- 1AixMarseille University, French Alternative Energies and Atomic Energy Commission (CEA), National Center for Scientific Research (CNRS), Bioscience and Biotechnology Institute of Aix-Marseille (BIAM), Saint-Paul Lez Durance, France
- 2Faculty of Life and Environmental Sciences University of Tsukuba, Tsukuba, Ibaraki, Japan
- 3Institute for Advanced Research, Nagoya University, Nagoya, Japan
- 4Center for Research in Isotopes and Environmental Dynamics, University of Tsukuba, Tsukuba, Ibaraki, Japan
Potassium (K+) is an essential macronutrient for plant growth. The transcriptional regulation of K+ transporter genes is one of the key mechanisms by which plants respond to K+ deficiency. Among the HAK/KUP/KT transporter family, HAK5, a high-affinity K+ transporter, is essential for root K+ uptake under low external K+ conditions. HAK5 expression in the root is highly induced by low external K+ concentration. While the molecular mechanisms of HAK5 regulation have been extensively studied, it remains unclear how plants sense and coordinates K+ uptake and translocation in response to changing environmental conditions. Using skor mutants, which have a defect in root-to-shoot K+ translocation, we have been able to determine how the internal K+ status affects the expression of HAK5. In skor mutant roots, under K+ deficiency, HAK5 expression was lower than in wild-type although the K+ concentration in roots was not significantly different. These results reveal that HAK5 is not only regulated by external K+ conditions but it is also regulated by internal K+ levels, which is in agreement with recent findings. Additionally, HAK5 plays a major role in the uptake of Cs+ in roots. Therefore, studying Cs+ in roots and having more detailed information about its uptake and translocation in the plant would be valuable. Radioactive tracing experiments revealed not only a reduction in the uptake of 137Cs+ and 42K+in skor mutants compared to wild-type but also a different distribution of 137Cs+ and 42K+ in tissues. In order to gain insight into the translocation, accumulation, and repartitioning of both K+ and Cs+ in plants, long-term treatment and split root experiments were conducted with the stable isotopes 133Cs+ and 85Rb+. Finally, our findings show that the K+ distribution in plant tissues regulates root uptake of K+ and Cs+ similarly, depending on HAK5; however, the translocation and accumulation of the two elements are different.
1 Introduction
To maximize growth in the environment, plants coordinate mineral uptake from the soil. Plant roots have transporters on their cell membranes that are regulated at both transcriptional and post-transcriptional levels. It is necessary to understand molecular mechanisms in order to control ion transport in plants so that they can be used for agricultural purposes such as fertilization and phytoremediation. In plants, potassium (K+) is among the most abundant macronutrients corresponding to between 2-6% of their dry mass (Leigh and Wyn Jones, 1984), and it plays a key role in the regulation of osmotic pressure, cytoplasmic pH, membrane potential, and metabolism catalytic activity (Ragel et al., 2019). In plant cells, the K+ concentration in the cytoplasm is usually maintained close to 100 mM (Walker et al., 1996). However, the concentration of K+ in the soil is highly fluctuating, ranging from micro-millimolar to millimolar (Rengel and Marschner, 2005; Maathuis, 2009). The uptake and distribution of K+ in plants are determined by a variety of K+ transport systems, which include channels and transporters with varying K+ affinity and localization. The K+ channels and transporters are composed of multiple gene families that function at different external K+ concentrations (Véry and Sentenac, 2003; Véry et al., 2014). In particular, the HAK5 high-affinity transporter is responsible for K+ uptake when external K+ is above 10 µM, whereas AKT1 is active at more than 100µM. In addition, non-selective cation channels are active at an external K+ concentration of more than 1 mM (Gierth et al., 2005; Pyo et al., 2010; Nieves-Cordones et al., 2014). HAK5 transcript levels rise rapidly during K+ deficiency in order to enhance high-affinity K+ uptake but decrease during K+ sufficiency (Ahn et al., 2004; Gierth et al., 2005). In addition, under low K+ conditions, root growth is impaired in HAK5 knockout mutants compared to wild-type (Qi et al., 2008). Also, HAK5, which belongs to the KT/KUP/HAK family, is a major component in mediating high-affinitty K+ uptake in Arabidopsis under low K+ conditions.
In addition, HAK5 is the main contributor to cesium (Cs+) uptake by plants (Qi et al., 2008). Then, the hak5-1 mutant strongly reduces Cs+ uptake and is more tolerant of Cs+. In contrast, the akt1-1 mutant is more sensitive to Cs+ than the wild-type due to an increased expression of HAK5 (Qi et al., 2008). Cs+ itself is not required for plant growth. However, the chemical properties of Cs+ are similar to those of K+, so it is taken up by the plant and perturbs cellular activity (Hampton et al., 2004; Adams et al., 2013). Due to the radioactive Cs+ (134Cs+ and 137Cs+) spreading from the explosion of the Fukushima Daiichi nuclear power plant, understanding the regulation of HAK5 activity is crucial to producing safe crops by reducing the entry of Cs+ into the food chain and utilizing phytoremediation technologies to remove Cs+ from the contaminated soil (Nieves-Cordones et al., 2017; Rai et al., 2017).
In the past decade, multiple regulation mechanisms of HAK5 transporter have been identified. Low environmental K+ conditions stimulate signaling and enhance the accumulation of transcripts and activity of HAK5. In addition, low external K+ concentrations induce plasma membrane hyperpolarization (Nieves-Cordones et al., 2008; Rubio et al., 2014) and extracellular acidification. In turn, extracellular acidification accelerates the H+-coupled transport of HAK5. Furthermore, under the low K+ conditions, ethylene increased and positively regulated reactive oxygen species (ROS), which in turn induced the transcription factor RAP2.11 gene expression, thereby positively regulating HAK5 (Shin et al., 2005; Kim et al., 2012). In a recent study, Hong et al. (2013) reported the identification of several transcription factors that could promote HAK5 expression. It has also been shown that the transcription factor, Auxin Response Factor 2 (ARF2) directly binds to the HAK5 promoter and repressed HAK5 expression under K+ sufficient conditions (Zhao et al., 2016). In response to low-K+ treatment, the DNA-binding activity of ARF2 to the HAK5 promoter is abolished, promoting HAK5 transcription (Zhao et al., 2016). HAK5 is also subjected to post-transcriptional regulation. Membrane hyperpolarization and ROS activate Ca2+ permeable channels, and this Ca2+ signal can be perceived and transduced downstream by a Ca2+ sensor such as CBL. CBL binds to CIPK23, a cytoplasmic kinase, which phosphorylates HAK5. The phosphorylation of HAK5 increases its affinity for K+ (Ragel et al., 2015).
In order to increase ion uptake and accumulation in plants, the overexpression of ion transporters has been promoted. Nevertheless, it does not always result in higher uptake and biomass (Ai et al., 2009). Therefore, further research is needed to unravel complex regulation mechanisms. At a whole plant scale, the phloem K+ concentration provides information about shoot K+ demand, and potassium-release channels of the xylem parenchyma take advantage of this signal to coordinate K+ uptake (Wegner and De Boer, 1997; Dreyer et al., 2017). In addition, it has been shown that the K+ translocation rate affects K+ uptake (Nieves-Cordones et al., 2019). It was therefore observed that skor knock-out mutants, which are defective in the highly selective outward-rectifying K+ channels responsible for releasing K+ into xylem sap toward the shoot prevented the uptake of Rb+ by plants under K+ deficiency conditions (Nieves-Cordones et al., 2019).
In previous studies, it has been found that low K+ environmental conditions are associated with a signaling network that is responsible for the regulation of HAK5 at the cellular level (Nieves-Cordones et al., 2008; Rubio et al., 2014; Ragel et al., 2015; Zhao et al., 2016). It is still necessary, however, to conduct further studies in order to be able to gain a better understanding of how K+ is sensed. As a whole plant, the roots are the main organs that are directly exposed to the external environment. The low K+ signal is first perceived at the plasma membrane of the root epidermal cells and then it is transduced into the cytoplasm of the cells. A short-term response occurs within a few hours without a noticeable change in the cytoplasmic K+ level, and a long-term response is stimulated by a decrease in the cytoplasmic K+ level. Only the deprivation of K+ produces functional HAK5-mediated K+ uptake in the root (Rubio et al., 2014). However, there is little information available on whether or not changes in internal K+ distribution and/or concentration affect the expression of transporters in roots. In Arabidopsis, SKOR (Stelar K+ Outward Rectifier) is expressed in root stele cells (pericycle and xylem parenchyma cells), where it is involved in mediating K+ secretion by the xylem parenchyma cells of roots and toward the xylem vessels (Gaymard et al., 1998). SKOR, being an outward-rectifying channel, opens upon membrane depolarization to allow cytosolic K+ to be released from the cell. Thus, SKOR plays a significant role in the transport of K+ over long distances, especially in the translocation of K+ from roots to shoots. Consequently, both skor knockout mutants prevented K+ from being transported from the root to the shoot, and the shoot K+ concentration decreased drastically by 50% compared to the wild-type genotype (Gaymard et al., 1998). In addition, NRT1.5/NPF7 has been described as a proton-coupled H+/K+ antiporter involved in the translocation of K+ from roots to shoots (Li et al., 2017). It should be noted, however, that NRT1.5/NPF7 is able to function under low availability regardless of the availability of K+, whereas SKOR is able to mediate K+ translocation from root to shoot when there is low K+ availability and high (Drechsler et al., 2015; Meng et al., 2016; Li et al., 2017).
In this study, we have decided to focus on how K+ translocation from roots to shoots impacts the K+ and Cs+ uptake mechanisms and distribution in plants in the short-term and the long-term responses. The present study demonstrated, using skor mutants, that the balance in the distribution of K+ between shoots and roots affects the expression of the high-affinity potassium transporter HAK5 gene in the roots. Furthermore, since HAK5 plays a crucial role in the uptake of Cs+, we have evaluated the transport properties of Cs+. Interestingly, using these experiments, the ion uptake capacity of AKT1 could be separated from that of HAK5 since AKT1 is not permeable to Cs+, therefore inhibiting its activity. (Schachtman, 2000; Adams et al., 2019). Finally, our study showed that SKOR mutation altered the distribution of K+ and Cs+ in the plant differently.
