
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci. , 26 January 2023
Sec. Plant Metabolism and Chemodiversity
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1116894
This article is part of the Research Topic Metabolic Architecture of Developing Seeds and Grains View all 7 articles
The world’s population is projected to increase by two billion by 2050, resulting in food and energy insecurity. Oilseed crops have been identified as key to address these challenges: they produce and store lipids in the seeds as triacylglycerols that can serve as a source of food/feed, renewable fuels, and other industrially-relevant chemicals. Therefore, improving seed oil content and composition has generated immense interest. Research efforts aiming to unravel the regulatory pathways involved in fatty acid synthesis and to identify targets for metabolic engineering have made tremendous progress. This review provides a summary of the current knowledge of oil metabolism and discusses how photochemical activity and unconventional pathways can contribute to high carbon conversion efficiency in seeds. It also highlights the importance of 13C-metabolic flux analysis as a tool to gain insights on the pathways that regulate oil biosynthesis in seeds. Finally, a list of key genes and regulators that have been recently targeted to enhance seed oil production are reviewed and additional possible targets in the metabolic pathways are proposed to achieve desirable oil content and quality.
Depending on plant species, seeds accumulate various proportions of biomass components, such as proteins, starch and lipids. In seeds, storage oils are mainly in the form of triacylglycerols (TGs), as an energy reserve utilized during germination and post-germinative growth. These oilseeds have a profound agricultural and industrial significance, utilized predominantly in food processing and preparation, and as a renewable resource for various industrial applications (Jaworski and Cahoon, 2003). Because of their structural similarity with long-chain hydrocarbons, TGs can replace petroleum-based products, such as diesel, lubricants, paints, coatings or inks (Cahoon et al., 2007; Durrett et al., 2008). The renewable biofuels derived from oilseeds produce ~85% less carcinogens during combustion than petroleum-based diesel fuels, presenting advantages in terms of sustainability, environment and health (Atadashi et al., 2012; Hasan and Rahman, 2017). Because of its popularity as a renewable resource, the consumption of seed oil has been increasing simultaneously with the rapidly growing population and upgraded standards of living (Marchive et al., 2014; Samarth and Mahanwar, 2015). To meet these rising demands, there is an urgent need to develop new oilseed cultivars with improved oil content and composition (Singer et al., 2013; Xu et al., 2018). Many research efforts have been focused into the improvement of seed oil over the years using conventional or molecular-assisted breeding approaches, as well as more targeted genetic manipulation.
The selection of candidate genes that can be engineered relies on understanding the biochemical regulations that control carbon partitioning during de novo fatty acid synthesis (FAS) in seeds. Biosynthesis of TGs starts from FAS in plastids which relies on a cycle of condensation, reduction and dehydration reactions that extend an acyl-chain linked to an acyl carrier protein (ACP) by two carbon units per cycle. These fatty acids (FAs) are then assembled into TGs in the endoplasmic reticulum (ER), or used in other metabolic processes, such as chain elongation and acyl editing. The carbon precursor for FAS is acetyl-CoA, which is generated from the oxidative decarboxylation of pyruvate through the pyruvate dehydrogenase complex (Johnston et al., 1997). Determining the efficiency of the developing embryo in converting this carbon source into oil and other biomass components (e.g. proteins, and carbohydrates) known as carbon conversion efficiency (CCE), is important to evaluate the potential for improving seed oil quality (Goffman et al., 2005; Alonso et al., 2007; Allen et al., 2009; Lonien and Schwender, 2009; Chen and Shachar-Hill, 2012; Cocuron et al., 2019; Carey et al., 2020; Tsogtbaatar et al., 2020). CCE results from the sum of all catabolic and anabolic metabolic processes which varies in developing embryos from different oilseeds, classified as “green” or “non-green”, depending on the presence or absence of chlorophyll during seed filling (Goffman et al., 2005; Allen et al., 2009; Lonien and Schwender, 2009; Carey et al., 2020; Tsogtbaatar et al., 2020). However, to decipher the biochemical pathways underlying the CCE in each species, a more quantitative analysis of the carbon fluxes through the central metabolism—which conducts the vast majority of biochemical carbon transformation—is needed. Steady state metabolic flux analysis (MFA) has been useful to gain a quantitative assessment of the carbon flux through central metabolism based on 13C-labeling, which may guide genetic engineering (Libourel & Shachar-Hill, 2008; Lee et al., 2011; Chen & Shachar-Hill, 2012; Kim et al., 2012; Kruger et al., 2012; O’Grady et al., 2012; Shachar-Hill, 2013). In parallel, several strategies have been employed to genetically manipulate FA composition and plant lipid metabolism in order to increase the FA content in oilseeds. To this end, the “push, pull, package, and protect” strategy has been implemented at various degrees; it consists of manipulating the expression of genes to boost the synthesis of FAs (“push”), or increase TG assembly reactions (“pull”), or improve the storage of FAs into lipid droplets (LDs) (“package”), or prevent the degradation of stored lipids (“protect”), or any combination of these.
This review focuses on FAS in seeds, highlighting the limitation and challenges involved in performing earlier experiments with isolated plastids and the relevance of 13C-MFA to decipher the pathways that contribute to oil biosynthesis, discussing how photochemical activity and unconventional pathways may contribute to higher efficiency of carbon conversion, assessing key genes and regulators that have been recently targeted to enhance seed oil content, and proposing alternative targets/strategies to achieve desirable oil content and quality.
The schematic mechanism of FAS and FA elongation for green and non-green embryos, and the possible sources of carbon precursors, energy, and reductants, are depicted in Figure 1. The disaccharide sucrose represents the major form in which photosynthetically assimilated carbon is transported into oil seeds. The hexose phosphates generated by the cleavage of sucrose can be metabolized through the glycolysis and the oxidative pentose phosphate pathway (OPPP) which can be found both in the cytosol and in the plastids. A major route for carbon going into FAS may involve the cytosolic glycolytic pathway until phosphoenolpyruvate (PEP) and pyruvate, which may be imported into the plastid and undergo decarboxylation to form acetyl-coenzyme A (acetyl-CoA) via the plastidic pyruvate dehydrogenase complex. Acetyl-CoA carboxylase (ACCase) is the first committed step for the FAS: it uses energy to carboxylate acetyl-CoA into malonyl-CoA which is transferred to the Acyl Carrier Protein (ACP) by the malonyl-CoA-ACP transacylase. Then, malonyl-ACP is condensed with acetyl-CoA by the 3-ketoacyl-ACP synthase III (KAS III), generating 3-ketobutyryl-ACP. The 3-ketoacyl-ACP reductase utilizes NADPH to reduce 3-ketobutyryl-ACP into 3-hydroxybutyryl-ACP which is dehydrated by 3-hydroxyacyl-ACP dehydratase to form trans-Δ2-butenoyl-ACP. The reduction of the double bond uses NAD(P)H to convert trans-Δ2-butenoyl-ACP into butyryl-ACP which in then condensed with malonyl-CoA by the 3-ketoacyl-ACP synthase I (KAS I) to generate 3-ketoacyl-ACP. This cycle is repeated to elongate saturated FA chains till 16:0-ACP, and then, the 3-ketoacyl-ACP synthase II (KAS II) performs the last elongation step to synthesize 18:0-ACP. The stearoyl-ACP Δ9-desaturase desaturates 18:0-ACP into 18:1-ACP. Plastidic de novo FAS ends when the FA thioesterase removes the ACP group from acyl backbones.
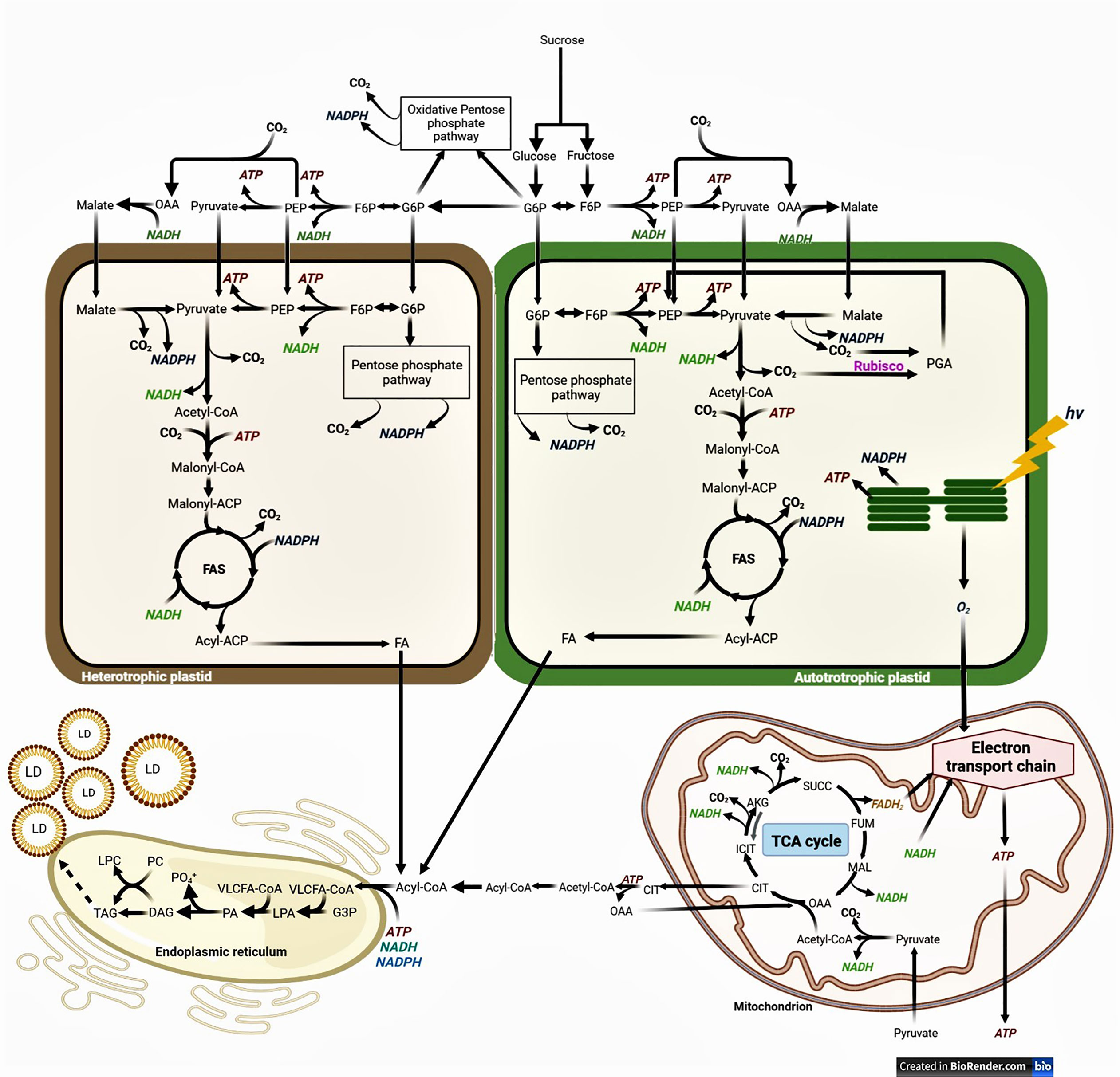
Figure 1 Simplified schematic biochemical pathway of fatty acid and TG synthesis in higher plants. Fatty acid synthesis (FAS) and lipid droplet (LD) formation in heterotrophic and in autotrophic embryos. ACP, acyl carrier protein; AKG, α-ketoglutarate; CIT, citrate; CoA, coenzyme A; DAG, diacylglycerol; FA, fatty acid; F6P, fructose 6-phosphate; FUM, fumarate; G6P, glucose 6-phosphate; hv, light; ICIT, isocitrate; LD, lipid droplet; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; MAL, malate; OAA, oxaloacetate; PA, phosphatidic acid; PC, phosphatidylcholine; PGA, phosphoglycerate; PEP, phosphoenolpyruvate; PGA, phosphoglycerate; SUCC, succinate; TCA, tricarboxylic acid; TG, triacylglycerol; VLCFA, very-long-chain fatty acid.
Beyond the complex network defined by interconnected OPPP and glycolytic pathways, additional metabolic routes may supply precursors for de novo FAS in the plastid (Figure 1). During the conversion of pyruvate into acetyl-CoA, there is a concomitant loss of the carboxylic group of the pyruvate (released as CO2) which has a significant impact on the CCE of developing embryos. In autotrophic plastids of green embryos, ribulose-1,5-bisphophate carboxylase/oxygenase (Rubisco) is able to re-fix the released CO2 apart from the Calvin cycle. This “Rubisco shunt” involves the conversion of hexose-phosphates and triose-phosphates to ribulose-1,5-bisphosphate (RuBP) by the non-oxidative reactions of the OPPP, and the subsequent fixation of CO2 onto RuBP by Rubisco and cleavage in two 3-phosphoglycerate (PGA) (Schwender et al., 2004). PGA can be further metabolized to pyruvate and then to FAs via acetyl-CoA. This metabolic route offsets the loss of CO2 at reactions, such as the OPPP and pyruvate dehydrogenase, thus increasing the CCE in developing embryos. Another route for the synthesis of plastidial pyruvate (and then acetyl-CoA) is through decarboxylation of imported malate by the plastidial NADP-dependent malic enzyme (pNADP-ME) (Smith et al., 1992; Shearer et al., 2004). Supply of malate relies on either the translocation of mitochondrial malate or the carboxylation of PEP into oxaloacetate (OAA) via the cytosolic phosphoenolpyruvate carboxylase, followed by the conversion of OAA into malate catalyzed by NAD-dependent malate dehydrogenase (King et al., 1998) which happens in both green and non-green seeds. The production of acetyl-CoA from imported malate requires the successive action of the pNADP-ME and the plastidic pyruvate dehydrogenase, which presents the disadvantage of generating 2 molecules of CO2 for each 4-carbon malate molecule (Figure 1).
FAS also requires stoichiometric amounts of ATP, NADPH, and NADH for each sequential addition of an acetyl unit to the growing chain of the fatty acid (Figure 1). ATP is required for the carboxylation of acetyl-CoA to malonyl-CoA by ACCase, whereas the two reductases of the FAS complex require NADPH and NADH, respectively. Plastids of oilseeds must either take up ATP produced by oxidative phosphorylation in the mitochondria or generate it internally. ATP can be produced in the cytosol via the glycolysis and through the mitochondrial oxidative phosphorylation, and then be imported into the plastids via a nucleotide transporter. A potential source for ATP and reductant within the plastids is generated by the oxidation of sugar phosphates via glycolytic enzymes: glyceraldehyde 3-phosphate dehydrogenase generates NADH whereas phosphoglycerate kinase and pyruvate kinase produce ATP. Likewise, the subsequent conversion of pyruvate into acetyl-CoA by the pyruvate dehydrogenase is accompanied by the production of NADH. The intraplastidial conversion of malate to pyruvate by pNADP-ME constitutes another potential source of NADPH. Finally, the oxidation of sugar phosphates by the plastidial OPPP may also contribute to the production of NADPH. In the case of green oilseeds, light reactions in the thylakoids produce ATP and NADPH that can be used for de novo FAS (Figure 1). The balance between cyclic and non-cyclic photophosphorylation may also adjust respective ATP and NADPH productions to metabolic requirements in autotrophic plastids (Allen et al., 2009).
Following FAS, acyl groups in FA are hydrolyzed by acyl-ACP thioesterases, releasing free acyl chains. These free acyl chains are then activated to CoA esters on the outer membrane of the plastid by long-chain acyl-CoA synthetases prior to their export toward the ER for their elongation into very-long chain fatty acids (VLCFA). Carbon for FA elongation comes from the cleavage of cytosolic citrate into OAA and acetyl-CoA by citrate lyase, reaction that requires ATP (Figure 1). This cytosolic acetyl-CoA is then used by acetyl-CoA carboxylase to synthesize malonyl-CoA, the carbon donor for FA elongation. The elongation of acyl-CoA in the ER involves four sequential enzymatic steps catalyzed by the fatty acid elongase complex: i) the ketoacyl-CoA synthase (KCS) requires energy under the form of ATP to condenses malonyl-CoA with the elongating acyl-CoA; ii) the 3-ketoacyl-CoA reductase (KCR) uses (NAD/PH) to reduce 3-ketoacyl-CoA; iii) the 3-hydroxy acyl-CoA dehydrate (HCD) catalyzes the dehydration of 3-hydroxyacyl-CoA; and iv) the enoyl-CoA reductase (ECR) uses NAD/PH to reduce enoyl-CoA. Similar to FAS, FA elongation requires energy and reductant. ATP may be produced by oxidative phosphorylation in the mitochondria and cytosolic glycolysis which may also provide NADH. The cytosolic pentose-phosphate pathway may contribute to NADPH production in developing embryos with the glucose-6-phosphate and 6-phosphogluconate dehydrogenases (Figure 1).
