- Guangdong Key Laboratory of Plant Adaptation and Molecular Design, Guangzhou Key Laboratory of Crop Gene Editing, Innovative Center of Molecular Genetics and Evolution, School of Life Sciences, Guangzhou University, Guangzhou Higher Education Mega Center, Guangzhou, China
Nutrition affects plant growth and development, including flowering. Flowering represents the transition from the vegetative period to the reproduction period and requires the consumption of nutrients. Moreover, nutrients (e.g., nitrate) act as signals that affect flowering. Regulation of flowering time is therefore intimately associated with both nutrient-use efficiency and crop yield. Here, we review current knowledge of the relationships between nutrients (primarily nitrogen, phosphorus, and potassium) and flowering, with the goal of deepening our understanding of how plant nutrition affects flowering.
1. Introduction
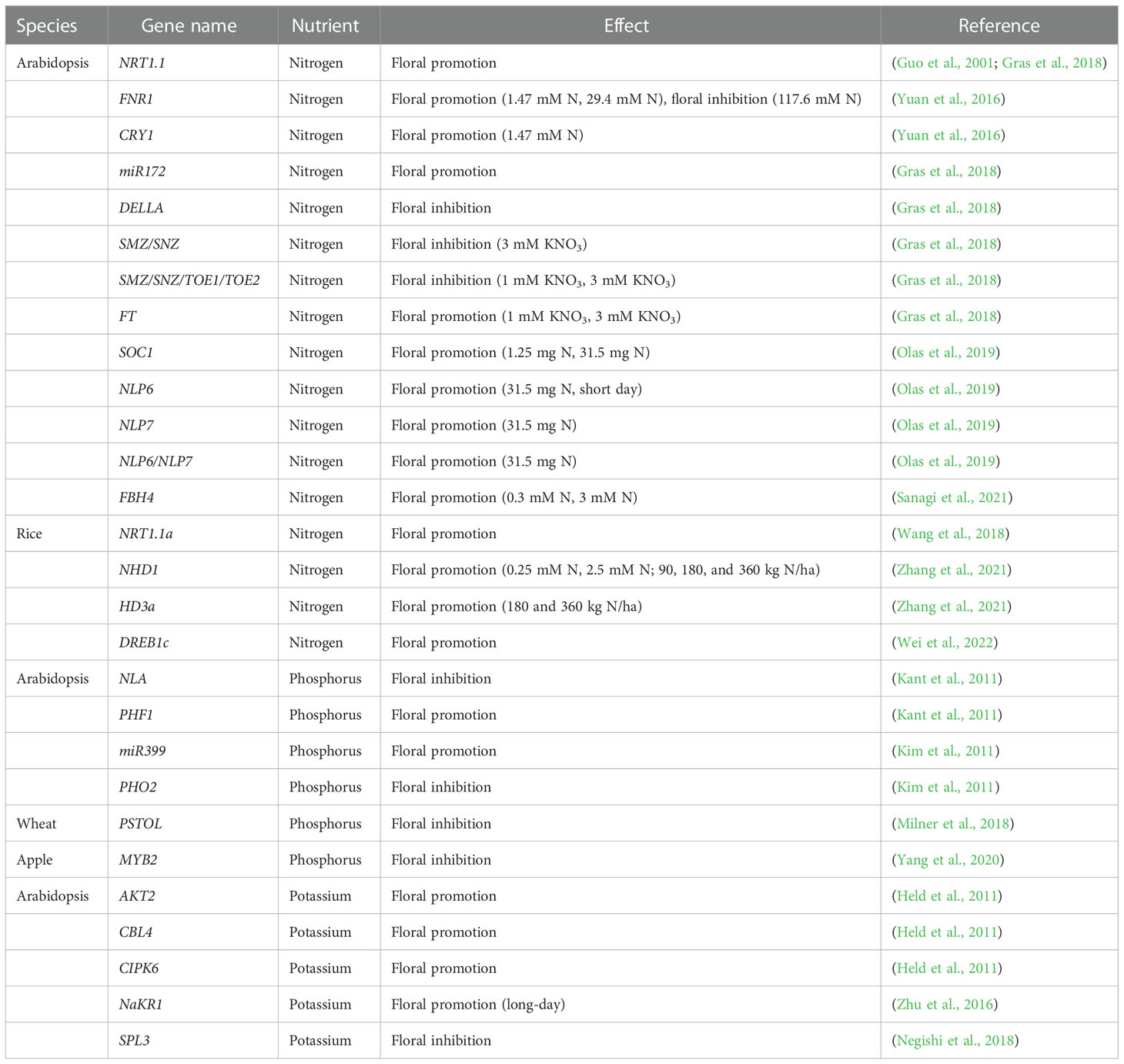
Plants must acquire at least 14 essential mineral elements for their growth, development, structure, physiology, and reproduction; these comprise both macronutrients and micronutrients (Epstein, 2005). Nutrient availability is tightly linked to flowering time, which requires resources for producing and sustaining sink tissues for reproduction (Sanagi et al., 2021). Deficiency or excess of nutrients results in a stress response that affects flowering time (Tanaka, 1986; Miyazaki et al., 2014; Sanagi et al., 2021). Flowering time control integrates external environmental factors (daylength, temperature, light, stress, and nutritional status) and endogenous signals from the plant itself (Kobayashi and Weigel, 2007; Ahmad et al., 2022; Khosa, 2022). The mechanisms by which several environmental factors (daylength, temperature, and stress) alter flowering time have been well characterized and reviewed (Cho et al., 2017; Fernández-Calleja et al., 2021; Freytes et al., 2021; Lin et al., 2021; Luo et al., 2021; Osnato et al., 2022; Preston and Fjellheim, 2022; Shi et al., 2022); however, there are fewer reports on how nutrients affect flowering time. In this review, we summarize what is known about the interactions between nutrients [primarily nitrogen (N), phosphorus (P), and potassium (K)] and flowering time. Genes previously reported to be involved in nutrient-mediated modulation of flowering time are summarized in Table 1.
2. Nutrients and flowering time
2.1. Nitrogen
Nitrogen (N) is the most important macronutrient for plant growth, needed for proper root morphology, shoot growth, stomatal opening, flowering, yield, and senescence (Bernier et al., 1993; Crawford, 1995; Marschner and Marschner, 1995; Leng et al., 2020; Sakuraba, 2022; Wei et al., 2022; Zinta et al., 2022). The influence of N on flowering time in Arabidopsis and rice can be visualized as a U-shaped trend (Lin and Tsay, 2017; Gras et al., 2018; Zhang et al., 2021), with both deficiency and sufficiency of N postponing flowering time.
