- 1Technology Center of China Tobacco Yunnan Industrial Co. Ltd., Kunming, China
- 2College of Plant Protection, Shandong Agricultural University, Tai’an, China
- 3Honghe Tobacco Company, Mile, China
- 4Yuxi Cigarette Factory, Hongta Tobacco Group Co. Ltd., Yuxi, China
Tobacco belongs to the family Solanaceae, which easily forms continuous cropping obstacles. Continuous cropping exacerbates the accumulation of autotoxins in tobacco rhizospheric soil, affects the normal metabolism and growth of plants, changes soil microecology, and severely reduces the yield and quality of tobacco. In this study, the types and composition of tobacco autotoxins under continuous cropping systems are summarized, and a model is proposed, suggesting that autotoxins can cause toxicity to tobacco plants at the cell level, plant-growth level, and physiological process level, negatively affecting soil microbial life activities, population number, and community structure and disrupting soil microecology. A combined strategy for managing tobacco autotoxicity is proposed based on the breeding of superior varieties, and this approach can be combined with adjustments to cropping systems, the induction of plant immunity, and the optimization of cultivation and biological control measures. Additionally, future research directions are suggested and challenges associated with autotoxicity are provided. This study aims to serve as a reference and provide inspirations needed to develop green and sustainable strategies and alleviate the continuous cropping obstacles of tobacco. It also acts as a reference for resolving continuous cropping challenges in other crops.
1 Introduction
Tobacco is an economically important crop with a long worldwide cultivation history, and it is widely studied as a significant model plant that helps lay a foundation for agricultural biotechnological research (Sierro et al., 2014). Due to limited farmland areas and a lack of scientific cultivation methods, continuous tobacco cropping is often subject to continuous cropping obstacles even in the absence of major challenges such as pests, fertility, or climate change, and these obstacles cause poor growth of seedlings and a significant decrease in crop yield and quality (Chi et al., 2013; Niu et al., 2017). The causes of sustained decline in tobacco yield and quality are multifaceted, but autotoxicity is considered the most important influencing factor (Sun, 2010; Deng et al., 2017b).
Allelopathy broadly exists in the competition of plants and organisms for light, water, nutrients, and space, exerting an effect on the renewal of organisms, community succession, and seed germination in an ecosystem. As a particular form of allelopathy, autotoxicity affects plant growth in multiple ways, such as influencing cell membrane permeability, ion absorption, photosynthesis, and enzymatic activity, making it the major cause of continuous cropping obstacles for tobacco (Liu et al., 2010; Zhang et al., 2018; Zhang et al., 2021). Tobacco is fundamentally different from other crops in that it contains special bioactive substances, such as the aromatic components in secondary metabolites, and these causes tobacco to be more susceptible to allelopathic autotoxicity (Farooq et al., 2014; Deng et al., 2017b).
Soil microorganisms participate in many vital processes in the dynamics of the soil ecosystem, including the nutrient cycle, organic matter turnover, soil structure maintenance, and toxin degradation (Brussaard et al., 2007). Due to the rapid response of soil microorganisms to environmental changes and agricultural practices, they are considered a critical biological indicator for the efficacy of soil fertility and land management measures and are also known as the second genome of plants (Avidano et al., 2005; Wu et al., 2017). Changes in soil microflora are closely associated with continuous cropping obstacles as they significantly impact those vital processes in the soil ecosystem (Brussaard et al., 2007). The long-term continuous cropping of tobacco causes changes in the number of soil microorganisms, an imbalance in soil microecosystems, and a reduction in soil fertility, thus severely damaging the physicochemical properties of soil and the ecological environment. Under such influences, tobacco tends to exhibit retarded growth, dwarfed plants, reduced leaf area, and worsened diseases and pests, causing a decline in both yield and quality (Elsas et al., 2002; Nayyar et al., 2010). Therefore, researching the interactions between autotoxins and rhizosphere microorganisms lays a theoretical foundation for identifying the formation mechanisms of continuous cropping obstacles and the patterns of succession in the rhizosphere microorganism community.
Currently, tobacco production mainly relies on the application of pesticides and fertilizers, which not only causes cost increases and degrade tobacco quality but also pollutes farmland soil and the water environment, ultimately threatening human health. Research focusing on inducing plant immunity, improving cultivation measures, and utilizing microbiological methods to reduce continuous cropping obstacles during tobacco production can provide significant guidance and new approaches for seeking effective technologies that can sustainably improve the growth of continuously cropped tobacco.
2 The concept of autotoxins and component analysis of tobacco autotoxins
Autotoxins can be generated by plant roots, stems, leaves, and fruits. These autotoxins contain a variety of carbon-based primary metabolites and more complex secondary compounds, such as root exudates, making them the largest inputs of chemical substances into the rhizosphere (Bertin et al., 2003; Hao et al., 2010; Huang et al., 2013). Autotoxins are thus considered the largest source of allelochemicals. These substances can be released into the environment through aboveground leaching, volatilization, root secretion, degradation and leaching, and some autotoxins, upon reaching a certain level of concentration, can cause autotoxicity in continuously cropped plants (Rial et al., 2014; Hisashi et al., 2017).
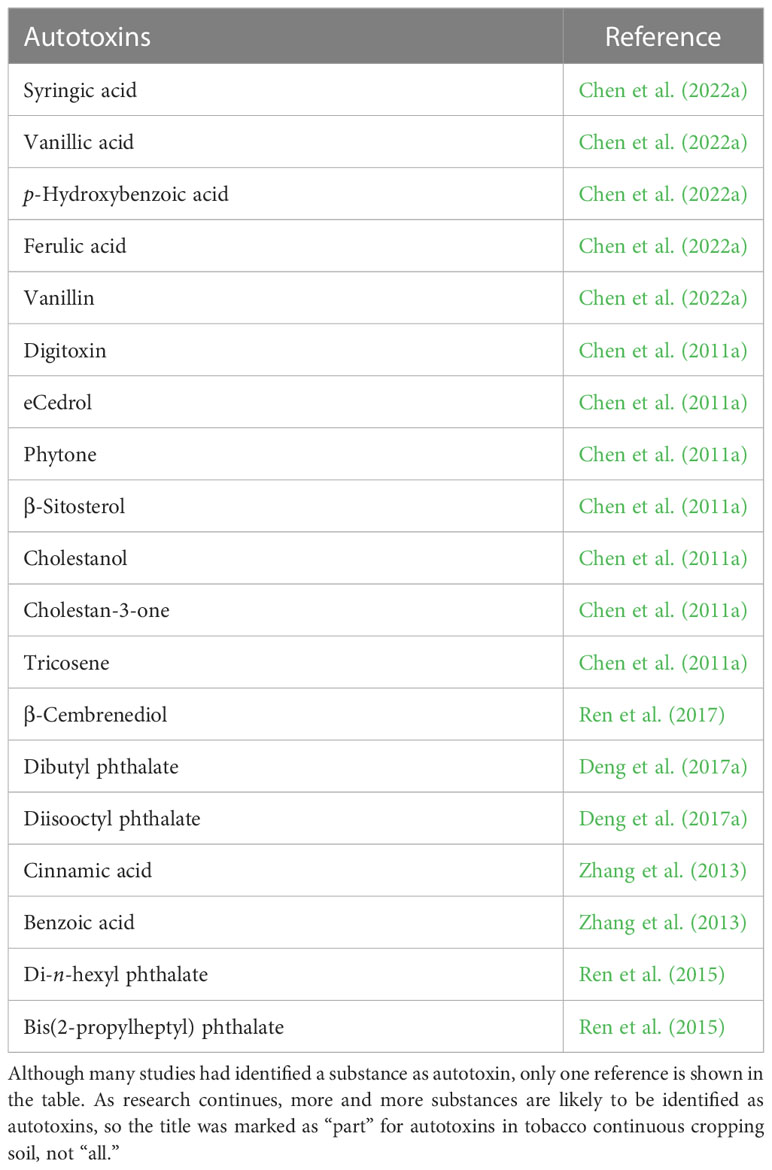
Autotoxicity poses a major threat to tobacco plants. On the one hand, it stimulates the growth of rhizospheric pathogenic bacteria while inhibiting that of beneficial microorganisms; on the other hand, it inhibits plant growth by affecting membrane systems, photosynthesis, and the enzymatic activity of plants, causing an allelopathic effect and inducing continuous cropping obstacles (Inderjit et al., 2006; Chen et al., 2022a). To clarify autotoxic and allelopathic effects, researchers have collected tobacco root exudates, and isolated, purified, and characterized autotoxins and evaluated their autotoxicity. Research indicates that autotoxins are mostly small molecules containing -OH, C=O, and S→O groups. They have simple structures and are difficult to degrade. These molecules contain oxygen atoms and easily excited double and triple bonds and are susceptible to release into the environment (Zhang et al., 2007b; Yu et al., 2015). Autotoxins are generally divided into water-soluble organic acids, linear alcohols, aliphatic aldehydes, and alkenes; simple phenols, benzoic acids, and their derivatives; simple unsaturated lactones, long-chain fatty acids, and polyacetylenes; naphthoquinone, anthraquinone, and quinone compounds; cinnamic acids and their derivatives; coumarins, tannins, terpenoids, and sterides; amino acids and polypeptides; alkaloids and cyanohydrins; sulfides and glucosinolates; and purines and nucleosides (Zhang et al., 2011b; Scavo et al., 2018; Blum, 2019). Many autotoxins associated with continuous cropping obstacles (p-hydroxybenzoic acid, homovanillic acid, vanillic acid, vanillin, cinnamic acid, ferulic acid, cumaric acid, benzoic acid, sesamin, momilactone B, etc.) have already been studied in different plant models (Kato-Noguchi et al., 2002; Nakano et al., 2006; Li et al., 2012; Ni et al., 2012; Yeasmin et al., 2014).