2 Materials and methods
2.1 Plant materials and growth conditions
Arabidopsis (Arabidopsis thaliana) ecotype Colombia (Col) and Wassilewskija (WS) were used in this study. skor 1-1 in WS ecotype were kindly provided by Dr. Anne-Alienor Very (INRAE, Montpellier). skor3-1 (GK391G12) in Col ecotype was obtained from the NASC (Nottingham Arabidopsis Stock Centre). hak5-1 (SALK_014177) in Col ecotype was obtained from the ABRC (Arabidopsis Biological Resource Centre). T-DNA insertion and homozygous lines were identified by PCR using primers T-DNA left border primer and a gene-specific primer (Supplementary Table 1).
For root phenotype assay, seeds were surface sterilized and sown in vitro on a Petri dish plate with a solid medium containing low-K+ (KCl 10 µM) and high-K+ (KCl 1000 µM) for 7 days. Solid medium is composed of nutrient solution (0.75 mM MgSO4, 2 mM Ca(NO3)2, 0.5 mM H3PO4, 9.25 µM H3BO3, 3.6 µM MnSO4, 3 µM ZnSO4, 0.785 µM CuSO4, 0.074 µM NH4Mo7O24, 3.5 mM MES, pH 5.8), 0.5% (w/v) Suc, and 0.8% (w/v) agar). For phenotype assay with Cs+, seeds were sown in vitro on a Petri plate with a solid medium containing low-K+ (KCl 10 µM) nutrient solution. Two days after germination, seedlings were transferred to a liquid medium KCl 10 µM with CsCl 0 or 100 µM for 4 days. For the radiotracer experiment, plants were grown for 14 days in solid medium low-K+ (KCl 10 µM) and high-K+ (KCl 1000 µM) containing nutrient solution. For measurements of K+ distribution phenotype between lines and qRT-PCR experiments, plants were grown in a solid medium containing 100 µM K+ and nutrient solution mix for 12 days, then transferred to sand culture with nutrient solution containing 100 µM K+ for 7 days, then transferred to hydroponic cultures with nutrient solution containing 0, 10, 100, and 1000 µM K+ for 4 days. For cold Cs+ and Rb+ trace experiments, plants were grown in a solid medium containing 100 µM K+ and nutrient solution for 12 days, then transferred to sand culture with nutrient solution containing 100 µM K+ for 10 days, then transferred to hydroponic cultures using a nutrient solution containing low-K+ (KCl 10 µM) and high-K+ (KCl 3000 µM) for 8 days, and then was transferred to a nutrient solution containing 10 µM K+ (12 plants per 15 L nutrient solution containing 10 µM K+) with 1 µM CsCl and 1 µM RbCl for 3 days. Plants were grown in a growth chamber set to 23°C and 8 h Light/16 h dark cycle.
2.2 Root morphology analyses
Pictures of the plates were taken with a camera to analyze lateral root density and root lengths. Root length was measured using the plugin NeuronJ (Meijering et al., 2004) for the ImageJ software.
2.3 ICP analysis
The roots of plants were washed in sterile distilled water, separated into shoots and roots, and dried for 3 days at 50°C. Dried samples were digested in HNO3 (concentrated) at 80°C overnight. After filtration, acid solutions were diluted with 1% HNO3. K+ concentrations in the solution were determined by ICP-OES (Agilent Technology 5800). Cs+ and Rb+ were determined by ICP-MS (OPTIMA 8300, Perkin Elmer).
2.4 RNA extraction and RT-qPCR
Total RNA was extracted from the shoot and root of 23-day-old plantlets, using Direct-zol RNA MiniPrep (ZYMO RESEARCH). cDNA was synthesized with the qScript cDNA SuperMix (Quanta). Quantitative real-time PCR was conducted with the Light Cycler 480 SYBR Green I Master PCR Mater (Roche) on a Light Cycler 480 (Roche) following the manufacturer’s protocols. The amplification reactions were performed in a total volume of 5 µl, which contained 2 µl cDNA, 2.5 µl SYBR Green premix, and 0.5 µl forward and reverse primers (1µM). The PCR was programmed as follows: 90°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. For each pair of primers, the PCR efficiency was around 100% and a threshold value was determined. The specificity of PCR amplification was examined by monitoring the presence of the single peak in the melting curves after RT–qPCRs. The relative expression of the gene in each sample was compared to the control sample and was calculated with the delta delta Ct (Ct) method using the following equation: relative expression = 2–ΔΔCt, with ΔCt = Ctsample – Ctcontrol and with Ct = Cttarget gene – Cthousekeeping gene, where Ct refers to the threshold cycle determined for each gene in the exponential phase of PCR amplification (Livak and Schmittgen, 2001). Using this analysis method, the relative expression of the gene in the control sample was equal to one, and the relative expression of the other treatments was then compared to the control plants. The housekeeping gene was the ROC3 gene (At2g16600) (Bonnot et al., 2016; Genies et al., 2021). The subsequent RT-qPCRs were performed in triplicate for each sample. Primer sequences are provided in Supplementary Table S1.
2.5 137Cs and 42K Uptake measurements
Plants 14 days old grown in solid medium-low-K+ (KCl 10 µM) or high-K+ (KCl 1000 µM) containing nutrient solution were incubated for 2 hours in hydroponic cultures using a nutrient solution containing 1 µM 39K+, 1 µM 133Cs, and 100 Bq/ml 137Cs or a mixed tracer of 42K and 43K 500 Bq/ml. At the end of the uptake period, plant roots were washed with 1 mM KCl and 1 mM CsCl solution. The radioactivity in plants was measured with a gamma counter (AccuFLEXγ8000, HITACHI.co). For the gamma-ray measurements of the 42K (half-life,12 hours) and 43K (half-life, 22 hours) RI mixture, only the spectrum of 43K was measured during the experiments due to its longer half-life.
2.6 Split root experiment
Plants were grown under the same conditions as those used for the cold trace experiments of Cs+ and Rb+. Then, the roots were washed in distilled water and separated into two parts. The two root parts were maintained in the same nutrient solution mix containing low -K+ (KCl 10 µM) for 8 days. The tracer experiment was conducted by placing two root parts in separate pots. Then, 1 µM CsCl or 1 µM RbCl was added to one part of the root for 3 days. Mineralization and measurement are the same as the method described in the ICP analysis.
2.7 Accession numbers
Sequence data for the genes described in this article are in the Arabidopsis TAIR database (https://www.arabidopsis.org/index.jsp) under the following accession numbers: At3g02850 for SKOR, At4g13420 for HAK5, At2g30070 for AtKUP1, At2g40540 for AtKUP2, At3g02050 for AtKUP3, At4g23640 for AtKUP4, At4g33530 for AtKUP5, At1g70300 for AtKUP6, At5g09400 for AtKUP7, At5g14880 for AtKUP8, At4g18860 for AtKUP9, At1g31120 for AtKUP10, At2g35060 for AtKUP11, and At1g60160 for AtKUP12.
3 Results
3.1 The skor mutant is tolerant to low K+ stress
We determined the root growth of skor mutants under different K+ conditions. This study used two independent knockout mutants of the highly selective outward-rectifying K+ channel SKOR including skor1-1 in the WS background (Gaymard et al., 1998) and skor3-1 in the Col background. As shown in Figure 1, both skor mutants showed similar root architecture to the wild type both under high (1000 µM) and low (10µM) K+ conditions. In contrast, in the hak5-1 mutant, primary root growth is strongly impaired and a significant increase in lateral root density is observed under low K+ conditions (10 µM) (Figure 1), as previously observed (Qi et al., 2008). These results show that the root morphology of the skor mutants is not modified by low K+ conditions compared to hak5-1.
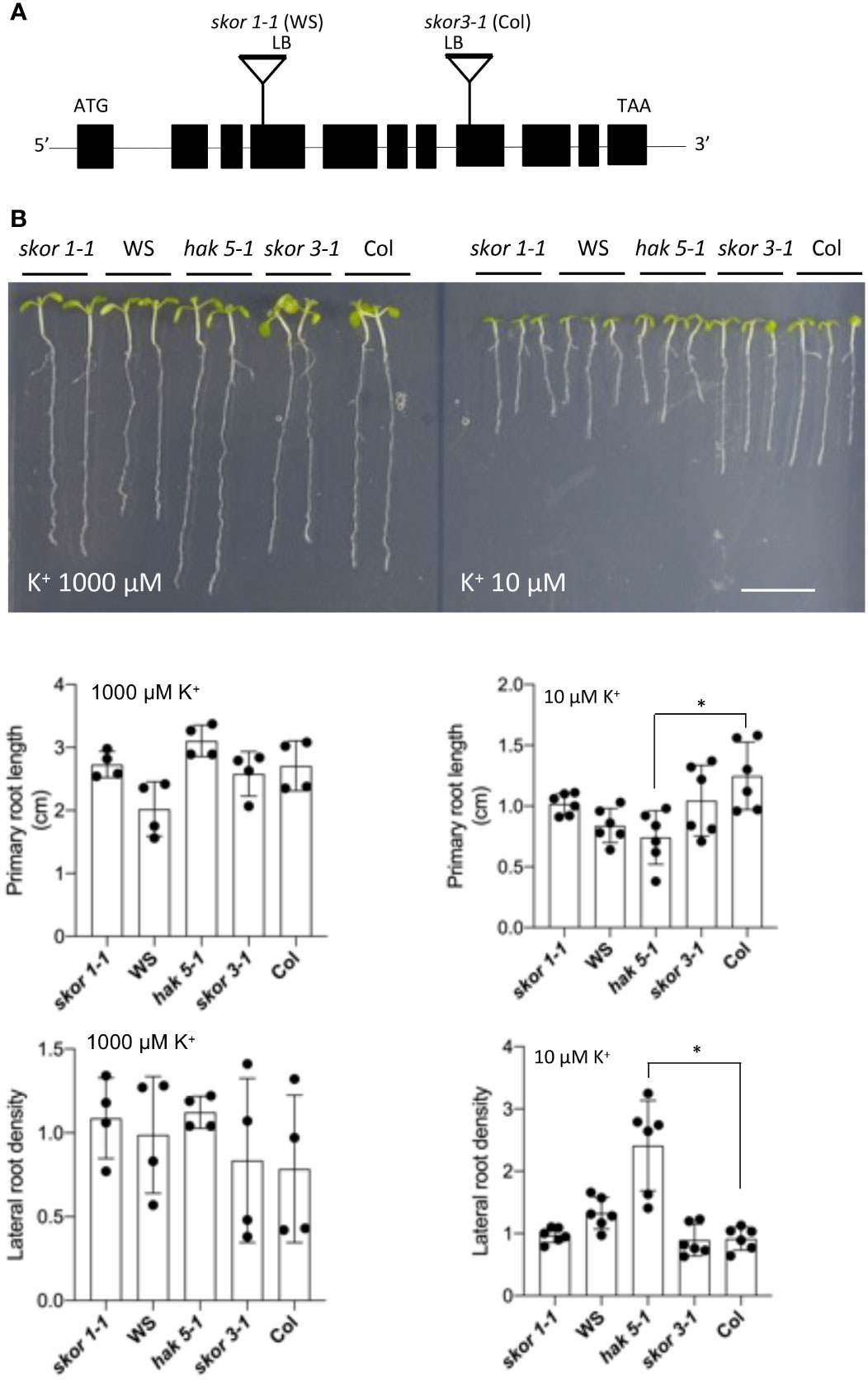
Figure 1 K+-dependent growth analysis of skor T-DNA insertion mutants. (A). A schematic representation of the T-DNA insertion in the SKOR gene (At3g02850.1) in the WS or Col-0 ecotypes. A solid line and black boxes indicate exons and introns, respectively. The T-DNA insertion sites in SKOR are represented as triangles, and the left border (LB) orientation is indicated. (B). In contrast to wild-type plants, both skor-1 and skor-3 mutants show no phenotype when grown for 7 days on a medium containing KCl at the indicated concentration. The scale bar is 1 cm. The lateral root density and the length of the primary roots were determined for wild-type and skor mutant plants after 7 days. Each bar represents the mean root length (n = 4-6) of seedlings ± SD. Statistical significance was determined by Student’s t-test. Significant differences between Col and hak5-1 mutants are indicated with asterisks (*P <0.05).