The first evidence that nitrate is involved in the regulation of flowering time in Arabidopsis was obtained from genetic studies showing that nia1nia2 mutants flower later than their wild-type controls (Tocquin et al., 2003; Seligman et al., 2008). Nitrate regulates the expression of flowering-related genes at the shoot apical meristem (SAM) to modulate flowering time; these genes include SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1), CONSTANS (CO), FLOWERING LOCUS C (FLC), LEAFY (LFY), APETALA1 (AP1), and FLOWERING LOCUS T (FT) (Kant et al., 2011; Gras et al., 2018). NIN-LIKE PROTEIN6 (NLP6) and NLP7 function in the regulation of signaling by binding to the promoter of SOC1-LIKE3 (SPL3) and SPL5 in the Arabidopsis SAM (Konishi and Yanagisawa, 2013; Guan et al., 2017; Olas et al., 2019). Nitrate greatly affects the vegetative growth of plants, so defining whether it has a direct influence on flowering is difficult (Hirel et al., 2007; Castro Marin et al., 2011). To separate the effects of nitrate on growth and flowering, a growth system using glutamine supplementation was established; low nitrate was still found to accelerate flowering in late-flowering Arabidopsis mutants with impaired photoperiod, temperature, GA, and autonomous flowering pathways (Castro Marin et al., 2011; Weber and Burow, 2018), suggesting that regulates flowering time independently of the autonomous, light, and gibberellin pathways in Arabidopsis (Castro Marin et al., 2011). Moreover, delayed flowering time in co, ft, fd (FLOWERING LOCUS D), and tsf (TWIN SISTER OF FT) mutants as well as plants overexpressing microRNA156 (miR156) under low-N conditions proves that -dependent flowering is indeed independent of age and photoperiod pathways (Gras et al., 2018; Olas et al., 2019). These observations indicate that -dependent flowering pathways are dependent on the concentration and source of nitrate used (KNO3, NH4NO3, mixed, or supplemented with glutamine) (Gras et al., 2018; Fredes et al., 2019).
Protein phosphorylation is important for the transmission of information regarding N availability (Ho et al., 2009; Menz et al., 2016; Liu et al., 2017; Fredes et al., 2019; Liu et al., 2020). Notably, Sanagi et al. (2021) reported that the phosphorylation state of FLOWERING BHLH4 (FBH4) is altered by changes in N conditions, clarifying a link between N availability and the regulation of flowering. The kinase activity of SNF1-RELATED KINASE1 (SnRK1) is inhibited under low-N concentrations, resulting in a decrease in the phosphorylation of its direct target, FBH4 (Sanagi et al., 2021). This in turn promotes nuclear localization of FBH4, increasing the transcription of the flowering time genes CO and FT (Sanagi et al., 2021).