Finally, the assembly of TGs involves the sequentially esterification of acyl-CoAs to glycerol 3-phosphate (G3P) backbone by membrane-bound acyltransferases (Figure 1). The first acylation yields lysophosphatidic acid (LPA), which in turn is acylated to produce the central metabolite phosphatidic acid (PA). PA is then converted to diacylglycerol (DAG) by the action of PA phosphatase (PAP). In the Kennedy pathway, a third FA is transferred to the vacant position of DAG by diacylglycerol acyltransferase (DGAT), the only enzymatic reaction of the pathway exclusively committed to TG biosynthesis (Cao and Huang, 1986; Cao and Huang, 1987; Li-Beisson et al., 2013). The acyl chains from phosphatidylcholine (PC) may also become available for TG synthesis through transfer of an acyl from PC to DAG, which is catalyzed by phospholipid:diacylglycerol acyltransferase (PDAT). Once TG assembly is achieved in the ER, TGs are accumulated between the two layers of phospholipids, resulting in the formation of structures called oil bodies or lipid droplets (LD). These spherical organelles comprise a matrix of TGs surrounded by a phospholipid monolayer where the aliphatic chains are oriented to the TG lumen and the phosphate groups toward the cytosol (Yatsu and Jacks, 1972; Chapman and Ohlrogge, 2012).
Besides ATP which can be produced by oxidative phosphorylation and can be translocated from one compartment to the other, the carbon precursor (acetyl-CoA) and reductant do not cross biological membranes, and have therefore to be synthesized in the plastids for FAS and/or the cytosol for FA elongation. As described above (Figure 1), several biochemical steps may lead to the production of acetyl-CoA and NAD(P)H, and their relative importance varies from one species to another. Determining the nature of carbon precursors and reducing power for FAS is crucial to understand and enhance oil synthesis in developing seeds, and also to identify potential bottlenecks.
Early experiments were performed by incubating isolated plastids with radiolabeled substrates to investigate which carbon precursors were stimulating the rate of de novo FAS, and which pathway were producing reductant. Feeding isolated plastids from Brassica napus with 14C-labeled substrates showed that pyruvate, glucose 6-phosphate (G6P), dihydroxyacetone phosphate (DHAP), malate, acetate, and phosphoenolpyruvate (PEP) were utilized as precursors for FAS (Kang and Rawsthorne, 1994; Kubis et al., 2004). Particularly, the utilization of G6P through the OPPP also provided reductant for FAS (Eastmond and Rawsthorne, 2000; Pleite et al., 2005). However, incubation of B. napus isolated plastids with 14C-PEP revealed that it did not only provide carbons, but also supplied additional ATP for FAS (Kubis et al., 2004). In contrast to B. napus, feeding sunflower isolated plastids with 14C-G6P indicated that there was no incorporation of labeling in FAs (Pleite et al., 2005). However, pyruvate utilization in combination with G6P dramatically increased FAS. Due to an insufficient NADPH pool, pyruvate feeding alone was not sufficient to enhance the rate of FAS. Moreover, malate has been demonstrated to be one of the most efficient precursors for FAS. Indeed, in isolated plastids from castor and sunflower embryos, supply of malate stimulated the rates of FAS (Smith et al., 1992; Pleite et al., 2005). In comparison to pyruvate and acetate, malate significantly increased the rate of FAS due to generation of additional NADPH via pNADP-ME (Pleite et al., 2005). However, supply of G6P with malate did not significantly alter the rate of FAS in isolated plastids.
Although experiments conducted with isolated plastids were key to identify the carbon precursors and reductant, these results did not completely reflect what happens in vivo, in the entire developing embryo. Initial experiments in vivo were carried out by incubating embryos with radioisotopes. Labeling studies performed with [U-14C4]-malate in sunflowers embryos in culture showed that malate could provide a source of carbon for the FAS (Alonso et al., 2007), corroborating the previous results on isolated plastids mentioned above (Pleite et al., 2005). However, malate is not a physiological substrate provided from the sunflower plant to the developing embryos. Using culture conditions that mimic the feeding and the development of the sunflower embryos in planta, isotopic steady state labeling experiments demonstrated that the flow of malate towards FAS was minor; the major source of carbon (91-95%) contributing to FAS was from triose phosphates (Alonso et al., 2007). Due to discrepancies between results on isolated plastids and whole embryos in culture, MFA has been the method of choice to study the metabolic pathways involved in FAS under conditions that are physiologically relevant.
The goal of MFA is to quantify all the in vivo metabolic fluxes in a given organ or cell, here developing embryos, which results in a metabolic flux map. The determination of intermediary carbon fluxes requires 13C-labeling. Therefore, establishing culture conditions that mimic the development of embryos in planta is decisive to build carbon flux maps. For instance, it has been found for multiple species of the Brassicaceae family that developing embryos readily grow in liquid cultures (Schwender and Ohlrogge, 2002; Lonien and Schwender, 2009; Chen and Shachar-Hill, 2012; Tsogtbaatar et al., 2020). Substrates, furnished by the mother plant, are unloaded in the endosperm liquid and taken up by the embryo. Therefore, the best way to design a liquid growth medium that mimics the in planta liquid environment is to analyze the constituents of the endosperm or the vascular tissue (Alonso et al., 2007; Alonso et al., 2010; Cocuron et al., 2019; Tsogtbaatar et al., 2020). Substrate composition, total osmotic pressure of the medium, and light intensity are important factors influencing plant tissue development (Goffman et al., 2005; Allen et al., 2007; Alonso et al., 2010), and hence have to be optimized. To meet requirements for 13C-MFA, it is important to maintain homeostasis and metabolic steady state in culture embryos. Therefore, photoperiod is not commonly applied to embryos in culture for 13C-MFA studies. Ideal culture conditions for plant embryos are validated when the dry weight gain and the biomass composition of embryos grown in culture are not significantly different from the ones grown in planta (Alonso et al., 2007; Alonso et al., 2010; Alonso et al., 2011; Cocuron et al., 2019; Tsogtbaatar et al., 2020). Parallel labeling experiments, using different 13C-substrates, are usually conducted to have a better coverage of the metabolic network (Schwender et al., 2006; Alonso et al., 2007; Alonso et al., 2010; Antoniewicz, 2015; Crown et al., 2016; Antoniewicz, 2018; Acket et al., 2019; Tsogtbaatar et al., 2020). Labeled embryos are harvested after they have reached an isotopic steady-state, meaning that the labeling in intermediary metabolites and products have reached a constant pattern. The resultant labeling in a range of metabolites is then determined using nuclear magnetic resonance and/or mass spectrometry (MS) (Ratcliffe and Shachar-Hill, 2006; Dieuaide-Noubhani and Alonso, 2014). Several sensitive gas chromatography-mass spectrometry (GC-MS) and liquid chromatography tandem mass spectrometry (LC-MS/MS) methods have been recently developed to follow the labeling directly in key metabolic intermediaries such as sugars, phosphorylated compounds, free amino acids, and organic acids (Alonso et al., 2010; Koubaa et al., 2013; Cocuron and Alonso, 2014; Cocuron et al., 2017; Acket et al., 2019; Cocuron et al., 2020). Compartmentalization in plant cells is usually considered by following different labeling patterns of a few metabolites and hydrolyzed macromolecules whose biosynthesis occur in one compartment (Allen et al., 2007; Allen et al., 2012; Dieuaide-Noubhani and Alonso, 2014; Cocuron et al., 2020). The complete labeling information is entered into a mathematical model describing the metabolic network. This mathematical model includes equations expressing metabolic and isotopic steady states. Finally, the fluxes through the network that correspond to the observed label distribution are calculated using mathematical algorithms that can be computed using available software (Wiechert and De Graaf, 1997; Wiechert and De Graaf, 1997; Möllney et al., 1999; Wiechert et al., 1999; Wiechert et al., 2001). 13C-based MFA has been successfully applied to plant systems to characterize the in vivo carbon fluxes in important metabolic pathways during FAS in developing embryos (Goffman et al., 2005; Alonso et al., 2007; Allen et al., 2009; Lonien and Schwender, 2009; Alonso et al., 2010; Cocuron et al., 2019; Carey et al., 2020; Tsogtbaatar et al., 2020).
Determining the efficiency of the developing embryo in converting carbon sources into oil and other biomass components (e.g. proteins, and carbohydrates) known as carbon conversion efficiency (CCE), is important to evaluate the potential for improving seed oil quantity (Goffman et al., 2005; Alonso et al., 2007; Allen et al., 2009; Lonien and Schwender, 2009; Chen and Shachar-Hill, 2012; Cocuron et al., 2019; Carey et al., 2020; Tsogtbaatar et al., 2020). CCE results from the sum of all catabolic and anabolic metabolic processes, which varies in developing embryos from different oilseeds (Goffman et al., 2005; Allen et al., 2009; Lonien and Schwender, 2009; Carey et al., 2020; Tsogtbaatar et al., 2020). To assess the CCE, embryos are grown for several days culture media and conditions that mimic their development in plant, as explained in the above section. There are two options to determine the percentage of carbon uptaken that was stored into biomass components. The first option uses 14C-labeled substrates in sealed flasks, and quantifies the radiolabeling released as 14CO2, and incorporated as 14C-oil, 14C-proteins, and 14C-carbohydrates (Goffman et al., 2005; Alonso et al., 2007; Allen et al., 2009; Alonso et al., 2010; Alonso et al., 2011; Carey et al., 2020). The second option is the quantify the substrate depletion from the media and the biomass stored during the incubation period (Cocuron et al., 2019; Tsogtbaatar et al., 2020). Table 1 summarizes the biomass composition and the CCE of different embryos from green and non-green oilseeds that have been studied and published so far. Under physiological conditions, the biomass composition of the developing embryos in culture differs among species. Oil content varied from 18% (w/w) for G. max to 56% for B. napus, proteins from 6% for Z. mays LH59 to 40% for G. max, and carbohydrates from 43% for G. max to 60% for Z. mays LH59. Under physiological conditions, T. arvense embryos were found to be the most efficient in converting carbon substrates into biomass (93%) while C. sativa were the least (32%). It is important to note that these differences in CCE reflect the amount of carbon loss as CO2 during the synthesis of biomass components: the lower the CCE, the higher is the loss of carbon as CO2, which has implications for metabolic engineering. For instance, improving oil content in C. sativa may be achieved by reducing the pathways producing CO2. However, this strategy would not work in T. arvense which is already extremely efficient. Instead, for T. arvense, one would have to redirect carbon from another biomass component to increase FAS.
Except C. sativa, embryos from green seeds had higher CCEs than those seeds that cannot use light (Table 1). The importance of light was further demonstrated by incubating developing embryos at different intensities. In general, higher light intensity significantly increased the CCE. This finding suggests that light has strong effects on the metabolism of developing green embryos—probably due to the additional production of NADPH and ATP via photosynthesis–resulting in faster growth and storage product accumulation, which improved the CCE (Goffman et al., 2005; Alonso et al., 2007; Allen et al., 2009; Lonien and Schwender, 2009; Chen and Shachar-Hill, 2012; Cocuron et al., 2019; Carey et al., 2020; Tsogtbaatar et al., 2020). Indeed, studies on these developing green embryos showed that all the major photosynthetic complexes (PSII, PSI and their antenna complexes, cytochrome b6f complex, and ATP synthase) are present at a necessary stoichiometric ratio, suggesting a high photochemical activity despite being partially blocked from light by the pod and seed coat (Asokanthan et al., 1997; Ruuska et al., 2004; Puthur et al., 2013; Allorent et al., 2015). The light reactions occurring in these green embryos have been associated with the rapid synthesis of ATP and NADPH needed for energetically-expensive FAS. It has also been reported that embryo photosynthesis contributes to a significant amount of oxygen, which fuels energy-generating biochemical pathways, including mitochondrial respiration (Ruuska et al., 2004; Borisjuk et al., 2005; Rolletschek et al., 2005; Tschiersch et al., 2011; Galili et al., 2014).
Differences in the flow of carbon through central metabolic pathways are responsible for the differences in biomass composition and CCE measured in developing embryos from various oilseed species (Table 1). 13C-MFA has been the method of choice to measure in vivo rates of carbon flow, quantifying the metabolic pathways that are active during FAS.
13C-MFA was applied to developing embryos from various oilseed species to determine the sources of carbon and reductant for FAS, and to unravel the occurrence of non-conventional pathways that improved the CCE (Table 2) (Schwender et al., 2004; Schwender et al., 2006; Alonso et al., 2007; Allen et al., 2009; Lonien and Schwender, 2009; Alonso et al., 2010; Hay et al., 2014; Cocuron et al., 2019; Acket et al., 2020; Carey et al., 2020; Tsogtbaatar et al., 2020). Glycolysis is a major source of pyruvate for de novo FAS (Figure 1). 13C-labeling and metabolic flux analysis performed in non-photosynthetic and photosynthetic embryos revealed the contribution of the plastidic NADP-dependent malic enzyme (pNADP-ME) for the production of plastidic pyruvate (pPYR) in Z. mays, H. annuus, G. max, T. arvense, and C. sativa (Table 2). The pNADP-ME provided up to 54% of pPYR in the high oil ALEX maize line (Cocuron et al., 2019). In plastids, pNADP-ME catalyzes the conversion of malate to pPYR with the production of CO2 and NADPH (Figure 1). In addition, pPDH further catalyzes the decarboxylation of pPYR to acetyl-CoA with the generation of CO2 and NADH. Although the overall equimolar conversion of malate into plastidic acetyl-CoA results in the production of valuable reductant necessary for FAS (NADH and NADPH), it leads, in return, to the loss of two carbons as CO2, which may affect the overall CCE. Interestingly, 13C-labeling in developing B. napus embryos demonstrated for the first time that Rubisco was fixing this plastidic 13CO2 to produce phosphoglycerate (pPGA) through an unconventional “Rubisco shunt” (Table 2) (Schwender et al., 2004). The fixation of pCO2 by Rubisco contributes to additional sources of carbon for FAS: it compensates for the decarboxylation steps, and channels more substrates into FAS, improving the CCE (Eastmond et al., 1996; Ruuska et al., 2004; Schwender et al., 2004). Indeed, studies in developing B. napus embryos showed a CCE of 86% under physiological conditions due to CO2 refixation by Rubisco into pPGA (Tables 1, 2) (Schwender et al., 2004). 13C-MFA demonstrated that up to 64% of pPGA was produced by Rubisco, contributing to the synthesis of acetyl-CoA for de novo FAS, and reducing by 40% the carbon lost as CO2 (Table 2) (Schwender et al., 2004). Similar processes have been described in developing embryos of G. max and T. arvense where Rubisco was found to contribute to 14% and 25% of the pPGA, respectively (Table 2) (Allen et al., 2009; Tsogtbaatar et al., 2020).
In addition to the role of Rubisco, 13C-labeling and MFA also revealed the non-canonical function of isocitrate dehydrogenase (IDH) in photosynthetic embryos (Table 2). This reaction, assumed to be thermodynamically irreversible, was reported to catalyze the carboxylation of α-ketoglutarate into isocitrate in vivo in developing B. napus embryos (Schwender et al., 2006). It is important to note that the CO2 fixation by IDH may also improve the CCE. This phenomenon was explained by a high demand in citrate for FA elongation in B. napus (Figure 1), and a high concentration of CO2 (40 mM) available in developing oilseeds (Goffman et al., 2004). Similarly, reversibility of IDH was measured in developing green embryos from G. max, even when the concentration of CO2 was lower (Allen et al., 2009), T. arvense (Tsogtbaatar et al., 2020), C. sativa (Schwender et al., 2006), and L. usitatissinum (Acket et al., 2019), but absent in heterotrophic embryos (Table 2). Interestingly, developing embryos that have an active Rubisco and reversible IDH were found to be the more efficient in converting their substrates into biomass (Tables 1, 2).
Besides carbon, FAS requires reductant that must be synthesized in the plastid. For each mole of acetyl-CoA produced, the plastidic pyruvate dehydrogenase complex generated one mole of NADH, which can be directly used for FAS (Figure 1). The plastidic production of NADPH necessary for de novo FAS may be ensured by the OPPP and/or pNADP-ME, and light reactions in the case of photosynthetic embryos (Figure 1). Knowing that the OPPP and the pNADP-ME generate CO2, their operation may affect negatively the CCE (Figure 1). 13C-labeling and MFA were used to measure the relative contribution of the OPPP and pNADP-ME to the production of NADPH in developing embryos from different species (Table 2). In general, the OPPP supported a higher production of NADPH than the pNADP-ME. For G. max and B. napus, these two combined pathways did not provide sufficient NADPH to support FAS in developing embryos. In those species, the remainder of NADPH may be supplied by the light reactions of photosynthesis and/or catabolism (Goffman et al., 2005; Schwender et al., 2006; Allen et al., 2009). For Z. mays, the production of NADPH by the OPPP and pNAPD-ME was just enough to support FAS in developing embryos (Table 2). More specifically, the pNAPD-ME was found to work at maximal capacity in vivo; it was identified as the limiting step in the provision of pPYR and NADPH for FAS in maize (Alonso et al., 2010; Cocuron et al., 2019). The embryos from the other species, H. annuus, C. sativa, and L. usitatissinum, were producing NADPH via the OPPP and pNADP-ME in excess of the requirements for FAS (Table 2), concomitantly generating CO2, which resulted in the lowest CCE for these species (Table 1).
Overall, the aforementioned studies have demonstrated that MFA is a valuable tool to quantify the carbon partitioning in vivo, and identify the sources of carbon skeletons and reductants necessary for FAS in the developing green and non-green embryos. Information gathered from MFA, combined with transcriptomics and metabolomics, will give insights on the genes that control oil synthesis and create novel approaches for the genetic engineering of oilseed crops. The following sections review some of the target genes and the common genetic engineering strategies for enhancing oil content and quality in seeds.
