- 1Academy of Animal Science and Veterinary Medicine, Qinghai University, Xining, Qinghai, China
- 2Qinghai Provincial Key Laboratory of Adaptive Management on Alpine Grassland, Qinghai University, Xining, Qinghai, China
- 3State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University, Xining, Qinghai, China
- 4College of Animal Science and Technology, Yangzhou University, Yangzhou, Jiangsu, China
- 5Desert Animal Adaptations and Husbandry, Wyler Department of Dryland Agriculture, Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Beer Sheva, Israel
- 6State Key Laboratory of Herbage Improvement and Grassland Agro-ecosystems, College of Ecology, Lanzhou University, Lanzhou, China
Three different herbivore grazing assemblages, namely, yak grazing (YG), Tibetan sheep grazing (SG) and yak and Tibetan sheep co-grazing (MG), are practiced in alpine meadows on the Qinghai-Tibetan Plateau (QTP), but the effects of the different herbivore assemblages on soil microbes are relatively unknown. The microbial community plays an important role in the functional stability of alpine grassland ecosystems. Therefore, it is important to understand how the microbial community structure of grassland ecosystems changes under different herbivore grazing assemblages to ensure their sustainable development. To fill this gap, a field study was carried out to investigate the effects of YG, SG, and MG on plant communities, soil physico-chemical properties and microbial communities under moderate grazing intensity in alpine meadows. Grazing increased the β-diversity of the bacteria community and decreased the β-diversity of the fungal community. The herbivore assemblage affected the microbial community diversity, but not the plant community diversity. Total phosphorus, soil bulk density, root biomass, and plant α-diversity were correlated with both the bacterial and fungal community composition, available phosphorus and soil moisture were correlated only with the bacterial community composition, while available potassium and above-ground net primary production (ANPP) were correlated only with the fungal community composition. Soil available nitrogen, soil available phosphorus and soil bulk density were highest in SG, while ANPP was highest in MG. It was concluded that MG can improve ANPP and stabilize the soil microbial community, suggesting that MG is an effective method for sustainable use and conservation of alpine meadows on the QTP.
1 Introduction
Grazing can alter the grassland ecosystems by affecting the soil physico-chemical properties (Liu et al., 2015; Jordon, 2021). For example, livestock trampling compacts soil to increase soil bulk density and alters soil water-holding capacity (Veldhuis et al., 2014; Byrnes et al., 2018). In addition, faeces and urine from herbivores increase soil nitrogen (N) availability and reduce soil pH (Zhou et al., 2017; Hong et al., 2021).
The changes in soil physico-chemical properties caused by grazing, trampling and faecal and urine deposition directly or indirectly affect the microbial community, which in turn affects grassland ecosystem function (Yang et al., 2013; Liu et al., 2015; Zhang et al., 2020) (Figure S1). In addition, grazing, especially selective grazing by different herbivores (Pan et al., 2019), not only alters the plant community composition and decreases the above-ground biomass, but also increases the below-ground biomass. This promotes an increase in plant root exudates, which include organic substances, polysaccharides and enzymes (Bardgett et al., 1998; Huhe et al., 2017; Gao et al., 2021). These root exudates influence microbial abundance, such as increasing the abundance of pathogenic bacteria, and also drive plant-soil-microbes feedbacks in response to plant needs, where plants can adjust their exudation patterns to tailor microbial recruitment to meet nutrient requirements (Zhao et al., 2021).
The alpine meadows of the Qinghai-Tibetan Plateau (QTP), one of the largest natural grasslands in China, cover an area of approximately 2.27 × 106 hm2, and provide important ecological functions, as well as social services for the inhabitants (Cao et al., 2021). The QTP is also a huge carbon (C) sink, with a total organic C stock of approximately 19.23 Pg C, accounting for 23% of China’s total C pool (Yang et al., 2017). Primary production on the plateau is generally low due to the unfavorable climate and short growing season, and the ecosystem is highly sensitive to external disturbances (Lu et al., 2017). In recent decades, the grasslands have been significantly degraded due to overgrazing, irrational management and global climate change (Harris, 2010; Wu et al., 2014; Xue et al., 2017). Poor grassland management has reduced plant diversity and soil fertility and lessened ecosystem function (Cao et al., 2011; Niu et al., 2019).
Grazing is the most common land use in alpine meadows and crucial for the livelihoods of local people, and is, therefore, essential for maintaining the biodiversity and ecosystem function of alpine meadows (Niu et al., 2016; Lu et al., 2017; Wang et al., 2019). Current research on alpine meadows has focused on the effects of grazing exclusion, grazing intensity and practices (rotational or deferred grazing) on plant community composition, plant productivity, soil respiration and microbes (Chen et al., 2016; Du et al., 2020; Jäschke et al., 2020).
To prevent further degradation of grasslands, the Chinese government has enacted a series of grassland use and protection policies, such as extensive fencing to exclude grazing (Sun et al., 2020). In addition, the government divided the grasslands so that each herder has private pasture for livestock. Some herders graze only Tibetan sheep or yaks, while others co-graze yaks and Tibetan sheep. The herbivore assemblage affects the soil biotic and abiotic properties, which then influences the plant species, bacteria and fungi communities and the grassland ecosystem function (Eldridge et al., 2021; El Mujtar et al., 2021). The key role of microbes in maintaining the sustainability of grassland ecosystems has been widely recognized (Rillig et al., 2019). However, soil microbes have also been identified as a critical factor in the degradation of alpine meadows (Chen et al., 2021). Therefore, understanding how soil microbial communities respond to different herbivore assemblages could provide important information for the management of degraded grasslands. However, there are very few studies on the effects of herbivore assemblages on soil microbial communities. To fill this knowledge gap, we designed a field study to assess: (a) how different herbivore assemblages affect the soil microbial community and composition; and (b) what environmental factors interact with the herbivore assemblage to impact the soil microbial communities. We hypothesize that the herbivore assemblage influences the environmental factors (soil physico-chemical properties and the composition of the plant community). To test this hypothesis, we examined three different herbivore grazing assemblages, namely, yak grazing, Tibetan sheep grazing and yak and Tibetan sheep co-grazing, under moderate grazing intensities to investigate the effects on the soil physico-chemical properties, microbial communities and plant communities on the QTP.
2 Materials and methods
2.1 The study site
This study site was situated in the Xihai Town, Qinghai Province (lat. 36°44′-37°39′ N, long. 100°23′-101°20′ E; 3000 m), in eastern QTP (Figure S2). The climate type is mountain plateau, with an average annual precipitation of 330~370 mm, mainly concentrated in May to September (growing season). The annual average temperature is 1.4°C, with the coldest monthly average temperature of -24.8°C in the non-growing season, and the hottest monthly average temperature of 12.5°C in the growing season. The grassland type is alpine meadows nd the soil type is clay loam. The main vegetation was Kobresia humilis, Elymus nutans, Stipa sareptana, Potentilla acaulis and Leymus secalinus (Yang X. et al., 2019).
2.2 Design of experiments
Grazing plots with different herbivore assemblages were established in June 2014 in an alpine meadow with flat terrain and relatively uniform environment and plant cover. Twelve plots were arranged in a randomized design, with three plots each for yak grazing (YG, 2600 m2), Tibetan sheep grazing (SG, 1700 m2), yak and Tibetan sheep co-grazing as 1:2 mixed grazing (MG, 4300 m2), and grazing exclusion (NG, 500 m2) treatments. Grazing intensity was moderate at 0.5 sheep units/ha, as it was previously reported that above-ground biomass and plant α-diversity were greatest in alpine meadows under moderate grazing intensity (Gao and Carmel, 2020). The yaks (~ 100 kg and 1.5-year-old males) and the Tibetan sheep (~30 kg and 1 year old males) were purchased each year from local herders at the start of the grazing season and were de-wormed prior to the study. The livestock grazed during the growing season (June to October) each year for 7 years, till August 2020, and the forage the grazed plots was 50% - 55%. There was no supplementary feed offered during grazing, but drinking water was available freely.