Using soils used for continuous tobacco cropping for 12 years, researchers comparatively examined the autotoxic potentials and differences in major chemical components between continuously cropped soils and controlled samples (Chen et al., 2011a). The study revealed that the rhizospheric soil of continuously cropped tobacco and its leach liquor had significant allelopathic autotoxicity against receiving plants such as lettuce and tobacco seedlings. GC-MS analysis showed that eight specific substances in the tobacco rhizospheric soil were associated with allelopathic autotoxicity, and vanillin showed relatively strong allelopathy; in contrast, only one alcohol with allelopathic autotoxicity was found in the control sample (Table 1) (Chen et al., 2011a). The root exudates of tobacco contain various secondary compounds, and some are capable of accumulating around the rhizosphere and causing autotoxicity (Walker et al., 2003; Xie et al., 2007). β-Cembrenediol is considered as an essential autotoxin in the root metabolites of tobacco, which affects plant mitosis, enhances the generation of reactive oxygen and induces oxidative damage, increases the degree of lipid peroxidation of membranes, inhibits root and stem elongation, reduces the content of chlorophyll, and causes cell death (Ren et al., 2017). Substances such as din-butyl phthalate (DBP) and diisobutyl phthalate (DIBP) have been confirmed to be major autotoxins. At concentrations greater than 0.5 mmol, both substances have significant inhibitory effects on seed germination and seedling growth in tobacco and exhibit a synergistic effect for autotoxicity (Zhang et al., 2015; Deng et al., 2017a). Similarly, ferulic acid, benzoic acid, phthalates, and phenolic acids generated from the degradation of organic residues may be important autotoxins that cause the degradation of tobacco leaves (Yi et al., 2012). Furthermore, insect attractants such as muscalure resulting from the long-term continuous cropping of tobacco can attract pests and cause damage to tobacco growth (Miao et al., 2004; Chen et al., 2011a).
3 Effects of autotoxins on the growth of tobacco
Autotoxicity is a special type of intraspecific competition, and it involves interactions between individuals using limited resources, which usually leads to density dependence or to self-thinning of plants. Autotoxin types vary by plant types (Tu et al., 2000), and factors such as the physicochemical properties of soil, abiotic stress, and microorganisms can cause intra- and interspecies differences in the types and concentrations of autotoxins. Different stimulation intensities of the various factors induce plant root systems to release different substances into the environment (Feng et al., 2010; Qin et al., 2021), including secretions, exudates, lysates, and mucilage. Specifically, substances that inhibit the growth of related plants are called autotoxins (Alías et al., 2006). Relative to plants in mature stages, those in the seed germination and seedling growth stages are considered more important for evaluating autotoxicity processes (Lara-Núñez et al., 2010; Margot et al., 2012), as plants are more susceptible to the effect of autotoxins during these stages (Callaway and Aschehoug, 2000; Callaway and Ridenour, 2004; Weir et al., 2004). Many autotoxins have been found to affect seed germination, seedling growth, photosynthesis, nutrient absorption, cell division, cytoskeleton formation, generation of reactive oxygen species, and the expression of functional genes (Figure 1)(Inderjit and Duke, 2003; Blum and Gerig, 2005; Zhang et al., 2010a; Soltys et al., 2011).

Figure 1 Effects of autotoxins on tobacco and soil microorganisms. Autotoxins affect tobacco at three levels of cell, growth, and physiological processes and affect soil microorganisms at three levels of life action, population, and community.
Autotoxins affect tobacco growth conditions in fields and its agronomic traits, causing a reduction in growth parameters such as plant height and leaf area coefficients during the vigorous growing and budding stages and to poor root growth and development (You et al., 2015a). Long-term continuous cropping of tobacco leads to an accumulation of large amounts of autotoxins, causing a decline in tobacco biomass, yield, and quality; a decrease in tobacco photosynthesis, transpiration rate, and potassium and sugar contents; an increase in nicotine content; and a degradation in aroma quality (Jing and Matsui, 1997; Yu et al., 2000; Chen et al., 2010; Zhang et al., 2011a; Chen et al., 2022b). A study indicated that as autotoxins accumulate, the weights of tobacco stems, roots, and leaves exhibit significant declining trends (Zhang et al., 2007a). Increasing the time of continuous tobacco cropping leads to a significant reduction in the total sugar level, reducing sugar and potassium levels, and to a downward trend of its major economic trait indicators, leading to adverse effects on smoking quality (Fu et al., 2018). A number of studies have shown that 1 year of continuous cropping causes a reduction in the total nitrogen content of tobacco, 2 years of continuous cropping causes an upward trend in nicotine content, and 3(+) years of continuous cropping significantly reduces the percentage of medium-grade tobacco and its qualities, as well as its Schmuck value, K/Cl ratio, and sugar-to-nicotine ratio (Jin et al., 2002; Jin et al., 2004; Zhao et al., 2008). The effect of autotoxins on tobacco varies based on their concentrations. Among the root metabolites of tobacco, benzoic acid, cinnamic acid, and p-hydroxybenzoic acid significantly inhibit the growth of tobacco radicles at concentrations higher than 100 μg/ml, whereas ferulic acid significantly inhibits tobacco seed germination, seedling growth, and radicle elongation (Zhang et al., 2013).
4 Interaction between autotoxins and soil microorganisms
The types and numbers of root exudates, which serve as the medium for interactions between plants and rhizosphere microorganisms, are important factors influencing the number, activity, and diversity of soil microorganisms (Bais et al., 2006). The carbohydrates, organic acids, amino acids, ectoenzymes, and autotoxins contained in root exudates not only provide energy, signaling molecules, and growth substrates for the growth and reproduction of rhizosphere microorganisms but also exert selective and facilitating effects on particular microbial populations (Badri and Vivanco, 2009; Gabriele and Kornelia, 2010; Huang et al., 2014; Rohrbacher and St-Arnaud, 2016). By regulating nutrient absorption, as well as the growth and development of plants and soil properties, autotoxins indirectly control the diversity of rhizosphere microorganisms (Broeckling et al., 2008). Such changes stimulate root systems to accumulate more autotoxins, simplifying the microbial population structure of the rhizospheric soil, reducing the types of dominant soil microorganisms populations, and making them mainly concentrated on Acidobacteria (Liu et al., 2016; Li, 2017; Chen et al., 2018b). In contrast, the dominant soil microorganism populations in tobacco rotation-cropped fields are primarily Acidobacteria, γ-proteobacteria, and α-proteobacteria, showing a high level of microbial diversity (Duan et al., 2012). The longer continuous cropping is practiced, the worse the tobacco diseases (Chen et al., 2022b). Dysfunctions or variations in the flora of soil microorganisms associated with tobacco plants cause a reduction in the number, abundance, and diversity of probiotic bacterial populations in soil (ammonificator and nitrifier), a decrease in the number of bacteria, and an increase in the number of fungi and actinomycetes (Wang et al., 2008), inducing a shift in the continuously cropped soil from highly fertile “bacterial” soil to less fertile “fungal” soil (Niu et al., 2017). This increases the number of pathogens and disease morbidity rates of tobacco, causing continuous cropping obstacles (Figure 1) (Duan et al., 2012). Black shank disease, tobacco mosaic, root-knot nematode, black root rot, tobacco black death disease, and tobacco bacterial wilt are all positively correlated with the accumulation of autotoxins (Zhang et al., 2011b). Autotoxins such as gallic acid, p-hydroxybenzoic acid, and ortho-hydroxybenzoic acid also stimulate the germination of spores of bacteria causing Fusarium wilt and Verticillium wilt (Zhang et al., 2012b). In addition, the activities of urease, acidic phosphatase, and saccharase in rhizospheric soil also gradually decrease, compared with a significant increase in the activity of catalase (Zhang et al., 2007c).