To improve the yield and FA composition in oilseed crops, it is critical to understand how assimilates are partitioned in favor of storage lipids. It is also of great importance to elucidate the mechanisms of TG biosynthesis in different oilseeds to further optimize their FA composition since it is a key determinant of oilseed nutritional value and industrial applications. Several strategies have been employed to genetically manipulate FA composition and plant lipid metabolism in order to increase the FA content oilseeds. To this end, the “push, pull, package, and protect” strategy has been implemented at various degrees; it consists of manipulating the expression of genes to boost the synthesis of FAs (“push”), or increase TG assembly reactions (“pull”), or improve the storage of FAs into lipid droplets (LDs) (“package”), or prevent the degradation of stored lipids (“protect”), or any combination of these (Figure 2) (Vanhercke et al., 2019). The production of novel/unusual FAs presents additional challenges because they may disrupt membrane stability. Therefore, seeds must incorporate and store novel/unusual FAs into TG. Tables 3–7 review the genes that were up- or down-regulated in several species, and when available, their effect in total seed oil content and FA composition.
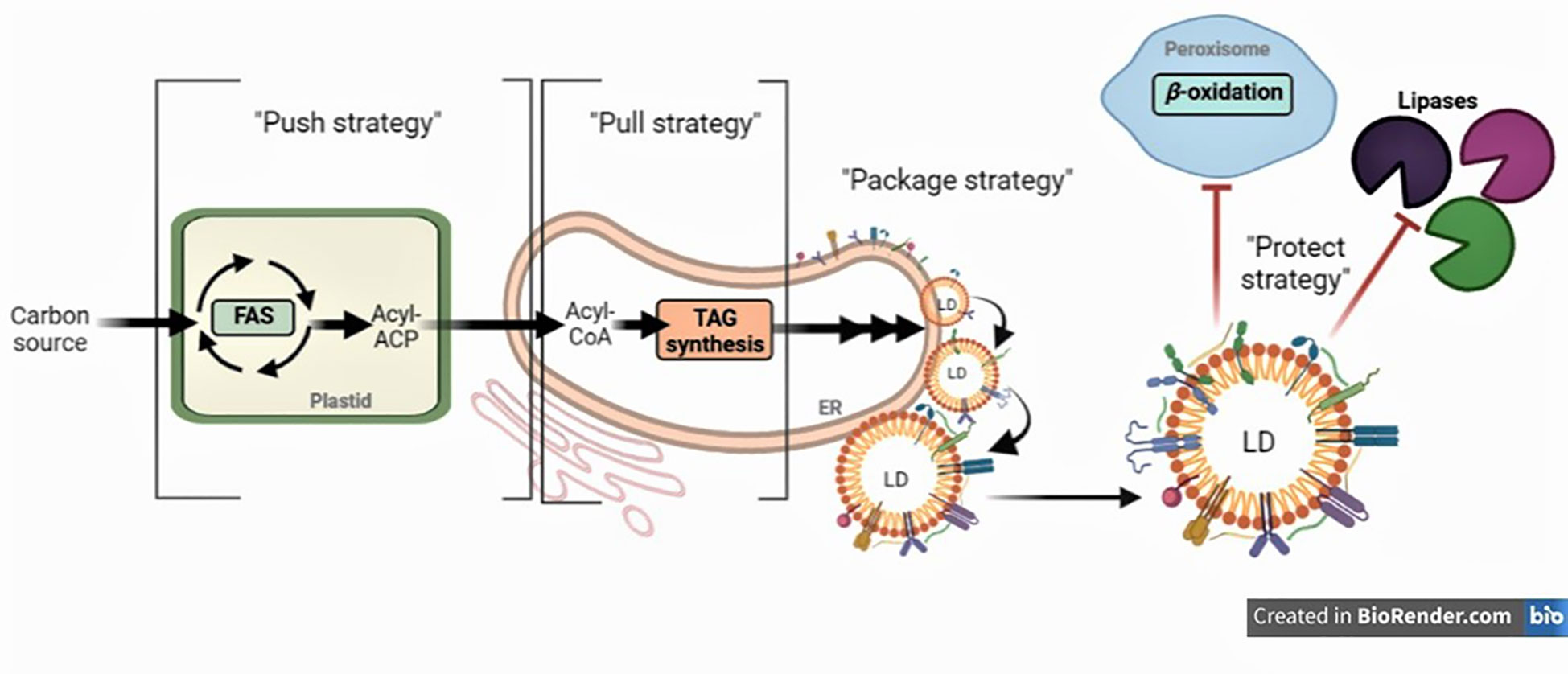
Figure 2 Improving oil quality in seeds by an integrated metabolic engineering approach. Broadly, strategies aim to (1) increase the carbon flux into de novo fatty acid synthesis (FAS) (“Push strategy”), (2) ensure efficient assembly of nascent acyl chains into triacylglycerol (TG) (“Pull strategy”), (3) facilitate lipid droplet (LD) biogenesis and maximize droplet stability by proper coating (“Package strategy”), and (4) minimize TG turnover (“Protect strategy”).
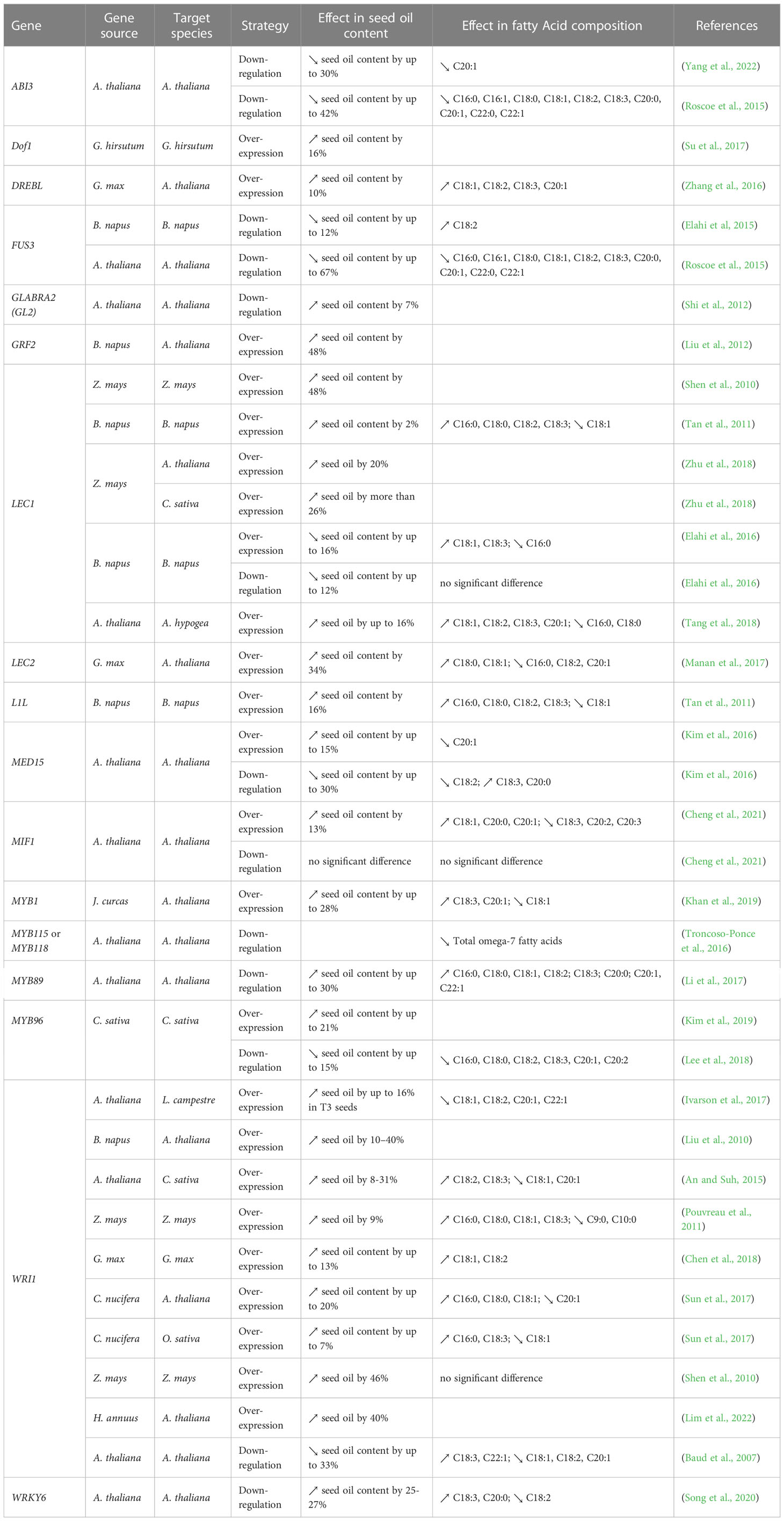
Table 3 List of genes encoding TFs manipulated to enhance oil yield and/or change the FA composition in seeds.
This strategy aims to enhance the flux of carbon through FAS to produce additional acyl chains in the plastids for subsequent assembly into TGs in the ER (Figure 2). The “push” strategy is considered to be one of the most extensively used to manipulate oil content. Commonly, over-expression (or even down-regulation) of transcription factors (TFs) offers a great advantage of controlling a number of reactions simultaneously in the oil biosynthetic pathway (Table 3). The best examples of such TFs are LEAFY COTYLEDON1 (LEC1), LEC1-LIKE (L1L), LEC2, and WRINKLED1 (WRI1) (Focks and Benning, 1998; Lotan et al., 1998; Stone et al., 2001; Kwong et al., 2003; Cernac and Benning, 2004; Mu et al., 2008). Other TFs, shown to regulate the expression of genes involved in FAS, have been tested, including dehydration-responsive element-binding (DREB) (Zhang et al., 2016), Dof-type transcription factor 1 (Dof1) (Su et al., 2017), FUSCA3 (FUS3) (Elahi et al, 2015), growth-regulating factor 2-like (GRF2) (Liu et al., 2012), GLABRA2 (GL2) (Shi et al., 2012), and MYB interaction factor 1 (MIF1) (Cheng et al., 2021). Among all the TFs, GRF2, LEC1, WRI1 were shown to be the most efficient in increasing seed oil content (40% to 48% higher compared to wild-type) (Table 3). In parallel, specific enzymes in the first committed steps of de novo FAS have also been targeted, such as acetyl-CoA carboxylase (ACCase) (Dong et al., 2002; Cui et al., 2017), and malonyl CoA-ACP malonyltransferase (MCAMT) (Jung et al., 2019) (Table 4). Finally, several studies demonstrated that overexpression of membrane-intrinsic proteins that mediate the export of FAs from plastids, such as FA export 1 (FAX1) (Li et al., 2015b; Tian et al., 2018; Li et al., 2020b; Cai et al., 2021), FAX2 and FAX4 (Li et al., 2015b; Li et al., 2016a; Li et al., 2020b), BILE ACID : SODIUM SYMPORTER FAMILY PROTEIN 2 (BASS2) (Lee et al., 2017), and fatty acyl-ACP thioesterases (FAT) (Bonaventure et al., 2003; Sinha et al., 2007; Parveez et al., 2010; Belide et al., 2012; Nguyen et al., 2015a; Ozseyhan et al., 2018; Ma et al., 2021; Liu et al., 2022) could also boost lipid accumulation in seeds (Table 4). Using the “push” strategy, the highest TG levels recorded in seed so far were achieved by over-expressing ACCase in maize (+65%) (Dong et al., 2002) (Table 4).
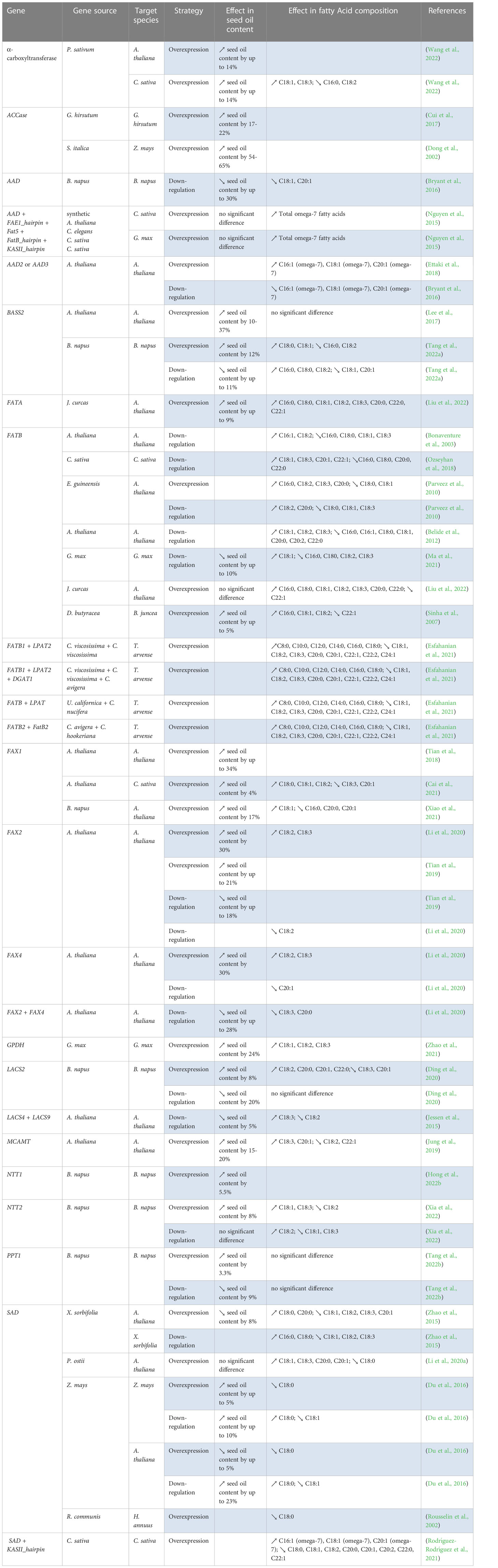
Table 4 List of “push” genes manipulated to enhance oil yield and/or change the FA composition in seeds.
The pull strategy aims to ensure efficient assembly of FAs generated in the plastid into TG at the ER (Figure 2). Most of the metabolic engineering studies that attempted to optimize this step in seeds have focused on the over-expression of diacylglycerol acyltransferase (DGAT) (He et al., 2004; Kroon et al., 2006; Lung and Weselake, 2006; Shockey et al., 2006; Yu et al., 2006; Burgal et al., 2008; Misra et al., 2013; Wang et al., 2014; Liu et al., 2015; Savadi et al., 2015; Zhou et al., 2020; Chen et al., 2022) and phospholipid: diacylglycerol acyltransferase 1 (PDAT1) (Zhang et al., 2010; van Erp et al., 2011; Guan et al., 2015; Zhou et al., 2020) which commit acyl chains to storage TGs. Manipulation of other genes encoding for “pull” proteins, such as glycerol-3-phosphate dehydrogenases (GPDH), glycerol-3-phosphate acyltransferase (GPAT), and lysophosphatidic acid acyltransferase (LPAT), have also been reported to increase TG levels in seeds (Liu et al., 2015) (Table 5). Using the “pull” strategy, the combined over-expression of DGAT, GPAT, and LPAT was shown to be the most efficient to increase total seed oil content (+25%) (Shockey et al., 2019) (Table 5).
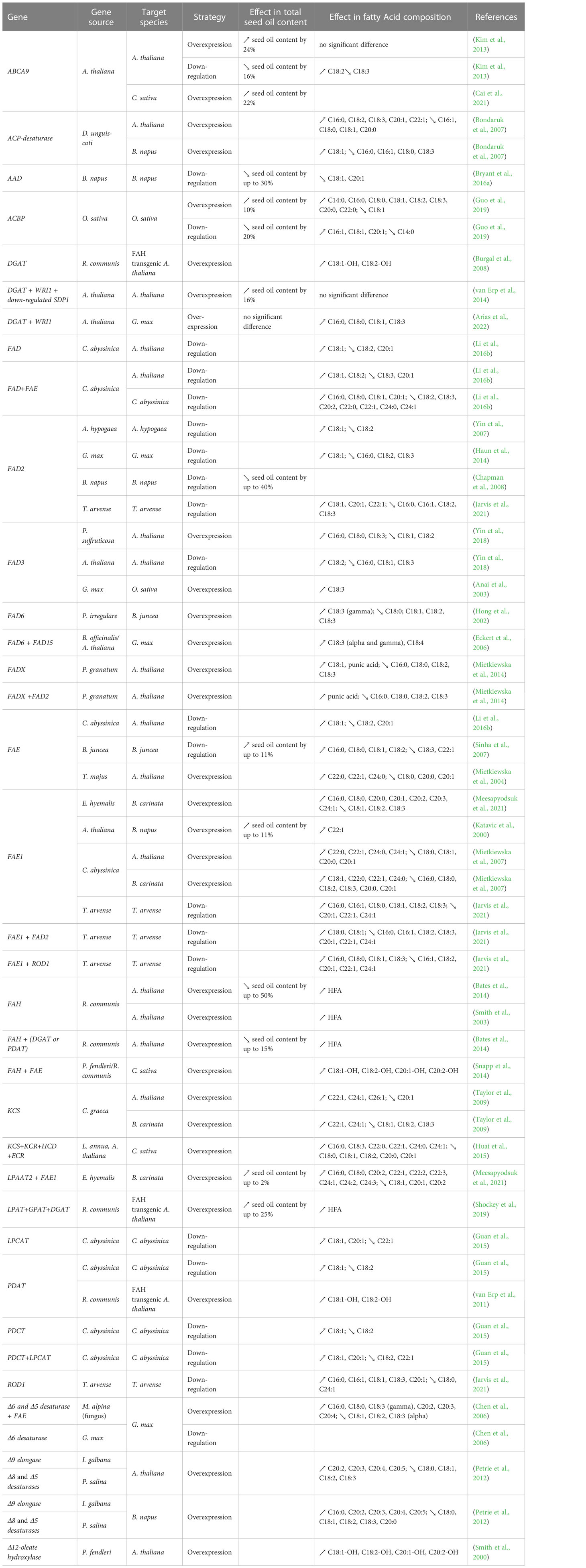
Table 5 List of “pull” genes manipulated to enhance oil yield and/or change FA composition in seeds.