Delaying flowering by applying N fertilizers to crops is common in agricultural production, but the underlying molecular mechanism of this delay is largely unclear. High N delays flowering by improving transcription levels of SCHNARCHZAPFEN (SNZ) and SCHLAFMUTZE (SMZ), which directly bind to the promoter of FT (Mathieu et al., 2009; Gras et al., 2018). Mutants lacking ferredoxin–NADP oxidoreductase (FNR1) flower later than wild-type plants, and fnr1 and cryptochrome1 (cry1) mutants display insensitivity to different levels of N at flowering time (Yuan et al., 2016). FNR1 is inhibited at high N levels, leading to upregulation of CRY1 and degradation of FNR1 in the nucleus, thereby reducing transcript levels of central circadian clock genes [e.g., TOC1 (TIMING OF CAB EXPRESSION1), CCA1 (CIRCADIAN CLOCK-ASSOCIATED1), and LHY (LATE ELONGATED HYPOCOTYL)] and some flowering-output genes [e.g., GIGANTEA (GI) and CO] to disturb the flowering process (Pathak et al., 2013; Yuan et al., 2016). N signaling therefore alters the abundance of CRY1 protein and is also involved in the pathway of the central circadian clock, which regulates flowering.
Breeding programs often aim to develop cultivars that tolerate high N inputs without delayed flowering. OsNRT1.1A increases N utilization without delaying flowering, offering potential for the development of crops with the combined traits of early maturation and high yield (Wang et al., 2018). Rice, a short-day plant, has special flowering pathways besides the conserved flowering genes shared with long-day plants, such as Arabidopsis (Shoko et al., 2002; Doi et al., 2004). Arabidopsis and other upland plants prefer to absorb N as nitrate, while paddy rice prefers ammonium (Li et al., 2008); hence, these species use different mechanisms for regulating flowering time in response to N forms and concentration. (Zhang et al., 2021) showed that N-mediated heading date1 (Nhd1), which shares a high sequence similarity with CCA1 and LHY, increases the levels of florigen Hd3a to control flowering time but downregulates Fd-GOGAT for N assimilation in rice. Recent studies have shown that transcription of OsDREB1C (dehydration-responsive element binding) is induced by light and low N, suggesting a function in N-use efficiency and flowering time (Wei et al., 2022). Plants lacking this gene display delayed flowering under long-day conditions because OsDREB1C binds to the exons of OsFTL1, activating its transcription (Wei et al., 2022). As orthologs of Nhd1 or DREB exist in other crops, the functions of Nhd1 and DREB in the regulation of flowering and N-use efficiency should be useful in other crops (Wei et al., 2022).
2.2. Phosphorus
Plants obtain phosphorus (P) mainly in the form of inorganic phosphate (Pi) (Marschner and Marschner, 1995). Low Pi availability generally delays flowering time in annual plants (Marschner and Marschner, 1995; Nord and Lynch, 2008); for example, the nla (NITROGEN LIMITATION ADAPTATION) mutant accumulates high levels of Pi and flowers significantly earlier than the wild type, while phf1 (PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1) mutant with lower Pi accumulation flowers later than the wild type (Kant et al., 2011). MiR399 and PHOSPHATE2 (PHO2) are known to play a role in the maintenance of Pi homeostasis, and miR399b-overexpressing plants and pho2 mutant accumulate high levels of Pi and exhibit early flowering phenotype via upregulation of TSF (Chiou et al., 2006; Kim et al., 2011). Moreover, the overexpression of TaPSTOL in transgenic wheat showed a significantly lower physiological P use efficiency and the P efficiency ratio than the wild type and were significantly later in flowering than the wild type, which revealing that P has a significant effect on flowering time (Milner et al., 2018). Transgenic expression of the Pi-responsive gene MdMYB2 postpones flowering in Arabidopsis by decreasing the transcription of flowering genes (CO, SOC1, LFY, and FT) (Yang et al., 2020). No further data are available regarding the molecular mechanisms by which Pi availability affects flowering in crops such as rice, maize (Zea mays), and soybean (Glycine max); thus, further work is required to understand these processes in crop plants.