The accumulation of beneficial rhizospheric substances may be an important factor in reducing autotoxin-induced damage. As important components that sustain the productivity of soil, rhizosphere microorganisms affect the structure, function, and processes of soil ecosystems (Chen et al., 2022b), inhibit soil-borne diseases in host plants, increase plant nutrient absorption and stress resistance, and decompose autotoxins, thereby facilitating plant growth. Research shows that inoculating plants with Pseudomonas putida helps decompose 99.47% of p-hydroxybenzoic acid in Hoagland’s nutrient solution within 72 h (Chen et al., 2015). Pseudomonas putida, Pseudomonas nitroreducens, and Rhodotorula glutinis can effectively decompose ferulic acid, p-hydroxybenzoic acid, and p-hydroxybenzaldehyde (Zhang et al., 2010b). Micrococcus lylae, Phyllobacterium myrsinacearum, and Leminorella grimontii can decompose oleic acid, hexadecanoic acid, and phthalic acid, respectively, and multistrain bacterial assemblages can achieve a degradation rate of 66.7% for allelochemicals (Zhao et al., 2016). Small molecular volatile compounds generated by microbial metabolism spread quickly in the atmosphere and soil (Hung et al., 2015). For example, signaling factors such as N-acyl-L-homoserine lactones significantly upregulate the expression of genes associated with vegetative storage proteins, γ-glutamyl hydrolase, and Rubisco large proteins, thus increasing the systemic resistance in plants (Timmusk et al., 2014; Vaishnav et al., 2015). Adipic acid, butyric acid, 2-undecanone, 7-hexanol, 3-methyl-butanol, and dimethyl disulfide produced by strains such as Alcaligenes faecalis and Paraburkholderia phytofirmans have also been confirmed to facilitate plant growth and induce stress tolerance (Bhattacharyya and Jha, 2012; Ledger et al., 2016).
5 Management of autotoxicity
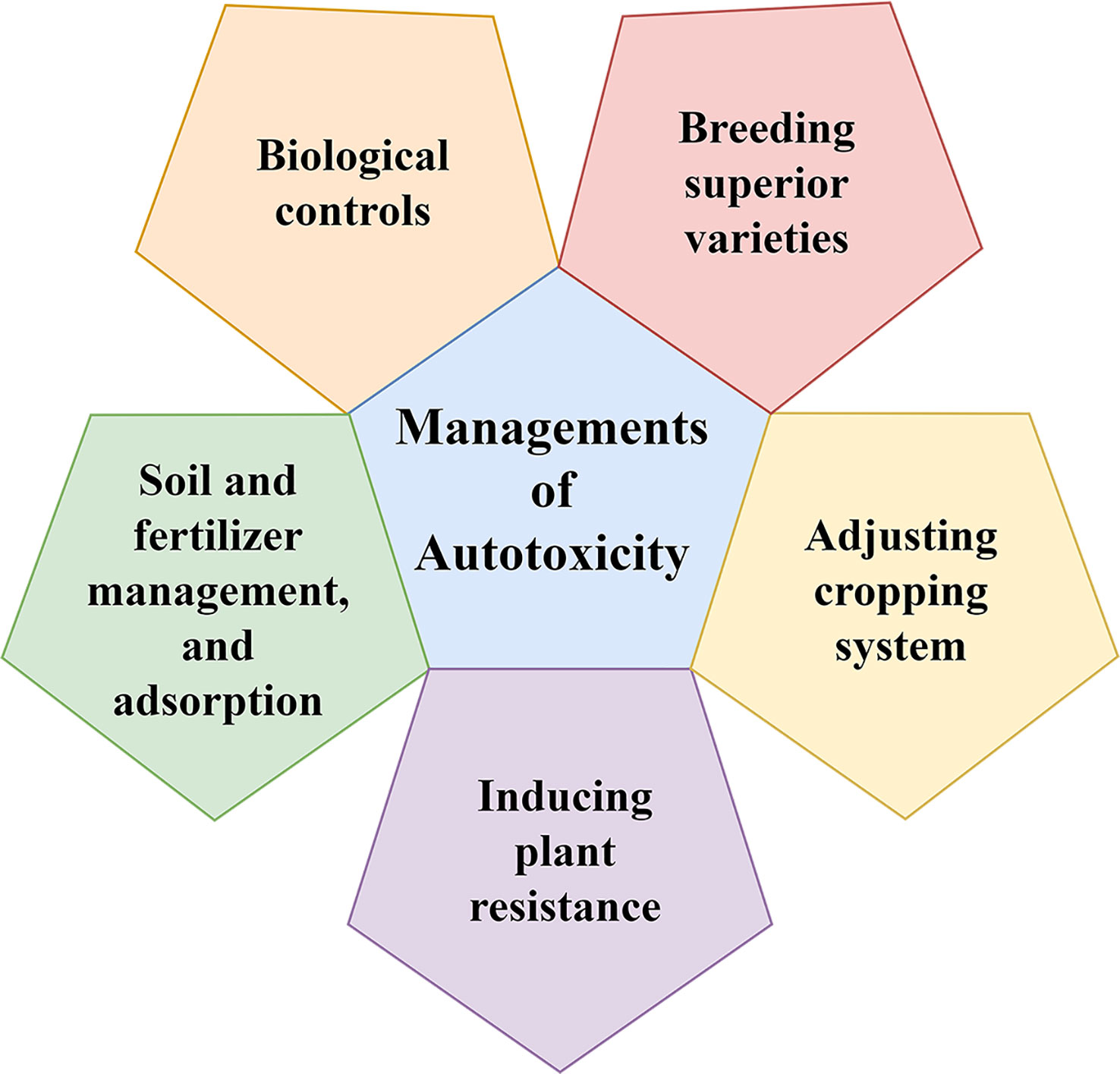
The objective of autotoxicity management is to reduce the production of autotoxins and to increase the elimination of produced autotoxins. To this end, we propose combined management strategies (Figure 2).
5.1 Breeding superior varieties
Researching the factors involved in continuous cropping obstacles and solutions is an essential undertaking for high-quality tobacco production. Since the implementation of “the Tobacco Genome Project,” scientists from China have cultivated batches of tobacco varieties that are easy to cure, have a pleasing aroma and high quality, produce a steady yield, and are fertilizer tolerant (Li et al., 2017; Chen et al., 2018a; Luo et al., 2019; Zhang et al., 2019). The promotion rate of self-breeding seeds has exceeded 80% (Yang et al., 2013; Sun et al., 2016), providing substantial support for tobacco production and cigarette manufacturers. Breeding tobacco varieties resistant to autotoxicity is an effective approach to preventing continuous cropping obstacles (Su et al., 2019). Utilizing interspecies allelopathy to address continuous cropping obstacles has become an effective approach (Li et al., 2018). However, at present, most tobacco planting areas grow monotonous varieties, lacking varieties that are resistant to continuous cropping and secrete less autotoxins.
5.2 Adjusting the cropping system
Establishing a reasonable cropping system and strengthening land maintenance measures can reduce tobacco autotoxicity to some extent (You et al., 2015b). Researchers have examined the difference in the diversity of soil microflora of tobacco under different land maintenance measures. They found that adopting rice straw return to soils significantly boosted microbial diversity in the rhizospheric soil, reduced the accumulation of phenolic acids around root systems, and alleviated tobacco autotoxicity. Under tobacco–rice continuous cropping conditions, fertility improvement and land maintenance measures in winter increased the diversity of beneficial microorganisms in the soil. Meanwhile, returning rice straw to soils also facilitated the growth of microorganisms that use amines as their carbon source, playing a significant role in alleviating damage caused by continuous cropping obstacles and improving tobacco quality (You et al., 2015b). Corn–tobacco rotational cropping promoted tobacco growth by increasing the contents of organic matter and nitrogen in the soil and inhibiting the accumulation of autotoxins and the occurrence of soil-borne diseases (e.g., tobacco black shank and tobacco bacterial wilt) (Zhang et al., 2012a; Niu et al., 2017). Studies also showed that reasonable rotation of alfalfa, corn, and wheat could significantly improve soil microbial ecology and reduce soil autotoxin content (Yin et al., 2019). The autotoxicity in the faba bean were effectively mitigated by the application of nitrogen fertilizer in a faba bean–wheat intercropping system (Guo et al., 2021; Cen et al., 2023).
5.3 Inducing plant resistance
Plant immune-induced resistance refers to the use of endogenous or exogenous substances to activate plant immune response, generate antibodies, and obtain or improve resistance to pathogens (Burketova et al., 2015; Liu et al., 2020; Ya Ayba et al., 2020). These substances are called plant immune inducers and include protein polypeptides, oligosaccharides, organic acids, inorganic compounds, and microorganisms (Newman et al., 2013; Qiu, 2016; Liu et al., 2020). Plant immune inducers can enter plants through various routes, causing a change in plant hydroxyproline-rich glycoprotein (HRGP) and resulting in the deposition of lignin in cell walls, to physically enhance the resistance of plants to pathogens (Qiu, 2016; Lavanya et al., 2018). In plants, plant immune inducers can cause the accumulation of endogenous hormones, induce plant anaphylaxis (HR), and induce cell death to resist further colonization by pathogens (Liu et al., 2020). Alternatively, by interacting with plants, plant immune inducers can trigger plant PTI and ETI reactions and enhance plant resistance to pathogens (Dodds and Rathjen, 2010). The early use of plant immune inducers to activate plant immune response and enhance plant growth also helps protect plants from autotoxicity. At the same time, some immune inducers may be used as carbon sources to recruit beneficial microorganisms that colonize and inhibit the proliferation of harmful microorganisms, also building another line of defense against autotoxins on the periphery of plant roots. Studies have shown that dimethyl disulfide, produced by Bacillus cereus C1L, can protect tobacco and corn plants against Botrytis cinerea and Cochliobolus heterostrophus, respectively, when applied through irrigation under greenhouse conditions (Huang et al., 2012). Similarly, the combined application of the metabolites of a Trichoderma sp. and brassinolide reduced gray mold on tomato leaves by approximately 70.0% (Li et al., 2020).
