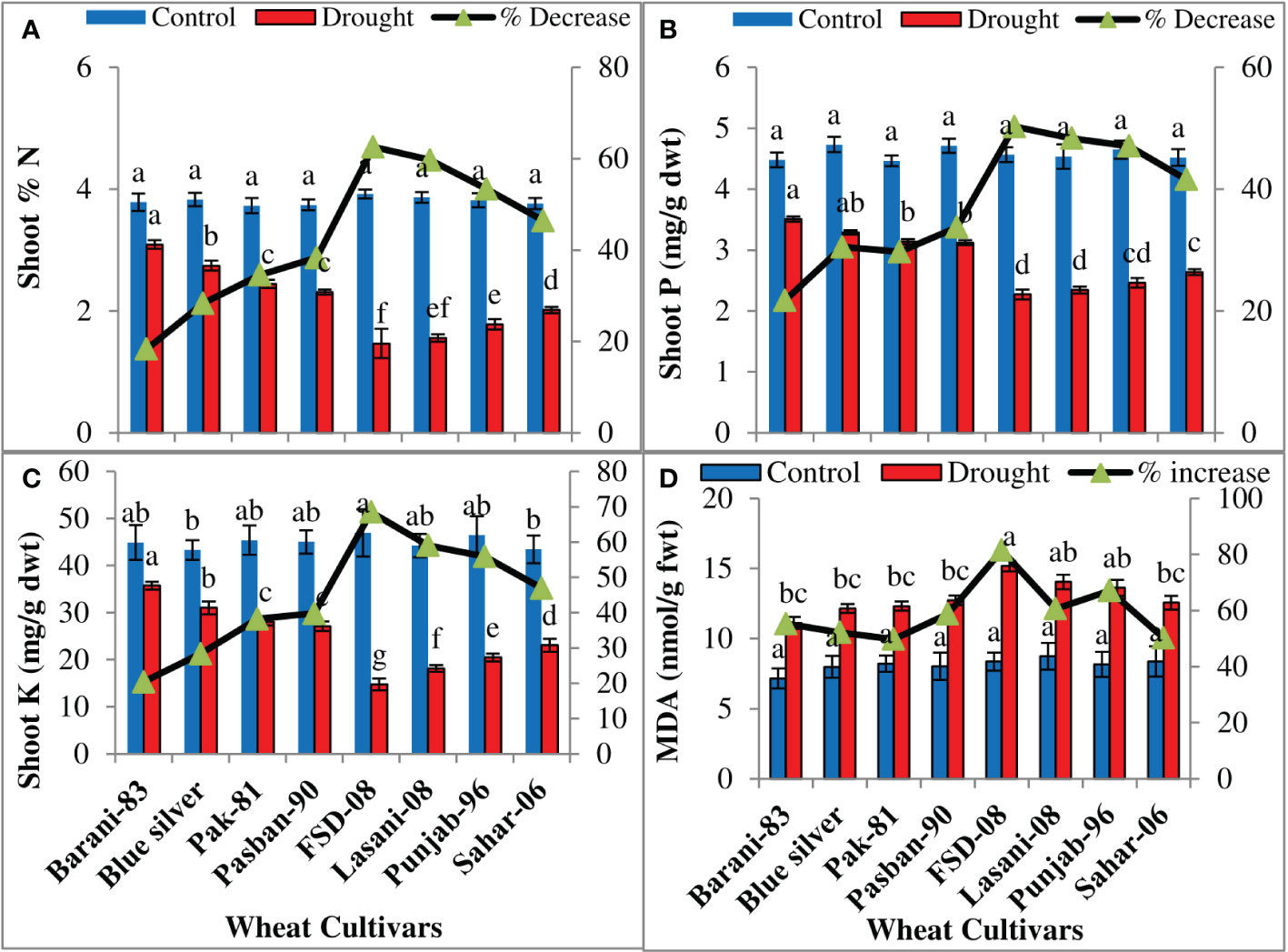
Figure 5 Accumulation of mineral nutrients and MDA in selected wheat cultivars subjected to control or drought stress at adult stage. Means (± SE; n=5) are presented on the primary vertical axis, while percent decrease value is presented on the secondary vertical axis. Different letters represent significant differences among cultivars at p < 0.05 according to LSD test. (A) Shoot N, (B) shoot P, (C) shoot K+, and (D) MDA.
Statistical results showed a significant (p ≤ 0.001) (Table 1) increase in total soluble sugars, proline, and free amino acids while a decrease in total soluble proteins in response to drought stress in all wheat cultivars. Cultivars differed considerably in accumulation of total soluble sugars under drought stress. A maximum increase (48%) in total soluble sugars was noted in cv. Barani-83 due to drought stress as compared to its control, while Blue Silver, Pak-81, and Pasban-90 with 37%, 38%, and 35% increase, respectively, come next to it. The lowest increase was displayed by FSD-08, i.e., 20%, followed by Lasani-08, Punjab-96, and Sahar-06 cultivars with ~25%–31% increase on facing drought stress (Figure 6A). A positive correlation was found between the degree of drought tolerance and proline accumulation. Barani-83 accumulated maximum proline and can be considered as the most drought tolerant, while FSD-08 with least increase in accumulation of proline (94%) was considered as drought sensitive (Figure 6B). Total soluble protein concentration diminished, while those of total free amino acids increase significantly (p ≤ 0.001) in all wheat cultivars under drought stress (Table 1). Barani-83, Blue Silver, Pak-81, and Pasban-90 exhibited decrease in total soluble proteins from ~14%–28%, while FSD-08, Lasani-08, Punjab-96, and Sahar-06 wheat cultivars showed greater decrease, i.e., 52%, 42%, 37%, and 33%, respectively, under dry conditions. Cultivars showing greater decrease in protein contents have greater increase in total free amino acids contents (Figures 6C, D).