Phenological delay under low-Pi conditions is beneficial because it gives plants more time for P absorption (Nord and Lynch, 2008). By contrast, plants generally flower early to complete their life cycles more quickly under low-N conditions (Kant et al., 2011). Notably, crosstalk between N and P shows their accumulated influence on flowering (Kant et al., 2011; Yang et al., 2020). MiR827 and NLA regulate Pi homeostasis in a N-dependent manner (Kant et al., 2011), and availability of both N and Pi affects flowering time by altering the expression of FLC, CO, FT, LFY, AP1, and some downstream genes of miR156–SPL (Kim et al., 2011; Vidal et al., 2014; Lei et al., 2016). The mechanisms by which N and P affect flowering time are worthy of further study, especially in crops.
2.3. Potassium
The concentration of potassium ions (K+) in plant cells can be as high as 100 mM, but the concentration in soil is only 100–1000 μM (Leigh and Jones, 1984; Wang and Wu, 2015; Wang et al., 2021). Absorption of K+ through roots requires K+ channels and transporters (Wang et al., 2021). In Arabidopsis, loss of AKT2/3 (Arabidopsis K+ channel) changes the flowering time phenotype, implying that, similar to N and P, K+ availability also affects flowering time (Held et al., 2011); however, the molecular mechanisms involved are not clear. Additional studies report that akt2, cbl4 (calcineurin B-like proteins), and cipk6 exhibit analogous late flowering under short-day treatment (Held et al., 2011), indicating that regulation of ion channels involved in the flowering pathway and the activity of K+ channels are modulated via a Ca2+ sensor kinase (Held et al., 2011). (Negishi et al., 2018) found that mutant plants lacking SODIUM POTASSIUM ROOT DEFECTIVE1 (NaKR1) over-accumulate Na+ and K+ and display late flowering. NaKR1 participates in the phloem transport of FT protein (Zhu et al., 2016) and increases the transcription level of FT under long-day treatment, dependent on K+ concentration. NaKR1 therefore regulates not only florigen production, but also transport (Negishi et al., 2018). It is thus clear that the miR156–SPL module might respond to N, P, and K, which deserves more in-depth study.
3. Perspective
Besides N, P, and K, there are at least 11 essential nutrients required by plants. Whether and how these nutrients affect flowering time require further exploration. (Yuan et al., 2016) suggested that FNR1 promotes flowering in response to N, and the level of FNR1 is also induced by sufficient iron (Fe) and sulfur (S) supply. Therefore, Fe and S may also regulate plant flowering time through FNR1. Recent studies have mainly focused on the absorption, translocation, and reuse of nutrients (Verma et al., 2021; Wani et al., 2021; Johnson et al., 2022; Lambers, 2022; Liu et al., 2022a; Liu et al., 2022b; Podar and Maathuis, 2022; Prathap et al., 2022; Ren et al., 2022; Vélez-Bermúdez and Schmidt, 2022; Xie et al., 2022), meaning that the potential mechanisms underlying nutrient-regulated flowering remain largely unknown in plants, especially crops. UV stress, drought, salt, cold, and heat also alter flowering (Martínez et al., 2004; Cho et al., 2017; Ionescu et al., 2017; Shim and Jang, 2020; Preston and Fjellheim, 2022). Improving nutrient-use efficiency by coordinating flowering time is an effective way to increase crop yield; thus, the effects of interaction between flowering time and nutrients on crop yield are in need of more in-depth study.
Author contributions
YZ and LC wrote this mini review, BL revised the mini review, FK and LC conceived the review. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (32001502, 31901499), and also supported by the China Postdoctoral Science Foundation (2019M652839, 2020M682655).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, S., Peng, D., Zhou, Y., Zhao, K. (2022). The genetic and hormonal inducers of continuous flowering in orchids: An emerging view. Cells 11 (4), 657. doi: 10.3390/cells11040657
Bernier, G., Havelange, A., Houssa, C., Petitjean, A., Lejeune, P. (1993). Physiological signals that induce flowering. Plant Cell 5 (10), 1147–1155. doi: 10.1105/tpc.5.10.1147
Castro Marin, I., Loef, I., Bartetzko, L., Searle, I., Coupland, G., Stitt, M., et al. (2011). Nitrate regulates floral induction in arabidopsis, acting independently of light, gibberellin and autonomous pathways. Planta 233 (3), 539–552. doi: 10.1007/s00425-010-1316-5
Chiou, T. J., Aung, K., Lin, S. I., Wu, C. C., Chiang, S. F., Su, C. L. (2006). Regulation of phosphate homeostasis by MicroRNA in arabidopsis. Plant Cell 18 (2), 412–421. doi: 10.1105/tpc.105.038943
Cho, L. H., Yoon, J., An, G. (2017). The control of flowering time by environmental factors. Plant J. 90 (4), 708–719. doi: 10.1111/tpj.13461
Crawford, N. M. (1995). Nitrate: nutrient and signal for plant growth. Plant Cell 7 (7), 859–868. doi: 10.1105/tpc.7.7.859
Doi, K., Izawa, T., Fuse, T., Yamanouchi, U., Kubo, T., Shimatani, Z., et al. (2004). Ehd1, a b-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev. 18 (8), 926–936. doi: 10.1101/gad.1189604
Epstein, E. (2005). Mineral nutrition of plants: principles and perspectives. 2nd ed. Ed. Bloom, A. J. (Sunderland, MassSinauer Associates, Inc).