5.4 Soil and fertilizer management and adsorption
Soil and fertilizer management is of great significance for alleviating damage caused by tobacco autotoxicity. Replacing and deep-plowing soil effectively improves extremely poor-quality soil and can be highly effective for removing soil autotoxicity, alleviating biotic or abiotic stresses, and preventing diseases and pests. However, these measures are not cost-effective, as they can consume colossal amounts of manpower, material, and financial resources and can easily cause damage to the soil structure (Wang et al., 2012). The selective absorption of soil nutrients and the improper use of fertilizers for successively cropped tobacco can easily lead to an imbalance in trace elements, causing nutritional deficiencies, increasing autotoxicity, and decreasing tobacco yield and quality (Zhang et al., 2015; Li et al., 2018; Chen et al., 2022b). Monitoring elements in the soil and supplementing Fe, Zn, Se, Mg, and other trace elements at appropriate times are significant measures for fertility recovery, for facilitating root growth and development, for enhancing water- and fertilizer-absorption abilities, and for inhibiting the release of autotoxins (Xun et al., 2016). Soil fertility improvement and maintenance help recover the abundance and numbers of microbial populations. Measures such as the application of organic fertilizers with the appropriate addition of non-organic fertilizers and reduction of topdressing help increase soil organic matter, microbial biomass, and eventually the yield and quality of tobacco (Dubey et al., 2019; Dubey et al., 2020; Dubey et al., 2021).
Physical adsorption is also used to reduce autotoxicity and improve plant growth. In recent years, biochar has been used mainly in agricultural production as a solid product produced by the pyrolysis of organic biomass at high temperatures in an anoxic environment (Elmer and Pignatello, 2011; Xia et al., 2019; Sadikshya et al., 2020). Biochar can absorb harmful substances from soils because of its high porosity and large specific surface area and is widely used for soil improvement (Fang et al., 2020; Wang et al., 2020a). Biochar application reduces autotoxin content in soils by adsorption, weakening the autotoxicity on plant growth, and increases the biomass, growth rate, and sporulation of probiotics (Wang et al., 2020b; Ma et al., 2021).
5.5 Biological controls
The biological control of autotoxins mainly depends on soil microorganisms that carry out autotoxin biodegradation (Mao et al., 2010; Xie and Dai, 2015; Wang et al., 2021). Bacteria isolated from soils have shown particular abilities to decompose autotoxins secreted by plants roots, especially when these bacteria were fed back into the soils from which they were isolated (Shen et al., 2020; Wang et al., 2021). Therefore, the use of beneficial microorganisms can also resolve or alleviate autotoxicity. Inoculation with disease-preventing and growth-promoting bacteria that are capable of decomposing autotoxins is an effective, ecological, and environmentally friendly measure to reduce autotoxins in soils (Su et al., 2020). Pathogenic microorganisms can change plants’ normal metabolism of major components such as amino acids, proteins, lipids, carbohydrates, and nucleic acids and stimulate root secretions (Rojas et al., 2014). Beneficial microorganisms compete with pathogenic bacteria for oxygen, water, growth factors, and trace elements and partially limit the proliferation of soil-borne pathogens through antagonistic action or mycoparasitism (Landa et al., 2002). For example, the inoculation of soils with Paenibacillus polymyxa, which has high levels of antagonism and phosphate-solubilizing activity, substantially contributes to the improvement of the content of organic carbon and available phosphorus. The results of quantitative PCR showed that the total number of bacteria in the treatment strain group was significantly higher than that in the control group, whereas the total number of fungi in the former group was significantly lower than that in the latter group (Sui et al., 2019). The functions of growth-promoting rhizobacteria, such as nitrogen fixation, phosphate and potassium solubilization, and phytohormone synthesis help improve plants’ abilities to absorb nutritive elements and water. For example, inoculation with Trichoderma harzianum helps achieve an 80% degradation rate of six phenolic allelopathic and autotoxic substances produced by plant roots, such as hydroxybenzoic acid, vanillic acid, and ferulic acid, to significantly boost plant growth (Chen et al., 2011b). In addition, the application of compound microbial agents also helps improve the microflora of continuously cropped soil and significantly increases enzymatic activity in these soils. In summary, microbial agents not only alleviate continuous cropping obstacles but also reduce the environmental pollution caused by the use of fertilizers and pesticides (Zhao et al., 2016).
6 Conclusions and prospects
Autotoxicity is a key factor that limits yield and quality improvements in tobacco, and it is a pressing agricultural problem to be addressed. In this study, the types and composition of tobacco autotoxins present under continuous cropping systems were summarized, and a model for the toxicity of autotoxins toward tobacco and soil microorganisms was proposed. This study also proposes a combination of management strategies for remediating tobacco autotoxicity.
Presently, studies focusing on the action mechanism of autotoxins have mostly been limited to phenomenological descriptions. To further explore tobacco–soil–microorganism interactions and develop more practical preventive measures against autotoxins, accelerate the promotion of autotoxin prevention technology, and reduce damage caused by tobacco autotoxicity, further studies are recommended from the following perspectives:
(1) The separation and determination of autotoxins is a necessary step in the study of autotoxicity. It is important to develop new and more reliable separation, extraction, and analysis technologies for autotoxicity. For example, sediment analysis technology can help identify whether a substance is autotoxin and monitor the source and dynamic change law.
(2) The secretion and accumulation of autotoxins causes tobacco to undergo multiple signal transduction pathways, and signaling factors such as auxin, gibberellin, abscisic acid, and cytokinin in tobacco plants are mutually promotive or inhibitive. The synergistic effects of different factors still need to be clarified. Research in this direction will help us gain a more comprehensive understanding of the regulatory mechanism of autotoxins, thus providing a theoretical basis for developing reliable autotoxin degradation approaches.
(3) Presently, the research on autotoxin-degrading bacteria is largely focused on the degradation rate of autotoxins under laboratory conditions, and the complex interactions of autotoxins with different microbiological species and the effects of critical microorganisms are still not clear. Moreover, the effects of bacterial strains on hosts in the field and their synergistic effects with other rhizosphere microorganisms and rhizospheric autotoxins have been researched to a much lower extent. Finding beneficial microflora that stably exist in the tobacco rhizosphere and analyzing their characteristics and action patterns using high-throughput sequencing, q-PCR, and other technologies can provide a theoretical foundation for better understanding the ecological functions of autotoxin-degrading bacteria in continuous tobacco cropping soils. In terms of physical and chemical degradation of autotoxins, the application potential of technologies or materials such as microwave, ultraviolet, and nanomaterials also has not been systematically evaluated and tested.
(4) While it is not difficult to obtain bacterial strains with autotoxin-degrading functions, intensive research is still needed to obtain strains that have high biological activity, can stably colonize the tobacco rhizosphere, and have a clear action mechanism, great application prospects, and good field experimental outcomes. Presently, most studies have been based on short-term artificial pot culture simulations, and little research exists on the biological activity and colonization stability of microorganisms in the rhizosphere of tobacco in field experiments, as well as on plant–soil–microorganism interactions and their industrialization potential.
(5) Some of aromatic compounds (signaling substances) are also tobacco autotoxins. Thus, improving tobacco quality may worsen tobacco allelopathy. Identifying the mechanisms of autotoxin generation, developing comprehensive measures to degrade autotoxins, promoting plant growth and regulating soil ecosystems from agronomic, chemical, and biomanipulative perspectives, and accelerating the integration and promotion of such technologies may be a best approach for addressing tobacco autotoxicity.
(6) The development of gene editing technology based on CRISPR/Cas9 has provided a powerful tool for the creation of resistant continuous cropping tobacco varieties. In the future, targeted gene mutations can be targeted at genes for the synthesis and secretion of autotoxins, tobacco root structure genes, nutrient absorption and utilization genes, and plant defense genes, so as to provide materials for the cultivation of new continuous cropping-resistant varieties with reduced autotoxin secretion, rapid plant growth and development, and outstanding resistance to disease and continuous cropping.
Author contributions
YC: conceptualization, visualization, writing—original draft preparation, writing—review and editing. LY: conceptualization, validation, funding acquisition, writing—original draft preparation. LZ: investigation, writing—review and editing. JL: investigation, writing—review and editing. YZ: visualization, writing—review and editing. WY: investigation, writing—review and editing. LD: investigation, writing—review and editing. QG: investigation, writing—review and editing. QM: supervision, writing—review and editing. XL: supervision, writing—review and editing. WZ: conceptualization, validation, writing—review and editing. XD: conceptualization, validation, writing—review and editing. HX: conceptualization, validation, funding acquisition, writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the Foundation of Shandong Province Modern Agricultural Technology System Innovation Team (SDAIT-25-01) and Scale Identification of Gene-Editing Homozygous and Cultivation of Excellent Breeding Materials for Red Flower Mammoth Gold (110202101033).