2.3 Plant and soil sampling
In 2020, three cages (0.7m × 0.7m) spaced more than 10 m apart were randomly placed in each plot before grazing, and the biomass in a 0.25 m2 quadrat in each cage was collected to measure the above-ground net primary productivity (ANPP). At the end of August, all above-ground biomass was harvested within the quadrats in the 36 cages (4 treatments × 3 replicates × 3 cages), and was dried at 85°C to constant weight (Wang et al., 2014). The plant community characteristics was surveyed in three 0.25 m2 random quadrats per plot, and the abundance, height, richness and cover of each species were recorded (Wang et al., 2021). The above-ground parts of the plants were pruned, and were dried at 85°C (24 h) in an oven. The plant species were divided into four functional groups based on plant characteristics: Gramineae, Cyperaceae, Leguminosae and forbs, respectively. Concomitantly, we collected the root biomass in the 0-10 cm (Yang F. et al., 2019).
Surface soil samples (0-10 cm) for DNA extraction and sequencing were collected from the same quadrats used for surveying plant species. Three random soil cores (0-10 cm) were taken in each quadrat using a 7 cm soil auger. The samples from each quadrat were mixed thoroughly to form one composite sample for the determination of relevant indicators. Mixing multiple soil samples reduces errors due to soil heterogeneity (Edwards, 2010), and many studies on soil microbiology and phyisco-chemical properties often use such an approach (Chen et al., 2010; Huhe et al., 2017; Xiao et al., 2020). These 36 soil samples were stored at 4°C and immediately brought back to the laboratory.
2.4 Plant and soil measurements
The importance value (IV) was calculated according to the following formula (Zhang et al., 2016): IV=(RHi + RCi)/2, RH was relative height and RC was relative cover in a community. The composite soil sample was sieved through a 2 mm screen to remove impurities such as stones and debris. The roots were washed and dried at 85°C (48 h) to constant weight to measure root biomass. The soil was then divided into three sub-samples for the determination of different indicators. One sub-sample was air-dried at room temperature (25°C) and used to measure the soil physico-chemical characteristics, the second subsample was kept at 4°C for measurements of soil nitrate () and ammonium () contents, and the third subsample was kept at -80°C for DNA extraction.
We followed the method of Ye to determine the soil total nitrogen (TN), soil total carbon (TC) (Ye et al., 2018). The available phosphorus (AP), available potassium (AK), nitrate (-N), ammonium (-N) contents and soil pH were measured following Huang (Huang et al., 2019). The soil 0-10 cm bulk density (SBD) was measured by the cutting ring (100 cm3) method (Wang et al., 2021). ANPP calculation was based on the above-ground biomass in a quadrat (Zheng et al., 2022).
2.5 High throughput sequencing of soil samples
Five samples were selected randomly from each treatment for microbiological analysis. Sequencing was done by the Gene Denovo Biotechnology Co., Ltd (Guangzhou, China) (Appendix S1).
2.6 Data analyses
Plant community α-diversity was assessed using Shannon, Simpson and species richness indices, and soil microbial community α-diversity was assessed using Sobs, Chao1, Shannon and Simpson indices. One-way ANOVA was used to test for differences among herbivore assemblages for plant and soil physico-chemical variables and relative abundances of different taxa and α-diversity indices (P<0.05). Plant species and soil microbial β-diversity in herbivore assemblages were analyzed by nonmetric multidimensional scaling analysis (NMDS) (Cao et al., 2022). Based on canonical correlation analysis (CCA) using ‘enfit’, the main environmental factors driving changes in the microbial community were identified. Linear discriminant analysis effect size (LEfSe) was used to identify the main taxa driving changes in the microbial community under different treatments (Segata et al., 2011). We identified the most refined classification levels for bacteria (phylum level) and fungi (family level; Figures 2, 3). A structural equation model (SEM) identified the relationships between soil bacterial and fungi β-diversity and environmental factors. All statistical analyses and drawings used R 4.0.2 for Windows and the “vegan”, “psych”, “piecewiseSEM” package.
3 Results
3.1 Effect of herbivore assemblage on plant traits and soil properties
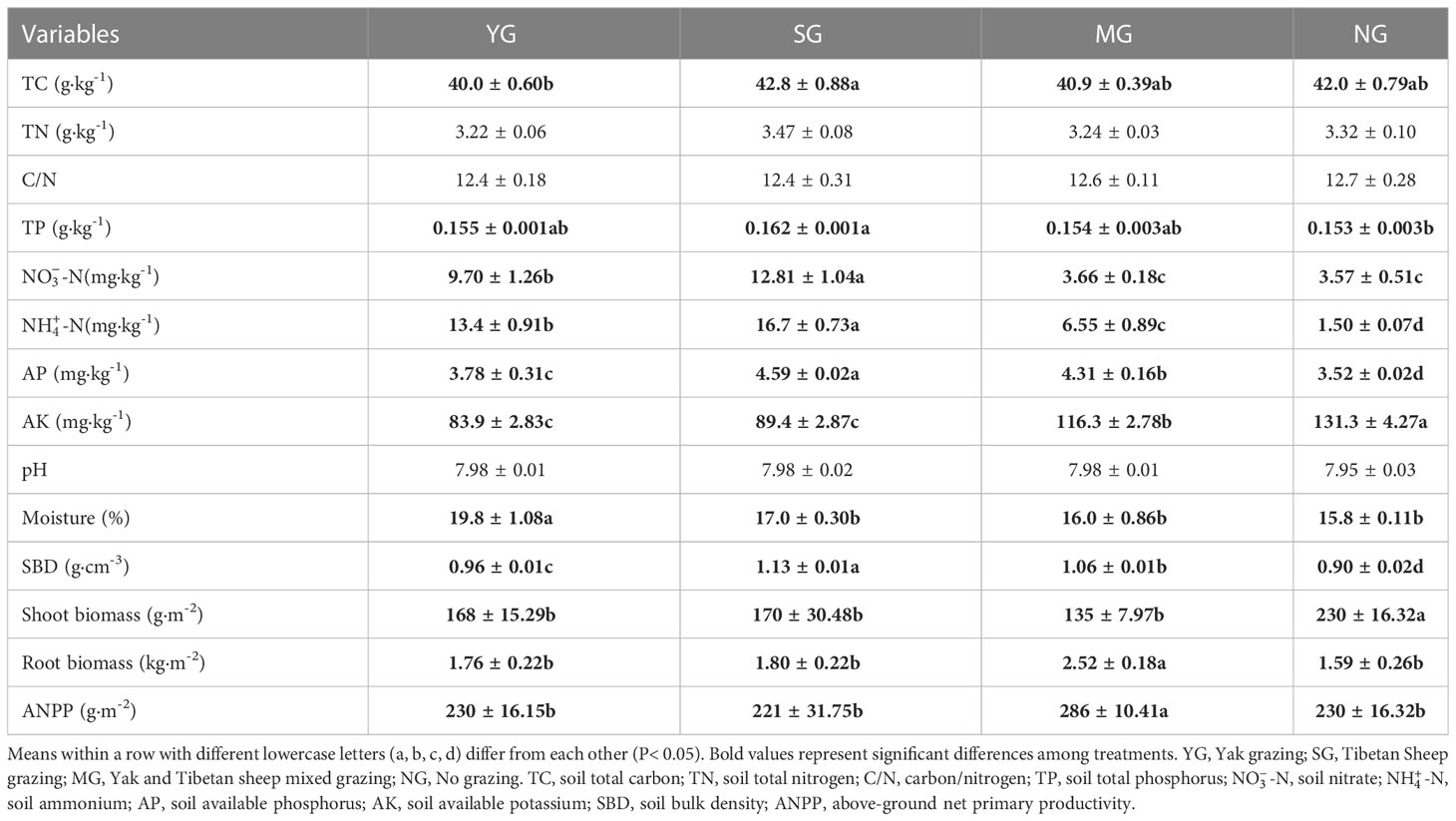
-N, -N and AP contents and SBD were highest in SG (P< 0.05), soil moisture was highest in YG (P< 0.05), root biomass and ANPP were highest in MG (P< 0.05), and AK content and shoot biomass were highest in NG. Herbivore assemblage had no effect on soil TN content, the C:N ratio and pH (Table 1).