As TGs accumulate within the ER phospholipid bilayer, the outer layer starts to expand into the cytosol, forming nascent LDs that bud off to form LDs (Figure 2). The “package” strategy aims to facilitate LD biogenesis and maximize droplet stability by proper coating. Proteomic analyses of seed LDs indicated that they contain 20–30 coat proteins which were found to be involved in LD formation and/or stability (Jolivet et al., 2004; Jolivet et al., 2009). These coat proteins might also help blocking the accessibility of the TG within the LD core to lipases allowing more LD formation. Recent evidence shows that seed-specific overexpression or down-regulation of some genes encoding these proteins increased LD size and/or number. Such proteins include lipid droplet-associated protein (LDAP) (Gidda et al., 2016), LDAP-interacting protein (LDIP) (Pyc et al., 2017), oleosin (Lu et al., 2006, Lu et al., 2018; Siloto et al., 2006; Shimada et al., 2008; Hu et al., 2009; Liu et al., 2013; Zhang et al., 2019), and seipins (Lunn et al., 2018) (Table 6). Interestingly, several studies also used mammalian “package” genes, such as fat-specific protein 27 (FSP27) and fat storage-inducing transmembrane protein 2 (FIT2) from mouse in Arabidopsis. These resulted not only in improvement of seed oil content but also stimulation of LD clustering and fusion (Cai et al., 2017; Price et al., 2020) (Table 6). Among all the mentioned “package” genes, over-expression of seipin in Arabidopsis gave the highest increase in seed oil content (+62%), which enhanced hydroxy-fatty acid (HFA) levels too (Lunn et al., 2018) (Table 6).
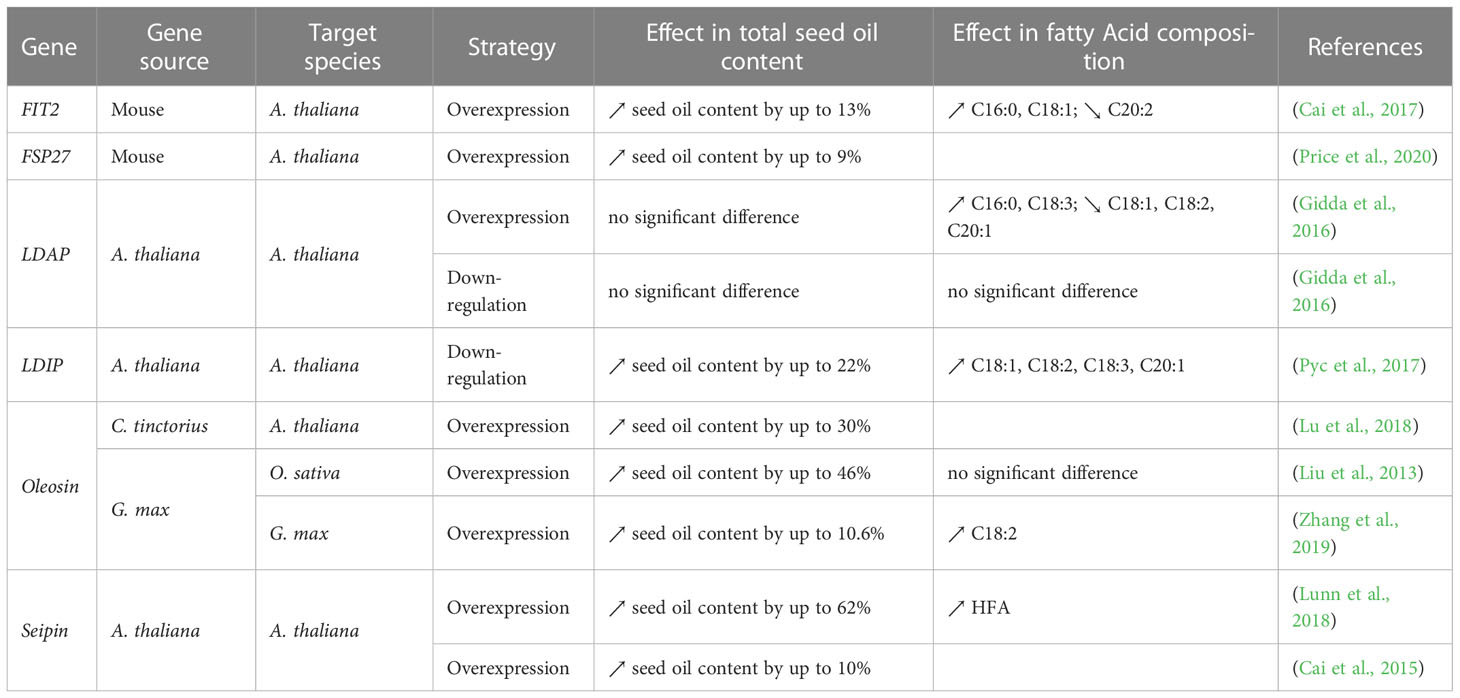
Table 6 List of “package” genes manipulated to enhance oil yield and/or change the FA composition in seeds.
Coat proteins can be hydrolyzed by endogenous proteases and TGs can be degraded into free FAs by various lipases to produce acetyl‐CoA via β‐oxidation particularly during seed germination and seedling growth (Pracharoenwattana and Smith, 2008; Li-Beisson et al., 2013; Borek et al., 2015) (Figure 2). Blocking the breakdowns of coat proteins and TGs (also known as “protect” strategy) is therefore an attractive strategy to increase oil content in seeds. The initial step in lipase-induced TG mobilization is the ubiquitination of the respective coat proteins, particularly oleosin and caleosin, and then subsequent digestion by the proteasome (Sorokin et al., 2009; Hsiao and Tzen, 2011; Deruyffelaere et al., 2015), which was extensively reviewed (Sorokin et al., 2009; Shao et al., 2019; Guzha et al., 2023). Important insights into the mechanism regulating the turnover of oleosins in plants has been recently published (Deruyffelaere et al., 2018; Kretzschmar et al., 2018). Two key components, PUX10 (a member of the plant ubiquitin regulatory X (UBX)-domain containing protein family) and CDC48A (the AAA ATPase, Cell Division Cycle 48) were identified. PUX10 localizes to LDs, binds to the ubiquitinated oleosins, and interacts with ubiquitin and CDC48A, respectively. As an adaptor, PUX10 recruits CDC48A to ubiquitinated oleosins, leading to dislocation of oleosins from LDs via the segregase activity of CDC48A (Deruyffelaere et al., 2018; Kretzschmar et al., 2018). In Arabidopsis pux10 mutant seeds, PUX10 deficiency impaired the degradation of ubiquitinated oleosins from LDs. However, this did not change the total FA content in seeds of mutant genotypes in comparison to wild-type (Deruyffelaere et al., 2018; Kretzschmar et al., 2018) (Table 7). On the other hand, suppression of TG degradation to enhance FA content was achieved by down-regulating the genes that code for TG breakdown enzymes. Such genes are seed fatty acid reducer (SFAR) (Karunarathna et al., 2020), Gly-Asp-Ser-Leu (GDSL)-motif lipases (Ding et al., 2019), plastid lipase1 (PLIP1), and Sugar-dependent1 (SDP1) (Kelly et al., 2013; Kim et al., 2014; Kanai et al., 2019) (Table 7). Interestingly, recent studies reported that the over-expression of several genes with major role in TG degradation have a positive effect on seed oil content, which may seem counterintuitive. Such genes include patatin‐related phospholipase (pPLAIIIδ) (Li et al., 2013; Li et al., 2015a), and nonspecific phospholipase C6 (NPC6) (Cai et al., 2020) (Table 7). An intriguing question arising from these studies is how the over-expressed proteins increased lipid accumulation in seeds. One hypothesis is that phospholipase-mediated phospholipid turnover facilitates the movement of FAs from the plastid to the ER. Phosphatidylcholine (PC) is the most abundant class of phospholipids in plants and plays pivotal roles in TG production (Wang, 2005; Chapman and Ohlrogge, 2012; Karki et al., 2019). When hydrolyzed, PC can serve as a substrate for FA desaturation and also provides free FAs or DAG for TG synthesis (Mhaske et al., 2005; Lu et al., 2009; Zhang et al., 2010; Pokotylo et al., 2013). Therefore, over-expression of these phospholipases could enhance the acyl flux into ER and increase the overall levels of TGs in seeds. Among the genes tested as part of the “protect” strategy, it was shown that the down-regulation of PLIP1 in Arabidopsis gave the highest increase in seed oil content (+45%) (Wang et al., 2017) (Table 7).
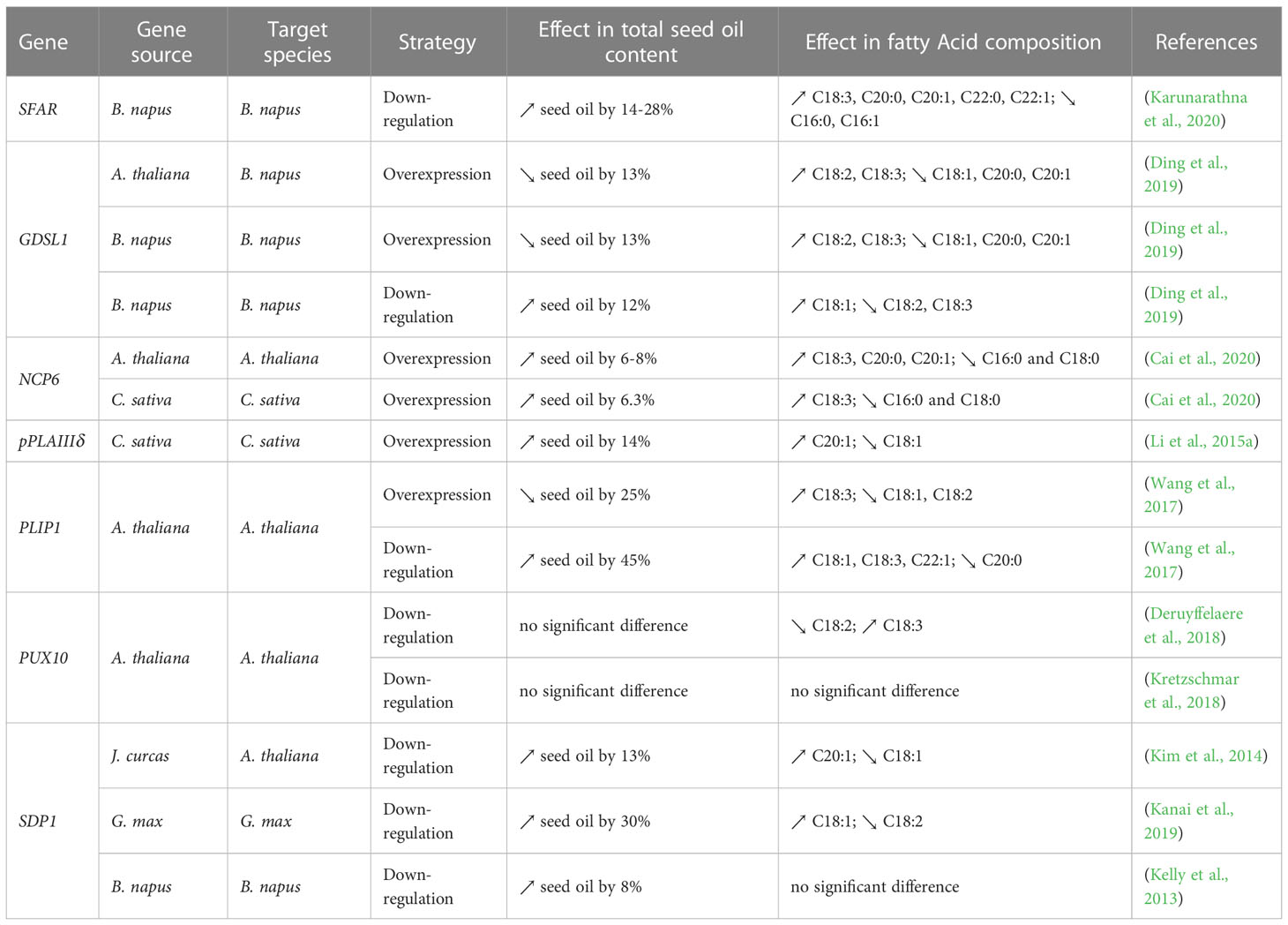
Table 7 List of “protect” genes used to enhance oil yield and/or change the FA composition in seeds.
Metabolic engineering strategies have been used to produce novel high-value FAs or to improve their synthesis in seeds of established crops and other model species (Tables 3-7). For instance, over-expression and down-regulation of desaturases, such as acyl-acyl carrier protein desaturase (AAD), fatty acid desaturases (FADs), and stearoyl-acyl carrier protein desaturase (SAD), and FAT, and/or down-regulation of fatty acid elongase (FAE) genes were attempted to divert the carbon flux towards the synthesis of beneficial FAs required for human health and nutrition, such as gamma-linolenic acid (C18:3), alpha-linolenic acid (C18:3), docosadienoic acid (C22:2), docosatrienoic acid (C22:3) (Hong et al., 2002; Anai et al., 2003; Eckert et al., 2006; Huai et al., 2015; Yin et al., 2018; Meesapyodsuk et al., 2021), and oils enriched in omega-7 monounsaturated FAs such as palmitoleic acid (C16:1) and its elongation products vaccenic acid (C18:1) and paullinic acid (C20:1) (Nguyen et al., 2015; Ettaki et al., 2018; Rodríguez-Rodríguez et al., 2021) (Tables 4-5). Also, several oilseed plants produce industrially-relevant FAs, such as erucic acid (C22:1), lesquerolic acid (C20:1-OH), and nervonic acid (C24:1), providing renewable alternatives to petrochemicals for the manufacture of lubricants, coatings, or polymers. However, most plants producing these FAs usually have undesirable traits and are not economically viable crops. It is hence important to improve the production of these valuable FAs in other established crops via metabolic engineering technology. One example is the improvement of the production of erucic and nervonic acids. VLCFAs, such as erucic acid (C22:1) and nervonic acid (C24:1), are important in plastic, cosmetic, nylon, and lubricant industries (Mastebroek and Marvin, 2000). Nervonic acid has only been found in the seed oils of a few known plants, and among these, only Lunaria annua has been considered as a niche crop for future development. However, this plant is a biennial with highly variable seed yields (800–2,000 kg/ha) and has a major problem of seed shattering, making it an uneconomical source of nervonic acid (Mastebroek and Marvin, 2000). Consequently, there is a high demanded to improve the production of this valuable FA in other oilseed crops via metabolic engineering technology (Taylor et al., 2009). Over-expression of 3-ketoacyl-CoA synthase (KCS) and FAE genes were shown to increase the production of erucic and nervonic acids in Brassica napus, Camelina sativa, and Arabidopsis thaliana (Katavic et al., 2000; Mietkiewska et al., 2004; Taylor et al., 2009; Huai et al., 2015) (Table 5). Another example is the production of HFAs in a toxin free oilseed crop to replace castor oil as a renewable source for numerous industrial applications. This was achieved through over-expression of fatty acid hydroxylase and elongase genes, and also acyltransferase genes from species producing HFAs, such as castor bean (Ricinus communis) and lesquerella (Physaria fendleri), in the seeds of the model species Arabidopsis and in the industrial oilseed crop Camelina (Smith et al., 2003; van Erp et al., 2011; Bates et al., 2014; Snapp et al., 2014; Shockey et al., 2019) (Table 5).
To further increase the TG levels in seed oil, several studies have combined genes from different strategies. It is important to note that this combinatorial strategy has been mostly used to increase TG content in non-seed organs, such as leaves and roots (Slocombe et al., 2009; Fan et al., 2013; Kelly et al., 2013; Vanhercke et al., 2013; Winichayakul et al., 2013), and were extensively reviewed (Cahoon et al., 2007; Taylor et al., 2011; Xu et al., 2018; Vanhercke et al., 2019; Singh et al., 2021). However, it was shown that seed-specific overexpression of AtWRI1 (“push”) and AtDGAT1 (“pull”) combined with suppression of the triacylglycerol lipase SUGAR- DEPENDENT1 (AtSDP1) (“protect”) resulted in a higher seed oil content than manipulation of each gene individually (van Erp et al., 2014a) (Table 5). Similarly, seed specific over-expression of Seipin (“package”) in transgenic Arabidopsis expressing R. communis fatty acid hydroxylase (FAH) (“pull”) not only increased the total oil content but also increased the HFA composition in seeds (Lunn et al., 2018) (Table 6). However, this combinatorial strategy is not guaranteed to succeed: several attempts did not increase TG in seeds, such as the over-expression of WRI1 (“push”) and DGAT1(“pull”) from Arabidopsis in soybean, suggesting that some species put in place counteracting processes to keep oil content stable (Arias et al., 2022) (Table 5). On the other hand, combining multiple strategies has also been successful to modify FA composition in seeds (Tables 4-5). For instance, co-expression of Cuphea viscosissima FATB (“Push”) and LPAT (“pull”) with Cuphea avigera DGAT (“pull”) increased the accumulation of TGs rich in medium-chain FAs (C6-C14) in pennycress for industrial, jet fuel and improved biodiesel applications (Esfahanian et al., 2021) (Table 4). Another example is the co-expression of Δ6 and Δ5 desaturases (“pull”) and FAE (“pull”) from a fungal species (Mortierella alpinia) in soybean, which caused the seed to accumulate novel FAs that are not naturally produced in soybean: γ-linolenic acid (GLA), eicosa-8, 11-dienoic acid (EDA), dihomo-γ-linolenic acid (DGLA), and arachidonic acid (C20:4), which are important for human health (Chen et al., 2006) (Table 5).