Conflict of interest
Authors YC, WY, LD, QG, QM, XL, WZ and HX are employed by China Tobacco Yunnan Industrial Co., Ltd. Author LZ is employed by Honghe Tobacco Company. Author JL is employed by Yuxi Cigarette Factory, Hongta Tobacco Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alías, J. C., Sosa, T., Escudero, J. C., Chaves, N. (2006). Autotoxicity against germination and seedling emergence in Cistus ladanifer l. Plant Soil 282, 327–332. doi: 10.1007/s11104-005-6066-y
Avidano, L., Gamalero, E., Cossa, G. P., Carraro, E. (2005). Characterization of soil health in an Italian polluted site by using microorganisms as bioindicators. Appl. Soil Ecol. 30, 21–33. doi: 10.1016/j.apsoil.2005.01.003
Badri, D. V., Vivanco, J. M. (2009). Regulation and function of root exudates. Plant Cell Environ. 32, 666–681. doi: 10.1111/j.1365-3040.2008.01926.x
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S., Vivanco, J. M. (2006). The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266. doi: 10.1146/annurev.arplant.57.032905.105159
Bertin, C., Yang, X., Weston, L. A. (2003). The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256, 67–83. doi: 10.1023/a:1026290508166
Bhattacharyya, P. N., Jha, D. K. (2012). Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol. Biotechnol. 28, 1327–1350. doi: 10.1007/s11274-011-0979-9
Blum, U. (2019). Reflections regarding plant-plant interactions, communications and allelopathic interactions with an emphasis on allelopathic interactions. plant-plant allelopathic interactions III: Partitioning and seedling effects of phenolic acids as related to their physicochemical and conditional properties (Cham: Springer International Publishing). doi: 10.1007/978-3-030-22098-3_1
Blum, U., Gerig, T. M. (2005). Relationships between phenolic acid concentrations, transpiration, water utilization, leaf area expansion, and uptake of phenolic acids: nutrient culture studies. J. Chem. Ecol. 31, 1907–1932. doi: 10.1007/s10886-005-5934-5
Broeckling, C. D., Broz, A. K., Bergelson, J., Manter, D. K., Vivanco, J. M. (2008). Root exudates regulate soil fungal community composition and diversity. Appl. Environ. Microbiol. 74, 738–744. doi: 10.1128/AEM.02188-07
Brussaard, L., De Ruiter, P. C., Brown, G. G. (2007). Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 121, 233–244. doi: 10.1016/j.agee.2006.12.013
Burketova, L., Trda, L., Ott, P. G., Valentova, O. (2015). Bio-based resistance inducers for sustainable plant protection against pathogens. Biotechnol. Adv. 33, 994–1004. doi: 10.1016/j.biotechadv.2015.01.004
Callaway, R. M., Aschehoug, E. T. (2000). Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290, 521–523. doi: 10.1126/science.290.5491.521
Callaway, R. M., Ridenour, W. M. (2004). Novel weapons: invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2, 436–443. doi: 10.1890/1540-9295(2004)002[0436:Nwisat]2.0.Co;2
Cen, Z., Zheng, Y., Guo, Y., Yang, S., Dong, Y. (2023). Nitrogen fertilization in a faba bean-wheat intercropping system can alleviate the autotoxic effects in faba bean. Plants 12, 1232. doi: 10.3390/plants12061232
Chen, Y., Du, J., Li, Y., Tang, H., Yin, Z., Yang, L., et al. (2022b). Evolutions and managements of soil microbial community structure drove by continuous cropping. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.839494
Chen, S. Y., Guo, L. Y., Bai, J. G., Zhang, Y., Wang, X. J. (2015). Biodegradation of p-hydroxybenzoic acid in soil by pseudomonas putida CSY-P1 isolated from cucumber rhizosphere soil. Plant Soil 389, 197–210. doi: 10.1007/s11104-014-2360-x
Chen, D. M., Ke, W. H., Chen, L. L., Huang, J. W., Wu, W. X., Chen, T., et al. (2010). Diversity of bacterial community in rhizosphere soils under effects of continuously planting burley tobacco. Chin. J. Appl. Ecol. 21, 1751–1758. doi: 10.13287/j.1001-9332.2010.0259
Chen, J., Li, S., Ma, Z., Li, M., Zhu, W., Li, J., et al. (2018a). Breeding and characterization of a new flue-cured tobacco variety yueyan 208. Chin. Tobacco Sci. 39, 1–6. doi: 10.13496/j.issn.1007-5119.2018.06.001
Chen, S., Qi, G., Luo, T., Zhang, H., Jiang, Q., Wang, R., et al. (2018b). Continuous-cropping tobacco caused variance of chemical properties and structure of bacterial network in soils. Land Degradation Dev. 29, 4106–4120. doi: 10.1002/ldr.3167
Chen, D., Yang, Y., Jin, Y., Wang, H., Duan, Y., You, C., et al. (2011a). Constituents of autotoxic chemical from rhizosphere soil under flue-cured tobacco continuous cropping. Pratacultural Sci. 28, 1766–1769.
Chen, L., Yang, X., Raza, W., Li, J., Liu, Y., Qiu, M., et al. (2011b). Trichoderma harzianum SQR-T037 rapidly degrades allelochemicals in rhizospheres of continuously cropped cucumbers. Appl. Microbiol. Biotechnol. 89, 1653–1663. doi: 10.1007/s00253-010-2948-x
Chen, D., Zhou, Y., Wang, M., Mujtaba Munir, M. A., Lian, J., Yu, S., et al. (2022a). Succession pattern in soil micro-ecology under tobacco (Nicotiana tabacum l.) continuous cropping circumstances in yunnan province of southwest China. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.785110
Chi, W. C., Chen, Y. A., Hsiung, Y. C., Fu, S. F., Chou, C. H., Trinh, N. N., et al. (2013). Autotoxicity mechanism of oryza sativa: transcriptome response in rice roots exposed to ferulic acid. BMC Genomics 14, 351. doi: 10.1186/1471-2164-14-351
Deng, J., Zhang, Y., Hu, J., Jiao, J., Hu, F., Li, H., et al. (2017b). Autotoxicity of phthalate esters in tobacco root exudates: Effects on seed germination and seedling growth. Pedosphere 27, 1073–1082. doi: 10.1016/S1002-0160(17)60374-6
Deng, J., Zhang, S., Zhang, F., Zhang, Y., Hu, F., Li, H. (2017a). Autotoxins exuded from roots and the effects of PAEs on antioxidant capacity in roots of tobacco seedlings. Acta Ecologica Sin. 37, 495–504. doi: 10.5846/stxb201508021630
Dodds, P. N., Rathjen, J. P. (2010). Plant immunity: towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 11, 539–548. doi: 10.1038/nrg2812
Duan, Y., Jin, Y., Chen, Z., Xia, Z., Yang, Y., Xu, Z. (2012). Comparison of bacteria diversity between tobacco plantation soils of rotational cropping and continuous cropping. Acta Tabacaria Sin. 18, 53–59. doi: 10.3969/j.issn.1004-5708.2012.06.011
Dubey, R. K., Dubey, P. K., Abhilash, P. C. (2019). Sustainable soil amendments for improving the soil quality, yield and nutrient content of brassica juncea (L.) grown in different agroecological zones of eastern uttar pradesh, India. Soil Tillage Res. 195. doi: 10.1016/j.still.2019.104418
Dubey, R. K., Dubey, P. K., Chaurasia, R., Rao, C. S., Abhilash, P. C. (2021). Impact of integrated agronomic practices on soil fertility and respiration on the indo-gangetic plain of north India. Agronomy 11, 402–419. doi: 10.3390/agronomy11020402
Dubey, R. K., Dubey, P. K., Chaurasia, R., Singh, H. B., Abhilash, P. C. (2020). Sustainable agronomic practices for enhancing the soil quality and yield of cicer arietinum l. under diverse agroecosystems. J. Environ. Manage. 262, 110284. doi: 10.1016/j.jenvman.2020.110284