3.2 HAK5 transcription in skor mutants decreased in low K+ conditions
To determine and compare the transcriptional regulation of the KUP/KT/HAK transporters in response to K+ concentration, plants were grown under different K+ conditions, ranging from 10 µM to 1000 µM. Among all members of the family, only HAK5 is highly induced in roots when the external K+ concentration is less than 100µM, and no expression is detected above this concentration (Supplementary Data 1, 2). Previous studies have reported similar results (Ahn et al., 2004, Shin and Schachtman, 2004, Gierth et al., 2005). In contrast, transcription of the SKOR gene, which was predominantly detected in roots, did not change in roots according to K+ conditions (Supplementary Data 2). However, under low (10µM) K+ environmental conditions, the expression level of HAK5 in roots is strongly decreased in skor mutants compared to the wild types (Figure 2B). These results suggest that a decrease in K+ concentrations in the shoots of skor mutant plants (Figure 2A) induces a modification of the potassium distribution between roots and shoots, increasing the root-to-shoot ratio. This increase might affect the HAK5 expression in roots. In order to determine whether the decrease in HAK5 transcription levels in skor mutants is associated with an increase in K+ concentration in roots, the K+ concentrations in the roots of plants exposed to various K+ concentrations were measured using ICP-OES, and the correlation between K+ concentrations and HAK5 transcription levels was calculated. Then, correlation analysis between the relative expression of HAK5 and the root K+ concentrations (Figure 2C) was studied and the Pearson’s Correlation Coefficient using z scores was determined using Excel Software (Microsoft) electronic datasheet. The results show a correlation between HAK5 expression and K+ concentrations in wild-type Col roots (r = - 0,57, p-value = 0,0005) and the wild-type WS roots (r = - 0,51, p-value = 0,0023). This linear regression, however, is lost in both skor mutants (Figure 2C), skor 3-1 (r = - 0,34, p-value = 0,4095) and skor1-1 (r = - 0,11, p-value = 0,7663). Overall, these results suggest that in addition to external K+ conditions, K+ root concentrations tightly regulate HAK5 expression in roots.
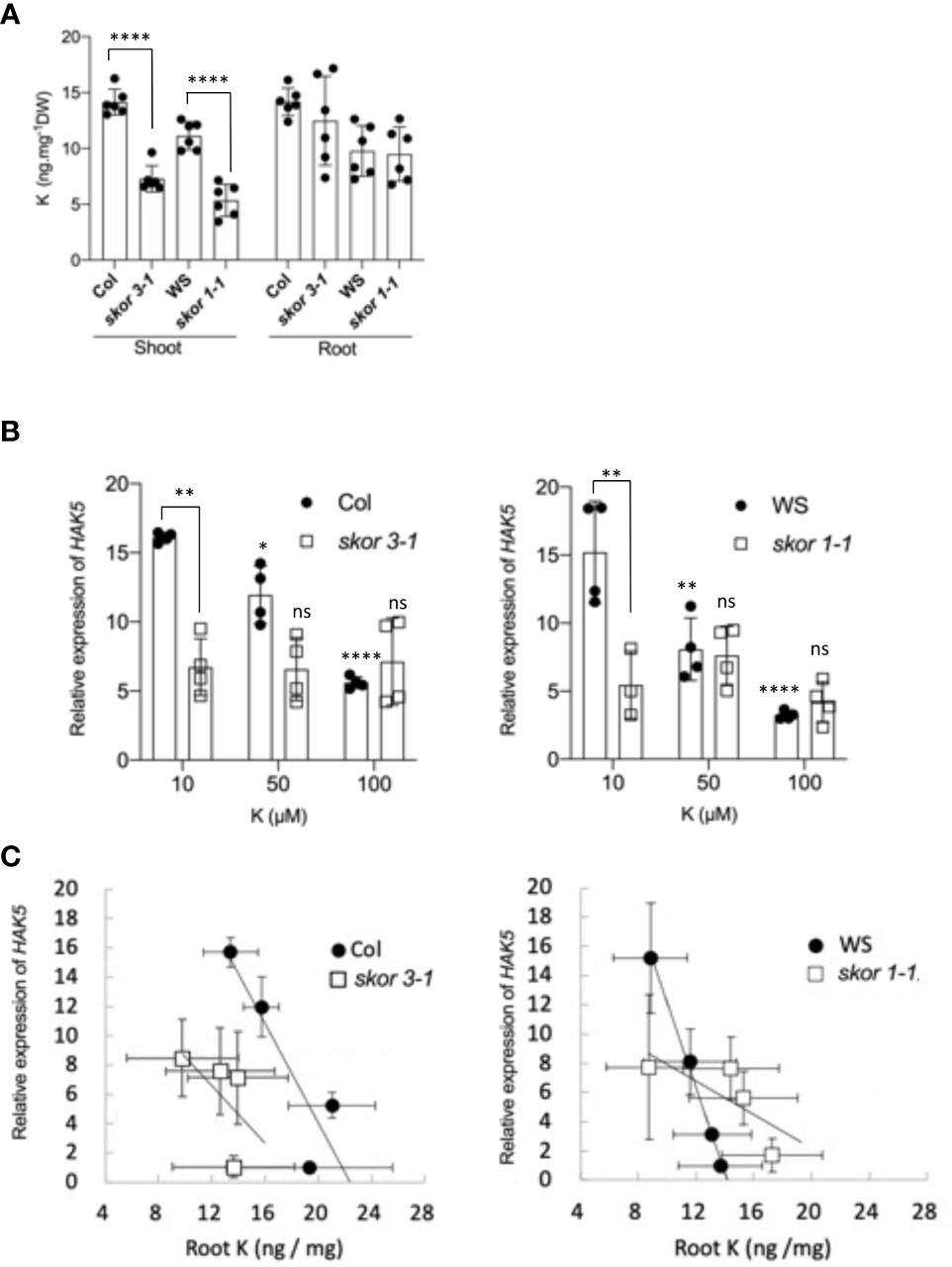
Figure 2 Both the external and internal concentrations of K+ affect the expression of HAK5 in roots. (A). SKOR mediates K+ translocation to the shoot. The K+ concentration of roots and shoots of different genotypes was measured using ICP-OES. Wild-type and mutant plants were grown in hydroponic cultures for 5 weeks in a medium containing 0.1 mM KCl. Data represent the mean ± SD (n = 6). Statistical significance was determined by Student’s t-test with Welch’s correction, and significant differences are indicated with asterisks (****P < 0.0001). (B). RT-qPCR analyses of HAK5 expression in roots of wild-type and skor mutants. HAK5 expression was negatively correlated with external potassium concentration in the roots of both wild-type ecotypes. Asterisks represent statistical significance between K+ treatments based on two-way ANOVA analysis (*p = 0.0227, **p = 0.0012, ****p < 0.0001) and statistical significance between genotypes bases on t-test with Welch's correction (**p < 0.01). (C). A comparison of the expression levels of HAK5 in WT and the skor mutant according to the K+ concentration in roots. The correlation analysis was performed, and Pearson’s Correlation Coefficient square using z scores was also calculated using Excel Software (Microsoft) electronic datasheet (Pearson’s Correlation Coefficient: Col r = - 0,57, p-value = 0,0005; skor3-1: r = - 0,34, p-value = 0,4095; WS: r = - 0,51, p-value = 0,0023; skor1-1: r = - 0,11, p-value = 0,7663). The expression of HAK5 is negatively correlated with the root’s internal potassium concentration in wild-type plants, whereas these correlations are lost in skor mutants.
3.3 skor and hak5 mutants are more Cs+ tolerant
As HAK5 is the major contributor to Cs+ uptake in Arabidopsis, we examined Cs+ tolerance in both skor and hak5 mutants and their corresponding wild types. Seedlings of wild-type (WS and Col), skor, and hak5-1 knockout mutants were grown in hydroponic cultures for 4 days in the presence of CsCl (100 µM). The skor and hak5 mutants were more resistant to Cs+ toxicity than wild-type plants, as shown in Figure 3A. In both wild-type ecotypes, Col and WS, the cotyledons bleached significantly in the presence of 100 µM Cs+, whereas the cotyledons of skor1-1, skor3-1, and hak5-1 remained green. As expected, and previously observed, the hak5-1 mutant is less susceptible to Cs+ toxicity due to its reduced uptake of Cs+ (Qi et al., 2008). Furthermore, both skor mutants exhibit enhanced resistance to Cs+ toxicity, similar to hak5-1. Our results were confirmed by measuring the Cs+ concentration of plants previously grown under different K+ conditions for 14 days. The roots of previously cultivated plants under different K+ conditions were exposed for 2 hours in the presence of 137Cs in order to determine the uptake rates of each mutant. Figure 3B shows that skor1-1 and skor3-1 mutant roots showed substantially and significantly reduced Cs+ concentration under low K+ conditions (10 µM K+). However, under high potassium conditions, there was no difference between wild-type and skor mutants in Cs+ uptake (Figure 3B).