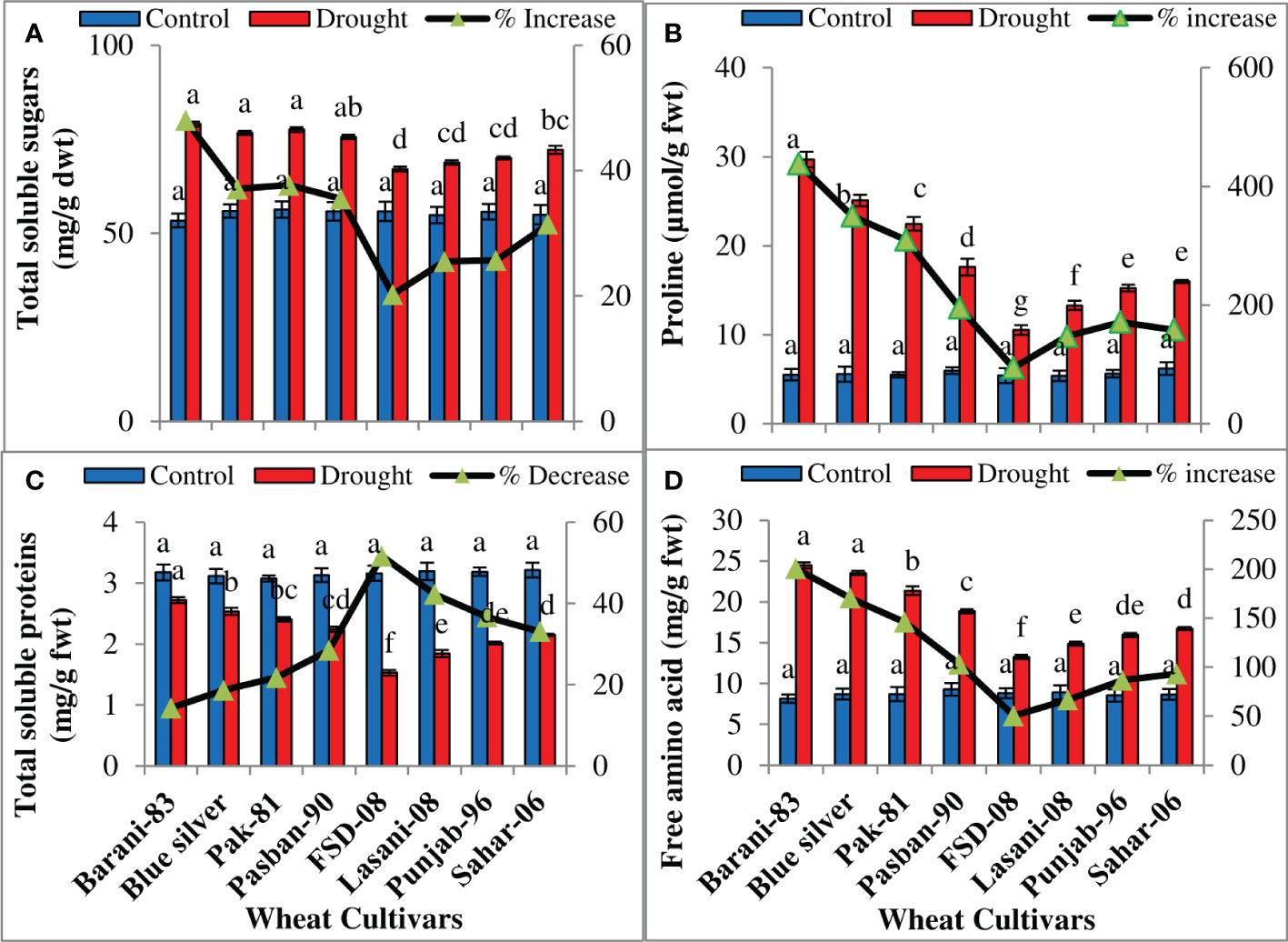
Figure 6 Accumulation of organic osmolytes in selected wheat cultivars subjected to control or drought stress at adult stage. Means (± SE; n=5) are presented on the primary vertical axis, while percent increase/decrease value is presented on the secondary vertical axis. Different letters represent significant differences among cultivars at p < 0.05 according to LSD test. (A) Total soluble sugars, (B) proline, (C) total soluble proteins, and (D) free amino acids.
Analysis of variance of the data showed significant (p ≤ 0.001) decrease in all yield parameters under water deficiency (Table 1). Under drought conditions, the highest grain yield (g/plant) with minimum decrease (24%) was recorded in Barani-83 followed by Blue Silver, Pak-81, and Pasban-90 with ~34%–45% decrease and may be considered as drought resistant, while the least grain yield was shown by FSD-08 with 76% decrease, followed by Lasani-08, Punjab-96, and Sahar-06 with 69%, 63%, and 57% decrease, respectively, and are thought to be drought-sensitive cultivars (Figure 7A). Similarly, a lesser decrease in 100-grain weight (g) was observed in Barani-83, Blue Silver, Pak-81, and Pasban-90, while a greater decrease was observed in FSD-08, i.e., 45%, followed by Lasani-08 (34%), Punjab-96 (31%), and Sahar-06 (30%) as depicted in Figure 7B. The number of grains per spike in Barani-83 decreased much less under drought stress followed by Blue Silver, Pak-81, and Pasban-90, however, a greater reduction was found in FSD-08 followed by Lasani-08, Punjab-96, and Sahar-06 (Figure 7C). The production potential of wheat is indicated by the number of spikelets per spike, which is adversely affected by drought stress in all wheat cultivars studied. Barani-83 with the least decrease (12%) in the number of spikelets per spike is followed by Blue Silver, Pak-81, and Pasban-90, whereas FSD-08 exhibited 43% decrease in the number of spikelets per spike, Lasani-08, Punjab-96, and Sahar-06 come after it (Figure 7D). The maximum length of spike was attained by the well-watered crop, which was substantially reduced under dry conditions. It was found that Barani-83 exhibited minimum percent reduction (23%) in spike length, while FSD-08 showed maximum percent reduction in spike length (36%) when plants faced water stress environment. The other cultivars were intermediate in reducing spike length on exposure to drought stress (Figure 7E). The number of fertile tillers per plant is an important indicator of plant yield, which is substantially decreased in all cultivars due to drought stress. Minimum percent reduction (44%) was shown by Barani-83 followed by Blue Silver, Pak-81, and Pasban-90 with 48%, 49%, and 51% decrease in the number of fertile tillers per plant, respectively. However, the maximum decrease (76%) was displayed by FSD-08 followed by Lasani-08, Punjab-96, and Sahar-06 with 71%, 60%, and 56% decrease, respectively, under drought stress (Figure 7F).
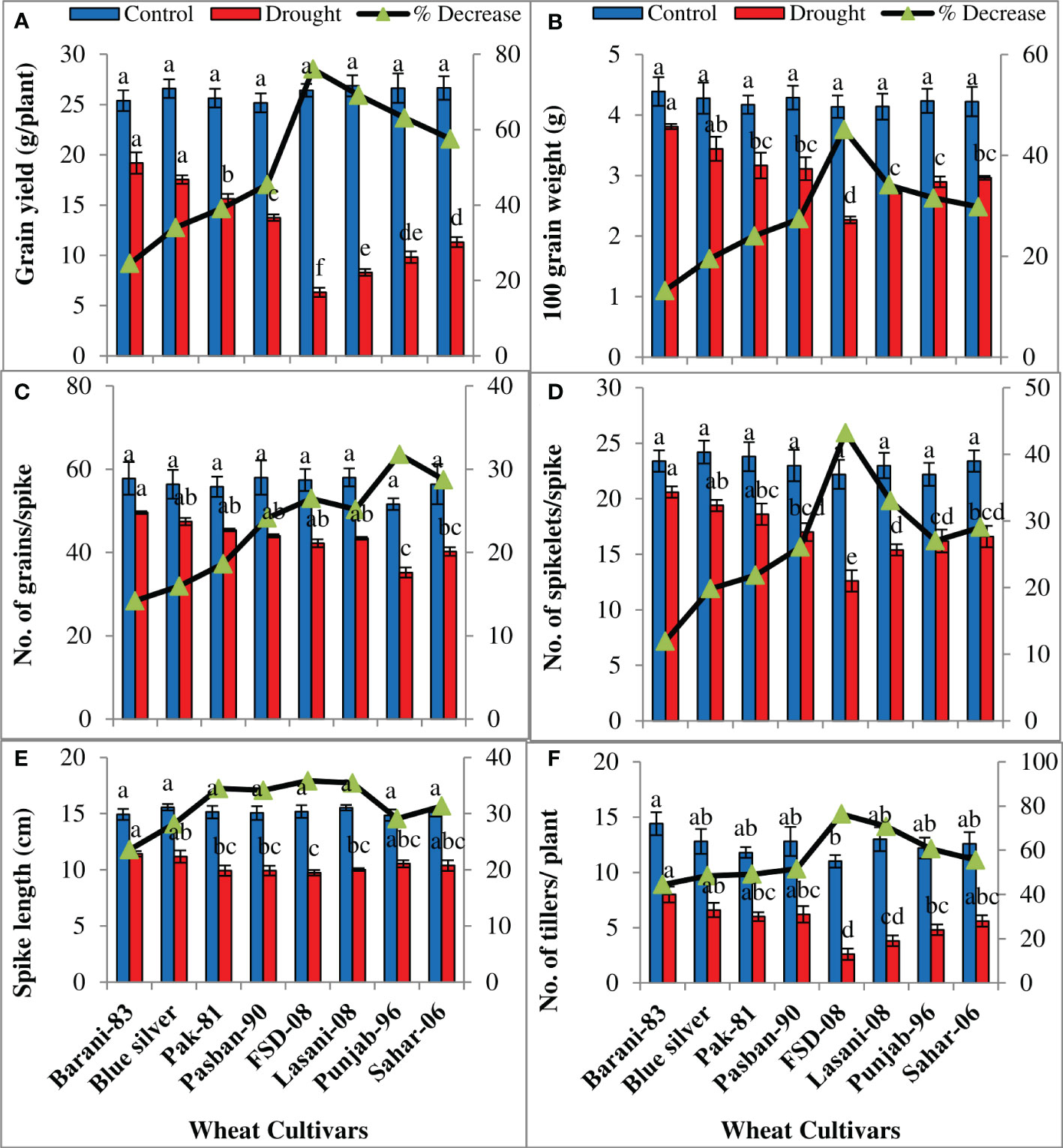
Figure 7 Yield attributes of selected wheat cultivars subjected to control or drought stress at adult stage. Means (± SE; n=5) are presented on the primary vertical axis, while percent decrease value is presented on secondary vertical axis. Different letters represent significant differences among cultivars at p < 0.05 according to LSD test. (A) Grain yield, (B) 100 grain weight, (C) number of grains/spike, (D) number of spikelets/spike, (E) spike length, and (F) number of tillers/plant.