Elmer, W. H., Pignatello, J. J. (2011). Effect of biochar amendments on mycorrhizal associations and fusarium crown and root rot of asparagus in replant soils. Plant Dis. 95, 960–966. doi: 10.1094/PDIS-10-10-0741
Elsas, J., Garbeva, P., Salles, J. (2002). Effects of agronomical measures on the microbial diversity of soils as related to the suppression of soil-borne plant pathogens. Biodegradation 13, 29–40. doi: 10.1023/A:1016393915414
Fang, W., Song, Z., Tao, S., Zhang, D., Huang, B., Ren, L., et al. (2020). Biochar mitigates the negative effect of chloropicrin fumigation on beneficial soil microorganisms. Sci. Total Environ. 738, 139880. doi: 10.1016/j.scitotenv.2020.139880
Farooq, M., Hussain, T., Wakeel, A., Cheema, Z. A. (2014). Differential response of maize and mungbean to tobacco allelopathy. Exp. Agric. 50, 611–624. doi: 10.1017/s0014479714000106
Feng, D., Yu, C., Bai, Y. (2010). Research of microbial-phytoremediation on petroleum-contaminated soil. Environ. Impact Assess. 32, 57–60. doi: 10.3969/j.issn.1674-2842.2010.06.016
Fu, Z., Zhang, X., Zhang, X., Zhou, H., Qin, Y., Ma, J., et al. (2018). Effect of continuous cropping on quality of flue-cured tobacco leaves and carbon pool in tobacco growing soil. J. Northwest A&F Univ. (Natural Sci. Edition) 46, 16–22. doi: 10.13207/j.cnki.jnwafu.2018.08.003
Gabriele, B., Kornelia, S. (2010). Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 68, 1–13. doi: 10.1111/j.1574-6941.2009.00654.x
Guo, Y., Lv, J., Dong, Y., Dong, K. (2021). Exploration of the potential mechanism of faba bean–wheat intercropping to control faba bean fusarium wilt due to allelopathic plant extracts. ACS Omega 6, 15590–15600. doi: 10.1021/acsomega.0c06120
Hao, W. Y., Ren, L. X., Ran, W., Shen, Q. R. (2010). Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f.sp. niveum. Plant Soil 336, 485–497. doi: 10.1007/s11104-010-0505-0
Hisashi, K.-N., Keisuke, N., Osamu, O., Kiyotake, S., Nobuyuki, O. (2017). Asparagus decline: autotoxicity and autotoxic compounds in asparagus rhizomes. J. Plant Physiol. 213, 23–29. doi: 10.1016/j.jplph.2017.02.011
Huang, X., Chaparro, J. M., Reardon, K. F., Zhang, R., Shen, Q., Vivanco, J. M. (2014). Rhizosphere interactions: root exudates, microbes, and microbial communities. Botany 92, 267–275. doi: 10.1139/cjb-2013-0225
Huang, L. F., Song, L. X., Xia, X. J., Mao, W. H., Shi, K., Zhou, Y. H., et al. (2013). Plant-soil feedbacks and soil sickness: from mechanisms to application in agriculture. J. Chem. Ecol. 39, 232–242. doi: 10.1007/s10886-013-0244-9
Huang, C.-J., Tsay, J.-F., Chang, S.-Y., Yang, H.-P., Wu, W.-S., Chen, C.-Y. (2012). Dimethyl disulfide is an induced systemic resistance elicitor produced by bacillus cereus C1L. Pest Manag Sci. 68, 1306–1310. doi: 10.1002/ps.3301
Hung, R., Lee, S., Bennett, J. W. (2015). Fungal volatile organic compounds and their role in ecosystems. Appl. Microbiol. Biotechnol. 99, 3395–3405. doi: 10.1007/s00253-015-6494-4
Inderjit, Callaway, R. M., Vivanco, J. M. (2006). Can plant biochemistry contribute to understanding of invasion ecology? Trends Plant Sci. 11, 574–580. doi: 10.1016/j.tplants.2006.10.004
Inderjit, and Duke, S. O. (2003). Ecophysiological aspects of allelopathy. Planta 217, 529–539. doi: 10.1007/s00425-003-1054-z
Jin, Y., Yang, Y., Duan, Y., Kong, G. (2004). Effect of rotational cropping and continuous cropping on yield and quality of flue-cured tobacco. Southwest China J. Of Agric. Sci. 17, 267–271. doi: 10.3969/j.issn.1001-4829.2004.z1.063
Jin, Y., Yang, Y., Duan, Y., Long, Y., Ye, C. (2002). Influence of continuous cropping on yield and quality of flue-cured tobacco. Tobacco Sci. Technol. 50, 25–30. doi: 10.3969/j.issn.1002-0861.2002.01.016
Jing, Q. Y., Matsui, Y. (1997). Effects of root exudates of cucumber (Cucumis sativus) and allelochemicals on ion uptake by cucumber seedlings. J. Chem. Ecol. 23, 817–827. doi: 10.1023/B:JOEC.0000006413.98507.55
Kato-Noguchi, H., Ino, T., Sata, N., Yamamura, S. (2002). Isolation and identification of a potent allelopathic substance in rice root exudates. Physiol. Plant 115, 401–405. doi: 10.1034/j.1399-3054.2002.1150310.x
Landa, B. B., Mavrodi, O. V., Raaijmakers, J. M., Mcspadden Gardener, B. B., Thomashow, L. S., Weller, D. M. (2002). Differential ability of genotypes of 2,4-diacetylphloroglucinol-producing pseudomonas fluorescens strains to colonize the roots of pea plants. Appl. Environ. Microbiol. 68, 3226–3237. doi: 10.1128/AEM.68.7.3226-3237.2002
Lara-Núñez, A., Sánchez-Nieto, S., Luisa Anaya, A., Cruz-Ortega, R. (2010). Phytotoxic effects of Sicyos deppei (Cucurbitaceae) in germinating tomato seeds. Physiol. Plant 136, 180–192. doi: 10.1111/j.1399-3054.2009.01228.x
Lavanya, S. N., Udayashankar, A. C., Raj, S. N., Mohan, C. D., Gupta, V. K., Tarasatyavati, C., et al. (2018). Lipopolysaccharide-induced priming enhances NO-mediated activation of defense responses in pearl millet challenged with Sclerospora graminicola. 3 Biotech. 8, 475. doi: 10.1007/s13205-018-1501-y
Ledger, T., Rojas, S., Timmermann, T., Pinedo, I., Poupin, M. J., Garrido, T., et al. (2016). Volatile-mediated effects predominate in paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.01838
Li, Y. (2017). Microbal diversity in continuous cropped tobacco field and application of microbial agents (Zhengzhou, Henan Province: Zhengzhou University).
Li, X., Sun, J., Ding, Y., Ping, W., Sun, H., Li, Y., et al. (2017). Breeding of flue-cured tobacco variety Yuyan13 and its characteristics. Chin. Tobacco Sci. 38, 17–22. doi: 10.13496/j.issn.1007-5119.2017.04.003
Li, Z. F., Yang, Y. Q., Xie, D. F., Zhu, L. F., Zhang, Z. G., Lin, W. X. (2012). Identification of autotoxic compounds in fibrous roots of rehmannia (Rehmannia glutinosa libosch.). PloS One 7, e28806. doi: 10.1371/journal.pone.0028806
Li, T., Zhang, J., Tang, J., Liu, Z., Li, Y., Chen, J., et al. (2020). Combined use of trichoderma atroviride CCTCCSBW0199 and brassinolide to control botrytis cinerea infection in tomato. Plant Dis. 104, 1298–1304. doi: 10.1094/pdis-07-19-1568-re
Li, S., Zhu, Q., Pei, Z., Chen, B., Zhuang, S. (2018). Reasons and countermeasures of tobacco successive cropping obstacle. Modern Agric. Sci. Technol. 58, 54–56. doi: 10.3969/j.issn.1007-5739.2018.04.036
Liu, Y., Jiang, Y., Wang, G., Zhang, Y., Yang, Y., Yue, M., et al. (2016). Effect of different continuous cropping years on tobacco-growing soil’s physical and chemical properties and microflora. Chin. Agric. Sci. Bull. 32, 136–140. doi: 10.11924/j.issn.1000-6850.casb15110015
Liu, J., Zhou, J., Deng, X., Wang, K., Yang, H., Li, C., et al. (2010). Allelopathic potentional of decomposing material of tobacco residues. J. Of Hunan Agric. University(Natural Sciences) 36, 26–29.