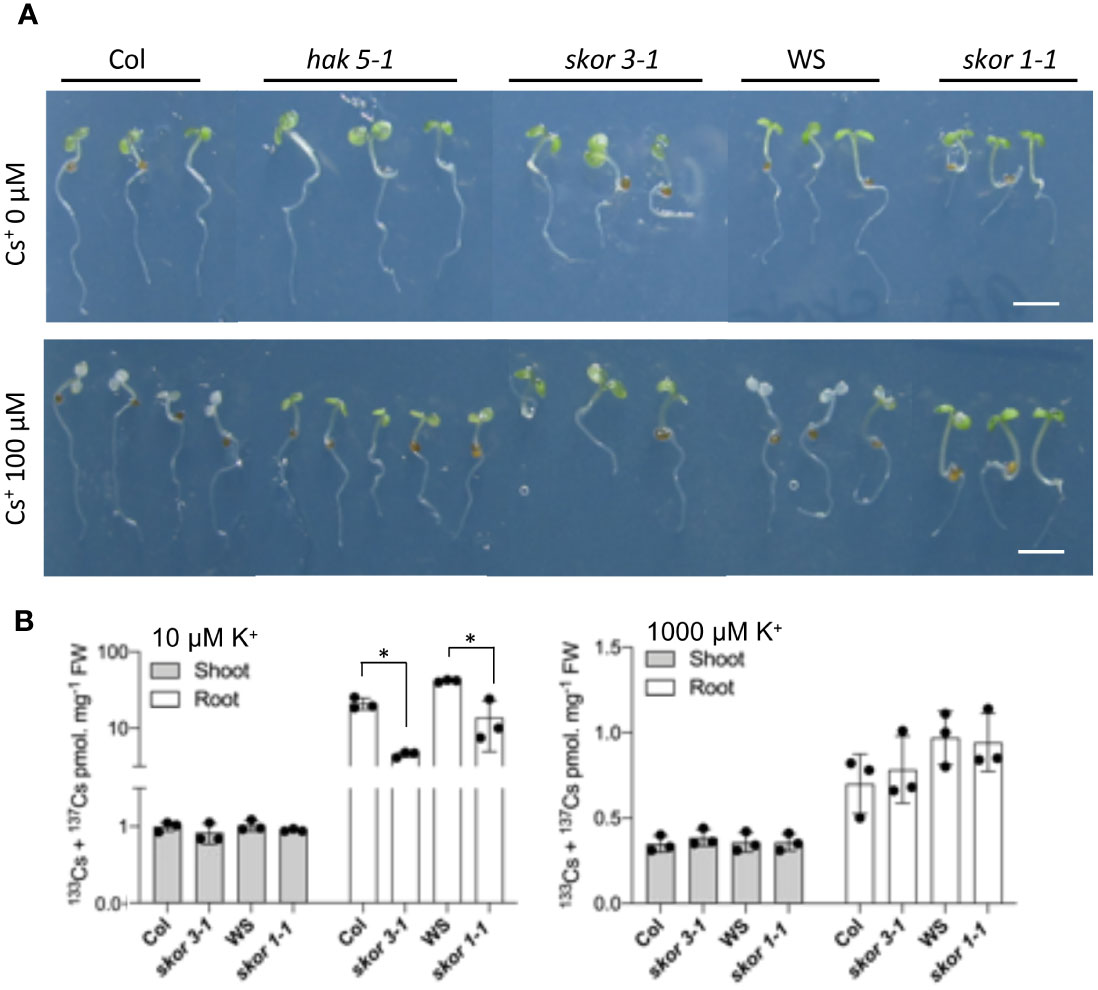
Figure 3 skor mutants are tolerant to Cs+ due to a decrease in Cs+ uptake. (A). Wild-type (Col, WS), both skor mutants skor3-1 (Col), skor1-1 (WS), and hak5-1 were germinated for 4 days and then transferred into a hydroponic medium containing different Cs+ concentrations for 4 days. The control plants showed chlorosis as a result of Cs+ toxicity, whereas the skor and hak5-1 mutants were more resistant. The scale bar was 0.5 cm. (B). Plants were grown on an agar medium containing different K+ concentrations for 14 days. Then, roots were incubated for 2 hours in a hydroponic medium containing K+ and 133Cs 1 μM + 137Cs 100 Bq/ml. Cs+ uptake is reduced in both skor mutants under low K+. Data are shown as means ± SD (n = 3). Statistical significance was determined by Student’s t-test with Welch’s correction. Significant differences between wild-types and skor mutants are indicated with asterisks (*P < 0.05).
3.4 The uptake and translocation of K+ and Cs+ are different
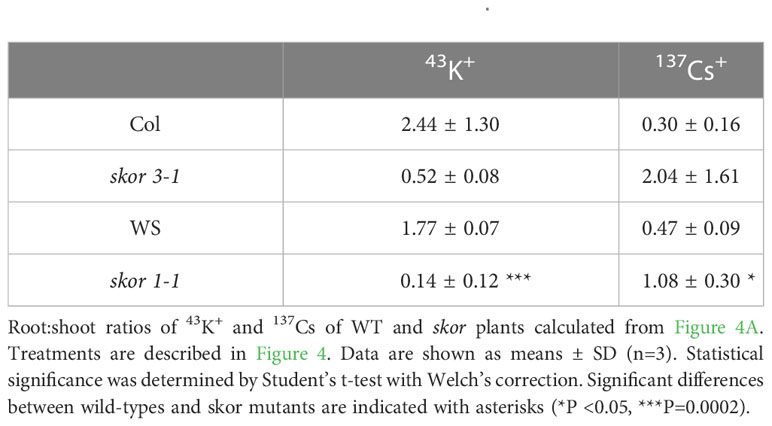
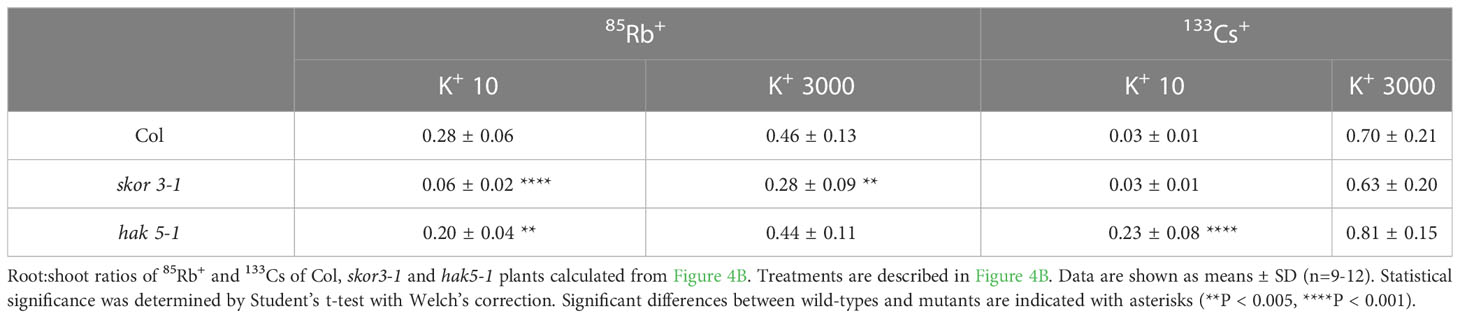
By mediating K+ secretion by the xylem parenchyma cells of roots and toward the xylem vessels, SKOR plays a role in root-to-shoot K+ translocation. In consequence, both skor knockout mutants significantly decreased K+ translocation from roots to shoots, resulting in a substantial decrease of more than 50% in shoot K+ concentration (Figure 2A). To determine whether SKOR affects Cs+ transport in a similar manner, we conducted experiments using radioactive tracers, 42K+ and 137Cs+, on plants grown under low potassium conditions to compare K+ and Cs+ uptake properties. In the skor1-1 shoot, 42K+ distributions are significantly impaired compared to the wild type (Figure 4A). The distribution pattern of 137Cs+ between shoots and roots, however, is different from that of 42K+, with a strong accumulation occurring in the roots of wild-type plants. This high accumulation of 137Cs+ in the roots is impaired in the skor1-1 mutant (Figure 4A). Interestingly, 137Cs+ translocation from roots to shoots is higher compared to 42K+ in skor mutants (Figure 4A). The shoot:root ratio of K+ is higher than that of Cs+ in the wild type, while the shoot:root ratio of skor is higher in Cs+ than in K+ (Table 1). According to these findings, we show that in skor mutants, Cs+ uptake in roots is dramatically reduced, while its translocation to the shoot is not affected. Thus, it is possible that under low K+ conditions, the decrease in HAK5 expression in the skor1-1 mutant could negatively affect Cs+ uptake; however, other factors are also involved in Cs+ translocation from roots to shoots. In order to better understand K+ and Cs+ translocation, accumulation, and repartitioning in plants, long-term treatments (3 days) were performed using 133Cs+ and 85Rb+ since 42K+ tracer has a short half-life of 12.4 hours and so cannot be used for long-term experiments. The roots and shoots of 35-day-old plants were collected separately after 3 days of treatment with 133Cs+ and 85Rb+. Plants grown in high K+ accumulate significantly less 133Cs+ and 85Rb+ than plants grown in low K+ (Figure 4B). Compared to wild-type shoots, the 85Rb+ xonxen in the hak5-1 mutant decreased under low K+ conditions, but this was not observed in roots. In the shoots in the skor3-1 mutant, the 85Rb+ concentration drastically decreases, as we observed previously, and increases slightly in the root (Figure 4B). These results confirm that the translocation of 85Rb+ from roots to shoots is reduced in the skor mutant compared to the wild type. However, this increase in root concentration was not observed in the 42K potassium uptake experiment (Figure 4A). Furthermore, the 85Rb+ translocation from roots to shoots is higher in the hak5-1 mutant than in the skor mutant. These results suggest that although 85Rb+ may be used as a tracer for potassium, the two elements may be transported differently. In contrast, the 133Cs+ concentration of roots and shoots of the hak5-1 mutant was significantly reduced. In the skor3-1 knockout mutant, the 133Cs+ concentration of roots and shoots was also significantly decreased, but the changes are less pronounced. It is interesting to note that in older plants of the skor mutants, not only the translocation of 133Cs+ from roots to shoots is reduced as observed in Figure 4A but also its concentration in the roots (Figure 4B, Table 2). Altogether, these results suggest that under low K+ conditions, root uptake of K+ and Cs+ is similar and may be affected by HAK5 expression; however, their translocation and their accumulation are different.
