
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 14 February 2023
Sec. Functional Plant Ecology
Volume 14 - 2023 | https://doi.org/10.3389/fpls.2023.1104632
This article is part of the Research TopicPlant Responses to Environmental Stresses Based on Physiological and Functional EcologyView all 31 articles
This study was aimed to clarify the effects of stumping on root and leaf traits as well as the tradeoffs and synergies of decaying Hippophae rhamnoides in feldspathic sandstone areas, and to select the optimal stump height that contributed to the recovery and growth of H. rhamnoides. variations and coordination between leaf traits and fine root traits of H. rhamnoides were studied at different stump heights (0, 10, 15, 20 cm, and no stumping) in feldspathic sandstone areas. All functional traits of the leaves and roots, except the leaf C content (LC) and the fine root C content (FRC), were significantly different among different stump heights. The total variation coefficient was the largest in the specific leaf area (SLA), which is therefore the most sensitive trait. Compared to non-stumping, SLA, leaf N content (LN), specific root length (SRL) and fine root N content (FRN) all improved significantly at stump height of 15 cm, but leaf tissue density (LTD), leaf dry matter content (LDMC), leaf carbon to nitrogen ratio (LC : LN), fine root tissue density (FRTD), fine root dry matter content (FRDMC) and fine root carbon to nitrogen ratio (FRC : FRN) all decreased significantly. The leaf traits of H. rhamnoides at different stump heights follow the leaf economic spectrum, and the fine roots show a similar trait syndrome to the leaves. SLA and LN are positively correlated with SRL and FRN and negatively with FRTD and FRC : FRN. LDMC and LC : LN are positively correlated with FRTD and FRC : FRN, and negatively correlated SRL and RN. The stumped H. rhamnoides changes to the ‘rapid investment–return type’ resource trade-offs strategy, and the growth rate is maximized at the stump height of 15 cm. Our findings are critical to the prevention and control of vegetation recovery and soil erosion in feldspathic sandstone areas.
The feldspathic sandstone zones of Inner Mongolia are one of the regions with the most severe soil erosion on the Loess Plateau. Feldspathic sandstone is characteristic of a low degree of diagenesis and can be easily invaded to form sand. The construction of artificial vegetation is an important measure of eco-environmental rehabilitation in this region. Hippophae rhamnoides is a critical soil and water-conserving plant in arid and semi-arid areas. With well-developed roots, it has strong tillering and germinating abilities and can rapidly spread out to produce large biomass. It has excellent soil water conservation abilities and is extremely dominant in the feldspathic sandstone areas of Inner Mongolia (Liu et al., 2022). However, artificial forests of H. rhamnoides in this region that grow to 10 years old will suffer a massive decline in growth and a decrease in productivity (Yang et al., 2014b; Cao et al., 2016), indicating that effective conservation is needed at this age. Since stumping will change functional traits, stumped shrubs can compensationarily recover and grow, and are stimulated to grow more sprouts and branches and thereby improve the net photosynthetic rate and primary productivity, changing the resource intake and use strategies of roots, and preventing the decay of shrub forests (Yang et al., 2020; Liu et al., 2022; Liu et al., 2023). The sprouting effect of stumping is affected by multiple factors, including the controllable stump height (Giambalvo et al., 2011; Langworthy et al., 2019).
Functional traits are used as the response indices of plants to environmental changes and can characterize the survival and resource utilization strategies of plants under unfavourable conditions (Dyer et al., 2001; Reich et al., 2003). The functional traits of leaves play a critical role in the assimilation of carbon, the relationships of moisture, and the energy balance of plants (Ackerly et al., 2002), and the traits of roots decide the absorption of nutrients and moisture that are essential for the survival and growth of plants (McCormack et al., 2015; Sun et al., 2018). However, existing research on plant functional traits is focused on forests and grasslands (He et al., 2008; Hosseini et al., 2019; Cui et al., 2022), and the root and leaf traits of H. rhamnoides before and after stumping in feldspathic sandstone areas are still unclear.
The spectrum theory of plant economics (Wright et al., 2004; Freschet et al., 2010) holds that there are associations in functional traits, biomass, construction consumption, and resource absorption between the aboveground and underground parts of plants living under the restrictions of environmental selection and biological–physical factors. This theory also demonstrates the tradeoff between rapid acquisition and resource saving for plants (Reich et al., 1999; Osnas et al., 2013). Apparently, there is a tradeoff and synergy among the functional traits of the leaves. Unlike the leaf trait syndrome, the traits of fine roots belong to be a more complex multidimensional economic space that reflects the diverse evolution pressures and tradeoffs in the underground part (Kong et al., 2014; Xia et al., 2021; Ning et al., 2022). The large specific root length, small diameter and low tissue density of fine roots are related to the high respiration rate and high turnover rate, and such trade-off is similar to the economic spectrum of leaves (Caplan et al., 2019; Laughlin et al., 2021). Other research shows that the traits of fine roots are mutually independent to a large extent. Furthermore, it is unclear whether the trait syndromes of the leaves and roots in H. rhamnoides exist before and after stumping.
To achieve the economic spectrum, the traits of different organs (e.g., leaves, roots) have to coordinate in accordance with the evolution and biophysical restraints (Reich, 2014; Weemstra et al., 2016). As reported, similar leaf and root traits are interrelated among species in the grasslands of the Inner-Mongolia Plateau or the Qinghai-Tibetan Plateau (Geng et al., 2014). Compared to forest species, species in arid areas tend to acquire underground resources rather than ground resources (Liu et al., 2010; Bardgett et al., 2014). However, it is unknown whether the existing theory applies to H. rhamnoides grown in feldspathic sandstone areas. In addition, there is little research on coordination in chemical tissue traits and morphological traits between the leaves and fine roots of H. rhamnoides in feldspathic sandstone areas.
For these reasons, in this study targeted at H. rhamnoides grown in feldspathic sandstone areas of Inner Mongolia, we analyzed the variation traits of roots and leaves at different stump heights as well as the tradeoffs and synergies between them. Specifically, we tested three hypotheses: (1) the leaf-trait syndrome in H. rhamnoides at different stump heights is in line with the global prediction of LES and is in parallel with the root-trait syndrome; (2) functional traits related to nutrient content and resource absorption are closely coordinated between leaves and fine roots; (3) stumped H. rhamnoides change to the ‘rapid investment–return type’ resource trade-off strategy.
The study area is located in the soil-water conservation science and technology demonstration zone in the feldspathic sandstone zone in Nuanshui Village Jungar Banner, Ordos, Inner Mongolia (Figure 1). This region (39°42’N -39°50’ N, 110°25’E -110°48’ E, 96 km2) has complex terrains with extensive gullies and fluctuating girders, and suffers soil erosion and soil-water loss. It has an average altitude of 800 - 1590 m, a precipitation of 400 mm (concentrated in July and August), a duration of sunlight duration above 3000 d, an evaporation of 2093 mm, and a temperature of 6.2-8.7°C on the annual average. The vegetation of this region is dominated by artificial vegetation, including H. rhamnoides, Pinus tableulaeformis, Caragana korshinskii, Medicago sativa and Prunus sibirica.
In the demonstration zone, we chose H. rhamnoides plantation land that had basically consistent site conditions and forest compositions and were in the decaying stage as the experimental site, which were under the northwest slope face at slope of 4°. On the same slope face, H. rhamnoides were planted at the row space 0f 2m × 4m, and its tree age was 10 years. H. rhamnoides were stumped at early March 2020 in the experimental site. The stump heights (the stumping distance from the ground) were 0, 10, 15 and 20 cm, which corresponded to the treatments H1, H2, H3 and H4, respectively (Figure 2). A stump-free plantation site was established as a control (CK). All treatment sample plots were 50m×50m in area. Each treatment was carried out in triplicate. Stumping was performed using electric saws and pruning shears, which ensured that the incisions were flat and smooth without burrs. The overall stumping mode was adopted. To decrease plant moisture dissipation, we painted stumped sites after stumping.