Liu, Y., Zhu, Y., Zhou, E. (2020). Research progress on the action mechanism and application of plant immune inducers. Mol. Plant Breed. 18, 1020–1026. doi: 10.13271/j.mpb.018.001020
Luo, J., Li, H., Li, Y., Zhou, L., Hu, R., Yu, J., et al. (2019). Breeding and characterization of a new flue-cured tobacco variety Xiangyan6. Chin. Tobacco Sci. 40 (1-6), 13. doi: 10.13496/j.issn.1007-5119.2019.04.001
Ma, Z. T., Wang, Q., Wang, X. W., Chen, X. S., Wang, Y. F., Mao, Z. Q. (2021). Effects of biochar on replant disease by amendment soil environment. Commun. Soil Sci. Plant Anal. 52, 673–685. doi: 10.1080/00103624.2020.1869758
Mao, N., Xue, Q., Tang, M. (2010). Biodegradation of benzoic acid and p-hydroxybenzoic acid in the strawberry planting soil by two strains of actinomyces. J. Northwest Agric. Forestry Univ 38, 143–148. doi: 10.13207/j.cnki.jnwafu.2010.05.007
Margot, S., Adriano, M., Vincenzo, T. (2012). BOA detoxification of four summer weeds during germination and seedling growth. J. Chem. Ecol. 38, 933–946. doi: 10.1007/s10886-012-0136-4
Miao, Z., Zhang, Z., Wang, P., Guo, Y., Sun, J. (2004). Response of the red turpentine beetle, Dendroctonus valens LeConte (Coleoptera: Scolytidae) to host semiochemicals and its implication in management. Acta Entomol. Sin. 47, 360–364. doi: 10.3321/j.issn:0454-6296.2004.03.014
Nakano, H., Morita, S., Shigemori, H., Hasegawa, K. (2006). Plant growth inhibitory compounds from aqueous leachate of wheat straw. Plant Growth Regul. 48, 215–219. doi: 10.1007/s10725-006-0006-6
Nayyar, A., Hamel, C., Lafond, G., Gossen, B. D., Hanson, K., Germida, J. (2010). Soil microbial quality associated with yield reduction in continuous-pea. Appl. Soil Ecol. 43, 115–121. doi: 10.1016/j.apsoil.2009.06.008
Newman, M. A., Sundelin, T., Nielsen, J. T., Erbs, G. (2013). MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front. Plant Sci. 4. doi: 10.3389/fpls.2013.00139
Ni, L., Acharya, K., Hao, X., Li, S. (2012). Isolation and identification of an anti-algal compound from Artemisia annua and mechanisms of inhibitory effect on algae. Chemosphere 88, 1051–1057. doi: 10.1016/j.chemosphere.2012.05.009
Niu, J., Jin, C., Xiao, Y., Wu, C., Dai, L. (2017). Insight into the effects of different cropping systems on soil bacterial community and tobacco bacterial wilt rate. J. Basic Microbiol. 57, 3–11. doi: 10.1002/jobm.201600222
Qin, G., Niu, Z., Yu, J., Li, Z., Ma, J., Xiang, P. (2021). Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 267, 129205. doi: 10.1016/j.chemosphere.2020.129205
Qiu, D. (2016). Research status and trend analysis of plant immune induction technology in China. Plant Protect 42, 10–14. doi: 10.3969/j.issn.0529-1542.2016.05.002
Ren, X., He, X., Zhang, Z., Yan, Z., Jin, H., Li, X., et al. (2015). Isolation, identification, and autotoxicity effect of allelochemicals from rhizosphere soils of flue-cured tobacco. J. Agric. Food Chem. 63, 8975–8980. doi: 10.1021/acs.jafc.5b03086
Ren, X., Yan, Z., He, X., Li, X., Qin, B. (2017). Allelopathic effect of β-cembrenediol and its mode of action: Induced oxidative stress in lettuce seedlings. Emirates J. Food Agric. 29, 441–449. doi: 10.9755/ejfa.2016-09-1263
Rial, C., Novaes, P., Varela, R. M., Molinillo, J. M., Macias, F. A. (2014). Phytotoxicity of cardoon (Cynara cardunculus) allelochemicals on standard target species and weeds. J. Agric. Food Chem. 62, 6699–6706. doi: 10.1021/jf501976h
Rohrbacher, F., St-Arnaud, M. (2016). Root exudation: The ecological driver of hydrocarbon rhizoremediation. Agronomy 6, 19. doi: 10.3390/agronomy6010019
Rojas, C., Senthil-Kumar, M., Tzin, V., Mysore, K. (2014). Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00017
Sadikshya, D., Gao, S., Duan, Y., Wang, D. (2020). Soil microbial community structure affected by biochar and fertilizer sources. Appl. Soil Ecol. 150, 103452. doi: 10.1016/j.apsoil.2019.103452
Scavo, A., Restuccia, A., Mauromicale, G. (2018). “Allelopathy: Principles and basic aspects for agroecosystem control,” in Sustainable agriculture reviews 28: Ecology for agriculture. Eds. Gaba, S., Smith, B., Lichtfouse, E. (Cham: Springer International Publishing). doi: 10.1007/978-3-319-90309-5_2
Shen, L., Zhu, G., Guo, S., Li, X., Xiao, S., Xu, J., et al. (2020). Isolation of a pseudomonas putida strain that degrades p-hydroxybenzoic acid from the soil of a panax ginseng field. doi: 10.21203/rs.3.rs-19021/v1
Sierro, N., Battey, J., Ouadi, S., Bakaher, N., Bovet, L., Willig, A., et al. (2014). The tobacco genome sequence and its comparison with those of tomato and potato. Nat. Commun. 5, 3833. doi: 10.1038/ncomms4833
Soltys, D., Rudzinska-Langwald, A., Kurek, W., Gniazdowska, A., Sliwinska, E., Bogatek, R. (2011). Cyanamide mode of action during inhibition of onion (Allium cepa l.) root growth involves disturbances in cell division and cytoskeleton formation. Planta 234, 609–621. doi: 10.1007/s00425-011-1429-5
Su, Y., Li, M., Chen, X., Qu, S. (2019). Research progress of crop continuous cropping obstacle and its prevention and control technology. Heilongjiang Anim. Sci. Veterinary Med. 573 (9), 44–48. doi: 10.13881/j.cnki.hljxmsy.2018.07.0060
Su, Y., Peng, F., Peng, S., Qu, S. (2020). Research progress on crop autotoxicity and its prevention and control. Guangxi Plant Prot. 33, 20–24. doi: 10.3969/j.issn.1003-8779.2020.01.007
Sui, J., Ji, C., Wang, X., Liu, Z., Sa, R., Hu, Y., et al. (2019). A plant growth-promoting bacterium alters the microbial community of continuous cropping poplar trees’ rhizosphere. J. Appl. Microbiol. 126, 1209–1220. doi: 10.1111/jam.14194
Sun, Y. (2010). Effects of continuous cropping of processed tomato on its growth and biological activity (Shihezi, Xinjiang Uygur Autonomous Region: Shihezi University). doi: 10.7666/d.y1839275
Sun, J., Wu, Z., Li, X., Sun, H., Ding, Y., Ping, W., et al. (2016). Analysis of regional variation and major varieties of flue-cured tobacco planted in China in the twenty-first century. Chin. Tobacco Sci. 37, 86–92. doi: 10.13496/j.issn.1007-5119.2016.03.015
Timmusk, S., Abd El-Daim, I. A., Copolovici, L., Tanilas, T., Kannaste, A., Behers, L., et al. (2014). Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PloS One 9, e96086. doi: 10.1371/journal.pone.0096086
Tu, S., Sun, J., Guo, Z., Gu, F. (2000). On relationship between root exudates and plant nutrition in rhizosphere. Ecol. Environ. Sci. 9, 64–67. doi: 10.3969/j.issn.1674-5906.2000.01.017
Vaishnav, A., Kumari, S., Jain, S., Varma, A., Choudhary, D. K. (2015). Putative bacterial volatile-mediated growth in soybean (Glycine max l. Merrill) and expression of induced proteins under salt stress. J. Appl. Microbiol. 119, 539–551. doi: 10.1111/jam.12866
Walker, T. S., Bais, H. P., Grotewold, E., Vivanco, J. M. (2003). Root exudation and rhizosphere biology. Plant Physiol. 132, 44–51. doi: 10.1104/pp.102.019661
Wang, J., Feng, X., Anderson, C. W., Xing, Y., Shang, L. (2012). Remediation of mercury contaminated sites - a review. J. Hazard Mater. 221-222, 1–18. doi: 10.1016/j.jhazmat.2012.04.035
Wang, M., Jiang, C., Pan, W., Xue, X., Chen, Y., Liang, Y. (2008). Study on physico-chemical properties and microbiological community in tobacco-growing soils under different continuous cropping years. J. Anhui Agric. Sci. 36, 5033–5034, 5052. doi: 10.13989/j.cnki.0517-6611.2008.12.066
Wang, H. H., Ren, T. B., Yang, H. J., Feng, Y. Q., Feng, H. L., Liu, G. S., et al. (2020a). Research and application of biochar in soil CO2 emission, fertility, and microorganisms: A sustainable solution to solve China’s agricultural straw burning problem. Sustainability 12, 1922. doi: 10.3390/su12051922
Wang, W., Wang, Z., Yang, K., Wang, P., Wang, H., Guo, L., et al. (2020b). Biochar application alleviated negative plant-soil feedback by modifying soil microbiome. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.00799
Wang, Y., Zhang, W., Zhang, Z., Wang, W., Xu, S., He, X. (2021). Isolation, identification and characterization of phenolic acid-degrading bacteria from soil. J. Appl. Microbiol. 131, 208–220. doi: 10.1111/jam.14956