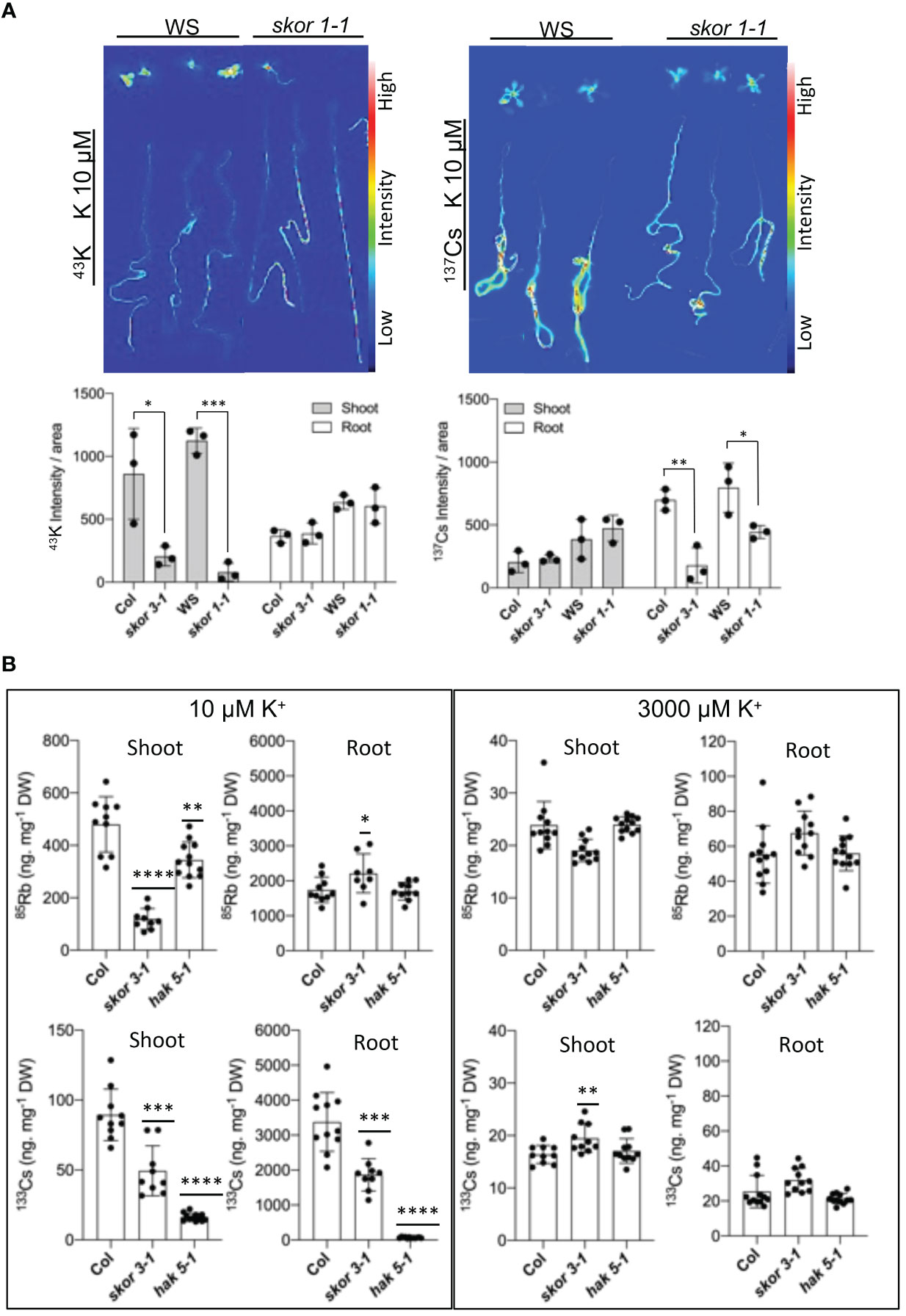
Figure 4 SKOR mutation affects K+ and Cs+ distribution in plants. (A). Differences in the distribution of K+ and Cs+ as determined by 137Cs and 42K. The roots of 12-day-old plants grown in low-K conditions (10 µM) were incubated for 2 hours in a hydroponic medium that contained 42K, 43K, or 137Cs. Data are shown as means ± SD (n = 3). Statistical significance was determined by Student’s t-test with Welch’s correction. Significant differences are between wild types and skor mutants indicated with asterisks (*P <0.05). (B). Plants 35 days old were transferred to different K+ conditions for 3 days with 1 µM CsCl and 1 µM RbCl in a hydroponic medium. By using ICP-MS, the concentrations of Cs+ and Rb+ were determined. A decrease in Rb+ concentration is observed in the shoots of skor. In addition, Cs+ concentration is reduced in both shoots and roots of the skor mutant. Data are shown as means ± SD (n = 9-12). Statistical significance was determined by Student’s t-test with Welch’s correction. Significant differences between wild types and mutants are indicated with asterisks (*P <0.05, **P = 0.0016, ***P = 0.0002, and ****P < 0.0001).
3.5 Ion circulation keeps K+ status higher in the root in the skor mutant.
There is a constant movement of potassium through the tissues and organs of plants, which is transported in both directions at the same time, upstream by the xylem and downstream by the phloem. Therefore, split root experiments were performed to determine the impact of K+ or Cs+ fluxes on the distribution of K+ and Cs+ between the roots and shoots (Figure 5, Supplementary Data 3). Split roots were cultivated in the solution containing 133Cs+ or 85Rb+ for 3 days, and subsequently collected in three parts: shoots, roots with tracer, and roots without tracer, and percentages were calculated for each part (Figure 5). The results reveal that wild-type plants showed similar distributions (%) of applied Rb+ and Cs+ in shoots and half roots (treated and untreated), indicating that both Cs+ and K+ circulate within the plant. In the skor3-1 mutant, there is a slight reduction in the total uptake of Rb+ or Cs+. In addition, less Rb+ was transported to the skor3-1 shoot, as previously observed, whereas it was similarly transferred to the untreated root as in the wild type. However, Cs+ transported to the shoots of skor3-1 was close to that of the wild type, whereas Cs+ transferred from the shoots to the untreated roots was reduced compared to the wild-type plants. In contrast, in the hak5-1 mutant, despite a slight decrease in the total Rb+ absorption, the Cs+ uptake has been dramatically reduced. In the presence of Rb+, hak5-1 plants exhibit broadly comparable distributions (%) to wild-type plants; however, this distribution is significantly altered when Cs+ is applied. In line with previous experiments, the percentage of Cs+ transported from the treated roots to the shoots was dramatically increased (70%) compared to the wild type (48%), but the percentage transported from the shoots to the untreated roots remained unchanged. In the present study, we found that when plants are exposed to low K+ conditions, HAK5 plays a critical role in the uptake and distribution of Cs+ and that SKOR participates in the distribution of Cs+ from the shoot to the roots.
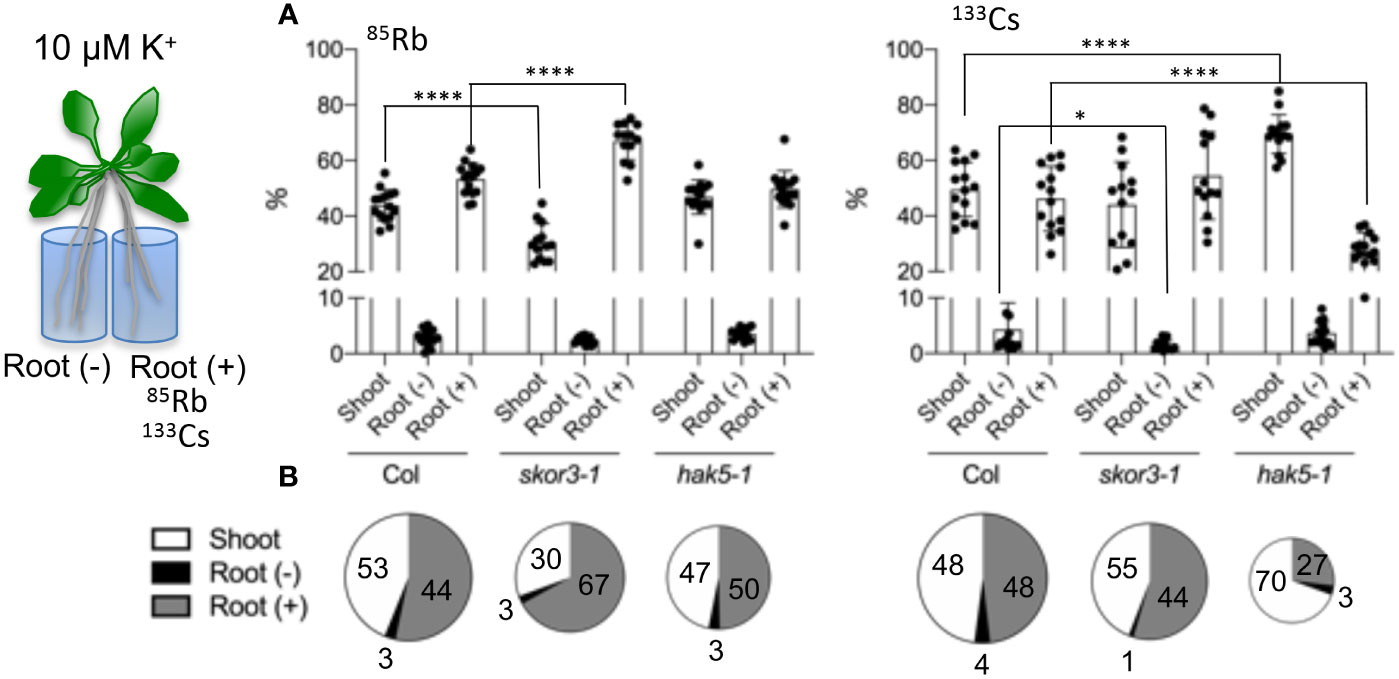
Figure 5 An analysis of the Rb+ and Cs+ distribution in mutants. A long-term study of ion distribution in plants. skor did not exhibit similar effects on the translocation of 133Cs and 85Rb from root to shoot. Plant roots 35 days old are divided into two parts, and one part is incubated with 1µM 133Cs or 1 µM 85Rb (Root +) for 3 days. The number of ions in the three parts was determined using ICP-MS. (A). Plant distribution (%) of 85Rb and 133Cs among the three parts of the plant: shoot, root (+) incubated with 1µM 133Cs or 1µM 85Rb, and root (-). (B). An illustration of the distribution of the data from A in a pie chart. According to the pie chart, the size corresponds to the total amount of ions absorbed. Data are shown as means ± SD (n=11-15). Statistical significance was determined by Student’s t-test with Welch’s correction. Significant differences between wild types and mutants are indicated with asterisks (*P =0.0457 and ****P <0.0001).