Fernández-Calleja, M., Casas, A. M., Igartua, E. (2021). Major flowering time genes of barley: allelic diversity, effects, and comparison with wheat. Theor. Appl. Genet. 134 (7), 1867–1897. doi: 10.1007/s00122-021-03824-z
Fredes, I., Moreno, S., Díaz, F. P., Gutiérrez, R. A. (2019). Nitrate signaling and the control of arabidopsis growth and development. Curr. Opin. Plant Biol. 47, 112–118. doi: 10.1016/j.pbi.2018.10.004
Freytes, S. N., Canelo, M., Cerdán, P. D. (2021). Regulation of flowering time: When and where? Curr. Opin. Plant Biol. 63, 102049. doi: 10.1016/j.pbi.2021.102049
Gras, D. E., Vidal, E. A., Undurraga, S. F., Riveras, E., Moreno, S., Dominguez-Figueroa, J., et al. (2018). SMZ/SNZ and gibberellin signaling are required for nitrate-elicited delay of flowering time in Arabidopsis thaliana. J. Exp. Bot. 69 (3), 619–631. doi: 10.1093/jxb/erx423
Guan, P., Ripoll, J. J., Wang, R., Vuong, L., Bailey-Steinitz, L. J., Ye, D., et al. (2017). Interacting TCP and NLP transcription factors control plant responses to nitrate availability. Proc. Natl. Acad. Sci. U.S.A. 114 (9), 2419–2424. doi: 10.1073/pnas.1615676114
Guo, F. Q., Wang, R., Chen, M., Crawford, N. M. (2001). The arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is activated and functions in nascent organ development during vegetative and reproductive growth. Plant Cell 13 (8), 1761–1777. doi: 10.11054/tpc.010126
Held, K., Pascaud, F., Eckert, C., Gajdanowicz, P., Hashimoto, K., Corratgé-Faillie, C., et al. (2011). Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 21 (7), 1116–1130. doi: 10.1038/cr.2011.50
Hirel, B., Le Gouis, J., Ney, B., Gallais, A. (2007). The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 58 (9), 2369–2387. doi: 10.1093/jxb/erm097
Ho, C. H., Lin, S. H., Hu, H. C., Tsay, Y. F. (2009). CHL1 functions as a nitrate sensor in plants. Cell 138 (6), 1184–1194. doi: 10.1016/j.cell.2009.07.004
Ionescu, I. A., Møller, B. L., Sánchez-Pérez, R. (2017). Chemical control of flowering time. J. Exp. Bot. 68 (3), 369–382. doi: 10.1093/jxb/erw427
Johnson, R., Vishwakarma, K., Hossen, M. S., Kumar, V., Shackira, A. M., Puthur, J. T., et al. (2022). Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 172, 56–69. doi: 10.1016/j.plaphy.2022.01.001
Kant, S., Peng, M., Rothstein, S. J. (2011). Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in arabidopsis. PloS Genet. 7 (3), e1002021. doi: 10.1371/journal.pgen.1002021
Khosa, J. S. (2022). Phospholipids and flowering regulation. Trends Plant Sci. 27 (7), 621–623. doi: 10.1016/j.tplants.2022.01.006
Kim, W., Ahn, H. J., Chiou, T. J., Ahn, J. H. (2011). The role of the miR399-PHO2 module in the regulation of flowering time in response to different ambient temperatures in Arabidopsis thaliana. Mol. Cells 32 (1), 83–88. doi: 10.1007/s10059-011-1043-1
Kobayashi, Y., Weigel, D. (2007). Move on up, it’s time for change–mobile signals controlling photoperiod-dependent flowering. Genes Dev. 21 (19), 2371–2384. doi: 10.1101/gad.1589007
Konishi, M., Yanagisawa, S. (2013). Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nat. Commun. 4, 1617. doi: 10.1038/ncomms2621
Lambers, H. (2022). Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 73, 17–42. doi: 10.1146/annurev-arplant-102720-125738
Leigh, R., Jones, R. G. W. (1984). A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 97, 1–13. doi: 10.1111/j.1469-8137.1984.tb04103.x
