- 1Pure and Applied Biology Programme, College of Agriculture, Engineering and Science, Bowen University, Iwo, Osun, Nigeria
- 2Department of Biological Sciences/Biotechnology Cluster, Covenant University, Ota, Ogun, Nigeria
- 3Department of Biological Sciences, Kings University, Ode-Omu, Osun, Nigeria
- 4Food Security and Safety Focus, Faculty of Natural and Agricultural Sciences, North-West University, Mmabatho, South Africa
- 5Department of Chemical and Food Sciences, College of Natural and Applied Sciences, Bells University of Technology, Ota, Ogun, Nigeria
- 6Department of Pure and Applied Botany, College of Biosciences, Federal University of Agriculture, Abeokuta, Nigeria
- 7Microbiology Programme, College of Agriculture, Engineering and Science, Bowen University, Iwo, Osun, Nigeria
- 8The Radcliffe Institute for Advanced Study, Harvard University, Cambridge, MA, United States
- 9Biology Unit, Faculty of Science, Air Force Institute of Technology, Kaduna, Nigeria
Globally, legumes are vital constituents of diet and perform critical roles in maintaining well-being owing to the dense nutritional contents and functional properties of their seeds. While much emphasis has been placed on the major grain legumes over the years, the neglected and underutilized legumes (NULs) are gaining significant recognition as probable crops to alleviate malnutrition and give a boost to food security in Africa. Consumption of these underutilized legumes has been associated with several health-promoting benefits and can be utilized as functional foods due to their rich dietary fibers, vitamins, polyunsaturated fatty acids (PUFAs), proteins/essential amino acids, micro-nutrients, and bioactive compounds. Despite the plethora of nutritional benefits, the underutilized legumes have not received much research attention compared to common mainstream grain legumes, thus hindering their adoption and utilization. Consequently, research efforts geared toward improvement, utilization, and incorporation into mainstream agriculture in Africa are more convincing than ever. This work reviews some selected NULs of Africa (Adzuki beans (Vigna angularis), African yam bean (Sphenostylis stenocarpa), Bambara groundnut (Vigna subterranea), Jack bean (Canavalia ensiformis), Kidney bean (Phaseolus vulgaris), Lima bean (Phaseolus lunatus), Marama bean (Tylosema esculentum), Mung bean, (Vigna radiata), Rice bean (Vigna Umbellata), and Winged bean (Psophocarpus tetragonolobus)), and their nutritional, and functional properties. Furthermore, we highlight the prospects and current challenges associated with the utilization of the NULs and discusses the strategies to facilitate their exploitation as not only sources of vital nutrients, but also their integration for the development of cheap and accessible functional foods.
1 Introduction
Legumes are a group of flowering plants and are classified under the Fabaceae family. This family is the third-largest in terms of angiosperm groups, consisting of over 800 different types and around 20,000 species. Within the Fabaceae family, there are three subfamilies known as Papilionoideae, Caesalpinioideae, and Mimosoideae. Of these, the edible legumes are grouped in the sub-family Papilionoideae. Globally, legumes are regarded as a valuable and inexpensive alternative protein sources and rank second after cereals as the most important food crop (Maphosa and Jideani, 2017). Apart from the rich protein and amino acid content, legume seeds provide a substantial amount of carbohydrates, minerals, and vitamins (Vadivel et al., 2012; Barman et al., 2018). In addition to having no cholesterol and gluten, legumes possess low fat and glycemic index and are rich in dietary fiber and antioxidants. These legumes possess bioactive compounds which possess antidiabetic, antimicrobial, anti-atherogenic, anti-thrombogenic, anti-hypertensive, and anticancer properties amongst others. Legumes also serve as fodder for livestock and fix atmospheric nitrogen in soils, thereby enhancing soil fertility and invariably promoting agricultural sustainability. They are also adapted to diverse agro-ecological zones and unfavorable environmental conditions, possessing structures for augmenting the sustainability of dry subtropical and tropical agricultural systems (Khoury, 2015).
It is interesting to note that some legumes also produce underground tubers in addition to edible seeds. However, only a few of these legumes are incorporated into the human diet. Such dual food legumes fall into the category of neglected and underutilized legumes (NULs) simply because they have not received much research focus and are still cultivated at the subsistence level by resource-poor farmers who hold the genetic resources of these plants. Tuberous underutilized legumes are gradually gaining recognition. These include the African yam bean (AYB) (Sphenostylis stenocarpa) cultivated in West Africa for the seeds and in East and Central Africa for the tubers (Adewale and Nnamani, 2022); winged bean (Psophocarpus tetragonolobus), grown and cultivated in Papua New Guinea Highland, northern Ghana, and Burma; the Marama bean (Tylosema esculentum) cultivated in the Southern Africa regions of Botswana, Namibia, Mozambique, Zambia, and in northern South Africa (Abberton et al., 2020a; Abberton et al., 2020b; Ojuederie et al., 2021; Sriwichai et al., 2021); Mexican yam bean (Pachyrhizus erosus); Zombi pea (Vigna vexillata) an underutilized legume with a pantropical distribution; hyacinth bean (Lablab purpureus) grown in North Africa; as well as Tala (Neoapaloxylon tuberosum) cultivated in Madagascar (Von Wettberg et al., 2021). Different tuber shapes and sizes of some tuberous underutilized legumes are presented in Figure 1.
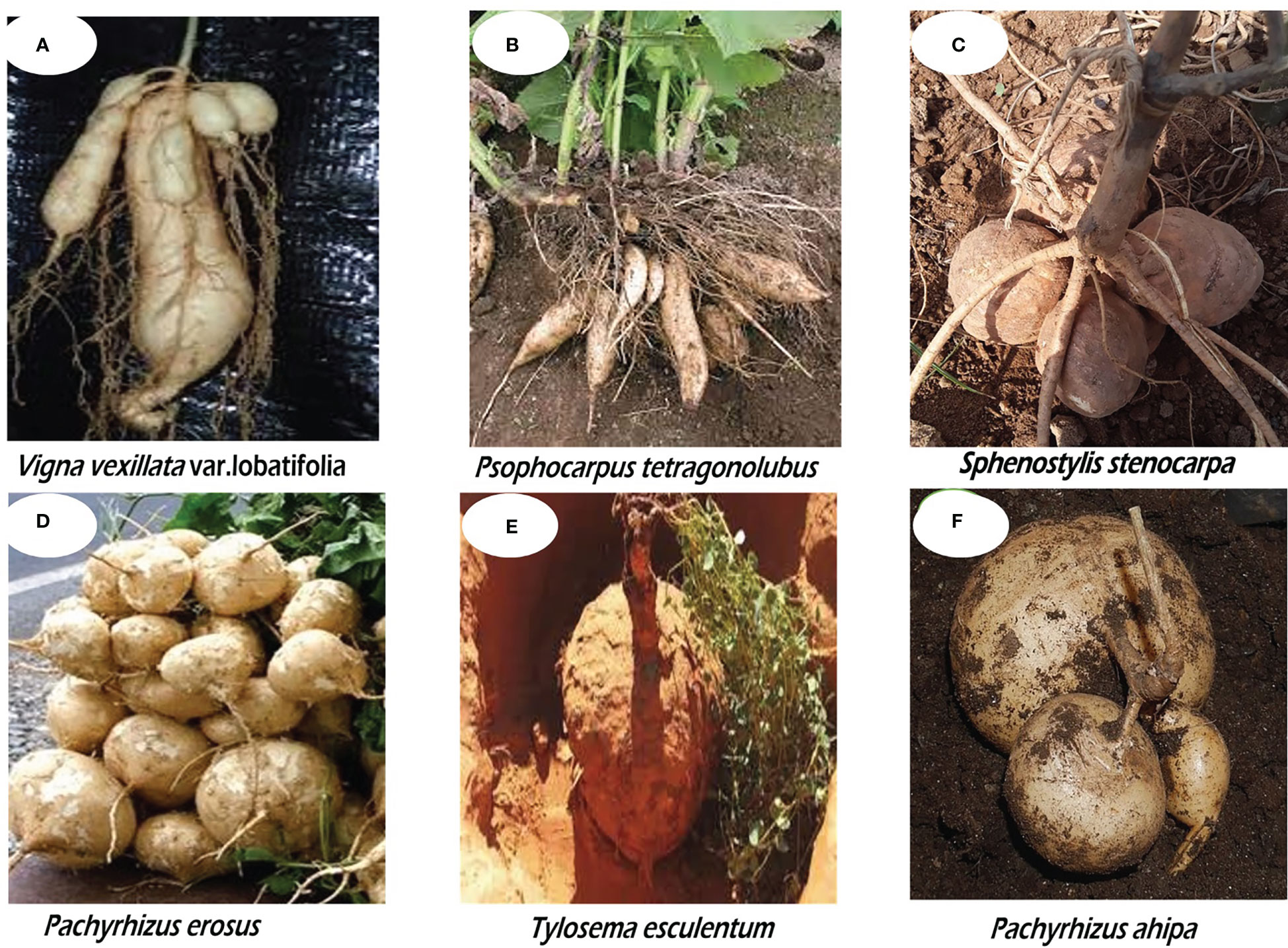
Figure 1 Different tuber shapes and sizes of some underutilized legumes: (A) Zombi pea (Vigna vexillata), (B) Winged bean (Psophocarpus tetragonolobus), (C) African yam bean (Sphenostylis stenocarpa), (D) Mexican yam bean (Pachyrhizus erosus), (E) Marama bean (Tylosema esculentum), (F) Ahipa (Pachyrhizus aphipa).
The Bambara groundnut (Vigna subterranea) is a crop that is extensively grown for its seeds in certain regions of West and Southern Africa. Nigeria has been reported to be the largest producer of this crop (Ojuederie et al., 2021; Popoola et al., 2022b; Arise et al., 2022). Tubers of Zombi peas are crispy, rich in protein (15%) and can be consumed raw (Tripathi et al., 2020; Von Wettberg et al., 2021). The seeds and tubers of many of the NULs are also rich in protein. For instance, AYB seeds contain 19.5% protein, while the tubers hold about 15.5% protein (Ojuederie and Balogun, 2017; Ojuederie and Balogun, 2019; Abberton et al., 2020a). In winged bean, the protein content of the seeds and tubers are 29.8% to 42.5% and 20% respectively (Abberton et al., 2020b). Negi and Gaur (1994) stated that Zombi peas contain 14.5% protein when their roots are dried. Nevertheless, a more recent study conducted by Tripathi et al. (2020) to analyze the nutritional content of seven different Zombi peas accessions found that the protein content of their tubers ranged from 7.64% to 9.93%. This is remarkable because it was seven to nine times higher than the protein content found in sweet potato and cassava tubers (Tripathi et al., 2020). Although Zombi peas is not considered in this review, its rich nutritional contents particularly the tubers call for more research attention (Tripathi et al., 2020). The edible tubers of AYB and winged beans are still propagated at the subsistence level with no genetic improvement. The mechanism behind tuberization in AYB is yet to be understood.
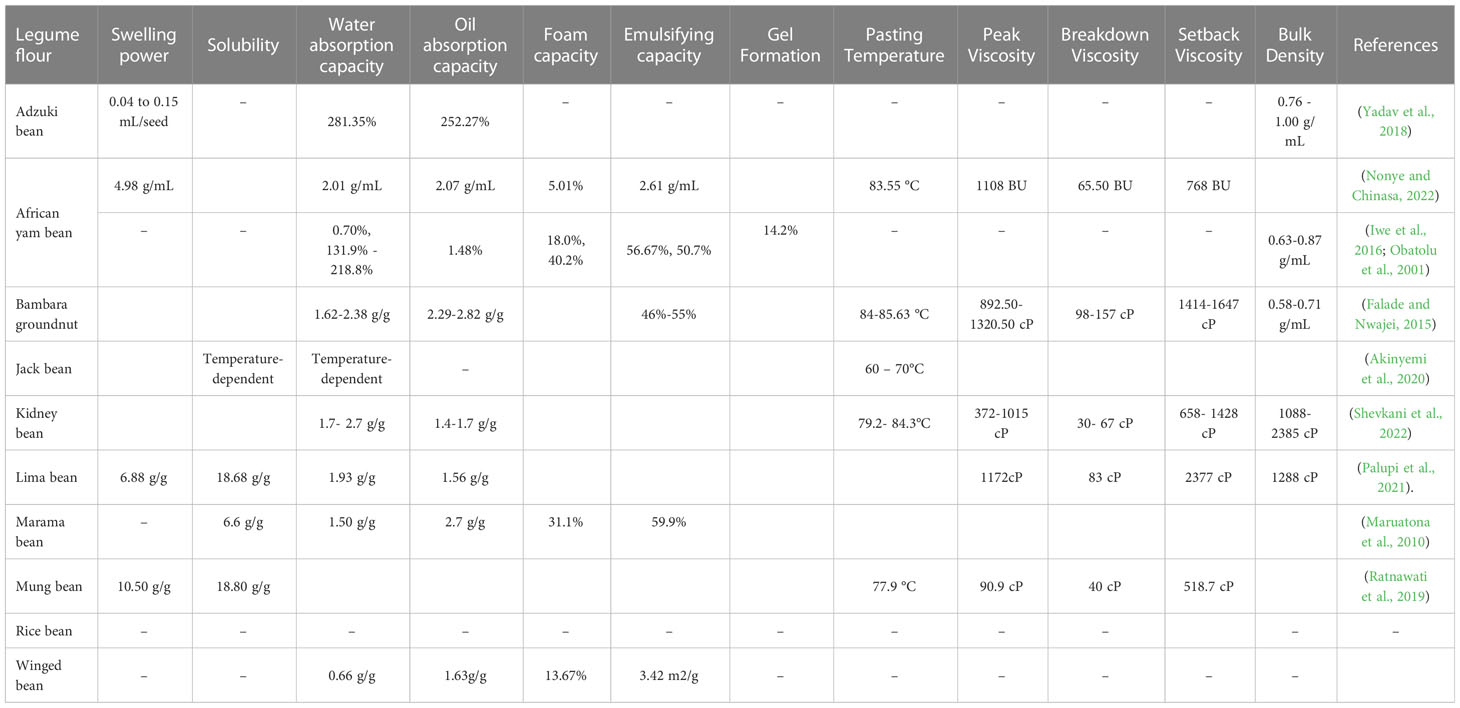
Bioactive compounds have been identified in NULs, but with little or no impact on the nutritional and food security in Africa. In plants, bioactives perform several functions, ranging from protection against herbivores and insect pest to attraction of pollinators during pollination and induction of essential functions (Chandrasekara and Josheph Kumar, 2016; Divekar et al., 2022). These bioactive compounds also exhibit pharmacological properties in humans and animals (Chandrasekara and Josheph Kumar, 2016) which forms a major part of this review. The bioactive components of the NULs are yet to be fully harnessed for improved health and well-being as many consumers in Africa are unaware of their nutritional and health benefits. In our previous review, we emphasized the need to integrate the NULs into food systems in sub-Saharan Africa (SSA) to cushion the negative effects of climate change, soil degradation, poverty, food insecurity, and malnourishment (Popoola et al., 2022b). This article attempts to present a broad review of some selected NULs, and their nutritional and functional properties. The selected NULs include Adzuki beans (Vigna angularis), African yam bean (Sphenostylis stenocarpa), Bambara groundnut (Vigna subterranea), Jack bean (Canavalia ensiformis), Kidney bean (Phaseolus vulgaris), Lima bean (Phaseolus lunatus), Marama bean (Tylosema esculentum), Mung bean, (Vigna radiata), Rice bean (Vigna Umbellata), and Winged bean (Psophocarpus tetragonolobus). In Africa, these NULs have been relegated to the status of “poor man’s food” with abysmally low level of cultivation, production, consumption, and utilization compared to the mainstream legumes. Consequently, the need to create awareness about their potential utility, health and nutritional benefits becomes imperative. Also, the relevance of the untapped bioactive compounds inherent in the seeds of these potential food and nutrition security crops are discussed. Furthermore, we highlight the prospects and current challenges associated with the utilization of these NULs and present strategies to facilitate their exploitation as not only sources of vital nutrients, but also integration for the development of cheap and accessible functional foods. The plant products and distribution of the selected underutilized legumes and center of diversity in Africa are presented in Table 1 and Figure 2.
Figure 2 Center of the diversity of some underutilized legumes in Africa Source: http://www.zipcodezoo.com/Plants/S/Sphenostylis%5Fstenocarpa/Default.asp (February 19, 2023).
2 Neglected and underutilized legumes
The term “neglected” or “underutilized” alludes to a class of legumes that are climate-smart, adapted to marginal areas, indigenously propagated with fewer or no ex-situ collections, and have not given priority by policymakers. The term also refers to legumes that have received little research attention, possess local significance in production and consumption, traded regionally or internationally and are usually cultivated on a small scale by rural families for subsistence, particularly under adverse environment conditions (Cullis and Kunert, 2017; Yang et al., 2018; Rathi et al., 2021; Popoola et al., 2022b). While these crops have received relatively little research and funding, their potential is well recognized (Cullis and Kunert, 2017; Yang et al., 2018). The NULs exhibit an array of genetic diversity and exist as wild or cultivated species across different regions of the world (Agbolade et al., 2019). These crops are primarily grown by traditional farmers in SSA, Asia, and North America (Alvarado-Lopez et al., 2019). The NULs are marked by unique characteristics such as ethno-uses, seed sizes, growth habits, and fruiting patterns that distinguish them from the common pea. Furthermore, they are of agricultural importance owing to their capability to augment soils via symbiotic nitrogen fixation (Agbolade et al., 2019; Hunter et al., 2019; Popoola et al., 2022b). Underutilized legumes are a great source of essential nutrients such as dietary fiber, vitamins, polyunsaturated fatty acids (PUFAs), proteins with essential amino acids, minerals, and bioactive compounds. These legumes are therefore considered functional foods that can have positive effects on our health (Popoola et al., 2020; Rai et al., 2021).