4 Discussion
Proper control of ion uptake is one of the essential elements for plant growth. It is nevertheless important to note that the regulation of ion transport is rather complex, particularly as a result of the redundancy of genes. For example, 71 K+ channels and transporters have already been identified in Arabidopsis thaliana (Mäser et al., 2001; Véry and Sentenac, 2003; Amtmann et al., 2018; Wang and Wu, 2013) and divided into six distinct gene families consisting of three channel families and three transporter families (Gierth and Mäser, 2007; Chanroj et al., 2012; Gomez-Porras et al., 2012). K+ is absorbed in the root by the epidermis and root hairs. In order to reach the shoot, it must pass through several layers of root cells in order to be transported into the xylem. In order to achieve this process, numerous K+ channels and transporters must be present in the epidermis and xylem, which ensures that K+ can flow from the epidermis to the xylem. AKT1 and HAK5, two high-affinity K+ transport proteins, have been characterized (Hirsch et al., 1998; Rubio et al., 2008), and their regulation mechanisms have been extensively investigated (Rubio et al., 2010; Nieves-Cordones et al., 2014; Feng et al., 2021; Huimin et al., 2021). Compared to K+ uptake, little has been known about K+ transport in the stele since the identification of SKOR (Gaymard et al., 1998) and NRT1.5/NPF7.3 (Li et al., 2017). Nevertheless, NRT1.5/NPF7.3 is thought to maintain root-to-shoot translocation of K+ only under limited availability (Drechsler et al., 2015; Meng et al., 2016; Li et al., 2017). During K+ deficiency, HAK5 exhibits crucial roles in K+ uptake and transport, while SKOR plays a prominent role in root-to-shoot K+ translocation. However, the details of the relationships between K+ uptake, translocation, and recycling are not completely understood. Accordingly, we investigated the molecular and physiological responses of Arabidopsis wild-type (Col and WS), hak5, and skor T-DNA insertion lines to various K+, Cs+, and Rb+ conditions.
4.1 HAK5 expression is regulated by internal K+ distribution
There is a close relationship between root uptake and xylem K+ loading. There is, however, little understanding of the molecular mechanisms involved in the coordination of these two processes. In this study, a correlation was found between the level of HAK5 expression and the concentration of K+ measured in roots. When the concentration of K+ in roots decreases, the expression of HAK5 increases (Figure 2). In addition, we observed that HAK5 was down-regulated in skor mutants. In these mutants, the translocation of K+ from root to shoot is impaired, resulting in a significant reduction in shoot K+ concentration (Figures 2, 4). Altogether, these results suggest the hypothesis that xylem K+ loading regulates K+ uptake through the regulation of HAK5 expression. Changes in the expression of HAK5 have also been observed in other mutants affected by K+ translocation including nrt1.5-5 (Drechsler et al., 2015; Meng et al., 2016) and in cpr5 mutants (Borghi et al., 2011). This transcriptional regulation provides a regulatory pathway for long-distance K+ signaling during low K+ stress and demonstrates that uptake and translocation of K+ are closely coordinated. It is noteworthy that AKT1 is not involved in this process as its role in K+ resorption from xylem vessels has only been observed under conditions in which K+ is sufficient (Nieves-Cordones et al., 2019). By combining genetics, radiotracer experiments, and ion concentration measurements, we have established that K+ tissue distribution tightly controls HAK5 expression.
4.2 SKOR does not transport Cs+ but contributes to Cs+ uptake through HAK5
The chemical properties of Cs+ are similar to those of K+, and early studies indicate that the mechanisms underlying Cs+ and K+ uptake in plants are similar (Collander, 1941; Epstein and Hagen, 1952). Furthermore, the level of K+ supply also impacts the contribution of each pathway to the uptake of Cs+. To date, Cs+ uptake has been demonstrated for several K+ transporters, including HAK5 (Nieves-Cordones et al., 2017; Rai et al., 2017; Rai and Kawabata, 2020). Additionally, SKOR, which mediates K+ xylem loading, has been suggested as a possible candidate for proteins that mediate Cs+ translocation from root to shoot. The results of our experiments clearly showed that K+ and Cs+ translocation rates were affected differentially in skor mutants in comparison to wild types (Figure 4). Electrophysiology studies indicate that the ratio of Cs+ to K+ permeability of the SKOR channel in Arabidopsis is 0.15 (Gaymard et al., 1998; Johansson et al., 2006). Thus, the Cs+ permeability of SKOR is significantly lower than the K+ permeability. In this study, we found that 137Cs uptake is strongly decreased in roots but not in shoots under low K+ conditions in both skor mutants. Thus, it is tempting to speculate that this decrease is caused by the down-regulation of HAK5 expression. Previous studies have demonstrated that Cs+ blocks AKT1 activity, so the role of AKT1 in this process can be excluded (Adams et al., 2019).
4.3 Difference of Rb+ and Cs+ movement
A difference in skor K+ transport was observed in Figure 4 when comparing results from short-term (2 hours) experiments on young plants and long-term (3 days) experiments on old plants. However, a difference in Cs+ accumulation in roots was only observed in old plants. This suggests that Cs+ transport to the roots may take longer than K+ or that the activity of the transporters may differ with plant age. The analyses of the relative distribution of Rb+ and Cs+ using split root experiments (Figure 5) indicated that the total amount of Rb+ decreased similarly in skor and hak5 mutants. In accordance with our radiotracer experiments, both mutants exhibit differential distributions between shoots and roots. Interestingly, there is an increase in the amount of Rb+ in the skor mutant that coincides with a decrease in HAK5 expression in the roots. The results of this study confirm the role played by SKOR in regulating K+ uptake and the existence of cross-regulation with HAK5 at low K+ levels. Alternatively, both mutants exhibit a different pattern of Cs+ distribution and amount. In the hak5 mutant, the Cs+ amount was drastically reduced and distributed differently compared to skor and Col. A hypothetical model illustrating the regulation mechanism of HAK5 expression in skor is proposed in Figure 6. Our results not only show that HAK5 plays a major role in Cs+ uptake in roots under low K+ conditions but also demonstrate that other transporters are involved in Cs+ uptake, translocation, and recycling. Interestingly, two ATP-binding cassette (ABC) proteins, ABCG37 and ABCG33, have been identified as high-affinity Cs+ transporters which are K+-independent (Ashraf et al., 2021). However, further research is needed to evaluate their contribution to Cs+ uptake compared to HAK5 under low K+ conditions. Among the candidates likely to be involved in the root-growth translocation of Cs+, the K+ transporter KUP7 is thought to be involved in K+ uptake and accumulation in the xylem parenchyma and affects K+ loading into the xylem (Han et al., 2016; Šustr et al., 2020). It is known that NRT1.5 is involved in potassium xylem loading, but its discrimination between Cs+ and K+ is unknown (Drechsler et al., 2015; Li et al., 2017). On the other hand, ZIFL2 (Zinc-induced facilitator-like transporter 2) plays a role in the partitioning of Cs+. A mutation in ZIFL2 results in the accumulation of Cs+ in shoots (Remy et al., 2015). KT/HAK/KUP transporters have recently been identified to accumulate potassium in rice xylem parenchyma, such as OsHAK5, which contributes to both potassium uptake and xylem loading (Yang et al., 2014). However, this transporter is not involved in Cs accumulation. Furthermore, it cannot be excluded that HAK5 also contributes to the loading of K+ and Cs+ into the xylem. Finally, we investigated the role of KUP6 in K+ translocation for Cs+ transport by using a kup6 T-DNA insertion mutant. Nevertheless, the kup6 single mutant showed no significant difference in Cs+ translocation (data not shown).