Chlorophyll a fluorescence transients (Ft) measured by JIP test explain that no considerable change in typical OJIP kinetics was observed in cv. Blue Silver, while a small decrease in chlorophyll fluorescence in cv. Barani-83 was found at I and P steps; however, in cvs. FSD-08 and Lasani-08, a greater decrease in fluorescence was observed at J, I, and P steps as clearly seen from Figures 8A-D. Kinetic differences between double normalized drought stressed and control plants for chlorophyll a fluorescence values over a time range of 20–300 μs (between O and K steps) is called ΔVOK or L-band, which expresses uncoupling of OEC. Its increased value in FSD-08, Lasani-08, and Blue Silver indicated uncoupling of OEC with core protein of PSII, while in Barani-83, no such L-band was observed (Figure 9A). ΔVOJ (K-band) represents kinetic differences between double-normalized drought stressed and control plants over a time range of 20–2,000 μs (between O and J steps) and expresses OEC activity. FSD-08 and Lasani-08 showed positive K-band values, while Blue Silver and Barani-83 showed negative values (Figure 9B). Fo and Fj values in cvs. FSD-08 and Lasani-08 significantly increased, whereas they remained unchanged in cvs. Blue Silver and Barani-83 under drought stress (Figure 10). A greater decrease in Fi, Fm, and Fv in FSD-08 and Lasani-08 as compared to Blue Silver and Barani-83 was observed under dry conditions. The relative variable chlorophyll fluorescence at J (Vj) was significantly higher in cvs. FSD-08 and Lasani-08 than that of cvs. Blue Silver and Barani-83 (Figure 10). The functional status of PSII is represented by various chlorophyll fluorescence ratios (Fm/Fo, Fv/Fo, and Fv/Fm). Under water scarcity, a greater decrease in all of these ratios was found in cvs. FSD-08 and Lasani-08 as compared to those of cvs. Blue Silver and Barani-83 (Figure 10). The quantum efficiency of primary photochemistry (ΦPo), trapped energy (Ψo), and electron transport (ΦEo) was greatly decreased in FSD-08 and Lasani-08, while a lesser decrease in these values in cvs. Blue Silver and Barani-83 was observed under water-deficient conditions (Figure 10). Increase in energy fluxes for absorption (ABS/RC), trapping (TRo/RC), and heat dissipation (DIo/RC) was observed in drought-stressed plants of cvs. FSD-08 and Lasani-08, whereas the energy fluxes for electron transport (ETo/RC) decreased due to imposition of drought stress (Figure 10). A greater decrease in PIABS (performance index of PSII) due to drought stress was observed in cvs. FSD-08 and Lasani-08 than that of cvs. Blue Silver and Barani-83 (Figure 10).
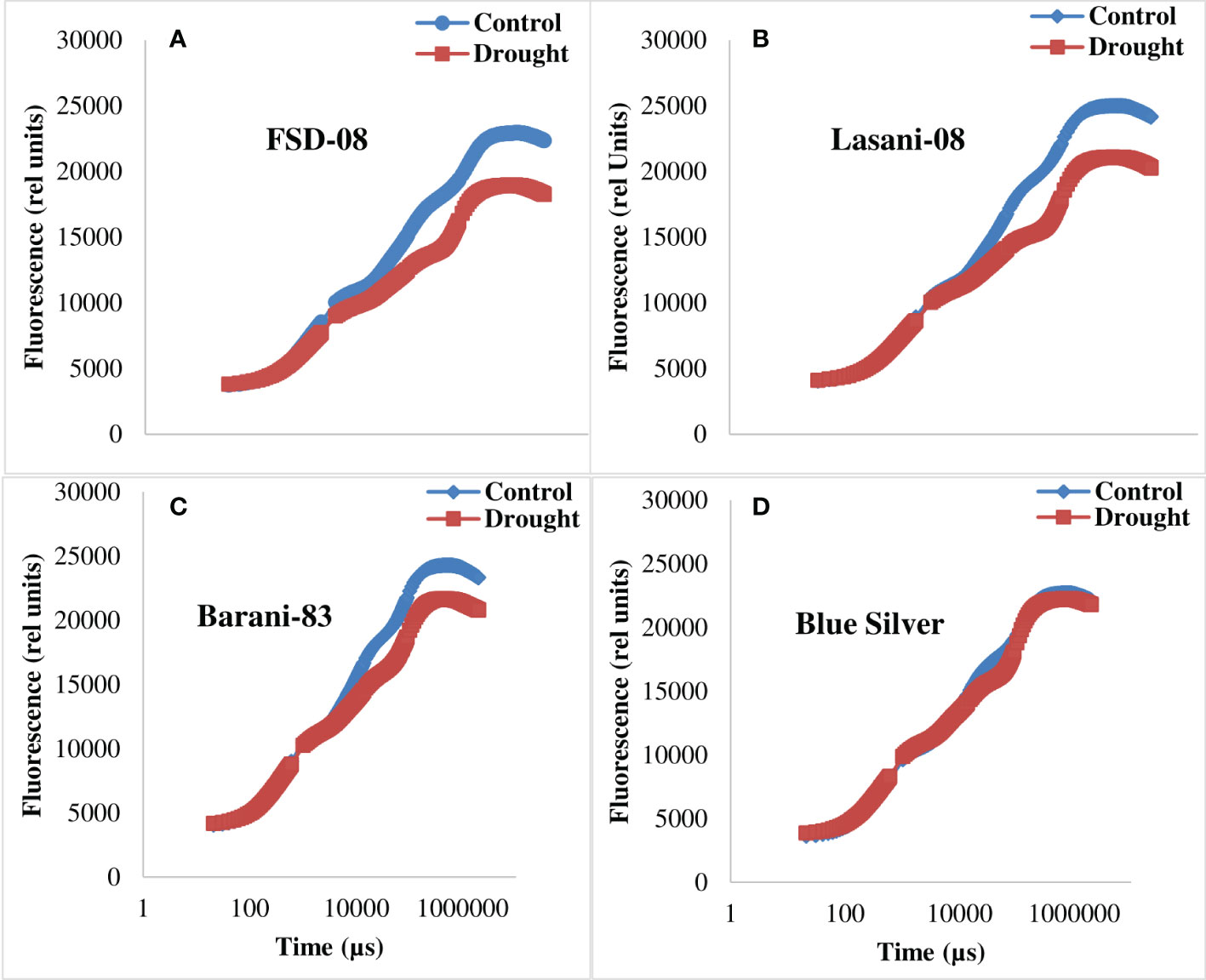
Figure 8 Raw OJIP chlorophyll a fluorescence curves (Ft) of the four selected wheat cultivars when plants were subjected to control or drought stress at adult stage. (A) FSD-08, (B) Lasani-08, (C) Barani-83, and (D) Blue Silver.