African yam bean, Bambara groundnut, lablab bean, lima bean, and the winged bean are examples of some commonly cultivated underutilized leguminous species in SSA (Agbolade et al., 2019; Popoola et al., 2020). These legumes have the potential to drive sustainable agri-food systems in the region given their diversity, climate resilience, nutrient-dense nature, and cultural attachment to the regional food habits of the communities of origin (Paliwal et al., 2021). A recent analysis of the utilization and cultivation of lesser known legumes brought attention to the significant traits and potential prospects of some of the crops (Popoola et al., 2022b).
3 Nutritional properties of NULs
Nutritionally, the seeds of the selected NULs are rich in proteins, carbohydrates, minerals (calcium, manganese, phosphorus amongst others), and a wide range of vitamins (Table 2). Nutritional information on the tubers is scanty and has not been studied in detail compared to the seeds (Ojuederie and Balogun, 2019; Tripathi et al., 2020). Nevertheless, a few reports on AYB, Zombi pea, and winged beans indicate that the tubers are nutritionally rich with varied carbohydrates, proteins, ash, dietary fiber, minerals, and vitamin contents when compared to those of cassava, potato, and yam (Adegboyega et al., 2019; Konyeme et al., 2020; Ojuederie et al., 2020; Tripathi et al., 2020). The studies of Konyeme et al. (2020) emphasized the rich nutritional value of the tubers, while the investigation of Ojuederie et al. (2020) confirmed their safe consumption by humans and livestock. Globally, the demand for food legumes is ever-increasing as one of the essential nutritional and conventional food with health and pharmacological relevance (Tadele and Bartels, 2019). The nutritional contents of the seeds are diverse and have been widely reported and discussed by many researchers (Gagné-Bourque et al., 2016; Huang et al., 2018; Omotayo and Aremu, 2021; Popoola et al., 2022a). The nutritional profile of the seeds varies in different accessions of the same and different species. This can be exploited by breeders to enhance yield, taste and value chain for the food and confectionary industries. The amount of carbohydrates found in the selected underutilized legumes range from 18.90g in Marama bean (MB) to 70.48g in Kersting’s groundnut (KG) (Table 2). In legumes, carbohydrates usually contain resistant starch sugars such as stachyose, raffinose, and fructooligosaccharides. These sugars have the ability to improve the microbial environment in our gastrointestinal tract and promote gut metabolism (Johnson et al., 2020). Adding such components to food systems can greatly improve health and ensure nutritional quality. The proteins are of high quality and range from 7.80g in Lima beans (LB) to 29.60g in winged beans (WG) while others also contain a good quantity of protein. The amino acids found in these legumes are valuable in boosting the immune system, regulating metabolic processes, and enhancing glucose and fatty-acid metabolism (Tjahjadi et al., 1988; Semba et al., 2021; Ayilara et al., 2022). Numerous studies, including those conducted by Gohara et al. (2016), Nnamani et al. (2017), Baiyeri et al. (2018), Adegboyega et al. (2020), and Abberton et al. (2022), have documented the functions of various vitamins and minerals. Selected underutilized legumes (NULs) have been found to contain thiamine, niacin, riboflavin, vitamins A, B6, C, D, E, K, and pantothenic acid, according to these studies (Table 2). Underutilized legumes have been found to contain various mineral elements such as sodium (Na), calcium (Ca), copper (Cu), magnesium (Mg), manganese (Mn), phosphorus (Ph), potassium (K), and zinc (Zn) (Baiyeri et al., 2018; Nnamani et al., 2018; Adegboyega et al., 2019; Adedayo et al., 2021). The vitamins and minerals are required for optimal health and growth, improved memory, and blood circulation. Nevertheless, allergenicity, digestibility, and antinutritional factors (ANF) are major constraints to their functional utilization. However, various methods such as steaming, boiling, fermentation, irradiation, and high-pressure cooking have been found to overcome these challenges, as indicated in studies conducted by (Maphosa and Jideani, 2017; Bessada et al., 2019; Tan et al., 2020). Despite this, underutilized legumes, especially tubers, have not been fully utilized and their nutritional content has not been fully exploited (Ojuederie et al., 2020; Ayilara et al., 2022; Popoola et al., 2022a). The nutritional composition of the selected underutilized legumes’ raw, mature seeds, with values per 100g, is presented in Table 2.
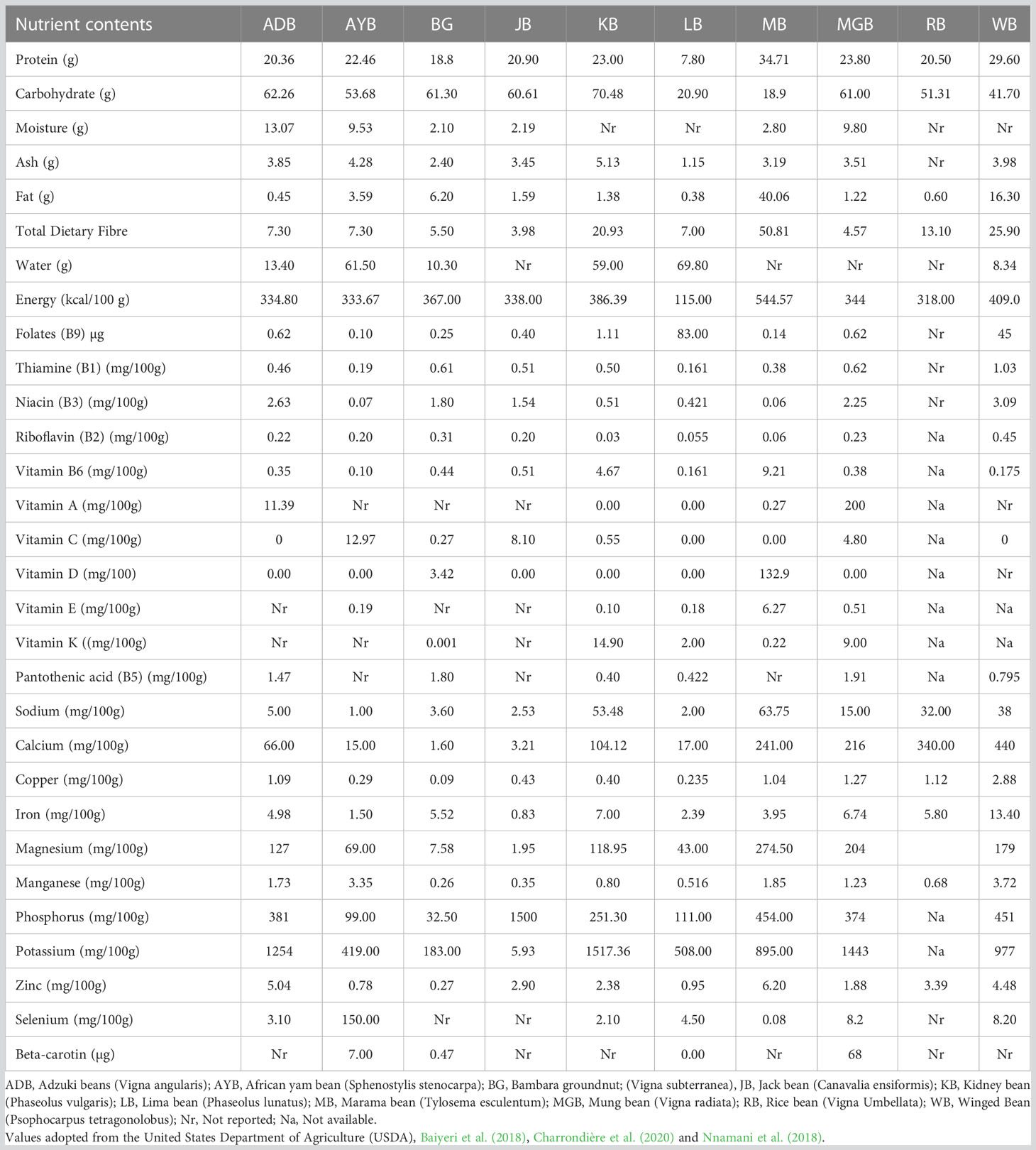
Table 2 Nutritional composition of the selected underutilized legumes' raw, mature seeds, with values per 100g.
4 Functional properties and probiotics of underutilized legumes
4.1 Functional properties of underutilized legumes
Underutilized legumes possess noteworthy functional properties which are beneficial to food systems. Functional properties such as solubility, hydration, emulsification, foaming stability, gel-forming index, and pasting properties govern the utilization of legumes as protein-rich gluten-free food additives. The functional properties of these substances should be taken into account when formulating and processing food, to develop innovative food products (Bessada et al., 2019). Moreover, the functionality of legumes is affected by protein including its molecular size, structure, and charge distribution as well as non-protein molecules such as carbohydrates, lipids, and salts.
The functional properties of the underutilized legumes considered in this review are presented in Table 3. The hydration properties of flour are swelling power, solubility, water, and oil absorption capacities. These properties influence the structural, rheological, thermal, and sensory characteristics of foods. Swelling power is a measure of both intragranular and intergranular water present in flour/starch under excess water and high thermal conditions (Iuga and Mironeasa, 2020). According to recent research by (Palupi et al., 2021), lima bean flour has a swelling power of 6.88 g/g, a solubility of 18.68%, a water absorption capacity of 1.93 g/g, and an oil absorption capacity of 1.56 g/g when exposed to a temperature of 87°C. The African yam bean seed flour displayed good gelation properties, while protein solubility varied with pH, with high solubilities in acid and alkali (Oshodi et al., 1997) (Table 2). Ratnawati et al. (2019) revealed that at 95°C, mung bean and red bean flours had significantly higher swelling powers (10.5 and 10.1 g/g, respectively) than soybean flour (4.8 g/g). The research of Yadav et al. (2018) indicated that adzuki beans had a hydration capacity range of 0.05 to 0.12 g/seed, a swelling capacity of 0.04 to 0.15 mL/seed when the cooking time is reduced (48.67 to 74.33 min). Typically, flour with high swelling power elicits high gelatinization and paste properties which is vital for the structural and textural development of baked foods (Onyango, 2016). In addition, the solubility of flour or starch provides an index of the hydrophilic behavior of amylose molecules under high moisture and thermal conditions (Dudu et al., 2019). Flour solubility has an impact on the clarity of drinks as well as foam formation and stabilization. It also affects emulsification, gelation, and retrogradation which influences crumb grain formation, texture, sensory properties, and staling of baked foods. Furthermore, water and oil absorption capacities are the maximum amount of intragranular water or oil present in flour under excess moisture and ambient temperature conditions (Iuga and Mironeasa, 2020). Emulsification properties consist of emulsifying activity and stability which are modulated by the ratio of hydrophobic to hydrophilic amino acids present in the legume flour. Emulsifying activity is a measure of the ability of flour to form a stable emulsion (oil-water interaction) by protein dispersion in the presence of oil. On the other hand, stability measures the strength of the emulsion formed (Nawaz et al., 2021). Most of the underutilized legumes have been reported to show good functional properties of solubility, emulsification, oil absorption capacity, gelation and forming properties (Okezie and Bello, 1988; Onimawo et al., 1998; Barac et al., 2015; Arogundade et al., 2016; Mubaiwa et al., 2018; Diedericks et al., 2020; Yang et al., 2022).
Foaming properties include foaming capacity and stability. Foaming capacity is the ability of protein or flour to add air when whipped, and foam stability is the capacity to stabilize foams (volume) over time (≤30 min) (Bessada et al., 2019). Iwe et al. (2016) revealed that African yam bean flour has a foaming capacity and stability of 18% and 92.6%, respectively. Pasting properties, mainly pasting temperature, peak, breakdown, and setback viscosities provide insights into swelling capacity, structural stability, and amylose retrogradation tendency of flour under combined high mechanical shearing and hydrothermal conditions. This is typically carried out in a rapid viscosity analyzer or a Brabender Visco-Amylo-Graph. These properties are critical to the functionality of flour in food and industrial systems. Pasting temperature is the thermal energy required to destroy flour granule structures leading to the onset of paste development. Associations between flour composition, pasting, viscosity, and bulk density of some underutilized legumes have been investigated (Du et al., 2014). Furthermore, some studies on legume flour highlighted the relationship of flour microstructure with pasting even though such studies are scanty for the underutilized legumes (Shevkani et al., 2021). The pasting properties of the processed lima bean flour showed a peak of 1172 cP, a breakdown of 83 cP, final of 2377 cP, and a setback viscosity of 1288 cp (Palupi et al., 2021). Ratnawati et al. (2019) revealed that the pasting temperature of red kidney bean flour (81.7 °C) was higher than that of mungbean flour (77.9 °C). Flours with low breakdown viscosity can serve as structuring agents in food where a minimal structural breakdown is required. Setback viscosity is the recovery of viscosity during the cooling of flour after being subjected to combined high mechanical shearing and hydrothermal conditions (Cornejo-Ramírez et al., 2018). It is an index of the retrogradation ability of flour, which is an important prerequisite for staling activity during product storage. Flours with low setback viscosity may be utilized in delaying retrogradation activity in cooked infant formulas, breakfast foods, and pasta products as well as prevent staling of baked products.
Bulk density is an index of the structural integrity of granules that relates to the packaging and raw material handling of flour (Adedayo et al., 2021). Iwe et al. (2016) revealed that AYB has a loose bulk density of 0.63 g/mL, repacked bulk density of 0.87g/mL, water absorption of 0.70%, and oil absorption of 1.48%, all of which are comparable to that of cowpea and rice. Bulk density in adzuki beans range 0.76 to 1.00 g/mL (Yadav et al., 2018). Nwajagu et al. (2021) showed that M. flagellipes seed flour has a bulk density of 0.8 mg/100g. Okechukwu-Ezike et al. (2020) revealed that black-eyed beans and black beans have higher bulk densities (0.6 g/cm3) than brown beans (0.4 g/cm3). Sattar et al. (2017) showed that black gram (Vigna mungo) flour, green gram (Vigna radiata) flour, and lentil (Lens culinaris) flour have bulk densities of 0.5 g/cm3, 0.5 g/cm3, and 0.6 g/cm3, respectively. Flours with high bulk density are suitable as thickeners, while those with low bulk density can be in complementary food formulas. The functional properties of the underutilized legumes considered in this review are presented in Table 3.
4.2 Probiotics and prebiotics potential of underutilized legumes
According to The International Scientific Association for Probiotics and Prebiotics (ISAPP), “Probiotics are live microorganisms, which when administered in adequate amounts, confer a health benefit on the host” (Marco et al., 2021). Moreover, prebiotics are “substrates that are selectively utilized by host microorganisms conferring a health benefit” (Sanders et al., 2019). In time past, the health benefits of probiotics were realized from the consumption of milk, soybean, and other dairy products. However, the problem of short shelf-life, allergenic milk proteins, high cholesterol content, lactose intolerance, consumer inclination towards veganism, and economic considerations for developing countries, have compelled the exploration of non-dairy alternatives with good nutritional profile and health-promoting factors (Panghal et al., 2018; Chaturvedi and Chakraborty, 2021; Chaturvedi and Chakraborty, 2022). In the last decade, the non-dairy food products market has received positive acceptance and is projected to reach an estimated 26 billion USD by the year 2025. The underutilized legumes hold an exceptional capacity to be utilized as probiotic carriers (Rasika et al., 2021; Chaturvedi and Chakraborty, 2022). These legumes constitute appropriate matrices for the production of non-dairy alternatives like plant-based beverages due to the presence of natural prebiotics including resistant starch, oligosaccharides, isoflavones, and polyphenols. These prebiotics exert a wide range of physiological functions such as immune system modulation, metabolic regulation, and anti-inflammatory and anti-cancer properties, and therefore offer enormous potential for the development of symbiotic foods (a blend of prebiotics and probiotics) using lactic acid bacteria (LAB) (Chaturvedi and Chakraborty, 2022; Cichońska and Ziarno, 2022).
Generally, research findings have shown that underutilized legumes such as adzuki beans, African yam bean, Bambara groundnut, and mung beans exhibit a low glycemic index due to their high resistant starch and amylose contents and have been shown to reduce the risks of high blood pressure and type-2 diabetes (Barac et al. 2015; Johnson et al., 2020; Adedayo et al., 2021; Johnson et al., 2022). Adeoye et al. (2021) reported the probiotic nutraceutical potential of Bambara groundnut. In the study, Lactobacillus delbrueckii, L. casei and L. brevis were the preponderant LAB found in isolates of fermented Bambara groundnut. The authors further demonstrated the in vitro antagonistic properties of the LAB isolates against pathogenic namely Salmonella sp, Escherichia coli, Staphylococcus sp, Shigella sp and Pseudomonas sp. Very recently, Chaturvedi and Chakraborty (2022) conducted a study to evaluate the prebiotic characteristics of synbiotic drinks made from legumes, specifically red kidney beans and green mung beans. The results indicated that these drinks had a considerable impact on promoting the growth of the probiotic Lactobacillus casei ATCC 335, while simultaneously hindering the colonization of the enteric pathogen Escherichia coli. The study established that the formulated beverage showed prebiotic and probiotic potentials that could serve as a veritable alternative to dairy symbiotic beverages. Thus, the functional and probiotic properties elicited by the above-mentioned underutilized legumes serve as a point of reference for their suitability and/or exploitation in food, industrial and pharmaceutical systems. However, more research is required in this regard to provide more insight into the probiotic and prebiotic potentials of underutilized legumes.