The demands in seed oils for food and feed is rapidly increasing with growing population, urbanization, and industrialization. Enhancing oil production and improving FA composition in oilseed crops through metabolic engineering is a promising venue to meet these demands; the challenge is in identifying the right target(s). 13C-MFA has played a pivotal role in advancing our understanding of plant FAS at the systems level. The flux maps of primary metabolism that have emerged from MFA provide information on regulatory steps and pathways to be assessed within the context of the whole network, leading to the identification of candidate genes to be engineered for oil improvement. Current advances in mass spectrometry imaging techniques coupled with 13C-isotopic pulse labeling would also allow to assess spatial and temporal resolution of metabolic fluxes (Romsdahl et al., 2021). Recent discoveries of a number of genes involved in the “push, pull, package, and protect” steps of oil synthesis have enabled successful engineering of oil content and composition in different crops. Effective strategies combined the overexpression of TFs that upregulate FAS and genes involved in TG assembly, combined with the downregulation of TG catabolic enzymes. The challenge is that a given strategy may work in a species but not in others: as demonstrated by 13C-MFA studies, developing embryos from different species use different pathways, sometimes even non-conventional reactions, for FAS. Finally, the comprehensive understanding of multi-”omics” technologies and advanced genome-editing capabilities offer the possibility of rapid assembly and introduction of multiple candidate genes for further improvements in seed oil quality. Popular genome-editing tools, such as CRISPR/Cas9, could be used to remove or minimize metabolic competition while directing metabolic flux toward TG biosynthesis, or to edit specific amino acids in oil biosynthesis enzymes to improve/modify enzymatic activities (Park and Kim, 2022).
AA conceived the idea. JS and UY drafted the manuscript and designed the figures. AA reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
This review was funded by the Agriculture and Food Research Initiative competitive grant # 2021-67013-33777 from the USDA National Institute of Food and Agriculture, the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, Genomic Science Program grant no. DE-SC0020325, and the United Soybean Board project no. 2332-203-0102 to AA.
We acknowledge Drs. Christopher Johnston and Cintia Arias at the UNT BioDiscovery Institute and Department of Biological Sciences for their helpful comments to improve the review. We also acknowledge the BioRender tool that we used to build the Figures 1 and 2.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acket, S., Degournay, A., Rossez, Y., Mottelet, S., Villon, P., Troncoso-Ponce, A., et al. (2019). 13c-metabolic flux analysis in developing flax (Linum usitatissinum l.) embryos to understand storage lipid biosynthesis. Metabolites 10, 14. doi: 10.3390/metabo10010014
Allen, D. K., Laclair, R. W., Ohlrogge, J. B., Shachar-Hill, Y. (2012). Isotope labelling of rubisco subunits provides in vivo information on subcellular biosynthesis and exchange of amino acids between compartments. Plant Cell Environ. 35, 1232–1244. doi: 10.1111/j.1365-3040.2012.02485.x
Allen, D. K., Ohlrogge, J. B., Shachar-Hill, Y. (2009). The role of light in soybean seed filling metabolism. Plant J. 58, 220–234. doi: 10.1111/j.1365-313X.2008.03771.x
Allen, D. K., Shachar-Hill, Y., Ohlrogge, J. B. (2007). Compartment-specific labeling information in 13C metabolic flux analysis of plants. Phytochemistry 68, 2197–2210. doi: 10.1016/j.phytochem.2007.04.010
Allorent, G., Osorio, S., Ly Vu, J., Falconet, D., Jouhet, J., Kuntz, M., et al. (2015). Adjustments of embryonic photosynthetic activity modulate seed fitness in arabidopsis thaliana. New Phytol. 205, 707–719. doi: 10.1111/nph.13044
Alonso, A. P., Dale, V. L., Shachar-Hill, Y. (2010). Understanding fatty acid synthesis in developing maize embryos using metabolic flux analysis. Metab. Eng. 12, 488–497. doi: 10.1016/j.ymben.2010.04.002
Alonso, A. P., Goffman, F. D., Ohlrogge, J. B., Shachar-Hill, Y. (2007). Carbon conversion efficiency and central metabolic fluxes in developing sunflower (Helianthus annuus l.) embryos. Plant J. 52, 296–308. doi: 10.1111/j.1365-313X.2007.03235.x
Alonso, A. P., Val, D. L., Shachar-Hill, Y. (2011). Central metabolic fluxes in the endosperm of developing maize seeds and their implications for metabolic engineering. Metab. Eng. 13, 96–107. doi: 10.1016/j.ymben.2010.10.002
Anai, T., Koga, M., Tanaka, H., Kinoshita, T., Rahman, S. M., Takagi, Y. (2003). Improvement of rice (Oryza sativa l.) seed oil quality through introduction of a soybean microsomal omega-3 fatty acid desaturase gene. Plant Cell Rep. 21, 988–992. doi: 10.1007/s00299-003-0609-6
An, D., Suh, M. C. (2015). Overexpression of arabidopsis WRI1 enhanced seed mass and storage oil content in camelina sativa. Plant Biotechnol. Rep. 9, 137–148. doi: 10.1007/s11816-015-0351-x
Antoniewicz, M. R. (2015). Parallel labeling experiments for pathway elucidation and 13C metabolic flux analysis. Curr. Opin. Biotechnol. 36, 91–97. doi: 10.1016/j.copbio.2015.08.014
Antoniewicz, M. R. (2018). A guide to 13C metabolic flux analysis for the cancer biologist. Exp. Mol. Med. 50, 1–13. doi: 10.1038/s12276-018-0060-y
Arias, C. L., Quach, T., Huynh, T., Nguyen, H., Moretti, A., Shi, Y., et al. (2022). Expression of AtWRI1 and AtDGAT1 during soybean embryo development influences oil and carbohydrate metabolism. Plant Biotechnol. J. 20, 1327–1345. doi: 10.1111/pbi.13810
Asokanthan, P. S., Johnson, R. W., Griffith, M., Krol, M. (1997). The photosynthetic potential of canola embryos. Physiol. Plant 101, 353–360. doi: 10.1111/j.1399-3054.1997.tb01008.x
Atadashi, I. M., Aroua, M. K., Abdul Aziz, A. R., Sulaiman, N. M. N. (2012). Production of biodiesel using high free fatty acid feedstocks. Renewable Sustain. Energy Rev. 16, 3275–3285. doi: 10.1016/j.rser.2012.02.063
Bates, P. D., Johnson, S. R., Cao, X., Li, J., Nam, J. W., Jaworski, J. G., et al. (2014). Fatty acid synthesis is inhibited by inefficient utilization of unusual fatty acids for glycerolipid assembly. Proc. Natl. Acad. Sci. U.S.A. 111, 1204–1209. doi: 10.1073/pnas.1318511111
Baud, S., Mendoza, M. S., To, A., Harscoët, E., Lepiniec, L., Dubreucq, B. (2007). WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in arabidopsis. Plant J. 50, 825–838. doi: 10.1111/j.1365-313X.2007.03092.x
Belide, S., Petrie, J. R., Shrestha, P., Singh, S. P. (2012). Modification of seed oil composition in arabidopsis by artificial microRNA-mediated gene silencing. Front. Plant Sci. 3. doi: 10.3389/fpls.2012.00168
Bonaventure, G., Salas, J. J., Pollard, M. R., Ohlrogge, J. B. (2003). Disruption of the FATB gene in arabidopsis demonstrates an essential role of saturated fatty acids in plant growth. Plant Cell 15, 1020–1033. doi: 10.1105/TPC.008946
Bondaruk, M., Johnson, S., Degafu, A., Boora, P., Bilodeau, P., Morris, J., et al. (2007). Expression of a cDNA encoding palmitoyl-acyl carrier protein desaturase from cat’s claw (Doxantha unguis-cati l.) in arabidopsis thaliana and brassica napus leads to accumulation of unusual unsaturated fatty acids and increased stearic acid content in the. Plant Breed. 126, 186–194. doi: 10.1111/j.1439-0523.2007.01316.x
Borek, S., Ratajczak, W., Ratajczak, L. (2015). Regulation of storage lipid metabolism in developing and germinating lupin (Lupinus spp.) seeds. Acta Physiol. Plant 37, 1–11. doi: 10.1007/S11738-015-1871-2/FIGURES/4
Borisjuk, L., Nguyen, T. H., Neuberger, T., Rutten, T., Tschiersch, H., Claus, B., et al. (2005). Gradients of lipid storage, photosynthesis and plastid differentiation in developing soybean seeds. New Phytol. 167, 761–776. doi: 10.1111/J.1469-8137.2005.01474.X
Bryant, F. M., Munoz-Azcarate, O., Kelly, A. A., Beaudoin, F., Kurup, S., Eastmond, P. J. (2016). ACYL-ACYL CARRIER PROTEIN DESATURASE2 and 3 are responsible for making omega-7 fatty acids in thearabidopsis aleurone. Plant Physiol. 172, 154–162. doi: 10.1104/pp.16.00836
Burgal, J., Shockey, J., Lu, C., Dyer, J., Larson, T., Graham, I., et al. (2008). Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol. J. 6, 819–831. doi: 10.1111/j.1467-7652.2008.00361.x
Cahoon, E. B., Shockey, J. M., Dietrich, C. R., Gidda, S. K., Mullen, R. T., Dyer, J. M. (2007). Engineering oilseeds for sustainable production of industrial and nutritional feedstocks: solving bottlenecks in fatty acid flux. Curr. Opin. Plant Biol. 10, 236–244. doi: 10.1016/j.pbi.2007.04.005
Cai, G., Fan, C., Liu, S., Yang, Q., Liu, D., Wu, J., et al. (2020). Nonspecific phospholipase C6 increases seed oil production in oilseed brassicaceae plants. New Phytol. 226, 1055–1073. doi: 10.1111/nph.16473
Cai, Y., Goodman, J. M., Pyc, M., Mullen, R. T., Dyer, J. M., Chapmana, K. D. (2015). Arabidopsis SEIPIN proteins modulate triacylglycerol accumulation and influence lipid droplet proliferation. Plant Cell 27, 2616–2636. doi: 10.1105/tpc.15.00588
Cai, Y., McClinchie, E., Price, A., Nguyen, T. N., Gidda, S. K., Watt, S. C., et al. (2017). Mouse fat storage-inducing transmembrane protein 2 (FIT2) promotes lipid droplet accumulation in plants. Plant Biotechnol. J. 15, 824–836. doi: 10.1111/pbi.12678
Cai, G., Wang, G., Kim, S. C., Li, J., Zhou, Y., Wang, X. (2021). Increased expression of fatty acid and ABC transporters enhances seed oil production in camelina. Biotechnol. Biofuels 14, 49. doi: 10.1186/s13068-021-01899-w
Cao, Y., Huang, A. H. C. (1986). Diacylglycerol acyltransferase in maturing oil seeds of maize and other species. Plant Physiol. 82, 813–820. doi: 10.1104/pp.82.3.813
Cao, Y.-Z., Huang, A. H. C. (1987). Acyl coenzyme a preference of diacylglycerol acyltransferase from the maturing seeds of cuphea , maize, rapeseed, and canola. Plant Physiol. 84, 762–765. doi: 10.1104/pp.84.3.762
Carey, L. M., Clark, T. J., Deshpande, R. R., Cocuron, J. C., Rustad, E. K., Shachar-Hill, Y. (2020). High flux through the oxidative pentose phosphate pathway lowers efficiency in developing camelina seeds. Plant Physiol. 182, 493–506. doi: 10.1104/pp.19.00740
Cernac, A., Benning, C. (2004). WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in arabidopsis. Plant J. 40, 575–585. doi: 10.1111/J.1365-313X.2004.02235.X
Chapman, K. D., Neogi, P. B., Hake, K. D., Stawska, A. A., Speed, T. R., Cotter, M. Q., et al. (2008). Reduced oil accumulation in cottonseeds transformed with a brassica nonfunctional allele of a delta-12 fatty acid desaturase (FAD2). Crop Sci. 48, 1470–1481. doi: 10.2135/cropsci2007.11.0618
Chapman, K. D., Ohlrogge, J. B. (2012). Compartmentation of triacylglycerol accumulation in plants. J. Biol. Chem. 287, 2288–2294. doi: 10.1074/jbc.R111.290072
Cheng, T., Zhao, P., Ren, Y., Zou, J., Sun, M. X. (2021). AtMIF1 increases seed oil content by attenuating GL2 inhibition. New Phytol. 229, 2152–2162. doi: 10.1111/nph.17016
Chen, G., Harwood., J. L., Lemieux, M. J., Stone, S. J., Weselakea, R. J. (2022). Acyl-CoA:diacylglycerol acyltransferase: Properties, physiological roles, metabolic engineering and intentional control. Prog. Lipid Res. 88, 101181. doi: 10.1016/j.plipres.2022.101181
Chen, R., Matsui, K., Ogawa, M., Oe, M., Ochiai, M., Kawashima, H., et al. (2006). Expression of Δ6, Δ5 desaturase and GLELO elongase genes from mortierella alpina for production of arachidonic acid in soybean [Glycine max (L.) Merrill] seeds. Plant Sci. 170, 399–406. doi: 10.1016/j.plantsci.2005.09.006
Chen, X., Shachar-Hill, Y. (2012). Insights into metabolic efficiency from flux analysis. J. Exp. Bot. 63, 2343–2351. doi: 10.1093/jxb/ers057
Chen, L., Zheng, Y., Dong, Z., Meng, F., Sun, X., Fan, X., et al. (2018). Soybean (Glycine max) WRINKLED1 transcription factor, GmWRI1a, positively regulates seed oil accumulation. Mol. Genet. Genomics 293, 401–415. doi: 10.1007/s00438-017-1393-2
Cocuron, J. C., Alonso, A. P. (2014). Liquid chromatography tandem mass spectrometry for measuring 13C-labeling in intermediates of the glycolysis and pentose phosphate pathway. Methods Mol. Biol. 1090, 131–142. doi: 10.1007/978-1-62703-688-7_9
Cocuron, J. C., Koubaa, M., Kimmelfield, R., Ross, Z., Alonso, A. P. (2019). A combined metabolomics and fluxomics analysis identifies steps limiting oil synthesis in maize embryos. Plant Physiol. 181, 961–975. doi: 10.1104/PP.19.00920
Cocuron, J.-C., Ross, Z., Alonso, A. P. (2020). Liquid chromatography tandem mass spectrometry quantification of 13C-labeling in sugars. Metabolites 10, 30. doi: 10.3390/metabo10010030
Cocuron, J. C., Tsogtbaatar, E., Alonso, A. P. (2017). High-throughput quantification of the levels and labeling abundance of free amino acids by liquid chromatography tandem mass spectrometry. J. Chromatogr A 1490, 148–155. doi: 10.1016/j.chroma.2017.02.028
Crown, S. B., Kelleher, J. K., Rouf, R., Muoio, D. M., Antoniewicz, M. R. (2016). Comprehensive metabolic modeling of multiple13C-isotopomer data sets to study metabolism in perfused working hearts. Am. J. Physiol. Heart Circ. Physiol. 311, H881–H891. doi: 10.1152/ajpheart.00428.2016
Cui, Y., Liu, Z., Zhao, Y., Wang, Y., Huang, Y., Li, L., et al. (2017). Overexpression of heteromeric GhACCase subunits enhanced oil accumulation in upland cotton. Plant Mol. Biol. Rep. 35, 287–297. doi: 10.1007/s11105-016-1022-y
Deruyffelaere, C., Bouchez, I., Morin, H., Guillot, A., Miquel, M., Froissard, M., et al. (2015). Ubiquitin-mediated proteasomal degradation of oleosins is involved in oil body mobilization during post-germinative seedling growth in arabidopsis. Plant Cell Physiol. 56, 1374–1387. doi: 10.1093/pcp/pcv056
Deruyffelaere, C., Purkrtova, Z., Bouchez, I., Collet, B., Cacas, J. L., Chardot, T., et al. (2018). PUX10 IS a CDC48A adaptor protein that regulates the extraction of ubiquitinated oleosins from seed lipid droplets in arabidopsis. Plant Cell 30, 2116–2136. doi: 10.1105/tpc.18.00275
Dieuaide-Noubhani, M., Alonso, A. P. (2014). Application of metabolic flux analysis to plants. Methods Mol. Biol. 1090, 1–17. doi: 10.1007/978-1-62703-688-7_1
Ding, L. N., Guo, X. J., Li, M., Fu, Z. L., Yan, S. Z., Zhu, K. M., et al. (2019). Improving seed germination and oil contents by regulating the GDSL transcriptional level in brassica napus. Plant Cell Rep. 38, 243–253. doi: 10.1007/s00299-018-2365-7