Lei, K. J., Lin, Y. M., Ren, J., Bai, L., Miao, Y. C., An, G. Y., et al. (2016). Modulation of the phosphate-deficient responses by MicroRNA156 and its targeted SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 3 in arabidopsis. Plant Cell Physiol. 57 (1), 192–203. doi: 10.1093/pcp/pcv197
Leng, Y., Gao, Y., Chen, L., Yang, Y., Huang, L., Dai, L., et al. (2020). Using heading date 1 preponderant alleles from indica cultivars to breed high-yield, high-quality japonica rice varieties for cultivation in south China. Plant Biotechnol. J. 18 (1), 119–128. doi: 10.1111/pbi.13177
Li, Y. L., Fan, X. R., Shen, Q. R. (2008). The relationship between rhizosphere nitrification and nitrogen-use efficiency in rice plants. Plant Cell Environ. 31 (1), 73–85. doi: 10.1111/j.1365-3040.2007.01737.x
Lin, X., Liu, B., Weller, J. L., Abe, J., Kong, F. (2021). Molecular mechanisms for the photoperiodic regulation of flowering in soybean. J. Integr. Plant Biol. 63 (6), 981–994. doi: 10.1111/jipb.13021
Lin, Y. L., Tsay, Y. F. (2017). Influence of differing nitrate and nitrogen availability on flowering control in arabidopsis. J. Exp. Bot. 68 (10), 2603–2609. doi: 10.1093/jxb/erx053
Liu, K. H., Diener, A., Lin, Z., Liu, C., Sheen, J. (2020). Primary nitrate responses mediated by calcium signalling and diverse protein phosphorylation. J. Exp. Bot. 71 (15), 4428–4441. doi: 10.1093/jxb/eraa047
Liu, X., Hu, B., Chu, C. (2022b). Nitrogen assimilation in plants: current status and future prospects. J. Genet. Genomics 49 (5), 394–404. doi: 10.1016/j.jgg.2021.12.006
Liu, K. H., Niu, Y., Konishi, M., Wu, Y., Du, H., Sun Chung, H., et al. (2017). Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 545 (7654), 311–316. doi: 10.1038/nature22077
Liu, Q., Wu, K., Song, W., Zhong, N., Wu, Y., Fu, X. (2022a). Improving crop nitrogen use efficiency toward sustainable green revolution. Annu. Rev. Plant Biol. 73, 523–551. doi: 10.1146/annurev-arplant-070121-015752
Luo, X., Yin, M., He, Y. (2021). Molecular genetic understanding of photoperiodic regulation of flowering time in arabidopsis and soybean. Int. J. Mol. Sci. 23 (1), 466. doi: 10.3390/ijms23010466
Marschner, H., Marschner, P. (1995). Mineral nutrition of higher plants (Burlington: Elsevier Science).
Martínez, C., Pons, E., Prats, G., León, J. (2004). Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J. 37 (2), 209–217. doi: 10.1046/j.1365-313x.2003.01954.x
Mathieu, J., Yant, L. J., Murdter, F., Kuttner, F., Schmid, M. (2009). Repression of flowering by the miR172 target SMZ. PloS Biol. 7 (7), e1000148. doi: 10.1371/journal.pbio.1000148
Menz, J., Li, Z., Schulze, W. X., Ludewig, U. (2016). Early nitrogen-deprivation responses in arabidopsis roots reveal distinct differences on transcriptome and (phospho-) proteome levels between nitrate and ammonium nutrition. Plant J. 88 (5), 717–734. doi: 10.1111/tpj.13272
Milner, M. J., Howells, R. M., Craze, M., Bowden, S., Graham, N., Wallington, E. J. (2018). A PSTOL-like gene, TaPSTOL, controls a number of agronomically important traits in wheat. BMC Plant Biol. 18 (1), 115. doi: 10.1186/s12870-018-1331-4
Miyazaki, Y., Maruyama, Y., Chiba, Y., Kobayashi, M. J., Joseph, B., Shimizu, K. K., et al. (2014). Nitrogen as a key regulator of flowering in Fagus crenata: understanding the physiological mechanism of masting by gene expression analysis. Ecol. Lett. 17 (10), 1299–1309. doi: 10.1111/ele.12338
Negishi, K., Endo, M., Abe, M., Araki, T. (2018). SODIUM POTASSIUM ROOT DEFECTIVE1 regulates FLOWERING LOCUS t expression via the microRNA156-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE3 module in response to potassium conditions. Plant Cell Physiol. 59 (2), 404–413. doi: 10.1093/pcp/pcx199