5 Bioactive components of underutilized legumes
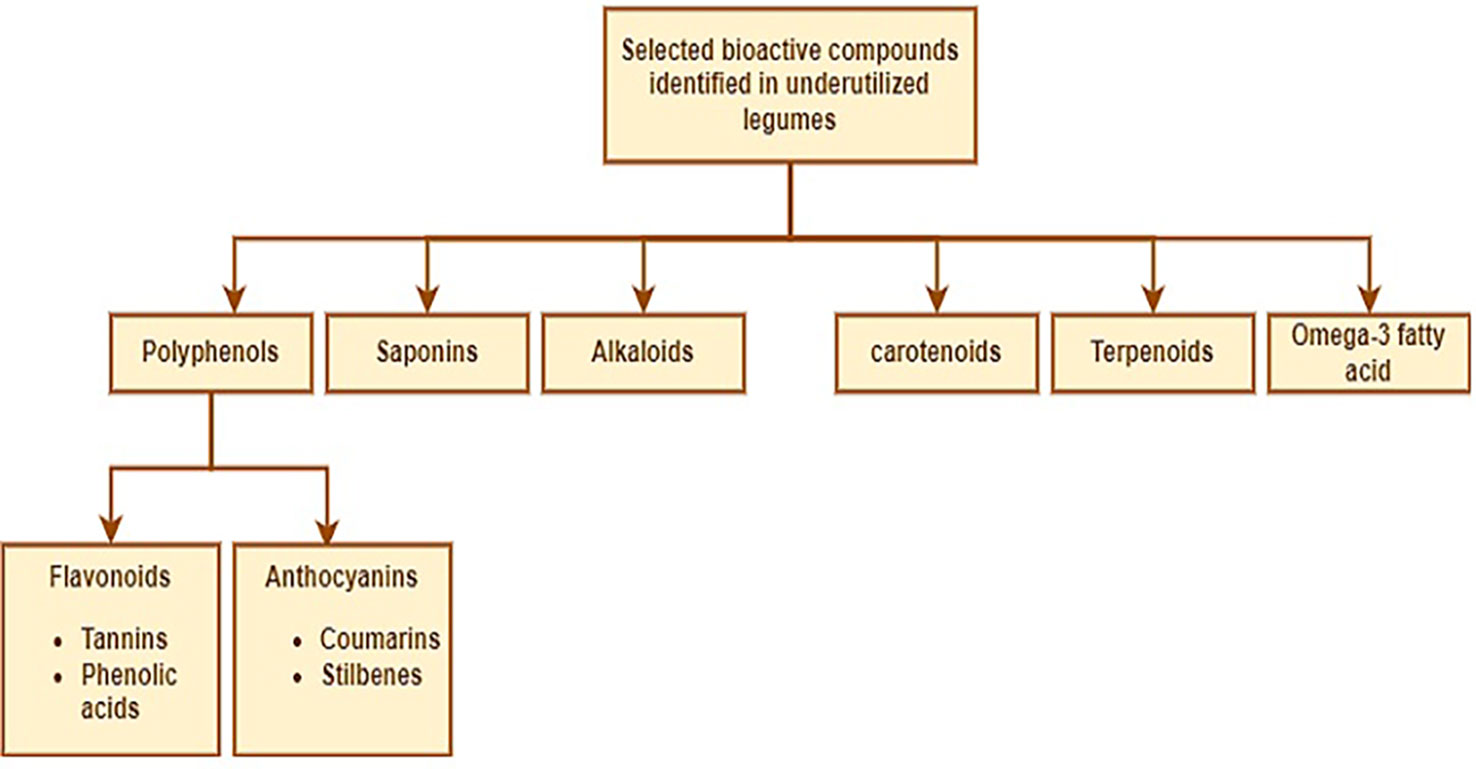
Bioactives are compounds that when ingested by humans/animals have some physiological contributions that could enhance healthy living and support a decrease in the occurrence of illness (James et al., 2020; Cui et al., 2021). Commonly, legumes including the underutilized ones are rich in polyphenols, alkaloids, saponins, carotenoids, terpenoids, omega-3 fatty acids, flavonoids, and anthocyanins amongst others (Figure 3). These substances have varying degrees of abilities to act as antioxidants, antimicrobials, anticancer agents, anti-tumor agents, anti-inflammatory agents, and neuroprotective agents (Zheng et al., 2019; Shevkani et al., 2022; Popoola et al., 2022a). Furthermore, it has been suggested that polyphenols, alkaloids, and saponins play a vital role in protecting the plant from herbivores and pathogens by serving as defense mechanisms. They also act as signaling molecules between the plant and its biotic environment (Divekar et al., 2022).
Several authors such as Oboh et al. (2015); Ajibola and Olapade (2016); Soetan (2017); Soetan and Atanda (2018); Soetan and Adeola (2018); Soetan et al. (2018); Adegboyega et al. (2019); Mayes et al. (2019); Ojuederie and Balogun (2019); Adegboyega et al. (2020); Adegboyega et al. (2021); Ikhajiagbe et al. (2021); Ojuederie et al. (2021) and Popoola et al. (2022a) have attempted to unravel the bioactive ingredients available in underutilized legume crops such as African yam bean, Bambara groundnut, Kersting’s groundnut, and winged bean which can be exploited as nutraceuticals. In general, the seeds contain bioactive compounds that can improve human health and provide several benefits, such as aiding digestion, promoting weight loss, and reducing the risk of heart diseases and type 2 diabetes (Alcázar-Valle et al., 2020; Bhadkaria et al., 2021). In addition to the dietary fiber, polyphenols, and natural antioxidants embedded in the seeds are of vital benefits to defend against free radicals (Amarowicz and Pegg, 2008; Xu et al., 2017). These components are urgently needed to be exploited for the benefit of man and animals particularly in the management of degenerative infections (Silva et al., 2007; Singh et al., 2017). The promising nature of these compounds (Figure 3) has afforded researchers to look into the possibilities of developing food-based therapy for disease management but more actions are still required in this direction (James et al., 2020).
Polyphenols and their derivatives such as flavonoids, anthocyanins, tannins, and tocopherol are among the essential bioactive compounds found in underutilized legumes. In a comparative study of antioxidants produced by Bambara groundnut (BG) using methanolic extract, Salawu (2016) identified the presence of several polyphenols in the raw and cooked BG seeds after quantification using HPLC-DAD. A recent study identified catechin, epicatechins, rutin, quercetin, iso-quercetin, kaempferol, luteolin, gallic acid, chlorogenic acid, caffeic acid, and ellagic acid as polyphenols found in BG (Okafor et al., 2022). Likewise, Harris et al. (2018) compared the level of flavonoids in red and brown BG hulls and observed that the brown hull had the highest amount of rutin (24.46 ± 0,23 mg g-1) and myricetin (1.80 ± 0.77 mg g-1) while the phytochemicals chlorogenic acid and ellagic acids which are tannins, had their highest concentrations in red BG hulls (0.12 ± 0.19 mg g-1) and brown BG (0.11 ± 0.08 mg g-1) respectively (Harris et al., 2018). Their findings revealed that the best source of flavonoids and tannins were found in the brown and red hulls rather than in the whole or dehulled BG seeds.
Apart from producing antioxidants, polyphenols are also known to possess anti-microbial, anti-viral, anti-inflammatory, anti-allergic as well as anti-mutagenic effects, scavenging free radicals that cause cell degeneration and death. The anti-cancer properties of some of these polyphenols have been previously tested and confirmed. For instance, ellagic acid, quercetin, catechin, and phenolic acid prevented several kinds of cancer that affect the skin, stomach, duodenum, mouth, colon, liver, lung, and mammary glands (Yang et al., 2001; Okafor et al., 2022). Ade-Omowaye et al. (2015) and Soetan et al. (2018) independently confirmed the presence of antioxidant-related phytochemicals: phenolics and flavonoids in AYB. Out of nine underutilized legumes studied by Ade-Omowaye et al. (2015), AYB had very high total polyphenolic contents of 293.23 mg 100 g-1 and 288.68 mg 100 g-1 with higher antioxidant activities, (1.00 mmoleTE 100 g-1 and 0.67 mmolTE 100 g-1), respectively, including a variety of BG (0.88 mmolTE 100 g-1). Akinyemi et al. (2020) confirmed the presence of flavonoids, tannins, alkaloids, saponins, and cardiac glycosides in Jack beans. According to several studies (Sowndhararajan et al., 2011; Solomon et al., 2018; Akinyemi et al., 2020), Jack beans have high levels of antioxidants that have been shown to possess numerous health benefits such as reducing the risk of type 2 diabetes, cancer, and inflammation, improving lipid metabolism, lowering bad cholesterol levels, preventing metabolic syndrome, and reducing the incidence of cardiovascular diseases. Legume seeds contain significant amounts of antioxidants due to their high phenolic, flavonoid, and anthocyanin contents. Consuming products made from these legumes may help prevent and manage various chronic and degenerative diseases, as well as address protein-calorie malnutrition (Foyer et al., 2016; Tsamo et al., 2020; Popoola et al., 2022a). Studies by Adefegha and Oboh (2012); Kennedy (2014); Adefegha et al. (2017); Gupta and Prakash (2019), and Adefegha (2018) have confirmed that these bioactive components of NULs could play significant roles in increasing the immune level and support prevention of common diseases such as malnutrition (severe and acute particularly in infants), sexual enhancers, obesity, diabetes, heart-related diseases amongst others.
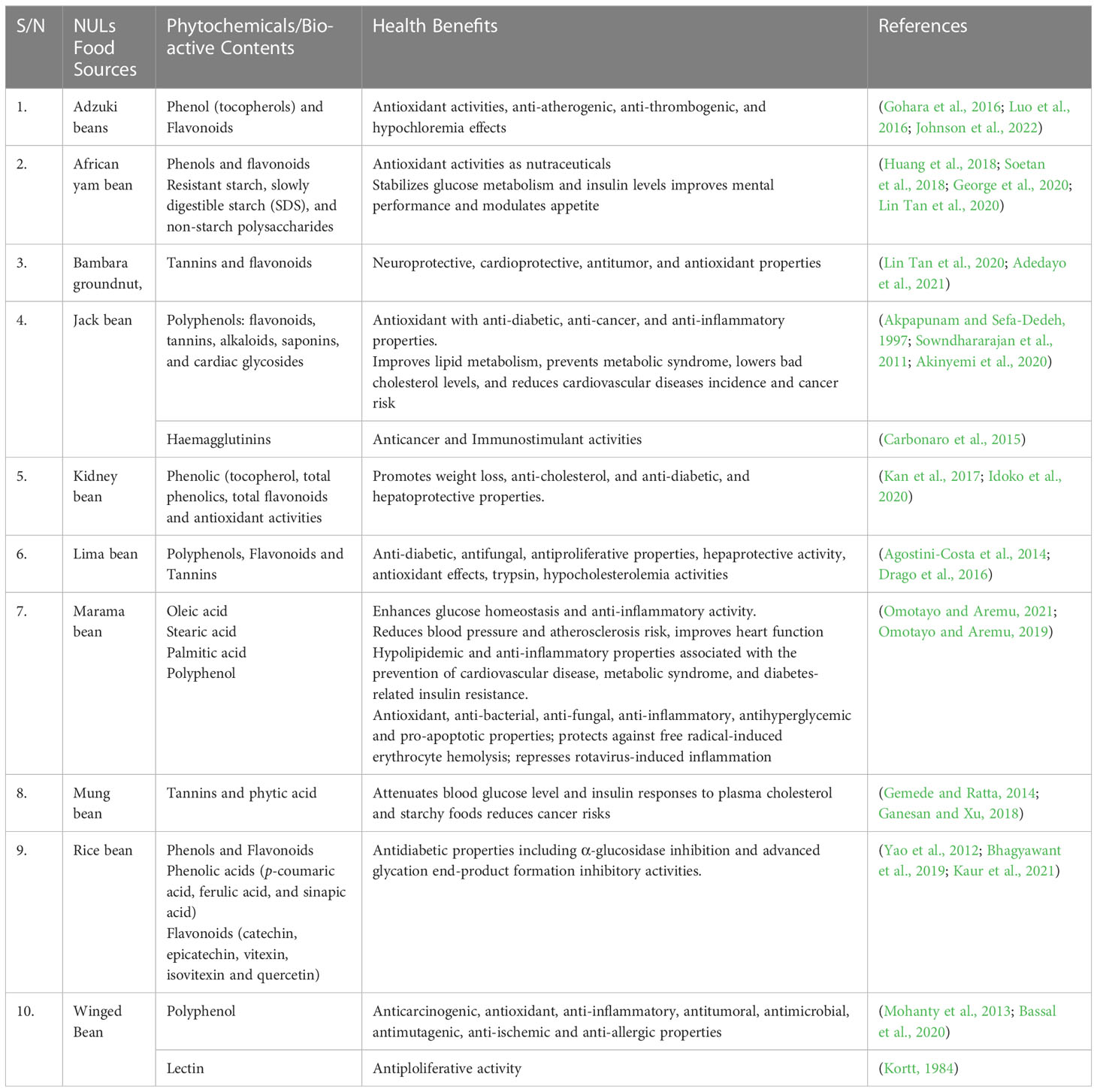
In terms of potentials as nutraceuticals, African yam bean (Sphenostylis stenocarpa), and Horse gram (Macrotyloma uniflorum) are legumes worthy of note, due to their anti-diabetic property, anti-urolithiasis effect, and role in the prevention and management of cardiovascular diseases, kidney stones, gastritis, pile, and urinary tract disease (Sharma et al., 2019; Vijayakumar, 2021). Extracts from mung bean, adzuki bean, black bean, rice bean, and lima bean have been documented to exert hepato-protective effects due to the presence of antioxidant and anti-inflammatory compounds (Vijayakumar, 2021). The bioactive components and health benefits of the selected NULs are presented in Table 4 and Figure 3.
Bioactive proteins and peptides are abundant in legume seeds (Kortt, 1984; Mojica and González de Meija, 2015; Makeri et al., 2016; Hussein et al., 2020). A notable bioactive protein in legume seeds is a lectin. Lectins possess anticancer and immunostimulatory activities. Lectins also help to reduce the risk of cardiovascular diseases in obsessed individuals (Roy et al., 2010; Carbonaro and Nucara, 2022).
Haemagglutinins from Jack bean possess anticancer and immunostimulatory properties (Carbonaro and Nucara, 2022). Jack bean produces a well-known lectin called Concanavalin A (Con. A) which has an extremely high anti-hepatoma activity arising from its resistance and structural stability to in vitro proteolysis and denaturation (Carbonaro et al., 2015; Huldani et al., 2022). The high level of lectins in winged bean has also been shown to have antiproliferative activity on human cancer cell lines. Two lectins (B2 and B3) were identified in winged bean which exhibited the same amino-terminal sequences and the sequence of lectin B3 to residue 40 reflected extensive homology with other legume lectins such as soybean lectin (Kortt, 1984).
Saponins are often regarded as antinutritional factors (ANTs) in grain legumes as they inhibit active transport and simultaneously increase the general permeability of enterocyte barrier (Bennetau-Pelissero, 2018). Thus, saponins increase the permeability of the small intestinal mucosal cells, facilitating the uptake of substances to which the gut would normally be impermeable. It also reduces the bioavailability of nutrients and decreases enzyme activity, resulting in an inhibition of growth. Notwithstanding, saponins have some positive health benefits as they contain a triterpenoid aglycone (sapogenin) linked to one or more oligosaccharide groups with the ability to absorb free radicals and activate antioxidant enzymes (Hai et al., 2021). Saponins in legume seeds contain two major components soya-saponin I (approximately 630 to 900 mg/kg) and dehydrosoyasaponin I (approximately 650 to 1300 mg/kg). The hilum portion of legume seeds has been identified as having the highest saponin content compared to the cotyledons (Hai et al., 2021). Research findings reveal that Japanese and Chinese populations have a lower risk for breast, colon, corpus uterine, and prostate cancers due to their high intakes of legumes and legume products, which are good sources of saponins (Messina et al., 1994; Shi et al., 2004). Thus, they tend to have a longer life span than Africans (Lu et al., 2017). Terpenoids are a sub-group of triterpenoids and have been implicated to reduce bad cholesterol level and possess anti-cancer and antimicrobial properties (Marrelli et al., 2016). To our knowledge, little or no research has been done on the benefits of saponins and terpenoids derived from African underutilized legumes. This is an aspect of research that should be promoted to enhance the livelihood of the African population and would aid in the attainment of the Sustainable Development Goal of the United Nation on better health and well-being.
Alkaloids just like saponins are considered ANTs, and have been reported in a few underutilized legumes but not in detail (Konyeme et al., 2020; Popoola et al., 2022a). Alkaloids are naturally essential as defense agents which make up approximately 20% of the known secondary metabolites available in plants (Kaur and Arora, 2015). Therapeutically, alkaloids are particularly well-known as antioxidants, anti-inflammatories, anesthetics, and cardioprotective agents (Kurek, 2019; Heinrich et al., 2021). The presence of alkaloids in some underutilized legumes (winged bean, AYB, and Kersting’s groundnut) suggests their potential application as anti-cancer, anti-inflammatory, antimicrobial, and analgesic agents amongst others (Kaur and Arora, 2015; Popoola et al., 2022a). Jack bean and AYB have been found to exhibit higher contents of alkaloids (0.645g/100g) and (22.195-183g\100g) (Konyeme et al., 2020). More investigations are needed on underutilized alkaloids, especially about their contents and variability as regards quinolizidine (QA) and pyrrolidone (PA).
Carotenoids such as β-carotene, lutein, and cryptoxanthin have been detected in most legumes though much lower compared to that of fruits and vegetables (Tee et al., 1995). Carotenoids are widely distributed in legumes and have been reported to exhibit health-promoting benefits such as antioxidants, better visual function, and reduction of cardiovascular diseases (Voutilainen et al., 2006; Ku et al., 2020; Maoka, 2020). In a study to evaluate bioactive components of selected underutilized legumes indigenous to Nigeria, James et al. (2020) found out that, fermentation and germination reduced carotenoid, anthocyanin, tannin, and flavonoid contents of the legumes.
Lipid profiles of underutilized legumes has been fairly documented but their effects on blood lipid levels are limited (Zhang et al., 2010; Adebowale et al., 2011). However, research has linked the consumption of these diets to a decreased risk of heart disease and obesity, according to (Hossain et al., 2016). Furthermore, Yao et al. (2015) discovered that the Ci12 landrace of Bambara groundnut from Côte d’Ivoire contained a high concentration of n-6 fatty acids, which are classified as polyunsaturated fatty acids (PUFAs) and include Omega-6 linoleic acid (C18:2, ώ-6) and Omega-3 alpha-linoleic acid (C18:3, ώ-3). These acids cannot be produced by the body and must be obtained through diet. Studies have also shown that consuming diets rich in Omega-6 fatty acids can reduce the incidence of cardiovascular disease and obesity, as noted by (Patterson et al., 2012; Djuricic and Calder, 2021). Oleic, stearic and palmitic acids have been recorded for Maraba bean (Omotayo and Aremu, 2021). The fatty acids components such as palmitic, palmitoleic, oleic, arachidonic, eicosapentaenoic, docosapentaenoic, lignoceric, docosahexaenoic and nervonic acids have not been studied extensively in African underutilized legumes. Further studies are required to unravel the PUFAs available in these lesser-known legumes.