Ding, L. N., Gu, S. L., Zhu, F. G., Ma, Z. Y., Li, J., Li, M., et al. (2020). Long-chain acyl-CoA synthetase 2 is involved in seed oil production in brassica napus. BMC Plant Biol. 20, 1–14. doi: 10.1186/s12870-020-2240-x
Dong, Z., Zhao, H., He, J., Huai, J., Lin, H., Zheng, J., et al. (2002). Overexpression of a foxtail millet acetyl-CoA carboxylase gene in maize increases sethoxydim resistance and oil conten. Afr J. Biotechnol. 10, 3986–3995. doi: 10.5897/AJB11.053
Du, H., Huang, M., Hu, J., Li, J. (2016). Modification of the fatty acid composition in arabidopsis and maize seeds using a stearoyl-acyl carrier protein desaturase-1 (ZmSAD1) gene. BMC Plant Biol. 16, 1–10. doi: 10.1186/s12870-016-0827-z
Durrett, T. P., Benning, C., Ohlrogge, J. (2008). Plant triacylglycerols as feedstocks for the production of biofuels. Plant J. 54, 593–607. doi: 10.1111/j.1365-313X.2008.03442.x
Eastmond, P., Koláčná, L., Rawsthorne, S. (1996). Photosynthesis by developing embryos of oilseed rape (Brassica napus l.). J. Exp. Bot. 47, 1763–1769. doi: 10.1093/JXB/47.11.1763
Eastmond, P. J., Rawsthorne, S. (2000). Coordinate changes in carbon partitioning and plastidial metabolism during the development of oilseed rape embryos. Plant Physiol. 112, 767–774. doi: 10.1104/pp.122.3.767
Eckert, H., LaVallee, B., Schweiger, B. J., Kinney, A. J., Cahoon, E. B., Clemente, T. (2006). Co-Expression of the borage Δ6 desaturase and the arabidopsis Δ15 desaturase results in high accumulation of stearidonic acid in the seeds of transgenic soybean. Planta 224, 1050–1057. doi: 10.1007/s00425-006-0291-3
Elahi, N., Duncan, R. W., Stasolla, C. (2015). Decreased seed oil production in FUSCA3 brassica napus mutant plants. Plant Physiol. Biochem. 96, 222–230. doi: 10.1016/j.plaphy.2015.08.002
Elahi, N., Duncan, R. W., Stasolla, C. (2016). Modification of oil and glucosinolate content in canola seeds with altered expression of brassica napus LEAFY COTYLEDON1. Plant Physiol. Biochem. 100, 52–63. doi: 10.1016/j.plaphy.2015.12.022
Esfahanian, M., Nazarenus, T. J., Freund, M. M., McIntosh, G., Phippen, W. B., Phippen, M. E., et al. (2021). Generating pennycress (Thlaspi arvense) seed triacylglycerols and acetyl-triacylglycerols containing medium-chain fatty acids. Front. Energy Res. 9. doi: 10.3389/fenrg.2021.620118
Ettaki, H., Troncoso-Ponce, M. A., To, A., Barthole, G., Lepiniec, L., Baud, S. (2018). Overexpression of MYB115, AAD2, or AAD3 in arabidopsis thaliana seeds yields contrasting omega-7 contents. PloS One 13, 1–19. doi: 10.1371/journal.pone.0192156
Fan, J., Yan, C., Zhang, X., Xu, C. (2013). Dual role for Phospholipid:Diacylglycerol acyltransferase: Enhancing fatty acid synthesis and diverting fatty acids from membrane lipids to triacylglycerol in arabidopsis leaves. Plant Cell 25, 3506–3518. doi: 10.1105/TPC.113.117358
Focks, N., Benning, C. (1998). wrinkled1: A novel, low-Seed-Oil mutant of arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol. 118, 91. doi: 10.1104/PP.118.1.91
Galili, G., Avin-Wittenberg, T., Angelovici, R., Fernie, A. R. (2014). The role of photosynthesis and amino acid metabolism in the energy status during seed development. Front. Plant Sci. 0. doi: 10.3389/FPLS.2014.00447
Gidda, S. K., Park, S., Pyc, M., Yurchenko, O., Cai, Y., Wu, P., et al. (2016). Lipid droplet-associated proteins (LDAPs) are required for the dynamic regulation of neutral lipid compartmentation in plant cells. Plant Physiol. 170, 2052–2071. doi: 10.1104/pp.15.01977
Goffman, F. D., Alonso, A. P., Schwender, J., Shachar-Hill, Y., Ohlrogge, J. B. (2005). Light enables a very high efficiency of carbon storage in developing embryos of rapeseed. Plant Physiol. 138, 2269–2279. doi: 10.1104/pp.105.063628
Goffman, F. D., Ruckle, M., Ohlrogge, J., Shachar-Hill, Y. (2004). Carbon dioxide concentrations are very high in developing oilseeds. Plant Physiol. Biochem. 42, 703–708. doi: 10.1016/J.PLAPHY.2004.07.003
Guan, R., Li, X., Hofvander, P., Zhou, X. R., Wang, D., Stymne, S., et al. (2015). RNAi targeting putative genes in phosphatidylcholine turnover results in significant change in fatty acid composition in crambe abyssinica seed oil. Lipids 50, 407–416. doi: 10.1007/s11745-015-4004-1
Guo, Z. H., Haslam, R. P., Michaelson, L., Yeung, E. C., Lung, S. C., Napier, J. A., et al. (2019). The overexpression of rice ACYL-CoA-BINDING PROTEIN2 increases grain size and bran oil content in transgenic rice. Plant J. 100, 1132–1147. doi: 10.1111/tpj.14503
Guzha, A., Whitehead, P., Ischebeck, T., Chapman, K. D. (2023). Lipid droplets: Packing hydrophobic molecules within the aqueous cytoplasm. 1–29. Annu. Rev. Plant Biol. 74, 1–29. doi: 10.1146/annurev-arplant-070122-021752
Hasan, M. M., Rahman, M. M. (2017). Performance and emission characteristics of biodiesel–diesel blend and environmental and economic impacts of biodiesel production: A review. Renewable Sustain. Energy Rev. 74, 938–948. doi: 10.1016/j.rser.2017.03.045
Haun, W., Coffman, A., Clasen, B. M., Demorest, Z. L., Lowy, A., Ray, E., et al. (2014). Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol. J. 12, 934–940. doi: 10.1111/pbi.12201
Hay, J. O., Shi, H., Heinzel, N., Hebbelmann, I., Rolletschek, H., Schwender, J. (2014). Integration of a constraint-based metabolic model of brassica napus developing seeds with 13C-metabolic flux analysis. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00724
He, X., Turner, C., Chen, G. Q., Lin, J. T., McKeon, T. A. (2004). Cloning and characterization of a cDNA encoding diacylglycerol acyltransferase from castor bean. Lipids 39, 311–318. doi: 10.1007/S11745-004-1234-2
Hong, H., Datla, N., Reed, D. W., Covello, P. S., MacKenzie, S. L., Qiu, X. (2002). High-level production of γ-linolenic acid in brassica juncea using a Δ6 desaturase from pythium irregulare. Plant Physiol. 129, 354–362. doi: 10.1104/pp.001495
Hong, Y., Xia, H., Li, X., Fan, R., Li, Q., Ouyang, Z., et al. (2022). Brassica napus BnaNTT1 modulates ATP homeostasis in plastids to sustain metabolism and growth. Cell Rep. 40, 111060. doi: 10.1016/j.celrep.2022.111060
Hsiao, E. S. L., Tzen, J. T. C. (2011). Ubiquitination of oleosin-h and caleosin in sesame oil bodies after seed germination. Plant Physiol. Biochem. 49, 77–81. doi: 10.1016/j.plaphy.2010.10.001
Huai, D., Zhang, Y., Zhang, C., Cahoon, E. B., Zhou, Y. (2015). Combinatorial effects of fatty acid elongase enzymes on nervonic acid production in camelina sativa. PloS One 10, e0131755. doi: 10.1371/journal.pone.0131755
Hu, Z., Wang, X., Zhan, G., Liu, G., Hua, W., Wang, H. (2009). Unusually large oilbodies are highly correlated with lower oil content in brassica napus. Plant Cell Rep. 28, 541–549. doi: 10.1007/S00299-008-0654-2
Ivarson, E., Leiva-Eriksson, N., Ahlman, A., Kanagarajan, S., Bülow, L., Zhu, L. H. (2017). Effects of overexpression of WRI1 and hemoglobin genes on the seed oil content of lepidium campestre. Front. Plant Sci. 7, 2032. doi: 10.3389/fpls.2016.02032
Jarvis, B. A., Romsdahl, T. B., McGinn, M. G., Nazarenus, T. J., Cahoon, E. B., Chapman, K. D., et al. (2021). CRISPR/Cas9-induced fad2 and rod1 mutations stacked with fae1 confer high oleic acid seed oil in pennycress (Thlaspi arvense l.). Front. Plant Sci. 12. doi: 10.3389/fpls.2021.652319
Jaworski, J., Cahoon, E. B. (2003). Industrial oils from transgenic plants. Curr. Opin. Plant Biol. 6, 178–184. doi: 10.1016/S1369-5266(03)00013-X
Jessen, D., Roth, C., Wiermer, M., Fulda, M. (2015). Two activities of long-chain acyl-coenzyme a synthetase are involved in lipid trafficking between the endoplasmic reticulum and the plastid in arabidopsis. Plant Physiol. 167, 351–366. doi: 10.1104/pp.114.250365
Johnston, M. L., Luethy, M. H., Miernyk, J. A., Randall, D. D. (1997). Cloning and molecular analyses of the arabidopsis thaliana plastid pyruvate dehydrogenase subunits. Biochim. Biophys. Acta Bioenerg 1321, 200–206. doi: 10.1016/S0005-2728(97)00059-5
Jolivet, P., Boulard, C., Bellamy, A., Larré, C., Barre, M., Rogniaux, H., et al. (2009). Protein composition of oil bodies from mature brassica napus seeds. Proteomics 9, 3268–3284. doi: 10.1002/PMIC.200800449
Jolivet, P., Roux, E., D’Andrea, S., Davanture, M., Negroni, L., Zivy, M., et al. (2004). Protein composition of oil bodies in arabidopsis thaliana ecotype WS. Plant Physiol. Biochem. 42, 501–509. doi: 10.1016/J.PLAPHY.2004.04.006
Jung, S. H., Kim, R. J., Kim, K. J., Lee, D. H., Suh, M. C. (2019). Plastidial and mitochondrial malonyl CoA-ACP malonyltransferase is essential for cell division and its overexpression increases storage oil content. Plant Cell Physiol. 60, 1239–1249. doi: 10.1093/pcp/pcz032
Kanai, M., Yamada, T., Hayashi, M., Mano, S., Nishimura, M. (2019). Soybean (Glycine max l.) triacylglycerol lipase GmSDP1 regulates the quality and quantity of seed oil. Sci. Rep. 9, 1–10. doi: 10.1038/s41598-019-45331-8
Kang, F., Rawsthorne, S. (1994). Starch and fatty acid synthesis in plastids from developing embryos of oilseed rape (Brassica napus l.). Plant J. 6, 795–805. doi: 10.1046/j.1365-313X.1994.6060795.x
Karki, N., Johnson, B. S., Bates, P. D. (2019). Metabolically distinct pools of phosphatidylcholine are involved in trafficking of fatty acids out of and into the chloroplast for membrane production. Plant Cell 31, 2768–2788. doi: 10.1105/tpc.19.00121
Karunarathna, N. L., Wang, H., Harloff, H. J., Jiang, L., Jung, C. (2020). Elevating seed oil content in a polyploid crop by induced mutations in SEED FATTY ACID REDUCER genes. Plant Biotechnol. J. 18, 2251–2266. doi: 10.1111/pbi.13381
Katavic, V., Friesen, W., Barton, D. L., Gossen, K. K., Giblin, E. M., Luciw, T., et al. (2000). “Utility of the arabidopsis FAEI and yeast SLCI-I genes for improvements in erucic acid and oil content in rapeseed,” in Biochemical society transactions (Portland Press), 935–937. doi: 10.1042/bst0280935
Kelly, A. A., Shaw, E., Powers, S. J., Kurup, S., Eastmond, P. J. (2013). Suppression of the SUGAR-DEPENDENT1 triacylglycerol lipase family during seed development enhances oil yield in oilseed rape (Brassica napus l.). Plant Biotechnol. J. 11, 355–361. doi: 10.1111/pbi.12021
Khan, K., Kumar, V., Niranjan, A., Shanware, A., Sane, V. A. (2019). JcMYB1, a jatropha R2R3MYB transcription factor gene, modulates lipid biosynthesis in transgenic plants. Plant Cell Physiol. 60, 462–475. doi: 10.1093/pcp/pcy223
Kim, M. J., Jang, I. C., Chua, N. H. (2016). The mediator complex MED15 subunit mediates activation of downstream lipid-related genes by the WRINKLED1 transcription factor. Plant Physiol. 171, 1951–1964. doi: 10.1104/pp.16.00664
Kim, R. J., Kim, H. U., Suh, M. C. (2019). Development of camelina enhanced with drought stress resistance and seed oil production by co-overexpression of MYB96A and DGAT1C. Ind. Crops Prod 138, 111475. doi: 10.1016/j.indcrop.2019.111475
Kim, T. Y., Sohn, S. B., Kim, Y., Kim, W. J., Lee, S. Y. (2012). Recent advances in reconstruction and applications of genome-scale metabolic models. Curr. Opin. Biotechnol. 23, 617–623. doi: 10.1016/j.copbio.2011.10.007
Kim, S., Yamaoka, Y., Ono, H., Kim, H., Shim, D., Maeshima, M., et al. (2013). AtABCA9 transporter supplies fatty acids for lipid synthesis to the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 110, 773–778. doi: 10.1073/pnas.1214159110
Kim, M. J., Yang, S. W., Mao, H. Z., Veena, S. P., Yin, J. L., Chua, N. H. (2014). Gene silencing of sugar-dependent 1 (JcSDP1), encoding a patatin-domain triacylglycerol lipase, enhances seed oil accumulation in jatropha curcas. Biotechnol. Biofuels 7, 1–16. doi: 10.1186/1754-6834-7-36
King, S. P., Badger, M. R., Furbank, R. T. (1998). CO2 refixation characteristics of developing canola seeds and silique wall. Aust. J. Plant Physiol. 25, 377–386. doi: 10.1071/PP97157
Koubaa, M., Cocuron, J. C., Thomasset, B., Alonso, A. P. (2013). Highlighting the tricarboxylic acid cycle: Liquid and gas chromatography-mass spectrometry analyses of 13C-labeled organic acids. Anal. Biochem. 436, 151–159. doi: 10.1016/j.ab.2013.01.027
Kretzschmar, F. K., Mengel, L. A., Müller, A. O., Schmitt, K., Blersch, K. F., Valerius, O., et al. (2018). PUX10 is a lipid droplet-localized scaffold protein that interacts with CELL DIVISION CYCLE48 and is involved in the degradation of lipid droplet proteins. Plant Cell 30, 2137–2160. doi: 10.1105/tpc.18.00276
Kroon, J. T. M., Wei, W., Simon, W. J., Slabas, A. R. (2006). Identification and functional expression of a type 2 acyl-CoA:diacylglycerol acyltransferase (DGAT2) in developing castor bean seeds which has high homology to the major triglyceride biosynthetic enzyme of fungi and animals. Phytochemistry 67, 2541–2549. doi: 10.1016/J.PHYTOCHEM.2006.09.020
Kruger, N. J., Masakapalli, S. K., Ratcliffe, R. G. (2012). Strategies for investigating the plant metabolic network with steady-state metabolic flux analysis: Lessons from an arabidopsis cell culture and other systems. J. Exp. Bot. 63, 2309–2323. doi: 10.1093/jxb/err382
Kubis, S. E., Pike, M. J., Everett, C. J., Hill, L. M., Rawsthorne, S. (2004). The import of phosphoenolpyruvate by plastids from developing embryos of oilseed rape, brassica napus (L.), and its potential as a substrate for fatty acid synthesis. J. Exp. Bot. 55, 1455–1462. doi: 10.1093/jxb/erh157
Kwong, R. W., Bui, A. Q., Lee, H., Kwong, L. W., Fischer, R. L., Goldberg, R. B., et al. (2003). LEAFY COTYLEDON1-LIKE defines a class of regulators essential for embryo development. Plant Cell 15, 5–18. doi: 10.1105/TPC.006973
Lee, H. G., Kim, H., Suh, M. C., Kim, H. U., Seo, P. J. (2018). The MYB96 transcription factor regulates triacylglycerol accumulation by activating DGAT1 and PDAT1 expression in arabidopsis seeds. Plant Cell Physiol. 59, 1432–1442. doi: 10.1093/pcp/pcy073
Lee, E. J., Oh, M., Hwang, J. U., Li-Beisson, Y., Nishida, I., Lee, Y. (2017). Seed-specific overexpression of the pyruvate transporter BASS2 increases oil content in arabidopsis seeds. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00194
Lee, S. Y., Park, J. M., Kim, T. Y. (2011). “Application of metabolic flux analysis in metabolic engineering,” in Methods in enzymology (Academic Press Inc), 67–93. doi: 10.1016/B978-0-12-385120-8.00004-8
Li, M., Bahn, S. C., Fan, C., Li, J., Phan, T., Ortiz, M., et al. (2013). Patatin-related phospholipase pPLAIIIδ increases seed oil content with long-chain fatty acids in arabidopsis. Plant Physiol. 162, 39–51. doi: 10.1104/PP.113.216994