The leaf and fine root traits in five samples from the plots treated at different stump heights were measured in the middle growing season (middle June to lower August) of 2022 (The growth of H. rhamnoides at different stump heights was listed in Table 1). A sample involved at least five representative and healthy plants under basically consistent growth status. From each plant, 10 healthy leaves in medium size were collected and used to measure morphology, C and N concentrations. Fine roots were collected by the digging method. The weeds and litter around the basal of each chosen sample were cleaned away. After the surface soils were mostly cleaned, the peri-root soils were carefully cleared away to find the main roots. When root branches were encountered in the growing direction of the main roots, we further dug along the branch roots until we reached the end of the root system. During sampling, the loss of terminal low-level roots was avoided to ensure root completeness. Then fine roots in diameter smaller than 2 mm were chosen (Cornelissen et al., 2003; Mitchell et al., 2017).
The roots collected as they were put in labeled preservation bags, which were then placed in a refrigerator at 2-3° C on the same day and taken back to our laboratory. The leaves and fine roots were washed with deionized water and scanned using an 11000XL scanner (Epson, Tokyo, Japan). Together with WinRHIZO Pro 2012b (Regent Instruments Inc., Quebec City, Canada), leaf areas, leaf volumes, total fine root length, and fine root volumes were measured. Subsequently, the leaves and roots in each sample were placed in water and stored away from light at 4°C for 24 h (Ning et al., 2022). After the water was saturated, the surface water of the leaves or fine roots was sucked using absorbent paper, and then the saturated fresh weight of the leaves or fine roots was measured. Then the leaves and fine roots were dried at 60°C for 48 h, and the dry weight of the leaves and the dry weight of the fine roots were monitored at constant weight (Cui et al., 2019).
Then the specific leaf area (SLA), leaf dry matter content (LDMC), leaf tissue density (LTD), specific root length of fine roots (SRL), fine root dry matter content (FRDMC), and fine root tissue density (FRLTD) were computed. SLA and SRL are the ratio of area to dry weight of leaves and the ratio of area to dry weight of fine roots respectively. LDMC and FRDMC are the ratio of dry weight to saturated water weight of leaves and the ratio of dry weight to saturated water weight of fine roots, respectively. LTD and FRTD stand for the ratio of dry weight to volume of leaves, and the ratio of dry weight to volume of fine roots respectively (Jiang et al., 2021). The dried leaves and fine roots were ground into powder using a PULVERISETTE 5 high flow ball milling system (Fritsch, Munich, Germany), which was passed through a 0.149 mm sieve for chemical analysis. Carbon and nitrogen content in fine roots and leaves were measured using a Vario MACRO cube elemental analyzer (Elementar, Hanau, Germany), and the carbon and nitrogen ratios in roots and leaves were determined.
Data were analyzed in SPSS 26.0. The data of leaves and fine roots were sent to descriptive statistics and analysis of the variation coefficient (variation coefficient =standard deviation/mean value ×100%). Differences in the traits of leaves and fine roots of H. rhamnoides among different stump heights were statistically analyzed by one-way analysis of variance (ANOVA). Significance was tested using Fisher’s least significant difference method at the level p < 0.05. The leaf and fine root variation characteristics at different stump heights, the Pearson heatmaps of fine roots and leaves, and the PCA of root and leaf coordination were plotted in Origin 2021.
Neither leaf C content (LC) nor fine root carbon content (FRC) was significantly different among different stump height treatments (p > 0.05) (Table 2; Figure 3). SLA, LTD, LDMC, LC, leaf nitrogen content (LN), leaf carbon to nitrogen ratio (LC : LN), SRL, FRID, FRMDC, FRC, fine root nitrogen content (FRN) and fine root carbon to nitrogen ratio (FRC : FRN) were very significantly different between treatments (p < 0.001).
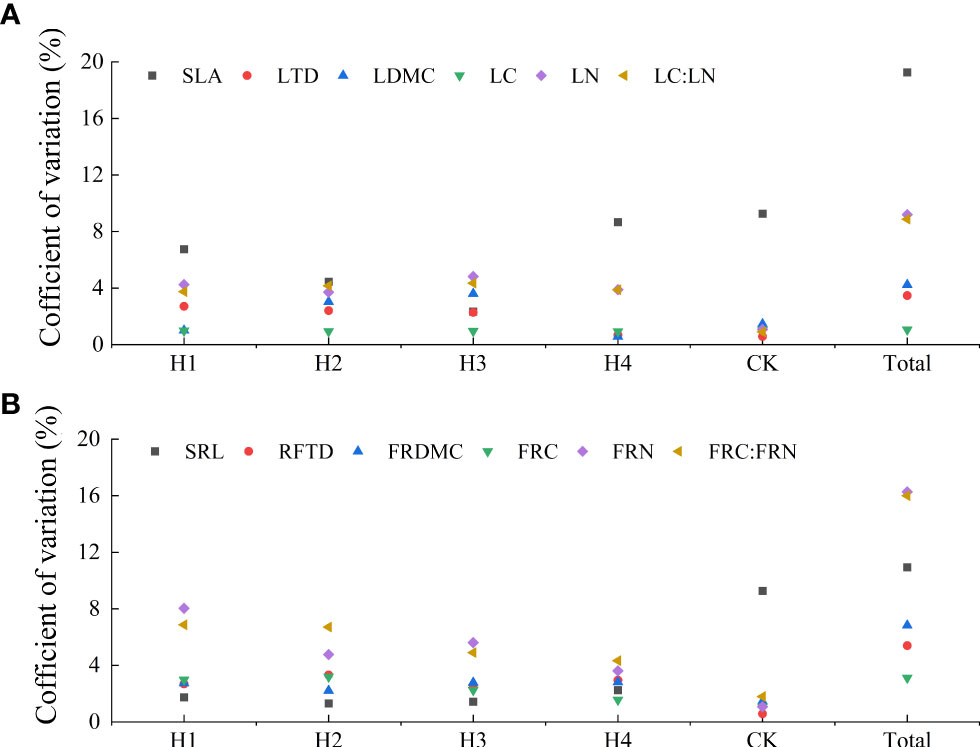
Figure 3 Variation traits of leaves (A) and fine roots (B) at different stump heights. Specific leaf area (SLA), Leaf tissue density (LTD), Leaf dry matter content (LDMC), Leaf C content (LC), Leaf N content (LN), Leaf C:N ratio (LC : LN), Specific fine root length (SRL), Fine root tissue density (FRTD), Fine root fry matter content (FRDMC), Fine root C content (FRC), Fine root N content, Fine root C:N ratio (FRC : FRN).
The total coefficient of variation among different stump heights was 1.05%–19.26%. Total coefficients of variation in SLA, FRN, FRC : FRN, and SRL were greater than 10%, with a maximum in SLA (19.26%). The total coefficients of variation in LN, LC : LN, RDMC, RTD, LDMC, LTD, FRC, and LC were all larger than 10%, with the minimum values of 1.05% in LC and 3.22% in FRC. The total coefficient of variation among different treatments was classified as SLA > FRN > FRNC : FRN > SRL > LN > LC : LN > FRDMC > FRTD > LMDC > LTD > FRC > LC. The coefficients of variation in all indices of H1, H2, H3, H4 and CK were all lower than 10% (Figure 3).
The SLA, LTD, LDMC, LC, LN, LC : LN, SRL, FRID, FRMDC, FRC, FRN, and FRC : FRN were all significantly different between H2 and H3 (p < 0.05). Compared to CK, SLA, LN, SRL and FRN improved significantly after stumping, but LTD, LDMC, LC : LN, FRTD, FRDMC and FRC : FRN significantly decreased. SLA, LN, SRL and LN ranked as H3 > H2 > H1 > H4 > CK. The changing rules of LTD, LDMC, LC : LN, FRTD, and FRDMC were the opposite and ranked as H3 < H2 < H1 < H4 < CK (Figure 4).