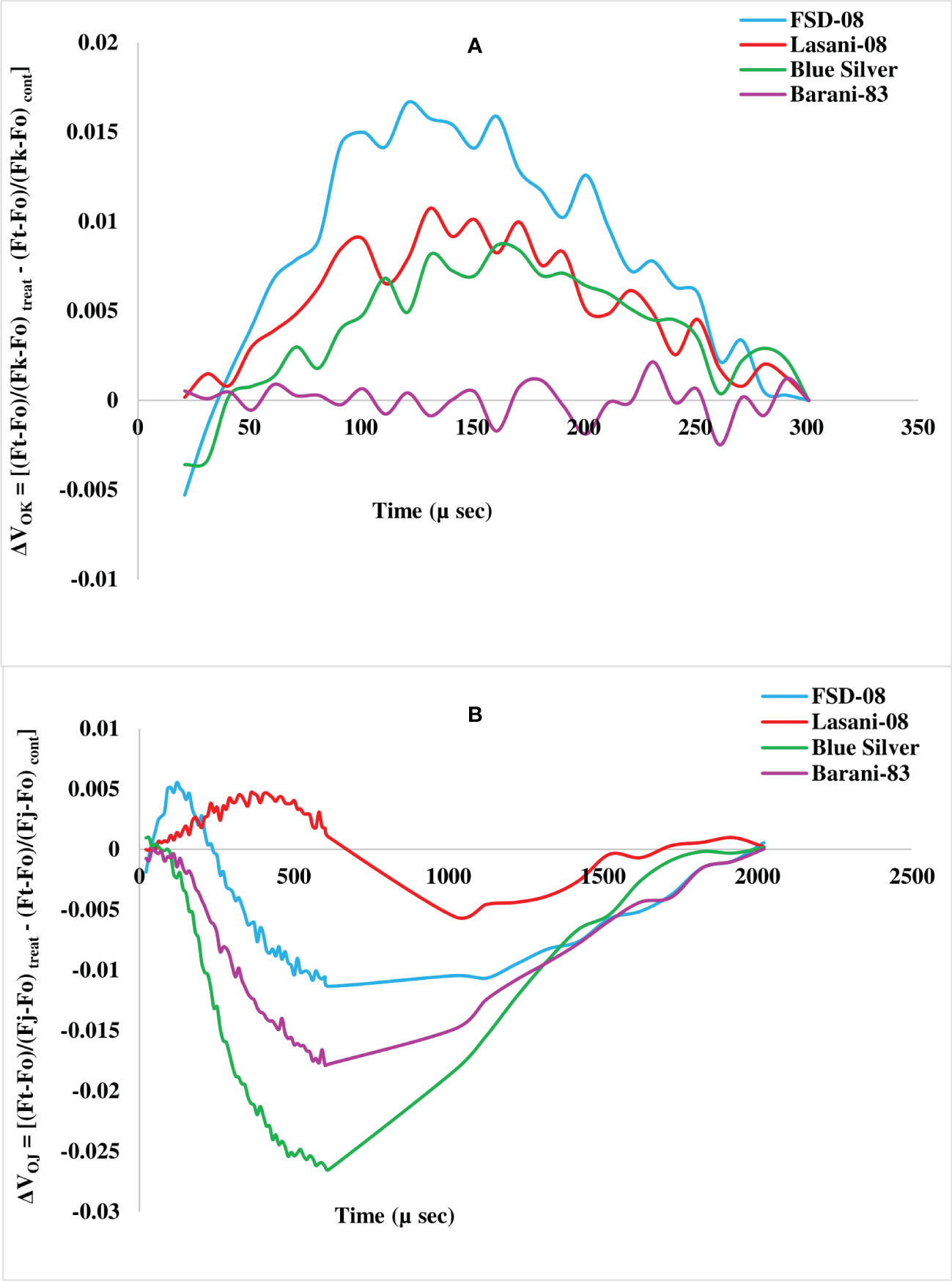
Figure 9 Kinetic differences of four selected wheat cultivars subjected to control or drought stress at adult stage. (A) Double normalized between Fo and Fk (L-band). (B) Double normalized between Fo and Fj (K band).