Li-Beisson, Y., Shorrosh, B., Beisson, F., Andersson, M. X., Arondel, V., Bates, P. D., et al. (2013). Acyl-lipid metabolism. Arabidopsis Book / Am. Soc. Plant Biologists 11, e0161. doi: 10.1199/TAB.0161
Libourel, I. G. L., Shachar-Hill, Y. (2008). Metabolic flux analysis in plants: From intelligent design to rational engineering. Annu. Rev. Plant Biol. 59, 625–650. doi: 10.1146/annurev.arplant.58.032806.103822
Li, N., Gügel, I. L., Giavalisco, P., Zeisler, V., Schreiber, L., Soll, J., et al. (2015b). FAX1, a novel membrane protein mediating plastid fatty acid export. PloS Biol. 13, e1002053. doi: 10.1371/JOURNAL.PBIO.1002053
Li, D., Jin, C., Duan, S., Zhu, Y., Qi, S., Liu, K., et al. (2017). MYB89 transcription factor represses seed oil accumulation. Plant Physiol. 173, 1211–1225. doi: 10.1104/pp.16.01634
Li, L., Li, Y., Wang, R., Chao, L., Xiu, Y., Wang, H. (2020a). Characterization of the stearoyl-ACP desaturase gene (PoSAD) from woody oil crop paeonia ostii var. lishizhenii in oleic acid biosynthesis. Phytochemistry 178, 112480. doi: 10.1016/j.phytochem.2020.112480
Li, X., Mei, D., Liu, Q., Fan, J., Singh, S., Green, A., et al. (2016). Down-regulation of crambe fatty acid desaturase and elongase in arabidopsis and crambe resulted in significantly increased oleic acid content in seed oil. Plant Biotechnol. J. 14, 323–331. doi: 10.1111/pbi.12386
Li, N., Meng, H., Li, S., Zhang, Z., Zhao, X., Wang, S., et al. (2020b). Two plastid fatty acid exporters contribute to seed oil accumulation in arabidopsis. Plant Physiol. 182, 1910–1919. doi: 10.1104/PP.19.01344
Lim, A. R. Q., Kong, Q., Singh, S. K., Guo, L., Yuan, L., Ma, W. (2022). Sunflower WRINKLED1 plays a key role in transcriptional regulation of oil biosynthesis. Int. J. Mol. Sci. 23, 3054. doi: 10.3390/ijms23063054
Liu, Y., Han, J., Li, Z., Jiang, Z., Luo, L., Zhang, Y., et al. (2022). Heterologous expression of jatropha curcas fatty acyl-ACP thioesterase a (JcFATA) and b (JcFATB) affects fatty acid accumulation and promotes plant growth and development in arabidopsis. Int. J. Mol. Sci. 23, 4209. doi: 10.3390/IJMS23084209/S1
Liu, J., Hua, W., Yang, H. L., Zhan, G. M., Li, R. J., Deng, L.B., et al. (2012). The BnGRF2 gene (GRF2-like gene from brassica napus) enhances seed oil production through regulating cell number and plant photosynthesis. J. Exp. Bot. 63, 3727–3740. doi: 10.1093/jxb/ers066
Liu, J., Hua, W., Zhan, G., Wei, F., Wang, X., Liu, G., et al. (2010). Increasing seed mass and oil content in transgenic arabidopsis by the overexpression of wri1-like gene from brassica napus. Plant Physiol. Biochem. 48, 9–15. doi: 10.1016/j.plaphy.2009.09.007
Liu, W. X., Liu, H. L., Qu, L. Q. (2013). Embryo-specific expression of soybean oleosin altered oil body morphogenesis and increased lipid content in transgenic rice seeds. Theor. Appl. Genet. 126, 2289–2297. doi: 10.1007/s00122-013-2135-4
Liu, F., Xia, Y., Wu, L., Fu, D., Hayward, A., Luo, J., et al. (2015). Enhanced seed oil content by overexpressing genes related to triacylglyceride synthesis. Gene 557, 163–171. doi: 10.1016/j.gene.2014.12.029
Li, M., Wei, F., Tawfall, A., Tang, M., Saettele, A., Wang, X. (2015a). Overexpression of patatin-related phospholipase AIIIδ altered plant growth and increased seed oil content in camelina. Plant Biotechnol. J. 13, 766–778. doi: 10.1111/pbi.12304
Li, N., Xu, C., Li-Beisson, Y., Philippar, K. (2016a). Fatty acid and lipid transport in plant cells. Trends Plant Sci. 21, 145–158. doi: 10.1016/j.tplants.2015.10.011
Lonien, J., Schwender, J. (2009). Analysis of metabolic flux phenotypes for two arabidopsis mutants with severe impairment in seed storage lipid synthesis. Plant Physiol. 151, 1617–1634. doi: 10.1104/pp.109.144121
Lotan, T., Ohto, M. A., Matsudaira Yee, K., West, M. A. L., Lo, R., Kwong, R. W., et al. (1998). Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93, 1195–1205. doi: 10.1016/S0092-8674(00)81463-4
Lu, Y., Chi, M., Li, L., Li, H., Noman, M., Yang, Y., et al. (2018). Genome-wide identification, expression profiling, and functional validation of oleosin gene family in carthamus tinctorius l. Front. Plant Sci. 9, 1393. doi: 10.3389/fpls.2018.01393
Lu, C., Fulda, M., Wallis, J. G., Browse, J. (2006). A high-throughput screen for genes from castor that boost hydroxy fatty acid accumulation in seed oils of transgenic arabidopsis. Plant J. 45, 847–856. doi: 10.1111/J.1365-313X.2005.02636.X
Lung, S. C., Weselake, R. J. (2006). Diacylglycerol acyltransferase: A key mediator of plant triacylglycerol synthesis. Lipids 41, 1073–1088. doi: 10.1007/S11745-006-5057-Y
Lunn, D., Wallis, J. G., Browse, J. (2018). Overexpression of Seipin1 increases oil in hydroxy fatty acid-accumulating seeds. Plant Cell Physiol. 59, 205–214. doi: 10.1093/pcp/pcx177
Lu, C., Xin, Z., Ren, Z., Miquel, M., Browse, J. (2009). An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 106, 18837–18842. doi: 10.1073/PNAS.0908848106
Manan, S., Ahmad, M. Z., Zhang, G., Chen, B., Haq, B. U., Yang, J., et al. (2017). Soybean LEC2 regulates subsets of genes involved in controlling the biosynthesis and catabolism of seed storage substances and seed development. Front. Plant Sci. 8, 1604. doi: 10.3389/fpls.2017.01604
Marchive, C., Nikovics, K., To, A., Lepiniec, L., Baud, S. (2014). Transcriptional regulation of fatty acid production in higher plants: Molecular bases and biotechnological outcomes. Eur. J. Lipid Sci. Technol. 116, 1332–1343. doi: 10.1002/ejlt.201400027
Mastebroek, H. D., Marvin, H. J. P. (2000). Breeding prospects of lunaria annua l. Ind. Crops Prod 11, 139–143. doi: 10.1016/S0926-6690(99)00056-4
Ma, J., Sun, S., Whelan, J., Shou, H. (2021). CRISPR/Cas9-mediated knockout of GmFATB1 significantly reduced the amount of saturated fatty acids in soybean seeds. Int. J. Mol. Sci. 22, 3877. doi: 10.3390/IJMS22083877/S1
Meesapyodsuk, D., Chen, Y., Ye, S., Chapman, R. G., Qiu, X. (2021). Co-Expressing eranthis hyemalis lysophosphatidic acid acyltransferase 2 and elongase improves two very long chain polyunsaturated fatty acid production in brassica carinata. Metab. Eng. Commun. 12, e00171. doi: 10.1016/j.mec.2021.e00171
Mhaske, V., Beldjilali, K., Ohlrogge, J., Pollard, M. (2005). Isolation and characterization of an arabidopsis thaliana knockout line for phospholipid: diacylglycerol transacylase gene (At5g13640). Plant Physiol. Biochem. 43, 413–417. doi: 10.1016/J.PLAPHY.2005.01.013
Mietkiewska, E., Brost, J. M., Giblin, E. M., Barton, D. L., Taylor, D. C. (2007). Cloning and functional characterization of the fatty acid elongase 1 (FAE1) gene from high erucic crambe abyssinica cv. prophet. Plant Biotechnol. J. 5, 636–645. doi: 10.1111/j.1467-7652.2007.00268.x
Mietkiewska, E., Giblin, E. M., Wang, S., Barton, D. L., Dirpaul, J., Brost, J. M., et al. (2004). Seed-specific heterologous expression of a nasturtium FAE gene in arabidopsis results in a dramatic increase in the proportion of erucic acid. Plant Physiol. 136, 2665–2675. doi: 10.1104/pp.104.046839
Mietkiewska, E., Miles, R., Wickramarathna, A., Sahibollah, A. F., Greer, M. S., Chen, G., et al. (2014). Combined transgenic expression of punica granatum conjugase (FADX) and FAD2 desaturase in high linoleic acid arabidopsis thaliana mutant leads to increased accumulation of punicic acid. Planta 240, 575–583. doi: 10.1007/s00425-014-2109-z
Misra, A., Khan, K., Niranjan, A., Nath, P., Sane, V. A. (2013). Over-expression of JcDGAT1 from jatropha curcas increases seed oil levels and alters oil quality in transgenic arabidopsis thaliana. Phytochemistry 96, 37–45. doi: 10.1016/j.phytochem.2013.09.020
Möllney, M., Wiechert, W., Kownatzki, D., de Graaf, A. A. (1999). Bidirectional reaction steps in metabolic networks: IV. optimal design of isotopomer labeling experiments. Biotechnol. Bioeng 66, 86–103. doi: 10.1002/(SICI)1097-0290(1999)66:2<86::AID-BIT2>3.0.CO;2-A
Mu, J., Tan, H., Zheng, Q., Fu, Y., Liang, Y., Zhang, J., et al. (2008). LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in arabidopsis. Plant Physiol. 148, 1042–1054. doi: 10.1104/pp.108.126342
Nguyen, H. T., Park, H., Koster, K. L., Cahoon, R. E., Nguyen, H. T. M., Shanklin, J., et al. (2015). Redirection of metabolic flux for high levels of omega-7 monounsaturated fatty acid accumulation in camelina seeds. Plant Biotechnol. J. 13, 38–50. doi: 10.1111/PBI.12233
O’Grady, J., Schwender, J., Shachar-Hill, Y., Morgan, J. A. (2012). Metabolic cartography: Experimental quantification of metabolic fluxes from isotopic labelling studies. J. Exp. Bot. 63, 2293–2308. doi: 10.1093/jxb/ers032
Ozseyhan, M. E., Li, P., Na, G. N., Li, Z., Wang, C., Lu, C. (2018). Improved fatty acid profiles in seeds of camelina sativa by artificial microRNA mediated FATB gene suppression. Biochem. Biophys. Res. Commun. 503, 621–624. doi: 10.1016/J.BBRC.2018.06.051
Park, M. E., Kim, H. U. (2022). Applications and prospects of genome editing in plant fatty acid and triacylglycerol biosynthesis. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.969844
Parveez, G. K. A., Othman, A., Ramin, N., Bohari, B. (2010)Functional analysis of oil palm palmitoyl-acp thioesterase (FatB) gene via down-regulation in a model plant: Arabidopsis thaliana (Accessed September 30, 2022).
Petrie, J. R., Shrestha, P., Belide, S., Mansour, M. P., Liu, Q., Horne, J., et al. (2012). Transgenic production of arachidonic acid in oilseeds. Transgenic Res. 21, 139–147. doi: 10.1007/s11248-011-9517-7
Pleite, R., Pike, M. J., Garcés, R., Martínez-Force, E., Rawsthorne, S. (2005). The sources of carbon and reducing power for fatty acid synthesis in the heterotrophic plastids of developing sunflower (Helianthus annuus l.) embryos. J. Exp. Bot. 56, 1297–1303. doi: 10.1093/jxb/eri130
Pokotylo, I., Pejchar, P., Potocký, M., Kocourková, D., Krčková, Z., Ruelland, E., et al. (2013). The plant non-specific phospholipase c gene family. novel competitors in lipid signalling. Prog. Lipid Res. 52, 62–79. doi: 10.1016/J.PLIPRES.2012.09.001
Pouvreau, B., Baud, S., Vernoud, V., Morin, V., Py, C., Gendrot, G., et al. (2011). Duplicate maize wrinkled1 transcription factors activate target genes involved in seed oil biosynthesis. Plant Physiol. 156, 674–686. doi: 10.1104/pp.111.173641
Pracharoenwattana, I., Smith, S. M. (2008). When is a peroxisome not a peroxisome? Trends Plant Sci. 13, 522–525. doi: 10.1016/J.TPLANTS.2008.07.003
Price, A. M., Doner, N. M., Gidda, S. K., Jambunathan, S., James, C. N., Schami, A., et al. (2020). Mouse fat-specific protein 27 (FSP27) expressed in plant cells localizes to lipid droplets and promotes lipid droplet accumulation and fusion. Biochimie 169, 41–53. doi: 10.1016/j.biochi.2019.08.002
Puthur, J. T., Shackira, A. M., Saradhi, P. P., Bartels, D. (2013). Chloroembryos: A unique photosynthesis system. J. Plant Physiol. 170, 1131–1138. doi: 10.1016/j.jplph.2013.04.011
Pyc, M., Cai, Y., Gidda, S. K., Yurchenko, O., Park, S., Kretzschmar, F. K., et al. (2017). Arabidopsis lipid droplet-associated protein (LDAP) – interacting protein ( LDIP ) influences lipid droplet size and neutral lipid homeostasis in both leaves and seeds. Plant J. 92, 1182–1201. doi: 10.1111/tpj.13754
Ratcliffe, R. G., Shachar-Hill, Y. (2006). Measuring multiple fluxes through plant metabolic networks. Plant J. 45, 490–511. doi: 10.1111/j.1365-313X.2005.02649.x
Rodríguez-Rodríguez, M. F., Moreno-Pérez, A. J., Makni, S., Troncoso-Ponce, M. A., Acket, S., Thomasset, B., et al. (2021). Lipid profiling and oil properties of camelina sativa seeds engineered to enhance the production of saturated and omega-7 fatty acids. Ind. Crops Prod 170, 113765. doi: 10.1016/j.indcrop.2021.113765
Rolletschek, H., Radchuk, R., Klukas, C., Schreiber, F., Wobus, U., Borisjuk, L. (2005). Evidence of a key role for photosynthetic oxygen release in oil storage in developing soybean seeds. New Phytol. 167, 777–786. doi: 10.1111/J.1469-8137.2005.01473.X
Romsdahl, T. B., Kambhampati, S., Koley, S., Yadav, U. P., Alonso, A. P., Allen, D. K., et al. (2021). Analyzing mass spectrometry imaging data of13c-labeled phospholipids in camelina sativa and thlaspi arvense (Pennycress) embryos. Metabolites. 11, 148. doi: 10.3390/metabo11030148
Roscoe, T. T., Guilleminot, J., Bessoule, J. J., Berger, F., Devic, M. (2015). Complementation of seed maturation phenotypes by ectopic expression of ABSCISIC ACID INSENSITIVE3, FUSCA3 and LEAFY COTYLEDON2 in arabidopsis. Plant Cell Physiol. 56, 1215–1228. doi: 10.1093/pcp/pcv049
Rousselin, P., Molinier, J., Himber, C., Schontz, D., Prieto-Dapena, P., Jordano, J., et al. (2002). Modification of sunflower oil quality by seed-specific expression of a heterologous Δ9-stearoyl-(acyl carrier protein) desaturase gene. Plant Breed. 121, 108–116. doi: 10.1046/j.1439-0523.2002.00682.x
Ruuska, S. A., Schwender, J., Ohlrogge, J. B. (2004). The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes. Plant Physiol. 136, 2700–2709. doi: 10.1104/pp.104.047977
Samarth, N. B., Mahanwar, P. A. (2015). Modified vegetable oil based additives as a future polymeric material–review. Open J. Organic Polymer Materials 05, 1–22. doi: 10.4236/ojopm.2015.51001
Savadi, S., Naresh, V., Kumar, V., Bhat, S. R. (2015). Seed-specific overexpression of arabidopsis DGAT1 in Indian mustard (Brassica juncea) increases seed oil content and seed weight. Botany 94, 177–184. doi: 10.1139/cjb-2015-0218
Schwender, J., Goffman, F., Ohlrogge, J. B., Shachar-Hill, Y. (2004). Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds. Nature 432, 779–782. doi: 10.1038/nature03145
Schwender, J., Ohlrogge, J. B. (2002). Probing in vivo metabolism by stable isotope labeling of storage lipids and proteins in developing brassica napus embryos. Plant Physiol. 130, 347–361. doi: 10.1104/pp.004275
Schwender, J., Shachar-Hill, Y., Ohlrogge, J. B. (2006). Mitochondrial metabolism in developing embryos of brassica napus. J. Biol. Chem. 281, 34040–34047. doi: 10.1074/jbc.M606266200
Shachar-Hill, Y. (2013). Metabolic network flux analysis for engineering plant systems. Curr. Opin. Biotechnol. 24, 247–255. doi: 10.1016/j.copbio.2013.01.004
Shao, Q., Liu, X., Su, T., Ma, C., Wang, P. (2019). New insights into the role of seed oil body proteins in metabolism and plant development. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01568