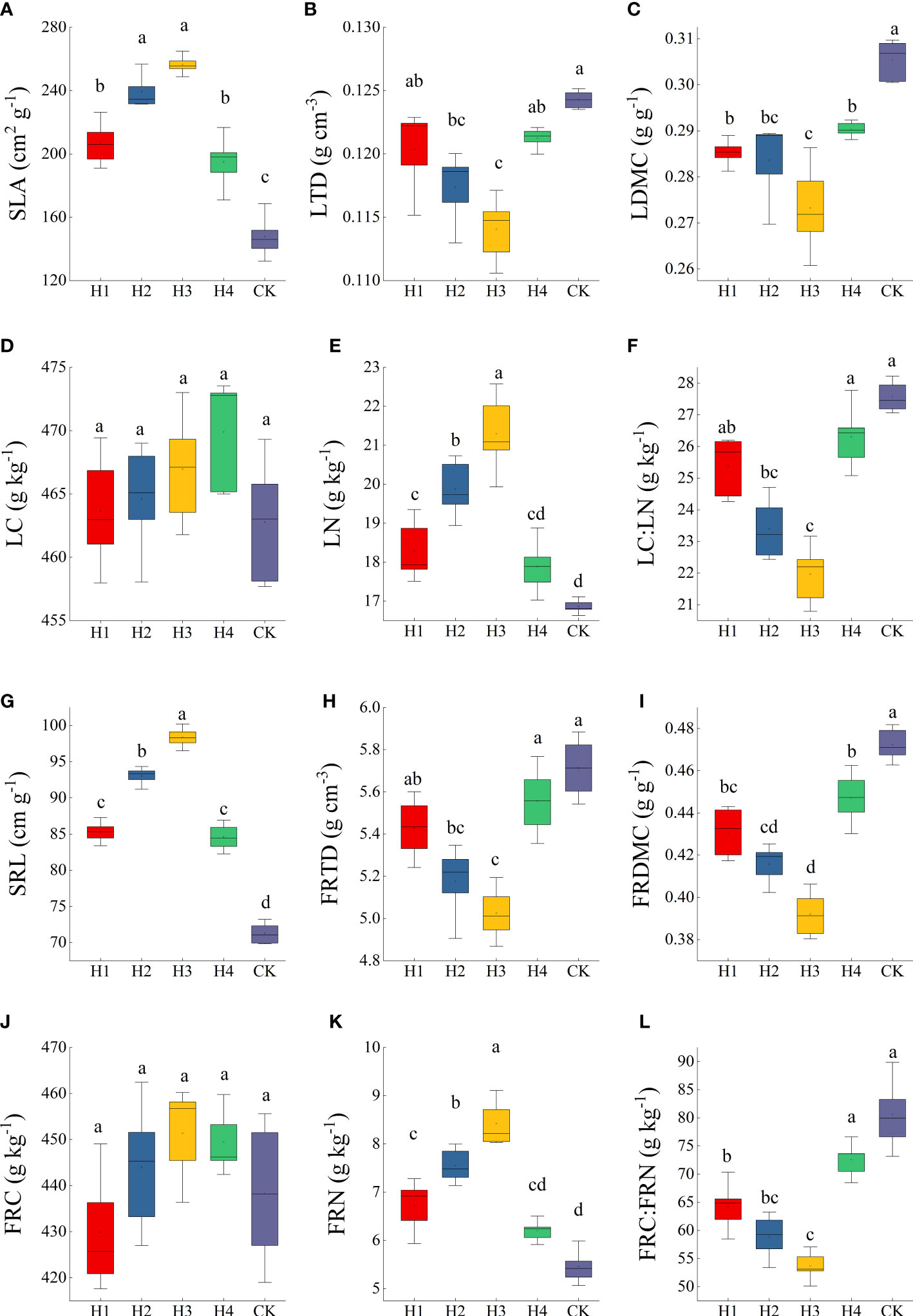
Figure 4 The traits of leaves (A–F) and fine roots (G–L) at different stump heights. Error bars represent ± SE of the mean. Significant differences are indicated by different lowercase letters at p < 0.05. (All abbreviations are shown in (Figure 3).
The leaf traits and the fine root traits were both significantly correlated among different stump heights (Figure 5). SLA in CK was negatively correlated with LTD, LDMC, and LC : LN, and positively correlated with LN. LDMC in CK was positively correlated with LTD and LC : LN (p < 0.05), and very significantly negatively correlated with LN (p < 0.01).The LN and LC : LN in CK were very significantly negatively correlated (p < 0.01, Figure 5E). The correlations of SLA with LTD and LDMC were weakened after stumping, but the correlation between SLA and LN or LC : LN was enhanced. In particular, the SLA in H1, H3 or H4 was highly significantly correlated positively with LN (p < 0.01) and negatively with LC : LN (both p < 0.01). After stumping, LTD was positively correlated with LDMC and negatively with LN (p < 0.05), and LN was very significantly positively correlated with LC : LN (p < 0.01, Figures 5A–D).
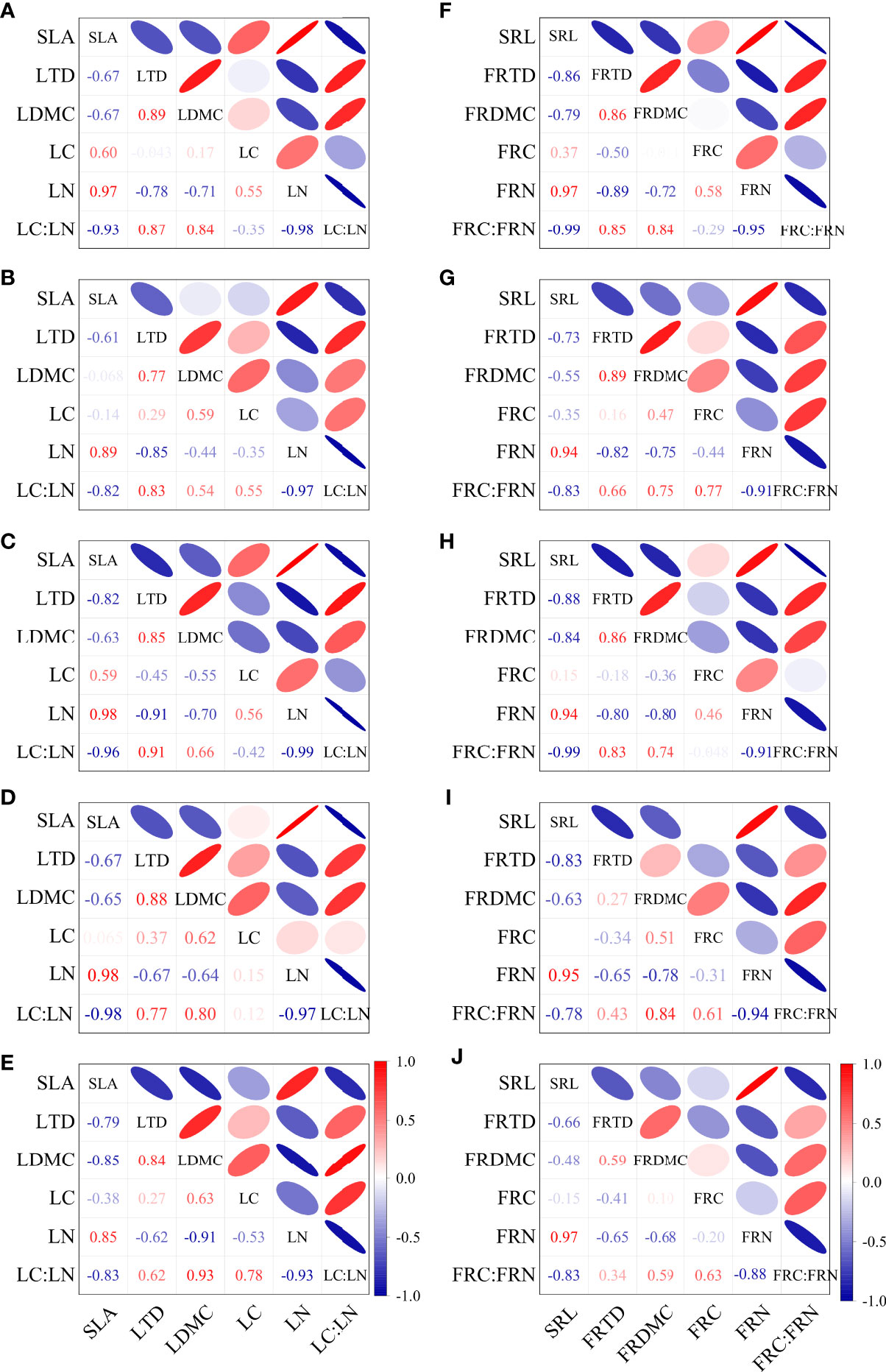
Figure 5 Correlations of leaf traits and of fine root traits at different stump heights (A–E). Correlations of leaf properties at 0, 10, 15, 20 cm and no-stumping (F–J). Correlations of fine root properties at 0, 10, 15, 20 cm and without stumping. (All abbreviations are shown in Figure 3).