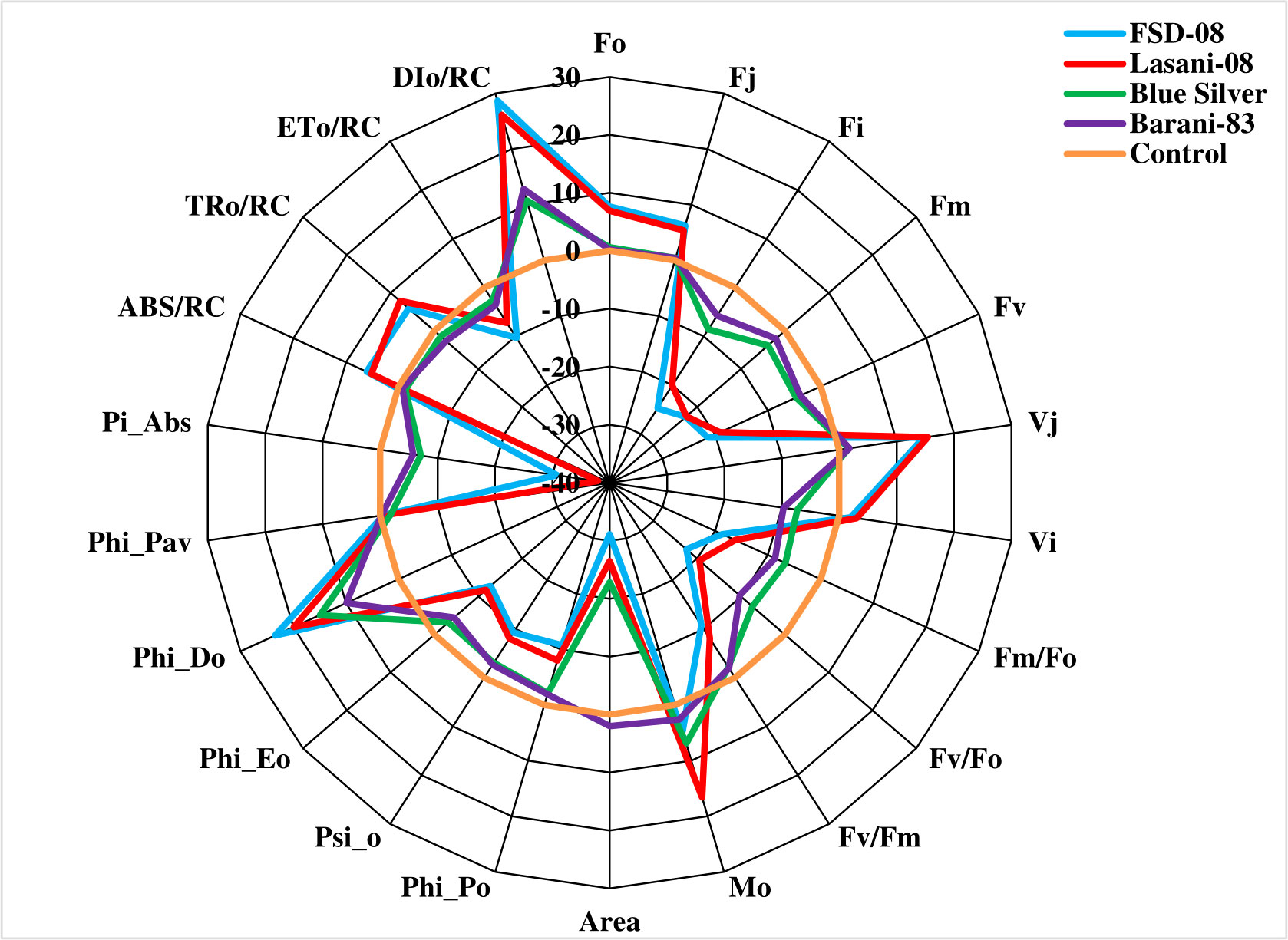
Figure 10 Radar plot of selected JIP test parameters showing changes in chlorophyll a fluorescence transients (normalized to control as reference) of four selected wheat cultivars subjected to drought stress at adult growth stage.
Drought-induced osmotic stress at vegetative, flowering, or grain filling stage adversely affects crop production. In the present study, PEG treatment decreased shoot and root fresh and dry weight of seedlings in all wheat cultivars (Supplementary Figure S2) as observed previously by Peršić et al. (2022) that morpho-physiological and biochemical traits of seedlings shoot and root are greatly affected by PEG-induced drought stress reducing growth. Different growth parameters (plant height, length of roots, and fresh and dry matter weight), number of branches, and plant yield in Cleome amblyocarp are significantly decreased due to increasing levels of water stress (Shahin et al., 2018a). Reduction in growth attributes such as fresh and dry biomass and RWC in Brassica napus was observed by Chen et al. (2022b). Our results indicated that water stress led to a decline in shoot and root fresh and dry weights, with FSD-08 as the most affected and sensitive cultivar in contrast to Barani-83, the least affected and most tolerant variety under drought stress conditions (Figures 3A-D). Previously, it was observed that drought stress destroys photosynthetic pigments, disturbs PSII activity, and reduces stomatal conduction, influx of CO2, and its fixation by Calvin cycle enzymes (Ashraf and Harris, 2013). In present studies, the decrease in plant height and leaf area under drought stress (Figures 3E, F) might be due to protoplasmic dehydration, which in turn decreases turgidity, cell enlargement, and cell division. A reduction in plant height and leaf area due to the decrease in cell elongation under drought stress was observed by Shahin et al. (2018b). Drought-linked decline in chlorophyll contents was due to greater activity of chlorophyllase enzyme or destruction of thylakoid membrane; as a result, photosynthetic performance in the form of quantum yield is also reduced (Shen et al., 2020). Drought-tolerant wheat cultivars in this experiment have higher leaf chlorophyll content and quantum yield of PSII than sensitive genotypes (Figures 3G, H) producing high yield under drought stress, indicating a positive correlation between chlorophyll content, quantum yield of PSII, and crop yield, in accordance with findings of Chowdhury et al. (2021) and Bano et al. (2021) that drought-tolerant wheat cultivars have greater chlorophyll content and quantum yield.
Wasaya et al. (2021) noted a reduction in spike length, number of grains per spike, and grain weight under drought stress in wheat cultivars, while a reduction in the number of tillers per plant, number of spikelet per spike, and number of grains per spike was observed by Panhwar et al. (2021). The results obtained from the present research suggest that Barani-83, Blue Silver, Pak-81, and Pasban-90 showed better response towards drought conditions, while FSD-08, Lasani-08, Punjab-96, and Sahar-06 cultivars showed poor performance in terms of yield attributes such as the decline in grain yield and 100 grain weight under water shortage is due to reduced production of photo-assimilates and their reduced absorption by sink while the reduction in the number of grains per spike was due to loss of pollen viability as explained in earlier studies by Chowdhury et al. (2021).
Plant water status is measured in terms of RWC, which reflects metabolic activities of plant tissues (Chowdhury et al., 2021). A reduction in RWC was found in all wheat cultivars under drought stress; less reduction in tolerant cultivars (Barani-83 and Blue Silver) while more in sensitive cultivars (FSD-08 and Lasani-08) were found in the present study. These results are in agreement with the previous studies by Belay et al. (2021) who found less reduction in RWC in drought-tolerant wheat cultivars under water-deficit conditions. The decrease in RWC might be due to reduced water uptake and greater loss of water from leaves, which reduces leaf turgor potential, inhibits cell elongation and cell division, and ultimately reduces growth and yield (Camaille et al., 2021). Under such conditions, plants modify their osmotic potential by lowering the water potential in a process called osmotic adjustment, which is carried out by accumulation of suitable solutes like soluble sugars, proline, free amino acids, soluble proteins, and glycine-betaine that increase the tolerance to all types of environmental stresses (Ashraf and Foolad, 2007). The conservation of desirable cellular turgidity under water scarcity permits the plant to keep its physio-metabolic functions such as opening of stomata, photosynthesis, cell expansion, and other developmental processes at optimum level (Serraj and Sinclair, 2002). Cultivars recognized as drought tolerant during the present study maintained water status by accumulation of total soluble sugars, proline, and amino acids (Figures 6A, B, D). Different studies have already explained that accumulation of osmo-protectants like soluble sugars, soluble proteins, and proline in leaves is involved in maintaining plant water status under stress conditions (Gontia-Mishra et al., 2016; Blum, 2017; Qayyum et al., 2021). Increase in proline production under drought stress is needed to supply energy for growth and survival to tolerate plants against drought stress (Shahin et al., 2018b). A greater concentration of proline plays an important role in osmotic adjustment, ROS scavenging, and stabilizing cellular components (Hussain et al., 2021). In wheat, the soluble sugars play the most important role in osmotic adjustment during drought stress (Camaille et al., 2021).