3.2 Effect of herbivore assemblage on plant and microbial community compositions
There was no effect of herbivore assemblage on plant α- diversity (P > 0.05). Bacteria α- diversity was highest in SG (P > 0.05), and fungi α- diversity, and the sobs and Chao1 were higher in SG than NG (P > 0.05, Table 2). In addition, the Shannon and Simpson indices were higher in MG than SG (P > 0.05). NMDS analysis revealed that the bacteria and fungi communities differed among herbivore assemblages, but the plant communities did not (Figure S3A; Figure 1).
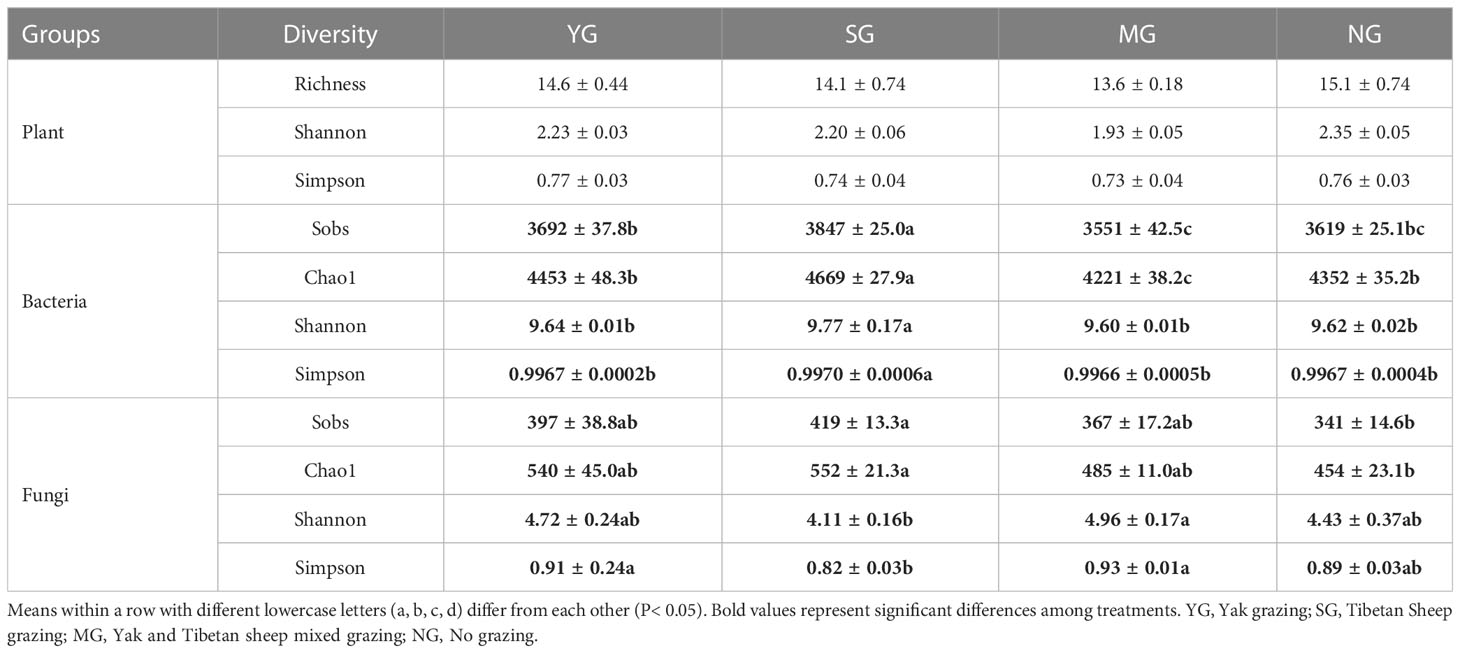
Table 2 α-diversity of plant, bacterial and fungal communities under different herbivore assemblages.

Figure 1 Non-metric multidimensional scaling (NMDS) ordination of all sampling units indicating the relative differences in bacterial (A) and fungal (B) community compositions. YG, Yak grazing; SG, Tibetan Sheep grazing; MG, Yak and Tibetan sheep mixed grazing; NG, No grazing.
At the phylum level, Actinobacteria, Proteobacteria, Acidobacteria, Planctomycetes and Chloroflexi dominated the soil bacterial communities (Figure 2A). Only Chloroflexi did not differ among herbivore assemblages (Figure 2C). At the family level, Clavicipitaceae, Nectriaceae, Pseudeurotiaceae, Saccharomycetaceae and Mortierellaceae dominated the soil fungal communities (Figure 2B). Only Mortierellaceae did not differ among herbivore assemblages, while the relative abundance of Clavicipitaceae was higher in SG than NG (P< 0.05, Figure 2D). Further NMDS analyses of bacteria and fungi that differed significantly in relative abundances among herbivore assemblages revealed significant differences in the β-diversity of bacterial communities, but not in the fungal communities (Figure S4; S5). For all treatments, the phyla that caused the main changes in bacterial communities were Acidobacteria and Proteobacteria. In addition, the phylum that caused the main changes in fungal communities was Ascomycota (Figure 3).