Nord, E. A., Lynch, J. P. (2008). Delayed reproduction in Arabidopsis thaliana improves fitness in soil with suboptimal phosphorus availability. Plant Cell Environ. 31 (10), 1432–1441. doi: 10.1111/j.1365-3040.2008.01857.x
Olas, J. J., Van Dingenen, J., Abel, C., Dzialo, M. A., Feil, R., Krapp, A., et al. (2019). Nitrate acts at the Arabidopsis thaliana shoot apical meristem to regulate flowering time. New Phytol. 223 (2), 814–827. doi: 10.1111/nph.15812
Osnato, M., Cota, I., Nebhnani, P., Cereijo, U., Pelaz, S. (2022). Photoperiod control of plant growth: flowering time genes beyond flowering. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.805635
Pathak, P., Li, T., Chiang, J. Y. (2013). Retinoic acid-related orphan receptor α regulates diurnal rhythm and fasting induction of sterol 12α-hydroxylase in bile acid synthesis. J. Biol. Chem. 288 (52), 37154–37165. doi: 10.1074/jbc.M113.485987
Podar, D., Maathuis, F. J. M. (2022). Primary nutrient sensors in plants. iScience 25 (4), 104029. doi: 10.1016/j.isci.2022.104029
Prathap, V., Kumar, A., Maheshwari, C., Tyagi, A. (2022). Phosphorus homeostasis: acquisition, sensing, and long-distance signaling in plants. Mol. Biol. Rep. 49 (8), 8071–8086. doi: 10.1007/s11033-022-07354-9
Preston, J. C., Fjellheim, S. (2022). Flowering time runs hot and cold. Plant Physiol. 190 (1), 5–18. doi: 10.1093/plphys/kiac111
Ren, X., Guo, R., Akami, M., Niu, C. (2022). Nitrogen acquisition strategies mediated by insect symbionts: A review of their mechanisms, methodologies, and case studies. Insects 13 (1), 84. doi: 10.3390/insects13010084
Sakuraba, Y. (2022). Molecular basis of nitrogen starvation-induced leaf senescence. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1013304
Sanagi, M., Aoyama, S., Kubo, A., Lu, Y., Sato, Y., Ito, S., et al. (2021). Low nitrogen conditions accelerate flowering by modulating the phosphorylation state of FLOWERING BHLH 4 in arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 118 (19), e2022942118. doi: 10.1073/pnas.2022942118
Seligman, K., Saviani, E. E., Oliveira, H. C., Pinto-Maglio, C. A., Salgado, I. (2008). Floral transition and nitric oxide emission during flower development in Arabidopsis thaliana is affected in nitrate reductase-deficient plants. Plant Cell Physiol. 49 (7), 1112–1121. doi: 10.1093/pcp/pcn089
Shim, J. S., Jang, G. (2020). Environmental signal-dependent regulation of flowering time in rice. Int. J. Mol. Sci. 21 (17), 6155. doi: 10.3390/ijms21176155
Shi, M., Wang, C., Wang, P., Zhang, M., Liao, W. (2022). Methylation in DNA, histone, and RNA during flowering under stress condition: A review. Plant Sci. 324, 111431. doi: 10.1016/j.plantsci.2022.111431
Shoko, K., Yuji, T., Yasushi, K., Lisa, M., Takuji, S., Takashi, A., et al. (2002). Hd3a, a rice ortholog of the arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 10), 1096–1105. doi: 10.1093/pcp/pcf156
Tanaka, O. (1986). Flower induction by nitrogen deficiency in Lemna paucicostata 6746. Plant Cell Physiol. 27 (5), 875–880. doi: 10.1093/oxfordjournals.pcp.a077173
Tocquin, P., Corbesier, L., Havelange, A., Pieltain, A., Kurtem, E., Bernier, G., et al. (2003). A novel high efficiency, low maintenance, hydroponic system for synchronous growth and flowering of Arabidopsis thaliana. BMC Plant Biol. 3, 2. doi: 10.1186/1471-2229-3-2