Weir, T. L., Park, S. W., Vivanco, J. M. (2004). Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant Biol. 7, 472–479. doi: 10.1016/j.pbi.2004.05.007
Wu, J., Jiao, Z., Zhou, J., Guo, F., Ding, Z., Qiu, Z. (2017). Analysis of bacterial communities in rhizosphere soil of continuously cropped healthy and diseased konjac. World J. Microbiol. Biotechnol. 33, 134. doi: 10.1007/s11274-017-2287-5
Xia, J., Ni, C., Liu, S. (2019). Research progress on application effect of biomass charcoal and its restoration of soil phenolic acid pollution. Plant Dis. pests 10, 5–9. doi: 10.19579/j.cnki.plant-d.p.2019.01.002
Xie, X., Dai, C. (2015). Biodegradation of a model allelochemical cinnamic acid by a novel endophytic fungus phomopsis liquidambari. Int. Biodeterior. Biodegrad. 104, 498–507. doi: 10.1016/j.ibiod.2015.08.004
Xie, X., Kusumoto, D., Takeuchi, Y., Yoneyama, K., Yamada, Y., Yoneyama, K. (2007). 2’-epi-orobanchol and solanacol, two unique strigolactones, germination stimulants for root parasitic weeds, produced by tobacco. J. Agric. Food Chem. 55, 8067–8072. doi: 10.1021/jf0715121
Xun, W., Xu, Z., Li, W., Ren, Y., Huang, T., Ran, W., et al. (2016). Long-term organic-inorganic fertilization ensures great soil productivity and bacterial diversity after natural-to-agricultural ecosystem conversion. J. Microbiol. 54, 611–617. doi: 10.1007/s12275-016-6143-3
Ya Ayba, L., Karpun, N. N., Mikhailova, Y. V., Pantiya, G. G. (2020). Inclusion of plant immunity inducers in the fruit crops protection system for the purpose of reducing the pesticide load. IOP Conf. Ser.: Earth Environ. Sci. 604, 012019. doi: 10.1088/1755-1315/604/1/012019
Yang, Z., Wang, Y., Liu, H., Wang, R., Wang, Z., Shi, Y., et al. (2013). Genetic relationship in major flue-cured tobacco cultivars in China and its implication in variety breeding. Acta Tabacaria Sin. 19, 34–41. doi: 10.3969/j.issn.1004-5708.2013.02.007
Yeasmin, R., Nakamatsu, K., Matsumoto, H., Motoki, S., Nishihara, E., Yamamoto, S. (2014). Inference of allelopathy and autotoxicity to varietal resistance of asparagus (‘Asparagus officinalis’ l.). Aust. J. Crop Sci. 8, 251–256. doi: 10.3316/informit.198804986959601
Yi, J., Jia, Z., Lin, Q., Lv, H., Shen, H. (2012). Allelopathic effects of decaying tobacco leaves on tobacco seedlings. Allelopathy J. 29, 51–62. doi: 10.1080/03650340.2010.528408
Yin, G., Cai, Z., Tao, R., Wu, F., Chen, J., Shi, S. (2019). Effects of different crop rotations on soil nutrient, microorganism abundance and soil allelochemical levels in alfalfa. Acta Prataculturae Sin. 28, 42–50. doi: 10.11686/cyxb2018408
You, C., Gao, F., Wang, F., Tang, S., Gu, L., Zhang, T., et al. (2015a). Effect of continuous cropping on rhizosphere micro-ecology as well as on yield and quality of flue-cured tobacco in yunnan. Acta Tabacaria Sin. 21, 60–67. doi: 10.16472/j.chinatobacco.2014.151
You, C., Zeng, W., Chen, D., Huang, J., Tang, L. (2015b). Effect of different soil management methods on functional diversity of microbial flora in rhizospheric soil for continuous tobacco cropping. Acta Tabacaria Sin. 21, 68–74. doi: 10.16472/j.chinatobacco.2014.341
Yu, J., Shou, S., Qian, Y., Zhu, Z., Hu, W. (2000). Autotoxic potential of cucurbit crops. Plant Soil 223, 149–153. doi: 10.1023/A:1004829512147
Yu, H., Song, X., Wang, S., Cao, L., Guo, L., Wang, X., et al. (2015). Effects of low molecular weight organic acids on soil enzymes activities and bacterial community structure. Scientia Agricultura Sin. 48, 4936–4947. doi: 10.3864/j.issn.0578-1752.2015.24.008
Zhang, Y., Chen, Y., Lei, F., Li, S., Shi, F., Dou, M., et al. (2018). Advances in research on allelopathic autotoxicity effects of medicinal plants. Chin. Traditional Herbal Drugs 49, 1946–1956. doi: 10.7501/j.issn.0253-2670.2018.08.032
Zhang, S., Guo, W., Huixin, L., Wang, J., Li, H., Wang, A., et al. (2015). Research progresses on continuous cropping obstacles of tobacco. Soils 47, 823–829. doi: 10.13758/j.cnki.tr.2015.05.001
Zhang, Y., Liu, Y., Wang, Y., Luo, C., Yang, A., Pan, X., et al. (2019). Breeding and characterization of a new flue-cured tobacco variety Zhongchuan208. Chin. Tobacco Sci. 40, 1–7. doi: 10.13496/j.issn.1007-5119.2019.05.001
Zhang, Y., Min, G., Kai, S., Yan, H. Z., Jing, Q. Y. (2010a). Effects of aqueous root extracts and hydrophobic root exudates of cucumber (Cucumis sativus l.) on nuclei DNA content and expression of cell cycle-related genes in cucumber radicles. Plant Soil 327, 455–463. doi: 10.1007/s11104-009-0075-1
Zhang, Z. Y., Pan, L. P., Li, H. H. (2010b). Isolation, identification and characterization of soil microbes which degrade phenolic allelochemicals. J. Appl. Microbiol. 108, 1839–1849. doi: 10.1111/j.1365-2672.2009.04589.x
Zhang, X., Pan, Z., Zhou, X., Ni, W. (2007b). Autotoxicity and continuous cropping obstacles: A review. Chin. J. Of Soil Sci. 38, 781–784. doi: 10.3321/j.issn:0564-3945.2007.04.033
Zhang, C., Wang, Z., Chen, Y., Pan, W., Huang, J. (2007a). The effect of continuous cropping on tobacco growth and soil nutrients. Guizhou Agric. Sci. 35, 62–65. doi: 10.3969/j.issn.1001-3601.2007.04.022
Zhang, Y., Wang, L., Yao, Y., Yan, J., He, Z. (2012b). Phenolic acid profiles of Chinese wheat cultivars. J. Cereal Sci. 56, 629–635. doi: 10.1016/j.jcs.2012.07.006
Zhang, Z., Wu, J., Xi, Y., Zhang, L., Gao, Q., Gefu, W.-P. (2021). Effects of autotoxicity on seed germination, gas exchange attributes and chlorophyll fluorescence in melon seedlings. J. Plant Growth Regul. 41 (30), 993–1001. doi: 10.1007/s00344-021-10355-w
Zhang, Z., Xie, X., Wang, Y., Chen, D., Xi, X., Chen, X., et al. (2011b). Study on allelopathic autotoxicity and continuous cropping obstacles of tobacco. Acta Tabacaria Sin. 17, 88–92. doi: 10.3969/j.issn.1004-5708.2011.04.017
Zhang, K., Xu, T., Shen, F., Shi, B., Gu, M., Shou, A., et al. (2013). Phenolic acids in Nicotiana tobacco l. root exudate and their autotoxicity effects. Southwest China J. Agric. Sci. 26, 2552–2557. doi: 10.16213/j.cnki.scjas.2013.06.062
Zhang, X., Zhang, E., Lang, D. (2011a). Autotoxic compounds from rhizosphere soil of l. extracts: Identification and biological activity. Agron. J. 103, 695–701. doi: 10.2134/agrnj2010.0425
Zhang, Y., Zhang, C., Wang, Z., HuanG, J. (2007c). The effects on the yields of flue-cured tobacco and activities of main soil enzymes. Chin. Agric. Sci. Bull. 23, 211–215, 23. doi: 10.3969/j.issn.1000-6850.2007.12.048
Zhang, J., Zheng, L., Shi, Y., Zhang, Z., Ma, X., Shen, G., et al. (2012a). Effects of different planting patterns on soil microbial community, yield and quality of flue-cured tobacco leaves. Trans. Chin. Soc. Agric. Eng. 28, 93–102. doi: 10.3969/j.issn.1002-6819.2012.19.013
Zhao, Y., Hua, X., Du, H., Xu, S., Cui, D. (2016). Research onthe application effect of compound bacterium agent for AllelochemicalsDegradation bacteria andAntagonisticBacteria resistance to continuous cropping. Chin. J. Soil Sci. 47, 599–604. doi: 10.19336/j.cnki.trtb.2016.03.14
Keywords: tobacco, autotoxins, continuous cropping obstacles, soil microorganisms, management of autotoxicity
Citation: Chen Y, Yang L, Zhang L, Li J, Zheng Y, Yang W, Deng L, Gao Q, Mi Q, Li X, Zeng W, Ding X and Xiang H (2023) Autotoxins in continuous tobacco cropping soils and their management. Front. Plant Sci. 14:1106033. doi: 10.3389/fpls.2023.1106033
Received: 23 November 2022; Accepted: 29 March 2023;
Published: 17 April 2023.
Edited by:
Xiang Tao, Sichuan Normal University, ChinaReviewed by:
Krishan K. Verma, Guangxi Academy of Agricultural Sciences, ChinaWeitao Jiang, Shandong Agricultural University, China
Yang Li, Shandong Agricultural University, China
Copyright © 2023 Chen, Yang, Zhang, Li, Zheng, Yang, Deng, Gao, Mi, Li, Zeng, Ding and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long Yang, bHlhbmdAc2RhdS5lZHUuY24=; Wanli Zeng, emVuZ3dsX2ttQDE2My5jb20=; Xinhua Ding, eGhkaW5nQHNkYXUuZWR1LmNu; Haiying Xiang, Y2FzZXhoeUAxMjYuY29t
†These authors have contributed equally to this work and share the first authorship
 Yudong Chen
Yudong Chen Long Yang
Long Yang Lumin Zhang3
Lumin Zhang3 Xinhua Ding
Xinhua Ding