SRL in CK was negatively correlated with RTD and FRC : FRN (p < 0.05), and was very significantly positively correlated with FRN (p < 0.01). FRN and FRTD in CK were positively correlated with FRDMC (p < 0.05), and were very significantly negatively correlated with FRC : FRN (p < 0.01, Figure 5J). SRL after stumping was negatively correlated with RTD and FRC : FRN (p < 0.05), but SRL in H1 or H3 was very significantly negatively correlated with FRC : FRN (p < 0.01). The FRN and FRTD after stumping were both positively correlated with FRDMC (p < 0.05), and the FRTD and FRDMC of H1, H2, H3 were positively correlated with FRC : FRN (p < 0.05).The FRN was still very significantly negatively correlated with FRC : FRN after stumping (p < 0.01, Figures 5F–I), and the correlation was unchanged. Clearly, the correlations between leaf traits and between fine root traits differ at different stump heights.
There was a significant correlation between the leaf and fine root traits of H. rhamnoide (Figure 6, p < 0.05).SLA is negatively correlated with LTD, LDMC and LC : LN, and positively with LN. LTD is positively correlated with LDMC and LC : LN, and negatively correlated with LN. LN and LC : LN are very significantly positively correlated (p < 0.01). SLA and LN are both positively correlated with SRL and FRN and negatively correlated with FRTD and FRC : FRN. LDMC and LC : LN are positively correlated with FRTD and FRC : FRN respectively, and are both negatively correlated with SRL and RN. The correlations of the fine root traits are similar to those of the leaves. SRL is negatively correlated with FRTD and FRC : FRN, and very significantly positively with FRN (P < 0.01). Both FRTD and FRDMC are negatively correlated with FRN and positively with FRC : FRN. FRN and FRC : FRN are negatively correlated.
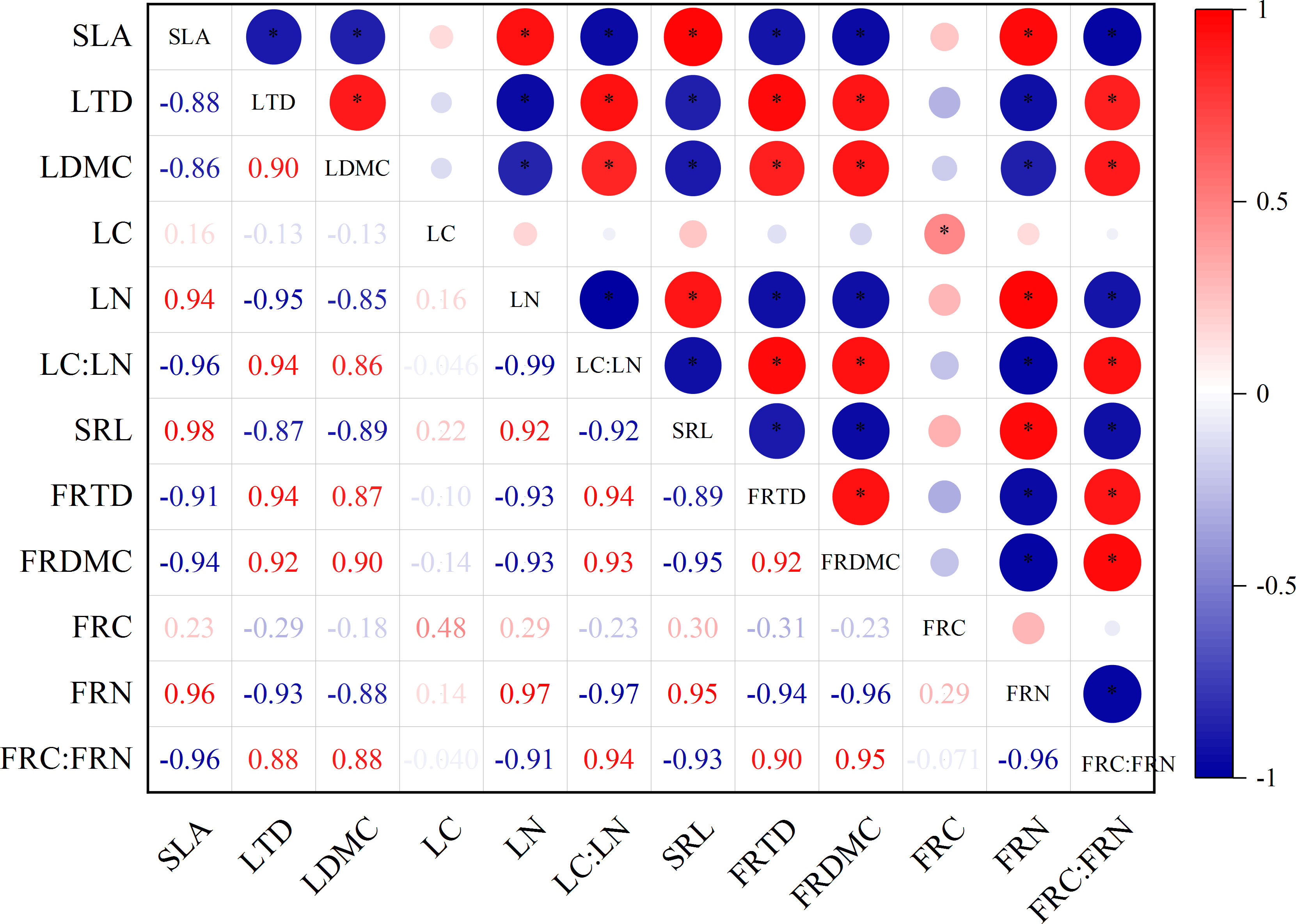
Figure 6 Correlations between leaves and fine roots. (All abbreviations are shown in (Figure 1).
The accumulative contribution rates of variance on axis 1 and axis 2 in the PCA at different stump heights were both above 80%, which can well reflect the relationship between the functional traits of leaves and fine roots. CK PC1 is a structural axis decided mainly by SLA, LN, LDMC, FRC : FRN, and LTD, and its PC2 is defined by LC, FRC, and FRTD. The closest correlations in CK were found between LC : LN and LN, between FRN and SRL, and between FRC : FRN and SLA (Figure 7E). The indices in H1, H2, H3 and H4 after stumping did not change much on the PC1 axis, but LC and FRC axis 2 in all indices and the correlations between root and leaf traits were enhanced. In particular, the associations of SLA or SRL with LN, LC : LN, FRN, and FRC : FRN were closer (Figures 7A–D). Although the confidence groups overlapped slightly at different stump heights, the root and leaf traits after different treatments aggregated and were mutually separated. The CK root and leaf traits were mainly distributed in the right half of axis 2, and the root and leaf traits of H1, H2, H3 and H4 shifted along axis 2 from right to left, and H3 was mainly distributed in the right half of PC2 (Figure 7F).