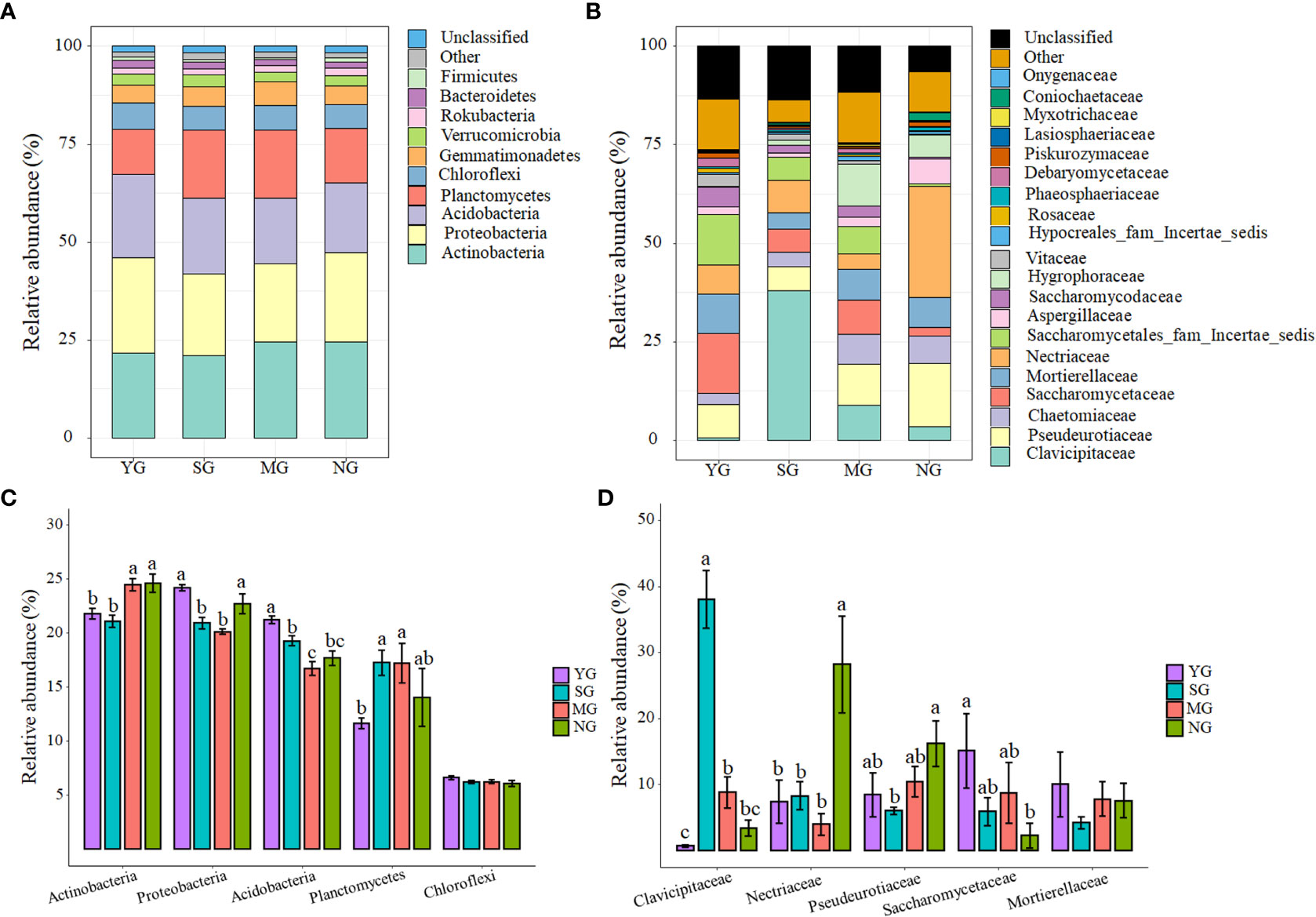
Figure 2 Relative abundances of bacterial phyla under different herbivore assemblages (A); relative abundances of fungal families under different herbivore assemblages (B); dominant bacterial phyla (C); dominant fungal families (D). Significance level: P< 0.05. YG, Yak grazing; SG, Tibetan Sheep grazing; MG, Yak and Tibetan sheep mixed grazing; NG, No grazing.
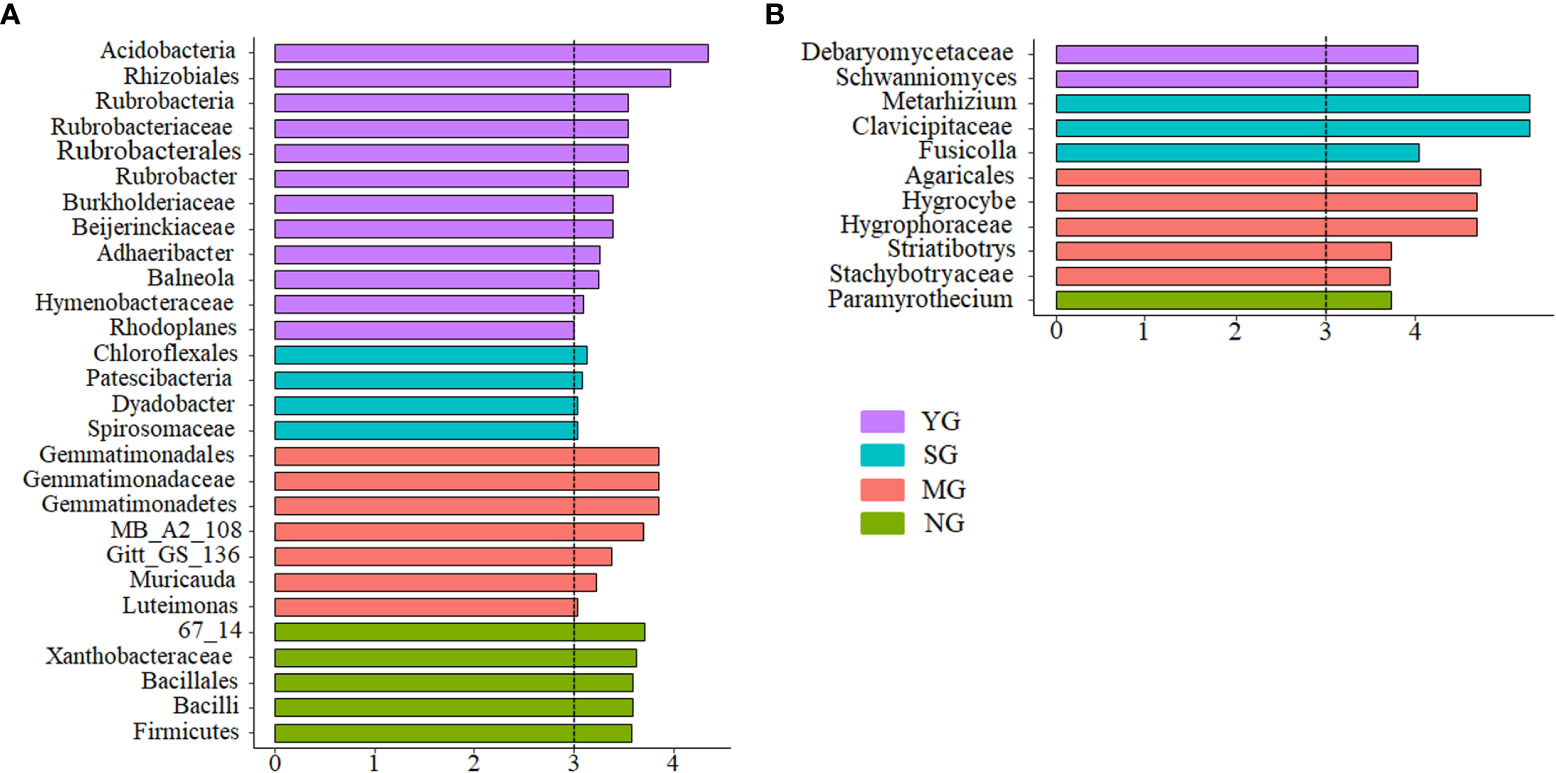
Figure 3 Indicator bacteria with LDA scores of 3 or greater in bacterial communities under different herbivore assemblages (A); indicator fungi with LDA scores of 3 or greater in fungal communities under different herbivore assemblages (B). YG, Yak grazing; SG, Tibetan Sheep grazing; MG, Yak and Tibetan sheep mixed grazing; NG, No grazing.
3.3 Key factors driving changes in microbial communities
The CCA revealed that the bacterial community composition was correlated (P< 0.05) to soil TP, AP, moisture content, SBD, root biomass and plant α diversity (Table S1, Figure 4A). The fungal community composition was correlated (P< 0.05) to TP, -N, -N, AK, SBD, root biomass, ANPP and plant α diversity (Table S1, Figure 4B).