6 Antimicrobial properties of underutilized legumes
Infections resulting from microbial sources are a great source of threat to plants, animals, and human health, which have necessitated the use of effective, safe, and sustainable biocontrol methods. This is particularly important due to the resistance of microbes to antibiotics and other control mechanisms as well as the search for novel antimicrobial agents (Udeh et al., 2020). Underutilized legumes are embedded with inherent antimicrobial abilities through the presence of different phytochemicals which include phytate, tannins, anthocyanin, flavonoids, etc. (Ayilara et al., 2022). These phytochemicals have been reported to be capable of controlling both gram-positive and gram-negative pathogenic bacteria (Ramatsetse et al., 2023). For instance, Bambara groundnut has been reported to inhibit the growth of different human pathogenic organisms which include Klebsiella pneumonia, Escherichia coli, Bacillus aureus, Pseudomonas aeruginosa, Candida albicans, Klebsiella aerogenes, Aspergillus niger and Staphylococcus aureus (Klompong and Benjakul, 2015; Wanyama et al., 2017; Oyeyinka et al., 2021) (Table 5). Antimicrobial properties of other selected underutilized legumes are shown in Table 5. The mechanism of action of the underutilized legumes as antimicrobial agents includes disruption of the microbial activity, chelation of crucial micro mineral elements (zinc and iron), suppression of the cell surface microbial enzymes, hydrophobic and electrostatic interaction with the cell membrane and cell wall (leading to the production of large pores and consequently its disintegration), induction of morphological changes in bacteria cells, increase in the permeability of cell wall which results to cell lysis and death, penetration of the cytoplasmic membrane, reduced intracellular ATP concentration and the prevention of spore germination and mycelial growth in fungi (Sitohy et al., 2013; Lopes and Brandelli, 2018; Udeh et al., 2020; Jia et al., 2021). On antifungal potentials, an array of proteins/peptides from mungbean, kidney bean, African yam bean, lima beans, brown kidney, winged beans), have elicited antifungal effect against plant and human pathogens including Fusarium oxysporum and Coprinus comatus, Verticillium dahlia, Botrytis cinerea, Setosphaeria turcica, Rhizoctonia solani, Mycosphaerella arachidicola, Helminthosporium maydis, Candida albicans, Gibberalla sanbinetti, Sclerotinia sclerotiorum, etc. (Mani-López et al., 2021). More research focus is required on arrays of antimicrobial properties of underutilized legumes of Africa which will possibly lead to cheaper means of drug discovery and good health care in Africa.
The species of legumes, the concentration of the extract, and the type of extractant (solvent) used are essential factors that affect the activity of underutilized legumes as antimicrobial agents (Shelke et al., 2022). Kaundal et al. (2019) reported that when different solvents (dichloromethane, 1-butanol, water and ethyl acetate) were used to assess the antimicrobial properties of Horse gram against human pathogenic organisms (Bacillus sp., E.coli, Shigella sp., Staphylococcus sp. and Salmonella sp.), ethyl acetate and dichloromethane extracts revealed antibacterial activities while the aqueous and 1-butanol extract showed no antibacterial properties (Kaundal et al., 2019). Hence, it is essential to carry out further research on different underutilized legumes to unravel the best extractant that can be used to extract the active ingredients in different NULs to promote their potential in the discovery of new drugs.
In addition, different plant parts are used in the production of plant extracts, these include the pods, seeds, flowers, hall, root, stem, tuber, and leaf, where different types and different forms of phytochemicals can be found (Henciya et al., 2017; Zhong et al., 2022). More research should be carried out on underutilized legumes to unravel the different parts of each NULs that can give a maximum recovery and variety of antimicrobial active compounds.
7 Prospects in harnessing the benefits of underutilized legumes
Too much reliance on a few staple crops to meet the food and nutritional needs of man is a potential threat to the global fight against food insecurity and to ensure that the zero hunger sustainable development goals (SDGs) are achieved by 2030. Traditional or indigenous food crops in Africa have major roles to play in realizing the SDGs 2 and 3 of the United Nations if given the utmost attention and necessary improvement for human consumption. The African populace needs to be sensitized to the benefits derived from her indigenous legumes. Furthermore, researchers in Africa must embark on collaborative research and give priority to these legumes in crop improvement programs using a holistic approach. Cellular oxidative stress has been implicated in the development of chronic diseases such as cardiovascular disease, cancer, arthritis, diabetes, and degenerative diseases in humans. Nevertheless, antioxidants in foods regulate and reduce oxidative destruction by inhibiting oxidation caused by reactive oxygen species (ROS), and improve the shelf-life and quality of these foods (Ames et al., 1993; Altemimi et al., 2017). The bioactive components of legume seeds possess antioxidant activity which could mitigate the effects of oxidative stress. However, these bioactive forms a small percentage of the nutritional components of legume seeds.
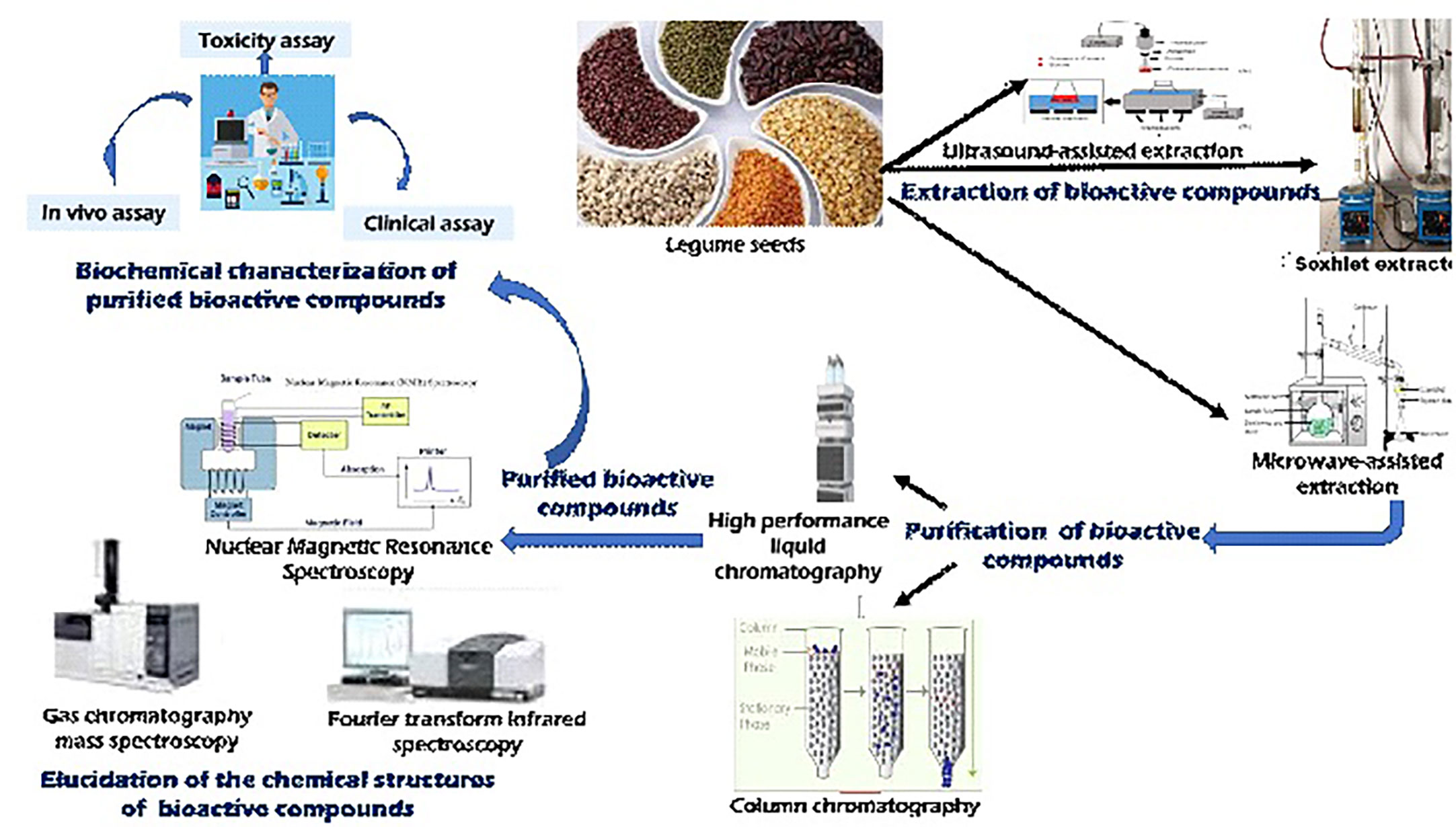
Singh et al. (2021) revealed that bioactive compounds are concentrated in different parts of the seeds of legumes. For instance, phenolic compounds such as flavonoids and dietary fibers occur in the seed coat while non-flavonoids such as oligosaccharides and dietary fiber occur in the cotyledons (Singh et al., 2021). Including dietary fibers from legume seeds and cotyledons in one’s diet has several positive effects on human health. These fibers can assist with digestion in the gastrointestinal tract by increasing water-holding capacity, viscosity, bulk, fermentability, and the ability to bind bile acids, as noted by Stosh and Yada (2010). In addition, it is known to reduce serum cholesterol in hypercholesterolemic people and postprandial glycemia. The dietary fiber in the seed testa has been reported to be significantly higher than the quantity in the cotyledons (Sergio et al., 2020). These bioactive compounds could also be used to design functional food products. Unfortunately, most of the bioactive components of the seeds of underutilized grain legumes are unknown. Therefore, there is a need for these bioactives present in Africa’s indigenous legumes to be extracted, purified, and characterized using biochemical approaches with the chemical structures elucidated with the aid of high-tech equipment (Figure 4). To extract the bioactive compounds from these NULs, modern extraction techniques can be employed for maximum efficiency. Once extracted, these components are further purified using chromatographic methods, such as high performance liquid chromatography (HPLC) or column chromatography, before being profiled using analytical techniques such as nuclear magnetic resonance (NMR), gas-chromatography-mass spectroscopy (GC-MS), GC-time of flight-MS (GC-TOF-MS) which improves resolved peaks, LC-MS, or Fourier Transform Infrared spectroscopy (FTIR) used to detect different functional groups of metabolites. These techniques help to identify and quantify a variety of primary and secondary metabolites in the purified bioactive compounds (Pandey et al., 2016).
Different methods have been used for the extraction of bioactive compounds; these include microwave-assisted extraction (MAE) soxhlet extraction (SE) using different solvents as well as ultrasonic/ultrasound-assisted extraction (UAE). Other modern methods also in use include supercritical fluid extraction (SFE), solid-phase extraction (SPE), enzyme-assisted extraction, pressurized liquid extraction (PLE) or accelerated solvent extraction (ASE), and extraction assisted by a pulsed electric field. These recent methods are efficient in the removal of flavonoids from plant products. Current reviews have discussed in detail, the use of these modern methods for the extraction of essential bioactives from plants (Selvamuthukumaran and Shi, 2017; Chaves et al., 2020).
The MAE is one of the modern extraction methods that has received much patronage due to several merits such as a reduction in utilization of solvents, enhanced yield recovery, better selectivity, reproducibility, reduced operation time, and less sample manipulation (Alara and Abdurahman, 2019; Alara et al., 2019; Nour et al., 2021). MAE utilizes microwave energy for faster heating which results from a range of electromagnetic spectrums of light (300 MHz to 300 GHz) with short wavelengths usually between 1 cm−1 and 1 m−1. The combined effect of increased temperature within the extraction medium and the effect of microwave electromagnetic radiation on vibrations of both the extraction solvents and the analytes being extracted enhances the extraction yield (Ameer et al., 2017; Chaves et al., 2020). It is essential to note that the efficiency of the extraction process is dependent on several factors such as the temperature and particle size, solvent-solid ratio, as well as the nature of the solvent used for extraction.
MAE utilizes microwave energy for rapid heating which results from a range of electromagnetic spectrums of light (300 MHz to 300 GHz) with short wavelengths usually between 1 cm−1 and 1 m−1. The combined effect of increased temperature within the extraction medium and the effect of microwave electromagnetic radiation on vibrations of both the extraction solvents and the analytes being extracted enhances the extraction yield (Ameer et al., 2017; Chaves et al., 2020). It is noteworthy that the efficiency of the extraction process is dependent on several factors such as the temperature and particle size, solvent-solid ratio, as well as the nature of the solvent used for extraction. The extraction efficiency is dependent on factors such as the nature of the solvent, solvent–solid ratio, temperature, as well as particle size. To enhance the extraction process, optimization of modern extraction methods is required. Synergistic effects could be obtained if there is a combination of the above novel extraction methods. Tsamo et al. (2020) used an ultra-performance liquid chromatographic system with a PDA detector coupled to a mass spectrophotometer detector (UPLC-qTOF-MS) to identify phenolic compounds in the seeds of Kersting’s groundnut. A total of 57 potential compounds were identified, among which were flavonoids; catechin, gallocatechin, quercetin, rutin, naringin, kaempferol, 7-rutinoside, and eriodictyol 7-rutinoside.Gallocatechin is known to enhance lipid metabolism and aids the prevention of metabolic syndrome as has been reported in some other legume seeds such as pea and lentils (Mirali et al., 2017; Jha et al., 2019; Tsamo et al., 2020). Table 6 shows different modern methods used in extraction of bioactive components of plants, including seeds.
With the advancement in sequencing techniques and omics technologies such as genomics, proteomics, transcriptomics, metabolomics, and the genome editing tools such as the CRISPER-Cas9 or TALEN, underutilized legumes can be genetically improved for better utilization and acceptance as these legumes are currently faced with some production constraints such as high antinutritional factors in the seeds which reduce the bioavailability of minerals, prolonged cooking time due to hardness of the seed coat of some pulses, and photoperiod sensitivity which also affects tuberization in tuberous legumes. The utilization of plant-based functional foods can be enhanced by the use of innovative technologies for the extraction and microencapsulation of bioactive compounds using novel technologies in metabolomics (Nayak et al., 2021; Pattnaik et al., 2021). Metabolomic studies can be used to identify rich value-added compounds from different parts of underutilized legumes such as the seeds, leaves, stems, or tubers, listing the main bioactive metabolites identified and the factors affecting their production. Metabolomics finds its usefulness in the identification of metabolites after the bioactives have been extracted using one of the modern methods discussed above. It highlights the expressions and changes of metabolites, as well as their interactions and resulting phenotypic traits in plants subjected to harsh environmental conditions. Under such stress, plants must adapt their metabolomic pathways to maintain metabolic homeostasis, a process referred to as acclimation (Joshi et al., 2021; Makhumbila et al., 2022).
Chen et al. (2020) studied the metabolomic profile of common bean and identified major findings related to amino acids, flavonoids, isoflavonoids, purines, and proline metabolism. These pathways enhanced the plant’s potential for defense against pathogens like Fusarium solani (FS). The study combined RNA sequencing and metabolomics techniques to investigate changes in gene expression and metabolic processes in common bean infected with FS. The results showed that metabolic pathways were enriched, leading to an increase in metabolites involved in plant defense response. Infected common bean seedlings responded with modifications to their cell walls, the generation of reactive oxygen species, and a synergistic hormone-driven defense response. The study also found that infected plants induced energy metabolism, nitrogen mobilization, accumulation of sugars, and arginine and proline metabolism (Chen et al., 2020; Makhumbila et al., 2022). Reliable software tools such as GCMS, LC-MS, and NMR are required to analyze the vast amounts of data generated by metabolomic technologies. These tools should be capable of visualizing, detecting peaks, normalizing/transforming sample data, annotating, identifying, quantifying, and statistically analyzing targeted and untargeted metabolite variations using algorithms for univariate and multivariate analysis (Sun and Weckwerth, 2012; Junot et al., 2014; Makhumbila et al., 2022). There are now several metabolomic pathway databases available online that group metabolites with similar functions. These databases include the Kyoto Encyclopedia of Genes and Genomes (KEGG), Cytoscape, MapMan, and iPath, which are relevant to plants. Cytoscape is an open-source software platform used to visualize complex networks and integrate them with any type of attribute data. MapMan is a user-driven tool that displays large datasets onto diagrams of metabolic pathways while iPath is a relevant tool for plants (Fukushima and Kusano, 2013; Patel et al., 2021).
With the advent of Next-generation Sequencing (NGS), the cost of sequencing has plummeted, making it possible to sequence large and complex genomes in a shorter period (Hamilton and Robin Buell, 2012; Kumar et al., 2021). Several whole genome sequencing studies are underway for some underutilized crops, and some have been completed. Once these sequences are available, they can be applied for in-depth structural and functional genomic studies to characterize and annotate the genes. Furthermore, the availability of the whole genome sequence will accelerate the development of genetic linkage maps of genomic regions that control particular traits of the plant, as well as the accumulation of bioactive compounds. The coupling of sequencing technologies with bioinformatics and high-through put phenotyping techniques genomic studies and bioinformatics tools could facilitate the improvement of the genetic pathways for the production of bioactive compounds and identification of genes that regulate essential agronomic traits relevant to the quality of NULs (Mochida and Shinozaki, 2011; Steinwand and Ronald, 2020; Kumar et al., 2021). Utilization of various genomics approaches such as genome-wide association studies (GWAS); marker-assisted selection (MAS) and genomic selection (GS) have been used to identify useful markers linked to nutritional traits and bioactives in various crops. For instance, a study on 94 chickpea genotypes from a diverse population using GWAS resulted in the identification of eight single nucleotide polymorphisms (SNPs) associated with Fe and Zn content in chickpea seeds (Diapari et al., 2014), while two closely associated SNPs markers for Fe and Zn were identified by GWAS in lentils (Khazaei et al., 2017). Following the identification of SNPs linked to these trace elements, marker-assisted selection can be applied for the introgression of these traits into underutilized legumes through the process of biofortification. This is particularly relevant in the current global pandemic as Zn is known to be an immune booster. Shreds of evidence have shown that Zn deficiency increases the risk of infectious diseases, autoimmune disorders, and cancer (Roohani et al., 2013; Haase and Schomburg, 2019; Wessels and Rink, 2020; Wessels et al., 2020). Although most of the NULs especially the African yam bean are good sources of Zn and Fe, the levels of these elements could be enhanced through biofortification. Legumes with high Fe and Ze levels could be harnessed to boost the immunity of risk groups mainly the elderly and patients with inflammatory or autoimmune diseases. With the use of modern breeding methods, an international organization located in Malaysia, Crops for the Future is spearheading research on some underutilized species such as bambara groundnut and winged bean to enhance food and nutritional security.