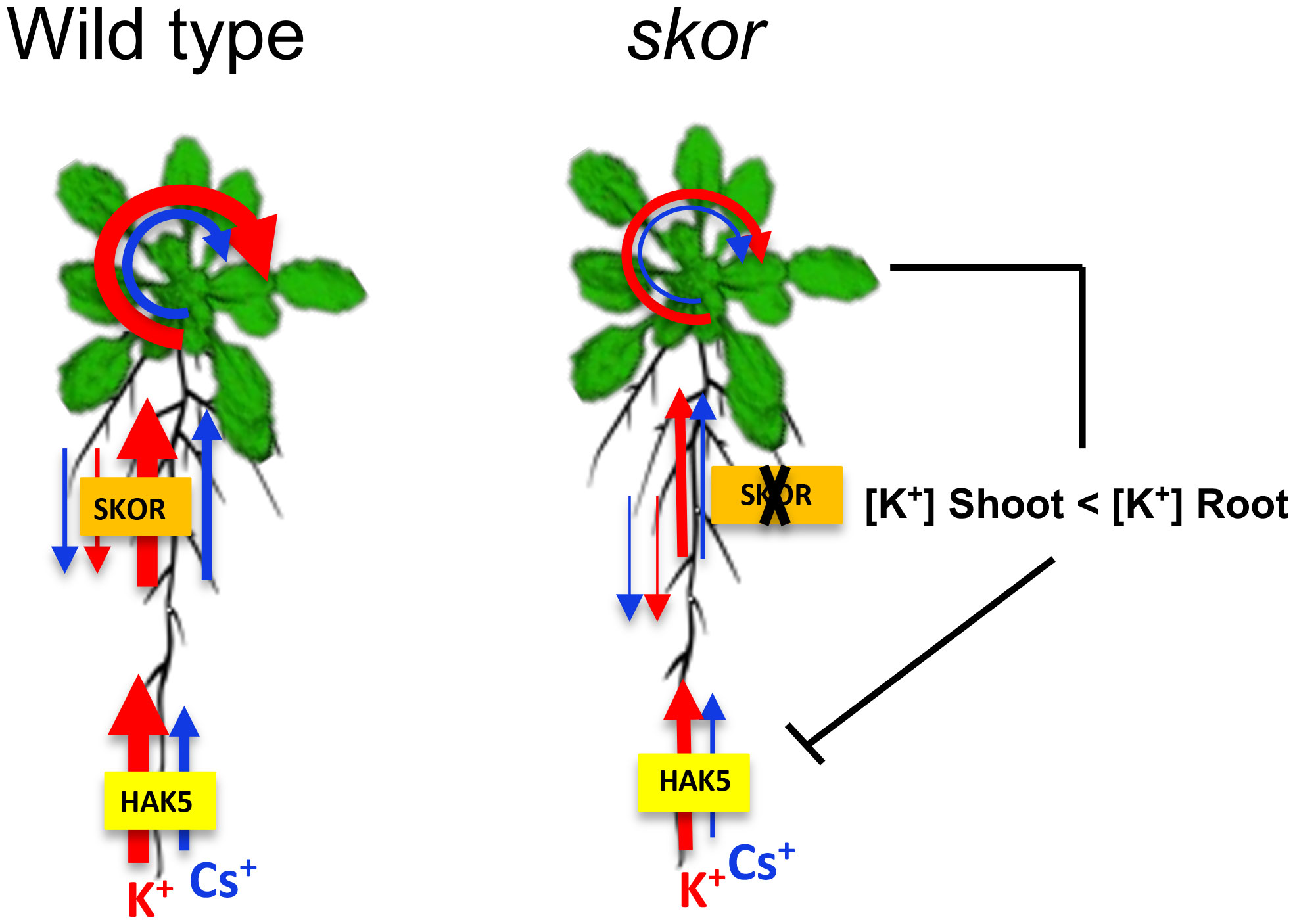
Figure 6 Hypothetical model of the regulation of HAK5 in skor mutants under low K+ conditions. This schematic representation illustrates the hypothetical role of SKOR on K+ and Cs+ uptake and translocation in plants. The size of the arrows represents K+ (in red) and Cs+ (in blue) fluxes. A lack of the SKOR gene reduced the translocation of K+ from root to shoot. Since potassium transport from shoot to root is the same in WT and skor, the K+ in the root is relatively higher in skor. As a result of the imbalance in K+ concentrations between shoots and roots, HAK5 was repressed in the roots of skor mutants affecting both K+ and Cs+ absorption. There is, however, a difference in the translocation and distribution of K+ and Cs+.
Finally, we present new insights regarding long-distance K+ and Cs+ transport, which may contribute to increased fertilizer efficiency and application for phytoremediation. Furthermore, our results indicate that modifying only the surface transporters in the root is not sufficient to improve fertilizer efficiency or facilitate phytoremediation. Further research is required to understand the regulatory mechanism of root surfaces and internal transporters in coordination with each other, which is crucial for the above applications.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
NL and AV designed the research. NL, SK, LM, NV, SC, TN, and JF performed the research. NL and SK analyzed the data and wrote the paper with the contribution of all authors. All authors contributed to the article and approved the submitted version.
Funding
The authors received financial support from the French “Programme Investissement d’Avenir” (ANR 11-RSNR-0005 DEMETERRES, https://anr.fr/ProjetIA-11-RSNR-0005), the French Alternative Energies and Atomic Energy Commission, and JSPS KAKENHI Grant Number 15K18764, and was supported by ERAN I-19-02, F-20-13, F-21-10.
Acknowledgments
The “42K and 43K” was supplied through the Supply Platform of Short-lived Radioisotopes, supported by the JSPS Grant-in-Aid for Scientific Research on Innovative Areas, Grant Number 16H06278.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1040118/full#supplementary-material
References
Adams, E., Abdollahi, P., Shin, R. (2013). Cesium inhibits plant growth through jasmonate signaling in arabidopsis thaliana. Int J Mol Sci. 14 (3),4545–4559. doi: 10.3390/ijms14034545
Adams, E., Miyazaki, T., Saito, S., Uozumi, N., Shin, R. (2019). Cesium inhibits plant growth primarily through reduction of potassium influx and accumulation in arabidopsis. Plant Cell Physiol. 60 (1), 63–76. doi: 10.1093/pcp/pcy188
Ahn, S. J., Shin, R., Schachtman, D. P. (2004). Expression of KT / KUP genes in arabidopsis and the role of root hairs in k+ uptake. Plant Physiol. 134 (3), 1135–1145. doi: 10.1104/pp.103.034660
Ai, P., Sun, S., Zhao, J., Fan, X., Xin, W., Guo, Q., et al. (2009). Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J. 57 (5), 798–809. doi: 10.1111/j.1365-313X.2008.03726.x
Amtmann, A., Armengaud, P., Volkov, V. (2018). “Potassium nutrition and salt stress,” in Annual plant reviews online. Ed. Roberts, J. A. (Chichester, UK: John Wiley & Sons, Ltd), 328–379. doi: 10.1002/9781119312994.apr0151
Ashraf, M. A., Akihiro, T., Ito, K., Kumagai, S., Sugita, R., Tanoi, K., et al. (2021). ATP binding cassette proteins ABCG37 and ABCG33 function as potassium-independent cesium uptake carriers in arabidopsis roots. Mol. Plant 14, 664–667. doi: 10.1016/j.molp.2021.02.002
Bonnot, C., Pinson, B., Clément, M., Bernillon, S., Chiarenza, S., Kanno, S., et al. (2016). A chemical genetic strategy identify the PHOSTIN, a synthetic molecule that triggers phosphate starvation responses in arabidopsis thaliana. New Phytol. 209, 161–176. doi: 10.1111/nph.13591
Borghi, M., Rus, A., Salt, D. E. (2011). Loss-of-Function of constitutive expresser of pathogenesis related Genes5 affects potassium homeostasis in arabidopsis thaliana. PloS One 6, e26360. doi: 10.1371/journal.pone.0026360
Chanroj, S., Wang, G., Venema, K., Zhang, M. W., Delwiche, C. F., Sze, H. (2012). Conserved and diversified gene families of monovalent cation/h(+) antiporters from algae to flowering plants. Front. Plant Sci. 3. doi: 10.3389/fpls.2012.00025
Collander, R. (1941). SELECTIVE ABSORPTION OF CATIONS BY HIGHER PLANTS. Plant Physiol. 16, 691–720. doi: 10.1104/pp.16.4.691
Drechsler, N., Zheng, Y., Bohner, A., Nobmann, B., von Wirén, N., Kunze, R., et al. (2015). Nitrate-dependent control of shoot K homeostasis by the nitrate Transporter1/Peptide transporter family member NPF7.3/NRT1.5 and the stelar k+ outward rectifier SKOR in arabidopsis. Plant Physiol. 169, 2832–2847. doi: 10.1104/pp.15.01152
Dreyer, I., Gomez-Porras, J. L., Riedelsberger, J. (2017). The potassium battery: a mobile energy source for transport processes in plant vascular tissues. New Phytol. 216 (4), 1049–1053. doi: 10.1111/nph.14667
Epstein, E., Hagen, C. E. (1952). A KINETIC STUDY OF THE ABSORPTION OF ALKALI CATIONS BY BARLEY ROOTS. Plant Physiol. 27, 457–474. doi: 10.1104/pp.27.3.457
Feng, C.-Z., Luo, Y.-X., Wang, P.-D., Gilliham, M., Long, Y. (2021). MYB77 regulates high-affinity potassium uptake by promoting expression of HAK5. New Phytol. 232, 176–189. doi: 10.1111/nph.17589
Gaymard, F., Pilot, G., Lacombe, B., Bouchez, D., Bruneau, D., Boucherez, J., et al. (1998). Identification and disruption of a plant shaker-like outward channel involved in k+ release into the xylem sap. Cell 94 (5), 647–655. doi: 10.1016/S0092-8674(00)81606-2
Genies, L., Martin, L., Kanno, S., Chiarenza, S., Carasco, L., Camilleri, V., et al. (2021). Disruption of AtHAK/KT/KUP9 enhances plant cesium accumulation under low potassium supply. Physiol Plant 173 (3), 1230–1243. doi: 10.1111/ppl.13518
Gierth, M., Mäser, P. (2007). Potassium transporters in plants–involvement in k+ acquisition, redistribution and homeostasis. FEBS Lett. 581, 2348–2356. doi: 10.1016/j.febslet.2007.03.035
Gierth, M., Mäser, P., Schroeder, J. I. (2005). The potassium transporter AtHAK5 functions in k+ deprivation-induced high-affinity k+ uptake and AKT1 k+ channel contribution to k+ uptake kinetics in arabidopsis roots. Plant Physiol. 137 (3), 1105–1114. doi: 10.1104/pp.104.057216
Gomez-Porras, J. L., Riaño-Pachón, D. M., Benito, B., Haro, R., Sklodowski, K., Rodríguez-Navarro, A., et al. (2012). Phylogenetic analysis of k(+) transporters in bryophytes, lycophytes, and flowering plants indicates a specialization of vascular plants. Front. Plant Sci. 3. doi: 10.3389/fpls.2012.00167
Hampton, C. R., Bowen, H. C., Broadley, M. R., Hammond, J. P., Mead, A., Payne, K. A., et al. (2004). Cesium toxicity in arabidopsis. Plant Physiol. 136 (3), 3824–3837. doi: 10.1104/pp.104.046672
Han, M., Wu, W., Wu, W.-H., Wang, Y. (2016). Potassium transporter KUP7 is involved in k(+) acquisition and translocation in arabidopsis root under k(+)-limited conditions. Mol. Plant 9, 437–446. doi: 10.1016/j.molp.2016.01.012
Hirsch, R. E., Lewis, B. D., Spalding, E. P., Sussman, M. R. (1998). A role for the AKT1 potassium channel in plant nutrition. Science 280, 918–921. doi: 10.1126/science.280.5365.918
Hong, J. P., Takeshi, Y., Kondou, Y., Schachtman, D. P., Matsui, M., Shin, R. (2013). Identification and characterization of transcription factors regulating arabidopsis HAK5. Plant Cell Physiol. 54 (9), 1478–1490. doi: 10.1093/pcp/pct094
Huimin, R., Hussain, J., Wenjie, L., Fenyong, Y., Junjun, G., Youhan, K., et al. (2021). The expression of constitutively active CPK3 impairs potassium uptake and transport in arabidopsis under low k+ stress. Cell Calcium 98, 102447. doi: 10.1016/j.ceca.2021.102447
Johansson, I., Wulfetange, K., Porée, F., Michard, E., Gajdanowicz, P., Lacombe, B., et al. (2006). External k+ modulates the activity of the arabidopsis potassium channel SKOR via an unusual mechanism. Plant J. 46, 269–281. doi: 10.1111/j.1365-313X.2006.02690.x