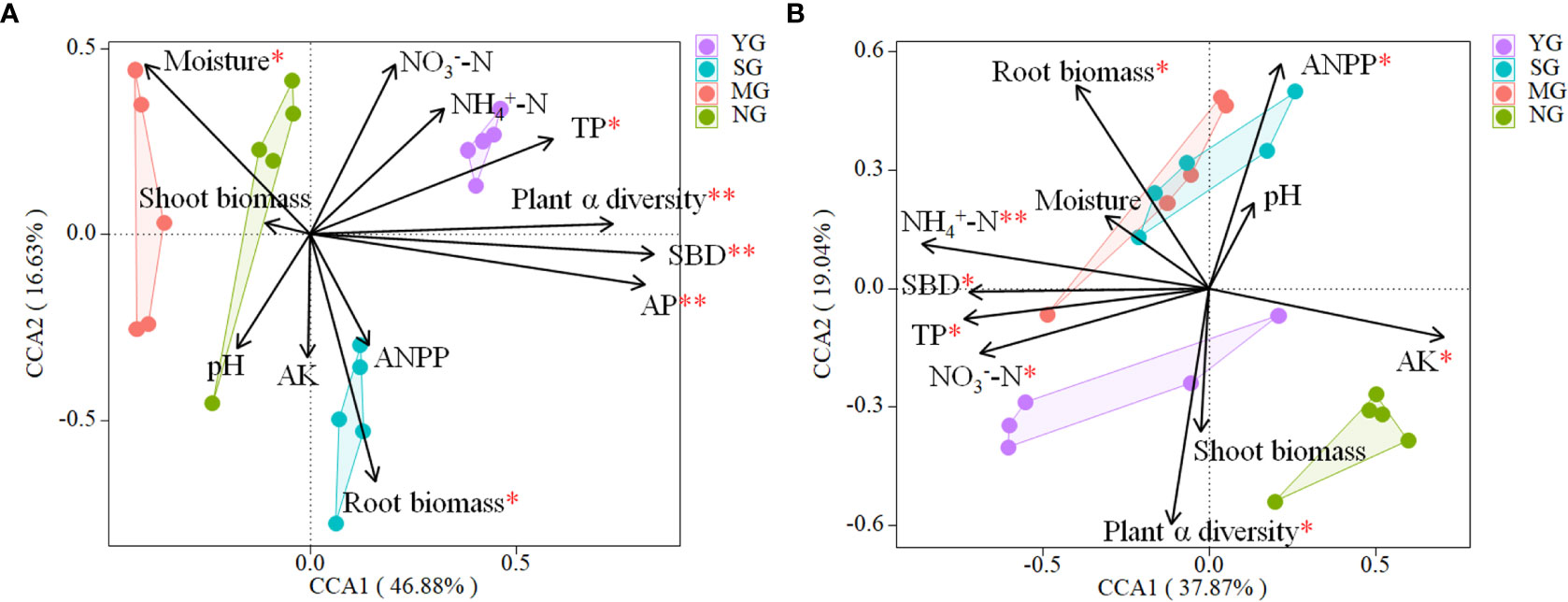
Figure 4 Canonical correspondence analysis using pooled data of bacterial (A) and fungal (B) communities and abiotic and biotic variables (arrows). The values of Axis 1 and 2 are percentages that the corresponding axis can explain. TC, soil total carbon; TN, soil total nitrogen; C/N, carbon/nitrogen; TP, soil total phosphorus; -N, soil nitrate; -N, soil ammonium; AP, soil available phosphorus; AK, soil available potassium; SBD, soil bulk density; ANPP, above-ground net primary productivity. YG, Yak grazing; SG, Tibetan Sheep grazing; MG, Yak and Tibetan sheep mixed grazing; NG, No grazing.
The variables which had significant effects on the microbial community compositions in CCA were selected as predictors to generate a piecewise SEM to identify the key drivers on soil microbial β-diversity. For bacteria, path analysis indicated that grazing, plant α diversity and root biomass had a direct impact on soil bacteria β-diversity, and grazing also increased root biomass and SBD. For fungi, the path analysis indicated that grazing, AK and SBD had direct impacts on soil fungi β-diversity, and grazing increased ANPP, root biomass, -N and SBD, but decreased AK (Figure 5).
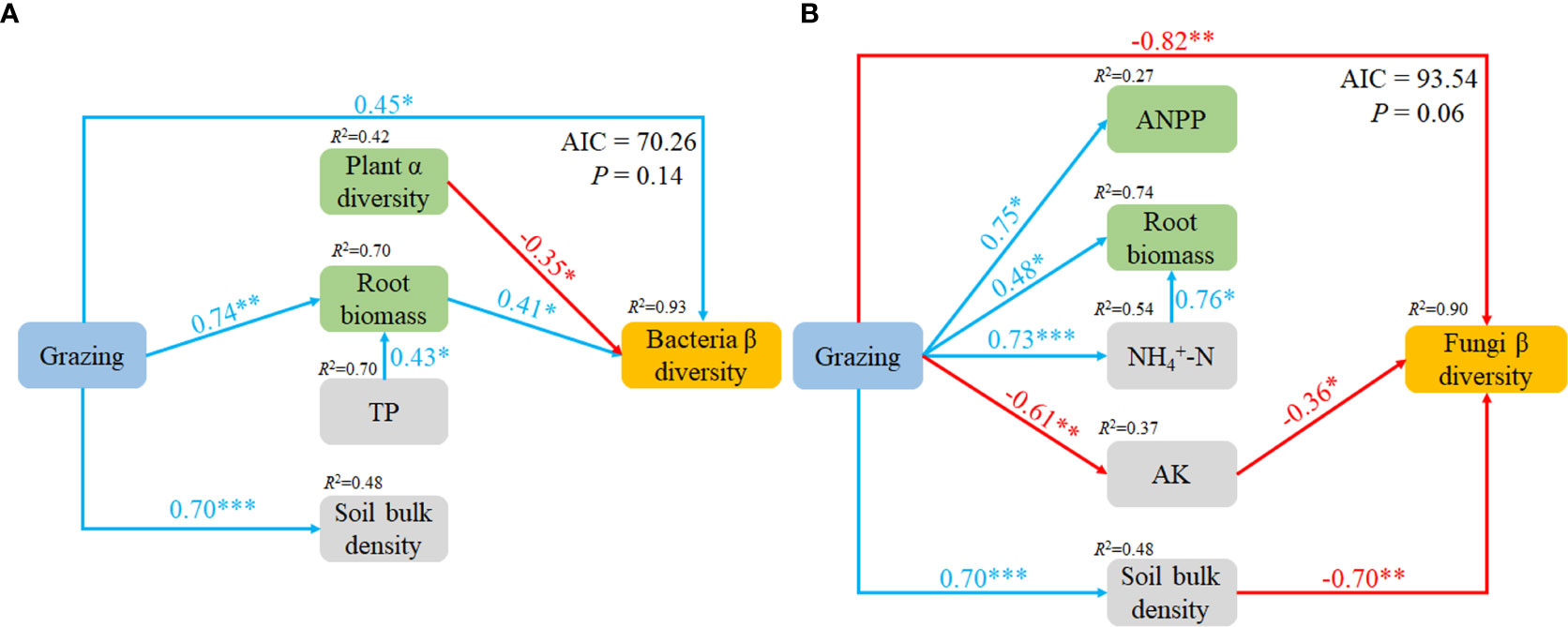
Figure 5 Piecewise structural equation model (SEM) describing the effects of grazing on soil bacteria (A) and fungi (B) β diversity. TP, soil total phosphorus; -N, soil ammonium; AK, soil available potassium.