Omic technologies should be employed to enhance the functional components of NULs towards ensuring food and nutritional security. The use of transcriptomic analysis for the identification of regulatory genes in biochemical pathways can assist researchers to gain significant insight into the functional mechanisms of plant’s biosynthetic pathways especially those involved in secondary metabolite synthesis. The transcriptomic analysis is usually carried out to study gene expression in plants. This is done using microarray technology or RNAseq analysis. Available transcriptome data of some model legumes could be applied to study NULs when the transcripts have been obtained as has been reported for Medicago trunculata using theCTDB (RNASeq) and MtGEA (Mocroarray) (Garg and Jain, 2013). Integrated use of omics technologies methods to enhance the nutrient potential of any crop, could influence nutritional security if applied in food processing and formulations (Tian et al., 2016; Nayak et al., 2021). If this is not done the rich bioactive compounds inherent in most of these indigenous legumes will remain unknown and untapped. Molecular studies should therefore be used for genetic dissection of antioxidant activities in NULs and nutrient-related traits. This has not received much research attention thus far. Apart from enhancing the nutritional contents of these legumes, omics technologies coupled with genome editing could aid the reduction of antinutrients like oxalate and phytic acid which affects the bioavailability of vital minerals, thereby enhancing human health.
TThe CRISPR/Cas 9 method of genetic manipulation in plants has gained a lot of attention and acceptance for crop improvement because it is straightforward, adaptable, and accurate (Bhowmik et al., 2021). It would be very useful for enhancing genetic improvement in NULs. This technology is constantly evolving and has a wide range of applications, such as producing knockouts, precise modifications, multiplex genome engineering, or controlling gene expression (Arora and Narula, 2017; Baloglu et al., 2022). CRISPR/Cas9 relies on two key components: a Cas9 endonuclease and a guide RNA (gRNA) consisting of two small RNA molecules: the CRISPR RNA (crRNA) which is a 20-nucleotide sequence that matches the target DNA, and the transactivating crRNA (tracrRNA), which serves as a binding scaffold (Bhowmik et al., 2021). The CRISPR/Cas9 technology is highly useful for improving genetic traits in plants. Though it has been utilized in gene editing of known legumes such as soybean, cowpea, and the model legume Medicago trunculata (Curtin et al., 2018; Al Amin et al., 2019; Bao et al., 2019; Ji et al., 2019; Bao et al., 2020; Juranic et al., 2020; Chen et al., 2020; Wolabu et al., 2020; Bhowmik et al., 2021), very few attempts have been made on genome editing in underutilized legumes. The bottleneck has been in getting the whole genome sequence of the underutilized legumes.
Meng et al. (2017) developed an efficient CRISPR/Cas9 system for inducing targeted mutations in the MtPDS gene in the model legume M. truncatula. Among the 309 T0 transgenic plants, 32 displayed the albino phenotype. To determine if the albino phenotype was due to the targeted mutation, 16 out of the 32 transgenic plants were randomly selected for sequence analysis. Results revealed that all the albino plants had mutations at the targeted site of the MtPDS gene. These findings were supported by Baloglu et al. (2022). Efforts are ongoing by the African Orphan Crops Consortium (AOCC) through a network of international to regional public-private partnerships and collaborators, to generate genomic sequences of some underutilized legumes (Faba bean, Mungbean, Bambara groundnut, Marama bean, Mungbean, and Lablab purpureus). The complete genome sequence of Mungbean published in 2014 permitted genomic research and molecular breeding of mung bean (Kang et al., 2014; Baloglu et al., 2022). Schafleitner et al. (2015) evaluated agronomic traits in 1481 Mungbean collections based on the availability of its whole genome sequence. This paves the way for genome editing to be used for the genetic improvement of this species to enhance its yield, nutritional content, and resistance to diseases. The lack of whole genome sequences in most of these beneficial underutilized legumes poses challenges to their improvement using CRISPER/Cas9. Despite the absence of a genome reference sequence for Faba bean, significant advancements have been made in genetic and genomic resources to aid molecular breeding. The robust synteny shared with the model legume M. trunculata allows for the use of omic technologies like transcriptomics and comparative genomics. These methods help identify single-nucleotide polymorphisms (SNPs), develop high-density consensus genetic maps, and predict the candidate genes responsible for various desirable traits. Bhowmik et al. (2021) discussed these approaches in their study. The genome editing technology no doubt has the potential to enhance crop improvement in various ways. However, some individuals are against its global acceptance pushing for regulations on its use. Nevertheless, genome editing technology differs from genetic engineering or modification which requires novel genes to be inserted into another organism of a different species. Across many countries and regions in the world, different regulatory approaches are being sought. In Africa, the National Biosafety Management Agency of Nigeria released the first gene editing guidelines which paves the way for its utilization in the improvement of economic crops in the country. Other African governments should take a cue from Nigeria and develop a regulatory framework for gene editing.
8 Conclusion
There is no doubt that underutilized legumes are rich sources of micronutrients and bioactive compounds with a great capacity to achieve zero hunger of the Sustainable Development Goals (SDG) by 2030. The rich bioactive compounds inherent in underutilized legumes which are yet to be tapped have great health benefits for man. Most phytochemicals in legumes are regarded as antinutritional components as they have no nutritional value. However, recent studies have shown that these non-nutrients such as tannins, glycosides, and saponins possess hypocholesterolemic and anticarcinogenic activity while flavonoids possess antioxidant activities which are essential for scavenging reactive oxygen species which cause oxidative stress in diseased conditions. If the abundant bioactive compounds in these underutilized legumes are identified and employed as therapeutics or used in the development of functional food products, it will greatly enhance human health, reduce the over utilization of the common legumes as well as help to increase food, protein, and nutrition security in Africa. The renewed efforts in this direction will be to evolve strong research and development between industries (pharma and foods) and the academia/research for appropriate food-based or pharmaceutical product developments. Thus, incorporating NULs rich in bioactive compounds into the diet of man will boost achieving the Sustainable Development Goal 3 of the United Nations on good health and well-being.
Author contributions
JP and OO – conceived the idea, wrote the first draft, searched the literature, and reviewed the manuscript. OA, LO, TA, MA, AO, SD, and AA – contributed to the writing and review of the manuscript. PA, SO, MA, and CO – contributed to the writing. JP – edited, fine-tuned, and approved the final draft. All authors contributed to the article and approved the submitted version.
Funding
The authors acknowledge Bowen University's Directorate of Research and Strategic Partnerships (DRSPs) for defraying the publication fees.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abberton, M., Adegboyega, T. T., Faloye, B., Paliwal, R., Oyatomi, O. (2020a). African Yam bean, Sphenostylis stenocarpa. Legume Perspect. 14. doi: 10.1155/2020/6569420
Abberton, M., Adegboyega, T. T., Faloye, B., Paliwal, R., Oyatomi, O. (2020b). Winged bean, Psophocarpus tetragonolobus. Legume Perspect. 27-28. doi: 10.1155/2020/3439620
Abberton, M., Paliwal, R., Faloye, B., Marimagne, T., Moriam, A., Oyatomi, O. (2022). Indigenous African orphan legumes: Potential for food and nutrition security in SSA. Front. Sustain. Food Syst. 6. doi: 10.3389/fsufs.2022.708124
Adebowale, Y. A., Schwarzenbolz, U., Henle, T. (2011). Protein isolates from bambara groundnut (Voandzeia subterranean l.): Chemical characterization and functional properties. Int. J. Food Prop 14, 758–775. doi: 10.1080/10942910903420743
Adedayo, B. C., Anyasi, T. A., Taylor, M. J. C., Rautenbauch, F., Le Roes-Hill, M., Jideani, V. A. (2021). Phytochemical composition and antioxidant properties of methanolic extracts of whole and dehulled bambara groundnut (Vigna subterranea) seeds. Sci. Rep. 11, 14116. doi: 10.1038/s41598-021-93525-w
Adefegha, S. A. (2018). Functional foods and nutraceuticals as dietary intervention in chronic diseases; Novel perspectives for health promotion and disease prevention. J. Diet. Suppl. 15, 977–1009. doi: 10.1080/19390211.2017.1401573
Adefegha, S. A., Oboh, G. (2012). Acetylcholinesterase (AChE) inhibitory activity, antioxidant properties and phenolic composition of two Aframomum species. J. Basic Clin. Physiol. Pharmacol. 23, 153–161. doi: 10.1515/jbcpp-2012-0029
Adefegha, S. A., Oboh, G., Oyeleye, S. I., Dada, F. A., Ejakpovi, I., Boligon, A. A. (2017). Cognitive enhancing and antioxidative potentials of velvet beans (Mucuna pruriens) and horseradish (Moringa oleifera) seeds extracts: A comparative study. J. Food Biochem. 41, e12292. doi: 10.1111/jfbc.12292
Adegboyega, T. T., Abberton, M. T., AbdelGadir, A. H., Dianda, M., Maziya-Dixon, B., Oyatomi, O. A., et al. (2020). Evaluation of nutritional and antinutritional properties of African yam bean (Sphenostylis stenocarpa (Hochst ex. a. rich.) harms.) seeds. J. Food Qual. 2020, 6569420. doi: 10.1155/2020/6569420
Adegboyega, T. T., Abberton, M. T., Abdelgadir, A. H., Dianda, M., Maziya-Dixon, B., Oyatomi, O. A., et al. (2019). Nutrient and antinutrient composition of winged bean (Psophocarpus tetragonolobus (L.) DC.) seeds and tubers. J. Food Qual. 2019, 3075208. doi: 10.1155/2019/3075208
Adegboyega, T. T., Mansurat, O. S., Anas, A. T. (2021). Proximate and antinutrient composition of selected West African bambara groundnut (Vigna subterranea (L.) verdc.) accessions. J. Underut. Leg 3, 13–25.
Ade-Omowaye, B., Tucker, G., Smetanska, I. (2015). Nutritional potential of nine underexploited legumes in southwest Nigeria. Int. Food Res. J. 22, 798.
Adeoye, B. K., Aransiola, E. F., Alebiowu, G., Bisi-Johnson, M. A., Olorunmola, F. O., Adepoju, O. A. (2021). The characterization and microbiological evaluation of probiotic isolated from bambara groundnut. Int. J. Appl. Sci. Biotechnol. 9 (1), 54–64. doi: 10.3126/ijasbt.v9i1.32959
Adewale, B. D., Nnamani, C. V. (2022). Introduction to food, feed, and health wealth in African yam bean, a locked-in African indigenous tuberouslegume. Front. Sustain. Food Syst. 6:72645. doi: 10.3389/fsufs.2022.726458
Agbolade, J., Olakunle, T., Popoola, K., Idowu, J., Isiaka, A., Aasa-Sadique, A. (2019). Genetic variability and diversity analysis in pod and seed characters of some neglected and underutilized legumes (NULs). Asian J. Biochem. Genet. Mole. Biol. 2, 1–8. doi: 10.9734/ajbgmb/2019/v2i330059
Aghajanian, S., Kazemi, S., Esmaeili, S., Aghajanian, S., Moghadamnia, A. A. (2020). Sequential microwave-assisted extraction for isolation of quercetin from red kidney bean. Int. J. Eng. 33, 12–17. doi: 10.5829/ije.2020.33.01a.02
Agostini-Costa, T., da, S., Teodoro, A. F. P., Alves, R., de B. das, N., Braga, L. R., et al. (2014). Total phenolics, flavonoids, tannins and antioxidant activity of lima beans conserved in a Brazilian genebank. Cienc. Rural 45, 335–341. doi: 10.1590/0103-8478cr20140030
Ajibola, G. O., Olapade, A. A. (2016). Physical, proximate and anti-nutritional composition of African yam bean (Sphenostylis stenocarpa) seeds varieties. J. Food Res. 5, 67. doi: 10.5539/jfr.v5n2p67
Akinyemi, F. A., Orishadipe, A. T., Ebun-Oluwa, O., Aladesanmi, O. A. (2020). Physico-chemical properties and functional characteristics of jack beans (Canavalia ensiformis) starch. World J. Biol. Pharm. Health Sci. 3 (2), 012–022. doi: 10.30574/wjbphs.2020.3.2.0018
Akpapunam, M. A., Sefa-Dedeh, S. (1997). Jack bean (Canavalia ensiformis): Nutrition related aspects and needed nutrition research. Plant Foods Hum. Nutr. 50, 93–99. doi: 10.1007/BF02436029
Al Amin, N., Ahmad, N., Wu, N., Pu, X., Ma, T., Du, Y., et al. (2019). CRISPR-Cas9 mediated targeted disruption of FAD2-2 microsomal omega-6 desaturase in soybean (Glycine max.L). BMC Biotechnol. 19, 9. doi: 10.1186/s12896-019-0501-2
Alara, O. R., Abdurahman, N. H. (2019). Microwave-assisted extraction of phenolics from Hibiscus sabdariffa calyces: Kinetic modelling and process intensification. Ind. Crop Prod. 137, 528–535. doi: 10.1016/j.indcrop.2019.05.053
Alara, O. R., Abdurahman, N. H., Abdul Mudalip, S. K. (2019). Optimizing microwave-assisted extraction conditions to obtain phenolic-rich extract from Chromolaena odorata leaves. Chem. Eng. Technol. 42, 1733–1740. doi: 10.1002/ceat.201800462
Alcázar-Valle, M., Lugo-Cervantes, E., Mojica, L., Morales-Hernández, N., Reyes-Ramírez, H., Enríquez-Vara, J. N., et al. (2020). Bioactive compounds, antioxidant activity, and antinutritional content of legumes: A comparison between four phaseolus species. Molecules 25, 3528. doi: 10.3390/molecules25153528
Altemimi, A., Lakhssassi, N., Baharlouei, A., Watson, D. G., Lightfoot, D. A. (2017). Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 6, 42. doi: 10.3390/plants6040042
Alvarado-López, A. N., Gómez-Oliván, L. M., Heredia, J. B., Baeza-Jiménez, R., Garcia-Galindo, H. S., Lopez-Martinez, L. X. (2019). Nutritional and bioactive characteristics of Ayocote bean (Phaseolus coccienus L.): An underutilized legume harvested in Mexico. CyTA-J. Food 17 (1), 199–206. doi: 10.1080/19476337.2019.1571530.