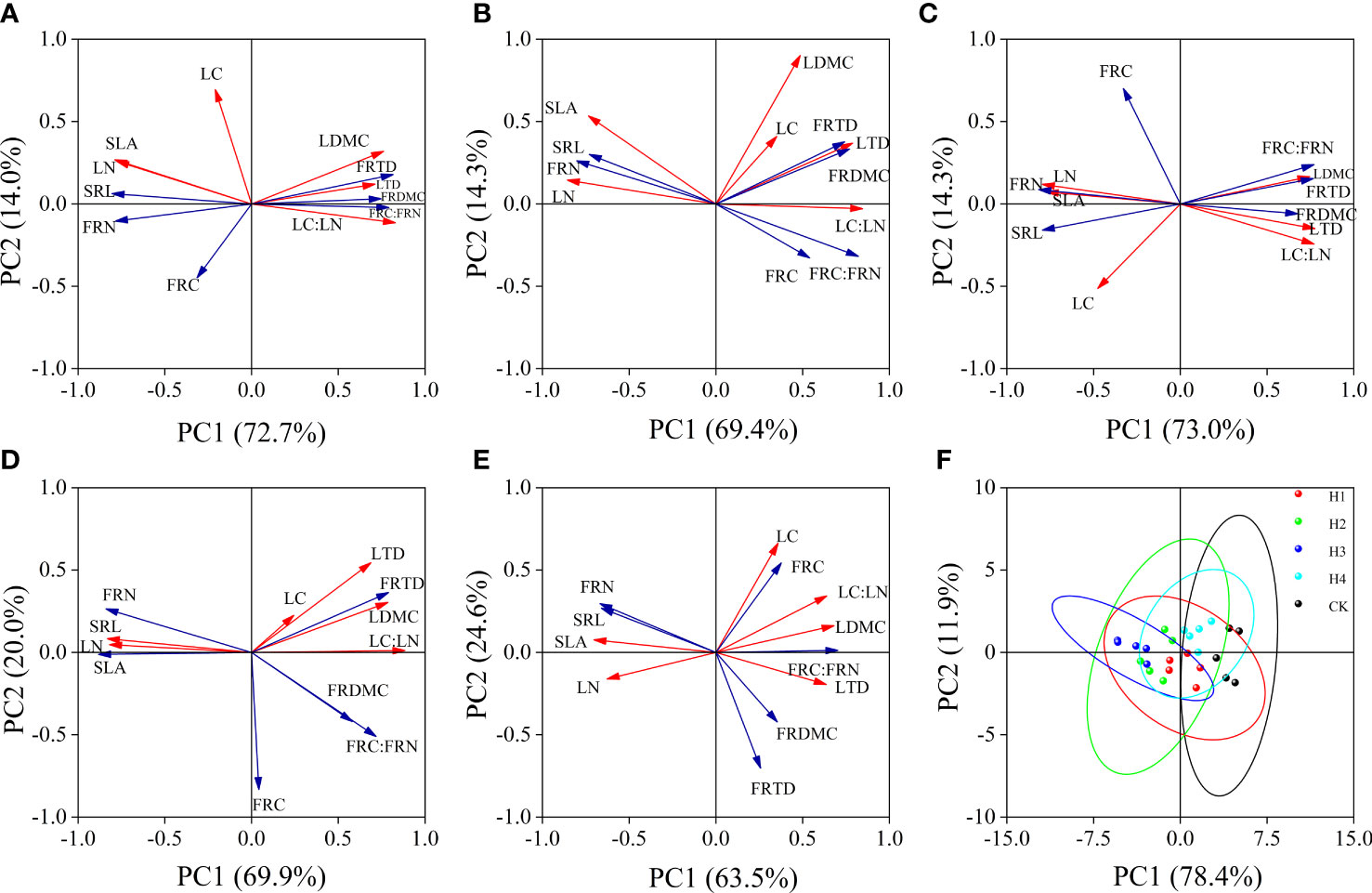
Figure 7 Coordination of root and leaf at different stump heights (A–E). Principal component analysis of leaves and fine roots at 0, 10, 15, 20 cm and without stumps respectively. (F) is the analysis of the principal component of the leaves and fine roots at different stump heights. (All abbreviations are shown in Figure 3).
The results showed that stumping significantly affected the morphological traits of both roots and leaves (Table 2). The coefficients of variation in the functional morphological traits of the roots and leaves at different stump heights fell within 3.47%–19.25%, which were all smaller than 20%. The coefficients of variation in SLA and SRL were large and very significantly different among different stump heights. These results indicate that these functional traits are very sensitive to stumping. The tissue densities and dry matter content of both roots and leaves are relatively stable variables on the resource acquisition axis, and thus the coefficients of variation are small (Figure 3).
According to leaf and root economics spectra (LES and RES respectively), SLA and SRL are two key traits reflecting the resource strategies of plants (Wright et al., 2004; Cheng et al., 2016). Specifically, the species with larger SLA had higher LN, leaf P content, faster photosynthetic rate, shorter leaf longevity, and lower LTD, which indicates the resource-acquisitive strategy. On the contrary, the species with lower SLA were conservative. The fine roots with large SRL, small diameter, low RTD and high N content are related to the low construction cost, fast respiration rate and high turnover rate. This pattern is similar to that of leaf trait correlations (Caplan et al., 2019; Reich, 2014).
The specific leaf area reflects the light receiving and capturing area per unit of leaf dry weight and indicates the abilities of plants to use environmental resources and to store the acquired resources. It is closely related to the rate of assimilation rate and the survival countermeasures of plants (Fajardo and Siefert, 2016). The specific fine root length is an important morphological structure that decides the water and nutrient absorption capacity of roots (Wang et al., 2019; Wang et al., 2020). Generally, the specific leaf area is larger in a resource-rich environment. Plants subjected to nutrient restriction or interspecific competition will increase the specific root length and root specific surface area to improve the acquisition capacity or competitiveness of nutrients (Kraft and Ackerly, 2010; Yang et al., 2014a). A decrease in tissue density or dry matter content in roots or leaves can rapidly accelerate plant growth turnover, so the loss of moisture and nutrients will decreased, thus improving the efficiency of use of moisture and nutrient and improving the defense force (Laughlin et al., 2021; Ning et al., 2022).
Our results showed that compared to CK, the SLA and SRL of H. rhamnoides were increased significantly after stumping, and LTD, LDMC, RTD, and RDMC were reduced (Figure 4), which are basically consistent with previous research. The reasons are that during compensationary recovery and growth after stumping, the resource acquisition conditions of H. rhamnoides are altered, as the cost of leaf construction is lowered. Consequently, the evaporation of vegetation decreases and the root branching ability and the soil nutrient absorption ability are enhanced, which promotes H. rhamnoides to adjust the adapting strategy to enhance its viability and to provide moisture and nutrients for the growth and metabolism of plants (Yuan et al., 2020; Liu et al., 2022), forming a growing strategy of high SLA and SRL and low LTD, LDMC, RTD and RDMC. This validates our first hypothesis. SLA, LN, SRL and LN all rank as H3 > H2 > H1 > H4 > CK. The changing rules of LTD, LDMC, LC : LN, FRTD, and FRDMC are the opposite and rank as H3 < H2 < H1 < H4 < CK (Figure 4).This compensation recovery and growth strategy was optimized at the stump height of 15 cm, above which the promoting effect was lowered.