Drought-tolerant cultivars Barani-83, Blue Silver, Pak-81, and Pasban-90 showed greater amounts of N, P, and K+ accumulated in the leaves as compared to FSD-08, Lasani-08, Punjab-96, and Sahar-06 cultivars under drought stress; previously, it was observed that plants exposed to drought stress have low N content in comparison to well-watered plants as found for rice and wheat due to restricted movement of N and P in the soil and less absorption by roots (Sun et al., 2012). Severe drought reduces N and P contents in leaves of wheat, canola, and soybean plants (Ashraf and Foolad, 2013). Noman et al. (2018) observed reduced levels of K, P, and Ca2+ in roots and shoots of wheat plants under drought stress. Oxidative stress refers to generation of ROS in response to drought stress due to shifting of Calvin cycle to photorespiration, which causes lipid peroxidation, chlorophyll etiolation, protein oxidation, and damage to cellular organelles, DNA, and RNA (Abedi and Pakniyat, 2010). Our experimental results are also in accordance with Outoukarte et al. (2019), who reported that enhanced lipid peroxidation and more ROS result in loss of membranes ability to control the rate of ion movement into and out of cells, an indication of plasma membrane damage as revealed by increased MDA content, osmotic stress, and reduced N, P, and K status of drought-sensitive wheat cultivars (FSD-08, Lasani-08, Punjab-96, and Sahar-06) that ultimately affect their final yield.
The effects of drought stress on the shape of raw OJIP curves (Ft) show that Fo and Fj under drought stress were significantly increased, while Fi and Fm were markedly reduced in cvs. FSD-08 and Lasani-08 (Figures 8A-D). However, all these fluorescence attributes either remained unchanged or slightly changed in cvs. Blue Silver and Barani-83 due to water stress, which suggests only minor structural damage to PSII at both donor and acceptor end of PSII in these cultivars and can be considered as drought tolerant. Sensitive cvs. FSD-08 and Lasani-08 exhibited increased Fo and decreased energy trapping capacity of PSII, while decreased Fm may be attributed to inactive reaction centers. Damage to oxygen-evolving complex (OEC) reducing Fv values and increasing Vj is an indication of lowering PSII ability to reduce plastoquinone in these cultivars. Similar findings in mung bean were observed by Bano et al. (2021). L-band expresses uncoupling of OEC; its increased value in FSD-08, Lasani-08, and Blue Silver indicated uncoupling of OEC with core protein of PSII, while in Barani-83, no such L-band was observed (Figure 9A). Negative K-band values for Blue Silver and Barani-83 show that plants maintain antenna complex and PSII reaction center under the influence of drought stress (Figure 9B), while its positive values for cvs. FSD-08 and Lasani-08 show reduced OEC performance due to disturbance in electron flow from OEC to PSII reaction center in accordance with the previous studies, which explain that K-step gives information about the destruction of OEC (Guo et al., 2020). Many studies explain that double normalized differential chlorophyll a fluorescence kinetics such as L- and K-bands indicate drought tolerance potential in plants (Brestic and Zivcak, 2013; Kalaji et al., 2018; Zhou et al., 2019). Drought stress caused minor changes in Fv/Fm in cvs. Blue Silver and Barani-83, which suggest no photo-inhibition, while the decrease in PSII efficiency of cvs. FSD-08 and Lasani-08 was mainly due to photoinhibition with greater decrease in Fv/Fm. The results are supported by the findings of Flexas et al. (2009) and Lawlor and Tezara (2009). The quantum yields of primary photochemistry (ΦPo) and the electron transfer from QA to PQ (ΦEo) are slightly affected in drought-tolerant cultivars (Blue Silver and Barani-83), while a greater decrease was observed in cvs. FSD-08 and Lasani-08. However, quantum yield for heat dissipation (ΦDo) increased only in drought-sensitive cultivars FSD-08 and Lasani-08. Fv/Fm and PIABS are important screening tools to investigate photosynthetic efficiency and effects of abiotic stresses on PSII in different plants. PIABS depends upon the concentration of active reaction centers of chlorophyll, efficiency of energy trapping, and electron transport from PSII to the plastoquinone pool (Botyanszka et al., 2020). Water stress increased the energy fluxes of absorption (ABS/RC), trapping (TRo/RC), and electron transport (ETo/RC) in FSD-08 and Lasani-08, while it decreased those in Blue Silver and Barani-83 cultivars. Strasser et al. (2000) explained that effective antenna size and absorbed energy are reduced in drought-tolerant cultivars, whereas drought-sensitive cultivars were unable to regulate the antenna size, so active reaction centers of PSII are damaged due to over-excitation. The absorbed energy by antenna proteins is dissipated as heat (DIo/RC) (Figure 10).
A great genetic variability was found among wheat cultivars in the present study. Based on maintaining growth, photosynthetic pigments, and RWC and good osmotic adjustment by accumulating proline, total soluble sugars, and proteins under water stress, Barani-83 and Blue silver were considered the most drought tolerant, while based on disruption of photosynthetic pigments and poor management of water status, FSD-08 and Lasani-08 were ranked as drought-sensitive cultivars. Chlorophyll a fluorescence transients confirmed better efficiency of photosystem II in tolerant cultivars (Barani-83 and Blue Silver) due to greater dissipation (DIo/RC) of absorbed energy (ABS/RC) and downregulation of electron transport per reaction center (ETo/RC) to prevent photosystem-II from photooxidative damage. Greater PSII stability and functional activity in drought-tolerant cultivars (Barani-83 and Blue Silver) was evident with greater performance index (PIABS) and greater quantum yield (Fv/Fm) of PSII.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conceptualization and designing the experiments, H-u-RA, RA, ZUZ, and SS. Execution of experiments, NH, AG, ZUZ, HB, AK, and MY. Data analysis, NH, MJ, RA, MI, SN, SS, and MZ. Data validation, H-u-RA. First draft writing, NH, AG, and KS. Supervision of the experiment, H-u-RA and ZUZ. Review and editing of the manuscript, H-u-RA, RA, SS, MJ, MI, AK, HB, and MZ. Proofing of the manuscript, H-u-RA, SN, SS, and RA. Providing resources and funding acquisition, RA and SS. All authors contributed to the article and approved the submitted version.
The research work is a part of PhD study of Abdul Ghaffar. The authors are thankful to all who contributed in making this research study possible.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1123080/full#supplementary-material
Abedi, T., Pakniyat, H. (2010). Antioxidant enzymes changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus l.). Czech J. Genet. Plant Breed. 46, 27–34. doi: 10.17221/67/2009-CJGPB
Ahmad, A., Aslam, Z., Javed, T., Hussain, S., Raza, A., Shabbir, R., et al. (2022). Screening of wheat (Triticum aestivum l.) genotypes for drought tolerance through agronomic and physiological response. Agronomy 12, 287. doi: 10.3390/agronomy12020287
Allen, S., Dobrenz, A., Schonhorst, M., Stoner, J. (1985). Heritability of NaCl tolerance in germinating alfalfa seeds 1. Agron. J. 77, 99–101. doi: 10.2134/agronj1985.00021962007700010023x
Ashraf, M., Foolad, M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59, 206–216. doi: 10.1016/j.envexpbot.2005.12.006
Ashraf, M., Foolad, M. R. (2013). Crop breeding for salt tolerance in the era of molecular markers and marker-assisted selection. Plant Breed. 132, 10–20. doi: 10.1111/pbr.12000
Ashraf, M., Harris, P. J. (2013). Photosynthesis under stressful environments: an overview. Photosynthetica 51, 163–190. doi: 10.1007/s11099-013-0021-6
Bano, H., Athar, H. U. R., Zafar, Z. U., Kalaji, H. M., Ashraf, M. (2021). Linking changes in chlorophyll a fluorescence with drought stress susceptibility in mung bean [Vigna radiata (L.) wilczek]. Physiol. Plantarum 172, 1244–1254. doi: 10.1111/ppl.13327
Bashir, N., Athar, H.-U.-R., Kalaji, H. M., Wróbel, J., Mahmood, S., Zafar, Z. U., et al. (2021). Is photoprotection of PSII one of the key mechanisms for drought tolerance in maize? Int. J. Mol. Sci. 22, 13490. doi: 10.3390/ijms222413490
Bates, L. S., Waldren, R. P., Teare, I. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. doi: 10.1007/BF00018060
Belay, G. A., Zhang, Z., Xu, P. (2021). Physio-morphological and biochemical trait-based evaluation of Ethiopian and Chinese wheat germplasm for drought tolerance at the seedling stage. Sustainability 13, 4605. doi: 10.3390/su13094605
Blum, A. (2016). Stress, strain, signaling, and adaptation–not just a matter of definition. J. Exp. Bot. 67, 562–565. doi: 10.1093/jxb/erv497
Blum, A. (2017). Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 40, 4–10. doi: 10.1111/pce.12800
Botyanszka, L., Zivcak, M., Chovancek, E., Sytar, O., Barek, V., Hauptvogel, P., et al. (2020). Chlorophyll fluorescence kinetics may be useful to identify early drought and irrigation effects on photosynthetic apparatus in field-grown wheat. Agronomy 10, 1275. doi: 10.3390/agronomy10091275
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Bremner, J. M. (1965). “Organic nitrogen in soils,” in Soil nitrogen. Eds. Bartholomew, W. V., Clark, F. E. (USA: The American Society of Agronomy, Inc).