Amarowicz, R., Pegg, R. B. (2008). Legumes as a source of natural antioxidants. Eur. J. Lipid Sci. Technol. 110, 865–878. doi: 10.1002/ejlt.200800114
Ameer, K., Shahbaz, H. M., Kwon, J. H. (2017). Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 16, 295–315. doi: 10.1111/1541-4337.12253
Ames, B. N., Shigenaga, M. K., Hagen, T. M. (1993). Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. U.S.As 90, 7915–7922. doi: 10.1073/pnas.90.17.7915
Arise, A. K., Malomo, S. A., Awaw, A. A., Arise, R. O. (2022). Quality attributes and consumer acceptability of custard supplemented with bambara groundnut protein isolates. App. Food Res. 2, 100056. doi: 10.1016/j.afres.2022.100056
Arogundade, L. A., Mu, T.-H., Akinhanmi, T. F. (2016). Structural, physicochemical and interfacial stabilisation properties of ultrafiltered African yam bean (Sphenostylis stenocarpa) protein isolate compared with those of isoelectric protein isolate. LWT - Food Sci. Tech. 69, 400–408. doi: 10.1016/j.lwt.2016.01.049
Arora, L., Narula, A. (2017). Gene editing and crop improvement using CRISPR-Cas9 system. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01932
Ayilara, M. S., Abberton, M., Oyatomi, O. A., Odeyemi, O., Babalola, O. O. (2022). Potentials of underutilized legumes in food security. Front. Soil Sci. 2. doi: 10.3389/fsoil.2022.1020193
Bai-Ngew, S., Chuensun, T., Wangtueai, S., Phongthai, S., Jantanasakulwong, K., Rachtanapun, P., et al. (2021). Antimicrobial activity of a crude peptide extract from lablab bean (Dolichos lablab) for semi-dried rice noodles shelf-life. Qual. Assur. Saf. Crop Food 13, 25–33. doi: 10.15586/qas.v13i2.882
Baiyeri, S. O., Uguru, M. I., Ogbonna, P. E., Samuel-Baiyeri, C. C. A., Okechukwu, R., Kumaga, F. K., et al. (2018). Evaluation of the nutritional composition of the seeds of some selected African yam bean (Sphenostylis stenocarpa hochst ex. a. rich (harms)) accessions. Agroscience 17, 37–44. doi: 10.4314/as.v17i2.5
Baloglu, M. C., Celik, A. Y., Baloglu, P., Yildiz, A. B., Türkölmez, N., Özden, et al. (2022). Gene-editing technologies and applications in legumes: Progress, evolution, and future prospects. Front. Genet. 13. doi: 10.3389/fgene.2022.859437
Bao, A., Chen, H., Chen, L., Chen, S., Hao, Q., Guo, W., et al. (2019). CRISPR/Cas9- mediated targeted mutagenesis of GmSPL9 genes alters plant architecture in soybean. BMC Plant Biol. 19, 131. doi: 10.1186/s12870-019-1746-6
Bao, A., Zhang, C., Huang, Y., Chen, H., Zhou, X., Cao, D. (2020). Genome editing technology and application in soybean improvement. Oil Crop Sci. 5 (1), 31–40. doi: 10.1016/j.ocsci.2020.03.001
Barac, M. B., Pesic, M. B., Stanojevic, S. P., Kostic, A. Z., Bivolarevic, V. (2015). Comparative study of the functional properties of three legume seed isolates: Adzuki, pea and soy bean. J. Food Sci. Technol. 52, 2779–2787. doi: 10.1007/s13197-014-1298-6
Barman, A., Marak, C. M., Barman, R. M., Sangma, C. S. (2018). “Nutraceutical properties of legume seeds and their impact on human health,” in Legume seed nutraceutical research (United Kingdom (UK): IntechOpen). doi: 10.5772/intechopen.78799
Bassal, H., Merah, O., Ali, A. M., Hijazi, A., El Omar, F. (2020). Psophocarpus tetragonolobus: An underused species with multiple potential uses. Plants 9, 1730. doi: 10.3390/plants9121730
Basu, S., Ghosh, M., Bhunia, R. K., Ganguly, J., Banik, B. K. (2017). Polysaccharides from Dolichos biflorus Linn and trachyspermum ammi Linn seeds: Isolation, characterization and remarkable antimicrobial activity. Chem. Cent. J. 11, 1–10. doi: 10.1186/s13065-017-0349-2
Bennetau-Pelissero, C. (2018). “Plant proteins from legumes,” in Bioactive molecules in food, reference series in phytochemistry. Eds. Mérillon, J. M., Ramawat, K. G. (Springer Nature). doi: 10.1007/978-3-319-54528-8_3-1
Bessada, S. M. F., Barreira, J. C. M., Oliveira, M. B. P. P. (2019). Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends Food Sci. Technol. 93, 53–68. doi: 10.1016/j.tifs.2019.08.022
Bhadkaria, A., Srivastava, N., Bhagyawant, S. S. (2021). A prospective of underutilized legume moth bean (Vigna aconitifolia (Jacq.) marechàl): Phytochemical profiling, bioactive compounds and in vitro pharmacological studies. Food Biosci. 42, 101088. doi: 10.1016/j.fbio.2021.101088
Bhagyawant, S. S., Bhadkaria, A., Narvekar, D. T., Srivastava, N. (2019). Multivariate biochemical characterization of rice bean (Vigna umbellata) seeds for nutritional enhancement. Biocatal. Agric. Biotechnol. 20, 101193. doi: 10.1016/j.bcab.2019.101193
Bhowmik, P., Konkin, D., Polowick, P., Hodgins, C. L., Subedi, M., Xiang, D., et al. (2021). CRISPR/Cas9 gene editing in legume crops: Opportunities and challenges. Legume Sci. 3 (3), e96. doi: 10.1002/leg3.96
Carbonaro, M., Maselli, P., Nucara, A. (2015). Structural aspects of legume proteins and nutraceutical properties. Food Res. Int. 76, 19–30. doi: 10.1016/j.foodres.2014.11.007
Carbonaro, M., Nucara, A. (2022). Legume proteins and peptides as compounds in nutraceuticals: A structural basis for dietary health effects. Nutrients 14 (6), 1188. doi: 10.3390/nu14061188
Carrero-Carralero, C., Mansukhani, D., Ruiz-Matute, A. I., Martínez-Castro, I., Ramos, L., Sanz, M. L. (2018). Extraction and characterization of low molecular weight bioactive carbohydrates from mung bean (Vigna radiata). Food Chem. 266, 146–154. doi: 10.1016/j.foodchem.2018.05.114
Chandrasekara, A., Josheph Kumar, T. (2016). Roots and tuber crops as functional foods: A review on phytochemical constituents and their potential health benefits. Int. J. Food Sci. 2016, 3631647. doi: 10.1155/2016/3631647
Charrondière, U. R., Vincent, A., Grande, F. (2020). "FAO/INFOODS Food Composition Table for Western Africa (2019): User Guide & Condensed Food Composition Table = Table de Composition Des Aliments FAO/INFOODS Pour l’Afrique de l’Ouest (2019): Guide d’utilisation & Table de Composition Des Aliments Condensée.” AGRIS: International Information System for the Agricultural Science and Technology. Available at: https://agris.fao.org/agrissearch/search.do?ecordID=XF2020000998. (Accessed March 10, 2023).
Chaturvedi, S., Chakraborty, S. (2021). Review on potential non-dairy synbiotic beverages: A preliminary approach using legumes. Int. J. Food Sci. Technol. 56 (5), 2068–2077. doi: 10.1111/ijfs.14779
Chaturvedi, S., Chakraborty, S. (2022). Evaluation of prebiotic properties of legume-based synbiotic beverages. J. Food Proc. Preserv. 46, e16685. doi: 10.1111/jfpp.16685
Chaves, J. O., De Souza, M. C., Da Silva, L. C., Lachos-Perez, D., Torres-Mayanga, P. C., Da Fonseca Machado, A. P., et al. (2020). Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 8. doi: 10.3389/fchem.2020.507887
Chen, L., Wu, Q., He, T., Lan, J., Ding, L., Liu, T., et al. (2020). Transcriptomic and metabolomic changes triggered by fusarium solani in common bean (Phaseolus vulgaris l.). Genes 11, 177. doi: 10.3390/genes11020177
Chingwaru, W., Vidmar, J., Kapewangolo, P. T., Mazimba, O., Jackson, J. (2015). Therapeutic and prophylactic potential of morama (Tylosema esculentum): A review. Phytother. Res. 29, 1423–1438. doi: 10.1002/ptr.5419
Cichońska, P., Ziarno, M. (2022). Legumes and legume-based beverages fermented with lactic acid bacteria as a potential carrier of probiotics and prebiotics. Microorganisms 10 (1), 91. doi: 10.3390/microorganisms1001009
Cornejo-Ramírez, Y. I., Martínez-Cruz, O., Del Toro-Sánchez, C. L., Wong-Corral, F. J., Borboa-Flores, J., Cinco-Moroyoqui, F. J. (2018). The structural characteristics of starches and their functional properties. CyTA-J. Food 16, 1003–1017. doi: 10.1080/19476337.2018.1518343
Cui, H., Abdel-Samie, M. A.-S., Lin, L., Jafari, S. M. (2021). “Application of antimicrobial-loaded nano/microcarriers in different food products,” in Application of Nano/Microencapsulated ingredients in food products (Elsevier), 469–517. doi: 10.1016/B978-0-12-815726-8.00012-X
Cullis, C., Kunert, K. J. (2017). Unlocking the potential of orphan legumes. J. exper. Bot. 68, 1895–1903. doi: 10.1093/jxb/erw437
Curtin, S. J., Xiong, Y., Michno, J.-M., Campbell, B. W., Stec, A. O., Čermák, T., et al. (2018). CRISPR/Cas9 and TALENs generate heritable mutations for genes involved in small rna processing of Glycine max and Medicago truncatula. Plant Biotechnol. J. 16, 1125–1137. doi: 10.1111/pbi.12857
Del Hierro, J. N., Herrera, T., García-Risco, M. R., Fornari, T., Reglero, G., Martin, D. (2018). Ultrasound-assisted extraction and bioaccessibility of saponins from edible seeds: Quinoa, lentil, fenugreek, soybean and lupin. Food Res. Int. 109, 440–447. doi: 10.1016/j.foodres.2018.04.058
Diapari, M., Sindhu, A., Bett, K., Deokar, A., Warkentin, T. D., Tar’an, B. (2014). Genetic diversity and association mapping of iron and zinc concentrations in chickpea (Cicer arietinum l.). Genome 57, 459–468. doi: 10.1139/gen-2014-0108
Diedericks, C. F., Shek, C., Jideani, V. A., Venema, P., van der Linden, E. (2020). Physicochemical properties and gelling behaviour of bambara groundnut protein isolates and protein-enriched fractions. Food Res. Inter. 138, 109773. doi: 10.1016/j.foodres.2020.109773
Divekar, P. A., Narayana, S., Divekar, B. A., Kumar, R., Gadratagi, B. G., Ray, A., et al. (2022). Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. IJMS 23, 2690. doi: 10.3390/ijms23052690
Djuricic, I., Calder, P. C. (2021). Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients 13, 2421. doi: 10.3390/nu13072421
Drago, S. R., Franco-Miranda, H., Cian, R. E., Betancur-Ancona, D., Chel-Guerrero, L. (2016). Bioactive properties of Phaseolus lunatus (Lima bean) and Vigna unguiculata (Cowpea) hydrolyzates incorporated into pasta. residual activity after pasta cooking. Plant Foods Hum. Nutr. 71, 339–345. doi: 10.1007/s11130-016-0565-2
Du, S., Jiang, H., Yu, X., Jane, J. (2014). Physicochemical and functional properties of whole legume flour. LWT - Food Sci. Tech. 55, 308–313. doi: 10.1016/j.lwt.2013.06.001
Dudu, O. E., Li, L., Oyedeji, A. B., Oyeyinka, S. A., Ma, Y. (2019). Structural and functional characteristics of optimised dry-heat-moisture treated cassava flour and starch. Int. J. Biol. Macromol. 133, 1219–1227. doi: 10.1016/j.ijbiomac.2019.04.202
Ebrahim, A. E., Abd El-Aziz, N. K., Elariny, E. Y., Shindia, A., Osman, A., Hozzein, W. N., et al. (2022). Antibacterial activity of bioactive compounds extracted from red kidney bean (Phaseolus vulgaris l.) seeds against multidrug-resistant enterobacterales. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1035586
Falade, K. O., Nwajei, C. P. (2015). Physical, proximate, functional and pasting properties of four non-and γ-irradiated bambara groundnut (Vigna subterranean) cultivars. Int. J. Food Sci. Technol. 50 (3), 640–651.
Foyer, C. H., Lam, H.-M., Nguyen, H. T., Siddique, K. H., Varshney, R. K., Colmer, T. D., et al. (2016). Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2, 16112. doi: 10.1038/nplants.2016.112
Fukushima, A., Kusano, M. (2013). Recent progress in the development of metabolome databases for plant systems biology. Front. Plant Sci. 4, 73. doi: 10.3389/fpls.2013.00073
Gagné-Bourque, F., Bertrand, A., Claessens, A., Aliferis, K. A., Jabaji, S. (2016). Alleviation of drought stress and metabolic changes in timothy (Phleum pratense l.) colonized with bacillus subtilis B26. Fron. Plant Sc. 7. doi: 10.3389/fpls.2016.00584
Ganesan, K., Xu, B. (2018). A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci. Hum. Well. 7, 11–33. doi: 10.1016/j.fshw.2017.11.002
Garg, R., Jain, M. (2013). Transcriptome analyses in legumes: A resource for functional genomics. Plant Genome 6. doi: 10.3835/plantgenome2013.04.0011
Gemede, H. F., Ratta, N. (2014). Antinutritional factors in plant foods: Potential health benefits and adverse effects. Int. J. Nutrit. Food Sci. 3, 284–289. doi: 10.11648/j.ijnfs.20140304.18
George, T. T., Obilana, A. O., Oyeyinka, S. A. (2020). The prospects of African yam bean: past and future importance. Heliyon 6, e05458. doi: 10.1016/j.heliyon.2020.e05458
Gohara, A. K., Souza, A. H. P., de Gomes, S. T. M., Souza, N. E., de, Visentainer, J. V., Matsushita, M. (2016). Nutritional and bioactive compounds of adzuki beans cultivars using chemometric approach. Ciênc. agrotec. 40, 104–113. doi: 10.1590/S1413-70542016000100010
Gupta, C., Prakash, D. (2019). “Nutraceuticals from microbes of marine sources,” in Nutraceuticals-past, present and future (United Kingdom (UK): Intech Open), 99. doi: 10.5772/intechopen.82369
Haase, H., Schomburg, L. (2019). "You’d better zinc–trace element homeostasis in infection and inflammation".). Nutrient 11 (9), 2078. doi: 10.3390/nu11092078
Hai, H., Thi, P., Nguyen, T. L. (2021). “Nutraceutical properties of legume seeds,” in Phytochemical compounds legume research, vol. 2. (United Kingdom (UK): Intech Open).
Hamilton, J. P., Robin Buell, C. (2012). Advances in plant genome sequencing. Plant J. 70, 177–190. doi: 10.1111/j.1365-313X.2012.04894.x
Harris, T., Jideani, V., Le Roes-Hill, M. (2018). Flavonoids and tannin composition of bambara groundnut (Vigna subterranea) of mpumalanga, south Africa. Heliyon 4, e00833. doi: 10.1016/j.heliyon.2018.e00833
Heinrich, M., Mah, J., Amirkia, V. (2021). Alkaloids used as medicines: Structural phytochemistry meets biodiversity–an update and forward look. Molecules 26, 1836. doi: 10.3390/molecules26071836
Henciya, S., Seturaman, P., James, A. R., Tsai, Y.-H., Nikam, R., Wu, Y.-C., et al. (2017). Biopharmaceutical potentials of Prosopis spp.(mimosaceae, leguminosa). J.f Food Drug Anal. 25, 187–196. doi: 10.1016/j.jfda.2016.11.001
Hossain, S., Ahmed, R., Bhowmick, S., Mamun, A. A., Hashimoto, M. (2016). Proximate composition and fatty acid analysis of Lablab purpureus (L.) legume seed: implicates to both protein and essential fatty acid supplementation. SpringerPlus 5(1), 1899. doi: 10.1186/s40064-016-3587-1
Huang, J., Yang, Q., Pu, H. (2018). “Slowly digestible starch,” in Functional starch and applications in food (United Kingdom (UK): Springer), 27–61.
Huldani, H., Rashid, A. I., Turaev, K. N., Opulencia, M. J. ,. C., Abdelbasset, W. K., Bokov, D. O., et al. (2022). Concanavalin a as a promising lectin-based anti-cancer agent: the molecular mechanisms and therapeutic potential. Cell Commun. Signal 20, 167. doi: 10.1186/s12964-022-00972-7
Hunter, D., Borelli, T., Beltrame, D. M., Oliveira, C. N., Coradin, L., Wasike, V. W., et al. (2019). The potential of neglected and underutilized species for improving diets and nutrition. Planta, 1–21. doi: 10.1007/s00425-019-03169-4
Hussein, H., Awad, S., El-Sayed, I., Ibrahim, A. (2020). Impact of chickpea as prebiotic, antioxidant and thickener agent of stirred bio-yoghurt. Annal. Agric. Sci. 65, 49–58. doi: 10.1016/j.aoas.2020.03.001
Idoko, A., Onyinye, A. O., Blessing, N. O., Ayomide, T., Phillip, O. C., Nwali, O. N. (2020). Heating effect on phytochemical and proximate contents of cooked aqueous extract of Phaseolus vulgaris (Kidney beans). Univ J. Pharm. Res 4 (6), 35-41. doi: 10.22270/ujpr.v4i6.334
Ikhajiagbe, B., Ogwu, M. C., Ogochukwu, O. F., Odozi, E. B., Adekunle, I. J., Omage, Z. E. (2021). The place of neglected and underutilized legumes in human nutrition and protein security in Nigeria. Crit. Rev. Food Sc. Nutr., 1–10. doi: 10.1080/10408398.2020.1871319
Iuga, M., Mironeasa, S. (2020). A review of the hydrothermal treatments impact on starch based systems properties. Crit. Rev.Food Sci. Nutr., 1–26. doi: 10.1080/10408398.2019.1664978
Iwe, M. O., Onyeukwu, U., Agiriga, A. N. (2016). Proximate, functional and pasting properties of FARO 44 rice, African yam bean and brown cowpea seeds composite flour. Cogent Food Agric. 2 (1), 1142409.
James, S., Nwabueze, T., Ndife, J., Onwuka, G., Usman, M., Audu, Y. (2020). Effects of treatments on some bioactive components of selected lesser known legumes indigenous to Nigeria. J. Food Chem. Nanotechnol. 6, 197–206. doi: 10.17756/jfcn.2020-102
Jha, A. B., Purves, R. W., Elessawy, F. M., Zhang, H., Vandenberg, A., Warkentin, T. D. (2019). Polyphenolic profile of seed components of white and purple flower pea lines. Crop Sci. 59, 2711–2719. doi: 10.2135/cropsci2019.04.0279
Ji, J., Zhang, C., Sun, Z., Wang, L., Duanmu, D., Fan, Q. (2019). Genome editing in cowpea (Vigna unguiculata (L.)) using CRISPR-Cas9. Int. J. Mol. Sci. 20 (10), 2471. doi: 10.3390/ijms20102471
Jia, R., Ge, S., Ren, S., Luo, Y., Xiu, L., Liu, H., et al. (2021). Antibacterial mechanism of adzuki bean seed coat polyphenols and their potential application in preservation of fresh raw beef. Int. J.f Food Sci. Technol. 56, 5025–5039. doi: 10.1111/ijfs.15292
Johnson, N., Johnson, C. R., Thavarajah, P., Kumar, S., Thavarajah, D. (2020). The roles and potential of lentil prebiotic carbohydrates in human and plant health. Plants People Planet 2, 310–319. doi: 10.1002/ppp3.10103
Johnson, J. B., Neupane, P., Bhattarai, S. P., Trotter, T., Naiker, M. (2022). Partitioning of nutritional and phytochemical constituents in nine adzuki bean genotypes from Australia. J. Agric. Food Res. 10, 100398. doi: 10.1016/j.jafr.2022.100398
Joshi, J., Hasnain, G., Logue, T., Lynch, M., Wu, S., Guan, J., et al. (2021). A core metabolome response of maize leaves subjected to long-duration abiotic stresses. Metabolites 11, 797. doi: 10.3390/metabo11110797
Junot, C., Fenaille, F., Colsch, B., Becher, F. (2014). High-resolution mass spectrometry-based techniques at the crossroads of metabolic pathways. Mass Spectrom. Rev. 33, 471–500. doi: 10.1002/mas.21401
Juranić, M., Nagahatenna, D. S., Salinas-Gamboa, R., Hand, M. L., Sánchez-León, N., Leong, W. H., et al. (2020). A detached leaf assay for testing transient gene expression and gene editing in cowpea (Vigna unguiculata [L.] walp.). Plant Methods 16 (1), 1–17.