The C element is the substrate and energy source of various physiological-biochemical processes of plants, and is the most important step to connect the external inorganic environment and organisms. C is also the basic framework of all organisms and is closely related to the photosynthesis and respiration of plants (Xu et al., 2018). N, a basic nutrient element of plants, and is an important compositional element and adjustment substance of diverse proteins and genetic materials (Cui et al., 2022). The C:N reflects the carbon assimilation ability of plants during the absorption of nutrient elements and indicates the use efficiency of nutrient elements. Generally, low C:N suggests that a plant grows fast (Hu et al., 2022).
We found that stumping significantly affected both N and C:N of leaves and roots, but leaf C contents were not significantly different among different stump heights (Table 2), and the root and leaf C contents were highly stable (Figure 3). This was because the organic carbon in the organs of plants is usually not directly involved in production activities, but acts as skeleton to provide plant activities with energy, and thus the organic carbon content in vivo is large and stable with low variation (Wang et al., 2018). Our results showed that the C : N was always larger in roots than in leaves at all stump heights, and the C : N in both leaves and roots was not significantly different among different stump heights (Figure 4), indicating the rate of N utilization is larger in roots than in leaves. The C and N contents in leaves are always larger than in roots regardless of the stump height, which is because the efficiency of C and N use of leaves is lower than that of roots. Hence, when cell division occurs rapidly due to the rapid growth of the leaves, the leaves demand largely for nutrient. Consequently, the C produced from photosynthesis gradually accumulates, and the roots transport more N to leaves, which is used in the synthesis of proteins and nucleic acids (Craine, 2006; Morales et al., 2015), so the C and N contents in the leaves of H. rhamnoides are higher.
Leaf nitrogen is a key factor for photosynthetic material metabolism and plant growth, and is an important component for the synthesis of chlorophylls and relevant photosynthetic proteins. Leaves with high N contents usually have fast photosynthetic rate (Jaikumar et al., 2013). Our results showed the leaf and root N contents after treatments H1, H2, H3, and H4 were all significantly larger compared to the CK (Figure 4). This was because the metabolism of roots was enhanced after the stumping, which promoted the nitrogen fixation ability of root nodules and improved the absorption and transportation of nitrogen nutrient elements by fine roots from the soils. As a result, the nitrogen contents in roots and leaves were significantly improved to maintain the rapid recovery and growth of plants (Yang et al., 2020; Liu et al., 2022; Liu et al., 2023). In addition, leaf area is an indicator of the photosynthesis ability of leaves. A larger leaf area is favorable for the interception of more solar light to produce organic matter (Osnas et al., 2013). Our calculations showed the leaf areas after stumping were significantly larger compared to CK. The increased leaf area and the increase nitrogen transport to leaves through roots after the stumping jointly promoted the efficiency of plant photosynthesis and accelerated the synthesis of abundant chlorophyll and photosynthetic proteins in leaves, which once again increased the leaf N content (Craine, 2006; Morales et al., 2015). This again validates our first hypothesis.
The C: N ratios in the roots or leaves after treatments H1, H2, H3, and H4 were all significantly lower than those without stumping (Figure 4), suggesting H. rhamnoides at the stump height of 15 cm can grow faster. In all, stumping can not only control the external growing morphology of plants to some extent, but can also indirectly change the internal physiological processes by adjusting the needed resources and environment, which will considerably affect plant growth.
The functional traits of plants are not mutually independent, but are coordinated or traded off to promote plant growth. Then the correlations among the leaf functional traits were compared between CK and H1, H2, H3 or H4. Commonness was found, as SLA and LN were positively correlated, and LC : LN was negatively correlated with both SLA and LN. It is speculated the coupling between the 2 above traits is the most stable among the leaf functional traits of H. rhamnoides.
The correlations of SLA with LTD and LDMC were weaker after the stumping, and the correlations between SLA and LN, and between LC : LN and LN were strengthened (Figures 5A–E). These correlations between traits are similar to the findings on leaf functional traits in accordance with the leaf economics spectrum (Wright et al., 2004; Marechaux et al., 2020), which reflects that the associations between the leaf functional traits are universal. However, we found the correlations among leaf morphological traits were weakened after the stumping, and the correlations with chemical characteristics of the tissue were enhanced. Adjusting leaf morphological structure, growing pattern and nutrient element allocation strategy after stumping will alter the correlations between traits, so the stumped plants can rapidly recover the lost aground branches, which will improve the nutrient absorbing capacity of roots and the nutrient transport ability to leaves (Yang et al., 2020; Liu et al., 2022). The morphological traits of leaves and the absorption of nutrient resource by leaves are directly and closely associated (Ackerly et al., 2002; Wright et al., 2004). This is also the reason for the increased. correlations between the leaf morphological traits and chemical tissue traits after the stumping. The change between morphological traits may be indirect after the chemical tissue traits of leaves affect a certain morphological trait. Therefore, during the compensationary recovery and growth of leaves, the correlations between the morphological traits of the leaves are weakened.
We found the correlations between fine root traits of H. rhamnoides were similar to the correlations between leaf traits, especially SRL and SLA that reflect the resource acquisition abilities of fine root and leaves respectively (Reich, 2014; Laughlin et al., 2021). The results showed that SRL was negatively correlated with RTD, and FRC : FRN, and very significantly positively correlated with FRN. FRTD and FRDMC were both positively correlated with FRN. FRN and FRC : FRN were very significantly negatively correlated (Figures 5F–J). All of these results basically consistent with previous studies. The correlations of root morphological traits after the stumping with FRN, and FRC : FRN were enhanced, which is consistent with the correlations between leaf traits after the stumping.
In a dry environment, the functional traits related to nutrition contents and resource absorption are closely coordinated between leaves and fine roots. This is because the moisture and nutrient restrictions for plant growth require that the functions of fine roots (namely, moisture and nutrient absorption) must match the functions of leaves (namely photosynthesis and transpiration) (Carvajal et al., 2019). Our root and leaf correlation analyzes prove this view: SLA and LN are both positively correlated with SRL and FRN, and negatively correlated with FRTD and FRC : FRN. LDMC and LC : LN are positively correlated with FRTD and FRC : FRN respectively, and negatively correlated with SRL and RN (Figure 6). Namely, nutrient absorption by the ground and underground organs is strongly associated. These results further prove that the aground traits and underground traits of H. rhamnoides are coordinated to some extent. Our results are consistent with previous studies on temperate grasslands (Craine et al., 2005), forests (Holdaway et al., 2011) and lawn (Liu et al., 2010; Zhao et al., 2016). This validates our second hypothesis.
According to the plant economics spectrum theory, functional traits of plants are important indices for measuring environmental resource trade-off strategies of plants (Reich et al., 2003; Freschet et al., 2010). When LTD, LDMC, RTD, RDMC, and C: N are low, SLA and SRL are large, and when the N contents in roots and leaves are large, the plants usually have a rapid photosynthetic rate and growth rate and become the ‘rapid investment–return’ type; otherwise, they approach the ‘slow investment –return’ type (Jiang et al., 2021; Pérez-Ramos et al., 2012). Compared with CK (Figure 7E), the LTD, LDMC, FRTD, FRDMC, LC: LN, and FRC : FRN significantly decreased, SLA, and SRL significantly rose, and LN and FRN were large after the stumping (Figures 4, 7A–D). It is indicated H. rhamnoides in an environment with limited resources is indicated to optimally allocate resources between the functional traits of leaves and roots by using a trade-off strategy. Compared with CK, the root and leaf economics spectra of stumped H. rhamnoides are closer to one end of ‘rapid investment–return type’ species (Figure 7F), which indicates the shift to the resource trade-off strategy of ‘rapid investment–return type’. This validates our third hypothesis. Our findings are significant for the prevention and control of revegetation and soil erosion in feldspathic sandstone areas.