4 Discussion
4.1 Effect of herbivore assemblage on plant and soil properties
The herbivore assemblage affected soil available nutrients, but not total nutrients. Studies at the eastern edge of the QTP reported similar conclusions (Liu et al., 2022). Soil nitrate, ammonium and AP contents and SBD were highest in SG. A study in the eastern Eurasian steppe reported that soil available N was closely related to the herbivore assemblage, and that the highest available N content was with sheep grazing (Zhang et al., 2020). This was also observed in the current study. The faeces and urine of small herbivores have a relatively high N content and are easily degraded, while those of large herbivores have a relatively low N content and are not easily degraded (Wang et al., 2018). Consequently, this could explain why soil nitrate and ammonium nitrate contents were higher in grazed grassland than in NG grassland, and higher in SG than in YG and MG.
Tibetan sheep graze over a greater area and defaecate more frequently than yaks. Soil AP was highest in SG and soil AK was highest in NG. This may be related to the spatial pattern use of the different livestock (Yamada et al., 2011). Tibetan sheep require better quality forage than yaks (Laca et al., 2010), and, thus, are more selective in their intake. This difference forces sheep to walk over greater distances than yaks in search of high quality forage. The source of soil AP is mainly from mineralization, biofiltration and eluviation (Walker and Syers, 1976), while soil AK is mainly released from the soil parent material during soil formation. The AK is highly soluble and is easily lost due to external disturbances (Wang et al., 2002). The greater movement by Tibetan sheep than yak reduces plant cover, disrupts the soil crust and leads to increased mineralization and eluviation (Zhang et al., 2020). This could explain why the highest content of soil AP and lowest content of soil AK were recorded in SG. In addition, grazing reduced shoot biomass, but both below-ground biomass and ANPP were highest in MG. Grazing can drive plant biomass allocation to below-ground, and when subjected to external stresses, vegetation employs special defense mechanisms to allot more biomass to below-ground (Zhou et al., 2021). In MG, more of the plant species were subjected to foraging stress, as the Tibetan sheep and yak overlapped in many of the plant species consumed, but each also consumed some unique plant species so the biomass allocation to the below-ground was more pronounced. The highest ANPP occurred in MG, which indicates that the plants had a strong compensatory growth at this time.
4.2 Effect of herbivore assemblage on microbial α and β diversity
Grazing can affect soil microbial communities directly by altering resource availability, or indirectly through its effects on plants. In the current study, bacterial α-diversity differed among herbivore assemblages, with the highest in SG, but fungal α-diversity did not differ among herbivore assemblages. This suggests that the bacterial community was more sensitive to herbivore assemblages than the fungal community, which is contrary to the findings of Yang X. et al. (2019), whose research indicated that winter and annual grazing did not affect microbial α-diversity. These conflicting findings could be due to different herbivore assemblages, different grazing intensities and different initial microbial communities.
The β-diversity of both soil bacteria and fungi displayed differences among herbivore assemblages. Further analysis of the top 4 microbes in terms of relative abundances revealed that the β-diversity of bacteria differed among treatments, whereas the fungi did not. These results indicate that the difference in bacterial β-diversity was due to the dominant species, whereas in fungi was due to rare species. Theoretically, different plant characteristics and soil environments can create different niches (Hamid et al., 2021), and, consequently, affect specific soil microbial communities (Pot et al., 2022). In the current study, bacterial β-diversity was driven mainly by plant α-diversity and root biomass, but fungal β-diversity was driven mainly by AK and SBD. Some of the differences could be due to the selective intake by the herbivores and excrement which can affect plant and soil properties and can cause the different responses of microbial communities to grazing. The different responses of microbial communities to the presence of herbivores warrant further investigation.
4.3 Effect of herbivore assemblage on microbial community composition
Herbivore assemblage had a greater effect on soil microbial β-diversity than soil microbial α-diversity, indicating that microbial β-diversity is more sensitive to herbivore assemblage than microbial α-diversity. Another study on the effects of grazing on alpine grasslands came to similar conclusions (Cao et al., 2022). Soil nutrient contents, soil moisture content, SBD, root biomass and plant α-diversity were the main drivers of soil microbial community composition for herbivore assemblages, which is consistent with several studies under different grazing intensities (Yang F. et al., 2019; Cao et al., 2021; Wang et al., 2021) and may be due to: (1) soil microbes are sensitive to soil nutrient and moisture availability, particularly due to nutrient changes caused by urine and manure from grazing livestock, resulting in a shift in soil microbial communities from one dominant species to another (Levy-Booth et al., 2016); and (2) feedback loops between plant communities, soil and microbial communities change markedly under different herbivore assemblages (Rigg et al., 2016). Furthermore, litter deposition and changes in SBD by livestock affect soil permeability, which in turn affects the composition of soil microbial communities (Che et al., 2020).
5 Conclusions
Different herbivore assemblages directly or indirectly influence changes in microbial community structure through their selective foraging, trampling and excreta return pathways, which in turn affect the stability of grassland ecosystem structure and function. The results of this study show that grazing increased bacterial community β-diversity and decreased fungal community β-diversity. Soil available nitrogen (AN), available phosphorus (AP) and soil bulk density (SBD) were highest in SG, while ANPP was highest in MG. YG and SG reduced the relative abundance of Actinobacteria among bacteria, while SG increased the relative abundance of Clavicipitaceae among fungi. From a management perspective, the results of this study indicate that MG can improve ANPP and is less damaging to the soil microbial community and its functions than SG and YG. Therefore, co-grazing Tibetan sheep and yaks should be considered as a viable option for sustainable development of alpine meadows on the QTP.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
QD and WL conceived the ideas and designed the study; YL, BF, QC, SS, CZ, XY, and YY collected the data; YL and XZ analyzed the data; YL wrote and revised the draft; and AD and ZS revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Basic Research Innovation-Team Program of Qinghai Provincial Science Foundation (2021-ZJ-901), the Joint Funds of the National Natural Science Foundation of China (U20A2007) and the National Key R&D Program of China (2021YFD1300504).