Brestic, M., Zivcak, M. (2013). “PSII fluorescence techniques for measurement of drought and high temperature stress signal in crop plants: protocols and applications,” in Molecular stress physiology of plants (Springer, India: Springer). doi: 10.1007/978-81-322-0807-5_4
Cakmak, I., Horst, W. J. (1991). Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plantarum 83, 463–468. doi: 10.1111/j.1399-3054.1991.tb00121.x
Camaille, M., Fabre, N., Clément, C., Ait Barka, E. (2021). Advances in wheat physiology in response to drought and the role of plant growth promoting rhizobacteria to trigger drought tolerance. Microorganisms 9, 687. doi: 10.3390/microorganisms9040687
Chen, W., Miao, Y., Ayyaz, A., Hannan, F., Huang, Q., Ulhassan, Z., et al. (2022b). Purple stem brassica napus exhibits higher photosynthetic efficiency, antioxidant potential and anthocyanin biosynthesis related gene expression against drought stress. Front. Plant Sci. 2606. doi: 10.3389/fpls.2022.936696
Chen, F., Wang, H., Zhao, F., Wang, R., Qi, Y., Zhang, K., et al. (2022a). The response mechanism and threshold of spring wheat to rapid drought. Atmosphere 13, 596. doi: 10.3390/atmos13040596
Chowdhury, M. K., Hasan, M., Bahadur, M., Islam, M. R., Hakim, M. A., Iqbal, M. A., et al. (2021). Evaluation of drought tolerance of some wheat (Triticum aestivum l.) genotypes through phenology, growth, and physiological indices. Agronomy 11, 1792. doi: 10.3390/agronomy11091792
Del Pozo, A., Yáñez, A., Matus, I. A., Tapia, G., Castillo, D., Sanchez-Jardón, L., et al. (2016). Physiological traits associated with wheat yield potential and performance under water-stress in a Mediterranean environment. Front. Plant Sci. 7, 987. doi: 10.3389/fpls.2016.00987
FAOSTAT (2022) License: CC BY-NC-SA 3.0 IGO. Available at: https://www.fao.org/faostat/en/#data/QCL/visualize.
FAO, UNICEF 2022, WFP, WHO (2022). The state of food security and nutrition in the world 2022. repurposing food and agricultural policies to make healthy diets more affordable (Rome: FAO. FAO, Rome).
Flexas, J., Barón, M., Bota, J., Ducruet, J.-M., Gallé, A., Galmés, J., et al. (2009). Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted vitis hybrid Richter-110 (V. berlandieri× v. rupestris). J. Exp. Bot. 60, 2361–2377. doi: 10.1093/jxb/erp069
Gontia-Mishra, I., Sapre, S., Sharma, A., Tiwari, S. (2016). Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 18, 992–1000. doi: 10.1111/plb.12505
Guo, Y., Lu, Y., Goltsev, V., Strasser, R. J., Kalaji, H. M., Wang, H., et al. (2020). Comparative effect of tenuazonic acid, diuron, bentazone, dibromothymoquinone and methyl viologen on the kinetics of chl a fluorescence rise OJIP and the MR820 signal. Plant Physiol. Biochem. 156, 39–48. doi: 10.1016/j.plaphy.2020.08.044
Halder, T., Choudhary, M., Liu, H., Chen, Y., Yan, G., Siddique, K. H. (2022). Wheat proteomics for abiotic stress tolerance and root system architecture: Current status and future prospects. Proteomes 10, 17. doi: 10.3390/proteomes10020017
Hamilton, P. B., Van Slyke, D. D., Lemish, S. (1943). The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J. Biol. Chem. 150, 231–250. doi: 10.1016/S0021-9258(18)51268-0
Hoagland, D. R., Arnon, D. I. (1950). “. the water-culture method for growing plants without soil,” in Circular, vol. 347. (Berkeley, USA: College of Agriculture, University of California).
Hussain, N., Ghaffar, A., Zafar, Z. U., Javed, M., Shah, K. H., Noreen, S., et al. (2021). Identification of novel source of salt tolerance in local bread wheat germplasm using morpho-physiological and biochemical attributes. Sci. Rep. 11, 1–12. doi: 10.1038/s41598-021-90280-w
Hussain, N., Sohail, Y., Shakeel, N., Javed, M., Bano, H., Gul, H. S., et al. (2022). Role of mineral nutrients, antioxidants, osmotic adjustment and PSII stability in salt tolerance of contrasting wheat genotypes. Sci. Rep. 12, 1–13. doi: 10.1038/s41598-022-16922-9
Jackson, M. (1958). Soil chemical analysis Englewood cliffs (New Jersey, USA: NT Prentice Hall Inc).
Jones, M. M., Turner, N. C. (1978). Osmotic adjustment in leaves of sorghum in response to water deficits. Plant Physiol. 61, 122–126. doi: 10.1104/pp.61.1.122
Kalaji, H. M., Račková, L., Paganová, V., Swoczyna, T., Rusinowski, S., Sitko, K. (2018). Can chlorophyll-a fluorescence parameters be used as bio-indicators to distinguish between drought and salinity stress in tilia cordata mill? Environ. Exp. Bot. 152, 149–157. doi: 10.1016/j.envexpbot.2017.11.001
Lawlor, D. W., Tezara, W. (2009). Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann. Bot. 103, 561–579. doi: 10.1093/aob/mcn244
Li, M., Kim, C. (2021). Chloroplast ROS and stress signaling. Plant Commun., 3(1), 100264. doi: 10.1016/j.xplc.2021.100264
Muller, J. (1991). Determining leaf surface area by means of a wheat osmoregulation water use: the challenge. Agric. Meteorol. 14, 311–320. doi: 10.25081/jpsp.2019.v5.3700
Nobel, P. (1991). Physicochemical and environmental plant physiology (New York, NY: Academic Press).