Kan, L., Nie, S., Hu, J., Wang, S., Cui, S. W., Li, Y., et al. (2017). Nutrients, phytochemicals and antioxidant activities of 26 kidney bean cultivars. Food Chem. Toxicol. 108, 467–477. doi: 10.1016/j.fct.2016.09.007
Kanatt, S. R., Arjun, K., Sharma, A. (2011). Antioxidant and antimicrobial activity of legume hulls. Food Res. Int. 44, 3182–3187. doi: 10.1016/j.foodres.2011.08.022
Kang, Y. J., Kim, S. K., Kim, M. Y., Lestari, P., Kim, K. H., Ha, B.-K., et al. (2014). Genome sequence of mungbean and insights into evolution within Vigna species. Nat. Commun. 5 (1), 1–9. doi: 10.1038/ncomms6443
Kaundal, S. P., Sharma, A., Kumar, R., Kumar, V., Kumar, R. (2019). Exploration of medicinal importance of an underutilized legume crop, Macrotyloma uniflorum (Lam.) Verdc.(Horse gram): A review. Int. J. Pharm. Sci. Res. 10, 3178–3186. doi: 10.13040/IJPSR.0975-8232.10(7).3178-86
Kaur, R., Arora, S. (2015). Alkaloids–important therapeutic secondary metabolites of plant origin. J. Crit. Rev. 2, 1–8.
Kaur, D., Rasane, P., Dhawan, K., Singh, J., Kaur, S., Gurumayum, S., et al. (2021). Rice bean (Vigna umbellata) based ready-to-eat geriatric premix: Optimization and analysis. J. Food Process. Preserv. 45. doi: 10.1111/jfpp.16075
Keawpeng, I., Paulraj, B., Venkatachalam, K. (2022). Antioxidant and antimicrobial properties of mung bean phyto-film combined with longkong pericarp extract and sonication. Membranes 12, 379. doi: 10.3390/membranes12040379
Kennedy, D. O. (2014). Polyphenols and the human brain: Plant “secondary metabolite” ecologic roles and endogenous signaling functions drive benefits. Adv. Nutr. 5, 515–533. doi: 10.3945/an.114.006320
Khazaei, H., Podder, R., Caron, C. T., Kundu, S. S., Diapari, M., Vandenberg, A., et al. (2017). Marker–trait association analysis of iron and zinc concentration in lentil (Lens culinaris medik.) seeds. Plant Genome 10(2). doi: 10.3835/plantgenome2017.02.0007
Khoury, C. K. (2015). The conservation and use of crop genetic resources for food security (United Kingdom (UK): Wageningen University and Research Centre).
Klompong, V., Benjakul, S. (2015). Antioxidative and antimicrobial activities of the extracts from the seed coat of bambara groundnut (Voandzeia subterranea). RSC Adv. 5, 9973–9985. doi: 10.1039/C4RA10955D
Konyeme, T., Nyananyo, B., Tanee, F. (2020). Diversity in proximate analysis of tubers of some African yam bean (Sphenostylis stenocarpa)(Hochst ex. a. rich.) harms (Fabaceae) accessions. J. Appl. Sci. Environ. Manage. 24, 1787–1793. doi: 10.4314/jasem.v24i10.12
Kortt, A. A. (1984). Purification and properties of the basic lectins from winged bean seed [Psophocarpus tetragonolobus (L.) DC]. Eur. J. Biochem. 138, 519–525. doi: 10.1111/j.1432-1033.1984.tb07946.x
Ku, Y.-S., Contador, C. A., Ng, M.-S., Yu, J., Chung, G., Lam, H.-M. (2020). The effects of domestication on secondary metabolite composition in legumes. Front. Genet. 11. doi: 10.3389/fgene.2020.581357
Kumar, A., Anju, T., Kumar, S., Chhapekar, S. S., Sreedharan, S., Singh, S., et al. (2021). Integrating omics and gene editing tools for rapid improvement of traditional food plants for diversified and sustainable food security. Int. J. Mol. Sci. 22, 8093. doi: 10.3390/ijms22158093
Kurek, J. (2019). “Introductory chapter: Alkaloids - their importance in nature and for human life,” in Alkaloids - their importance in nature and human life. Ed. Kurek, J. (United Kingdom (UK): IntechOpen). doi: 10.5772/intechopen.85400
Latha, L. Y., Sasidharan, S., Zuraini, Z., Suryani, S., Shirley, L., Sangetha, S. (2007). Antibacterial activity and toxicity of psophocarpus tetragonolobus. Pharm. Biol. 45 (1), 31–36. doi: 10.1080/13880200601026317
Lin Tan, X., Azam-Ali, S., Goh, E. V., Mustafa, M. A., Chai, H. H., Kuan Ho, W., et al. (2020). Bambara groundnut: An underutilized leguminous crop for global food security and nutrition. Front. Nutr. 7, 276. doi: 10.3389/fnut.2020.601496
Lopes, N. A., Brandelli, A. (2018). Nanostructures for delivery of natural antimicrobials in food. Crit. Rev. Food Sci. Nutr. 58, 2202–2212. doi: 10.1080/10408398.2017.1308915
Lu, S., Qian, Y., Huang, X., Yu, H., Yang, J., Han, R., et al. (2017). The association of dietary pattern and breast cancer in jiangsu, China: A population-based case-control study. PloS One 12 (9), e0184453. doi: 10.1371/journal.pone.0184453
Luo, J., Cai, W., Wu, T., Xu, B. (2016). Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis l.) and mung bean (Vigna radiate l.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem. 201, 350–360. doi: 10.1016/j.foodchem.2016.01.101
Makeri, M. U., Karim, R., Adbulkarim, M. S., Ghazali, H. M., Miskandar, M. S., Muhammad, K. (2016). Comparative analysis of the physiochemical, thermal, and oxidative properties of winged bean and soybean oils. Int. J. Food Prop. 19, 2769–2278. doi: 10.1080/10942912.2015.1031246
Makhumbila, P., Rauwane, M., Muedi, H., Figlan, S. (2022). Metabolome profiling: A breeding prediction tool for legume performance under biotic stress conditions. Plants 11, 1756. doi: 10.3390/plants11131756
Mani-López, E., Palou, E., López-Malo, A. (2021). Legume proteins, peptides, water extracts, and crude protein extracts as antifungals for food applications. Trend. Food Sci. Technol. 112, 16–24. doi: 10.1016/j.tifs.2021.03.035
Maoka, T. (2020). Carotenoids as natural functional pigments. J. Nat. Med. 74, 1–16. doi: 10.1007/s11418-019-01364-x
Maphosa, Y., Jideani, V. A. (2017). “The role of legumes in human nutrition,” in Functional food-improve health through adequate food, vol. 1. (United Kingdom (UK): Intech Open), 13. doi: 10.5772/intechopen.69127
Marco, M. L., Sanders, M. E., Gänzle, M., Arrieta, M. C., Cotter, P. D., De Vuyst, L., et al. (2021). The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastro. Hepatol. 18 (3), 196–208. doi: 10.1038/s41575-020-00390-5
Marrelli, M., Conforti, F., Araniti, F., Statti, G. A. (2016). Effects of saponins on lipid metabolism: a review of potential health benefits in the treatment of obesity. Molecules 21, 1404. doi: 10.3390/molecules21101404
Maruatona, G. N., Duodu, K. G., Minnaar, A. (2010). Physicochemical, nutritional and functional properties of marama bean flour. Food Chem. 121, 400–405. doi: 10.1016/j.foodchem.2009.12.054
Mayes, S., Ho, W. K., Chai, H. H., Gao, X., Kundy, A. C., Mateva, K. I., et al. (2019). Bambara groundnut: An exemplar underutilized legume for resilience under climate change. Planta 250, 803–820. doi: 10.1007/s00425-019-03191-6
Meng, Y., Hou, Y., Wang, H., Ji, R., Liu, B., Wen, J., et al. (2017). Targeted mutagenesis by CRISPR/Cas9 system in the model legume medicago truncatula. Plant Cell Rep. 36, 371–374. doi: 10.1007/s00299-016-2069-9
Messina, M. J., Persky, V., Setchell, K. D. R., Barnes, S. (1994). Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr. Cancer 21, 113–131.
Mirali, M., Purves, R. W., Vandenberg, A. (2017). Profiling the phenolic compounds of the four major seed coat types and their relation to color genes in lentil. J. Nat. Prod 80, 1310–1317. doi: 10.1021/acs.jnatprod.6b00872
Mochida, K., Shinozaki, K. (2011). Advances in omics and bioinformatics tools for systems analyses of plant functions. Plant Cell Physiol. 52, 2017–2038. doi: 10.1093/pcp/pcr153
Mohanty, C. S., Verma, S., Singh, V., Khan, S., Gaur, P., Gupta, P., et al. (2013). Characterization of winged bean (Psophocarpus tetragonolobus (L.) DC.) based on molecular, chemical and physiological parameters. AJMB 03, 187–197. doi: 10.4236/ajmb.2013.34025
Mojica, L., González de Meija, E. (2015). Characterization and comparison of protein and peptide profiles and their biological activities of improved common bean cultivars (Phaseolus vulgaris l.) from Mexico and Brazil. Plant Foods Hum. Nutr. 70, 105–112. doi: 10.1007/s11130-015-0477-6
Mubaiwa, J., Fogliano, V., Chidewe, C., Linnemann, A. R. (2018). Bambara groundnut (Vigna subterranea (L.) verdc.) flour: A functional ingredient to favour the use of an unexploited sustainable protein source. PloS One 13, e0205776. doi: 10.1371/journal.pone.0205776
Nawaz, H., Aslam, M., Rehman, T., Mehmood, R. (2021). Modification of emulsifying properties of cereal flours by blending with legume flours. Asian J. Dairy Food Res., 1–6. doi: 10.18805/ajdfr.DR-223
Nayak, S., Malavalli, S. S., Bharati, P., Hefferon, K. L., Kole, C., Puppala, N. (2021). Omics technologies to enhance plant based functional foods: An overview. Front. Genet. 12. doi: 10.3389/fgene.2021.742095
Negi, K. S., Gaur, R. D. (1994). Principle wild food plants of western himalaya, uttar pradesh, India. Hishar Plants Indian Subconti-nent (India) pp, 1–78.
Nnamani, C. V., Ajayi, S. A., Oselebe, H. O., Atkinson, C. J., Adewale, D. B., Igwe, D. O., et al. (2018). Updates on nutritional diversity in Sphenostylis stenocarpa (Hoechst ex. a. rich.) harms. for food security and conservation. Am. J. Agric. Biol. Sci. 13, 38–49. doi: 10.3844/ajabssp.2018.38.49
Nnamani, C. V., Ajayi, S. A., Oselebe, H. O., Atkinson, C. J., Igboabuchi, A. N., Ezigbo, E. C. (2017). Sphenostylis stenocarpa (ex. a. rich.) harms., a fading genetic resource in a changing climate: prerequisite for conservation and sustainability. Plan. Theory 6, 30. doi: 10.3390/plants6030030
Nonye, H. U. H., Chinasa, O. R. (2022). Evaluation of pasting and functional properties of flour blends made from African yam bean (Sphenostylis stenocarpa) and corn (Zea mays) seed. World J. Innovat. Res. 12 (3), 19–24.
Nour, A. H., Oluwaseun, A. R., Nour, A. H., Omer, M. S., Ahmed, N. (2021). “Microwave-assisted extraction of bioactive compounds,” in Microwave heating. Ed. Churyumov, G. L. (United Kingdom (UK): IntechOpen), (Ed). doi: 10.5772/intechopen.96092
Nwajagu, I. U., Garba, A., Nzelibe, H. C., Chukwuekezie, N. E., Abah, C. R., Umar, A. T., et al. (2021). Effect of processing on the nutrient, anti-nutrient and functional properties of Mucuna flagellipes (Ox-eyed bean) seed flour; An underutilized legume in Nigeria. Ame. J. Food Nutr. 9, 49–59. doi: 10.12691/ajfn-9-1-7
Obatolu, V. A., Fasoyiro, S. B., Ogunsumi, L. (2001). Effect of processing on functional properties of yam beans (Sphenostylis stenocarpa). Food Sci. Techn. Res. 7 (4), 319–322. doi: 10.3136/fstr.7.319
Oboh, G., Agunloye, O. M., Adefegha, S. A., Akinyemi, A. J., Ademiluyi, A. O. (2015). Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): a comparative study. J. Basic Clin. Physiol. Pharmacol. 26, 165–170. doi: 10.1515/jbcpp-2013-0141
Ojuederie, O. B., Ajiboye, J. A., Babalola, O. O. (2020). Biochemical and histopathological studies of key tissues in healthy Male wistar rats fed on African yam bean seed and tuber meals. J. Food Qual. 2020, 8892618. doi: 10.1155/2020/8892618
Ojuederie, O., Balogun, M. (2017). Genetic variation in nutritional properties of African yam bean Sphenostylis stenocarpa (Hochst ex. a. rich. harms) accessions. Nigerian J. Agric. Food Environ. 13, 180–187.
Ojuederie, O., Balogun, M. (2019). African Yam bean (Sphenostylis stenocarpa) tubers for nutritional security. J. Underut. Leg. 1, 56–68.
Ojuederie, O. B., Popoola, J. O., Aremu, C., Babalola, O. O. (2021). “Harnessing the hidden treasures in African yam bean (Sphenostylis stenocarpa), an underutilized grain legume with food security potentials,” in Food security and safety. Ed. Babalola, O. O. (Cham: Springer), 1–20. doi: 10.1007/978-3-030-50672-8_1
Okafor, J. N., Jideani, V. A., Meyer, M., Le Roes-Hill, M. (2022). Bioactive components in bambara groundnut (Vigna subterraenea (L.) verdc) as a potential source of nutraceutical ingredients. Heliyon 8(3), e09024. doi: 10.1016/j.heliyon.2022.e09024
Okechukwu-Ezike, N., Munonye, J., Esiegwu, A. (2020). Comparative evaluation of the proximate composition, anti-nutrients and functional properties of some underutilized pulses. Food Sci. Qual. Manage. 95, 72–76.
Okezie, B.O., Bello, A. B. (1988). Physicochemical and functional properties of winged bean flour and isolate compared with soy isolate. J. Food Sci. 53, 450–454. doi: 10.1111/j.1365-2621.1988.tb07728.x
Omotayo, A. O., Aremu, A. O. (2019). “Nutritional, phytochemical and economic potential of marama bean,” in The ‘green gold’ of southern Africa (Boston, USA: Harvard University). doi: 10.13140/RG.2.2.22809.36961
Omotayo, A. O., Aremu, A. O. (2021). Marama bean [Tylosema esculentum (Burch.) a. schreib.]: an indigenous plant with potential for food, nutrition, and economic sustainability. Food Funct. 12, 2389–2403. doi: 10.1039/D0FO01937B
Onimawo, I. A., Momoh, A. H., Usman, A. (1998). Proximate composition and functional properties of four cultivars of bambara groundnut (Voandezeia subterranea). Plant Foods Hum. Nutri. 53, 153–158. doi: 10.1023/A:1008027030095
Onyango, C. (2016). Starch and modified starch in bread making: A review. Afr. J.f Food Sci. 10, 344–351. doi: 10.5897/AJFS2016.1481
Oshodi, A. A., Ipinmoroti, K. O., Adeyeye, E. I. (1997). Functional properties of some varieties of African yam bean (Sphenostylis stenocarpa) flour — III. Int. J. Food Sci. Nutr. 48, 243–250. doi: 10.3109/09637489709028568
Oyeyinka, S. A., Abdulsalam, A. O., Ahmed El-Imam, A. M., Oyeyinka, A. T., Olagunju, O. F., Kolawole, F. L., et al. (2021). Total phenolic content, antioxidant, anti-inflammatory and anti-microbial potentials of bambara groundnut (Vigna subterranea l.) seed extract. Brit. Food J. 123, 3421–3435. doi: 10.1108/BFJ-07-2020-0637
Paliwal, R., Adegboyega, T. T., Abberton, M., Faloye, B., Oyatomi, O. (2021). Potential of genomics for the improvement of underutilized legumes in sub-Saharan Africa. Legume Sci. 3 (2), 1–16. doi: 10.1002/leg3.69
Palupi, H. T., Estiasih, T., Yunianta, Sutrisno, A. (2021). Characterization of nutritional and functional properties of Lima bean flour (Phaseolus lunatus l.). IOP Conf. Ser.: Earth Environ. Sci. 924, 12033. doi: 10.1088/1755-1315/924/1/012033
Pandey, M. K., Roorkiwal, M., Singh, V. K., Ramalingam, A., Kudapa, H., Thudi, M., et al. (2016). Emerging genomic tools for legume breeding: current status and future prospects. Front. Plant Sci. 7, 455. doi: 10.3389/fpls.2016.00455
Panghal, A., Janghu, S., Virkar, K., Gat, Y., Kumar, V., Chhikara, N. (2018). Potential non-dairy probiotic products–a healthy approach. Food Biosci. 21, 80–89. doi: 10.1016/j.fbio.2017.12.003
Patel, M., Pandey, S., Kumar, M., Haque, M., Pal, S., Yadav, N. (2021). Plants metabolome study: Emerging tools and techniques. Plants 10, 2409. doi: 10.3390/plants10112409
Patterson, E., Wall, R., Fitzgerald, G. F., Ross, R. P., Stanton, C. (2012). Health implications of high dietary omega-6 polyunsaturated fatty acids. J. nutri. Metabol. 2012, 539426. doi: 10.1155/2012/539426
Pattnaik, M., Pandey, P., Martin, G. J., Mishra, H. N., Ashokkumar, M. (2021). Innovative technologies for extraction and microencapsulation of bioactives from plant-based food waste and their applications in functional food development. Foods 10, 279. doi: 10.3390/foods10020279
Popoola, J. O., Ajani, O. C., Oyesola, O. L., Oniha, M. (2022a). Comparative analysis of phytochemical and proximate contents of three orphan legume species. Asian J. Plant Sci. 21, 520–528. doi: 10.3923/ajps.2022.520.528
Popoola, J. O., Aworunse, O. S., Ojuederie, O. B., Adewale, B. D., Ajani, O. C., Oyatomi, O. A., et al. (2022b). The exploitation of orphan legumes for food, income, and nutrition security in Sub-Saharan Africa. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.782140
Popoola, J., Ojuederie, O., Omonhinmin, C., Adegbite, A. (2020). “Neglected and underutilized legume crops: Improvement and future prospects,” in Recent advances in grain crops research. Eds. Shah, F., Khan, Z., Iqbal, A., Turan, M., Olgun, M. (United Kingdom (UK): IntechOpen). doi: 10.5772/intechopen.87069
Rai, K. K., Pandey, N., Meena, R. P., Rai, S. P. (2021). Biotechnological strategies for enhancing heavy metal tolerance in neglected and underutilized legume crops: A comprehensive review. Ecotoxicol. Environ. Safe. 208, 111750. doi: 10.1016/j.ecoenv.2020.111750
Ramatsetse, K. E., Ramashia, S. E., Mashau, M. E. (2023). A review on health benefits, antimicrobial and antioxidant properties of bambara groundnut (Vigna subterranean). Int. J. Food Prop. 26, 91–107. doi: 10.1080/10942912.2022.2153864
Rasika, D. M. D., Vidanarachchi, J. K., Rocha, R. S., Balthazar, C. F., Cruz, A. G., Sant’ana, A. S., et al. (2021). Plant-based milk substitutes as emerging probiotic carriers. Curr. Opin. Food Sci. 38, 8–20. doi: 10.1016/j.cofs.2020.10.025
Rathi, D., Chakraborty, S., Chakraborty, N. (2021). Grasspea, a critical recruit among neglected and underutilized legumes, for tapping genomic resources. Curr. Plant Biol., 100200. doi: 10.1016/j.cpb.2021.100200
Ratnawati, L., Desnilasari, D., Surahman, D. N., Kumalasari, R. (2019). Evaluation of physicochemical, functional and pasting properties of soybean, mung bean and red kidney bean flour as ingredient in biscuit. IOP Conf. Ser.: Earth Environ. Sci. 251, 12026. doi: 10.1088/1755-1315/251/1/012026
Roohani, N., Hurrell, R., Kelishadi, R., Schulin, R. (2013). Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 18 (2), 144–157.