Kim, M. J., Ruzicka, D., Shin, R., Schachtman, D. P. (2012). The arabidopsis AP2/ERF transcription factor RAP2.11 modulates plant response to low-potassium conditions. Mol. Plant 5 (5), 1042–1057. doi: 10.1093/mp/sss003
Leigh, R. A., Wyn Jones, R. G. (1984). A hypothesis relating critical potassium concentration for growth to the distribution. New Phytol. 97, 1–13. doi: 10.1111/j.1469-8137.1984.tb04103.x
Li, J., Wu, W.-H., Wang, Y. (2017). Potassium channel AKT1 is involved in the auxin-mediated root growth inhibition in arabidopsis response to low k+ stress. J. Integr. Plant Biol. 59, 895–909. doi: 10.1111/jipb.12575
Livak, K. J., Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Maathuis, F. J. (2009). Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 12 (3), 250–258. doi: 10.1016/j.pbi.2009.04.003
Mäser, P., Thomine, S., Schroeder, J. I., Ward, J. M., Hirschi, K., Sze, H., et al. (2001). Phylogenetic relationships within cation transporter families of arabidopsis. Plant Physiol. 126, 1646–1667. doi: 10.1104/pp.126.4.1646
Meijering, E., Jacob, M., Sarria, J.-C. F., Steiner, P., Hirling, H., Unser, M. (2004). Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry 58A, 167–176. doi: 10.1002/cyto.a.20022
Meng, S., Peng, J.-S., He, Y.-N., Zhang, G.-B., Yi, H.-Y., Fu, Y.-L., et al. (2016). Arabidopsis NRT1.5 mediates the suppression of nitrate starvation-induced leaf senescence by modulating foliar potassium level. Mol. Plant 9, 461–470. doi: 10.1016/j.molp.2015.12.015
Nieves-Cordones, M., Alemán, F., Martínez, V., Rubio, F. (2014). K+ uptake in plant roots. the systems involved, their regulation and parallels in other organisms. J. Plant Physiol. 171 (9), 688–695. doi: 10.1016/j.jplph.2013.09.021
Nieves-Cordones, M., Lara, A., Ródenas, R., Amo, J., Rivero, R., Martínez, V., et al. (2019). Modulation of k+ translocation by AKT1 and AtHAK5 in arabidopsis plants. Plant Cell Environ. 42 (8), 2357–2371. doi: 10.1111/pce.13573
Nieves-Cordones, M., Miller, A. J., Alemán, F., Martínez, V., Rubio, F. (2008). A putative role for the plasma membrane potential in the control of the expression of the gene encoding the tomato high-affinity potassium transporter HAK5. Plant Mol. Biol. 68 (6), 521–532. doi: 10.1007/s11103-008-9388-3
Nieves-Cordones, M., Mohamed, S., Tanoi, K., Kobayashi, N. I., Takagi, K., Vernet, A., et al. (2017). Production of low-cs(+) rice plants by inactivation of the k(+) transporter OsHAK1 with the CRISPR-cas system. Plant J. 92, 43–56. doi: 10.1111/tpj.13632
Pyo, Y. J., Gierth, M., Schroeder, J. I., Cho, M. H. (2010). High-affinity k+ transport in arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol. 153 (2), 863–875. doi: 10.1104/pp.110.154369
Qi, Z., Hampton, C. R., Shin, R., Barkla, B. J., White, P. J., Schachtman, D. P. (2008). The high affinity K + transporter AtHAK5 plays a physiological role in planta at very low K + concentrations and provides a caesium uptake pathway in arabidopsis. J. Exp. Bot. 59 (3), 595–607. doi: 10.1093/jxb/erm330
Ragel, P., Raddatz, N., Quintero, F., Pardo, J., Leidi, E. (2019). Regulation of K + nutrition in plants. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00281
Ragel, P., Ródenas, R., García-Martín, E., Andrés, Z., Villalta, I., Nieves-Cordones, M., et al. (2015). CIPK23 regulates HAK5-mediated high-affinity k+ uptake in arabidopsis roots. Plant Physiol. 169 (December), 1401. doi: 10.1104/pp.15.01401
Rai, H., Kawabata, M. (2020). The dynamics of radio-cesium in soils and mechanism of cesium uptake into higher Plants : newly elucidated mechanism of cesium uptake into rice plants 11, May, 80–90. doi: 10.3389/fpls.2020.00528
Rai, H., Yokoyama, S., Satoh-Nagasawa, N., Furukawa, J., Nomi, T., Ito, Y., et al. (2017). Cesium uptake by rice roots largely depends upon a single gene, HAK1, which encodes a potassium transporter. Plant Cell Physiol. 58 (11), 2041. doi: 10.1093/pcp/pcx137
Remy, E., Cabrito, T. R., Batista, R. A., Teixeira, M. C., Sá-Correia, I., Duque, P. (2015). The major facilitator superfamily transporter ZIFL2 modulates cesium and potassium homeostasis in arabidopsis. Plant Cell Physiol. 56, 148–162. doi: 10.1093/pcp/pcu157
Rengel, Z., Marschner, P. (2005). Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol. 168 (2), 305–312. doi: 10.1111/j.1469-8137.2005.01558.x
Rubio, F., Alemán, F., Nieves-Cordones, M., Martínez, V. (2010). Studies on arabidopsis athak5, atakt1 double mutants disclose the range of concentrations at which AtHAK5, AtAKT1 and unknown systems mediate k+ uptake. Physiol. Plant. 139 (2), 220–228. doi: 10.1111/j.1399-3054.2010.01354.x
Rubio, F., Fon, M., Ródenas, R., Nieves-Cordones, M., Alemán, F., Rivero, R. M., et al. (2014). A low k+ signal is required for functional high-affinity k+ uptake through HAK5 transporters. Physiol. Plant. 152 (3), 558–570. doi: 10.1111/ppl.12205
Rubio, F., Nieves-Cordones, M., Alemán, F., Martínez, V. (2008). Relative contribution of AtHAK5 and AtAKT1 to k+ uptake in the high-affinity range of concentrations. Physiol. Plant 134, 598–608. doi: 10.1111/j.1399-3054.2008.01168.x
Schachtman, D. P. (2000). Molecular insights into the structure and function of plant k+ transport mechanisms. Biochim. Biophys. Acta - Biomembranes 1465 (1–2), 127–139. doi: 10.1016/S0005-2736(00)00134-6
Shin, R., Berg, R. H., Schachtman, D. P. (2005). Reactive oxygen species and root hairs in arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 46 (8), 1350–1357. doi: 10.1093/pcp/pci145
Šustr, M., Doksanská, T., Doležalová, B., Soukup, A., Tylová, E. (2020). 134Cs uptake and growth at various cs+ and k+ levels in arabidopsis AtKUP7 mutants. Plants (Basel) 9, E1525. doi: 10.3390/plants9111525
Véry, A., Nieves-cordones, M., Daly, M., Khan, I. (2014). Molecular biology of K + transport across the plant cell membrane : what do we learn from comparison between plant species? J. Plant Physiol. 171 (9), 748–769. doi: 10.1016/j.jplph.2014.01.011
Véry, A.-A., Sentenac, H. (2003). MOLECULAR MECHANISMS AND REGULATION OF K + TRANSPORT IN HIGHER PLANTS. Annu. Rev. Plant Biol. 54, 575–603. doi: 10.1146/annurev.plant.54.031902.134831
Walker, D. J., Leigh, R. A., Miller, A. J. (1996). Potassium homeostasis in vacuolate plant cells (cytosolic k+/cytosolic pH/plant vacuole). Proc Natl Acad Sci U S A 93 (19), 10510–10514. doi: 10.1073/pnas.93.19.10510
Wang, Y., Wu, W.-H. (2013). Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 64, 451–476. doi: 10.1146/annurev-arplant-050312-120153
Wegner, L. H., De Boer, A. H. (1997). Properties of two outward-rectifying channels in root xylem parenchyma cells suggest a role in k+ homeostasis and long-distance signaling. Plant Physiol. 115 (4), 1707–1719. doi: 10.1104/pp.115.4.1707
Yang, T., Zhang, S., Hu, Y., Wu, F., Hu, Q., Chen, G., et al. (2014). The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 166, 945–959. doi: 10.1104/pp.114.246520
Keywords: Arabidopsis thaliana, HAK5, SKOR, potassium, cesium, transporter
Citation: Kanno S, Martin L, Vallier N, Chiarenza S, Nobori T, Furukawa J, Nussaume L, Vavasseur A and Leonhardt N (2023) Xylem K+ loading modulates K+ and Cs+ absorption and distribution in Arabidopsis under K+-limited conditions. Front. Plant Sci. 14:1040118. doi: 10.3389/fpls.2023.1040118
Received: 08 September 2022; Accepted: 28 June 2023;
Published: 22 September 2023.
Edited by:
Jose M. Mulet, Universitat Politècnica de València, SpainReviewed by:
Reyes Rodenas, UMR5546 Laboratoire de Recherche en Sciences Vegetales (LRSV), FranceSho Nishida, Saga University, Japan
Copyright © 2023 Kanno, Martin, Vallier, Chiarenza, Nobori, Furukawa, Nussaume, Vavasseur and Leonhardt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nathalie Leonhardt, bmF0aGFsaWUubGVvbmhhcmR0QGNlYS5mcg==
 Satomi Kanno
Satomi Kanno Ludovic Martin
Ludovic Martin Natacha Vallier
Natacha Vallier Serge Chiarenza1
Serge Chiarenza1 Tatsuya Nobori
Tatsuya Nobori Jun Furukawa
Jun Furukawa Laurent Nussaume
Laurent Nussaume Alain Vavasseur
Alain Vavasseur Nathalie Leonhardt
Nathalie Leonhardt