Acknowledgments
The authors are grateful to the Platform of the Adaptive Management of the Alpine Grassland-livestock System Base of Qinghai University for supporting the field work.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1117372/full#supplementary-material
References
Bardgett, R. D., Wardle, D. A., Yeates, G. W. (1998). Linking above-ground and below-ground interactions: How plant responses to foliar herbivory influence soil organisms. Soil Biol. Biochem. 30 (14), 1867–1878. doi: 10.1016/S0038-0717(98)00069-8
Byrnes, R. C., Eastburn, D. J., Tate, K. W., Roche, L. M. (2018). A global meta-analysis of grazing impacts on soil health indicators. J. Environ. Qual. 47 (4), 758–765. doi: 10.2134/jeq2017.08.0313
Cao, J., Holden, N. M., Lü, X. T., Du, G. (2011). The effect of grazing management on plant species richness on the qinghai-tibetan plateau. Grass Forage Sci. 66 (3), 333–336. doi: 10.1111/j.1365-2494.2011.00793.x
Cao, J., Jiao, Y., Che, R., Holden, N. M., Zhang, X., Biswas, A., et al. (2022). The effects of grazer exclosure duration on soil microbial communities on the qinghai-tibetan plateau. Sci. Total Environ. 839, 156238. doi: 10.1016/j.scitotenv.2022.156238
Cao, J., Wang, H., Holden, N. M., Adamowski, J. F., Biswas, A., Zhang, X., et al. (2021). Soil properties and microbiome of annual and perennial cultivated grasslands on the qinghai–tibetan plateau. Land Degrad. Dev. 32 (18), 5306–5321. doi: 10.1002/ldr.4110
Che, R., Liu, D., Qin, J., Wang, F., Wang, W., Xu, Z., et al. (2020). Increased litter input significantly changed the total and active microbial communities in degraded grassland soils. J. Soils Sediments 20 (7), 2804–2816. doi: 10.1007/s11368-020-02619-x
Chen, L., Jiang, L., Jing, X., Wang, J., Shi, Y., Chu, H., et al. (2021). Above- and belowground biodiversity jointly drive ecosystem stability in natural alpine grasslands on the tibetan plateau. Global Ecol. Biogeogr. 30 (7), 1418–1429. doi: 10.1111/geb.13307
Chen, D. D., Zhang, S. H., Dong, S. K., Wang, X. T., Du, G. Z. (2010). Effect of land-use on soil nutrients and microbial biomass of an alpine region on the northeastern tibetan plateau, china. Land Degrad. Dev. 21 (5), 446–452. doi: 10.1002/ldr.990
Chen, J., Zhou, X., Wang, J., Hruska, T., Shi, W., Cao, J., et al. (2016). Grazing exclusion reduced soil respiration but increased its temperature sensitivity in a meadow grassland on the tibetan plateau. Ecol. Evol. 6 (3), 675–687. doi: 10.1002/ece3.1867
Du, Y., Ke, X., Dai, L., Cao, G., Zhou, H., Guo, X. (2020). Moderate grazing increased alpine meadow soils bacterial abundance and diversity index on the tibetan plateau. Ecol. Evol. 10 (16), 8681–8687. doi: 10.1002/ece3.6563
Edwards, A. C. (2010). Soil sampling and sample preparation. Trace elem. soils, 39–51. doi: 10.1002/9781444319477.ch3
Eldridge, D. J., Travers, S. K., Val, J., Ding, J., Wang, J.-T., Singh, B. K., et al. (2021). Experimental evidence of strong relationships between soil microbial communities and plant germination. J. Ecol. 109 (6), 2488–2498. doi: 10.1111/1365-2745.13660
El Mujtar, V. A., Gregorutti, V. C., Eclesia, R. P., Wingeyer, A., Lezana, L., Canavelli, S. B., et al. (2021). Assessing soil microbial biodiversity as affected by grazing and woody vegetation cover in a temperate savannah. Ann. Appl. Biol. 179 (2), 231–245. doi: 10.1111/aab.12695
Gao, J., Carmel, Y. (2020). Can the intermediate disturbance hypothesis explain grazing–diversity relations at a global scale? Oikos 129 (4), 493–502. doi: 10.1111/oik.06338
Gao, X., Dong, S., Xu, Y., Fry, E. L., Li, Y., Li, S., et al. (2021). Plant biomass allocation and driving factors of grassland revegetation in a qinghai-tibetan plateau chronosequence. Land Degrad. Dev. 32 (4), 1732–1741. doi: 10.1002/ldr.3819
Hamid, M., Khuroo, A. A., Malik, A. H., Ahmad, R., Singh, C. P. (2021). Elevation and aspect determine the differences in soil properties and plant species diversity on himalayan mountain summits. Ecol. Res. 36 (2), 340–352. doi: 10.1111/1440-1703.12202
Harris, R. B. (2010). Rangeland degradation on the qinghai-tibetan plateau: A review of the evidence of its magnitude and causes. J. Arid Environ. 74 (1), 1–12. doi: 10.1016/j.jaridenv.2009.06.014
Hong, J., Xu, X., Pang, B., Ma, X., Wang, X. (2021). Significant soil acidification caused by grazing exclusion across china’s grassland areas. Land Degrad. Dev. 32 (2), 535–545. doi: 10.1002/ldr.3722
Huang, X., Su, J., Li, S., Liu, W., Lang, X. (2019). Functional diversity drives ecosystem multifunctionality in a pinus yunnanensis natural secondary forest. Sci. Rep. 9 (1), 6979. doi: 10.1038/s41598-019-43475-1
Huhe, X., Hou, F., Wu, Y., Cheng, Y. (2017). Bacterial and fungal community structures in loess plateau grasslands with different grazing intensities. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00606
Jäschke, Y., Heberling, G., Wesche, K. (2020). Environmental controls override grazing effects on plant functional traits in tibetan rangelands. Funct. Ecol. 34 (3), 747–760. doi: 10.1111/1365-2435.13492
Jordon, M. W. (2021). Does mixed vs separate sheep and cattle grazing reduce soil compaction? Soil Use Manage. 37 (4), 822–831. doi: 10.1111/sum.12659
Laca, E. A., Sokolow, S., Galli, J. R., Cangiano, C. A. (2010). Allometry and spatial scales of foraging in mammalian herbivores. Ecol. Lett. 13 (3), 311–320. doi: 10.1111/j.1461-0248.2009.01423.x
Levy-Booth, D. J., Prescott, C. E., Christiansen, J. R., Grayston, S. J. (2016). Site preparation and fertilization of wet forests alter soil bacterial and fungal abundance, community profiles and co2 fluxes. For. Ecol. Manage. 375, 159–171. doi: 10.1016/j.foreco.2016.05.033
Liu, N., Kan, H. M., Yang, G. W., Zhang, Y. J. (2015). Changes in plant, soil, and microbes in a typical steppe from simulated grazing: Explaining potential change in soil c. Ecol. Monogr. 85 (2), 269–286. doi: 10.1890/14-1368.1
Liu, Y., Sun, C., Liu, W., Yang, X., Feng, B., Shi, G., et al. (2022). Response of keystone species changes in alpine grasslands plant communities to different herbivore assemblage grazing. Acta Ecol. Sin. 42 (18), 7529–7540. doi: 10.5846/stxb202110072757
Lu, X., Kelsey, K. C., Yan, Y., Sun, J., Wang, X., Cheng, G., et al. (2017). Effects of grazing on ecosystem structure and function of alpine grasslands in qinghai–tibetan plateau: A synthesis. Ecosphere 8 (1), e01656. doi: 10.1002/ecs2.1656
Niu, K., He, J.-S., Lechowicz, M. J. (2016). Grazing-induced shifts in community functional composition and soil nutrient availability in tibetan alpine meadows. J. Appl. Ecol. 53 (5), 1554–1564. doi: 10.1111/1365-2664.12727
Niu, Y., Zhu, H., Yang, S., Ma, S., Zhou, J., Chu, B., et al. (2019). Overgrazing leads to soil cracking that later triggers the severe degradation of alpine meadows on the tibetan plateau. Land Degrad. Dev. 30 (10), 1243–1257. doi: 10.1002/ldr.3312
Pan, D., Li, X., De, K., Wang, L., Wang, D., Guo, Q., et al. (2019). Food and habitat provisions jointly determine competitive and facilitative interactions among distantly related herbivores. Funct. Ecol. 33 (12), 2381–2390. doi: 10.1111/1365-2435.13456
Pot, V., Portell, X., Otten, W., Garnier, P., Monga, O., Baveye, P. C. (2022). Understanding the joint impacts of soil architecture and microbial dynamics on soil functions: Insights derived from microscale models. Eur. J. Soil Sci. 73 (3), e13256. doi: 10.1111/ejss.13256
Rigg, J. L., Offord, C. A., Singh, B. K., Anderson, I., Clarke, S., Powell, J. R. (2016). Soil microbial communities influence seedling growth of a rare conifer independent of plant–soil feedback. Ecology 97 (12), 3346–3358. doi: 10.1002/ecy.1594
Rillig, M. C., Ryo, M., Lehmann, A., Aguilar-Trigueros, C. A., Yang, G. (2019). The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 366 (6467), 886–890. doi: 10.1126/SCIENCE.AAY2832
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12 (6), R60. doi: 10.1186/gb-2011-12-6-r60
Sun, J., Liu, M., Fu, B., Kemp, D., Zhao, W., Liu, G., et al. (2020). Reconsidering the efficiency of grazing exclusion using fences on the tibetan plateau. Sci. Bull. 65 (16), 1405–1414. doi: 10.1016/j.scib.2020.04.035
Veldhuis, M. P., Howison, R. A., Fokkema, R. W., Tielens, E., Olff, H. (2014). A novel mechanism for grazing lawn formation: Large herbivore-induced modification of the plant–soil water balance. J. Ecol. 102 (6), 1506–1517. doi: 10.1111/1365-2745.12322
Walker, T. W., Syers, J. K. (1976). The fate of phosphorus during pedogenesis. Geoderma 15 (1), 1–19. doi: 10.1016/0016-7061(76)90066-5
Wang, Z., Hou, X., Schellenberg, M. P., Qin, Y., Yun, X., Wei, Z., et al. (2014). Different responses of plant species to deferment of sheep grazing in a desert steppe of inner mongolia, china. Rangeland J. 36 (6), 583–592. doi: 10.1016/S2095-3119(13)60660-7
Wang, J., Li, W., Cao, W., Atio Abalori, T., Liu, Y., Xin, Y., et al. (2021). Soil bacterial community responses to short-term grazing exclusion in a degraded alpine shrubland – grassland ecotone. Ecol. Indic. 130, 108043. doi: 10.1016/j.ecolind.2021.108043
Wang, J., Wang, D., Li, C., Seastedt, T. R., Liang, C., Wang, L., et al. (2018). Feces nitrogen release induced by different large herbivores in a dry grassland. Ecol. Appl. 28 (1), 201–211. doi: 10.1002/eap.1640
Wang, D.-B., Wang, X.-Y., Wu, Y., Lin, H.-L. (2019). Grazing buffers the effect of climate change on the species diversity of seedlings in an alpine meadow on the tibetan plateau. Ecol. Evol. 9 (3), 1119–1126. doi: 10.1002/ece3.4799
Wang, S., Zhou, G., Lv, Y., Zou, J. (2002). Distribution of soil carbon, nitrogen and phosphorus along northeast chaina transect (nect) and their relationships with climate factors. Chin. J. Plant Ecol. 26 (5), 513–517.