The variation and coordination between the traits of the roots and leaves were analyzed at different stump heights. The results show that the leaf traits of H. rhamnoides at different stump heights agree with the global prediction of LES, and are in parallel with the root-trait syndrome. The H. rhamnoides after stumping has a high SLA, SRL, LN, and FRN, and low LTD, LDMC, LC : LN, FRTD, FRDMC, and FRC : FRN. It is indicated that H. rhamnoides in an environment with limited resources can optimally allocate resources between functional traits of leaves and roots using a trade-off strategy, and thus H. rhamnoides shifts to the resource trade-off strategy of ‘rapid investment-return type’. The coordination between the leaves and the fine roots is stronger in terms of the chemical tissue traits compared to the morphological traits. Generally, stumped H. rhamnoides can grow faster compared to unstumped shrubs, and the optimal stump height is 15 cm. Thus, to improve the decaying of H. rhamnoides forests in feldspathic sandstone areas, shrubs can be stumped at a height of 15 cm.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
LL: Conceptualization, Methodology, Writing–original Draft, Visualization, Data curation, Software, Investigation, Formal analysis. YG: Conceptualization, Methodology, Writing –original Draft, Writing–review and editing, Supervision, Project administration. XL: Conceptualization, Methodology, Writing–original Draft, Data curation, Software, Investigation, Formal analysis. YY: Conceptualization, Writing–original Draft, Writing–review and editing, Supervision, Project administration. WQ: Conceptualization, Writing–original Draft, Visualization, Investigation, Formal analysis. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (31960329); the Autonomous Region Application Technology Research and Development Fund Program (2021GG0085); the Natural Science Foundation of Inner Mongolian Autonomous Region (2022MS03029); the Inner Mongolian Autonomous Region Directly Affiliated Universities Basic Scientific Research Operating Expenses Project (BR22-15-01); the Ordos Science and Technology Cooperation Key Project (2021EEDSCXQDFZ011); the Inner Mongolian Ordos Application the Research and Technology Development Project (2021YY SHE 106-55); and the Autonomous Region Application Technology Research and Development Fund Program (2019GG004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ackerly, D. D., Knight, C. A., Weiss, S. B., Barton, K., Starmer, K. P. (2002). Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: Contrasting patterns in species level and community level analyses. Oecologia 130, 449–457. doi: 10.1007/s004420100805
Bardgett, R. D., Mommer, L., Vries, F. T. (2014). Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 29, 692–699. doi: 10.1016/j.tree.2014.10.006
Cao, Z. L., Li, T. J., Li, G. Q., Liu, C. H., Gao, H. Y., Dai, G. H., et al. (2016). Modular growth and clonal propagation of Hippophae rhamnoides subsp sinensis in response to irrigation intensity. J. Forestry Res. 27, 1019–1028. doi: 10.1007/s11676-016-0236-z
Caplan, J. S., Meiners, S. J., Flores-Moreno, H., McCormack, M. L. (2019). Fine-root traits are linked to species dynamics in a successional plant community. Ecology 100, e02588. doi: 10.1002/ecy.2588
Carvajal, D. E., Loayza, A. P., Rios, R. S., Delpiano, C. A., Squeo, F. A. (2019). A hyper-arid environment shapes an inverse pattern of the fast–slow plant economics spectrum for above-, but not below-ground resource acquisition strategies. J. Ecol. 107, 1079–1092. doi: 10.1111/1365-2745.13092
Cheng, J., Chu, P., Chen, D., Bai, Y. (2016). Functional correlations between specific leaf area and specific root length along a regional environmental gradient in inner Mongolia grasslands. Funct. Ecol. 30, 985–997. doi: 10.1111/1365-2435.12569
Cornelissen, J. H. C., Lavorel, S., Garnier, E., Díaz, S., Buchmann, N., Gurvich, D. E., et al. (2003). A handbook of protocols for standardized and easy measurement of plant functional traits worldwide. Aust. J. Bot. 51, 335–380. doi: 10.1071/BT02124
Craine, J. M. (2006). Competition for nutrients and optimal root allocation. Plant Soil 285, 171–185. doi: 10.1007/s11104-006-9002-x
Craine, J. M., Lee, W. G., Bond, W. J., Williams, R. J., Johnson, L. C. (2005). Environmental constraints on a global relationship among leaf and root traits of grasses. Ecology 86, 12–19. doi: 10.1890/04-1075
Cui, E. Q., Lu, R. L., Xu, X. N., Sun, H. F., Qiao, Y., Ping, J. Y., et al. (2022). Soil phosphorus drives plant trait variations in a mature subtropical forest. Global Change Biol. 28, 3310–3320. doi: 10.1111/gcb.16148
Cui, Z., Wu, G. L., Huang, Z., Liu, Y. (2019). Fine roots determine soil infiltration potential than soil water content in semi-arid grassland soils. J. Hydrol. 578, 124023. doi: 10.1016/j.jhydrol.2019.124023
Dyer, A. R., Goldberg, D. E., Turkington, R., Sayre, C. (2001). Effects of growing conditions and source habitat on plant traits and functional group definition. Funct. Ecol. 15, 85–95. doi: 10.1046/j.1365-2435.2001.00487.x
Fajardo, A., Siefert, A. (2016). Phenological variation of leaf functional traits within species. Oecologia 180, 951–959. doi: 10.1007/s00442-016-3545-1
Freschet, G. T., Cornelissen, J. H. C., van Logtestijn, R. S. P., Aerts, R. A. F. (2010). Evidence of the ‘plant economics spectrum’ in a subarctic flora. J. Ecol. 98, 362–373. doi: 10.1111/j.1365-2745.2009.01615.x
Geng, Y., Wang, L., Jin, D., Liu, H., He, J. S. (2014). Alpine climate alters the relationship between leaf and root morphological traits but not chemical traits. Oecologia 175, 445–455. doi: 10.1007/s00442-014-2919-5
Giambalvo, D., Amato, G., Stringi, L. (2011). Effects of stubble height and cutting frequency on regrowth of berseem clover in a Mediterranean semiarid environment. Crop Sci. 51, 1808–1814. doi: 10.2135/cropsci2010.05.0271
He, J. S., Wang, L., Flynn, D. F. B., Wang, X., Ma, W., Fang, J. (2008). Leaf nitrogen: Phosphorus stoichiometry across Chinese grassland biomes. Oecologia 155, 301–310. doi: 10.1007/s00442-007-0912-y
Holdaway, R. J., Richardson, S. J., Dickie, I. A., Peltzer, D. A., Coomes, D. A. (2011). Species- and community-level patterns in fine root traits along a 120000-year soil chronosequence in temperate rain forest. J. Ecol. 99, 954–963. doi: 10.1111/j.1365-2745.2011.01821.x
Hosseini, A., Hosseini, S. M., Linares, J. C. (2019). Linking morphological and ecophysiological leaf traits to canopy dieback in Persian oak trees from central zagros. J. For. Res. 30, 1755–1764. doi: 10.1007/s11676-018-0805-4
Hu, M. Y., Ma, Z. L., Chen, H. Y. H. (2022). Intensive plantations decouple fine root C:N:P in subtropical forests. For. Ecol. Manage. 505, 119901. doi: 10.1016/j.foreco.2021.119901
Jaikumar, N. S., Snapp, S. S., Sharkey, T. D. (2013). Life history and resource acquisition: photosynthetic traits in selected accessions of three perennial cereal species compared with annual wheat and rye. Am. J. Bot. 100, 2468–2477. doi: 10.3732/ajb.1300122
Jiang, X., Jia, X., Gao, S., Jiang, Y., Wei, N., Han, C., et al. (2021). Plant nutrient contents rather than physical traits are coordinated between leaves and roots in a desert shrubland. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.734775