Vélez-Bermúdez, I. C., Schmidt, W. (2022). How plants recalibrate cellular iron homeostasis. Plant Cell Physiol. 36 (2), 154–162. doi: 10.1093/pcp/pcab166
Verma, P., Sanyal, S. K., Pandey, G. K. (2021). Ca2+-CBL-CIPK: A modulator system for efficient nutrient acquisition. Plant Cell Rep. 40 (11), 2111–2122. doi: 10.1007/s00299-021-02772-8
Vidal, E. A., Moyano, T. C., Canales, J., Gutierrez, R. A. (2014). Nitrogen control of developmental phase transitions in Arabidopsis thaliana. J. Exp. Bot. 65 (19), 5611–5618. doi: 10.1093/jxb/eru326
Wang, Y., Chen, Y. F., Wu, W. H. (2021). Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 63 (1), 34–52. doi: 10.1111/jipb.13053
Wang, W., Hu, B., Yuan, D., Liu, Y., Che, R., Hu, Y., et al. (2018). Expression of the nitrate transporter gene OsNRT1.1A/OsNPF6.3 confers high yield and early maturation in rice. Plant Cell 30 (3), 638–651. doi: 10.1105/tpc.17.00809
Wang, Y., Wu, W. H. (2015). Genetic approaches for improvement of the crop potassium acquisition and utilization efficiency. Curr. Opin. Plant Biol. 25, 46–52. doi: 10.1016/j.pbi.2015.04.007
Wani, S. H., Vijayan, R., Choudhary, M., Kumar, A., Zaid, A., Singh, V., et al. (2021). Nitrogen use efficiency (NUE): elucidated mechanisms, mapped genes and gene networks in maize (Zea mays l.). Physiol. Mol. Biol. Plants 27 (12), 2875–2891. doi: 10.1007/s12298-021-01113-z
Weber, K., Burow, M. (2018). Nitrogen - essential macronutrient and signal controlling flowering time. Physiol. Plant 162 (2), 251–260. doi: 10.1111/ppl.12664
Wei, S., Li, X., Lu, Z., Zhang, H., Ye, X., Zhou, Y., et al. (2022). A transcriptional regulator that boosts grain yields and shortens the growth duration of rice. Science 377 (6604), eabi8455. doi: 10.1126/science.abi8455
Xie, K., Ren, Y., Chen, A., Yang, C., Zheng, Q., Chen, J., et al. (2022). Plant nitrogen nutrition: The roles of arbuscular mycorrhizal fungi. J. Plant Physiol. 269, 153591. doi: 10.1016/j.jplph.2021.153591
Yang, Y. Y., Ren, Y. R., Zheng, P. F., Qu, F. J., Song, L. Q., You, C. X., et al. (2020). Functional identification of apple MdMYB2 gene in phosphate-starvation response. J. Plant Physiol. 244, 153089. doi: 10.1016/j.jplph.2019.153089
Yuan, S., Zhang, Z. W., Zheng, C., Zhao, Z. Y., Wang, Y., Feng, L. Y., et al. (2016). Arabidopsis cryptochrome 1 functions in nitrogen regulation of flowering. Proc. Natl. Acad. Sci. U.S.A. 113 (27), 7661–7666. doi: 10.1073/pnas.1602004113
Zhang, S., Zhang, Y., Li, K., Yan, M., Zhang, J., Yu, M., et al. (2021). Nitrogen mediates flowering time and nitrogen use efficiency via floral regulators in rice. Curr. Biol. 31671–683 (4), e675. doi: 10.1016/j.cub.2020.10.095
Zhu, Y., Liu, L., Shen, L., Yu, H. (2016). NaKR1 regulates long-distance movement of FLOWERING LOCUS T in arabidopsis. Nat. Plants 2 (6), 16075. doi: 10.1038/nplants.2016.75
Keywords: nutrient, flowering time, nitrogen, phosphorus, potassium
Citation: Zhang Y, Liu B, Kong F and Chen L (2023) Nutrient-mediated modulation of flowering time. Front. Plant Sci. 14:1101611. doi: 10.3389/fpls.2023.1101611
Received: 18 November 2022; Accepted: 05 January 2023;
Published: 19 January 2023.
Edited by:
Gaetano Distefano, University of Catania, ItalyReviewed by:
Yongqiang Zhang, Fujian Agriculture and Forestry University, ChinaCopyright © 2023 Zhang, Liu, Kong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyu Chen, Y2hlbmxpeXUxNzE1QGd6aHUuZWR1LmNu; Fanjiang Kong, a29uZ2ZqQGd6aHUuZWR1LmNu
 Yuhang Zhang
Yuhang Zhang Baohui Liu
Baohui Liu Fanjiang Kong
Fanjiang Kong Liyu Chen
Liyu Chen