Shearer, H. L., Turpin, D. H., Dennis, D. T. (2004). Characterization of NADP-dependent malic enzyme from developing castor oil seed endosperm. Arch. Biochem. Biophys. 429, 134–144. doi: 10.1016/j.abb.2004.07.001
Shen, B., Allen, W. B., Zheng, P., Li, C., Glassman, K., Ranch, J., et al. (2010). Expression of ZmLEC1 and ZmWRI1 increases seed oil production in maize. Plant Physiol. 153, 980–987. doi: 10.1104/pp.110.157537
Shi, L., Katavic, V., Yu, Y., Kunst, L., Haughn, G. (2012). Arabidopsis glabra2 mutant seeds deficient in mucilage biosynthesis produce more oil. Plant J. 69, 37–46. doi: 10.1111/j.1365-313X.2011.04768.x
Shimada, T. L., Shimada, T., Takahashi, H., Fukao, Y., Hara-Nishimura, I. (2008). A novel role for oleosins in freezing tolerance of oilseeds in arabidopsis thaliana. Plant J. 55, 798–809. doi: 10.1111/J.1365-313X.2008.03553.X
Shockey, J. M., Gidda, S. K., Chapital, D. C., Kuan, J. C., Dhanoa, P. K., Bland, J. M., et al. (2006). Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18, 2294–2313. doi: 10.1105/TPC.106.043695
Shockey, J., Lager, I., Stymne, S., Kotapati, H. K., Sheffield, J., Mason, C., et al. (2019). Specialized lysophosphatidic acid acyltransferases contribute to unusual fatty acid accumulation in exotic euphorbiaceae seed oils. Planta 249, 1285–1299. doi: 10.1007/S00425-018-03086-Y/FIGURES/8
Siloto, R. M. P., Findlay, K., Lopez-Villalobos, A., Yeung, E. C., Nykiforuk, C. L., Moloney, M. M. (2006). The accumulation of oleosins determines the size of seed oilbodies in arabidopsis. Plant Cell 18, 1961–1974. doi: 10.1105/TPC.106.041269
Singer, S. D., Greer, M. S., Mietkiewska, E., Pan, X., Weselake, R. J. (2013). “Genetic engineering of lipid biosynthesis in seeds,” in Biotechnology of crucifers (Springer New York), 111–149. doi: 10.1007/978-1-4614-7795-2_7
Singh, R., Arora, A., Singh, V. (2021). Biodiesel from oil produced in vegetative tissues of biomass – a review. Bioresour Technol. 326, 124772. doi: 10.1016/j.biortech.2021.124772
Sinha, S., Jha, J. K., Maiti, M. K., Basu, A., Mukhopadhyay, U. K., Sen, S. K. (2007). Metabolic engineering of fatty acid biosynthesis in Indian mustard (Brassica juncea) improves nutritional quality of seed oil. Plant Biotechnol. Rep. 1, 185–197. doi: 10.1007/s11816-007-0032-5
Slocombe, S. P., Cornah, J., Pinfield-Wells, H., Soady, K., Zhang, Q., Gilday, A., et al. (2009). Oil accumulation in leaves directed by modification of fatty acid breakdown and lipid synthesis pathways. Plant Biotechnol. J. 7, 694–703. doi: 10.1111/j.1467-7652.2009.00435.x
Smith, R. G., Gauthier, D. A., Dennis, D. T., Turpin, D. H. (1992). Malate- and pyruvate-dependent fatty acid synthesis in leucoplasts from developing castor endosperm. Plant Physiol. 98, 1233–1238. doi: 10.1104/pp.98.4.1233
Smith, M. A., Moon, H., Chowrira, G., Kunst, L. (2003). Heterologous expression of a fatty acid hydroxylase gene in developing seeds of arabidopsis thaliana. Planta 217, 507–516. doi: 10.1007/s00425-003-1015-6
Smith, M., Moon, H., Kunst, L. (2000). “Production of hydroxy fatty acids in the seeds of arabidopsis thaliana,” in Biochemical society transactions (Portland Press), 947–950. doi: 10.1042/bst0280947
Snapp, A. R., Kang, J., Qi, X., Lu, C. (2014). A fatty acid condensing enzyme from physaria fendleri increases hydroxy fatty acid accumulation in transgenic oilseeds of camelina sativa. Planta 240, 599–610. doi: 10.1007/s00425-014-2122-2
Song, G., Li, X., Munir, R., Khan, A. R., Azhar, W., Yasin, M. U., et al. (2020). The WRKY6 transcription factor affects seed oil accumulation and alters fatty acid compositions in Arabidopsis thaliana. Physiol. Plant 169, 612–624. doi: 10.1111/ppl.13082
Sorokin, A., Kim, E. R., Ovchinnikov, L. P. (2009). Proteasome system of protein degradation and processing. Biochem. (Moscow) 74, 1411–1442. doi: 10.1134/S000629790913001X
Stone, S. L., Kwong, L. W., Yee, K. M., Pelletier, J., Lepiniec, L., Fischer, R. L., et al. (2001). LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc. Natl. Acad. Sci. U.S.A. 98, 11806–11811. doi: 10.1073/PNAS.201413498/ASSET/33629AFE-C5C7-4684-9F25-24BA60D45D9F/ASSETS/GRAPHIC/PQ2014134006.JPEG
Su, Y., Liang, W., Liu, Z., Wang, Y., Zhao, Y., Ijaz, B., et al. (2017). Overexpression of GhDof1 improved salt and cold tolerance and seed oil content in gossypium hirsutum. J. Plant Physiol. 218, 222–234. doi: 10.1016/j.jplph.2017.07.017
Sun, R. H., Ye, R., Gao, L., Zhang, L., Wang, R., Mao, T., et al. (2017). Characterization and ectopic expression of CoWR1, an AP2/EREBP domain-containing transcription factor from coconut (cocos nucifera l.) endosperm, changes the seeds oil content in transgenic arabidopsis thaliana and rice (oryza sativa l.). Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00063
Tang, S., Guo, N., Tang, Q., Peng, F., Liu, Y., Xia, H., et al. (2022a). Pyruvate transporter BnaBASS2 impacts seed oil accumulation in brassica napus. Plant Biotechnol. J. 20, 2406–2417. doi: 10.1111/pbi.13922
Tang, S., Peng, F., Tang, Q., Liu, Y., Xia, H., Yao, X., et al. (2022b). BnaPPT1 is essential for chloroplast development and seed oil accumulation in brassica napus. J. Adv. Res. 42, 29–40. doi: 10.1016/j.jare.2022.07.008
Tang, G., Xu, P., Ma, W., Wang, F., Liu, Z., Wan, S., et al. (2018). Seed-specific expression of AtLEC1 increased oil content and altered fatty acid composition in seeds of peanut (Arachis hypogaea l.). Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00260
Tan, H., Yang, X., Zhang, F., Zheng, X., Qu, C., Mu, J., et al. (2011). Enhanced seed oil production in canola by conditional expression of brassica napus LEAFY COTYLEDON1 and LEC1-LIKE in developing seeds. Plant Physiol. 156, 1577–1588. doi: 10.1104/pp.111.175000
Taylor, D. C., Francis, T., Guo, Y., Brost, J. M., Katavic, V., Mietkiewska, E., et al. (2009). Molecular cloning and characterization of a KCS gene from cardamine graeca and its heterologous expression in brassica oilseeds to engineer high nervonic acid oils for potential medical and industrial use. Plant Biotechnol. J. 7, 925–938. doi: 10.1111/j.1467-7652.2009.00454.x
Taylor, D. C., Smith, M. A., Fobert, P., Mietkiewska, E., Weselake, R. J. (2011). “Metabolic engineering of higher plants to produce bio-industrial oils,” in Comprehensive biotechnology, second edition (Elsevier Inc). doi: 10.1016/B978-0-08-088504-9.00256-7
Tian, Y., Lv, X., Xie, G., Wang, L., Dai, T., Qin, X., et al. (2019). FAX2 mediates fatty acid export from plastids in developing arabidopsis seeds. Plant Cell Physiol. 60, 2231–2242. doi: 10.1093/pcp/pcz117
Tian, Y., Lv, X., Xie, G., Zhang, J., Xu, Y., Chen, F. (2018). Seed-specific overexpression of AtFAX1 increases seed oil content in arabidopsis. Biochem. Biophys. Res. Commun. 500, 370–375. doi: 10.1016/j.bbrc.2018.04.081
Troncoso-Ponce, M. A., Barthole, G., Tremblais, G., To, A., Miquel, M., Lepiniec, L., et al. (2016). Transcriptional activation of two delta-9 palmitoyl-acp desaturase genes by myb115 and myb118 is critical for biosynthesis of omega-7 monounsaturated fatty acids in the endosperm of arabidopsis seeds. Plant Cell 28, 2666–2682. doi: 10.1105/tpc.16.00612
Tschiersch, H., Borisjuk, L., Rutten, T., Rolletschek, H. (2011). Gradients of seed photosynthesis and its role for oxygen balancing. BioSystems 103, 302–308. doi: 10.1016/j.biosystems.2010.08.007
Tsogtbaatar, E., Cocuron, J.-C., Alonso, A. P. (2020). Non-conventional pathways enable pennycress (Thlaspi arvense l.) embryos to achieve high efficiency of oil biosynthesis. J. Exp. Bot. 71, 3037–3051. doi: 10.1093/jxb/eraa060
van Erp, H., Bates, P. D., Burgal, J., Shockey, J., Browse, J. (2011). Castor phospholipid:Diacylglycerol acyltransferase facilitates efficient metabolism of hydroxy fatty acids in transgenic arabidopsis. Plant Physiol. 155, 683–693. doi: 10.1104/pp.110.167239
van Erp, H., Kelly, A. A., Menard, G., Eastmond, P. J. (2014). Multigene engineering of triacylglycerol metabolism boosts seed oil content in arabidopsis. Plant Physiol. 165, 30–36. doi: 10.1104/pp.114.236430
Vanhercke, T., Dyer, J. M., Mullen, R. T., Kilaru, A., Rahman, M. M., Petrie, J. R., et al. (2019). Metabolic engineering for enhanced oil in biomass. Prog. Lipid Res. 74, 103–129. doi: 10.1016/j.plipres.2019.02.002
Vanhercke, T., el Tahchy, A., Shrestha, P., Zhou, X. R., Singh, S. P., Petrie, J. R. (2013). Synergistic effect of WRI1 and DGAT1 coexpression on triacylglycerol biosynthesis in plants. FEBS Lett. 587, 364–369. doi: 10.1016/j.febslet.2012.12.018
Wang, X. (2005). Regulatory functions of phospholipase d and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol. 139, 566–573. doi: 10.1104/pp.105.068809
Wang, K., Froehlich, J. E., Zienkiewicz, A., Hersh, H. L., Benning, C. (2017). A plastid phosphatidylglycerol lipase contributes to the export of acyl groups from plastids for seed oil biosynthesis. Plant Cell 29, 1678–1696. doi: 10.1105/tpc.17.00397
Wang, M., Garneau, M. G., Poudel, A. N., Lamm, D., Koo, A. J., Bates, P. D., et al. (2022). Overexpression of pea α-carboxyltransferase in arabidopsis and camelina increases fatty acid synthesis leading to improved seed oil content. Plant J. 110, 1035–1046. doi: 10.1111/tpj.15721
Wang, Z., Huang, W., Chang, J., Sebastian, A., Li, Y., Li, H., et al. (2014). Overexpression of SiDGAT1, a gene encoding acyl-CoA:diacylglycerol acyltransferase from sesamum indicum l. increases oil content in transgenic arabidopsis and soybean. Plant Cell Tissue Organ Cult 119, 399–410. doi: 10.1007/s11240-014-0543-z
Wiechert, W., De Graaf, A. A. (1997). Bidirectional reaction steps in metabolic networks: I. modeling and simulation of carbon isotope labeling experiments. Biotechnol. Bioeng 55, 101–117. doi: 10.1002/(SICI)1097-0290(19970705)55:1
Wiechert, W., Möllney, M., Isermann, N., Wurzel, M., de Graaf, A. A. (1999). Bidirectional reaction steps in metabolic networks: III. explicit solution and analysis of isotopomer labeling systems. Sons Inc. Biotechnol. Bioeng 66, 69–85. doi: 10.1002/(SICI)1097-0290(1999)66:2<69::AID-BIT1>3.0.CO;2-6
Wiechert, W., Möllney, M., Petersen, S., de Graaf, A. A. (2001). A universal framework for 13C metabolic flux analysis. Metab. Eng. 3, 265–283. doi: 10.1006/mben.2001.0188
Winichayakul, S., William Scott, R., Roldan, M., Bertrand Hatier, J. H., Livingston, S., Cookson, R., et al. (2013). In vivo packaging of triacylglycerols enhances arabidopsis leaf biomass and energy density. Plant Physiol. 162, 626–639. doi: 10.1104/pp.113.216820
Xia, H., Hong, Y., Li, X., Fan, R., Li, Q., Ouyang, Z., et al. (2022). BnaNTT2 regulates ATP homeostasis in plastid to sustain lipid metabolism and plant growth in brassica napus. Mol. Breed. 42, 1–19. doi: 10.1007/s11032-022-01322-8
Xiao, Z., Tang, F., Zhang, L., Li, S., Wang, S., Huo, Q., et al. (2021). The brassica napus fatty acid exporter FAX1-1 contributes to biological yield, seed oil content, and oil quality. Biotechnol. Biofuels 14, 190. doi: 10.1186/s13068-021-02035-4
Xu, X. Y., Yang, H. K., Singh, S. P., Sharp, P. J., Liu, Q. (2018). Genetic manipulation of non-classic oilseed plants for enhancement of their potential as a biofactory for triacylglycerol production. Engineering 4, 523–533. doi: 10.1016/j.eng.2018.07.002
Yang, Z., Liu, X., Wang, K., Li, Z., Jia, Q., Zhao, C., et al. (2022). ABA-INSENSITIVE 3 with or without FUSCA3 highly up-regulates lipid droplet proteins and activates oil accumulation. J. Exp. Bot. 73, 2077–2092. doi: 10.1093/jxb/erab524
Yatsu, L. Y., Jacks, T. J. (1972). Spherosome membranes. Plant Physiol. 49, 937–943. doi: 10.1104/pp.49.6.937
Yin, D., Deng, S., Zhan, K., Cui, D. (2007). High-oleic peanut oils produced by HpRNA-mediated gene silencing of oleate desaturase. Plant Mol. Biol. Rep. 25, 154–163. doi: 10.1007/s11105-007-0017-0
Yin, D. D., Xu, W. Z., Shu, Q. Y., Li, S. S., Wu, Q., Feng, C. Y., et al. (2018). Fatty acid desaturase 3 (PsFAD3) from paeonia suffruticosa reveals high α-linolenic acid accumulation. Plant Sci. 274, 212–222. doi: 10.1016/j.plantsci.2018.05.027
Yu, K., McCracken, C. T., Li, R., Hildebrand, D. F. (2006). Diacylglycerol acyltransferases from vernonia and stokesia prefer substrates with vernolic acid. Lipids 41, 557–566. doi: 10.1007/S11745-006-5005-X
Zhang, M., Fan, J., Taylor, D. C., Ohlrogge, J. B. (2010). DGAT1 and PDAT1 acyltransferases have overlapping functions in arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell 21, 3885–3901. doi: 10.1105/TPC.109.071795
Zhang, Y. Q., Lu, X., Zhao, F. Y., Li, Q. T., Niu, S. L., Wei, W., et al. (2016). Soybean GmDREBL increases lipid content in seeds of transgenic arabidopsis. Sci. Rep. 6, 1–13. doi: 10.1038/srep34307
Zhang, D., Zhang, H., Hu, Z., Chu, S., Yu, K., Lv, L., et al. (2019). Artificial selection on GmOLEO1 contributes to the increase in seed oil during soybean domestication. PloS Genet. 15, 1008267. doi: 10.1371/journal.pgen.1008267
Zhao, Y., Cao, P., Cui, Y., Liu, D., Li, J., Zhao, Y., et al. (2021). Enhanced production of seed oil with improved fatty acid composition by overexpressing NAD+-dependent glycerol-3-phosphate dehydrogenase in soybean. J. Integr. Plant Biol. 63, 1036–1053. doi: 10.1111/jipb.13094
Zhao, N., Zhang, Y., Li, Q., Li, R., Xia, X., Qin, X., et al. (2015). Identification and expression of a stearoyl-ACP desaturase gene responsible for oleic acid accumulation in xanthoceras sorbifolia seeds. Plant Physiol. Biochem. 87, 9–16. doi: 10.1016/j.plaphy.2014.12.009
Zhou, B., Fei, W., Yang, S., Yang, F., Qu, G., Tang, W., et al. (2020). Alteration of the fatty acid composition of brassica napus l. via overexpression of phospholipid: Diacylglycerol acyltransferase 1 from sapium sebiferum (L.) roxb. Plant Sci. 298, 110562. doi: 10.1016/j.plantsci.2020.110562
Keywords: carbon conversion efficiency, embryo culture, fatty acid synthesis, isolated plastids, lipid storage, metabolic flux analysis, oilseed, triacylglycerol
Citation: Sagun JV, Yadav UP and Alonso AP (2023) Progress in understanding and improving oil content and quality in seeds. Front. Plant Sci. 14:1116894. doi: 10.3389/fpls.2023.1116894
Received: 05 December 2022; Accepted: 09 January 2023;
Published: 26 January 2023.
Edited by:
Frederic Beaudoin, Rothamsted Research, United KingdomReviewed by:
Adrian Troncoso, University of Technology Compiegne, FranceCopyright © 2023 Sagun, Yadav and Alonso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Paula Alonso, QW5hcGF1bGEuQWxvbnNvQHVudC5lZHU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.