Kong, D., Ma, C., Zhang, Q., Li, L., Chen, X., Zeng, H., et al. (2014). Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 203, 863–872. doi: 10.1111/nph.12842
Kraft, N. J. B., Ackerly, D. D. (2010). Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecol. Monogr. 80, 401–422. doi: 10.1890/09-1672.1
Langworthy, A. D., Rawnsley, R. P., Freeman, M. J., Corkrey, R., Harrison, M. T., Pembleton, K. G., et al. (2019). Effect of stubble-height management on crown temperature of perennial ryegrass, tall fescue and chicory. Crop Pasture Sci. 70, 183–194. doi: 10.1071/CP18313
Laughlin, D. C., Mommer, L., Sabatini, F. M., Bruelheide, H., Kuyper, T. W., Mccormack, M. L., et al. (2021). Root traits explain plant species distributions along climatic gradients yet challenge the nature of ecological trade-offs. Nat. Ecol. Evol. 5, 1–12. doi: 10.1038/s41559-021-01471-7
Liu, G., Freschet, G. T., Pan, X., Cornelissen, J. H. C., Li, Y., Dong, M. (2010). Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytol. 188, 543–553. doi: 10.1111/j.1469-8137.2010.03388.x
Liu, L., Guo, Y. F., Liu, X. Y., Yao, Y. F., Qi, W. (2022). Stump height after regenerative cutting of sea-buckthorn (Hippophae rhamnoides) affects fine root architecture and rhizosphere soil stoichiometric properties. Rhizosphere 24, 100602. doi: 10.1016/j.rhisph.2022.100602
Liu, L., Guo, Y.F., Liu, X.Y., Yao, Y.F., Qi, W. (2023). Relationship between the roots of Hippophae rhamnoides at different stump heights and the root microenvironment in feldspathic sandstone areas. Peerj 11, e14819. doi: 10.7717/peerj.14819
Marechaux, I., Saint-Andre, L., Bartlett, M. K., Sack, L., Chave, J. (2020). Leaf drought tolerance cannot be inferred from classic leaf traits in a tropical rainforest. J. Ecol. 108, 1030–1045. doi: 10.1111/1365-2745.13321
McCormack, M. L., Dickie, I. A., Eissenstat, D. M., Fahey, T. J., Fernandez, C. W., Guo, D., et al. (2015). Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 207, 505–518. doi: 10.1111/nph.13363
Mitchell, R. M., Wright, J. P., Ames, G. M. (2017). Intraspecific variability improves environmental matching, but does not increase ecological breadth along a wet-to-dry ecotone. Oikos 126, 988–995. doi: 10.1111/oik.04001
Morales, J., Squeo, F. A., Tracol, Y., Armas, C., Gutiérrez, J. R. (2015). Resource economics and coordination among above-and below-ground functional traits of three dominant shrubs from the Chilean coastal desert. J. Plant Ecol. 8, 70–78. doi: 10.1093/jpe/rtu010
Ning, Z., Li, Y., Zhao, X., Han, D., Zhan, J. (2022). Comparison of leaf and fine root traits between annuals and perennials, implicating the mechanism of species changes in desertified grasslands. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.778547
Osnas, J. L. D., Lichstein, J. W., Reich, P. B., Pacala, S. W. (2013). Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science 340, 741–744. doi: 10.1126/science.1231574
Pérez-Ramos, I. M., Roumet, C., Cruz, P., Blanchard, A., Autran, P., Garnier, E. (2012). Evidence for a ‘plant community economics spectrum’ driven by nutrient and water limitations in a Mediterranean rangeland of southern France. J. Ecol. 100, 1315–1327. doi: 10.1111/1365-2745.12000
Reich, P. B. (2014). The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 102, 275–301. doi: 10.1111/1365-2745.12211
Reich, P. B., Ellsworth, D. S., Walters, M. B., Vose, J. M., Gresham, C., Volin, J. C., et al. (1999). Generality of leaf trait relationships: A test across six biomes. Ecology 80, 1955–1969. doi: 10.2307/176671
Reich, P., Wright, I. J., Cavender-Bares, J., Craine, J. M., Oleksyn, J., Westoby, M., et al. (2003). The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 164, S143–S164. doi: 10.1086/374368
Sun, T., Hobbie, S. E., Berg, B., Zhang, H., Wang, Q., Wang, Z., et al. (2018). Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. P Natl. Acad. Sci. U.S.A. 115, 10392–10397. doi: 10.1073/pnas.1716595115
Wang, Y. N., Gao, G. Q., Wang, N., Wang, Z. Q., Gu, J. C. (2019). Effects of morphology and stand structure on root biomass and length differed between absorptive and transport roots in temperate trees. Plant Soil. 442, 355–367. doi: 10.1007/s11104-019-04206-7
Wang, G., Lada, H., Jia, J., Miklavcic, S. J., Srivastava, A. (2020). Statistical analysis and modeling of the geometry and topology of plant roots. J. Theor. Biol. 486, 110108. doi: 10.1016/j.jtbi.2019.110108
Wang, R., Wang, Q., Zhao, N., Xu, Z., Zhu, X., Jiao, C., et al. (2018). Different phylogenetic and environmental controls of first-order root morphological and nutrient traits: evidence of multidimensional root traits. Funct. Ecol. 32, 29–39. doi: 10.1111/1365-2435.12983
Weemstra, M., Mommer, L., Visser, E. J. W., van Ruijven, J., Kuyper, T. W., Mohren, G. M. J., et al. (2016). Towards a multidimensional root trait framework: a tree root review. New Phytol. 211, 1159–1169. doi: 10.1111/nph.14003
Wright, I. J., Reich, P. B., Westoby, M., Ackerly, D. D., Baruch, Z., Bongers, F., et al. (2004). The worldwide leaf economics spectrum. Nature 428, 821–827. doi: 10.1038/nature02403
Xia, M., Valverde-Barrantes, O. J., Suseela, V., Blackwood, C. B., Tharayil, N. (2021). Coordination between compound-specific chemistry and morphology in plant roots aligns with ancestral mycorrhizal association in woody angiosperms. New Phytol. 232, 1259–1271. doi: 10.1111/nph.17561
Xu, B., Xu, W., Wang, Z. (2018). Accumulation of n and p in the legume Lespedeza davurica in controlled mixtures with the grass Bothriochloa ischaemum under varying water and fertilization conditions. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00165
Yang, F. S., Bi, C. F., Cao, M. M., Li, H. E., Wang, X. H., Wu, W. (2014b). Simulation of sediment retention effects of the double seabuckthorn plant flexible dams in the pisha sandstone area of China. Ecol. Eng. 71, 21–31. doi: 10.1016/j.ecoleng.2014.07.050
Yang, J., Ci, X. Q., Lu, M. M., Zhang, G. C., Cao, M., Li, J., et al. (2014a). Functional traits of tree species with phylogenetic signal co-vary with environmental niches in two large forest dynamics plots. J. Plant Ecol. 7, 11–125. doi: 10.1093/jpe/rtt070
Yang, Z. P., Minggagud, H., Baoyin, T. G. T., Li, F. Y. H. (2020). Plant production decreases whereas nutrients concentration increases in response to the decrease of mowing stubble height. J. Environ. Manage. 253, 109745. doi: 10.1016/j.jenvman.2019.109745
Yuan, J. H., Li, H. Y., Yang, Y. F. (2020). The compensatory tillering in the forage grass Hordeum brevisubulatum after simulated grazing of different severity. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00792
Keywords: leaves, fine root, plant economics spectrum, stump height, conservation
Citation: Liu L, Guo Y, Liu X, Yao Y and Qi W (2023) Coordinated variation in root and leaf functional traits of Hippophae rhamnoides treated at different stump heights in feldspathic sandstone areas of Inner Mongolia. Front. Plant Sci. 14:1104632. doi: 10.3389/fpls.2023.1104632
Received: 23 November 2022; Accepted: 03 February 2023;
Published: 14 February 2023.
Edited by:
Kaixiong Xing, Hainan Normal University, ChinaReviewed by:
Lin Zhang, Institute of Tibetan Plateau Research (CAS), ChinaCopyright © 2023 Liu, Guo, Liu, Yao and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuefeng Guo, c2hhbW9sQGVtYWlscy5pbWF1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.