Wu, G.-L., Ren, G.-H., Dong, Q.-M., Shi, J.-J., Wang, Y.-L. (2014). Above- and belowground response along degradation gradient in an alpine grassland of the qinghai-tibetan plateau. CLEAN – Soil Air Water 42 (3), 319–323. doi: 10.1002/clen.201200084
Xiao, Y., Li, C., Yang, Y., Peng, Y., Yang, Y., Zhou, G. (2020). Soil fungal community composition, not assembly process, was altered by nitrogen addition and precipitation changes at an alpine steppe. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.579072
Xue, X., You, Q., Peng, F., Dong, S., Duan, H. (2017). Experimental warming aggravates degradation-induced topsoil drought in alpine meadows of the qinghai–tibetan plateau. Land Degrad. Dev. 28 (8), 2343–2353. doi: 10.1002/ldr.2763
Yamada, D., Higashiyama, M., Yamaguchi, M., Shibuya, T., Shindo, K., Tejima, S. (2011). Spatial distribution of cattle dung excretion and dung nutrients on a sloping pasture. Japan. J. Grassland Sci. 57 (3), 129–135. doi: 10.14941/grass.57.129
Yang, X., Dong, Q., Chu, H., Ding, C., Yu, Y., Zhang, C., et al. (2019). Different responses of soil element contents and their stoichiometry (c:N:P) to yak grazing and tibetan sheep grazing in an alpine grassland on the eastern qinghai – tibetan plateau’. Agricult. Ecosyst. Environ. 285, 106628. doi: 10.1016/j.agee.2019.106628
Yang, F., Niu, K., Collins, C. G., Yan, X., Ji, Y., Ling, N., et al. (2019). Grazing practices affect the soil microbial community composition in a tibetan alpine meadow. Land Degrad. Dev. 30 (1), 49–59. doi: 10.1002/ldr.3189
Yang, G., Peng, C., Chen, H., Dong, F., Wu, N., Yang, Y., et al. (2017). Qinghai–tibetan plateau peatland sustainable utilization under anthropogenic disturbances and climate change. Ecosyst. Health Sustain. 3 (3), e01263. doi: 10.1002/ehs2.1263
Yang, Y., Wu, L., Lin, Q., Yuan, M., Xu, D., Yu, H., et al. (2013). Responses of the functional structure of soil microbial community to livestock grazing in the tibetan alpine grassland. Global Change Biol. 19 (2), 637–648. doi: 10.1111/gcb.12065
Ye, C., Chen, D., Hall, S. J., Pan, S., Yan, X., Bai, T., et al. (2018). Reconciling multiple impacts of nitrogen enrichment on soil carbon: Plant, microbial and geochemical controls. Ecol. Lett. 21 (8), 1162–1173. doi: 10.1111/ele.13083
Zhang, Y., Dong, S., Gao, Q., Liu, S., Liang, Y., Cao, X. (2016). Responses of alpine vegetation and soils to the disturbance of plateau pika (ochotona curzoniae) at burrow level on the qinghai–tibetan plateau of china. Ecol. Eng. 88, 232–236. doi: 10.1016/j.ecoleng.2015.12.034
Zhang, M., Li, G., Liu, B., Liu, J., Wang, L., Wang, D. (2020). Effects of herbivore assemblage on the spatial heterogeneity of soil nitrogen in eastern eurasian steppe. J. Appl. Ecol. 57 (8), 1551–1560. doi: 10.1111/1365-2664.13655
Zhao, M., Zhao, J., Yuan, J., Hale, L., Wen, T., Huang, Q., et al. (2021). Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 44 (2), 613–628. doi: 10.1111/pce.13928
Zheng, S., Chi, Y., Yang, X., Li, W., Lan, Z., Bai, Y. (2022). Direct and indirect effects of nitrogen enrichment and grazing on grassland productivity through intraspecific trait variability. J. Appl. Ecol. 59 (2), 598–610. doi: 10.1111/1365-2664.14078
Zhou, T., Sun, J., Zong, N., Hou, G., Shi, P. (2021). Community species diversity mediates the trade-off between aboveground and belowground biomass for grasses and forbs in degraded alpine meadow, tibetan plateau. Ecol. Evol. 11 (19), 13259–13267. doi: 10.1002/ece3.8048
Keywords: herbivore assemblage, soil microbial community, plant community, Qinghai-Tibetan Plateau, β-diversity of soil microbes
Citation: Liu Y, Zhao X, Liu W, Yang X, Feng B, Zhang C, Yu Y, Cao Q, Sun S, Degen AA, Shang Z and Dong Q (2023) Herbivore assemblages affect soil microbial communities by altering root biomass and available nutrients in an alpine meadow. Front. Plant Sci. 14:1117372. doi: 10.3389/fpls.2023.1117372
Received: 06 December 2022; Accepted: 14 February 2023;
Published: 02 March 2023.
Edited by:
James Millner, Massey University, New ZealandReviewed by:
Yanfu Bai, Sichuan Agricultural University, ChinaYuanming Xiao, Northwest Institute of Plateau Biology (CAS), China
Copyright © 2023 Liu, Zhao, Liu, Yang, Feng, Zhang, Yu, Cao, Sun, Degen, Shang and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quanmin Dong, cW1kb25nQHFodS5lZHUuY24=
 Yuzhen Liu1,2
Yuzhen Liu1,2 Wenting Liu
Wenting Liu Quan Cao
Quan Cao Shengnan Sun
Shengnan Sun A. Allan Degen
A. Allan Degen Zhanhuan Shang
Zhanhuan Shang Quanmin Dong
Quanmin Dong