Roy, F., Boye, J. I., Simpson, B. K. (2010). Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 43, 432–442. doi: 10.1016/j.foodres.2009.09.002
Salawu, S. (2016). Comparative study of the antioxidant activities of methanolic extract and simulated gastrointestinal enzyme digest of bambara nut (Vigna subterranea) FUTA. J. Res.Sci. 1, 107–120.
Sanders, M. E., Merenstein, D. J., Reid, G., Gibson, G. R., Rastall, R. A. (2019). Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastro. Hepatol. 16 (10), 605–616. doi: 10.1038/s41575-019-0173-3
Sattar, D., Ali, T. M., Hasnain, A. (2017). Effect of germination on enzymatic, functional and bioactive attributes of different Pakistani legume cultivars. J. Food Meas. Charact. 11, 2076–2086. doi: 10.1007/s11694-017-9591-5
Schafleitner, R., Nair, R. M., Rathore, A., Wang, Y.-w., Lin, C.-y., Chu, S.-h., et al. (2015). The AVRDC–the world vegetable center mungbean (Vigna radiata) core and mini core collections. BMC Genomics 16, 344. doi: 10.1186/s12864-015-1556-7
Selvamuthukumaran, M., Shi, J. (2017). Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Safe. 1, 61–81. doi: 10.1093/fqs/fyx004
Semba, R. D., Ramsing, R., Rahman, N., Kraemer, K., Bloem, M. W. (2021). Legumes as a sustainable source of protein in human diets. Global Food Secur. 28, 100520. doi: 10.1016/j.gfs.2021.100520
Sergio, O., Saldívar, S., Hernández, D. S. (2020). “Dietary fiber in cereals, legumes,pseudocereals and other seeds,” in Science and technology of fibers in food systems, food engineering series. Ed. Welti-Chanes, J., Sergio, O. S, Osvaldo, C., Viridiana, T. (Switzerland AG: Springer Nature). doi: 10.1007/978-3-030-38654-2_5
Sharma, N., Bisht, S. S., Gupta, S., Rana, M., Kumar, A. (2019). Nutraceutical evaluation of horse gram (Macrotyloma uniflorum) cultivated in high altitudes of uttarakhand himalaya, India. Indian J. Pure Appl. Biosci. 7, 190–202.
Shelke, D. B., Tayade, S., Gawande, P., Sonawane, H. B. (2022). GC-MS analysis and antioxidant potential of wild underutilized medicinally important legume, velvet bean (Mucuna pruriens l. DC.). Not. Sci. Biol. 14, 11098–11098. doi: 10.15835/nsb14111098
Shevkani, K., Kaur, M., Singh, N. (2021). Composition, pasting, functional, and microstructural properties of flours from different split dehulled pulses (dhals). J. Food Process. Preserv. 45(6). doi: 10.1111/jfpp.15485
Shevkani, K., Singh, N., Patil, C., Awasthi, A., Paul, M. (2022). Antioxidative and antimicrobial properties of pulse proteins and their applications in gluten-free foods and sports nutrition. Int. J. Food Sci. Tech 57, 5571–5584. doi: 10.1111/ijfs.15666
Shi, J., Arunasalam, K., Yeung, D., Kakuda, Y., Mittal, G., Jiang, Y. (2004). Saponins from edible legumes: Chemistry, processing, and health benefits. J. Med. Food 7 (1), 67–78. doi: 10.1089/109662004322984734
Silva, E., Souza, J., Rogez, H., Rees, J.-F., Larondelle, Y. (2007). Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 101, 1012–1018. doi: 10.1016/j.foodchem.2006.02.055
Singh, J. P., Singh, B., Kaur, A. (2021). Bioactive compounds of legume seeds. Bioactive Compounds Underutilized Vegetables Legumes, 645–665. doi: 10.1007/978-3-030-57415-4_33
Singh, B., Singh, J. P., Shevkani, K., Singh, N., Kaur, A. (2017). Bioactive constituents in pulses and their health benefits. J. Food Sci. Technol. 54, 858–870. doi: 10.1007/s13197-016-2391-9
Sitohy, M., Mahgoub, S., Osman, A., El-Masry, R., Al-Gaby, A. (2013). Extent and mode of action of cationic legume proteins against Listeria monocytogenes and Salmonella enteritidis. Probiotics Antimicrob. Proteins 5, 195–205. doi: 10.1007/s12602-013-9134-2
Soetan, K. O. (2017). Preliminary studies on the nutritional and antinutritional constituents of six accessions of African yam bean (Sphenostylis stenocarpa): An underutilized legume. J.Appl. Res. Child. 8.
Soetan, K., Adeola, A. (2018). Comparative nutritional and functional properties of selected underutilized legumes. Nigerian J. Anim. Product. 45, 96–106-196–106.
Soetan, K., Atanda, T. (2018). Proximate composition, phytochemical screening and some antinutritional factors of three accessions of lima beans (Phaseolus lunatus) an underutilized legume. Nigerian J. Anim. Product. 45, 71–78-71–78.
Soetan, K. O., Olaiya, C. O., Karigidi, K. O. (2018). Comparative in vitro antioxidant activities of six accessions of African yam beans (Sphenostylis stenocarpa l.). Ann. Food Sci. Technol. 19, 455–461.
Solarte, D. A., Ruiz-Matute, A. I., Chito-Trujillo, D. M., Rada-Mendoza, M., Sanz, M. L. (2021). Microwave assisted extraction of bioactive carbohydrates from different morphological parts of alfalfa (Medicago sativa l.). Foods 10, 346. doi: 10.3390/foods10020346
Solomon, S. G., Okomoda, V. T., Oguche, O. (2018). Nutritional value of raw Canavalia ensiformis and its utilization as partial replacement for soybean meal in the diet of Clarias gariepinus (Burchell 1822) fingerlings. Food Sci. Nutr. 6, 207–213. doi: 10.1002/fsn3.548
Sowndhararajan, K., Siddhuraju, P., Manian, S. (2011). Antioxidant activity of the differentially processed seeds of jack bean (Canavalia ensiformis l. DC). Food Sci. Biotechnol. 20, 585–591. doi: 10.1007/s10068-011-0083-9
Sriwichai, S., Monkham, T., Sanitchon, J., Jogloy, S., Chankaew, S. (2021). Dual-purpose of the winged bean (Psophocarpus tetragonolobus (L.) DC.), the neglected tropical legume, based on pod and tuber yields. Plants 10, 1746. doi: 10.3390/plants10081746
Steinwand, M. A., Ronald, P. C. (2020). Crop biotechnology and the future of food. Nat. Food 1, 273–283. doi: 10.1038/s43016-020-0072-3
Stosh, S. M., Yada, S. (2010). Dietary fibres in pulse seeds and fractions: Characterization, functional attributes, and applications. Food Res. Int. 43, 450–460. doi: 10.1016/j.foodres.2009.09.005
Sun, X., Weckwerth, W. (2012). COVAIN: A toolbox for uni- and multivariate statistics, time-series and correlation network analysis and inverse estimation of the differential Jacobian from metabolomics covariance data. Metabolomics 28, 81–93. doi: 10.1007/s11306-012-0399-3
Sutivisedsak, N., Cheng, H., Willett, J., Lesch, W., Tangsrud, R., Biswas, A. (2010). Microwave-assisted extraction of phenolics from bean (Phaseolus vulgaris l.). Food Res. Int. 43, 516–519. doi: 10.1016/j.foodres.2009.09.014
Tadele, Z., Bartels, D. (2019). Promoting orphan crops research and development. Planta 250, 675–676. doi: 10.1007/s00425-019-03235-x
Tan, S. S., Tan, C. X., Tan, S. T. (2020). “Cold pressed peanut (Arachis hypogaea l.) oil,” in Cold pressed oils (United States: Elsevier), 357–364. doi: 10.1016/B978-0-12-818188-1.00032-3
Tee, E. S., Goh, A. H., Khor, S. C. (1995). Carotenoid composition and content of legumes, tubers and roots by HPLC. Malaysian J. nutri. 1 (1), 51–61.
Tian, J., Bryksa, B., Yada, R. (2016). Feeding the world into the future–food and nutrition security: the role of food science and technology. Front. Life Sci. 9 (3), 155–166. doi: 10.1080/21553769.2016.1174958
Tjahjadi, C., Lin, S., Breene, W. M. (1988). Isolation and characterization of adzuki bean (Vigna angularis cv takara) proteins. J. Food Sci. 53, 1438–1443. doi: 10.1111/j.1365-2621.1988.tb09294.x
Tripathi, K., Gore, P. G., Pandey, A., Nayar, E. R., Gayacharan, C., Pamarthi, R. K., et al. (2020). Morphological and nutritional assessment of Vigna vexillata (L.) a. rich.: a potential tuberous legume of India. Genet. Resour Crop Evol. 68, 397–408. doi: 10.1007/s10722-020-01023-1
Tsamo, A. T., Mohammed, M., Dakora, F. D. (2020). Metabolite fingerprinting of kersting’s groundnut [Macrotyloma geocarpum (Harms) maréchal & baudet] seeds using UPLC-qTOF-MS reveals the nutraceutical and antioxidant potentials of the orphan legume. Front. Nutr. 7, 306. doi: 10.3389/fnut.2020.593436
Udeh, E. L., Nyila, M. A., Kanu, S. A. (2020). Nutraceutical and antimicrobial potentials of bambara groundnut (Vigna subterranean): A review. Heliyon 6, e05205. doi: 10.1016/j.heliyon.2020.e05205
Vadivel, V., Patel, A., Biesalski, H. K. (2012). Effect of traditional processing methods on the antioxidant, α-amylase and α-glucosidase enzyme inhibition properties of sesbania sesban Merrill seeds. CyTA-J. Food 10, 128–136. doi: 10.1080/19476337.2011.601427
Vijayakumar, V. (2021). “Nutraceutical legumes: A brief review on the nutritional and medicinal values,” in Sustainable agriculture reviews, vol. 51 . Eds. Guleria, P., Kumar, V., Lichtfouse, E. (Cham: Springer), 1–28. doi: 10.1007/978-3-030-68828-8_1
Von Wettberg, E. B., Naito, K., Kur, A., Ludidi, N. (2021). The unusual biogeography of zombi pea, Vigna vexillata. Legumes 8-9.
Voutilainen, S., Nurmi, T., Mursu, J., Rissanen, T. H. (2006). Carotenoids and cardiovascular health. Amer. J. Clin. Nutr. 83, 1265–1271. doi: 10.1093/ajcn/83.6.1265
Wanyama, A., Orwa, J., Njenga, P., Irungu, B. (2017). Evaluation of cytotoxicity, antimicrobial activities and minerals composition of Vigna subterranea (L.) verdc.(Bambara groundnut) extracts. Afr. J. Health Sci. 30, 88–104.
Wessels, I., Rink, L. (2020). Micronutrients in autoimmune diseases: Possible therapeutic benefits of zinc and vitamin d. J. Nutrit. Biochem. 77, 108240. doi: 10.1016/j.jnutbio.2019.108240
Wessels, I., Rolles, B., Rink, L. (2020). The potential impact of zinc supplementation on COVID-19 pathogenesis. Front. Immunol. 11, 1712. doi: 10.3389/fimmu.2020.01712
Wolabu, T. W., Park, J. J., Chen, M., Cong, L., Ge, Y., Jiang, Q., et al (2020). Improving the genome editing efficiency of CRISPR/Cas9 in Arabidopsis and Medicago truncatula. Planta 252, 15. doi: 10.1007/s00425-020-03415-0
Xu, D.-P., Li, Y., Meng, X., Zhou, T., Zhou, Y., Zheng, J., et al. (2017). Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. IJMS 18, 96. doi: 10.3390/ijms18010096
Yadav, U., Singh, N., Kaur, A., Thakur, S. (2018). Physico-chemical, hydration, cooking, textural and pasting properties of different adzuki bean (Vigna angularis) accessions. J. Food Sci. Technol. 55, 802–810. doi: 10.1007/s13197-017-2994-9
Yang, J., de Wit, A., Diedericks, C. F., Venema, P., van der Linden, E., Sagis, L. M. C. (2022). Foaming and emulsifying properties of extensively and mildly extracted bambara groundnut proteins: A comparison of legumin, vicilin and albumin protein. Food Hydrocolloids 123, 107190. doi: 10.1016/j.foodhyd.2021.107190
Yang, S., Grall, A., Chapman, M. A. (2018). Origin and diversification of winged bean (Psophocarpus tetragonolobus (L.) DC.), a multipurpose underutilized legume. Am. J. Bot. 105, 888–897. doi: 10.1002/ajb2.1093
Yang, C. S., Landau, J. M., Huang, M.-T., Newmark, H. L. (2001). Inhibition of carcinogenesis by dietary polyphenolic compounds. Ann. Rev. Nutrit. 21, 381. doi: 10.1146/annurev.nutr.21.1.381
Yao, Y., Cheng, X.-Z., Wang, L.-X., Wang, S.-H., Ren, G. (2012). Major phenolic compounds, antioxidant capacity and antidiabetic potential of rice bean (Vigna umbellata l.) in China. IJMS 13, 2707–2716. doi: 10.3390/ijms13032707
Yao, D. N., Kouassi, K. N., Erba, D., Scazzina, F., Pellegrini, N., Casiraghi, M. C. (2015). Nutritive evaluation of the bambara groundnut Ci12 landrace [Vigna subterranea (L.) verdc. (Fabaceae)] produced in côte d’Ivoire. Int. J. Mol. Sci. 16, 21428–21441. doi: 10.3390/ijms160921428
Yao, X., Zheng, X., Zhao, R., Li, Z., Shen, H., Li, T., et al. (2021). Quality formation of adzuki bean baked: From acrylamide to volatiles under microwave heating and drum roasting. Foods 10, 2762. doi: 10.3390/foods10112762
Zhang, Z., Lanza, E., Kris-Etherton, P. M., Colburn, N. H., Bagshaw, D., Rovine, M. J., et al. (2010). A high legume low glycemic index diet improves serum lipid profiles in men. Lipids 45 (9), 765–775. doi: 10.1007/s11745-010-3463-7
Zheng, Z., Li, J., Li, J., Sun, H., Liu, Y. (2019). Physicochemical and antioxidative characteristics of black bean protein hydrolysates obtained from different enzymes. Food Hydrocolloids 97, 105222. doi: 10.1016/j.foodhyd.2019.105222
Keywords: antioxidants, bioactive compounds, functional food products, under-exploited legumes, sustainable development
Citation: Popoola JO, Ojuederie OB, Aworunse OS, Adelekan A, Oyelakin AS, Oyesola OL, Akinduti PA, Dahunsi SO, Adegboyega TT, Oranusi SU, Ayilara MS and Omonhinmin CA (2023) Nutritional, functional, and bioactive properties of african underutilized legumes. Front. Plant Sci. 14:1105364. doi: 10.3389/fpls.2023.1105364
Received: 22 November 2022; Accepted: 16 March 2023;
Published: 14 April 2023.
Edited by:
María Serrano, Miguel Hernández University of Elche, SpainReviewed by:
Kuldeep Tripathi, National Bureau of Plant Genetic Resources, Indian Council of Agricultural Research (ICAR), IndiaSergio Medina Godoy, National Polytechnic Institute (IPN), Mexico
Copyright © 2023 Popoola, Ojuederie, Aworunse, Adelekan, Oyelakin, Oyesola, Akinduti, Dahunsi, Adegboyega, Oranusi, Ayilara and Omonhinmin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacob Olagbenro Popoola, amFjb2IucG9wb29sYUBib3dlbi5lZHUubmc=; Omena B. Ojuederie, b2Iub2p1ZWRlcmllQGtpbmdzdW5pdmVyc2l0eS5lZHUubmc=
†ORCID: Jacob Olagbenro Popoola, orcid.org/0000-0001-5302-4856Omena B. Ojuederie, orcid.org/0000-0003-0474-6697
 Jacob Olagbenro Popoola
Jacob Olagbenro Popoola Omena B. Ojuederie
Omena B. Ojuederie Oluwadurotimi Samuel Aworunse
Oluwadurotimi Samuel Aworunse Aminat Adelekan5
Aminat Adelekan5 Olusola Luke Oyesola
Olusola Luke Oyesola Paul A. Akinduti
Paul A. Akinduti Samuel Olatunde Dahunsi
Samuel Olatunde Dahunsi Modupe S. Ayilara
Modupe S. Ayilara