Noman, A., Ali, Q., Naseem, J., Javed, M. T., Kanwal, H., Islam, W., et al. (2018). Sugar beet extract acts as a natural bio-stimulant for physio-biochemical attributes in water stressed wheat (Triticum aestivum l.). Acta Physiol. Plantarum 40, 1–17. doi: 10.1007/s11738-018-2681-0
Outoukarte, I., El Keroumi, A., Dihazi, A., Naamani, K. (2019). Use of morpho-physiological parameters and biochemical markers to select drought tolerant genotypes of durum wheat. J. Plant Stress Physiol., 01–07. doi: 10.25081/jpsp.2019.v5.3700
Ozturk, M., Unal Turkyilmaz, B., García-Caparrós, P., Khursheed, A., Gul, A., Hasanuzzaman, M. (2021). Osmoregulation and its actions during the drought stress in plants. Physiol. Plantarum 172, 1321–1335. doi: 10.1111/ppl.13297
Panhwar, N. A., Mierzwa-Hersztek, M., Baloch, G. M., Soomro, Z. A., Sial, M. A., Demiraj, E., et al. (2021). Water stress affects the some morpho-physiological traits of twenty wheat (Triticum aestivum l.) genotypes under field condition. Sustainability 13, 13736. doi: 10.3390/su132413736
Peršić, V., Ament, A., Drezner, G., Cesar, V. (2022). PEG-induced physiological drought for screening winter wheat genotypes sensitivity–integrated biochemical and chlorophyll a fluorescence analysis. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.987702
Qayyum, A., Al Ayoubi, S., Sher, A., Bibi, Y., Ahmad, S., Shen, Z., et al. (2021). Improvement in drought tolerance in bread wheat is related to an improvement in osmolyte production, antioxidant enzyme activities, and gaseous exchange. Saudi J. Biol. Sci. 28, 5238–5249. doi: 10.1016/j.sjbs.2021.05.040
Riyazuddin, R., Nisha, N., Singh, K., Verma, R., Gupta, R. (2022). Involvement of dehydrin proteins in mitigating the negative effects of drought stress in plants. Plant Cell Rep. 41, 519–533. doi: 10.1007/s00299-021-02720-6
Sallam, A., Alqudah, A. M., Dawood, M. F., Baenziger, P. S., Börner, A. (2019). Drought stress tolerance in wheat and barley: advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 20, 3137. doi: 10.3390/ijms20133137
Scholander, P. F., Hammel, H., Hemmingsen, E., Bradstreet, E. (1964). Hydrostatic pressure and osmotic potential in leaves of mangroves and some other plants. Proc. Natl. Acad. Sci. United States America 52, 119. doi: 10.1073/pnas.52.1.119
Sekher, T. (2022) in World Population Day-2022 Symposium & Launch of United Nations World Population Prospects-2022, Conference Proceedings Columbia University.
Serraj, R., Sinclair, T. (2002). Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ. 25, 333–341. doi: 10.1046/j.1365-3040.2002.00754.x
Shahin, S., Kurup, S., Cheruth, J., Lennartz, F., Salem, M. (2018b). Growth, yield, and physiological responses of cleome amblyocarpa barr. & murb. under varied irrigation levels in sandy soils. J. Food Agric. Environ. 16, 124–134. doi: 10.1234/4.2018.5508
Shahin, S., Kurup, S., Cheruth, A.-J., Salem, M. (2018a). Chemical composition of cleome amblyocarpa barr. & murb. essential oils under different irrigation levels in sandy soils with antioxidant activity. J. Essential Oil Bearing Plants 21, 1235–1256. doi: 10.1080/0972060X.2018.1512422
Shen, J., Guo, M.-J., Wang, Y.-G., Yuan, X.-Y., Wen, Y.-Y., Song, X.-E., et al. (2020). Humic acid improves the physiological and photosynthetic characteristics of millet seedlings under drought stress. Plant Signaling Behav. 15, 1774212. doi: 10.1080/15592324.2020.1774212
Snedecor, G. W., Cochran, W. G. (1989). Statistical methods. eight edition (Ames, Iowa: Iowa state University press).
Strasser, R. J., Srivastava, A., Tsimilli-Michael, M. (2000). “The fluorescence transient as a tool to characterize and screen photosynthetic samples,” in Probing Photosynthesis: Mechanism, Regulation and Adaptation eds Yunus, M., Pathre, U., Mohanty, P. (London, UK: CRC Press, Taylor and Francis Group) 445–483.
Strasser, R. J., Tsimilli-Michael, M., Srivastava, A. (2004). “Analysis of the chlorophyll a fluorescence transient,” in Chlorophyll a fluorescence (New York, USA: Springer).
Sun, M., Gao, Z.-Q., Yang, Z.-P., He, L.-H. (2012). Absorption and accumulation characteristics of nitrogen in different wheat cultivars under irrigated and dryland conditions. Aust. J. Crop Sci. 6, 613. doi: 10.3316/informit.362102772665611
Tefera, A., Kebede, M., Tadesse, K., Getahun, T. (2021). Morphological, physiological, and biochemical characterization of drought-tolerant wheat (Triticum spp.) varieties. Int. J. Agron. 2021, 1–12. doi: 10.1155/2021/8811749
Tsimilli-Michael, M. (2020). Special issue in honour of Prof. reto j. strasser–revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 58, 275–292. doi: 10.32615/ps.2019.150
Wasaya, A., Manzoor, S., Yasir, T. A., Sarwar, N., Mubeen, K., Ismail, I. A., et al. (2021). Evaluation of fourteen bread wheat (Triticum aestivum l.) genotypes by observing gas exchange parameters, relative water and chlorophyll content, and yield attributes under drought stress. Sustainability 13, 4799. doi: 10.3390/su13094799
Yemm, E., Willis, A. (1954). The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 57, 508–514. doi: 10.1042/bj0570508
Zhang, X., Yang, H., Du, T. (2022). Osmotic adjustment of tomato under mild soil salinity can enhance drought resistance. Environ. Exp. Bot. 202, 105004. doi: 10.1016/j.envexpbot.2022.105004
Zhou, R., Kan, X., Chen, J., Hua, H., Li, Y., Ren, J., et al. (2019). Drought-induced changes in photosynthetic electron transport in maize probed by prompt fluorescence, delayed fluorescence, P700 and cyclic electron flow signals. Environ. Exp. Bot. 158, 51–62. doi: 10.1016/j.envexpbot.2018.11.005
Keywords: drought stress, mineral nutrients, osmotic adjustment, Triticum aestivum, chlorophyll fluorescence, water relations
Citation: Ghaffar A, Hussain N, Ajaj R, Shahin SM, Bano H, Javed M, Khalid A, Yasmin M, Shah KH, Zaheer M, Iqbal M, Zafar ZU and Athar H-u-R (2023) Photosynthetic activity and metabolic profiling of bread wheat cultivars contrasting in drought tolerance. Front. Plant Sci. 14:1123080. doi: 10.3389/fpls.2023.1123080
Received: 13 December 2022; Accepted: 18 January 2023;
Published: 02 February 2023.
Edited by:
Muhammad Waseem, Hainan University, ChinaReviewed by:
Rehana Sardar, University of the Punjab, PakistanCopyright © 2023 Ghaffar, Hussain, Ajaj, Shahin, Bano, Javed, Khalid, Yasmin, Shah, Zaheer, Iqbal, Zafar and Athar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nadeem Hussain, bmFkZWVtaHVzc2FpbjM5NkB5YWhvby5jb20=; Abdul Ghaffar, YWJkdWxnaGFmZmFyNDY3QHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.